User login
To the Editor:
Cutaneous collagenous vasculopathy (CCV) is an uncommon microangiopathy that presents with progressive telangiectases on the lower extremities that can eventually spread to involve the upper extremities and trunk. Systemic involvement is uncommon. The diagnosis is confirmed by biopsy, which demonstrates dilated capillaries and postcapillary venules with eosinophilic hyalinized walls. Treatment generally has focused on the use of vascular lasers.1 We report a patient with advanced CCV and ocular involvement that responded to a combination of pulsed dye laser (PDL) therapy and sclerotherapy for cutaneous lesions.
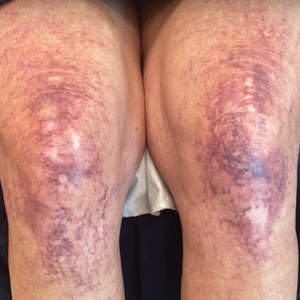
A 63-year-old woman presented with partially blanchable, purple-black patches on the lower extremities (Figure 1). The upper extremities had minimal involvement at the time of presentation. A medical history revealed the lesions presented on the legs 10 years prior but were beginning to form on the arms. She had a history of hypertension and bleeding in the retina.
Histopathology revealed prominent dilation of postcapillary venules with eosinophilic collagenous materials in the vessel walls that was positive on periodic acid–Schiff stain, confirming the diagnosis of CCV. The perivascular collagenous material failed to stain with Congo red. Laboratory testing for serum protein electrophoresis, antinuclear antibodies, and baseline hematologic and metabolic panels revealed no abnormalities.
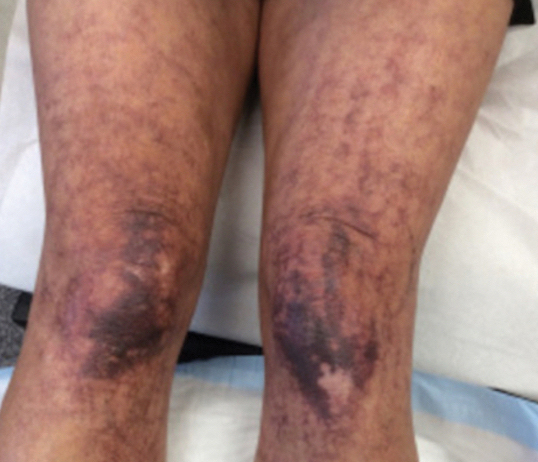
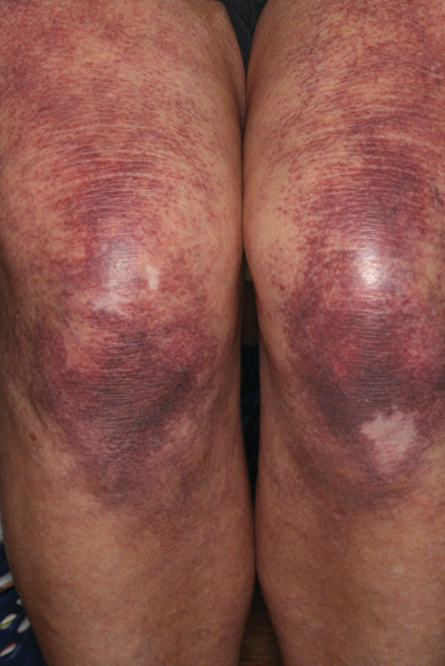
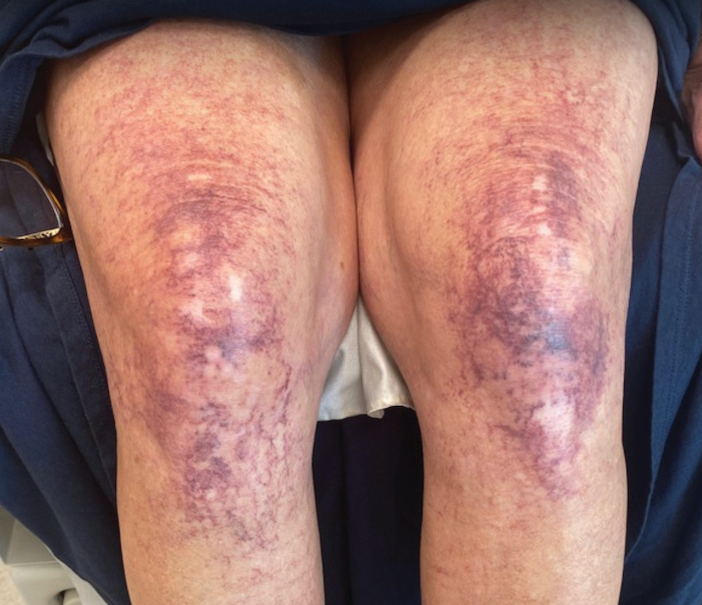
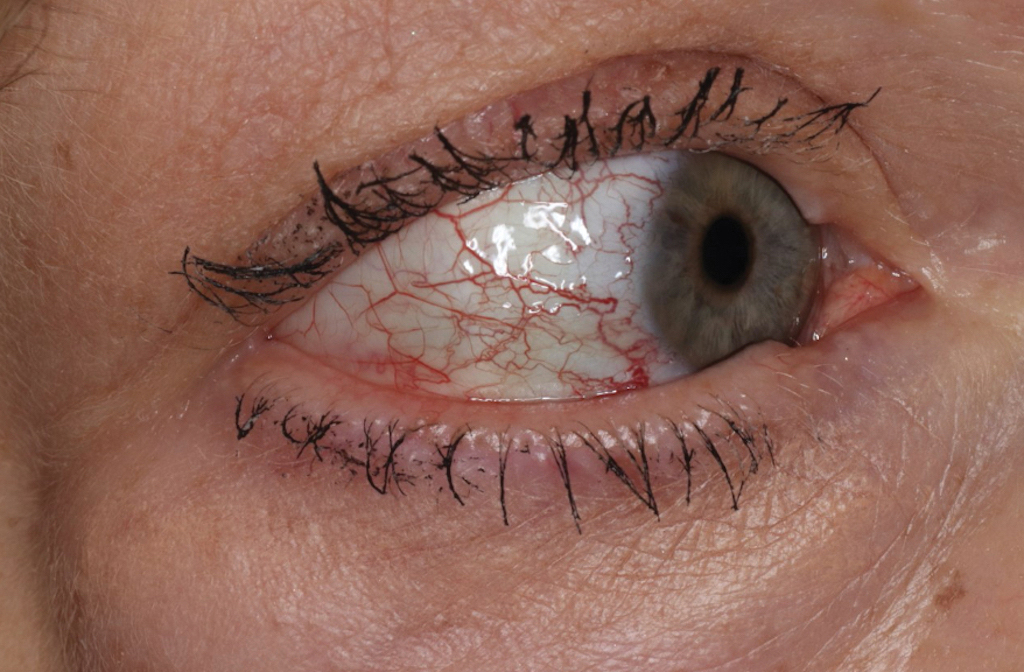
Over 3 years of treatment with PDL, most of the black patches resolved, but prominent telangiectatic vessels remained (Figure 2). Sclerotherapy with polidocanol (10 mg/mL) resulted in clearance of the majority of telangiectatic vessels. After each sclerotherapy treatment, Unna boots were applied for a minimum of 24 hours. The patient had no adverse effects from either PDL or sclerotherapy and was pleased with the results (Figure 3). An ophthalmologist had attributed the retinal bleeding to central serous chorioretinopathy, but tortuosity of superficial scleral and episcleral vessels progressed, suggesting CCV as the more likely cause (Figure 4). Currently, she is being followed for visual changes and further retinal bleeding.
