User login
The 2 cardinal properties of stem cells are the ability to self-renew and the ability to differentiate into distinctive end-stage cell types. The work of Caplan1 captured our early attention, with cells cultured from bone marrow differentiating into a number of different cell types of orthopedic interest. Our latest attention has been captured by the additional abilities of these cells to mobilize, monitor, and interact with their surrounding environment.2-4 In response to their environment, stem cells are able to release a broad spectrum of macromolecules with trophic, chemotactic, and immunomodulatory potential, which allows them to participate in injury response, tissue healing, and tissue regeneration.4 These cells are innate to the body’s monitoring, maintenance, repair, and stress response systems.2,4-11 Basic science and animal studies have illustrated the potential of cells with stem potential regardless of their environment/source of harvest.
Where Can We Get Stem Cells?
Cells with stem properties are present in many environmental niches, including the bone marrow, peripheral circulatory system, adipose tissue, synovial tissue, muscle tissue, and tendon tissue.12-15 A number of cell types with stem properties populate the bone marrow niche, including hematopoietic stem/progenitor cells (HSPC), perivascular stromal cells (PSC), endothelial stem cells (ESC), and immature cells with qualities like embryonal stem cells termed very small embryonal-like stem cells (VESL).12,15-19 All of these cells have stem properties and have been shown to differentiate to tissues of orthopedic interest.The interplay, interaction, and potential of these cell types is complex and incompletely understood.12,15-19 When bone marrow is aspirated for culturing purposes, it is unclear which cell line produces the plastic-adherent multipotent cells grown in culture, which are often referred to as mesenchymal stem cells (MSCs). Researches propose that HSPC and/or VESL circulate peripherally in small numbers but leave the bone marrow in certain mobilization instances and are important for the monitoring and maintenance of the majority of tissues in our bodies.5,16 Current clinical utilization of these cell types by the orthopedic community primarily utilizes point-of-care bone marrow aspiration and concentration, while the hematology oncology community mobilizes cells from the bone marrow to the blood stream with pharmaceutical agents and harvests cells via apheresis. Bone marrow aspiration produces variable numbers of stem cells, with studies ranging from 1 stem cell per mL of tissue collected to 300,000 stem cells per mL of tissue collected.20Mobilization and apheresis can produce large volumes of peripheral blood-derived cells with 600,000 HSPC per mL and 2.32 million PSC per mL of tissue collected.21
In adipose tissue, cells adherent to the abluminal side of blood vessels known as pericytes also carry stem qualities. Aspiration and processing of adipose tissue can access these stem cells, producing a product often referred to as stromal vascular fraction (SVF). Processing of lipoaspirate to create stromal vascular fraction requires mechanical or enzymatic processing. This also produces variable numbers of stem cells, with quantitative studies ranging from 5000 to 1.5 million stem cells per mL of tissue collected.20 Similar to adipose-derived stem cells, synovial-derived and muscle-derived stem cells also require mechanical or enzymatic processing. For applications where it is believed that a large number of cells is necessary, investigators often utilize culturing techniques for all sources with the exception of mobilization and apheresis harvest. As clinicians, 3 challenges have proven more important than which cell type to utilize: 1) patient-care logistics regarding collection and application; 2) the undefined dose-response curve regarding stem cell treatments; and 3) evolving government/community regulation.
Regulation of Stem Cell Therapies
The regulation of stem cell technologies is a double-edged sword for development. While loose regulation encourages clinical application and experimentation, patient safety and efficacy concerns are raised, and a technology’s worth is not proven before clinical application. Tight regulation temporarily hampers progress, yet ensures the proof of safety and efficacy prior to widespread implementation. Within the United States, the Food and Drug Administration (FDA) has tightened regulation, established precedent, and intervened in the ability of clinicians to utilize stem cell therapies in humans, through “warning letters,” “untitled letters,” and industry guidance documents.22-30
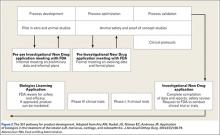
The FDA categorizes stem cell therapies as human cells, tissues, and cellular- and tissue-based products (HCT/Ps). Section 361 of the Public Health Safety (PHS) Act established and outlined the authority of the FDA to regulate low-risk HCT/Ps in order to prevent the introduction, transmission, and spread of communicable disease. Section 361 provided standards for safety without requiring preclinical development. The FDA established 4 principles to determine the risk of HCT/Ps: the extent of manipulation involved in manufacture, the metabolic activity/autologous nature of the product, whether the product represents a tissue combined with another product, and whether the product is utilized in a fashion homologous with its original function (Figure 1). If a product/therapy meets requirements around all 4 of these principles, then it is deemed a low-risk product and regulated under Section 361 alone. If a product/therapy does not meet requirements around all 4 of these principles, then the FDA regulates the product/therapy under additional codes including Section 351 of the PHS Act. Section 351 outlines a developmental process including preclinical animal trials, phased clinical study, and premarket review by the FDA prior to offering the product/treatment in clinical practice. The developmental process requires investigators and/or industry developers to initiate an Investigational New Drug (IND) program whose end goal is to present data from all developmental study and obtain a Biologic License Application (BLA) approval to market the product.22-23 To establish safety and efficacy, the traditional IND program involves a preclinical animal study, a small pilot human study (Phase I), a small initial randomized controlled trial (Phase II), followed by a large multicenter randomized controlled trial (Phase III) (Figure 2). The FDA has recognized little to no stem cell treatments as products regulated by Section 361 alone. Additionally, the FDA has established precedent regarding allograft stem cells, cells obtained from fat harvest, amniotic/placental products, and cultured cells, suggesting that these products are not low risk and require an IND pathway outlined in Section 351.24-30
Bone Marrow Aspiration
Surprisingly, the FDA has not moved to regulate the point-of-care use of bone marrow aspirate or platelet-rich plasma and has labeled these as “not HCTPs.” The stem cell concentration of bone marrow aspirate is technique-dependent, declines with age, and has been found to be an important factor for clinical benefit.31 While it is possible to aspirate from multiple sites, posterior iliac crest harvest produces the highest stem cell yield.32-34 Hernigou and colleagues35-36 have outlined safe zones for trocar placement and illustrated that strong aspiration with small-volume syringes, 10-mL syringes, optimizes stem cell harvest. Additionally, studies by Hernigou and colleagues31,37-38 involving tibial nonunion, avascular necrosis of the femur, and augmentation of rotator cuff repair are guideposts to clinicians utilizing bone marrow aspirate.
