User login
Several important developments in the palliative care of patients with lung cancer have occurred over the past few years, including publication of a landmark study comparing early with as-needed palliative care, the release of new data on the treatment of cancer-related anorexia, elucidation of new mechanisms and treatment options for dyspnea, and the availability of buprenorphine. This article reviews these emerging concepts.
LUNG CANCER SYMPTOMS: COMMON AND SEVERE
The symptom burden of lung cancer is usually great. At least 80% of patients experience fatigue, 65% suffer loss of appetite, 77% have cough, 73% report dyspnea (both from local symptoms and weight loss), 57% have chest pain, and 17% have hemoptysis.1
When symptoms are present, they are usually severe. Thirty-eight percent of the patients who report fatigue have severe fatigue, 47% have inadequate appetite to the point of requiring intervention, and more than one-half of patients who have chest pain require opioids for relief.1
Symptom frequency and severity are worse in individuals who survive 3 months or less.1 Increasing symptom burden is therefore prognostically important, particularly in patients with advanced stages of lung cancer. As a result, self-assessment of quality of life has a significant ability to predict survival in patients with advanced non–small cell lung cancer (NSCLC).2
Patients with lung cancer tend to suffer from groups of symptoms or symptom clusters. Lutz et al1 found that 79% of patients reported three or more symptoms; these results were similar to the findings of a study by Hollen et al,3 in which 81% of patients suffered from three or more symptoms, all them severe except for cough.
EARLY PALLIATIVE CARE HAS CLINICAL BENEFITS
A landmark study by Temel et al4 examined the benefits of early palliative care integrated with standard oncologic care versus standard oncologic care and palliative care only “as needed” on patient-reported outcomes, the use of health services, and the quality of end-of-life care among patients with metastatic NSCLC. The study was a prospective, nonblinded, randomized, controlled trial of outpatients conducted at a single center. The intervention was based on guidelines from the National Consensus Project for Quality Palliative Care, with specific attention to symptom management, goals of care, decision-making regarding treatment, and coordination of care. Patients assigned to the intervention met monthly with both a palliative care service and an oncologist, and 90% of the patients randomized to intervention complied with at least 50% of the visits.
Measures of health-related quality of life and mood were obtained using the Functional Assessment of Cancer Therapy-Lung (FACT-L), the Hospital Anxiety and Depression Scale, and the 9-item depression scale of the Patient Health Questionnaire.
Measures of health care service utilization included use of antitumor therapy within 14 days of death, late or no referral to hospice, hospital admissions, and emergency room visits. Patients were considered to have received aggressive care if they met any one of the following three criteria: chemotherapy within 14 days of death, no hospice care, or admission to hospice within 3 days of death.
Compared with standard care, early palliative care was associated with an increase in the number of advance directives, earlier hospice referral (11 days vs 4 days), fewer hospitalizations and emergency room visits, and fewer instances of inappropriate oncologic care (defined as chemotherapy within 14 days of death). The percentage of patients with depressed mood was also lower among those assigned to early palliative care versus standard care (16% vs 38%).
A 2.7-month difference in median survival (P = .02) in favor of the group assigned to early palliative care was also observed, although survival was not a primary end point of the trial. This outcome needs to be validated in future studies.
CANCER-RELATED ANOREXIA AND CACHEXIA: TREATMENT IMPROVES APPETITE
The main hallmark of cancer-related anorexia and cachexia is weight loss; this symptom cluster is most often associated with hypophagia. The coexistence of anorexia and appetite-related anhedonia is common in lung cancer patients, such that 25% of lung cancer patients with anorexia report no distress with not eating, nor do they derive pleasure from eating. Others report that early satiety and changes in taste dramatically affect appetite. To some, anorexia is a distressful reminder of progression of their cancer.
Megestrol acetate and medroxyprogesterone acetate at least partially improve appetite in a subset of anorectic cancer patients. The use of medroxyprogesterone acetate has resulted in weight gain but not muscle mass in some patients with cancer-related anorexia, but has had less effect on fatigue and quality of life in these patients.
Olanzapine is an atypical antipsychotic with an affinity for multiple neurotransmitter receptors. Several of these, such as the serotonin receptors 5-HT2 and 5-HT3, histamine receptors, and dopamine receptors, are implicated in anorexia, nausea, and vomiting. Case reports suggest that olanzapine has antiemetic activity in patients with advanced cancer and usefulness as prophylaxis against chemotherapy-related nausea and vomiting.5 Reduced risk of extrapyramidal symptoms compared with standard antiemetics enhances the value of olanzapine for prevention of cancer-related anorexia.
Navari et al6 conducted a randomized trial to determine the effectiveness of megestrol acetate and olanzapine for the treatment of cancer-related anorexia. Eighty patients were randomized to receive oral megestrol acetate 800 mg/d, or oral megestrol acetate 800 mg/d plus olanzapine 5 mg once nightly, for 8 weeks. Patients were removed from the study if they did not take the study medication for a 48-hour period or if intolerable toxicity developed that was attributable to the study agents.
The MD Anderson Symptom Inventory (MDASI) was completed weekly to assess key symptom outcome variables. A change of 3 cm on the visual analog scale over two separate time periods for a symptom was considered sufficient to define a change in the symptom.
Quality of life was measured using a valid 28-item self-reported instrument (Functional Assessment of Cancer Therapy-General). Patients were examined by their physicians every 2 weeks.
In the group assigned to megestrol acetate, 15 patients had a weight gain of at least 5%—a change that was considered significant. Appetite improved in two patients, nausea decreased in three patients, and quality of life improved in five patients at both 4 weeks and 8 weeks. The improvements in appetite, nausea, and quality of life for the whole group on megestrol acetate alone were not significant, and there was no improvement in mean symptom scores measured by the MDASI.
There were incremental improvements of all measures in patients randomized to megestrol acetate plus olanzapine. Among patients receiving the combination, 33 had a weight gain of at least 5%; 25 reported an improvement in appetite, 21 experienced a reduction in nausea, and 23 had an improvement in quality of life at both 4 weeks and 8 weeks. All outcome variables were improved on the MDASI
CANCER AND DYSPNEA: NUMEROUS INTERVENTIONS HAVE BEEN ASSESSED
Reduced inspiratory capacity caused by weakened inspiratory muscles results in an increased Borg rating of perceived exertion (RPE) relative to oxygen levels. Both central nervous system activation of muscle and loss of muscle tissue contribute to dyspnea and fatigue in lung cancer patients.7 Cancer fatigue, also measured by the Borg RPE scale, appears to be a “central” mechanism that stems from a mismatch between efferent output for afferent inputs in the setting of ventilatory muscle weakness, thereby increasing the perception of dyspnea. Several interventions have been used to relieve dyspnea, ranging from oxygen therapy to treatment with opioids.
