User login
- Update on Fertility
G. David Adamson, MD; Mary E. Abusief, MD (February 2012) - Ovarian stimulation ups risk of ovarian tumors in later life
(November 2011) - Can ovulation induction be accelerated in women who have PCOS-related infertility?
Richard S. Legro, MD (Examining the Evidence, September 2009)
CASE Infertility Dx: PCOS, anovulation
You have been treating a 33-year-old G0P0 woman who has polycystic ovary syndrome (PCOS) and anovulatory infertility with clomiphene citrate for three cycles, at escalating doses of 50 mg, 100 mg, and 150 mg daily. She has not ovulated, however, as determined by appropriately timed serum progesterone measurement.
What is your next step?
Anovulation is a common cause of infertility; approximately 70% of cases of anovulatory infertility are caused by PCOS. For women who have PCOS and anovulatory infertility, approaches to ovulation induction include:
- weight loss
- clomiphene
- metformin
- follicle-stimulating hormone (FSH) injection
- laparoscopic ovarian drilling
- in vitro fertilization (IVF).
Women who fail to ovulate at standard dosages of clomiphene are labeled “clomiphene-resistant.” A consensus panel of expert fertility specialists recommended that, for such women, the most appropriate next steps in treatment include FSH injection or laparoscopic ovarian drilling.1 For many women, however, those options are prohibitively expensive.
For a clomiphene-resistant woman who has PCOS, then, what affordable treatment can you prescribe?
One answer is that many clomiphene-resistant women will ovulate if they are treated with a combination of clomiphene and dexamethasone.2-16 Here is a stepwise approach to using clomiphene with dexamethasone.
STEP 1 Review the infertility work-up
The standard infertility work-up includes hysterosalpingography, semen analysis, and test of ovulation. If the work-up shows severely abnormal semen parameters or bilateral tubal disease, consultation with a fertility specialist, and IVF, might be a more appropriate course than undertaking ovulation induction with clomiphene.
Further work-up of anovulatory infertility. First obtain measurements of serum thyroid-stimulating hormone (TSH), follicle-stimulating hormone (FSH), and prolactin; abnormalities of these hormones might contraindicate clomiphene for ovulation induction.
Next, measurement of total serum testosterone and dehydroepiandrosterone sulfate (DHEAS) might be useful to determine if your patient’s clomiphene resistance is caused by significantly elevated androgen levels.
Then, if your clomiphene resistant patient has a normal hysterosalpingogram (HSG) and normal TSH, FSH, and prolactin test results, and her partner has a normal semen analysis, consider that she might benefit from treatment with a combination of clomiphene and dexamethasone.
STEP 2 Induce ovulation with clomiphene plus dexamethasone
One cause of clomiphene resistance is an elevated serum testosterone level.7 Other causes include an elevated body-mass index and advanced age. Dexamethasone can improve the efficacy of clomiphene by reducing androgen levels.
What trials have demonstrated. Two randomized clinical trials have shown that, in clomiphene-resistant women, dexamethasone plus clomiphene increases ovulation and the pregnancy rate, compared with clomiphene alone.2,3
One regimen that has been reported to be successful is to treat the clomiphene-resistant woman with clomiphene, 100 mg daily, for cycle Days 3 to 7, and simultaneously treat her with dexamethasone, 2 mg daily, for cycle Days 3 to 12 (FIGURE).2 Treatment with dexamethasone reduces the serum concentration of androgens, thereby increasing the efficacy of clomiphene.
In the randomized trial that used this regimen to treat clomiphene-resistant women, the ovulation rate was 75% in the clomiphene plus dexamethasone group and 15% in the clomiphene-only group (P <.001). The pregnancy rate was 40% in the clomiphene plus dexamethasone group and 5% in the clomiphene-only group (P <.05).
