User login
Overexpression of HOXA9 and activated JAK/STAT signaling cooperate to drive the development of T-cell acute lymphoblastic leukemia (ALL), according to researchers.
The team found that JAK3 mutations are significantly associated with elevated HOXA9 expression in T-ALL, and co-expression of HOXA9 and JAK3 mutations prompt “rapid” leukemia development in mice.
In addition, STAT5 and HOXA9 occupy similar genetic loci, which results in increased JAK-STAT signaling in leukemia cells.
These discoveries, and results of subsequent experiments, suggested that PIM1 and JAK1 are potential therapeutic targets for T-ALL.
Jan Cools, PhD, of VIB-KU Leuven Center for Cancer Biology in Leuven, Belgium, and his colleagues described this research in Cancer Discovery.
“JAK3/STAT5 mutations are important in ALL since they stimulate the growth of the cells,” Dr Cools said. “[W]e found that JAK3/STAT5 mutations frequently occur together with HOXA9 mutations.”
In analyzing data from 2 cohorts of T-ALL patients, the researchers found that IL7R/JAK/STAT5 mutations were more frequent in HOXA+ cases, and HOXA9 was “the most important upregulated gene of the HOXA cluster.” (HOXA9 expression levels were significantly higher than HOXA10 or HOXA11 levels.)
“We examined the cooperation between JAK3/STAT5 mutation and HOXA9, [and] we observed that HOXA9 boosts the effects of other genes, leading to tumor development,” said study author Charles de Bock, PhD, of VIB-KU Leuven.
“As a result, when JAK3/STAT5 mutations and HOXA9 are both present, leukemia develops more rapidly and aggressively.”
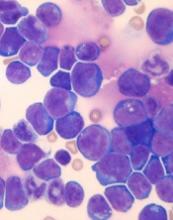
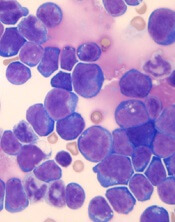
The researchers found that co-expression of HOXA9 and the JAK3 M511I mutation led to rapid leukemia development in mice. Animals with co-expression of HOXA9 and JAK3 (M511I) developed leukemia that was characterized by an increase in peripheral white blood cell counts that exceeded 10,000 cells/mm3 within 30 days.
In addition, these mice had a significant decrease in disease-free survival compared to mice with JAK3 (M511I) alone. The median disease-free survival was 25 days and 126.5 days, respectively (P<0.0001).
Further analysis revealed co-localization of HOXA9 and STAT5. The researchers also found that HOXA9 enhances transcriptional activity of STAT5 in leukemia cells.
The team said this reconfirms STAT5 as “a major central player” in T-ALL, and it suggests that STAT5 target genes such as PIM1 could be therapeutic targets for T-ALL.
To test this theory, the researchers inhibited both PIM1 and JAK1 in JAK/STAT mutant T-ALL. The PIM1 inhibitor AZD1208 and the JAK1/2 inhibitor ruxolitinib demonstrated synergy and significantly reduced leukemia burden in vivo.
Overexpression of HOXA9 and activated JAK/STAT signaling cooperate to drive the development of T-cell acute lymphoblastic leukemia (ALL), according to researchers.
The team found that JAK3 mutations are significantly associated with elevated HOXA9 expression in T-ALL, and co-expression of HOXA9 and JAK3 mutations prompt “rapid” leukemia development in mice.
In addition, STAT5 and HOXA9 occupy similar genetic loci, which results in increased JAK-STAT signaling in leukemia cells.
These discoveries, and results of subsequent experiments, suggested that PIM1 and JAK1 are potential therapeutic targets for T-ALL.
Jan Cools, PhD, of VIB-KU Leuven Center for Cancer Biology in Leuven, Belgium, and his colleagues described this research in Cancer Discovery.
“JAK3/STAT5 mutations are important in ALL since they stimulate the growth of the cells,” Dr Cools said. “[W]e found that JAK3/STAT5 mutations frequently occur together with HOXA9 mutations.”
In analyzing data from 2 cohorts of T-ALL patients, the researchers found that IL7R/JAK/STAT5 mutations were more frequent in HOXA+ cases, and HOXA9 was “the most important upregulated gene of the HOXA cluster.” (HOXA9 expression levels were significantly higher than HOXA10 or HOXA11 levels.)