Oxygen saturation
The association between hypoxemia and dyspnea is poor.8 In a randomized prospective trial, Abernethy et al9 found no benefit to oxygen therapy compared with medical air without added supplemental oxygen in individuals who had normal oxygen saturation but symptomatic dyspnea.
Bilevel positive airway pressure
Bilevel positive airway pressure has been shown to reduce the need for invasive ventilation; improve oxygen saturation; and reduce dynamic hyperinflation, thus relieving dyspnea.10 It has been effective in dyspneic patients with motor neuron disease, cancer, heart failure, status asthmaticus, stroke, drug overdose, and interstitial lung disease.
Indwelling pleural catheters
Tunneled pleural catheters reduce the severity of dyspnea in 95% of patients.11 These catheters are inserted on an outpatient basis, allowing for outpatient drainage. Autopleurodesis occurs in about 45% of patients, in which case the catheter can be removed. Adverse reactions are few (incidence < 10%), but consist of empyema, pneumothorax, cellulitis, or catheter obstruction. The disadvantage is the expense of catheter maintenance.
Nebulized furosemide
Case reports suggest that inhalation of nebulized furosemide, 20 mg four times daily, dramatically improves dyspnea in patients with advanced cancer and severe shortness of breath that is unresponsive to opioids.12 Nebulized furosemide appears to have a direct effect on either pulmonary stretch receptors or irritant receptors in the airways; it also has a diuretic effect. Response occurs quickly with an onset of effect in 20 to 30 minutes.
B-type natriuretic peptide
The level of N-terminal precursor of B-type natriuretic peptide (NT-pro-BNP) can predict response to sunitinib in renal cancer,13 and the BNP level predicts 30-day mortality in pulmonary embolism.14 Measurement of BNP to detect dyspnea in patients with lung cancer is not useful, however, because the BNP level increases with cardiac and pericardial metastases. The BNP level is also persistently elevated after chest radiation therapy, and it increases with anthracycline cardiotoxicity. It is not a useful marker for distinguishing pulmonary from nonpulmonary or cardiac from noncardiac causes of dyspnea.
Lung ultrasound
Portable diagnostic lung ultrasound can be used to detect pneumonia, pleural effusions, pulmonary emboli, pneumothorax, atelectasis, and lung abscesses as potential causes of dyspnea.15–18 In addition to the advantage of portability, there is no radiation exposure and the technology permits echocardiography to be conducted.
Opioids
Evidence supports opioids for pharmacologic relief of dyspnea in the palliative care of patients with chronic obstructive pulmonary disease and cancer. Studies have been conducted with morphine sulfate, hydromorphone, dihydrocodeine, intranasal and transmucosal fentanyl, oxycodone, and diamorphine.19–21
The response to opioids is unrelated to the severity of dyspnea.22 Responses and safe administration occur even in patients with reduced oxygen saturation or elevated carbon dioxide partial pressure.20 Opioids can be used safely in the opioid-naïve population.20 Recommended dosages in these patients are 2.5 to 5.0 mg of morphine sulfate every 4 hours, 5 mg of oxycodone every 4 hours as needed, and 1 mg of hydromorphone every 4 hours in the opioid-naïve. In opioid-tolerant patients, it is recommended that therapy start with these doses and then be increased in 25% increments every 24 hours, as needed.
BUPRENORPHINE: UNIQUE OPIOID
Buprenorphine is a mu- and nociceptin (ORL-1)-receptor partial agonist with intravenous, subcutaneous, sublingual, transdermal, and intranasal routes of delivery.23 An agent that acts as an ORL-1 agonist can induce analgesia by blocking nociceptive responses at the level of the spinal cord. It is a kappa antagonist (depending upon the kappa ligand used in the assay), which may contribute to its antihyperalgesia. The parent drug has a high affinity and low intrinsic efficacy for the mu receptor. The main metabolite, norbuprenorphine, is a delta opioid-receptor agonist.
Secondary hyperalgesia is an increased sensitivity to painful stimuli around an area of injury and occurs frequently following injury. The increased pain sensation is a result of central sensitization derived from brainstem neurons that facilitate pain; it is not derived from afferent signals from the primary site. Secondary hyperalgesia is less responsive to opioids than primary hyperalgesia at the site of injury.
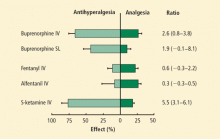
Pain is improved with buprenorphine predominantly through modulation of central sensitization and less so at the primary site. Koppert et al25 demonstrated in human volunteers that buprenorphine reduced the area and duration of secondary hyperalgesia more than pain at the site of injury (half-life of 171 minutes vs 288 minutes, respectively). Buprenorphine had a much greater antihyperalgesic effect than analgesic effect compared with potent opioids such as fentanyl. In contrast, the analgesic effects with fentanyl and alfentanil were much greater than their antihyperalgesic effects (Figure), suggesting the possibility of a combination of opioid therapy for superior pain relief or choices based on pain phenotype (eg, secondary or primary hyperalgesia).
SUMMARY
Early palliative care improves quality of life and decision-making in patients with advanced lung cancer and may improve survival, although survival data need to be confirmed. Olanzapine and megestrol acetate are superior to megestrol acetate alone for the treatment of anorexia. Oxygen is no better than medical air in the management of dyspnea associated with normal oxygen saturation. Buprenorphine is a unique opioid that has value for pharmacologic relief in patients at risk for respiratory depression.
- Lutz S, Norrell R, Bertucio C, et al. Symptom frequency and severity in patients with metastatic or locally recurrent lung cancer: a prospective study using the Lung Cancer Symptom Scale in a community hospital. J Palliat Med 2001; 4:157–165.
- Perrone F, Gridelli C, Cigolari S, et al. Baseline assessment of quality of life (QOL) is a strong prognostic factor for survival of elderly patients with advanced non-small cell lung cancer (NSCLC): a secondary analysis of the MILES study [ASCO abstract 1346]. Proc Am Soc Clin Oncol 2002;21.
- Hollen PJ, Gralla RJ, Kris MG, et al. Measurement of quality of life in patients with lung cancer in multicenter trials of new therapies: psychometric assessment of the Lung Cancer Symptom Scale. Cancer 1994; 73:2087–2098.
- Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non–small-cell lung cancer. N Engl J Med 2010; 363:733–742.
- Navari RM, Einhorn LH, Loehrer PJ, et al. A phase II trial of olanzapine, dexamethasone, and palonosetron for the prevention of chemotherapy-induced nausea and vomiting: a Hoosier oncology group study [published online ahead of print March 21, 2007]. Support Care Cancer 2007; 15:1285–1291. doi: 10.1007/s00520-007-0248-5
- Navari RM, Brenner MC. Treatment of cancer-related anorexia with olanzapine and megestrol acetate: a randomized trial. Support Care Cancer 2010; 18:951–956.
