User login
The presence of urate crystals in synovial fluid is the gold standard for diagnosing gout,1 yet clinicians—both primary care physicians and rheumatologists—may not routinely perform synovial fluid analysis even when evaluating a patient who presents with an acute inflammatory arthritis.2 This paper discusses the various reasons why this is so and reviews several important resulting clinical issues: how a presumptive diagnosis of gout is made, when to measure the serum urate level, and special considerations in the differential diagnosis.
SYNOVIAL FLUID ANALYSIS: WHY IS THE GOLD STANDARD NOT MORE ROUTINE?
Occasionally, the aspirated joint does not appear to contain any joint fluid and the clinician may be concerned about the possibility of a “dry tap.” Other possible reasons include lack of experience with synovial fluid aspiration and evaluation, or limited access to the polarizing microscopes used to examine synovial fluid. Time is another factor; in a busy primary care practice, where patients are usually seen approximately every 7 to 11 minutes, there may not be time to aspirate a joint. The urgency of fluid examination is another issue, as synovial fluid must be examined immediately, since the crystals can become smaller, less numerous, and less birefringent with time.4
THE CLINICAL, OR PRESUMPTIVE, DIAGNOSIS
In the appropriate clinical scenario, a presumptive diagnosis of gout can be made on the basis of typical clinical features and the presence of hyperuricemia.1,2
Expert societies offer guidance, but no validation studies to date
Evidence-based recommendations for the diagnosis of gout from the European League Against Rheumatism (EULAR) state that in acute attacks, the rapid development of severe pain, swelling, and tenderness that peaks within 6 to 12 hours, especially with overlying erythema, is highly suggestive of crystal inflammation although not specific for gout.5 These recommendations further state that for typical presentations of gout (such as recurrent podagra [gouty pain in the great toe] with hyperuricemia), a clinical diagnosis alone is reasonably accurate.5
- The presence of urate crystals in joint fluid
- A tophus containing urate crystals
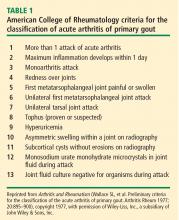
- Fulfillment of 6 or more of the criteria in Table 1.
No subsequent studies have been published on the validity or usefulness of any of these diagnostic criteria.
What must inform the presumptive diagnosis
Both the EULAR recommendations and the ACR criteria state that although the gold standard for diagnosing gout is the presence of urate crystals on synovial fluid analysis, a clinical diagnosis of gout can be made on the basis of certain patient criteria. This clinical, or presumptive, diagnosis of gout should be made based on the following:
- A careful patient and family history, including questions regarding comorbid conditions frequently associated with gout (such as hypertriglyceridemia, diabetes, coronary heart disease, hypertension, and the metabolic syndrome) and whether the patient has had previous similar episodes of acute joint pain and swelling in the absence of trauma
- Thorough identification of all current medications, some of which may be associated with hyperuricemia
- A thorough physical examination.
THE PHYSICAL EXAMINATION FOR GOUT
Examination of patients with a history suggestive of gout should include not only the joints but also the extensor surface of the forearms and feet. When patients are seen for a visit and gout is suspected, they should be instructed to remove their shoes and socks and roll up their sleeves to allow examination for evidence of tophi, which would suggest a past history of gouty arthritis. The ear, knee, and olecranon bursa are other common sites for tophi,3 so patients should also be asked to roll up their pants and sleeves and remove any head coverings.7 In the late stages of gouty arthritis, multiple joints may be involved, which can cause the condition to be confused with other diagnoses such as psoriatic arthritis or erosive osteoarthritis.7
ACUTE PRESENTATIONS OF GOUT
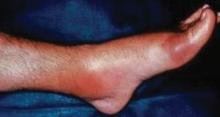
The typical gout presentation is remarkable for very intense pain that often occurs at night when the extremities are colder. Precipitation of urate in the distal extremities can occur when the extremities are horizontal and tend to become cold.8
Approximately 90% of initial gout attacks are monoarticular, leaving only 10% of cases that are oligoarticular or polyarticular.7 If more than one joint is involved, especially if the patient has a family history suggestive of gout or takes a medication that causes hyperuricemia, gout should be considered in the differential diagnosis even if the patient denies having a prior gout attack.
Frequently, patients will call their primary care physician during a gout attack but are not be able to schedule an appointment until after the attack has resolved. When possible, patients should be seen during the attack to confirm whether the attack is due to gout. A diagnosis of gout should not be made over the phone when a patient describes pain in the great toe, as only 50% of initial gout attacks occur in the great toe7 and it is not known what proportion of acute pain episodes in the great toe are attributable to gout. The most common cause of pain in the great toe is osteoarthritis.
SPECIAL CONSIDERATIONS FOR THE PRESUMPTIVE DIAGNOSIS OF GOUT
How long have acute attacks been occurring?
In a clinical scenario in which synovial fluid aspiration cannot be performed, the appropriateness of a presumptive diagnosis can be assessed by a discussion with the patient about how long he or she has been experiencing acute attacks of joint pain. If the attacks have occurred for more than 10 years, tophi will likely be present.3 After even longer periods, gout may become polyarticular.7 In postmenopausal women, the distal interphalangeal joints may be involved,3 which may lead to a misdiagnosis of osteoarthritis, as these joints are typically affected by osteoarthritis.
