User login
Uric acid—urate in most physiologic fluids—is an end product of purine degradation. The serum urate level in a given patient is determined by the amount of purines synthesized and ingested, the amount of urate produced from purines, and the amount of uric acid excreted by the kidney (and, to a lesser degree, from the gastrointestinal tract).1 A major source of circulating urate is the metabolized endogenous purine. Renal excretion is likely determined by genetic factors that dictate expression of uric acid transporters, as well as by the presence of organic acids, certain drugs, hormones, and the glomerular filtration rate. A small minority of patients will have increased production of urate as a result of enzymopathies, chronic hemolysis, or rapidly dividing tumors, psoriasis, or other disorders characterized by increased turnover of cells.
Humans do not have a functional enzyme (uricase) to break down urate into allantoin, which is more soluble and readily excreted. There may have been genetic pressures that explain why functional uricase was lost and why humans have relatively high urate levels compared with other species.2 If higher levels of serum urate are clinically detrimental, one would think that humans could have evolved an efficient way to excrete it. Instead, we excrete uric acid inefficiently as a result of active reabsorption in the proximal renal tubule. We have higher levels of serum urate than most other species, and we are predisposed to develop gouty arthritis and perhaps other sequelae of hyperuricemia, including hypertension, the metabolic syndrome, and coronary artery disease.
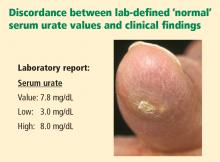
CLINICALLY SIGNIFICANT HYPERURICEMIA VS LAB-DEFINED HYPERURICEMIA
Clinically significant hyperuricemia is a serum urate level greater than 6.8 mg/dL, although the population-defined “normal” urate level indicated by the clinical laboratory is higher. At levels above 6.8 mg/dL, urate exceeds its solubility in most biological fluids.
SERUM URATE CAN VARY BY SEX, AGE, DIET
Men generally have higher serum urate levels than premenopausal women; serum urate levels increase in women after menopause. For years these findings were attributed to an estrogen effect, but the mechanism was not well understood. Recently a specific transporter (urate transporter 1 [URAT1]) has been identified in the proximal tubule of the kidney3 that seems primarily responsible for the reabsorption of uric acid. Estrogen has a direct effect on the expression of this transporter. It also seems that the hypouricemic effects of probenecid and losartan, as well as the hyperuricemic effects of organic acids and high insulin levels, may be mediated via modulation of URAT1 activity.
Urate values tend to be lower in children, and urate levels are generally affected only modestly by diet.4 Epidemiologic studies, however, have linked increased ingestion of red meats and low ingestion of dairy foods with an increased incidence of gout.5 Acute alcohol ingestion can cause fluctuations in the serum urate levels and may precipitate acute gout attacks.
HYPERURICEMIA LEADING TO GOUT
Urate concentrations greater than 6.8 mg/dL may result in the deposition of urate crystals in the tissues around joints and in other soft tissue structures (tophi). Why this occurs in only some patients is not known. Crystals, when mobilized from these deposits, can provoke the acute gouty flare. The tophi are not usually hot or tender. Biopsy of a tophus reveals a chronic granulomatous inflammatory response around the sequestered crystals. However, the tophi are not inert; the uric acid can be mobilized by mass action effect if the urate in surrounding fluid is reduced. If tophi are adjacent to bone, erosion into bone may occur.
CLINICAL PROGRESSION OF HYPERURICEMIA AND GOUT: FOUR STAGES OF A CHRONIC DISEASE
Although there is significant heterogeneity in the expression of gout, we can conceptualize a prototypic progression from asymptomatic hyperuricemia to chronic gouty arthritis.
Stage 1: Asymptomatic hyperuricemia. At a serum urate concentration greater than 6.8 mg/dL, urate crystals may start to deposit. During this period of asymptomatic hyperuricemia, urate deposits may directly contribute to organ damage. This does not occur in everyone, however, and at present there is no evidence that treatment is warranted for asymptomatic hyperuricemia.
Stages 2 and 3: Acute gout and intercritical periods. If sufficient urate deposits develop around joints, and if the local milieu or some trauma triggers the release of crystals into the joint space, a patient will suffer acute attacks of gout. These flares are self-resolving but are likely to recur. The intervals between attacks are termed “intercritical periods.” During these periods, crystals may still be present at a low level in the fluid, and are certainly present in the periarticular and synovial tissue, providing a nidus for future attacks.
Stage 4: Advanced gout. If crystal deposits continue to accumulate, patients may develop chronically stiff and swollen joints. This advanced stage of gout is relatively uncommon but is avoidable with therapy.
