User login
Biomarker use in ARDS resulting from COVID-19 infection
There is renewed interest in the use of immunomodulator therapies in patients with acute hypoxemic respiratory failure.
Beyond COVID-19, studies have also shown corticosteroid therapy improves clinical outcomes in patients with severe community-acquired pneumonia.3 However, the overwhelming majority of studies identifying plasma biomarkers that are associated with clinical outcomes in severe lung injury predate the routine use of corticosteroids.4 Two investigators at Massachusetts General Hospital, Jehan W. Alladina, MD, and George A. Alba, MD, performed a study to assess whether plasma biomarkers previously associated with clinical outcomes in ARDS maintained their predictive value in the setting of widespread immunomodulator therapy in the ICU. Drs. Alladina and Alba are physician-scientists and codirectors of the Program for Advancing Critical Care Translational Science at Massachusetts General Hospital in Boston.
In a study published in CHEST®Critical Care earlier this year, they prospectively enrolled patients with ARDS due to confirmed SARS-CoV-2 infection during the second wave of the COVID-19 pandemic from December 31, 2020, to March 31, 2021, at Massachusetts General Hospital.5 Plasma samples were collected within 24 hours of intubation for mechanical ventilation for protein analysis in 69 patients. Baseline demographics included a mean age of 62 plus or minus 15 years and a BMI of 31 plus or minus 8, and 45% were female. The median PaO2 to FiO2 ratio was 174 mm Hg, consistent with moderate ARDS, and the median duration of ventilation was 17 days. The patients had a median modified sequential organ failure assessment score of 8.5, and in-hospital mortality was 44% by 60 days. Notably, all patients in this cohort received steroids during their ICU stay.
Interestingly, the study investigators found no association between clinical outcomes and circulating proteins implicated in inflammation (eg, interleukin [IL]-6, IL-8), epithelial injury (eg, soluble receptor for advanced glycation end products, surfactant protein D), or coagulation (eg, D-dimer, tissue factor). However, four endothelial biomarkers—von Willebrand factor A2 domain; angiopoietin-2; syndecan-1; and neural precursor cell expressed, developmentally downregulated 9 (NEDD9)—were associated with 60-day mortality after adjusting for age, sex, and severity of illness. A sensitivity analysis, in which patients treated with the IL-6 inhibitor tocilizumab (n=4) were excluded, showed similar results.
Of the endothelial proteins, NEDD9 demonstrated the greatest effect size in its association with mortality in patients with ARDS due to COVID-19 who were treated with immunomodulators. NEDD9 is a scaffolding protein highly expressed in the pulmonary vascular endothelium, but its role in ARDS is not well known. In pulmonary vascular disease, plasma levels are associated with adverse pulmonary hemodynamics and clinical outcomes. Pulmonary artery endothelial NEDD9 is upregulated by cellular hypoxia and can mediate platelet-endothelial adhesion by interacting with P-selectin on the surface of activated platelets.6 Additionally, there is evidence of increased pulmonary endothelial NEDD9 expression and colocalization with fibrin within pulmonary arteries in lung tissue of patients who died from ARDS due to COVID-19.7 Thus, NEDD9 may be an important mediator of pulmonary vascular dysfunction observed in ARDS and could be a novel biomarker for patient subphenotyping and prognostication of clinical outcomes.
In summary, in a cohort of patients with COVID-19 ARDS uniformly treated with corticosteroids, plasma biomarkers of inflammation, coagulation, and epithelial injury were not associated with clinical outcomes, but endothelial biomarkers remained prognostic. It is biologically plausible that immunomodulators could attenuate the association between inflammatory biomarkers and patient outcomes. The findings of this study highlight the association of endothelial biomarkers with clinical outcomes in patients with COVID-19 ARDS treated with immunomodulators and warrant prospective validation, especially with the increasing evidence-based use of antiinflammatory therapy in acute lung injury. However, there are several important limitations to consider, including a small sample size from a single institution that precludes any definitive conclusions regarding any negative associations. Moreover, the single time point studied (the day of initiation of mechanical ventilation) and absence of a comparator group do not allow a comprehensive evaluation of the impact of antiinflammatory therapies across the trajectory of disease. Whether the findings are generalizable to all patients with ARDS treated with immunomodulators also remains unknown.
Overall, these data suggest that circulating signatures previously associated with ARDS, particularly those related to systemic inflammation, may have limited prognostic utility in the era of increasing immunomodulator use in critical illness. A deeper understanding of the pathobiology of ARDS, including the complex interplay with systemic immunomodulation, is needed to identify prognostic biomarkers and targeted therapies that improve patient outcomes.
Both authors work in the Division of Pulmonary and Critical Care Medicine, Massachusetts General Hospital, Harvard Medical School, in Boston.
References
1. Horby P, Lim WS, Emberson JR, et al; RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384(8):693-704.
2. Tomazini BM, Maia IS, Cavalcanti AB, et al. Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19. JAMA. 2020;324(13):1-11.
3. Dequin P-F, Meziani F, Quenot J-P, et al. Hydrocortisone in severe community-acquired pneumonia. N Engl J Med. 2023;388(21):1931-1941.
4. Del Valle DM, Kim-Schulze S, Huang H-H, et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat Med. 2020;26(10):1636-1643.
5. Alladina JW, Giacona FL, Haring AM, et al. Circulating biomarkers of endothelial dysfunction associated with ventilatory ratio and mortality in ARDS resulting from SARS-CoV-2 infection treated with antiinflammatory therapies. CHEST Crit Care. 2024;2(2):100054.
6. Alba GA, Samokhin AO, Wang R-S, et al. NEDD9 is a novel and modifiable mediator of platelet-endothelial adhesion in the pulmonary circulation. Am J Respir Crit Care Med. 2021;203(12):1533-1545.
7. Alba GA, Samokhin AO, Wang R-S, et al. Pulmonary endothelial NEDD9 and the prothrombotic pathophenotype of acute respiratory distress syndrome due to SARS‐CoV‐2 infection. Pulm Circ. 2022;12(2):e12071.
There is renewed interest in the use of immunomodulator therapies in patients with acute hypoxemic respiratory failure.
Beyond COVID-19, studies have also shown corticosteroid therapy improves clinical outcomes in patients with severe community-acquired pneumonia.3 However, the overwhelming majority of studies identifying plasma biomarkers that are associated with clinical outcomes in severe lung injury predate the routine use of corticosteroids.4 Two investigators at Massachusetts General Hospital, Jehan W. Alladina, MD, and George A. Alba, MD, performed a study to assess whether plasma biomarkers previously associated with clinical outcomes in ARDS maintained their predictive value in the setting of widespread immunomodulator therapy in the ICU. Drs. Alladina and Alba are physician-scientists and codirectors of the Program for Advancing Critical Care Translational Science at Massachusetts General Hospital in Boston.
In a study published in CHEST®Critical Care earlier this year, they prospectively enrolled patients with ARDS due to confirmed SARS-CoV-2 infection during the second wave of the COVID-19 pandemic from December 31, 2020, to March 31, 2021, at Massachusetts General Hospital.5 Plasma samples were collected within 24 hours of intubation for mechanical ventilation for protein analysis in 69 patients. Baseline demographics included a mean age of 62 plus or minus 15 years and a BMI of 31 plus or minus 8, and 45% were female. The median PaO2 to FiO2 ratio was 174 mm Hg, consistent with moderate ARDS, and the median duration of ventilation was 17 days. The patients had a median modified sequential organ failure assessment score of 8.5, and in-hospital mortality was 44% by 60 days. Notably, all patients in this cohort received steroids during their ICU stay.
Interestingly, the study investigators found no association between clinical outcomes and circulating proteins implicated in inflammation (eg, interleukin [IL]-6, IL-8), epithelial injury (eg, soluble receptor for advanced glycation end products, surfactant protein D), or coagulation (eg, D-dimer, tissue factor). However, four endothelial biomarkers—von Willebrand factor A2 domain; angiopoietin-2; syndecan-1; and neural precursor cell expressed, developmentally downregulated 9 (NEDD9)—were associated with 60-day mortality after adjusting for age, sex, and severity of illness. A sensitivity analysis, in which patients treated with the IL-6 inhibitor tocilizumab (n=4) were excluded, showed similar results.
Of the endothelial proteins, NEDD9 demonstrated the greatest effect size in its association with mortality in patients with ARDS due to COVID-19 who were treated with immunomodulators. NEDD9 is a scaffolding protein highly expressed in the pulmonary vascular endothelium, but its role in ARDS is not well known. In pulmonary vascular disease, plasma levels are associated with adverse pulmonary hemodynamics and clinical outcomes. Pulmonary artery endothelial NEDD9 is upregulated by cellular hypoxia and can mediate platelet-endothelial adhesion by interacting with P-selectin on the surface of activated platelets.6 Additionally, there is evidence of increased pulmonary endothelial NEDD9 expression and colocalization with fibrin within pulmonary arteries in lung tissue of patients who died from ARDS due to COVID-19.7 Thus, NEDD9 may be an important mediator of pulmonary vascular dysfunction observed in ARDS and could be a novel biomarker for patient subphenotyping and prognostication of clinical outcomes.
In summary, in a cohort of patients with COVID-19 ARDS uniformly treated with corticosteroids, plasma biomarkers of inflammation, coagulation, and epithelial injury were not associated with clinical outcomes, but endothelial biomarkers remained prognostic. It is biologically plausible that immunomodulators could attenuate the association between inflammatory biomarkers and patient outcomes. The findings of this study highlight the association of endothelial biomarkers with clinical outcomes in patients with COVID-19 ARDS treated with immunomodulators and warrant prospective validation, especially with the increasing evidence-based use of antiinflammatory therapy in acute lung injury. However, there are several important limitations to consider, including a small sample size from a single institution that precludes any definitive conclusions regarding any negative associations. Moreover, the single time point studied (the day of initiation of mechanical ventilation) and absence of a comparator group do not allow a comprehensive evaluation of the impact of antiinflammatory therapies across the trajectory of disease. Whether the findings are generalizable to all patients with ARDS treated with immunomodulators also remains unknown.
Overall, these data suggest that circulating signatures previously associated with ARDS, particularly those related to systemic inflammation, may have limited prognostic utility in the era of increasing immunomodulator use in critical illness. A deeper understanding of the pathobiology of ARDS, including the complex interplay with systemic immunomodulation, is needed to identify prognostic biomarkers and targeted therapies that improve patient outcomes.
Both authors work in the Division of Pulmonary and Critical Care Medicine, Massachusetts General Hospital, Harvard Medical School, in Boston.
References
1. Horby P, Lim WS, Emberson JR, et al; RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384(8):693-704.
2. Tomazini BM, Maia IS, Cavalcanti AB, et al. Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19. JAMA. 2020;324(13):1-11.
3. Dequin P-F, Meziani F, Quenot J-P, et al. Hydrocortisone in severe community-acquired pneumonia. N Engl J Med. 2023;388(21):1931-1941.
4. Del Valle DM, Kim-Schulze S, Huang H-H, et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat Med. 2020;26(10):1636-1643.
5. Alladina JW, Giacona FL, Haring AM, et al. Circulating biomarkers of endothelial dysfunction associated with ventilatory ratio and mortality in ARDS resulting from SARS-CoV-2 infection treated with antiinflammatory therapies. CHEST Crit Care. 2024;2(2):100054.
6. Alba GA, Samokhin AO, Wang R-S, et al. NEDD9 is a novel and modifiable mediator of platelet-endothelial adhesion in the pulmonary circulation. Am J Respir Crit Care Med. 2021;203(12):1533-1545.
7. Alba GA, Samokhin AO, Wang R-S, et al. Pulmonary endothelial NEDD9 and the prothrombotic pathophenotype of acute respiratory distress syndrome due to SARS‐CoV‐2 infection. Pulm Circ. 2022;12(2):e12071.
There is renewed interest in the use of immunomodulator therapies in patients with acute hypoxemic respiratory failure.
Beyond COVID-19, studies have also shown corticosteroid therapy improves clinical outcomes in patients with severe community-acquired pneumonia.3 However, the overwhelming majority of studies identifying plasma biomarkers that are associated with clinical outcomes in severe lung injury predate the routine use of corticosteroids.4 Two investigators at Massachusetts General Hospital, Jehan W. Alladina, MD, and George A. Alba, MD, performed a study to assess whether plasma biomarkers previously associated with clinical outcomes in ARDS maintained their predictive value in the setting of widespread immunomodulator therapy in the ICU. Drs. Alladina and Alba are physician-scientists and codirectors of the Program for Advancing Critical Care Translational Science at Massachusetts General Hospital in Boston.
In a study published in CHEST®Critical Care earlier this year, they prospectively enrolled patients with ARDS due to confirmed SARS-CoV-2 infection during the second wave of the COVID-19 pandemic from December 31, 2020, to March 31, 2021, at Massachusetts General Hospital.5 Plasma samples were collected within 24 hours of intubation for mechanical ventilation for protein analysis in 69 patients. Baseline demographics included a mean age of 62 plus or minus 15 years and a BMI of 31 plus or minus 8, and 45% were female. The median PaO2 to FiO2 ratio was 174 mm Hg, consistent with moderate ARDS, and the median duration of ventilation was 17 days. The patients had a median modified sequential organ failure assessment score of 8.5, and in-hospital mortality was 44% by 60 days. Notably, all patients in this cohort received steroids during their ICU stay.
Interestingly, the study investigators found no association between clinical outcomes and circulating proteins implicated in inflammation (eg, interleukin [IL]-6, IL-8), epithelial injury (eg, soluble receptor for advanced glycation end products, surfactant protein D), or coagulation (eg, D-dimer, tissue factor). However, four endothelial biomarkers—von Willebrand factor A2 domain; angiopoietin-2; syndecan-1; and neural precursor cell expressed, developmentally downregulated 9 (NEDD9)—were associated with 60-day mortality after adjusting for age, sex, and severity of illness. A sensitivity analysis, in which patients treated with the IL-6 inhibitor tocilizumab (n=4) were excluded, showed similar results.
Of the endothelial proteins, NEDD9 demonstrated the greatest effect size in its association with mortality in patients with ARDS due to COVID-19 who were treated with immunomodulators. NEDD9 is a scaffolding protein highly expressed in the pulmonary vascular endothelium, but its role in ARDS is not well known. In pulmonary vascular disease, plasma levels are associated with adverse pulmonary hemodynamics and clinical outcomes. Pulmonary artery endothelial NEDD9 is upregulated by cellular hypoxia and can mediate platelet-endothelial adhesion by interacting with P-selectin on the surface of activated platelets.6 Additionally, there is evidence of increased pulmonary endothelial NEDD9 expression and colocalization with fibrin within pulmonary arteries in lung tissue of patients who died from ARDS due to COVID-19.7 Thus, NEDD9 may be an important mediator of pulmonary vascular dysfunction observed in ARDS and could be a novel biomarker for patient subphenotyping and prognostication of clinical outcomes.
In summary, in a cohort of patients with COVID-19 ARDS uniformly treated with corticosteroids, plasma biomarkers of inflammation, coagulation, and epithelial injury were not associated with clinical outcomes, but endothelial biomarkers remained prognostic. It is biologically plausible that immunomodulators could attenuate the association between inflammatory biomarkers and patient outcomes. The findings of this study highlight the association of endothelial biomarkers with clinical outcomes in patients with COVID-19 ARDS treated with immunomodulators and warrant prospective validation, especially with the increasing evidence-based use of antiinflammatory therapy in acute lung injury. However, there are several important limitations to consider, including a small sample size from a single institution that precludes any definitive conclusions regarding any negative associations. Moreover, the single time point studied (the day of initiation of mechanical ventilation) and absence of a comparator group do not allow a comprehensive evaluation of the impact of antiinflammatory therapies across the trajectory of disease. Whether the findings are generalizable to all patients with ARDS treated with immunomodulators also remains unknown.
Overall, these data suggest that circulating signatures previously associated with ARDS, particularly those related to systemic inflammation, may have limited prognostic utility in the era of increasing immunomodulator use in critical illness. A deeper understanding of the pathobiology of ARDS, including the complex interplay with systemic immunomodulation, is needed to identify prognostic biomarkers and targeted therapies that improve patient outcomes.
Both authors work in the Division of Pulmonary and Critical Care Medicine, Massachusetts General Hospital, Harvard Medical School, in Boston.
References
1. Horby P, Lim WS, Emberson JR, et al; RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384(8):693-704.
2. Tomazini BM, Maia IS, Cavalcanti AB, et al. Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19. JAMA. 2020;324(13):1-11.
3. Dequin P-F, Meziani F, Quenot J-P, et al. Hydrocortisone in severe community-acquired pneumonia. N Engl J Med. 2023;388(21):1931-1941.
4. Del Valle DM, Kim-Schulze S, Huang H-H, et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat Med. 2020;26(10):1636-1643.
5. Alladina JW, Giacona FL, Haring AM, et al. Circulating biomarkers of endothelial dysfunction associated with ventilatory ratio and mortality in ARDS resulting from SARS-CoV-2 infection treated with antiinflammatory therapies. CHEST Crit Care. 2024;2(2):100054.
6. Alba GA, Samokhin AO, Wang R-S, et al. NEDD9 is a novel and modifiable mediator of platelet-endothelial adhesion in the pulmonary circulation. Am J Respir Crit Care Med. 2021;203(12):1533-1545.
7. Alba GA, Samokhin AO, Wang R-S, et al. Pulmonary endothelial NEDD9 and the prothrombotic pathophenotype of acute respiratory distress syndrome due to SARS‐CoV‐2 infection. Pulm Circ. 2022;12(2):e12071.
The language of AI and its applications in health care
AI is a group of nonhuman techniques that utilize automated learning methods to extract information from datasets through generalization, classification, prediction, and association. In other words, AI is the simulation of human intelligence processes by machines. The branches of AI include natural language processing, speech recognition, machine vision, and expert systems. AI can make clinical care more efficient; however, many find its confusing terminology to be a barrier.1 This article provides concise definitions of AI terms and is intended to help physicians better understand how AI methods can be applied to clinical care. The clinical application of natural language processing and machine vision applications are more clinically intuitive than the roles of machine learning algorithms.
Machine learning and algorithms
Machine learning is a branch of AI that uses data and algorithms to mimic human reasoning through classification, pattern recognition, and prediction. Supervised and unsupervised machine-learning algorithms can analyze data and recognize undetected associations and relationships.
Supervised learning involves training models to make predictions using data sets that have correct outcome parameters called labels using predictive fields called features. Machine learning uses iterative analysis including random forest, decision tree, and gradient boosting methods that minimize predictive error metrics (see Table 1). This approach is widely used to improve diagnoses, predict disease progression or exacerbation, and personalize treatment plan modifications.
Supervised machine learning methods can be particularly effective for processing large volumes of medical information to identify patterns and make accurate predictions. In contrast, unsupervised learning techniques can analyze unlabeled data and help clinicians uncover hidden patterns or undetected groupings. Techniques including clustering, exploratory analysis, and anomaly detection are common applications. Both of these machine-learning approaches can be used to extract novel and helpful insights.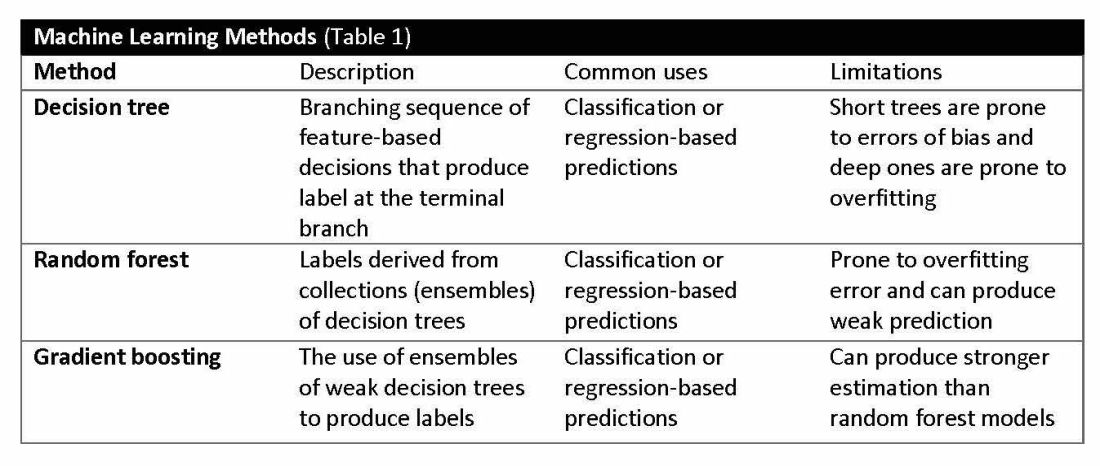
The utility of machine learning analyses depends on the size and accuracy of the available datasets. Small datasets can limit usability, while large datasets require substantial computational power. Predictive models are generated using training datasets and evaluated using separate evaluation datasets. Deep learning models, a subset of machine learning, can automatically readjust themselves to maintain or improve accuracy when analyzing new observations that include accurate labels.
Challenges of algorithms and calibration
Machine learning algorithms vary in complexity and accuracy. For example, a simple logistic regression model using time, date, latitude, and indoor/outdoor location can accurately recommend sunscreen application. This model identifies when solar radiation is high enough to warrant sunscreen use, avoiding unnecessary recommendations during nighttime hours or indoor locations. A more complex model might suffer from model overfitting and inappropriately suggest sunscreen before a tanning salon visit.
Complex machine learning models, like support vector machine (SVM) and decision tree methods, are useful when many features have predictive power. SVMs are useful for small but complex datasets. Features are manipulated in a multidimensional space to maximize the “margins” separating 2 groups. Decision tree analyses are useful when more than 2 groups are being analyzed. SVM and decision tree models can also lose accuracy by data overfitting.
Consider the development of an SVM analysis to predict whether an individual is a fellow or a senior faculty member. One could use high gray hair density feature values to identify senior faculty. When this algorithm is applied to an individual with alopecia, no amount of model adjustment can achieve high levels of discrimination because no hair is present. Rather than overfitting the model by adding more nonpredictive features, individuals with alopecia are analyzed by their own algorithm (tree) that uses the skin wrinkle/solar damage rather than the gray hair density feature.
