User login
Facial aging is the result of the interplay between loss of skin elasticity, changes in subcutaneous fat and other soft-tissue layers, and skeletal remodeling with chronological age.1 Dermal fillers are effective for the treatment of rhytides, facial scars, and lipoatrophy, as well as facial contouring and augmentation. Given that multiple filler options exist, updated reviews are necessary to inform clinicians of the choices that are available. We provide a detailed review of the clinical efficacy and safety of the dermal fillers with the most recent approvals by the US Food and Drug Administration (FDA).
Polymethylmethacrylate
Polymethylmethacrylate (PMMA) microspheres suspended in bovine collagen and lidocaine 0.3% were approved in 2006 for use in nasolabial folds (NLFs) and in 2014 for acne scars. Now branded as Bellafill (Suneva Medical, Inc), it is the only permanent injectable filler currently available. Once injected, the particles are not reabsorbed and can only be removed by procedural extraction (eg, liposuction of the surrounding fat); however, the permanence of PMMA does not extend to facial rejuvenation, which can last up to 5 years. Prior to use, skin testing for bovine collagen reaction is necessary. In a clinical trial of 147 patients with moderate to severe acne scarring, patients were randomized to receive PMMA in collagen (n=97) or saline (n=50).2 Injections were administered using a linear threading or serial puncture technique, and patients were reevaluated after 4 weeks for touch-up injections. After 6 months, 64% of patients treated with PMMA in collagen achieved improvement in acne scars by 2 points or more on the acne scar rating scale versus 33% of the control group (P=.0005).2
Treatment-related adverse events (AEs) include injection-site pain, bruising, swelling, erythema, and more rarely pruritus and lumps/granulomas.3 A 5-year longitudinal safety investigation of 871 patients initially treated with PMMA in collagen for NLF correction revealed that 17 patients (2.0%) had biopsy-confirmed granulomas with half of these retained at study end.4 Fifteen of these patients were treated with intralesional corticosteroids alone or in combination with intralesional 5-fluorouracil, oral antibiotics, or topical calcineurin inhibitors; 1 patient was untreated and another used topical corticosteroids. The authors noted no correlation between treatment method and granuloma response.4 Polymethylmethacrylate in collagen is contraindicated in patients with lidocaine or bovine collagen sensitivity and is not indicated for use in lip augmentation due to high rates of nodule formation.3
Hyaluronic Acid
Hyaluronic acid (HA) is a naturally occurring glycosaminoglycan polymer found in the extracellular matrix of the dermis. Hyaluronic acid fillers are bacteria derived and come in gel form. A useful advantage of HA fillers compared to other dermal fillers is the commercial availability of hyaluronidase to correct injections. Preinjection skin testing is not necessary.5
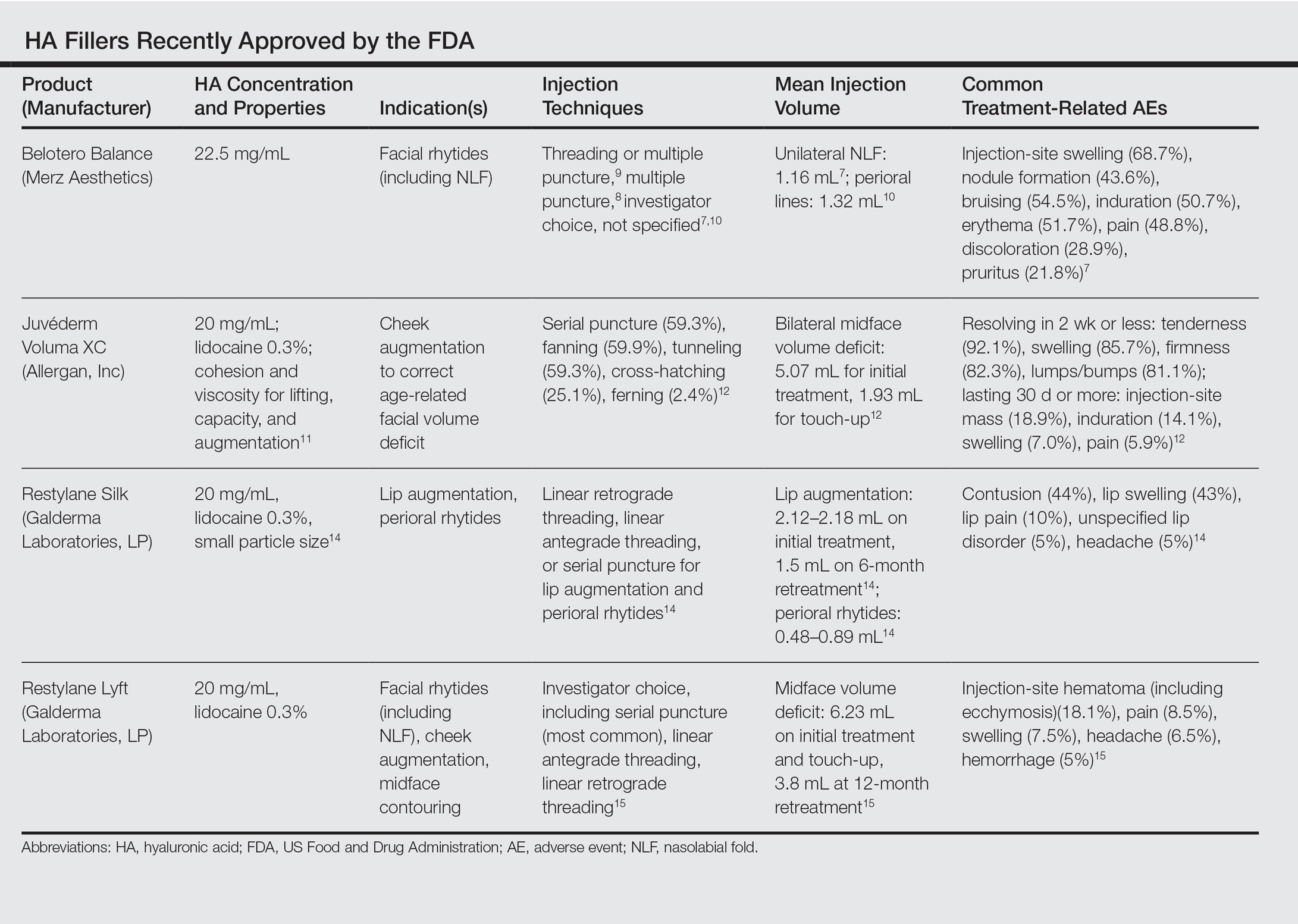
This category of nonpermanent dermal fillers has the most robust market choices. Older HA dermal fillers with reliable and proven efficacy are Restylane (Galderma Laboratories, LP)(facial rhytides, lip augmentation), Juvéderm (Ultra/Ultra XC/Ultra Plus/Ultra Plus XC [Allergan, Inc])(facial rhytides, lip augmentation), Hydrelle (Anika Therapeutics, Inc)(facial rhytides), and Prevelle Silk (Mentor Corporation)(facial rhytides); they will not be reviewed here. Newer agents include Belotero Balance (Merz Aesthetics), Juvéderm Voluma XC (Allergan, Inc), Restylane Silk (Galderma Laboratories, LP), and Restylane Lyft (Galderma Laboratories, LP).
