User login
ABSTRACT
Soft tissue defects associated with exposed tendon pose difficult reconstructive problems because of tendon adhesions, poor range of motion, poor cosmetic appearance, and donor site morbidity. Dermal regeneration template is a skin substitute widely used in reconstructive surgery, including the occasional coverage of tendons. However, postoperative functionality of the tendons has not been well documented. We report a case of using dermal regeneration template for soft tissue reconstruction overlying tendons with loss of paratenon in a patient with Dupuytren’s contracture. Dermal regeneration template may offer an alternative option for immediate tendon coverage in the hand.
Soft tissue defects overlying exposed tendon with loss of paratenon often precipitate poor clinical outcomes because of the dichotomous demands of both closing the overlying soft-tissue defect and providing a gliding surface for the underlying tendons.1 Although avoidance of adhesions and restoration of function are the primary goals of the procedure, satisfactory appearance is also desirable. Likewise, any form of coverage should ideally provide good vasculature required for complete healing and an early form of closure following débridement.2 Simple skin grafts do not adequately meet these demands because they result in a high rate of tendon adhesions,3 and also are limited in patients with limited donor skin availability or questionable underlying wound bed viability, such as in scleroderma.
In order to reduce the frequency of tendon adhesions by creating a gliding surface, the use of interpositional materials, both artificial and biologic, has been employed with varying degrees of success, including cellophane, chitosan membrane, fibrin sealant, autogenous fascial flaps, and autogenous venous grafts.4-7 Many of the autogenous flaps and grafts have been employed with good success.8 However, complications and donor site morbidity encourage alternative procedures, including the use of artificial substances.2,8-10
We present our clinical experience with a patient who underwent successful placement of Integra (Integra LifeSciences) Dermal Regeneration Template (DRT) directly over exposed tendons with a subsequent full-thickness skin graft several weeks later. The procedures were performed per the manufacturer’s specifications, resulting in 2 stages of reconstruction. In our experience, DRT can offer immediate coverage unrestricted by wound size, and provides shorter operative time and decreased donor site and surgical morbidity compared with flap coverage, while demonstrating good cosmetic results. The patient provided written informed consent for print and electronic publication of this case report.
CASE
A 74-year-old right-handed man with Dupuytren’s contracture was evaluated for recurrent symptomatic contracture causing difficulty with daily activities. He reported palpable cords and contractures in the ring and small fingers of the right hand. He had 2 prior open surgical procedures, including palmar and digital fasciectomy of both hands. On the right hand, the ring and small fingers demonstrated 90° proximal interphalangeal (PIP) and 60° metacarpophalangeal (MCP) flexion contractures. Palpable central cords were present on the flexor surfaces of both the ring and small fingers. A well-healed surgical incision, performed 22 years earlier, was present over the palmar aspect of the ring finger.
Continue to: With consideration given...
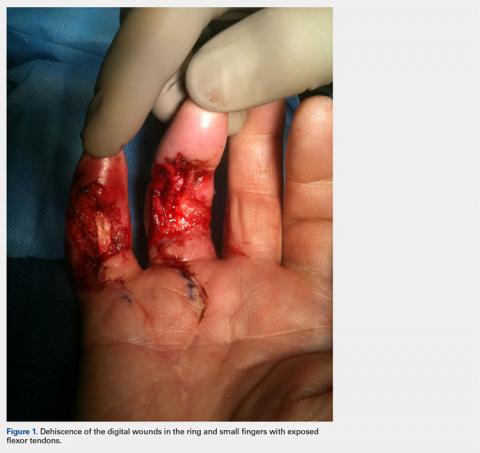
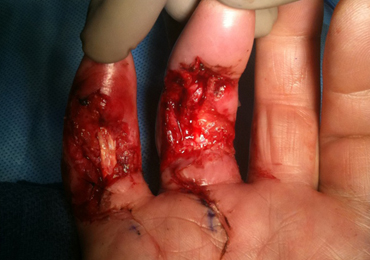
With consideration given to the patient’s recurrent contracture after a prior surgical procedure, we discussed surgical excision of the diseased cords in order to eliminate the possibility of a second recurrence and maximize the gain of motion. Following discussion with the patient, we performed palmar and digital fasciectomy of the ring and small finger contractures. Postoperatively, the patient was followed closely for wound complications and vascular status. On his return to our clinic 11 days later, the patient was noted to have dehiscence of the digital wounds in the ring and small fingers (Figure 1).
