User login
What is the difference between palliative care and hospice care?
Hospice care generally falls under the category of palliative care, despite being an older subspecialty. However, the two have different indications and goals and are often provided in different settings.
ORIGINS OF PALLIATIVE CARE
Prompted by what he perceived as neglect of dying patients in the acute care setting, Dr. Balfour Mount opened the first acute inpatient palliative care unit in Royal Victoria Hospital in Montréal, Québec, in 1976.1 His purpose was to provide a crisis-intervention service for patients who were actively dying, and this continues to be the main reason for consulting palliative care services in the hospital.
Palliative care has evolved since the 1970s and is now used in a variety of situations:
- A life-limiting illness in a patient who is not terminally ill
- A life-threatening illness in a patient who has symptoms but with the potential to recover
- A chronic illness such as heart failure or chronic obstructive pulmonary disease in a patient who is on disease-modifying therapy but has symptoms and will eventually succumb to the illness, but is expected to live longer than someone with advanced cancer.2
PALLIATIVE CARE IN CANCER PATIENTS
In patients with advanced cancer, palliative care is utilized earlier in the course of serious and life-limiting illness and is even involved in patient care when cure is the goal. Importantly, it now includes outpatient clinics to provide patients seamless care in conjunction with their oncologist’s care.3
Because palliative care focuses on the patient’s experience of the illness (sickness) rather than on disease itself (pathology), symptom management, psychosocial support, and assistance in decision-making are foremost. Initiating palliative care early in advanced cancer improves multiple outcomes and limits overly aggressive, ineffective therapies at the end of life (eg, late chemotherapy, late referral to hospice care, death in the intensive care unit), without hastening death. In fact, it may prolong life.3,4
Palliative care is indicated in a number of situations in oncology:
- Symptomatic presentations of cancer, even when curative treatments are available
- At the time of a sentinel event such as recurrence or unanticipated hospitalization
- When palliative radiation is needed
- When changes in chemotherapy are needed because of disease progression.
Also, cancer patients may develop symptoms that require a palliative procedure such as thoracentesis for pleural effusion, paracentesis for ascites, or surgery for a fracture or spinal cord compression. A palliative care consultation is also appropriate when patients change their goals of care (ie, palliation rather than cure), and when an oncologic crisis occurs and there is a need to offer support to the family and to clarify the goals of care.
PALLIATIVE CARE IN OTHER DISEASES
For patients with illnesses other than cancer, palliative care may be helpful when disease-modifying therapy becomes burdensome or ineffective, or when patients are symptomatic despite maximum therapy. Palliative care should also be considered when goals of care need to be explored, when a second opinion is needed on goals of care, or if the primary care provider and family are at odds.
WHEN A CONSULT IS INAPPROPRIATE
Palliative care consultation is inappropriate when used in lieu of an oncology consult in advanced cancer. Palliative care specialists are not experts in cancer care, whereas oncologists are familiar with rapid advancements in cancer care, including targeted agents that may offer benefit to patients with advanced cancer.
Palliative care consultation is also inappropriate if the patient does not want to see a palliative care specialist, or if the consult is used as a way to convince a patient to change advance directives or to choose not to be resuscitated. Also, cancer patients who are asymptomatic are unlikely to benefit from palliative care initially. The decision to consult palliative care should not depend on prognosis, and palliative care is more cost-effective when utilized early rather than as a crisis intervention near the end of life.3
THE PALLIATIVE CARE EVALUATION
The initial palliative care consultation usually involves an evaluation of the patient’s symptoms and concerns. Symptoms are targeted based on the patient’s priorities and on an assessment using validated questionnaires. A validated questionnaire is a better way to comprehensively gauge symptom burden than depending on patients to volunteer symptoms.5
As the relationship develops between patient, family, and palliative care specialist and as the disease takes its course, advance directives, prognosis, and end-of-life care goals can be addressed in follow-up consultations.3 Patients want to know about their prognosis, and they usually complete advance directives based on clinical circumstances rather than viewing them as an extension of patient autonomy, as originally intended.6
REIMBURSEMENT FOR PALLIATIVE CARE
Reimbursement for palliative care is similar to that for acute care and falls within the All Patient Refined Diagnosis-Related Group, or APR-DRG, system, and palliative care has its own V code for identification. Codes are used to designate disease, stage or location of metastases, disease complications, and symptoms, as well as for the discussion of goals of care.
WHAT PALLIATIVE CARE IS NOT
Palliative care has too often been tied to end-of-life care.7 The two often appear together in titles of reports in the literature. As a result, patients and physicians may be confused and, thus, reluctant to utilize palliative care services. To avoid the confusion, certain programs have included the term “supportive” oncology care in their title. This appears to facilitate palliative care referral, but may be misleading.8
WHAT IS HOSPICE CARE?
Hospice care is a service funded and capitated under Medicare part A and is largely provided as outpatient home care for those deemed terminally ill.9 An illness must be certified as terminal by two physicians. Medicare defines terminal illness as a life expectancy of 6 months or less if the illness runs its normal course.
The philosophy of hospice care is to provide comfort through intensive nurse management and home-based follow-up. In some cases, disease-modifying therapies are continued to control symptoms—eg, continuing angiotensin-converting enzyme inhibitors in heart failure patients. Hospice care is typically delivered at home, but it is also delivered in nursing homes, in hospital inpatient units, and at private or nonprofit hospice facilities.
Inpatient palliative care units are often mistaken for hospices. The purpose of hospice care is to provide quality of life and comfort and to avoid overly aggressive, expensive, and futile care at the end of life. The focus is on intensive, hands-on, personalized symptom care and family support at home. The goal is to provide a comfortable and dignified death among friends and family. The use of palliative radiation, transfusions, and antibiotics in hospice varies among hospice programs and is considered on a case-by-case basis.10
The Medicare per diem payment limits what hospices can afford, so they must be fiscally responsible. Hospice agencies are capitated and are responsible for providing medications and durable equipment necessary to treat symptoms related to the terminal illness. They also provide bereavement services for family members at no charge. Enrollment in hospice care can be revoked depending on circumstances and then reinstituted later as the goals of care change.
Care for nonterminal comorbid illnesses can be continued by a general practitioner or internist. This care is not covered under the Medicare hospice benefit, but it is covered under Medicare part B.
The patient and family can choose the hospice physician, who may be a family practitioner, internist, oncologist, or palliative care specialist, or may designate the hospice medical director as the hospice physician.
Criteria for hospice admission have been established for noncancer terminal illnesses and should be considered when practitioners decide to consult hospice.11–13
HOME-BASED PALLIATIVE CARE
Programs such as advanced illness management or home-based palliative care aim to improve the quality of care at home and prevent rehospitalization, particularly for patients with repeated hospitalizations.14 Home-based palliative care services are provided either by a clinician who makes home visits or by a certified home health care agency. Services are particularly useful for patients with serious illnesses who do not qualify for hospice services but are homebound. Consultations are obtained for ongoing supportive care at home, assessment for medication compliance, and disease monitoring at home. Consultations are scheduled at the time of hospital discharge.
Unlike hospice care, home-based palliative care does not include 24-hour on-call service. Comprehensive services (eg, home health aide, durable equipment, medications) are not provided as they are under hospice care: patients must qualify under Medicare stipulations for such services outside of hospice care. For example, home oxygen can only be supplied if the patient's oxygen saturation is less than 90%, while under the hospice benefit it is provided without regard to oxygen saturation and is based on symptom need. For home-based palliative care, patients must be largely homebound or unable to be seen regularly in the outpatient clinic. This type of care can be a bridge to hospice care for patients who feel they are not ready for hospice care at the time of discharge from acute care. Those who receive palliative care at home are less likely to be hospitalized at the end of life, are more likely to be transitioned to hospice at an appropriate time, and are more likely to have relief of symptoms.15
- Mount BM. The problem of caring for the dying in a general hospital; the palliative care unit as a possible solution. Can Med Assoc J 1976; 115:119–121.
- Higginson I. Palliative care: a review of past changes and future trends. J Public Health Med 1993; 15:3–8.
- Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med 2010; 363:733–742.
- Zimmermann C, Riechelmann R, Krzyzanowska M, Rodin G, Tannock I. Effectiveness of specialized palliative care: a systematic review. JAMA 2008; 299:1698–1709.
- Homsi J, Walsh D, Rivera N, et al. Symptom evaluation in palliative medicine: patient report vs systematic assessment. Support Care Cancer 2006; 14:444–453.
- Tang ST, Liu TW, Lai MS, Liu LN, Chen CH, Koong SL. Congruence of knowledge, experiences, and p for disclosure of diagnosis and prognosis between terminally-ill cancer patients and their family caregivers in Taiwan. Cancer Invest 2006; 24:360–366.
- Bakitas M, Lyons KD, Hegel MT, Ahles T. Oncologists’ perspectives on concurrent palliative care in a National Cancer Institute-designated comprehensive cancer center. Palliat Support Care 2013; 11:415–423.
- Fadul N, Elsayem A, Palmer JL, et al. Supportive versus palliative care: what’s in a name: a survey of medical oncologists and midlevel providers at a comprehensive cancer center. Cancer 2009; 115:2013–2021.
- Rinaldo MJ. Medicare to cover hospice services. J Med Soc NJ 1982; 79:1015–1016.
- Enck RE. Palliative radiation therapy in hospice care. Am J Hosp Palliat Care 2002; 19:151–152.
- Luchins DJ, Hanrahan P, Murphy K. Criteria for enrolling dementia patients in hospice. J Am Geriatr Soc 1997; 45:1054–1059.
- Fox E, Landrum-McNiff K, Zhong Z, Dawson NV, Wu AW, Lynn J. Evaluation of prognostic criteria for determining hospice eligibility in patients with advanced lung, heart, or liver disease. JAMA 1999; 282:1638–1645.
- Stuart B. The NHO medical guidelines for non-cancer disease and local medical review policy: hospice access for patients with diseases other than cancer. Hosp J 1999; 14:139–154.
- McKinney M. Beyond hospice. New models of care focus on advanced illnesses. Mod Healthc 2013; 43:14–15.
- Gomes B, Calanzani N, Curiale V, McCrone P, Higginson IJ. Effectiveness and cost-effectiveness of home palliative care services for adults with advanced illness and their caregivers. Cochrane Database Syst Rev 2013; 6:CD007760.
Hospice care generally falls under the category of palliative care, despite being an older subspecialty. However, the two have different indications and goals and are often provided in different settings.
ORIGINS OF PALLIATIVE CARE
Prompted by what he perceived as neglect of dying patients in the acute care setting, Dr. Balfour Mount opened the first acute inpatient palliative care unit in Royal Victoria Hospital in Montréal, Québec, in 1976.1 His purpose was to provide a crisis-intervention service for patients who were actively dying, and this continues to be the main reason for consulting palliative care services in the hospital.
Palliative care has evolved since the 1970s and is now used in a variety of situations:
- A life-limiting illness in a patient who is not terminally ill
- A life-threatening illness in a patient who has symptoms but with the potential to recover
- A chronic illness such as heart failure or chronic obstructive pulmonary disease in a patient who is on disease-modifying therapy but has symptoms and will eventually succumb to the illness, but is expected to live longer than someone with advanced cancer.2
PALLIATIVE CARE IN CANCER PATIENTS
In patients with advanced cancer, palliative care is utilized earlier in the course of serious and life-limiting illness and is even involved in patient care when cure is the goal. Importantly, it now includes outpatient clinics to provide patients seamless care in conjunction with their oncologist’s care.3
Because palliative care focuses on the patient’s experience of the illness (sickness) rather than on disease itself (pathology), symptom management, psychosocial support, and assistance in decision-making are foremost. Initiating palliative care early in advanced cancer improves multiple outcomes and limits overly aggressive, ineffective therapies at the end of life (eg, late chemotherapy, late referral to hospice care, death in the intensive care unit), without hastening death. In fact, it may prolong life.3,4
Palliative care is indicated in a number of situations in oncology:
- Symptomatic presentations of cancer, even when curative treatments are available
- At the time of a sentinel event such as recurrence or unanticipated hospitalization
- When palliative radiation is needed
- When changes in chemotherapy are needed because of disease progression.
Also, cancer patients may develop symptoms that require a palliative procedure such as thoracentesis for pleural effusion, paracentesis for ascites, or surgery for a fracture or spinal cord compression. A palliative care consultation is also appropriate when patients change their goals of care (ie, palliation rather than cure), and when an oncologic crisis occurs and there is a need to offer support to the family and to clarify the goals of care.
PALLIATIVE CARE IN OTHER DISEASES
For patients with illnesses other than cancer, palliative care may be helpful when disease-modifying therapy becomes burdensome or ineffective, or when patients are symptomatic despite maximum therapy. Palliative care should also be considered when goals of care need to be explored, when a second opinion is needed on goals of care, or if the primary care provider and family are at odds.
WHEN A CONSULT IS INAPPROPRIATE
Palliative care consultation is inappropriate when used in lieu of an oncology consult in advanced cancer. Palliative care specialists are not experts in cancer care, whereas oncologists are familiar with rapid advancements in cancer care, including targeted agents that may offer benefit to patients with advanced cancer.
Palliative care consultation is also inappropriate if the patient does not want to see a palliative care specialist, or if the consult is used as a way to convince a patient to change advance directives or to choose not to be resuscitated. Also, cancer patients who are asymptomatic are unlikely to benefit from palliative care initially. The decision to consult palliative care should not depend on prognosis, and palliative care is more cost-effective when utilized early rather than as a crisis intervention near the end of life.3
THE PALLIATIVE CARE EVALUATION
The initial palliative care consultation usually involves an evaluation of the patient’s symptoms and concerns. Symptoms are targeted based on the patient’s priorities and on an assessment using validated questionnaires. A validated questionnaire is a better way to comprehensively gauge symptom burden than depending on patients to volunteer symptoms.5
As the relationship develops between patient, family, and palliative care specialist and as the disease takes its course, advance directives, prognosis, and end-of-life care goals can be addressed in follow-up consultations.3 Patients want to know about their prognosis, and they usually complete advance directives based on clinical circumstances rather than viewing them as an extension of patient autonomy, as originally intended.6
REIMBURSEMENT FOR PALLIATIVE CARE
Reimbursement for palliative care is similar to that for acute care and falls within the All Patient Refined Diagnosis-Related Group, or APR-DRG, system, and palliative care has its own V code for identification. Codes are used to designate disease, stage or location of metastases, disease complications, and symptoms, as well as for the discussion of goals of care.
WHAT PALLIATIVE CARE IS NOT
Palliative care has too often been tied to end-of-life care.7 The two often appear together in titles of reports in the literature. As a result, patients and physicians may be confused and, thus, reluctant to utilize palliative care services. To avoid the confusion, certain programs have included the term “supportive” oncology care in their title. This appears to facilitate palliative care referral, but may be misleading.8
WHAT IS HOSPICE CARE?
Hospice care is a service funded and capitated under Medicare part A and is largely provided as outpatient home care for those deemed terminally ill.9 An illness must be certified as terminal by two physicians. Medicare defines terminal illness as a life expectancy of 6 months or less if the illness runs its normal course.