Is the patient taking a urate-raising medication?
Certain medications have been associated with hyper-uricemia, including cyclosporine and thiazide diuretics.9 If a patient has been taking one of these medications, gout should be considered in the differential diagnosis if the patient presents with acute joint pain.
It has been argued that a reduction in joint pain and swelling after the use of colchicine confirms a diagnosis of gout. However, other conditions—such as tendonitis, calcium pyrophosphate dihydrate (CPPD) crystal deposition disease (pseudogout),3 and rheumatoid arthritis (RA)—can also improve after treatment with colchicine.1
Be vigilant for fever
Another consideration in making a clinical diagnosis of gout is the association with a low-grade fever; these patients may feel as if they have the flu.8 Acute gout may also cause a high fever and an elevated white blood cell (WBC) count;3 in this situation, synovial fluid aspiration must be performed to exclude septic arthritis, either alone or in the presence of gouty arthritis. In situations where septic arthritis is suspected, an emergency visit to a rheumatologist is indicated for synovial fluid aspiration to be performed, as gout and sepsis can coexist.5 In such instances, Gram staining and culture of the synovial fluid should still be performed even if monosodium urate crystals are identified.5
MEASUREMENT OF SERUM URATE LEVELS
Measuring serum urate levels during an acute attack, treating the acute attack with anti-inflammatory medications, and reevaluating the patient in the office 2 weeks after the acute attack are all recommended in the management of a patient with gout. If the serum urate level was not elevated during the acute attack, it is likely to be elevated 2 weeks later if the patient has gout.10 Elevated levels of serum urate during the intercritical periods are predictive of future gout attacks.11 Measuring serum urate during the initial attack and then 2 weeks later yields two serum urate levels that can be compared to assist in considering a presumed diagnosis of gout. A study by Rigby and Wood concluded that in patients with low serum urate levels (< 4 mg/dL) 2 weeks following an inflammatory arthritis attack, a diagnosis of gout is unlikely.12
DIFFERENTIAL DIAGNOSIS OF GOUT
Rheumatoid arthritis
CPPD crystal deposition disease (pseudogout)
CPPD crystal deposition disease, or pseudogout, must also be included in the differential diagnosis of gout. This disease usually occurs in joints previously affected by osteoarthritis or joints that have been injured in the past.15 Attacks of CPPD crystal deposition disease commonly occur in the knee, in the wrist at the base of the thumb, or in the shoulder.15 Radiographic examination may reveal a line of calcification along the cartilage outlining the joint.15 Like gout, pseudogout attacks can occur spontaneously or after trauma, surgery, or a severe illness such as myocardial infarction or stroke.16
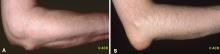
The presentation of pseudogout can be very similar to an acute attack of gout. The difference is seen when evaluating the crystals through a polarizing microscope. CPPD crystals are weakly positively birefringent (Figure 1B), in contrast to the negatively birefringent crystals seen with gout (Figure 1A).7 If a polarizing microscope is not available, the crystals usually can be distinguished by their differing shapes: urate crystals are fine and needlelike, whereas CPPD crystals are rhomboid (Figure 1).
Septic arthritis
When the differential diagnosis includes septic arthritis, the joint must be aspirated; a presumed diagnosis cannot be made. Among patients with an acute gouty attack, low-grade fever is reported during the attack in 29% of gout patients and 38% of patients with CPPD crystal deposition disease.14 Temperatures of 101°F or higher are not usually seen in patients with gout or CPPD crystal deposition disease and suggest an infection, although patients with septic arthritis may be afebrile, especially if they are taking immunosuppressive therapy or glucocorticoids, which can inhibit a febrile response. Synovial fluid analysis in patients with gout and septic arthritis can reveal WBC counts above 100,000 per mm3, whereas synovial fluid WBC counts above 50,000 per mm3 are more common in infection.
As noted earlier, gout and septic arthritis can coexist. In a patient presenting with a fever and a warm erythematous swollen joint, synovial fluid aspiration must be performed and evaluated for the presence of crystals and bacteria. The patient may require treatment for both causes of acute monoarticular arthritis.
In a patient undergoing renal dialysis, where gout or pseudogout can occur and where there is frequent intravascular manipulation, a septic joint can occur simultaneously.3,14 In this situation, not only must joint aspiration be performed, but the synovial fluid also needs to be evaluated for both crystals and bacteria. Again, the patient may require treatment for both causes of acute monoarticular arthritis.
CONCLUSIONS
The gold standard for diagnosing gout remains synovial fluid aspiration and analysis. In clinical situations when joint aspiration cannot be performed, the EULAR recommendations5 and the ACR criteria6 provide guidance for making a clinical or presumptive diagnosis of gout. A thorough patient history—both personal and family—and physical examination are critical in making a presumed diagnosis of gout. If the patient presents during an acute attack, serum urate measurement may be useful in making a clinical diagnosis if it reveals an elevated level. When the patient returns for follow-up 2 weeks later, a second serum urate measurement should be taken to allow comparison of the two levels. If the serum urate level is elevated at the follow-up visit, the EULAR recommendations state that a clinical diagnosis of gout can be made if the patient had an acute attack of arthritis in the great toe.