Early CCV typically appears as blanchable pink or red macules, telangiectases, or petechiae on the lower extremities, progressing to involve the trunk and upper extremity.1-3 In rare cases, CCV presents in a papular or annular variant instead of the typical telangiectatic form.4,5 As the lesions progress, they often darken in appearance. Bleeding can occur, and the progressive patches are disfiguring.6,7 Middle-aged to older adults typically present with CCV (range, 16–83 years), with a mean age of 62 years.1,2,6 This disease affects both males and females, predominantly in White individuals.1 Extracutaneous manifestations are rare.1,2,6 One case of mucosal involvement was described in a patient with glossitis and oral erosions.8 We found no prior reports of nail or eye changes.1,2
The etiology of CCV is unknown, but different theories have been proposed. One is that CCV is due to a genetic defect that changes collagen synthesis in the cutaneous microvasculature. Another more widely held belief is that CCV originates from an injury that occurs to the microvasculature endothelial cells. Regardless of the cause of the triggering injury, the result is induced intravascular occlusive microthrombi that cause perivascular fibrosis and endothelial hyperplasia.2,6,7,9
Cutaneous collagenous vasculopathy may be influenced by systemic diseases. The most common comorbidities are hypertension, cardiovascular disease, diabetes mellitus, and hyperlipidemia.1,3,6-8 The presentation of CCV with a malignancy is rare; 1 patient was diagnosed with multiple myeloma 18 months after CCV, and another patient’s cutaneous presentation led to discovery of pancreatic cancer with metastasis.8,10 In this setting, the increased growth factors or hypercoagulability of malignancy may play a role in endothelial cell damage and hyperplasia. Autoimmune vascular injury also has been suggested to trigger CCV; 1 case involved antiribonucleoprotein antibodies, while another case involved anti–endothelial cell antibody assays.11 In addition, CCV has been reported in hypercoagulable patients, demonstrating another route for endothelial damage, with 1 patient being heterozygous for prothrombin G20210A, a report of CCV in a patient with cryofibrinogenemia, and another patient being found positive for lupus anticoagulant.11,12 Drugs also have been thought to influence CCV, including corticosteroids, lithium, thiothixene, interferon, isotretinoin, calcium channel blockers, antibiotics, hydroxyurea, and antidepressants.7,11
The diagnosis of CCV is confirmed using light microscopy and collagen-specific immunostaining. Examination shows hyaline eosinophilic deposition of type IV collagen around the affected vessels, with the postcapillary venules showing characteristic duplication of the basal lamina.3,9 The material stains positive with periodic acid-Schiff and Masson trichrome.3
Underreporting may contribute to the low incidence of CCV. The clinical presentation of CCV is similar to generalized essential telangiectasia, with biopsy distinguishing the two. Other diagnoses in the differential include hereditary hemorrhagic telangiectasia, which typically would have mucosal involvement; radiating telangiectatic mats and a strong family history; and hereditary benign telangiectasia, which typically presents in younger patients aged 1 year to adolescence.1
Treatment with vascular lasers has been the main focus, using either the 595-nm PDL or the 1064-nm Nd:YAG laser.6,13 Pulsed dye laser or intense pulsed light devices can improve patient well-being1,2; intense pulsed light allows for a larger spot size and may be preferred in patients with a larger body surface area involved.13 However, a few other treatments have been proposed. One case report noted poor response to sclerotherapy.1 In another case, a patient treated with a chemotherapy agent, bortezomib, for their concurrent multiple myeloma showed notable CCV cutaneous improvement. The proposed mechanism for bortezomib improving CCV is through its antiproliferative effect on endothelial cells of the superficial dermal vessels.8 Our patient did not achieve an adequate response with PDL, but the addition of sclerotherapy with polidocanol induced a successful response.
Patients should be examined for evidence of ocular involvement and referred to an ophthalmologist for appropriate care. Although there is no definite association with systemic illnesses or mediation, recent associations with an autoimmune disorder or underlying malignancy have been noted.8,10,11 Age-appropriate cancer screening and attention to associated signs and symptoms are recommended.
