User login
Since Neer and colleagues1 first reported in 1982, glenoid loosening persists as a common cause of anatomic total shoulder arthroplasty (TSA) failure.1-4 Currently, cemented, all-polyethylene glenoid components are the gold standard, and minimum clinical survival of 10 to 15 years is expected.3,5 Several clinical studies5-9 and in vitro biomechanical studies10 have suggested an advantage of pegged over keeled glenoid components, but glenoid component loosening remains a frequent complication,11 with the cement–implant interface suggested as the weak link of fixation.10,12 In addition to mechanical loosening, poor cement penetration and heat-induced necrosis have been postulated as contributing to glenoid component loosening.13,14
Because of these potential complications, there is a growing consideration to minimize or abandon cement fixation and rely on biological fixation to polyethylene for long-term component stability.15 A newer pegged glenoid component design consists of traditional, peripherally located pegs designed for cement fixation as well as a central, uncemented, fluted, interference-fit peg that allows for bony ingrowth. Short-term clinical studies have shown that bony ingrowth into the space between the flutes can be achieved with a hybrid cementation technique and that, when that occurs, excellent outcomes are likely.13,16-19 The immediate in vivo stability of this implant design upon initial implantation, before the cement has cured, has prompted some surgeons to consider implanting the device without cement. In a recent series in which this implant design was used without cement, clinical and radiographic results were promising.15
Despite the widespread clinical use, little biomechanical work has been done to characterize initial fixation of all-polyethylene pegged glenoid implants. We conducted a study to compare glenoid micromotion in an all-polyethylene, centrally fluted pegged glenoid component as a function of 3 fixation techniques: cementless interference-fit fixation, hybrid partial cementation based on manufacturer recommendations, and full cementation to simulate a gold-standard, traditional, cemented, pegged design.
Materials and MethodsBiomechanical Testing
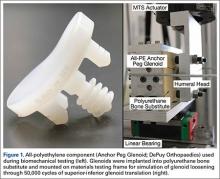
The biomechanical testing methodology used in this study was based on previous studies20-23 and on ASTM standard F2028-1224 using polyurethane bone substitute 0.24 g/cm3 (Pacific Research Laboratories) with ultimate strength of 4.9 MPa and compressive modulus of 123 MPa for component implantation. This material was selected because its mechanical properties are similar to those of cancellous glenoid bone in primary shoulder arthroplasty,25 and it minimizes variability with use of cadaveric specimens. Components were mounted on an MTS 858 Mini-Bionix II materials testing frame (Figure 1). A static compressive load of 756 N (170 lb) was applied via a mass-pulley system simulating the joint compressive force the shoulder is likely to experience during higher load activities.24,26 The glenoid component was positioned on a linear bearing to allow for joint compression.
Test Groups and Cement Fixation Techniques
All-polyethylene pegged glenoid components (Anchor Peg Glenoid, size 44; DePuy Orthopaedics) were used for biomechanical testing (Figure 1). Polyurethane blocks were reamed with a size 44 reamer until the superior-inferior distance reached 33 mm, ensuring complete seating of implant. Three fixation-technique groups were formed: interference-fit, hybrid cement, and fully cemented. Interference-fit fixation was done without polymethylmethacrylate (PMMA) cement. In hybrid fixation, 2 cm3 of PMMA (SpeedSet Cement, Stryker Orthopaedics) was injected (using a catheter tip syringe) into the peripheral peg holes and manually pressurized; the central peg was press-fit into polyurethane bone substitute. In the fully cemented group, both peripheral and central peg holes received PMMA; the peripheral peg holes were cemented as in hybrid fixation, and the central peg hole was injected with 3 cm3 of PMMA, which was then manually pressurized. The humeral head component (Global Advantage, 44×18 mm; DePuy Synthes) was mounted on the test frame actuator and centrally located within the glenoid at the start of the test.
Determination of Humeral Head Translation via Subluxation Testing
Humeral head subluxation distance, simulating a humeral head rim loading event, was calculated on the basis of preliminary tests outlined in the ASTM standard.24 Three glenoids (1 per fixation technique) were mounted on the test frame with a humeral head positioned centrally within the glenoid. After the joint compressive force was applied, the humeral head was translated along the true superior axis of the glenoid at a rate of 50 mm/min. Testing software was used to record humeral head displacement and load data at a frequency of 100 Hz. Humeral head subluxation displacement was determined at the end of the linear region of the force versus displacement response. This distance, averaged from the 3 subluxation tests, was used as the subluxation distance during cyclic testing.
Determination of Glenoid Component Motion via Cyclic Testing
After subluxation displacement was determined, glenoid components were mounted on the test frame (5 per fixation technique) and subjected to 50,000 cycles of humeral head translation at a frequency of 2 Hz. Amplitude of the humeral head displacement against the glenoid component followed a sinusoidal pattern with maxima and minima represented by the subluxation displacement (positive and negative, respectively). Glenoid edge compression/distraction of the superior edge and glenoid inferior/superior translation were monitored with 2 variable resistance reluctance transducers (Microminiature DVRT; 4.5-µm resolution; MicroStrain) secured to the glenoid component and testing fixture.
Microminiature DVRT measurements of glenoid motion were taken for 5 consecutive cycles at cycles 1, 20, 100, 500, 1000, 5000, 10,000, 15,000, 20,000, 30,000, 40,000, and 50,000. Distraction-compression displacement and superior-inferior translation measurements were recorded relative to the glenoid position with the humeral head at the neutral position at a given cycle. Final glenoid micromotion data were calculated from the mean of consecutive cycles at each cycle time point.
Statistical Analysis
Glenoid motion results are reported as means and standard deviations. Comparisons with 2 factors of fixation technique and number of cycles for glenoid distraction, glenoid compression, and absolute glenoid translation were characterized with 2-way analysis of variance (SigmaPlot Version 11.0; Systat Software), with the Holm-Šídák test used for post hoc determination of significant relationships.
Results
Under subluxation testing, the humeral head translation distance at the end of the linear region was determined to be 0.50 mm. Subsequently for cyclic testing, the humeral head was then translated 0.50 mm from the neutral position of the humeral head along both the superior and inferior axes of the glenoid. All glenoids successfully completed the entire 50,000 cycles of testing. For the glenoid component, Figure 2 depicts distraction and compression, and Figure 3 depicts superior-inferior translation.
Glenoid Component Distraction
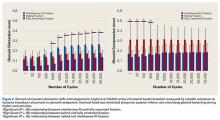
Overall, mean (SD) glenoid distraction was significantly higher for interference-fit fixation, 0.21 (0.10) mm, than for hybrid cement fixation, 0.16 (0.05) mm (P < .001), and fully cemented fixation, 0.09 (0.07) mm (P < .001). It was also significantly higher for hybrid fixation than fully cemented fixation (P < .001). From cycle 1000 to cycle 50,000, distraction was significantly higher for interference-fit fixation than for fully cemented fixation at each time point (P < .05).
Glenoid Component Compression
Mean (SD) compression was significantly higher for hybrid cement fixation, 0.31 (0.13) mm, than for interference-fit fixation, 0.17 (0.07) mm (P < .001), and fully cemented fixation, 0.17 (0.08) mm (P < .001). No significant difference was found between interference-fit and fully cemented fixation (P = .793) (Figure 2). At cycles 1, 20, 100, and 500, compression was significantly higher for hybrid fixation than for fully cemented fixation (P < .05). In addition, at cycle 500, it was significantly higher for hybrid fixation than for interference-fit fixation (P < .05).