The philosophy of hospice care is to provide comfort through intensive nurse management and home-based follow-up. In some cases, disease-modifying therapies are continued to control symptoms—eg, continuing angiotensin-converting enzyme inhibitors in heart failure patients. Hospice care is typically delivered at home, but it is also delivered in nursing homes, in hospital inpatient units, and at private or nonprofit hospice facilities.
Inpatient palliative care units are often mistaken for hospices. The purpose of hospice care is to provide quality of life and comfort and to avoid overly aggressive, expensive, and futile care at the end of life. The focus is on intensive, hands-on, personalized symptom care and family support at home. The goal is to provide a comfortable and dignified death among friends and family. The use of palliative radiation, transfusions, and antibiotics in hospice varies among hospice programs and is considered on a case-by-case basis.10
The Medicare per diem payment limits what hospices can afford, so they must be fiscally responsible. Hospice agencies are capitated and are responsible for providing medications and durable equipment necessary to treat symptoms related to the terminal illness. They also provide bereavement services for family members at no charge. Enrollment in hospice care can be revoked depending on circumstances and then reinstituted later as the goals of care change.
Care for nonterminal comorbid illnesses can be continued by a general practitioner or internist. This care is not covered under the Medicare hospice benefit, but it is covered under Medicare part B.
The patient and family can choose the hospice physician, who may be a family practitioner, internist, oncologist, or palliative care specialist, or may designate the hospice medical director as the hospice physician.
Criteria for hospice admission have been established for noncancer terminal illnesses and should be considered when practitioners decide to consult hospice.11–13
HOME-BASED PALLIATIVE CARE
Programs such as advanced illness management or home-based palliative care aim to improve the quality of care at home and prevent rehospitalization, particularly for patients with repeated hospitalizations.14 Home-based palliative care services are provided either by a clinician who makes home visits or by a certified home health care agency. Services are particularly useful for patients with serious illnesses who do not qualify for hospice services but are homebound. Consultations are obtained for ongoing supportive care at home, assessment for medication compliance, and disease monitoring at home. Consultations are scheduled at the time of hospital discharge.
Unlike hospice care, home-based palliative care does not include 24-hour on-call service. Comprehensive services (eg, home health aide, durable equipment, medications) are not provided as they are under hospice care: patients must qualify under Medicare stipulations for such services outside of hospice care. For example, home oxygen can only be supplied if the patient's oxygen saturation is less than 90%, while under the hospice benefit it is provided without regard to oxygen saturation and is based on symptom need. For home-based palliative care, patients must be largely homebound or unable to be seen regularly in the outpatient clinic. This type of care can be a bridge to hospice care for patients who feel they are not ready for hospice care at the time of discharge from acute care. Those who receive palliative care at home are less likely to be hospitalized at the end of life, are more likely to be transitioned to hospice at an appropriate time, and are more likely to have relief of symptoms.15
Hospice care generally falls under the category of palliative care, despite being an older subspecialty. However, the two have different indications and goals and are often provided in different settings.
ORIGINS OF PALLIATIVE CARE
Prompted by what he perceived as neglect of dying patients in the acute care setting, Dr. Balfour Mount opened the first acute inpatient palliative care unit in Royal Victoria Hospital in Montréal, Québec, in 1976.1 His purpose was to provide a crisis-intervention service for patients who were actively dying, and this continues to be the main reason for consulting palliative care services in the hospital.
Palliative care has evolved since the 1970s and is now used in a variety of situations:
- A life-limiting illness in a patient who is not terminally ill
- A life-threatening illness in a patient who has symptoms but with the potential to recover
- A chronic illness such as heart failure or chronic obstructive pulmonary disease in a patient who is on disease-modifying therapy but has symptoms and will eventually succumb to the illness, but is expected to live longer than someone with advanced cancer.2
PALLIATIVE CARE IN CANCER PATIENTS
In patients with advanced cancer, palliative care is utilized earlier in the course of serious and life-limiting illness and is even involved in patient care when cure is the goal. Importantly, it now includes outpatient clinics to provide patients seamless care in conjunction with their oncologist’s care.3
Because palliative care focuses on the patient’s experience of the illness (sickness) rather than on disease itself (pathology), symptom management, psychosocial support, and assistance in decision-making are foremost. Initiating palliative care early in advanced cancer improves multiple outcomes and limits overly aggressive, ineffective therapies at the end of life (eg, late chemotherapy, late referral to hospice care, death in the intensive care unit), without hastening death. In fact, it may prolong life.3,4
Palliative care is indicated in a number of situations in oncology:
- Symptomatic presentations of cancer, even when curative treatments are available
- At the time of a sentinel event such as recurrence or unanticipated hospitalization
- When palliative radiation is needed
- When changes in chemotherapy are needed because of disease progression.
Also, cancer patients may develop symptoms that require a palliative procedure such as thoracentesis for pleural effusion, paracentesis for ascites, or surgery for a fracture or spinal cord compression. A palliative care consultation is also appropriate when patients change their goals of care (ie, palliation rather than cure), and when an oncologic crisis occurs and there is a need to offer support to the family and to clarify the goals of care.
PALLIATIVE CARE IN OTHER DISEASES
For patients with illnesses other than cancer, palliative care may be helpful when disease-modifying therapy becomes burdensome or ineffective, or when patients are symptomatic despite maximum therapy. Palliative care should also be considered when goals of care need to be explored, when a second opinion is needed on goals of care, or if the primary care provider and family are at odds.
WHEN A CONSULT IS INAPPROPRIATE
Palliative care consultation is inappropriate when used in lieu of an oncology consult in advanced cancer. Palliative care specialists are not experts in cancer care, whereas oncologists are familiar with rapid advancements in cancer care, including targeted agents that may offer benefit to patients with advanced cancer.
Palliative care consultation is also inappropriate if the patient does not want to see a palliative care specialist, or if the consult is used as a way to convince a patient to change advance directives or to choose not to be resuscitated. Also, cancer patients who are asymptomatic are unlikely to benefit from palliative care initially. The decision to consult palliative care should not depend on prognosis, and palliative care is more cost-effective when utilized early rather than as a crisis intervention near the end of life.3
THE PALLIATIVE CARE EVALUATION
The initial palliative care consultation usually involves an evaluation of the patient’s symptoms and concerns. Symptoms are targeted based on the patient’s priorities and on an assessment using validated questionnaires. A validated questionnaire is a better way to comprehensively gauge symptom burden than depending on patients to volunteer symptoms.5
As the relationship develops between patient, family, and palliative care specialist and as the disease takes its course, advance directives, prognosis, and end-of-life care goals can be addressed in follow-up consultations.3 Patients want to know about their prognosis, and they usually complete advance directives based on clinical circumstances rather than viewing them as an extension of patient autonomy, as originally intended.6
REIMBURSEMENT FOR PALLIATIVE CARE
Reimbursement for palliative care is similar to that for acute care and falls within the All Patient Refined Diagnosis-Related Group, or APR-DRG, system, and palliative care has its own V code for identification. Codes are used to designate disease, stage or location of metastases, disease complications, and symptoms, as well as for the discussion of goals of care.
WHAT PALLIATIVE CARE IS NOT
Palliative care has too often been tied to end-of-life care.7 The two often appear together in titles of reports in the literature. As a result, patients and physicians may be confused and, thus, reluctant to utilize palliative care services. To avoid the confusion, certain programs have included the term “supportive” oncology care in their title. This appears to facilitate palliative care referral, but may be misleading.8
WHAT IS HOSPICE CARE?
Hospice care is a service funded and capitated under Medicare part A and is largely provided as outpatient home care for those deemed terminally ill.9 An illness must be certified as terminal by two physicians. Medicare defines terminal illness as a life expectancy of 6 months or less if the illness runs its normal course.
The philosophy of hospice care is to provide comfort through intensive nurse management and home-based follow-up. In some cases, disease-modifying therapies are continued to control symptoms—eg, continuing angiotensin-converting enzyme inhibitors in heart failure patients. Hospice care is typically delivered at home, but it is also delivered in nursing homes, in hospital inpatient units, and at private or nonprofit hospice facilities.
Inpatient palliative care units are often mistaken for hospices. The purpose of hospice care is to provide quality of life and comfort and to avoid overly aggressive, expensive, and futile care at the end of life. The focus is on intensive, hands-on, personalized symptom care and family support at home. The goal is to provide a comfortable and dignified death among friends and family. The use of palliative radiation, transfusions, and antibiotics in hospice varies among hospice programs and is considered on a case-by-case basis.10
The Medicare per diem payment limits what hospices can afford, so they must be fiscally responsible. Hospice agencies are capitated and are responsible for providing medications and durable equipment necessary to treat symptoms related to the terminal illness. They also provide bereavement services for family members at no charge. Enrollment in hospice care can be revoked depending on circumstances and then reinstituted later as the goals of care change.
Care for nonterminal comorbid illnesses can be continued by a general practitioner or internist. This care is not covered under the Medicare hospice benefit, but it is covered under Medicare part B.
The patient and family can choose the hospice physician, who may be a family practitioner, internist, oncologist, or palliative care specialist, or may designate the hospice medical director as the hospice physician.
Criteria for hospice admission have been established for noncancer terminal illnesses and should be considered when practitioners decide to consult hospice.11–13
HOME-BASED PALLIATIVE CARE
Programs such as advanced illness management or home-based palliative care aim to improve the quality of care at home and prevent rehospitalization, particularly for patients with repeated hospitalizations.14 Home-based palliative care services are provided either by a clinician who makes home visits or by a certified home health care agency. Services are particularly useful for patients with serious illnesses who do not qualify for hospice services but are homebound. Consultations are obtained for ongoing supportive care at home, assessment for medication compliance, and disease monitoring at home. Consultations are scheduled at the time of hospital discharge.
Unlike hospice care, home-based palliative care does not include 24-hour on-call service. Comprehensive services (eg, home health aide, durable equipment, medications) are not provided as they are under hospice care: patients must qualify under Medicare stipulations for such services outside of hospice care. For example, home oxygen can only be supplied if the patient's oxygen saturation is less than 90%, while under the hospice benefit it is provided without regard to oxygen saturation and is based on symptom need. For home-based palliative care, patients must be largely homebound or unable to be seen regularly in the outpatient clinic. This type of care can be a bridge to hospice care for patients who feel they are not ready for hospice care at the time of discharge from acute care. Those who receive palliative care at home are less likely to be hospitalized at the end of life, are more likely to be transitioned to hospice at an appropriate time, and are more likely to have relief of symptoms.15
- Mount BM. The problem of caring for the dying in a general hospital; the palliative care unit as a possible solution. Can Med Assoc J 1976; 115:119–121.
- Higginson I. Palliative care: a review of past changes and future trends. J Public Health Med 1993; 15:3–8.
- Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med 2010; 363:733–742.
- Zimmermann C, Riechelmann R, Krzyzanowska M, Rodin G, Tannock I. Effectiveness of specialized palliative care: a systematic review. JAMA 2008; 299:1698–1709.
- Homsi J, Walsh D, Rivera N, et al. Symptom evaluation in palliative medicine: patient report vs systematic assessment. Support Care Cancer 2006; 14:444–453.
- Tang ST, Liu TW, Lai MS, Liu LN, Chen CH, Koong SL. Congruence of knowledge, experiences, and p for disclosure of diagnosis and prognosis between terminally-ill cancer patients and their family caregivers in Taiwan. Cancer Invest 2006; 24:360–366.
- Bakitas M, Lyons KD, Hegel MT, Ahles T. Oncologists’ perspectives on concurrent palliative care in a National Cancer Institute-designated comprehensive cancer center. Palliat Support Care 2013; 11:415–423.
- Fadul N, Elsayem A, Palmer JL, et al. Supportive versus palliative care: what’s in a name: a survey of medical oncologists and midlevel providers at a comprehensive cancer center. Cancer 2009; 115:2013–2021.
- Rinaldo MJ. Medicare to cover hospice services. J Med Soc NJ 1982; 79:1015–1016.
- Enck RE. Palliative radiation therapy in hospice care. Am J Hosp Palliat Care 2002; 19:151–152.
- Luchins DJ, Hanrahan P, Murphy K. Criteria for enrolling dementia patients in hospice. J Am Geriatr Soc 1997; 45:1054–1059.
- Fox E, Landrum-McNiff K, Zhong Z, Dawson NV, Wu AW, Lynn J. Evaluation of prognostic criteria for determining hospice eligibility in patients with advanced lung, heart, or liver disease. JAMA 1999; 282:1638–1645.
- Stuart B. The NHO medical guidelines for non-cancer disease and local medical review policy: hospice access for patients with diseases other than cancer. Hosp J 1999; 14:139–154.
- McKinney M. Beyond hospice. New models of care focus on advanced illnesses. Mod Healthc 2013; 43:14–15.
- Gomes B, Calanzani N, Curiale V, McCrone P, Higginson IJ. Effectiveness and cost-effectiveness of home palliative care services for adults with advanced illness and their caregivers. Cochrane Database Syst Rev 2013; 6:CD007760.
- Mount BM. The problem of caring for the dying in a general hospital; the palliative care unit as a possible solution. Can Med Assoc J 1976; 115:119–121.
- Higginson I. Palliative care: a review of past changes and future trends. J Public Health Med 1993; 15:3–8.
- Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med 2010; 363:733–742.
- Zimmermann C, Riechelmann R, Krzyzanowska M, Rodin G, Tannock I. Effectiveness of specialized palliative care: a systematic review. JAMA 2008; 299:1698–1709.
- Homsi J, Walsh D, Rivera N, et al. Symptom evaluation in palliative medicine: patient report vs systematic assessment. Support Care Cancer 2006; 14:444–453.
- Tang ST, Liu TW, Lai MS, Liu LN, Chen CH, Koong SL. Congruence of knowledge, experiences, and p for disclosure of diagnosis and prognosis between terminally-ill cancer patients and their family caregivers in Taiwan. Cancer Invest 2006; 24:360–366.
- Bakitas M, Lyons KD, Hegel MT, Ahles T. Oncologists’ perspectives on concurrent palliative care in a National Cancer Institute-designated comprehensive cancer center. Palliat Support Care 2013; 11:415–423.
- Fadul N, Elsayem A, Palmer JL, et al. Supportive versus palliative care: what’s in a name: a survey of medical oncologists and midlevel providers at a comprehensive cancer center. Cancer 2009; 115:2013–2021.
- Rinaldo MJ. Medicare to cover hospice services. J Med Soc NJ 1982; 79:1015–1016.
- Enck RE. Palliative radiation therapy in hospice care. Am J Hosp Palliat Care 2002; 19:151–152.
- Luchins DJ, Hanrahan P, Murphy K. Criteria for enrolling dementia patients in hospice. J Am Geriatr Soc 1997; 45:1054–1059.
- Fox E, Landrum-McNiff K, Zhong Z, Dawson NV, Wu AW, Lynn J. Evaluation of prognostic criteria for determining hospice eligibility in patients with advanced lung, heart, or liver disease. JAMA 1999; 282:1638–1645.