Amniotic Stem Cell Technologies and Adipose-Derived Stem Cells
While some argue that there is regulatory confusion around amniotic/placental-derived tissues and adipose-derived products, the FDA has clearly established precedent establishing these as products requiring Section 351 development.26-29 Companies are marketing products derived from perinatal byproducts, yet there are multiple FDA letters suggesting that these are not products regulated solely under PHS Act 361 because they do not meet the criteria of homologous use and are not autologous.28-29 Use of these products places risk upon the clinician and the patient. Some argue that adipose-derived stem cell products are 361 products. While the FDA has approved devices for the mechanical processing of lipoaspirate, they have established precedent suggesting that they consider orthopedic applications nonhomologous and any processing that “alters the original relevant characteristics of adipose tissue relating to the tissue’s utility for reconstruction, repair, or replacement” as more than minimal manipulation.26,27 The FDA originally planned an open forum for discussion with clinicians and industry for April 2016. This open forum was delayed due to the volume of interest, and a workshop has been planned for Fall 2016.
Future Regulation of Stem Cell Technologies
While many countries have mirrored the FDA with tight regulatory mechanisms, a few countries have established modern regulatory mechanisms aimed at the promotion of conscientious development, including South Korea, Japan, and England. For example, in 2014 Japan labeled stem cell technologies as “regenerative medicine products,” setting them apart from pharmaceuticals, and implemented a new approval system allowing early observed commercialization with reimbursement after less stringent safety and efficacy milestones.22The observed commercialization lowers time and financial hurdles for development while still requiring the proof of the technology’s worth. Countries that have effected change have positioned themselves to be pioneers in this emerging field.
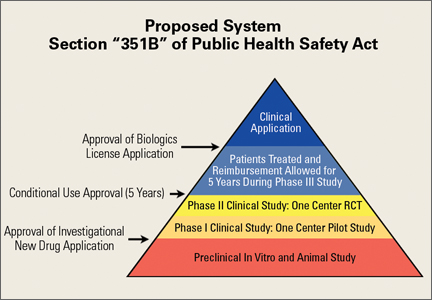
In March 2016, the Reliable and Effective Growth for Regenerative Health Options that Improve Wellness (REGROW) Act of 2016 (S. 2689 / H.R. 4762) was introduced into the United States Congress. This bipartisan, bicameral legislation was introduced, read twice, and referred to subcommittee. Its goal is to reduce barriers and accelerate development of biologic therapies while keeping the frame work set forth under Sections 351 and 361 of the PHS Act.39 Similar to the pathway in Japan, the REGROW Act would establish a conditional approval pathway that would ensure products are safe and effective while also evolving the regulatory pathway towards progress (Figure 3). Development would still require an IND application after preclinical animal study. However, after safety was established with human Phase I data and preliminary evidence of efficacy with Phase II data, patients could be treated with the investigational therapies and reimbursement collected for a limited period of time (5 years) prior to a large Phase III human clinical trial. Patients treated with the new therapy would be monitored closely. All results would be reported to the FDA in a BLA. This change in legislation would lower but not remove regulatory hurdles necessary for development.
Conclusion
The future of stem cell treatments hinges upon the creation of new favorable regulatory mechanisms that will promote clinical application while ensuring that safety and efficacy milestones are reached. Clinical researchers require freedom to develop these technologies while protecting patients and ensuring the validity of treatments. The coordination of research and regulatory affairs on a global level is necessary focusing on the harmonization of guidelines, regulations, and mechanisms for simultaneous adoption in different countries. The global orthopedic community has made strides regarding the science of stem cell technologies; it is time for us to initiate progressive change regarding regulation so that we can determine what is effective clinically.
1. Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9(5):641-650.
2. Wright DE, Wagers AJ, Gulati AP, Johnson FL, Weissman IL. Physiological migration of hematopoietic stem and progenitor cells. Science. 2001;294(5548):1933-1936.
3. Caplan AI. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol. 2007;213(2):341-347.
4. Murphy MB, Moncivais K, Caplan AI. Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine. Exp Mol Med. 2013;45:e54.
5. Ogawa M, LaRue AC, Mehrotra M. Hematopoietic stem cells are pluripotent and not just “hematopoietic.” Blood Cells Mol Dis. 2013;51(1):3-8.
6. Cesselli D, Beltrami AP, Rigo S, et al. Multipotent progenitor cells are present in human peripheral blood. Circ Res. 2009;104(10):1225-1234.
7. Massberg S, Schaerli P, Knezevic-Maramica I, et al. Immunosurveillance by hematopoietic progenitor cells trafficking through blood, lymph, and peripheral tissues. Cell. 2007;131(5):994-1008.
8. Wang Y, Johnsen HE, Mortensen S, et al. Changes in circulating mesenchymal stem cells, stem cell homing factor, and vascular growth factors in patients with acute ST elevation myocardial infarction treated with primary percutaneous coronary intervention. Heart. 2006;92(6):768-774.
9. Mansilla E, Marín GH, Drago H, et al. Bloodstream cells phenotypically identical to human mesenchymal bone marrow stem cells circulate in large amounts under the influence of acute large skin damage: new evidence for their use in regenerative medicine. Transplant Proc. 2006;38(3):967-969.
10. Rankin SM. Impact of bone marrow on respiratory disease. Curr Opin Pharmacol. 2008;8(3):236-241.
11. Rochefort GY, Delorme B, Lopez A, et al. Multipotential mesenchymal stem cells are mobilized into peripheral blood by hypoxia. Stem Cells. 2006;24(10):2202-2208.
12. Ugarte F, Forsberg EC. Haematopoietic stem cell niches: new insights inspire new questions. EMBO J. 2013;32(19):2535-2547.
13. Harvanová D, Tóthová T, Sarišský M, Amrichová J, Rosocha J. Isolation and characterization of synovial mesenchymal stem cells. Folia Biol (Praha). 2011;57(3):119-124.
14. Crisan M, Yap S, Casteilla L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3(3):301-313.
15. Frenette PS, Pinho S, Lucas D, Scheiermann C. Mesenchymal stem cell: keystone of the hematopoietic stem cell niche and a stepping-stone for regenerative medicine. Annu Rev Immunol. 2013;31:285-316.
16. Bonig H, Papayannopoulou T. Hematopoietic stem cell mobilization: updated conceptual renditions. Leukemia. 2013;27(1):24-31.
17. Ratajczak MZ, Marycz K, Poniewierska-Baran A, Fiedorowicz K, Zbucka-Kretowska M, Moniuszko M. Very small embryonic-like stem cells as a novel developmental concept and the hierarchy of the stem cell compartment. Adv Med Sci. 2014;59(2):273-280.
18. Smith JN, Calvi LM. Concise review: current concepts in bone marrow microenvironmental regulation of hematopoietic stem and progenitor cells. Stem Cells. 2013;31(6):1044-1050.
19. Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature. 2014;505(7483):327-334.