“We examined the cooperation between JAK3/STAT5 mutation and HOXA9, [and] we observed that HOXA9 boosts the effects of other genes, leading to tumor development,” said study author Charles de Bock, PhD, of VIB-KU Leuven.
“As a result, when JAK3/STAT5 mutations and HOXA9 are both present, leukemia develops more rapidly and aggressively.”
The researchers found that co-expression of HOXA9 and the JAK3 M511I mutation led to rapid leukemia development in mice. Animals with co-expression of HOXA9 and JAK3 (M511I) developed leukemia that was characterized by an increase in peripheral white blood cell counts that exceeded 10,000 cells/mm3 within 30 days.
In addition, these mice had a significant decrease in disease-free survival compared to mice with JAK3 (M511I) alone. The median disease-free survival was 25 days and 126.5 days, respectively (P<0.0001).
Further analysis revealed co-localization of HOXA9 and STAT5. The researchers also found that HOXA9 enhances transcriptional activity of STAT5 in leukemia cells.
The team said this reconfirms STAT5 as “a major central player” in T-ALL, and it suggests that STAT5 target genes such as PIM1 could be therapeutic targets for T-ALL.
To test this theory, the researchers inhibited both PIM1 and JAK1 in JAK/STAT mutant T-ALL. The PIM1 inhibitor AZD1208 and the JAK1/2 inhibitor ruxolitinib demonstrated synergy and significantly reduced leukemia burden in vivo.
Overexpression of HOXA9 and activated JAK/STAT signaling cooperate to drive the development of T-cell acute lymphoblastic leukemia (ALL), according to researchers.
The team found that JAK3 mutations are significantly associated with elevated HOXA9 expression in T-ALL, and co-expression of HOXA9 and JAK3 mutations prompt “rapid” leukemia development in mice.
In addition, STAT5 and HOXA9 occupy similar genetic loci, which results in increased JAK-STAT signaling in leukemia cells.
These discoveries, and results of subsequent experiments, suggested that PIM1 and JAK1 are potential therapeutic targets for T-ALL.
Jan Cools, PhD, of VIB-KU Leuven Center for Cancer Biology in Leuven, Belgium, and his colleagues described this research in Cancer Discovery.
“JAK3/STAT5 mutations are important in ALL since they stimulate the growth of the cells,” Dr Cools said. “[W]e found that JAK3/STAT5 mutations frequently occur together with HOXA9 mutations.”
In analyzing data from 2 cohorts of T-ALL patients, the researchers found that IL7R/JAK/STAT5 mutations were more frequent in HOXA+ cases, and HOXA9 was “the most important upregulated gene of the HOXA cluster.” (HOXA9 expression levels were significantly higher than HOXA10 or HOXA11 levels.)
“We examined the cooperation between JAK3/STAT5 mutation and HOXA9, [and] we observed that HOXA9 boosts the effects of other genes, leading to tumor development,” said study author Charles de Bock, PhD, of VIB-KU Leuven.
“As a result, when JAK3/STAT5 mutations and HOXA9 are both present, leukemia develops more rapidly and aggressively.”
The researchers found that co-expression of HOXA9 and the JAK3 M511I mutation led to rapid leukemia development in mice. Animals with co-expression of HOXA9 and JAK3 (M511I) developed leukemia that was characterized by an increase in peripheral white blood cell counts that exceeded 10,000 cells/mm3 within 30 days.
In addition, these mice had a significant decrease in disease-free survival compared to mice with JAK3 (M511I) alone. The median disease-free survival was 25 days and 126.5 days, respectively (P<0.0001).
Further analysis revealed co-localization of HOXA9 and STAT5. The researchers also found that HOXA9 enhances transcriptional activity of STAT5 in leukemia cells.
The team said this reconfirms STAT5 as “a major central player” in T-ALL, and it suggests that STAT5 target genes such as PIM1 could be therapeutic targets for T-ALL.
To test this theory, the researchers inhibited both PIM1 and JAK1 in JAK/STAT mutant T-ALL. The PIM1 inhibitor AZD1208 and the JAK1/2 inhibitor ruxolitinib demonstrated synergy and significantly reduced leukemia burden in vivo.