User login
Ms. L, age 37, presents to psychiatric emergency services with command auditory hallucinations, ideas of reference, and suicidal ideation.
Ms. L has a 22-year history of schizophrenia. Additionally, she has a history of cocaine use disorder (in remission for 12 years), cannabis use disorder (in remission for 6 months), type 2 diabetes mellitus, and hypertension. Her psychotic symptoms are well controlled on a regimen of
On interview, Ms. L reports smoking cannabis each day for the past month and using $400 worth of cocaine over 2 days. She is experiencing intense guilt over these relapses and is admitted to the inpatient adult psychiatry unit. On admission, Ms. L’s clozapine and norclozapine trough levels (drawn approximately 12 hours after last administration documented by the ACT member) are 300 and 275 ng/mL, respectively. Generally, clozapine levels >350 to 420 ng/mL are considered therapeutic, and a clozapine-to-norclozapine ratio of 2:1 is desirable for maximum efficacy and tolerability. Because Ms. L’s clozapine level is <350 and her ratio is approximately 1:1, her clozapine treatment is subtherapeutic.
Because Ms. L has a history of documented adherence to and benefit from her current medication regimen, no changes are made during her 3-week hospital stay. She notices a gradual reduction in auditory hallucinations, no longer wants to harm herself, and is motivated to regain sobriety.
At the time of discharge, Ms. L’s clozapine and norclozapine trough levels are 550 and 250 ng/mL, respectively, which indicates a more favorable clozapine-to-norclozapine ratio of approximately 2:1 and a clozapine level greater than the recommended minimum threshold of 350 ng/mL. While cocaine ingestion presumably played a role in her acute decompensation, the treatment team determined that Ms. L’s relapse to cannabis use likely contributed to low clozapine levels by induction of cytochrome P450 (CYP) 1A2, and subsequently led to the delayed recovery of symptom control.1
The use of illicit substances is a widespread, growing problem. According to the 2017 National Survey on Drug Use and Health, 11.5% of Americans age ≥12 had used an illicit substance (ie, use of marijuana, cocaine, heroin, hallucinogens, inhalants, or methamphetamine, or misuse of prescription psychotherapeutics) in the past month.2 While illicit substance use is of particular public health interest due to a known increase in mortality and health care spending, there has been little discussion of the impact of illicit drug use on concurrent pharmacologic therapy. Just as prescription medications have pharmacokinetic drug–drug interactions with each other, so do illicit substances, though far less is known about their impact on the treatment of medical conditions.
Pharmacokinetic interactions
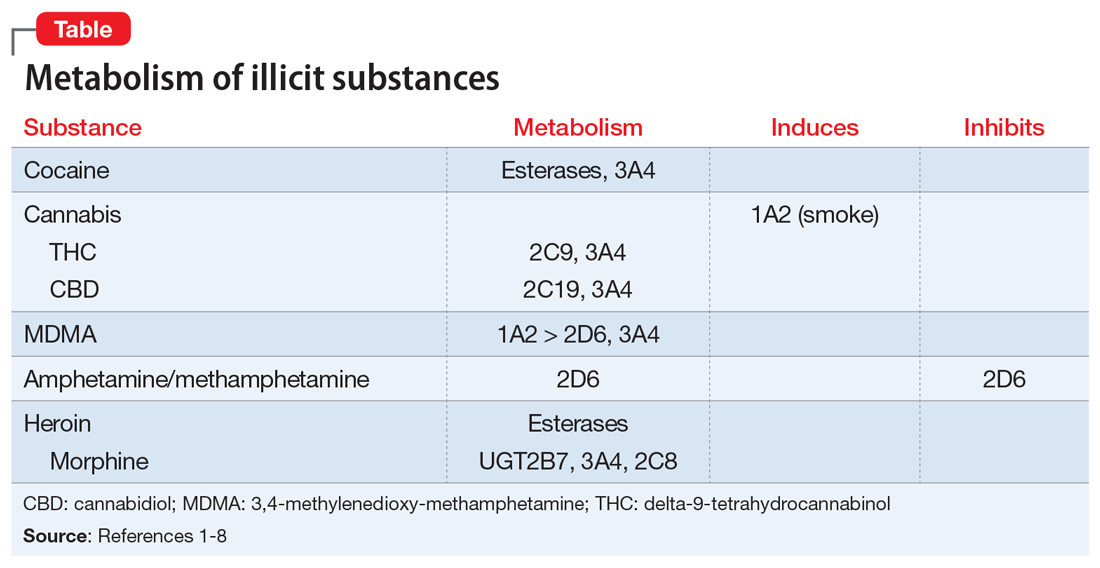
Key pharmacokinetic interactions have been reported with cocaine, marijuana, amphetamines, and opioids. The Table1-8 summarizes the metabolism of illicit substances.