STAGE 1
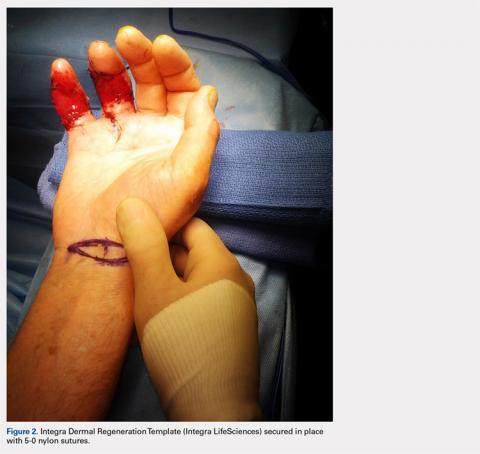
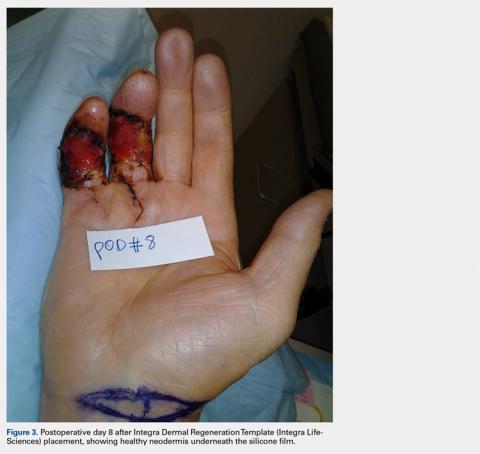
During the first stage, completed 14 days following the index procedure, débridement of the wounds was performed, followed by provisional DRT coverage of the tendons, secured with 5-0 nylon sutures (Figure 2).
STAGE 2
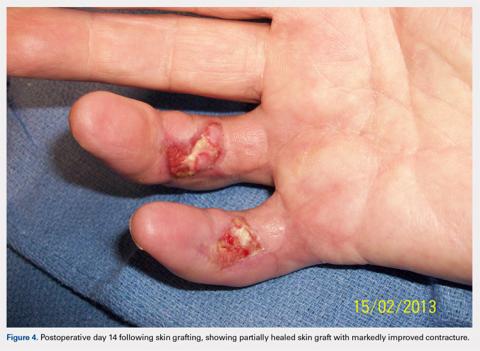
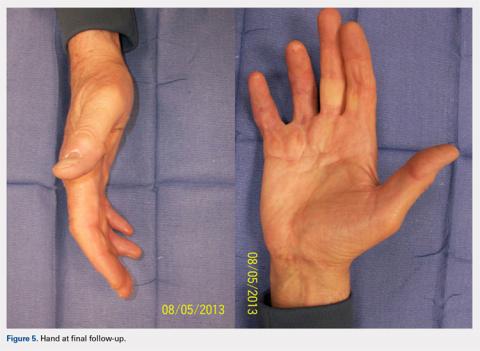
At approximately 2 weeks after application of the DRT, a full-thickness skin graft was applied. The thickness of the graft was chosen to allow for durable coverage of the palmar skin defects. Upon successful completion of the second stage, the patient was followed and evaluated for complete wound healing. On performing an examination 14 days after surgery, the ring and small fingers demonstrated only partially healed skin graft but significantly improved range of motion (ROM), with 40° to 90° arc of motion in the PIP joint and 25° to 90° arc of motion in the MCP joint (Figure 4). Owing to their limited size, the wounds were treated with dressing changes until successful healing (Figure 5).
Hand therapy was instituted to achieve maximum mobility for covered soft tissue and tendons and to maximize tendon gliding. At 1-year follow-up, the skin was fully healed and the patient’s active PIP motion was 30° to 90°, active MCP motion was 0° to 90°, and grip strength was 90 lb on both sides. The tendons glided under a well-vascularized tissue at the DRT placement site, and no secondary tenolysis procedure was deemed necessary.
DISCUSSION
Soft tissue defects with exposed tendons may offer a number of challenges for coverage. The primary concern is the creation of a gliding surface and the restoration of a functional tendon without adhesions.2 However, surgeons must use their own clinical judgment when choosing the method of coverage so as to minimize the effects of donor site morbidity and maximize the overall functional and cosmetic outcomes. All options must be considered while selecting a material or flap that is likely to survive in the relatively avascular tendon plane.2,8,11 When considering the reconstructive ladder, skin grafts may not represent a viable option in the presence of a nonvascularized wound bed, such as exposed tendon or bone, where paratenon or periosteum have been damaged. That leaves the surgeon with local flaps, regional flaps, free flaps, and skin substitutes.
Continue to : Before planning closure...
Before planning closure, wound conditions should be optimized, including wound bed quality, vascularization, and bacterial loads. Experimental data suggest that the bacterial load should be brought down below a critical level of 105 bacteria per g of tissue to allow a skin graft to take. This may be problematic from a practical standpoint because quantitative bacterial cultures take about 48 hours to obtain the result, long after a decision to graft is made. As a result, the surgeon may take an aggressive approach to wound débridement, making sure that all necrotic material has been sharply débrided prior to coverage.
As Levin12 noted in 1993, decisions regarding repair of any soft tissue defect may follow a well-delineated ladder beginning with the primary choice of split-thickness skin grafts and ending with free flaps. When treating tissue defects in the hand complex, flaps are an excellent option as they replace like with like, allow minimal scarring and early rehabilitation. 13,14 Nevertheless, a few general disadvantages are inherent in flap procedure: increase in operating time, risk of flap loss, and in case of free flaps, knowledge, experience, and microsurgical ability.2 In reference to complications, the rate of flap loss found by Khouri and colleagues15 was 4.1% with a 12.1% chance of incurring some measured complication, including wound dehiscence, arterial insufficiency, and flap necrosis.