Progression is variable
The progression from asymptomatic hyperuricemia to advanced gout is quite variable from person to person. In most people it takes many years to progress, if it does so at all. In patients treated with cyclosporine following an organ transplant, the progression can be accelerated, although the reasons are not fully understood.
ASYMPTOMATIC HYPERURICEMIA: TO TREAT OR NOT TO TREAT?
Clues for predicting the likelihood that an individual patient with asymptomatic hyperuricemia will develop articular gout are elusive. Campion and colleagues presented data on men without a history of gout who were grouped by serum urate level and followed over a 5-year period.6 The higher the patient’s urate level, the more likely that he would have a gouty attack during the 5 years. In this relatively young population of hyperuricemic men (average age of 42 years), less than 30% developed gout over this short period.
The dilemma is how to predict who is most likely to get gout and will benefit from early urate-lowering treatment, and who will not. Currently, clinicians have no reliable way of predicting the likelihood of gout development in a given hyperuricemic patient. A history of organ transplantation, the continued need for diuretics, an extremely high urate level, alcohol ingestion, low dietary consumption of dairy products, high consumption of meat and seafood, and a family history of gout at a young age suggest a higher risk of gouty arthritis. At present, treatment of asymptomatic hyperuricemia in order to prevent gouty arthritis is not generally recommended.
ACUTE GOUT FLARES: PAINFUL, UNPREDICTABLE, HIGHLY LIKELY TO RECUR
Acute flares of gouty arthritis are characterized by warmth, swelling, redness, and often severe pain. Pain frequently begins in the middle of the night or early morning. Many patients will describe awakening with pain in the foot that is so intense that they are unable to support their own weight. Patients may report fever and a flulike malaise. Fever and constitutional features are sequelae of the release of cytokines such as tumor necrosis factor, interleukin-1, and interleukin-6 following phagocytosis of crystals and activation of the intracellular inflammasome complex.7 Untreated, the initial attack will usually resolve in 3 to 14 days. Subsequent attacks tend to last longer and may involve more joints or tendons.
Where flares occur
It has been estimated that 90% of first attacks are monoarticular. However, the first recognized attack can be oligoarticular or even polyarticular. This seems particularly true in postmenopausal women and in transplant recipients. Gout attacks initially tend to occur in the lower extremities: midfoot, first metatarsophalangeal joint, ankle, or knee. Over time, gout tends to include additional joints, including those of the upper extremities. Axial joints are far less commonly involved. The initial (or subsequent) attack may be in the instep of the foot, not a well-defined joint. Patients may recall “ankle sprains,” often ascribed to an event such as “stepping off the curb wrong,” with delayed ankle swelling. These may have been attacks of gout that were not recognized by the patient and thus not reported to his or her physician. Bunion pain may be incorrectly attributed to gout (and vice versa). Therefore, we need to accept the limitations of historical recognition of gout attacks.
Acute flares also occur in periarticular structures, including bursae and tendons. The olecranon bursa, the tendons around the ankle, and the bursae around the knee are among the locations where acute attacks of gout can occur.
Risk of recurrence and implications for treatment
Based on historical data, the estimated flare recurrence rate is approximately 60% within 1 year after the initial attack, 78% within 2 years, and 84% within 3 years. Less than 10% of patients will not have a recurrence over a 10-year period. Untreated, some patients with gout will continue to have attacks and accrue chronic joint damage, stiffness, and tophi. However, that does not imply that published outcome data support treating every patient with urate-lowering therapy following an initial gout attack or even several attacks. There are no outcome data from appropriately controlled, long-term trials to validate such a treatment approach. Nonetheless, in some gouty patients, if hyperuricemia is not addressed, morbidity and joint damage will accrue. The decision as to when to intervene with urate-lowering therapy should be individualized, taking into consideration comorbidities, estimation of the likelihood of continued attacks, the impact of attacks on the patient’s lifestyle, and the potential complications of needing to use medications to treat acute attacks.
