User login
Morcellation in the News
- Philadelphia hospital restricts 'morcellation' procedure Wall Street Journal, February 20, 2014
- Society of Gynecologic Surgeons releases position statement on morcellation
- Uterine surgical technique is linked to abnormal growths and cancer spread New York Times, February 6, 2014
- Evaluating the risks of electric uterine morcellation JAMA, February 6, 2014. doi:10.1001/jama.2014.1093
- Critics of fibroid removal procedure question risks it may pose for women with undetected uterine cancer JAMA, February 6, 2014. doi:10.001/jama.2014.27
Uterine leiomyomata, also known as fibroids, are the most common pelvic tumor among women. In some women fibroids may be associated with no symptoms. In these cases, the fibroids are often managed expectantly. For many women, however, fibroids cause problems, including heavy uterine bleeding, pelvic pain, and infertility. In these cases removal of the fibroids, either by myomectomy or by hysterectomy, is a common treatment. In the United States, the two most common reasons for performing a hysterectomy are uterine fibroids or abnormal uterine bleeding.1
New minimally invasive gynecologic surgery (MIGS) technology and advanced surgical skills now permit the removal of very large uterine fibroids through small laparoscopic surgery incisions. There are many advantages to MIGS removal of uterine fibroids. The reported benefits include2:
- no large abdominal laparotomy incision
- faster healing and recovery from surgery
- less postoperative pain
- lower risk of surgical site infection
- more rapid return to full activities.
Related Article: Update: Minimally invasive surgery Amy Garcia, MD (April 2012)
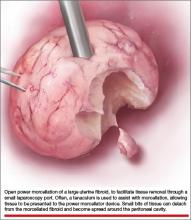
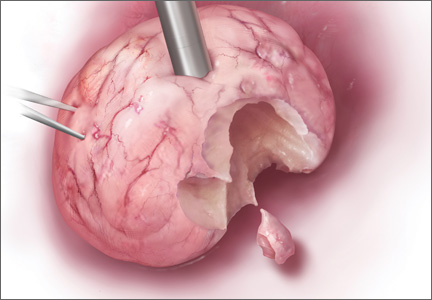
Laparoscopic surgeons often select open power morcellation of the fibroid or fibroid-laden uterus to remove the mass of tissue through a small laparoscopy port. In current MIGS practice, power morcellation typically occurs outside of a closed environment, such as a bag. Open power morcellation of uterine tissue has an important pitfall. This practice permits small bits of tissue to spread around the peritoneal cavity (FIGURE). Given the enhanced growth potential of uterine tumor cells, the postoperative growth of nodules of tissue generated by morcellation is a worrisome possibility.
Morcellation of uterine fibroids and the possibility of causing disseminated leiomyomatosis
Uterine fibroids are clonal tumors that arise from a somatic mutation in a precursor cell. Tumors with clonal somatic mutations have enhanced growth potential because normal mechanisms that control cell growth have been disrupted at the level of cell DNA. Many uterine fibroids demonstrate an abnormal karyotype. Fibroids with a karyotype showing an exchange of genetic material between chromosomes 12 and 14 (t(12;14) (q13-15, q23-24)) are often large.3 The t(12;14) (q13-15, q23-24) translocation is associated with an increased expression of HMGIC, a gene that stimulates cell growth.4
Open morcellation of uterine fibroids may result in the dispersion of bits of tissue around the peritoneal cavity and surgical ports. If the fibroid contains genetic mutations that significantly increase the growth potential of fibroid cells, fibroid nodules may grow postoperatively in the pelvis, abdomen, and surgical ports.5–15 Investigators have called these disseminated nodules of fibroid tumor, “morcellomas.”16
Related Article: Ins and outs of straight-stick laparoscopic myomectomy James Robinson, MD, MS, and Gaby Moawad, MD (September 2012)
Ordulu and colleagues recently reported a case of a woman with uterine fibroids who underwent supracervical hysterectomy using morcellation and 7 years later developed disseminated peritoneal leiomyomatosis (DPL) requiring a second operation.17 Five anatomically distinct DPL masses were karyotyped and showed cytogenetic abnormalities often observed in uterine fibroids with enhanced growth potential, including the t(12;14) (q13-15, q23-24) chromosome translocation. As noted above, the t(12;14) (q13-15, q23-24) translocation is associated with enhanced growth of fibroid tumor cells due, in part, to overexpression of HMGIC.4
Morcellation of occult uterine leiomyosarcomas
In some cases, a uterine tumor thought to be a fibroid is surgically excised, but histologic analysis shows that the tumor is actually a uterine leiomyosarcoma. In surgical case series, approximately 0.08% to 0.13% of uterine masses thought to be due to fibroids are proven by postoperative histology to be a uterine leiomyosarcoma.18–20 Most leiomyosarcomas are Stage I,21 but if the sarcoma tissue is open power morcellated in the peritoneal cavity, bits of tissue can be spread, upstaging the tumor and worsening the prognosis.22
In one retrospective study of women with leiomyosarcoma, the 5-year estimated survival was 73% if the tumor was removed without morcellation and 46% if the tumor was removed using morcellation.22 If a “fibroid” or “fibroid uterus” is power morcellated, but the final histology reveals leiomyosarcoma, surgical re-exploration will be recommended to detect any spread of cancer, and chemotherapy may be necessary if tumor spread is detected.23,24
Many experts recommend a standardized preoperative evaluation of all cases of presumed uterine fibroid tumors where open power morcellation of tissue is planned. Recommended preoperative studies include25:
- imaging studies such as MRI or sonography,
- endometrial biopsy in cases with abnormal uterine bleeding
- cervical cytology.
These studies are recommended because they may identify an occult cervical or endometrial cancer or precancer. If the preoperative evaluation detects an occult cervical or endometrial cancer it is best to avoid open power morcellation of the uterus. These standard preoperative studies are unlikely to detect an occult leiomyosarcoma. There are currently no established imaging studies that can reliably detect a uterine sarcoma preoperatively,26,27 and endometrial biopsy has a low sensitivity for detecting leiomyosarcoma.