Glenoid Component Translation
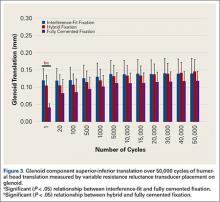
Mean (SD) glenoid translation was significantly lower for fully cemented fixation, 0.10 (0.04) mm, than for interference-fit fixation, 0.13 (0.04) mm (P < .001), and hybrid cement fixation, 0.13 (0.03) mm (P < .001), with all time points considered. There was no significant difference between interference-fit and hybrid fixation (P = .343). Initial translation at cycle 1 was significantly higher for interference-fit and hybid fixation than for fully cemented fixation.
Discussion
Despite advances in glenoid component design, glenoid loosening remains the most common cause of anatomical TSA failure. Recent implants have been designed to take advantage of an all-polyethylene component while providing long-lasting fixation through bony ingrowth into a central peg. In a study of the hybrid cementation technique drescribed here, Groh17 found no glenoid loosening or radiolucent lines but discovered fingerlike projections of bone between the flanges of the implant in 24 (29%) of 83 cases. Churchill and colleagues16 also reported bony ingrowth into the central peg in 15 (75%) of 20 patients. Furthermore, Arnold and colleagues13 reported complete bony ingrowth (6/6 inter-fin compartments) in 23 (71%) of 35 shoulders at a mean of 43 months. Wirth and colleagues19 reported increased radiodensity between the flanges of the central peg in 30 of 44 cases (68%) and osteolysis around the central peg in 3 of 44 cases (7%) at 3 years.
There are also reports of successful bony ingrowth associated with all-polyethylene components implanted without cement. In a canine study using an early ingrowth implant design, Wirth and colleagues27 showed that, though initial fixation was superior with a cemented, keeled implant, pullout strength of the uncemented, pegged implant improved over time and eventually far surpassed that of the cemented, keeled implant owing to both the loosening of the cemented component and the bony ingrowth into the central peg component. Furthermore, Anglin and colleagues10 confirmed that component micromotion was lower with pegged glenoid components than with keeled components in a biomechanical model. De Wilde and colleagues15 recently reported on a series of uncemented, central fluted peg glenoids implanted in 34 patients followed clinically and with computed tomography for a minimum of 24 months. The investigators found bony ingrowth into the central peg in 27 (79%) of 34 patients and no signs of loosening in 30 (88%) of 34 patients. Incomplete lucencies around 1 or 2 peripheral pegs were found in 2 (6%) of 34 patients, and complete lucencies around 2 or more peripheral pegs were found in 2 (6%) of 34 patients. However, there was no statistical difference in clinical outcome between patients with and without loosening.
With this type of implant, initial fixation that provides stability while minimizing micromotion under biomechanical loading likely is crucial for attaining bony growth within the central peg flanges. To our knowledge, this is the first biomechanical study to compare micromotion using 3 different fixation methods with a central fluted peg glenoid component design. Of all these fixation methods, fully cemented fixation yielded the most stable glenoid throughout testing with respect to the evaluated parameters. However, this method is not necessarily clinically applicable, as a fully cemented glenoid would inhibit any bony growth within the central flange, which is necessary for long-term biological fixation. Our data showed that, though glenoid distraction was significantly lower with hybrid cement fixation, this fixation method exhibited significantly higher glenoid compression. In addition, there were no significant differences between glenoid components with hybrid fixation and glenoid components with interference-fit fixation with respect to component translation in the superior-inferior direction. These findings may indicate that initial fixation is not significantly improved by the addition of cement to the peripheral pegs in a glenoid component with a central fluted peg design.
The interference fit of the central peg is primarily responsible for conferring long-term implant stability,13,27 which is ultimately achieved by bony formation between the flutes of the peg. Other authors have reported that, for bony ingrowth to occur, micromotion between the bone–implant interface must not exceed 20 to 150 µm.28-30 Other than for interference-fit distraction at more than 1000 cycles and hybrid cement fixation compression at each time point throughout testing, our data fall within the reported upper limits of micromotion to support bony ingrowth. Increased micromotion in the interference-fit fixation group is seen at later time points and may be caused by the inability to simulate the potential fixation gained from bony ingrowth allowed with this surgical technique. Research is needed to further explain this increase in distraction.
Results from this study must be interpreted with caution because of limitations of the in vitro testing methodology. This biomechanical model using bone substitute characterizes glenoid fixation at time zero, directly after implantation, followed by repetitive cyclic loading simulating 5 years of implant service. This differs from the clinical scenario in which the shoulder undergoes postoperative immobilization or protected motion during which the early phases of bony remodeling are likely occurring. Furthermore, simulation of 5 years of implant service may not be necessary for an implant that is expected to achieve ultimate fixation by bony ingrowth within the first several months after implantation. Use of this implant without cement is classified off-label, and surgeons should take this into consideration during implantation. Last, this study could not simulate the effect of bony ingrowth on fixation, though our experimental technique of cementing the central peg may be a gross approximation of a fully ingrown central peg and its expected rigid fixation.
Fully cemented fixation of a polyethylene glenoid is superior to hybrid cement fixation and interference-fit fixation with respect to early glenoid micromotion. However, the long-term stability of a fully cemented polyethylene glenoid component remains a clinical concern, as fixation is achieved by bony ingrowth around the central fluted peg of the implant. In this study, interference-fit and hybrid fixation had equivocal component micromotion in biomechanical testing. Our findings suggest that cementation of the peripheral pegs confers no additional initial stability over an uncemented interference-fit technique in a biomechanical model. More research is needed to further evaluate interference-fit fixation as a viable option for implantation of a central fluted, all-polyethylene glenoid component.
1. Neer CS 2nd, Watson KC, Stanton FJ. Recent experience in total shoulder replacement. J Bone Joint Surg Am. 1982;64(3):319-337.
2. Sperling JW, Cofield RH, O’Driscoll SW, Torchia ME, Rowland CM. Radiographic assessment of ingrowth total shoulder arthroplasty. J Shoulder Elbow Surg. 2000;9(6):507-513.
3. Torchia ME, Cofield RH, Settergren CR. Total shoulder arthroplasty with the Neer prosthesis: long-term results. J Shoulder Elbow Surg. 1997;6(6):495-505.
4. Wirth MA, Rockwood CA Jr. Complications of total shoulder-replacement arthroplasty. J Bone Joint Surg Am. 1996;78(4):603-616.
5. Fox TJ, Cil A, Sperling JW, Sanchez-Sotelo J, Schleck CD, Cofield RH. Survival of the glenoid component in shoulder arthroplasty. J Shoulder Elbow Surg. 2009;18(6):859-863.
6. Edwards TB, Labriola JE, Stanley RJ, O’Connor DP, Elkousy HA, Gartsman GM. Radiographic comparison of pegged and keeled glenoid components using modern cementing techniques: a prospective randomized study. J Shoulder Elbow Surg. 2010;19(2):251-257.