Many clinicians instruct their patients to take the dexamethasone at night to maximally blunt the early morning adrenocorticotropic (ACTH) surge, which stimulates adrenal androgen production. I have found, however, that, for many women, a nighttime dose of dexamethasone energizes them and causes difficulty falling, and remaining, asleep. I recommend that patients take dexamethasone in the morning.
Note: Before initiating a clomiphene plus dexamethasone cycle, many experts 1) obtain a pregnancy test to rule out ongoing pregnancy and then 2) prescribe progestin withdrawal. A commonly used agent for progestin withdrawal is medroxyprogesterone acetate (Provera), 10 mg/d for 5 days. The first day of full withdrawal flow after cessation of progestin treatment is considered Day 1 of the cycle.
During the clomiphene plus dexamethasone treatment cycle, the patient can take urine luteinizing hormone (LH) measurements at home to identify the preovulatory LH surge, which typically occurs 5 to 12 days after the last day of clomiphene medication (FIGURE). The woman’s maximal fertile span is the day before the LH surge, the day of the LH surge, and the day following the LH surge. Coitus should occur on at least 2 of these 3 days.
If the patient prefers not to measure urine LH, recommend that she have coitus every other day for 8 days, beginning 5 days after the last clomiphene tablet.
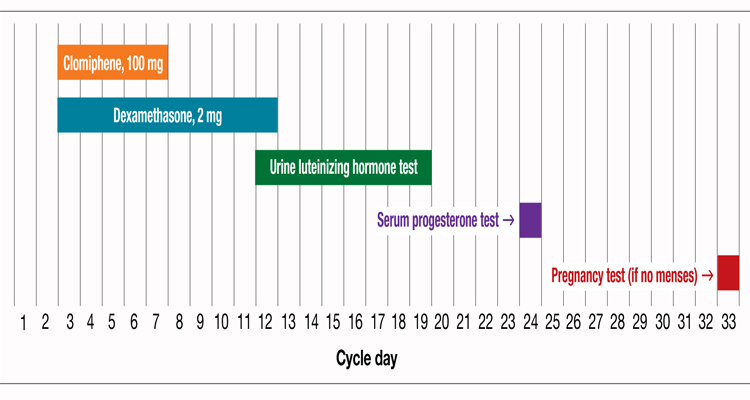
Plotting a cycle of clomiphene plus dexamethasone, and accompanying testing
STEP 3 Measure serum progesterone approximately 7 to 10 days after the LH surge
Evidence of successful ovulation is an appropriately drawn serum progesterone level >8 ng/mL; in most clomiphene cycles in which successful ovulation has occurred, the serum progesterone level is >20 ng/mL.
If menses does not occur within 17 days after the LH surge, obtain a pregnancy test. If the combination of clomiphene, 100 mg/d for 5 days, plus dexamethasone does not cause ovulation, prescribe a cycle of clomiphene, 150 mg/d for cycle Days 3 to 7, plus dexamethasone.
If that regimen does not cause ovulation, advise the patient to consider other options for ovulation induction—such as weight loss, FSH injection, laparoscopic ovarian drilling, and IVF.
Caution on the duration of therapy. Experts recommend that clomiphene therapy for infertility be limited to no more than approximately 6 to 12 cycles; the concerns are that prolonged clomiphene treatment may increase the risk of ovarian neoplasm8 and that the pregnancy rate per cycle may decrease with prolonged use of clomiphene.
Women who use clomiphene should also be aware that approximately 8% of clomiphene-induced pregnancies are twin gestations and <0.5% are triplet gestations.
CASE Resolved, on labor and delivery
The clomiphene-resistant woman described at the beginning of the Editorial underwent HSG that showed bilaterally patent tubes, of normal caliber. Her partner’s semen analysis was normal.
She was treated with clomiphene plus dexamethasone, and ovulated. She became pregnant, and delivered a singleton newborn, at term.
