Article
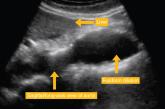
How best to diagnose and manage abdominal aortic aneurysms
- Author:
- Nicholas LeFevre, MD, MSAM, FAAFP
- Brandon Chase, MD
The evidence summarized here can help guide your approach to this life-threatening condition that often goes undetected until rupture.
Article
Subclinical hypothyroidism: Let the evidence be your guide
- Author:
- Nicholas LeFevre, MD, MSAM, FAAFP
- Alexander Zweig, MD, MSAM
Patient age, time of day, and supplement use influence screening results; repeat testing is advised. Avoid treating to improve mood, cognition,...
