User login
Case
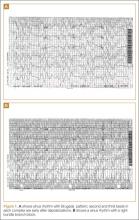
A 50-year-old man ingests two handfuls of small, red berries that he picked from a shrub in front of his apartment building, with the belief that they would have medicinal value. Two hours later, he developed abdominal cramping and vomited multiple times, followed shortly thereafter by profuse diaphoresis, lethargy, and ataxia. His concerned family brought him to the ED where his vital signs on presentation were: blood pressure (BP), 78/43 mm Hg; heart rate (HR), 50 beats/minute; respiratory rate (RR), 12 breaths/minute; temperature (T), 97.8°F. With the exception of bradycardia, the patient’s cardiac, pulmonary, and abdominal examinations were normal. His skin was diaphoretic, and he had no focal motor or sensory deficits or tremor. Initial laboratory values were: hemoglobin, 12.6 g/dL; sodium, 137 mEq/L; potassium, 4.6 mEq/L; bicarbonate, 20 mEq/L; blood urea nitrogen, 17 mg/dL; creatinine, 2.2 mg/dL; glucose, 288 mg/dL. The patient’s troponin I level was slightly elevated at 0.06 ng/mL; electrocardiogram (ECG) results are shown in Figure 1.
Why do plant poisonings occur?
There is the general belief that what is natural is not only healthful but also safe. This is clearly not true: cyanide, uranium, and king cobras are all natural but hardly safe. While most plants chosen for their purported medicinal properties are generally harmless in most patients when taken in low doses, there are plants that are sufficiently poisonous to be consequential with even relatively small exposures. Some people, often unknowingly vulnerable due to genetic or other causes, are uniquely susceptible to even minute doses.
Humans probably learned about plant toxicity early on—most likely the hard way. To this day, however, the Internet is replete with traditional and avant-garde natural healing remedies involving the use of naturally-derived plant products. These numerous bioactive compounds are often sold in plant form or as extracts, the latter being more concerning given their more concentrated formulation.
Plant misidentification is a common cause of poisoning, whether the intended use is for food or medicine. For example, some mistake “deadly nightshade” (Atropa belladonna) berries, which are deep blue, for blueberries, or pokeweed roots for horseradish roots due to their similar appearances.1
Alternatively, even when a plant is correctly identified, patients may experience adverse effects if they exceed the “therapeutic dose” (eg, dysrhythmia from aconite roots used in traditional Chinese medicine) or if the plant is improperly prepared (eg, hypoglycemia from consuming unripe ackee fruit).2 In addition, a toxic plant such as Jimson weed (Datura stramonium) or coca leaf extract may be intentionally ingested for its psychoactive hallucinatory effects.2 Although rare in the United States, in certain parts of Asia, persons intent on self-harm may consume toxic plants.1
When ingested, what plants cause bradycardia and hypotension, and why do these effects occur?
The two broad classes of plant-derived toxins that can cause these findings are cardioactive steroids and sodium channel active agents.
Cardioactive Steroids
There are numerous botanical sources of cardioactive steroids (sometimes called cardiac glycosides) such as Digitalis lanata, from which digoxin is derived; and Digitalis purpurea, the source of digitoxin. Poisoning by Digitalis spp, squill, lily of the valley, oleander, yellow oleander, and Cerbera manghas are clinically similar. Cardioactive steroids act pharmacologically to block the sodium-potassium ATPase pump on the myocardial cell membrane. This in turn increases intracellular sodium, which subsequently inhibits the exchange of extracellular sodium for intracellular calcium, leading to inotropy. Clinical manifestations of toxicity include nausea, vomiting, hyperkalemia, bradycardia, cardiac dysrhythmias, and occasionally hypotension—some of which can be life-threatening.
Sodium Channel Active Agents
Several plant toxins affect the flow of sodium by blocking or activating the sodium channel. Both effects alter the rate and strength of cardiac contraction, causing cardiac dysrhythmias.
Aconite is often used in traditional Chinese medicine. In North America, it is mainly derived from Aconitinum napellus, commonly called monkshood, helmet flower, or wolfsbane. It effectively holds open the voltage-dependent sodium channel, increasing cellular excitability. By prolonging the sodium current influx, neuronal and cardiac repolarization eventually slow due to sodium overload, leading to bradycardia and hypotension, as well as neurological effects. Its cardiotoxicity resembles that caused by cardiac glycosides, though a history of paresthesias or muscle weakness may help to differentiate the two toxins.
