User login
Getting PrEP to the patients who need it
More than 1.2 million Americans are living with HIV, and more than 30,000 new cases are diagnosed each year. While total incidence has declined since 2016, HIV remains a nationwide epidemic.1
Medications that prevent HIV acquisition, termed preexposure prophylaxis (PrEP), are an important tool to initiate in the primary care setting to reduce HIV transmission. However, while there are an estimated 1.2 million people eligible for PrEP, only 36% have received PrEP prescriptions.2 Several barriers that have impeded its widespread adoption include a lack of clinician knowledge and clinical resources for testing, high medication costs, and stigma around sexual health and intravenous (IV) drug use.
The value of PrEP
PrEP is chemoprophylaxis against the acquisition of HIV infection through the administration of an oral or injectable medication to people at risk for HIV. This practice began in the early 2000s, with the first oral regimen approved in 2012, and since has become an important tool in preventing HIV transmission.
When taken as prescribed, PrEP medications reduce the risk for acquiring HIV through sex by approximately 99% and can reduce the risk for acquiring HIV from injection drug use by approximately 74%.3 The US Preventive Services Task Force issued a Grade “A” recommendation to offer PrEP to people at high risk for HIV acquisition in June 2019 and reaffirmed it in a 2023 update.4
PrEP is notably distinct from postexposure prophylaxis (PEP), which is the administration of medication to prevent HIV infection after a possible exposure.
The available regimens
Regimens for PrEP include oral tablets or intramuscular (IM) injections.5 There are 3 PrEP regimens approved by the US Food and Drug Administration (FDA): tenofovir disoproxil fumarate/emtricitabine (Truvada), tenofovir alafenamide/emtricitabine (Descovy), and cabotegravir (Apretude).
Truvada is once-daily oral PrEP that was approved in 2012 and is now available in a generic formulation. Notable adverse effects of Truvada include a small negative impact on renal function and small reductions in bone mineral density; these have been noted in individual trials, but in meta-analyses such differences were not found to be statistically significant.6-8 The most common adverse effects of Truvada, experienced by up to 6% of patients, are gastrointestinal symptoms, fatigue, headache/dizziness, depression, and insomnia; most symptoms resolve within weeks.
Continue to: Descovy
Descovy is daily oral PrEP that was approved in 2019. Descovy is associated with increases in LDL and triglycerides but has less impact on renal and bone health.9 The most common adverse effect of Descovy, experienced by about 5% of patients, is diarrhea, followed by nausea.
Apretude was approved in 2021 and is a 600-mg IM injection given monthly for 2 months, then every 2 months (± 7 days). The advantages of Apretude are frequency and discreteness of dosing and the ability to use in patients with estimated creatinine clearance (eCrCl) > 15 mL/min. The most common adverse effects of Apretude are injection-site reactions, which occur in 30% to 80% of patients but are rarely significant enough to lead to discontinuation (< 2% of patients discontinue use due to injection-site reactions).10
Who should take PrEP?
The latest Centers for Disease Control and Prevention (CDC) guidelines recommend that all sexually active adults receive information about PrEP.5 Indications for PrEP are broad and summarized in the FIGURE.5
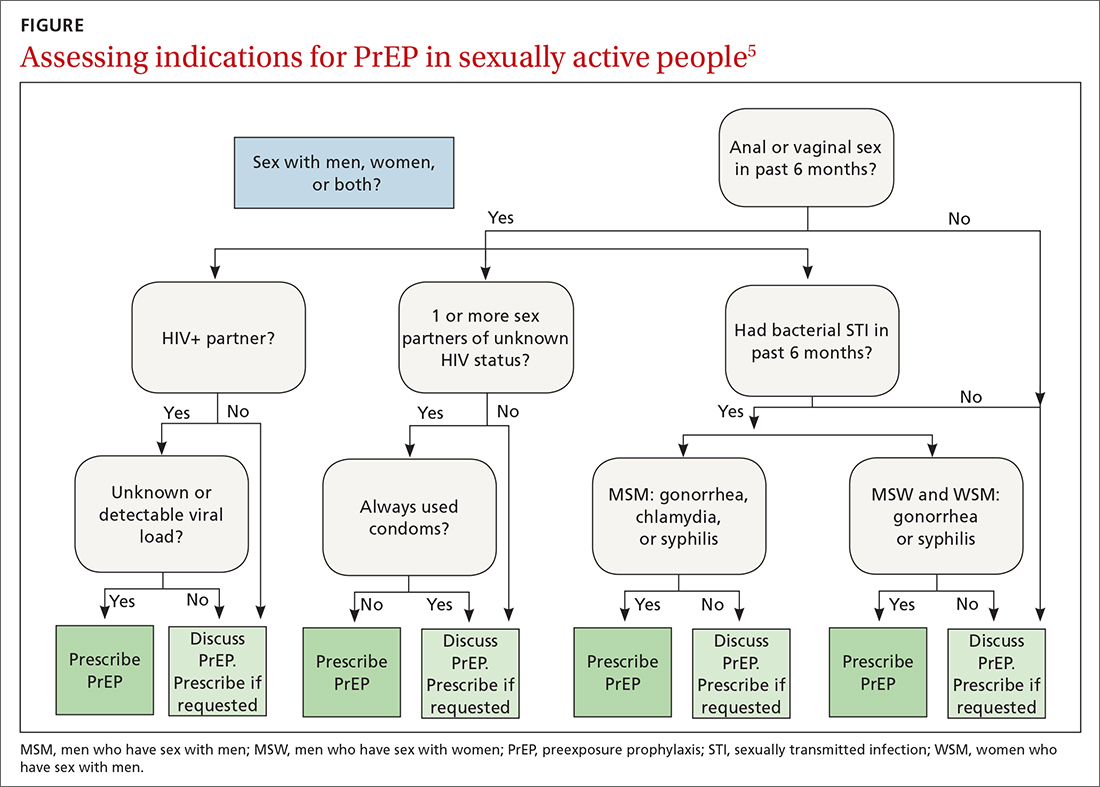
PrEP is indicated in patients who report sexual or injection drug use behaviors that place them at substantial ongoing risk for HIV exposure. Specific indications include patients with sexual partner(s) with unknown HIV status with whom they have inconsistent or no condom use, a history of bacterial sexually transmitted infection (STI) in the past 6 months, an HIV-positive sexual partner, or the sharing of injection drug equipment.
Hepatitis B infection is not a contraindication for PrEP use, but knowledge of infection status is essential. All current oral medications used for PrEP have activity against hepatitis B. Incomplete adherence to or abrupt discontinuation of oral PrEP could precipitate a hepatitis B flare. Hepatitis B surface antigen should be tested at the time of PrEP initiation, although PrEP can begin while testing is in process.
Continue to: How to use PrEP
How to use PrEP
At PrEP initiation, acute or chronic HIV infection must be excluded with a documented negative HIV antigen/antibody test within 1 week of prescribing PrEP.5 The CDC guidelines provide an updated HIV testing algorithm (www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2021.pdf, p 30-31, Figures 4a and 4b), which considers whether patients have received PrEP recently.
Patients with recent high-risk exposures or symptoms of acute HIV at the time of desired PrEP initiation should have an HIV-1 viral load checked with negative results before PrEP is prescribed. Additional criteria for PrEP include weight > 35 kg; screening for hepatitis B virus infection; screening for drug interactions; and drug-specific eCrCl cutoffs of > 60 mL/min for Truvada, > 30 mL/min for Descovy, and > 15 mL/min for Apretude.5
Studies regarding time to medication effectiveness are limited. Pharmacokinetic studies of Truvada demonstrate sufficient drug concentrations should be present in peripheral blood mononuclear cells and rectal tissue within 7 days of initiation of oral dosing and around 20 days in vaginal tissue.
Of note, while expedited partner therapy is used as a harm-reduction strategy to treat the sexual partners of patients diagnosed with certain STIs, PrEP is not recommended to be used in this way.
Ongoing monitoring with PrEP. Once oral PrEP is started, STI risk assessment and HIV testing via 4th generation antibody/antigen test should be completed at least every 3 months. PrEP oral prescription refills should be limited to 3 months. For patients receiving IM PrEP (Apretude), HIV testing via viral load and antibody/antigen testing should be done at the time of each injection (every 2 months).5
Continue to: With oral PrEP...
With oral PrEP, renal function should be checked every 6 months in patients older than 50 years or those with eCrCl < 90 mL/min at initiation. For patients younger than 50 years with no baseline renal dysfunction, the latest guidelines now recommend monitoring every 12 months instead of 6 months.5
For patients on Descovy, a lipid panel is recommended at PrEP initiation and every 12 months. Testing for other STIs can be considered on this schedule, based on clinical assessment. The TABLE5 summarizes recommended monitoring for patients taking oral PrEP.
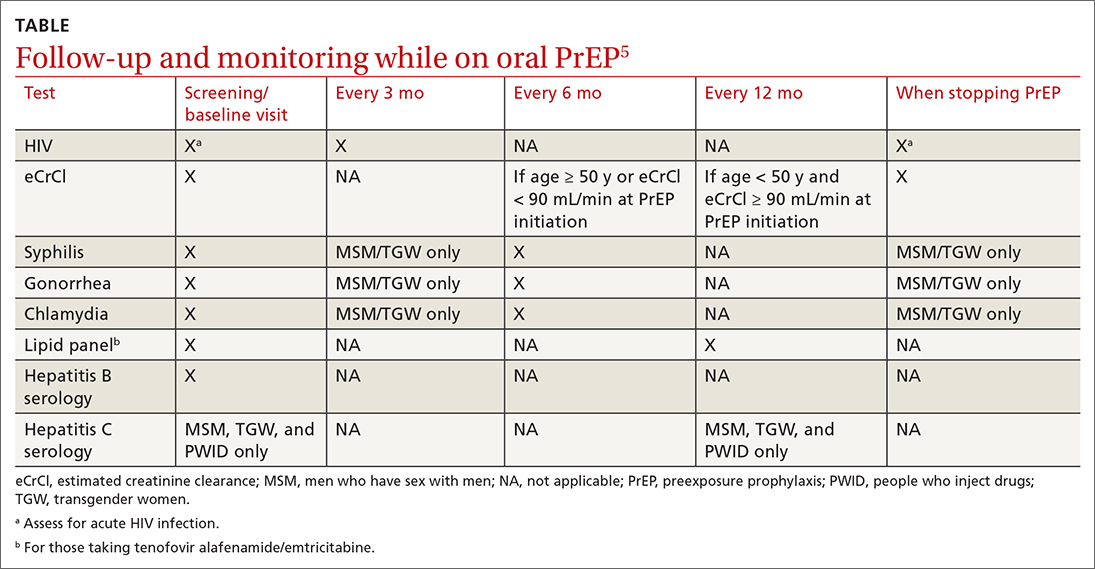
Recommended follow-up provides an opportunity to have frequent contact with a potentially high-risk population, and PrEP should be one part of a comprehensive HIV prevention and risk reduction plan. Many patients at high risk for HIV acquisition may benefit from frequent follow-up to address screening, referral, and treatment of substance use disorders, mental health conditions, and chronic medical conditions (including hepatitis C infection) and provide ongoing preventive health care.
Special uses of PrEP
Same-day PrEP. Starting PrEP on the day of the initial appointment may be appropriate based on patient risk factors and barriers to care, such as a high risk for contracting HIV before the subsequent appointment for a prescription of PrEP or an inability to return to the clinic in a timely fashion due to transportation or work constraints, or clinician availability. For these patients, assuming there is a low concern for acute or chronic HIV infection, PrEP can be initiated on the day of the initial visit.5
In these cases, point-of-care HIV and creatinine testing with same-day results should be completed. Antigen/antibody fingerstick testing or HIV-1 RNA test are preferred; oral fluid HIV testing should not be used for same-day PrEP due to its lower sensitivity for HIV detection. If same-day testing is unavailable, blood should be drawn at the visit so that HIV and creatinine testing can be completed as soon as possible.
Continue to: In addition to initial laboratory testing...
In addition to initial laboratory testing, clinics offering same-day PrEP should be able to provide: (1) assistance for patients to enroll in health insurance or a medication assistance program (eg, Ready, Set, PrEP) for those ineligible for insurance coverage, (2) rapid follow-up on all laboratory results with reliable patient contact information, and (3) follow-up appointments with clinicians able to prescribe and administer PrEP medications.
Off-label “on-demand” PrEP. An off-label treatment regimen for men who have sex with men (MSM) is termed “on-demand” PrEP or “2-1-1 PrEP” and is included in the CDC guidelines for consideration by clinicians.5 This alternative dosing schedule can be used for individuals who have sex less frequently and in a more planned fashion.
On-demand PrEP requires a patient to take 2 tablets of Truvada 2 to 24 hours before sex, followed by 1 tablet 24 hours and 1 tablet 48 hours after sexual activity. If a sexual act occurs at 48 hours, the patient should extend the daily dose for 48 additional hours, such that PrEP is always used daily for 48 hours after the last sex act.
This method has been studied with Truvada in MSM in Europe and Canada through the IPERGAY and PREVENIR trials and shown to have ≥ 86% efficacy in preventing HIV acquisition.11,12 The only US-based study showed lower efficacy; however, based on the currently available data, the International Antiviral Society-USA Panel has recommended it as an alternative regimen.13,14
PrEP via telehealth. Visits for PrEP initiation and continuation can be completed via telehealth.5 Patients then can complete necessary laboratory tests by going to a physical laboratory location or using mailed specimen kits in which they can self-collect urine, oral/rectal swabs, and fingerstick blood samples.
Continue to: PrEP use in specific populations
PrEP use in specific populations
Adolescents
Truvada, Descovy, and Apretude all are now approved for use in adolescents weighing ≥ 35 kg. Two important considerations when prescribing to this population are the effects of Truvada on bone health and the unique barriers to access.
In studies of adolescent MSM using Truvada for PrEP, bone mineral density declined, especially among those ages 15 to 19 years.15 As such, the clinical impact of decreased bone mineral density should be weighed against the risk for HIV acquisition; however, bone mineral density monitoring is not recommended in the current guidelines. CDC guidelines suggest considering Descovy for male adolescents given its potential lower impact on bone mineral density.5
Confidentiality and legal issues exist when prescribing PrEP to minors. In terms of parental/guardian involvement, clinicians who are prescribing PrEP for patients younger than 18 years should consult the CDC website for guidance on local and state regulations that govern prescribing and confidentiality (www.cdc.gov/hiv/policies/law/states/minors.html).
Insurance billing statements may lead to inadvertent disclosure of a minor’s decision to take PrEP to their legal guardian.16 Generic Truvada costs less than $100 for a 3-month supply when using goodrx.com, which may offer an alternative to insurance for medication payment.
Peripartum patients
The increased risk for HIV acquisition in the peripartum period for female patients is well documented.17 Guidelines recommend offering PrEP with Truvada to female patients at risk for conception, currently pregnant, or breastfeeding when that patient’s partner has HIV and the partner’s viral load is unknown or detectable. Descovy is not recommended for pregnant or breastfeeding patients.5 Cabotegravir-containing regimens (Apretude) have not been approved by the FDA for pregnant or breastfeeding patients.5
Continue to: Data on the impact of...
Data on the impact of Truvada for PrEP on fetal health are still emerging. A large study in Kenya showed no significant differences in preterm birth, low birth weight, or early infant growth, and a randomized, noninferiority trial in South Africa showed no association between Truvada for PrEP and preterm birth or the birth of small-for-gestational-age infants.18,19 There are no definitive studies of breastfeeding infants exposed to Truvada, but data from previous trials of breastfeeding mothers who were taking the individual components that are combined in the Truvada pill indicated there is minimal medication exposure to the infant.5
PrEP studies in the peripartum period to date have been conducted exclusively among cisgender women, and data do not yet reflect the experiences of transgender men, genderqueer people, and nonbinary individuals in the peripartum period.5
Transgender people
Transgender women should be strongly considered candidates for PrEP as they are at an extremely high risk for HIV acquisition. The most recent National HIV Behavioral Surveillance survey found that approximately 42% of transgender women were living with HIV.20 The survey revealed stark racial and ethnic disparities among transgender women living with HIV: 62% identified as Black/African American, compared with 35% Hispanic/Latina and 17% White.20
Transgender women report high rates of sexual assault, unprotected receptive anal sex, commercial sex work, homelessness, mental health disorders, and substance use, putting them at increased risk for HIV acquisition.21 However, transgender women are less likely to have discussed PrEP with a clinician, are less likely to be on PrEP even when interested in starting, and have higher rates of medication nonadherence compared with cisgender MSM.21,22 PrEP has not been found to decrease levels of feminizing hormones; however, studies are mixed as to whether feminizing hormones decrease Truvada concentrations in rectal mucosa, so clinicians should emphasize the importance of daily medication adherence.23
Transgender men have not been included in any PrEP trials, so no specific recommendations are available.
Continue to: Disparities in PrEP access and use exist
Disparities in PrEP access and use exist
The lifetime risk for HIV acquisition is 9% among White MSM, 50% among Black MSM, and 20% among Hispanic MSM.24 Despite this large disparity in disease burden, Black and Hispanic individuals are less likely to be aware of PrEP, have discussed PrEP with a health care professional, or used PrEP compared with their White counterparts.25 As a result, in 2020, PrEP coverage for eligible White individuals was 61%, while coverage among eligible Black and Hispanic/Latino individuals was just 8% and 14%, respectively.26
Surveillance data comparing male and female PrEP coverage reveal further disparities between the sexes, with PrEP coverage for eligible female-at-birth patients estimated to be 9% compared with 25.8% for male-at-birth patients.26 The gap between the risk for HIV infection and the access to and uptake of PrEP coverage is most pronounced among Black women, for whom the rate of new HIV diagnosis is > 10 times higher than it is for White women, but who have some of the lowest awareness and utilization rates of all demographics.27
The rural population at risk. Disparities in HIV awareness and PrEP use also exist between rural and urban populations, as well as by health insurance status. Rural areas have been shown to lag behind urban areas in PrEP awareness and use. Two potential explanations for this disparity are differences in HIV- and drug use–associated stigma and health insurance status. Greater stigma against drug use and HIV in rural areas has been associated with lower rates of PrEP use.28
Individuals younger than 65 years in rural areas are less likely to have private health insurance and more likely to be uninsured compared with their urban counterparts, which may impact access to clinicians knowledgeable about PrEP.29 Notably, MSM who live in states that have expanded Medicaid have higher rates of PrEP use compared with MSM living in states that have not expanded Medicaid.30
Health insurers in the United States are required to cover PrEP medication, clinician visits, and associated blood work with no patient cost-sharing, although implementation barriers such as prior authorizations still exist.
Conclusion
Family physicians are well positioned to identify patients at risk for HIV infection, prescribe PrEP, organize comprehensive follow-up care, and partner with their health systems and local communities to reduce barriers to care. Those who can leverage existing relationships with local health departments, school-based health clinics, congregate housing programs, LGBTQIA+ advocacy groups, harm-reduction coalitions, and other community-based organizations to raise PrEP awareness play a critical role in preventing HIV transmission and reducing health care disparities in their communities.
CORRESPONDENCE
Andrew V.A. Foley, MD, MPH, Erie Family Health Centers, 2418 W Division Street, Chicago, IL 60622; andrewvafoley@gmail.com
1. CDC. Estimated HIV incidence and prevalence in the United States 2017–2021. HIV Surveill Supplemental Rep. 2023;28. Accessed October 23, 2023. https://www.cdc.gov/hiv/library/reports/hiv-surveillance/vol-28-no-3/index.html
2. CDC. Core indicators for monitoring the Ending the HIV Epidemic initiative (preliminary data): National HIV Surveillance System data reported through March 2023; and preexposure prophylaxis (PrEP) data reported through December 2022. HIV Surveill Data Tables. 2023;4. Published June 2023. Accessed October 23, 2023. https://www.cdc.gov/hiv/library/reports/surveillance-data-tables/
3. CDC. Division of HIV Prevention, National Center for HIV, Viral Hepatitis, STD, and TB Prevention. PrEP effectiveness. Updated June 2022. Accessed October 23, 2023. https://www.cdc.gov/hiv/basics/prep/prep-effectiveness.html
4. US Preventive Services Task Force. Prevention of acquisition of HIV: preexposure prophylaxis. Final recommendation statement. August 22, 2023. Accessed October 23, 2023. https://uspreventiveservicestaskforce.org/uspstf/recommendation/prevention-of-human-immunodeficiency-virus-hiv-infection-pre-exposure-prophylaxis
5. CDC. Preexposure prophylaxis for the prevention of HIV infection in the United States—2021 update: a clinical practice guideline. Accessed October 23, 2023. www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2021.pdf
6. Mugwanya KK, Wyatt C, Celum C, et al. Changes in glomerular kidney function among HIV-1-uninfected men and women receiving emtricitabine-tenofovir disoproxil fumarate preexposure prophylaxis: a randomized clinical trial. JAMA Intern Med. 2015;175:246-254. doi: 10.1001/jamainternmed.2014.6786
7. Havens PL, Stephensen CB, Van Loan MD, et al. Decline in bone mass with tenofovir disoproxil fumarate/emtricitabine is associated with hormonal changes in the absence of renal impairment when used by HIV-uninfected adolescent boys and young men for HIV preexposure prophylaxis. Clin Infect Dis. 2017;64:317-325. doi: 10.1093/cid/ciw765
8. Pilkington V, Hill A, Hughes S, et al. How safe is TDF/FTC as PrEP? A systematic review and meta-analysis of the risk of adverse events in 13 randomised trials of PrEP. J Virus Erad. 2018;4:215-224.
9. Mayer KH, Molina JM, Thompson MA, et al. Emtricitabine and tenofovir alafenamide vs emtricitabine and tenofovir disoproxil fumarate for HIV pre-exposure prophylaxis (DISCOVER): primary results from a randomised, double-blind, multicentre, active-controlled, phase 3, non-inferiority trial. Lancet. 2020;396:239-254. doi: 10.1016/S0140-6736(20)31065-5
10. Liegeon G, Ghosn, J. Long-acting injectable cabotegravir for PrEP: a game-changer in HIV prevention. HIV Med. 2022;24:653-663. doi: 10.1111/hiv.13451
11. Molina JM, Capitant C, Spire B, et al. On-demand preexposure prophylaxis in men at high risk for HIV-1 infection. N Engl J Med. 2015;373:2237-2246. doi: 10.1056/NEJMoa1506273
12. Molina JM, Ghosn J, Assoumou L, et al. Daily and on-demand HIV pre-exposure prophylaxis with emtricitabine and tenofovir disoproxil (ANRS PREVENIR): a prospective observational cohort study. Lancet HIV. 2022;9:e554-e562. doi: 10.1016/S2352-3018(22)00133-3
13. Dimitrov D, Moore JR, Wood D, et al. Predicted effectiveness of daily and nondaily preexposure prophylaxis for men who have sex with men based on sex and pill-taking patterns from the Human Immuno Virus Prevention Trials Network 067/ADAPT Study. Clin Infect Dis. 2020;71:249-255. doi: 10.1093/cid/ciz799
14. Saag MS, Gandhi RT, Hoy JF, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2020 recommendations of the International Antiviral Society-USA Panel. JAMA. 2020;324:1651-1669. doi: 10.1001/jama.2020.17025
15. Havens PL, Perumean-Chaney SE, Patki A, et al. Changes in bone mass after discontinuation of preexposure prophylaxis with tenofovir disoproxil fumarate/emtricitabine in young men who have sex with men: extension phase results of Adolescent Trials Network Protocols 110 and 113. Clin Infect Dis. 2020;70:687-691. doi: 10.1093/cid/ciz486
16. Neilan AM, Salvant Valentine S, Knopf AS. Case 27-2021: a 16-year-old boy seeking human immunodeficiency virus prophylaxis. N Engl J Med. 2021;385:1034-1041. doi: 10.1056/NEJMcpc1909626
17. Thomson KA, Hughes J, Baeten JM, et al. Increased risk of HIV acquisition among women throughout pregnancy and during the postpartum period: a prospective per-coital-act analysis among women with HIV-infected partners. J Infect Dis. 2018;218:16-25. doi: 10.1093/infdis/jiy113
18. Dettinger JC, Kinuthia J, Pintye J, et al. Perinatal outcomes following maternal pre-exposure prophylaxis (PrEP) use during pregnancy: results from a large PrEP implementation program in Kenya. J Int AIDS Soc. 2019;22:e25378. doi: 10.1002/jia2.25378
19. Moodley D, Lombard C, Govender V, et al. Pregnancy and neonatal safety outcomes of timing of initiation of daily oral tenofovir disoproxil fumarate and emtricitabine pre-exposure prophylaxis for HIV prevention (CAP016): an open-label, randomised, non-inferiority trial. Lancet HIV. 2023;10:e154-e163. doi: 10.1016/S2352-3018(22)00369-1
20. CDC. HIV Infection, Risk, Prevention, and Testing Behaviors Among Transgender Women—National HIV Behavioral Surveillance, 7 U.S. Cities, 2019–2020. HIV Surveillance Special Report 27. April 2021. Accessed October 23, 2023. www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-special-report-number-27.pdf
21. Wilson EC, Turner CM, Arayasirikul S, et al. Disparities in the PrEP continuum for trans women compared to MSM in San Francisco, California: results from population-based cross-sectional behavioural surveillance studies. J Int AIDS Soc. 2020;23:e25539. doi: 10.1002/jia2.25539
22. Poteat T, Wirtz A, Malik M, et al. A gap between willingness and uptake: findings from mixed methods research on HIV prevention among Black and Latina transgender women. J Acquir Immune Defic Syndr. 2019;82:131-140. doi: 10.1097/QAI.0000000000002112
23. Cottrell ML, Prince HM, Schauer AP, et al. Decreased tenofovir diphosphate concentrations in a transgender female cohort: implications for human immunodeficiency virus preexposure prophylaxis. Clin Infect Dis. 2019;69:2201-2204. doi: 10.1093/cid/ciz290
24. Hess KL, Hu X, Lansky A, et al. Lifetime risk of a diagnosis of HIV infection in the United States. Ann Epidemiol. 2017;27:238-243. doi: 10.1016/j.annepidem.2017.02.003
25. Kanny D, Jeffries WL 4th, Chapin-Bardales J, et al. Racial/ethnic disparities in HIV preexposure prophylaxis among men who have sex with men—23 urban areas, 2017. MMWR Morb Mortal Wkly Rep. 2019;68:801-806. doi: 10.15585/mmwr.mm6837a2
26. CDC. Core indicators for monitoring the Ending the HIV Epidemic initiative (early release): National HIV Surveillance System data reported through December 2020; and preexposure prophylaxis (PrEP) data reported through September 2020. HIV Surveill Data Tables. 2021;2. Accessed October 23, 2023. www.cdc.gov/hiv/pdf/library/reports/surveillance-data-tables/vol-2-no-2/cdc-hiv-surveillance-tables-vol-2-no-2.pdf
27. CDC. Diagnoses of HIV infection in the United States and dependent areas 2021: special focus profiles. Updated May 23, 2023. Accessed October 23, 2023. www.cdc.gov/hiv/library/reports/hiv-surveillance/vol-34/content/special-focus-profiles.html
28. Walters SM, Frank D, Van Ham B, et al. PrEP care continuum engagement among persons who inject drugs: rural and urban differences in stigma and social infrastructure. AIDS Behav. 2021;26:1308-1320. doi: 10.1007/s10461-021-03488-2
29. Foutz J, Artiga S, Garfield R. The role of Medicaid in rural America [issue brief]. April 25, 2017. Accessed August 16, 2023. www.kff.org/medicaid/issue-brief/the-role-of-medicaid-in-rural-america/
30. Baugher AR, Finlayson T, Lewis R, et al. Health care coverage and preexposure prophylaxis (PrEP) use among men who have sex with men living in 22 US cities with vs without Medicaid expansion, 2017. Am J Public Health. 2021;111:743-751. doi: 10.2105/AJPH.2020.306035
More than 1.2 million Americans are living with HIV, and more than 30,000 new cases are diagnosed each year. While total incidence has declined since 2016, HIV remains a nationwide epidemic.1
Medications that prevent HIV acquisition, termed preexposure prophylaxis (PrEP), are an important tool to initiate in the primary care setting to reduce HIV transmission. However, while there are an estimated 1.2 million people eligible for PrEP, only 36% have received PrEP prescriptions.2 Several barriers that have impeded its widespread adoption include a lack of clinician knowledge and clinical resources for testing, high medication costs, and stigma around sexual health and intravenous (IV) drug use.
The value of PrEP
PrEP is chemoprophylaxis against the acquisition of HIV infection through the administration of an oral or injectable medication to people at risk for HIV. This practice began in the early 2000s, with the first oral regimen approved in 2012, and since has become an important tool in preventing HIV transmission.
When taken as prescribed, PrEP medications reduce the risk for acquiring HIV through sex by approximately 99% and can reduce the risk for acquiring HIV from injection drug use by approximately 74%.3 The US Preventive Services Task Force issued a Grade “A” recommendation to offer PrEP to people at high risk for HIV acquisition in June 2019 and reaffirmed it in a 2023 update.4
PrEP is notably distinct from postexposure prophylaxis (PEP), which is the administration of medication to prevent HIV infection after a possible exposure.
The available regimens
Regimens for PrEP include oral tablets or intramuscular (IM) injections.5 There are 3 PrEP regimens approved by the US Food and Drug Administration (FDA): tenofovir disoproxil fumarate/emtricitabine (Truvada), tenofovir alafenamide/emtricitabine (Descovy), and cabotegravir (Apretude).
Truvada is once-daily oral PrEP that was approved in 2012 and is now available in a generic formulation. Notable adverse effects of Truvada include a small negative impact on renal function and small reductions in bone mineral density; these have been noted in individual trials, but in meta-analyses such differences were not found to be statistically significant.6-8 The most common adverse effects of Truvada, experienced by up to 6% of patients, are gastrointestinal symptoms, fatigue, headache/dizziness, depression, and insomnia; most symptoms resolve within weeks.
Continue to: Descovy
Descovy is daily oral PrEP that was approved in 2019. Descovy is associated with increases in LDL and triglycerides but has less impact on renal and bone health.9 The most common adverse effect of Descovy, experienced by about 5% of patients, is diarrhea, followed by nausea.
Apretude was approved in 2021 and is a 600-mg IM injection given monthly for 2 months, then every 2 months (± 7 days). The advantages of Apretude are frequency and discreteness of dosing and the ability to use in patients with estimated creatinine clearance (eCrCl) > 15 mL/min. The most common adverse effects of Apretude are injection-site reactions, which occur in 30% to 80% of patients but are rarely significant enough to lead to discontinuation (< 2% of patients discontinue use due to injection-site reactions).10
Who should take PrEP?
The latest Centers for Disease Control and Prevention (CDC) guidelines recommend that all sexually active adults receive information about PrEP.5 Indications for PrEP are broad and summarized in the FIGURE.5

PrEP is indicated in patients who report sexual or injection drug use behaviors that place them at substantial ongoing risk for HIV exposure. Specific indications include patients with sexual partner(s) with unknown HIV status with whom they have inconsistent or no condom use, a history of bacterial sexually transmitted infection (STI) in the past 6 months, an HIV-positive sexual partner, or the sharing of injection drug equipment.
Hepatitis B infection is not a contraindication for PrEP use, but knowledge of infection status is essential. All current oral medications used for PrEP have activity against hepatitis B. Incomplete adherence to or abrupt discontinuation of oral PrEP could precipitate a hepatitis B flare. Hepatitis B surface antigen should be tested at the time of PrEP initiation, although PrEP can begin while testing is in process.
Continue to: How to use PrEP
How to use PrEP
At PrEP initiation, acute or chronic HIV infection must be excluded with a documented negative HIV antigen/antibody test within 1 week of prescribing PrEP.5 The CDC guidelines provide an updated HIV testing algorithm (www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2021.pdf, p 30-31, Figures 4a and 4b), which considers whether patients have received PrEP recently.
Patients with recent high-risk exposures or symptoms of acute HIV at the time of desired PrEP initiation should have an HIV-1 viral load checked with negative results before PrEP is prescribed. Additional criteria for PrEP include weight > 35 kg; screening for hepatitis B virus infection; screening for drug interactions; and drug-specific eCrCl cutoffs of > 60 mL/min for Truvada, > 30 mL/min for Descovy, and > 15 mL/min for Apretude.5
Studies regarding time to medication effectiveness are limited. Pharmacokinetic studies of Truvada demonstrate sufficient drug concentrations should be present in peripheral blood mononuclear cells and rectal tissue within 7 days of initiation of oral dosing and around 20 days in vaginal tissue.
Of note, while expedited partner therapy is used as a harm-reduction strategy to treat the sexual partners of patients diagnosed with certain STIs, PrEP is not recommended to be used in this way.
Ongoing monitoring with PrEP. Once oral PrEP is started, STI risk assessment and HIV testing via 4th generation antibody/antigen test should be completed at least every 3 months. PrEP oral prescription refills should be limited to 3 months. For patients receiving IM PrEP (Apretude), HIV testing via viral load and antibody/antigen testing should be done at the time of each injection (every 2 months).5
Continue to: With oral PrEP...
With oral PrEP, renal function should be checked every 6 months in patients older than 50 years or those with eCrCl < 90 mL/min at initiation. For patients younger than 50 years with no baseline renal dysfunction, the latest guidelines now recommend monitoring every 12 months instead of 6 months.5
For patients on Descovy, a lipid panel is recommended at PrEP initiation and every 12 months. Testing for other STIs can be considered on this schedule, based on clinical assessment. The TABLE5 summarizes recommended monitoring for patients taking oral PrEP.

Recommended follow-up provides an opportunity to have frequent contact with a potentially high-risk population, and PrEP should be one part of a comprehensive HIV prevention and risk reduction plan. Many patients at high risk for HIV acquisition may benefit from frequent follow-up to address screening, referral, and treatment of substance use disorders, mental health conditions, and chronic medical conditions (including hepatitis C infection) and provide ongoing preventive health care.
Special uses of PrEP
Same-day PrEP. Starting PrEP on the day of the initial appointment may be appropriate based on patient risk factors and barriers to care, such as a high risk for contracting HIV before the subsequent appointment for a prescription of PrEP or an inability to return to the clinic in a timely fashion due to transportation or work constraints, or clinician availability. For these patients, assuming there is a low concern for acute or chronic HIV infection, PrEP can be initiated on the day of the initial visit.5
In these cases, point-of-care HIV and creatinine testing with same-day results should be completed. Antigen/antibody fingerstick testing or HIV-1 RNA test are preferred; oral fluid HIV testing should not be used for same-day PrEP due to its lower sensitivity for HIV detection. If same-day testing is unavailable, blood should be drawn at the visit so that HIV and creatinine testing can be completed as soon as possible.
Continue to: In addition to initial laboratory testing...
In addition to initial laboratory testing, clinics offering same-day PrEP should be able to provide: (1) assistance for patients to enroll in health insurance or a medication assistance program (eg, Ready, Set, PrEP) for those ineligible for insurance coverage, (2) rapid follow-up on all laboratory results with reliable patient contact information, and (3) follow-up appointments with clinicians able to prescribe and administer PrEP medications.
Off-label “on-demand” PrEP. An off-label treatment regimen for men who have sex with men (MSM) is termed “on-demand” PrEP or “2-1-1 PrEP” and is included in the CDC guidelines for consideration by clinicians.5 This alternative dosing schedule can be used for individuals who have sex less frequently and in a more planned fashion.
On-demand PrEP requires a patient to take 2 tablets of Truvada 2 to 24 hours before sex, followed by 1 tablet 24 hours and 1 tablet 48 hours after sexual activity. If a sexual act occurs at 48 hours, the patient should extend the daily dose for 48 additional hours, such that PrEP is always used daily for 48 hours after the last sex act.
This method has been studied with Truvada in MSM in Europe and Canada through the IPERGAY and PREVENIR trials and shown to have ≥ 86% efficacy in preventing HIV acquisition.11,12 The only US-based study showed lower efficacy; however, based on the currently available data, the International Antiviral Society-USA Panel has recommended it as an alternative regimen.13,14
PrEP via telehealth. Visits for PrEP initiation and continuation can be completed via telehealth.5 Patients then can complete necessary laboratory tests by going to a physical laboratory location or using mailed specimen kits in which they can self-collect urine, oral/rectal swabs, and fingerstick blood samples.
Continue to: PrEP use in specific populations
PrEP use in specific populations
Adolescents
Truvada, Descovy, and Apretude all are now approved for use in adolescents weighing ≥ 35 kg. Two important considerations when prescribing to this population are the effects of Truvada on bone health and the unique barriers to access.
In studies of adolescent MSM using Truvada for PrEP, bone mineral density declined, especially among those ages 15 to 19 years.15 As such, the clinical impact of decreased bone mineral density should be weighed against the risk for HIV acquisition; however, bone mineral density monitoring is not recommended in the current guidelines. CDC guidelines suggest considering Descovy for male adolescents given its potential lower impact on bone mineral density.5
Confidentiality and legal issues exist when prescribing PrEP to minors. In terms of parental/guardian involvement, clinicians who are prescribing PrEP for patients younger than 18 years should consult the CDC website for guidance on local and state regulations that govern prescribing and confidentiality (www.cdc.gov/hiv/policies/law/states/minors.html).
Insurance billing statements may lead to inadvertent disclosure of a minor’s decision to take PrEP to their legal guardian.16 Generic Truvada costs less than $100 for a 3-month supply when using goodrx.com, which may offer an alternative to insurance for medication payment.
Peripartum patients
The increased risk for HIV acquisition in the peripartum period for female patients is well documented.17 Guidelines recommend offering PrEP with Truvada to female patients at risk for conception, currently pregnant, or breastfeeding when that patient’s partner has HIV and the partner’s viral load is unknown or detectable. Descovy is not recommended for pregnant or breastfeeding patients.5 Cabotegravir-containing regimens (Apretude) have not been approved by the FDA for pregnant or breastfeeding patients.5
Continue to: Data on the impact of...
Data on the impact of Truvada for PrEP on fetal health are still emerging. A large study in Kenya showed no significant differences in preterm birth, low birth weight, or early infant growth, and a randomized, noninferiority trial in South Africa showed no association between Truvada for PrEP and preterm birth or the birth of small-for-gestational-age infants.18,19 There are no definitive studies of breastfeeding infants exposed to Truvada, but data from previous trials of breastfeeding mothers who were taking the individual components that are combined in the Truvada pill indicated there is minimal medication exposure to the infant.5
PrEP studies in the peripartum period to date have been conducted exclusively among cisgender women, and data do not yet reflect the experiences of transgender men, genderqueer people, and nonbinary individuals in the peripartum period.5
Transgender people
Transgender women should be strongly considered candidates for PrEP as they are at an extremely high risk for HIV acquisition. The most recent National HIV Behavioral Surveillance survey found that approximately 42% of transgender women were living with HIV.20 The survey revealed stark racial and ethnic disparities among transgender women living with HIV: 62% identified as Black/African American, compared with 35% Hispanic/Latina and 17% White.20
Transgender women report high rates of sexual assault, unprotected receptive anal sex, commercial sex work, homelessness, mental health disorders, and substance use, putting them at increased risk for HIV acquisition.21 However, transgender women are less likely to have discussed PrEP with a clinician, are less likely to be on PrEP even when interested in starting, and have higher rates of medication nonadherence compared with cisgender MSM.21,22 PrEP has not been found to decrease levels of feminizing hormones; however, studies are mixed as to whether feminizing hormones decrease Truvada concentrations in rectal mucosa, so clinicians should emphasize the importance of daily medication adherence.23
Transgender men have not been included in any PrEP trials, so no specific recommendations are available.
Continue to: Disparities in PrEP access and use exist
Disparities in PrEP access and use exist
The lifetime risk for HIV acquisition is 9% among White MSM, 50% among Black MSM, and 20% among Hispanic MSM.24 Despite this large disparity in disease burden, Black and Hispanic individuals are less likely to be aware of PrEP, have discussed PrEP with a health care professional, or used PrEP compared with their White counterparts.25 As a result, in 2020, PrEP coverage for eligible White individuals was 61%, while coverage among eligible Black and Hispanic/Latino individuals was just 8% and 14%, respectively.26
Surveillance data comparing male and female PrEP coverage reveal further disparities between the sexes, with PrEP coverage for eligible female-at-birth patients estimated to be 9% compared with 25.8% for male-at-birth patients.26 The gap between the risk for HIV infection and the access to and uptake of PrEP coverage is most pronounced among Black women, for whom the rate of new HIV diagnosis is > 10 times higher than it is for White women, but who have some of the lowest awareness and utilization rates of all demographics.27
The rural population at risk. Disparities in HIV awareness and PrEP use also exist between rural and urban populations, as well as by health insurance status. Rural areas have been shown to lag behind urban areas in PrEP awareness and use. Two potential explanations for this disparity are differences in HIV- and drug use–associated stigma and health insurance status. Greater stigma against drug use and HIV in rural areas has been associated with lower rates of PrEP use.28
Individuals younger than 65 years in rural areas are less likely to have private health insurance and more likely to be uninsured compared with their urban counterparts, which may impact access to clinicians knowledgeable about PrEP.29 Notably, MSM who live in states that have expanded Medicaid have higher rates of PrEP use compared with MSM living in states that have not expanded Medicaid.30
Health insurers in the United States are required to cover PrEP medication, clinician visits, and associated blood work with no patient cost-sharing, although implementation barriers such as prior authorizations still exist.
Conclusion
Family physicians are well positioned to identify patients at risk for HIV infection, prescribe PrEP, organize comprehensive follow-up care, and partner with their health systems and local communities to reduce barriers to care. Those who can leverage existing relationships with local health departments, school-based health clinics, congregate housing programs, LGBTQIA+ advocacy groups, harm-reduction coalitions, and other community-based organizations to raise PrEP awareness play a critical role in preventing HIV transmission and reducing health care disparities in their communities.
CORRESPONDENCE
Andrew V.A. Foley, MD, MPH, Erie Family Health Centers, 2418 W Division Street, Chicago, IL 60622; andrewvafoley@gmail.com
More than 1.2 million Americans are living with HIV, and more than 30,000 new cases are diagnosed each year. While total incidence has declined since 2016, HIV remains a nationwide epidemic.1
Medications that prevent HIV acquisition, termed preexposure prophylaxis (PrEP), are an important tool to initiate in the primary care setting to reduce HIV transmission. However, while there are an estimated 1.2 million people eligible for PrEP, only 36% have received PrEP prescriptions.2 Several barriers that have impeded its widespread adoption include a lack of clinician knowledge and clinical resources for testing, high medication costs, and stigma around sexual health and intravenous (IV) drug use.
The value of PrEP
PrEP is chemoprophylaxis against the acquisition of HIV infection through the administration of an oral or injectable medication to people at risk for HIV. This practice began in the early 2000s, with the first oral regimen approved in 2012, and since has become an important tool in preventing HIV transmission.
When taken as prescribed, PrEP medications reduce the risk for acquiring HIV through sex by approximately 99% and can reduce the risk for acquiring HIV from injection drug use by approximately 74%.3 The US Preventive Services Task Force issued a Grade “A” recommendation to offer PrEP to people at high risk for HIV acquisition in June 2019 and reaffirmed it in a 2023 update.4
PrEP is notably distinct from postexposure prophylaxis (PEP), which is the administration of medication to prevent HIV infection after a possible exposure.
The available regimens
Regimens for PrEP include oral tablets or intramuscular (IM) injections.5 There are 3 PrEP regimens approved by the US Food and Drug Administration (FDA): tenofovir disoproxil fumarate/emtricitabine (Truvada), tenofovir alafenamide/emtricitabine (Descovy), and cabotegravir (Apretude).
Truvada is once-daily oral PrEP that was approved in 2012 and is now available in a generic formulation. Notable adverse effects of Truvada include a small negative impact on renal function and small reductions in bone mineral density; these have been noted in individual trials, but in meta-analyses such differences were not found to be statistically significant.6-8 The most common adverse effects of Truvada, experienced by up to 6% of patients, are gastrointestinal symptoms, fatigue, headache/dizziness, depression, and insomnia; most symptoms resolve within weeks.
Continue to: Descovy
Descovy is daily oral PrEP that was approved in 2019. Descovy is associated with increases in LDL and triglycerides but has less impact on renal and bone health.9 The most common adverse effect of Descovy, experienced by about 5% of patients, is diarrhea, followed by nausea.
Apretude was approved in 2021 and is a 600-mg IM injection given monthly for 2 months, then every 2 months (± 7 days). The advantages of Apretude are frequency and discreteness of dosing and the ability to use in patients with estimated creatinine clearance (eCrCl) > 15 mL/min. The most common adverse effects of Apretude are injection-site reactions, which occur in 30% to 80% of patients but are rarely significant enough to lead to discontinuation (< 2% of patients discontinue use due to injection-site reactions).10
Who should take PrEP?
The latest Centers for Disease Control and Prevention (CDC) guidelines recommend that all sexually active adults receive information about PrEP.5 Indications for PrEP are broad and summarized in the FIGURE.5

PrEP is indicated in patients who report sexual or injection drug use behaviors that place them at substantial ongoing risk for HIV exposure. Specific indications include patients with sexual partner(s) with unknown HIV status with whom they have inconsistent or no condom use, a history of bacterial sexually transmitted infection (STI) in the past 6 months, an HIV-positive sexual partner, or the sharing of injection drug equipment.
Hepatitis B infection is not a contraindication for PrEP use, but knowledge of infection status is essential. All current oral medications used for PrEP have activity against hepatitis B. Incomplete adherence to or abrupt discontinuation of oral PrEP could precipitate a hepatitis B flare. Hepatitis B surface antigen should be tested at the time of PrEP initiation, although PrEP can begin while testing is in process.
Continue to: How to use PrEP
How to use PrEP
At PrEP initiation, acute or chronic HIV infection must be excluded with a documented negative HIV antigen/antibody test within 1 week of prescribing PrEP.5 The CDC guidelines provide an updated HIV testing algorithm (www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2021.pdf, p 30-31, Figures 4a and 4b), which considers whether patients have received PrEP recently.
Patients with recent high-risk exposures or symptoms of acute HIV at the time of desired PrEP initiation should have an HIV-1 viral load checked with negative results before PrEP is prescribed. Additional criteria for PrEP include weight > 35 kg; screening for hepatitis B virus infection; screening for drug interactions; and drug-specific eCrCl cutoffs of > 60 mL/min for Truvada, > 30 mL/min for Descovy, and > 15 mL/min for Apretude.5
Studies regarding time to medication effectiveness are limited. Pharmacokinetic studies of Truvada demonstrate sufficient drug concentrations should be present in peripheral blood mononuclear cells and rectal tissue within 7 days of initiation of oral dosing and around 20 days in vaginal tissue.
Of note, while expedited partner therapy is used as a harm-reduction strategy to treat the sexual partners of patients diagnosed with certain STIs, PrEP is not recommended to be used in this way.
Ongoing monitoring with PrEP. Once oral PrEP is started, STI risk assessment and HIV testing via 4th generation antibody/antigen test should be completed at least every 3 months. PrEP oral prescription refills should be limited to 3 months. For patients receiving IM PrEP (Apretude), HIV testing via viral load and antibody/antigen testing should be done at the time of each injection (every 2 months).5
Continue to: With oral PrEP...
With oral PrEP, renal function should be checked every 6 months in patients older than 50 years or those with eCrCl < 90 mL/min at initiation. For patients younger than 50 years with no baseline renal dysfunction, the latest guidelines now recommend monitoring every 12 months instead of 6 months.5
For patients on Descovy, a lipid panel is recommended at PrEP initiation and every 12 months. Testing for other STIs can be considered on this schedule, based on clinical assessment. The TABLE5 summarizes recommended monitoring for patients taking oral PrEP.

