User login
Mrs. C, age 68, is experiencing worsening paranoid delusions. She believes that because she lied about her income when she was younger, the FBI is tracking her and wants to poison her food and spray dangerous fumes in her house. Her paranoia has made it hard for her to sleep, eat, or feel safe in her home.
Mrs. C’s daughter reports that her mother’s delusions started 3 years ago and have worsened in recent months. Mrs. C has no psychiatric history. Her medical history is significant for renal transplant in 2015, type 2 diabetes, hyperlipidemia, hypertension, and hypothyroidism. She is currently normotensive. Mrs. C’s home medications include cyclosporine, which is a calcineurin inhibitor, 125 mg twice daily (trough level = 138 mcg/L); mycophenolate mofetil, 500 mg twice daily; cinacalcet, 30 mg 3 times a week; metformin, 500 mg twice daily; amlodipine, 5 mg twice daily; levothyroxine, 112 mcg/d; and atorvastatin, 40 mg at bedtime.
As you review her medications, you wonder if the cyclosporine she began receiving after her kidney transplant could be causing or contributing to her worsening paranoid delusions.
Kidney transplantation has become an ideal treatment for patients with end-stage renal disease. In 2020, 22,817 kidney transplants were performed in the United States.1 Compared with dialysis, kidney transplant allows patients the chance to return to a satisfactory quality of life.2 However, to ensure a successful and long-lasting transplant, patients must be started and maintained on lifelong immunosuppressant agents that have potential adverse effects, including nephrotoxicity and hypertension. Further, immunosuppressant agents—particularly calcineurin inhibitors—are associated with potential adverse effects on mental health. The most commonly documented mental health-related adverse effects include insomnia, anxiety, depression, and confusion.3 In this article, we discuss the risk of severe psychosis associated with the use of calcineurin inhibitors.
Calcineurin inhibitors and psychiatric symptoms
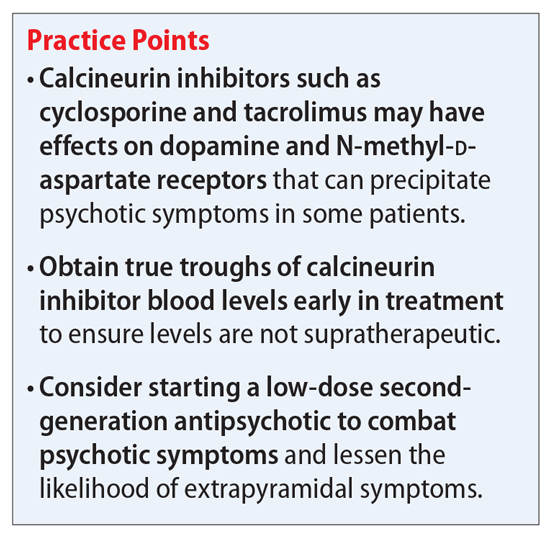
Cyclosporine and tacrolimus are calcineurin inhibitors that are commonly used as immunosuppressant agents after kidney transplantation. They primarily work by specifically and competitively binding to and inhibiting the calcineurin protein to reduce the transcriptional activation of cytokine genes for interleukin-2, tumor necrosis factor-alpha, interleukin-3, interleukin-4, CD40L (CD40 ligand), granulocyte-macrophage colony-stimulating factor, and interferon-gamma.4,5 The ultimate downstream effect is reduced proliferation of T lymphocytes and thereby an immunosuppressed state that will protect against organ rejection. However, this is not the only downstream effect that can occur from inhibiting calcineurin. Cyclosporine and tacrolimus may modulate the activity of dopamine and N-methyl-
An increased effect of dopamine in the mesocortical dopaminergic pathway has long been a suspected cause for psychotic symptoms. A study conducted in rodents suggested that tacrolimus selectively modifies the responsivity and sensitivity of postsynaptic dopamine-2 (D2) and dopamine-3 (D3) receptors.9 These receptors are important when discussing psychosis because antipsychotic medications work primarily by blocking dopamine D2, while many also block the D3 receptor. We hypothesize that modifying the responsivity and sensitivity of these 2 receptors could increase the risk of a person developing psychosis. It may also provide insight into how to best treat a psychotic episode.
