User login
Case Report
A 74-year-old man with a history of squamous cell carcinoma (SCC) on the right side of the temple that was treated with Mohs micrographic surgery (MMS) 3 years prior presented with a burning and tingling sensation of 3 months’ duration in the medial border of the repair scar. The patient denied prior anesthesia or muscle weakness of the face as well as any loss or change in vision.
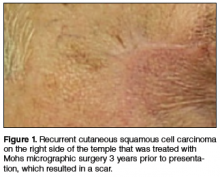
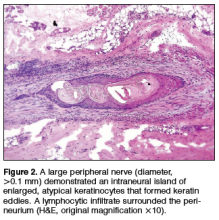
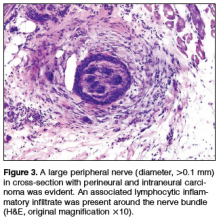
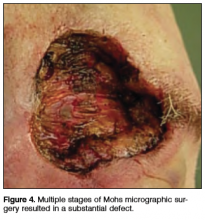
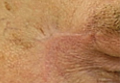
Physical examination revealed a well-healed advancement flap scar with induration at the medial border (Figure 1). Biopsy results were positive for recurrent SCC. Based on anatomic location, clinical symptoms, and tumor recurrence, treatment with MMS was initiated. Mohs sections demonstrated perineural invasion (PNI) (Figures 2 and 3). Multiple treatment stages were required for tumor clearance following the retrograde course of a nerve, which resulted in a substantial defect (Figure 4). The defect was allowed to heal by second intention followed by radiation therapy.
Comment
Incidence and Pathogenesis—Perineural invasion was first described by Cruveilhier1 in a report of invasion of the facial nerve in a patient with mammary carcinoma. Neumann2 reported the first case of a primary cutaneous lesion exhibiting PNI in a patient with a primary carcinoma of the lower lip with invasion and spread along the mental nerve. Perineural invasion is seen in approximately 5% of 200,000 total cases of cutaneous SCC reported annually in the United States.3,4 Other malignancies exhibit PNI more frequently, such as microcystic adnexal carcinoma of the skin, which has been reported to have an 80% rate of perineural growth.5
Perineural invasion can involve nerves of variable thickness, but invasion of larger nerves typically portends a poorer prognosis.6 Characteristics of cutaneous SCC that predispose the lesion to PNI include size greater than 2 cm, male gender, location on the face, and prior treatment of the lesion.6,7 In a study of cutaneous SCC, Leibovitch et al7 found PNI in 4.7% (36/772) of primary lesions and 6.9% (34/491) of recurrent lesions. In another study of 180 SCC tumors of the head and neck with PNI, Carter et al8 found that PNI was most commonly seen in tumors that were greater than 2.5 cm, suggesting that larger lesions have an increased predisposition for PNI.
The mechanism(s) by which PNI develops from these malignancies has not been fully elucidated, but some clues have been found. Vural et al9 showed a statistically significant difference (P<.01) in expression of neural cell adhesion molecules with 93% (38/41) of SCCs with PNI showing evidence of expression versus 36% (9/25) of SCCs without PNI. Chen-Tsai et al10 also suggested that levels of neural cell adhesion molecules may be a factor in determining the metastatic potential of cutaneous SCCs and that levels of neurotrophic tyrosine kinase receptor type 1 (TrkA) may predict PNI, but their study results lacked statistical power to form a firm conclusion.
Diagnosis and Prognosis—Perineural invasion can be diagnosed clinically, radiologically, or microscopically. On clinical examination, PNI is suggested by findings of neuropathy most frequently in cranial nerves V and/or VII, likely due to their extensive subcutaneous distribution.11 Common symptoms include pain, loss of motor skills, anesthesia, dysesthesia, and/or paresthesia (ie, tingling, burning, pricking, numbness).12,13 In a study of 72 cases, Goepfert et al14 found that only 40% (29/72) of patients with pathologically confirmed PNI presented with clinical symptoms and these patients had a poorer prognosis.
Radiologically, PNI can be identified via computed tomography or magnetic resonance imaging through findings of enlargement or abnormal enhancement of the nerve, obliteration of the normal fat plane surrounding the nerve, or erosion or enlargement of its related foramen.15 Magnetic resonance imaging is the preferred method for assessing enhancement of the nerve, while computed tomography is preferred to assess involvement of bone.16,17 Microscopically, there is some debate as to what defines PNI. Suggested findings include the presence of cells inside the epineurium, involvement of nerves outside the main bulk of the tumor, or presence of tumor cells surrounding a nerve.18
These definitions have prognostic significance. Mendenhall et al16 found that patients with radiologic evidence of PNI without clinical symptoms had a higher cure rate using surgery and postoperative irradiation compared to patients with clinical symptoms (80% vs 45%). Although prognosis generally is good in patients with cutaneous SCC without PNI, prognosis is notably poorer when PNI is present due to the association of this finding with increased tumor recurrence and both local and distant metastasis.13 Most frequently, cutaneous SCC with PNI spreads proximally, which can lead to invasion into the base of the brain, but also can extend distally, leading to increased local burden.12,19 In a study of 64 patients with mucosal SCC, Soo et al20 found that patients with lesions that exhibited PNI had a 5-year survival of 16% versus 44% in those without PNI. In their study of SCC of the head and neck, Goepfert et al14 reported that 46% (33/72) of patients with PNI had died or were alive with recurrence at 2 years’ follow-up versus 9.1% (41/448) of patients without PNI. In a systematic review of outcomes, Jambusaria-Pahlajani et al21 reported a disease-specific death rate of 16% for cutaneous SCC with PNI compared to 4% for SCC without PNI.