INTERCRITICAL PERIODS: CRYSTAL DEPOSITION CONTINUES SILENTLY
Immediately after an attack of gout, patients may be apt to have another if anti-inflammatory therapy is not provided for a long enough period, ie, until several days after an attack has completely resolved. Subsequently, there may be a prolonged period before another attack occurs. During this time, uric acid deposits may continue to increase silently. The factors that control the rate, location, and degree of ongoing deposition in a specific patient are not well defined. Crystals may still be found in the synovial fluid of previously involved joints until the serum urate level is reduced for a significant period to a level significantly less than 6.8 mg/dL.8
ADVANCED GOUT: DIFFERENTIATION FROM RHEUMATOID ARTHRITIS IS KEY
Tissue stores of urate may continue to increase if hyperuricemia persists at biologically significant levels (> 6.8 mg/dL). Crystal deposition can cause chronic polyarthritis. Some patients, especially as they age, develop rheumatoid factor positivity. Chronic gout, involving multiple joints, can mimic rheumatoid arthritis. Patients can develop subcutaneous tophi in areas of friction or trauma. These tophi, as well as periarticular ones, can be mistaken for rheumatoid nodules. It is unclear why only some people with hyperuricemia develop tophi. The presence of urate crystals in the aspirate of a nodule (tophus) or synovial fluid will distinguish gout from rheumatoid arthritis. Radiographs can also be of diagnostic use.
Unlike radiographic findings in rheumatoid arthritis, in gout there is a prominent, proliferative bony reaction, and tophi can cause bone destruction away from the joint. There may be a characteristic “overhanging edge” of proliferating bone surrounding a gout erosion (see Figure 3 in preceding article by Schumacher). These radiographic findings, although distinct from those of rheumatoid arthritis, can be confused with psoriatic arthritis, which also can be erosive with a proliferative bone response. Gout, however, is less likely to cause joint space narrowing than is either psoriatic arthritis or rheumatoid arthritis.
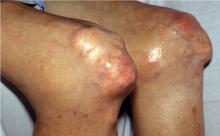
Intradermal tophi (Figure 1) are asymptomatic and frequently not recognized, yet are not that rare in severe untreated gout. Such tophi may be particularly common in transplant patients and appear as white or yellowish deposits with the overlying skin pulled taut.
POSTSCRIPT: GOUT IS NOT SO EASILY RECOGNIZED AFTER ALL
- Choi HK, Mount DB, Reginato AM. Pathogenesis of gout. Ann Intern Med 2005; 143:499–516.
- Oda M, Satta Y, Takenaka O, Takahata N. Loss of urate oxidase activity in hominoids and its evolutionary implications. Mol Biol Evol 2002; 19:640–653.
- Enomoto A, Kimura H, Chairoungdua A, et al. Molecular identification of a renal urate anion exchanger that regulates blood urate levels. Nature 2002; 417:447–452.
- Fam AG. Gout: excess calories, purines, and alcohol intake and beyond. Response to a urate-lowering diet. J Rheumatol 2005; 32:773–777.
- Choi HK, Atkinson K, Karlson EW, Willett W, Curhan G. Purine-rich foods, dairy and protein intake, and the risk of gout in men. N Engl J Med 2004; 350:1093–1103.
- Campion EW, Glynn RJ, DeLabry LO. Asymptomatic hyperuricemia. Risks and consequences in the Normative Aging Study. Am J Med 1987; 82:421–426.
- Martinon F, Pétrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 2006; 440:237–241.
- Pascual E, Sivera F. Time required for disappearance of urate crystals from synovial fluid after successful hypouricaemic treatment relates to the duration of gout. Ann Rheum Dis 2007; 66:1056–1058.
Uric acid—urate in most physiologic fluids—is an end product of purine degradation. The serum urate level in a given patient is determined by the amount of purines synthesized and ingested, the amount of urate produced from purines, and the amount of uric acid excreted by the kidney (and, to a lesser degree, from the gastrointestinal tract).1 A major source of circulating urate is the metabolized endogenous purine. Renal excretion is likely determined by genetic factors that dictate expression of uric acid transporters, as well as by the presence of organic acids, certain drugs, hormones, and the glomerular filtration rate. A small minority of patients will have increased production of urate as a result of enzymopathies, chronic hemolysis, or rapidly dividing tumors, psoriasis, or other disorders characterized by increased turnover of cells.
Humans do not have a functional enzyme (uricase) to break down urate into allantoin, which is more soluble and readily excreted. There may have been genetic pressures that explain why functional uricase was lost and why humans have relatively high urate levels compared with other species.2 If higher levels of serum urate are clinically detrimental, one would think that humans could have evolved an efficient way to excrete it. Instead, we excrete uric acid inefficiently as a result of active reabsorption in the proximal renal tubule. We have higher levels of serum urate than most other species, and we are predisposed to develop gouty arthritis and perhaps other sequelae of hyperuricemia, including hypertension, the metabolic syndrome, and coronary artery disease.
CLINICALLY SIGNIFICANT HYPERURICEMIA VS LAB-DEFINED HYPERURICEMIA
Clinically significant hyperuricemia is a serum urate level greater than 6.8 mg/dL, although the population-defined “normal” urate level indicated by the clinical laboratory is higher. At levels above 6.8 mg/dL, urate exceeds its solubility in most biological fluids.