Tacrolimus has been shown to elicit inhibition of NMDA-induced neurotransmitter release and augmentation of depolarization-induced neurotransmitter release.10 In theory, this potential inhibition at the NMDA receptors may lead to a compensatory and excessive release of glutamate. Elevated glutamate levels in the brain could lead to psychiatric symptoms, including psychosis. This is supported by the psychosis caused by many NMDA receptor antagonists, such as phencyclidine (PCP) and ketamine. Furthermore, a study examining calcineurin in knockout mice showed that the spectrum of behavioral abnormalities was strikingly similar to those in schizophrenia models.11 These mice displayed impaired working memory, impaired attentional function, social withdrawal, and psychomotor agitation. This further supports the idea that calcineurin inhibition can play a significant role in causing psychiatric symptoms by affecting both dopamine and NMDA receptors.
Continue to: How to address calcineurin inhibitor–induced psychosis...
How to address calcineurin inhibitor–induced psychosis
Here we outline a potential treatment strategy to combat psychosis secondary to calcineurin inhibitors. First, evaluate the patient’s calcineurin inhibitor level (either cyclosporine or tacrolimus). Levels should be drawn as a true trough and doses adjusted if necessary via appropriate consultation with a transplant specialist. Because many of the adverse effects associated with these agents are dose-dependent, we suspect that the risk of psychosis and other mental health–related adverse effects may also follow this trend.
Assuming that the calcineurin inhibitor cannot be stopped, changed to a different agent, or subject to a dose decrease, we recommend initiating an antipsychotic medication to control psychotic symptoms. Given the potential effect of calcineurin inhibitors on dopamine, we suggest trialing a second-generation antipsychotic with relatively high affinity for dopamine D2 receptors, such as risperidone or paliperidone. However, compared with patients with schizophrenia, patients receiving a calcineurin inhibitor may be more likely to develop extrapyramidal symptoms (EPS). Therefore, to avoid potential adverse effects, consider using a lower starting dose or an antipsychotic medication with less dopamine D2 affinity, such as quetiapine, olanzapine, or aripiprazole. Furthermore, because post-transplant patients may be at a higher risk for depression, which may be secondary to medication adverse effects, we suggest avoiding first-generation antipsychotics (FGAs) such as haloperidol because FGAs may worsen depressive symptoms.
We recommend initiating risperidone, 1 mg twice a day, for patients with psychosis secondary to a calcineurin inhibitor. If the patient develops EPS, consider switching to an antipsychotic medication with a less potent dopamine D2 blockade, such as quetiapine, olanzapine, or aripiprazole. We recommend an antipsychotic switch rather than adding benztropine or diphenhydramine to the regimen because many transplant recipients may be older patients, and adding anticholinergic medications can be problematic for this population. However, if the patient is younger or has responded particularly well to risperidone, the benefit of adding an anticholinergic medication may outweigh the risks. This decision should be made on an individual basis and may include other options, such as a switch to quetiapine, olanzapine, or aripiprazole. While these agents may not block the D2 receptor as strongly as risperidone, they all are effective and approved for adjunct therapy in major depressive disorder. We recommend quetiapine and olanzapine as second-line agents because of their potential for sedation and significant weight gain. While aripiprazole has a great metabolic adverse effect profile, its mechanism of action as a partial D2 agonist may make it difficult to control psychotic symptoms in this patient population compared with true D2 antagonists.
Continue to: CASE CONTINUED...
CASE CONTINUED
Mrs. C is admitted to the inpatient psychiatric unit and started on risperidone, 1 mg twice daily. Initially, she complains of lightheadedness at night due to the risperidone, so her dose is changed to 2 mg at bedtime. Gradually, she begins to show mild improvement. Previously, she reported feeling frightened of staff members, but after a few days she reports that she feels safe at the hospital. However, her delusions of being monitored by the FBI persist.
