User login
Lamotrigine interactions with oral contraceptives
Ms. A, age 20, presents to the clinic after experiencing difficulty sleeping, depressed mood, fatigue, and difficulty concentrating. Her psychiatric history includes bipolar II disorder (BD II), predominantly with depressive episodes. Ms. A’s current medications include a combination of lamotrigine 200 mg/d and bupropion extended-release 450 mg/d, and her symptoms were well maintained until 2 weeks ago. When her psychiatrist performs a medication reconciliation at her medication management appointment, Ms. A indicates she started taking an oral contraceptive, ethinyl estradiol and norgestimate, approximately 1 month ago for management of endometriosis symptoms. She is not currently taking any other medications or supplements.
Lamotrigine is indicated for epilepsy and as maintenance treatment for BD I. It is also used off-label to treat other mood disorders. After oral administration, lamotrigine is rapidly and fully absorbed with a high bioavailability (98%).The principal metabolic pathway is via glucuronic acid conjugation, leading to the major inactive metabolite 2-N-glucuronide. Minor metabolites include 5-N-glucuronide and a 2-N-glucuronide metabolite.1
Combined oral contraceptives contain an estrogen component, typically ethinyl estradiol, and a progestin component, which varies based on the specific formulation. The metabolism of ethinyl estradiol occurs through cytochrome P450 (CYP)3A4, CYP2C9, sulfation, and glucuronidation. For progestin—the second component of combined oral contraceptives and the lone component of progestin-only oral contraceptives—metabolism occurs via CYP3A4 and conjugation reactions.2 This article focuses on lamotrigine interactions specifically with oral contraceptives, but it is important to note that other formulations of combined hormonal contraceptives, such as the combined contraceptive patch (Ortho Evra) and vaginal ring (NuvaRing), would be expected to interact in the same way as oral formulations.3
Bidirectional interaction
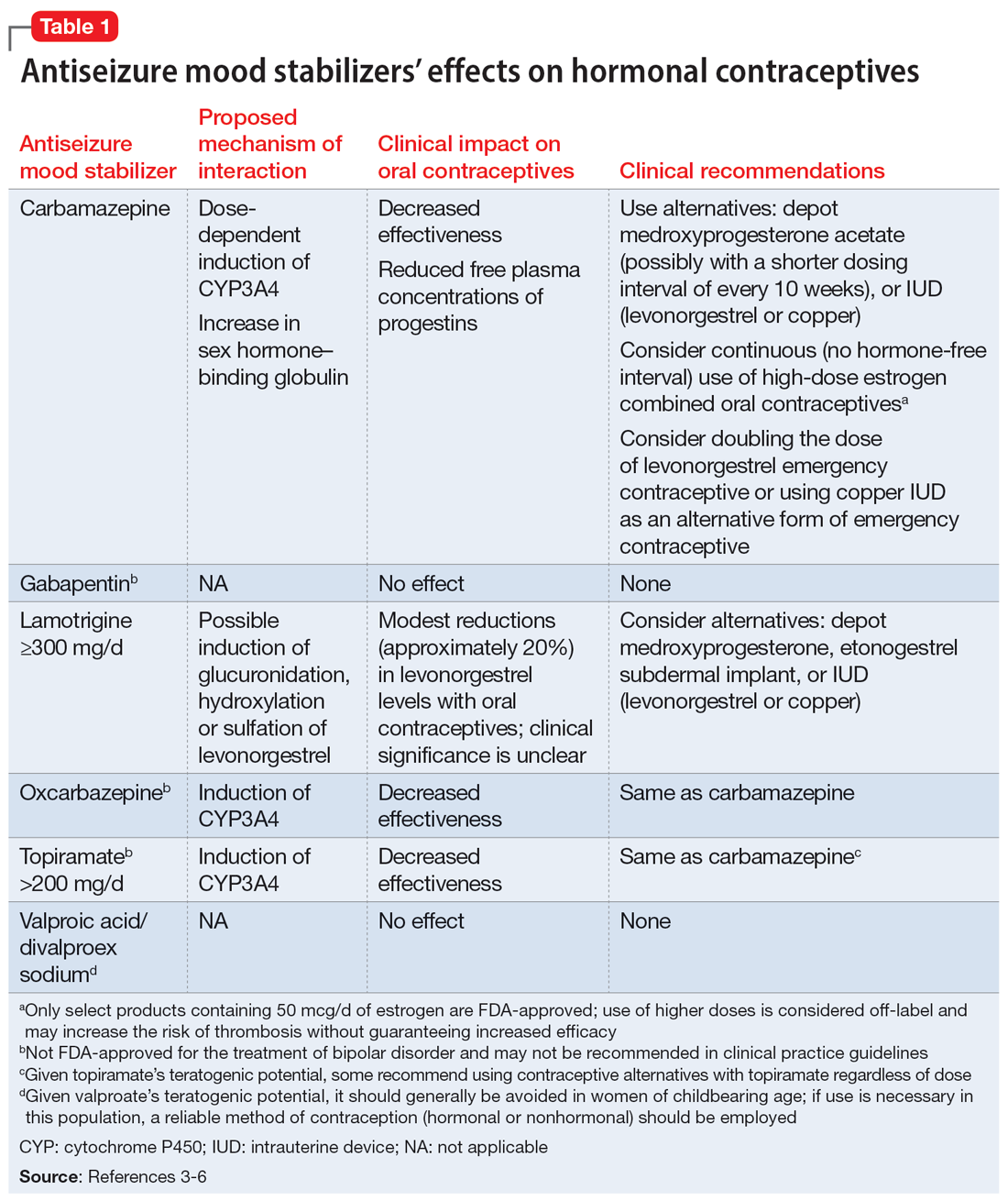
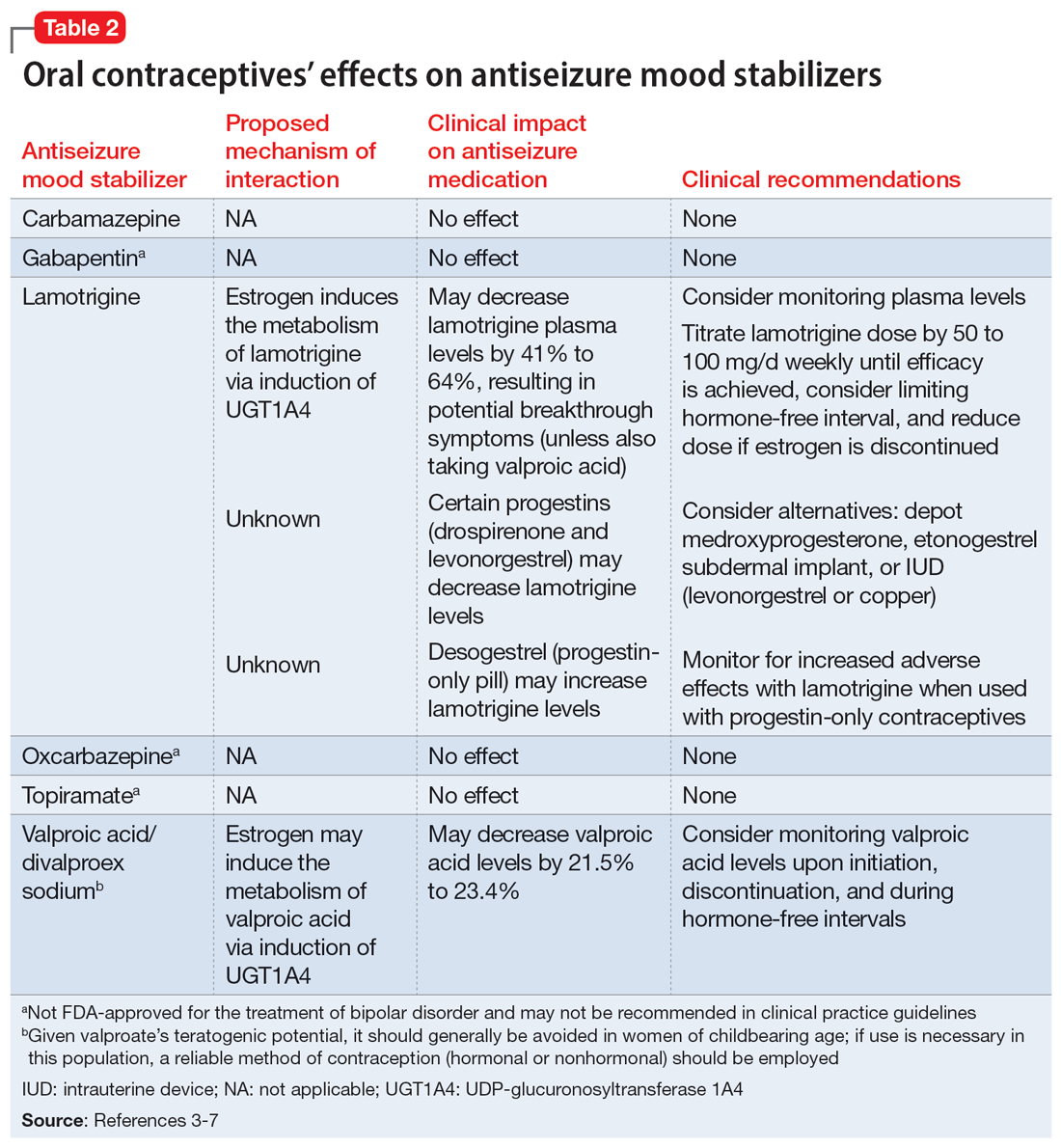