- Travers J, Dudgeon DJ, Amjadi K, et al. Mechanisms of exertional dyspnea in patients with cancer. J Appl Physiol 2008; 104:57–66.
- Galbraith S, Fagan P, Perkins P, Lynch A, Booth S. Does the use of a handheld fan improve chronic dyspnea? A randomized, controlled, crossover trial. J Pain Symptom Manage 2010; 39:831–838.
- Abernethy AP, McDonald CF, Frith PA, et al. Effect of palliative oxygen versus room air in relief of breathlessness in patients with refractory dyspnoea: a double-blind, randomised controlled trial. Lancet 2010; 376:784–793.
- Sellner-Pogány T, Lahrmann H. BIPAP-mask-ventilation in terminal amyotrophic lateral sclerosis (ALS) [in German]. Wien Med Wochenschr 2009; 159:604–607.
- Van Meter ME, McKee KY, Kohlwes RJ. Efficacy and safety of tunneled pleural catheters in adults with malignant pleural effusions: a systematic review [published online ahead of print August 10, 2010]. J Gen Intern Med 2011; 26:70–76. doi: 10.1007/s11606-010-1472-0
- Shimoyama N, Shimoyama M. Nebulized furosemide as a novel treatment for dyspnea in terminal cancer patients. J Pain Symptom Manage 2002; 23:73–76.
- Papazisis KT, Kontovinis LF, Papandreou CN, et al. Brain natriuretic peptide precursor (NT-pro-BNP) levels predict for clinical benefit to sunitinib treatment in patients with metastatic renal cell carcinoma. BMC Cancer 2010; 10:489.
- Cavallazzi R, Nair A, Vasu T, Marik PE. Natriuretic peptides in acute pulmonary embolism: a systematic review [published online ahead of print July 15, 2008]. Intensive Care Med 2008; 34:2147–2156. doi: 10.1007/s00134-008-1214-5
- Chen H-J, Yu Y-H, Tu C-Y, Chen C-H, Hsia T-C, Tsai K-D. Ultrasound in peripheral pulmonary air-fluid lesions: color Doppler imaging as an aid in differentiating empyema and abscess. Chest 2009; 135:1426–1432.
- Lichtenstein DA, Mezière GA, Lagoueyte J-F, Biderman P, Goldstein I, Gepner A. A-lines and B-lines: lung ultrasound as a bedside tool for predicting pulmonary artery occlusion pressure in the critically ill. Chest 2009; 136:1014–1020.
- Lichtenstein DA. Ultrasound examination of the lungs in the intensive care unit. Pediatr Crit Care Med 2009; 10:693–698.
- Reissig A, Heyne J-P, Kroegel C. Sonography of lung and pleura in pulmonary embolism: sonomorphologic characterization and comparison with spiral CT scanning. Chest 2001; 120:1977–1983.
- Kallet RH. The role of inhaled opioids and furosemide for the treatment of dyspnea. Respir Care 2007; 52:900–910.
- Clemens KE, Quednau I, Klaschik E. Is there a higher risk of respiratory depression in opioid-naïve palliative care patients during symptomatic therapy of dyspnea with strong opioids? J Palliat Med 2008; 11:204–216.
- Varkey B. Opioids for palliation of refractory dyspnea in chronic obstructive pulmonary disease patients. Curr Opin Pulm Med 2010; 16:150–154.
- Currow DC, Plummer J, Frith P, Abernethy AP. Can we predict which patients with refractory dyspnea will respond to opioids? J Palliat Med 2007; 10:1031–1036.
- Davis MP. Buprenorphine in cancer pain. Support Care Cancer 2005; 13:878–887.
- Watson PJQ, McQuay HJ, Bullingham RES, Allen MC, Moore RA. Single-dose comparison of buprenorphine 0.3 and 0.6 mg i.v. given after operation: clinical effects and plasma concentrations. Br J Anaesth 1982; 54:37–43.
- Koppert W, Ihmsen H, Körber N, et al. Different profiles of buprenorphine-induced analgesia and antihyperalgesia in a human pain model. Pain 2005; 118:15–22.
- Sittl R, Likar R, Nautrup BP. Equipotent doses of transdermal fentanyl and transdermal buprenorphine in patients with cancer and noncancer pain: results of a retrospective cohort study. Clin Ther 2005; 27:225–237.
- Sittl R. Transdermal buprenorphine in cancer pain and palliative care. Palliat Med 2006; 20 (suppl 1):s25–s30.
Several important developments in the palliative care of patients with lung cancer have occurred over the past few years, including publication of a landmark study comparing early with as-needed palliative care, the release of new data on the treatment of cancer-related anorexia, elucidation of new mechanisms and treatment options for dyspnea, and the availability of buprenorphine. This article reviews these emerging concepts.
LUNG CANCER SYMPTOMS: COMMON AND SEVERE
The symptom burden of lung cancer is usually great. At least 80% of patients experience fatigue, 65% suffer loss of appetite, 77% have cough, 73% report dyspnea (both from local symptoms and weight loss), 57% have chest pain, and 17% have hemoptysis.1
When symptoms are present, they are usually severe. Thirty-eight percent of the patients who report fatigue have severe fatigue, 47% have inadequate appetite to the point of requiring intervention, and more than one-half of patients who have chest pain require opioids for relief.1
Symptom frequency and severity are worse in individuals who survive 3 months or less.1 Increasing symptom burden is therefore prognostically important, particularly in patients with advanced stages of lung cancer. As a result, self-assessment of quality of life has a significant ability to predict survival in patients with advanced non–small cell lung cancer (NSCLC).2
Patients with lung cancer tend to suffer from groups of symptoms or symptom clusters. Lutz et al1 found that 79% of patients reported three or more symptoms; these results were similar to the findings of a study by Hollen et al,3 in which 81% of patients suffered from three or more symptoms, all them severe except for cough.
EARLY PALLIATIVE CARE HAS CLINICAL BENEFITS
A landmark study by Temel et al4 examined the benefits of early palliative care integrated with standard oncologic care versus standard oncologic care and palliative care only “as needed” on patient-reported outcomes, the use of health services, and the quality of end-of-life care among patients with metastatic NSCLC. The study was a prospective, nonblinded, randomized, controlled trial of outpatients conducted at a single center. The intervention was based on guidelines from the National Consensus Project for Quality Palliative Care, with specific attention to symptom management, goals of care, decision-making regarding treatment, and coordination of care. Patients assigned to the intervention met monthly with both a palliative care service and an oncologist, and 90% of the patients randomized to intervention complied with at least 50% of the visits.