Belotero Balance
Belotero Balance is used to treat fine lines and wrinkles, especially NLFs.6 The initial pivotal studies that led to FDA approval in 2011 demonstrated noninferiority and superiority to bovine collagen for use in the treatment of NLFs.7,8 One hundred eighteen patients with bilateral NLFs that were rated as 2 (moderate) or 3 (severe) on the wrinkle severity rating scale (WSRS) were randomized to split-face injection of Belotero Balance in one NLF and bovine collagen in the contralateral NLF.7 An additional injection at week 2 was allowed for optimal correction. Belotero Balance was noninferior to bovine collagen at week 2, with mean improvement in WSRS of 1.52 versus 1.57 (P=.50). Belotero Balance was superior to bovine collagen in mean WSRS improvement at weeks 12 (1.25 vs 0.26; P<.001), 16 (1.09 vs 0.66; P<.001), and 24 (1.08 vs 0.50; P<.001).7 In a subsequent open-label extension study, which included 95 of 118 patients who received Belotero Balance injections in both NLFs at week 24, 80.2% of patients showed sustained improvement in WSRS from baseline for 48 weeks without further injection.8
The first comparative study of Belotero Balance with other established HA fillers at the time—Restylane and Juvéderm Ultra 3/Ultra Plus XC—to treat NLFs demonstrated noninferiority.9 Forty patients with bilateral, moderate to severe NLFs (rated 3 or 4 on the Merz severity scale) were randomized to split-face groups of Belotero Balance versus Restylane or Belotero Balance versus Juvéderm. At 12 months, NLF severity improved from 2.3 to 1.5 in the Restylane group and from 2.3 to 1.6 in the Juvéderm group.9
Belotero Balance has been compared to Juvéderm Ultra XC for use in perioral lines.10 The study included 136 patients with moderate to severe perioral lines, according to the perioral lines severity scale, who were randomized (1:1 ratio) to receive injections of Belotero Balance or Juvéderm Ultra XC to correct upper and lower perioral lines, with assessment at week 2 for optimization. After 6 months, 87% of Juvéderm-treated patients compared to 72% of Belotero Balance–treated patients had 1-point improvement in perioral lines (P<.04). Juvéderm-treated patients also reported significantly less pain than Belotero Balance–treated patients (P<.001).10
Treatment-related AEs are described in the Table, with the majority occurring at lower rates compared to a collagen control group and self-resolving within 2 weeks.7
Juvéderm Voluma XC
Juvéderm Voluma XC was FDA approved in 2013 for cheek augmentation to correct age-related volume deficit restoration by subcutaneous or subperiosteal injections. In its landmark multicenter investigation, 282 patients with moderate to severe midface (eg, zygomaticomalar, anteromedial cheek, submalar regions) volume deficit measured on a validated midface volume deficit scale (MFVDS) were treated with Juvéderm Voluma XC (n=235) or control (n=47).11 Patients were reevaluated at 30 days and 81.9% received touch-up injections. At a 6-month primary evaluation, 86% of the Juvéderm-treated patients versus 39% of the control patients showed 1-point improvement on the MFVDS (P<.001). At 24-months’ follow-up, 44.6% of patients sustained efficacy.11 Of these aforementioned patients, 167 received repeat treatment due to lost correction or patient request and 91.1% improved by 1 point or more on the MFVDS on evaluation 12 months after repeat treatment.12 For this same population of patients, a 2-year extended follow-up of patient-reported outcomes revealed that 49% of patients felt fulfilled in their treatment goals 2 years after treatment and 79% of patients rated improvement from baseline based on the global aesthetic improvement scale.13 Efficacy studies involving Juvéderm Voluma XC are currently ongoing for facial temporal aging (registered at www.clinicaltrials.gov with the identifier NCT02437903) and recruiting for mandibular hypoplasia (NCT02330016).
Common treatment-related AEs are detailed in the Table. Two patients required treatment with hyaluronidase for chronic lumpiness and nodularity following non–treatment-related cellulitis.11 The product is contraindicated in patients with allergy to lidocaine.
