User login
The incidence of cutaneous melanoma (CM) has increased, warranting further study of new risk factors.1,2 Hereditary risk factors for CM include light-colored eyes; fair skin; light brown, blonde, or red hair; tendency to burn; high density of freckles; history of other types of skin cancer; high number of common, atypical, and/or congenital nevi; and family history of skin cancer, as well as risks related to the presence of CDKN2A, BRAF, and MC1R gene mutations. Environmental risk factors include UV exposure from sunlight or tanning beds, among others.3-5
Nutritional factors also have been suggested as possible modifiable risk factors for CM.6 Evidence from epidemiological studies show that diets rich in fruits and vegetables are associated with lower risks for several types of cancer.7,8 A growing number of studies have assessed the effects of diet and the intake of nutrients on the prevention of cancer, specifically the use of dietary supplements to protect the skin from the adverse effects of UV light.6
Preformed vitamin A (ie, retinol) is necessary for the regulation of cell differentiation and also can reduce the incidence of skin tumors in animals exposed to UV light. Certain carotenoids such as α-carotene and β-carotene are metabolized to retinol. These retinol precursors, along with antioxidant nutrients, are important components of fruits and vegetables and may account for the observed anticancer effects of these foods.8
The aim of this study was to assess the relationship between dietary intake and the risk for CM.
Methods
Participants
A case-control study was carried out between 2012 and 2013 at 3 reference centers in Porto Alegre, Brazil—Universidade Federal de Ciências da Saúde de Porto Alegre, Pontifícia Universidade Católica do Rio Grande do Sul, and Hospital de Clínicas de Porto Alegre—for the treatment of patients with CM. Enrolled patients were 18 years and older with a diagnosis of primary CM confirmed by histology. Controls were selected from patients at the same centers, and they were enrolled and matched by institution. Controls were frequency matched to cases by sex and age (+/– 5 years). Exclusion criteria for controls were those presenting with suspicious lesions and those needing radiation therapy or chemotherapy due to other diseases. The study was approved by the ethics committees of the participating centers and informed consent was obtained from all participants. A total of 191 participants (95 cases; 96 controls) were enrolled in the study.
Data Collection
After informed consent was obtained, participants were interviewed and were clinically examined by an experienced dermatologist (C.B.H. and M.M.S.). The questionnaire included sociodemographic variables, medical history, phenotypic characteristics (ie, Fitzpatrick skin type, skin/hair/eye color), family history of skin cancer, history of sunlight exposure, history of sunburns, use of artificial tanning, sunscreen use, and detailed dietary intake. Physical examination included the assessment of several melanocytic lesions (nevi, freckles/ephelides, lentigines, and café au lait spots), actinic keratoses, solar elastosis, and nonmelanocytic tumors following the International Agency for Research on Cancer (IARC) protocol.9
Using a food frequency questionnaire, participants were asked to report their usual frequency of consumption of each food from a list of 36 foods. The frequency of intake of all groups of food and beverages was defined according to the following scale: never, rarely (less than once monthly), once or twice weekly, 3 to 4 times weekly, 5 to 7 times weekly, and more than 7 times weekly. Combination of categories was based on the overall distribution among controls. Therefore, for some items such as mussels and fresh herbs, only 2 categories were used.
Statistical Analysis
A descriptive statistical analysis of the results was performed using SPSS version 20.0 with absolute and relative frequencies for the categorical variables, and mean, SD, and median for the continuous variables. The symmetry of distributions was investigated using the Kolmogorov-Smirnov test.
A t test for independent groups was applied for the continuous variables, while the Pearson χ2 test was used for the categorical variables. The Fisher exact test was used in situations in which at least 25% of the values of the cells presented an expected frequency of less than 5. Monte Carlo simulation was used when at least 1 variable had a polytomic characteristic. Odds ratio (OR) was used to estimate the strength of the association between exposures and outcome. An unconditional binary logistic regression was used to study the association between dietary variables and the risk for CM. To obtain unbiased estimates, multivariate analyses were performed controlling for 1 or more confounding variables. Using low exposure as a base category, the risks and 95% CIs were calculated for the high-exposure categories. Based on the results of bivariate analyses, variables with P≤.25 or lower were included in the models. The likelihood ratio test was used to decide which covariates should be maintained in the model. To test the goodness of fit of the models, the Hosmer-Lemeshow statistic was used.
Potential confounding factors considered in the logistic regression model were sex; age; education level; skin, hair, and eye color; Fitzpatrick skin type; presence of freckles, solar lentigines, and actinic keratosis; history of nonmelanoma skin cancer; number of melanocytic nevi; family history of skin cancer; sunburns in adulthood (≥6 episodes a year); occupational sun exposure; and history of sunscreen use in adulthood.
Results
A total of 191 participants were enrolled in the study (95 [49.7%] cases; 96 [50.3%] controls). Most participants were female (60.0% of cases; 59.4% of controls). The mean age (SD) of cases and controls was 56.8 (13.9) years and 56.5 (13.2) years, respectively. Mean body mass index (SD) did not differ between cases (27.2 [4.6]) and controls (28.2 [6.5]). Education levels of 8 years or less predominated in both groups (64.2% of cases; 57.3% of controls). No statistical difference was found for sex, age, education, or body mass index. The most frequent anatomic sites of CM were the trunk (54.7%) and arms (20.0%), and the most frequent histological type was superficial spreading (62.8%). The median Breslow thickness was 0.90 mm. Ulceration was observed in 20.9% of the cases, and 67% of participants with CM had a high mitotic rate (≥1 mitosis per square millimeter).
Phenotypic characteristics associated with an increased risk for melanoma were light brown hair (OR, 6.73; 95% CI, 3.30-14.2), blonde/red hair (OR, 21.7; 95% CI, 7.51-63.1), light-colored eyes (eg, blue, gray, green)(OR, 13.2; 95% CI, 6.13-28.7), light brown eyes (OR, 5.01; 95% CI, 2.24-11.5), and Fitzpatrick skin types I and II (OR, 7.37; 95% CI, 2.90-26.1). Family history of skin cancer was associated with an increased risk for CM (OR, 4.31; 95% CI, 1.86-10.7) as well as sunburns in adulthood (OR, 1.64; 95% CI, 1.17-1.99). Regular sunscreen use in adulthood had a 5-fold increased risk for CM compared to not using sunscreen regularly (OR, 5.6; 95% CI, 2.85-10.7). Regarding pigmented lesions, the presence of solar lentigines (OR, 4.8; 95% CI, 2.2-11.2), 60 or more nevi (OR, 5.4; 95% CI, 2.4-12.7), and freckles (OR, 3.7; 95% CI, 1.82-7.64) were all associated with an increased risk for CM. Solar elastosis (OR, 2.5; 95% CI, 1.08-5.85), actinic keratosis (OR, 9.1, 95% CI, 3.97-20.84), and occupational exposure to sun (OR, 2.57; 95% CI, 1.23-5.38) also were associated with an increased risk for melanoma.
