User login
- Advise patients to monitor blood glucose levels frequently and learn to correlate drops in glucose to symptoms, which vary among patients.
- Ask patients at each visit about awareness of hypoglycemic episodes, their severity and timing, and how events relate to dosing, meals, and activities.
- When using oral agents, consider insulin sensitizers or newer sulfonylureas or meglitinides to reduce risk of hypoglycemia.
- If adding basal insulin to an oral regimen, the analog glargine has proven superior to NPH insulin in avoiding hypoglycemia.
Signs and symptoms of hypoglycemia vary considerably among patients with type 2 diabetes, making the condition easy to miss. Moreover, the most common symptoms are not necessarily the first symptoms.
If hypoglycemia occurs repeatedly, it can start a vicious cycle of physiologic reactions that mask or diminish the symptoms that warn patients of an impending episode. This may lead to hypoglycemia unawareness and hypoglycemic episodes of increasing severity. Fear of hypoglycemia, particularly of nocturnal events, may discourage patients from more intensive glycemic control, particularly using insulin. Such fear may even lead them to reduce their antidiabetic medication dosage, resulting in poor glycemic control.1
Breaking this cycle and restoring normal physiologic responses is one focus of this article, as is teaching patients how to monitor their blood glucose levels and how to correlate low blood glucose with the signs and symptoms of hypoglycemia.
Other therapies and strategies that we discuss in this article:
- Newer insulin analogs and the associated risk of hypoglycemia with each
- Appropriate combination of insulin with oral antidiabetic medications
- The long-acting analog insulin glargine used as basal insulin to lower the incidence of hypoglycemia, including nocturnal and severe hypoglycemia
- Rapid-acting insulin analogs (aspart, glulisine, and lispro) used in basalprandial insulin regimens.
First symptoms vary among individuals
Symptoms of hypoglycemia result primarily from a lowered glucose level in the brain and its effects on the central and autonomic nervous systems (FIGURE 1). A decrease in glucose below physiologic levels has acute consequences for brain function because the brain has an immediate requirement for glucose and little capacity for storage.
Two types of symptoms
Neuroglycopenia and the inhibition of neuronal metabolism causes sensations of warmth, weakness, fatigue, difficulty concentrating, confusion, behavioral changes, and in the most severe cases, a loss of consciousness, seizures, brain damage, and even death.2-4
Neurogenic symptoms are mediated by the hormones and neurotransmitters secreted in response to low brain glucose levels (FIGURE 1). The gluconeogenic actions of the autonomic nervous system produce the classic warning symptoms—tremulousness, pounding heart, anxiety, sweating, hunger, and tingling sensations—that usually precede the symptoms of hypoglycemia.2-4 This is particularly so in iatrogenic hypoglycemia.
These direct symptoms of neuroglycopenia are the ones patients typically identify with hypoglycemia. The most common symptoms of hypoglycemia are therefore not necessarily the first symptoms of hypoglycemia (TABLE 1).5 For example, though most patients experience sweating as a symptom of hypoglycemia, the first symptom might be trembling or anxiety, depending on the individual.5
TABLE 1
Signs and symptoms most commonly associated with hypoglycemia are not always the first to appear
| SYMPTOM | FREQUENCY (%) | FIRST SYMPTOM (%) |
|---|---|---|
| Sweating | 78 | 25 |
| Trembling | 62 | 6 |
| Inability to concentrate | 49 | 6 |
| Confusion | 40 | 3 |
| Weakness | 36 | 4 |
| Dry mouth | 35 | 0 |
| Blurred vision | 34 | 3 |
| Hunger | 33 | 3 |
| Anxiety | 26 | 1 |
| Headache | 21 | 0 |
| Difficulty walking | 21 | 3 |
| Pounding heart | 20 | 0 |
| Tingling around mouth | 20 | 6 |
| Difficulty speaking | 17 | 0 |
| Drowsiness | 15 | 0 |
| Odd behavior | 13 | 1 |
| Nausea | 13 | 0 |
| Adapted from Hepburn DA, MacLeod KM, Pell AC, et al. Diabet Med 1993;10:231–237.5 | ||
Factors influencing frequency and severity of hypoglycemia
Aggressive diabetes management commonly causes mild-to-moderate hypoglycemia, defined as a blood glucose value <60 mg/dL, that can be managed by the patient without assistance.
Severe hypoglycemia—a blood glucose value <50 mg/dL—is relatively uncommon in type 2 diabetes and requires the assistance of another person to manage, since neurological impairment may render patients unable to treat themselves.2,6 Severe hypoglycemia, whether in patients with type 1 or type 2 diabetes, can have debilitating consequences, including seizures or coma or even death.7
Long-standing type 2 disease. Hypoglycemia is more common in patients with type 1 diabetes than in those with type 2, but it can occur in type 2 diabetes patients who require insulin or are treated intensively with combinations of oral agents.6 As type 2 diabetes progresses,8 the incidence of hypoglycemic events increases, as endogenously produced insulin declines and is replaced by exogenous insulin.5,9 In fact, the prevalence of severe episodes (eg, requiring assistance of another person to administer glucose or glucagon) in patients with type 2 diabetes was comparable to that exhibited among patients with type 1 diabetes if they had been on insulin therapy for the same length of time.5,10
Nocturnal hypoglycemia. This event poses a special concern because the warning signs of hypoglycemia may be blunted during sleep. It has been reported that as many as 29% to 56% of all adult patients treated with insulin have an overnight glucose profile that indicates hypoglycemia occurs at night.11-13 However, it is important to note that the extent of the problem of nocturnal hypoglycemia is difficult to assess since overnight monitoring of glucose levels is required.
Additional insights from the UKPDS
Hypoglycemia in type 2 diabetes has not received rigorous attention in clinical trials. However, the United Kingdom Prospective Diabetes Study (UKPDS) was a large longitudinal trial in type 2 diabetes that included hypoglycemia as an outcome measure and thus provides some helpful information.
Events with insulin>sulfonylureas>diet. The 6-year follow-up revealed that the cumulative proportion of patients reporting 1 or more hypoglycemic events (of any type) was 76% for those using insulin, 45% among those taking sulfonylureas, and 3% for those on diet alone. Expressed as events per patient year, this was 37%, 17%, and 0.9%, respectively. When only “major” events (those requiring third-party assistance or hospital admission) were considered, the proportion of patients per year reporting 1 or more such events, was 2.3% for insulin, 0.7% for sulfonylureas, and 0.03% for diet alone. The cumulative proportion over 6 years was 3.3% of participants using sulfonylureas, 11.2% of those using insulin, and 0.15% of those on diet therapy.8
Metformin increases risk. The cumulative proportion of obese patients reporting any hypoglycemic event was 17.6% for those taking metformin vs 2.8% for those on diet. Severe hypoglycemia (as defined earlier) occurred in 2.4% of participants using metformin compared with 0.4% of those on diet therapy.8
Findings from other studies
Interestingly, in a recent systematic review of randomized controlled trials comparing insulin monotherapy with insulin plus oral antidiabetic agents, 13 of 14 studies reporting hypoglycemia demonstrated no difference in events.14
The occurrence of hypoglycemia among patients on metformin monotherapy in the UKPDS study is notable since, theoretically, hypoglycemia should not occur with agents whose mechanisms of action do not increase insulin secretion (biguanides, thiazolidinediones [TZDs], or α-glucosidase inhibitors),1 Since newer classes such as TZDs, α-glucosidase inhibitors, and meglitinides were not available when UKPDS was initiated, the trial does not provide data on these classes.
In a small comparative study of insulin combined with either metformin or a TZD, it appeared that metformin combination was associated with fewer occurrences of hypoglycemia; however, the small patient sample limits generalizability of the finding.15
How hypoglycemia occurs
Normally, as blood glucose levels (red) rise, insulin secretion increases, circulating insulin levels (blue) rise, and hepatic glucose production is inhibited. As glucose is disposed and circulating levels decrease due to insulin action, insulin levels then drop and hepatic glucose production begins again.
Hypoglycemia in diabetes can result from an excess of endogenous or exogenous insulin (iatrogenic hypoglycemia). In healthy patients, high insulin and falling glucose levels suppress insulin production and stimulate a hormone-mediated burst of glucose production. In patients with diabetes, the loss of physiologic control of insulin secretion coupled with exogenous administration of insulin or insulin secretagogues can interfere with the normal physiologic response to low blood glucose levels, resulting in hypoglycemia.1
With intense insulin regimens, the incidence of hypoglycemia can be as high as 30%, in contrast to 12% for patients treated with diet alone and 16% for those taking oral agents.6
With secretagogues, it has been suggested that the incidence of hypoglycemia is higher with the older, longer acting sulfonylurea agents.1,6,16-19 Although populationbased data on hypoglycemic rates associated with combination therapy with oral antidiabetic agents are not available, numerous clinical studies have reported rates of 10% to 20% for any hypoglycemic event.20-23
Heightening patient awareness, and yours
Because the signs of hypoglycemia vary considerably among individuals, they can easily be missed.3 In addition, repeated episodes of hypoglycemia can alter the normal regulatory responses and diminish the most important signs of a drop in glucose levels.1,2,24,25 The loss of the physiologic warning signs is thought to stem from dampening and eventual loss of the neuroadrenal response to low glucose levels in the brain (FIGURE 1). A vicious cycle is set up, whereby reduction in the neurogenic response attenuates hypoglycemic symptoms, causing more episodes to occur and become more severe as they are repeated. This cycle can be broken, and the normal physiologic response restored, if hypoglycemic events can be avoided for just a few weeks.2,26
Key points for patients. The main strategy for managing hypoglycemia is educating patients about the early symptoms of hypoglycemia and how to self-treat when they occur. Reinforce the need to time meals consistently and to limit the amount of carbohydrate ingested.