Restylane Silk
Restylane Silk was approved in 2014 for lip augmentation and perioral rhytides. Efficacy and safety was demonstrated in a large multicenter randomized investigation in which 221 patients seeking lip augmentation received either Restylane Silk (n=177) injected submucosally for treatment of the upper and lower lips and/or intradermally for perioral rhytides or no treatment (n=44).14 Restylane treatment group patients optionally received touch-up at 2 weeks for optimization. All patients, including the control group, received injections at 6 months. At the 2-month primary end point, 80.2% of the treatment group exhibited at least 1-point improvement in upper lip fullness on the Medicis lip fullness scale compared to 11.9% (P<.001) of the control group; response rates for the lower lips were 84.2% versus 18.4% (P<.001). Patients in the treatment group receiving injections for perioral rhytides showed significant improvement in perioral rhytides through week 24 compared to patients treated for lip augmentation only (P<.001).14 Restylane Silk currently is undergoing investigation for cheek rejuvenation (NCT02636894, NCT02679924) and treatment of hand photoaging (NCT02780258).
The most common AEs are listed in the Table. No lip disorders were considered clinically concerning on evaluation. Concomitant lip augmentation and treatment of perioral rhytides yielded similar rates of AEs.14 Restylane Silk is not to be used in patients with known lidocaine allergy.
Restylane Lyft
Restylane Lyft (formerly known as Perlane-L) was approved in 2010 for use in facial rhytides, including NLFs, and gained approval in 2015 for use in cheek augmentation and midface contouring. Only its efficacy and safety for the more recent indication will be reviewed here.
In an evaluator-blinded investigation of 200 patients with mild to substantial bilateral midface deficiency based on the Medicis midface volume scale (MMVS), patients were randomized to receive supraperiosteal and subcutaneous treatment with Restylane Lyft (n=150) or no treatment (n=50).15 Touch-up injections at week 2 or month 12 were available to treatment group patients and all patients were given either an initial treatment or retreatment at 12 months. Primary end point evaluation at week 8 showed that 89% of treatment group patients had at least 1 grade MMVS improvement compared to 16% of the control group (P<.001). Although the percentage of these MMVS responders in the treatment group decreased with each follow-up period to 54.3% at month 12, retreatment was effective in reproducing a similar MMVS response rate as with initial treatment.15 Restylane Lyft is under ongoing investigation for dorsal hand rejuvenation (NCT02650921).
In addition to the common treatment-related AEs listed in the Table, 2 patients reported serious AEs, including bilateral implant-site inflammation and unilateral implant-site hematoma and infection (organism not described), all of which resolved with unspecified treatment.15 Lidocaine allergies are contraindications for use.
Conclusion
Several new options in dermal fillers have been approved in recent years and have demonstrated efficacy and acceptable safety in various cosmetic rejuvenation applications. Restylane Silk and Restylane Lyft are undergoing further studies to evaluate use in hand rejuvenation, an area that currently has few cosmetic filler treatment options. As technology continues to progress and new formulations of dermal fillers with varied properties and benefits are available, clinicians should expect multiple options for use in rhytides, volume deficits, and contouring.
ADDENDUM
After the manuscript was accepted for publication, Juvéderm Volbella XC (Allergan, Inc) was approved by the FDA for use in lip augmentation and thus is not included in this review.
- Fitzgerald R, Graivier MH, Kane M, et al. Update on facial aging. Aesthet Surg J. 2010;30(suppl):S11-S24.
- Karnik J, Baumann L, Bruce S, et al. A double-blind, randomized, multicenter, controlled trial of suspended polymethylmethacrylate microspheres for the correction of atrophic facial acne scars. J Am Acad Dermatol. 2014;71:77-83.
- Bellafill [package insert]. San Diego, CA: Suneva Medical, Inc; 2015.
- Cohen S, Dover J, Monheit G, et al. Five-year safety and satisfaction study of PMMA-collagen in the correction of nasolabial folds. Dermatol Surg. 2015;41(suppl 1):S302-S313.
- Greene JJ, Sidle DM. The hyaluronic acid fillers: current understanding of the tissue device interface. Facial Plast Surg Clin North Am. 2015;23:423-432.
- Lorenc ZP, Fagien S, Flynn TC, et al. Review of key Belotero Balance safety and efficacy trials. Plast Reconstr Surg. 2013;132(4, suppl 2):33S-40S.
- Narins RS, Coleman W, Donofrio L, et al. Nonanimal sourced hyaluronic acid–based dermal filler using a cohesive polydensified matrix technology is superior to bovine collagen in the correction of moderate to severe nasolabial folds: results from a 6-month, randomized, blinded, controlled, multicenter study. Dermatol Surg. 2010;36(suppl 1):730-740.
- Narins RS, Coleman WP 3rd, Donofrio LM, et al. Improvement in nasolabial folds with a hyaluronic acid filler using a cohesive polydensified matrix technology: results from an 18-month open-label extension trial. Dermatol Surg. 2010;36(suppl 3):1800-1808.
- Prager W, Wissmueller E, Havermann I, et al. A prospective, split-face, randomized, comparative study of safety and 12-month longevity of three formulations of hyaluronic acid dermal filler for treatment of nasolabial folds. Dermatol Surg. 2012;38(7, pt 2):1143-1150.
- Butterwick K, Marmur E, Narurkar V, et al. HYC-24L demonstrates greater effectiveness with less pain than CPM-22.5 for treatment of perioral lines in a randomized controlled trial. Dermatol Surg. 2015;41:1351-1360.
- Jones D, Murphy DK. Volumizing hyaluronic acid filler for midface volume deficit: 2-year results from a pivotal single-blind randomized controlled study. Dermatol Surg. 2013;39:1602-1612.
- Baumann L, Narins RS, Beer K, et al. Volumizing hyaluronic acid filler for midface volume deficit: results after repeat treatment. Dermatol Surg. 2015;41(suppl 1):S284-S292.
- Few J, Cox SE, Paradkar-Mitragotri D, et al. A multicenter, single-blind randomized, controlled study of a volumizing hyaluronic acid filler for midface volume deficit: patient-reported outcomes at 2 years. Aesthet Surg J. 2015;35:589-599.