Likewise, some of the conventional local and free flaps, including cutaneous and muscular flaps, prove ineffective in preventing tendon adhesions, create unsightly postoperative contours, or increase the area of trauma on the wounded hand, encouraging the use of free fascial flaps.11 Among the wide array of potential free fascial flaps, the temporoparietal, scapular, lateral arm, radial forearm, and free serratus fascial flaps are some of the most popular for hand defects.8,9 However, these procedures require an additional surgical site, meticulous dissection, microsurgical technique at times, and increased operating cost and time.2,8-10 Furthermore, free fascial flaps have demonstrated occasional partial flap loss and a decreased survival of the overlying skin graft, leading some to advocate delayed skin graft placement.10,16,17
On the basis of these complications, Bray and colleagues11 noted that the utility of free flaps may be limited in smaller clinical settings. The primary disadvantage of using DRTs is the necessity for a second operative procedure to harvest and place the skin graft. Traditionally, this is performed 2 to 3 weeks after the initial DRT application. Nevertheless, a 1-stage procedure can be performed in an outpatient setup, minimizing the burden to the patient and the medical costs, followed by secondary intention healing.
In response to critics of the 2-stage technique, Sanger and colleagues18 described single-stage use of DRT with split-thickness skin grafts with placement of an overlying wound vacuum-assisted closure to help speed incorporation of the DRT and improve survival of the immediately grafted skin. Another viable alternative is the McCash open-palm technique.19 In the open-palm technique, a Brunner zigzag incision is made in the affected digit. A transverse incision is made in the palm. A partial fasciectomy is performed in the palm and digit. After release, the digital incision is closed, and the palmar incision is left open. Although this well-studied and well-reported technique is known to reduce the risk of flap necrosis due to tension and hematoma,20 its main application is in the palm, as the name implies. Because in our patient the defect was palmar-digital with exposed “white structures,” we elected to use DRT.
Continue to: Although there is still...
Although there is still no perfect answer for wound coverage and closure in the hand with exposed or damaged tendons, DRT certainly performs well as a primary choice by minimizing adhesions; allowing a good ROM; and providing a durable, satisfactory cosmetic outcome. Likewise, an initial treatment with DRT does not preclude later, more elaborate reconstructive efforts, such as local or free flaps, if they continue to be indicated. DRT also does not diminish the ability to revise a tendon reconstruction if a secondary procedure is necessary. In our patient, tendon revision has not been necessary. DRT gives the surgeon a minimally invasive, efficient initial alternative to more labor-intensive, potentially morbid reconstructive procedures, without sacrificing outcome. Therefore, DRT can offer an alternative procedure in the surgeon’s armamentarium for tendon coverage in complex hand defects.
1. Flügel A. Kehrer C. Heitmann C, German G, Sauerbier M. Coverage of soft tissue defects of the hand with free fascial flaps. Microsurgery.2005;25(1):47-53.
2. Chen H, Buchman MT, Wei FC. Free flaps for soft tissue coverage in the hand and fingers. Hand Clin. 1999;15(4):541-554.
3. Chia J, Lim A, Peng YP. Use of an arterialized venous flap for resurfacing a circumferential soft tissue defect of a digit. Microsurgery. 2001; 21(8):374-378.
4. Wheeldon T. The use of cellophane as a permanent tendon sheath. J Bone J Surg Am; 1939;21(2):393-396.
5. Frykman E, Jacobsson S, Widenfalk B. Fibrin sealant in prevention of flexor tendon adhesions: an experimental study in the rabbit. J Hand Surg Am. 1993;18(1):68-75.
6. Jones NF, Lister GD. Free skin and composite flaps. In: Wolfe SW, Hotchkiss RN, Pederson WC, Kozin SH, eds. Green’s Operative hand surgery. 6th ed. New York, NY: Churchill Livingstone; 2011:1721-1756.
7. Yan D, Shi X, Lui Q. Reconstruction of tendon sheath by autogenous vein graft in preventing adhesion. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 1997;11(1):38-39.
8. Pederson WC. Upper extremity microsurgery. Plast Reconstr Surg. 2001;107(6):1524-1537; discussion 1538-15399, 1540-1543.
9. WintschK, Helaly P. Free flap of gliding tissue. J Reconstr Microsurg. 1986;2(3):143-151.
10. Meland NB, Weimar R. Microsurgical reconstruction: experience with free fascia flaps. Ann Plast Surg. 1991;27(1):1-8.
11. Bray PW, Boyer MI, Bowen CV. Complex injuries of the forearm. Coverage considerations. Hand Clin. 1997;13(2):263-278.
12. Levin LS. The reconstructive ladder: an orthoplastic approach. Ortho Clin North Am. 1993; 24(3):393-409.
13. Hallock GG. Utility of both muscle and fascia flaps in severe lower extremity trauma. J Trauma. 2000;48 (5):913-917. doi:10.1097/00005373-200005000-00016.
14. Hallock GG. The utility of both muscle and fascia flaps in severe upper extremity trauma. J Trauma. 2002;53(1):61-65. doi:10.1097/00005373-200207000-00013.
15. Khouri RK, Cooley BC, Kunselman AR, et al. A prospective study of microvascular free-flap surgery and outcome. Plast Reconstr Surg. 1998;102(3):711-721.
16. Woods JM 4th, Shack RB, Hagan KF. Free temporoparietal fascia flap in reconstruction of the lower extremity. Ann Plast Surg. 1995;34(5):501-506. doi:10.1097/00000637-199505000-00008.
