User login
The American Academy of Orthopaedic Surgeons has adopted clinical practice guidelines that assign evidence-based ratings for common strategies used to diagnose and treat carpal tunnel syndrome (CTS).
The 982-page comprehensive guidelines have been endorsed by the American Society for the Surgery of the Hand and the American College of Radiology. The guidelines address the burden of CTS, the second most common cause of sick days from work, according to AAOS, and its etiology, risk factors, emotional and physical impact, potential benefits, harms, contraindications, and future research. The document is available on the OrthoGuidelines Web-based app at orthoguidelines.org.
The assessments of evidence are based upon a systematic review of the current scientific and clinical information and accepted approaches to treatment and/or diagnosis of carpal tunnel syndrome. In addition to a concise summary, the report includes an exhaustive list of studies used to establish levels of evidence and a summary of the evidence in each. Also included is a list of studies not included, many because of poor study design or very small samples.
The guidelines make recommendations on practices to diagnose and manage CTS based on four levels of evidence:
• Strong: Supported by two or more “high-quality” studies with consistent findings.
• Moderate: Supported by two or more “moderate-quality” studies or one “high-quality” study.
• Limited: Supported by two or more “low-quality” studies or one “moderate-quality” study, or the evidence is considered insufficient or conflicting.
• Consensus: No supporting evidence but the guidelines development group made a recommendation based on clinical opinion.
Diagnosis and risk evidence
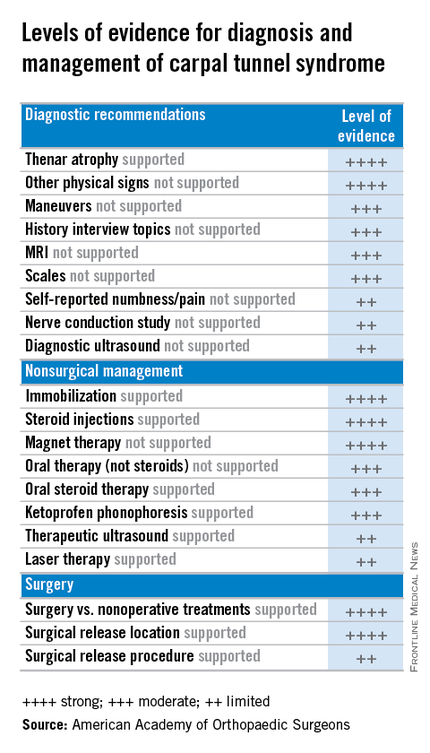
For diagnosis of CTS, the guidelines rate the evidence for the value of both observation and physical signs as strong, but assign ratings of moderate to MRI and limited to ultrasound. Evidence is strong for thenar atrophy, or diminished thumb muscle mass, being associated with CTS, but a lack of thenar atrophy is not enough to rule out a diagnosis. Common evaluation tools such the Phalen test, Tinel sign, Flick sign, or Upper-Limb Neurodynamic/Nerve Tension test (ULNT) are weakly supported as independent physical examination maneuvers to rule in or rule out carpal tunnel and the guidelines suggest that they not be used as sole diagnostic tools.
Moderate evidence supports exercise and physical activity to reduce the risk of developing CTS. The guidelines consider obesity a strong risk factor for CTS, but assign moderate ratings to evidence for a host of other factors, perimenopausal status, wrist ratio/index, rheumatoid arthritis, psychosocial factors, and activities such as gardening and computer use among them.
Treatment evidence
For treatment, the guidelines evaluate evidence for both surgical and nonsurgical strategies. In general, evidence for the efficacy of splinting, steroids (oral or injection), the use of ketoprofen phonophoresis gel, and magnetic therapy is strong. But therapeutic ultrasound and laser therapy are backed up with only limited evidence from the literature.
As might be expected, the evidence is strong for the efficacy of surgery to release the transverse carpal ligament. “Strong evidence supports that surgical treatment of carpal tunnel syndrome should have a greater treatment benefit at 6 and 12 months as compared to splinting, NSAIDs/therapy, and a single steroid injection.” But the value of adjunctive techniques such as epineurotomy, neurolysis, flexor tenosynovectomy, and lengthening/reconstruction of the flexor retinaculum (transverse carpal ligament) is not supported with strong evidence at this point. And the superiority of the endoscopic surgical approach is supported with only limited evidence.
“The impetus for this came from trying to help physicians cull through literally thousands and thousands of published research papers concerning various diagnoses,” said Dr. Allan E. Peljovich, vice-chair of the Guideline Work Group and AAOS representative to the group. It’s a tool to help orthopedic surgeons and other practitioners “understand what our best evidence tells us about diagnosing and treating a variety of conditions,” he said.