Measures of health-related quality of life and mood were obtained using the Functional Assessment of Cancer Therapy-Lung (FACT-L), the Hospital Anxiety and Depression Scale, and the 9-item depression scale of the Patient Health Questionnaire.
Measures of health care service utilization included use of antitumor therapy within 14 days of death, late or no referral to hospice, hospital admissions, and emergency room visits. Patients were considered to have received aggressive care if they met any one of the following three criteria: chemotherapy within 14 days of death, no hospice care, or admission to hospice within 3 days of death.
Compared with standard care, early palliative care was associated with an increase in the number of advance directives, earlier hospice referral (11 days vs 4 days), fewer hospitalizations and emergency room visits, and fewer instances of inappropriate oncologic care (defined as chemotherapy within 14 days of death). The percentage of patients with depressed mood was also lower among those assigned to early palliative care versus standard care (16% vs 38%).
A 2.7-month difference in median survival (P = .02) in favor of the group assigned to early palliative care was also observed, although survival was not a primary end point of the trial. This outcome needs to be validated in future studies.
CANCER-RELATED ANOREXIA AND CACHEXIA: TREATMENT IMPROVES APPETITE
The main hallmark of cancer-related anorexia and cachexia is weight loss; this symptom cluster is most often associated with hypophagia. The coexistence of anorexia and appetite-related anhedonia is common in lung cancer patients, such that 25% of lung cancer patients with anorexia report no distress with not eating, nor do they derive pleasure from eating. Others report that early satiety and changes in taste dramatically affect appetite. To some, anorexia is a distressful reminder of progression of their cancer.
Megestrol acetate and medroxyprogesterone acetate at least partially improve appetite in a subset of anorectic cancer patients. The use of medroxyprogesterone acetate has resulted in weight gain but not muscle mass in some patients with cancer-related anorexia, but has had less effect on fatigue and quality of life in these patients.
Olanzapine is an atypical antipsychotic with an affinity for multiple neurotransmitter receptors. Several of these, such as the serotonin receptors 5-HT2 and 5-HT3, histamine receptors, and dopamine receptors, are implicated in anorexia, nausea, and vomiting. Case reports suggest that olanzapine has antiemetic activity in patients with advanced cancer and usefulness as prophylaxis against chemotherapy-related nausea and vomiting.5 Reduced risk of extrapyramidal symptoms compared with standard antiemetics enhances the value of olanzapine for prevention of cancer-related anorexia.
Navari et al6 conducted a randomized trial to determine the effectiveness of megestrol acetate and olanzapine for the treatment of cancer-related anorexia. Eighty patients were randomized to receive oral megestrol acetate 800 mg/d, or oral megestrol acetate 800 mg/d plus olanzapine 5 mg once nightly, for 8 weeks. Patients were removed from the study if they did not take the study medication for a 48-hour period or if intolerable toxicity developed that was attributable to the study agents.
The MD Anderson Symptom Inventory (MDASI) was completed weekly to assess key symptom outcome variables. A change of 3 cm on the visual analog scale over two separate time periods for a symptom was considered sufficient to define a change in the symptom.
Quality of life was measured using a valid 28-item self-reported instrument (Functional Assessment of Cancer Therapy-General). Patients were examined by their physicians every 2 weeks.
In the group assigned to megestrol acetate, 15 patients had a weight gain of at least 5%—a change that was considered significant. Appetite improved in two patients, nausea decreased in three patients, and quality of life improved in five patients at both 4 weeks and 8 weeks. The improvements in appetite, nausea, and quality of life for the whole group on megestrol acetate alone were not significant, and there was no improvement in mean symptom scores measured by the MDASI.
There were incremental improvements of all measures in patients randomized to megestrol acetate plus olanzapine. Among patients receiving the combination, 33 had a weight gain of at least 5%; 25 reported an improvement in appetite, 21 experienced a reduction in nausea, and 23 had an improvement in quality of life at both 4 weeks and 8 weeks. All outcome variables were improved on the MDASI
CANCER AND DYSPNEA: NUMEROUS INTERVENTIONS HAVE BEEN ASSESSED
Reduced inspiratory capacity caused by weakened inspiratory muscles results in an increased Borg rating of perceived exertion (RPE) relative to oxygen levels. Both central nervous system activation of muscle and loss of muscle tissue contribute to dyspnea and fatigue in lung cancer patients.7 Cancer fatigue, also measured by the Borg RPE scale, appears to be a “central” mechanism that stems from a mismatch between efferent output for afferent inputs in the setting of ventilatory muscle weakness, thereby increasing the perception of dyspnea. Several interventions have been used to relieve dyspnea, ranging from oxygen therapy to treatment with opioids.
Oxygen saturation
The association between hypoxemia and dyspnea is poor.8 In a randomized prospective trial, Abernethy et al9 found no benefit to oxygen therapy compared with medical air without added supplemental oxygen in individuals who had normal oxygen saturation but symptomatic dyspnea.
Bilevel positive airway pressure
Bilevel positive airway pressure has been shown to reduce the need for invasive ventilation; improve oxygen saturation; and reduce dynamic hyperinflation, thus relieving dyspnea.10 It has been effective in dyspneic patients with motor neuron disease, cancer, heart failure, status asthmaticus, stroke, drug overdose, and interstitial lung disease.
Indwelling pleural catheters
Tunneled pleural catheters reduce the severity of dyspnea in 95% of patients.11 These catheters are inserted on an outpatient basis, allowing for outpatient drainage. Autopleurodesis occurs in about 45% of patients, in which case the catheter can be removed. Adverse reactions are few (incidence < 10%), but consist of empyema, pneumothorax, cellulitis, or catheter obstruction. The disadvantage is the expense of catheter maintenance.
Nebulized furosemide
Case reports suggest that inhalation of nebulized furosemide, 20 mg four times daily, dramatically improves dyspnea in patients with advanced cancer and severe shortness of breath that is unresponsive to opioids.12 Nebulized furosemide appears to have a direct effect on either pulmonary stretch receptors or irritant receptors in the airways; it also has a diuretic effect. Response occurs quickly with an onset of effect in 20 to 30 minutes.
B-type natriuretic peptide
The level of N-terminal precursor of B-type natriuretic peptide (NT-pro-BNP) can predict response to sunitinib in renal cancer,13 and the BNP level predicts 30-day mortality in pulmonary embolism.14 Measurement of BNP to detect dyspnea in patients with lung cancer is not useful, however, because the BNP level increases with cardiac and pericardial metastases. The BNP level is also persistently elevated after chest radiation therapy, and it increases with anthracycline cardiotoxicity. It is not a useful marker for distinguishing pulmonary from nonpulmonary or cardiac from noncardiac causes of dyspnea.