Continue to: Cocaine
Cocaine is largely metabolized by serum esterases such as pseudocholinesterase, human carboxylesterase-1 (hCE-1), and human carboxylesterase-2 (hCE-2), to inactive metabolites benzoylecgonine (35% to 45%) and ecgonine (32% to 49%).2 However, a smaller portion (2.6% to 6.2%) undergoes hepatic N-demethylation by CYP3A4 to norcocaine.3 Norcocaine is an active metabolite responsible for some of the toxic effects of cocaine (eg, hepatotoxicity).4,5 Several commonly prescribed medications are known inducers of CYP3A4 (eg, phenytoin, carbamazepine) and may lead to increased levels of the toxic metabolite when used concurrently with cocaine. Additionally, the use of cocaine with acetylcholinesterase inhibitors, such as donepezil, may lead to reduction of serum esterases and shunt cocaine metabolism toward the hepatic pathway, thus increasing norcocaine formation.3
Cannabis. The metabolism and drug–drug interactions of cannabis can be separated by its 2 main components: delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD). A review conducted in 2014 concluded that THC is primarily metabolized by CYP2C9 and 3A4, while CBD is metabolized by CYP2C19 and 3A4.6 Oral administration of ketoconazole, a CYP3A4 inhibitor, along with cannabis extract has been shown to increase the maximum concentration (Cmax) and area under the curve (AUC) of THC by 1.2- and 1.8-fold, respectively, while increasing both Cmaxand AUC of CBD by 2-fold.6 In addition, CYP2C9 poor metabolizers have been shown to experience significant increases in THC exposure and reductions in metabolite formation, further supporting the role of CYP enzymes in cannabis metabolism.6
There is also evidence of enzyme induction by cannabis. Individuals who reported smoking marijuana experienced greater clearance of theophylline, a substrate of CYP1A2, than did those who reported not smoking marijuana.1,6 As with cigarette smoking, this effect appears to be a direct result of the hydrocarbons found in marijuana smoke rather than the cannabis itself, as there is a lack of evidence for enzyme induction when the drug is orally ingested.6
Amphetamine and methamphetamine appear to be both substrates and competitive inhibitors of CYP2D6.7 Rats administered quinidine (a strong 2D6 inhibitor) had 2-fold elevations in AUC and decreased clearance of amphetamine and its metabolites.8 Amphetamine-related recreational drugs, such as 3,4-methylenedioxy-methamphetamine (MDMA) and 3,4-methylenedioxyamphetamine (MDA), are substrates of CYP2D6 and CYP3A4, while MDMA also undergoes substantial metabolism by CYP1A2.3,7,9
Opioids. Heroin is metabolized to 6‑monoacetylmorphine (6-MAM) and morphine by hCE-1, hCE-2, and pseudocholinesterase, and has minimal impact on CYP enzymes. However, while morphine is primarily metabolized to inactive metabolites by UGT2B7, it does undergo minor metabolism through CYP3A4 and 2C8 pathways, creating potential for drug interactions with medications that inhibit or induce CYP3A4.10
Continue to: An underappreciated risk of illicit substance use
An underappreciated risk of illicit substance use
There is a paucity of evidence regarding the metabolism and pharmacokinetic interactions with illicit substances, and further research is needed. Despite the absence of comprehensive data on the subject, the available information indicates the use of illicit substances may have a significant impact on medications used to treat comorbid conditions. Alternatively, those medications may affect the kinetics of recreationally used substances. The risk of adverse consequences of drug–drug interactions is yet another reason patients should be encouraged to avoid use of substances and seek treatment for substance use disorders. When determining the most appropriate therapy for comorbid conditions for patients who are using illicit substances and are likely to continue to do so, clinicians should take into consideration potential interactions among prescription medications and the specific illicit substances the patient uses.