The effort to develop the CTS guidelines started February 2013 and involved the Guideline Work Group formulating a set of questions that, as Dr. Peljovich explained, were “the most pertinent questions that anybody interested in a particular diagnosis would want to have answered.” Then a team of statisticians and epidemiologists culled through the “incredible expanse of English language literature” to correlate data to answer those questions.
In May 2015 the work group then met to review the evidence and draft final recommendations. After a period of editing, the draft was submitted for peer review in September. The AAOS board of directors adopted the guidelines in February.
“The guidelines are not intended to be a cookbook on how to treat a condition,” Dr. Peljovich said. “They are really designed to tell you what the best evidence says about a particular set of questions. It helps you to be as updated as you want to be; it’s not designed to tell you this is the only way to do anything. ... It’s an educational tool.”
Members of the Guideline Work Group, AAOS staff, and contributing members submitted their disclosures to the AAOS.
The American Academy of Orthopaedic Surgeons has adopted clinical practice guidelines that assign evidence-based ratings for common strategies used to diagnose and treat carpal tunnel syndrome (CTS).
The 982-page comprehensive guidelines have been endorsed by the American Society for the Surgery of the Hand and the American College of Radiology. The guidelines address the burden of CTS, the second most common cause of sick days from work, according to AAOS, and its etiology, risk factors, emotional and physical impact, potential benefits, harms, contraindications, and future research. The document is available on the OrthoGuidelines Web-based app at orthoguidelines.org.
The assessments of evidence are based upon a systematic review of the current scientific and clinical information and accepted approaches to treatment and/or diagnosis of carpal tunnel syndrome. In addition to a concise summary, the report includes an exhaustive list of studies used to establish levels of evidence and a summary of the evidence in each. Also included is a list of studies not included, many because of poor study design or very small samples.
The guidelines make recommendations on practices to diagnose and manage CTS based on four levels of evidence:
• Strong: Supported by two or more “high-quality” studies with consistent findings.
• Moderate: Supported by two or more “moderate-quality” studies or one “high-quality” study.
• Limited: Supported by two or more “low-quality” studies or one “moderate-quality” study, or the evidence is considered insufficient or conflicting.
• Consensus: No supporting evidence but the guidelines development group made a recommendation based on clinical opinion.
Diagnosis and risk evidence
For diagnosis of CTS, the guidelines rate the evidence for the value of both observation and physical signs as strong, but assign ratings of moderate to MRI and limited to ultrasound. Evidence is strong for thenar atrophy, or diminished thumb muscle mass, being associated with CTS, but a lack of thenar atrophy is not enough to rule out a diagnosis. Common evaluation tools such the Phalen test, Tinel sign, Flick sign, or Upper-Limb Neurodynamic/Nerve Tension test (ULNT) are weakly supported as independent physical examination maneuvers to rule in or rule out carpal tunnel and the guidelines suggest that they not be used as sole diagnostic tools.
Moderate evidence supports exercise and physical activity to reduce the risk of developing CTS. The guidelines consider obesity a strong risk factor for CTS, but assign moderate ratings to evidence for a host of other factors, perimenopausal status, wrist ratio/index, rheumatoid arthritis, psychosocial factors, and activities such as gardening and computer use among them.

Treatment evidence
For treatment, the guidelines evaluate evidence for both surgical and nonsurgical strategies. In general, evidence for the efficacy of splinting, steroids (oral or injection), the use of ketoprofen phonophoresis gel, and magnetic therapy is strong. But therapeutic ultrasound and laser therapy are backed up with only limited evidence from the literature.
As might be expected, the evidence is strong for the efficacy of surgery to release the transverse carpal ligament. “Strong evidence supports that surgical treatment of carpal tunnel syndrome should have a greater treatment benefit at 6 and 12 months as compared to splinting, NSAIDs/therapy, and a single steroid injection.” But the value of adjunctive techniques such as epineurotomy, neurolysis, flexor tenosynovectomy, and lengthening/reconstruction of the flexor retinaculum (transverse carpal ligament) is not supported with strong evidence at this point. And the superiority of the endoscopic surgical approach is supported with only limited evidence.
“The impetus for this came from trying to help physicians cull through literally thousands and thousands of published research papers concerning various diagnoses,” said Dr. Allan E. Peljovich, vice-chair of the Guideline Work Group and AAOS representative to the group. It’s a tool to help orthopedic surgeons and other practitioners “understand what our best evidence tells us about diagnosing and treating a variety of conditions,” he said.
The effort to develop the CTS guidelines started February 2013 and involved the Guideline Work Group formulating a set of questions that, as Dr. Peljovich explained, were “the most pertinent questions that anybody interested in a particular diagnosis would want to have answered.” Then a team of statisticians and epidemiologists culled through the “incredible expanse of English language literature” to correlate data to answer those questions.
In May 2015 the work group then met to review the evidence and draft final recommendations. After a period of editing, the draft was submitted for peer review in September. The AAOS board of directors adopted the guidelines in February.