Consider estrogen-progestin for 2 months, leading up to a clomiphene cycle
Another treatment option that can enhance the efficacy of clomiphene in women who are resistant to the drug is to prescribe 2 months of an estrogen–progestin contraceptive, then stop the contraceptive and prescribe a standard cycle of clomiphene. A randomized trial has demonstrated the effectiveness of this regimen for clomiphene-resistant patients.1
In the report of the trial by Branigan and Estes,1 women who failed to ovulate with clomiphene, 150 mg/d for 5 days, were randomized to:
- 42 to 50 days of ethinyl estradiol, 0.03 mg, plus desogestrel (Desogen), 0.15 mg, before a clomiphene cycle (Group 1) or
- no treatment before a clomiphene cycle (Group 2, controls).
After a withdrawal bleed (Group 1) or spontaneous menses (Group 2), all subjects were treated with clomiphene, 100 mg/d, for cycle Days 5 to 9. Women in Group 1 exhibited a 55% mean decrease in serum testosterone (P <.001); women in the control group had a 6% mean decrease in serum testosterone (no significant change). Across six treatment cycles, the pregnancy rate was 54% (Group 1) and 4% (Group 2) (P <.001).
The researchers’ conclusion? Lowering serum testosterone with estrogen–progestin pretreatment might improve responsiveness to clomiphene-induced ovulation.
Reference
A woman seeing pregnancy has PCOS and anovulatory infertility, and is clomiphene-resistant. What is your preferred next step in management?
1. The Thessalonkiki ESHRE/ASRM-sponsored PCOS Consensus Workshop Group. Consensus on infertility treatment related to polycystic ovary syndrome. Fertil Steril. 2008;89(3):505-522.
2. Elnashar A, Abdelmageed E, Fayed M, Sharaf M. Clomiphene citrate and dexamethasone in treatment of clomiphene citrate-resistant polycystic ovary syndrome: a prospective placebo-controlled study. Hum Reprod. 2006;21(7):1805-1808.
3. Parsanezhad ME, Alborzi S, Motazedian S, Omrani G. Use of dexamethasone and clomiphene citrate in the treatment of clomiphene citrate-resistant patients with polycystic ovary syndrome and normal dehydroepiandrosterone sulfate levels: placebo-controlled trial. Fertil Steril. 2002;78(5):1001-1004.
4. Isaacs JD, Lincoln SR, Cowan BD. Extended clomiphene citrate (CC) and prednisone for the treatment of chronic anovulation resistant to CC alone. Fertil Steril. 1997;67(4):641-643.
5. Daly DC, Walters CA, Soto-Albors CE, Tohan N, Riddick DH. A randomized study of dexamethasone in ovulation induction with clomiphene citrate. Fertil Steril. 1984;41(6):844-848.
6. Brown J, Farquhar C, Beck J, Boothroyd C, Hughes E. Clomiphene and anti-estrogens for ovulation induction in PCOS. Cochrane Database Syst Rev. 2009;(4):CD002249.-
7. Imani B, Eijkemans MJ, te Velde ER, Habbema JDF, Fauser BC. A nomogram to predict the probability of live birth after clomiphene citrate induction of ovulation in normogonadotropic oligoamenorrheic infertility. Fertil Steril. 2002;77(1):91-97.
8. Rossing MA, Daling JR, Weiss NS, Moore DE, Self SG. Ovarian tumors in a cohort of infertile women. N Engl J Med. 1994;331(12):771-776.
- Update on Fertility
G. David Adamson, MD; Mary E. Abusief, MD (February 2012) - Ovarian stimulation ups risk of ovarian tumors in later life
(November 2011) - Can ovulation induction be accelerated in women who have PCOS-related infertility?
Richard S. Legro, MD (Examining the Evidence, September 2009)
CASE Infertility Dx: PCOS, anovulation
You have been treating a 33-year-old G0P0 woman who has polycystic ovary syndrome (PCOS) and anovulatory infertility with clomiphene citrate for three cycles, at escalating doses of 50 mg, 100 mg, and 150 mg daily. She has not ovulated, however, as determined by appropriately timed serum progesterone measurement.