Related Resources
- Lindsey W, Stewart D, Childress D. Drug interactions between common illicit drugs and prescription therapies. Am J Drug Alcohol Abuse. 2012;38(4):334-343.
- Maurer H, Sauer C, Theobald D. Toxicokinetics of drugs of abuse: current knowledge of the isoenzymes involved in the human metabolism of tetrahydrocannabinol, cocaine, heroin, morphine, and codeine. Ther Drug Monit. 2006;28(3):447-453.
- Dean A. Illicit drugs and drug interactions. Pharmacist. 2006;25(9):684-689.
Drug Brand Names
Carbamazepine • Carbatrol, Tegretol
Clozapine • Clozaril
Donepezil • Aricept
Ketoconazole • Nizoral
Paliperidone palmitate • Invega sustenna
Phenytoin • Dilantin, Phenytek
Quinidine • Cardioquin, Duraquin
Theophylline • Elixophylline, Theochron
1. Jusko WJ, Schentag JJ, Clark JH, et al. Enhanced biotransformation of theophylline in marihuana and tobacco smokers. Clin Pharmacol Ther. 1978;24(4):405-410.
2. Substance Abuse and Mental Health Services Administration. Results from the 2017 National Survey on Drug Use and Health: Detailed Tables. https://www.samhsa.gov/data/sites/default/files/cbhsq-reports/NSDUHDetailedTabs2017/NSDUHDetailedTabs2017.htm#tab1-1A. Published 2019. Accessed February 7, 2020.
3. Quinn D, Wodak A, Day R. Pharmacokinetic and pharmacodynamic principles of illicit drug use and treatment of illicit drug users. Clin Pharmacokinet. 1997;33(5):344-400.
4. Ndikum-Moffor FM, Schoeb TR, Roberts SM. Liver toxicity from norcocaine nitroxide, an N-oxidative metabolite of cocaine. J Pharmacol Exp Ther. 1998;284(1):413-419.
5. Pellinen P, Honkakoski P, Stenbäck F, et al. Cocaine N-demethylation and the metabolism-related hepatotoxicity can be prevented by cytochrome P450 3A inhibitors. Eur J Pharmacol. 1994;270(1):35-43.
6. Stout S, Cimino N. Exogenous cannabinoids as substrates, inhibitors, and inducers of human drug metabolizing enzymes: a systematic review. Drug Metab Rev. 2013;46(1):86-95.
7. Kraemer T, Maurer H. Toxicokinetics of amphetamines: metabolism and toxicokinetic data of designer drugs, amphetamine, methamphetamine, and their N-alkyl derivatives. Ther Drug Monit. 2002;24(2):277-289.
8. Markowitz J, Patrick K. Pharmacokinetic and pharmacodynamic drug interactions in the treatment of attention-deficit hyperactivity disorder. Clin Pharmacokinet. 2001;40(10):753-772.
9. Kreth K, Kovar K, Schwab M, et al. Identification of the human cytochromes P450 involved in the oxidative metabolism of “ecstasy”-related designer drugs. Biochem Pharmacol. 2000;59(12):1563-1571.