- Beer K, Glogau RG, Dover JS, et al. A randomized, evaluator-blinded, controlled study of effectiveness and safety of small particle hyaluronic acid plus lidocaine for lip augmentation and perioral rhytides. Dermatol Surg. 2015;41(suppl 1):S127-S136.
- Weiss RA, Moradi A, Bank D, et al. Effectiveness and safety of large gel particle hyaluronic acid with lidocaine for correction of midface volume deficit or contour deficiency. Dermatol Surg. 2016;42:699-709.
Facial aging is the result of the interplay between loss of skin elasticity, changes in subcutaneous fat and other soft-tissue layers, and skeletal remodeling with chronological age.1 Dermal fillers are effective for the treatment of rhytides, facial scars, and lipoatrophy, as well as facial contouring and augmentation. Given that multiple filler options exist, updated reviews are necessary to inform clinicians of the choices that are available. We provide a detailed review of the clinical efficacy and safety of the dermal fillers with the most recent approvals by the US Food and Drug Administration (FDA).
Polymethylmethacrylate
Polymethylmethacrylate (PMMA) microspheres suspended in bovine collagen and lidocaine 0.3% were approved in 2006 for use in nasolabial folds (NLFs) and in 2014 for acne scars. Now branded as Bellafill (Suneva Medical, Inc), it is the only permanent injectable filler currently available. Once injected, the particles are not reabsorbed and can only be removed by procedural extraction (eg, liposuction of the surrounding fat); however, the permanence of PMMA does not extend to facial rejuvenation, which can last up to 5 years. Prior to use, skin testing for bovine collagen reaction is necessary. In a clinical trial of 147 patients with moderate to severe acne scarring, patients were randomized to receive PMMA in collagen (n=97) or saline (n=50).2 Injections were administered using a linear threading or serial puncture technique, and patients were reevaluated after 4 weeks for touch-up injections. After 6 months, 64% of patients treated with PMMA in collagen achieved improvement in acne scars by 2 points or more on the acne scar rating scale versus 33% of the control group (P=.0005).2
Treatment-related adverse events (AEs) include injection-site pain, bruising, swelling, erythema, and more rarely pruritus and lumps/granulomas.3 A 5-year longitudinal safety investigation of 871 patients initially treated with PMMA in collagen for NLF correction revealed that 17 patients (2.0%) had biopsy-confirmed granulomas with half of these retained at study end.4 Fifteen of these patients were treated with intralesional corticosteroids alone or in combination with intralesional 5-fluorouracil, oral antibiotics, or topical calcineurin inhibitors; 1 patient was untreated and another used topical corticosteroids. The authors noted no correlation between treatment method and granuloma response.4 Polymethylmethacrylate in collagen is contraindicated in patients with lidocaine or bovine collagen sensitivity and is not indicated for use in lip augmentation due to high rates of nodule formation.3
Hyaluronic Acid
Hyaluronic acid (HA) is a naturally occurring glycosaminoglycan polymer found in the extracellular matrix of the dermis. Hyaluronic acid fillers are bacteria derived and come in gel form. A useful advantage of HA fillers compared to other dermal fillers is the commercial availability of hyaluronidase to correct injections. Preinjection skin testing is not necessary.5
This category of nonpermanent dermal fillers has the most robust market choices. Older HA dermal fillers with reliable and proven efficacy are Restylane (Galderma Laboratories, LP)(facial rhytides, lip augmentation), Juvéderm (Ultra/Ultra XC/Ultra Plus/Ultra Plus XC [Allergan, Inc])(facial rhytides, lip augmentation), Hydrelle (Anika Therapeutics, Inc)(facial rhytides), and Prevelle Silk (Mentor Corporation)(facial rhytides); they will not be reviewed here. Newer agents include Belotero Balance (Merz Aesthetics), Juvéderm Voluma XC (Allergan, Inc), Restylane Silk (Galderma Laboratories, LP), and Restylane Lyft (Galderma Laboratories, LP).
Belotero Balance
Belotero Balance is used to treat fine lines and wrinkles, especially NLFs.6 The initial pivotal studies that led to FDA approval in 2011 demonstrated noninferiority and superiority to bovine collagen for use in the treatment of NLFs.7,8 One hundred eighteen patients with bilateral NLFs that were rated as 2 (moderate) or 3 (severe) on the wrinkle severity rating scale (WSRS) were randomized to split-face injection of Belotero Balance in one NLF and bovine collagen in the contralateral NLF.7 An additional injection at week 2 was allowed for optimal correction. Belotero Balance was noninferior to bovine collagen at week 2, with mean improvement in WSRS of 1.52 versus 1.57 (P=.50). Belotero Balance was superior to bovine collagen in mean WSRS improvement at weeks 12 (1.25 vs 0.26; P<.001), 16 (1.09 vs 0.66; P<.001), and 24 (1.08 vs 0.50; P<.001).7 In a subsequent open-label extension study, which included 95 of 118 patients who received Belotero Balance injections in both NLFs at week 24, 80.2% of patients showed sustained improvement in WSRS from baseline for 48 weeks without further injection.8
The first comparative study of Belotero Balance with other established HA fillers at the time—Restylane and Juvéderm Ultra 3/Ultra Plus XC—to treat NLFs demonstrated noninferiority.9 Forty patients with bilateral, moderate to severe NLFs (rated 3 or 4 on the Merz severity scale) were randomized to split-face groups of Belotero Balance versus Restylane or Belotero Balance versus Juvéderm. At 12 months, NLF severity improved from 2.3 to 1.5 in the Restylane group and from 2.3 to 1.6 in the Juvéderm group.9
Belotero Balance has been compared to Juvéderm Ultra XC for use in perioral lines.10 The study included 136 patients with moderate to severe perioral lines, according to the perioral lines severity scale, who were randomized (1:1 ratio) to receive injections of Belotero Balance or Juvéderm Ultra XC to correct upper and lower perioral lines, with assessment at week 2 for optimization. After 6 months, 87% of Juvéderm-treated patients compared to 72% of Belotero Balance–treated patients had 1-point improvement in perioral lines (P<.04). Juvéderm-treated patients also reported significantly less pain than Belotero Balance–treated patients (P<.001).10