What is your next step?
Anovulation is a common cause of infertility; approximately 70% of cases of anovulatory infertility are caused by PCOS. For women who have PCOS and anovulatory infertility, approaches to ovulation induction include:
- weight loss
- clomiphene
- metformin
- follicle-stimulating hormone (FSH) injection
- laparoscopic ovarian drilling
- in vitro fertilization (IVF).
Women who fail to ovulate at standard dosages of clomiphene are labeled “clomiphene-resistant.” A consensus panel of expert fertility specialists recommended that, for such women, the most appropriate next steps in treatment include FSH injection or laparoscopic ovarian drilling.1 For many women, however, those options are prohibitively expensive.
For a clomiphene-resistant woman who has PCOS, then, what affordable treatment can you prescribe?
One answer is that many clomiphene-resistant women will ovulate if they are treated with a combination of clomiphene and dexamethasone.2-16 Here is a stepwise approach to using clomiphene with dexamethasone.
STEP 1 Review the infertility work-up
The standard infertility work-up includes hysterosalpingography, semen analysis, and test of ovulation. If the work-up shows severely abnormal semen parameters or bilateral tubal disease, consultation with a fertility specialist, and IVF, might be a more appropriate course than undertaking ovulation induction with clomiphene.
Further work-up of anovulatory infertility. First obtain measurements of serum thyroid-stimulating hormone (TSH), follicle-stimulating hormone (FSH), and prolactin; abnormalities of these hormones might contraindicate clomiphene for ovulation induction.
Next, measurement of total serum testosterone and dehydroepiandrosterone sulfate (DHEAS) might be useful to determine if your patient’s clomiphene resistance is caused by significantly elevated androgen levels.
Then, if your clomiphene resistant patient has a normal hysterosalpingogram (HSG) and normal TSH, FSH, and prolactin test results, and her partner has a normal semen analysis, consider that she might benefit from treatment with a combination of clomiphene and dexamethasone.
STEP 2 Induce ovulation with clomiphene plus dexamethasone
One cause of clomiphene resistance is an elevated serum testosterone level.7 Other causes include an elevated body-mass index and advanced age. Dexamethasone can improve the efficacy of clomiphene by reducing androgen levels.
What trials have demonstrated. Two randomized clinical trials have shown that, in clomiphene-resistant women, dexamethasone plus clomiphene increases ovulation and the pregnancy rate, compared with clomiphene alone.2,3
One regimen that has been reported to be successful is to treat the clomiphene-resistant woman with clomiphene, 100 mg daily, for cycle Days 3 to 7, and simultaneously treat her with dexamethasone, 2 mg daily, for cycle Days 3 to 12 (FIGURE).2 Treatment with dexamethasone reduces the serum concentration of androgens, thereby increasing the efficacy of clomiphene.
In the randomized trial that used this regimen to treat clomiphene-resistant women, the ovulation rate was 75% in the clomiphene plus dexamethasone group and 15% in the clomiphene-only group (P <.001). The pregnancy rate was 40% in the clomiphene plus dexamethasone group and 5% in the clomiphene-only group (P <.05).
Many clinicians instruct their patients to take the dexamethasone at night to maximally blunt the early morning adrenocorticotropic (ACTH) surge, which stimulates adrenal androgen production. I have found, however, that, for many women, a nighttime dose of dexamethasone energizes them and causes difficulty falling, and remaining, asleep. I recommend that patients take dexamethasone in the morning.
Note: Before initiating a clomiphene plus dexamethasone cycle, many experts 1) obtain a pregnancy test to rule out ongoing pregnancy and then 2) prescribe progestin withdrawal. A commonly used agent for progestin withdrawal is medroxyprogesterone acetate (Provera), 10 mg/d for 5 days. The first day of full withdrawal flow after cessation of progestin treatment is considered Day 1 of the cycle.