17. Chung KC, Cederna PS. Endoscopic harvest of temporoparietal fascial free flaps for coverage of hand wounds. J Hand Surg Am. 2002;27(3):525-533.
18. Sanger C, Molnar JA, Newman CE, et al. Immediate skin grafting of an engineered dermal substitute: P37. Plast Reconstr Surg. 2005;116(3S):165.
19. McCash CR. The open palm technique in Dupuytren’s contracture. Br J Plast Surg. 1964;17:271-280.
20. Shaw DL, Wise DI, Holms W. Dupuytren's disease treated by palmar fasciectomy and an open palm technique. J Hand Surg Br. 1996;21(4):484-485.
ABSTRACT
Soft tissue defects associated with exposed tendon pose difficult reconstructive problems because of tendon adhesions, poor range of motion, poor cosmetic appearance, and donor site morbidity. Dermal regeneration template is a skin substitute widely used in reconstructive surgery, including the occasional coverage of tendons. However, postoperative functionality of the tendons has not been well documented. We report a case of using dermal regeneration template for soft tissue reconstruction overlying tendons with loss of paratenon in a patient with Dupuytren’s contracture. Dermal regeneration template may offer an alternative option for immediate tendon coverage in the hand.
Soft tissue defects overlying exposed tendon with loss of paratenon often precipitate poor clinical outcomes because of the dichotomous demands of both closing the overlying soft-tissue defect and providing a gliding surface for the underlying tendons.1 Although avoidance of adhesions and restoration of function are the primary goals of the procedure, satisfactory appearance is also desirable. Likewise, any form of coverage should ideally provide good vasculature required for complete healing and an early form of closure following débridement.2 Simple skin grafts do not adequately meet these demands because they result in a high rate of tendon adhesions,3 and also are limited in patients with limited donor skin availability or questionable underlying wound bed viability, such as in scleroderma.
In order to reduce the frequency of tendon adhesions by creating a gliding surface, the use of interpositional materials, both artificial and biologic, has been employed with varying degrees of success, including cellophane, chitosan membrane, fibrin sealant, autogenous fascial flaps, and autogenous venous grafts.4-7 Many of the autogenous flaps and grafts have been employed with good success.8 However, complications and donor site morbidity encourage alternative procedures, including the use of artificial substances.2,8-10
We present our clinical experience with a patient who underwent successful placement of Integra (Integra LifeSciences) Dermal Regeneration Template (DRT) directly over exposed tendons with a subsequent full-thickness skin graft several weeks later. The procedures were performed per the manufacturer’s specifications, resulting in 2 stages of reconstruction. In our experience, DRT can offer immediate coverage unrestricted by wound size, and provides shorter operative time and decreased donor site and surgical morbidity compared with flap coverage, while demonstrating good cosmetic results. The patient provided written informed consent for print and electronic publication of this case report.
CASE
A 74-year-old right-handed man with Dupuytren’s contracture was evaluated for recurrent symptomatic contracture causing difficulty with daily activities. He reported palpable cords and contractures in the ring and small fingers of the right hand. He had 2 prior open surgical procedures, including palmar and digital fasciectomy of both hands. On the right hand, the ring and small fingers demonstrated 90° proximal interphalangeal (PIP) and 60° metacarpophalangeal (MCP) flexion contractures. Palpable central cords were present on the flexor surfaces of both the ring and small fingers. A well-healed surgical incision, performed 22 years earlier, was present over the palmar aspect of the ring finger.
Continue to: With consideration given...
With consideration given to the patient’s recurrent contracture after a prior surgical procedure, we discussed surgical excision of the diseased cords in order to eliminate the possibility of a second recurrence and maximize the gain of motion. Following discussion with the patient, we performed palmar and digital fasciectomy of the ring and small finger contractures. Postoperatively, the patient was followed closely for wound complications and vascular status. On his return to our clinic 11 days later, the patient was noted to have dehiscence of the digital wounds in the ring and small fingers (Figure 1).
STAGE 1
During the first stage, completed 14 days following the index procedure, débridement of the wounds was performed, followed by provisional DRT coverage of the tendons, secured with 5-0 nylon sutures (Figure 2).
STAGE 2
At approximately 2 weeks after application of the DRT, a full-thickness skin graft was applied. The thickness of the graft was chosen to allow for durable coverage of the palmar skin defects. Upon successful completion of the second stage, the patient was followed and evaluated for complete wound healing. On performing an examination 14 days after surgery, the ring and small fingers demonstrated only partially healed skin graft but significantly improved range of motion (ROM), with 40° to 90° arc of motion in the PIP joint and 25° to 90° arc of motion in the MCP joint (Figure 4). Owing to their limited size, the wounds were treated with dressing changes until successful healing (Figure 5).
Hand therapy was instituted to achieve maximum mobility for covered soft tissue and tendons and to maximize tendon gliding. At 1-year follow-up, the skin was fully healed and the patient’s active PIP motion was 30° to 90°, active MCP motion was 0° to 90°, and grip strength was 90 lb on both sides. The tendons glided under a well-vascularized tissue at the DRT placement site, and no secondary tenolysis procedure was deemed necessary.