7. Gartsman GM, Elkousy HA, Warnock KM, Edwards TB, O’Connor DP. Radiographic comparison of pegged and keeled glenoid components. J Shoulder Elbow Surg. 2005;14(3):252-257.
8. Klepps S, Chiang AS, Miller S, Jiang CY, Hazrati Y, Flatow EL. Incidence of early radiolucent glenoid lines in patients having total shoulder replacements. Clin Orthop Relat Res. 2005;(435):118-125.
9. Lazarus MD, Jensen KL, Southworth C, Matsen FA 3rd. The radiographic evaluation of keeled and pegged glenoid component insertion. J Bone Joint Surg Am. 2002;84(7):1174-1182.
10. Anglin C, Wyss UP, Nyffeler RW, Gerber C. Loosening performance of cemented glenoid prosthesis design pairs. Clin Biomech. 2001;16(2):144-150.
11. Walch G, Young AA, Melis B, Gazielly D, Loew M, Boileau P. Results of a convex-back cemented keeled glenoid component in primary osteoarthritis: multicenter study with a follow-up greater than 5 years. J Shoulder Elbow Surg. 2011;20(3):385-394.
12. Gregory T, Hansen U, Taillieu F, et al. Glenoid loosening after total shoulder arthroplasty: an in vitro CT-scan study. J Orthop Res. 2009;27(12):1589-1595.
13. Arnold RM, High RR, Grosshans KT, Walker CW, Fehringer EV. Bone presence between the central peg’s radial fins of a partially cemented pegged all poly glenoid component suggest few radiolucencies. J Shoulder Elbow Surg. 2011;20(2):315-321.
14. Churchill RS, Boorman RS, Fehringer EV, Matsen FA 3rd. Glenoid cementing may generate sufficient heat to endanger the surrounding bone. Clin Orthop Relat Res. 2004;(419):76-79.
15. De Wilde L, Dayerizadeh N, De Neve F, Basamania C, Van Tongel A. Fully uncemented glenoid component in total shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(10):e1-e7.
16. Churchill RS, Zellmer C, Zimmers HJ, Ruggero R. Clinical and radiographic analysis of a partially cemented glenoid implant: five-year minimum follow-up. J Shoulder Elbow Surg. 2010;19(7):1091-1097.
17. Groh GI. Survival and radiographic analysis of a glenoid component with a cementless fluted central peg. J Shoulder Elbow Surg. 2010;19(8):1265-1268.
18. Vidil A, Valenti P, Guichoux F, Barthas JH. CT scan evaluation of glenoid component fixation: a prospective study of 27 minimally cemented shoulder arthroplasties. Eur J Orthop Surg Traumatol. 2012;23(5):521-525.
19. Wirth MA, Loredo R, Garcia G, Rockwood CA Jr, Southworth C, Iannotti JP. Total shoulder arthroplasty with an all-polyethylene pegged bone-ingrowth glenoid component: a clinical and radiographic outcome study. J Bone Joint Surg Am. 2012;94(3):260-267.
20. Anglin C, Wyss UP, Pichora DR. Mechanical testing of shoulder prostheses and recommendations for glenoid design. J Shoulder Elbow Surg. 2000;9(4):323-331.
21. Hoenig MP, Loeffler B, Brown S, et al. Reverse glenoid component fixation: is a posterior screw necessary? J Shoulder Elbow Surg. 2010;19(4):544-549.
22. Sarah J, Sanjay G, Sanjay S, et al. Failure mechanism of the all-polyethylene glenoid implant. J Biomech. 2010;43(4):714-719.
23. Suárez DR, Nerkens W, Valstar ER, Rozing PM, van Keulen F. Interface micromotions increase with less-conforming cementless glenoid components. J Shoulder Elbow Surg. 2012;21(4):474-482.
24. ASTM International. Standard Test Methods for Dynamic Evaluation of Glenoid Loosening or Disassociation. West Conshocken, PA: ASTM International; 2012. ASTM F2028-08.
25. Anglin C, Tolhurst P, Wyss UP, Pichora DR. Glenoid cancellous bone strength and modulus. J Biomech. 1999;32(10):1091-1097.
26. Anglin C, Wyss U, Pichora D. Glenohumeral contact forces. Proc Inst Mech Eng H. 2000;214(6):637-644.
27. Wirth MA, Korvick DL, Basamania CJ, Toro F, Aufdemorte TB, Rockwood CA Jr. Radiologic, mechanical, and histologic evaluation of 2 glenoid prosthesis designs in a canine model. J Shoulder Elbow Surg. 2001;10(2):140-148.
28. Pilliar RM, Lee JM, Maniatopoulos C. Observations on the effect of movement on bone ingrowth into porous-surfaced implants. Clin Orthop Relat Res. 1986;(208):108-113.
29. Ramamurti BS, Orr TE, Bragdon CR, Lowenstein JD, Jasty M, Harris WH. Factors influencing stability at the interface between a porous surface and cancellous bone: a finite element analysis of a canine in vivo micromotion experiment. J Biomed Mater Res. 1997;36(2):274-280.
30. Şahin S, Cehreli MC, Yalçın E. The influence of functional forces on the biomechanics of implant-supported prostheses—a review. J Dent. 2002;30(7-8):271-282.
Since Neer and colleagues1 first reported in 1982, glenoid loosening persists as a common cause of anatomic total shoulder arthroplasty (TSA) failure.1-4 Currently, cemented, all-polyethylene glenoid components are the gold standard, and minimum clinical survival of 10 to 15 years is expected.3,5 Several clinical studies5-9 and in vitro biomechanical studies10 have suggested an advantage of pegged over keeled glenoid components, but glenoid component loosening remains a frequent complication,11 with the cement–implant interface suggested as the weak link of fixation.10,12 In addition to mechanical loosening, poor cement penetration and heat-induced necrosis have been postulated as contributing to glenoid component loosening.13,14
Because of these potential complications, there is a growing consideration to minimize or abandon cement fixation and rely on biological fixation to polyethylene for long-term component stability.15 A newer pegged glenoid component design consists of traditional, peripherally located pegs designed for cement fixation as well as a central, uncemented, fluted, interference-fit peg that allows for bony ingrowth. Short-term clinical studies have shown that bony ingrowth into the space between the flutes can be achieved with a hybrid cementation technique and that, when that occurs, excellent outcomes are likely.13,16-19 The immediate in vivo stability of this implant design upon initial implantation, before the cement has cured, has prompted some surgeons to consider implanting the device without cement. In a recent series in which this implant design was used without cement, clinical and radiographic results were promising.15
Despite the widespread clinical use, little biomechanical work has been done to characterize initial fixation of all-polyethylene pegged glenoid implants. We conducted a study to compare glenoid micromotion in an all-polyethylene, centrally fluted pegged glenoid component as a function of 3 fixation techniques: cementless interference-fit fixation, hybrid partial cementation based on manufacturer recommendations, and full cementation to simulate a gold-standard, traditional, cemented, pegged design.
