User login
A 60-year-old man presented to our dermatology clinic with a chronic, recurrent pruritic rash on his hands and neck. He noted that the rash developed into blisters, which he would pick until they scabbed over. The rash only manifested on sun-exposed areas.
The patient did not take any medications. He admitted to drinking alcohol (4 beers/d on average) and had roughly a 50-pack year history of smoking. There was no family history of similar symptoms.
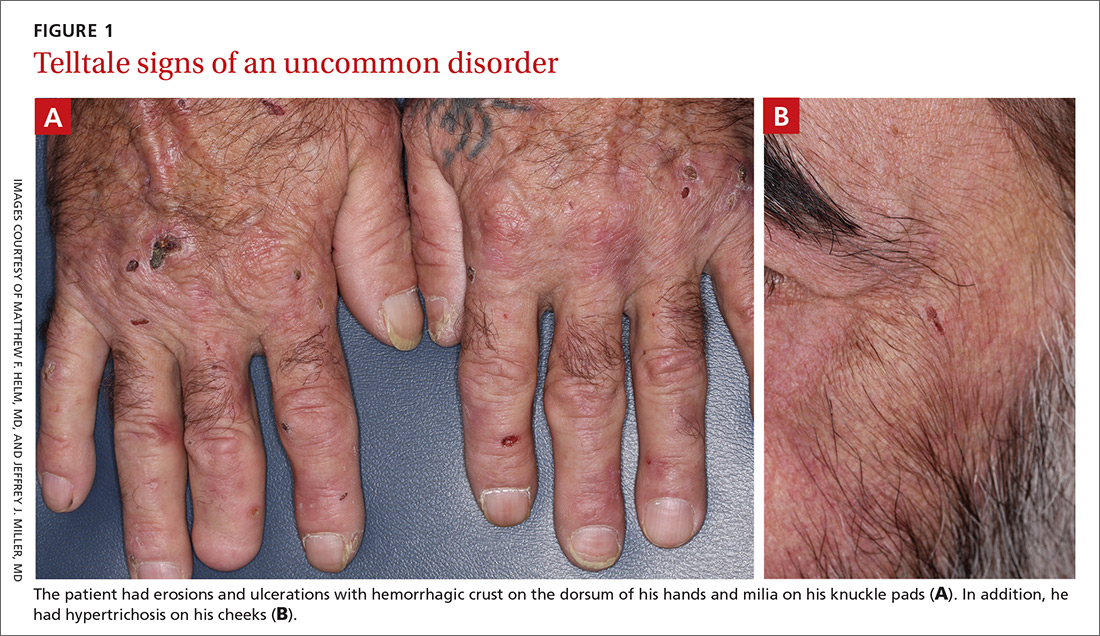
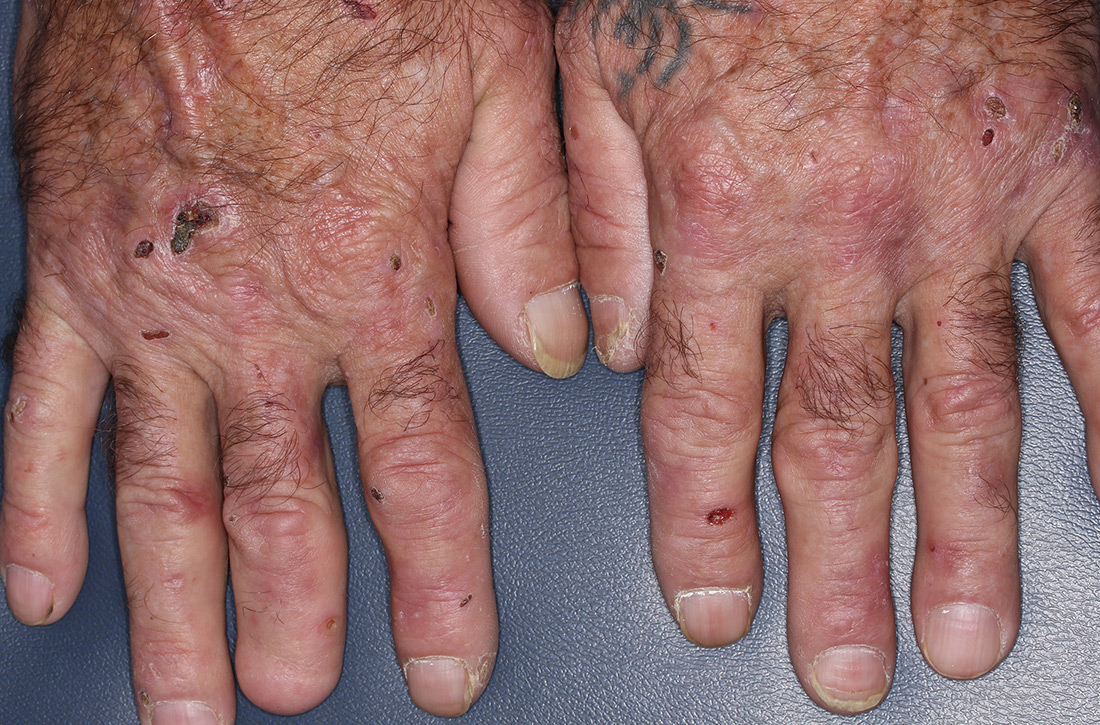
On physical exam, we noted erosions and ulcerations with hemorrhagic crust on the dorsal aspect of his hands, along with milia on the knuckle pads (FIGURE 1A). Further skin examination revealed hypopigmented scars on his shoulders and lower extremities bilaterally, with hypertrichosis of the cheeks (FIGURE 1B).
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Porphyria cutanea tarda
Based on the clinical presentation and the patient’s history of smoking and alcohol consumption, we suspected that this was a case of porphyria cutanea tarda (PCT). Laboratory studies, including a complete blood count, basic metabolic panel, iron studies, and liver function tests, were ordered. These revealed elevated levels of serum alanine transaminase (116 IU/L; reference range, 20-60 IU/L), aspartate aminotransferase (184 IU/L; reference range, 6-34 IU/L in men), and ferritin (1594 ng/mL; reference range, 12-300 ng/mL in men), consistent with PCT. Total porphyrins were then measured and found to be elevated (128.5 mcg/dL; reference range, 0 to 1 mcg/dL), which confirmed the diagnosis. Further testing revealed that the patient was positive for both hepatitis C virus (HCV) and hepatitis B virus infection.