Veratrum spp include false hellebore, Indian poke, and California hellebore. These plants are occasionally mistaken for leeks (ramps) and can cause vomiting, bradycardia, and hypotension by a mechanism of action similar to aconitine.
Grayanotoxins, a group of diterpenoid toxins found in death camas, azalea, Rhododendron spp, and mountain laurel, can become concentrated in honey made from these plants. Depending on the specific toxin, they variably open or close the sodium channel. In addition to causing bradycardia and hypotension, patients may exhibit mental status changes (“mad honey” poisoning) and seizures.2
Case Continuation
After rapid infusion of 1-liter of normal saline, the patient’s BP was 80/63 mm Hg and HR was 52 beats/minute. His wife arrived to the ED 30-minutes later with a plastic bag containing the red berries the patient had ingested. The emergency physician identified them as Taxus baccata, or more commonly, yew berries. The patient stated that he ingested both the red fleshy aril and chewed the hard central seed.
How is cardiotoxicity from yew berries treated?
Within hours of ingestion, toxicity progresses from nausea, abdominal pain, paresthesias, and ataxia, to bradycardia, cardiac conduction delays, wide-complex ventricular dysrhythmias and mental status changes.3 Although toxicity of Taxus has been known since antiquity, no antidote exists. Ventricular dysrhythmias causing hemodynamic instability should be electrically cardioverted, although there is no evidence to support the safety or efficacy of such therapy. Since the serum, and therefore cardiac concentration of taxine will be identical after cardioversion to its value prior, recurrent dysrhythmias are common.1 Sodium bicarbonate has been inconsistently effective in the treatment of wide-complex tachydysrhythmias,4 but its use seems counterintuitive for most cases. There may be merit to raising the sodium gradient on an already sodium overloaded myocyte, but short-term gain may lead to unintended consequences. Success with antidysrhythmics has been limited: although amiodarone is often used to treat wide-complex tachydysrhythmias, its efficacy in Taxus toxicity has been conflicting.4-6
There have been a few reported cases of yew alkaloid crossreactivity with digoxin assays, suggesting that digoxin-specific antibody fragments may bind taxine.7 There is no evidence, however, that cardioactive steroids are present in yew, and empiric use of antidigoxin Fab-fragments cannot be recommended. A single case report demonstrated that hemodialysis was ineffective in the removal of taxines, likely due to the toxin’s large volume of distribution.8 As a last resort, extracorporeal life support with membrane oxygenation is described favorably in two cases of yew berry poisoning refractory to conventional therapy.9,10
Case Conclusion
The patient’s ECGs showed a morphologically abnormal rhythm, possibly with a Brugada pattern, which are representative of the dysrhythmias caused by taxine’s inhibitory effects on the sodium and calcium channels. Despite an attempt at electrical cardioversion, the dysrhythmia persisted. He was given intravenous boluses of fluids and started on an amiodarone infusion. The patient’s BP gradually improved over the following 2 hours, and the dysrhythmia resolved with hemodynamic improvement. The amiodarone infusion was then discontinued, and he was admitted to the hospital for further testing. Echocardiography, electrophysiology studies, and cardiac catheterization were all normal. The absence of structural, dysrhythmogenic, and ischemic abnormalities supported the toxic etiology of his hemodynamic aberrations. He was discharged from the hospital 3 days later without report of sequelae.
Dr Nguyen is a medical toxicology fellow in the department of emergency medicine at New York University Langone Medical Center. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
- Bruneton J. Toxic Plants; Dangerous to Humans and Animals. Paris, France: Lavoisier Publishing; 1999:4-752.
- Palmer ME, Betz JM. Plants. In: Nelson LS, Lewin NA, Howland MA, Hoffman RS, Goldfrank LR, Flomenbaum NE. In: Goldfrank’s Toxicologic Emergencies. 9th ed. New York, NY: McGraw Hill; 2010:1537-1560.
- Nelson LS, Shih RD, Balick MJ. Handbook of Poisonous and Injurious Plants. 2nd ed. New York, NY: Springer/New York Botanical Garden; 2007:288-290.