Materials and MethodsBiomechanical Testing
The biomechanical testing methodology used in this study was based on previous studies20-23 and on ASTM standard F2028-1224 using polyurethane bone substitute 0.24 g/cm3 (Pacific Research Laboratories) with ultimate strength of 4.9 MPa and compressive modulus of 123 MPa for component implantation. This material was selected because its mechanical properties are similar to those of cancellous glenoid bone in primary shoulder arthroplasty,25 and it minimizes variability with use of cadaveric specimens. Components were mounted on an MTS 858 Mini-Bionix II materials testing frame (Figure 1). A static compressive load of 756 N (170 lb) was applied via a mass-pulley system simulating the joint compressive force the shoulder is likely to experience during higher load activities.24,26 The glenoid component was positioned on a linear bearing to allow for joint compression.
Test Groups and Cement Fixation Techniques
All-polyethylene pegged glenoid components (Anchor Peg Glenoid, size 44; DePuy Orthopaedics) were used for biomechanical testing (Figure 1). Polyurethane blocks were reamed with a size 44 reamer until the superior-inferior distance reached 33 mm, ensuring complete seating of implant. Three fixation-technique groups were formed: interference-fit, hybrid cement, and fully cemented. Interference-fit fixation was done without polymethylmethacrylate (PMMA) cement. In hybrid fixation, 2 cm3 of PMMA (SpeedSet Cement, Stryker Orthopaedics) was injected (using a catheter tip syringe) into the peripheral peg holes and manually pressurized; the central peg was press-fit into polyurethane bone substitute. In the fully cemented group, both peripheral and central peg holes received PMMA; the peripheral peg holes were cemented as in hybrid fixation, and the central peg hole was injected with 3 cm3 of PMMA, which was then manually pressurized. The humeral head component (Global Advantage, 44×18 mm; DePuy Synthes) was mounted on the test frame actuator and centrally located within the glenoid at the start of the test.
Determination of Humeral Head Translation via Subluxation Testing
Humeral head subluxation distance, simulating a humeral head rim loading event, was calculated on the basis of preliminary tests outlined in the ASTM standard.24 Three glenoids (1 per fixation technique) were mounted on the test frame with a humeral head positioned centrally within the glenoid. After the joint compressive force was applied, the humeral head was translated along the true superior axis of the glenoid at a rate of 50 mm/min. Testing software was used to record humeral head displacement and load data at a frequency of 100 Hz. Humeral head subluxation displacement was determined at the end of the linear region of the force versus displacement response. This distance, averaged from the 3 subluxation tests, was used as the subluxation distance during cyclic testing.
Determination of Glenoid Component Motion via Cyclic Testing
After subluxation displacement was determined, glenoid components were mounted on the test frame (5 per fixation technique) and subjected to 50,000 cycles of humeral head translation at a frequency of 2 Hz. Amplitude of the humeral head displacement against the glenoid component followed a sinusoidal pattern with maxima and minima represented by the subluxation displacement (positive and negative, respectively). Glenoid edge compression/distraction of the superior edge and glenoid inferior/superior translation were monitored with 2 variable resistance reluctance transducers (Microminiature DVRT; 4.5-µm resolution; MicroStrain) secured to the glenoid component and testing fixture.
Microminiature DVRT measurements of glenoid motion were taken for 5 consecutive cycles at cycles 1, 20, 100, 500, 1000, 5000, 10,000, 15,000, 20,000, 30,000, 40,000, and 50,000. Distraction-compression displacement and superior-inferior translation measurements were recorded relative to the glenoid position with the humeral head at the neutral position at a given cycle. Final glenoid micromotion data were calculated from the mean of consecutive cycles at each cycle time point.
Statistical Analysis
Glenoid motion results are reported as means and standard deviations. Comparisons with 2 factors of fixation technique and number of cycles for glenoid distraction, glenoid compression, and absolute glenoid translation were characterized with 2-way analysis of variance (SigmaPlot Version 11.0; Systat Software), with the Holm-Šídák test used for post hoc determination of significant relationships.
Results
Under subluxation testing, the humeral head translation distance at the end of the linear region was determined to be 0.50 mm. Subsequently for cyclic testing, the humeral head was then translated 0.50 mm from the neutral position of the humeral head along both the superior and inferior axes of the glenoid. All glenoids successfully completed the entire 50,000 cycles of testing. For the glenoid component, Figure 2 depicts distraction and compression, and Figure 3 depicts superior-inferior translation.
Glenoid Component Distraction
Overall, mean (SD) glenoid distraction was significantly higher for interference-fit fixation, 0.21 (0.10) mm, than for hybrid cement fixation, 0.16 (0.05) mm (P < .001), and fully cemented fixation, 0.09 (0.07) mm (P < .001). It was also significantly higher for hybrid fixation than fully cemented fixation (P < .001). From cycle 1000 to cycle 50,000, distraction was significantly higher for interference-fit fixation than for fully cemented fixation at each time point (P < .05).
Glenoid Component Compression
Mean (SD) compression was significantly higher for hybrid cement fixation, 0.31 (0.13) mm, than for interference-fit fixation, 0.17 (0.07) mm (P < .001), and fully cemented fixation, 0.17 (0.08) mm (P < .001). No significant difference was found between interference-fit and fully cemented fixation (P = .793) (Figure 2). At cycles 1, 20, 100, and 500, compression was significantly higher for hybrid fixation than for fully cemented fixation (P < .05). In addition, at cycle 500, it was significantly higher for hybrid fixation than for interference-fit fixation (P < .05).
Glenoid Component Translation
Mean (SD) glenoid translation was significantly lower for fully cemented fixation, 0.10 (0.04) mm, than for interference-fit fixation, 0.13 (0.04) mm (P < .001), and hybrid cement fixation, 0.13 (0.03) mm (P < .001), with all time points considered. There was no significant difference between interference-fit and hybrid fixation (P = .343). Initial translation at cycle 1 was significantly higher for interference-fit and hybid fixation than for fully cemented fixation.
Discussion
Despite advances in glenoid component design, glenoid loosening remains the most common cause of anatomical TSA failure. Recent implants have been designed to take advantage of an all-polyethylene component while providing long-lasting fixation through bony ingrowth into a central peg. In a study of the hybrid cementation technique drescribed here, Groh17 found no glenoid loosening or radiolucent lines but discovered fingerlike projections of bone between the flanges of the implant in 24 (29%) of 83 cases. Churchill and colleagues16 also reported bony ingrowth into the central peg in 15 (75%) of 20 patients. Furthermore, Arnold and colleagues13 reported complete bony ingrowth (6/6 inter-fin compartments) in 23 (71%) of 35 shoulders at a mean of 43 months. Wirth and colleagues19 reported increased radiodensity between the flanges of the central peg in 30 of 44 cases (68%) and osteolysis around the central peg in 3 of 44 cases (7%) at 3 years.
There are also reports of successful bony ingrowth associated with all-polyethylene components implanted without cement. In a canine study using an early ingrowth implant design, Wirth and colleagues27 showed that, though initial fixation was superior with a cemented, keeled implant, pullout strength of the uncemented, pegged implant improved over time and eventually far surpassed that of the cemented, keeled implant owing to both the loosening of the cemented component and the bony ingrowth into the central peg component. Furthermore, Anglin and colleagues10 confirmed that component micromotion was lower with pegged glenoid components than with keeled components in a biomechanical model. De Wilde and colleagues15 recently reported on a series of uncemented, central fluted peg glenoids implanted in 34 patients followed clinically and with computed tomography for a minimum of 24 months. The investigators found bony ingrowth into the central peg in 27 (79%) of 34 patients and no signs of loosening in 30 (88%) of 34 patients. Incomplete lucencies around 1 or 2 peripheral pegs were found in 2 (6%) of 34 patients, and complete lucencies around 2 or more peripheral pegs were found in 2 (6%) of 34 patients. However, there was no statistical difference in clinical outcome between patients with and without loosening.