- Stuart B. The NHO medical guidelines for non-cancer disease and local medical review policy: hospice access for patients with diseases other than cancer. Hosp J 1999; 14:139–154.
- McKinney M. Beyond hospice. New models of care focus on advanced illnesses. Mod Healthc 2013; 43:14–15.
- Gomes B, Calanzani N, Curiale V, McCrone P, Higginson IJ. Effectiveness and cost-effectiveness of home palliative care services for adults with advanced illness and their caregivers. Cochrane Database Syst Rev 2013; 6:CD007760.
Comprehensive wound malodor management: Win the RACE
Wounds that fail to heal become more than mere skin lesions. Pain, malodor, and the accompanying psychological distress often complicate nonhealing wounds and impair quality of life.1 Management of malodor requires perseverance, sensitivity, and familiarity with tools and procedures that range from surgical debridement to medical-grade honey.
Chronic, nonhealing wounds are defined as persisting for more than 6 months.2 These lesions are incapable of undergoing anatomic and functional repair on their own. Commonly encountered nonhealing wounds include pressure ulcers, venous stasis ulcers, arterial insufficiency ulcers, and malignant cutaneous wounds.
Typically, the patient with a nonhealing wound is frail, debilitated, medically complex, and often faced with one or more life-limiting illnesses. Complete wound healing may therefore be unrealistic, and optimal wound management becomes the goal of care.3,4
Healthcare providers encounter nonhealing wounds in varied settings—acute inpatient, outpatient, long-term, and home care. For instance, in the home care setting, a study of 383 patients enrolled in hospice found that 35% had skin ulcers and wounds.3 Half of those affected had pressure ulcers, 20% had ischemic ulcers, and 30% had other skin disorders such as stasis ulcers, burns, skin tears, and tumors. A larger study, also in hospice patients, found that 26% had pressure ulcers and 10% more developed them within 6 months.5
While pressure ulcers are the most common nonhealing wounds, malignant or fungating wounds are found in 5% to 10% of patients with metastatic disease, usually with cancers of the breast, head, and neck.6
Maximizing wound care provides comfort, relieves suffering, and promotes quality of life.3,7 To achieve these goals, clinicians must be familiar with strategies to manage complications associated with nonhealing wounds such as pain, malodor, and psychosocial adverse effects. Of these complications, malodor has been pointed out by both patients and caregivers as the most distressing.8
This article focuses on wound malodor, discusses the processes that cause wounds to emit an offensive smell, and outlines a comprehensive management approach.
MRS. A., AGE 61, WITH STAGE IV BREAST CANCER
Mrs. A., 61 years old, had a fungating mass in her left breast, which began as a small nodule and progressively enlarged to deform her breast over several months. Her oncologist subsequently staged the extent of her cancer as stage IV after workup revealed lung metastasis. Mrs. A. and her family decided to forgo cancer treatment, including radiotherapy, and to transition to hospice care after discussions with the oncologist.
Mrs. A. lived at home with her husband. Her daughter and three grandchildren all lived nearby.
When her hospice physician arrived at her home to meet her, a strong, pungent, and nauseating smell greeted him as he entered her bedroom. The patient said that for the past few months she had been increasingly distressed by the revolting odor. She rarely left home and had been ashamed to have people visit her, including her family.
On examination, the physician noticed a large fungating mass with yellowish discharge and necrotic tissue in her left breast. In addition to mild pain, she was immensely bothered by the strong odor coming from her breast.
THE IMPACT OF MALODOR
As seen in the case of Mrs. A., malodor has grave effects, both physical and psychological. Patients experience impaired or socially unacceptable body image, social rejection, personal shame, and embarrassment.9,10 Feelings of fear, anxiety, and depression are common. If left uncontrolled, malodor results in social isolation, reluctance to engage in social activities, diminished appetite, and nausea. In addition, malodor is a constant reminder of patients’ pain and cancer, and it results in further suffering.11
Reactions of family members and caregivers can worsen the situation.9,12 Expressions of revulsion limit contact and inhibit intimacy, especially near the end of life. Caregivers are often frustrated and distressed over their inability to control the malodor. The environment becomes uninhabitable, and the malodor can permeate clothing, furniture, and living quarters.
Managing malodor can be emotionally draining, physically daunting, and frustrating for healthcare professionals, as several methods are usually employed, often in a trial-and-error approach, to achieve an acceptable degree of odor control. In addition, clinicians must face the challenge of treating malodorous wounds at very close distance without reacting in a way that offends or alarms patients and family members.13
MALODOR PRODUCTION: WHERE IS THAT SMELL COMING FROM?
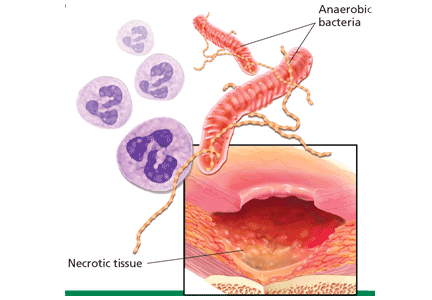
All wounds can produce an odor.14 Wounds that are expected to heal typically emit a faint but not unpleasant odor, akin to fresh blood. Wounds colonized by Pseudomonas aeruginosa produce a fruity or grapelike odor that is tolerable. Malodor occurs with wounds infected by other gram-negative organisms or anaerobic bacteria.15 Similarly, wounds covered by necrotic tissue smell like decaying flesh.
Three major causes
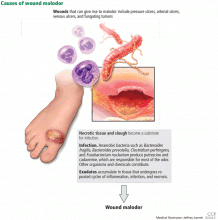
The three major causes of wound malodor are slough, infection, and exudate (Figure 1).
Slough is dead or necrotic tissue, usually resulting from vascular compromise. Arterial ulcers, pressure ulcers, and malignant wounds all form slough from capillary occlusion, subsequent ischemia, and tissue necrosis.
Infection. Devitalized tissue, an ideal medium in which bacteria thrive, becomes the source of infection. Anaerobic bacteria are usually implicated in malodor. These include Bacteroides fragilis, Bacteroides prevotella, Clostridium perfringens, and Fusobacterium nucleatum.16,17 Anaerobic organisms produce putrescine and cadaverine, which are largely responsible for the offensive odor.16,18 Volatile fatty acids such as propionic, butyric, isovaleric, and valeric acid are formed from lipid catabolism by anaerobes and add to malodor.17 Aerobic bacteria such as Proteus, Klebsiella, and Pseudomonas species supercolonize necrotic tissue as well and contribute to malodor.17,18
Exudate. Since nonhealing wounds undergo repeated cycles of inflammation, infection, and necrosis, accumulation of exudate becomes inevitable. Exudate typically is a pus-like fluid containing serum, fibrin, and white blood cells, which leak from blood vessels. In addition, bacteria that colonize chronic wounds filled with necrotic tissue activate proteases that degrade and liquefy dead tissue, thereby forming extensive amounts of exudate.19
Apart from slough, infection, and exudate, poor general hygiene and dressings left on for too long may contribute to malodor.16 Moisture-retentive dressings such as hydrocolloids leave an odor after removal. Dressings that liquefy upon contact with the wound surface leave a pus-like, potentially malodorous material.
MALODOR ASSESSMENT: DO YOU SMELL SOMETHING?
Various ways to document wound malodor can prove useful in guiding assessment and treatment. Descriptions such as “foul,” “putrid,” “fishy,” or “filled the room” vividly portray the initial presentation. A 10-point numerical scale similar to a numerical pain scale or a visual analogue scale can be used as a subjective measure.
Other grading methods, which to the authors’ knowledge are not validated, may be helpful. In a study that focused on patients suffering from malodorous gynecologic malignancies, von Gruenigen et al20 used a 0-to-3 scale:
- 0 Absent
- 1 Not offensive
- 2 Offensive but tolerable
- 3 Offensive and intolerable.
A scale often adapted by other authors was devised by Baker and Haig,21 which clearly defines four classes:
- 1 Strong—odor is evident upon entering the room (6 to 10 feet from the patient) with the dressing intact
- 2 Moderate—odor is evident upon entering the room with dressing removed
- 3 Slight—odor is evident at close proximity to the patient when the dressing is removed
- 4 No odor—no odor is evident, even at the patient’s bedside with the dressing removed.
COMPREHENSIVE MANAGEMENT: HOW DO WE WIN THE ‘RACE’?
The acronym RACE outlines an approach to dealing with malodor. It stands for removal of necrotic tissue; antibacterials; odor concealers; and education and support (Table 1).
Remove necrotic tissue
An important step in eliminating malodor is to remove necrotic tissue. This starts with debridement, which decreases the incidence of infection and hastens wound closure.22,23 Table 2 compares the different types of debridement.
Sharp or surgical debridement involves the use of a scalpel or scissors. This type of debridement may increase the risk of bleeding, pain, and malignant cell seeding in fungating wounds.4,24
Enzymatic debridement employs chemicals with proteolytic action (eg, collagenase) to digest extracellular proteins in wounds.18,25
Mechanical debridement involves aggressive therapies such as forceful irrigation and hydrotherapy, which may fail to discriminate between necrotic and viable tissues.18,26
Biological debridement using maggots, which ingest bacteria and devitalized tissue, may cause increased wound bleeding and may be unacceptable for patients and families.24,27
Autolytic debridement is often recommended, particularly if complete healing is not the primary goal.17,24,28,29 Autolysis uses proteolytic enzymes and phagocytic cells present in the wound bed and wound fluid to clear devitalized tissue. It is easy, inexpensive, noninvasive, and painless,4 and it requires less frequent dressing changes relative to standard dressing or wet-to-dry dressing.
Autolytic debridement is commonly accomplished using hydrocolloid and hydrogel dressings.15,29 Hydrocolloids are adhesive, occlusive, and conformable dressings that are suitable for wounds with low to moderate amounts of exudate. Upon contact with the wound surface, the dressing absorbs the exudate, forms a gel layer, and maintains a moist environment. Hydrocolloids are not recommended for infected wounds or for those with copious exudate as they may lead to maceration around the wound. A disadvantage of hydrocolloid dressings is their tendency to generate brown, often malodorous exudate when removed.
On the other hand, hydrogels in amorphous gel, dressing, sheet, or impregnated gauze form are water-based products that create a moist environment similar to hydrocolloids. Aside from causing minimal trauma to the wound bed when removed, the dressing’s cooling effect may bring some pain relief. Hydrogels are appropriate for dry wounds and for those with minimal exudate.
After debridement, the wound is cleansed and irrigated. A number of cleansers and solutions are available, but normal saline is a cheap alternative. To irrigate, experts recommend an 18- or 20-gauge intravenous catheter attached to a 30- or 60-mL syringe.15 This technique provides 8 to 15 psi of pressure, enough to cleanse the wound without causing tissue trauma.
Antibacterials and absorption
Antibacterials. Topical antibiotics have several advantages over systemic antibiotics in treating chronic wounds.30,31 These include a high and sustained concentration of the antimicrobial at the site of infection, limited potential for systemic absorption and toxicity, reduced potential for antibiotic resistance, and drawing of the patient’s and caregiver’s attention to the wound.
Metronidazole is the most widely used topical antibacterial for malodor management. Its efficacy is likely due to the predominant involvement of anaerobic bacteria in foul-smelling wounds. Topical metronidazole is available as a gel and as a cream. A systematic review showed that on average, topical metronidazole was used once daily for 14 consecutive days.19 The layer of topical metronidazole is typically covered with a nonadherent primary dressing followed by an absorbent secondary dressing.
The best clinical evidence for topical metronidazole consists of case reports and series.32–35 The largest of these studies was done by Finlay et al, who treated 47 patients with malodorous benign and malignant cutaneous wounds with 0.75% metronidazole gel daily.32 Forty-five (96%) of the patients reported significantly decreased odor by 14 days, as well as decreased pain, discharge, and surrounding cellulitis.
A randomized, placebo-controlled trial conducted by Bale et al had equivocal findings.9 All 41 patients who received metronidazole gel reported a decrease in malodor within 3 days of starting it. However, 76% of patients who received placebo also reported malodor control; in the final analysis, no significant difference was noted in the success rate between the two groups.
Metronidazole tablets can be crushed and sprinkled over the wound. As with metronidazole gel or cream, the crushed tablets are applied daily and covered by a primary nonadherent dressing and an absorbent secondary dressing. This off-label use of metronidazole serves as a cheaper alternative to commercially available topical preparations. To our knowledge, there has been no head-to-head trial comparing the two topical strategies.
Systemic metronidazole, often given orally, has been recommended if evidence of deep tissue or systemic infection is noted15 and in cases of fungating wounds with fistulas invading either the gastrointestinal or genitourinary tracts.18 Side effects such as nausea, neuropathy, and alcohol intolerance (ie, disulfiram reaction) may occur, which are not seen with topical metronidazole.
Both topical and systemic metronidazole can be used together on a time-limited basis for extensive malodorous wounds, such as fungating malignant wounds or stage IV sacral pressure ulcers.
Other antimicrobial agents used to treat malodor include silver-containing products, iodine-containing topical agents, mupirocin, bacitracin, neomycin, and polymyxin B.
Honey was used for wound care by the ancient Egyptians, and it is still used.36 Its beneficial effects include antimicrobial, debriding, deodorizing, anti-inflammatory, and granulation tissue-stimulating. Honey has even been shown to significantly decrease skin colonization with various kinds of bacteria, including methicillin-resistant Staphylococcus aureus.37 Medical-grade honey is preferred over table honey, as the latter is nonsterile and can contain Clostridium spores, which contaminate the wound.38
Yogurt and buttermilk lower the pH of the wound and control bacterial proliferation to control malodor.39,40 Either is applied for 10 to 15 minutes after the wound is cleansed and is then washed off thoroughly.
Absorbent dressings are used either over a layer of topical metronidazole and a nonadherent primary dressing or as a primary dressing itself. An absorbent dressing containing activated charcoal is used for rapid improvement, although cost may be prohibitive, especially in developing countries.13,19 Another type of absorbent dressing, composed of polyester impregnated with sodium chloride, has been found to be useful in malodor control.41 An important pointer is to maintain a tight seal around the absorbent dressing to prevent leakage of exudate.
Concealers
Aromatics used to conceal malodor include scented candles, incense, fragrant flowers and plants, and air-freshener sprays. When circumstances allow, candles are good options since they conceal malodor by emitting fragrance, and the flame burns off foul-smelling chemicals. Aromatics such as coffee beans, vanilla beans, and cider vinegar can be placed in a pan and left under the patient’s bed or close to it. Drops of peppermint oil or oil of wintergreen can be placed on wound dressings.
Other odor concealers are adsorbent materials that attract and cause ions and molecules to adhere to their surface. Examples are charcoal, baking soda, and cat litter. As with other aromatics, these materials are placed in pans and left under the bed or near the patient.