Related Article: Tips and techniques for robot-assisted laparoscopic myomectomy Arnold P. Advincula, MD, and Bich-Van Tran, MD (Surgical Technique, August 2013)
During the surgical consent process the issue of power morcellating an occult leiomyosarcoma and the consequences of causing the spread of cancer cells should be discussed with the patient. The patient may decide that the small risk of power morcellating an occult leiomyosarcoma is sufficiently worrisome that she would prefer an alternative procedure. These alternatives include abdominal hysterectomy, vaginal hysterectomy, or a hybrid operation involving removal of the uterine tissue through a mini-laparotomy or colpotomy incision.
Morcellation in a bag and removal of the bag through an abdominal or vaginal incision
Open power morcellation of uterine tumors creates the potential pitfall of spreading cells with enhanced growth potential around the peritoneal cavity. One approach to reducing the pitfall of open power morcellation is to place the uterine tumor in a bag, hand morcellate the tissue within the bag, and remove the bag and tissue through an abdominal incision (mini-laparotomy) or a colpotomy.28,29
If transabdominal removal of the tumor tissue is planned, the surgical port incision typically would need to be enlarged to permit hand morcellation and removal of the tissue. If a supracervical hysterectomy is planned, it may be difficult to remove a very large tumor through a small colpotomy.
Development of new technology
A major advance in gynecology would be the development of imaging studies that would permit the identification of uterine leiomyosarcomas prior to hysterectomy using open power morcellation. A preliminary study reported that diffusion-weighted imaging may be capable of differentiating leiomyosarcomas and leiomyomas30 but this study would require replication in multiple centers before it could be reliably utilized in surgical planning.
Another advance would be to develop power morcellation technology that occurs within a bag, with the removal of the bag and the enclosed morcellated tumor through a laparoscopy port. Morcellation of tissue within a bag would reduce the risk of spreading bits of tumor tissue around the peritoneal cavity.
The pitfalls of open morcellation are less likely to be clinically significant when surgery is planned for women who are aged 35 years and younger because leiomyosarcoma rarely occurs in young women. The pitfalls of open morcellation are more likely to occur when the woman is perimenopausal or postmenopausal with a very large uterus. This is because the incidence of uterine leiomyosarcoma increases with age, with the diagnosis often being made between 40 and 60 years of age.21,31
Open power morcellation has enhanced the ability of surgeons to remove large tumors through small incisions. The benefits to the patient are faster healing, faster postoperative recovery, and more rapid return to full activities. Hundreds of thousands of women have benefited from these advances in gynecologic surgery.
However, for an occasional woman, open power morcellation may be associated with the intraperitoneal spread of an occult leiomyosarcoma. This may result in the upstaging of the cancer and may cause premature death. The benefits and risks of open power morcellation need to be weighed carefully with each woman.
INSTANT POLL
Do you think that open power morcellation of uterine tumors thought to be fibroids can result in the dissemination of cancer cells throughout the peritoneal cavity, should an occult leiomyosarcoma be present?
Tell us—at rbarbieri@frontlinemedcom.com. Please include your name and city and state.
- Wright JD, Herzog TJ, Tsui J, Ananth CV, et al. Nationwide trends in the performance of inpatient hysterectomy in the United States. Obstet Gynecol. 2013;122(2 Pt 1):233–241.
- Johnson N, Barlow D, Leghaby A, Tavender E, Curr E, Garry R. Surgical approach to hysterectomy for benign gynaecological disease. Cochrane Database Syst Rev. 2006;(2):CD003677.
- Rein MS, Powell WL, Walters FC, et al. Cytogenetic abnormalities in uterine myomas are associated with myoma size. Mol Hum Reprod. 1998;4(1):83–86.
- Gattas GJF, Quade BJ, Nowak RA, Morton CC. HMGIC expression in human adult and fetal tissues and in uterine leiomyomata. Genes Chromosomes Cancer. 1999;25(4):316–322.
- Ostrzenski A. Uterine leiomyoma particle growth in an abdominal wall incision after laparoscopic retrieval. Obstet Gynecol. 1997;89 (5 Pt 2):853–854.
- Paul PG, Koshy AK. Multiple peritoneal parasitic myomas after laparoscopic myomectomy and morcellation. Fertil Steril. 2006;85(2):492–493.
- Takeda A, Mori M, Sakai K, Misui T, Nakamura H. Parasitic peritoneal leiomyomatosis diagnosed 6 years after laparoscopic myomectomy with electric tissue morcellation: Report of a case and review of the literature. J Minim Invasive Gyencol. 2007;14(6):770–775.
- Kumar S, Sharma JB, Verma D, Gupta P, Roy KK, Malhotra N. Disseminated peritoneal leiomyomyomatosis: An unusual complication of laparoscopic myomectomy. Arch Gynecol Obstet. 2008;278(1):93–95.
- Miyake T, Enomoto T, Ueda Y, et al. A case of disseminated peritoneal leiomyomatosis developing after laparoscope-assisted myomectomy. Gynecol Obstet Invest. 2009;67(2):96–102.
- Thian YL, Tan KH, Kwek JW, Wang J, Chern B, Yam KL. Leiomyomatosis peritonealis disseminate and subcutaneous myoma—a rare complication of laparoscopic myomectomy. Abdom Imaging. 2009;34(2):235–238.
- Al-Talib A, Tulandi T. Pathophysiology and possible iatrogenic cause of leiomyomatosis peritonealis disseminata. Gynecol Obstet Invest. 2010;69(4):239–244.
- Larrain D, Rabischong B, Khoo CK, Botchorishvili R, Canis M, Mage G. Iatrogenic parasitic myomas: Unusual late complication of laparoscopic morcellation procedures. J Minim Invasive Gynecol. 2010;17(6):719–724.
- Cucinella G, Granese R, Calagna G, Somigliana E, Perino A. Parasitic myomas after laparoscopic surgery: An emerging complication in the use of morcellator? Description of four cases. Fertil Steril. 2011;96(2):e90–e96.
