User login
Monoamine oxidase inhibitors and tricyclic antidepressants for MDD
Ms. B, age 45, has a history of major depressive disorder (MDD) and migraines. She is admitted after presenting with anhedonia, hopelessness, and hypersomnia. These symptoms have become more severe over the last few weeks. Ms. B describes a past suicide attempt via overdose on doxylamine for which she required treatment in the intensive care unit. The only activity she enjoys is her weekly girls’ night, during which she drinks a few glasses of wine. Ms. B’s current medications are dextromethorphan/bupropion 45/105 mg twice daily and aripiprazole 5 mg/d, which she has taken for 3 months. She states she has “been on every antidepressant there is.”
When clinicians review Ms. B’s medication history, it is clear she has had adequate trials of selective serotonin reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake inhibitors (SNRIs), intranasal esketamine, multiple augmentation strategies, and electroconvulsive therapy (ECT). Ms. B seeks an alternative medication to improve her depressive symptoms.
Treatment-resistant depression (TRD) is commonly defined as depression that has not responded to ≥2 adequate trials of an antidepressant.1 Some guidelines recommend monoamine oxidase inhibitors (MAOIs) and tricyclic antidepressants (TCAs) as second- or even third-line options for MDD,2 while others recommend reserving them for patients with insufficient responses to alternative treatment modalities.3,4 Although MAOIs and TCAs have been available since the 1950s, prescribing these medications has become less prevalent due to safety concerns, the availability of other pharmacologic options, and a lack of clinical training and comfort.5,6 Most research notes that MAOIs are superior for treating atypical depression while TCAs are more effective for melancholic depression.2-4 In a review of 20 studies, Thase et al7 found that 50% of TCA nonresponders benefited from an MAOI. In the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial, monotherapy with the MAOI tranylcypromine was associated with a lower remission rate than the TCA nortriptyline; many argue the dose of tranylcypromine was suboptimal, and few participants completed an adequate trial in the last level.8,9 A more recent study by Kim et al10 found MAOIs to be “generally more effective” than TCAs for TRD, particularly in patients with fewer antidepressant trials; however, this was a small retrospective exploratory trial. A network meta-analysis found both classes to be “competitive” with SSRIs based on efficacy and tolerability, which leads to the question of whether these medications should be considered earlier in therapy.11 Considering patient-specific factors and particular medication properties is an effective strategy when prescribing an MAOI or TCA.
Monoamine oxidase inhibitors
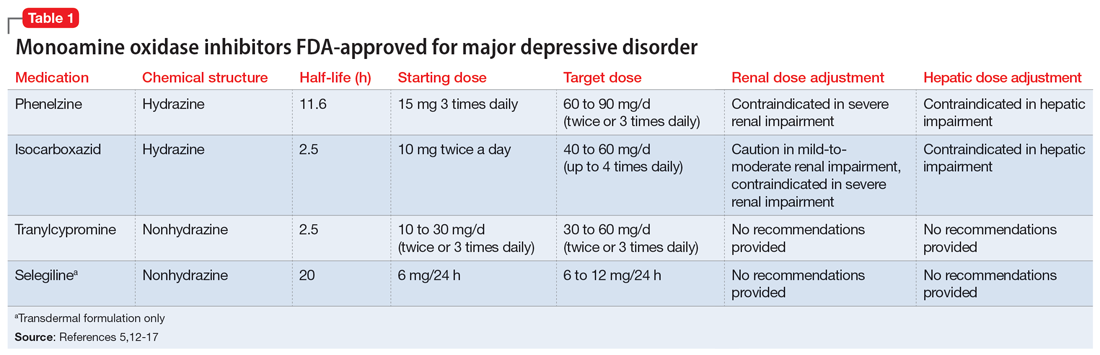
Four MAOIs are FDA-approved for treating MDD (Table 15,12-17): phenelzine, isocarboxazid, tranylcypromine, and selegiline. These medications irreversibly inhibit MAO, which exists as isomers A and B. MAO-A primarily metabolizes serotonin and norepinephrine, which is largely responsible for these medications’ antidepressant effects. Both isomers equally metabolize dopamine.5,12,18 It is best to avoid using MAOIs in patients with cerebrovascular disease, hepatic disease, or pheochromocytoma. Patients with active substance use disorders (particularly sympathomimetics and hallucinogens) are at an increased risk for hypertensive crises and serotonin syndrome, respectively. The most common adverse effects are orthostatic hypotension (despite more well-known concerns regarding hypertension), alterations in sleep patterns (insomnia or hypersomnia, depending on the agent), gastrointestinal issues, and anticholinergic adverse effects such as dry mouth and constipation.13,19-21
In one review and meta-analysis, phenelzine displayed the highest efficacy across all MAOIs.11 It likely requires high doses to achieve adequate MAO inhibition.11 A metabolite of phenelzine inhibits gamma-aminobutyric acid transaminase and may be helpful for patients with comorbid anxiety disorders or MDD with anxious distress.18,21 Additional considerations include phenelzine’s propensity for orthostasis (with rapid titrations and higher doses), sedation, weight gain, sexual dysfunction, and a rare adverse effect of vitamin B6 deficiency.5,13,14,20-22
Use of isocarboxazid in clinical practice is rare. Its adverse effects are similar to those of phenelzine but isocarboxazid is less studied. Tranylcypromine has a similar chemical structure to amphetamine. It can be stimulating at higher doses, potentially benefitting patients with comorbid attention-deficit/hyperactivity disorder (ADHD) or significant apathy.13,23 Selegiline’s distinct quality is its availability as a transdermal patch, which may be useful for patients who struggle to take oral medications. At low doses (6 mg/24 h), the selegiline transdermal patch allows patients to disregard a dietary tyramine restriction because it avoids first-pass metabolism. It inhibits both MAO isomers in the brain but is only selective for MAO-B once concentrations are distributed to the liver. Higher doses require a tyramine-restricted diet because there is still some MAO-A inhibition in the gut. Selegiline is also stimulating because it is converted to amphetamine and methamphetamine.5,12,13,17,19,24
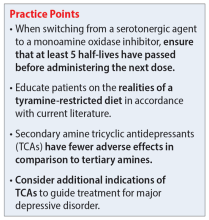
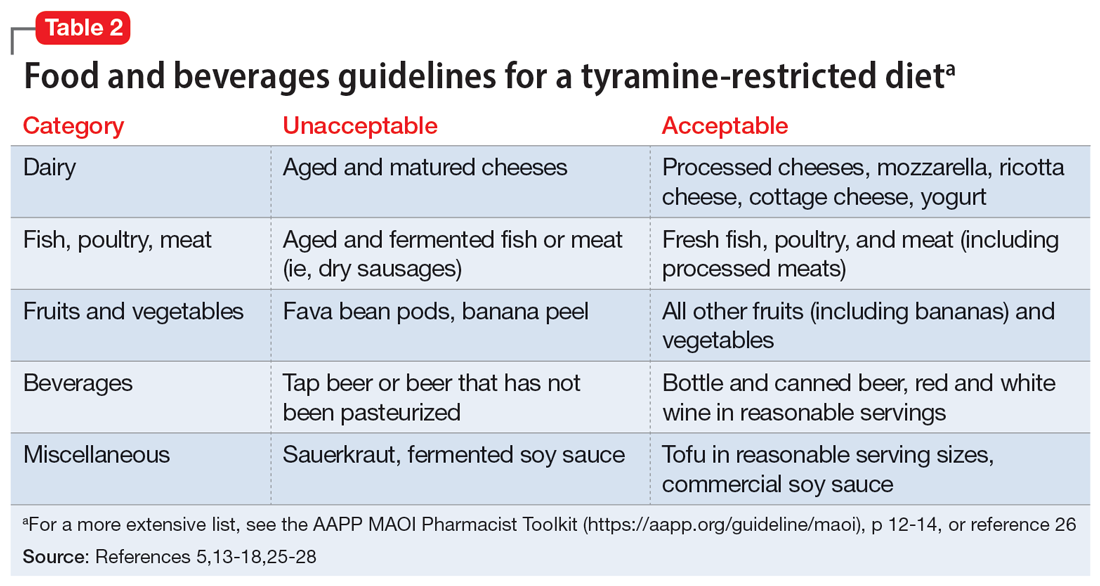
Despite promising results from the use of MAOIs, physicians and patients may be reluctant to use these medications due to perceived limitations. One prominent barrier is the infamous “cheese reaction.” Tyramine, an amino acid found in certain food and beverages (Table 25,13-18,25-28), is broken down by MAO-A in the gut. When this enzyme is inhibited, higher concentrations of tyramine reach systemic circulation. Tyramine’s release of norepinephrine (which now cannot be broken down) can lead to a hypertensive crisis. Consequently, a tyramine-restricted diet is recommended for patients taking an MAOI. However, the common notion that cheese, wine, and beer must be avoided is false, because most of the dietary restrictions developed following the discovery of MAOIs are antiquated.5,12,25-28 Patients who take an MAOI only need to slightly adjust their diet, as outlined in Table 2.5,13-18,25-28 A reasonable serving size of most foods and beverages containing tyramine is unlikely to elicit this “pressor” response. Of the 4 MAOIs FDA-approved for MDD, tranylcypromine appears to be the most sensitive to tyramine.21 Transient postdose hypertension (regardless of tyramine) may occur after taking an MAOI.29 Encourage patients to monitor their blood pressure.
Continue to: Additional hurdles include...
Additional hurdles include the required washout period from serotonergic medications and interactions with sympathomimetics. MAOIs pose the highest risk of serotonin syndrome; however, this usually occurs if given concomitantly with other serotonergic agents. The standard recommendation is a 14-day washout period from SSRIs (5 weeks for fluoxetine and 3 weeks for vortioxetine), SNRIs, mirtazapine, and other antidepressants. It can be distressing for patients to be without medication during that period. Because some antidepressants have much shorter half-lives, waiting 5 half-lives (typically 5 to 7 days) for the discontinued medication to be excreted is feasible if patients are closely monitored.5,12,13,25,27,30 There are rare instances where a TCA may be combined with an MAOI (typically initiated within 1 to 2 days of each other), but never clomipramine or imipramine due to their potent serotonin reuptake inhibition.31 If switching to an alternative MAOI, waiting 7 to 14 days is recommended to allow adequate time for the inhibited enzyme to regenerate.14-17,32 Taking medications that increase dopamine and norepinephrine (eg, stimulants or oral over-the-counter decongestants) with an MAOI is typically not recommended due to the risk of hypertensive crisis.25,27 In severe TRD or comorbid ADHD, successful simultaneous use of methylphenidate or amphetamine—typically at low doses—with close blood pressure monitoring has been reported.33 There have also been positive cases of the use of modafinil in combination with an MAOI; however, this should be done with caution.34,35 Clinicians must use clinical judgment when considering a combination of medications that pose a higher risk.
Tricyclic antidepressants
TCAs work differently than MAOIs to increase monoamines. They inhibit presynaptic serotonin and norepinephrine transporters in the CNS to increase levels of these chemicals in the synaptic cleft. While all TCAs inhibit these transporters, they do so at varying levels (Table 336-51). Based on their chemical structure, TCAs can be categorized into secondary and tertiary amines. Tertiary amines are metabolized via demethylation into their derivatives (Table 336-51). Patients who have recently suffered a myocardial infarction (MI) should avoid tertiary amines. TCAs can reduce heart rate variability, which is already decreased after an MI, thus presenting the potential for cardiac arrhythmias. TCAs should also be avoided in patients with cardiac conduction abnormalities.38-46,52 Patients with a prior baseline cardiac conduction defect, such as a bundle branch block, are at higher risk for further cardiac abnormalities. In those with a preexisting first-degree heart block, TCAs can still be used, but electrocardiogram monitoring is recommended.52,53 TCAs have also been reported to decrease the seizure threshold.38-46 They can be used with caution in patients who have a history of epilepsy or head trauma, or with concomitant medications that lower the seizure threshold.38-46
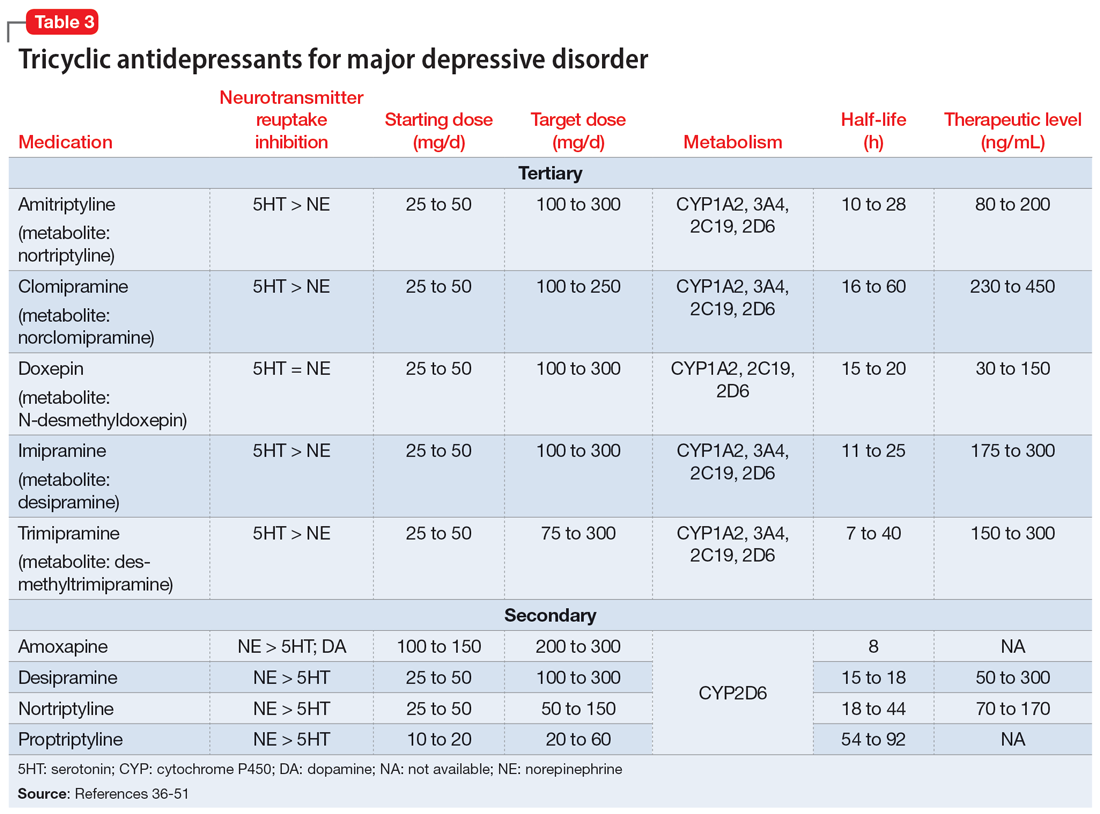
Overdose risk is a concern with TCAs because ingestion of 10 to 20 mg/kg can lead to significant toxicity.54 This is due to their blockage of voltage-gated sodium channels found in the CNS and heart, which contributes to overdose symptoms such as a widened QRS complex and seizures. Symptoms usually develop within 2 hours but may be delayed up to 6 hours.55 Patients with a history of overdose must be carefully assessed before initiating a TCA. Prescribing a limited supply of these medications may be valuable. The use of TCAs has often been limited due to their adverse effects, most of which are associated with their respective affinities for alpha 1, muscarinic 1, and histamine 1 receptors. Inhibition of the alpha 1 receptor is associated with hypotension, muscarinic 1 with anticholinergic adverse effects, and histamine 1 with sedation and weight gain. Tertiary amines have a higher affinity for these receptors compared to secondary amines, leading to a more significant adverse effect profile.36,50 Among TCAs, amitriptyline is the most likely to cause hypotension, whereas desipramine and nortriptyline are least likely. Amitriptyline and clomipramine are most likely to cause anticholinergic adverse effects, whereas desipramine and nortriptyline are the least likely. Amitriptyline, doxepin, and imipramine have the highest propensity for QTc prolongation.36
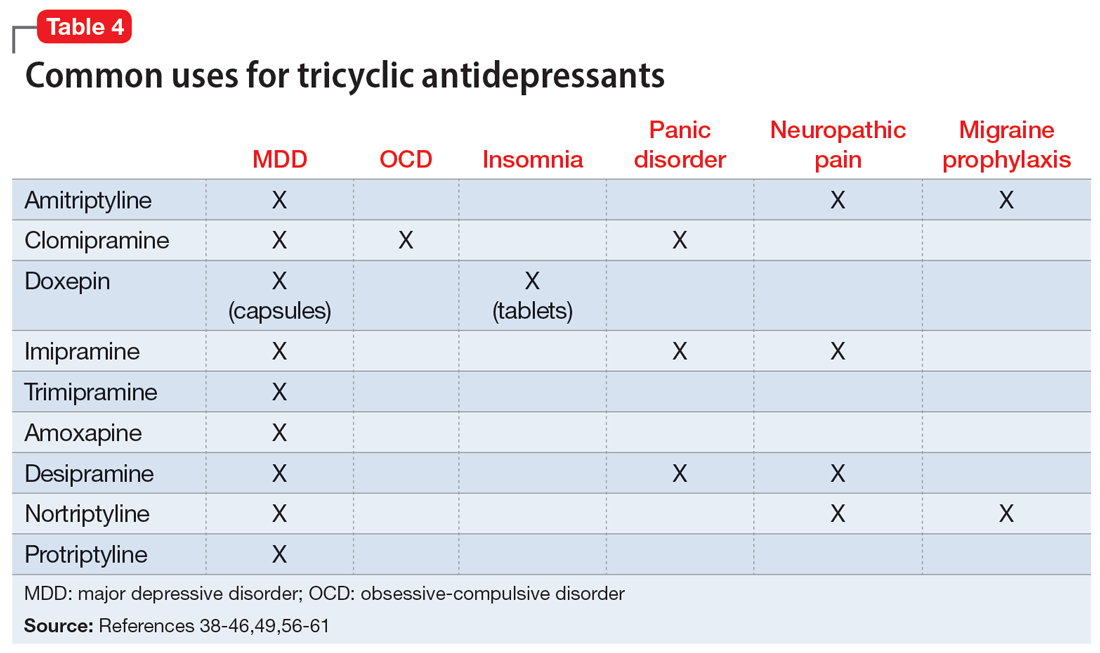
Beyond treating MDD, TCAs have shown benefits for treating other disease states (Table 438-46,49,56-61).These differing indications may help psychiatrists determine the best TCA to prescribe for a given patient. Amitriptyline is the most studied TCA for MDD; however, nortriptyline is typically preferred due to its favorable tolerability profile.4,62 Nortriptyline also has data supporting its use in ECT to prevent relapse.63 Amitriptyline and nortriptyline have shown benefits in patients with neuropathic pain and for migraine prophylaxis.56-60 Although frequently used for MDD, clomipramine is not FDA-approved for this indication, but is for obsessive-compulsive disorder.39 Doxepin is FDA-approved for insomnia at lower doses and for MDD at higher doses.40 Therefore, it may benefit patients with sleep difficulties secondary to depression. Desipramine has been used off-label to treat ADHD in children and has shown some benefits in adults.64-66 Protriptyline, trimipramine, and amoxapine are infrequently used in clinical practice.
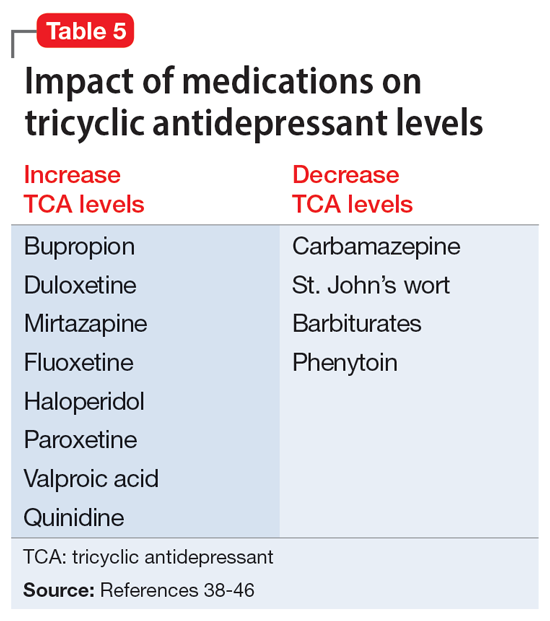
A unique feature of TCAs is the ability to monitor serum concentrations (Table 336-51).Guidelines recommend therapeutic drug monitoring (TDM) with amitriptyline, clomipramine, imipramine, and nortriptyline for routine use. TDM is still recommended for doxepin, desipramine, and trimipramine, but its utility is largely for treatment failure or resistance.37 These plasma levels can be altered based on coadministered medications (Table 538-46) and should be closely monitored. Physicians should obtain a trough level after at least 5 half-lives and before the next dose is due, and use TDM as indicated to optimize dosing.
Continue to: CASE CONTINUED
CASE CONTINUED
Ms. B’s outpatient psychiatrist provides collateral information about her medical history and confirms her long-standing MDD with multiple medication trials, though she has never received an MAOI or TCA. Ms. B is adamant she does not want a medication-free period between treatments and refuses to adjust her diet, despite being educated on the few changes necessary. She has no contraindications for TCAs and may benefit from a TCA for her comorbid migraines. The care team expresses concern for TCA overdose to Ms. B and her family. Ms. B’s sister reassures the team they will have someone monitor and dispense her medications at home. They decide to discontinue her current psychiatric regimen, and Ms. B is started on nortriptyline 50 mg/d at night, with plans to titrate based on tolerability.
Related Resources
- Meyer JM. A concise guide to monoamine oxidase inhibitors. Current Psychiatry. 2017;16(12):14-16,18-23,47,A.
- Espejo GD. Treating major depressive disorder after limited response to an initial agent. Current Psychiatry. 2021;20(10):51-53. doi:10.12788/cp.0178
- American Association of Psychiatric Pharmacists (AAPP) MAOI Pharmacist Toolkit. https://aapp.org/guideline/maoi
Drug Brand Names
Amitriptyline • Elavil
Amphetamine • Adzenys, Dyanavel
Aripiprazole • Abilify
Clomipramine • Anafranil
Desipramine • Norpramin
Dextromethorphan/bupropion • Auvelity
Doxepin • Sinequan, Adapin
Esketamine • Spravato
Fluoxetine • Prozac
Imipramine • Tofranil
Isocarboxazid • Marplan
Methamphetamine • Desoxyn
Mirtazapine • Remeron
Modafinil • Provigil
Nortriptyline • Pamelor
Phenelzine • Nardil
Protriptyline • Vivactil
Selegiline • Emsam
Tranylcypromine • Parnate
Trimipramine • Surmontil
Vortioxetine • Trintellix
1. Gaynes BN, Lux L, Gartlehner G, et al. Defining treatment-resistant depression. Depress Anxiety. 2020;37(2):134-145. doi:10.1002/da.22968
2. Kennedy SH, Lam RW, McIntyre RS, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: section 3. Pharmacological treatments. Can J Psychiatry. 2016;61(9):540-560. doi:10.1177/0706743716659417
3. VA/DoD clinical practice guideline for the management of major depressive disorder. Veterans Health Administration and Department of Defense; 2016. https://www.healthquality.va.gov/guidelines/MH/mdd/VADoDMDDCPGFINAL82916.pdf
4. Gelenberg AJ, Freeman MP, Markowitz JC, et al. Practice guideline for the treatment of patients with major depressive disorder. Am J Psychiatry. 2010;167(Suppl 10):9-118.
5. Meyer JM. A concise guide to monoamine oxidase inhibitors. Current Psychiatry. 2017;16(12):14-16,18-23,47,A.
6. Taylor D. Selective serotonin reuptake inhibitors and tricyclic antidepressants in combination. Interactions and therapeutic uses. Br J Psychiatry. 1995;167(5):575-580. doi:10.1192/bjp.167.5.575
7. Thase ME, Trivedi MH, Rush AJ. MAOIs in the contemporary treatment of depression. Neuropsychopharmacology. 1995;12(3):185-219. doi:10.1016/0893-133X(94)00058-8
8. Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905-1917. doi:10.1176/ajp.2006.163.11.1905
9. McGrath PJ, Stewart JW, Fava M, et al. Tranylcypromine versus venlafaxine plus mirtazapine following three failed antidepressant medication trials for depression: a STAR*D report. Am J Psychiatry. 2006;163(9):1531-1666. doi:10.1176/ajp.2006.163.9.1531
10. Kim T, Xu C, Amsterdam JD. Relative effectiveness of tricyclic antidepressant versus monoamine oxidase inhibitor monotherapy for treatment-resistant depression. J Affect Disord. 2019;250:199-203. doi:10.1016/j.jad.2019.03.028
11. Suchting R, Tirumalajaru V, Gareeb R, et al. Revisiting monoamine oxidase inhibitors for the treatment of depressive disorders: a systematic review and network meta-analysis. J Affect Disord. 2021;282:1153-1160. doi:10.1016/j.jad.2021.01.021
12. Stahl SM, Felker A. Monoamine oxidase inhibitors: a modern guide to an unrequited class of antidepressants. CNS Spectr. 2008;13(10):855-870. doi:10.1017/s1092852900016965
13. Chamberlain SR, Baldwin DS. Monoamine oxidase inhibitors (MAOIs) in psychiatric practice: how to use them safely and effectively. CNS Drugs. 2021;35(7):703-716. doi:10.1007/s40263-021-00832-x
14. Nardil [package insert]. New York, NY: Parke-Davis; 2009.
15. Marplan [package insert]. Parsippany, NJ: Validus Pharmaceuticals LLC; 2020.
16. Parnate [package insert]. Saint Michael, Barbados: Concordia Pharmaceuticals; 2015.
17. Emsam [package insert]. Morgantown, WV: Mylan Specialty LP; 2014.
18. Shulman KI, Herrmann N, Walker SE. Current place of monoamine oxidase inhibitors in the treatment of depression. CNS Drugs. 2013;27(10):789-797. doi:10.1007/s40263-013-0097-3
19. Sub Laban T, Saadabadi A. Monoamine oxidase inhibitors (MAOI). StatPearls Publishing; 2023. https://www.ncbi.nlm.nih.gov/books/NBK539848/
20. Rabkin JG, Quitkin FM, McGrath P, et al. Adverse reactions to monoamine oxidase inhibitors. Part II. Treatment correlates and clinical management. J Clin Psychopharmacol. 1985;5(1):2-9.
21. Gillman PK. Advances pertaining to the pharmacology and interactions of irreversible nonselective monoamine oxidase inhibitors. J Clin Psychopharmacol. 2011;31(1):66-74. doi:10.1097/JCP.0b013e31820469ea
22. Sidhu G, Marwaha R. Phenelzine. StatPearls Publishing; 2023. https://www.ncbi.nlm.nih.gov/books/NBK554508/
23. Frieling H, Bleich S. Tranylcypromine: new perspectives on an “old” drug. Eur Arch Psychiatry Clin Neurosci. 2006;256(5):268-273. doi:10.1007/s00406-006-0660-8
24. Goodnick PJ. Seligiline transdermal system in depression. Expert Opin Pharmacother. 2007;8(1):59-64. doi:10.1517/14656566.8.1.59
25. Edinoff AN, Swinford CR, Odisho AS, et al. Clinically relevant drug interactions with monoamine oxidase inhibitors. Health Psychol Res. 2022;10(4):39576. doi:10.52965/001c.39576
26. Gillman PK. A reassessment of the safety profile of monoamine oxidase inhibitors: elucidating tired old tyramine myths. J Neural Transm (Vienna). 2018;125(11):1707-1717. doi:10.1007/s00702-018-1932-y
27. Flockhart DA. Dietary restrictions and drug interactions with monoamine oxidase inhibitors: an update. J Clin Psychiatry. 2012;73 Suppl 1:17-24. doi:10.4088/JCP.11096su1c.03
28. McCabe-Sellers BJ, Staggs CG, Bogle ML. Tyramine in foods and monoamine oxidase inhibitor drugs: a crossroad where medicine, nutrition, pharmacy, and food industry converge. J Food Composit Anal. 2006;19:S58-S65. doi:10.1016/j.jfca.2005.12.008
29. Keck PE Jr, Vuckovic A, Pope HG Jr, et al. Acute cardiovascular response to monoamine oxidase inhibitors: a prospective assessment. J Clin Psychopharmacol. 1989;9(3):203-206.
30. Bodkin JA, Dunlop BW. Moving on with monoamine oxidase inhibitors. Focus (Am Psychiatr Publ). 2021;19(1):50-52. doi:10.1176/appi.focus.20200046
31. Amsterdam JD, Kim TT. Relative effectiveness of monoamine oxidase inhibitor and tricyclic antidepressant combination therapy for treatment-resistant depression. J Clin Psychopharmacol. 2019;39(6):649-652. doi:10.1097/JCP.0000000000001130
32. Keks N, Hope J, Keogh S. Switching and stopping antidepressants. Aust Prescr. 2016;39(3):76-83. doi:10.18773/austprescr.2016.039
33. Israel JA. Combining stimulants and monoamine oxidase inhibitors: a reexamination of the literature and a report of a new treatment combination. Prim Care Companion CNS Disord. 2015;17(6):10.4088/PCC.15br01836. doi:10.4088/PCC.15br01836
34. Clemons WE, Makela E, Young J. Concomitant use of modafinil and tranylcypromine in a patient with narcolepsy: a case report. Sleep Med. 2004;5(5):509-511. doi:10.1016/j.sleep.2004.06.006
35. Ashton AK. Modafinil augmentation of phenelzine for residual fatigue in dysthymia. Am J Psychiatry. 2004;161(9):1716-1717. doi:10.1176/appi.ajp.161.9.1716-a
36. O’Donnell JM, Bies RR, Shelton RC. Drug therapy of depression and anxiety disorders. In: Brunton LL, Hilal-Dandan R, Knollmann BC, eds. Goodman & Gilman’s: The Pharmacological Basis of Therapeutics. 13th ed. McGraw Hill; 2017. Accessed June 4, 2023. https://accessanesthesiology.mhmedical.com/content.aspx?bookid=2189§ionid=169518711
37. Hiemke C, Bergemann N, Clement HW, et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry. 2018;51(1-02):9-62. doi:10.1055/s-0043-116492
38. Amitriptyline hydrochloride [package insert]. East Brunswick, NJ: Unichem Pharmaceuticals (USA); 2021.
39. Clomipramine hydrochloride [package insert]. East Windsor, NJ: Aurobindo Pharma Limited; 2023.
40. Doxepin hydrochloride capsules, USP [package insert]. Bedminster, NJ: Alembic Pharmaceuticals Inc; 2021.
41. Imipramine hydrochloride tablet [package insert]. Fairfield, NJ: Leading Pharma LLC USA; 2022.
42. Trimipramine maleate [package insert]. Northvale, NJ: Elite Laboratories Inc; 2021.
43. Amoxapine [package insert]. Parsippany, NJ: Actavis Pharma Inc; 2015.
44. Desipramine hydrochloride tablets [package insert]. Bedminster, NJ: Alembic Pharmaceuticals Inc; 2023.
45. Nortriptyline hydrochloride capsules, USP [package insert]. Parsippany, NJ: Teva Pharmaceuticals Inc; 2021.
46. Protriptyline hydrochloride [package insert]. Bensalem, PA: Sigmapharm Laboratories, LLC; 2023.
47. Calvo B, García MJ, Pedraz JL, et al. Pharmacokinetics of amoxapine and its active metabolites. Int J Clin Pharmacol Ther Toxicol. 1985;23(4):180-185.
48. Ziegler VE, Biggs JT, Wylie LT, et al. Protriptyline kinetics. Clin Pharmacol Ther. 1978;23(5):580-584. doi:10.1002/cpt1978235580
49. Cleare A, Pariante CM, Young AH, et al. Evidence-based guidelines for treating depressive disorders with antidepressants: a revision of the 2008 British Association for Psychopharmacology guidelines. J Psychopharmacol. 2015;29(5):459-525. doi:10.1177/0269881115581093
50. Richelson E. Synaptic effects of antidepressants. J Clin Psychopharmacol. 1996;16(3 Suppl 2):1S-9S. doi:10.1097/00004714-199606002-00001
51. Vos CF, Aarnoutse RE, Op de Coul MJM, et al. Tricyclic antidepressants for major depressive disorder: a comprehensive evaluation of current practice in the Netherlands. BMC Psychiatry. 2021;21(1):481. doi:10.1186/s12888-021-03490-x
52. Alvarez W Jr, Pickworth KK. Safety of antidepressant drugs in the patient with cardiac disease: a review of the literature. Pharmacotherapy. 2003;23(6):754-771. doi:10.1592/phco.23.6.754.32185
53. Dietch JT, Fine M. The effect of nortriptyline in elderly patients with cardiac conduction disease. J Clin Psychiatry. 1990;51(2):65-67.
54. Valento M, Liebelt EL. Cyclic antidepressants. In: Nelson LS, Howland M, Lewin NA, et al, eds. Goldfrank’s Toxicologic Emergencies. 9th ed. McGraw Hill; 2011. Accessed June 10, 2023. https://accesspharmacy.mhmedical.com/content.aspx?bookid=2569§ionid=210274664
55. Woolf AD, Erdman AR, Nelson LS, et al. Tricyclic antidepressant poisoning: an evidence-based consensus guideline for out-of-hospital management. Clin Toxicol (Phila). 2007;45(3):203-233. doi:10.1080/15563650701226192
56. Baldwin DS, Anderson IM, Nutt DJ, et al. Evidence-based pharmacological treatment of anxiety disorders, post-traumatic stress disorder and obsessive-compulsive disorder: a revision of the 2005 guidelines from the British Association for Psychopharmacology. J Psychopharmacol. 2014;28(5):403-439. doi:10.1177/0269881114525674
57. Moulin DE, Clark AJ, Gilron I, et al. Pharmacological management of chronic neuropathic pain - consensus statement and guidelines from the Canadian Pain Society. Pain Res Manag. 2007;12(1):13-21. doi:10.1155/2007/730785
58. Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14(2):162-173. doi:10.1016/S1474-4422(14)70251-0
59. Saarto T, Wiffen PJ. Antidepressants for neuropathic pain. Cochrane Database Syst Rev. 2007;(4):CD005454. doi:10.1002/14651858.CD005454.pub2
60. Burch R. Antidepressants for preventive treatment of migraine. Curr Treat Options Neurol. 2019;21(4):18. doi:10.1007/s11940-019-0557-2
61. Ailani J, Burch RC, Robbins MS; Board of Directors of the American Headache Society. The American Headache Society consensus statement: update on integrating new migraine treatments into clinical practice. Headache. 2021;61(7):1021-1039. doi:10.1111/head.14153
62. Bauer M, Pfennig A, Severus E, et al. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of unipolar depressive disorders, part 1: update 2013 on the acute and continuation treatment of unipolar depressive disorders. World J Biol Psychiatry. 2013;14(5):334-385. doi:10.3109/15622975.2013.804195
63. Jelovac A, Kolshus E, McLoughlin DM. Relapse following successful electroconvulsive therapy for major depression: a meta-analysis. Neuropsychopharmacology. 2013;38(12):2467-2474. doi:10.1038/npp.2013.149
64. Spencer T, Biederman J, Coffey B, et al. A double-blind comparison of desipramine and placebo in children and adolescents with chronic tic disorder and comorbid attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2002;59(7):649-656. doi:10.1001/archpsyc.59.7.649
65. Spencer T, Biederman J, Wilens T, et al. Pharmacotherapy of attention-deficit hyperactivity disorder across the life cycle. J Am Acad Child Adolesc Psychiatry. 1996;35(4):409-432. doi:10.1097/00004583-199604000-00008
66. Wilens TE, Biederman J, Prince J, et al. Six-week, double-blind, placebo-controlled study of desipramine for adult attention deficit hyperactivity disorder. Am J Psychiatry. 1996;153(9):1147-1153. doi:10.1176/ajp.153.9.1147
Ms. B, age 45, has a history of major depressive disorder (MDD) and migraines. She is admitted after presenting with anhedonia, hopelessness, and hypersomnia. These symptoms have become more severe over the last few weeks. Ms. B describes a past suicide attempt via overdose on doxylamine for which she required treatment in the intensive care unit. The only activity she enjoys is her weekly girls’ night, during which she drinks a few glasses of wine. Ms. B’s current medications are dextromethorphan/bupropion 45/105 mg twice daily and aripiprazole 5 mg/d, which she has taken for 3 months. She states she has “been on every antidepressant there is.”
When clinicians review Ms. B’s medication history, it is clear she has had adequate trials of selective serotonin reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake inhibitors (SNRIs), intranasal esketamine, multiple augmentation strategies, and electroconvulsive therapy (ECT). Ms. B seeks an alternative medication to improve her depressive symptoms.
Treatment-resistant depression (TRD) is commonly defined as depression that has not responded to ≥2 adequate trials of an antidepressant.1 Some guidelines recommend monoamine oxidase inhibitors (MAOIs) and tricyclic antidepressants (TCAs) as second- or even third-line options for MDD,2 while others recommend reserving them for patients with insufficient responses to alternative treatment modalities.3,4 Although MAOIs and TCAs have been available since the 1950s, prescribing these medications has become less prevalent due to safety concerns, the availability of other pharmacologic options, and a lack of clinical training and comfort.5,6 Most research notes that MAOIs are superior for treating atypical depression while TCAs are more effective for melancholic depression.2-4 In a review of 20 studies, Thase et al7 found that 50% of TCA nonresponders benefited from an MAOI. In the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial, monotherapy with the MAOI tranylcypromine was associated with a lower remission rate than the TCA nortriptyline; many argue the dose of tranylcypromine was suboptimal, and few participants completed an adequate trial in the last level.8,9 A more recent study by Kim et al10 found MAOIs to be “generally more effective” than TCAs for TRD, particularly in patients with fewer antidepressant trials; however, this was a small retrospective exploratory trial. A network meta-analysis found both classes to be “competitive” with SSRIs based on efficacy and tolerability, which leads to the question of whether these medications should be considered earlier in therapy.11 Considering patient-specific factors and particular medication properties is an effective strategy when prescribing an MAOI or TCA.
Monoamine oxidase inhibitors
Four MAOIs are FDA-approved for treating MDD (Table 15,12-17): phenelzine, isocarboxazid, tranylcypromine, and selegiline. These medications irreversibly inhibit MAO, which exists as isomers A and B. MAO-A primarily metabolizes serotonin and norepinephrine, which is largely responsible for these medications’ antidepressant effects. Both isomers equally metabolize dopamine.5,12,18 It is best to avoid using MAOIs in patients with cerebrovascular disease, hepatic disease, or pheochromocytoma. Patients with active substance use disorders (particularly sympathomimetics and hallucinogens) are at an increased risk for hypertensive crises and serotonin syndrome, respectively. The most common adverse effects are orthostatic hypotension (despite more well-known concerns regarding hypertension), alterations in sleep patterns (insomnia or hypersomnia, depending on the agent), gastrointestinal issues, and anticholinergic adverse effects such as dry mouth and constipation.13,19-21

