User login
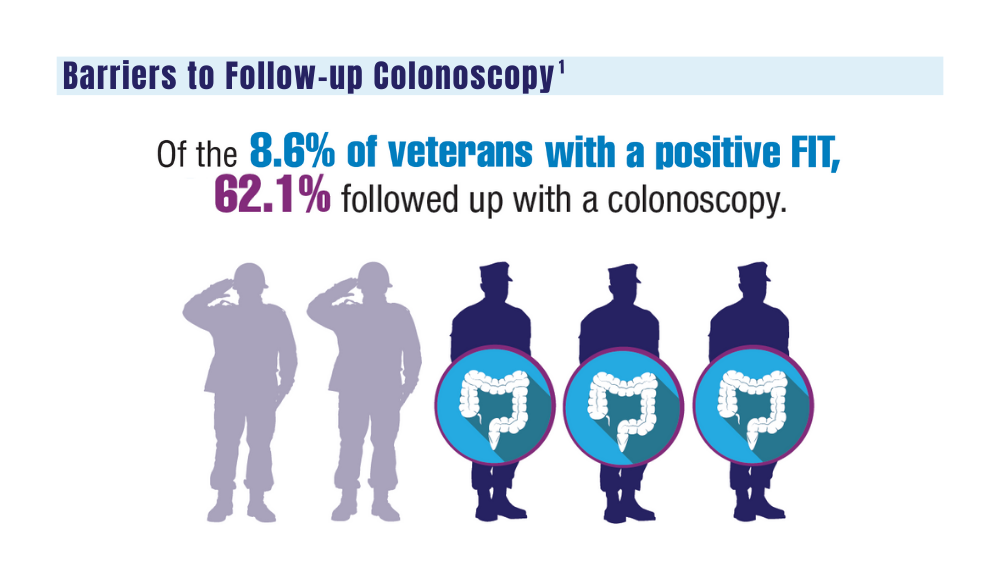
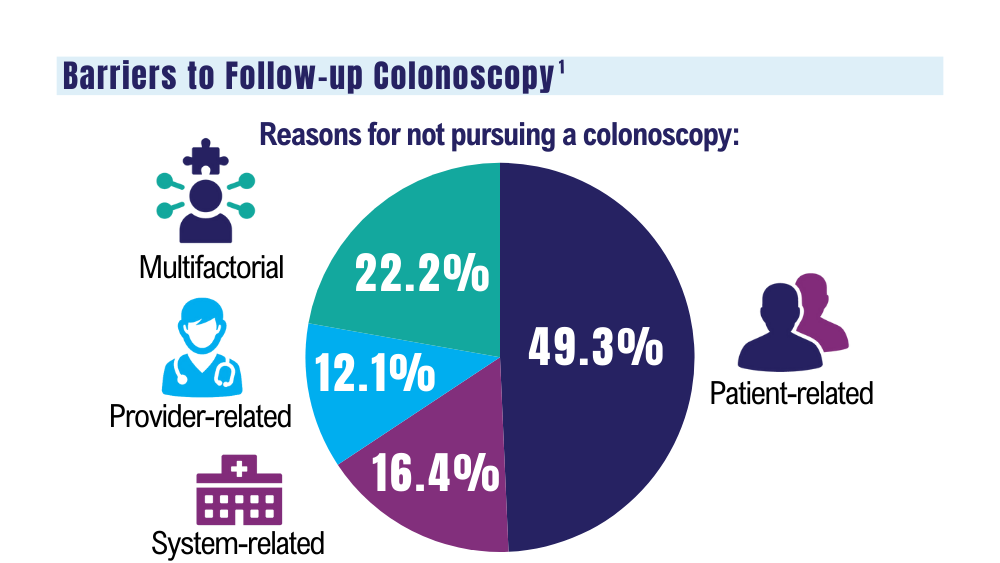
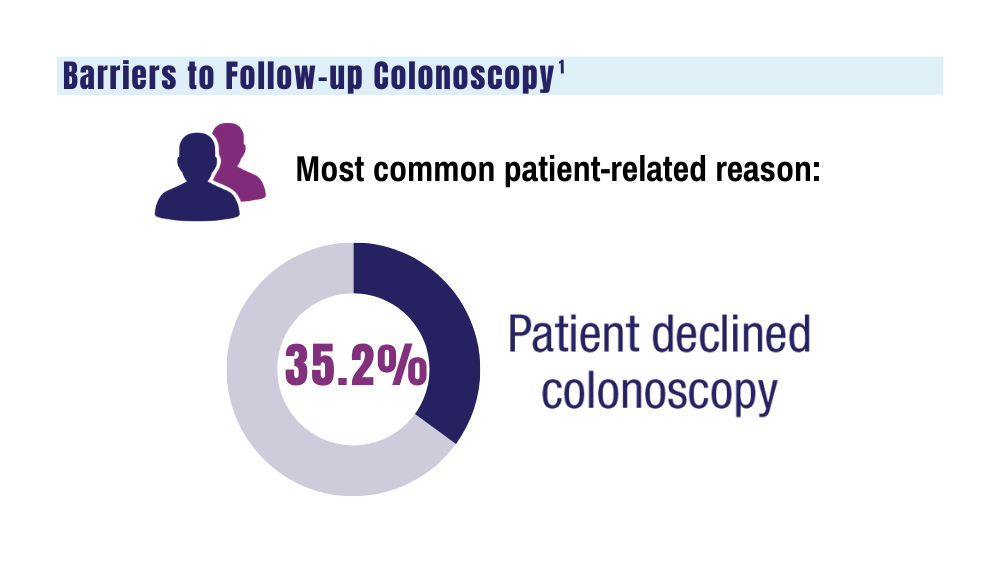
1. May FP, Yano EM, Provenzale D, et al. Barriers to follow-up colonoscopies for patients with positive results from fecal immunochemical tests during colorectal cancer screening. Clin Gastroenterol Hepatol. 2019;17(3):469-476. doi:10.1016/j.cgh.2018.05.022