DISCUSSION
Soft tissue defects with exposed tendons may offer a number of challenges for coverage. The primary concern is the creation of a gliding surface and the restoration of a functional tendon without adhesions.2 However, surgeons must use their own clinical judgment when choosing the method of coverage so as to minimize the effects of donor site morbidity and maximize the overall functional and cosmetic outcomes. All options must be considered while selecting a material or flap that is likely to survive in the relatively avascular tendon plane.2,8,11 When considering the reconstructive ladder, skin grafts may not represent a viable option in the presence of a nonvascularized wound bed, such as exposed tendon or bone, where paratenon or periosteum have been damaged. That leaves the surgeon with local flaps, regional flaps, free flaps, and skin substitutes.
Continue to : Before planning closure...
Before planning closure, wound conditions should be optimized, including wound bed quality, vascularization, and bacterial loads. Experimental data suggest that the bacterial load should be brought down below a critical level of 105 bacteria per g of tissue to allow a skin graft to take. This may be problematic from a practical standpoint because quantitative bacterial cultures take about 48 hours to obtain the result, long after a decision to graft is made. As a result, the surgeon may take an aggressive approach to wound débridement, making sure that all necrotic material has been sharply débrided prior to coverage.
As Levin12 noted in 1993, decisions regarding repair of any soft tissue defect may follow a well-delineated ladder beginning with the primary choice of split-thickness skin grafts and ending with free flaps. When treating tissue defects in the hand complex, flaps are an excellent option as they replace like with like, allow minimal scarring and early rehabilitation. 13,14 Nevertheless, a few general disadvantages are inherent in flap procedure: increase in operating time, risk of flap loss, and in case of free flaps, knowledge, experience, and microsurgical ability.2 In reference to complications, the rate of flap loss found by Khouri and colleagues15 was 4.1% with a 12.1% chance of incurring some measured complication, including wound dehiscence, arterial insufficiency, and flap necrosis.
Likewise, some of the conventional local and free flaps, including cutaneous and muscular flaps, prove ineffective in preventing tendon adhesions, create unsightly postoperative contours, or increase the area of trauma on the wounded hand, encouraging the use of free fascial flaps.11 Among the wide array of potential free fascial flaps, the temporoparietal, scapular, lateral arm, radial forearm, and free serratus fascial flaps are some of the most popular for hand defects.8,9 However, these procedures require an additional surgical site, meticulous dissection, microsurgical technique at times, and increased operating cost and time.2,8-10 Furthermore, free fascial flaps have demonstrated occasional partial flap loss and a decreased survival of the overlying skin graft, leading some to advocate delayed skin graft placement.10,16,17
On the basis of these complications, Bray and colleagues11 noted that the utility of free flaps may be limited in smaller clinical settings. The primary disadvantage of using DRTs is the necessity for a second operative procedure to harvest and place the skin graft. Traditionally, this is performed 2 to 3 weeks after the initial DRT application. Nevertheless, a 1-stage procedure can be performed in an outpatient setup, minimizing the burden to the patient and the medical costs, followed by secondary intention healing.
In response to critics of the 2-stage technique, Sanger and colleagues18 described single-stage use of DRT with split-thickness skin grafts with placement of an overlying wound vacuum-assisted closure to help speed incorporation of the DRT and improve survival of the immediately grafted skin. Another viable alternative is the McCash open-palm technique.19 In the open-palm technique, a Brunner zigzag incision is made in the affected digit. A transverse incision is made in the palm. A partial fasciectomy is performed in the palm and digit. After release, the digital incision is closed, and the palmar incision is left open. Although this well-studied and well-reported technique is known to reduce the risk of flap necrosis due to tension and hematoma,20 its main application is in the palm, as the name implies. Because in our patient the defect was palmar-digital with exposed “white structures,” we elected to use DRT.
Continue to: Although there is still...
Although there is still no perfect answer for wound coverage and closure in the hand with exposed or damaged tendons, DRT certainly performs well as a primary choice by minimizing adhesions; allowing a good ROM; and providing a durable, satisfactory cosmetic outcome. Likewise, an initial treatment with DRT does not preclude later, more elaborate reconstructive efforts, such as local or free flaps, if they continue to be indicated. DRT also does not diminish the ability to revise a tendon reconstruction if a secondary procedure is necessary. In our patient, tendon revision has not been necessary. DRT gives the surgeon a minimally invasive, efficient initial alternative to more labor-intensive, potentially morbid reconstructive procedures, without sacrificing outcome. Therefore, DRT can offer an alternative procedure in the surgeon’s armamentarium for tendon coverage in complex hand defects.
ABSTRACT
Soft tissue defects associated with exposed tendon pose difficult reconstructive problems because of tendon adhesions, poor range of motion, poor cosmetic appearance, and donor site morbidity. Dermal regeneration template is a skin substitute widely used in reconstructive surgery, including the occasional coverage of tendons. However, postoperative functionality of the tendons has not been well documented. We report a case of using dermal regeneration template for soft tissue reconstruction overlying tendons with loss of paratenon in a patient with Dupuytren’s contracture. Dermal regeneration template may offer an alternative option for immediate tendon coverage in the hand.
Soft tissue defects overlying exposed tendon with loss of paratenon often precipitate poor clinical outcomes because of the dichotomous demands of both closing the overlying soft-tissue defect and providing a gliding surface for the underlying tendons.1 Although avoidance of adhesions and restoration of function are the primary goals of the procedure, satisfactory appearance is also desirable. Likewise, any form of coverage should ideally provide good vasculature required for complete healing and an early form of closure following débridement.2 Simple skin grafts do not adequately meet these demands because they result in a high rate of tendon adhesions,3 and also are limited in patients with limited donor skin availability or questionable underlying wound bed viability, such as in scleroderma.
