User login
Psoriasis is a chronic inflammatory disorder associated with increased expression of proinflammatory mediators such as tumor necrosis factor (TNF) α.1 Anti-TNF drugs (eg, etanercept, adalimumab, infliximab) were proven to be highly effective for the treatment of psoriasis over the last 2 decades.2 Interestingly, TNF inhibitors have been thought to be effective in improving insulin resistance in patients with type 2 diabetes mellitus (DM) by blocking TNF, which is involved in the inflammatory condition in DM.
Type 2 DM is a common chronic condition characterized by hyperglycemia resulting from a combination of peripheral and hepatic insulin resistance and impaired insulin secretion.3 It is characterized by defects in both insulin secretion and insulin sensitivity.4,5 Type 2 DM has been linked with a marked increase in cardiovascular disease, morbidity, and mortality.6 Evidence-based literature regarding the role of chronic inflammation as an important pathogenetic factor in type 2 DM has been growing.7-9 It also has been suggested that pharmacological strategies to reduce this underlying associated silent inflammation are useful in treating DM, which also is true for other conditions such as obesity, metabolic syndrome, and cardiovascular diseases.10
Psoriasis predisposes patients to insulin resistance and may put them at risk for developing DM.11,12 The association between psoriasis and DM suggests that systemic immunosuppression also may diminish the risk for developing DM. Several longitudinal studies have found that TNF inhibitors improve insulin resistance.13,14 Dandona et al15 reported a considerable decrease of TNF-α levels with the concurrent restoration of insulin sensitivity during weight loss.
Pereira et al16 found a notable connection between psoriasis, DM, and insulin resistance with an odds ratio of 2.63 of abnormal glucose homeostasis in patients with psoriasis compared to controls. Yazdani-Biuki et al17 proved that extended administration of anti–TNF-α antibody was able to improve insulin sensitivity in insulin-resistant patients. The same finding was established by Kiortsis et al.14
In this prospective controlled study, we evaluated the effects of anti-TNF agents on insulin resistance and sensitivity in psoriasis patients with type 2 DM treated with anti-TNF agents.
Methods
A total of 70 patients attending the dermatological outpatient clinics at Farwaniya Hospital (Kuwait City, Kuwait) between January 2012 and September 2014 were enrolled in the study and were randomly distributed into 2 equal groups (n=35 each). The study was approved by the hospital ethics committee. Patients were included in the study if they had moderate to severe psoriasis (ie, psoriasis area severity index score ≥10) with documented type 2 DM and high fasting plasma glucose (FPG) levels (ie, >10 mmol/L). Patients who were currently being treated with oral hypoglycemic agents but not insulin therapy were included in the study. Patients were excluded if they had phototherapy within the last 4 weeks, prior biologic therapy, current and prior insulin therapy, a change in oral hypoglycemic drug dosage in the last 2 months, other serious systemic illness (eg, malignancy, hepatitis B or C virus, metabolic or endocrine disease), and/or abnormal laboratory investigations (eg, liver/kidney profile, chest radiograph abnormality, positive Mantoux test). All of the patients enrolled in the study provided informed consent and underwent routine baseline investigations including complete blood cell counts, general health profile, chest radiograph, antinuclear antibody test, Mantoux test, FPG and insulin levels, glycated hemoglobin (HbA1C), homeostasis model assessment (HOMA), routine urine examination, and enzyme-linked immunosorbent assay for tuberculosis. The study group was treated with anti-TNF agents, and the control group received conventional antipsoriatic medications.
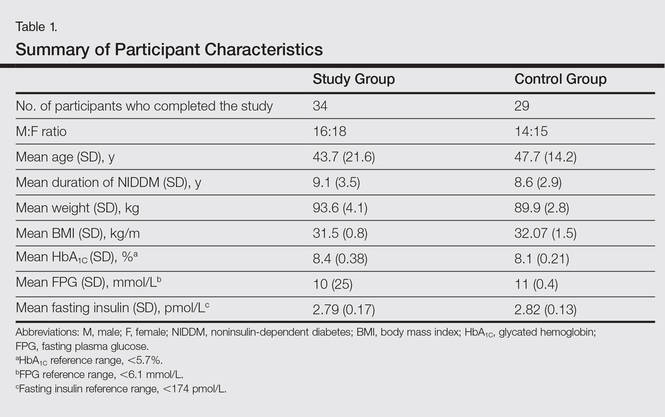
All the patients included in the study had high FPG levels (ie, >10 mmol/L) at the time of enrollment and were currently being treated with oral hypoglycemic agents. The dose of oral hypoglycemic agents was unchanged for at least 2 months before entry into the run-in period and throughout the 24-week study period. Demographic details including sex, age, medical history (eg, type of psoriasis, prior and concomitant treatments) were collected from the participants’ clinical histories. Participants from both groups were appropriately matched in terms of age, sex, body weight, body mass index, and duration of type 2 DM (Table 1). The primary end point of the study was to analyze and compare clinical and serum data collected at baseline and after 24 weeks of therapy.
A complete biochemical profile was repeated in both groups after 24 weeks of treatment. Each participant underwent a baseline short insulin sensitivity test immediately before treatment and at 4 and 24 weeks of treatment. We assessed insulin resistance via HOMA, calculated as follows: FPG [mmol/L] × fasting serum insulin [pmol/L] / 22.5.18 Oral glucose tolerance tests were performed to calculate the HOMA of insulin resistance. Serum insulin concentration was determined via enzyme-linked immunosorbent assay.
Statistical analysis was performed using SPSS software (version 12.0). Continuous patient characteristics were analyzed using mean and SD as well as discrete data as counts and proportions. Association was examined using χ2 tests for categorical variables and 2-sided t test/Wilcoxon rank sum test for continuous variables. Analysis of variance was used to compare the results in 3 different anti-TNF agents used in the study group.
Results
Of the 35 participants enrolled in the study group, 34 (97.1%) completed the study and were evaluated. The study group included 16 men and 18 women aged 19 to 63 years (mean age [SD], 43.7 [21.6] years) who were treated with TNF-α inhibitors—8 participants with etanercept, 14 with adalimumab, and 12 with infliximab—according to the standard dosage schedule for 24 weeks.
Of the 35 participants enrolled in the control group, 29 (82.9%) completed the study and were evaluated. Six patients did not follow up for the complete duration of the 24-week study period and were not evaluated. The control group included 14 men and 15 women aged 18 to 65 years (mean age [SD], 47.7 [14.2] years) who were treated with other systemic therapies—8 participants with topical corticosteroids or calcipotriol only, 7 with cyclosporine A, and 14 with methotrexate. The dose of the drug was kept stable throughout the 24-week study period.
Demographic and baseline characteristics for all participants are shown in Table 1. There were no significant differences in demographic or baseline characteristics among the study group versus the control group, and all participants were similar in age; body mass index; as well as FPG, fasting insulin, and HbA1C levels.
At baseline, both study and control participants had elevated mean (SD) FPG levels (10 [25] mmol/L and 11 [0.4] mmol/L, respectively), fasting insulin levels (2.79 [0.17] pmol/L and 2.82 [0.13] pmol/L, respectively), and HbA1C levels (8.4% [0.38%] and 8.1% [0.21%], respectively)(Table 1).
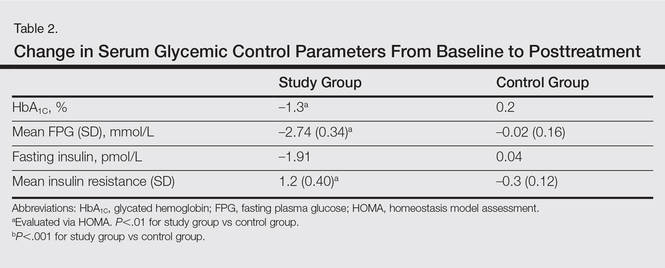
The study group showed significant improvements in glycemic control at the end of the study (Table 2). At week 24, study group participants had a mean (SD) decrease in FPG levels of 2.74 (0.34) mmol/L versus 0.02 (0.16) mmol/L in the control group. This difference between the 2 groups after 24 weeks was found to be statistically significant (P<.01). On further analysis of the study group, no statistically significant difference (P>.01) was noted in the 3 anti-TNF agents used. Compared to the control group, the study group showed a significant decrease from baseline values of FPG and HbA1C (P<.01). Fasting insulin levels decreased significantly for study group participants as compared with control (–1.91 pmol/L vs 0.04 pmol/L)(P<.001)(Table 2). However, on analysis of the 3 anti-TNF agents, no statistically significant difference was found (P>.05). Participants in the control group showed no significant change in fasting insulin and FPG levels.
