User login
Monoamine oxidase inhibitors and tricyclic antidepressants for MDD
Ms. B, age 45, has a history of major depressive disorder (MDD) and migraines. She is admitted after presenting with anhedonia, hopelessness, and hypersomnia. These symptoms have become more severe over the last few weeks. Ms. B describes a past suicide attempt via overdose on doxylamine for which she required treatment in the intensive care unit. The only activity she enjoys is her weekly girls’ night, during which she drinks a few glasses of wine. Ms. B’s current medications are dextromethorphan/bupropion 45/105 mg twice daily and aripiprazole 5 mg/d, which she has taken for 3 months. She states she has “been on every antidepressant there is.”
When clinicians review Ms. B’s medication history, it is clear she has had adequate trials of selective serotonin reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake inhibitors (SNRIs), intranasal esketamine, multiple augmentation strategies, and electroconvulsive therapy (ECT). Ms. B seeks an alternative medication to improve her depressive symptoms.
Treatment-resistant depression (TRD) is commonly defined as depression that has not responded to ≥2 adequate trials of an antidepressant.1 Some guidelines recommend monoamine oxidase inhibitors (MAOIs) and tricyclic antidepressants (TCAs) as second- or even third-line options for MDD,2 while others recommend reserving them for patients with insufficient responses to alternative treatment modalities.3,4 Although MAOIs and TCAs have been available since the 1950s, prescribing these medications has become less prevalent due to safety concerns, the availability of other pharmacologic options, and a lack of clinical training and comfort.5,6 Most research notes that MAOIs are superior for treating atypical depression while TCAs are more effective for melancholic depression.2-4 In a review of 20 studies, Thase et al7 found that 50% of TCA nonresponders benefited from an MAOI. In the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial, monotherapy with the MAOI tranylcypromine was associated with a lower remission rate than the TCA nortriptyline; many argue the dose of tranylcypromine was suboptimal, and few participants completed an adequate trial in the last level.8,9 A more recent study by Kim et al10 found MAOIs to be “generally more effective” than TCAs for TRD, particularly in patients with fewer antidepressant trials; however, this was a small retrospective exploratory trial. A network meta-analysis found both classes to be “competitive” with SSRIs based on efficacy and tolerability, which leads to the question of whether these medications should be considered earlier in therapy.11 Considering patient-specific factors and particular medication properties is an effective strategy when prescribing an MAOI or TCA.
Monoamine oxidase inhibitors
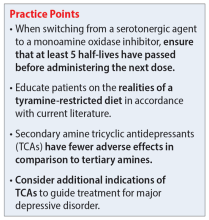
Four MAOIs are FDA-approved for treating MDD (Table 15,12-17): phenelzine, isocarboxazid, tranylcypromine, and selegiline. These medications irreversibly inhibit MAO, which exists as isomers A and B. MAO-A primarily metabolizes serotonin and norepinephrine, which is largely responsible for these medications’ antidepressant effects. Both isomers equally metabolize dopamine.5,12,18 It is best to avoid using MAOIs in patients with cerebrovascular disease, hepatic disease, or pheochromocytoma. Patients with active substance use disorders (particularly sympathomimetics and hallucinogens) are at an increased risk for hypertensive crises and serotonin syndrome, respectively. The most common adverse effects are orthostatic hypotension (despite more well-known concerns regarding hypertension), alterations in sleep patterns (insomnia or hypersomnia, depending on the agent), gastrointestinal issues, and anticholinergic adverse effects such as dry mouth and constipation.13,19-21
In one review and meta-analysis, phenelzine displayed the highest efficacy across all MAOIs.11 It likely requires high doses to achieve adequate MAO inhibition.11 A metabolite of phenelzine inhibits gamma-aminobutyric acid transaminase and may be helpful for patients with comorbid anxiety disorders or MDD with anxious distress.18,21 Additional considerations include phenelzine’s propensity for orthostasis (with rapid titrations and higher doses), sedation, weight gain, sexual dysfunction, and a rare adverse effect of vitamin B6 deficiency.5,13,14,20-22
Use of isocarboxazid in clinical practice is rare. Its adverse effects are similar to those of phenelzine but isocarboxazid is less studied. Tranylcypromine has a similar chemical structure to amphetamine. It can be stimulating at higher doses, potentially benefitting patients with comorbid attention-deficit/hyperactivity disorder (ADHD) or significant apathy.13,23 Selegiline’s distinct quality is its availability as a transdermal patch, which may be useful for patients who struggle to take oral medications. At low doses (6 mg/24 h), the selegiline transdermal patch allows patients to disregard a dietary tyramine restriction because it avoids first-pass metabolism. It inhibits both MAO isomers in the brain but is only selective for MAO-B once concentrations are distributed to the liver. Higher doses require a tyramine-restricted diet because there is still some MAO-A inhibition in the gut. Selegiline is also stimulating because it is converted to amphetamine and methamphetamine.5,12,13,17,19,24
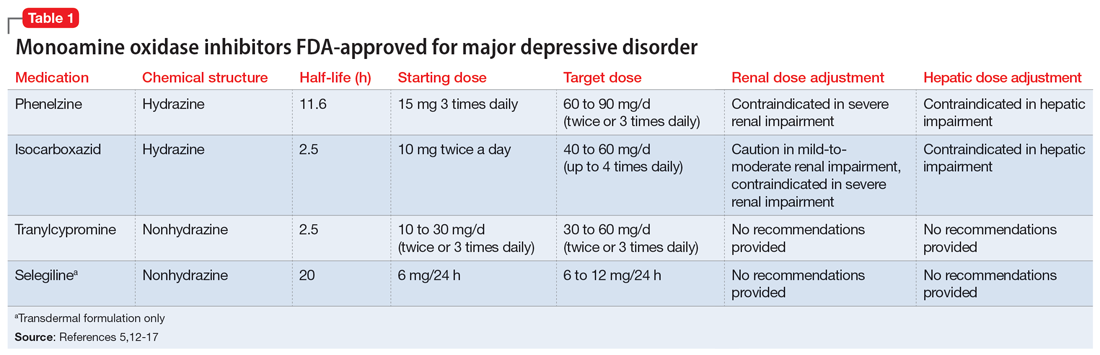
Despite promising results from the use of MAOIs, physicians and patients may be reluctant to use these medications due to perceived limitations. One prominent barrier is the infamous “cheese reaction.” Tyramine, an amino acid found in certain food and beverages (Table 25,13-18,25-28), is broken down by MAO-A in the gut. When this enzyme is inhibited, higher concentrations of tyramine reach systemic circulation. Tyramine’s release of norepinephrine (which now cannot be broken down) can lead to a hypertensive crisis. Consequently, a tyramine-restricted diet is recommended for patients taking an MAOI. However, the common notion that cheese, wine, and beer must be avoided is false, because most of the dietary restrictions developed following the discovery of MAOIs are antiquated.5,12,25-28 Patients who take an MAOI only need to slightly adjust their diet, as outlined in Table 2.5,13-18,25-28 A reasonable serving size of most foods and beverages containing tyramine is unlikely to elicit this “pressor” response. Of the 4 MAOIs FDA-approved for MDD, tranylcypromine appears to be the most sensitive to tyramine.21 Transient postdose hypertension (regardless of tyramine) may occur after taking an MAOI.29 Encourage patients to monitor their blood pressure.
Continue to: Additional hurdles include...
Additional hurdles include the required washout period from serotonergic medications and interactions with sympathomimetics. MAOIs pose the highest risk of serotonin syndrome; however, this usually occurs if given concomitantly with other serotonergic agents. The standard recommendation is a 14-day washout period from SSRIs (5 weeks for fluoxetine and 3 weeks for vortioxetine), SNRIs, mirtazapine, and other antidepressants. It can be distressing for patients to be without medication during that period. Because some antidepressants have much shorter half-lives, waiting 5 half-lives (typically 5 to 7 days) for the discontinued medication to be excreted is feasible if patients are closely monitored.5,12,13,25,27,30 There are rare instances where a TCA may be combined with an MAOI (typically initiated within 1 to 2 days of each other), but never clomipramine or imipramine due to their potent serotonin reuptake inhibition.31 If switching to an alternative MAOI, waiting 7 to 14 days is recommended to allow adequate time for the inhibited enzyme to regenerate.14-17,32 Taking medications that increase dopamine and norepinephrine (eg, stimulants or oral over-the-counter decongestants) with an MAOI is typically not recommended due to the risk of hypertensive crisis.25,27 In severe TRD or comorbid ADHD, successful simultaneous use of methylphenidate or amphetamine—typically at low doses—with close blood pressure monitoring has been reported.33 There have also been positive cases of the use of modafinil in combination with an MAOI; however, this should be done with caution.34,35 Clinicians must use clinical judgment when considering a combination of medications that pose a higher risk.