SERUM URATE CAN VARY BY SEX, AGE, DIET
Men generally have higher serum urate levels than premenopausal women; serum urate levels increase in women after menopause. For years these findings were attributed to an estrogen effect, but the mechanism was not well understood. Recently a specific transporter (urate transporter 1 [URAT1]) has been identified in the proximal tubule of the kidney3 that seems primarily responsible for the reabsorption of uric acid. Estrogen has a direct effect on the expression of this transporter. It also seems that the hypouricemic effects of probenecid and losartan, as well as the hyperuricemic effects of organic acids and high insulin levels, may be mediated via modulation of URAT1 activity.
Urate values tend to be lower in children, and urate levels are generally affected only modestly by diet.4 Epidemiologic studies, however, have linked increased ingestion of red meats and low ingestion of dairy foods with an increased incidence of gout.5 Acute alcohol ingestion can cause fluctuations in the serum urate levels and may precipitate acute gout attacks.
HYPERURICEMIA LEADING TO GOUT
Urate concentrations greater than 6.8 mg/dL may result in the deposition of urate crystals in the tissues around joints and in other soft tissue structures (tophi). Why this occurs in only some patients is not known. Crystals, when mobilized from these deposits, can provoke the acute gouty flare. The tophi are not usually hot or tender. Biopsy of a tophus reveals a chronic granulomatous inflammatory response around the sequestered crystals. However, the tophi are not inert; the uric acid can be mobilized by mass action effect if the urate in surrounding fluid is reduced. If tophi are adjacent to bone, erosion into bone may occur.
CLINICAL PROGRESSION OF HYPERURICEMIA AND GOUT: FOUR STAGES OF A CHRONIC DISEASE
Although there is significant heterogeneity in the expression of gout, we can conceptualize a prototypic progression from asymptomatic hyperuricemia to chronic gouty arthritis.
Stage 1: Asymptomatic hyperuricemia. At a serum urate concentration greater than 6.8 mg/dL, urate crystals may start to deposit. During this period of asymptomatic hyperuricemia, urate deposits may directly contribute to organ damage. This does not occur in everyone, however, and at present there is no evidence that treatment is warranted for asymptomatic hyperuricemia.
Stages 2 and 3: Acute gout and intercritical periods. If sufficient urate deposits develop around joints, and if the local milieu or some trauma triggers the release of crystals into the joint space, a patient will suffer acute attacks of gout. These flares are self-resolving but are likely to recur. The intervals between attacks are termed “intercritical periods.” During these periods, crystals may still be present at a low level in the fluid, and are certainly present in the periarticular and synovial tissue, providing a nidus for future attacks.
Stage 4: Advanced gout. If crystal deposits continue to accumulate, patients may develop chronically stiff and swollen joints. This advanced stage of gout is relatively uncommon but is avoidable with therapy.
Progression is variable
The progression from asymptomatic hyperuricemia to advanced gout is quite variable from person to person. In most people it takes many years to progress, if it does so at all. In patients treated with cyclosporine following an organ transplant, the progression can be accelerated, although the reasons are not fully understood.
ASYMPTOMATIC HYPERURICEMIA: TO TREAT OR NOT TO TREAT?
Clues for predicting the likelihood that an individual patient with asymptomatic hyperuricemia will develop articular gout are elusive. Campion and colleagues presented data on men without a history of gout who were grouped by serum urate level and followed over a 5-year period.6 The higher the patient’s urate level, the more likely that he would have a gouty attack during the 5 years. In this relatively young population of hyperuricemic men (average age of 42 years), less than 30% developed gout over this short period.
The dilemma is how to predict who is most likely to get gout and will benefit from early urate-lowering treatment, and who will not. Currently, clinicians have no reliable way of predicting the likelihood of gout development in a given hyperuricemic patient. A history of organ transplantation, the continued need for diuretics, an extremely high urate level, alcohol ingestion, low dietary consumption of dairy products, high consumption of meat and seafood, and a family history of gout at a young age suggest a higher risk of gouty arthritis. At present, treatment of asymptomatic hyperuricemia in order to prevent gouty arthritis is not generally recommended.