- Beck MM, Biswas B, D’Souza A, Kuma R. Benign metastasizing leiomyoma after hysterectomy and bilateral salpingooophorectomy. Hong Kong Med J. 2012;18:153–155.
- Bogusiewicz M, Rosinska-Bogusiewicz K, Walczyna B, Drop A, Rechberger T. Leiomyomatosis peritonealis disseminata with formation of endometrial cysts within tumors arising after supracervical laparoscopic hysterectomy. Ginekol Pol. 2013;84(1):68–71.
- Shakir F, Hill N. A case of multiple morcelloma formation following laparoscopic subtotal hysterectomy. J Obstet Gynecol. 2012;32(7):713–714.
- Ordulu Z, Dal Cin P, Chong WWS, et al. Disseminated peritoneal leiomyomatosis after laparoscopic supracervical hysterectomy with characteristic molecular cytogenetic findings of uterine leiomyoma. Genes Chromosomes Cancer. 2010;49(12):1152–1160.
- Theben JU, Schellong ARM, Altgassen C, Kelling K, Schneider S, Grobe-Drieling D. Unexpected malignancies after laparoscopic-assisted supracervical hysterectomies (LASH): An analysis of 1,584 LASH cases. Arch Gynecol Obstet. 2013;287(3):455–462.
- Parker WH, Fu YS, Berek JS. Uterine sarcoma in patients operated on for presumed leiomyoma and rapidly growing leiomyoma. Obstet Gynecol. 1994;83(3):414–418.
- Seidman MA, Oduyebo T, Muto MG, Crum CP, Nucci MR, Quade BJ. Peritoneal dissemination complicating morcellation of uterine mesenchymal neoplasms. PLOS One. 2012;7(11):e50058.
- Brooks SE, Zhan M, Cote T, Baquet CR. Surveillance, epidemiology, and end results analysis of 2677 cases of uterine sarcoma 1989 to 1999. Gyn Oncol. 2004;93(1):204–208.
- Park JY, Park SK, Kim DY, et al. The impact of tumor morcellation during surgery on the prognosis of patients with apparently early uterine leiomyosarcoma. Gynecol Oncol. 2011;122(2):255–259.
- Einstein MH, Barakat RR, Chi DS, et al. Management of uterine malignancy found incidentally after supracervical hysterectomy or uterine morcellation for presumed benign disease. Int J Gynecol Cancer. 2008;18(5):1065–1070.
- Oduyebo T, Rauh-Hain AJ, Meserve E, et al. The value of re-exploration in patients with inadvertently morcellated uterine sarcoma [published online ahead of print December 1, 2013]. Gynecol Oncol. doi:10.1016/j.ygyno.2013.11.024.
- Hagemann IS, Hagemann AR, LiVolsi VA, Montone KT, Chu CS. Risk of occult malignancy in morcellated hysterectomy: A case series. Int J Obstet Gynecol. 2011;304(5):476–483.
- Rha SE, Byun JY, Jung SE, et al. CT and MRI of uterine sarcomas and their mimickers. AJR. 2003;181(5):1369–1374 .
- Kitajima K, Murakami K, Kajhi Y, Sugimura K. Spectrum of FDG PET/CT findings of uterine tumors. AJR. 2010;195(3):737–743.
- Serur E, Lakhi N. Laparoscopic hysterectomy with manual morcellation of the uterus: An original technique that permits the safe and quick removal of a large uterus. Am J Obstet Gynecol. 2011;204(6):566.e1–e2.
- Favero G, Anton C, Silva e Silva A, et al. Vaginal morcellation: A new strategy for large gynecological malignant tumor extraction: A pilot study. Gynecol Oncol. 2012;126(3):443–447.
- Sato K, Yuasa N, Fujita M, Fukushima Y. Clinical application of diffusion-weighted imaging for preoperative differentiation between uterine leiomyoma and leiomyosarcomas [published online ahead of print December 22, 2013]. Am J Obstet Gynecol. doi:10.1016/j.ajog.2013.12.028.
- Rauh-Hain JA, Oduyebo T, Diver EJ, et al. Uterine leiomyosarcoma: An updated series. Int J Gynecol Cancer. 2013;23(6):1036–1043.
Morcellation in the News
- Philadelphia hospital restricts 'morcellation' procedure Wall Street Journal, February 20, 2014
- Society of Gynecologic Surgeons releases position statement on morcellation
- Uterine surgical technique is linked to abnormal growths and cancer spread New York Times, February 6, 2014
- Evaluating the risks of electric uterine morcellation JAMA, February 6, 2014. doi:10.1001/jama.2014.1093
- Critics of fibroid removal procedure question risks it may pose for women with undetected uterine cancer JAMA, February 6, 2014. doi:10.001/jama.2014.27
Uterine leiomyomata, also known as fibroids, are the most common pelvic tumor among women. In some women fibroids may be associated with no symptoms. In these cases, the fibroids are often managed expectantly. For many women, however, fibroids cause problems, including heavy uterine bleeding, pelvic pain, and infertility. In these cases removal of the fibroids, either by myomectomy or by hysterectomy, is a common treatment. In the United States, the two most common reasons for performing a hysterectomy are uterine fibroids or abnormal uterine bleeding.1
New minimally invasive gynecologic surgery (MIGS) technology and advanced surgical skills now permit the removal of very large uterine fibroids through small laparoscopic surgery incisions. There are many advantages to MIGS removal of uterine fibroids. The reported benefits include2:
- no large abdominal laparotomy incision
- faster healing and recovery from surgery
- less postoperative pain
- lower risk of surgical site infection
- more rapid return to full activities.
Related Article: Update: Minimally invasive surgery Amy Garcia, MD (April 2012)
Laparoscopic surgeons often select open power morcellation of the fibroid or fibroid-laden uterus to remove the mass of tissue through a small laparoscopy port. In current MIGS practice, power morcellation typically occurs outside of a closed environment, such as a bag. Open power morcellation of uterine tissue has an important pitfall. This practice permits small bits of tissue to spread around the peritoneal cavity (FIGURE). Given the enhanced growth potential of uterine tumor cells, the postoperative growth of nodules of tissue generated by morcellation is a worrisome possibility.
