User login
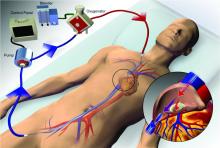
Extracorporeal membrane oxygenation (ECMO) has become increasingly accepted as a rescue therapy for severe respiratory failure from a variety of conditions, though most commonly, the acute respiratory distress syndrome (ARDS) (Thiagarajan R, et al. ASAIO. 2017;63[1]:60). ECMO can provide respiratory or cardiorespiratory support for failing lungs, heart, or both. The most common ECMO configuration used in ARDS is venovenous ECMO, in which blood is withdrawn from a catheter placed in a central vein, pumped through a gas exchange device known as an oxygenator, and returned to the venous system via another catheter. The blood flowing through the oxygenator is separated from a continuous supply of oxygen-rich sweep gas by a semipermeable membrane, across which diffusion-mediated gas exchange occurs, so that the blood exiting it is rich in oxygen and low in carbon dioxide. As venovenous ECMO functions in series with the native circulation, the well-oxygenated blood exiting the ECMO circuit mixes with poorly oxygenated blood flowing through the lungs. Therefore, oxygenation is dependent on native cardiac output to achieve systemic oxygen delivery (Figure 1).
ECMO been used successfully in adults with ARDS since the early 1970s (Hill JD, et al. N Engl J Med. 1972;286[12]:629-34) but, until recently, was limited to small numbers of patients at select global centers and associated with a high-risk profile. In the last decade, however, driven by improvements in ECMO circuit components making the device safer and easier to use, encouraging worldwide experience during the 2009 influenza A (H1N1) pandemic (Davies A, et al. JAMA. 2009;302[17]1888-95), and publication of the Efficacy and Economic Assessment of Conventional Ventilatory Support versus Extracorporeal Membrane Oxygenation for Severe Adult Respiratory Failure (CESAR) trial (Peek GJ, et al. Lancet. 2009;374[9698]:1351-63), ECMO use has markedly increased.
Despite its rapid growth, however, rigorous evidence supporting the use of ECMO has been lacking. The CESAR trial, while impressive in execution, had methodological issues that limited the strength of its conclusions. CESAR was a pragmatic trial that randomized 180 adults with severe respiratory failure from multiple etiologies to conventional management or transfer to an experienced, ECMO-capable center. CESAR met its primary outcome of improved survival without disability in the ECMO-referred group (63% vs 47%, relative risk [RR] 0.69; 95% confidence interval [CI] 0.05 to 0.97, P=.03), but not all patients in that group ultimately received ECMO. In addition, the use of lung protective ventilation was significantly higher in the ECMO-referred group, making it difficult to separate its benefit from that of ECMO. A conservative interpretation is that CESAR showed the clinical benefit of treatment at an ECMO-capable center, experienced in the management of patients with severe respiratory failure.
Not until the release of the Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome (EOLIA) trial earlier this year (Combes A, et al. N Engl J Med. 2018;378[21]:1965-75), did a modern, randomized controlled trial evaluating the use of ECMO itself exist. The EOLIA trial addressed the limitations of CESAR and randomized adult patients with early, severe ARDS to conventional, standard of care management that included a protocolized lung protective strategy in the control group vs immediate initiation of ECMO combined with an ultra-lung protective strategy (targeting end-inspiratory plateau pressure ≤24 cmH2O) in the intervention group. The primary outcome was all-cause mortality at 60 days. Of note, patients enrolled in EOLIA met entry criteria despite greater than 90% of patients receiving neuromuscular blockade and around 60% treated with prone positioning at the time of randomization (importantly, 90% of control group patients ultimately underwent prone positioning).
EOLIA was powered to detect a 20% decrease in mortality in the ECMO group. Based on trial design and the results of the fourth interim analysis, the trial was stopped for futility to reach that endpoint after enrollment of 249 of a maximum 331 patients. Although a 20% mortality reduction was not achieved, 60-day mortality was notably lower in the ECMO-treated group (35% vs 46%, RR 0.76, 95% CI 0.55 to 1.04, P=.09). The key secondary outcome of risk of treatment failure (defined as death in the ECMO group and death or crossover to ECMO in the control group) favored the ECMO group with a RR for mortality of 0.62 (95% CI, 0.47 to 0.82; P<.001), as did other secondary endpoints such as days free of renal and other organ failure.