While PCT is the most common porphyria worldwide, it is nonetheless a rare disorder that results from deficient activity (< 20% of normal) of uroporphyrinogen decarboxylase (UROD), the fifth enzyme in the heme synthetic pathway.1,2 It is typically (~75% cases) an acquired disorder of mid- to late adulthood and more commonly affects males.1 In the remainder of cases, patients have a genetic predisposition—a mutation of the UROD or HFE gene. Patients with a genetic predisposition may present earlier.2,3 Susceptibility factors for both forms of PCT include chronic alcohol use, HCV and/or human immunodeficiency virus (HIV) infection, estrogen therapy, and a history of chronic/heavy smoking.1,4
Cutaneous manifestations of PCT are caused by the accumulation of porphyrins, which are photo-oxidized in the skin.1 Findings include photosensitivity, skin fragility, blistering, scarring, hypo- or hyperpigmentation, and milia in sun-exposed areas, such as the dorsum of the hands, forearms, face, ears, neck, and feet.1,2 Hypertrichosis can occur, particularly on the cheeks and forearms.1 Elevated transaminases often accompany cutaneous findings, due to porphyrin accumulation in hepatocytes and the hepatotoxic effects of alcohol, HCV infection, or iron overload.5 Iron overload, in part due to dysregulation of hepcidin, can lead to increased serum ferritin, iron, and transferrin saturation.1
Differential includes autoimmune and autosomal conditions
Diseases that manifest with blistering, elevated porphyrins or porphyrin precursors, and iron overload should be included in the differential diagnosis.
Bullous pemphigoid is an autoimmune subepithelial blistering disorder that occurs when antibodies attack hemidesmosomes in the epidermis. It commonly manifests in the elderly and classically presents with tense bullae, typically on the trunk, abdomen, and proximal extremities. Serologic testing and biopsy can confirm the diagnosis.6
Continue to: Pseudoporphyria...