Morcellation of uterine fibroids and the possibility of causing disseminated leiomyomatosis
Uterine fibroids are clonal tumors that arise from a somatic mutation in a precursor cell. Tumors with clonal somatic mutations have enhanced growth potential because normal mechanisms that control cell growth have been disrupted at the level of cell DNA. Many uterine fibroids demonstrate an abnormal karyotype. Fibroids with a karyotype showing an exchange of genetic material between chromosomes 12 and 14 (t(12;14) (q13-15, q23-24)) are often large.3 The t(12;14) (q13-15, q23-24) translocation is associated with an increased expression of HMGIC, a gene that stimulates cell growth.4
Open morcellation of uterine fibroids may result in the dispersion of bits of tissue around the peritoneal cavity and surgical ports. If the fibroid contains genetic mutations that significantly increase the growth potential of fibroid cells, fibroid nodules may grow postoperatively in the pelvis, abdomen, and surgical ports.5–15 Investigators have called these disseminated nodules of fibroid tumor, “morcellomas.”16
Related Article: Ins and outs of straight-stick laparoscopic myomectomy James Robinson, MD, MS, and Gaby Moawad, MD (September 2012)
Ordulu and colleagues recently reported a case of a woman with uterine fibroids who underwent supracervical hysterectomy using morcellation and 7 years later developed disseminated peritoneal leiomyomatosis (DPL) requiring a second operation.17 Five anatomically distinct DPL masses were karyotyped and showed cytogenetic abnormalities often observed in uterine fibroids with enhanced growth potential, including the t(12;14) (q13-15, q23-24) chromosome translocation. As noted above, the t(12;14) (q13-15, q23-24) translocation is associated with enhanced growth of fibroid tumor cells due, in part, to overexpression of HMGIC.4
Morcellation of occult uterine leiomyosarcomas
In some cases, a uterine tumor thought to be a fibroid is surgically excised, but histologic analysis shows that the tumor is actually a uterine leiomyosarcoma. In surgical case series, approximately 0.08% to 0.13% of uterine masses thought to be due to fibroids are proven by postoperative histology to be a uterine leiomyosarcoma.18–20 Most leiomyosarcomas are Stage I,21 but if the sarcoma tissue is open power morcellated in the peritoneal cavity, bits of tissue can be spread, upstaging the tumor and worsening the prognosis.22
In one retrospective study of women with leiomyosarcoma, the 5-year estimated survival was 73% if the tumor was removed without morcellation and 46% if the tumor was removed using morcellation.22 If a “fibroid” or “fibroid uterus” is power morcellated, but the final histology reveals leiomyosarcoma, surgical re-exploration will be recommended to detect any spread of cancer, and chemotherapy may be necessary if tumor spread is detected.23,24
Many experts recommend a standardized preoperative evaluation of all cases of presumed uterine fibroid tumors where open power morcellation of tissue is planned. Recommended preoperative studies include25:
- imaging studies such as MRI or sonography,
- endometrial biopsy in cases with abnormal uterine bleeding
- cervical cytology.
These studies are recommended because they may identify an occult cervical or endometrial cancer or precancer. If the preoperative evaluation detects an occult cervical or endometrial cancer it is best to avoid open power morcellation of the uterus. These standard preoperative studies are unlikely to detect an occult leiomyosarcoma. There are currently no established imaging studies that can reliably detect a uterine sarcoma preoperatively,26,27 and endometrial biopsy has a low sensitivity for detecting leiomyosarcoma.
Related Article: Tips and techniques for robot-assisted laparoscopic myomectomy Arnold P. Advincula, MD, and Bich-Van Tran, MD (Surgical Technique, August 2013)
During the surgical consent process the issue of power morcellating an occult leiomyosarcoma and the consequences of causing the spread of cancer cells should be discussed with the patient. The patient may decide that the small risk of power morcellating an occult leiomyosarcoma is sufficiently worrisome that she would prefer an alternative procedure. These alternatives include abdominal hysterectomy, vaginal hysterectomy, or a hybrid operation involving removal of the uterine tissue through a mini-laparotomy or colpotomy incision.
Morcellation in a bag and removal of the bag through an abdominal or vaginal incision
Open power morcellation of uterine tumors creates the potential pitfall of spreading cells with enhanced growth potential around the peritoneal cavity. One approach to reducing the pitfall of open power morcellation is to place the uterine tumor in a bag, hand morcellate the tissue within the bag, and remove the bag and tissue through an abdominal incision (mini-laparotomy) or a colpotomy.28,29
If transabdominal removal of the tumor tissue is planned, the surgical port incision typically would need to be enlarged to permit hand morcellation and removal of the tissue. If a supracervical hysterectomy is planned, it may be difficult to remove a very large tumor through a small colpotomy.
Development of new technology
A major advance in gynecology would be the development of imaging studies that would permit the identification of uterine leiomyosarcomas prior to hysterectomy using open power morcellation. A preliminary study reported that diffusion-weighted imaging may be capable of differentiating leiomyosarcomas and leiomyomas30 but this study would require replication in multiple centers before it could be reliably utilized in surgical planning.
Another advance would be to develop power morcellation technology that occurs within a bag, with the removal of the bag and the enclosed morcellated tumor through a laparoscopy port. Morcellation of tissue within a bag would reduce the risk of spreading bits of tumor tissue around the peritoneal cavity.
The pitfalls of open morcellation are less likely to be clinically significant when surgery is planned for women who are aged 35 years and younger because leiomyosarcoma rarely occurs in young women. The pitfalls of open morcellation are more likely to occur when the woman is perimenopausal or postmenopausal with a very large uterus. This is because the incidence of uterine leiomyosarcoma increases with age, with the diagnosis often being made between 40 and 60 years of age.21,31
Open power morcellation has enhanced the ability of surgeons to remove large tumors through small incisions. The benefits to the patient are faster healing, faster postoperative recovery, and more rapid return to full activities. Hundreds of thousands of women have benefited from these advances in gynecologic surgery.