“The guidelines are not intended to be a cookbook on how to treat a condition,” Dr. Peljovich said. “They are really designed to tell you what the best evidence says about a particular set of questions. It helps you to be as updated as you want to be; it’s not designed to tell you this is the only way to do anything. ... It’s an educational tool.”
Members of the Guideline Work Group, AAOS staff, and contributing members submitted their disclosures to the AAOS.
The American Academy of Orthopaedic Surgeons has adopted clinical practice guidelines that assign evidence-based ratings for common strategies used to diagnose and treat carpal tunnel syndrome (CTS).
The 982-page comprehensive guidelines have been endorsed by the American Society for the Surgery of the Hand and the American College of Radiology. The guidelines address the burden of CTS, the second most common cause of sick days from work, according to AAOS, and its etiology, risk factors, emotional and physical impact, potential benefits, harms, contraindications, and future research. The document is available on the OrthoGuidelines Web-based app at orthoguidelines.org.
The assessments of evidence are based upon a systematic review of the current scientific and clinical information and accepted approaches to treatment and/or diagnosis of carpal tunnel syndrome. In addition to a concise summary, the report includes an exhaustive list of studies used to establish levels of evidence and a summary of the evidence in each. Also included is a list of studies not included, many because of poor study design or very small samples.
The guidelines make recommendations on practices to diagnose and manage CTS based on four levels of evidence:
• Strong: Supported by two or more “high-quality” studies with consistent findings.
• Moderate: Supported by two or more “moderate-quality” studies or one “high-quality” study.
• Limited: Supported by two or more “low-quality” studies or one “moderate-quality” study, or the evidence is considered insufficient or conflicting.
• Consensus: No supporting evidence but the guidelines development group made a recommendation based on clinical opinion.
Diagnosis and risk evidence
For diagnosis of CTS, the guidelines rate the evidence for the value of both observation and physical signs as strong, but assign ratings of moderate to MRI and limited to ultrasound. Evidence is strong for thenar atrophy, or diminished thumb muscle mass, being associated with CTS, but a lack of thenar atrophy is not enough to rule out a diagnosis. Common evaluation tools such the Phalen test, Tinel sign, Flick sign, or Upper-Limb Neurodynamic/Nerve Tension test (ULNT) are weakly supported as independent physical examination maneuvers to rule in or rule out carpal tunnel and the guidelines suggest that they not be used as sole diagnostic tools.
Moderate evidence supports exercise and physical activity to reduce the risk of developing CTS. The guidelines consider obesity a strong risk factor for CTS, but assign moderate ratings to evidence for a host of other factors, perimenopausal status, wrist ratio/index, rheumatoid arthritis, psychosocial factors, and activities such as gardening and computer use among them.

Treatment evidence
For treatment, the guidelines evaluate evidence for both surgical and nonsurgical strategies. In general, evidence for the efficacy of splinting, steroids (oral or injection), the use of ketoprofen phonophoresis gel, and magnetic therapy is strong. But therapeutic ultrasound and laser therapy are backed up with only limited evidence from the literature.
As might be expected, the evidence is strong for the efficacy of surgery to release the transverse carpal ligament. “Strong evidence supports that surgical treatment of carpal tunnel syndrome should have a greater treatment benefit at 6 and 12 months as compared to splinting, NSAIDs/therapy, and a single steroid injection.” But the value of adjunctive techniques such as epineurotomy, neurolysis, flexor tenosynovectomy, and lengthening/reconstruction of the flexor retinaculum (transverse carpal ligament) is not supported with strong evidence at this point. And the superiority of the endoscopic surgical approach is supported with only limited evidence.
“The impetus for this came from trying to help physicians cull through literally thousands and thousands of published research papers concerning various diagnoses,” said Dr. Allan E. Peljovich, vice-chair of the Guideline Work Group and AAOS representative to the group. It’s a tool to help orthopedic surgeons and other practitioners “understand what our best evidence tells us about diagnosing and treating a variety of conditions,” he said.
The effort to develop the CTS guidelines started February 2013 and involved the Guideline Work Group formulating a set of questions that, as Dr. Peljovich explained, were “the most pertinent questions that anybody interested in a particular diagnosis would want to have answered.” Then a team of statisticians and epidemiologists culled through the “incredible expanse of English language literature” to correlate data to answer those questions.
In May 2015 the work group then met to review the evidence and draft final recommendations. After a period of editing, the draft was submitted for peer review in September. The AAOS board of directors adopted the guidelines in February.
“The guidelines are not intended to be a cookbook on how to treat a condition,” Dr. Peljovich said. “They are really designed to tell you what the best evidence says about a particular set of questions. It helps you to be as updated as you want to be; it’s not designed to tell you this is the only way to do anything. ... It’s an educational tool.”
Members of the Guideline Work Group, AAOS staff, and contributing members submitted their disclosures to the AAOS.