In one review and meta-analysis, phenelzine displayed the highest efficacy across all MAOIs.11 It likely requires high doses to achieve adequate MAO inhibition.11 A metabolite of phenelzine inhibits gamma-aminobutyric acid transaminase and may be helpful for patients with comorbid anxiety disorders or MDD with anxious distress.18,21 Additional considerations include phenelzine’s propensity for orthostasis (with rapid titrations and higher doses), sedation, weight gain, sexual dysfunction, and a rare adverse effect of vitamin B6 deficiency.5,13,14,20-22
Use of isocarboxazid in clinical practice is rare. Its adverse effects are similar to those of phenelzine but isocarboxazid is less studied. Tranylcypromine has a similar chemical structure to amphetamine. It can be stimulating at higher doses, potentially benefitting patients with comorbid attention-deficit/hyperactivity disorder (ADHD) or significant apathy.13,23 Selegiline’s distinct quality is its availability as a transdermal patch, which may be useful for patients who struggle to take oral medications. At low doses (6 mg/24 h), the selegiline transdermal patch allows patients to disregard a dietary tyramine restriction because it avoids first-pass metabolism. It inhibits both MAO isomers in the brain but is only selective for MAO-B once concentrations are distributed to the liver. Higher doses require a tyramine-restricted diet because there is still some MAO-A inhibition in the gut. Selegiline is also stimulating because it is converted to amphetamine and methamphetamine.5,12,13,17,19,24
Despite promising results from the use of MAOIs, physicians and patients may be reluctant to use these medications due to perceived limitations. One prominent barrier is the infamous “cheese reaction.” Tyramine, an amino acid found in certain food and beverages (Table 25,13-18,25-28), is broken down by MAO-A in the gut. When this enzyme is inhibited, higher concentrations of tyramine reach systemic circulation. Tyramine’s release of norepinephrine (which now cannot be broken down) can lead to a hypertensive crisis. Consequently, a tyramine-restricted diet is recommended for patients taking an MAOI. However, the common notion that cheese, wine, and beer must be avoided is false, because most of the dietary restrictions developed following the discovery of MAOIs are antiquated.5,12,25-28 Patients who take an MAOI only need to slightly adjust their diet, as outlined in Table 2.5,13-18,25-28 A reasonable serving size of most foods and beverages containing tyramine is unlikely to elicit this “pressor” response. Of the 4 MAOIs FDA-approved for MDD, tranylcypromine appears to be the most sensitive to tyramine.21 Transient postdose hypertension (regardless of tyramine) may occur after taking an MAOI.29 Encourage patients to monitor their blood pressure.

Continue to: Additional hurdles include...
Additional hurdles include the required washout period from serotonergic medications and interactions with sympathomimetics. MAOIs pose the highest risk of serotonin syndrome; however, this usually occurs if given concomitantly with other serotonergic agents. The standard recommendation is a 14-day washout period from SSRIs (5 weeks for fluoxetine and 3 weeks for vortioxetine), SNRIs, mirtazapine, and other antidepressants. It can be distressing for patients to be without medication during that period. Because some antidepressants have much shorter half-lives, waiting 5 half-lives (typically 5 to 7 days) for the discontinued medication to be excreted is feasible if patients are closely monitored.5,12,13,25,27,30 There are rare instances where a TCA may be combined with an MAOI (typically initiated within 1 to 2 days of each other), but never clomipramine or imipramine due to their potent serotonin reuptake inhibition.31 If switching to an alternative MAOI, waiting 7 to 14 days is recommended to allow adequate time for the inhibited enzyme to regenerate.14-17,32 Taking medications that increase dopamine and norepinephrine (eg, stimulants or oral over-the-counter decongestants) with an MAOI is typically not recommended due to the risk of hypertensive crisis.25,27 In severe TRD or comorbid ADHD, successful simultaneous use of methylphenidate or amphetamine—typically at low doses—with close blood pressure monitoring has been reported.33 There have also been positive cases of the use of modafinil in combination with an MAOI; however, this should be done with caution.34,35 Clinicians must use clinical judgment when considering a combination of medications that pose a higher risk.
Tricyclic antidepressants
TCAs work differently than MAOIs to increase monoamines. They inhibit presynaptic serotonin and norepinephrine transporters in the CNS to increase levels of these chemicals in the synaptic cleft. While all TCAs inhibit these transporters, they do so at varying levels (Table 336-51). Based on their chemical structure, TCAs can be categorized into secondary and tertiary amines. Tertiary amines are metabolized via demethylation into their derivatives (Table 336-51). Patients who have recently suffered a myocardial infarction (MI) should avoid tertiary amines. TCAs can reduce heart rate variability, which is already decreased after an MI, thus presenting the potential for cardiac arrhythmias. TCAs should also be avoided in patients with cardiac conduction abnormalities.38-46,52 Patients with a prior baseline cardiac conduction defect, such as a bundle branch block, are at higher risk for further cardiac abnormalities. In those with a preexisting first-degree heart block, TCAs can still be used, but electrocardiogram monitoring is recommended.52,53 TCAs have also been reported to decrease the seizure threshold.38-46 They can be used with caution in patients who have a history of epilepsy or head trauma, or with concomitant medications that lower the seizure threshold.38-46

Overdose risk is a concern with TCAs because ingestion of 10 to 20 mg/kg can lead to significant toxicity.54 This is due to their blockage of voltage-gated sodium channels found in the CNS and heart, which contributes to overdose symptoms such as a widened QRS complex and seizures. Symptoms usually develop within 2 hours but may be delayed up to 6 hours.55 Patients with a history of overdose must be carefully assessed before initiating a TCA. Prescribing a limited supply of these medications may be valuable. The use of TCAs has often been limited due to their adverse effects, most of which are associated with their respective affinities for alpha 1, muscarinic 1, and histamine 1 receptors. Inhibition of the alpha 1 receptor is associated with hypotension, muscarinic 1 with anticholinergic adverse effects, and histamine 1 with sedation and weight gain. Tertiary amines have a higher affinity for these receptors compared to secondary amines, leading to a more significant adverse effect profile.36,50 Among TCAs, amitriptyline is the most likely to cause hypotension, whereas desipramine and nortriptyline are least likely. Amitriptyline and clomipramine are most likely to cause anticholinergic adverse effects, whereas desipramine and nortriptyline are the least likely. Amitriptyline, doxepin, and imipramine have the highest propensity for QTc prolongation.36
Beyond treating MDD, TCAs have shown benefits for treating other disease states (Table 438-46,49,56-61).These differing indications may help psychiatrists determine the best TCA to prescribe for a given patient. Amitriptyline is the most studied TCA for MDD; however, nortriptyline is typically preferred due to its favorable tolerability profile.4,62 Nortriptyline also has data supporting its use in ECT to prevent relapse.63 Amitriptyline and nortriptyline have shown benefits in patients with neuropathic pain and for migraine prophylaxis.56-60 Although frequently used for MDD, clomipramine is not FDA-approved for this indication, but is for obsessive-compulsive disorder.39 Doxepin is FDA-approved for insomnia at lower doses and for MDD at higher doses.40 Therefore, it may benefit patients with sleep difficulties secondary to depression. Desipramine has been used off-label to treat ADHD in children and has shown some benefits in adults.64-66 Protriptyline, trimipramine, and amoxapine are infrequently used in clinical practice.

A unique feature of TCAs is the ability to monitor serum concentrations (Table 336-51).Guidelines recommend therapeutic drug monitoring (TDM) with amitriptyline, clomipramine, imipramine, and nortriptyline for routine use. TDM is still recommended for doxepin, desipramine, and trimipramine, but its utility is largely for treatment failure or resistance.37 These plasma levels can be altered based on coadministered medications (Table 538-46) and should be closely monitored. Physicians should obtain a trough level after at least 5 half-lives and before the next dose is due, and use TDM as indicated to optimize dosing.

Continue to: CASE CONTINUED
CASE CONTINUED
Ms. B’s outpatient psychiatrist provides collateral information about her medical history and confirms her long-standing MDD with multiple medication trials, though she has never received an MAOI or TCA. Ms. B is adamant she does not want a medication-free period between treatments and refuses to adjust her diet, despite being educated on the few changes necessary. She has no contraindications for TCAs and may benefit from a TCA for her comorbid migraines. The care team expresses concern for TCA overdose to Ms. B and her family. Ms. B’s sister reassures the team they will have someone monitor and dispense her medications at home. They decide to discontinue her current psychiatric regimen, and Ms. B is started on nortriptyline 50 mg/d at night, with plans to titrate based on tolerability.
Related Resources
- Meyer JM. A concise guide to monoamine oxidase inhibitors. Current Psychiatry. 2017;16(12):14-16,18-23,47,A.
- Espejo GD. Treating major depressive disorder after limited response to an initial agent. Current Psychiatry. 2021;20(10):51-53. doi:10.12788/cp.0178
- American Association of Psychiatric Pharmacists (AAPP) MAOI Pharmacist Toolkit. https://aapp.org/guideline/maoi
Drug Brand Names
Amitriptyline • Elavil
Amphetamine • Adzenys, Dyanavel
Aripiprazole • Abilify
Clomipramine • Anafranil
Desipramine • Norpramin
Dextromethorphan/bupropion • Auvelity
Doxepin • Sinequan, Adapin
Esketamine • Spravato
Fluoxetine • Prozac
Imipramine • Tofranil
Isocarboxazid • Marplan
Methamphetamine • Desoxyn
Mirtazapine • Remeron
Modafinil • Provigil
Nortriptyline • Pamelor
Phenelzine • Nardil
Protriptyline • Vivactil
Selegiline • Emsam
Tranylcypromine • Parnate
Trimipramine • Surmontil
Vortioxetine • Trintellix
Ms. B, age 45, has a history of major depressive disorder (MDD) and migraines. She is admitted after presenting with anhedonia, hopelessness, and hypersomnia. These symptoms have become more severe over the last few weeks. Ms. B describes a past suicide attempt via overdose on doxylamine for which she required treatment in the intensive care unit. The only activity she enjoys is her weekly girls’ night, during which she drinks a few glasses of wine. Ms. B’s current medications are dextromethorphan/bupropion 45/105 mg twice daily and aripiprazole 5 mg/d, which she has taken for 3 months. She states she has “been on every antidepressant there is.”
When clinicians review Ms. B’s medication history, it is clear she has had adequate trials of selective serotonin reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake inhibitors (SNRIs), intranasal esketamine, multiple augmentation strategies, and electroconvulsive therapy (ECT). Ms. B seeks an alternative medication to improve her depressive symptoms.
Treatment-resistant depression (TRD) is commonly defined as depression that has not responded to ≥2 adequate trials of an antidepressant.1 Some guidelines recommend monoamine oxidase inhibitors (MAOIs) and tricyclic antidepressants (TCAs) as second- or even third-line options for MDD,2 while others recommend reserving them for patients with insufficient responses to alternative treatment modalities.3,4 Although MAOIs and TCAs have been available since the 1950s, prescribing these medications has become less prevalent due to safety concerns, the availability of other pharmacologic options, and a lack of clinical training and comfort.5,6 Most research notes that MAOIs are superior for treating atypical depression while TCAs are more effective for melancholic depression.2-4 In a review of 20 studies, Thase et al7 found that 50% of TCA nonresponders benefited from an MAOI. In the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial, monotherapy with the MAOI tranylcypromine was associated with a lower remission rate than the TCA nortriptyline; many argue the dose of tranylcypromine was suboptimal, and few participants completed an adequate trial in the last level.8,9 A more recent study by Kim et al10 found MAOIs to be “generally more effective” than TCAs for TRD, particularly in patients with fewer antidepressant trials; however, this was a small retrospective exploratory trial. A network meta-analysis found both classes to be “competitive” with SSRIs based on efficacy and tolerability, which leads to the question of whether these medications should be considered earlier in therapy.11 Considering patient-specific factors and particular medication properties is an effective strategy when prescribing an MAOI or TCA.
Monoamine oxidase inhibitors
Four MAOIs are FDA-approved for treating MDD (Table 15,12-17): phenelzine, isocarboxazid, tranylcypromine, and selegiline. These medications irreversibly inhibit MAO, which exists as isomers A and B. MAO-A primarily metabolizes serotonin and norepinephrine, which is largely responsible for these medications’ antidepressant effects. Both isomers equally metabolize dopamine.5,12,18 It is best to avoid using MAOIs in patients with cerebrovascular disease, hepatic disease, or pheochromocytoma. Patients with active substance use disorders (particularly sympathomimetics and hallucinogens) are at an increased risk for hypertensive crises and serotonin syndrome, respectively. The most common adverse effects are orthostatic hypotension (despite more well-known concerns regarding hypertension), alterations in sleep patterns (insomnia or hypersomnia, depending on the agent), gastrointestinal issues, and anticholinergic adverse effects such as dry mouth and constipation.13,19-21

In one review and meta-analysis, phenelzine displayed the highest efficacy across all MAOIs.11 It likely requires high doses to achieve adequate MAO inhibition.11 A metabolite of phenelzine inhibits gamma-aminobutyric acid transaminase and may be helpful for patients with comorbid anxiety disorders or MDD with anxious distress.18,21 Additional considerations include phenelzine’s propensity for orthostasis (with rapid titrations and higher doses), sedation, weight gain, sexual dysfunction, and a rare adverse effect of vitamin B6 deficiency.5,13,14,20-22
Use of isocarboxazid in clinical practice is rare. Its adverse effects are similar to those of phenelzine but isocarboxazid is less studied. Tranylcypromine has a similar chemical structure to amphetamine. It can be stimulating at higher doses, potentially benefitting patients with comorbid attention-deficit/hyperactivity disorder (ADHD) or significant apathy.13,23 Selegiline’s distinct quality is its availability as a transdermal patch, which may be useful for patients who struggle to take oral medications. At low doses (6 mg/24 h), the selegiline transdermal patch allows patients to disregard a dietary tyramine restriction because it avoids first-pass metabolism. It inhibits both MAO isomers in the brain but is only selective for MAO-B once concentrations are distributed to the liver. Higher doses require a tyramine-restricted diet because there is still some MAO-A inhibition in the gut. Selegiline is also stimulating because it is converted to amphetamine and methamphetamine.5,12,13,17,19,24
Despite promising results from the use of MAOIs, physicians and patients may be reluctant to use these medications due to perceived limitations. One prominent barrier is the infamous “cheese reaction.” Tyramine, an amino acid found in certain food and beverages (Table 25,13-18,25-28), is broken down by MAO-A in the gut. When this enzyme is inhibited, higher concentrations of tyramine reach systemic circulation. Tyramine’s release of norepinephrine (which now cannot be broken down) can lead to a hypertensive crisis. Consequently, a tyramine-restricted diet is recommended for patients taking an MAOI. However, the common notion that cheese, wine, and beer must be avoided is false, because most of the dietary restrictions developed following the discovery of MAOIs are antiquated.5,12,25-28 Patients who take an MAOI only need to slightly adjust their diet, as outlined in Table 2.5,13-18,25-28 A reasonable serving size of most foods and beverages containing tyramine is unlikely to elicit this “pressor” response. Of the 4 MAOIs FDA-approved for MDD, tranylcypromine appears to be the most sensitive to tyramine.21 Transient postdose hypertension (regardless of tyramine) may occur after taking an MAOI.29 Encourage patients to monitor their blood pressure.

Continue to: Additional hurdles include...
Additional hurdles include the required washout period from serotonergic medications and interactions with sympathomimetics. MAOIs pose the highest risk of serotonin syndrome; however, this usually occurs if given concomitantly with other serotonergic agents. The standard recommendation is a 14-day washout period from SSRIs (5 weeks for fluoxetine and 3 weeks for vortioxetine), SNRIs, mirtazapine, and other antidepressants. It can be distressing for patients to be without medication during that period. Because some antidepressants have much shorter half-lives, waiting 5 half-lives (typically 5 to 7 days) for the discontinued medication to be excreted is feasible if patients are closely monitored.5,12,13,25,27,30 There are rare instances where a TCA may be combined with an MAOI (typically initiated within 1 to 2 days of each other), but never clomipramine or imipramine due to their potent serotonin reuptake inhibition.31 If switching to an alternative MAOI, waiting 7 to 14 days is recommended to allow adequate time for the inhibited enzyme to regenerate.14-17,32 Taking medications that increase dopamine and norepinephrine (eg, stimulants or oral over-the-counter decongestants) with an MAOI is typically not recommended due to the risk of hypertensive crisis.25,27 In severe TRD or comorbid ADHD, successful simultaneous use of methylphenidate or amphetamine—typically at low doses—with close blood pressure monitoring has been reported.33 There have also been positive cases of the use of modafinil in combination with an MAOI; however, this should be done with caution.34,35 Clinicians must use clinical judgment when considering a combination of medications that pose a higher risk.
Tricyclic antidepressants
TCAs work differently than MAOIs to increase monoamines. They inhibit presynaptic serotonin and norepinephrine transporters in the CNS to increase levels of these chemicals in the synaptic cleft. While all TCAs inhibit these transporters, they do so at varying levels (Table 336-51). Based on their chemical structure, TCAs can be categorized into secondary and tertiary amines. Tertiary amines are metabolized via demethylation into their derivatives (Table 336-51). Patients who have recently suffered a myocardial infarction (MI) should avoid tertiary amines. TCAs can reduce heart rate variability, which is already decreased after an MI, thus presenting the potential for cardiac arrhythmias. TCAs should also be avoided in patients with cardiac conduction abnormalities.38-46,52 Patients with a prior baseline cardiac conduction defect, such as a bundle branch block, are at higher risk for further cardiac abnormalities. In those with a preexisting first-degree heart block, TCAs can still be used, but electrocardiogram monitoring is recommended.52,53 TCAs have also been reported to decrease the seizure threshold.38-46 They can be used with caution in patients who have a history of epilepsy or head trauma, or with concomitant medications that lower the seizure threshold.38-46

Overdose risk is a concern with TCAs because ingestion of 10 to 20 mg/kg can lead to significant toxicity.54 This is due to their blockage of voltage-gated sodium channels found in the CNS and heart, which contributes to overdose symptoms such as a widened QRS complex and seizures. Symptoms usually develop within 2 hours but may be delayed up to 6 hours.55 Patients with a history of overdose must be carefully assessed before initiating a TCA. Prescribing a limited supply of these medications may be valuable. The use of TCAs has often been limited due to their adverse effects, most of which are associated with their respective affinities for alpha 1, muscarinic 1, and histamine 1 receptors. Inhibition of the alpha 1 receptor is associated with hypotension, muscarinic 1 with anticholinergic adverse effects, and histamine 1 with sedation and weight gain. Tertiary amines have a higher affinity for these receptors compared to secondary amines, leading to a more significant adverse effect profile.36,50 Among TCAs, amitriptyline is the most likely to cause hypotension, whereas desipramine and nortriptyline are least likely. Amitriptyline and clomipramine are most likely to cause anticholinergic adverse effects, whereas desipramine and nortriptyline are the least likely. Amitriptyline, doxepin, and imipramine have the highest propensity for QTc prolongation.36
Beyond treating MDD, TCAs have shown benefits for treating other disease states (Table 438-46,49,56-61).These differing indications may help psychiatrists determine the best TCA to prescribe for a given patient. Amitriptyline is the most studied TCA for MDD; however, nortriptyline is typically preferred due to its favorable tolerability profile.4,62 Nortriptyline also has data supporting its use in ECT to prevent relapse.63 Amitriptyline and nortriptyline have shown benefits in patients with neuropathic pain and for migraine prophylaxis.56-60 Although frequently used for MDD, clomipramine is not FDA-approved for this indication, but is for obsessive-compulsive disorder.39 Doxepin is FDA-approved for insomnia at lower doses and for MDD at higher doses.40 Therefore, it may benefit patients with sleep difficulties secondary to depression. Desipramine has been used off-label to treat ADHD in children and has shown some benefits in adults.64-66 Protriptyline, trimipramine, and amoxapine are infrequently used in clinical practice.

A unique feature of TCAs is the ability to monitor serum concentrations (Table 336-51).Guidelines recommend therapeutic drug monitoring (TDM) with amitriptyline, clomipramine, imipramine, and nortriptyline for routine use. TDM is still recommended for doxepin, desipramine, and trimipramine, but its utility is largely for treatment failure or resistance.37 These plasma levels can be altered based on coadministered medications (Table 538-46) and should be closely monitored. Physicians should obtain a trough level after at least 5 half-lives and before the next dose is due, and use TDM as indicated to optimize dosing.