- Pierog J, Kane B, Kane K, Donovan JW. Management of isolated yew berry toxicity with sodium bicarbonate: a case report in treatment efficacy. J Med Toxicol. 2009;5(2):84-89.
- Jones R, Jones J, Causer J, Ewins D, Goenka N, Joseph F. Yew tree poisoning: a near-fatal lesson from history. Clin Med. 2011;11(2):173-175.
- Willaert W, Claessens P, Vankelecom B, Vanderheyden M. Intoxication with Taxus baccata: cardiac arrhythmias following yew leaves ingestion. Pacing Clin Electrophysiol. 2002;25(4 Pt 1):511,512.
- Cummins RO, Haulman J, Quan L, Graves JR, Peterson D, Horan S. Near-fatal yew berry intoxication treated with external cardiac pacing and digoxin-specific FAB antibody fragments. Ann Emerg Med. 1990;19(1):38-43
- Dahlqvist M, Venzin R, König S, et al. Haemodialysis in Taxus baccata poisoning: a case report. QJM. 2012;105(4):359-361.
- Panzeri C, Bacis G, Ferri F, et al. Extracorporeal life support in severe Taxus baccata poisoning. Clin Toxicol. 2010;48(5):463-465.
- Soumagne N, Chauvet S, Chatellier D, Robert R, Charrière JM, Menu P. Treatment of yew leaf intoxication with extracorporeal circulation. Am J Emerg Med. 2011;29(3):354.e5-6.
Case
A 50-year-old man ingests two handfuls of small, red berries that he picked from a shrub in front of his apartment building, with the belief that they would have medicinal value. Two hours later, he developed abdominal cramping and vomited multiple times, followed shortly thereafter by profuse diaphoresis, lethargy, and ataxia. His concerned family brought him to the ED where his vital signs on presentation were: blood pressure (BP), 78/43 mm Hg; heart rate (HR), 50 beats/minute; respiratory rate (RR), 12 breaths/minute; temperature (T), 97.8°F. With the exception of bradycardia, the patient’s cardiac, pulmonary, and abdominal examinations were normal. His skin was diaphoretic, and he had no focal motor or sensory deficits or tremor. Initial laboratory values were: hemoglobin, 12.6 g/dL; sodium, 137 mEq/L; potassium, 4.6 mEq/L; bicarbonate, 20 mEq/L; blood urea nitrogen, 17 mg/dL; creatinine, 2.2 mg/dL; glucose, 288 mg/dL. The patient’s troponin I level was slightly elevated at 0.06 ng/mL; electrocardiogram (ECG) results are shown in Figure 1.
Why do plant poisonings occur?
There is the general belief that what is natural is not only healthful but also safe. This is clearly not true: cyanide, uranium, and king cobras are all natural but hardly safe. While most plants chosen for their purported medicinal properties are generally harmless in most patients when taken in low doses, there are plants that are sufficiently poisonous to be consequential with even relatively small exposures. Some people, often unknowingly vulnerable due to genetic or other causes, are uniquely susceptible to even minute doses.
Humans probably learned about plant toxicity early on—most likely the hard way. To this day, however, the Internet is replete with traditional and avant-garde natural healing remedies involving the use of naturally-derived plant products. These numerous bioactive compounds are often sold in plant form or as extracts, the latter being more concerning given their more concentrated formulation.
Plant misidentification is a common cause of poisoning, whether the intended use is for food or medicine. For example, some mistake “deadly nightshade” (Atropa belladonna) berries, which are deep blue, for blueberries, or pokeweed roots for horseradish roots due to their similar appearances.1
Alternatively, even when a plant is correctly identified, patients may experience adverse effects if they exceed the “therapeutic dose” (eg, dysrhythmia from aconite roots used in traditional Chinese medicine) or if the plant is improperly prepared (eg, hypoglycemia from consuming unripe ackee fruit).2 In addition, a toxic plant such as Jimson weed (Datura stramonium) or coca leaf extract may be intentionally ingested for its psychoactive hallucinatory effects.2 Although rare in the United States, in certain parts of Asia, persons intent on self-harm may consume toxic plants.1
When ingested, what plants cause bradycardia and hypotension, and why do these effects occur?