Recommended follow-up provides an opportunity to have frequent contact with a potentially high-risk population, and PrEP should be one part of a comprehensive HIV prevention and risk reduction plan. Many patients at high risk for HIV acquisition may benefit from frequent follow-up to address screening, referral, and treatment of substance use disorders, mental health conditions, and chronic medical conditions (including hepatitis C infection) and provide ongoing preventive health care.
Special uses of PrEP
Same-day PrEP. Starting PrEP on the day of the initial appointment may be appropriate based on patient risk factors and barriers to care, such as a high risk for contracting HIV before the subsequent appointment for a prescription of PrEP or an inability to return to the clinic in a timely fashion due to transportation or work constraints, or clinician availability. For these patients, assuming there is a low concern for acute or chronic HIV infection, PrEP can be initiated on the day of the initial visit.5
In these cases, point-of-care HIV and creatinine testing with same-day results should be completed. Antigen/antibody fingerstick testing or HIV-1 RNA test are preferred; oral fluid HIV testing should not be used for same-day PrEP due to its lower sensitivity for HIV detection. If same-day testing is unavailable, blood should be drawn at the visit so that HIV and creatinine testing can be completed as soon as possible.
Continue to: In addition to initial laboratory testing...
In addition to initial laboratory testing, clinics offering same-day PrEP should be able to provide: (1) assistance for patients to enroll in health insurance or a medication assistance program (eg, Ready, Set, PrEP) for those ineligible for insurance coverage, (2) rapid follow-up on all laboratory results with reliable patient contact information, and (3) follow-up appointments with clinicians able to prescribe and administer PrEP medications.
Off-label “on-demand” PrEP. An off-label treatment regimen for men who have sex with men (MSM) is termed “on-demand” PrEP or “2-1-1 PrEP” and is included in the CDC guidelines for consideration by clinicians.5 This alternative dosing schedule can be used for individuals who have sex less frequently and in a more planned fashion.
On-demand PrEP requires a patient to take 2 tablets of Truvada 2 to 24 hours before sex, followed by 1 tablet 24 hours and 1 tablet 48 hours after sexual activity. If a sexual act occurs at 48 hours, the patient should extend the daily dose for 48 additional hours, such that PrEP is always used daily for 48 hours after the last sex act.
This method has been studied with Truvada in MSM in Europe and Canada through the IPERGAY and PREVENIR trials and shown to have ≥ 86% efficacy in preventing HIV acquisition.11,12 The only US-based study showed lower efficacy; however, based on the currently available data, the International Antiviral Society-USA Panel has recommended it as an alternative regimen.13,14
PrEP via telehealth. Visits for PrEP initiation and continuation can be completed via telehealth.5 Patients then can complete necessary laboratory tests by going to a physical laboratory location or using mailed specimen kits in which they can self-collect urine, oral/rectal swabs, and fingerstick blood samples.
Continue to: PrEP use in specific populations
PrEP use in specific populations
Adolescents
Truvada, Descovy, and Apretude all are now approved for use in adolescents weighing ≥ 35 kg. Two important considerations when prescribing to this population are the effects of Truvada on bone health and the unique barriers to access.
In studies of adolescent MSM using Truvada for PrEP, bone mineral density declined, especially among those ages 15 to 19 years.15 As such, the clinical impact of decreased bone mineral density should be weighed against the risk for HIV acquisition; however, bone mineral density monitoring is not recommended in the current guidelines. CDC guidelines suggest considering Descovy for male adolescents given its potential lower impact on bone mineral density.5
Confidentiality and legal issues exist when prescribing PrEP to minors. In terms of parental/guardian involvement, clinicians who are prescribing PrEP for patients younger than 18 years should consult the CDC website for guidance on local and state regulations that govern prescribing and confidentiality (www.cdc.gov/hiv/policies/law/states/minors.html).
Insurance billing statements may lead to inadvertent disclosure of a minor’s decision to take PrEP to their legal guardian.16 Generic Truvada costs less than $100 for a 3-month supply when using goodrx.com, which may offer an alternative to insurance for medication payment.
Peripartum patients
The increased risk for HIV acquisition in the peripartum period for female patients is well documented.17 Guidelines recommend offering PrEP with Truvada to female patients at risk for conception, currently pregnant, or breastfeeding when that patient’s partner has HIV and the partner’s viral load is unknown or detectable. Descovy is not recommended for pregnant or breastfeeding patients.5 Cabotegravir-containing regimens (Apretude) have not been approved by the FDA for pregnant or breastfeeding patients.5
Continue to: Data on the impact of...
Data on the impact of Truvada for PrEP on fetal health are still emerging. A large study in Kenya showed no significant differences in preterm birth, low birth weight, or early infant growth, and a randomized, noninferiority trial in South Africa showed no association between Truvada for PrEP and preterm birth or the birth of small-for-gestational-age infants.18,19 There are no definitive studies of breastfeeding infants exposed to Truvada, but data from previous trials of breastfeeding mothers who were taking the individual components that are combined in the Truvada pill indicated there is minimal medication exposure to the infant.5
PrEP studies in the peripartum period to date have been conducted exclusively among cisgender women, and data do not yet reflect the experiences of transgender men, genderqueer people, and nonbinary individuals in the peripartum period.5
Transgender people
Transgender women should be strongly considered candidates for PrEP as they are at an extremely high risk for HIV acquisition. The most recent National HIV Behavioral Surveillance survey found that approximately 42% of transgender women were living with HIV.20 The survey revealed stark racial and ethnic disparities among transgender women living with HIV: 62% identified as Black/African American, compared with 35% Hispanic/Latina and 17% White.20
Transgender women report high rates of sexual assault, unprotected receptive anal sex, commercial sex work, homelessness, mental health disorders, and substance use, putting them at increased risk for HIV acquisition.21 However, transgender women are less likely to have discussed PrEP with a clinician, are less likely to be on PrEP even when interested in starting, and have higher rates of medication nonadherence compared with cisgender MSM.21,22 PrEP has not been found to decrease levels of feminizing hormones; however, studies are mixed as to whether feminizing hormones decrease Truvada concentrations in rectal mucosa, so clinicians should emphasize the importance of daily medication adherence.23
Transgender men have not been included in any PrEP trials, so no specific recommendations are available.
Continue to: Disparities in PrEP access and use exist
Disparities in PrEP access and use exist
The lifetime risk for HIV acquisition is 9% among White MSM, 50% among Black MSM, and 20% among Hispanic MSM.24 Despite this large disparity in disease burden, Black and Hispanic individuals are less likely to be aware of PrEP, have discussed PrEP with a health care professional, or used PrEP compared with their White counterparts.25 As a result, in 2020, PrEP coverage for eligible White individuals was 61%, while coverage among eligible Black and Hispanic/Latino individuals was just 8% and 14%, respectively.26
Surveillance data comparing male and female PrEP coverage reveal further disparities between the sexes, with PrEP coverage for eligible female-at-birth patients estimated to be 9% compared with 25.8% for male-at-birth patients.26 The gap between the risk for HIV infection and the access to and uptake of PrEP coverage is most pronounced among Black women, for whom the rate of new HIV diagnosis is > 10 times higher than it is for White women, but who have some of the lowest awareness and utilization rates of all demographics.27
The rural population at risk. Disparities in HIV awareness and PrEP use also exist between rural and urban populations, as well as by health insurance status. Rural areas have been shown to lag behind urban areas in PrEP awareness and use. Two potential explanations for this disparity are differences in HIV- and drug use–associated stigma and health insurance status. Greater stigma against drug use and HIV in rural areas has been associated with lower rates of PrEP use.28
Individuals younger than 65 years in rural areas are less likely to have private health insurance and more likely to be uninsured compared with their urban counterparts, which may impact access to clinicians knowledgeable about PrEP.29 Notably, MSM who live in states that have expanded Medicaid have higher rates of PrEP use compared with MSM living in states that have not expanded Medicaid.30
Health insurers in the United States are required to cover PrEP medication, clinician visits, and associated blood work with no patient cost-sharing, although implementation barriers such as prior authorizations still exist.
Conclusion
Family physicians are well positioned to identify patients at risk for HIV infection, prescribe PrEP, organize comprehensive follow-up care, and partner with their health systems and local communities to reduce barriers to care. Those who can leverage existing relationships with local health departments, school-based health clinics, congregate housing programs, LGBTQIA+ advocacy groups, harm-reduction coalitions, and other community-based organizations to raise PrEP awareness play a critical role in preventing HIV transmission and reducing health care disparities in their communities.
CORRESPONDENCE
Andrew V.A. Foley, MD, MPH, Erie Family Health Centers, 2418 W Division Street, Chicago, IL 60622; andrewvafoley@gmail.com
1. CDC. Estimated HIV incidence and prevalence in the United States 2017–2021. HIV Surveill Supplemental Rep. 2023;28. Accessed October 23, 2023. https://www.cdc.gov/hiv/library/reports/hiv-surveillance/vol-28-no-3/index.html
2. CDC. Core indicators for monitoring the Ending the HIV Epidemic initiative (preliminary data): National HIV Surveillance System data reported through March 2023; and preexposure prophylaxis (PrEP) data reported through December 2022. HIV Surveill Data Tables. 2023;4. Published June 2023. Accessed October 23, 2023. https://www.cdc.gov/hiv/library/reports/surveillance-data-tables/
3. CDC. Division of HIV Prevention, National Center for HIV, Viral Hepatitis, STD, and TB Prevention. PrEP effectiveness. Updated June 2022. Accessed October 23, 2023. https://www.cdc.gov/hiv/basics/prep/prep-effectiveness.html
4. US Preventive Services Task Force. Prevention of acquisition of HIV: preexposure prophylaxis. Final recommendation statement. August 22, 2023. Accessed October 23, 2023. https://uspreventiveservicestaskforce.org/uspstf/recommendation/prevention-of-human-immunodeficiency-virus-hiv-infection-pre-exposure-prophylaxis
5. CDC. Preexposure prophylaxis for the prevention of HIV infection in the United States—2021 update: a clinical practice guideline. Accessed October 23, 2023. www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2021.pdf
6. Mugwanya KK, Wyatt C, Celum C, et al. Changes in glomerular kidney function among HIV-1-uninfected men and women receiving emtricitabine-tenofovir disoproxil fumarate preexposure prophylaxis: a randomized clinical trial. JAMA Intern Med. 2015;175:246-254. doi: 10.1001/jamainternmed.2014.6786
7. Havens PL, Stephensen CB, Van Loan MD, et al. Decline in bone mass with tenofovir disoproxil fumarate/emtricitabine is associated with hormonal changes in the absence of renal impairment when used by HIV-uninfected adolescent boys and young men for HIV preexposure prophylaxis. Clin Infect Dis. 2017;64:317-325. doi: 10.1093/cid/ciw765
8. Pilkington V, Hill A, Hughes S, et al. How safe is TDF/FTC as PrEP? A systematic review and meta-analysis of the risk of adverse events in 13 randomised trials of PrEP. J Virus Erad. 2018;4:215-224.
9. Mayer KH, Molina JM, Thompson MA, et al. Emtricitabine and tenofovir alafenamide vs emtricitabine and tenofovir disoproxil fumarate for HIV pre-exposure prophylaxis (DISCOVER): primary results from a randomised, double-blind, multicentre, active-controlled, phase 3, non-inferiority trial. Lancet. 2020;396:239-254. doi: 10.1016/S0140-6736(20)31065-5
10. Liegeon G, Ghosn, J. Long-acting injectable cabotegravir for PrEP: a game-changer in HIV prevention. HIV Med. 2022;24:653-663. doi: 10.1111/hiv.13451
11. Molina JM, Capitant C, Spire B, et al. On-demand preexposure prophylaxis in men at high risk for HIV-1 infection. N Engl J Med. 2015;373:2237-2246. doi: 10.1056/NEJMoa1506273
12. Molina JM, Ghosn J, Assoumou L, et al. Daily and on-demand HIV pre-exposure prophylaxis with emtricitabine and tenofovir disoproxil (ANRS PREVENIR): a prospective observational cohort study. Lancet HIV. 2022;9:e554-e562. doi: 10.1016/S2352-3018(22)00133-3
13. Dimitrov D, Moore JR, Wood D, et al. Predicted effectiveness of daily and nondaily preexposure prophylaxis for men who have sex with men based on sex and pill-taking patterns from the Human Immuno Virus Prevention Trials Network 067/ADAPT Study. Clin Infect Dis. 2020;71:249-255. doi: 10.1093/cid/ciz799
14. Saag MS, Gandhi RT, Hoy JF, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2020 recommendations of the International Antiviral Society-USA Panel. JAMA. 2020;324:1651-1669. doi: 10.1001/jama.2020.17025
15. Havens PL, Perumean-Chaney SE, Patki A, et al. Changes in bone mass after discontinuation of preexposure prophylaxis with tenofovir disoproxil fumarate/emtricitabine in young men who have sex with men: extension phase results of Adolescent Trials Network Protocols 110 and 113. Clin Infect Dis. 2020;70:687-691. doi: 10.1093/cid/ciz486
16. Neilan AM, Salvant Valentine S, Knopf AS. Case 27-2021: a 16-year-old boy seeking human immunodeficiency virus prophylaxis. N Engl J Med. 2021;385:1034-1041. doi: 10.1056/NEJMcpc1909626
17. Thomson KA, Hughes J, Baeten JM, et al. Increased risk of HIV acquisition among women throughout pregnancy and during the postpartum period: a prospective per-coital-act analysis among women with HIV-infected partners. J Infect Dis. 2018;218:16-25. doi: 10.1093/infdis/jiy113
18. Dettinger JC, Kinuthia J, Pintye J, et al. Perinatal outcomes following maternal pre-exposure prophylaxis (PrEP) use during pregnancy: results from a large PrEP implementation program in Kenya. J Int AIDS Soc. 2019;22:e25378. doi: 10.1002/jia2.25378
19. Moodley D, Lombard C, Govender V, et al. Pregnancy and neonatal safety outcomes of timing of initiation of daily oral tenofovir disoproxil fumarate and emtricitabine pre-exposure prophylaxis for HIV prevention (CAP016): an open-label, randomised, non-inferiority trial. Lancet HIV. 2023;10:e154-e163. doi: 10.1016/S2352-3018(22)00369-1
20. CDC. HIV Infection, Risk, Prevention, and Testing Behaviors Among Transgender Women—National HIV Behavioral Surveillance, 7 U.S. Cities, 2019–2020. HIV Surveillance Special Report 27. April 2021. Accessed October 23, 2023. www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-special-report-number-27.pdf
21. Wilson EC, Turner CM, Arayasirikul S, et al. Disparities in the PrEP continuum for trans women compared to MSM in San Francisco, California: results from population-based cross-sectional behavioural surveillance studies. J Int AIDS Soc. 2020;23:e25539. doi: 10.1002/jia2.25539
22. Poteat T, Wirtz A, Malik M, et al. A gap between willingness and uptake: findings from mixed methods research on HIV prevention among Black and Latina transgender women. J Acquir Immune Defic Syndr. 2019;82:131-140. doi: 10.1097/QAI.0000000000002112
23. Cottrell ML, Prince HM, Schauer AP, et al. Decreased tenofovir diphosphate concentrations in a transgender female cohort: implications for human immunodeficiency virus preexposure prophylaxis. Clin Infect Dis. 2019;69:2201-2204. doi: 10.1093/cid/ciz290
24. Hess KL, Hu X, Lansky A, et al. Lifetime risk of a diagnosis of HIV infection in the United States. Ann Epidemiol. 2017;27:238-243. doi: 10.1016/j.annepidem.2017.02.003
25. Kanny D, Jeffries WL 4th, Chapin-Bardales J, et al. Racial/ethnic disparities in HIV preexposure prophylaxis among men who have sex with men—23 urban areas, 2017. MMWR Morb Mortal Wkly Rep. 2019;68:801-806. doi: 10.15585/mmwr.mm6837a2
26. CDC. Core indicators for monitoring the Ending the HIV Epidemic initiative (early release): National HIV Surveillance System data reported through December 2020; and preexposure prophylaxis (PrEP) data reported through September 2020. HIV Surveill Data Tables. 2021;2. Accessed October 23, 2023. www.cdc.gov/hiv/pdf/library/reports/surveillance-data-tables/vol-2-no-2/cdc-hiv-surveillance-tables-vol-2-no-2.pdf
27. CDC. Diagnoses of HIV infection in the United States and dependent areas 2021: special focus profiles. Updated May 23, 2023. Accessed October 23, 2023. www.cdc.gov/hiv/library/reports/hiv-surveillance/vol-34/content/special-focus-profiles.html
28. Walters SM, Frank D, Van Ham B, et al. PrEP care continuum engagement among persons who inject drugs: rural and urban differences in stigma and social infrastructure. AIDS Behav. 2021;26:1308-1320. doi: 10.1007/s10461-021-03488-2
29. Foutz J, Artiga S, Garfield R. The role of Medicaid in rural America [issue brief]. April 25, 2017. Accessed August 16, 2023. www.kff.org/medicaid/issue-brief/the-role-of-medicaid-in-rural-america/
30. Baugher AR, Finlayson T, Lewis R, et al. Health care coverage and preexposure prophylaxis (PrEP) use among men who have sex with men living in 22 US cities with vs without Medicaid expansion, 2017. Am J Public Health. 2021;111:743-751. doi: 10.2105/AJPH.2020.306035
1. CDC. Estimated HIV incidence and prevalence in the United States 2017–2021. HIV Surveill Supplemental Rep. 2023;28. Accessed October 23, 2023. https://www.cdc.gov/hiv/library/reports/hiv-surveillance/vol-28-no-3/index.html
2. CDC. Core indicators for monitoring the Ending the HIV Epidemic initiative (preliminary data): National HIV Surveillance System data reported through March 2023; and preexposure prophylaxis (PrEP) data reported through December 2022. HIV Surveill Data Tables. 2023;4. Published June 2023. Accessed October 23, 2023. https://www.cdc.gov/hiv/library/reports/surveillance-data-tables/
3. CDC. Division of HIV Prevention, National Center for HIV, Viral Hepatitis, STD, and TB Prevention. PrEP effectiveness. Updated June 2022. Accessed October 23, 2023. https://www.cdc.gov/hiv/basics/prep/prep-effectiveness.html
4. US Preventive Services Task Force. Prevention of acquisition of HIV: preexposure prophylaxis. Final recommendation statement. August 22, 2023. Accessed October 23, 2023. https://uspreventiveservicestaskforce.org/uspstf/recommendation/prevention-of-human-immunodeficiency-virus-hiv-infection-pre-exposure-prophylaxis
5. CDC. Preexposure prophylaxis for the prevention of HIV infection in the United States—2021 update: a clinical practice guideline. Accessed October 23, 2023. www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2021.pdf
6. Mugwanya KK, Wyatt C, Celum C, et al. Changes in glomerular kidney function among HIV-1-uninfected men and women receiving emtricitabine-tenofovir disoproxil fumarate preexposure prophylaxis: a randomized clinical trial. JAMA Intern Med. 2015;175:246-254. doi: 10.1001/jamainternmed.2014.6786
7. Havens PL, Stephensen CB, Van Loan MD, et al. Decline in bone mass with tenofovir disoproxil fumarate/emtricitabine is associated with hormonal changes in the absence of renal impairment when used by HIV-uninfected adolescent boys and young men for HIV preexposure prophylaxis. Clin Infect Dis. 2017;64:317-325. doi: 10.1093/cid/ciw765
8. Pilkington V, Hill A, Hughes S, et al. How safe is TDF/FTC as PrEP? A systematic review and meta-analysis of the risk of adverse events in 13 randomised trials of PrEP. J Virus Erad. 2018;4:215-224.
9. Mayer KH, Molina JM, Thompson MA, et al. Emtricitabine and tenofovir alafenamide vs emtricitabine and tenofovir disoproxil fumarate for HIV pre-exposure prophylaxis (DISCOVER): primary results from a randomised, double-blind, multicentre, active-controlled, phase 3, non-inferiority trial. Lancet. 2020;396:239-254. doi: 10.1016/S0140-6736(20)31065-5
10. Liegeon G, Ghosn, J. Long-acting injectable cabotegravir for PrEP: a game-changer in HIV prevention. HIV Med. 2022;24:653-663. doi: 10.1111/hiv.13451
11. Molina JM, Capitant C, Spire B, et al. On-demand preexposure prophylaxis in men at high risk for HIV-1 infection. N Engl J Med. 2015;373:2237-2246. doi: 10.1056/NEJMoa1506273
12. Molina JM, Ghosn J, Assoumou L, et al. Daily and on-demand HIV pre-exposure prophylaxis with emtricitabine and tenofovir disoproxil (ANRS PREVENIR): a prospective observational cohort study. Lancet HIV. 2022;9:e554-e562. doi: 10.1016/S2352-3018(22)00133-3
13. Dimitrov D, Moore JR, Wood D, et al. Predicted effectiveness of daily and nondaily preexposure prophylaxis for men who have sex with men based on sex and pill-taking patterns from the Human Immuno Virus Prevention Trials Network 067/ADAPT Study. Clin Infect Dis. 2020;71:249-255. doi: 10.1093/cid/ciz799
14. Saag MS, Gandhi RT, Hoy JF, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2020 recommendations of the International Antiviral Society-USA Panel. JAMA. 2020;324:1651-1669. doi: 10.1001/jama.2020.17025
15. Havens PL, Perumean-Chaney SE, Patki A, et al. Changes in bone mass after discontinuation of preexposure prophylaxis with tenofovir disoproxil fumarate/emtricitabine in young men who have sex with men: extension phase results of Adolescent Trials Network Protocols 110 and 113. Clin Infect Dis. 2020;70:687-691. doi: 10.1093/cid/ciz486
16. Neilan AM, Salvant Valentine S, Knopf AS. Case 27-2021: a 16-year-old boy seeking human immunodeficiency virus prophylaxis. N Engl J Med. 2021;385:1034-1041. doi: 10.1056/NEJMcpc1909626
17. Thomson KA, Hughes J, Baeten JM, et al. Increased risk of HIV acquisition among women throughout pregnancy and during the postpartum period: a prospective per-coital-act analysis among women with HIV-infected partners. J Infect Dis. 2018;218:16-25. doi: 10.1093/infdis/jiy113
18. Dettinger JC, Kinuthia J, Pintye J, et al. Perinatal outcomes following maternal pre-exposure prophylaxis (PrEP) use during pregnancy: results from a large PrEP implementation program in Kenya. J Int AIDS Soc. 2019;22:e25378. doi: 10.1002/jia2.25378
19. Moodley D, Lombard C, Govender V, et al. Pregnancy and neonatal safety outcomes of timing of initiation of daily oral tenofovir disoproxil fumarate and emtricitabine pre-exposure prophylaxis for HIV prevention (CAP016): an open-label, randomised, non-inferiority trial. Lancet HIV. 2023;10:e154-e163. doi: 10.1016/S2352-3018(22)00369-1
20. CDC. HIV Infection, Risk, Prevention, and Testing Behaviors Among Transgender Women—National HIV Behavioral Surveillance, 7 U.S. Cities, 2019–2020. HIV Surveillance Special Report 27. April 2021. Accessed October 23, 2023. www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-special-report-number-27.pdf
21. Wilson EC, Turner CM, Arayasirikul S, et al. Disparities in the PrEP continuum for trans women compared to MSM in San Francisco, California: results from population-based cross-sectional behavioural surveillance studies. J Int AIDS Soc. 2020;23:e25539. doi: 10.1002/jia2.25539
22. Poteat T, Wirtz A, Malik M, et al. A gap between willingness and uptake: findings from mixed methods research on HIV prevention among Black and Latina transgender women. J Acquir Immune Defic Syndr. 2019;82:131-140. doi: 10.1097/QAI.0000000000002112
23. Cottrell ML, Prince HM, Schauer AP, et al. Decreased tenofovir diphosphate concentrations in a transgender female cohort: implications for human immunodeficiency virus preexposure prophylaxis. Clin Infect Dis. 2019;69:2201-2204. doi: 10.1093/cid/ciz290
24. Hess KL, Hu X, Lansky A, et al. Lifetime risk of a diagnosis of HIV infection in the United States. Ann Epidemiol. 2017;27:238-243. doi: 10.1016/j.annepidem.2017.02.003
25. Kanny D, Jeffries WL 4th, Chapin-Bardales J, et al. Racial/ethnic disparities in HIV preexposure prophylaxis among men who have sex with men—23 urban areas, 2017. MMWR Morb Mortal Wkly Rep. 2019;68:801-806. doi: 10.15585/mmwr.mm6837a2
26. CDC. Core indicators for monitoring the Ending the HIV Epidemic initiative (early release): National HIV Surveillance System data reported through December 2020; and preexposure prophylaxis (PrEP) data reported through September 2020. HIV Surveill Data Tables. 2021;2. Accessed October 23, 2023. www.cdc.gov/hiv/pdf/library/reports/surveillance-data-tables/vol-2-no-2/cdc-hiv-surveillance-tables-vol-2-no-2.pdf
27. CDC. Diagnoses of HIV infection in the United States and dependent areas 2021: special focus profiles. Updated May 23, 2023. Accessed October 23, 2023. www.cdc.gov/hiv/library/reports/hiv-surveillance/vol-34/content/special-focus-profiles.html
28. Walters SM, Frank D, Van Ham B, et al. PrEP care continuum engagement among persons who inject drugs: rural and urban differences in stigma and social infrastructure. AIDS Behav. 2021;26:1308-1320. doi: 10.1007/s10461-021-03488-2
29. Foutz J, Artiga S, Garfield R. The role of Medicaid in rural America [issue brief]. April 25, 2017. Accessed August 16, 2023. www.kff.org/medicaid/issue-brief/the-role-of-medicaid-in-rural-america/
30. Baugher AR, Finlayson T, Lewis R, et al. Health care coverage and preexposure prophylaxis (PrEP) use among men who have sex with men living in 22 US cities with vs without Medicaid expansion, 2017. Am J Public Health. 2021;111:743-751. doi: 10.2105/AJPH.2020.306035
PRACTICE RECOMMENDATIONS
› Perform routine screening of patients for preexposure prophylaxis (PrEP) eligibility. B
› Prescribe oral or intramuscular PrEP for eligible patients after screening for HIV, other sexually transmitted infections, and hepatitis B, and establishing baseline renal function. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
An FP’s guide to caring for patients with seizure and epilepsy
Managing first-time seizures and epilepsy often requires consultation with a neurologist or epileptologist for diagnosis and subsequent management, including when medical treatment fails or in determining whether patients may benefit from surgery. However, given the high prevalence of epilepsy and even higher incidence of a single seizure, family physicians contribute significantly to the management of these patients. The main issues are managing a first-time seizure, making the diagnosis, establishing a treatment plan, and exploring triggers and mitigating factors.
Seizure vs epilepsy
All patients with epilepsy experience seizures, but not every person who experiences a seizure has (or will develop) epilepsy. Nearly 10% of the population has one seizure during their lifetime,whereas the risk for epilepsy is just 3%.1 Therefore, a first-time seizure may not herald epilepsy, defined as repetitive (≥ 2) unprovoked seizures more than 24 hours apart.2 Seizures can be provoked (acute symptomatic) or unprovoked; a clear distinction between these 2 occurrences—as well as between single and recurrent seizures—is critical for proper management. A close look at the circumstances of a first-time seizure is imperative to define the nature of the event and the possibility of further seizures before devising a treatment plan.
Provoked seizures are due to an acute brain insult such as toxic-metabolic disorders, concussion, alcohol withdrawal, an adverse effect of a medication or its withdrawal, or photic stimulation presumably by disrupting the brain’s metabolic homeostasis or integrity. The key factor is that provoked seizures always happen in close temporal association with an acute insult. A single provoked seizure happens each year in 29 to 39 individuals per 100,000.3 While these seizures typically occur singly, there is a small risk they may recur if the triggering insult persists or repeats.1 Therefore, more than 1 seizure per se may not indicate epilepsy.3
Unprovoked seizures reflect an underlying brain dysfunction. A single unprovoked seizure happens in 23 to 61 individuals per 100,000 per year, often in men in either younger or older age groups.3 Unprovoked seizures may occur only once or may recur (ie, evolve into epilepsy). The latter scenario happens in only about half of cases; the overall risk for a recurrent seizure within 2 years of a first seizure is estimated at 42% (24% to 65%, depending on the etiology and electroencephalogram [EEG] findings).4 More specifically, without treatment the relapse rate will be 36% at 1 year and 47% at 2 years.4 Further, a second unprovoked seizure, if untreated, would increase the risk for third and fourth seizures to 73% and 76%, respectively, within 4 years.3
Evaluating the first-time seizure
Ask the patient or observers about the circumstances of the event to differentiate provoked from unprovoked onset. For one thing, not all “spells” are seizures. The differential diagnoses may include syncope, psychogenic nonepileptic events, drug intoxication or withdrawal, migraine, panic attacks, sleep disorders (parasomnia), transient global amnesia, concussion, and transient ischemic attack. EEG, neuroimaging, and other relevant diagnostic tests often are needed (eg, electrocardiogram/echocardiogram/Holter monitoring to evaluate for syncope/cardiac arrhythmia). Clinically, syncopal episodes tend to be brief with rapid recovery and no confusion, speech problems, aura, or lateralizing signs such as hand posturing or lip smacking that are typical with focal seizures. However, cases of convulsive syncope can be challenging to assess without diagnostic tests.
True convulsive seizures do not have the variability in clinical signs seen with psychogenic nonepileptic events (eg, alternating body parts involved or direction of movements). Transient global amnesia is a rare condition with no established diagnostic test and is considered a diagnosis of exclusion, although bitemporal hyperintensities on magnetic resonance imaging (MRI) may appear 12 to 48 hours after the clinical episode.5 Blood work is needed in patients with medical issues treated with multiple medications to evaluate for metabolic derangements; otherwise, routine blood work provides minimal information in stable patients.
Region-specific causes. Neurocysticercosis is common in some regions, such as Latin America; therefore, attention should be paid to this aspect of patient history.
Continue to: Is it really a first-time seizure?
Is it really a first-time seizure? A “first,” usually dramatic, generalized tonic-clonic seizure that triggers the diagnostic work-up may not be the very first seizure. Evidence suggests that many patients have experienced prior undiagnosed seizures. Subtle prior events often missed include episodes of deja vu, transient feelings of fear or unusual smells, speech difficulties, staring spells, or myoclonic jerks.1 A routine EEG to record epileptiform discharges and a high-resolution brain MRI to rule out any intracranial pathology are indicated. However, if the EEG indicates a primary generalized (as opposed to focal-onset) epilepsy, a brain MRI may not be needed. If a routine EEG is unrevealing, long-term video-EEG monitoring may be needed to detect an abnormality.
Accuracy of EEG and MRI. Following a first unprovoked seizure, routine EEG to detect epileptiform discharges in adults has yielded a sensitivity of 17.3% and specificity of 94.7%. In evaluating children, these values are 57.8% and 69.6%, respectively.6 If results are equivocal, a 24-hour EEG can increase the likelihood of detecting epileptiform discharges to 89% of patients.7 Brain MRI may detect an abnormality in 12% to 14% of patients with newly diagnosed epilepsy, and in up to 80% of those with recurrent seizures.8 In confirming hippocampus sclerosis, MRI has demonstrated a sensitivity of 93% and specificity of 86%.9
When to treat a first-time seizure. Available data and prediction models identify risk factors that would help determine whether to start an antiseizure medication after a first unprovoked seizure:
Epilepsy diagnosis
The International League Against Epilepsy (ILAE) previously defined epilepsy as 2 unprovoked seizures more than 24 hours apart. However, a more recent ILAE task force modified this definition: even a single unprovoked seizure would be enough to diagnose epilepsy if there is high probability of further seizures—eg, in the presence of definitive epileptiform discharges on EEG or presence of a brain tumor or a remote brain insult on imaging, since such conditions induce an enduring predisposition to generate epileptic seizures. 2 Also, a single unprovoked seizure is enough to diagnose epilepsy if it is part of an epileptic syndrome such as juvenile myoclonic epilepsy. Further, a time limit was added to the definition—ie, epilepsy is considered resolved if a patient remains seizure free for 10 years without use of antiseizure medications during the past 5 years. However, given the multitude of variables and evidence, the task force acknowledged the need for individualized considerations. 2
Seizure classification
Classification of seizure type is based on the site of seizure onset and its spread pattern—ie, focal, generalized, or unknown onset.
Continue to: Focal-onset seizures
Focal-onset seizures originate “within networks limited to one hemisphere,” although possibly in more than 1 region (ie, multifocal, and presence or absence of loss of awareness). 12 Focal seizures may then be further classified into “motor onset” or “nonmotor onset” (eg, autonomic, emotional, sensory). 2
Generalized seizures are those “originating at some point within, and rapidly engaging, bilaterally distributed networks.” 13 Unlike focal-onset seizures, generalized seizures are not classified based on awareness, as most generalized seizures involve loss of awareness (absence) or total loss of consciousness (generalized tonic-clonic). They are instead categorized based on the presence of motor vs nonmotor features (eg, tonic-clonic, myoclonic, atonic). Epilepsy classification is quite dynamic and constantly updated based on new genetic, electroencephalographic, and neuroimaging discoveries.
Treatment of epilepsy
Antiseizure medications
Treatment with antiseizure medications (ASMs; formerly known as antiepileptic drugs ) is the mainstay of epilepsy management. Achieving efficacy (seizure freedom) and tolerability (minimal adverse effects) are the primary goals of treatment. Factors that should govern the selection of an ASM include the seizure type/epilepsy syndrome, adverse effect profile of the ASM, pharmacodynamic/pharmacokinetic considerations, and patient comorbidities.
The Standard and New Antiepileptic Drugs (SANAD I and II) trials provide data from direct, unblinded, and longitudinal comparisons of existing and new ASMs and their utility in different seizure types. In the SANAD I cohort of patients with generalized and unclassified epilepsies, valproate was superior to lamotrigine and topiramate for 12-month remission and treatment failure rates, respectively.14 However, valproate generally is avoided in women of childbearing age due its potential adverse effects during pregnancy. In focal epilepsies, lamotrigine was superior to carbamazepine, gabapentin, and topiramate with respect to treatment failure, and noninferior to carbamazepine for 12-month remission.15 In the SANAD II trial, levetiracetam was noninferior to valproate for incidence of adverse events in patients with generalized and unclassified epilepsies although was found to be neither more clinically effective nor more cost effective.16 For patients of childbearing potential with generalized and unclassified epilepsies, there is evidence to support the safe and effective use of levetiracetam.17In focal epilepsies, lamotrigine was superior to levetiracetam and zonisamide with respect to treatment failures and adverse events and was noninferior to zonisamide for 12-month remission.18 In summary, levetiracetam and valproate (not to be used in women of childbearing potential) are considered appropriate first-line agents for generalized and unclassified epilepsies while lamotrigine is deemed an appropriate first-line agent for focal epilepsies (TABLE 119-28).
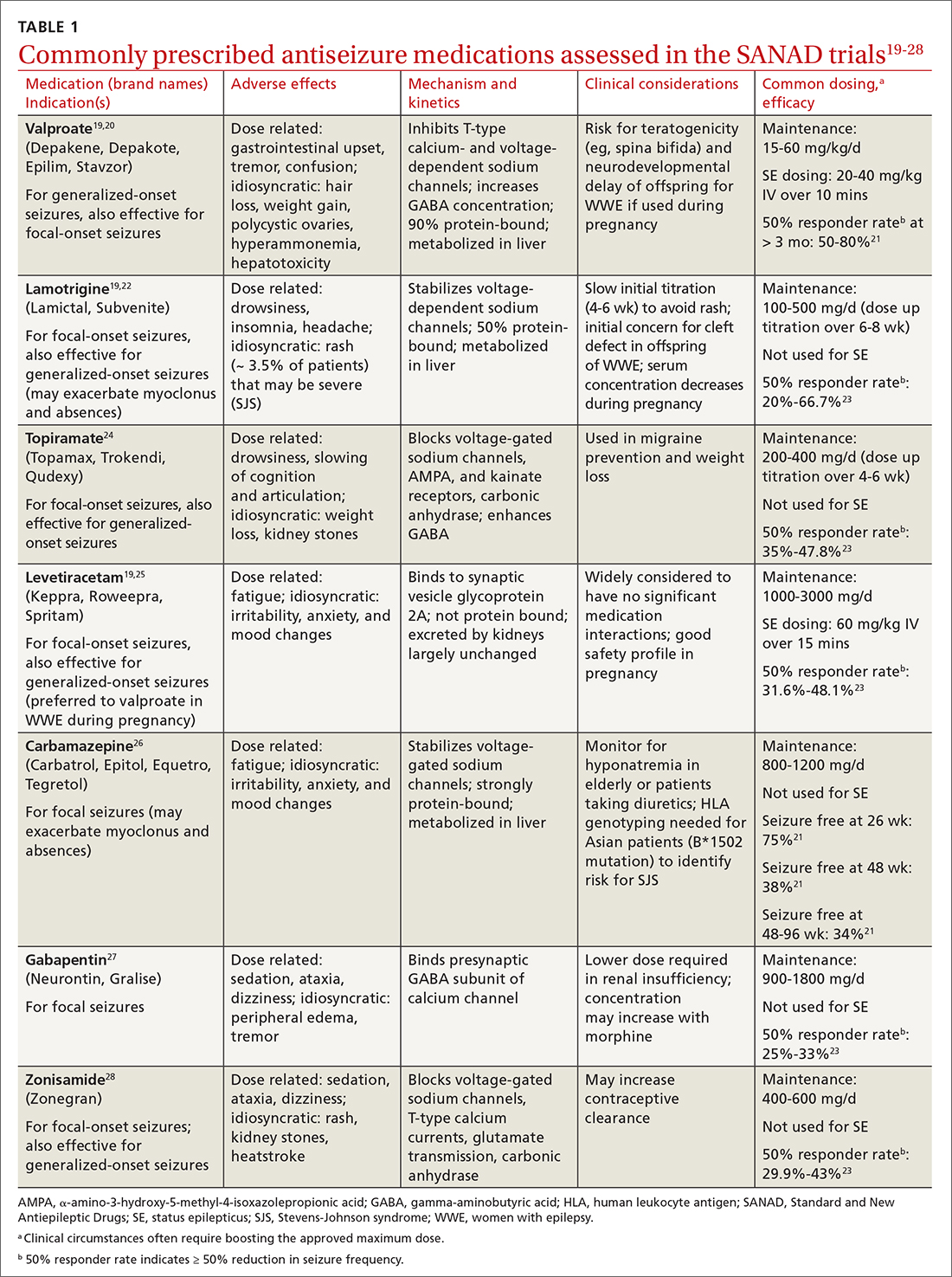
Drug level monitoring. It is standard practice to periodically monitor serum levels in patients taking first-generation ASMs such as phenytoin, carbamazepine, phenobarbital, and valproic acid because of their narrow therapeutic range and the potential for overdose or interaction with other medications or foods (eg, grapefruit juice may increase carbamazepine serum level by inhibiting CYP3A4, the enzyme that metabolizes the drug). Patients taking newer ASMs may not require regular serum level monitoring except during titration, with hepatic or renal dosing, when concomitantly used with estrogen-based oral contraceptives (eg, lamotrigine), before or during pregnancy, or when nonadherence is suspected.
Continue to: Can antiseizure treatment be stopped?
Can antiseizure treatment be stopped?
Current evidence favors continuing ASM therapy in patients whose seizures are under control, although the decision should be tailored to an individual’s circumstances. According to the 2021 American Academy of Neurology (AAN) guidelines, adults who have been seizure free for at least 2 years and discontinue ASMs are possibly still at higher risk for seizure recurrence in the long term (24-60 months), compared with those who continue treatment.29 On the other hand, for adults who have been seizure free for at least 12 months, ASM withdrawal may not increase their risk for status epilepticus, and there are insufficient data to support or refute an effect on mortality or quality of life with ASM withdrawal in this population. The decision to taper or maintain ASM therapy in seizure-free patients also should take into consideration other clinically relevant outcome measures such as the patient’s lifestyle and medication adverse effects. Therefore, this decision should be made after sufficient discussion with patients and their caregivers. (Information for patients can be found at: www.epilepsy.com/treatment/medicines/stopping-medication.)
For children, the AAN guideline panel recommends discussing with family the small risk (2%) for becoming medication resistant if seizures recur during or after ASM withdrawal. 29 For children who have been seizure free for 18 to 24 months, there is probably not a significant long-term (24-48 months) difference in seizure recurrence in those who taper ASMs vs those who do not. However, presence of epileptiform discharges on EEG before discontinuation of an ASM indicates increased risk for seizure recurrence. 29
Intractable (refractory) epilepsy
While most patients with epilepsy attain complete seizure control with appropriate drug therapy, approximately 30% continue to experience seizures (“drug-resistant” epilepsy, also termed intractable or refractory ). 30 In 2010, the ILAE defined drug-resistant epilepsy as “failure of adequate trials of two tolerated, appropriately chosen and used anti-epileptic drug schedules (whether as monotherapy or in combination) to achieve sustained seizure freedom” (defined as cessation of seizures for at least 3 times the longest pre-intervention inter-seizure interval or 12 months, whichever is longer). 21,31 It should be noted that drug withdrawal due to adverse effects is not counted as failure of that ASM. Recognition of drug-resistant epilepsy may prompt referral to an epileptologist who can consider rational combination drug therapy or surgical resection of the seizure focus, vagus nerve stimulation, electrical stimulation of the seizure focus, or deep brain (thalamic) stimulation.
Seizure triggers and mitigating factors
Epilepsy mostly affects patients during seizure episodes; however, the unpredictability of these events adds significantly to the burden of disease. There are no reliable methods for predicting seizure other than knowing of the several potential risks and recognizing and avoiding these triggers.
Noncompliance with antiseizure medications is a common seizure trigger affecting up to one-half of patients with epilepsy.32
Continue to: Medications
Medications may provoke seizures in susceptible individuals
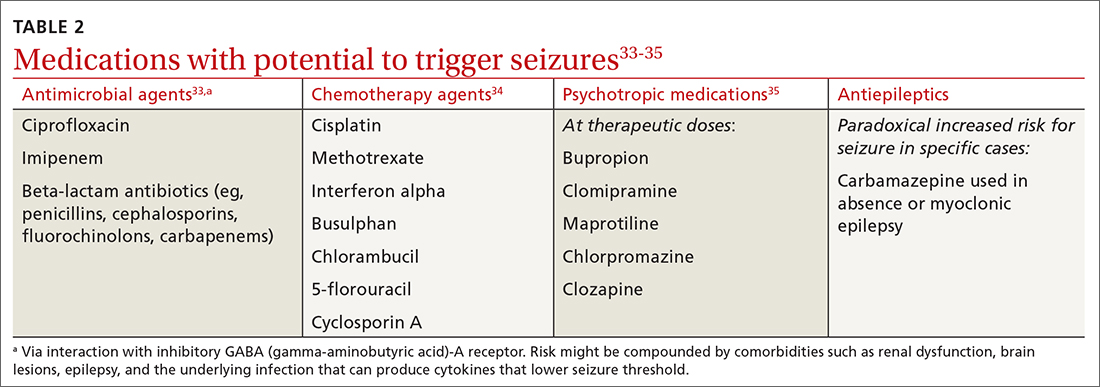
Sleep deprivation is a potential seizure trigger in people with epilepsy based on observational studies, case reports, patient surveys, and EEG-based studies, although data from randomized controlled studies are limited.36 The standard best practice is to encourage appropriate sleep hygiene, which involves getting at least 7 hours of sleep per night.37
Alcohol is a GABAergic substance like benzodiazepines with antiseizure effects. However, it acts as a potential precipitant of seizures in cases of withdrawal or acute intoxication, or when it leads to sleep disruption or nonadherence to antiseizure medications. Therefore, advise patients with alcohol use disorder to slowly taper consumption (best done through a support program) and avoid sudden withdrawal. However, complete abstinence from alcohol use is not often recommended except in special circumstances (eg, a history of alcohol-related seizures). Several studies have demonstrated that modest alcohol use (1-2 drinks per occasion) does not increase seizure frequency or significantly alter serum concentrations of commonly used ASMs.38
Cannabis and other substances. The 2 main biologically active components of marijuana are delta-9-tetrahydrocannibinol (THC), the main psychoactive constituent, and cannabidiol (CBD). Animal and human studies have demonstrated anticonvulsant properties of THC and CBD. But THC, in high amounts, can result in adverse cognitive effects and worsening seizures.39 A purified 98% oil-based CBD extract (Epidiolex) has been approved as an adjunctive treatment for certain medically refractory epilepsy syndromes in children and young adults—ie, Dravet syndrome, Lennox-Gastaut syndrome, and tuberous sclerosis complex syndrome.40 There are no reliable data on the effect of recreational use of marijuana on seizure control. Other illicit substances such as cocaine may lower seizure threshold by their stimulatory and disruptive effects on sleep, diet, and healthy routines.
Special clinical cases
Pregnancy and epilepsy
Despite the potential adverse effects of ASMs on fetal health, the current global consensus is to continue treatment during pregnancy, given that the potential harm of convulsive seizures outweighs the potential risks associated with in-utero exposure to ASMs. There is not enough evidence to indicate significant harm to the fetus caused by focal, absence, or myoclonic seizures. Low-dose folic acid is used to minimize the risks of ASMs during pregnancy.
Continue to: As the fetus develops...
As the fetus develops, there are changes in volume of ASM distribution, renal clearance, protein binding, and hepatic metabolism, which require checking serum levels at regular intervals and making dosage adjustments.
The ongoing study evaluating Maternal Outcomes and Neurodevelopmental Effects of Antiepileptic Drugs (MONEAD)41 has led to multiple landmark studies guiding the choice of preferred ASMs during pregnancy in patients with epilepsy.42,43 This has culminated in today’s use of lamotrigine and levetiracetam as the 2 preferred agents (while avoiding valproate) in pregnant patients with epilepsy.44
Psychogenic nonepileptic seizures
A form of conversion disorder, psychogenic nonepileptic seizures (PNES) manifests as abnormal motor or behavioral events mimicking seizures but without associated epileptiform discharges on EEG. This is observed in 10% of patients seen in epilepsy clinics and even more often in those admitted to epilepsy monitoring units (25%-40%).45 Diagnosis of PNES requires EEG monitoring both for confirmation and for discernment from true epileptic seizures, in particular frontal lobe epilepsy that may clinically mimic PNES. PNES often is associated with underlying psychological tensions or comorbid conditions such as depression, anxiety, or traumatic life experiences. There is no treatment for PNES per se, and its management is focused on controlling any underlying psychological comorbidities that may not always be obvious. There is some evidence suggesting that these patients experience an innate inability to verbally express their emotions and instead subconsciously resort to psychosomatics to express them in a somatic dimension.46,47
Status epilepticus
Defined as prolonged seizures (> 5 min) or 2 consecutive seizures without regaining aware ness in between, status epilepticus (SE) is a potentially fatal condition. Subclinical nonconvulsive SE, especially in comatose patients, can be diagnosed only via EEG monitoring. Untreated SE may manifest as a diagnostic dilemma in unresponsive or critically ill patients and can increase the risk for mortality. 48
Febrile seizures
Febrile seizures affect 2% to 5% of children most often in the second year of life.49 The use of preventive antiseizure medication is not recommended; instead, the key is to investigate the underlying febrile illness. Lumbar puncture is indicated if there are signs and symptoms of meningitis (25% of children with bacterial meningitis present with seizures).49 Febrile seizures often are self-limited, but there is risk for SE in up to 15% of cases.50 If convulsive febrile seizures last longer than 5 minutes, initiate benzodiazepines followed by the standard protocol used for the management of SE.51
Continue to: Epilepsy as a spectrum disorder
Epilepsy as a spectrum disorder
The higher prevalence of comorbid cognitive and psychiatric conditions in patients with epilepsy, affecting about half of patients, 52 suggests that seizures may constitute only one aspect of a multifaceted disease that otherwise should be considered a spectrum disorder. Among such conditions are memory deficits, depression, and anxiety. Conversely, epilepsy is more common in patients with depression than in those without. 52
Social impact of epilepsy
Vehicle driving regulations. Patients with epilepsy are required to follow state law regarding driving restrictions. Different states have different rules and regulations about driving restrictions and reporting requirements (by patients or their physicians). Refer patients to the Department of Motor Vehicles (DMV) in their state of residence for up-to-date instructions.53 The Epilepsy Foundation (epilepsy.com) can serve as a resource for each state’s DMV website.
Employment assistance. Having epilepsy should not preclude patients from seeking employment and pursuing meaningful careers. The Americans with Disabilities Act (ADA) and the US Equal Employment Opportunity Commission (EEOC) forbid discrimination against qualified people with disabilities, including those with epilepsy, and require reasonable accommodations in the workplace (www.eeoc.gov/laws/guidance/epilepsy-workplace-and-ada).54
CORRESPONDENCE
Gholam K. Motamedi, MD, Department of Neurology, PHC 7, Georgetown University Hospital, 3800 Reservoir Road, NW, Washington, DC 20007; motamedi@georgetown.edu
1. Hauser WA, Annegers JF, Rocca WA. Descriptive epidemiology of epilepsy: contributions of population-based studies from Rochester, Minnesota. Mayo Clin Proc. 1996;71:576-586. doi: 10.4065/71.6.576
2. Fisher RS, Acevedo C, Arzimanoglou A, et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia. 2014;55:475-482. doi: 10.1111/epi.12550.
3. Hauser WA, Beghi E. First seizure definitions and worldwide incidence and mortality. Epilepsia. 2008;49:8-12. doi: 10.1111/j.1528-1167.2008.01443.x
4. Berg AT, Shinnar S. The risk of seizure recurrence following a first unprovoked seizure: a quantitative review. Neurology. 1991;41:965-972. doi: 10.1212/wnl.41.7.965
5. Ropper AH. Transient global amnesia. N Engl J Med. 2023;388:635-540. doi: 10.1056/NEJMra2213867
6. Bouma HK, Labos C, Gore GC, et al. The diagnostic accuracy of routine electroencephalography after a first unprovoked seizure. Eur J Neurol. 2016;23:455-463. doi: 10.1111/ene.12739
7. Narayanan JT, Labar DR, Schaul N. Latency to first spike in the EEG of epilepsy patients. Seizure. 2008;17:34-41. doi: 10.1016/j.seizure.2007.06.003
8. Salmenpera TM, Duncan JS. Imaging in epilepsy. J Neurol Neurosurg Psychiatry. 2005;76:iii2-iii10. doi: 10.1136/jnnp.2005.075135
9. Jackson GD, Berkovic SF, Tress , et al Hippocampal sclerosis can be reliably detected by magnetic resonance imaging. Neurology. 1990;40:1869-1875. doi: 10.1212/wnl.40.12.1869
10. Bonnett LJ, Kim, L, Johnson A, et al. Risk of seizure recurrence in people with single seizures and early epilepsy - model development and external validation. Seizure. 2022;94:26-32. doi: 10.1016/j.seizure.2021.11.007
11. Krumholz A, Wiebe S, Gronseth GS, et al. Evidence-based guideline: management of an unprovoked first seizure in adults: Report of the Guideline Development Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology. 2015;84:1705-1713. doi: 10.1212/WNL.0000000000001487
12. Fisher RS, Cross JH, French JA, et al. Operational classification of seizure types by the International League Against Epilepsy: position paper of the ILAE Commission for Classification and terminology. Epilepsia. 2017;58:522-530. doi: 10.1111/epi.13670
13. Berg AT, Berkovic SF, Brodie MJ, et al. Revised terminology and concepts for organization of seizures and epilepsy: report of the ILAE Commission on Classification and Terminology, 2005-2009. Epilepsia. 2010;51:676-685. doi: 10.1111/j.1528-1167.2010.02522.x
14. Marson AG, Al-Kharusi AM, Alwaidh M, et al. The SANAD study of effectiveness of valproate, lamotrigine, or topiramate for generalized and unclassifiable epilepsy: an unblinded randomized controlled trial. Lancet. 2007;369:1016-1026. doi: 10.1016/S0140-6736(07)60461-9
15. Marson AG, Al-Kharusi AM, Alwaidh M, et al. The SANAD study of effectiveness of carbamazepine, gabapentin, lamotrigine, oxcarbazepine, or topiramate for treatment of partial epilepsy: an unblinded randomized controlled trial. Lancet 2007;369:1000-1015. doi: 10.1016/S0140-6736(07)60460-7
16. Marson A, Burnside G, Appleton R, et al. The SANAD II study of the effectiveness and cost-effectiveness of valproate versus levetiracetam for newly diagnosed generalized and unclassified epilepsy: an open-label, non-inferiority, multicentre, phase 4, randomized controlled trial. Lancet. 2021;397:1375-1386. doi: 10.1016/S0140-6736(21)00246-4
17. Mawhinney E, Craig J, Morrow J. Levetiracetam in pregnancy: results from the UK and Ireland epilepsy and pregnancy registers. Neurology. 2013;80:400-405.
18. Marson A, Burnside G, Appleton R, et al. The SANAD II study of the effectiveness and cost-effectiveness of levetiracetam, zonisamide, or lamotrigine for newly diagnosed focal epilepsy: an open-label, non-inferiority, multicentre, phase 4, randomized controlled trial. Lancet. 2021;397:1363-1374. doi: 10.1016/S0140-6736(21)00247-6
19. Smith PE. Initial management of seizure in adults. N Engl J Med. 2021;385:251-263. doi: 10.1056/NEJMcp2024526
20. Depakene (valproic acid). Package insert. Abbott Laboratories; 2011. Accessed October 6, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2011/018081s046_18082s031lbl.pdf
21. Greenberg RG, Melloni C, Wu H, et al. Therapeutic index estimation of antiepileptic drugs: a systematic literature review approach. Clin Neuropharmacol. 2016;39:232-240. doi: 10.1097/WNF.0000000000000172
22. Lamictal (lamotrigine). Package insert. GlaxoSmithKline; 2009. Accessed October 6, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2009/020241s037s038,020764s030s031lbl.pdf
23. LaRoche SM, Helmers SL. The new antiepileptic drugs: scientific review. JAMA. 2004;291:605-614. doi: 10.1001/jama.291.5.605
24. Topamax (topiramate). Package insert. Janssen Pharmaceuticals, Inc. Accessed October 6, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2012/020844s041lbl.pdf
25. Keppra (levetiracetam). Package insert. UCB, Inc.; 2009. Accessed October 6, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2009/021035s078s080%2C021505s021s024lbl.pdf
26. Carbatrol (carbamazepine). Package insert. Shire US Inc; 2013. Accessed October 6, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2013/020712s032s035lbl.pdf
27.Neurontin (gabapentin). Package insert. Pfizer; 2017. Accessed October 6, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2017/020235s064_020882s047_021129s046lbl.pdf
28.Zonegran (zonisamide). Package insert. Eisai Inc; 2006. Accessed October 6, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2006/020789s019lbl.pdf
29.Gloss D, Paragon K, Pack A, et al. Antiseizure medication withdrawal in seizure-free patients: practice advisory update. Report of the AAN Guideline Subcommittee. Neurology. 2021;97:1072-1081. doi: 10.1212/WNL.0000000000012944
30.Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med. 2000:342:314-319. doi: 10.1056/NEJM200002033420503
31.Kwan P, Arzimanoglou A, Berg AT, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia. 2010;51:1069-1077. doi: 10.1111/j.1528-1167.2009.02397.x
Compliance during treatment of epilepsy. Epilepsia 1988;29(suppl 2):S79-S84.
33.utter R, Rüegg S, Tschudin-Sutter S. Seizures as adverse events of antibiotic drugs: a systematic review. Neurology. 2015;13;85:1332-1341. doi: 10.1212/WNL.0000000000002023
34.Singh G, Rees JH, Sander JW. Seizures and epilepsy in oncological practice: causes, course, mechanisms and treatment. JNNP. 2007;78:342-349. doi: 10.1136/jnnp.2006.106211
35.Pisani F, Oteri G, Costa C., et al. Effects of psychotropic drugs on seizure threshold. Drug Safety. 2002;25:91-110.
36.Rossi KC, Joe J, Makhjia M, et al. Insufficient sleep, electroencephalogram activation, and seizure risk: re-evaluating the evidence. Ann Neurol. 2020;86:798-806. doi: 10.1002/ana.25710
37.Watson NF, Badr MS, Belenky G, et al. Recommended amount of sleep for a healthy adult: a Joint Consensus Statement of the American Academy of Sleep Medicine and Sleep Research Society. Sleep. 2015;38:843-844. doi: 10.5665/sleep.4716
38.Höppener RJ, Kuyer A, van der Lugt PJ. Epilepsy and alcohol: the influence of social alcohol intake on seizures and treatment in epilepsy. Epilepsia. 1983;24:459-471. doi: 10.1111/j.1528-1157.1983.tb04917.x
39.Keeler MH, Reifler CB. Grand mal convulsions subsequent to marijuana use. Case report. Dis Nerv Syst. 1967:28:474-475.
40.Epidiolex (cannabidiol). Package insert. Greenwich Biosciences Inc; 2018. Accessed September 27, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2018/210365lbl.pdf
41.ClinicalTrials.gov. Maternal Outcomes and Neurodevelopmental Effects of Antiseizure Drugs (MONEAD). Accessed September 24, 2023. https://classic.clinicaltrials.gov/ct2/show/NCT01730170
42.Meador KJ, Baker GA, Finnell RH, et al. In utero antiepileptic drug exposure: fetal death and malformations. Neurology. 2006;67:407-412. doi: 10.1212/01.wnl.0000227919.81208.b2
43.Meador K, Reynolds MW, Crean S. Pregnancy outcomes in women with epilepsy: a systematic review and meta-analysis of published pregnancy registries and cohorts. Epilepsy Res. 2008;81:1-13. doi:10.1016/j.eplepsyres.2008.04.022
44.Marxer CA, Rüegg S, Rauch A review of the evidence on the risk of congenital malformations and neurodevelopmental disorders in association with antiseizure medications during pregnancy. Expert Opin Drug Saf. 2021;20:1487-1499. doi: 10.1080/14740338.2021.1943355
Asadi-Pooya AA, Sperling MR. Epidemiology of psychogenic nonepileptic seizures. Epilepsy Behav. 2015;46:60-65. doi: 10.1016/j.yebeh.2015.03.015
Evaluation and treatment of psychogenic nonepileptic seizures. Neurol Clin. 2022;40:799-820. doi: 10.1016/j.ncl.2022.03.017
47.Motamedi GK. Psychogenic nonepileptic seizures: a disconnect between body and mind. Epilepsy Behav. 2018;78:293-294. doi: 10.1016/j.yebeh.2017.10.016
, Nonconvulsive status epilepticus. Emerg Med Clin North Am. 2011;29:65-72. doi: 10.1016/j.emc.2010.08.006
doi: 10.1542/peds.2010-3318
Drug management for acute tonic-clonic convulsions including convulsive status epilepticus in children. Cochrane Database Sys Rev. 2018;1(1):CD001905. doi: 10.1002/14651858.CD001905.pub3
52.Jensen FE. Epilepsy as a spectrum disorder: implications from novel clinical and basic neuroscience. Epilepsia. 2011;52(suppl 1):1-6. doi: 10.1111/j.1528-1167.2010.02904.x
53.Kass JS, Rose RV. Driving and epilepsy: ethical, legal, and health care policy challenges. Continuum (Minneap Minn). 2019;25:537-542. doi: 10.1212/CON.0000000000000714
54.Troxell J. Epilepsy and employment: the Americans with Disabilities Act and its protections against employment discrimination. Med Law. 1997;16:375-384.
Managing first-time seizures and epilepsy often requires consultation with a neurologist or epileptologist for diagnosis and subsequent management, including when medical treatment fails or in determining whether patients may benefit from surgery. However, given the high prevalence of epilepsy and even higher incidence of a single seizure, family physicians contribute significantly to the management of these patients. The main issues are managing a first-time seizure, making the diagnosis, establishing a treatment plan, and exploring triggers and mitigating factors.
Seizure vs epilepsy
All patients with epilepsy experience seizures, but not every person who experiences a seizure has (or will develop) epilepsy. Nearly 10% of the population has one seizure during their lifetime,whereas the risk for epilepsy is just 3%.1 Therefore, a first-time seizure may not herald epilepsy, defined as repetitive (≥ 2) unprovoked seizures more than 24 hours apart.2 Seizures can be provoked (acute symptomatic) or unprovoked; a clear distinction between these 2 occurrences—as well as between single and recurrent seizures—is critical for proper management. A close look at the circumstances of a first-time seizure is imperative to define the nature of the event and the possibility of further seizures before devising a treatment plan.
Provoked seizures are due to an acute brain insult such as toxic-metabolic disorders, concussion, alcohol withdrawal, an adverse effect of a medication or its withdrawal, or photic stimulation presumably by disrupting the brain’s metabolic homeostasis or integrity. The key factor is that provoked seizures always happen in close temporal association with an acute insult. A single provoked seizure happens each year in 29 to 39 individuals per 100,000.3 While these seizures typically occur singly, there is a small risk they may recur if the triggering insult persists or repeats.1 Therefore, more than 1 seizure per se may not indicate epilepsy.3
Unprovoked seizures reflect an underlying brain dysfunction. A single unprovoked seizure happens in 23 to 61 individuals per 100,000 per year, often in men in either younger or older age groups.3 Unprovoked seizures may occur only once or may recur (ie, evolve into epilepsy). The latter scenario happens in only about half of cases; the overall risk for a recurrent seizure within 2 years of a first seizure is estimated at 42% (24% to 65%, depending on the etiology and electroencephalogram [EEG] findings).4 More specifically, without treatment the relapse rate will be 36% at 1 year and 47% at 2 years.4 Further, a second unprovoked seizure, if untreated, would increase the risk for third and fourth seizures to 73% and 76%, respectively, within 4 years.3
Evaluating the first-time seizure
Ask the patient or observers about the circumstances of the event to differentiate provoked from unprovoked onset. For one thing, not all “spells” are seizures. The differential diagnoses may include syncope, psychogenic nonepileptic events, drug intoxication or withdrawal, migraine, panic attacks, sleep disorders (parasomnia), transient global amnesia, concussion, and transient ischemic attack. EEG, neuroimaging, and other relevant diagnostic tests often are needed (eg, electrocardiogram/echocardiogram/Holter monitoring to evaluate for syncope/cardiac arrhythmia). Clinically, syncopal episodes tend to be brief with rapid recovery and no confusion, speech problems, aura, or lateralizing signs such as hand posturing or lip smacking that are typical with focal seizures. However, cases of convulsive syncope can be challenging to assess without diagnostic tests.
True convulsive seizures do not have the variability in clinical signs seen with psychogenic nonepileptic events (eg, alternating body parts involved or direction of movements). Transient global amnesia is a rare condition with no established diagnostic test and is considered a diagnosis of exclusion, although bitemporal hyperintensities on magnetic resonance imaging (MRI) may appear 12 to 48 hours after the clinical episode.5 Blood work is needed in patients with medical issues treated with multiple medications to evaluate for metabolic derangements; otherwise, routine blood work provides minimal information in stable patients.
Region-specific causes. Neurocysticercosis is common in some regions, such as Latin America; therefore, attention should be paid to this aspect of patient history.
Continue to: Is it really a first-time seizure?
Is it really a first-time seizure? A “first,” usually dramatic, generalized tonic-clonic seizure that triggers the diagnostic work-up may not be the very first seizure. Evidence suggests that many patients have experienced prior undiagnosed seizures. Subtle prior events often missed include episodes of deja vu, transient feelings of fear or unusual smells, speech difficulties, staring spells, or myoclonic jerks.1 A routine EEG to record epileptiform discharges and a high-resolution brain MRI to rule out any intracranial pathology are indicated. However, if the EEG indicates a primary generalized (as opposed to focal-onset) epilepsy, a brain MRI may not be needed. If a routine EEG is unrevealing, long-term video-EEG monitoring may be needed to detect an abnormality.
Accuracy of EEG and MRI. Following a first unprovoked seizure, routine EEG to detect epileptiform discharges in adults has yielded a sensitivity of 17.3% and specificity of 94.7%. In evaluating children, these values are 57.8% and 69.6%, respectively.6 If results are equivocal, a 24-hour EEG can increase the likelihood of detecting epileptiform discharges to 89% of patients.7 Brain MRI may detect an abnormality in 12% to 14% of patients with newly diagnosed epilepsy, and in up to 80% of those with recurrent seizures.8 In confirming hippocampus sclerosis, MRI has demonstrated a sensitivity of 93% and specificity of 86%.9
When to treat a first-time seizure. Available data and prediction models identify risk factors that would help determine whether to start an antiseizure medication after a first unprovoked seizure:
Epilepsy diagnosis
The International League Against Epilepsy (ILAE) previously defined epilepsy as 2 unprovoked seizures more than 24 hours apart. However, a more recent ILAE task force modified this definition: even a single unprovoked seizure would be enough to diagnose epilepsy if there is high probability of further seizures—eg, in the presence of definitive epileptiform discharges on EEG or presence of a brain tumor or a remote brain insult on imaging, since such conditions induce an enduring predisposition to generate epileptic seizures. 2 Also, a single unprovoked seizure is enough to diagnose epilepsy if it is part of an epileptic syndrome such as juvenile myoclonic epilepsy. Further, a time limit was added to the definition—ie, epilepsy is considered resolved if a patient remains seizure free for 10 years without use of antiseizure medications during the past 5 years. However, given the multitude of variables and evidence, the task force acknowledged the need for individualized considerations. 2
Seizure classification
Classification of seizure type is based on the site of seizure onset and its spread pattern—ie, focal, generalized, or unknown onset.
Continue to: Focal-onset seizures
Focal-onset seizures originate “within networks limited to one hemisphere,” although possibly in more than 1 region (ie, multifocal, and presence or absence of loss of awareness). 12 Focal seizures may then be further classified into “motor onset” or “nonmotor onset” (eg, autonomic, emotional, sensory). 2
Generalized seizures are those “originating at some point within, and rapidly engaging, bilaterally distributed networks.” 13 Unlike focal-onset seizures, generalized seizures are not classified based on awareness, as most generalized seizures involve loss of awareness (absence) or total loss of consciousness (generalized tonic-clonic). They are instead categorized based on the presence of motor vs nonmotor features (eg, tonic-clonic, myoclonic, atonic). Epilepsy classification is quite dynamic and constantly updated based on new genetic, electroencephalographic, and neuroimaging discoveries.
Treatment of epilepsy
Antiseizure medications
Treatment with antiseizure medications (ASMs; formerly known as antiepileptic drugs ) is the mainstay of epilepsy management. Achieving efficacy (seizure freedom) and tolerability (minimal adverse effects) are the primary goals of treatment. Factors that should govern the selection of an ASM include the seizure type/epilepsy syndrome, adverse effect profile of the ASM, pharmacodynamic/pharmacokinetic considerations, and patient comorbidities.
The Standard and New Antiepileptic Drugs (SANAD I and II) trials provide data from direct, unblinded, and longitudinal comparisons of existing and new ASMs and their utility in different seizure types. In the SANAD I cohort of patients with generalized and unclassified epilepsies, valproate was superior to lamotrigine and topiramate for 12-month remission and treatment failure rates, respectively.14 However, valproate generally is avoided in women of childbearing age due its potential adverse effects during pregnancy. In focal epilepsies, lamotrigine was superior to carbamazepine, gabapentin, and topiramate with respect to treatment failure, and noninferior to carbamazepine for 12-month remission.15 In the SANAD II trial, levetiracetam was noninferior to valproate for incidence of adverse events in patients with generalized and unclassified epilepsies although was found to be neither more clinically effective nor more cost effective.16 For patients of childbearing potential with generalized and unclassified epilepsies, there is evidence to support the safe and effective use of levetiracetam.17In focal epilepsies, lamotrigine was superior to levetiracetam and zonisamide with respect to treatment failures and adverse events and was noninferior to zonisamide for 12-month remission.18 In summary, levetiracetam and valproate (not to be used in women of childbearing potential) are considered appropriate first-line agents for generalized and unclassified epilepsies while lamotrigine is deemed an appropriate first-line agent for focal epilepsies (TABLE 119-28).

Drug level monitoring. It is standard practice to periodically monitor serum levels in patients taking first-generation ASMs such as phenytoin, carbamazepine, phenobarbital, and valproic acid because of their narrow therapeutic range and the potential for overdose or interaction with other medications or foods (eg, grapefruit juice may increase carbamazepine serum level by inhibiting CYP3A4, the enzyme that metabolizes the drug). Patients taking newer ASMs may not require regular serum level monitoring except during titration, with hepatic or renal dosing, when concomitantly used with estrogen-based oral contraceptives (eg, lamotrigine), before or during pregnancy, or when nonadherence is suspected.
Continue to: Can antiseizure treatment be stopped?
Can antiseizure treatment be stopped?
Current evidence favors continuing ASM therapy in patients whose seizures are under control, although the decision should be tailored to an individual’s circumstances. According to the 2021 American Academy of Neurology (AAN) guidelines, adults who have been seizure free for at least 2 years and discontinue ASMs are possibly still at higher risk for seizure recurrence in the long term (24-60 months), compared with those who continue treatment.29 On the other hand, for adults who have been seizure free for at least 12 months, ASM withdrawal may not increase their risk for status epilepticus, and there are insufficient data to support or refute an effect on mortality or quality of life with ASM withdrawal in this population. The decision to taper or maintain ASM therapy in seizure-free patients also should take into consideration other clinically relevant outcome measures such as the patient’s lifestyle and medication adverse effects. Therefore, this decision should be made after sufficient discussion with patients and their caregivers. (Information for patients can be found at: www.epilepsy.com/treatment/medicines/stopping-medication.)
For children, the AAN guideline panel recommends discussing with family the small risk (2%) for becoming medication resistant if seizures recur during or after ASM withdrawal. 29 For children who have been seizure free for 18 to 24 months, there is probably not a significant long-term (24-48 months) difference in seizure recurrence in those who taper ASMs vs those who do not. However, presence of epileptiform discharges on EEG before discontinuation of an ASM indicates increased risk for seizure recurrence. 29
Intractable (refractory) epilepsy
While most patients with epilepsy attain complete seizure control with appropriate drug therapy, approximately 30% continue to experience seizures (“drug-resistant” epilepsy, also termed intractable or refractory ). 30 In 2010, the ILAE defined drug-resistant epilepsy as “failure of adequate trials of two tolerated, appropriately chosen and used anti-epileptic drug schedules (whether as monotherapy or in combination) to achieve sustained seizure freedom” (defined as cessation of seizures for at least 3 times the longest pre-intervention inter-seizure interval or 12 months, whichever is longer). 21,31 It should be noted that drug withdrawal due to adverse effects is not counted as failure of that ASM. Recognition of drug-resistant epilepsy may prompt referral to an epileptologist who can consider rational combination drug therapy or surgical resection of the seizure focus, vagus nerve stimulation, electrical stimulation of the seizure focus, or deep brain (thalamic) stimulation.
Seizure triggers and mitigating factors
Epilepsy mostly affects patients during seizure episodes; however, the unpredictability of these events adds significantly to the burden of disease. There are no reliable methods for predicting seizure other than knowing of the several potential risks and recognizing and avoiding these triggers.
Noncompliance with antiseizure medications is a common seizure trigger affecting up to one-half of patients with epilepsy.32
Continue to: Medications
Medications may provoke seizures in susceptible individuals

Sleep deprivation is a potential seizure trigger in people with epilepsy based on observational studies, case reports, patient surveys, and EEG-based studies, although data from randomized controlled studies are limited.36 The standard best practice is to encourage appropriate sleep hygiene, which involves getting at least 7 hours of sleep per night.37
Alcohol is a GABAergic substance like benzodiazepines with antiseizure effects. However, it acts as a potential precipitant of seizures in cases of withdrawal or acute intoxication, or when it leads to sleep disruption or nonadherence to antiseizure medications. Therefore, advise patients with alcohol use disorder to slowly taper consumption (best done through a support program) and avoid sudden withdrawal. However, complete abstinence from alcohol use is not often recommended except in special circumstances (eg, a history of alcohol-related seizures). Several studies have demonstrated that modest alcohol use (1-2 drinks per occasion) does not increase seizure frequency or significantly alter serum concentrations of commonly used ASMs.38
Cannabis and other substances. The 2 main biologically active components of marijuana are delta-9-tetrahydrocannibinol (THC), the main psychoactive constituent, and cannabidiol (CBD). Animal and human studies have demonstrated anticonvulsant properties of THC and CBD. But THC, in high amounts, can result in adverse cognitive effects and worsening seizures.39 A purified 98% oil-based CBD extract (Epidiolex) has been approved as an adjunctive treatment for certain medically refractory epilepsy syndromes in children and young adults—ie, Dravet syndrome, Lennox-Gastaut syndrome, and tuberous sclerosis complex syndrome.40 There are no reliable data on the effect of recreational use of marijuana on seizure control. Other illicit substances such as cocaine may lower seizure threshold by their stimulatory and disruptive effects on sleep, diet, and healthy routines.
Special clinical cases
Pregnancy and epilepsy
Despite the potential adverse effects of ASMs on fetal health, the current global consensus is to continue treatment during pregnancy, given that the potential harm of convulsive seizures outweighs the potential risks associated with in-utero exposure to ASMs. There is not enough evidence to indicate significant harm to the fetus caused by focal, absence, or myoclonic seizures. Low-dose folic acid is used to minimize the risks of ASMs during pregnancy.
Continue to: As the fetus develops...
As the fetus develops, there are changes in volume of ASM distribution, renal clearance, protein binding, and hepatic metabolism, which require checking serum levels at regular intervals and making dosage adjustments.
The ongoing study evaluating Maternal Outcomes and Neurodevelopmental Effects of Antiepileptic Drugs (MONEAD)41 has led to multiple landmark studies guiding the choice of preferred ASMs during pregnancy in patients with epilepsy.42,43 This has culminated in today’s use of lamotrigine and levetiracetam as the 2 preferred agents (while avoiding valproate) in pregnant patients with epilepsy.44
Psychogenic nonepileptic seizures
A form of conversion disorder, psychogenic nonepileptic seizures (PNES) manifests as abnormal motor or behavioral events mimicking seizures but without associated epileptiform discharges on EEG. This is observed in 10% of patients seen in epilepsy clinics and even more often in those admitted to epilepsy monitoring units (25%-40%).45 Diagnosis of PNES requires EEG monitoring both for confirmation and for discernment from true epileptic seizures, in particular frontal lobe epilepsy that may clinically mimic PNES. PNES often is associated with underlying psychological tensions or comorbid conditions such as depression, anxiety, or traumatic life experiences. There is no treatment for PNES per se, and its management is focused on controlling any underlying psychological comorbidities that may not always be obvious. There is some evidence suggesting that these patients experience an innate inability to verbally express their emotions and instead subconsciously resort to psychosomatics to express them in a somatic dimension.46,47
Status epilepticus
Defined as prolonged seizures (> 5 min) or 2 consecutive seizures without regaining aware ness in between, status epilepticus (SE) is a potentially fatal condition. Subclinical nonconvulsive SE, especially in comatose patients, can be diagnosed only via EEG monitoring. Untreated SE may manifest as a diagnostic dilemma in unresponsive or critically ill patients and can increase the risk for mortality. 48
Febrile seizures
Febrile seizures affect 2% to 5% of children most often in the second year of life.49 The use of preventive antiseizure medication is not recommended; instead, the key is to investigate the underlying febrile illness. Lumbar puncture is indicated if there are signs and symptoms of meningitis (25% of children with bacterial meningitis present with seizures).49 Febrile seizures often are self-limited, but there is risk for SE in up to 15% of cases.50 If convulsive febrile seizures last longer than 5 minutes, initiate benzodiazepines followed by the standard protocol used for the management of SE.51
Continue to: Epilepsy as a spectrum disorder
Epilepsy as a spectrum disorder
The higher prevalence of comorbid cognitive and psychiatric conditions in patients with epilepsy, affecting about half of patients, 52 suggests that seizures may constitute only one aspect of a multifaceted disease that otherwise should be considered a spectrum disorder. Among such conditions are memory deficits, depression, and anxiety. Conversely, epilepsy is more common in patients with depression than in those without. 52
Social impact of epilepsy
Vehicle driving regulations. Patients with epilepsy are required to follow state law regarding driving restrictions. Different states have different rules and regulations about driving restrictions and reporting requirements (by patients or their physicians). Refer patients to the Department of Motor Vehicles (DMV) in their state of residence for up-to-date instructions.53 The Epilepsy Foundation (epilepsy.com) can serve as a resource for each state’s DMV website.
Employment assistance. Having epilepsy should not preclude patients from seeking employment and pursuing meaningful careers. The Americans with Disabilities Act (ADA) and the US Equal Employment Opportunity Commission (EEOC) forbid discrimination against qualified people with disabilities, including those with epilepsy, and require reasonable accommodations in the workplace (www.eeoc.gov/laws/guidance/epilepsy-workplace-and-ada).54
CORRESPONDENCE
Gholam K. Motamedi, MD, Department of Neurology, PHC 7, Georgetown University Hospital, 3800 Reservoir Road, NW, Washington, DC 20007; motamedi@georgetown.edu
Managing first-time seizures and epilepsy often requires consultation with a neurologist or epileptologist for diagnosis and subsequent management, including when medical treatment fails or in determining whether patients may benefit from surgery. However, given the high prevalence of epilepsy and even higher incidence of a single seizure, family physicians contribute significantly to the management of these patients. The main issues are managing a first-time seizure, making the diagnosis, establishing a treatment plan, and exploring triggers and mitigating factors.
Seizure vs epilepsy
All patients with epilepsy experience seizures, but not every person who experiences a seizure has (or will develop) epilepsy. Nearly 10% of the population has one seizure during their lifetime,whereas the risk for epilepsy is just 3%.1 Therefore, a first-time seizure may not herald epilepsy, defined as repetitive (≥ 2) unprovoked seizures more than 24 hours apart.2 Seizures can be provoked (acute symptomatic) or unprovoked; a clear distinction between these 2 occurrences—as well as between single and recurrent seizures—is critical for proper management. A close look at the circumstances of a first-time seizure is imperative to define the nature of the event and the possibility of further seizures before devising a treatment plan.
Provoked seizures are due to an acute brain insult such as toxic-metabolic disorders, concussion, alcohol withdrawal, an adverse effect of a medication or its withdrawal, or photic stimulation presumably by disrupting the brain’s metabolic homeostasis or integrity. The key factor is that provoked seizures always happen in close temporal association with an acute insult. A single provoked seizure happens each year in 29 to 39 individuals per 100,000.3 While these seizures typically occur singly, there is a small risk they may recur if the triggering insult persists or repeats.1 Therefore, more than 1 seizure per se may not indicate epilepsy.3
Unprovoked seizures reflect an underlying brain dysfunction. A single unprovoked seizure happens in 23 to 61 individuals per 100,000 per year, often in men in either younger or older age groups.3 Unprovoked seizures may occur only once or may recur (ie, evolve into epilepsy). The latter scenario happens in only about half of cases; the overall risk for a recurrent seizure within 2 years of a first seizure is estimated at 42% (24% to 65%, depending on the etiology and electroencephalogram [EEG] findings).4 More specifically, without treatment the relapse rate will be 36% at 1 year and 47% at 2 years.4 Further, a second unprovoked seizure, if untreated, would increase the risk for third and fourth seizures to 73% and 76%, respectively, within 4 years.3
Evaluating the first-time seizure
Ask the patient or observers about the circumstances of the event to differentiate provoked from unprovoked onset. For one thing, not all “spells” are seizures. The differential diagnoses may include syncope, psychogenic nonepileptic events, drug intoxication or withdrawal, migraine, panic attacks, sleep disorders (parasomnia), transient global amnesia, concussion, and transient ischemic attack. EEG, neuroimaging, and other relevant diagnostic tests often are needed (eg, electrocardiogram/echocardiogram/Holter monitoring to evaluate for syncope/cardiac arrhythmia). Clinically, syncopal episodes tend to be brief with rapid recovery and no confusion, speech problems, aura, or lateralizing signs such as hand posturing or lip smacking that are typical with focal seizures. However, cases of convulsive syncope can be challenging to assess without diagnostic tests.
True convulsive seizures do not have the variability in clinical signs seen with psychogenic nonepileptic events (eg, alternating body parts involved or direction of movements). Transient global amnesia is a rare condition with no established diagnostic test and is considered a diagnosis of exclusion, although bitemporal hyperintensities on magnetic resonance imaging (MRI) may appear 12 to 48 hours after the clinical episode.5 Blood work is needed in patients with medical issues treated with multiple medications to evaluate for metabolic derangements; otherwise, routine blood work provides minimal information in stable patients.
Region-specific causes. Neurocysticercosis is common in some regions, such as Latin America; therefore, attention should be paid to this aspect of patient history.
Continue to: Is it really a first-time seizure?
Is it really a first-time seizure? A “first,” usually dramatic, generalized tonic-clonic seizure that triggers the diagnostic work-up may not be the very first seizure. Evidence suggests that many patients have experienced prior undiagnosed seizures. Subtle prior events often missed include episodes of deja vu, transient feelings of fear or unusual smells, speech difficulties, staring spells, or myoclonic jerks.1 A routine EEG to record epileptiform discharges and a high-resolution brain MRI to rule out any intracranial pathology are indicated. However, if the EEG indicates a primary generalized (as opposed to focal-onset) epilepsy, a brain MRI may not be needed. If a routine EEG is unrevealing, long-term video-EEG monitoring may be needed to detect an abnormality.
Accuracy of EEG and MRI. Following a first unprovoked seizure, routine EEG to detect epileptiform discharges in adults has yielded a sensitivity of 17.3% and specificity of 94.7%. In evaluating children, these values are 57.8% and 69.6%, respectively.6 If results are equivocal, a 24-hour EEG can increase the likelihood of detecting epileptiform discharges to 89% of patients.7 Brain MRI may detect an abnormality in 12% to 14% of patients with newly diagnosed epilepsy, and in up to 80% of those with recurrent seizures.8 In confirming hippocampus sclerosis, MRI has demonstrated a sensitivity of 93% and specificity of 86%.9
When to treat a first-time seizure. Available data and prediction models identify risk factors that would help determine whether to start an antiseizure medication after a first unprovoked seizure:
Epilepsy diagnosis
The International League Against Epilepsy (ILAE) previously defined epilepsy as 2 unprovoked seizures more than 24 hours apart. However, a more recent ILAE task force modified this definition: even a single unprovoked seizure would be enough to diagnose epilepsy if there is high probability of further seizures—eg, in the presence of definitive epileptiform discharges on EEG or presence of a brain tumor or a remote brain insult on imaging, since such conditions induce an enduring predisposition to generate epileptic seizures. 2 Also, a single unprovoked seizure is enough to diagnose epilepsy if it is part of an epileptic syndrome such as juvenile myoclonic epilepsy. Further, a time limit was added to the definition—ie, epilepsy is considered resolved if a patient remains seizure free for 10 years without use of antiseizure medications during the past 5 years. However, given the multitude of variables and evidence, the task force acknowledged the need for individualized considerations. 2
Seizure classification
Classification of seizure type is based on the site of seizure onset and its spread pattern—ie, focal, generalized, or unknown onset.
Continue to: Focal-onset seizures
Focal-onset seizures originate “within networks limited to one hemisphere,” although possibly in more than 1 region (ie, multifocal, and presence or absence of loss of awareness). 12 Focal seizures may then be further classified into “motor onset” or “nonmotor onset” (eg, autonomic, emotional, sensory). 2
Generalized seizures are those “originating at some point within, and rapidly engaging, bilaterally distributed networks.” 13 Unlike focal-onset seizures, generalized seizures are not classified based on awareness, as most generalized seizures involve loss of awareness (absence) or total loss of consciousness (generalized tonic-clonic). They are instead categorized based on the presence of motor vs nonmotor features (eg, tonic-clonic, myoclonic, atonic). Epilepsy classification is quite dynamic and constantly updated based on new genetic, electroencephalographic, and neuroimaging discoveries.
Treatment of epilepsy
Antiseizure medications
Treatment with antiseizure medications (ASMs; formerly known as antiepileptic drugs ) is the mainstay of epilepsy management. Achieving efficacy (seizure freedom) and tolerability (minimal adverse effects) are the primary goals of treatment. Factors that should govern the selection of an ASM include the seizure type/epilepsy syndrome, adverse effect profile of the ASM, pharmacodynamic/pharmacokinetic considerations, and patient comorbidities.
The Standard and New Antiepileptic Drugs (SANAD I and II) trials provide data from direct, unblinded, and longitudinal comparisons of existing and new ASMs and their utility in different seizure types. In the SANAD I cohort of patients with generalized and unclassified epilepsies, valproate was superior to lamotrigine and topiramate for 12-month remission and treatment failure rates, respectively.14 However, valproate generally is avoided in women of childbearing age due its potential adverse effects during pregnancy. In focal epilepsies, lamotrigine was superior to carbamazepine, gabapentin, and topiramate with respect to treatment failure, and noninferior to carbamazepine for 12-month remission.15 In the SANAD II trial, levetiracetam was noninferior to valproate for incidence of adverse events in patients with generalized and unclassified epilepsies although was found to be neither more clinically effective nor more cost effective.16 For patients of childbearing potential with generalized and unclassified epilepsies, there is evidence to support the safe and effective use of levetiracetam.17In focal epilepsies, lamotrigine was superior to levetiracetam and zonisamide with respect to treatment failures and adverse events and was noninferior to zonisamide for 12-month remission.18 In summary, levetiracetam and valproate (not to be used in women of childbearing potential) are considered appropriate first-line agents for generalized and unclassified epilepsies while lamotrigine is deemed an appropriate first-line agent for focal epilepsies (TABLE 119-28).

Drug level monitoring. It is standard practice to periodically monitor serum levels in patients taking first-generation ASMs such as phenytoin, carbamazepine, phenobarbital, and valproic acid because of their narrow therapeutic range and the potential for overdose or interaction with other medications or foods (eg, grapefruit juice may increase carbamazepine serum level by inhibiting CYP3A4, the enzyme that metabolizes the drug). Patients taking newer ASMs may not require regular serum level monitoring except during titration, with hepatic or renal dosing, when concomitantly used with estrogen-based oral contraceptives (eg, lamotrigine), before or during pregnancy, or when nonadherence is suspected.
Continue to: Can antiseizure treatment be stopped?
Can antiseizure treatment be stopped?
Current evidence favors continuing ASM therapy in patients whose seizures are under control, although the decision should be tailored to an individual’s circumstances. According to the 2021 American Academy of Neurology (AAN) guidelines, adults who have been seizure free for at least 2 years and discontinue ASMs are possibly still at higher risk for seizure recurrence in the long term (24-60 months), compared with those who continue treatment.29 On the other hand, for adults who have been seizure free for at least 12 months, ASM withdrawal may not increase their risk for status epilepticus, and there are insufficient data to support or refute an effect on mortality or quality of life with ASM withdrawal in this population. The decision to taper or maintain ASM therapy in seizure-free patients also should take into consideration other clinically relevant outcome measures such as the patient’s lifestyle and medication adverse effects. Therefore, this decision should be made after sufficient discussion with patients and their caregivers. (Information for patients can be found at: www.epilepsy.com/treatment/medicines/stopping-medication.)
For children, the AAN guideline panel recommends discussing with family the small risk (2%) for becoming medication resistant if seizures recur during or after ASM withdrawal. 29 For children who have been seizure free for 18 to 24 months, there is probably not a significant long-term (24-48 months) difference in seizure recurrence in those who taper ASMs vs those who do not. However, presence of epileptiform discharges on EEG before discontinuation of an ASM indicates increased risk for seizure recurrence. 29
Intractable (refractory) epilepsy
While most patients with epilepsy attain complete seizure control with appropriate drug therapy, approximately 30% continue to experience seizures (“drug-resistant” epilepsy, also termed intractable or refractory ). 30 In 2010, the ILAE defined drug-resistant epilepsy as “failure of adequate trials of two tolerated, appropriately chosen and used anti-epileptic drug schedules (whether as monotherapy or in combination) to achieve sustained seizure freedom” (defined as cessation of seizures for at least 3 times the longest pre-intervention inter-seizure interval or 12 months, whichever is longer). 21,31 It should be noted that drug withdrawal due to adverse effects is not counted as failure of that ASM. Recognition of drug-resistant epilepsy may prompt referral to an epileptologist who can consider rational combination drug therapy or surgical resection of the seizure focus, vagus nerve stimulation, electrical stimulation of the seizure focus, or deep brain (thalamic) stimulation.
Seizure triggers and mitigating factors
Epilepsy mostly affects patients during seizure episodes; however, the unpredictability of these events adds significantly to the burden of disease. There are no reliable methods for predicting seizure other than knowing of the several potential risks and recognizing and avoiding these triggers.
Noncompliance with antiseizure medications is a common seizure trigger affecting up to one-half of patients with epilepsy.32
Continue to: Medications
Medications may provoke seizures in susceptible individuals

Sleep deprivation is a potential seizure trigger in people with epilepsy based on observational studies, case reports, patient surveys, and EEG-based studies, although data from randomized controlled studies are limited.36 The standard best practice is to encourage appropriate sleep hygiene, which involves getting at least 7 hours of sleep per night.37
Alcohol is a GABAergic substance like benzodiazepines with antiseizure effects. However, it acts as a potential precipitant of seizures in cases of withdrawal or acute intoxication, or when it leads to sleep disruption or nonadherence to antiseizure medications. Therefore, advise patients with alcohol use disorder to slowly taper consumption (best done through a support program) and avoid sudden withdrawal. However, complete abstinence from alcohol use is not often recommended except in special circumstances (eg, a history of alcohol-related seizures). Several studies have demonstrated that modest alcohol use (1-2 drinks per occasion) does not increase seizure frequency or significantly alter serum concentrations of commonly used ASMs.38
Cannabis and other substances. The 2 main biologically active components of marijuana are delta-9-tetrahydrocannibinol (THC), the main psychoactive constituent, and cannabidiol (CBD). Animal and human studies have demonstrated anticonvulsant properties of THC and CBD. But THC, in high amounts, can result in adverse cognitive effects and worsening seizures.39 A purified 98% oil-based CBD extract (Epidiolex) has been approved as an adjunctive treatment for certain medically refractory epilepsy syndromes in children and young adults—ie, Dravet syndrome, Lennox-Gastaut syndrome, and tuberous sclerosis complex syndrome.40 There are no reliable data on the effect of recreational use of marijuana on seizure control. Other illicit substances such as cocaine may lower seizure threshold by their stimulatory and disruptive effects on sleep, diet, and healthy routines.
Special clinical cases
Pregnancy and epilepsy
Despite the potential adverse effects of ASMs on fetal health, the current global consensus is to continue treatment during pregnancy, given that the potential harm of convulsive seizures outweighs the potential risks associated with in-utero exposure to ASMs. There is not enough evidence to indicate significant harm to the fetus caused by focal, absence, or myoclonic seizures. Low-dose folic acid is used to minimize the risks of ASMs during pregnancy.
Continue to: As the fetus develops...
As the fetus develops, there are changes in volume of ASM distribution, renal clearance, protein binding, and hepatic metabolism, which require checking serum levels at regular intervals and making dosage adjustments.
The ongoing study evaluating Maternal Outcomes and Neurodevelopmental Effects of Antiepileptic Drugs (MONEAD)41 has led to multiple landmark studies guiding the choice of preferred ASMs during pregnancy in patients with epilepsy.42,43 This has culminated in today’s use of lamotrigine and levetiracetam as the 2 preferred agents (while avoiding valproate) in pregnant patients with epilepsy.44
Psychogenic nonepileptic seizures
A form of conversion disorder, psychogenic nonepileptic seizures (PNES) manifests as abnormal motor or behavioral events mimicking seizures but without associated epileptiform discharges on EEG. This is observed in 10% of patients seen in epilepsy clinics and even more often in those admitted to epilepsy monitoring units (25%-40%).45 Diagnosis of PNES requires EEG monitoring both for confirmation and for discernment from true epileptic seizures, in particular frontal lobe epilepsy that may clinically mimic PNES. PNES often is associated with underlying psychological tensions or comorbid conditions such as depression, anxiety, or traumatic life experiences. There is no treatment for PNES per se, and its management is focused on controlling any underlying psychological comorbidities that may not always be obvious. There is some evidence suggesting that these patients experience an innate inability to verbally express their emotions and instead subconsciously resort to psychosomatics to express them in a somatic dimension.46,47
Status epilepticus
Defined as prolonged seizures (> 5 min) or 2 consecutive seizures without regaining aware ness in between, status epilepticus (SE) is a potentially fatal condition. Subclinical nonconvulsive SE, especially in comatose patients, can be diagnosed only via EEG monitoring. Untreated SE may manifest as a diagnostic dilemma in unresponsive or critically ill patients and can increase the risk for mortality. 48
Febrile seizures
Febrile seizures affect 2% to 5% of children most often in the second year of life.49 The use of preventive antiseizure medication is not recommended; instead, the key is to investigate the underlying febrile illness. Lumbar puncture is indicated if there are signs and symptoms of meningitis (25% of children with bacterial meningitis present with seizures).49 Febrile seizures often are self-limited, but there is risk for SE in up to 15% of cases.50 If convulsive febrile seizures last longer than 5 minutes, initiate benzodiazepines followed by the standard protocol used for the management of SE.51
Continue to: Epilepsy as a spectrum disorder
Epilepsy as a spectrum disorder
The higher prevalence of comorbid cognitive and psychiatric conditions in patients with epilepsy, affecting about half of patients, 52 suggests that seizures may constitute only one aspect of a multifaceted disease that otherwise should be considered a spectrum disorder. Among such conditions are memory deficits, depression, and anxiety. Conversely, epilepsy is more common in patients with depression than in those without. 52
Social impact of epilepsy
Vehicle driving regulations. Patients with epilepsy are required to follow state law regarding driving restrictions. Different states have different rules and regulations about driving restrictions and reporting requirements (by patients or their physicians). Refer patients to the Department of Motor Vehicles (DMV) in their state of residence for up-to-date instructions.53 The Epilepsy Foundation (epilepsy.com) can serve as a resource for each state’s DMV website.
Employment assistance. Having epilepsy should not preclude patients from seeking employment and pursuing meaningful careers. The Americans with Disabilities Act (ADA) and the US Equal Employment Opportunity Commission (EEOC) forbid discrimination against qualified people with disabilities, including those with epilepsy, and require reasonable accommodations in the workplace (www.eeoc.gov/laws/guidance/epilepsy-workplace-and-ada).54
CORRESPONDENCE
Gholam K. Motamedi, MD, Department of Neurology, PHC 7, Georgetown University Hospital, 3800 Reservoir Road, NW, Washington, DC 20007; motamedi@georgetown.edu
1. Hauser WA, Annegers JF, Rocca WA. Descriptive epidemiology of epilepsy: contributions of population-based studies from Rochester, Minnesota. Mayo Clin Proc. 1996;71:576-586. doi: 10.4065/71.6.576
2. Fisher RS, Acevedo C, Arzimanoglou A, et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia. 2014;55:475-482. doi: 10.1111/epi.12550.
3. Hauser WA, Beghi E. First seizure definitions and worldwide incidence and mortality. Epilepsia. 2008;49:8-12. doi: 10.1111/j.1528-1167.2008.01443.x
4. Berg AT, Shinnar S. The risk of seizure recurrence following a first unprovoked seizure: a quantitative review. Neurology. 1991;41:965-972. doi: 10.1212/wnl.41.7.965
5. Ropper AH. Transient global amnesia. N Engl J Med. 2023;388:635-540. doi: 10.1056/NEJMra2213867
6. Bouma HK, Labos C, Gore GC, et al. The diagnostic accuracy of routine electroencephalography after a first unprovoked seizure. Eur J Neurol. 2016;23:455-463. doi: 10.1111/ene.12739
7. Narayanan JT, Labar DR, Schaul N. Latency to first spike in the EEG of epilepsy patients. Seizure. 2008;17:34-41. doi: 10.1016/j.seizure.2007.06.003
8. Salmenpera TM, Duncan JS. Imaging in epilepsy. J Neurol Neurosurg Psychiatry. 2005;76:iii2-iii10. doi: 10.1136/jnnp.2005.075135
9. Jackson GD, Berkovic SF, Tress , et al Hippocampal sclerosis can be reliably detected by magnetic resonance imaging. Neurology. 1990;40:1869-1875. doi: 10.1212/wnl.40.12.1869
10. Bonnett LJ, Kim, L, Johnson A, et al. Risk of seizure recurrence in people with single seizures and early epilepsy - model development and external validation. Seizure. 2022;94:26-32. doi: 10.1016/j.seizure.2021.11.007
11. Krumholz A, Wiebe S, Gronseth GS, et al. Evidence-based guideline: management of an unprovoked first seizure in adults: Report of the Guideline Development Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology. 2015;84:1705-1713. doi: 10.1212/WNL.0000000000001487
12. Fisher RS, Cross JH, French JA, et al. Operational classification of seizure types by the International League Against Epilepsy: position paper of the ILAE Commission for Classification and terminology. Epilepsia. 2017;58:522-530. doi: 10.1111/epi.13670
13. Berg AT, Berkovic SF, Brodie MJ, et al. Revised terminology and concepts for organization of seizures and epilepsy: report of the ILAE Commission on Classification and Terminology, 2005-2009. Epilepsia. 2010;51:676-685. doi: 10.1111/j.1528-1167.2010.02522.x
14. Marson AG, Al-Kharusi AM, Alwaidh M, et al. The SANAD study of effectiveness of valproate, lamotrigine, or topiramate for generalized and unclassifiable epilepsy: an unblinded randomized controlled trial. Lancet. 2007;369:1016-1026. doi: 10.1016/S0140-6736(07)60461-9
15. Marson AG, Al-Kharusi AM, Alwaidh M, et al. The SANAD study of effectiveness of carbamazepine, gabapentin, lamotrigine, oxcarbazepine, or topiramate for treatment of partial epilepsy: an unblinded randomized controlled trial. Lancet 2007;369:1000-1015. doi: 10.1016/S0140-6736(07)60460-7
16. Marson A, Burnside G, Appleton R, et al. The SANAD II study of the effectiveness and cost-effectiveness of valproate versus levetiracetam for newly diagnosed generalized and unclassified epilepsy: an open-label, non-inferiority, multicentre, phase 4, randomized controlled trial. Lancet. 2021;397:1375-1386. doi: 10.1016/S0140-6736(21)00246-4
17. Mawhinney E, Craig J, Morrow J. Levetiracetam in pregnancy: results from the UK and Ireland epilepsy and pregnancy registers. Neurology. 2013;80:400-405.
18. Marson A, Burnside G, Appleton R, et al. The SANAD II study of the effectiveness and cost-effectiveness of levetiracetam, zonisamide, or lamotrigine for newly diagnosed focal epilepsy: an open-label, non-inferiority, multicentre, phase 4, randomized controlled trial. Lancet. 2021;397:1363-1374. doi: 10.1016/S0140-6736(21)00247-6
19. Smith PE. Initial management of seizure in adults. N Engl J Med. 2021;385:251-263. doi: 10.1056/NEJMcp2024526
20. Depakene (valproic acid). Package insert. Abbott Laboratories; 2011. Accessed October 6, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2011/018081s046_18082s031lbl.pdf
21. Greenberg RG, Melloni C, Wu H, et al. Therapeutic index estimation of antiepileptic drugs: a systematic literature review approach. Clin Neuropharmacol. 2016;39:232-240. doi: 10.1097/WNF.0000000000000172
22. Lamictal (lamotrigine). Package insert. GlaxoSmithKline; 2009. Accessed October 6, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2009/020241s037s038,020764s030s031lbl.pdf
23. LaRoche SM, Helmers SL. The new antiepileptic drugs: scientific review. JAMA. 2004;291:605-614. doi: 10.1001/jama.291.5.605
24. Topamax (topiramate). Package insert. Janssen Pharmaceuticals, Inc. Accessed October 6, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2012/020844s041lbl.pdf
25. Keppra (levetiracetam). Package insert. UCB, Inc.; 2009. Accessed October 6, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2009/021035s078s080%2C021505s021s024lbl.pdf
26. Carbatrol (carbamazepine). Package insert. Shire US Inc; 2013. Accessed October 6, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2013/020712s032s035lbl.pdf
27.Neurontin (gabapentin). Package insert. Pfizer; 2017. Accessed October 6, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2017/020235s064_020882s047_021129s046lbl.pdf
28.Zonegran (zonisamide). Package insert. Eisai Inc; 2006. Accessed October 6, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2006/020789s019lbl.pdf
29.Gloss D, Paragon K, Pack A, et al. Antiseizure medication withdrawal in seizure-free patients: practice advisory update. Report of the AAN Guideline Subcommittee. Neurology. 2021;97:1072-1081. doi: 10.1212/WNL.0000000000012944
30.Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med. 2000:342:314-319. doi: 10.1056/NEJM200002033420503
31.Kwan P, Arzimanoglou A, Berg AT, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia. 2010;51:1069-1077. doi: 10.1111/j.1528-1167.2009.02397.x
Compliance during treatment of epilepsy. Epilepsia 1988;29(suppl 2):S79-S84.
33.utter R, Rüegg S, Tschudin-Sutter S. Seizures as adverse events of antibiotic drugs: a systematic review. Neurology. 2015;13;85:1332-1341. doi: 10.1212/WNL.0000000000002023
34.Singh G, Rees JH, Sander JW. Seizures and epilepsy in oncological practice: causes, course, mechanisms and treatment. JNNP. 2007;78:342-349. doi: 10.1136/jnnp.2006.106211
35.Pisani F, Oteri G, Costa C., et al. Effects of psychotropic drugs on seizure threshold. Drug Safety. 2002;25:91-110.
36.Rossi KC, Joe J, Makhjia M, et al. Insufficient sleep, electroencephalogram activation, and seizure risk: re-evaluating the evidence. Ann Neurol. 2020;86:798-806. doi: 10.1002/ana.25710
37.Watson NF, Badr MS, Belenky G, et al. Recommended amount of sleep for a healthy adult: a Joint Consensus Statement of the American Academy of Sleep Medicine and Sleep Research Society. Sleep. 2015;38:843-844. doi: 10.5665/sleep.4716
38.Höppener RJ, Kuyer A, van der Lugt PJ. Epilepsy and alcohol: the influence of social alcohol intake on seizures and treatment in epilepsy. Epilepsia. 1983;24:459-471. doi: 10.1111/j.1528-1157.1983.tb04917.x
39.Keeler MH, Reifler CB. Grand mal convulsions subsequent to marijuana use. Case report. Dis Nerv Syst. 1967:28:474-475.
40.Epidiolex (cannabidiol). Package insert. Greenwich Biosciences Inc; 2018. Accessed September 27, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2018/210365lbl.pdf
41.ClinicalTrials.gov. Maternal Outcomes and Neurodevelopmental Effects of Antiseizure Drugs (MONEAD). Accessed September 24, 2023. https://classic.clinicaltrials.gov/ct2/show/NCT01730170
42.Meador KJ, Baker GA, Finnell RH, et al. In utero antiepileptic drug exposure: fetal death and malformations. Neurology. 2006;67:407-412. doi: 10.1212/01.wnl.0000227919.81208.b2
43.Meador K, Reynolds MW, Crean S. Pregnancy outcomes in women with epilepsy: a systematic review and meta-analysis of published pregnancy registries and cohorts. Epilepsy Res. 2008;81:1-13. doi:10.1016/j.eplepsyres.2008.04.022
44.Marxer CA, Rüegg S, Rauch A review of the evidence on the risk of congenital malformations and neurodevelopmental disorders in association with antiseizure medications during pregnancy. Expert Opin Drug Saf. 2021;20:1487-1499. doi: 10.1080/14740338.2021.1943355
Asadi-Pooya AA, Sperling MR. Epidemiology of psychogenic nonepileptic seizures. Epilepsy Behav. 2015;46:60-65. doi: 10.1016/j.yebeh.2015.03.015
Evaluation and treatment of psychogenic nonepileptic seizures. Neurol Clin. 2022;40:799-820. doi: 10.1016/j.ncl.2022.03.017
47.Motamedi GK. Psychogenic nonepileptic seizures: a disconnect between body and mind. Epilepsy Behav. 2018;78:293-294. doi: 10.1016/j.yebeh.2017.10.016
, Nonconvulsive status epilepticus. Emerg Med Clin North Am. 2011;29:65-72. doi: 10.1016/j.emc.2010.08.006
doi: 10.1542/peds.2010-3318
Drug management for acute tonic-clonic convulsions including convulsive status epilepticus in children. Cochrane Database Sys Rev. 2018;1(1):CD001905. doi: 10.1002/14651858.CD001905.pub3
52.Jensen FE. Epilepsy as a spectrum disorder: implications from novel clinical and basic neuroscience. Epilepsia. 2011;52(suppl 1):1-6. doi: 10.1111/j.1528-1167.2010.02904.x
53.Kass JS, Rose RV. Driving and epilepsy: ethical, legal, and health care policy challenges. Continuum (Minneap Minn). 2019;25:537-542. doi: 10.1212/CON.0000000000000714
54.Troxell J. Epilepsy and employment: the Americans with Disabilities Act and its protections against employment discrimination. Med Law. 1997;16:375-384.
1. Hauser WA, Annegers JF, Rocca WA. Descriptive epidemiology of epilepsy: contributions of population-based studies from Rochester, Minnesota. Mayo Clin Proc. 1996;71:576-586. doi: 10.4065/71.6.576
2. Fisher RS, Acevedo C, Arzimanoglou A, et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia. 2014;55:475-482. doi: 10.1111/epi.12550.
3. Hauser WA, Beghi E. First seizure definitions and worldwide incidence and mortality. Epilepsia. 2008;49:8-12. doi: 10.1111/j.1528-1167.2008.01443.x
4. Berg AT, Shinnar S. The risk of seizure recurrence following a first unprovoked seizure: a quantitative review. Neurology. 1991;41:965-972. doi: 10.1212/wnl.41.7.965
5. Ropper AH. Transient global amnesia. N Engl J Med. 2023;388:635-540. doi: 10.1056/NEJMra2213867
6. Bouma HK, Labos C, Gore GC, et al. The diagnostic accuracy of routine electroencephalography after a first unprovoked seizure. Eur J Neurol. 2016;23:455-463. doi: 10.1111/ene.12739
7. Narayanan JT, Labar DR, Schaul N. Latency to first spike in the EEG of epilepsy patients. Seizure. 2008;17:34-41. doi: 10.1016/j.seizure.2007.06.003
8. Salmenpera TM, Duncan JS. Imaging in epilepsy. J Neurol Neurosurg Psychiatry. 2005;76:iii2-iii10. doi: 10.1136/jnnp.2005.075135
9. Jackson GD, Berkovic SF, Tress , et al Hippocampal sclerosis can be reliably detected by magnetic resonance imaging. Neurology. 1990;40:1869-1875. doi: 10.1212/wnl.40.12.1869
10. Bonnett LJ, Kim, L, Johnson A, et al. Risk of seizure recurrence in people with single seizures and early epilepsy - model development and external validation. Seizure. 2022;94:26-32. doi: 10.1016/j.seizure.2021.11.007
11. Krumholz A, Wiebe S, Gronseth GS, et al. Evidence-based guideline: management of an unprovoked first seizure in adults: Report of the Guideline Development Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology. 2015;84:1705-1713. doi: 10.1212/WNL.0000000000001487
12. Fisher RS, Cross JH, French JA, et al. Operational classification of seizure types by the International League Against Epilepsy: position paper of the ILAE Commission for Classification and terminology. Epilepsia. 2017;58:522-530. doi: 10.1111/epi.13670
13. Berg AT, Berkovic SF, Brodie MJ, et al. Revised terminology and concepts for organization of seizures and epilepsy: report of the ILAE Commission on Classification and Terminology, 2005-2009. Epilepsia. 2010;51:676-685. doi: 10.1111/j.1528-1167.2010.02522.x
14. Marson AG, Al-Kharusi AM, Alwaidh M, et al. The SANAD study of effectiveness of valproate, lamotrigine, or topiramate for generalized and unclassifiable epilepsy: an unblinded randomized controlled trial. Lancet. 2007;369:1016-1026. doi: 10.1016/S0140-6736(07)60461-9
15. Marson AG, Al-Kharusi AM, Alwaidh M, et al. The SANAD study of effectiveness of carbamazepine, gabapentin, lamotrigine, oxcarbazepine, or topiramate for treatment of partial epilepsy: an unblinded randomized controlled trial. Lancet 2007;369:1000-1015. doi: 10.1016/S0140-6736(07)60460-7
16. Marson A, Burnside G, Appleton R, et al. The SANAD II study of the effectiveness and cost-effectiveness of valproate versus levetiracetam for newly diagnosed generalized and unclassified epilepsy: an open-label, non-inferiority, multicentre, phase 4, randomized controlled trial. Lancet. 2021;397:1375-1386. doi: 10.1016/S0140-6736(21)00246-4
17. Mawhinney E, Craig J, Morrow J. Levetiracetam in pregnancy: results from the UK and Ireland epilepsy and pregnancy registers. Neurology. 2013;80:400-405.
18. Marson A, Burnside G, Appleton R, et al. The SANAD II study of the effectiveness and cost-effectiveness of levetiracetam, zonisamide, or lamotrigine for newly diagnosed focal epilepsy: an open-label, non-inferiority, multicentre, phase 4, randomized controlled trial. Lancet. 2021;397:1363-1374. doi: 10.1016/S0140-6736(21)00247-6
19. Smith PE. Initial management of seizure in adults. N Engl J Med. 2021;385:251-263. doi: 10.1056/NEJMcp2024526
20. Depakene (valproic acid). Package insert. Abbott Laboratories; 2011. Accessed October 6, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2011/018081s046_18082s031lbl.pdf
21. Greenberg RG, Melloni C, Wu H, et al. Therapeutic index estimation of antiepileptic drugs: a systematic literature review approach. Clin Neuropharmacol. 2016;39:232-240. doi: 10.1097/WNF.0000000000000172
22. Lamictal (lamotrigine). Package insert. GlaxoSmithKline; 2009. Accessed October 6, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2009/020241s037s038,020764s030s031lbl.pdf
23. LaRoche SM, Helmers SL. The new antiepileptic drugs: scientific review. JAMA. 2004;291:605-614. doi: 10.1001/jama.291.5.605
24. Topamax (topiramate). Package insert. Janssen Pharmaceuticals, Inc. Accessed October 6, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2012/020844s041lbl.pdf
25. Keppra (levetiracetam). Package insert. UCB, Inc.; 2009. Accessed October 6, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2009/021035s078s080%2C021505s021s024lbl.pdf
26. Carbatrol (carbamazepine). Package insert. Shire US Inc; 2013. Accessed October 6, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2013/020712s032s035lbl.pdf
27.Neurontin (gabapentin). Package insert. Pfizer; 2017. Accessed October 6, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2017/020235s064_020882s047_021129s046lbl.pdf
28.Zonegran (zonisamide). Package insert. Eisai Inc; 2006. Accessed October 6, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2006/020789s019lbl.pdf
29.Gloss D, Paragon K, Pack A, et al. Antiseizure medication withdrawal in seizure-free patients: practice advisory update. Report of the AAN Guideline Subcommittee. Neurology. 2021;97:1072-1081. doi: 10.1212/WNL.0000000000012944
30.Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med. 2000:342:314-319. doi: 10.1056/NEJM200002033420503
31.Kwan P, Arzimanoglou A, Berg AT, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia. 2010;51:1069-1077. doi: 10.1111/j.1528-1167.2009.02397.x
Compliance during treatment of epilepsy. Epilepsia 1988;29(suppl 2):S79-S84.
33.utter R, Rüegg S, Tschudin-Sutter S. Seizures as adverse events of antibiotic drugs: a systematic review. Neurology. 2015;13;85:1332-1341. doi: 10.1212/WNL.0000000000002023
34.Singh G, Rees JH, Sander JW. Seizures and epilepsy in oncological practice: causes, course, mechanisms and treatment. JNNP. 2007;78:342-349. doi: 10.1136/jnnp.2006.106211
35.Pisani F, Oteri G, Costa C., et al. Effects of psychotropic drugs on seizure threshold. Drug Safety. 2002;25:91-110.
36.Rossi KC, Joe J, Makhjia M, et al. Insufficient sleep, electroencephalogram activation, and seizure risk: re-evaluating the evidence. Ann Neurol. 2020;86:798-806. doi: 10.1002/ana.25710
37.Watson NF, Badr MS, Belenky G, et al. Recommended amount of sleep for a healthy adult: a Joint Consensus Statement of the American Academy of Sleep Medicine and Sleep Research Society. Sleep. 2015;38:843-844. doi: 10.5665/sleep.4716
38.Höppener RJ, Kuyer A, van der Lugt PJ. Epilepsy and alcohol: the influence of social alcohol intake on seizures and treatment in epilepsy. Epilepsia. 1983;24:459-471. doi: 10.1111/j.1528-1157.1983.tb04917.x
39.Keeler MH, Reifler CB. Grand mal convulsions subsequent to marijuana use. Case report. Dis Nerv Syst. 1967:28:474-475.
40.Epidiolex (cannabidiol). Package insert. Greenwich Biosciences Inc; 2018. Accessed September 27, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2018/210365lbl.pdf
41.ClinicalTrials.gov. Maternal Outcomes and Neurodevelopmental Effects of Antiseizure Drugs (MONEAD). Accessed September 24, 2023. https://classic.clinicaltrials.gov/ct2/show/NCT01730170
42.Meador KJ, Baker GA, Finnell RH, et al. In utero antiepileptic drug exposure: fetal death and malformations. Neurology. 2006;67:407-412. doi: 10.1212/01.wnl.0000227919.81208.b2
43.Meador K, Reynolds MW, Crean S. Pregnancy outcomes in women with epilepsy: a systematic review and meta-analysis of published pregnancy registries and cohorts. Epilepsy Res. 2008;81:1-13. doi:10.1016/j.eplepsyres.2008.04.022
44.Marxer CA, Rüegg S, Rauch A review of the evidence on the risk of congenital malformations and neurodevelopmental disorders in association with antiseizure medications during pregnancy. Expert Opin Drug Saf. 2021;20:1487-1499. doi: 10.1080/14740338.2021.1943355
Asadi-Pooya AA, Sperling MR. Epidemiology of psychogenic nonepileptic seizures. Epilepsy Behav. 2015;46:60-65. doi: 10.1016/j.yebeh.2015.03.015
Evaluation and treatment of psychogenic nonepileptic seizures. Neurol Clin. 2022;40:799-820. doi: 10.1016/j.ncl.2022.03.017
47.Motamedi GK. Psychogenic nonepileptic seizures: a disconnect between body and mind. Epilepsy Behav. 2018;78:293-294. doi: 10.1016/j.yebeh.2017.10.016
, Nonconvulsive status epilepticus. Emerg Med Clin North Am. 2011;29:65-72. doi: 10.1016/j.emc.2010.08.006
doi: 10.1542/peds.2010-3318
Drug management for acute tonic-clonic convulsions including convulsive status epilepticus in children. Cochrane Database Sys Rev. 2018;1(1):CD001905. doi: 10.1002/14651858.CD001905.pub3
52.Jensen FE. Epilepsy as a spectrum disorder: implications from novel clinical and basic neuroscience. Epilepsia. 2011;52(suppl 1):1-6. doi: 10.1111/j.1528-1167.2010.02904.x
53.Kass JS, Rose RV. Driving and epilepsy: ethical, legal, and health care policy challenges. Continuum (Minneap Minn). 2019;25:537-542. doi: 10.1212/CON.0000000000000714
54.Troxell J. Epilepsy and employment: the Americans with Disabilities Act and its protections against employment discrimination. Med Law. 1997;16:375-384.
PRACTICE RECOMMENDATIONS
› Consider treating a first-time seizure if electroencephalography shows particular epileptiform activity, if the neurologic exam or computerized tomography or magnetic resonance imaging results are abnormal, if the seizure is focal or nocturnal, or if there is a family history of seizures. A
› Consider valproate (except for women of childbearing age) and levetiracetam as first-line agents for generalized or unclassified epilepsy, and lamotrigine for focal epilepsies. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Essential oils: How safe? How effective?
Essential oils (EOs), which are concentrated plant-based oils, have become ubiquitous over the past decade. Given the far reach of EOs and their longtime use in traditional, complementary, alternative, and integrative medicine, it is imperative that clinicians have some knowledge of the potential benefits, risks, and overall efficacy.
Commonly used for aromatic benefits (aromatherapy), EOs are now also incorporated into a multitude of products promoting health and wellness. EOs are sold as individual products and can be a component in consumer goods such as cosmetics, body care/hygiene/beauty products, laundry detergents, insect repellents, over-the-counter medications, and food.
The review that follows presents the most current evidence available. With that said, it’s important to keep in mind some caveats that relate to this evidence. First, the studies cited tend to have a small sample size. Second, a majority of these studies were conducted in countries where there appears to be a significant culture of EO use, which could contribute to confirmation bias. Finally, in a number of the studies, there is concern for publication bias as well as a discrepancy between calculated statistical significance and actual clinical relevance.