20. Vangsness CT Jr, Sternberg H, Harris L. Umbilical cord tissue offers the greatest number of harvestable mesenchymal stem cells for research and clinical application: a literature review of different harvest sites. Arthroscopy. 2015;31(9):1836-1843.
21. Saw KY, Anz A, Merican S, et al. Articular cartilage regeneration with autologous peripheral blood progenitor cells and hyaluronic acid after arthroscopic subchondral drilling: a report of 5 cases with histology. Arthroscopy. 2011;27(4):493-506.
22. Board on Health Sciences Policy; Board on Life Sciences; Division on Earth and Life Studies; Institute of Medicine; National Academy of Sciences. Stem Cell Therapies: Opportunities for Ensuring the Quality and Safety of Clinical Offerings: Summary of a Joint Workshop. Washington, DC: National Academies Press (US); 2014.
23. US Food and Drug Administration. Minimal manipulation of human cells, tissues, and cellular and tissue-based products: draft guidance for industry and food and drug administration staff. http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/CellularandGeneTherapy/ucm427692.htm. Updated February 3, 2015. Accessed June 10, 2016.
24. US Food and Drug Administration. PureGen™ osteoprogenitor cell allograft, parcell laboratories, LLC - untitled letter. http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/ComplianceActivities/Enforcement/UntitledLetters/ucm264011.htm. Published June 23, 2011. Accessed June 10, 2016.
25. US Food and Drug Administration. Map3 chips allograft-untitled letter. http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/ComplianceActivities/Enforcement/UntitledLetters/ucm418126.htm. Updated December 30, 2014. Accessed June 10, 2016.
26. US Food and Drug Administration. Irvine stem cell treatment center 12/30/15: warning letter. http://www.fda.gov/ICECI/EnforcementActions/WarningLetters/2015/ucm479837.htm. Published December 30, 2015. Accessed June 10, 2016.
27. US Food and Drug Administration. IntelliCell Biosciences, Inc. 3/13/12: warning letter. http://www.fda.gov/ICECI/EnforcementActions/WarningLetters/2012/ucm297245.htm. Published March 13, 2012. Accessed June 10, 2016.
28. US Food and Drug Administration. Osiris Therapeutics, Inc. - untitled letter. http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/ComplianceActivities/Enforcement/UntitledLetters/ucm371540.htm. Updated October 21, 2013. Accessed June 10, 2016.
29. US Food and Drug Administration. BioD- untitled letter. http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/ComplianceActivities/Enforcement/UntitledLetters/UCM452862.pdf. Published June 22, 2015. Accessed June 10, 2016.
30. US Food and Drug Administration. Regenerative Sciences, Inc. http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/ComplianceActivities/Enforcement/UntitledLetters/ucm091991.htm. Published July 25, 2008. Accessed June 10, 2016.
31. Hernigou P, Poignard A, Beaujean F, Rouard H. Percutaneous autologous bone-marrow grafting for nonunions. Influence of the number and concentration of progenitor cells. J Bone Joint Surg Am. 2005;87(7):1430-1437.
32. Narbona-Carceles J, Vaquero J, Suárez-Sancho S, Forriol F, Fernández-Santos ME. Bone marrow mesenchymal stem cell aspirates from alternative sources: is the knee as good as the iliac crest? Injury. 2014;45 Suppl 4:S42-S47.
33. Hyer CF, Berlet GC, Bussewitz BW, Hankins T, Ziegler HL, Philbin TM. Quantitative assessment of the yield of osteoblastic connective tissue progenitors in bone marrow aspirate from the iliac crest, tibia, and calcaneus. J Bone Joint Surg Am. 2013;95(14):1312-1316.
34. Pierini M, Di Bella C, Dozza B, et al. The posterior iliac crest outperforms the anterior iliac crest when obtaining mesenchymal stem cells from bone marrow. J Bone Joint Surg Am. 2013;95(12):1101-1107.
35. Hernigou J, Picard L, Alves A, Silvera J, Homma Y, Hernigou P. Understanding bone safety zones during bone marrow aspiration from the iliac crest: the sector rule. Int Orthop. 2014;38(11):2377-2384.
36. Hernigou P, Homma Y, Flouzat Lachaniette CH, et al. Benefits of small volume and small syringe for bone marrow aspirations of mesenchymal stem cells. Int Orthop. 2013;37(11):2279-2287.
37. Hernigou P, Flouzat Lachaniette CH, Delambre J, et al. Biologic augmentation of rotator cuff repair with mesenchymal stem cells during arthroscopy improves healing and prevents further tears: a case-controlled study. Int Orthop. 2014;38(9):1811-1818.
38. Hernigou P, Poignard A, Zilber S, Rouard H. Cell therapy of hip osteonecrosis with autologous bone marrow grafting. Indian J Orthop. 2009;43(1):40-45.
39. 114th Congress (2015-2016). S.2689 - REGROW Act. https://www.congress.gov/bill/114th-congress/senate-bill/2689/text. Accessed June 10, 2016.
The 2 cardinal properties of stem cells are the ability to self-renew and the ability to differentiate into distinctive end-stage cell types. The work of Caplan1 captured our early attention, with cells cultured from bone marrow differentiating into a number of different cell types of orthopedic interest. Our latest attention has been captured by the additional abilities of these cells to mobilize, monitor, and interact with their surrounding environment.2-4 In response to their environment, stem cells are able to release a broad spectrum of macromolecules with trophic, chemotactic, and immunomodulatory potential, which allows them to participate in injury response, tissue healing, and tissue regeneration.4 These cells are innate to the body’s monitoring, maintenance, repair, and stress response systems.2,4-11 Basic science and animal studies have illustrated the potential of cells with stem potential regardless of their environment/source of harvest.
Where Can We Get Stem Cells?