While many antiseizure medications are known to interact with and potentially decrease the efficacy of oral contraceptives (Table 13-6), the interactions between lamotrigine and oral contraceptives is uniquely bidirectional. Combined oral contraceptives are thought to interact with lamotrigine primarily via the estrogen component, which causes increased metabolism of lamotrigine through induction of glucuronidation. This drug interaction decreases the plasma concentrations of lamotrigine in the body by up to 2-fold, resulting in an increased risk of seizures or inadequate mood stabilization.1 This effect on metabolism is very rapid, resulting in decreases in lamotrigine concentrations within 1 week.4,7 A recent study suggested that certain progestins may also contribute to decreased plasma levels of lamotrigine, but the mechanism for this is unknown (Table 23-7).8
Clinicians should consider increasing the lamotrigine dose (potentially as much as 2-fold) in a patient who initiates treatment with a combined hormonal contraceptive. Dose increases should not be >50 to 100 mg/d every week.1 Collect lamotrigine blood levels before starting a hormonal contraceptive and during dose titration. While there is not a well-established therapeutic range for lamotrigine in BD, expert consensus recommends a range of 1 to 6 mcg/mL.8
The lamotrigine dose should be decreased if combined hormonal contraceptives are discontinued. Dose decreases should not exceed 25% of the total daily dose per week.1 Desogestrel, a progestin-only medication, may increase exposure to lamotrigine, but this has not been observed in research with other progestins.5,9 When starting a progestin-only pill, monitor patients for signs of lamotrigine toxicity (ataxia, diplopia, dizziness) and consider monitoring their blood levels.
An important consideration to note with combined oral contraceptives is the hormone-free interval, also known as the pill-free week. Due to the rapid effect of estrogens, the lamotrigine concentrations have been shown to rise, even double, during this hormone-free interval, so patients should be closely monitored for adverse effects.3 Some recommend use of an extended cycle regimen (with a limited hormone-free interval), or continuous cycle regimen (with no hormone-free interval) to avoid fluctuations in lamotrigine levels.3,5 Additionally, data suggest that in patients taking lamotrigine and valproate, which inhibits glucuronidation, oral contraceptives do not cause reductions in lamotrigine concentrations.2,5 In these instances, dose increases of lamotrigine are not needed.