Perineural invasion can be further classified as clinical or microscopic (incidental) for prognostic purposes. A study by Garcia-Serra et al13 found that patients with clinical PNI had a notably poorer prognosis than those with microscopic (incidental) PNI. The clinical group achieved a local control rate of 55% at 5 years’ follow-up versus 87% in the microscopic group. McCord et al22 found a 5-year local control rate of 78% for microscopic (incidental) PNI versus 50% for clinical PNI; they also found that patients with radiologic evidence of PNI had a worse prognosis, noting that patients with radiologic evidence of PNI were nearly all clinically symptomatic.
Prognosis also is altered by the diameter of the nerve involved. In a study of 48 patients, Ross et al23 found that patients with cutaneous SCC involving small-caliber nerves (diameter, ≤0.1 mm) had a 0% disease-specific death rate versus 32% in those with large-caliber nerves (>0.1 mm). Perineural involvement of small-caliber nerves (<0.1 mm) was a positive prognostic indicator in that it was associated with smaller tumor diameter, more shallow invasion, and increased likelihood to be primary tumors.23 In a recent study, Jambusaria-Pahlajani et al24 investigated tumor staging for cutaneous SCC and reported that PNI is a statistically independent prognostic risk factor for nodal metastasis (subhazard ratio, 2.2 [95% confidence interval, 0.9-5.1]) and disease-specific death (subhazard ratio, 3.4 [95% confidence interval, 0.9-13.3]). Of interest, this increased risk applied only to PNI in nerves that were greater than 0.1 mm.24
Treatment Options—Management of confirmed cases of cutaneous SCC with PNI is difficult because of the nature of the lesions, including their increased propensity for metastasis, increased frequency of poorly differentiated cell types, highly aggressive nature, and the unique challenge of skip lesions.4,16 Skip lesions are found microscopically and show (or appear to show) neoplastic cells invading a nerve in a discontinuous fashion. This phenomenon has been suggested as one explanation for the relatively higher postsurgical recurrence rate of SCC with PNI compared to lesions without PNI.7 They are of particular interest when removing cutaneous SCC with PNI using MMS and attempting to define clear margins. Despite this limitation, MMS generally is accepted as the primary mode of excision of cutaneous SCCs with PNI, as it has the highest known cure rate.7 Cottel4 did not report any cases of local recurrence over 1 to 42 months in 17 patients who were treated with MMS, in contrast to Rowe et al25 who demonstrated that traditional surgical excision had a 47% (34/72) local recurrence rate; however, it bears noting that the varying follow-up periods in the Cottel4 study may underestimate recurrence rate. Leibovitch et al7 had similar findings in their prospective case series study of 70 patients, which revealed an 8% recurrence rate within 5 years in patients treated with MMS, a rate lower than other non-MMS modalities. In this same study, the authors noted that some researchers believe an additional level should be taken with MMS beyond the appearance of free margins in cases with PNI.7
Jambusaria-Pahlajani et al21 reported that PNI is one of the most common reasons cited for using adjuvant radiation therapy for cases of cutaneous SCC because of the known propensity of local recurrence; however, in 74 reviewed cases, there was no statistically significant difference in outcomes in cases of surgery alone versus surgery and adjuvant irradiation. Radiation therapy is a possible alternative primary treatment of cutaneous SCC with PNI, especially in cases of perineural involvement that is extensive or affects proximal portions of cranial nerves when surgery is a less viable option.17 Mendenhall et al16 suggested that patients with positive margins after excision who display extensive PNI should be treated with adjuvant irradiation locally and along the course of the involved nerve to the skull base.
Conclusion
Physicians should recognize the importance of early detection of PNI in cases of cutaneous SCC. A thorough history with good neurologic examination of the head and neck in patients with cutaneous SCC is imperative so patients can be treated earlier in the course of the lesion, increasing the likelihood of local control, minimizing the risk for future recurrence, and decreasing mortality.
1. Cruveilhier J. Maladies des nerfs. In: Cruveilhier J, ed. Anatomie Pathologique du Corps Humain. 2nd ed. Paris, France: JB Bailliere; 1835:1-3.
2. Neumann E. Secondare cancroid infiltration des nervus mentalis bei einem fall von lippincroid. Arch Pathol Anat. 1862;24:201-205.
3. Salasche S. Epidemiology of actinic keratoses and squamous cell carcinoma. J Am Acad Dermatol. 2000;42(1, pt 2):4-7.
4. Cottel WI. Perineural invasion by squamous-cell carcinoma. J Dermatol Surg Oncol. 1982;8:589-600.
5. Cooper PH, Mills SE, Leonard DD, et al. Sclerosing sweat duct (syringomatous) carcinoma. Am J Surg Pathol. 1985;9:422-433.
6. Ross AS, Whalen FM, Elenitsas R, et al. Diameter of involved nerves predicts outcomes in cutaneous squamous cell carcinoma with perineural invasion: an investigator-blinded retrospective cohort study. Dermatol Surg. 2009;35:1859-1866.
7. Leibovitch I, Huilgol SC, Selva D, et al. Cutaneous squamous cell carcinoma treated with Mohs micrographic surgery in Australia II. perineural invasion. J Am Acad Dermatol. 2005;53:261-266.