Continue to: CASE CONTINUED
CASE CONTINUED
Ms. B’s outpatient psychiatrist provides collateral information about her medical history and confirms her long-standing MDD with multiple medication trials, though she has never received an MAOI or TCA. Ms. B is adamant she does not want a medication-free period between treatments and refuses to adjust her diet, despite being educated on the few changes necessary. She has no contraindications for TCAs and may benefit from a TCA for her comorbid migraines. The care team expresses concern for TCA overdose to Ms. B and her family. Ms. B’s sister reassures the team they will have someone monitor and dispense her medications at home. They decide to discontinue her current psychiatric regimen, and Ms. B is started on nortriptyline 50 mg/d at night, with plans to titrate based on tolerability.
Related Resources
- Meyer JM. A concise guide to monoamine oxidase inhibitors. Current Psychiatry. 2017;16(12):14-16,18-23,47,A.
- Espejo GD. Treating major depressive disorder after limited response to an initial agent. Current Psychiatry. 2021;20(10):51-53. doi:10.12788/cp.0178
- American Association of Psychiatric Pharmacists (AAPP) MAOI Pharmacist Toolkit. https://aapp.org/guideline/maoi
Drug Brand Names
Amitriptyline • Elavil
Amphetamine • Adzenys, Dyanavel
Aripiprazole • Abilify
Clomipramine • Anafranil
Desipramine • Norpramin
Dextromethorphan/bupropion • Auvelity
Doxepin • Sinequan, Adapin
Esketamine • Spravato
Fluoxetine • Prozac
Imipramine • Tofranil
Isocarboxazid • Marplan
Methamphetamine • Desoxyn
Mirtazapine • Remeron
Modafinil • Provigil
Nortriptyline • Pamelor
Phenelzine • Nardil
Protriptyline • Vivactil
Selegiline • Emsam
Tranylcypromine • Parnate
Trimipramine • Surmontil
Vortioxetine • Trintellix
1. Gaynes BN, Lux L, Gartlehner G, et al. Defining treatment-resistant depression. Depress Anxiety. 2020;37(2):134-145. doi:10.1002/da.22968
2. Kennedy SH, Lam RW, McIntyre RS, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: section 3. Pharmacological treatments. Can J Psychiatry. 2016;61(9):540-560. doi:10.1177/0706743716659417
3. VA/DoD clinical practice guideline for the management of major depressive disorder. Veterans Health Administration and Department of Defense; 2016. https://www.healthquality.va.gov/guidelines/MH/mdd/VADoDMDDCPGFINAL82916.pdf
4. Gelenberg AJ, Freeman MP, Markowitz JC, et al. Practice guideline for the treatment of patients with major depressive disorder. Am J Psychiatry. 2010;167(Suppl 10):9-118.
5. Meyer JM. A concise guide to monoamine oxidase inhibitors. Current Psychiatry. 2017;16(12):14-16,18-23,47,A.
6. Taylor D. Selective serotonin reuptake inhibitors and tricyclic antidepressants in combination. Interactions and therapeutic uses. Br J Psychiatry. 1995;167(5):575-580. doi:10.1192/bjp.167.5.575
7. Thase ME, Trivedi MH, Rush AJ. MAOIs in the contemporary treatment of depression. Neuropsychopharmacology. 1995;12(3):185-219. doi:10.1016/0893-133X(94)00058-8
8. Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905-1917. doi:10.1176/ajp.2006.163.11.1905
9. McGrath PJ, Stewart JW, Fava M, et al. Tranylcypromine versus venlafaxine plus mirtazapine following three failed antidepressant medication trials for depression: a STAR*D report. Am J Psychiatry. 2006;163(9):1531-1666. doi:10.1176/ajp.2006.163.9.1531
10. Kim T, Xu C, Amsterdam JD. Relative effectiveness of tricyclic antidepressant versus monoamine oxidase inhibitor monotherapy for treatment-resistant depression. J Affect Disord. 2019;250:199-203. doi:10.1016/j.jad.2019.03.028
11. Suchting R, Tirumalajaru V, Gareeb R, et al. Revisiting monoamine oxidase inhibitors for the treatment of depressive disorders: a systematic review and network meta-analysis. J Affect Disord. 2021;282:1153-1160. doi:10.1016/j.jad.2021.01.021
12. Stahl SM, Felker A. Monoamine oxidase inhibitors: a modern guide to an unrequited class of antidepressants. CNS Spectr. 2008;13(10):855-870. doi:10.1017/s1092852900016965
13. Chamberlain SR, Baldwin DS. Monoamine oxidase inhibitors (MAOIs) in psychiatric practice: how to use them safely and effectively. CNS Drugs. 2021;35(7):703-716. doi:10.1007/s40263-021-00832-x
14. Nardil [package insert]. New York, NY: Parke-Davis; 2009.
15. Marplan [package insert]. Parsippany, NJ: Validus Pharmaceuticals LLC; 2020.
16. Parnate [package insert]. Saint Michael, Barbados: Concordia Pharmaceuticals; 2015.
17. Emsam [package insert]. Morgantown, WV: Mylan Specialty LP; 2014.
18. Shulman KI, Herrmann N, Walker SE. Current place of monoamine oxidase inhibitors in the treatment of depression. CNS Drugs. 2013;27(10):789-797. doi:10.1007/s40263-013-0097-3
19. Sub Laban T, Saadabadi A. Monoamine oxidase inhibitors (MAOI). StatPearls Publishing; 2023. https://www.ncbi.nlm.nih.gov/books/NBK539848/
20. Rabkin JG, Quitkin FM, McGrath P, et al. Adverse reactions to monoamine oxidase inhibitors. Part II. Treatment correlates and clinical management. J Clin Psychopharmacol. 1985;5(1):2-9.
21. Gillman PK. Advances pertaining to the pharmacology and interactions of irreversible nonselective monoamine oxidase inhibitors. J Clin Psychopharmacol. 2011;31(1):66-74. doi:10.1097/JCP.0b013e31820469ea
22. Sidhu G, Marwaha R. Phenelzine. StatPearls Publishing; 2023. https://www.ncbi.nlm.nih.gov/books/NBK554508/
23. Frieling H, Bleich S. Tranylcypromine: new perspectives on an “old” drug. Eur Arch Psychiatry Clin Neurosci. 2006;256(5):268-273. doi:10.1007/s00406-006-0660-8
24. Goodnick PJ. Seligiline transdermal system in depression. Expert Opin Pharmacother. 2007;8(1):59-64. doi:10.1517/14656566.8.1.59
25. Edinoff AN, Swinford CR, Odisho AS, et al. Clinically relevant drug interactions with monoamine oxidase inhibitors. Health Psychol Res. 2022;10(4):39576. doi:10.52965/001c.39576
26. Gillman PK. A reassessment of the safety profile of monoamine oxidase inhibitors: elucidating tired old tyramine myths. J Neural Transm (Vienna). 2018;125(11):1707-1717. doi:10.1007/s00702-018-1932-y
27. Flockhart DA. Dietary restrictions and drug interactions with monoamine oxidase inhibitors: an update. J Clin Psychiatry. 2012;73 Suppl 1:17-24. doi:10.4088/JCP.11096su1c.03
28. McCabe-Sellers BJ, Staggs CG, Bogle ML. Tyramine in foods and monoamine oxidase inhibitor drugs: a crossroad where medicine, nutrition, pharmacy, and food industry converge. J Food Composit Anal. 2006;19:S58-S65. doi:10.1016/j.jfca.2005.12.008
29. Keck PE Jr, Vuckovic A, Pope HG Jr, et al. Acute cardiovascular response to monoamine oxidase inhibitors: a prospective assessment. J Clin Psychopharmacol. 1989;9(3):203-206.
30. Bodkin JA, Dunlop BW. Moving on with monoamine oxidase inhibitors. Focus (Am Psychiatr Publ). 2021;19(1):50-52. doi:10.1176/appi.focus.20200046
31. Amsterdam JD, Kim TT. Relative effectiveness of monoamine oxidase inhibitor and tricyclic antidepressant combination therapy for treatment-resistant depression. J Clin Psychopharmacol. 2019;39(6):649-652. doi:10.1097/JCP.0000000000001130
32. Keks N, Hope J, Keogh S. Switching and stopping antidepressants. Aust Prescr. 2016;39(3):76-83. doi:10.18773/austprescr.2016.039
33. Israel JA. Combining stimulants and monoamine oxidase inhibitors: a reexamination of the literature and a report of a new treatment combination. Prim Care Companion CNS Disord. 2015;17(6):10.4088/PCC.15br01836. doi:10.4088/PCC.15br01836
34. Clemons WE, Makela E, Young J. Concomitant use of modafinil and tranylcypromine in a patient with narcolepsy: a case report. Sleep Med. 2004;5(5):509-511. doi:10.1016/j.sleep.2004.06.006
35. Ashton AK. Modafinil augmentation of phenelzine for residual fatigue in dysthymia. Am J Psychiatry. 2004;161(9):1716-1717. doi:10.1176/appi.ajp.161.9.1716-a
36. O’Donnell JM, Bies RR, Shelton RC. Drug therapy of depression and anxiety disorders. In: Brunton LL, Hilal-Dandan R, Knollmann BC, eds. Goodman & Gilman’s: The Pharmacological Basis of Therapeutics. 13th ed. McGraw Hill; 2017. Accessed June 4, 2023. https://accessanesthesiology.mhmedical.com/content.aspx?bookid=2189§ionid=169518711
37. Hiemke C, Bergemann N, Clement HW, et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry. 2018;51(1-02):9-62. doi:10.1055/s-0043-116492
38. Amitriptyline hydrochloride [package insert]. East Brunswick, NJ: Unichem Pharmaceuticals (USA); 2021.
39. Clomipramine hydrochloride [package insert]. East Windsor, NJ: Aurobindo Pharma Limited; 2023.
40. Doxepin hydrochloride capsules, USP [package insert]. Bedminster, NJ: Alembic Pharmaceuticals Inc; 2021.
41. Imipramine hydrochloride tablet [package insert]. Fairfield, NJ: Leading Pharma LLC USA; 2022.
42. Trimipramine maleate [package insert]. Northvale, NJ: Elite Laboratories Inc; 2021.
43. Amoxapine [package insert]. Parsippany, NJ: Actavis Pharma Inc; 2015.
44. Desipramine hydrochloride tablets [package insert]. Bedminster, NJ: Alembic Pharmaceuticals Inc; 2023.
45. Nortriptyline hydrochloride capsules, USP [package insert]. Parsippany, NJ: Teva Pharmaceuticals Inc; 2021.
46. Protriptyline hydrochloride [package insert]. Bensalem, PA: Sigmapharm Laboratories, LLC; 2023.
47. Calvo B, García MJ, Pedraz JL, et al. Pharmacokinetics of amoxapine and its active metabolites. Int J Clin Pharmacol Ther Toxicol. 1985;23(4):180-185.
48. Ziegler VE, Biggs JT, Wylie LT, et al. Protriptyline kinetics. Clin Pharmacol Ther. 1978;23(5):580-584. doi:10.1002/cpt1978235580
49. Cleare A, Pariante CM, Young AH, et al. Evidence-based guidelines for treating depressive disorders with antidepressants: a revision of the 2008 British Association for Psychopharmacology guidelines. J Psychopharmacol. 2015;29(5):459-525. doi:10.1177/0269881115581093
50. Richelson E. Synaptic effects of antidepressants. J Clin Psychopharmacol. 1996;16(3 Suppl 2):1S-9S. doi:10.1097/00004714-199606002-00001
51. Vos CF, Aarnoutse RE, Op de Coul MJM, et al. Tricyclic antidepressants for major depressive disorder: a comprehensive evaluation of current practice in the Netherlands. BMC Psychiatry. 2021;21(1):481. doi:10.1186/s12888-021-03490-x
52. Alvarez W Jr, Pickworth KK. Safety of antidepressant drugs in the patient with cardiac disease: a review of the literature. Pharmacotherapy. 2003;23(6):754-771. doi:10.1592/phco.23.6.754.32185
53. Dietch JT, Fine M. The effect of nortriptyline in elderly patients with cardiac conduction disease. J Clin Psychiatry. 1990;51(2):65-67.
54. Valento M, Liebelt EL. Cyclic antidepressants. In: Nelson LS, Howland M, Lewin NA, et al, eds. Goldfrank’s Toxicologic Emergencies. 9th ed. McGraw Hill; 2011. Accessed June 10, 2023. https://accesspharmacy.mhmedical.com/content.aspx?bookid=2569§ionid=210274664
55. Woolf AD, Erdman AR, Nelson LS, et al. Tricyclic antidepressant poisoning: an evidence-based consensus guideline for out-of-hospital management. Clin Toxicol (Phila). 2007;45(3):203-233. doi:10.1080/15563650701226192
56. Baldwin DS, Anderson IM, Nutt DJ, et al. Evidence-based pharmacological treatment of anxiety disorders, post-traumatic stress disorder and obsessive-compulsive disorder: a revision of the 2005 guidelines from the British Association for Psychopharmacology. J Psychopharmacol. 2014;28(5):403-439. doi:10.1177/0269881114525674
57. Moulin DE, Clark AJ, Gilron I, et al. Pharmacological management of chronic neuropathic pain - consensus statement and guidelines from the Canadian Pain Society. Pain Res Manag. 2007;12(1):13-21. doi:10.1155/2007/730785
58. Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14(2):162-173. doi:10.1016/S1474-4422(14)70251-0
59. Saarto T, Wiffen PJ. Antidepressants for neuropathic pain. Cochrane Database Syst Rev. 2007;(4):CD005454. doi:10.1002/14651858.CD005454.pub2
60. Burch R. Antidepressants for preventive treatment of migraine. Curr Treat Options Neurol. 2019;21(4):18. doi:10.1007/s11940-019-0557-2
61. Ailani J, Burch RC, Robbins MS; Board of Directors of the American Headache Society. The American Headache Society consensus statement: update on integrating new migraine treatments into clinical practice. Headache. 2021;61(7):1021-1039. doi:10.1111/head.14153
62. Bauer M, Pfennig A, Severus E, et al. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of unipolar depressive disorders, part 1: update 2013 on the acute and continuation treatment of unipolar depressive disorders. World J Biol Psychiatry. 2013;14(5):334-385. doi:10.3109/15622975.2013.804195
63. Jelovac A, Kolshus E, McLoughlin DM. Relapse following successful electroconvulsive therapy for major depression: a meta-analysis. Neuropsychopharmacology. 2013;38(12):2467-2474. doi:10.1038/npp.2013.149
64. Spencer T, Biederman J, Coffey B, et al. A double-blind comparison of desipramine and placebo in children and adolescents with chronic tic disorder and comorbid attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2002;59(7):649-656. doi:10.1001/archpsyc.59.7.649
65. Spencer T, Biederman J, Wilens T, et al. Pharmacotherapy of attention-deficit hyperactivity disorder across the life cycle. J Am Acad Child Adolesc Psychiatry. 1996;35(4):409-432. doi:10.1097/00004583-199604000-00008
66. Wilens TE, Biederman J, Prince J, et al. Six-week, double-blind, placebo-controlled study of desipramine for adult attention deficit hyperactivity disorder. Am J Psychiatry. 1996;153(9):1147-1153. doi:10.1176/ajp.153.9.1147
1. Gaynes BN, Lux L, Gartlehner G, et al. Defining treatment-resistant depression. Depress Anxiety. 2020;37(2):134-145. doi:10.1002/da.22968
2. Kennedy SH, Lam RW, McIntyre RS, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: section 3. Pharmacological treatments. Can J Psychiatry. 2016;61(9):540-560. doi:10.1177/0706743716659417
3. VA/DoD clinical practice guideline for the management of major depressive disorder. Veterans Health Administration and Department of Defense; 2016. https://www.healthquality.va.gov/guidelines/MH/mdd/VADoDMDDCPGFINAL82916.pdf
4. Gelenberg AJ, Freeman MP, Markowitz JC, et al. Practice guideline for the treatment of patients with major depressive disorder. Am J Psychiatry. 2010;167(Suppl 10):9-118.
5. Meyer JM. A concise guide to monoamine oxidase inhibitors. Current Psychiatry. 2017;16(12):14-16,18-23,47,A.
6. Taylor D. Selective serotonin reuptake inhibitors and tricyclic antidepressants in combination. Interactions and therapeutic uses. Br J Psychiatry. 1995;167(5):575-580. doi:10.1192/bjp.167.5.575
7. Thase ME, Trivedi MH, Rush AJ. MAOIs in the contemporary treatment of depression. Neuropsychopharmacology. 1995;12(3):185-219. doi:10.1016/0893-133X(94)00058-8
8. Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905-1917. doi:10.1176/ajp.2006.163.11.1905
9. McGrath PJ, Stewart JW, Fava M, et al. Tranylcypromine versus venlafaxine plus mirtazapine following three failed antidepressant medication trials for depression: a STAR*D report. Am J Psychiatry. 2006;163(9):1531-1666. doi:10.1176/ajp.2006.163.9.1531
10. Kim T, Xu C, Amsterdam JD. Relative effectiveness of tricyclic antidepressant versus monoamine oxidase inhibitor monotherapy for treatment-resistant depression. J Affect Disord. 2019;250:199-203. doi:10.1016/j.jad.2019.03.028
11. Suchting R, Tirumalajaru V, Gareeb R, et al. Revisiting monoamine oxidase inhibitors for the treatment of depressive disorders: a systematic review and network meta-analysis. J Affect Disord. 2021;282:1153-1160. doi:10.1016/j.jad.2021.01.021
12. Stahl SM, Felker A. Monoamine oxidase inhibitors: a modern guide to an unrequited class of antidepressants. CNS Spectr. 2008;13(10):855-870. doi:10.1017/s1092852900016965
13. Chamberlain SR, Baldwin DS. Monoamine oxidase inhibitors (MAOIs) in psychiatric practice: how to use them safely and effectively. CNS Drugs. 2021;35(7):703-716. doi:10.1007/s40263-021-00832-x
14. Nardil [package insert]. New York, NY: Parke-Davis; 2009.
15. Marplan [package insert]. Parsippany, NJ: Validus Pharmaceuticals LLC; 2020.
16. Parnate [package insert]. Saint Michael, Barbados: Concordia Pharmaceuticals; 2015.
17. Emsam [package insert]. Morgantown, WV: Mylan Specialty LP; 2014.
18. Shulman KI, Herrmann N, Walker SE. Current place of monoamine oxidase inhibitors in the treatment of depression. CNS Drugs. 2013;27(10):789-797. doi:10.1007/s40263-013-0097-3
19. Sub Laban T, Saadabadi A. Monoamine oxidase inhibitors (MAOI). StatPearls Publishing; 2023. https://www.ncbi.nlm.nih.gov/books/NBK539848/
20. Rabkin JG, Quitkin FM, McGrath P, et al. Adverse reactions to monoamine oxidase inhibitors. Part II. Treatment correlates and clinical management. J Clin Psychopharmacol. 1985;5(1):2-9.
21. Gillman PK. Advances pertaining to the pharmacology and interactions of irreversible nonselective monoamine oxidase inhibitors. J Clin Psychopharmacol. 2011;31(1):66-74. doi:10.1097/JCP.0b013e31820469ea
22. Sidhu G, Marwaha R. Phenelzine. StatPearls Publishing; 2023. https://www.ncbi.nlm.nih.gov/books/NBK554508/
23. Frieling H, Bleich S. Tranylcypromine: new perspectives on an “old” drug. Eur Arch Psychiatry Clin Neurosci. 2006;256(5):268-273. doi:10.1007/s00406-006-0660-8
24. Goodnick PJ. Seligiline transdermal system in depression. Expert Opin Pharmacother. 2007;8(1):59-64. doi:10.1517/14656566.8.1.59
25. Edinoff AN, Swinford CR, Odisho AS, et al. Clinically relevant drug interactions with monoamine oxidase inhibitors. Health Psychol Res. 2022;10(4):39576. doi:10.52965/001c.39576
26. Gillman PK. A reassessment of the safety profile of monoamine oxidase inhibitors: elucidating tired old tyramine myths. J Neural Transm (Vienna). 2018;125(11):1707-1717. doi:10.1007/s00702-018-1932-y
27. Flockhart DA. Dietary restrictions and drug interactions with monoamine oxidase inhibitors: an update. J Clin Psychiatry. 2012;73 Suppl 1:17-24. doi:10.4088/JCP.11096su1c.03
28. McCabe-Sellers BJ, Staggs CG, Bogle ML. Tyramine in foods and monoamine oxidase inhibitor drugs: a crossroad where medicine, nutrition, pharmacy, and food industry converge. J Food Composit Anal. 2006;19:S58-S65. doi:10.1016/j.jfca.2005.12.008
29. Keck PE Jr, Vuckovic A, Pope HG Jr, et al. Acute cardiovascular response to monoamine oxidase inhibitors: a prospective assessment. J Clin Psychopharmacol. 1989;9(3):203-206.
30. Bodkin JA, Dunlop BW. Moving on with monoamine oxidase inhibitors. Focus (Am Psychiatr Publ). 2021;19(1):50-52. doi:10.1176/appi.focus.20200046
31. Amsterdam JD, Kim TT. Relative effectiveness of monoamine oxidase inhibitor and tricyclic antidepressant combination therapy for treatment-resistant depression. J Clin Psychopharmacol. 2019;39(6):649-652. doi:10.1097/JCP.0000000000001130
32. Keks N, Hope J, Keogh S. Switching and stopping antidepressants. Aust Prescr. 2016;39(3):76-83. doi:10.18773/austprescr.2016.039
33. Israel JA. Combining stimulants and monoamine oxidase inhibitors: a reexamination of the literature and a report of a new treatment combination. Prim Care Companion CNS Disord. 2015;17(6):10.4088/PCC.15br01836. doi:10.4088/PCC.15br01836
34. Clemons WE, Makela E, Young J. Concomitant use of modafinil and tranylcypromine in a patient with narcolepsy: a case report. Sleep Med. 2004;5(5):509-511. doi:10.1016/j.sleep.2004.06.006
35. Ashton AK. Modafinil augmentation of phenelzine for residual fatigue in dysthymia. Am J Psychiatry. 2004;161(9):1716-1717. doi:10.1176/appi.ajp.161.9.1716-a
36. O’Donnell JM, Bies RR, Shelton RC. Drug therapy of depression and anxiety disorders. In: Brunton LL, Hilal-Dandan R, Knollmann BC, eds. Goodman & Gilman’s: The Pharmacological Basis of Therapeutics. 13th ed. McGraw Hill; 2017. Accessed June 4, 2023. https://accessanesthesiology.mhmedical.com/content.aspx?bookid=2189§ionid=169518711
37. Hiemke C, Bergemann N, Clement HW, et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry. 2018;51(1-02):9-62. doi:10.1055/s-0043-116492
38. Amitriptyline hydrochloride [package insert]. East Brunswick, NJ: Unichem Pharmaceuticals (USA); 2021.
39. Clomipramine hydrochloride [package insert]. East Windsor, NJ: Aurobindo Pharma Limited; 2023.
40. Doxepin hydrochloride capsules, USP [package insert]. Bedminster, NJ: Alembic Pharmaceuticals Inc; 2021.
41. Imipramine hydrochloride tablet [package insert]. Fairfield, NJ: Leading Pharma LLC USA; 2022.
42. Trimipramine maleate [package insert]. Northvale, NJ: Elite Laboratories Inc; 2021.
43. Amoxapine [package insert]. Parsippany, NJ: Actavis Pharma Inc; 2015.
44. Desipramine hydrochloride tablets [package insert]. Bedminster, NJ: Alembic Pharmaceuticals Inc; 2023.
45. Nortriptyline hydrochloride capsules, USP [package insert]. Parsippany, NJ: Teva Pharmaceuticals Inc; 2021.
46. Protriptyline hydrochloride [package insert]. Bensalem, PA: Sigmapharm Laboratories, LLC; 2023.
47. Calvo B, García MJ, Pedraz JL, et al. Pharmacokinetics of amoxapine and its active metabolites. Int J Clin Pharmacol Ther Toxicol. 1985;23(4):180-185.
48. Ziegler VE, Biggs JT, Wylie LT, et al. Protriptyline kinetics. Clin Pharmacol Ther. 1978;23(5):580-584. doi:10.1002/cpt1978235580
49. Cleare A, Pariante CM, Young AH, et al. Evidence-based guidelines for treating depressive disorders with antidepressants: a revision of the 2008 British Association for Psychopharmacology guidelines. J Psychopharmacol. 2015;29(5):459-525. doi:10.1177/0269881115581093
50. Richelson E. Synaptic effects of antidepressants. J Clin Psychopharmacol. 1996;16(3 Suppl 2):1S-9S. doi:10.1097/00004714-199606002-00001
51. Vos CF, Aarnoutse RE, Op de Coul MJM, et al. Tricyclic antidepressants for major depressive disorder: a comprehensive evaluation of current practice in the Netherlands. BMC Psychiatry. 2021;21(1):481. doi:10.1186/s12888-021-03490-x
52. Alvarez W Jr, Pickworth KK. Safety of antidepressant drugs in the patient with cardiac disease: a review of the literature. Pharmacotherapy. 2003;23(6):754-771. doi:10.1592/phco.23.6.754.32185
53. Dietch JT, Fine M. The effect of nortriptyline in elderly patients with cardiac conduction disease. J Clin Psychiatry. 1990;51(2):65-67.
54. Valento M, Liebelt EL. Cyclic antidepressants. In: Nelson LS, Howland M, Lewin NA, et al, eds. Goldfrank’s Toxicologic Emergencies. 9th ed. McGraw Hill; 2011. Accessed June 10, 2023. https://accesspharmacy.mhmedical.com/content.aspx?bookid=2569§ionid=210274664
55. Woolf AD, Erdman AR, Nelson LS, et al. Tricyclic antidepressant poisoning: an evidence-based consensus guideline for out-of-hospital management. Clin Toxicol (Phila). 2007;45(3):203-233. doi:10.1080/15563650701226192
56. Baldwin DS, Anderson IM, Nutt DJ, et al. Evidence-based pharmacological treatment of anxiety disorders, post-traumatic stress disorder and obsessive-compulsive disorder: a revision of the 2005 guidelines from the British Association for Psychopharmacology. J Psychopharmacol. 2014;28(5):403-439. doi:10.1177/0269881114525674
57. Moulin DE, Clark AJ, Gilron I, et al. Pharmacological management of chronic neuropathic pain - consensus statement and guidelines from the Canadian Pain Society. Pain Res Manag. 2007;12(1):13-21. doi:10.1155/2007/730785
58. Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14(2):162-173. doi:10.1016/S1474-4422(14)70251-0
59. Saarto T, Wiffen PJ. Antidepressants for neuropathic pain. Cochrane Database Syst Rev. 2007;(4):CD005454. doi:10.1002/14651858.CD005454.pub2
60. Burch R. Antidepressants for preventive treatment of migraine. Curr Treat Options Neurol. 2019;21(4):18. doi:10.1007/s11940-019-0557-2
61. Ailani J, Burch RC, Robbins MS; Board of Directors of the American Headache Society. The American Headache Society consensus statement: update on integrating new migraine treatments into clinical practice. Headache. 2021;61(7):1021-1039. doi:10.1111/head.14153
62. Bauer M, Pfennig A, Severus E, et al. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of unipolar depressive disorders, part 1: update 2013 on the acute and continuation treatment of unipolar depressive disorders. World J Biol Psychiatry. 2013;14(5):334-385. doi:10.3109/15622975.2013.804195
63. Jelovac A, Kolshus E, McLoughlin DM. Relapse following successful electroconvulsive therapy for major depression: a meta-analysis. Neuropsychopharmacology. 2013;38(12):2467-2474. doi:10.1038/npp.2013.149
64. Spencer T, Biederman J, Coffey B, et al. A double-blind comparison of desipramine and placebo in children and adolescents with chronic tic disorder and comorbid attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2002;59(7):649-656. doi:10.1001/archpsyc.59.7.649
65. Spencer T, Biederman J, Wilens T, et al. Pharmacotherapy of attention-deficit hyperactivity disorder across the life cycle. J Am Acad Child Adolesc Psychiatry. 1996;35(4):409-432. doi:10.1097/00004583-199604000-00008
66. Wilens TE, Biederman J, Prince J, et al. Six-week, double-blind, placebo-controlled study of desipramine for adult attention deficit hyperactivity disorder. Am J Psychiatry. 1996;153(9):1147-1153. doi:10.1176/ajp.153.9.1147
Managing psychotropic-induced hyperhidrosis
Ms. K, age 32, presents to the psychiatric clinic for a routine follow-up. Her history includes agoraphobia, attention-deficit/hyperactivity disorder, and schizoaffective disorder. Ms. K’s current medications are oral hydroxyzine 50 mg 4 times daily as needed for anxiety and paliperidone palmitate 234 mg IM monthly. Since her last follow-up, she has been switched from oral sertraline 150 mg/d to oral paroxetine 20 mg/d. Ms. K reports having constipation (which improves by taking oral docusate 100 mg twice daily) and generalized hyperhidrosis. She wants to alleviate the hyperhidrosis without changing her paroxetine because that medication improved her symptoms.
Hyperhidrosis—excessive sweating not needed to maintain a normal body temperature—is an uncommon and uncomfortable adverse effect of many medications, including psychotropics.1 This long-term adverse effect typically is not dose-related and does not remit with continued therapy.2Table 11-3 lists psychotropic medications associated with hyperhidrosis as well as postulated mechanisms.
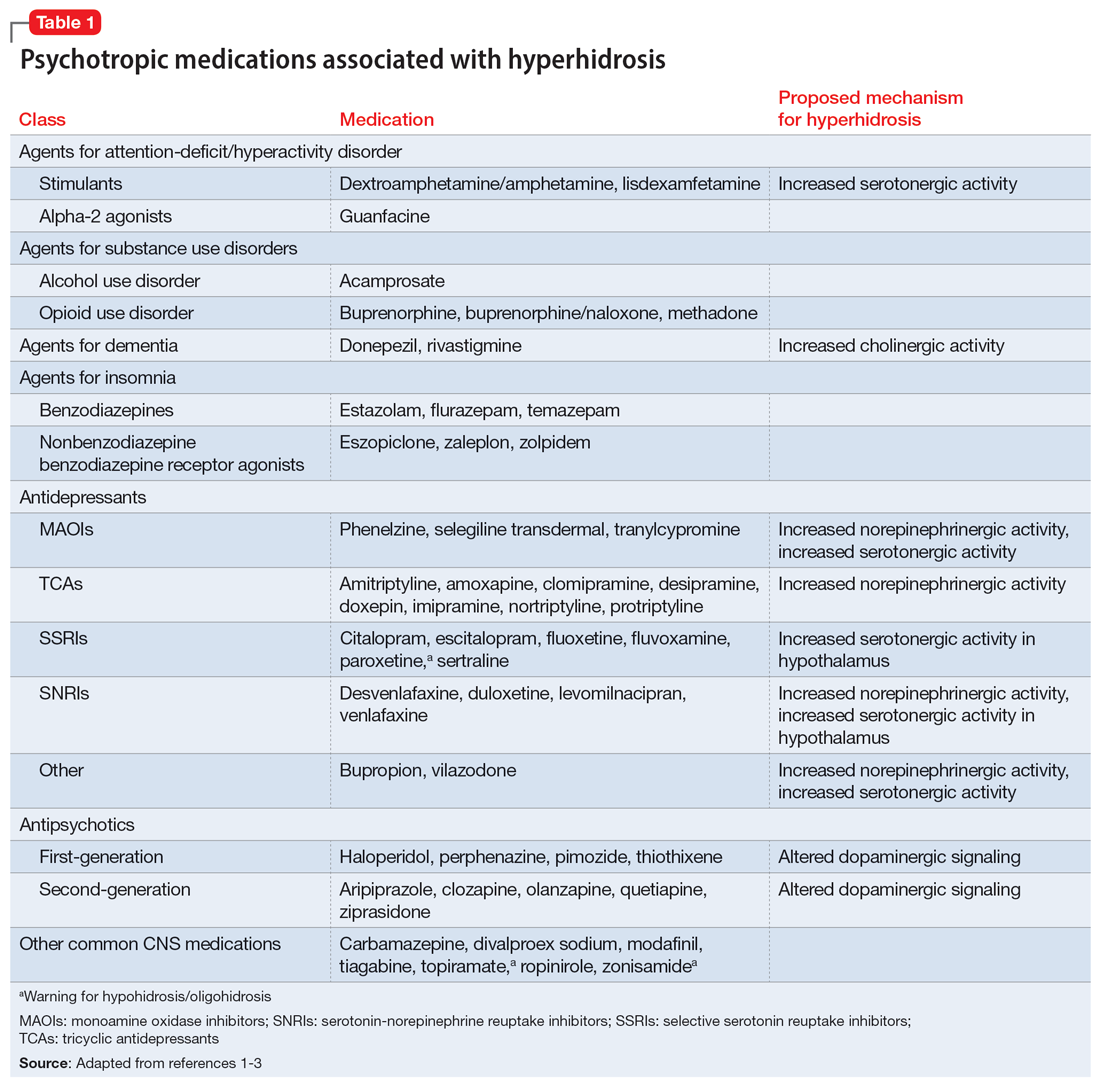
The incidence of medication-induced hyperhidrosis is unknown,but for psychotropic medications it is estimated to be 5% to 20%.3 Patients may not report hyperhidrosis due to embarrassment; in clinical trials, reporting measures may be inconsistent and, in some cases, misleading. For example, it is possible hyperhidrosis that appears to be associated with buprenorphine is actually a symptom of the withdrawal syndrome rather than a direct effect of the medication. Also, some medications, including certain psychotropics (eg, paroxetine4 and topiramate3) may cause either hyperhidrosis or hypohidrosis (decreased sweating). Few medications carry labeled warnings for hypohidrosis; the condition generally is not of clinical concern unless patients experience heat intolerance or hyperthermia.3
Psychotropic-induced hyperhidrosis is likely an idiopathic effect. There are few known predisposing factors, but some medications carry a greater risk than others. In a meta-analysis, Beyer et al2 found certain selective serotonin reuptake inhibitors (SSRIs), such as sertraline and paroxetine, had a higher risk of causing hyperhidrosis. Fluvoxamine, bupropion, and vortioxetine had the lowest risk. The class risk for SSRIs was comparable to that of serotonin-norepinephrine reuptake inhibitors (SNRIs), which all carried a comparable risk. In this analysis, neither indication nor dose were reliable indicators of risk of causing hyperhidrosis. However, the study found that for both SSRIs and SNRIs, increased affinity for the dopamine transporter was correlated with an increased risk of hyperhidrosis.2
Treatment
Treatment of hyperhidrosis depends on its cause and presentation.5 Hyperhidrosis may be categorized as primary (idiopathic) or secondary (also termed diaphoresis), and either focal or generalized.6 Many treatment recommendations focus on primary or focal hyperhidrosis and prioritize topical therapies.5 Because medication-induced hyperhidrosis most commonly presents as generalized3 and thus affects a large body surface area, the use of topical therapies is precluded. Topical therapy for psychotropic-induced hyperhidrosis should be pursued only if the patient’s sweating is localized.
Treating medication-induced hyperhidrosis becomes more complicated if it is not possible to alter the inciting medication (ie, because the medication is effective or the patient is resistant to change). In such scenarios, discontinuing the medication and initiating an alternative therapy may not be effective or feasible.2 For generalized presentations of medication-induced hyperhidrosis, if the inciting medication cannot be altered, initiating an oral systemic therapy is the preferred treatment.3,5
Oral anticholinergic medications (eg, benztropine, glycopyrrolate, and oxybutynin),4-6 act directly on muscarinic receptors within the eccrine sweat glands to decrease or stop sweating. They are considered first-line for generalized hyperhidrosis but may be inappropriate for psychotropic-induced hyperhidrosis because many psychotropics (eg, tricyclic antidepressants, paroxetine, olanzapine, quetiapine, and clozapine) have anticholinergic properties. Adding an anticholinergic medication to these patients’ regimens may increase the adverse effect burden and worsen cognitive deficits. Additionally, approximately one-third of patients discontinue anticholinergic medications due to tolerability issues (eg, dry mouth).
Continue to: However, anticholinergic medications...
However, anticholinergic medications may still have a role in treating psychotropic-induced hyperhidrosis. Benztropine3,7,8 and cyproheptadine2,3,9 may be effective options, though their role in treating psychotropic-induced hyperhidrosis should be limited and reserved for patients who have another compelling indication for these medications (eg, extrapyramidal symptoms) or when other treatment options are ineffective or intolerable.
Avoiding anticholinergic medications can also be justified based on the proposed mechanism of psychotropic-induced hyperhidrosis as an extension of the medication’s toxic effects. Conceptualizing psychotropic-induced hyperhidrosis as similar to the diaphoresis and hyperthermia observed in neuroleptic malignant syndrome and serotonin syndrome offers a clearer target for treatment. Though the specifics of the mechanisms remain unknown,2 many medications that cause hyperhidrosis do so by increasing sweat gland secretions, either directly by increasing cholinergic activity or indirectly via increased sympathetic transmission.
Considering this pathophysiology, another target for psychotropic-induced hyperhidrosis may be altered and/or excessive catecholamine activity. The use of medications such as clonidine,3-6 propranolol,4-6 or terazosin2,3,10 should be considered given their beneficial effects on the activation of the sympathetic nervous system, although clonidine also possesses anticholinergic activity. The calcium channel blocker diltiazem can improve hyperhidrosis symptoms by interfering with the calcium signaling necessary for normal sweat gland function.4,5 Comorbid cardiovascular diseases and tachycardia, an adverse effect of many psychotropic medications, may also be managed with these treatment options. Some research suggests using benzodiazepines to treat psychotropic-induced hyperhidrosis.4-6 As is the case for anticholinergic medications, the use of benzodiazepines would require another compelling indication for long-term use.
Table 23,4,6-8,10 provides recommended dosing and caveats for the use of these medications and other potentially appropriate medications.
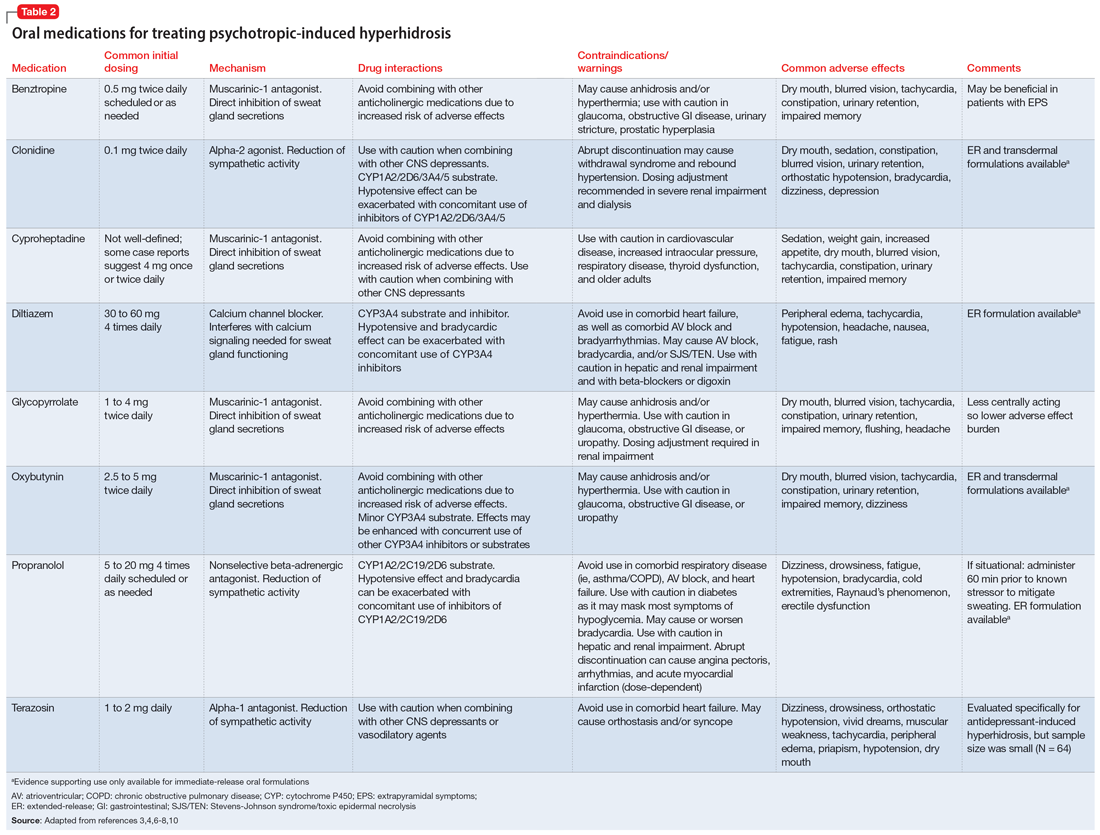
Research of investigational treatments for generalized hyperhidrosis is ongoing. It is possible some of these medications may have a future role in the treatment of psychotropic-induced hyperhidrosis, with improved efficacy and better tolerability.
Continue to: CASE CONTINUED
CASE CONTINUED
Because Ms. K’s medication-induced hyperhidrosis is generalized and therefore ineligible for topical therapies, and because the inciting medication (paroxetine) cannot be switched to an alternative, the treatment team considers adding an oral medication. Treatment with an anticholinergic medication, such as benztropine, is not preferred due to the anticholinergic activity associated with paroxetine and Ms. K’s history of constipation. After discussing other oral treatment options with Ms. K, the team ultimately decides to initiate propranolol at a low dose (5 mg twice daily) to minimize the chances of an interaction with paroxetine, and titrate based on efficacy and tolerability.
Related Resources
- International Hyperhidrosis Society. Hyperhidrosis treatment overview. www.sweathelp.org/hyperhidrosis-treatments/treatment-overview.html
Drug Brand Names
Acamprosate • Campral
Aripiprazole • Abilify
Buprenorphine • Sublocade
Buprenorphine/naloxone • Zubsolv
Bupropion • Wellbutrin
Carbamazepine • Tegretol
Citalopram • Celexa
Clomipramine • Anafranil
Clonidine • Catapres
Clozapine • Clozaril
Desipramine • Norpramin
Desvenlafaxine • Pristiq
Dextroamphetamine/amphetamine • Adderall
Diltiazem • Cardizem
Divalproex • Depakote
Donepezil • Aricept
Doxepin • Silenor
Duloxetine • Cymbalta
Escitalopram • Lexapro
Eszopiclone • Lunesta
Fluoxetine • Prozac
Fluvoxamine • Luvox
Guanfacine • Intuniv
Glycopyrrolate • Cuvposa
Hydroxyzine • Vistaril
Imipramine • Tofranil
Levomilnacipran • Fetzima
Lisdexamfetamine • Vyvanse
Methadone • Dolophine, Methadose
Modafinil • Provigil
Nortriptyline • Pamelor
Olanzapine • Zyprexa
Paliperidone palmitate • Invega Sustenna
Paroxetine • Paxil
Phenelzine • Nardil
Pimozide • Orap
Protriptyline • Vivactil
Quetiapine • Seroquel
Rivastigmine • Exelon
Selegiline transdermal • Emsam
Sertraline • Zoloft
Temazepam • Restoril
Thiothixene • Navane
Tiagabine • Gabitril
Topiramate • Topamax
Tranylcypromine • Parnate
Vilazodone • Viibryd
Vortioxetine • Trintellix
Zaleplon • Sonata
Ziprasidone • Geodon
Zolpidem • Ambien
Zonisamide • Zonegran
1. International Hyperhidrosis Society. Drugs/medications known to cause hyperhidrosis. Sweathelp.org. 2022. Accessed September 6, 2022. https://www.sweathelp.org/pdf/drugs_2009.pdf
2. Beyer C, Cappetta K, Johnson JA, et al. Meta-analysis: risk of hyperhidrosis with second-generation antidepressants. Depress Anxiety. 2017;34(12):1134-1146. doi:10.1002/da.22680
3. Cheshire WP, Fealey RD. Drug-induced hyperhidrosis and hypohidrosis: incidence, prevention and management. Drug Saf. 2008;31(2):109-126. doi:10.2165/00002018-200831020-00002
4. del Boz J. Systemic treatment of hyperhidrosis. Actas Dermosifiliogr. 2015;106(4):271-277. doi:10.1016/j.ad.2014.11.012
5. Nawrocki S, Cha J. The etiology, diagnosis, and management of hyperhidrosis: a comprehensive review: therapeutic options. J Am Acad Dermatol. 2019;81(3):669-680. doi:10.1016/j.jaad2018.11.066
6. Glaser DA. Oral medications. Dermatol Clin. 2014;32(4):527-532. doi:10.1016/j.det.2014.06.002
7. Garber A, Gregory RJ. Benztropine in the treatment of venlafaxine-induced sweating. J Clin Psychiatry. 1997;58(4):176-177. doi:10.4088/jcp.v58n0407e
8. Kolli V, Ramaswamy S. Improvement of antidepressant-induced sweating with as-required benztropine. Innov Clin Neurosci. 2013;10(11-12):10-11.
9. Ashton AK, Weinstein WL. Cyproheptadine for drug-induced sweating. Am J Psychiatry. 2002;159(5):875. doi:10.1176/APPI.AJP.159.5.874-A
10. Ghaleiha A, Shahidi KM, Afzali S, et al. Effect of terazosin on sweating in patients with major depressive disorder receiving sertraline: a randomized controlled trial. Int J Psychiatry Clin Pract. 2013;17(1):44-47. doi:10.3109/13651501.2012.687449
Ms. K, age 32, presents to the psychiatric clinic for a routine follow-up. Her history includes agoraphobia, attention-deficit/hyperactivity disorder, and schizoaffective disorder. Ms. K’s current medications are oral hydroxyzine 50 mg 4 times daily as needed for anxiety and paliperidone palmitate 234 mg IM monthly. Since her last follow-up, she has been switched from oral sertraline 150 mg/d to oral paroxetine 20 mg/d. Ms. K reports having constipation (which improves by taking oral docusate 100 mg twice daily) and generalized hyperhidrosis. She wants to alleviate the hyperhidrosis without changing her paroxetine because that medication improved her symptoms.
Hyperhidrosis—excessive sweating not needed to maintain a normal body temperature—is an uncommon and uncomfortable adverse effect of many medications, including psychotropics.1 This long-term adverse effect typically is not dose-related and does not remit with continued therapy.2Table 11-3 lists psychotropic medications associated with hyperhidrosis as well as postulated mechanisms.

