User login
Contraception and family planning have improved the health of all people by reducing maternal mortality, improving maternal and child health through birth spacing, supporting full education attainment, and advancing workforce participation.1 Contraception is cost-effective and should be supported by all health insurers. One economic study reported that depending on the contraceptive method utilized, up to $7 of health care costs were saved for each dollar spent on contraceptive services and supplies.2
Progestin-only pills (POPs) are an important contraceptive option for people in the following situations who3:
- have a contraindication to estrogen-containing contraceptives
- are actively breastfeeding
- are less than 21 days since birth
- have a preference to avoid estrogen.
POPs are contraindicated for women who have breast cancer, abnormal uterine bleeding, or active liver disease and for women who are pregnant. A history of bariatric surgery with a malabsorption procedure (Roux-en-Y and biliopancreatic diversion) and the use of antiepileptic medications that are strong enzyme inducers are additional situations where the risk of POP may outweigh the benefit.3 Alternative progestin-only options include the subdermal etonogestrel implant, depot medroxyprogesterone acetate, and levonorgestrel-releasing intrauterine devices. These 3 options provide superior contraceptive efficacy to POP.
As a contraceptive, norethindrone at a dose of 0.35 mg daily has two major flaws:
- it does not reliably inhibit ovulation
- it has a short half-life.
In clinical studies, norethindrone inhibits ovulation in approximately 50% of cycles.4,5 Because norethindrone at a dose of 0.35 mg does not reliably inhibit ovulation it relies on additional mechanisms for contraceptive efficacy, including thickening of the cervical mucus to block sperm entry into the upper reproductive tract, reduced fallopian tube motility, and thinning of the endometrium.6
Norethindrone POP is formulated in packs of 28 pills containing 0.35 mg intended for daily continuous administration and no medication-free intervals. One rationale for the low dose of 0.35 mg in norethindrone POP is that it approximates the lowest dose with contraceptive efficacy for breastfeeding women, which has the benefit of minimizing exposure of the baby to the medication. Estrogen-progestin birth control pills containing norethindrone as the progestin reliably inhibit ovulation and have a minimum of 1 mg of norethindrone in each hormone pill. A POP with 1 mg of norethindrone per pill would likely have greater contraceptive efficacy. When taken daily, norethindrone acetate 5 mg (Aygestin) suppresses ovarian estrogen production, ovulation, and often causes cessation of uterine bleeding.7 The short half-life of norethindrone (7.7 hours) further exacerbates the problem of an insufficient daily dose.6 The standard guidance is that norethindrone must be taken at the same time every day, a goal that is nearly impossible to achieve. If a dose of norethindrone is taken >3 hours late, backup contraception is recommended for 48 hours.6
Drospirenone is a chemical analogue of spironolactone. Drospirenone is a progestin that suppresses LH and FSH and has anti-androgenic and partial anti-mineralocorticoid effects.8 Drospirenone POP contains 4 mg of a nonmicronized formulation that is believed to provide a pharmacologically similar area under the curve in drug metabolism studies to the 3 mg of micronized drospirenone, present in drospirenone-containing estrogen-progestin contraceptives.8 It is provided in a pack of 28 pills with 24 drospirenone pills and 4 pills without hormone. Drospirenone has a long half-life of 30 to 34 hours.8 If ≥2 drospirenone pills are missed, backup contraception is recommended for 7 days.9 The contraceptive effectiveness of drospirenone POP is thought to be similar to estrogen-progestin pills.8 Theoretically, drospirenone, acting as an anti-mineralocorticoid, can cause hyperkalemia. People with renal and adrenal insufficiency are most vulnerable to this adverse effect and should not be prescribed drospirenone. Women taking drospirenone and a medication that strongly inhibits CYP3A4, an enzyme involved in drospirenone degradation—including ketoconazole, indinavir, boceprevir, and clarithromycin—may have increased circulating levels of drospirenone and be at an increased risk of hyperkalemia. The US Food and Drug Administration (FDA) suggests that clinicians consider monitoring potassium concentration in women taking drospirenone who are also prescribed a strong CYP3A4 inhibitor.9 In people with normal renal and adrenal function, drospirenone-induced hyperkalemia is not commonly observed.9