The two broad classes of plant-derived toxins that can cause these findings are cardioactive steroids and sodium channel active agents.
Cardioactive Steroids
There are numerous botanical sources of cardioactive steroids (sometimes called cardiac glycosides) such as Digitalis lanata, from which digoxin is derived; and Digitalis purpurea, the source of digitoxin. Poisoning by Digitalis spp, squill, lily of the valley, oleander, yellow oleander, and Cerbera manghas are clinically similar. Cardioactive steroids act pharmacologically to block the sodium-potassium ATPase pump on the myocardial cell membrane. This in turn increases intracellular sodium, which subsequently inhibits the exchange of extracellular sodium for intracellular calcium, leading to inotropy. Clinical manifestations of toxicity include nausea, vomiting, hyperkalemia, bradycardia, cardiac dysrhythmias, and occasionally hypotension—some of which can be life-threatening.
Sodium Channel Active Agents
Several plant toxins affect the flow of sodium by blocking or activating the sodium channel. Both effects alter the rate and strength of cardiac contraction, causing cardiac dysrhythmias.
Aconite is often used in traditional Chinese medicine. In North America, it is mainly derived from Aconitinum napellus, commonly called monkshood, helmet flower, or wolfsbane. It effectively holds open the voltage-dependent sodium channel, increasing cellular excitability. By prolonging the sodium current influx, neuronal and cardiac repolarization eventually slow due to sodium overload, leading to bradycardia and hypotension, as well as neurological effects. Its cardiotoxicity resembles that caused by cardiac glycosides, though a history of paresthesias or muscle weakness may help to differentiate the two toxins.
Veratrum spp include false hellebore, Indian poke, and California hellebore. These plants are occasionally mistaken for leeks (ramps) and can cause vomiting, bradycardia, and hypotension by a mechanism of action similar to aconitine.
Grayanotoxins, a group of diterpenoid toxins found in death camas, azalea, Rhododendron spp, and mountain laurel, can become concentrated in honey made from these plants. Depending on the specific toxin, they variably open or close the sodium channel. In addition to causing bradycardia and hypotension, patients may exhibit mental status changes (“mad honey” poisoning) and seizures.2
Case Continuation
After rapid infusion of 1-liter of normal saline, the patient’s BP was 80/63 mm Hg and HR was 52 beats/minute. His wife arrived to the ED 30-minutes later with a plastic bag containing the red berries the patient had ingested. The emergency physician identified them as Taxus baccata, or more commonly, yew berries. The patient stated that he ingested both the red fleshy aril and chewed the hard central seed.
How is cardiotoxicity from yew berries treated?
Within hours of ingestion, toxicity progresses from nausea, abdominal pain, paresthesias, and ataxia, to bradycardia, cardiac conduction delays, wide-complex ventricular dysrhythmias and mental status changes.3 Although toxicity of Taxus has been known since antiquity, no antidote exists. Ventricular dysrhythmias causing hemodynamic instability should be electrically cardioverted, although there is no evidence to support the safety or efficacy of such therapy. Since the serum, and therefore cardiac concentration of taxine will be identical after cardioversion to its value prior, recurrent dysrhythmias are common.1 Sodium bicarbonate has been inconsistently effective in the treatment of wide-complex tachydysrhythmias,4 but its use seems counterintuitive for most cases. There may be merit to raising the sodium gradient on an already sodium overloaded myocyte, but short-term gain may lead to unintended consequences. Success with antidysrhythmics has been limited: although amiodarone is often used to treat wide-complex tachydysrhythmias, its efficacy in Taxus toxicity has been conflicting.4-6
There have been a few reported cases of yew alkaloid crossreactivity with digoxin assays, suggesting that digoxin-specific antibody fragments may bind taxine.7 There is no evidence, however, that cardioactive steroids are present in yew, and empiric use of antidigoxin Fab-fragments cannot be recommended. A single case report demonstrated that hemodialysis was ineffective in the removal of taxines, likely due to the toxin’s large volume of distribution.8 As a last resort, extracorporeal life support with membrane oxygenation is described favorably in two cases of yew berry poisoning refractory to conventional therapy.9,10
Case Conclusion
The patient’s ECGs showed a morphologically abnormal rhythm, possibly with a Brugada pattern, which are representative of the dysrhythmias caused by taxine’s inhibitory effects on the sodium and calcium channels. Despite an attempt at electrical cardioversion, the dysrhythmia persisted. He was given intravenous boluses of fluids and started on an amiodarone infusion. The patient’s BP gradually improved over the following 2 hours, and the dysrhythmia resolved with hemodynamic improvement. The amiodarone infusion was then discontinued, and he was admitted to the hospital for further testing. Echocardiography, electrophysiology studies, and cardiac catheterization were all normal. The absence of structural, dysrhythmogenic, and ischemic abnormalities supported the toxic etiology of his hemodynamic aberrations. He was discharged from the hospital 3 days later without report of sequelae.