As noted in the EULAR recommendations, the future research agenda should include validating the clinical manifestations of gout against a diagnosis established by identification of urate crystals on synovial fluid analysis.5 Until this task can be completed, clinicians should become familiarized with the technique of joint aspiration so that in situations where a clinical or presumptive diagnosis of gout cannot be made—including cases where the differential diagnosis includes a septic joint—clinicians will be able to perform aspiration with confidence.
- Jelley MJ, Wortmann R. Practical steps in the diagnosis and management of gout. BioDrugs 2000; 14:99–107.
- Eggebeen AT. Gout: an update. Am Fam Physician 2007; 76:801–808, 811–812.
- Rott KT, Agudelo CA. Gout. JAMA 2003; 289:2857–2860.
- Poór G, Mituszova M. History, classification and epidemiology of crystal-related arthropathies. In: Hochberg MC, Silman AJ, Smolen JS, Weinblatt ME, Weisman MH, eds. Rheumatology. 3rd ed, vol 2. Edinburgh: Mosby; 2003:1893–1901.
- Zhang W, Doherty M, Pascual E, et al. EULAR evidence based recommendations for gout. Part I: Diagnosis. Report of a task force of the Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis 2006; 65:1301–1311.
- Wallace SL, Robinson H, Masi AT, Decker JL, McCarty DJ, Yü TF. Preliminary criteria for the classification of the acute arthritis of primary gout. Arthritis Rheum 1977; 20:895–900.
- Harris MD, Siegel LB, Alloway JA. Gout and hyperuricemia. Am Fam Physician 1999; 59:925–934.
- Saag KG, Mikuls TR. Recent advances in the epidemiology of gout. Curr Rheumatol Rep 2005; 7:235–241.
- Edwards NL. Gout. B. Clinical and laboratory features. In: Klippel JH, Crofford LJ, Stone JH, Weyand CM, eds. Primer on the Rheumatic Diseases. 12th ed. Atlanta, GA: Arthritis Foundation; 2001:313–319.
- Urano W, Yamanaka H, Tsutani H, et al. The inflammatory process in the mechanism of decreased serum uric acid concentrations during acute gouty arthritis. J Rheumatol 2002; 29:1950–1953.
- Shoji A, Yamanaka H, Kamatani N. A retrospective study of the relationship between serum urate level and recurrent attacks of gouty arthritis: evidence for reduction of recurrent gouty arthritis with anti-hyperuricemic therapy. Arthritis Rheum 2004; 51:321–325.
- Rigby AS, Wood PH. Serum uric acid levels and gout: what does this herald for the population? Clin Exp Rheumatol 1994; 12:395–400.
- George DL. Skin and rheumatic disease. In: Hochberg MC, Silman AJ, Smolen JS, Weinblatt ME, Weisman MH, eds. Rheumatology. 3rd ed, vol 1. Edinburgh: Mosby; 2003:279–292.
- Ho G Jr, DeNuccio M. Gout and pseudogout in hospitalized patients. Arch Intern Med 1993; 153:2787–2790.
- Agarwal AK. Gout and pseudogout. Prim Care 1993; 20:839–855.
- Halverson PB, Ryan LM. Arthritis associated with calcium-containing crystals. In: Klippel JH, Crofford LJ, Stone JH, Weyand CM, eds. Primer on the Rheumatic Diseases. 12th ed. Atlanta, GA: Arthritis Foundation; 2001:299–306.
The presence of urate crystals in synovial fluid is the gold standard for diagnosing gout,1 yet clinicians—both primary care physicians and rheumatologists—may not routinely perform synovial fluid analysis even when evaluating a patient who presents with an acute inflammatory arthritis.2 This paper discusses the various reasons why this is so and reviews several important resulting clinical issues: how a presumptive diagnosis of gout is made, when to measure the serum urate level, and special considerations in the differential diagnosis.
SYNOVIAL FLUID ANALYSIS: WHY IS THE GOLD STANDARD NOT MORE ROUTINE?
Occasionally, the aspirated joint does not appear to contain any joint fluid and the clinician may be concerned about the possibility of a “dry tap.” Other possible reasons include lack of experience with synovial fluid aspiration and evaluation, or limited access to the polarizing microscopes used to examine synovial fluid. Time is another factor; in a busy primary care practice, where patients are usually seen approximately every 7 to 11 minutes, there may not be time to aspirate a joint. The urgency of fluid examination is another issue, as synovial fluid must be examined immediately, since the crystals can become smaller, less numerous, and less birefringent with time.4
THE CLINICAL, OR PRESUMPTIVE, DIAGNOSIS
In the appropriate clinical scenario, a presumptive diagnosis of gout can be made on the basis of typical clinical features and the presence of hyperuricemia.1,2
Expert societies offer guidance, but no validation studies to date
Evidence-based recommendations for the diagnosis of gout from the European League Against Rheumatism (EULAR) state that in acute attacks, the rapid development of severe pain, swelling, and tenderness that peaks within 6 to 12 hours, especially with overlying erythema, is highly suggestive of crystal inflammation although not specific for gout.5 These recommendations further state that for typical presentations of gout (such as recurrent podagra [gouty pain in the great toe] with hyperuricemia), a clinical diagnosis alone is reasonably accurate.5
- The presence of urate crystals in joint fluid
- A tophus containing urate crystals
- Fulfillment of 6 or more of the criteria in Table 1.