Drospirenone 4 mg has been reported to not affect the natural balance of pro- and anti-coagulation factors in women.10 Drospirenone 4 mg daily has been reported to cause a modest decrease in systolic (-8 mm Hg) and diastolic (-5 mm Hg) blood pressure for women with a baseline blood pressure ≥130 mm Hg. Drospirenone 4 mg daily did not change blood pressure measurement in women with a baseline systolic blood pressure <130 mm Hg.11 For women using drospirenone POP, circulating estradiol concentration is usually >30 pg/mL, with a mean concentration of 51 pg/mL.12,13 Drospirenone POP does not result in a significant change in body weight.14 Preliminary studies suggest that drospirenone is an effective contraceptive in women with a BMI >30 kg/m2.14,15 Drospirenone enters breast milk and the relative infant dose is reported to be 1.5%.9 In general, breastfeeding is considered reasonably safe when the relative infant dose of a medication is <10%.16
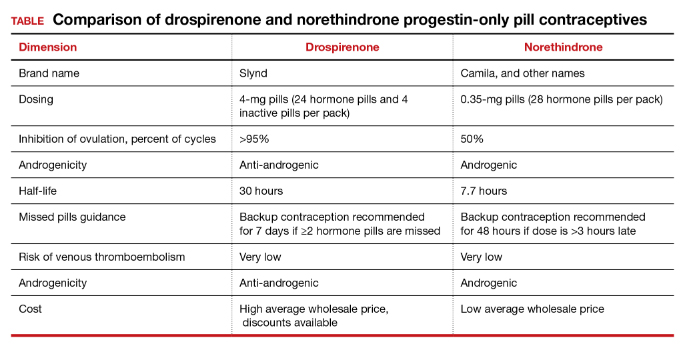
The most common adverse effect reported with both norethindrone and drospirenone POP is unscheduled uterine bleeding. With norethindrone POP about 50% of users have a relatively preserved monthly bleeding pattern and approximately 50% have bleeding between periods, spotting and/or prolonged bleeding.17,18 A similar frequency of unscheduled uterine bleeding has been reported with drospirenone POP.14,19 Unscheduled and bothersome uterine bleeding is a common reason people discontinue POP. For drospirenone POP, the FDA reports a Pearl Index of 4.9 Other studies report a Pearl Index of 0.73 (95% confidence interval [CI], 0.31 to 1.43) for drospirenone POP.14 For norethindrone POP, the FDA reports that in typical use about 5% of people using the contraceptive method would become pregnant.6 The TABLE provides a comparison of the key features of the two available POP contraceptives. My assessment is that drospirenone has superior contraceptive properties over norethindrone POP. However, a head-to-head clinical trial would be necessary to determine the relative contraceptive effectiveness of drospirenone versus norethindrone POP.
Maintaining contraception access
Access to contraception without a copayment is an important component of a comprehensive and equitable insurance program.20 The American College of Obstetricians and Gynecologists (ACOG) advocates that all people “should have unhindered and affordable access to all U.S. Food and Drug Administration-approved contraceptives.”21 ACOG also calls for the “full implementation of the Affordable Care Act requirement that new and revised private health insurance plans cover all U.S. Food and Drug Administration approved contraceptives without cost sharing, including nonequivalent options within one method category.” The National Women’s Law Center22 provides helpful resources to ensure access to legislated contraceptive benefits, including a phone script for speaking with an insurance benefits agent23 and a toolkit for advocating for your contraceptive choice.24 We need to ensure that people have unfettered access to all FDA-approved contraceptives because access to contraception is an important component of public health. Although drospirenone is more costly than norethindrone POP, drospirenone contraception should be available to all patients seeking POP contraception. ●
- Kavanaugh ML, Andreson RM. Contraception and beyond: the health benefits of services provided at family planning centers, NY. Guttmacher Institute. 2013. www.gutmacher.org/pubs/helth-benefits.pdf. Accessed January 13, 2022.
- Foster DG, Rostovtseva DP, Brindis CD, et al. Cost savings from the provision of specific methods of contraception in a publicly funded program. Am J Pub Health. 2009;99:446-451.
- Curtis M, Tepper NK, Jatlaoui TC, et al. U.S. Medical eligibility criteria for contraceptive use, 2016. MMWR Recomm Rep. 2016;65:1-103.
- Rice CF, Killick SR, Dieben T, et al. A comparison of the inhibition of ovulation achieved by desogestrel 75 µg and levonorgestrel 30 µg daily. Human Reprod. 1999;14:982-985.
- Milsom I, Korver T. Ovulation incidence with oral contraceptives: a literature review. J Fam Plann Reprod Health Care. 2008;34:237-246.