Pseudoporphyria has a similar presentation to PCT but with no abnormalities in porphyrin metabolism. Risk factors include UV radiation exposure; use of medications such as nonsteroidal anti-inflammatory drugs, diuretics, and retinoids; chronic renal failure; and hemodialysis.7
Acute intermittent porphyria is an autosomal dominant disorder due to deficiency of porphobilinogen deaminase, a heme biosynthetic enzyme. Clinical manifestations usually arise in adulthood and include neurovisceral attacks (eg, abdominal pain, vomiting, muscle weakness). Diagnosis during an acute attack can be made by measuring urinary 5-aminolaevulinc acid and porphobilinogen.1
Hereditary hemochromatosis is an autosomal recessive disorder most commonly due to mutations in the HFE gene. Patients typically have iron overload and abnormal liver function test results. The main cutaneous finding is skin hyperpigmentation. Patients also may develop diabetes mellitus, arthropathy, cardiac disease, and hypopituitarism, although most are diagnosed with asymptomatic disease following routine laboratory studies.8
Confirm the diagnosis with total porphyrin measurement
The preferred initial test to confirm the diagnosis of PCT is measurement of plasma or urine total porphyrins, which will be elevated.1 Further testing is then performed to discern PCT from the other, less common cutaneous porphyrias.1 If needed, biopsy can be done to exclude other diagnoses. Testing for HIV and viral hepatitis infection may be performed when clinical suspicion is high.1 Testing for UROD and HFE mutations may also be advised.1
Treatment choice is guided by iron levels
For patients with normal iron levels, low-dose hydroxychloroquine 100 mg or chloroquine 125 mg twice per week can be used until restoration of normal plasma or urine porphyrin levels has been achieved for several months.1 For those with iron excess (serum ferritin > 600 ng/dL), repeat phlebotomy is the preferred treatment; a unit of blood (350-500 mL) is typically removed, as tolerated, until iron stores return to normal.1 In severe cases of PCT, these therapies can be used in combination.1 Clinical remission with these methods can be expected within 6 to 9 months.9
Continue to: In addition...
In addition, it is important to provide patient education regarding proper sun protection and risk factor modification.1 Underlying HIV and viral hepatitis infection should be managed appropriately by the relevant specialists.
Our patient was counseled on proper sun protection and encouraged to cease alcohol consumption and smoking. We subsequently referred him to Hepatology for the treatment of his liver disease. Given that the patient’s ferritin level was so high (1594 ng/mL), serial phlebotomy was initiated twice monthly until levels reached the lower limit of normal. He was also started on direct-acting antiviral therapy with Epclusa (sofosbuvir/velpatasvir) for 12 weeks for treatment of his HCV and is currently in remission.
CORRESPONDENCE
Christopher G. Bazewicz, MD, Department of Dermatology, Penn State Milton S. Hershey Medical Center, 500 University Drive, Hershey, PA 17033; bazewicz@uchc.edu
1. Puy H, Gouya L, Deybach JC. Porphyrias. Lancet. 2010;375:924-937.
2. Méndez M, Poblete-Gutiérrez P, García-Bravo M, et al. Molecular heterogeneity of familial porphyria cutanea tarda in Spain: characterization of 10 novel mutations in the UROD gene. Br J Dermatol. 2007;157:501-507.
3. Brady JJ, Jackson HA, Roberts AG, et al. Co-inheritance of mutations in the uroporphyrinogen decarboxylase and hemochromatosis genes accelerates the onset of porphyria cutanea tarda. J Invest Dermatol. 2000;115:868-874.
4. Jalil S, Grady JJ, Lee C, et al. Associations among behavior-related susceptibility factors in porphyria cutanea tarda. Clin Gastroenterol Hepatol. 2010;8:297-302, 302.e1.
5. Gisbert JP, García-Buey L, Alonso A, et al. Hepatocellular carcinoma risk in patients with porphyria cutanea tarda. Eur J Gastroenterol Hepatol. 2004;16:689-692.