A major limitation of the trial was that 35 (28%) of control group patients ultimately crossed over to ECMO, which diluted the effect of ECMO observed in the intention-to-treat analysis. Crossover occurred at clinician discretion an average of 6.5 days after randomization and after stringent criteria for crossover was met. These patients were incredibly ill, with a median oxygen saturation of 77%, rapidly worsening inotropic scores, and lactic acidosis; nine individuals had already suffered cardiac arrest, and six had received ECMO as part of extracorporeal cardiopulmonary resuscitation (ECPR), the initiation of venoarterial ECMO during cardiac arrest in attempt to restore spontaneous circulation. Mortality was considerably worse in the crossover group than in conventionally managed cohort overall, and, notably, 33% of patients crossed over to ECMO still survived.
In order to estimate the effect of ECMO on survival times if crossover had not occurred, the authors performed a post-hoc, rank-preserving structural failure time analysis. Though this relies on some assessment regarding the effect of the treatment itself, it showed a hazard ratio for mortality in the ECMO group of 0.51 (95% CI 0.24 to 1.02, P=.055). Although the EOLIA trial was not positive by traditional interpretation, all three major analyses and all secondary endpoints suggest some degree of benefit in patients with severe ARDS managed with ECMO.
Importantly, ECMO was well tolerated (at least when performed at expert centers, as done in this trial). There were significantly more bleeding events and cases of severe thrombocytopenia in the ECMO-treated group, but massive hemorrhage, ischemic and hemorrhagic stroke, arrhythmias, and other complications were similar.
Where do we go from here? Based on the totality of information, it is reasonable to consider ECMO for cases of severe ARDS not responsive to conventional measures, such as a lung protective ventilator strategy, neuromuscular blockade, and prone positioning. Initiation of ECMO may be reasonable prior to implementation of standard of care therapies, in order to permit safe transfer to an experienced center from a center not able to provide them.
Two take-away points: First, it is important to recognize that much of the clinical benefit derived from ECMO may be beyond its ability to normalize gas exchange and be due, at least in part, to the fact that ECMO allows the enhancement of proven lung protective ventilatory strategies. Initiation of ECMO and the “lung rest” it permits reduce the mechanical power applied to the injured alveoli and may attenuate ventilator-induced lung injury, cytokine release, and multiorgan failure that portend poor clinical outcomes in ARDS. Second, ECMO in EOLIA was conducted at expert centers with relatively low rates of complications.
It is too early to know how the critical care community will view ECMO for ARDS in light of EOLIA as well as a growing body of global ECMO experience, or how its wider application may impact the distribution and organization of ECMO centers. Regardless, of paramount importance in using ECMO as a treatment modality is optimizing patient management both prior to and after its initiation.
Dr. Agerstrand is Assistant Professor of Medicine, Director of the Medical ECMO Program, Columbia University College of Physicians and Surgeons, New York-Presbyterian Hospital.
Extracorporeal membrane oxygenation (ECMO) has become increasingly accepted as a rescue therapy for severe respiratory failure from a variety of conditions, though most commonly, the acute respiratory distress syndrome (ARDS) (Thiagarajan R, et al. ASAIO. 2017;63[1]:60). ECMO can provide respiratory or cardiorespiratory support for failing lungs, heart, or both. The most common ECMO configuration used in ARDS is venovenous ECMO, in which blood is withdrawn from a catheter placed in a central vein, pumped through a gas exchange device known as an oxygenator, and returned to the venous system via another catheter. The blood flowing through the oxygenator is separated from a continuous supply of oxygen-rich sweep gas by a semipermeable membrane, across which diffusion-mediated gas exchange occurs, so that the blood exiting it is rich in oxygen and low in carbon dioxide. As venovenous ECMO functions in series with the native circulation, the well-oxygenated blood exiting the ECMO circuit mixes with poorly oxygenated blood flowing through the lungs. Therefore, oxygenation is dependent on native cardiac output to achieve systemic oxygen delivery (Figure 1).