Treatment-related AEs are described in the Table, with the majority occurring at lower rates compared to a collagen control group and self-resolving within 2 weeks.7
Juvéderm Voluma XC
Juvéderm Voluma XC was FDA approved in 2013 for cheek augmentation to correct age-related volume deficit restoration by subcutaneous or subperiosteal injections. In its landmark multicenter investigation, 282 patients with moderate to severe midface (eg, zygomaticomalar, anteromedial cheek, submalar regions) volume deficit measured on a validated midface volume deficit scale (MFVDS) were treated with Juvéderm Voluma XC (n=235) or control (n=47).11 Patients were reevaluated at 30 days and 81.9% received touch-up injections. At a 6-month primary evaluation, 86% of the Juvéderm-treated patients versus 39% of the control patients showed 1-point improvement on the MFVDS (P<.001). At 24-months’ follow-up, 44.6% of patients sustained efficacy.11 Of these aforementioned patients, 167 received repeat treatment due to lost correction or patient request and 91.1% improved by 1 point or more on the MFVDS on evaluation 12 months after repeat treatment.12 For this same population of patients, a 2-year extended follow-up of patient-reported outcomes revealed that 49% of patients felt fulfilled in their treatment goals 2 years after treatment and 79% of patients rated improvement from baseline based on the global aesthetic improvement scale.13 Efficacy studies involving Juvéderm Voluma XC are currently ongoing for facial temporal aging (registered at www.clinicaltrials.gov with the identifier NCT02437903) and recruiting for mandibular hypoplasia (NCT02330016).
Common treatment-related AEs are detailed in the Table. Two patients required treatment with hyaluronidase for chronic lumpiness and nodularity following non–treatment-related cellulitis.11 The product is contraindicated in patients with allergy to lidocaine.
Restylane Silk
Restylane Silk was approved in 2014 for lip augmentation and perioral rhytides. Efficacy and safety was demonstrated in a large multicenter randomized investigation in which 221 patients seeking lip augmentation received either Restylane Silk (n=177) injected submucosally for treatment of the upper and lower lips and/or intradermally for perioral rhytides or no treatment (n=44).14 Restylane treatment group patients optionally received touch-up at 2 weeks for optimization. All patients, including the control group, received injections at 6 months. At the 2-month primary end point, 80.2% of the treatment group exhibited at least 1-point improvement in upper lip fullness on the Medicis lip fullness scale compared to 11.9% (P<.001) of the control group; response rates for the lower lips were 84.2% versus 18.4% (P<.001). Patients in the treatment group receiving injections for perioral rhytides showed significant improvement in perioral rhytides through week 24 compared to patients treated for lip augmentation only (P<.001).14 Restylane Silk currently is undergoing investigation for cheek rejuvenation (NCT02636894, NCT02679924) and treatment of hand photoaging (NCT02780258).
The most common AEs are listed in the Table. No lip disorders were considered clinically concerning on evaluation. Concomitant lip augmentation and treatment of perioral rhytides yielded similar rates of AEs.14 Restylane Silk is not to be used in patients with known lidocaine allergy.
Restylane Lyft
Restylane Lyft (formerly known as Perlane-L) was approved in 2010 for use in facial rhytides, including NLFs, and gained approval in 2015 for use in cheek augmentation and midface contouring. Only its efficacy and safety for the more recent indication will be reviewed here.
In an evaluator-blinded investigation of 200 patients with mild to substantial bilateral midface deficiency based on the Medicis midface volume scale (MMVS), patients were randomized to receive supraperiosteal and subcutaneous treatment with Restylane Lyft (n=150) or no treatment (n=50).15 Touch-up injections at week 2 or month 12 were available to treatment group patients and all patients were given either an initial treatment or retreatment at 12 months. Primary end point evaluation at week 8 showed that 89% of treatment group patients had at least 1 grade MMVS improvement compared to 16% of the control group (P<.001). Although the percentage of these MMVS responders in the treatment group decreased with each follow-up period to 54.3% at month 12, retreatment was effective in reproducing a similar MMVS response rate as with initial treatment.15 Restylane Lyft is under ongoing investigation for dorsal hand rejuvenation (NCT02650921).
In addition to the common treatment-related AEs listed in the Table, 2 patients reported serious AEs, including bilateral implant-site inflammation and unilateral implant-site hematoma and infection (organism not described), all of which resolved with unspecified treatment.15 Lidocaine allergies are contraindications for use.
Conclusion
Several new options in dermal fillers have been approved in recent years and have demonstrated efficacy and acceptable safety in various cosmetic rejuvenation applications. Restylane Silk and Restylane Lyft are undergoing further studies to evaluate use in hand rejuvenation, an area that currently has few cosmetic filler treatment options. As technology continues to progress and new formulations of dermal fillers with varied properties and benefits are available, clinicians should expect multiple options for use in rhytides, volume deficits, and contouring.
ADDENDUM
After the manuscript was accepted for publication, Juvéderm Volbella XC (Allergan, Inc) was approved by the FDA for use in lip augmentation and thus is not included in this review.
Facial aging is the result of the interplay between loss of skin elasticity, changes in subcutaneous fat and other soft-tissue layers, and skeletal remodeling with chronological age.1 Dermal fillers are effective for the treatment of rhytides, facial scars, and lipoatrophy, as well as facial contouring and augmentation. Given that multiple filler options exist, updated reviews are necessary to inform clinicians of the choices that are available. We provide a detailed review of the clinical efficacy and safety of the dermal fillers with the most recent approvals by the US Food and Drug Administration (FDA).