Continue to: The metabolism of ethinyl estradiol...
The metabolism of ethinyl estradiol and progestin are susceptible to CYP3A4 induction and increased glucuronidation. Serum concentrations may be reduced by ≥50% when used concomitantly with CYP enzyme–inducing medications, which could possibly result in subtherapeutic levels and unplanned pregnancy.3 CYP3A4 induction occurs for up to 4 weeks after discontinuation of an enzyme-inducing agent, pointing to the need for alternative or backup contraception during this time.3 Lamotrigine is not a CYP enzyme–inducing medication; it is unlikely to affect the efficacy of oral contraceptives in the same manner as other antiseizure medications. However, a study of lamotrigine and the combined hormonal contraceptive ethinyl estradiol and levonorgestrel demonstrated reduced exposure to levonorgestrel, resulting in breakthrough bleeding.5
In a study on the coadministration of lamotrigine and combined oral contraceptives, Sidhu et al4 observed a small mean reduction (20%) in progestin concentrations when lamotrigine was used at a dose of 300 mg/d. Although there is no research suggesting decreased effectiveness in preventing pregnancy when lamotrigine is used with combined oral contraceptives, progestin-only oral contraceptives, or progestin implants, additional or alternative contraceptive methods may be considered based on this pharmacokinetic data, particularly in patients who require lamotrigine doses ≥300 mg/d.5
CASE CONTINUED
Given when Ms. A started the oral contraceptive, the treatment team determines it is likely that an interaction with lamotrigine is causing her resurgence of depressive symptoms. Her care team decides to titrate the lamotrigine gradually to 300 mg/d, then 400 mg/d if needed, while carefully monitoring for signs of a serious rash. This dosage increase may help Ms. A achieve symptom remission. Monitoring plasma levels may be considered, although it is unknown what plasma level was effective for Ms. A before she started the oral contraceptive. Ms. A would need to be counseled regarding the effect of higher doses of lamotrigine on the effectiveness of the oral contraceptive.