Advise patients to monitor blood glucose levels frequently, and to learn to relate a drop in glucose levels to hypoglycemic symptoms.2
Counsel patients to eat a snack or, preferably, drink fruit juice to counteract hypoglycemia. Patients may also carry glucose tablets, which are convenient and less tempting than candy.27
Glucagon is indicated for severe cases.
Whenever possible, a patient’s family members (particularly in the case of children) should be educated too.
Ask regularly about episodes. Finally, act to identify problems by querying patients and family members at every visit about hypoglycemia episodes, probing for information about awareness, severity and timing of the episodes, and how these events relate to dosing, meals, and activities.27 If hypoglycemia recurs, analyze the dosing regimen and consider flexible insulin dosing.1
Anti-hypoglycemia strategies for each new phase of therapy
As the course of diabetes therapy moves, typically, from oral medications to insulin to combination regimens, drug selections can be made in part to reduce the risk of hypoglycemia.
Oral agents: Insulin sensitizers, newer agents generally better
As noted earlier, among oral agents, insulin sensitizers are generally thought to have lower rates of hypoglycemia.
Newer sulfonylureas such as glimepiride and the rapid-acting meglitinides may also cause fewer hypoglycemic events.
Given the progressive decline of endogenous insulin secretion, combination therapy with secretagogues or insulin is eventually required for most patients.
Insulin analogs
A number of rapid-, short-, intermediateand long-acting insulin analogs have been introduced, and many of them make it possible to mimic different phases of physiologic insulin secretion (FIGURE 2). One of the newer analogs less likely to cause hypoglycemia is glargine, a long-acting insulin with a steady, relatively consistent action profile over a 24-hour period, closely mimicking normal basal pancreatic insulin secretion.28
FIGURE 2
Plasma insulin levels with newer analogs
N=20 type 1 diabetic patients; mean±SE
CSII, continuous subcutaneous insulin infusion; NPH, neutral protamine Hagedorn.
Copyright © 2000 American Diabetes Association. From Diabetes 2000; 49:2142–2148.28 Reprinted with permission from American Diabetes Association.
TABLE 2
Strategies for avoiding and addressing hypoglycemia
|
Insulin mixtures helpful when meal times guaranteed
Other insulins include mixtures of regular insulin and long-acting insulin available in split mixed or premixed formulations. These mixtures are intended to cover insulin peaks at mealtimes with twice-daily administration.
Mixed insulin formulations are often perceived as relatively convenient and simple to use, but they require meals to be taken within set time frames, without a great deal of flexibility. Since the ratios of the insulin components are fixed, and designed to work with meals consumed on a fixed schedule, hypoglycemia can occur if patients miss a meal. In addition, the time-activity profile of the insulin may not match the postprandial glucose peak even if the meal is consumed, and will increase the chance of postprandial hypoglycemia.
Basal insulin plus oral regimens
For patients with type 2 diabetes, adding basal insulin to oral regimens can significantly improve glycemic control. Ideally, basal insulin therapy provides a sustained and relatively constant concentration of insulin throughout the day. In the past, neutral protamine Hagedorn (NPH) insulin, a longer-acting insulin, was used for basal insulin therapy, and regular insulin was used to cover prandial insulin needs. Ultralente, also used as a basal insulin, has a relatively unpredictable timeactivity profile.28
Insulin glargine superior to NPH. In a recent clinical trial, patients with type 2 diabetes whose glucose levels were inadequately controlled on oral antidiabetic medications were given bedtime insulin glargine or NPH insulin.29 The insulin doses were titrated using a simple algorithm targeting a fasting plasma glucose (FPG) of ≤100 mg/dL to reach recommended glycosylated hemoglobin (Hb A1c) levels. Though no significant difference in glycemic control was found between insulin glargine and NPH insulin, significantly fewer hypoglycemic episodes occurred with insulin glargine therapy. The 24-hour distribution per patient-year of hypoglycemia for glargine vs NPH is shown in FIGURE 3.
Specifically, nearly 25% more patients treated with insulin glargine than with NPH insulin reached target Hb A1c levels of ≤7.0% without nocturnal hypoglycemia. Moreover, the overall incidence of any hypoglycemic event (eg, plasma-referenced glucose ≤72 mg/dL) and severe hypoglycemia (eg, patient required assistance of another person, and had a glucose level <56 mg/dL or prompt recovery after glucose or glucagons) was lower with insulin glargine than with NPH insulin. Results from other studies and a recent metaanalysis have been similar.30-32
Thus, using insulin glargine as basal insulin allows patients to reach recommended targets with fewer episodes of hypoglycemia, and can help address patients’ fear that can be a barrier to initiating insulin therapy in type 2 diabetes. Two recent studies have reported that dosing of insulin glargine can be flexible—morning or bedtime administration yields comparable low rates of hypoglycemia.30,31
FIGURE 3
Hourly hypoglycemia rate with glargine much less than with NPH
*P<.05 (between treatment).
Copyright © 2003 American Diabetes Association. From Diabetes Care 2003; 26:3080–3086.29 Reprinted with permission from American Diabetes Association.
Basal insulin plus prandial insulin
For patients who cannot otherwise reach Hb A1c goals, basal insulin therapy may be supplemented with prandial insulin. Newer, rapid-acting analogs used for the prandial component are insulin lispro, insulin glulisine, or insulin aspart. Although this approach is physiologically more rational than regimens using conventional insulins, data are limited for use in type 2 diabetes.
The incidence of nocturnal hypoglycemia was evaluated in a study of patients with type 1 diabetes and impaired hypoglycemic awareness who were treated with 1 of 2 regimens: insulin lispro in a basal-prandial combination with NPH insulin, or twice-daily, premixed NPH/regular insulin.33 Results showed that the incidence of nocturnal hypoglycemia was lower in patients receiving the insulin lispro regimen.
Another study, comparing insulin aspart and regular insulin as the prandial component in a basal-prandial regimen with NPH, showed that postprandial glucose control and Hb A1c levels were significantly better after 1 year of treatment in the insulin aspart group than in the group receiving regular insulin, without an increased risk for hypoglycemia.34 These results suggest that treatment with rapidacting insulin analogs could be helpful in avoiding hypoglycemia in patients with type 2 diabetes when a basal-prandial insulin regimen is indicated.
Avoiding hypoglycemia in the elderly
Elderly patients may be at increased risk for iatrogenic hypoglycemia. A populationbased study of patients presenting to an emergency room with severe hypoglycemic symptoms reported that rates of such events among elderly patients with type 2 diabetes and multiple comorbidities approached the rates among patients with type 1 diabetes.35
Creatinine clearance is often decreased in elderly patients, slowing elimination of oral agents and insulin and potentially resulting in sustained pharmacological action and creating a greater risk for hypoglycemia.
Furthermore, there is evidence that the neurogenic symptoms of hypoglycemia are reduced in elderly patients, diminishing awareness of hypoglycemia.36
In the demented elderly, malnutrition, weight loss, and anorexia may exacerbate the risk for hypoglycemia. For elderly patients with tertiary disease (eg, cerebrovascular accident, myocardial infarction, congestive heart failure, blindness, chronic renal failure), the risk for hypoglycemia and subsequent comorbidity may outweigh the benefits of strict glycemic control.3,37 Elderly patients may have comorbid conditions that increase risk of falls (eg, poor vision, neurologic conditions), and hypoglycemic episodes may further increase the risk of falls and lead to morbidity (eg, fragility fracture in patients with osteopenia or osteoporosis).
Because the elderly are at a greater risk for hypoglycemia, a switch to a less restrictive diet, such as a “no concentrated sweets” diet, is an option, with control of glucose levels through the administration of oral agents or insulin.36 This may also promote a better quality of life, considering that many of these patients already have secondary and tertiary complications of diabetes, prevention of which is not a realistic goal.
CORRESPONDING AUTHOR
William Cefalu, MD, Professor and Chief, Division of Nutrition and Chronic Diseases, Pennington Biomedical Research Center, Louisiana State University, 6400 Perkins Road, Baton Rouge, LA 70808. Email: cefaluwt@pbrc.edu
1. Cryer PE, Childs BP. Negotiating the barrier of hypoglycemia in diabetes. Diabetes Spectrum 2002;15:20-27.
2. Cryer PE, ed. Hypoglycemia: Pathophysiology, Diagnosis, and Treatment. New York, NY: Oxford University Press; 1997.
3. McAulay V, Deary IJ, Frier BM. Symptoms of hypoglycaemia in people with diabetes. Diabet Med 2001;18:690-705.
4. Towler DA, Havlin CE, Craft S, Cryer P. Mechanism of awareness of hypoglycemia. Perception of neurogenic (predominantly cholinergic) rather than neuroglycopenic symptoms. Diabetes 1993;42:1791-1798.
5. Hepburn DA, MacLeod KM, Pell AC, Scougal IJ, Frier BM. Frequency and symptoms of hypoglycaemia experienced by patients with type 2 diabetes treated with insulin. Diabet Med 1993;10:231-237.
6. Miller CD, Phillips LS, Ziemer DC, Gallina DL, Cook CB, El-Kebbi IM. Hypoglycemia in patients with type 2 diabetes mellitus. Arch Intern Med 2001;161:1653-1659.
7. The DCCT Research Group. Epidemiology of severe hypoglycemia in the diabetes control and complications trial. Am J Med 1991;90:450-459.
8. UK Prospective Diabetes Study Group. Perspectives in diabetes. UK Prospective Diabetes Study 16: overview of 6 years’ therapy of type II diabetes: a progressive disease. Diabetes 1995;44:1249-1258.
9. United Kingdom Prospective Diabetes Study Group. UKPDS 24: a 6-year, randomized, controlled trial comparing sulfonylurea, insulin, and metformin therapy in patients with newly diagnosed type 2 diabetes that could not be controlled with diet therapy. Ann Intern Med 1998;128:165-175.