Decision tree ensemble algorithms like random forest and gradient boosting use feature-based decision trees to process and classify data. Random forests are robust, scalable, and versatile, providing classifications and predictions while protecting against inaccurate data and outliers and have the advantage of being able to handle both categorical and continuous features. Gradient boosting, which uses an ensemble of weak decision trees, often outperforms random forests when individual trees perform only slightly better than random chance. This method incrementally builds the model by optimizing the residual errors of previous trees, leading to more accurate predictions.
In practice, gradient boosting can be used to fine-tune diagnostic models, improving their precision and reliability. A recent example of how gradient boosting of random forest predictions yielded highly accurate predictions for unplanned vasopressor initiation and intubation events 2 to 4 hours before an ICU adult became unstable.2
Assessing the accuracy of algorithms
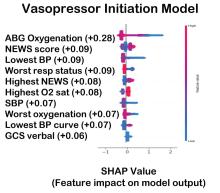
The value of the data set is directly related to the accuracy of its labels. Traditional methods that measure model performance, such as sensitivity, specificity, and predictive values (PPV and NPV), have important limitations. They provide little insight into how a complex model made its prediction. Understanding which individual features drive model accuracy is key to fostering trust in model predictions. This can be done by comparing model output with and without including individual features. The results of all possible combinations are aggregated according to feature importance, which is summarized in the Shapley value for each model feature. Higher values indicate greater relative importance. SHAP plots help identify how much and how often specific features change the model output, presenting values of individual model estimates with and without a specific feature (see Figure 1).
Promoting AI use
AI and machine learning algorithms are coming to patient care. Understanding the language of AI helps caregivers integrate these tools into their practices. The science of AI faces serious challenges. Algorithms must be recalibrated to keep pace as therapies advance, disease prevalence changes, and our population ages. AI must address new challenges as they confront those suffering from respiratory diseases. This resource encourages clinicians with novel approaches by using AI methodologies to advance their development. We can better address future health care needs by promoting the equitable use of AI technologies, especially among socially disadvantaged developers.
References
1. Lilly CM, Soni AV, Dunlap D, et al. Advancing point of care testing by application of machine learning techniques and artificial intelligence. Chest. 2024 (in press).
2. Lilly CM, Kirk D, Pessach IM, et al. Application of machine learning models to biomedical and information system signals from critically ill adults. Chest. 2024;165(5):1139-1148.
AI is a group of nonhuman techniques that utilize automated learning methods to extract information from datasets through generalization, classification, prediction, and association. In other words, AI is the simulation of human intelligence processes by machines. The branches of AI include natural language processing, speech recognition, machine vision, and expert systems. AI can make clinical care more efficient; however, many find its confusing terminology to be a barrier.1 This article provides concise definitions of AI terms and is intended to help physicians better understand how AI methods can be applied to clinical care. The clinical application of natural language processing and machine vision applications are more clinically intuitive than the roles of machine learning algorithms.
Machine learning and algorithms
Machine learning is a branch of AI that uses data and algorithms to mimic human reasoning through classification, pattern recognition, and prediction. Supervised and unsupervised machine-learning algorithms can analyze data and recognize undetected associations and relationships.
Supervised learning involves training models to make predictions using data sets that have correct outcome parameters called labels using predictive fields called features. Machine learning uses iterative analysis including random forest, decision tree, and gradient boosting methods that minimize predictive error metrics (see Table 1). This approach is widely used to improve diagnoses, predict disease progression or exacerbation, and personalize treatment plan modifications.
Supervised machine learning methods can be particularly effective for processing large volumes of medical information to identify patterns and make accurate predictions. In contrast, unsupervised learning techniques can analyze unlabeled data and help clinicians uncover hidden patterns or undetected groupings. Techniques including clustering, exploratory analysis, and anomaly detection are common applications. Both of these machine-learning approaches can be used to extract novel and helpful insights.
The utility of machine learning analyses depends on the size and accuracy of the available datasets. Small datasets can limit usability, while large datasets require substantial computational power. Predictive models are generated using training datasets and evaluated using separate evaluation datasets. Deep learning models, a subset of machine learning, can automatically readjust themselves to maintain or improve accuracy when analyzing new observations that include accurate labels.
Challenges of algorithms and calibration
Machine learning algorithms vary in complexity and accuracy. For example, a simple logistic regression model using time, date, latitude, and indoor/outdoor location can accurately recommend sunscreen application. This model identifies when solar radiation is high enough to warrant sunscreen use, avoiding unnecessary recommendations during nighttime hours or indoor locations. A more complex model might suffer from model overfitting and inappropriately suggest sunscreen before a tanning salon visit.
Complex machine learning models, like support vector machine (SVM) and decision tree methods, are useful when many features have predictive power. SVMs are useful for small but complex datasets. Features are manipulated in a multidimensional space to maximize the “margins” separating 2 groups. Decision tree analyses are useful when more than 2 groups are being analyzed. SVM and decision tree models can also lose accuracy by data overfitting.
Consider the development of an SVM analysis to predict whether an individual is a fellow or a senior faculty member. One could use high gray hair density feature values to identify senior faculty. When this algorithm is applied to an individual with alopecia, no amount of model adjustment can achieve high levels of discrimination because no hair is present. Rather than overfitting the model by adding more nonpredictive features, individuals with alopecia are analyzed by their own algorithm (tree) that uses the skin wrinkle/solar damage rather than the gray hair density feature.
Decision tree ensemble algorithms like random forest and gradient boosting use feature-based decision trees to process and classify data. Random forests are robust, scalable, and versatile, providing classifications and predictions while protecting against inaccurate data and outliers and have the advantage of being able to handle both categorical and continuous features. Gradient boosting, which uses an ensemble of weak decision trees, often outperforms random forests when individual trees perform only slightly better than random chance. This method incrementally builds the model by optimizing the residual errors of previous trees, leading to more accurate predictions.
In practice, gradient boosting can be used to fine-tune diagnostic models, improving their precision and reliability. A recent example of how gradient boosting of random forest predictions yielded highly accurate predictions for unplanned vasopressor initiation and intubation events 2 to 4 hours before an ICU adult became unstable.2
Assessing the accuracy of algorithms
The value of the data set is directly related to the accuracy of its labels. Traditional methods that measure model performance, such as sensitivity, specificity, and predictive values (PPV and NPV), have important limitations. They provide little insight into how a complex model made its prediction. Understanding which individual features drive model accuracy is key to fostering trust in model predictions. This can be done by comparing model output with and without including individual features. The results of all possible combinations are aggregated according to feature importance, which is summarized in the Shapley value for each model feature. Higher values indicate greater relative importance. SHAP plots help identify how much and how often specific features change the model output, presenting values of individual model estimates with and without a specific feature (see Figure 1).
Promoting AI use
AI and machine learning algorithms are coming to patient care. Understanding the language of AI helps caregivers integrate these tools into their practices. The science of AI faces serious challenges. Algorithms must be recalibrated to keep pace as therapies advance, disease prevalence changes, and our population ages. AI must address new challenges as they confront those suffering from respiratory diseases. This resource encourages clinicians with novel approaches by using AI methodologies to advance their development. We can better address future health care needs by promoting the equitable use of AI technologies, especially among socially disadvantaged developers.
References
1. Lilly CM, Soni AV, Dunlap D, et al. Advancing point of care testing by application of machine learning techniques and artificial intelligence. Chest. 2024 (in press).
2. Lilly CM, Kirk D, Pessach IM, et al. Application of machine learning models to biomedical and information system signals from critically ill adults. Chest. 2024;165(5):1139-1148.
AI is a group of nonhuman techniques that utilize automated learning methods to extract information from datasets through generalization, classification, prediction, and association. In other words, AI is the simulation of human intelligence processes by machines. The branches of AI include natural language processing, speech recognition, machine vision, and expert systems. AI can make clinical care more efficient; however, many find its confusing terminology to be a barrier.1 This article provides concise definitions of AI terms and is intended to help physicians better understand how AI methods can be applied to clinical care. The clinical application of natural language processing and machine vision applications are more clinically intuitive than the roles of machine learning algorithms.
Machine learning and algorithms
Machine learning is a branch of AI that uses data and algorithms to mimic human reasoning through classification, pattern recognition, and prediction. Supervised and unsupervised machine-learning algorithms can analyze data and recognize undetected associations and relationships.
Supervised learning involves training models to make predictions using data sets that have correct outcome parameters called labels using predictive fields called features. Machine learning uses iterative analysis including random forest, decision tree, and gradient boosting methods that minimize predictive error metrics (see Table 1). This approach is widely used to improve diagnoses, predict disease progression or exacerbation, and personalize treatment plan modifications.
Supervised machine learning methods can be particularly effective for processing large volumes of medical information to identify patterns and make accurate predictions. In contrast, unsupervised learning techniques can analyze unlabeled data and help clinicians uncover hidden patterns or undetected groupings. Techniques including clustering, exploratory analysis, and anomaly detection are common applications. Both of these machine-learning approaches can be used to extract novel and helpful insights.
The utility of machine learning analyses depends on the size and accuracy of the available datasets. Small datasets can limit usability, while large datasets require substantial computational power. Predictive models are generated using training datasets and evaluated using separate evaluation datasets. Deep learning models, a subset of machine learning, can automatically readjust themselves to maintain or improve accuracy when analyzing new observations that include accurate labels.
Challenges of algorithms and calibration
Machine learning algorithms vary in complexity and accuracy. For example, a simple logistic regression model using time, date, latitude, and indoor/outdoor location can accurately recommend sunscreen application. This model identifies when solar radiation is high enough to warrant sunscreen use, avoiding unnecessary recommendations during nighttime hours or indoor locations. A more complex model might suffer from model overfitting and inappropriately suggest sunscreen before a tanning salon visit.
Complex machine learning models, like support vector machine (SVM) and decision tree methods, are useful when many features have predictive power. SVMs are useful for small but complex datasets. Features are manipulated in a multidimensional space to maximize the “margins” separating 2 groups. Decision tree analyses are useful when more than 2 groups are being analyzed. SVM and decision tree models can also lose accuracy by data overfitting.
Consider the development of an SVM analysis to predict whether an individual is a fellow or a senior faculty member. One could use high gray hair density feature values to identify senior faculty. When this algorithm is applied to an individual with alopecia, no amount of model adjustment can achieve high levels of discrimination because no hair is present. Rather than overfitting the model by adding more nonpredictive features, individuals with alopecia are analyzed by their own algorithm (tree) that uses the skin wrinkle/solar damage rather than the gray hair density feature.
Decision tree ensemble algorithms like random forest and gradient boosting use feature-based decision trees to process and classify data. Random forests are robust, scalable, and versatile, providing classifications and predictions while protecting against inaccurate data and outliers and have the advantage of being able to handle both categorical and continuous features. Gradient boosting, which uses an ensemble of weak decision trees, often outperforms random forests when individual trees perform only slightly better than random chance. This method incrementally builds the model by optimizing the residual errors of previous trees, leading to more accurate predictions.
In practice, gradient boosting can be used to fine-tune diagnostic models, improving their precision and reliability. A recent example of how gradient boosting of random forest predictions yielded highly accurate predictions for unplanned vasopressor initiation and intubation events 2 to 4 hours before an ICU adult became unstable.2
Assessing the accuracy of algorithms
The value of the data set is directly related to the accuracy of its labels. Traditional methods that measure model performance, such as sensitivity, specificity, and predictive values (PPV and NPV), have important limitations. They provide little insight into how a complex model made its prediction. Understanding which individual features drive model accuracy is key to fostering trust in model predictions. This can be done by comparing model output with and without including individual features. The results of all possible combinations are aggregated according to feature importance, which is summarized in the Shapley value for each model feature. Higher values indicate greater relative importance. SHAP plots help identify how much and how often specific features change the model output, presenting values of individual model estimates with and without a specific feature (see Figure 1).
Promoting AI use
AI and machine learning algorithms are coming to patient care. Understanding the language of AI helps caregivers integrate these tools into their practices. The science of AI faces serious challenges. Algorithms must be recalibrated to keep pace as therapies advance, disease prevalence changes, and our population ages. AI must address new challenges as they confront those suffering from respiratory diseases. This resource encourages clinicians with novel approaches by using AI methodologies to advance their development. We can better address future health care needs by promoting the equitable use of AI technologies, especially among socially disadvantaged developers.
References
1. Lilly CM, Soni AV, Dunlap D, et al. Advancing point of care testing by application of machine learning techniques and artificial intelligence. Chest. 2024 (in press).
2. Lilly CM, Kirk D, Pessach IM, et al. Application of machine learning models to biomedical and information system signals from critically ill adults. Chest. 2024;165(5):1139-1148.
Use of albumin in critically ill patients
Intravenous albumin is a human-derived blood product studied widely in a variety of patient populations. Despite its frequent use in critical care, few high-quality studies have demonstrated improvements in patient-important outcomes. Compared with crystalloids, albumin increases the risk of fluid overload and bleeding and infections in patients undergoing cardiac surgery.1,2 In addition, albumin is costly, and its production is fraught with donor supply chain ethical concerns (the majority of albumin is derived from paid plasma donors).
Albumin use is highly variable between countries, hospitals, and even clinicians within the same specialty due to several factors, including the perception of minimal risk with albumin, concerns regarding insufficient short-term hemodynamic response to crystalloid, and lack of high-quality evidence to inform clinical practice. We will discuss when intensivists should consider albumin use (with prescription personalized to patient context) and when it should be avoided due to the concerns for patient harm.
An intensivist might consider albumin as a reasonable treatment option in patients with cirrhosis undergoing large volume paracentesis to prevent paracentesis-induced circulatory dysfunction, and in patients with cirrhosis and spontaneous bacterial peritonitis (SBP), as data suggests use in this setting leads to a reduction in mortality.3 Clinicians should be aware that even for these widely accepted albumin indications, which are supported by published guidelines, the certainty of evidence is low, recommendations are weak (conditional), and, therefore, albumin should always be personalized to the patient based on volume of paracentesis fluid removed, prior history of hypotension after procedures, and degree of renal dysfunction.4
There are also several conditions for which an intensivist might consider albumin and for which albumin is commonly administered but lacks high-quality studies to support its use either as a frontline or rescue fluid therapy. One such condition is type 1 hepatorenal syndrome (HRS), for which albumin is widely used; however, there are no randomized controlled trials that have compared albumin with placebo. 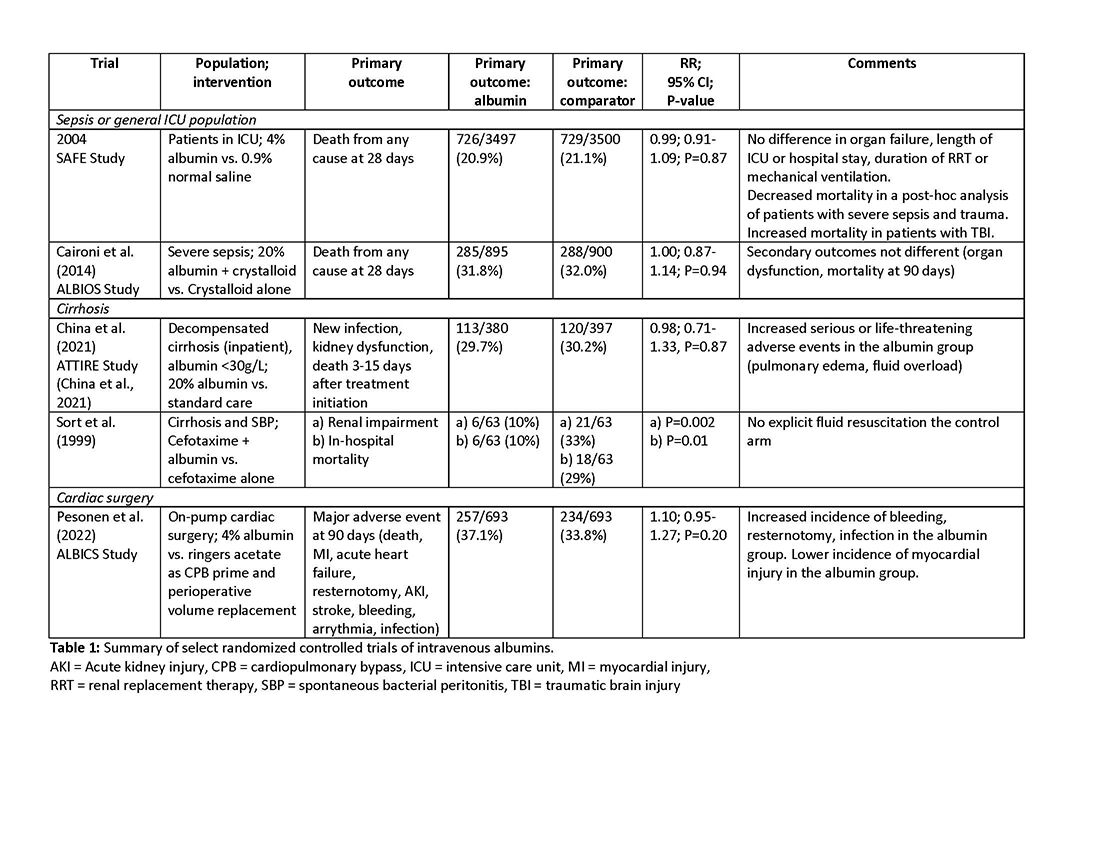
As with any intervention, the use of albumin is associated with risks. In patients undergoing on-pump cardiac surgery, the ALBICS study showed that albumin did not reduce the risk of major adverse events and, instead, increased risk of bleeding, resternotomy, and infection.2 The ATTIRE trial showed that in patients hospitalized with decompensated cirrhosis and serum albumin <30 g/L, albumin failed to reduce infection, renal impairment, or mortality while increasing life-threatening adverse events, including pulmonary edema and fluid overload.1 Similarly, in patients with cirrhosis and extraperitoneal infections, albumin showed no benefit in reducing renal impairment or mortality, and its use was associated with higher rates of pulmonary edema.6 Lastly, critically ill patients with traumatic brain injury (TBI) who received fluid resuscitation with albumin have been shown to experience higher mortality compared with saline.7 Thus, based on current evidence, intravenous albumin is not recommended for patients undergoing cardiac surgery (priming of the bypass circuit or volume replacement), patients hospitalized with decompensated cirrhosis and hypoalbuminemia, patients hospitalized with cirrhosis and extraperitoneal infections, and critically ill patients with TBI.4
Overall, intravenous albumin prescription in critical care patients requires a personalized approach informed by current best evidence and is not without potential harm. 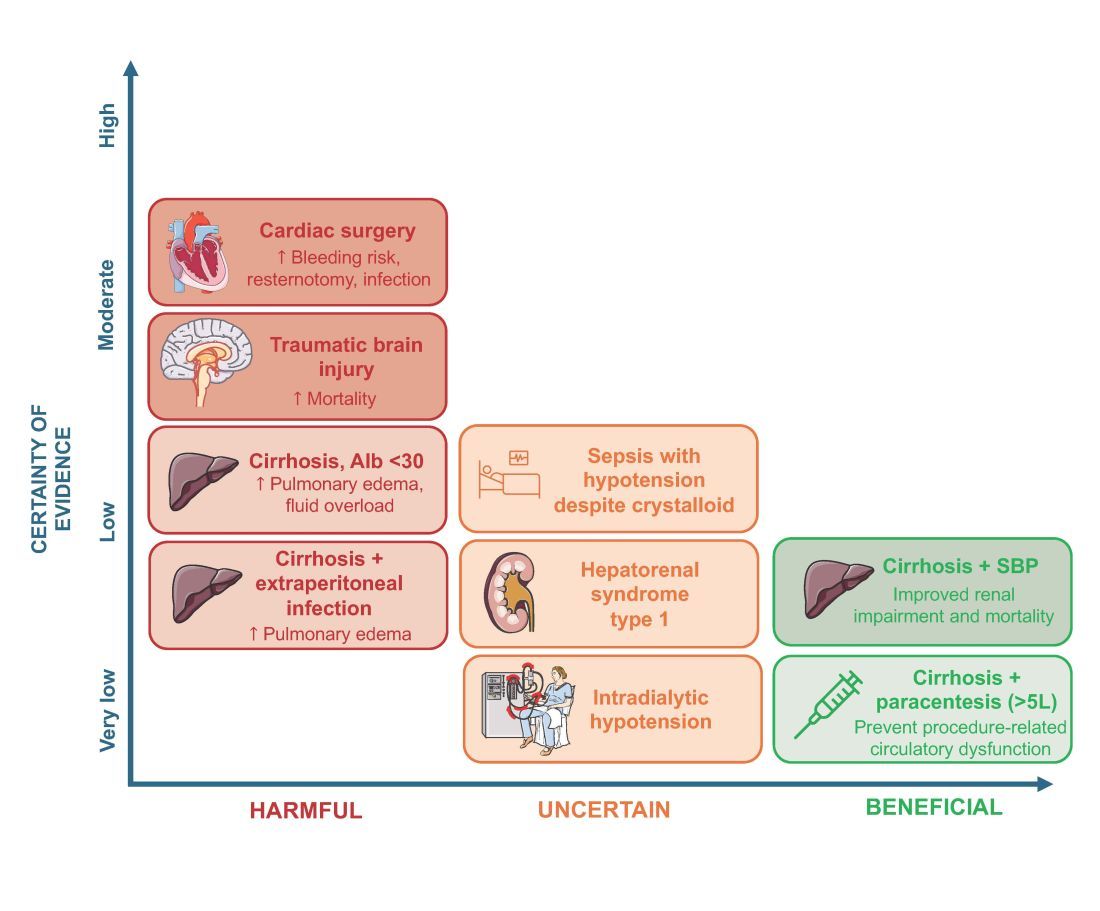
High-quality evidence is currently lacking in many clinical settings, and large randomized controlled trials are underway to provide further insights into the utility of albumin. These trials will address albumin use in the following: acute kidney injury requiring renal replacement therapy (ALTER-AKI, NCT04705896), inpatients with community-acquired pneumonia (NCT04071041), high-risk cardiac surgery (ACTRN1261900135516703), and septic shock (NCT03869385).