Although it does not appear Ms. A is using the oral contraceptive specifically to prevent pregnancy, the team informs her about the possibility of unintended pregnancy with this medication combination. If Ms. A was also using the medication for this indication, alternative contraceptive options would include medroxyprogesterone acetate, levonorgestrel implants, or an intrauterine device (levonorgestrel or copper, though copper would not be effective for endometriosis symptom management). Ms. A should consult with her gynecologist regarding the most appropriate option for her endometriosis. If the decision is made to discontinue her oral contraceptive in the future, the lamotrigine dose should be decreased to her previously effective dose of 200 mg/d.
Related Resources
- Makino KK, Hatters Friedman S, Amin J. Emergency contraception for psychiatric patients. Current Psychiatry. 2022;21(11):34-39,44-45. doi:10.12788/cp.0300
- MGH Center for Women’s Mental Health. You asked: is there an interaction between lamotrigine and oral contraceptives? September 29, 2015. https://womensmentalhealth.org/posts/you-asked-is-there-an-interaction-between-lamotrigine-andoral-contraceptives/
Drug Brand Names
Bupropion extended-release • Wellbutrin XL
Carbamazepine • Equetro, Tegretol
Desogestrel • Cerazette
Divalproex sodium • Depakote
Ethinyl estradiol and etonogestrel • NuvaRing
Ethinyl estradiol and norelgestromin • Ortho Evra
Ethinyl estradiol and norgestimate • Ortho Tri-Cyclen, TriNessa, others
Etonogestrel • Implanon, Nexplanon
Gabapentin • Neurontin
Lamotrigine • Lamictal
Levonorgestrel emergency contraceptive pill • AfterPill, Plan B
Levonorgestrel intrauterine device • Mirena, Skyla
Medroxyprogesterone acetate • Depo-Provera
Oxcarbazepine • Trileptal
Topiramate • Topamax
Valproic acid • Depakene
1. Lamictal [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2020.
2. Lee CR. Drug interactions and hormonal contraception. Trends in Urology Gynaecology & Sexual Health. 2009;14(3):23-26.
3. Williams D. Antiepileptic drugs and contraception. US Pharm. 2014;39(1):39-42.
4. Sidhu J, Job S, Singh S, et al. The pharmacokinetic and pharmacodynamic consequences of the co-administration of lamotrigine and a combined oral contraceptive in healthy female subjects. Br J Clin Pharmacol. 2006;61(2):191-199. doi:10.1111/j.1365-2125.2005.02539.x
5. Faculty of Sexual & Reproductive Healthcare. Clinical guidance: drug interactions with hormonal contraception. Published May 9, 2022. Accessed September 28, 2022. https://www.fsrh.org/documents/ceu-clinical-guidance-drug-interactions-with-hormonal/
6. Johnston CA, Crawford PM. Anti-epileptic drugs and hormonal treatments. Curr Treat Options Neurol. 2014;16(5):288. doi:10.1007/s11940-014-0288-3
7. Christensen J, Petrenaite V, Atterman J, et al. Oral contraceptives induce lamotrigine metabolism: evidence from a double-blind, placebo-controlled trial. Epilepsia. 2007;48(3):484-489. doi:10.1111/j.1528-1167.2007.00997.x
8. Hiemke C, Bergemann N, Clement HW, et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry. 2018;51(1-02):9-62. doi:10.1055/s-0043-116492
9. Rauchenzauner M, Deichmann S, Pittschieler, et al. Bidirectional interaction between oral contraception and lamotrigine in women with epilepsy – role of progestins. Seizure. 2020;74:89-92. doi:10.1016/j.seizure.2019.11.011
Ms. A, age 20, presents to the clinic after experiencing difficulty sleeping, depressed mood, fatigue, and difficulty concentrating. Her psychiatric history includes bipolar II disorder (BD II), predominantly with depressive episodes. Ms. A’s current medications include a combination of lamotrigine 200 mg/d and bupropion extended-release 450 mg/d, and her symptoms were well maintained until 2 weeks ago. When her psychiatrist performs a medication reconciliation at her medication management appointment, Ms. A indicates she started taking an oral contraceptive, ethinyl estradiol and norgestimate, approximately 1 month ago for management of endometriosis symptoms. She is not currently taking any other medications or supplements.