Polymethylmethacrylate
Polymethylmethacrylate (PMMA) microspheres suspended in bovine collagen and lidocaine 0.3% were approved in 2006 for use in nasolabial folds (NLFs) and in 2014 for acne scars. Now branded as Bellafill (Suneva Medical, Inc), it is the only permanent injectable filler currently available. Once injected, the particles are not reabsorbed and can only be removed by procedural extraction (eg, liposuction of the surrounding fat); however, the permanence of PMMA does not extend to facial rejuvenation, which can last up to 5 years. Prior to use, skin testing for bovine collagen reaction is necessary. In a clinical trial of 147 patients with moderate to severe acne scarring, patients were randomized to receive PMMA in collagen (n=97) or saline (n=50).2 Injections were administered using a linear threading or serial puncture technique, and patients were reevaluated after 4 weeks for touch-up injections. After 6 months, 64% of patients treated with PMMA in collagen achieved improvement in acne scars by 2 points or more on the acne scar rating scale versus 33% of the control group (P=.0005).2
Treatment-related adverse events (AEs) include injection-site pain, bruising, swelling, erythema, and more rarely pruritus and lumps/granulomas.3 A 5-year longitudinal safety investigation of 871 patients initially treated with PMMA in collagen for NLF correction revealed that 17 patients (2.0%) had biopsy-confirmed granulomas with half of these retained at study end.4 Fifteen of these patients were treated with intralesional corticosteroids alone or in combination with intralesional 5-fluorouracil, oral antibiotics, or topical calcineurin inhibitors; 1 patient was untreated and another used topical corticosteroids. The authors noted no correlation between treatment method and granuloma response.4 Polymethylmethacrylate in collagen is contraindicated in patients with lidocaine or bovine collagen sensitivity and is not indicated for use in lip augmentation due to high rates of nodule formation.3
Hyaluronic Acid
Hyaluronic acid (HA) is a naturally occurring glycosaminoglycan polymer found in the extracellular matrix of the dermis. Hyaluronic acid fillers are bacteria derived and come in gel form. A useful advantage of HA fillers compared to other dermal fillers is the commercial availability of hyaluronidase to correct injections. Preinjection skin testing is not necessary.5
This category of nonpermanent dermal fillers has the most robust market choices. Older HA dermal fillers with reliable and proven efficacy are Restylane (Galderma Laboratories, LP)(facial rhytides, lip augmentation), Juvéderm (Ultra/Ultra XC/Ultra Plus/Ultra Plus XC [Allergan, Inc])(facial rhytides, lip augmentation), Hydrelle (Anika Therapeutics, Inc)(facial rhytides), and Prevelle Silk (Mentor Corporation)(facial rhytides); they will not be reviewed here. Newer agents include Belotero Balance (Merz Aesthetics), Juvéderm Voluma XC (Allergan, Inc), Restylane Silk (Galderma Laboratories, LP), and Restylane Lyft (Galderma Laboratories, LP).
Belotero Balance
Belotero Balance is used to treat fine lines and wrinkles, especially NLFs.6 The initial pivotal studies that led to FDA approval in 2011 demonstrated noninferiority and superiority to bovine collagen for use in the treatment of NLFs.7,8 One hundred eighteen patients with bilateral NLFs that were rated as 2 (moderate) or 3 (severe) on the wrinkle severity rating scale (WSRS) were randomized to split-face injection of Belotero Balance in one NLF and bovine collagen in the contralateral NLF.7 An additional injection at week 2 was allowed for optimal correction. Belotero Balance was noninferior to bovine collagen at week 2, with mean improvement in WSRS of 1.52 versus 1.57 (P=.50). Belotero Balance was superior to bovine collagen in mean WSRS improvement at weeks 12 (1.25 vs 0.26; P<.001), 16 (1.09 vs 0.66; P<.001), and 24 (1.08 vs 0.50; P<.001).7 In a subsequent open-label extension study, which included 95 of 118 patients who received Belotero Balance injections in both NLFs at week 24, 80.2% of patients showed sustained improvement in WSRS from baseline for 48 weeks without further injection.8
The first comparative study of Belotero Balance with other established HA fillers at the time—Restylane and Juvéderm Ultra 3/Ultra Plus XC—to treat NLFs demonstrated noninferiority.9 Forty patients with bilateral, moderate to severe NLFs (rated 3 or 4 on the Merz severity scale) were randomized to split-face groups of Belotero Balance versus Restylane or Belotero Balance versus Juvéderm. At 12 months, NLF severity improved from 2.3 to 1.5 in the Restylane group and from 2.3 to 1.6 in the Juvéderm group.9
Belotero Balance has been compared to Juvéderm Ultra XC for use in perioral lines.10 The study included 136 patients with moderate to severe perioral lines, according to the perioral lines severity scale, who were randomized (1:1 ratio) to receive injections of Belotero Balance or Juvéderm Ultra XC to correct upper and lower perioral lines, with assessment at week 2 for optimization. After 6 months, 87% of Juvéderm-treated patients compared to 72% of Belotero Balance–treated patients had 1-point improvement in perioral lines (P<.04). Juvéderm-treated patients also reported significantly less pain than Belotero Balance–treated patients (P<.001).10
Treatment-related AEs are described in the Table, with the majority occurring at lower rates compared to a collagen control group and self-resolving within 2 weeks.7
Juvéderm Voluma XC
Juvéderm Voluma XC was FDA approved in 2013 for cheek augmentation to correct age-related volume deficit restoration by subcutaneous or subperiosteal injections. In its landmark multicenter investigation, 282 patients with moderate to severe midface (eg, zygomaticomalar, anteromedial cheek, submalar regions) volume deficit measured on a validated midface volume deficit scale (MFVDS) were treated with Juvéderm Voluma XC (n=235) or control (n=47).11 Patients were reevaluated at 30 days and 81.9% received touch-up injections. At a 6-month primary evaluation, 86% of the Juvéderm-treated patients versus 39% of the control patients showed 1-point improvement on the MFVDS (P<.001). At 24-months’ follow-up, 44.6% of patients sustained efficacy.11 Of these aforementioned patients, 167 received repeat treatment due to lost correction or patient request and 91.1% improved by 1 point or more on the MFVDS on evaluation 12 months after repeat treatment.12 For this same population of patients, a 2-year extended follow-up of patient-reported outcomes revealed that 49% of patients felt fulfilled in their treatment goals 2 years after treatment and 79% of patients rated improvement from baseline based on the global aesthetic improvement scale.13 Efficacy studies involving Juvéderm Voluma XC are currently ongoing for facial temporal aging (registered at www.clinicaltrials.gov with the identifier NCT02437903) and recruiting for mandibular hypoplasia (NCT02330016).