- OrthoMicronor [package insert]. OrthoMcNeil: Raritan, New Jersey. June 2008.
- Brown JB, Fotherby K, Loraine JA. The effect of norethisterone and its acetate on ovarian and pituitary function during the menstrual cycle. J Endocrinol. 1962;25:331-341.
- Romer T, Bitzer J, Egarter C, et al. Oral progestins in hormonal contraception: importance and future perspectives of a new progestin only-pill containing 4 mg drospirenone. Geburtsch Frauenheilk. 2021;81:1021-1030.
- Slynd [package insert]. Exeltis: Florham Park, New Jersey. May 2019.
- Regidor PA, Colli E, Schindlre AE. Drospirenone as estrogen-free pill and hemostasis: coagulatory study results comparing a novel 4 mg formulation in a 24+4 cycle with desogestrel 75 µg per day. Gynecol Endocrinol. 2016;32:749-751.
- Palacios S, Colli E, Regidor PA. Efficacy and cardiovascular safety of the new estrogen-free contraceptive pill containing 4 mg drospirenone alone in a 24/4 regime. BMC Womens Health. 2020;20:218.
- Hadji P, Colli E, Regidor PA. Bone health in estrogen-free contraception. Osteoporosis Int. 2019;30:2391-2400.
- Mitchell VE, Welling LM. Not all progestins are created equally: considering unique progestins individually in psychobehavioral research. Adapt Human Behav Physiol. 2020;6:381-412.
- Palacios S, Colli E, Regidor PA. Multicenter, phase III trials on the contraceptive efficacy, tolerability and safety of a new drospirenone-only pill. Acta Obstet Gynecol Scand. 2019;98:1549-1557.
- Archer DF, Ahrendt HJ, Drouin D. Drospirenone-only oral contraceptive: results from a multicenter noncomparative trial of efficacy, safety and tolerability. Contraception. 2015;92:439-444.
- Anderson PO, Sauberan JB. Modeling drug passage into human milk. Clin Pharmacol Ther. 2016;100:42-52. doi: 10.1002/cpt.377.
- Belsey EM. Vaginal bleeding patterns among women using one natural and eight hormonal methods of contraception. Contraception. 1988;38:181-206.
- Broome M, Fotherby K. Clinical experience with the progestin-only pill. Contraception. 1990;42:489-495.
- Apter D, Colli E, Gemzell-Danielsson K, et al. Multicenter, open-label trial to assess the safety and tolerability of drospirenone 4.0 mg over 6 cycles in female adolescents with a 7-cycle extension phase. Contraception. 2020;101:412.
- Birth control benefits. Healthcare.gov website. https://www.healthcare.gov/coverage/birth-control-benefits/. Accessed January 13, 2022.
- American College of Obstetricians and Gynecologists. Access to contraception. Committee Opinion No. 615. Obstet Gynecol. 2015;125:250-256.
- Health care and reproductive rights. National Women’s Law Center website. https://nwlc.org/issue/health-care. Accessed January 13, 2022.
- How to find out if your health plan covers birth control at no cost to you. National Women’s Law Center website. https://nwlc.org/sites/default/files/072014-insuranceflowchart_vupdated.pdf. Accessed January 13, 2022.
- Toolkit: Getting the coverage you deserve. National Women’s Law Center website. https://nwlc.org/sites/default/files/pdfs/final_nwlclogo_preventive servicestoolkit_9-25-13.pdf. Accessed January 13, 2022.
Contraception and family planning have improved the health of all people by reducing maternal mortality, improving maternal and child health through birth spacing, supporting full education attainment, and advancing workforce participation.1 Contraception is cost-effective and should be supported by all health insurers. One economic study reported that depending on the contraceptive method utilized, up to $7 of health care costs were saved for each dollar spent on contraceptive services and supplies.2
Progestin-only pills (POPs) are an important contraceptive option for people in the following situations who3:
- have a contraindication to estrogen-containing contraceptives
- are actively breastfeeding
- are less than 21 days since birth
- have a preference to avoid estrogen.
POPs are contraindicated for women who have breast cancer, abnormal uterine bleeding, or active liver disease and for women who are pregnant. A history of bariatric surgery with a malabsorption procedure (Roux-en-Y and biliopancreatic diversion) and the use of antiepileptic medications that are strong enzyme inducers are additional situations where the risk of POP may outweigh the benefit.3 Alternative progestin-only options include the subdermal etonogestrel implant, depot medroxyprogesterone acetate, and levonorgestrel-releasing intrauterine devices. These 3 options provide superior contraceptive efficacy to POP.