To confirm that there was a change in insulin sensitivity in response to TNF-α inhibitors, we analyzed FPG and fasting insulin values using the HOMA method. There was no change in mean relative insulin resistance in the control group in response to therapy (mean [SD], 5.4 [0.31] vs 5.6 [0.15], before vs after therapy), while there was mild improvement in relative insulin resistance in the study group (5.9 [0.52] vs 4.8 [0.34], before vs after therapy). There also was a significant difference in the change in relative insulin resistance in response to treatment between the study and control groups (1.2 [0.40] vs –0.3 [0.12]; P<.01)(Table 2).
Comment
There has been an unprecedented rise in the rate of obesity and associated metabolic diseases such as type 2 DM. Following the current trend, it is estimated that the world will have approximately 592 million cases of type 2 DM by the year 2035.19 Almost two-thirds of these patients are estimated to die of cardiovascular diseases.
Although the pathophysiology of type 2 DM is not known, insulin resistance in the muscles and liver as well as failure of pancreatic β cells represent the core of the complex pathophysiology. The associated underlying silent inflammation was thought to have a key role in both insulin resistance and insulin secretory defects seen in type 2 DM. Furthermore, recent data suggest the central role of TNF-α, IL-1, and IL-6 pathways in this inflammation.10 Tumor necrosis factor α has been shown to have a dual effect on insulin resistance as well as pancreatic β cell function. It blocks the function of insulin at the receptor level and has been implicated as a causative factor in obesity-associated insulin resistance and also in the pathogenesis of type 2 DM.20,21 Furthermore, cytokines that activate nuclear factor κβ (a nuclear transcription factor closely involved in the regulation of cellular inflammatory response), such as TNF-α, are thought to be a common denominator for β-cell apoptosis in types 1 and 2 DM.22 Additionally, it has been suggested that TNF-α is a powerful regulator of adipose tissue.23 Neutralizing TNF-α in obese Zucker rats has shown increased insulin sensitivity.3 Tumor necrosis factor α and IL-6 as well as C-reactive protein and plasminogen activator inhibitor 1 are negatively associated with insulin sensitivity.24-27 These findings have led researchers to investigate the role of anti-TNF agents for the management of type 2 DM.28
Psoriasis has now come to be known as a systemic inflammatory disorder and is associated with increased expression of TNF-α. It predisposes patients to insulin resistance and places them at higher risk for developing DM.11,12 Systematic reports recommend that there is a link between psoriasis and DM featured by helper T cell (TH1) cytokines.29 This link can stimulate insulin resistance and metabolic syndrome as well as inflammatory cytokines identified to motivate psoriasis.29,30 The association between psoriasis and type 2 DM proposes a possible pathophysiologic connection between the 2 diseases. Patients with psoriasis have altered T-cell subtype 1 pathways and dysregulated oxidative and angiogenic mechanisms.31,32 Many of these immune pathways may similarly predispose psoriasis patients to impaired glucose tolerance and DM. Inflammation may cause insulin resistance and DM through numerous mechanisms. Systemic inflammation linked with psoriasis may lead to high levels of circulating IL-1, IL-6, and TNF-α that predispose patients to impaired glucose tolerance and type 2 DM.33
Several longitudinal investigations have found that TNF inhibitors improve insulin resistance.13,14,34-38 Gonzalez-Gay et al13 confirmed a rapid beneficial effect of infliximab on insulin resistance and insulin sensitivity in rheumatoid arthritis (RA) patients, which might support the long-term use of drugs that act by blocking TNF-α to diminish the mechanisms implicated in the development of atherosclerosis in patients with RA. Kiortsis et al14 performed a complete biochemical profile before and after 6 months of treatment with infliximab in 17 patients with ankylosing spondylitis and 28 patients with RA. The researchers found a significant decrease of the HOMA index in the percentile of their patients with the highest insulin resistance (P<.01).14
Stagakis et al34 found that 12 weeks of treatment with anti-TNF agents may improve insulin resistance in patients with active RA and high insulin resistance. Treatment with anti-TNF agents was shown to restore the phosphorylation status of serine phosphorylation of insulin receptor substrate 1 (Ser312-IRS-1) and AKT (protein kinase B), which are important mediators in the insulin signaling cascade. The investigators concluded that treatment with anti-TNF agents may improve insulin resistance and sensitivity in RA patients with active disease and high insulin resistance.34
Solomon et al35 studied the link between disease-modifying antirheumatic drugs and DM risk in patients with RA and psoriasis. The authors proposed that initiation of treatment with TNF inhibitors in psoriasis patients was associated with a reduced incidence of DM. The results showed a lower risk for developing DM in patients with psoriasis who were treated with a TNF inhibitor compared with numerous other drugs.35
Marra et al36 studied the effects of etanercept on insulin sensitivity in 9 patients with psoriasis. They reported a decrease in insulin resistance evaluated by HOMA after 24 weeks of etanercept treatment.36 Wambier et al37 reported severe hypoglycemia after initiation of anti-TNF therapy with etanercept in a patient with generalized pustular psoriasis and type 2 DM.
Yazdani-Biuki et al38 reported the case of a patient who demonstrated a relapse of type 2 DM after an interruption of prolonged treatment with infliximab, an anti–TNF-α antibody for psoriatic arthritis. The improvement in insulin sensitivity of this patient has been reported along with post hoc evidence that chronic administration of infliximab improves insulin resistance in a small sample of patients with inflammatory joint diseases.17
Other studies on the effects of TNF inhibitors on insulin resistance and sensitivity have yielded conflicting results. Martínez-Abundis et al39 studied the effects of etanercept on insulin resistance and sensitivity in a randomized trial of psoriatic patients at risk for developing type 2 DM. Results indicated that anti-TNF therapy had no significant influence on insulin sensitivity measured using a hyperinsulinemic clamp during 2 weeks of etanercept treatment in psoriatic patients with risk factors for type 2 DM. The explanation of this discrepancy may be due to the short duration of the study period.
It is still unknown if psoriasis treatment affects a patient’s risk for developing DM. However, Solomon et al35 evaluated the association of incidental DM among patients with prescribed TNF inhibitors or methotrexate and proposed that initiation of treatment with TNF inhibitors was associated with a diminished incidence of DM.
Our study supports and confirms that psoriasis patients treated with TNF-α inhibitors showed improved glycemic indices and insulin resistance compared with control patients treated with other common systemic drugs for psoriasis. We did not take into consideration other conventional risk factors such as hypertension and coronary artery disease. The number of participants included in the current study was not large enough to evaluate each of the anti-TNF agents in a separate group, and participants were not followed up long enough to see the impact of the biochemical changes noted in the results on long-term morbidity or mortality.
Conclusion
Our study confirms a beneficial effect of TNF-α inhibitors on insulin resistance and insulin sensitivity in psoriasis patients with type 2 DM. Treatment with TNF-α inhibitors may have beneficial effects on insulin sensitivity in even the most insulin-resistant patients with psoriasis. The study results may support the hypothesis that long-term use of TNF inhibitors may reduce the mechanisms involved in the development of DM in patients with psoriasis. The improvement in insulin sensitivity may in turn decrease the coronary artery disease risk in these patients. Additional large, prospective, multicenter studies are required to further analyze the effects of anti–TNF-α antibodies on insulin sensitivity and β cell function in insulin-resistant or diabetic psoriasis patients.
- Lowes MA, Bowcock AM, Krueger JG. Pathogenesis and therapy of psoriasis. Nature. 2007;445:866-873.
- Boehncke WH, Prinz J, Gottlieb AB. Biologic therapies for psoriasis. a systematic review. J Rheumatol. 2006;33:1447-1451.
- DeFronzo RA, Bonadonna RC, Ferrannini E. Pathogenesis of NIDDM. a balanced overview. Diabetes Care. 1992;15:318-368.
- DeFronzo RA. Pathogenesis of type 2 diabetes: metabolic and molecular implications for identifying diabetes genes. Diabetes Rev. 1997;4:177-269.
- Reaven GM. Banting Lecture 1988. role of insulin resistance in human disease. Diabetes. 1988;37:1595-1607.
- Erkelens DW. Insulin resistance syndrome and type 2 diabetes mellitus. Am J Cardiol. 2001;88(7B):38J-42J.
- Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87-91.
- Hotamisligil GS, Spiegelman BM. Tumor necrosis factor alpha: a key component of the obesity-diabetes link. Diabetes. 1994;43:1271-1278.
- Van der Poll T, Romijn JA, Endert E, et al. Tumor necrosis factor mimics the metabolic response to acute infection in healthy humans. Am J Physiol. 1991;261:457-465.
- Tabas I, Glass CK. Anti-inflammatory therapy in chronic disease: challenges and opportunities. Science. 2013;339:166-172.
- Qureshi AA, Choi HK, Setty AR, et al. Psoriasis and the risk of diabetes and hypertension: a prospective study of US female nurses. Arch Dermatol. 2009;145:379-382.
- Solomon DH, Love TJ, Canning C, et al. Risk of diabetes among patients with rheumatoid arthritis, psoriatic arthritis and psoriasis. Ann Rheum Dis. 2010;69:2114-2117.
- Gonzalez-Gay MA, De Matias JM, Gonzalez-Juanatey C, et al. Anti-tumor necrosis factor-alpha blockade improves insulin resistance in patients with rheumatoid arthritis. Clin Exp Rheumatol. 2006;24:83-86.
- Kiortsis DN, Mavridis AK, Vasakos S, et al. Effects of infliximab treatment on insulin resistance in patients with rheumatoid arthritis and ankylosing spondylitis. Ann Rheum Dis. 2005;64:765-766.
- Dandona P, Weinstock R, Thusu K, et al. Tumor necrosis factor-α in sera of obese patients: fall with weight loss. J Clin Endocrinol Metabol. 1998;83:2907-2910.
- Pereira RR, Amladi ST, Varthakavi PK. A study of the prevalence of diabetes, insulin resistance, lipid abnormalities, and cardiovascular risk factors in patients with chronic plaque psoriasis. Ind J Dermatol. 2011;56:520-526.
- Yazdani-Biuki B, Stelzl H, Brezinschek HP, et al. Improvement of insulin sensitivity in insulin resistant subjects during prolonged treatment with the anti-TNF-α antibody infliximab. Eur J Clin Invest. 2004;34:641-642.
- Bonora E, Kiechl S, Willeit J, et al. Prevalence of insulin resistance in metabolic disorders. The Bruneck Study. Diabetes. 1998;47:1643-1649.
- Ryden L, Grant PJ, Anker SD, et al. ESC Guidelines on diabetes, prediabetes, and cardiovascular diseases developed in collaboration with the EASD: the Task Force on diabetes, prediabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD). Eur Heart J. 2013;34:3035-3087.
- Peraldi P, Spiegelman B. TNF-alpha and insulin resistance: summary and future prospects. Mol Cell Biochem. 1998;182:169-175.
- Moller DE. Potential role of TNF-alpha in the pathogenesis of insulin resistance and type 2 diabetes. Trends Endocrinol Metab. 2000;11:212-217.
- Mandrup-Poulsen T. Apoptotic signal transduction pathways in diabetes. Biochem Pharmacol. 2003;66:1433-1440.
- Coppack SW. Pro-inflammatory cytokines and adipose tissue. Proc Nutr Soc. 2001;60:349-356.
- Pradhan AD, Manson JE, Rifai N, et al. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327-334.
- Festa A, D’Agostino R Jr, Tracy RP, et al. Insulin Resistance Atherosclerosis Study. elevated levels of acute-phase proteins and plasminogen activator inhibitor-1 predict the development of type 2 diabetes: the insulin resistance atherosclerosis study. Diabetes. 2002;51:1131-1137.
- Meigs JB, O’Donnell CJ, Tofler GH, et al. Hemostatic markers of endothelial dysfunction and risk of incident type 2 diabetes: the Framingham Offspring Study. Diabetes. 2006;55:530-537.
- Liu S, Tinker L, Song Y, et al. A prospective study of inflammatory cytokines and diabetes mellitus in a multiethnic cohort of postmenopausal women. Arch Intern Med. 2007;167:1676-1685.
- Esser N, Paquot N, Scheen AJ. Anti-inflammatory agents to treat or prevent type 2 diabetes, metabolic syndrome and cardiovascular disease. Exp Opin Invest Drugs. 2014;24:1-25.
- Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111-1119.
- Karadag AS, Yavuz B, Ertugrul DT, et al. Is psoriasis a pre-atherosclerotic disease? increased insulin resistance and impaired endothelial function in patients with psoriasis. Int J Dermatol. 2010;49:642-646.
- Armstrong AW, Voyles SV, Armstrong EJ, et al. A tale of two plaques: convergent mechanisms of T-cell–mediated inflammation in psoriasis and atherosclerosis. Exp Dermatol. 2011;20:544-549.
- Armstrong AW, Voyles SV, Armstrong EJ, et al. Angiogenesis and oxidative stress: common mechanisms linking psoriasis with atherosclerosis. J Dermatol Sci. 2011;63:1-9.
- Boehncke S, Thaci D, Beschmann H, et al. Psoriasis patients show signs of insulin resistance. Br J Dermatol. 2007;157:1249-1251.
- Stagakis I, Bertsias G, Karvounaris S, et al. Anti-tumor necrosis factor therapy improves insulin resistance, beta cell function and insulin signaling in active rheumatoid arthritis patients with high insulin resistance. Arthritis Res Ther. 2012;14:R141.
- Solomon DH, Massarotti E, Garg R, et al. Association between disease-modifying antirheumatic drugs and diabetes risk in patients with rheumatoid arthritis and psoriasis. JAMA. 2011;305:2525-2531.
- Marra M, Campanati A, Testa R, et al. Effect of etanercept on insulin sensitivity in nine patients with psoriasis. Int J Immunopathol Pharmacol. 2007;20:731-736.
- Wambier CG, Foss-Freitas MC, Paschoal RS, et al. Severe hypoglycemia after initiation of anti-tumor necrosis factor therapy with etanercept in a patient with generalized pustular psoriasis and type 2 diabetes mellitus. J Am Acad Dermatol. 2009;60:883-885.
- Yazdani-Biuki B, Mueller T, Brezinschek HP, et al. Relapse of diabetes after interruption of chronic administration of anti-tumor necrosis factor-alpha antibody infliximab: a case observation. Diabetes Care. 2006;29:1712.
- Martínez-Abundis E, Reynoso-von Drateln C, Hernández-Salazar E, et al. Effect of etanercept on insulin secretion and insulin sensitivity in a randomized trial with psoriatic patients at risk for developing type 2 diabetes mellitus. Arch Dermatol Res. 2007;299:461-465.
Psoriasis is a chronic inflammatory disorder associated with increased expression of proinflammatory mediators such as tumor necrosis factor (TNF) α.1 Anti-TNF drugs (eg, etanercept, adalimumab, infliximab) were proven to be highly effective for the treatment of psoriasis over the last 2 decades.2 Interestingly, TNF inhibitors have been thought to be effective in improving insulin resistance in patients with type 2 diabetes mellitus (DM) by blocking TNF, which is involved in the inflammatory condition in DM.
Type 2 DM is a common chronic condition characterized by hyperglycemia resulting from a combination of peripheral and hepatic insulin resistance and impaired insulin secretion.3 It is characterized by defects in both insulin secretion and insulin sensitivity.4,5 Type 2 DM has been linked with a marked increase in cardiovascular disease, morbidity, and mortality.6 Evidence-based literature regarding the role of chronic inflammation as an important pathogenetic factor in type 2 DM has been growing.7-9 It also has been suggested that pharmacological strategies to reduce this underlying associated silent inflammation are useful in treating DM, which also is true for other conditions such as obesity, metabolic syndrome, and cardiovascular diseases.10
Psoriasis predisposes patients to insulin resistance and may put them at risk for developing DM.11,12 The association between psoriasis and DM suggests that systemic immunosuppression also may diminish the risk for developing DM. Several longitudinal studies have found that TNF inhibitors improve insulin resistance.13,14 Dandona et al15 reported a considerable decrease of TNF-α levels with the concurrent restoration of insulin sensitivity during weight loss.
Pereira et al16 found a notable connection between psoriasis, DM, and insulin resistance with an odds ratio of 2.63 of abnormal glucose homeostasis in patients with psoriasis compared to controls. Yazdani-Biuki et al17 proved that extended administration of anti–TNF-α antibody was able to improve insulin sensitivity in insulin-resistant patients. The same finding was established by Kiortsis et al.14
In this prospective controlled study, we evaluated the effects of anti-TNF agents on insulin resistance and sensitivity in psoriasis patients with type 2 DM treated with anti-TNF agents.