What are essential oils?
EOs generally are made by extracting the oil from leaves, bark, flowers, seeds/fruit, rinds, and/or roots by steaming or pressing parts of a plant. It can take several pounds of plant material to produce a single bottle of EO, which usually contains ≥ 15 to 30 mL (.5 to 1 oz).1
Some commonly used EOs in the United States are lavender, peppermint, rose, clary sage, tea tree, eucalyptus, and citrus; however, there are approximately 300 EOs available.2 EOs are used most often via topical application, inhalation, or ingestion.
As with any botanical agent, EOs are complex substances often containing a multitude of chemical compounds.1 Because of the complex makeup of EOs, which often contain up to 100 volatile organic compounds, and their wide-ranging potential effects, applying the scientific method to study effectiveness poses a challenge that has limited their adoption in evidence-based practice.2
Availability and cost. EOs can be purchased at large retailers (eg, grocery stores, drug stores) and smaller health food stores, as well as on the Internet. Various EO vehicles, such as inhalers and topical creams, also can be purchased at these stores.
Continue to: The cost varies...
The cost varies enormously by manufacturer and type of plant used to make the EO. Common EOs such as peppermint and lavender oil generally cost $10 to $25, while rarer plant oils can cost $80 or more per bottle.
How safe are essential oils?
Patients may assume EOs are harmless because they are derived from natural plants and have been used medicinally for centuries. However, care must be taken with their use.
The safest way to use EOs is topically, although due to their highly concentrated nature, EOs should be diluted in an unscented neutral carrier oil such as coconut, jojoba, olive, or sweet almond.3 Ingestion of certain oils can cause hepatotoxicity, seizures, and even death.3 In fact, patients should speak with a knowledgeable physician before purchasing any oral EO capsules.
Whether used topically or ingested, all EOs carry risk for skin irritation and allergic reactions, and oral ingestion may result in some negative gastrointestinal (GI) adverse effects.4 A case report of 3 patients published in 2007 identified the potential for lavender and tea tree EOs to be endocrine disruptors.5
Inhalation of EOs may be harmful, as they emit many volatile organic compounds, some of which are considered potentially hazardous.6 At this time, there is insufficient evidence regarding inhaled EOs and their direct connection to respiratory health. It is reasonable to suggest, however, that the prolonged use of EOs and their use by patients who have lung conditions such as asthma or COPD should be avoided.7
Continue to: How are quality and purity assessed?
How are quality and purity assessed?
Like other dietary supplements, EOs are not regulated. No US regulatory agencies (eg, the US Food and Drug Administration [FDA] or Department of Agriculture [USDA]) certify or approve EOs for quality and purity. Bottles labeled with “QAI” for Quality Assurance International or “USDA Organic” will ensure the plant constituents used in the EO are from organic farming but do not attest to quality or purity.
Manufacturers commonly use marketing terms such as “therapeutic grade” or “pure” to sell products, but again, these terms do not reflect the product’s quality or purity. A labeled single EO may contain contaminants, alcohol, or additional ingredients.7 When choosing to use EOs, identifying reputable brands is essential; one resource is the independent testing organization ConsumerLab.com.
It is important to assess the manufacturer and read ingredient labels before purchasing an EO to understand what the product contains. Reputable companies will identify the plant ingredient, usually by the formal Latin binomial name, and explain the extraction process. A more certain way to assess the quality and purity of an EO is to ask the manufacturer to provide a certificate of analysis and gas chromatography/mass spectroscopy (GC/MS) data for the specific product. Some manufacturers offer GC/MS test results on their website Quality page.8 Others have detailed information on quality and testing, and GC/MS test reports can be obtained.9 Yet another manufacturer has test results on a product page matching reports to batch codes.10
Which conditions have evidence of benefit from essential oils?
EOs currently are being studied for treatment of many conditions—including pain, GI disorders, behavioral health disorders, and women’s health issues. The TABLE summarizes the conditions treated, outcomes, and practical applications of EOs.11-44
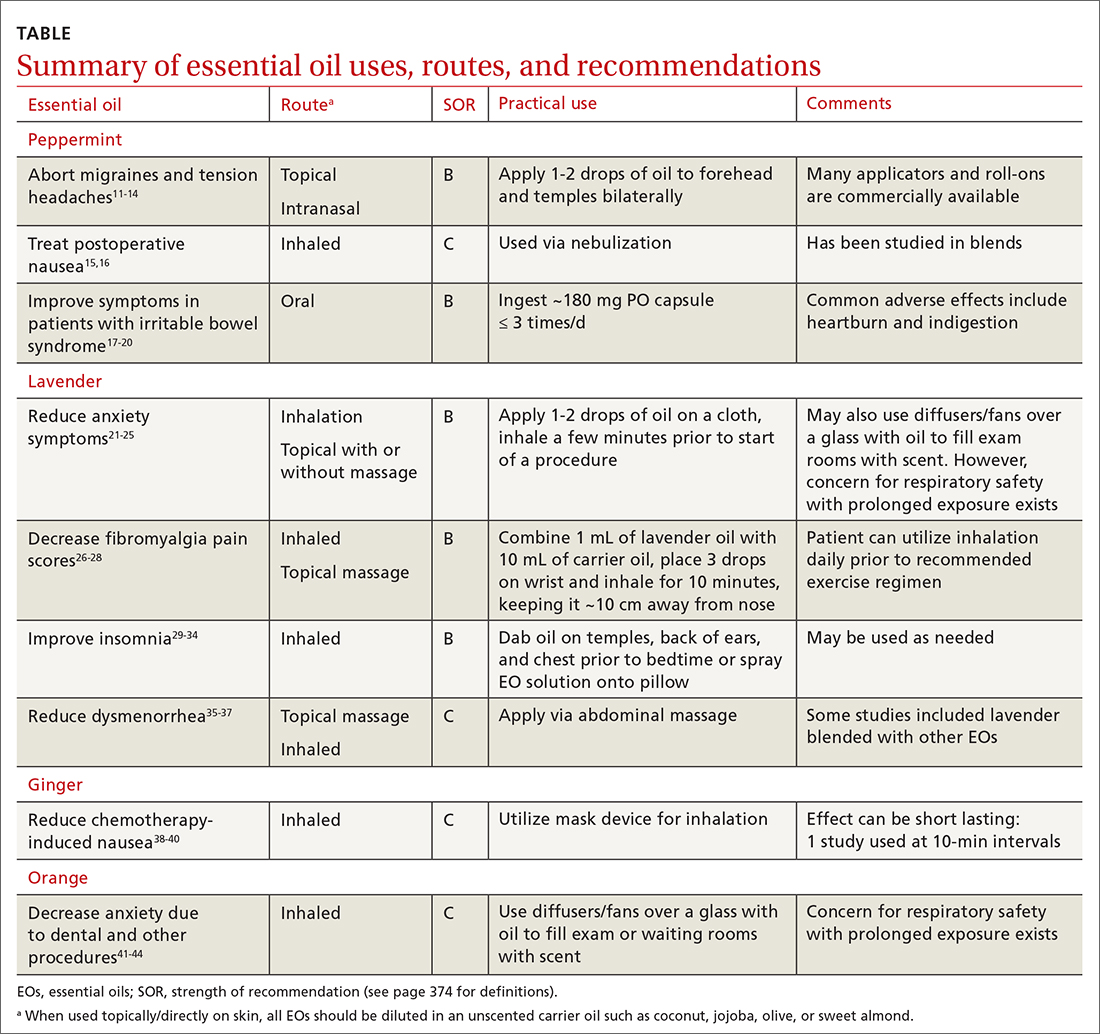
Pain
Headache. As an adjunct to available medications and procedures for headache treatment, EOs are one of the nonpharmacologic modalities that patients and clinicians have at their disposal for both migraine and tension-type headaches. A systematic review of 19 randomized controlled trials (RCTs) examining the effects of herbal ingredients for the acute treatment or prophylaxis of migraines found certain topically applied or inhaled EOs, such as peppermint and chamomile, to be effective for migraine pain alleviation; however, topically applied rose oil was not effective.11-13 Note: “topical application” in these studies implies application of the EO to ≥ 1 of the following areas: temples, forehead, behind ears, or above upper lip/below the nose.
Continue to: One RCT with 120 patients...
One RCT with 120 patients evaluated diluted intranasal peppermint oil and found that it reduced migraine intensity at similar rates to intranasal lidocaine.13 In this study, patients were randomized to receive one of the following: 4% lidocaine, 1.5% peppermint EO, or placebo. Two drops of the intranasal intervention were self-administered while the patient was in a supine position with their head suspended off the edge of the surface on which they were lying. They were instructed to stay in this position for at least 30 seconds after administration.
With regard to tension headache treatment, there is limited literature on the use of EOs. One study found that a preparation of peppermint oil applied topically to the temples and forehead of study participants resulted in significant analgesic effect.14
Fibromyalgia. Usual treatments for fibromyalgia include exercise, antidepressant and anticonvulsant medications, and stress management. Evidence also supports the use of inhaled and topically applied (with and without massage) lavender oil to improve symptoms.26 Positive effects may be related to the analgesic, anti-inflammatory, sleep-regulating, and anxiety-reducing effects of the major volatile compounds contained in lavender oil.
In one RCT with 42 patients with fibromyalgia, the use of inhaled lavender oil was shown to increase the perception of well-being (assessed on the validated SF-36 Health Survey Questionnaire) after 4 weeks.27 In this study, the patient applied 3 drops of an oil mixture, comprising 1 mL lavender EO and 10 mL of fixed neutral base oil, to the wrist and inhaled for 10 minutes before going to bed.
The use of a topical oil blend labeled “Oil 24” (containing camphor, rosemary, eucalyptus, peppermint, aloe vera, and lemon/orange) also has been shown to be more effective than placebo in managing fibromyalgia symptoms. A randomized controlled pilot study of 153 participants found that regular application of Oil 24 improved scores on pain scales and the Fibromyalgia Impact Questionnaire.28
Continue to: GI disorders
GI disorders
Irritable bowel syndrome. Peppermint oil relaxes GI smooth muscle, which has led to investigation of its use in irritable bowel syndrome (IBS) symptom amelioration.17 One meta-analysis including 12 RCTs with 835 patients with undifferentiated IBS found that orally ingested peppermint EO capsules reduced patient-reported symptoms of either abdominal pain or global symptoms.18
One study utilized the Total IBS Symptom Score to evaluate symptom reduction in patients with IBS-D (with diarrhea) and IBS-M (mixed) using 180-mg peppermint EO capsules ingested 3 times daily. There was a significant improvement in abdominal pain/discomfort, bloating/distension, pain at evacuation, and bowel urgency.19 A reduction in symptoms was observed after the first 24 hours of treatment and at the end of the 4-week treatment period.
In another study, among the 190 patients meeting Rome IV criteria for general (nonspecific) IBS who were treated with 182-mg peppermint EO capsules, no statistically significant reduction in overall symptom relief was found (based on outcome measures by the FDA and European Medicines Agency). However, in a secondary outcome analysis, peppermint oil produced greater improvements than placebo for the alleviation of abdominal pain, discomfort, and general IBS severity.20
Chemotherapy-induced nausea and vomiting. Patients with cancer undergoing chemotherapy often explore integrative medicine approaches, including aromatherapy, to ameliorate adverse effects and improve quality of life.38 A few small studies have shown potential for the use of inhaled ginger oil to reduce nausea and vomiting severity and improve health-related quality-of-life measures in these patients.
For example, a study with 60 participants found that inhaling ginger EO for 10 minutes was beneficial for reducing both nausea and vomiting.39 A single-blind, controlled, randomized crossover study of 60 patients with breast cancer receiving chemotherapy showed that ginger EO inhaled 3 times per day for 2 minutes at a time can decrease the severity of nausea but had no effect on vomiting. The same study showed that health-related quality of life improved with the ginger oil treatment.40
Continue to: Other EOs such as cardamom...
Other EOs such as cardamom and peppermint show promise as an adjunctive treatment for chemotherapy-induced nausea and vomiting as well.38
Postoperative nausea. A 2013 randomized trial of 303 patients examined the use of ginger EO, a blend of EOs (including ginger, spearmint, peppermint, and cardamom), and isopropyl alcohol. Both the single EO and EO blend significantly reduced the symptom of nausea. The number of antiemetic medications requested by patients receiving an EO also was significantly reduced compared to those receiving saline.15
The use of EOs to reduce nausea after cardiac operations was reviewed in an RCT of 60 surgical candidates using 10% peppermint oil via nebulization for 10 minutes.16 This technique was effective in reducing nausea during cardiac postoperative periods. Although the evidence for the use of EOs for postoperative nausea is not robust, it may be a useful and generally safe approach for this common issue.
Behavioral health
Insomnia. EOs have been used as a treatment for insomnia traditionally and in complementary, alternative, and integrative medicine. A 2014 systematic review of 15 quantitative studies, including 11 RCTs, evaluated the hypnotic effects of EOs through inhalation, finding the strongest evidence for lavender, jasmine, and peppermint oils.29 The majority of the studies in the systematic review used the Pittsburgh Sleep Quality Index (PSQI) to evaluate EO effectiveness. A more recent 2021 systematic review and meta-analysis that evaluated 34 RCTs found that inhalation of EOs, most notably lavender aromatherapy, is effective in improving sleep problems such as insomnia.30
Findings from multiple smaller RCTs were consistent with those of the aforementioned systematic reviews. For example, in a well-conducted parallel randomized double-blind placebo-controlled trial of 100 people using orally ingested lemon verbena, the authors concluded that this intervention can be a complementary therapy for improving sleep quality and reducing insomnia severity.31 Another RCT with 60 participants evaluated an inhaled EO blend (lemon, eucalyptus, tea tree, and peppermint) over 4 weeks and found lowered perceived stress and depression as well as better sleep quality, but no influence on objective physiologic data such as stress indices or immune states.32
Continue to: In a 2020 randomized crossover...
In a 2020 randomized crossover placebocontrolled trial of 37 participants with diabetes reporting insomnia, inhaled lavender improved sleep quality and quantity, quality of life, and mood but not physiologic or metabolic measures, such as fasting glucose.33 Findings were similar in a cohort of cardiac rehabilitation patients (n = 37) who were treated with either an inhaled combination of lavender, bergamot, and ylang ylang, or placebo; cotton balls infused with the intervention oil or placebo oil were placed at the patient’s bedside for 5 nights. Sleep quality of participants receiving intervention oil was significantly better than the sleep quality of participants receiving the placebo oil as measured by participant completion of the PSQI.34
Anxiety is a common disorder that can be managed with nonpharmacologic treatments such as yoga, deep breathing, meditation, and EO therapy.21,22 In a systematic review and meta-analysis, the inhaled and topical use (with or without massage) of lavender EO was shown to improve psychological and physical manifestations of anxiety.23 Lavender EO is purported to affect the parasympathetic nervous system via anxiolytic, sedative, analgesic, and anticonvulsant properties.24 One systematic review and meta-analysis evaluating the anxiolytic effect of both inhaled and topical lavender EO found improvement in several biomarkers and physiologic data including blood pressure, heart rate, and cortisol levels, as well as a reduction in self-reported levels of anxiety, compared with placebo.25
Anxiety related to dental procedures is another area of study for the use of EOs. Two RCTs demonstrate statistically significant improvement in anxiety-related physiologic markers such as heart rate, blood pressure, and salivary cortisol levels in children who inhaled lavender EO during dental procedures.41,42 In 1 of the RCTs, the intervention was described as 3 drops of 100% lavender EO applied to a cloth and inhaled over the course of 3 minutes.41 Additionally, 2 studies found that orange EO was beneficial for dental procedure–induced anxiety, reducing pulse rates, cortisol levels, and self-reported anxiety.43,44
Dementia-related behavioral disturbances. A small, poorly designed study examining 2 EO blends—rosemary with lemon and lavender with orange—found some potential for improving cognitive function, especially in patients with Alzheimer disease.45 A Cochrane review of 13 RCTs totaling 708 patients concluded that it is not certain from the available evidence that EO therapy benefits patients with dementia in long-term-care facilities and hospital wards.46 Given that reporting of adverse events in the trials was poor, it is not possible to make conclusions about the risk vs benefit of EO therapy in this population.
Women’s health
Dysmenorrhea.
Continue to: In a randomized, double-blind clinical trial...
In a randomized, double-blind clinical trial of 48 women, a cream-based blend of lavender, clary sage, and marjoram EO (used topically in a 2:1:1 ratio diluted in unscented cream at 3% concentration and applied daily via abdominal massage) reduced participants’ reported menstrual pain symptoms and duration of pain.36 In a meta-analysis of 6 studies, topical abdominal application of EO (mainly lavender with or without other oils) with massage showed superiority over massage with placebo oils in reducing menstrual pain.37 A reduction in pain, mood symptoms, and fatigue in women with premenstrual symptoms was seen in an RCT of 77 patients using 3 drops of inhaled lavender EO.47
Labor. There is limited evidence for the use of EOs during labor. In an RCT of 104 women, patient-selected diffused EOs, including lavender, rose geranium, citrus, or jasmine, were found to help lower pain scores during the latent and early active phase of labor. There were no differences in labor augmentation, length of labor, perinatal outcomes, or need for additional pain medication.48
Other uses
Antimicrobial support. Some common EOs that have demonstrated antimicrobial properties are oregano, thyme, clove, lavender, clary sage, garlic, and cinnamon.49,50 Topical lemongrass and tea tree EOs have shown some degree of efficacy as an alternative treatment for acne, decolonization of methicillin-resistant Staphylococcus aureus, and superficial fungal infections.51 Support for an oral mixture of EOs labeled Myrtol (containing eucalyptus, citrus myrtle, and lavender) for viral acute bronchitis and sinusitis was found in a review of 7 studies.52 More research needs to be done before clear recommendations can be made on the use of EOs as antimicrobials, but the current data are encouraging.
Insect repellent. Reviews of the insect-repellent properties of EOs have shown promise and are in the public’s interest due to increasing awareness of the potential health and environmental hazards of synthetic repellents.53 Individual compounds present in EOs such as citronella/lemongrass, basil, and eucalyptus species demonstrate high repellent activity.54 Since EOs require frequent reapplication for efficacy due to their highly volatile nature, scientists are currently developing a means to prolong their protection time through cream-based formulations.55
The bottom line
Because of the ubiquity of EOs, family physicians will undoubtedly be asked about them by patients, and it would be beneficial to feel comfortable discussing their most common uses. For most adult patients, the topical and periodic inhaled usage of EOs is generally safe.56
There is existing evidence of efficacy for a number of EOs, most strongly for lavender and peppermint. Future research into EOs should include higher-powered and higher-quality studies in order to provide more conclusive evidence regarding the continued use of EOs for many common conditions. More evidence-based information on dosing, application/use regimens, and safety in long-term use also will help providers better instruct patients on how to utilize EOs effectively and safely.
CORRESPONDENCE
Pooja Amy Shah, MD, Columbia University College of Physicians & Surgeons, 610 West 158th Street, New York, NY 10032; pas2176@cumc.columbia.edu
1. Butnariu M, Sarac I. Essential oils from plants. J Biotechnol Biomed Sci. 2018;1:35-43. doi: 10.14302/issn.2576-6694.jbbs-18-2489
2. Singh B, Sellam P, Majumder, J, et al. Floral essential oils : importance and uses for mankind. HortFlora Res Spectr. 2014;3:7-13. www.academia.edu/6707801/Floral_essential_oils_Importance_and_uses_for_mankind
3. Posadzki P, Alotaibi A, Ernst E. Adverse effects of aromatherapy: a systematic review of case reports and case series. Int J Risk Saf Med. 2012;24:147-161. doi: 10.3233/JRS-2012-0568
4. Sharmeen JB, Mahomoodally FM, Zengin G, et al. Essential oils as natural sources of fragrance compounds for cosmetics and cosmeceuticals. Molecules. 2021;26:666. doi: 10.3390/molecules26030666
5. Henley DV, Lipson N, Korach KS, et al. Prepubertal gynecomastia linked to lavender and tea tree oils. N Engl J Med. 2007;356:479-485. doi: 10.1056/NEJMoa064725
6. Nematollahi N, Weinberg JL, Flattery J, et al. Volatile chemical emissions from essential oils with therapeutic claims. Air Qual Atmosphere Health. 2021;14:365-369. doi: 10.1007/s11869-020-00941-4
7. Balekian D, Long A. Essential oil diffusers and asthma. Published February 24, 2020. Accessed September 22, 2023. www.aaaai.org/Allergist-Resources/Ask-the-Expert/Answers/Old-Ask-the-Experts/oil-diffusers-asthma
8. Aura Cacia. Quality. Accessed September 22, 2023. www.auracacia.com/quality
9. Now. Essential oil identity & purity testing. Accessed September 22, 2023. www.nowfoods.com/quality-safety/essential-oil-identity-purity-testing
10. Aura Cacia. GCMS documents. Accessed September 22, 2023. www.auracacia.com/aura-cacia-gcms-documents
11. Lopresti AL, Smith SJ, Drummond PD. Herbal treatments for migraine: a systematic review of randomised-controlled studies. Phytother Res. 2020;34:2493-2517. doi: 10.1002/ptr.6701
12. Niazi M, Hashempur MH, Taghizadeh M, et al. Efficacy of topical Rose (Rosa damascena Mill.) oil for migraine headache: A randomized double-blinded placebo-controlled cross-over trial. Complement Ther Med. 2017;34:35-41. doi: 10.1016/j.ctim. 2017.07.009
13. Rafieian-Kopaei M, Hasanpour-Dehkordi A, Lorigooini Z, et al. Comparing the effect of intranasal lidocaine 4% with peppermint essential oil drop 1.5% on migraine attacks: a double-blind clinical trial. Int J Prev Med. 2019;10:121. doi: 10.4103/ijpvm.IJPVM_530_17
14. Göbel H, Fresenius J, Heinze A, et al. [Effectiveness of Oleum menthae piperitae and paracetamol in therapy of headache of the tension type]. Nervenarzt. 1996;67:672-681. doi: 10.1007/s001150050040
15. Hunt R, Dienemann J, Norton HJ, et al. Aromatherapy as treatment for postoperative nausea: a randomized trial. Anesth Analg. 2013;117:597-604. doi: 10.1213/ANE.0b013e31824a0b1c
16. Maghami M, Afazel MR, Azizi-Fini I, et al. The effect of aromatherapy with peppermint essential oil on nausea and vomiting after cardiac surgery: a randomized clinical trial. Complement Ther Clin Pract. 2020;40:101199. doi: 10.1016/j.ctcp.2020.101199
17. Hills JM, Aaronson PI. The mechanism of action of peppermint oil on gastrointestinal smooth muscle. An analysis using patch clamp electrophysiology and isolated tissue pharmacology in rabbit and guinea pig. Gastroenterology. 1991;101:55-65. doi: 10.1016/0016-5085(91)90459-x
18. Alammar N, Wang L, Saberi B, et al. The impact of peppermint oil on the irritable bowel syndrome: a meta-analysis of the pooled clinical data. BMC Complement Altern Med. 2019;19:21. doi: 10.1186/s12906-018-2409-0
19. Cash BD, Epstein MS, Shah SM. A novel delivery system of peppermint oil is an effective therapy for irritable bowel syndrome symptoms. Dig Dis Sci. 2016;61:560-571. doi: 10.1007/s10620-015-3858-7
20. Weerts ZZRM, Masclee AAM, Witteman BJM, et al. Efficacy and safety of peppermint oil in a randomized, double-blind trial of patients with irritable bowel syndrome. Gastroenterology. 2020;158:123-136. doi: 10.1053/j.gastro.2019.08.026
21. Ma X, Yue ZQ, Gong ZQ, et al. The effect of diaphragmatic breathing on attention, negative affect and stress in healthy adults. Front Psychol. 2017;8:874. doi: 10.3389/fpsyg.2017.00874
22. Cabral P, Meyer HB, Ames D. Effectiveness of yoga therapy as a complementary treatment for major psychiatric disorders: a meta-analysis. Prim Care Companion CNS Disord. Published July 7, 2011. doi: 10.4088/PCC.10r01068
23. Donelli D, Antonelli M, Bellinazzi C, et ala. Effects of lavender on anxiety: systematic review and meta-analysis. Phytomedicine Int J Phytother Phytopharm. 2019;65:153099. doi: 10.1016/j.phymed.2019.153099
24. Koulivand PH, Khaleghi Ghadiri M, Gorji A. Lavender and the nervous system. Evid Based Complement Alternat Med. 2013;2013:1-10. doi: 10.1155/2013/681304
25. Kang HJ, Nam ES, Lee Y, et al. How strong is the evidence for the anxiolytic efficacy of lavender? Systematic review and meta-analysis of randomized controlled trials. Asian Nurs Res. 2019;13:295-305. doi: 10.1016/j.anr.2019.11.003
26. Barão Paixão VL, Freire de Carvalho J. Essential oil therapy in rheumatic diseases: a systematic review. Complement Ther Clin Pract. 2021;43:101391. doi: 10.1016/j.ctcp.2021.101391
27. Yasa Ozturk G, Bashan I. The effect of aromatherapy with lavender oil on the health-related quality of life in patients with fibromyalgia. J Food Qual. 2021;2021:1-5. doi: 10.1155/2021/9938630
28. Ko GD, Hum A, Traitses G, et al. Effects of topical O24 essential oils on patients with fibromyalgia syndrome: a randomized, placebo controlled pilot study. J Musculoskelet Pain. 2007;15:11-19. doi: 10.1300/J094v15n01_03
29. Lillehei AS, Halcon LL. A systematic review of the effect of inhaled essential oils on sleep. J Altern Complement Med. 2014;20:441-451. doi: 10.1089/acm.2013.0311
30. Cheong MJ, Kim S, Kim JS, et al. A systematic literature review and meta-analysis of the clinical effects of aroma inhalation therapy on sleep problems. Medicine (Baltimore). 2021;100:e24652. doi: 10.1097/MD.0000000000024652
31. Afrasiabian F, Mirabzadeh Ardakani M, Rahmani K, et al. Aloysia citriodora Paláu (lemon verbena) for insomnia patients: a randomized, double-blind, placebo-controlled clinical trial of efficacy and safety. Phytother Res PTR. 2019;33:350-359. doi: 10.1002/ptr.6228
32. Lee M, Lim S, Song JA, et al. The effects of aromatherapy essential oil inhalation on stress, sleep quality and immunity in healthy adults: randomized controlled trial. Eur J Integr Med. 2017;12:79-86. doi: 10.1016/j.eujim.2017.04.009
33. Nasiri Lari Z, Hajimonfarednejad M, Riasatian M, et al. Efficacy of inhaled Lavandula angustifolia Mill. Essential oil on sleep quality, quality of life and metabolic control in patients with diabetes mellitus type II and insomnia. J Ethnopharmacol. 2020;251:112560. doi: 10.1016/j.jep.2020.112560
34. McDonnell B, Newcomb P. Trial of essential oils to improve sleep for patients in cardiac rehabilitation. J Altern Complement Med N Y N. 2019;25:1193-1199. doi: 10.1089/acm.2019.0222
35. Song JA, Lee MK, Min E, et al. Effects of aromatherapy on dysmenorrhea: a systematic review and meta-analysis. Int J Nurs Stud. 2018;84:1-11. doi: 10.1016/j.ijnurstu.2018.01.016
36. Ou MC, Hsu TF, Lai AC, et al. Pain relief assessment by aromatic essential oil massage on outpatients with primary dysmenorrhea: a randomized, double-blind clinical trial: PD pain relief by aromatic oil massage. J Obstet Gynaecol Res. 2012;38:817-822. doi: 10.1111/j.1447-0756.2011.01802.x
37. Sut N, Kahyaoglu-Sut H. Effect of aromatherapy massage on pain in primary dysmenorrhea: a meta-analysis. Complement Ther Clin Pract. 2017;27:5-10. doi: 10.1016/j.ctcp.2017.01.001
38. Keyhanmehr AS, Kolouri S, Heydarirad G, et al. Aromatherapy for the management of cancer complications: a narrative review. Complement Ther Clin Pract. 2018;31:175-180. doi: 10.1016/j.ctcp.2018.02.009
39. Sriningsih I, Elisa E, Lestari KP. Aromatherapy ginger use in patients with nausea & vomiting on post cervical cancer chemotherapy. KEMAS J Kesehat Masy. 2017;13:59-68. doi: 10.15294/kemas.v13i1.5367
40. Lua PL, Salihah N, Mazlan N. Effects of inhaled ginger aromatherapy on chemotherapy-induced nausea and vomiting and health-related quality of life in women with breast cancer. Complement Ther Med. 2015;23:396-404. doi: 10.1016/j.ctim.2015.03.009
41. Arslan I, Aydinoglu S, Karan NB. Can lavender oil inhalation help to overcome dental anxiety and pain in children? A randomized clinical trial. Eur J Pediatr. 2020;179:985-992. doi: 10.1007/s00431-020-03595-7
42. Ghaderi F, Solhjou N. The effects of lavender aromatherapy on stress and pain perception in children during dental treatment: a randomized clinical trial. Complement Ther Clin Pract. 2020;40:101182. doi: 10.1016/j.ctcp.2020.101182
43. Jafarzadeh M, Arman S, Pour FF. Effect of aromatherapy with orange essential oil on salivary cortisol and pulse rate in children during dental treatment: a randomized controlled clinical trial. Adv Biomed Res. 2013;2:10. doi: 10.4103/2277-9175.107968
44. Lehrner J, Eckersberger C, Walla P, et al. Ambient odor of orange in a dental office reduces anxiety and improves mood in female patients. Physiol Behav. 2000;71:83-86. doi: 10.1016/S0031-9384(00)00308-5
45. Jimbo D, Kimura Y, Taniguchi M, et al. Effect of aromatherapy on patients with Alzheimer’s disease. Psychogeriatrics. 2009;9:173-179. doi: 10.1111/j.1479-8301.2009.00299.x
46. Ball EL, Owen-Booth B, Gray A, et al. Aromatherapy for dementia. Cochrane Database Syst Rev. 2020;(8). doi: 10.1002/14651858.CD003150.pub3
47. Uzunçakmak T, Ayaz Alkaya S. Effect of aromatherapy on coping with premenstrual syndrome: a randomized controlled trial. Complement Ther Med. 2018;36:63-67. doi: 10.1016/j.ctim.2017.11.022
48. Tanvisut R, Traisrisilp K, Tongsong T. Efficacy of aromatherapy for reducing pain during labor: a randomized controlled trial. Arch Gynecol Obstet. 2018;297:1145-1150. doi: 10.1007/s00404-018-4700-1
49. Ramsey JT, Shropshire BC, Nagy TR, et al. Essential oils and health. Yale J Biol Med. 2020;93:291-305.
50. Puškárová A, Bučková M, Kraková L, et al. The antibacterial and antifungal activity of six essential oils and their cyto/genotoxicity to human HEL 12469 cells. Sci Rep. 2017;7:8211. doi: 10.1038/s41598-017-08673-9
51. Deyno S, Mtewa AG, Abebe A, et al. Essential oils as topical anti-infective agents: a systematic review and meta-analysis. Complement Ther Med. 2019;47:102224. doi: 10.1016/j.ctim.2019.102224
52. Prall S, Bowles EJ, Bennett K, et al. Effects of essential oils on symptoms and course (duration and severity) of viral respiratory infections in humans: a rapid review. Adv Integr Med. 2020;7:218-221. doi: 10.1016/j.aimed.2020.07.005
53. Weeks JA, Guiney PD, Nikiforov AI. Assessment of the environmental fate and ecotoxicity of N,N-diethyl-m-toluamide (DEET). Integr Environ Assess Manag. 2012;8:120-134. doi: 10.1002/ieam.1246
54. Nerio LS, Olivero-Verbel J, Stashenko E. Repellent activity of essential oils: a review. Bioresour Technol. 2010;101:372-378. doi: 10.1016/j.biortech.2009.07.048
55. Lee MY. Essential oils as repellents against arthropods. BioMed Res Int. 2018;2018:6860271. doi: 10.1155/2018/6860271
56. Göbel H, Heinze A, Heinze-Kuhn K, et al. [Peppermint oil in the acute treatment of tension-type headache]. Schmerz Berl Ger. 2016;30:295-310. doi: 10.1007/s00482-016-0109-6
Essential oils (EOs), which are concentrated plant-based oils, have become ubiquitous over the past decade. Given the far reach of EOs and their longtime use in traditional, complementary, alternative, and integrative medicine, it is imperative that clinicians have some knowledge of the potential benefits, risks, and overall efficacy.
Commonly used for aromatic benefits (aromatherapy), EOs are now also incorporated into a multitude of products promoting health and wellness. EOs are sold as individual products and can be a component in consumer goods such as cosmetics, body care/hygiene/beauty products, laundry detergents, insect repellents, over-the-counter medications, and food.
The review that follows presents the most current evidence available. With that said, it’s important to keep in mind some caveats that relate to this evidence. First, the studies cited tend to have a small sample size. Second, a majority of these studies were conducted in countries where there appears to be a significant culture of EO use, which could contribute to confirmation bias. Finally, in a number of the studies, there is concern for publication bias as well as a discrepancy between calculated statistical significance and actual clinical relevance.

What are essential oils?
EOs generally are made by extracting the oil from leaves, bark, flowers, seeds/fruit, rinds, and/or roots by steaming or pressing parts of a plant. It can take several pounds of plant material to produce a single bottle of EO, which usually contains ≥ 15 to 30 mL (.5 to 1 oz).1
Some commonly used EOs in the United States are lavender, peppermint, rose, clary sage, tea tree, eucalyptus, and citrus; however, there are approximately 300 EOs available.2 EOs are used most often via topical application, inhalation, or ingestion.
As with any botanical agent, EOs are complex substances often containing a multitude of chemical compounds.1 Because of the complex makeup of EOs, which often contain up to 100 volatile organic compounds, and their wide-ranging potential effects, applying the scientific method to study effectiveness poses a challenge that has limited their adoption in evidence-based practice.2
Availability and cost. EOs can be purchased at large retailers (eg, grocery stores, drug stores) and smaller health food stores, as well as on the Internet. Various EO vehicles, such as inhalers and topical creams, also can be purchased at these stores.
Continue to: The cost varies...
The cost varies enormously by manufacturer and type of plant used to make the EO. Common EOs such as peppermint and lavender oil generally cost $10 to $25, while rarer plant oils can cost $80 or more per bottle.
How safe are essential oils?
Patients may assume EOs are harmless because they are derived from natural plants and have been used medicinally for centuries. However, care must be taken with their use.
The safest way to use EOs is topically, although due to their highly concentrated nature, EOs should be diluted in an unscented neutral carrier oil such as coconut, jojoba, olive, or sweet almond.3 Ingestion of certain oils can cause hepatotoxicity, seizures, and even death.3 In fact, patients should speak with a knowledgeable physician before purchasing any oral EO capsules.
Whether used topically or ingested, all EOs carry risk for skin irritation and allergic reactions, and oral ingestion may result in some negative gastrointestinal (GI) adverse effects.4 A case report of 3 patients published in 2007 identified the potential for lavender and tea tree EOs to be endocrine disruptors.5
Inhalation of EOs may be harmful, as they emit many volatile organic compounds, some of which are considered potentially hazardous.6 At this time, there is insufficient evidence regarding inhaled EOs and their direct connection to respiratory health. It is reasonable to suggest, however, that the prolonged use of EOs and their use by patients who have lung conditions such as asthma or COPD should be avoided.7
Continue to: How are quality and purity assessed?
How are quality and purity assessed?
Like other dietary supplements, EOs are not regulated. No US regulatory agencies (eg, the US Food and Drug Administration [FDA] or Department of Agriculture [USDA]) certify or approve EOs for quality and purity. Bottles labeled with “QAI” for Quality Assurance International or “USDA Organic” will ensure the plant constituents used in the EO are from organic farming but do not attest to quality or purity.
Manufacturers commonly use marketing terms such as “therapeutic grade” or “pure” to sell products, but again, these terms do not reflect the product’s quality or purity. A labeled single EO may contain contaminants, alcohol, or additional ingredients.7 When choosing to use EOs, identifying reputable brands is essential; one resource is the independent testing organization ConsumerLab.com.
It is important to assess the manufacturer and read ingredient labels before purchasing an EO to understand what the product contains. Reputable companies will identify the plant ingredient, usually by the formal Latin binomial name, and explain the extraction process. A more certain way to assess the quality and purity of an EO is to ask the manufacturer to provide a certificate of analysis and gas chromatography/mass spectroscopy (GC/MS) data for the specific product. Some manufacturers offer GC/MS test results on their website Quality page.8 Others have detailed information on quality and testing, and GC/MS test reports can be obtained.9 Yet another manufacturer has test results on a product page matching reports to batch codes.10
Which conditions have evidence of benefit from essential oils?
EOs currently are being studied for treatment of many conditions—including pain, GI disorders, behavioral health disorders, and women’s health issues. The TABLE summarizes the conditions treated, outcomes, and practical applications of EOs.11-44

Pain
Headache. As an adjunct to available medications and procedures for headache treatment, EOs are one of the nonpharmacologic modalities that patients and clinicians have at their disposal for both migraine and tension-type headaches. A systematic review of 19 randomized controlled trials (RCTs) examining the effects of herbal ingredients for the acute treatment or prophylaxis of migraines found certain topically applied or inhaled EOs, such as peppermint and chamomile, to be effective for migraine pain alleviation; however, topically applied rose oil was not effective.11-13 Note: “topical application” in these studies implies application of the EO to ≥ 1 of the following areas: temples, forehead, behind ears, or above upper lip/below the nose.
Continue to: One RCT with 120 patients...
One RCT with 120 patients evaluated diluted intranasal peppermint oil and found that it reduced migraine intensity at similar rates to intranasal lidocaine.13 In this study, patients were randomized to receive one of the following: 4% lidocaine, 1.5% peppermint EO, or placebo. Two drops of the intranasal intervention were self-administered while the patient was in a supine position with their head suspended off the edge of the surface on which they were lying. They were instructed to stay in this position for at least 30 seconds after administration.
With regard to tension headache treatment, there is limited literature on the use of EOs. One study found that a preparation of peppermint oil applied topically to the temples and forehead of study participants resulted in significant analgesic effect.14
Fibromyalgia. Usual treatments for fibromyalgia include exercise, antidepressant and anticonvulsant medications, and stress management. Evidence also supports the use of inhaled and topically applied (with and without massage) lavender oil to improve symptoms.26 Positive effects may be related to the analgesic, anti-inflammatory, sleep-regulating, and anxiety-reducing effects of the major volatile compounds contained in lavender oil.
In one RCT with 42 patients with fibromyalgia, the use of inhaled lavender oil was shown to increase the perception of well-being (assessed on the validated SF-36 Health Survey Questionnaire) after 4 weeks.27 In this study, the patient applied 3 drops of an oil mixture, comprising 1 mL lavender EO and 10 mL of fixed neutral base oil, to the wrist and inhaled for 10 minutes before going to bed.
The use of a topical oil blend labeled “Oil 24” (containing camphor, rosemary, eucalyptus, peppermint, aloe vera, and lemon/orange) also has been shown to be more effective than placebo in managing fibromyalgia symptoms. A randomized controlled pilot study of 153 participants found that regular application of Oil 24 improved scores on pain scales and the Fibromyalgia Impact Questionnaire.28
Continue to: GI disorders
GI disorders
Irritable bowel syndrome. Peppermint oil relaxes GI smooth muscle, which has led to investigation of its use in irritable bowel syndrome (IBS) symptom amelioration.17 One meta-analysis including 12 RCTs with 835 patients with undifferentiated IBS found that orally ingested peppermint EO capsules reduced patient-reported symptoms of either abdominal pain or global symptoms.18
One study utilized the Total IBS Symptom Score to evaluate symptom reduction in patients with IBS-D (with diarrhea) and IBS-M (mixed) using 180-mg peppermint EO capsules ingested 3 times daily. There was a significant improvement in abdominal pain/discomfort, bloating/distension, pain at evacuation, and bowel urgency.19 A reduction in symptoms was observed after the first 24 hours of treatment and at the end of the 4-week treatment period.
In another study, among the 190 patients meeting Rome IV criteria for general (nonspecific) IBS who were treated with 182-mg peppermint EO capsules, no statistically significant reduction in overall symptom relief was found (based on outcome measures by the FDA and European Medicines Agency). However, in a secondary outcome analysis, peppermint oil produced greater improvements than placebo for the alleviation of abdominal pain, discomfort, and general IBS severity.20
Chemotherapy-induced nausea and vomiting. Patients with cancer undergoing chemotherapy often explore integrative medicine approaches, including aromatherapy, to ameliorate adverse effects and improve quality of life.38 A few small studies have shown potential for the use of inhaled ginger oil to reduce nausea and vomiting severity and improve health-related quality-of-life measures in these patients.
For example, a study with 60 participants found that inhaling ginger EO for 10 minutes was beneficial for reducing both nausea and vomiting.39 A single-blind, controlled, randomized crossover study of 60 patients with breast cancer receiving chemotherapy showed that ginger EO inhaled 3 times per day for 2 minutes at a time can decrease the severity of nausea but had no effect on vomiting. The same study showed that health-related quality of life improved with the ginger oil treatment.40
Continue to: Other EOs such as cardamom...
Other EOs such as cardamom and peppermint show promise as an adjunctive treatment for chemotherapy-induced nausea and vomiting as well.38
Postoperative nausea. A 2013 randomized trial of 303 patients examined the use of ginger EO, a blend of EOs (including ginger, spearmint, peppermint, and cardamom), and isopropyl alcohol. Both the single EO and EO blend significantly reduced the symptom of nausea. The number of antiemetic medications requested by patients receiving an EO also was significantly reduced compared to those receiving saline.15
The use of EOs to reduce nausea after cardiac operations was reviewed in an RCT of 60 surgical candidates using 10% peppermint oil via nebulization for 10 minutes.16 This technique was effective in reducing nausea during cardiac postoperative periods. Although the evidence for the use of EOs for postoperative nausea is not robust, it may be a useful and generally safe approach for this common issue.
Behavioral health
Insomnia. EOs have been used as a treatment for insomnia traditionally and in complementary, alternative, and integrative medicine. A 2014 systematic review of 15 quantitative studies, including 11 RCTs, evaluated the hypnotic effects of EOs through inhalation, finding the strongest evidence for lavender, jasmine, and peppermint oils.29 The majority of the studies in the systematic review used the Pittsburgh Sleep Quality Index (PSQI) to evaluate EO effectiveness. A more recent 2021 systematic review and meta-analysis that evaluated 34 RCTs found that inhalation of EOs, most notably lavender aromatherapy, is effective in improving sleep problems such as insomnia.30
Findings from multiple smaller RCTs were consistent with those of the aforementioned systematic reviews. For example, in a well-conducted parallel randomized double-blind placebo-controlled trial of 100 people using orally ingested lemon verbena, the authors concluded that this intervention can be a complementary therapy for improving sleep quality and reducing insomnia severity.31 Another RCT with 60 participants evaluated an inhaled EO blend (lemon, eucalyptus, tea tree, and peppermint) over 4 weeks and found lowered perceived stress and depression as well as better sleep quality, but no influence on objective physiologic data such as stress indices or immune states.32
Continue to: In a 2020 randomized crossover...
In a 2020 randomized crossover placebocontrolled trial of 37 participants with diabetes reporting insomnia, inhaled lavender improved sleep quality and quantity, quality of life, and mood but not physiologic or metabolic measures, such as fasting glucose.33 Findings were similar in a cohort of cardiac rehabilitation patients (n = 37) who were treated with either an inhaled combination of lavender, bergamot, and ylang ylang, or placebo; cotton balls infused with the intervention oil or placebo oil were placed at the patient’s bedside for 5 nights. Sleep quality of participants receiving intervention oil was significantly better than the sleep quality of participants receiving the placebo oil as measured by participant completion of the PSQI.34
Anxiety is a common disorder that can be managed with nonpharmacologic treatments such as yoga, deep breathing, meditation, and EO therapy.21,22 In a systematic review and meta-analysis, the inhaled and topical use (with or without massage) of lavender EO was shown to improve psychological and physical manifestations of anxiety.23 Lavender EO is purported to affect the parasympathetic nervous system via anxiolytic, sedative, analgesic, and anticonvulsant properties.24 One systematic review and meta-analysis evaluating the anxiolytic effect of both inhaled and topical lavender EO found improvement in several biomarkers and physiologic data including blood pressure, heart rate, and cortisol levels, as well as a reduction in self-reported levels of anxiety, compared with placebo.25
Anxiety related to dental procedures is another area of study for the use of EOs. Two RCTs demonstrate statistically significant improvement in anxiety-related physiologic markers such as heart rate, blood pressure, and salivary cortisol levels in children who inhaled lavender EO during dental procedures.41,42 In 1 of the RCTs, the intervention was described as 3 drops of 100% lavender EO applied to a cloth and inhaled over the course of 3 minutes.41 Additionally, 2 studies found that orange EO was beneficial for dental procedure–induced anxiety, reducing pulse rates, cortisol levels, and self-reported anxiety.43,44
Dementia-related behavioral disturbances. A small, poorly designed study examining 2 EO blends—rosemary with lemon and lavender with orange—found some potential for improving cognitive function, especially in patients with Alzheimer disease.45 A Cochrane review of 13 RCTs totaling 708 patients concluded that it is not certain from the available evidence that EO therapy benefits patients with dementia in long-term-care facilities and hospital wards.46 Given that reporting of adverse events in the trials was poor, it is not possible to make conclusions about the risk vs benefit of EO therapy in this population.
Women’s health
Dysmenorrhea.
Continue to: In a randomized, double-blind clinical trial...
In a randomized, double-blind clinical trial of 48 women, a cream-based blend of lavender, clary sage, and marjoram EO (used topically in a 2:1:1 ratio diluted in unscented cream at 3% concentration and applied daily via abdominal massage) reduced participants’ reported menstrual pain symptoms and duration of pain.36 In a meta-analysis of 6 studies, topical abdominal application of EO (mainly lavender with or without other oils) with massage showed superiority over massage with placebo oils in reducing menstrual pain.37 A reduction in pain, mood symptoms, and fatigue in women with premenstrual symptoms was seen in an RCT of 77 patients using 3 drops of inhaled lavender EO.47
Labor. There is limited evidence for the use of EOs during labor. In an RCT of 104 women, patient-selected diffused EOs, including lavender, rose geranium, citrus, or jasmine, were found to help lower pain scores during the latent and early active phase of labor. There were no differences in labor augmentation, length of labor, perinatal outcomes, or need for additional pain medication.48
Other uses
Antimicrobial support. Some common EOs that have demonstrated antimicrobial properties are oregano, thyme, clove, lavender, clary sage, garlic, and cinnamon.49,50 Topical lemongrass and tea tree EOs have shown some degree of efficacy as an alternative treatment for acne, decolonization of methicillin-resistant Staphylococcus aureus, and superficial fungal infections.51 Support for an oral mixture of EOs labeled Myrtol (containing eucalyptus, citrus myrtle, and lavender) for viral acute bronchitis and sinusitis was found in a review of 7 studies.52 More research needs to be done before clear recommendations can be made on the use of EOs as antimicrobials, but the current data are encouraging.
Insect repellent. Reviews of the insect-repellent properties of EOs have shown promise and are in the public’s interest due to increasing awareness of the potential health and environmental hazards of synthetic repellents.53 Individual compounds present in EOs such as citronella/lemongrass, basil, and eucalyptus species demonstrate high repellent activity.54 Since EOs require frequent reapplication for efficacy due to their highly volatile nature, scientists are currently developing a means to prolong their protection time through cream-based formulations.55
The bottom line
Because of the ubiquity of EOs, family physicians will undoubtedly be asked about them by patients, and it would be beneficial to feel comfortable discussing their most common uses. For most adult patients, the topical and periodic inhaled usage of EOs is generally safe.56
There is existing evidence of efficacy for a number of EOs, most strongly for lavender and peppermint. Future research into EOs should include higher-powered and higher-quality studies in order to provide more conclusive evidence regarding the continued use of EOs for many common conditions. More evidence-based information on dosing, application/use regimens, and safety in long-term use also will help providers better instruct patients on how to utilize EOs effectively and safely.
CORRESPONDENCE
Pooja Amy Shah, MD, Columbia University College of Physicians & Surgeons, 610 West 158th Street, New York, NY 10032; pas2176@cumc.columbia.edu
Essential oils (EOs), which are concentrated plant-based oils, have become ubiquitous over the past decade. Given the far reach of EOs and their longtime use in traditional, complementary, alternative, and integrative medicine, it is imperative that clinicians have some knowledge of the potential benefits, risks, and overall efficacy.
Commonly used for aromatic benefits (aromatherapy), EOs are now also incorporated into a multitude of products promoting health and wellness. EOs are sold as individual products and can be a component in consumer goods such as cosmetics, body care/hygiene/beauty products, laundry detergents, insect repellents, over-the-counter medications, and food.
The review that follows presents the most current evidence available. With that said, it’s important to keep in mind some caveats that relate to this evidence. First, the studies cited tend to have a small sample size. Second, a majority of these studies were conducted in countries where there appears to be a significant culture of EO use, which could contribute to confirmation bias. Finally, in a number of the studies, there is concern for publication bias as well as a discrepancy between calculated statistical significance and actual clinical relevance.

What are essential oils?
EOs generally are made by extracting the oil from leaves, bark, flowers, seeds/fruit, rinds, and/or roots by steaming or pressing parts of a plant. It can take several pounds of plant material to produce a single bottle of EO, which usually contains ≥ 15 to 30 mL (.5 to 1 oz).1
Some commonly used EOs in the United States are lavender, peppermint, rose, clary sage, tea tree, eucalyptus, and citrus; however, there are approximately 300 EOs available.2 EOs are used most often via topical application, inhalation, or ingestion.
As with any botanical agent, EOs are complex substances often containing a multitude of chemical compounds.1 Because of the complex makeup of EOs, which often contain up to 100 volatile organic compounds, and their wide-ranging potential effects, applying the scientific method to study effectiveness poses a challenge that has limited their adoption in evidence-based practice.2
Availability and cost. EOs can be purchased at large retailers (eg, grocery stores, drug stores) and smaller health food stores, as well as on the Internet. Various EO vehicles, such as inhalers and topical creams, also can be purchased at these stores.
Continue to: The cost varies...
The cost varies enormously by manufacturer and type of plant used to make the EO. Common EOs such as peppermint and lavender oil generally cost $10 to $25, while rarer plant oils can cost $80 or more per bottle.
How safe are essential oils?
Patients may assume EOs are harmless because they are derived from natural plants and have been used medicinally for centuries. However, care must be taken with their use.
The safest way to use EOs is topically, although due to their highly concentrated nature, EOs should be diluted in an unscented neutral carrier oil such as coconut, jojoba, olive, or sweet almond.3 Ingestion of certain oils can cause hepatotoxicity, seizures, and even death.3 In fact, patients should speak with a knowledgeable physician before purchasing any oral EO capsules.
Whether used topically or ingested, all EOs carry risk for skin irritation and allergic reactions, and oral ingestion may result in some negative gastrointestinal (GI) adverse effects.4 A case report of 3 patients published in 2007 identified the potential for lavender and tea tree EOs to be endocrine disruptors.5
Inhalation of EOs may be harmful, as they emit many volatile organic compounds, some of which are considered potentially hazardous.6 At this time, there is insufficient evidence regarding inhaled EOs and their direct connection to respiratory health. It is reasonable to suggest, however, that the prolonged use of EOs and their use by patients who have lung conditions such as asthma or COPD should be avoided.7
Continue to: How are quality and purity assessed?
How are quality and purity assessed?
Like other dietary supplements, EOs are not regulated. No US regulatory agencies (eg, the US Food and Drug Administration [FDA] or Department of Agriculture [USDA]) certify or approve EOs for quality and purity. Bottles labeled with “QAI” for Quality Assurance International or “USDA Organic” will ensure the plant constituents used in the EO are from organic farming but do not attest to quality or purity.
Manufacturers commonly use marketing terms such as “therapeutic grade” or “pure” to sell products, but again, these terms do not reflect the product’s quality or purity. A labeled single EO may contain contaminants, alcohol, or additional ingredients.7 When choosing to use EOs, identifying reputable brands is essential; one resource is the independent testing organization ConsumerLab.com.
It is important to assess the manufacturer and read ingredient labels before purchasing an EO to understand what the product contains. Reputable companies will identify the plant ingredient, usually by the formal Latin binomial name, and explain the extraction process. A more certain way to assess the quality and purity of an EO is to ask the manufacturer to provide a certificate of analysis and gas chromatography/mass spectroscopy (GC/MS) data for the specific product. Some manufacturers offer GC/MS test results on their website Quality page.8 Others have detailed information on quality and testing, and GC/MS test reports can be obtained.9 Yet another manufacturer has test results on a product page matching reports to batch codes.10
Which conditions have evidence of benefit from essential oils?
EOs currently are being studied for treatment of many conditions—including pain, GI disorders, behavioral health disorders, and women’s health issues. The TABLE summarizes the conditions treated, outcomes, and practical applications of EOs.11-44