6. Di Zenzo G, Della Torre R, Zambruno G, et al. Bullous pemphigoid: from the clinic to the bench. Clin Dermatol. 2012;30:3-16.
7. Green JJ, Manders SM. Pseudoporphyria. J Am Acad Dermatol. 2001;44:100-108.
8. Crownover BK, Covey CJ. Hereditary hemochromatosis. Am Fam Physician. 2013;87:183-190.
9. Sarkany RP. The management of porphyria cutanea tarda. Clin Exp Dermatol. 2001;26:225-232.
A 60-year-old man presented to our dermatology clinic with a chronic, recurrent pruritic rash on his hands and neck. He noted that the rash developed into blisters, which he would pick until they scabbed over. The rash only manifested on sun-exposed areas.
The patient did not take any medications. He admitted to drinking alcohol (4 beers/d on average) and had roughly a 50-pack year history of smoking. There was no family history of similar symptoms.
On physical exam, we noted erosions and ulcerations with hemorrhagic crust on the dorsal aspect of his hands, along with milia on the knuckle pads (FIGURE 1A). Further skin examination revealed hypopigmented scars on his shoulders and lower extremities bilaterally, with hypertrichosis of the cheeks (FIGURE 1B).
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Porphyria cutanea tarda
Based on the clinical presentation and the patient’s history of smoking and alcohol consumption, we suspected that this was a case of porphyria cutanea tarda (PCT). Laboratory studies, including a complete blood count, basic metabolic panel, iron studies, and liver function tests, were ordered. These revealed elevated levels of serum alanine transaminase (116 IU/L; reference range, 20-60 IU/L), aspartate aminotransferase (184 IU/L; reference range, 6-34 IU/L in men), and ferritin (1594 ng/mL; reference range, 12-300 ng/mL in men), consistent with PCT. Total porphyrins were then measured and found to be elevated (128.5 mcg/dL; reference range, 0 to 1 mcg/dL), which confirmed the diagnosis. Further testing revealed that the patient was positive for both hepatitis C virus (HCV) and hepatitis B virus infection.
While PCT is the most common porphyria worldwide, it is nonetheless a rare disorder that results from deficient activity (< 20% of normal) of uroporphyrinogen decarboxylase (UROD), the fifth enzyme in the heme synthetic pathway.1,2 It is typically (~75% cases) an acquired disorder of mid- to late adulthood and more commonly affects males.1 In the remainder of cases, patients have a genetic predisposition—a mutation of the UROD or HFE gene. Patients with a genetic predisposition may present earlier.2,3 Susceptibility factors for both forms of PCT include chronic alcohol use, HCV and/or human immunodeficiency virus (HIV) infection, estrogen therapy, and a history of chronic/heavy smoking.1,4
Cutaneous manifestations of PCT are caused by the accumulation of porphyrins, which are photo-oxidized in the skin.1 Findings include photosensitivity, skin fragility, blistering, scarring, hypo- or hyperpigmentation, and milia in sun-exposed areas, such as the dorsum of the hands, forearms, face, ears, neck, and feet.1,2 Hypertrichosis can occur, particularly on the cheeks and forearms.1 Elevated transaminases often accompany cutaneous findings, due to porphyrin accumulation in hepatocytes and the hepatotoxic effects of alcohol, HCV infection, or iron overload.5 Iron overload, in part due to dysregulation of hepcidin, can lead to increased serum ferritin, iron, and transferrin saturation.1
Differential includes autoimmune and autosomal conditions
Diseases that manifest with blistering, elevated porphyrins or porphyrin precursors, and iron overload should be included in the differential diagnosis.
Bullous pemphigoid is an autoimmune subepithelial blistering disorder that occurs when antibodies attack hemidesmosomes in the epidermis. It commonly manifests in the elderly and classically presents with tense bullae, typically on the trunk, abdomen, and proximal extremities. Serologic testing and biopsy can confirm the diagnosis.6
Continue to: Pseudoporphyria...