Lamotrigine is indicated for epilepsy and as maintenance treatment for BD I. It is also used off-label to treat other mood disorders. After oral administration, lamotrigine is rapidly and fully absorbed with a high bioavailability (98%).The principal metabolic pathway is via glucuronic acid conjugation, leading to the major inactive metabolite 2-N-glucuronide. Minor metabolites include 5-N-glucuronide and a 2-N-glucuronide metabolite.1
Combined oral contraceptives contain an estrogen component, typically ethinyl estradiol, and a progestin component, which varies based on the specific formulation. The metabolism of ethinyl estradiol occurs through cytochrome P450 (CYP)3A4, CYP2C9, sulfation, and glucuronidation. For progestin—the second component of combined oral contraceptives and the lone component of progestin-only oral contraceptives—metabolism occurs via CYP3A4 and conjugation reactions.2 This article focuses on lamotrigine interactions specifically with oral contraceptives, but it is important to note that other formulations of combined hormonal contraceptives, such as the combined contraceptive patch (Ortho Evra) and vaginal ring (NuvaRing), would be expected to interact in the same way as oral formulations.3
Bidirectional interaction
While many antiseizure medications are known to interact with and potentially decrease the efficacy of oral contraceptives (Table 13-6), the interactions between lamotrigine and oral contraceptives is uniquely bidirectional. Combined oral contraceptives are thought to interact with lamotrigine primarily via the estrogen component, which causes increased metabolism of lamotrigine through induction of glucuronidation. This drug interaction decreases the plasma concentrations of lamotrigine in the body by up to 2-fold, resulting in an increased risk of seizures or inadequate mood stabilization.1 This effect on metabolism is very rapid, resulting in decreases in lamotrigine concentrations within 1 week.4,7 A recent study suggested that certain progestins may also contribute to decreased plasma levels of lamotrigine, but the mechanism for this is unknown (Table 23-7).8

Clinicians should consider increasing the lamotrigine dose (potentially as much as 2-fold) in a patient who initiates treatment with a combined hormonal contraceptive. Dose increases should not be >50 to 100 mg/d every week.1 Collect lamotrigine blood levels before starting a hormonal contraceptive and during dose titration. While there is not a well-established therapeutic range for lamotrigine in BD, expert consensus recommends a range of 1 to 6 mcg/mL.8

The lamotrigine dose should be decreased if combined hormonal contraceptives are discontinued. Dose decreases should not exceed 25% of the total daily dose per week.1 Desogestrel, a progestin-only medication, may increase exposure to lamotrigine, but this has not been observed in research with other progestins.5,9 When starting a progestin-only pill, monitor patients for signs of lamotrigine toxicity (ataxia, diplopia, dizziness) and consider monitoring their blood levels.
An important consideration to note with combined oral contraceptives is the hormone-free interval, also known as the pill-free week. Due to the rapid effect of estrogens, the lamotrigine concentrations have been shown to rise, even double, during this hormone-free interval, so patients should be closely monitored for adverse effects.3 Some recommend use of an extended cycle regimen (with a limited hormone-free interval), or continuous cycle regimen (with no hormone-free interval) to avoid fluctuations in lamotrigine levels.3,5 Additionally, data suggest that in patients taking lamotrigine and valproate, which inhibits glucuronidation, oral contraceptives do not cause reductions in lamotrigine concentrations.2,5 In these instances, dose increases of lamotrigine are not needed.
Continue to: The metabolism of ethinyl estradiol...
The metabolism of ethinyl estradiol and progestin are susceptible to CYP3A4 induction and increased glucuronidation. Serum concentrations may be reduced by ≥50% when used concomitantly with CYP enzyme–inducing medications, which could possibly result in subtherapeutic levels and unplanned pregnancy.3 CYP3A4 induction occurs for up to 4 weeks after discontinuation of an enzyme-inducing agent, pointing to the need for alternative or backup contraception during this time.3 Lamotrigine is not a CYP enzyme–inducing medication; it is unlikely to affect the efficacy of oral contraceptives in the same manner as other antiseizure medications. However, a study of lamotrigine and the combined hormonal contraceptive ethinyl estradiol and levonorgestrel demonstrated reduced exposure to levonorgestrel, resulting in breakthrough bleeding.5
In a study on the coadministration of lamotrigine and combined oral contraceptives, Sidhu et al4 observed a small mean reduction (20%) in progestin concentrations when lamotrigine was used at a dose of 300 mg/d. Although there is no research suggesting decreased effectiveness in preventing pregnancy when lamotrigine is used with combined oral contraceptives, progestin-only oral contraceptives, or progestin implants, additional or alternative contraceptive methods may be considered based on this pharmacokinetic data, particularly in patients who require lamotrigine doses ≥300 mg/d.5
CASE CONTINUED
Given when Ms. A started the oral contraceptive, the treatment team determines it is likely that an interaction with lamotrigine is causing her resurgence of depressive symptoms. Her care team decides to titrate the lamotrigine gradually to 300 mg/d, then 400 mg/d if needed, while carefully monitoring for signs of a serious rash. This dosage increase may help Ms. A achieve symptom remission. Monitoring plasma levels may be considered, although it is unknown what plasma level was effective for Ms. A before she started the oral contraceptive. Ms. A would need to be counseled regarding the effect of higher doses of lamotrigine on the effectiveness of the oral contraceptive.