- Brady BG, Ortleb M, Boyd AS, et al. Cutaneous collagenous vasculopathy. J Clin Aesthet Dermatol. 2015;8:49-52. https://doi.org/10.1097/dad.0000000000000194
- Castiñeiras-Mato I, Rodríguez-Lojo R, Fernández-Díaz ML, et al. Cutaneous collagenous vasculopathy: a case report and review of the literature. Actas Dermosifiliogr. 2016;107:444-447. https://doi.org/10.1016/j.ad.2015.11.006
- Rambhia KD, Hadawale SD, Khopkar US. Cutaneous collagenous vasculopathy: a rare case report. Indian Dermatol Online J. 2016;7:40-42. https://doi.org/10.4103/2229-5178.174327
- Conde-Ferreirós A, Roncero-Riesco M, Cañueto J, et al. Cutaneous collagenous vasculopathy: papular form [published online August 15, 2019]. Dermatol Online J. https://doi.org/10.5070/d3258045128
- García-Martínez P, Gomez-Martin I, Lloreta J, et al. Multiple progressive annular telangiectasias: a clinicopathological variant of cutaneous collagenous vasculopathy? J Cutan Pathol. 2017;44:982-985. https://doi.org/10.1111/cup.13029
- Sartori DS, de Almeida Jr HL, Dorn TV, et al. Cutaneous collagenous vasculopathy: light and transmission electron microscopy. An Bras Dermatol. 2019;94:211-213. https://doi.org/10.1590/abd1806-4841.20198166
- Basso D, Ribero S, Blazek C, et al. Cutaneous collagenous vasculopathy: a rare form of microangiopathy successfully treated with a combination of multiplex laser and optimized pulsed light with a review of the literature. Dermatology. 2016;232:107-111. https://doi.org/10.1159/000439126
- Dura M, Pock L, Cetkovska P, et al. A case of cutaneous collagenous vasculopathy associated with multiple myeloma and with a pathogenic variant of the glucocerebrosidase gene. J Cutan Pathol. 2022;49:717-721. https://doi.org/10.1111/cup.14227
- Salama S, Chorneyko K, Belovic B. Cutaneous collagenous vasculopathy associated with intravascular occlusive fibrin thrombi. J Cutan Pathol. 2014;41:386-393. https://doi.org/10.1111/cup.12285
- Holder E, Schreckenberg C, Lipsker D. Cutaneous collagenous vasculopathy leading to the diagnosis of an advanced pancreatic cancer. J Eur Acad Dermatol Venereol. 2022;36:E699-E701. https://doi.org/10.1111/jdv.18152
- Grossman ME, Cohen M, Ravits M, et al. Cutaneous collagenous vasculopathy: a report of three cases. J Cutan Pathol. 2022;49:491-495. https://doi.org/10.1111/cup.14192
- Eldik H, Leisenring NH, Al-Rohil RN, et al. Cutaneous collagenous vasculopathy in a middle-aged woman with a history of prothrombin G20210A thrombophilia. J Cutan Pathol. 2022;49:679-682. https://doi.org/10.1111/cup.13895
- Weiss E, Lazzara DR. Commentary on clinical improvement of cutaneous collagenous vasculopathy with intense pulsed light therapy. Dermatol Surg. 2021;47:1412. https://doi.org/10.1097/DSS.0000000000003209
To the Editor:
Cutaneous collagenous vasculopathy (CCV) is an uncommon microangiopathy that presents with progressive telangiectases on the lower extremities that can eventually spread to involve the upper extremities and trunk. Systemic involvement is uncommon. The diagnosis is confirmed by biopsy, which demonstrates dilated capillaries and postcapillary venules with eosinophilic hyalinized walls. Treatment generally has focused on the use of vascular lasers.1 We report a patient with advanced CCV and ocular involvement that responded to a combination of pulsed dye laser (PDL) therapy and sclerotherapy for cutaneous lesions.
A 63-year-old woman presented with partially blanchable, purple-black patches on the lower extremities (Figure 1). The upper extremities had minimal involvement at the time of presentation. A medical history revealed the lesions presented on the legs 10 years prior but were beginning to form on the arms. She had a history of hypertension and bleeding in the retina.

Histopathology revealed prominent dilation of postcapillary venules with eosinophilic collagenous materials in the vessel walls that was positive on periodic acid–Schiff stain, confirming the diagnosis of CCV. The perivascular collagenous material failed to stain with Congo red. Laboratory testing for serum protein electrophoresis, antinuclear antibodies, and baseline hematologic and metabolic panels revealed no abnormalities.
Over 3 years of treatment with PDL, most of the black patches resolved, but prominent telangiectatic vessels remained (Figure 2). Sclerotherapy with polidocanol (10 mg/mL) resulted in clearance of the majority of telangiectatic vessels. After each sclerotherapy treatment, Unna boots were applied for a minimum of 24 hours. The patient had no adverse effects from either PDL or sclerotherapy and was pleased with the results (Figure 3). An ophthalmologist had attributed the retinal bleeding to central serous chorioretinopathy, but tortuosity of superficial scleral and episcleral vessels progressed, suggesting CCV as the more likely cause (Figure 4). Currently, she is being followed for visual changes and further retinal bleeding.