Pseudoporphyria has a similar presentation to PCT but with no abnormalities in porphyrin metabolism. Risk factors include UV radiation exposure; use of medications such as nonsteroidal anti-inflammatory drugs, diuretics, and retinoids; chronic renal failure; and hemodialysis.7
Acute intermittent porphyria is an autosomal dominant disorder due to deficiency of porphobilinogen deaminase, a heme biosynthetic enzyme. Clinical manifestations usually arise in adulthood and include neurovisceral attacks (eg, abdominal pain, vomiting, muscle weakness). Diagnosis during an acute attack can be made by measuring urinary 5-aminolaevulinc acid and porphobilinogen.1
Hereditary hemochromatosis is an autosomal recessive disorder most commonly due to mutations in the HFE gene. Patients typically have iron overload and abnormal liver function test results. The main cutaneous finding is skin hyperpigmentation. Patients also may develop diabetes mellitus, arthropathy, cardiac disease, and hypopituitarism, although most are diagnosed with asymptomatic disease following routine laboratory studies.8
Confirm the diagnosis with total porphyrin measurement
The preferred initial test to confirm the diagnosis of PCT is measurement of plasma or urine total porphyrins, which will be elevated.1 Further testing is then performed to discern PCT from the other, less common cutaneous porphyrias.1 If needed, biopsy can be done to exclude other diagnoses. Testing for HIV and viral hepatitis infection may be performed when clinical suspicion is high.1 Testing for UROD and HFE mutations may also be advised.1
Treatment choice is guided by iron levels
For patients with normal iron levels, low-dose hydroxychloroquine 100 mg or chloroquine 125 mg twice per week can be used until restoration of normal plasma or urine porphyrin levels has been achieved for several months.1 For those with iron excess (serum ferritin > 600 ng/dL), repeat phlebotomy is the preferred treatment; a unit of blood (350-500 mL) is typically removed, as tolerated, until iron stores return to normal.1 In severe cases of PCT, these therapies can be used in combination.1 Clinical remission with these methods can be expected within 6 to 9 months.9
Continue to: In addition...
In addition, it is important to provide patient education regarding proper sun protection and risk factor modification.1 Underlying HIV and viral hepatitis infection should be managed appropriately by the relevant specialists.
Our patient was counseled on proper sun protection and encouraged to cease alcohol consumption and smoking. We subsequently referred him to Hepatology for the treatment of his liver disease. Given that the patient’s ferritin level was so high (1594 ng/mL), serial phlebotomy was initiated twice monthly until levels reached the lower limit of normal. He was also started on direct-acting antiviral therapy with Epclusa (sofosbuvir/velpatasvir) for 12 weeks for treatment of his HCV and is currently in remission.
CORRESPONDENCE
Christopher G. Bazewicz, MD, Department of Dermatology, Penn State Milton S. Hershey Medical Center, 500 University Drive, Hershey, PA 17033; bazewicz@uchc.edu
A 60-year-old man presented to our dermatology clinic with a chronic, recurrent pruritic rash on his hands and neck. He noted that the rash developed into blisters, which he would pick until they scabbed over. The rash only manifested on sun-exposed areas.
The patient did not take any medications. He admitted to drinking alcohol (4 beers/d on average) and had roughly a 50-pack year history of smoking. There was no family history of similar symptoms.
On physical exam, we noted erosions and ulcerations with hemorrhagic crust on the dorsal aspect of his hands, along with milia on the knuckle pads (FIGURE 1A). Further skin examination revealed hypopigmented scars on his shoulders and lower extremities bilaterally, with hypertrichosis of the cheeks (FIGURE 1B).
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Porphyria cutanea tarda
Based on the clinical presentation and the patient’s history of smoking and alcohol consumption, we suspected that this was a case of porphyria cutanea tarda (PCT). Laboratory studies, including a complete blood count, basic metabolic panel, iron studies, and liver function tests, were ordered. These revealed elevated levels of serum alanine transaminase (116 IU/L; reference range, 20-60 IU/L), aspartate aminotransferase (184 IU/L; reference range, 6-34 IU/L in men), and ferritin (1594 ng/mL; reference range, 12-300 ng/mL in men), consistent with PCT. Total porphyrins were then measured and found to be elevated (128.5 mcg/dL; reference range, 0 to 1 mcg/dL), which confirmed the diagnosis. Further testing revealed that the patient was positive for both hepatitis C virus (HCV) and hepatitis B virus infection.