Methods
A total of 70 patients attending the dermatological outpatient clinics at Farwaniya Hospital (Kuwait City, Kuwait) between January 2012 and September 2014 were enrolled in the study and were randomly distributed into 2 equal groups (n=35 each). The study was approved by the hospital ethics committee. Patients were included in the study if they had moderate to severe psoriasis (ie, psoriasis area severity index score ≥10) with documented type 2 DM and high fasting plasma glucose (FPG) levels (ie, >10 mmol/L). Patients who were currently being treated with oral hypoglycemic agents but not insulin therapy were included in the study. Patients were excluded if they had phototherapy within the last 4 weeks, prior biologic therapy, current and prior insulin therapy, a change in oral hypoglycemic drug dosage in the last 2 months, other serious systemic illness (eg, malignancy, hepatitis B or C virus, metabolic or endocrine disease), and/or abnormal laboratory investigations (eg, liver/kidney profile, chest radiograph abnormality, positive Mantoux test). All of the patients enrolled in the study provided informed consent and underwent routine baseline investigations including complete blood cell counts, general health profile, chest radiograph, antinuclear antibody test, Mantoux test, FPG and insulin levels, glycated hemoglobin (HbA1C), homeostasis model assessment (HOMA), routine urine examination, and enzyme-linked immunosorbent assay for tuberculosis. The study group was treated with anti-TNF agents, and the control group received conventional antipsoriatic medications.
All the patients included in the study had high FPG levels (ie, >10 mmol/L) at the time of enrollment and were currently being treated with oral hypoglycemic agents. The dose of oral hypoglycemic agents was unchanged for at least 2 months before entry into the run-in period and throughout the 24-week study period. Demographic details including sex, age, medical history (eg, type of psoriasis, prior and concomitant treatments) were collected from the participants’ clinical histories. Participants from both groups were appropriately matched in terms of age, sex, body weight, body mass index, and duration of type 2 DM (Table 1). The primary end point of the study was to analyze and compare clinical and serum data collected at baseline and after 24 weeks of therapy.

A complete biochemical profile was repeated in both groups after 24 weeks of treatment. Each participant underwent a baseline short insulin sensitivity test immediately before treatment and at 4 and 24 weeks of treatment. We assessed insulin resistance via HOMA, calculated as follows: FPG [mmol/L] × fasting serum insulin [pmol/L] / 22.5.18 Oral glucose tolerance tests were performed to calculate the HOMA of insulin resistance. Serum insulin concentration was determined via enzyme-linked immunosorbent assay.
Statistical analysis was performed using SPSS software (version 12.0). Continuous patient characteristics were analyzed using mean and SD as well as discrete data as counts and proportions. Association was examined using χ2 tests for categorical variables and 2-sided t test/Wilcoxon rank sum test for continuous variables. Analysis of variance was used to compare the results in 3 different anti-TNF agents used in the study group.
Results
Of the 35 participants enrolled in the study group, 34 (97.1%) completed the study and were evaluated. The study group included 16 men and 18 women aged 19 to 63 years (mean age [SD], 43.7 [21.6] years) who were treated with TNF-α inhibitors—8 participants with etanercept, 14 with adalimumab, and 12 with infliximab—according to the standard dosage schedule for 24 weeks.
Of the 35 participants enrolled in the control group, 29 (82.9%) completed the study and were evaluated. Six patients did not follow up for the complete duration of the 24-week study period and were not evaluated. The control group included 14 men and 15 women aged 18 to 65 years (mean age [SD], 47.7 [14.2] years) who were treated with other systemic therapies—8 participants with topical corticosteroids or calcipotriol only, 7 with cyclosporine A, and 14 with methotrexate. The dose of the drug was kept stable throughout the 24-week study period.
Demographic and baseline characteristics for all participants are shown in Table 1. There were no significant differences in demographic or baseline characteristics among the study group versus the control group, and all participants were similar in age; body mass index; as well as FPG, fasting insulin, and HbA1C levels.
At baseline, both study and control participants had elevated mean (SD) FPG levels (10 [25] mmol/L and 11 [0.4] mmol/L, respectively), fasting insulin levels (2.79 [0.17] pmol/L and 2.82 [0.13] pmol/L, respectively), and HbA1C levels (8.4% [0.38%] and 8.1% [0.21%], respectively)(Table 1).
The study group showed significant improvements in glycemic control at the end of the study (Table 2). At week 24, study group participants had a mean (SD) decrease in FPG levels of 2.74 (0.34) mmol/L versus 0.02 (0.16) mmol/L in the control group. This difference between the 2 groups after 24 weeks was found to be statistically significant (P<.01). On further analysis of the study group, no statistically significant difference (P>.01) was noted in the 3 anti-TNF agents used. Compared to the control group, the study group showed a significant decrease from baseline values of FPG and HbA1C (P<.01). Fasting insulin levels decreased significantly for study group participants as compared with control (–1.91 pmol/L vs 0.04 pmol/L)(P<.001)(Table 2). However, on analysis of the 3 anti-TNF agents, no statistically significant difference was found (P>.05). Participants in the control group showed no significant change in fasting insulin and FPG levels.
To confirm that there was a change in insulin sensitivity in response to TNF-α inhibitors, we analyzed FPG and fasting insulin values using the HOMA method. There was no change in mean relative insulin resistance in the control group in response to therapy (mean [SD], 5.4 [0.31] vs 5.6 [0.15], before vs after therapy), while there was mild improvement in relative insulin resistance in the study group (5.9 [0.52] vs 4.8 [0.34], before vs after therapy). There also was a significant difference in the change in relative insulin resistance in response to treatment between the study and control groups (1.2 [0.40] vs –0.3 [0.12]; P<.01)(Table 2).

Comment
There has been an unprecedented rise in the rate of obesity and associated metabolic diseases such as type 2 DM. Following the current trend, it is estimated that the world will have approximately 592 million cases of type 2 DM by the year 2035.19 Almost two-thirds of these patients are estimated to die of cardiovascular diseases.
Although the pathophysiology of type 2 DM is not known, insulin resistance in the muscles and liver as well as failure of pancreatic β cells represent the core of the complex pathophysiology. The associated underlying silent inflammation was thought to have a key role in both insulin resistance and insulin secretory defects seen in type 2 DM. Furthermore, recent data suggest the central role of TNF-α, IL-1, and IL-6 pathways in this inflammation.10 Tumor necrosis factor α has been shown to have a dual effect on insulin resistance as well as pancreatic β cell function. It blocks the function of insulin at the receptor level and has been implicated as a causative factor in obesity-associated insulin resistance and also in the pathogenesis of type 2 DM.20,21 Furthermore, cytokines that activate nuclear factor κβ (a nuclear transcription factor closely involved in the regulation of cellular inflammatory response), such as TNF-α, are thought to be a common denominator for β-cell apoptosis in types 1 and 2 DM.22 Additionally, it has been suggested that TNF-α is a powerful regulator of adipose tissue.23 Neutralizing TNF-α in obese Zucker rats has shown increased insulin sensitivity.3 Tumor necrosis factor α and IL-6 as well as C-reactive protein and plasminogen activator inhibitor 1 are negatively associated with insulin sensitivity.24-27 These findings have led researchers to investigate the role of anti-TNF agents for the management of type 2 DM.28
Psoriasis has now come to be known as a systemic inflammatory disorder and is associated with increased expression of TNF-α. It predisposes patients to insulin resistance and places them at higher risk for developing DM.11,12 Systematic reports recommend that there is a link between psoriasis and DM featured by helper T cell (TH1) cytokines.29 This link can stimulate insulin resistance and metabolic syndrome as well as inflammatory cytokines identified to motivate psoriasis.29,30 The association between psoriasis and type 2 DM proposes a possible pathophysiologic connection between the 2 diseases. Patients with psoriasis have altered T-cell subtype 1 pathways and dysregulated oxidative and angiogenic mechanisms.31,32 Many of these immune pathways may similarly predispose psoriasis patients to impaired glucose tolerance and DM. Inflammation may cause insulin resistance and DM through numerous mechanisms. Systemic inflammation linked with psoriasis may lead to high levels of circulating IL-1, IL-6, and TNF-α that predispose patients to impaired glucose tolerance and type 2 DM.33
Several longitudinal investigations have found that TNF inhibitors improve insulin resistance.13,14,34-38 Gonzalez-Gay et al13 confirmed a rapid beneficial effect of infliximab on insulin resistance and insulin sensitivity in rheumatoid arthritis (RA) patients, which might support the long-term use of drugs that act by blocking TNF-α to diminish the mechanisms implicated in the development of atherosclerosis in patients with RA. Kiortsis et al14 performed a complete biochemical profile before and after 6 months of treatment with infliximab in 17 patients with ankylosing spondylitis and 28 patients with RA. The researchers found a significant decrease of the HOMA index in the percentile of their patients with the highest insulin resistance (P<.01).14
Stagakis et al34 found that 12 weeks of treatment with anti-TNF agents may improve insulin resistance in patients with active RA and high insulin resistance. Treatment with anti-TNF agents was shown to restore the phosphorylation status of serine phosphorylation of insulin receptor substrate 1 (Ser312-IRS-1) and AKT (protein kinase B), which are important mediators in the insulin signaling cascade. The investigators concluded that treatment with anti-TNF agents may improve insulin resistance and sensitivity in RA patients with active disease and high insulin resistance.34
Solomon et al35 studied the link between disease-modifying antirheumatic drugs and DM risk in patients with RA and psoriasis. The authors proposed that initiation of treatment with TNF inhibitors in psoriasis patients was associated with a reduced incidence of DM. The results showed a lower risk for developing DM in patients with psoriasis who were treated with a TNF inhibitor compared with numerous other drugs.35
Marra et al36 studied the effects of etanercept on insulin sensitivity in 9 patients with psoriasis. They reported a decrease in insulin resistance evaluated by HOMA after 24 weeks of etanercept treatment.36 Wambier et al37 reported severe hypoglycemia after initiation of anti-TNF therapy with etanercept in a patient with generalized pustular psoriasis and type 2 DM.