Cells with stem properties are present in many environmental niches, including the bone marrow, peripheral circulatory system, adipose tissue, synovial tissue, muscle tissue, and tendon tissue.12-15 A number of cell types with stem properties populate the bone marrow niche, including hematopoietic stem/progenitor cells (HSPC), perivascular stromal cells (PSC), endothelial stem cells (ESC), and immature cells with qualities like embryonal stem cells termed very small embryonal-like stem cells (VESL).12,15-19 All of these cells have stem properties and have been shown to differentiate to tissues of orthopedic interest.The interplay, interaction, and potential of these cell types is complex and incompletely understood.12,15-19 When bone marrow is aspirated for culturing purposes, it is unclear which cell line produces the plastic-adherent multipotent cells grown in culture, which are often referred to as mesenchymal stem cells (MSCs). Researches propose that HSPC and/or VESL circulate peripherally in small numbers but leave the bone marrow in certain mobilization instances and are important for the monitoring and maintenance of the majority of tissues in our bodies.5,16 Current clinical utilization of these cell types by the orthopedic community primarily utilizes point-of-care bone marrow aspiration and concentration, while the hematology oncology community mobilizes cells from the bone marrow to the blood stream with pharmaceutical agents and harvests cells via apheresis. Bone marrow aspiration produces variable numbers of stem cells, with studies ranging from 1 stem cell per mL of tissue collected to 300,000 stem cells per mL of tissue collected.20Mobilization and apheresis can produce large volumes of peripheral blood-derived cells with 600,000 HSPC per mL and 2.32 million PSC per mL of tissue collected.21
In adipose tissue, cells adherent to the abluminal side of blood vessels known as pericytes also carry stem qualities. Aspiration and processing of adipose tissue can access these stem cells, producing a product often referred to as stromal vascular fraction (SVF). Processing of lipoaspirate to create stromal vascular fraction requires mechanical or enzymatic processing. This also produces variable numbers of stem cells, with quantitative studies ranging from 5000 to 1.5 million stem cells per mL of tissue collected.20 Similar to adipose-derived stem cells, synovial-derived and muscle-derived stem cells also require mechanical or enzymatic processing. For applications where it is believed that a large number of cells is necessary, investigators often utilize culturing techniques for all sources with the exception of mobilization and apheresis harvest. As clinicians, 3 challenges have proven more important than which cell type to utilize: 1) patient-care logistics regarding collection and application; 2) the undefined dose-response curve regarding stem cell treatments; and 3) evolving government/community regulation.
Regulation of Stem Cell Therapies
The regulation of stem cell technologies is a double-edged sword for development. While loose regulation encourages clinical application and experimentation, patient safety and efficacy concerns are raised, and a technology’s worth is not proven before clinical application. Tight regulation temporarily hampers progress, yet ensures the proof of safety and efficacy prior to widespread implementation. Within the United States, the Food and Drug Administration (FDA) has tightened regulation, established precedent, and intervened in the ability of clinicians to utilize stem cell therapies in humans, through “warning letters,” “untitled letters,” and industry guidance documents.22-30
The FDA categorizes stem cell therapies as human cells, tissues, and cellular- and tissue-based products (HCT/Ps). Section 361 of the Public Health Safety (PHS) Act established and outlined the authority of the FDA to regulate low-risk HCT/Ps in order to prevent the introduction, transmission, and spread of communicable disease. Section 361 provided standards for safety without requiring preclinical development. The FDA established 4 principles to determine the risk of HCT/Ps: the extent of manipulation involved in manufacture, the metabolic activity/autologous nature of the product, whether the product represents a tissue combined with another product, and whether the product is utilized in a fashion homologous with its original function (Figure 1). If a product/therapy meets requirements around all 4 of these principles, then it is deemed a low-risk product and regulated under Section 361 alone. If a product/therapy does not meet requirements around all 4 of these principles, then the FDA regulates the product/therapy under additional codes including Section 351 of the PHS Act. Section 351 outlines a developmental process including preclinical animal trials, phased clinical study, and premarket review by the FDA prior to offering the product/treatment in clinical practice. The developmental process requires investigators and/or industry developers to initiate an Investigational New Drug (IND) program whose end goal is to present data from all developmental study and obtain a Biologic License Application (BLA) approval to market the product.22-23 To establish safety and efficacy, the traditional IND program involves a preclinical animal study, a small pilot human study (Phase I), a small initial randomized controlled trial (Phase II), followed by a large multicenter randomized controlled trial (Phase III) (Figure 2). The FDA has recognized little to no stem cell treatments as products regulated by Section 361 alone. Additionally, the FDA has established precedent regarding allograft stem cells, cells obtained from fat harvest, amniotic/placental products, and cultured cells, suggesting that these products are not low risk and require an IND pathway outlined in Section 351.24-30
Bone Marrow Aspiration
Surprisingly, the FDA has not moved to regulate the point-of-care use of bone marrow aspirate or platelet-rich plasma and has labeled these as “not HCTPs.” The stem cell concentration of bone marrow aspirate is technique-dependent, declines with age, and has been found to be an important factor for clinical benefit.31 While it is possible to aspirate from multiple sites, posterior iliac crest harvest produces the highest stem cell yield.32-34 Hernigou and colleagues35-36 have outlined safe zones for trocar placement and illustrated that strong aspiration with small-volume syringes, 10-mL syringes, optimizes stem cell harvest. Additionally, studies by Hernigou and colleagues31,37-38 involving tibial nonunion, avascular necrosis of the femur, and augmentation of rotator cuff repair are guideposts to clinicians utilizing bone marrow aspirate.
Amniotic Stem Cell Technologies and Adipose-Derived Stem Cells
While some argue that there is regulatory confusion around amniotic/placental-derived tissues and adipose-derived products, the FDA has clearly established precedent establishing these as products requiring Section 351 development.26-29 Companies are marketing products derived from perinatal byproducts, yet there are multiple FDA letters suggesting that these are not products regulated solely under PHS Act 361 because they do not meet the criteria of homologous use and are not autologous.28-29 Use of these products places risk upon the clinician and the patient. Some argue that adipose-derived stem cell products are 361 products. While the FDA has approved devices for the mechanical processing of lipoaspirate, they have established precedent suggesting that they consider orthopedic applications nonhomologous and any processing that “alters the original relevant characteristics of adipose tissue relating to the tissue’s utility for reconstruction, repair, or replacement” as more than minimal manipulation.26,27 The FDA originally planned an open forum for discussion with clinicians and industry for April 2016. This open forum was delayed due to the volume of interest, and a workshop has been planned for Fall 2016.
Future Regulation of Stem Cell Technologies
While many countries have mirrored the FDA with tight regulatory mechanisms, a few countries have established modern regulatory mechanisms aimed at the promotion of conscientious development, including South Korea, Japan, and England. For example, in 2014 Japan labeled stem cell technologies as “regenerative medicine products,” setting them apart from pharmaceuticals, and implemented a new approval system allowing early observed commercialization with reimbursement after less stringent safety and efficacy milestones.22The observed commercialization lowers time and financial hurdles for development while still requiring the proof of the technology’s worth. Countries that have effected change have positioned themselves to be pioneers in this emerging field.
In March 2016, the Reliable and Effective Growth for Regenerative Health Options that Improve Wellness (REGROW) Act of 2016 (S. 2689 / H.R. 4762) was introduced into the United States Congress. This bipartisan, bicameral legislation was introduced, read twice, and referred to subcommittee. Its goal is to reduce barriers and accelerate development of biologic therapies while keeping the frame work set forth under Sections 351 and 361 of the PHS Act.39 Similar to the pathway in Japan, the REGROW Act would establish a conditional approval pathway that would ensure products are safe and effective while also evolving the regulatory pathway towards progress (Figure 3). Development would still require an IND application after preclinical animal study. However, after safety was established with human Phase I data and preliminary evidence of efficacy with Phase II data, patients could be treated with the investigational therapies and reimbursement collected for a limited period of time (5 years) prior to a large Phase III human clinical trial. Patients treated with the new therapy would be monitored closely. All results would be reported to the FDA in a BLA. This change in legislation would lower but not remove regulatory hurdles necessary for development.