Dr Nguyen is a medical toxicology fellow in the department of emergency medicine at New York University Langone Medical Center. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
Case
A 50-year-old man ingests two handfuls of small, red berries that he picked from a shrub in front of his apartment building, with the belief that they would have medicinal value. Two hours later, he developed abdominal cramping and vomited multiple times, followed shortly thereafter by profuse diaphoresis, lethargy, and ataxia. His concerned family brought him to the ED where his vital signs on presentation were: blood pressure (BP), 78/43 mm Hg; heart rate (HR), 50 beats/minute; respiratory rate (RR), 12 breaths/minute; temperature (T), 97.8°F. With the exception of bradycardia, the patient’s cardiac, pulmonary, and abdominal examinations were normal. His skin was diaphoretic, and he had no focal motor or sensory deficits or tremor. Initial laboratory values were: hemoglobin, 12.6 g/dL; sodium, 137 mEq/L; potassium, 4.6 mEq/L; bicarbonate, 20 mEq/L; blood urea nitrogen, 17 mg/dL; creatinine, 2.2 mg/dL; glucose, 288 mg/dL. The patient’s troponin I level was slightly elevated at 0.06 ng/mL; electrocardiogram (ECG) results are shown in Figure 1.
Why do plant poisonings occur?
There is the general belief that what is natural is not only healthful but also safe. This is clearly not true: cyanide, uranium, and king cobras are all natural but hardly safe. While most plants chosen for their purported medicinal properties are generally harmless in most patients when taken in low doses, there are plants that are sufficiently poisonous to be consequential with even relatively small exposures. Some people, often unknowingly vulnerable due to genetic or other causes, are uniquely susceptible to even minute doses.
Humans probably learned about plant toxicity early on—most likely the hard way. To this day, however, the Internet is replete with traditional and avant-garde natural healing remedies involving the use of naturally-derived plant products. These numerous bioactive compounds are often sold in plant form or as extracts, the latter being more concerning given their more concentrated formulation.
Plant misidentification is a common cause of poisoning, whether the intended use is for food or medicine. For example, some mistake “deadly nightshade” (Atropa belladonna) berries, which are deep blue, for blueberries, or pokeweed roots for horseradish roots due to their similar appearances.1
Alternatively, even when a plant is correctly identified, patients may experience adverse effects if they exceed the “therapeutic dose” (eg, dysrhythmia from aconite roots used in traditional Chinese medicine) or if the plant is improperly prepared (eg, hypoglycemia from consuming unripe ackee fruit).2 In addition, a toxic plant such as Jimson weed (Datura stramonium) or coca leaf extract may be intentionally ingested for its psychoactive hallucinatory effects.2 Although rare in the United States, in certain parts of Asia, persons intent on self-harm may consume toxic plants.1
When ingested, what plants cause bradycardia and hypotension, and why do these effects occur?
The two broad classes of plant-derived toxins that can cause these findings are cardioactive steroids and sodium channel active agents.
Cardioactive Steroids
There are numerous botanical sources of cardioactive steroids (sometimes called cardiac glycosides) such as Digitalis lanata, from which digoxin is derived; and Digitalis purpurea, the source of digitoxin. Poisoning by Digitalis spp, squill, lily of the valley, oleander, yellow oleander, and Cerbera manghas are clinically similar. Cardioactive steroids act pharmacologically to block the sodium-potassium ATPase pump on the myocardial cell membrane. This in turn increases intracellular sodium, which subsequently inhibits the exchange of extracellular sodium for intracellular calcium, leading to inotropy. Clinical manifestations of toxicity include nausea, vomiting, hyperkalemia, bradycardia, cardiac dysrhythmias, and occasionally hypotension—some of which can be life-threatening.