Lung ultrasound
Portable diagnostic lung ultrasound can be used to detect pneumonia, pleural effusions, pulmonary emboli, pneumothorax, atelectasis, and lung abscesses as potential causes of dyspnea.15–18 In addition to the advantage of portability, there is no radiation exposure and the technology permits echocardiography to be conducted.
Opioids
Evidence supports opioids for pharmacologic relief of dyspnea in the palliative care of patients with chronic obstructive pulmonary disease and cancer. Studies have been conducted with morphine sulfate, hydromorphone, dihydrocodeine, intranasal and transmucosal fentanyl, oxycodone, and diamorphine.19–21
The response to opioids is unrelated to the severity of dyspnea.22 Responses and safe administration occur even in patients with reduced oxygen saturation or elevated carbon dioxide partial pressure.20 Opioids can be used safely in the opioid-naïve population.20 Recommended dosages in these patients are 2.5 to 5.0 mg of morphine sulfate every 4 hours, 5 mg of oxycodone every 4 hours as needed, and 1 mg of hydromorphone every 4 hours in the opioid-naïve. In opioid-tolerant patients, it is recommended that therapy start with these doses and then be increased in 25% increments every 24 hours, as needed.
BUPRENORPHINE: UNIQUE OPIOID
Buprenorphine is a mu- and nociceptin (ORL-1)-receptor partial agonist with intravenous, subcutaneous, sublingual, transdermal, and intranasal routes of delivery.23 An agent that acts as an ORL-1 agonist can induce analgesia by blocking nociceptive responses at the level of the spinal cord. It is a kappa antagonist (depending upon the kappa ligand used in the assay), which may contribute to its antihyperalgesia. The parent drug has a high affinity and low intrinsic efficacy for the mu receptor. The main metabolite, norbuprenorphine, is a delta opioid-receptor agonist.
Secondary hyperalgesia is an increased sensitivity to painful stimuli around an area of injury and occurs frequently following injury. The increased pain sensation is a result of central sensitization derived from brainstem neurons that facilitate pain; it is not derived from afferent signals from the primary site. Secondary hyperalgesia is less responsive to opioids than primary hyperalgesia at the site of injury.
Pain is improved with buprenorphine predominantly through modulation of central sensitization and less so at the primary site. Koppert et al25 demonstrated in human volunteers that buprenorphine reduced the area and duration of secondary hyperalgesia more than pain at the site of injury (half-life of 171 minutes vs 288 minutes, respectively). Buprenorphine had a much greater antihyperalgesic effect than analgesic effect compared with potent opioids such as fentanyl. In contrast, the analgesic effects with fentanyl and alfentanil were much greater than their antihyperalgesic effects (Figure), suggesting the possibility of a combination of opioid therapy for superior pain relief or choices based on pain phenotype (eg, secondary or primary hyperalgesia).
SUMMARY
Early palliative care improves quality of life and decision-making in patients with advanced lung cancer and may improve survival, although survival data need to be confirmed. Olanzapine and megestrol acetate are superior to megestrol acetate alone for the treatment of anorexia. Oxygen is no better than medical air in the management of dyspnea associated with normal oxygen saturation. Buprenorphine is a unique opioid that has value for pharmacologic relief in patients at risk for respiratory depression.
Several important developments in the palliative care of patients with lung cancer have occurred over the past few years, including publication of a landmark study comparing early with as-needed palliative care, the release of new data on the treatment of cancer-related anorexia, elucidation of new mechanisms and treatment options for dyspnea, and the availability of buprenorphine. This article reviews these emerging concepts.
LUNG CANCER SYMPTOMS: COMMON AND SEVERE
The symptom burden of lung cancer is usually great. At least 80% of patients experience fatigue, 65% suffer loss of appetite, 77% have cough, 73% report dyspnea (both from local symptoms and weight loss), 57% have chest pain, and 17% have hemoptysis.1
When symptoms are present, they are usually severe. Thirty-eight percent of the patients who report fatigue have severe fatigue, 47% have inadequate appetite to the point of requiring intervention, and more than one-half of patients who have chest pain require opioids for relief.1
Symptom frequency and severity are worse in individuals who survive 3 months or less.1 Increasing symptom burden is therefore prognostically important, particularly in patients with advanced stages of lung cancer. As a result, self-assessment of quality of life has a significant ability to predict survival in patients with advanced non–small cell lung cancer (NSCLC).2
Patients with lung cancer tend to suffer from groups of symptoms or symptom clusters. Lutz et al1 found that 79% of patients reported three or more symptoms; these results were similar to the findings of a study by Hollen et al,3 in which 81% of patients suffered from three or more symptoms, all them severe except for cough.
EARLY PALLIATIVE CARE HAS CLINICAL BENEFITS
A landmark study by Temel et al4 examined the benefits of early palliative care integrated with standard oncologic care versus standard oncologic care and palliative care only “as needed” on patient-reported outcomes, the use of health services, and the quality of end-of-life care among patients with metastatic NSCLC. The study was a prospective, nonblinded, randomized, controlled trial of outpatients conducted at a single center. The intervention was based on guidelines from the National Consensus Project for Quality Palliative Care, with specific attention to symptom management, goals of care, decision-making regarding treatment, and coordination of care. Patients assigned to the intervention met monthly with both a palliative care service and an oncologist, and 90% of the patients randomized to intervention complied with at least 50% of the visits.
Measures of health-related quality of life and mood were obtained using the Functional Assessment of Cancer Therapy-Lung (FACT-L), the Hospital Anxiety and Depression Scale, and the 9-item depression scale of the Patient Health Questionnaire.
Measures of health care service utilization included use of antitumor therapy within 14 days of death, late or no referral to hospice, hospital admissions, and emergency room visits. Patients were considered to have received aggressive care if they met any one of the following three criteria: chemotherapy within 14 days of death, no hospice care, or admission to hospice within 3 days of death.
Compared with standard care, early palliative care was associated with an increase in the number of advance directives, earlier hospice referral (11 days vs 4 days), fewer hospitalizations and emergency room visits, and fewer instances of inappropriate oncologic care (defined as chemotherapy within 14 days of death). The percentage of patients with depressed mood was also lower among those assigned to early palliative care versus standard care (16% vs 38%).
A 2.7-month difference in median survival (P = .02) in favor of the group assigned to early palliative care was also observed, although survival was not a primary end point of the trial. This outcome needs to be validated in future studies.
CANCER-RELATED ANOREXIA AND CACHEXIA: TREATMENT IMPROVES APPETITE
The main hallmark of cancer-related anorexia and cachexia is weight loss; this symptom cluster is most often associated with hypophagia. The coexistence of anorexia and appetite-related anhedonia is common in lung cancer patients, such that 25% of lung cancer patients with anorexia report no distress with not eating, nor do they derive pleasure from eating. Others report that early satiety and changes in taste dramatically affect appetite. To some, anorexia is a distressful reminder of progression of their cancer.