As a contraceptive, norethindrone at a dose of 0.35 mg daily has two major flaws:
- it does not reliably inhibit ovulation
- it has a short half-life.
In clinical studies, norethindrone inhibits ovulation in approximately 50% of cycles.4,5 Because norethindrone at a dose of 0.35 mg does not reliably inhibit ovulation it relies on additional mechanisms for contraceptive efficacy, including thickening of the cervical mucus to block sperm entry into the upper reproductive tract, reduced fallopian tube motility, and thinning of the endometrium.6
Norethindrone POP is formulated in packs of 28 pills containing 0.35 mg intended for daily continuous administration and no medication-free intervals. One rationale for the low dose of 0.35 mg in norethindrone POP is that it approximates the lowest dose with contraceptive efficacy for breastfeeding women, which has the benefit of minimizing exposure of the baby to the medication. Estrogen-progestin birth control pills containing norethindrone as the progestin reliably inhibit ovulation and have a minimum of 1 mg of norethindrone in each hormone pill. A POP with 1 mg of norethindrone per pill would likely have greater contraceptive efficacy. When taken daily, norethindrone acetate 5 mg (Aygestin) suppresses ovarian estrogen production, ovulation, and often causes cessation of uterine bleeding.7 The short half-life of norethindrone (7.7 hours) further exacerbates the problem of an insufficient daily dose.6 The standard guidance is that norethindrone must be taken at the same time every day, a goal that is nearly impossible to achieve. If a dose of norethindrone is taken >3 hours late, backup contraception is recommended for 48 hours.6
Drospirenone is a chemical analogue of spironolactone. Drospirenone is a progestin that suppresses LH and FSH and has anti-androgenic and partial anti-mineralocorticoid effects.8 Drospirenone POP contains 4 mg of a nonmicronized formulation that is believed to provide a pharmacologically similar area under the curve in drug metabolism studies to the 3 mg of micronized drospirenone, present in drospirenone-containing estrogen-progestin contraceptives.8 It is provided in a pack of 28 pills with 24 drospirenone pills and 4 pills without hormone. Drospirenone has a long half-life of 30 to 34 hours.8 If ≥2 drospirenone pills are missed, backup contraception is recommended for 7 days.9 The contraceptive effectiveness of drospirenone POP is thought to be similar to estrogen-progestin pills.8 Theoretically, drospirenone, acting as an anti-mineralocorticoid, can cause hyperkalemia. People with renal and adrenal insufficiency are most vulnerable to this adverse effect and should not be prescribed drospirenone. Women taking drospirenone and a medication that strongly inhibits CYP3A4, an enzyme involved in drospirenone degradation—including ketoconazole, indinavir, boceprevir, and clarithromycin—may have increased circulating levels of drospirenone and be at an increased risk of hyperkalemia. The US Food and Drug Administration (FDA) suggests that clinicians consider monitoring potassium concentration in women taking drospirenone who are also prescribed a strong CYP3A4 inhibitor.9 In people with normal renal and adrenal function, drospirenone-induced hyperkalemia is not commonly observed.9
Drospirenone 4 mg has been reported to not affect the natural balance of pro- and anti-coagulation factors in women.10 Drospirenone 4 mg daily has been reported to cause a modest decrease in systolic (-8 mm Hg) and diastolic (-5 mm Hg) blood pressure for women with a baseline blood pressure ≥130 mm Hg. Drospirenone 4 mg daily did not change blood pressure measurement in women with a baseline systolic blood pressure <130 mm Hg.11 For women using drospirenone POP, circulating estradiol concentration is usually >30 pg/mL, with a mean concentration of 51 pg/mL.12,13 Drospirenone POP does not result in a significant change in body weight.14 Preliminary studies suggest that drospirenone is an effective contraceptive in women with a BMI >30 kg/m2.14,15 Drospirenone enters breast milk and the relative infant dose is reported to be 1.5%.9 In general, breastfeeding is considered reasonably safe when the relative infant dose of a medication is <10%.16
The most common adverse effect reported with both norethindrone and drospirenone POP is unscheduled uterine bleeding. With norethindrone POP about 50% of users have a relatively preserved monthly bleeding pattern and approximately 50% have bleeding between periods, spotting and/or prolonged bleeding.17,18 A similar frequency of unscheduled uterine bleeding has been reported with drospirenone POP.14,19 Unscheduled and bothersome uterine bleeding is a common reason people discontinue POP. For drospirenone POP, the FDA reports a Pearl Index of 4.9 Other studies report a Pearl Index of 0.73 (95% confidence interval [CI], 0.31 to 1.43) for drospirenone POP.14 For norethindrone POP, the FDA reports that in typical use about 5% of people using the contraceptive method would become pregnant.6 The TABLE provides a comparison of the key features of the two available POP contraceptives. My assessment is that drospirenone has superior contraceptive properties over norethindrone POP. However, a head-to-head clinical trial would be necessary to determine the relative contraceptive effectiveness of drospirenone versus norethindrone POP.