Financial/nonfinancial disclosures
Nicole Relke: None. Mark Hewitt: None. Bram Rochwerg: None. Jeannie Callum: Research support from Canadian Blood Services and Octapharma.
References
1. China L, Freemantle N, Forrest E, et al. A randomized trial of albumin infusions in hospitalized patients with cirrhosis. N Engl J Med. 2021;384(9):808-817. doi:10.1056/NEJMoa2022166
2. Pesonen E, Vlasov H, Suojaranta R, et al. Effect of 4% albumin solution vs ringer acetate on major adverse events in patients undergoing cardiac surgery with cardiopulmonary bypass: a randomized clinical trial. JAMA. 2022;328(3):251-258. doi:10.1001/jama.2022.10461
3. Sort P, Navasa M, Arroyo V, et al. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. NEJM. 1999;341:403-409.
4. Callum J, Skubas NJ, Bathla A, et al. Use of intravenous albumin: a guideline from the international collaboration for transfusion medicine guidelines. Chest. 2024:S0012-3692(24)00285-X. doi:10.1016/j.chest.2024.02.049
5. Torp N. High doses of albumin increases mortality and complications in terlipressin treated patients with cirrhosis: insights from the ATTIRE trial. Paper presented at the AASLD; 2023; San Diego, CA. https://www.aasld.org/the-liver-meeting/high-doses-albumin-increases-mortality-and-complications-terlipressin-treated
6. Wong YJ, Qiu TY, Tam YC, Mohan BP, Gallegos-Orozco JF, Adler DG. Efficacy and safety of IV albumin for non-spontaneous bacterial peritonitis infection among patients with cirrhosis: a systematic review and meta-analysis. Dig Liver Dis. 2020;52(10):1137-1142. doi:10.1016/j.dld.2020.05.047
7. Myburgh J, Cooper JD, Finfer S, et al. Saline or albumin for fluid resuscitation in patients with traumatic brain injury. N Engl J Med. 2007;357(9):874-884.
Intravenous albumin is a human-derived blood product studied widely in a variety of patient populations. Despite its frequent use in critical care, few high-quality studies have demonstrated improvements in patient-important outcomes. Compared with crystalloids, albumin increases the risk of fluid overload and bleeding and infections in patients undergoing cardiac surgery.1,2 In addition, albumin is costly, and its production is fraught with donor supply chain ethical concerns (the majority of albumin is derived from paid plasma donors).
Albumin use is highly variable between countries, hospitals, and even clinicians within the same specialty due to several factors, including the perception of minimal risk with albumin, concerns regarding insufficient short-term hemodynamic response to crystalloid, and lack of high-quality evidence to inform clinical practice. We will discuss when intensivists should consider albumin use (with prescription personalized to patient context) and when it should be avoided due to the concerns for patient harm.
An intensivist might consider albumin as a reasonable treatment option in patients with cirrhosis undergoing large volume paracentesis to prevent paracentesis-induced circulatory dysfunction, and in patients with cirrhosis and spontaneous bacterial peritonitis (SBP), as data suggests use in this setting leads to a reduction in mortality.3 Clinicians should be aware that even for these widely accepted albumin indications, which are supported by published guidelines, the certainty of evidence is low, recommendations are weak (conditional), and, therefore, albumin should always be personalized to the patient based on volume of paracentesis fluid removed, prior history of hypotension after procedures, and degree of renal dysfunction.4
There are also several conditions for which an intensivist might consider albumin and for which albumin is commonly administered but lacks high-quality studies to support its use either as a frontline or rescue fluid therapy. One such condition is type 1 hepatorenal syndrome (HRS), for which albumin is widely used; however, there are no randomized controlled trials that have compared albumin with placebo. 
As with any intervention, the use of albumin is associated with risks. In patients undergoing on-pump cardiac surgery, the ALBICS study showed that albumin did not reduce the risk of major adverse events and, instead, increased risk of bleeding, resternotomy, and infection.2 The ATTIRE trial showed that in patients hospitalized with decompensated cirrhosis and serum albumin <30 g/L, albumin failed to reduce infection, renal impairment, or mortality while increasing life-threatening adverse events, including pulmonary edema and fluid overload.1 Similarly, in patients with cirrhosis and extraperitoneal infections, albumin showed no benefit in reducing renal impairment or mortality, and its use was associated with higher rates of pulmonary edema.6 Lastly, critically ill patients with traumatic brain injury (TBI) who received fluid resuscitation with albumin have been shown to experience higher mortality compared with saline.7 Thus, based on current evidence, intravenous albumin is not recommended for patients undergoing cardiac surgery (priming of the bypass circuit or volume replacement), patients hospitalized with decompensated cirrhosis and hypoalbuminemia, patients hospitalized with cirrhosis and extraperitoneal infections, and critically ill patients with TBI.4
Overall, intravenous albumin prescription in critical care patients requires a personalized approach informed by current best evidence and is not without potential harm. 
High-quality evidence is currently lacking in many clinical settings, and large randomized controlled trials are underway to provide further insights into the utility of albumin. These trials will address albumin use in the following: acute kidney injury requiring renal replacement therapy (ALTER-AKI, NCT04705896), inpatients with community-acquired pneumonia (NCT04071041), high-risk cardiac surgery (ACTRN1261900135516703), and septic shock (NCT03869385).
Financial/nonfinancial disclosures
Nicole Relke: None. Mark Hewitt: None. Bram Rochwerg: None. Jeannie Callum: Research support from Canadian Blood Services and Octapharma.
References
1. China L, Freemantle N, Forrest E, et al. A randomized trial of albumin infusions in hospitalized patients with cirrhosis. N Engl J Med. 2021;384(9):808-817. doi:10.1056/NEJMoa2022166
2. Pesonen E, Vlasov H, Suojaranta R, et al. Effect of 4% albumin solution vs ringer acetate on major adverse events in patients undergoing cardiac surgery with cardiopulmonary bypass: a randomized clinical trial. JAMA. 2022;328(3):251-258. doi:10.1001/jama.2022.10461
3. Sort P, Navasa M, Arroyo V, et al. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. NEJM. 1999;341:403-409.
4. Callum J, Skubas NJ, Bathla A, et al. Use of intravenous albumin: a guideline from the international collaboration for transfusion medicine guidelines. Chest. 2024:S0012-3692(24)00285-X. doi:10.1016/j.chest.2024.02.049
5. Torp N. High doses of albumin increases mortality and complications in terlipressin treated patients with cirrhosis: insights from the ATTIRE trial. Paper presented at the AASLD; 2023; San Diego, CA. https://www.aasld.org/the-liver-meeting/high-doses-albumin-increases-mortality-and-complications-terlipressin-treated
6. Wong YJ, Qiu TY, Tam YC, Mohan BP, Gallegos-Orozco JF, Adler DG. Efficacy and safety of IV albumin for non-spontaneous bacterial peritonitis infection among patients with cirrhosis: a systematic review and meta-analysis. Dig Liver Dis. 2020;52(10):1137-1142. doi:10.1016/j.dld.2020.05.047
7. Myburgh J, Cooper JD, Finfer S, et al. Saline or albumin for fluid resuscitation in patients with traumatic brain injury. N Engl J Med. 2007;357(9):874-884.
Intravenous albumin is a human-derived blood product studied widely in a variety of patient populations. Despite its frequent use in critical care, few high-quality studies have demonstrated improvements in patient-important outcomes. Compared with crystalloids, albumin increases the risk of fluid overload and bleeding and infections in patients undergoing cardiac surgery.1,2 In addition, albumin is costly, and its production is fraught with donor supply chain ethical concerns (the majority of albumin is derived from paid plasma donors).
Albumin use is highly variable between countries, hospitals, and even clinicians within the same specialty due to several factors, including the perception of minimal risk with albumin, concerns regarding insufficient short-term hemodynamic response to crystalloid, and lack of high-quality evidence to inform clinical practice. We will discuss when intensivists should consider albumin use (with prescription personalized to patient context) and when it should be avoided due to the concerns for patient harm.
An intensivist might consider albumin as a reasonable treatment option in patients with cirrhosis undergoing large volume paracentesis to prevent paracentesis-induced circulatory dysfunction, and in patients with cirrhosis and spontaneous bacterial peritonitis (SBP), as data suggests use in this setting leads to a reduction in mortality.3 Clinicians should be aware that even for these widely accepted albumin indications, which are supported by published guidelines, the certainty of evidence is low, recommendations are weak (conditional), and, therefore, albumin should always be personalized to the patient based on volume of paracentesis fluid removed, prior history of hypotension after procedures, and degree of renal dysfunction.4
There are also several conditions for which an intensivist might consider albumin and for which albumin is commonly administered but lacks high-quality studies to support its use either as a frontline or rescue fluid therapy. One such condition is type 1 hepatorenal syndrome (HRS), for which albumin is widely used; however, there are no randomized controlled trials that have compared albumin with placebo. 
As with any intervention, the use of albumin is associated with risks. In patients undergoing on-pump cardiac surgery, the ALBICS study showed that albumin did not reduce the risk of major adverse events and, instead, increased risk of bleeding, resternotomy, and infection.2 The ATTIRE trial showed that in patients hospitalized with decompensated cirrhosis and serum albumin <30 g/L, albumin failed to reduce infection, renal impairment, or mortality while increasing life-threatening adverse events, including pulmonary edema and fluid overload.1 Similarly, in patients with cirrhosis and extraperitoneal infections, albumin showed no benefit in reducing renal impairment or mortality, and its use was associated with higher rates of pulmonary edema.6 Lastly, critically ill patients with traumatic brain injury (TBI) who received fluid resuscitation with albumin have been shown to experience higher mortality compared with saline.7 Thus, based on current evidence, intravenous albumin is not recommended for patients undergoing cardiac surgery (priming of the bypass circuit or volume replacement), patients hospitalized with decompensated cirrhosis and hypoalbuminemia, patients hospitalized with cirrhosis and extraperitoneal infections, and critically ill patients with TBI.4
Overall, intravenous albumin prescription in critical care patients requires a personalized approach informed by current best evidence and is not without potential harm. 
High-quality evidence is currently lacking in many clinical settings, and large randomized controlled trials are underway to provide further insights into the utility of albumin. These trials will address albumin use in the following: acute kidney injury requiring renal replacement therapy (ALTER-AKI, NCT04705896), inpatients with community-acquired pneumonia (NCT04071041), high-risk cardiac surgery (ACTRN1261900135516703), and septic shock (NCT03869385).
Financial/nonfinancial disclosures
Nicole Relke: None. Mark Hewitt: None. Bram Rochwerg: None. Jeannie Callum: Research support from Canadian Blood Services and Octapharma.
References
1. China L, Freemantle N, Forrest E, et al. A randomized trial of albumin infusions in hospitalized patients with cirrhosis. N Engl J Med. 2021;384(9):808-817. doi:10.1056/NEJMoa2022166
2. Pesonen E, Vlasov H, Suojaranta R, et al. Effect of 4% albumin solution vs ringer acetate on major adverse events in patients undergoing cardiac surgery with cardiopulmonary bypass: a randomized clinical trial. JAMA. 2022;328(3):251-258. doi:10.1001/jama.2022.10461
3. Sort P, Navasa M, Arroyo V, et al. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. NEJM. 1999;341:403-409.
4. Callum J, Skubas NJ, Bathla A, et al. Use of intravenous albumin: a guideline from the international collaboration for transfusion medicine guidelines. Chest. 2024:S0012-3692(24)00285-X. doi:10.1016/j.chest.2024.02.049
5. Torp N. High doses of albumin increases mortality and complications in terlipressin treated patients with cirrhosis: insights from the ATTIRE trial. Paper presented at the AASLD; 2023; San Diego, CA. https://www.aasld.org/the-liver-meeting/high-doses-albumin-increases-mortality-and-complications-terlipressin-treated
6. Wong YJ, Qiu TY, Tam YC, Mohan BP, Gallegos-Orozco JF, Adler DG. Efficacy and safety of IV albumin for non-spontaneous bacterial peritonitis infection among patients with cirrhosis: a systematic review and meta-analysis. Dig Liver Dis. 2020;52(10):1137-1142. doi:10.1016/j.dld.2020.05.047
7. Myburgh J, Cooper JD, Finfer S, et al. Saline or albumin for fluid resuscitation in patients with traumatic brain injury. N Engl J Med. 2007;357(9):874-884.
Hospital-onset sepsis: Why the brouhaha?
A 47-year-old woman with a history of cirrhosis is admitted with an acute kidney injury and altered mental status. On the initial workup, there are no signs of infection, and dehydration is determined to be the cause of the kidney injury. There are signs of improvement in the kidney injury with hydration. On hospital day 3, the patient develops a fever (101.9 oF) with accompanying leukocytosis to 14,000. Concerned for infection, the team starts empiric broad spectrum antibiotics for presumed spontaneous bacterial peritonitis. The next day (hospital day 4), a rapid response evaluation is activated as the patient is demonstrating increasing confusion, hypotension with a systolic blood pressure of 70 mm Hg, and elevated lactic acid. The patient receives 1 L of normal saline and transfers to the ICU. The new critical care fellow, who has just read up on sepsis early management bundles, and specifically the Severe Sepsis and Septic Shock Management Bundle (SEP-1), is reviewing the chart and notices a history of multidrug-resistant organisms in her urine cultures from an admission 2 months ago. They ask of the transferring team, “When was time zero, and was the 3-hour bundle completed?”
1 The excessive cost, both of human life and monetary, has led to the commitment of significant resources to sepsis care. Improved recognition and timely intervention for sepsis have led to noteworthy improvement in mortality. Most of this effort has been directed toward patients with sepsis diagnosed in the emergency department (ED) who are presenting with community-onset sepsis (COS). A new entity, called hospital-onset sepsis (HOS), has been described recently, defined by the Centers for Disease Control and Prevention (CDC) as both infection and organ dysfunction developing more than 48 hours after hospital admission.2
A systematic review of 51 studies found approximately 23.6% of all sepsis cases are HOS. The proportion of HOS is even higher (more than 45%) in patients admitted to the ICU with sepsis.3 The outcome for this group remains comparatively poor. The hospital mortality among patients with HOS is 35%, which increases to 52% with progression to septic shock compared with 25% with COS.3 Even after adjusting for baseline factors that make one prone to developing infection in the hospital, a patient developing HOS has three-times a higher risk of dying compared with a patient who never developed sepsis and two-times a higher risk of dying compared with patients with COS.4Furthermore, HOS utilizes more resources with significantly longer ICU and hospital stays and has five-times the hospital cost compared with COS.4
The two most crucial factors in improving sepsis outcomes, as identified by the Surviving Sepsis Campaign guidelines, are: 1) prompt identification and treatment within the first few hours of onset and 2) regular reevaluation of the patient’s response to treatment.
Prompt identification
Diagnosing sepsis in the patient who is hospitalized is challenging. Patients admitted to the hospital often have competing comorbidities, have existing organ failure, or are in a postoperative/intervention state that clouds the application and interpretation of vital sign triggers customarily used to identify sepsis. The positive predictive value for all existing sepsis definitions and diagnostic criteria is dismally low. 5 And while automated electronic sepsis alerts may improve processes of care, they still have poor positive predictive value and have not impacted patient-centered outcomes (mortality or length of stay). Furthermore, the causative microorganisms often associated with hospital-acquired infections are complex, are drug-resistant, and can have courses which further delay identification. Finally, cognitive errors, such as anchoring biases or premature diagnosis closure, can contribute to provider-level identification delays that are only further exacerbated by system issues, such as capacity constraints, staffing issues, and differing paces between wards that tend to impede time-sensitive evaluations and interventions. 4,6,7
Management
The SEP-1 core measure uses a framework of early recognition of infection and completion of the sepsis bundles in a timely manner to improve outcomes. Patients with HOS are less likely than those with COS to receive Centers for Medicare & Medicaid Services SEP-1-compliant care, including timely blood culture collection, initial and repeat lactate testing, and fluid resuscitation.8 The Surviving Sepsis Campaign has explored barriers to managing HOS. Among caregivers, these include delay in recognition, poor communication regarding change in patient status, not prioritizing treatment for sepsis, failure to measure lactate, delayed or no antimicrobial administration, and inadequate fluid resuscitation. In one study, the adherence to SEP-1 for HOS was reported at 13% compared with 39.9% in COS. The differences in initial sepsis management included timing of antimicrobials and fluid resuscitation, which accounted for 23% of observed greater mortality risk among patients with HOS compared with COS.6,8 It remains unclear how these recommendations should be applied and whether some of these recommendations confer the same benefits for patients with HOS as for those with COS. For example, administration of fluids conferred no additional benefit to patients with HOS, while rapid antimicrobial administration was shown to be associated with improved mortality in patients with HOS. Although, the optimal timing for treatment initiation and microbial coverage has not been established.
The path forward
Effective HOS management requires both individual and systematic approaches. How clinicians identify a patient with sepsis must be context-dependent. Although standard criteria exist for defining sepsis, the approach to a patient presenting to the ED from home should differ from that of a patient who has been hospitalized for several days, is postoperative, or is in the ICU on multiple forms of life support. Clinical medicine is context-dependent, and the same principles apply to sepsis management. To address the diagnostic uncertainty of the syndrome, providers must remain vigilant and maintain a clinical “iterative urgency” in diagnosing and managing sepsis. While machine learning algorithms have potential, they still rely on human intervention and interaction to navigate the complexities of HOS diagnosis.
At the system level, survival from sepsis is determined by the speed with which complex medical care is delivered and the effectiveness with which resources and personnel are mobilized and coordinated. The Hospital Sepsis Program Core Elements, released by the CDC, serves as an initial playbook to aid hospitals in establishing comprehensive sepsis improvement programs.
A second invaluable resource for hospitals in sepsis management is the rapid response team (RRT). Studies have shown that resolute RRTs can enhance patient outcomes and compliance with sepsis bundles; though, the composition and scope of these teams are crucial to their effectiveness. Responding to in-hospital emergencies and urgencies without conflicting responsibilities is an essential feature of a successful RRT. Often, they are familiar with bundles, protocols, and documentation, and members of these teams can offer clinical and/or technical expertise as well as support active participation and reengagement with bedside staff, which fosters trust and collaboration. This partnership is key, as these interactions instill a common mission and foster a culture of sepsis improvement that is required to achieve sustained success and improved patient outcomes.
Dr. Dugar is Director, Point-of-Care Ultrasound, Department of Critical Care, Respiratory Institute, Assistant Professor, Cleveland Clinic Lerner College of Medicine, Cleveland, OH. Dr. Jayaprakash is Associate Medical Director, Quality, Emergency Medicine, Physician Lead, Henry Ford Health Sepsis Program. Dr. Reilkoff is Executive Medical Director of Critical Care, M Health Fairview Intensive Care Units, Director of Acting Internship in Critical Care, University of Minnesota Medical School, Associate Professor of Medicine and Surgery, University of Minnesota. Dr. Duggal is Vice-Chair, Department of Critical Care, Respiratory Institute, Director, Critical Care Clinical Research, Associate Professor, Cleveland Clinic Lerner College of Medicine, Cleveland, OH
References
1. Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016;315(8):801-810.
2. Ginestra JC, Coz Yataco AO, Dugar SP, Dettmer MR. Hospital-onset sepsis warrants expanded investigation and consideration as a unique clinical entity. Chest. 2024;S0012-3692(24):00039-4.
3. Markwart R, Saito H, Harder T, et al. Epidemiology and burden of sepsis acquired in hospitals and intensive care units: a systematic review and meta-analysis. Intensive Care Med. 2020;46(8):1536-1551.
4. Rhee C, Wang R, Zhang Z, et al. Epidemiology of hospital-onset versus community-onset sepsis in U.S. hospitals and association with mortality: a retrospective analysis using electronic clinical data. Crit Care Med. 2019;47(9):1169-1176.
5. Wong A, Otles E, Donnelly JP, et al. External validation of a widely implemented proprietary sepsis prediction model in hospitalized patients. JAMA Intern Med. 2021;181(8):1065-1070.
6. Baghdadi JD, Brook RH, Uslan DZ, et al. Association of a care bundle for early sepsis management with mortality among patients with hospital-onset or community-onset sepsis. JAMA Intern Med. 2020;180(5):707-716.
7. Baghdadi JD, Wong MD, Uslan DZ, et al. Adherence to the SEP-1 sepsis bundle in hospital-onset v. community-onset sepsis: a multicenter retrospective cohort study. J Gen Intern Med. 2020;35(4):1153-1160.
8. Basheer A. Patients with hospital-onset sepsis are less likely to receive sepsis bundle care than those with community-onset sepsis. Evid Based Nurs. 2021;24(3):99.
A 47-year-old woman with a history of cirrhosis is admitted with an acute kidney injury and altered mental status. On the initial workup, there are no signs of infection, and dehydration is determined to be the cause of the kidney injury. There are signs of improvement in the kidney injury with hydration. On hospital day 3, the patient develops a fever (101.9 oF) with accompanying leukocytosis to 14,000. Concerned for infection, the team starts empiric broad spectrum antibiotics for presumed spontaneous bacterial peritonitis. The next day (hospital day 4), a rapid response evaluation is activated as the patient is demonstrating increasing confusion, hypotension with a systolic blood pressure of 70 mm Hg, and elevated lactic acid. The patient receives 1 L of normal saline and transfers to the ICU. The new critical care fellow, who has just read up on sepsis early management bundles, and specifically the Severe Sepsis and Septic Shock Management Bundle (SEP-1), is reviewing the chart and notices a history of multidrug-resistant organisms in her urine cultures from an admission 2 months ago. They ask of the transferring team, “When was time zero, and was the 3-hour bundle completed?”