8. Carter RL, Foster CS, Dinsdale EA, et al. Perineural spread by squamous carcinomas of the head and neck: a morphological study using antiaxonal and antimyelin monoclonal antibodies. J Clin Pathol. 1983;36:269-275.
9. Vural E, Hutcheson J, Korourian S, et al. Correlation of neural cell adhesion molecules with perineural spread of squamous cell carcinoma of the head and neck. Otolaryngol Head Neck Surg. 2000;122:717-720.
10. Chen-Tsai CP, Colome-Grimmer M, Wagner RF Jr. Correlations among neural cell adhesion molecule, nerve growth factor, and its receptors, TrkA, TrkB, TrkC, and p75, in perineural invasion by basal cell and cutaneous squamous cell carcinomas. Dermatol Surg. 2004;30:1009-1016.
11. McCord M, Mendenhall WM, Parsons JT, et al. Skin cancer of the head and neck with clinical perineural invasion. Int J Radiat Oncol Biol Phys. 2000;47:89-93.
12. Ampil FL, Hardin JC, Peskind SP, et al. Perineural invasion in skin cancer of the head and neck: a review of nine cases. J Oral Maxillofac Surg. 1995;53:34-38.
13. Garcia-Serra A, Hinerman RW, Mendenhall WM, et al. Carcinoma of the skin with perineural invasion. Head Neck. 2003;25:1027-1033.
14. Goepfert H, Dichtel WJ, Medina JE, et al. Perineural invasion in squamous cell skin carcinoma of the head and neck. Am J Surg. 1984;148:542-547.
15. Galloway TJ, Morris CG, Mancuso AA, et al. Impact of radiographic findings on prognosis for skin carcinoma with clinical perineural invasion. Cancer. 2005;103:1254-1257.
16. Mendenhall WM, Amdur RJ, Williams LS, et al. Carcinoma of the skin of the head and neck with perineural invasion. Head Neck. 2002;24:78-83.
17. Williams LS, Mancuso AA, Mendenhall WM. Perineural spread of cutaneous squamous and basal cell carcinoma: CT and MR detection and its impact on patient management and prognosis. Int J Radiat Oncol Biol Phys. 2001;49:1061-1069.
18. Veness MJ, Biankin S. Perineural spread leading to orbital invasion from skin cancer. Australasian Radiol. 2000;44:296-302.
19. Feasel AM, Brown TJ, Bogle MA, et al. Perineural invasion of cutaneous malignancies. Dermatol Surg. 2001;27:531-542.
20. Soo K, Carter RL, O’Brien CJ, et al. Prognostic implications of perineural spread in squamous carcinomas of the head and neck. Laryngoscope. 1986;96:1145-1148.
21. Jambusaria-Pahlajani A, Miller CJ, Quon H, et al. Surgical monotherapy versus surgery plus adjuvant radiotherapy in high-risk cutaneous squamous cell carcinoma: a systematic review of outcomes. Dermatol Surg. 2009;35:574-584.
22. McCord MW, Mendenhall WM, Parsons JT, et al. Skin cancer of the head and neck with incidental microscopic perineural invasion. Int J Radiat Oncol Biol Phys. 1999;43:591-595.
23. Ross AS, Whalen FM, Elenitsas R, et al. Diameter of involved nerves predicts outcomes in cutaneous squamous cell carcinoma with perineural invasion: an investigator-blinded retrospective cohort study. Dermatol Surg. 2009;35:1859-1866.
24. Jambusaria-Pahlajani A, Kanetsky PA, Karia PS, et al. Evaluation of AJCC tumor staging for cutaneous squamous cell carcinoma and a proposed alternative tumor staging system. JAMA Dermatol. 2013;149:402-410.
25. Rowe DE, Carroll RJ, Day CL. Prognostic factors for local recurrence, metastasis, and survival rates in squamous cell carcinoma of the skin, ear, and lip. J Am Acad Dermatol. 1992;26:976-990.
Case Report
A 74-year-old man with a history of squamous cell carcinoma (SCC) on the right side of the temple that was treated with Mohs micrographic surgery (MMS) 3 years prior presented with a burning and tingling sensation of 3 months’ duration in the medial border of the repair scar. The patient denied prior anesthesia or muscle weakness of the face as well as any loss or change in vision.
Physical examination revealed a well-healed advancement flap scar with induration at the medial border (Figure 1). Biopsy results were positive for recurrent SCC. Based on anatomic location, clinical symptoms, and tumor recurrence, treatment with MMS was initiated. Mohs sections demonstrated perineural invasion (PNI) (Figures 2 and 3). Multiple treatment stages were required for tumor clearance following the retrograde course of a nerve, which resulted in a substantial defect (Figure 4). The defect was allowed to heal by second intention followed by radiation therapy.
Comment
Incidence and Pathogenesis—Perineural invasion was first described by Cruveilhier1 in a report of invasion of the facial nerve in a patient with mammary carcinoma. Neumann2 reported the first case of a primary cutaneous lesion exhibiting PNI in a patient with a primary carcinoma of the lower lip with invasion and spread along the mental nerve. Perineural invasion is seen in approximately 5% of 200,000 total cases of cutaneous SCC reported annually in the United States.3,4 Other malignancies exhibit PNI more frequently, such as microcystic adnexal carcinoma of the skin, which has been reported to have an 80% rate of perineural growth.5
Perineural invasion can involve nerves of variable thickness, but invasion of larger nerves typically portends a poorer prognosis.6 Characteristics of cutaneous SCC that predispose the lesion to PNI include size greater than 2 cm, male gender, location on the face, and prior treatment of the lesion.6,7 In a study of cutaneous SCC, Leibovitch et al7 found PNI in 4.7% (36/772) of primary lesions and 6.9% (34/491) of recurrent lesions. In another study of 180 SCC tumors of the head and neck with PNI, Carter et al8 found that PNI was most commonly seen in tumors that were greater than 2.5 cm, suggesting that larger lesions have an increased predisposition for PNI.