ACUTE GOUT FLARES: PAINFUL, UNPREDICTABLE, HIGHLY LIKELY TO RECUR
Acute flares of gouty arthritis are characterized by warmth, swelling, redness, and often severe pain. Pain frequently begins in the middle of the night or early morning. Many patients will describe awakening with pain in the foot that is so intense that they are unable to support their own weight. Patients may report fever and a flulike malaise. Fever and constitutional features are sequelae of the release of cytokines such as tumor necrosis factor, interleukin-1, and interleukin-6 following phagocytosis of crystals and activation of the intracellular inflammasome complex.7 Untreated, the initial attack will usually resolve in 3 to 14 days. Subsequent attacks tend to last longer and may involve more joints or tendons.
Where flares occur
It has been estimated that 90% of first attacks are monoarticular. However, the first recognized attack can be oligoarticular or even polyarticular. This seems particularly true in postmenopausal women and in transplant recipients. Gout attacks initially tend to occur in the lower extremities: midfoot, first metatarsophalangeal joint, ankle, or knee. Over time, gout tends to include additional joints, including those of the upper extremities. Axial joints are far less commonly involved. The initial (or subsequent) attack may be in the instep of the foot, not a well-defined joint. Patients may recall “ankle sprains,” often ascribed to an event such as “stepping off the curb wrong,” with delayed ankle swelling. These may have been attacks of gout that were not recognized by the patient and thus not reported to his or her physician. Bunion pain may be incorrectly attributed to gout (and vice versa). Therefore, we need to accept the limitations of historical recognition of gout attacks.
Acute flares also occur in periarticular structures, including bursae and tendons. The olecranon bursa, the tendons around the ankle, and the bursae around the knee are among the locations where acute attacks of gout can occur.
Risk of recurrence and implications for treatment
Based on historical data, the estimated flare recurrence rate is approximately 60% within 1 year after the initial attack, 78% within 2 years, and 84% within 3 years. Less than 10% of patients will not have a recurrence over a 10-year period. Untreated, some patients with gout will continue to have attacks and accrue chronic joint damage, stiffness, and tophi. However, that does not imply that published outcome data support treating every patient with urate-lowering therapy following an initial gout attack or even several attacks. There are no outcome data from appropriately controlled, long-term trials to validate such a treatment approach. Nonetheless, in some gouty patients, if hyperuricemia is not addressed, morbidity and joint damage will accrue. The decision as to when to intervene with urate-lowering therapy should be individualized, taking into consideration comorbidities, estimation of the likelihood of continued attacks, the impact of attacks on the patient’s lifestyle, and the potential complications of needing to use medications to treat acute attacks.
INTERCRITICAL PERIODS: CRYSTAL DEPOSITION CONTINUES SILENTLY
Immediately after an attack of gout, patients may be apt to have another if anti-inflammatory therapy is not provided for a long enough period, ie, until several days after an attack has completely resolved. Subsequently, there may be a prolonged period before another attack occurs. During this time, uric acid deposits may continue to increase silently. The factors that control the rate, location, and degree of ongoing deposition in a specific patient are not well defined. Crystals may still be found in the synovial fluid of previously involved joints until the serum urate level is reduced for a significant period to a level significantly less than 6.8 mg/dL.8
ADVANCED GOUT: DIFFERENTIATION FROM RHEUMATOID ARTHRITIS IS KEY
Tissue stores of urate may continue to increase if hyperuricemia persists at biologically significant levels (> 6.8 mg/dL). Crystal deposition can cause chronic polyarthritis. Some patients, especially as they age, develop rheumatoid factor positivity. Chronic gout, involving multiple joints, can mimic rheumatoid arthritis. Patients can develop subcutaneous tophi in areas of friction or trauma. These tophi, as well as periarticular ones, can be mistaken for rheumatoid nodules. It is unclear why only some people with hyperuricemia develop tophi. The presence of urate crystals in the aspirate of a nodule (tophus) or synovial fluid will distinguish gout from rheumatoid arthritis. Radiographs can also be of diagnostic use.
Unlike radiographic findings in rheumatoid arthritis, in gout there is a prominent, proliferative bony reaction, and tophi can cause bone destruction away from the joint. There may be a characteristic “overhanging edge” of proliferating bone surrounding a gout erosion (see Figure 3 in preceding article by Schumacher). These radiographic findings, although distinct from those of rheumatoid arthritis, can be confused with psoriatic arthritis, which also can be erosive with a proliferative bone response. Gout, however, is less likely to cause joint space narrowing than is either psoriatic arthritis or rheumatoid arthritis.
Intradermal tophi (Figure 1) are asymptomatic and frequently not recognized, yet are not that rare in severe untreated gout. Such tophi may be particularly common in transplant patients and appear as white or yellowish deposits with the overlying skin pulled taut.