Early CCV typically appears as blanchable pink or red macules, telangiectases, or petechiae on the lower extremities, progressing to involve the trunk and upper extremity.1-3 In rare cases, CCV presents in a papular or annular variant instead of the typical telangiectatic form.4,5 As the lesions progress, they often darken in appearance. Bleeding can occur, and the progressive patches are disfiguring.6,7 Middle-aged to older adults typically present with CCV (range, 16–83 years), with a mean age of 62 years.1,2,6 This disease affects both males and females, predominantly in White individuals.1 Extracutaneous manifestations are rare.1,2,6 One case of mucosal involvement was described in a patient with glossitis and oral erosions.8 We found no prior reports of nail or eye changes.1,2

The etiology of CCV is unknown, but different theories have been proposed. One is that CCV is due to a genetic defect that changes collagen synthesis in the cutaneous microvasculature. Another more widely held belief is that CCV originates from an injury that occurs to the microvasculature endothelial cells. Regardless of the cause of the triggering injury, the result is induced intravascular occlusive microthrombi that cause perivascular fibrosis and endothelial hyperplasia.2,6,7,9

Cutaneous collagenous vasculopathy may be influenced by systemic diseases. The most common comorbidities are hypertension, cardiovascular disease, diabetes mellitus, and hyperlipidemia.1,3,6-8 The presentation of CCV with a malignancy is rare; 1 patient was diagnosed with multiple myeloma 18 months after CCV, and another patient’s cutaneous presentation led to discovery of pancreatic cancer with metastasis.8,10 In this setting, the increased growth factors or hypercoagulability of malignancy may play a role in endothelial cell damage and hyperplasia. Autoimmune vascular injury also has been suggested to trigger CCV; 1 case involved antiribonucleoprotein antibodies, while another case involved anti–endothelial cell antibody assays.11 In addition, CCV has been reported in hypercoagulable patients, demonstrating another route for endothelial damage, with 1 patient being heterozygous for prothrombin G20210A, a report of CCV in a patient with cryofibrinogenemia, and another patient being found positive for lupus anticoagulant.11,12 Drugs also have been thought to influence CCV, including corticosteroids, lithium, thiothixene, interferon, isotretinoin, calcium channel blockers, antibiotics, hydroxyurea, and antidepressants.7,11
The diagnosis of CCV is confirmed using light microscopy and collagen-specific immunostaining. Examination shows hyaline eosinophilic deposition of type IV collagen around the affected vessels, with the postcapillary venules showing characteristic duplication of the basal lamina.3,9 The material stains positive with periodic acid-Schiff and Masson trichrome.3
Underreporting may contribute to the low incidence of CCV. The clinical presentation of CCV is similar to generalized essential telangiectasia, with biopsy distinguishing the two. Other diagnoses in the differential include hereditary hemorrhagic telangiectasia, which typically would have mucosal involvement; radiating telangiectatic mats and a strong family history; and hereditary benign telangiectasia, which typically presents in younger patients aged 1 year to adolescence.1
Treatment with vascular lasers has been the main focus, using either the 595-nm PDL or the 1064-nm Nd:YAG laser.6,13 Pulsed dye laser or intense pulsed light devices can improve patient well-being1,2; intense pulsed light allows for a larger spot size and may be preferred in patients with a larger body surface area involved.13 However, a few other treatments have been proposed. One case report noted poor response to sclerotherapy.1 In another case, a patient treated with a chemotherapy agent, bortezomib, for their concurrent multiple myeloma showed notable CCV cutaneous improvement. The proposed mechanism for bortezomib improving CCV is through its antiproliferative effect on endothelial cells of the superficial dermal vessels.8 Our patient did not achieve an adequate response with PDL, but the addition of sclerotherapy with polidocanol induced a successful response.
Patients should be examined for evidence of ocular involvement and referred to an ophthalmologist for appropriate care. Although there is no definite association with systemic illnesses or mediation, recent associations with an autoimmune disorder or underlying malignancy have been noted.8,10,11 Age-appropriate cancer screening and attention to associated signs and symptoms are recommended.