While PCT is the most common porphyria worldwide, it is nonetheless a rare disorder that results from deficient activity (< 20% of normal) of uroporphyrinogen decarboxylase (UROD), the fifth enzyme in the heme synthetic pathway.1,2 It is typically (~75% cases) an acquired disorder of mid- to late adulthood and more commonly affects males.1 In the remainder of cases, patients have a genetic predisposition—a mutation of the UROD or HFE gene. Patients with a genetic predisposition may present earlier.2,3 Susceptibility factors for both forms of PCT include chronic alcohol use, HCV and/or human immunodeficiency virus (HIV) infection, estrogen therapy, and a history of chronic/heavy smoking.1,4
Cutaneous manifestations of PCT are caused by the accumulation of porphyrins, which are photo-oxidized in the skin.1 Findings include photosensitivity, skin fragility, blistering, scarring, hypo- or hyperpigmentation, and milia in sun-exposed areas, such as the dorsum of the hands, forearms, face, ears, neck, and feet.1,2 Hypertrichosis can occur, particularly on the cheeks and forearms.1 Elevated transaminases often accompany cutaneous findings, due to porphyrin accumulation in hepatocytes and the hepatotoxic effects of alcohol, HCV infection, or iron overload.5 Iron overload, in part due to dysregulation of hepcidin, can lead to increased serum ferritin, iron, and transferrin saturation.1
Differential includes autoimmune and autosomal conditions
Diseases that manifest with blistering, elevated porphyrins or porphyrin precursors, and iron overload should be included in the differential diagnosis.
Bullous pemphigoid is an autoimmune subepithelial blistering disorder that occurs when antibodies attack hemidesmosomes in the epidermis. It commonly manifests in the elderly and classically presents with tense bullae, typically on the trunk, abdomen, and proximal extremities. Serologic testing and biopsy can confirm the diagnosis.6
Continue to: Pseudoporphyria...
Pseudoporphyria has a similar presentation to PCT but with no abnormalities in porphyrin metabolism. Risk factors include UV radiation exposure; use of medications such as nonsteroidal anti-inflammatory drugs, diuretics, and retinoids; chronic renal failure; and hemodialysis.7
Acute intermittent porphyria is an autosomal dominant disorder due to deficiency of porphobilinogen deaminase, a heme biosynthetic enzyme. Clinical manifestations usually arise in adulthood and include neurovisceral attacks (eg, abdominal pain, vomiting, muscle weakness). Diagnosis during an acute attack can be made by measuring urinary 5-aminolaevulinc acid and porphobilinogen.1
Hereditary hemochromatosis is an autosomal recessive disorder most commonly due to mutations in the HFE gene. Patients typically have iron overload and abnormal liver function test results. The main cutaneous finding is skin hyperpigmentation. Patients also may develop diabetes mellitus, arthropathy, cardiac disease, and hypopituitarism, although most are diagnosed with asymptomatic disease following routine laboratory studies.8
Confirm the diagnosis with total porphyrin measurement
The preferred initial test to confirm the diagnosis of PCT is measurement of plasma or urine total porphyrins, which will be elevated.1 Further testing is then performed to discern PCT from the other, less common cutaneous porphyrias.1 If needed, biopsy can be done to exclude other diagnoses. Testing for HIV and viral hepatitis infection may be performed when clinical suspicion is high.1 Testing for UROD and HFE mutations may also be advised.1
Treatment choice is guided by iron levels
For patients with normal iron levels, low-dose hydroxychloroquine 100 mg or chloroquine 125 mg twice per week can be used until restoration of normal plasma or urine porphyrin levels has been achieved for several months.1 For those with iron excess (serum ferritin > 600 ng/dL), repeat phlebotomy is the preferred treatment; a unit of blood (350-500 mL) is typically removed, as tolerated, until iron stores return to normal.1 In severe cases of PCT, these therapies can be used in combination.1 Clinical remission with these methods can be expected within 6 to 9 months.9
Continue to: In addition...