Tricyclic antidepressants
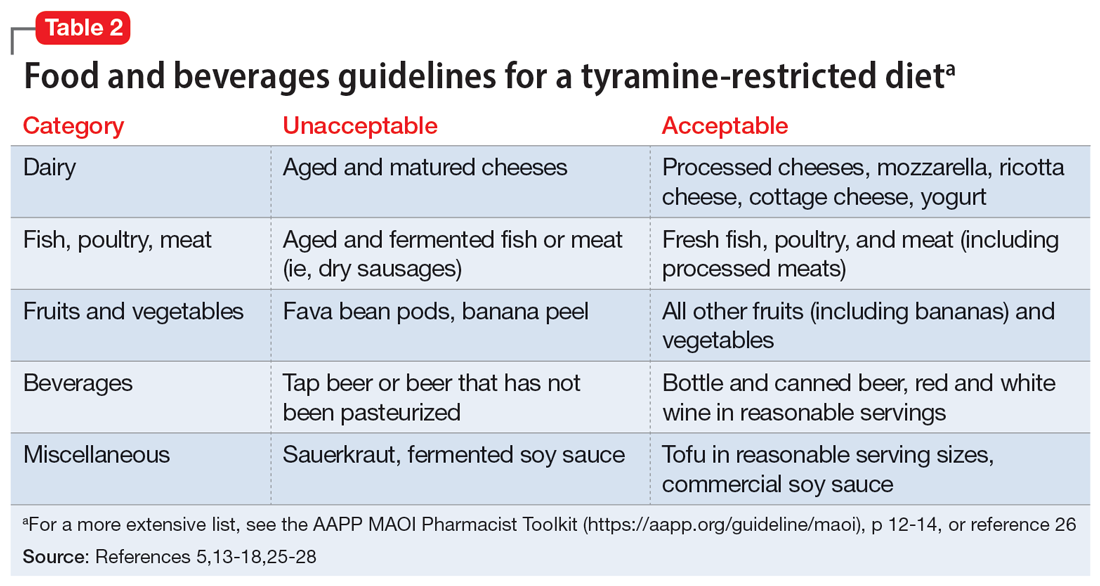
TCAs work differently than MAOIs to increase monoamines. They inhibit presynaptic serotonin and norepinephrine transporters in the CNS to increase levels of these chemicals in the synaptic cleft. While all TCAs inhibit these transporters, they do so at varying levels (Table 336-51). Based on their chemical structure, TCAs can be categorized into secondary and tertiary amines. Tertiary amines are metabolized via demethylation into their derivatives (Table 336-51). Patients who have recently suffered a myocardial infarction (MI) should avoid tertiary amines. TCAs can reduce heart rate variability, which is already decreased after an MI, thus presenting the potential for cardiac arrhythmias. TCAs should also be avoided in patients with cardiac conduction abnormalities.38-46,52 Patients with a prior baseline cardiac conduction defect, such as a bundle branch block, are at higher risk for further cardiac abnormalities. In those with a preexisting first-degree heart block, TCAs can still be used, but electrocardiogram monitoring is recommended.52,53 TCAs have also been reported to decrease the seizure threshold.38-46 They can be used with caution in patients who have a history of epilepsy or head trauma, or with concomitant medications that lower the seizure threshold.38-46
Overdose risk is a concern with TCAs because ingestion of 10 to 20 mg/kg can lead to significant toxicity.54 This is due to their blockage of voltage-gated sodium channels found in the CNS and heart, which contributes to overdose symptoms such as a widened QRS complex and seizures. Symptoms usually develop within 2 hours but may be delayed up to 6 hours.55 Patients with a history of overdose must be carefully assessed before initiating a TCA. Prescribing a limited supply of these medications may be valuable. The use of TCAs has often been limited due to their adverse effects, most of which are associated with their respective affinities for alpha 1, muscarinic 1, and histamine 1 receptors. Inhibition of the alpha 1 receptor is associated with hypotension, muscarinic 1 with anticholinergic adverse effects, and histamine 1 with sedation and weight gain. Tertiary amines have a higher affinity for these receptors compared to secondary amines, leading to a more significant adverse effect profile.36,50 Among TCAs, amitriptyline is the most likely to cause hypotension, whereas desipramine and nortriptyline are least likely. Amitriptyline and clomipramine are most likely to cause anticholinergic adverse effects, whereas desipramine and nortriptyline are the least likely. Amitriptyline, doxepin, and imipramine have the highest propensity for QTc prolongation.36
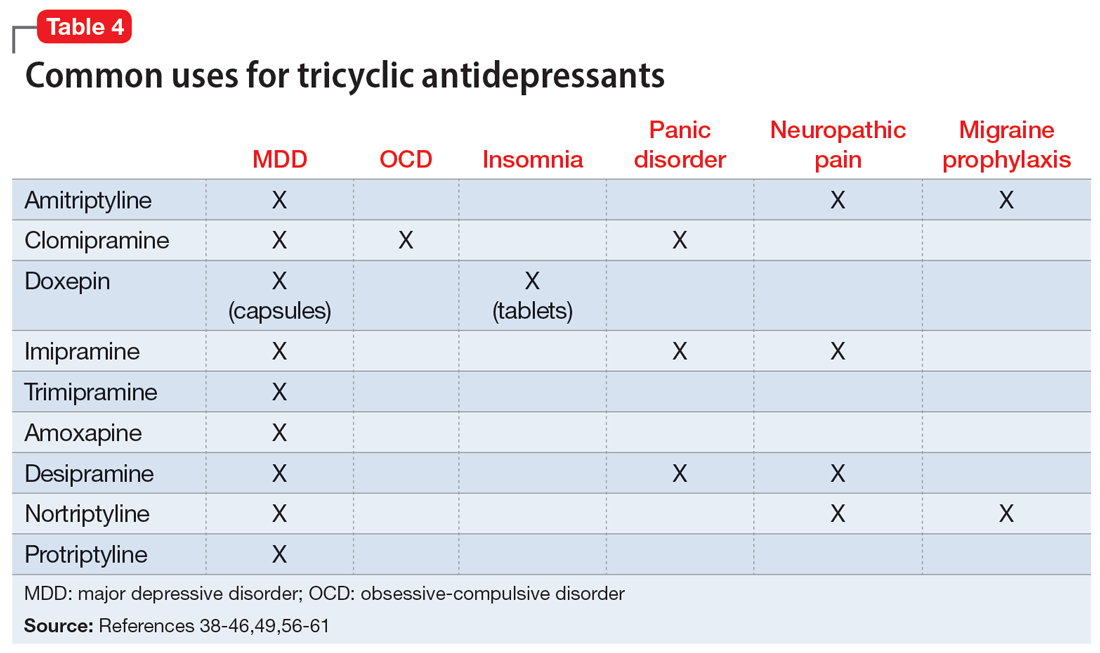
Beyond treating MDD, TCAs have shown benefits for treating other disease states (Table 438-46,49,56-61).These differing indications may help psychiatrists determine the best TCA to prescribe for a given patient. Amitriptyline is the most studied TCA for MDD; however, nortriptyline is typically preferred due to its favorable tolerability profile.4,62 Nortriptyline also has data supporting its use in ECT to prevent relapse.63 Amitriptyline and nortriptyline have shown benefits in patients with neuropathic pain and for migraine prophylaxis.56-60 Although frequently used for MDD, clomipramine is not FDA-approved for this indication, but is for obsessive-compulsive disorder.39 Doxepin is FDA-approved for insomnia at lower doses and for MDD at higher doses.40 Therefore, it may benefit patients with sleep difficulties secondary to depression. Desipramine has been used off-label to treat ADHD in children and has shown some benefits in adults.64-66 Protriptyline, trimipramine, and amoxapine are infrequently used in clinical practice.
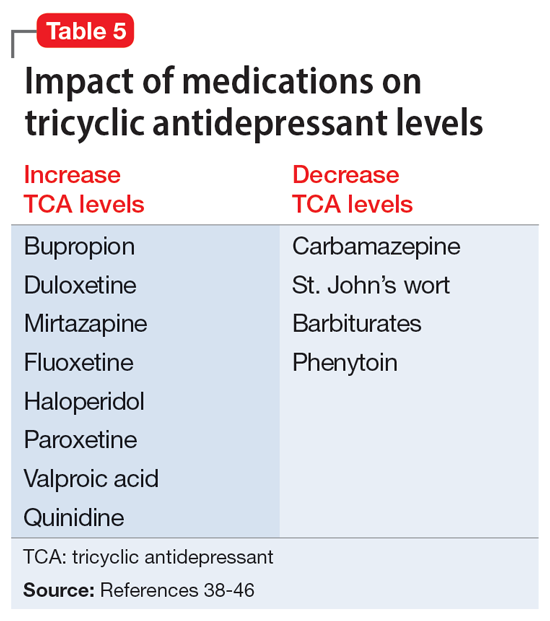
A unique feature of TCAs is the ability to monitor serum concentrations (Table 336-51).Guidelines recommend therapeutic drug monitoring (TDM) with amitriptyline, clomipramine, imipramine, and nortriptyline for routine use. TDM is still recommended for doxepin, desipramine, and trimipramine, but its utility is largely for treatment failure or resistance.37 These plasma levels can be altered based on coadministered medications (Table 538-46) and should be closely monitored. Physicians should obtain a trough level after at least 5 half-lives and before the next dose is due, and use TDM as indicated to optimize dosing.
Continue to: CASE CONTINUED
CASE CONTINUED
Ms. B’s outpatient psychiatrist provides collateral information about her medical history and confirms her long-standing MDD with multiple medication trials, though she has never received an MAOI or TCA. Ms. B is adamant she does not want a medication-free period between treatments and refuses to adjust her diet, despite being educated on the few changes necessary. She has no contraindications for TCAs and may benefit from a TCA for her comorbid migraines. The care team expresses concern for TCA overdose to Ms. B and her family. Ms. B’s sister reassures the team they will have someone monitor and dispense her medications at home. They decide to discontinue her current psychiatric regimen, and Ms. B is started on nortriptyline 50 mg/d at night, with plans to titrate based on tolerability.
Related Resources
- Meyer JM. A concise guide to monoamine oxidase inhibitors. Current Psychiatry. 2017;16(12):14-16,18-23,47,A.
- Espejo GD. Treating major depressive disorder after limited response to an initial agent. Current Psychiatry. 2021;20(10):51-53. doi:10.12788/cp.0178
- American Association of Psychiatric Pharmacists (AAPP) MAOI Pharmacist Toolkit. https://aapp.org/guideline/maoi
Drug Brand Names
Amitriptyline • Elavil
Amphetamine • Adzenys, Dyanavel
Aripiprazole • Abilify
Clomipramine • Anafranil
Desipramine • Norpramin
Dextromethorphan/bupropion • Auvelity
Doxepin • Sinequan, Adapin
Esketamine • Spravato
Fluoxetine • Prozac
Imipramine • Tofranil
Isocarboxazid • Marplan
Methamphetamine • Desoxyn
Mirtazapine • Remeron
Modafinil • Provigil
Nortriptyline • Pamelor
Phenelzine • Nardil
Protriptyline • Vivactil
Selegiline • Emsam
Tranylcypromine • Parnate
Trimipramine • Surmontil
Vortioxetine • Trintellix
1. Gaynes BN, Lux L, Gartlehner G, et al. Defining treatment-resistant depression. Depress Anxiety. 2020;37(2):134-145. doi:10.1002/da.22968
2. Kennedy SH, Lam RW, McIntyre RS, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: section 3. Pharmacological treatments. Can J Psychiatry. 2016;61(9):540-560. doi:10.1177/0706743716659417
3. VA/DoD clinical practice guideline for the management of major depressive disorder. Veterans Health Administration and Department of Defense; 2016. https://www.healthquality.va.gov/guidelines/MH/mdd/VADoDMDDCPGFINAL82916.pdf
4. Gelenberg AJ, Freeman MP, Markowitz JC, et al. Practice guideline for the treatment of patients with major depressive disorder. Am J Psychiatry. 2010;167(Suppl 10):9-118.