To the Editor:
Cutaneous collagenous vasculopathy (CCV) is an uncommon microangiopathy that presents with progressive telangiectases on the lower extremities that can eventually spread to involve the upper extremities and trunk. Systemic involvement is uncommon. The diagnosis is confirmed by biopsy, which demonstrates dilated capillaries and postcapillary venules with eosinophilic hyalinized walls. Treatment generally has focused on the use of vascular lasers.1 We report a patient with advanced CCV and ocular involvement that responded to a combination of pulsed dye laser (PDL) therapy and sclerotherapy for cutaneous lesions.
A 63-year-old woman presented with partially blanchable, purple-black patches on the lower extremities (Figure 1). The upper extremities had minimal involvement at the time of presentation. A medical history revealed the lesions presented on the legs 10 years prior but were beginning to form on the arms. She had a history of hypertension and bleeding in the retina.

Histopathology revealed prominent dilation of postcapillary venules with eosinophilic collagenous materials in the vessel walls that was positive on periodic acid–Schiff stain, confirming the diagnosis of CCV. The perivascular collagenous material failed to stain with Congo red. Laboratory testing for serum protein electrophoresis, antinuclear antibodies, and baseline hematologic and metabolic panels revealed no abnormalities.
Over 3 years of treatment with PDL, most of the black patches resolved, but prominent telangiectatic vessels remained (Figure 2). Sclerotherapy with polidocanol (10 mg/mL) resulted in clearance of the majority of telangiectatic vessels. After each sclerotherapy treatment, Unna boots were applied for a minimum of 24 hours. The patient had no adverse effects from either PDL or sclerotherapy and was pleased with the results (Figure 3). An ophthalmologist had attributed the retinal bleeding to central serous chorioretinopathy, but tortuosity of superficial scleral and episcleral vessels progressed, suggesting CCV as the more likely cause (Figure 4). Currently, she is being followed for visual changes and further retinal bleeding.

Early CCV typically appears as blanchable pink or red macules, telangiectases, or petechiae on the lower extremities, progressing to involve the trunk and upper extremity.1-3 In rare cases, CCV presents in a papular or annular variant instead of the typical telangiectatic form.4,5 As the lesions progress, they often darken in appearance. Bleeding can occur, and the progressive patches are disfiguring.6,7 Middle-aged to older adults typically present with CCV (range, 16–83 years), with a mean age of 62 years.1,2,6 This disease affects both males and females, predominantly in White individuals.1 Extracutaneous manifestations are rare.1,2,6 One case of mucosal involvement was described in a patient with glossitis and oral erosions.8 We found no prior reports of nail or eye changes.1,2

The etiology of CCV is unknown, but different theories have been proposed. One is that CCV is due to a genetic defect that changes collagen synthesis in the cutaneous microvasculature. Another more widely held belief is that CCV originates from an injury that occurs to the microvasculature endothelial cells. Regardless of the cause of the triggering injury, the result is induced intravascular occlusive microthrombi that cause perivascular fibrosis and endothelial hyperplasia.2,6,7,9

Cutaneous collagenous vasculopathy may be influenced by systemic diseases. The most common comorbidities are hypertension, cardiovascular disease, diabetes mellitus, and hyperlipidemia.1,3,6-8 The presentation of CCV with a malignancy is rare; 1 patient was diagnosed with multiple myeloma 18 months after CCV, and another patient’s cutaneous presentation led to discovery of pancreatic cancer with metastasis.8,10 In this setting, the increased growth factors or hypercoagulability of malignancy may play a role in endothelial cell damage and hyperplasia. Autoimmune vascular injury also has been suggested to trigger CCV; 1 case involved antiribonucleoprotein antibodies, while another case involved anti–endothelial cell antibody assays.11 In addition, CCV has been reported in hypercoagulable patients, demonstrating another route for endothelial damage, with 1 patient being heterozygous for prothrombin G20210A, a report of CCV in a patient with cryofibrinogenemia, and another patient being found positive for lupus anticoagulant.11,12 Drugs also have been thought to influence CCV, including corticosteroids, lithium, thiothixene, interferon, isotretinoin, calcium channel blockers, antibiotics, hydroxyurea, and antidepressants.7,11