Megestrol acetate and medroxyprogesterone acetate at least partially improve appetite in a subset of anorectic cancer patients. The use of medroxyprogesterone acetate has resulted in weight gain but not muscle mass in some patients with cancer-related anorexia, but has had less effect on fatigue and quality of life in these patients.
Olanzapine is an atypical antipsychotic with an affinity for multiple neurotransmitter receptors. Several of these, such as the serotonin receptors 5-HT2 and 5-HT3, histamine receptors, and dopamine receptors, are implicated in anorexia, nausea, and vomiting. Case reports suggest that olanzapine has antiemetic activity in patients with advanced cancer and usefulness as prophylaxis against chemotherapy-related nausea and vomiting.5 Reduced risk of extrapyramidal symptoms compared with standard antiemetics enhances the value of olanzapine for prevention of cancer-related anorexia.
Navari et al6 conducted a randomized trial to determine the effectiveness of megestrol acetate and olanzapine for the treatment of cancer-related anorexia. Eighty patients were randomized to receive oral megestrol acetate 800 mg/d, or oral megestrol acetate 800 mg/d plus olanzapine 5 mg once nightly, for 8 weeks. Patients were removed from the study if they did not take the study medication for a 48-hour period or if intolerable toxicity developed that was attributable to the study agents.
The MD Anderson Symptom Inventory (MDASI) was completed weekly to assess key symptom outcome variables. A change of 3 cm on the visual analog scale over two separate time periods for a symptom was considered sufficient to define a change in the symptom.
Quality of life was measured using a valid 28-item self-reported instrument (Functional Assessment of Cancer Therapy-General). Patients were examined by their physicians every 2 weeks.
In the group assigned to megestrol acetate, 15 patients had a weight gain of at least 5%—a change that was considered significant. Appetite improved in two patients, nausea decreased in three patients, and quality of life improved in five patients at both 4 weeks and 8 weeks. The improvements in appetite, nausea, and quality of life for the whole group on megestrol acetate alone were not significant, and there was no improvement in mean symptom scores measured by the MDASI.
There were incremental improvements of all measures in patients randomized to megestrol acetate plus olanzapine. Among patients receiving the combination, 33 had a weight gain of at least 5%; 25 reported an improvement in appetite, 21 experienced a reduction in nausea, and 23 had an improvement in quality of life at both 4 weeks and 8 weeks. All outcome variables were improved on the MDASI
CANCER AND DYSPNEA: NUMEROUS INTERVENTIONS HAVE BEEN ASSESSED
Reduced inspiratory capacity caused by weakened inspiratory muscles results in an increased Borg rating of perceived exertion (RPE) relative to oxygen levels. Both central nervous system activation of muscle and loss of muscle tissue contribute to dyspnea and fatigue in lung cancer patients.7 Cancer fatigue, also measured by the Borg RPE scale, appears to be a “central” mechanism that stems from a mismatch between efferent output for afferent inputs in the setting of ventilatory muscle weakness, thereby increasing the perception of dyspnea. Several interventions have been used to relieve dyspnea, ranging from oxygen therapy to treatment with opioids.
Oxygen saturation
The association between hypoxemia and dyspnea is poor.8 In a randomized prospective trial, Abernethy et al9 found no benefit to oxygen therapy compared with medical air without added supplemental oxygen in individuals who had normal oxygen saturation but symptomatic dyspnea.
Bilevel positive airway pressure
Bilevel positive airway pressure has been shown to reduce the need for invasive ventilation; improve oxygen saturation; and reduce dynamic hyperinflation, thus relieving dyspnea.10 It has been effective in dyspneic patients with motor neuron disease, cancer, heart failure, status asthmaticus, stroke, drug overdose, and interstitial lung disease.
Indwelling pleural catheters
Tunneled pleural catheters reduce the severity of dyspnea in 95% of patients.11 These catheters are inserted on an outpatient basis, allowing for outpatient drainage. Autopleurodesis occurs in about 45% of patients, in which case the catheter can be removed. Adverse reactions are few (incidence < 10%), but consist of empyema, pneumothorax, cellulitis, or catheter obstruction. The disadvantage is the expense of catheter maintenance.
Nebulized furosemide
Case reports suggest that inhalation of nebulized furosemide, 20 mg four times daily, dramatically improves dyspnea in patients with advanced cancer and severe shortness of breath that is unresponsive to opioids.12 Nebulized furosemide appears to have a direct effect on either pulmonary stretch receptors or irritant receptors in the airways; it also has a diuretic effect. Response occurs quickly with an onset of effect in 20 to 30 minutes.
B-type natriuretic peptide
The level of N-terminal precursor of B-type natriuretic peptide (NT-pro-BNP) can predict response to sunitinib in renal cancer,13 and the BNP level predicts 30-day mortality in pulmonary embolism.14 Measurement of BNP to detect dyspnea in patients with lung cancer is not useful, however, because the BNP level increases with cardiac and pericardial metastases. The BNP level is also persistently elevated after chest radiation therapy, and it increases with anthracycline cardiotoxicity. It is not a useful marker for distinguishing pulmonary from nonpulmonary or cardiac from noncardiac causes of dyspnea.
Lung ultrasound
Portable diagnostic lung ultrasound can be used to detect pneumonia, pleural effusions, pulmonary emboli, pneumothorax, atelectasis, and lung abscesses as potential causes of dyspnea.15–18 In addition to the advantage of portability, there is no radiation exposure and the technology permits echocardiography to be conducted.
Opioids
Evidence supports opioids for pharmacologic relief of dyspnea in the palliative care of patients with chronic obstructive pulmonary disease and cancer. Studies have been conducted with morphine sulfate, hydromorphone, dihydrocodeine, intranasal and transmucosal fentanyl, oxycodone, and diamorphine.19–21
The response to opioids is unrelated to the severity of dyspnea.22 Responses and safe administration occur even in patients with reduced oxygen saturation or elevated carbon dioxide partial pressure.20 Opioids can be used safely in the opioid-naïve population.20 Recommended dosages in these patients are 2.5 to 5.0 mg of morphine sulfate every 4 hours, 5 mg of oxycodone every 4 hours as needed, and 1 mg of hydromorphone every 4 hours in the opioid-naïve. In opioid-tolerant patients, it is recommended that therapy start with these doses and then be increased in 25% increments every 24 hours, as needed.