Yazdani-Biuki et al38 reported the case of a patient who demonstrated a relapse of type 2 DM after an interruption of prolonged treatment with infliximab, an anti–TNF-α antibody for psoriatic arthritis. The improvement in insulin sensitivity of this patient has been reported along with post hoc evidence that chronic administration of infliximab improves insulin resistance in a small sample of patients with inflammatory joint diseases.17
Other studies on the effects of TNF inhibitors on insulin resistance and sensitivity have yielded conflicting results. Martínez-Abundis et al39 studied the effects of etanercept on insulin resistance and sensitivity in a randomized trial of psoriatic patients at risk for developing type 2 DM. Results indicated that anti-TNF therapy had no significant influence on insulin sensitivity measured using a hyperinsulinemic clamp during 2 weeks of etanercept treatment in psoriatic patients with risk factors for type 2 DM. The explanation of this discrepancy may be due to the short duration of the study period.
It is still unknown if psoriasis treatment affects a patient’s risk for developing DM. However, Solomon et al35 evaluated the association of incidental DM among patients with prescribed TNF inhibitors or methotrexate and proposed that initiation of treatment with TNF inhibitors was associated with a diminished incidence of DM.
Our study supports and confirms that psoriasis patients treated with TNF-α inhibitors showed improved glycemic indices and insulin resistance compared with control patients treated with other common systemic drugs for psoriasis. We did not take into consideration other conventional risk factors such as hypertension and coronary artery disease. The number of participants included in the current study was not large enough to evaluate each of the anti-TNF agents in a separate group, and participants were not followed up long enough to see the impact of the biochemical changes noted in the results on long-term morbidity or mortality.
Conclusion
Our study confirms a beneficial effect of TNF-α inhibitors on insulin resistance and insulin sensitivity in psoriasis patients with type 2 DM. Treatment with TNF-α inhibitors may have beneficial effects on insulin sensitivity in even the most insulin-resistant patients with psoriasis. The study results may support the hypothesis that long-term use of TNF inhibitors may reduce the mechanisms involved in the development of DM in patients with psoriasis. The improvement in insulin sensitivity may in turn decrease the coronary artery disease risk in these patients. Additional large, prospective, multicenter studies are required to further analyze the effects of anti–TNF-α antibodies on insulin sensitivity and β cell function in insulin-resistant or diabetic psoriasis patients.
Psoriasis is a chronic inflammatory disorder associated with increased expression of proinflammatory mediators such as tumor necrosis factor (TNF) α.1 Anti-TNF drugs (eg, etanercept, adalimumab, infliximab) were proven to be highly effective for the treatment of psoriasis over the last 2 decades.2 Interestingly, TNF inhibitors have been thought to be effective in improving insulin resistance in patients with type 2 diabetes mellitus (DM) by blocking TNF, which is involved in the inflammatory condition in DM.
Type 2 DM is a common chronic condition characterized by hyperglycemia resulting from a combination of peripheral and hepatic insulin resistance and impaired insulin secretion.3 It is characterized by defects in both insulin secretion and insulin sensitivity.4,5 Type 2 DM has been linked with a marked increase in cardiovascular disease, morbidity, and mortality.6 Evidence-based literature regarding the role of chronic inflammation as an important pathogenetic factor in type 2 DM has been growing.7-9 It also has been suggested that pharmacological strategies to reduce this underlying associated silent inflammation are useful in treating DM, which also is true for other conditions such as obesity, metabolic syndrome, and cardiovascular diseases.10
Psoriasis predisposes patients to insulin resistance and may put them at risk for developing DM.11,12 The association between psoriasis and DM suggests that systemic immunosuppression also may diminish the risk for developing DM. Several longitudinal studies have found that TNF inhibitors improve insulin resistance.13,14 Dandona et al15 reported a considerable decrease of TNF-α levels with the concurrent restoration of insulin sensitivity during weight loss.
Pereira et al16 found a notable connection between psoriasis, DM, and insulin resistance with an odds ratio of 2.63 of abnormal glucose homeostasis in patients with psoriasis compared to controls. Yazdani-Biuki et al17 proved that extended administration of anti–TNF-α antibody was able to improve insulin sensitivity in insulin-resistant patients. The same finding was established by Kiortsis et al.14
In this prospective controlled study, we evaluated the effects of anti-TNF agents on insulin resistance and sensitivity in psoriasis patients with type 2 DM treated with anti-TNF agents.
Methods
A total of 70 patients attending the dermatological outpatient clinics at Farwaniya Hospital (Kuwait City, Kuwait) between January 2012 and September 2014 were enrolled in the study and were randomly distributed into 2 equal groups (n=35 each). The study was approved by the hospital ethics committee. Patients were included in the study if they had moderate to severe psoriasis (ie, psoriasis area severity index score ≥10) with documented type 2 DM and high fasting plasma glucose (FPG) levels (ie, >10 mmol/L). Patients who were currently being treated with oral hypoglycemic agents but not insulin therapy were included in the study. Patients were excluded if they had phototherapy within the last 4 weeks, prior biologic therapy, current and prior insulin therapy, a change in oral hypoglycemic drug dosage in the last 2 months, other serious systemic illness (eg, malignancy, hepatitis B or C virus, metabolic or endocrine disease), and/or abnormal laboratory investigations (eg, liver/kidney profile, chest radiograph abnormality, positive Mantoux test). All of the patients enrolled in the study provided informed consent and underwent routine baseline investigations including complete blood cell counts, general health profile, chest radiograph, antinuclear antibody test, Mantoux test, FPG and insulin levels, glycated hemoglobin (HbA1C), homeostasis model assessment (HOMA), routine urine examination, and enzyme-linked immunosorbent assay for tuberculosis. The study group was treated with anti-TNF agents, and the control group received conventional antipsoriatic medications.
All the patients included in the study had high FPG levels (ie, >10 mmol/L) at the time of enrollment and were currently being treated with oral hypoglycemic agents. The dose of oral hypoglycemic agents was unchanged for at least 2 months before entry into the run-in period and throughout the 24-week study period. Demographic details including sex, age, medical history (eg, type of psoriasis, prior and concomitant treatments) were collected from the participants’ clinical histories. Participants from both groups were appropriately matched in terms of age, sex, body weight, body mass index, and duration of type 2 DM (Table 1). The primary end point of the study was to analyze and compare clinical and serum data collected at baseline and after 24 weeks of therapy.

A complete biochemical profile was repeated in both groups after 24 weeks of treatment. Each participant underwent a baseline short insulin sensitivity test immediately before treatment and at 4 and 24 weeks of treatment. We assessed insulin resistance via HOMA, calculated as follows: FPG [mmol/L] × fasting serum insulin [pmol/L] / 22.5.18 Oral glucose tolerance tests were performed to calculate the HOMA of insulin resistance. Serum insulin concentration was determined via enzyme-linked immunosorbent assay.