10. Meyer M, Maurer H. Absorption, distribution, metabolism and excretion pharmacogenomics of drugs of abuse. Pharmacogenomics. 2011;12(2):215-233.
Ms. L, age 37, presents to psychiatric emergency services with command auditory hallucinations, ideas of reference, and suicidal ideation.
Ms. L has a 22-year history of schizophrenia. Additionally, she has a history of cocaine use disorder (in remission for 12 years), cannabis use disorder (in remission for 6 months), type 2 diabetes mellitus, and hypertension. Her psychotic symptoms are well controlled on a regimen of
On interview, Ms. L reports smoking cannabis each day for the past month and using $400 worth of cocaine over 2 days. She is experiencing intense guilt over these relapses and is admitted to the inpatient adult psychiatry unit. On admission, Ms. L’s clozapine and norclozapine trough levels (drawn approximately 12 hours after last administration documented by the ACT member) are 300 and 275 ng/mL, respectively. Generally, clozapine levels >350 to 420 ng/mL are considered therapeutic, and a clozapine-to-norclozapine ratio of 2:1 is desirable for maximum efficacy and tolerability. Because Ms. L’s clozapine level is <350 and her ratio is approximately 1:1, her clozapine treatment is subtherapeutic.
Because Ms. L has a history of documented adherence to and benefit from her current medication regimen, no changes are made during her 3-week hospital stay. She notices a gradual reduction in auditory hallucinations, no longer wants to harm herself, and is motivated to regain sobriety.
At the time of discharge, Ms. L’s clozapine and norclozapine trough levels are 550 and 250 ng/mL, respectively, which indicates a more favorable clozapine-to-norclozapine ratio of approximately 2:1 and a clozapine level greater than the recommended minimum threshold of 350 ng/mL. While cocaine ingestion presumably played a role in her acute decompensation, the treatment team determined that Ms. L’s relapse to cannabis use likely contributed to low clozapine levels by induction of cytochrome P450 (CYP) 1A2, and subsequently led to the delayed recovery of symptom control.1
The use of illicit substances is a widespread, growing problem. According to the 2017 National Survey on Drug Use and Health, 11.5% of Americans age ≥12 had used an illicit substance (ie, use of marijuana, cocaine, heroin, hallucinogens, inhalants, or methamphetamine, or misuse of prescription psychotherapeutics) in the past month.2 While illicit substance use is of particular public health interest due to a known increase in mortality and health care spending, there has been little discussion of the impact of illicit drug use on concurrent pharmacologic therapy. Just as prescription medications have pharmacokinetic drug–drug interactions with each other, so do illicit substances, though far less is known about their impact on the treatment of medical conditions.
Pharmacokinetic interactions
Key pharmacokinetic interactions have been reported with cocaine, marijuana, amphetamines, and opioids. The Table1-8 summarizes the metabolism of illicit substances.
