User login
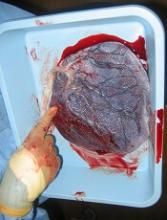
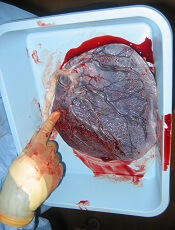
Placenta-derived decidua stromal cells (DSCs) can treat severe acute graft-versus-host disease (GVHD), according to a small study.
When given in the optimal way, DSCs produced GVHD responses in all patients, and the 1-year survival rate was 76%.
Steroid-refractory (SR) patients who received DSCs had superior 1-year survival rates compared to SR historical controls and SR patients who received mesenchymal stem cells (MSCs).
Olle Ringdén, MD, PhD, of Karolinska Institutet in Huddinge, Sweden, and his colleagues reported these findings in STEM CELLS Translational Medicine.
“There were a couple of things that led us to be curious about [DSCs as a treatment for GVHD],” Dr Ringdén said. “First, placenta plays an important role in helping the mother’s body tolerate the developing fetus.”
“[S]econd, placenta has been used in Africa for 100 years to successfully treat burn injuries. This speaks somewhat to its effectiveness and safety. We also found that placenta-derived DSCs are immunosuppressive in vitro and in vivo, which led us to wonder if they might cure severe acute GVHD.”
To test their theory, the team conducted a study of 38 patients with severe acute GVHD, including 25 SR patients.
The patients received DSCs in 1 of 2 groups. In group 1 (n=17), DSCs were infused in buffer supplemented with 10% AB plasma. In group 2 (n=21), the buffer was supplemented with 5% albumin.
Group 1 received significantly fewer infusions than group 2—1 (range, 1-5) and 2 (range, 1-6), respectively (P=0.002). But group 1 had a significantly higher median cell dose—2.0 x 106 DSCs/kg (range, 0.9-2.8) vs 1.2 x 106 DSCs/kg (range, 0.9-2.9; P<0.001).
Cell passage was significantly lower in group 1 than group 2—2 (range, 2-4) and 4 (range, 2-4), respectively (P<0.001). And cell viability was significantly lower in group 1 than 2—90% (range, 70-97) and 95% (range, 69-100), respectively (P<0.001).
Patients
There were no significant differences in baseline characteristics between groups 1 and 2. The median ages were 54.5 (range, 0.9-65.6) and 48.9 (range, 1.6-72.4), respectively.
Most patients were male (9 in group 1 and 16 in group 2), and most had malignant disease (14 and 17, respectively).
The most common graft source was peripheral blood stem cells (14 in group 1 and 16 in group 2), and most patients had a matched, unrelated donor (10 and 14, respectively).
Most patients received reduced-intensity conditioning (9 in group 1 and 17 in group 2) and GVHD prophylaxis with cyclosporine/methotrexate (13 in both groups).
All cases of GVHD were localized to the gut. Fifteen patients in each group had grade 3 GVHD. Two patients in group 1 and 6 in group 2 had grade 2 GVHD.
Results
Responses and survival rates were superior in group 2, but there was no significant difference in relapse or chronic GVHD between the groups.
In group 1, 7 patients did not respond, 5 had a partial response, and 5 had a complete response. In group 2, all patients responded, 10 with a partial response and 11 with a complete response (between-group difference, P=0.01).
The 1-year survival rate was 47% in group 1 and 76% in group 2 (P=0.016). The rate of GVHD-related mortality was 41% and 5%, respectively (P=0.003).
The cumulative incidence of chronic GVHD at 1.5 years was 36% in group 1 and 31% in group 2. The relapse rate was 29% and 18%, respectively.
The researchers compared SR patients in groups 1 (n=13) and 2 (n=11) to SR patients treated with bone marrow-derived MSCs (n=15) and SR historical controls (n=32).
The 1-year survival rate was 73% in SR group 2, which was significantly higher than the 31% survival rate in SR group 1 (P=0.02), the 20% rate in SR MSC recipients (P=0.0015), and the 3% rate in SR historical controls (P<0.001).
Severe adverse events in the DSC recipients included relapse (n=8), pneumonia (n=5), proven or probable invasive fungal infection (n=6), bacterial infection (n=2), graft failure (n=3), multiple organ failure (n=1), viral infection (n=2), central nervous system complications (n=2), septicemia (n=2), skin squamous cell carcinoma (n=2), and acute pancreatitis (n=1).
Causes of death in DSC recipients included acute GVHD (n=9), relapse (n=2), bacterial infection (n=2), invasive fungal infection (n=1), liver failure (n=1), hemorrhage (n=1), and secondary malignancy (n=1).
“Collectively, we think these data demonstrate that DSCs are a promising treatment for severe acute GVHD,” Dr Ringdén said. “But it was a small patient group, so, to further assess safety and efficacy, a larger, prospective trial will be necessary.”