After 9 days of hospitalization, Mrs. C is discharged home to the care of her daughter. At first, she does well, but unfortunately she begins to refuse to take her medication and returns to her baseline.
Related Resources
- Gok F, Eroglu MZ. Acute psychotic disorder associated with immunosuppressive agent use after renal transplantation: a case report. Psychiatry and Clinical Psychopharmacology. 2017;3:314-316.
- Bersani G, Marino P, Valerani G, et al. Manic-like psychosis associated with elevated trough tacrolimus blood concentrations 17 years after kidney transplant. Case Rep Psychiatry. 2013;2013:926395. doi: 10.1155/2013/926395
Drug Brand Names
Amlodipine • Norvasc
Aripiprazole • Abilify
Atorvastatin • Lipitor
Benztropine • Cogentin
Cinacalcet • Sensipar
Cyclosporine • Gengraf
Haloperidol • Haldol
Ketamine • Ketalar
Levothyroxine • Synthroid
Metformin • Glucophage
Mycophenolate mofetil • CellCept
Olanzapine • Zyprexa
Quetiapine • Seroquel
Paliperidone • Invega
Risperidone • Risperdal
Tacrolimus • Prograf
1. Health Resources & Services Administration. US Government Information on Organ Donor Transplantation. Organ Donation Statistics. Updated October 1, 2020. Accessed October 8, 2021. https://www.organdonor.gov/learn/organ-donation-statistics/detailed-description#fig1
2. De Pasquale C, Veroux M, Indelicato L, et al. Psychopathological aspects of kidney transplantation: efficacy of a multidisciplinary team. World J Transplant. 2014;4(4):267-275.
3. Gengraf capsules [package insert]. North Chicago, IL: AbbVie Inc; 2017.
4. Wiederrecht G, Lam E, Hung S, et al. The mechanism of action of FK-506 and cyclosporin A. Ann N Y Acad Sci. 1993;696:9-19.
5. Schreiber SL, Crabtree GR. The mechanism of action of cyclosporin A and FK506. Immunol Today. 1992;13(4):136-142.
6. Scherrer U, Vissing SF, Morgan BJ, et al. Cyclosporine-induced sympathetic activation and hypertension after heart transplantation. N Engl J Med. 1990;323(11):693-699.
7. Fulya G, Meliha ZE. Acute psychotic disorder associated with immunosuppressive agent use after renal transplantation: a case report. Psychiatry and Clinical Psychopharmacology. 2017;27(3):314-316.
8. Tan TC, Robinson PJ. Mechanisms of calcineurin inhibitor-induced neurotoxicity. Transplant Rev. 2006;20(1):49-60.
9. Masatsuna S, Norio M, Nori Takei, et al. Tacrolimus, a specific inhibitor of calcineurin, modifies the locomotor activity of quinpirole, but not that of SKF82958, in male rats. Eur J Pharmacol. 2002;438(1-2):93-97.
10. Gold BG. FK506 and the role of immunophilins in nerve regeneration. Mol Neurobiol. 1997;15(3):285-306.
11. Miyakawa T, Leiter LM, Gerber DJ. Conditional calcineurin knockout mice exhibit multiple abnormal behaviors related to schizophrenia. Proc Natl Acad Sci U S A. 2003;100(15): 8987-8992.
Mrs. C, age 68, is experiencing worsening paranoid delusions. She believes that because she lied about her income when she was younger, the FBI is tracking her and wants to poison her food and spray dangerous fumes in her house. Her paranoia has made it hard for her to sleep, eat, or feel safe in her home.
Mrs. C’s daughter reports that her mother’s delusions started 3 years ago and have worsened in recent months. Mrs. C has no psychiatric history. Her medical history is significant for renal transplant in 2015, type 2 diabetes, hyperlipidemia, hypertension, and hypothyroidism. She is currently normotensive. Mrs. C’s home medications include cyclosporine, which is a calcineurin inhibitor, 125 mg twice daily (trough level = 138 mcg/L); mycophenolate mofetil, 500 mg twice daily; cinacalcet, 30 mg 3 times a week; metformin, 500 mg twice daily; amlodipine, 5 mg twice daily; levothyroxine, 112 mcg/d; and atorvastatin, 40 mg at bedtime.