BUPRENORPHINE: UNIQUE OPIOID
Buprenorphine is a mu- and nociceptin (ORL-1)-receptor partial agonist with intravenous, subcutaneous, sublingual, transdermal, and intranasal routes of delivery.23 An agent that acts as an ORL-1 agonist can induce analgesia by blocking nociceptive responses at the level of the spinal cord. It is a kappa antagonist (depending upon the kappa ligand used in the assay), which may contribute to its antihyperalgesia. The parent drug has a high affinity and low intrinsic efficacy for the mu receptor. The main metabolite, norbuprenorphine, is a delta opioid-receptor agonist.
Secondary hyperalgesia is an increased sensitivity to painful stimuli around an area of injury and occurs frequently following injury. The increased pain sensation is a result of central sensitization derived from brainstem neurons that facilitate pain; it is not derived from afferent signals from the primary site. Secondary hyperalgesia is less responsive to opioids than primary hyperalgesia at the site of injury.
Pain is improved with buprenorphine predominantly through modulation of central sensitization and less so at the primary site. Koppert et al25 demonstrated in human volunteers that buprenorphine reduced the area and duration of secondary hyperalgesia more than pain at the site of injury (half-life of 171 minutes vs 288 minutes, respectively). Buprenorphine had a much greater antihyperalgesic effect than analgesic effect compared with potent opioids such as fentanyl. In contrast, the analgesic effects with fentanyl and alfentanil were much greater than their antihyperalgesic effects (Figure), suggesting the possibility of a combination of opioid therapy for superior pain relief or choices based on pain phenotype (eg, secondary or primary hyperalgesia).
SUMMARY
Early palliative care improves quality of life and decision-making in patients with advanced lung cancer and may improve survival, although survival data need to be confirmed. Olanzapine and megestrol acetate are superior to megestrol acetate alone for the treatment of anorexia. Oxygen is no better than medical air in the management of dyspnea associated with normal oxygen saturation. Buprenorphine is a unique opioid that has value for pharmacologic relief in patients at risk for respiratory depression.
- Lutz S, Norrell R, Bertucio C, et al. Symptom frequency and severity in patients with metastatic or locally recurrent lung cancer: a prospective study using the Lung Cancer Symptom Scale in a community hospital. J Palliat Med 2001; 4:157–165.
- Perrone F, Gridelli C, Cigolari S, et al. Baseline assessment of quality of life (QOL) is a strong prognostic factor for survival of elderly patients with advanced non-small cell lung cancer (NSCLC): a secondary analysis of the MILES study [ASCO abstract 1346]. Proc Am Soc Clin Oncol 2002;21.
- Hollen PJ, Gralla RJ, Kris MG, et al. Measurement of quality of life in patients with lung cancer in multicenter trials of new therapies: psychometric assessment of the Lung Cancer Symptom Scale. Cancer 1994; 73:2087–2098.
- Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non–small-cell lung cancer. N Engl J Med 2010; 363:733–742.
- Navari RM, Einhorn LH, Loehrer PJ, et al. A phase II trial of olanzapine, dexamethasone, and palonosetron for the prevention of chemotherapy-induced nausea and vomiting: a Hoosier oncology group study [published online ahead of print March 21, 2007]. Support Care Cancer 2007; 15:1285–1291. doi: 10.1007/s00520-007-0248-5
- Navari RM, Brenner MC. Treatment of cancer-related anorexia with olanzapine and megestrol acetate: a randomized trial. Support Care Cancer 2010; 18:951–956.
- Travers J, Dudgeon DJ, Amjadi K, et al. Mechanisms of exertional dyspnea in patients with cancer. J Appl Physiol 2008; 104:57–66.
- Galbraith S, Fagan P, Perkins P, Lynch A, Booth S. Does the use of a handheld fan improve chronic dyspnea? A randomized, controlled, crossover trial. J Pain Symptom Manage 2010; 39:831–838.
- Abernethy AP, McDonald CF, Frith PA, et al. Effect of palliative oxygen versus room air in relief of breathlessness in patients with refractory dyspnoea: a double-blind, randomised controlled trial. Lancet 2010; 376:784–793.
- Sellner-Pogány T, Lahrmann H. BIPAP-mask-ventilation in terminal amyotrophic lateral sclerosis (ALS) [in German]. Wien Med Wochenschr 2009; 159:604–607.
- Van Meter ME, McKee KY, Kohlwes RJ. Efficacy and safety of tunneled pleural catheters in adults with malignant pleural effusions: a systematic review [published online ahead of print August 10, 2010]. J Gen Intern Med 2011; 26:70–76. doi: 10.1007/s11606-010-1472-0
- Shimoyama N, Shimoyama M. Nebulized furosemide as a novel treatment for dyspnea in terminal cancer patients. J Pain Symptom Manage 2002; 23:73–76.
- Papazisis KT, Kontovinis LF, Papandreou CN, et al. Brain natriuretic peptide precursor (NT-pro-BNP) levels predict for clinical benefit to sunitinib treatment in patients with metastatic renal cell carcinoma. BMC Cancer 2010; 10:489.
- Cavallazzi R, Nair A, Vasu T, Marik PE. Natriuretic peptides in acute pulmonary embolism: a systematic review [published online ahead of print July 15, 2008]. Intensive Care Med 2008; 34:2147–2156. doi: 10.1007/s00134-008-1214-5
- Chen H-J, Yu Y-H, Tu C-Y, Chen C-H, Hsia T-C, Tsai K-D. Ultrasound in peripheral pulmonary air-fluid lesions: color Doppler imaging as an aid in differentiating empyema and abscess. Chest 2009; 135:1426–1432.
- Lichtenstein DA, Mezière GA, Lagoueyte J-F, Biderman P, Goldstein I, Gepner A. A-lines and B-lines: lung ultrasound as a bedside tool for predicting pulmonary artery occlusion pressure in the critically ill. Chest 2009; 136:1014–1020.
- Lichtenstein DA. Ultrasound examination of the lungs in the intensive care unit. Pediatr Crit Care Med 2009; 10:693–698.
- Reissig A, Heyne J-P, Kroegel C. Sonography of lung and pleura in pulmonary embolism: sonomorphologic characterization and comparison with spiral CT scanning. Chest 2001; 120:1977–1983.
- Kallet RH. The role of inhaled opioids and furosemide for the treatment of dyspnea. Respir Care 2007; 52:900–910.
- Clemens KE, Quednau I, Klaschik E. Is there a higher risk of respiratory depression in opioid-naïve palliative care patients during symptomatic therapy of dyspnea with strong opioids? J Palliat Med 2008; 11:204–216.
- Varkey B. Opioids for palliation of refractory dyspnea in chronic obstructive pulmonary disease patients. Curr Opin Pulm Med 2010; 16:150–154.
- Currow DC, Plummer J, Frith P, Abernethy AP. Can we predict which patients with refractory dyspnea will respond to opioids? J Palliat Med 2007; 10:1031–1036.
- Davis MP. Buprenorphine in cancer pain. Support Care Cancer 2005; 13:878–887.