10. MacLeod KM, Hepburn DA, Frier BM. Frequency and morbidity of severe hypoglycaemia in insulin-treated diabetic patients. Diabet Med 1993;10:238-245.
11. Vervoort G, Goldschmidt HM, van Doorn LG. Nocturnal blood glucose profiles in patients with type 1 diabetes mellitus on multiple (>or=4) daily insulin injection regimens. Diabet Med 1996;13:794-799.
12. Gale EA, Tattersall RB. Unrecognised nocturnal hypoglycaemia in insulin-treated diabetics. Lancet 1979;1:1049-1052.
13. Pramming S, Thorsteinsson B, Bendtson I, Ronn B, Binder C. Nocturnal hypoglycaemia in patients receiving conventional treatment with insulin. Br Med J (Clin Res Ed) 1985;291:376-379.
14. Goudswaard AN, Furlong NJ, Valk GD, Stolk RP, Rutten GEHM. Insulin monotherapy versus combinations of insulin with oral hypoglycaemic agents in patients with type 2 diabetes mellitus. Cochrane Database Syst Rev 2004;4:1-51.
15. Strowig SM, Avilés-Santa ML, Raskin P. Comparison of insulin monotherapy and combination therapy with insulin and metformin or insulin and troglitazone in type 2 diabetes. Diabetes Care 2002;25:1691-1698.
16. Campbell IW. Hypoglycaemia and type 2 diabetes: sulphonylureas. In: Frier BM, Fisher BM, eds. Hypoglycaemia and Diabetes: Clinical and Physiological Aspects. London, UK: Edward Arnold, 1993;387-392.
17. Shorr RI, Ray WA, Daugherty JR, Griffin MR. Individual sulfonylureas and serious hypoglycemia in older people. J Am Geriatr Soc 1996;44:751-755.
18. Holstein A, Egberts EH. Risk of hypoglycaemia with oral antidiabetic agents in patients with Type 2 diabetes. Exp Clin Endocrinol Diabetes 2003;111:405-414.
19. Holstein A, Plaschke A, Egberts E-H. Lower incidence of severe hypoglycaemia in patients with type 2 diabetes treated with glimepiride versus glibenclamide. Diabetes Metab Res Rev 2001;17:467-473.
20. Aljabri K, Kozak SE, Thompson DM. Addition of pioglitazone or bedtime insulin to maximal doses of sulfonylurea and metformin in type 2 diabetes patients with poor glucose control: a prospective, randomized trial. Am J Med 2004;116:230-235.
21. Wolffenbuttel BHR, Landgraf R. A 1-year multicenter randomized double-blind comparison of repaglinide and glyburide for the treatment of type 2 diabetes. Dutch and German Repaglinide Study Group. Diabetes Care 1999;22:463-467.
22. Raskin P, McGill J, Saad MF, et al. Combination therapy for type 2 diabetes: repaglinide plus rosiglitazone. Diabetes Med 2004;21:329-335.
23. Dailey GE, III, Noor MA, Park JS, Bruce S, Fiedorek FT. Glycemic control with glyburide/metformin tablets in combination with rosiglitazone in patients with type 2 diabetes: a randomized, double-blind trial. Am J Med 2004;116:223-229.
24. Korytkowski MT, Mokan M, Veneman TF, Mitrakou A, Cryer PE, Gerich JE. Reduced beta-adrenergic sensitivity in patients with type 1 diabetes and hypoglycemia unawareness. Diabetes Care 1998;21:1939-1943.
25. Fritsche A, Stumvoll M, Grub M, et al. Effect of hypoglycemia on beta-adrenergic sensitivity in normal and type 1 diabetic subjects. Diabetes Care 1998;21:1505-1510.
26. Fritsche A, Stefan N, Haring H, Gerich J, Stumvoll M. Avoidance of hypoglycemia restores hypoglycemia awareness by increasing beta-adrenergic sensitivity in type 1 diabetes. Ann Intern Med 2001;134:729-736.
27. Cryer PE, Davis SN, Shamoon H. Hypoglycemia in diabetes. Diabetes Care 2003;26:1902-1912.
28. Lepore M, Pampanelli S, Fanelli C, et al. Pharmacokinetics and pharmacodynamics of subcutaneous injection of long-acting human insulin analog glargine, NPH insulin, and ultralente human insulin and continuous subcutaneous infusion of insulin lispro. Diabetes 2000;49:2142-2148.
29. Riddle MC, Rosenstock J, Gerich J. The Treat-to-Target Trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care 2003;26:3080-3086.
30. Fritsche A, Schweitzer MA, Häring HU. Glimepiride combined with morning insulin glargine, bedtime neutral protamine Hagedorn insulin, or bedtime insulin glargine in patients with type 2 diabetes: a randomized, controlled trial. Ann Intern Med 2003;138:952-959.
31. Standl E, Maxeiner S, Raptis S, Karimi-Anderesi Z, Schweitzer MA. Good glycemic control with flexibility in timing of basal insulin supply: a 24-week comparison of insulin glargine given once daily in the morning or at bedtime in combination with morning glimepiride. Diabetes Care 2005;28:419-420.
32. Rosenstock J, Dailey G, Massi Benedetti M, Fritsche A, Lin Z, Salzman A. Reduced hypoglycemia risk with insulin glargine. A meta-analysis comparing insulin glargine with human NPH insulin in type 2 diabetes. Diabetes Care 2005;28:950-955.
33. Ferguson SC, Strachan MW, Janes JM, Frier BM. Severe hypoglycaemia in patients with type 1 diabetes and impaired awareness of hypoglycaemia: a comparative study of insulin lispro and regular human insulin. Diabetes Metab Res Rev 2001;17:285-291.
34. Raskin P, Guthrie RA, Leiter L, Riis A, Jovanovic L. Use of insulin aspart, a fast-acting insulin analog, as the mealtime insulin in the management of patients with type 1 diabetes. Diabetes Care 2000;23:583-558.
35. Holstein A, Plaschke A, Egberts EH. Clinical characterisation of severe hypoglycaemia-a prospective population-based study. Exp Clin Endocrinol Diabetes 2003;111:364-369.
36. Meneilly GS, Cheung E, Tuokko H. Counterregulatory hormone responses to hypoglycemia in the elderly patient with diabetes. Diabetes 1994;43:403-410.
37. American Diabetes Association. Standards of medical care for patients with diabetes mellitus. Diabetes Care 2003;26(suppl 1):S33-S50.
- Advise patients to monitor blood glucose levels frequently and learn to correlate drops in glucose to symptoms, which vary among patients.
- Ask patients at each visit about awareness of hypoglycemic episodes, their severity and timing, and how events relate to dosing, meals, and activities.
- When using oral agents, consider insulin sensitizers or newer sulfonylureas or meglitinides to reduce risk of hypoglycemia.
- If adding basal insulin to an oral regimen, the analog glargine has proven superior to NPH insulin in avoiding hypoglycemia.
Signs and symptoms of hypoglycemia vary considerably among patients with type 2 diabetes, making the condition easy to miss. Moreover, the most common symptoms are not necessarily the first symptoms.
If hypoglycemia occurs repeatedly, it can start a vicious cycle of physiologic reactions that mask or diminish the symptoms that warn patients of an impending episode. This may lead to hypoglycemia unawareness and hypoglycemic episodes of increasing severity. Fear of hypoglycemia, particularly of nocturnal events, may discourage patients from more intensive glycemic control, particularly using insulin. Such fear may even lead them to reduce their antidiabetic medication dosage, resulting in poor glycemic control.1
Breaking this cycle and restoring normal physiologic responses is one focus of this article, as is teaching patients how to monitor their blood glucose levels and how to correlate low blood glucose with the signs and symptoms of hypoglycemia.
Other therapies and strategies that we discuss in this article:
- Newer insulin analogs and the associated risk of hypoglycemia with each
- Appropriate combination of insulin with oral antidiabetic medications
- The long-acting analog insulin glargine used as basal insulin to lower the incidence of hypoglycemia, including nocturnal and severe hypoglycemia
- Rapid-acting insulin analogs (aspart, glulisine, and lispro) used in basalprandial insulin regimens.
First symptoms vary among individuals
Symptoms of hypoglycemia result primarily from a lowered glucose level in the brain and its effects on the central and autonomic nervous systems (FIGURE 1). A decrease in glucose below physiologic levels has acute consequences for brain function because the brain has an immediate requirement for glucose and little capacity for storage.
Two types of symptoms
Neuroglycopenia and the inhibition of neuronal metabolism causes sensations of warmth, weakness, fatigue, difficulty concentrating, confusion, behavioral changes, and in the most severe cases, a loss of consciousness, seizures, brain damage, and even death.2-4
Neurogenic symptoms are mediated by the hormones and neurotransmitters secreted in response to low brain glucose levels (FIGURE 1). The gluconeogenic actions of the autonomic nervous system produce the classic warning symptoms—tremulousness, pounding heart, anxiety, sweating, hunger, and tingling sensations—that usually precede the symptoms of hypoglycemia.2-4 This is particularly so in iatrogenic hypoglycemia.