Aromatics can have disadvantages, as certain scents, especially strong ones, can be nauseating for patients. Some fragrances trigger asthma or skin irritation. Patients and caregivers can be left with an unpleasant association of certain fragrances with malodor by conditioning.15,17,18
Education and support
Concerns of the patient and family members need to be heard, addressed promptly, and reassessed with each visit, since uncontrolled malodor can be a chief source of caregiver fatigue.
Foremost in formulating a patient- and family-centered malodor management strategy is to commit to controlling malodor as much as possible. Regular follow-up appointments should be made, whether in the office or at home, to check on the patient’s progress and address new and ongoing concerns. Symptoms accompanying malodor, such as pain, bleeding, and sleep disturbance, need to be addressed, as they all affect quality of life.1 Audience-appropriate educational materials should be made available.26 Online resources that patients and families can explore include the websites of the Wound Ostomy and Continence Nurses Society (www.wocn.org) and the Association for the Advancement of Wound Care (aawconline.org).
Healthcare professionals need to be prepared to deal with problems and complications involving patients and family members that may arise in the course of treatment.12 Problems include the cost and local unavailability of dressing supplies, insurance coverage for dressings and topical agents, lack of assistance at home, and fear of changing dressings. A cardinal rule for healthcare providers is to avoid expressing distress at odors in front of or within hearing of patients and families.
OTHER STRATEGIES: WHAT ELSE CAN WE DO?
Curcumin, the main biologically active compound in the herb turmeric, applied directly to wounds three times daily as an ointment, has been shown to have odor-controlling properties.42
Sugar paste has been reported to control malodor by drawing out exudative and tissue fluid osmotically, and inhibiting bacterial growth.16,17 Water is mixed with sugar (ie, granulated, caster, or powdered) to form a paste, with additives like glycerin and polyethylene glycol used to alter the consistency. Thick clay-like paste is good for wounds with large cavities, while thin paste is useful for wounds with small or superficial openings. The paste is applied twice daily and is covered by an absorbent dressing.
Pressure relief is vital in managing pressure ulcers.18,43 Repositioning every 2 hours and using special devices, such as mattress overlays, alternating pressure mattresses, and low air loss mattresses, are frequently employed techniques.
If circumstances permit and when congruent with the patient’s goals of care, intra-arterial chemotherapy and radiotherapy can be contemplated for malignant fungating wounds.44,45
Other strategies include opening the windows during dressing changes, increasing the frequency of dressing changes, promptly removing used dressings from the house, and ensuring good general hygiene.
CASE RESOLUTION
After telling her that he was committed to control the malodor or, if possible, eliminate it, Mrs. A.’s doctor prepared two lists of materials—one for himself and one for Mrs. A.’s husband. He returned the next day, brought out his supplies, asked Mrs. A. to lie in bed, and invited her husband to assist him.
He cleansed and irrigated the breast lesion with normal saline, making sure to remove as much dead tissue as he could. He applied a layer of metronidazole cream to the wound cavity, then covered it with a nonadherent dressing. He then covered the wound with gauze, sealed the edges with medical adhesive tape, and applied a few drops of oil of wintergreen to the surface. A pan of charcoal briquettes was put under the bed, and a candle with Mrs. A.’s favorite scent was lit by the bedside. The physician then instructed Mrs. A.’s husband to repeat the procedure once daily for 1 week.
After 2 weeks, Mrs. A. and her husband said the foul odor had greatly decreased. She appeared more cheerful and energetic, especially after her grandchildren visited a few days earlier. The physician then instructed the husband to stop using metronidazole cream and to apply a hydrocolloid dressing every 3 days instead. He advised them to continue the rest of the process of applying a few drops of oil of wintergreen on the dressing surface, placing a pan of charcoal briquettes under the bed, and lighting a scented candle by the bedside.
FINISH THE RACE!
Complex nonhealing wounds are encountered across various healthcare settings. Wound malodor is an important component of nonhealing wounds, which adversely affects patients, families, and healthcare providers. Infection, slough, and exudate are the major causes of wound malodor. The essential steps to reduce malodor are to remove necrotic tissue, use antibacterial and odor-absorbing agents, apply appropriate odor “concealers,” educate families, and formulate a patient- and family-centered strategy (Table 1).
Acknowledgment: The authors would like to thank Sue Reif, CNP, for her assistance in completing the manuscript.
- Lo SF, Hayter M, Hu WY, Tai CY, Hsu MY, Li YF. Symptom burden and quality of life in patients with malignant fungating wounds. J Adv Nurs 2012; 68:1312–1321.
- Lazarus GS, Cooper DM, Knighton DR, et al. Definitions and guidelines for assessment of wounds and evaluation of healing. Arch Dermatol 1994; 130:489–493.
- Tippett AW. Wounds at the end of life. Wounds 2005; 17:91–98.
- Burt T. Palliative care of pressure ulcers in long-term care. Ann Long-Term Care 2013; 21:20–28.
- Reifsnyder J, Magee HS. Development of pressure ulcers in patients receiving home hospice care. Wounds 2005; 17:74–79.
- Haisfield-Wolfe ME, Rund C. Malignant cutaneous wounds: a management protocol. Ostomy Wound Manage 1997; 43:56–66.
- O’Brien C. Malignant wounds: managing odour. Can Fam Physician 2012; 58:272–274.
- Gethin G, Grocott P, Probst S, Clarke E. Current practice in the management of wound odour: an international survey. Int J Nurs Stud 2014; 51:865–874.
- Bale S, Tebble N, Price P. A topical metronidazole gel used to treat malodorous wounds. Br J Nurs 2004; 13:S4–S11.
- Hack A. Malodorous wounds—taking the patient’s perspective into account. J Wound Care 2003; 12:319–321.
- Price E. Wound care. The stigma of smell. Nurs Times 1996; 92:71–72.
- Paul JC, Pieper BA. Topical metronidazole for the treatment of wound odor: a review of the literature. Ostomy Wound Manage 2008; 54:18–27.
- Lee G, Anand SC, Rajendran S, Walker I. Overview of current practice and future trends in the evaluation of dressings for malodorous wounds. J Wound Care 2006; 15:344–346.
- Cutting K, Harding K. Criteria for identifying wound infection. J Wound Care 1994; 3:198–201.
- McDonald A, Lesage P. Palliative management of pressure ulcers and malignant wounds in patients with advanced illness. J Palliat Med 2006; 9:285–295.
- Holloway S. Recognising and treating the causes of chronic malodorous wounds. Prof Nurse 2004; 19:380–384.
- Haughton W, Young T. Common problems in wound care: malodorous wounds. Br J Nurs 1995; 4:959–963.
- Alvarez OM, Kalinski C, Nusbaum J, et al. Incorporating wound healing strategies to improve palliation (symptom management) in patients with chronic wounds. J Palliat Med 2007; 10:1161–1189.
- da Costa Santos CM, de Mattos Pimenta CA, Nobre MR. A systematic review of topical treatments to control the odor of malignant fungating wounds. J Pain Symptom Manage 2010; 39:1065–1076.
- Von Gruenigen VE, Coleman RL, et al. Bacteriology and treatment of malodorous lower reproductive tract in gynecologic cancer patients. Obstet Gynecol 2000; 96:23–27.
- Baker PG, Haig G. Metronidazole in the treatment of chronic pressure sores and ulcers: a comparison with standard treatment in general practice. Practitioner 1981; 225:569–573.
- Whitney J, Phillips L, Aslam R, et al. Guidelines for the treatment of pressure ulcers. Wound Repair Regen 2006; 14:663–679.
- Williams D, Enoch S, Miller D, Harris K, Price P, Harding KG. Effect of sharp debridement using curette on recalcitrant nonhealing venous ulcers: a concurrently controlled, prospective cohort study. Wound Repair Regen 2005; 13:131–137.
- Bergstrom KJ. Assessment and management of fungating wounds. J Wound Ostomy Continence Nurs 2011: 38:31–37.
- Sinclair RD, Ryan TJ. Proteolytic enzymes in wound healing: the role of enzymatic debridement. Australas J Dermatol 1994; 35:35–41.
- Enoch S, Harding KG. Wound bed preparation: the science behind the removal of barriers to healing. Wounds 2003;15:213–229.
- Mumcuoglu KY. Clinical applications for maggots in wound care. Am J Clin Dermatol 2001; 2:219–227.
- Langemo DK, Black J; National Pressure Ulcer Advisory Panel. Pressure ulcers in individuals receiving palliative care: a National Pressure Ulcer Advisory Panel white paper. Adv Skin Wound Care 2010; 23:59–72.
- Fonder MA, Lazarus GS, Cowan DA, Aronson-Cook B, Kohli AR, Mamelak AJ. Treating the chronic wound: a practical approach to the care of nonhealing wounds and wound care dressings. J Am Acad Dermatol 2008; 58:185–206.
- Lio PA, Kaye ET. Topical antibacterial agents. Infect Dis Clin North Am 2004; 18:717–733.
- Gelmetti C. Local antibiotics in dermatology. Dermatol Ther 2008; 21:187–195.
- Finlay IG, Bowszyc J, Ramlau C, Gwiezdzinski Z. The effect of topical 0.75% metronidazole gel on malodorous cutaneous ulcers. J Pain Symptom Manage 1996; 11:158–162.
- Bower M, Stein R, Evans TR, Hedley A, Pert P, Coombes RC. A double-blind study of the efficacy of metronidazole gel in the treatment of malodorous fungating tumours. Eur J Cancer 1992; 28A:888–889.
- Kalinski C, Schnepf M, Laboy D, et al. Effectiveness of a topical formulation containing metronidazole for wound odor and exudate control. Wounds 2005; 17:84–90.
- Kuge S, Tokuda Y, Ohta M, et al. Use of metronidazole gel to control malodor in advanced and recurrent breast cancer. Jpn J Clin Oncol 1996; 26:207–210.
- Belcher J. A review of medical-grade honey in wound care. Br J Nurs 2012: 21:S4–S9.
- Kwakman PH, Van den Akker JP, Güçlü A, et al. Medical-grade honey kills antibiotic-resistant bacteria in vitro and eradicates skin colonization. Clin Infect Dis 2008; 46:1677–1682.
- Cooper RA, Jenkins L. A comparison between medical grade honey and table honeys in relation to antimicrobial efficacy. Wounds 2009; 21:29–36.
- Patel B, Cox-Hayley D. Managing wound odor #218. J Palliat Med 2010; 13:1286–1287.
- Schulte MJ. Yogurt helps to control wound odor. Oncol Nurs Forum 1993; 20:1262.
- Upright CA, Salton C, Roberts F, Murphy J. Evaluation of Mesalt dressings and continuous wet saline dressings in ulcerating metastatic skin lesions. Cancer Nurs 1994; 17:149–155.
- Kuttan R, Sudheeran PC, Josph CD. Turmeric and curcumin as topical agents in cancer therapy. Tumori 1987; 73:29–31.
- Bass MJ, Phillips LG. Pressure sores. Curr Probl Surg 2007; 44:101–143.
- Bufill JA, Grace WR, Neff R. Intra-arterial chemotherapy for palliation of fungating breast cancer: a case report and review of the literature. Am J Clin Oncol 1994; 17:118–124.
- Murakami M, Kuroda Y, Sano A, et al. Validity of local treatment including intraarterial infusion chemotherapy and radiotherapy for fungating adenocarcinoma of the breast: case report of more than 8-year survival. Am J Clin Oncol 2001; 24:388–391.
Wounds that fail to heal become more than mere skin lesions. Pain, malodor, and the accompanying psychological distress often complicate nonhealing wounds and impair quality of life.1 Management of malodor requires perseverance, sensitivity, and familiarity with tools and procedures that range from surgical debridement to medical-grade honey.
Chronic, nonhealing wounds are defined as persisting for more than 6 months.2 These lesions are incapable of undergoing anatomic and functional repair on their own. Commonly encountered nonhealing wounds include pressure ulcers, venous stasis ulcers, arterial insufficiency ulcers, and malignant cutaneous wounds.
Typically, the patient with a nonhealing wound is frail, debilitated, medically complex, and often faced with one or more life-limiting illnesses. Complete wound healing may therefore be unrealistic, and optimal wound management becomes the goal of care.3,4
Healthcare providers encounter nonhealing wounds in varied settings—acute inpatient, outpatient, long-term, and home care. For instance, in the home care setting, a study of 383 patients enrolled in hospice found that 35% had skin ulcers and wounds.3 Half of those affected had pressure ulcers, 20% had ischemic ulcers, and 30% had other skin disorders such as stasis ulcers, burns, skin tears, and tumors. A larger study, also in hospice patients, found that 26% had pressure ulcers and 10% more developed them within 6 months.5
While pressure ulcers are the most common nonhealing wounds, malignant or fungating wounds are found in 5% to 10% of patients with metastatic disease, usually with cancers of the breast, head, and neck.6
Maximizing wound care provides comfort, relieves suffering, and promotes quality of life.3,7 To achieve these goals, clinicians must be familiar with strategies to manage complications associated with nonhealing wounds such as pain, malodor, and psychosocial adverse effects. Of these complications, malodor has been pointed out by both patients and caregivers as the most distressing.8
This article focuses on wound malodor, discusses the processes that cause wounds to emit an offensive smell, and outlines a comprehensive management approach.
MRS. A., AGE 61, WITH STAGE IV BREAST CANCER
Mrs. A., 61 years old, had a fungating mass in her left breast, which began as a small nodule and progressively enlarged to deform her breast over several months. Her oncologist subsequently staged the extent of her cancer as stage IV after workup revealed lung metastasis. Mrs. A. and her family decided to forgo cancer treatment, including radiotherapy, and to transition to hospice care after discussions with the oncologist.
Mrs. A. lived at home with her husband. Her daughter and three grandchildren all lived nearby.
When her hospice physician arrived at her home to meet her, a strong, pungent, and nauseating smell greeted him as he entered her bedroom. The patient said that for the past few months she had been increasingly distressed by the revolting odor. She rarely left home and had been ashamed to have people visit her, including her family.
On examination, the physician noticed a large fungating mass with yellowish discharge and necrotic tissue in her left breast. In addition to mild pain, she was immensely bothered by the strong odor coming from her breast.
THE IMPACT OF MALODOR
As seen in the case of Mrs. A., malodor has grave effects, both physical and psychological. Patients experience impaired or socially unacceptable body image, social rejection, personal shame, and embarrassment.9,10 Feelings of fear, anxiety, and depression are common. If left uncontrolled, malodor results in social isolation, reluctance to engage in social activities, diminished appetite, and nausea. In addition, malodor is a constant reminder of patients’ pain and cancer, and it results in further suffering.11
Reactions of family members and caregivers can worsen the situation.9,12 Expressions of revulsion limit contact and inhibit intimacy, especially near the end of life. Caregivers are often frustrated and distressed over their inability to control the malodor. The environment becomes uninhabitable, and the malodor can permeate clothing, furniture, and living quarters.
Managing malodor can be emotionally draining, physically daunting, and frustrating for healthcare professionals, as several methods are usually employed, often in a trial-and-error approach, to achieve an acceptable degree of odor control. In addition, clinicians must face the challenge of treating malodorous wounds at very close distance without reacting in a way that offends or alarms patients and family members.13
MALODOR PRODUCTION: WHERE IS THAT SMELL COMING FROM?