During the clomiphene plus dexamethasone treatment cycle, the patient can take urine luteinizing hormone (LH) measurements at home to identify the preovulatory LH surge, which typically occurs 5 to 12 days after the last day of clomiphene medication (FIGURE). The woman’s maximal fertile span is the day before the LH surge, the day of the LH surge, and the day following the LH surge. Coitus should occur on at least 2 of these 3 days.
If the patient prefers not to measure urine LH, recommend that she have coitus every other day for 8 days, beginning 5 days after the last clomiphene tablet.

Plotting a cycle of clomiphene plus dexamethasone, and accompanying testing
STEP 3 Measure serum progesterone approximately 7 to 10 days after the LH surge
Evidence of successful ovulation is an appropriately drawn serum progesterone level >8 ng/mL; in most clomiphene cycles in which successful ovulation has occurred, the serum progesterone level is >20 ng/mL.
If menses does not occur within 17 days after the LH surge, obtain a pregnancy test. If the combination of clomiphene, 100 mg/d for 5 days, plus dexamethasone does not cause ovulation, prescribe a cycle of clomiphene, 150 mg/d for cycle Days 3 to 7, plus dexamethasone.
If that regimen does not cause ovulation, advise the patient to consider other options for ovulation induction—such as weight loss, FSH injection, laparoscopic ovarian drilling, and IVF.
Caution on the duration of therapy. Experts recommend that clomiphene therapy for infertility be limited to no more than approximately 6 to 12 cycles; the concerns are that prolonged clomiphene treatment may increase the risk of ovarian neoplasm8 and that the pregnancy rate per cycle may decrease with prolonged use of clomiphene.
Women who use clomiphene should also be aware that approximately 8% of clomiphene-induced pregnancies are twin gestations and <0.5% are triplet gestations.
CASE Resolved, on labor and delivery
The clomiphene-resistant woman described at the beginning of the Editorial underwent HSG that showed bilaterally patent tubes, of normal caliber. Her partner’s semen analysis was normal.
She was treated with clomiphene plus dexamethasone, and ovulated. She became pregnant, and delivered a singleton newborn, at term.
Consider estrogen-progestin for 2 months, leading up to a clomiphene cycle
Another treatment option that can enhance the efficacy of clomiphene in women who are resistant to the drug is to prescribe 2 months of an estrogen–progestin contraceptive, then stop the contraceptive and prescribe a standard cycle of clomiphene. A randomized trial has demonstrated the effectiveness of this regimen for clomiphene-resistant patients.1
In the report of the trial by Branigan and Estes,1 women who failed to ovulate with clomiphene, 150 mg/d for 5 days, were randomized to:
- 42 to 50 days of ethinyl estradiol, 0.03 mg, plus desogestrel (Desogen), 0.15 mg, before a clomiphene cycle (Group 1) or
- no treatment before a clomiphene cycle (Group 2, controls).
After a withdrawal bleed (Group 1) or spontaneous menses (Group 2), all subjects were treated with clomiphene, 100 mg/d, for cycle Days 5 to 9. Women in Group 1 exhibited a 55% mean decrease in serum testosterone (P <.001); women in the control group had a 6% mean decrease in serum testosterone (no significant change). Across six treatment cycles, the pregnancy rate was 54% (Group 1) and 4% (Group 2) (P <.001).
The researchers’ conclusion? Lowering serum testosterone with estrogen–progestin pretreatment might improve responsiveness to clomiphene-induced ovulation.
Reference
A woman seeing pregnancy has PCOS and anovulatory infertility, and is clomiphene-resistant. What is your preferred next step in management?
- Update on Fertility
G. David Adamson, MD; Mary E. Abusief, MD (February 2012) - Ovarian stimulation ups risk of ovarian tumors in later life
(November 2011) - Can ovulation induction be accelerated in women who have PCOS-related infertility?