The intake of most of the foods and beverages included in the study showed no association with CM. High frequency of butter intake (more than daily) was a protective factor for CM (OR, 0.33; 95% CI, 0.16-0.70) compared to low-frequency consumption (daily and less than daily). Consumption of mussels (OR, 0.53; 95% CI, 0.29-0.97) and oregano (OR, 0.28; 95% CI, 0.12-0.66) also were shown to be protective against CM (OR, 0.53; 95% CI, 0.29-0.97). Regarding beverages, those in the highest categories of consumption—liquor (OR, 2.12; 95% CI, 1.09-4.12) and spirits (OR, 2.23; 95% CI, 1.16-4.68)—were associated with an increased risk for CM.
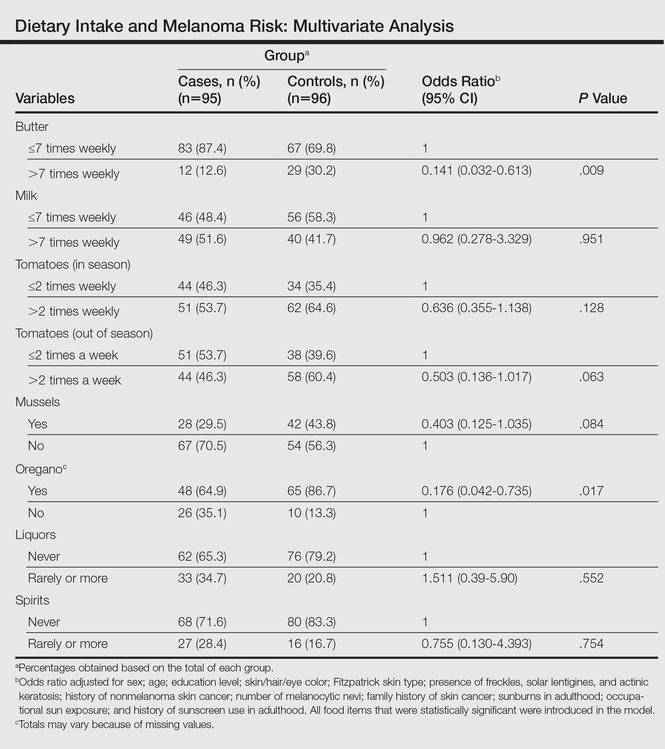
To identify the relationship between CM and the consumption of some foods that were relevant on bivariate analysis, we performed a multivariate model. When adjustments were made, the association remained for butter (OR, 0.141; 95% CI, 0.032-0.613) and oregano (OR, 0.176; 95% CI, 0.042-0.735), while the risk associated with the consumption of both liquor (OR, 1.511; 95% CI, 0.39-5.90) and spirits (OR, 0.755; 95% CI, 0.130-4.393) disappeared (Table).
Comment
Observational studies show that diets rich in fruits and vegetables are associated with a lower risk for different types of cancers.7,8 According to some studies, more than 30% of cancers in adulthood could be prevented or delayed by appropriate dietary intake and physical activity.10 However, there are still limited data on some specific cancers such as CM.
Substantial differences in the incidence of CM among different populations have suggested that environmental factors may play an etiological role in the development of CM and diet could be one of the modifiable risk factors.11-13
Initially, we assessed the already known risk factors for CM, and results showed a significantly increased risk for participants with light brown, blonde, or red hair (P<.0001); light-colored and light brown eyes (P<.0001); Fitzpatrick skin types I and II (P<.0001); positive family history of skin cancer (P=.001); the presence of solar lentigines (P<.001), freckles (P<.001), and actinic keratosis (P<.0001); and high number of nevi (P<.0001). Sunburns in adulthood (P<.001) were associated with an increased risk for CM, and our findings are in agreement with the literature.12
Besides confirming the well-known risk factors for CM, our study also showed that some foods (eg, butter, oregano) may act as important protective factors in CM. It could be argued that the increased risks associated with the well-known risk factors (eg, Fitzpatrick skin type, number of sunburns) might not be as strong and/or could be modulated by dietary factors. To further elucidate this critical issue, we analyzed our data by examining the joint relationship between dietary consumption, individual characteristics, sun exposure, and melanoma. We conducted a multivariable analysis controlling for the well-known risk factors and our findings suggest that both butter and oregano, foods that are rich in vitamins A and D, are independent and protective risk factors for melanoma.
Vitamin A (retinol) is a fat-soluble, organic compound that cannot be synthesized by humans but is necessary for normal physiological function and therefore is classified as an essential nutrient. The main source of vitamin A in the human diet is from retinyl esters, mostly from animal products such as dairy products (eg, butter) as well as from plant-based, provitamin A carotenoids (α-carotene, β-carotene) that can be converted to retinol in the intestines.14
Some case-control studies have investigated the association of vitamin A intake and CM risk, reporting mixed findings. Naldi et al15 found a notable inverse association between vitamin A intake and CM risk. Le Marchand et al16 found no inverse association for carotenoids or retinol. Kirkpatrick et al17 found no evidence of a protective effect for vitamin A or carotenoids on CM. However, the Nurses’ Health Study and the Nurses’ Health Study II reported inverse associations between CM and retinol from foods and dietary supplements.8
Dairy products such as butter contain several components considered to be potentially anticarcinogenic, such as calcium, vitamin D, butyric acid, conjugated linoleic acid, sphingolipids, and probiotic bacteria. Some studies found an inverted association between melanoma and high intake of dairy products or other dietary sources of vitamin D, while some investigators showed no association.6,18
Fortes et al18 assessed the role of diet on CM and found no protective effects of butter intake against the development of melanoma; however, a protective effect was found for carrots, which are rich in provitamin A (β-carotene) and for the regular intake of herbs rich in polyphenols (eg, rosemary). In our study, we found a protective effect against CM for butter but not for other dairy products. These findings could be explained by the high content of vitamin A in butter in comparison to other dairy products. Habitual intake of oregano also was associated with a protective effect for CM. Oregano is rich in polyphenols such as carvacrol, thymol, and rosmarinic acid, which are known for their antioxidant capacities and the inhibition of cyclooxygenase.19-21 At experimental levels, both carvacrol and thymol have been shown to inhibit the growth of melanoma cells.19,20 Rosmarinic acid, contained by both rosemary and oregano, have been shown at experimental levels to have photoprotective effects against melanoma.21
The relationship between dietary and nutritional intake and CM has a great potential that should be further explored. Tong and Young22 showed that proanthocyanidins found in grape seeds, epigallocatechin-3-gallate, resveratrol, rosmarinic acid, lycopene, and fig latex have demonstrated clear anticancer effects toward melanoma.