With this type of implant, initial fixation that provides stability while minimizing micromotion under biomechanical loading likely is crucial for attaining bony growth within the central peg flanges. To our knowledge, this is the first biomechanical study to compare micromotion using 3 different fixation methods with a central fluted peg glenoid component design. Of all these fixation methods, fully cemented fixation yielded the most stable glenoid throughout testing with respect to the evaluated parameters. However, this method is not necessarily clinically applicable, as a fully cemented glenoid would inhibit any bony growth within the central flange, which is necessary for long-term biological fixation. Our data showed that, though glenoid distraction was significantly lower with hybrid cement fixation, this fixation method exhibited significantly higher glenoid compression. In addition, there were no significant differences between glenoid components with hybrid fixation and glenoid components with interference-fit fixation with respect to component translation in the superior-inferior direction. These findings may indicate that initial fixation is not significantly improved by the addition of cement to the peripheral pegs in a glenoid component with a central fluted peg design.
The interference fit of the central peg is primarily responsible for conferring long-term implant stability,13,27 which is ultimately achieved by bony formation between the flutes of the peg. Other authors have reported that, for bony ingrowth to occur, micromotion between the bone–implant interface must not exceed 20 to 150 µm.28-30 Other than for interference-fit distraction at more than 1000 cycles and hybrid cement fixation compression at each time point throughout testing, our data fall within the reported upper limits of micromotion to support bony ingrowth. Increased micromotion in the interference-fit fixation group is seen at later time points and may be caused by the inability to simulate the potential fixation gained from bony ingrowth allowed with this surgical technique. Research is needed to further explain this increase in distraction.
Results from this study must be interpreted with caution because of limitations of the in vitro testing methodology. This biomechanical model using bone substitute characterizes glenoid fixation at time zero, directly after implantation, followed by repetitive cyclic loading simulating 5 years of implant service. This differs from the clinical scenario in which the shoulder undergoes postoperative immobilization or protected motion during which the early phases of bony remodeling are likely occurring. Furthermore, simulation of 5 years of implant service may not be necessary for an implant that is expected to achieve ultimate fixation by bony ingrowth within the first several months after implantation. Use of this implant without cement is classified off-label, and surgeons should take this into consideration during implantation. Last, this study could not simulate the effect of bony ingrowth on fixation, though our experimental technique of cementing the central peg may be a gross approximation of a fully ingrown central peg and its expected rigid fixation.
Fully cemented fixation of a polyethylene glenoid is superior to hybrid cement fixation and interference-fit fixation with respect to early glenoid micromotion. However, the long-term stability of a fully cemented polyethylene glenoid component remains a clinical concern, as fixation is achieved by bony ingrowth around the central fluted peg of the implant. In this study, interference-fit and hybrid fixation had equivocal component micromotion in biomechanical testing. Our findings suggest that cementation of the peripheral pegs confers no additional initial stability over an uncemented interference-fit technique in a biomechanical model. More research is needed to further evaluate interference-fit fixation as a viable option for implantation of a central fluted, all-polyethylene glenoid component.
Since Neer and colleagues1 first reported in 1982, glenoid loosening persists as a common cause of anatomic total shoulder arthroplasty (TSA) failure.1-4 Currently, cemented, all-polyethylene glenoid components are the gold standard, and minimum clinical survival of 10 to 15 years is expected.3,5 Several clinical studies5-9 and in vitro biomechanical studies10 have suggested an advantage of pegged over keeled glenoid components, but glenoid component loosening remains a frequent complication,11 with the cement–implant interface suggested as the weak link of fixation.10,12 In addition to mechanical loosening, poor cement penetration and heat-induced necrosis have been postulated as contributing to glenoid component loosening.13,14
Because of these potential complications, there is a growing consideration to minimize or abandon cement fixation and rely on biological fixation to polyethylene for long-term component stability.15 A newer pegged glenoid component design consists of traditional, peripherally located pegs designed for cement fixation as well as a central, uncemented, fluted, interference-fit peg that allows for bony ingrowth. Short-term clinical studies have shown that bony ingrowth into the space between the flutes can be achieved with a hybrid cementation technique and that, when that occurs, excellent outcomes are likely.13,16-19 The immediate in vivo stability of this implant design upon initial implantation, before the cement has cured, has prompted some surgeons to consider implanting the device without cement. In a recent series in which this implant design was used without cement, clinical and radiographic results were promising.15
Despite the widespread clinical use, little biomechanical work has been done to characterize initial fixation of all-polyethylene pegged glenoid implants. We conducted a study to compare glenoid micromotion in an all-polyethylene, centrally fluted pegged glenoid component as a function of 3 fixation techniques: cementless interference-fit fixation, hybrid partial cementation based on manufacturer recommendations, and full cementation to simulate a gold-standard, traditional, cemented, pegged design.
Materials and MethodsBiomechanical Testing
The biomechanical testing methodology used in this study was based on previous studies20-23 and on ASTM standard F2028-1224 using polyurethane bone substitute 0.24 g/cm3 (Pacific Research Laboratories) with ultimate strength of 4.9 MPa and compressive modulus of 123 MPa for component implantation. This material was selected because its mechanical properties are similar to those of cancellous glenoid bone in primary shoulder arthroplasty,25 and it minimizes variability with use of cadaveric specimens. Components were mounted on an MTS 858 Mini-Bionix II materials testing frame (Figure 1). A static compressive load of 756 N (170 lb) was applied via a mass-pulley system simulating the joint compressive force the shoulder is likely to experience during higher load activities.24,26 The glenoid component was positioned on a linear bearing to allow for joint compression.
Test Groups and Cement Fixation Techniques
All-polyethylene pegged glenoid components (Anchor Peg Glenoid, size 44; DePuy Orthopaedics) were used for biomechanical testing (Figure 1). Polyurethane blocks were reamed with a size 44 reamer until the superior-inferior distance reached 33 mm, ensuring complete seating of implant. Three fixation-technique groups were formed: interference-fit, hybrid cement, and fully cemented. Interference-fit fixation was done without polymethylmethacrylate (PMMA) cement. In hybrid fixation, 2 cm3 of PMMA (SpeedSet Cement, Stryker Orthopaedics) was injected (using a catheter tip syringe) into the peripheral peg holes and manually pressurized; the central peg was press-fit into polyurethane bone substitute. In the fully cemented group, both peripheral and central peg holes received PMMA; the peripheral peg holes were cemented as in hybrid fixation, and the central peg hole was injected with 3 cm3 of PMMA, which was then manually pressurized. The humeral head component (Global Advantage, 44×18 mm; DePuy Synthes) was mounted on the test frame actuator and centrally located within the glenoid at the start of the test.