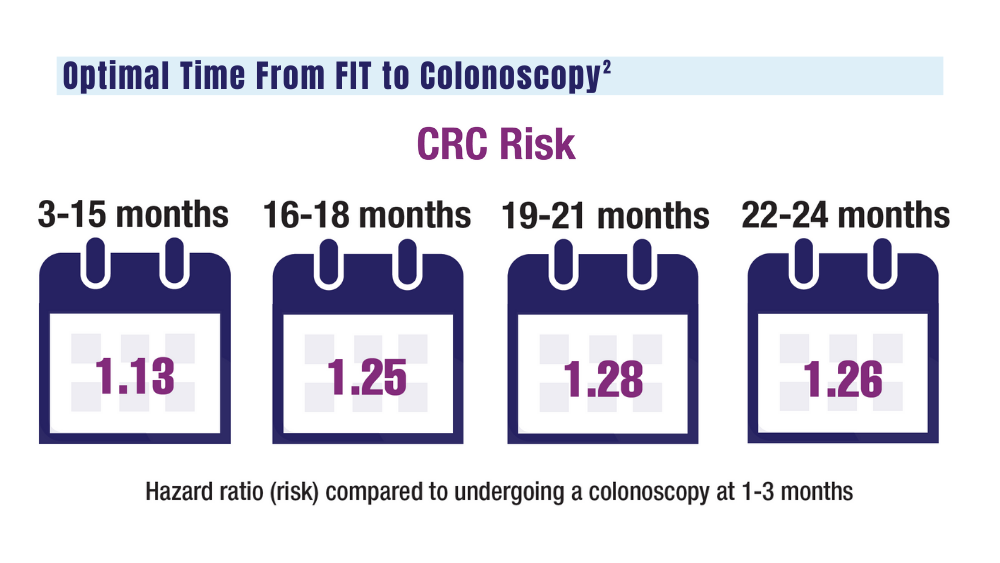
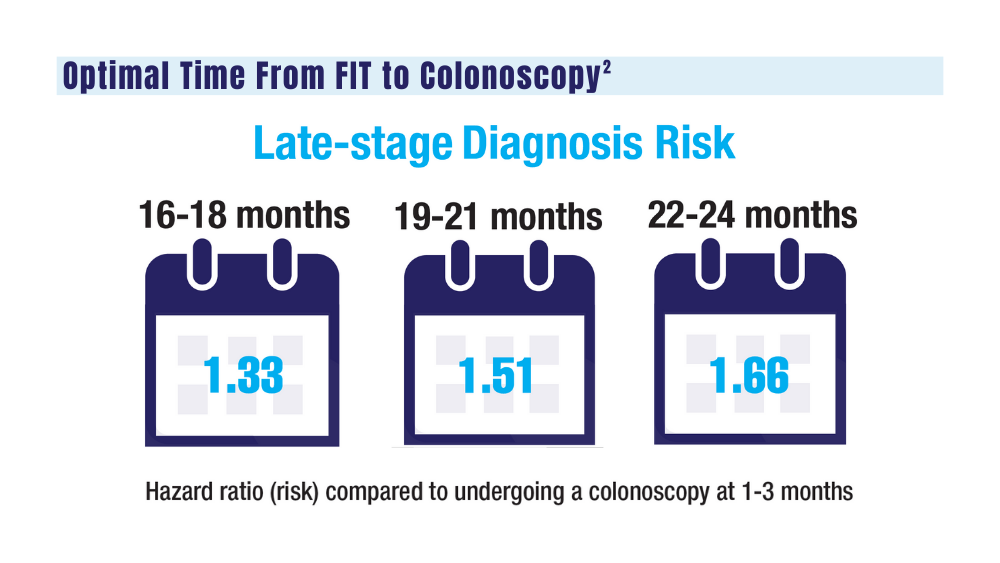
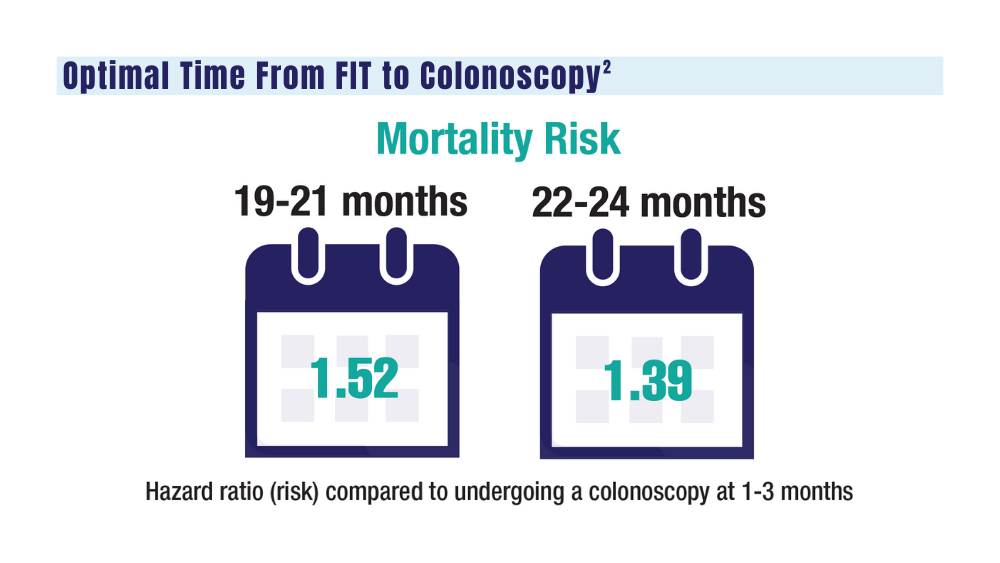
2. San Miguel Y, Demb J, Martinez ME, Gupta S, May FP. Time to colonoscopy after abnormal stool-based screening and risk for colorectal cancer incidence and mortality. Gastroenterology. 2021;160(6):1997-2005.e3. doi:10.1053/j.gastro.2021.01.219