POSTSCRIPT: GOUT IS NOT SO EASILY RECOGNIZED AFTER ALL
Uric acid—urate in most physiologic fluids—is an end product of purine degradation. The serum urate level in a given patient is determined by the amount of purines synthesized and ingested, the amount of urate produced from purines, and the amount of uric acid excreted by the kidney (and, to a lesser degree, from the gastrointestinal tract).1 A major source of circulating urate is the metabolized endogenous purine. Renal excretion is likely determined by genetic factors that dictate expression of uric acid transporters, as well as by the presence of organic acids, certain drugs, hormones, and the glomerular filtration rate. A small minority of patients will have increased production of urate as a result of enzymopathies, chronic hemolysis, or rapidly dividing tumors, psoriasis, or other disorders characterized by increased turnover of cells.
Humans do not have a functional enzyme (uricase) to break down urate into allantoin, which is more soluble and readily excreted. There may have been genetic pressures that explain why functional uricase was lost and why humans have relatively high urate levels compared with other species.2 If higher levels of serum urate are clinically detrimental, one would think that humans could have evolved an efficient way to excrete it. Instead, we excrete uric acid inefficiently as a result of active reabsorption in the proximal renal tubule. We have higher levels of serum urate than most other species, and we are predisposed to develop gouty arthritis and perhaps other sequelae of hyperuricemia, including hypertension, the metabolic syndrome, and coronary artery disease.
CLINICALLY SIGNIFICANT HYPERURICEMIA VS LAB-DEFINED HYPERURICEMIA
Clinically significant hyperuricemia is a serum urate level greater than 6.8 mg/dL, although the population-defined “normal” urate level indicated by the clinical laboratory is higher. At levels above 6.8 mg/dL, urate exceeds its solubility in most biological fluids.
SERUM URATE CAN VARY BY SEX, AGE, DIET
Men generally have higher serum urate levels than premenopausal women; serum urate levels increase in women after menopause. For years these findings were attributed to an estrogen effect, but the mechanism was not well understood. Recently a specific transporter (urate transporter 1 [URAT1]) has been identified in the proximal tubule of the kidney3 that seems primarily responsible for the reabsorption of uric acid. Estrogen has a direct effect on the expression of this transporter. It also seems that the hypouricemic effects of probenecid and losartan, as well as the hyperuricemic effects of organic acids and high insulin levels, may be mediated via modulation of URAT1 activity.
Urate values tend to be lower in children, and urate levels are generally affected only modestly by diet.4 Epidemiologic studies, however, have linked increased ingestion of red meats and low ingestion of dairy foods with an increased incidence of gout.5 Acute alcohol ingestion can cause fluctuations in the serum urate levels and may precipitate acute gout attacks.
HYPERURICEMIA LEADING TO GOUT
Urate concentrations greater than 6.8 mg/dL may result in the deposition of urate crystals in the tissues around joints and in other soft tissue structures (tophi). Why this occurs in only some patients is not known. Crystals, when mobilized from these deposits, can provoke the acute gouty flare. The tophi are not usually hot or tender. Biopsy of a tophus reveals a chronic granulomatous inflammatory response around the sequestered crystals. However, the tophi are not inert; the uric acid can be mobilized by mass action effect if the urate in surrounding fluid is reduced. If tophi are adjacent to bone, erosion into bone may occur.
CLINICAL PROGRESSION OF HYPERURICEMIA AND GOUT: FOUR STAGES OF A CHRONIC DISEASE
Although there is significant heterogeneity in the expression of gout, we can conceptualize a prototypic progression from asymptomatic hyperuricemia to chronic gouty arthritis.
Stage 1: Asymptomatic hyperuricemia. At a serum urate concentration greater than 6.8 mg/dL, urate crystals may start to deposit. During this period of asymptomatic hyperuricemia, urate deposits may directly contribute to organ damage. This does not occur in everyone, however, and at present there is no evidence that treatment is warranted for asymptomatic hyperuricemia.
Stages 2 and 3: Acute gout and intercritical periods. If sufficient urate deposits develop around joints, and if the local milieu or some trauma triggers the release of crystals into the joint space, a patient will suffer acute attacks of gout. These flares are self-resolving but are likely to recur. The intervals between attacks are termed “intercritical periods.” During these periods, crystals may still be present at a low level in the fluid, and are certainly present in the periarticular and synovial tissue, providing a nidus for future attacks.
Stage 4: Advanced gout. If crystal deposits continue to accumulate, patients may develop chronically stiff and swollen joints. This advanced stage of gout is relatively uncommon but is avoidable with therapy.