Conclusion
The future of stem cell treatments hinges upon the creation of new favorable regulatory mechanisms that will promote clinical application while ensuring that safety and efficacy milestones are reached. Clinical researchers require freedom to develop these technologies while protecting patients and ensuring the validity of treatments. The coordination of research and regulatory affairs on a global level is necessary focusing on the harmonization of guidelines, regulations, and mechanisms for simultaneous adoption in different countries. The global orthopedic community has made strides regarding the science of stem cell technologies; it is time for us to initiate progressive change regarding regulation so that we can determine what is effective clinically.
The 2 cardinal properties of stem cells are the ability to self-renew and the ability to differentiate into distinctive end-stage cell types. The work of Caplan1 captured our early attention, with cells cultured from bone marrow differentiating into a number of different cell types of orthopedic interest. Our latest attention has been captured by the additional abilities of these cells to mobilize, monitor, and interact with their surrounding environment.2-4 In response to their environment, stem cells are able to release a broad spectrum of macromolecules with trophic, chemotactic, and immunomodulatory potential, which allows them to participate in injury response, tissue healing, and tissue regeneration.4 These cells are innate to the body’s monitoring, maintenance, repair, and stress response systems.2,4-11 Basic science and animal studies have illustrated the potential of cells with stem potential regardless of their environment/source of harvest.
Where Can We Get Stem Cells?
Cells with stem properties are present in many environmental niches, including the bone marrow, peripheral circulatory system, adipose tissue, synovial tissue, muscle tissue, and tendon tissue.12-15 A number of cell types with stem properties populate the bone marrow niche, including hematopoietic stem/progenitor cells (HSPC), perivascular stromal cells (PSC), endothelial stem cells (ESC), and immature cells with qualities like embryonal stem cells termed very small embryonal-like stem cells (VESL).12,15-19 All of these cells have stem properties and have been shown to differentiate to tissues of orthopedic interest.The interplay, interaction, and potential of these cell types is complex and incompletely understood.12,15-19 When bone marrow is aspirated for culturing purposes, it is unclear which cell line produces the plastic-adherent multipotent cells grown in culture, which are often referred to as mesenchymal stem cells (MSCs). Researches propose that HSPC and/or VESL circulate peripherally in small numbers but leave the bone marrow in certain mobilization instances and are important for the monitoring and maintenance of the majority of tissues in our bodies.5,16 Current clinical utilization of these cell types by the orthopedic community primarily utilizes point-of-care bone marrow aspiration and concentration, while the hematology oncology community mobilizes cells from the bone marrow to the blood stream with pharmaceutical agents and harvests cells via apheresis. Bone marrow aspiration produces variable numbers of stem cells, with studies ranging from 1 stem cell per mL of tissue collected to 300,000 stem cells per mL of tissue collected.20Mobilization and apheresis can produce large volumes of peripheral blood-derived cells with 600,000 HSPC per mL and 2.32 million PSC per mL of tissue collected.21
In adipose tissue, cells adherent to the abluminal side of blood vessels known as pericytes also carry stem qualities. Aspiration and processing of adipose tissue can access these stem cells, producing a product often referred to as stromal vascular fraction (SVF). Processing of lipoaspirate to create stromal vascular fraction requires mechanical or enzymatic processing. This also produces variable numbers of stem cells, with quantitative studies ranging from 5000 to 1.5 million stem cells per mL of tissue collected.20 Similar to adipose-derived stem cells, synovial-derived and muscle-derived stem cells also require mechanical or enzymatic processing. For applications where it is believed that a large number of cells is necessary, investigators often utilize culturing techniques for all sources with the exception of mobilization and apheresis harvest. As clinicians, 3 challenges have proven more important than which cell type to utilize: 1) patient-care logistics regarding collection and application; 2) the undefined dose-response curve regarding stem cell treatments; and 3) evolving government/community regulation.
Regulation of Stem Cell Therapies
The regulation of stem cell technologies is a double-edged sword for development. While loose regulation encourages clinical application and experimentation, patient safety and efficacy concerns are raised, and a technology’s worth is not proven before clinical application. Tight regulation temporarily hampers progress, yet ensures the proof of safety and efficacy prior to widespread implementation. Within the United States, the Food and Drug Administration (FDA) has tightened regulation, established precedent, and intervened in the ability of clinicians to utilize stem cell therapies in humans, through “warning letters,” “untitled letters,” and industry guidance documents.22-30
The FDA categorizes stem cell therapies as human cells, tissues, and cellular- and tissue-based products (HCT/Ps). Section 361 of the Public Health Safety (PHS) Act established and outlined the authority of the FDA to regulate low-risk HCT/Ps in order to prevent the introduction, transmission, and spread of communicable disease. Section 361 provided standards for safety without requiring preclinical development. The FDA established 4 principles to determine the risk of HCT/Ps: the extent of manipulation involved in manufacture, the metabolic activity/autologous nature of the product, whether the product represents a tissue combined with another product, and whether the product is utilized in a fashion homologous with its original function (Figure 1). If a product/therapy meets requirements around all 4 of these principles, then it is deemed a low-risk product and regulated under Section 361 alone. If a product/therapy does not meet requirements around all 4 of these principles, then the FDA regulates the product/therapy under additional codes including Section 351 of the PHS Act. Section 351 outlines a developmental process including preclinical animal trials, phased clinical study, and premarket review by the FDA prior to offering the product/treatment in clinical practice. The developmental process requires investigators and/or industry developers to initiate an Investigational New Drug (IND) program whose end goal is to present data from all developmental study and obtain a Biologic License Application (BLA) approval to market the product.22-23 To establish safety and efficacy, the traditional IND program involves a preclinical animal study, a small pilot human study (Phase I), a small initial randomized controlled trial (Phase II), followed by a large multicenter randomized controlled trial (Phase III) (Figure 2). The FDA has recognized little to no stem cell treatments as products regulated by Section 361 alone. Additionally, the FDA has established precedent regarding allograft stem cells, cells obtained from fat harvest, amniotic/placental products, and cultured cells, suggesting that these products are not low risk and require an IND pathway outlined in Section 351.24-30
Bone Marrow Aspiration
Surprisingly, the FDA has not moved to regulate the point-of-care use of bone marrow aspirate or platelet-rich plasma and has labeled these as “not HCTPs.” The stem cell concentration of bone marrow aspirate is technique-dependent, declines with age, and has been found to be an important factor for clinical benefit.31 While it is possible to aspirate from multiple sites, posterior iliac crest harvest produces the highest stem cell yield.32-34 Hernigou and colleagues35-36 have outlined safe zones for trocar placement and illustrated that strong aspiration with small-volume syringes, 10-mL syringes, optimizes stem cell harvest. Additionally, studies by Hernigou and colleagues31,37-38 involving tibial nonunion, avascular necrosis of the femur, and augmentation of rotator cuff repair are guideposts to clinicians utilizing bone marrow aspirate.