“If an effective therapy for severe acute GVHD is indeed found and validated, it will increase the usefulness of stem cell transplantation with a possible broadening of indications.”
Placenta-derived decidua stromal cells (DSCs) can treat severe acute graft-versus-host disease (GVHD), according to a small study.
When given in the optimal way, DSCs produced GVHD responses in all patients, and the 1-year survival rate was 76%.
Steroid-refractory (SR) patients who received DSCs had superior 1-year survival rates compared to SR historical controls and SR patients who received mesenchymal stem cells (MSCs).
Olle Ringdén, MD, PhD, of Karolinska Institutet in Huddinge, Sweden, and his colleagues reported these findings in STEM CELLS Translational Medicine.
“There were a couple of things that led us to be curious about [DSCs as a treatment for GVHD],” Dr Ringdén said. “First, placenta plays an important role in helping the mother’s body tolerate the developing fetus.”
“[S]econd, placenta has been used in Africa for 100 years to successfully treat burn injuries. This speaks somewhat to its effectiveness and safety. We also found that placenta-derived DSCs are immunosuppressive in vitro and in vivo, which led us to wonder if they might cure severe acute GVHD.”
To test their theory, the team conducted a study of 38 patients with severe acute GVHD, including 25 SR patients.
The patients received DSCs in 1 of 2 groups. In group 1 (n=17), DSCs were infused in buffer supplemented with 10% AB plasma. In group 2 (n=21), the buffer was supplemented with 5% albumin.
Group 1 received significantly fewer infusions than group 2—1 (range, 1-5) and 2 (range, 1-6), respectively (P=0.002). But group 1 had a significantly higher median cell dose—2.0 x 106 DSCs/kg (range, 0.9-2.8) vs 1.2 x 106 DSCs/kg (range, 0.9-2.9; P<0.001).
Cell passage was significantly lower in group 1 than group 2—2 (range, 2-4) and 4 (range, 2-4), respectively (P<0.001). And cell viability was significantly lower in group 1 than 2—90% (range, 70-97) and 95% (range, 69-100), respectively (P<0.001).
Patients
There were no significant differences in baseline characteristics between groups 1 and 2. The median ages were 54.5 (range, 0.9-65.6) and 48.9 (range, 1.6-72.4), respectively.
Most patients were male (9 in group 1 and 16 in group 2), and most had malignant disease (14 and 17, respectively).
The most common graft source was peripheral blood stem cells (14 in group 1 and 16 in group 2), and most patients had a matched, unrelated donor (10 and 14, respectively).
Most patients received reduced-intensity conditioning (9 in group 1 and 17 in group 2) and GVHD prophylaxis with cyclosporine/methotrexate (13 in both groups).
All cases of GVHD were localized to the gut. Fifteen patients in each group had grade 3 GVHD. Two patients in group 1 and 6 in group 2 had grade 2 GVHD.
Results
Responses and survival rates were superior in group 2, but there was no significant difference in relapse or chronic GVHD between the groups.
In group 1, 7 patients did not respond, 5 had a partial response, and 5 had a complete response. In group 2, all patients responded, 10 with a partial response and 11 with a complete response (between-group difference, P=0.01).
The 1-year survival rate was 47% in group 1 and 76% in group 2 (P=0.016). The rate of GVHD-related mortality was 41% and 5%, respectively (P=0.003).
The cumulative incidence of chronic GVHD at 1.5 years was 36% in group 1 and 31% in group 2. The relapse rate was 29% and 18%, respectively.
The researchers compared SR patients in groups 1 (n=13) and 2 (n=11) to SR patients treated with bone marrow-derived MSCs (n=15) and SR historical controls (n=32).
The 1-year survival rate was 73% in SR group 2, which was significantly higher than the 31% survival rate in SR group 1 (P=0.02), the 20% rate in SR MSC recipients (P=0.0015), and the 3% rate in SR historical controls (P<0.001).
Severe adverse events in the DSC recipients included relapse (n=8), pneumonia (n=5), proven or probable invasive fungal infection (n=6), bacterial infection (n=2), graft failure (n=3), multiple organ failure (n=1), viral infection (n=2), central nervous system complications (n=2), septicemia (n=2), skin squamous cell carcinoma (n=2), and acute pancreatitis (n=1).
Causes of death in DSC recipients included acute GVHD (n=9), relapse (n=2), bacterial infection (n=2), invasive fungal infection (n=1), liver failure (n=1), hemorrhage (n=1), and secondary malignancy (n=1).
“Collectively, we think these data demonstrate that DSCs are a promising treatment for severe acute GVHD,” Dr Ringdén said. “But it was a small patient group, so, to further assess safety and efficacy, a larger, prospective trial will be necessary.”