These direct symptoms of neuroglycopenia are the ones patients typically identify with hypoglycemia. The most common symptoms of hypoglycemia are therefore not necessarily the first symptoms of hypoglycemia (TABLE 1).5 For example, though most patients experience sweating as a symptom of hypoglycemia, the first symptom might be trembling or anxiety, depending on the individual.5
TABLE 1
Signs and symptoms most commonly associated with hypoglycemia are not always the first to appear
| SYMPTOM | FREQUENCY (%) | FIRST SYMPTOM (%) |
|---|---|---|
| Sweating | 78 | 25 |
| Trembling | 62 | 6 |
| Inability to concentrate | 49 | 6 |
| Confusion | 40 | 3 |
| Weakness | 36 | 4 |
| Dry mouth | 35 | 0 |
| Blurred vision | 34 | 3 |
| Hunger | 33 | 3 |
| Anxiety | 26 | 1 |
| Headache | 21 | 0 |
| Difficulty walking | 21 | 3 |
| Pounding heart | 20 | 0 |
| Tingling around mouth | 20 | 6 |
| Difficulty speaking | 17 | 0 |
| Drowsiness | 15 | 0 |
| Odd behavior | 13 | 1 |
| Nausea | 13 | 0 |
| Adapted from Hepburn DA, MacLeod KM, Pell AC, et al. Diabet Med 1993;10:231–237.5 | ||
Factors influencing frequency and severity of hypoglycemia
Aggressive diabetes management commonly causes mild-to-moderate hypoglycemia, defined as a blood glucose value <60 mg/dL, that can be managed by the patient without assistance.
Severe hypoglycemia—a blood glucose value <50 mg/dL—is relatively uncommon in type 2 diabetes and requires the assistance of another person to manage, since neurological impairment may render patients unable to treat themselves.2,6 Severe hypoglycemia, whether in patients with type 1 or type 2 diabetes, can have debilitating consequences, including seizures or coma or even death.7
Long-standing type 2 disease. Hypoglycemia is more common in patients with type 1 diabetes than in those with type 2, but it can occur in type 2 diabetes patients who require insulin or are treated intensively with combinations of oral agents.6 As type 2 diabetes progresses,8 the incidence of hypoglycemic events increases, as endogenously produced insulin declines and is replaced by exogenous insulin.5,9 In fact, the prevalence of severe episodes (eg, requiring assistance of another person to administer glucose or glucagon) in patients with type 2 diabetes was comparable to that exhibited among patients with type 1 diabetes if they had been on insulin therapy for the same length of time.5,10
Nocturnal hypoglycemia. This event poses a special concern because the warning signs of hypoglycemia may be blunted during sleep. It has been reported that as many as 29% to 56% of all adult patients treated with insulin have an overnight glucose profile that indicates hypoglycemia occurs at night.11-13 However, it is important to note that the extent of the problem of nocturnal hypoglycemia is difficult to assess since overnight monitoring of glucose levels is required.
Additional insights from the UKPDS
Hypoglycemia in type 2 diabetes has not received rigorous attention in clinical trials. However, the United Kingdom Prospective Diabetes Study (UKPDS) was a large longitudinal trial in type 2 diabetes that included hypoglycemia as an outcome measure and thus provides some helpful information.
Events with insulin>sulfonylureas>diet. The 6-year follow-up revealed that the cumulative proportion of patients reporting 1 or more hypoglycemic events (of any type) was 76% for those using insulin, 45% among those taking sulfonylureas, and 3% for those on diet alone. Expressed as events per patient year, this was 37%, 17%, and 0.9%, respectively. When only “major” events (those requiring third-party assistance or hospital admission) were considered, the proportion of patients per year reporting 1 or more such events, was 2.3% for insulin, 0.7% for sulfonylureas, and 0.03% for diet alone. The cumulative proportion over 6 years was 3.3% of participants using sulfonylureas, 11.2% of those using insulin, and 0.15% of those on diet therapy.8
Metformin increases risk. The cumulative proportion of obese patients reporting any hypoglycemic event was 17.6% for those taking metformin vs 2.8% for those on diet. Severe hypoglycemia (as defined earlier) occurred in 2.4% of participants using metformin compared with 0.4% of those on diet therapy.8
Findings from other studies
Interestingly, in a recent systematic review of randomized controlled trials comparing insulin monotherapy with insulin plus oral antidiabetic agents, 13 of 14 studies reporting hypoglycemia demonstrated no difference in events.14
The occurrence of hypoglycemia among patients on metformin monotherapy in the UKPDS study is notable since, theoretically, hypoglycemia should not occur with agents whose mechanisms of action do not increase insulin secretion (biguanides, thiazolidinediones [TZDs], or α-glucosidase inhibitors),1 Since newer classes such as TZDs, α-glucosidase inhibitors, and meglitinides were not available when UKPDS was initiated, the trial does not provide data on these classes.
In a small comparative study of insulin combined with either metformin or a TZD, it appeared that metformin combination was associated with fewer occurrences of hypoglycemia; however, the small patient sample limits generalizability of the finding.15
How hypoglycemia occurs
Normally, as blood glucose levels (red) rise, insulin secretion increases, circulating insulin levels (blue) rise, and hepatic glucose production is inhibited. As glucose is disposed and circulating levels decrease due to insulin action, insulin levels then drop and hepatic glucose production begins again.
Hypoglycemia in diabetes can result from an excess of endogenous or exogenous insulin (iatrogenic hypoglycemia). In healthy patients, high insulin and falling glucose levels suppress insulin production and stimulate a hormone-mediated burst of glucose production. In patients with diabetes, the loss of physiologic control of insulin secretion coupled with exogenous administration of insulin or insulin secretagogues can interfere with the normal physiologic response to low blood glucose levels, resulting in hypoglycemia.1
With intense insulin regimens, the incidence of hypoglycemia can be as high as 30%, in contrast to 12% for patients treated with diet alone and 16% for those taking oral agents.6
With secretagogues, it has been suggested that the incidence of hypoglycemia is higher with the older, longer acting sulfonylurea agents.1,6,16-19 Although populationbased data on hypoglycemic rates associated with combination therapy with oral antidiabetic agents are not available, numerous clinical studies have reported rates of 10% to 20% for any hypoglycemic event.20-23
Heightening patient awareness, and yours
Because the signs of hypoglycemia vary considerably among individuals, they can easily be missed.3 In addition, repeated episodes of hypoglycemia can alter the normal regulatory responses and diminish the most important signs of a drop in glucose levels.1,2,24,25 The loss of the physiologic warning signs is thought to stem from dampening and eventual loss of the neuroadrenal response to low glucose levels in the brain (FIGURE 1). A vicious cycle is set up, whereby reduction in the neurogenic response attenuates hypoglycemic symptoms, causing more episodes to occur and become more severe as they are repeated. This cycle can be broken, and the normal physiologic response restored, if hypoglycemic events can be avoided for just a few weeks.2,26
Key points for patients. The main strategy for managing hypoglycemia is educating patients about the early symptoms of hypoglycemia and how to self-treat when they occur. Reinforce the need to time meals consistently and to limit the amount of carbohydrate ingested.
Advise patients to monitor blood glucose levels frequently, and to learn to relate a drop in glucose levels to hypoglycemic symptoms.2
Counsel patients to eat a snack or, preferably, drink fruit juice to counteract hypoglycemia. Patients may also carry glucose tablets, which are convenient and less tempting than candy.27
Glucagon is indicated for severe cases.
Whenever possible, a patient’s family members (particularly in the case of children) should be educated too.
Ask regularly about episodes. Finally, act to identify problems by querying patients and family members at every visit about hypoglycemia episodes, probing for information about awareness, severity and timing of the episodes, and how these events relate to dosing, meals, and activities.27 If hypoglycemia recurs, analyze the dosing regimen and consider flexible insulin dosing.1
Anti-hypoglycemia strategies for each new phase of therapy
As the course of diabetes therapy moves, typically, from oral medications to insulin to combination regimens, drug selections can be made in part to reduce the risk of hypoglycemia.
Oral agents: Insulin sensitizers, newer agents generally better
As noted earlier, among oral agents, insulin sensitizers are generally thought to have lower rates of hypoglycemia.
Newer sulfonylureas such as glimepiride and the rapid-acting meglitinides may also cause fewer hypoglycemic events.
Given the progressive decline of endogenous insulin secretion, combination therapy with secretagogues or insulin is eventually required for most patients.
Insulin analogs
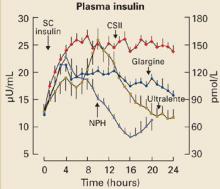
A number of rapid-, short-, intermediateand long-acting insulin analogs have been introduced, and many of them make it possible to mimic different phases of physiologic insulin secretion (FIGURE 2). One of the newer analogs less likely to cause hypoglycemia is glargine, a long-acting insulin with a steady, relatively consistent action profile over a 24-hour period, closely mimicking normal basal pancreatic insulin secretion.28
FIGURE 2
Plasma insulin levels with newer analogs
N=20 type 1 diabetic patients; mean±SE
CSII, continuous subcutaneous insulin infusion; NPH, neutral protamine Hagedorn.
Copyright © 2000 American Diabetes Association. From Diabetes 2000; 49:2142–2148.28 Reprinted with permission from American Diabetes Association.
TABLE 2
Strategies for avoiding and addressing hypoglycemia
|
Insulin mixtures helpful when meal times guaranteed
Other insulins include mixtures of regular insulin and long-acting insulin available in split mixed or premixed formulations. These mixtures are intended to cover insulin peaks at mealtimes with twice-daily administration.
Mixed insulin formulations are often perceived as relatively convenient and simple to use, but they require meals to be taken within set time frames, without a great deal of flexibility. Since the ratios of the insulin components are fixed, and designed to work with meals consumed on a fixed schedule, hypoglycemia can occur if patients miss a meal. In addition, the time-activity profile of the insulin may not match the postprandial glucose peak even if the meal is consumed, and will increase the chance of postprandial hypoglycemia.