5. Meyer JM. A concise guide to monoamine oxidase inhibitors. Current Psychiatry. 2017;16(12):14-16,18-23,47,A.
6. Taylor D. Selective serotonin reuptake inhibitors and tricyclic antidepressants in combination. Interactions and therapeutic uses. Br J Psychiatry. 1995;167(5):575-580. doi:10.1192/bjp.167.5.575
7. Thase ME, Trivedi MH, Rush AJ. MAOIs in the contemporary treatment of depression. Neuropsychopharmacology. 1995;12(3):185-219. doi:10.1016/0893-133X(94)00058-8
8. Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905-1917. doi:10.1176/ajp.2006.163.11.1905
9. McGrath PJ, Stewart JW, Fava M, et al. Tranylcypromine versus venlafaxine plus mirtazapine following three failed antidepressant medication trials for depression: a STAR*D report. Am J Psychiatry. 2006;163(9):1531-1666. doi:10.1176/ajp.2006.163.9.1531
10. Kim T, Xu C, Amsterdam JD. Relative effectiveness of tricyclic antidepressant versus monoamine oxidase inhibitor monotherapy for treatment-resistant depression. J Affect Disord. 2019;250:199-203. doi:10.1016/j.jad.2019.03.028
11. Suchting R, Tirumalajaru V, Gareeb R, et al. Revisiting monoamine oxidase inhibitors for the treatment of depressive disorders: a systematic review and network meta-analysis. J Affect Disord. 2021;282:1153-1160. doi:10.1016/j.jad.2021.01.021
12. Stahl SM, Felker A. Monoamine oxidase inhibitors: a modern guide to an unrequited class of antidepressants. CNS Spectr. 2008;13(10):855-870. doi:10.1017/s1092852900016965
13. Chamberlain SR, Baldwin DS. Monoamine oxidase inhibitors (MAOIs) in psychiatric practice: how to use them safely and effectively. CNS Drugs. 2021;35(7):703-716. doi:10.1007/s40263-021-00832-x
14. Nardil [package insert]. New York, NY: Parke-Davis; 2009.
15. Marplan [package insert]. Parsippany, NJ: Validus Pharmaceuticals LLC; 2020.
16. Parnate [package insert]. Saint Michael, Barbados: Concordia Pharmaceuticals; 2015.
17. Emsam [package insert]. Morgantown, WV: Mylan Specialty LP; 2014.
18. Shulman KI, Herrmann N, Walker SE. Current place of monoamine oxidase inhibitors in the treatment of depression. CNS Drugs. 2013;27(10):789-797. doi:10.1007/s40263-013-0097-3
19. Sub Laban T, Saadabadi A. Monoamine oxidase inhibitors (MAOI). StatPearls Publishing; 2023. https://www.ncbi.nlm.nih.gov/books/NBK539848/
20. Rabkin JG, Quitkin FM, McGrath P, et al. Adverse reactions to monoamine oxidase inhibitors. Part II. Treatment correlates and clinical management. J Clin Psychopharmacol. 1985;5(1):2-9.
21. Gillman PK. Advances pertaining to the pharmacology and interactions of irreversible nonselective monoamine oxidase inhibitors. J Clin Psychopharmacol. 2011;31(1):66-74. doi:10.1097/JCP.0b013e31820469ea
22. Sidhu G, Marwaha R. Phenelzine. StatPearls Publishing; 2023. https://www.ncbi.nlm.nih.gov/books/NBK554508/
23. Frieling H, Bleich S. Tranylcypromine: new perspectives on an “old” drug. Eur Arch Psychiatry Clin Neurosci. 2006;256(5):268-273. doi:10.1007/s00406-006-0660-8
24. Goodnick PJ. Seligiline transdermal system in depression. Expert Opin Pharmacother. 2007;8(1):59-64. doi:10.1517/14656566.8.1.59
25. Edinoff AN, Swinford CR, Odisho AS, et al. Clinically relevant drug interactions with monoamine oxidase inhibitors. Health Psychol Res. 2022;10(4):39576. doi:10.52965/001c.39576
26. Gillman PK. A reassessment of the safety profile of monoamine oxidase inhibitors: elucidating tired old tyramine myths. J Neural Transm (Vienna). 2018;125(11):1707-1717. doi:10.1007/s00702-018-1932-y
27. Flockhart DA. Dietary restrictions and drug interactions with monoamine oxidase inhibitors: an update. J Clin Psychiatry. 2012;73 Suppl 1:17-24. doi:10.4088/JCP.11096su1c.03
28. McCabe-Sellers BJ, Staggs CG, Bogle ML. Tyramine in foods and monoamine oxidase inhibitor drugs: a crossroad where medicine, nutrition, pharmacy, and food industry converge. J Food Composit Anal. 2006;19:S58-S65. doi:10.1016/j.jfca.2005.12.008
29. Keck PE Jr, Vuckovic A, Pope HG Jr, et al. Acute cardiovascular response to monoamine oxidase inhibitors: a prospective assessment. J Clin Psychopharmacol. 1989;9(3):203-206.
30. Bodkin JA, Dunlop BW. Moving on with monoamine oxidase inhibitors. Focus (Am Psychiatr Publ). 2021;19(1):50-52. doi:10.1176/appi.focus.20200046
31. Amsterdam JD, Kim TT. Relative effectiveness of monoamine oxidase inhibitor and tricyclic antidepressant combination therapy for treatment-resistant depression. J Clin Psychopharmacol. 2019;39(6):649-652. doi:10.1097/JCP.0000000000001130
32. Keks N, Hope J, Keogh S. Switching and stopping antidepressants. Aust Prescr. 2016;39(3):76-83. doi:10.18773/austprescr.2016.039
33. Israel JA. Combining stimulants and monoamine oxidase inhibitors: a reexamination of the literature and a report of a new treatment combination. Prim Care Companion CNS Disord. 2015;17(6):10.4088/PCC.15br01836. doi:10.4088/PCC.15br01836
34. Clemons WE, Makela E, Young J. Concomitant use of modafinil and tranylcypromine in a patient with narcolepsy: a case report. Sleep Med. 2004;5(5):509-511. doi:10.1016/j.sleep.2004.06.006
35. Ashton AK. Modafinil augmentation of phenelzine for residual fatigue in dysthymia. Am J Psychiatry. 2004;161(9):1716-1717. doi:10.1176/appi.ajp.161.9.1716-a
36. O’Donnell JM, Bies RR, Shelton RC. Drug therapy of depression and anxiety disorders. In: Brunton LL, Hilal-Dandan R, Knollmann BC, eds. Goodman & Gilman’s: The Pharmacological Basis of Therapeutics. 13th ed. McGraw Hill; 2017. Accessed June 4, 2023. https://accessanesthesiology.mhmedical.com/content.aspx?bookid=2189§ionid=169518711
37. Hiemke C, Bergemann N, Clement HW, et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry. 2018;51(1-02):9-62. doi:10.1055/s-0043-116492
38. Amitriptyline hydrochloride [package insert]. East Brunswick, NJ: Unichem Pharmaceuticals (USA); 2021.
39. Clomipramine hydrochloride [package insert]. East Windsor, NJ: Aurobindo Pharma Limited; 2023.
40. Doxepin hydrochloride capsules, USP [package insert]. Bedminster, NJ: Alembic Pharmaceuticals Inc; 2021.
41. Imipramine hydrochloride tablet [package insert]. Fairfield, NJ: Leading Pharma LLC USA; 2022.
42. Trimipramine maleate [package insert]. Northvale, NJ: Elite Laboratories Inc; 2021.
43. Amoxapine [package insert]. Parsippany, NJ: Actavis Pharma Inc; 2015.
44. Desipramine hydrochloride tablets [package insert]. Bedminster, NJ: Alembic Pharmaceuticals Inc; 2023.
45. Nortriptyline hydrochloride capsules, USP [package insert]. Parsippany, NJ: Teva Pharmaceuticals Inc; 2021.
46. Protriptyline hydrochloride [package insert]. Bensalem, PA: Sigmapharm Laboratories, LLC; 2023.
47. Calvo B, García MJ, Pedraz JL, et al. Pharmacokinetics of amoxapine and its active metabolites. Int J Clin Pharmacol Ther Toxicol. 1985;23(4):180-185.
48. Ziegler VE, Biggs JT, Wylie LT, et al. Protriptyline kinetics. Clin Pharmacol Ther. 1978;23(5):580-584. doi:10.1002/cpt1978235580
49. Cleare A, Pariante CM, Young AH, et al. Evidence-based guidelines for treating depressive disorders with antidepressants: a revision of the 2008 British Association for Psychopharmacology guidelines. J Psychopharmacol. 2015;29(5):459-525. doi:10.1177/0269881115581093
50. Richelson E. Synaptic effects of antidepressants. J Clin Psychopharmacol. 1996;16(3 Suppl 2):1S-9S. doi:10.1097/00004714-199606002-00001
51. Vos CF, Aarnoutse RE, Op de Coul MJM, et al. Tricyclic antidepressants for major depressive disorder: a comprehensive evaluation of current practice in the Netherlands. BMC Psychiatry. 2021;21(1):481. doi:10.1186/s12888-021-03490-x
52. Alvarez W Jr, Pickworth KK. Safety of antidepressant drugs in the patient with cardiac disease: a review of the literature. Pharmacotherapy. 2003;23(6):754-771. doi:10.1592/phco.23.6.754.32185
53. Dietch JT, Fine M. The effect of nortriptyline in elderly patients with cardiac conduction disease. J Clin Psychiatry. 1990;51(2):65-67.
54. Valento M, Liebelt EL. Cyclic antidepressants. In: Nelson LS, Howland M, Lewin NA, et al, eds. Goldfrank’s Toxicologic Emergencies. 9th ed. McGraw Hill; 2011. Accessed June 10, 2023. https://accesspharmacy.mhmedical.com/content.aspx?bookid=2569§ionid=210274664
55. Woolf AD, Erdman AR, Nelson LS, et al. Tricyclic antidepressant poisoning: an evidence-based consensus guideline for out-of-hospital management. Clin Toxicol (Phila). 2007;45(3):203-233. doi:10.1080/15563650701226192
56. Baldwin DS, Anderson IM, Nutt DJ, et al. Evidence-based pharmacological treatment of anxiety disorders, post-traumatic stress disorder and obsessive-compulsive disorder: a revision of the 2005 guidelines from the British Association for Psychopharmacology. J Psychopharmacol. 2014;28(5):403-439. doi:10.1177/0269881114525674
57. Moulin DE, Clark AJ, Gilron I, et al. Pharmacological management of chronic neuropathic pain - consensus statement and guidelines from the Canadian Pain Society. Pain Res Manag. 2007;12(1):13-21. doi:10.1155/2007/730785
58. Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14(2):162-173. doi:10.1016/S1474-4422(14)70251-0
59. Saarto T, Wiffen PJ. Antidepressants for neuropathic pain. Cochrane Database Syst Rev. 2007;(4):CD005454. doi:10.1002/14651858.CD005454.pub2
60. Burch R. Antidepressants for preventive treatment of migraine. Curr Treat Options Neurol. 2019;21(4):18. doi:10.1007/s11940-019-0557-2
61. Ailani J, Burch RC, Robbins MS; Board of Directors of the American Headache Society. The American Headache Society consensus statement: update on integrating new migraine treatments into clinical practice. Headache. 2021;61(7):1021-1039. doi:10.1111/head.14153
62. Bauer M, Pfennig A, Severus E, et al. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of unipolar depressive disorders, part 1: update 2013 on the acute and continuation treatment of unipolar depressive disorders. World J Biol Psychiatry. 2013;14(5):334-385. doi:10.3109/15622975.2013.804195
63. Jelovac A, Kolshus E, McLoughlin DM. Relapse following successful electroconvulsive therapy for major depression: a meta-analysis. Neuropsychopharmacology. 2013;38(12):2467-2474. doi:10.1038/npp.2013.149
64. Spencer T, Biederman J, Coffey B, et al. A double-blind comparison of desipramine and placebo in children and adolescents with chronic tic disorder and comorbid attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2002;59(7):649-656. doi:10.1001/archpsyc.59.7.649
65. Spencer T, Biederman J, Wilens T, et al. Pharmacotherapy of attention-deficit hyperactivity disorder across the life cycle. J Am Acad Child Adolesc Psychiatry. 1996;35(4):409-432. doi:10.1097/00004583-199604000-00008
66. Wilens TE, Biederman J, Prince J, et al. Six-week, double-blind, placebo-controlled study of desipramine for adult attention deficit hyperactivity disorder. Am J Psychiatry. 1996;153(9):1147-1153. doi:10.1176/ajp.153.9.1147
Ms. B, age 45, has a history of major depressive disorder (MDD) and migraines. She is admitted after presenting with anhedonia, hopelessness, and hypersomnia. These symptoms have become more severe over the last few weeks. Ms. B describes a past suicide attempt via overdose on doxylamine for which she required treatment in the intensive care unit. The only activity she enjoys is her weekly girls’ night, during which she drinks a few glasses of wine. Ms. B’s current medications are dextromethorphan/bupropion 45/105 mg twice daily and aripiprazole 5 mg/d, which she has taken for 3 months. She states she has “been on every antidepressant there is.”