As you review her medications, you wonder if the cyclosporine she began receiving after her kidney transplant could be causing or contributing to her worsening paranoid delusions.
Kidney transplantation has become an ideal treatment for patients with end-stage renal disease. In 2020, 22,817 kidney transplants were performed in the United States.1 Compared with dialysis, kidney transplant allows patients the chance to return to a satisfactory quality of life.2 However, to ensure a successful and long-lasting transplant, patients must be started and maintained on lifelong immunosuppressant agents that have potential adverse effects, including nephrotoxicity and hypertension. Further, immunosuppressant agents—particularly calcineurin inhibitors—are associated with potential adverse effects on mental health. The most commonly documented mental health-related adverse effects include insomnia, anxiety, depression, and confusion.3 In this article, we discuss the risk of severe psychosis associated with the use of calcineurin inhibitors.

Calcineurin inhibitors and psychiatric symptoms
Cyclosporine and tacrolimus are calcineurin inhibitors that are commonly used as immunosuppressant agents after kidney transplantation. They primarily work by specifically and competitively binding to and inhibiting the calcineurin protein to reduce the transcriptional activation of cytokine genes for interleukin-2, tumor necrosis factor-alpha, interleukin-3, interleukin-4, CD40L (CD40 ligand), granulocyte-macrophage colony-stimulating factor, and interferon-gamma.4,5 The ultimate downstream effect is reduced proliferation of T lymphocytes and thereby an immunosuppressed state that will protect against organ rejection. However, this is not the only downstream effect that can occur from inhibiting calcineurin. Cyclosporine and tacrolimus may modulate the activity of dopamine and N-methyl-
An increased effect of dopamine in the mesocortical dopaminergic pathway has long been a suspected cause for psychotic symptoms. A study conducted in rodents suggested that tacrolimus selectively modifies the responsivity and sensitivity of postsynaptic dopamine-2 (D2) and dopamine-3 (D3) receptors.9 These receptors are important when discussing psychosis because antipsychotic medications work primarily by blocking dopamine D2, while many also block the D3 receptor. We hypothesize that modifying the responsivity and sensitivity of these 2 receptors could increase the risk of a person developing psychosis. It may also provide insight into how to best treat a psychotic episode.
Tacrolimus has been shown to elicit inhibition of NMDA-induced neurotransmitter release and augmentation of depolarization-induced neurotransmitter release.10 In theory, this potential inhibition at the NMDA receptors may lead to a compensatory and excessive release of glutamate. Elevated glutamate levels in the brain could lead to psychiatric symptoms, including psychosis. This is supported by the psychosis caused by many NMDA receptor antagonists, such as phencyclidine (PCP) and ketamine. Furthermore, a study examining calcineurin in knockout mice showed that the spectrum of behavioral abnormalities was strikingly similar to those in schizophrenia models.11 These mice displayed impaired working memory, impaired attentional function, social withdrawal, and psychomotor agitation. This further supports the idea that calcineurin inhibition can play a significant role in causing psychiatric symptoms by affecting both dopamine and NMDA receptors.
Continue to: How to address calcineurin inhibitor–induced psychosis...
How to address calcineurin inhibitor–induced psychosis
Here we outline a potential treatment strategy to combat psychosis secondary to calcineurin inhibitors. First, evaluate the patient’s calcineurin inhibitor level (either cyclosporine or tacrolimus). Levels should be drawn as a true trough and doses adjusted if necessary via appropriate consultation with a transplant specialist. Because many of the adverse effects associated with these agents are dose-dependent, we suspect that the risk of psychosis and other mental health–related adverse effects may also follow this trend.