The strength of this study is the high response rate of both cases and controls and the use of incidence melanoma cases that decrease recall bias. A limitation of our study is that food portions were based on average portion size for each food item and therefore it can capture habitual consumption but not calculate actual nutrient intake. Misclassification of dietary exposure also could be a problem. Part of this misclassification is a result of a food frequency questionnaire being an imperfect measure of dietary history; however, we evaluated the reproducibility of the food frequency questionnaire used in this case-control study. Overall, there was a fair to good reproducibility between answers in 2 different periods (12 months apart). For example, agreement for frequency of intake of fresh herbs, tomatoes, and butter were 90.8%, 83.1%, and 83.3%, respectively.
Our sample size had sufficient statistical power to detect the effects of diet on CM.
Conclusion
Our study indicates that butter and oregano intake seem to have a protective role against the development of CM. Further studies are needed to confirm these findings.
- Gilchrest B, Eller MS, Geller AC, et al. The pathogenesis of melanoma induced by ultraviolet radiation. N Engl J Med. 1999;340:1341-1347.
- Lotti T, Bruscino N, Hercogova J, et al. Controversial issues on melanoma. Dermatol Ther. 2012;25:458-462.
- Ródenas JM, Delgado-Rodríguez M, Herranz MT, et al. Sun exposure, pigmentary traits, and risk of cutaneous malignant melanoma: a case-control study in a Mediterranean population. Cancer Causes Control. 1996;7:275-283.
- Autier P, Doré JF. Influence of sun exposures during childhood and during adulthood on melanoma risk. EEPIMEL and EORTC. Melanoma Cooperative Group. European Organization for research and treatment of cancer. Int J Cancer. 1998;77:533-537.
- Fortes C, Mastroeni S, Melchi F, et al. The association between residential pesticide use and cutaneous melanoma. Eur J Cancer. 2007;43:1066-1075.
- Jensen JD, Wing GJ, Dellavalle RP. Nutrition and melanoma prevention. Clin Dermatol. 2010;28:644-649.
- Millen AE, Tucker MA, Hartge P, et al. Diet and melanoma in a case-control study. Cancer Epidemiol Biomarkers Prev. 2004;13:1042-1051.
- Feskanich D, Willett WC, Hunter DJ, et al. Dietary intakes of vitamins A, C, and E and risk of melanoma in two cohorts of women. Br J Cancer. 2003;88:1381-1387.
- English DR, Mac Lennan R, Rivers J, et al. Epidemiological studies of melanocytic naevi: protocol for identifying and recording naevi. International Agency for Research on Cancer (IARC) internal report. No. 90/002. Lyon, France: IARC; 1990.
- Cancer preventability statistics. World Cancer Research Fund website. http://www.wcrf-uk.org/uk/preventing-cancer/cancer-preventability-statistics. Accessed May 24, 2016.
- Gandini S, Raimondi S, Gnagnarella P, et al. Vitamin D and skin cancer: a meta-analysis. Eur J Cancer. 2009;45:634-641.
- Gandini S, Sera F, Cattaruzza MS, et al. Meta-analysis of risk factors for cutaneous melanoma: II. sun exposure. Eur J Cancer. 2005;41:45-60.
- Volkovova K, Bilanicova D, Bartonova A, et al. Associations between environmental factors and incidence of cutaneous melanoma. review. Environ Health. 2012;11(11, suppl 1):S12.
- Asgari MM, Brasky TM, White E. Association of vitamin A and carotenoid intake with melanoma risk in a large prospective cohort. J Invest Dermatol. 2012;132:1573-1582.
- Naldi L, Gallus S, Tavani A, et al. Risk of melanoma and vitamin A, coffee and alcohol: a case-control study from Italy. Eur J Cancer Prev. 2004;13:503-508.
- Le Marchand L, Saltzman BS, Hankin JH, et al. Sun exposure, diet, and melanoma in Hawaii Caucasians. Am J Epidemiol. 2006;164:232-245.
- Kirkpatrick CS, White E, Lee JA. Case-control study of malignant melanoma in Washington State. II. diet, alcohol, and obesity. Am J Epidemiol. 1994;139:869-880.
- Fortes C, Mastroeni S, Melchi F, et al. A protective effect of the Mediterranean diet for cutaneous melanoma. Int J Epidemiol. 2008;37:1018-1029.
- Landa P, Kokoska L, Pribylova M, et al. In vitro anti-inflammatory activity of carvacrol: inhibitory effect on COX-2 catalyzed prostaglandin E(2) biosynthesis. Arch Pharm Res. 2009;32:75-78.
- He L, Mo H, Hadisusilo S, et al. Isoprenoids suppress the growth of murine B16 melanomas in vitro and in vivo. J Nutr. 1997;127:668-674.
- Sánchez-Campillo M, Gabaldon JA, Castillo J, et al. Rosmarinic acid, a photo-protective agent against UV and other ionizing radiations. Food Chem Toxicol. 2009;47:386-392.
- Tong LX, Young LC. Nutrition: the future of melanoma prevention? J Am Acad Dermatol. 2014;71:151-160.
The incidence of cutaneous melanoma (CM) has increased, warranting further study of new risk factors.1,2 Hereditary risk factors for CM include light-colored eyes; fair skin; light brown, blonde, or red hair; tendency to burn; high density of freckles; history of other types of skin cancer; high number of common, atypical, and/or congenital nevi; and family history of skin cancer, as well as risks related to the presence of CDKN2A, BRAF, and MC1R gene mutations. Environmental risk factors include UV exposure from sunlight or tanning beds, among others.3-5
Nutritional factors also have been suggested as possible modifiable risk factors for CM.6 Evidence from epidemiological studies show that diets rich in fruits and vegetables are associated with lower risks for several types of cancer.7,8 A growing number of studies have assessed the effects of diet and the intake of nutrients on the prevention of cancer, specifically the use of dietary supplements to protect the skin from the adverse effects of UV light.6
Preformed vitamin A (ie, retinol) is necessary for the regulation of cell differentiation and also can reduce the incidence of skin tumors in animals exposed to UV light. Certain carotenoids such as α-carotene and β-carotene are metabolized to retinol. These retinol precursors, along with antioxidant nutrients, are important components of fruits and vegetables and may account for the observed anticancer effects of these foods.8
The aim of this study was to assess the relationship between dietary intake and the risk for CM.