1 The excessive cost, both of human life and monetary, has led to the commitment of significant resources to sepsis care. Improved recognition and timely intervention for sepsis have led to noteworthy improvement in mortality. Most of this effort has been directed toward patients with sepsis diagnosed in the emergency department (ED) who are presenting with community-onset sepsis (COS). A new entity, called hospital-onset sepsis (HOS), has been described recently, defined by the Centers for Disease Control and Prevention (CDC) as both infection and organ dysfunction developing more than 48 hours after hospital admission.2
A systematic review of 51 studies found approximately 23.6% of all sepsis cases are HOS. The proportion of HOS is even higher (more than 45%) in patients admitted to the ICU with sepsis.3 The outcome for this group remains comparatively poor. The hospital mortality among patients with HOS is 35%, which increases to 52% with progression to septic shock compared with 25% with COS.3 Even after adjusting for baseline factors that make one prone to developing infection in the hospital, a patient developing HOS has three-times a higher risk of dying compared with a patient who never developed sepsis and two-times a higher risk of dying compared with patients with COS.4Furthermore, HOS utilizes more resources with significantly longer ICU and hospital stays and has five-times the hospital cost compared with COS.4
The two most crucial factors in improving sepsis outcomes, as identified by the Surviving Sepsis Campaign guidelines, are: 1) prompt identification and treatment within the first few hours of onset and 2) regular reevaluation of the patient’s response to treatment.
Prompt identification
Diagnosing sepsis in the patient who is hospitalized is challenging. Patients admitted to the hospital often have competing comorbidities, have existing organ failure, or are in a postoperative/intervention state that clouds the application and interpretation of vital sign triggers customarily used to identify sepsis. The positive predictive value for all existing sepsis definitions and diagnostic criteria is dismally low. 5 And while automated electronic sepsis alerts may improve processes of care, they still have poor positive predictive value and have not impacted patient-centered outcomes (mortality or length of stay). Furthermore, the causative microorganisms often associated with hospital-acquired infections are complex, are drug-resistant, and can have courses which further delay identification. Finally, cognitive errors, such as anchoring biases or premature diagnosis closure, can contribute to provider-level identification delays that are only further exacerbated by system issues, such as capacity constraints, staffing issues, and differing paces between wards that tend to impede time-sensitive evaluations and interventions. 4,6,7
Management
The SEP-1 core measure uses a framework of early recognition of infection and completion of the sepsis bundles in a timely manner to improve outcomes. Patients with HOS are less likely than those with COS to receive Centers for Medicare & Medicaid Services SEP-1-compliant care, including timely blood culture collection, initial and repeat lactate testing, and fluid resuscitation.8 The Surviving Sepsis Campaign has explored barriers to managing HOS. Among caregivers, these include delay in recognition, poor communication regarding change in patient status, not prioritizing treatment for sepsis, failure to measure lactate, delayed or no antimicrobial administration, and inadequate fluid resuscitation. In one study, the adherence to SEP-1 for HOS was reported at 13% compared with 39.9% in COS. The differences in initial sepsis management included timing of antimicrobials and fluid resuscitation, which accounted for 23% of observed greater mortality risk among patients with HOS compared with COS.6,8 It remains unclear how these recommendations should be applied and whether some of these recommendations confer the same benefits for patients with HOS as for those with COS. For example, administration of fluids conferred no additional benefit to patients with HOS, while rapid antimicrobial administration was shown to be associated with improved mortality in patients with HOS. Although, the optimal timing for treatment initiation and microbial coverage has not been established.
The path forward
Effective HOS management requires both individual and systematic approaches. How clinicians identify a patient with sepsis must be context-dependent. Although standard criteria exist for defining sepsis, the approach to a patient presenting to the ED from home should differ from that of a patient who has been hospitalized for several days, is postoperative, or is in the ICU on multiple forms of life support. Clinical medicine is context-dependent, and the same principles apply to sepsis management. To address the diagnostic uncertainty of the syndrome, providers must remain vigilant and maintain a clinical “iterative urgency” in diagnosing and managing sepsis. While machine learning algorithms have potential, they still rely on human intervention and interaction to navigate the complexities of HOS diagnosis.
At the system level, survival from sepsis is determined by the speed with which complex medical care is delivered and the effectiveness with which resources and personnel are mobilized and coordinated. The Hospital Sepsis Program Core Elements, released by the CDC, serves as an initial playbook to aid hospitals in establishing comprehensive sepsis improvement programs.
A second invaluable resource for hospitals in sepsis management is the rapid response team (RRT). Studies have shown that resolute RRTs can enhance patient outcomes and compliance with sepsis bundles; though, the composition and scope of these teams are crucial to their effectiveness. Responding to in-hospital emergencies and urgencies without conflicting responsibilities is an essential feature of a successful RRT. Often, they are familiar with bundles, protocols, and documentation, and members of these teams can offer clinical and/or technical expertise as well as support active participation and reengagement with bedside staff, which fosters trust and collaboration. This partnership is key, as these interactions instill a common mission and foster a culture of sepsis improvement that is required to achieve sustained success and improved patient outcomes.
Dr. Dugar is Director, Point-of-Care Ultrasound, Department of Critical Care, Respiratory Institute, Assistant Professor, Cleveland Clinic Lerner College of Medicine, Cleveland, OH. Dr. Jayaprakash is Associate Medical Director, Quality, Emergency Medicine, Physician Lead, Henry Ford Health Sepsis Program. Dr. Reilkoff is Executive Medical Director of Critical Care, M Health Fairview Intensive Care Units, Director of Acting Internship in Critical Care, University of Minnesota Medical School, Associate Professor of Medicine and Surgery, University of Minnesota. Dr. Duggal is Vice-Chair, Department of Critical Care, Respiratory Institute, Director, Critical Care Clinical Research, Associate Professor, Cleveland Clinic Lerner College of Medicine, Cleveland, OH
References
1. Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016;315(8):801-810.
2. Ginestra JC, Coz Yataco AO, Dugar SP, Dettmer MR. Hospital-onset sepsis warrants expanded investigation and consideration as a unique clinical entity. Chest. 2024;S0012-3692(24):00039-4.
3. Markwart R, Saito H, Harder T, et al. Epidemiology and burden of sepsis acquired in hospitals and intensive care units: a systematic review and meta-analysis. Intensive Care Med. 2020;46(8):1536-1551.
4. Rhee C, Wang R, Zhang Z, et al. Epidemiology of hospital-onset versus community-onset sepsis in U.S. hospitals and association with mortality: a retrospective analysis using electronic clinical data. Crit Care Med. 2019;47(9):1169-1176.
5. Wong A, Otles E, Donnelly JP, et al. External validation of a widely implemented proprietary sepsis prediction model in hospitalized patients. JAMA Intern Med. 2021;181(8):1065-1070.
6. Baghdadi JD, Brook RH, Uslan DZ, et al. Association of a care bundle for early sepsis management with mortality among patients with hospital-onset or community-onset sepsis. JAMA Intern Med. 2020;180(5):707-716.
7. Baghdadi JD, Wong MD, Uslan DZ, et al. Adherence to the SEP-1 sepsis bundle in hospital-onset v. community-onset sepsis: a multicenter retrospective cohort study. J Gen Intern Med. 2020;35(4):1153-1160.
8. Basheer A. Patients with hospital-onset sepsis are less likely to receive sepsis bundle care than those with community-onset sepsis. Evid Based Nurs. 2021;24(3):99.
A 47-year-old woman with a history of cirrhosis is admitted with an acute kidney injury and altered mental status. On the initial workup, there are no signs of infection, and dehydration is determined to be the cause of the kidney injury. There are signs of improvement in the kidney injury with hydration. On hospital day 3, the patient develops a fever (101.9 oF) with accompanying leukocytosis to 14,000. Concerned for infection, the team starts empiric broad spectrum antibiotics for presumed spontaneous bacterial peritonitis. The next day (hospital day 4), a rapid response evaluation is activated as the patient is demonstrating increasing confusion, hypotension with a systolic blood pressure of 70 mm Hg, and elevated lactic acid. The patient receives 1 L of normal saline and transfers to the ICU. The new critical care fellow, who has just read up on sepsis early management bundles, and specifically the Severe Sepsis and Septic Shock Management Bundle (SEP-1), is reviewing the chart and notices a history of multidrug-resistant organisms in her urine cultures from an admission 2 months ago. They ask of the transferring team, “When was time zero, and was the 3-hour bundle completed?”
1 The excessive cost, both of human life and monetary, has led to the commitment of significant resources to sepsis care. Improved recognition and timely intervention for sepsis have led to noteworthy improvement in mortality. Most of this effort has been directed toward patients with sepsis diagnosed in the emergency department (ED) who are presenting with community-onset sepsis (COS). A new entity, called hospital-onset sepsis (HOS), has been described recently, defined by the Centers for Disease Control and Prevention (CDC) as both infection and organ dysfunction developing more than 48 hours after hospital admission.2
A systematic review of 51 studies found approximately 23.6% of all sepsis cases are HOS. The proportion of HOS is even higher (more than 45%) in patients admitted to the ICU with sepsis.3 The outcome for this group remains comparatively poor. The hospital mortality among patients with HOS is 35%, which increases to 52% with progression to septic shock compared with 25% with COS.3 Even after adjusting for baseline factors that make one prone to developing infection in the hospital, a patient developing HOS has three-times a higher risk of dying compared with a patient who never developed sepsis and two-times a higher risk of dying compared with patients with COS.4Furthermore, HOS utilizes more resources with significantly longer ICU and hospital stays and has five-times the hospital cost compared with COS.4
The two most crucial factors in improving sepsis outcomes, as identified by the Surviving Sepsis Campaign guidelines, are: 1) prompt identification and treatment within the first few hours of onset and 2) regular reevaluation of the patient’s response to treatment.
Prompt identification
Diagnosing sepsis in the patient who is hospitalized is challenging. Patients admitted to the hospital often have competing comorbidities, have existing organ failure, or are in a postoperative/intervention state that clouds the application and interpretation of vital sign triggers customarily used to identify sepsis. The positive predictive value for all existing sepsis definitions and diagnostic criteria is dismally low. 5 And while automated electronic sepsis alerts may improve processes of care, they still have poor positive predictive value and have not impacted patient-centered outcomes (mortality or length of stay). Furthermore, the causative microorganisms often associated with hospital-acquired infections are complex, are drug-resistant, and can have courses which further delay identification. Finally, cognitive errors, such as anchoring biases or premature diagnosis closure, can contribute to provider-level identification delays that are only further exacerbated by system issues, such as capacity constraints, staffing issues, and differing paces between wards that tend to impede time-sensitive evaluations and interventions. 4,6,7
Management
The SEP-1 core measure uses a framework of early recognition of infection and completion of the sepsis bundles in a timely manner to improve outcomes. Patients with HOS are less likely than those with COS to receive Centers for Medicare & Medicaid Services SEP-1-compliant care, including timely blood culture collection, initial and repeat lactate testing, and fluid resuscitation.8 The Surviving Sepsis Campaign has explored barriers to managing HOS. Among caregivers, these include delay in recognition, poor communication regarding change in patient status, not prioritizing treatment for sepsis, failure to measure lactate, delayed or no antimicrobial administration, and inadequate fluid resuscitation. In one study, the adherence to SEP-1 for HOS was reported at 13% compared with 39.9% in COS. The differences in initial sepsis management included timing of antimicrobials and fluid resuscitation, which accounted for 23% of observed greater mortality risk among patients with HOS compared with COS.6,8 It remains unclear how these recommendations should be applied and whether some of these recommendations confer the same benefits for patients with HOS as for those with COS. For example, administration of fluids conferred no additional benefit to patients with HOS, while rapid antimicrobial administration was shown to be associated with improved mortality in patients with HOS. Although, the optimal timing for treatment initiation and microbial coverage has not been established.
The path forward
Effective HOS management requires both individual and systematic approaches. How clinicians identify a patient with sepsis must be context-dependent. Although standard criteria exist for defining sepsis, the approach to a patient presenting to the ED from home should differ from that of a patient who has been hospitalized for several days, is postoperative, or is in the ICU on multiple forms of life support. Clinical medicine is context-dependent, and the same principles apply to sepsis management. To address the diagnostic uncertainty of the syndrome, providers must remain vigilant and maintain a clinical “iterative urgency” in diagnosing and managing sepsis. While machine learning algorithms have potential, they still rely on human intervention and interaction to navigate the complexities of HOS diagnosis.
At the system level, survival from sepsis is determined by the speed with which complex medical care is delivered and the effectiveness with which resources and personnel are mobilized and coordinated. The Hospital Sepsis Program Core Elements, released by the CDC, serves as an initial playbook to aid hospitals in establishing comprehensive sepsis improvement programs.
A second invaluable resource for hospitals in sepsis management is the rapid response team (RRT). Studies have shown that resolute RRTs can enhance patient outcomes and compliance with sepsis bundles; though, the composition and scope of these teams are crucial to their effectiveness. Responding to in-hospital emergencies and urgencies without conflicting responsibilities is an essential feature of a successful RRT. Often, they are familiar with bundles, protocols, and documentation, and members of these teams can offer clinical and/or technical expertise as well as support active participation and reengagement with bedside staff, which fosters trust and collaboration. This partnership is key, as these interactions instill a common mission and foster a culture of sepsis improvement that is required to achieve sustained success and improved patient outcomes.
Dr. Dugar is Director, Point-of-Care Ultrasound, Department of Critical Care, Respiratory Institute, Assistant Professor, Cleveland Clinic Lerner College of Medicine, Cleveland, OH. Dr. Jayaprakash is Associate Medical Director, Quality, Emergency Medicine, Physician Lead, Henry Ford Health Sepsis Program. Dr. Reilkoff is Executive Medical Director of Critical Care, M Health Fairview Intensive Care Units, Director of Acting Internship in Critical Care, University of Minnesota Medical School, Associate Professor of Medicine and Surgery, University of Minnesota. Dr. Duggal is Vice-Chair, Department of Critical Care, Respiratory Institute, Director, Critical Care Clinical Research, Associate Professor, Cleveland Clinic Lerner College of Medicine, Cleveland, OH
References
1. Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016;315(8):801-810.
2. Ginestra JC, Coz Yataco AO, Dugar SP, Dettmer MR. Hospital-onset sepsis warrants expanded investigation and consideration as a unique clinical entity. Chest. 2024;S0012-3692(24):00039-4.
3. Markwart R, Saito H, Harder T, et al. Epidemiology and burden of sepsis acquired in hospitals and intensive care units: a systematic review and meta-analysis. Intensive Care Med. 2020;46(8):1536-1551.
4. Rhee C, Wang R, Zhang Z, et al. Epidemiology of hospital-onset versus community-onset sepsis in U.S. hospitals and association with mortality: a retrospective analysis using electronic clinical data. Crit Care Med. 2019;47(9):1169-1176.
5. Wong A, Otles E, Donnelly JP, et al. External validation of a widely implemented proprietary sepsis prediction model in hospitalized patients. JAMA Intern Med. 2021;181(8):1065-1070.
6. Baghdadi JD, Brook RH, Uslan DZ, et al. Association of a care bundle for early sepsis management with mortality among patients with hospital-onset or community-onset sepsis. JAMA Intern Med. 2020;180(5):707-716.
7. Baghdadi JD, Wong MD, Uslan DZ, et al. Adherence to the SEP-1 sepsis bundle in hospital-onset v. community-onset sepsis: a multicenter retrospective cohort study. J Gen Intern Med. 2020;35(4):1153-1160.
8. Basheer A. Patients with hospital-onset sepsis are less likely to receive sepsis bundle care than those with community-onset sepsis. Evid Based Nurs. 2021;24(3):99.
Implementing a critical care TEE program at your institution
Starting from the ground up!
Bedside-focused cardiac ultrasound assessment, or cardiac point-of-care ultrasound (POCUS), has become common in intensive care units throughout the US and the world.
However, obtaining images adequate for decision making via standard transthoracic echo (TTE) is not possible in a significant number of patients; as high as 30% of critically ill patients, according to The American Society of Echocardiography (ASE) guidelines.1 Factors common to critically ill patients, such as invasive mechanical ventilation, external dressings, and limited mobility, contribute to poor image acquisition.
In almost all these cases, the factors limiting image acquisition can be eliminated by utilizing a transesophageal approach. In a recent study, researchers were able to demonstrate that adding transesophageal echocardiography (TEE) to TTE in critically ill patients yielded a new diagnosis or a change in management about 45% of the time.2
Using transesophageal ultrasound for a focused cardiac assessment in hemodynamically unstable patients is not new—and is often referred to as rescue TEE or resuscitative TEE. A broader term, transesophageal ultrasound, has also been used to include sonographic evaluation of the lungs in patients with poor acoustic windows. At my institution, we use the term critical care TEE to define TEE performed by a noncardiology-trained intensivist in an intubated critically ill patient.
Regardless of the term, the use of transesophageal ultrasound by the noncardiologist in the ICU appears to be a developing trend. As with other uses of POCUS, ultrasound machines continue to be able to “do more” at a lower price point. In 2024, several cart-based ultrasound machines are compatible with transesophageal probes and contain software packages capable of common cardiac measurements.
Despite this growing interest, intensivists are likely to encounter barriers to implementing critical care TEE. Our division recently implemented adding TEE to our practice. Our practice involves two separate systems: a Veterans Administration hospital and a university-based county hospital. Our division has integrated the use of TEE in the medical ICU at both institutions. Having navigated the process at both institutions, I can offer some guidance in navigating barriers.
The development of a critical care TEE program must start with a strong base in transthoracic cardiac POCUS, at least for the foreseeable future. Having a strong background in TTE gives learners a solid foundation in cardiac anatomy, cardiac function, and ultrasound properties. Obtaining testamur status or board certification in critical care echocardiography is not an absolute must but is a definite benefit. Having significant experience in TTE image acquisition and interpretation will flatten the learning curve for TEE. Interestingly, image acquisition in TEE is often easier than in TTE, so the paradigm of learning TTE before TEE may reverse in the years to come.
Two barriers often work together to create a vicious cycle that stops the development of a TEE program at its start. These barriers include the lack of training and lack of equipment, specifically a TEE probe. Those who do not understand the value of TEE may ask, “Why purchase equipment for a procedure that you do not yet know how to do?” The opposite question can also be asked, “Why get trained to do something you don’t have the equipment to perform?”
My best advice to break this cycle is to “dive in” to whichever barrier seems easier to overcome first. I started with obtaining knowledge and training. Obtaining training and education in a procedure that is historically not done in your specialty is challenging but is not impossible. It takes a combination of high levels of self-motivation and at least one colleague with the training to support you. I approached a cardiac anesthesiologist, whom I knew from the surgical ICU. Cardiologists can also be a resource, but working with cardiac anesthesiologists offers several advantages. TEEs done by cardiac anesthesiologists are similar to those done in ICU patients (ie, all patients are intubated and sedated). The procedures are also scheduled several days in advance, making it easier to integrate training into your daily work schedule. Lastly, the TEE probe remains in place for several hours, so repeating the probe manipulations again as a learner does not add additional risk to the patient. In my case, we somewhat arbitrarily agreed that I participate in 25 TEE exams. (CME courses, both online and in-person simulation, exist and greatly supplement self-study.)
Obtaining equipment is also a common barrier, though this has become less restrictive in the last several years. As previously mentioned, many cart-based ultrasound machines can accommodate a TEE probe. This changes the request from purchasing a new machine to “just a probe.” Despite the higher cost than most other probes, those in charge of purchasing are often more open to purchasing “a probe” than to purchasing an ultrasound machine.
Additionally, the purchasing decision regarding probes may fall to a different person than it does for an ultrasound machine. If available, POCUS image archiving into the medical record can help offset the cost of equipment, both by increasing revenue via billing and by demonstrating that equipment is being used. If initially declined, continue to ask and work to integrate the purchase into the next year’s budget. Inquire about the process of making a formal request and follow that process. This will often involve obtaining a quote or quotes from the ultrasound manufacturer(s).
Keep in mind that the probe will require a special storage cabinet specifically designed for TEE probes. It is prudent to include this in budget requests. If needed, the echocardiography lab can be a useful resource for additional information regarding the cabinet requirements. It is strongly recommended to discuss TEE probe models with sterile processing before any purchasing. If options are available, it is wise to choose a model the hospital already uses, as the cleaning protocol is well established. Our unit purchased a model that did not have an established protocol, which took nearly 6 months to develop. If probe options are limited, involving sterile processing early to start developing a protocol will help decrease delays.
Obtaining hospital privileges is also a common barrier, though this may not be as challenging as expected. Hospitals typically have well-outlined policies on obtaining privileges for established procedures. One of our hospital systems had four different options; the most straightforward required 20 hours of CME specific to TEE and 10 supervised cases by a proctor currently holding TEE privileges (see Table 1).
Discussions about obtaining privileges should involve your division chief, chair of medicine, and the cardiology division chief. Clearly outlining the plan to perform this procedure only in critically ill patients who are already intubated for other reasons made these conversations go much more smoothly. In the development of delineation of privileges, we used the term critical care TEE to clearly define this patient population. During these conversations, highlight the safety of the procedure; ASE guidelines3 estimate a severe complication rate of less than 1 in 10,000 cases and explain the anticipated benefits to critically ill patients.
In conclusion, at an institution that is already adept at the use of POCUS in the ICU, the additional of critical care TEE within 1 to 2 years is a very realistic achievement. It will undoubtedly require patience, persistence, and self-motivation, but the barriers are becoming smaller every day. Stay motivated!
Dr. Proud is Associate Professor of Medicine, Division of Pulmonary and Critical Care Medicine, Pulmonary and Critical Care Medicine Program Director, UT Health San Antonio.
References:
1. Porter TR, Abdelmoneim S, Belcik FT, et al. Guidelines for the cardiac sonographer in the performance of contrast echocardiography: a focused update from the American Society of Echocardiography. J Am Soc Echocardiogr. 2024;27(8):797-810.
2. Si X, Ma J, Cao DY, et al. Transesophageal echocardiography instead or in addition to transthoracic echocardiography in evaluating haemodynamic problems in intubated critically ill patients. Ann Transl Med. 2020;8(12):785.
3. Hahn RT, Abraham T, Adams MS, et al. Guidelines for performing a cmprehensive transesophageal echocardiographic examination: recommendations from the American Society of Echocardioraphy and the Society of Cardiovascular Anesthesiologists. J Am Soc Echocardiogr. 2013;26(9):921-964.
Starting from the ground up!