However, for an occasional woman, open power morcellation may be associated with the intraperitoneal spread of an occult leiomyosarcoma. This may result in the upstaging of the cancer and may cause premature death. The benefits and risks of open power morcellation need to be weighed carefully with each woman.
INSTANT POLL
Do you think that open power morcellation of uterine tumors thought to be fibroids can result in the dissemination of cancer cells throughout the peritoneal cavity, should an occult leiomyosarcoma be present?
Tell us—at rbarbieri@frontlinemedcom.com. Please include your name and city and state.
Morcellation in the News
- Philadelphia hospital restricts 'morcellation' procedure Wall Street Journal, February 20, 2014
- Society of Gynecologic Surgeons releases position statement on morcellation
- Uterine surgical technique is linked to abnormal growths and cancer spread New York Times, February 6, 2014
- Evaluating the risks of electric uterine morcellation JAMA, February 6, 2014. doi:10.1001/jama.2014.1093
- Critics of fibroid removal procedure question risks it may pose for women with undetected uterine cancer JAMA, February 6, 2014. doi:10.001/jama.2014.27
Uterine leiomyomata, also known as fibroids, are the most common pelvic tumor among women. In some women fibroids may be associated with no symptoms. In these cases, the fibroids are often managed expectantly. For many women, however, fibroids cause problems, including heavy uterine bleeding, pelvic pain, and infertility. In these cases removal of the fibroids, either by myomectomy or by hysterectomy, is a common treatment. In the United States, the two most common reasons for performing a hysterectomy are uterine fibroids or abnormal uterine bleeding.1
New minimally invasive gynecologic surgery (MIGS) technology and advanced surgical skills now permit the removal of very large uterine fibroids through small laparoscopic surgery incisions. There are many advantages to MIGS removal of uterine fibroids. The reported benefits include2:
- no large abdominal laparotomy incision
- faster healing and recovery from surgery
- less postoperative pain
- lower risk of surgical site infection
- more rapid return to full activities.
Related Article: Update: Minimally invasive surgery Amy Garcia, MD (April 2012)
Laparoscopic surgeons often select open power morcellation of the fibroid or fibroid-laden uterus to remove the mass of tissue through a small laparoscopy port. In current MIGS practice, power morcellation typically occurs outside of a closed environment, such as a bag. Open power morcellation of uterine tissue has an important pitfall. This practice permits small bits of tissue to spread around the peritoneal cavity (FIGURE). Given the enhanced growth potential of uterine tumor cells, the postoperative growth of nodules of tissue generated by morcellation is a worrisome possibility.
Morcellation of uterine fibroids and the possibility of causing disseminated leiomyomatosis
Uterine fibroids are clonal tumors that arise from a somatic mutation in a precursor cell. Tumors with clonal somatic mutations have enhanced growth potential because normal mechanisms that control cell growth have been disrupted at the level of cell DNA. Many uterine fibroids demonstrate an abnormal karyotype. Fibroids with a karyotype showing an exchange of genetic material between chromosomes 12 and 14 (t(12;14) (q13-15, q23-24)) are often large.3 The t(12;14) (q13-15, q23-24) translocation is associated with an increased expression of HMGIC, a gene that stimulates cell growth.4
Open morcellation of uterine fibroids may result in the dispersion of bits of tissue around the peritoneal cavity and surgical ports. If the fibroid contains genetic mutations that significantly increase the growth potential of fibroid cells, fibroid nodules may grow postoperatively in the pelvis, abdomen, and surgical ports.5–15 Investigators have called these disseminated nodules of fibroid tumor, “morcellomas.”16
Related Article: Ins and outs of straight-stick laparoscopic myomectomy James Robinson, MD, MS, and Gaby Moawad, MD (September 2012)
Ordulu and colleagues recently reported a case of a woman with uterine fibroids who underwent supracervical hysterectomy using morcellation and 7 years later developed disseminated peritoneal leiomyomatosis (DPL) requiring a second operation.17 Five anatomically distinct DPL masses were karyotyped and showed cytogenetic abnormalities often observed in uterine fibroids with enhanced growth potential, including the t(12;14) (q13-15, q23-24) chromosome translocation. As noted above, the t(12;14) (q13-15, q23-24) translocation is associated with enhanced growth of fibroid tumor cells due, in part, to overexpression of HMGIC.4
Morcellation of occult uterine leiomyosarcomas
In some cases, a uterine tumor thought to be a fibroid is surgically excised, but histologic analysis shows that the tumor is actually a uterine leiomyosarcoma. In surgical case series, approximately 0.08% to 0.13% of uterine masses thought to be due to fibroids are proven by postoperative histology to be a uterine leiomyosarcoma.18–20 Most leiomyosarcomas are Stage I,21 but if the sarcoma tissue is open power morcellated in the peritoneal cavity, bits of tissue can be spread, upstaging the tumor and worsening the prognosis.22
In one retrospective study of women with leiomyosarcoma, the 5-year estimated survival was 73% if the tumor was removed without morcellation and 46% if the tumor was removed using morcellation.22 If a “fibroid” or “fibroid uterus” is power morcellated, but the final histology reveals leiomyosarcoma, surgical re-exploration will be recommended to detect any spread of cancer, and chemotherapy may be necessary if tumor spread is detected.23,24
Many experts recommend a standardized preoperative evaluation of all cases of presumed uterine fibroid tumors where open power morcellation of tissue is planned. Recommended preoperative studies include25:
- imaging studies such as MRI or sonography,
- endometrial biopsy in cases with abnormal uterine bleeding
- cervical cytology.
These studies are recommended because they may identify an occult cervical or endometrial cancer or precancer. If the preoperative evaluation detects an occult cervical or endometrial cancer it is best to avoid open power morcellation of the uterus. These standard preoperative studies are unlikely to detect an occult leiomyosarcoma. There are currently no established imaging studies that can reliably detect a uterine sarcoma preoperatively,26,27 and endometrial biopsy has a low sensitivity for detecting leiomyosarcoma.