Statistical analysis was performed using SPSS software (version 12.0). Continuous patient characteristics were analyzed using mean and SD as well as discrete data as counts and proportions. Association was examined using χ2 tests for categorical variables and 2-sided t test/Wilcoxon rank sum test for continuous variables. Analysis of variance was used to compare the results in 3 different anti-TNF agents used in the study group.
Results
Of the 35 participants enrolled in the study group, 34 (97.1%) completed the study and were evaluated. The study group included 16 men and 18 women aged 19 to 63 years (mean age [SD], 43.7 [21.6] years) who were treated with TNF-α inhibitors—8 participants with etanercept, 14 with adalimumab, and 12 with infliximab—according to the standard dosage schedule for 24 weeks.
Of the 35 participants enrolled in the control group, 29 (82.9%) completed the study and were evaluated. Six patients did not follow up for the complete duration of the 24-week study period and were not evaluated. The control group included 14 men and 15 women aged 18 to 65 years (mean age [SD], 47.7 [14.2] years) who were treated with other systemic therapies—8 participants with topical corticosteroids or calcipotriol only, 7 with cyclosporine A, and 14 with methotrexate. The dose of the drug was kept stable throughout the 24-week study period.
Demographic and baseline characteristics for all participants are shown in Table 1. There were no significant differences in demographic or baseline characteristics among the study group versus the control group, and all participants were similar in age; body mass index; as well as FPG, fasting insulin, and HbA1C levels.
At baseline, both study and control participants had elevated mean (SD) FPG levels (10 [25] mmol/L and 11 [0.4] mmol/L, respectively), fasting insulin levels (2.79 [0.17] pmol/L and 2.82 [0.13] pmol/L, respectively), and HbA1C levels (8.4% [0.38%] and 8.1% [0.21%], respectively)(Table 1).
The study group showed significant improvements in glycemic control at the end of the study (Table 2). At week 24, study group participants had a mean (SD) decrease in FPG levels of 2.74 (0.34) mmol/L versus 0.02 (0.16) mmol/L in the control group. This difference between the 2 groups after 24 weeks was found to be statistically significant (P<.01). On further analysis of the study group, no statistically significant difference (P>.01) was noted in the 3 anti-TNF agents used. Compared to the control group, the study group showed a significant decrease from baseline values of FPG and HbA1C (P<.01). Fasting insulin levels decreased significantly for study group participants as compared with control (–1.91 pmol/L vs 0.04 pmol/L)(P<.001)(Table 2). However, on analysis of the 3 anti-TNF agents, no statistically significant difference was found (P>.05). Participants in the control group showed no significant change in fasting insulin and FPG levels.
To confirm that there was a change in insulin sensitivity in response to TNF-α inhibitors, we analyzed FPG and fasting insulin values using the HOMA method. There was no change in mean relative insulin resistance in the control group in response to therapy (mean [SD], 5.4 [0.31] vs 5.6 [0.15], before vs after therapy), while there was mild improvement in relative insulin resistance in the study group (5.9 [0.52] vs 4.8 [0.34], before vs after therapy). There also was a significant difference in the change in relative insulin resistance in response to treatment between the study and control groups (1.2 [0.40] vs –0.3 [0.12]; P<.01)(Table 2).

Comment
There has been an unprecedented rise in the rate of obesity and associated metabolic diseases such as type 2 DM. Following the current trend, it is estimated that the world will have approximately 592 million cases of type 2 DM by the year 2035.19 Almost two-thirds of these patients are estimated to die of cardiovascular diseases.
Although the pathophysiology of type 2 DM is not known, insulin resistance in the muscles and liver as well as failure of pancreatic β cells represent the core of the complex pathophysiology. The associated underlying silent inflammation was thought to have a key role in both insulin resistance and insulin secretory defects seen in type 2 DM. Furthermore, recent data suggest the central role of TNF-α, IL-1, and IL-6 pathways in this inflammation.10 Tumor necrosis factor α has been shown to have a dual effect on insulin resistance as well as pancreatic β cell function. It blocks the function of insulin at the receptor level and has been implicated as a causative factor in obesity-associated insulin resistance and also in the pathogenesis of type 2 DM.20,21 Furthermore, cytokines that activate nuclear factor κβ (a nuclear transcription factor closely involved in the regulation of cellular inflammatory response), such as TNF-α, are thought to be a common denominator for β-cell apoptosis in types 1 and 2 DM.22 Additionally, it has been suggested that TNF-α is a powerful regulator of adipose tissue.23 Neutralizing TNF-α in obese Zucker rats has shown increased insulin sensitivity.3 Tumor necrosis factor α and IL-6 as well as C-reactive protein and plasminogen activator inhibitor 1 are negatively associated with insulin sensitivity.24-27 These findings have led researchers to investigate the role of anti-TNF agents for the management of type 2 DM.28
Psoriasis has now come to be known as a systemic inflammatory disorder and is associated with increased expression of TNF-α. It predisposes patients to insulin resistance and places them at higher risk for developing DM.11,12 Systematic reports recommend that there is a link between psoriasis and DM featured by helper T cell (TH1) cytokines.29 This link can stimulate insulin resistance and metabolic syndrome as well as inflammatory cytokines identified to motivate psoriasis.29,30 The association between psoriasis and type 2 DM proposes a possible pathophysiologic connection between the 2 diseases. Patients with psoriasis have altered T-cell subtype 1 pathways and dysregulated oxidative and angiogenic mechanisms.31,32 Many of these immune pathways may similarly predispose psoriasis patients to impaired glucose tolerance and DM. Inflammation may cause insulin resistance and DM through numerous mechanisms. Systemic inflammation linked with psoriasis may lead to high levels of circulating IL-1, IL-6, and TNF-α that predispose patients to impaired glucose tolerance and type 2 DM.33
Several longitudinal investigations have found that TNF inhibitors improve insulin resistance.13,14,34-38 Gonzalez-Gay et al13 confirmed a rapid beneficial effect of infliximab on insulin resistance and insulin sensitivity in rheumatoid arthritis (RA) patients, which might support the long-term use of drugs that act by blocking TNF-α to diminish the mechanisms implicated in the development of atherosclerosis in patients with RA. Kiortsis et al14 performed a complete biochemical profile before and after 6 months of treatment with infliximab in 17 patients with ankylosing spondylitis and 28 patients with RA. The researchers found a significant decrease of the HOMA index in the percentile of their patients with the highest insulin resistance (P<.01).14
Stagakis et al34 found that 12 weeks of treatment with anti-TNF agents may improve insulin resistance in patients with active RA and high insulin resistance. Treatment with anti-TNF agents was shown to restore the phosphorylation status of serine phosphorylation of insulin receptor substrate 1 (Ser312-IRS-1) and AKT (protein kinase B), which are important mediators in the insulin signaling cascade. The investigators concluded that treatment with anti-TNF agents may improve insulin resistance and sensitivity in RA patients with active disease and high insulin resistance.34
Solomon et al35 studied the link between disease-modifying antirheumatic drugs and DM risk in patients with RA and psoriasis. The authors proposed that initiation of treatment with TNF inhibitors in psoriasis patients was associated with a reduced incidence of DM. The results showed a lower risk for developing DM in patients with psoriasis who were treated with a TNF inhibitor compared with numerous other drugs.35
Marra et al36 studied the effects of etanercept on insulin sensitivity in 9 patients with psoriasis. They reported a decrease in insulin resistance evaluated by HOMA after 24 weeks of etanercept treatment.36 Wambier et al37 reported severe hypoglycemia after initiation of anti-TNF therapy with etanercept in a patient with generalized pustular psoriasis and type 2 DM.
Yazdani-Biuki et al38 reported the case of a patient who demonstrated a relapse of type 2 DM after an interruption of prolonged treatment with infliximab, an anti–TNF-α antibody for psoriatic arthritis. The improvement in insulin sensitivity of this patient has been reported along with post hoc evidence that chronic administration of infliximab improves insulin resistance in a small sample of patients with inflammatory joint diseases.17
Other studies on the effects of TNF inhibitors on insulin resistance and sensitivity have yielded conflicting results. Martínez-Abundis et al39 studied the effects of etanercept on insulin resistance and sensitivity in a randomized trial of psoriatic patients at risk for developing type 2 DM. Results indicated that anti-TNF therapy had no significant influence on insulin sensitivity measured using a hyperinsulinemic clamp during 2 weeks of etanercept treatment in psoriatic patients with risk factors for type 2 DM. The explanation of this discrepancy may be due to the short duration of the study period.