ECMO been used successfully in adults with ARDS since the early 1970s (Hill JD, et al. N Engl J Med. 1972;286[12]:629-34) but, until recently, was limited to small numbers of patients at select global centers and associated with a high-risk profile. In the last decade, however, driven by improvements in ECMO circuit components making the device safer and easier to use, encouraging worldwide experience during the 2009 influenza A (H1N1) pandemic (Davies A, et al. JAMA. 2009;302[17]1888-95), and publication of the Efficacy and Economic Assessment of Conventional Ventilatory Support versus Extracorporeal Membrane Oxygenation for Severe Adult Respiratory Failure (CESAR) trial (Peek GJ, et al. Lancet. 2009;374[9698]:1351-63), ECMO use has markedly increased.
Despite its rapid growth, however, rigorous evidence supporting the use of ECMO has been lacking. The CESAR trial, while impressive in execution, had methodological issues that limited the strength of its conclusions. CESAR was a pragmatic trial that randomized 180 adults with severe respiratory failure from multiple etiologies to conventional management or transfer to an experienced, ECMO-capable center. CESAR met its primary outcome of improved survival without disability in the ECMO-referred group (63% vs 47%, relative risk [RR] 0.69; 95% confidence interval [CI] 0.05 to 0.97, P=.03), but not all patients in that group ultimately received ECMO. In addition, the use of lung protective ventilation was significantly higher in the ECMO-referred group, making it difficult to separate its benefit from that of ECMO. A conservative interpretation is that CESAR showed the clinical benefit of treatment at an ECMO-capable center, experienced in the management of patients with severe respiratory failure.
Not until the release of the Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome (EOLIA) trial earlier this year (Combes A, et al. N Engl J Med. 2018;378[21]:1965-75), did a modern, randomized controlled trial evaluating the use of ECMO itself exist. The EOLIA trial addressed the limitations of CESAR and randomized adult patients with early, severe ARDS to conventional, standard of care management that included a protocolized lung protective strategy in the control group vs immediate initiation of ECMO combined with an ultra-lung protective strategy (targeting end-inspiratory plateau pressure ≤24 cmH2O) in the intervention group. The primary outcome was all-cause mortality at 60 days. Of note, patients enrolled in EOLIA met entry criteria despite greater than 90% of patients receiving neuromuscular blockade and around 60% treated with prone positioning at the time of randomization (importantly, 90% of control group patients ultimately underwent prone positioning).
EOLIA was powered to detect a 20% decrease in mortality in the ECMO group. Based on trial design and the results of the fourth interim analysis, the trial was stopped for futility to reach that endpoint after enrollment of 249 of a maximum 331 patients. Although a 20% mortality reduction was not achieved, 60-day mortality was notably lower in the ECMO-treated group (35% vs 46%, RR 0.76, 95% CI 0.55 to 1.04, P=.09). The key secondary outcome of risk of treatment failure (defined as death in the ECMO group and death or crossover to ECMO in the control group) favored the ECMO group with a RR for mortality of 0.62 (95% CI, 0.47 to 0.82; P<.001), as did other secondary endpoints such as days free of renal and other organ failure.
A major limitation of the trial was that 35 (28%) of control group patients ultimately crossed over to ECMO, which diluted the effect of ECMO observed in the intention-to-treat analysis. Crossover occurred at clinician discretion an average of 6.5 days after randomization and after stringent criteria for crossover was met. These patients were incredibly ill, with a median oxygen saturation of 77%, rapidly worsening inotropic scores, and lactic acidosis; nine individuals had already suffered cardiac arrest, and six had received ECMO as part of extracorporeal cardiopulmonary resuscitation (ECPR), the initiation of venoarterial ECMO during cardiac arrest in attempt to restore spontaneous circulation. Mortality was considerably worse in the crossover group than in conventionally managed cohort overall, and, notably, 33% of patients crossed over to ECMO still survived.
In order to estimate the effect of ECMO on survival times if crossover had not occurred, the authors performed a post-hoc, rank-preserving structural failure time analysis. Though this relies on some assessment regarding the effect of the treatment itself, it showed a hazard ratio for mortality in the ECMO group of 0.51 (95% CI 0.24 to 1.02, P=.055). Although the EOLIA trial was not positive by traditional interpretation, all three major analyses and all secondary endpoints suggest some degree of benefit in patients with severe ARDS managed with ECMO.
Importantly, ECMO was well tolerated (at least when performed at expert centers, as done in this trial). There were significantly more bleeding events and cases of severe thrombocytopenia in the ECMO-treated group, but massive hemorrhage, ischemic and hemorrhagic stroke, arrhythmias, and other complications were similar.