Although it does not appear Ms. A is using the oral contraceptive specifically to prevent pregnancy, the team informs her about the possibility of unintended pregnancy with this medication combination. If Ms. A was also using the medication for this indication, alternative contraceptive options would include medroxyprogesterone acetate, levonorgestrel implants, or an intrauterine device (levonorgestrel or copper, though copper would not be effective for endometriosis symptom management). Ms. A should consult with her gynecologist regarding the most appropriate option for her endometriosis. If the decision is made to discontinue her oral contraceptive in the future, the lamotrigine dose should be decreased to her previously effective dose of 200 mg/d.
Related Resources
- Makino KK, Hatters Friedman S, Amin J. Emergency contraception for psychiatric patients. Current Psychiatry. 2022;21(11):34-39,44-45. doi:10.12788/cp.0300
- MGH Center for Women’s Mental Health. You asked: is there an interaction between lamotrigine and oral contraceptives? September 29, 2015. https://womensmentalhealth.org/posts/you-asked-is-there-an-interaction-between-lamotrigine-andoral-contraceptives/
Drug Brand Names
Bupropion extended-release • Wellbutrin XL
Carbamazepine • Equetro, Tegretol
Desogestrel • Cerazette
Divalproex sodium • Depakote
Ethinyl estradiol and etonogestrel • NuvaRing
Ethinyl estradiol and norelgestromin • Ortho Evra
Ethinyl estradiol and norgestimate • Ortho Tri-Cyclen, TriNessa, others
Etonogestrel • Implanon, Nexplanon
Gabapentin • Neurontin
Lamotrigine • Lamictal
Levonorgestrel emergency contraceptive pill • AfterPill, Plan B
Levonorgestrel intrauterine device • Mirena, Skyla
Medroxyprogesterone acetate • Depo-Provera
Oxcarbazepine • Trileptal
Topiramate • Topamax
Valproic acid • Depakene
Ms. A, age 20, presents to the clinic after experiencing difficulty sleeping, depressed mood, fatigue, and difficulty concentrating. Her psychiatric history includes bipolar II disorder (BD II), predominantly with depressive episodes. Ms. A’s current medications include a combination of lamotrigine 200 mg/d and bupropion extended-release 450 mg/d, and her symptoms were well maintained until 2 weeks ago. When her psychiatrist performs a medication reconciliation at her medication management appointment, Ms. A indicates she started taking an oral contraceptive, ethinyl estradiol and norgestimate, approximately 1 month ago for management of endometriosis symptoms. She is not currently taking any other medications or supplements.
Lamotrigine is indicated for epilepsy and as maintenance treatment for BD I. It is also used off-label to treat other mood disorders. After oral administration, lamotrigine is rapidly and fully absorbed with a high bioavailability (98%).The principal metabolic pathway is via glucuronic acid conjugation, leading to the major inactive metabolite 2-N-glucuronide. Minor metabolites include 5-N-glucuronide and a 2-N-glucuronide metabolite.1
Combined oral contraceptives contain an estrogen component, typically ethinyl estradiol, and a progestin component, which varies based on the specific formulation. The metabolism of ethinyl estradiol occurs through cytochrome P450 (CYP)3A4, CYP2C9, sulfation, and glucuronidation. For progestin—the second component of combined oral contraceptives and the lone component of progestin-only oral contraceptives—metabolism occurs via CYP3A4 and conjugation reactions.2 This article focuses on lamotrigine interactions specifically with oral contraceptives, but it is important to note that other formulations of combined hormonal contraceptives, such as the combined contraceptive patch (Ortho Evra) and vaginal ring (NuvaRing), would be expected to interact in the same way as oral formulations.3
Bidirectional interaction
While many antiseizure medications are known to interact with and potentially decrease the efficacy of oral contraceptives (Table 13-6), the interactions between lamotrigine and oral contraceptives is uniquely bidirectional. Combined oral contraceptives are thought to interact with lamotrigine primarily via the estrogen component, which causes increased metabolism of lamotrigine through induction of glucuronidation. This drug interaction decreases the plasma concentrations of lamotrigine in the body by up to 2-fold, resulting in an increased risk of seizures or inadequate mood stabilization.1 This effect on metabolism is very rapid, resulting in decreases in lamotrigine concentrations within 1 week.4,7 A recent study suggested that certain progestins may also contribute to decreased plasma levels of lamotrigine, but the mechanism for this is unknown (Table 23-7).8

Clinicians should consider increasing the lamotrigine dose (potentially as much as 2-fold) in a patient who initiates treatment with a combined hormonal contraceptive. Dose increases should not be >50 to 100 mg/d every week.1 Collect lamotrigine blood levels before starting a hormonal contraceptive and during dose titration. While there is not a well-established therapeutic range for lamotrigine in BD, expert consensus recommends a range of 1 to 6 mcg/mL.8

The lamotrigine dose should be decreased if combined hormonal contraceptives are discontinued. Dose decreases should not exceed 25% of the total daily dose per week.1 Desogestrel, a progestin-only medication, may increase exposure to lamotrigine, but this has not been observed in research with other progestins.5,9 When starting a progestin-only pill, monitor patients for signs of lamotrigine toxicity (ataxia, diplopia, dizziness) and consider monitoring their blood levels.