The diagnosis of CCV is confirmed using light microscopy and collagen-specific immunostaining. Examination shows hyaline eosinophilic deposition of type IV collagen around the affected vessels, with the postcapillary venules showing characteristic duplication of the basal lamina.3,9 The material stains positive with periodic acid-Schiff and Masson trichrome.3
Underreporting may contribute to the low incidence of CCV. The clinical presentation of CCV is similar to generalized essential telangiectasia, with biopsy distinguishing the two. Other diagnoses in the differential include hereditary hemorrhagic telangiectasia, which typically would have mucosal involvement; radiating telangiectatic mats and a strong family history; and hereditary benign telangiectasia, which typically presents in younger patients aged 1 year to adolescence.1
Treatment with vascular lasers has been the main focus, using either the 595-nm PDL or the 1064-nm Nd:YAG laser.6,13 Pulsed dye laser or intense pulsed light devices can improve patient well-being1,2; intense pulsed light allows for a larger spot size and may be preferred in patients with a larger body surface area involved.13 However, a few other treatments have been proposed. One case report noted poor response to sclerotherapy.1 In another case, a patient treated with a chemotherapy agent, bortezomib, for their concurrent multiple myeloma showed notable CCV cutaneous improvement. The proposed mechanism for bortezomib improving CCV is through its antiproliferative effect on endothelial cells of the superficial dermal vessels.8 Our patient did not achieve an adequate response with PDL, but the addition of sclerotherapy with polidocanol induced a successful response.
Patients should be examined for evidence of ocular involvement and referred to an ophthalmologist for appropriate care. Although there is no definite association with systemic illnesses or mediation, recent associations with an autoimmune disorder or underlying malignancy have been noted.8,10,11 Age-appropriate cancer screening and attention to associated signs and symptoms are recommended.
- Brady BG, Ortleb M, Boyd AS, et al. Cutaneous collagenous vasculopathy. J Clin Aesthet Dermatol. 2015;8:49-52. https://doi.org/10.1097/dad.0000000000000194
- Castiñeiras-Mato I, Rodríguez-Lojo R, Fernández-Díaz ML, et al. Cutaneous collagenous vasculopathy: a case report and review of the literature. Actas Dermosifiliogr. 2016;107:444-447. https://doi.org/10.1016/j.ad.2015.11.006
- Rambhia KD, Hadawale SD, Khopkar US. Cutaneous collagenous vasculopathy: a rare case report. Indian Dermatol Online J. 2016;7:40-42. https://doi.org/10.4103/2229-5178.174327
- Conde-Ferreirós A, Roncero-Riesco M, Cañueto J, et al. Cutaneous collagenous vasculopathy: papular form [published online August 15, 2019]. Dermatol Online J. https://doi.org/10.5070/d3258045128
- García-Martínez P, Gomez-Martin I, Lloreta J, et al. Multiple progressive annular telangiectasias: a clinicopathological variant of cutaneous collagenous vasculopathy? J Cutan Pathol. 2017;44:982-985. https://doi.org/10.1111/cup.13029
- Sartori DS, de Almeida Jr HL, Dorn TV, et al. Cutaneous collagenous vasculopathy: light and transmission electron microscopy. An Bras Dermatol. 2019;94:211-213. https://doi.org/10.1590/abd1806-4841.20198166
- Basso D, Ribero S, Blazek C, et al. Cutaneous collagenous vasculopathy: a rare form of microangiopathy successfully treated with a combination of multiplex laser and optimized pulsed light with a review of the literature. Dermatology. 2016;232:107-111. https://doi.org/10.1159/000439126