Amniotic Stem Cell Technologies and Adipose-Derived Stem Cells
While some argue that there is regulatory confusion around amniotic/placental-derived tissues and adipose-derived products, the FDA has clearly established precedent establishing these as products requiring Section 351 development.26-29 Companies are marketing products derived from perinatal byproducts, yet there are multiple FDA letters suggesting that these are not products regulated solely under PHS Act 361 because they do not meet the criteria of homologous use and are not autologous.28-29 Use of these products places risk upon the clinician and the patient. Some argue that adipose-derived stem cell products are 361 products. While the FDA has approved devices for the mechanical processing of lipoaspirate, they have established precedent suggesting that they consider orthopedic applications nonhomologous and any processing that “alters the original relevant characteristics of adipose tissue relating to the tissue’s utility for reconstruction, repair, or replacement” as more than minimal manipulation.26,27 The FDA originally planned an open forum for discussion with clinicians and industry for April 2016. This open forum was delayed due to the volume of interest, and a workshop has been planned for Fall 2016.
Future Regulation of Stem Cell Technologies
While many countries have mirrored the FDA with tight regulatory mechanisms, a few countries have established modern regulatory mechanisms aimed at the promotion of conscientious development, including South Korea, Japan, and England. For example, in 2014 Japan labeled stem cell technologies as “regenerative medicine products,” setting them apart from pharmaceuticals, and implemented a new approval system allowing early observed commercialization with reimbursement after less stringent safety and efficacy milestones.22The observed commercialization lowers time and financial hurdles for development while still requiring the proof of the technology’s worth. Countries that have effected change have positioned themselves to be pioneers in this emerging field.
In March 2016, the Reliable and Effective Growth for Regenerative Health Options that Improve Wellness (REGROW) Act of 2016 (S. 2689 / H.R. 4762) was introduced into the United States Congress. This bipartisan, bicameral legislation was introduced, read twice, and referred to subcommittee. Its goal is to reduce barriers and accelerate development of biologic therapies while keeping the frame work set forth under Sections 351 and 361 of the PHS Act.39 Similar to the pathway in Japan, the REGROW Act would establish a conditional approval pathway that would ensure products are safe and effective while also evolving the regulatory pathway towards progress (Figure 3). Development would still require an IND application after preclinical animal study. However, after safety was established with human Phase I data and preliminary evidence of efficacy with Phase II data, patients could be treated with the investigational therapies and reimbursement collected for a limited period of time (5 years) prior to a large Phase III human clinical trial. Patients treated with the new therapy would be monitored closely. All results would be reported to the FDA in a BLA. This change in legislation would lower but not remove regulatory hurdles necessary for development.
Conclusion
The future of stem cell treatments hinges upon the creation of new favorable regulatory mechanisms that will promote clinical application while ensuring that safety and efficacy milestones are reached. Clinical researchers require freedom to develop these technologies while protecting patients and ensuring the validity of treatments. The coordination of research and regulatory affairs on a global level is necessary focusing on the harmonization of guidelines, regulations, and mechanisms for simultaneous adoption in different countries. The global orthopedic community has made strides regarding the science of stem cell technologies; it is time for us to initiate progressive change regarding regulation so that we can determine what is effective clinically.
1. Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9(5):641-650.
2. Wright DE, Wagers AJ, Gulati AP, Johnson FL, Weissman IL. Physiological migration of hematopoietic stem and progenitor cells. Science. 2001;294(5548):1933-1936.
3. Caplan AI. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol. 2007;213(2):341-347.
4. Murphy MB, Moncivais K, Caplan AI. Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine. Exp Mol Med. 2013;45:e54.
5. Ogawa M, LaRue AC, Mehrotra M. Hematopoietic stem cells are pluripotent and not just “hematopoietic.” Blood Cells Mol Dis. 2013;51(1):3-8.
6. Cesselli D, Beltrami AP, Rigo S, et al. Multipotent progenitor cells are present in human peripheral blood. Circ Res. 2009;104(10):1225-1234.
7. Massberg S, Schaerli P, Knezevic-Maramica I, et al. Immunosurveillance by hematopoietic progenitor cells trafficking through blood, lymph, and peripheral tissues. Cell. 2007;131(5):994-1008.
8. Wang Y, Johnsen HE, Mortensen S, et al. Changes in circulating mesenchymal stem cells, stem cell homing factor, and vascular growth factors in patients with acute ST elevation myocardial infarction treated with primary percutaneous coronary intervention. Heart. 2006;92(6):768-774.
9. Mansilla E, Marín GH, Drago H, et al. Bloodstream cells phenotypically identical to human mesenchymal bone marrow stem cells circulate in large amounts under the influence of acute large skin damage: new evidence for their use in regenerative medicine. Transplant Proc. 2006;38(3):967-969.
10. Rankin SM. Impact of bone marrow on respiratory disease. Curr Opin Pharmacol. 2008;8(3):236-241.
11. Rochefort GY, Delorme B, Lopez A, et al. Multipotential mesenchymal stem cells are mobilized into peripheral blood by hypoxia. Stem Cells. 2006;24(10):2202-2208.
12. Ugarte F, Forsberg EC. Haematopoietic stem cell niches: new insights inspire new questions. EMBO J. 2013;32(19):2535-2547.
13. Harvanová D, Tóthová T, Sarišský M, Amrichová J, Rosocha J. Isolation and characterization of synovial mesenchymal stem cells. Folia Biol (Praha). 2011;57(3):119-124.
14. Crisan M, Yap S, Casteilla L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3(3):301-313.
15. Frenette PS, Pinho S, Lucas D, Scheiermann C. Mesenchymal stem cell: keystone of the hematopoietic stem cell niche and a stepping-stone for regenerative medicine. Annu Rev Immunol. 2013;31:285-316.
16. Bonig H, Papayannopoulou T. Hematopoietic stem cell mobilization: updated conceptual renditions. Leukemia. 2013;27(1):24-31.
17. Ratajczak MZ, Marycz K, Poniewierska-Baran A, Fiedorowicz K, Zbucka-Kretowska M, Moniuszko M. Very small embryonic-like stem cells as a novel developmental concept and the hierarchy of the stem cell compartment. Adv Med Sci. 2014;59(2):273-280.