Richard S. Legro, MD (Examining the Evidence, September 2009)
CASE Infertility Dx: PCOS, anovulation
You have been treating a 33-year-old G0P0 woman who has polycystic ovary syndrome (PCOS) and anovulatory infertility with clomiphene citrate for three cycles, at escalating doses of 50 mg, 100 mg, and 150 mg daily. She has not ovulated, however, as determined by appropriately timed serum progesterone measurement.
What is your next step?
Anovulation is a common cause of infertility; approximately 70% of cases of anovulatory infertility are caused by PCOS. For women who have PCOS and anovulatory infertility, approaches to ovulation induction include:
- weight loss
- clomiphene
- metformin
- follicle-stimulating hormone (FSH) injection
- laparoscopic ovarian drilling
- in vitro fertilization (IVF).
Women who fail to ovulate at standard dosages of clomiphene are labeled “clomiphene-resistant.” A consensus panel of expert fertility specialists recommended that, for such women, the most appropriate next steps in treatment include FSH injection or laparoscopic ovarian drilling.1 For many women, however, those options are prohibitively expensive.
For a clomiphene-resistant woman who has PCOS, then, what affordable treatment can you prescribe?
One answer is that many clomiphene-resistant women will ovulate if they are treated with a combination of clomiphene and dexamethasone.2-16 Here is a stepwise approach to using clomiphene with dexamethasone.
STEP 1 Review the infertility work-up
The standard infertility work-up includes hysterosalpingography, semen analysis, and test of ovulation. If the work-up shows severely abnormal semen parameters or bilateral tubal disease, consultation with a fertility specialist, and IVF, might be a more appropriate course than undertaking ovulation induction with clomiphene.
Further work-up of anovulatory infertility. First obtain measurements of serum thyroid-stimulating hormone (TSH), follicle-stimulating hormone (FSH), and prolactin; abnormalities of these hormones might contraindicate clomiphene for ovulation induction.
Next, measurement of total serum testosterone and dehydroepiandrosterone sulfate (DHEAS) might be useful to determine if your patient’s clomiphene resistance is caused by significantly elevated androgen levels.
Then, if your clomiphene resistant patient has a normal hysterosalpingogram (HSG) and normal TSH, FSH, and prolactin test results, and her partner has a normal semen analysis, consider that she might benefit from treatment with a combination of clomiphene and dexamethasone.
STEP 2 Induce ovulation with clomiphene plus dexamethasone
One cause of clomiphene resistance is an elevated serum testosterone level.7 Other causes include an elevated body-mass index and advanced age. Dexamethasone can improve the efficacy of clomiphene by reducing androgen levels.
What trials have demonstrated. Two randomized clinical trials have shown that, in clomiphene-resistant women, dexamethasone plus clomiphene increases ovulation and the pregnancy rate, compared with clomiphene alone.2,3
One regimen that has been reported to be successful is to treat the clomiphene-resistant woman with clomiphene, 100 mg daily, for cycle Days 3 to 7, and simultaneously treat her with dexamethasone, 2 mg daily, for cycle Days 3 to 12 (FIGURE).2 Treatment with dexamethasone reduces the serum concentration of androgens, thereby increasing the efficacy of clomiphene.
In the randomized trial that used this regimen to treat clomiphene-resistant women, the ovulation rate was 75% in the clomiphene plus dexamethasone group and 15% in the clomiphene-only group (P <.001). The pregnancy rate was 40% in the clomiphene plus dexamethasone group and 5% in the clomiphene-only group (P <.05).
Many clinicians instruct their patients to take the dexamethasone at night to maximally blunt the early morning adrenocorticotropic (ACTH) surge, which stimulates adrenal androgen production. I have found, however, that, for many women, a nighttime dose of dexamethasone energizes them and causes difficulty falling, and remaining, asleep. I recommend that patients take dexamethasone in the morning.