- Dura M, Pock L, Cetkovska P, et al. A case of cutaneous collagenous vasculopathy associated with multiple myeloma and with a pathogenic variant of the glucocerebrosidase gene. J Cutan Pathol. 2022;49:717-721. https://doi.org/10.1111/cup.14227
- Salama S, Chorneyko K, Belovic B. Cutaneous collagenous vasculopathy associated with intravascular occlusive fibrin thrombi. J Cutan Pathol. 2014;41:386-393. https://doi.org/10.1111/cup.12285
- Holder E, Schreckenberg C, Lipsker D. Cutaneous collagenous vasculopathy leading to the diagnosis of an advanced pancreatic cancer. J Eur Acad Dermatol Venereol. 2022;36:E699-E701. https://doi.org/10.1111/jdv.18152
- Grossman ME, Cohen M, Ravits M, et al. Cutaneous collagenous vasculopathy: a report of three cases. J Cutan Pathol. 2022;49:491-495. https://doi.org/10.1111/cup.14192
- Eldik H, Leisenring NH, Al-Rohil RN, et al. Cutaneous collagenous vasculopathy in a middle-aged woman with a history of prothrombin G20210A thrombophilia. J Cutan Pathol. 2022;49:679-682. https://doi.org/10.1111/cup.13895
- Weiss E, Lazzara DR. Commentary on clinical improvement of cutaneous collagenous vasculopathy with intense pulsed light therapy. Dermatol Surg. 2021;47:1412. https://doi.org/10.1097/DSS.0000000000003209
- Brady BG, Ortleb M, Boyd AS, et al. Cutaneous collagenous vasculopathy. J Clin Aesthet Dermatol. 2015;8:49-52. https://doi.org/10.1097/dad.0000000000000194
- Castiñeiras-Mato I, Rodríguez-Lojo R, Fernández-Díaz ML, et al. Cutaneous collagenous vasculopathy: a case report and review of the literature. Actas Dermosifiliogr. 2016;107:444-447. https://doi.org/10.1016/j.ad.2015.11.006
- Rambhia KD, Hadawale SD, Khopkar US. Cutaneous collagenous vasculopathy: a rare case report. Indian Dermatol Online J. 2016;7:40-42. https://doi.org/10.4103/2229-5178.174327
- Conde-Ferreirós A, Roncero-Riesco M, Cañueto J, et al. Cutaneous collagenous vasculopathy: papular form [published online August 15, 2019]. Dermatol Online J. https://doi.org/10.5070/d3258045128
- García-Martínez P, Gomez-Martin I, Lloreta J, et al. Multiple progressive annular telangiectasias: a clinicopathological variant of cutaneous collagenous vasculopathy? J Cutan Pathol. 2017;44:982-985. https://doi.org/10.1111/cup.13029
- Sartori DS, de Almeida Jr HL, Dorn TV, et al. Cutaneous collagenous vasculopathy: light and transmission electron microscopy. An Bras Dermatol. 2019;94:211-213. https://doi.org/10.1590/abd1806-4841.20198166
- Basso D, Ribero S, Blazek C, et al. Cutaneous collagenous vasculopathy: a rare form of microangiopathy successfully treated with a combination of multiplex laser and optimized pulsed light with a review of the literature. Dermatology. 2016;232:107-111. https://doi.org/10.1159/000439126
- Dura M, Pock L, Cetkovska P, et al. A case of cutaneous collagenous vasculopathy associated with multiple myeloma and with a pathogenic variant of the glucocerebrosidase gene. J Cutan Pathol. 2022;49:717-721. https://doi.org/10.1111/cup.14227
- Salama S, Chorneyko K, Belovic B. Cutaneous collagenous vasculopathy associated with intravascular occlusive fibrin thrombi. J Cutan Pathol. 2014;41:386-393. https://doi.org/10.1111/cup.12285
- Holder E, Schreckenberg C, Lipsker D. Cutaneous collagenous vasculopathy leading to the diagnosis of an advanced pancreatic cancer. J Eur Acad Dermatol Venereol. 2022;36:E699-E701. https://doi.org/10.1111/jdv.18152
- Grossman ME, Cohen M, Ravits M, et al. Cutaneous collagenous vasculopathy: a report of three cases. J Cutan Pathol. 2022;49:491-495. https://doi.org/10.1111/cup.14192
- Eldik H, Leisenring NH, Al-Rohil RN, et al. Cutaneous collagenous vasculopathy in a middle-aged woman with a history of prothrombin G20210A thrombophilia. J Cutan Pathol. 2022;49:679-682. https://doi.org/10.1111/cup.13895
- Weiss E, Lazzara DR. Commentary on clinical improvement of cutaneous collagenous vasculopathy with intense pulsed light therapy. Dermatol Surg. 2021;47:1412. https://doi.org/10.1097/DSS.0000000000003209
Practice Points
- Collagenous vasculopathy is an underrecognized entity.
- Although most patients exhibit only cutaneous disease, systemic involvement also should be assessed.