Basal insulin plus oral regimens
For patients with type 2 diabetes, adding basal insulin to oral regimens can significantly improve glycemic control. Ideally, basal insulin therapy provides a sustained and relatively constant concentration of insulin throughout the day. In the past, neutral protamine Hagedorn (NPH) insulin, a longer-acting insulin, was used for basal insulin therapy, and regular insulin was used to cover prandial insulin needs. Ultralente, also used as a basal insulin, has a relatively unpredictable timeactivity profile.28
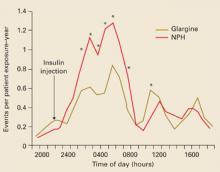
Insulin glargine superior to NPH. In a recent clinical trial, patients with type 2 diabetes whose glucose levels were inadequately controlled on oral antidiabetic medications were given bedtime insulin glargine or NPH insulin.29 The insulin doses were titrated using a simple algorithm targeting a fasting plasma glucose (FPG) of ≤100 mg/dL to reach recommended glycosylated hemoglobin (Hb A1c) levels. Though no significant difference in glycemic control was found between insulin glargine and NPH insulin, significantly fewer hypoglycemic episodes occurred with insulin glargine therapy. The 24-hour distribution per patient-year of hypoglycemia for glargine vs NPH is shown in FIGURE 3.
Specifically, nearly 25% more patients treated with insulin glargine than with NPH insulin reached target Hb A1c levels of ≤7.0% without nocturnal hypoglycemia. Moreover, the overall incidence of any hypoglycemic event (eg, plasma-referenced glucose ≤72 mg/dL) and severe hypoglycemia (eg, patient required assistance of another person, and had a glucose level <56 mg/dL or prompt recovery after glucose or glucagons) was lower with insulin glargine than with NPH insulin. Results from other studies and a recent metaanalysis have been similar.30-32
Thus, using insulin glargine as basal insulin allows patients to reach recommended targets with fewer episodes of hypoglycemia, and can help address patients’ fear that can be a barrier to initiating insulin therapy in type 2 diabetes. Two recent studies have reported that dosing of insulin glargine can be flexible—morning or bedtime administration yields comparable low rates of hypoglycemia.30,31
FIGURE 3
Hourly hypoglycemia rate with glargine much less than with NPH
*P<.05 (between treatment).
Copyright © 2003 American Diabetes Association. From Diabetes Care 2003; 26:3080–3086.29 Reprinted with permission from American Diabetes Association.
Basal insulin plus prandial insulin
For patients who cannot otherwise reach Hb A1c goals, basal insulin therapy may be supplemented with prandial insulin. Newer, rapid-acting analogs used for the prandial component are insulin lispro, insulin glulisine, or insulin aspart. Although this approach is physiologically more rational than regimens using conventional insulins, data are limited for use in type 2 diabetes.
The incidence of nocturnal hypoglycemia was evaluated in a study of patients with type 1 diabetes and impaired hypoglycemic awareness who were treated with 1 of 2 regimens: insulin lispro in a basal-prandial combination with NPH insulin, or twice-daily, premixed NPH/regular insulin.33 Results showed that the incidence of nocturnal hypoglycemia was lower in patients receiving the insulin lispro regimen.
Another study, comparing insulin aspart and regular insulin as the prandial component in a basal-prandial regimen with NPH, showed that postprandial glucose control and Hb A1c levels were significantly better after 1 year of treatment in the insulin aspart group than in the group receiving regular insulin, without an increased risk for hypoglycemia.34 These results suggest that treatment with rapidacting insulin analogs could be helpful in avoiding hypoglycemia in patients with type 2 diabetes when a basal-prandial insulin regimen is indicated.
Avoiding hypoglycemia in the elderly
Elderly patients may be at increased risk for iatrogenic hypoglycemia. A populationbased study of patients presenting to an emergency room with severe hypoglycemic symptoms reported that rates of such events among elderly patients with type 2 diabetes and multiple comorbidities approached the rates among patients with type 1 diabetes.35
Creatinine clearance is often decreased in elderly patients, slowing elimination of oral agents and insulin and potentially resulting in sustained pharmacological action and creating a greater risk for hypoglycemia.
Furthermore, there is evidence that the neurogenic symptoms of hypoglycemia are reduced in elderly patients, diminishing awareness of hypoglycemia.36
In the demented elderly, malnutrition, weight loss, and anorexia may exacerbate the risk for hypoglycemia. For elderly patients with tertiary disease (eg, cerebrovascular accident, myocardial infarction, congestive heart failure, blindness, chronic renal failure), the risk for hypoglycemia and subsequent comorbidity may outweigh the benefits of strict glycemic control.3,37 Elderly patients may have comorbid conditions that increase risk of falls (eg, poor vision, neurologic conditions), and hypoglycemic episodes may further increase the risk of falls and lead to morbidity (eg, fragility fracture in patients with osteopenia or osteoporosis).
Because the elderly are at a greater risk for hypoglycemia, a switch to a less restrictive diet, such as a “no concentrated sweets” diet, is an option, with control of glucose levels through the administration of oral agents or insulin.36 This may also promote a better quality of life, considering that many of these patients already have secondary and tertiary complications of diabetes, prevention of which is not a realistic goal.
CORRESPONDING AUTHOR
William Cefalu, MD, Professor and Chief, Division of Nutrition and Chronic Diseases, Pennington Biomedical Research Center, Louisiana State University, 6400 Perkins Road, Baton Rouge, LA 70808. Email: cefaluwt@pbrc.edu
- Advise patients to monitor blood glucose levels frequently and learn to correlate drops in glucose to symptoms, which vary among patients.
- Ask patients at each visit about awareness of hypoglycemic episodes, their severity and timing, and how events relate to dosing, meals, and activities.
- When using oral agents, consider insulin sensitizers or newer sulfonylureas or meglitinides to reduce risk of hypoglycemia.
- If adding basal insulin to an oral regimen, the analog glargine has proven superior to NPH insulin in avoiding hypoglycemia.
Signs and symptoms of hypoglycemia vary considerably among patients with type 2 diabetes, making the condition easy to miss. Moreover, the most common symptoms are not necessarily the first symptoms.
If hypoglycemia occurs repeatedly, it can start a vicious cycle of physiologic reactions that mask or diminish the symptoms that warn patients of an impending episode. This may lead to hypoglycemia unawareness and hypoglycemic episodes of increasing severity. Fear of hypoglycemia, particularly of nocturnal events, may discourage patients from more intensive glycemic control, particularly using insulin. Such fear may even lead them to reduce their antidiabetic medication dosage, resulting in poor glycemic control.1
Breaking this cycle and restoring normal physiologic responses is one focus of this article, as is teaching patients how to monitor their blood glucose levels and how to correlate low blood glucose with the signs and symptoms of hypoglycemia.
Other therapies and strategies that we discuss in this article:
- Newer insulin analogs and the associated risk of hypoglycemia with each
- Appropriate combination of insulin with oral antidiabetic medications
- The long-acting analog insulin glargine used as basal insulin to lower the incidence of hypoglycemia, including nocturnal and severe hypoglycemia
- Rapid-acting insulin analogs (aspart, glulisine, and lispro) used in basalprandial insulin regimens.
First symptoms vary among individuals
Symptoms of hypoglycemia result primarily from a lowered glucose level in the brain and its effects on the central and autonomic nervous systems (FIGURE 1). A decrease in glucose below physiologic levels has acute consequences for brain function because the brain has an immediate requirement for glucose and little capacity for storage.
Two types of symptoms
Neuroglycopenia and the inhibition of neuronal metabolism causes sensations of warmth, weakness, fatigue, difficulty concentrating, confusion, behavioral changes, and in the most severe cases, a loss of consciousness, seizures, brain damage, and even death.2-4
Neurogenic symptoms are mediated by the hormones and neurotransmitters secreted in response to low brain glucose levels (FIGURE 1). The gluconeogenic actions of the autonomic nervous system produce the classic warning symptoms—tremulousness, pounding heart, anxiety, sweating, hunger, and tingling sensations—that usually precede the symptoms of hypoglycemia.2-4 This is particularly so in iatrogenic hypoglycemia.
These direct symptoms of neuroglycopenia are the ones patients typically identify with hypoglycemia. The most common symptoms of hypoglycemia are therefore not necessarily the first symptoms of hypoglycemia (TABLE 1).5 For example, though most patients experience sweating as a symptom of hypoglycemia, the first symptom might be trembling or anxiety, depending on the individual.5
TABLE 1
Signs and symptoms most commonly associated with hypoglycemia are not always the first to appear
| SYMPTOM | FREQUENCY (%) | FIRST SYMPTOM (%) |
|---|---|---|
| Sweating | 78 | 25 |
| Trembling | 62 | 6 |
| Inability to concentrate | 49 | 6 |
| Confusion | 40 | 3 |
| Weakness | 36 | 4 |
| Dry mouth | 35 | 0 |
| Blurred vision | 34 | 3 |
| Hunger | 33 | 3 |
| Anxiety | 26 | 1 |
| Headache | 21 | 0 |
| Difficulty walking | 21 | 3 |
| Pounding heart | 20 | 0 |
| Tingling around mouth | 20 | 6 |
| Difficulty speaking | 17 | 0 |
| Drowsiness | 15 | 0 |
| Odd behavior | 13 | 1 |
| Nausea | 13 | 0 |
| Adapted from Hepburn DA, MacLeod KM, Pell AC, et al. Diabet Med 1993;10:231–237.5 | ||
Factors influencing frequency and severity of hypoglycemia
Aggressive diabetes management commonly causes mild-to-moderate hypoglycemia, defined as a blood glucose value <60 mg/dL, that can be managed by the patient without assistance.