All wounds can produce an odor.14 Wounds that are expected to heal typically emit a faint but not unpleasant odor, akin to fresh blood. Wounds colonized by Pseudomonas aeruginosa produce a fruity or grapelike odor that is tolerable. Malodor occurs with wounds infected by other gram-negative organisms or anaerobic bacteria.15 Similarly, wounds covered by necrotic tissue smell like decaying flesh.
Three major causes
The three major causes of wound malodor are slough, infection, and exudate (Figure 1).
Slough is dead or necrotic tissue, usually resulting from vascular compromise. Arterial ulcers, pressure ulcers, and malignant wounds all form slough from capillary occlusion, subsequent ischemia, and tissue necrosis.
Infection. Devitalized tissue, an ideal medium in which bacteria thrive, becomes the source of infection. Anaerobic bacteria are usually implicated in malodor. These include Bacteroides fragilis, Bacteroides prevotella, Clostridium perfringens, and Fusobacterium nucleatum.16,17 Anaerobic organisms produce putrescine and cadaverine, which are largely responsible for the offensive odor.16,18 Volatile fatty acids such as propionic, butyric, isovaleric, and valeric acid are formed from lipid catabolism by anaerobes and add to malodor.17 Aerobic bacteria such as Proteus, Klebsiella, and Pseudomonas species supercolonize necrotic tissue as well and contribute to malodor.17,18
Exudate. Since nonhealing wounds undergo repeated cycles of inflammation, infection, and necrosis, accumulation of exudate becomes inevitable. Exudate typically is a pus-like fluid containing serum, fibrin, and white blood cells, which leak from blood vessels. In addition, bacteria that colonize chronic wounds filled with necrotic tissue activate proteases that degrade and liquefy dead tissue, thereby forming extensive amounts of exudate.19
Apart from slough, infection, and exudate, poor general hygiene and dressings left on for too long may contribute to malodor.16 Moisture-retentive dressings such as hydrocolloids leave an odor after removal. Dressings that liquefy upon contact with the wound surface leave a pus-like, potentially malodorous material.
MALODOR ASSESSMENT: DO YOU SMELL SOMETHING?
Various ways to document wound malodor can prove useful in guiding assessment and treatment. Descriptions such as “foul,” “putrid,” “fishy,” or “filled the room” vividly portray the initial presentation. A 10-point numerical scale similar to a numerical pain scale or a visual analogue scale can be used as a subjective measure.
Other grading methods, which to the authors’ knowledge are not validated, may be helpful. In a study that focused on patients suffering from malodorous gynecologic malignancies, von Gruenigen et al20 used a 0-to-3 scale:
- 0 Absent
- 1 Not offensive
- 2 Offensive but tolerable
- 3 Offensive and intolerable.
A scale often adapted by other authors was devised by Baker and Haig,21 which clearly defines four classes:
- 1 Strong—odor is evident upon entering the room (6 to 10 feet from the patient) with the dressing intact
- 2 Moderate—odor is evident upon entering the room with dressing removed
- 3 Slight—odor is evident at close proximity to the patient when the dressing is removed
- 4 No odor—no odor is evident, even at the patient’s bedside with the dressing removed.
COMPREHENSIVE MANAGEMENT: HOW DO WE WIN THE ‘RACE’?
The acronym RACE outlines an approach to dealing with malodor. It stands for removal of necrotic tissue; antibacterials; odor concealers; and education and support (Table 1).
Remove necrotic tissue
An important step in eliminating malodor is to remove necrotic tissue. This starts with debridement, which decreases the incidence of infection and hastens wound closure.22,23 Table 2 compares the different types of debridement.
Sharp or surgical debridement involves the use of a scalpel or scissors. This type of debridement may increase the risk of bleeding, pain, and malignant cell seeding in fungating wounds.4,24
Enzymatic debridement employs chemicals with proteolytic action (eg, collagenase) to digest extracellular proteins in wounds.18,25
Mechanical debridement involves aggressive therapies such as forceful irrigation and hydrotherapy, which may fail to discriminate between necrotic and viable tissues.18,26
Biological debridement using maggots, which ingest bacteria and devitalized tissue, may cause increased wound bleeding and may be unacceptable for patients and families.24,27
Autolytic debridement is often recommended, particularly if complete healing is not the primary goal.17,24,28,29 Autolysis uses proteolytic enzymes and phagocytic cells present in the wound bed and wound fluid to clear devitalized tissue. It is easy, inexpensive, noninvasive, and painless,4 and it requires less frequent dressing changes relative to standard dressing or wet-to-dry dressing.
Autolytic debridement is commonly accomplished using hydrocolloid and hydrogel dressings.15,29 Hydrocolloids are adhesive, occlusive, and conformable dressings that are suitable for wounds with low to moderate amounts of exudate. Upon contact with the wound surface, the dressing absorbs the exudate, forms a gel layer, and maintains a moist environment. Hydrocolloids are not recommended for infected wounds or for those with copious exudate as they may lead to maceration around the wound. A disadvantage of hydrocolloid dressings is their tendency to generate brown, often malodorous exudate when removed.
On the other hand, hydrogels in amorphous gel, dressing, sheet, or impregnated gauze form are water-based products that create a moist environment similar to hydrocolloids. Aside from causing minimal trauma to the wound bed when removed, the dressing’s cooling effect may bring some pain relief. Hydrogels are appropriate for dry wounds and for those with minimal exudate.
After debridement, the wound is cleansed and irrigated. A number of cleansers and solutions are available, but normal saline is a cheap alternative. To irrigate, experts recommend an 18- or 20-gauge intravenous catheter attached to a 30- or 60-mL syringe.15 This technique provides 8 to 15 psi of pressure, enough to cleanse the wound without causing tissue trauma.
Antibacterials and absorption
Antibacterials. Topical antibiotics have several advantages over systemic antibiotics in treating chronic wounds.30,31 These include a high and sustained concentration of the antimicrobial at the site of infection, limited potential for systemic absorption and toxicity, reduced potential for antibiotic resistance, and drawing of the patient’s and caregiver’s attention to the wound.
Metronidazole is the most widely used topical antibacterial for malodor management. Its efficacy is likely due to the predominant involvement of anaerobic bacteria in foul-smelling wounds. Topical metronidazole is available as a gel and as a cream. A systematic review showed that on average, topical metronidazole was used once daily for 14 consecutive days.19 The layer of topical metronidazole is typically covered with a nonadherent primary dressing followed by an absorbent secondary dressing.
The best clinical evidence for topical metronidazole consists of case reports and series.32–35 The largest of these studies was done by Finlay et al, who treated 47 patients with malodorous benign and malignant cutaneous wounds with 0.75% metronidazole gel daily.32 Forty-five (96%) of the patients reported significantly decreased odor by 14 days, as well as decreased pain, discharge, and surrounding cellulitis.
A randomized, placebo-controlled trial conducted by Bale et al had equivocal findings.9 All 41 patients who received metronidazole gel reported a decrease in malodor within 3 days of starting it. However, 76% of patients who received placebo also reported malodor control; in the final analysis, no significant difference was noted in the success rate between the two groups.
Metronidazole tablets can be crushed and sprinkled over the wound. As with metronidazole gel or cream, the crushed tablets are applied daily and covered by a primary nonadherent dressing and an absorbent secondary dressing. This off-label use of metronidazole serves as a cheaper alternative to commercially available topical preparations. To our knowledge, there has been no head-to-head trial comparing the two topical strategies.
Systemic metronidazole, often given orally, has been recommended if evidence of deep tissue or systemic infection is noted15 and in cases of fungating wounds with fistulas invading either the gastrointestinal or genitourinary tracts.18 Side effects such as nausea, neuropathy, and alcohol intolerance (ie, disulfiram reaction) may occur, which are not seen with topical metronidazole.
Both topical and systemic metronidazole can be used together on a time-limited basis for extensive malodorous wounds, such as fungating malignant wounds or stage IV sacral pressure ulcers.
Other antimicrobial agents used to treat malodor include silver-containing products, iodine-containing topical agents, mupirocin, bacitracin, neomycin, and polymyxin B.
Honey was used for wound care by the ancient Egyptians, and it is still used.36 Its beneficial effects include antimicrobial, debriding, deodorizing, anti-inflammatory, and granulation tissue-stimulating. Honey has even been shown to significantly decrease skin colonization with various kinds of bacteria, including methicillin-resistant Staphylococcus aureus.37 Medical-grade honey is preferred over table honey, as the latter is nonsterile and can contain Clostridium spores, which contaminate the wound.38
Yogurt and buttermilk lower the pH of the wound and control bacterial proliferation to control malodor.39,40 Either is applied for 10 to 15 minutes after the wound is cleansed and is then washed off thoroughly.
Absorbent dressings are used either over a layer of topical metronidazole and a nonadherent primary dressing or as a primary dressing itself. An absorbent dressing containing activated charcoal is used for rapid improvement, although cost may be prohibitive, especially in developing countries.13,19 Another type of absorbent dressing, composed of polyester impregnated with sodium chloride, has been found to be useful in malodor control.41 An important pointer is to maintain a tight seal around the absorbent dressing to prevent leakage of exudate.
Concealers
Aromatics used to conceal malodor include scented candles, incense, fragrant flowers and plants, and air-freshener sprays. When circumstances allow, candles are good options since they conceal malodor by emitting fragrance, and the flame burns off foul-smelling chemicals. Aromatics such as coffee beans, vanilla beans, and cider vinegar can be placed in a pan and left under the patient’s bed or close to it. Drops of peppermint oil or oil of wintergreen can be placed on wound dressings.
Other odor concealers are adsorbent materials that attract and cause ions and molecules to adhere to their surface. Examples are charcoal, baking soda, and cat litter. As with other aromatics, these materials are placed in pans and left under the bed or near the patient.
Aromatics can have disadvantages, as certain scents, especially strong ones, can be nauseating for patients. Some fragrances trigger asthma or skin irritation. Patients and caregivers can be left with an unpleasant association of certain fragrances with malodor by conditioning.15,17,18
Education and support
Concerns of the patient and family members need to be heard, addressed promptly, and reassessed with each visit, since uncontrolled malodor can be a chief source of caregiver fatigue.
Foremost in formulating a patient- and family-centered malodor management strategy is to commit to controlling malodor as much as possible. Regular follow-up appointments should be made, whether in the office or at home, to check on the patient’s progress and address new and ongoing concerns. Symptoms accompanying malodor, such as pain, bleeding, and sleep disturbance, need to be addressed, as they all affect quality of life.1 Audience-appropriate educational materials should be made available.26 Online resources that patients and families can explore include the websites of the Wound Ostomy and Continence Nurses Society (www.wocn.org) and the Association for the Advancement of Wound Care (aawconline.org).
Healthcare professionals need to be prepared to deal with problems and complications involving patients and family members that may arise in the course of treatment.12 Problems include the cost and local unavailability of dressing supplies, insurance coverage for dressings and topical agents, lack of assistance at home, and fear of changing dressings. A cardinal rule for healthcare providers is to avoid expressing distress at odors in front of or within hearing of patients and families.
OTHER STRATEGIES: WHAT ELSE CAN WE DO?
Curcumin, the main biologically active compound in the herb turmeric, applied directly to wounds three times daily as an ointment, has been shown to have odor-controlling properties.42
Sugar paste has been reported to control malodor by drawing out exudative and tissue fluid osmotically, and inhibiting bacterial growth.16,17 Water is mixed with sugar (ie, granulated, caster, or powdered) to form a paste, with additives like glycerin and polyethylene glycol used to alter the consistency. Thick clay-like paste is good for wounds with large cavities, while thin paste is useful for wounds with small or superficial openings. The paste is applied twice daily and is covered by an absorbent dressing.
Pressure relief is vital in managing pressure ulcers.18,43 Repositioning every 2 hours and using special devices, such as mattress overlays, alternating pressure mattresses, and low air loss mattresses, are frequently employed techniques.
If circumstances permit and when congruent with the patient’s goals of care, intra-arterial chemotherapy and radiotherapy can be contemplated for malignant fungating wounds.44,45
Other strategies include opening the windows during dressing changes, increasing the frequency of dressing changes, promptly removing used dressings from the house, and ensuring good general hygiene.
CASE RESOLUTION
After telling her that he was committed to control the malodor or, if possible, eliminate it, Mrs. A.’s doctor prepared two lists of materials—one for himself and one for Mrs. A.’s husband. He returned the next day, brought out his supplies, asked Mrs. A. to lie in bed, and invited her husband to assist him.
He cleansed and irrigated the breast lesion with normal saline, making sure to remove as much dead tissue as he could. He applied a layer of metronidazole cream to the wound cavity, then covered it with a nonadherent dressing. He then covered the wound with gauze, sealed the edges with medical adhesive tape, and applied a few drops of oil of wintergreen to the surface. A pan of charcoal briquettes was put under the bed, and a candle with Mrs. A.’s favorite scent was lit by the bedside. The physician then instructed Mrs. A.’s husband to repeat the procedure once daily for 1 week.
After 2 weeks, Mrs. A. and her husband said the foul odor had greatly decreased. She appeared more cheerful and energetic, especially after her grandchildren visited a few days earlier. The physician then instructed the husband to stop using metronidazole cream and to apply a hydrocolloid dressing every 3 days instead. He advised them to continue the rest of the process of applying a few drops of oil of wintergreen on the dressing surface, placing a pan of charcoal briquettes under the bed, and lighting a scented candle by the bedside.
FINISH THE RACE!
Complex nonhealing wounds are encountered across various healthcare settings. Wound malodor is an important component of nonhealing wounds, which adversely affects patients, families, and healthcare providers. Infection, slough, and exudate are the major causes of wound malodor. The essential steps to reduce malodor are to remove necrotic tissue, use antibacterial and odor-absorbing agents, apply appropriate odor “concealers,” educate families, and formulate a patient- and family-centered strategy (Table 1).
Acknowledgment: The authors would like to thank Sue Reif, CNP, for her assistance in completing the manuscript.
Wounds that fail to heal become more than mere skin lesions. Pain, malodor, and the accompanying psychological distress often complicate nonhealing wounds and impair quality of life.1 Management of malodor requires perseverance, sensitivity, and familiarity with tools and procedures that range from surgical debridement to medical-grade honey.
Chronic, nonhealing wounds are defined as persisting for more than 6 months.2 These lesions are incapable of undergoing anatomic and functional repair on their own. Commonly encountered nonhealing wounds include pressure ulcers, venous stasis ulcers, arterial insufficiency ulcers, and malignant cutaneous wounds.