Maintaining contraception access
Access to contraception without a copayment is an important component of a comprehensive and equitable insurance program.20 The American College of Obstetricians and Gynecologists (ACOG) advocates that all people “should have unhindered and affordable access to all U.S. Food and Drug Administration-approved contraceptives.”21 ACOG also calls for the “full implementation of the Affordable Care Act requirement that new and revised private health insurance plans cover all U.S. Food and Drug Administration approved contraceptives without cost sharing, including nonequivalent options within one method category.” The National Women’s Law Center22 provides helpful resources to ensure access to legislated contraceptive benefits, including a phone script for speaking with an insurance benefits agent23 and a toolkit for advocating for your contraceptive choice.24 We need to ensure that people have unfettered access to all FDA-approved contraceptives because access to contraception is an important component of public health. Although drospirenone is more costly than norethindrone POP, drospirenone contraception should be available to all patients seeking POP contraception. ●
Contraception and family planning have improved the health of all people by reducing maternal mortality, improving maternal and child health through birth spacing, supporting full education attainment, and advancing workforce participation.1 Contraception is cost-effective and should be supported by all health insurers. One economic study reported that depending on the contraceptive method utilized, up to $7 of health care costs were saved for each dollar spent on contraceptive services and supplies.2
Progestin-only pills (POPs) are an important contraceptive option for people in the following situations who3:
- have a contraindication to estrogen-containing contraceptives
- are actively breastfeeding
- are less than 21 days since birth
- have a preference to avoid estrogen.
POPs are contraindicated for women who have breast cancer, abnormal uterine bleeding, or active liver disease and for women who are pregnant. A history of bariatric surgery with a malabsorption procedure (Roux-en-Y and biliopancreatic diversion) and the use of antiepileptic medications that are strong enzyme inducers are additional situations where the risk of POP may outweigh the benefit.3 Alternative progestin-only options include the subdermal etonogestrel implant, depot medroxyprogesterone acetate, and levonorgestrel-releasing intrauterine devices. These 3 options provide superior contraceptive efficacy to POP.
As a contraceptive, norethindrone at a dose of 0.35 mg daily has two major flaws:
- it does not reliably inhibit ovulation
- it has a short half-life.
In clinical studies, norethindrone inhibits ovulation in approximately 50% of cycles.4,5 Because norethindrone at a dose of 0.35 mg does not reliably inhibit ovulation it relies on additional mechanisms for contraceptive efficacy, including thickening of the cervical mucus to block sperm entry into the upper reproductive tract, reduced fallopian tube motility, and thinning of the endometrium.6
Norethindrone POP is formulated in packs of 28 pills containing 0.35 mg intended for daily continuous administration and no medication-free intervals. One rationale for the low dose of 0.35 mg in norethindrone POP is that it approximates the lowest dose with contraceptive efficacy for breastfeeding women, which has the benefit of minimizing exposure of the baby to the medication. Estrogen-progestin birth control pills containing norethindrone as the progestin reliably inhibit ovulation and have a minimum of 1 mg of norethindrone in each hormone pill. A POP with 1 mg of norethindrone per pill would likely have greater contraceptive efficacy. When taken daily, norethindrone acetate 5 mg (Aygestin) suppresses ovarian estrogen production, ovulation, and often causes cessation of uterine bleeding.7 The short half-life of norethindrone (7.7 hours) further exacerbates the problem of an insufficient daily dose.6 The standard guidance is that norethindrone must be taken at the same time every day, a goal that is nearly impossible to achieve. If a dose of norethindrone is taken >3 hours late, backup contraception is recommended for 48 hours.6