Assuming that the calcineurin inhibitor cannot be stopped, changed to a different agent, or subject to a dose decrease, we recommend initiating an antipsychotic medication to control psychotic symptoms. Given the potential effect of calcineurin inhibitors on dopamine, we suggest trialing a second-generation antipsychotic with relatively high affinity for dopamine D2 receptors, such as risperidone or paliperidone. However, compared with patients with schizophrenia, patients receiving a calcineurin inhibitor may be more likely to develop extrapyramidal symptoms (EPS). Therefore, to avoid potential adverse effects, consider using a lower starting dose or an antipsychotic medication with less dopamine D2 affinity, such as quetiapine, olanzapine, or aripiprazole. Furthermore, because post-transplant patients may be at a higher risk for depression, which may be secondary to medication adverse effects, we suggest avoiding first-generation antipsychotics (FGAs) such as haloperidol because FGAs may worsen depressive symptoms.
We recommend initiating risperidone, 1 mg twice a day, for patients with psychosis secondary to a calcineurin inhibitor. If the patient develops EPS, consider switching to an antipsychotic medication with a less potent dopamine D2 blockade, such as quetiapine, olanzapine, or aripiprazole. We recommend an antipsychotic switch rather than adding benztropine or diphenhydramine to the regimen because many transplant recipients may be older patients, and adding anticholinergic medications can be problematic for this population. However, if the patient is younger or has responded particularly well to risperidone, the benefit of adding an anticholinergic medication may outweigh the risks. This decision should be made on an individual basis and may include other options, such as a switch to quetiapine, olanzapine, or aripiprazole. While these agents may not block the D2 receptor as strongly as risperidone, they all are effective and approved for adjunct therapy in major depressive disorder. We recommend quetiapine and olanzapine as second-line agents because of their potential for sedation and significant weight gain. While aripiprazole has a great metabolic adverse effect profile, its mechanism of action as a partial D2 agonist may make it difficult to control psychotic symptoms in this patient population compared with true D2 antagonists.
Continue to: CASE CONTINUED...
CASE CONTINUED
Mrs. C is admitted to the inpatient psychiatric unit and started on risperidone, 1 mg twice daily. Initially, she complains of lightheadedness at night due to the risperidone, so her dose is changed to 2 mg at bedtime. Gradually, she begins to show mild improvement. Previously, she reported feeling frightened of staff members, but after a few days she reports that she feels safe at the hospital. However, her delusions of being monitored by the FBI persist.
After 9 days of hospitalization, Mrs. C is discharged home to the care of her daughter. At first, she does well, but unfortunately she begins to refuse to take her medication and returns to her baseline.
Related Resources
- Gok F, Eroglu MZ. Acute psychotic disorder associated with immunosuppressive agent use after renal transplantation: a case report. Psychiatry and Clinical Psychopharmacology. 2017;3:314-316.
- Bersani G, Marino P, Valerani G, et al. Manic-like psychosis associated with elevated trough tacrolimus blood concentrations 17 years after kidney transplant. Case Rep Psychiatry. 2013;2013:926395. doi: 10.1155/2013/926395
Drug Brand Names
Amlodipine • Norvasc
Aripiprazole • Abilify
Atorvastatin • Lipitor
Benztropine • Cogentin
Cinacalcet • Sensipar
Cyclosporine • Gengraf
Haloperidol • Haldol
Ketamine • Ketalar
Levothyroxine • Synthroid
Metformin • Glucophage
Mycophenolate mofetil • CellCept
Olanzapine • Zyprexa
Quetiapine • Seroquel
Paliperidone • Invega
Risperidone • Risperdal
Tacrolimus • Prograf
Mrs. C, age 68, is experiencing worsening paranoid delusions. She believes that because she lied about her income when she was younger, the FBI is tracking her and wants to poison her food and spray dangerous fumes in her house. Her paranoia has made it hard for her to sleep, eat, or feel safe in her home.