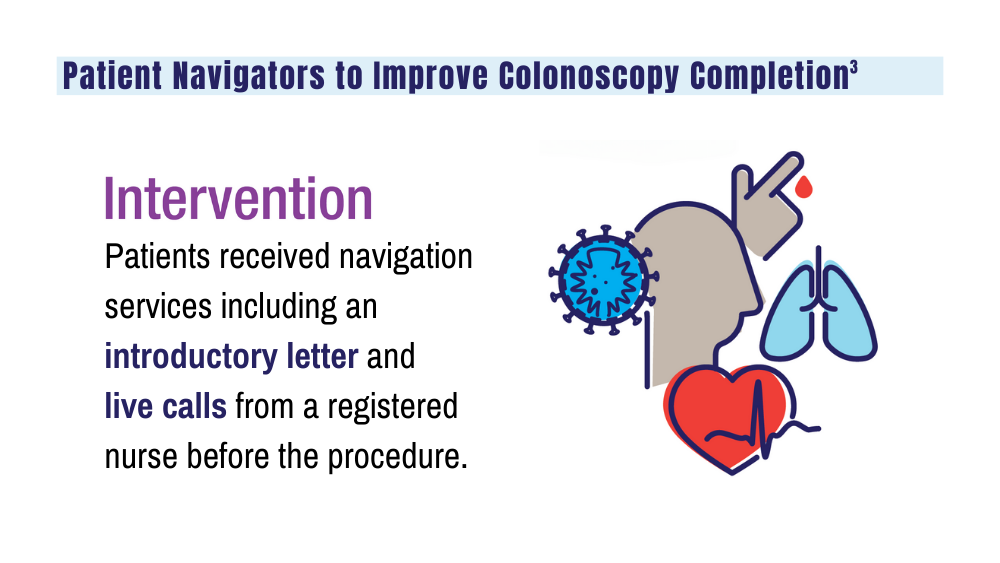
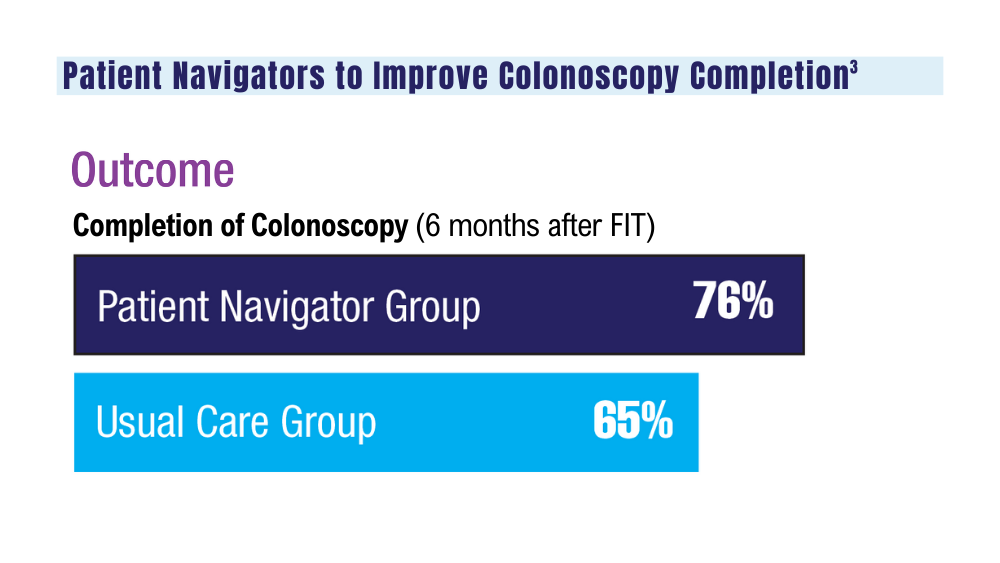
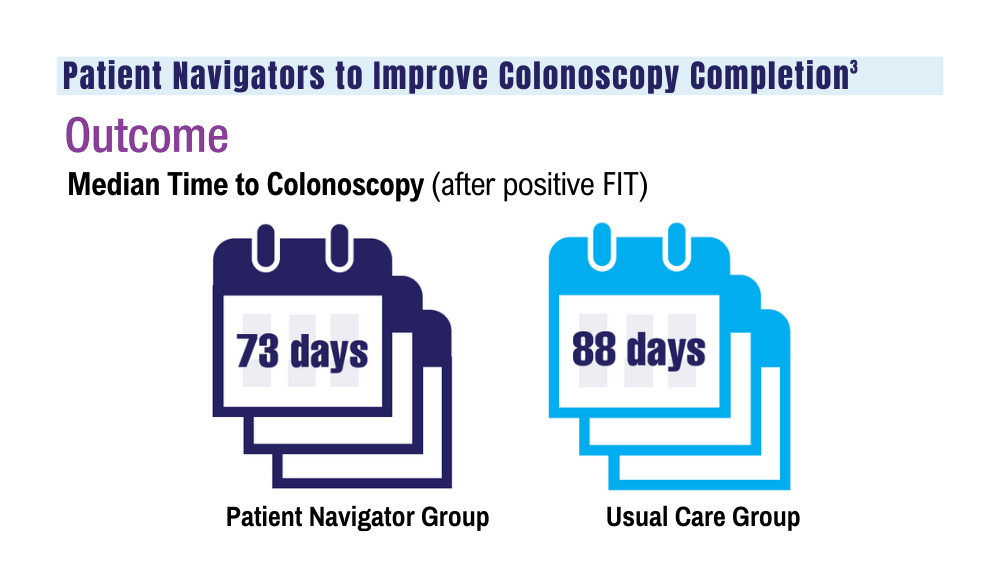
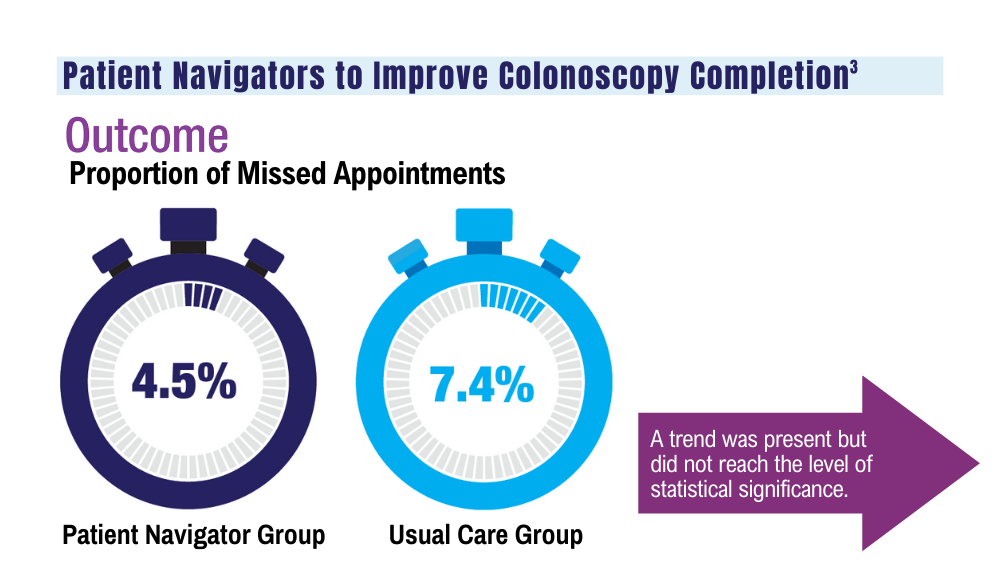
3. Coronado GD, Rawlings AM, Petrik AF, et al. Precision patient navigation to improve rates of follow-up colonoscopy, an individual randomized effectiveness trial. Cancer Epidemiol