Drospirenone is a chemical analogue of spironolactone. Drospirenone is a progestin that suppresses LH and FSH and has anti-androgenic and partial anti-mineralocorticoid effects.8 Drospirenone POP contains 4 mg of a nonmicronized formulation that is believed to provide a pharmacologically similar area under the curve in drug metabolism studies to the 3 mg of micronized drospirenone, present in drospirenone-containing estrogen-progestin contraceptives.8 It is provided in a pack of 28 pills with 24 drospirenone pills and 4 pills without hormone. Drospirenone has a long half-life of 30 to 34 hours.8 If ≥2 drospirenone pills are missed, backup contraception is recommended for 7 days.9 The contraceptive effectiveness of drospirenone POP is thought to be similar to estrogen-progestin pills.8 Theoretically, drospirenone, acting as an anti-mineralocorticoid, can cause hyperkalemia. People with renal and adrenal insufficiency are most vulnerable to this adverse effect and should not be prescribed drospirenone. Women taking drospirenone and a medication that strongly inhibits CYP3A4, an enzyme involved in drospirenone degradation—including ketoconazole, indinavir, boceprevir, and clarithromycin—may have increased circulating levels of drospirenone and be at an increased risk of hyperkalemia. The US Food and Drug Administration (FDA) suggests that clinicians consider monitoring potassium concentration in women taking drospirenone who are also prescribed a strong CYP3A4 inhibitor.9 In people with normal renal and adrenal function, drospirenone-induced hyperkalemia is not commonly observed.9
Drospirenone 4 mg has been reported to not affect the natural balance of pro- and anti-coagulation factors in women.10 Drospirenone 4 mg daily has been reported to cause a modest decrease in systolic (-8 mm Hg) and diastolic (-5 mm Hg) blood pressure for women with a baseline blood pressure ≥130 mm Hg. Drospirenone 4 mg daily did not change blood pressure measurement in women with a baseline systolic blood pressure <130 mm Hg.11 For women using drospirenone POP, circulating estradiol concentration is usually >30 pg/mL, with a mean concentration of 51 pg/mL.12,13 Drospirenone POP does not result in a significant change in body weight.14 Preliminary studies suggest that drospirenone is an effective contraceptive in women with a BMI >30 kg/m2.14,15 Drospirenone enters breast milk and the relative infant dose is reported to be 1.5%.9 In general, breastfeeding is considered reasonably safe when the relative infant dose of a medication is <10%.16
The most common adverse effect reported with both norethindrone and drospirenone POP is unscheduled uterine bleeding. With norethindrone POP about 50% of users have a relatively preserved monthly bleeding pattern and approximately 50% have bleeding between periods, spotting and/or prolonged bleeding.17,18 A similar frequency of unscheduled uterine bleeding has been reported with drospirenone POP.14,19 Unscheduled and bothersome uterine bleeding is a common reason people discontinue POP. For drospirenone POP, the FDA reports a Pearl Index of 4.9 Other studies report a Pearl Index of 0.73 (95% confidence interval [CI], 0.31 to 1.43) for drospirenone POP.14 For norethindrone POP, the FDA reports that in typical use about 5% of people using the contraceptive method would become pregnant.6 The TABLE provides a comparison of the key features of the two available POP contraceptives. My assessment is that drospirenone has superior contraceptive properties over norethindrone POP. However, a head-to-head clinical trial would be necessary to determine the relative contraceptive effectiveness of drospirenone versus norethindrone POP.

Maintaining contraception access
Access to contraception without a copayment is an important component of a comprehensive and equitable insurance program.20 The American College of Obstetricians and Gynecologists (ACOG) advocates that all people “should have unhindered and affordable access to all U.S. Food and Drug Administration-approved contraceptives.”21 ACOG also calls for the “full implementation of the Affordable Care Act requirement that new and revised private health insurance plans cover all U.S. Food and Drug Administration approved contraceptives without cost sharing, including nonequivalent options within one method category.” The National Women’s Law Center22 provides helpful resources to ensure access to legislated contraceptive benefits, including a phone script for speaking with an insurance benefits agent23 and a toolkit for advocating for your contraceptive choice.24 We need to ensure that people have unfettered access to all FDA-approved contraceptives because access to contraception is an important component of public health. Although drospirenone is more costly than norethindrone POP, drospirenone contraception should be available to all patients seeking POP contraception. ●
- Kavanaugh ML, Andreson RM. Contraception and beyond: the health benefits of services provided at family planning centers, NY. Guttmacher Institute. 2013. www.gutmacher.org/pubs/helth-benefits.pdf. Accessed January 13, 2022.