An important consideration to note with combined oral contraceptives is the hormone-free interval, also known as the pill-free week. Due to the rapid effect of estrogens, the lamotrigine concentrations have been shown to rise, even double, during this hormone-free interval, so patients should be closely monitored for adverse effects.3 Some recommend use of an extended cycle regimen (with a limited hormone-free interval), or continuous cycle regimen (with no hormone-free interval) to avoid fluctuations in lamotrigine levels.3,5 Additionally, data suggest that in patients taking lamotrigine and valproate, which inhibits glucuronidation, oral contraceptives do not cause reductions in lamotrigine concentrations.2,5 In these instances, dose increases of lamotrigine are not needed.
Continue to: The metabolism of ethinyl estradiol...
The metabolism of ethinyl estradiol and progestin are susceptible to CYP3A4 induction and increased glucuronidation. Serum concentrations may be reduced by ≥50% when used concomitantly with CYP enzyme–inducing medications, which could possibly result in subtherapeutic levels and unplanned pregnancy.3 CYP3A4 induction occurs for up to 4 weeks after discontinuation of an enzyme-inducing agent, pointing to the need for alternative or backup contraception during this time.3 Lamotrigine is not a CYP enzyme–inducing medication; it is unlikely to affect the efficacy of oral contraceptives in the same manner as other antiseizure medications. However, a study of lamotrigine and the combined hormonal contraceptive ethinyl estradiol and levonorgestrel demonstrated reduced exposure to levonorgestrel, resulting in breakthrough bleeding.5
In a study on the coadministration of lamotrigine and combined oral contraceptives, Sidhu et al4 observed a small mean reduction (20%) in progestin concentrations when lamotrigine was used at a dose of 300 mg/d. Although there is no research suggesting decreased effectiveness in preventing pregnancy when lamotrigine is used with combined oral contraceptives, progestin-only oral contraceptives, or progestin implants, additional or alternative contraceptive methods may be considered based on this pharmacokinetic data, particularly in patients who require lamotrigine doses ≥300 mg/d.5
CASE CONTINUED
Given when Ms. A started the oral contraceptive, the treatment team determines it is likely that an interaction with lamotrigine is causing her resurgence of depressive symptoms. Her care team decides to titrate the lamotrigine gradually to 300 mg/d, then 400 mg/d if needed, while carefully monitoring for signs of a serious rash. This dosage increase may help Ms. A achieve symptom remission. Monitoring plasma levels may be considered, although it is unknown what plasma level was effective for Ms. A before she started the oral contraceptive. Ms. A would need to be counseled regarding the effect of higher doses of lamotrigine on the effectiveness of the oral contraceptive.
Although it does not appear Ms. A is using the oral contraceptive specifically to prevent pregnancy, the team informs her about the possibility of unintended pregnancy with this medication combination. If Ms. A was also using the medication for this indication, alternative contraceptive options would include medroxyprogesterone acetate, levonorgestrel implants, or an intrauterine device (levonorgestrel or copper, though copper would not be effective for endometriosis symptom management). Ms. A should consult with her gynecologist regarding the most appropriate option for her endometriosis. If the decision is made to discontinue her oral contraceptive in the future, the lamotrigine dose should be decreased to her previously effective dose of 200 mg/d.