Pain
Headache. As an adjunct to available medications and procedures for headache treatment, EOs are one of the nonpharmacologic modalities that patients and clinicians have at their disposal for both migraine and tension-type headaches. A systematic review of 19 randomized controlled trials (RCTs) examining the effects of herbal ingredients for the acute treatment or prophylaxis of migraines found certain topically applied or inhaled EOs, such as peppermint and chamomile, to be effective for migraine pain alleviation; however, topically applied rose oil was not effective.11-13 Note: “topical application” in these studies implies application of the EO to ≥ 1 of the following areas: temples, forehead, behind ears, or above upper lip/below the nose.
Continue to: One RCT with 120 patients...
One RCT with 120 patients evaluated diluted intranasal peppermint oil and found that it reduced migraine intensity at similar rates to intranasal lidocaine.13 In this study, patients were randomized to receive one of the following: 4% lidocaine, 1.5% peppermint EO, or placebo. Two drops of the intranasal intervention were self-administered while the patient was in a supine position with their head suspended off the edge of the surface on which they were lying. They were instructed to stay in this position for at least 30 seconds after administration.
With regard to tension headache treatment, there is limited literature on the use of EOs. One study found that a preparation of peppermint oil applied topically to the temples and forehead of study participants resulted in significant analgesic effect.14
Fibromyalgia. Usual treatments for fibromyalgia include exercise, antidepressant and anticonvulsant medications, and stress management. Evidence also supports the use of inhaled and topically applied (with and without massage) lavender oil to improve symptoms.26 Positive effects may be related to the analgesic, anti-inflammatory, sleep-regulating, and anxiety-reducing effects of the major volatile compounds contained in lavender oil.
In one RCT with 42 patients with fibromyalgia, the use of inhaled lavender oil was shown to increase the perception of well-being (assessed on the validated SF-36 Health Survey Questionnaire) after 4 weeks.27 In this study, the patient applied 3 drops of an oil mixture, comprising 1 mL lavender EO and 10 mL of fixed neutral base oil, to the wrist and inhaled for 10 minutes before going to bed.
The use of a topical oil blend labeled “Oil 24” (containing camphor, rosemary, eucalyptus, peppermint, aloe vera, and lemon/orange) also has been shown to be more effective than placebo in managing fibromyalgia symptoms. A randomized controlled pilot study of 153 participants found that regular application of Oil 24 improved scores on pain scales and the Fibromyalgia Impact Questionnaire.28
Continue to: GI disorders
GI disorders
Irritable bowel syndrome. Peppermint oil relaxes GI smooth muscle, which has led to investigation of its use in irritable bowel syndrome (IBS) symptom amelioration.17 One meta-analysis including 12 RCTs with 835 patients with undifferentiated IBS found that orally ingested peppermint EO capsules reduced patient-reported symptoms of either abdominal pain or global symptoms.18
One study utilized the Total IBS Symptom Score to evaluate symptom reduction in patients with IBS-D (with diarrhea) and IBS-M (mixed) using 180-mg peppermint EO capsules ingested 3 times daily. There was a significant improvement in abdominal pain/discomfort, bloating/distension, pain at evacuation, and bowel urgency.19 A reduction in symptoms was observed after the first 24 hours of treatment and at the end of the 4-week treatment period.
In another study, among the 190 patients meeting Rome IV criteria for general (nonspecific) IBS who were treated with 182-mg peppermint EO capsules, no statistically significant reduction in overall symptom relief was found (based on outcome measures by the FDA and European Medicines Agency). However, in a secondary outcome analysis, peppermint oil produced greater improvements than placebo for the alleviation of abdominal pain, discomfort, and general IBS severity.20
Chemotherapy-induced nausea and vomiting. Patients with cancer undergoing chemotherapy often explore integrative medicine approaches, including aromatherapy, to ameliorate adverse effects and improve quality of life.38 A few small studies have shown potential for the use of inhaled ginger oil to reduce nausea and vomiting severity and improve health-related quality-of-life measures in these patients.
For example, a study with 60 participants found that inhaling ginger EO for 10 minutes was beneficial for reducing both nausea and vomiting.39 A single-blind, controlled, randomized crossover study of 60 patients with breast cancer receiving chemotherapy showed that ginger EO inhaled 3 times per day for 2 minutes at a time can decrease the severity of nausea but had no effect on vomiting. The same study showed that health-related quality of life improved with the ginger oil treatment.40
Continue to: Other EOs such as cardamom...
Other EOs such as cardamom and peppermint show promise as an adjunctive treatment for chemotherapy-induced nausea and vomiting as well.38
Postoperative nausea. A 2013 randomized trial of 303 patients examined the use of ginger EO, a blend of EOs (including ginger, spearmint, peppermint, and cardamom), and isopropyl alcohol. Both the single EO and EO blend significantly reduced the symptom of nausea. The number of antiemetic medications requested by patients receiving an EO also was significantly reduced compared to those receiving saline.15
The use of EOs to reduce nausea after cardiac operations was reviewed in an RCT of 60 surgical candidates using 10% peppermint oil via nebulization for 10 minutes.16 This technique was effective in reducing nausea during cardiac postoperative periods. Although the evidence for the use of EOs for postoperative nausea is not robust, it may be a useful and generally safe approach for this common issue.
Behavioral health
Insomnia. EOs have been used as a treatment for insomnia traditionally and in complementary, alternative, and integrative medicine. A 2014 systematic review of 15 quantitative studies, including 11 RCTs, evaluated the hypnotic effects of EOs through inhalation, finding the strongest evidence for lavender, jasmine, and peppermint oils.29 The majority of the studies in the systematic review used the Pittsburgh Sleep Quality Index (PSQI) to evaluate EO effectiveness. A more recent 2021 systematic review and meta-analysis that evaluated 34 RCTs found that inhalation of EOs, most notably lavender aromatherapy, is effective in improving sleep problems such as insomnia.30
Findings from multiple smaller RCTs were consistent with those of the aforementioned systematic reviews. For example, in a well-conducted parallel randomized double-blind placebo-controlled trial of 100 people using orally ingested lemon verbena, the authors concluded that this intervention can be a complementary therapy for improving sleep quality and reducing insomnia severity.31 Another RCT with 60 participants evaluated an inhaled EO blend (lemon, eucalyptus, tea tree, and peppermint) over 4 weeks and found lowered perceived stress and depression as well as better sleep quality, but no influence on objective physiologic data such as stress indices or immune states.32
Continue to: In a 2020 randomized crossover...
In a 2020 randomized crossover placebocontrolled trial of 37 participants with diabetes reporting insomnia, inhaled lavender improved sleep quality and quantity, quality of life, and mood but not physiologic or metabolic measures, such as fasting glucose.33 Findings were similar in a cohort of cardiac rehabilitation patients (n = 37) who were treated with either an inhaled combination of lavender, bergamot, and ylang ylang, or placebo; cotton balls infused with the intervention oil or placebo oil were placed at the patient’s bedside for 5 nights. Sleep quality of participants receiving intervention oil was significantly better than the sleep quality of participants receiving the placebo oil as measured by participant completion of the PSQI.34
Anxiety is a common disorder that can be managed with nonpharmacologic treatments such as yoga, deep breathing, meditation, and EO therapy.21,22 In a systematic review and meta-analysis, the inhaled and topical use (with or without massage) of lavender EO was shown to improve psychological and physical manifestations of anxiety.23 Lavender EO is purported to affect the parasympathetic nervous system via anxiolytic, sedative, analgesic, and anticonvulsant properties.24 One systematic review and meta-analysis evaluating the anxiolytic effect of both inhaled and topical lavender EO found improvement in several biomarkers and physiologic data including blood pressure, heart rate, and cortisol levels, as well as a reduction in self-reported levels of anxiety, compared with placebo.25
Anxiety related to dental procedures is another area of study for the use of EOs. Two RCTs demonstrate statistically significant improvement in anxiety-related physiologic markers such as heart rate, blood pressure, and salivary cortisol levels in children who inhaled lavender EO during dental procedures.41,42 In 1 of the RCTs, the intervention was described as 3 drops of 100% lavender EO applied to a cloth and inhaled over the course of 3 minutes.41 Additionally, 2 studies found that orange EO was beneficial for dental procedure–induced anxiety, reducing pulse rates, cortisol levels, and self-reported anxiety.43,44
Dementia-related behavioral disturbances. A small, poorly designed study examining 2 EO blends—rosemary with lemon and lavender with orange—found some potential for improving cognitive function, especially in patients with Alzheimer disease.45 A Cochrane review of 13 RCTs totaling 708 patients concluded that it is not certain from the available evidence that EO therapy benefits patients with dementia in long-term-care facilities and hospital wards.46 Given that reporting of adverse events in the trials was poor, it is not possible to make conclusions about the risk vs benefit of EO therapy in this population.
Women’s health
Dysmenorrhea.
Continue to: In a randomized, double-blind clinical trial...
In a randomized, double-blind clinical trial of 48 women, a cream-based blend of lavender, clary sage, and marjoram EO (used topically in a 2:1:1 ratio diluted in unscented cream at 3% concentration and applied daily via abdominal massage) reduced participants’ reported menstrual pain symptoms and duration of pain.36 In a meta-analysis of 6 studies, topical abdominal application of EO (mainly lavender with or without other oils) with massage showed superiority over massage with placebo oils in reducing menstrual pain.37 A reduction in pain, mood symptoms, and fatigue in women with premenstrual symptoms was seen in an RCT of 77 patients using 3 drops of inhaled lavender EO.47
Labor. There is limited evidence for the use of EOs during labor. In an RCT of 104 women, patient-selected diffused EOs, including lavender, rose geranium, citrus, or jasmine, were found to help lower pain scores during the latent and early active phase of labor. There were no differences in labor augmentation, length of labor, perinatal outcomes, or need for additional pain medication.48
Other uses
Antimicrobial support. Some common EOs that have demonstrated antimicrobial properties are oregano, thyme, clove, lavender, clary sage, garlic, and cinnamon.49,50 Topical lemongrass and tea tree EOs have shown some degree of efficacy as an alternative treatment for acne, decolonization of methicillin-resistant Staphylococcus aureus, and superficial fungal infections.51 Support for an oral mixture of EOs labeled Myrtol (containing eucalyptus, citrus myrtle, and lavender) for viral acute bronchitis and sinusitis was found in a review of 7 studies.52 More research needs to be done before clear recommendations can be made on the use of EOs as antimicrobials, but the current data are encouraging.
Insect repellent. Reviews of the insect-repellent properties of EOs have shown promise and are in the public’s interest due to increasing awareness of the potential health and environmental hazards of synthetic repellents.53 Individual compounds present in EOs such as citronella/lemongrass, basil, and eucalyptus species demonstrate high repellent activity.54 Since EOs require frequent reapplication for efficacy due to their highly volatile nature, scientists are currently developing a means to prolong their protection time through cream-based formulations.55
The bottom line
Because of the ubiquity of EOs, family physicians will undoubtedly be asked about them by patients, and it would be beneficial to feel comfortable discussing their most common uses. For most adult patients, the topical and periodic inhaled usage of EOs is generally safe.56
There is existing evidence of efficacy for a number of EOs, most strongly for lavender and peppermint. Future research into EOs should include higher-powered and higher-quality studies in order to provide more conclusive evidence regarding the continued use of EOs for many common conditions. More evidence-based information on dosing, application/use regimens, and safety in long-term use also will help providers better instruct patients on how to utilize EOs effectively and safely.
CORRESPONDENCE
Pooja Amy Shah, MD, Columbia University College of Physicians & Surgeons, 610 West 158th Street, New York, NY 10032; pas2176@cumc.columbia.edu
1. Butnariu M, Sarac I. Essential oils from plants. J Biotechnol Biomed Sci. 2018;1:35-43. doi: 10.14302/issn.2576-6694.jbbs-18-2489
2. Singh B, Sellam P, Majumder, J, et al. Floral essential oils : importance and uses for mankind. HortFlora Res Spectr. 2014;3:7-13. www.academia.edu/6707801/Floral_essential_oils_Importance_and_uses_for_mankind
3. Posadzki P, Alotaibi A, Ernst E. Adverse effects of aromatherapy: a systematic review of case reports and case series. Int J Risk Saf Med. 2012;24:147-161. doi: 10.3233/JRS-2012-0568
4. Sharmeen JB, Mahomoodally FM, Zengin G, et al. Essential oils as natural sources of fragrance compounds for cosmetics and cosmeceuticals. Molecules. 2021;26:666. doi: 10.3390/molecules26030666
5. Henley DV, Lipson N, Korach KS, et al. Prepubertal gynecomastia linked to lavender and tea tree oils. N Engl J Med. 2007;356:479-485. doi: 10.1056/NEJMoa064725
6. Nematollahi N, Weinberg JL, Flattery J, et al. Volatile chemical emissions from essential oils with therapeutic claims. Air Qual Atmosphere Health. 2021;14:365-369. doi: 10.1007/s11869-020-00941-4
7. Balekian D, Long A. Essential oil diffusers and asthma. Published February 24, 2020. Accessed September 22, 2023. www.aaaai.org/Allergist-Resources/Ask-the-Expert/Answers/Old-Ask-the-Experts/oil-diffusers-asthma
8. Aura Cacia. Quality. Accessed September 22, 2023. www.auracacia.com/quality
9. Now. Essential oil identity & purity testing. Accessed September 22, 2023. www.nowfoods.com/quality-safety/essential-oil-identity-purity-testing
10. Aura Cacia. GCMS documents. Accessed September 22, 2023. www.auracacia.com/aura-cacia-gcms-documents
11. Lopresti AL, Smith SJ, Drummond PD. Herbal treatments for migraine: a systematic review of randomised-controlled studies. Phytother Res. 2020;34:2493-2517. doi: 10.1002/ptr.6701
12. Niazi M, Hashempur MH, Taghizadeh M, et al. Efficacy of topical Rose (Rosa damascena Mill.) oil for migraine headache: A randomized double-blinded placebo-controlled cross-over trial. Complement Ther Med. 2017;34:35-41. doi: 10.1016/j.ctim. 2017.07.009
13. Rafieian-Kopaei M, Hasanpour-Dehkordi A, Lorigooini Z, et al. Comparing the effect of intranasal lidocaine 4% with peppermint essential oil drop 1.5% on migraine attacks: a double-blind clinical trial. Int J Prev Med. 2019;10:121. doi: 10.4103/ijpvm.IJPVM_530_17
14. Göbel H, Fresenius J, Heinze A, et al. [Effectiveness of Oleum menthae piperitae and paracetamol in therapy of headache of the tension type]. Nervenarzt. 1996;67:672-681. doi: 10.1007/s001150050040
15. Hunt R, Dienemann J, Norton HJ, et al. Aromatherapy as treatment for postoperative nausea: a randomized trial. Anesth Analg. 2013;117:597-604. doi: 10.1213/ANE.0b013e31824a0b1c
16. Maghami M, Afazel MR, Azizi-Fini I, et al. The effect of aromatherapy with peppermint essential oil on nausea and vomiting after cardiac surgery: a randomized clinical trial. Complement Ther Clin Pract. 2020;40:101199. doi: 10.1016/j.ctcp.2020.101199
17. Hills JM, Aaronson PI. The mechanism of action of peppermint oil on gastrointestinal smooth muscle. An analysis using patch clamp electrophysiology and isolated tissue pharmacology in rabbit and guinea pig. Gastroenterology. 1991;101:55-65. doi: 10.1016/0016-5085(91)90459-x
18. Alammar N, Wang L, Saberi B, et al. The impact of peppermint oil on the irritable bowel syndrome: a meta-analysis of the pooled clinical data. BMC Complement Altern Med. 2019;19:21. doi: 10.1186/s12906-018-2409-0
19. Cash BD, Epstein MS, Shah SM. A novel delivery system of peppermint oil is an effective therapy for irritable bowel syndrome symptoms. Dig Dis Sci. 2016;61:560-571. doi: 10.1007/s10620-015-3858-7
20. Weerts ZZRM, Masclee AAM, Witteman BJM, et al. Efficacy and safety of peppermint oil in a randomized, double-blind trial of patients with irritable bowel syndrome. Gastroenterology. 2020;158:123-136. doi: 10.1053/j.gastro.2019.08.026
21. Ma X, Yue ZQ, Gong ZQ, et al. The effect of diaphragmatic breathing on attention, negative affect and stress in healthy adults. Front Psychol. 2017;8:874. doi: 10.3389/fpsyg.2017.00874
22. Cabral P, Meyer HB, Ames D. Effectiveness of yoga therapy as a complementary treatment for major psychiatric disorders: a meta-analysis. Prim Care Companion CNS Disord. Published July 7, 2011. doi: 10.4088/PCC.10r01068
23. Donelli D, Antonelli M, Bellinazzi C, et ala. Effects of lavender on anxiety: systematic review and meta-analysis. Phytomedicine Int J Phytother Phytopharm. 2019;65:153099. doi: 10.1016/j.phymed.2019.153099
24. Koulivand PH, Khaleghi Ghadiri M, Gorji A. Lavender and the nervous system. Evid Based Complement Alternat Med. 2013;2013:1-10. doi: 10.1155/2013/681304
25. Kang HJ, Nam ES, Lee Y, et al. How strong is the evidence for the anxiolytic efficacy of lavender? Systematic review and meta-analysis of randomized controlled trials. Asian Nurs Res. 2019;13:295-305. doi: 10.1016/j.anr.2019.11.003
26. Barão Paixão VL, Freire de Carvalho J. Essential oil therapy in rheumatic diseases: a systematic review. Complement Ther Clin Pract. 2021;43:101391. doi: 10.1016/j.ctcp.2021.101391
27. Yasa Ozturk G, Bashan I. The effect of aromatherapy with lavender oil on the health-related quality of life in patients with fibromyalgia. J Food Qual. 2021;2021:1-5. doi: 10.1155/2021/9938630
28. Ko GD, Hum A, Traitses G, et al. Effects of topical O24 essential oils on patients with fibromyalgia syndrome: a randomized, placebo controlled pilot study. J Musculoskelet Pain. 2007;15:11-19. doi: 10.1300/J094v15n01_03
29. Lillehei AS, Halcon LL. A systematic review of the effect of inhaled essential oils on sleep. J Altern Complement Med. 2014;20:441-451. doi: 10.1089/acm.2013.0311
30. Cheong MJ, Kim S, Kim JS, et al. A systematic literature review and meta-analysis of the clinical effects of aroma inhalation therapy on sleep problems. Medicine (Baltimore). 2021;100:e24652. doi: 10.1097/MD.0000000000024652
31. Afrasiabian F, Mirabzadeh Ardakani M, Rahmani K, et al. Aloysia citriodora Paláu (lemon verbena) for insomnia patients: a randomized, double-blind, placebo-controlled clinical trial of efficacy and safety. Phytother Res PTR. 2019;33:350-359. doi: 10.1002/ptr.6228
32. Lee M, Lim S, Song JA, et al. The effects of aromatherapy essential oil inhalation on stress, sleep quality and immunity in healthy adults: randomized controlled trial. Eur J Integr Med. 2017;12:79-86. doi: 10.1016/j.eujim.2017.04.009
33. Nasiri Lari Z, Hajimonfarednejad M, Riasatian M, et al. Efficacy of inhaled Lavandula angustifolia Mill. Essential oil on sleep quality, quality of life and metabolic control in patients with diabetes mellitus type II and insomnia. J Ethnopharmacol. 2020;251:112560. doi: 10.1016/j.jep.2020.112560
34. McDonnell B, Newcomb P. Trial of essential oils to improve sleep for patients in cardiac rehabilitation. J Altern Complement Med N Y N. 2019;25:1193-1199. doi: 10.1089/acm.2019.0222
35. Song JA, Lee MK, Min E, et al. Effects of aromatherapy on dysmenorrhea: a systematic review and meta-analysis. Int J Nurs Stud. 2018;84:1-11. doi: 10.1016/j.ijnurstu.2018.01.016
36. Ou MC, Hsu TF, Lai AC, et al. Pain relief assessment by aromatic essential oil massage on outpatients with primary dysmenorrhea: a randomized, double-blind clinical trial: PD pain relief by aromatic oil massage. J Obstet Gynaecol Res. 2012;38:817-822. doi: 10.1111/j.1447-0756.2011.01802.x
37. Sut N, Kahyaoglu-Sut H. Effect of aromatherapy massage on pain in primary dysmenorrhea: a meta-analysis. Complement Ther Clin Pract. 2017;27:5-10. doi: 10.1016/j.ctcp.2017.01.001
38. Keyhanmehr AS, Kolouri S, Heydarirad G, et al. Aromatherapy for the management of cancer complications: a narrative review. Complement Ther Clin Pract. 2018;31:175-180. doi: 10.1016/j.ctcp.2018.02.009
39. Sriningsih I, Elisa E, Lestari KP. Aromatherapy ginger use in patients with nausea & vomiting on post cervical cancer chemotherapy. KEMAS J Kesehat Masy. 2017;13:59-68. doi: 10.15294/kemas.v13i1.5367
40. Lua PL, Salihah N, Mazlan N. Effects of inhaled ginger aromatherapy on chemotherapy-induced nausea and vomiting and health-related quality of life in women with breast cancer. Complement Ther Med. 2015;23:396-404. doi: 10.1016/j.ctim.2015.03.009
41. Arslan I, Aydinoglu S, Karan NB. Can lavender oil inhalation help to overcome dental anxiety and pain in children? A randomized clinical trial. Eur J Pediatr. 2020;179:985-992. doi: 10.1007/s00431-020-03595-7
42. Ghaderi F, Solhjou N. The effects of lavender aromatherapy on stress and pain perception in children during dental treatment: a randomized clinical trial. Complement Ther Clin Pract. 2020;40:101182. doi: 10.1016/j.ctcp.2020.101182
43. Jafarzadeh M, Arman S, Pour FF. Effect of aromatherapy with orange essential oil on salivary cortisol and pulse rate in children during dental treatment: a randomized controlled clinical trial. Adv Biomed Res. 2013;2:10. doi: 10.4103/2277-9175.107968
44. Lehrner J, Eckersberger C, Walla P, et al. Ambient odor of orange in a dental office reduces anxiety and improves mood in female patients. Physiol Behav. 2000;71:83-86. doi: 10.1016/S0031-9384(00)00308-5
45. Jimbo D, Kimura Y, Taniguchi M, et al. Effect of aromatherapy on patients with Alzheimer’s disease. Psychogeriatrics. 2009;9:173-179. doi: 10.1111/j.1479-8301.2009.00299.x
46. Ball EL, Owen-Booth B, Gray A, et al. Aromatherapy for dementia. Cochrane Database Syst Rev. 2020;(8). doi: 10.1002/14651858.CD003150.pub3
47. Uzunçakmak T, Ayaz Alkaya S. Effect of aromatherapy on coping with premenstrual syndrome: a randomized controlled trial. Complement Ther Med. 2018;36:63-67. doi: 10.1016/j.ctim.2017.11.022
48. Tanvisut R, Traisrisilp K, Tongsong T. Efficacy of aromatherapy for reducing pain during labor: a randomized controlled trial. Arch Gynecol Obstet. 2018;297:1145-1150. doi: 10.1007/s00404-018-4700-1
49. Ramsey JT, Shropshire BC, Nagy TR, et al. Essential oils and health. Yale J Biol Med. 2020;93:291-305.
50. Puškárová A, Bučková M, Kraková L, et al. The antibacterial and antifungal activity of six essential oils and their cyto/genotoxicity to human HEL 12469 cells. Sci Rep. 2017;7:8211. doi: 10.1038/s41598-017-08673-9
51. Deyno S, Mtewa AG, Abebe A, et al. Essential oils as topical anti-infective agents: a systematic review and meta-analysis. Complement Ther Med. 2019;47:102224. doi: 10.1016/j.ctim.2019.102224
52. Prall S, Bowles EJ, Bennett K, et al. Effects of essential oils on symptoms and course (duration and severity) of viral respiratory infections in humans: a rapid review. Adv Integr Med. 2020;7:218-221. doi: 10.1016/j.aimed.2020.07.005
53. Weeks JA, Guiney PD, Nikiforov AI. Assessment of the environmental fate and ecotoxicity of N,N-diethyl-m-toluamide (DEET). Integr Environ Assess Manag. 2012;8:120-134. doi: 10.1002/ieam.1246
54. Nerio LS, Olivero-Verbel J, Stashenko E. Repellent activity of essential oils: a review. Bioresour Technol. 2010;101:372-378. doi: 10.1016/j.biortech.2009.07.048
55. Lee MY. Essential oils as repellents against arthropods. BioMed Res Int. 2018;2018:6860271. doi: 10.1155/2018/6860271
56. Göbel H, Heinze A, Heinze-Kuhn K, et al. [Peppermint oil in the acute treatment of tension-type headache]. Schmerz Berl Ger. 2016;30:295-310. doi: 10.1007/s00482-016-0109-6
1. Butnariu M, Sarac I. Essential oils from plants. J Biotechnol Biomed Sci. 2018;1:35-43. doi: 10.14302/issn.2576-6694.jbbs-18-2489
2. Singh B, Sellam P, Majumder, J, et al. Floral essential oils : importance and uses for mankind. HortFlora Res Spectr. 2014;3:7-13. www.academia.edu/6707801/Floral_essential_oils_Importance_and_uses_for_mankind
3. Posadzki P, Alotaibi A, Ernst E. Adverse effects of aromatherapy: a systematic review of case reports and case series. Int J Risk Saf Med. 2012;24:147-161. doi: 10.3233/JRS-2012-0568
4. Sharmeen JB, Mahomoodally FM, Zengin G, et al. Essential oils as natural sources of fragrance compounds for cosmetics and cosmeceuticals. Molecules. 2021;26:666. doi: 10.3390/molecules26030666
5. Henley DV, Lipson N, Korach KS, et al. Prepubertal gynecomastia linked to lavender and tea tree oils. N Engl J Med. 2007;356:479-485. doi: 10.1056/NEJMoa064725
6. Nematollahi N, Weinberg JL, Flattery J, et al. Volatile chemical emissions from essential oils with therapeutic claims. Air Qual Atmosphere Health. 2021;14:365-369. doi: 10.1007/s11869-020-00941-4
7. Balekian D, Long A. Essential oil diffusers and asthma. Published February 24, 2020. Accessed September 22, 2023. www.aaaai.org/Allergist-Resources/Ask-the-Expert/Answers/Old-Ask-the-Experts/oil-diffusers-asthma
8. Aura Cacia. Quality. Accessed September 22, 2023. www.auracacia.com/quality
9. Now. Essential oil identity & purity testing. Accessed September 22, 2023. www.nowfoods.com/quality-safety/essential-oil-identity-purity-testing
10. Aura Cacia. GCMS documents. Accessed September 22, 2023. www.auracacia.com/aura-cacia-gcms-documents
11. Lopresti AL, Smith SJ, Drummond PD. Herbal treatments for migraine: a systematic review of randomised-controlled studies. Phytother Res. 2020;34:2493-2517. doi: 10.1002/ptr.6701
12. Niazi M, Hashempur MH, Taghizadeh M, et al. Efficacy of topical Rose (Rosa damascena Mill.) oil for migraine headache: A randomized double-blinded placebo-controlled cross-over trial. Complement Ther Med. 2017;34:35-41. doi: 10.1016/j.ctim. 2017.07.009
13. Rafieian-Kopaei M, Hasanpour-Dehkordi A, Lorigooini Z, et al. Comparing the effect of intranasal lidocaine 4% with peppermint essential oil drop 1.5% on migraine attacks: a double-blind clinical trial. Int J Prev Med. 2019;10:121. doi: 10.4103/ijpvm.IJPVM_530_17
14. Göbel H, Fresenius J, Heinze A, et al. [Effectiveness of Oleum menthae piperitae and paracetamol in therapy of headache of the tension type]. Nervenarzt. 1996;67:672-681. doi: 10.1007/s001150050040
15. Hunt R, Dienemann J, Norton HJ, et al. Aromatherapy as treatment for postoperative nausea: a randomized trial. Anesth Analg. 2013;117:597-604. doi: 10.1213/ANE.0b013e31824a0b1c
16. Maghami M, Afazel MR, Azizi-Fini I, et al. The effect of aromatherapy with peppermint essential oil on nausea and vomiting after cardiac surgery: a randomized clinical trial. Complement Ther Clin Pract. 2020;40:101199. doi: 10.1016/j.ctcp.2020.101199
17. Hills JM, Aaronson PI. The mechanism of action of peppermint oil on gastrointestinal smooth muscle. An analysis using patch clamp electrophysiology and isolated tissue pharmacology in rabbit and guinea pig. Gastroenterology. 1991;101:55-65. doi: 10.1016/0016-5085(91)90459-x
18. Alammar N, Wang L, Saberi B, et al. The impact of peppermint oil on the irritable bowel syndrome: a meta-analysis of the pooled clinical data. BMC Complement Altern Med. 2019;19:21. doi: 10.1186/s12906-018-2409-0
19. Cash BD, Epstein MS, Shah SM. A novel delivery system of peppermint oil is an effective therapy for irritable bowel syndrome symptoms. Dig Dis Sci. 2016;61:560-571. doi: 10.1007/s10620-015-3858-7
20. Weerts ZZRM, Masclee AAM, Witteman BJM, et al. Efficacy and safety of peppermint oil in a randomized, double-blind trial of patients with irritable bowel syndrome. Gastroenterology. 2020;158:123-136. doi: 10.1053/j.gastro.2019.08.026
21. Ma X, Yue ZQ, Gong ZQ, et al. The effect of diaphragmatic breathing on attention, negative affect and stress in healthy adults. Front Psychol. 2017;8:874. doi: 10.3389/fpsyg.2017.00874
22. Cabral P, Meyer HB, Ames D. Effectiveness of yoga therapy as a complementary treatment for major psychiatric disorders: a meta-analysis. Prim Care Companion CNS Disord. Published July 7, 2011. doi: 10.4088/PCC.10r01068
23. Donelli D, Antonelli M, Bellinazzi C, et ala. Effects of lavender on anxiety: systematic review and meta-analysis. Phytomedicine Int J Phytother Phytopharm. 2019;65:153099. doi: 10.1016/j.phymed.2019.153099
24. Koulivand PH, Khaleghi Ghadiri M, Gorji A. Lavender and the nervous system. Evid Based Complement Alternat Med. 2013;2013:1-10. doi: 10.1155/2013/681304
25. Kang HJ, Nam ES, Lee Y, et al. How strong is the evidence for the anxiolytic efficacy of lavender? Systematic review and meta-analysis of randomized controlled trials. Asian Nurs Res. 2019;13:295-305. doi: 10.1016/j.anr.2019.11.003
26. Barão Paixão VL, Freire de Carvalho J. Essential oil therapy in rheumatic diseases: a systematic review. Complement Ther Clin Pract. 2021;43:101391. doi: 10.1016/j.ctcp.2021.101391
27. Yasa Ozturk G, Bashan I. The effect of aromatherapy with lavender oil on the health-related quality of life in patients with fibromyalgia. J Food Qual. 2021;2021:1-5. doi: 10.1155/2021/9938630
28. Ko GD, Hum A, Traitses G, et al. Effects of topical O24 essential oils on patients with fibromyalgia syndrome: a randomized, placebo controlled pilot study. J Musculoskelet Pain. 2007;15:11-19. doi: 10.1300/J094v15n01_03
29. Lillehei AS, Halcon LL. A systematic review of the effect of inhaled essential oils on sleep. J Altern Complement Med. 2014;20:441-451. doi: 10.1089/acm.2013.0311
30. Cheong MJ, Kim S, Kim JS, et al. A systematic literature review and meta-analysis of the clinical effects of aroma inhalation therapy on sleep problems. Medicine (Baltimore). 2021;100:e24652. doi: 10.1097/MD.0000000000024652
31. Afrasiabian F, Mirabzadeh Ardakani M, Rahmani K, et al. Aloysia citriodora Paláu (lemon verbena) for insomnia patients: a randomized, double-blind, placebo-controlled clinical trial of efficacy and safety. Phytother Res PTR. 2019;33:350-359. doi: 10.1002/ptr.6228
32. Lee M, Lim S, Song JA, et al. The effects of aromatherapy essential oil inhalation on stress, sleep quality and immunity in healthy adults: randomized controlled trial. Eur J Integr Med. 2017;12:79-86. doi: 10.1016/j.eujim.2017.04.009
33. Nasiri Lari Z, Hajimonfarednejad M, Riasatian M, et al. Efficacy of inhaled Lavandula angustifolia Mill. Essential oil on sleep quality, quality of life and metabolic control in patients with diabetes mellitus type II and insomnia. J Ethnopharmacol. 2020;251:112560. doi: 10.1016/j.jep.2020.112560
34. McDonnell B, Newcomb P. Trial of essential oils to improve sleep for patients in cardiac rehabilitation. J Altern Complement Med N Y N. 2019;25:1193-1199. doi: 10.1089/acm.2019.0222
35. Song JA, Lee MK, Min E, et al. Effects of aromatherapy on dysmenorrhea: a systematic review and meta-analysis. Int J Nurs Stud. 2018;84:1-11. doi: 10.1016/j.ijnurstu.2018.01.016
36. Ou MC, Hsu TF, Lai AC, et al. Pain relief assessment by aromatic essential oil massage on outpatients with primary dysmenorrhea: a randomized, double-blind clinical trial: PD pain relief by aromatic oil massage. J Obstet Gynaecol Res. 2012;38:817-822. doi: 10.1111/j.1447-0756.2011.01802.x
37. Sut N, Kahyaoglu-Sut H. Effect of aromatherapy massage on pain in primary dysmenorrhea: a meta-analysis. Complement Ther Clin Pract. 2017;27:5-10. doi: 10.1016/j.ctcp.2017.01.001
38. Keyhanmehr AS, Kolouri S, Heydarirad G, et al. Aromatherapy for the management of cancer complications: a narrative review. Complement Ther Clin Pract. 2018;31:175-180. doi: 10.1016/j.ctcp.2018.02.009
39. Sriningsih I, Elisa E, Lestari KP. Aromatherapy ginger use in patients with nausea & vomiting on post cervical cancer chemotherapy. KEMAS J Kesehat Masy. 2017;13:59-68. doi: 10.15294/kemas.v13i1.5367
40. Lua PL, Salihah N, Mazlan N. Effects of inhaled ginger aromatherapy on chemotherapy-induced nausea and vomiting and health-related quality of life in women with breast cancer. Complement Ther Med. 2015;23:396-404. doi: 10.1016/j.ctim.2015.03.009
41. Arslan I, Aydinoglu S, Karan NB. Can lavender oil inhalation help to overcome dental anxiety and pain in children? A randomized clinical trial. Eur J Pediatr. 2020;179:985-992. doi: 10.1007/s00431-020-03595-7
42. Ghaderi F, Solhjou N. The effects of lavender aromatherapy on stress and pain perception in children during dental treatment: a randomized clinical trial. Complement Ther Clin Pract. 2020;40:101182. doi: 10.1016/j.ctcp.2020.101182
43. Jafarzadeh M, Arman S, Pour FF. Effect of aromatherapy with orange essential oil on salivary cortisol and pulse rate in children during dental treatment: a randomized controlled clinical trial. Adv Biomed Res. 2013;2:10. doi: 10.4103/2277-9175.107968
44. Lehrner J, Eckersberger C, Walla P, et al. Ambient odor of orange in a dental office reduces anxiety and improves mood in female patients. Physiol Behav. 2000;71:83-86. doi: 10.1016/S0031-9384(00)00308-5
45. Jimbo D, Kimura Y, Taniguchi M, et al. Effect of aromatherapy on patients with Alzheimer’s disease. Psychogeriatrics. 2009;9:173-179. doi: 10.1111/j.1479-8301.2009.00299.x
46. Ball EL, Owen-Booth B, Gray A, et al. Aromatherapy for dementia. Cochrane Database Syst Rev. 2020;(8). doi: 10.1002/14651858.CD003150.pub3
47. Uzunçakmak T, Ayaz Alkaya S. Effect of aromatherapy on coping with premenstrual syndrome: a randomized controlled trial. Complement Ther Med. 2018;36:63-67. doi: 10.1016/j.ctim.2017.11.022
48. Tanvisut R, Traisrisilp K, Tongsong T. Efficacy of aromatherapy for reducing pain during labor: a randomized controlled trial. Arch Gynecol Obstet. 2018;297:1145-1150. doi: 10.1007/s00404-018-4700-1
49. Ramsey JT, Shropshire BC, Nagy TR, et al. Essential oils and health. Yale J Biol Med. 2020;93:291-305.
50. Puškárová A, Bučková M, Kraková L, et al. The antibacterial and antifungal activity of six essential oils and their cyto/genotoxicity to human HEL 12469 cells. Sci Rep. 2017;7:8211. doi: 10.1038/s41598-017-08673-9
51. Deyno S, Mtewa AG, Abebe A, et al. Essential oils as topical anti-infective agents: a systematic review and meta-analysis. Complement Ther Med. 2019;47:102224. doi: 10.1016/j.ctim.2019.102224
52. Prall S, Bowles EJ, Bennett K, et al. Effects of essential oils on symptoms and course (duration and severity) of viral respiratory infections in humans: a rapid review. Adv Integr Med. 2020;7:218-221. doi: 10.1016/j.aimed.2020.07.005
53. Weeks JA, Guiney PD, Nikiforov AI. Assessment of the environmental fate and ecotoxicity of N,N-diethyl-m-toluamide (DEET). Integr Environ Assess Manag. 2012;8:120-134. doi: 10.1002/ieam.1246
54. Nerio LS, Olivero-Verbel J, Stashenko E. Repellent activity of essential oils: a review. Bioresour Technol. 2010;101:372-378. doi: 10.1016/j.biortech.2009.07.048
55. Lee MY. Essential oils as repellents against arthropods. BioMed Res Int. 2018;2018:6860271. doi: 10.1155/2018/6860271
56. Göbel H, Heinze A, Heinze-Kuhn K, et al. [Peppermint oil in the acute treatment of tension-type headache]. Schmerz Berl Ger. 2016;30:295-310. doi: 10.1007/s00482-016-0109-6
PRACTICE RECOMMENDATIONS
› Utilize lavender essential oil as an adjunctive treatment for fibromyalgia, dysmenorrhea, anxiety, and insomnia symptoms. B
› Recommend peppermint essential oil as an adjunctive treatment for irritable bowel syndrome, chemotherapy-induced nausea, and headache. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
How best to diagnose and manage abdominal aortic aneurysms
Ruptured abdominal aortic aneurysms (AAAs) caused about 6000 deaths annually in the United States between 2014 and 20201 and are associated with a pooled mortality rate of 81%.2 They result from a distinct degenerative process of the layers of the aortic wall.2 An AAA is defined as an abdominal aorta whose dilation is > 50% normal (more commonly, a diameter > 3 cm).3,4 The risk for rupture correlates closely with size; most ruptures occur in aneurysms > 5.5 cm3,4 (TABLE 15).
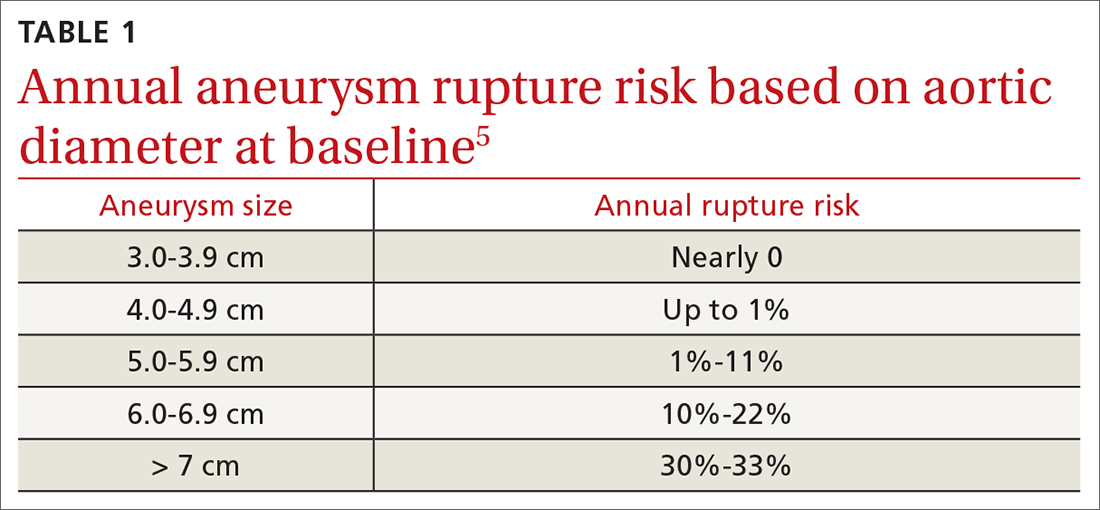
Most AAAs are asymptomatic and often go undetected until rupture, resulting in poor outcomes. Because of a low and declining prevalence of AAA and ruptured AAA in developed countries, screening recommendations target high-risk groups rather than the general population.4,6-8 This review summarizes risk factors, prevalence, and current evidence-based screening and management recommendations for AAA.
Who’s at risk?
Age is the most significant nonmodifiable risk factor, with AAA rupture uncommon in patients younger than 55 years.9 One retrospective study found the odds ratio (OR) for diagnosing AAA was 9.41 in adults ages 65 to 69 years (95% CI, 8.76-10.12; P < .0001) and 14.46 (95% CI, 13.45-15.55; P < .0001) in adults ages 70 to 74 years, compared to adults younger than 55 years.10
Smoking is the most potent modifiable risk factor for AAA. Among patients with AAA, > 90% have a history of smoking.4 The association between smoking and AAA is dose dependent, with an OR of 2.61 (95% CI, 2.47-2.74) in patients with a pack-per-year history < 5 years and 12.13 (95% CI, 11.66-12.61) in patients with a pack-per-year history > 35 years, compared to nonsmokers.10 The risk for AAA increases with smoking duration but decreases with cessation duration.4,10 Smoking cessation remains an important intervention, as active smokers have higher AAA rupture rates.11
Other risk factors for AAA include concomitant cardiovascular disease (CVD) such as coronary artery disease (CAD), cerebrovascular disease, atherosclerosis, dyslipidemia, and hypertension.10 Factors associated with reduced risk for AAA include African American race, Hispanic ethnicity, Asian ethnicity, diabetes, smoking cessation, consuming fruits and vegetables > 3 times per week, and exercising more than once per week.6,10
Prevalence declines but sex-based disparities in outcomes persist
The prevalence of AAA has declined in the United States and Europe in recent decades, correlating with declining rates of smoking.4,12 Reports published between 2011 and 2019 estimate that AAA prevalence in men older than 60 years has declined over time, with a prevalence of 1.2% to 3.3%.6 The prevalence of AAA has also decreased in women,6,13,14 estimated in 1 study to be as low as 0.74%.13 Similarly, deaths from ruptured AAA have declined markedly in the United States—by 70% between 1999 and 2016 according to 1 analysis.9
One striking difference in the male-female data is that although AAAs are more common in men, there is a 2- to 4-fold higher risk for rupture in women, who account for nearly half of all AAA-related deaths.9,10,15-17 The reasons for this heightened risk to women despite lower prevalence are not fully understood but are likely multifactorial and related to a general lack of screening for AAA in women, tendency for AAA to rupture at smaller diameters in women, rupture at an older age in women, and a history of worse surgical outcomes in women than men (though the gap in surgical outcomes appears to be closing).9,10,18
Continue to: While declines in AAA and AAA-related...
While declines in AAA and AAA-related death are largely attributed to lower smoking rates, other likely contributing factors include the implementation of screening programs, incidental detection during cross-sectional imaging, and improved surgical techniques and management of CV risk factors (eg, hypertension, hyperlipidemia).9,10
The benefits of screening older men
Randomized controlled trials (RCTs) have demonstrated the benefits of AAA screening programs. A meta-analysis of 4 populationbased RCTs of AAA screening in men ≥ 65 years demonstrated statistically significant reductions in AAA rupture (OR = 0.62; 95% CI, 0.55-0.70) and death from AAA (OR = 0.65; 95% CI, 0.57-0.74) over 12 to 15 years, with a number needed to screen (NNS) of 305 (95% CI, 248-411) to prevent 1 AAA-related death.18 The study also found screening decreases the rate of emergent surgeries for AAA (OR = 0.57; 95% CI, 0.48-0.68) while increasing the number of elective surgeries (OR = 1.44; 95% CI, 1.34-1.55) over 4 to 15 years.18
Only 1 study has demonstrated an improvement in all-cause mortality with screening programs, with a relatively small benefit (OR = 0.97; 95% CI, 0.94-0.99).19 Only 1 of the studies included women and, while underpowered, showed no difference in AAA-related death or rupture.20 Guidelines and recommendations of various countries and professional societies focus screening on subgroups at highest risk for AAA.4,6-8,18
Screening recommendations from USPSTF and others
The US Preventive Services Task Force (USPSTF) currently recommends one-time ultrasound screening for AAA in men ages 65 to 75 years who have ever smoked (commonly defined as having smoked > 100 cigarettes) in their lifetime.6 This grade “B” recommendation, initially made in 2005 and reaffirmed in the 2014 and 2019 USPSTF updates, recommends screening the highest-risk segment of the population (ie, older male smokers).
In men ages 65 to 75 years with no smoking history, rather than routine screening, the USPSTF recommends selectively offering screening based on the patient’s medical history, family history, risk factors, and personal values (with a “C” grade).6 The USPSTF continues to recommend against screening for AAA in women with no smoking history and no family history of AAA.6 According to the USPSTF, the evidence is insufficient to recommend for or against screening women ages 65 to 75 years who have ever smoked or have a family history of AAA (“I” statement).6
Continue to: One critique of the USPSTF recommendations
One critique of the USPSTF recommendations is that they fail to detect a significant portion of patients with AAA and AAA rupture. For example, in a retrospective analysis of 55,197 patients undergoing AAA repair, only 33% would have been detected by the USPSTF grade “B” recommendation to screen male smokers ages 65 to 75 years, and an analysis of AAA-related fatalities found 43% would be missed by USPSTF criteria.9,21
Screening guidelines from the Society for Vascular Surgery (SVS) are broader than those of the USPSTF, in an attempt to capture a larger percentage of the population at risk for AAA-related disease by extrapolating from epidemiologic data. The SVS guidelines include screening for women ages 65 to 75 years with a smoking history, screening men and women ages 65 to 75 years who have a first-degree relative with AAA, and consideration of screening patients older than 75 years if they are in good health and have a first-degree relative with AAA or a smoking history and have not been previously screened.4 However, these expanded recommendations are not supported by patient-oriented evidence.6
Attempts to broaden screening guidelines must be tempered by potential risks for harm, primarily overdiagnosis (ie, diagnosing AAAs that would not otherwise rise to clinical significance) and overtreatment (ie, resulting in unnecessary imaging, appointments, anxiety, or surgery). Negative psychological effects on quality of life after a diagnosis of AAA have not been shown to cause significant harm.6,18
A recent UK analysis found that screening programs for AAA in women modeled after those in men are not cost effective, with an NNS to prevent 1 death of 3900 in women vs 700 in men.15,18 Another recent trial of ultrasound screening in 5200 high-risk women ages 65 to 74 years found an AAA incidence of 0.29% (95% CI, 0.18%-0.48%) in which only 3 large aneurysms were identified.22
In the United States, rates of screening for AAA remain low.23 One study has shown electronic medical record–based reminders increased screening rates from 48% to 80%.24 Point-of-care bedside ultrasound performed by clinicians also could improve screening rates. Multiple studies have demonstrated that screening and diagnosis of AAA can be performed safely and effectively at the bedside by nonradiologists such as family physicians and emergency physicians.25-28 In 1 study, such exams added < 4 minutes to the patient encounter.26 Follow-up surveillance schedules for those identified as having a AAA are summarized in TABLE 2.4
Continue to: Management options
Management options: Immediate repair or surveillance?
After diagnosing AAA, important decisions must be made regarding management, including indications for surgical repair, appropriate follow-up surveillance, and medications for secondary prevention and cardiovascular risk reduction.
EVAR vs open repair
The 2 main surgical strategies for aneurysm repair are open repair and endovascular repair (EVAR). In the United States, EVAR is becoming the more common approach and was used to repair asymptomatic aneurysms in > 80% of patients and ruptured aneurysms in 50% of patients.6 There have been multiple RCTs assessing EVAR and open repair for large and small aneurysms.29-34 Findings across these studies consistently show EVAR is associated with lower immediate (ie, 30-day) morbidity and mortality but no longer-term survival benefit compared to open repair.
EVAR procedures require ongoing long-term surveillance for endovascular leakage and other complications, resulting in an increased need for re-intervention.31,33,35 For these reasons, the National Institute for Health and Care Excellence (NICE) guidelines suggest open repair as the preferred modality.7 However, SVS and the American College of Cardiology Foundation/American Heart Association guidance support either EVAR or open repair, noting that open repair may be preferable in patients unable to engage in long-term follow-up surveillance.36

Indications for repair. In general, repair is indicated when an aneurysm reaches or exceeds 5.5 cm.4,7 Both SVS and NICE also recommend clinicians consider surgical repair of smaller, rapidly expanding aneurysms (> 1 cm over a 1-year period).4,7 Based on evidence suggesting a higher risk for rupture in women with smaller aneurysms,14,37 SVS recommends clinicians consider surgical repair in women with an AAA ≥ 5.0 cm. Several RCTs evaluating the benefits of immediate repair for smaller-sized aneurysms (4.0-5.5 cm) favored surveillance.38,39 Accepted indications for surgical repair are summarized in TABLE 3.4,7,34Surgical repair recommendations also are based on aneurysm morphology, which can be fusiform or saccular (FIGURE). More than 90% of AAAs are fusiform.40 Although saccular AAAs are less common, some studies suggest they are more prone to rupture than fusiform AAAs, and SVS guidelines suggest surgical repair of saccular aneurysms regardless of size.4,41,42
Perioperative and long-term risks. Both EVAR and open repair of AAA carry a high perioperative and long-term risk for death, as patients often have multiple comorbidities. A 2019 trial comparing EVAR to open repair with 14 years of follow-up reported death in 68% of patients in the EVAR group and 70% in the open repair group. 31 Among these deaths, 2.7% in the EVAR group and 3.7% in the open repair group were aneurysm related.31 The study also found a second surgical intervention was required in 19.8% of patients in the open repair group and 26.7% in the EVAR group.31
Continue to: When assessing perioperative risk...
When assessing perioperative risk, SVS guidelines recommend clinicians employ a shared decision-making approach with patients that incorporates Vascular Quality Initiative (VQI) mortality risk score.4 (VQI risk calculators are available at https://qxmd.com/vascular-study-group-new-england-decision-support-tools.43)
Medication management
Based on the close association of aortic aneurysm with atherosclerotic CVD (ASCVD), professional societies such as the European Society of Cardiology and European Atherosclerosis Society (ESC/EAS) have suggested aortic aneurysm is equivalent to ASCVD and should be managed medically in a similar manner to peripheral arterial disease.44 Indeed, many patients with AAA may have concomitant CAD or other arterial vascular diseases (eg, carotid, lower extremity).
Statins. In its guidelines, the ESC/EAS consider patients with AAA at “very high risk” for adverse CV events and suggest pharmacotherapy with high-intensity statins, adding ezetimibe or proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors if needed, to reduce low-density lipoprotein cholesterol ≥ 50% from baseline, with a goal of < 55 mg/dL.44 Statin therapy additionally lowers all-cause postoperative mortality in patients undergoing AAA repair but does not affect the rate of aneurysm expansion.45
Aspirin and other anticoagulants. Although aspirin therapy may be indicated for the secondary prevention of other cardiovascular events that may coexist with AAA, it does not appear to affect the rate of growth or prevent rupture of aneurysms.46,47 In addition to aspirin, anticoagulants such as clopidogrel, enoxaparin, and warfarin are not recommended when the presence of AAA is the only indication.4
Other medications. Angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, beta-blockers, and antibiotics (eg, doxycycline) have been studied as a treatment for AAA. However, none has shown benefit in reducing aneurysm growth or rupture and they are not recommended for that sole purpose.4,48
Metformin. There is a negative association between diabetes and AAA expansion and rupture. Several cohort studies have indicated that this may be an independent effect driven primarily by exposure to metformin. While it is not unreasonable to consider this another important indication for metformin use in patients with diabetes, RCT evidence has yet to establish a role for metformin in patients without diabetes who have AAA.48,49
ACKNOWLEDGEMENT
The authors thank Gwen Wilson, MLS, AHIP, for her assistance with the literature searches performed in the preparation of this manuscript.
CORRESPONDENCE
Nicholas LeFevre, MD, Family and Community Medicine, University of Missouri–Columbia School of Medicine, One Hospital Drive, M224 Medical Science Building, Columbia, MO 65212; nlefevre@health.missouri.edu
1. CDC. Wide-ranging Online Data for Epidemiologic Research (WONDER) database. Accessed August 30, 2023. https://wonder.cdc.gov/ucd-icd10.html
2. Reimerink JJ, van der Laan MJ, Koelemay MJ, et al. Systematic review and meta-analysis of population-based mortality from ruptured abdominal aortic aneurysm. Br J Surg. 2013;100:1405-1413. doi: 10.1002/bjs.9235
3. Kent KC. Clinical practice. Abdominal aortic aneurysms. N Engl J Med. 2014;371:2101-2108. doi: 10.1056/NEJMcp1401430
4. Chaikof EL, Dalman RL, Eskandari MK, et al. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg. 2018;67:2-77.e2. doi: 10.1016/j.jvs.2017.10.044
5. Moll FL, Powell JT, Fraedrich G, et al. Management of abdominal aortic aneurysms clinical practice guidelines of the European society for vascular surgery. Eur J Vasc Endovasc Surg. 2011;41 suppl 1:S1-S58. doi: 10.1016/j.ejvs.2010.09.011
6. Owens DK, Davidson KW, Krist AH, et al; US Preventive Services Task Force. Screening for abdominal aortic aneurysm: US Preventive Services Task Force recommendation statement. JAMA. 2019;322:2211-2218. doi: 10.1001/jama.2019.18928
7. National Institute for Health and Care Excellence. Abdominal aortic aneurysm: diagnosis and management. NICE guideline [NG156]. March 19, 2020. Accessed June 30, 2023. www.nice.org.uk/guidance/ng156/chapter/recommendations
8. Canadian Task Force on Preventive Health Care. Recommendations on screening for abdominal aortic aneurysm in primary care. CMAJ. 2017;189:E1137-E1145. doi: 10.1503/cmaj.170118
9. Abdulameer H, Al Taii H, Al-Kindi SG, et al. Epidemiology of fatal ruptured aortic aneurysms in the United States (1999-2016). J Vasc Surg. 2019;69:378-384.e2. doi: 10.1016/j.jvs.2018.03.435
10. Kent KC, Zwolak RM, Egorova NN, et al. Analysis of risk factors for abdominal aortic aneurysm in a cohort of more than 3 million individuals. J Vasc Surg. 2010;52:539-548. doi: 10.1016/j.jvs.2010.05.090
11. [No authors listed] Smoking, lung function and the prognosis of abdominal aortic aneurysm. The UK Small Aneurysm Trial Participants. Eur J Vasc Endovasc Surg. 2000;19:636-642. doi: 10.1053/ejvs.2000.1066
12. Oliver-Williams C, Sweeting MJ, Turton G, et al. Lessons learned about prevalence and growth rates of abdominal aortic aneurysms from a 25-year ultrasound population screening programme. Br J Surg. 2018;105:68-74. doi: 10.1002/bjs.10715
13. Ulug P, Powell JT, Sweeting MJ, et al. Meta-analysis of the current prevalence of screen-detected abdominal aortic aneurysm in women. Br J Surg. 2016;103:1097-1104. doi: 10.1002/bjs.10225
14. Chabok M, Nicolaides A, Aslam M, et al. Risk factors associated with increased prevalence of abdominal aortic aneurysm in women. Br J Surg. 2016;103:1132-1138. doi: 10.1002/bjs.10179
15. Sweeting, MJ, Masconi KL, Jones E, et al. Analysis of clinical benefit, harms, and cost-effectiveness of screening women for abdominal aortic aneurysm. Lancet. 2018;392:487-495. doi: 10.1016/S0140-6736(18)31222-4
16. Sweeting MJ, Thompson SG, Brown LC, et al; RESCAN collaborators. Meta-analysis of individual patient data to examine factors affecting growth and rupture of small abdominal aortic aneurysms. Br J Surg. 2012;99:655-665. doi: 10.1002/bjs.8707
17. Skibba AA, Evans JR, Hopkins SP, et al. Reconsidering gender relative to risk of rupture in the contemporary management of abdominal aortic aneurysms. J Vasc Surg. 2015;62:1429-1436. doi: 10.1016/j.jvs.2015.07.079
18. Guirguis-Blake JM, Beil TL, Senger CA, et al. Primary care screening for abdominal aortic aneurysm: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2019;322:2219-2238. doi: 10.1001/jama.2019.17021
19. Thompson SG, Ashton HA, Gao L, et al; Multicentre Aneurysm Screening Study (MASS) Group. Final follow-up of the Multicentre Aneurysm Screening Study (MASS) randomized trial of abdominal aortic aneurysm screening. Br J Surg. 2012;99:1649-1656. doi: 10.1002/bjs.8897
20. Ashton HA, Gao L, Kim LG, et al. Fifteen-year follow-up of a randomized clinical trial of ultrasonographic screening for abdominal aortic aneurysms. Br J Surg. 2007;94:696-701. doi: 10.1002/bjs.5780
21. Carnevale ML, Koleilat I, Lipsitz EC, et al. Extended screening guidelines for the diagnosis of abdominal aortic aneurysm. J Vasc Surg. 2020;72:1917-1926. doi: 10.1016/j.jvs.2020.03.047
22. Duncan A, Maslen C, Gibson C, et al. Ultrasound screening for abdominal aortic aneurysm in high-risk women. Br J Surg. 2021;108:1192-1198. doi: 10.1093/bjs/znab220
23. Shreibati JB, Baker LC, Hlatky MA, et al. Impact of the Screening Abdominal Aortic Aneurysms Very Efficiently (SAAAVE) Act on abdominal ultrasonography use among Medicare beneficiaries. Arch Intern Med. 2012;172:1456-1462. doi: 10.1001/archinternmed.2012.4268
24. Hye RJ, Smith AE, Wong GH, et al. Leveraging the electronic medical record to implement an abdominal aortic aneurysm screening program. J Vasc Surg. 2014;59:1535-1542. doi: 10.1016/j.jvs.2013.12.016
25. Rubano E, Mehta N, Caputo W, et al., Systematic review: emergency department bedside ultrasonography for diagnosing suspected abdominal aortic aneurysm. Acad Emerg Med. 2013. 20:128-138. doi: 10.1111/acem.12080
26. Blois B. Office-based ultrasound screening for abdominal aortic aneurysm. Can Fam Physician. 2012;58:e172-e178.
27. Arnold MJ, Jonas CE, Carter RE. Point-of-care ultrasonography. Am Fam Physician. 2020;101:275-285.
28. Nixon G, Blattner K, Muirhead J, et al. Point-of-care ultrasound for FAST and AAA in rural New Zealand: quality and impact on patient care. Rural Remote Health. 2019;19:5027. doi: 10.22605/RRH5027
29. Lederle FA, Wilson SE, Johnson GR, et al. Immediate repair compared with surveillance of small abdominal aortic aneurysms. N Engl J Med. 2002;346:1437-1444. doi: 10.1056/NEJMoa012573
30. Filardo G, Lederle FA, Ballard DJ, et al. Immediate open repair vs surveillance in patients with small abdominal aortic aneurysms: survival differences by aneurysm size. Mayo Clin Proc. 2013;88:910-919. doi: 10.1016/j.mayocp.2013.05.014
31. Lederle FA, Kyriakides TC, Stroupe KT, et al. Open versus endovascular repair of abdominal aortic aneurysm. N Engl J Med. 2019;380:2126-2135. doi: 10.1056/NEJMoa1715955
32. Patel R, Sweeting MJ, Powell JT, et al., Endovascular versus open repair of abdominal aortic aneurysm in 15-years’ follow-up of the UK endovascular aneurysm repair trial 1 (EVAR trial 1): a randomised controlled trial. Lancet. 2016;388:2366-2374. doi: 10.1016/S0140-6736(16)31135-7
33. van Schaik TG, Yeung KK, Verhagen HJ, et al. Long-term survival and secondary procedures after open or endovascular repair of abdominal aortic aneurysms. J Vasc Surg. 2017;66:1379-1389. doi: 10.1016/j.jvs.2017.05.122
34. Powell JT, Brady AR, Brown, LC, et al; United Kingdom Small Aneurysm Trial Participants. Long-term outcomes of immediate repair compared with surveillance of small abdominal aortic aneurysms. N Engl J Med. 2002;346:1445-1452. doi: 10.1056/NEJMoa013527
35. Paravastu SC, Jayarajasingam R, Cottam R, et al. Endovascular repair of abdominal aortic aneurysm. Cochrane Database Syst Rev. 2014:CD004178. doi: 10.1002/14651858.CD004178.pub2
36. Rooke TW, Hirsch AT, Misra S, et al. 2011 ACCF/AHA focused update of the guideline for the management of patients with peripheral artery disease (updating the 2005 guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2011;58:2020-2045. doi: 10.1016/j.jacc.2011.08.023
37. Bhak RH, Wininger M, Johnson GR, et al. Factors associated with small abdominal aortic aneurysm expansion rate. JAMA Surg. 2015;150:44-50. doi: 10.1001/jamasurg.2014.2025
38. Ouriel K, Clair DG, Kent KC, et al; Positive Impact of Endovascular Options for treating Aneurysms Early (PIVOTAL) Investigators. Endovascular repair compared with surveillance for patients with small abdominal aortic aneurysms. J Vasc Surg. 2010;51:1081-1087. doi: 10.1016/j.jvs.2009.10.113
39. Cao P, De Rango P, Verzini F, et al. Comparison of surveillance versus aortic endografting for small aneurysm repair (CAESAR): results from a randomised trial. Eur J Vasc Endovasc Surg. 2011;41:13-25. doi: 10.1016/j.ejvs.2010.08.026
40. Karthaus EG, Tong TML, Vahl A, et al; Dutch Society of Vascular Surgery, the Steering Committee of the Dutch Surgical Aneurysm Audit and the Dutch Institute for Clinical Auditing. Saccular abdominal aortic aneurysms: patient characteristics, clinical presentation, treatment, and outcomes in the Netherlands. Ann Surg. 2019;270:852-858. doi: 10.1097/SLA.0000000000003529
41. Nathan DP, Xu C, Pouch AM, et al. Increased wall stress of saccular versus fusiform aneurysms of the descending thoracic aorta. Ann Vasc Surg. 2011;25:1129-2237. doi: 10.1016/j.avsg.2011.07.008
42. Durojaye MS, Adeniyi TO, Alagbe OA. Multiple saccular aneurysms of the abdominal aorta: a case report and short review of risk factors for rupture on CT Scan. Ann Ib Postgrad Med. 2020;18:178-180.
43. Bertges DJ, Neal D, Schanzer A, et al. The Vascular Quality Initiative Cardiac Risk Index for prediction of myocardial infarction after vascular surgery. J Vasc Surg. 2016;64:1411-1421.e4. doi: 10.1016/j.jvs.2016.04.045
44. Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41:111-188. doi: 10.1093/eurheartj/ehz455
45. Twine CP, Williams IM. Systematic review and meta-analysis of the effects of statin therapy on abdominal aortic aneurysms. Br J Surg. 2011;98:346-353. doi: 10.1002/bjs.7343
46. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:e596-e646. doi: 10.1161/CIR.0000000000000678
47. Erbel R, Aboyans V, Boileau C, et al. 2014 ESC guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur Heart J. 2014;35:2873-2926. doi: 10.1093/eurheartj/ehu281
48. Lederle FA, Noorbaloochi S, Nugent S, et al. Multicentre study of abdominal aortic aneurysm measurement and enlargement. Br J Surg. 2015;102:1480-1487. doi: 10.1002/bjs.9895
49. Itoga NK, Rothenberg KA, Suarez P, et al. Metformin prescription status and abdominal aortic aneurysm disease progression in the U.S. veteran population. J Vasc Surg. 2019;69:710-716.e3. doi: 10.1016/j.jvs.2018.06.19
Ruptured abdominal aortic aneurysms (AAAs) caused about 6000 deaths annually in the United States between 2014 and 20201 and are associated with a pooled mortality rate of 81%.2 They result from a distinct degenerative process of the layers of the aortic wall.2 An AAA is defined as an abdominal aorta whose dilation is > 50% normal (more commonly, a diameter > 3 cm).3,4 The risk for rupture correlates closely with size; most ruptures occur in aneurysms > 5.5 cm3,4 (TABLE 15).

Most AAAs are asymptomatic and often go undetected until rupture, resulting in poor outcomes. Because of a low and declining prevalence of AAA and ruptured AAA in developed countries, screening recommendations target high-risk groups rather than the general population.4,6-8 This review summarizes risk factors, prevalence, and current evidence-based screening and management recommendations for AAA.
Who’s at risk?
Age is the most significant nonmodifiable risk factor, with AAA rupture uncommon in patients younger than 55 years.9 One retrospective study found the odds ratio (OR) for diagnosing AAA was 9.41 in adults ages 65 to 69 years (95% CI, 8.76-10.12; P < .0001) and 14.46 (95% CI, 13.45-15.55; P < .0001) in adults ages 70 to 74 years, compared to adults younger than 55 years.10
Smoking is the most potent modifiable risk factor for AAA. Among patients with AAA, > 90% have a history of smoking.4 The association between smoking and AAA is dose dependent, with an OR of 2.61 (95% CI, 2.47-2.74) in patients with a pack-per-year history < 5 years and 12.13 (95% CI, 11.66-12.61) in patients with a pack-per-year history > 35 years, compared to nonsmokers.10 The risk for AAA increases with smoking duration but decreases with cessation duration.4,10 Smoking cessation remains an important intervention, as active smokers have higher AAA rupture rates.11
Other risk factors for AAA include concomitant cardiovascular disease (CVD) such as coronary artery disease (CAD), cerebrovascular disease, atherosclerosis, dyslipidemia, and hypertension.10 Factors associated with reduced risk for AAA include African American race, Hispanic ethnicity, Asian ethnicity, diabetes, smoking cessation, consuming fruits and vegetables > 3 times per week, and exercising more than once per week.6,10
Prevalence declines but sex-based disparities in outcomes persist
The prevalence of AAA has declined in the United States and Europe in recent decades, correlating with declining rates of smoking.4,12 Reports published between 2011 and 2019 estimate that AAA prevalence in men older than 60 years has declined over time, with a prevalence of 1.2% to 3.3%.6 The prevalence of AAA has also decreased in women,6,13,14 estimated in 1 study to be as low as 0.74%.13 Similarly, deaths from ruptured AAA have declined markedly in the United States—by 70% between 1999 and 2016 according to 1 analysis.9
One striking difference in the male-female data is that although AAAs are more common in men, there is a 2- to 4-fold higher risk for rupture in women, who account for nearly half of all AAA-related deaths.9,10,15-17 The reasons for this heightened risk to women despite lower prevalence are not fully understood but are likely multifactorial and related to a general lack of screening for AAA in women, tendency for AAA to rupture at smaller diameters in women, rupture at an older age in women, and a history of worse surgical outcomes in women than men (though the gap in surgical outcomes appears to be closing).9,10,18
Continue to: While declines in AAA and AAA-related...
While declines in AAA and AAA-related death are largely attributed to lower smoking rates, other likely contributing factors include the implementation of screening programs, incidental detection during cross-sectional imaging, and improved surgical techniques and management of CV risk factors (eg, hypertension, hyperlipidemia).9,10
The benefits of screening older men
Randomized controlled trials (RCTs) have demonstrated the benefits of AAA screening programs. A meta-analysis of 4 populationbased RCTs of AAA screening in men ≥ 65 years demonstrated statistically significant reductions in AAA rupture (OR = 0.62; 95% CI, 0.55-0.70) and death from AAA (OR = 0.65; 95% CI, 0.57-0.74) over 12 to 15 years, with a number needed to screen (NNS) of 305 (95% CI, 248-411) to prevent 1 AAA-related death.18 The study also found screening decreases the rate of emergent surgeries for AAA (OR = 0.57; 95% CI, 0.48-0.68) while increasing the number of elective surgeries (OR = 1.44; 95% CI, 1.34-1.55) over 4 to 15 years.18
Only 1 study has demonstrated an improvement in all-cause mortality with screening programs, with a relatively small benefit (OR = 0.97; 95% CI, 0.94-0.99).19 Only 1 of the studies included women and, while underpowered, showed no difference in AAA-related death or rupture.20 Guidelines and recommendations of various countries and professional societies focus screening on subgroups at highest risk for AAA.4,6-8,18
Screening recommendations from USPSTF and others
The US Preventive Services Task Force (USPSTF) currently recommends one-time ultrasound screening for AAA in men ages 65 to 75 years who have ever smoked (commonly defined as having smoked > 100 cigarettes) in their lifetime.6 This grade “B” recommendation, initially made in 2005 and reaffirmed in the 2014 and 2019 USPSTF updates, recommends screening the highest-risk segment of the population (ie, older male smokers).
In men ages 65 to 75 years with no smoking history, rather than routine screening, the USPSTF recommends selectively offering screening based on the patient’s medical history, family history, risk factors, and personal values (with a “C” grade).6 The USPSTF continues to recommend against screening for AAA in women with no smoking history and no family history of AAA.6 According to the USPSTF, the evidence is insufficient to recommend for or against screening women ages 65 to 75 years who have ever smoked or have a family history of AAA (“I” statement).6
Continue to: One critique of the USPSTF recommendations
One critique of the USPSTF recommendations is that they fail to detect a significant portion of patients with AAA and AAA rupture. For example, in a retrospective analysis of 55,197 patients undergoing AAA repair, only 33% would have been detected by the USPSTF grade “B” recommendation to screen male smokers ages 65 to 75 years, and an analysis of AAA-related fatalities found 43% would be missed by USPSTF criteria.9,21
Screening guidelines from the Society for Vascular Surgery (SVS) are broader than those of the USPSTF, in an attempt to capture a larger percentage of the population at risk for AAA-related disease by extrapolating from epidemiologic data. The SVS guidelines include screening for women ages 65 to 75 years with a smoking history, screening men and women ages 65 to 75 years who have a first-degree relative with AAA, and consideration of screening patients older than 75 years if they are in good health and have a first-degree relative with AAA or a smoking history and have not been previously screened.4 However, these expanded recommendations are not supported by patient-oriented evidence.6
Attempts to broaden screening guidelines must be tempered by potential risks for harm, primarily overdiagnosis (ie, diagnosing AAAs that would not otherwise rise to clinical significance) and overtreatment (ie, resulting in unnecessary imaging, appointments, anxiety, or surgery). Negative psychological effects on quality of life after a diagnosis of AAA have not been shown to cause significant harm.6,18
A recent UK analysis found that screening programs for AAA in women modeled after those in men are not cost effective, with an NNS to prevent 1 death of 3900 in women vs 700 in men.15,18 Another recent trial of ultrasound screening in 5200 high-risk women ages 65 to 74 years found an AAA incidence of 0.29% (95% CI, 0.18%-0.48%) in which only 3 large aneurysms were identified.22
In the United States, rates of screening for AAA remain low.23 One study has shown electronic medical record–based reminders increased screening rates from 48% to 80%.24 Point-of-care bedside ultrasound performed by clinicians also could improve screening rates. Multiple studies have demonstrated that screening and diagnosis of AAA can be performed safely and effectively at the bedside by nonradiologists such as family physicians and emergency physicians.25-28 In 1 study, such exams added < 4 minutes to the patient encounter.26 Follow-up surveillance schedules for those identified as having a AAA are summarized in TABLE 2.4

Continue to: Management options
Management options: Immediate repair or surveillance?
After diagnosing AAA, important decisions must be made regarding management, including indications for surgical repair, appropriate follow-up surveillance, and medications for secondary prevention and cardiovascular risk reduction.
EVAR vs open repair
The 2 main surgical strategies for aneurysm repair are open repair and endovascular repair (EVAR). In the United States, EVAR is becoming the more common approach and was used to repair asymptomatic aneurysms in > 80% of patients and ruptured aneurysms in 50% of patients.6 There have been multiple RCTs assessing EVAR and open repair for large and small aneurysms.29-34 Findings across these studies consistently show EVAR is associated with lower immediate (ie, 30-day) morbidity and mortality but no longer-term survival benefit compared to open repair.
EVAR procedures require ongoing long-term surveillance for endovascular leakage and other complications, resulting in an increased need for re-intervention.31,33,35 For these reasons, the National Institute for Health and Care Excellence (NICE) guidelines suggest open repair as the preferred modality.7 However, SVS and the American College of Cardiology Foundation/American Heart Association guidance support either EVAR or open repair, noting that open repair may be preferable in patients unable to engage in long-term follow-up surveillance.36

Indications for repair. In general, repair is indicated when an aneurysm reaches or exceeds 5.5 cm.4,7 Both SVS and NICE also recommend clinicians consider surgical repair of smaller, rapidly expanding aneurysms (> 1 cm over a 1-year period).4,7 Based on evidence suggesting a higher risk for rupture in women with smaller aneurysms,14,37 SVS recommends clinicians consider surgical repair in women with an AAA ≥ 5.0 cm. Several RCTs evaluating the benefits of immediate repair for smaller-sized aneurysms (4.0-5.5 cm) favored surveillance.38,39 Accepted indications for surgical repair are summarized in TABLE 3.4,7,34Surgical repair recommendations also are based on aneurysm morphology, which can be fusiform or saccular (FIGURE). More than 90% of AAAs are fusiform.40 Although saccular AAAs are less common, some studies suggest they are more prone to rupture than fusiform AAAs, and SVS guidelines suggest surgical repair of saccular aneurysms regardless of size.4,41,42

Perioperative and long-term risks. Both EVAR and open repair of AAA carry a high perioperative and long-term risk for death, as patients often have multiple comorbidities. A 2019 trial comparing EVAR to open repair with 14 years of follow-up reported death in 68% of patients in the EVAR group and 70% in the open repair group. 31 Among these deaths, 2.7% in the EVAR group and 3.7% in the open repair group were aneurysm related.31 The study also found a second surgical intervention was required in 19.8% of patients in the open repair group and 26.7% in the EVAR group.31
Continue to: When assessing perioperative risk...
When assessing perioperative risk, SVS guidelines recommend clinicians employ a shared decision-making approach with patients that incorporates Vascular Quality Initiative (VQI) mortality risk score.4 (VQI risk calculators are available at https://qxmd.com/vascular-study-group-new-england-decision-support-tools.43)
Medication management
Based on the close association of aortic aneurysm with atherosclerotic CVD (ASCVD), professional societies such as the European Society of Cardiology and European Atherosclerosis Society (ESC/EAS) have suggested aortic aneurysm is equivalent to ASCVD and should be managed medically in a similar manner to peripheral arterial disease.44 Indeed, many patients with AAA may have concomitant CAD or other arterial vascular diseases (eg, carotid, lower extremity).
Statins. In its guidelines, the ESC/EAS consider patients with AAA at “very high risk” for adverse CV events and suggest pharmacotherapy with high-intensity statins, adding ezetimibe or proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors if needed, to reduce low-density lipoprotein cholesterol ≥ 50% from baseline, with a goal of < 55 mg/dL.44 Statin therapy additionally lowers all-cause postoperative mortality in patients undergoing AAA repair but does not affect the rate of aneurysm expansion.45
Aspirin and other anticoagulants. Although aspirin therapy may be indicated for the secondary prevention of other cardiovascular events that may coexist with AAA, it does not appear to affect the rate of growth or prevent rupture of aneurysms.46,47 In addition to aspirin, anticoagulants such as clopidogrel, enoxaparin, and warfarin are not recommended when the presence of AAA is the only indication.4
Other medications. Angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, beta-blockers, and antibiotics (eg, doxycycline) have been studied as a treatment for AAA. However, none has shown benefit in reducing aneurysm growth or rupture and they are not recommended for that sole purpose.4,48
Metformin. There is a negative association between diabetes and AAA expansion and rupture. Several cohort studies have indicated that this may be an independent effect driven primarily by exposure to metformin. While it is not unreasonable to consider this another important indication for metformin use in patients with diabetes, RCT evidence has yet to establish a role for metformin in patients without diabetes who have AAA.48,49
ACKNOWLEDGEMENT
The authors thank Gwen Wilson, MLS, AHIP, for her assistance with the literature searches performed in the preparation of this manuscript.
CORRESPONDENCE
Nicholas LeFevre, MD, Family and Community Medicine, University of Missouri–Columbia School of Medicine, One Hospital Drive, M224 Medical Science Building, Columbia, MO 65212; nlefevre@health.missouri.edu
Ruptured abdominal aortic aneurysms (AAAs) caused about 6000 deaths annually in the United States between 2014 and 20201 and are associated with a pooled mortality rate of 81%.2 They result from a distinct degenerative process of the layers of the aortic wall.2 An AAA is defined as an abdominal aorta whose dilation is > 50% normal (more commonly, a diameter > 3 cm).3,4 The risk for rupture correlates closely with size; most ruptures occur in aneurysms > 5.5 cm3,4 (TABLE 15).