It is still unknown if psoriasis treatment affects a patient’s risk for developing DM. However, Solomon et al35 evaluated the association of incidental DM among patients with prescribed TNF inhibitors or methotrexate and proposed that initiation of treatment with TNF inhibitors was associated with a diminished incidence of DM.
Our study supports and confirms that psoriasis patients treated with TNF-α inhibitors showed improved glycemic indices and insulin resistance compared with control patients treated with other common systemic drugs for psoriasis. We did not take into consideration other conventional risk factors such as hypertension and coronary artery disease. The number of participants included in the current study was not large enough to evaluate each of the anti-TNF agents in a separate group, and participants were not followed up long enough to see the impact of the biochemical changes noted in the results on long-term morbidity or mortality.
Conclusion
Our study confirms a beneficial effect of TNF-α inhibitors on insulin resistance and insulin sensitivity in psoriasis patients with type 2 DM. Treatment with TNF-α inhibitors may have beneficial effects on insulin sensitivity in even the most insulin-resistant patients with psoriasis. The study results may support the hypothesis that long-term use of TNF inhibitors may reduce the mechanisms involved in the development of DM in patients with psoriasis. The improvement in insulin sensitivity may in turn decrease the coronary artery disease risk in these patients. Additional large, prospective, multicenter studies are required to further analyze the effects of anti–TNF-α antibodies on insulin sensitivity and β cell function in insulin-resistant or diabetic psoriasis patients.
- Lowes MA, Bowcock AM, Krueger JG. Pathogenesis and therapy of psoriasis. Nature. 2007;445:866-873.
- Boehncke WH, Prinz J, Gottlieb AB. Biologic therapies for psoriasis. a systematic review. J Rheumatol. 2006;33:1447-1451.
- DeFronzo RA, Bonadonna RC, Ferrannini E. Pathogenesis of NIDDM. a balanced overview. Diabetes Care. 1992;15:318-368.
- DeFronzo RA. Pathogenesis of type 2 diabetes: metabolic and molecular implications for identifying diabetes genes. Diabetes Rev. 1997;4:177-269.
- Reaven GM. Banting Lecture 1988. role of insulin resistance in human disease. Diabetes. 1988;37:1595-1607.
- Erkelens DW. Insulin resistance syndrome and type 2 diabetes mellitus. Am J Cardiol. 2001;88(7B):38J-42J.
- Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87-91.
- Hotamisligil GS, Spiegelman BM. Tumor necrosis factor alpha: a key component of the obesity-diabetes link. Diabetes. 1994;43:1271-1278.
- Van der Poll T, Romijn JA, Endert E, et al. Tumor necrosis factor mimics the metabolic response to acute infection in healthy humans. Am J Physiol. 1991;261:457-465.
- Tabas I, Glass CK. Anti-inflammatory therapy in chronic disease: challenges and opportunities. Science. 2013;339:166-172.
- Qureshi AA, Choi HK, Setty AR, et al. Psoriasis and the risk of diabetes and hypertension: a prospective study of US female nurses. Arch Dermatol. 2009;145:379-382.
- Solomon DH, Love TJ, Canning C, et al. Risk of diabetes among patients with rheumatoid arthritis, psoriatic arthritis and psoriasis. Ann Rheum Dis. 2010;69:2114-2117.
- Gonzalez-Gay MA, De Matias JM, Gonzalez-Juanatey C, et al. Anti-tumor necrosis factor-alpha blockade improves insulin resistance in patients with rheumatoid arthritis. Clin Exp Rheumatol. 2006;24:83-86.
- Kiortsis DN, Mavridis AK, Vasakos S, et al. Effects of infliximab treatment on insulin resistance in patients with rheumatoid arthritis and ankylosing spondylitis. Ann Rheum Dis. 2005;64:765-766.
- Dandona P, Weinstock R, Thusu K, et al. Tumor necrosis factor-α in sera of obese patients: fall with weight loss. J Clin Endocrinol Metabol. 1998;83:2907-2910.
- Pereira RR, Amladi ST, Varthakavi PK. A study of the prevalence of diabetes, insulin resistance, lipid abnormalities, and cardiovascular risk factors in patients with chronic plaque psoriasis. Ind J Dermatol. 2011;56:520-526.
- Yazdani-Biuki B, Stelzl H, Brezinschek HP, et al. Improvement of insulin sensitivity in insulin resistant subjects during prolonged treatment with the anti-TNF-α antibody infliximab. Eur J Clin Invest. 2004;34:641-642.
- Bonora E, Kiechl S, Willeit J, et al. Prevalence of insulin resistance in metabolic disorders. The Bruneck Study. Diabetes. 1998;47:1643-1649.
- Ryden L, Grant PJ, Anker SD, et al. ESC Guidelines on diabetes, prediabetes, and cardiovascular diseases developed in collaboration with the EASD: the Task Force on diabetes, prediabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD). Eur Heart J. 2013;34:3035-3087.
- Peraldi P, Spiegelman B. TNF-alpha and insulin resistance: summary and future prospects. Mol Cell Biochem. 1998;182:169-175.
- Moller DE. Potential role of TNF-alpha in the pathogenesis of insulin resistance and type 2 diabetes. Trends Endocrinol Metab. 2000;11:212-217.
- Mandrup-Poulsen T. Apoptotic signal transduction pathways in diabetes. Biochem Pharmacol. 2003;66:1433-1440.
- Coppack SW. Pro-inflammatory cytokines and adipose tissue. Proc Nutr Soc. 2001;60:349-356.
- Pradhan AD, Manson JE, Rifai N, et al. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327-334.
- Festa A, D’Agostino R Jr, Tracy RP, et al. Insulin Resistance Atherosclerosis Study. elevated levels of acute-phase proteins and plasminogen activator inhibitor-1 predict the development of type 2 diabetes: the insulin resistance atherosclerosis study. Diabetes. 2002;51:1131-1137.
- Meigs JB, O’Donnell CJ, Tofler GH, et al. Hemostatic markers of endothelial dysfunction and risk of incident type 2 diabetes: the Framingham Offspring Study. Diabetes. 2006;55:530-537.
- Liu S, Tinker L, Song Y, et al. A prospective study of inflammatory cytokines and diabetes mellitus in a multiethnic cohort of postmenopausal women. Arch Intern Med. 2007;167:1676-1685.
- Esser N, Paquot N, Scheen AJ. Anti-inflammatory agents to treat or prevent type 2 diabetes, metabolic syndrome and cardiovascular disease. Exp Opin Invest Drugs. 2014;24:1-25.
- Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111-1119.
- Karadag AS, Yavuz B, Ertugrul DT, et al. Is psoriasis a pre-atherosclerotic disease? increased insulin resistance and impaired endothelial function in patients with psoriasis. Int J Dermatol. 2010;49:642-646.
- Armstrong AW, Voyles SV, Armstrong EJ, et al. A tale of two plaques: convergent mechanisms of T-cell–mediated inflammation in psoriasis and atherosclerosis. Exp Dermatol. 2011;20:544-549.
- Armstrong AW, Voyles SV, Armstrong EJ, et al. Angiogenesis and oxidative stress: common mechanisms linking psoriasis with atherosclerosis. J Dermatol Sci. 2011;63:1-9.
- Boehncke S, Thaci D, Beschmann H, et al. Psoriasis patients show signs of insulin resistance. Br J Dermatol. 2007;157:1249-1251.
- Stagakis I, Bertsias G, Karvounaris S, et al. Anti-tumor necrosis factor therapy improves insulin resistance, beta cell function and insulin signaling in active rheumatoid arthritis patients with high insulin resistance. Arthritis Res Ther. 2012;14:R141.
- Solomon DH, Massarotti E, Garg R, et al. Association between disease-modifying antirheumatic drugs and diabetes risk in patients with rheumatoid arthritis and psoriasis. JAMA. 2011;305:2525-2531.
- Marra M, Campanati A, Testa R, et al. Effect of etanercept on insulin sensitivity in nine patients with psoriasis. Int J Immunopathol Pharmacol. 2007;20:731-736.