Determination of Humeral Head Translation via Subluxation Testing
Humeral head subluxation distance, simulating a humeral head rim loading event, was calculated on the basis of preliminary tests outlined in the ASTM standard.24 Three glenoids (1 per fixation technique) were mounted on the test frame with a humeral head positioned centrally within the glenoid. After the joint compressive force was applied, the humeral head was translated along the true superior axis of the glenoid at a rate of 50 mm/min. Testing software was used to record humeral head displacement and load data at a frequency of 100 Hz. Humeral head subluxation displacement was determined at the end of the linear region of the force versus displacement response. This distance, averaged from the 3 subluxation tests, was used as the subluxation distance during cyclic testing.
Determination of Glenoid Component Motion via Cyclic Testing
After subluxation displacement was determined, glenoid components were mounted on the test frame (5 per fixation technique) and subjected to 50,000 cycles of humeral head translation at a frequency of 2 Hz. Amplitude of the humeral head displacement against the glenoid component followed a sinusoidal pattern with maxima and minima represented by the subluxation displacement (positive and negative, respectively). Glenoid edge compression/distraction of the superior edge and glenoid inferior/superior translation were monitored with 2 variable resistance reluctance transducers (Microminiature DVRT; 4.5-µm resolution; MicroStrain) secured to the glenoid component and testing fixture.
Microminiature DVRT measurements of glenoid motion were taken for 5 consecutive cycles at cycles 1, 20, 100, 500, 1000, 5000, 10,000, 15,000, 20,000, 30,000, 40,000, and 50,000. Distraction-compression displacement and superior-inferior translation measurements were recorded relative to the glenoid position with the humeral head at the neutral position at a given cycle. Final glenoid micromotion data were calculated from the mean of consecutive cycles at each cycle time point.
Statistical Analysis
Glenoid motion results are reported as means and standard deviations. Comparisons with 2 factors of fixation technique and number of cycles for glenoid distraction, glenoid compression, and absolute glenoid translation were characterized with 2-way analysis of variance (SigmaPlot Version 11.0; Systat Software), with the Holm-Šídák test used for post hoc determination of significant relationships.
Results
Under subluxation testing, the humeral head translation distance at the end of the linear region was determined to be 0.50 mm. Subsequently for cyclic testing, the humeral head was then translated 0.50 mm from the neutral position of the humeral head along both the superior and inferior axes of the glenoid. All glenoids successfully completed the entire 50,000 cycles of testing. For the glenoid component, Figure 2 depicts distraction and compression, and Figure 3 depicts superior-inferior translation.
Glenoid Component Distraction
Overall, mean (SD) glenoid distraction was significantly higher for interference-fit fixation, 0.21 (0.10) mm, than for hybrid cement fixation, 0.16 (0.05) mm (P < .001), and fully cemented fixation, 0.09 (0.07) mm (P < .001). It was also significantly higher for hybrid fixation than fully cemented fixation (P < .001). From cycle 1000 to cycle 50,000, distraction was significantly higher for interference-fit fixation than for fully cemented fixation at each time point (P < .05).
Glenoid Component Compression
Mean (SD) compression was significantly higher for hybrid cement fixation, 0.31 (0.13) mm, than for interference-fit fixation, 0.17 (0.07) mm (P < .001), and fully cemented fixation, 0.17 (0.08) mm (P < .001). No significant difference was found between interference-fit and fully cemented fixation (P = .793) (Figure 2). At cycles 1, 20, 100, and 500, compression was significantly higher for hybrid fixation than for fully cemented fixation (P < .05). In addition, at cycle 500, it was significantly higher for hybrid fixation than for interference-fit fixation (P < .05).
Glenoid Component Translation
Mean (SD) glenoid translation was significantly lower for fully cemented fixation, 0.10 (0.04) mm, than for interference-fit fixation, 0.13 (0.04) mm (P < .001), and hybrid cement fixation, 0.13 (0.03) mm (P < .001), with all time points considered. There was no significant difference between interference-fit and hybrid fixation (P = .343). Initial translation at cycle 1 was significantly higher for interference-fit and hybid fixation than for fully cemented fixation.
Discussion
Despite advances in glenoid component design, glenoid loosening remains the most common cause of anatomical TSA failure. Recent implants have been designed to take advantage of an all-polyethylene component while providing long-lasting fixation through bony ingrowth into a central peg. In a study of the hybrid cementation technique drescribed here, Groh17 found no glenoid loosening or radiolucent lines but discovered fingerlike projections of bone between the flanges of the implant in 24 (29%) of 83 cases. Churchill and colleagues16 also reported bony ingrowth into the central peg in 15 (75%) of 20 patients. Furthermore, Arnold and colleagues13 reported complete bony ingrowth (6/6 inter-fin compartments) in 23 (71%) of 35 shoulders at a mean of 43 months. Wirth and colleagues19 reported increased radiodensity between the flanges of the central peg in 30 of 44 cases (68%) and osteolysis around the central peg in 3 of 44 cases (7%) at 3 years.
There are also reports of successful bony ingrowth associated with all-polyethylene components implanted without cement. In a canine study using an early ingrowth implant design, Wirth and colleagues27 showed that, though initial fixation was superior with a cemented, keeled implant, pullout strength of the uncemented, pegged implant improved over time and eventually far surpassed that of the cemented, keeled implant owing to both the loosening of the cemented component and the bony ingrowth into the central peg component. Furthermore, Anglin and colleagues10 confirmed that component micromotion was lower with pegged glenoid components than with keeled components in a biomechanical model. De Wilde and colleagues15 recently reported on a series of uncemented, central fluted peg glenoids implanted in 34 patients followed clinically and with computed tomography for a minimum of 24 months. The investigators found bony ingrowth into the central peg in 27 (79%) of 34 patients and no signs of loosening in 30 (88%) of 34 patients. Incomplete lucencies around 1 or 2 peripheral pegs were found in 2 (6%) of 34 patients, and complete lucencies around 2 or more peripheral pegs were found in 2 (6%) of 34 patients. However, there was no statistical difference in clinical outcome between patients with and without loosening.
With this type of implant, initial fixation that provides stability while minimizing micromotion under biomechanical loading likely is crucial for attaining bony growth within the central peg flanges. To our knowledge, this is the first biomechanical study to compare micromotion using 3 different fixation methods with a central fluted peg glenoid component design. Of all these fixation methods, fully cemented fixation yielded the most stable glenoid throughout testing with respect to the evaluated parameters. However, this method is not necessarily clinically applicable, as a fully cemented glenoid would inhibit any bony growth within the central flange, which is necessary for long-term biological fixation. Our data showed that, though glenoid distraction was significantly lower with hybrid cement fixation, this fixation method exhibited significantly higher glenoid compression. In addition, there were no significant differences between glenoid components with hybrid fixation and glenoid components with interference-fit fixation with respect to component translation in the superior-inferior direction. These findings may indicate that initial fixation is not significantly improved by the addition of cement to the peripheral pegs in a glenoid component with a central fluted peg design.
The interference fit of the central peg is primarily responsible for conferring long-term implant stability,13,27 which is ultimately achieved by bony formation between the flutes of the peg. Other authors have reported that, for bony ingrowth to occur, micromotion between the bone–implant interface must not exceed 20 to 150 µm.28-30 Other than for interference-fit distraction at more than 1000 cycles and hybrid cement fixation compression at each time point throughout testing, our data fall within the reported upper limits of micromotion to support bony ingrowth. Increased micromotion in the interference-fit fixation group is seen at later time points and may be caused by the inability to simulate the potential fixation gained from bony ingrowth allowed with this surgical technique. Research is needed to further explain this increase in distraction.