Most AAAs are asymptomatic and often go undetected until rupture, resulting in poor outcomes. Because of a low and declining prevalence of AAA and ruptured AAA in developed countries, screening recommendations target high-risk groups rather than the general population.4,6-8 This review summarizes risk factors, prevalence, and current evidence-based screening and management recommendations for AAA.
Who’s at risk?
Age is the most significant nonmodifiable risk factor, with AAA rupture uncommon in patients younger than 55 years.9 One retrospective study found the odds ratio (OR) for diagnosing AAA was 9.41 in adults ages 65 to 69 years (95% CI, 8.76-10.12; P < .0001) and 14.46 (95% CI, 13.45-15.55; P < .0001) in adults ages 70 to 74 years, compared to adults younger than 55 years.10
Smoking is the most potent modifiable risk factor for AAA. Among patients with AAA, > 90% have a history of smoking.4 The association between smoking and AAA is dose dependent, with an OR of 2.61 (95% CI, 2.47-2.74) in patients with a pack-per-year history < 5 years and 12.13 (95% CI, 11.66-12.61) in patients with a pack-per-year history > 35 years, compared to nonsmokers.10 The risk for AAA increases with smoking duration but decreases with cessation duration.4,10 Smoking cessation remains an important intervention, as active smokers have higher AAA rupture rates.11
Other risk factors for AAA include concomitant cardiovascular disease (CVD) such as coronary artery disease (CAD), cerebrovascular disease, atherosclerosis, dyslipidemia, and hypertension.10 Factors associated with reduced risk for AAA include African American race, Hispanic ethnicity, Asian ethnicity, diabetes, smoking cessation, consuming fruits and vegetables > 3 times per week, and exercising more than once per week.6,10
Prevalence declines but sex-based disparities in outcomes persist
The prevalence of AAA has declined in the United States and Europe in recent decades, correlating with declining rates of smoking.4,12 Reports published between 2011 and 2019 estimate that AAA prevalence in men older than 60 years has declined over time, with a prevalence of 1.2% to 3.3%.6 The prevalence of AAA has also decreased in women,6,13,14 estimated in 1 study to be as low as 0.74%.13 Similarly, deaths from ruptured AAA have declined markedly in the United States—by 70% between 1999 and 2016 according to 1 analysis.9
One striking difference in the male-female data is that although AAAs are more common in men, there is a 2- to 4-fold higher risk for rupture in women, who account for nearly half of all AAA-related deaths.9,10,15-17 The reasons for this heightened risk to women despite lower prevalence are not fully understood but are likely multifactorial and related to a general lack of screening for AAA in women, tendency for AAA to rupture at smaller diameters in women, rupture at an older age in women, and a history of worse surgical outcomes in women than men (though the gap in surgical outcomes appears to be closing).9,10,18
Continue to: While declines in AAA and AAA-related...
While declines in AAA and AAA-related death are largely attributed to lower smoking rates, other likely contributing factors include the implementation of screening programs, incidental detection during cross-sectional imaging, and improved surgical techniques and management of CV risk factors (eg, hypertension, hyperlipidemia).9,10
The benefits of screening older men
Randomized controlled trials (RCTs) have demonstrated the benefits of AAA screening programs. A meta-analysis of 4 populationbased RCTs of AAA screening in men ≥ 65 years demonstrated statistically significant reductions in AAA rupture (OR = 0.62; 95% CI, 0.55-0.70) and death from AAA (OR = 0.65; 95% CI, 0.57-0.74) over 12 to 15 years, with a number needed to screen (NNS) of 305 (95% CI, 248-411) to prevent 1 AAA-related death.18 The study also found screening decreases the rate of emergent surgeries for AAA (OR = 0.57; 95% CI, 0.48-0.68) while increasing the number of elective surgeries (OR = 1.44; 95% CI, 1.34-1.55) over 4 to 15 years.18
Only 1 study has demonstrated an improvement in all-cause mortality with screening programs, with a relatively small benefit (OR = 0.97; 95% CI, 0.94-0.99).19 Only 1 of the studies included women and, while underpowered, showed no difference in AAA-related death or rupture.20 Guidelines and recommendations of various countries and professional societies focus screening on subgroups at highest risk for AAA.4,6-8,18
Screening recommendations from USPSTF and others
The US Preventive Services Task Force (USPSTF) currently recommends one-time ultrasound screening for AAA in men ages 65 to 75 years who have ever smoked (commonly defined as having smoked > 100 cigarettes) in their lifetime.6 This grade “B” recommendation, initially made in 2005 and reaffirmed in the 2014 and 2019 USPSTF updates, recommends screening the highest-risk segment of the population (ie, older male smokers).
In men ages 65 to 75 years with no smoking history, rather than routine screening, the USPSTF recommends selectively offering screening based on the patient’s medical history, family history, risk factors, and personal values (with a “C” grade).6 The USPSTF continues to recommend against screening for AAA in women with no smoking history and no family history of AAA.6 According to the USPSTF, the evidence is insufficient to recommend for or against screening women ages 65 to 75 years who have ever smoked or have a family history of AAA (“I” statement).6
Continue to: One critique of the USPSTF recommendations
One critique of the USPSTF recommendations is that they fail to detect a significant portion of patients with AAA and AAA rupture. For example, in a retrospective analysis of 55,197 patients undergoing AAA repair, only 33% would have been detected by the USPSTF grade “B” recommendation to screen male smokers ages 65 to 75 years, and an analysis of AAA-related fatalities found 43% would be missed by USPSTF criteria.9,21
Screening guidelines from the Society for Vascular Surgery (SVS) are broader than those of the USPSTF, in an attempt to capture a larger percentage of the population at risk for AAA-related disease by extrapolating from epidemiologic data. The SVS guidelines include screening for women ages 65 to 75 years with a smoking history, screening men and women ages 65 to 75 years who have a first-degree relative with AAA, and consideration of screening patients older than 75 years if they are in good health and have a first-degree relative with AAA or a smoking history and have not been previously screened.4 However, these expanded recommendations are not supported by patient-oriented evidence.6
Attempts to broaden screening guidelines must be tempered by potential risks for harm, primarily overdiagnosis (ie, diagnosing AAAs that would not otherwise rise to clinical significance) and overtreatment (ie, resulting in unnecessary imaging, appointments, anxiety, or surgery). Negative psychological effects on quality of life after a diagnosis of AAA have not been shown to cause significant harm.6,18
A recent UK analysis found that screening programs for AAA in women modeled after those in men are not cost effective, with an NNS to prevent 1 death of 3900 in women vs 700 in men.15,18 Another recent trial of ultrasound screening in 5200 high-risk women ages 65 to 74 years found an AAA incidence of 0.29% (95% CI, 0.18%-0.48%) in which only 3 large aneurysms were identified.22
In the United States, rates of screening for AAA remain low.23 One study has shown electronic medical record–based reminders increased screening rates from 48% to 80%.24 Point-of-care bedside ultrasound performed by clinicians also could improve screening rates. Multiple studies have demonstrated that screening and diagnosis of AAA can be performed safely and effectively at the bedside by nonradiologists such as family physicians and emergency physicians.25-28 In 1 study, such exams added < 4 minutes to the patient encounter.26 Follow-up surveillance schedules for those identified as having a AAA are summarized in TABLE 2.4

Continue to: Management options
Management options: Immediate repair or surveillance?
After diagnosing AAA, important decisions must be made regarding management, including indications for surgical repair, appropriate follow-up surveillance, and medications for secondary prevention and cardiovascular risk reduction.
EVAR vs open repair
The 2 main surgical strategies for aneurysm repair are open repair and endovascular repair (EVAR). In the United States, EVAR is becoming the more common approach and was used to repair asymptomatic aneurysms in > 80% of patients and ruptured aneurysms in 50% of patients.6 There have been multiple RCTs assessing EVAR and open repair for large and small aneurysms.29-34 Findings across these studies consistently show EVAR is associated with lower immediate (ie, 30-day) morbidity and mortality but no longer-term survival benefit compared to open repair.
EVAR procedures require ongoing long-term surveillance for endovascular leakage and other complications, resulting in an increased need for re-intervention.31,33,35 For these reasons, the National Institute for Health and Care Excellence (NICE) guidelines suggest open repair as the preferred modality.7 However, SVS and the American College of Cardiology Foundation/American Heart Association guidance support either EVAR or open repair, noting that open repair may be preferable in patients unable to engage in long-term follow-up surveillance.36

Indications for repair. In general, repair is indicated when an aneurysm reaches or exceeds 5.5 cm.4,7 Both SVS and NICE also recommend clinicians consider surgical repair of smaller, rapidly expanding aneurysms (> 1 cm over a 1-year period).4,7 Based on evidence suggesting a higher risk for rupture in women with smaller aneurysms,14,37 SVS recommends clinicians consider surgical repair in women with an AAA ≥ 5.0 cm. Several RCTs evaluating the benefits of immediate repair for smaller-sized aneurysms (4.0-5.5 cm) favored surveillance.38,39 Accepted indications for surgical repair are summarized in TABLE 3.4,7,34Surgical repair recommendations also are based on aneurysm morphology, which can be fusiform or saccular (FIGURE). More than 90% of AAAs are fusiform.40 Although saccular AAAs are less common, some studies suggest they are more prone to rupture than fusiform AAAs, and SVS guidelines suggest surgical repair of saccular aneurysms regardless of size.4,41,42

Perioperative and long-term risks. Both EVAR and open repair of AAA carry a high perioperative and long-term risk for death, as patients often have multiple comorbidities. A 2019 trial comparing EVAR to open repair with 14 years of follow-up reported death in 68% of patients in the EVAR group and 70% in the open repair group. 31 Among these deaths, 2.7% in the EVAR group and 3.7% in the open repair group were aneurysm related.31 The study also found a second surgical intervention was required in 19.8% of patients in the open repair group and 26.7% in the EVAR group.31
Continue to: When assessing perioperative risk...
When assessing perioperative risk, SVS guidelines recommend clinicians employ a shared decision-making approach with patients that incorporates Vascular Quality Initiative (VQI) mortality risk score.4 (VQI risk calculators are available at https://qxmd.com/vascular-study-group-new-england-decision-support-tools.43)
Medication management
Based on the close association of aortic aneurysm with atherosclerotic CVD (ASCVD), professional societies such as the European Society of Cardiology and European Atherosclerosis Society (ESC/EAS) have suggested aortic aneurysm is equivalent to ASCVD and should be managed medically in a similar manner to peripheral arterial disease.44 Indeed, many patients with AAA may have concomitant CAD or other arterial vascular diseases (eg, carotid, lower extremity).
Statins. In its guidelines, the ESC/EAS consider patients with AAA at “very high risk” for adverse CV events and suggest pharmacotherapy with high-intensity statins, adding ezetimibe or proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors if needed, to reduce low-density lipoprotein cholesterol ≥ 50% from baseline, with a goal of < 55 mg/dL.44 Statin therapy additionally lowers all-cause postoperative mortality in patients undergoing AAA repair but does not affect the rate of aneurysm expansion.45
Aspirin and other anticoagulants. Although aspirin therapy may be indicated for the secondary prevention of other cardiovascular events that may coexist with AAA, it does not appear to affect the rate of growth or prevent rupture of aneurysms.46,47 In addition to aspirin, anticoagulants such as clopidogrel, enoxaparin, and warfarin are not recommended when the presence of AAA is the only indication.4
Other medications. Angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, beta-blockers, and antibiotics (eg, doxycycline) have been studied as a treatment for AAA. However, none has shown benefit in reducing aneurysm growth or rupture and they are not recommended for that sole purpose.4,48
Metformin. There is a negative association between diabetes and AAA expansion and rupture. Several cohort studies have indicated that this may be an independent effect driven primarily by exposure to metformin. While it is not unreasonable to consider this another important indication for metformin use in patients with diabetes, RCT evidence has yet to establish a role for metformin in patients without diabetes who have AAA.48,49
ACKNOWLEDGEMENT
The authors thank Gwen Wilson, MLS, AHIP, for her assistance with the literature searches performed in the preparation of this manuscript.
CORRESPONDENCE
Nicholas LeFevre, MD, Family and Community Medicine, University of Missouri–Columbia School of Medicine, One Hospital Drive, M224 Medical Science Building, Columbia, MO 65212; nlefevre@health.missouri.edu
1. CDC. Wide-ranging Online Data for Epidemiologic Research (WONDER) database. Accessed August 30, 2023. https://wonder.cdc.gov/ucd-icd10.html
2. Reimerink JJ, van der Laan MJ, Koelemay MJ, et al. Systematic review and meta-analysis of population-based mortality from ruptured abdominal aortic aneurysm. Br J Surg. 2013;100:1405-1413. doi: 10.1002/bjs.9235
3. Kent KC. Clinical practice. Abdominal aortic aneurysms. N Engl J Med. 2014;371:2101-2108. doi: 10.1056/NEJMcp1401430
4. Chaikof EL, Dalman RL, Eskandari MK, et al. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg. 2018;67:2-77.e2. doi: 10.1016/j.jvs.2017.10.044
5. Moll FL, Powell JT, Fraedrich G, et al. Management of abdominal aortic aneurysms clinical practice guidelines of the European society for vascular surgery. Eur J Vasc Endovasc Surg. 2011;41 suppl 1:S1-S58. doi: 10.1016/j.ejvs.2010.09.011
6. Owens DK, Davidson KW, Krist AH, et al; US Preventive Services Task Force. Screening for abdominal aortic aneurysm: US Preventive Services Task Force recommendation statement. JAMA. 2019;322:2211-2218. doi: 10.1001/jama.2019.18928
7. National Institute for Health and Care Excellence. Abdominal aortic aneurysm: diagnosis and management. NICE guideline [NG156]. March 19, 2020. Accessed June 30, 2023. www.nice.org.uk/guidance/ng156/chapter/recommendations
8. Canadian Task Force on Preventive Health Care. Recommendations on screening for abdominal aortic aneurysm in primary care. CMAJ. 2017;189:E1137-E1145. doi: 10.1503/cmaj.170118
9. Abdulameer H, Al Taii H, Al-Kindi SG, et al. Epidemiology of fatal ruptured aortic aneurysms in the United States (1999-2016). J Vasc Surg. 2019;69:378-384.e2. doi: 10.1016/j.jvs.2018.03.435
10. Kent KC, Zwolak RM, Egorova NN, et al. Analysis of risk factors for abdominal aortic aneurysm in a cohort of more than 3 million individuals. J Vasc Surg. 2010;52:539-548. doi: 10.1016/j.jvs.2010.05.090
11. [No authors listed] Smoking, lung function and the prognosis of abdominal aortic aneurysm. The UK Small Aneurysm Trial Participants. Eur J Vasc Endovasc Surg. 2000;19:636-642. doi: 10.1053/ejvs.2000.1066
12. Oliver-Williams C, Sweeting MJ, Turton G, et al. Lessons learned about prevalence and growth rates of abdominal aortic aneurysms from a 25-year ultrasound population screening programme. Br J Surg. 2018;105:68-74. doi: 10.1002/bjs.10715
13. Ulug P, Powell JT, Sweeting MJ, et al. Meta-analysis of the current prevalence of screen-detected abdominal aortic aneurysm in women. Br J Surg. 2016;103:1097-1104. doi: 10.1002/bjs.10225
14. Chabok M, Nicolaides A, Aslam M, et al. Risk factors associated with increased prevalence of abdominal aortic aneurysm in women. Br J Surg. 2016;103:1132-1138. doi: 10.1002/bjs.10179
15. Sweeting, MJ, Masconi KL, Jones E, et al. Analysis of clinical benefit, harms, and cost-effectiveness of screening women for abdominal aortic aneurysm. Lancet. 2018;392:487-495. doi: 10.1016/S0140-6736(18)31222-4
16. Sweeting MJ, Thompson SG, Brown LC, et al; RESCAN collaborators. Meta-analysis of individual patient data to examine factors affecting growth and rupture of small abdominal aortic aneurysms. Br J Surg. 2012;99:655-665. doi: 10.1002/bjs.8707
17. Skibba AA, Evans JR, Hopkins SP, et al. Reconsidering gender relative to risk of rupture in the contemporary management of abdominal aortic aneurysms. J Vasc Surg. 2015;62:1429-1436. doi: 10.1016/j.jvs.2015.07.079
18. Guirguis-Blake JM, Beil TL, Senger CA, et al. Primary care screening for abdominal aortic aneurysm: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2019;322:2219-2238. doi: 10.1001/jama.2019.17021
19. Thompson SG, Ashton HA, Gao L, et al; Multicentre Aneurysm Screening Study (MASS) Group. Final follow-up of the Multicentre Aneurysm Screening Study (MASS) randomized trial of abdominal aortic aneurysm screening. Br J Surg. 2012;99:1649-1656. doi: 10.1002/bjs.8897
20. Ashton HA, Gao L, Kim LG, et al. Fifteen-year follow-up of a randomized clinical trial of ultrasonographic screening for abdominal aortic aneurysms. Br J Surg. 2007;94:696-701. doi: 10.1002/bjs.5780
21. Carnevale ML, Koleilat I, Lipsitz EC, et al. Extended screening guidelines for the diagnosis of abdominal aortic aneurysm. J Vasc Surg. 2020;72:1917-1926. doi: 10.1016/j.jvs.2020.03.047
22. Duncan A, Maslen C, Gibson C, et al. Ultrasound screening for abdominal aortic aneurysm in high-risk women. Br J Surg. 2021;108:1192-1198. doi: 10.1093/bjs/znab220
23. Shreibati JB, Baker LC, Hlatky MA, et al. Impact of the Screening Abdominal Aortic Aneurysms Very Efficiently (SAAAVE) Act on abdominal ultrasonography use among Medicare beneficiaries. Arch Intern Med. 2012;172:1456-1462. doi: 10.1001/archinternmed.2012.4268
24. Hye RJ, Smith AE, Wong GH, et al. Leveraging the electronic medical record to implement an abdominal aortic aneurysm screening program. J Vasc Surg. 2014;59:1535-1542. doi: 10.1016/j.jvs.2013.12.016
25. Rubano E, Mehta N, Caputo W, et al., Systematic review: emergency department bedside ultrasonography for diagnosing suspected abdominal aortic aneurysm. Acad Emerg Med. 2013. 20:128-138. doi: 10.1111/acem.12080
26. Blois B. Office-based ultrasound screening for abdominal aortic aneurysm. Can Fam Physician. 2012;58:e172-e178.
27. Arnold MJ, Jonas CE, Carter RE. Point-of-care ultrasonography. Am Fam Physician. 2020;101:275-285.
28. Nixon G, Blattner K, Muirhead J, et al. Point-of-care ultrasound for FAST and AAA in rural New Zealand: quality and impact on patient care. Rural Remote Health. 2019;19:5027. doi: 10.22605/RRH5027
29. Lederle FA, Wilson SE, Johnson GR, et al. Immediate repair compared with surveillance of small abdominal aortic aneurysms. N Engl J Med. 2002;346:1437-1444. doi: 10.1056/NEJMoa012573
30. Filardo G, Lederle FA, Ballard DJ, et al. Immediate open repair vs surveillance in patients with small abdominal aortic aneurysms: survival differences by aneurysm size. Mayo Clin Proc. 2013;88:910-919. doi: 10.1016/j.mayocp.2013.05.014
31. Lederle FA, Kyriakides TC, Stroupe KT, et al. Open versus endovascular repair of abdominal aortic aneurysm. N Engl J Med. 2019;380:2126-2135. doi: 10.1056/NEJMoa1715955
32. Patel R, Sweeting MJ, Powell JT, et al., Endovascular versus open repair of abdominal aortic aneurysm in 15-years’ follow-up of the UK endovascular aneurysm repair trial 1 (EVAR trial 1): a randomised controlled trial. Lancet. 2016;388:2366-2374. doi: 10.1016/S0140-6736(16)31135-7
33. van Schaik TG, Yeung KK, Verhagen HJ, et al. Long-term survival and secondary procedures after open or endovascular repair of abdominal aortic aneurysms. J Vasc Surg. 2017;66:1379-1389. doi: 10.1016/j.jvs.2017.05.122
34. Powell JT, Brady AR, Brown, LC, et al; United Kingdom Small Aneurysm Trial Participants. Long-term outcomes of immediate repair compared with surveillance of small abdominal aortic aneurysms. N Engl J Med. 2002;346:1445-1452. doi: 10.1056/NEJMoa013527
35. Paravastu SC, Jayarajasingam R, Cottam R, et al. Endovascular repair of abdominal aortic aneurysm. Cochrane Database Syst Rev. 2014:CD004178. doi: 10.1002/14651858.CD004178.pub2
36. Rooke TW, Hirsch AT, Misra S, et al. 2011 ACCF/AHA focused update of the guideline for the management of patients with peripheral artery disease (updating the 2005 guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2011;58:2020-2045. doi: 10.1016/j.jacc.2011.08.023
37. Bhak RH, Wininger M, Johnson GR, et al. Factors associated with small abdominal aortic aneurysm expansion rate. JAMA Surg. 2015;150:44-50. doi: 10.1001/jamasurg.2014.2025
38. Ouriel K, Clair DG, Kent KC, et al; Positive Impact of Endovascular Options for treating Aneurysms Early (PIVOTAL) Investigators. Endovascular repair compared with surveillance for patients with small abdominal aortic aneurysms. J Vasc Surg. 2010;51:1081-1087. doi: 10.1016/j.jvs.2009.10.113
39. Cao P, De Rango P, Verzini F, et al. Comparison of surveillance versus aortic endografting for small aneurysm repair (CAESAR): results from a randomised trial. Eur J Vasc Endovasc Surg. 2011;41:13-25. doi: 10.1016/j.ejvs.2010.08.026
40. Karthaus EG, Tong TML, Vahl A, et al; Dutch Society of Vascular Surgery, the Steering Committee of the Dutch Surgical Aneurysm Audit and the Dutch Institute for Clinical Auditing. Saccular abdominal aortic aneurysms: patient characteristics, clinical presentation, treatment, and outcomes in the Netherlands. Ann Surg. 2019;270:852-858. doi: 10.1097/SLA.0000000000003529
41. Nathan DP, Xu C, Pouch AM, et al. Increased wall stress of saccular versus fusiform aneurysms of the descending thoracic aorta. Ann Vasc Surg. 2011;25:1129-2237. doi: 10.1016/j.avsg.2011.07.008
42. Durojaye MS, Adeniyi TO, Alagbe OA. Multiple saccular aneurysms of the abdominal aorta: a case report and short review of risk factors for rupture on CT Scan. Ann Ib Postgrad Med. 2020;18:178-180.
43. Bertges DJ, Neal D, Schanzer A, et al. The Vascular Quality Initiative Cardiac Risk Index for prediction of myocardial infarction after vascular surgery. J Vasc Surg. 2016;64:1411-1421.e4. doi: 10.1016/j.jvs.2016.04.045
44. Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41:111-188. doi: 10.1093/eurheartj/ehz455
45. Twine CP, Williams IM. Systematic review and meta-analysis of the effects of statin therapy on abdominal aortic aneurysms. Br J Surg. 2011;98:346-353. doi: 10.1002/bjs.7343
46. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:e596-e646. doi: 10.1161/CIR.0000000000000678
47. Erbel R, Aboyans V, Boileau C, et al. 2014 ESC guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur Heart J. 2014;35:2873-2926. doi: 10.1093/eurheartj/ehu281
48. Lederle FA, Noorbaloochi S, Nugent S, et al. Multicentre study of abdominal aortic aneurysm measurement and enlargement. Br J Surg. 2015;102:1480-1487. doi: 10.1002/bjs.9895
49. Itoga NK, Rothenberg KA, Suarez P, et al. Metformin prescription status and abdominal aortic aneurysm disease progression in the U.S. veteran population. J Vasc Surg. 2019;69:710-716.e3. doi: 10.1016/j.jvs.2018.06.19
1. CDC. Wide-ranging Online Data for Epidemiologic Research (WONDER) database. Accessed August 30, 2023. https://wonder.cdc.gov/ucd-icd10.html
2. Reimerink JJ, van der Laan MJ, Koelemay MJ, et al. Systematic review and meta-analysis of population-based mortality from ruptured abdominal aortic aneurysm. Br J Surg. 2013;100:1405-1413. doi: 10.1002/bjs.9235
3. Kent KC. Clinical practice. Abdominal aortic aneurysms. N Engl J Med. 2014;371:2101-2108. doi: 10.1056/NEJMcp1401430
4. Chaikof EL, Dalman RL, Eskandari MK, et al. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg. 2018;67:2-77.e2. doi: 10.1016/j.jvs.2017.10.044
5. Moll FL, Powell JT, Fraedrich G, et al. Management of abdominal aortic aneurysms clinical practice guidelines of the European society for vascular surgery. Eur J Vasc Endovasc Surg. 2011;41 suppl 1:S1-S58. doi: 10.1016/j.ejvs.2010.09.011
6. Owens DK, Davidson KW, Krist AH, et al; US Preventive Services Task Force. Screening for abdominal aortic aneurysm: US Preventive Services Task Force recommendation statement. JAMA. 2019;322:2211-2218. doi: 10.1001/jama.2019.18928
7. National Institute for Health and Care Excellence. Abdominal aortic aneurysm: diagnosis and management. NICE guideline [NG156]. March 19, 2020. Accessed June 30, 2023. www.nice.org.uk/guidance/ng156/chapter/recommendations
8. Canadian Task Force on Preventive Health Care. Recommendations on screening for abdominal aortic aneurysm in primary care. CMAJ. 2017;189:E1137-E1145. doi: 10.1503/cmaj.170118
9. Abdulameer H, Al Taii H, Al-Kindi SG, et al. Epidemiology of fatal ruptured aortic aneurysms in the United States (1999-2016). J Vasc Surg. 2019;69:378-384.e2. doi: 10.1016/j.jvs.2018.03.435
10. Kent KC, Zwolak RM, Egorova NN, et al. Analysis of risk factors for abdominal aortic aneurysm in a cohort of more than 3 million individuals. J Vasc Surg. 2010;52:539-548. doi: 10.1016/j.jvs.2010.05.090
11. [No authors listed] Smoking, lung function and the prognosis of abdominal aortic aneurysm. The UK Small Aneurysm Trial Participants. Eur J Vasc Endovasc Surg. 2000;19:636-642. doi: 10.1053/ejvs.2000.1066
12. Oliver-Williams C, Sweeting MJ, Turton G, et al. Lessons learned about prevalence and growth rates of abdominal aortic aneurysms from a 25-year ultrasound population screening programme. Br J Surg. 2018;105:68-74. doi: 10.1002/bjs.10715
13. Ulug P, Powell JT, Sweeting MJ, et al. Meta-analysis of the current prevalence of screen-detected abdominal aortic aneurysm in women. Br J Surg. 2016;103:1097-1104. doi: 10.1002/bjs.10225
14. Chabok M, Nicolaides A, Aslam M, et al. Risk factors associated with increased prevalence of abdominal aortic aneurysm in women. Br J Surg. 2016;103:1132-1138. doi: 10.1002/bjs.10179
15. Sweeting, MJ, Masconi KL, Jones E, et al. Analysis of clinical benefit, harms, and cost-effectiveness of screening women for abdominal aortic aneurysm. Lancet. 2018;392:487-495. doi: 10.1016/S0140-6736(18)31222-4
16. Sweeting MJ, Thompson SG, Brown LC, et al; RESCAN collaborators. Meta-analysis of individual patient data to examine factors affecting growth and rupture of small abdominal aortic aneurysms. Br J Surg. 2012;99:655-665. doi: 10.1002/bjs.8707
17. Skibba AA, Evans JR, Hopkins SP, et al. Reconsidering gender relative to risk of rupture in the contemporary management of abdominal aortic aneurysms. J Vasc Surg. 2015;62:1429-1436. doi: 10.1016/j.jvs.2015.07.079
18. Guirguis-Blake JM, Beil TL, Senger CA, et al. Primary care screening for abdominal aortic aneurysm: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2019;322:2219-2238. doi: 10.1001/jama.2019.17021
19. Thompson SG, Ashton HA, Gao L, et al; Multicentre Aneurysm Screening Study (MASS) Group. Final follow-up of the Multicentre Aneurysm Screening Study (MASS) randomized trial of abdominal aortic aneurysm screening. Br J Surg. 2012;99:1649-1656. doi: 10.1002/bjs.8897
20. Ashton HA, Gao L, Kim LG, et al. Fifteen-year follow-up of a randomized clinical trial of ultrasonographic screening for abdominal aortic aneurysms. Br J Surg. 2007;94:696-701. doi: 10.1002/bjs.5780
21. Carnevale ML, Koleilat I, Lipsitz EC, et al. Extended screening guidelines for the diagnosis of abdominal aortic aneurysm. J Vasc Surg. 2020;72:1917-1926. doi: 10.1016/j.jvs.2020.03.047
22. Duncan A, Maslen C, Gibson C, et al. Ultrasound screening for abdominal aortic aneurysm in high-risk women. Br J Surg. 2021;108:1192-1198. doi: 10.1093/bjs/znab220
23. Shreibati JB, Baker LC, Hlatky MA, et al. Impact of the Screening Abdominal Aortic Aneurysms Very Efficiently (SAAAVE) Act on abdominal ultrasonography use among Medicare beneficiaries. Arch Intern Med. 2012;172:1456-1462. doi: 10.1001/archinternmed.2012.4268
24. Hye RJ, Smith AE, Wong GH, et al. Leveraging the electronic medical record to implement an abdominal aortic aneurysm screening program. J Vasc Surg. 2014;59:1535-1542. doi: 10.1016/j.jvs.2013.12.016
25. Rubano E, Mehta N, Caputo W, et al., Systematic review: emergency department bedside ultrasonography for diagnosing suspected abdominal aortic aneurysm. Acad Emerg Med. 2013. 20:128-138. doi: 10.1111/acem.12080
26. Blois B. Office-based ultrasound screening for abdominal aortic aneurysm. Can Fam Physician. 2012;58:e172-e178.
27. Arnold MJ, Jonas CE, Carter RE. Point-of-care ultrasonography. Am Fam Physician. 2020;101:275-285.
28. Nixon G, Blattner K, Muirhead J, et al. Point-of-care ultrasound for FAST and AAA in rural New Zealand: quality and impact on patient care. Rural Remote Health. 2019;19:5027. doi: 10.22605/RRH5027
29. Lederle FA, Wilson SE, Johnson GR, et al. Immediate repair compared with surveillance of small abdominal aortic aneurysms. N Engl J Med. 2002;346:1437-1444. doi: 10.1056/NEJMoa012573
30. Filardo G, Lederle FA, Ballard DJ, et al. Immediate open repair vs surveillance in patients with small abdominal aortic aneurysms: survival differences by aneurysm size. Mayo Clin Proc. 2013;88:910-919. doi: 10.1016/j.mayocp.2013.05.014
31. Lederle FA, Kyriakides TC, Stroupe KT, et al. Open versus endovascular repair of abdominal aortic aneurysm. N Engl J Med. 2019;380:2126-2135. doi: 10.1056/NEJMoa1715955
32. Patel R, Sweeting MJ, Powell JT, et al., Endovascular versus open repair of abdominal aortic aneurysm in 15-years’ follow-up of the UK endovascular aneurysm repair trial 1 (EVAR trial 1): a randomised controlled trial. Lancet. 2016;388:2366-2374. doi: 10.1016/S0140-6736(16)31135-7
33. van Schaik TG, Yeung KK, Verhagen HJ, et al. Long-term survival and secondary procedures after open or endovascular repair of abdominal aortic aneurysms. J Vasc Surg. 2017;66:1379-1389. doi: 10.1016/j.jvs.2017.05.122
34. Powell JT, Brady AR, Brown, LC, et al; United Kingdom Small Aneurysm Trial Participants. Long-term outcomes of immediate repair compared with surveillance of small abdominal aortic aneurysms. N Engl J Med. 2002;346:1445-1452. doi: 10.1056/NEJMoa013527
35. Paravastu SC, Jayarajasingam R, Cottam R, et al. Endovascular repair of abdominal aortic aneurysm. Cochrane Database Syst Rev. 2014:CD004178. doi: 10.1002/14651858.CD004178.pub2
36. Rooke TW, Hirsch AT, Misra S, et al. 2011 ACCF/AHA focused update of the guideline for the management of patients with peripheral artery disease (updating the 2005 guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2011;58:2020-2045. doi: 10.1016/j.jacc.2011.08.023
37. Bhak RH, Wininger M, Johnson GR, et al. Factors associated with small abdominal aortic aneurysm expansion rate. JAMA Surg. 2015;150:44-50. doi: 10.1001/jamasurg.2014.2025
38. Ouriel K, Clair DG, Kent KC, et al; Positive Impact of Endovascular Options for treating Aneurysms Early (PIVOTAL) Investigators. Endovascular repair compared with surveillance for patients with small abdominal aortic aneurysms. J Vasc Surg. 2010;51:1081-1087. doi: 10.1016/j.jvs.2009.10.113
39. Cao P, De Rango P, Verzini F, et al. Comparison of surveillance versus aortic endografting for small aneurysm repair (CAESAR): results from a randomised trial. Eur J Vasc Endovasc Surg. 2011;41:13-25. doi: 10.1016/j.ejvs.2010.08.026
40. Karthaus EG, Tong TML, Vahl A, et al; Dutch Society of Vascular Surgery, the Steering Committee of the Dutch Surgical Aneurysm Audit and the Dutch Institute for Clinical Auditing. Saccular abdominal aortic aneurysms: patient characteristics, clinical presentation, treatment, and outcomes in the Netherlands. Ann Surg. 2019;270:852-858. doi: 10.1097/SLA.0000000000003529
41. Nathan DP, Xu C, Pouch AM, et al. Increased wall stress of saccular versus fusiform aneurysms of the descending thoracic aorta. Ann Vasc Surg. 2011;25:1129-2237. doi: 10.1016/j.avsg.2011.07.008
42. Durojaye MS, Adeniyi TO, Alagbe OA. Multiple saccular aneurysms of the abdominal aorta: a case report and short review of risk factors for rupture on CT Scan. Ann Ib Postgrad Med. 2020;18:178-180.
43. Bertges DJ, Neal D, Schanzer A, et al. The Vascular Quality Initiative Cardiac Risk Index for prediction of myocardial infarction after vascular surgery. J Vasc Surg. 2016;64:1411-1421.e4. doi: 10.1016/j.jvs.2016.04.045
44. Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41:111-188. doi: 10.1093/eurheartj/ehz455
45. Twine CP, Williams IM. Systematic review and meta-analysis of the effects of statin therapy on abdominal aortic aneurysms. Br J Surg. 2011;98:346-353. doi: 10.1002/bjs.7343
46. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:e596-e646. doi: 10.1161/CIR.0000000000000678
47. Erbel R, Aboyans V, Boileau C, et al. 2014 ESC guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur Heart J. 2014;35:2873-2926. doi: 10.1093/eurheartj/ehu281
48. Lederle FA, Noorbaloochi S, Nugent S, et al. Multicentre study of abdominal aortic aneurysm measurement and enlargement. Br J Surg. 2015;102:1480-1487. doi: 10.1002/bjs.9895
49. Itoga NK, Rothenberg KA, Suarez P, et al. Metformin prescription status and abdominal aortic aneurysm disease progression in the U.S. veteran population. J Vasc Surg. 2019;69:710-716.e3. doi: 10.1016/j.jvs.2018.06.19
PRACTICE RECOMMENDATIONS
› Perform a one-time abdominal aortic aneurysm (AAA) screening ultrasound in men ages 65 to 75 years who have ever smoked. B
› Consider performing a one-time AAA screening ultrasound in women ages 65 to 75 years who have ever smoked. C
› Prescribe high-intensity statin therapy for men and women with atherosclerotic AAA. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Inadequate sleep & obesity: Breaking the vicious cycle
Sleep is fundamental to overall health and longevity, with the average person spending about one-third of their life sleeping.1 Adequate sleep is critical for optimal cognition, memory consolidation, mood regulation, metabolism, appetite regulation, and immune and hormone functioning. According to the American Academy of Sleep Medicine and the Sleep Research Society, adults should sleep at least 7 hours per night on a regular basis “to promote optimal health.”2 Yet, between 2013 and 2020, only about 65% of adults in the United States were meeting this amount.3 Insufficient sleep is associated with an increased risk for chronic health conditions, including obesity, diabetes, cardiovascular diseases, and even premature death.4

In a population-based longitudinal study of sleep disorders, short sleep duration was associated with increased body mass index (BMI), low blood levels of leptin, and high ghrelin levels.5 In addition to physical impairments, poor sleep can impair cognitive performance and lead to vehicular accidents and increased accidents at work.4 The potential economic impact that this may have is significant, and includes increased costs and loss of productivity in the workplace.6
Many factors may contribute to short sleep duration: environment, mental and physical condition, and social influences such as occupation, family responsibilities, travel, group activities, and personal care. Furthermore, the rapidly evolving and developing media, communication, and entertainment industries are already strongly implicated in poor sleep quality and quantity, both contributing to excessive daytime sleepiness.7 Poor sleep quality is most notable in modern societies, and it correlates with the increasing prevalence of obesity, likely due to sleep’s effect on food consumption and physical activity.8 Optimizing a person’s sleep will improve overall health and longevity by inhibiting the development of chronic disease.
How insufficient sleep raises the risk for obesity
Not only is sleep beneficial for brain health, memory, learning, and growth, its effect on food consumption and physical activity likely correlates with the increased prevalence of obesity in modern society. Yet the optimal amount of sleep is controversial, and current recommendations of 7 or more hours of sleep per night for adults are derived from expert panels only.2 The recommended sleep duration for children is longer, and it varies by age.9 The quality of sleep and its impact on neuroendocrine hormones, not just the quantity of sleep, needs to be factored into these recommendations.
Sleep restriction activates the orexigenic system via the hormones leptin and ghrelin. These hormones control the food reward system, essentially increasing hunger and food intake. Leptin, created by white adipose tissue, is responsible for satiety and decreased food consumption.10 Ghrelin, made by oxyntic glands in the stomach, is responsible for the sensation of hunger.
In a 2004 study by Spiegel et al,11 leptin and ghrelin levels were measured during 2 days of sleep restriction (4 hours in bed) and sleep extension (10 hours in bed). Sleep restriction was associated with a decrease in leptin levels and an increase in ghrelin levels. The researchers reported that participants experienced an increase in hunger and appetite—especially for calorie-dense foods with high carbohydrate content.
Although research design has limitations with predominantly self-reported sleep data, studies have shown that short sleep time leads to increased food intake by increasing hunger signals and craving of unhealthy foods, and by providing more opportunities to eat while awake. It also may lead to decreased physical activity, creating a sedentary lifestyle that further encourages obesity.8 Reduced sleep is even correlated to decreased efficacy of weight-loss treatments.12
Continue to: Other sleep characteristics weakly correlated with obesity
Other sleep characteristics weakly correlated with obesity are sleep variability, timing, efficiency, quality, and daytime napping.8 Sleep variability causes dysregulation of eating patterns, leading to increased food intake. A shift to later sleep and waking times often results in higher consumption of calories after 8
Poor sleep efficiency and quality decreases N3-stage (deep non-REM) sleep, affects the autonomic nervous system, and has been associated with increased abdominal obesity. Daytime napping, which can cause irregular circadian rhythms and sleep schedules, is associated with increased obesity.15 Thus, each component of sleep needs to be assessed to promote optimal regulation of the orexigenic system.
Another study showed that inadequate sleep not only promotes unhealthy lifestyle habits that can lead to obesity but also decreases the ability to lose weight.16 This small study with 10 overweight patients provided its subjects with a controlled caloric intake over 2 weeks. Patients spent two 14-day periods 3 months apart in the laboratory, divided into 2 time-in-bed arms of 8.5 and 5.5 hours per night. Neuroendocrine changes caused by decreased sleep were associated with a significant lean body mass loss while conserving energy-dense fat.16 This study highlights the importance of sleep hygiene counseling when developing a weight-management plan with patients.
Sleep, and its many components, play an integral role in the prevention and treatment of obesity.17 Poor sleep will increase the risk for obesity and hinder its treatment. Therefore, sleep quality and duration are vital components of obesity management.
The sleep–obesity link in children and the elderly
Childhood obesity is linked to several chronic diseases in adulthood, including type 2 diabetes, cardiovascular disease, nonalcoholic fatty liver disease, asthma, and obstructive sleep apnea (OSA).18 According to 2017-2018 NHANES (National Health and Nutrition Examination Surveys) data, obesity (BMI ≥ 95th percentile) prevalence among children and adolescents was reported at 19.3% and severe obesity (BMI ≥ 120% of the 95th percentile) at 6.1%. Pediatric overweight prevalence (≥ 85th percentile and < 95th percentile) was 16.1%.19
Continue to: Although poor sleep is associated...
Although poor sleep is associated with increased risk for obesity, there is no proven cause-effect relationship.20 Nutrition and physical activity have been identified as 2 critical factors in childhood obesity, but sleep health also needs to be investigated. Shorter sleep duration is strongly associated with the development of obesity. Furthermore, children with obesity are more likely to have shorter sleep duration.21 A short sleep duration alters plasma levels of insulin, low-density lipoprotein, and high-sensitivity C-reactive protein. It is associated with lower diet quality, an increased intake of nutrient-poor foods, and a lower intake of vegetables and fruits.22 Recent studies have shown that interventions to promote earlier bedtimes can improve sleep duration in children.
Older adults have many sleeping issues, including insomnia, circadian rhythm sleep-wake disorders, sleep-related movement disorders, and sleep-breathing disorders. Additionally, the older population has increased sleep latency, decreased sleep efficiency and total sleep time, decreased REM sleep, more frequent nighttime awakenings, and more daytime napping.23 The increased sleep disturbance with age is mainly related to higher risk factors for sleep disorders than the aging process itself. Sleeping 5 or fewer hours is associated with an increased risk for obesity and central abdominal fat compared with those who sleep 7 to 8 hours per night.24 Similar to children and youth, older adults also show a strong correlation between inadequate sleep and obesity.24
The consequence: A vicious cycle
Obesity in turn leads to shorter sleep duration and more disruptions. This negatively affects the orexigenic system, and the resulting hormonal derangement promotes worsening obesity. It is a cycle of poor sleep causing obesity and obesity causing poor sleep. Insomnia, in combination with shorter (and longer) sleep times, also has been linked with obesity.25 These patients experience more daytime sleepiness, fatigue, and nighttime sleep disturbances, all correlated with decreased quality of life and higher prevalence of medical comorbidities.8,26 Additional comorbidities secondary to obesity, including gastroesophageal reflux, depression, and asthma, also have been linked to sleep disturbances.8
OSA is a common sleep complication associated with obesity. With the increasing prevalence of obesity, the prevalence of OSA is rising.8,27 Factors that heighten the risk for OSA are male sex, age 40 to 70 years, postmenopausal status, elevated BMI, and craniofacial and upper airway abnormality.28 However, the US Preventive Services Task Force found insufficient evidence to screen for or treat OSA in asymptomatic adults.28 Signs and symptoms of OSA include nighttime awakenings with choking, loud snoring, and feeling unrefreshed after sleep.29
OSA is caused by the intermittent narrowing and obstruction of the pharyngeal airway due to anatomical and structural irregularities or neuromuscular impairments. Untreated OSA is associated with cardiovascular disease and cardiac arrhythmias such as atrial fibrillation. Even with this correlation between obesity and sleep, it is estimated that 80% of OSA remains undiagnosed.30 Approximately half of primary care clinicians do not screen at-risk patients for OSA, and 90% do not use validated OSA screening tools.31 Screening tools that have been validated are the STOP, STOP-BANG, Epworth Sleepiness Scale, and 4-Variable Screening Tool. However, the US Department of Veterans Affairs and the US Department of Defense have a more recent guideline recommending STOP as an easier-to-administer screen for OSA.32 A positive result with a screening tool should be confirmed with polysomnography.32
Continue to: Intervention for OSA
Intervention for OSA. The longest randomized controlled study to date, Sleep AHEAD, evaluated over a period of 10 years the effect of weight loss on OSA severity achieved with either an intensive lifestyle intervention (ILI) or with diabetes support and education (DSE).33 OSA severity is rated on an Apnea-Hypopnea Index (AHI), with scores reflecting the number of sleep apnea events per hour. This study demonstrated that weight loss was associated with decreased OSA severity. At 4-year follow-up, the greater the weight loss with ILI intervention, the lower the patients’ OSA severity scores. The study found an average decrease in AHI of 0.68 events per hour for every kilogram of weight loss in the ILI group (P < .0001).33,34 Over the follow-up visits, the ILI participants had 7.4 events per hour, a more significantly reduced AHI than the DSE participants (P < .0001).33,34
Additionally, a small cohort of study participants achieved OSA remission (ILI, 34.4%; DSE, 22.2%), indicated by a low AHI score (< 5 events per hour). At the conclusion of the study, OSA severity decreased to a greater degree with ILI intervention.33,34
Alcohol and drug use can negatively influence sleep patterns and obesity. Higher alcohol consumption is associated with poorer sleep quality and higher chances of developing short sleep duration and snoring.35 Alcohol, a muscle relaxant, causes upper airway narrowing and reduced tongue muscle tone, thereby increasing snoring and OSA as demonstrated by increased AHI on polysomnography after alcohol intake. Alcohol also changes sleep architecture by increasing slow-wave sleep, decreasing REM sleep duration, and increasing sleep arousal in the second half of the night.36 Disrupted circadian rhythm after alcohol consumption was correlated with increased adenosine neurotransmitters derived from ethanol metabolism.37 Alcohol dependence may be related to other psychiatric symptoms, and chronic alcohol use eventually alters sleep mechanisms leading to persistent insomnia, further perpetuating adverse outcomes such as suicidal ideation.36 There are positive associations between beer drinking and measures of abdominal adiposity in men, and “the combination of short sleep duration [and] disinhibited eating … is associated with greater alcohol intake and excess weight.”38
Therefore, counsel patients to avoid alcohol since it is a modifiable risk factor with pervasive adverse health effects.
Many drugs have a profound effect on sleep patterns. Illicit drug use in particular can affect the brain’s neurotransmitter serotonin system. For example, ecstasy users have an increased risk for OSA.39 People with cocaine and heroin use disorder tend to have more sleep-maintenance insomnia.40
Continue to: In contrast, those with alcohol...
In contrast, those with alcohol or cannabis use disorder tend to have more sleep-onset insomnia.40 Not only do illicit drugs interrupt sleep, but daily tobacco use also has been correlated with increased insomnia and shorter sleep duration since nicotine is a stimulant.41
Insomnia is commonly treated with sedative antidepressants and hypnotics—eg, mirtazapine and olanzapine—that contribute to weight gain.42 In addition, other common pharmaceuticals used for sleep disorders, such as diphenhydramine, have sedative properties and tend to lead to weight gain.43 Because so many medications affect sleep and weight, carefully review patients’ medication lists and switch offending agents to weight-neutral drugs if possible.
Treatment and tools to improve sleep in patients with obesity
Given the strong correlation between obesity and sleep disorders, validated screening tools should be used to assess sleep quality, including onset and potential symptoms associated with poor sleep (TABLE 144). For weight management to succeed in patients with obesity, it is crucial to address sleep in addition to nutrition and physical activity.17,45
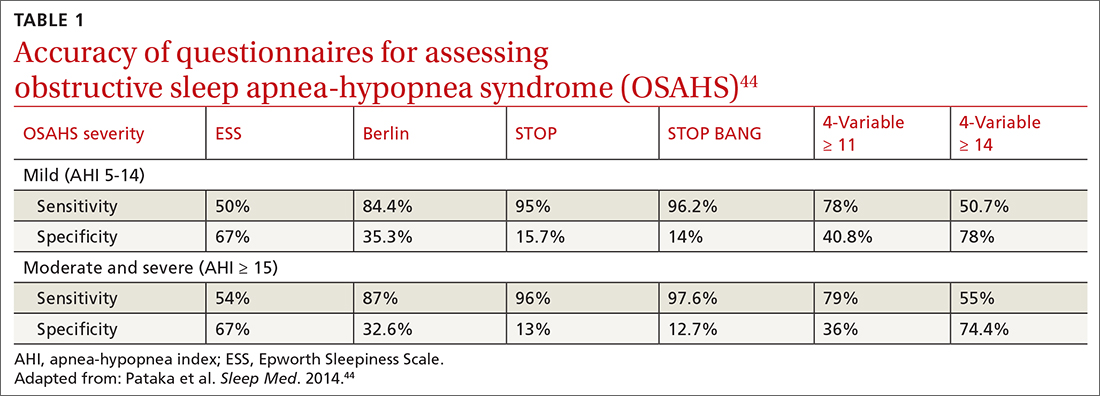
Physical activity has many benefits to overall health, especially for chronic diseases such as type 2 diabetes and hypertension. The Centers for Disease Control and Prevention recommends at least 150 minutes of moderate-intensity aerobic activity or 75 minutes of vigorous-intensity aerobic exercise per week in addition to muscle-strengthening activities 2 or more days per week.46 However, approximately 300 minutes of moderate-
Physical activity and diet in combination are vital, but diet restriction has a more substantial effect on weight loss than physical activity alone.48 Still, physical activity is essential in helping maintain and prevent weight regain.
Continue to: Nonpharmacologic interventions
Nonpharmacologic interventions include promoting greater sleep quality and quantity by emphasizing good sleep hygiene practices. Developing a practical and effective bedtime routine, creating a quiet sleep environment, and practicing healthy daily habits are essential components to sleep hygiene(TABLE 249,50). Relaxation techniques and cognitive behavioral therapy (CBT) also can help. CBT for insomnia (CBT-I) is the first-line intervention for chronic insomnia.51 Sleep restriction is a type of CBT used to treat insomnia, encouraging short-term sleep loss in the hopes of improving insomnia. A trial by Logue et al showed that patients with overweight and obesity randomized to undergo CBT with better sleep hygiene (nonpharmacologic) interventions had a greater mean weight loss percentage (5% vs 2%; P = .04) than did those who received CBT alone.52
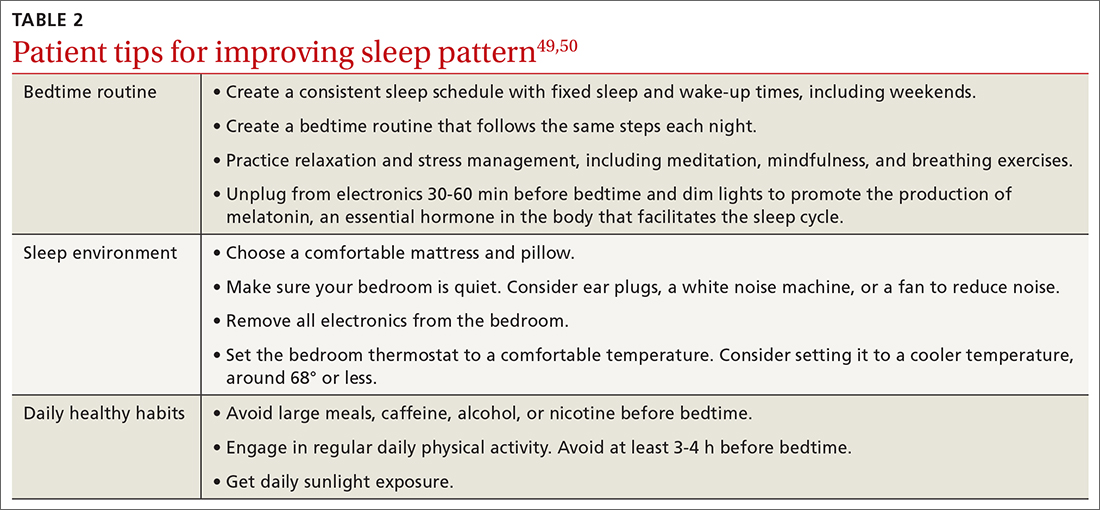
Eastern medicine including herbal interventions lack evidence of efficacy and safety. Further studies need to be done on the effects that chamomile, kava, valerian root (Valeriana officinalis), tryptophan, and Wu Ling (from mycelia Xylaria nigripes) might have on sleep.53
Proceed cautiously with medication. The American College of Physicians recommends a shared decision-making approach when considering pharmacologic therapy for chronic insomnia and the American Academy of Sleep Medicine (AASM) offers guidance on options.51,54 However, the evidence behind AASM sleep pharmacologic recommendations is weak, implying a lesser degree of confidence in the outcome and, therefore, in its appropriateness. Thus, it falls upon the clinician and patient to weigh the benefits and burdens of the pharmacologic treatments of insomnia. If indicated, medications suggested to treat sleep onset and sleep maintenance insomnia are eszopiclone, zolpidem, and temazepam. Zaleplon, triazolam, and ramelteon may improve sleep initiation. Suvorexant and doxepin are used for sleep-maintenance insomnia.54 Exploring patient preferences, cost of treatment, health care options, and available resources should all be considered.
CORRESPONDENCE
Ecler Ercole Jaqua, MD, MBA, FAAFP, AGSF, FACLM, DipABOM, Loma Linda University Health, 25455 Barton Road, Suite 206A, Loma Linda, CA 92354; ejaqua@llu.edu
1. Aminoff MJ, Boller F, Swaab DF. We spend about one-third of our life either sleeping or attempting to do so. Handb Clin Neurol. 2011;98:vii. doi: 10.1016/B978-0-444-52006-7.00047-2
2. Watson NF, Badr MS, Belenky G, et al. Recommended amount of sleep for a healthy adult: a joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society. Sleep. 2015;38:843-844. doi: 10.5665/sleep.4716
3. CDC. Sleep and sleep disorders, adults. Accessed September 21, 2023. www.cdc.gov/sleep/data-and-statistics/adults.html
4. Chattu VK, Manzar MD, Kumary S. The global problem of insufficient sleep and its serious public health implications. Healthcare (Basel). 2019;7:1. doi: 10.3390/healthcare7010001
5. Taheri S, Lin L, Austin D, et al. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004;1:e62. doi: 10.1371/journal.pmed.0010062
6. Hafner M, Stepanek M, Taylor J, et al. Why sleep matters—the economic costs of insufficient sleep. Rand Health Q. 2017;6:11.
7. Hisler G, Twenge JM, Krizan Z. Associations between screen time and short sleep duration among adolescents varies by media type: evidence from a cohort study. Sleep Med. 2020;66:92-102. doi: 10.1016/j.sleep.2019.08.007
8. Ogilvie RP, Patel SR. The epidemiology of sleep and obesity. Sleep Health. 2017;3:383-388. doi: 10.1016/j.sleh.2017.07.013
9. CDC. Sleep and sleep disorders: How much sleep do I need? Accessed September 21, 2023. www.cdc.gov/sleep/about_sleep/how_much_sleep.html
10. van Egmond LT, Meth EMS, Engström J, et al. Effects of acute sleep loss on leptin, ghrelin, and adiponectin in adults with healthy weight and obesity: a laboratory study. Obesity (Silver Spring). 2023;31:635-641. doi: 10.1002/oby.23616
11. Spiegel K, Tasali E, Penev P, et al. Brief communication: sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004;141:846-850. doi: 10.7326/0003-4819-141-11-200412070-00008
12. Antza C, Kostopoulos G, Mostafa S, et al. The links between sleep duration, obesity and type 2 diabetes mellitus. J Endocrinol. 2021;252:125-141. doi: 10.1530/JOE-21-0155
13. Baron KG, Reid KJ, Kern AS, et al. Role of sleep timing in caloric intake and BMI. Obesity (Silver Spring). 2011;19:1374-1381. doi: 10.1038/oby.2011.100
14. Liu XY, Zheng CL, Xu C, et al. Nighttime snacking is associated with risk of obesity and hyperglycemia in adults: a cross-sectional survey from Chinese adult teachers J Biomed Res. 2017;31:541-547. doi: 10.7555/JBR.31.20160083
15. Cai Z, Yang Y, Zhang J, et al. The relationship between daytime napping and obesity: a systematic review and meta-analysis. Sci Rep. 2023.13:12124. doi: 10.1038/s41598-023-37883-7
16. Nedeltcheva AV, Kilkus JM, Imperial J, et al. Insufficient sleep undermines dietary efforts to reduce adiposity. Ann Intern Med. 2010;153:435-441. doi: 10.7326/0003-4819-153-7-201010050-00006
17. Chaput JP, Tremblay A. Adequate sleep to improve the treatment of obesity. CMAJ. 2012;184:1975-1976. doi: 10.1503/cmaj.120876
18. Kelsey MM, Zaepfel A, Bjornstad P, et al. Age-related consequences of childhood obesity. Gerontology. 2014;60:222-228. doi: 10.1159/000356023
19. Fryar CD, Carroll MD, Afful J. Prevalence of overweight, obesity, and severe obesity among children and adolescents aged 2-19 years: United States, 1963-1965 through 2017-2018. National Center for Health Statistics Health E-Stats. Updated January 29, 2021. Accessed September 21, 2021. www.cdc.gov/nchs/data/hestat/obesity-child-17-18/overweight-obesity-child-H.pdf
20. Fatima Y, Doi SAR, Mamun AA. Sleep quality and obesity in young subjects: a meta-analysis. Obes Rev. 2016;17:1154-1166. doi: 10.1111/obr.12444
21. Gohil A, Hannon TS. Poor sleep and obesity: concurrent epidemics in adolescent youth. Front Endocrinol. 2018;9:364. doi: 10.3389/fendo.2018.00364
22. Golley RK, Maher CA, Matricciani L, et al. Sleep duration or bedtime? Exploring the association between sleep timing behaviour, diet and BMI in children and adolescents. Int J Obes (Lond). 2013;37:546-551. doi: 10.1038/ijo.2012.212
23. Alessi CA. Sleep issues. In: Harper GM, Lyons WL, Potter JF, eds. Geriatrics Review Syllabus (GRS 10). Updated January 2021. Accessed August 29, 2023. http://geriatricscareonline.org
24. Patel SR, Blackwell T, Redline S, et al. The association between sleep duration and obesity in older adults. Int J Obes (Lond). 2008;32:1825-1834. doi: 10.1038/ijo.2008.198
25. Cai GH, Theorell-Haglöw J, Janson C, et al. Insomnia symptoms and sleep duration and their combined effects in relation to associations with obesity and central obesity. Sleep Med. 2018;46:81-87. doi: 10.1016/j.sleep.2018.03.009
26. Beccuti G, Pannain S. Sleep and obesity. Curr Opin Clin Nutr Metab Care. 2011;14:402-412. doi: 10.1097/MCO.0b013 e3283479109
27. Franklin KA, Lindberg E. Obstructive sleep apnea is a common disorder in the population–a review on the epidemiology of sleep apnea. J Thorac Dis. 2015;7:1311-1322. doi: 10.3978/j.issn.2072-1439.2015.06.11
28. USPSTF. Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for obstructive sleep apnea in adults: US Preventive Services Task Force recommendation statement. JAMA. 2017;317:407-414. doi: 10.1001/jama.2016.20325
29. Goyal M, Johnson J. Obstructive sleep apnea diagnosis and management. Mo Med. 2017;114:120-124.
30. American Academy of Sleep Medicine. Hidden health crisis costing America billions: underdiagnosing and undertreating obstructive sleep apnea draining healthcare system. 2016. Accessed September 25, 2023. https://aasm.org/wp-content/uploads/2017/10/sleep-apnea-economic-crisis.pdf
31. Devaraj, NK. Knowledge, attitude, and practice regarding obstructive sleep apnea among primary care physicians. Sleep Breath. 2020;24:1581-1590. doi: 10.1007/s11325-020-02040-1
32. Mysliwiec V, Martin JL, Ulmer CS, et al. The management of chronic insomnia disorder and obstructive sleep apnea: synopsis of the 2019 U.S. Department of Veterans Affairs and U.S. Department of Defense Clinical Practice Guidelines. Ann Intern Med. 2020;172:325-336. doi: 10.7326/M19-3575
33. Kuna ST, Reboussin DM, Strotmeyer ES, et al. Effects of weight loss on obstructive sleep apnea severity. Ten-year results of the Sleep AHEAD study. Am J Respir Crit Care Med. 2021;203:221-229. doi: 10.1164/rccm.201912-2511OC
34. St-Onge MP, Tasali E. Weight loss is integral to obstructive sleep apnea management. Ten-year follow-up in Sleep AHEAD. Am J Respir Crit Care Med. 2021;203:161-162. doi: 10.1164/rccm.202007-2906ED
35. Zheng D, Yuan X, Ma C, et al. Alcohol consumption and sleep quality: a community-based study. Public Health Nutr. 2021;24:4851-4858. doi: 10.1017/S1368980020004553
36. Chakravorty S, Chaudhary NS, Brower KJ. Alcohol dependence and its relationship with insomnia and other sleep disorders. Alcohol Clin Exp Res. 2016;40:2271-2282. doi: 10.1111/acer.13217
37. Elmenhorst EM, Elmenhorst D, Benderoth S, et al. Cognitive impairments by alcohol and sleep deprivation indicate trait characteristics and a potential role for adenosine A1 receptors. Proc Natl Acad Sci U S A. 2018;115:8009-8014. doi: 10.1073/pnas.1803770115
38. Traversy G, Chaput JP. Alcohol consumption and obesity: an update. Curr Obes Rep. 2015;4:122-130. doi: 10.1007/s13679-014-0129-4
39. McCann UD, Sgambati FP, Schwartz AR, et al. Sleep apnea in young abstinent recreational MDMA (“ecstasy”) consumers. Neurology. 2009;73:2011-2017. doi: 10.1212/WNL.0b013e3181c51a62
40. Grau-López L, Grau-López L, Daigre C, et al. Insomnia symptoms in patients with substance use disorders during detoxification and associated clinical features. Front Psychiatry. 2020;11:540022. doi: 10.3389/fpsyt.2020.540022
41. Boehm MA, Lei QM, Lloyd RM, et al. Depression, anxiety, and tobacco use: overlapping impediments to sleep in a national sample of college students. J Am Coll Health. 2016;64:565-574. doi: 10.1080/07448481.2016.1205073
42. Gracious BL, Meyer AE. Psychotropic-induced weight gain and potential pharmacologic treatment strategies. Psychiatry (Edgmont). 2005;2:36-42.
43. Ratliff JC, Barber JA, Palmese LB, et al. Association of prescription H1 antihistamine use with obesity: results from the National Health and Nutrition Examination Survey. Obesity (Silver Spring). 2010;18:2398-2400. doi: 10.1038/oby.2010.176
44. Pataka A, Daskalopoulou E, Kalamaras G, et al. Evaluation of five different questionnaires for assessing sleep apnea syndrome in a sleep clinic. Sleep Med. 2014;15:776-781. doi: 10.1016/j.sleep.2014.03.012
45. Kline CE, Chasens ER, Bizhanova Z, et al. The association between sleep health and weight change during a 12-month behavioral weight loss intervention. Int J Obes (Lond). 2021;45:639-649. doi: 10.1038/s41366-020-00728-8
46. CDC. How much physical activity do adults need? Accessed August 23, 2023. www.cdc.gov/physicalactivity/basics/adults/index.htm
47. Flack KD, Hays HM, Moreland J, et al. Exercise for weight loss: further evaluating energy compensation with exercise. Med Sci Sports Exerc. 2020;52:2466-2475. doi: 10.1249/MSS.0000000000002376
48. Swift DL, Johannsen NM, Lavie CJ, et al. The role of exercise and physical activity in weight loss and maintenance. Prog Cardiovasc Dis. 2014;56:441-447. doi: 10.1016/j.pcad.2013.09.012
49. Irish LA, Kline CE, Gunn HE, et al. The role of sleep hygiene in promoting public health: a review of empirical evidence. Sleep Med Rev. 2015;22:23-36. doi: 10.1016/j.smrv.2014.10.001
50. CDC. Tips for better sleep. 2022. Accessed August 4, 2023. www.cdc.gov/sleep/about_sleep/sleep_hygiene.html
51. Qaseem A, Kansagara D, Forciea MA, et al. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165:125-133. doi: 10.7326/M15-2175
52. Logue EE, Bourguet CC, Palmieri PA, et al. The better weight-better sleep study: a pilot intervention in primary care. Am J Health Behav. 2012;36:319-334. doi: 10.5993/AJHB.36.3.4
53. Leach MJ, Page AT. Herbal medicine for insomnia: a systematic review and meta-analysis. Sleep Med Rev. 2015;24:1-12. doi: 10.1016/j.smrv.2014.12.003
54. Sateia MJ, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13:307-349. doi: 10.5664/jcsm.6470
Sleep is fundamental to overall health and longevity, with the average person spending about one-third of their life sleeping.1 Adequate sleep is critical for optimal cognition, memory consolidation, mood regulation, metabolism, appetite regulation, and immune and hormone functioning. According to the American Academy of Sleep Medicine and the Sleep Research Society, adults should sleep at least 7 hours per night on a regular basis “to promote optimal health.”2 Yet, between 2013 and 2020, only about 65% of adults in the United States were meeting this amount.3 Insufficient sleep is associated with an increased risk for chronic health conditions, including obesity, diabetes, cardiovascular diseases, and even premature death.4

In a population-based longitudinal study of sleep disorders, short sleep duration was associated with increased body mass index (BMI), low blood levels of leptin, and high ghrelin levels.5 In addition to physical impairments, poor sleep can impair cognitive performance and lead to vehicular accidents and increased accidents at work.4 The potential economic impact that this may have is significant, and includes increased costs and loss of productivity in the workplace.6
Many factors may contribute to short sleep duration: environment, mental and physical condition, and social influences such as occupation, family responsibilities, travel, group activities, and personal care. Furthermore, the rapidly evolving and developing media, communication, and entertainment industries are already strongly implicated in poor sleep quality and quantity, both contributing to excessive daytime sleepiness.7 Poor sleep quality is most notable in modern societies, and it correlates with the increasing prevalence of obesity, likely due to sleep’s effect on food consumption and physical activity.8 Optimizing a person’s sleep will improve overall health and longevity by inhibiting the development of chronic disease.
How insufficient sleep raises the risk for obesity
Not only is sleep beneficial for brain health, memory, learning, and growth, its effect on food consumption and physical activity likely correlates with the increased prevalence of obesity in modern society. Yet the optimal amount of sleep is controversial, and current recommendations of 7 or more hours of sleep per night for adults are derived from expert panels only.2 The recommended sleep duration for children is longer, and it varies by age.9 The quality of sleep and its impact on neuroendocrine hormones, not just the quantity of sleep, needs to be factored into these recommendations.
Sleep restriction activates the orexigenic system via the hormones leptin and ghrelin. These hormones control the food reward system, essentially increasing hunger and food intake. Leptin, created by white adipose tissue, is responsible for satiety and decreased food consumption.10 Ghrelin, made by oxyntic glands in the stomach, is responsible for the sensation of hunger.
In a 2004 study by Spiegel et al,11 leptin and ghrelin levels were measured during 2 days of sleep restriction (4 hours in bed) and sleep extension (10 hours in bed). Sleep restriction was associated with a decrease in leptin levels and an increase in ghrelin levels. The researchers reported that participants experienced an increase in hunger and appetite—especially for calorie-dense foods with high carbohydrate content.
Although research design has limitations with predominantly self-reported sleep data, studies have shown that short sleep time leads to increased food intake by increasing hunger signals and craving of unhealthy foods, and by providing more opportunities to eat while awake. It also may lead to decreased physical activity, creating a sedentary lifestyle that further encourages obesity.8 Reduced sleep is even correlated to decreased efficacy of weight-loss treatments.12
Continue to: Other sleep characteristics weakly correlated with obesity
Other sleep characteristics weakly correlated with obesity are sleep variability, timing, efficiency, quality, and daytime napping.8 Sleep variability causes dysregulation of eating patterns, leading to increased food intake. A shift to later sleep and waking times often results in higher consumption of calories after 8
Poor sleep efficiency and quality decreases N3-stage (deep non-REM) sleep, affects the autonomic nervous system, and has been associated with increased abdominal obesity. Daytime napping, which can cause irregular circadian rhythms and sleep schedules, is associated with increased obesity.15 Thus, each component of sleep needs to be assessed to promote optimal regulation of the orexigenic system.
Another study showed that inadequate sleep not only promotes unhealthy lifestyle habits that can lead to obesity but also decreases the ability to lose weight.16 This small study with 10 overweight patients provided its subjects with a controlled caloric intake over 2 weeks. Patients spent two 14-day periods 3 months apart in the laboratory, divided into 2 time-in-bed arms of 8.5 and 5.5 hours per night. Neuroendocrine changes caused by decreased sleep were associated with a significant lean body mass loss while conserving energy-dense fat.16 This study highlights the importance of sleep hygiene counseling when developing a weight-management plan with patients.
Sleep, and its many components, play an integral role in the prevention and treatment of obesity.17 Poor sleep will increase the risk for obesity and hinder its treatment. Therefore, sleep quality and duration are vital components of obesity management.
The sleep–obesity link in children and the elderly
Childhood obesity is linked to several chronic diseases in adulthood, including type 2 diabetes, cardiovascular disease, nonalcoholic fatty liver disease, asthma, and obstructive sleep apnea (OSA).18 According to 2017-2018 NHANES (National Health and Nutrition Examination Surveys) data, obesity (BMI ≥ 95th percentile) prevalence among children and adolescents was reported at 19.3% and severe obesity (BMI ≥ 120% of the 95th percentile) at 6.1%. Pediatric overweight prevalence (≥ 85th percentile and < 95th percentile) was 16.1%.19
Continue to: Although poor sleep is associated...
Although poor sleep is associated with increased risk for obesity, there is no proven cause-effect relationship.20 Nutrition and physical activity have been identified as 2 critical factors in childhood obesity, but sleep health also needs to be investigated. Shorter sleep duration is strongly associated with the development of obesity. Furthermore, children with obesity are more likely to have shorter sleep duration.21 A short sleep duration alters plasma levels of insulin, low-density lipoprotein, and high-sensitivity C-reactive protein. It is associated with lower diet quality, an increased intake of nutrient-poor foods, and a lower intake of vegetables and fruits.22 Recent studies have shown that interventions to promote earlier bedtimes can improve sleep duration in children.
Older adults have many sleeping issues, including insomnia, circadian rhythm sleep-wake disorders, sleep-related movement disorders, and sleep-breathing disorders. Additionally, the older population has increased sleep latency, decreased sleep efficiency and total sleep time, decreased REM sleep, more frequent nighttime awakenings, and more daytime napping.23 The increased sleep disturbance with age is mainly related to higher risk factors for sleep disorders than the aging process itself. Sleeping 5 or fewer hours is associated with an increased risk for obesity and central abdominal fat compared with those who sleep 7 to 8 hours per night.24 Similar to children and youth, older adults also show a strong correlation between inadequate sleep and obesity.24
The consequence: A vicious cycle
Obesity in turn leads to shorter sleep duration and more disruptions. This negatively affects the orexigenic system, and the resulting hormonal derangement promotes worsening obesity. It is a cycle of poor sleep causing obesity and obesity causing poor sleep. Insomnia, in combination with shorter (and longer) sleep times, also has been linked with obesity.25 These patients experience more daytime sleepiness, fatigue, and nighttime sleep disturbances, all correlated with decreased quality of life and higher prevalence of medical comorbidities.8,26 Additional comorbidities secondary to obesity, including gastroesophageal reflux, depression, and asthma, also have been linked to sleep disturbances.8
OSA is a common sleep complication associated with obesity. With the increasing prevalence of obesity, the prevalence of OSA is rising.8,27 Factors that heighten the risk for OSA are male sex, age 40 to 70 years, postmenopausal status, elevated BMI, and craniofacial and upper airway abnormality.28 However, the US Preventive Services Task Force found insufficient evidence to screen for or treat OSA in asymptomatic adults.28 Signs and symptoms of OSA include nighttime awakenings with choking, loud snoring, and feeling unrefreshed after sleep.29
OSA is caused by the intermittent narrowing and obstruction of the pharyngeal airway due to anatomical and structural irregularities or neuromuscular impairments. Untreated OSA is associated with cardiovascular disease and cardiac arrhythmias such as atrial fibrillation. Even with this correlation between obesity and sleep, it is estimated that 80% of OSA remains undiagnosed.30 Approximately half of primary care clinicians do not screen at-risk patients for OSA, and 90% do not use validated OSA screening tools.31 Screening tools that have been validated are the STOP, STOP-BANG, Epworth Sleepiness Scale, and 4-Variable Screening Tool. However, the US Department of Veterans Affairs and the US Department of Defense have a more recent guideline recommending STOP as an easier-to-administer screen for OSA.32 A positive result with a screening tool should be confirmed with polysomnography.32
Continue to: Intervention for OSA
Intervention for OSA. The longest randomized controlled study to date, Sleep AHEAD, evaluated over a period of 10 years the effect of weight loss on OSA severity achieved with either an intensive lifestyle intervention (ILI) or with diabetes support and education (DSE).33 OSA severity is rated on an Apnea-Hypopnea Index (AHI), with scores reflecting the number of sleep apnea events per hour. This study demonstrated that weight loss was associated with decreased OSA severity. At 4-year follow-up, the greater the weight loss with ILI intervention, the lower the patients’ OSA severity scores. The study found an average decrease in AHI of 0.68 events per hour for every kilogram of weight loss in the ILI group (P < .0001).33,34 Over the follow-up visits, the ILI participants had 7.4 events per hour, a more significantly reduced AHI than the DSE participants (P < .0001).33,34
Additionally, a small cohort of study participants achieved OSA remission (ILI, 34.4%; DSE, 22.2%), indicated by a low AHI score (< 5 events per hour). At the conclusion of the study, OSA severity decreased to a greater degree with ILI intervention.33,34
Alcohol and drug use can negatively influence sleep patterns and obesity. Higher alcohol consumption is associated with poorer sleep quality and higher chances of developing short sleep duration and snoring.35 Alcohol, a muscle relaxant, causes upper airway narrowing and reduced tongue muscle tone, thereby increasing snoring and OSA as demonstrated by increased AHI on polysomnography after alcohol intake. Alcohol also changes sleep architecture by increasing slow-wave sleep, decreasing REM sleep duration, and increasing sleep arousal in the second half of the night.36 Disrupted circadian rhythm after alcohol consumption was correlated with increased adenosine neurotransmitters derived from ethanol metabolism.37 Alcohol dependence may be related to other psychiatric symptoms, and chronic alcohol use eventually alters sleep mechanisms leading to persistent insomnia, further perpetuating adverse outcomes such as suicidal ideation.36 There are positive associations between beer drinking and measures of abdominal adiposity in men, and “the combination of short sleep duration [and] disinhibited eating … is associated with greater alcohol intake and excess weight.”38
Therefore, counsel patients to avoid alcohol since it is a modifiable risk factor with pervasive adverse health effects.
Many drugs have a profound effect on sleep patterns. Illicit drug use in particular can affect the brain’s neurotransmitter serotonin system. For example, ecstasy users have an increased risk for OSA.39 People with cocaine and heroin use disorder tend to have more sleep-maintenance insomnia.40
Continue to: In contrast, those with alcohol...
In contrast, those with alcohol or cannabis use disorder tend to have more sleep-onset insomnia.40 Not only do illicit drugs interrupt sleep, but daily tobacco use also has been correlated with increased insomnia and shorter sleep duration since nicotine is a stimulant.41
Insomnia is commonly treated with sedative antidepressants and hypnotics—eg, mirtazapine and olanzapine—that contribute to weight gain.42 In addition, other common pharmaceuticals used for sleep disorders, such as diphenhydramine, have sedative properties and tend to lead to weight gain.43 Because so many medications affect sleep and weight, carefully review patients’ medication lists and switch offending agents to weight-neutral drugs if possible.
Treatment and tools to improve sleep in patients with obesity
Given the strong correlation between obesity and sleep disorders, validated screening tools should be used to assess sleep quality, including onset and potential symptoms associated with poor sleep (TABLE 144). For weight management to succeed in patients with obesity, it is crucial to address sleep in addition to nutrition and physical activity.17,45

Physical activity has many benefits to overall health, especially for chronic diseases such as type 2 diabetes and hypertension. The Centers for Disease Control and Prevention recommends at least 150 minutes of moderate-intensity aerobic activity or 75 minutes of vigorous-intensity aerobic exercise per week in addition to muscle-strengthening activities 2 or more days per week.46 However, approximately 300 minutes of moderate-
Physical activity and diet in combination are vital, but diet restriction has a more substantial effect on weight loss than physical activity alone.48 Still, physical activity is essential in helping maintain and prevent weight regain.
Continue to: Nonpharmacologic interventions
Nonpharmacologic interventions include promoting greater sleep quality and quantity by emphasizing good sleep hygiene practices. Developing a practical and effective bedtime routine, creating a quiet sleep environment, and practicing healthy daily habits are essential components to sleep hygiene(TABLE 249,50). Relaxation techniques and cognitive behavioral therapy (CBT) also can help. CBT for insomnia (CBT-I) is the first-line intervention for chronic insomnia.51 Sleep restriction is a type of CBT used to treat insomnia, encouraging short-term sleep loss in the hopes of improving insomnia. A trial by Logue et al showed that patients with overweight and obesity randomized to undergo CBT with better sleep hygiene (nonpharmacologic) interventions had a greater mean weight loss percentage (5% vs 2%; P = .04) than did those who received CBT alone.52

Eastern medicine including herbal interventions lack evidence of efficacy and safety. Further studies need to be done on the effects that chamomile, kava, valerian root (Valeriana officinalis), tryptophan, and Wu Ling (from mycelia Xylaria nigripes) might have on sleep.53
Proceed cautiously with medication. The American College of Physicians recommends a shared decision-making approach when considering pharmacologic therapy for chronic insomnia and the American Academy of Sleep Medicine (AASM) offers guidance on options.51,54 However, the evidence behind AASM sleep pharmacologic recommendations is weak, implying a lesser degree of confidence in the outcome and, therefore, in its appropriateness. Thus, it falls upon the clinician and patient to weigh the benefits and burdens of the pharmacologic treatments of insomnia. If indicated, medications suggested to treat sleep onset and sleep maintenance insomnia are eszopiclone, zolpidem, and temazepam. Zaleplon, triazolam, and ramelteon may improve sleep initiation. Suvorexant and doxepin are used for sleep-maintenance insomnia.54 Exploring patient preferences, cost of treatment, health care options, and available resources should all be considered.
CORRESPONDENCE
Ecler Ercole Jaqua, MD, MBA, FAAFP, AGSF, FACLM, DipABOM, Loma Linda University Health, 25455 Barton Road, Suite 206A, Loma Linda, CA 92354; ejaqua@llu.edu
Sleep is fundamental to overall health and longevity, with the average person spending about one-third of their life sleeping.1 Adequate sleep is critical for optimal cognition, memory consolidation, mood regulation, metabolism, appetite regulation, and immune and hormone functioning. According to the American Academy of Sleep Medicine and the Sleep Research Society, adults should sleep at least 7 hours per night on a regular basis “to promote optimal health.”2 Yet, between 2013 and 2020, only about 65% of adults in the United States were meeting this amount.3 Insufficient sleep is associated with an increased risk for chronic health conditions, including obesity, diabetes, cardiovascular diseases, and even premature death.4

In a population-based longitudinal study of sleep disorders, short sleep duration was associated with increased body mass index (BMI), low blood levels of leptin, and high ghrelin levels.5 In addition to physical impairments, poor sleep can impair cognitive performance and lead to vehicular accidents and increased accidents at work.4 The potential economic impact that this may have is significant, and includes increased costs and loss of productivity in the workplace.6
Many factors may contribute to short sleep duration: environment, mental and physical condition, and social influences such as occupation, family responsibilities, travel, group activities, and personal care. Furthermore, the rapidly evolving and developing media, communication, and entertainment industries are already strongly implicated in poor sleep quality and quantity, both contributing to excessive daytime sleepiness.7 Poor sleep quality is most notable in modern societies, and it correlates with the increasing prevalence of obesity, likely due to sleep’s effect on food consumption and physical activity.8 Optimizing a person’s sleep will improve overall health and longevity by inhibiting the development of chronic disease.
How insufficient sleep raises the risk for obesity
Not only is sleep beneficial for brain health, memory, learning, and growth, its effect on food consumption and physical activity likely correlates with the increased prevalence of obesity in modern society. Yet the optimal amount of sleep is controversial, and current recommendations of 7 or more hours of sleep per night for adults are derived from expert panels only.2 The recommended sleep duration for children is longer, and it varies by age.9 The quality of sleep and its impact on neuroendocrine hormones, not just the quantity of sleep, needs to be factored into these recommendations.
Sleep restriction activates the orexigenic system via the hormones leptin and ghrelin. These hormones control the food reward system, essentially increasing hunger and food intake. Leptin, created by white adipose tissue, is responsible for satiety and decreased food consumption.10 Ghrelin, made by oxyntic glands in the stomach, is responsible for the sensation of hunger.
In a 2004 study by Spiegel et al,11 leptin and ghrelin levels were measured during 2 days of sleep restriction (4 hours in bed) and sleep extension (10 hours in bed). Sleep restriction was associated with a decrease in leptin levels and an increase in ghrelin levels. The researchers reported that participants experienced an increase in hunger and appetite—especially for calorie-dense foods with high carbohydrate content.
Although research design has limitations with predominantly self-reported sleep data, studies have shown that short sleep time leads to increased food intake by increasing hunger signals and craving of unhealthy foods, and by providing more opportunities to eat while awake. It also may lead to decreased physical activity, creating a sedentary lifestyle that further encourages obesity.8 Reduced sleep is even correlated to decreased efficacy of weight-loss treatments.12
Continue to: Other sleep characteristics weakly correlated with obesity
Other sleep characteristics weakly correlated with obesity are sleep variability, timing, efficiency, quality, and daytime napping.8 Sleep variability causes dysregulation of eating patterns, leading to increased food intake. A shift to later sleep and waking times often results in higher consumption of calories after 8
Poor sleep efficiency and quality decreases N3-stage (deep non-REM) sleep, affects the autonomic nervous system, and has been associated with increased abdominal obesity. Daytime napping, which can cause irregular circadian rhythms and sleep schedules, is associated with increased obesity.15 Thus, each component of sleep needs to be assessed to promote optimal regulation of the orexigenic system.
Another study showed that inadequate sleep not only promotes unhealthy lifestyle habits that can lead to obesity but also decreases the ability to lose weight.16 This small study with 10 overweight patients provided its subjects with a controlled caloric intake over 2 weeks. Patients spent two 14-day periods 3 months apart in the laboratory, divided into 2 time-in-bed arms of 8.5 and 5.5 hours per night. Neuroendocrine changes caused by decreased sleep were associated with a significant lean body mass loss while conserving energy-dense fat.16 This study highlights the importance of sleep hygiene counseling when developing a weight-management plan with patients.
Sleep, and its many components, play an integral role in the prevention and treatment of obesity.17 Poor sleep will increase the risk for obesity and hinder its treatment. Therefore, sleep quality and duration are vital components of obesity management.
The sleep–obesity link in children and the elderly
Childhood obesity is linked to several chronic diseases in adulthood, including type 2 diabetes, cardiovascular disease, nonalcoholic fatty liver disease, asthma, and obstructive sleep apnea (OSA).18 According to 2017-2018 NHANES (National Health and Nutrition Examination Surveys) data, obesity (BMI ≥ 95th percentile) prevalence among children and adolescents was reported at 19.3% and severe obesity (BMI ≥ 120% of the 95th percentile) at 6.1%. Pediatric overweight prevalence (≥ 85th percentile and < 95th percentile) was 16.1%.19
Continue to: Although poor sleep is associated...
Although poor sleep is associated with increased risk for obesity, there is no proven cause-effect relationship.20 Nutrition and physical activity have been identified as 2 critical factors in childhood obesity, but sleep health also needs to be investigated. Shorter sleep duration is strongly associated with the development of obesity. Furthermore, children with obesity are more likely to have shorter sleep duration.21 A short sleep duration alters plasma levels of insulin, low-density lipoprotein, and high-sensitivity C-reactive protein. It is associated with lower diet quality, an increased intake of nutrient-poor foods, and a lower intake of vegetables and fruits.22 Recent studies have shown that interventions to promote earlier bedtimes can improve sleep duration in children.
Older adults have many sleeping issues, including insomnia, circadian rhythm sleep-wake disorders, sleep-related movement disorders, and sleep-breathing disorders. Additionally, the older population has increased sleep latency, decreased sleep efficiency and total sleep time, decreased REM sleep, more frequent nighttime awakenings, and more daytime napping.23 The increased sleep disturbance with age is mainly related to higher risk factors for sleep disorders than the aging process itself. Sleeping 5 or fewer hours is associated with an increased risk for obesity and central abdominal fat compared with those who sleep 7 to 8 hours per night.24 Similar to children and youth, older adults also show a strong correlation between inadequate sleep and obesity.24
The consequence: A vicious cycle
Obesity in turn leads to shorter sleep duration and more disruptions. This negatively affects the orexigenic system, and the resulting hormonal derangement promotes worsening obesity. It is a cycle of poor sleep causing obesity and obesity causing poor sleep. Insomnia, in combination with shorter (and longer) sleep times, also has been linked with obesity.25 These patients experience more daytime sleepiness, fatigue, and nighttime sleep disturbances, all correlated with decreased quality of life and higher prevalence of medical comorbidities.8,26 Additional comorbidities secondary to obesity, including gastroesophageal reflux, depression, and asthma, also have been linked to sleep disturbances.8
OSA is a common sleep complication associated with obesity. With the increasing prevalence of obesity, the prevalence of OSA is rising.8,27 Factors that heighten the risk for OSA are male sex, age 40 to 70 years, postmenopausal status, elevated BMI, and craniofacial and upper airway abnormality.28 However, the US Preventive Services Task Force found insufficient evidence to screen for or treat OSA in asymptomatic adults.28 Signs and symptoms of OSA include nighttime awakenings with choking, loud snoring, and feeling unrefreshed after sleep.29
OSA is caused by the intermittent narrowing and obstruction of the pharyngeal airway due to anatomical and structural irregularities or neuromuscular impairments. Untreated OSA is associated with cardiovascular disease and cardiac arrhythmias such as atrial fibrillation. Even with this correlation between obesity and sleep, it is estimated that 80% of OSA remains undiagnosed.30 Approximately half of primary care clinicians do not screen at-risk patients for OSA, and 90% do not use validated OSA screening tools.31 Screening tools that have been validated are the STOP, STOP-BANG, Epworth Sleepiness Scale, and 4-Variable Screening Tool. However, the US Department of Veterans Affairs and the US Department of Defense have a more recent guideline recommending STOP as an easier-to-administer screen for OSA.32 A positive result with a screening tool should be confirmed with polysomnography.32
Continue to: Intervention for OSA
Intervention for OSA. The longest randomized controlled study to date, Sleep AHEAD, evaluated over a period of 10 years the effect of weight loss on OSA severity achieved with either an intensive lifestyle intervention (ILI) or with diabetes support and education (DSE).33 OSA severity is rated on an Apnea-Hypopnea Index (AHI), with scores reflecting the number of sleep apnea events per hour. This study demonstrated that weight loss was associated with decreased OSA severity. At 4-year follow-up, the greater the weight loss with ILI intervention, the lower the patients’ OSA severity scores. The study found an average decrease in AHI of 0.68 events per hour for every kilogram of weight loss in the ILI group (P < .0001).33,34 Over the follow-up visits, the ILI participants had 7.4 events per hour, a more significantly reduced AHI than the DSE participants (P < .0001).33,34
Additionally, a small cohort of study participants achieved OSA remission (ILI, 34.4%; DSE, 22.2%), indicated by a low AHI score (< 5 events per hour). At the conclusion of the study, OSA severity decreased to a greater degree with ILI intervention.33,34
Alcohol and drug use can negatively influence sleep patterns and obesity. Higher alcohol consumption is associated with poorer sleep quality and higher chances of developing short sleep duration and snoring.35 Alcohol, a muscle relaxant, causes upper airway narrowing and reduced tongue muscle tone, thereby increasing snoring and OSA as demonstrated by increased AHI on polysomnography after alcohol intake. Alcohol also changes sleep architecture by increasing slow-wave sleep, decreasing REM sleep duration, and increasing sleep arousal in the second half of the night.36 Disrupted circadian rhythm after alcohol consumption was correlated with increased adenosine neurotransmitters derived from ethanol metabolism.37 Alcohol dependence may be related to other psychiatric symptoms, and chronic alcohol use eventually alters sleep mechanisms leading to persistent insomnia, further perpetuating adverse outcomes such as suicidal ideation.36 There are positive associations between beer drinking and measures of abdominal adiposity in men, and “the combination of short sleep duration [and] disinhibited eating … is associated with greater alcohol intake and excess weight.”38
Therefore, counsel patients to avoid alcohol since it is a modifiable risk factor with pervasive adverse health effects.
Many drugs have a profound effect on sleep patterns. Illicit drug use in particular can affect the brain’s neurotransmitter serotonin system. For example, ecstasy users have an increased risk for OSA.39 People with cocaine and heroin use disorder tend to have more sleep-maintenance insomnia.40
Continue to: In contrast, those with alcohol...
In contrast, those with alcohol or cannabis use disorder tend to have more sleep-onset insomnia.40 Not only do illicit drugs interrupt sleep, but daily tobacco use also has been correlated with increased insomnia and shorter sleep duration since nicotine is a stimulant.41
Insomnia is commonly treated with sedative antidepressants and hypnotics—eg, mirtazapine and olanzapine—that contribute to weight gain.42 In addition, other common pharmaceuticals used for sleep disorders, such as diphenhydramine, have sedative properties and tend to lead to weight gain.43 Because so many medications affect sleep and weight, carefully review patients’ medication lists and switch offending agents to weight-neutral drugs if possible.
Treatment and tools to improve sleep in patients with obesity
Given the strong correlation between obesity and sleep disorders, validated screening tools should be used to assess sleep quality, including onset and potential symptoms associated with poor sleep (TABLE 144). For weight management to succeed in patients with obesity, it is crucial to address sleep in addition to nutrition and physical activity.17,45