Where do we go from here? Based on the totality of information, it is reasonable to consider ECMO for cases of severe ARDS not responsive to conventional measures, such as a lung protective ventilator strategy, neuromuscular blockade, and prone positioning. Initiation of ECMO may be reasonable prior to implementation of standard of care therapies, in order to permit safe transfer to an experienced center from a center not able to provide them.
Two take-away points: First, it is important to recognize that much of the clinical benefit derived from ECMO may be beyond its ability to normalize gas exchange and be due, at least in part, to the fact that ECMO allows the enhancement of proven lung protective ventilatory strategies. Initiation of ECMO and the “lung rest” it permits reduce the mechanical power applied to the injured alveoli and may attenuate ventilator-induced lung injury, cytokine release, and multiorgan failure that portend poor clinical outcomes in ARDS. Second, ECMO in EOLIA was conducted at expert centers with relatively low rates of complications.
It is too early to know how the critical care community will view ECMO for ARDS in light of EOLIA as well as a growing body of global ECMO experience, or how its wider application may impact the distribution and organization of ECMO centers. Regardless, of paramount importance in using ECMO as a treatment modality is optimizing patient management both prior to and after its initiation.
Dr. Agerstrand is Assistant Professor of Medicine, Director of the Medical ECMO Program, Columbia University College of Physicians and Surgeons, New York-Presbyterian Hospital.
Extracorporeal membrane oxygenation (ECMO) has become increasingly accepted as a rescue therapy for severe respiratory failure from a variety of conditions, though most commonly, the acute respiratory distress syndrome (ARDS) (Thiagarajan R, et al. ASAIO. 2017;63[1]:60). ECMO can provide respiratory or cardiorespiratory support for failing lungs, heart, or both. The most common ECMO configuration used in ARDS is venovenous ECMO, in which blood is withdrawn from a catheter placed in a central vein, pumped through a gas exchange device known as an oxygenator, and returned to the venous system via another catheter. The blood flowing through the oxygenator is separated from a continuous supply of oxygen-rich sweep gas by a semipermeable membrane, across which diffusion-mediated gas exchange occurs, so that the blood exiting it is rich in oxygen and low in carbon dioxide. As venovenous ECMO functions in series with the native circulation, the well-oxygenated blood exiting the ECMO circuit mixes with poorly oxygenated blood flowing through the lungs. Therefore, oxygenation is dependent on native cardiac output to achieve systemic oxygen delivery (Figure 1).
ECMO been used successfully in adults with ARDS since the early 1970s (Hill JD, et al. N Engl J Med. 1972;286[12]:629-34) but, until recently, was limited to small numbers of patients at select global centers and associated with a high-risk profile. In the last decade, however, driven by improvements in ECMO circuit components making the device safer and easier to use, encouraging worldwide experience during the 2009 influenza A (H1N1) pandemic (Davies A, et al. JAMA. 2009;302[17]1888-95), and publication of the Efficacy and Economic Assessment of Conventional Ventilatory Support versus Extracorporeal Membrane Oxygenation for Severe Adult Respiratory Failure (CESAR) trial (Peek GJ, et al. Lancet. 2009;374[9698]:1351-63), ECMO use has markedly increased.
Despite its rapid growth, however, rigorous evidence supporting the use of ECMO has been lacking. The CESAR trial, while impressive in execution, had methodological issues that limited the strength of its conclusions. CESAR was a pragmatic trial that randomized 180 adults with severe respiratory failure from multiple etiologies to conventional management or transfer to an experienced, ECMO-capable center. CESAR met its primary outcome of improved survival without disability in the ECMO-referred group (63% vs 47%, relative risk [RR] 0.69; 95% confidence interval [CI] 0.05 to 0.97, P=.03), but not all patients in that group ultimately received ECMO. In addition, the use of lung protective ventilation was significantly higher in the ECMO-referred group, making it difficult to separate its benefit from that of ECMO. A conservative interpretation is that CESAR showed the clinical benefit of treatment at an ECMO-capable center, experienced in the management of patients with severe respiratory failure.