Results from this study must be interpreted with caution because of limitations of the in vitro testing methodology. This biomechanical model using bone substitute characterizes glenoid fixation at time zero, directly after implantation, followed by repetitive cyclic loading simulating 5 years of implant service. This differs from the clinical scenario in which the shoulder undergoes postoperative immobilization or protected motion during which the early phases of bony remodeling are likely occurring. Furthermore, simulation of 5 years of implant service may not be necessary for an implant that is expected to achieve ultimate fixation by bony ingrowth within the first several months after implantation. Use of this implant without cement is classified off-label, and surgeons should take this into consideration during implantation. Last, this study could not simulate the effect of bony ingrowth on fixation, though our experimental technique of cementing the central peg may be a gross approximation of a fully ingrown central peg and its expected rigid fixation.
Fully cemented fixation of a polyethylene glenoid is superior to hybrid cement fixation and interference-fit fixation with respect to early glenoid micromotion. However, the long-term stability of a fully cemented polyethylene glenoid component remains a clinical concern, as fixation is achieved by bony ingrowth around the central fluted peg of the implant. In this study, interference-fit and hybrid fixation had equivocal component micromotion in biomechanical testing. Our findings suggest that cementation of the peripheral pegs confers no additional initial stability over an uncemented interference-fit technique in a biomechanical model. More research is needed to further evaluate interference-fit fixation as a viable option for implantation of a central fluted, all-polyethylene glenoid component.
1. Neer CS 2nd, Watson KC, Stanton FJ. Recent experience in total shoulder replacement. J Bone Joint Surg Am. 1982;64(3):319-337.
2. Sperling JW, Cofield RH, O’Driscoll SW, Torchia ME, Rowland CM. Radiographic assessment of ingrowth total shoulder arthroplasty. J Shoulder Elbow Surg. 2000;9(6):507-513.
3. Torchia ME, Cofield RH, Settergren CR. Total shoulder arthroplasty with the Neer prosthesis: long-term results. J Shoulder Elbow Surg. 1997;6(6):495-505.
4. Wirth MA, Rockwood CA Jr. Complications of total shoulder-replacement arthroplasty. J Bone Joint Surg Am. 1996;78(4):603-616.
5. Fox TJ, Cil A, Sperling JW, Sanchez-Sotelo J, Schleck CD, Cofield RH. Survival of the glenoid component in shoulder arthroplasty. J Shoulder Elbow Surg. 2009;18(6):859-863.
6. Edwards TB, Labriola JE, Stanley RJ, O’Connor DP, Elkousy HA, Gartsman GM. Radiographic comparison of pegged and keeled glenoid components using modern cementing techniques: a prospective randomized study. J Shoulder Elbow Surg. 2010;19(2):251-257.
7. Gartsman GM, Elkousy HA, Warnock KM, Edwards TB, O’Connor DP. Radiographic comparison of pegged and keeled glenoid components. J Shoulder Elbow Surg. 2005;14(3):252-257.
8. Klepps S, Chiang AS, Miller S, Jiang CY, Hazrati Y, Flatow EL. Incidence of early radiolucent glenoid lines in patients having total shoulder replacements. Clin Orthop Relat Res. 2005;(435):118-125.
9. Lazarus MD, Jensen KL, Southworth C, Matsen FA 3rd. The radiographic evaluation of keeled and pegged glenoid component insertion. J Bone Joint Surg Am. 2002;84(7):1174-1182.
10. Anglin C, Wyss UP, Nyffeler RW, Gerber C. Loosening performance of cemented glenoid prosthesis design pairs. Clin Biomech. 2001;16(2):144-150.
11. Walch G, Young AA, Melis B, Gazielly D, Loew M, Boileau P. Results of a convex-back cemented keeled glenoid component in primary osteoarthritis: multicenter study with a follow-up greater than 5 years. J Shoulder Elbow Surg. 2011;20(3):385-394.
12. Gregory T, Hansen U, Taillieu F, et al. Glenoid loosening after total shoulder arthroplasty: an in vitro CT-scan study. J Orthop Res. 2009;27(12):1589-1595.
13. Arnold RM, High RR, Grosshans KT, Walker CW, Fehringer EV. Bone presence between the central peg’s radial fins of a partially cemented pegged all poly glenoid component suggest few radiolucencies. J Shoulder Elbow Surg. 2011;20(2):315-321.
14. Churchill RS, Boorman RS, Fehringer EV, Matsen FA 3rd. Glenoid cementing may generate sufficient heat to endanger the surrounding bone. Clin Orthop Relat Res. 2004;(419):76-79.
15. De Wilde L, Dayerizadeh N, De Neve F, Basamania C, Van Tongel A. Fully uncemented glenoid component in total shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(10):e1-e7.
16. Churchill RS, Zellmer C, Zimmers HJ, Ruggero R. Clinical and radiographic analysis of a partially cemented glenoid implant: five-year minimum follow-up. J Shoulder Elbow Surg. 2010;19(7):1091-1097.
17. Groh GI. Survival and radiographic analysis of a glenoid component with a cementless fluted central peg. J Shoulder Elbow Surg. 2010;19(8):1265-1268.
18. Vidil A, Valenti P, Guichoux F, Barthas JH. CT scan evaluation of glenoid component fixation: a prospective study of 27 minimally cemented shoulder arthroplasties. Eur J Orthop Surg Traumatol. 2012;23(5):521-525.
19. Wirth MA, Loredo R, Garcia G, Rockwood CA Jr, Southworth C, Iannotti JP. Total shoulder arthroplasty with an all-polyethylene pegged bone-ingrowth glenoid component: a clinical and radiographic outcome study. J Bone Joint Surg Am. 2012;94(3):260-267.
20. Anglin C, Wyss UP, Pichora DR. Mechanical testing of shoulder prostheses and recommendations for glenoid design. J Shoulder Elbow Surg. 2000;9(4):323-331.
21. Hoenig MP, Loeffler B, Brown S, et al. Reverse glenoid component fixation: is a posterior screw necessary? J Shoulder Elbow Surg. 2010;19(4):544-549.
22. Sarah J, Sanjay G, Sanjay S, et al. Failure mechanism of the all-polyethylene glenoid implant. J Biomech. 2010;43(4):714-719.
23. Suárez DR, Nerkens W, Valstar ER, Rozing PM, van Keulen F. Interface micromotions increase with less-conforming cementless glenoid components. J Shoulder Elbow Surg. 2012;21(4):474-482.
24. ASTM International. Standard Test Methods for Dynamic Evaluation of Glenoid Loosening or Disassociation. West Conshocken, PA: ASTM International; 2012. ASTM F2028-08.
25. Anglin C, Tolhurst P, Wyss UP, Pichora DR. Glenoid cancellous bone strength and modulus. J Biomech. 1999;32(10):1091-1097.
26. Anglin C, Wyss U, Pichora D. Glenohumeral contact forces. Proc Inst Mech Eng H. 2000;214(6):637-644.
27. Wirth MA, Korvick DL, Basamania CJ, Toro F, Aufdemorte TB, Rockwood CA Jr. Radiologic, mechanical, and histologic evaluation of 2 glenoid prosthesis designs in a canine model. J Shoulder Elbow Surg. 2001;10(2):140-148.