Continue to: Cocaine
Cocaine is largely metabolized by serum esterases such as pseudocholinesterase, human carboxylesterase-1 (hCE-1), and human carboxylesterase-2 (hCE-2), to inactive metabolites benzoylecgonine (35% to 45%) and ecgonine (32% to 49%).2 However, a smaller portion (2.6% to 6.2%) undergoes hepatic N-demethylation by CYP3A4 to norcocaine.3 Norcocaine is an active metabolite responsible for some of the toxic effects of cocaine (eg, hepatotoxicity).4,5 Several commonly prescribed medications are known inducers of CYP3A4 (eg, phenytoin, carbamazepine) and may lead to increased levels of the toxic metabolite when used concurrently with cocaine. Additionally, the use of cocaine with acetylcholinesterase inhibitors, such as donepezil, may lead to reduction of serum esterases and shunt cocaine metabolism toward the hepatic pathway, thus increasing norcocaine formation.3
Cannabis. The metabolism and drug–drug interactions of cannabis can be separated by its 2 main components: delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD). A review conducted in 2014 concluded that THC is primarily metabolized by CYP2C9 and 3A4, while CBD is metabolized by CYP2C19 and 3A4.6 Oral administration of ketoconazole, a CYP3A4 inhibitor, along with cannabis extract has been shown to increase the maximum concentration (Cmax) and area under the curve (AUC) of THC by 1.2- and 1.8-fold, respectively, while increasing both Cmaxand AUC of CBD by 2-fold.6 In addition, CYP2C9 poor metabolizers have been shown to experience significant increases in THC exposure and reductions in metabolite formation, further supporting the role of CYP enzymes in cannabis metabolism.6
There is also evidence of enzyme induction by cannabis. Individuals who reported smoking marijuana experienced greater clearance of theophylline, a substrate of CYP1A2, than did those who reported not smoking marijuana.1,6 As with cigarette smoking, this effect appears to be a direct result of the hydrocarbons found in marijuana smoke rather than the cannabis itself, as there is a lack of evidence for enzyme induction when the drug is orally ingested.6
Amphetamine and methamphetamine appear to be both substrates and competitive inhibitors of CYP2D6.7 Rats administered quinidine (a strong 2D6 inhibitor) had 2-fold elevations in AUC and decreased clearance of amphetamine and its metabolites.8 Amphetamine-related recreational drugs, such as 3,4-methylenedioxy-methamphetamine (MDMA) and 3,4-methylenedioxyamphetamine (MDA), are substrates of CYP2D6 and CYP3A4, while MDMA also undergoes substantial metabolism by CYP1A2.3,7,9
Opioids. Heroin is metabolized to 6‑monoacetylmorphine (6-MAM) and morphine by hCE-1, hCE-2, and pseudocholinesterase, and has minimal impact on CYP enzymes. However, while morphine is primarily metabolized to inactive metabolites by UGT2B7, it does undergo minor metabolism through CYP3A4 and 2C8 pathways, creating potential for drug interactions with medications that inhibit or induce CYP3A4.10
Continue to: An underappreciated risk of illicit substance use
An underappreciated risk of illicit substance use
There is a paucity of evidence regarding the metabolism and pharmacokinetic interactions with illicit substances, and further research is needed. Despite the absence of comprehensive data on the subject, the available information indicates the use of illicit substances may have a significant impact on medications used to treat comorbid conditions. Alternatively, those medications may affect the kinetics of recreationally used substances. The risk of adverse consequences of drug–drug interactions is yet another reason patients should be encouraged to avoid use of substances and seek treatment for substance use disorders. When determining the most appropriate therapy for comorbid conditions for patients who are using illicit substances and are likely to continue to do so, clinicians should take into consideration potential interactions among prescription medications and the specific illicit substances the patient uses.
Related Resources
- Lindsey W, Stewart D, Childress D. Drug interactions between common illicit drugs and prescription therapies. Am J Drug Alcohol Abuse. 2012;38(4):334-343.
- Maurer H, Sauer C, Theobald D. Toxicokinetics of drugs of abuse: current knowledge of the isoenzymes involved in the human metabolism of tetrahydrocannabinol, cocaine, heroin, morphine, and codeine. Ther Drug Monit. 2006;28(3):447-453.
- Dean A. Illicit drugs and drug interactions. Pharmacist. 2006;25(9):684-689.
Drug Brand Names
Carbamazepine • Carbatrol, Tegretol
Clozapine • Clozaril
Donepezil • Aricept
Ketoconazole • Nizoral
Paliperidone palmitate • Invega sustenna
Phenytoin • Dilantin, Phenytek
Quinidine • Cardioquin, Duraquin
Theophylline • Elixophylline, Theochron
Ms. L, age 37, presents to psychiatric emergency services with command auditory hallucinations, ideas of reference, and suicidal ideation.