Mrs. C’s daughter reports that her mother’s delusions started 3 years ago and have worsened in recent months. Mrs. C has no psychiatric history. Her medical history is significant for renal transplant in 2015, type 2 diabetes, hyperlipidemia, hypertension, and hypothyroidism. She is currently normotensive. Mrs. C’s home medications include cyclosporine, which is a calcineurin inhibitor, 125 mg twice daily (trough level = 138 mcg/L); mycophenolate mofetil, 500 mg twice daily; cinacalcet, 30 mg 3 times a week; metformin, 500 mg twice daily; amlodipine, 5 mg twice daily; levothyroxine, 112 mcg/d; and atorvastatin, 40 mg at bedtime.
As you review her medications, you wonder if the cyclosporine she began receiving after her kidney transplant could be causing or contributing to her worsening paranoid delusions.
Kidney transplantation has become an ideal treatment for patients with end-stage renal disease. In 2020, 22,817 kidney transplants were performed in the United States.1 Compared with dialysis, kidney transplant allows patients the chance to return to a satisfactory quality of life.2 However, to ensure a successful and long-lasting transplant, patients must be started and maintained on lifelong immunosuppressant agents that have potential adverse effects, including nephrotoxicity and hypertension. Further, immunosuppressant agents—particularly calcineurin inhibitors—are associated with potential adverse effects on mental health. The most commonly documented mental health-related adverse effects include insomnia, anxiety, depression, and confusion.3 In this article, we discuss the risk of severe psychosis associated with the use of calcineurin inhibitors.

Calcineurin inhibitors and psychiatric symptoms
Cyclosporine and tacrolimus are calcineurin inhibitors that are commonly used as immunosuppressant agents after kidney transplantation. They primarily work by specifically and competitively binding to and inhibiting the calcineurin protein to reduce the transcriptional activation of cytokine genes for interleukin-2, tumor necrosis factor-alpha, interleukin-3, interleukin-4, CD40L (CD40 ligand), granulocyte-macrophage colony-stimulating factor, and interferon-gamma.4,5 The ultimate downstream effect is reduced proliferation of T lymphocytes and thereby an immunosuppressed state that will protect against organ rejection. However, this is not the only downstream effect that can occur from inhibiting calcineurin. Cyclosporine and tacrolimus may modulate the activity of dopamine and N-methyl-
An increased effect of dopamine in the mesocortical dopaminergic pathway has long been a suspected cause for psychotic symptoms. A study conducted in rodents suggested that tacrolimus selectively modifies the responsivity and sensitivity of postsynaptic dopamine-2 (D2) and dopamine-3 (D3) receptors.9 These receptors are important when discussing psychosis because antipsychotic medications work primarily by blocking dopamine D2, while many also block the D3 receptor. We hypothesize that modifying the responsivity and sensitivity of these 2 receptors could increase the risk of a person developing psychosis. It may also provide insight into how to best treat a psychotic episode.
Tacrolimus has been shown to elicit inhibition of NMDA-induced neurotransmitter release and augmentation of depolarization-induced neurotransmitter release.10 In theory, this potential inhibition at the NMDA receptors may lead to a compensatory and excessive release of glutamate. Elevated glutamate levels in the brain could lead to psychiatric symptoms, including psychosis. This is supported by the psychosis caused by many NMDA receptor antagonists, such as phencyclidine (PCP) and ketamine. Furthermore, a study examining calcineurin in knockout mice showed that the spectrum of behavioral abnormalities was strikingly similar to those in schizophrenia models.11 These mice displayed impaired working memory, impaired attentional function, social withdrawal, and psychomotor agitation. This further supports the idea that calcineurin inhibition can play a significant role in causing psychiatric symptoms by affecting both dopamine and NMDA receptors.
Continue to: How to address calcineurin inhibitor–induced psychosis...
How to address calcineurin inhibitor–induced psychosis
Here we outline a potential treatment strategy to combat psychosis secondary to calcineurin inhibitors. First, evaluate the patient’s calcineurin inhibitor level (either cyclosporine or tacrolimus). Levels should be drawn as a true trough and doses adjusted if necessary via appropriate consultation with a transplant specialist. Because many of the adverse effects associated with these agents are dose-dependent, we suspect that the risk of psychosis and other mental health–related adverse effects may also follow this trend.