When clinicians review Ms. B’s medication history, it is clear she has had adequate trials of selective serotonin reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake inhibitors (SNRIs), intranasal esketamine, multiple augmentation strategies, and electroconvulsive therapy (ECT). Ms. B seeks an alternative medication to improve her depressive symptoms.
Treatment-resistant depression (TRD) is commonly defined as depression that has not responded to ≥2 adequate trials of an antidepressant.1 Some guidelines recommend monoamine oxidase inhibitors (MAOIs) and tricyclic antidepressants (TCAs) as second- or even third-line options for MDD,2 while others recommend reserving them for patients with insufficient responses to alternative treatment modalities.3,4 Although MAOIs and TCAs have been available since the 1950s, prescribing these medications has become less prevalent due to safety concerns, the availability of other pharmacologic options, and a lack of clinical training and comfort.5,6 Most research notes that MAOIs are superior for treating atypical depression while TCAs are more effective for melancholic depression.2-4 In a review of 20 studies, Thase et al7 found that 50% of TCA nonresponders benefited from an MAOI. In the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial, monotherapy with the MAOI tranylcypromine was associated with a lower remission rate than the TCA nortriptyline; many argue the dose of tranylcypromine was suboptimal, and few participants completed an adequate trial in the last level.8,9 A more recent study by Kim et al10 found MAOIs to be “generally more effective” than TCAs for TRD, particularly in patients with fewer antidepressant trials; however, this was a small retrospective exploratory trial. A network meta-analysis found both classes to be “competitive” with SSRIs based on efficacy and tolerability, which leads to the question of whether these medications should be considered earlier in therapy.11 Considering patient-specific factors and particular medication properties is an effective strategy when prescribing an MAOI or TCA.
Monoamine oxidase inhibitors
Four MAOIs are FDA-approved for treating MDD (Table 15,12-17): phenelzine, isocarboxazid, tranylcypromine, and selegiline. These medications irreversibly inhibit MAO, which exists as isomers A and B. MAO-A primarily metabolizes serotonin and norepinephrine, which is largely responsible for these medications’ antidepressant effects. Both isomers equally metabolize dopamine.5,12,18 It is best to avoid using MAOIs in patients with cerebrovascular disease, hepatic disease, or pheochromocytoma. Patients with active substance use disorders (particularly sympathomimetics and hallucinogens) are at an increased risk for hypertensive crises and serotonin syndrome, respectively. The most common adverse effects are orthostatic hypotension (despite more well-known concerns regarding hypertension), alterations in sleep patterns (insomnia or hypersomnia, depending on the agent), gastrointestinal issues, and anticholinergic adverse effects such as dry mouth and constipation.13,19-21

In one review and meta-analysis, phenelzine displayed the highest efficacy across all MAOIs.11 It likely requires high doses to achieve adequate MAO inhibition.11 A metabolite of phenelzine inhibits gamma-aminobutyric acid transaminase and may be helpful for patients with comorbid anxiety disorders or MDD with anxious distress.18,21 Additional considerations include phenelzine’s propensity for orthostasis (with rapid titrations and higher doses), sedation, weight gain, sexual dysfunction, and a rare adverse effect of vitamin B6 deficiency.5,13,14,20-22
Use of isocarboxazid in clinical practice is rare. Its adverse effects are similar to those of phenelzine but isocarboxazid is less studied. Tranylcypromine has a similar chemical structure to amphetamine. It can be stimulating at higher doses, potentially benefitting patients with comorbid attention-deficit/hyperactivity disorder (ADHD) or significant apathy.13,23 Selegiline’s distinct quality is its availability as a transdermal patch, which may be useful for patients who struggle to take oral medications. At low doses (6 mg/24 h), the selegiline transdermal patch allows patients to disregard a dietary tyramine restriction because it avoids first-pass metabolism. It inhibits both MAO isomers in the brain but is only selective for MAO-B once concentrations are distributed to the liver. Higher doses require a tyramine-restricted diet because there is still some MAO-A inhibition in the gut. Selegiline is also stimulating because it is converted to amphetamine and methamphetamine.5,12,13,17,19,24
Despite promising results from the use of MAOIs, physicians and patients may be reluctant to use these medications due to perceived limitations. One prominent barrier is the infamous “cheese reaction.” Tyramine, an amino acid found in certain food and beverages (Table 25,13-18,25-28), is broken down by MAO-A in the gut. When this enzyme is inhibited, higher concentrations of tyramine reach systemic circulation. Tyramine’s release of norepinephrine (which now cannot be broken down) can lead to a hypertensive crisis. Consequently, a tyramine-restricted diet is recommended for patients taking an MAOI. However, the common notion that cheese, wine, and beer must be avoided is false, because most of the dietary restrictions developed following the discovery of MAOIs are antiquated.5,12,25-28 Patients who take an MAOI only need to slightly adjust their diet, as outlined in Table 2.5,13-18,25-28 A reasonable serving size of most foods and beverages containing tyramine is unlikely to elicit this “pressor” response. Of the 4 MAOIs FDA-approved for MDD, tranylcypromine appears to be the most sensitive to tyramine.21 Transient postdose hypertension (regardless of tyramine) may occur after taking an MAOI.29 Encourage patients to monitor their blood pressure.

Continue to: Additional hurdles include...
Additional hurdles include the required washout period from serotonergic medications and interactions with sympathomimetics. MAOIs pose the highest risk of serotonin syndrome; however, this usually occurs if given concomitantly with other serotonergic agents. The standard recommendation is a 14-day washout period from SSRIs (5 weeks for fluoxetine and 3 weeks for vortioxetine), SNRIs, mirtazapine, and other antidepressants. It can be distressing for patients to be without medication during that period. Because some antidepressants have much shorter half-lives, waiting 5 half-lives (typically 5 to 7 days) for the discontinued medication to be excreted is feasible if patients are closely monitored.5,12,13,25,27,30 There are rare instances where a TCA may be combined with an MAOI (typically initiated within 1 to 2 days of each other), but never clomipramine or imipramine due to their potent serotonin reuptake inhibition.31 If switching to an alternative MAOI, waiting 7 to 14 days is recommended to allow adequate time for the inhibited enzyme to regenerate.14-17,32 Taking medications that increase dopamine and norepinephrine (eg, stimulants or oral over-the-counter decongestants) with an MAOI is typically not recommended due to the risk of hypertensive crisis.25,27 In severe TRD or comorbid ADHD, successful simultaneous use of methylphenidate or amphetamine—typically at low doses—with close blood pressure monitoring has been reported.33 There have also been positive cases of the use of modafinil in combination with an MAOI; however, this should be done with caution.34,35 Clinicians must use clinical judgment when considering a combination of medications that pose a higher risk.
Tricyclic antidepressants
TCAs work differently than MAOIs to increase monoamines. They inhibit presynaptic serotonin and norepinephrine transporters in the CNS to increase levels of these chemicals in the synaptic cleft. While all TCAs inhibit these transporters, they do so at varying levels (Table 336-51). Based on their chemical structure, TCAs can be categorized into secondary and tertiary amines. Tertiary amines are metabolized via demethylation into their derivatives (Table 336-51). Patients who have recently suffered a myocardial infarction (MI) should avoid tertiary amines. TCAs can reduce heart rate variability, which is already decreased after an MI, thus presenting the potential for cardiac arrhythmias. TCAs should also be avoided in patients with cardiac conduction abnormalities.38-46,52 Patients with a prior baseline cardiac conduction defect, such as a bundle branch block, are at higher risk for further cardiac abnormalities. In those with a preexisting first-degree heart block, TCAs can still be used, but electrocardiogram monitoring is recommended.52,53 TCAs have also been reported to decrease the seizure threshold.38-46 They can be used with caution in patients who have a history of epilepsy or head trauma, or with concomitant medications that lower the seizure threshold.38-46

Overdose risk is a concern with TCAs because ingestion of 10 to 20 mg/kg can lead to significant toxicity.54 This is due to their blockage of voltage-gated sodium channels found in the CNS and heart, which contributes to overdose symptoms such as a widened QRS complex and seizures. Symptoms usually develop within 2 hours but may be delayed up to 6 hours.55 Patients with a history of overdose must be carefully assessed before initiating a TCA. Prescribing a limited supply of these medications may be valuable. The use of TCAs has often been limited due to their adverse effects, most of which are associated with their respective affinities for alpha 1, muscarinic 1, and histamine 1 receptors. Inhibition of the alpha 1 receptor is associated with hypotension, muscarinic 1 with anticholinergic adverse effects, and histamine 1 with sedation and weight gain. Tertiary amines have a higher affinity for these receptors compared to secondary amines, leading to a more significant adverse effect profile.36,50 Among TCAs, amitriptyline is the most likely to cause hypotension, whereas desipramine and nortriptyline are least likely. Amitriptyline and clomipramine are most likely to cause anticholinergic adverse effects, whereas desipramine and nortriptyline are the least likely. Amitriptyline, doxepin, and imipramine have the highest propensity for QTc prolongation.36
Beyond treating MDD, TCAs have shown benefits for treating other disease states (Table 438-46,49,56-61).These differing indications may help psychiatrists determine the best TCA to prescribe for a given patient. Amitriptyline is the most studied TCA for MDD; however, nortriptyline is typically preferred due to its favorable tolerability profile.4,62 Nortriptyline also has data supporting its use in ECT to prevent relapse.63 Amitriptyline and nortriptyline have shown benefits in patients with neuropathic pain and for migraine prophylaxis.56-60 Although frequently used for MDD, clomipramine is not FDA-approved for this indication, but is for obsessive-compulsive disorder.39 Doxepin is FDA-approved for insomnia at lower doses and for MDD at higher doses.40 Therefore, it may benefit patients with sleep difficulties secondary to depression. Desipramine has been used off-label to treat ADHD in children and has shown some benefits in adults.64-66 Protriptyline, trimipramine, and amoxapine are infrequently used in clinical practice.