Starting from the ground up!
Bedside-focused cardiac ultrasound assessment, or cardiac point-of-care ultrasound (POCUS), has become common in intensive care units throughout the US and the world.
However, obtaining images adequate for decision making via standard transthoracic echo (TTE) is not possible in a significant number of patients; as high as 30% of critically ill patients, according to The American Society of Echocardiography (ASE) guidelines.1 Factors common to critically ill patients, such as invasive mechanical ventilation, external dressings, and limited mobility, contribute to poor image acquisition.
In almost all these cases, the factors limiting image acquisition can be eliminated by utilizing a transesophageal approach. In a recent study, researchers were able to demonstrate that adding transesophageal echocardiography (TEE) to TTE in critically ill patients yielded a new diagnosis or a change in management about 45% of the time.2
Using transesophageal ultrasound for a focused cardiac assessment in hemodynamically unstable patients is not new—and is often referred to as rescue TEE or resuscitative TEE. A broader term, transesophageal ultrasound, has also been used to include sonographic evaluation of the lungs in patients with poor acoustic windows. At my institution, we use the term critical care TEE to define TEE performed by a noncardiology-trained intensivist in an intubated critically ill patient.
Regardless of the term, the use of transesophageal ultrasound by the noncardiologist in the ICU appears to be a developing trend. As with other uses of POCUS, ultrasound machines continue to be able to “do more” at a lower price point. In 2024, several cart-based ultrasound machines are compatible with transesophageal probes and contain software packages capable of common cardiac measurements.
Despite this growing interest, intensivists are likely to encounter barriers to implementing critical care TEE. Our division recently implemented adding TEE to our practice. Our practice involves two separate systems: a Veterans Administration hospital and a university-based county hospital. Our division has integrated the use of TEE in the medical ICU at both institutions. Having navigated the process at both institutions, I can offer some guidance in navigating barriers.
The development of a critical care TEE program must start with a strong base in transthoracic cardiac POCUS, at least for the foreseeable future. Having a strong background in TTE gives learners a solid foundation in cardiac anatomy, cardiac function, and ultrasound properties. Obtaining testamur status or board certification in critical care echocardiography is not an absolute must but is a definite benefit. Having significant experience in TTE image acquisition and interpretation will flatten the learning curve for TEE. Interestingly, image acquisition in TEE is often easier than in TTE, so the paradigm of learning TTE before TEE may reverse in the years to come.
Two barriers often work together to create a vicious cycle that stops the development of a TEE program at its start. These barriers include the lack of training and lack of equipment, specifically a TEE probe. Those who do not understand the value of TEE may ask, “Why purchase equipment for a procedure that you do not yet know how to do?” The opposite question can also be asked, “Why get trained to do something you don’t have the equipment to perform?”
My best advice to break this cycle is to “dive in” to whichever barrier seems easier to overcome first. I started with obtaining knowledge and training. Obtaining training and education in a procedure that is historically not done in your specialty is challenging but is not impossible. It takes a combination of high levels of self-motivation and at least one colleague with the training to support you. I approached a cardiac anesthesiologist, whom I knew from the surgical ICU. Cardiologists can also be a resource, but working with cardiac anesthesiologists offers several advantages. TEEs done by cardiac anesthesiologists are similar to those done in ICU patients (ie, all patients are intubated and sedated). The procedures are also scheduled several days in advance, making it easier to integrate training into your daily work schedule. Lastly, the TEE probe remains in place for several hours, so repeating the probe manipulations again as a learner does not add additional risk to the patient. In my case, we somewhat arbitrarily agreed that I participate in 25 TEE exams. (CME courses, both online and in-person simulation, exist and greatly supplement self-study.)
Obtaining equipment is also a common barrier, though this has become less restrictive in the last several years. As previously mentioned, many cart-based ultrasound machines can accommodate a TEE probe. This changes the request from purchasing a new machine to “just a probe.” Despite the higher cost than most other probes, those in charge of purchasing are often more open to purchasing “a probe” than to purchasing an ultrasound machine.
Additionally, the purchasing decision regarding probes may fall to a different person than it does for an ultrasound machine. If available, POCUS image archiving into the medical record can help offset the cost of equipment, both by increasing revenue via billing and by demonstrating that equipment is being used. If initially declined, continue to ask and work to integrate the purchase into the next year’s budget. Inquire about the process of making a formal request and follow that process. This will often involve obtaining a quote or quotes from the ultrasound manufacturer(s).
Keep in mind that the probe will require a special storage cabinet specifically designed for TEE probes. It is prudent to include this in budget requests. If needed, the echocardiography lab can be a useful resource for additional information regarding the cabinet requirements. It is strongly recommended to discuss TEE probe models with sterile processing before any purchasing. If options are available, it is wise to choose a model the hospital already uses, as the cleaning protocol is well established. Our unit purchased a model that did not have an established protocol, which took nearly 6 months to develop. If probe options are limited, involving sterile processing early to start developing a protocol will help decrease delays.
Obtaining hospital privileges is also a common barrier, though this may not be as challenging as expected. Hospitals typically have well-outlined policies on obtaining privileges for established procedures. One of our hospital systems had four different options; the most straightforward required 20 hours of CME specific to TEE and 10 supervised cases by a proctor currently holding TEE privileges (see Table 1).
Discussions about obtaining privileges should involve your division chief, chair of medicine, and the cardiology division chief. Clearly outlining the plan to perform this procedure only in critically ill patients who are already intubated for other reasons made these conversations go much more smoothly. In the development of delineation of privileges, we used the term critical care TEE to clearly define this patient population. During these conversations, highlight the safety of the procedure; ASE guidelines3 estimate a severe complication rate of less than 1 in 10,000 cases and explain the anticipated benefits to critically ill patients.
In conclusion, at an institution that is already adept at the use of POCUS in the ICU, the additional of critical care TEE within 1 to 2 years is a very realistic achievement. It will undoubtedly require patience, persistence, and self-motivation, but the barriers are becoming smaller every day. Stay motivated!
Dr. Proud is Associate Professor of Medicine, Division of Pulmonary and Critical Care Medicine, Pulmonary and Critical Care Medicine Program Director, UT Health San Antonio.
References:
1. Porter TR, Abdelmoneim S, Belcik FT, et al. Guidelines for the cardiac sonographer in the performance of contrast echocardiography: a focused update from the American Society of Echocardiography. J Am Soc Echocardiogr. 2024;27(8):797-810.
2. Si X, Ma J, Cao DY, et al. Transesophageal echocardiography instead or in addition to transthoracic echocardiography in evaluating haemodynamic problems in intubated critically ill patients. Ann Transl Med. 2020;8(12):785.
3. Hahn RT, Abraham T, Adams MS, et al. Guidelines for performing a cmprehensive transesophageal echocardiographic examination: recommendations from the American Society of Echocardioraphy and the Society of Cardiovascular Anesthesiologists. J Am Soc Echocardiogr. 2013;26(9):921-964.
Bedside-focused cardiac ultrasound assessment, or cardiac point-of-care ultrasound (POCUS), has become common in intensive care units throughout the US and the world.
However, obtaining images adequate for decision making via standard transthoracic echo (TTE) is not possible in a significant number of patients; as high as 30% of critically ill patients, according to The American Society of Echocardiography (ASE) guidelines.1 Factors common to critically ill patients, such as invasive mechanical ventilation, external dressings, and limited mobility, contribute to poor image acquisition.
In almost all these cases, the factors limiting image acquisition can be eliminated by utilizing a transesophageal approach. In a recent study, researchers were able to demonstrate that adding transesophageal echocardiography (TEE) to TTE in critically ill patients yielded a new diagnosis or a change in management about 45% of the time.2
Using transesophageal ultrasound for a focused cardiac assessment in hemodynamically unstable patients is not new—and is often referred to as rescue TEE or resuscitative TEE. A broader term, transesophageal ultrasound, has also been used to include sonographic evaluation of the lungs in patients with poor acoustic windows. At my institution, we use the term critical care TEE to define TEE performed by a noncardiology-trained intensivist in an intubated critically ill patient.
Regardless of the term, the use of transesophageal ultrasound by the noncardiologist in the ICU appears to be a developing trend. As with other uses of POCUS, ultrasound machines continue to be able to “do more” at a lower price point. In 2024, several cart-based ultrasound machines are compatible with transesophageal probes and contain software packages capable of common cardiac measurements.
Despite this growing interest, intensivists are likely to encounter barriers to implementing critical care TEE. Our division recently implemented adding TEE to our practice. Our practice involves two separate systems: a Veterans Administration hospital and a university-based county hospital. Our division has integrated the use of TEE in the medical ICU at both institutions. Having navigated the process at both institutions, I can offer some guidance in navigating barriers.
The development of a critical care TEE program must start with a strong base in transthoracic cardiac POCUS, at least for the foreseeable future. Having a strong background in TTE gives learners a solid foundation in cardiac anatomy, cardiac function, and ultrasound properties. Obtaining testamur status or board certification in critical care echocardiography is not an absolute must but is a definite benefit. Having significant experience in TTE image acquisition and interpretation will flatten the learning curve for TEE. Interestingly, image acquisition in TEE is often easier than in TTE, so the paradigm of learning TTE before TEE may reverse in the years to come.
Two barriers often work together to create a vicious cycle that stops the development of a TEE program at its start. These barriers include the lack of training and lack of equipment, specifically a TEE probe. Those who do not understand the value of TEE may ask, “Why purchase equipment for a procedure that you do not yet know how to do?” The opposite question can also be asked, “Why get trained to do something you don’t have the equipment to perform?”
My best advice to break this cycle is to “dive in” to whichever barrier seems easier to overcome first. I started with obtaining knowledge and training. Obtaining training and education in a procedure that is historically not done in your specialty is challenging but is not impossible. It takes a combination of high levels of self-motivation and at least one colleague with the training to support you. I approached a cardiac anesthesiologist, whom I knew from the surgical ICU. Cardiologists can also be a resource, but working with cardiac anesthesiologists offers several advantages. TEEs done by cardiac anesthesiologists are similar to those done in ICU patients (ie, all patients are intubated and sedated). The procedures are also scheduled several days in advance, making it easier to integrate training into your daily work schedule. Lastly, the TEE probe remains in place for several hours, so repeating the probe manipulations again as a learner does not add additional risk to the patient. In my case, we somewhat arbitrarily agreed that I participate in 25 TEE exams. (CME courses, both online and in-person simulation, exist and greatly supplement self-study.)
Obtaining equipment is also a common barrier, though this has become less restrictive in the last several years. As previously mentioned, many cart-based ultrasound machines can accommodate a TEE probe. This changes the request from purchasing a new machine to “just a probe.” Despite the higher cost than most other probes, those in charge of purchasing are often more open to purchasing “a probe” than to purchasing an ultrasound machine.
Additionally, the purchasing decision regarding probes may fall to a different person than it does for an ultrasound machine. If available, POCUS image archiving into the medical record can help offset the cost of equipment, both by increasing revenue via billing and by demonstrating that equipment is being used. If initially declined, continue to ask and work to integrate the purchase into the next year’s budget. Inquire about the process of making a formal request and follow that process. This will often involve obtaining a quote or quotes from the ultrasound manufacturer(s).
Keep in mind that the probe will require a special storage cabinet specifically designed for TEE probes. It is prudent to include this in budget requests. If needed, the echocardiography lab can be a useful resource for additional information regarding the cabinet requirements. It is strongly recommended to discuss TEE probe models with sterile processing before any purchasing. If options are available, it is wise to choose a model the hospital already uses, as the cleaning protocol is well established. Our unit purchased a model that did not have an established protocol, which took nearly 6 months to develop. If probe options are limited, involving sterile processing early to start developing a protocol will help decrease delays.
Obtaining hospital privileges is also a common barrier, though this may not be as challenging as expected. Hospitals typically have well-outlined policies on obtaining privileges for established procedures. One of our hospital systems had four different options; the most straightforward required 20 hours of CME specific to TEE and 10 supervised cases by a proctor currently holding TEE privileges (see Table 1).
Discussions about obtaining privileges should involve your division chief, chair of medicine, and the cardiology division chief. Clearly outlining the plan to perform this procedure only in critically ill patients who are already intubated for other reasons made these conversations go much more smoothly. In the development of delineation of privileges, we used the term critical care TEE to clearly define this patient population. During these conversations, highlight the safety of the procedure; ASE guidelines3 estimate a severe complication rate of less than 1 in 10,000 cases and explain the anticipated benefits to critically ill patients.
In conclusion, at an institution that is already adept at the use of POCUS in the ICU, the additional of critical care TEE within 1 to 2 years is a very realistic achievement. It will undoubtedly require patience, persistence, and self-motivation, but the barriers are becoming smaller every day. Stay motivated!
Dr. Proud is Associate Professor of Medicine, Division of Pulmonary and Critical Care Medicine, Pulmonary and Critical Care Medicine Program Director, UT Health San Antonio.
References:
1. Porter TR, Abdelmoneim S, Belcik FT, et al. Guidelines for the cardiac sonographer in the performance of contrast echocardiography: a focused update from the American Society of Echocardiography. J Am Soc Echocardiogr. 2024;27(8):797-810.
2. Si X, Ma J, Cao DY, et al. Transesophageal echocardiography instead or in addition to transthoracic echocardiography in evaluating haemodynamic problems in intubated critically ill patients. Ann Transl Med. 2020;8(12):785.
3. Hahn RT, Abraham T, Adams MS, et al. Guidelines for performing a cmprehensive transesophageal echocardiographic examination: recommendations from the American Society of Echocardioraphy and the Society of Cardiovascular Anesthesiologists. J Am Soc Echocardiogr. 2013;26(9):921-964.
Should intensivists place PEG tubes in critically ill patients?
The practice of initiating early and adequate nutrition in critically ill patients is a cornerstone of ICU management. Adequate nutrition combats the dangerous catabolic state that accompanies critical illness. A few of the benefits of this practice are a decrease in disease severity with resultant lessened hospital and ICU lengths of stay, reduced infection rates, and a decrease in hospital mortality. Enteral nutrition (EN) is the route of nutritional support most associated with safe and effective provision of enhanced immunologic function and the ability to preserve the patient’s lean body mass while avoiding metabolic and infectious complications.
Since its inception in 1980, percutaneous endoscopic gastrostomy (PEG) tubes have become the preferred method for delivering EN in ICUs across the United States. When comparing PEG and nasogastric tubes (NGTs), evidence shows reduced bleeding events, less tube dislodgement, and decreased tube obstructions with a faster rate of recovery of previous swallowing function that prevents delays in medical care and increased mortality rate. Although PEG tubes do not entirely prevent acid reflux or aspiration events, they are positively correlated to significantly reduced rates of both which result in a survival benefit seen in a 2012 study (Psychiatry Clin Neurosci. 2012 Aug;66[5]:418).
The majority of PEG tubes placed in the United States has unquestionably shifted to the ICU patient population since 2014 according to the largest health care database search on this topic published in 2019 (Ann Am Thorac Soc. 2019 Jun;16[6]:724). The safety and efficacy of this procedure has only improved, yet the delayed timing of placement remains problematic and often exceeds what is medically necessary or financially feasible.
To understand this issue, it is important to consider that despite intensivists being globally recognized as procedurally sound with enhanced ultrasound expertise, their endoscopic experience is usually limited to bronchoscopy without formal training in upper gastrointestinal endoscopy. This is the leading theory to explain why intensivists are performing their own percutaneous tracheostomies but not gastrostomies. Fortunately, the FDA-approved Point of Care Ultrasound Magnet Aligned Gastrostomy (PUMA-G) System has shown analogous safety and efficacy when compared with the traditional endoscopically placed PEG tube technique (J Intensive Care Med. 2022 May;37[5]:641).
A case series was published in 2021 that included three intensivists who underwent a 3-hour cadaver-based training course for the PUMA-G System with a mandatory minimum successful placement of three gastric tubes (J Clin Ultrasound. 2021 Jan;49[1]:28). Once they demonstrated competence in the technique, the procedure was performed on mechanically ventilated and sedated patients without any reported complications peri-procedurally or over the next 30 days. The evidence that intensivists can use their current skillset to rapidly become competent in this ultrasound-guided bedside procedure is without question.
PEG tube placement by intensivists is a procedure that will undoubtedly benefit patients in the ICU and assist in offloading the operation costs of a significant number of critical care units and their associated organizations. This is an area ripe for growth with further education and research.
The practice of initiating early and adequate nutrition in critically ill patients is a cornerstone of ICU management. Adequate nutrition combats the dangerous catabolic state that accompanies critical illness. A few of the benefits of this practice are a decrease in disease severity with resultant lessened hospital and ICU lengths of stay, reduced infection rates, and a decrease in hospital mortality. Enteral nutrition (EN) is the route of nutritional support most associated with safe and effective provision of enhanced immunologic function and the ability to preserve the patient’s lean body mass while avoiding metabolic and infectious complications.
Since its inception in 1980, percutaneous endoscopic gastrostomy (PEG) tubes have become the preferred method for delivering EN in ICUs across the United States. When comparing PEG and nasogastric tubes (NGTs), evidence shows reduced bleeding events, less tube dislodgement, and decreased tube obstructions with a faster rate of recovery of previous swallowing function that prevents delays in medical care and increased mortality rate. Although PEG tubes do not entirely prevent acid reflux or aspiration events, they are positively correlated to significantly reduced rates of both which result in a survival benefit seen in a 2012 study (Psychiatry Clin Neurosci. 2012 Aug;66[5]:418).
The majority of PEG tubes placed in the United States has unquestionably shifted to the ICU patient population since 2014 according to the largest health care database search on this topic published in 2019 (Ann Am Thorac Soc. 2019 Jun;16[6]:724). The safety and efficacy of this procedure has only improved, yet the delayed timing of placement remains problematic and often exceeds what is medically necessary or financially feasible.
To understand this issue, it is important to consider that despite intensivists being globally recognized as procedurally sound with enhanced ultrasound expertise, their endoscopic experience is usually limited to bronchoscopy without formal training in upper gastrointestinal endoscopy. This is the leading theory to explain why intensivists are performing their own percutaneous tracheostomies but not gastrostomies. Fortunately, the FDA-approved Point of Care Ultrasound Magnet Aligned Gastrostomy (PUMA-G) System has shown analogous safety and efficacy when compared with the traditional endoscopically placed PEG tube technique (J Intensive Care Med. 2022 May;37[5]:641).
A case series was published in 2021 that included three intensivists who underwent a 3-hour cadaver-based training course for the PUMA-G System with a mandatory minimum successful placement of three gastric tubes (J Clin Ultrasound. 2021 Jan;49[1]:28). Once they demonstrated competence in the technique, the procedure was performed on mechanically ventilated and sedated patients without any reported complications peri-procedurally or over the next 30 days. The evidence that intensivists can use their current skillset to rapidly become competent in this ultrasound-guided bedside procedure is without question.
PEG tube placement by intensivists is a procedure that will undoubtedly benefit patients in the ICU and assist in offloading the operation costs of a significant number of critical care units and their associated organizations. This is an area ripe for growth with further education and research.
The practice of initiating early and adequate nutrition in critically ill patients is a cornerstone of ICU management. Adequate nutrition combats the dangerous catabolic state that accompanies critical illness. A few of the benefits of this practice are a decrease in disease severity with resultant lessened hospital and ICU lengths of stay, reduced infection rates, and a decrease in hospital mortality. Enteral nutrition (EN) is the route of nutritional support most associated with safe and effective provision of enhanced immunologic function and the ability to preserve the patient’s lean body mass while avoiding metabolic and infectious complications.
Since its inception in 1980, percutaneous endoscopic gastrostomy (PEG) tubes have become the preferred method for delivering EN in ICUs across the United States. When comparing PEG and nasogastric tubes (NGTs), evidence shows reduced bleeding events, less tube dislodgement, and decreased tube obstructions with a faster rate of recovery of previous swallowing function that prevents delays in medical care and increased mortality rate. Although PEG tubes do not entirely prevent acid reflux or aspiration events, they are positively correlated to significantly reduced rates of both which result in a survival benefit seen in a 2012 study (Psychiatry Clin Neurosci. 2012 Aug;66[5]:418).
The majority of PEG tubes placed in the United States has unquestionably shifted to the ICU patient population since 2014 according to the largest health care database search on this topic published in 2019 (Ann Am Thorac Soc. 2019 Jun;16[6]:724). The safety and efficacy of this procedure has only improved, yet the delayed timing of placement remains problematic and often exceeds what is medically necessary or financially feasible.
To understand this issue, it is important to consider that despite intensivists being globally recognized as procedurally sound with enhanced ultrasound expertise, their endoscopic experience is usually limited to bronchoscopy without formal training in upper gastrointestinal endoscopy. This is the leading theory to explain why intensivists are performing their own percutaneous tracheostomies but not gastrostomies. Fortunately, the FDA-approved Point of Care Ultrasound Magnet Aligned Gastrostomy (PUMA-G) System has shown analogous safety and efficacy when compared with the traditional endoscopically placed PEG tube technique (J Intensive Care Med. 2022 May;37[5]:641).
A case series was published in 2021 that included three intensivists who underwent a 3-hour cadaver-based training course for the PUMA-G System with a mandatory minimum successful placement of three gastric tubes (J Clin Ultrasound. 2021 Jan;49[1]:28). Once they demonstrated competence in the technique, the procedure was performed on mechanically ventilated and sedated patients without any reported complications peri-procedurally or over the next 30 days. The evidence that intensivists can use their current skillset to rapidly become competent in this ultrasound-guided bedside procedure is without question.
PEG tube placement by intensivists is a procedure that will undoubtedly benefit patients in the ICU and assist in offloading the operation costs of a significant number of critical care units and their associated organizations. This is an area ripe for growth with further education and research.
Sedative use in older adults after critical illness
Modifications to medication regimens may include stopping home medications for chronic conditions, dose adjustments for altered organ function, or initiating new treatments for acute illness(es). Common examples of changes to a critically ill patient’s medication regimen are stopping a chronic antihypertensive drug in the setting of shock, holding an oral medication that cannot be crushed or administered through a feeding tube, and initiating sedatives and analgesics to support invasive mechanical ventilation. Medication regimens are especially vulnerable to errors and omissions at transition points (i.e., ICU to ward transfers and home discharge). As critical illness resolves and patients transition to different care teams, the hospital discharge medication regimen may differ from the preadmission list with the omission of prehospital medications and/or the continuation of acute medications no longer needed without thorough medication review and reconciliation.