Assuming that the calcineurin inhibitor cannot be stopped, changed to a different agent, or subject to a dose decrease, we recommend initiating an antipsychotic medication to control psychotic symptoms. Given the potential effect of calcineurin inhibitors on dopamine, we suggest trialing a second-generation antipsychotic with relatively high affinity for dopamine D2 receptors, such as risperidone or paliperidone. However, compared with patients with schizophrenia, patients receiving a calcineurin inhibitor may be more likely to develop extrapyramidal symptoms (EPS). Therefore, to avoid potential adverse effects, consider using a lower starting dose or an antipsychotic medication with less dopamine D2 affinity, such as quetiapine, olanzapine, or aripiprazole. Furthermore, because post-transplant patients may be at a higher risk for depression, which may be secondary to medication adverse effects, we suggest avoiding first-generation antipsychotics (FGAs) such as haloperidol because FGAs may worsen depressive symptoms.
We recommend initiating risperidone, 1 mg twice a day, for patients with psychosis secondary to a calcineurin inhibitor. If the patient develops EPS, consider switching to an antipsychotic medication with a less potent dopamine D2 blockade, such as quetiapine, olanzapine, or aripiprazole. We recommend an antipsychotic switch rather than adding benztropine or diphenhydramine to the regimen because many transplant recipients may be older patients, and adding anticholinergic medications can be problematic for this population. However, if the patient is younger or has responded particularly well to risperidone, the benefit of adding an anticholinergic medication may outweigh the risks. This decision should be made on an individual basis and may include other options, such as a switch to quetiapine, olanzapine, or aripiprazole. While these agents may not block the D2 receptor as strongly as risperidone, they all are effective and approved for adjunct therapy in major depressive disorder. We recommend quetiapine and olanzapine as second-line agents because of their potential for sedation and significant weight gain. While aripiprazole has a great metabolic adverse effect profile, its mechanism of action as a partial D2 agonist may make it difficult to control psychotic symptoms in this patient population compared with true D2 antagonists.
Continue to: CASE CONTINUED...
CASE CONTINUED
Mrs. C is admitted to the inpatient psychiatric unit and started on risperidone, 1 mg twice daily. Initially, she complains of lightheadedness at night due to the risperidone, so her dose is changed to 2 mg at bedtime. Gradually, she begins to show mild improvement. Previously, she reported feeling frightened of staff members, but after a few days she reports that she feels safe at the hospital. However, her delusions of being monitored by the FBI persist.
After 9 days of hospitalization, Mrs. C is discharged home to the care of her daughter. At first, she does well, but unfortunately she begins to refuse to take her medication and returns to her baseline.
Related Resources
- Gok F, Eroglu MZ. Acute psychotic disorder associated with immunosuppressive agent use after renal transplantation: a case report. Psychiatry and Clinical Psychopharmacology. 2017;3:314-316.
- Bersani G, Marino P, Valerani G, et al. Manic-like psychosis associated with elevated trough tacrolimus blood concentrations 17 years after kidney transplant. Case Rep Psychiatry. 2013;2013:926395. doi: 10.1155/2013/926395
Drug Brand Names
Amlodipine • Norvasc
Aripiprazole • Abilify
Atorvastatin • Lipitor
Benztropine • Cogentin
Cinacalcet • Sensipar
Cyclosporine • Gengraf
Haloperidol • Haldol
Ketamine • Ketalar
Levothyroxine • Synthroid
Metformin • Glucophage
Mycophenolate mofetil • CellCept
Olanzapine • Zyprexa
Quetiapine • Seroquel
Paliperidone • Invega
Risperidone • Risperdal
Tacrolimus • Prograf
1. Health Resources & Services Administration. US Government Information on Organ Donor Transplantation. Organ Donation Statistics. Updated October 1, 2020. Accessed October 8, 2021. https://www.organdonor.gov/learn/organ-donation-statistics/detailed-description#fig1
2. De Pasquale C, Veroux M, Indelicato L, et al. Psychopathological aspects of kidney transplantation: efficacy of a multidisciplinary team. World J Transplant. 2014;4(4):267-275.