The mechanism(s) by which PNI develops from these malignancies has not been fully elucidated, but some clues have been found. Vural et al9 showed a statistically significant difference (P<.01) in expression of neural cell adhesion molecules with 93% (38/41) of SCCs with PNI showing evidence of expression versus 36% (9/25) of SCCs without PNI. Chen-Tsai et al10 also suggested that levels of neural cell adhesion molecules may be a factor in determining the metastatic potential of cutaneous SCCs and that levels of neurotrophic tyrosine kinase receptor type 1 (TrkA) may predict PNI, but their study results lacked statistical power to form a firm conclusion.
Diagnosis and Prognosis—Perineural invasion can be diagnosed clinically, radiologically, or microscopically. On clinical examination, PNI is suggested by findings of neuropathy most frequently in cranial nerves V and/or VII, likely due to their extensive subcutaneous distribution.11 Common symptoms include pain, loss of motor skills, anesthesia, dysesthesia, and/or paresthesia (ie, tingling, burning, pricking, numbness).12,13 In a study of 72 cases, Goepfert et al14 found that only 40% (29/72) of patients with pathologically confirmed PNI presented with clinical symptoms and these patients had a poorer prognosis.
Radiologically, PNI can be identified via computed tomography or magnetic resonance imaging through findings of enlargement or abnormal enhancement of the nerve, obliteration of the normal fat plane surrounding the nerve, or erosion or enlargement of its related foramen.15 Magnetic resonance imaging is the preferred method for assessing enhancement of the nerve, while computed tomography is preferred to assess involvement of bone.16,17 Microscopically, there is some debate as to what defines PNI. Suggested findings include the presence of cells inside the epineurium, involvement of nerves outside the main bulk of the tumor, or presence of tumor cells surrounding a nerve.18
These definitions have prognostic significance. Mendenhall et al16 found that patients with radiologic evidence of PNI without clinical symptoms had a higher cure rate using surgery and postoperative irradiation compared to patients with clinical symptoms (80% vs 45%). Although prognosis generally is good in patients with cutaneous SCC without PNI, prognosis is notably poorer when PNI is present due to the association of this finding with increased tumor recurrence and both local and distant metastasis.13 Most frequently, cutaneous SCC with PNI spreads proximally, which can lead to invasion into the base of the brain, but also can extend distally, leading to increased local burden.12,19 In a study of 64 patients with mucosal SCC, Soo et al20 found that patients with lesions that exhibited PNI had a 5-year survival of 16% versus 44% in those without PNI. In their study of SCC of the head and neck, Goepfert et al14 reported that 46% (33/72) of patients with PNI had died or were alive with recurrence at 2 years’ follow-up versus 9.1% (41/448) of patients without PNI. In a systematic review of outcomes, Jambusaria-Pahlajani et al21 reported a disease-specific death rate of 16% for cutaneous SCC with PNI compared to 4% for SCC without PNI.
Perineural invasion can be further classified as clinical or microscopic (incidental) for prognostic purposes. A study by Garcia-Serra et al13 found that patients with clinical PNI had a notably poorer prognosis than those with microscopic (incidental) PNI. The clinical group achieved a local control rate of 55% at 5 years’ follow-up versus 87% in the microscopic group. McCord et al22 found a 5-year local control rate of 78% for microscopic (incidental) PNI versus 50% for clinical PNI; they also found that patients with radiologic evidence of PNI had a worse prognosis, noting that patients with radiologic evidence of PNI were nearly all clinically symptomatic.