Related Resources
- Makino KK, Hatters Friedman S, Amin J. Emergency contraception for psychiatric patients. Current Psychiatry. 2022;21(11):34-39,44-45. doi:10.12788/cp.0300
- MGH Center for Women’s Mental Health. You asked: is there an interaction between lamotrigine and oral contraceptives? September 29, 2015. https://womensmentalhealth.org/posts/you-asked-is-there-an-interaction-between-lamotrigine-andoral-contraceptives/
Drug Brand Names
Bupropion extended-release • Wellbutrin XL
Carbamazepine • Equetro, Tegretol
Desogestrel • Cerazette
Divalproex sodium • Depakote
Ethinyl estradiol and etonogestrel • NuvaRing
Ethinyl estradiol and norelgestromin • Ortho Evra
Ethinyl estradiol and norgestimate • Ortho Tri-Cyclen, TriNessa, others
Etonogestrel • Implanon, Nexplanon
Gabapentin • Neurontin
Lamotrigine • Lamictal
Levonorgestrel emergency contraceptive pill • AfterPill, Plan B
Levonorgestrel intrauterine device • Mirena, Skyla
Medroxyprogesterone acetate • Depo-Provera
Oxcarbazepine • Trileptal
Topiramate • Topamax
Valproic acid • Depakene
1. Lamictal [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2020.
2. Lee CR. Drug interactions and hormonal contraception. Trends in Urology Gynaecology & Sexual Health. 2009;14(3):23-26.
3. Williams D. Antiepileptic drugs and contraception. US Pharm. 2014;39(1):39-42.
4. Sidhu J, Job S, Singh S, et al. The pharmacokinetic and pharmacodynamic consequences of the co-administration of lamotrigine and a combined oral contraceptive in healthy female subjects. Br J Clin Pharmacol. 2006;61(2):191-199. doi:10.1111/j.1365-2125.2005.02539.x
5. Faculty of Sexual & Reproductive Healthcare. Clinical guidance: drug interactions with hormonal contraception. Published May 9, 2022. Accessed September 28, 2022. https://www.fsrh.org/documents/ceu-clinical-guidance-drug-interactions-with-hormonal/
6. Johnston CA, Crawford PM. Anti-epileptic drugs and hormonal treatments. Curr Treat Options Neurol. 2014;16(5):288. doi:10.1007/s11940-014-0288-3
7. Christensen J, Petrenaite V, Atterman J, et al. Oral contraceptives induce lamotrigine metabolism: evidence from a double-blind, placebo-controlled trial. Epilepsia. 2007;48(3):484-489. doi:10.1111/j.1528-1167.2007.00997.x
8. Hiemke C, Bergemann N, Clement HW, et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry. 2018;51(1-02):9-62. doi:10.1055/s-0043-116492
9. Rauchenzauner M, Deichmann S, Pittschieler, et al. Bidirectional interaction between oral contraception and lamotrigine in women with epilepsy – role of progestins. Seizure. 2020;74:89-92. doi:10.1016/j.seizure.2019.11.011
1. Lamictal [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2020.
2. Lee CR. Drug interactions and hormonal contraception. Trends in Urology Gynaecology & Sexual Health. 2009;14(3):23-26.
3. Williams D. Antiepileptic drugs and contraception. US Pharm. 2014;39(1):39-42.
4. Sidhu J, Job S, Singh S, et al. The pharmacokinetic and pharmacodynamic consequences of the co-administration of lamotrigine and a combined oral contraceptive in healthy female subjects. Br J Clin Pharmacol. 2006;61(2):191-199. doi:10.1111/j.1365-2125.2005.02539.x
5. Faculty of Sexual & Reproductive Healthcare. Clinical guidance: drug interactions with hormonal contraception. Published May 9, 2022. Accessed September 28, 2022. https://www.fsrh.org/documents/ceu-clinical-guidance-drug-interactions-with-hormonal/
6. Johnston CA, Crawford PM. Anti-epileptic drugs and hormonal treatments. Curr Treat Options Neurol. 2014;16(5):288. doi:10.1007/s11940-014-0288-3
7. Christensen J, Petrenaite V, Atterman J, et al. Oral contraceptives induce lamotrigine metabolism: evidence from a double-blind, placebo-controlled trial. Epilepsia. 2007;48(3):484-489. doi:10.1111/j.1528-1167.2007.00997.x
8. Hiemke C, Bergemann N, Clement HW, et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry. 2018;51(1-02):9-62. doi:10.1055/s-0043-116492
9. Rauchenzauner M, Deichmann S, Pittschieler, et al. Bidirectional interaction between oral contraception and lamotrigine in women with epilepsy – role of progestins. Seizure. 2020;74:89-92. doi:10.1016/j.seizure.2019.11.011