Biomarkers Prev. 2021;30(12):2327-2333. doi:10.1158/1055-9965.EPI-20-1793
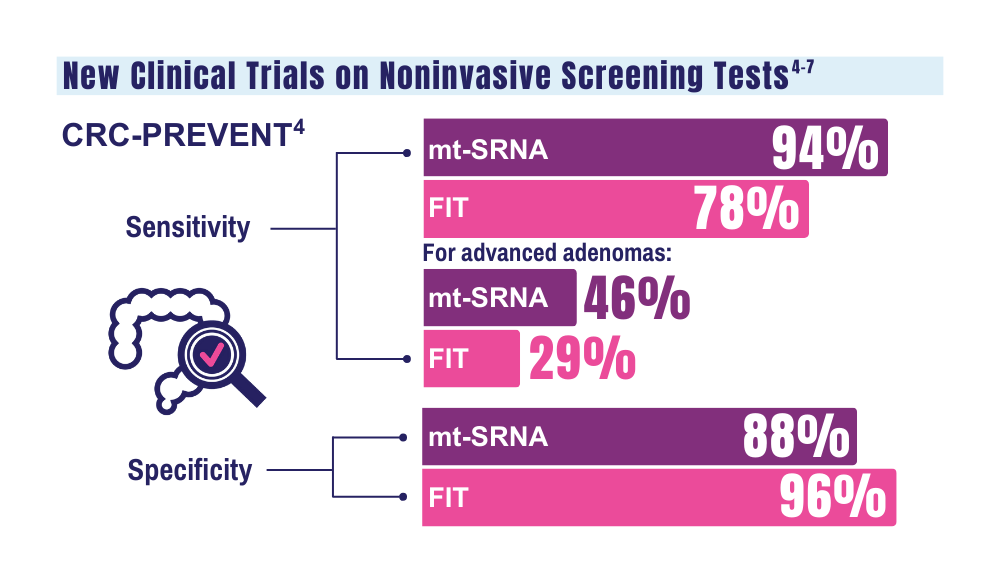
4. Barnell EK, Wurtzler EM, La Rocca J, et al. Multitarget stool RNA test for colorectal cancer screening. JAMA. 2023;330(18):1760- 1768. doi:10.1001/jama.2023.22231
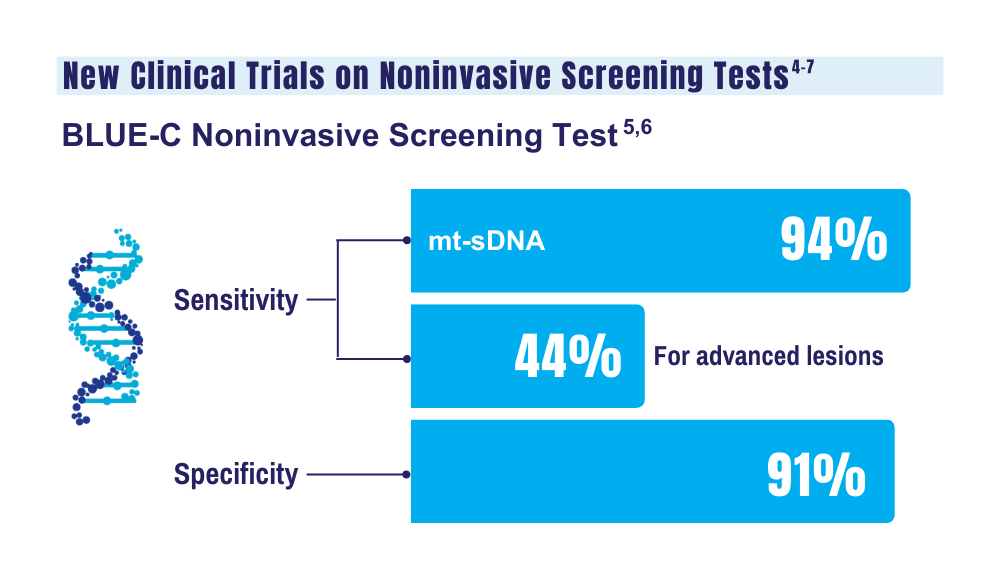