The incidence of medication-induced hyperhidrosis is unknown,but for psychotropic medications it is estimated to be 5% to 20%.3 Patients may not report hyperhidrosis due to embarrassment; in clinical trials, reporting measures may be inconsistent and, in some cases, misleading. For example, it is possible hyperhidrosis that appears to be associated with buprenorphine is actually a symptom of the withdrawal syndrome rather than a direct effect of the medication. Also, some medications, including certain psychotropics (eg, paroxetine4 and topiramate3) may cause either hyperhidrosis or hypohidrosis (decreased sweating). Few medications carry labeled warnings for hypohidrosis; the condition generally is not of clinical concern unless patients experience heat intolerance or hyperthermia.3
Psychotropic-induced hyperhidrosis is likely an idiopathic effect. There are few known predisposing factors, but some medications carry a greater risk than others. In a meta-analysis, Beyer et al2 found certain selective serotonin reuptake inhibitors (SSRIs), such as sertraline and paroxetine, had a higher risk of causing hyperhidrosis. Fluvoxamine, bupropion, and vortioxetine had the lowest risk. The class risk for SSRIs was comparable to that of serotonin-norepinephrine reuptake inhibitors (SNRIs), which all carried a comparable risk. In this analysis, neither indication nor dose were reliable indicators of risk of causing hyperhidrosis. However, the study found that for both SSRIs and SNRIs, increased affinity for the dopamine transporter was correlated with an increased risk of hyperhidrosis.2
Treatment
Treatment of hyperhidrosis depends on its cause and presentation.5 Hyperhidrosis may be categorized as primary (idiopathic) or secondary (also termed diaphoresis), and either focal or generalized.6 Many treatment recommendations focus on primary or focal hyperhidrosis and prioritize topical therapies.5 Because medication-induced hyperhidrosis most commonly presents as generalized3 and thus affects a large body surface area, the use of topical therapies is precluded. Topical therapy for psychotropic-induced hyperhidrosis should be pursued only if the patient’s sweating is localized.
Treating medication-induced hyperhidrosis becomes more complicated if it is not possible to alter the inciting medication (ie, because the medication is effective or the patient is resistant to change). In such scenarios, discontinuing the medication and initiating an alternative therapy may not be effective or feasible.2 For generalized presentations of medication-induced hyperhidrosis, if the inciting medication cannot be altered, initiating an oral systemic therapy is the preferred treatment.3,5
Oral anticholinergic medications (eg, benztropine, glycopyrrolate, and oxybutynin),4-6 act directly on muscarinic receptors within the eccrine sweat glands to decrease or stop sweating. They are considered first-line for generalized hyperhidrosis but may be inappropriate for psychotropic-induced hyperhidrosis because many psychotropics (eg, tricyclic antidepressants, paroxetine, olanzapine, quetiapine, and clozapine) have anticholinergic properties. Adding an anticholinergic medication to these patients’ regimens may increase the adverse effect burden and worsen cognitive deficits. Additionally, approximately one-third of patients discontinue anticholinergic medications due to tolerability issues (eg, dry mouth).
Continue to: However, anticholinergic medications...
However, anticholinergic medications may still have a role in treating psychotropic-induced hyperhidrosis. Benztropine3,7,8 and cyproheptadine2,3,9 may be effective options, though their role in treating psychotropic-induced hyperhidrosis should be limited and reserved for patients who have another compelling indication for these medications (eg, extrapyramidal symptoms) or when other treatment options are ineffective or intolerable.
Avoiding anticholinergic medications can also be justified based on the proposed mechanism of psychotropic-induced hyperhidrosis as an extension of the medication’s toxic effects. Conceptualizing psychotropic-induced hyperhidrosis as similar to the diaphoresis and hyperthermia observed in neuroleptic malignant syndrome and serotonin syndrome offers a clearer target for treatment. Though the specifics of the mechanisms remain unknown,2 many medications that cause hyperhidrosis do so by increasing sweat gland secretions, either directly by increasing cholinergic activity or indirectly via increased sympathetic transmission.
Considering this pathophysiology, another target for psychotropic-induced hyperhidrosis may be altered and/or excessive catecholamine activity. The use of medications such as clonidine,3-6 propranolol,4-6 or terazosin2,3,10 should be considered given their beneficial effects on the activation of the sympathetic nervous system, although clonidine also possesses anticholinergic activity. The calcium channel blocker diltiazem can improve hyperhidrosis symptoms by interfering with the calcium signaling necessary for normal sweat gland function.4,5 Comorbid cardiovascular diseases and tachycardia, an adverse effect of many psychotropic medications, may also be managed with these treatment options. Some research suggests using benzodiazepines to treat psychotropic-induced hyperhidrosis.4-6 As is the case for anticholinergic medications, the use of benzodiazepines would require another compelling indication for long-term use.
Table 23,4,6-8,10 provides recommended dosing and caveats for the use of these medications and other potentially appropriate medications.

Research of investigational treatments for generalized hyperhidrosis is ongoing. It is possible some of these medications may have a future role in the treatment of psychotropic-induced hyperhidrosis, with improved efficacy and better tolerability.
Continue to: CASE CONTINUED
CASE CONTINUED
Because Ms. K’s medication-induced hyperhidrosis is generalized and therefore ineligible for topical therapies, and because the inciting medication (paroxetine) cannot be switched to an alternative, the treatment team considers adding an oral medication. Treatment with an anticholinergic medication, such as benztropine, is not preferred due to the anticholinergic activity associated with paroxetine and Ms. K’s history of constipation. After discussing other oral treatment options with Ms. K, the team ultimately decides to initiate propranolol at a low dose (5 mg twice daily) to minimize the chances of an interaction with paroxetine, and titrate based on efficacy and tolerability.
Related Resources
- International Hyperhidrosis Society. Hyperhidrosis treatment overview. www.sweathelp.org/hyperhidrosis-treatments/treatment-overview.html
Drug Brand Names
Acamprosate • Campral
Aripiprazole • Abilify
Buprenorphine • Sublocade
Buprenorphine/naloxone • Zubsolv
Bupropion • Wellbutrin
Carbamazepine • Tegretol
Citalopram • Celexa
Clomipramine • Anafranil
Clonidine • Catapres
Clozapine • Clozaril
Desipramine • Norpramin
Desvenlafaxine • Pristiq
Dextroamphetamine/amphetamine • Adderall
Diltiazem • Cardizem
Divalproex • Depakote
Donepezil • Aricept
Doxepin • Silenor
Duloxetine • Cymbalta
Escitalopram • Lexapro
Eszopiclone • Lunesta
Fluoxetine • Prozac
Fluvoxamine • Luvox
Guanfacine • Intuniv
Glycopyrrolate • Cuvposa
Hydroxyzine • Vistaril
Imipramine • Tofranil
Levomilnacipran • Fetzima
Lisdexamfetamine • Vyvanse
Methadone • Dolophine, Methadose
Modafinil • Provigil
Nortriptyline • Pamelor
Olanzapine • Zyprexa
Paliperidone palmitate • Invega Sustenna
Paroxetine • Paxil
Phenelzine • Nardil
Pimozide • Orap
Protriptyline • Vivactil
Quetiapine • Seroquel
Rivastigmine • Exelon
Selegiline transdermal • Emsam
Sertraline • Zoloft
Temazepam • Restoril
Thiothixene • Navane
Tiagabine • Gabitril
Topiramate • Topamax
Tranylcypromine • Parnate
Vilazodone • Viibryd
Vortioxetine • Trintellix
Zaleplon • Sonata
Ziprasidone • Geodon
Zolpidem • Ambien
Zonisamide • Zonegran
Ms. K, age 32, presents to the psychiatric clinic for a routine follow-up. Her history includes agoraphobia, attention-deficit/hyperactivity disorder, and schizoaffective disorder. Ms. K’s current medications are oral hydroxyzine 50 mg 4 times daily as needed for anxiety and paliperidone palmitate 234 mg IM monthly. Since her last follow-up, she has been switched from oral sertraline 150 mg/d to oral paroxetine 20 mg/d. Ms. K reports having constipation (which improves by taking oral docusate 100 mg twice daily) and generalized hyperhidrosis. She wants to alleviate the hyperhidrosis without changing her paroxetine because that medication improved her symptoms.
Hyperhidrosis—excessive sweating not needed to maintain a normal body temperature—is an uncommon and uncomfortable adverse effect of many medications, including psychotropics.1 This long-term adverse effect typically is not dose-related and does not remit with continued therapy.2Table 11-3 lists psychotropic medications associated with hyperhidrosis as well as postulated mechanisms.

The incidence of medication-induced hyperhidrosis is unknown,but for psychotropic medications it is estimated to be 5% to 20%.3 Patients may not report hyperhidrosis due to embarrassment; in clinical trials, reporting measures may be inconsistent and, in some cases, misleading. For example, it is possible hyperhidrosis that appears to be associated with buprenorphine is actually a symptom of the withdrawal syndrome rather than a direct effect of the medication. Also, some medications, including certain psychotropics (eg, paroxetine4 and topiramate3) may cause either hyperhidrosis or hypohidrosis (decreased sweating). Few medications carry labeled warnings for hypohidrosis; the condition generally is not of clinical concern unless patients experience heat intolerance or hyperthermia.3
Psychotropic-induced hyperhidrosis is likely an idiopathic effect. There are few known predisposing factors, but some medications carry a greater risk than others. In a meta-analysis, Beyer et al2 found certain selective serotonin reuptake inhibitors (SSRIs), such as sertraline and paroxetine, had a higher risk of causing hyperhidrosis. Fluvoxamine, bupropion, and vortioxetine had the lowest risk. The class risk for SSRIs was comparable to that of serotonin-norepinephrine reuptake inhibitors (SNRIs), which all carried a comparable risk. In this analysis, neither indication nor dose were reliable indicators of risk of causing hyperhidrosis. However, the study found that for both SSRIs and SNRIs, increased affinity for the dopamine transporter was correlated with an increased risk of hyperhidrosis.2
Treatment
Treatment of hyperhidrosis depends on its cause and presentation.5 Hyperhidrosis may be categorized as primary (idiopathic) or secondary (also termed diaphoresis), and either focal or generalized.6 Many treatment recommendations focus on primary or focal hyperhidrosis and prioritize topical therapies.5 Because medication-induced hyperhidrosis most commonly presents as generalized3 and thus affects a large body surface area, the use of topical therapies is precluded. Topical therapy for psychotropic-induced hyperhidrosis should be pursued only if the patient’s sweating is localized.
Treating medication-induced hyperhidrosis becomes more complicated if it is not possible to alter the inciting medication (ie, because the medication is effective or the patient is resistant to change). In such scenarios, discontinuing the medication and initiating an alternative therapy may not be effective or feasible.2 For generalized presentations of medication-induced hyperhidrosis, if the inciting medication cannot be altered, initiating an oral systemic therapy is the preferred treatment.3,5
Oral anticholinergic medications (eg, benztropine, glycopyrrolate, and oxybutynin),4-6 act directly on muscarinic receptors within the eccrine sweat glands to decrease or stop sweating. They are considered first-line for generalized hyperhidrosis but may be inappropriate for psychotropic-induced hyperhidrosis because many psychotropics (eg, tricyclic antidepressants, paroxetine, olanzapine, quetiapine, and clozapine) have anticholinergic properties. Adding an anticholinergic medication to these patients’ regimens may increase the adverse effect burden and worsen cognitive deficits. Additionally, approximately one-third of patients discontinue anticholinergic medications due to tolerability issues (eg, dry mouth).
Continue to: However, anticholinergic medications...
However, anticholinergic medications may still have a role in treating psychotropic-induced hyperhidrosis. Benztropine3,7,8 and cyproheptadine2,3,9 may be effective options, though their role in treating psychotropic-induced hyperhidrosis should be limited and reserved for patients who have another compelling indication for these medications (eg, extrapyramidal symptoms) or when other treatment options are ineffective or intolerable.
Avoiding anticholinergic medications can also be justified based on the proposed mechanism of psychotropic-induced hyperhidrosis as an extension of the medication’s toxic effects. Conceptualizing psychotropic-induced hyperhidrosis as similar to the diaphoresis and hyperthermia observed in neuroleptic malignant syndrome and serotonin syndrome offers a clearer target for treatment. Though the specifics of the mechanisms remain unknown,2 many medications that cause hyperhidrosis do so by increasing sweat gland secretions, either directly by increasing cholinergic activity or indirectly via increased sympathetic transmission.
Considering this pathophysiology, another target for psychotropic-induced hyperhidrosis may be altered and/or excessive catecholamine activity. The use of medications such as clonidine,3-6 propranolol,4-6 or terazosin2,3,10 should be considered given their beneficial effects on the activation of the sympathetic nervous system, although clonidine also possesses anticholinergic activity. The calcium channel blocker diltiazem can improve hyperhidrosis symptoms by interfering with the calcium signaling necessary for normal sweat gland function.4,5 Comorbid cardiovascular diseases and tachycardia, an adverse effect of many psychotropic medications, may also be managed with these treatment options. Some research suggests using benzodiazepines to treat psychotropic-induced hyperhidrosis.4-6 As is the case for anticholinergic medications, the use of benzodiazepines would require another compelling indication for long-term use.
Table 23,4,6-8,10 provides recommended dosing and caveats for the use of these medications and other potentially appropriate medications.