Severe hypoglycemia—a blood glucose value <50 mg/dL—is relatively uncommon in type 2 diabetes and requires the assistance of another person to manage, since neurological impairment may render patients unable to treat themselves.2,6 Severe hypoglycemia, whether in patients with type 1 or type 2 diabetes, can have debilitating consequences, including seizures or coma or even death.7
Long-standing type 2 disease. Hypoglycemia is more common in patients with type 1 diabetes than in those with type 2, but it can occur in type 2 diabetes patients who require insulin or are treated intensively with combinations of oral agents.6 As type 2 diabetes progresses,8 the incidence of hypoglycemic events increases, as endogenously produced insulin declines and is replaced by exogenous insulin.5,9 In fact, the prevalence of severe episodes (eg, requiring assistance of another person to administer glucose or glucagon) in patients with type 2 diabetes was comparable to that exhibited among patients with type 1 diabetes if they had been on insulin therapy for the same length of time.5,10
Nocturnal hypoglycemia. This event poses a special concern because the warning signs of hypoglycemia may be blunted during sleep. It has been reported that as many as 29% to 56% of all adult patients treated with insulin have an overnight glucose profile that indicates hypoglycemia occurs at night.11-13 However, it is important to note that the extent of the problem of nocturnal hypoglycemia is difficult to assess since overnight monitoring of glucose levels is required.
Additional insights from the UKPDS
Hypoglycemia in type 2 diabetes has not received rigorous attention in clinical trials. However, the United Kingdom Prospective Diabetes Study (UKPDS) was a large longitudinal trial in type 2 diabetes that included hypoglycemia as an outcome measure and thus provides some helpful information.
Events with insulin>sulfonylureas>diet. The 6-year follow-up revealed that the cumulative proportion of patients reporting 1 or more hypoglycemic events (of any type) was 76% for those using insulin, 45% among those taking sulfonylureas, and 3% for those on diet alone. Expressed as events per patient year, this was 37%, 17%, and 0.9%, respectively. When only “major” events (those requiring third-party assistance or hospital admission) were considered, the proportion of patients per year reporting 1 or more such events, was 2.3% for insulin, 0.7% for sulfonylureas, and 0.03% for diet alone. The cumulative proportion over 6 years was 3.3% of participants using sulfonylureas, 11.2% of those using insulin, and 0.15% of those on diet therapy.8
Metformin increases risk. The cumulative proportion of obese patients reporting any hypoglycemic event was 17.6% for those taking metformin vs 2.8% for those on diet. Severe hypoglycemia (as defined earlier) occurred in 2.4% of participants using metformin compared with 0.4% of those on diet therapy.8
Findings from other studies
Interestingly, in a recent systematic review of randomized controlled trials comparing insulin monotherapy with insulin plus oral antidiabetic agents, 13 of 14 studies reporting hypoglycemia demonstrated no difference in events.14
The occurrence of hypoglycemia among patients on metformin monotherapy in the UKPDS study is notable since, theoretically, hypoglycemia should not occur with agents whose mechanisms of action do not increase insulin secretion (biguanides, thiazolidinediones [TZDs], or α-glucosidase inhibitors),1 Since newer classes such as TZDs, α-glucosidase inhibitors, and meglitinides were not available when UKPDS was initiated, the trial does not provide data on these classes.
In a small comparative study of insulin combined with either metformin or a TZD, it appeared that metformin combination was associated with fewer occurrences of hypoglycemia; however, the small patient sample limits generalizability of the finding.15
How hypoglycemia occurs
Normally, as blood glucose levels (red) rise, insulin secretion increases, circulating insulin levels (blue) rise, and hepatic glucose production is inhibited. As glucose is disposed and circulating levels decrease due to insulin action, insulin levels then drop and hepatic glucose production begins again.
Hypoglycemia in diabetes can result from an excess of endogenous or exogenous insulin (iatrogenic hypoglycemia). In healthy patients, high insulin and falling glucose levels suppress insulin production and stimulate a hormone-mediated burst of glucose production. In patients with diabetes, the loss of physiologic control of insulin secretion coupled with exogenous administration of insulin or insulin secretagogues can interfere with the normal physiologic response to low blood glucose levels, resulting in hypoglycemia.1
With intense insulin regimens, the incidence of hypoglycemia can be as high as 30%, in contrast to 12% for patients treated with diet alone and 16% for those taking oral agents.6
With secretagogues, it has been suggested that the incidence of hypoglycemia is higher with the older, longer acting sulfonylurea agents.1,6,16-19 Although populationbased data on hypoglycemic rates associated with combination therapy with oral antidiabetic agents are not available, numerous clinical studies have reported rates of 10% to 20% for any hypoglycemic event.20-23
Heightening patient awareness, and yours
Because the signs of hypoglycemia vary considerably among individuals, they can easily be missed.3 In addition, repeated episodes of hypoglycemia can alter the normal regulatory responses and diminish the most important signs of a drop in glucose levels.1,2,24,25 The loss of the physiologic warning signs is thought to stem from dampening and eventual loss of the neuroadrenal response to low glucose levels in the brain (FIGURE 1). A vicious cycle is set up, whereby reduction in the neurogenic response attenuates hypoglycemic symptoms, causing more episodes to occur and become more severe as they are repeated. This cycle can be broken, and the normal physiologic response restored, if hypoglycemic events can be avoided for just a few weeks.2,26
Key points for patients. The main strategy for managing hypoglycemia is educating patients about the early symptoms of hypoglycemia and how to self-treat when they occur. Reinforce the need to time meals consistently and to limit the amount of carbohydrate ingested.
Advise patients to monitor blood glucose levels frequently, and to learn to relate a drop in glucose levels to hypoglycemic symptoms.2
Counsel patients to eat a snack or, preferably, drink fruit juice to counteract hypoglycemia. Patients may also carry glucose tablets, which are convenient and less tempting than candy.27
Glucagon is indicated for severe cases.
Whenever possible, a patient’s family members (particularly in the case of children) should be educated too.
Ask regularly about episodes. Finally, act to identify problems by querying patients and family members at every visit about hypoglycemia episodes, probing for information about awareness, severity and timing of the episodes, and how these events relate to dosing, meals, and activities.27 If hypoglycemia recurs, analyze the dosing regimen and consider flexible insulin dosing.1
Anti-hypoglycemia strategies for each new phase of therapy
As the course of diabetes therapy moves, typically, from oral medications to insulin to combination regimens, drug selections can be made in part to reduce the risk of hypoglycemia.
Oral agents: Insulin sensitizers, newer agents generally better
As noted earlier, among oral agents, insulin sensitizers are generally thought to have lower rates of hypoglycemia.
Newer sulfonylureas such as glimepiride and the rapid-acting meglitinides may also cause fewer hypoglycemic events.
Given the progressive decline of endogenous insulin secretion, combination therapy with secretagogues or insulin is eventually required for most patients.
Insulin analogs
A number of rapid-, short-, intermediateand long-acting insulin analogs have been introduced, and many of them make it possible to mimic different phases of physiologic insulin secretion (FIGURE 2). One of the newer analogs less likely to cause hypoglycemia is glargine, a long-acting insulin with a steady, relatively consistent action profile over a 24-hour period, closely mimicking normal basal pancreatic insulin secretion.28
FIGURE 2
Plasma insulin levels with newer analogs
N=20 type 1 diabetic patients; mean±SE
CSII, continuous subcutaneous insulin infusion; NPH, neutral protamine Hagedorn.
Copyright © 2000 American Diabetes Association. From Diabetes 2000; 49:2142–2148.28 Reprinted with permission from American Diabetes Association.
TABLE 2
Strategies for avoiding and addressing hypoglycemia
|
Insulin mixtures helpful when meal times guaranteed
Other insulins include mixtures of regular insulin and long-acting insulin available in split mixed or premixed formulations. These mixtures are intended to cover insulin peaks at mealtimes with twice-daily administration.
Mixed insulin formulations are often perceived as relatively convenient and simple to use, but they require meals to be taken within set time frames, without a great deal of flexibility. Since the ratios of the insulin components are fixed, and designed to work with meals consumed on a fixed schedule, hypoglycemia can occur if patients miss a meal. In addition, the time-activity profile of the insulin may not match the postprandial glucose peak even if the meal is consumed, and will increase the chance of postprandial hypoglycemia.
Basal insulin plus oral regimens
For patients with type 2 diabetes, adding basal insulin to oral regimens can significantly improve glycemic control. Ideally, basal insulin therapy provides a sustained and relatively constant concentration of insulin throughout the day. In the past, neutral protamine Hagedorn (NPH) insulin, a longer-acting insulin, was used for basal insulin therapy, and regular insulin was used to cover prandial insulin needs. Ultralente, also used as a basal insulin, has a relatively unpredictable timeactivity profile.28
Insulin glargine superior to NPH. In a recent clinical trial, patients with type 2 diabetes whose glucose levels were inadequately controlled on oral antidiabetic medications were given bedtime insulin glargine or NPH insulin.29 The insulin doses were titrated using a simple algorithm targeting a fasting plasma glucose (FPG) of ≤100 mg/dL to reach recommended glycosylated hemoglobin (Hb A1c) levels. Though no significant difference in glycemic control was found between insulin glargine and NPH insulin, significantly fewer hypoglycemic episodes occurred with insulin glargine therapy. The 24-hour distribution per patient-year of hypoglycemia for glargine vs NPH is shown in FIGURE 3.
Specifically, nearly 25% more patients treated with insulin glargine than with NPH insulin reached target Hb A1c levels of ≤7.0% without nocturnal hypoglycemia. Moreover, the overall incidence of any hypoglycemic event (eg, plasma-referenced glucose ≤72 mg/dL) and severe hypoglycemia (eg, patient required assistance of another person, and had a glucose level <56 mg/dL or prompt recovery after glucose or glucagons) was lower with insulin glargine than with NPH insulin. Results from other studies and a recent metaanalysis have been similar.30-32
Thus, using insulin glargine as basal insulin allows patients to reach recommended targets with fewer episodes of hypoglycemia, and can help address patients’ fear that can be a barrier to initiating insulin therapy in type 2 diabetes. Two recent studies have reported that dosing of insulin glargine can be flexible—morning or bedtime administration yields comparable low rates of hypoglycemia.30,31
FIGURE 3
Hourly hypoglycemia rate with glargine much less than with NPH
*P<.05 (between treatment).