- Foster DG, Rostovtseva DP, Brindis CD, et al. Cost savings from the provision of specific methods of contraception in a publicly funded program. Am J Pub Health. 2009;99:446-451.
- Curtis M, Tepper NK, Jatlaoui TC, et al. U.S. Medical eligibility criteria for contraceptive use, 2016. MMWR Recomm Rep. 2016;65:1-103.
- Rice CF, Killick SR, Dieben T, et al. A comparison of the inhibition of ovulation achieved by desogestrel 75 µg and levonorgestrel 30 µg daily. Human Reprod. 1999;14:982-985.
- Milsom I, Korver T. Ovulation incidence with oral contraceptives: a literature review. J Fam Plann Reprod Health Care. 2008;34:237-246.
- OrthoMicronor [package insert]. OrthoMcNeil: Raritan, New Jersey. June 2008.
- Brown JB, Fotherby K, Loraine JA. The effect of norethisterone and its acetate on ovarian and pituitary function during the menstrual cycle. J Endocrinol. 1962;25:331-341.
- Romer T, Bitzer J, Egarter C, et al. Oral progestins in hormonal contraception: importance and future perspectives of a new progestin only-pill containing 4 mg drospirenone. Geburtsch Frauenheilk. 2021;81:1021-1030.
- Slynd [package insert]. Exeltis: Florham Park, New Jersey. May 2019.
- Regidor PA, Colli E, Schindlre AE. Drospirenone as estrogen-free pill and hemostasis: coagulatory study results comparing a novel 4 mg formulation in a 24+4 cycle with desogestrel 75 µg per day. Gynecol Endocrinol. 2016;32:749-751.
- Palacios S, Colli E, Regidor PA. Efficacy and cardiovascular safety of the new estrogen-free contraceptive pill containing 4 mg drospirenone alone in a 24/4 regime. BMC Womens Health. 2020;20:218.
- Hadji P, Colli E, Regidor PA. Bone health in estrogen-free contraception. Osteoporosis Int. 2019;30:2391-2400.
- Mitchell VE, Welling LM. Not all progestins are created equally: considering unique progestins individually in psychobehavioral research. Adapt Human Behav Physiol. 2020;6:381-412.
- Palacios S, Colli E, Regidor PA. Multicenter, phase III trials on the contraceptive efficacy, tolerability and safety of a new drospirenone-only pill. Acta Obstet Gynecol Scand. 2019;98:1549-1557.
- Archer DF, Ahrendt HJ, Drouin D. Drospirenone-only oral contraceptive: results from a multicenter noncomparative trial of efficacy, safety and tolerability. Contraception. 2015;92:439-444.
- Anderson PO, Sauberan JB. Modeling drug passage into human milk. Clin Pharmacol Ther. 2016;100:42-52. doi: 10.1002/cpt.377.
- Belsey EM. Vaginal bleeding patterns among women using one natural and eight hormonal methods of contraception. Contraception. 1988;38:181-206.
- Broome M, Fotherby K. Clinical experience with the progestin-only pill. Contraception. 1990;42:489-495.
- Apter D, Colli E, Gemzell-Danielsson K, et al. Multicenter, open-label trial to assess the safety and tolerability of drospirenone 4.0 mg over 6 cycles in female adolescents with a 7-cycle extension phase. Contraception. 2020;101:412.
- Birth control benefits. Healthcare.gov website. https://www.healthcare.gov/coverage/birth-control-benefits/. Accessed January 13, 2022.
- American College of Obstetricians and Gynecologists. Access to contraception. Committee Opinion No. 615. Obstet Gynecol. 2015;125:250-256.
- Health care and reproductive rights. National Women’s Law Center website. https://nwlc.org/issue/health-care. Accessed January 13, 2022.
- How to find out if your health plan covers birth control at no cost to you. National Women’s Law Center website. https://nwlc.org/sites/default/files/072014-insuranceflowchart_vupdated.pdf. Accessed January 13, 2022.
- Toolkit: Getting the coverage you deserve. National Women’s Law Center website. https://nwlc.org/sites/default/files/pdfs/final_nwlclogo_preventive servicestoolkit_9-25-13.pdf. Accessed January 13, 2022.