Research of investigational treatments for generalized hyperhidrosis is ongoing. It is possible some of these medications may have a future role in the treatment of psychotropic-induced hyperhidrosis, with improved efficacy and better tolerability.
Continue to: CASE CONTINUED
CASE CONTINUED
Because Ms. K’s medication-induced hyperhidrosis is generalized and therefore ineligible for topical therapies, and because the inciting medication (paroxetine) cannot be switched to an alternative, the treatment team considers adding an oral medication. Treatment with an anticholinergic medication, such as benztropine, is not preferred due to the anticholinergic activity associated with paroxetine and Ms. K’s history of constipation. After discussing other oral treatment options with Ms. K, the team ultimately decides to initiate propranolol at a low dose (5 mg twice daily) to minimize the chances of an interaction with paroxetine, and titrate based on efficacy and tolerability.
Related Resources
- International Hyperhidrosis Society. Hyperhidrosis treatment overview. www.sweathelp.org/hyperhidrosis-treatments/treatment-overview.html
Drug Brand Names
Acamprosate • Campral
Aripiprazole • Abilify
Buprenorphine • Sublocade
Buprenorphine/naloxone • Zubsolv
Bupropion • Wellbutrin
Carbamazepine • Tegretol
Citalopram • Celexa
Clomipramine • Anafranil
Clonidine • Catapres
Clozapine • Clozaril
Desipramine • Norpramin
Desvenlafaxine • Pristiq
Dextroamphetamine/amphetamine • Adderall
Diltiazem • Cardizem
Divalproex • Depakote
Donepezil • Aricept
Doxepin • Silenor
Duloxetine • Cymbalta
Escitalopram • Lexapro
Eszopiclone • Lunesta
Fluoxetine • Prozac
Fluvoxamine • Luvox
Guanfacine • Intuniv
Glycopyrrolate • Cuvposa
Hydroxyzine • Vistaril
Imipramine • Tofranil
Levomilnacipran • Fetzima
Lisdexamfetamine • Vyvanse
Methadone • Dolophine, Methadose
Modafinil • Provigil
Nortriptyline • Pamelor
Olanzapine • Zyprexa
Paliperidone palmitate • Invega Sustenna
Paroxetine • Paxil
Phenelzine • Nardil
Pimozide • Orap
Protriptyline • Vivactil
Quetiapine • Seroquel
Rivastigmine • Exelon
Selegiline transdermal • Emsam
Sertraline • Zoloft
Temazepam • Restoril
Thiothixene • Navane
Tiagabine • Gabitril
Topiramate • Topamax
Tranylcypromine • Parnate
Vilazodone • Viibryd
Vortioxetine • Trintellix
Zaleplon • Sonata
Ziprasidone • Geodon
Zolpidem • Ambien
Zonisamide • Zonegran
1. International Hyperhidrosis Society. Drugs/medications known to cause hyperhidrosis. Sweathelp.org. 2022. Accessed September 6, 2022. https://www.sweathelp.org/pdf/drugs_2009.pdf
2. Beyer C, Cappetta K, Johnson JA, et al. Meta-analysis: risk of hyperhidrosis with second-generation antidepressants. Depress Anxiety. 2017;34(12):1134-1146. doi:10.1002/da.22680
3. Cheshire WP, Fealey RD. Drug-induced hyperhidrosis and hypohidrosis: incidence, prevention and management. Drug Saf. 2008;31(2):109-126. doi:10.2165/00002018-200831020-00002
4. del Boz J. Systemic treatment of hyperhidrosis. Actas Dermosifiliogr. 2015;106(4):271-277. doi:10.1016/j.ad.2014.11.012
5. Nawrocki S, Cha J. The etiology, diagnosis, and management of hyperhidrosis: a comprehensive review: therapeutic options. J Am Acad Dermatol. 2019;81(3):669-680. doi:10.1016/j.jaad2018.11.066
6. Glaser DA. Oral medications. Dermatol Clin. 2014;32(4):527-532. doi:10.1016/j.det.2014.06.002
7. Garber A, Gregory RJ. Benztropine in the treatment of venlafaxine-induced sweating. J Clin Psychiatry. 1997;58(4):176-177. doi:10.4088/jcp.v58n0407e
8. Kolli V, Ramaswamy S. Improvement of antidepressant-induced sweating with as-required benztropine. Innov Clin Neurosci. 2013;10(11-12):10-11.
9. Ashton AK, Weinstein WL. Cyproheptadine for drug-induced sweating. Am J Psychiatry. 2002;159(5):875. doi:10.1176/APPI.AJP.159.5.874-A
10. Ghaleiha A, Shahidi KM, Afzali S, et al. Effect of terazosin on sweating in patients with major depressive disorder receiving sertraline: a randomized controlled trial. Int J Psychiatry Clin Pract. 2013;17(1):44-47. doi:10.3109/13651501.2012.687449
1. International Hyperhidrosis Society. Drugs/medications known to cause hyperhidrosis. Sweathelp.org. 2022. Accessed September 6, 2022. https://www.sweathelp.org/pdf/drugs_2009.pdf
2. Beyer C, Cappetta K, Johnson JA, et al. Meta-analysis: risk of hyperhidrosis with second-generation antidepressants. Depress Anxiety. 2017;34(12):1134-1146. doi:10.1002/da.22680
3. Cheshire WP, Fealey RD. Drug-induced hyperhidrosis and hypohidrosis: incidence, prevention and management. Drug Saf. 2008;31(2):109-126. doi:10.2165/00002018-200831020-00002
4. del Boz J. Systemic treatment of hyperhidrosis. Actas Dermosifiliogr. 2015;106(4):271-277. doi:10.1016/j.ad.2014.11.012
5. Nawrocki S, Cha J. The etiology, diagnosis, and management of hyperhidrosis: a comprehensive review: therapeutic options. J Am Acad Dermatol. 2019;81(3):669-680. doi:10.1016/j.jaad2018.11.066
6. Glaser DA. Oral medications. Dermatol Clin. 2014;32(4):527-532. doi:10.1016/j.det.2014.06.002
7. Garber A, Gregory RJ. Benztropine in the treatment of venlafaxine-induced sweating. J Clin Psychiatry. 1997;58(4):176-177. doi:10.4088/jcp.v58n0407e
8. Kolli V, Ramaswamy S. Improvement of antidepressant-induced sweating with as-required benztropine. Innov Clin Neurosci. 2013;10(11-12):10-11.
9. Ashton AK, Weinstein WL. Cyproheptadine for drug-induced sweating. Am J Psychiatry. 2002;159(5):875. doi:10.1176/APPI.AJP.159.5.874-A
10. Ghaleiha A, Shahidi KM, Afzali S, et al. Effect of terazosin on sweating in patients with major depressive disorder receiving sertraline: a randomized controlled trial. Int J Psychiatry Clin Pract. 2013;17(1):44-47. doi:10.3109/13651501.2012.687449
Abnormal sexual behaviors in frontotemporal dementia
Mr. S, age 77, is admitted to a long-term care facility due to progressive cognitive impairment and sexually inappropriate behavior. He has a history of sexual assault of medical staff. His medical history includes significant frontotemporal dementia (FTD) with behavioral disturbances, abnormal sexual behaviors, subclinical hypothyroidism, schizoid personality disorder, Parkinson disease, posttraumatic stress disorder, and hyperammonemia.
Upon admission, Mr. S’s vital signs are within normal limits except for an elevated thyroid-stimulating hormone (4.54 mIU/L; reference range 0.40 to 4.50 mIU/L). Prior cognitive testing results and updated ammonia levels are unavailable. Mr. S’s current medications include acetaminophen 650 mg every 4 hours as needed for pain, calcium carbonate/vitamin D twice daily for bone health, carbidopa/levodopa 25/100 mg twice daily for Parkinson disease, melatonin 3 mg/d at bedtime for insomnia, quetiapine 25 mg twice daily for psychosis with disturbance of behavior and 12.5 mg every 4 hours as needed for agitation, and trazodone 50 mg/d at bedtime for insomnia. Before Mr. S was admitted, previous therapy with selective serotonin reuptake inhibitors (SSRIs) had been tapered and discontinued. Mr. S had also started antipsychotic therapy at another facility due to worsening behaviors.
In patients with dementia, the brain is experiencing neurodegeneration. Progressively, neurons may stop functioning, lose connections with other neurons, and ultimately face cell death. The specific dementia diagnosis and its clinical features depend on the type of neurons and region of the brain affected.1,2
FTD occurs in response to damage to the frontal and temporal lobes. The frontal lobe correlates to executive functioning, while the temporal lobe plays a role in speech and comprehension. Damage to these areas may result in loss of movement, trouble speaking, difficulty solving complex problems, and problems with social behavior. Specifically, damage to the orbital frontal cortex may cause disinhibition and abnormal behaviors, including emotional lability, vulgarity, and indifference to social nuances.1 Within an FTD diagnosis, there are 3 disorders: behavioral-variant FTD (bvFTD), semantic dementia, and progressive nonfluent aphasia.1 Specifically, bvFTD can result in abnormal sexual behaviors such as making sexually inappropriate statements, masturbating in public, undressing in public, inappropriately or aggressively touching others, or confusing another individual as an intimate partner. In addition to cognitive impairment, these neurobehavioral symptoms can significantly impact an individual’s quality of life while increasing caregiver burden.2
Occurring at a similar frequency to Alzheimer’s disease in patients age <65, FTD is one of the more common causes of early-onset dementia. The mean age of onset is 58 and onset after age 75 is particularly unusual. Memory may not be affected early in the course of the disease, but social changes are likely. As FTD progresses, symptoms will resemble those of Alzheimer’s disease and patients will require assistance with activities of daily living. In later stages of FTD, patients will exhibit language and behavior symptoms. Due to its unique progression, FTD can be commonly misdiagnosed as other mental illnesses or neurocognitive disorders.1
Approaches to treatment: What to consider
Both nonpharmacologic and pharmacologic interventions are appropriate for addressing FTD. Because nonpharmacologic options improve patient safety and overall physical health, they should be used whenever practical. These interventions include safe driving measures, exercise, speech therapy, redirection, offering simple choices when making decisions, and managing environmental cues for behaviors that should be encouraged or discouraged.3
There are no FDA-approved medications to cure or slow the progression of FTD. Therefore, treatment is focused on alleviating neurobehavioral symptoms. The symptoms depend on the type of FTD the patient has; they include cognitive impairment, anxiety, insomnia or sleep disturbances, compulsive behaviors, speech and language problems, and agitation. While many medications have been commonly used for symptomatic relief, evidence for the efficacy of these treatments in FTD is limited.2
Continue to: A review of the literature...
A review of the literature on potential treatments for cognitive impairment and behavioral symptoms of FTD identified 2 trials and 1 case series (Table 14-6) in addition to a 2014 review article7 of current pharmacologic treatments. These trials evaluated cognitive improvement with rivastigmine, memantine, galantamine, and donepezil. None of the trials found a significant benefit from any of these medications for cognitive improvement in FTD. Data were conflicting on whether these medications improved or worsened behavioral symptoms. For example, the case series of 3 patients by Swanberg6 suggested improvement in behavior with memantine, while an open-label study analyzed in a 2014 review article7 found that donepezil may have worsened behaviors. Use of cholinesterase inhibitors or memantine in FTD is not recommended unless it is not certain if the patient has FTD or Alzheimer’s disease.7
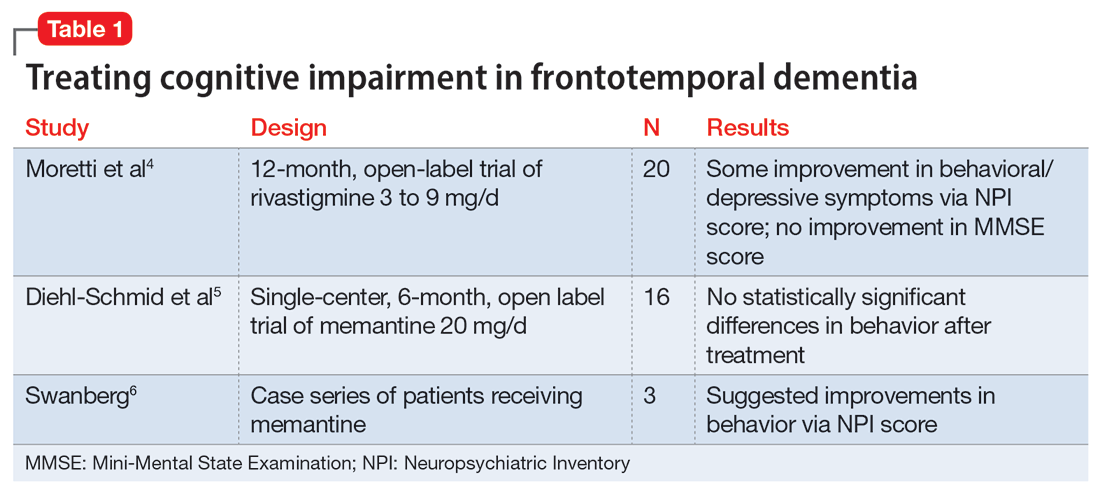
Addressing sexual behaviors. Creating a treatment regimen for FTD behavioral symptoms—specifically for abnormal sexual behaviors—can be challenging. Before starting pharmacotherapy directed at behavioral symptoms secondary to FTD, other causes of symptoms such as delirium, pain, or discomfort should be excluded. Nonpharmacologic approaches should be aimed at the type of sexual behavior and likely underlying environmental cause. For example, patients may inappropriately disrobe themselves. To address this behavior, hospital staff or caregivers should first eliminate environmental causes by ensuring the room is at a comfortable temperature, dressing the patient in light, breathable clothing, or checking if the patient needs to use the bathroom. If no environmental causes are found, a one-piece jumpsuit with closures on the back of the garment could be utilized to increase the difficulty of undressing.
Other nonpharmacologic methods include providing private areas for patients who are behaving inappropriately or removing potentially stimulating television or media from the environment. Another option is to increase the use of positive, pleasant stimuli. One approach that has shown benefit is music therapy, utilizing popular music genres from the patient’s youth.3
Evidence for pharmacotherapy is limited and largely from case reports and case series. A 2020 meta-analysis by Trieu et al8 reviewed 23 studies to expand on current clinical guidance for patients with bvFTD. These studies showed improvements in behavioral symptoms and reductions in caregiver fatigue with citalopram, trazodone, paroxetine, and fluvoxamine. Six of the trials included in this meta-analysis that evaluated these 4 medications are summarized in Table 2.9-14
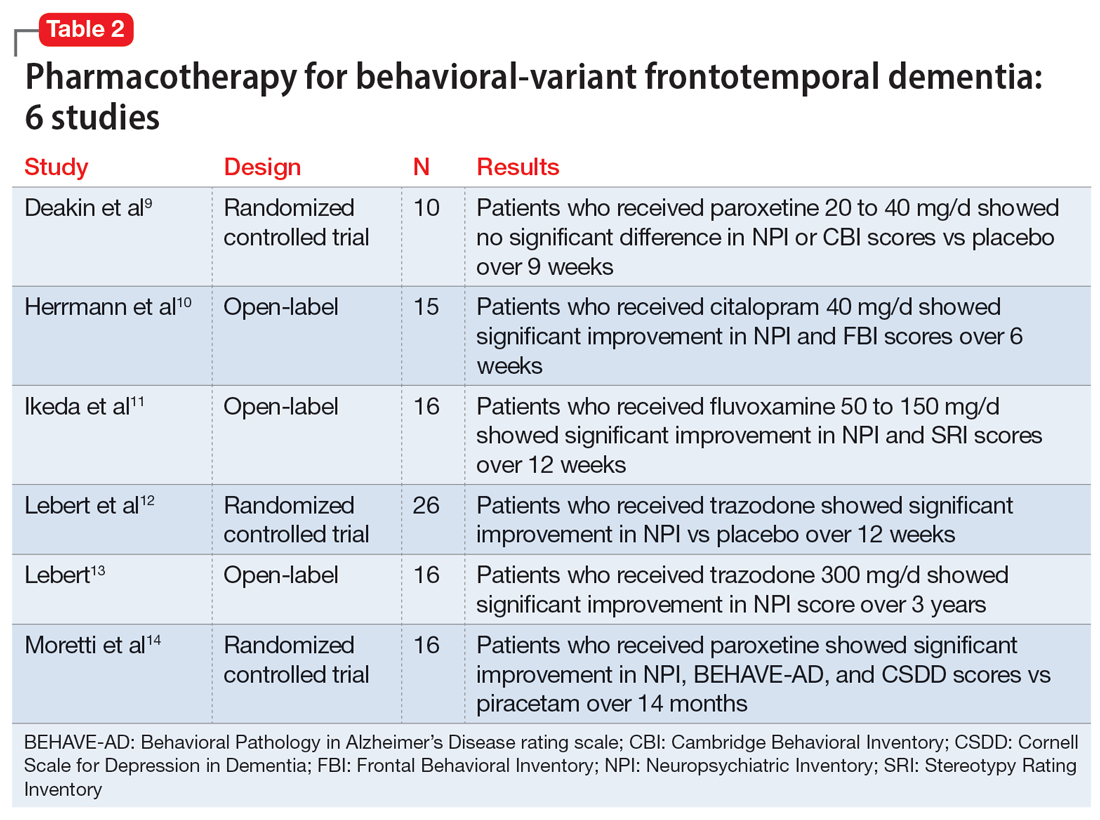
Due to the lower risk of adverse effects and favorable safety profiles, SSRIs and trazodone are considered first-line treatment options. Benefit from these medications is theorized to be a result of their serotonergic effects, because serotonin abnormalities and dysfunction have been linked to FTD symptoms. For example, in a patient experiencing hypersexuality, the common adverse effect of low libido associated with SSRIs can be particularly beneficial.8
Continue to: Other medication classes studied in patients...
Other medication classes studied in patients with FTD include antipsychotics, stimulants, anticonvulsants, benzodiazepines, and hormonal therapies. In addition to a black box warning for increased mortality in older patients with dementia-related psychosis, antipsychotics are associated with other serious adverse effects and should be used with caution.7
FTD is a debilitating disease that has a major impact on quality of life, particularly when behavioral symptoms accompany cognitive decline. Though some therapies may possibly improve behavioral symptoms, their routine use remains controversial due to a lack of clear evidence of benefit. In caring for patients with FTD and behavioral symptoms, a multimodal, team-based approach is vital.1
CASE CONTINUED
The treatment team starts Mr. S on several of the modalities discussed in this article over the span of 2 years, with limited efficacy. Nonpharmacologic methods do not provide much benefit because Mr. S is extremely difficult to redirect. Given Mr. S’s past trials of SSRIs prior to admission, sertraline was retrialed and titrated over 2 years. The highest dose utilized during his admission was 200 mg/d. The team starts estrogen therapy but tapers and discontinues it due to ineffectiveness. Mr. S’s use of carbidopa/levodopa is thought to be contributing to his behavioral abnormalities, so the team tapers it to discontinuation; however, Mr. S’s sexually inappropriate behaviors and agitation continue. The team initiates a plan to reduce the dose of quetiapine and switch to gabapentin, but Mr. S fails gradual dose reduction due to his worsening behaviors. He starts gabapentin. The team gradually increases the dose of gabapentin to decrease libido and agitation, respectively. The increase in sertraline dose and use of nonpharmacologic modalities causes Mr. S’s use of as-needed antipsychotics to decrease.
Related Resources
- Ellison JM. What are the stages of frontotemporal dementia? BrightFocus Foundation. July 5, 2021. Accessed July 7, 2023. https://www.brightfocus.org/alzheimers/article/what-are-stages-frontotemporal-dementia
- Dementia and sexually inappropriate behavior. ReaDementia. January 31, 2022. Accessed July 7, 2023. https://readementia.com/dementia-and-sexually-inappropriate-behavior/
Drug Brand Names
Carbidopa/levodopa • Sinemet
Citalopram • Celexa
Donepezil • Aricept
Fluvoxamine • Luvox
Gabapentin • Neurontin
Galantamine • Razadyne
Memantine • Namenda
Paroxetine • Paxil
Quetiapine • Seroquel
Rivastigmine • Exelon
Sertraline • Zoloft
Trazodone • Desyrel
1. Grossman M. Frontotemporal dementia: a review. J Int Neuropsychol Soc. 2002;8(4):566-583. doi:10.1017/s1355617702814357
2. The Johns Hopkins University. Frontotemporal dementia. Johns Hopkins Medicine. Accessed September 12, 2021. https://www.hopkinsmedicine.org/health/conditions-and-diseases/dementia/frontotemporal-dementia
3. Shinagawa S, Nakajima S, Plitman E, et al. Non-pharmacological management for patients with frontotemporal dementia: a systematic review. J Alzheimers Dis. 2015;45(1):283-293. doi:10.3233/JAD-142109
4. Moretti R, Torre P, Antonello RM, et al. Rivastigmine in frontotemporal dementia: an open-label study. Drugs Aging. 2004;21(14):931-937. doi:10.2165/00002512-200421140-00003
5. Diehl-Schmid J, Förstl H, Perneczky R, et al. A 6-month, open-label study for memantine in patients with frontotemporal dementia. In J Geriatr Psychiatry. 2008;23(7):754-759. doi:10.1002/gps.1973
6. Swanberg MM. Memantine for behavioral disturbances in frontotemporal dementia: a case series. Alzheimer Dis Assoc Disord. 2007;21(2):164-166. doi:10.1097/WAD.0b013e318047df5d
7. Tsai RM, Boxer AL. Treatment of frontotemporal dementia. Curr Treat Options Neurol. 2014;16(11):319. doi:10.1007/s11940-014-0319-0
8. Trieu C, Gossink F, Stek ML, et al. Effectiveness of pharmacological interventions for symptoms of behavioral variant frontotemporal dementia: a systematic review. Cogn Behav Neurol. 2020;33(1):1-15. doi:10.1097/WNN.0000000000000217
9. Deakin JB, Rahman S, Nestor PJ, et al. Paroxetine does not improve symptoms and impairs cognition in frontotemporal dementia: a double-blind randomized controlled trial. Psychopharmacology (Berl). 2004;172(4):400-408. doi:10.1007/s00213-003-1686-5
10. Herrmann N, Black SE, Chow T, et al. Serotonergic function and treatment of behavioral and psychological symptoms of frontotemporal dementia. Am J Geriatr Psychiatry. 2012;20(9):789-797. doi:10.1097/JGP.0b013e31823033f3
11. Ikeda M, Shigenobu K, Fukuhara R, et al. Efficacy of fluvoxamine as a treatment for behavioral symptoms in frontotemporal lobar degeneration patients. Dement Geriatr Cogn Disord. 2004;17(3):117-121. doi:10.1159/000076343
12. Lebert F, Stekke W, Hasenbroekx C, et al. Frontotemporal dementia: a randomised, controlled trial with trazodone. Dement Geriatr Cogn Disord. 2004;17(4):355-359. doi:10.1159/000077171
13. Lebert F. Behavioral benefits of trazodone are sustained for the long term in frontotemporal dementia. Therapy. 2006;3(1):93-96. doi:10.1586/14750708.3.1.93
14. Moretti R, Torre P, Antonello RM, et al. Frontotemporal dementia: paroxetine as a possible treatment of behavior symptoms. A randomized, controlled, open 14-month study. Eur Neurol. 2003;49(1):13-19. doi:10.1159/000067021
Mr. S, age 77, is admitted to a long-term care facility due to progressive cognitive impairment and sexually inappropriate behavior. He has a history of sexual assault of medical staff. His medical history includes significant frontotemporal dementia (FTD) with behavioral disturbances, abnormal sexual behaviors, subclinical hypothyroidism, schizoid personality disorder, Parkinson disease, posttraumatic stress disorder, and hyperammonemia.
Upon admission, Mr. S’s vital signs are within normal limits except for an elevated thyroid-stimulating hormone (4.54 mIU/L; reference range 0.40 to 4.50 mIU/L). Prior cognitive testing results and updated ammonia levels are unavailable. Mr. S’s current medications include acetaminophen 650 mg every 4 hours as needed for pain, calcium carbonate/vitamin D twice daily for bone health, carbidopa/levodopa 25/100 mg twice daily for Parkinson disease, melatonin 3 mg/d at bedtime for insomnia, quetiapine 25 mg twice daily for psychosis with disturbance of behavior and 12.5 mg every 4 hours as needed for agitation, and trazodone 50 mg/d at bedtime for insomnia. Before Mr. S was admitted, previous therapy with selective serotonin reuptake inhibitors (SSRIs) had been tapered and discontinued. Mr. S had also started antipsychotic therapy at another facility due to worsening behaviors.
In patients with dementia, the brain is experiencing neurodegeneration. Progressively, neurons may stop functioning, lose connections with other neurons, and ultimately face cell death. The specific dementia diagnosis and its clinical features depend on the type of neurons and region of the brain affected.1,2
FTD occurs in response to damage to the frontal and temporal lobes. The frontal lobe correlates to executive functioning, while the temporal lobe plays a role in speech and comprehension. Damage to these areas may result in loss of movement, trouble speaking, difficulty solving complex problems, and problems with social behavior. Specifically, damage to the orbital frontal cortex may cause disinhibition and abnormal behaviors, including emotional lability, vulgarity, and indifference to social nuances.1 Within an FTD diagnosis, there are 3 disorders: behavioral-variant FTD (bvFTD), semantic dementia, and progressive nonfluent aphasia.1 Specifically, bvFTD can result in abnormal sexual behaviors such as making sexually inappropriate statements, masturbating in public, undressing in public, inappropriately or aggressively touching others, or confusing another individual as an intimate partner. In addition to cognitive impairment, these neurobehavioral symptoms can significantly impact an individual’s quality of life while increasing caregiver burden.2
Occurring at a similar frequency to Alzheimer’s disease in patients age <65, FTD is one of the more common causes of early-onset dementia. The mean age of onset is 58 and onset after age 75 is particularly unusual. Memory may not be affected early in the course of the disease, but social changes are likely. As FTD progresses, symptoms will resemble those of Alzheimer’s disease and patients will require assistance with activities of daily living. In later stages of FTD, patients will exhibit language and behavior symptoms. Due to its unique progression, FTD can be commonly misdiagnosed as other mental illnesses or neurocognitive disorders.1
Approaches to treatment: What to consider
Both nonpharmacologic and pharmacologic interventions are appropriate for addressing FTD. Because nonpharmacologic options improve patient safety and overall physical health, they should be used whenever practical. These interventions include safe driving measures, exercise, speech therapy, redirection, offering simple choices when making decisions, and managing environmental cues for behaviors that should be encouraged or discouraged.3
There are no FDA-approved medications to cure or slow the progression of FTD. Therefore, treatment is focused on alleviating neurobehavioral symptoms. The symptoms depend on the type of FTD the patient has; they include cognitive impairment, anxiety, insomnia or sleep disturbances, compulsive behaviors, speech and language problems, and agitation. While many medications have been commonly used for symptomatic relief, evidence for the efficacy of these treatments in FTD is limited.2
Continue to: A review of the literature...
A review of the literature on potential treatments for cognitive impairment and behavioral symptoms of FTD identified 2 trials and 1 case series (Table 14-6) in addition to a 2014 review article7 of current pharmacologic treatments. These trials evaluated cognitive improvement with rivastigmine, memantine, galantamine, and donepezil. None of the trials found a significant benefit from any of these medications for cognitive improvement in FTD. Data were conflicting on whether these medications improved or worsened behavioral symptoms. For example, the case series of 3 patients by Swanberg6 suggested improvement in behavior with memantine, while an open-label study analyzed in a 2014 review article7 found that donepezil may have worsened behaviors. Use of cholinesterase inhibitors or memantine in FTD is not recommended unless it is not certain if the patient has FTD or Alzheimer’s disease.7

Addressing sexual behaviors. Creating a treatment regimen for FTD behavioral symptoms—specifically for abnormal sexual behaviors—can be challenging. Before starting pharmacotherapy directed at behavioral symptoms secondary to FTD, other causes of symptoms such as delirium, pain, or discomfort should be excluded. Nonpharmacologic approaches should be aimed at the type of sexual behavior and likely underlying environmental cause. For example, patients may inappropriately disrobe themselves. To address this behavior, hospital staff or caregivers should first eliminate environmental causes by ensuring the room is at a comfortable temperature, dressing the patient in light, breathable clothing, or checking if the patient needs to use the bathroom. If no environmental causes are found, a one-piece jumpsuit with closures on the back of the garment could be utilized to increase the difficulty of undressing.
Other nonpharmacologic methods include providing private areas for patients who are behaving inappropriately or removing potentially stimulating television or media from the environment. Another option is to increase the use of positive, pleasant stimuli. One approach that has shown benefit is music therapy, utilizing popular music genres from the patient’s youth.3
Evidence for pharmacotherapy is limited and largely from case reports and case series. A 2020 meta-analysis by Trieu et al8 reviewed 23 studies to expand on current clinical guidance for patients with bvFTD. These studies showed improvements in behavioral symptoms and reductions in caregiver fatigue with citalopram, trazodone, paroxetine, and fluvoxamine. Six of the trials included in this meta-analysis that evaluated these 4 medications are summarized in Table 2.9-14