Common treatment-related AEs are detailed in the Table. Two patients required treatment with hyaluronidase for chronic lumpiness and nodularity following non–treatment-related cellulitis.11 The product is contraindicated in patients with allergy to lidocaine.
Restylane Silk
Restylane Silk was approved in 2014 for lip augmentation and perioral rhytides. Efficacy and safety was demonstrated in a large multicenter randomized investigation in which 221 patients seeking lip augmentation received either Restylane Silk (n=177) injected submucosally for treatment of the upper and lower lips and/or intradermally for perioral rhytides or no treatment (n=44).14 Restylane treatment group patients optionally received touch-up at 2 weeks for optimization. All patients, including the control group, received injections at 6 months. At the 2-month primary end point, 80.2% of the treatment group exhibited at least 1-point improvement in upper lip fullness on the Medicis lip fullness scale compared to 11.9% (P<.001) of the control group; response rates for the lower lips were 84.2% versus 18.4% (P<.001). Patients in the treatment group receiving injections for perioral rhytides showed significant improvement in perioral rhytides through week 24 compared to patients treated for lip augmentation only (P<.001).14 Restylane Silk currently is undergoing investigation for cheek rejuvenation (NCT02636894, NCT02679924) and treatment of hand photoaging (NCT02780258).
The most common AEs are listed in the Table. No lip disorders were considered clinically concerning on evaluation. Concomitant lip augmentation and treatment of perioral rhytides yielded similar rates of AEs.14 Restylane Silk is not to be used in patients with known lidocaine allergy.
Restylane Lyft
Restylane Lyft (formerly known as Perlane-L) was approved in 2010 for use in facial rhytides, including NLFs, and gained approval in 2015 for use in cheek augmentation and midface contouring. Only its efficacy and safety for the more recent indication will be reviewed here.
In an evaluator-blinded investigation of 200 patients with mild to substantial bilateral midface deficiency based on the Medicis midface volume scale (MMVS), patients were randomized to receive supraperiosteal and subcutaneous treatment with Restylane Lyft (n=150) or no treatment (n=50).15 Touch-up injections at week 2 or month 12 were available to treatment group patients and all patients were given either an initial treatment or retreatment at 12 months. Primary end point evaluation at week 8 showed that 89% of treatment group patients had at least 1 grade MMVS improvement compared to 16% of the control group (P<.001). Although the percentage of these MMVS responders in the treatment group decreased with each follow-up period to 54.3% at month 12, retreatment was effective in reproducing a similar MMVS response rate as with initial treatment.15 Restylane Lyft is under ongoing investigation for dorsal hand rejuvenation (NCT02650921).
In addition to the common treatment-related AEs listed in the Table, 2 patients reported serious AEs, including bilateral implant-site inflammation and unilateral implant-site hematoma and infection (organism not described), all of which resolved with unspecified treatment.15 Lidocaine allergies are contraindications for use.
Conclusion
Several new options in dermal fillers have been approved in recent years and have demonstrated efficacy and acceptable safety in various cosmetic rejuvenation applications. Restylane Silk and Restylane Lyft are undergoing further studies to evaluate use in hand rejuvenation, an area that currently has few cosmetic filler treatment options. As technology continues to progress and new formulations of dermal fillers with varied properties and benefits are available, clinicians should expect multiple options for use in rhytides, volume deficits, and contouring.
ADDENDUM
After the manuscript was accepted for publication, Juvéderm Volbella XC (Allergan, Inc) was approved by the FDA for use in lip augmentation and thus is not included in this review.
- Fitzgerald R, Graivier MH, Kane M, et al. Update on facial aging. Aesthet Surg J. 2010;30(suppl):S11-S24.
- Karnik J, Baumann L, Bruce S, et al. A double-blind, randomized, multicenter, controlled trial of suspended polymethylmethacrylate microspheres for the correction of atrophic facial acne scars. J Am Acad Dermatol. 2014;71:77-83.
- Bellafill [package insert]. San Diego, CA: Suneva Medical, Inc; 2015.
- Cohen S, Dover J, Monheit G, et al. Five-year safety and satisfaction study of PMMA-collagen in the correction of nasolabial folds. Dermatol Surg. 2015;41(suppl 1):S302-S313.
- Greene JJ, Sidle DM. The hyaluronic acid fillers: current understanding of the tissue device interface. Facial Plast Surg Clin North Am. 2015;23:423-432.
- Lorenc ZP, Fagien S, Flynn TC, et al. Review of key Belotero Balance safety and efficacy trials. Plast Reconstr Surg. 2013;132(4, suppl 2):33S-40S.
- Narins RS, Coleman W, Donofrio L, et al. Nonanimal sourced hyaluronic acid–based dermal filler using a cohesive polydensified matrix technology is superior to bovine collagen in the correction of moderate to severe nasolabial folds: results from a 6-month, randomized, blinded, controlled, multicenter study. Dermatol Surg. 2010;36(suppl 1):730-740.