No subsequent studies have been published on the validity or usefulness of any of these diagnostic criteria.
What must inform the presumptive diagnosis
Both the EULAR recommendations and the ACR criteria state that although the gold standard for diagnosing gout is the presence of urate crystals on synovial fluid analysis, a clinical diagnosis of gout can be made on the basis of certain patient criteria. This clinical, or presumptive, diagnosis of gout should be made based on the following:
- A careful patient and family history, including questions regarding comorbid conditions frequently associated with gout (such as hypertriglyceridemia, diabetes, coronary heart disease, hypertension, and the metabolic syndrome) and whether the patient has had previous similar episodes of acute joint pain and swelling in the absence of trauma
- Thorough identification of all current medications, some of which may be associated with hyperuricemia
- A thorough physical examination.
THE PHYSICAL EXAMINATION FOR GOUT
Examination of patients with a history suggestive of gout should include not only the joints but also the extensor surface of the forearms and feet. When patients are seen for a visit and gout is suspected, they should be instructed to remove their shoes and socks and roll up their sleeves to allow examination for evidence of tophi, which would suggest a past history of gouty arthritis. The ear, knee, and olecranon bursa are other common sites for tophi,3 so patients should also be asked to roll up their pants and sleeves and remove any head coverings.7 In the late stages of gouty arthritis, multiple joints may be involved, which can cause the condition to be confused with other diagnoses such as psoriatic arthritis or erosive osteoarthritis.7
ACUTE PRESENTATIONS OF GOUT
The typical gout presentation is remarkable for very intense pain that often occurs at night when the extremities are colder. Precipitation of urate in the distal extremities can occur when the extremities are horizontal and tend to become cold.8
Approximately 90% of initial gout attacks are monoarticular, leaving only 10% of cases that are oligoarticular or polyarticular.7 If more than one joint is involved, especially if the patient has a family history suggestive of gout or takes a medication that causes hyperuricemia, gout should be considered in the differential diagnosis even if the patient denies having a prior gout attack.
Frequently, patients will call their primary care physician during a gout attack but are not be able to schedule an appointment until after the attack has resolved. When possible, patients should be seen during the attack to confirm whether the attack is due to gout. A diagnosis of gout should not be made over the phone when a patient describes pain in the great toe, as only 50% of initial gout attacks occur in the great toe7 and it is not known what proportion of acute pain episodes in the great toe are attributable to gout. The most common cause of pain in the great toe is osteoarthritis.
SPECIAL CONSIDERATIONS FOR THE PRESUMPTIVE DIAGNOSIS OF GOUT
How long have acute attacks been occurring?
In a clinical scenario in which synovial fluid aspiration cannot be performed, the appropriateness of a presumptive diagnosis can be assessed by a discussion with the patient about how long he or she has been experiencing acute attacks of joint pain. If the attacks have occurred for more than 10 years, tophi will likely be present.3 After even longer periods, gout may become polyarticular.7 In postmenopausal women, the distal interphalangeal joints may be involved,3 which may lead to a misdiagnosis of osteoarthritis, as these joints are typically affected by osteoarthritis.
Is the patient taking a urate-raising medication?
Certain medications have been associated with hyper-uricemia, including cyclosporine and thiazide diuretics.9 If a patient has been taking one of these medications, gout should be considered in the differential diagnosis if the patient presents with acute joint pain.
It has been argued that a reduction in joint pain and swelling after the use of colchicine confirms a diagnosis of gout. However, other conditions—such as tendonitis, calcium pyrophosphate dihydrate (CPPD) crystal deposition disease (pseudogout),3 and rheumatoid arthritis (RA)—can also improve after treatment with colchicine.1
Be vigilant for fever
Another consideration in making a clinical diagnosis of gout is the association with a low-grade fever; these patients may feel as if they have the flu.8 Acute gout may also cause a high fever and an elevated white blood cell (WBC) count;3 in this situation, synovial fluid aspiration must be performed to exclude septic arthritis, either alone or in the presence of gouty arthritis. In situations where septic arthritis is suspected, an emergency visit to a rheumatologist is indicated for synovial fluid aspiration to be performed, as gout and sepsis can coexist.5 In such instances, Gram staining and culture of the synovial fluid should still be performed even if monosodium urate crystals are identified.5
MEASUREMENT OF SERUM URATE LEVELS
Measuring serum urate levels during an acute attack, treating the acute attack with anti-inflammatory medications, and reevaluating the patient in the office 2 weeks after the acute attack are all recommended in the management of a patient with gout. If the serum urate level was not elevated during the acute attack, it is likely to be elevated 2 weeks later if the patient has gout.10 Elevated levels of serum urate during the intercritical periods are predictive of future gout attacks.11 Measuring serum urate during the initial attack and then 2 weeks later yields two serum urate levels that can be compared to assist in considering a presumed diagnosis of gout. A study by Rigby and Wood concluded that in patients with low serum urate levels (< 4 mg/dL) 2 weeks following an inflammatory arthritis attack, a diagnosis of gout is unlikely.12
DIFFERENTIAL DIAGNOSIS OF GOUT
Rheumatoid arthritis
CPPD crystal deposition disease (pseudogout)
CPPD crystal deposition disease, or pseudogout, must also be included in the differential diagnosis of gout. This disease usually occurs in joints previously affected by osteoarthritis or joints that have been injured in the past.15 Attacks of CPPD crystal deposition disease commonly occur in the knee, in the wrist at the base of the thumb, or in the shoulder.15 Radiographic examination may reveal a line of calcification along the cartilage outlining the joint.15 Like gout, pseudogout attacks can occur spontaneously or after trauma, surgery, or a severe illness such as myocardial infarction or stroke.16
The presentation of pseudogout can be very similar to an acute attack of gout. The difference is seen when evaluating the crystals through a polarizing microscope. CPPD crystals are weakly positively birefringent (Figure 1B), in contrast to the negatively birefringent crystals seen with gout (Figure 1A).7 If a polarizing microscope is not available, the crystals usually can be distinguished by their differing shapes: urate crystals are fine and needlelike, whereas CPPD crystals are rhomboid (Figure 1).