Due to the lower risk of adverse effects and favorable safety profiles, SSRIs and trazodone are considered first-line treatment options. Benefit from these medications is theorized to be a result of their serotonergic effects, because serotonin abnormalities and dysfunction have been linked to FTD symptoms. For example, in a patient experiencing hypersexuality, the common adverse effect of low libido associated with SSRIs can be particularly beneficial.8
Continue to: Other medication classes studied in patients...
Other medication classes studied in patients with FTD include antipsychotics, stimulants, anticonvulsants, benzodiazepines, and hormonal therapies. In addition to a black box warning for increased mortality in older patients with dementia-related psychosis, antipsychotics are associated with other serious adverse effects and should be used with caution.7
FTD is a debilitating disease that has a major impact on quality of life, particularly when behavioral symptoms accompany cognitive decline. Though some therapies may possibly improve behavioral symptoms, their routine use remains controversial due to a lack of clear evidence of benefit. In caring for patients with FTD and behavioral symptoms, a multimodal, team-based approach is vital.1
CASE CONTINUED
The treatment team starts Mr. S on several of the modalities discussed in this article over the span of 2 years, with limited efficacy. Nonpharmacologic methods do not provide much benefit because Mr. S is extremely difficult to redirect. Given Mr. S’s past trials of SSRIs prior to admission, sertraline was retrialed and titrated over 2 years. The highest dose utilized during his admission was 200 mg/d. The team starts estrogen therapy but tapers and discontinues it due to ineffectiveness. Mr. S’s use of carbidopa/levodopa is thought to be contributing to his behavioral abnormalities, so the team tapers it to discontinuation; however, Mr. S’s sexually inappropriate behaviors and agitation continue. The team initiates a plan to reduce the dose of quetiapine and switch to gabapentin, but Mr. S fails gradual dose reduction due to his worsening behaviors. He starts gabapentin. The team gradually increases the dose of gabapentin to decrease libido and agitation, respectively. The increase in sertraline dose and use of nonpharmacologic modalities causes Mr. S’s use of as-needed antipsychotics to decrease.
Related Resources
- Ellison JM. What are the stages of frontotemporal dementia? BrightFocus Foundation. July 5, 2021. Accessed July 7, 2023. https://www.brightfocus.org/alzheimers/article/what-are-stages-frontotemporal-dementia
- Dementia and sexually inappropriate behavior. ReaDementia. January 31, 2022. Accessed July 7, 2023. https://readementia.com/dementia-and-sexually-inappropriate-behavior/
Drug Brand Names
Carbidopa/levodopa • Sinemet
Citalopram • Celexa
Donepezil • Aricept
Fluvoxamine • Luvox
Gabapentin • Neurontin
Galantamine • Razadyne
Memantine • Namenda
Paroxetine • Paxil
Quetiapine • Seroquel
Rivastigmine • Exelon
Sertraline • Zoloft
Trazodone • Desyrel
Mr. S, age 77, is admitted to a long-term care facility due to progressive cognitive impairment and sexually inappropriate behavior. He has a history of sexual assault of medical staff. His medical history includes significant frontotemporal dementia (FTD) with behavioral disturbances, abnormal sexual behaviors, subclinical hypothyroidism, schizoid personality disorder, Parkinson disease, posttraumatic stress disorder, and hyperammonemia.
Upon admission, Mr. S’s vital signs are within normal limits except for an elevated thyroid-stimulating hormone (4.54 mIU/L; reference range 0.40 to 4.50 mIU/L). Prior cognitive testing results and updated ammonia levels are unavailable. Mr. S’s current medications include acetaminophen 650 mg every 4 hours as needed for pain, calcium carbonate/vitamin D twice daily for bone health, carbidopa/levodopa 25/100 mg twice daily for Parkinson disease, melatonin 3 mg/d at bedtime for insomnia, quetiapine 25 mg twice daily for psychosis with disturbance of behavior and 12.5 mg every 4 hours as needed for agitation, and trazodone 50 mg/d at bedtime for insomnia. Before Mr. S was admitted, previous therapy with selective serotonin reuptake inhibitors (SSRIs) had been tapered and discontinued. Mr. S had also started antipsychotic therapy at another facility due to worsening behaviors.
In patients with dementia, the brain is experiencing neurodegeneration. Progressively, neurons may stop functioning, lose connections with other neurons, and ultimately face cell death. The specific dementia diagnosis and its clinical features depend on the type of neurons and region of the brain affected.1,2
FTD occurs in response to damage to the frontal and temporal lobes. The frontal lobe correlates to executive functioning, while the temporal lobe plays a role in speech and comprehension. Damage to these areas may result in loss of movement, trouble speaking, difficulty solving complex problems, and problems with social behavior. Specifically, damage to the orbital frontal cortex may cause disinhibition and abnormal behaviors, including emotional lability, vulgarity, and indifference to social nuances.1 Within an FTD diagnosis, there are 3 disorders: behavioral-variant FTD (bvFTD), semantic dementia, and progressive nonfluent aphasia.1 Specifically, bvFTD can result in abnormal sexual behaviors such as making sexually inappropriate statements, masturbating in public, undressing in public, inappropriately or aggressively touching others, or confusing another individual as an intimate partner. In addition to cognitive impairment, these neurobehavioral symptoms can significantly impact an individual’s quality of life while increasing caregiver burden.2
Occurring at a similar frequency to Alzheimer’s disease in patients age <65, FTD is one of the more common causes of early-onset dementia. The mean age of onset is 58 and onset after age 75 is particularly unusual. Memory may not be affected early in the course of the disease, but social changes are likely. As FTD progresses, symptoms will resemble those of Alzheimer’s disease and patients will require assistance with activities of daily living. In later stages of FTD, patients will exhibit language and behavior symptoms. Due to its unique progression, FTD can be commonly misdiagnosed as other mental illnesses or neurocognitive disorders.1
Approaches to treatment: What to consider
Both nonpharmacologic and pharmacologic interventions are appropriate for addressing FTD. Because nonpharmacologic options improve patient safety and overall physical health, they should be used whenever practical. These interventions include safe driving measures, exercise, speech therapy, redirection, offering simple choices when making decisions, and managing environmental cues for behaviors that should be encouraged or discouraged.3
There are no FDA-approved medications to cure or slow the progression of FTD. Therefore, treatment is focused on alleviating neurobehavioral symptoms. The symptoms depend on the type of FTD the patient has; they include cognitive impairment, anxiety, insomnia or sleep disturbances, compulsive behaviors, speech and language problems, and agitation. While many medications have been commonly used for symptomatic relief, evidence for the efficacy of these treatments in FTD is limited.2
Continue to: A review of the literature...
A review of the literature on potential treatments for cognitive impairment and behavioral symptoms of FTD identified 2 trials and 1 case series (Table 14-6) in addition to a 2014 review article7 of current pharmacologic treatments. These trials evaluated cognitive improvement with rivastigmine, memantine, galantamine, and donepezil. None of the trials found a significant benefit from any of these medications for cognitive improvement in FTD. Data were conflicting on whether these medications improved or worsened behavioral symptoms. For example, the case series of 3 patients by Swanberg6 suggested improvement in behavior with memantine, while an open-label study analyzed in a 2014 review article7 found that donepezil may have worsened behaviors. Use of cholinesterase inhibitors or memantine in FTD is not recommended unless it is not certain if the patient has FTD or Alzheimer’s disease.7

Addressing sexual behaviors. Creating a treatment regimen for FTD behavioral symptoms—specifically for abnormal sexual behaviors—can be challenging. Before starting pharmacotherapy directed at behavioral symptoms secondary to FTD, other causes of symptoms such as delirium, pain, or discomfort should be excluded. Nonpharmacologic approaches should be aimed at the type of sexual behavior and likely underlying environmental cause. For example, patients may inappropriately disrobe themselves. To address this behavior, hospital staff or caregivers should first eliminate environmental causes by ensuring the room is at a comfortable temperature, dressing the patient in light, breathable clothing, or checking if the patient needs to use the bathroom. If no environmental causes are found, a one-piece jumpsuit with closures on the back of the garment could be utilized to increase the difficulty of undressing.
Other nonpharmacologic methods include providing private areas for patients who are behaving inappropriately or removing potentially stimulating television or media from the environment. Another option is to increase the use of positive, pleasant stimuli. One approach that has shown benefit is music therapy, utilizing popular music genres from the patient’s youth.3
Evidence for pharmacotherapy is limited and largely from case reports and case series. A 2020 meta-analysis by Trieu et al8 reviewed 23 studies to expand on current clinical guidance for patients with bvFTD. These studies showed improvements in behavioral symptoms and reductions in caregiver fatigue with citalopram, trazodone, paroxetine, and fluvoxamine. Six of the trials included in this meta-analysis that evaluated these 4 medications are summarized in Table 2.9-14

Due to the lower risk of adverse effects and favorable safety profiles, SSRIs and trazodone are considered first-line treatment options. Benefit from these medications is theorized to be a result of their serotonergic effects, because serotonin abnormalities and dysfunction have been linked to FTD symptoms. For example, in a patient experiencing hypersexuality, the common adverse effect of low libido associated with SSRIs can be particularly beneficial.8
Continue to: Other medication classes studied in patients...
Other medication classes studied in patients with FTD include antipsychotics, stimulants, anticonvulsants, benzodiazepines, and hormonal therapies. In addition to a black box warning for increased mortality in older patients with dementia-related psychosis, antipsychotics are associated with other serious adverse effects and should be used with caution.7
FTD is a debilitating disease that has a major impact on quality of life, particularly when behavioral symptoms accompany cognitive decline. Though some therapies may possibly improve behavioral symptoms, their routine use remains controversial due to a lack of clear evidence of benefit. In caring for patients with FTD and behavioral symptoms, a multimodal, team-based approach is vital.1
CASE CONTINUED
The treatment team starts Mr. S on several of the modalities discussed in this article over the span of 2 years, with limited efficacy. Nonpharmacologic methods do not provide much benefit because Mr. S is extremely difficult to redirect. Given Mr. S’s past trials of SSRIs prior to admission, sertraline was retrialed and titrated over 2 years. The highest dose utilized during his admission was 200 mg/d. The team starts estrogen therapy but tapers and discontinues it due to ineffectiveness. Mr. S’s use of carbidopa/levodopa is thought to be contributing to his behavioral abnormalities, so the team tapers it to discontinuation; however, Mr. S’s sexually inappropriate behaviors and agitation continue. The team initiates a plan to reduce the dose of quetiapine and switch to gabapentin, but Mr. S fails gradual dose reduction due to his worsening behaviors. He starts gabapentin. The team gradually increases the dose of gabapentin to decrease libido and agitation, respectively. The increase in sertraline dose and use of nonpharmacologic modalities causes Mr. S’s use of as-needed antipsychotics to decrease.
Related Resources
- Ellison JM. What are the stages of frontotemporal dementia? BrightFocus Foundation. July 5, 2021. Accessed July 7, 2023. https://www.brightfocus.org/alzheimers/article/what-are-stages-frontotemporal-dementia
- Dementia and sexually inappropriate behavior. ReaDementia. January 31, 2022. Accessed July 7, 2023. https://readementia.com/dementia-and-sexually-inappropriate-behavior/
Drug Brand Names
Carbidopa/levodopa • Sinemet
Citalopram • Celexa
Donepezil • Aricept
Fluvoxamine • Luvox
Gabapentin • Neurontin
Galantamine • Razadyne
Memantine • Namenda
Paroxetine • Paxil
Quetiapine • Seroquel
Rivastigmine • Exelon
Sertraline • Zoloft
Trazodone • Desyrel
1. Grossman M. Frontotemporal dementia: a review. J Int Neuropsychol Soc. 2002;8(4):566-583. doi:10.1017/s1355617702814357
2. The Johns Hopkins University. Frontotemporal dementia. Johns Hopkins Medicine. Accessed September 12, 2021. https://www.hopkinsmedicine.org/health/conditions-and-diseases/dementia/frontotemporal-dementia
3. Shinagawa S, Nakajima S, Plitman E, et al. Non-pharmacological management for patients with frontotemporal dementia: a systematic review. J Alzheimers Dis. 2015;45(1):283-293. doi:10.3233/JAD-142109
4. Moretti R, Torre P, Antonello RM, et al. Rivastigmine in frontotemporal dementia: an open-label study. Drugs Aging. 2004;21(14):931-937. doi:10.2165/00002512-200421140-00003
5. Diehl-Schmid J, Förstl H, Perneczky R, et al. A 6-month, open-label study for memantine in patients with frontotemporal dementia. In J Geriatr Psychiatry. 2008;23(7):754-759. doi:10.1002/gps.1973
6. Swanberg MM. Memantine for behavioral disturbances in frontotemporal dementia: a case series. Alzheimer Dis Assoc Disord. 2007;21(2):164-166. doi:10.1097/WAD.0b013e318047df5d
7. Tsai RM, Boxer AL. Treatment of frontotemporal dementia. Curr Treat Options Neurol. 2014;16(11):319. doi:10.1007/s11940-014-0319-0
8. Trieu C, Gossink F, Stek ML, et al. Effectiveness of pharmacological interventions for symptoms of behavioral variant frontotemporal dementia: a systematic review. Cogn Behav Neurol. 2020;33(1):1-15. doi:10.1097/WNN.0000000000000217
9. Deakin JB, Rahman S, Nestor PJ, et al. Paroxetine does not improve symptoms and impairs cognition in frontotemporal dementia: a double-blind randomized controlled trial. Psychopharmacology (Berl). 2004;172(4):400-408. doi:10.1007/s00213-003-1686-5
10. Herrmann N, Black SE, Chow T, et al. Serotonergic function and treatment of behavioral and psychological symptoms of frontotemporal dementia. Am J Geriatr Psychiatry. 2012;20(9):789-797. doi:10.1097/JGP.0b013e31823033f3
11. Ikeda M, Shigenobu K, Fukuhara R, et al. Efficacy of fluvoxamine as a treatment for behavioral symptoms in frontotemporal lobar degeneration patients. Dement Geriatr Cogn Disord. 2004;17(3):117-121. doi:10.1159/000076343
12. Lebert F, Stekke W, Hasenbroekx C, et al. Frontotemporal dementia: a randomised, controlled trial with trazodone. Dement Geriatr Cogn Disord. 2004;17(4):355-359. doi:10.1159/000077171
13. Lebert F. Behavioral benefits of trazodone are sustained for the long term in frontotemporal dementia. Therapy. 2006;3(1):93-96. doi:10.1586/14750708.3.1.93
14. Moretti R, Torre P, Antonello RM, et al. Frontotemporal dementia: paroxetine as a possible treatment of behavior symptoms. A randomized, controlled, open 14-month study. Eur Neurol. 2003;49(1):13-19. doi:10.1159/000067021
1. Grossman M. Frontotemporal dementia: a review. J Int Neuropsychol Soc. 2002;8(4):566-583. doi:10.1017/s1355617702814357
2. The Johns Hopkins University. Frontotemporal dementia. Johns Hopkins Medicine. Accessed September 12, 2021. https://www.hopkinsmedicine.org/health/conditions-and-diseases/dementia/frontotemporal-dementia
3. Shinagawa S, Nakajima S, Plitman E, et al. Non-pharmacological management for patients with frontotemporal dementia: a systematic review. J Alzheimers Dis. 2015;45(1):283-293. doi:10.3233/JAD-142109
4. Moretti R, Torre P, Antonello RM, et al. Rivastigmine in frontotemporal dementia: an open-label study. Drugs Aging. 2004;21(14):931-937. doi:10.2165/00002512-200421140-00003
5. Diehl-Schmid J, Förstl H, Perneczky R, et al. A 6-month, open-label study for memantine in patients with frontotemporal dementia. In J Geriatr Psychiatry. 2008;23(7):754-759. doi:10.1002/gps.1973
6. Swanberg MM. Memantine for behavioral disturbances in frontotemporal dementia: a case series. Alzheimer Dis Assoc Disord. 2007;21(2):164-166. doi:10.1097/WAD.0b013e318047df5d
7. Tsai RM, Boxer AL. Treatment of frontotemporal dementia. Curr Treat Options Neurol. 2014;16(11):319. doi:10.1007/s11940-014-0319-0
8. Trieu C, Gossink F, Stek ML, et al. Effectiveness of pharmacological interventions for symptoms of behavioral variant frontotemporal dementia: a systematic review. Cogn Behav Neurol. 2020;33(1):1-15. doi:10.1097/WNN.0000000000000217
9. Deakin JB, Rahman S, Nestor PJ, et al. Paroxetine does not improve symptoms and impairs cognition in frontotemporal dementia: a double-blind randomized controlled trial. Psychopharmacology (Berl). 2004;172(4):400-408. doi:10.1007/s00213-003-1686-5
10. Herrmann N, Black SE, Chow T, et al. Serotonergic function and treatment of behavioral and psychological symptoms of frontotemporal dementia. Am J Geriatr Psychiatry. 2012;20(9):789-797. doi:10.1097/JGP.0b013e31823033f3
11. Ikeda M, Shigenobu K, Fukuhara R, et al. Efficacy of fluvoxamine as a treatment for behavioral symptoms in frontotemporal lobar degeneration patients. Dement Geriatr Cogn Disord. 2004;17(3):117-121. doi:10.1159/000076343
12. Lebert F, Stekke W, Hasenbroekx C, et al. Frontotemporal dementia: a randomised, controlled trial with trazodone. Dement Geriatr Cogn Disord. 2004;17(4):355-359. doi:10.1159/000077171
13. Lebert F. Behavioral benefits of trazodone are sustained for the long term in frontotemporal dementia. Therapy. 2006;3(1):93-96. doi:10.1586/14750708.3.1.93
14. Moretti R, Torre P, Antonello RM, et al. Frontotemporal dementia: paroxetine as a possible treatment of behavior symptoms. A randomized, controlled, open 14-month study. Eur Neurol. 2003;49(1):13-19. doi:10.1159/000067021
Lamotrigine interactions with oral contraceptives
Ms. A, age 20, presents to the clinic after experiencing difficulty sleeping, depressed mood, fatigue, and difficulty concentrating. Her psychiatric history includes bipolar II disorder (BD II), predominantly with depressive episodes. Ms. A’s current medications include a combination of lamotrigine 200 mg/d and bupropion extended-release 450 mg/d, and her symptoms were well maintained until 2 weeks ago. When her psychiatrist performs a medication reconciliation at her medication management appointment, Ms. A indicates she started taking an oral contraceptive, ethinyl estradiol and norgestimate, approximately 1 month ago for management of endometriosis symptoms. She is not currently taking any other medications or supplements.
Lamotrigine is indicated for epilepsy and as maintenance treatment for BD I. It is also used off-label to treat other mood disorders. After oral administration, lamotrigine is rapidly and fully absorbed with a high bioavailability (98%).The principal metabolic pathway is via glucuronic acid conjugation, leading to the major inactive metabolite 2-N-glucuronide. Minor metabolites include 5-N-glucuronide and a 2-N-glucuronide metabolite.1
Combined oral contraceptives contain an estrogen component, typically ethinyl estradiol, and a progestin component, which varies based on the specific formulation. The metabolism of ethinyl estradiol occurs through cytochrome P450 (CYP)3A4, CYP2C9, sulfation, and glucuronidation. For progestin—the second component of combined oral contraceptives and the lone component of progestin-only oral contraceptives—metabolism occurs via CYP3A4 and conjugation reactions.2 This article focuses on lamotrigine interactions specifically with oral contraceptives, but it is important to note that other formulations of combined hormonal contraceptives, such as the combined contraceptive patch (Ortho Evra) and vaginal ring (NuvaRing), would be expected to interact in the same way as oral formulations.3
Bidirectional interaction
While many antiseizure medications are known to interact with and potentially decrease the efficacy of oral contraceptives (Table 13-6), the interactions between lamotrigine and oral contraceptives is uniquely bidirectional. Combined oral contraceptives are thought to interact with lamotrigine primarily via the estrogen component, which causes increased metabolism of lamotrigine through induction of glucuronidation. This drug interaction decreases the plasma concentrations of lamotrigine in the body by up to 2-fold, resulting in an increased risk of seizures or inadequate mood stabilization.1 This effect on metabolism is very rapid, resulting in decreases in lamotrigine concentrations within 1 week.4,7 A recent study suggested that certain progestins may also contribute to decreased plasma levels of lamotrigine, but the mechanism for this is unknown (Table 23-7).8
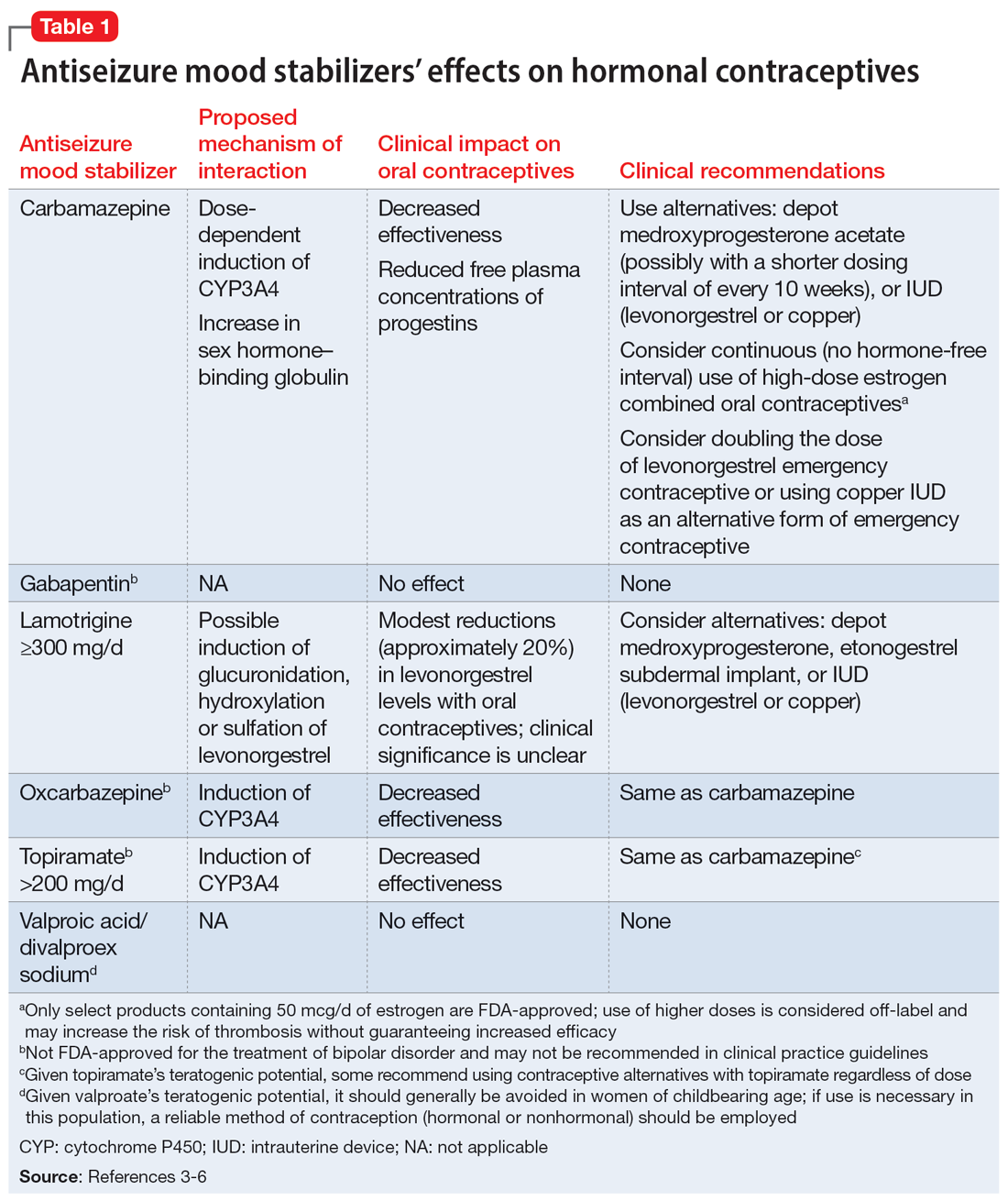
Clinicians should consider increasing the lamotrigine dose (potentially as much as 2-fold) in a patient who initiates treatment with a combined hormonal contraceptive. Dose increases should not be >50 to 100 mg/d every week.1 Collect lamotrigine blood levels before starting a hormonal contraceptive and during dose titration. While there is not a well-established therapeutic range for lamotrigine in BD, expert consensus recommends a range of 1 to 6 mcg/mL.8
The lamotrigine dose should be decreased if combined hormonal contraceptives are discontinued. Dose decreases should not exceed 25% of the total daily dose per week.1 Desogestrel, a progestin-only medication, may increase exposure to lamotrigine, but this has not been observed in research with other progestins.5,9 When starting a progestin-only pill, monitor patients for signs of lamotrigine toxicity (ataxia, diplopia, dizziness) and consider monitoring their blood levels.
An important consideration to note with combined oral contraceptives is the hormone-free interval, also known as the pill-free week. Due to the rapid effect of estrogens, the lamotrigine concentrations have been shown to rise, even double, during this hormone-free interval, so patients should be closely monitored for adverse effects.3 Some recommend use of an extended cycle regimen (with a limited hormone-free interval), or continuous cycle regimen (with no hormone-free interval) to avoid fluctuations in lamotrigine levels.3,5 Additionally, data suggest that in patients taking lamotrigine and valproate, which inhibits glucuronidation, oral contraceptives do not cause reductions in lamotrigine concentrations.2,5 In these instances, dose increases of lamotrigine are not needed.
Continue to: The metabolism of ethinyl estradiol...
The metabolism of ethinyl estradiol and progestin are susceptible to CYP3A4 induction and increased glucuronidation. Serum concentrations may be reduced by ≥50% when used concomitantly with CYP enzyme–inducing medications, which could possibly result in subtherapeutic levels and unplanned pregnancy.3 CYP3A4 induction occurs for up to 4 weeks after discontinuation of an enzyme-inducing agent, pointing to the need for alternative or backup contraception during this time.3 Lamotrigine is not a CYP enzyme–inducing medication; it is unlikely to affect the efficacy of oral contraceptives in the same manner as other antiseizure medications. However, a study of lamotrigine and the combined hormonal contraceptive ethinyl estradiol and levonorgestrel demonstrated reduced exposure to levonorgestrel, resulting in breakthrough bleeding.5
In a study on the coadministration of lamotrigine and combined oral contraceptives, Sidhu et al4 observed a small mean reduction (20%) in progestin concentrations when lamotrigine was used at a dose of 300 mg/d. Although there is no research suggesting decreased effectiveness in preventing pregnancy when lamotrigine is used with combined oral contraceptives, progestin-only oral contraceptives, or progestin implants, additional or alternative contraceptive methods may be considered based on this pharmacokinetic data, particularly in patients who require lamotrigine doses ≥300 mg/d.5
CASE CONTINUED
Given when Ms. A started the oral contraceptive, the treatment team determines it is likely that an interaction with lamotrigine is causing her resurgence of depressive symptoms. Her care team decides to titrate the lamotrigine gradually to 300 mg/d, then 400 mg/d if needed, while carefully monitoring for signs of a serious rash. This dosage increase may help Ms. A achieve symptom remission. Monitoring plasma levels may be considered, although it is unknown what plasma level was effective for Ms. A before she started the oral contraceptive. Ms. A would need to be counseled regarding the effect of higher doses of lamotrigine on the effectiveness of the oral contraceptive.
Although it does not appear Ms. A is using the oral contraceptive specifically to prevent pregnancy, the team informs her about the possibility of unintended pregnancy with this medication combination. If Ms. A was also using the medication for this indication, alternative contraceptive options would include medroxyprogesterone acetate, levonorgestrel implants, or an intrauterine device (levonorgestrel or copper, though copper would not be effective for endometriosis symptom management). Ms. A should consult with her gynecologist regarding the most appropriate option for her endometriosis. If the decision is made to discontinue her oral contraceptive in the future, the lamotrigine dose should be decreased to her previously effective dose of 200 mg/d.
Related Resources
- Makino KK, Hatters Friedman S, Amin J. Emergency contraception for psychiatric patients. Current Psychiatry. 2022;21(11):34-39,44-45. doi:10.12788/cp.0300
- MGH Center for Women’s Mental Health. You asked: is there an interaction between lamotrigine and oral contraceptives? September 29, 2015. https://womensmentalhealth.org/posts/you-asked-is-there-an-interaction-between-lamotrigine-andoral-contraceptives/
Drug Brand Names
Bupropion extended-release • Wellbutrin XL
Carbamazepine • Equetro, Tegretol
Desogestrel • Cerazette
Divalproex sodium • Depakote
Ethinyl estradiol and etonogestrel • NuvaRing
Ethinyl estradiol and norelgestromin • Ortho Evra
Ethinyl estradiol and norgestimate • Ortho Tri-Cyclen, TriNessa, others
Etonogestrel • Implanon, Nexplanon
Gabapentin • Neurontin
Lamotrigine • Lamictal
Levonorgestrel emergency contraceptive pill • AfterPill, Plan B
Levonorgestrel intrauterine device • Mirena, Skyla
Medroxyprogesterone acetate • Depo-Provera
Oxcarbazepine • Trileptal
Topiramate • Topamax
Valproic acid • Depakene
1. Lamictal [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2020.
2. Lee CR. Drug interactions and hormonal contraception. Trends in Urology Gynaecology & Sexual Health. 2009;14(3):23-26.
3. Williams D. Antiepileptic drugs and contraception. US Pharm. 2014;39(1):39-42.
4. Sidhu J, Job S, Singh S, et al. The pharmacokinetic and pharmacodynamic consequences of the co-administration of lamotrigine and a combined oral contraceptive in healthy female subjects. Br J Clin Pharmacol. 2006;61(2):191-199. doi:10.1111/j.1365-2125.2005.02539.x
5. Faculty of Sexual & Reproductive Healthcare. Clinical guidance: drug interactions with hormonal contraception. Published May 9, 2022. Accessed September 28, 2022. https://www.fsrh.org/documents/ceu-clinical-guidance-drug-interactions-with-hormonal/
6. Johnston CA, Crawford PM. Anti-epileptic drugs and hormonal treatments. Curr Treat Options Neurol. 2014;16(5):288. doi:10.1007/s11940-014-0288-3
7. Christensen J, Petrenaite V, Atterman J, et al. Oral contraceptives induce lamotrigine metabolism: evidence from a double-blind, placebo-controlled trial. Epilepsia. 2007;48(3):484-489. doi:10.1111/j.1528-1167.2007.00997.x
8. Hiemke C, Bergemann N, Clement HW, et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry. 2018;51(1-02):9-62. doi:10.1055/s-0043-116492
9. Rauchenzauner M, Deichmann S, Pittschieler, et al. Bidirectional interaction between oral contraception and lamotrigine in women with epilepsy – role of progestins. Seizure. 2020;74:89-92. doi:10.1016/j.seizure.2019.11.011
Ms. A, age 20, presents to the clinic after experiencing difficulty sleeping, depressed mood, fatigue, and difficulty concentrating. Her psychiatric history includes bipolar II disorder (BD II), predominantly with depressive episodes. Ms. A’s current medications include a combination of lamotrigine 200 mg/d and bupropion extended-release 450 mg/d, and her symptoms were well maintained until 2 weeks ago. When her psychiatrist performs a medication reconciliation at her medication management appointment, Ms. A indicates she started taking an oral contraceptive, ethinyl estradiol and norgestimate, approximately 1 month ago for management of endometriosis symptoms. She is not currently taking any other medications or supplements.
Lamotrigine is indicated for epilepsy and as maintenance treatment for BD I. It is also used off-label to treat other mood disorders. After oral administration, lamotrigine is rapidly and fully absorbed with a high bioavailability (98%).The principal metabolic pathway is via glucuronic acid conjugation, leading to the major inactive metabolite 2-N-glucuronide. Minor metabolites include 5-N-glucuronide and a 2-N-glucuronide metabolite.1
Combined oral contraceptives contain an estrogen component, typically ethinyl estradiol, and a progestin component, which varies based on the specific formulation. The metabolism of ethinyl estradiol occurs through cytochrome P450 (CYP)3A4, CYP2C9, sulfation, and glucuronidation. For progestin—the second component of combined oral contraceptives and the lone component of progestin-only oral contraceptives—metabolism occurs via CYP3A4 and conjugation reactions.2 This article focuses on lamotrigine interactions specifically with oral contraceptives, but it is important to note that other formulations of combined hormonal contraceptives, such as the combined contraceptive patch (Ortho Evra) and vaginal ring (NuvaRing), would be expected to interact in the same way as oral formulations.3
Bidirectional interaction
While many antiseizure medications are known to interact with and potentially decrease the efficacy of oral contraceptives (Table 13-6), the interactions between lamotrigine and oral contraceptives is uniquely bidirectional. Combined oral contraceptives are thought to interact with lamotrigine primarily via the estrogen component, which causes increased metabolism of lamotrigine through induction of glucuronidation. This drug interaction decreases the plasma concentrations of lamotrigine in the body by up to 2-fold, resulting in an increased risk of seizures or inadequate mood stabilization.1 This effect on metabolism is very rapid, resulting in decreases in lamotrigine concentrations within 1 week.4,7 A recent study suggested that certain progestins may also contribute to decreased plasma levels of lamotrigine, but the mechanism for this is unknown (Table 23-7).8