18. Smith JN, Calvi LM. Concise review: current concepts in bone marrow microenvironmental regulation of hematopoietic stem and progenitor cells. Stem Cells. 2013;31(6):1044-1050.
19. Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature. 2014;505(7483):327-334.
20. Vangsness CT Jr, Sternberg H, Harris L. Umbilical cord tissue offers the greatest number of harvestable mesenchymal stem cells for research and clinical application: a literature review of different harvest sites. Arthroscopy. 2015;31(9):1836-1843.
21. Saw KY, Anz A, Merican S, et al. Articular cartilage regeneration with autologous peripheral blood progenitor cells and hyaluronic acid after arthroscopic subchondral drilling: a report of 5 cases with histology. Arthroscopy. 2011;27(4):493-506.
22. Board on Health Sciences Policy; Board on Life Sciences; Division on Earth and Life Studies; Institute of Medicine; National Academy of Sciences. Stem Cell Therapies: Opportunities for Ensuring the Quality and Safety of Clinical Offerings: Summary of a Joint Workshop. Washington, DC: National Academies Press (US); 2014.
23. US Food and Drug Administration. Minimal manipulation of human cells, tissues, and cellular and tissue-based products: draft guidance for industry and food and drug administration staff. http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/CellularandGeneTherapy/ucm427692.htm. Updated February 3, 2015. Accessed June 10, 2016.
24. US Food and Drug Administration. PureGen™ osteoprogenitor cell allograft, parcell laboratories, LLC - untitled letter. http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/ComplianceActivities/Enforcement/UntitledLetters/ucm264011.htm. Published June 23, 2011. Accessed June 10, 2016.
25. US Food and Drug Administration. Map3 chips allograft-untitled letter. http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/ComplianceActivities/Enforcement/UntitledLetters/ucm418126.htm. Updated December 30, 2014. Accessed June 10, 2016.
26. US Food and Drug Administration. Irvine stem cell treatment center 12/30/15: warning letter. http://www.fda.gov/ICECI/EnforcementActions/WarningLetters/2015/ucm479837.htm. Published December 30, 2015. Accessed June 10, 2016.
27. US Food and Drug Administration. IntelliCell Biosciences, Inc. 3/13/12: warning letter. http://www.fda.gov/ICECI/EnforcementActions/WarningLetters/2012/ucm297245.htm. Published March 13, 2012. Accessed June 10, 2016.
28. US Food and Drug Administration. Osiris Therapeutics, Inc. - untitled letter. http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/ComplianceActivities/Enforcement/UntitledLetters/ucm371540.htm. Updated October 21, 2013. Accessed June 10, 2016.
29. US Food and Drug Administration. BioD- untitled letter. http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/ComplianceActivities/Enforcement/UntitledLetters/UCM452862.pdf. Published June 22, 2015. Accessed June 10, 2016.
30. US Food and Drug Administration. Regenerative Sciences, Inc. http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/ComplianceActivities/Enforcement/UntitledLetters/ucm091991.htm. Published July 25, 2008. Accessed June 10, 2016.
31. Hernigou P, Poignard A, Beaujean F, Rouard H. Percutaneous autologous bone-marrow grafting for nonunions. Influence of the number and concentration of progenitor cells. J Bone Joint Surg Am. 2005;87(7):1430-1437.
32. Narbona-Carceles J, Vaquero J, Suárez-Sancho S, Forriol F, Fernández-Santos ME. Bone marrow mesenchymal stem cell aspirates from alternative sources: is the knee as good as the iliac crest? Injury. 2014;45 Suppl 4:S42-S47.
33. Hyer CF, Berlet GC, Bussewitz BW, Hankins T, Ziegler HL, Philbin TM. Quantitative assessment of the yield of osteoblastic connective tissue progenitors in bone marrow aspirate from the iliac crest, tibia, and calcaneus. J Bone Joint Surg Am. 2013;95(14):1312-1316.
34. Pierini M, Di Bella C, Dozza B, et al. The posterior iliac crest outperforms the anterior iliac crest when obtaining mesenchymal stem cells from bone marrow. J Bone Joint Surg Am. 2013;95(12):1101-1107.
35. Hernigou J, Picard L, Alves A, Silvera J, Homma Y, Hernigou P. Understanding bone safety zones during bone marrow aspiration from the iliac crest: the sector rule. Int Orthop. 2014;38(11):2377-2384.
36. Hernigou P, Homma Y, Flouzat Lachaniette CH, et al. Benefits of small volume and small syringe for bone marrow aspirations of mesenchymal stem cells. Int Orthop. 2013;37(11):2279-2287.
37. Hernigou P, Flouzat Lachaniette CH, Delambre J, et al. Biologic augmentation of rotator cuff repair with mesenchymal stem cells during arthroscopy improves healing and prevents further tears: a case-controlled study. Int Orthop. 2014;38(9):1811-1818.
38. Hernigou P, Poignard A, Zilber S, Rouard H. Cell therapy of hip osteonecrosis with autologous bone marrow grafting. Indian J Orthop. 2009;43(1):40-45.
39. 114th Congress (2015-2016). S.2689 - REGROW Act. https://www.congress.gov/bill/114th-congress/senate-bill/2689/text. Accessed June 10, 2016.
1. Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9(5):641-650.
2. Wright DE, Wagers AJ, Gulati AP, Johnson FL, Weissman IL. Physiological migration of hematopoietic stem and progenitor cells. Science. 2001;294(5548):1933-1936.
3. Caplan AI. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol. 2007;213(2):341-347.
4. Murphy MB, Moncivais K, Caplan AI. Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine. Exp Mol Med. 2013;45:e54.
5. Ogawa M, LaRue AC, Mehrotra M. Hematopoietic stem cells are pluripotent and not just “hematopoietic.” Blood Cells Mol Dis. 2013;51(1):3-8.
6. Cesselli D, Beltrami AP, Rigo S, et al. Multipotent progenitor cells are present in human peripheral blood. Circ Res. 2009;104(10):1225-1234.
7. Massberg S, Schaerli P, Knezevic-Maramica I, et al. Immunosurveillance by hematopoietic progenitor cells trafficking through blood, lymph, and peripheral tissues. Cell. 2007;131(5):994-1008.
8. Wang Y, Johnsen HE, Mortensen S, et al. Changes in circulating mesenchymal stem cells, stem cell homing factor, and vascular growth factors in patients with acute ST elevation myocardial infarction treated with primary percutaneous coronary intervention. Heart. 2006;92(6):768-774.
9. Mansilla E, Marín GH, Drago H, et al. Bloodstream cells phenotypically identical to human mesenchymal bone marrow stem cells circulate in large amounts under the influence of acute large skin damage: new evidence for their use in regenerative medicine. Transplant Proc. 2006;38(3):967-969.