Methods
Participants
A case-control study was carried out between 2012 and 2013 at 3 reference centers in Porto Alegre, Brazil—Universidade Federal de Ciências da Saúde de Porto Alegre, Pontifícia Universidade Católica do Rio Grande do Sul, and Hospital de Clínicas de Porto Alegre—for the treatment of patients with CM. Enrolled patients were 18 years and older with a diagnosis of primary CM confirmed by histology. Controls were selected from patients at the same centers, and they were enrolled and matched by institution. Controls were frequency matched to cases by sex and age (+/– 5 years). Exclusion criteria for controls were those presenting with suspicious lesions and those needing radiation therapy or chemotherapy due to other diseases. The study was approved by the ethics committees of the participating centers and informed consent was obtained from all participants. A total of 191 participants (95 cases; 96 controls) were enrolled in the study.
Data Collection
After informed consent was obtained, participants were interviewed and were clinically examined by an experienced dermatologist (C.B.H. and M.M.S.). The questionnaire included sociodemographic variables, medical history, phenotypic characteristics (ie, Fitzpatrick skin type, skin/hair/eye color), family history of skin cancer, history of sunlight exposure, history of sunburns, use of artificial tanning, sunscreen use, and detailed dietary intake. Physical examination included the assessment of several melanocytic lesions (nevi, freckles/ephelides, lentigines, and café au lait spots), actinic keratoses, solar elastosis, and nonmelanocytic tumors following the International Agency for Research on Cancer (IARC) protocol.9
Using a food frequency questionnaire, participants were asked to report their usual frequency of consumption of each food from a list of 36 foods. The frequency of intake of all groups of food and beverages was defined according to the following scale: never, rarely (less than once monthly), once or twice weekly, 3 to 4 times weekly, 5 to 7 times weekly, and more than 7 times weekly. Combination of categories was based on the overall distribution among controls. Therefore, for some items such as mussels and fresh herbs, only 2 categories were used.
Statistical Analysis
A descriptive statistical analysis of the results was performed using SPSS version 20.0 with absolute and relative frequencies for the categorical variables, and mean, SD, and median for the continuous variables. The symmetry of distributions was investigated using the Kolmogorov-Smirnov test.
A t test for independent groups was applied for the continuous variables, while the Pearson χ2 test was used for the categorical variables. The Fisher exact test was used in situations in which at least 25% of the values of the cells presented an expected frequency of less than 5. Monte Carlo simulation was used when at least 1 variable had a polytomic characteristic. Odds ratio (OR) was used to estimate the strength of the association between exposures and outcome. An unconditional binary logistic regression was used to study the association between dietary variables and the risk for CM. To obtain unbiased estimates, multivariate analyses were performed controlling for 1 or more confounding variables. Using low exposure as a base category, the risks and 95% CIs were calculated for the high-exposure categories. Based on the results of bivariate analyses, variables with P≤.25 or lower were included in the models. The likelihood ratio test was used to decide which covariates should be maintained in the model. To test the goodness of fit of the models, the Hosmer-Lemeshow statistic was used.
Potential confounding factors considered in the logistic regression model were sex; age; education level; skin, hair, and eye color; Fitzpatrick skin type; presence of freckles, solar lentigines, and actinic keratosis; history of nonmelanoma skin cancer; number of melanocytic nevi; family history of skin cancer; sunburns in adulthood (≥6 episodes a year); occupational sun exposure; and history of sunscreen use in adulthood.
Results
A total of 191 participants were enrolled in the study (95 [49.7%] cases; 96 [50.3%] controls). Most participants were female (60.0% of cases; 59.4% of controls). The mean age (SD) of cases and controls was 56.8 (13.9) years and 56.5 (13.2) years, respectively. Mean body mass index (SD) did not differ between cases (27.2 [4.6]) and controls (28.2 [6.5]). Education levels of 8 years or less predominated in both groups (64.2% of cases; 57.3% of controls). No statistical difference was found for sex, age, education, or body mass index. The most frequent anatomic sites of CM were the trunk (54.7%) and arms (20.0%), and the most frequent histological type was superficial spreading (62.8%). The median Breslow thickness was 0.90 mm. Ulceration was observed in 20.9% of the cases, and 67% of participants with CM had a high mitotic rate (≥1 mitosis per square millimeter).
Phenotypic characteristics associated with an increased risk for melanoma were light brown hair (OR, 6.73; 95% CI, 3.30-14.2), blonde/red hair (OR, 21.7; 95% CI, 7.51-63.1), light-colored eyes (eg, blue, gray, green)(OR, 13.2; 95% CI, 6.13-28.7), light brown eyes (OR, 5.01; 95% CI, 2.24-11.5), and Fitzpatrick skin types I and II (OR, 7.37; 95% CI, 2.90-26.1). Family history of skin cancer was associated with an increased risk for CM (OR, 4.31; 95% CI, 1.86-10.7) as well as sunburns in adulthood (OR, 1.64; 95% CI, 1.17-1.99). Regular sunscreen use in adulthood had a 5-fold increased risk for CM compared to not using sunscreen regularly (OR, 5.6; 95% CI, 2.85-10.7). Regarding pigmented lesions, the presence of solar lentigines (OR, 4.8; 95% CI, 2.2-11.2), 60 or more nevi (OR, 5.4; 95% CI, 2.4-12.7), and freckles (OR, 3.7; 95% CI, 1.82-7.64) were all associated with an increased risk for CM. Solar elastosis (OR, 2.5; 95% CI, 1.08-5.85), actinic keratosis (OR, 9.1, 95% CI, 3.97-20.84), and occupational exposure to sun (OR, 2.57; 95% CI, 1.23-5.38) also were associated with an increased risk for melanoma.
The intake of most of the foods and beverages included in the study showed no association with CM. High frequency of butter intake (more than daily) was a protective factor for CM (OR, 0.33; 95% CI, 0.16-0.70) compared to low-frequency consumption (daily and less than daily). Consumption of mussels (OR, 0.53; 95% CI, 0.29-0.97) and oregano (OR, 0.28; 95% CI, 0.12-0.66) also were shown to be protective against CM (OR, 0.53; 95% CI, 0.29-0.97). Regarding beverages, those in the highest categories of consumption—liquor (OR, 2.12; 95% CI, 1.09-4.12) and spirits (OR, 2.23; 95% CI, 1.16-4.68)—were associated with an increased risk for CM.