Septic arthritis
When the differential diagnosis includes septic arthritis, the joint must be aspirated; a presumed diagnosis cannot be made. Among patients with an acute gouty attack, low-grade fever is reported during the attack in 29% of gout patients and 38% of patients with CPPD crystal deposition disease.14 Temperatures of 101°F or higher are not usually seen in patients with gout or CPPD crystal deposition disease and suggest an infection, although patients with septic arthritis may be afebrile, especially if they are taking immunosuppressive therapy or glucocorticoids, which can inhibit a febrile response. Synovial fluid analysis in patients with gout and septic arthritis can reveal WBC counts above 100,000 per mm3, whereas synovial fluid WBC counts above 50,000 per mm3 are more common in infection.
As noted earlier, gout and septic arthritis can coexist. In a patient presenting with a fever and a warm erythematous swollen joint, synovial fluid aspiration must be performed and evaluated for the presence of crystals and bacteria. The patient may require treatment for both causes of acute monoarticular arthritis.
In a patient undergoing renal dialysis, where gout or pseudogout can occur and where there is frequent intravascular manipulation, a septic joint can occur simultaneously.3,14 In this situation, not only must joint aspiration be performed, but the synovial fluid also needs to be evaluated for both crystals and bacteria. Again, the patient may require treatment for both causes of acute monoarticular arthritis.
CONCLUSIONS
The gold standard for diagnosing gout remains synovial fluid aspiration and analysis. In clinical situations when joint aspiration cannot be performed, the EULAR recommendations5 and the ACR criteria6 provide guidance for making a clinical or presumptive diagnosis of gout. A thorough patient history—both personal and family—and physical examination are critical in making a presumed diagnosis of gout. If the patient presents during an acute attack, serum urate measurement may be useful in making a clinical diagnosis if it reveals an elevated level. When the patient returns for follow-up 2 weeks later, a second serum urate measurement should be taken to allow comparison of the two levels. If the serum urate level is elevated at the follow-up visit, the EULAR recommendations state that a clinical diagnosis of gout can be made if the patient had an acute attack of arthritis in the great toe.
As noted in the EULAR recommendations, the future research agenda should include validating the clinical manifestations of gout against a diagnosis established by identification of urate crystals on synovial fluid analysis.5 Until this task can be completed, clinicians should become familiarized with the technique of joint aspiration so that in situations where a clinical or presumptive diagnosis of gout cannot be made—including cases where the differential diagnosis includes a septic joint—clinicians will be able to perform aspiration with confidence.
The presence of urate crystals in synovial fluid is the gold standard for diagnosing gout,1 yet clinicians—both primary care physicians and rheumatologists—may not routinely perform synovial fluid analysis even when evaluating a patient who presents with an acute inflammatory arthritis.2 This paper discusses the various reasons why this is so and reviews several important resulting clinical issues: how a presumptive diagnosis of gout is made, when to measure the serum urate level, and special considerations in the differential diagnosis.
SYNOVIAL FLUID ANALYSIS: WHY IS THE GOLD STANDARD NOT MORE ROUTINE?
Occasionally, the aspirated joint does not appear to contain any joint fluid and the clinician may be concerned about the possibility of a “dry tap.” Other possible reasons include lack of experience with synovial fluid aspiration and evaluation, or limited access to the polarizing microscopes used to examine synovial fluid. Time is another factor; in a busy primary care practice, where patients are usually seen approximately every 7 to 11 minutes, there may not be time to aspirate a joint. The urgency of fluid examination is another issue, as synovial fluid must be examined immediately, since the crystals can become smaller, less numerous, and less birefringent with time.4
THE CLINICAL, OR PRESUMPTIVE, DIAGNOSIS
In the appropriate clinical scenario, a presumptive diagnosis of gout can be made on the basis of typical clinical features and the presence of hyperuricemia.1,2
Expert societies offer guidance, but no validation studies to date
Evidence-based recommendations for the diagnosis of gout from the European League Against Rheumatism (EULAR) state that in acute attacks, the rapid development of severe pain, swelling, and tenderness that peaks within 6 to 12 hours, especially with overlying erythema, is highly suggestive of crystal inflammation although not specific for gout.5 These recommendations further state that for typical presentations of gout (such as recurrent podagra [gouty pain in the great toe] with hyperuricemia), a clinical diagnosis alone is reasonably accurate.5
- The presence of urate crystals in joint fluid
- A tophus containing urate crystals
- Fulfillment of 6 or more of the criteria in Table 1.