In order to reduce the frequency of tendon adhesions by creating a gliding surface, the use of interpositional materials, both artificial and biologic, has been employed with varying degrees of success, including cellophane, chitosan membrane, fibrin sealant, autogenous fascial flaps, and autogenous venous grafts.4-7 Many of the autogenous flaps and grafts have been employed with good success.8 However, complications and donor site morbidity encourage alternative procedures, including the use of artificial substances.2,8-10
We present our clinical experience with a patient who underwent successful placement of Integra (Integra LifeSciences) Dermal Regeneration Template (DRT) directly over exposed tendons with a subsequent full-thickness skin graft several weeks later. The procedures were performed per the manufacturer’s specifications, resulting in 2 stages of reconstruction. In our experience, DRT can offer immediate coverage unrestricted by wound size, and provides shorter operative time and decreased donor site and surgical morbidity compared with flap coverage, while demonstrating good cosmetic results. The patient provided written informed consent for print and electronic publication of this case report.
CASE
A 74-year-old right-handed man with Dupuytren’s contracture was evaluated for recurrent symptomatic contracture causing difficulty with daily activities. He reported palpable cords and contractures in the ring and small fingers of the right hand. He had 2 prior open surgical procedures, including palmar and digital fasciectomy of both hands. On the right hand, the ring and small fingers demonstrated 90° proximal interphalangeal (PIP) and 60° metacarpophalangeal (MCP) flexion contractures. Palpable central cords were present on the flexor surfaces of both the ring and small fingers. A well-healed surgical incision, performed 22 years earlier, was present over the palmar aspect of the ring finger.
Continue to: With consideration given...
With consideration given to the patient’s recurrent contracture after a prior surgical procedure, we discussed surgical excision of the diseased cords in order to eliminate the possibility of a second recurrence and maximize the gain of motion. Following discussion with the patient, we performed palmar and digital fasciectomy of the ring and small finger contractures. Postoperatively, the patient was followed closely for wound complications and vascular status. On his return to our clinic 11 days later, the patient was noted to have dehiscence of the digital wounds in the ring and small fingers (Figure 1).
STAGE 1
During the first stage, completed 14 days following the index procedure, débridement of the wounds was performed, followed by provisional DRT coverage of the tendons, secured with 5-0 nylon sutures (Figure 2).
STAGE 2
At approximately 2 weeks after application of the DRT, a full-thickness skin graft was applied. The thickness of the graft was chosen to allow for durable coverage of the palmar skin defects. Upon successful completion of the second stage, the patient was followed and evaluated for complete wound healing. On performing an examination 14 days after surgery, the ring and small fingers demonstrated only partially healed skin graft but significantly improved range of motion (ROM), with 40° to 90° arc of motion in the PIP joint and 25° to 90° arc of motion in the MCP joint (Figure 4). Owing to their limited size, the wounds were treated with dressing changes until successful healing (Figure 5).
Hand therapy was instituted to achieve maximum mobility for covered soft tissue and tendons and to maximize tendon gliding. At 1-year follow-up, the skin was fully healed and the patient’s active PIP motion was 30° to 90°, active MCP motion was 0° to 90°, and grip strength was 90 lb on both sides. The tendons glided under a well-vascularized tissue at the DRT placement site, and no secondary tenolysis procedure was deemed necessary.
DISCUSSION
Soft tissue defects with exposed tendons may offer a number of challenges for coverage. The primary concern is the creation of a gliding surface and the restoration of a functional tendon without adhesions.2 However, surgeons must use their own clinical judgment when choosing the method of coverage so as to minimize the effects of donor site morbidity and maximize the overall functional and cosmetic outcomes. All options must be considered while selecting a material or flap that is likely to survive in the relatively avascular tendon plane.2,8,11 When considering the reconstructive ladder, skin grafts may not represent a viable option in the presence of a nonvascularized wound bed, such as exposed tendon or bone, where paratenon or periosteum have been damaged. That leaves the surgeon with local flaps, regional flaps, free flaps, and skin substitutes.
Continue to : Before planning closure...
Before planning closure, wound conditions should be optimized, including wound bed quality, vascularization, and bacterial loads. Experimental data suggest that the bacterial load should be brought down below a critical level of 105 bacteria per g of tissue to allow a skin graft to take. This may be problematic from a practical standpoint because quantitative bacterial cultures take about 48 hours to obtain the result, long after a decision to graft is made. As a result, the surgeon may take an aggressive approach to wound débridement, making sure that all necrotic material has been sharply débrided prior to coverage.
As Levin12 noted in 1993, decisions regarding repair of any soft tissue defect may follow a well-delineated ladder beginning with the primary choice of split-thickness skin grafts and ending with free flaps. When treating tissue defects in the hand complex, flaps are an excellent option as they replace like with like, allow minimal scarring and early rehabilitation. 13,14 Nevertheless, a few general disadvantages are inherent in flap procedure: increase in operating time, risk of flap loss, and in case of free flaps, knowledge, experience, and microsurgical ability.2 In reference to complications, the rate of flap loss found by Khouri and colleagues15 was 4.1% with a 12.1% chance of incurring some measured complication, including wound dehiscence, arterial insufficiency, and flap necrosis.