5. McNamara D. Two multitarget stool tests show promise for CRC screening: studies. Medscape. Published October 22, 2023. Accessed December 20, 2023. https://www.medscape.com/viewarticle/997609#vp_1
6. Clinical validation of an optimized multi-target stool DNA (Mt-sDNA 2.0) test, for colorectal cancer screening “BLUE-C.” ClinicalTrials. gov identifier: NCT04144738. Updated September 1, 2023. Accessed December 20, 2023. https://www.clinicaltrials.gov/study/ NCT04144738
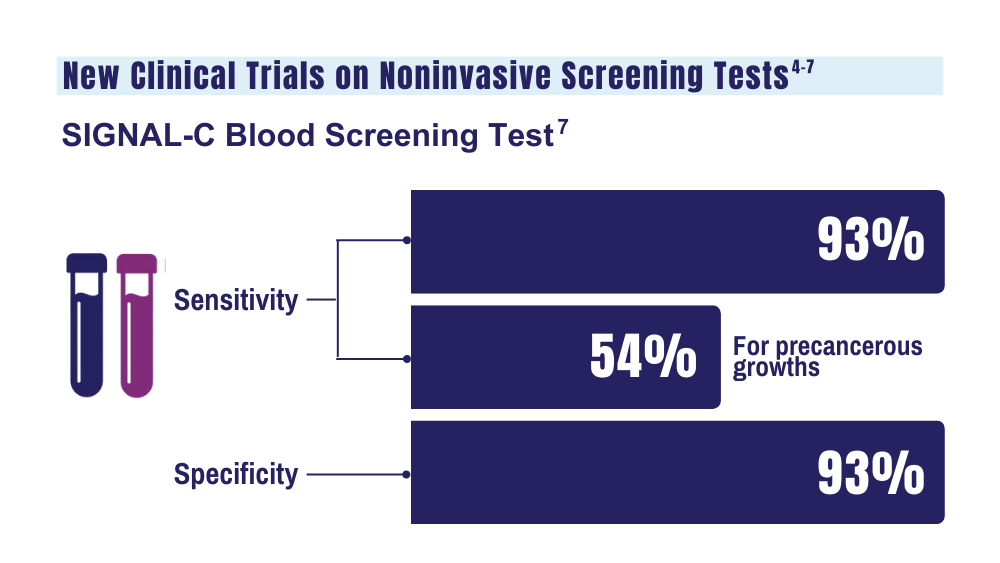
7. Universal Diagnostics. Universal DX Presents Data from Large, 1,000-patient, Multi-Cohort Study Proving 93% Sensitivity for Colorectal Cancer and 54% Sensitivity for Advanced Adenoma at 92% Specificity. Press Release. Published May 2023. Accessed January 26. 2024.