Copyright © 2003 American Diabetes Association. From Diabetes Care 2003; 26:3080–3086.29 Reprinted with permission from American Diabetes Association.
Basal insulin plus prandial insulin
For patients who cannot otherwise reach Hb A1c goals, basal insulin therapy may be supplemented with prandial insulin. Newer, rapid-acting analogs used for the prandial component are insulin lispro, insulin glulisine, or insulin aspart. Although this approach is physiologically more rational than regimens using conventional insulins, data are limited for use in type 2 diabetes.
The incidence of nocturnal hypoglycemia was evaluated in a study of patients with type 1 diabetes and impaired hypoglycemic awareness who were treated with 1 of 2 regimens: insulin lispro in a basal-prandial combination with NPH insulin, or twice-daily, premixed NPH/regular insulin.33 Results showed that the incidence of nocturnal hypoglycemia was lower in patients receiving the insulin lispro regimen.
Another study, comparing insulin aspart and regular insulin as the prandial component in a basal-prandial regimen with NPH, showed that postprandial glucose control and Hb A1c levels were significantly better after 1 year of treatment in the insulin aspart group than in the group receiving regular insulin, without an increased risk for hypoglycemia.34 These results suggest that treatment with rapidacting insulin analogs could be helpful in avoiding hypoglycemia in patients with type 2 diabetes when a basal-prandial insulin regimen is indicated.
Avoiding hypoglycemia in the elderly
Elderly patients may be at increased risk for iatrogenic hypoglycemia. A populationbased study of patients presenting to an emergency room with severe hypoglycemic symptoms reported that rates of such events among elderly patients with type 2 diabetes and multiple comorbidities approached the rates among patients with type 1 diabetes.35
Creatinine clearance is often decreased in elderly patients, slowing elimination of oral agents and insulin and potentially resulting in sustained pharmacological action and creating a greater risk for hypoglycemia.
Furthermore, there is evidence that the neurogenic symptoms of hypoglycemia are reduced in elderly patients, diminishing awareness of hypoglycemia.36
In the demented elderly, malnutrition, weight loss, and anorexia may exacerbate the risk for hypoglycemia. For elderly patients with tertiary disease (eg, cerebrovascular accident, myocardial infarction, congestive heart failure, blindness, chronic renal failure), the risk for hypoglycemia and subsequent comorbidity may outweigh the benefits of strict glycemic control.3,37 Elderly patients may have comorbid conditions that increase risk of falls (eg, poor vision, neurologic conditions), and hypoglycemic episodes may further increase the risk of falls and lead to morbidity (eg, fragility fracture in patients with osteopenia or osteoporosis).
Because the elderly are at a greater risk for hypoglycemia, a switch to a less restrictive diet, such as a “no concentrated sweets” diet, is an option, with control of glucose levels through the administration of oral agents or insulin.36 This may also promote a better quality of life, considering that many of these patients already have secondary and tertiary complications of diabetes, prevention of which is not a realistic goal.
CORRESPONDING AUTHOR
William Cefalu, MD, Professor and Chief, Division of Nutrition and Chronic Diseases, Pennington Biomedical Research Center, Louisiana State University, 6400 Perkins Road, Baton Rouge, LA 70808. Email: cefaluwt@pbrc.edu
1. Cryer PE, Childs BP. Negotiating the barrier of hypoglycemia in diabetes. Diabetes Spectrum 2002;15:20-27.
2. Cryer PE, ed. Hypoglycemia: Pathophysiology, Diagnosis, and Treatment. New York, NY: Oxford University Press; 1997.
3. McAulay V, Deary IJ, Frier BM. Symptoms of hypoglycaemia in people with diabetes. Diabet Med 2001;18:690-705.
4. Towler DA, Havlin CE, Craft S, Cryer P. Mechanism of awareness of hypoglycemia. Perception of neurogenic (predominantly cholinergic) rather than neuroglycopenic symptoms. Diabetes 1993;42:1791-1798.
5. Hepburn DA, MacLeod KM, Pell AC, Scougal IJ, Frier BM. Frequency and symptoms of hypoglycaemia experienced by patients with type 2 diabetes treated with insulin. Diabet Med 1993;10:231-237.
6. Miller CD, Phillips LS, Ziemer DC, Gallina DL, Cook CB, El-Kebbi IM. Hypoglycemia in patients with type 2 diabetes mellitus. Arch Intern Med 2001;161:1653-1659.
7. The DCCT Research Group. Epidemiology of severe hypoglycemia in the diabetes control and complications trial. Am J Med 1991;90:450-459.
8. UK Prospective Diabetes Study Group. Perspectives in diabetes. UK Prospective Diabetes Study 16: overview of 6 years’ therapy of type II diabetes: a progressive disease. Diabetes 1995;44:1249-1258.
9. United Kingdom Prospective Diabetes Study Group. UKPDS 24: a 6-year, randomized, controlled trial comparing sulfonylurea, insulin, and metformin therapy in patients with newly diagnosed type 2 diabetes that could not be controlled with diet therapy. Ann Intern Med 1998;128:165-175.
10. MacLeod KM, Hepburn DA, Frier BM. Frequency and morbidity of severe hypoglycaemia in insulin-treated diabetic patients. Diabet Med 1993;10:238-245.
11. Vervoort G, Goldschmidt HM, van Doorn LG. Nocturnal blood glucose profiles in patients with type 1 diabetes mellitus on multiple (>or=4) daily insulin injection regimens. Diabet Med 1996;13:794-799.
12. Gale EA, Tattersall RB. Unrecognised nocturnal hypoglycaemia in insulin-treated diabetics. Lancet 1979;1:1049-1052.
13. Pramming S, Thorsteinsson B, Bendtson I, Ronn B, Binder C. Nocturnal hypoglycaemia in patients receiving conventional treatment with insulin. Br Med J (Clin Res Ed) 1985;291:376-379.
14. Goudswaard AN, Furlong NJ, Valk GD, Stolk RP, Rutten GEHM. Insulin monotherapy versus combinations of insulin with oral hypoglycaemic agents in patients with type 2 diabetes mellitus. Cochrane Database Syst Rev 2004;4:1-51.
15. Strowig SM, Avilés-Santa ML, Raskin P. Comparison of insulin monotherapy and combination therapy with insulin and metformin or insulin and troglitazone in type 2 diabetes. Diabetes Care 2002;25:1691-1698.
16. Campbell IW. Hypoglycaemia and type 2 diabetes: sulphonylureas. In: Frier BM, Fisher BM, eds. Hypoglycaemia and Diabetes: Clinical and Physiological Aspects. London, UK: Edward Arnold, 1993;387-392.
17. Shorr RI, Ray WA, Daugherty JR, Griffin MR. Individual sulfonylureas and serious hypoglycemia in older people. J Am Geriatr Soc 1996;44:751-755.
18. Holstein A, Egberts EH. Risk of hypoglycaemia with oral antidiabetic agents in patients with Type 2 diabetes. Exp Clin Endocrinol Diabetes 2003;111:405-414.
19. Holstein A, Plaschke A, Egberts E-H. Lower incidence of severe hypoglycaemia in patients with type 2 diabetes treated with glimepiride versus glibenclamide. Diabetes Metab Res Rev 2001;17:467-473.
20. Aljabri K, Kozak SE, Thompson DM. Addition of pioglitazone or bedtime insulin to maximal doses of sulfonylurea and metformin in type 2 diabetes patients with poor glucose control: a prospective, randomized trial. Am J Med 2004;116:230-235.
21. Wolffenbuttel BHR, Landgraf R. A 1-year multicenter randomized double-blind comparison of repaglinide and glyburide for the treatment of type 2 diabetes. Dutch and German Repaglinide Study Group. Diabetes Care 1999;22:463-467.
22. Raskin P, McGill J, Saad MF, et al. Combination therapy for type 2 diabetes: repaglinide plus rosiglitazone. Diabetes Med 2004;21:329-335.
23. Dailey GE, III, Noor MA, Park JS, Bruce S, Fiedorek FT. Glycemic control with glyburide/metformin tablets in combination with rosiglitazone in patients with type 2 diabetes: a randomized, double-blind trial. Am J Med 2004;116:223-229.
24. Korytkowski MT, Mokan M, Veneman TF, Mitrakou A, Cryer PE, Gerich JE. Reduced beta-adrenergic sensitivity in patients with type 1 diabetes and hypoglycemia unawareness. Diabetes Care 1998;21:1939-1943.
25. Fritsche A, Stumvoll M, Grub M, et al. Effect of hypoglycemia on beta-adrenergic sensitivity in normal and type 1 diabetic subjects. Diabetes Care 1998;21:1505-1510.
26. Fritsche A, Stefan N, Haring H, Gerich J, Stumvoll M. Avoidance of hypoglycemia restores hypoglycemia awareness by increasing beta-adrenergic sensitivity in type 1 diabetes. Ann Intern Med 2001;134:729-736.
27. Cryer PE, Davis SN, Shamoon H. Hypoglycemia in diabetes. Diabetes Care 2003;26:1902-1912.
28. Lepore M, Pampanelli S, Fanelli C, et al. Pharmacokinetics and pharmacodynamics of subcutaneous injection of long-acting human insulin analog glargine, NPH insulin, and ultralente human insulin and continuous subcutaneous infusion of insulin lispro. Diabetes 2000;49:2142-2148.
29. Riddle MC, Rosenstock J, Gerich J. The Treat-to-Target Trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care 2003;26:3080-3086.
30. Fritsche A, Schweitzer MA, Häring HU. Glimepiride combined with morning insulin glargine, bedtime neutral protamine Hagedorn insulin, or bedtime insulin glargine in patients with type 2 diabetes: a randomized, controlled trial. Ann Intern Med 2003;138:952-959.