Related Article: Tips and techniques for robot-assisted laparoscopic myomectomy Arnold P. Advincula, MD, and Bich-Van Tran, MD (Surgical Technique, August 2013)
During the surgical consent process the issue of power morcellating an occult leiomyosarcoma and the consequences of causing the spread of cancer cells should be discussed with the patient. The patient may decide that the small risk of power morcellating an occult leiomyosarcoma is sufficiently worrisome that she would prefer an alternative procedure. These alternatives include abdominal hysterectomy, vaginal hysterectomy, or a hybrid operation involving removal of the uterine tissue through a mini-laparotomy or colpotomy incision.
Morcellation in a bag and removal of the bag through an abdominal or vaginal incision
Open power morcellation of uterine tumors creates the potential pitfall of spreading cells with enhanced growth potential around the peritoneal cavity. One approach to reducing the pitfall of open power morcellation is to place the uterine tumor in a bag, hand morcellate the tissue within the bag, and remove the bag and tissue through an abdominal incision (mini-laparotomy) or a colpotomy.28,29
If transabdominal removal of the tumor tissue is planned, the surgical port incision typically would need to be enlarged to permit hand morcellation and removal of the tissue. If a supracervical hysterectomy is planned, it may be difficult to remove a very large tumor through a small colpotomy.
Development of new technology
A major advance in gynecology would be the development of imaging studies that would permit the identification of uterine leiomyosarcomas prior to hysterectomy using open power morcellation. A preliminary study reported that diffusion-weighted imaging may be capable of differentiating leiomyosarcomas and leiomyomas30 but this study would require replication in multiple centers before it could be reliably utilized in surgical planning.
Another advance would be to develop power morcellation technology that occurs within a bag, with the removal of the bag and the enclosed morcellated tumor through a laparoscopy port. Morcellation of tissue within a bag would reduce the risk of spreading bits of tumor tissue around the peritoneal cavity.
The pitfalls of open morcellation are less likely to be clinically significant when surgery is planned for women who are aged 35 years and younger because leiomyosarcoma rarely occurs in young women. The pitfalls of open morcellation are more likely to occur when the woman is perimenopausal or postmenopausal with a very large uterus. This is because the incidence of uterine leiomyosarcoma increases with age, with the diagnosis often being made between 40 and 60 years of age.21,31
Open power morcellation has enhanced the ability of surgeons to remove large tumors through small incisions. The benefits to the patient are faster healing, faster postoperative recovery, and more rapid return to full activities. Hundreds of thousands of women have benefited from these advances in gynecologic surgery.
However, for an occasional woman, open power morcellation may be associated with the intraperitoneal spread of an occult leiomyosarcoma. This may result in the upstaging of the cancer and may cause premature death. The benefits and risks of open power morcellation need to be weighed carefully with each woman.
INSTANT POLL
Do you think that open power morcellation of uterine tumors thought to be fibroids can result in the dissemination of cancer cells throughout the peritoneal cavity, should an occult leiomyosarcoma be present?
Tell us—at rbarbieri@frontlinemedcom.com. Please include your name and city and state.
- Wright JD, Herzog TJ, Tsui J, Ananth CV, et al. Nationwide trends in the performance of inpatient hysterectomy in the United States. Obstet Gynecol. 2013;122(2 Pt 1):233–241.
- Johnson N, Barlow D, Leghaby A, Tavender E, Curr E, Garry R. Surgical approach to hysterectomy for benign gynaecological disease. Cochrane Database Syst Rev. 2006;(2):CD003677.
- Rein MS, Powell WL, Walters FC, et al. Cytogenetic abnormalities in uterine myomas are associated with myoma size. Mol Hum Reprod. 1998;4(1):83–86.
- Gattas GJF, Quade BJ, Nowak RA, Morton CC. HMGIC expression in human adult and fetal tissues and in uterine leiomyomata. Genes Chromosomes Cancer. 1999;25(4):316–322.
- Ostrzenski A. Uterine leiomyoma particle growth in an abdominal wall incision after laparoscopic retrieval. Obstet Gynecol. 1997;89 (5 Pt 2):853–854.
- Paul PG, Koshy AK. Multiple peritoneal parasitic myomas after laparoscopic myomectomy and morcellation. Fertil Steril. 2006;85(2):492–493.
- Takeda A, Mori M, Sakai K, Misui T, Nakamura H. Parasitic peritoneal leiomyomatosis diagnosed 6 years after laparoscopic myomectomy with electric tissue morcellation: Report of a case and review of the literature. J Minim Invasive Gyencol. 2007;14(6):770–775.
- Kumar S, Sharma JB, Verma D, Gupta P, Roy KK, Malhotra N. Disseminated peritoneal leiomyomyomatosis: An unusual complication of laparoscopic myomectomy. Arch Gynecol Obstet. 2008;278(1):93–95.
- Miyake T, Enomoto T, Ueda Y, et al. A case of disseminated peritoneal leiomyomatosis developing after laparoscope-assisted myomectomy. Gynecol Obstet Invest. 2009;67(2):96–102.
- Thian YL, Tan KH, Kwek JW, Wang J, Chern B, Yam KL. Leiomyomatosis peritonealis disseminate and subcutaneous myoma—a rare complication of laparoscopic myomectomy. Abdom Imaging. 2009;34(2):235–238.
- Al-Talib A, Tulandi T. Pathophysiology and possible iatrogenic cause of leiomyomatosis peritonealis disseminata. Gynecol Obstet Invest. 2010;69(4):239–244.
- Larrain D, Rabischong B, Khoo CK, Botchorishvili R, Canis M, Mage G. Iatrogenic parasitic myomas: Unusual late complication of laparoscopic morcellation procedures. J Minim Invasive Gynecol. 2010;17(6):719–724.
- Cucinella G, Granese R, Calagna G, Somigliana E, Perino A. Parasitic myomas after laparoscopic surgery: An emerging complication in the use of morcellator? Description of four cases. Fertil Steril. 2011;96(2):e90–e96.