1. May FP, Yano EM, Provenzale D, et al. Barriers to follow-up colonoscopies for patients with positive results from fecal immunochemical tests during colorectal cancer screening. Clin Gastroenterol Hepatol. 2019;17(3):469-476. doi:10.1016/j.cgh.2018.05.022
2. San Miguel Y, Demb J, Martinez ME, Gupta S, May FP. Time to colonoscopy after abnormal stool-based screening and risk for colorectal cancer incidence and mortality. Gastroenterology. 2021;160(6):1997-2005.e3. doi:10.1053/j.gastro.2021.01.219
3. Coronado GD, Rawlings AM, Petrik AF, et al. Precision patient navigation to improve rates of follow-up colonoscopy, an individual randomized effectiveness trial. Cancer Epidemiol Biomarkers Prev. 2021;30(12):2327-2333. doi:10.1158/1055-9965.EPI-20-1793
4. Barnell EK, Wurtzler EM, La Rocca J, et al. Multitarget stool RNA test for colorectal cancer screening. JAMA. 2023;330(18):1760- 1768. doi:10.1001/jama.2023.22231
5. McNamara D. Two multitarget stool tests show promise for CRC screening: studies. Medscape. Published October 22, 2023. Accessed December 20, 2023. https://www.medscape.com/viewarticle/997609#vp_1
6. Clinical validation of an optimized multi-target stool DNA (Mt-sDNA 2.0) test, for colorectal cancer screening “BLUE-C.” ClinicalTrials. gov identifier: NCT04144738. Updated September 1, 2023. Accessed December 20, 2023. https://www.clinicaltrials.gov/study/ NCT04144738
7. Universal Diagnostics. Universal DX Presents Data from Large, 1,000-patient, Multi-Cohort Study Proving 93% Sensitivity for Colorectal Cancer and 54% Sensitivity for Advanced Adenoma at 92% Specificity. Press Release. Published May 2023. Accessed January 26. 2024.
1. May FP, Yano EM, Provenzale D, et al. Barriers to follow-up colonoscopies for patients with positive results from fecal immunochemical tests during colorectal cancer screening. Clin Gastroenterol Hepatol. 2019;17(3):469-476. doi:10.1016/j.cgh.2018.05.022
2. San Miguel Y, Demb J, Martinez ME, Gupta S, May FP. Time to colonoscopy after abnormal stool-based screening and risk for colorectal cancer incidence and mortality. Gastroenterology. 2021;160(6):1997-2005.e3. doi:10.1053/j.gastro.2021.01.219
3. Coronado GD, Rawlings AM, Petrik AF, et al. Precision patient navigation to improve rates of follow-up colonoscopy, an individual randomized effectiveness trial. Cancer Epidemiol Biomarkers Prev. 2021;30(12):2327-2333. doi:10.1158/1055-9965.EPI-20-1793
4. Barnell EK, Wurtzler EM, La Rocca J, et al. Multitarget stool RNA test for colorectal cancer screening. JAMA. 2023;330(18):1760- 1768. doi:10.1001/jama.2023.22231
5. McNamara D. Two multitarget stool tests show promise for CRC screening: studies. Medscape. Published October 22, 2023. Accessed December 20, 2023. https://www.medscape.com/viewarticle/997609#vp_1
6. Clinical validation of an optimized multi-target stool DNA (Mt-sDNA 2.0) test, for colorectal cancer screening “BLUE-C.” ClinicalTrials. gov identifier: NCT04144738. Updated September 1, 2023. Accessed December 20, 2023. https://www.clinicaltrials.gov/study/ NCT04144738
7. Universal Diagnostics. Universal DX Presents Data from Large, 1,000-patient, Multi-Cohort Study Proving 93% Sensitivity for Colorectal Cancer and 54% Sensitivity for Advanced Adenoma at 92% Specificity. Press Release. Published May 2023. Accessed January 26. 2024.