To identify the relationship between CM and the consumption of some foods that were relevant on bivariate analysis, we performed a multivariate model. When adjustments were made, the association remained for butter (OR, 0.141; 95% CI, 0.032-0.613) and oregano (OR, 0.176; 95% CI, 0.042-0.735), while the risk associated with the consumption of both liquor (OR, 1.511; 95% CI, 0.39-5.90) and spirits (OR, 0.755; 95% CI, 0.130-4.393) disappeared (Table).

Comment
Observational studies show that diets rich in fruits and vegetables are associated with a lower risk for different types of cancers.7,8 According to some studies, more than 30% of cancers in adulthood could be prevented or delayed by appropriate dietary intake and physical activity.10 However, there are still limited data on some specific cancers such as CM.
Substantial differences in the incidence of CM among different populations have suggested that environmental factors may play an etiological role in the development of CM and diet could be one of the modifiable risk factors.11-13
Initially, we assessed the already known risk factors for CM, and results showed a significantly increased risk for participants with light brown, blonde, or red hair (P<.0001); light-colored and light brown eyes (P<.0001); Fitzpatrick skin types I and II (P<.0001); positive family history of skin cancer (P=.001); the presence of solar lentigines (P<.001), freckles (P<.001), and actinic keratosis (P<.0001); and high number of nevi (P<.0001). Sunburns in adulthood (P<.001) were associated with an increased risk for CM, and our findings are in agreement with the literature.12
Besides confirming the well-known risk factors for CM, our study also showed that some foods (eg, butter, oregano) may act as important protective factors in CM. It could be argued that the increased risks associated with the well-known risk factors (eg, Fitzpatrick skin type, number of sunburns) might not be as strong and/or could be modulated by dietary factors. To further elucidate this critical issue, we analyzed our data by examining the joint relationship between dietary consumption, individual characteristics, sun exposure, and melanoma. We conducted a multivariable analysis controlling for the well-known risk factors and our findings suggest that both butter and oregano, foods that are rich in vitamins A and D, are independent and protective risk factors for melanoma.
Vitamin A (retinol) is a fat-soluble, organic compound that cannot be synthesized by humans but is necessary for normal physiological function and therefore is classified as an essential nutrient. The main source of vitamin A in the human diet is from retinyl esters, mostly from animal products such as dairy products (eg, butter) as well as from plant-based, provitamin A carotenoids (α-carotene, β-carotene) that can be converted to retinol in the intestines.14
Some case-control studies have investigated the association of vitamin A intake and CM risk, reporting mixed findings. Naldi et al15 found a notable inverse association between vitamin A intake and CM risk. Le Marchand et al16 found no inverse association for carotenoids or retinol. Kirkpatrick et al17 found no evidence of a protective effect for vitamin A or carotenoids on CM. However, the Nurses’ Health Study and the Nurses’ Health Study II reported inverse associations between CM and retinol from foods and dietary supplements.8
Dairy products such as butter contain several components considered to be potentially anticarcinogenic, such as calcium, vitamin D, butyric acid, conjugated linoleic acid, sphingolipids, and probiotic bacteria. Some studies found an inverted association between melanoma and high intake of dairy products or other dietary sources of vitamin D, while some investigators showed no association.6,18
Fortes et al18 assessed the role of diet on CM and found no protective effects of butter intake against the development of melanoma; however, a protective effect was found for carrots, which are rich in provitamin A (β-carotene) and for the regular intake of herbs rich in polyphenols (eg, rosemary). In our study, we found a protective effect against CM for butter but not for other dairy products. These findings could be explained by the high content of vitamin A in butter in comparison to other dairy products. Habitual intake of oregano also was associated with a protective effect for CM. Oregano is rich in polyphenols such as carvacrol, thymol, and rosmarinic acid, which are known for their antioxidant capacities and the inhibition of cyclooxygenase.19-21 At experimental levels, both carvacrol and thymol have been shown to inhibit the growth of melanoma cells.19,20 Rosmarinic acid, contained by both rosemary and oregano, have been shown at experimental levels to have photoprotective effects against melanoma.21
The relationship between dietary and nutritional intake and CM has a great potential that should be further explored. Tong and Young22 showed that proanthocyanidins found in grape seeds, epigallocatechin-3-gallate, resveratrol, rosmarinic acid, lycopene, and fig latex have demonstrated clear anticancer effects toward melanoma.
The strength of this study is the high response rate of both cases and controls and the use of incidence melanoma cases that decrease recall bias. A limitation of our study is that food portions were based on average portion size for each food item and therefore it can capture habitual consumption but not calculate actual nutrient intake. Misclassification of dietary exposure also could be a problem. Part of this misclassification is a result of a food frequency questionnaire being an imperfect measure of dietary history; however, we evaluated the reproducibility of the food frequency questionnaire used in this case-control study. Overall, there was a fair to good reproducibility between answers in 2 different periods (12 months apart). For example, agreement for frequency of intake of fresh herbs, tomatoes, and butter were 90.8%, 83.1%, and 83.3%, respectively.
Our sample size had sufficient statistical power to detect the effects of diet on CM.
Conclusion
Our study indicates that butter and oregano intake seem to have a protective role against the development of CM. Further studies are needed to confirm these findings.
The incidence of cutaneous melanoma (CM) has increased, warranting further study of new risk factors.1,2 Hereditary risk factors for CM include light-colored eyes; fair skin; light brown, blonde, or red hair; tendency to burn; high density of freckles; history of other types of skin cancer; high number of common, atypical, and/or congenital nevi; and family history of skin cancer, as well as risks related to the presence of CDKN2A, BRAF, and MC1R gene mutations. Environmental risk factors include UV exposure from sunlight or tanning beds, among others.3-5
Nutritional factors also have been suggested as possible modifiable risk factors for CM.6 Evidence from epidemiological studies show that diets rich in fruits and vegetables are associated with lower risks for several types of cancer.7,8 A growing number of studies have assessed the effects of diet and the intake of nutrients on the prevention of cancer, specifically the use of dietary supplements to protect the skin from the adverse effects of UV light.6
Preformed vitamin A (ie, retinol) is necessary for the regulation of cell differentiation and also can reduce the incidence of skin tumors in animals exposed to UV light. Certain carotenoids such as α-carotene and β-carotene are metabolized to retinol. These retinol precursors, along with antioxidant nutrients, are important components of fruits and vegetables and may account for the observed anticancer effects of these foods.8
The aim of this study was to assess the relationship between dietary intake and the risk for CM.