- Narins RS, Coleman WP 3rd, Donofrio LM, et al. Improvement in nasolabial folds with a hyaluronic acid filler using a cohesive polydensified matrix technology: results from an 18-month open-label extension trial. Dermatol Surg. 2010;36(suppl 3):1800-1808.
- Prager W, Wissmueller E, Havermann I, et al. A prospective, split-face, randomized, comparative study of safety and 12-month longevity of three formulations of hyaluronic acid dermal filler for treatment of nasolabial folds. Dermatol Surg. 2012;38(7, pt 2):1143-1150.
- Butterwick K, Marmur E, Narurkar V, et al. HYC-24L demonstrates greater effectiveness with less pain than CPM-22.5 for treatment of perioral lines in a randomized controlled trial. Dermatol Surg. 2015;41:1351-1360.
- Jones D, Murphy DK. Volumizing hyaluronic acid filler for midface volume deficit: 2-year results from a pivotal single-blind randomized controlled study. Dermatol Surg. 2013;39:1602-1612.
- Baumann L, Narins RS, Beer K, et al. Volumizing hyaluronic acid filler for midface volume deficit: results after repeat treatment. Dermatol Surg. 2015;41(suppl 1):S284-S292.
- Few J, Cox SE, Paradkar-Mitragotri D, et al. A multicenter, single-blind randomized, controlled study of a volumizing hyaluronic acid filler for midface volume deficit: patient-reported outcomes at 2 years. Aesthet Surg J. 2015;35:589-599.
- Beer K, Glogau RG, Dover JS, et al. A randomized, evaluator-blinded, controlled study of effectiveness and safety of small particle hyaluronic acid plus lidocaine for lip augmentation and perioral rhytides. Dermatol Surg. 2015;41(suppl 1):S127-S136.
- Weiss RA, Moradi A, Bank D, et al. Effectiveness and safety of large gel particle hyaluronic acid with lidocaine for correction of midface volume deficit or contour deficiency. Dermatol Surg. 2016;42:699-709.
- Fitzgerald R, Graivier MH, Kane M, et al. Update on facial aging. Aesthet Surg J. 2010;30(suppl):S11-S24.
- Karnik J, Baumann L, Bruce S, et al. A double-blind, randomized, multicenter, controlled trial of suspended polymethylmethacrylate microspheres for the correction of atrophic facial acne scars. J Am Acad Dermatol. 2014;71:77-83.
- Bellafill [package insert]. San Diego, CA: Suneva Medical, Inc; 2015.
- Cohen S, Dover J, Monheit G, et al. Five-year safety and satisfaction study of PMMA-collagen in the correction of nasolabial folds. Dermatol Surg. 2015;41(suppl 1):S302-S313.
- Greene JJ, Sidle DM. The hyaluronic acid fillers: current understanding of the tissue device interface. Facial Plast Surg Clin North Am. 2015;23:423-432.
- Lorenc ZP, Fagien S, Flynn TC, et al. Review of key Belotero Balance safety and efficacy trials. Plast Reconstr Surg. 2013;132(4, suppl 2):33S-40S.
- Narins RS, Coleman W, Donofrio L, et al. Nonanimal sourced hyaluronic acid–based dermal filler using a cohesive polydensified matrix technology is superior to bovine collagen in the correction of moderate to severe nasolabial folds: results from a 6-month, randomized, blinded, controlled, multicenter study. Dermatol Surg. 2010;36(suppl 1):730-740.
- Narins RS, Coleman WP 3rd, Donofrio LM, et al. Improvement in nasolabial folds with a hyaluronic acid filler using a cohesive polydensified matrix technology: results from an 18-month open-label extension trial. Dermatol Surg. 2010;36(suppl 3):1800-1808.
- Prager W, Wissmueller E, Havermann I, et al. A prospective, split-face, randomized, comparative study of safety and 12-month longevity of three formulations of hyaluronic acid dermal filler for treatment of nasolabial folds. Dermatol Surg. 2012;38(7, pt 2):1143-1150.
- Butterwick K, Marmur E, Narurkar V, et al. HYC-24L demonstrates greater effectiveness with less pain than CPM-22.5 for treatment of perioral lines in a randomized controlled trial. Dermatol Surg. 2015;41:1351-1360.
- Jones D, Murphy DK. Volumizing hyaluronic acid filler for midface volume deficit: 2-year results from a pivotal single-blind randomized controlled study. Dermatol Surg. 2013;39:1602-1612.
- Baumann L, Narins RS, Beer K, et al. Volumizing hyaluronic acid filler for midface volume deficit: results after repeat treatment. Dermatol Surg. 2015;41(suppl 1):S284-S292.
- Few J, Cox SE, Paradkar-Mitragotri D, et al. A multicenter, single-blind randomized, controlled study of a volumizing hyaluronic acid filler for midface volume deficit: patient-reported outcomes at 2 years. Aesthet Surg J. 2015;35:589-599.
- Beer K, Glogau RG, Dover JS, et al. A randomized, evaluator-blinded, controlled study of effectiveness and safety of small particle hyaluronic acid plus lidocaine for lip augmentation and perioral rhytides. Dermatol Surg. 2015;41(suppl 1):S127-S136.
- Weiss RA, Moradi A, Bank D, et al. Effectiveness and safety of large gel particle hyaluronic acid with lidocaine for correction of midface volume deficit or contour deficiency. Dermatol Surg. 2016;42:699-709.
Practice Points
- The merits of new dermal fillers approved by the US Food and Drug Administration should be weighed with an understanding of aesthetic indications of use, duration of efficacy, and common adverse effects, in line with patient preference.
- The most common adverse effects are injection-site contusion, swelling, and pain, usually self-resolving within days to 2 weeks. Patient quality of care can be improved with forewarning and emphasis on alleviating symptoms.