A unique feature of TCAs is the ability to monitor serum concentrations (Table 336-51).Guidelines recommend therapeutic drug monitoring (TDM) with amitriptyline, clomipramine, imipramine, and nortriptyline for routine use. TDM is still recommended for doxepin, desipramine, and trimipramine, but its utility is largely for treatment failure or resistance.37 These plasma levels can be altered based on coadministered medications (Table 538-46) and should be closely monitored. Physicians should obtain a trough level after at least 5 half-lives and before the next dose is due, and use TDM as indicated to optimize dosing.

Continue to: CASE CONTINUED
CASE CONTINUED
Ms. B’s outpatient psychiatrist provides collateral information about her medical history and confirms her long-standing MDD with multiple medication trials, though she has never received an MAOI or TCA. Ms. B is adamant she does not want a medication-free period between treatments and refuses to adjust her diet, despite being educated on the few changes necessary. She has no contraindications for TCAs and may benefit from a TCA for her comorbid migraines. The care team expresses concern for TCA overdose to Ms. B and her family. Ms. B’s sister reassures the team they will have someone monitor and dispense her medications at home. They decide to discontinue her current psychiatric regimen, and Ms. B is started on nortriptyline 50 mg/d at night, with plans to titrate based on tolerability.
Related Resources
- Meyer JM. A concise guide to monoamine oxidase inhibitors. Current Psychiatry. 2017;16(12):14-16,18-23,47,A.
- Espejo GD. Treating major depressive disorder after limited response to an initial agent. Current Psychiatry. 2021;20(10):51-53. doi:10.12788/cp.0178
- American Association of Psychiatric Pharmacists (AAPP) MAOI Pharmacist Toolkit. https://aapp.org/guideline/maoi
Drug Brand Names
Amitriptyline • Elavil
Amphetamine • Adzenys, Dyanavel
Aripiprazole • Abilify
Clomipramine • Anafranil
Desipramine • Norpramin
Dextromethorphan/bupropion • Auvelity
Doxepin • Sinequan, Adapin
Esketamine • Spravato
Fluoxetine • Prozac
Imipramine • Tofranil
Isocarboxazid • Marplan
Methamphetamine • Desoxyn
Mirtazapine • Remeron
Modafinil • Provigil
Nortriptyline • Pamelor
Phenelzine • Nardil
Protriptyline • Vivactil
Selegiline • Emsam
Tranylcypromine • Parnate
Trimipramine • Surmontil
Vortioxetine • Trintellix
Ms. B, age 45, has a history of major depressive disorder (MDD) and migraines. She is admitted after presenting with anhedonia, hopelessness, and hypersomnia. These symptoms have become more severe over the last few weeks. Ms. B describes a past suicide attempt via overdose on doxylamine for which she required treatment in the intensive care unit. The only activity she enjoys is her weekly girls’ night, during which she drinks a few glasses of wine. Ms. B’s current medications are dextromethorphan/bupropion 45/105 mg twice daily and aripiprazole 5 mg/d, which she has taken for 3 months. She states she has “been on every antidepressant there is.”
When clinicians review Ms. B’s medication history, it is clear she has had adequate trials of selective serotonin reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake inhibitors (SNRIs), intranasal esketamine, multiple augmentation strategies, and electroconvulsive therapy (ECT). Ms. B seeks an alternative medication to improve her depressive symptoms.
Treatment-resistant depression (TRD) is commonly defined as depression that has not responded to ≥2 adequate trials of an antidepressant.1 Some guidelines recommend monoamine oxidase inhibitors (MAOIs) and tricyclic antidepressants (TCAs) as second- or even third-line options for MDD,2 while others recommend reserving them for patients with insufficient responses to alternative treatment modalities.3,4 Although MAOIs and TCAs have been available since the 1950s, prescribing these medications has become less prevalent due to safety concerns, the availability of other pharmacologic options, and a lack of clinical training and comfort.5,6 Most research notes that MAOIs are superior for treating atypical depression while TCAs are more effective for melancholic depression.2-4 In a review of 20 studies, Thase et al7 found that 50% of TCA nonresponders benefited from an MAOI. In the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial, monotherapy with the MAOI tranylcypromine was associated with a lower remission rate than the TCA nortriptyline; many argue the dose of tranylcypromine was suboptimal, and few participants completed an adequate trial in the last level.8,9 A more recent study by Kim et al10 found MAOIs to be “generally more effective” than TCAs for TRD, particularly in patients with fewer antidepressant trials; however, this was a small retrospective exploratory trial. A network meta-analysis found both classes to be “competitive” with SSRIs based on efficacy and tolerability, which leads to the question of whether these medications should be considered earlier in therapy.11 Considering patient-specific factors and particular medication properties is an effective strategy when prescribing an MAOI or TCA.
Monoamine oxidase inhibitors
Four MAOIs are FDA-approved for treating MDD (Table 15,12-17): phenelzine, isocarboxazid, tranylcypromine, and selegiline. These medications irreversibly inhibit MAO, which exists as isomers A and B. MAO-A primarily metabolizes serotonin and norepinephrine, which is largely responsible for these medications’ antidepressant effects. Both isomers equally metabolize dopamine.5,12,18 It is best to avoid using MAOIs in patients with cerebrovascular disease, hepatic disease, or pheochromocytoma. Patients with active substance use disorders (particularly sympathomimetics and hallucinogens) are at an increased risk for hypertensive crises and serotonin syndrome, respectively. The most common adverse effects are orthostatic hypotension (despite more well-known concerns regarding hypertension), alterations in sleep patterns (insomnia or hypersomnia, depending on the agent), gastrointestinal issues, and anticholinergic adverse effects such as dry mouth and constipation.13,19-21

In one review and meta-analysis, phenelzine displayed the highest efficacy across all MAOIs.11 It likely requires high doses to achieve adequate MAO inhibition.11 A metabolite of phenelzine inhibits gamma-aminobutyric acid transaminase and may be helpful for patients with comorbid anxiety disorders or MDD with anxious distress.18,21 Additional considerations include phenelzine’s propensity for orthostasis (with rapid titrations and higher doses), sedation, weight gain, sexual dysfunction, and a rare adverse effect of vitamin B6 deficiency.5,13,14,20-22
Use of isocarboxazid in clinical practice is rare. Its adverse effects are similar to those of phenelzine but isocarboxazid is less studied. Tranylcypromine has a similar chemical structure to amphetamine. It can be stimulating at higher doses, potentially benefitting patients with comorbid attention-deficit/hyperactivity disorder (ADHD) or significant apathy.13,23 Selegiline’s distinct quality is its availability as a transdermal patch, which may be useful for patients who struggle to take oral medications. At low doses (6 mg/24 h), the selegiline transdermal patch allows patients to disregard a dietary tyramine restriction because it avoids first-pass metabolism. It inhibits both MAO isomers in the brain but is only selective for MAO-B once concentrations are distributed to the liver. Higher doses require a tyramine-restricted diet because there is still some MAO-A inhibition in the gut. Selegiline is also stimulating because it is converted to amphetamine and methamphetamine.5,12,13,17,19,24
Despite promising results from the use of MAOIs, physicians and patients may be reluctant to use these medications due to perceived limitations. One prominent barrier is the infamous “cheese reaction.” Tyramine, an amino acid found in certain food and beverages (Table 25,13-18,25-28), is broken down by MAO-A in the gut. When this enzyme is inhibited, higher concentrations of tyramine reach systemic circulation. Tyramine’s release of norepinephrine (which now cannot be broken down) can lead to a hypertensive crisis. Consequently, a tyramine-restricted diet is recommended for patients taking an MAOI. However, the common notion that cheese, wine, and beer must be avoided is false, because most of the dietary restrictions developed following the discovery of MAOIs are antiquated.5,12,25-28 Patients who take an MAOI only need to slightly adjust their diet, as outlined in Table 2.5,13-18,25-28 A reasonable serving size of most foods and beverages containing tyramine is unlikely to elicit this “pressor” response. Of the 4 MAOIs FDA-approved for MDD, tranylcypromine appears to be the most sensitive to tyramine.21 Transient postdose hypertension (regardless of tyramine) may occur after taking an MAOI.29 Encourage patients to monitor their blood pressure.

Continue to: Additional hurdles include...
Additional hurdles include the required washout period from serotonergic medications and interactions with sympathomimetics. MAOIs pose the highest risk of serotonin syndrome; however, this usually occurs if given concomitantly with other serotonergic agents. The standard recommendation is a 14-day washout period from SSRIs (5 weeks for fluoxetine and 3 weeks for vortioxetine), SNRIs, mirtazapine, and other antidepressants. It can be distressing for patients to be without medication during that period. Because some antidepressants have much shorter half-lives, waiting 5 half-lives (typically 5 to 7 days) for the discontinued medication to be excreted is feasible if patients are closely monitored.5,12,13,25,27,30 There are rare instances where a TCA may be combined with an MAOI (typically initiated within 1 to 2 days of each other), but never clomipramine or imipramine due to their potent serotonin reuptake inhibition.31 If switching to an alternative MAOI, waiting 7 to 14 days is recommended to allow adequate time for the inhibited enzyme to regenerate.14-17,32 Taking medications that increase dopamine and norepinephrine (eg, stimulants or oral over-the-counter decongestants) with an MAOI is typically not recommended due to the risk of hypertensive crisis.25,27 In severe TRD or comorbid ADHD, successful simultaneous use of methylphenidate or amphetamine—typically at low doses—with close blood pressure monitoring has been reported.33 There have also been positive cases of the use of modafinil in combination with an MAOI; however, this should be done with caution.34,35 Clinicians must use clinical judgment when considering a combination of medications that pose a higher risk.