Sodium Channel Active Agents
Several plant toxins affect the flow of sodium by blocking or activating the sodium channel. Both effects alter the rate and strength of cardiac contraction, causing cardiac dysrhythmias.
Aconite is often used in traditional Chinese medicine. In North America, it is mainly derived from Aconitinum napellus, commonly called monkshood, helmet flower, or wolfsbane. It effectively holds open the voltage-dependent sodium channel, increasing cellular excitability. By prolonging the sodium current influx, neuronal and cardiac repolarization eventually slow due to sodium overload, leading to bradycardia and hypotension, as well as neurological effects. Its cardiotoxicity resembles that caused by cardiac glycosides, though a history of paresthesias or muscle weakness may help to differentiate the two toxins.
Veratrum spp include false hellebore, Indian poke, and California hellebore. These plants are occasionally mistaken for leeks (ramps) and can cause vomiting, bradycardia, and hypotension by a mechanism of action similar to aconitine.
Grayanotoxins, a group of diterpenoid toxins found in death camas, azalea, Rhododendron spp, and mountain laurel, can become concentrated in honey made from these plants. Depending on the specific toxin, they variably open or close the sodium channel. In addition to causing bradycardia and hypotension, patients may exhibit mental status changes (“mad honey” poisoning) and seizures.2
Case Continuation
After rapid infusion of 1-liter of normal saline, the patient’s BP was 80/63 mm Hg and HR was 52 beats/minute. His wife arrived to the ED 30-minutes later with a plastic bag containing the red berries the patient had ingested. The emergency physician identified them as Taxus baccata, or more commonly, yew berries. The patient stated that he ingested both the red fleshy aril and chewed the hard central seed.
How is cardiotoxicity from yew berries treated?
Within hours of ingestion, toxicity progresses from nausea, abdominal pain, paresthesias, and ataxia, to bradycardia, cardiac conduction delays, wide-complex ventricular dysrhythmias and mental status changes.3 Although toxicity of Taxus has been known since antiquity, no antidote exists. Ventricular dysrhythmias causing hemodynamic instability should be electrically cardioverted, although there is no evidence to support the safety or efficacy of such therapy. Since the serum, and therefore cardiac concentration of taxine will be identical after cardioversion to its value prior, recurrent dysrhythmias are common.1 Sodium bicarbonate has been inconsistently effective in the treatment of wide-complex tachydysrhythmias,4 but its use seems counterintuitive for most cases. There may be merit to raising the sodium gradient on an already sodium overloaded myocyte, but short-term gain may lead to unintended consequences. Success with antidysrhythmics has been limited: although amiodarone is often used to treat wide-complex tachydysrhythmias, its efficacy in Taxus toxicity has been conflicting.4-6
There have been a few reported cases of yew alkaloid crossreactivity with digoxin assays, suggesting that digoxin-specific antibody fragments may bind taxine.7 There is no evidence, however, that cardioactive steroids are present in yew, and empiric use of antidigoxin Fab-fragments cannot be recommended. A single case report demonstrated that hemodialysis was ineffective in the removal of taxines, likely due to the toxin’s large volume of distribution.8 As a last resort, extracorporeal life support with membrane oxygenation is described favorably in two cases of yew berry poisoning refractory to conventional therapy.9,10
Case Conclusion
The patient’s ECGs showed a morphologically abnormal rhythm, possibly with a Brugada pattern, which are representative of the dysrhythmias caused by taxine’s inhibitory effects on the sodium and calcium channels. Despite an attempt at electrical cardioversion, the dysrhythmia persisted. He was given intravenous boluses of fluids and started on an amiodarone infusion. The patient’s BP gradually improved over the following 2 hours, and the dysrhythmia resolved with hemodynamic improvement. The amiodarone infusion was then discontinued, and he was admitted to the hospital for further testing. Echocardiography, electrophysiology studies, and cardiac catheterization were all normal. The absence of structural, dysrhythmogenic, and ischemic abnormalities supported the toxic etiology of his hemodynamic aberrations. He was discharged from the hospital 3 days later without report of sequelae.