- Beck MM, Biswas B, D’Souza A, Kuma R. Benign metastasizing leiomyoma after hysterectomy and bilateral salpingooophorectomy. Hong Kong Med J. 2012;18:153–155.
- Bogusiewicz M, Rosinska-Bogusiewicz K, Walczyna B, Drop A, Rechberger T. Leiomyomatosis peritonealis disseminata with formation of endometrial cysts within tumors arising after supracervical laparoscopic hysterectomy. Ginekol Pol. 2013;84(1):68–71.
- Shakir F, Hill N. A case of multiple morcelloma formation following laparoscopic subtotal hysterectomy. J Obstet Gynecol. 2012;32(7):713–714.
- Ordulu Z, Dal Cin P, Chong WWS, et al. Disseminated peritoneal leiomyomatosis after laparoscopic supracervical hysterectomy with characteristic molecular cytogenetic findings of uterine leiomyoma. Genes Chromosomes Cancer. 2010;49(12):1152–1160.
- Theben JU, Schellong ARM, Altgassen C, Kelling K, Schneider S, Grobe-Drieling D. Unexpected malignancies after laparoscopic-assisted supracervical hysterectomies (LASH): An analysis of 1,584 LASH cases. Arch Gynecol Obstet. 2013;287(3):455–462.
- Parker WH, Fu YS, Berek JS. Uterine sarcoma in patients operated on for presumed leiomyoma and rapidly growing leiomyoma. Obstet Gynecol. 1994;83(3):414–418.
- Seidman MA, Oduyebo T, Muto MG, Crum CP, Nucci MR, Quade BJ. Peritoneal dissemination complicating morcellation of uterine mesenchymal neoplasms. PLOS One. 2012;7(11):e50058.
- Brooks SE, Zhan M, Cote T, Baquet CR. Surveillance, epidemiology, and end results analysis of 2677 cases of uterine sarcoma 1989 to 1999. Gyn Oncol. 2004;93(1):204–208.
- Park JY, Park SK, Kim DY, et al. The impact of tumor morcellation during surgery on the prognosis of patients with apparently early uterine leiomyosarcoma. Gynecol Oncol. 2011;122(2):255–259.
- Einstein MH, Barakat RR, Chi DS, et al. Management of uterine malignancy found incidentally after supracervical hysterectomy or uterine morcellation for presumed benign disease. Int J Gynecol Cancer. 2008;18(5):1065–1070.
- Oduyebo T, Rauh-Hain AJ, Meserve E, et al. The value of re-exploration in patients with inadvertently morcellated uterine sarcoma [published online ahead of print December 1, 2013]. Gynecol Oncol. doi:10.1016/j.ygyno.2013.11.024.
- Hagemann IS, Hagemann AR, LiVolsi VA, Montone KT, Chu CS. Risk of occult malignancy in morcellated hysterectomy: A case series. Int J Obstet Gynecol. 2011;304(5):476–483.
- Rha SE, Byun JY, Jung SE, et al. CT and MRI of uterine sarcomas and their mimickers. AJR. 2003;181(5):1369–1374 .
- Kitajima K, Murakami K, Kajhi Y, Sugimura K. Spectrum of FDG PET/CT findings of uterine tumors. AJR. 2010;195(3):737–743.
- Serur E, Lakhi N. Laparoscopic hysterectomy with manual morcellation of the uterus: An original technique that permits the safe and quick removal of a large uterus. Am J Obstet Gynecol. 2011;204(6):566.e1–e2.
- Favero G, Anton C, Silva e Silva A, et al. Vaginal morcellation: A new strategy for large gynecological malignant tumor extraction: A pilot study. Gynecol Oncol. 2012;126(3):443–447.
- Sato K, Yuasa N, Fujita M, Fukushima Y. Clinical application of diffusion-weighted imaging for preoperative differentiation between uterine leiomyoma and leiomyosarcomas [published online ahead of print December 22, 2013]. Am J Obstet Gynecol. doi:10.1016/j.ajog.2013.12.028.
- Rauh-Hain JA, Oduyebo T, Diver EJ, et al. Uterine leiomyosarcoma: An updated series. Int J Gynecol Cancer. 2013;23(6):1036–1043.
- Wright JD, Herzog TJ, Tsui J, Ananth CV, et al. Nationwide trends in the performance of inpatient hysterectomy in the United States. Obstet Gynecol. 2013;122(2 Pt 1):233–241.
- Johnson N, Barlow D, Leghaby A, Tavender E, Curr E, Garry R. Surgical approach to hysterectomy for benign gynaecological disease. Cochrane Database Syst Rev. 2006;(2):CD003677.
- Rein MS, Powell WL, Walters FC, et al. Cytogenetic abnormalities in uterine myomas are associated with myoma size. Mol Hum Reprod. 1998;4(1):83–86.
- Gattas GJF, Quade BJ, Nowak RA, Morton CC. HMGIC expression in human adult and fetal tissues and in uterine leiomyomata. Genes Chromosomes Cancer. 1999;25(4):316–322.
- Ostrzenski A. Uterine leiomyoma particle growth in an abdominal wall incision after laparoscopic retrieval. Obstet Gynecol. 1997;89 (5 Pt 2):853–854.
- Paul PG, Koshy AK. Multiple peritoneal parasitic myomas after laparoscopic myomectomy and morcellation. Fertil Steril. 2006;85(2):492–493.
- Takeda A, Mori M, Sakai K, Misui T, Nakamura H. Parasitic peritoneal leiomyomatosis diagnosed 6 years after laparoscopic myomectomy with electric tissue morcellation: Report of a case and review of the literature. J Minim Invasive Gyencol. 2007;14(6):770–775.
- Kumar S, Sharma JB, Verma D, Gupta P, Roy KK, Malhotra N. Disseminated peritoneal leiomyomyomatosis: An unusual complication of laparoscopic myomectomy. Arch Gynecol Obstet. 2008;278(1):93–95.