Tricyclic antidepressants
TCAs work differently than MAOIs to increase monoamines. They inhibit presynaptic serotonin and norepinephrine transporters in the CNS to increase levels of these chemicals in the synaptic cleft. While all TCAs inhibit these transporters, they do so at varying levels (Table 336-51). Based on their chemical structure, TCAs can be categorized into secondary and tertiary amines. Tertiary amines are metabolized via demethylation into their derivatives (Table 336-51). Patients who have recently suffered a myocardial infarction (MI) should avoid tertiary amines. TCAs can reduce heart rate variability, which is already decreased after an MI, thus presenting the potential for cardiac arrhythmias. TCAs should also be avoided in patients with cardiac conduction abnormalities.38-46,52 Patients with a prior baseline cardiac conduction defect, such as a bundle branch block, are at higher risk for further cardiac abnormalities. In those with a preexisting first-degree heart block, TCAs can still be used, but electrocardiogram monitoring is recommended.52,53 TCAs have also been reported to decrease the seizure threshold.38-46 They can be used with caution in patients who have a history of epilepsy or head trauma, or with concomitant medications that lower the seizure threshold.38-46

Overdose risk is a concern with TCAs because ingestion of 10 to 20 mg/kg can lead to significant toxicity.54 This is due to their blockage of voltage-gated sodium channels found in the CNS and heart, which contributes to overdose symptoms such as a widened QRS complex and seizures. Symptoms usually develop within 2 hours but may be delayed up to 6 hours.55 Patients with a history of overdose must be carefully assessed before initiating a TCA. Prescribing a limited supply of these medications may be valuable. The use of TCAs has often been limited due to their adverse effects, most of which are associated with their respective affinities for alpha 1, muscarinic 1, and histamine 1 receptors. Inhibition of the alpha 1 receptor is associated with hypotension, muscarinic 1 with anticholinergic adverse effects, and histamine 1 with sedation and weight gain. Tertiary amines have a higher affinity for these receptors compared to secondary amines, leading to a more significant adverse effect profile.36,50 Among TCAs, amitriptyline is the most likely to cause hypotension, whereas desipramine and nortriptyline are least likely. Amitriptyline and clomipramine are most likely to cause anticholinergic adverse effects, whereas desipramine and nortriptyline are the least likely. Amitriptyline, doxepin, and imipramine have the highest propensity for QTc prolongation.36
Beyond treating MDD, TCAs have shown benefits for treating other disease states (Table 438-46,49,56-61).These differing indications may help psychiatrists determine the best TCA to prescribe for a given patient. Amitriptyline is the most studied TCA for MDD; however, nortriptyline is typically preferred due to its favorable tolerability profile.4,62 Nortriptyline also has data supporting its use in ECT to prevent relapse.63 Amitriptyline and nortriptyline have shown benefits in patients with neuropathic pain and for migraine prophylaxis.56-60 Although frequently used for MDD, clomipramine is not FDA-approved for this indication, but is for obsessive-compulsive disorder.39 Doxepin is FDA-approved for insomnia at lower doses and for MDD at higher doses.40 Therefore, it may benefit patients with sleep difficulties secondary to depression. Desipramine has been used off-label to treat ADHD in children and has shown some benefits in adults.64-66 Protriptyline, trimipramine, and amoxapine are infrequently used in clinical practice.

A unique feature of TCAs is the ability to monitor serum concentrations (Table 336-51).Guidelines recommend therapeutic drug monitoring (TDM) with amitriptyline, clomipramine, imipramine, and nortriptyline for routine use. TDM is still recommended for doxepin, desipramine, and trimipramine, but its utility is largely for treatment failure or resistance.37 These plasma levels can be altered based on coadministered medications (Table 538-46) and should be closely monitored. Physicians should obtain a trough level after at least 5 half-lives and before the next dose is due, and use TDM as indicated to optimize dosing.

Continue to: CASE CONTINUED
CASE CONTINUED
Ms. B’s outpatient psychiatrist provides collateral information about her medical history and confirms her long-standing MDD with multiple medication trials, though she has never received an MAOI or TCA. Ms. B is adamant she does not want a medication-free period between treatments and refuses to adjust her diet, despite being educated on the few changes necessary. She has no contraindications for TCAs and may benefit from a TCA for her comorbid migraines. The care team expresses concern for TCA overdose to Ms. B and her family. Ms. B’s sister reassures the team they will have someone monitor and dispense her medications at home. They decide to discontinue her current psychiatric regimen, and Ms. B is started on nortriptyline 50 mg/d at night, with plans to titrate based on tolerability.
Related Resources
- Meyer JM. A concise guide to monoamine oxidase inhibitors. Current Psychiatry. 2017;16(12):14-16,18-23,47,A.
- Espejo GD. Treating major depressive disorder after limited response to an initial agent. Current Psychiatry. 2021;20(10):51-53. doi:10.12788/cp.0178
- American Association of Psychiatric Pharmacists (AAPP) MAOI Pharmacist Toolkit. https://aapp.org/guideline/maoi
Drug Brand Names
Amitriptyline • Elavil
Amphetamine • Adzenys, Dyanavel
Aripiprazole • Abilify
Clomipramine • Anafranil
Desipramine • Norpramin
Dextromethorphan/bupropion • Auvelity
Doxepin • Sinequan, Adapin
Esketamine • Spravato
Fluoxetine • Prozac
Imipramine • Tofranil
Isocarboxazid • Marplan
Methamphetamine • Desoxyn
Mirtazapine • Remeron
Modafinil • Provigil
Nortriptyline • Pamelor
Phenelzine • Nardil
Protriptyline • Vivactil
Selegiline • Emsam
Tranylcypromine • Parnate
Trimipramine • Surmontil
Vortioxetine • Trintellix
1. Gaynes BN, Lux L, Gartlehner G, et al. Defining treatment-resistant depression. Depress Anxiety. 2020;37(2):134-145. doi:10.1002/da.22968
2. Kennedy SH, Lam RW, McIntyre RS, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: section 3. Pharmacological treatments. Can J Psychiatry. 2016;61(9):540-560. doi:10.1177/0706743716659417
3. VA/DoD clinical practice guideline for the management of major depressive disorder. Veterans Health Administration and Department of Defense; 2016. https://www.healthquality.va.gov/guidelines/MH/mdd/VADoDMDDCPGFINAL82916.pdf
4. Gelenberg AJ, Freeman MP, Markowitz JC, et al. Practice guideline for the treatment of patients with major depressive disorder. Am J Psychiatry. 2010;167(Suppl 10):9-118.
5. Meyer JM. A concise guide to monoamine oxidase inhibitors. Current Psychiatry. 2017;16(12):14-16,18-23,47,A.
6. Taylor D. Selective serotonin reuptake inhibitors and tricyclic antidepressants in combination. Interactions and therapeutic uses. Br J Psychiatry. 1995;167(5):575-580. doi:10.1192/bjp.167.5.575
7. Thase ME, Trivedi MH, Rush AJ. MAOIs in the contemporary treatment of depression. Neuropsychopharmacology. 1995;12(3):185-219. doi:10.1016/0893-133X(94)00058-8
8. Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905-1917. doi:10.1176/ajp.2006.163.11.1905
9. McGrath PJ, Stewart JW, Fava M, et al. Tranylcypromine versus venlafaxine plus mirtazapine following three failed antidepressant medication trials for depression: a STAR*D report. Am J Psychiatry. 2006;163(9):1531-1666. doi:10.1176/ajp.2006.163.9.1531
10. Kim T, Xu C, Amsterdam JD. Relative effectiveness of tricyclic antidepressant versus monoamine oxidase inhibitor monotherapy for treatment-resistant depression. J Affect Disord. 2019;250:199-203. doi:10.1016/j.jad.2019.03.028
11. Suchting R, Tirumalajaru V, Gareeb R, et al. Revisiting monoamine oxidase inhibitors for the treatment of depressive disorders: a systematic review and network meta-analysis. J Affect Disord. 2021;282:1153-1160. doi:10.1016/j.jad.2021.01.021
12. Stahl SM, Felker A. Monoamine oxidase inhibitors: a modern guide to an unrequited class of antidepressants. CNS Spectr. 2008;13(10):855-870. doi:10.1017/s1092852900016965
13. Chamberlain SR, Baldwin DS. Monoamine oxidase inhibitors (MAOIs) in psychiatric practice: how to use them safely and effectively. CNS Drugs. 2021;35(7):703-716. doi:10.1007/s40263-021-00832-x
14. Nardil [package insert]. New York, NY: Parke-Davis; 2009.
15. Marplan [package insert]. Parsippany, NJ: Validus Pharmaceuticals LLC; 2020.
16. Parnate [package insert]. Saint Michael, Barbados: Concordia Pharmaceuticals; 2015.
17. Emsam [package insert]. Morgantown, WV: Mylan Specialty LP; 2014.
18. Shulman KI, Herrmann N, Walker SE. Current place of monoamine oxidase inhibitors in the treatment of depression. CNS Drugs. 2013;27(10):789-797. doi:10.1007/s40263-013-0097-3
19. Sub Laban T, Saadabadi A. Monoamine oxidase inhibitors (MAOI). StatPearls Publishing; 2023. https://www.ncbi.nlm.nih.gov/books/NBK539848/
20. Rabkin JG, Quitkin FM, McGrath P, et al. Adverse reactions to monoamine oxidase inhibitors. Part II. Treatment correlates and clinical management. J Clin Psychopharmacol. 1985;5(1):2-9.
21. Gillman PK. Advances pertaining to the pharmacology and interactions of irreversible nonselective monoamine oxidase inhibitors. J Clin Psychopharmacol. 2011;31(1):66-74. doi:10.1097/JCP.0b013e31820469ea
22. Sidhu G, Marwaha R. Phenelzine. StatPearls Publishing; 2023. https://www.ncbi.nlm.nih.gov/books/NBK554508/
23. Frieling H, Bleich S. Tranylcypromine: new perspectives on an “old” drug. Eur Arch Psychiatry Clin Neurosci. 2006;256(5):268-273. doi:10.1007/s00406-006-0660-8
24. Goodnick PJ. Seligiline transdermal system in depression. Expert Opin Pharmacother. 2007;8(1):59-64. doi:10.1517/14656566.8.1.59
25. Edinoff AN, Swinford CR, Odisho AS, et al. Clinically relevant drug interactions with monoamine oxidase inhibitors. Health Psychol Res. 2022;10(4):39576. doi:10.52965/001c.39576
26. Gillman PK. A reassessment of the safety profile of monoamine oxidase inhibitors: elucidating tired old tyramine myths. J Neural Transm (Vienna). 2018;125(11):1707-1717. doi:10.1007/s00702-018-1932-y
27. Flockhart DA. Dietary restrictions and drug interactions with monoamine oxidase inhibitors: an update. J Clin Psychiatry. 2012;73 Suppl 1:17-24. doi:10.4088/JCP.11096su1c.03
28. McCabe-Sellers BJ, Staggs CG, Bogle ML. Tyramine in foods and monoamine oxidase inhibitor drugs: a crossroad where medicine, nutrition, pharmacy, and food industry converge. J Food Composit Anal. 2006;19:S58-S65. doi:10.1016/j.jfca.2005.12.008
29. Keck PE Jr, Vuckovic A, Pope HG Jr, et al. Acute cardiovascular response to monoamine oxidase inhibitors: a prospective assessment. J Clin Psychopharmacol. 1989;9(3):203-206.