Physical activity has many benefits to overall health, especially for chronic diseases such as type 2 diabetes and hypertension. The Centers for Disease Control and Prevention recommends at least 150 minutes of moderate-intensity aerobic activity or 75 minutes of vigorous-intensity aerobic exercise per week in addition to muscle-strengthening activities 2 or more days per week.46 However, approximately 300 minutes of moderate-
Physical activity and diet in combination are vital, but diet restriction has a more substantial effect on weight loss than physical activity alone.48 Still, physical activity is essential in helping maintain and prevent weight regain.
Continue to: Nonpharmacologic interventions
Nonpharmacologic interventions include promoting greater sleep quality and quantity by emphasizing good sleep hygiene practices. Developing a practical and effective bedtime routine, creating a quiet sleep environment, and practicing healthy daily habits are essential components to sleep hygiene(TABLE 249,50). Relaxation techniques and cognitive behavioral therapy (CBT) also can help. CBT for insomnia (CBT-I) is the first-line intervention for chronic insomnia.51 Sleep restriction is a type of CBT used to treat insomnia, encouraging short-term sleep loss in the hopes of improving insomnia. A trial by Logue et al showed that patients with overweight and obesity randomized to undergo CBT with better sleep hygiene (nonpharmacologic) interventions had a greater mean weight loss percentage (5% vs 2%; P = .04) than did those who received CBT alone.52

Eastern medicine including herbal interventions lack evidence of efficacy and safety. Further studies need to be done on the effects that chamomile, kava, valerian root (Valeriana officinalis), tryptophan, and Wu Ling (from mycelia Xylaria nigripes) might have on sleep.53
Proceed cautiously with medication. The American College of Physicians recommends a shared decision-making approach when considering pharmacologic therapy for chronic insomnia and the American Academy of Sleep Medicine (AASM) offers guidance on options.51,54 However, the evidence behind AASM sleep pharmacologic recommendations is weak, implying a lesser degree of confidence in the outcome and, therefore, in its appropriateness. Thus, it falls upon the clinician and patient to weigh the benefits and burdens of the pharmacologic treatments of insomnia. If indicated, medications suggested to treat sleep onset and sleep maintenance insomnia are eszopiclone, zolpidem, and temazepam. Zaleplon, triazolam, and ramelteon may improve sleep initiation. Suvorexant and doxepin are used for sleep-maintenance insomnia.54 Exploring patient preferences, cost of treatment, health care options, and available resources should all be considered.
CORRESPONDENCE
Ecler Ercole Jaqua, MD, MBA, FAAFP, AGSF, FACLM, DipABOM, Loma Linda University Health, 25455 Barton Road, Suite 206A, Loma Linda, CA 92354; ejaqua@llu.edu
1. Aminoff MJ, Boller F, Swaab DF. We spend about one-third of our life either sleeping or attempting to do so. Handb Clin Neurol. 2011;98:vii. doi: 10.1016/B978-0-444-52006-7.00047-2
2. Watson NF, Badr MS, Belenky G, et al. Recommended amount of sleep for a healthy adult: a joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society. Sleep. 2015;38:843-844. doi: 10.5665/sleep.4716
3. CDC. Sleep and sleep disorders, adults. Accessed September 21, 2023. www.cdc.gov/sleep/data-and-statistics/adults.html
4. Chattu VK, Manzar MD, Kumary S. The global problem of insufficient sleep and its serious public health implications. Healthcare (Basel). 2019;7:1. doi: 10.3390/healthcare7010001
5. Taheri S, Lin L, Austin D, et al. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004;1:e62. doi: 10.1371/journal.pmed.0010062
6. Hafner M, Stepanek M, Taylor J, et al. Why sleep matters—the economic costs of insufficient sleep. Rand Health Q. 2017;6:11.
7. Hisler G, Twenge JM, Krizan Z. Associations between screen time and short sleep duration among adolescents varies by media type: evidence from a cohort study. Sleep Med. 2020;66:92-102. doi: 10.1016/j.sleep.2019.08.007
8. Ogilvie RP, Patel SR. The epidemiology of sleep and obesity. Sleep Health. 2017;3:383-388. doi: 10.1016/j.sleh.2017.07.013
9. CDC. Sleep and sleep disorders: How much sleep do I need? Accessed September 21, 2023. www.cdc.gov/sleep/about_sleep/how_much_sleep.html
10. van Egmond LT, Meth EMS, Engström J, et al. Effects of acute sleep loss on leptin, ghrelin, and adiponectin in adults with healthy weight and obesity: a laboratory study. Obesity (Silver Spring). 2023;31:635-641. doi: 10.1002/oby.23616
11. Spiegel K, Tasali E, Penev P, et al. Brief communication: sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004;141:846-850. doi: 10.7326/0003-4819-141-11-200412070-00008
12. Antza C, Kostopoulos G, Mostafa S, et al. The links between sleep duration, obesity and type 2 diabetes mellitus. J Endocrinol. 2021;252:125-141. doi: 10.1530/JOE-21-0155
13. Baron KG, Reid KJ, Kern AS, et al. Role of sleep timing in caloric intake and BMI. Obesity (Silver Spring). 2011;19:1374-1381. doi: 10.1038/oby.2011.100
14. Liu XY, Zheng CL, Xu C, et al. Nighttime snacking is associated with risk of obesity and hyperglycemia in adults: a cross-sectional survey from Chinese adult teachers J Biomed Res. 2017;31:541-547. doi: 10.7555/JBR.31.20160083
15. Cai Z, Yang Y, Zhang J, et al. The relationship between daytime napping and obesity: a systematic review and meta-analysis. Sci Rep. 2023.13:12124. doi: 10.1038/s41598-023-37883-7
16. Nedeltcheva AV, Kilkus JM, Imperial J, et al. Insufficient sleep undermines dietary efforts to reduce adiposity. Ann Intern Med. 2010;153:435-441. doi: 10.7326/0003-4819-153-7-201010050-00006
17. Chaput JP, Tremblay A. Adequate sleep to improve the treatment of obesity. CMAJ. 2012;184:1975-1976. doi: 10.1503/cmaj.120876
18. Kelsey MM, Zaepfel A, Bjornstad P, et al. Age-related consequences of childhood obesity. Gerontology. 2014;60:222-228. doi: 10.1159/000356023
19. Fryar CD, Carroll MD, Afful J. Prevalence of overweight, obesity, and severe obesity among children and adolescents aged 2-19 years: United States, 1963-1965 through 2017-2018. National Center for Health Statistics Health E-Stats. Updated January 29, 2021. Accessed September 21, 2021. www.cdc.gov/nchs/data/hestat/obesity-child-17-18/overweight-obesity-child-H.pdf
20. Fatima Y, Doi SAR, Mamun AA. Sleep quality and obesity in young subjects: a meta-analysis. Obes Rev. 2016;17:1154-1166. doi: 10.1111/obr.12444
21. Gohil A, Hannon TS. Poor sleep and obesity: concurrent epidemics in adolescent youth. Front Endocrinol. 2018;9:364. doi: 10.3389/fendo.2018.00364
22. Golley RK, Maher CA, Matricciani L, et al. Sleep duration or bedtime? Exploring the association between sleep timing behaviour, diet and BMI in children and adolescents. Int J Obes (Lond). 2013;37:546-551. doi: 10.1038/ijo.2012.212
23. Alessi CA. Sleep issues. In: Harper GM, Lyons WL, Potter JF, eds. Geriatrics Review Syllabus (GRS 10). Updated January 2021. Accessed August 29, 2023. http://geriatricscareonline.org
24. Patel SR, Blackwell T, Redline S, et al. The association between sleep duration and obesity in older adults. Int J Obes (Lond). 2008;32:1825-1834. doi: 10.1038/ijo.2008.198
25. Cai GH, Theorell-Haglöw J, Janson C, et al. Insomnia symptoms and sleep duration and their combined effects in relation to associations with obesity and central obesity. Sleep Med. 2018;46:81-87. doi: 10.1016/j.sleep.2018.03.009
26. Beccuti G, Pannain S. Sleep and obesity. Curr Opin Clin Nutr Metab Care. 2011;14:402-412. doi: 10.1097/MCO.0b013 e3283479109
27. Franklin KA, Lindberg E. Obstructive sleep apnea is a common disorder in the population–a review on the epidemiology of sleep apnea. J Thorac Dis. 2015;7:1311-1322. doi: 10.3978/j.issn.2072-1439.2015.06.11
28. USPSTF. Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for obstructive sleep apnea in adults: US Preventive Services Task Force recommendation statement. JAMA. 2017;317:407-414. doi: 10.1001/jama.2016.20325
29. Goyal M, Johnson J. Obstructive sleep apnea diagnosis and management. Mo Med. 2017;114:120-124.
30. American Academy of Sleep Medicine. Hidden health crisis costing America billions: underdiagnosing and undertreating obstructive sleep apnea draining healthcare system. 2016. Accessed September 25, 2023. https://aasm.org/wp-content/uploads/2017/10/sleep-apnea-economic-crisis.pdf
31. Devaraj, NK. Knowledge, attitude, and practice regarding obstructive sleep apnea among primary care physicians. Sleep Breath. 2020;24:1581-1590. doi: 10.1007/s11325-020-02040-1
32. Mysliwiec V, Martin JL, Ulmer CS, et al. The management of chronic insomnia disorder and obstructive sleep apnea: synopsis of the 2019 U.S. Department of Veterans Affairs and U.S. Department of Defense Clinical Practice Guidelines. Ann Intern Med. 2020;172:325-336. doi: 10.7326/M19-3575
33. Kuna ST, Reboussin DM, Strotmeyer ES, et al. Effects of weight loss on obstructive sleep apnea severity. Ten-year results of the Sleep AHEAD study. Am J Respir Crit Care Med. 2021;203:221-229. doi: 10.1164/rccm.201912-2511OC
34. St-Onge MP, Tasali E. Weight loss is integral to obstructive sleep apnea management. Ten-year follow-up in Sleep AHEAD. Am J Respir Crit Care Med. 2021;203:161-162. doi: 10.1164/rccm.202007-2906ED
35. Zheng D, Yuan X, Ma C, et al. Alcohol consumption and sleep quality: a community-based study. Public Health Nutr. 2021;24:4851-4858. doi: 10.1017/S1368980020004553
36. Chakravorty S, Chaudhary NS, Brower KJ. Alcohol dependence and its relationship with insomnia and other sleep disorders. Alcohol Clin Exp Res. 2016;40:2271-2282. doi: 10.1111/acer.13217
37. Elmenhorst EM, Elmenhorst D, Benderoth S, et al. Cognitive impairments by alcohol and sleep deprivation indicate trait characteristics and a potential role for adenosine A1 receptors. Proc Natl Acad Sci U S A. 2018;115:8009-8014. doi: 10.1073/pnas.1803770115
38. Traversy G, Chaput JP. Alcohol consumption and obesity: an update. Curr Obes Rep. 2015;4:122-130. doi: 10.1007/s13679-014-0129-4
39. McCann UD, Sgambati FP, Schwartz AR, et al. Sleep apnea in young abstinent recreational MDMA (“ecstasy”) consumers. Neurology. 2009;73:2011-2017. doi: 10.1212/WNL.0b013e3181c51a62
40. Grau-López L, Grau-López L, Daigre C, et al. Insomnia symptoms in patients with substance use disorders during detoxification and associated clinical features. Front Psychiatry. 2020;11:540022. doi: 10.3389/fpsyt.2020.540022
41. Boehm MA, Lei QM, Lloyd RM, et al. Depression, anxiety, and tobacco use: overlapping impediments to sleep in a national sample of college students. J Am Coll Health. 2016;64:565-574. doi: 10.1080/07448481.2016.1205073
42. Gracious BL, Meyer AE. Psychotropic-induced weight gain and potential pharmacologic treatment strategies. Psychiatry (Edgmont). 2005;2:36-42.
43. Ratliff JC, Barber JA, Palmese LB, et al. Association of prescription H1 antihistamine use with obesity: results from the National Health and Nutrition Examination Survey. Obesity (Silver Spring). 2010;18:2398-2400. doi: 10.1038/oby.2010.176
44. Pataka A, Daskalopoulou E, Kalamaras G, et al. Evaluation of five different questionnaires for assessing sleep apnea syndrome in a sleep clinic. Sleep Med. 2014;15:776-781. doi: 10.1016/j.sleep.2014.03.012
45. Kline CE, Chasens ER, Bizhanova Z, et al. The association between sleep health and weight change during a 12-month behavioral weight loss intervention. Int J Obes (Lond). 2021;45:639-649. doi: 10.1038/s41366-020-00728-8
46. CDC. How much physical activity do adults need? Accessed August 23, 2023. www.cdc.gov/physicalactivity/basics/adults/index.htm
47. Flack KD, Hays HM, Moreland J, et al. Exercise for weight loss: further evaluating energy compensation with exercise. Med Sci Sports Exerc. 2020;52:2466-2475. doi: 10.1249/MSS.0000000000002376
48. Swift DL, Johannsen NM, Lavie CJ, et al. The role of exercise and physical activity in weight loss and maintenance. Prog Cardiovasc Dis. 2014;56:441-447. doi: 10.1016/j.pcad.2013.09.012
49. Irish LA, Kline CE, Gunn HE, et al. The role of sleep hygiene in promoting public health: a review of empirical evidence. Sleep Med Rev. 2015;22:23-36. doi: 10.1016/j.smrv.2014.10.001
50. CDC. Tips for better sleep. 2022. Accessed August 4, 2023. www.cdc.gov/sleep/about_sleep/sleep_hygiene.html
51. Qaseem A, Kansagara D, Forciea MA, et al. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165:125-133. doi: 10.7326/M15-2175
52. Logue EE, Bourguet CC, Palmieri PA, et al. The better weight-better sleep study: a pilot intervention in primary care. Am J Health Behav. 2012;36:319-334. doi: 10.5993/AJHB.36.3.4
53. Leach MJ, Page AT. Herbal medicine for insomnia: a systematic review and meta-analysis. Sleep Med Rev. 2015;24:1-12. doi: 10.1016/j.smrv.2014.12.003
54. Sateia MJ, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13:307-349. doi: 10.5664/jcsm.6470
1. Aminoff MJ, Boller F, Swaab DF. We spend about one-third of our life either sleeping or attempting to do so. Handb Clin Neurol. 2011;98:vii. doi: 10.1016/B978-0-444-52006-7.00047-2
2. Watson NF, Badr MS, Belenky G, et al. Recommended amount of sleep for a healthy adult: a joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society. Sleep. 2015;38:843-844. doi: 10.5665/sleep.4716
3. CDC. Sleep and sleep disorders, adults. Accessed September 21, 2023. www.cdc.gov/sleep/data-and-statistics/adults.html
4. Chattu VK, Manzar MD, Kumary S. The global problem of insufficient sleep and its serious public health implications. Healthcare (Basel). 2019;7:1. doi: 10.3390/healthcare7010001
5. Taheri S, Lin L, Austin D, et al. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004;1:e62. doi: 10.1371/journal.pmed.0010062
6. Hafner M, Stepanek M, Taylor J, et al. Why sleep matters—the economic costs of insufficient sleep. Rand Health Q. 2017;6:11.
7. Hisler G, Twenge JM, Krizan Z. Associations between screen time and short sleep duration among adolescents varies by media type: evidence from a cohort study. Sleep Med. 2020;66:92-102. doi: 10.1016/j.sleep.2019.08.007
8. Ogilvie RP, Patel SR. The epidemiology of sleep and obesity. Sleep Health. 2017;3:383-388. doi: 10.1016/j.sleh.2017.07.013
9. CDC. Sleep and sleep disorders: How much sleep do I need? Accessed September 21, 2023. www.cdc.gov/sleep/about_sleep/how_much_sleep.html
10. van Egmond LT, Meth EMS, Engström J, et al. Effects of acute sleep loss on leptin, ghrelin, and adiponectin in adults with healthy weight and obesity: a laboratory study. Obesity (Silver Spring). 2023;31:635-641. doi: 10.1002/oby.23616
11. Spiegel K, Tasali E, Penev P, et al. Brief communication: sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004;141:846-850. doi: 10.7326/0003-4819-141-11-200412070-00008
12. Antza C, Kostopoulos G, Mostafa S, et al. The links between sleep duration, obesity and type 2 diabetes mellitus. J Endocrinol. 2021;252:125-141. doi: 10.1530/JOE-21-0155
13. Baron KG, Reid KJ, Kern AS, et al. Role of sleep timing in caloric intake and BMI. Obesity (Silver Spring). 2011;19:1374-1381. doi: 10.1038/oby.2011.100
14. Liu XY, Zheng CL, Xu C, et al. Nighttime snacking is associated with risk of obesity and hyperglycemia in adults: a cross-sectional survey from Chinese adult teachers J Biomed Res. 2017;31:541-547. doi: 10.7555/JBR.31.20160083
15. Cai Z, Yang Y, Zhang J, et al. The relationship between daytime napping and obesity: a systematic review and meta-analysis. Sci Rep. 2023.13:12124. doi: 10.1038/s41598-023-37883-7
16. Nedeltcheva AV, Kilkus JM, Imperial J, et al. Insufficient sleep undermines dietary efforts to reduce adiposity. Ann Intern Med. 2010;153:435-441. doi: 10.7326/0003-4819-153-7-201010050-00006
17. Chaput JP, Tremblay A. Adequate sleep to improve the treatment of obesity. CMAJ. 2012;184:1975-1976. doi: 10.1503/cmaj.120876
18. Kelsey MM, Zaepfel A, Bjornstad P, et al. Age-related consequences of childhood obesity. Gerontology. 2014;60:222-228. doi: 10.1159/000356023
19. Fryar CD, Carroll MD, Afful J. Prevalence of overweight, obesity, and severe obesity among children and adolescents aged 2-19 years: United States, 1963-1965 through 2017-2018. National Center for Health Statistics Health E-Stats. Updated January 29, 2021. Accessed September 21, 2021. www.cdc.gov/nchs/data/hestat/obesity-child-17-18/overweight-obesity-child-H.pdf
20. Fatima Y, Doi SAR, Mamun AA. Sleep quality and obesity in young subjects: a meta-analysis. Obes Rev. 2016;17:1154-1166. doi: 10.1111/obr.12444
21. Gohil A, Hannon TS. Poor sleep and obesity: concurrent epidemics in adolescent youth. Front Endocrinol. 2018;9:364. doi: 10.3389/fendo.2018.00364
22. Golley RK, Maher CA, Matricciani L, et al. Sleep duration or bedtime? Exploring the association between sleep timing behaviour, diet and BMI in children and adolescents. Int J Obes (Lond). 2013;37:546-551. doi: 10.1038/ijo.2012.212
23. Alessi CA. Sleep issues. In: Harper GM, Lyons WL, Potter JF, eds. Geriatrics Review Syllabus (GRS 10). Updated January 2021. Accessed August 29, 2023. http://geriatricscareonline.org
24. Patel SR, Blackwell T, Redline S, et al. The association between sleep duration and obesity in older adults. Int J Obes (Lond). 2008;32:1825-1834. doi: 10.1038/ijo.2008.198
25. Cai GH, Theorell-Haglöw J, Janson C, et al. Insomnia symptoms and sleep duration and their combined effects in relation to associations with obesity and central obesity. Sleep Med. 2018;46:81-87. doi: 10.1016/j.sleep.2018.03.009
26. Beccuti G, Pannain S. Sleep and obesity. Curr Opin Clin Nutr Metab Care. 2011;14:402-412. doi: 10.1097/MCO.0b013 e3283479109
27. Franklin KA, Lindberg E. Obstructive sleep apnea is a common disorder in the population–a review on the epidemiology of sleep apnea. J Thorac Dis. 2015;7:1311-1322. doi: 10.3978/j.issn.2072-1439.2015.06.11
28. USPSTF. Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for obstructive sleep apnea in adults: US Preventive Services Task Force recommendation statement. JAMA. 2017;317:407-414. doi: 10.1001/jama.2016.20325
29. Goyal M, Johnson J. Obstructive sleep apnea diagnosis and management. Mo Med. 2017;114:120-124.
30. American Academy of Sleep Medicine. Hidden health crisis costing America billions: underdiagnosing and undertreating obstructive sleep apnea draining healthcare system. 2016. Accessed September 25, 2023. https://aasm.org/wp-content/uploads/2017/10/sleep-apnea-economic-crisis.pdf
31. Devaraj, NK. Knowledge, attitude, and practice regarding obstructive sleep apnea among primary care physicians. Sleep Breath. 2020;24:1581-1590. doi: 10.1007/s11325-020-02040-1
32. Mysliwiec V, Martin JL, Ulmer CS, et al. The management of chronic insomnia disorder and obstructive sleep apnea: synopsis of the 2019 U.S. Department of Veterans Affairs and U.S. Department of Defense Clinical Practice Guidelines. Ann Intern Med. 2020;172:325-336. doi: 10.7326/M19-3575
33. Kuna ST, Reboussin DM, Strotmeyer ES, et al. Effects of weight loss on obstructive sleep apnea severity. Ten-year results of the Sleep AHEAD study. Am J Respir Crit Care Med. 2021;203:221-229. doi: 10.1164/rccm.201912-2511OC
34. St-Onge MP, Tasali E. Weight loss is integral to obstructive sleep apnea management. Ten-year follow-up in Sleep AHEAD. Am J Respir Crit Care Med. 2021;203:161-162. doi: 10.1164/rccm.202007-2906ED
35. Zheng D, Yuan X, Ma C, et al. Alcohol consumption and sleep quality: a community-based study. Public Health Nutr. 2021;24:4851-4858. doi: 10.1017/S1368980020004553
36. Chakravorty S, Chaudhary NS, Brower KJ. Alcohol dependence and its relationship with insomnia and other sleep disorders. Alcohol Clin Exp Res. 2016;40:2271-2282. doi: 10.1111/acer.13217
37. Elmenhorst EM, Elmenhorst D, Benderoth S, et al. Cognitive impairments by alcohol and sleep deprivation indicate trait characteristics and a potential role for adenosine A1 receptors. Proc Natl Acad Sci U S A. 2018;115:8009-8014. doi: 10.1073/pnas.1803770115
38. Traversy G, Chaput JP. Alcohol consumption and obesity: an update. Curr Obes Rep. 2015;4:122-130. doi: 10.1007/s13679-014-0129-4
39. McCann UD, Sgambati FP, Schwartz AR, et al. Sleep apnea in young abstinent recreational MDMA (“ecstasy”) consumers. Neurology. 2009;73:2011-2017. doi: 10.1212/WNL.0b013e3181c51a62
40. Grau-López L, Grau-López L, Daigre C, et al. Insomnia symptoms in patients with substance use disorders during detoxification and associated clinical features. Front Psychiatry. 2020;11:540022. doi: 10.3389/fpsyt.2020.540022
41. Boehm MA, Lei QM, Lloyd RM, et al. Depression, anxiety, and tobacco use: overlapping impediments to sleep in a national sample of college students. J Am Coll Health. 2016;64:565-574. doi: 10.1080/07448481.2016.1205073
42. Gracious BL, Meyer AE. Psychotropic-induced weight gain and potential pharmacologic treatment strategies. Psychiatry (Edgmont). 2005;2:36-42.
43. Ratliff JC, Barber JA, Palmese LB, et al. Association of prescription H1 antihistamine use with obesity: results from the National Health and Nutrition Examination Survey. Obesity (Silver Spring). 2010;18:2398-2400. doi: 10.1038/oby.2010.176
44. Pataka A, Daskalopoulou E, Kalamaras G, et al. Evaluation of five different questionnaires for assessing sleep apnea syndrome in a sleep clinic. Sleep Med. 2014;15:776-781. doi: 10.1016/j.sleep.2014.03.012
45. Kline CE, Chasens ER, Bizhanova Z, et al. The association between sleep health and weight change during a 12-month behavioral weight loss intervention. Int J Obes (Lond). 2021;45:639-649. doi: 10.1038/s41366-020-00728-8
46. CDC. How much physical activity do adults need? Accessed August 23, 2023. www.cdc.gov/physicalactivity/basics/adults/index.htm
47. Flack KD, Hays HM, Moreland J, et al. Exercise for weight loss: further evaluating energy compensation with exercise. Med Sci Sports Exerc. 2020;52:2466-2475. doi: 10.1249/MSS.0000000000002376
48. Swift DL, Johannsen NM, Lavie CJ, et al. The role of exercise and physical activity in weight loss and maintenance. Prog Cardiovasc Dis. 2014;56:441-447. doi: 10.1016/j.pcad.2013.09.012
49. Irish LA, Kline CE, Gunn HE, et al. The role of sleep hygiene in promoting public health: a review of empirical evidence. Sleep Med Rev. 2015;22:23-36. doi: 10.1016/j.smrv.2014.10.001
50. CDC. Tips for better sleep. 2022. Accessed August 4, 2023. www.cdc.gov/sleep/about_sleep/sleep_hygiene.html
51. Qaseem A, Kansagara D, Forciea MA, et al. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165:125-133. doi: 10.7326/M15-2175
52. Logue EE, Bourguet CC, Palmieri PA, et al. The better weight-better sleep study: a pilot intervention in primary care. Am J Health Behav. 2012;36:319-334. doi: 10.5993/AJHB.36.3.4
53. Leach MJ, Page AT. Herbal medicine for insomnia: a systematic review and meta-analysis. Sleep Med Rev. 2015;24:1-12. doi: 10.1016/j.smrv.2014.12.003
54. Sateia MJ, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13:307-349. doi: 10.5664/jcsm.6470
PRACTICE RECOMMENDATIONS
› Consider cognitive behaviorial therapy for insomnia (CBT-I) first-line treatment for insomnia. A
› Carefully review patients’ medication lists, as many pharmaceuticals can affect weight and sleep. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Migraine headache: When to consider these newer agents
Migraine is a headache disorder that often causes unilateral pain, photophobia, phonophobia, nausea, and vomiting. More than 70% of office visits for migraine are made to primary care physicians.1 Recent data suggest migraine may be caused primarily by neuronal dysfunction and only secondarily by vasodilation.2 Although there are numerous classes of drugs used for migraine prevention and treatment, their success has been limited by inadequate efficacy, tolerability, and patient adherence.3 The discovery of pro-inflammatory markers such as calcitonin gene-related peptide (CGRP) has led to the development of new medications to prevent and treat migraine.4

Pathophysiology, Dx and triggers, indications for pharmacotherapy
Pathophysiology. A migraine is thought to be caused by cortical spreading depression (CSD), a depolarization of glial and neuronal cell membranes.5
Dx and triggers. In 2018, the International Headache Society revised its guidelines for the diagnosis of migraine.7 According to the 3rd edition of The International Classification of Headache Disorders (ICHD-3), the diagnosis of migraine is made when a patient has at least 5 headache attacks that last 4 to 72 hours and have at least 2 of the following characteristics: (1) unilateral location, (2) pulsating quality, (3) moderate-to-severe pain intensity, and (4) aggravated by or causing avoidance of routine physical activity.7 The headache attacks also should have (1) associated nausea or vomiting or (2) photophobia and phonophobia.7 The presence of atypical signs or symptoms as indicated by the SNNOOP10 mnemonic raises concerns for secondary headaches and the need for further investigation into the cause of the headache (TABLE 1).8 It is not possible to detect every secondary headache with standard neuroimaging, but the SNNOOP10 red flags can help determine when imaging may be indicated.8 Potential triggers for migraine can be found in TABLE 2.9
Indications for pharmacotherapy. All patients receiving a diagnosis of migraine should be offered acute pharmacologic treatment. Consider preventive therapy anytime there are ≥ 4 headache days per month, debilitating attacks despite acute therapy, overuse of acute medication (> 2 d/wk), difficulty tolerating acute medication, patient preference, or presence of certain migraine subtypes.7,10

Acute treatments
Abortive therapies for migraine include analgesics such as nonsteroidal anti-inflammatory drugs (NSAIDs) and acetaminophen, and ergot alkaloids, triptans, or small-molecule CGRP receptor antagonists (gepants). Prompt administration increases the chance of success with acute therapy. Medications with the highest levels of efficacy based on the 2015 guidelines from the American Headache Society (AHS) are given in TABLE 3.11 Lasmiditan (Reyvow) is not included in the 2015 guidelines, as it was approved after publication of the guidelines.
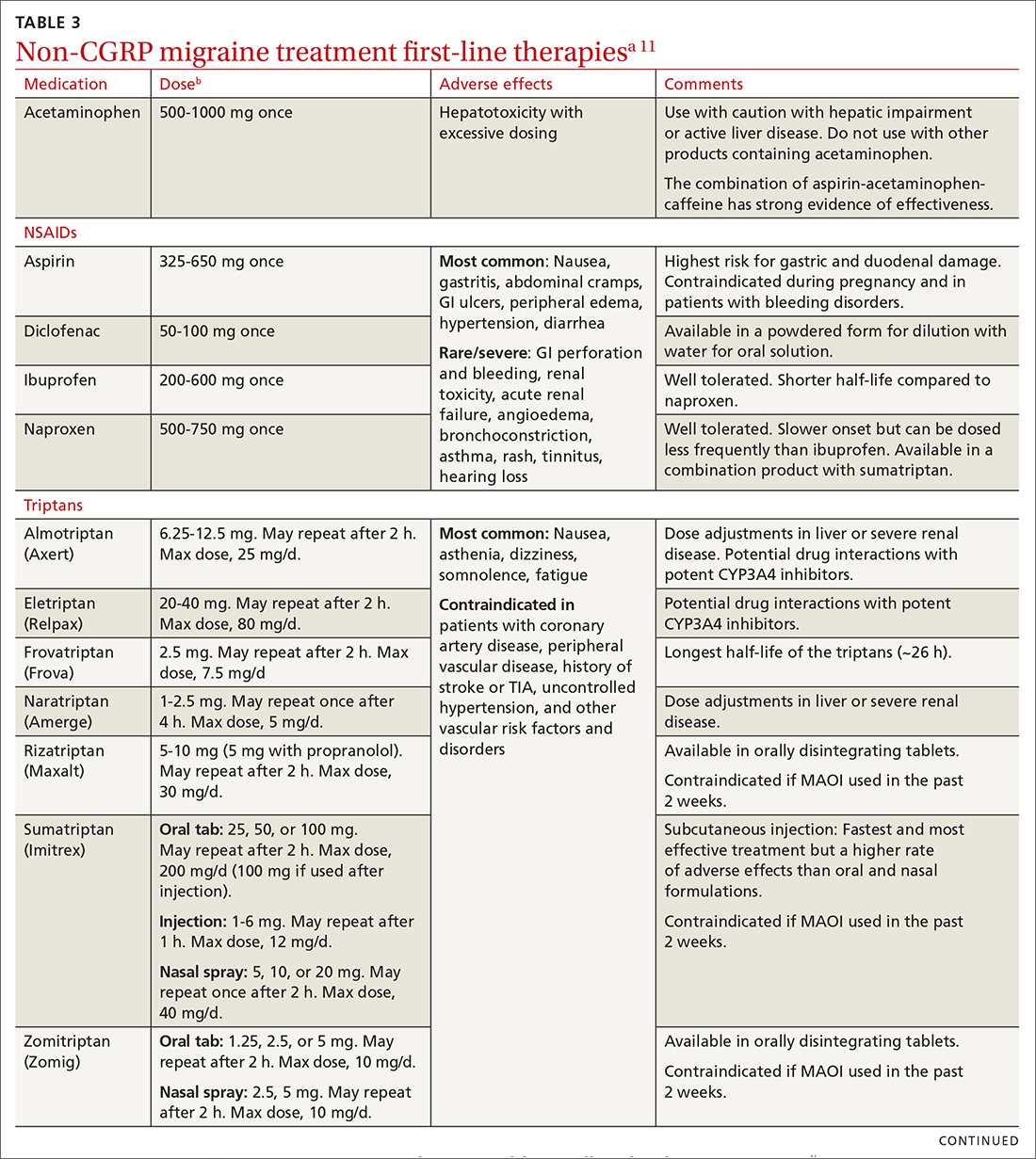
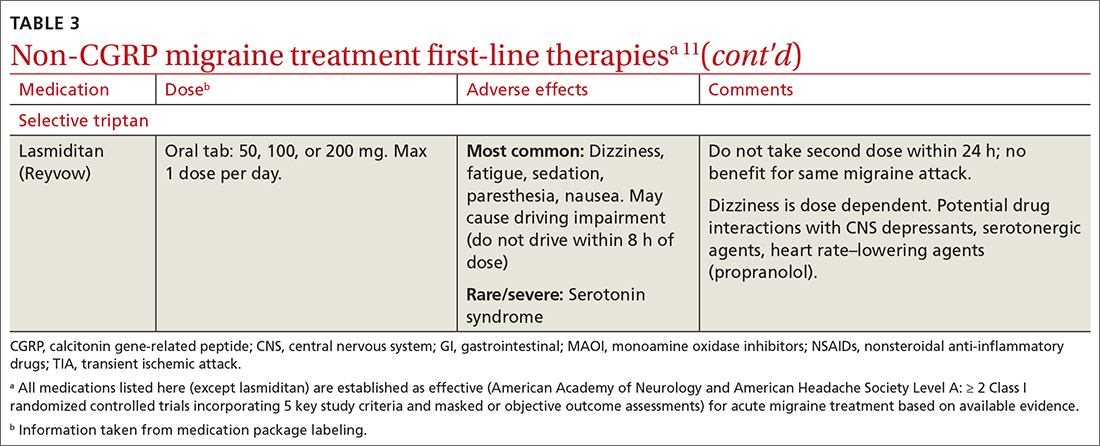
Non-CGRP first-line therapies
NSAIDs and acetaminophen. NSAIDs such as aspirin, diclofenac, ibuprofen, and naproxen have a high level of evidence to support their use as first-line treatments for mild-to-moderate migraine attacks. Trials consistently demonstrate their superiority to placebo in headache relief and complete pain relief at 2 hours. There is no recommendation for selecting one NSAID over another; however, consider their frequency of dosing and adverse effect profiles. The number needed to treat for complete pain relief at 2 hours ranges from 7 to 10 for most NSAIDs.11,12 In some placebo-controlled studies, acetaminophen was less effective than NSAIDs, but was safer because it did not cause gastric irritation or antiplatelet effects.12
Triptans inhibit 5-HT1B/1D receptors. Consider formulation, route of administration, cost, and pharmacokinetics when selecting a triptan. Patients who do not respond well to one triptan may respond favorably to another. A meta-analysis of the effectiveness of the 7 available agents found that triptans at standard doses provided pain relief within 2 hours in 42% to 76% of patients, and sustained freedom from pain for 2 hours in 18% to 50% of patients.13 Lasmiditan is a selective serotonin receptor (5-HT1F) agonist that lacks vasoconstrictor activity. This is an option for patients with relative contraindications to triptans due to cardiovascular risk factors.10
Continue to: Second-line therapies
Second-line therapies
Intranasal dihydroergotamine has a favorable adverse event profile and greater evidence for efficacy compared with ergotamine. Compared with triptans, intranasal dihydroergotamine has a high level of efficacy but causes more adverse effects.14 Severe nausea is common, and dihydroergotamine often is used in combination with an antiemetic drug. Dihydroergotamine should not be used within 24 hours of taking a triptan, and it is contraindicated for patients who have hypertension or ischemic heart disease or who are pregnant or breastfeeding. There is also the potential for adverse drug interactions.15
Antiemetics may be helpful for migraine associated with severe nausea or vomiting. The dopamine antagonists metoclopramide, prochlorperazine, and chlorpromazine have demonstrated benefit in randomized placebo-controlled trials.11 Ondansetron has not been studied extensively, but sometimes is used in clinical practice. Nonoral routes of administration may be useful in patients having trouble swallowing medications or in those experiencing significant nausea or vomiting early during migraine attacks.
Due to the high potential for abuse, opioids should not be used routinely for the treatment of migraine.12 There is no high-quality evidence supporting the efficacy of barbiturates (ie, butalbital-containing compounds) for acute migraine treatment.11 Moreover, use of these agents may increase the likelihood of progression from episodic to chronic migraine.16
Gepants for acute migraine treatment
Neuropeptide CGRP is released from trigeminal nerves and is a potent dilator of cerebral and dural vessels, playing a key role in regulating blood flow to the brain. Other roles of CGRP include the release of inflammatory agents from mast cells and the transmission of painful stimuli from intracranial vessels.17 The CGRP receptor or ligand can be targeted by small-molecule receptor antagonists for acute and preventive migraine treatment (and by monoclonal antibodies solely for prevention, discussed later). It has been theorized that gepants bind to CGRP receptors, resulting in decreased blood flow to the brain, inhibition of neurogenic inflammation, and reduced pain signaling.17 Unlike triptans and ergotamine derivatives, these novel treatments do not constrict blood vessels and may have a unique role in patients with contraindications to triptans.
The 3 gepants approved for acute treatment—ubrogepant (Ubrelvy),18 rimegepant (Nurtec),19 and zavegepant (Zavzpret)20—were compared with placebo in clinical trials and were shown to increase the number of patients who were completely pain free at 2 hours, were free of the most bothersome associated symptom (photophobia, phonophobia, or nausea) at 2 hours, and remained pain free at 24 hours (TABLE 418-24).
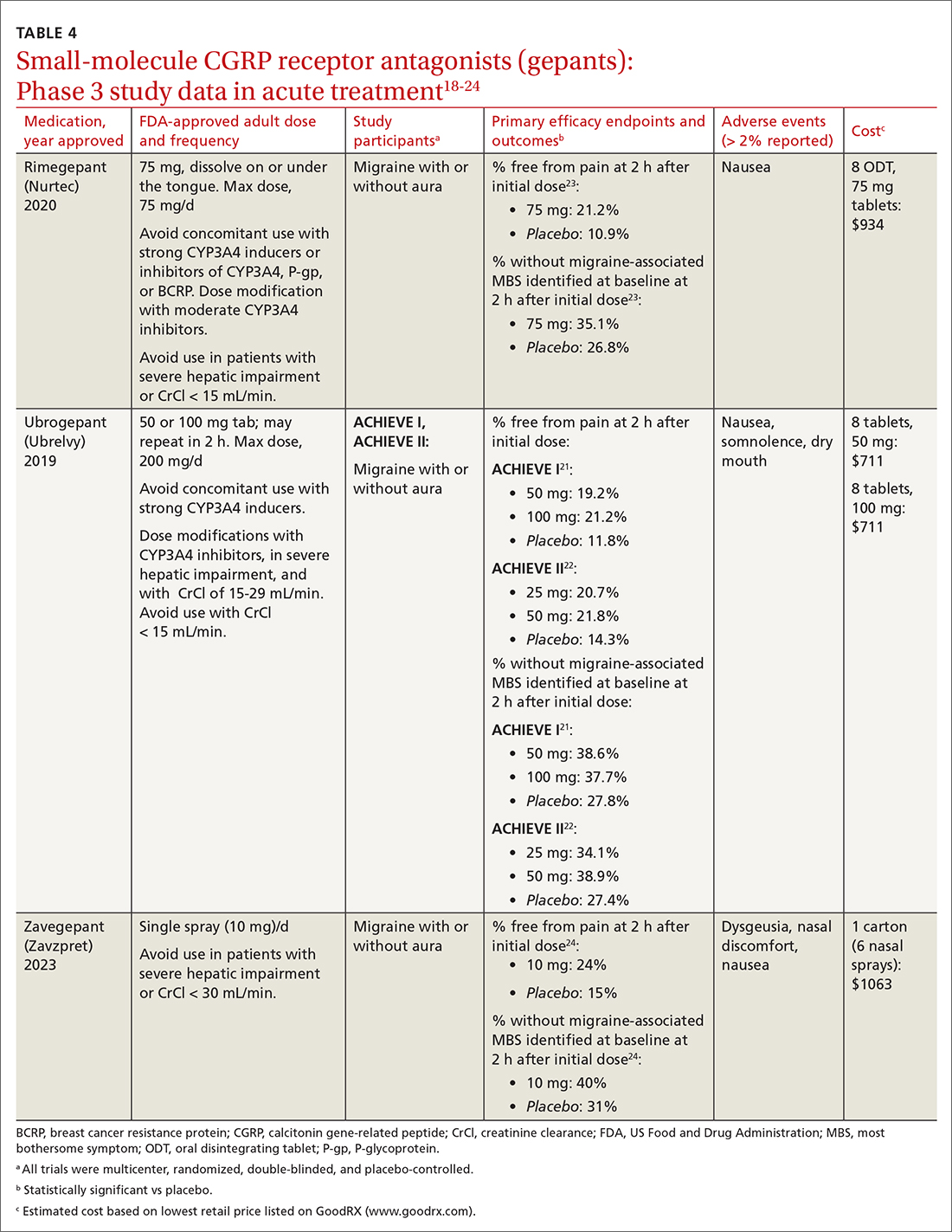
Continue to: Ubrogrepant
Ubrogepant, in 2 Phase 3 trials (ACHIEVE I and ACHIEVE II) demonstrated effectiveness compared with placebo.21,22 The most common adverse effects reported were nausea and somnolence at very low rates. Pain-relief rates at 2 hours post dose (> 60% of participants) were higher than pain-free rates, and a significantly higher percentage (> 40%) of ubrogepant-treated participants reported ability to function normally on the Functional Disability Scale.25
Rimegepant was also superior to placebo (59% vs 43%) in pain relief at 2 hours post dose and other secondary endpoints.23 Rimegepant also has potential drug interactions
Zavegepant, approved in March 2023, is administered once daily as a 10-mg nasal spray. In its Phase 3 trial, zavegepant was significantly superior to placebo at 2 hours post dose in freedom from pain (24% v 15%), and in freedom from the most bothersome symptom (40% v 31%).24 Dosage modifications are not needed with mild-to-moderate renal or hepatic disease.20
Worth noting. The safety of using ubrogepant to treat more than 8 migraine episodes in a 30-day period has not been established. The safety of using more than 18 doses of zavegepant in a 30-day period also has not been established. With ubrogepant and rimegepant, there are dosing modifications for concomitant use with specific drugs (CYP3A4 inhibitors and inducers) due to potential interactions and in patients with hepatic or renal impairment.18,19
There are no trials comparing efficacy of CGRP antagonists to triptans. Recognizing that these newer medications would be costly, the AHS position statement released in 2019 recommends that gepants be considered for those with contraindications to triptans or for whom at least 2 oral triptans have failed (as determined by a validated patient outcome questionnaire).10 Step therapy with documentation of previous trials and therapy failures is often required by insurance companies prior to gepant coverage.
Continue to: Preventive therapies
Preventive therapies
Preventive migraine therapies are used to reduce duration, frequency, and severity of attacks, the need for acute treatment, and overall headache disability.26 Medications typically are chosen based on efficacy, adverse effect profile, and patient comorbidities. Barriers to successful use include poor patient adherence and tolerability, the need for slow dose titration, and long-term use (minimum of 2 months) at maximum tolerated or minimum effective doses. Medications with established efficacy (Level Aa) based on the 2012 guidelines from the American Academy of Neurology (AAN) and the AHS are given in TABLE 5.27-29
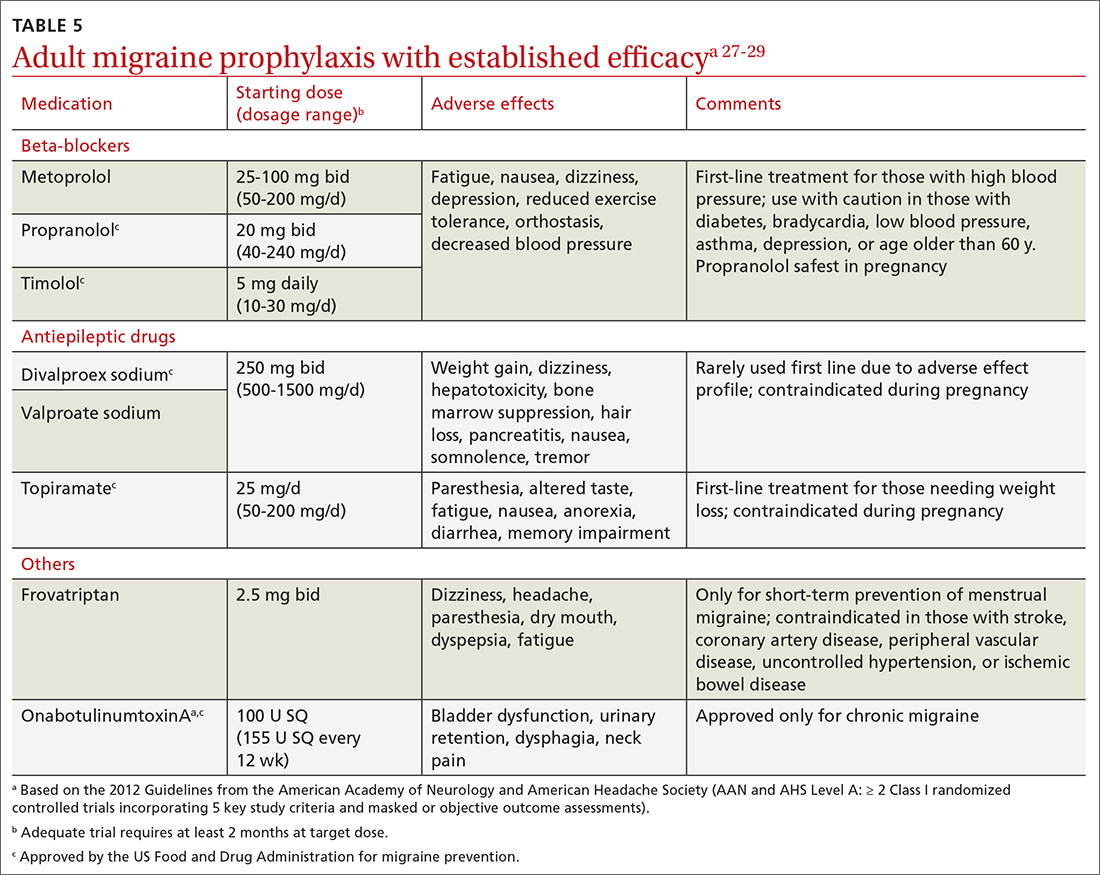
Drugs having received the strongest level of evidence for migraine prevention are metoprolol, propranolol, timolol, topiramate, valproate sodium, divalproex sodium, and onabotulinumtoxinA (Botox), and frovatriptan for menstrual migraine prevention. Because these guidelines were last updated in 2012, they did not cover gepants (which will be discussed shortly). The AHS released a position statement in 2019 supporting the use of
CGRP-targeted prevention
Four anti-CGRP mAbs and 2 gepants have been approved for migraine prevention in the United States. Differences between products include targets (ligand vs receptor), antibody IgG subtype, bioavailability, route of administration, and frequency of administration.28 As noted in the Phase 3 studies (TABLE 619,30-47), these therapies are highly efficacious, safe, and tolerable.
Gepants. Rimegepant, discussed earlier for migraine treatment, is one of the CGRP receptor antagonists approved for prevention. The other is atogepant (Qulipta), approved only for prevention. Ubrogepant is not approved for prevention.
Anti-CGRP mAb is the only medication class specifically created for migraine prevention.10,26 As already noted, several efficacious non-CGRP treatment options are available for migraine prevention. However, higher doses of those agents, if needed,
Continue to: The targeted anti-CGRP approach...
The targeted anti-CGRP approach, which can be used by patients with liver or kidney disease, results in decreased toxicity and minimal drug interactions. Long half-lives allow for monthly or quarterly injections, possibly resulting in increased compliance.28 Dose titration is not needed, allowing for more rapid symptom management. The large molecular size of a mAb limits its transfer across the blood-brain barrier, making central nervous system adverse effects unlikely.28 Despite the compelling mAb pharmacologic properties, their use may be limited by a lack of long-term safety data and the need for parenteral administration. Although immunogenicity—the development of neutralizing antibodies—can limit long-term tolerability or efficacy of mAbs generally,26,28 anti-CGRP mAbs were engineered to minimally activate the immune system and have not been associated with immune suppression, opportunistic infections, malignancies, or decreased efficacy.28
A pooled meta-analysis including 4 trials (3166 patients) found that CGRP mAbs compared with placebo significantly improved patient response rates, defined as at least a 50% and 75% reduction in monthly headache/migraine days from baseline to Weeks 9 to 12.48 Another meta-analysis including 8 trials (2292 patients) found a significant reduction from baseline in monthly migraine days and monthly acute migraine medication consumption among patients taking CGRP mAbs compared with those taking placebo.49 Open-label extension studies have shown progressive and cumulative benefits in individuals who respond to anti-CGRP mAbs. Therefore, several treatment cycles may be necessary to determine overall efficacy of therapy.10,28
Cost initially can be a barrier. Insurance companies often require step therapy before agreeing to cover mAb therapy, which aligns with the 2019 AHS position statement.10
When combination treatment may be appropriate
Monotherapy is the usual approach to preventing migraine due to advantages of efficacy, simplified regimens, lower cost, and reduced adverse effects.51 However, if a patient does not benefit from monotherapy even after trying dose titrations as tolerated or switching therapies, trying complementary combination therapy is appropriate. Despite a shortage of clinical trials supporting the use of 2 or more preventive medications with different mechanisms of action, this strategy is used clinically.10 Consider combination therapy in those with refractory disease, partial responses, or intolerance to recommended doses.52 Articles reporting on case study reviews have rationalized the combined use of onabotulinumtoxinA and anti-CGRP mAbs, noting better migraine control.51,53 The 2019 AHS position statement recommends adding a mAb to an existing preventive treatment regimen with no other changes until mAb effectiveness is determined, as the risk for drug interactions on dual therapy is low.10 Safety and efficacy also have been demonstrated with the combination of preventive anti-CGRP mAbs and acute treatment with gepants as needed.54
CORRESPONDENCE
Emily Peterson, PharmD, BCACP, 3640 Middlebury Road, Iowa City, IA 52242; Emily-a-peterson@uiowa.edu
1. Lipton RB, Nicholson RA, Reed ML, et al. Diagnosis, consultation, treatment, and impact of migraine in the US: results of the OVERCOME (US) study. Headache. 2022;62:122-140. doi: 10.1111/head.14259
2. Burstein R, Noseda R, Borsook D. Migraine: multiple processes; complext pathophysiology. J Neurosci. 2015;35:6619-6629. doi: 10.1523/JNEUROSCI.0373-15.2015
3. Edvinsson L, Haanes KA, Warfvinge K, et al. CGRP as the target of new migraine therapies - successful translation from bench to clinic. Nat Rev Neurol. 2018;14:338-350. doi: 10.1038/s41582-018-0003-1
4. McGrath K, Rague A, Thesing C, et al. Migraine: expanding our Tx arsenal. J Fam Pract. 2019;68:10-14;16-24.
5. Dodick DW. Migraine. Lancet. 2018;391:1315-1330. doi: 10.1016/S0140-6736(18)30478-1
6. Agostoni EC, Barbanti P, Calabresi P, et al. Current and emerging evidence-based treatment options in chronic migraine: a narrative review. J Headache Pain. 2019;20:92. doi: 10.1186/s10194-019-1038-4
7. IHS. Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38:1-211. doi: 10.1177/0333102417738202
8. Do TP, Remmers A, Schytz HW, et al. Red and orange flags for secondary headaches in clinical practice: SNNOOP10 list. Neurology. 2019;92:134-144. doi: 10.1212/WNL.0000000000006697
9. NIH. Migraine. Accessed July 30, 2023.
10. AHS. The American Headache Society position statement on integrating new migraine treatments into clinical practice. Headache. 2019;59:1-18. doi: 10.1111/head.13456
11. Marmura MJ, Silberstein SD, Schwedt TJ. The acute treatment of migraine in adults: the American Headache Society evidence assessment of migraine pharmacotherapies. Headache. 2015;55:3-20. doi: 10.1111/head.12499
12. Mayans L, Walling A. Acute migraine headache: treatment strategies. Am Fam Physician. 2018;97:243-251.
13. Cameron C, Kelly S, Hsieh SC, et al. Triptans in the acute treatment of migraine: a systematic review and network meta-analysis. Headache. 2015;55(suppl 4):221-235. doi: 10.1111/head.12601
14. Becker WJ. Acute migraine treatment. Continuum (Minneap Minn). 2015;21:953-972. doi: 10.1212/CON.0000000000000192
15. Migranal (dihydroergotamine mesylate) Package insert. Valeant Pharmaceuticals North America; 2019. Accessed June 17, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2019/020148Orig1s025lbl.pdf
16. Minen MT, Tanev K, Friedman BW. Evaluation and treatment of migraine in the emergency department: a review. Headache. 2014;54:1131-45. doi: 10.1111/head.12399
17. Durham PL. CGRP-receptor antagonists--a fresh approach to migraine therapy? N Engl J Med. 2004;350:1073-1075. doi: 10.1056/NEJMp048016
18. Ubrelvy (ubrogepant). Package insert. Allergan, Inc.; 2019. Accessed June 19, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2019/211765s000lbl.pdf
19. Nurtec ODT (rimegepant sulfate). Package insert. Biohaven Pharmaceuticals, Inc.; 2021. Accessed June 19, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2021/212728s006lbl.pdf
20. Zavzpret (zavegepant). Package insert. Pfizer Labs.; 2023. Accessed July 15, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2023/216386s000lbl.pdf
21. Dodick DW, Lipton RB, Ailani J, et al. Ubrogepant for the treatment of migraine. N Engl J Med. 2019;381:2230-2241. doi: 10.1056/NEJMoa1813049
22. Lipton RB, Dodick DW, Ailani J, et al. Effect of ubrogepant vs placebo on pain and the most bothersome associated symptom in the acute treatment of migraine: the ACHIEVE II randomized clinical trial. JAMA. 2019;322:1887-1898. doi: 10.1001/jama.2019.16711
23. Croop R, Goadsby PJ, Stock DA, et al. Efficacy, safety, and tolerability of rimegepant orally disintegrating tablet for the acute treatment of migraine: a randomised, phase 3, double-blind, placebo-controlled trial. Lancet. 2019;394:737-745. doi: 10.1016/S0140-6736(19)31606-X
24. Lipton RB, Croop R, Stock DA, et al. Safety, tolerability, and efficacy of zavegepant 10 mg nasal spray for the acute treatment of migraine in the USA: a phase 3, double-blind, randomised, placebo-controlled multicentre trial. Lancet Neurol. 2023;22:209-217. doi: 10.1016/S1474-4422(22)00517-8
25. Dodick DW, Lipton RB, Ailani J, et al. Ubrogepant, an acute treatment for migraine, improved patient-reported functional disability and satisfaction in 2 single-attack phase 3 randomized trials, ACHIEVE I and II. Headache. 2020;60:686-700. doi: 10.1111/head.13766
26. Burch R. Migraine and tension-type headache: diagnosis and treatment. Med Clin North Am. 2019;103:215-233. doi:10.1016/j.mcna.2018.10.003
27. Silberstein SD, Holland S, Freitag F, et al. Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1337-1345. doi: 10.1212/WNL.0b013e3182535d20
28. Dodick DW. CGRP ligand and receptor monoclonal antibodies for migraine prevention: evidence review and clinical implications. Cephalalgia. 2019;39:445-458. doi: 10.1177/ 0333102418821662
29. Pringsheim T, Davenport WJ, Becker WJ. Prophylaxis of migraine headache. CMAJ. 2010;182:E269-276. doi: 10.1503/cmaj.081657
30. Vyepti (eptinezumab-jjmr). Package insert. Lundbeck Pharmaceuticals LLV; 2020. Accessed June 19, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2020/761119s000lbl.pdf
31. Aimovig (erenumab-aooe). Package insert. Amgen Inc.; 2021. Accessed June 19, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2021/761077s009lbl.pdf
32. Ajovy (fremanezumab-vfrm). Package insert. Teva Pharmaceuticals USA, Inc.; 2018. Accessed June 19, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2018/761089s000lbl.pdf
33. Emgality (galcanezumab-gnlm). Package insert. Eli Lilly and Company; 2018. Accessed June 19, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2018/761063s000lbl.pdf
34. Ashina M, Saper J, Cady R, et al. Eptinezumab in episodic migraine: a randomized, double-blind, placebo-controlled study (PROMISE-1). Cephalalgia. 2020;40:241-254. doi: 10.1177/0333102420905132
35. Lipton RB, Goadsby PJ, Smith J, et al. Efficacy and safety of eptinezumab in patients with chronic migraine: PROMISE-2. Neurology. 2020;94:e1365-e1377. doi: 10.1212/WNL.0000000000009169
36. Dodick DW, Ashina M, Brandes JL, et al. ARISE: a phase 3 randomized trial of erenumab for episodic migraine. Cephalalgia. 2018;38:1026-1037. doi: 10.1177/0333102418759786
37. Goadsby PJ, Reuter U, Hallström Y, et al. A controlled trial of erenumab for episodic migraine. N Engl J Med. 2017;377:2123-2132. doi: 10.1056/NEJMoa1705848
38. Reuter U, Goadsby PJ, Lanteri-Minet M, et al. Efficacy and tolerability of erenumab in patients with episodic migraine in whom two-to-four previous preventive treatments were unsuccessful: a randomised, double-blind, placebo-controlled, phase 3b study. Lancet. 2018;392:2280-2287. doi: 10.1016/S0140-6736(18)32534-0
39. Silberstein SD, Dodick DW, Bigal ME, et al. Fremanezumab for the preventive treatment of chronic migraine. N Engl J Med. 2017; 377:2113-2122. doi: 10.1056/NEJMoa1709038
40. Dodick DW, Silberstein SD, Bigal ME, et al. Effect of fremanezumab compared with placebo for prevention of episodic migraine: a randomized clinical trial. JAMA. 2018;319:1999-2008. doi: 10.1001/jama.2018.4853
41. Stauffer VL, Dodick DW, Zhang Q, et al. Evaluation of galcanezumab for the prevention of episodic migraine: the EVOLVE-1 randomized clinical trial. JAMA Neurol. 2018;75:1080-1088. doi: 10.1001/jamaneurol.2018.1212
42. Skljarevski V, Matharu M, Millen BA, et al. Efficacy and safety of galcanezumab for the prevention of episodic migraine: results of the EVOLVE-2 phase 3 randomized controlled clinical trial. Cephalalgia. 2018;38:1442-1454. doi: 10.1177/0333102418779543
43. Detke HC, Goadsby PJ, Wang S, et al. Galcanezumab in chronic migraine: the randomized, double-blind, placebo-controlled REGAIN study. Neurology. 2018;91:e2211-e2221. doi: 10.1212/WNL.0000000000006640
44. Goadsby PJ, Dodick DW, Leone M, at al. Trial of galcanezumab in prevention of episodic cluster headache. N Engl J Med. 2019; 381:132-141. doi: 10.1056/NEJMoa1813440
45. Croop R, Lipton RB, Kudrow D, et al. Oral rimegepant for preventive treatment of migraine: a phase 2/3, randomised, double-blind, placebo-controlled trial. Lancet. 2021;397:51-60. doi: 10.1016/S0140-6736(20)32544-7
46. Ailani J, Lipton RB, Goadsby PJ, et al. Atogepant for the preventive treatment of migraine. N Engl J Med. 2021;385:695-706. doi: 10.1056/NEJMoa2035908
47. Qulipta (atogepant). Package insert. AbbVie; 2021. Accessed June 19, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2021/215206Orig1s000lbl.pdf
48. Han L, Liu Y, Xiong H, et al. CGRP monoclonal antibody for preventive treatment of chronic migraine: an update of meta-analysis. Brain Behav. 2019;9:e01215. doi: 10.1002/brb3.1215
49. Zhu Y, Liu Y, Zhao J, et al. The efficacy and safety of calcitonin gene-related peptide monoclonal antibody for episodic migraine: a meta-analysis. Neurol Sci. 2018;39:2097-2106. doi: 10.1007/s10072-018-3547-3
50. Szperka CL, VanderPluym J, Orr SL, et al. Recommendations on the use of anti-CGRP monoclonal antibodies in children and adolescents. Headache. 2018;58:1658-1669. doi: 10.1111/head.13414
51. Pellesi L, Do TP, Ashina H, et al. Dual therapy with anti-CGRP monoclonal antibodies and botulinum toxin for migraine prevention: is there a rationale? Headache. 2020;60:1056-1065. doi: 10.1111/head.13843
52. D’Antona L, Matharu M. Identifying and managing refractory migraine: barriers and opportunities? J Headache Pain. 2019;20:89. doi: 10.1186/s10194-019-1040-x
53. Cohen F, Armand C, Lipton RB, et al. Efficacy and tolerability of calcitonin gene-related peptide targeted monoclonal antibody medications as add-on therapy to onabotulinumtoxinA in patients with chronic migraine. Pain Med. 2021;1857-1863. doi: 10.1093/pm/pnab093
54. Berman G, Croop R, Kudrow D, et al. Safety of rimegepant, an oral CGRP receptor antagonist, plus CGRP monoclonal antibodies for migraine. Headache. 2020;60:1734-1742. doi: 10.1111/head.13930
Migraine is a headache disorder that often causes unilateral pain, photophobia, phonophobia, nausea, and vomiting. More than 70% of office visits for migraine are made to primary care physicians.1 Recent data suggest migraine may be caused primarily by neuronal dysfunction and only secondarily by vasodilation.2 Although there are numerous classes of drugs used for migraine prevention and treatment, their success has been limited by inadequate efficacy, tolerability, and patient adherence.3 The discovery of pro-inflammatory markers such as calcitonin gene-related peptide (CGRP) has led to the development of new medications to prevent and treat migraine.4

Pathophysiology, Dx and triggers, indications for pharmacotherapy
Pathophysiology. A migraine is thought to be caused by cortical spreading depression (CSD), a depolarization of glial and neuronal cell membranes.5
Dx and triggers. In 2018, the International Headache Society revised its guidelines for the diagnosis of migraine.7 According to the 3rd edition of The International Classification of Headache Disorders (ICHD-3), the diagnosis of migraine is made when a patient has at least 5 headache attacks that last 4 to 72 hours and have at least 2 of the following characteristics: (1) unilateral location, (2) pulsating quality, (3) moderate-to-severe pain intensity, and (4) aggravated by or causing avoidance of routine physical activity.7 The headache attacks also should have (1) associated nausea or vomiting or (2) photophobia and phonophobia.7 The presence of atypical signs or symptoms as indicated by the SNNOOP10 mnemonic raises concerns for secondary headaches and the need for further investigation into the cause of the headache (TABLE 1).8 It is not possible to detect every secondary headache with standard neuroimaging, but the SNNOOP10 red flags can help determine when imaging may be indicated.8 Potential triggers for migraine can be found in TABLE 2.9

Indications for pharmacotherapy. All patients receiving a diagnosis of migraine should be offered acute pharmacologic treatment. Consider preventive therapy anytime there are ≥ 4 headache days per month, debilitating attacks despite acute therapy, overuse of acute medication (> 2 d/wk), difficulty tolerating acute medication, patient preference, or presence of certain migraine subtypes.7,10

Acute treatments
Abortive therapies for migraine include analgesics such as nonsteroidal anti-inflammatory drugs (NSAIDs) and acetaminophen, and ergot alkaloids, triptans, or small-molecule CGRP receptor antagonists (gepants). Prompt administration increases the chance of success with acute therapy. Medications with the highest levels of efficacy based on the 2015 guidelines from the American Headache Society (AHS) are given in TABLE 3.11 Lasmiditan (Reyvow) is not included in the 2015 guidelines, as it was approved after publication of the guidelines.


Non-CGRP first-line therapies
NSAIDs and acetaminophen. NSAIDs such as aspirin, diclofenac, ibuprofen, and naproxen have a high level of evidence to support their use as first-line treatments for mild-to-moderate migraine attacks. Trials consistently demonstrate their superiority to placebo in headache relief and complete pain relief at 2 hours. There is no recommendation for selecting one NSAID over another; however, consider their frequency of dosing and adverse effect profiles. The number needed to treat for complete pain relief at 2 hours ranges from 7 to 10 for most NSAIDs.11,12 In some placebo-controlled studies, acetaminophen was less effective than NSAIDs, but was safer because it did not cause gastric irritation or antiplatelet effects.12
Triptans inhibit 5-HT1B/1D receptors. Consider formulation, route of administration, cost, and pharmacokinetics when selecting a triptan. Patients who do not respond well to one triptan may respond favorably to another. A meta-analysis of the effectiveness of the 7 available agents found that triptans at standard doses provided pain relief within 2 hours in 42% to 76% of patients, and sustained freedom from pain for 2 hours in 18% to 50% of patients.13 Lasmiditan is a selective serotonin receptor (5-HT1F) agonist that lacks vasoconstrictor activity. This is an option for patients with relative contraindications to triptans due to cardiovascular risk factors.10
Continue to: Second-line therapies
Second-line therapies
Intranasal dihydroergotamine has a favorable adverse event profile and greater evidence for efficacy compared with ergotamine. Compared with triptans, intranasal dihydroergotamine has a high level of efficacy but causes more adverse effects.14 Severe nausea is common, and dihydroergotamine often is used in combination with an antiemetic drug. Dihydroergotamine should not be used within 24 hours of taking a triptan, and it is contraindicated for patients who have hypertension or ischemic heart disease or who are pregnant or breastfeeding. There is also the potential for adverse drug interactions.15
Antiemetics may be helpful for migraine associated with severe nausea or vomiting. The dopamine antagonists metoclopramide, prochlorperazine, and chlorpromazine have demonstrated benefit in randomized placebo-controlled trials.11 Ondansetron has not been studied extensively, but sometimes is used in clinical practice. Nonoral routes of administration may be useful in patients having trouble swallowing medications or in those experiencing significant nausea or vomiting early during migraine attacks.
Due to the high potential for abuse, opioids should not be used routinely for the treatment of migraine.12 There is no high-quality evidence supporting the efficacy of barbiturates (ie, butalbital-containing compounds) for acute migraine treatment.11 Moreover, use of these agents may increase the likelihood of progression from episodic to chronic migraine.16
Gepants for acute migraine treatment
Neuropeptide CGRP is released from trigeminal nerves and is a potent dilator of cerebral and dural vessels, playing a key role in regulating blood flow to the brain. Other roles of CGRP include the release of inflammatory agents from mast cells and the transmission of painful stimuli from intracranial vessels.17 The CGRP receptor or ligand can be targeted by small-molecule receptor antagonists for acute and preventive migraine treatment (and by monoclonal antibodies solely for prevention, discussed later). It has been theorized that gepants bind to CGRP receptors, resulting in decreased blood flow to the brain, inhibition of neurogenic inflammation, and reduced pain signaling.17 Unlike triptans and ergotamine derivatives, these novel treatments do not constrict blood vessels and may have a unique role in patients with contraindications to triptans.
The 3 gepants approved for acute treatment—ubrogepant (Ubrelvy),18 rimegepant (Nurtec),19 and zavegepant (Zavzpret)20—were compared with placebo in clinical trials and were shown to increase the number of patients who were completely pain free at 2 hours, were free of the most bothersome associated symptom (photophobia, phonophobia, or nausea) at 2 hours, and remained pain free at 24 hours (TABLE 418-24).

Continue to: Ubrogrepant
Ubrogepant, in 2 Phase 3 trials (ACHIEVE I and ACHIEVE II) demonstrated effectiveness compared with placebo.21,22 The most common adverse effects reported were nausea and somnolence at very low rates. Pain-relief rates at 2 hours post dose (> 60% of participants) were higher than pain-free rates, and a significantly higher percentage (> 40%) of ubrogepant-treated participants reported ability to function normally on the Functional Disability Scale.25
Rimegepant was also superior to placebo (59% vs 43%) in pain relief at 2 hours post dose and other secondary endpoints.23 Rimegepant also has potential drug interactions
Zavegepant, approved in March 2023, is administered once daily as a 10-mg nasal spray. In its Phase 3 trial, zavegepant was significantly superior to placebo at 2 hours post dose in freedom from pain (24% v 15%), and in freedom from the most bothersome symptom (40% v 31%).24 Dosage modifications are not needed with mild-to-moderate renal or hepatic disease.20
Worth noting. The safety of using ubrogepant to treat more than 8 migraine episodes in a 30-day period has not been established. The safety of using more than 18 doses of zavegepant in a 30-day period also has not been established. With ubrogepant and rimegepant, there are dosing modifications for concomitant use with specific drugs (CYP3A4 inhibitors and inducers) due to potential interactions and in patients with hepatic or renal impairment.18,19
There are no trials comparing efficacy of CGRP antagonists to triptans. Recognizing that these newer medications would be costly, the AHS position statement released in 2019 recommends that gepants be considered for those with contraindications to triptans or for whom at least 2 oral triptans have failed (as determined by a validated patient outcome questionnaire).10 Step therapy with documentation of previous trials and therapy failures is often required by insurance companies prior to gepant coverage.
Continue to: Preventive therapies
Preventive therapies
Preventive migraine therapies are used to reduce duration, frequency, and severity of attacks, the need for acute treatment, and overall headache disability.26 Medications typically are chosen based on efficacy, adverse effect profile, and patient comorbidities. Barriers to successful use include poor patient adherence and tolerability, the need for slow dose titration, and long-term use (minimum of 2 months) at maximum tolerated or minimum effective doses. Medications with established efficacy (Level Aa) based on the 2012 guidelines from the American Academy of Neurology (AAN) and the AHS are given in TABLE 5.27-29

Drugs having received the strongest level of evidence for migraine prevention are metoprolol, propranolol, timolol, topiramate, valproate sodium, divalproex sodium, and onabotulinumtoxinA (Botox), and frovatriptan for menstrual migraine prevention. Because these guidelines were last updated in 2012, they did not cover gepants (which will be discussed shortly). The AHS released a position statement in 2019 supporting the use of
CGRP-targeted prevention
Four anti-CGRP mAbs and 2 gepants have been approved for migraine prevention in the United States. Differences between products include targets (ligand vs receptor), antibody IgG subtype, bioavailability, route of administration, and frequency of administration.28 As noted in the Phase 3 studies (TABLE 619,30-47), these therapies are highly efficacious, safe, and tolerable.
Gepants. Rimegepant, discussed earlier for migraine treatment, is one of the CGRP receptor antagonists approved for prevention. The other is atogepant (Qulipta), approved only for prevention. Ubrogepant is not approved for prevention.
Anti-CGRP mAb is the only medication class specifically created for migraine prevention.10,26 As already noted, several efficacious non-CGRP treatment options are available for migraine prevention. However, higher doses of those agents, if needed,
Continue to: The targeted anti-CGRP approach...
The targeted anti-CGRP approach, which can be used by patients with liver or kidney disease, results in decreased toxicity and minimal drug interactions. Long half-lives allow for monthly or quarterly injections, possibly resulting in increased compliance.28 Dose titration is not needed, allowing for more rapid symptom management. The large molecular size of a mAb limits its transfer across the blood-brain barrier, making central nervous system adverse effects unlikely.28 Despite the compelling mAb pharmacologic properties, their use may be limited by a lack of long-term safety data and the need for parenteral administration. Although immunogenicity—the development of neutralizing antibodies—can limit long-term tolerability or efficacy of mAbs generally,26,28 anti-CGRP mAbs were engineered to minimally activate the immune system and have not been associated with immune suppression, opportunistic infections, malignancies, or decreased efficacy.28
A pooled meta-analysis including 4 trials (3166 patients) found that CGRP mAbs compared with placebo significantly improved patient response rates, defined as at least a 50% and 75% reduction in monthly headache/migraine days from baseline to Weeks 9 to 12.48 Another meta-analysis including 8 trials (2292 patients) found a significant reduction from baseline in monthly migraine days and monthly acute migraine medication consumption among patients taking CGRP mAbs compared with those taking placebo.49 Open-label extension studies have shown progressive and cumulative benefits in individuals who respond to anti-CGRP mAbs. Therefore, several treatment cycles may be necessary to determine overall efficacy of therapy.10,28
Cost initially can be a barrier. Insurance companies often require step therapy before agreeing to cover mAb therapy, which aligns with the 2019 AHS position statement.10
When combination treatment may be appropriate
Monotherapy is the usual approach to preventing migraine due to advantages of efficacy, simplified regimens, lower cost, and reduced adverse effects.51 However, if a patient does not benefit from monotherapy even after trying dose titrations as tolerated or switching therapies, trying complementary combination therapy is appropriate. Despite a shortage of clinical trials supporting the use of 2 or more preventive medications with different mechanisms of action, this strategy is used clinically.10 Consider combination therapy in those with refractory disease, partial responses, or intolerance to recommended doses.52 Articles reporting on case study reviews have rationalized the combined use of onabotulinumtoxinA and anti-CGRP mAbs, noting better migraine control.51,53 The 2019 AHS position statement recommends adding a mAb to an existing preventive treatment regimen with no other changes until mAb effectiveness is determined, as the risk for drug interactions on dual therapy is low.10 Safety and efficacy also have been demonstrated with the combination of preventive anti-CGRP mAbs and acute treatment with gepants as needed.54
CORRESPONDENCE
Emily Peterson, PharmD, BCACP, 3640 Middlebury Road, Iowa City, IA 52242; Emily-a-peterson@uiowa.edu
Migraine is a headache disorder that often causes unilateral pain, photophobia, phonophobia, nausea, and vomiting. More than 70% of office visits for migraine are made to primary care physicians.1 Recent data suggest migraine may be caused primarily by neuronal dysfunction and only secondarily by vasodilation.2 Although there are numerous classes of drugs used for migraine prevention and treatment, their success has been limited by inadequate efficacy, tolerability, and patient adherence.3 The discovery of pro-inflammatory markers such as calcitonin gene-related peptide (CGRP) has led to the development of new medications to prevent and treat migraine.4

Pathophysiology, Dx and triggers, indications for pharmacotherapy
Pathophysiology. A migraine is thought to be caused by cortical spreading depression (CSD), a depolarization of glial and neuronal cell membranes.5
Dx and triggers. In 2018, the International Headache Society revised its guidelines for the diagnosis of migraine.7 According to the 3rd edition of The International Classification of Headache Disorders (ICHD-3), the diagnosis of migraine is made when a patient has at least 5 headache attacks that last 4 to 72 hours and have at least 2 of the following characteristics: (1) unilateral location, (2) pulsating quality, (3) moderate-to-severe pain intensity, and (4) aggravated by or causing avoidance of routine physical activity.7 The headache attacks also should have (1) associated nausea or vomiting or (2) photophobia and phonophobia.7 The presence of atypical signs or symptoms as indicated by the SNNOOP10 mnemonic raises concerns for secondary headaches and the need for further investigation into the cause of the headache (TABLE 1).8 It is not possible to detect every secondary headache with standard neuroimaging, but the SNNOOP10 red flags can help determine when imaging may be indicated.8 Potential triggers for migraine can be found in TABLE 2.9

Indications for pharmacotherapy. All patients receiving a diagnosis of migraine should be offered acute pharmacologic treatment. Consider preventive therapy anytime there are ≥ 4 headache days per month, debilitating attacks despite acute therapy, overuse of acute medication (> 2 d/wk), difficulty tolerating acute medication, patient preference, or presence of certain migraine subtypes.7,10

Acute treatments
Abortive therapies for migraine include analgesics such as nonsteroidal anti-inflammatory drugs (NSAIDs) and acetaminophen, and ergot alkaloids, triptans, or small-molecule CGRP receptor antagonists (gepants). Prompt administration increases the chance of success with acute therapy. Medications with the highest levels of efficacy based on the 2015 guidelines from the American Headache Society (AHS) are given in TABLE 3.11 Lasmiditan (Reyvow) is not included in the 2015 guidelines, as it was approved after publication of the guidelines.