- Watson PJQ, McQuay HJ, Bullingham RES, Allen MC, Moore RA. Single-dose comparison of buprenorphine 0.3 and 0.6 mg i.v. given after operation: clinical effects and plasma concentrations. Br J Anaesth 1982; 54:37–43.
- Koppert W, Ihmsen H, Körber N, et al. Different profiles of buprenorphine-induced analgesia and antihyperalgesia in a human pain model. Pain 2005; 118:15–22.
- Sittl R, Likar R, Nautrup BP. Equipotent doses of transdermal fentanyl and transdermal buprenorphine in patients with cancer and noncancer pain: results of a retrospective cohort study. Clin Ther 2005; 27:225–237.
- Sittl R. Transdermal buprenorphine in cancer pain and palliative care. Palliat Med 2006; 20 (suppl 1):s25–s30.
- Lutz S, Norrell R, Bertucio C, et al. Symptom frequency and severity in patients with metastatic or locally recurrent lung cancer: a prospective study using the Lung Cancer Symptom Scale in a community hospital. J Palliat Med 2001; 4:157–165.
- Perrone F, Gridelli C, Cigolari S, et al. Baseline assessment of quality of life (QOL) is a strong prognostic factor for survival of elderly patients with advanced non-small cell lung cancer (NSCLC): a secondary analysis of the MILES study [ASCO abstract 1346]. Proc Am Soc Clin Oncol 2002;21.
- Hollen PJ, Gralla RJ, Kris MG, et al. Measurement of quality of life in patients with lung cancer in multicenter trials of new therapies: psychometric assessment of the Lung Cancer Symptom Scale. Cancer 1994; 73:2087–2098.
- Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non–small-cell lung cancer. N Engl J Med 2010; 363:733–742.
- Navari RM, Einhorn LH, Loehrer PJ, et al. A phase II trial of olanzapine, dexamethasone, and palonosetron for the prevention of chemotherapy-induced nausea and vomiting: a Hoosier oncology group study [published online ahead of print March 21, 2007]. Support Care Cancer 2007; 15:1285–1291. doi: 10.1007/s00520-007-0248-5
- Navari RM, Brenner MC. Treatment of cancer-related anorexia with olanzapine and megestrol acetate: a randomized trial. Support Care Cancer 2010; 18:951–956.
- Travers J, Dudgeon DJ, Amjadi K, et al. Mechanisms of exertional dyspnea in patients with cancer. J Appl Physiol 2008; 104:57–66.
- Galbraith S, Fagan P, Perkins P, Lynch A, Booth S. Does the use of a handheld fan improve chronic dyspnea? A randomized, controlled, crossover trial. J Pain Symptom Manage 2010; 39:831–838.
- Abernethy AP, McDonald CF, Frith PA, et al. Effect of palliative oxygen versus room air in relief of breathlessness in patients with refractory dyspnoea: a double-blind, randomised controlled trial. Lancet 2010; 376:784–793.
- Sellner-Pogány T, Lahrmann H. BIPAP-mask-ventilation in terminal amyotrophic lateral sclerosis (ALS) [in German]. Wien Med Wochenschr 2009; 159:604–607.
- Van Meter ME, McKee KY, Kohlwes RJ. Efficacy and safety of tunneled pleural catheters in adults with malignant pleural effusions: a systematic review [published online ahead of print August 10, 2010]. J Gen Intern Med 2011; 26:70–76. doi: 10.1007/s11606-010-1472-0
- Shimoyama N, Shimoyama M. Nebulized furosemide as a novel treatment for dyspnea in terminal cancer patients. J Pain Symptom Manage 2002; 23:73–76.
- Papazisis KT, Kontovinis LF, Papandreou CN, et al. Brain natriuretic peptide precursor (NT-pro-BNP) levels predict for clinical benefit to sunitinib treatment in patients with metastatic renal cell carcinoma. BMC Cancer 2010; 10:489.
- Cavallazzi R, Nair A, Vasu T, Marik PE. Natriuretic peptides in acute pulmonary embolism: a systematic review [published online ahead of print July 15, 2008]. Intensive Care Med 2008; 34:2147–2156. doi: 10.1007/s00134-008-1214-5
- Chen H-J, Yu Y-H, Tu C-Y, Chen C-H, Hsia T-C, Tsai K-D. Ultrasound in peripheral pulmonary air-fluid lesions: color Doppler imaging as an aid in differentiating empyema and abscess. Chest 2009; 135:1426–1432.
- Lichtenstein DA, Mezière GA, Lagoueyte J-F, Biderman P, Goldstein I, Gepner A. A-lines and B-lines: lung ultrasound as a bedside tool for predicting pulmonary artery occlusion pressure in the critically ill. Chest 2009; 136:1014–1020.
- Lichtenstein DA. Ultrasound examination of the lungs in the intensive care unit. Pediatr Crit Care Med 2009; 10:693–698.
- Reissig A, Heyne J-P, Kroegel C. Sonography of lung and pleura in pulmonary embolism: sonomorphologic characterization and comparison with spiral CT scanning. Chest 2001; 120:1977–1983.
- Kallet RH. The role of inhaled opioids and furosemide for the treatment of dyspnea. Respir Care 2007; 52:900–910.
- Clemens KE, Quednau I, Klaschik E. Is there a higher risk of respiratory depression in opioid-naïve palliative care patients during symptomatic therapy of dyspnea with strong opioids? J Palliat Med 2008; 11:204–216.
- Varkey B. Opioids for palliation of refractory dyspnea in chronic obstructive pulmonary disease patients. Curr Opin Pulm Med 2010; 16:150–154.
- Currow DC, Plummer J, Frith P, Abernethy AP. Can we predict which patients with refractory dyspnea will respond to opioids? J Palliat Med 2007; 10:1031–1036.
- Davis MP. Buprenorphine in cancer pain. Support Care Cancer 2005; 13:878–887.
- Watson PJQ, McQuay HJ, Bullingham RES, Allen MC, Moore RA. Single-dose comparison of buprenorphine 0.3 and 0.6 mg i.v. given after operation: clinical effects and plasma concentrations. Br J Anaesth 1982; 54:37–43.
- Koppert W, Ihmsen H, Körber N, et al. Different profiles of buprenorphine-induced analgesia and antihyperalgesia in a human pain model. Pain 2005; 118:15–22.
- Sittl R, Likar R, Nautrup BP. Equipotent doses of transdermal fentanyl and transdermal buprenorphine in patients with cancer and noncancer pain: results of a retrospective cohort study. Clin Ther 2005; 27:225–237.
- Sittl R. Transdermal buprenorphine in cancer pain and palliative care. Palliat Med 2006; 20 (suppl 1):s25–s30.