Methods
Participants
A case-control study was carried out between 2012 and 2013 at 3 reference centers in Porto Alegre, Brazil—Universidade Federal de Ciências da Saúde de Porto Alegre, Pontifícia Universidade Católica do Rio Grande do Sul, and Hospital de Clínicas de Porto Alegre—for the treatment of patients with CM. Enrolled patients were 18 years and older with a diagnosis of primary CM confirmed by histology. Controls were selected from patients at the same centers, and they were enrolled and matched by institution. Controls were frequency matched to cases by sex and age (+/– 5 years). Exclusion criteria for controls were those presenting with suspicious lesions and those needing radiation therapy or chemotherapy due to other diseases. The study was approved by the ethics committees of the participating centers and informed consent was obtained from all participants. A total of 191 participants (95 cases; 96 controls) were enrolled in the study.
Data Collection
After informed consent was obtained, participants were interviewed and were clinically examined by an experienced dermatologist (C.B.H. and M.M.S.). The questionnaire included sociodemographic variables, medical history, phenotypic characteristics (ie, Fitzpatrick skin type, skin/hair/eye color), family history of skin cancer, history of sunlight exposure, history of sunburns, use of artificial tanning, sunscreen use, and detailed dietary intake. Physical examination included the assessment of several melanocytic lesions (nevi, freckles/ephelides, lentigines, and café au lait spots), actinic keratoses, solar elastosis, and nonmelanocytic tumors following the International Agency for Research on Cancer (IARC) protocol.9
Using a food frequency questionnaire, participants were asked to report their usual frequency of consumption of each food from a list of 36 foods. The frequency of intake of all groups of food and beverages was defined according to the following scale: never, rarely (less than once monthly), once or twice weekly, 3 to 4 times weekly, 5 to 7 times weekly, and more than 7 times weekly. Combination of categories was based on the overall distribution among controls. Therefore, for some items such as mussels and fresh herbs, only 2 categories were used.
Statistical Analysis
A descriptive statistical analysis of the results was performed using SPSS version 20.0 with absolute and relative frequencies for the categorical variables, and mean, SD, and median for the continuous variables. The symmetry of distributions was investigated using the Kolmogorov-Smirnov test.
A t test for independent groups was applied for the continuous variables, while the Pearson χ2 test was used for the categorical variables. The Fisher exact test was used in situations in which at least 25% of the values of the cells presented an expected frequency of less than 5. Monte Carlo simulation was used when at least 1 variable had a polytomic characteristic. Odds ratio (OR) was used to estimate the strength of the association between exposures and outcome. An unconditional binary logistic regression was used to study the association between dietary variables and the risk for CM. To obtain unbiased estimates, multivariate analyses were performed controlling for 1 or more confounding variables. Using low exposure as a base category, the risks and 95% CIs were calculated for the high-exposure categories. Based on the results of bivariate analyses, variables with P≤.25 or lower were included in the models. The likelihood ratio test was used to decide which covariates should be maintained in the model. To test the goodness of fit of the models, the Hosmer-Lemeshow statistic was used.
Potential confounding factors considered in the logistic regression model were sex; age; education level; skin, hair, and eye color; Fitzpatrick skin type; presence of freckles, solar lentigines, and actinic keratosis; history of nonmelanoma skin cancer; number of melanocytic nevi; family history of skin cancer; sunburns in adulthood (≥6 episodes a year); occupational sun exposure; and history of sunscreen use in adulthood.
Results
A total of 191 participants were enrolled in the study (95 [49.7%] cases; 96 [50.3%] controls). Most participants were female (60.0% of cases; 59.4% of controls). The mean age (SD) of cases and controls was 56.8 (13.9) years and 56.5 (13.2) years, respectively. Mean body mass index (SD) did not differ between cases (27.2 [4.6]) and controls (28.2 [6.5]). Education levels of 8 years or less predominated in both groups (64.2% of cases; 57.3% of controls). No statistical difference was found for sex, age, education, or body mass index. The most frequent anatomic sites of CM were the trunk (54.7%) and arms (20.0%), and the most frequent histological type was superficial spreading (62.8%). The median Breslow thickness was 0.90 mm. Ulceration was observed in 20.9% of the cases, and 67% of participants with CM had a high mitotic rate (≥1 mitosis per square millimeter).
Phenotypic characteristics associated with an increased risk for melanoma were light brown hair (OR, 6.73; 95% CI, 3.30-14.2), blonde/red hair (OR, 21.7; 95% CI, 7.51-63.1), light-colored eyes (eg, blue, gray, green)(OR, 13.2; 95% CI, 6.13-28.7), light brown eyes (OR, 5.01; 95% CI, 2.24-11.5), and Fitzpatrick skin types I and II (OR, 7.37; 95% CI, 2.90-26.1). Family history of skin cancer was associated with an increased risk for CM (OR, 4.31; 95% CI, 1.86-10.7) as well as sunburns in adulthood (OR, 1.64; 95% CI, 1.17-1.99). Regular sunscreen use in adulthood had a 5-fold increased risk for CM compared to not using sunscreen regularly (OR, 5.6; 95% CI, 2.85-10.7). Regarding pigmented lesions, the presence of solar lentigines (OR, 4.8; 95% CI, 2.2-11.2), 60 or more nevi (OR, 5.4; 95% CI, 2.4-12.7), and freckles (OR, 3.7; 95% CI, 1.82-7.64) were all associated with an increased risk for CM. Solar elastosis (OR, 2.5; 95% CI, 1.08-5.85), actinic keratosis (OR, 9.1, 95% CI, 3.97-20.84), and occupational exposure to sun (OR, 2.57; 95% CI, 1.23-5.38) also were associated with an increased risk for melanoma.
The intake of most of the foods and beverages included in the study showed no association with CM. High frequency of butter intake (more than daily) was a protective factor for CM (OR, 0.33; 95% CI, 0.16-0.70) compared to low-frequency consumption (daily and less than daily). Consumption of mussels (OR, 0.53; 95% CI, 0.29-0.97) and oregano (OR, 0.28; 95% CI, 0.12-0.66) also were shown to be protective against CM (OR, 0.53; 95% CI, 0.29-0.97). Regarding beverages, those in the highest categories of consumption—liquor (OR, 2.12; 95% CI, 1.09-4.12) and spirits (OR, 2.23; 95% CI, 1.16-4.68)—were associated with an increased risk for CM.
To identify the relationship between CM and the consumption of some foods that were relevant on bivariate analysis, we performed a multivariate model. When adjustments were made, the association remained for butter (OR, 0.141; 95% CI, 0.032-0.613) and oregano (OR, 0.176; 95% CI, 0.042-0.735), while the risk associated with the consumption of both liquor (OR, 1.511; 95% CI, 0.39-5.90) and spirits (OR, 0.755; 95% CI, 0.130-4.393) disappeared (Table).