Typically, the patient with a nonhealing wound is frail, debilitated, medically complex, and often faced with one or more life-limiting illnesses. Complete wound healing may therefore be unrealistic, and optimal wound management becomes the goal of care.3,4
Healthcare providers encounter nonhealing wounds in varied settings—acute inpatient, outpatient, long-term, and home care. For instance, in the home care setting, a study of 383 patients enrolled in hospice found that 35% had skin ulcers and wounds.3 Half of those affected had pressure ulcers, 20% had ischemic ulcers, and 30% had other skin disorders such as stasis ulcers, burns, skin tears, and tumors. A larger study, also in hospice patients, found that 26% had pressure ulcers and 10% more developed them within 6 months.5
While pressure ulcers are the most common nonhealing wounds, malignant or fungating wounds are found in 5% to 10% of patients with metastatic disease, usually with cancers of the breast, head, and neck.6
Maximizing wound care provides comfort, relieves suffering, and promotes quality of life.3,7 To achieve these goals, clinicians must be familiar with strategies to manage complications associated with nonhealing wounds such as pain, malodor, and psychosocial adverse effects. Of these complications, malodor has been pointed out by both patients and caregivers as the most distressing.8
This article focuses on wound malodor, discusses the processes that cause wounds to emit an offensive smell, and outlines a comprehensive management approach.
MRS. A., AGE 61, WITH STAGE IV BREAST CANCER
Mrs. A., 61 years old, had a fungating mass in her left breast, which began as a small nodule and progressively enlarged to deform her breast over several months. Her oncologist subsequently staged the extent of her cancer as stage IV after workup revealed lung metastasis. Mrs. A. and her family decided to forgo cancer treatment, including radiotherapy, and to transition to hospice care after discussions with the oncologist.
Mrs. A. lived at home with her husband. Her daughter and three grandchildren all lived nearby.
When her hospice physician arrived at her home to meet her, a strong, pungent, and nauseating smell greeted him as he entered her bedroom. The patient said that for the past few months she had been increasingly distressed by the revolting odor. She rarely left home and had been ashamed to have people visit her, including her family.
On examination, the physician noticed a large fungating mass with yellowish discharge and necrotic tissue in her left breast. In addition to mild pain, she was immensely bothered by the strong odor coming from her breast.
THE IMPACT OF MALODOR
As seen in the case of Mrs. A., malodor has grave effects, both physical and psychological. Patients experience impaired or socially unacceptable body image, social rejection, personal shame, and embarrassment.9,10 Feelings of fear, anxiety, and depression are common. If left uncontrolled, malodor results in social isolation, reluctance to engage in social activities, diminished appetite, and nausea. In addition, malodor is a constant reminder of patients’ pain and cancer, and it results in further suffering.11
Reactions of family members and caregivers can worsen the situation.9,12 Expressions of revulsion limit contact and inhibit intimacy, especially near the end of life. Caregivers are often frustrated and distressed over their inability to control the malodor. The environment becomes uninhabitable, and the malodor can permeate clothing, furniture, and living quarters.
Managing malodor can be emotionally draining, physically daunting, and frustrating for healthcare professionals, as several methods are usually employed, often in a trial-and-error approach, to achieve an acceptable degree of odor control. In addition, clinicians must face the challenge of treating malodorous wounds at very close distance without reacting in a way that offends or alarms patients and family members.13
MALODOR PRODUCTION: WHERE IS THAT SMELL COMING FROM?
All wounds can produce an odor.14 Wounds that are expected to heal typically emit a faint but not unpleasant odor, akin to fresh blood. Wounds colonized by Pseudomonas aeruginosa produce a fruity or grapelike odor that is tolerable. Malodor occurs with wounds infected by other gram-negative organisms or anaerobic bacteria.15 Similarly, wounds covered by necrotic tissue smell like decaying flesh.
Three major causes
The three major causes of wound malodor are slough, infection, and exudate (Figure 1).
Slough is dead or necrotic tissue, usually resulting from vascular compromise. Arterial ulcers, pressure ulcers, and malignant wounds all form slough from capillary occlusion, subsequent ischemia, and tissue necrosis.
Infection. Devitalized tissue, an ideal medium in which bacteria thrive, becomes the source of infection. Anaerobic bacteria are usually implicated in malodor. These include Bacteroides fragilis, Bacteroides prevotella, Clostridium perfringens, and Fusobacterium nucleatum.16,17 Anaerobic organisms produce putrescine and cadaverine, which are largely responsible for the offensive odor.16,18 Volatile fatty acids such as propionic, butyric, isovaleric, and valeric acid are formed from lipid catabolism by anaerobes and add to malodor.17 Aerobic bacteria such as Proteus, Klebsiella, and Pseudomonas species supercolonize necrotic tissue as well and contribute to malodor.17,18
Exudate. Since nonhealing wounds undergo repeated cycles of inflammation, infection, and necrosis, accumulation of exudate becomes inevitable. Exudate typically is a pus-like fluid containing serum, fibrin, and white blood cells, which leak from blood vessels. In addition, bacteria that colonize chronic wounds filled with necrotic tissue activate proteases that degrade and liquefy dead tissue, thereby forming extensive amounts of exudate.19
Apart from slough, infection, and exudate, poor general hygiene and dressings left on for too long may contribute to malodor.16 Moisture-retentive dressings such as hydrocolloids leave an odor after removal. Dressings that liquefy upon contact with the wound surface leave a pus-like, potentially malodorous material.
MALODOR ASSESSMENT: DO YOU SMELL SOMETHING?
Various ways to document wound malodor can prove useful in guiding assessment and treatment. Descriptions such as “foul,” “putrid,” “fishy,” or “filled the room” vividly portray the initial presentation. A 10-point numerical scale similar to a numerical pain scale or a visual analogue scale can be used as a subjective measure.
Other grading methods, which to the authors’ knowledge are not validated, may be helpful. In a study that focused on patients suffering from malodorous gynecologic malignancies, von Gruenigen et al20 used a 0-to-3 scale:
- 0 Absent
- 1 Not offensive
- 2 Offensive but tolerable
- 3 Offensive and intolerable.
A scale often adapted by other authors was devised by Baker and Haig,21 which clearly defines four classes:
- 1 Strong—odor is evident upon entering the room (6 to 10 feet from the patient) with the dressing intact
- 2 Moderate—odor is evident upon entering the room with dressing removed
- 3 Slight—odor is evident at close proximity to the patient when the dressing is removed
- 4 No odor—no odor is evident, even at the patient’s bedside with the dressing removed.
COMPREHENSIVE MANAGEMENT: HOW DO WE WIN THE ‘RACE’?
The acronym RACE outlines an approach to dealing with malodor. It stands for removal of necrotic tissue; antibacterials; odor concealers; and education and support (Table 1).
Remove necrotic tissue
An important step in eliminating malodor is to remove necrotic tissue. This starts with debridement, which decreases the incidence of infection and hastens wound closure.22,23 Table 2 compares the different types of debridement.
Sharp or surgical debridement involves the use of a scalpel or scissors. This type of debridement may increase the risk of bleeding, pain, and malignant cell seeding in fungating wounds.4,24
Enzymatic debridement employs chemicals with proteolytic action (eg, collagenase) to digest extracellular proteins in wounds.18,25
Mechanical debridement involves aggressive therapies such as forceful irrigation and hydrotherapy, which may fail to discriminate between necrotic and viable tissues.18,26
Biological debridement using maggots, which ingest bacteria and devitalized tissue, may cause increased wound bleeding and may be unacceptable for patients and families.24,27
Autolytic debridement is often recommended, particularly if complete healing is not the primary goal.17,24,28,29 Autolysis uses proteolytic enzymes and phagocytic cells present in the wound bed and wound fluid to clear devitalized tissue. It is easy, inexpensive, noninvasive, and painless,4 and it requires less frequent dressing changes relative to standard dressing or wet-to-dry dressing.
Autolytic debridement is commonly accomplished using hydrocolloid and hydrogel dressings.15,29 Hydrocolloids are adhesive, occlusive, and conformable dressings that are suitable for wounds with low to moderate amounts of exudate. Upon contact with the wound surface, the dressing absorbs the exudate, forms a gel layer, and maintains a moist environment. Hydrocolloids are not recommended for infected wounds or for those with copious exudate as they may lead to maceration around the wound. A disadvantage of hydrocolloid dressings is their tendency to generate brown, often malodorous exudate when removed.
On the other hand, hydrogels in amorphous gel, dressing, sheet, or impregnated gauze form are water-based products that create a moist environment similar to hydrocolloids. Aside from causing minimal trauma to the wound bed when removed, the dressing’s cooling effect may bring some pain relief. Hydrogels are appropriate for dry wounds and for those with minimal exudate.
After debridement, the wound is cleansed and irrigated. A number of cleansers and solutions are available, but normal saline is a cheap alternative. To irrigate, experts recommend an 18- or 20-gauge intravenous catheter attached to a 30- or 60-mL syringe.15 This technique provides 8 to 15 psi of pressure, enough to cleanse the wound without causing tissue trauma.
Antibacterials and absorption
Antibacterials. Topical antibiotics have several advantages over systemic antibiotics in treating chronic wounds.30,31 These include a high and sustained concentration of the antimicrobial at the site of infection, limited potential for systemic absorption and toxicity, reduced potential for antibiotic resistance, and drawing of the patient’s and caregiver’s attention to the wound.
Metronidazole is the most widely used topical antibacterial for malodor management. Its efficacy is likely due to the predominant involvement of anaerobic bacteria in foul-smelling wounds. Topical metronidazole is available as a gel and as a cream. A systematic review showed that on average, topical metronidazole was used once daily for 14 consecutive days.19 The layer of topical metronidazole is typically covered with a nonadherent primary dressing followed by an absorbent secondary dressing.
The best clinical evidence for topical metronidazole consists of case reports and series.32–35 The largest of these studies was done by Finlay et al, who treated 47 patients with malodorous benign and malignant cutaneous wounds with 0.75% metronidazole gel daily.32 Forty-five (96%) of the patients reported significantly decreased odor by 14 days, as well as decreased pain, discharge, and surrounding cellulitis.
A randomized, placebo-controlled trial conducted by Bale et al had equivocal findings.9 All 41 patients who received metronidazole gel reported a decrease in malodor within 3 days of starting it. However, 76% of patients who received placebo also reported malodor control; in the final analysis, no significant difference was noted in the success rate between the two groups.
Metronidazole tablets can be crushed and sprinkled over the wound. As with metronidazole gel or cream, the crushed tablets are applied daily and covered by a primary nonadherent dressing and an absorbent secondary dressing. This off-label use of metronidazole serves as a cheaper alternative to commercially available topical preparations. To our knowledge, there has been no head-to-head trial comparing the two topical strategies.
Systemic metronidazole, often given orally, has been recommended if evidence of deep tissue or systemic infection is noted15 and in cases of fungating wounds with fistulas invading either the gastrointestinal or genitourinary tracts.18 Side effects such as nausea, neuropathy, and alcohol intolerance (ie, disulfiram reaction) may occur, which are not seen with topical metronidazole.
Both topical and systemic metronidazole can be used together on a time-limited basis for extensive malodorous wounds, such as fungating malignant wounds or stage IV sacral pressure ulcers.
Other antimicrobial agents used to treat malodor include silver-containing products, iodine-containing topical agents, mupirocin, bacitracin, neomycin, and polymyxin B.
Honey was used for wound care by the ancient Egyptians, and it is still used.36 Its beneficial effects include antimicrobial, debriding, deodorizing, anti-inflammatory, and granulation tissue-stimulating. Honey has even been shown to significantly decrease skin colonization with various kinds of bacteria, including methicillin-resistant Staphylococcus aureus.37 Medical-grade honey is preferred over table honey, as the latter is nonsterile and can contain Clostridium spores, which contaminate the wound.38
Yogurt and buttermilk lower the pH of the wound and control bacterial proliferation to control malodor.39,40 Either is applied for 10 to 15 minutes after the wound is cleansed and is then washed off thoroughly.
Absorbent dressings are used either over a layer of topical metronidazole and a nonadherent primary dressing or as a primary dressing itself. An absorbent dressing containing activated charcoal is used for rapid improvement, although cost may be prohibitive, especially in developing countries.13,19 Another type of absorbent dressing, composed of polyester impregnated with sodium chloride, has been found to be useful in malodor control.41 An important pointer is to maintain a tight seal around the absorbent dressing to prevent leakage of exudate.
Concealers
Aromatics used to conceal malodor include scented candles, incense, fragrant flowers and plants, and air-freshener sprays. When circumstances allow, candles are good options since they conceal malodor by emitting fragrance, and the flame burns off foul-smelling chemicals. Aromatics such as coffee beans, vanilla beans, and cider vinegar can be placed in a pan and left under the patient’s bed or close to it. Drops of peppermint oil or oil of wintergreen can be placed on wound dressings.
Other odor concealers are adsorbent materials that attract and cause ions and molecules to adhere to their surface. Examples are charcoal, baking soda, and cat litter. As with other aromatics, these materials are placed in pans and left under the bed or near the patient.
Aromatics can have disadvantages, as certain scents, especially strong ones, can be nauseating for patients. Some fragrances trigger asthma or skin irritation. Patients and caregivers can be left with an unpleasant association of certain fragrances with malodor by conditioning.15,17,18
Education and support
Concerns of the patient and family members need to be heard, addressed promptly, and reassessed with each visit, since uncontrolled malodor can be a chief source of caregiver fatigue.
Foremost in formulating a patient- and family-centered malodor management strategy is to commit to controlling malodor as much as possible. Regular follow-up appointments should be made, whether in the office or at home, to check on the patient’s progress and address new and ongoing concerns. Symptoms accompanying malodor, such as pain, bleeding, and sleep disturbance, need to be addressed, as they all affect quality of life.1 Audience-appropriate educational materials should be made available.26 Online resources that patients and families can explore include the websites of the Wound Ostomy and Continence Nurses Society (www.wocn.org) and the Association for the Advancement of Wound Care (aawconline.org).
Healthcare professionals need to be prepared to deal with problems and complications involving patients and family members that may arise in the course of treatment.12 Problems include the cost and local unavailability of dressing supplies, insurance coverage for dressings and topical agents, lack of assistance at home, and fear of changing dressings. A cardinal rule for healthcare providers is to avoid expressing distress at odors in front of or within hearing of patients and families.
OTHER STRATEGIES: WHAT ELSE CAN WE DO?
Curcumin, the main biologically active compound in the herb turmeric, applied directly to wounds three times daily as an ointment, has been shown to have odor-controlling properties.42
Sugar paste has been reported to control malodor by drawing out exudative and tissue fluid osmotically, and inhibiting bacterial growth.16,17 Water is mixed with sugar (ie, granulated, caster, or powdered) to form a paste, with additives like glycerin and polyethylene glycol used to alter the consistency. Thick clay-like paste is good for wounds with large cavities, while thin paste is useful for wounds with small or superficial openings. The paste is applied twice daily and is covered by an absorbent dressing.
Pressure relief is vital in managing pressure ulcers.18,43 Repositioning every 2 hours and using special devices, such as mattress overlays, alternating pressure mattresses, and low air loss mattresses, are frequently employed techniques.