No subsequent studies have been published on the validity or usefulness of any of these diagnostic criteria.
What must inform the presumptive diagnosis
Both the EULAR recommendations and the ACR criteria state that although the gold standard for diagnosing gout is the presence of urate crystals on synovial fluid analysis, a clinical diagnosis of gout can be made on the basis of certain patient criteria. This clinical, or presumptive, diagnosis of gout should be made based on the following:
- A careful patient and family history, including questions regarding comorbid conditions frequently associated with gout (such as hypertriglyceridemia, diabetes, coronary heart disease, hypertension, and the metabolic syndrome) and whether the patient has had previous similar episodes of acute joint pain and swelling in the absence of trauma
- Thorough identification of all current medications, some of which may be associated with hyperuricemia
- A thorough physical examination.
THE PHYSICAL EXAMINATION FOR GOUT
Examination of patients with a history suggestive of gout should include not only the joints but also the extensor surface of the forearms and feet. When patients are seen for a visit and gout is suspected, they should be instructed to remove their shoes and socks and roll up their sleeves to allow examination for evidence of tophi, which would suggest a past history of gouty arthritis. The ear, knee, and olecranon bursa are other common sites for tophi,3 so patients should also be asked to roll up their pants and sleeves and remove any head coverings.7 In the late stages of gouty arthritis, multiple joints may be involved, which can cause the condition to be confused with other diagnoses such as psoriatic arthritis or erosive osteoarthritis.7
ACUTE PRESENTATIONS OF GOUT
The typical gout presentation is remarkable for very intense pain that often occurs at night when the extremities are colder. Precipitation of urate in the distal extremities can occur when the extremities are horizontal and tend to become cold.8
Approximately 90% of initial gout attacks are monoarticular, leaving only 10% of cases that are oligoarticular or polyarticular.7 If more than one joint is involved, especially if the patient has a family history suggestive of gout or takes a medication that causes hyperuricemia, gout should be considered in the differential diagnosis even if the patient denies having a prior gout attack.
Frequently, patients will call their primary care physician during a gout attack but are not be able to schedule an appointment until after the attack has resolved. When possible, patients should be seen during the attack to confirm whether the attack is due to gout. A diagnosis of gout should not be made over the phone when a patient describes pain in the great toe, as only 50% of initial gout attacks occur in the great toe7 and it is not known what proportion of acute pain episodes in the great toe are attributable to gout. The most common cause of pain in the great toe is osteoarthritis.
SPECIAL CONSIDERATIONS FOR THE PRESUMPTIVE DIAGNOSIS OF GOUT
How long have acute attacks been occurring?
In a clinical scenario in which synovial fluid aspiration cannot be performed, the appropriateness of a presumptive diagnosis can be assessed by a discussion with the patient about how long he or she has been experiencing acute attacks of joint pain. If the attacks have occurred for more than 10 years, tophi will likely be present.3 After even longer periods, gout may become polyarticular.7 In postmenopausal women, the distal interphalangeal joints may be involved,3 which may lead to a misdiagnosis of osteoarthritis, as these joints are typically affected by osteoarthritis.
Is the patient taking a urate-raising medication?
Certain medications have been associated with hyper-uricemia, including cyclosporine and thiazide diuretics.9 If a patient has been taking one of these medications, gout should be considered in the differential diagnosis if the patient presents with acute joint pain.
It has been argued that a reduction in joint pain and swelling after the use of colchicine confirms a diagnosis of gout. However, other conditions—such as tendonitis, calcium pyrophosphate dihydrate (CPPD) crystal deposition disease (pseudogout),3 and rheumatoid arthritis (RA)—can also improve after treatment with colchicine.1
Be vigilant for fever
Another consideration in making a clinical diagnosis of gout is the association with a low-grade fever; these patients may feel as if they have the flu.8 Acute gout may also cause a high fever and an elevated white blood cell (WBC) count;3 in this situation, synovial fluid aspiration must be performed to exclude septic arthritis, either alone or in the presence of gouty arthritis. In situations where septic arthritis is suspected, an emergency visit to a rheumatologist is indicated for synovial fluid aspiration to be performed, as gout and sepsis can coexist.5 In such instances, Gram staining and culture of the synovial fluid should still be performed even if monosodium urate crystals are identified.5
MEASUREMENT OF SERUM URATE LEVELS
Measuring serum urate levels during an acute attack, treating the acute attack with anti-inflammatory medications, and reevaluating the patient in the office 2 weeks after the acute attack are all recommended in the management of a patient with gout. If the serum urate level was not elevated during the acute attack, it is likely to be elevated 2 weeks later if the patient has gout.10 Elevated levels of serum urate during the intercritical periods are predictive of future gout attacks.11 Measuring serum urate during the initial attack and then 2 weeks later yields two serum urate levels that can be compared to assist in considering a presumed diagnosis of gout. A study by Rigby and Wood concluded that in patients with low serum urate levels (< 4 mg/dL) 2 weeks following an inflammatory arthritis attack, a diagnosis of gout is unlikely.12
DIFFERENTIAL DIAGNOSIS OF GOUT
Rheumatoid arthritis
CPPD crystal deposition disease (pseudogout)
CPPD crystal deposition disease, or pseudogout, must also be included in the differential diagnosis of gout. This disease usually occurs in joints previously affected by osteoarthritis or joints that have been injured in the past.15 Attacks of CPPD crystal deposition disease commonly occur in the knee, in the wrist at the base of the thumb, or in the shoulder.15 Radiographic examination may reveal a line of calcification along the cartilage outlining the joint.15 Like gout, pseudogout attacks can occur spontaneously or after trauma, surgery, or a severe illness such as myocardial infarction or stroke.16
The presentation of pseudogout can be very similar to an acute attack of gout. The difference is seen when evaluating the crystals through a polarizing microscope. CPPD crystals are weakly positively birefringent (Figure 1B), in contrast to the negatively birefringent crystals seen with gout (Figure 1A).7 If a polarizing microscope is not available, the crystals usually can be distinguished by their differing shapes: urate crystals are fine and needlelike, whereas CPPD crystals are rhomboid (Figure 1).