Prognosis also is altered by the diameter of the nerve involved. In a study of 48 patients, Ross et al23 found that patients with cutaneous SCC involving small-caliber nerves (diameter, ≤0.1 mm) had a 0% disease-specific death rate versus 32% in those with large-caliber nerves (>0.1 mm). Perineural involvement of small-caliber nerves (<0.1 mm) was a positive prognostic indicator in that it was associated with smaller tumor diameter, more shallow invasion, and increased likelihood to be primary tumors.23 In a recent study, Jambusaria-Pahlajani et al24 investigated tumor staging for cutaneous SCC and reported that PNI is a statistically independent prognostic risk factor for nodal metastasis (subhazard ratio, 2.2 [95% confidence interval, 0.9-5.1]) and disease-specific death (subhazard ratio, 3.4 [95% confidence interval, 0.9-13.3]). Of interest, this increased risk applied only to PNI in nerves that were greater than 0.1 mm.24
Treatment Options—Management of confirmed cases of cutaneous SCC with PNI is difficult because of the nature of the lesions, including their increased propensity for metastasis, increased frequency of poorly differentiated cell types, highly aggressive nature, and the unique challenge of skip lesions.4,16 Skip lesions are found microscopically and show (or appear to show) neoplastic cells invading a nerve in a discontinuous fashion. This phenomenon has been suggested as one explanation for the relatively higher postsurgical recurrence rate of SCC with PNI compared to lesions without PNI.7 They are of particular interest when removing cutaneous SCC with PNI using MMS and attempting to define clear margins. Despite this limitation, MMS generally is accepted as the primary mode of excision of cutaneous SCCs with PNI, as it has the highest known cure rate.7 Cottel4 did not report any cases of local recurrence over 1 to 42 months in 17 patients who were treated with MMS, in contrast to Rowe et al25 who demonstrated that traditional surgical excision had a 47% (34/72) local recurrence rate; however, it bears noting that the varying follow-up periods in the Cottel4 study may underestimate recurrence rate. Leibovitch et al7 had similar findings in their prospective case series study of 70 patients, which revealed an 8% recurrence rate within 5 years in patients treated with MMS, a rate lower than other non-MMS modalities. In this same study, the authors noted that some researchers believe an additional level should be taken with MMS beyond the appearance of free margins in cases with PNI.7
Jambusaria-Pahlajani et al21 reported that PNI is one of the most common reasons cited for using adjuvant radiation therapy for cases of cutaneous SCC because of the known propensity of local recurrence; however, in 74 reviewed cases, there was no statistically significant difference in outcomes in cases of surgery alone versus surgery and adjuvant irradiation. Radiation therapy is a possible alternative primary treatment of cutaneous SCC with PNI, especially in cases of perineural involvement that is extensive or affects proximal portions of cranial nerves when surgery is a less viable option.17 Mendenhall et al16 suggested that patients with positive margins after excision who display extensive PNI should be treated with adjuvant irradiation locally and along the course of the involved nerve to the skull base.
Conclusion
Physicians should recognize the importance of early detection of PNI in cases of cutaneous SCC. A thorough history with good neurologic examination of the head and neck in patients with cutaneous SCC is imperative so patients can be treated earlier in the course of the lesion, increasing the likelihood of local control, minimizing the risk for future recurrence, and decreasing mortality.
Case Report
A 74-year-old man with a history of squamous cell carcinoma (SCC) on the right side of the temple that was treated with Mohs micrographic surgery (MMS) 3 years prior presented with a burning and tingling sensation of 3 months’ duration in the medial border of the repair scar. The patient denied prior anesthesia or muscle weakness of the face as well as any loss or change in vision.
Physical examination revealed a well-healed advancement flap scar with induration at the medial border (Figure 1). Biopsy results were positive for recurrent SCC. Based on anatomic location, clinical symptoms, and tumor recurrence, treatment with MMS was initiated. Mohs sections demonstrated perineural invasion (PNI) (Figures 2 and 3). Multiple treatment stages were required for tumor clearance following the retrograde course of a nerve, which resulted in a substantial defect (Figure 4). The defect was allowed to heal by second intention followed by radiation therapy.
Comment
Incidence and Pathogenesis—Perineural invasion was first described by Cruveilhier1 in a report of invasion of the facial nerve in a patient with mammary carcinoma. Neumann2 reported the first case of a primary cutaneous lesion exhibiting PNI in a patient with a primary carcinoma of the lower lip with invasion and spread along the mental nerve. Perineural invasion is seen in approximately 5% of 200,000 total cases of cutaneous SCC reported annually in the United States.3,4 Other malignancies exhibit PNI more frequently, such as microcystic adnexal carcinoma of the skin, which has been reported to have an 80% rate of perineural growth.5
Perineural invasion can involve nerves of variable thickness, but invasion of larger nerves typically portends a poorer prognosis.6 Characteristics of cutaneous SCC that predispose the lesion to PNI include size greater than 2 cm, male gender, location on the face, and prior treatment of the lesion.6,7 In a study of cutaneous SCC, Leibovitch et al7 found PNI in 4.7% (36/772) of primary lesions and 6.9% (34/491) of recurrent lesions. In another study of 180 SCC tumors of the head and neck with PNI, Carter et al8 found that PNI was most commonly seen in tumors that were greater than 2.5 cm, suggesting that larger lesions have an increased predisposition for PNI.
The mechanism(s) by which PNI develops from these malignancies has not been fully elucidated, but some clues have been found. Vural et al9 showed a statistically significant difference (P<.01) in expression of neural cell adhesion molecules with 93% (38/41) of SCCs with PNI showing evidence of expression versus 36% (9/25) of SCCs without PNI. Chen-Tsai et al10 also suggested that levels of neural cell adhesion molecules may be a factor in determining the metastatic potential of cutaneous SCCs and that levels of neurotrophic tyrosine kinase receptor type 1 (TrkA) may predict PNI, but their study results lacked statistical power to form a firm conclusion.
Diagnosis and Prognosis—Perineural invasion can be diagnosed clinically, radiologically, or microscopically. On clinical examination, PNI is suggested by findings of neuropathy most frequently in cranial nerves V and/or VII, likely due to their extensive subcutaneous distribution.11 Common symptoms include pain, loss of motor skills, anesthesia, dysesthesia, and/or paresthesia (ie, tingling, burning, pricking, numbness).12,13 In a study of 72 cases, Goepfert et al14 found that only 40% (29/72) of patients with pathologically confirmed PNI presented with clinical symptoms and these patients had a poorer prognosis.