If circumstances permit and when congruent with the patient’s goals of care, intra-arterial chemotherapy and radiotherapy can be contemplated for malignant fungating wounds.44,45
Other strategies include opening the windows during dressing changes, increasing the frequency of dressing changes, promptly removing used dressings from the house, and ensuring good general hygiene.
CASE RESOLUTION
After telling her that he was committed to control the malodor or, if possible, eliminate it, Mrs. A.’s doctor prepared two lists of materials—one for himself and one for Mrs. A.’s husband. He returned the next day, brought out his supplies, asked Mrs. A. to lie in bed, and invited her husband to assist him.
He cleansed and irrigated the breast lesion with normal saline, making sure to remove as much dead tissue as he could. He applied a layer of metronidazole cream to the wound cavity, then covered it with a nonadherent dressing. He then covered the wound with gauze, sealed the edges with medical adhesive tape, and applied a few drops of oil of wintergreen to the surface. A pan of charcoal briquettes was put under the bed, and a candle with Mrs. A.’s favorite scent was lit by the bedside. The physician then instructed Mrs. A.’s husband to repeat the procedure once daily for 1 week.
After 2 weeks, Mrs. A. and her husband said the foul odor had greatly decreased. She appeared more cheerful and energetic, especially after her grandchildren visited a few days earlier. The physician then instructed the husband to stop using metronidazole cream and to apply a hydrocolloid dressing every 3 days instead. He advised them to continue the rest of the process of applying a few drops of oil of wintergreen on the dressing surface, placing a pan of charcoal briquettes under the bed, and lighting a scented candle by the bedside.
FINISH THE RACE!
Complex nonhealing wounds are encountered across various healthcare settings. Wound malodor is an important component of nonhealing wounds, which adversely affects patients, families, and healthcare providers. Infection, slough, and exudate are the major causes of wound malodor. The essential steps to reduce malodor are to remove necrotic tissue, use antibacterial and odor-absorbing agents, apply appropriate odor “concealers,” educate families, and formulate a patient- and family-centered strategy (Table 1).
Acknowledgment: The authors would like to thank Sue Reif, CNP, for her assistance in completing the manuscript.
- Lo SF, Hayter M, Hu WY, Tai CY, Hsu MY, Li YF. Symptom burden and quality of life in patients with malignant fungating wounds. J Adv Nurs 2012; 68:1312–1321.
- Lazarus GS, Cooper DM, Knighton DR, et al. Definitions and guidelines for assessment of wounds and evaluation of healing. Arch Dermatol 1994; 130:489–493.
- Tippett AW. Wounds at the end of life. Wounds 2005; 17:91–98.
- Burt T. Palliative care of pressure ulcers in long-term care. Ann Long-Term Care 2013; 21:20–28.
- Reifsnyder J, Magee HS. Development of pressure ulcers in patients receiving home hospice care. Wounds 2005; 17:74–79.
- Haisfield-Wolfe ME, Rund C. Malignant cutaneous wounds: a management protocol. Ostomy Wound Manage 1997; 43:56–66.
- O’Brien C. Malignant wounds: managing odour. Can Fam Physician 2012; 58:272–274.
- Gethin G, Grocott P, Probst S, Clarke E. Current practice in the management of wound odour: an international survey. Int J Nurs Stud 2014; 51:865–874.
- Bale S, Tebble N, Price P. A topical metronidazole gel used to treat malodorous wounds. Br J Nurs 2004; 13:S4–S11.
- Hack A. Malodorous wounds—taking the patient’s perspective into account. J Wound Care 2003; 12:319–321.
- Price E. Wound care. The stigma of smell. Nurs Times 1996; 92:71–72.
- Paul JC, Pieper BA. Topical metronidazole for the treatment of wound odor: a review of the literature. Ostomy Wound Manage 2008; 54:18–27.
- Lee G, Anand SC, Rajendran S, Walker I. Overview of current practice and future trends in the evaluation of dressings for malodorous wounds. J Wound Care 2006; 15:344–346.
- Cutting K, Harding K. Criteria for identifying wound infection. J Wound Care 1994; 3:198–201.
- McDonald A, Lesage P. Palliative management of pressure ulcers and malignant wounds in patients with advanced illness. J Palliat Med 2006; 9:285–295.
- Holloway S. Recognising and treating the causes of chronic malodorous wounds. Prof Nurse 2004; 19:380–384.
- Haughton W, Young T. Common problems in wound care: malodorous wounds. Br J Nurs 1995; 4:959–963.
- Alvarez OM, Kalinski C, Nusbaum J, et al. Incorporating wound healing strategies to improve palliation (symptom management) in patients with chronic wounds. J Palliat Med 2007; 10:1161–1189.
- da Costa Santos CM, de Mattos Pimenta CA, Nobre MR. A systematic review of topical treatments to control the odor of malignant fungating wounds. J Pain Symptom Manage 2010; 39:1065–1076.
- Von Gruenigen VE, Coleman RL, et al. Bacteriology and treatment of malodorous lower reproductive tract in gynecologic cancer patients. Obstet Gynecol 2000; 96:23–27.
- Baker PG, Haig G. Metronidazole in the treatment of chronic pressure sores and ulcers: a comparison with standard treatment in general practice. Practitioner 1981; 225:569–573.
- Whitney J, Phillips L, Aslam R, et al. Guidelines for the treatment of pressure ulcers. Wound Repair Regen 2006; 14:663–679.
- Williams D, Enoch S, Miller D, Harris K, Price P, Harding KG. Effect of sharp debridement using curette on recalcitrant nonhealing venous ulcers: a concurrently controlled, prospective cohort study. Wound Repair Regen 2005; 13:131–137.
- Bergstrom KJ. Assessment and management of fungating wounds. J Wound Ostomy Continence Nurs 2011: 38:31–37.
- Sinclair RD, Ryan TJ. Proteolytic enzymes in wound healing: the role of enzymatic debridement. Australas J Dermatol 1994; 35:35–41.
- Enoch S, Harding KG. Wound bed preparation: the science behind the removal of barriers to healing. Wounds 2003;15:213–229.
- Mumcuoglu KY. Clinical applications for maggots in wound care. Am J Clin Dermatol 2001; 2:219–227.
- Langemo DK, Black J; National Pressure Ulcer Advisory Panel. Pressure ulcers in individuals receiving palliative care: a National Pressure Ulcer Advisory Panel white paper. Adv Skin Wound Care 2010; 23:59–72.
- Fonder MA, Lazarus GS, Cowan DA, Aronson-Cook B, Kohli AR, Mamelak AJ. Treating the chronic wound: a practical approach to the care of nonhealing wounds and wound care dressings. J Am Acad Dermatol 2008; 58:185–206.
- Lio PA, Kaye ET. Topical antibacterial agents. Infect Dis Clin North Am 2004; 18:717–733.
- Gelmetti C. Local antibiotics in dermatology. Dermatol Ther 2008; 21:187–195.
- Finlay IG, Bowszyc J, Ramlau C, Gwiezdzinski Z. The effect of topical 0.75% metronidazole gel on malodorous cutaneous ulcers. J Pain Symptom Manage 1996; 11:158–162.
- Bower M, Stein R, Evans TR, Hedley A, Pert P, Coombes RC. A double-blind study of the efficacy of metronidazole gel in the treatment of malodorous fungating tumours. Eur J Cancer 1992; 28A:888–889.
- Kalinski C, Schnepf M, Laboy D, et al. Effectiveness of a topical formulation containing metronidazole for wound odor and exudate control. Wounds 2005; 17:84–90.
- Kuge S, Tokuda Y, Ohta M, et al. Use of metronidazole gel to control malodor in advanced and recurrent breast cancer. Jpn J Clin Oncol 1996; 26:207–210.
- Belcher J. A review of medical-grade honey in wound care. Br J Nurs 2012: 21:S4–S9.
- Kwakman PH, Van den Akker JP, Güçlü A, et al. Medical-grade honey kills antibiotic-resistant bacteria in vitro and eradicates skin colonization. Clin Infect Dis 2008; 46:1677–1682.
- Cooper RA, Jenkins L. A comparison between medical grade honey and table honeys in relation to antimicrobial efficacy. Wounds 2009; 21:29–36.
- Patel B, Cox-Hayley D. Managing wound odor #218. J Palliat Med 2010; 13:1286–1287.
- Schulte MJ. Yogurt helps to control wound odor. Oncol Nurs Forum 1993; 20:1262.
- Upright CA, Salton C, Roberts F, Murphy J. Evaluation of Mesalt dressings and continuous wet saline dressings in ulcerating metastatic skin lesions. Cancer Nurs 1994; 17:149–155.
- Kuttan R, Sudheeran PC, Josph CD. Turmeric and curcumin as topical agents in cancer therapy. Tumori 1987; 73:29–31.
- Bass MJ, Phillips LG. Pressure sores. Curr Probl Surg 2007; 44:101–143.
- Bufill JA, Grace WR, Neff R. Intra-arterial chemotherapy for palliation of fungating breast cancer: a case report and review of the literature. Am J Clin Oncol 1994; 17:118–124.
- Murakami M, Kuroda Y, Sano A, et al. Validity of local treatment including intraarterial infusion chemotherapy and radiotherapy for fungating adenocarcinoma of the breast: case report of more than 8-year survival. Am J Clin Oncol 2001; 24:388–391.
- Lo SF, Hayter M, Hu WY, Tai CY, Hsu MY, Li YF. Symptom burden and quality of life in patients with malignant fungating wounds. J Adv Nurs 2012; 68:1312–1321.
- Lazarus GS, Cooper DM, Knighton DR, et al. Definitions and guidelines for assessment of wounds and evaluation of healing. Arch Dermatol 1994; 130:489–493.
- Tippett AW. Wounds at the end of life. Wounds 2005; 17:91–98.
- Burt T. Palliative care of pressure ulcers in long-term care. Ann Long-Term Care 2013; 21:20–28.
- Reifsnyder J, Magee HS. Development of pressure ulcers in patients receiving home hospice care. Wounds 2005; 17:74–79.
- Haisfield-Wolfe ME, Rund C. Malignant cutaneous wounds: a management protocol. Ostomy Wound Manage 1997; 43:56–66.
- O’Brien C. Malignant wounds: managing odour. Can Fam Physician 2012; 58:272–274.
- Gethin G, Grocott P, Probst S, Clarke E. Current practice in the management of wound odour: an international survey. Int J Nurs Stud 2014; 51:865–874.
- Bale S, Tebble N, Price P. A topical metronidazole gel used to treat malodorous wounds. Br J Nurs 2004; 13:S4–S11.
- Hack A. Malodorous wounds—taking the patient’s perspective into account. J Wound Care 2003; 12:319–321.
- Price E. Wound care. The stigma of smell. Nurs Times 1996; 92:71–72.
- Paul JC, Pieper BA. Topical metronidazole for the treatment of wound odor: a review of the literature. Ostomy Wound Manage 2008; 54:18–27.
- Lee G, Anand SC, Rajendran S, Walker I. Overview of current practice and future trends in the evaluation of dressings for malodorous wounds. J Wound Care 2006; 15:344–346.
- Cutting K, Harding K. Criteria for identifying wound infection. J Wound Care 1994; 3:198–201.
- McDonald A, Lesage P. Palliative management of pressure ulcers and malignant wounds in patients with advanced illness. J Palliat Med 2006; 9:285–295.
- Holloway S. Recognising and treating the causes of chronic malodorous wounds. Prof Nurse 2004; 19:380–384.
- Haughton W, Young T. Common problems in wound care: malodorous wounds. Br J Nurs 1995; 4:959–963.
- Alvarez OM, Kalinski C, Nusbaum J, et al. Incorporating wound healing strategies to improve palliation (symptom management) in patients with chronic wounds. J Palliat Med 2007; 10:1161–1189.
- da Costa Santos CM, de Mattos Pimenta CA, Nobre MR. A systematic review of topical treatments to control the odor of malignant fungating wounds. J Pain Symptom Manage 2010; 39:1065–1076.
- Von Gruenigen VE, Coleman RL, et al. Bacteriology and treatment of malodorous lower reproductive tract in gynecologic cancer patients. Obstet Gynecol 2000; 96:23–27.
- Baker PG, Haig G. Metronidazole in the treatment of chronic pressure sores and ulcers: a comparison with standard treatment in general practice. Practitioner 1981; 225:569–573.
- Whitney J, Phillips L, Aslam R, et al. Guidelines for the treatment of pressure ulcers. Wound Repair Regen 2006; 14:663–679.
- Williams D, Enoch S, Miller D, Harris K, Price P, Harding KG. Effect of sharp debridement using curette on recalcitrant nonhealing venous ulcers: a concurrently controlled, prospective cohort study. Wound Repair Regen 2005; 13:131–137.
- Bergstrom KJ. Assessment and management of fungating wounds. J Wound Ostomy Continence Nurs 2011: 38:31–37.
- Sinclair RD, Ryan TJ. Proteolytic enzymes in wound healing: the role of enzymatic debridement. Australas J Dermatol 1994; 35:35–41.
- Enoch S, Harding KG. Wound bed preparation: the science behind the removal of barriers to healing. Wounds 2003;15:213–229.
- Mumcuoglu KY. Clinical applications for maggots in wound care. Am J Clin Dermatol 2001; 2:219–227.
- Langemo DK, Black J; National Pressure Ulcer Advisory Panel. Pressure ulcers in individuals receiving palliative care: a National Pressure Ulcer Advisory Panel white paper. Adv Skin Wound Care 2010; 23:59–72.
- Fonder MA, Lazarus GS, Cowan DA, Aronson-Cook B, Kohli AR, Mamelak AJ. Treating the chronic wound: a practical approach to the care of nonhealing wounds and wound care dressings. J Am Acad Dermatol 2008; 58:185–206.
- Lio PA, Kaye ET. Topical antibacterial agents. Infect Dis Clin North Am 2004; 18:717–733.
- Gelmetti C. Local antibiotics in dermatology. Dermatol Ther 2008; 21:187–195.
- Finlay IG, Bowszyc J, Ramlau C, Gwiezdzinski Z. The effect of topical 0.75% metronidazole gel on malodorous cutaneous ulcers. J Pain Symptom Manage 1996; 11:158–162.
- Bower M, Stein R, Evans TR, Hedley A, Pert P, Coombes RC. A double-blind study of the efficacy of metronidazole gel in the treatment of malodorous fungating tumours. Eur J Cancer 1992; 28A:888–889.
- Kalinski C, Schnepf M, Laboy D, et al. Effectiveness of a topical formulation containing metronidazole for wound odor and exudate control. Wounds 2005; 17:84–90.
- Kuge S, Tokuda Y, Ohta M, et al. Use of metronidazole gel to control malodor in advanced and recurrent breast cancer. Jpn J Clin Oncol 1996; 26:207–210.
- Belcher J. A review of medical-grade honey in wound care. Br J Nurs 2012: 21:S4–S9.
- Kwakman PH, Van den Akker JP, Güçlü A, et al. Medical-grade honey kills antibiotic-resistant bacteria in vitro and eradicates skin colonization. Clin Infect Dis 2008; 46:1677–1682.