10. Rankin SM. Impact of bone marrow on respiratory disease. Curr Opin Pharmacol. 2008;8(3):236-241.
11. Rochefort GY, Delorme B, Lopez A, et al. Multipotential mesenchymal stem cells are mobilized into peripheral blood by hypoxia. Stem Cells. 2006;24(10):2202-2208.
12. Ugarte F, Forsberg EC. Haematopoietic stem cell niches: new insights inspire new questions. EMBO J. 2013;32(19):2535-2547.
13. Harvanová D, Tóthová T, Sarišský M, Amrichová J, Rosocha J. Isolation and characterization of synovial mesenchymal stem cells. Folia Biol (Praha). 2011;57(3):119-124.
14. Crisan M, Yap S, Casteilla L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3(3):301-313.
15. Frenette PS, Pinho S, Lucas D, Scheiermann C. Mesenchymal stem cell: keystone of the hematopoietic stem cell niche and a stepping-stone for regenerative medicine. Annu Rev Immunol. 2013;31:285-316.
16. Bonig H, Papayannopoulou T. Hematopoietic stem cell mobilization: updated conceptual renditions. Leukemia. 2013;27(1):24-31.
17. Ratajczak MZ, Marycz K, Poniewierska-Baran A, Fiedorowicz K, Zbucka-Kretowska M, Moniuszko M. Very small embryonic-like stem cells as a novel developmental concept and the hierarchy of the stem cell compartment. Adv Med Sci. 2014;59(2):273-280.
18. Smith JN, Calvi LM. Concise review: current concepts in bone marrow microenvironmental regulation of hematopoietic stem and progenitor cells. Stem Cells. 2013;31(6):1044-1050.
19. Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature. 2014;505(7483):327-334.
20. Vangsness CT Jr, Sternberg H, Harris L. Umbilical cord tissue offers the greatest number of harvestable mesenchymal stem cells for research and clinical application: a literature review of different harvest sites. Arthroscopy. 2015;31(9):1836-1843.
21. Saw KY, Anz A, Merican S, et al. Articular cartilage regeneration with autologous peripheral blood progenitor cells and hyaluronic acid after arthroscopic subchondral drilling: a report of 5 cases with histology. Arthroscopy. 2011;27(4):493-506.
22. Board on Health Sciences Policy; Board on Life Sciences; Division on Earth and Life Studies; Institute of Medicine; National Academy of Sciences. Stem Cell Therapies: Opportunities for Ensuring the Quality and Safety of Clinical Offerings: Summary of a Joint Workshop. Washington, DC: National Academies Press (US); 2014.
23. US Food and Drug Administration. Minimal manipulation of human cells, tissues, and cellular and tissue-based products: draft guidance for industry and food and drug administration staff. http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/CellularandGeneTherapy/ucm427692.htm. Updated February 3, 2015. Accessed June 10, 2016.
24. US Food and Drug Administration. PureGen™ osteoprogenitor cell allograft, parcell laboratories, LLC - untitled letter. http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/ComplianceActivities/Enforcement/UntitledLetters/ucm264011.htm. Published June 23, 2011. Accessed June 10, 2016.
25. US Food and Drug Administration. Map3 chips allograft-untitled letter. http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/ComplianceActivities/Enforcement/UntitledLetters/ucm418126.htm. Updated December 30, 2014. Accessed June 10, 2016.
26. US Food and Drug Administration. Irvine stem cell treatment center 12/30/15: warning letter. http://www.fda.gov/ICECI/EnforcementActions/WarningLetters/2015/ucm479837.htm. Published December 30, 2015. Accessed June 10, 2016.
27. US Food and Drug Administration. IntelliCell Biosciences, Inc. 3/13/12: warning letter. http://www.fda.gov/ICECI/EnforcementActions/WarningLetters/2012/ucm297245.htm. Published March 13, 2012. Accessed June 10, 2016.
28. US Food and Drug Administration. Osiris Therapeutics, Inc. - untitled letter. http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/ComplianceActivities/Enforcement/UntitledLetters/ucm371540.htm. Updated October 21, 2013. Accessed June 10, 2016.
29. US Food and Drug Administration. BioD- untitled letter. http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/ComplianceActivities/Enforcement/UntitledLetters/UCM452862.pdf. Published June 22, 2015. Accessed June 10, 2016.
30. US Food and Drug Administration. Regenerative Sciences, Inc. http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/ComplianceActivities/Enforcement/UntitledLetters/ucm091991.htm. Published July 25, 2008. Accessed June 10, 2016.
31. Hernigou P, Poignard A, Beaujean F, Rouard H. Percutaneous autologous bone-marrow grafting for nonunions. Influence of the number and concentration of progenitor cells. J Bone Joint Surg Am. 2005;87(7):1430-1437.
32. Narbona-Carceles J, Vaquero J, Suárez-Sancho S, Forriol F, Fernández-Santos ME. Bone marrow mesenchymal stem cell aspirates from alternative sources: is the knee as good as the iliac crest? Injury. 2014;45 Suppl 4:S42-S47.
33. Hyer CF, Berlet GC, Bussewitz BW, Hankins T, Ziegler HL, Philbin TM. Quantitative assessment of the yield of osteoblastic connective tissue progenitors in bone marrow aspirate from the iliac crest, tibia, and calcaneus. J Bone Joint Surg Am. 2013;95(14):1312-1316.
34. Pierini M, Di Bella C, Dozza B, et al. The posterior iliac crest outperforms the anterior iliac crest when obtaining mesenchymal stem cells from bone marrow. J Bone Joint Surg Am. 2013;95(12):1101-1107.
35. Hernigou J, Picard L, Alves A, Silvera J, Homma Y, Hernigou P. Understanding bone safety zones during bone marrow aspiration from the iliac crest: the sector rule. Int Orthop. 2014;38(11):2377-2384.
36. Hernigou P, Homma Y, Flouzat Lachaniette CH, et al. Benefits of small volume and small syringe for bone marrow aspirations of mesenchymal stem cells. Int Orthop. 2013;37(11):2279-2287.
37. Hernigou P, Flouzat Lachaniette CH, Delambre J, et al. Biologic augmentation of rotator cuff repair with mesenchymal stem cells during arthroscopy improves healing and prevents further tears: a case-controlled study. Int Orthop. 2014;38(9):1811-1818.
38. Hernigou P, Poignard A, Zilber S, Rouard H. Cell therapy of hip osteonecrosis with autologous bone marrow grafting. Indian J Orthop. 2009;43(1):40-45.
39. 114th Congress (2015-2016). S.2689 - REGROW Act. https://www.congress.gov/bill/114th-congress/senate-bill/2689/text. Accessed June 10, 2016.