Ms. L has a 22-year history of schizophrenia. Additionally, she has a history of cocaine use disorder (in remission for 12 years), cannabis use disorder (in remission for 6 months), type 2 diabetes mellitus, and hypertension. Her psychotic symptoms are well controlled on a regimen of
On interview, Ms. L reports smoking cannabis each day for the past month and using $400 worth of cocaine over 2 days. She is experiencing intense guilt over these relapses and is admitted to the inpatient adult psychiatry unit. On admission, Ms. L’s clozapine and norclozapine trough levels (drawn approximately 12 hours after last administration documented by the ACT member) are 300 and 275 ng/mL, respectively. Generally, clozapine levels >350 to 420 ng/mL are considered therapeutic, and a clozapine-to-norclozapine ratio of 2:1 is desirable for maximum efficacy and tolerability. Because Ms. L’s clozapine level is <350 and her ratio is approximately 1:1, her clozapine treatment is subtherapeutic.
Because Ms. L has a history of documented adherence to and benefit from her current medication regimen, no changes are made during her 3-week hospital stay. She notices a gradual reduction in auditory hallucinations, no longer wants to harm herself, and is motivated to regain sobriety.
At the time of discharge, Ms. L’s clozapine and norclozapine trough levels are 550 and 250 ng/mL, respectively, which indicates a more favorable clozapine-to-norclozapine ratio of approximately 2:1 and a clozapine level greater than the recommended minimum threshold of 350 ng/mL. While cocaine ingestion presumably played a role in her acute decompensation, the treatment team determined that Ms. L’s relapse to cannabis use likely contributed to low clozapine levels by induction of cytochrome P450 (CYP) 1A2, and subsequently led to the delayed recovery of symptom control.1
The use of illicit substances is a widespread, growing problem. According to the 2017 National Survey on Drug Use and Health, 11.5% of Americans age ≥12 had used an illicit substance (ie, use of marijuana, cocaine, heroin, hallucinogens, inhalants, or methamphetamine, or misuse of prescription psychotherapeutics) in the past month.2 While illicit substance use is of particular public health interest due to a known increase in mortality and health care spending, there has been little discussion of the impact of illicit drug use on concurrent pharmacologic therapy. Just as prescription medications have pharmacokinetic drug–drug interactions with each other, so do illicit substances, though far less is known about their impact on the treatment of medical conditions.
Pharmacokinetic interactions
Key pharmacokinetic interactions have been reported with cocaine, marijuana, amphetamines, and opioids. The Table1-8 summarizes the metabolism of illicit substances.
Continue to: Cocaine
Cocaine is largely metabolized by serum esterases such as pseudocholinesterase, human carboxylesterase-1 (hCE-1), and human carboxylesterase-2 (hCE-2), to inactive metabolites benzoylecgonine (35% to 45%) and ecgonine (32% to 49%).2 However, a smaller portion (2.6% to 6.2%) undergoes hepatic N-demethylation by CYP3A4 to norcocaine.3 Norcocaine is an active metabolite responsible for some of the toxic effects of cocaine (eg, hepatotoxicity).4,5 Several commonly prescribed medications are known inducers of CYP3A4 (eg, phenytoin, carbamazepine) and may lead to increased levels of the toxic metabolite when used concurrently with cocaine. Additionally, the use of cocaine with acetylcholinesterase inhibitors, such as donepezil, may lead to reduction of serum esterases and shunt cocaine metabolism toward the hepatic pathway, thus increasing norcocaine formation.3
Cannabis. The metabolism and drug–drug interactions of cannabis can be separated by its 2 main components: delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD). A review conducted in 2014 concluded that THC is primarily metabolized by CYP2C9 and 3A4, while CBD is metabolized by CYP2C19 and 3A4.6 Oral administration of ketoconazole, a CYP3A4 inhibitor, along with cannabis extract has been shown to increase the maximum concentration (Cmax) and area under the curve (AUC) of THC by 1.2- and 1.8-fold, respectively, while increasing both Cmaxand AUC of CBD by 2-fold.6 In addition, CYP2C9 poor metabolizers have been shown to experience significant increases in THC exposure and reductions in metabolite formation, further supporting the role of CYP enzymes in cannabis metabolism.6