- Miyake T, Enomoto T, Ueda Y, et al. A case of disseminated peritoneal leiomyomatosis developing after laparoscope-assisted myomectomy. Gynecol Obstet Invest. 2009;67(2):96–102.
- Thian YL, Tan KH, Kwek JW, Wang J, Chern B, Yam KL. Leiomyomatosis peritonealis disseminate and subcutaneous myoma—a rare complication of laparoscopic myomectomy. Abdom Imaging. 2009;34(2):235–238.
- Al-Talib A, Tulandi T. Pathophysiology and possible iatrogenic cause of leiomyomatosis peritonealis disseminata. Gynecol Obstet Invest. 2010;69(4):239–244.
- Larrain D, Rabischong B, Khoo CK, Botchorishvili R, Canis M, Mage G. Iatrogenic parasitic myomas: Unusual late complication of laparoscopic morcellation procedures. J Minim Invasive Gynecol. 2010;17(6):719–724.
- Cucinella G, Granese R, Calagna G, Somigliana E, Perino A. Parasitic myomas after laparoscopic surgery: An emerging complication in the use of morcellator? Description of four cases. Fertil Steril. 2011;96(2):e90–e96.
- Beck MM, Biswas B, D’Souza A, Kuma R. Benign metastasizing leiomyoma after hysterectomy and bilateral salpingooophorectomy. Hong Kong Med J. 2012;18:153–155.
- Bogusiewicz M, Rosinska-Bogusiewicz K, Walczyna B, Drop A, Rechberger T. Leiomyomatosis peritonealis disseminata with formation of endometrial cysts within tumors arising after supracervical laparoscopic hysterectomy. Ginekol Pol. 2013;84(1):68–71.
- Shakir F, Hill N. A case of multiple morcelloma formation following laparoscopic subtotal hysterectomy. J Obstet Gynecol. 2012;32(7):713–714.
- Ordulu Z, Dal Cin P, Chong WWS, et al. Disseminated peritoneal leiomyomatosis after laparoscopic supracervical hysterectomy with characteristic molecular cytogenetic findings of uterine leiomyoma. Genes Chromosomes Cancer. 2010;49(12):1152–1160.
- Theben JU, Schellong ARM, Altgassen C, Kelling K, Schneider S, Grobe-Drieling D. Unexpected malignancies after laparoscopic-assisted supracervical hysterectomies (LASH): An analysis of 1,584 LASH cases. Arch Gynecol Obstet. 2013;287(3):455–462.
- Parker WH, Fu YS, Berek JS. Uterine sarcoma in patients operated on for presumed leiomyoma and rapidly growing leiomyoma. Obstet Gynecol. 1994;83(3):414–418.
- Seidman MA, Oduyebo T, Muto MG, Crum CP, Nucci MR, Quade BJ. Peritoneal dissemination complicating morcellation of uterine mesenchymal neoplasms. PLOS One. 2012;7(11):e50058.
- Brooks SE, Zhan M, Cote T, Baquet CR. Surveillance, epidemiology, and end results analysis of 2677 cases of uterine sarcoma 1989 to 1999. Gyn Oncol. 2004;93(1):204–208.
- Park JY, Park SK, Kim DY, et al. The impact of tumor morcellation during surgery on the prognosis of patients with apparently early uterine leiomyosarcoma. Gynecol Oncol. 2011;122(2):255–259.
- Einstein MH, Barakat RR, Chi DS, et al. Management of uterine malignancy found incidentally after supracervical hysterectomy or uterine morcellation for presumed benign disease. Int J Gynecol Cancer. 2008;18(5):1065–1070.
- Oduyebo T, Rauh-Hain AJ, Meserve E, et al. The value of re-exploration in patients with inadvertently morcellated uterine sarcoma [published online ahead of print December 1, 2013]. Gynecol Oncol. doi:10.1016/j.ygyno.2013.11.024.
- Hagemann IS, Hagemann AR, LiVolsi VA, Montone KT, Chu CS. Risk of occult malignancy in morcellated hysterectomy: A case series. Int J Obstet Gynecol. 2011;304(5):476–483.
- Rha SE, Byun JY, Jung SE, et al. CT and MRI of uterine sarcomas and their mimickers. AJR. 2003;181(5):1369–1374 .
- Kitajima K, Murakami K, Kajhi Y, Sugimura K. Spectrum of FDG PET/CT findings of uterine tumors. AJR. 2010;195(3):737–743.
- Serur E, Lakhi N. Laparoscopic hysterectomy with manual morcellation of the uterus: An original technique that permits the safe and quick removal of a large uterus. Am J Obstet Gynecol. 2011;204(6):566.e1–e2.
- Favero G, Anton C, Silva e Silva A, et al. Vaginal morcellation: A new strategy for large gynecological malignant tumor extraction: A pilot study. Gynecol Oncol. 2012;126(3):443–447.
- Sato K, Yuasa N, Fujita M, Fukushima Y. Clinical application of diffusion-weighted imaging for preoperative differentiation between uterine leiomyoma and leiomyosarcomas [published online ahead of print December 22, 2013]. Am J Obstet Gynecol. doi:10.1016/j.ajog.2013.12.028.
- Rauh-Hain JA, Oduyebo T, Diver EJ, et al. Uterine leiomyosarcoma: An updated series. Int J Gynecol Cancer. 2013;23(6):1036–1043.
![]()
Sequelae of open power morcellation
Video courtesy of Javier F. Magrina, MD, Professor, Obstetrics-Gynecology, Mayo Clinic, Phoenix, Arizona; Immediate Past President, AAGL
Vaginal approach to open hand morcellation using a colpotomy incision during hysterectomy
Video courtesy of Javier F. Magrina, MD, Professor, Obstetrics-Gynecology, Mayo Clinic, Phoenix, Arizona; Immediate Past President, AAGL
Vaginal approach to open hand morcellation of a large uterus using a colpotomy incision during hysterectomy
Video courtesy of John B. Gebhart, MD, MS, Associate Professor of Obstetrics and Gynecology, and Fellowship Program Director, Mayo Clinic, Rochester, Minnesota