Note: Before initiating a clomiphene plus dexamethasone cycle, many experts 1) obtain a pregnancy test to rule out ongoing pregnancy and then 2) prescribe progestin withdrawal. A commonly used agent for progestin withdrawal is medroxyprogesterone acetate (Provera), 10 mg/d for 5 days. The first day of full withdrawal flow after cessation of progestin treatment is considered Day 1 of the cycle.
During the clomiphene plus dexamethasone treatment cycle, the patient can take urine luteinizing hormone (LH) measurements at home to identify the preovulatory LH surge, which typically occurs 5 to 12 days after the last day of clomiphene medication (FIGURE). The woman’s maximal fertile span is the day before the LH surge, the day of the LH surge, and the day following the LH surge. Coitus should occur on at least 2 of these 3 days.
If the patient prefers not to measure urine LH, recommend that she have coitus every other day for 8 days, beginning 5 days after the last clomiphene tablet.

Plotting a cycle of clomiphene plus dexamethasone, and accompanying testing
STEP 3 Measure serum progesterone approximately 7 to 10 days after the LH surge
Evidence of successful ovulation is an appropriately drawn serum progesterone level >8 ng/mL; in most clomiphene cycles in which successful ovulation has occurred, the serum progesterone level is >20 ng/mL.
If menses does not occur within 17 days after the LH surge, obtain a pregnancy test. If the combination of clomiphene, 100 mg/d for 5 days, plus dexamethasone does not cause ovulation, prescribe a cycle of clomiphene, 150 mg/d for cycle Days 3 to 7, plus dexamethasone.
If that regimen does not cause ovulation, advise the patient to consider other options for ovulation induction—such as weight loss, FSH injection, laparoscopic ovarian drilling, and IVF.
Caution on the duration of therapy. Experts recommend that clomiphene therapy for infertility be limited to no more than approximately 6 to 12 cycles; the concerns are that prolonged clomiphene treatment may increase the risk of ovarian neoplasm8 and that the pregnancy rate per cycle may decrease with prolonged use of clomiphene.
Women who use clomiphene should also be aware that approximately 8% of clomiphene-induced pregnancies are twin gestations and <0.5% are triplet gestations.
CASE Resolved, on labor and delivery
The clomiphene-resistant woman described at the beginning of the Editorial underwent HSG that showed bilaterally patent tubes, of normal caliber. Her partner’s semen analysis was normal.
She was treated with clomiphene plus dexamethasone, and ovulated. She became pregnant, and delivered a singleton newborn, at term.
Consider estrogen-progestin for 2 months, leading up to a clomiphene cycle
Another treatment option that can enhance the efficacy of clomiphene in women who are resistant to the drug is to prescribe 2 months of an estrogen–progestin contraceptive, then stop the contraceptive and prescribe a standard cycle of clomiphene. A randomized trial has demonstrated the effectiveness of this regimen for clomiphene-resistant patients.1
In the report of the trial by Branigan and Estes,1 women who failed to ovulate with clomiphene, 150 mg/d for 5 days, were randomized to:
- 42 to 50 days of ethinyl estradiol, 0.03 mg, plus desogestrel (Desogen), 0.15 mg, before a clomiphene cycle (Group 1) or
- no treatment before a clomiphene cycle (Group 2, controls).
After a withdrawal bleed (Group 1) or spontaneous menses (Group 2), all subjects were treated with clomiphene, 100 mg/d, for cycle Days 5 to 9. Women in Group 1 exhibited a 55% mean decrease in serum testosterone (P <.001); women in the control group had a 6% mean decrease in serum testosterone (no significant change). Across six treatment cycles, the pregnancy rate was 54% (Group 1) and 4% (Group 2) (P <.001).
The researchers’ conclusion? Lowering serum testosterone with estrogen–progestin pretreatment might improve responsiveness to clomiphene-induced ovulation.
Reference
A woman seeing pregnancy has PCOS and anovulatory infertility, and is clomiphene-resistant. What is your preferred next step in management?