Progression is variable
The progression from asymptomatic hyperuricemia to advanced gout is quite variable from person to person. In most people it takes many years to progress, if it does so at all. In patients treated with cyclosporine following an organ transplant, the progression can be accelerated, although the reasons are not fully understood.
ASYMPTOMATIC HYPERURICEMIA: TO TREAT OR NOT TO TREAT?
Clues for predicting the likelihood that an individual patient with asymptomatic hyperuricemia will develop articular gout are elusive. Campion and colleagues presented data on men without a history of gout who were grouped by serum urate level and followed over a 5-year period.6 The higher the patient’s urate level, the more likely that he would have a gouty attack during the 5 years. In this relatively young population of hyperuricemic men (average age of 42 years), less than 30% developed gout over this short period.
The dilemma is how to predict who is most likely to get gout and will benefit from early urate-lowering treatment, and who will not. Currently, clinicians have no reliable way of predicting the likelihood of gout development in a given hyperuricemic patient. A history of organ transplantation, the continued need for diuretics, an extremely high urate level, alcohol ingestion, low dietary consumption of dairy products, high consumption of meat and seafood, and a family history of gout at a young age suggest a higher risk of gouty arthritis. At present, treatment of asymptomatic hyperuricemia in order to prevent gouty arthritis is not generally recommended.
ACUTE GOUT FLARES: PAINFUL, UNPREDICTABLE, HIGHLY LIKELY TO RECUR
Acute flares of gouty arthritis are characterized by warmth, swelling, redness, and often severe pain. Pain frequently begins in the middle of the night or early morning. Many patients will describe awakening with pain in the foot that is so intense that they are unable to support their own weight. Patients may report fever and a flulike malaise. Fever and constitutional features are sequelae of the release of cytokines such as tumor necrosis factor, interleukin-1, and interleukin-6 following phagocytosis of crystals and activation of the intracellular inflammasome complex.7 Untreated, the initial attack will usually resolve in 3 to 14 days. Subsequent attacks tend to last longer and may involve more joints or tendons.
Where flares occur
It has been estimated that 90% of first attacks are monoarticular. However, the first recognized attack can be oligoarticular or even polyarticular. This seems particularly true in postmenopausal women and in transplant recipients. Gout attacks initially tend to occur in the lower extremities: midfoot, first metatarsophalangeal joint, ankle, or knee. Over time, gout tends to include additional joints, including those of the upper extremities. Axial joints are far less commonly involved. The initial (or subsequent) attack may be in the instep of the foot, not a well-defined joint. Patients may recall “ankle sprains,” often ascribed to an event such as “stepping off the curb wrong,” with delayed ankle swelling. These may have been attacks of gout that were not recognized by the patient and thus not reported to his or her physician. Bunion pain may be incorrectly attributed to gout (and vice versa). Therefore, we need to accept the limitations of historical recognition of gout attacks.
Acute flares also occur in periarticular structures, including bursae and tendons. The olecranon bursa, the tendons around the ankle, and the bursae around the knee are among the locations where acute attacks of gout can occur.
Risk of recurrence and implications for treatment
Based on historical data, the estimated flare recurrence rate is approximately 60% within 1 year after the initial attack, 78% within 2 years, and 84% within 3 years. Less than 10% of patients will not have a recurrence over a 10-year period. Untreated, some patients with gout will continue to have attacks and accrue chronic joint damage, stiffness, and tophi. However, that does not imply that published outcome data support treating every patient with urate-lowering therapy following an initial gout attack or even several attacks. There are no outcome data from appropriately controlled, long-term trials to validate such a treatment approach. Nonetheless, in some gouty patients, if hyperuricemia is not addressed, morbidity and joint damage will accrue. The decision as to when to intervene with urate-lowering therapy should be individualized, taking into consideration comorbidities, estimation of the likelihood of continued attacks, the impact of attacks on the patient’s lifestyle, and the potential complications of needing to use medications to treat acute attacks.
INTERCRITICAL PERIODS: CRYSTAL DEPOSITION CONTINUES SILENTLY
Immediately after an attack of gout, patients may be apt to have another if anti-inflammatory therapy is not provided for a long enough period, ie, until several days after an attack has completely resolved. Subsequently, there may be a prolonged period before another attack occurs. During this time, uric acid deposits may continue to increase silently. The factors that control the rate, location, and degree of ongoing deposition in a specific patient are not well defined. Crystals may still be found in the synovial fluid of previously involved joints until the serum urate level is reduced for a significant period to a level significantly less than 6.8 mg/dL.8
ADVANCED GOUT: DIFFERENTIATION FROM RHEUMATOID ARTHRITIS IS KEY
Tissue stores of urate may continue to increase if hyperuricemia persists at biologically significant levels (> 6.8 mg/dL). Crystal deposition can cause chronic polyarthritis. Some patients, especially as they age, develop rheumatoid factor positivity. Chronic gout, involving multiple joints, can mimic rheumatoid arthritis. Patients can develop subcutaneous tophi in areas of friction or trauma. These tophi, as well as periarticular ones, can be mistaken for rheumatoid nodules. It is unclear why only some people with hyperuricemia develop tophi. The presence of urate crystals in the aspirate of a nodule (tophus) or synovial fluid will distinguish gout from rheumatoid arthritis. Radiographs can also be of diagnostic use.