3. Gengraf capsules [package insert]. North Chicago, IL: AbbVie Inc; 2017.
4. Wiederrecht G, Lam E, Hung S, et al. The mechanism of action of FK-506 and cyclosporin A. Ann N Y Acad Sci. 1993;696:9-19.
5. Schreiber SL, Crabtree GR. The mechanism of action of cyclosporin A and FK506. Immunol Today. 1992;13(4):136-142.
6. Scherrer U, Vissing SF, Morgan BJ, et al. Cyclosporine-induced sympathetic activation and hypertension after heart transplantation. N Engl J Med. 1990;323(11):693-699.
7. Fulya G, Meliha ZE. Acute psychotic disorder associated with immunosuppressive agent use after renal transplantation: a case report. Psychiatry and Clinical Psychopharmacology. 2017;27(3):314-316.
8. Tan TC, Robinson PJ. Mechanisms of calcineurin inhibitor-induced neurotoxicity. Transplant Rev. 2006;20(1):49-60.
9. Masatsuna S, Norio M, Nori Takei, et al. Tacrolimus, a specific inhibitor of calcineurin, modifies the locomotor activity of quinpirole, but not that of SKF82958, in male rats. Eur J Pharmacol. 2002;438(1-2):93-97.
10. Gold BG. FK506 and the role of immunophilins in nerve regeneration. Mol Neurobiol. 1997;15(3):285-306.
11. Miyakawa T, Leiter LM, Gerber DJ. Conditional calcineurin knockout mice exhibit multiple abnormal behaviors related to schizophrenia. Proc Natl Acad Sci U S A. 2003;100(15): 8987-8992.
1. Health Resources & Services Administration. US Government Information on Organ Donor Transplantation. Organ Donation Statistics. Updated October 1, 2020. Accessed October 8, 2021. https://www.organdonor.gov/learn/organ-donation-statistics/detailed-description#fig1
2. De Pasquale C, Veroux M, Indelicato L, et al. Psychopathological aspects of kidney transplantation: efficacy of a multidisciplinary team. World J Transplant. 2014;4(4):267-275.
3. Gengraf capsules [package insert]. North Chicago, IL: AbbVie Inc; 2017.
4. Wiederrecht G, Lam E, Hung S, et al. The mechanism of action of FK-506 and cyclosporin A. Ann N Y Acad Sci. 1993;696:9-19.
5. Schreiber SL, Crabtree GR. The mechanism of action of cyclosporin A and FK506. Immunol Today. 1992;13(4):136-142.
6. Scherrer U, Vissing SF, Morgan BJ, et al. Cyclosporine-induced sympathetic activation and hypertension after heart transplantation. N Engl J Med. 1990;323(11):693-699.
7. Fulya G, Meliha ZE. Acute psychotic disorder associated with immunosuppressive agent use after renal transplantation: a case report. Psychiatry and Clinical Psychopharmacology. 2017;27(3):314-316.
8. Tan TC, Robinson PJ. Mechanisms of calcineurin inhibitor-induced neurotoxicity. Transplant Rev. 2006;20(1):49-60.
9. Masatsuna S, Norio M, Nori Takei, et al. Tacrolimus, a specific inhibitor of calcineurin, modifies the locomotor activity of quinpirole, but not that of SKF82958, in male rats. Eur J Pharmacol. 2002;438(1-2):93-97.
10. Gold BG. FK506 and the role of immunophilins in nerve regeneration. Mol Neurobiol. 1997;15(3):285-306.
11. Miyakawa T, Leiter LM, Gerber DJ. Conditional calcineurin knockout mice exhibit multiple abnormal behaviors related to schizophrenia. Proc Natl Acad Sci U S A. 2003;100(15): 8987-8992.