- Kavanaugh ML, Andreson RM. Contraception and beyond: the health benefits of services provided at family planning centers, NY. Guttmacher Institute. 2013. www.gutmacher.org/pubs/helth-benefits.pdf. Accessed January 13, 2022.
- Foster DG, Rostovtseva DP, Brindis CD, et al. Cost savings from the provision of specific methods of contraception in a publicly funded program. Am J Pub Health. 2009;99:446-451.
- Curtis M, Tepper NK, Jatlaoui TC, et al. U.S. Medical eligibility criteria for contraceptive use, 2016. MMWR Recomm Rep. 2016;65:1-103.
- Rice CF, Killick SR, Dieben T, et al. A comparison of the inhibition of ovulation achieved by desogestrel 75 µg and levonorgestrel 30 µg daily. Human Reprod. 1999;14:982-985.
- Milsom I, Korver T. Ovulation incidence with oral contraceptives: a literature review. J Fam Plann Reprod Health Care. 2008;34:237-246.
- OrthoMicronor [package insert]. OrthoMcNeil: Raritan, New Jersey. June 2008.
- Brown JB, Fotherby K, Loraine JA. The effect of norethisterone and its acetate on ovarian and pituitary function during the menstrual cycle. J Endocrinol. 1962;25:331-341.
- Romer T, Bitzer J, Egarter C, et al. Oral progestins in hormonal contraception: importance and future perspectives of a new progestin only-pill containing 4 mg drospirenone. Geburtsch Frauenheilk. 2021;81:1021-1030.
- Slynd [package insert]. Exeltis: Florham Park, New Jersey. May 2019.
- Regidor PA, Colli E, Schindlre AE. Drospirenone as estrogen-free pill and hemostasis: coagulatory study results comparing a novel 4 mg formulation in a 24+4 cycle with desogestrel 75 µg per day. Gynecol Endocrinol. 2016;32:749-751.
- Palacios S, Colli E, Regidor PA. Efficacy and cardiovascular safety of the new estrogen-free contraceptive pill containing 4 mg drospirenone alone in a 24/4 regime. BMC Womens Health. 2020;20:218.
- Hadji P, Colli E, Regidor PA. Bone health in estrogen-free contraception. Osteoporosis Int. 2019;30:2391-2400.
- Mitchell VE, Welling LM. Not all progestins are created equally: considering unique progestins individually in psychobehavioral research. Adapt Human Behav Physiol. 2020;6:381-412.
- Palacios S, Colli E, Regidor PA. Multicenter, phase III trials on the contraceptive efficacy, tolerability and safety of a new drospirenone-only pill. Acta Obstet Gynecol Scand. 2019;98:1549-1557.
- Archer DF, Ahrendt HJ, Drouin D. Drospirenone-only oral contraceptive: results from a multicenter noncomparative trial of efficacy, safety and tolerability. Contraception. 2015;92:439-444.
- Anderson PO, Sauberan JB. Modeling drug passage into human milk. Clin Pharmacol Ther. 2016;100:42-52. doi: 10.1002/cpt.377.
- Belsey EM. Vaginal bleeding patterns among women using one natural and eight hormonal methods of contraception. Contraception. 1988;38:181-206.
- Broome M, Fotherby K. Clinical experience with the progestin-only pill. Contraception. 1990;42:489-495.
- Apter D, Colli E, Gemzell-Danielsson K, et al. Multicenter, open-label trial to assess the safety and tolerability of drospirenone 4.0 mg over 6 cycles in female adolescents with a 7-cycle extension phase. Contraception. 2020;101:412.
- Birth control benefits. Healthcare.gov website. https://www.healthcare.gov/coverage/birth-control-benefits/. Accessed January 13, 2022.
- American College of Obstetricians and Gynecologists. Access to contraception. Committee Opinion No. 615. Obstet Gynecol. 2015;125:250-256.
- Health care and reproductive rights. National Women’s Law Center website. https://nwlc.org/issue/health-care. Accessed January 13, 2022.
- How to find out if your health plan covers birth control at no cost to you. National Women’s Law Center website. https://nwlc.org/sites/default/files/072014-insuranceflowchart_vupdated.pdf. Accessed January 13, 2022.
- Toolkit: Getting the coverage you deserve. National Women’s Law Center website. https://nwlc.org/sites/default/files/pdfs/final_nwlclogo_preventive servicestoolkit_9-25-13.pdf. Accessed January 13, 2022.