- Wambier CG, Foss-Freitas MC, Paschoal RS, et al. Severe hypoglycemia after initiation of anti-tumor necrosis factor therapy with etanercept in a patient with generalized pustular psoriasis and type 2 diabetes mellitus. J Am Acad Dermatol. 2009;60:883-885.
- Yazdani-Biuki B, Mueller T, Brezinschek HP, et al. Relapse of diabetes after interruption of chronic administration of anti-tumor necrosis factor-alpha antibody infliximab: a case observation. Diabetes Care. 2006;29:1712.
- Martínez-Abundis E, Reynoso-von Drateln C, Hernández-Salazar E, et al. Effect of etanercept on insulin secretion and insulin sensitivity in a randomized trial with psoriatic patients at risk for developing type 2 diabetes mellitus. Arch Dermatol Res. 2007;299:461-465.
- Lowes MA, Bowcock AM, Krueger JG. Pathogenesis and therapy of psoriasis. Nature. 2007;445:866-873.
- Boehncke WH, Prinz J, Gottlieb AB. Biologic therapies for psoriasis. a systematic review. J Rheumatol. 2006;33:1447-1451.
- DeFronzo RA, Bonadonna RC, Ferrannini E. Pathogenesis of NIDDM. a balanced overview. Diabetes Care. 1992;15:318-368.
- DeFronzo RA. Pathogenesis of type 2 diabetes: metabolic and molecular implications for identifying diabetes genes. Diabetes Rev. 1997;4:177-269.
- Reaven GM. Banting Lecture 1988. role of insulin resistance in human disease. Diabetes. 1988;37:1595-1607.
- Erkelens DW. Insulin resistance syndrome and type 2 diabetes mellitus. Am J Cardiol. 2001;88(7B):38J-42J.
- Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87-91.
- Hotamisligil GS, Spiegelman BM. Tumor necrosis factor alpha: a key component of the obesity-diabetes link. Diabetes. 1994;43:1271-1278.
- Van der Poll T, Romijn JA, Endert E, et al. Tumor necrosis factor mimics the metabolic response to acute infection in healthy humans. Am J Physiol. 1991;261:457-465.
- Tabas I, Glass CK. Anti-inflammatory therapy in chronic disease: challenges and opportunities. Science. 2013;339:166-172.
- Qureshi AA, Choi HK, Setty AR, et al. Psoriasis and the risk of diabetes and hypertension: a prospective study of US female nurses. Arch Dermatol. 2009;145:379-382.
- Solomon DH, Love TJ, Canning C, et al. Risk of diabetes among patients with rheumatoid arthritis, psoriatic arthritis and psoriasis. Ann Rheum Dis. 2010;69:2114-2117.
- Gonzalez-Gay MA, De Matias JM, Gonzalez-Juanatey C, et al. Anti-tumor necrosis factor-alpha blockade improves insulin resistance in patients with rheumatoid arthritis. Clin Exp Rheumatol. 2006;24:83-86.
- Kiortsis DN, Mavridis AK, Vasakos S, et al. Effects of infliximab treatment on insulin resistance in patients with rheumatoid arthritis and ankylosing spondylitis. Ann Rheum Dis. 2005;64:765-766.
- Dandona P, Weinstock R, Thusu K, et al. Tumor necrosis factor-α in sera of obese patients: fall with weight loss. J Clin Endocrinol Metabol. 1998;83:2907-2910.
- Pereira RR, Amladi ST, Varthakavi PK. A study of the prevalence of diabetes, insulin resistance, lipid abnormalities, and cardiovascular risk factors in patients with chronic plaque psoriasis. Ind J Dermatol. 2011;56:520-526.
- Yazdani-Biuki B, Stelzl H, Brezinschek HP, et al. Improvement of insulin sensitivity in insulin resistant subjects during prolonged treatment with the anti-TNF-α antibody infliximab. Eur J Clin Invest. 2004;34:641-642.
- Bonora E, Kiechl S, Willeit J, et al. Prevalence of insulin resistance in metabolic disorders. The Bruneck Study. Diabetes. 1998;47:1643-1649.
- Ryden L, Grant PJ, Anker SD, et al. ESC Guidelines on diabetes, prediabetes, and cardiovascular diseases developed in collaboration with the EASD: the Task Force on diabetes, prediabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD). Eur Heart J. 2013;34:3035-3087.
- Peraldi P, Spiegelman B. TNF-alpha and insulin resistance: summary and future prospects. Mol Cell Biochem. 1998;182:169-175.
- Moller DE. Potential role of TNF-alpha in the pathogenesis of insulin resistance and type 2 diabetes. Trends Endocrinol Metab. 2000;11:212-217.
- Mandrup-Poulsen T. Apoptotic signal transduction pathways in diabetes. Biochem Pharmacol. 2003;66:1433-1440.
- Coppack SW. Pro-inflammatory cytokines and adipose tissue. Proc Nutr Soc. 2001;60:349-356.
- Pradhan AD, Manson JE, Rifai N, et al. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327-334.
- Festa A, D’Agostino R Jr, Tracy RP, et al. Insulin Resistance Atherosclerosis Study. elevated levels of acute-phase proteins and plasminogen activator inhibitor-1 predict the development of type 2 diabetes: the insulin resistance atherosclerosis study. Diabetes. 2002;51:1131-1137.
- Meigs JB, O’Donnell CJ, Tofler GH, et al. Hemostatic markers of endothelial dysfunction and risk of incident type 2 diabetes: the Framingham Offspring Study. Diabetes. 2006;55:530-537.
- Liu S, Tinker L, Song Y, et al. A prospective study of inflammatory cytokines and diabetes mellitus in a multiethnic cohort of postmenopausal women. Arch Intern Med. 2007;167:1676-1685.
- Esser N, Paquot N, Scheen AJ. Anti-inflammatory agents to treat or prevent type 2 diabetes, metabolic syndrome and cardiovascular disease. Exp Opin Invest Drugs. 2014;24:1-25.
- Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111-1119.
- Karadag AS, Yavuz B, Ertugrul DT, et al. Is psoriasis a pre-atherosclerotic disease? increased insulin resistance and impaired endothelial function in patients with psoriasis. Int J Dermatol. 2010;49:642-646.
- Armstrong AW, Voyles SV, Armstrong EJ, et al. A tale of two plaques: convergent mechanisms of T-cell–mediated inflammation in psoriasis and atherosclerosis. Exp Dermatol. 2011;20:544-549.
- Armstrong AW, Voyles SV, Armstrong EJ, et al. Angiogenesis and oxidative stress: common mechanisms linking psoriasis with atherosclerosis. J Dermatol Sci. 2011;63:1-9.
- Boehncke S, Thaci D, Beschmann H, et al. Psoriasis patients show signs of insulin resistance. Br J Dermatol. 2007;157:1249-1251.
- Stagakis I, Bertsias G, Karvounaris S, et al. Anti-tumor necrosis factor therapy improves insulin resistance, beta cell function and insulin signaling in active rheumatoid arthritis patients with high insulin resistance. Arthritis Res Ther. 2012;14:R141.
- Solomon DH, Massarotti E, Garg R, et al. Association between disease-modifying antirheumatic drugs and diabetes risk in patients with rheumatoid arthritis and psoriasis. JAMA. 2011;305:2525-2531.
- Marra M, Campanati A, Testa R, et al. Effect of etanercept on insulin sensitivity in nine patients with psoriasis. Int J Immunopathol Pharmacol. 2007;20:731-736.
- Wambier CG, Foss-Freitas MC, Paschoal RS, et al. Severe hypoglycemia after initiation of anti-tumor necrosis factor therapy with etanercept in a patient with generalized pustular psoriasis and type 2 diabetes mellitus. J Am Acad Dermatol. 2009;60:883-885.
- Yazdani-Biuki B, Mueller T, Brezinschek HP, et al. Relapse of diabetes after interruption of chronic administration of anti-tumor necrosis factor-alpha antibody infliximab: a case observation. Diabetes Care. 2006;29:1712.
- Martínez-Abundis E, Reynoso-von Drateln C, Hernández-Salazar E, et al. Effect of etanercept on insulin secretion and insulin sensitivity in a randomized trial with psoriatic patients at risk for developing type 2 diabetes mellitus. Arch Dermatol Res. 2007;299:461-465.
Practice Points
- Psoriasis is associated with an increased incidence of insulin resistance and type 2 diabetes mellitus (DM).
- Anti–tumor necrosis factor drugs, which are effective for the treatment of psoriasis, were found to improve insulin resistance in psoriasis patients with type 2 DM.