Septic arthritis
When the differential diagnosis includes septic arthritis, the joint must be aspirated; a presumed diagnosis cannot be made. Among patients with an acute gouty attack, low-grade fever is reported during the attack in 29% of gout patients and 38% of patients with CPPD crystal deposition disease.14 Temperatures of 101°F or higher are not usually seen in patients with gout or CPPD crystal deposition disease and suggest an infection, although patients with septic arthritis may be afebrile, especially if they are taking immunosuppressive therapy or glucocorticoids, which can inhibit a febrile response. Synovial fluid analysis in patients with gout and septic arthritis can reveal WBC counts above 100,000 per mm3, whereas synovial fluid WBC counts above 50,000 per mm3 are more common in infection.
As noted earlier, gout and septic arthritis can coexist. In a patient presenting with a fever and a warm erythematous swollen joint, synovial fluid aspiration must be performed and evaluated for the presence of crystals and bacteria. The patient may require treatment for both causes of acute monoarticular arthritis.
In a patient undergoing renal dialysis, where gout or pseudogout can occur and where there is frequent intravascular manipulation, a septic joint can occur simultaneously.3,14 In this situation, not only must joint aspiration be performed, but the synovial fluid also needs to be evaluated for both crystals and bacteria. Again, the patient may require treatment for both causes of acute monoarticular arthritis.
CONCLUSIONS
The gold standard for diagnosing gout remains synovial fluid aspiration and analysis. In clinical situations when joint aspiration cannot be performed, the EULAR recommendations5 and the ACR criteria6 provide guidance for making a clinical or presumptive diagnosis of gout. A thorough patient history—both personal and family—and physical examination are critical in making a presumed diagnosis of gout. If the patient presents during an acute attack, serum urate measurement may be useful in making a clinical diagnosis if it reveals an elevated level. When the patient returns for follow-up 2 weeks later, a second serum urate measurement should be taken to allow comparison of the two levels. If the serum urate level is elevated at the follow-up visit, the EULAR recommendations state that a clinical diagnosis of gout can be made if the patient had an acute attack of arthritis in the great toe.
As noted in the EULAR recommendations, the future research agenda should include validating the clinical manifestations of gout against a diagnosis established by identification of urate crystals on synovial fluid analysis.5 Until this task can be completed, clinicians should become familiarized with the technique of joint aspiration so that in situations where a clinical or presumptive diagnosis of gout cannot be made—including cases where the differential diagnosis includes a septic joint—clinicians will be able to perform aspiration with confidence.
- Jelley MJ, Wortmann R. Practical steps in the diagnosis and management of gout. BioDrugs 2000; 14:99–107.
- Eggebeen AT. Gout: an update. Am Fam Physician 2007; 76:801–808, 811–812.
- Rott KT, Agudelo CA. Gout. JAMA 2003; 289:2857–2860.
- Poór G, Mituszova M. History, classification and epidemiology of crystal-related arthropathies. In: Hochberg MC, Silman AJ, Smolen JS, Weinblatt ME, Weisman MH, eds. Rheumatology. 3rd ed, vol 2. Edinburgh: Mosby; 2003:1893–1901.
- Zhang W, Doherty M, Pascual E, et al. EULAR evidence based recommendations for gout. Part I: Diagnosis. Report of a task force of the Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis 2006; 65:1301–1311.
- Wallace SL, Robinson H, Masi AT, Decker JL, McCarty DJ, Yü TF. Preliminary criteria for the classification of the acute arthritis of primary gout. Arthritis Rheum 1977; 20:895–900.
- Harris MD, Siegel LB, Alloway JA. Gout and hyperuricemia. Am Fam Physician 1999; 59:925–934.
- Saag KG, Mikuls TR. Recent advances in the epidemiology of gout. Curr Rheumatol Rep 2005; 7:235–241.