Comment
Observational studies show that diets rich in fruits and vegetables are associated with a lower risk for different types of cancers.7,8 According to some studies, more than 30% of cancers in adulthood could be prevented or delayed by appropriate dietary intake and physical activity.10 However, there are still limited data on some specific cancers such as CM.
Substantial differences in the incidence of CM among different populations have suggested that environmental factors may play an etiological role in the development of CM and diet could be one of the modifiable risk factors.11-13
Initially, we assessed the already known risk factors for CM, and results showed a significantly increased risk for participants with light brown, blonde, or red hair (P<.0001); light-colored and light brown eyes (P<.0001); Fitzpatrick skin types I and II (P<.0001); positive family history of skin cancer (P=.001); the presence of solar lentigines (P<.001), freckles (P<.001), and actinic keratosis (P<.0001); and high number of nevi (P<.0001). Sunburns in adulthood (P<.001) were associated with an increased risk for CM, and our findings are in agreement with the literature.12
Besides confirming the well-known risk factors for CM, our study also showed that some foods (eg, butter, oregano) may act as important protective factors in CM. It could be argued that the increased risks associated with the well-known risk factors (eg, Fitzpatrick skin type, number of sunburns) might not be as strong and/or could be modulated by dietary factors. To further elucidate this critical issue, we analyzed our data by examining the joint relationship between dietary consumption, individual characteristics, sun exposure, and melanoma. We conducted a multivariable analysis controlling for the well-known risk factors and our findings suggest that both butter and oregano, foods that are rich in vitamins A and D, are independent and protective risk factors for melanoma.
Vitamin A (retinol) is a fat-soluble, organic compound that cannot be synthesized by humans but is necessary for normal physiological function and therefore is classified as an essential nutrient. The main source of vitamin A in the human diet is from retinyl esters, mostly from animal products such as dairy products (eg, butter) as well as from plant-based, provitamin A carotenoids (α-carotene, β-carotene) that can be converted to retinol in the intestines.14
Some case-control studies have investigated the association of vitamin A intake and CM risk, reporting mixed findings. Naldi et al15 found a notable inverse association between vitamin A intake and CM risk. Le Marchand et al16 found no inverse association for carotenoids or retinol. Kirkpatrick et al17 found no evidence of a protective effect for vitamin A or carotenoids on CM. However, the Nurses’ Health Study and the Nurses’ Health Study II reported inverse associations between CM and retinol from foods and dietary supplements.8
Dairy products such as butter contain several components considered to be potentially anticarcinogenic, such as calcium, vitamin D, butyric acid, conjugated linoleic acid, sphingolipids, and probiotic bacteria. Some studies found an inverted association between melanoma and high intake of dairy products or other dietary sources of vitamin D, while some investigators showed no association.6,18
Fortes et al18 assessed the role of diet on CM and found no protective effects of butter intake against the development of melanoma; however, a protective effect was found for carrots, which are rich in provitamin A (β-carotene) and for the regular intake of herbs rich in polyphenols (eg, rosemary). In our study, we found a protective effect against CM for butter but not for other dairy products. These findings could be explained by the high content of vitamin A in butter in comparison to other dairy products. Habitual intake of oregano also was associated with a protective effect for CM. Oregano is rich in polyphenols such as carvacrol, thymol, and rosmarinic acid, which are known for their antioxidant capacities and the inhibition of cyclooxygenase.19-21 At experimental levels, both carvacrol and thymol have been shown to inhibit the growth of melanoma cells.19,20 Rosmarinic acid, contained by both rosemary and oregano, have been shown at experimental levels to have photoprotective effects against melanoma.21
The relationship between dietary and nutritional intake and CM has a great potential that should be further explored. Tong and Young22 showed that proanthocyanidins found in grape seeds, epigallocatechin-3-gallate, resveratrol, rosmarinic acid, lycopene, and fig latex have demonstrated clear anticancer effects toward melanoma.
The strength of this study is the high response rate of both cases and controls and the use of incidence melanoma cases that decrease recall bias. A limitation of our study is that food portions were based on average portion size for each food item and therefore it can capture habitual consumption but not calculate actual nutrient intake. Misclassification of dietary exposure also could be a problem. Part of this misclassification is a result of a food frequency questionnaire being an imperfect measure of dietary history; however, we evaluated the reproducibility of the food frequency questionnaire used in this case-control study. Overall, there was a fair to good reproducibility between answers in 2 different periods (12 months apart). For example, agreement for frequency of intake of fresh herbs, tomatoes, and butter were 90.8%, 83.1%, and 83.3%, respectively.
Our sample size had sufficient statistical power to detect the effects of diet on CM.
Conclusion
Our study indicates that butter and oregano intake seem to have a protective role against the development of CM. Further studies are needed to confirm these findings.
- Gilchrest B, Eller MS, Geller AC, et al. The pathogenesis of melanoma induced by ultraviolet radiation. N Engl J Med. 1999;340:1341-1347.
- Lotti T, Bruscino N, Hercogova J, et al. Controversial issues on melanoma. Dermatol Ther. 2012;25:458-462.
- Ródenas JM, Delgado-Rodríguez M, Herranz MT, et al. Sun exposure, pigmentary traits, and risk of cutaneous malignant melanoma: a case-control study in a Mediterranean population. Cancer Causes Control. 1996;7:275-283.
- Autier P, Doré JF. Influence of sun exposures during childhood and during adulthood on melanoma risk. EEPIMEL and EORTC. Melanoma Cooperative Group. European Organization for research and treatment of cancer. Int J Cancer. 1998;77:533-537.
- Fortes C, Mastroeni S, Melchi F, et al. The association between residential pesticide use and cutaneous melanoma. Eur J Cancer. 2007;43:1066-1075.
- Jensen JD, Wing GJ, Dellavalle RP. Nutrition and melanoma prevention. Clin Dermatol. 2010;28:644-649.
- Millen AE, Tucker MA, Hartge P, et al. Diet and melanoma in a case-control study. Cancer Epidemiol Biomarkers Prev. 2004;13:1042-1051.
- Feskanich D, Willett WC, Hunter DJ, et al. Dietary intakes of vitamins A, C, and E and risk of melanoma in two cohorts of women. Br J Cancer. 2003;88:1381-1387.
- English DR, Mac Lennan R, Rivers J, et al. Epidemiological studies of melanocytic naevi: protocol for identifying and recording naevi. International Agency for Research on Cancer (IARC) internal report. No. 90/002. Lyon, France: IARC; 1990.
- Cancer preventability statistics. World Cancer Research Fund website. http://www.wcrf-uk.org/uk/preventing-cancer/cancer-preventability-statistics. Accessed May 24, 2016.