Radiologically, PNI can be identified via computed tomography or magnetic resonance imaging through findings of enlargement or abnormal enhancement of the nerve, obliteration of the normal fat plane surrounding the nerve, or erosion or enlargement of its related foramen.15 Magnetic resonance imaging is the preferred method for assessing enhancement of the nerve, while computed tomography is preferred to assess involvement of bone.16,17 Microscopically, there is some debate as to what defines PNI. Suggested findings include the presence of cells inside the epineurium, involvement of nerves outside the main bulk of the tumor, or presence of tumor cells surrounding a nerve.18
These definitions have prognostic significance. Mendenhall et al16 found that patients with radiologic evidence of PNI without clinical symptoms had a higher cure rate using surgery and postoperative irradiation compared to patients with clinical symptoms (80% vs 45%). Although prognosis generally is good in patients with cutaneous SCC without PNI, prognosis is notably poorer when PNI is present due to the association of this finding with increased tumor recurrence and both local and distant metastasis.13 Most frequently, cutaneous SCC with PNI spreads proximally, which can lead to invasion into the base of the brain, but also can extend distally, leading to increased local burden.12,19 In a study of 64 patients with mucosal SCC, Soo et al20 found that patients with lesions that exhibited PNI had a 5-year survival of 16% versus 44% in those without PNI. In their study of SCC of the head and neck, Goepfert et al14 reported that 46% (33/72) of patients with PNI had died or were alive with recurrence at 2 years’ follow-up versus 9.1% (41/448) of patients without PNI. In a systematic review of outcomes, Jambusaria-Pahlajani et al21 reported a disease-specific death rate of 16% for cutaneous SCC with PNI compared to 4% for SCC without PNI.
Perineural invasion can be further classified as clinical or microscopic (incidental) for prognostic purposes. A study by Garcia-Serra et al13 found that patients with clinical PNI had a notably poorer prognosis than those with microscopic (incidental) PNI. The clinical group achieved a local control rate of 55% at 5 years’ follow-up versus 87% in the microscopic group. McCord et al22 found a 5-year local control rate of 78% for microscopic (incidental) PNI versus 50% for clinical PNI; they also found that patients with radiologic evidence of PNI had a worse prognosis, noting that patients with radiologic evidence of PNI were nearly all clinically symptomatic.
Prognosis also is altered by the diameter of the nerve involved. In a study of 48 patients, Ross et al23 found that patients with cutaneous SCC involving small-caliber nerves (diameter, ≤0.1 mm) had a 0% disease-specific death rate versus 32% in those with large-caliber nerves (>0.1 mm). Perineural involvement of small-caliber nerves (<0.1 mm) was a positive prognostic indicator in that it was associated with smaller tumor diameter, more shallow invasion, and increased likelihood to be primary tumors.23 In a recent study, Jambusaria-Pahlajani et al24 investigated tumor staging for cutaneous SCC and reported that PNI is a statistically independent prognostic risk factor for nodal metastasis (subhazard ratio, 2.2 [95% confidence interval, 0.9-5.1]) and disease-specific death (subhazard ratio, 3.4 [95% confidence interval, 0.9-13.3]). Of interest, this increased risk applied only to PNI in nerves that were greater than 0.1 mm.24
Treatment Options—Management of confirmed cases of cutaneous SCC with PNI is difficult because of the nature of the lesions, including their increased propensity for metastasis, increased frequency of poorly differentiated cell types, highly aggressive nature, and the unique challenge of skip lesions.4,16 Skip lesions are found microscopically and show (or appear to show) neoplastic cells invading a nerve in a discontinuous fashion. This phenomenon has been suggested as one explanation for the relatively higher postsurgical recurrence rate of SCC with PNI compared to lesions without PNI.7 They are of particular interest when removing cutaneous SCC with PNI using MMS and attempting to define clear margins. Despite this limitation, MMS generally is accepted as the primary mode of excision of cutaneous SCCs with PNI, as it has the highest known cure rate.7 Cottel4 did not report any cases of local recurrence over 1 to 42 months in 17 patients who were treated with MMS, in contrast to Rowe et al25 who demonstrated that traditional surgical excision had a 47% (34/72) local recurrence rate; however, it bears noting that the varying follow-up periods in the Cottel4 study may underestimate recurrence rate. Leibovitch et al7 had similar findings in their prospective case series study of 70 patients, which revealed an 8% recurrence rate within 5 years in patients treated with MMS, a rate lower than other non-MMS modalities. In this same study, the authors noted that some researchers believe an additional level should be taken with MMS beyond the appearance of free margins in cases with PNI.7
Jambusaria-Pahlajani et al21 reported that PNI is one of the most common reasons cited for using adjuvant radiation therapy for cases of cutaneous SCC because of the known propensity of local recurrence; however, in 74 reviewed cases, there was no statistically significant difference in outcomes in cases of surgery alone versus surgery and adjuvant irradiation. Radiation therapy is a possible alternative primary treatment of cutaneous SCC with PNI, especially in cases of perineural involvement that is extensive or affects proximal portions of cranial nerves when surgery is a less viable option.17 Mendenhall et al16 suggested that patients with positive margins after excision who display extensive PNI should be treated with adjuvant irradiation locally and along the course of the involved nerve to the skull base.
Conclusion
Physicians should recognize the importance of early detection of PNI in cases of cutaneous SCC. A thorough history with good neurologic examination of the head and neck in patients with cutaneous SCC is imperative so patients can be treated earlier in the course of the lesion, increasing the likelihood of local control, minimizing the risk for future recurrence, and decreasing mortality.