Non-CGRP first-line therapies
NSAIDs and acetaminophen. NSAIDs such as aspirin, diclofenac, ibuprofen, and naproxen have a high level of evidence to support their use as first-line treatments for mild-to-moderate migraine attacks. Trials consistently demonstrate their superiority to placebo in headache relief and complete pain relief at 2 hours. There is no recommendation for selecting one NSAID over another; however, consider their frequency of dosing and adverse effect profiles. The number needed to treat for complete pain relief at 2 hours ranges from 7 to 10 for most NSAIDs.11,12 In some placebo-controlled studies, acetaminophen was less effective than NSAIDs, but was safer because it did not cause gastric irritation or antiplatelet effects.12
Triptans inhibit 5-HT1B/1D receptors. Consider formulation, route of administration, cost, and pharmacokinetics when selecting a triptan. Patients who do not respond well to one triptan may respond favorably to another. A meta-analysis of the effectiveness of the 7 available agents found that triptans at standard doses provided pain relief within 2 hours in 42% to 76% of patients, and sustained freedom from pain for 2 hours in 18% to 50% of patients.13 Lasmiditan is a selective serotonin receptor (5-HT1F) agonist that lacks vasoconstrictor activity. This is an option for patients with relative contraindications to triptans due to cardiovascular risk factors.10
Continue to: Second-line therapies
Second-line therapies
Intranasal dihydroergotamine has a favorable adverse event profile and greater evidence for efficacy compared with ergotamine. Compared with triptans, intranasal dihydroergotamine has a high level of efficacy but causes more adverse effects.14 Severe nausea is common, and dihydroergotamine often is used in combination with an antiemetic drug. Dihydroergotamine should not be used within 24 hours of taking a triptan, and it is contraindicated for patients who have hypertension or ischemic heart disease or who are pregnant or breastfeeding. There is also the potential for adverse drug interactions.15
Antiemetics may be helpful for migraine associated with severe nausea or vomiting. The dopamine antagonists metoclopramide, prochlorperazine, and chlorpromazine have demonstrated benefit in randomized placebo-controlled trials.11 Ondansetron has not been studied extensively, but sometimes is used in clinical practice. Nonoral routes of administration may be useful in patients having trouble swallowing medications or in those experiencing significant nausea or vomiting early during migraine attacks.
Due to the high potential for abuse, opioids should not be used routinely for the treatment of migraine.12 There is no high-quality evidence supporting the efficacy of barbiturates (ie, butalbital-containing compounds) for acute migraine treatment.11 Moreover, use of these agents may increase the likelihood of progression from episodic to chronic migraine.16
Gepants for acute migraine treatment
Neuropeptide CGRP is released from trigeminal nerves and is a potent dilator of cerebral and dural vessels, playing a key role in regulating blood flow to the brain. Other roles of CGRP include the release of inflammatory agents from mast cells and the transmission of painful stimuli from intracranial vessels.17 The CGRP receptor or ligand can be targeted by small-molecule receptor antagonists for acute and preventive migraine treatment (and by monoclonal antibodies solely for prevention, discussed later). It has been theorized that gepants bind to CGRP receptors, resulting in decreased blood flow to the brain, inhibition of neurogenic inflammation, and reduced pain signaling.17 Unlike triptans and ergotamine derivatives, these novel treatments do not constrict blood vessels and may have a unique role in patients with contraindications to triptans.
The 3 gepants approved for acute treatment—ubrogepant (Ubrelvy),18 rimegepant (Nurtec),19 and zavegepant (Zavzpret)20—were compared with placebo in clinical trials and were shown to increase the number of patients who were completely pain free at 2 hours, were free of the most bothersome associated symptom (photophobia, phonophobia, or nausea) at 2 hours, and remained pain free at 24 hours (TABLE 418-24).

Continue to: Ubrogrepant
Ubrogepant, in 2 Phase 3 trials (ACHIEVE I and ACHIEVE II) demonstrated effectiveness compared with placebo.21,22 The most common adverse effects reported were nausea and somnolence at very low rates. Pain-relief rates at 2 hours post dose (> 60% of participants) were higher than pain-free rates, and a significantly higher percentage (> 40%) of ubrogepant-treated participants reported ability to function normally on the Functional Disability Scale.25
Rimegepant was also superior to placebo (59% vs 43%) in pain relief at 2 hours post dose and other secondary endpoints.23 Rimegepant also has potential drug interactions
Zavegepant, approved in March 2023, is administered once daily as a 10-mg nasal spray. In its Phase 3 trial, zavegepant was significantly superior to placebo at 2 hours post dose in freedom from pain (24% v 15%), and in freedom from the most bothersome symptom (40% v 31%).24 Dosage modifications are not needed with mild-to-moderate renal or hepatic disease.20
Worth noting. The safety of using ubrogepant to treat more than 8 migraine episodes in a 30-day period has not been established. The safety of using more than 18 doses of zavegepant in a 30-day period also has not been established. With ubrogepant and rimegepant, there are dosing modifications for concomitant use with specific drugs (CYP3A4 inhibitors and inducers) due to potential interactions and in patients with hepatic or renal impairment.18,19
There are no trials comparing efficacy of CGRP antagonists to triptans. Recognizing that these newer medications would be costly, the AHS position statement released in 2019 recommends that gepants be considered for those with contraindications to triptans or for whom at least 2 oral triptans have failed (as determined by a validated patient outcome questionnaire).10 Step therapy with documentation of previous trials and therapy failures is often required by insurance companies prior to gepant coverage.
Continue to: Preventive therapies
Preventive therapies
Preventive migraine therapies are used to reduce duration, frequency, and severity of attacks, the need for acute treatment, and overall headache disability.26 Medications typically are chosen based on efficacy, adverse effect profile, and patient comorbidities. Barriers to successful use include poor patient adherence and tolerability, the need for slow dose titration, and long-term use (minimum of 2 months) at maximum tolerated or minimum effective doses. Medications with established efficacy (Level Aa) based on the 2012 guidelines from the American Academy of Neurology (AAN) and the AHS are given in TABLE 5.27-29

Drugs having received the strongest level of evidence for migraine prevention are metoprolol, propranolol, timolol, topiramate, valproate sodium, divalproex sodium, and onabotulinumtoxinA (Botox), and frovatriptan for menstrual migraine prevention. Because these guidelines were last updated in 2012, they did not cover gepants (which will be discussed shortly). The AHS released a position statement in 2019 supporting the use of
CGRP-targeted prevention
Four anti-CGRP mAbs and 2 gepants have been approved for migraine prevention in the United States. Differences between products include targets (ligand vs receptor), antibody IgG subtype, bioavailability, route of administration, and frequency of administration.28 As noted in the Phase 3 studies (TABLE 619,30-47), these therapies are highly efficacious, safe, and tolerable.
Gepants. Rimegepant, discussed earlier for migraine treatment, is one of the CGRP receptor antagonists approved for prevention. The other is atogepant (Qulipta), approved only for prevention. Ubrogepant is not approved for prevention.
Anti-CGRP mAb is the only medication class specifically created for migraine prevention.10,26 As already noted, several efficacious non-CGRP treatment options are available for migraine prevention. However, higher doses of those agents, if needed,
Continue to: The targeted anti-CGRP approach...
The targeted anti-CGRP approach, which can be used by patients with liver or kidney disease, results in decreased toxicity and minimal drug interactions. Long half-lives allow for monthly or quarterly injections, possibly resulting in increased compliance.28 Dose titration is not needed, allowing for more rapid symptom management. The large molecular size of a mAb limits its transfer across the blood-brain barrier, making central nervous system adverse effects unlikely.28 Despite the compelling mAb pharmacologic properties, their use may be limited by a lack of long-term safety data and the need for parenteral administration. Although immunogenicity—the development of neutralizing antibodies—can limit long-term tolerability or efficacy of mAbs generally,26,28 anti-CGRP mAbs were engineered to minimally activate the immune system and have not been associated with immune suppression, opportunistic infections, malignancies, or decreased efficacy.28
A pooled meta-analysis including 4 trials (3166 patients) found that CGRP mAbs compared with placebo significantly improved patient response rates, defined as at least a 50% and 75% reduction in monthly headache/migraine days from baseline to Weeks 9 to 12.48 Another meta-analysis including 8 trials (2292 patients) found a significant reduction from baseline in monthly migraine days and monthly acute migraine medication consumption among patients taking CGRP mAbs compared with those taking placebo.49 Open-label extension studies have shown progressive and cumulative benefits in individuals who respond to anti-CGRP mAbs. Therefore, several treatment cycles may be necessary to determine overall efficacy of therapy.10,28
Cost initially can be a barrier. Insurance companies often require step therapy before agreeing to cover mAb therapy, which aligns with the 2019 AHS position statement.10
When combination treatment may be appropriate
Monotherapy is the usual approach to preventing migraine due to advantages of efficacy, simplified regimens, lower cost, and reduced adverse effects.51 However, if a patient does not benefit from monotherapy even after trying dose titrations as tolerated or switching therapies, trying complementary combination therapy is appropriate. Despite a shortage of clinical trials supporting the use of 2 or more preventive medications with different mechanisms of action, this strategy is used clinically.10 Consider combination therapy in those with refractory disease, partial responses, or intolerance to recommended doses.52 Articles reporting on case study reviews have rationalized the combined use of onabotulinumtoxinA and anti-CGRP mAbs, noting better migraine control.51,53 The 2019 AHS position statement recommends adding a mAb to an existing preventive treatment regimen with no other changes until mAb effectiveness is determined, as the risk for drug interactions on dual therapy is low.10 Safety and efficacy also have been demonstrated with the combination of preventive anti-CGRP mAbs and acute treatment with gepants as needed.54
CORRESPONDENCE
Emily Peterson, PharmD, BCACP, 3640 Middlebury Road, Iowa City, IA 52242; Emily-a-peterson@uiowa.edu
1. Lipton RB, Nicholson RA, Reed ML, et al. Diagnosis, consultation, treatment, and impact of migraine in the US: results of the OVERCOME (US) study. Headache. 2022;62:122-140. doi: 10.1111/head.14259
2. Burstein R, Noseda R, Borsook D. Migraine: multiple processes; complext pathophysiology. J Neurosci. 2015;35:6619-6629. doi: 10.1523/JNEUROSCI.0373-15.2015
3. Edvinsson L, Haanes KA, Warfvinge K, et al. CGRP as the target of new migraine therapies - successful translation from bench to clinic. Nat Rev Neurol. 2018;14:338-350. doi: 10.1038/s41582-018-0003-1
4. McGrath K, Rague A, Thesing C, et al. Migraine: expanding our Tx arsenal. J Fam Pract. 2019;68:10-14;16-24.
5. Dodick DW. Migraine. Lancet. 2018;391:1315-1330. doi: 10.1016/S0140-6736(18)30478-1
6. Agostoni EC, Barbanti P, Calabresi P, et al. Current and emerging evidence-based treatment options in chronic migraine: a narrative review. J Headache Pain. 2019;20:92. doi: 10.1186/s10194-019-1038-4
7. IHS. Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38:1-211. doi: 10.1177/0333102417738202
8. Do TP, Remmers A, Schytz HW, et al. Red and orange flags for secondary headaches in clinical practice: SNNOOP10 list. Neurology. 2019;92:134-144. doi: 10.1212/WNL.0000000000006697
9. NIH. Migraine. Accessed July 30, 2023.
10. AHS. The American Headache Society position statement on integrating new migraine treatments into clinical practice. Headache. 2019;59:1-18. doi: 10.1111/head.13456
11. Marmura MJ, Silberstein SD, Schwedt TJ. The acute treatment of migraine in adults: the American Headache Society evidence assessment of migraine pharmacotherapies. Headache. 2015;55:3-20. doi: 10.1111/head.12499
12. Mayans L, Walling A. Acute migraine headache: treatment strategies. Am Fam Physician. 2018;97:243-251.
13. Cameron C, Kelly S, Hsieh SC, et al. Triptans in the acute treatment of migraine: a systematic review and network meta-analysis. Headache. 2015;55(suppl 4):221-235. doi: 10.1111/head.12601
14. Becker WJ. Acute migraine treatment. Continuum (Minneap Minn). 2015;21:953-972. doi: 10.1212/CON.0000000000000192
15. Migranal (dihydroergotamine mesylate) Package insert. Valeant Pharmaceuticals North America; 2019. Accessed June 17, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2019/020148Orig1s025lbl.pdf
16. Minen MT, Tanev K, Friedman BW. Evaluation and treatment of migraine in the emergency department: a review. Headache. 2014;54:1131-45. doi: 10.1111/head.12399
17. Durham PL. CGRP-receptor antagonists--a fresh approach to migraine therapy? N Engl J Med. 2004;350:1073-1075. doi: 10.1056/NEJMp048016
18. Ubrelvy (ubrogepant). Package insert. Allergan, Inc.; 2019. Accessed June 19, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2019/211765s000lbl.pdf
19. Nurtec ODT (rimegepant sulfate). Package insert. Biohaven Pharmaceuticals, Inc.; 2021. Accessed June 19, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2021/212728s006lbl.pdf
20. Zavzpret (zavegepant). Package insert. Pfizer Labs.; 2023. Accessed July 15, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2023/216386s000lbl.pdf
21. Dodick DW, Lipton RB, Ailani J, et al. Ubrogepant for the treatment of migraine. N Engl J Med. 2019;381:2230-2241. doi: 10.1056/NEJMoa1813049
22. Lipton RB, Dodick DW, Ailani J, et al. Effect of ubrogepant vs placebo on pain and the most bothersome associated symptom in the acute treatment of migraine: the ACHIEVE II randomized clinical trial. JAMA. 2019;322:1887-1898. doi: 10.1001/jama.2019.16711
23. Croop R, Goadsby PJ, Stock DA, et al. Efficacy, safety, and tolerability of rimegepant orally disintegrating tablet for the acute treatment of migraine: a randomised, phase 3, double-blind, placebo-controlled trial. Lancet. 2019;394:737-745. doi: 10.1016/S0140-6736(19)31606-X
24. Lipton RB, Croop R, Stock DA, et al. Safety, tolerability, and efficacy of zavegepant 10 mg nasal spray for the acute treatment of migraine in the USA: a phase 3, double-blind, randomised, placebo-controlled multicentre trial. Lancet Neurol. 2023;22:209-217. doi: 10.1016/S1474-4422(22)00517-8
25. Dodick DW, Lipton RB, Ailani J, et al. Ubrogepant, an acute treatment for migraine, improved patient-reported functional disability and satisfaction in 2 single-attack phase 3 randomized trials, ACHIEVE I and II. Headache. 2020;60:686-700. doi: 10.1111/head.13766
26. Burch R. Migraine and tension-type headache: diagnosis and treatment. Med Clin North Am. 2019;103:215-233. doi:10.1016/j.mcna.2018.10.003
27. Silberstein SD, Holland S, Freitag F, et al. Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1337-1345. doi: 10.1212/WNL.0b013e3182535d20
28. Dodick DW. CGRP ligand and receptor monoclonal antibodies for migraine prevention: evidence review and clinical implications. Cephalalgia. 2019;39:445-458. doi: 10.1177/ 0333102418821662
29. Pringsheim T, Davenport WJ, Becker WJ. Prophylaxis of migraine headache. CMAJ. 2010;182:E269-276. doi: 10.1503/cmaj.081657
30. Vyepti (eptinezumab-jjmr). Package insert. Lundbeck Pharmaceuticals LLV; 2020. Accessed June 19, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2020/761119s000lbl.pdf
31. Aimovig (erenumab-aooe). Package insert. Amgen Inc.; 2021. Accessed June 19, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2021/761077s009lbl.pdf
32. Ajovy (fremanezumab-vfrm). Package insert. Teva Pharmaceuticals USA, Inc.; 2018. Accessed June 19, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2018/761089s000lbl.pdf
33. Emgality (galcanezumab-gnlm). Package insert. Eli Lilly and Company; 2018. Accessed June 19, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2018/761063s000lbl.pdf
34. Ashina M, Saper J, Cady R, et al. Eptinezumab in episodic migraine: a randomized, double-blind, placebo-controlled study (PROMISE-1). Cephalalgia. 2020;40:241-254. doi: 10.1177/0333102420905132
35. Lipton RB, Goadsby PJ, Smith J, et al. Efficacy and safety of eptinezumab in patients with chronic migraine: PROMISE-2. Neurology. 2020;94:e1365-e1377. doi: 10.1212/WNL.0000000000009169
36. Dodick DW, Ashina M, Brandes JL, et al. ARISE: a phase 3 randomized trial of erenumab for episodic migraine. Cephalalgia. 2018;38:1026-1037. doi: 10.1177/0333102418759786
37. Goadsby PJ, Reuter U, Hallström Y, et al. A controlled trial of erenumab for episodic migraine. N Engl J Med. 2017;377:2123-2132. doi: 10.1056/NEJMoa1705848
38. Reuter U, Goadsby PJ, Lanteri-Minet M, et al. Efficacy and tolerability of erenumab in patients with episodic migraine in whom two-to-four previous preventive treatments were unsuccessful: a randomised, double-blind, placebo-controlled, phase 3b study. Lancet. 2018;392:2280-2287. doi: 10.1016/S0140-6736(18)32534-0
39. Silberstein SD, Dodick DW, Bigal ME, et al. Fremanezumab for the preventive treatment of chronic migraine. N Engl J Med. 2017; 377:2113-2122. doi: 10.1056/NEJMoa1709038
40. Dodick DW, Silberstein SD, Bigal ME, et al. Effect of fremanezumab compared with placebo for prevention of episodic migraine: a randomized clinical trial. JAMA. 2018;319:1999-2008. doi: 10.1001/jama.2018.4853
41. Stauffer VL, Dodick DW, Zhang Q, et al. Evaluation of galcanezumab for the prevention of episodic migraine: the EVOLVE-1 randomized clinical trial. JAMA Neurol. 2018;75:1080-1088. doi: 10.1001/jamaneurol.2018.1212
42. Skljarevski V, Matharu M, Millen BA, et al. Efficacy and safety of galcanezumab for the prevention of episodic migraine: results of the EVOLVE-2 phase 3 randomized controlled clinical trial. Cephalalgia. 2018;38:1442-1454. doi: 10.1177/0333102418779543
43. Detke HC, Goadsby PJ, Wang S, et al. Galcanezumab in chronic migraine: the randomized, double-blind, placebo-controlled REGAIN study. Neurology. 2018;91:e2211-e2221. doi: 10.1212/WNL.0000000000006640
44. Goadsby PJ, Dodick DW, Leone M, at al. Trial of galcanezumab in prevention of episodic cluster headache. N Engl J Med. 2019; 381:132-141. doi: 10.1056/NEJMoa1813440
45. Croop R, Lipton RB, Kudrow D, et al. Oral rimegepant for preventive treatment of migraine: a phase 2/3, randomised, double-blind, placebo-controlled trial. Lancet. 2021;397:51-60. doi: 10.1016/S0140-6736(20)32544-7
46. Ailani J, Lipton RB, Goadsby PJ, et al. Atogepant for the preventive treatment of migraine. N Engl J Med. 2021;385:695-706. doi: 10.1056/NEJMoa2035908
47. Qulipta (atogepant). Package insert. AbbVie; 2021. Accessed June 19, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2021/215206Orig1s000lbl.pdf
48. Han L, Liu Y, Xiong H, et al. CGRP monoclonal antibody for preventive treatment of chronic migraine: an update of meta-analysis. Brain Behav. 2019;9:e01215. doi: 10.1002/brb3.1215
49. Zhu Y, Liu Y, Zhao J, et al. The efficacy and safety of calcitonin gene-related peptide monoclonal antibody for episodic migraine: a meta-analysis. Neurol Sci. 2018;39:2097-2106. doi: 10.1007/s10072-018-3547-3
50. Szperka CL, VanderPluym J, Orr SL, et al. Recommendations on the use of anti-CGRP monoclonal antibodies in children and adolescents. Headache. 2018;58:1658-1669. doi: 10.1111/head.13414
51. Pellesi L, Do TP, Ashina H, et al. Dual therapy with anti-CGRP monoclonal antibodies and botulinum toxin for migraine prevention: is there a rationale? Headache. 2020;60:1056-1065. doi: 10.1111/head.13843
52. D’Antona L, Matharu M. Identifying and managing refractory migraine: barriers and opportunities? J Headache Pain. 2019;20:89. doi: 10.1186/s10194-019-1040-x
53. Cohen F, Armand C, Lipton RB, et al. Efficacy and tolerability of calcitonin gene-related peptide targeted monoclonal antibody medications as add-on therapy to onabotulinumtoxinA in patients with chronic migraine. Pain Med. 2021;1857-1863. doi: 10.1093/pm/pnab093
54. Berman G, Croop R, Kudrow D, et al. Safety of rimegepant, an oral CGRP receptor antagonist, plus CGRP monoclonal antibodies for migraine. Headache. 2020;60:1734-1742. doi: 10.1111/head.13930
1. Lipton RB, Nicholson RA, Reed ML, et al. Diagnosis, consultation, treatment, and impact of migraine in the US: results of the OVERCOME (US) study. Headache. 2022;62:122-140. doi: 10.1111/head.14259
2. Burstein R, Noseda R, Borsook D. Migraine: multiple processes; complext pathophysiology. J Neurosci. 2015;35:6619-6629. doi: 10.1523/JNEUROSCI.0373-15.2015
3. Edvinsson L, Haanes KA, Warfvinge K, et al. CGRP as the target of new migraine therapies - successful translation from bench to clinic. Nat Rev Neurol. 2018;14:338-350. doi: 10.1038/s41582-018-0003-1
4. McGrath K, Rague A, Thesing C, et al. Migraine: expanding our Tx arsenal. J Fam Pract. 2019;68:10-14;16-24.
5. Dodick DW. Migraine. Lancet. 2018;391:1315-1330. doi: 10.1016/S0140-6736(18)30478-1
6. Agostoni EC, Barbanti P, Calabresi P, et al. Current and emerging evidence-based treatment options in chronic migraine: a narrative review. J Headache Pain. 2019;20:92. doi: 10.1186/s10194-019-1038-4
7. IHS. Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38:1-211. doi: 10.1177/0333102417738202
8. Do TP, Remmers A, Schytz HW, et al. Red and orange flags for secondary headaches in clinical practice: SNNOOP10 list. Neurology. 2019;92:134-144. doi: 10.1212/WNL.0000000000006697
9. NIH. Migraine. Accessed July 30, 2023.
10. AHS. The American Headache Society position statement on integrating new migraine treatments into clinical practice. Headache. 2019;59:1-18. doi: 10.1111/head.13456
11. Marmura MJ, Silberstein SD, Schwedt TJ. The acute treatment of migraine in adults: the American Headache Society evidence assessment of migraine pharmacotherapies. Headache. 2015;55:3-20. doi: 10.1111/head.12499
12. Mayans L, Walling A. Acute migraine headache: treatment strategies. Am Fam Physician. 2018;97:243-251.
13. Cameron C, Kelly S, Hsieh SC, et al. Triptans in the acute treatment of migraine: a systematic review and network meta-analysis. Headache. 2015;55(suppl 4):221-235. doi: 10.1111/head.12601
14. Becker WJ. Acute migraine treatment. Continuum (Minneap Minn). 2015;21:953-972. doi: 10.1212/CON.0000000000000192
15. Migranal (dihydroergotamine mesylate) Package insert. Valeant Pharmaceuticals North America; 2019. Accessed June 17, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2019/020148Orig1s025lbl.pdf
16. Minen MT, Tanev K, Friedman BW. Evaluation and treatment of migraine in the emergency department: a review. Headache. 2014;54:1131-45. doi: 10.1111/head.12399
17. Durham PL. CGRP-receptor antagonists--a fresh approach to migraine therapy? N Engl J Med. 2004;350:1073-1075. doi: 10.1056/NEJMp048016
18. Ubrelvy (ubrogepant). Package insert. Allergan, Inc.; 2019. Accessed June 19, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2019/211765s000lbl.pdf
19. Nurtec ODT (rimegepant sulfate). Package insert. Biohaven Pharmaceuticals, Inc.; 2021. Accessed June 19, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2021/212728s006lbl.pdf
20. Zavzpret (zavegepant). Package insert. Pfizer Labs.; 2023. Accessed July 15, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2023/216386s000lbl.pdf
21. Dodick DW, Lipton RB, Ailani J, et al. Ubrogepant for the treatment of migraine. N Engl J Med. 2019;381:2230-2241. doi: 10.1056/NEJMoa1813049
22. Lipton RB, Dodick DW, Ailani J, et al. Effect of ubrogepant vs placebo on pain and the most bothersome associated symptom in the acute treatment of migraine: the ACHIEVE II randomized clinical trial. JAMA. 2019;322:1887-1898. doi: 10.1001/jama.2019.16711
23. Croop R, Goadsby PJ, Stock DA, et al. Efficacy, safety, and tolerability of rimegepant orally disintegrating tablet for the acute treatment of migraine: a randomised, phase 3, double-blind, placebo-controlled trial. Lancet. 2019;394:737-745. doi: 10.1016/S0140-6736(19)31606-X
24. Lipton RB, Croop R, Stock DA, et al. Safety, tolerability, and efficacy of zavegepant 10 mg nasal spray for the acute treatment of migraine in the USA: a phase 3, double-blind, randomised, placebo-controlled multicentre trial. Lancet Neurol. 2023;22:209-217. doi: 10.1016/S1474-4422(22)00517-8
25. Dodick DW, Lipton RB, Ailani J, et al. Ubrogepant, an acute treatment for migraine, improved patient-reported functional disability and satisfaction in 2 single-attack phase 3 randomized trials, ACHIEVE I and II. Headache. 2020;60:686-700. doi: 10.1111/head.13766
26. Burch R. Migraine and tension-type headache: diagnosis and treatment. Med Clin North Am. 2019;103:215-233. doi:10.1016/j.mcna.2018.10.003
27. Silberstein SD, Holland S, Freitag F, et al. Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1337-1345. doi: 10.1212/WNL.0b013e3182535d20
28. Dodick DW. CGRP ligand and receptor monoclonal antibodies for migraine prevention: evidence review and clinical implications. Cephalalgia. 2019;39:445-458. doi: 10.1177/ 0333102418821662
29. Pringsheim T, Davenport WJ, Becker WJ. Prophylaxis of migraine headache. CMAJ. 2010;182:E269-276. doi: 10.1503/cmaj.081657
30. Vyepti (eptinezumab-jjmr). Package insert. Lundbeck Pharmaceuticals LLV; 2020. Accessed June 19, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2020/761119s000lbl.pdf
31. Aimovig (erenumab-aooe). Package insert. Amgen Inc.; 2021. Accessed June 19, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2021/761077s009lbl.pdf
32. Ajovy (fremanezumab-vfrm). Package insert. Teva Pharmaceuticals USA, Inc.; 2018. Accessed June 19, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2018/761089s000lbl.pdf
33. Emgality (galcanezumab-gnlm). Package insert. Eli Lilly and Company; 2018. Accessed June 19, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2018/761063s000lbl.pdf
34. Ashina M, Saper J, Cady R, et al. Eptinezumab in episodic migraine: a randomized, double-blind, placebo-controlled study (PROMISE-1). Cephalalgia. 2020;40:241-254. doi: 10.1177/0333102420905132
35. Lipton RB, Goadsby PJ, Smith J, et al. Efficacy and safety of eptinezumab in patients with chronic migraine: PROMISE-2. Neurology. 2020;94:e1365-e1377. doi: 10.1212/WNL.0000000000009169
36. Dodick DW, Ashina M, Brandes JL, et al. ARISE: a phase 3 randomized trial of erenumab for episodic migraine. Cephalalgia. 2018;38:1026-1037. doi: 10.1177/0333102418759786
37. Goadsby PJ, Reuter U, Hallström Y, et al. A controlled trial of erenumab for episodic migraine. N Engl J Med. 2017;377:2123-2132. doi: 10.1056/NEJMoa1705848
38. Reuter U, Goadsby PJ, Lanteri-Minet M, et al. Efficacy and tolerability of erenumab in patients with episodic migraine in whom two-to-four previous preventive treatments were unsuccessful: a randomised, double-blind, placebo-controlled, phase 3b study. Lancet. 2018;392:2280-2287. doi: 10.1016/S0140-6736(18)32534-0
39. Silberstein SD, Dodick DW, Bigal ME, et al. Fremanezumab for the preventive treatment of chronic migraine. N Engl J Med. 2017; 377:2113-2122. doi: 10.1056/NEJMoa1709038
40. Dodick DW, Silberstein SD, Bigal ME, et al. Effect of fremanezumab compared with placebo for prevention of episodic migraine: a randomized clinical trial. JAMA. 2018;319:1999-2008. doi: 10.1001/jama.2018.4853
41. Stauffer VL, Dodick DW, Zhang Q, et al. Evaluation of galcanezumab for the prevention of episodic migraine: the EVOLVE-1 randomized clinical trial. JAMA Neurol. 2018;75:1080-1088. doi: 10.1001/jamaneurol.2018.1212
42. Skljarevski V, Matharu M, Millen BA, et al. Efficacy and safety of galcanezumab for the prevention of episodic migraine: results of the EVOLVE-2 phase 3 randomized controlled clinical trial. Cephalalgia. 2018;38:1442-1454. doi: 10.1177/0333102418779543
43. Detke HC, Goadsby PJ, Wang S, et al. Galcanezumab in chronic migraine: the randomized, double-blind, placebo-controlled REGAIN study. Neurology. 2018;91:e2211-e2221. doi: 10.1212/WNL.0000000000006640
44. Goadsby PJ, Dodick DW, Leone M, at al. Trial of galcanezumab in prevention of episodic cluster headache. N Engl J Med. 2019; 381:132-141. doi: 10.1056/NEJMoa1813440
45. Croop R, Lipton RB, Kudrow D, et al. Oral rimegepant for preventive treatment of migraine: a phase 2/3, randomised, double-blind, placebo-controlled trial. Lancet. 2021;397:51-60. doi: 10.1016/S0140-6736(20)32544-7
46. Ailani J, Lipton RB, Goadsby PJ, et al. Atogepant for the preventive treatment of migraine. N Engl J Med. 2021;385:695-706. doi: 10.1056/NEJMoa2035908
47. Qulipta (atogepant). Package insert. AbbVie; 2021. Accessed June 19, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2021/215206Orig1s000lbl.pdf
48. Han L, Liu Y, Xiong H, et al. CGRP monoclonal antibody for preventive treatment of chronic migraine: an update of meta-analysis. Brain Behav. 2019;9:e01215. doi: 10.1002/brb3.1215
49. Zhu Y, Liu Y, Zhao J, et al. The efficacy and safety of calcitonin gene-related peptide monoclonal antibody for episodic migraine: a meta-analysis. Neurol Sci. 2018;39:2097-2106. doi: 10.1007/s10072-018-3547-3
50. Szperka CL, VanderPluym J, Orr SL, et al. Recommendations on the use of anti-CGRP monoclonal antibodies in children and adolescents. Headache. 2018;58:1658-1669. doi: 10.1111/head.13414
51. Pellesi L, Do TP, Ashina H, et al. Dual therapy with anti-CGRP monoclonal antibodies and botulinum toxin for migraine prevention: is there a rationale? Headache. 2020;60:1056-1065. doi: 10.1111/head.13843
52. D’Antona L, Matharu M. Identifying and managing refractory migraine: barriers and opportunities? J Headache Pain. 2019;20:89. doi: 10.1186/s10194-019-1040-x
53. Cohen F, Armand C, Lipton RB, et al. Efficacy and tolerability of calcitonin gene-related peptide targeted monoclonal antibody medications as add-on therapy to onabotulinumtoxinA in patients with chronic migraine. Pain Med. 2021;1857-1863. doi: 10.1093/pm/pnab093
54. Berman G, Croop R, Kudrow D, et al. Safety of rimegepant, an oral CGRP receptor antagonist, plus CGRP monoclonal antibodies for migraine. Headache. 2020;60:1734-1742. doi: 10.1111/head.13930
PRACTICE RECOMMENDATIONS
› Consider small-molecule calcitonin gene-related peptide (CGRP) receptor antagonists (gepants) for acute migraine treatment after treatment failure of at least 2 non-CGRP first-line therapies. A
› Consider anti-CGRP monoclonal antibodies or gepants for migraine prevention if traditional therapies have proven ineffective or are contraindicated or intolerable to the patient. A
› Add an anti-CGRP monoclonal antibody or gepant to existing preventive treatment if the patient continues to experience migraine. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
An STI upsurge requires a nimble approach to care
Except for a drop in the number of sexually transmitted infections (STIs) early in the COVID-19 pandemic (March and April 2020), the incidence of STIs has been rising throughout this century.1 In 2018, 1 in 5 people in the United States had an STI; 26 million new cases were reported that year, resulting in direct costs of $16 billion—85% of which was for the care of HIV infection.2 Also that year, infection with Chlamydia trachomatis (chlamydia), Trichomonas vaginalis (trichomoniasis), herpesvirus type 2 (genital herpes), and/or human papillomavirus (condylomata acuminata) constituted 97.6% of all prevalent and 93.1% of all incident STIs.3 Almost half (45.5%) of new cases of STIs occur in people between the ages of 15 and 24 years.3
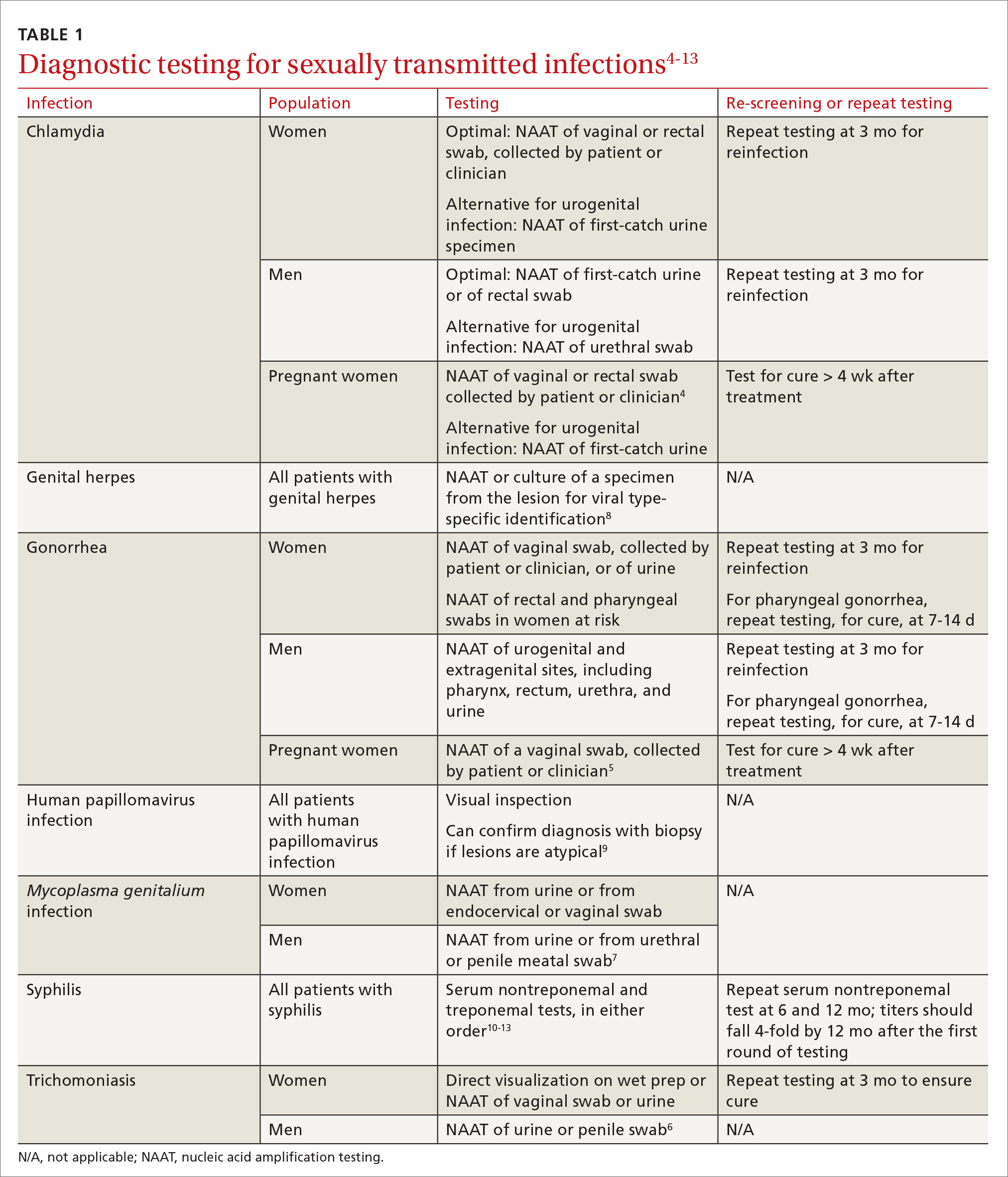
Three factors—changing social patterns, including the increase of social networking; the ability of antiviral therapy to decrease the spread of HIV, leading to a reduction in condom use; and increasing antibiotic resistance—have converged to force changes in screening and treatment recommendations. In this article, we summarize updated guidance for primary care clinicians from several sources—including the Centers for Disease Control and Prevention (CDC), the US Preventive Services Task Force (USPSTF), and the American Society for Colposcopy and Cervical Pathology (ASCCP)—on diagnosing STIs (TABLE 14-13) and providing guideline-based treatment (Table 214). Because of the breadth and complexity of HIV disease, it is not addressed here.
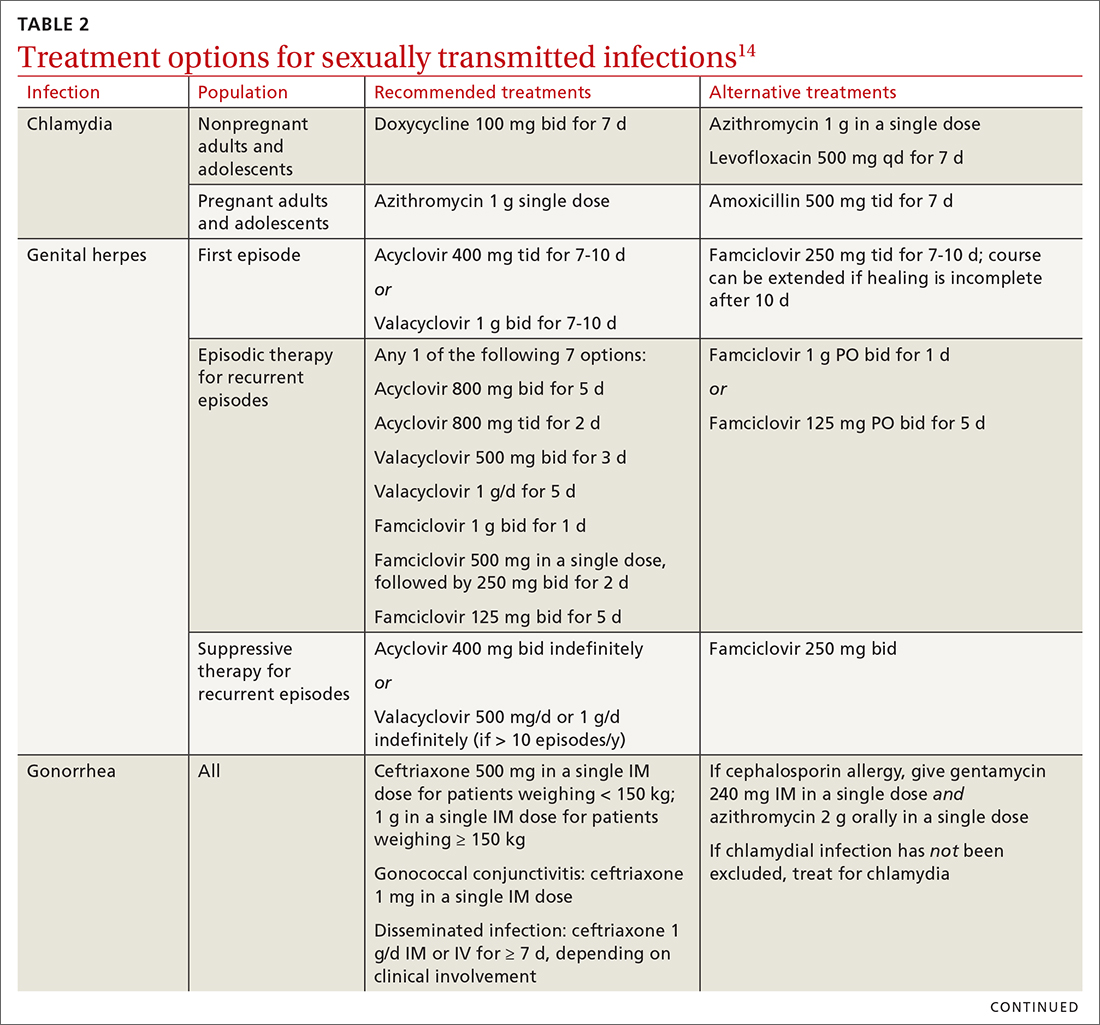
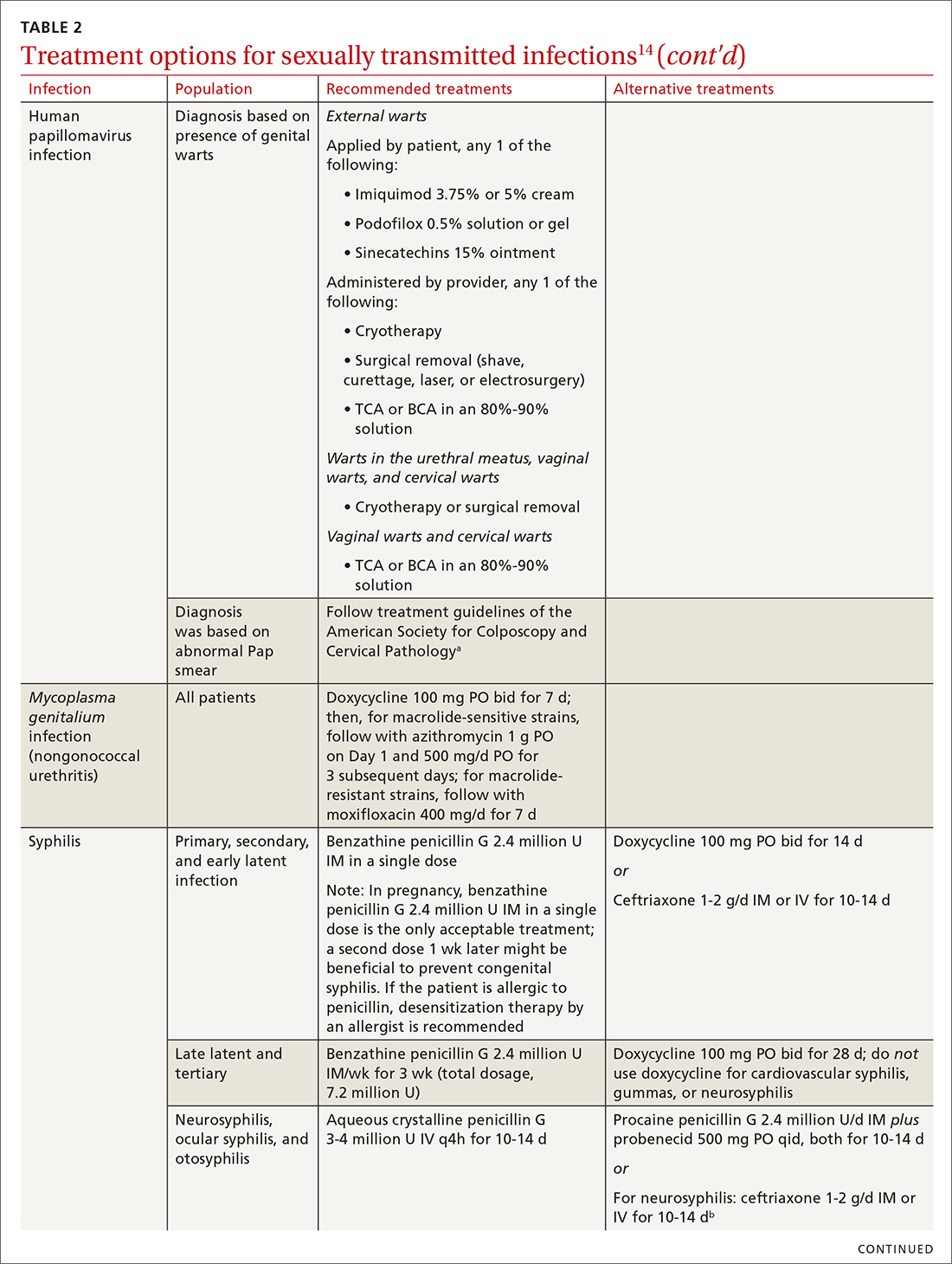
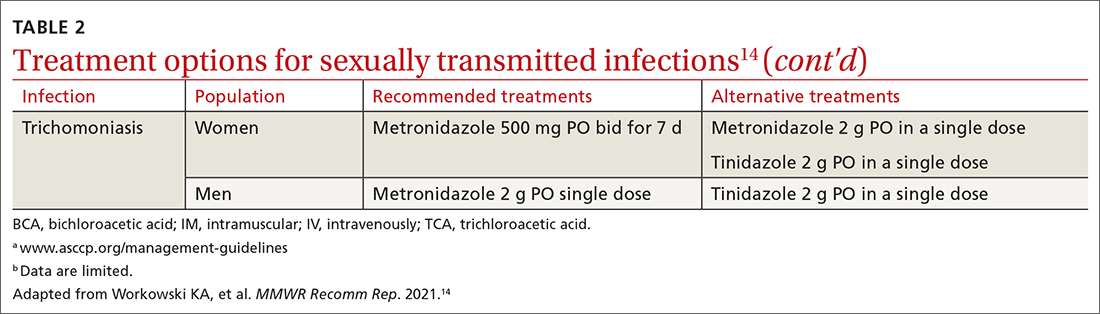
Chlamydia
Infection with Chlamydia trachomatis—the most commonly reported bacterial STI in the United States—primarily causes cervicitis in women and proctitis in men, and can cause urethritis and pharyngitis in men and women. Prevalence is highest in sexually active people younger than 24 years.15
Because most infected people are asymptomatic and show no signs of illness on physical exam, screening is recommended for all sexually active women younger than 25 years and all men who have sex with men (MSM).4 No studies have established proper screening intervals; a reasonable approach, therefore, is to repeat screening for patients who have a sexual history that confers a new or persistent risk for infection since their last negative result.
Depending on the location of the infection, symptoms of chlamydia can include vaginal or penile irritation or discharge, dysuria, pelvic or rectal pain, and sore throat. Breakthrough bleeding in a patient who is taking an oral contraceptive should raise suspicion for chlamydia.
Untreated chlamydia can lead to pelvic inflammatory disease (PID), tubo-ovarian abscess, tubal factor infertility, ectopic pregnancy, and chronic pelvic pain. Infection can be transmitted vertically (mother to baby) antenatally, which can cause ophthalmia neonatorum and pneumonia in these newborns.
Diagnosis. The diagnosis of chlamydia is made using nucleic acid amplification testing (NAAT). Specimens can be collected by the clinician or the patient (self collected) using a vaginal, rectal, or oropharyngeal swab, or a combination of these, and can be obtained from urine or liquid-based cytology material.16
Continue to: Treatment
Treatment. Recommendations for treating chlamydia were updated by the CDC in its 2021 treatment guidelines (Table 214). Doxycycline 100 mg bid for 7 days is the preferred regimen; alternative regiments are (1) azithromycin 1 g in a single dose and (2) levofloxacin 500 mg daily for 7 days.4 A meta-analysis17 and a Cochrane review18 showed that the rate of treatment failure was higher among men when they were treated with azithromycin instead of doxycycline; furthermore, a randomized controlled trial demonstrated that doxycycline is more effective than azithromycin (cure rate, 100%, compared to 74%) at treating rectal chlamydia in MSM.19
Azithromycin is efficacious for urogenital infection in women; however, there is concern that the 33% to 83% of women who have concomitant rectal infection (despite reporting no receptive anorectal sexual activity) would be insufficiently treated. Outside pregnancy, the CDC does not recommend a test of cure but does recommend follow-up testing for reinfection in 3 months. Patients should abstain from sexual activity until 7 days after all sexual partners have been treated.
Expedited partner therapy (EPT) is the practice of treating sexual partners of patients with known chlamydia (and patients with gonococcal infection). Unless prohibited by law in your state, offer EPT to patients with chlamydia if they cannot ensure that their sexual partners from the past 60 days will seek timely treatment.a
Evidence to support EPT comes from 3 US clinical trials, whose subjects comprised heterosexual men and women with chlamydia or gonorrhea.21-23 The role of EPT for MSM is unclear; data are limited. Shared decision-making is recommended to determine whether EPT should be provided, to ensure that co-infection with other bacterial STIs (eg, syphilis) or HIV is not missed.24-26
a Visit www.cdc.gov/std/ept to read updated information about laws and regulations regarding EPT in your state.20
Gonorrhea
Gonorrhea is the second most-reported bacterial communicable disease.5 Infection with Neisseria gonorrhoeae causes urethral discharge in men, leading them to seek treatment; infected women, however, are often asymptomatic. Infected men and women might not recognize symptoms until they have transmitted the disease. Women have a slower natural clearance of gonococcal infection, which might explain their higher prevalence.27 Delayed recognition of symptoms can result in complications, including PID.5
Diagnosis. Specimens for NAAT can be obtained from urine, endocervical, vaginal, rectal, pharyngeal, and male urethral specimens. Reported sexual behaviors and exposures of women and transgender or gender-diverse people should be taken into consideration to determine whether rectal or pharyngeal testing, or both, should be performed.28 MSM should be screened annually at sites of contact, including the urethra, rectum, and pharynx.28 All patients with urogenital or rectal gonorrhea should be asked about oral sexual exposure; if reported, pharyngeal testing should be performed.5
NAAT of urine is at least as sensitive as testing of an endocervical specimen; the same specimen can be used to test for chlamydia and gonorrhea. Patient-collected specimens are a reasonable alternative to clinician-collected swab specimens.29
Continue to: Treatment
Treatment is complicated by the ability of gonorrhea to develop resistance. Intramuscular ceftriaxone 500 mg in a single dose cures 98% to 99% of infections in the United States; however, monitoring local resistance patterns in the community is an important component of treatment.28 (See Table 214 for an alternative regimen for cephalosporin-allergic patients and for treating gonococcal conjunctivitis and disseminated infection.)
In 2007, the CDC identified widespread quinolone-resistant gonococcal strains; therefore, fluoroquinolones no longer are recommended for treating gonorrhea.30 Cefixime has demonstrated only limited success in treating pharyngeal gonorrhea and does not attain a bactericidal level as high as ceftriaxone does; cefixime therefore is recommended only if ceftriaxone is unavailable.28 The national Gonococcal Isolate Surveillance Project is finding emerging evidence of the reduced susceptibility of N gonorrhoeae to azithromycin—making dual therapy for gonococcal infection no longer a recommendation.28
Patients should abstain from sex until 7 days after all sex partners have been treated for gonorrhea. As with chlamydia, the CDC does not recommend a test of cure for uncomplicated urogenital or rectal gonorrhea unless the patient is pregnant, but does recommend testing for reinfection 3 months after treatment.14 For patients with pharyngeal gonorrhea, a test of cure is recommended 7 to 14 days after initial treatment, due to challenges in treatment and because this site of infection is a potential source of antibiotic resistance.28
Trichomoniasis
T vaginalis, the most common nonviral STI worldwide,31 can manifest as a yellow-green vaginal discharge with or without vaginal discomfort, dysuria, epididymitis, and prostatitis; most cases, however, are asymptomatic. On examination, the cervix might be erythematous with punctate lesions (known as strawberry cervix).
Unlike most STIs, trichomoniasis is as common in women older than 24 years as it is in younger women. Infection is associated with a lower educational level, lower socioeconomic status, and having ≥ 2 sexual partners in the past year.32 Prevalence is approximately 10 times as high in Black women as it is in White women.
T vaginalis infection is associated with an increase in the risk for preterm birth, premature rupture of membranes, cervical cancer, and HIV infection. With a lack of high-quality clinical trials on the efficacy of screening, women with HIV are the only group for whom routine screening is recommended.6
Diagnosis. NAAT for trichomoniasis is now available in conjunction with gonorrhea and chlamydia testing of specimens on vaginal or urethral swabs and of urine specimens and liquid Pap smears.
Continue to: Treatment
Treatment. Because of greater efficacy, the treatment recommendation for women has changed from a single 2-g dose of oral metronidazole to 500 mg twice daily for 7 days. The 2-g single oral dose is still recommended for men7 (Table 214 lists alternative regimens).
Mycoplasma genitalium
Infection with M genitalium is common and often asymptomatic. The disease causes approximately 20% of all cases of nongonococcal and nonchlamydial urethritis in men and about 40% of persistent or recurrent infections. M genitalium is present in approximately 20% of women with cervicitis and has been associated with PID, preterm delivery, spontaneous abortion, and infertility.
There are limited and conflicting data regarding outcomes in infected patients other than those with persistent or recurrent infection; furthermore, resistance to azithromycin is increasing rapidly, resulting in an increase in treatment failures. Screening therefore is not recommended, and testing is recommended only in men with nongonococcal urethritis.33,34
Diagnosis. NAAT can be performed on urine or on a urethral, penile meatal, endocervical, or vaginal swab; men with recurrent urethritis or women with recurrent cervicitis should be tested. NAAT also can be considered in women with PID. Testing the specimen for the microorganism’s resistance to macrolide antibiotics is recommended (if such testing is available).
Treatment is initiated with doxycycline 100 mg twice daily for 7 days. If the organism is macrolide sensitive, follow with azithromycin 1 g orally on Day 1, then 500 mg/d for 3 more days. If the organism is macrolide resistant or testing is unavailable, follow doxycycline with oral moxifloxacin 400 mg/d for 7 days.33
Genital herpes (mostly herpesvirus type 2)
Genital herpes, characterized by painful, recurrent outbreaks of genital and anal lesions,35 is a lifelong infection that increases in prevalence with age.8 Because many infected people have disease that is undiagnosed or mild or have unrecognizable symptoms during viral shedding, most genital herpes infections are transmitted by people who are unaware that they are contagious.36 Herpesvirus type 2 (HSV-2) causes most cases of genital herpes, although an increasing percentage of cases are attributed to HSV type 1 (HSV-1) through receptive oral sex from a person who has an oral HSV-1 lesion.
Importantly, HSV-2–infected people are 2 to 3 times more likely to become infected with HIV than people who are not HSV-2 infected.37 This is becauseCD4+ T cells concentrate at the site of HSV lesions and express a higher level of cell-surface receptors that HIV uses to enter cells. HIV replicates 3 to 5 times more quickly in HSV-infected tissue.38
Continue to: HSV can become disseminated...
HSV can become disseminated, particularly in immunosuppressed people, and can manifest as encephalitis, hepatitis, and pneumonitis. Beyond its significant burden on health, HSV carries significant psychosocial consequences.9
Diagnosis. Clinical diagnosis can be challenging if classic lesions are absent at evaluation. If genital lesions are present, HSV can be identified by NAAT or culture of a specimen of those lesions. False-negative antibody results might be more frequent in early stages of infection; repeating antibody testing 12 weeks after presumed time of acquisition might therefore be indicated, based on clinical judgment. HSV-2 antibody positivity implies anogenital infection because almost all HSV-2 infections are sexually acquired.
HSV-1 antibody positivity alone is more difficult to interpret because this finding does not distinguish between oral and genital lesions, and most HSV-1 seropositivity is acquired during childhood.36 HSV polymerase chain reaction (PCR) testing of blood should not be performed to diagnose genital herpes infection, except in settings in which there is concern about disseminated infection.
Treatment. Management should address the acute episode and the chronic nature of genital herpes. Antivirals will not eradicate latent
- attenuate current infection
- prevent recurrence
- improve quality of life
- suppress the virus to prevent transmission to sexual partners.
All patients experiencing an initial episode of genital herpes should be treated, regardless of symptoms, due to the potential for prolonged or severe symptoms during recurrent episodes.9 Three drugs—acyclovir, valacyclovir, and famciclovir—are approved by the US Food and Drug Administration (FDA) to treat genital herpes and appear equally effective (TABLE 214).
Antiviral therapy for recurrent genital HSV infection can be administered either as suppressive therapy to reduce the frequency of recurrences or episodically to shorten the duration of lesions:
- Suppressive therapy reduces the frequency of recurrence by 70% to 80% among patients with frequent outbreaks. Long-term safety and efficacy are well established.
- Episodic therapy is most effective if started within 1 day after onset of lesions or during the prodrome.36
There is no specific recommendation for when to choose suppressive over episodic therapy; most patients prefer suppressive therapy because it improves quality of life. Use shared clinical decision-making to determine the best option for an individual patient.
Continue to: Human papillomavirus
Human papillomavirus
Condylomata acuminata (genital warts) are caused by human papillomavirus (HPV), most commonly types 6 and 11, which manifest as soft papules or plaques on the external genitalia, perineum, perianal skin, and groin. The warts are usually asymptomatic but can be painful or pruritic, depending on size and location.
Diagnosis is made by visual inspection and can be confirmed by biopsy if lesions are atypical. Lesions can resolve spontaneously, remain unchanged, or grow in size or number.
Treatment. The aim of treatment is relief of symptoms and removal of warts. Treatment does not eradicate HPV infection. Multiple treatments are available that can be applied by the patient as a cream, gel, or ointment or administered by the provider, including cryotherapy, surgical removal, and solutions. The decision on how to treat should be based on the number, size, and
HPV-associated cancers and precancers. This is a broad (and separate) topic. HPV types 16 and 18 cause most cases of cervical, penile, vulvar, vaginal, anal, and oropharyngeal cancer and precancer.39 The USPSTF, the American Cancer Society, and the American College of Obstetricians and Gynecologists all have recommendations for cervical cancer screening in the United States.40 Refer to guidelines of the ASCCP for recommendations on abnormal screening tests.41
Prevention of genital warts. The 9-valent HPV vaccine available in the United States is safe and effective and helps protect against viral types 6, 11, 16, 18, 31, 33, 45, 52, and 58. Types 6 and 11 are the principal causes of genital warts. Types 16 and 18 cause 66% of cervical cancer. The vaccination series can be started at age 9 years and is recommended for everyone through age 26 years. Only 2 doses are needed if the first dose is given prior to age 15 years; given after that age, a 3-dose series is utilized. Refer to CDC vaccine guidelines42 for details on the exact timing of vaccination.
Vaccination for women ages 27 to 45 years is not universally recommended because most people have been exposed to HPV by that age. However, the vaccine can still be administered, depending on clinical circumstances and the risk for new infection.42
Syphilis
Caused by the spirochete Treponema pallidum, syphilis manifests across a spectrum—from congenital to tertiary. The inability of medical science to develop a method for culturing the spirochete has confounded diagnosis and treatment.
Continue to: Since reaching a historic...
Since reaching a historic nadir of incidence in 2000 (5979 cases in the United States), there has been an increasingly rapid rise in that number: to 130,000 in 2020. More than 50% of cases are in MSM; however, the number of cases in heterosexual women is rapidly increasing.43
Routine screening for syphilis should be performed in any person who is at risk: all pregnant women in the first trimester (and in the third trimester and at delivery if they are at risk or live in a community where prevalence is high) and annually in sexually active MSM or anyone with HIV infection.10
Diagnosis. Examination by dark-field microscopy, testing by PCR, and direct fluorescent antibody assay for T pallidum from lesion tissue or exudate provide definitive diagnosis for early and congenital syphilis, but are often unavailable.
Presumptive diagnosis requires 2 serologic tests:
- Nontreponemal tests (the VDRL and rapid plasma reagin tests) identify anticardiolipin antibodies released during syphilis infection, although results also can be elevated in autoimmune disease or after certain immunizations, including the COVID-19 vaccine.
- Treponemal tests (the fluorescent treponemal antibody absorbed assay, T pallidum particulate agglutination assay, enzyme immunoassay, and chemiluminescence immunoassay) are specific antibody tests.
Historically, reactive nontreponemal tests, which are less expensive and easier to perform, were followed by a treponemal test to confirm the presumptive diagnosis. This method continues to be reasonable when screening patients in a low-prevalence population.11 The reverse sequence screening algorithm (ie, begin with a treponemal test) is now frequently used. With this method, a positive treponemal test must be confirmed with a nontreponemal test. If the treponemal test is positive and the nontreponemal test is negative, another treponemal test must be positive to confirm the diagnosis. This algorithm is useful in high-risk populations because it provides earlier detection of recently acquired syphilis and enhanced detection of late latent syphilis.12,13,44 The CDC has not stated a diagnostic preference.
Once the diagnosis is made, a complete history (including a sexual history and a history of syphilis testing and treatment) and a physical exam are necessary to confirm stage of disease.45
Special circumstances. Neurosyphilis, ocular syphilis, and otosyphilis refer to the site of infection and can occur at any stage of disease. The nervous system usually is infected within hours of initial infection, but symptoms might take weeks or years to develop—or might never manifest. Any time a patient develops neurologic, ophthalmologic, or audiologic symptoms, careful neurologic and ophthalmologic evaluation should be performed and the patient should be tested for HIV.
Continue to: Lumbar puncture is warranted...
Lumbar puncture is warranted for evaluation of cerebrospinal fluid if neurologic symptoms are present but is not necessary for isolated ocular syphilis or otosyphilis without neurologic findings. Treatment should not be delayed for test results if ocular syphilis is suspected because permanent blindness can develop. Any patient at high risk for an STI who presents with neurologic or ophthalmologic symptoms should be tested for syphilis and HIV.45
Pregnant women who have a diagnosis of syphilis should be treated with penicillin immediately because treatment ≥ 30 days prior to delivery is likely to prevent most cases of congenital syphilis. However, a course of penicillin might not prevent stillbirth or congenital syphilis in a gravely infected fetus, evidenced by fetal syphilis on a sonogram at the time of treatment. Additional doses of penicillin in pregnant women with early syphilis might be indicated if there is evidence of fetal syphilis on ultrasonography. All women who deliver a stillborn infant (≥ 20 weeks’ gestation) should be tested for syphilis at delivery.46
All patients in whom primary or secondary syphilis has been diagnosed should be tested for HIV at the time of diagnosis and treatment; if the result is negative, they should be offered preexposure prophylaxis (PrEP; discussed shortly). If the incidence of HIV in your community is high, repeat testing for HIV in 3 months. Clinical and serologic evaluation should be performed 6 and 12 months after treatment.47
Treatment. Penicillin remains the standard treatment for syphilis. Primary, secondary, and early tertiary stages (including in pregnancy) are treated with benzathine penicillin G 2.4 million units intramuscular (IM) in a single dose. For pregnant patients, repeating that dose in 1 week generally is recommended. Patients in the late latent (> 1 year) or tertiary stage receive the same dose of penicillin, which is then repeated weekly, for a total of 3 doses. Doxycycline and ceftriaxone are alternatives, except in pregnancy.
Warn patients of the Jarisch-Herxheimer reaction: fever, headache, and myalgias associated with initiation of treatment in the presence of the high bacterial load seen in early syphilis. Treatment is symptomatic, but the Jarisch-Herxheimer reaction can cause fetal distress in pregnancy.
Otosyphilis, ocular syphilis, and neurosyphilis require intravenous (IV) aqueous crystalline penicillin G 3 to 4 million U every 4 hours for 10 to 14 days.45 Alternatively, procaine penicillin G 2.4 million U/d IM can be given daily with oral probenecid 500 mg qid, both for 10 to 14 days (TABLE 214).
Screening andprevention of STIs
Screening recommendations
Follow USPSTF screening guidelines for STIs.10,48-54 Screen annually for:
- gonorrhea and chlamydia in women ages 15 to 24 years and in women older than 25 years if they are at increased risk
- gonorrhea, chlamydia, syphilis, and HIV in MSM, and hepatitis C if they are HIV positive
- trichomoniasis in women who are HIV positive.
Continue to: Consider the community in which...
Consider the community in which you practice when determining risk; you might want to consult local public health authorities for information about local epidemiology and guidance on determining which of your patients are at increased risk.
Preexposure prophylaxis
According to the CDC, all sexually active adults and adolescents should be informed about the availability of PrEP to prevent HIV infection. PrEP should be (1) available to anyone who requests it and (2) recommended for anyone who is sexually active and who practices sexual behaviors that place them at substantial risk for exposure to or acquisition of HIV, or both.
The recommended treatment protocol for men and women who have either an HIV-positive partner or inconsistent condom use or who have had a bacterial STI in the previous 6 months is oral emtricitabine 200 mg plus tenofovir disoproxil fumarate 300 mg/d (sold as Truvada-F/TDF). Men and transgender women (ie, assigned male at birth) with at-risk behaviors also can use emtricitabine plus tenofovir alafenamide 25 mg/d (sold as Descovy-F/TAF).
In addition, cabotegravir plus rilpirivine (sold as Cabenuva), IM every 2 months, was approved by the FDA for PrEP in 2021.
Creatinine clearance should be assessed at baseline and yearly (every 6 months for those older than 50 years) in patients taking PrEP. All patients must be tested for HIV at initiation of treatment and every 3 months thereafter (every 4 months for cabotegravir plus rilpirivine). Patients should be screened for bacterial STIs every 6 months (every 3 months for MSM and transgender women); screening for chlamydia should be done yearly. For patients being treated with emtricitabine plus tenofovir alafenamide, weight and a lipid profile (cholesterol and triglycerides) should be assessed annually.55
Postexposure prophylaxis
The sharp rise in the incidence of STIs in the past few years has brought renewed interest in postexposure prophylaxis (PEP) for STIs. Although PEP should be standard in cases of sexual assault, this protocol also can be considered in other instances of high-risk exposure.
CDC recommendations for PEP in cases of assault are56:
- ceftriaxone 500 mg IM in a single dose (1 g if weight is ≥ 150 kg) plus
- doxycycline 100 mg bid for 7 days plus
- metronidazole 2 g bid for 7 days (for vaginal exposure)
- pregnancy evaluation and emergency contraception
- hepatitis B risk evaluation and vaccination, with or without hepatitis B immune globulin
- HIV risk evaluation, based on CDC guidelines, and possible HIV prophylaxis (PrEP)
- HPV vaccination for patients ages 9 to 26 years if they are not already fully vaccinated.
CORRESPONDENCE
Belinda Vail, MD, 3901 Rainbow Boulevard, Mail Stop 4010, Kansas City, KS 66160; bvail@kumc.edu
1. Pagaoa M, Grey J, Torrone E, et al. Trends in nationally notifiable sexually transmitted disease case reports during the US COVID-19 pandemic, January to December 2020. Sex Transm Dis. 2021;48:798-804. doi: 10.1097/OLQ.0000000000001506
2. Chesson HW, Spicknall IH; Bingham A, et al. The estimated direct lifetime medical costs of sexually transmitted infections acquired in the United States in 2018. Sex Transm Dis. 2021;48:215-221. doi: 10.1097/OLQ.0000000000001380
3. Kreisel KM, Spicknall IH, Gargano JW, et al. Sexually transmitted infections among US women and men: prevalence and incidence estimates, 2018. Sex Transm Dis. 2021;48:208-214. doi: 10.1097/OLQ.0000000000001355
4. CDC. Sexually transmitted infections treatment guidelines, 2021: Chlamydial infections among adolescents and adults. US Department of Health and Human Services. July 21, 2021. Accessed April 19, 2023. www.cdc.gov/std/treatment-guidelines/chlamydia.htm
5. CDC. Sexually transmitted infections treatment guidelines, 2021: Gonococcal infections among adolescents and adults. US Department of Health and Human Services. September 21, 2022. Accessed April 23, 2023. www.cdc.gov/std/treatment-guidelines/gonorrhea-adults.htm
6. Van Gerwen OT, Muzny CA. Recent advances in the epidemiology, diagnosis, and management of Trichomonas vaginalis infection. F1000Res. 2019;
7. CDC. Sexually transmitted infections treatment guidelines, 2021. Trichomoniasis. US Department of Health and Human Services. September 21, 2022. Accessed April 23, 2023. December 27, 2021. www.cdc.gov/std/treatment-guidelines/trichomoniasis.htm
8. Spicknall IH, Flagg EW, Torrone EA. Estimates of the prevalence and incidence of genital herpes, United States, 2018. Sex Transm Dis. 2021;48:260-265. doi: 10.1097/OLQ.0000000000001375
9. Mark H, Gilbert L, Nanda J. Psychosocial well-being and quality of life among women newly diagnosed with genital herpes. J Obstet Gynecol Neonatal Nurs.
10. Bibbins-Domingo K, Grossman DC, Curry SJ, et al; US Preventive Services Task Force. Screening for syphilis infection in nonpregnant adults and adolescents: US Preventive Services Task Force recommendation statement. JAMA. 2016;315:2321-2327. doi: 10.1001/jama.2016.5824
11. Ricco J, Westby A. Syphilis: far from ancient history. Am Fam Physician. 2020;102:91-98.
12. Goza M, Kulwicki B, Akers JM, et al. Syphilis screening: a review of the Syphilis Health Check rapid immunochromatographic test. J Pharm Technol. 2017;33:53-59. doi:10.1177/8755122517691308
13. Henao- AF, Johnson SC. Diagnostic tests for syphilis: new tests and new algorithms. Neurol Clin Pract. 2014;4:114-122. doi: 10.1212/01.CPJ.0000435752.17621.48
14. Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep. 2021;70:1-187. doi:10.15585/mmwr.rr7004a1
15. CDC. Sexually Transmitted Disease Surveillance 2021. National overview of STDs. US Department of Health and Human Services. April 2023. Accessed May 9, 2023. www.cdc.gov/std/statistics/2021/overview.htm#Chlamydia
16. CDC. Recommendations for the laboratory-based detection of Chlamydia trachomatis and Neisseria gonorrhoeae—2014. MMWR Recomm Rep. 2014;63:1-19.
17. Kong FYS, Tabrizi SN, Law M, et al. Azithromycin versus doxycycline for the treatment of genital chlamydia infection: a meta-analysis of randomized controlled trials. Clin Infect Dis. 2014;59:193-205. doi: 10.1093/cid/ciu220
18. Páez-Canro C, Alzate JP, González LM, et al. Antibiotics for treating urogenital Chlamydia trachomatis infection in men and non-pregnant women. Cochrane Database Syst Rev. 2019;1:CD010871. doi: 10.1002/14651858.CD010871.pub2
19. Dombrowski JC, Wierzbicki MR, Newman LM, et al. Doxycycline versus azithromycin for the treatment of rectal chlamydia in men who have sex with men: a randomized controlled trial. Clin Infect Dis. 2021;73:824-831. doi: 10.1093/cid/ciab153
20. CDC. Sexually transmitted infections treatment guidelines, 2021: Expedited partner therapy. US Department of Health and Human Services. July 22, 2021. Accessed April 19, 2023. www.cdc.gov/std/treatment-guidelines/clinical-EPT.htm
21. Golden MR, Whittington WLH, Handsfield HH, et al. Effect of expedited treatment of sex partners on recurrent or persistent gonorrhea or chlamydial infection. N Engl J Med. 2005;352:676-685. doi: 10.1056/NEJMoa041681
22. Schillinger JA, Kissinger P, Calvet H, et al. Patient-delivered partner treatment with azithromycin to prevent repeated Chlamydia trachomatis infection among women: a randomized, controlled trial. Sex Transm Dis. 2003;30:49-56. doi: 10.1097/00007435-200301000-00011
23. Cameron ST, Glasier A, Scott G, et al. Novel interventions to reduce re-infection in women with chlamydia: a randomized controlled trial. Hum Reprod. 2009;24:888-895. doi: 10.1093/humrep/den475
24. McNulty A, Teh MF, Freedman E. Patient delivered partner therapy for chlamydial infection—what would be missed? Sex Transm Dis. 2008;35:834-836. doi: 10.1097/OLQ.0b013e3181761993
25. Stekler J, Bachmann L, Brotman RM, et al. Concurrent sexually transmitted infections (STIs) in sex partners of patients with selected STIs: implications for patient-delivered partner therapy. Clin Infect Dis. 2005;40:787-793. doi: 10.1086/428043
26. Schillinger JA, Kissinger P, Calvet H, et al. Patient-delivered partner treatment with azithromycin to prevent repeated Chlamydia trachomatis infection among women: a randomized, controlled trial. Sex Transm Dis. 2003;30:49-56. doi: 10.1097/00007435-200301000-00011
27. Stupiansky NW, Van der Pol B, Williams JA, et al. The natural history of incident gonococcal infection in adolescent women. Sex Transm Dis. 2011;38:750-754. doi: 10.1097/OLQ.0b013e31820ff9a4
28. CDC. Sexually transmitted infections treatment guidelines, 2021: Screening recommendations and considerations referenced in treatment guidelines and original sources. US Department of Health and Human Services. June 6, 2022. Accessed May 9, 2023. www.cdc.gov/std/treatment-guidelines/screening-recommen dations.htm
29. Cantor A, Dana T, Griffen JC, et al. Screening for chlamydial and gonococcal infections: a systematic review update for the US Preventive Services Task Force. Evidence Synthesis No. 206. AHRQ Report No. 21-05275-EF-1. Agency for Healthcare Research and Quality. September 2021. www.ncbi.nlm.nih.gov/books/NBK574045
30. CDC. Update to CDC’s sexually transmitted diseases treatment guidelines, 2006: fluoroquinolones no longer recommended for treatment of gonococcal infections. MMWR Morb Mortal Wkly Rep. 2007;56:332-336.
31. Rowley J, Vander Hoorn S, Korenromp E, et al. Chlamydia, gonorrhoea, trichomoniasis and syphilis: global prevalence and incidence estimates, 2016. Bull World Health Organ. 2019:97:548-562P. doi: 10.2471/BLT.18.228486
32. Patel EU, Gaydos CA, Packman ZR, et al. Prevalence and correlates of Trichomonas vaginalis infection among men and women in the United States. Clin Infect Dis. 2018;67:211-217. doi: 10.1093/cid/ciy079
33. CDC. Sexually transmitted infections treatment guidelines, 2021. Mycoplasma genitalium. US Department of Health and Human Services. July 22, 2021. Accessed April 23, 2023. www.cdc.gov/std/treatment-guidelines/mycoplasmagenitalium.htm
34. Manhart LE, Broad JM, Bolden MR. Mycoplasma genitalium: should we treat and how? Clin Infect Dis. 2011;53(suppl 3):S129-S142. doi:10.1093/cid/cir702.
35. Corey L, Wald A. Genital herpes. In: Holmes KK, Sparling PF, Stamm WE, et al, eds. Sexually Transmitted Diseases. 4th ed. McGraw-Hill; 2008:399-437.
36. CDC. Sexually transmitted infections treatment guidelines, 2021: Genital herpes. US Department of Health and Human Services. September 21, 2022. Accessed April 23, 2023. www.cdc.gov/std/treatment-guidelines/herpes.htm
37. Looker KJ, Elmes JAR, Gottlieb SL, et al. Effect of HSV-2 infection on subsequent HIV acquisition: an updated systematic review and meta-analysis. Lancet Infect Dis. 2017;17:1303-1316. doi: 10.1016/S1473-3099(17)30405-X
38. Rollenhagen C, Lathrop M, Macura SL, et al. Herpes simplex virus type-2 stimulates HIV-1 replication in cervical tissues: implications for HIV-1 transmission and efficacy of anti-HIV-1 microbicides. Mucosal Immunol. 2014;7:1165-1174. doi: 10.1038/mi.2014.3
39. Cogliano V, Baan R, Straif K, et al; WHO International Agency for Research on Cancer. Carcinogenicity of human papillomaviruses. Lancet Oncol.
40. Simon MA, Tseng CW, Wong JB. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686. doi:10.1001/jama.2018.10897
41. Perkins RB, Guido RS, Castle PE, et al; . 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2020;24:102-131. doi: 10.1097/LGT.0000000000000525
42. Meites E, Szilagyi PG, Chesson HW, et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68:698-702. doi: 10.15585/mmwr.mm6832a3
43. Schmidt R, Carson PJ, Jansen RJ. Resurgence of syphilis in the United States: an assessment of contributing factors. Infect Dis (Auckl). 2019;12:1178633719883282. doi: 10.1177/1178633719883282
44. Boog GHP, Lopes JVZ, Mahler JV, et al. Diagnostic tools for neurosyphilis: a systematic review. BMC Infect Dis. 2021;21:568. doi: 10.1186/s12879-021-06264-8
45. CDC. Sexually transmitted infections treatment guidelines, 2021. Syphilis. US Department of Health and Human Services. April 20, 2023. Accessed April 24, 2023. www.cdc.gov/std/treatment-guidelines/syphilis.htm
46. Matthias JM, Rahman MM, Newman DR, et al. Effectiveness of prenatal screening and treatment to prevent congenital syphilis, Louisiana and Florida, 2013-2014. Sex Transm Dis. 2017;44:498-502. doi: 10.1097/OLQ.0000000000000638
47. Clement ME, Okeke NL, Hicks CB. Treatment of syphilis: a systematic review. JAMA. 2014;312:1905-1917. doi: 10.1001/jama.2014.13259
48. Davidson KW, Barry MJ, Mangione CM, et al; US Preventive Services Task Force. Screening for chlamydia and gonorrhea: US Preventive Services Task Force recommendation statement. JAMA. 2021;326:949-956. doi: 10.1001/jama.2021.14081
49. Krist AH, Davidson KW, Mangione CM, et al; US Preventive Services Task Force. Screening for hepatitis B virus infection in adolescents and adults: US Preventive Services Task Force recommendation statement. JAMA. 2020;324:2415-2422. doi: 10.1001/jama.2020.22980
50. Owens DK, Davidson KW, Krist AH, et al; US Preventive Services Task Force. Screening for hepatitis C virus infection in adolescents and adults: US Preventive Services Task Force recommendation statement. JAMA. 2020;323:970-975. doi: 10.1001/jama.2020.1123
51. Bibbins-Domingo K, Grossman DC, Curry SJ, et al; US Preventive Services Task Force. Serologic screening for genital herpes infection: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:2525-2530. doi: 10.1001/jama.2016.16776
52. Curry SJ, Krist AH, Owens DK, et al; US Preventive Services Task Force. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686. doi: 10.1001/jama.2018.10897
53. Owens DK, Davidson KW, Krist AH, et al; US Preventive Services Task Force. Screening for HIV infection: US Preventive Services Task Force recommendation statement. JAMA. 2019;321:2326-2336. doi: 10.1001/jama.2019.6587
54. Farley TA, Cohen DA, Elkins W. Asymptomatic sexually transmitted diseases: the case for screening. Prev Med. 2003;36:502-509. doi: 10.1016/s0091-7435(02)00058-0
55. US Public Health Service. Preexposure prophylaxis for the prevention of HIV infection in the United States—2021 update. A clinical practice guideline. Centers for Disease Control and Prevention. Accessed April 24, 2023. www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2021.pdf
56. CDC. Sexually transmitted infections treatment guidelines, 2021: Sexual assault and abuse and STIs—adolescents and adults, 2021. US Department of Health and Human Services. July 22, 2021. Accessed April 24, 2023. www.cdc.gov/std/treatment-guidelines/sexual-assault-adults.htm
Except for a drop in the number of sexually transmitted infections (STIs) early in the COVID-19 pandemic (March and April 2020), the incidence of STIs has been rising throughout this century.1 In 2018, 1 in 5 people in the United States had an STI; 26 million new cases were reported that year, resulting in direct costs of $16 billion—85% of which was for the care of HIV infection.2 Also that year, infection with Chlamydia trachomatis (chlamydia), Trichomonas vaginalis (trichomoniasis), herpesvirus type 2 (genital herpes), and/or human papillomavirus (condylomata acuminata) constituted 97.6% of all prevalent and 93.1% of all incident STIs.3 Almost half (45.5%) of new cases of STIs occur in people between the ages of 15 and 24 years.3

Three factors—changing social patterns, including the increase of social networking; the ability of antiviral therapy to decrease the spread of HIV, leading to a reduction in condom use; and increasing antibiotic resistance—have converged to force changes in screening and treatment recommendations. In this article, we summarize updated guidance for primary care clinicians from several sources—including the Centers for Disease Control and Prevention (CDC), the US Preventive Services Task Force (USPSTF), and the American Society for Colposcopy and Cervical Pathology (ASCCP)—on diagnosing STIs (TABLE 14-13) and providing guideline-based treatment (Table 214). Because of the breadth and complexity of HIV disease, it is not addressed here.