31. Standl E, Maxeiner S, Raptis S, Karimi-Anderesi Z, Schweitzer MA. Good glycemic control with flexibility in timing of basal insulin supply: a 24-week comparison of insulin glargine given once daily in the morning or at bedtime in combination with morning glimepiride. Diabetes Care 2005;28:419-420.
32. Rosenstock J, Dailey G, Massi Benedetti M, Fritsche A, Lin Z, Salzman A. Reduced hypoglycemia risk with insulin glargine. A meta-analysis comparing insulin glargine with human NPH insulin in type 2 diabetes. Diabetes Care 2005;28:950-955.
33. Ferguson SC, Strachan MW, Janes JM, Frier BM. Severe hypoglycaemia in patients with type 1 diabetes and impaired awareness of hypoglycaemia: a comparative study of insulin lispro and regular human insulin. Diabetes Metab Res Rev 2001;17:285-291.
34. Raskin P, Guthrie RA, Leiter L, Riis A, Jovanovic L. Use of insulin aspart, a fast-acting insulin analog, as the mealtime insulin in the management of patients with type 1 diabetes. Diabetes Care 2000;23:583-558.
35. Holstein A, Plaschke A, Egberts EH. Clinical characterisation of severe hypoglycaemia-a prospective population-based study. Exp Clin Endocrinol Diabetes 2003;111:364-369.
36. Meneilly GS, Cheung E, Tuokko H. Counterregulatory hormone responses to hypoglycemia in the elderly patient with diabetes. Diabetes 1994;43:403-410.
37. American Diabetes Association. Standards of medical care for patients with diabetes mellitus. Diabetes Care 2003;26(suppl 1):S33-S50.
1. Cryer PE, Childs BP. Negotiating the barrier of hypoglycemia in diabetes. Diabetes Spectrum 2002;15:20-27.
2. Cryer PE, ed. Hypoglycemia: Pathophysiology, Diagnosis, and Treatment. New York, NY: Oxford University Press; 1997.
3. McAulay V, Deary IJ, Frier BM. Symptoms of hypoglycaemia in people with diabetes. Diabet Med 2001;18:690-705.
4. Towler DA, Havlin CE, Craft S, Cryer P. Mechanism of awareness of hypoglycemia. Perception of neurogenic (predominantly cholinergic) rather than neuroglycopenic symptoms. Diabetes 1993;42:1791-1798.
5. Hepburn DA, MacLeod KM, Pell AC, Scougal IJ, Frier BM. Frequency and symptoms of hypoglycaemia experienced by patients with type 2 diabetes treated with insulin. Diabet Med 1993;10:231-237.
6. Miller CD, Phillips LS, Ziemer DC, Gallina DL, Cook CB, El-Kebbi IM. Hypoglycemia in patients with type 2 diabetes mellitus. Arch Intern Med 2001;161:1653-1659.
7. The DCCT Research Group. Epidemiology of severe hypoglycemia in the diabetes control and complications trial. Am J Med 1991;90:450-459.
8. UK Prospective Diabetes Study Group. Perspectives in diabetes. UK Prospective Diabetes Study 16: overview of 6 years’ therapy of type II diabetes: a progressive disease. Diabetes 1995;44:1249-1258.
9. United Kingdom Prospective Diabetes Study Group. UKPDS 24: a 6-year, randomized, controlled trial comparing sulfonylurea, insulin, and metformin therapy in patients with newly diagnosed type 2 diabetes that could not be controlled with diet therapy. Ann Intern Med 1998;128:165-175.
10. MacLeod KM, Hepburn DA, Frier BM. Frequency and morbidity of severe hypoglycaemia in insulin-treated diabetic patients. Diabet Med 1993;10:238-245.
11. Vervoort G, Goldschmidt HM, van Doorn LG. Nocturnal blood glucose profiles in patients with type 1 diabetes mellitus on multiple (>or=4) daily insulin injection regimens. Diabet Med 1996;13:794-799.
12. Gale EA, Tattersall RB. Unrecognised nocturnal hypoglycaemia in insulin-treated diabetics. Lancet 1979;1:1049-1052.
13. Pramming S, Thorsteinsson B, Bendtson I, Ronn B, Binder C. Nocturnal hypoglycaemia in patients receiving conventional treatment with insulin. Br Med J (Clin Res Ed) 1985;291:376-379.
14. Goudswaard AN, Furlong NJ, Valk GD, Stolk RP, Rutten GEHM. Insulin monotherapy versus combinations of insulin with oral hypoglycaemic agents in patients with type 2 diabetes mellitus. Cochrane Database Syst Rev 2004;4:1-51.
15. Strowig SM, Avilés-Santa ML, Raskin P. Comparison of insulin monotherapy and combination therapy with insulin and metformin or insulin and troglitazone in type 2 diabetes. Diabetes Care 2002;25:1691-1698.
16. Campbell IW. Hypoglycaemia and type 2 diabetes: sulphonylureas. In: Frier BM, Fisher BM, eds. Hypoglycaemia and Diabetes: Clinical and Physiological Aspects. London, UK: Edward Arnold, 1993;387-392.
17. Shorr RI, Ray WA, Daugherty JR, Griffin MR. Individual sulfonylureas and serious hypoglycemia in older people. J Am Geriatr Soc 1996;44:751-755.
18. Holstein A, Egberts EH. Risk of hypoglycaemia with oral antidiabetic agents in patients with Type 2 diabetes. Exp Clin Endocrinol Diabetes 2003;111:405-414.
19. Holstein A, Plaschke A, Egberts E-H. Lower incidence of severe hypoglycaemia in patients with type 2 diabetes treated with glimepiride versus glibenclamide. Diabetes Metab Res Rev 2001;17:467-473.
20. Aljabri K, Kozak SE, Thompson DM. Addition of pioglitazone or bedtime insulin to maximal doses of sulfonylurea and metformin in type 2 diabetes patients with poor glucose control: a prospective, randomized trial. Am J Med 2004;116:230-235.
21. Wolffenbuttel BHR, Landgraf R. A 1-year multicenter randomized double-blind comparison of repaglinide and glyburide for the treatment of type 2 diabetes. Dutch and German Repaglinide Study Group. Diabetes Care 1999;22:463-467.
22. Raskin P, McGill J, Saad MF, et al. Combination therapy for type 2 diabetes: repaglinide plus rosiglitazone. Diabetes Med 2004;21:329-335.
23. Dailey GE, III, Noor MA, Park JS, Bruce S, Fiedorek FT. Glycemic control with glyburide/metformin tablets in combination with rosiglitazone in patients with type 2 diabetes: a randomized, double-blind trial. Am J Med 2004;116:223-229.
24. Korytkowski MT, Mokan M, Veneman TF, Mitrakou A, Cryer PE, Gerich JE. Reduced beta-adrenergic sensitivity in patients with type 1 diabetes and hypoglycemia unawareness. Diabetes Care 1998;21:1939-1943.
25. Fritsche A, Stumvoll M, Grub M, et al. Effect of hypoglycemia on beta-adrenergic sensitivity in normal and type 1 diabetic subjects. Diabetes Care 1998;21:1505-1510.
26. Fritsche A, Stefan N, Haring H, Gerich J, Stumvoll M. Avoidance of hypoglycemia restores hypoglycemia awareness by increasing beta-adrenergic sensitivity in type 1 diabetes. Ann Intern Med 2001;134:729-736.
27. Cryer PE, Davis SN, Shamoon H. Hypoglycemia in diabetes. Diabetes Care 2003;26:1902-1912.
28. Lepore M, Pampanelli S, Fanelli C, et al. Pharmacokinetics and pharmacodynamics of subcutaneous injection of long-acting human insulin analog glargine, NPH insulin, and ultralente human insulin and continuous subcutaneous infusion of insulin lispro. Diabetes 2000;49:2142-2148.
29. Riddle MC, Rosenstock J, Gerich J. The Treat-to-Target Trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care 2003;26:3080-3086.
30. Fritsche A, Schweitzer MA, Häring HU. Glimepiride combined with morning insulin glargine, bedtime neutral protamine Hagedorn insulin, or bedtime insulin glargine in patients with type 2 diabetes: a randomized, controlled trial. Ann Intern Med 2003;138:952-959.
31. Standl E, Maxeiner S, Raptis S, Karimi-Anderesi Z, Schweitzer MA. Good glycemic control with flexibility in timing of basal insulin supply: a 24-week comparison of insulin glargine given once daily in the morning or at bedtime in combination with morning glimepiride. Diabetes Care 2005;28:419-420.
32. Rosenstock J, Dailey G, Massi Benedetti M, Fritsche A, Lin Z, Salzman A. Reduced hypoglycemia risk with insulin glargine. A meta-analysis comparing insulin glargine with human NPH insulin in type 2 diabetes. Diabetes Care 2005;28:950-955.
33. Ferguson SC, Strachan MW, Janes JM, Frier BM. Severe hypoglycaemia in patients with type 1 diabetes and impaired awareness of hypoglycaemia: a comparative study of insulin lispro and regular human insulin. Diabetes Metab Res Rev 2001;17:285-291.
34. Raskin P, Guthrie RA, Leiter L, Riis A, Jovanovic L. Use of insulin aspart, a fast-acting insulin analog, as the mealtime insulin in the management of patients with type 1 diabetes. Diabetes Care 2000;23:583-558.
35. Holstein A, Plaschke A, Egberts EH. Clinical characterisation of severe hypoglycaemia-a prospective population-based study. Exp Clin Endocrinol Diabetes 2003;111:364-369.
36. Meneilly GS, Cheung E, Tuokko H. Counterregulatory hormone responses to hypoglycemia in the elderly patient with diabetes. Diabetes 1994;43:403-410.
37. American Diabetes Association. Standards of medical care for patients with diabetes mellitus. Diabetes Care 2003;26(suppl 1):S33-S50.