28. Pilliar RM, Lee JM, Maniatopoulos C. Observations on the effect of movement on bone ingrowth into porous-surfaced implants. Clin Orthop Relat Res. 1986;(208):108-113.
29. Ramamurti BS, Orr TE, Bragdon CR, Lowenstein JD, Jasty M, Harris WH. Factors influencing stability at the interface between a porous surface and cancellous bone: a finite element analysis of a canine in vivo micromotion experiment. J Biomed Mater Res. 1997;36(2):274-280.
30. Şahin S, Cehreli MC, Yalçın E. The influence of functional forces on the biomechanics of implant-supported prostheses—a review. J Dent. 2002;30(7-8):271-282.
1. Neer CS 2nd, Watson KC, Stanton FJ. Recent experience in total shoulder replacement. J Bone Joint Surg Am. 1982;64(3):319-337.
2. Sperling JW, Cofield RH, O’Driscoll SW, Torchia ME, Rowland CM. Radiographic assessment of ingrowth total shoulder arthroplasty. J Shoulder Elbow Surg. 2000;9(6):507-513.
3. Torchia ME, Cofield RH, Settergren CR. Total shoulder arthroplasty with the Neer prosthesis: long-term results. J Shoulder Elbow Surg. 1997;6(6):495-505.
4. Wirth MA, Rockwood CA Jr. Complications of total shoulder-replacement arthroplasty. J Bone Joint Surg Am. 1996;78(4):603-616.
5. Fox TJ, Cil A, Sperling JW, Sanchez-Sotelo J, Schleck CD, Cofield RH. Survival of the glenoid component in shoulder arthroplasty. J Shoulder Elbow Surg. 2009;18(6):859-863.
6. Edwards TB, Labriola JE, Stanley RJ, O’Connor DP, Elkousy HA, Gartsman GM. Radiographic comparison of pegged and keeled glenoid components using modern cementing techniques: a prospective randomized study. J Shoulder Elbow Surg. 2010;19(2):251-257.
7. Gartsman GM, Elkousy HA, Warnock KM, Edwards TB, O’Connor DP. Radiographic comparison of pegged and keeled glenoid components. J Shoulder Elbow Surg. 2005;14(3):252-257.
8. Klepps S, Chiang AS, Miller S, Jiang CY, Hazrati Y, Flatow EL. Incidence of early radiolucent glenoid lines in patients having total shoulder replacements. Clin Orthop Relat Res. 2005;(435):118-125.
9. Lazarus MD, Jensen KL, Southworth C, Matsen FA 3rd. The radiographic evaluation of keeled and pegged glenoid component insertion. J Bone Joint Surg Am. 2002;84(7):1174-1182.
10. Anglin C, Wyss UP, Nyffeler RW, Gerber C. Loosening performance of cemented glenoid prosthesis design pairs. Clin Biomech. 2001;16(2):144-150.
11. Walch G, Young AA, Melis B, Gazielly D, Loew M, Boileau P. Results of a convex-back cemented keeled glenoid component in primary osteoarthritis: multicenter study with a follow-up greater than 5 years. J Shoulder Elbow Surg. 2011;20(3):385-394.
12. Gregory T, Hansen U, Taillieu F, et al. Glenoid loosening after total shoulder arthroplasty: an in vitro CT-scan study. J Orthop Res. 2009;27(12):1589-1595.
13. Arnold RM, High RR, Grosshans KT, Walker CW, Fehringer EV. Bone presence between the central peg’s radial fins of a partially cemented pegged all poly glenoid component suggest few radiolucencies. J Shoulder Elbow Surg. 2011;20(2):315-321.
14. Churchill RS, Boorman RS, Fehringer EV, Matsen FA 3rd. Glenoid cementing may generate sufficient heat to endanger the surrounding bone. Clin Orthop Relat Res. 2004;(419):76-79.
15. De Wilde L, Dayerizadeh N, De Neve F, Basamania C, Van Tongel A. Fully uncemented glenoid component in total shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(10):e1-e7.
16. Churchill RS, Zellmer C, Zimmers HJ, Ruggero R. Clinical and radiographic analysis of a partially cemented glenoid implant: five-year minimum follow-up. J Shoulder Elbow Surg. 2010;19(7):1091-1097.
17. Groh GI. Survival and radiographic analysis of a glenoid component with a cementless fluted central peg. J Shoulder Elbow Surg. 2010;19(8):1265-1268.
18. Vidil A, Valenti P, Guichoux F, Barthas JH. CT scan evaluation of glenoid component fixation: a prospective study of 27 minimally cemented shoulder arthroplasties. Eur J Orthop Surg Traumatol. 2012;23(5):521-525.
19. Wirth MA, Loredo R, Garcia G, Rockwood CA Jr, Southworth C, Iannotti JP. Total shoulder arthroplasty with an all-polyethylene pegged bone-ingrowth glenoid component: a clinical and radiographic outcome study. J Bone Joint Surg Am. 2012;94(3):260-267.
20. Anglin C, Wyss UP, Pichora DR. Mechanical testing of shoulder prostheses and recommendations for glenoid design. J Shoulder Elbow Surg. 2000;9(4):323-331.
21. Hoenig MP, Loeffler B, Brown S, et al. Reverse glenoid component fixation: is a posterior screw necessary? J Shoulder Elbow Surg. 2010;19(4):544-549.
22. Sarah J, Sanjay G, Sanjay S, et al. Failure mechanism of the all-polyethylene glenoid implant. J Biomech. 2010;43(4):714-719.
23. Suárez DR, Nerkens W, Valstar ER, Rozing PM, van Keulen F. Interface micromotions increase with less-conforming cementless glenoid components. J Shoulder Elbow Surg. 2012;21(4):474-482.
24. ASTM International. Standard Test Methods for Dynamic Evaluation of Glenoid Loosening or Disassociation. West Conshocken, PA: ASTM International; 2012. ASTM F2028-08.
25. Anglin C, Tolhurst P, Wyss UP, Pichora DR. Glenoid cancellous bone strength and modulus. J Biomech. 1999;32(10):1091-1097.
26. Anglin C, Wyss U, Pichora D. Glenohumeral contact forces. Proc Inst Mech Eng H. 2000;214(6):637-644.
27. Wirth MA, Korvick DL, Basamania CJ, Toro F, Aufdemorte TB, Rockwood CA Jr. Radiologic, mechanical, and histologic evaluation of 2 glenoid prosthesis designs in a canine model. J Shoulder Elbow Surg. 2001;10(2):140-148.
28. Pilliar RM, Lee JM, Maniatopoulos C. Observations on the effect of movement on bone ingrowth into porous-surfaced implants. Clin Orthop Relat Res. 1986;(208):108-113.
29. Ramamurti BS, Orr TE, Bragdon CR, Lowenstein JD, Jasty M, Harris WH. Factors influencing stability at the interface between a porous surface and cancellous bone: a finite element analysis of a canine in vivo micromotion experiment. J Biomed Mater Res. 1997;36(2):274-280.
30. Şahin S, Cehreli MC, Yalçın E. The influence of functional forces on the biomechanics of implant-supported prostheses—a review. J Dent. 2002;30(7-8):271-282.