While admitted to ICU, many critically ill patients – particularly those who are mechanically ventilated – receive intravenous or enteral sedatives such as benzodiazepines and antipsychotics. Sedatives are prescribed to more than two-thirds of critically ill patients for disturbing symptoms of agitation, delirium, anxiety, and insomnia and to facilitate invasive procedures (Burry LD, et al. J Crit Care. 2017;42:268). Current sedation practice guidelines endorse the use of sedatives when indicated for the shortest duration possible, given the known associated serious short- and long-term adverse drug events (Devlin JW, et al. Crit Care Med. 2018;46[9]:e825). Previous research has demonstrated that benzodiazepines initiated in-hospital are often continued on discharge for older adults and that patients from the ICU are at greater risk of benzodiazepine continuation than patients hospitalized without an ICU admission (Scales DC, et al. J Gen Intern Med. 2016;31[2]:196; Bell C, et al. J Gen Intern Med. 2007;22[7]:1024). This is particularly concerning for older adults as sedatives have been associated with serious adverse events in community-dwelling older adults, including falls and cognitive impairment (American Geriatrics Society. J Am Geriatr Soc. 2015;63[11]:2227)
The cohort of patients included all adults aged 66 years or more, who were discharged alive from the hospital and who were sedative-naive prior to hospitalization. Sedative-naive status was defined as no sedative prescription filled for any class, dose, or duration in the 180 days before hospital admission. The proportion of ICU survivors who filled a sedative prescription within 1 week of hospital discharge was the primary outcome. The secondary outcomes were the proportion of patients that filled each sedative class (e.g., antipsychotic, benzodiazepine, nonbenzodiazepine sedative) within 1 week of hospital discharge and persistent sedative prescription (additional prescriptions filled within 6 months after discharge).
The cohort included 250,428 sedative-naive older adults. The mean age was 75.8 years, 61.0% were male, 26.3% received invasive mechanical ventilation, and 14.8% had sepsis. In total, 6.1% (n=15,277) of patients filled a sedative prescription within 1 week of discharge; 57.7% (n = 8824) filled a benzodiazepine, 18.0% (n = 2749) filled a non-benzodiazepine sedative, 17.9% (n = 2745) filled an antipsychotic, and 6.2% (n = 959) filled more than 1 sedative drug class. Most patients filled prescriptions on the day of discharge (median 0 days (interquartile range (IQR) 0-3). The study found considerable variation in the primary outcome across the 153 hospitals: 2.1% (95% confidence interval [CI] 1.2% to 2.8%) to 44.0% (95% CI 3.0% to –57.8%) filled a sedative prescription within a week of hospital discharge. The factors strongly associated with an increased odds of a sedative prescription filled within a week of discharge included: discharge to long-term care (adjusted OR (aOR) 4.00, 95% CI 3.72 to 4.31), receipt of inpatient geriatric (aOR 1.95, 95% CI 1.80 to 2.10) or psychiatry consultation (aOR 2.76, 95% CI 2.62, 2.91), mechanical ventilation (aOR 1.59, 95% CI 1.53 to 1.66), and admitted ≥ 7 days to the ICU (aOR 1.50, 95% CI 1.42 to 1.58). Among hospital factors, a community hospital (vs academic) (aOR 1.40, 95% CI 1.16 to 1.70) and rural location (vs urban) (aOR 1.67, 95% CI 1.36 to 2.05) were also associated with new sedative prescriptions. Even after adjusting for patient and site characteristics, there was considerable remaining variability between sites quantified by the median odds ratio (aMOR) of 1.43. By drug class, there were similar findings with the exception of different associations for sex and frailty. For benzodiazepine prescriptions, female sex was associated with increased odds of a prescription (aOR 1.13, 95% CI 1.08 to 1.18), while frailty was inversely associated (aOR 0.82, 95% CI 0.75 to 0.89). The opposite associations were identified for antipsychotics: female sex (aOR 0.75, 95% CI 0.69 to 0.81) and frailty (aOR 1.41, 95% CI 1.28 to 1.55). No associations were identified for sex and frailty and non-benzodiazepine sedative prescriptions.
Persistent sedative prescription was common as 55% met the definition of persistence, filling a median of 2 prescriptions (IQR 1,3) in the 6 months after hospital discharge. The factors associated with persistent sedative prescriptions were similar to those identified above except female sex was associated with persistent sedative prescription (sHR 1.07, 95% CI 1.02 to 1.13). Those who filled an antipsychotic prescription (sHR 1.45, 95% CI 1.35 to 1.56), a non-benzodiazepine sedative prescription (sHR 1.44, 955 CI 1.34 to 1.53), or prescriptions for more than 1 sedative class filled (sHR 2.16, 95% CI 1.97 to 2.37) were more likely to fill persistent prescriptions compared with those who filled a prescription for a benzodiazepine alone as their first sedative.
In summary, 1 in 15 sedative-naive older adults filled a sedative prescription within a week of hospital discharge following a critical illness, and many continued to fill sedative prescriptions in the next 6 months. We were able to identify factors associated with new sedative prescriptions that could be targeted for stewardship programs or quality improvement projects that focus on medication safety and reconciliation. Medication stewardship and reconciliation processes have been broadly studied in many patient care settings but not the ICU. There is still much to determine regarding de-escalating and discontinuing sedatives as critical illness resolves and patients are liberated from intensive clinical interventions as well as the consequences of sedative exposure after hospital discharge for this population.
Dr. Burry is with the Departments of Pharmacy and Medicine, Sinai Health; Leslie Dan Faculty of Pharmacy and Interdepartmental Division of Critical Care, University of Toronto, Toronto, Canada. Dr. Williamson is with the Faculté de Pharmacie, Université de Montréal; Pharmacy Département, Hôpital du Sacré-Cœur de Montréal; and Research center, CIUSSS du Nord-de-l’Île-de-Montréal, Canada.
Modifications to medication regimens may include stopping home medications for chronic conditions, dose adjustments for altered organ function, or initiating new treatments for acute illness(es). Common examples of changes to a critically ill patient’s medication regimen are stopping a chronic antihypertensive drug in the setting of shock, holding an oral medication that cannot be crushed or administered through a feeding tube, and initiating sedatives and analgesics to support invasive mechanical ventilation. Medication regimens are especially vulnerable to errors and omissions at transition points (i.e., ICU to ward transfers and home discharge). As critical illness resolves and patients transition to different care teams, the hospital discharge medication regimen may differ from the preadmission list with the omission of prehospital medications and/or the continuation of acute medications no longer needed without thorough medication review and reconciliation.
While admitted to ICU, many critically ill patients – particularly those who are mechanically ventilated – receive intravenous or enteral sedatives such as benzodiazepines and antipsychotics. Sedatives are prescribed to more than two-thirds of critically ill patients for disturbing symptoms of agitation, delirium, anxiety, and insomnia and to facilitate invasive procedures (Burry LD, et al. J Crit Care. 2017;42:268). Current sedation practice guidelines endorse the use of sedatives when indicated for the shortest duration possible, given the known associated serious short- and long-term adverse drug events (Devlin JW, et al. Crit Care Med. 2018;46[9]:e825). Previous research has demonstrated that benzodiazepines initiated in-hospital are often continued on discharge for older adults and that patients from the ICU are at greater risk of benzodiazepine continuation than patients hospitalized without an ICU admission (Scales DC, et al. J Gen Intern Med. 2016;31[2]:196; Bell C, et al. J Gen Intern Med. 2007;22[7]:1024). This is particularly concerning for older adults as sedatives have been associated with serious adverse events in community-dwelling older adults, including falls and cognitive impairment (American Geriatrics Society. J Am Geriatr Soc. 2015;63[11]:2227)
The cohort of patients included all adults aged 66 years or more, who were discharged alive from the hospital and who were sedative-naive prior to hospitalization. Sedative-naive status was defined as no sedative prescription filled for any class, dose, or duration in the 180 days before hospital admission. The proportion of ICU survivors who filled a sedative prescription within 1 week of hospital discharge was the primary outcome. The secondary outcomes were the proportion of patients that filled each sedative class (e.g., antipsychotic, benzodiazepine, nonbenzodiazepine sedative) within 1 week of hospital discharge and persistent sedative prescription (additional prescriptions filled within 6 months after discharge).
The cohort included 250,428 sedative-naive older adults. The mean age was 75.8 years, 61.0% were male, 26.3% received invasive mechanical ventilation, and 14.8% had sepsis. In total, 6.1% (n=15,277) of patients filled a sedative prescription within 1 week of discharge; 57.7% (n = 8824) filled a benzodiazepine, 18.0% (n = 2749) filled a non-benzodiazepine sedative, 17.9% (n = 2745) filled an antipsychotic, and 6.2% (n = 959) filled more than 1 sedative drug class. Most patients filled prescriptions on the day of discharge (median 0 days (interquartile range (IQR) 0-3). The study found considerable variation in the primary outcome across the 153 hospitals: 2.1% (95% confidence interval [CI] 1.2% to 2.8%) to 44.0% (95% CI 3.0% to –57.8%) filled a sedative prescription within a week of hospital discharge. The factors strongly associated with an increased odds of a sedative prescription filled within a week of discharge included: discharge to long-term care (adjusted OR (aOR) 4.00, 95% CI 3.72 to 4.31), receipt of inpatient geriatric (aOR 1.95, 95% CI 1.80 to 2.10) or psychiatry consultation (aOR 2.76, 95% CI 2.62, 2.91), mechanical ventilation (aOR 1.59, 95% CI 1.53 to 1.66), and admitted ≥ 7 days to the ICU (aOR 1.50, 95% CI 1.42 to 1.58). Among hospital factors, a community hospital (vs academic) (aOR 1.40, 95% CI 1.16 to 1.70) and rural location (vs urban) (aOR 1.67, 95% CI 1.36 to 2.05) were also associated with new sedative prescriptions. Even after adjusting for patient and site characteristics, there was considerable remaining variability between sites quantified by the median odds ratio (aMOR) of 1.43. By drug class, there were similar findings with the exception of different associations for sex and frailty. For benzodiazepine prescriptions, female sex was associated with increased odds of a prescription (aOR 1.13, 95% CI 1.08 to 1.18), while frailty was inversely associated (aOR 0.82, 95% CI 0.75 to 0.89). The opposite associations were identified for antipsychotics: female sex (aOR 0.75, 95% CI 0.69 to 0.81) and frailty (aOR 1.41, 95% CI 1.28 to 1.55). No associations were identified for sex and frailty and non-benzodiazepine sedative prescriptions.
Persistent sedative prescription was common as 55% met the definition of persistence, filling a median of 2 prescriptions (IQR 1,3) in the 6 months after hospital discharge. The factors associated with persistent sedative prescriptions were similar to those identified above except female sex was associated with persistent sedative prescription (sHR 1.07, 95% CI 1.02 to 1.13). Those who filled an antipsychotic prescription (sHR 1.45, 95% CI 1.35 to 1.56), a non-benzodiazepine sedative prescription (sHR 1.44, 955 CI 1.34 to 1.53), or prescriptions for more than 1 sedative class filled (sHR 2.16, 95% CI 1.97 to 2.37) were more likely to fill persistent prescriptions compared with those who filled a prescription for a benzodiazepine alone as their first sedative.
In summary, 1 in 15 sedative-naive older adults filled a sedative prescription within a week of hospital discharge following a critical illness, and many continued to fill sedative prescriptions in the next 6 months. We were able to identify factors associated with new sedative prescriptions that could be targeted for stewardship programs or quality improvement projects that focus on medication safety and reconciliation. Medication stewardship and reconciliation processes have been broadly studied in many patient care settings but not the ICU. There is still much to determine regarding de-escalating and discontinuing sedatives as critical illness resolves and patients are liberated from intensive clinical interventions as well as the consequences of sedative exposure after hospital discharge for this population.
Dr. Burry is with the Departments of Pharmacy and Medicine, Sinai Health; Leslie Dan Faculty of Pharmacy and Interdepartmental Division of Critical Care, University of Toronto, Toronto, Canada. Dr. Williamson is with the Faculté de Pharmacie, Université de Montréal; Pharmacy Département, Hôpital du Sacré-Cœur de Montréal; and Research center, CIUSSS du Nord-de-l’Île-de-Montréal, Canada.
Modifications to medication regimens may include stopping home medications for chronic conditions, dose adjustments for altered organ function, or initiating new treatments for acute illness(es). Common examples of changes to a critically ill patient’s medication regimen are stopping a chronic antihypertensive drug in the setting of shock, holding an oral medication that cannot be crushed or administered through a feeding tube, and initiating sedatives and analgesics to support invasive mechanical ventilation. Medication regimens are especially vulnerable to errors and omissions at transition points (i.e., ICU to ward transfers and home discharge). As critical illness resolves and patients transition to different care teams, the hospital discharge medication regimen may differ from the preadmission list with the omission of prehospital medications and/or the continuation of acute medications no longer needed without thorough medication review and reconciliation.
While admitted to ICU, many critically ill patients – particularly those who are mechanically ventilated – receive intravenous or enteral sedatives such as benzodiazepines and antipsychotics. Sedatives are prescribed to more than two-thirds of critically ill patients for disturbing symptoms of agitation, delirium, anxiety, and insomnia and to facilitate invasive procedures (Burry LD, et al. J Crit Care. 2017;42:268). Current sedation practice guidelines endorse the use of sedatives when indicated for the shortest duration possible, given the known associated serious short- and long-term adverse drug events (Devlin JW, et al. Crit Care Med. 2018;46[9]:e825). Previous research has demonstrated that benzodiazepines initiated in-hospital are often continued on discharge for older adults and that patients from the ICU are at greater risk of benzodiazepine continuation than patients hospitalized without an ICU admission (Scales DC, et al. J Gen Intern Med. 2016;31[2]:196; Bell C, et al. J Gen Intern Med. 2007;22[7]:1024). This is particularly concerning for older adults as sedatives have been associated with serious adverse events in community-dwelling older adults, including falls and cognitive impairment (American Geriatrics Society. J Am Geriatr Soc. 2015;63[11]:2227)
The cohort of patients included all adults aged 66 years or more, who were discharged alive from the hospital and who were sedative-naive prior to hospitalization. Sedative-naive status was defined as no sedative prescription filled for any class, dose, or duration in the 180 days before hospital admission. The proportion of ICU survivors who filled a sedative prescription within 1 week of hospital discharge was the primary outcome. The secondary outcomes were the proportion of patients that filled each sedative class (e.g., antipsychotic, benzodiazepine, nonbenzodiazepine sedative) within 1 week of hospital discharge and persistent sedative prescription (additional prescriptions filled within 6 months after discharge).
The cohort included 250,428 sedative-naive older adults. The mean age was 75.8 years, 61.0% were male, 26.3% received invasive mechanical ventilation, and 14.8% had sepsis. In total, 6.1% (n=15,277) of patients filled a sedative prescription within 1 week of discharge; 57.7% (n = 8824) filled a benzodiazepine, 18.0% (n = 2749) filled a non-benzodiazepine sedative, 17.9% (n = 2745) filled an antipsychotic, and 6.2% (n = 959) filled more than 1 sedative drug class. Most patients filled prescriptions on the day of discharge (median 0 days (interquartile range (IQR) 0-3). The study found considerable variation in the primary outcome across the 153 hospitals: 2.1% (95% confidence interval [CI] 1.2% to 2.8%) to 44.0% (95% CI 3.0% to –57.8%) filled a sedative prescription within a week of hospital discharge. The factors strongly associated with an increased odds of a sedative prescription filled within a week of discharge included: discharge to long-term care (adjusted OR (aOR) 4.00, 95% CI 3.72 to 4.31), receipt of inpatient geriatric (aOR 1.95, 95% CI 1.80 to 2.10) or psychiatry consultation (aOR 2.76, 95% CI 2.62, 2.91), mechanical ventilation (aOR 1.59, 95% CI 1.53 to 1.66), and admitted ≥ 7 days to the ICU (aOR 1.50, 95% CI 1.42 to 1.58). Among hospital factors, a community hospital (vs academic) (aOR 1.40, 95% CI 1.16 to 1.70) and rural location (vs urban) (aOR 1.67, 95% CI 1.36 to 2.05) were also associated with new sedative prescriptions. Even after adjusting for patient and site characteristics, there was considerable remaining variability between sites quantified by the median odds ratio (aMOR) of 1.43. By drug class, there were similar findings with the exception of different associations for sex and frailty. For benzodiazepine prescriptions, female sex was associated with increased odds of a prescription (aOR 1.13, 95% CI 1.08 to 1.18), while frailty was inversely associated (aOR 0.82, 95% CI 0.75 to 0.89). The opposite associations were identified for antipsychotics: female sex (aOR 0.75, 95% CI 0.69 to 0.81) and frailty (aOR 1.41, 95% CI 1.28 to 1.55). No associations were identified for sex and frailty and non-benzodiazepine sedative prescriptions.
Persistent sedative prescription was common as 55% met the definition of persistence, filling a median of 2 prescriptions (IQR 1,3) in the 6 months after hospital discharge. The factors associated with persistent sedative prescriptions were similar to those identified above except female sex was associated with persistent sedative prescription (sHR 1.07, 95% CI 1.02 to 1.13). Those who filled an antipsychotic prescription (sHR 1.45, 95% CI 1.35 to 1.56), a non-benzodiazepine sedative prescription (sHR 1.44, 955 CI 1.34 to 1.53), or prescriptions for more than 1 sedative class filled (sHR 2.16, 95% CI 1.97 to 2.37) were more likely to fill persistent prescriptions compared with those who filled a prescription for a benzodiazepine alone as their first sedative.
In summary, 1 in 15 sedative-naive older adults filled a sedative prescription within a week of hospital discharge following a critical illness, and many continued to fill sedative prescriptions in the next 6 months. We were able to identify factors associated with new sedative prescriptions that could be targeted for stewardship programs or quality improvement projects that focus on medication safety and reconciliation. Medication stewardship and reconciliation processes have been broadly studied in many patient care settings but not the ICU. There is still much to determine regarding de-escalating and discontinuing sedatives as critical illness resolves and patients are liberated from intensive clinical interventions as well as the consequences of sedative exposure after hospital discharge for this population.
Dr. Burry is with the Departments of Pharmacy and Medicine, Sinai Health; Leslie Dan Faculty of Pharmacy and Interdepartmental Division of Critical Care, University of Toronto, Toronto, Canada. Dr. Williamson is with the Faculté de Pharmacie, Université de Montréal; Pharmacy Département, Hôpital du Sacré-Cœur de Montréal; and Research center, CIUSSS du Nord-de-l’Île-de-Montréal, Canada.
Celebrating the inaugural issues of CHEST’s new open access journals
After much anticipation, the inaugural issues of both CHEST®Critical Care and CHEST®Pulmonary officially launched in late June. – promoting transparency, inclusiveness, and collaboration in research – and offer authors more avenues to share their practice-changing research.
The first issue of CHEST Critical Care featured research into ICU mortality across prepandemic and pandemic cohorts in resource-limited settings in South Africa, an exploration into symptom trajectory in recipients of hematopoietic stem-cell transplantation, a narrative review of post-intensive care syndrome, and an investigation into early echocardiographic and ultrasonographic findings in critically ill patients with COVID-19.
In addition, an editorial from Hayley Gershengorn, MD, Editor in Chief of CHEST Critical Care, offers readers more insights into the need for a publication focused on the breadth of clinical topics in critical care and her goals for the new publication.
“I’m ecstatic for this launch. We are grateful to our authors for the trust they put in us and are excited to share their work with our critical care colleagues around the world,” Dr. Gershengorn said. “The editorial team and the American College of Chest Physicians staff have worked tirelessly on this journal, and it’s incredibly gratifying to see the first issue publish.”
Read the full issue and new research from the journal at www.chestcc.org.
In his own editorial featured in the inaugural issue of CHEST Pulmonary, Editor in Chief Matthew Miles, MD, MEd, FCCP, shares how the flagship journal’s proud heritage of sharing impactful clinical research – and the need to target areas of pulmonary and sleep medicine research not covered by other journals – inspired the creation of this new publication.
The issue also includes research into mobile health opportunities for asthma management, an exploration into telemedicine for patients with interstitial lung diseases, an in-depth review into the rare and often underdiagnosed disorder primary ciliary dyskinesia, research on the impact of the social vulnerability index on pulmonary embolism mortality, and an investigation into pneumothorax complications after percutaneous lung biopsy.
“I am deeply grateful to our authors, reviewers, editorial board, and staff who have contributed to the launch of our first issue,” Dr. Miles said. “The journal CHEST is known for excellence in clinically relevant research and patient management guidance. CHEST Pulmonary expands the CHEST portfolio with additional opportunity for researchers to share their work in an exclusively open access format to reach the broadest possible audience. I know our readers will enjoy learning from the research and reviews in issue one.”
Review the full issue and new articles from CHEST Pulmonary at www.chestpulmonary.org.
After much anticipation, the inaugural issues of both CHEST®Critical Care and CHEST®Pulmonary officially launched in late June. – promoting transparency, inclusiveness, and collaboration in research – and offer authors more avenues to share their practice-changing research.
The first issue of CHEST Critical Care featured research into ICU mortality across prepandemic and pandemic cohorts in resource-limited settings in South Africa, an exploration into symptom trajectory in recipients of hematopoietic stem-cell transplantation, a narrative review of post-intensive care syndrome, and an investigation into early echocardiographic and ultrasonographic findings in critically ill patients with COVID-19.
In addition, an editorial from Hayley Gershengorn, MD, Editor in Chief of CHEST Critical Care, offers readers more insights into the need for a publication focused on the breadth of clinical topics in critical care and her goals for the new publication.
“I’m ecstatic for this launch. We are grateful to our authors for the trust they put in us and are excited to share their work with our critical care colleagues around the world,” Dr. Gershengorn said. “The editorial team and the American College of Chest Physicians staff have worked tirelessly on this journal, and it’s incredibly gratifying to see the first issue publish.”