1. The Thessalonkiki ESHRE/ASRM-sponsored PCOS Consensus Workshop Group. Consensus on infertility treatment related to polycystic ovary syndrome. Fertil Steril. 2008;89(3):505-522.
2. Elnashar A, Abdelmageed E, Fayed M, Sharaf M. Clomiphene citrate and dexamethasone in treatment of clomiphene citrate-resistant polycystic ovary syndrome: a prospective placebo-controlled study. Hum Reprod. 2006;21(7):1805-1808.
3. Parsanezhad ME, Alborzi S, Motazedian S, Omrani G. Use of dexamethasone and clomiphene citrate in the treatment of clomiphene citrate-resistant patients with polycystic ovary syndrome and normal dehydroepiandrosterone sulfate levels: placebo-controlled trial. Fertil Steril. 2002;78(5):1001-1004.
4. Isaacs JD, Lincoln SR, Cowan BD. Extended clomiphene citrate (CC) and prednisone for the treatment of chronic anovulation resistant to CC alone. Fertil Steril. 1997;67(4):641-643.
5. Daly DC, Walters CA, Soto-Albors CE, Tohan N, Riddick DH. A randomized study of dexamethasone in ovulation induction with clomiphene citrate. Fertil Steril. 1984;41(6):844-848.
6. Brown J, Farquhar C, Beck J, Boothroyd C, Hughes E. Clomiphene and anti-estrogens for ovulation induction in PCOS. Cochrane Database Syst Rev. 2009;(4):CD002249.-
7. Imani B, Eijkemans MJ, te Velde ER, Habbema JDF, Fauser BC. A nomogram to predict the probability of live birth after clomiphene citrate induction of ovulation in normogonadotropic oligoamenorrheic infertility. Fertil Steril. 2002;77(1):91-97.
8. Rossing MA, Daling JR, Weiss NS, Moore DE, Self SG. Ovarian tumors in a cohort of infertile women. N Engl J Med. 1994;331(12):771-776.
1. The Thessalonkiki ESHRE/ASRM-sponsored PCOS Consensus Workshop Group. Consensus on infertility treatment related to polycystic ovary syndrome. Fertil Steril. 2008;89(3):505-522.
2. Elnashar A, Abdelmageed E, Fayed M, Sharaf M. Clomiphene citrate and dexamethasone in treatment of clomiphene citrate-resistant polycystic ovary syndrome: a prospective placebo-controlled study. Hum Reprod. 2006;21(7):1805-1808.
3. Parsanezhad ME, Alborzi S, Motazedian S, Omrani G. Use of dexamethasone and clomiphene citrate in the treatment of clomiphene citrate-resistant patients with polycystic ovary syndrome and normal dehydroepiandrosterone sulfate levels: placebo-controlled trial. Fertil Steril. 2002;78(5):1001-1004.
4. Isaacs JD, Lincoln SR, Cowan BD. Extended clomiphene citrate (CC) and prednisone for the treatment of chronic anovulation resistant to CC alone. Fertil Steril. 1997;67(4):641-643.
5. Daly DC, Walters CA, Soto-Albors CE, Tohan N, Riddick DH. A randomized study of dexamethasone in ovulation induction with clomiphene citrate. Fertil Steril. 1984;41(6):844-848.
6. Brown J, Farquhar C, Beck J, Boothroyd C, Hughes E. Clomiphene and anti-estrogens for ovulation induction in PCOS. Cochrane Database Syst Rev. 2009;(4):CD002249.-
7. Imani B, Eijkemans MJ, te Velde ER, Habbema JDF, Fauser BC. A nomogram to predict the probability of live birth after clomiphene citrate induction of ovulation in normogonadotropic oligoamenorrheic infertility. Fertil Steril. 2002;77(1):91-97.
8. Rossing MA, Daling JR, Weiss NS, Moore DE, Self SG. Ovarian tumors in a cohort of infertile women. N Engl J Med. 1994;331(12):771-776.