Dr Nguyen is a medical toxicology fellow in the department of emergency medicine at New York University Langone Medical Center. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
- Bruneton J. Toxic Plants; Dangerous to Humans and Animals. Paris, France: Lavoisier Publishing; 1999:4-752.
- Palmer ME, Betz JM. Plants. In: Nelson LS, Lewin NA, Howland MA, Hoffman RS, Goldfrank LR, Flomenbaum NE. In: Goldfrank’s Toxicologic Emergencies. 9th ed. New York, NY: McGraw Hill; 2010:1537-1560.
- Nelson LS, Shih RD, Balick MJ. Handbook of Poisonous and Injurious Plants. 2nd ed. New York, NY: Springer/New York Botanical Garden; 2007:288-290.
- Pierog J, Kane B, Kane K, Donovan JW. Management of isolated yew berry toxicity with sodium bicarbonate: a case report in treatment efficacy. J Med Toxicol. 2009;5(2):84-89.
- Jones R, Jones J, Causer J, Ewins D, Goenka N, Joseph F. Yew tree poisoning: a near-fatal lesson from history. Clin Med. 2011;11(2):173-175.
- Willaert W, Claessens P, Vankelecom B, Vanderheyden M. Intoxication with Taxus baccata: cardiac arrhythmias following yew leaves ingestion. Pacing Clin Electrophysiol. 2002;25(4 Pt 1):511,512.
- Cummins RO, Haulman J, Quan L, Graves JR, Peterson D, Horan S. Near-fatal yew berry intoxication treated with external cardiac pacing and digoxin-specific FAB antibody fragments. Ann Emerg Med. 1990;19(1):38-43
- Dahlqvist M, Venzin R, König S, et al. Haemodialysis in Taxus baccata poisoning: a case report. QJM. 2012;105(4):359-361.
- Panzeri C, Bacis G, Ferri F, et al. Extracorporeal life support in severe Taxus baccata poisoning. Clin Toxicol. 2010;48(5):463-465.
- Soumagne N, Chauvet S, Chatellier D, Robert R, Charrière JM, Menu P. Treatment of yew leaf intoxication with extracorporeal circulation. Am J Emerg Med. 2011;29(3):354.e5-6.
- Bruneton J. Toxic Plants; Dangerous to Humans and Animals. Paris, France: Lavoisier Publishing; 1999:4-752.
- Palmer ME, Betz JM. Plants. In: Nelson LS, Lewin NA, Howland MA, Hoffman RS, Goldfrank LR, Flomenbaum NE. In: Goldfrank’s Toxicologic Emergencies. 9th ed. New York, NY: McGraw Hill; 2010:1537-1560.
- Nelson LS, Shih RD, Balick MJ. Handbook of Poisonous and Injurious Plants. 2nd ed. New York, NY: Springer/New York Botanical Garden; 2007:288-290.
- Pierog J, Kane B, Kane K, Donovan JW. Management of isolated yew berry toxicity with sodium bicarbonate: a case report in treatment efficacy. J Med Toxicol. 2009;5(2):84-89.
- Jones R, Jones J, Causer J, Ewins D, Goenka N, Joseph F. Yew tree poisoning: a near-fatal lesson from history. Clin Med. 2011;11(2):173-175.
- Willaert W, Claessens P, Vankelecom B, Vanderheyden M. Intoxication with Taxus baccata: cardiac arrhythmias following yew leaves ingestion. Pacing Clin Electrophysiol. 2002;25(4 Pt 1):511,512.
- Cummins RO, Haulman J, Quan L, Graves JR, Peterson D, Horan S. Near-fatal yew berry intoxication treated with external cardiac pacing and digoxin-specific FAB antibody fragments. Ann Emerg Med. 1990;19(1):38-43
- Dahlqvist M, Venzin R, König S, et al. Haemodialysis in Taxus baccata poisoning: a case report. QJM. 2012;105(4):359-361.
- Panzeri C, Bacis G, Ferri F, et al. Extracorporeal life support in severe Taxus baccata poisoning. Clin Toxicol. 2010;48(5):463-465.
- Soumagne N, Chauvet S, Chatellier D, Robert R, Charrière JM, Menu P. Treatment of yew leaf intoxication with extracorporeal circulation. Am J Emerg Med. 2011;29(3):354.e5-6.