- Gandini S, Raimondi S, Gnagnarella P, et al. Vitamin D and skin cancer: a meta-analysis. Eur J Cancer. 2009;45:634-641.
- Gandini S, Sera F, Cattaruzza MS, et al. Meta-analysis of risk factors for cutaneous melanoma: II. sun exposure. Eur J Cancer. 2005;41:45-60.
- Volkovova K, Bilanicova D, Bartonova A, et al. Associations between environmental factors and incidence of cutaneous melanoma. review. Environ Health. 2012;11(11, suppl 1):S12.
- Asgari MM, Brasky TM, White E. Association of vitamin A and carotenoid intake with melanoma risk in a large prospective cohort. J Invest Dermatol. 2012;132:1573-1582.
- Naldi L, Gallus S, Tavani A, et al. Risk of melanoma and vitamin A, coffee and alcohol: a case-control study from Italy. Eur J Cancer Prev. 2004;13:503-508.
- Le Marchand L, Saltzman BS, Hankin JH, et al. Sun exposure, diet, and melanoma in Hawaii Caucasians. Am J Epidemiol. 2006;164:232-245.
- Kirkpatrick CS, White E, Lee JA. Case-control study of malignant melanoma in Washington State. II. diet, alcohol, and obesity. Am J Epidemiol. 1994;139:869-880.
- Fortes C, Mastroeni S, Melchi F, et al. A protective effect of the Mediterranean diet for cutaneous melanoma. Int J Epidemiol. 2008;37:1018-1029.
- Landa P, Kokoska L, Pribylova M, et al. In vitro anti-inflammatory activity of carvacrol: inhibitory effect on COX-2 catalyzed prostaglandin E(2) biosynthesis. Arch Pharm Res. 2009;32:75-78.
- He L, Mo H, Hadisusilo S, et al. Isoprenoids suppress the growth of murine B16 melanomas in vitro and in vivo. J Nutr. 1997;127:668-674.
- Sánchez-Campillo M, Gabaldon JA, Castillo J, et al. Rosmarinic acid, a photo-protective agent against UV and other ionizing radiations. Food Chem Toxicol. 2009;47:386-392.
- Tong LX, Young LC. Nutrition: the future of melanoma prevention? J Am Acad Dermatol. 2014;71:151-160.
- Gilchrest B, Eller MS, Geller AC, et al. The pathogenesis of melanoma induced by ultraviolet radiation. N Engl J Med. 1999;340:1341-1347.
- Lotti T, Bruscino N, Hercogova J, et al. Controversial issues on melanoma. Dermatol Ther. 2012;25:458-462.
- Ródenas JM, Delgado-Rodríguez M, Herranz MT, et al. Sun exposure, pigmentary traits, and risk of cutaneous malignant melanoma: a case-control study in a Mediterranean population. Cancer Causes Control. 1996;7:275-283.
- Autier P, Doré JF. Influence of sun exposures during childhood and during adulthood on melanoma risk. EEPIMEL and EORTC. Melanoma Cooperative Group. European Organization for research and treatment of cancer. Int J Cancer. 1998;77:533-537.
- Fortes C, Mastroeni S, Melchi F, et al. The association between residential pesticide use and cutaneous melanoma. Eur J Cancer. 2007;43:1066-1075.
- Jensen JD, Wing GJ, Dellavalle RP. Nutrition and melanoma prevention. Clin Dermatol. 2010;28:644-649.
- Millen AE, Tucker MA, Hartge P, et al. Diet and melanoma in a case-control study. Cancer Epidemiol Biomarkers Prev. 2004;13:1042-1051.
- Feskanich D, Willett WC, Hunter DJ, et al. Dietary intakes of vitamins A, C, and E and risk of melanoma in two cohorts of women. Br J Cancer. 2003;88:1381-1387.
- English DR, Mac Lennan R, Rivers J, et al. Epidemiological studies of melanocytic naevi: protocol for identifying and recording naevi. International Agency for Research on Cancer (IARC) internal report. No. 90/002. Lyon, France: IARC; 1990.
- Cancer preventability statistics. World Cancer Research Fund website. http://www.wcrf-uk.org/uk/preventing-cancer/cancer-preventability-statistics. Accessed May 24, 2016.
- Gandini S, Raimondi S, Gnagnarella P, et al. Vitamin D and skin cancer: a meta-analysis. Eur J Cancer. 2009;45:634-641.
- Gandini S, Sera F, Cattaruzza MS, et al. Meta-analysis of risk factors for cutaneous melanoma: II. sun exposure. Eur J Cancer. 2005;41:45-60.
- Volkovova K, Bilanicova D, Bartonova A, et al. Associations between environmental factors and incidence of cutaneous melanoma. review. Environ Health. 2012;11(11, suppl 1):S12.
- Asgari MM, Brasky TM, White E. Association of vitamin A and carotenoid intake with melanoma risk in a large prospective cohort. J Invest Dermatol. 2012;132:1573-1582.
- Naldi L, Gallus S, Tavani A, et al. Risk of melanoma and vitamin A, coffee and alcohol: a case-control study from Italy. Eur J Cancer Prev. 2004;13:503-508.
- Le Marchand L, Saltzman BS, Hankin JH, et al. Sun exposure, diet, and melanoma in Hawaii Caucasians. Am J Epidemiol. 2006;164:232-245.
- Kirkpatrick CS, White E, Lee JA. Case-control study of malignant melanoma in Washington State. II. diet, alcohol, and obesity. Am J Epidemiol. 1994;139:869-880.
- Fortes C, Mastroeni S, Melchi F, et al. A protective effect of the Mediterranean diet for cutaneous melanoma. Int J Epidemiol. 2008;37:1018-1029.
- Landa P, Kokoska L, Pribylova M, et al. In vitro anti-inflammatory activity of carvacrol: inhibitory effect on COX-2 catalyzed prostaglandin E(2) biosynthesis. Arch Pharm Res. 2009;32:75-78.
- He L, Mo H, Hadisusilo S, et al. Isoprenoids suppress the growth of murine B16 melanomas in vitro and in vivo. J Nutr. 1997;127:668-674.
- Sánchez-Campillo M, Gabaldon JA, Castillo J, et al. Rosmarinic acid, a photo-protective agent against UV and other ionizing radiations. Food Chem Toxicol. 2009;47:386-392.
- Tong LX, Young LC. Nutrition: the future of melanoma prevention? J Am Acad Dermatol. 2014;71:151-160.
Practice Points
- Hereditary and environmental risk factors have been identified for cutaneous melanoma (CM). Nutritional factors have been suggested as possible modifiable risk factors.
- Foods rich in vitamins A and D may be protective risk factors for CM.