Unlike radiographic findings in rheumatoid arthritis, in gout there is a prominent, proliferative bony reaction, and tophi can cause bone destruction away from the joint. There may be a characteristic “overhanging edge” of proliferating bone surrounding a gout erosion (see Figure 3 in preceding article by Schumacher). These radiographic findings, although distinct from those of rheumatoid arthritis, can be confused with psoriatic arthritis, which also can be erosive with a proliferative bone response. Gout, however, is less likely to cause joint space narrowing than is either psoriatic arthritis or rheumatoid arthritis.
Intradermal tophi (Figure 1) are asymptomatic and frequently not recognized, yet are not that rare in severe untreated gout. Such tophi may be particularly common in transplant patients and appear as white or yellowish deposits with the overlying skin pulled taut.
POSTSCRIPT: GOUT IS NOT SO EASILY RECOGNIZED AFTER ALL
- Choi HK, Mount DB, Reginato AM. Pathogenesis of gout. Ann Intern Med 2005; 143:499–516.
- Oda M, Satta Y, Takenaka O, Takahata N. Loss of urate oxidase activity in hominoids and its evolutionary implications. Mol Biol Evol 2002; 19:640–653.
- Enomoto A, Kimura H, Chairoungdua A, et al. Molecular identification of a renal urate anion exchanger that regulates blood urate levels. Nature 2002; 417:447–452.
- Fam AG. Gout: excess calories, purines, and alcohol intake and beyond. Response to a urate-lowering diet. J Rheumatol 2005; 32:773–777.
- Choi HK, Atkinson K, Karlson EW, Willett W, Curhan G. Purine-rich foods, dairy and protein intake, and the risk of gout in men. N Engl J Med 2004; 350:1093–1103.
- Campion EW, Glynn RJ, DeLabry LO. Asymptomatic hyperuricemia. Risks and consequences in the Normative Aging Study. Am J Med 1987; 82:421–426.
- Martinon F, Pétrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 2006; 440:237–241.
- Pascual E, Sivera F. Time required for disappearance of urate crystals from synovial fluid after successful hypouricaemic treatment relates to the duration of gout. Ann Rheum Dis 2007; 66:1056–1058.
- Choi HK, Mount DB, Reginato AM. Pathogenesis of gout. Ann Intern Med 2005; 143:499–516.
- Oda M, Satta Y, Takenaka O, Takahata N. Loss of urate oxidase activity in hominoids and its evolutionary implications. Mol Biol Evol 2002; 19:640–653.
- Enomoto A, Kimura H, Chairoungdua A, et al. Molecular identification of a renal urate anion exchanger that regulates blood urate levels. Nature 2002; 417:447–452.
- Fam AG. Gout: excess calories, purines, and alcohol intake and beyond. Response to a urate-lowering diet. J Rheumatol 2005; 32:773–777.
- Choi HK, Atkinson K, Karlson EW, Willett W, Curhan G. Purine-rich foods, dairy and protein intake, and the risk of gout in men. N Engl J Med 2004; 350:1093–1103.
- Campion EW, Glynn RJ, DeLabry LO. Asymptomatic hyperuricemia. Risks and consequences in the Normative Aging Study. Am J Med 1987; 82:421–426.
- Martinon F, Pétrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 2006; 440:237–241.
- Pascual E, Sivera F. Time required for disappearance of urate crystals from synovial fluid after successful hypouricaemic treatment relates to the duration of gout. Ann Rheum Dis 2007; 66:1056–1058.
KEY POINTS
- Clinically significant hyperuricemia includes serum urate levels that fall within the population-defined “normal” range of many clinical laboratories.
- There is no reliable way to predict the likelihood that gout will develop in a given hyperuricemic patient. Treatment of asymptomatic hyperuricemia is not generally recommended.
- Untreated, an initial acute gout attack resolves within 3 to 14 days. Subsequent attacks tend to last longer and may involve more joints.
- Chronic gout can mimic rheumatoid or psoriatic arthritis.