1. Cruveilhier J. Maladies des nerfs. In: Cruveilhier J, ed. Anatomie Pathologique du Corps Humain. 2nd ed. Paris, France: JB Bailliere; 1835:1-3.
2. Neumann E. Secondare cancroid infiltration des nervus mentalis bei einem fall von lippincroid. Arch Pathol Anat. 1862;24:201-205.
3. Salasche S. Epidemiology of actinic keratoses and squamous cell carcinoma. J Am Acad Dermatol. 2000;42(1, pt 2):4-7.
4. Cottel WI. Perineural invasion by squamous-cell carcinoma. J Dermatol Surg Oncol. 1982;8:589-600.
5. Cooper PH, Mills SE, Leonard DD, et al. Sclerosing sweat duct (syringomatous) carcinoma. Am J Surg Pathol. 1985;9:422-433.
6. Ross AS, Whalen FM, Elenitsas R, et al. Diameter of involved nerves predicts outcomes in cutaneous squamous cell carcinoma with perineural invasion: an investigator-blinded retrospective cohort study. Dermatol Surg. 2009;35:1859-1866.
7. Leibovitch I, Huilgol SC, Selva D, et al. Cutaneous squamous cell carcinoma treated with Mohs micrographic surgery in Australia II. perineural invasion. J Am Acad Dermatol. 2005;53:261-266.
8. Carter RL, Foster CS, Dinsdale EA, et al. Perineural spread by squamous carcinomas of the head and neck: a morphological study using antiaxonal and antimyelin monoclonal antibodies. J Clin Pathol. 1983;36:269-275.
9. Vural E, Hutcheson J, Korourian S, et al. Correlation of neural cell adhesion molecules with perineural spread of squamous cell carcinoma of the head and neck. Otolaryngol Head Neck Surg. 2000;122:717-720.
10. Chen-Tsai CP, Colome-Grimmer M, Wagner RF Jr. Correlations among neural cell adhesion molecule, nerve growth factor, and its receptors, TrkA, TrkB, TrkC, and p75, in perineural invasion by basal cell and cutaneous squamous cell carcinomas. Dermatol Surg. 2004;30:1009-1016.
11. McCord M, Mendenhall WM, Parsons JT, et al. Skin cancer of the head and neck with clinical perineural invasion. Int J Radiat Oncol Biol Phys. 2000;47:89-93.
12. Ampil FL, Hardin JC, Peskind SP, et al. Perineural invasion in skin cancer of the head and neck: a review of nine cases. J Oral Maxillofac Surg. 1995;53:34-38.
13. Garcia-Serra A, Hinerman RW, Mendenhall WM, et al. Carcinoma of the skin with perineural invasion. Head Neck. 2003;25:1027-1033.
14. Goepfert H, Dichtel WJ, Medina JE, et al. Perineural invasion in squamous cell skin carcinoma of the head and neck. Am J Surg. 1984;148:542-547.
15. Galloway TJ, Morris CG, Mancuso AA, et al. Impact of radiographic findings on prognosis for skin carcinoma with clinical perineural invasion. Cancer. 2005;103:1254-1257.
16. Mendenhall WM, Amdur RJ, Williams LS, et al. Carcinoma of the skin of the head and neck with perineural invasion. Head Neck. 2002;24:78-83.
17. Williams LS, Mancuso AA, Mendenhall WM. Perineural spread of cutaneous squamous and basal cell carcinoma: CT and MR detection and its impact on patient management and prognosis. Int J Radiat Oncol Biol Phys. 2001;49:1061-1069.
18. Veness MJ, Biankin S. Perineural spread leading to orbital invasion from skin cancer. Australasian Radiol. 2000;44:296-302.
19. Feasel AM, Brown TJ, Bogle MA, et al. Perineural invasion of cutaneous malignancies. Dermatol Surg. 2001;27:531-542.
20. Soo K, Carter RL, O’Brien CJ, et al. Prognostic implications of perineural spread in squamous carcinomas of the head and neck. Laryngoscope. 1986;96:1145-1148.
21. Jambusaria-Pahlajani A, Miller CJ, Quon H, et al. Surgical monotherapy versus surgery plus adjuvant radiotherapy in high-risk cutaneous squamous cell carcinoma: a systematic review of outcomes. Dermatol Surg. 2009;35:574-584.
22. McCord MW, Mendenhall WM, Parsons JT, et al. Skin cancer of the head and neck with incidental microscopic perineural invasion. Int J Radiat Oncol Biol Phys. 1999;43:591-595.
23. Ross AS, Whalen FM, Elenitsas R, et al. Diameter of involved nerves predicts outcomes in cutaneous squamous cell carcinoma with perineural invasion: an investigator-blinded retrospective cohort study. Dermatol Surg. 2009;35:1859-1866.
24. Jambusaria-Pahlajani A, Kanetsky PA, Karia PS, et al. Evaluation of AJCC tumor staging for cutaneous squamous cell carcinoma and a proposed alternative tumor staging system. JAMA Dermatol. 2013;149:402-410.
25. Rowe DE, Carroll RJ, Day CL. Prognostic factors for local recurrence, metastasis, and survival rates in squamous cell carcinoma of the skin, ear, and lip. J Am Acad Dermatol. 1992;26:976-990.
1. Cruveilhier J. Maladies des nerfs. In: Cruveilhier J, ed. Anatomie Pathologique du Corps Humain. 2nd ed. Paris, France: JB Bailliere; 1835:1-3.