“If an effective therapy for severe acute GVHD is indeed found and validated, it will increase the usefulness of stem cell transplantation with a possible broadening of indications.”
Placenta-derived decidua stromal cells (DSCs) can treat severe acute graft-versus-host disease (GVHD), according to a small study.
When given in the optimal way, DSCs produced GVHD responses in all patients, and the 1-year survival rate was 76%.
Steroid-refractory (SR) patients who received DSCs had superior 1-year survival rates compared to SR historical controls and SR patients who received mesenchymal stem cells (MSCs).
Olle Ringdén, MD, PhD, of Karolinska Institutet in Huddinge, Sweden, and his colleagues reported these findings in STEM CELLS Translational Medicine.
“There were a couple of things that led us to be curious about [DSCs as a treatment for GVHD],” Dr Ringdén said. “First, placenta plays an important role in helping the mother’s body tolerate the developing fetus.”
“[S]econd, placenta has been used in Africa for 100 years to successfully treat burn injuries. This speaks somewhat to its effectiveness and safety. We also found that placenta-derived DSCs are immunosuppressive in vitro and in vivo, which led us to wonder if they might cure severe acute GVHD.”
To test their theory, the team conducted a study of 38 patients with severe acute GVHD, including 25 SR patients.
The patients received DSCs in 1 of 2 groups. In group 1 (n=17), DSCs were infused in buffer supplemented with 10% AB plasma. In group 2 (n=21), the buffer was supplemented with 5% albumin.
Group 1 received significantly fewer infusions than group 2—1 (range, 1-5) and 2 (range, 1-6), respectively (P=0.002). But group 1 had a significantly higher median cell dose—2.0 x 106 DSCs/kg (range, 0.9-2.8) vs 1.2 x 106 DSCs/kg (range, 0.9-2.9; P<0.001).
Cell passage was significantly lower in group 1 than group 2—2 (range, 2-4) and 4 (range, 2-4), respectively (P<0.001). And cell viability was significantly lower in group 1 than 2—90% (range, 70-97) and 95% (range, 69-100), respectively (P<0.001).
Patients
There were no significant differences in baseline characteristics between groups 1 and 2. The median ages were 54.5 (range, 0.9-65.6) and 48.9 (range, 1.6-72.4), respectively.
Most patients were male (9 in group 1 and 16 in group 2), and most had malignant disease (14 and 17, respectively).
The most common graft source was peripheral blood stem cells (14 in group 1 and 16 in group 2), and most patients had a matched, unrelated donor (10 and 14, respectively).
Most patients received reduced-intensity conditioning (9 in group 1 and 17 in group 2) and GVHD prophylaxis with cyclosporine/methotrexate (13 in both groups).
All cases of GVHD were localized to the gut. Fifteen patients in each group had grade 3 GVHD. Two patients in group 1 and 6 in group 2 had grade 2 GVHD.
Results
Responses and survival rates were superior in group 2, but there was no significant difference in relapse or chronic GVHD between the groups.
In group 1, 7 patients did not respond, 5 had a partial response, and 5 had a complete response. In group 2, all patients responded, 10 with a partial response and 11 with a complete response (between-group difference, P=0.01).
The 1-year survival rate was 47% in group 1 and 76% in group 2 (P=0.016). The rate of GVHD-related mortality was 41% and 5%, respectively (P=0.003).
The cumulative incidence of chronic GVHD at 1.5 years was 36% in group 1 and 31% in group 2. The relapse rate was 29% and 18%, respectively.
The researchers compared SR patients in groups 1 (n=13) and 2 (n=11) to SR patients treated with bone marrow-derived MSCs (n=15) and SR historical controls (n=32).
The 1-year survival rate was 73% in SR group 2, which was significantly higher than the 31% survival rate in SR group 1 (P=0.02), the 20% rate in SR MSC recipients (P=0.0015), and the 3% rate in SR historical controls (P<0.001).
Severe adverse events in the DSC recipients included relapse (n=8), pneumonia (n=5), proven or probable invasive fungal infection (n=6), bacterial infection (n=2), graft failure (n=3), multiple organ failure (n=1), viral infection (n=2), central nervous system complications (n=2), septicemia (n=2), skin squamous cell carcinoma (n=2), and acute pancreatitis (n=1).
Causes of death in DSC recipients included acute GVHD (n=9), relapse (n=2), bacterial infection (n=2), invasive fungal infection (n=1), liver failure (n=1), hemorrhage (n=1), and secondary malignancy (n=1).
“Collectively, we think these data demonstrate that DSCs are a promising treatment for severe acute GVHD,” Dr Ringdén said. “But it was a small patient group, so, to further assess safety and efficacy, a larger, prospective trial will be necessary.”
“If an effective therapy for severe acute GVHD is indeed found and validated, it will increase the usefulness of stem cell transplantation with a possible broadening of indications.”