Read the full issue and new research from the journal at www.chestcc.org.
In his own editorial featured in the inaugural issue of CHEST Pulmonary, Editor in Chief Matthew Miles, MD, MEd, FCCP, shares how the flagship journal’s proud heritage of sharing impactful clinical research – and the need to target areas of pulmonary and sleep medicine research not covered by other journals – inspired the creation of this new publication.
The issue also includes research into mobile health opportunities for asthma management, an exploration into telemedicine for patients with interstitial lung diseases, an in-depth review into the rare and often underdiagnosed disorder primary ciliary dyskinesia, research on the impact of the social vulnerability index on pulmonary embolism mortality, and an investigation into pneumothorax complications after percutaneous lung biopsy.
“I am deeply grateful to our authors, reviewers, editorial board, and staff who have contributed to the launch of our first issue,” Dr. Miles said. “The journal CHEST is known for excellence in clinically relevant research and patient management guidance. CHEST Pulmonary expands the CHEST portfolio with additional opportunity for researchers to share their work in an exclusively open access format to reach the broadest possible audience. I know our readers will enjoy learning from the research and reviews in issue one.”
Review the full issue and new articles from CHEST Pulmonary at www.chestpulmonary.org.
After much anticipation, the inaugural issues of both CHEST®Critical Care and CHEST®Pulmonary officially launched in late June. – promoting transparency, inclusiveness, and collaboration in research – and offer authors more avenues to share their practice-changing research.
The first issue of CHEST Critical Care featured research into ICU mortality across prepandemic and pandemic cohorts in resource-limited settings in South Africa, an exploration into symptom trajectory in recipients of hematopoietic stem-cell transplantation, a narrative review of post-intensive care syndrome, and an investigation into early echocardiographic and ultrasonographic findings in critically ill patients with COVID-19.
In addition, an editorial from Hayley Gershengorn, MD, Editor in Chief of CHEST Critical Care, offers readers more insights into the need for a publication focused on the breadth of clinical topics in critical care and her goals for the new publication.
“I’m ecstatic for this launch. We are grateful to our authors for the trust they put in us and are excited to share their work with our critical care colleagues around the world,” Dr. Gershengorn said. “The editorial team and the American College of Chest Physicians staff have worked tirelessly on this journal, and it’s incredibly gratifying to see the first issue publish.”
Read the full issue and new research from the journal at www.chestcc.org.
In his own editorial featured in the inaugural issue of CHEST Pulmonary, Editor in Chief Matthew Miles, MD, MEd, FCCP, shares how the flagship journal’s proud heritage of sharing impactful clinical research – and the need to target areas of pulmonary and sleep medicine research not covered by other journals – inspired the creation of this new publication.
The issue also includes research into mobile health opportunities for asthma management, an exploration into telemedicine for patients with interstitial lung diseases, an in-depth review into the rare and often underdiagnosed disorder primary ciliary dyskinesia, research on the impact of the social vulnerability index on pulmonary embolism mortality, and an investigation into pneumothorax complications after percutaneous lung biopsy.
“I am deeply grateful to our authors, reviewers, editorial board, and staff who have contributed to the launch of our first issue,” Dr. Miles said. “The journal CHEST is known for excellence in clinically relevant research and patient management guidance. CHEST Pulmonary expands the CHEST portfolio with additional opportunity for researchers to share their work in an exclusively open access format to reach the broadest possible audience. I know our readers will enjoy learning from the research and reviews in issue one.”
Review the full issue and new articles from CHEST Pulmonary at www.chestpulmonary.org.
Use the SCAI stages to identify and treat cardiogenic shock
Cardiogenic shock (CS) is being recognized more often in critically ill patients. This increased prevalence is likely due to a better understanding of CS and the benefit of improving cardiac output (CO) to ensure adequate oxygen delivery (DO2).
CS is often, but not always, caused by a cardiac dysfunction. The heart is not able to provide adequate DO2 to the tissues. Hypoperfusion ensues. The body attempts to compensate for the poor perfusion by increasing heart rate, vasoconstriction, and shunting blood flow to vital organs. These compensatory mechanisms worsen perfusion by increasing myocardial ischemia which further worsens cardiac dysfunction. This is known as the downward spiral of CS (Ann Intern Med. 1999 Jul 6;131[1]).
There is a number of different etiologies for CS. Historically, acute myocardial infarctions (AMI) was the most common cause. In the last 20 years, AMI-induced CS has become less prevalent due to more aggressive reperfusion strategies. CS due to etiologies such as cardiomyopathy, myocarditis, right ventricle failure, and valvular pathologies have become more common. While the overarching goal is to restore DO2 to the tissue, the optimal treatment may differ based on the etiology of the CS. The Society for Cardiovascular Angiography and Intervention (SCAI) published CS classification stages in 2019 and then updated the stages 2022 (J Am Coll Cardiol. 2022 Mar 8;79[9]:933-46). In addition to the stages, there is now a three-axis model to address risk stratification. These classifications are a practically means of identifying and treating patients presenting with or concern for acute CS.
Stage A (At Risk) patients are not experiencing CS, but they are the at risk population. The patient’s hemodynamics, physical exam, and markers of hypoperfusion are normal. Stage A includes patients who have had a recent AMI or have heart failure.
Stage B (Beginning) patients have evidence of hemodynamic instability but are able to maintain tissue perfusion. These patients will have true or relative hypotension or tachycardia (in an attempt to maintain CO). Distal perfusion is adequate, but signs of ensuing decompensation (eg, elevated jugular venous pressure [JVP]) are present. Lactate is <2.0 mmol/L. Clinicians must be vigilant and treat these patients aggressively, so they do not decompensate further. It can be difficult to identify these patients because their blood pressure may be “normal,” but upon investigation, the blood pressure is actually a drop from the patient’s baseline.
Chronic heart failure patients with a history of depressed cardiac function will often have periods of cardiac decompensation between stages A and B. These patients are able to maintain perfusion for longer periods of time before further decompensation with hypoperfusion. If and when they do decompensate, they will often have a steep downward trajectory, so it is advantageous to the patient to be aggressive early.
Stage C (Classic) patients have evidence of tissue hypoperfusion. While these patients will often have true or relative hypotension, it is not a definition of stage C. These patients have evidence of volume overload with elevated JVP and rales throughout their lung fields. They will have poor distal perfusion and cool extremities that may become mottled. Lactate is ≥ 2 mmol/L. B-type natriuretic peptide (BNP) and liver function test (LFTs) results are elevated, and urine output is diminished. If a pulmonary arterial catheter is placed (highly recommended), the cardiac index (CI) is < 2.2 L/min/m2 and the pulmonary capillary wedge pressure (PCWP) is > 15 mm Hg. These patients look like what many clinicians think of when they think of CS.
These patients need better tissue perfusion. Inotropic support is needed to augment CO and DO2. Pharmacologic support is often the initial step. These patients also benefit from volume removal. This is usually accomplished with aggressive diuresis with a loop diuretic.
Stage D (Deteriorating) patients have failed initial treatment with single inotropic support. Hypoperfusion is not getting better and is often worsening. Lactate is staying > 2 mmol/L or rising. BNP and LFTs are also rising. These patients require additional inotropes and usually need vasopressors. Mechanical cardiac support (MCS) is often needed in addition to pharmacologic inotropic support.
Stage E (Extremis) patients have actual or impending circulatory collapse. These patients are peri-arrest with profound hypotension, lactic acidosis (often > 8 mmol/L), and unconsciousness. These patients are worsening despite multiple strategies to augment CO and DO2. These patients will likely die without emergent veno-arterial (VA) extracorporeal membrane oxygenation (ECMO). The goal of treatment is to stabilize the patient as quickly as possible to prevent cardiac arrest.
In addition to the stage of CS, SCAI developed the three-axis model of risk stratification as a conceptual model to be used for evaluation and prognostication. Etiology and phenotype, shock severity, and risk modifiers are factors related to patient outcomes from CS. This model is a way to individualize treatment to a specific patient.
Shock severity: What is the patient’s shock stage? What are the hemodynamics and metabolic abnormalities? What are the doses of the inotropes or vasopressors? Risk goes up with higher shock stages and vasoactive agent doses and worsening metabolic disturbances or hemodynamics.
Phenotype and etiology: what is the clinical etiology of the patient’s CS? Is this acute or acute on chronic? Which ventricle is involved? Is this cardiac driven or are other organs the driving factor? Single ventricle involvement is better than bi-ventricular failure. Cardiogenic collapse due to an overdose may have a better outcome than a massive AMI.
Risk modifiers: how old is the patient? What are the comorbidities? Did the patient have a cardiac arrest? What is the patient’s mental status? Some factors are modifiable, but others are not. The concept of chronologic vs. physiologic age may come into play. A frail 40 year old with stage 4 cancer and end stage renal failure may be assessed differently than a 70 year old with mild hypertension and an AMI.
The SCAI stages of CS are a pragmatic way to assess patients with an acute presentation of CS. These stages have defined criteria and treatment recommendations for all patients. The three-axis model allows the clinician to individualize patient care based on shock severity, etiology/phenotype, and risk modification. The goal of these stages is to identify and aggressively treat patients with CS, as well as identify when treatment is failing and additional therapies may be needed.
Dr. Gaillard is Associate Professor in the Departments of Anesthesiology, Section on Critical Care; Internal Medicine, Section on Pulmonology, Critical Care, Allergy, and Immunologic Diseases; and Emergency Medicine; Wake Forest School of Medicine, Winston-Salem, N.C.
Cardiogenic shock (CS) is being recognized more often in critically ill patients. This increased prevalence is likely due to a better understanding of CS and the benefit of improving cardiac output (CO) to ensure adequate oxygen delivery (DO2).
CS is often, but not always, caused by a cardiac dysfunction. The heart is not able to provide adequate DO2 to the tissues. Hypoperfusion ensues. The body attempts to compensate for the poor perfusion by increasing heart rate, vasoconstriction, and shunting blood flow to vital organs. These compensatory mechanisms worsen perfusion by increasing myocardial ischemia which further worsens cardiac dysfunction. This is known as the downward spiral of CS (Ann Intern Med. 1999 Jul 6;131[1]).
There is a number of different etiologies for CS. Historically, acute myocardial infarctions (AMI) was the most common cause. In the last 20 years, AMI-induced CS has become less prevalent due to more aggressive reperfusion strategies. CS due to etiologies such as cardiomyopathy, myocarditis, right ventricle failure, and valvular pathologies have become more common. While the overarching goal is to restore DO2 to the tissue, the optimal treatment may differ based on the etiology of the CS. The Society for Cardiovascular Angiography and Intervention (SCAI) published CS classification stages in 2019 and then updated the stages 2022 (J Am Coll Cardiol. 2022 Mar 8;79[9]:933-46). In addition to the stages, there is now a three-axis model to address risk stratification. These classifications are a practically means of identifying and treating patients presenting with or concern for acute CS.
Stage A (At Risk) patients are not experiencing CS, but they are the at risk population. The patient’s hemodynamics, physical exam, and markers of hypoperfusion are normal. Stage A includes patients who have had a recent AMI or have heart failure.
Stage B (Beginning) patients have evidence of hemodynamic instability but are able to maintain tissue perfusion. These patients will have true or relative hypotension or tachycardia (in an attempt to maintain CO). Distal perfusion is adequate, but signs of ensuing decompensation (eg, elevated jugular venous pressure [JVP]) are present. Lactate is <2.0 mmol/L. Clinicians must be vigilant and treat these patients aggressively, so they do not decompensate further. It can be difficult to identify these patients because their blood pressure may be “normal,” but upon investigation, the blood pressure is actually a drop from the patient’s baseline.
Chronic heart failure patients with a history of depressed cardiac function will often have periods of cardiac decompensation between stages A and B. These patients are able to maintain perfusion for longer periods of time before further decompensation with hypoperfusion. If and when they do decompensate, they will often have a steep downward trajectory, so it is advantageous to the patient to be aggressive early.
Stage C (Classic) patients have evidence of tissue hypoperfusion. While these patients will often have true or relative hypotension, it is not a definition of stage C. These patients have evidence of volume overload with elevated JVP and rales throughout their lung fields. They will have poor distal perfusion and cool extremities that may become mottled. Lactate is ≥ 2 mmol/L. B-type natriuretic peptide (BNP) and liver function test (LFTs) results are elevated, and urine output is diminished. If a pulmonary arterial catheter is placed (highly recommended), the cardiac index (CI) is < 2.2 L/min/m2 and the pulmonary capillary wedge pressure (PCWP) is > 15 mm Hg. These patients look like what many clinicians think of when they think of CS.
These patients need better tissue perfusion. Inotropic support is needed to augment CO and DO2. Pharmacologic support is often the initial step. These patients also benefit from volume removal. This is usually accomplished with aggressive diuresis with a loop diuretic.
Stage D (Deteriorating) patients have failed initial treatment with single inotropic support. Hypoperfusion is not getting better and is often worsening. Lactate is staying > 2 mmol/L or rising. BNP and LFTs are also rising. These patients require additional inotropes and usually need vasopressors. Mechanical cardiac support (MCS) is often needed in addition to pharmacologic inotropic support.
Stage E (Extremis) patients have actual or impending circulatory collapse. These patients are peri-arrest with profound hypotension, lactic acidosis (often > 8 mmol/L), and unconsciousness. These patients are worsening despite multiple strategies to augment CO and DO2. These patients will likely die without emergent veno-arterial (VA) extracorporeal membrane oxygenation (ECMO). The goal of treatment is to stabilize the patient as quickly as possible to prevent cardiac arrest.
In addition to the stage of CS, SCAI developed the three-axis model of risk stratification as a conceptual model to be used for evaluation and prognostication. Etiology and phenotype, shock severity, and risk modifiers are factors related to patient outcomes from CS. This model is a way to individualize treatment to a specific patient.
Shock severity: What is the patient’s shock stage? What are the hemodynamics and metabolic abnormalities? What are the doses of the inotropes or vasopressors? Risk goes up with higher shock stages and vasoactive agent doses and worsening metabolic disturbances or hemodynamics.
Phenotype and etiology: what is the clinical etiology of the patient’s CS? Is this acute or acute on chronic? Which ventricle is involved? Is this cardiac driven or are other organs the driving factor? Single ventricle involvement is better than bi-ventricular failure. Cardiogenic collapse due to an overdose may have a better outcome than a massive AMI.
Risk modifiers: how old is the patient? What are the comorbidities? Did the patient have a cardiac arrest? What is the patient’s mental status? Some factors are modifiable, but others are not. The concept of chronologic vs. physiologic age may come into play. A frail 40 year old with stage 4 cancer and end stage renal failure may be assessed differently than a 70 year old with mild hypertension and an AMI.
The SCAI stages of CS are a pragmatic way to assess patients with an acute presentation of CS. These stages have defined criteria and treatment recommendations for all patients. The three-axis model allows the clinician to individualize patient care based on shock severity, etiology/phenotype, and risk modification. The goal of these stages is to identify and aggressively treat patients with CS, as well as identify when treatment is failing and additional therapies may be needed.
Dr. Gaillard is Associate Professor in the Departments of Anesthesiology, Section on Critical Care; Internal Medicine, Section on Pulmonology, Critical Care, Allergy, and Immunologic Diseases; and Emergency Medicine; Wake Forest School of Medicine, Winston-Salem, N.C.
Cardiogenic shock (CS) is being recognized more often in critically ill patients. This increased prevalence is likely due to a better understanding of CS and the benefit of improving cardiac output (CO) to ensure adequate oxygen delivery (DO2).
CS is often, but not always, caused by a cardiac dysfunction. The heart is not able to provide adequate DO2 to the tissues. Hypoperfusion ensues. The body attempts to compensate for the poor perfusion by increasing heart rate, vasoconstriction, and shunting blood flow to vital organs. These compensatory mechanisms worsen perfusion by increasing myocardial ischemia which further worsens cardiac dysfunction. This is known as the downward spiral of CS (Ann Intern Med. 1999 Jul 6;131[1]).
There is a number of different etiologies for CS. Historically, acute myocardial infarctions (AMI) was the most common cause. In the last 20 years, AMI-induced CS has become less prevalent due to more aggressive reperfusion strategies. CS due to etiologies such as cardiomyopathy, myocarditis, right ventricle failure, and valvular pathologies have become more common. While the overarching goal is to restore DO2 to the tissue, the optimal treatment may differ based on the etiology of the CS. The Society for Cardiovascular Angiography and Intervention (SCAI) published CS classification stages in 2019 and then updated the stages 2022 (J Am Coll Cardiol. 2022 Mar 8;79[9]:933-46). In addition to the stages, there is now a three-axis model to address risk stratification. These classifications are a practically means of identifying and treating patients presenting with or concern for acute CS.
Stage A (At Risk) patients are not experiencing CS, but they are the at risk population. The patient’s hemodynamics, physical exam, and markers of hypoperfusion are normal. Stage A includes patients who have had a recent AMI or have heart failure.
Stage B (Beginning) patients have evidence of hemodynamic instability but are able to maintain tissue perfusion. These patients will have true or relative hypotension or tachycardia (in an attempt to maintain CO). Distal perfusion is adequate, but signs of ensuing decompensation (eg, elevated jugular venous pressure [JVP]) are present. Lactate is <2.0 mmol/L. Clinicians must be vigilant and treat these patients aggressively, so they do not decompensate further. It can be difficult to identify these patients because their blood pressure may be “normal,” but upon investigation, the blood pressure is actually a drop from the patient’s baseline.
Chronic heart failure patients with a history of depressed cardiac function will often have periods of cardiac decompensation between stages A and B. These patients are able to maintain perfusion for longer periods of time before further decompensation with hypoperfusion. If and when they do decompensate, they will often have a steep downward trajectory, so it is advantageous to the patient to be aggressive early.
Stage C (Classic) patients have evidence of tissue hypoperfusion. While these patients will often have true or relative hypotension, it is not a definition of stage C. These patients have evidence of volume overload with elevated JVP and rales throughout their lung fields. They will have poor distal perfusion and cool extremities that may become mottled. Lactate is ≥ 2 mmol/L. B-type natriuretic peptide (BNP) and liver function test (LFTs) results are elevated, and urine output is diminished. If a pulmonary arterial catheter is placed (highly recommended), the cardiac index (CI) is < 2.2 L/min/m2 and the pulmonary capillary wedge pressure (PCWP) is > 15 mm Hg. These patients look like what many clinicians think of when they think of CS.
These patients need better tissue perfusion. Inotropic support is needed to augment CO and DO2. Pharmacologic support is often the initial step. These patients also benefit from volume removal. This is usually accomplished with aggressive diuresis with a loop diuretic.
Stage D (Deteriorating) patients have failed initial treatment with single inotropic support. Hypoperfusion is not getting better and is often worsening. Lactate is staying > 2 mmol/L or rising. BNP and LFTs are also rising. These patients require additional inotropes and usually need vasopressors. Mechanical cardiac support (MCS) is often needed in addition to pharmacologic inotropic support.
Stage E (Extremis) patients have actual or impending circulatory collapse. These patients are peri-arrest with profound hypotension, lactic acidosis (often > 8 mmol/L), and unconsciousness. These patients are worsening despite multiple strategies to augment CO and DO2. These patients will likely die without emergent veno-arterial (VA) extracorporeal membrane oxygenation (ECMO). The goal of treatment is to stabilize the patient as quickly as possible to prevent cardiac arrest.
In addition to the stage of CS, SCAI developed the three-axis model of risk stratification as a conceptual model to be used for evaluation and prognostication. Etiology and phenotype, shock severity, and risk modifiers are factors related to patient outcomes from CS. This model is a way to individualize treatment to a specific patient.
Shock severity: What is the patient’s shock stage? What are the hemodynamics and metabolic abnormalities? What are the doses of the inotropes or vasopressors? Risk goes up with higher shock stages and vasoactive agent doses and worsening metabolic disturbances or hemodynamics.
Phenotype and etiology: what is the clinical etiology of the patient’s CS? Is this acute or acute on chronic? Which ventricle is involved? Is this cardiac driven or are other organs the driving factor? Single ventricle involvement is better than bi-ventricular failure. Cardiogenic collapse due to an overdose may have a better outcome than a massive AMI.
Risk modifiers: how old is the patient? What are the comorbidities? Did the patient have a cardiac arrest? What is the patient’s mental status? Some factors are modifiable, but others are not. The concept of chronologic vs. physiologic age may come into play. A frail 40 year old with stage 4 cancer and end stage renal failure may be assessed differently than a 70 year old with mild hypertension and an AMI.
The SCAI stages of CS are a pragmatic way to assess patients with an acute presentation of CS. These stages have defined criteria and treatment recommendations for all patients. The three-axis model allows the clinician to individualize patient care based on shock severity, etiology/phenotype, and risk modification. The goal of these stages is to identify and aggressively treat patients with CS, as well as identify when treatment is failing and additional therapies may be needed.
Dr. Gaillard is Associate Professor in the Departments of Anesthesiology, Section on Critical Care; Internal Medicine, Section on Pulmonology, Critical Care, Allergy, and Immunologic Diseases; and Emergency Medicine; Wake Forest School of Medicine, Winston-Salem, N.C.
Fluids or vasopressors: Is sepsis management that simple?
In recent months, we have seen the results of the much awaited Crystalloid Liberal or Vasopressors Early Resuscitation in Sepsis (CLOVERS) trial showing that a restrictive fluid and early vasopressor strategy initiated on arrival of patients with sepsis and hypotension in the ED did not result in decreased mortality compared with a liberal fluid approach (PETAL Network. www.nejm.org/doi/10.1056/NEJMoa2202707). The March 2023 issue of CHEST Physician provided a synopsis of the trial highlighting several limitations (Splete H. CHEST Physician. 2023;18[3]:1). Last year in 2022, the Conservative versus Liberal Approach to Fluid Therapy in Septic Shock (CLASSIC) trial also showed no difference in mortality with restrictive fluid compared with standard fluid in patients with septic shock in the ICU already receiving vasopressor therapy (Meyhoff TS, et al. N Engl J Med. 2022;386[26]:2459). Did their results suggest a “you can do what you want” approach? Is the management of sepsis and septic shock limited to fluids vs vasopressors? Hopefully, the ongoing studies ARISE FLUIDS (NCT04569942), EVIS (NCT05179499), FRESHLY (NCT05453565), 1BED (NCT05273034), and REDUCE (NCT04931485) will further address these questions.