In addition, it is important to provide patient education regarding proper sun protection and risk factor modification.1 Underlying HIV and viral hepatitis infection should be managed appropriately by the relevant specialists.
Our patient was counseled on proper sun protection and encouraged to cease alcohol consumption and smoking. We subsequently referred him to Hepatology for the treatment of his liver disease. Given that the patient’s ferritin level was so high (1594 ng/mL), serial phlebotomy was initiated twice monthly until levels reached the lower limit of normal. He was also started on direct-acting antiviral therapy with Epclusa (sofosbuvir/velpatasvir) for 12 weeks for treatment of his HCV and is currently in remission.
CORRESPONDENCE
Christopher G. Bazewicz, MD, Department of Dermatology, Penn State Milton S. Hershey Medical Center, 500 University Drive, Hershey, PA 17033; bazewicz@uchc.edu
1. Puy H, Gouya L, Deybach JC. Porphyrias. Lancet. 2010;375:924-937.
2. Méndez M, Poblete-Gutiérrez P, García-Bravo M, et al. Molecular heterogeneity of familial porphyria cutanea tarda in Spain: characterization of 10 novel mutations in the UROD gene. Br J Dermatol. 2007;157:501-507.
3. Brady JJ, Jackson HA, Roberts AG, et al. Co-inheritance of mutations in the uroporphyrinogen decarboxylase and hemochromatosis genes accelerates the onset of porphyria cutanea tarda. J Invest Dermatol. 2000;115:868-874.
4. Jalil S, Grady JJ, Lee C, et al. Associations among behavior-related susceptibility factors in porphyria cutanea tarda. Clin Gastroenterol Hepatol. 2010;8:297-302, 302.e1.
5. Gisbert JP, García-Buey L, Alonso A, et al. Hepatocellular carcinoma risk in patients with porphyria cutanea tarda. Eur J Gastroenterol Hepatol. 2004;16:689-692.
6. Di Zenzo G, Della Torre R, Zambruno G, et al. Bullous pemphigoid: from the clinic to the bench. Clin Dermatol. 2012;30:3-16.
7. Green JJ, Manders SM. Pseudoporphyria. J Am Acad Dermatol. 2001;44:100-108.
8. Crownover BK, Covey CJ. Hereditary hemochromatosis. Am Fam Physician. 2013;87:183-190.
9. Sarkany RP. The management of porphyria cutanea tarda. Clin Exp Dermatol. 2001;26:225-232.
1. Puy H, Gouya L, Deybach JC. Porphyrias. Lancet. 2010;375:924-937.
2. Méndez M, Poblete-Gutiérrez P, García-Bravo M, et al. Molecular heterogeneity of familial porphyria cutanea tarda in Spain: characterization of 10 novel mutations in the UROD gene. Br J Dermatol. 2007;157:501-507.
3. Brady JJ, Jackson HA, Roberts AG, et al. Co-inheritance of mutations in the uroporphyrinogen decarboxylase and hemochromatosis genes accelerates the onset of porphyria cutanea tarda. J Invest Dermatol. 2000;115:868-874.
4. Jalil S, Grady JJ, Lee C, et al. Associations among behavior-related susceptibility factors in porphyria cutanea tarda. Clin Gastroenterol Hepatol. 2010;8:297-302, 302.e1.
5. Gisbert JP, García-Buey L, Alonso A, et al. Hepatocellular carcinoma risk in patients with porphyria cutanea tarda. Eur J Gastroenterol Hepatol. 2004;16:689-692.
6. Di Zenzo G, Della Torre R, Zambruno G, et al. Bullous pemphigoid: from the clinic to the bench. Clin Dermatol. 2012;30:3-16.
7. Green JJ, Manders SM. Pseudoporphyria. J Am Acad Dermatol. 2001;44:100-108.
8. Crownover BK, Covey CJ. Hereditary hemochromatosis. Am Fam Physician. 2013;87:183-190.
9. Sarkany RP. The management of porphyria cutanea tarda. Clin Exp Dermatol. 2001;26:225-232.