2. Neumann E. Secondare cancroid infiltration des nervus mentalis bei einem fall von lippincroid. Arch Pathol Anat. 1862;24:201-205.
3. Salasche S. Epidemiology of actinic keratoses and squamous cell carcinoma. J Am Acad Dermatol. 2000;42(1, pt 2):4-7.
4. Cottel WI. Perineural invasion by squamous-cell carcinoma. J Dermatol Surg Oncol. 1982;8:589-600.
5. Cooper PH, Mills SE, Leonard DD, et al. Sclerosing sweat duct (syringomatous) carcinoma. Am J Surg Pathol. 1985;9:422-433.
6. Ross AS, Whalen FM, Elenitsas R, et al. Diameter of involved nerves predicts outcomes in cutaneous squamous cell carcinoma with perineural invasion: an investigator-blinded retrospective cohort study. Dermatol Surg. 2009;35:1859-1866.
7. Leibovitch I, Huilgol SC, Selva D, et al. Cutaneous squamous cell carcinoma treated with Mohs micrographic surgery in Australia II. perineural invasion. J Am Acad Dermatol. 2005;53:261-266.
8. Carter RL, Foster CS, Dinsdale EA, et al. Perineural spread by squamous carcinomas of the head and neck: a morphological study using antiaxonal and antimyelin monoclonal antibodies. J Clin Pathol. 1983;36:269-275.
9. Vural E, Hutcheson J, Korourian S, et al. Correlation of neural cell adhesion molecules with perineural spread of squamous cell carcinoma of the head and neck. Otolaryngol Head Neck Surg. 2000;122:717-720.
10. Chen-Tsai CP, Colome-Grimmer M, Wagner RF Jr. Correlations among neural cell adhesion molecule, nerve growth factor, and its receptors, TrkA, TrkB, TrkC, and p75, in perineural invasion by basal cell and cutaneous squamous cell carcinomas. Dermatol Surg. 2004;30:1009-1016.
11. McCord M, Mendenhall WM, Parsons JT, et al. Skin cancer of the head and neck with clinical perineural invasion. Int J Radiat Oncol Biol Phys. 2000;47:89-93.
12. Ampil FL, Hardin JC, Peskind SP, et al. Perineural invasion in skin cancer of the head and neck: a review of nine cases. J Oral Maxillofac Surg. 1995;53:34-38.
13. Garcia-Serra A, Hinerman RW, Mendenhall WM, et al. Carcinoma of the skin with perineural invasion. Head Neck. 2003;25:1027-1033.
14. Goepfert H, Dichtel WJ, Medina JE, et al. Perineural invasion in squamous cell skin carcinoma of the head and neck. Am J Surg. 1984;148:542-547.
15. Galloway TJ, Morris CG, Mancuso AA, et al. Impact of radiographic findings on prognosis for skin carcinoma with clinical perineural invasion. Cancer. 2005;103:1254-1257.
16. Mendenhall WM, Amdur RJ, Williams LS, et al. Carcinoma of the skin of the head and neck with perineural invasion. Head Neck. 2002;24:78-83.
17. Williams LS, Mancuso AA, Mendenhall WM. Perineural spread of cutaneous squamous and basal cell carcinoma: CT and MR detection and its impact on patient management and prognosis. Int J Radiat Oncol Biol Phys. 2001;49:1061-1069.
18. Veness MJ, Biankin S. Perineural spread leading to orbital invasion from skin cancer. Australasian Radiol. 2000;44:296-302.
19. Feasel AM, Brown TJ, Bogle MA, et al. Perineural invasion of cutaneous malignancies. Dermatol Surg. 2001;27:531-542.
20. Soo K, Carter RL, O’Brien CJ, et al. Prognostic implications of perineural spread in squamous carcinomas of the head and neck. Laryngoscope. 1986;96:1145-1148.
21. Jambusaria-Pahlajani A, Miller CJ, Quon H, et al. Surgical monotherapy versus surgery plus adjuvant radiotherapy in high-risk cutaneous squamous cell carcinoma: a systematic review of outcomes. Dermatol Surg. 2009;35:574-584.
22. McCord MW, Mendenhall WM, Parsons JT, et al. Skin cancer of the head and neck with incidental microscopic perineural invasion. Int J Radiat Oncol Biol Phys. 1999;43:591-595.
23. Ross AS, Whalen FM, Elenitsas R, et al. Diameter of involved nerves predicts outcomes in cutaneous squamous cell carcinoma with perineural invasion: an investigator-blinded retrospective cohort study. Dermatol Surg. 2009;35:1859-1866.
24. Jambusaria-Pahlajani A, Kanetsky PA, Karia PS, et al. Evaluation of AJCC tumor staging for cutaneous squamous cell carcinoma and a proposed alternative tumor staging system. JAMA Dermatol. 2013;149:402-410.
25. Rowe DE, Carroll RJ, Day CL. Prognostic factors for local recurrence, metastasis, and survival rates in squamous cell carcinoma of the skin, ear, and lip. J Am Acad Dermatol. 1992;26:976-990.
Practice Points
• Patients with suspected cutaneous squamous cell carcinoma should be asked about neurological symptoms including pain, loss of motor skills, anesthesia, dysesthesia, and/or paresthesia, which may indicate perineural invasion.
• Patients with perineural invasion carry a much higher risk for local and distant recurrence and may require more aggressive treatment including Mohs micrographic surgery and adjuvant radiation.