Likewise, some of the conventional local and free flaps, including cutaneous and muscular flaps, prove ineffective in preventing tendon adhesions, create unsightly postoperative contours, or increase the area of trauma on the wounded hand, encouraging the use of free fascial flaps.11 Among the wide array of potential free fascial flaps, the temporoparietal, scapular, lateral arm, radial forearm, and free serratus fascial flaps are some of the most popular for hand defects.8,9 However, these procedures require an additional surgical site, meticulous dissection, microsurgical technique at times, and increased operating cost and time.2,8-10 Furthermore, free fascial flaps have demonstrated occasional partial flap loss and a decreased survival of the overlying skin graft, leading some to advocate delayed skin graft placement.10,16,17
On the basis of these complications, Bray and colleagues11 noted that the utility of free flaps may be limited in smaller clinical settings. The primary disadvantage of using DRTs is the necessity for a second operative procedure to harvest and place the skin graft. Traditionally, this is performed 2 to 3 weeks after the initial DRT application. Nevertheless, a 1-stage procedure can be performed in an outpatient setup, minimizing the burden to the patient and the medical costs, followed by secondary intention healing.
In response to critics of the 2-stage technique, Sanger and colleagues18 described single-stage use of DRT with split-thickness skin grafts with placement of an overlying wound vacuum-assisted closure to help speed incorporation of the DRT and improve survival of the immediately grafted skin. Another viable alternative is the McCash open-palm technique.19 In the open-palm technique, a Brunner zigzag incision is made in the affected digit. A transverse incision is made in the palm. A partial fasciectomy is performed in the palm and digit. After release, the digital incision is closed, and the palmar incision is left open. Although this well-studied and well-reported technique is known to reduce the risk of flap necrosis due to tension and hematoma,20 its main application is in the palm, as the name implies. Because in our patient the defect was palmar-digital with exposed “white structures,” we elected to use DRT.
Continue to: Although there is still...
Although there is still no perfect answer for wound coverage and closure in the hand with exposed or damaged tendons, DRT certainly performs well as a primary choice by minimizing adhesions; allowing a good ROM; and providing a durable, satisfactory cosmetic outcome. Likewise, an initial treatment with DRT does not preclude later, more elaborate reconstructive efforts, such as local or free flaps, if they continue to be indicated. DRT also does not diminish the ability to revise a tendon reconstruction if a secondary procedure is necessary. In our patient, tendon revision has not been necessary. DRT gives the surgeon a minimally invasive, efficient initial alternative to more labor-intensive, potentially morbid reconstructive procedures, without sacrificing outcome. Therefore, DRT can offer an alternative procedure in the surgeon’s armamentarium for tendon coverage in complex hand defects.
1. Flügel A. Kehrer C. Heitmann C, German G, Sauerbier M. Coverage of soft tissue defects of the hand with free fascial flaps. Microsurgery.2005;25(1):47-53.
2. Chen H, Buchman MT, Wei FC. Free flaps for soft tissue coverage in the hand and fingers. Hand Clin. 1999;15(4):541-554.
3. Chia J, Lim A, Peng YP. Use of an arterialized venous flap for resurfacing a circumferential soft tissue defect of a digit. Microsurgery. 2001; 21(8):374-378.
4. Wheeldon T. The use of cellophane as a permanent tendon sheath. J Bone J Surg Am; 1939;21(2):393-396.
5. Frykman E, Jacobsson S, Widenfalk B. Fibrin sealant in prevention of flexor tendon adhesions: an experimental study in the rabbit. J Hand Surg Am. 1993;18(1):68-75.
6. Jones NF, Lister GD. Free skin and composite flaps. In: Wolfe SW, Hotchkiss RN, Pederson WC, Kozin SH, eds. Green’s Operative hand surgery. 6th ed. New York, NY: Churchill Livingstone; 2011:1721-1756.
7. Yan D, Shi X, Lui Q. Reconstruction of tendon sheath by autogenous vein graft in preventing adhesion. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 1997;11(1):38-39.
8. Pederson WC. Upper extremity microsurgery. Plast Reconstr Surg. 2001;107(6):1524-1537; discussion 1538-15399, 1540-1543.
9. WintschK, Helaly P. Free flap of gliding tissue. J Reconstr Microsurg. 1986;2(3):143-151.
10. Meland NB, Weimar R. Microsurgical reconstruction: experience with free fascia flaps. Ann Plast Surg. 1991;27(1):1-8.
11. Bray PW, Boyer MI, Bowen CV. Complex injuries of the forearm. Coverage considerations. Hand Clin. 1997;13(2):263-278.
12. Levin LS. The reconstructive ladder: an orthoplastic approach. Ortho Clin North Am. 1993; 24(3):393-409.
13. Hallock GG. Utility of both muscle and fascia flaps in severe lower extremity trauma. J Trauma. 2000;48 (5):913-917. doi:10.1097/00005373-200005000-00016.
14. Hallock GG. The utility of both muscle and fascia flaps in severe upper extremity trauma. J Trauma. 2002;53(1):61-65. doi:10.1097/00005373-200207000-00013.