There is also evidence of enzyme induction by cannabis. Individuals who reported smoking marijuana experienced greater clearance of theophylline, a substrate of CYP1A2, than did those who reported not smoking marijuana.1,6 As with cigarette smoking, this effect appears to be a direct result of the hydrocarbons found in marijuana smoke rather than the cannabis itself, as there is a lack of evidence for enzyme induction when the drug is orally ingested.6
Amphetamine and methamphetamine appear to be both substrates and competitive inhibitors of CYP2D6.7 Rats administered quinidine (a strong 2D6 inhibitor) had 2-fold elevations in AUC and decreased clearance of amphetamine and its metabolites.8 Amphetamine-related recreational drugs, such as 3,4-methylenedioxy-methamphetamine (MDMA) and 3,4-methylenedioxyamphetamine (MDA), are substrates of CYP2D6 and CYP3A4, while MDMA also undergoes substantial metabolism by CYP1A2.3,7,9
Opioids. Heroin is metabolized to 6‑monoacetylmorphine (6-MAM) and morphine by hCE-1, hCE-2, and pseudocholinesterase, and has minimal impact on CYP enzymes. However, while morphine is primarily metabolized to inactive metabolites by UGT2B7, it does undergo minor metabolism through CYP3A4 and 2C8 pathways, creating potential for drug interactions with medications that inhibit or induce CYP3A4.10
Continue to: An underappreciated risk of illicit substance use
An underappreciated risk of illicit substance use
There is a paucity of evidence regarding the metabolism and pharmacokinetic interactions with illicit substances, and further research is needed. Despite the absence of comprehensive data on the subject, the available information indicates the use of illicit substances may have a significant impact on medications used to treat comorbid conditions. Alternatively, those medications may affect the kinetics of recreationally used substances. The risk of adverse consequences of drug–drug interactions is yet another reason patients should be encouraged to avoid use of substances and seek treatment for substance use disorders. When determining the most appropriate therapy for comorbid conditions for patients who are using illicit substances and are likely to continue to do so, clinicians should take into consideration potential interactions among prescription medications and the specific illicit substances the patient uses.
Related Resources
- Lindsey W, Stewart D, Childress D. Drug interactions between common illicit drugs and prescription therapies. Am J Drug Alcohol Abuse. 2012;38(4):334-343.
- Maurer H, Sauer C, Theobald D. Toxicokinetics of drugs of abuse: current knowledge of the isoenzymes involved in the human metabolism of tetrahydrocannabinol, cocaine, heroin, morphine, and codeine. Ther Drug Monit. 2006;28(3):447-453.
- Dean A. Illicit drugs and drug interactions. Pharmacist. 2006;25(9):684-689.
Drug Brand Names
Carbamazepine • Carbatrol, Tegretol
Clozapine • Clozaril
Donepezil • Aricept
Ketoconazole • Nizoral
Paliperidone palmitate • Invega sustenna
Phenytoin • Dilantin, Phenytek
Quinidine • Cardioquin, Duraquin
Theophylline • Elixophylline, Theochron
1. Jusko WJ, Schentag JJ, Clark JH, et al. Enhanced biotransformation of theophylline in marihuana and tobacco smokers. Clin Pharmacol Ther. 1978;24(4):405-410.
2. Substance Abuse and Mental Health Services Administration. Results from the 2017 National Survey on Drug Use and Health: Detailed Tables. https://www.samhsa.gov/data/sites/default/files/cbhsq-reports/NSDUHDetailedTabs2017/NSDUHDetailedTabs2017.htm#tab1-1A. Published 2019. Accessed February 7, 2020.
3. Quinn D, Wodak A, Day R. Pharmacokinetic and pharmacodynamic principles of illicit drug use and treatment of illicit drug users. Clin Pharmacokinet. 1997;33(5):344-400.