Clinicians should consider increasing the lamotrigine dose (potentially as much as 2-fold) in a patient who initiates treatment with a combined hormonal contraceptive. Dose increases should not be >50 to 100 mg/d every week.1 Collect lamotrigine blood levels before starting a hormonal contraceptive and during dose titration. While there is not a well-established therapeutic range for lamotrigine in BD, expert consensus recommends a range of 1 to 6 mcg/mL.8

The lamotrigine dose should be decreased if combined hormonal contraceptives are discontinued. Dose decreases should not exceed 25% of the total daily dose per week.1 Desogestrel, a progestin-only medication, may increase exposure to lamotrigine, but this has not been observed in research with other progestins.5,9 When starting a progestin-only pill, monitor patients for signs of lamotrigine toxicity (ataxia, diplopia, dizziness) and consider monitoring their blood levels.
An important consideration to note with combined oral contraceptives is the hormone-free interval, also known as the pill-free week. Due to the rapid effect of estrogens, the lamotrigine concentrations have been shown to rise, even double, during this hormone-free interval, so patients should be closely monitored for adverse effects.3 Some recommend use of an extended cycle regimen (with a limited hormone-free interval), or continuous cycle regimen (with no hormone-free interval) to avoid fluctuations in lamotrigine levels.3,5 Additionally, data suggest that in patients taking lamotrigine and valproate, which inhibits glucuronidation, oral contraceptives do not cause reductions in lamotrigine concentrations.2,5 In these instances, dose increases of lamotrigine are not needed.
Continue to: The metabolism of ethinyl estradiol...
The metabolism of ethinyl estradiol and progestin are susceptible to CYP3A4 induction and increased glucuronidation. Serum concentrations may be reduced by ≥50% when used concomitantly with CYP enzyme–inducing medications, which could possibly result in subtherapeutic levels and unplanned pregnancy.3 CYP3A4 induction occurs for up to 4 weeks after discontinuation of an enzyme-inducing agent, pointing to the need for alternative or backup contraception during this time.3 Lamotrigine is not a CYP enzyme–inducing medication; it is unlikely to affect the efficacy of oral contraceptives in the same manner as other antiseizure medications. However, a study of lamotrigine and the combined hormonal contraceptive ethinyl estradiol and levonorgestrel demonstrated reduced exposure to levonorgestrel, resulting in breakthrough bleeding.5
In a study on the coadministration of lamotrigine and combined oral contraceptives, Sidhu et al4 observed a small mean reduction (20%) in progestin concentrations when lamotrigine was used at a dose of 300 mg/d. Although there is no research suggesting decreased effectiveness in preventing pregnancy when lamotrigine is used with combined oral contraceptives, progestin-only oral contraceptives, or progestin implants, additional or alternative contraceptive methods may be considered based on this pharmacokinetic data, particularly in patients who require lamotrigine doses ≥300 mg/d.5
CASE CONTINUED
Given when Ms. A started the oral contraceptive, the treatment team determines it is likely that an interaction with lamotrigine is causing her resurgence of depressive symptoms. Her care team decides to titrate the lamotrigine gradually to 300 mg/d, then 400 mg/d if needed, while carefully monitoring for signs of a serious rash. This dosage increase may help Ms. A achieve symptom remission. Monitoring plasma levels may be considered, although it is unknown what plasma level was effective for Ms. A before she started the oral contraceptive. Ms. A would need to be counseled regarding the effect of higher doses of lamotrigine on the effectiveness of the oral contraceptive.
Although it does not appear Ms. A is using the oral contraceptive specifically to prevent pregnancy, the team informs her about the possibility of unintended pregnancy with this medication combination. If Ms. A was also using the medication for this indication, alternative contraceptive options would include medroxyprogesterone acetate, levonorgestrel implants, or an intrauterine device (levonorgestrel or copper, though copper would not be effective for endometriosis symptom management). Ms. A should consult with her gynecologist regarding the most appropriate option for her endometriosis. If the decision is made to discontinue her oral contraceptive in the future, the lamotrigine dose should be decreased to her previously effective dose of 200 mg/d.
Related Resources
- Makino KK, Hatters Friedman S, Amin J. Emergency contraception for psychiatric patients. Current Psychiatry. 2022;21(11):34-39,44-45. doi:10.12788/cp.0300
- MGH Center for Women’s Mental Health. You asked: is there an interaction between lamotrigine and oral contraceptives? September 29, 2015. https://womensmentalhealth.org/posts/you-asked-is-there-an-interaction-between-lamotrigine-andoral-contraceptives/
Drug Brand Names
Bupropion extended-release • Wellbutrin XL
Carbamazepine • Equetro, Tegretol
Desogestrel • Cerazette
Divalproex sodium • Depakote
Ethinyl estradiol and etonogestrel • NuvaRing
Ethinyl estradiol and norelgestromin • Ortho Evra
Ethinyl estradiol and norgestimate • Ortho Tri-Cyclen, TriNessa, others
Etonogestrel • Implanon, Nexplanon
Gabapentin • Neurontin
Lamotrigine • Lamictal
Levonorgestrel emergency contraceptive pill • AfterPill, Plan B
Levonorgestrel intrauterine device • Mirena, Skyla
Medroxyprogesterone acetate • Depo-Provera
Oxcarbazepine • Trileptal
Topiramate • Topamax
Valproic acid • Depakene
Ms. A, age 20, presents to the clinic after experiencing difficulty sleeping, depressed mood, fatigue, and difficulty concentrating. Her psychiatric history includes bipolar II disorder (BD II), predominantly with depressive episodes. Ms. A’s current medications include a combination of lamotrigine 200 mg/d and bupropion extended-release 450 mg/d, and her symptoms were well maintained until 2 weeks ago. When her psychiatrist performs a medication reconciliation at her medication management appointment, Ms. A indicates she started taking an oral contraceptive, ethinyl estradiol and norgestimate, approximately 1 month ago for management of endometriosis symptoms. She is not currently taking any other medications or supplements.
Lamotrigine is indicated for epilepsy and as maintenance treatment for BD I. It is also used off-label to treat other mood disorders. After oral administration, lamotrigine is rapidly and fully absorbed with a high bioavailability (98%).The principal metabolic pathway is via glucuronic acid conjugation, leading to the major inactive metabolite 2-N-glucuronide. Minor metabolites include 5-N-glucuronide and a 2-N-glucuronide metabolite.1
Combined oral contraceptives contain an estrogen component, typically ethinyl estradiol, and a progestin component, which varies based on the specific formulation. The metabolism of ethinyl estradiol occurs through cytochrome P450 (CYP)3A4, CYP2C9, sulfation, and glucuronidation. For progestin—the second component of combined oral contraceptives and the lone component of progestin-only oral contraceptives—metabolism occurs via CYP3A4 and conjugation reactions.2 This article focuses on lamotrigine interactions specifically with oral contraceptives, but it is important to note that other formulations of combined hormonal contraceptives, such as the combined contraceptive patch (Ortho Evra) and vaginal ring (NuvaRing), would be expected to interact in the same way as oral formulations.3
Bidirectional interaction
While many antiseizure medications are known to interact with and potentially decrease the efficacy of oral contraceptives (Table 13-6), the interactions between lamotrigine and oral contraceptives is uniquely bidirectional. Combined oral contraceptives are thought to interact with lamotrigine primarily via the estrogen component, which causes increased metabolism of lamotrigine through induction of glucuronidation. This drug interaction decreases the plasma concentrations of lamotrigine in the body by up to 2-fold, resulting in an increased risk of seizures or inadequate mood stabilization.1 This effect on metabolism is very rapid, resulting in decreases in lamotrigine concentrations within 1 week.4,7 A recent study suggested that certain progestins may also contribute to decreased plasma levels of lamotrigine, but the mechanism for this is unknown (Table 23-7).8

Clinicians should consider increasing the lamotrigine dose (potentially as much as 2-fold) in a patient who initiates treatment with a combined hormonal contraceptive. Dose increases should not be >50 to 100 mg/d every week.1 Collect lamotrigine blood levels before starting a hormonal contraceptive and during dose titration. While there is not a well-established therapeutic range for lamotrigine in BD, expert consensus recommends a range of 1 to 6 mcg/mL.8

The lamotrigine dose should be decreased if combined hormonal contraceptives are discontinued. Dose decreases should not exceed 25% of the total daily dose per week.1 Desogestrel, a progestin-only medication, may increase exposure to lamotrigine, but this has not been observed in research with other progestins.5,9 When starting a progestin-only pill, monitor patients for signs of lamotrigine toxicity (ataxia, diplopia, dizziness) and consider monitoring their blood levels.
An important consideration to note with combined oral contraceptives is the hormone-free interval, also known as the pill-free week. Due to the rapid effect of estrogens, the lamotrigine concentrations have been shown to rise, even double, during this hormone-free interval, so patients should be closely monitored for adverse effects.3 Some recommend use of an extended cycle regimen (with a limited hormone-free interval), or continuous cycle regimen (with no hormone-free interval) to avoid fluctuations in lamotrigine levels.3,5 Additionally, data suggest that in patients taking lamotrigine and valproate, which inhibits glucuronidation, oral contraceptives do not cause reductions in lamotrigine concentrations.2,5 In these instances, dose increases of lamotrigine are not needed.
Continue to: The metabolism of ethinyl estradiol...
The metabolism of ethinyl estradiol and progestin are susceptible to CYP3A4 induction and increased glucuronidation. Serum concentrations may be reduced by ≥50% when used concomitantly with CYP enzyme–inducing medications, which could possibly result in subtherapeutic levels and unplanned pregnancy.3 CYP3A4 induction occurs for up to 4 weeks after discontinuation of an enzyme-inducing agent, pointing to the need for alternative or backup contraception during this time.3 Lamotrigine is not a CYP enzyme–inducing medication; it is unlikely to affect the efficacy of oral contraceptives in the same manner as other antiseizure medications. However, a study of lamotrigine and the combined hormonal contraceptive ethinyl estradiol and levonorgestrel demonstrated reduced exposure to levonorgestrel, resulting in breakthrough bleeding.5
In a study on the coadministration of lamotrigine and combined oral contraceptives, Sidhu et al4 observed a small mean reduction (20%) in progestin concentrations when lamotrigine was used at a dose of 300 mg/d. Although there is no research suggesting decreased effectiveness in preventing pregnancy when lamotrigine is used with combined oral contraceptives, progestin-only oral contraceptives, or progestin implants, additional or alternative contraceptive methods may be considered based on this pharmacokinetic data, particularly in patients who require lamotrigine doses ≥300 mg/d.5
CASE CONTINUED
Given when Ms. A started the oral contraceptive, the treatment team determines it is likely that an interaction with lamotrigine is causing her resurgence of depressive symptoms. Her care team decides to titrate the lamotrigine gradually to 300 mg/d, then 400 mg/d if needed, while carefully monitoring for signs of a serious rash. This dosage increase may help Ms. A achieve symptom remission. Monitoring plasma levels may be considered, although it is unknown what plasma level was effective for Ms. A before she started the oral contraceptive. Ms. A would need to be counseled regarding the effect of higher doses of lamotrigine on the effectiveness of the oral contraceptive.
Although it does not appear Ms. A is using the oral contraceptive specifically to prevent pregnancy, the team informs her about the possibility of unintended pregnancy with this medication combination. If Ms. A was also using the medication for this indication, alternative contraceptive options would include medroxyprogesterone acetate, levonorgestrel implants, or an intrauterine device (levonorgestrel or copper, though copper would not be effective for endometriosis symptom management). Ms. A should consult with her gynecologist regarding the most appropriate option for her endometriosis. If the decision is made to discontinue her oral contraceptive in the future, the lamotrigine dose should be decreased to her previously effective dose of 200 mg/d.
Related Resources
- Makino KK, Hatters Friedman S, Amin J. Emergency contraception for psychiatric patients. Current Psychiatry. 2022;21(11):34-39,44-45. doi:10.12788/cp.0300
- MGH Center for Women’s Mental Health. You asked: is there an interaction between lamotrigine and oral contraceptives? September 29, 2015. https://womensmentalhealth.org/posts/you-asked-is-there-an-interaction-between-lamotrigine-andoral-contraceptives/
Drug Brand Names
Bupropion extended-release • Wellbutrin XL
Carbamazepine • Equetro, Tegretol
Desogestrel • Cerazette
Divalproex sodium • Depakote
Ethinyl estradiol and etonogestrel • NuvaRing
Ethinyl estradiol and norelgestromin • Ortho Evra
Ethinyl estradiol and norgestimate • Ortho Tri-Cyclen, TriNessa, others
Etonogestrel • Implanon, Nexplanon
Gabapentin • Neurontin
Lamotrigine • Lamictal
Levonorgestrel emergency contraceptive pill • AfterPill, Plan B
Levonorgestrel intrauterine device • Mirena, Skyla
Medroxyprogesterone acetate • Depo-Provera
Oxcarbazepine • Trileptal
Topiramate • Topamax
Valproic acid • Depakene
1. Lamictal [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2020.
2. Lee CR. Drug interactions and hormonal contraception. Trends in Urology Gynaecology & Sexual Health. 2009;14(3):23-26.
3. Williams D. Antiepileptic drugs and contraception. US Pharm. 2014;39(1):39-42.
4. Sidhu J, Job S, Singh S, et al. The pharmacokinetic and pharmacodynamic consequences of the co-administration of lamotrigine and a combined oral contraceptive in healthy female subjects. Br J Clin Pharmacol. 2006;61(2):191-199. doi:10.1111/j.1365-2125.2005.02539.x
5. Faculty of Sexual & Reproductive Healthcare. Clinical guidance: drug interactions with hormonal contraception. Published May 9, 2022. Accessed September 28, 2022. https://www.fsrh.org/documents/ceu-clinical-guidance-drug-interactions-with-hormonal/
6. Johnston CA, Crawford PM. Anti-epileptic drugs and hormonal treatments. Curr Treat Options Neurol. 2014;16(5):288. doi:10.1007/s11940-014-0288-3
7. Christensen J, Petrenaite V, Atterman J, et al. Oral contraceptives induce lamotrigine metabolism: evidence from a double-blind, placebo-controlled trial. Epilepsia. 2007;48(3):484-489. doi:10.1111/j.1528-1167.2007.00997.x
8. Hiemke C, Bergemann N, Clement HW, et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry. 2018;51(1-02):9-62. doi:10.1055/s-0043-116492
9. Rauchenzauner M, Deichmann S, Pittschieler, et al. Bidirectional interaction between oral contraception and lamotrigine in women with epilepsy – role of progestins. Seizure. 2020;74:89-92. doi:10.1016/j.seizure.2019.11.011
1. Lamictal [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2020.
2. Lee CR. Drug interactions and hormonal contraception. Trends in Urology Gynaecology & Sexual Health. 2009;14(3):23-26.
3. Williams D. Antiepileptic drugs and contraception. US Pharm. 2014;39(1):39-42.
4. Sidhu J, Job S, Singh S, et al. The pharmacokinetic and pharmacodynamic consequences of the co-administration of lamotrigine and a combined oral contraceptive in healthy female subjects. Br J Clin Pharmacol. 2006;61(2):191-199. doi:10.1111/j.1365-2125.2005.02539.x
5. Faculty of Sexual & Reproductive Healthcare. Clinical guidance: drug interactions with hormonal contraception. Published May 9, 2022. Accessed September 28, 2022. https://www.fsrh.org/documents/ceu-clinical-guidance-drug-interactions-with-hormonal/
6. Johnston CA, Crawford PM. Anti-epileptic drugs and hormonal treatments. Curr Treat Options Neurol. 2014;16(5):288. doi:10.1007/s11940-014-0288-3
7. Christensen J, Petrenaite V, Atterman J, et al. Oral contraceptives induce lamotrigine metabolism: evidence from a double-blind, placebo-controlled trial. Epilepsia. 2007;48(3):484-489. doi:10.1111/j.1528-1167.2007.00997.x
8. Hiemke C, Bergemann N, Clement HW, et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry. 2018;51(1-02):9-62. doi:10.1055/s-0043-116492
9. Rauchenzauner M, Deichmann S, Pittschieler, et al. Bidirectional interaction between oral contraception and lamotrigine in women with epilepsy – role of progestins. Seizure. 2020;74:89-92. doi:10.1016/j.seizure.2019.11.011
High-dose stimulants for adult ADHD
Ms. H, age 30, presents to the outpatient clinic for a follow-up visit, where she reports difficulty paying attention to conversations, starting and completing tasks, and meeting deadlines. These challenges occur at work and home. Her psychiatric history includes attention-deficit/hyperactivity disorder (ADHD), major depressive disorder, and generalized anxiety disorder. Approximately 10 years ago, she underwent Roux-en-Y gastric bypass surgery. Following surgery, Ms. H’s care team prescribed liquid formulations of medications whenever possible to minimize malabsorption. Ms. H may be a rapid metabolizer; she says the effects of her prescribed stimulants only last briefly, so she has to frequently redose. As a result, she often runs out of her monthly stimulant allotment earlier than expected.
Ms. H’s current medications include dextroamphetamine/amphetamine immediate-release (IR) 30 mg 3 times daily, atenolol 50 mg/d, and escitalopram oral solution 10 mg/d. Previous unsuccessful medication trials for her ADHD include methylphenidate IR 20 mg 3 times daily and lisdexamfetamine 70 mg/d. Ms. H reports that when her responsibilities increased at work or home, she took methylphenidate IR 20 mg up to 6 times daily to relieve her symptoms.
In the United States, ADHD affects an estimated 4.4% of adults age 18 to 44.1 The actual rate may be higher, however, as recent research has called into question the hypothesis that approximately 50% of cases of childhood ADHD remit by adulthood.2 Prevalence estimates relying on DSM-IV criteria (which were designed with children in mind) can underestimate this condition in adults. Newer data suggest that up to 90% of individuals with ADHD in childhood continue to experience significant ADHD symptoms into adulthood.2
Unless contraindications are present, methylphenidate or amphetamine-based stimulants are the medications of choice for treating adult ADHD.3 Many formulations of both medications are available,4 which allows clinicians to better tailor therapy to each patient’s pharmacokinetics and daily schedule. Although there can be differences in response and tolerability, methylphenidate and amphetamine offer comparable efficacy and a similar adverse effect profile.5
Because amphetamine is more potent than methylphenidate, clinicians commonly start treatment with an amphetamine dose that is one-half to two-thirds the dose of methylphenidate.6 While both classes of stimulants inhibit the reuptake of dopamine and norepinephrine into presynaptic neurons, amphetamines also promote the release of dopamine and norepinephrine from their storage sites in presynaptic nerve terminals.3
Methylphenidate
Methylphenidate IR has an average onset of action of 30 to 45 minutes and its effects last approximately 3 to 4 hours. The extended-release (XR) formulations have varying onsets of action, with durations of action up to 12 hours (Table 13,7).4 The XR products usually immediately release a certain percentage of the medication, eliminating the need for an additional IR tablet. One methylphenidate XR product (Jornay) as well as serdexmethylphenidate/dexmethylphenidate (Azstarys) offer durations of action of 24 to 36 hours. Methylphenidate is primarily metabolized by carboxylesterase 1 (CES1) to the inactive metabolite ritalinic acid. Most of the medication (60% to 80%) is excreted in the urine as ritalinic acid.4 Theoretically, genetic variations in the CES1 and concomitant use of medications that compete with or alter this pathway may impact methylphenidate pharmacokinetics.8 However, plasma levels have not yet shown to be helpful in guiding treatment selection or dosing.4

Amphetamine
Dextroamphetamine/amphetamine IR has an average onset of action of 30 to 45 minutes and its effects last approximately 4 to 6 hours. XR formulations have varying onsets of action, with durations of action up to 13 hours (Table 23,7,9).4 One XR product, mixed salts of single amphetamine entity (Mydayis), has a duration of action of 16 hours. In XR formulations, a certain percentage of the medication is typically released immediately, eliminating the need for an additional IR tablet. Amphetamine is primarily metabolized by cytochrome P450 (CYP) 2D6 hydroxylation and oxidative deamination. Genetic variability in amphetamine metabolism may be relevant due to CYP2D6 polymorphisms. Ultra-rapid metabolizers might need higher doses, while poor metabolizers might require smaller amounts and may be more susceptible to adverse effects.4 However, there is currently insufficient data supporting gene/medication concentration relationships. As is the case with methylphenidate, plasma levels have not yet shown to be helpful in guiding treatment selection or dosing.6