15. Khouri RK, Cooley BC, Kunselman AR, et al. A prospective study of microvascular free-flap surgery and outcome. Plast Reconstr Surg. 1998;102(3):711-721.
16. Woods JM 4th, Shack RB, Hagan KF. Free temporoparietal fascia flap in reconstruction of the lower extremity. Ann Plast Surg. 1995;34(5):501-506. doi:10.1097/00000637-199505000-00008.
17. Chung KC, Cederna PS. Endoscopic harvest of temporoparietal fascial free flaps for coverage of hand wounds. J Hand Surg Am. 2002;27(3):525-533.
18. Sanger C, Molnar JA, Newman CE, et al. Immediate skin grafting of an engineered dermal substitute: P37. Plast Reconstr Surg. 2005;116(3S):165.
19. McCash CR. The open palm technique in Dupuytren’s contracture. Br J Plast Surg. 1964;17:271-280.
20. Shaw DL, Wise DI, Holms W. Dupuytren's disease treated by palmar fasciectomy and an open palm technique. J Hand Surg Br. 1996;21(4):484-485.
1. Flügel A. Kehrer C. Heitmann C, German G, Sauerbier M. Coverage of soft tissue defects of the hand with free fascial flaps. Microsurgery.2005;25(1):47-53.
2. Chen H, Buchman MT, Wei FC. Free flaps for soft tissue coverage in the hand and fingers. Hand Clin. 1999;15(4):541-554.
3. Chia J, Lim A, Peng YP. Use of an arterialized venous flap for resurfacing a circumferential soft tissue defect of a digit. Microsurgery. 2001; 21(8):374-378.
4. Wheeldon T. The use of cellophane as a permanent tendon sheath. J Bone J Surg Am; 1939;21(2):393-396.
5. Frykman E, Jacobsson S, Widenfalk B. Fibrin sealant in prevention of flexor tendon adhesions: an experimental study in the rabbit. J Hand Surg Am. 1993;18(1):68-75.
6. Jones NF, Lister GD. Free skin and composite flaps. In: Wolfe SW, Hotchkiss RN, Pederson WC, Kozin SH, eds. Green’s Operative hand surgery. 6th ed. New York, NY: Churchill Livingstone; 2011:1721-1756.
7. Yan D, Shi X, Lui Q. Reconstruction of tendon sheath by autogenous vein graft in preventing adhesion. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 1997;11(1):38-39.
8. Pederson WC. Upper extremity microsurgery. Plast Reconstr Surg. 2001;107(6):1524-1537; discussion 1538-15399, 1540-1543.
9. WintschK, Helaly P. Free flap of gliding tissue. J Reconstr Microsurg. 1986;2(3):143-151.
10. Meland NB, Weimar R. Microsurgical reconstruction: experience with free fascia flaps. Ann Plast Surg. 1991;27(1):1-8.
11. Bray PW, Boyer MI, Bowen CV. Complex injuries of the forearm. Coverage considerations. Hand Clin. 1997;13(2):263-278.
12. Levin LS. The reconstructive ladder: an orthoplastic approach. Ortho Clin North Am. 1993; 24(3):393-409.
13. Hallock GG. Utility of both muscle and fascia flaps in severe lower extremity trauma. J Trauma. 2000;48 (5):913-917. doi:10.1097/00005373-200005000-00016.
14. Hallock GG. The utility of both muscle and fascia flaps in severe upper extremity trauma. J Trauma. 2002;53(1):61-65. doi:10.1097/00005373-200207000-00013.
15. Khouri RK, Cooley BC, Kunselman AR, et al. A prospective study of microvascular free-flap surgery and outcome. Plast Reconstr Surg. 1998;102(3):711-721.
16. Woods JM 4th, Shack RB, Hagan KF. Free temporoparietal fascia flap in reconstruction of the lower extremity. Ann Plast Surg. 1995;34(5):501-506. doi:10.1097/00000637-199505000-00008.
17. Chung KC, Cederna PS. Endoscopic harvest of temporoparietal fascial free flaps for coverage of hand wounds. J Hand Surg Am. 2002;27(3):525-533.
18. Sanger C, Molnar JA, Newman CE, et al. Immediate skin grafting of an engineered dermal substitute: P37. Plast Reconstr Surg. 2005;116(3S):165.
19. McCash CR. The open palm technique in Dupuytren’s contracture. Br J Plast Surg. 1964;17:271-280.
20. Shaw DL, Wise DI, Holms W. Dupuytren's disease treated by palmar fasciectomy and an open palm technique. J Hand Surg Br. 1996;21(4):484-485.
TAKE-HOME POINTS
- Full thickness skin grafts are generally considered unreliable for coverage of 3-dimensional defects of the hand with tendon exposure.
- Integra (Integra LifeSciences) is a bilayer skin substitute. The “dermal” (lower) layer is a bovine collagen base with glycosaminoglycan chondroitin-6-sulfate while the upper layer is a silicone sheet that acts as a temporary epidermis.
- Despite its popularity of Integra in burn reconstruction, little has been published regarding its utility in complex hand wounds with exposed tendons.
- Small areas of exposed tendons without remaining paratenon can be successfully grafted with Integra.
- In the presence of a healthy wound bed and no necrotic tissue or infection, Integra offers a reconstructive option that allows immediate coverage of complex hand wounds.