30. Bodkin JA, Dunlop BW. Moving on with monoamine oxidase inhibitors. Focus (Am Psychiatr Publ). 2021;19(1):50-52. doi:10.1176/appi.focus.20200046
31. Amsterdam JD, Kim TT. Relative effectiveness of monoamine oxidase inhibitor and tricyclic antidepressant combination therapy for treatment-resistant depression. J Clin Psychopharmacol. 2019;39(6):649-652. doi:10.1097/JCP.0000000000001130
32. Keks N, Hope J, Keogh S. Switching and stopping antidepressants. Aust Prescr. 2016;39(3):76-83. doi:10.18773/austprescr.2016.039
33. Israel JA. Combining stimulants and monoamine oxidase inhibitors: a reexamination of the literature and a report of a new treatment combination. Prim Care Companion CNS Disord. 2015;17(6):10.4088/PCC.15br01836. doi:10.4088/PCC.15br01836
34. Clemons WE, Makela E, Young J. Concomitant use of modafinil and tranylcypromine in a patient with narcolepsy: a case report. Sleep Med. 2004;5(5):509-511. doi:10.1016/j.sleep.2004.06.006
35. Ashton AK. Modafinil augmentation of phenelzine for residual fatigue in dysthymia. Am J Psychiatry. 2004;161(9):1716-1717. doi:10.1176/appi.ajp.161.9.1716-a
36. O’Donnell JM, Bies RR, Shelton RC. Drug therapy of depression and anxiety disorders. In: Brunton LL, Hilal-Dandan R, Knollmann BC, eds. Goodman & Gilman’s: The Pharmacological Basis of Therapeutics. 13th ed. McGraw Hill; 2017. Accessed June 4, 2023. https://accessanesthesiology.mhmedical.com/content.aspx?bookid=2189§ionid=169518711
37. Hiemke C, Bergemann N, Clement HW, et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry. 2018;51(1-02):9-62. doi:10.1055/s-0043-116492
38. Amitriptyline hydrochloride [package insert]. East Brunswick, NJ: Unichem Pharmaceuticals (USA); 2021.
39. Clomipramine hydrochloride [package insert]. East Windsor, NJ: Aurobindo Pharma Limited; 2023.
40. Doxepin hydrochloride capsules, USP [package insert]. Bedminster, NJ: Alembic Pharmaceuticals Inc; 2021.
41. Imipramine hydrochloride tablet [package insert]. Fairfield, NJ: Leading Pharma LLC USA; 2022.
42. Trimipramine maleate [package insert]. Northvale, NJ: Elite Laboratories Inc; 2021.
43. Amoxapine [package insert]. Parsippany, NJ: Actavis Pharma Inc; 2015.
44. Desipramine hydrochloride tablets [package insert]. Bedminster, NJ: Alembic Pharmaceuticals Inc; 2023.
45. Nortriptyline hydrochloride capsules, USP [package insert]. Parsippany, NJ: Teva Pharmaceuticals Inc; 2021.
46. Protriptyline hydrochloride [package insert]. Bensalem, PA: Sigmapharm Laboratories, LLC; 2023.
47. Calvo B, García MJ, Pedraz JL, et al. Pharmacokinetics of amoxapine and its active metabolites. Int J Clin Pharmacol Ther Toxicol. 1985;23(4):180-185.
48. Ziegler VE, Biggs JT, Wylie LT, et al. Protriptyline kinetics. Clin Pharmacol Ther. 1978;23(5):580-584. doi:10.1002/cpt1978235580
49. Cleare A, Pariante CM, Young AH, et al. Evidence-based guidelines for treating depressive disorders with antidepressants: a revision of the 2008 British Association for Psychopharmacology guidelines. J Psychopharmacol. 2015;29(5):459-525. doi:10.1177/0269881115581093
50. Richelson E. Synaptic effects of antidepressants. J Clin Psychopharmacol. 1996;16(3 Suppl 2):1S-9S. doi:10.1097/00004714-199606002-00001
51. Vos CF, Aarnoutse RE, Op de Coul MJM, et al. Tricyclic antidepressants for major depressive disorder: a comprehensive evaluation of current practice in the Netherlands. BMC Psychiatry. 2021;21(1):481. doi:10.1186/s12888-021-03490-x
52. Alvarez W Jr, Pickworth KK. Safety of antidepressant drugs in the patient with cardiac disease: a review of the literature. Pharmacotherapy. 2003;23(6):754-771. doi:10.1592/phco.23.6.754.32185
53. Dietch JT, Fine M. The effect of nortriptyline in elderly patients with cardiac conduction disease. J Clin Psychiatry. 1990;51(2):65-67.
54. Valento M, Liebelt EL. Cyclic antidepressants. In: Nelson LS, Howland M, Lewin NA, et al, eds. Goldfrank’s Toxicologic Emergencies. 9th ed. McGraw Hill; 2011. Accessed June 10, 2023. https://accesspharmacy.mhmedical.com/content.aspx?bookid=2569§ionid=210274664
55. Woolf AD, Erdman AR, Nelson LS, et al. Tricyclic antidepressant poisoning: an evidence-based consensus guideline for out-of-hospital management. Clin Toxicol (Phila). 2007;45(3):203-233. doi:10.1080/15563650701226192
56. Baldwin DS, Anderson IM, Nutt DJ, et al. Evidence-based pharmacological treatment of anxiety disorders, post-traumatic stress disorder and obsessive-compulsive disorder: a revision of the 2005 guidelines from the British Association for Psychopharmacology. J Psychopharmacol. 2014;28(5):403-439. doi:10.1177/0269881114525674
57. Moulin DE, Clark AJ, Gilron I, et al. Pharmacological management of chronic neuropathic pain - consensus statement and guidelines from the Canadian Pain Society. Pain Res Manag. 2007;12(1):13-21. doi:10.1155/2007/730785
58. Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14(2):162-173. doi:10.1016/S1474-4422(14)70251-0
59. Saarto T, Wiffen PJ. Antidepressants for neuropathic pain. Cochrane Database Syst Rev. 2007;(4):CD005454. doi:10.1002/14651858.CD005454.pub2
60. Burch R. Antidepressants for preventive treatment of migraine. Curr Treat Options Neurol. 2019;21(4):18. doi:10.1007/s11940-019-0557-2
61. Ailani J, Burch RC, Robbins MS; Board of Directors of the American Headache Society. The American Headache Society consensus statement: update on integrating new migraine treatments into clinical practice. Headache. 2021;61(7):1021-1039. doi:10.1111/head.14153
62. Bauer M, Pfennig A, Severus E, et al. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of unipolar depressive disorders, part 1: update 2013 on the acute and continuation treatment of unipolar depressive disorders. World J Biol Psychiatry. 2013;14(5):334-385. doi:10.3109/15622975.2013.804195
63. Jelovac A, Kolshus E, McLoughlin DM. Relapse following successful electroconvulsive therapy for major depression: a meta-analysis. Neuropsychopharmacology. 2013;38(12):2467-2474. doi:10.1038/npp.2013.149
64. Spencer T, Biederman J, Coffey B, et al. A double-blind comparison of desipramine and placebo in children and adolescents with chronic tic disorder and comorbid attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2002;59(7):649-656. doi:10.1001/archpsyc.59.7.649
65. Spencer T, Biederman J, Wilens T, et al. Pharmacotherapy of attention-deficit hyperactivity disorder across the life cycle. J Am Acad Child Adolesc Psychiatry. 1996;35(4):409-432. doi:10.1097/00004583-199604000-00008
66. Wilens TE, Biederman J, Prince J, et al. Six-week, double-blind, placebo-controlled study of desipramine for adult attention deficit hyperactivity disorder. Am J Psychiatry. 1996;153(9):1147-1153. doi:10.1176/ajp.153.9.1147
1. Gaynes BN, Lux L, Gartlehner G, et al. Defining treatment-resistant depression. Depress Anxiety. 2020;37(2):134-145. doi:10.1002/da.22968
2. Kennedy SH, Lam RW, McIntyre RS, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: section 3. Pharmacological treatments. Can J Psychiatry. 2016;61(9):540-560. doi:10.1177/0706743716659417
3. VA/DoD clinical practice guideline for the management of major depressive disorder. Veterans Health Administration and Department of Defense; 2016. https://www.healthquality.va.gov/guidelines/MH/mdd/VADoDMDDCPGFINAL82916.pdf
4. Gelenberg AJ, Freeman MP, Markowitz JC, et al. Practice guideline for the treatment of patients with major depressive disorder. Am J Psychiatry. 2010;167(Suppl 10):9-118.
5. Meyer JM. A concise guide to monoamine oxidase inhibitors. Current Psychiatry. 2017;16(12):14-16,18-23,47,A.
6. Taylor D. Selective serotonin reuptake inhibitors and tricyclic antidepressants in combination. Interactions and therapeutic uses. Br J Psychiatry. 1995;167(5):575-580. doi:10.1192/bjp.167.5.575
7. Thase ME, Trivedi MH, Rush AJ. MAOIs in the contemporary treatment of depression. Neuropsychopharmacology. 1995;12(3):185-219. doi:10.1016/0893-133X(94)00058-8
8. Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905-1917. doi:10.1176/ajp.2006.163.11.1905
9. McGrath PJ, Stewart JW, Fava M, et al. Tranylcypromine versus venlafaxine plus mirtazapine following three failed antidepressant medication trials for depression: a STAR*D report. Am J Psychiatry. 2006;163(9):1531-1666. doi:10.1176/ajp.2006.163.9.1531
10. Kim T, Xu C, Amsterdam JD. Relative effectiveness of tricyclic antidepressant versus monoamine oxidase inhibitor monotherapy for treatment-resistant depression. J Affect Disord. 2019;250:199-203. doi:10.1016/j.jad.2019.03.028
11. Suchting R, Tirumalajaru V, Gareeb R, et al. Revisiting monoamine oxidase inhibitors for the treatment of depressive disorders: a systematic review and network meta-analysis. J Affect Disord. 2021;282:1153-1160. doi:10.1016/j.jad.2021.01.021
12. Stahl SM, Felker A. Monoamine oxidase inhibitors: a modern guide to an unrequited class of antidepressants. CNS Spectr. 2008;13(10):855-870. doi:10.1017/s1092852900016965
13. Chamberlain SR, Baldwin DS. Monoamine oxidase inhibitors (MAOIs) in psychiatric practice: how to use them safely and effectively. CNS Drugs. 2021;35(7):703-716. doi:10.1007/s40263-021-00832-x
14. Nardil [package insert]. New York, NY: Parke-Davis; 2009.
15. Marplan [package insert]. Parsippany, NJ: Validus Pharmaceuticals LLC; 2020.
16. Parnate [package insert]. Saint Michael, Barbados: Concordia Pharmaceuticals; 2015.
17. Emsam [package insert]. Morgantown, WV: Mylan Specialty LP; 2014.
18. Shulman KI, Herrmann N, Walker SE. Current place of monoamine oxidase inhibitors in the treatment of depression. CNS Drugs. 2013;27(10):789-797. doi:10.1007/s40263-013-0097-3
19. Sub Laban T, Saadabadi A. Monoamine oxidase inhibitors (MAOI). StatPearls Publishing; 2023. https://www.ncbi.nlm.nih.gov/books/NBK539848/
20. Rabkin JG, Quitkin FM, McGrath P, et al. Adverse reactions to monoamine oxidase inhibitors. Part II. Treatment correlates and clinical management. J Clin Psychopharmacol. 1985;5(1):2-9.