- Edwards NL. Gout. B. Clinical and laboratory features. In: Klippel JH, Crofford LJ, Stone JH, Weyand CM, eds. Primer on the Rheumatic Diseases. 12th ed. Atlanta, GA: Arthritis Foundation; 2001:313–319.
- Urano W, Yamanaka H, Tsutani H, et al. The inflammatory process in the mechanism of decreased serum uric acid concentrations during acute gouty arthritis. J Rheumatol 2002; 29:1950–1953.
- Shoji A, Yamanaka H, Kamatani N. A retrospective study of the relationship between serum urate level and recurrent attacks of gouty arthritis: evidence for reduction of recurrent gouty arthritis with anti-hyperuricemic therapy. Arthritis Rheum 2004; 51:321–325.
- Rigby AS, Wood PH. Serum uric acid levels and gout: what does this herald for the population? Clin Exp Rheumatol 1994; 12:395–400.
- George DL. Skin and rheumatic disease. In: Hochberg MC, Silman AJ, Smolen JS, Weinblatt ME, Weisman MH, eds. Rheumatology. 3rd ed, vol 1. Edinburgh: Mosby; 2003:279–292.
- Ho G Jr, DeNuccio M. Gout and pseudogout in hospitalized patients. Arch Intern Med 1993; 153:2787–2790.
- Agarwal AK. Gout and pseudogout. Prim Care 1993; 20:839–855.
- Halverson PB, Ryan LM. Arthritis associated with calcium-containing crystals. In: Klippel JH, Crofford LJ, Stone JH, Weyand CM, eds. Primer on the Rheumatic Diseases. 12th ed. Atlanta, GA: Arthritis Foundation; 2001:299–306.
- Jelley MJ, Wortmann R. Practical steps in the diagnosis and management of gout. BioDrugs 2000; 14:99–107.
- Eggebeen AT. Gout: an update. Am Fam Physician 2007; 76:801–808, 811–812.
- Rott KT, Agudelo CA. Gout. JAMA 2003; 289:2857–2860.
- Poór G, Mituszova M. History, classification and epidemiology of crystal-related arthropathies. In: Hochberg MC, Silman AJ, Smolen JS, Weinblatt ME, Weisman MH, eds. Rheumatology. 3rd ed, vol 2. Edinburgh: Mosby; 2003:1893–1901.
- Zhang W, Doherty M, Pascual E, et al. EULAR evidence based recommendations for gout. Part I: Diagnosis. Report of a task force of the Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis 2006; 65:1301–1311.
- Wallace SL, Robinson H, Masi AT, Decker JL, McCarty DJ, Yü TF. Preliminary criteria for the classification of the acute arthritis of primary gout. Arthritis Rheum 1977; 20:895–900.
- Harris MD, Siegel LB, Alloway JA. Gout and hyperuricemia. Am Fam Physician 1999; 59:925–934.
- Saag KG, Mikuls TR. Recent advances in the epidemiology of gout. Curr Rheumatol Rep 2005; 7:235–241.
- Edwards NL. Gout. B. Clinical and laboratory features. In: Klippel JH, Crofford LJ, Stone JH, Weyand CM, eds. Primer on the Rheumatic Diseases. 12th ed. Atlanta, GA: Arthritis Foundation; 2001:313–319.
- Urano W, Yamanaka H, Tsutani H, et al. The inflammatory process in the mechanism of decreased serum uric acid concentrations during acute gouty arthritis. J Rheumatol 2002; 29:1950–1953.
- Shoji A, Yamanaka H, Kamatani N. A retrospective study of the relationship between serum urate level and recurrent attacks of gouty arthritis: evidence for reduction of recurrent gouty arthritis with anti-hyperuricemic therapy. Arthritis Rheum 2004; 51:321–325.
- Rigby AS, Wood PH. Serum uric acid levels and gout: what does this herald for the population? Clin Exp Rheumatol 1994; 12:395–400.
- George DL. Skin and rheumatic disease. In: Hochberg MC, Silman AJ, Smolen JS, Weinblatt ME, Weisman MH, eds. Rheumatology. 3rd ed, vol 1. Edinburgh: Mosby; 2003:279–292.
- Ho G Jr, DeNuccio M. Gout and pseudogout in hospitalized patients. Arch Intern Med 1993; 153:2787–2790.
- Agarwal AK. Gout and pseudogout. Prim Care 1993; 20:839–855.
- Halverson PB, Ryan LM. Arthritis associated with calcium-containing crystals. In: Klippel JH, Crofford LJ, Stone JH, Weyand CM, eds. Primer on the Rheumatic Diseases. 12th ed. Atlanta, GA: Arthritis Foundation; 2001:299–306.
KEY POINTS
- If the serum urate level was not elevated when measured during an acute attack of arthritis, it will likely be elevated at 2-week follow-up if the patient does indeed have gout.
- Gouty tophi are typically found in the olecranon bursa, whereas rheumatoid nodules are usually located on the extensor surface of the forearm.
- Urate crystals of gout are negatively bifringent and fine and needlelike in shape, whereas the crystals of pseudogout are weakly positively birefringent and rhomboid.
- Gout and septic arthritis can coexist; when the differential diagnosis includes septic arthritis, joint aspiration is required.
- Until criteria for the presumptive diagnosis of gout are validated, clinicians should become familiar with the technique of joint aspiration.