Not until the release of the Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome (EOLIA) trial earlier this year (Combes A, et al. N Engl J Med. 2018;378[21]:1965-75), did a modern, randomized controlled trial evaluating the use of ECMO itself exist. The EOLIA trial addressed the limitations of CESAR and randomized adult patients with early, severe ARDS to conventional, standard of care management that included a protocolized lung protective strategy in the control group vs immediate initiation of ECMO combined with an ultra-lung protective strategy (targeting end-inspiratory plateau pressure ≤24 cmH2O) in the intervention group. The primary outcome was all-cause mortality at 60 days. Of note, patients enrolled in EOLIA met entry criteria despite greater than 90% of patients receiving neuromuscular blockade and around 60% treated with prone positioning at the time of randomization (importantly, 90% of control group patients ultimately underwent prone positioning).
EOLIA was powered to detect a 20% decrease in mortality in the ECMO group. Based on trial design and the results of the fourth interim analysis, the trial was stopped for futility to reach that endpoint after enrollment of 249 of a maximum 331 patients. Although a 20% mortality reduction was not achieved, 60-day mortality was notably lower in the ECMO-treated group (35% vs 46%, RR 0.76, 95% CI 0.55 to 1.04, P=.09). The key secondary outcome of risk of treatment failure (defined as death in the ECMO group and death or crossover to ECMO in the control group) favored the ECMO group with a RR for mortality of 0.62 (95% CI, 0.47 to 0.82; P<.001), as did other secondary endpoints such as days free of renal and other organ failure.
A major limitation of the trial was that 35 (28%) of control group patients ultimately crossed over to ECMO, which diluted the effect of ECMO observed in the intention-to-treat analysis. Crossover occurred at clinician discretion an average of 6.5 days after randomization and after stringent criteria for crossover was met. These patients were incredibly ill, with a median oxygen saturation of 77%, rapidly worsening inotropic scores, and lactic acidosis; nine individuals had already suffered cardiac arrest, and six had received ECMO as part of extracorporeal cardiopulmonary resuscitation (ECPR), the initiation of venoarterial ECMO during cardiac arrest in attempt to restore spontaneous circulation. Mortality was considerably worse in the crossover group than in conventionally managed cohort overall, and, notably, 33% of patients crossed over to ECMO still survived.
In order to estimate the effect of ECMO on survival times if crossover had not occurred, the authors performed a post-hoc, rank-preserving structural failure time analysis. Though this relies on some assessment regarding the effect of the treatment itself, it showed a hazard ratio for mortality in the ECMO group of 0.51 (95% CI 0.24 to 1.02, P=.055). Although the EOLIA trial was not positive by traditional interpretation, all three major analyses and all secondary endpoints suggest some degree of benefit in patients with severe ARDS managed with ECMO.
Importantly, ECMO was well tolerated (at least when performed at expert centers, as done in this trial). There were significantly more bleeding events and cases of severe thrombocytopenia in the ECMO-treated group, but massive hemorrhage, ischemic and hemorrhagic stroke, arrhythmias, and other complications were similar.
Where do we go from here? Based on the totality of information, it is reasonable to consider ECMO for cases of severe ARDS not responsive to conventional measures, such as a lung protective ventilator strategy, neuromuscular blockade, and prone positioning. Initiation of ECMO may be reasonable prior to implementation of standard of care therapies, in order to permit safe transfer to an experienced center from a center not able to provide them.
Two take-away points: First, it is important to recognize that much of the clinical benefit derived from ECMO may be beyond its ability to normalize gas exchange and be due, at least in part, to the fact that ECMO allows the enhancement of proven lung protective ventilatory strategies. Initiation of ECMO and the “lung rest” it permits reduce the mechanical power applied to the injured alveoli and may attenuate ventilator-induced lung injury, cytokine release, and multiorgan failure that portend poor clinical outcomes in ARDS. Second, ECMO in EOLIA was conducted at expert centers with relatively low rates of complications.
It is too early to know how the critical care community will view ECMO for ARDS in light of EOLIA as well as a growing body of global ECMO experience, or how its wider application may impact the distribution and organization of ECMO centers. Regardless, of paramount importance in using ECMO as a treatment modality is optimizing patient management both prior to and after its initiation.
Dr. Agerstrand is Assistant Professor of Medicine, Director of the Medical ECMO Program, Columbia University College of Physicians and Surgeons, New York-Presbyterian Hospital.