21. Gillman PK. Advances pertaining to the pharmacology and interactions of irreversible nonselective monoamine oxidase inhibitors. J Clin Psychopharmacol. 2011;31(1):66-74. doi:10.1097/JCP.0b013e31820469ea
22. Sidhu G, Marwaha R. Phenelzine. StatPearls Publishing; 2023. https://www.ncbi.nlm.nih.gov/books/NBK554508/
23. Frieling H, Bleich S. Tranylcypromine: new perspectives on an “old” drug. Eur Arch Psychiatry Clin Neurosci. 2006;256(5):268-273. doi:10.1007/s00406-006-0660-8
24. Goodnick PJ. Seligiline transdermal system in depression. Expert Opin Pharmacother. 2007;8(1):59-64. doi:10.1517/14656566.8.1.59
25. Edinoff AN, Swinford CR, Odisho AS, et al. Clinically relevant drug interactions with monoamine oxidase inhibitors. Health Psychol Res. 2022;10(4):39576. doi:10.52965/001c.39576
26. Gillman PK. A reassessment of the safety profile of monoamine oxidase inhibitors: elucidating tired old tyramine myths. J Neural Transm (Vienna). 2018;125(11):1707-1717. doi:10.1007/s00702-018-1932-y
27. Flockhart DA. Dietary restrictions and drug interactions with monoamine oxidase inhibitors: an update. J Clin Psychiatry. 2012;73 Suppl 1:17-24. doi:10.4088/JCP.11096su1c.03
28. McCabe-Sellers BJ, Staggs CG, Bogle ML. Tyramine in foods and monoamine oxidase inhibitor drugs: a crossroad where medicine, nutrition, pharmacy, and food industry converge. J Food Composit Anal. 2006;19:S58-S65. doi:10.1016/j.jfca.2005.12.008
29. Keck PE Jr, Vuckovic A, Pope HG Jr, et al. Acute cardiovascular response to monoamine oxidase inhibitors: a prospective assessment. J Clin Psychopharmacol. 1989;9(3):203-206.
30. Bodkin JA, Dunlop BW. Moving on with monoamine oxidase inhibitors. Focus (Am Psychiatr Publ). 2021;19(1):50-52. doi:10.1176/appi.focus.20200046
31. Amsterdam JD, Kim TT. Relative effectiveness of monoamine oxidase inhibitor and tricyclic antidepressant combination therapy for treatment-resistant depression. J Clin Psychopharmacol. 2019;39(6):649-652. doi:10.1097/JCP.0000000000001130
32. Keks N, Hope J, Keogh S. Switching and stopping antidepressants. Aust Prescr. 2016;39(3):76-83. doi:10.18773/austprescr.2016.039
33. Israel JA. Combining stimulants and monoamine oxidase inhibitors: a reexamination of the literature and a report of a new treatment combination. Prim Care Companion CNS Disord. 2015;17(6):10.4088/PCC.15br01836. doi:10.4088/PCC.15br01836
34. Clemons WE, Makela E, Young J. Concomitant use of modafinil and tranylcypromine in a patient with narcolepsy: a case report. Sleep Med. 2004;5(5):509-511. doi:10.1016/j.sleep.2004.06.006
35. Ashton AK. Modafinil augmentation of phenelzine for residual fatigue in dysthymia. Am J Psychiatry. 2004;161(9):1716-1717. doi:10.1176/appi.ajp.161.9.1716-a
36. O’Donnell JM, Bies RR, Shelton RC. Drug therapy of depression and anxiety disorders. In: Brunton LL, Hilal-Dandan R, Knollmann BC, eds. Goodman & Gilman’s: The Pharmacological Basis of Therapeutics. 13th ed. McGraw Hill; 2017. Accessed June 4, 2023. https://accessanesthesiology.mhmedical.com/content.aspx?bookid=2189§ionid=169518711
37. Hiemke C, Bergemann N, Clement HW, et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry. 2018;51(1-02):9-62. doi:10.1055/s-0043-116492
38. Amitriptyline hydrochloride [package insert]. East Brunswick, NJ: Unichem Pharmaceuticals (USA); 2021.
39. Clomipramine hydrochloride [package insert]. East Windsor, NJ: Aurobindo Pharma Limited; 2023.
40. Doxepin hydrochloride capsules, USP [package insert]. Bedminster, NJ: Alembic Pharmaceuticals Inc; 2021.
41. Imipramine hydrochloride tablet [package insert]. Fairfield, NJ: Leading Pharma LLC USA; 2022.
42. Trimipramine maleate [package insert]. Northvale, NJ: Elite Laboratories Inc; 2021.
43. Amoxapine [package insert]. Parsippany, NJ: Actavis Pharma Inc; 2015.
44. Desipramine hydrochloride tablets [package insert]. Bedminster, NJ: Alembic Pharmaceuticals Inc; 2023.
45. Nortriptyline hydrochloride capsules, USP [package insert]. Parsippany, NJ: Teva Pharmaceuticals Inc; 2021.
46. Protriptyline hydrochloride [package insert]. Bensalem, PA: Sigmapharm Laboratories, LLC; 2023.
47. Calvo B, García MJ, Pedraz JL, et al. Pharmacokinetics of amoxapine and its active metabolites. Int J Clin Pharmacol Ther Toxicol. 1985;23(4):180-185.
48. Ziegler VE, Biggs JT, Wylie LT, et al. Protriptyline kinetics. Clin Pharmacol Ther. 1978;23(5):580-584. doi:10.1002/cpt1978235580
49. Cleare A, Pariante CM, Young AH, et al. Evidence-based guidelines for treating depressive disorders with antidepressants: a revision of the 2008 British Association for Psychopharmacology guidelines. J Psychopharmacol. 2015;29(5):459-525. doi:10.1177/0269881115581093
50. Richelson E. Synaptic effects of antidepressants. J Clin Psychopharmacol. 1996;16(3 Suppl 2):1S-9S. doi:10.1097/00004714-199606002-00001
51. Vos CF, Aarnoutse RE, Op de Coul MJM, et al. Tricyclic antidepressants for major depressive disorder: a comprehensive evaluation of current practice in the Netherlands. BMC Psychiatry. 2021;21(1):481. doi:10.1186/s12888-021-03490-x
52. Alvarez W Jr, Pickworth KK. Safety of antidepressant drugs in the patient with cardiac disease: a review of the literature. Pharmacotherapy. 2003;23(6):754-771. doi:10.1592/phco.23.6.754.32185
53. Dietch JT, Fine M. The effect of nortriptyline in elderly patients with cardiac conduction disease. J Clin Psychiatry. 1990;51(2):65-67.
54. Valento M, Liebelt EL. Cyclic antidepressants. In: Nelson LS, Howland M, Lewin NA, et al, eds. Goldfrank’s Toxicologic Emergencies. 9th ed. McGraw Hill; 2011. Accessed June 10, 2023. https://accesspharmacy.mhmedical.com/content.aspx?bookid=2569§ionid=210274664
55. Woolf AD, Erdman AR, Nelson LS, et al. Tricyclic antidepressant poisoning: an evidence-based consensus guideline for out-of-hospital management. Clin Toxicol (Phila). 2007;45(3):203-233. doi:10.1080/15563650701226192
56. Baldwin DS, Anderson IM, Nutt DJ, et al. Evidence-based pharmacological treatment of anxiety disorders, post-traumatic stress disorder and obsessive-compulsive disorder: a revision of the 2005 guidelines from the British Association for Psychopharmacology. J Psychopharmacol. 2014;28(5):403-439. doi:10.1177/0269881114525674
57. Moulin DE, Clark AJ, Gilron I, et al. Pharmacological management of chronic neuropathic pain - consensus statement and guidelines from the Canadian Pain Society. Pain Res Manag. 2007;12(1):13-21. doi:10.1155/2007/730785
58. Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14(2):162-173. doi:10.1016/S1474-4422(14)70251-0
59. Saarto T, Wiffen PJ. Antidepressants for neuropathic pain. Cochrane Database Syst Rev. 2007;(4):CD005454. doi:10.1002/14651858.CD005454.pub2
60. Burch R. Antidepressants for preventive treatment of migraine. Curr Treat Options Neurol. 2019;21(4):18. doi:10.1007/s11940-019-0557-2
61. Ailani J, Burch RC, Robbins MS; Board of Directors of the American Headache Society. The American Headache Society consensus statement: update on integrating new migraine treatments into clinical practice. Headache. 2021;61(7):1021-1039. doi:10.1111/head.14153
62. Bauer M, Pfennig A, Severus E, et al. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of unipolar depressive disorders, part 1: update 2013 on the acute and continuation treatment of unipolar depressive disorders. World J Biol Psychiatry. 2013;14(5):334-385. doi:10.3109/15622975.2013.804195
63. Jelovac A, Kolshus E, McLoughlin DM. Relapse following successful electroconvulsive therapy for major depression: a meta-analysis. Neuropsychopharmacology. 2013;38(12):2467-2474. doi:10.1038/npp.2013.149
64. Spencer T, Biederman J, Coffey B, et al. A double-blind comparison of desipramine and placebo in children and adolescents with chronic tic disorder and comorbid attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2002;59(7):649-656. doi:10.1001/archpsyc.59.7.649
65. Spencer T, Biederman J, Wilens T, et al. Pharmacotherapy of attention-deficit hyperactivity disorder across the life cycle. J Am Acad Child Adolesc Psychiatry. 1996;35(4):409-432. doi:10.1097/00004583-199604000-00008
66. Wilens TE, Biederman J, Prince J, et al. Six-week, double-blind, placebo-controlled study of desipramine for adult attention deficit hyperactivity disorder. Am J Psychiatry. 1996;153(9):1147-1153. doi:10.1176/ajp.153.9.1147