In the meantime, I continue to admit and care for patients with sepsis in the ICU. One example was a 72-year-old woman with a history of stroke, coronary artery disease, diabetes, and chronic kidney disease presenting with 3 days of progressive cough and dyspnea. In the ED, temperature was 38.2° C, heart rate 120 beats per min, respiratory rate 28/min, blood pressure 82/48 mm Hg, and weight 92 kg. She had audible crackles in the left lower lung. Her laboratory and imaging results supported a diagnosis of sepsis due to severe community-acquired pneumonia, including the following values: white blood cell 18.2 million/mm3; lactate 3.8 mmol/L; and creatinine 4.3 mg/dL.
While in the ED, the patient received 1 liter of crystalloid fluids and appropriate broad spectrum antibiotics. Repeat lactate value was 2.8 mmol/L. Patient’s blood pressure then decreased to 85/42 mm Hg. Norepinephrine was started peripherally and titrated to 6 mcg/min to achieve blood pressure 104/56 mm Hg. No further fluid administration was given, and the patient was admitted to the medical ICU. On admission, a repeat lactate had increased to 3.4 mmol/L with blood pressure of 80/45 mm Hg. Instead of further escalating vasopressor administration, she received 2 L of fluid and continued at 150 mL/h. Shortly after, norepinephrine was titrated off. Fluid resuscitation was then deescalated. We transfered the patient to the general ward within 12 hours of ICU admission.
Could we have avoided ICU admission and critical care resource utilization if the patient had received more optimal fluid resuscitation in the ED?
While our fear of fluids (or hydrophobia) may be unwarranted, the management of this patient was a common example of fluid restriction in sepsis (Jaehne AK, et al. Crit Care Med. 2016;44[12]:2263). By clinical criteria, she was in septic shock (requiring vasopressor) and appropriately required ICU admission. But, I would posit that the patient had severe sepsis based on pre-Sepsis 3 criteria. Optimal initial fluid resuscitation would have prevented her from requiring vasopressor and progressing to septic shock with ICU admission. Unfortunately, the patient’s care reflected the objective of CLOVERS and its results. Other than the lack of decreased mortality, decreased ventilator use, decreased renal replacement therapy, and decreased hospital length of stay, restricting fluids resulted in an increase of 8.1% (95% confidence interval 3.3 to 12.8) ICU utilization. Furthermore, the data and safety monitoring committee halted the trial for futility at two-thirds of enrollment. One must wonder if CLOVERS had completed its intended enrollment of 2,320 patients, negative outcomes would have occurred.
Should an astute clinician interpret the results of the CLOVERS and CLASSIC trials as “Fluids, it doesn’t matter, so I can do what I want?” Absolutely not! The literature is abundant with studies showing that increasing dose and/or number of vasopressors is associated with higher mortality in septic shock. One example is a recent multicenter prospective cohort study examining the association of vasopressor dosing during the first 24 hours and 30-day mortality in septic shock over 33 hospitals (Roberts RJ, et al. Crit Care Med. 2020;48[10]:1445).
Six hundred and sixteen patients were enrolled with 31% 30-day mortality. In 24 hours after shock diagnosis, patients received a median of 3.4 (1.9-5.3) L of fluids and 8.5 mcg/min norepinephrine equivalent. During the first 6 hours, increasing vasopressor dosing was associated with increased odds of mortality. Every 10 mcg/min increase in norepinephrine over the 24-hour period was associated with a 33% increased odds of mortality. Patients who received no fluids but 35 mcg/min norepinephrine in 6 hours had the highest mortality of 50%. As fluid volume increased, the association between vasopressor dosing and mortality decreased, such that at least 2 L of fluid during the first 6 hours was required for this association to become nonsignificant. Based on these results and a number of past studies, we should be cautious in believing that a resuscitation strategy favoring vasopressors would result in a better outcome.
Shock resuscitation is complex, and there is no one-size-fits-all approach. With the present climate, the success of resuscitation has been simplified to assessing fluid responsiveness. Trainees learn to identify the inferior vena cava and lung B-lines by ultrasound. With more advanced technology, stroke volume variation is considered. And, let us not forget the passive leg raise. Rarely can our fellows and residents recite the components of oxygen delivery as targets of shock resuscitation: preload, afterload, contractility, hemoglobin, and oxygen saturation. Another patient example comes to mind when fluid responsiveness alone is inadequate.
Our patient was a 46-year-old man now day 4 in the ICU with Klebsiella bacteremia and acute cholecystitis undergoing medical management. His comorbidities included diabetes, obesity, hypertension, and cardiomyopathy with ejection fraction 35%. He was supported sson mechanical ventilation, norepinephrine 20 mcg/min, and receiving appropriate antibiotics. For hemodynamic monitoring, a central venous and arterial catheter have been placed. The patient had a heart rate 92 beats per min, mean arterial pressure (MAP) 57 mm Hg, central venous pressure (CVP) 26 mm Hg, stroke volume variation (SVV) 9%, cardiac output (CO) 2.5 L/min, and central venous oxygen saturation (ScvO2) 42%.
Based on these parameters, we initiated dobutamine at 2.5 mcg/kg/min, which was then titrated to 20 mcg/kg/min over 2 hours to achieve ScvO2 72%. Interestingly, CVP had decreased to 18 mm Hg, SVV increased to 16%, with CO 4.5 L/min. MAP also increased to 68 mm Hg. We then administered 1-L fluid bolus with the elevated SVV. Given the patient’s underlying cardiomyopathy, CVP < 20 mm Hg appeared to indicate a state of fluid responsiveness. After our fluid administration, heart rate 98 beats per min, MAP 70 mm Hg, CVP increased to 21 mm Hg, SVV 12%, CO 4.7 L/min, and ScvO2 74%. In acknowledging a mixed hypovolemic, cardiogenic, and septic shock, we had optimized his hemodynamic state. Importantly, during this exercise of hemodynamic manipulation, we were able to decrease norepinephrine to 8 mcg/min, maintaining dobutamine at 20 mcg/kg/min.
The above case illustrates that the hemodynamic perturbations in sepsis and septic shock are not simple. Patients do not present with a single shock state. An infection progressing to shock often is confounded by hypovolemia and underlying comorbidities, such as cardiac dysfunction. Without considering the complex physiology, our desire to continue the debate of fluids vs vasopressors is on the brink of taking us back several decades when the management of sepsis was to start a fluid bolus, administer “Rocephin,” and initiate dopamine. But I remind myself that we have made advances – now it’s 1 L lactated Ringer’s, administer “vanco and zosyn,” and initiate norepinephrine.
In recent months, we have seen the results of the much awaited Crystalloid Liberal or Vasopressors Early Resuscitation in Sepsis (CLOVERS) trial showing that a restrictive fluid and early vasopressor strategy initiated on arrival of patients with sepsis and hypotension in the ED did not result in decreased mortality compared with a liberal fluid approach (PETAL Network. www.nejm.org/doi/10.1056/NEJMoa2202707). The March 2023 issue of CHEST Physician provided a synopsis of the trial highlighting several limitations (Splete H. CHEST Physician. 2023;18[3]:1). Last year in 2022, the Conservative versus Liberal Approach to Fluid Therapy in Septic Shock (CLASSIC) trial also showed no difference in mortality with restrictive fluid compared with standard fluid in patients with septic shock in the ICU already receiving vasopressor therapy (Meyhoff TS, et al. N Engl J Med. 2022;386[26]:2459). Did their results suggest a “you can do what you want” approach? Is the management of sepsis and septic shock limited to fluids vs vasopressors? Hopefully, the ongoing studies ARISE FLUIDS (NCT04569942), EVIS (NCT05179499), FRESHLY (NCT05453565), 1BED (NCT05273034), and REDUCE (NCT04931485) will further address these questions.
In the meantime, I continue to admit and care for patients with sepsis in the ICU. One example was a 72-year-old woman with a history of stroke, coronary artery disease, diabetes, and chronic kidney disease presenting with 3 days of progressive cough and dyspnea. In the ED, temperature was 38.2° C, heart rate 120 beats per min, respiratory rate 28/min, blood pressure 82/48 mm Hg, and weight 92 kg. She had audible crackles in the left lower lung. Her laboratory and imaging results supported a diagnosis of sepsis due to severe community-acquired pneumonia, including the following values: white blood cell 18.2 million/mm3; lactate 3.8 mmol/L; and creatinine 4.3 mg/dL.
While in the ED, the patient received 1 liter of crystalloid fluids and appropriate broad spectrum antibiotics. Repeat lactate value was 2.8 mmol/L. Patient’s blood pressure then decreased to 85/42 mm Hg. Norepinephrine was started peripherally and titrated to 6 mcg/min to achieve blood pressure 104/56 mm Hg. No further fluid administration was given, and the patient was admitted to the medical ICU. On admission, a repeat lactate had increased to 3.4 mmol/L with blood pressure of 80/45 mm Hg. Instead of further escalating vasopressor administration, she received 2 L of fluid and continued at 150 mL/h. Shortly after, norepinephrine was titrated off. Fluid resuscitation was then deescalated. We transfered the patient to the general ward within 12 hours of ICU admission.
Could we have avoided ICU admission and critical care resource utilization if the patient had received more optimal fluid resuscitation in the ED?
While our fear of fluids (or hydrophobia) may be unwarranted, the management of this patient was a common example of fluid restriction in sepsis (Jaehne AK, et al. Crit Care Med. 2016;44[12]:2263). By clinical criteria, she was in septic shock (requiring vasopressor) and appropriately required ICU admission. But, I would posit that the patient had severe sepsis based on pre-Sepsis 3 criteria. Optimal initial fluid resuscitation would have prevented her from requiring vasopressor and progressing to septic shock with ICU admission. Unfortunately, the patient’s care reflected the objective of CLOVERS and its results. Other than the lack of decreased mortality, decreased ventilator use, decreased renal replacement therapy, and decreased hospital length of stay, restricting fluids resulted in an increase of 8.1% (95% confidence interval 3.3 to 12.8) ICU utilization. Furthermore, the data and safety monitoring committee halted the trial for futility at two-thirds of enrollment. One must wonder if CLOVERS had completed its intended enrollment of 2,320 patients, negative outcomes would have occurred.
Should an astute clinician interpret the results of the CLOVERS and CLASSIC trials as “Fluids, it doesn’t matter, so I can do what I want?” Absolutely not! The literature is abundant with studies showing that increasing dose and/or number of vasopressors is associated with higher mortality in septic shock. One example is a recent multicenter prospective cohort study examining the association of vasopressor dosing during the first 24 hours and 30-day mortality in septic shock over 33 hospitals (Roberts RJ, et al. Crit Care Med. 2020;48[10]:1445).
Six hundred and sixteen patients were enrolled with 31% 30-day mortality. In 24 hours after shock diagnosis, patients received a median of 3.4 (1.9-5.3) L of fluids and 8.5 mcg/min norepinephrine equivalent. During the first 6 hours, increasing vasopressor dosing was associated with increased odds of mortality. Every 10 mcg/min increase in norepinephrine over the 24-hour period was associated with a 33% increased odds of mortality. Patients who received no fluids but 35 mcg/min norepinephrine in 6 hours had the highest mortality of 50%. As fluid volume increased, the association between vasopressor dosing and mortality decreased, such that at least 2 L of fluid during the first 6 hours was required for this association to become nonsignificant. Based on these results and a number of past studies, we should be cautious in believing that a resuscitation strategy favoring vasopressors would result in a better outcome.
Shock resuscitation is complex, and there is no one-size-fits-all approach. With the present climate, the success of resuscitation has been simplified to assessing fluid responsiveness. Trainees learn to identify the inferior vena cava and lung B-lines by ultrasound. With more advanced technology, stroke volume variation is considered. And, let us not forget the passive leg raise. Rarely can our fellows and residents recite the components of oxygen delivery as targets of shock resuscitation: preload, afterload, contractility, hemoglobin, and oxygen saturation. Another patient example comes to mind when fluid responsiveness alone is inadequate.
Our patient was a 46-year-old man now day 4 in the ICU with Klebsiella bacteremia and acute cholecystitis undergoing medical management. His comorbidities included diabetes, obesity, hypertension, and cardiomyopathy with ejection fraction 35%. He was supported sson mechanical ventilation, norepinephrine 20 mcg/min, and receiving appropriate antibiotics. For hemodynamic monitoring, a central venous and arterial catheter have been placed. The patient had a heart rate 92 beats per min, mean arterial pressure (MAP) 57 mm Hg, central venous pressure (CVP) 26 mm Hg, stroke volume variation (SVV) 9%, cardiac output (CO) 2.5 L/min, and central venous oxygen saturation (ScvO2) 42%.
Based on these parameters, we initiated dobutamine at 2.5 mcg/kg/min, which was then titrated to 20 mcg/kg/min over 2 hours to achieve ScvO2 72%. Interestingly, CVP had decreased to 18 mm Hg, SVV increased to 16%, with CO 4.5 L/min. MAP also increased to 68 mm Hg. We then administered 1-L fluid bolus with the elevated SVV. Given the patient’s underlying cardiomyopathy, CVP < 20 mm Hg appeared to indicate a state of fluid responsiveness. After our fluid administration, heart rate 98 beats per min, MAP 70 mm Hg, CVP increased to 21 mm Hg, SVV 12%, CO 4.7 L/min, and ScvO2 74%. In acknowledging a mixed hypovolemic, cardiogenic, and septic shock, we had optimized his hemodynamic state. Importantly, during this exercise of hemodynamic manipulation, we were able to decrease norepinephrine to 8 mcg/min, maintaining dobutamine at 20 mcg/kg/min.
The above case illustrates that the hemodynamic perturbations in sepsis and septic shock are not simple. Patients do not present with a single shock state. An infection progressing to shock often is confounded by hypovolemia and underlying comorbidities, such as cardiac dysfunction. Without considering the complex physiology, our desire to continue the debate of fluids vs vasopressors is on the brink of taking us back several decades when the management of sepsis was to start a fluid bolus, administer “Rocephin,” and initiate dopamine. But I remind myself that we have made advances – now it’s 1 L lactated Ringer’s, administer “vanco and zosyn,” and initiate norepinephrine.
In recent months, we have seen the results of the much awaited Crystalloid Liberal or Vasopressors Early Resuscitation in Sepsis (CLOVERS) trial showing that a restrictive fluid and early vasopressor strategy initiated on arrival of patients with sepsis and hypotension in the ED did not result in decreased mortality compared with a liberal fluid approach (PETAL Network. www.nejm.org/doi/10.1056/NEJMoa2202707). The March 2023 issue of CHEST Physician provided a synopsis of the trial highlighting several limitations (Splete H. CHEST Physician. 2023;18[3]:1). Last year in 2022, the Conservative versus Liberal Approach to Fluid Therapy in Septic Shock (CLASSIC) trial also showed no difference in mortality with restrictive fluid compared with standard fluid in patients with septic shock in the ICU already receiving vasopressor therapy (Meyhoff TS, et al. N Engl J Med. 2022;386[26]:2459). Did their results suggest a “you can do what you want” approach? Is the management of sepsis and septic shock limited to fluids vs vasopressors? Hopefully, the ongoing studies ARISE FLUIDS (NCT04569942), EVIS (NCT05179499), FRESHLY (NCT05453565), 1BED (NCT05273034), and REDUCE (NCT04931485) will further address these questions.
In the meantime, I continue to admit and care for patients with sepsis in the ICU. One example was a 72-year-old woman with a history of stroke, coronary artery disease, diabetes, and chronic kidney disease presenting with 3 days of progressive cough and dyspnea. In the ED, temperature was 38.2° C, heart rate 120 beats per min, respiratory rate 28/min, blood pressure 82/48 mm Hg, and weight 92 kg. She had audible crackles in the left lower lung. Her laboratory and imaging results supported a diagnosis of sepsis due to severe community-acquired pneumonia, including the following values: white blood cell 18.2 million/mm3; lactate 3.8 mmol/L; and creatinine 4.3 mg/dL.
While in the ED, the patient received 1 liter of crystalloid fluids and appropriate broad spectrum antibiotics. Repeat lactate value was 2.8 mmol/L. Patient’s blood pressure then decreased to 85/42 mm Hg. Norepinephrine was started peripherally and titrated to 6 mcg/min to achieve blood pressure 104/56 mm Hg. No further fluid administration was given, and the patient was admitted to the medical ICU. On admission, a repeat lactate had increased to 3.4 mmol/L with blood pressure of 80/45 mm Hg. Instead of further escalating vasopressor administration, she received 2 L of fluid and continued at 150 mL/h. Shortly after, norepinephrine was titrated off. Fluid resuscitation was then deescalated. We transfered the patient to the general ward within 12 hours of ICU admission.
Could we have avoided ICU admission and critical care resource utilization if the patient had received more optimal fluid resuscitation in the ED?
While our fear of fluids (or hydrophobia) may be unwarranted, the management of this patient was a common example of fluid restriction in sepsis (Jaehne AK, et al. Crit Care Med. 2016;44[12]:2263). By clinical criteria, she was in septic shock (requiring vasopressor) and appropriately required ICU admission. But, I would posit that the patient had severe sepsis based on pre-Sepsis 3 criteria. Optimal initial fluid resuscitation would have prevented her from requiring vasopressor and progressing to septic shock with ICU admission. Unfortunately, the patient’s care reflected the objective of CLOVERS and its results. Other than the lack of decreased mortality, decreased ventilator use, decreased renal replacement therapy, and decreased hospital length of stay, restricting fluids resulted in an increase of 8.1% (95% confidence interval 3.3 to 12.8) ICU utilization. Furthermore, the data and safety monitoring committee halted the trial for futility at two-thirds of enrollment. One must wonder if CLOVERS had completed its intended enrollment of 2,320 patients, negative outcomes would have occurred.
Should an astute clinician interpret the results of the CLOVERS and CLASSIC trials as “Fluids, it doesn’t matter, so I can do what I want?” Absolutely not! The literature is abundant with studies showing that increasing dose and/or number of vasopressors is associated with higher mortality in septic shock. One example is a recent multicenter prospective cohort study examining the association of vasopressor dosing during the first 24 hours and 30-day mortality in septic shock over 33 hospitals (Roberts RJ, et al. Crit Care Med. 2020;48[10]:1445).
Six hundred and sixteen patients were enrolled with 31% 30-day mortality. In 24 hours after shock diagnosis, patients received a median of 3.4 (1.9-5.3) L of fluids and 8.5 mcg/min norepinephrine equivalent. During the first 6 hours, increasing vasopressor dosing was associated with increased odds of mortality. Every 10 mcg/min increase in norepinephrine over the 24-hour period was associated with a 33% increased odds of mortality. Patients who received no fluids but 35 mcg/min norepinephrine in 6 hours had the highest mortality of 50%. As fluid volume increased, the association between vasopressor dosing and mortality decreased, such that at least 2 L of fluid during the first 6 hours was required for this association to become nonsignificant. Based on these results and a number of past studies, we should be cautious in believing that a resuscitation strategy favoring vasopressors would result in a better outcome.
Shock resuscitation is complex, and there is no one-size-fits-all approach. With the present climate, the success of resuscitation has been simplified to assessing fluid responsiveness. Trainees learn to identify the inferior vena cava and lung B-lines by ultrasound. With more advanced technology, stroke volume variation is considered. And, let us not forget the passive leg raise. Rarely can our fellows and residents recite the components of oxygen delivery as targets of shock resuscitation: preload, afterload, contractility, hemoglobin, and oxygen saturation. Another patient example comes to mind when fluid responsiveness alone is inadequate.
Our patient was a 46-year-old man now day 4 in the ICU with Klebsiella bacteremia and acute cholecystitis undergoing medical management. His comorbidities included diabetes, obesity, hypertension, and cardiomyopathy with ejection fraction 35%. He was supported sson mechanical ventilation, norepinephrine 20 mcg/min, and receiving appropriate antibiotics. For hemodynamic monitoring, a central venous and arterial catheter have been placed. The patient had a heart rate 92 beats per min, mean arterial pressure (MAP) 57 mm Hg, central venous pressure (CVP) 26 mm Hg, stroke volume variation (SVV) 9%, cardiac output (CO) 2.5 L/min, and central venous oxygen saturation (ScvO2) 42%.
Based on these parameters, we initiated dobutamine at 2.5 mcg/kg/min, which was then titrated to 20 mcg/kg/min over 2 hours to achieve ScvO2 72%. Interestingly, CVP had decreased to 18 mm Hg, SVV increased to 16%, with CO 4.5 L/min. MAP also increased to 68 mm Hg. We then administered 1-L fluid bolus with the elevated SVV. Given the patient’s underlying cardiomyopathy, CVP < 20 mm Hg appeared to indicate a state of fluid responsiveness. After our fluid administration, heart rate 98 beats per min, MAP 70 mm Hg, CVP increased to 21 mm Hg, SVV 12%, CO 4.7 L/min, and ScvO2 74%. In acknowledging a mixed hypovolemic, cardiogenic, and septic shock, we had optimized his hemodynamic state. Importantly, during this exercise of hemodynamic manipulation, we were able to decrease norepinephrine to 8 mcg/min, maintaining dobutamine at 20 mcg/kg/min.
The above case illustrates that the hemodynamic perturbations in sepsis and septic shock are not simple. Patients do not present with a single shock state. An infection progressing to shock often is confounded by hypovolemia and underlying comorbidities, such as cardiac dysfunction. Without considering the complex physiology, our desire to continue the debate of fluids vs vasopressors is on the brink of taking us back several decades when the management of sepsis was to start a fluid bolus, administer “Rocephin,” and initiate dopamine. But I remind myself that we have made advances – now it’s 1 L lactated Ringer’s, administer “vanco and zosyn,” and initiate norepinephrine.