4. Ndikum-Moffor FM, Schoeb TR, Roberts SM. Liver toxicity from norcocaine nitroxide, an N-oxidative metabolite of cocaine. J Pharmacol Exp Ther. 1998;284(1):413-419.
5. Pellinen P, Honkakoski P, Stenbäck F, et al. Cocaine N-demethylation and the metabolism-related hepatotoxicity can be prevented by cytochrome P450 3A inhibitors. Eur J Pharmacol. 1994;270(1):35-43.
6. Stout S, Cimino N. Exogenous cannabinoids as substrates, inhibitors, and inducers of human drug metabolizing enzymes: a systematic review. Drug Metab Rev. 2013;46(1):86-95.
7. Kraemer T, Maurer H. Toxicokinetics of amphetamines: metabolism and toxicokinetic data of designer drugs, amphetamine, methamphetamine, and their N-alkyl derivatives. Ther Drug Monit. 2002;24(2):277-289.
8. Markowitz J, Patrick K. Pharmacokinetic and pharmacodynamic drug interactions in the treatment of attention-deficit hyperactivity disorder. Clin Pharmacokinet. 2001;40(10):753-772.
9. Kreth K, Kovar K, Schwab M, et al. Identification of the human cytochromes P450 involved in the oxidative metabolism of “ecstasy”-related designer drugs. Biochem Pharmacol. 2000;59(12):1563-1571.
10. Meyer M, Maurer H. Absorption, distribution, metabolism and excretion pharmacogenomics of drugs of abuse. Pharmacogenomics. 2011;12(2):215-233.
1. Jusko WJ, Schentag JJ, Clark JH, et al. Enhanced biotransformation of theophylline in marihuana and tobacco smokers. Clin Pharmacol Ther. 1978;24(4):405-410.
2. Substance Abuse and Mental Health Services Administration. Results from the 2017 National Survey on Drug Use and Health: Detailed Tables. https://www.samhsa.gov/data/sites/default/files/cbhsq-reports/NSDUHDetailedTabs2017/NSDUHDetailedTabs2017.htm#tab1-1A. Published 2019. Accessed February 7, 2020.
3. Quinn D, Wodak A, Day R. Pharmacokinetic and pharmacodynamic principles of illicit drug use and treatment of illicit drug users. Clin Pharmacokinet. 1997;33(5):344-400.
4. Ndikum-Moffor FM, Schoeb TR, Roberts SM. Liver toxicity from norcocaine nitroxide, an N-oxidative metabolite of cocaine. J Pharmacol Exp Ther. 1998;284(1):413-419.
5. Pellinen P, Honkakoski P, Stenbäck F, et al. Cocaine N-demethylation and the metabolism-related hepatotoxicity can be prevented by cytochrome P450 3A inhibitors. Eur J Pharmacol. 1994;270(1):35-43.
6. Stout S, Cimino N. Exogenous cannabinoids as substrates, inhibitors, and inducers of human drug metabolizing enzymes: a systematic review. Drug Metab Rev. 2013;46(1):86-95.
7. Kraemer T, Maurer H. Toxicokinetics of amphetamines: metabolism and toxicokinetic data of designer drugs, amphetamine, methamphetamine, and their N-alkyl derivatives. Ther Drug Monit. 2002;24(2):277-289.
8. Markowitz J, Patrick K. Pharmacokinetic and pharmacodynamic drug interactions in the treatment of attention-deficit hyperactivity disorder. Clin Pharmacokinet. 2001;40(10):753-772.
9. Kreth K, Kovar K, Schwab M, et al. Identification of the human cytochromes P450 involved in the oxidative metabolism of “ecstasy”-related designer drugs. Biochem Pharmacol. 2000;59(12):1563-1571.
10. Meyer M, Maurer H. Absorption, distribution, metabolism and excretion pharmacogenomics of drugs of abuse. Pharmacogenomics. 2011;12(2):215-233.