Continue to: Impaired medication absorption after bariatric surgery
Impaired medication absorption after bariatric surgery
Medication malabsorption following bariatric surgery is a significant concern. In a systematic review of 22 studies, Padwal et al10 found that in one-third of these studies, decreased absorption following bariatric surgery may be present in patients taking medications that have poor absorption, high lipophilicity, or enterohepatic recirculation. Childress et al11 found that methylphenidate IR and dextroamphetamine/amphetamine are both well absorbed, with bioavailability percentages of 100% and 90%, respectively. Additional research shows both stimulants have rapid absorption rates but relatively poor bioavailability.12 In one study analyzing the dissolution of common psychiatric medications, methylphenidate was shown to dissolve slightly more in the Roux-en-Y gastric bypass surgery model (80 mg) compared to controls (70 mg).13 One case indicated potential methylphenidate toxicity following Roux-en-Y gastric bypass surgery,14 while another suggested impaired absorption following the same procedure.15 A case-control design study assessing the impact of Roux-en-Y gastric bypass surgery on the pharmacokinetic properties of lisdexamfetamine found no significant differences between the Roux-en-Y group (n = 10) and nonsurgical controls (n = 10). The investigators concluded that while data suggest adjusting lisdexamfetamine dosing following Roux-en-Y gastric bypass surgery is unnecessary, there may be interindividual differences, and individualized dosing regimens may be needed.16
When managing patients who might be experiencing medication malabsorption, it may be helpful to use dosage forms that avoid disintegration, acidic environments, and slow dissolution. Because they are more rapidly absorbed and not susceptible to disintegration and dissolution, liquid formulations are recommended.17 For medications that are not available as a liquid, an IR formulation is recommended.18
Using nonoral routes of administration that avoid the anatomical changes of the gastrointestinal tract should be considered for patients who have undergone Roux-en-Y gastric bypass surgery.17 The methylphenidate transdermal patch, a medication delivery system that avoids gut and hepatic first-pass metabolism, can improve medication bioavailability, reduce dose frequency, and stabilize medication delivery. It is available in 4 sizes/dosages: 10 mg/9 hours, 15 mg/9 hours, 20 mg/9 hours, and 30 mg/9 hours. Methylphenidate is delivered at a steady rate based upon patch size. The onset of action of the patch is approximately 2 hours, and patients should wear the patch for 9 hours, then remove it. Methylphenidate will still be absorbed up to 2 to 3 hours after patch removal. Appropriate application and removal of the patch is important for optimal effectiveness and to avoid adverse effects.4
In March 2022, t
CASE CONTINUED
Ms. H emphasizes her desire to maintain functionality in all areas of life, while her care team reiterates the risks of continuing to take high-dose stimulants. Both Ms. H and her care team acknowledge that stimulant usage could be worsening her anxiety, and that Roux-en-Y gastric bypass surgery may be a possible explanation for her dosing challenges.
Continue to: Following consultation with the pharmacist...
Following consultation with the pharmacist, the care team explains the possible pharmacokinetic benefits of using the methylphenidate transdermal patch. After completing the prior authorization paperwork, Ms. H is started on the 30 mg/d patch. This dose was selected because she previously tolerated high-dose stimulants, including methylphenidate IR 20 mg up to 6 times daily. At a follow-up visit 1 month after starting the patch, Ms. H reports an improvement in her ADHD symptoms and says she is not experiencing any adverse effects.
Related Resources
- DeMarco R, Rana R, Powell K, et al. How bariatric surgery affects psychotropic drug absorption. Current Psychiatry. 2022;21(8):39-44. doi:10.12788/cp.0271
- Santos MG, Majarwitz DJ, Saeed SA. Adult ADHD: 6 studies of pharmacologic interventions. Current Psychiatry. 2023;22(4):16-27. doi:10.12788/cp.0344
Drug Brand Names
Amphetamine sulfate • Adzenys ER, Adzenys XR-ODT, Dyanavel XR, Evekeo
Atenolol • Tenormin
Dexmethylphenidate • Focalin, Focalin XR
Dextroamphetamine transdermal • Xelstrym
Dextroamphetamine • Dexedrine, Dexedrine Spansule, ProCentra, Zenzedi
Escitalopram • Lexapro
Lisdexamfetamine • Vyvanse
Methylphenidate • Aptensio XR, Adhansia XR, Concerta, Cotempla, Jornay PM, Metadate CD, Metadate ER, Methylin, Qullichew ER, Quillivant XR, Relexxii, Ritalin, Ritalin LA
Methylphenidate transdermal • Daytrana
Mixed amphetamine salts • Adderall, Adderall XR
Mixed salts of a single-entity amphetamine • Mydayis
Serdexmethylphenidate and dexmethylphenidate • Azstarys
1. Kessler RC, Adler L, Barkley R, et al. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry. 2006;163(4):716-723. doi:10.1176/ajp.2006.163.4.716
2. Sibley MH, Arnold LE, Swanson JM, et al. Variable patterns of remission from ADHD in the multimodal treatment study of ADHD. Am J Psychiatry. 2022;179(2):142-151. doi:10.1176/appi.ajp.2021.21010032
3. Cleveland KW, Boyle J, Robinson RF. Attention-deficit/hyperactivity disorder. In: Chisholm-Burns MA, Schwinghammer TL, Malone PM, et al, eds. Pharmacotherapy Principles & Practice. 6th ed. McGraw Hill; 2022. Accessed December 1, 2022. https://ppp.mhmedical.com/content.aspx?bookid=3114§ionid=261474885
4. Steingard R, Taskiran S, Connor DF, et al. New formulations of stimulants: an update for clinicians. J Child Adolesc Psychopharmacol. 2019;29(5):324-339. doi:10.1089/cap.2019.0043
5. Faraone SV. The pharmacology of amphetamine and methylphenidate: relevance to the neurobiology of attention-deficit/hyperactivity disorder and other psychiatric comorbidities. Neurosci Biobehav Rev. 2018;87:255-270. doi:10.1016/j.neubiorev.2018.02.001
6. Markowitz JS, Patrick KS. The clinical pharmacokinetics of amphetamines utilized in the treatment of attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2017;27(8):678-689. doi:10.1089/cap.2017.0071
7. Mullen S. Medication Table 2: Attention Deficit Hyperactivity Disorder. In: English C, ed. CPNP Psychiatric Pharmacotherapy Review Course. 2022-2023 ed. College of Psychiatric and Neurologic Pharmacists; 2022.
8. Zhu HJ, Patrick KS, Yuan HJ, et al. Two CES1 gene mutations lead to dysfunctional carboxylesterase 1 activity in man: clinical significance and molecular basis. Am J Hum Genet. 2008;82(6):1241-1248. doi:10.1016/j.ajhg.2008.04.015
9. Xelstrym [package insert]. Miami, FL: Noven Pharmaceuticals, Inc.; 2022.
10. Padwal R, Brocks D, Sharma AM. A systematic review of drug absorption following bariatric surgery and its theoretical implications. Obes Rev. 2010;11(1):41-50. doi:10.1111/j.1467-789X.2009.00614.x
11. Childress AC, Komolova M, Sallee FR. An update on the pharmacokinetic considerations in the treatment of ADHD with long-acting methylphenidate and amphetamine formulations. Expert Opin Drug Metab Toxicol. 2019;15(11):937-974. doi:10.1080/17425255.2019.1675636
12. Markowitz JS, Melchert PW. The pharmacokinetics and pharmacogenomics of psychostimulants. Child Adolesc Psychiatr Clin N Am. 2022;31(3):393-416. doi:10.1016/j.chc.2022.03.003
13. Seaman JS, Bowers SP, Dixon P, et al. Dissolution of common psychiatric medications in a Roux-en-Y gastric bypass model. Psychosomatics. 2005;46(3):250-253. doi:10.1176/appi.psy.46.3.250
14. Ludvigsson M, Haenni A. Methylphenidate toxicity after Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2016;12(5):e55-e57. doi:10.1016/j.soard.2016.03.015
15. Azran C, Langguth P, Dahan A. Impaired oral absorption of methylphenidate after Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2017;13(7):1245-1247. doi:10.1016/j.soard.2017.03.003
16. Steffen KJ, Mohammad AS, Roerig JL, et al. Lisdexamfetamine pharmacokinetic comparison between patients who underwent Roux-en-Y gastric bypass and nonsurgical controls. Obes Surg. 2021;31(10):4289-4294. doi:10.1007/s11695-020-04969-4
17. Buxton ILO. Pharmacokinetics: the dynamics of drug absorption, distribution, metabolism, and elimination. In: Brunton LL, Knollmann BC, eds. Goodman & Gilman’s: The Pharmacological Basis of Therapeutics. 14th ed. McGraw Hill; 2023. Accessed December 1, 2022. https://accesspharmacy.mhmedical.com/content.aspx?bookid=2189§ionid=166182905
18. DeMarco R, Rana R, Powell K, et al. How bariatric surgery affects psychotropic drug absorption. Current Psychiatry. 2022;21(8):39-44. doi:10.12788/cp.0271
Ms. H, age 30, presents to the outpatient clinic for a follow-up visit, where she reports difficulty paying attention to conversations, starting and completing tasks, and meeting deadlines. These challenges occur at work and home. Her psychiatric history includes attention-deficit/hyperactivity disorder (ADHD), major depressive disorder, and generalized anxiety disorder. Approximately 10 years ago, she underwent Roux-en-Y gastric bypass surgery. Following surgery, Ms. H’s care team prescribed liquid formulations of medications whenever possible to minimize malabsorption. Ms. H may be a rapid metabolizer; she says the effects of her prescribed stimulants only last briefly, so she has to frequently redose. As a result, she often runs out of her monthly stimulant allotment earlier than expected.
Ms. H’s current medications include dextroamphetamine/amphetamine immediate-release (IR) 30 mg 3 times daily, atenolol 50 mg/d, and escitalopram oral solution 10 mg/d. Previous unsuccessful medication trials for her ADHD include methylphenidate IR 20 mg 3 times daily and lisdexamfetamine 70 mg/d. Ms. H reports that when her responsibilities increased at work or home, she took methylphenidate IR 20 mg up to 6 times daily to relieve her symptoms.
In the United States, ADHD affects an estimated 4.4% of adults age 18 to 44.1 The actual rate may be higher, however, as recent research has called into question the hypothesis that approximately 50% of cases of childhood ADHD remit by adulthood.2 Prevalence estimates relying on DSM-IV criteria (which were designed with children in mind) can underestimate this condition in adults. Newer data suggest that up to 90% of individuals with ADHD in childhood continue to experience significant ADHD symptoms into adulthood.2
Unless contraindications are present, methylphenidate or amphetamine-based stimulants are the medications of choice for treating adult ADHD.3 Many formulations of both medications are available,4 which allows clinicians to better tailor therapy to each patient’s pharmacokinetics and daily schedule. Although there can be differences in response and tolerability, methylphenidate and amphetamine offer comparable efficacy and a similar adverse effect profile.5
Because amphetamine is more potent than methylphenidate, clinicians commonly start treatment with an amphetamine dose that is one-half to two-thirds the dose of methylphenidate.6 While both classes of stimulants inhibit the reuptake of dopamine and norepinephrine into presynaptic neurons, amphetamines also promote the release of dopamine and norepinephrine from their storage sites in presynaptic nerve terminals.3
Methylphenidate
Methylphenidate IR has an average onset of action of 30 to 45 minutes and its effects last approximately 3 to 4 hours. The extended-release (XR) formulations have varying onsets of action, with durations of action up to 12 hours (Table 13,7).4 The XR products usually immediately release a certain percentage of the medication, eliminating the need for an additional IR tablet. One methylphenidate XR product (Jornay) as well as serdexmethylphenidate/dexmethylphenidate (Azstarys) offer durations of action of 24 to 36 hours. Methylphenidate is primarily metabolized by carboxylesterase 1 (CES1) to the inactive metabolite ritalinic acid. Most of the medication (60% to 80%) is excreted in the urine as ritalinic acid.4 Theoretically, genetic variations in the CES1 and concomitant use of medications that compete with or alter this pathway may impact methylphenidate pharmacokinetics.8 However, plasma levels have not yet shown to be helpful in guiding treatment selection or dosing.4

Amphetamine
Dextroamphetamine/amphetamine IR has an average onset of action of 30 to 45 minutes and its effects last approximately 4 to 6 hours. XR formulations have varying onsets of action, with durations of action up to 13 hours (Table 23,7,9).4 One XR product, mixed salts of single amphetamine entity (Mydayis), has a duration of action of 16 hours. In XR formulations, a certain percentage of the medication is typically released immediately, eliminating the need for an additional IR tablet. Amphetamine is primarily metabolized by cytochrome P450 (CYP) 2D6 hydroxylation and oxidative deamination. Genetic variability in amphetamine metabolism may be relevant due to CYP2D6 polymorphisms. Ultra-rapid metabolizers might need higher doses, while poor metabolizers might require smaller amounts and may be more susceptible to adverse effects.4 However, there is currently insufficient data supporting gene/medication concentration relationships. As is the case with methylphenidate, plasma levels have not yet shown to be helpful in guiding treatment selection or dosing.6

Continue to: Impaired medication absorption after bariatric surgery
Impaired medication absorption after bariatric surgery
Medication malabsorption following bariatric surgery is a significant concern. In a systematic review of 22 studies, Padwal et al10 found that in one-third of these studies, decreased absorption following bariatric surgery may be present in patients taking medications that have poor absorption, high lipophilicity, or enterohepatic recirculation. Childress et al11 found that methylphenidate IR and dextroamphetamine/amphetamine are both well absorbed, with bioavailability percentages of 100% and 90%, respectively. Additional research shows both stimulants have rapid absorption rates but relatively poor bioavailability.12 In one study analyzing the dissolution of common psychiatric medications, methylphenidate was shown to dissolve slightly more in the Roux-en-Y gastric bypass surgery model (80 mg) compared to controls (70 mg).13 One case indicated potential methylphenidate toxicity following Roux-en-Y gastric bypass surgery,14 while another suggested impaired absorption following the same procedure.15 A case-control design study assessing the impact of Roux-en-Y gastric bypass surgery on the pharmacokinetic properties of lisdexamfetamine found no significant differences between the Roux-en-Y group (n = 10) and nonsurgical controls (n = 10). The investigators concluded that while data suggest adjusting lisdexamfetamine dosing following Roux-en-Y gastric bypass surgery is unnecessary, there may be interindividual differences, and individualized dosing regimens may be needed.16
When managing patients who might be experiencing medication malabsorption, it may be helpful to use dosage forms that avoid disintegration, acidic environments, and slow dissolution. Because they are more rapidly absorbed and not susceptible to disintegration and dissolution, liquid formulations are recommended.17 For medications that are not available as a liquid, an IR formulation is recommended.18
Using nonoral routes of administration that avoid the anatomical changes of the gastrointestinal tract should be considered for patients who have undergone Roux-en-Y gastric bypass surgery.17 The methylphenidate transdermal patch, a medication delivery system that avoids gut and hepatic first-pass metabolism, can improve medication bioavailability, reduce dose frequency, and stabilize medication delivery. It is available in 4 sizes/dosages: 10 mg/9 hours, 15 mg/9 hours, 20 mg/9 hours, and 30 mg/9 hours. Methylphenidate is delivered at a steady rate based upon patch size. The onset of action of the patch is approximately 2 hours, and patients should wear the patch for 9 hours, then remove it. Methylphenidate will still be absorbed up to 2 to 3 hours after patch removal. Appropriate application and removal of the patch is important for optimal effectiveness and to avoid adverse effects.4
In March 2022, t
CASE CONTINUED
Ms. H emphasizes her desire to maintain functionality in all areas of life, while her care team reiterates the risks of continuing to take high-dose stimulants. Both Ms. H and her care team acknowledge that stimulant usage could be worsening her anxiety, and that Roux-en-Y gastric bypass surgery may be a possible explanation for her dosing challenges.
Continue to: Following consultation with the pharmacist...
Following consultation with the pharmacist, the care team explains the possible pharmacokinetic benefits of using the methylphenidate transdermal patch. After completing the prior authorization paperwork, Ms. H is started on the 30 mg/d patch. This dose was selected because she previously tolerated high-dose stimulants, including methylphenidate IR 20 mg up to 6 times daily. At a follow-up visit 1 month after starting the patch, Ms. H reports an improvement in her ADHD symptoms and says she is not experiencing any adverse effects.
Related Resources
- DeMarco R, Rana R, Powell K, et al. How bariatric surgery affects psychotropic drug absorption. Current Psychiatry. 2022;21(8):39-44. doi:10.12788/cp.0271
- Santos MG, Majarwitz DJ, Saeed SA. Adult ADHD: 6 studies of pharmacologic interventions. Current Psychiatry. 2023;22(4):16-27. doi:10.12788/cp.0344
Drug Brand Names
Amphetamine sulfate • Adzenys ER, Adzenys XR-ODT, Dyanavel XR, Evekeo
Atenolol • Tenormin
Dexmethylphenidate • Focalin, Focalin XR
Dextroamphetamine transdermal • Xelstrym
Dextroamphetamine • Dexedrine, Dexedrine Spansule, ProCentra, Zenzedi
Escitalopram • Lexapro
Lisdexamfetamine • Vyvanse
Methylphenidate • Aptensio XR, Adhansia XR, Concerta, Cotempla, Jornay PM, Metadate CD, Metadate ER, Methylin, Qullichew ER, Quillivant XR, Relexxii, Ritalin, Ritalin LA
Methylphenidate transdermal • Daytrana
Mixed amphetamine salts • Adderall, Adderall XR
Mixed salts of a single-entity amphetamine • Mydayis
Serdexmethylphenidate and dexmethylphenidate • Azstarys
Ms. H, age 30, presents to the outpatient clinic for a follow-up visit, where she reports difficulty paying attention to conversations, starting and completing tasks, and meeting deadlines. These challenges occur at work and home. Her psychiatric history includes attention-deficit/hyperactivity disorder (ADHD), major depressive disorder, and generalized anxiety disorder. Approximately 10 years ago, she underwent Roux-en-Y gastric bypass surgery. Following surgery, Ms. H’s care team prescribed liquid formulations of medications whenever possible to minimize malabsorption. Ms. H may be a rapid metabolizer; she says the effects of her prescribed stimulants only last briefly, so she has to frequently redose. As a result, she often runs out of her monthly stimulant allotment earlier than expected.
Ms. H’s current medications include dextroamphetamine/amphetamine immediate-release (IR) 30 mg 3 times daily, atenolol 50 mg/d, and escitalopram oral solution 10 mg/d. Previous unsuccessful medication trials for her ADHD include methylphenidate IR 20 mg 3 times daily and lisdexamfetamine 70 mg/d. Ms. H reports that when her responsibilities increased at work or home, she took methylphenidate IR 20 mg up to 6 times daily to relieve her symptoms.
In the United States, ADHD affects an estimated 4.4% of adults age 18 to 44.1 The actual rate may be higher, however, as recent research has called into question the hypothesis that approximately 50% of cases of childhood ADHD remit by adulthood.2 Prevalence estimates relying on DSM-IV criteria (which were designed with children in mind) can underestimate this condition in adults. Newer data suggest that up to 90% of individuals with ADHD in childhood continue to experience significant ADHD symptoms into adulthood.2
Unless contraindications are present, methylphenidate or amphetamine-based stimulants are the medications of choice for treating adult ADHD.3 Many formulations of both medications are available,4 which allows clinicians to better tailor therapy to each patient’s pharmacokinetics and daily schedule. Although there can be differences in response and tolerability, methylphenidate and amphetamine offer comparable efficacy and a similar adverse effect profile.5
Because amphetamine is more potent than methylphenidate, clinicians commonly start treatment with an amphetamine dose that is one-half to two-thirds the dose of methylphenidate.6 While both classes of stimulants inhibit the reuptake of dopamine and norepinephrine into presynaptic neurons, amphetamines also promote the release of dopamine and norepinephrine from their storage sites in presynaptic nerve terminals.3
Methylphenidate
Methylphenidate IR has an average onset of action of 30 to 45 minutes and its effects last approximately 3 to 4 hours. The extended-release (XR) formulations have varying onsets of action, with durations of action up to 12 hours (Table 13,7).4 The XR products usually immediately release a certain percentage of the medication, eliminating the need for an additional IR tablet. One methylphenidate XR product (Jornay) as well as serdexmethylphenidate/dexmethylphenidate (Azstarys) offer durations of action of 24 to 36 hours. Methylphenidate is primarily metabolized by carboxylesterase 1 (CES1) to the inactive metabolite ritalinic acid. Most of the medication (60% to 80%) is excreted in the urine as ritalinic acid.4 Theoretically, genetic variations in the CES1 and concomitant use of medications that compete with or alter this pathway may impact methylphenidate pharmacokinetics.8 However, plasma levels have not yet shown to be helpful in guiding treatment selection or dosing.4

Amphetamine
Dextroamphetamine/amphetamine IR has an average onset of action of 30 to 45 minutes and its effects last approximately 4 to 6 hours. XR formulations have varying onsets of action, with durations of action up to 13 hours (Table 23,7,9).4 One XR product, mixed salts of single amphetamine entity (Mydayis), has a duration of action of 16 hours. In XR formulations, a certain percentage of the medication is typically released immediately, eliminating the need for an additional IR tablet. Amphetamine is primarily metabolized by cytochrome P450 (CYP) 2D6 hydroxylation and oxidative deamination. Genetic variability in amphetamine metabolism may be relevant due to CYP2D6 polymorphisms. Ultra-rapid metabolizers might need higher doses, while poor metabolizers might require smaller amounts and may be more susceptible to adverse effects.4 However, there is currently insufficient data supporting gene/medication concentration relationships. As is the case with methylphenidate, plasma levels have not yet shown to be helpful in guiding treatment selection or dosing.6

Continue to: Impaired medication absorption after bariatric surgery
Impaired medication absorption after bariatric surgery
Medication malabsorption following bariatric surgery is a significant concern. In a systematic review of 22 studies, Padwal et al10 found that in one-third of these studies, decreased absorption following bariatric surgery may be present in patients taking medications that have poor absorption, high lipophilicity, or enterohepatic recirculation. Childress et al11 found that methylphenidate IR and dextroamphetamine/amphetamine are both well absorbed, with bioavailability percentages of 100% and 90%, respectively. Additional research shows both stimulants have rapid absorption rates but relatively poor bioavailability.12 In one study analyzing the dissolution of common psychiatric medications, methylphenidate was shown to dissolve slightly more in the Roux-en-Y gastric bypass surgery model (80 mg) compared to controls (70 mg).13 One case indicated potential methylphenidate toxicity following Roux-en-Y gastric bypass surgery,14 while another suggested impaired absorption following the same procedure.15 A case-control design study assessing the impact of Roux-en-Y gastric bypass surgery on the pharmacokinetic properties of lisdexamfetamine found no significant differences between the Roux-en-Y group (n = 10) and nonsurgical controls (n = 10). The investigators concluded that while data suggest adjusting lisdexamfetamine dosing following Roux-en-Y gastric bypass surgery is unnecessary, there may be interindividual differences, and individualized dosing regimens may be needed.16
When managing patients who might be experiencing medication malabsorption, it may be helpful to use dosage forms that avoid disintegration, acidic environments, and slow dissolution. Because they are more rapidly absorbed and not susceptible to disintegration and dissolution, liquid formulations are recommended.17 For medications that are not available as a liquid, an IR formulation is recommended.18
Using nonoral routes of administration that avoid the anatomical changes of the gastrointestinal tract should be considered for patients who have undergone Roux-en-Y gastric bypass surgery.17 The methylphenidate transdermal patch, a medication delivery system that avoids gut and hepatic first-pass metabolism, can improve medication bioavailability, reduce dose frequency, and stabilize medication delivery. It is available in 4 sizes/dosages: 10 mg/9 hours, 15 mg/9 hours, 20 mg/9 hours, and 30 mg/9 hours. Methylphenidate is delivered at a steady rate based upon patch size. The onset of action of the patch is approximately 2 hours, and patients should wear the patch for 9 hours, then remove it. Methylphenidate will still be absorbed up to 2 to 3 hours after patch removal. Appropriate application and removal of the patch is important for optimal effectiveness and to avoid adverse effects.4
In March 2022, t
CASE CONTINUED
Ms. H emphasizes her desire to maintain functionality in all areas of life, while her care team reiterates the risks of continuing to take high-dose stimulants. Both Ms. H and her care team acknowledge that stimulant usage could be worsening her anxiety, and that Roux-en-Y gastric bypass surgery may be a possible explanation for her dosing challenges.
Continue to: Following consultation with the pharmacist...
Following consultation with the pharmacist, the care team explains the possible pharmacokinetic benefits of using the methylphenidate transdermal patch. After completing the prior authorization paperwork, Ms. H is started on the 30 mg/d patch. This dose was selected because she previously tolerated high-dose stimulants, including methylphenidate IR 20 mg up to 6 times daily. At a follow-up visit 1 month after starting the patch, Ms. H reports an improvement in her ADHD symptoms and says she is not experiencing any adverse effects.
Related Resources
- DeMarco R, Rana R, Powell K, et al. How bariatric surgery affects psychotropic drug absorption. Current Psychiatry. 2022;21(8):39-44. doi:10.12788/cp.0271
- Santos MG, Majarwitz DJ, Saeed SA. Adult ADHD: 6 studies of pharmacologic interventions. Current Psychiatry. 2023;22(4):16-27. doi:10.12788/cp.0344
Drug Brand Names
Amphetamine sulfate • Adzenys ER, Adzenys XR-ODT, Dyanavel XR, Evekeo
Atenolol • Tenormin
Dexmethylphenidate • Focalin, Focalin XR
Dextroamphetamine transdermal • Xelstrym
Dextroamphetamine • Dexedrine, Dexedrine Spansule, ProCentra, Zenzedi
Escitalopram • Lexapro
Lisdexamfetamine • Vyvanse
Methylphenidate • Aptensio XR, Adhansia XR, Concerta, Cotempla, Jornay PM, Metadate CD, Metadate ER, Methylin, Qullichew ER, Quillivant XR, Relexxii, Ritalin, Ritalin LA
Methylphenidate transdermal • Daytrana
Mixed amphetamine salts • Adderall, Adderall XR
Mixed salts of a single-entity amphetamine • Mydayis
Serdexmethylphenidate and dexmethylphenidate • Azstarys
1. Kessler RC, Adler L, Barkley R, et al. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry. 2006;163(4):716-723. doi:10.1176/ajp.2006.163.4.716
2. Sibley MH, Arnold LE, Swanson JM, et al. Variable patterns of remission from ADHD in the multimodal treatment study of ADHD. Am J Psychiatry. 2022;179(2):142-151. doi:10.1176/appi.ajp.2021.21010032
3. Cleveland KW, Boyle J, Robinson RF. Attention-deficit/hyperactivity disorder. In: Chisholm-Burns MA, Schwinghammer TL, Malone PM, et al, eds. Pharmacotherapy Principles & Practice. 6th ed. McGraw Hill; 2022. Accessed December 1, 2022. https://ppp.mhmedical.com/content.aspx?bookid=3114§ionid=261474885
4. Steingard R, Taskiran S, Connor DF, et al. New formulations of stimulants: an update for clinicians. J Child Adolesc Psychopharmacol. 2019;29(5):324-339. doi:10.1089/cap.2019.0043
5. Faraone SV. The pharmacology of amphetamine and methylphenidate: relevance to the neurobiology of attention-deficit/hyperactivity disorder and other psychiatric comorbidities. Neurosci Biobehav Rev. 2018;87:255-270. doi:10.1016/j.neubiorev.2018.02.001
6. Markowitz JS, Patrick KS. The clinical pharmacokinetics of amphetamines utilized in the treatment of attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2017;27(8):678-689. doi:10.1089/cap.2017.0071
7. Mullen S. Medication Table 2: Attention Deficit Hyperactivity Disorder. In: English C, ed. CPNP Psychiatric Pharmacotherapy Review Course. 2022-2023 ed. College of Psychiatric and Neurologic Pharmacists; 2022.
8. Zhu HJ, Patrick KS, Yuan HJ, et al. Two CES1 gene mutations lead to dysfunctional carboxylesterase 1 activity in man: clinical significance and molecular basis. Am J Hum Genet. 2008;82(6):1241-1248. doi:10.1016/j.ajhg.2008.04.015
9. Xelstrym [package insert]. Miami, FL: Noven Pharmaceuticals, Inc.; 2022.
10. Padwal R, Brocks D, Sharma AM. A systematic review of drug absorption following bariatric surgery and its theoretical implications. Obes Rev. 2010;11(1):41-50. doi:10.1111/j.1467-789X.2009.00614.x
11. Childress AC, Komolova M, Sallee FR. An update on the pharmacokinetic considerations in the treatment of ADHD with long-acting methylphenidate and amphetamine formulations. Expert Opin Drug Metab Toxicol. 2019;15(11):937-974. doi:10.1080/17425255.2019.1675636
12. Markowitz JS, Melchert PW. The pharmacokinetics and pharmacogenomics of psychostimulants. Child Adolesc Psychiatr Clin N Am. 2022;31(3):393-416. doi:10.1016/j.chc.2022.03.003
13. Seaman JS, Bowers SP, Dixon P, et al. Dissolution of common psychiatric medications in a Roux-en-Y gastric bypass model. Psychosomatics. 2005;46(3):250-253. doi:10.1176/appi.psy.46.3.250
14. Ludvigsson M, Haenni A. Methylphenidate toxicity after Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2016;12(5):e55-e57. doi:10.1016/j.soard.2016.03.015
15. Azran C, Langguth P, Dahan A. Impaired oral absorption of methylphenidate after Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2017;13(7):1245-1247. doi:10.1016/j.soard.2017.03.003
16. Steffen KJ, Mohammad AS, Roerig JL, et al. Lisdexamfetamine pharmacokinetic comparison between patients who underwent Roux-en-Y gastric bypass and nonsurgical controls. Obes Surg. 2021;31(10):4289-4294. doi:10.1007/s11695-020-04969-4
17. Buxton ILO. Pharmacokinetics: the dynamics of drug absorption, distribution, metabolism, and elimination. In: Brunton LL, Knollmann BC, eds. Goodman & Gilman’s: The Pharmacological Basis of Therapeutics. 14th ed. McGraw Hill; 2023. Accessed December 1, 2022. https://accesspharmacy.mhmedical.com/content.aspx?bookid=2189§ionid=166182905
18. DeMarco R, Rana R, Powell K, et al. How bariatric surgery affects psychotropic drug absorption. Current Psychiatry. 2022;21(8):39-44. doi:10.12788/cp.0271
1. Kessler RC, Adler L, Barkley R, et al. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry. 2006;163(4):716-723. doi:10.1176/ajp.2006.163.4.716
2. Sibley MH, Arnold LE, Swanson JM, et al. Variable patterns of remission from ADHD in the multimodal treatment study of ADHD. Am J Psychiatry. 2022;179(2):142-151. doi:10.1176/appi.ajp.2021.21010032
3. Cleveland KW, Boyle J, Robinson RF. Attention-deficit/hyperactivity disorder. In: Chisholm-Burns MA, Schwinghammer TL, Malone PM, et al, eds. Pharmacotherapy Principles & Practice. 6th ed. McGraw Hill; 2022. Accessed December 1, 2022. https://ppp.mhmedical.com/content.aspx?bookid=3114§ionid=261474885
4. Steingard R, Taskiran S, Connor DF, et al. New formulations of stimulants: an update for clinicians. J Child Adolesc Psychopharmacol. 2019;29(5):324-339. doi:10.1089/cap.2019.0043
5. Faraone SV. The pharmacology of amphetamine and methylphenidate: relevance to the neurobiology of attention-deficit/hyperactivity disorder and other psychiatric comorbidities. Neurosci Biobehav Rev. 2018;87:255-270. doi:10.1016/j.neubiorev.2018.02.001
6. Markowitz JS, Patrick KS. The clinical pharmacokinetics of amphetamines utilized in the treatment of attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2017;27(8):678-689. doi:10.1089/cap.2017.0071
7. Mullen S. Medication Table 2: Attention Deficit Hyperactivity Disorder. In: English C, ed. CPNP Psychiatric Pharmacotherapy Review Course. 2022-2023 ed. College of Psychiatric and Neurologic Pharmacists; 2022.
8. Zhu HJ, Patrick KS, Yuan HJ, et al. Two CES1 gene mutations lead to dysfunctional carboxylesterase 1 activity in man: clinical significance and molecular basis. Am J Hum Genet. 2008;82(6):1241-1248. doi:10.1016/j.ajhg.2008.04.015
9. Xelstrym [package insert]. Miami, FL: Noven Pharmaceuticals, Inc.; 2022.
10. Padwal R, Brocks D, Sharma AM. A systematic review of drug absorption following bariatric surgery and its theoretical implications. Obes Rev. 2010;11(1):41-50. doi:10.1111/j.1467-789X.2009.00614.x
11. Childress AC, Komolova M, Sallee FR. An update on the pharmacokinetic considerations in the treatment of ADHD with long-acting methylphenidate and amphetamine formulations. Expert Opin Drug Metab Toxicol. 2019;15(11):937-974. doi:10.1080/17425255.2019.1675636
12. Markowitz JS, Melchert PW. The pharmacokinetics and pharmacogenomics of psychostimulants. Child Adolesc Psychiatr Clin N Am. 2022;31(3):393-416. doi:10.1016/j.chc.2022.03.003
13. Seaman JS, Bowers SP, Dixon P, et al. Dissolution of common psychiatric medications in a Roux-en-Y gastric bypass model. Psychosomatics. 2005;46(3):250-253. doi:10.1176/appi.psy.46.3.250
14. Ludvigsson M, Haenni A. Methylphenidate toxicity after Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2016;12(5):e55-e57. doi:10.1016/j.soard.2016.03.015
15. Azran C, Langguth P, Dahan A. Impaired oral absorption of methylphenidate after Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2017;13(7):1245-1247. doi:10.1016/j.soard.2017.03.003
16. Steffen KJ, Mohammad AS, Roerig JL, et al. Lisdexamfetamine pharmacokinetic comparison between patients who underwent Roux-en-Y gastric bypass and nonsurgical controls. Obes Surg. 2021;31(10):4289-4294. doi:10.1007/s11695-020-04969-4
17. Buxton ILO. Pharmacokinetics: the dynamics of drug absorption, distribution, metabolism, and elimination. In: Brunton LL, Knollmann BC, eds. Goodman & Gilman’s: The Pharmacological Basis of Therapeutics. 14th ed. McGraw Hill; 2023. Accessed December 1, 2022. https://accesspharmacy.mhmedical.com/content.aspx?bookid=2189§ionid=166182905
18. DeMarco R, Rana R, Powell K, et al. How bariatric surgery affects psychotropic drug absorption. Current Psychiatry. 2022;21(8):39-44. doi:10.12788/cp.0271