Chlamydia
Infection with Chlamydia trachomatis—the most commonly reported bacterial STI in the United States—primarily causes cervicitis in women and proctitis in men, and can cause urethritis and pharyngitis in men and women. Prevalence is highest in sexually active people younger than 24 years.15
Because most infected people are asymptomatic and show no signs of illness on physical exam, screening is recommended for all sexually active women younger than 25 years and all men who have sex with men (MSM).4 No studies have established proper screening intervals; a reasonable approach, therefore, is to repeat screening for patients who have a sexual history that confers a new or persistent risk for infection since their last negative result.
Depending on the location of the infection, symptoms of chlamydia can include vaginal or penile irritation or discharge, dysuria, pelvic or rectal pain, and sore throat. Breakthrough bleeding in a patient who is taking an oral contraceptive should raise suspicion for chlamydia.
Untreated chlamydia can lead to pelvic inflammatory disease (PID), tubo-ovarian abscess, tubal factor infertility, ectopic pregnancy, and chronic pelvic pain. Infection can be transmitted vertically (mother to baby) antenatally, which can cause ophthalmia neonatorum and pneumonia in these newborns.
Diagnosis. The diagnosis of chlamydia is made using nucleic acid amplification testing (NAAT). Specimens can be collected by the clinician or the patient (self collected) using a vaginal, rectal, or oropharyngeal swab, or a combination of these, and can be obtained from urine or liquid-based cytology material.16
Continue to: Treatment
Treatment. Recommendations for treating chlamydia were updated by the CDC in its 2021 treatment guidelines (Table 214). Doxycycline 100 mg bid for 7 days is the preferred regimen; alternative regiments are (1) azithromycin 1 g in a single dose and (2) levofloxacin 500 mg daily for 7 days.4 A meta-analysis17 and a Cochrane review18 showed that the rate of treatment failure was higher among men when they were treated with azithromycin instead of doxycycline; furthermore, a randomized controlled trial demonstrated that doxycycline is more effective than azithromycin (cure rate, 100%, compared to 74%) at treating rectal chlamydia in MSM.19
Azithromycin is efficacious for urogenital infection in women; however, there is concern that the 33% to 83% of women who have concomitant rectal infection (despite reporting no receptive anorectal sexual activity) would be insufficiently treated. Outside pregnancy, the CDC does not recommend a test of cure but does recommend follow-up testing for reinfection in 3 months. Patients should abstain from sexual activity until 7 days after all sexual partners have been treated.
Expedited partner therapy (EPT) is the practice of treating sexual partners of patients with known chlamydia (and patients with gonococcal infection). Unless prohibited by law in your state, offer EPT to patients with chlamydia if they cannot ensure that their sexual partners from the past 60 days will seek timely treatment.a
Evidence to support EPT comes from 3 US clinical trials, whose subjects comprised heterosexual men and women with chlamydia or gonorrhea.21-23 The role of EPT for MSM is unclear; data are limited. Shared decision-making is recommended to determine whether EPT should be provided, to ensure that co-infection with other bacterial STIs (eg, syphilis) or HIV is not missed.24-26
a Visit www.cdc.gov/std/ept to read updated information about laws and regulations regarding EPT in your state.20
Gonorrhea
Gonorrhea is the second most-reported bacterial communicable disease.5 Infection with Neisseria gonorrhoeae causes urethral discharge in men, leading them to seek treatment; infected women, however, are often asymptomatic. Infected men and women might not recognize symptoms until they have transmitted the disease. Women have a slower natural clearance of gonococcal infection, which might explain their higher prevalence.27 Delayed recognition of symptoms can result in complications, including PID.5
Diagnosis. Specimens for NAAT can be obtained from urine, endocervical, vaginal, rectal, pharyngeal, and male urethral specimens. Reported sexual behaviors and exposures of women and transgender or gender-diverse people should be taken into consideration to determine whether rectal or pharyngeal testing, or both, should be performed.28 MSM should be screened annually at sites of contact, including the urethra, rectum, and pharynx.28 All patients with urogenital or rectal gonorrhea should be asked about oral sexual exposure; if reported, pharyngeal testing should be performed.5
NAAT of urine is at least as sensitive as testing of an endocervical specimen; the same specimen can be used to test for chlamydia and gonorrhea. Patient-collected specimens are a reasonable alternative to clinician-collected swab specimens.29
Continue to: Treatment
Treatment is complicated by the ability of gonorrhea to develop resistance. Intramuscular ceftriaxone 500 mg in a single dose cures 98% to 99% of infections in the United States; however, monitoring local resistance patterns in the community is an important component of treatment.28 (See Table 214 for an alternative regimen for cephalosporin-allergic patients and for treating gonococcal conjunctivitis and disseminated infection.)
In 2007, the CDC identified widespread quinolone-resistant gonococcal strains; therefore, fluoroquinolones no longer are recommended for treating gonorrhea.30 Cefixime has demonstrated only limited success in treating pharyngeal gonorrhea and does not attain a bactericidal level as high as ceftriaxone does; cefixime therefore is recommended only if ceftriaxone is unavailable.28 The national Gonococcal Isolate Surveillance Project is finding emerging evidence of the reduced susceptibility of N gonorrhoeae to azithromycin—making dual therapy for gonococcal infection no longer a recommendation.28
Patients should abstain from sex until 7 days after all sex partners have been treated for gonorrhea. As with chlamydia, the CDC does not recommend a test of cure for uncomplicated urogenital or rectal gonorrhea unless the patient is pregnant, but does recommend testing for reinfection 3 months after treatment.14 For patients with pharyngeal gonorrhea, a test of cure is recommended 7 to 14 days after initial treatment, due to challenges in treatment and because this site of infection is a potential source of antibiotic resistance.28
Trichomoniasis
T vaginalis, the most common nonviral STI worldwide,31 can manifest as a yellow-green vaginal discharge with or without vaginal discomfort, dysuria, epididymitis, and prostatitis; most cases, however, are asymptomatic. On examination, the cervix might be erythematous with punctate lesions (known as strawberry cervix).
Unlike most STIs, trichomoniasis is as common in women older than 24 years as it is in younger women. Infection is associated with a lower educational level, lower socioeconomic status, and having ≥ 2 sexual partners in the past year.32 Prevalence is approximately 10 times as high in Black women as it is in White women.
T vaginalis infection is associated with an increase in the risk for preterm birth, premature rupture of membranes, cervical cancer, and HIV infection. With a lack of high-quality clinical trials on the efficacy of screening, women with HIV are the only group for whom routine screening is recommended.6
Diagnosis. NAAT for trichomoniasis is now available in conjunction with gonorrhea and chlamydia testing of specimens on vaginal or urethral swabs and of urine specimens and liquid Pap smears.
Continue to: Treatment
Treatment. Because of greater efficacy, the treatment recommendation for women has changed from a single 2-g dose of oral metronidazole to 500 mg twice daily for 7 days. The 2-g single oral dose is still recommended for men7 (Table 214 lists alternative regimens).
Mycoplasma genitalium
Infection with M genitalium is common and often asymptomatic. The disease causes approximately 20% of all cases of nongonococcal and nonchlamydial urethritis in men and about 40% of persistent or recurrent infections. M genitalium is present in approximately 20% of women with cervicitis and has been associated with PID, preterm delivery, spontaneous abortion, and infertility.
There are limited and conflicting data regarding outcomes in infected patients other than those with persistent or recurrent infection; furthermore, resistance to azithromycin is increasing rapidly, resulting in an increase in treatment failures. Screening therefore is not recommended, and testing is recommended only in men with nongonococcal urethritis.33,34
Diagnosis. NAAT can be performed on urine or on a urethral, penile meatal, endocervical, or vaginal swab; men with recurrent urethritis or women with recurrent cervicitis should be tested. NAAT also can be considered in women with PID. Testing the specimen for the microorganism’s resistance to macrolide antibiotics is recommended (if such testing is available).
Treatment is initiated with doxycycline 100 mg twice daily for 7 days. If the organism is macrolide sensitive, follow with azithromycin 1 g orally on Day 1, then 500 mg/d for 3 more days. If the organism is macrolide resistant or testing is unavailable, follow doxycycline with oral moxifloxacin 400 mg/d for 7 days.33
Genital herpes (mostly herpesvirus type 2)
Genital herpes, characterized by painful, recurrent outbreaks of genital and anal lesions,35 is a lifelong infection that increases in prevalence with age.8 Because many infected people have disease that is undiagnosed or mild or have unrecognizable symptoms during viral shedding, most genital herpes infections are transmitted by people who are unaware that they are contagious.36 Herpesvirus type 2 (HSV-2) causes most cases of genital herpes, although an increasing percentage of cases are attributed to HSV type 1 (HSV-1) through receptive oral sex from a person who has an oral HSV-1 lesion.
Importantly, HSV-2–infected people are 2 to 3 times more likely to become infected with HIV than people who are not HSV-2 infected.37 This is becauseCD4+ T cells concentrate at the site of HSV lesions and express a higher level of cell-surface receptors that HIV uses to enter cells. HIV replicates 3 to 5 times more quickly in HSV-infected tissue.38
Continue to: HSV can become disseminated...
HSV can become disseminated, particularly in immunosuppressed people, and can manifest as encephalitis, hepatitis, and pneumonitis. Beyond its significant burden on health, HSV carries significant psychosocial consequences.9
Diagnosis. Clinical diagnosis can be challenging if classic lesions are absent at evaluation. If genital lesions are present, HSV can be identified by NAAT or culture of a specimen of those lesions. False-negative antibody results might be more frequent in early stages of infection; repeating antibody testing 12 weeks after presumed time of acquisition might therefore be indicated, based on clinical judgment. HSV-2 antibody positivity implies anogenital infection because almost all HSV-2 infections are sexually acquired.
HSV-1 antibody positivity alone is more difficult to interpret because this finding does not distinguish between oral and genital lesions, and most HSV-1 seropositivity is acquired during childhood.36 HSV polymerase chain reaction (PCR) testing of blood should not be performed to diagnose genital herpes infection, except in settings in which there is concern about disseminated infection.
Treatment. Management should address the acute episode and the chronic nature of genital herpes. Antivirals will not eradicate latent
- attenuate current infection
- prevent recurrence
- improve quality of life
- suppress the virus to prevent transmission to sexual partners.
All patients experiencing an initial episode of genital herpes should be treated, regardless of symptoms, due to the potential for prolonged or severe symptoms during recurrent episodes.9 Three drugs—acyclovir, valacyclovir, and famciclovir—are approved by the US Food and Drug Administration (FDA) to treat genital herpes and appear equally effective (TABLE 214).
Antiviral therapy for recurrent genital HSV infection can be administered either as suppressive therapy to reduce the frequency of recurrences or episodically to shorten the duration of lesions:
- Suppressive therapy reduces the frequency of recurrence by 70% to 80% among patients with frequent outbreaks. Long-term safety and efficacy are well established.
- Episodic therapy is most effective if started within 1 day after onset of lesions or during the prodrome.36
There is no specific recommendation for when to choose suppressive over episodic therapy; most patients prefer suppressive therapy because it improves quality of life. Use shared clinical decision-making to determine the best option for an individual patient.
Continue to: Human papillomavirus
Human papillomavirus
Condylomata acuminata (genital warts) are caused by human papillomavirus (HPV), most commonly types 6 and 11, which manifest as soft papules or plaques on the external genitalia, perineum, perianal skin, and groin. The warts are usually asymptomatic but can be painful or pruritic, depending on size and location.
Diagnosis is made by visual inspection and can be confirmed by biopsy if lesions are atypical. Lesions can resolve spontaneously, remain unchanged, or grow in size or number.
Treatment. The aim of treatment is relief of symptoms and removal of warts. Treatment does not eradicate HPV infection. Multiple treatments are available that can be applied by the patient as a cream, gel, or ointment or administered by the provider, including cryotherapy, surgical removal, and solutions. The decision on how to treat should be based on the number, size, and
HPV-associated cancers and precancers. This is a broad (and separate) topic. HPV types 16 and 18 cause most cases of cervical, penile, vulvar, vaginal, anal, and oropharyngeal cancer and precancer.39 The USPSTF, the American Cancer Society, and the American College of Obstetricians and Gynecologists all have recommendations for cervical cancer screening in the United States.40 Refer to guidelines of the ASCCP for recommendations on abnormal screening tests.41
Prevention of genital warts. The 9-valent HPV vaccine available in the United States is safe and effective and helps protect against viral types 6, 11, 16, 18, 31, 33, 45, 52, and 58. Types 6 and 11 are the principal causes of genital warts. Types 16 and 18 cause 66% of cervical cancer. The vaccination series can be started at age 9 years and is recommended for everyone through age 26 years. Only 2 doses are needed if the first dose is given prior to age 15 years; given after that age, a 3-dose series is utilized. Refer to CDC vaccine guidelines42 for details on the exact timing of vaccination.
Vaccination for women ages 27 to 45 years is not universally recommended because most people have been exposed to HPV by that age. However, the vaccine can still be administered, depending on clinical circumstances and the risk for new infection.42
Syphilis
Caused by the spirochete Treponema pallidum, syphilis manifests across a spectrum—from congenital to tertiary. The inability of medical science to develop a method for culturing the spirochete has confounded diagnosis and treatment.
Continue to: Since reaching a historic...
Since reaching a historic nadir of incidence in 2000 (5979 cases in the United States), there has been an increasingly rapid rise in that number: to 130,000 in 2020. More than 50% of cases are in MSM; however, the number of cases in heterosexual women is rapidly increasing.43
Routine screening for syphilis should be performed in any person who is at risk: all pregnant women in the first trimester (and in the third trimester and at delivery if they are at risk or live in a community where prevalence is high) and annually in sexually active MSM or anyone with HIV infection.10
Diagnosis. Examination by dark-field microscopy, testing by PCR, and direct fluorescent antibody assay for T pallidum from lesion tissue or exudate provide definitive diagnosis for early and congenital syphilis, but are often unavailable.
Presumptive diagnosis requires 2 serologic tests:
- Nontreponemal tests (the VDRL and rapid plasma reagin tests) identify anticardiolipin antibodies released during syphilis infection, although results also can be elevated in autoimmune disease or after certain immunizations, including the COVID-19 vaccine.
- Treponemal tests (the fluorescent treponemal antibody absorbed assay, T pallidum particulate agglutination assay, enzyme immunoassay, and chemiluminescence immunoassay) are specific antibody tests.
Historically, reactive nontreponemal tests, which are less expensive and easier to perform, were followed by a treponemal test to confirm the presumptive diagnosis. This method continues to be reasonable when screening patients in a low-prevalence population.11 The reverse sequence screening algorithm (ie, begin with a treponemal test) is now frequently used. With this method, a positive treponemal test must be confirmed with a nontreponemal test. If the treponemal test is positive and the nontreponemal test is negative, another treponemal test must be positive to confirm the diagnosis. This algorithm is useful in high-risk populations because it provides earlier detection of recently acquired syphilis and enhanced detection of late latent syphilis.12,13,44 The CDC has not stated a diagnostic preference.
Once the diagnosis is made, a complete history (including a sexual history and a history of syphilis testing and treatment) and a physical exam are necessary to confirm stage of disease.45
Special circumstances. Neurosyphilis, ocular syphilis, and otosyphilis refer to the site of infection and can occur at any stage of disease. The nervous system usually is infected within hours of initial infection, but symptoms might take weeks or years to develop—or might never manifest. Any time a patient develops neurologic, ophthalmologic, or audiologic symptoms, careful neurologic and ophthalmologic evaluation should be performed and the patient should be tested for HIV.
Continue to: Lumbar puncture is warranted...
Lumbar puncture is warranted for evaluation of cerebrospinal fluid if neurologic symptoms are present but is not necessary for isolated ocular syphilis or otosyphilis without neurologic findings. Treatment should not be delayed for test results if ocular syphilis is suspected because permanent blindness can develop. Any patient at high risk for an STI who presents with neurologic or ophthalmologic symptoms should be tested for syphilis and HIV.45
Pregnant women who have a diagnosis of syphilis should be treated with penicillin immediately because treatment ≥ 30 days prior to delivery is likely to prevent most cases of congenital syphilis. However, a course of penicillin might not prevent stillbirth or congenital syphilis in a gravely infected fetus, evidenced by fetal syphilis on a sonogram at the time of treatment. Additional doses of penicillin in pregnant women with early syphilis might be indicated if there is evidence of fetal syphilis on ultrasonography. All women who deliver a stillborn infant (≥ 20 weeks’ gestation) should be tested for syphilis at delivery.46
All patients in whom primary or secondary syphilis has been diagnosed should be tested for HIV at the time of diagnosis and treatment; if the result is negative, they should be offered preexposure prophylaxis (PrEP; discussed shortly). If the incidence of HIV in your community is high, repeat testing for HIV in 3 months. Clinical and serologic evaluation should be performed 6 and 12 months after treatment.47
Treatment. Penicillin remains the standard treatment for syphilis. Primary, secondary, and early tertiary stages (including in pregnancy) are treated with benzathine penicillin G 2.4 million units intramuscular (IM) in a single dose. For pregnant patients, repeating that dose in 1 week generally is recommended. Patients in the late latent (> 1 year) or tertiary stage receive the same dose of penicillin, which is then repeated weekly, for a total of 3 doses. Doxycycline and ceftriaxone are alternatives, except in pregnancy.
Warn patients of the Jarisch-Herxheimer reaction: fever, headache, and myalgias associated with initiation of treatment in the presence of the high bacterial load seen in early syphilis. Treatment is symptomatic, but the Jarisch-Herxheimer reaction can cause fetal distress in pregnancy.
Otosyphilis, ocular syphilis, and neurosyphilis require intravenous (IV) aqueous crystalline penicillin G 3 to 4 million U every 4 hours for 10 to 14 days.45 Alternatively, procaine penicillin G 2.4 million U/d IM can be given daily with oral probenecid 500 mg qid, both for 10 to 14 days (TABLE 214).
Screening andprevention of STIs
Screening recommendations
Follow USPSTF screening guidelines for STIs.10,48-54 Screen annually for:
- gonorrhea and chlamydia in women ages 15 to 24 years and in women older than 25 years if they are at increased risk
- gonorrhea, chlamydia, syphilis, and HIV in MSM, and hepatitis C if they are HIV positive
- trichomoniasis in women who are HIV positive.
Continue to: Consider the community in which...
Consider the community in which you practice when determining risk; you might want to consult local public health authorities for information about local epidemiology and guidance on determining which of your patients are at increased risk.
Preexposure prophylaxis
According to the CDC, all sexually active adults and adolescents should be informed about the availability of PrEP to prevent HIV infection. PrEP should be (1) available to anyone who requests it and (2) recommended for anyone who is sexually active and who practices sexual behaviors that place them at substantial risk for exposure to or acquisition of HIV, or both.
The recommended treatment protocol for men and women who have either an HIV-positive partner or inconsistent condom use or who have had a bacterial STI in the previous 6 months is oral emtricitabine 200 mg plus tenofovir disoproxil fumarate 300 mg/d (sold as Truvada-F/TDF). Men and transgender women (ie, assigned male at birth) with at-risk behaviors also can use emtricitabine plus tenofovir alafenamide 25 mg/d (sold as Descovy-F/TAF).
In addition, cabotegravir plus rilpirivine (sold as Cabenuva), IM every 2 months, was approved by the FDA for PrEP in 2021.
Creatinine clearance should be assessed at baseline and yearly (every 6 months for those older than 50 years) in patients taking PrEP. All patients must be tested for HIV at initiation of treatment and every 3 months thereafter (every 4 months for cabotegravir plus rilpirivine). Patients should be screened for bacterial STIs every 6 months (every 3 months for MSM and transgender women); screening for chlamydia should be done yearly. For patients being treated with emtricitabine plus tenofovir alafenamide, weight and a lipid profile (cholesterol and triglycerides) should be assessed annually.55
Postexposure prophylaxis
The sharp rise in the incidence of STIs in the past few years has brought renewed interest in postexposure prophylaxis (PEP) for STIs. Although PEP should be standard in cases of sexual assault, this protocol also can be considered in other instances of high-risk exposure.
CDC recommendations for PEP in cases of assault are56:
- ceftriaxone 500 mg IM in a single dose (1 g if weight is ≥ 150 kg) plus
- doxycycline 100 mg bid for 7 days plus
- metronidazole 2 g bid for 7 days (for vaginal exposure)
- pregnancy evaluation and emergency contraception
- hepatitis B risk evaluation and vaccination, with or without hepatitis B immune globulin
- HIV risk evaluation, based on CDC guidelines, and possible HIV prophylaxis (PrEP)
- HPV vaccination for patients ages 9 to 26 years if they are not already fully vaccinated.
CORRESPONDENCE
Belinda Vail, MD, 3901 Rainbow Boulevard, Mail Stop 4010, Kansas City, KS 66160; bvail@kumc.edu
Except for a drop in the number of sexually transmitted infections (STIs) early in the COVID-19 pandemic (March and April 2020), the incidence of STIs has been rising throughout this century.1 In 2018, 1 in 5 people in the United States had an STI; 26 million new cases were reported that year, resulting in direct costs of $16 billion—85% of which was for the care of HIV infection.2 Also that year, infection with Chlamydia trachomatis (chlamydia), Trichomonas vaginalis (trichomoniasis), herpesvirus type 2 (genital herpes), and/or human papillomavirus (condylomata acuminata) constituted 97.6% of all prevalent and 93.1% of all incident STIs.3 Almost half (45.5%) of new cases of STIs occur in people between the ages of 15 and 24 years.3

Three factors—changing social patterns, including the increase of social networking; the ability of antiviral therapy to decrease the spread of HIV, leading to a reduction in condom use; and increasing antibiotic resistance—have converged to force changes in screening and treatment recommendations. In this article, we summarize updated guidance for primary care clinicians from several sources—including the Centers for Disease Control and Prevention (CDC), the US Preventive Services Task Force (USPSTF), and the American Society for Colposcopy and Cervical Pathology (ASCCP)—on diagnosing STIs (TABLE 14-13) and providing guideline-based treatment (Table 214). Because of the breadth and complexity of HIV disease, it is not addressed here.



Chlamydia
Infection with Chlamydia trachomatis—the most commonly reported bacterial STI in the United States—primarily causes cervicitis in women and proctitis in men, and can cause urethritis and pharyngitis in men and women. Prevalence is highest in sexually active people younger than 24 years.15
Because most infected people are asymptomatic and show no signs of illness on physical exam, screening is recommended for all sexually active women younger than 25 years and all men who have sex with men (MSM).4 No studies have established proper screening intervals; a reasonable approach, therefore, is to repeat screening for patients who have a sexual history that confers a new or persistent risk for infection since their last negative result.
Depending on the location of the infection, symptoms of chlamydia can include vaginal or penile irritation or discharge, dysuria, pelvic or rectal pain, and sore throat. Breakthrough bleeding in a patient who is taking an oral contraceptive should raise suspicion for chlamydia.
Untreated chlamydia can lead to pelvic inflammatory disease (PID), tubo-ovarian abscess, tubal factor infertility, ectopic pregnancy, and chronic pelvic pain. Infection can be transmitted vertically (mother to baby) antenatally, which can cause ophthalmia neonatorum and pneumonia in these newborns.
Diagnosis. The diagnosis of chlamydia is made using nucleic acid amplification testing (NAAT). Specimens can be collected by the clinician or the patient (self collected) using a vaginal, rectal, or oropharyngeal swab, or a combination of these, and can be obtained from urine or liquid-based cytology material.16
Continue to: Treatment
Treatment. Recommendations for treating chlamydia were updated by the CDC in its 2021 treatment guidelines (Table 214). Doxycycline 100 mg bid for 7 days is the preferred regimen; alternative regiments are (1) azithromycin 1 g in a single dose and (2) levofloxacin 500 mg daily for 7 days.4 A meta-analysis17 and a Cochrane review18 showed that the rate of treatment failure was higher among men when they were treated with azithromycin instead of doxycycline; furthermore, a randomized controlled trial demonstrated that doxycycline is more effective than azithromycin (cure rate, 100%, compared to 74%) at treating rectal chlamydia in MSM.19
Azithromycin is efficacious for urogenital infection in women; however, there is concern that the 33% to 83% of women who have concomitant rectal infection (despite reporting no receptive anorectal sexual activity) would be insufficiently treated. Outside pregnancy, the CDC does not recommend a test of cure but does recommend follow-up testing for reinfection in 3 months. Patients should abstain from sexual activity until 7 days after all sexual partners have been treated.
Expedited partner therapy (EPT) is the practice of treating sexual partners of patients with known chlamydia (and patients with gonococcal infection). Unless prohibited by law in your state, offer EPT to patients with chlamydia if they cannot ensure that their sexual partners from the past 60 days will seek timely treatment.a
Evidence to support EPT comes from 3 US clinical trials, whose subjects comprised heterosexual men and women with chlamydia or gonorrhea.21-23 The role of EPT for MSM is unclear; data are limited. Shared decision-making is recommended to determine whether EPT should be provided, to ensure that co-infection with other bacterial STIs (eg, syphilis) or HIV is not missed.24-26
a Visit www.cdc.gov/std/ept to read updated information about laws and regulations regarding EPT in your state.20
Gonorrhea
Gonorrhea is the second most-reported bacterial communicable disease.5 Infection with Neisseria gonorrhoeae causes urethral discharge in men, leading them to seek treatment; infected women, however, are often asymptomatic. Infected men and women might not recognize symptoms until they have transmitted the disease. Women have a slower natural clearance of gonococcal infection, which might explain their higher prevalence.27 Delayed recognition of symptoms can result in complications, including PID.5
Diagnosis. Specimens for NAAT can be obtained from urine, endocervical, vaginal, rectal, pharyngeal, and male urethral specimens. Reported sexual behaviors and exposures of women and transgender or gender-diverse people should be taken into consideration to determine whether rectal or pharyngeal testing, or both, should be performed.28 MSM should be screened annually at sites of contact, including the urethra, rectum, and pharynx.28 All patients with urogenital or rectal gonorrhea should be asked about oral sexual exposure; if reported, pharyngeal testing should be performed.5
NAAT of urine is at least as sensitive as testing of an endocervical specimen; the same specimen can be used to test for chlamydia and gonorrhea. Patient-collected specimens are a reasonable alternative to clinician-collected swab specimens.29
Continue to: Treatment
Treatment is complicated by the ability of gonorrhea to develop resistance. Intramuscular ceftriaxone 500 mg in a single dose cures 98% to 99% of infections in the United States; however, monitoring local resistance patterns in the community is an important component of treatment.28 (See Table 214 for an alternative regimen for cephalosporin-allergic patients and for treating gonococcal conjunctivitis and disseminated infection.)
In 2007, the CDC identified widespread quinolone-resistant gonococcal strains; therefore, fluoroquinolones no longer are recommended for treating gonorrhea.30 Cefixime has demonstrated only limited success in treating pharyngeal gonorrhea and does not attain a bactericidal level as high as ceftriaxone does; cefixime therefore is recommended only if ceftriaxone is unavailable.28 The national Gonococcal Isolate Surveillance Project is finding emerging evidence of the reduced susceptibility of N gonorrhoeae to azithromycin—making dual therapy for gonococcal infection no longer a recommendation.28
Patients should abstain from sex until 7 days after all sex partners have been treated for gonorrhea. As with chlamydia, the CDC does not recommend a test of cure for uncomplicated urogenital or rectal gonorrhea unless the patient is pregnant, but does recommend testing for reinfection 3 months after treatment.14 For patients with pharyngeal gonorrhea, a test of cure is recommended 7 to 14 days after initial treatment, due to challenges in treatment and because this site of infection is a potential source of antibiotic resistance.28
Trichomoniasis
T vaginalis, the most common nonviral STI worldwide,31 can manifest as a yellow-green vaginal discharge with or without vaginal discomfort, dysuria, epididymitis, and prostatitis; most cases, however, are asymptomatic. On examination, the cervix might be erythematous with punctate lesions (known as strawberry cervix).
Unlike most STIs, trichomoniasis is as common in women older than 24 years as it is in younger women. Infection is associated with a lower educational level, lower socioeconomic status, and having ≥ 2 sexual partners in the past year.32 Prevalence is approximately 10 times as high in Black women as it is in White women.
T vaginalis infection is associated with an increase in the risk for preterm birth, premature rupture of membranes, cervical cancer, and HIV infection. With a lack of high-quality clinical trials on the efficacy of screening, women with HIV are the only group for whom routine screening is recommended.6
Diagnosis. NAAT for trichomoniasis is now available in conjunction with gonorrhea and chlamydia testing of specimens on vaginal or urethral swabs and of urine specimens and liquid Pap smears.
Continue to: Treatment
Treatment. Because of greater efficacy, the treatment recommendation for women has changed from a single 2-g dose of oral metronidazole to 500 mg twice daily for 7 days. The 2-g single oral dose is still recommended for men7 (Table 214 lists alternative regimens).
Mycoplasma genitalium
Infection with M genitalium is common and often asymptomatic. The disease causes approximately 20% of all cases of nongonococcal and nonchlamydial urethritis in men and about 40% of persistent or recurrent infections. M genitalium is present in approximately 20% of women with cervicitis and has been associated with PID, preterm delivery, spontaneous abortion, and infertility.
There are limited and conflicting data regarding outcomes in infected patients other than those with persistent or recurrent infection; furthermore, resistance to azithromycin is increasing rapidly, resulting in an increase in treatment failures. Screening therefore is not recommended, and testing is recommended only in men with nongonococcal urethritis.33,34
Diagnosis. NAAT can be performed on urine or on a urethral, penile meatal, endocervical, or vaginal swab; men with recurrent urethritis or women with recurrent cervicitis should be tested. NAAT also can be considered in women with PID. Testing the specimen for the microorganism’s resistance to macrolide antibiotics is recommended (if such testing is available).
Treatment is initiated with doxycycline 100 mg twice daily for 7 days. If the organism is macrolide sensitive, follow with azithromycin 1 g orally on Day 1, then 500 mg/d for 3 more days. If the organism is macrolide resistant or testing is unavailable, follow doxycycline with oral moxifloxacin 400 mg/d for 7 days.33
Genital herpes (mostly herpesvirus type 2)
Genital herpes, characterized by painful, recurrent outbreaks of genital and anal lesions,35 is a lifelong infection that increases in prevalence with age.8 Because many infected people have disease that is undiagnosed or mild or have unrecognizable symptoms during viral shedding, most genital herpes infections are transmitted by people who are unaware that they are contagious.36 Herpesvirus type 2 (HSV-2) causes most cases of genital herpes, although an increasing percentage of cases are attributed to HSV type 1 (HSV-1) through receptive oral sex from a person who has an oral HSV-1 lesion.
Importantly, HSV-2–infected people are 2 to 3 times more likely to become infected with HIV than people who are not HSV-2 infected.37 This is becauseCD4+ T cells concentrate at the site of HSV lesions and express a higher level of cell-surface receptors that HIV uses to enter cells. HIV replicates 3 to 5 times more quickly in HSV-infected tissue.38
Continue to: HSV can become disseminated...
HSV can become disseminated, particularly in immunosuppressed people, and can manifest as encephalitis, hepatitis, and pneumonitis. Beyond its significant burden on health, HSV carries significant psychosocial consequences.9
Diagnosis. Clinical diagnosis can be challenging if classic lesions are absent at evaluation. If genital lesions are present, HSV can be identified by NAAT or culture of a specimen of those lesions. False-negative antibody results might be more frequent in early stages of infection; repeating antibody testing 12 weeks after presumed time of acquisition might therefore be indicated, based on clinical judgment. HSV-2 antibody positivity implies anogenital infection because almost all HSV-2 infections are sexually acquired.
HSV-1 antibody positivity alone is more difficult to interpret because this finding does not distinguish between oral and genital lesions, and most HSV-1 seropositivity is acquired during childhood.36 HSV polymerase chain reaction (PCR) testing of blood should not be performed to diagnose genital herpes infection, except in settings in which there is concern about disseminated infection.
Treatment. Management should address the acute episode and the chronic nature of genital herpes. Antivirals will not eradicate latent
- attenuate current infection
- prevent recurrence
- improve quality of life
- suppress the virus to prevent transmission to sexual partners.
All patients experiencing an initial episode of genital herpes should be treated, regardless of symptoms, due to the potential for prolonged or severe symptoms during recurrent episodes.9 Three drugs—acyclovir, valacyclovir, and famciclovir—are approved by the US Food and Drug Administration (FDA) to treat genital herpes and appear equally effective (TABLE 214).
Antiviral therapy for recurrent genital HSV infection can be administered either as suppressive therapy to reduce the frequency of recurrences or episodically to shorten the duration of lesions:
- Suppressive therapy reduces the frequency of recurrence by 70% to 80% among patients with frequent outbreaks. Long-term safety and efficacy are well established.
- Episodic therapy is most effective if started within 1 day after onset of lesions or during the prodrome.36
There is no specific recommendation for when to choose suppressive over episodic therapy; most patients prefer suppressive therapy because it improves quality of life. Use shared clinical decision-making to determine the best option for an individual patient.
Continue to: Human papillomavirus
Human papillomavirus
Condylomata acuminata (genital warts) are caused by human papillomavirus (HPV), most commonly types 6 and 11, which manifest as soft papules or plaques on the external genitalia, perineum, perianal skin, and groin. The warts are usually asymptomatic but can be painful or pruritic, depending on size and location.
Diagnosis is made by visual inspection and can be confirmed by biopsy if lesions are atypical. Lesions can resolve spontaneously, remain unchanged, or grow in size or number.
Treatment. The aim of treatment is relief of symptoms and removal of warts. Treatment does not eradicate HPV infection. Multiple treatments are available that can be applied by the patient as a cream, gel, or ointment or administered by the provider, including cryotherapy, surgical removal, and solutions. The decision on how to treat should be based on the number, size, and
HPV-associated cancers and precancers. This is a broad (and separate) topic. HPV types 16 and 18 cause most cases of cervical, penile, vulvar, vaginal, anal, and oropharyngeal cancer and precancer.39 The USPSTF, the American Cancer Society, and the American College of Obstetricians and Gynecologists all have recommendations for cervical cancer screening in the United States.40 Refer to guidelines of the ASCCP for recommendations on abnormal screening tests.41
Prevention of genital warts. The 9-valent HPV vaccine available in the United States is safe and effective and helps protect against viral types 6, 11, 16, 18, 31, 33, 45, 52, and 58. Types 6 and 11 are the principal causes of genital warts. Types 16 and 18 cause 66% of cervical cancer. The vaccination series can be started at age 9 years and is recommended for everyone through age 26 years. Only 2 doses are needed if the first dose is given prior to age 15 years; given after that age, a 3-dose series is utilized. Refer to CDC vaccine guidelines42 for details on the exact timing of vaccination.
Vaccination for women ages 27 to 45 years is not universally recommended because most people have been exposed to HPV by that age. However, the vaccine can still be administered, depending on clinical circumstances and the risk for new infection.42
Syphilis
Caused by the spirochete Treponema pallidum, syphilis manifests across a spectrum—from congenital to tertiary. The inability of medical science to develop a method for culturing the spirochete has confounded diagnosis and treatment.
Continue to: Since reaching a historic...
Since reaching a historic nadir of incidence in 2000 (5979 cases in the United States), there has been an increasingly rapid rise in that number: to 130,000 in 2020. More than 50% of cases are in MSM; however, the number of cases in heterosexual women is rapidly increasing.43
Routine screening for syphilis should be performed in any person who is at risk: all pregnant women in the first trimester (and in the third trimester and at delivery if they are at risk or live in a community where prevalence is high) and annually in sexually active MSM or anyone with HIV infection.10
Diagnosis. Examination by dark-field microscopy, testing by PCR, and direct fluorescent antibody assay for T pallidum from lesion tissue or exudate provide definitive diagnosis for early and congenital syphilis, but are often unavailable.
Presumptive diagnosis requires 2 serologic tests:
- Nontreponemal tests (the VDRL and rapid plasma reagin tests) identify anticardiolipin antibodies released during syphilis infection, although results also can be elevated in autoimmune disease or after certain immunizations, including the COVID-19 vaccine.
- Treponemal tests (the fluorescent treponemal antibody absorbed assay, T pallidum particulate agglutination assay, enzyme immunoassay, and chemiluminescence immunoassay) are specific antibody tests.
Historically, reactive nontreponemal tests, which are less expensive and easier to perform, were followed by a treponemal test to confirm the presumptive diagnosis. This method continues to be reasonable when screening patients in a low-prevalence population.11 The reverse sequence screening algorithm (ie, begin with a treponemal test) is now frequently used. With this method, a positive treponemal test must be confirmed with a nontreponemal test. If the treponemal test is positive and the nontreponemal test is negative, another treponemal test must be positive to confirm the diagnosis. This algorithm is useful in high-risk populations because it provides earlier detection of recently acquired syphilis and enhanced detection of late latent syphilis.12,13,44 The CDC has not stated a diagnostic preference.
Once the diagnosis is made, a complete history (including a sexual history and a history of syphilis testing and treatment) and a physical exam are necessary to confirm stage of disease.45
Special circumstances. Neurosyphilis, ocular syphilis, and otosyphilis refer to the site of infection and can occur at any stage of disease. The nervous system usually is infected within hours of initial infection, but symptoms might take weeks or years to develop—or might never manifest. Any time a patient develops neurologic, ophthalmologic, or audiologic symptoms, careful neurologic and ophthalmologic evaluation should be performed and the patient should be tested for HIV.
Continue to: Lumbar puncture is warranted...
Lumbar puncture is warranted for evaluation of cerebrospinal fluid if neurologic symptoms are present but is not necessary for isolated ocular syphilis or otosyphilis without neurologic findings. Treatment should not be delayed for test results if ocular syphilis is suspected because permanent blindness can develop. Any patient at high risk for an STI who presents with neurologic or ophthalmologic symptoms should be tested for syphilis and HIV.45
Pregnant women who have a diagnosis of syphilis should be treated with penicillin immediately because treatment ≥ 30 days prior to delivery is likely to prevent most cases of congenital syphilis. However, a course of penicillin might not prevent stillbirth or congenital syphilis in a gravely infected fetus, evidenced by fetal syphilis on a sonogram at the time of treatment. Additional doses of penicillin in pregnant women with early syphilis might be indicated if there is evidence of fetal syphilis on ultrasonography. All women who deliver a stillborn infant (≥ 20 weeks’ gestation) should be tested for syphilis at delivery.46
All patients in whom primary or secondary syphilis has been diagnosed should be tested for HIV at the time of diagnosis and treatment; if the result is negative, they should be offered preexposure prophylaxis (PrEP; discussed shortly). If the incidence of HIV in your community is high, repeat testing for HIV in 3 months. Clinical and serologic evaluation should be performed 6 and 12 months after treatment.47
Treatment. Penicillin remains the standard treatment for syphilis. Primary, secondary, and early tertiary stages (including in pregnancy) are treated with benzathine penicillin G 2.4 million units intramuscular (IM) in a single dose. For pregnant patients, repeating that dose in 1 week generally is recommended. Patients in the late latent (> 1 year) or tertiary stage receive the same dose of penicillin, which is then repeated weekly, for a total of 3 doses. Doxycycline and ceftriaxone are alternatives, except in pregnancy.
Warn patients of the Jarisch-Herxheimer reaction: fever, headache, and myalgias associated with initiation of treatment in the presence of the high bacterial load seen in early syphilis. Treatment is symptomatic, but the Jarisch-Herxheimer reaction can cause fetal distress in pregnancy.
Otosyphilis, ocular syphilis, and neurosyphilis require intravenous (IV) aqueous crystalline penicillin G 3 to 4 million U every 4 hours for 10 to 14 days.45 Alternatively, procaine penicillin G 2.4 million U/d IM can be given daily with oral probenecid 500 mg qid, both for 10 to 14 days (TABLE 214).
Screening andprevention of STIs
Screening recommendations
Follow USPSTF screening guidelines for STIs.10,48-54 Screen annually for:
- gonorrhea and chlamydia in women ages 15 to 24 years and in women older than 25 years if they are at increased risk
- gonorrhea, chlamydia, syphilis, and HIV in MSM, and hepatitis C if they are HIV positive
- trichomoniasis in women who are HIV positive.
Continue to: Consider the community in which...
Consider the community in which you practice when determining risk; you might want to consult local public health authorities for information about local epidemiology and guidance on determining which of your patients are at increased risk.
Preexposure prophylaxis
According to the CDC, all sexually active adults and adolescents should be informed about the availability of PrEP to prevent HIV infection. PrEP should be (1) available to anyone who requests it and (2) recommended for anyone who is sexually active and who practices sexual behaviors that place them at substantial risk for exposure to or acquisition of HIV, or both.
The recommended treatment protocol for men and women who have either an HIV-positive partner or inconsistent condom use or who have had a bacterial STI in the previous 6 months is oral emtricitabine 200 mg plus tenofovir disoproxil fumarate 300 mg/d (sold as Truvada-F/TDF). Men and transgender women (ie, assigned male at birth) with at-risk behaviors also can use emtricitabine plus tenofovir alafenamide 25 mg/d (sold as Descovy-F/TAF).
In addition, cabotegravir plus rilpirivine (sold as Cabenuva), IM every 2 months, was approved by the FDA for PrEP in 2021.
Creatinine clearance should be assessed at baseline and yearly (every 6 months for those older than 50 years) in patients taking PrEP. All patients must be tested for HIV at initiation of treatment and every 3 months thereafter (every 4 months for cabotegravir plus rilpirivine). Patients should be screened for bacterial STIs every 6 months (every 3 months for MSM and transgender women); screening for chlamydia should be done yearly. For patients being treated with emtricitabine plus tenofovir alafenamide, weight and a lipid profile (cholesterol and triglycerides) should be assessed annually.55
Postexposure prophylaxis
The sharp rise in the incidence of STIs in the past few years has brought renewed interest in postexposure prophylaxis (PEP) for STIs. Although PEP should be standard in cases of sexual assault, this protocol also can be considered in other instances of high-risk exposure.
CDC recommendations for PEP in cases of assault are56:
- ceftriaxone 500 mg IM in a single dose (1 g if weight is ≥ 150 kg) plus
- doxycycline 100 mg bid for 7 days plus
- metronidazole 2 g bid for 7 days (for vaginal exposure)
- pregnancy evaluation and emergency contraception
- hepatitis B risk evaluation and vaccination, with or without hepatitis B immune globulin
- HIV risk evaluation, based on CDC guidelines, and possible HIV prophylaxis (PrEP)
- HPV vaccination for patients ages 9 to 26 years if they are not already fully vaccinated.
CORRESPONDENCE
Belinda Vail, MD, 3901 Rainbow Boulevard, Mail Stop 4010, Kansas City, KS 66160; bvail@kumc.edu
1. Pagaoa M, Grey J, Torrone E, et al. Trends in nationally notifiable sexually transmitted disease case reports during the US COVID-19 pandemic, January to December 2020. Sex Transm Dis. 2021;48:798-804. doi: 10.1097/OLQ.0000000000001506
2. Chesson HW, Spicknall IH; Bingham A, et al. The estimated direct lifetime medical costs of sexually transmitted infections acquired in the United States in 2018. Sex Transm Dis. 2021;48:215-221. doi: 10.1097/OLQ.0000000000001380
3. Kreisel KM, Spicknall IH, Gargano JW, et al. Sexually transmitted infections among US women and men: prevalence and incidence estimates, 2018. Sex Transm Dis. 2021;48:208-214. doi: 10.1097/OLQ.0000000000001355
4. CDC. Sexually transmitted infections treatment guidelines, 2021: Chlamydial infections among adolescents and adults. US Department of Health and Human Services. July 21, 2021. Accessed April 19, 2023. www.cdc.gov/std/treatment-guidelines/chlamydia.htm
5. CDC. Sexually transmitted infections treatment guidelines, 2021: Gonococcal infections among adolescents and adults. US Department of Health and Human Services. September 21, 2022. Accessed April 23, 2023. www.cdc.gov/std/treatment-guidelines/gonorrhea-adults.htm
6. Van Gerwen OT, Muzny CA. Recent advances in the epidemiology, diagnosis, and management of Trichomonas vaginalis infection. F1000Res. 2019;
7. CDC. Sexually transmitted infections treatment guidelines, 2021. Trichomoniasis. US Department of Health and Human Services. September 21, 2022. Accessed April 23, 2023. December 27, 2021. www.cdc.gov/std/treatment-guidelines/trichomoniasis.htm
8. Spicknall IH, Flagg EW, Torrone EA. Estimates of the prevalence and incidence of genital herpes, United States, 2018. Sex Transm Dis. 2021;48:260-265. doi: 10.1097/OLQ.0000000000001375
9. Mark H, Gilbert L, Nanda J. Psychosocial well-being and quality of life among women newly diagnosed with genital herpes. J Obstet Gynecol Neonatal Nurs.
10. Bibbins-Domingo K, Grossman DC, Curry SJ, et al; US Preventive Services Task Force. Screening for syphilis infection in nonpregnant adults and adolescents: US Preventive Services Task Force recommendation statement. JAMA. 2016;315:2321-2327. doi: 10.1001/jama.2016.5824
11. Ricco J, Westby A. Syphilis: far from ancient history. Am Fam Physician. 2020;102:91-98.
12. Goza M, Kulwicki B, Akers JM, et al. Syphilis screening: a review of the Syphilis Health Check rapid immunochromatographic test. J Pharm Technol. 2017;33:53-59. doi:10.1177/8755122517691308
13. Henao- AF, Johnson SC. Diagnostic tests for syphilis: new tests and new algorithms. Neurol Clin Pract. 2014;4:114-122. doi: 10.1212/01.CPJ.0000435752.17621.48
14. Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep. 2021;70:1-187. doi:10.15585/mmwr.rr7004a1
15. CDC. Sexually Transmitted Disease Surveillance 2021. National overview of STDs. US Department of Health and Human Services. April 2023. Accessed May 9, 2023. www.cdc.gov/std/statistics/2021/overview.htm#Chlamydia
16. CDC. Recommendations for the laboratory-based detection of Chlamydia trachomatis and Neisseria gonorrhoeae—2014. MMWR Recomm Rep. 2014;63:1-19.
17. Kong FYS, Tabrizi SN, Law M, et al. Azithromycin versus doxycycline for the treatment of genital chlamydia infection: a meta-analysis of randomized controlled trials. Clin Infect Dis. 2014;59:193-205. doi: 10.1093/cid/ciu220
18. Páez-Canro C, Alzate JP, González LM, et al. Antibiotics for treating urogenital Chlamydia trachomatis infection in men and non-pregnant women. Cochrane Database Syst Rev. 2019;1:CD010871. doi: 10.1002/14651858.CD010871.pub2
19. Dombrowski JC, Wierzbicki MR, Newman LM, et al. Doxycycline versus azithromycin for the treatment of rectal chlamydia in men who have sex with men: a randomized controlled trial. Clin Infect Dis. 2021;73:824-831. doi: 10.1093/cid/ciab153
20. CDC. Sexually transmitted infections treatment guidelines, 2021: Expedited partner therapy. US Department of Health and Human Services. July 22, 2021. Accessed April 19, 2023. www.cdc.gov/std/treatment-guidelines/clinical-EPT.htm
21. Golden MR, Whittington WLH, Handsfield HH, et al. Effect of expedited treatment of sex partners on recurrent or persistent gonorrhea or chlamydial infection. N Engl J Med. 2005;352:676-685. doi: 10.1056/NEJMoa041681
22. Schillinger JA, Kissinger P, Calvet H, et al. Patient-delivered partner treatment with azithromycin to prevent repeated Chlamydia trachomatis infection among women: a randomized, controlled trial. Sex Transm Dis. 2003;30:49-56. doi: 10.1097/00007435-200301000-00011
23. Cameron ST, Glasier A, Scott G, et al. Novel interventions to reduce re-infection in women with chlamydia: a randomized controlled trial. Hum Reprod. 2009;24:888-895. doi: 10.1093/humrep/den475
24. McNulty A, Teh MF, Freedman E. Patient delivered partner therapy for chlamydial infection—what would be missed? Sex Transm Dis. 2008;35:834-836. doi: 10.1097/OLQ.0b013e3181761993
25. Stekler J, Bachmann L, Brotman RM, et al. Concurrent sexually transmitted infections (STIs) in sex partners of patients with selected STIs: implications for patient-delivered partner therapy. Clin Infect Dis. 2005;40:787-793. doi: 10.1086/428043
26. Schillinger JA, Kissinger P, Calvet H, et al. Patient-delivered partner treatment with azithromycin to prevent repeated Chlamydia trachomatis infection among women: a randomized, controlled trial. Sex Transm Dis. 2003;30:49-56. doi: 10.1097/00007435-200301000-00011
27. Stupiansky NW, Van der Pol B, Williams JA, et al. The natural history of incident gonococcal infection in adolescent women. Sex Transm Dis. 2011;38:750-754. doi: 10.1097/OLQ.0b013e31820ff9a4
28. CDC. Sexually transmitted infections treatment guidelines, 2021: Screening recommendations and considerations referenced in treatment guidelines and original sources. US Department of Health and Human Services. June 6, 2022. Accessed May 9, 2023. www.cdc.gov/std/treatment-guidelines/screening-recommen dations.htm
29. Cantor A, Dana T, Griffen JC, et al. Screening for chlamydial and gonococcal infections: a systematic review update for the US Preventive Services Task Force. Evidence Synthesis No. 206. AHRQ Report No. 21-05275-EF-1. Agency for Healthcare Research and Quality. September 2021. www.ncbi.nlm.nih.gov/books/NBK574045
30. CDC. Update to CDC’s sexually transmitted diseases treatment guidelines, 2006: fluoroquinolones no longer recommended for treatment of gonococcal infections. MMWR Morb Mortal Wkly Rep. 2007;56:332-336.
31. Rowley J, Vander Hoorn S, Korenromp E, et al. Chlamydia, gonorrhoea, trichomoniasis and syphilis: global prevalence and incidence estimates, 2016. Bull World Health Organ. 2019:97:548-562P. doi: 10.2471/BLT.18.228486
32. Patel EU, Gaydos CA, Packman ZR, et al. Prevalence and correlates of Trichomonas vaginalis infection among men and women in the United States. Clin Infect Dis. 2018;67:211-217. doi: 10.1093/cid/ciy079
33. CDC. Sexually transmitted infections treatment guidelines, 2021. Mycoplasma genitalium. US Department of Health and Human Services. July 22, 2021. Accessed April 23, 2023. www.cdc.gov/std/treatment-guidelines/mycoplasmagenitalium.htm
34. Manhart LE, Broad JM, Bolden MR. Mycoplasma genitalium: should we treat and how? Clin Infect Dis. 2011;53(suppl 3):S129-S142. doi:10.1093/cid/cir702.
35. Corey L, Wald A. Genital herpes. In: Holmes KK, Sparling PF, Stamm WE, et al, eds. Sexually Transmitted Diseases. 4th ed. McGraw-Hill; 2008:399-437.
36. CDC. Sexually transmitted infections treatment guidelines, 2021: Genital herpes. US Department of Health and Human Services. September 21, 2022. Accessed April 23, 2023. www.cdc.gov/std/treatment-guidelines/herpes.htm
37. Looker KJ, Elmes JAR, Gottlieb SL, et al. Effect of HSV-2 infection on subsequent HIV acquisition: an updated systematic review and meta-analysis. Lancet Infect Dis. 2017;17:1303-1316. doi: 10.1016/S1473-3099(17)30405-X
38. Rollenhagen C, Lathrop M, Macura SL, et al. Herpes simplex virus type-2 stimulates HIV-1 replication in cervical tissues: implications for HIV-1 transmission and efficacy of anti-HIV-1 microbicides. Mucosal Immunol. 2014;7:1165-1174. doi: 10.1038/mi.2014.3
39. Cogliano V, Baan R, Straif K, et al; WHO International Agency for Research on Cancer. Carcinogenicity of human papillomaviruses. Lancet Oncol.
40. Simon MA, Tseng CW, Wong JB. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686. doi:10.1001/jama.2018.10897
41. Perkins RB, Guido RS, Castle PE, et al; . 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2020;24:102-131. doi: 10.1097/LGT.0000000000000525
42. Meites E, Szilagyi PG, Chesson HW, et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68:698-702. doi: 10.15585/mmwr.mm6832a3
43. Schmidt R, Carson PJ, Jansen RJ. Resurgence of syphilis in the United States: an assessment of contributing factors. Infect Dis (Auckl). 2019;12:1178633719883282. doi: 10.1177/1178633719883282
44. Boog GHP, Lopes JVZ, Mahler JV, et al. Diagnostic tools for neurosyphilis: a systematic review. BMC Infect Dis. 2021;21:568. doi: 10.1186/s12879-021-06264-8
45. CDC. Sexually transmitted infections treatment guidelines, 2021. Syphilis. US Department of Health and Human Services. April 20, 2023. Accessed April 24, 2023. www.cdc.gov/std/treatment-guidelines/syphilis.htm
46. Matthias JM, Rahman MM, Newman DR, et al. Effectiveness of prenatal screening and treatment to prevent congenital syphilis, Louisiana and Florida, 2013-2014. Sex Transm Dis. 2017;44:498-502. doi: 10.1097/OLQ.0000000000000638
47. Clement ME, Okeke NL, Hicks CB. Treatment of syphilis: a systematic review. JAMA. 2014;312:1905-1917. doi: 10.1001/jama.2014.13259
48. Davidson KW, Barry MJ, Mangione CM, et al; US Preventive Services Task Force. Screening for chlamydia and gonorrhea: US Preventive Services Task Force recommendation statement. JAMA. 2021;326:949-956. doi: 10.1001/jama.2021.14081
49. Krist AH, Davidson KW, Mangione CM, et al; US Preventive Services Task Force. Screening for hepatitis B virus infection in adolescents and adults: US Preventive Services Task Force recommendation statement. JAMA. 2020;324:2415-2422. doi: 10.1001/jama.2020.22980
50. Owens DK, Davidson KW, Krist AH, et al; US Preventive Services Task Force. Screening for hepatitis C virus infection in adolescents and adults: US Preventive Services Task Force recommendation statement. JAMA. 2020;323:970-975. doi: 10.1001/jama.2020.1123
51. Bibbins-Domingo K, Grossman DC, Curry SJ, et al; US Preventive Services Task Force. Serologic screening for genital herpes infection: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:2525-2530. doi: 10.1001/jama.2016.16776
52. Curry SJ, Krist AH, Owens DK, et al; US Preventive Services Task Force. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686. doi: 10.1001/jama.2018.10897
53. Owens DK, Davidson KW, Krist AH, et al; US Preventive Services Task Force. Screening for HIV infection: US Preventive Services Task Force recommendation statement. JAMA. 2019;321:2326-2336. doi: 10.1001/jama.2019.6587
54. Farley TA, Cohen DA, Elkins W. Asymptomatic sexually transmitted diseases: the case for screening. Prev Med. 2003;36:502-509. doi: 10.1016/s0091-7435(02)00058-0
55. US Public Health Service. Preexposure prophylaxis for the prevention of HIV infection in the United States—2021 update. A clinical practice guideline. Centers for Disease Control and Prevention. Accessed April 24, 2023. www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2021.pdf
56. CDC. Sexually transmitted infections treatment guidelines, 2021: Sexual assault and abuse and STIs—adolescents and adults, 2021. US Department of Health and Human Services. July 22, 2021. Accessed April 24, 2023. www.cdc.gov/std/treatment-guidelines/sexual-assault-adults.htm
1. Pagaoa M, Grey J, Torrone E, et al. Trends in nationally notifiable sexually transmitted disease case reports during the US COVID-19 pandemic, January to December 2020. Sex Transm Dis. 2021;48:798-804. doi: 10.1097/OLQ.0000000000001506
2. Chesson HW, Spicknall IH; Bingham A, et al. The estimated direct lifetime medical costs of sexually transmitted infections acquired in the United States in 2018. Sex Transm Dis. 2021;48:215-221. doi: 10.1097/OLQ.0000000000001380
3. Kreisel KM, Spicknall IH, Gargano JW, et al. Sexually transmitted infections among US women and men: prevalence and incidence estimates, 2018. Sex Transm Dis. 2021;48:208-214. doi: 10.1097/OLQ.0000000000001355
4. CDC. Sexually transmitted infections treatment guidelines, 2021: Chlamydial infections among adolescents and adults. US Department of Health and Human Services. July 21, 2021. Accessed April 19, 2023. www.cdc.gov/std/treatment-guidelines/chlamydia.htm
5. CDC. Sexually transmitted infections treatment guidelines, 2021: Gonococcal infections among adolescents and adults. US Department of Health and Human Services. September 21, 2022. Accessed April 23, 2023. www.cdc.gov/std/treatment-guidelines/gonorrhea-adults.htm
6. Van Gerwen OT, Muzny CA. Recent advances in the epidemiology, diagnosis, and management of Trichomonas vaginalis infection. F1000Res. 2019;
7. CDC. Sexually transmitted infections treatment guidelines, 2021. Trichomoniasis. US Department of Health and Human Services. September 21, 2022. Accessed April 23, 2023. December 27, 2021. www.cdc.gov/std/treatment-guidelines/trichomoniasis.htm
8. Spicknall IH, Flagg EW, Torrone EA. Estimates of the prevalence and incidence of genital herpes, United States, 2018. Sex Transm Dis. 2021;48:260-265. doi: 10.1097/OLQ.0000000000001375
9. Mark H, Gilbert L, Nanda J. Psychosocial well-being and quality of life among women newly diagnosed with genital herpes. J Obstet Gynecol Neonatal Nurs.
10. Bibbins-Domingo K, Grossman DC, Curry SJ, et al; US Preventive Services Task Force. Screening for syphilis infection in nonpregnant adults and adolescents: US Preventive Services Task Force recommendation statement. JAMA. 2016;315:2321-2327. doi: 10.1001/jama.2016.5824
11. Ricco J, Westby A. Syphilis: far from ancient history. Am Fam Physician. 2020;102:91-98.
12. Goza M, Kulwicki B, Akers JM, et al. Syphilis screening: a review of the Syphilis Health Check rapid immunochromatographic test. J Pharm Technol. 2017;33:53-59. doi:10.1177/8755122517691308
13. Henao- AF, Johnson SC. Diagnostic tests for syphilis: new tests and new algorithms. Neurol Clin Pract. 2014;4:114-122. doi: 10.1212/01.CPJ.0000435752.17621.48
14. Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep. 2021;70:1-187. doi:10.15585/mmwr.rr7004a1
15. CDC. Sexually Transmitted Disease Surveillance 2021. National overview of STDs. US Department of Health and Human Services. April 2023. Accessed May 9, 2023. www.cdc.gov/std/statistics/2021/overview.htm#Chlamydia
16. CDC. Recommendations for the laboratory-based detection of Chlamydia trachomatis and Neisseria gonorrhoeae—2014. MMWR Recomm Rep. 2014;63:1-19.
17. Kong FYS, Tabrizi SN, Law M, et al. Azithromycin versus doxycycline for the treatment of genital chlamydia infection: a meta-analysis of randomized controlled trials. Clin Infect Dis. 2014;59:193-205. doi: 10.1093/cid/ciu220
18. Páez-Canro C, Alzate JP, González LM, et al. Antibiotics for treating urogenital Chlamydia trachomatis infection in men and non-pregnant women. Cochrane Database Syst Rev. 2019;1:CD010871. doi: 10.1002/14651858.CD010871.pub2
19. Dombrowski JC, Wierzbicki MR, Newman LM, et al. Doxycycline versus azithromycin for the treatment of rectal chlamydia in men who have sex with men: a randomized controlled trial. Clin Infect Dis. 2021;73:824-831. doi: 10.1093/cid/ciab153
20. CDC. Sexually transmitted infections treatment guidelines, 2021: Expedited partner therapy. US Department of Health and Human Services. July 22, 2021. Accessed April 19, 2023. www.cdc.gov/std/treatment-guidelines/clinical-EPT.htm
21. Golden MR, Whittington WLH, Handsfield HH, et al. Effect of expedited treatment of sex partners on recurrent or persistent gonorrhea or chlamydial infection. N Engl J Med. 2005;352:676-685. doi: 10.1056/NEJMoa041681
22. Schillinger JA, Kissinger P, Calvet H, et al. Patient-delivered partner treatment with azithromycin to prevent repeated Chlamydia trachomatis infection among women: a randomized, controlled trial. Sex Transm Dis. 2003;30:49-56. doi: 10.1097/00007435-200301000-00011
23. Cameron ST, Glasier A, Scott G, et al. Novel interventions to reduce re-infection in women with chlamydia: a randomized controlled trial. Hum Reprod. 2009;24:888-895. doi: 10.1093/humrep/den475
24. McNulty A, Teh MF, Freedman E. Patient delivered partner therapy for chlamydial infection—what would be missed? Sex Transm Dis. 2008;35:834-836. doi: 10.1097/OLQ.0b013e3181761993
25. Stekler J, Bachmann L, Brotman RM, et al. Concurrent sexually transmitted infections (STIs) in sex partners of patients with selected STIs: implications for patient-delivered partner therapy. Clin Infect Dis. 2005;40:787-793. doi: 10.1086/428043
26. Schillinger JA, Kissinger P, Calvet H, et al. Patient-delivered partner treatment with azithromycin to prevent repeated Chlamydia trachomatis infection among women: a randomized, controlled trial. Sex Transm Dis. 2003;30:49-56. doi: 10.1097/00007435-200301000-00011
27. Stupiansky NW, Van der Pol B, Williams JA, et al. The natural history of incident gonococcal infection in adolescent women. Sex Transm Dis. 2011;38:750-754. doi: 10.1097/OLQ.0b013e31820ff9a4
28. CDC. Sexually transmitted infections treatment guidelines, 2021: Screening recommendations and considerations referenced in treatment guidelines and original sources. US Department of Health and Human Services. June 6, 2022. Accessed May 9, 2023. www.cdc.gov/std/treatment-guidelines/screening-recommen dations.htm
29. Cantor A, Dana T, Griffen JC, et al. Screening for chlamydial and gonococcal infections: a systematic review update for the US Preventive Services Task Force. Evidence Synthesis No. 206. AHRQ Report No. 21-05275-EF-1. Agency for Healthcare Research and Quality. September 2021. www.ncbi.nlm.nih.gov/books/NBK574045
30. CDC. Update to CDC’s sexually transmitted diseases treatment guidelines, 2006: fluoroquinolones no longer recommended for treatment of gonococcal infections. MMWR Morb Mortal Wkly Rep. 2007;56:332-336.
31. Rowley J, Vander Hoorn S, Korenromp E, et al. Chlamydia, gonorrhoea, trichomoniasis and syphilis: global prevalence and incidence estimates, 2016. Bull World Health Organ. 2019:97:548-562P. doi: 10.2471/BLT.18.228486
32. Patel EU, Gaydos CA, Packman ZR, et al. Prevalence and correlates of Trichomonas vaginalis infection among men and women in the United States. Clin Infect Dis. 2018;67:211-217. doi: 10.1093/cid/ciy079
33. CDC. Sexually transmitted infections treatment guidelines, 2021. Mycoplasma genitalium. US Department of Health and Human Services. July 22, 2021. Accessed April 23, 2023. www.cdc.gov/std/treatment-guidelines/mycoplasmagenitalium.htm
34. Manhart LE, Broad JM, Bolden MR. Mycoplasma genitalium: should we treat and how? Clin Infect Dis. 2011;53(suppl 3):S129-S142. doi:10.1093/cid/cir702.
35. Corey L, Wald A. Genital herpes. In: Holmes KK, Sparling PF, Stamm WE, et al, eds. Sexually Transmitted Diseases. 4th ed. McGraw-Hill; 2008:399-437.
36. CDC. Sexually transmitted infections treatment guidelines, 2021: Genital herpes. US Department of Health and Human Services. September 21, 2022. Accessed April 23, 2023. www.cdc.gov/std/treatment-guidelines/herpes.htm
37. Looker KJ, Elmes JAR, Gottlieb SL, et al. Effect of HSV-2 infection on subsequent HIV acquisition: an updated systematic review and meta-analysis. Lancet Infect Dis. 2017;17:1303-1316. doi: 10.1016/S1473-3099(17)30405-X
38. Rollenhagen C, Lathrop M, Macura SL, et al. Herpes simplex virus type-2 stimulates HIV-1 replication in cervical tissues: implications for HIV-1 transmission and efficacy of anti-HIV-1 microbicides. Mucosal Immunol. 2014;7:1165-1174. doi: 10.1038/mi.2014.3
39. Cogliano V, Baan R, Straif K, et al; WHO International Agency for Research on Cancer. Carcinogenicity of human papillomaviruses. Lancet Oncol.
40. Simon MA, Tseng CW, Wong JB. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686. doi:10.1001/jama.2018.10897
41. Perkins RB, Guido RS, Castle PE, et al; . 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2020;24:102-131. doi: 10.1097/LGT.0000000000000525
42. Meites E, Szilagyi PG, Chesson HW, et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68:698-702. doi: 10.15585/mmwr.mm6832a3
43. Schmidt R, Carson PJ, Jansen RJ. Resurgence of syphilis in the United States: an assessment of contributing factors. Infect Dis (Auckl). 2019;12:1178633719883282. doi: 10.1177/1178633719883282
44. Boog GHP, Lopes JVZ, Mahler JV, et al. Diagnostic tools for neurosyphilis: a systematic review. BMC Infect Dis. 2021;21:568. doi: 10.1186/s12879-021-06264-8
45. CDC. Sexually transmitted infections treatment guidelines, 2021. Syphilis. US Department of Health and Human Services. April 20, 2023. Accessed April 24, 2023. www.cdc.gov/std/treatment-guidelines/syphilis.htm
46. Matthias JM, Rahman MM, Newman DR, et al. Effectiveness of prenatal screening and treatment to prevent congenital syphilis, Louisiana and Florida, 2013-2014. Sex Transm Dis. 2017;44:498-502. doi: 10.1097/OLQ.0000000000000638
47. Clement ME, Okeke NL, Hicks CB. Treatment of syphilis: a systematic review. JAMA. 2014;312:1905-1917. doi: 10.1001/jama.2014.13259
48. Davidson KW, Barry MJ, Mangione CM, et al; US Preventive Services Task Force. Screening for chlamydia and gonorrhea: US Preventive Services Task Force recommendation statement. JAMA. 2021;326:949-956. doi: 10.1001/jama.2021.14081
49. Krist AH, Davidson KW, Mangione CM, et al; US Preventive Services Task Force. Screening for hepatitis B virus infection in adolescents and adults: US Preventive Services Task Force recommendation statement. JAMA. 2020;324:2415-2422. doi: 10.1001/jama.2020.22980
50. Owens DK, Davidson KW, Krist AH, et al; US Preventive Services Task Force. Screening for hepatitis C virus infection in adolescents and adults: US Preventive Services Task Force recommendation statement. JAMA. 2020;323:970-975. doi: 10.1001/jama.2020.1123
51. Bibbins-Domingo K, Grossman DC, Curry SJ, et al; US Preventive Services Task Force. Serologic screening for genital herpes infection: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:2525-2530. doi: 10.1001/jama.2016.16776
52. Curry SJ, Krist AH, Owens DK, et al; US Preventive Services Task Force. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686. doi: 10.1001/jama.2018.10897
53. Owens DK, Davidson KW, Krist AH, et al; US Preventive Services Task Force. Screening for HIV infection: US Preventive Services Task Force recommendation statement. JAMA. 2019;321:2326-2336. doi: 10.1001/jama.2019.6587
54. Farley TA, Cohen DA, Elkins W. Asymptomatic sexually transmitted diseases: the case for screening. Prev Med. 2003;36:502-509. doi: 10.1016/s0091-7435(02)00058-0
55. US Public Health Service. Preexposure prophylaxis for the prevention of HIV infection in the United States—2021 update. A clinical practice guideline. Centers for Disease Control and Prevention. Accessed April 24, 2023. www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2021.pdf
56. CDC. Sexually transmitted infections treatment guidelines, 2021: Sexual assault and abuse and STIs—adolescents and adults, 2021. US Department of Health and Human Services. July 22, 2021. Accessed April 24, 2023. www.cdc.gov/std/treatment-guidelines/sexual-assault-adults.htm
PRACTICE RECOMMENDATIONS
› Focus efforts to prevent sexually transmitted infections (STIs) on patients ages 15 to 24 years—because half of new STIs in the United States occur in this age group. A
› Screen for other STIs, including HIV infection, if a person tests positive for a single STI. A
› Treat STIs by following updated (2021) guidelines developed by the Centers for Disease Control and Prevention. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series