- Cooper RA, Jenkins L. A comparison between medical grade honey and table honeys in relation to antimicrobial efficacy. Wounds 2009; 21:29–36.
- Patel B, Cox-Hayley D. Managing wound odor #218. J Palliat Med 2010; 13:1286–1287.
- Schulte MJ. Yogurt helps to control wound odor. Oncol Nurs Forum 1993; 20:1262.
- Upright CA, Salton C, Roberts F, Murphy J. Evaluation of Mesalt dressings and continuous wet saline dressings in ulcerating metastatic skin lesions. Cancer Nurs 1994; 17:149–155.
- Kuttan R, Sudheeran PC, Josph CD. Turmeric and curcumin as topical agents in cancer therapy. Tumori 1987; 73:29–31.
- Bass MJ, Phillips LG. Pressure sores. Curr Probl Surg 2007; 44:101–143.
- Bufill JA, Grace WR, Neff R. Intra-arterial chemotherapy for palliation of fungating breast cancer: a case report and review of the literature. Am J Clin Oncol 1994; 17:118–124.
- Murakami M, Kuroda Y, Sano A, et al. Validity of local treatment including intraarterial infusion chemotherapy and radiotherapy for fungating adenocarcinoma of the breast: case report of more than 8-year survival. Am J Clin Oncol 2001; 24:388–391.
KEY POINTS
- Necrotic tissue is a substrate for bacterial growth and should be debrided. A variety of methods can be used.
- Malodor is most often from infection with anaerobic organisms, which topical metronidazole and other agents can help control.
- An absorbent dressing should be used either as a primary dressing, or over a layer of topical metronidazole and a nonadherent primary dressing.
- Foremost in formulating a patient- and family-centered malodor management strategy is to commit to controlling it as much as possible.
The emerging role of palliative medicine in the treatment of lung cancer patients
Several important developments in the palliative care of patients with lung cancer have occurred over the past few years, including publication of a landmark study comparing early with as-needed palliative care, the release of new data on the treatment of cancer-related anorexia, elucidation of new mechanisms and treatment options for dyspnea, and the availability of buprenorphine. This article reviews these emerging concepts.
LUNG CANCER SYMPTOMS: COMMON AND SEVERE
The symptom burden of lung cancer is usually great. At least 80% of patients experience fatigue, 65% suffer loss of appetite, 77% have cough, 73% report dyspnea (both from local symptoms and weight loss), 57% have chest pain, and 17% have hemoptysis.1
When symptoms are present, they are usually severe. Thirty-eight percent of the patients who report fatigue have severe fatigue, 47% have inadequate appetite to the point of requiring intervention, and more than one-half of patients who have chest pain require opioids for relief.1
Symptom frequency and severity are worse in individuals who survive 3 months or less.1 Increasing symptom burden is therefore prognostically important, particularly in patients with advanced stages of lung cancer. As a result, self-assessment of quality of life has a significant ability to predict survival in patients with advanced non–small cell lung cancer (NSCLC).2
Patients with lung cancer tend to suffer from groups of symptoms or symptom clusters. Lutz et al1 found that 79% of patients reported three or more symptoms; these results were similar to the findings of a study by Hollen et al,3 in which 81% of patients suffered from three or more symptoms, all them severe except for cough.
EARLY PALLIATIVE CARE HAS CLINICAL BENEFITS
A landmark study by Temel et al4 examined the benefits of early palliative care integrated with standard oncologic care versus standard oncologic care and palliative care only “as needed” on patient-reported outcomes, the use of health services, and the quality of end-of-life care among patients with metastatic NSCLC. The study was a prospective, nonblinded, randomized, controlled trial of outpatients conducted at a single center. The intervention was based on guidelines from the National Consensus Project for Quality Palliative Care, with specific attention to symptom management, goals of care, decision-making regarding treatment, and coordination of care. Patients assigned to the intervention met monthly with both a palliative care service and an oncologist, and 90% of the patients randomized to intervention complied with at least 50% of the visits.
Measures of health-related quality of life and mood were obtained using the Functional Assessment of Cancer Therapy-Lung (FACT-L), the Hospital Anxiety and Depression Scale, and the 9-item depression scale of the Patient Health Questionnaire.
Measures of health care service utilization included use of antitumor therapy within 14 days of death, late or no referral to hospice, hospital admissions, and emergency room visits. Patients were considered to have received aggressive care if they met any one of the following three criteria: chemotherapy within 14 days of death, no hospice care, or admission to hospice within 3 days of death.
Compared with standard care, early palliative care was associated with an increase in the number of advance directives, earlier hospice referral (11 days vs 4 days), fewer hospitalizations and emergency room visits, and fewer instances of inappropriate oncologic care (defined as chemotherapy within 14 days of death). The percentage of patients with depressed mood was also lower among those assigned to early palliative care versus standard care (16% vs 38%).
A 2.7-month difference in median survival (P = .02) in favor of the group assigned to early palliative care was also observed, although survival was not a primary end point of the trial. This outcome needs to be validated in future studies.
CANCER-RELATED ANOREXIA AND CACHEXIA: TREATMENT IMPROVES APPETITE
The main hallmark of cancer-related anorexia and cachexia is weight loss; this symptom cluster is most often associated with hypophagia. The coexistence of anorexia and appetite-related anhedonia is common in lung cancer patients, such that 25% of lung cancer patients with anorexia report no distress with not eating, nor do they derive pleasure from eating. Others report that early satiety and changes in taste dramatically affect appetite. To some, anorexia is a distressful reminder of progression of their cancer.
Megestrol acetate and medroxyprogesterone acetate at least partially improve appetite in a subset of anorectic cancer patients. The use of medroxyprogesterone acetate has resulted in weight gain but not muscle mass in some patients with cancer-related anorexia, but has had less effect on fatigue and quality of life in these patients.
Olanzapine is an atypical antipsychotic with an affinity for multiple neurotransmitter receptors. Several of these, such as the serotonin receptors 5-HT2 and 5-HT3, histamine receptors, and dopamine receptors, are implicated in anorexia, nausea, and vomiting. Case reports suggest that olanzapine has antiemetic activity in patients with advanced cancer and usefulness as prophylaxis against chemotherapy-related nausea and vomiting.5 Reduced risk of extrapyramidal symptoms compared with standard antiemetics enhances the value of olanzapine for prevention of cancer-related anorexia.
Navari et al6 conducted a randomized trial to determine the effectiveness of megestrol acetate and olanzapine for the treatment of cancer-related anorexia. Eighty patients were randomized to receive oral megestrol acetate 800 mg/d, or oral megestrol acetate 800 mg/d plus olanzapine 5 mg once nightly, for 8 weeks. Patients were removed from the study if they did not take the study medication for a 48-hour period or if intolerable toxicity developed that was attributable to the study agents.
The MD Anderson Symptom Inventory (MDASI) was completed weekly to assess key symptom outcome variables. A change of 3 cm on the visual analog scale over two separate time periods for a symptom was considered sufficient to define a change in the symptom.
Quality of life was measured using a valid 28-item self-reported instrument (Functional Assessment of Cancer Therapy-General). Patients were examined by their physicians every 2 weeks.
In the group assigned to megestrol acetate, 15 patients had a weight gain of at least 5%—a change that was considered significant. Appetite improved in two patients, nausea decreased in three patients, and quality of life improved in five patients at both 4 weeks and 8 weeks. The improvements in appetite, nausea, and quality of life for the whole group on megestrol acetate alone were not significant, and there was no improvement in mean symptom scores measured by the MDASI.
There were incremental improvements of all measures in patients randomized to megestrol acetate plus olanzapine. Among patients receiving the combination, 33 had a weight gain of at least 5%; 25 reported an improvement in appetite, 21 experienced a reduction in nausea, and 23 had an improvement in quality of life at both 4 weeks and 8 weeks. All outcome variables were improved on the MDASI
CANCER AND DYSPNEA: NUMEROUS INTERVENTIONS HAVE BEEN ASSESSED
Reduced inspiratory capacity caused by weakened inspiratory muscles results in an increased Borg rating of perceived exertion (RPE) relative to oxygen levels. Both central nervous system activation of muscle and loss of muscle tissue contribute to dyspnea and fatigue in lung cancer patients.7 Cancer fatigue, also measured by the Borg RPE scale, appears to be a “central” mechanism that stems from a mismatch between efferent output for afferent inputs in the setting of ventilatory muscle weakness, thereby increasing the perception of dyspnea. Several interventions have been used to relieve dyspnea, ranging from oxygen therapy to treatment with opioids.
Oxygen saturation
The association between hypoxemia and dyspnea is poor.8 In a randomized prospective trial, Abernethy et al9 found no benefit to oxygen therapy compared with medical air without added supplemental oxygen in individuals who had normal oxygen saturation but symptomatic dyspnea.
Bilevel positive airway pressure
Bilevel positive airway pressure has been shown to reduce the need for invasive ventilation; improve oxygen saturation; and reduce dynamic hyperinflation, thus relieving dyspnea.10 It has been effective in dyspneic patients with motor neuron disease, cancer, heart failure, status asthmaticus, stroke, drug overdose, and interstitial lung disease.
Indwelling pleural catheters
Tunneled pleural catheters reduce the severity of dyspnea in 95% of patients.11 These catheters are inserted on an outpatient basis, allowing for outpatient drainage. Autopleurodesis occurs in about 45% of patients, in which case the catheter can be removed. Adverse reactions are few (incidence < 10%), but consist of empyema, pneumothorax, cellulitis, or catheter obstruction. The disadvantage is the expense of catheter maintenance.
Nebulized furosemide
Case reports suggest that inhalation of nebulized furosemide, 20 mg four times daily, dramatically improves dyspnea in patients with advanced cancer and severe shortness of breath that is unresponsive to opioids.12 Nebulized furosemide appears to have a direct effect on either pulmonary stretch receptors or irritant receptors in the airways; it also has a diuretic effect. Response occurs quickly with an onset of effect in 20 to 30 minutes.
B-type natriuretic peptide
The level of N-terminal precursor of B-type natriuretic peptide (NT-pro-BNP) can predict response to sunitinib in renal cancer,13 and the BNP level predicts 30-day mortality in pulmonary embolism.14 Measurement of BNP to detect dyspnea in patients with lung cancer is not useful, however, because the BNP level increases with cardiac and pericardial metastases. The BNP level is also persistently elevated after chest radiation therapy, and it increases with anthracycline cardiotoxicity. It is not a useful marker for distinguishing pulmonary from nonpulmonary or cardiac from noncardiac causes of dyspnea.
Lung ultrasound
Portable diagnostic lung ultrasound can be used to detect pneumonia, pleural effusions, pulmonary emboli, pneumothorax, atelectasis, and lung abscesses as potential causes of dyspnea.15–18 In addition to the advantage of portability, there is no radiation exposure and the technology permits echocardiography to be conducted.
Opioids
Evidence supports opioids for pharmacologic relief of dyspnea in the palliative care of patients with chronic obstructive pulmonary disease and cancer. Studies have been conducted with morphine sulfate, hydromorphone, dihydrocodeine, intranasal and transmucosal fentanyl, oxycodone, and diamorphine.19–21
The response to opioids is unrelated to the severity of dyspnea.22 Responses and safe administration occur even in patients with reduced oxygen saturation or elevated carbon dioxide partial pressure.20 Opioids can be used safely in the opioid-naïve population.20 Recommended dosages in these patients are 2.5 to 5.0 mg of morphine sulfate every 4 hours, 5 mg of oxycodone every 4 hours as needed, and 1 mg of hydromorphone every 4 hours in the opioid-naïve. In opioid-tolerant patients, it is recommended that therapy start with these doses and then be increased in 25% increments every 24 hours, as needed.
BUPRENORPHINE: UNIQUE OPIOID
Buprenorphine is a mu- and nociceptin (ORL-1)-receptor partial agonist with intravenous, subcutaneous, sublingual, transdermal, and intranasal routes of delivery.23 An agent that acts as an ORL-1 agonist can induce analgesia by blocking nociceptive responses at the level of the spinal cord. It is a kappa antagonist (depending upon the kappa ligand used in the assay), which may contribute to its antihyperalgesia. The parent drug has a high affinity and low intrinsic efficacy for the mu receptor. The main metabolite, norbuprenorphine, is a delta opioid-receptor agonist.
Secondary hyperalgesia is an increased sensitivity to painful stimuli around an area of injury and occurs frequently following injury. The increased pain sensation is a result of central sensitization derived from brainstem neurons that facilitate pain; it is not derived from afferent signals from the primary site. Secondary hyperalgesia is less responsive to opioids than primary hyperalgesia at the site of injury.
Pain is improved with buprenorphine predominantly through modulation of central sensitization and less so at the primary site. Koppert et al25 demonstrated in human volunteers that buprenorphine reduced the area and duration of secondary hyperalgesia more than pain at the site of injury (half-life of 171 minutes vs 288 minutes, respectively). Buprenorphine had a much greater antihyperalgesic effect than analgesic effect compared with potent opioids such as fentanyl. In contrast, the analgesic effects with fentanyl and alfentanil were much greater than their antihyperalgesic effects (Figure), suggesting the possibility of a combination of opioid therapy for superior pain relief or choices based on pain phenotype (eg, secondary or primary hyperalgesia).
SUMMARY
Early palliative care improves quality of life and decision-making in patients with advanced lung cancer and may improve survival, although survival data need to be confirmed. Olanzapine and megestrol acetate are superior to megestrol acetate alone for the treatment of anorexia. Oxygen is no better than medical air in the management of dyspnea associated with normal oxygen saturation. Buprenorphine is a unique opioid that has value for pharmacologic relief in patients at risk for respiratory depression.
- Lutz S, Norrell R, Bertucio C, et al. Symptom frequency and severity in patients with metastatic or locally recurrent lung cancer: a prospective study using the Lung Cancer Symptom Scale in a community hospital. J Palliat Med 2001; 4:157–165.
- Perrone F, Gridelli C, Cigolari S, et al. Baseline assessment of quality of life (QOL) is a strong prognostic factor for survival of elderly patients with advanced non-small cell lung cancer (NSCLC): a secondary analysis of the MILES study [ASCO abstract 1346]. Proc Am Soc Clin Oncol 2002;21.
- Hollen PJ, Gralla RJ, Kris MG, et al. Measurement of quality of life in patients with lung cancer in multicenter trials of new therapies: psychometric assessment of the Lung Cancer Symptom Scale. Cancer 1994; 73:2087–2098.
- Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non–small-cell lung cancer. N Engl J Med 2010; 363:733–742.
- Navari RM, Einhorn LH, Loehrer PJ, et al. A phase II trial of olanzapine, dexamethasone, and palonosetron for the prevention of chemotherapy-induced nausea and vomiting: a Hoosier oncology group study [published online ahead of print March 21, 2007]. Support Care Cancer 2007; 15:1285–1291. doi: 10.1007/s00520-007-0248-5
- Navari RM, Brenner MC. Treatment of cancer-related anorexia with olanzapine and megestrol acetate: a randomized trial. Support Care Cancer 2010; 18:951–956.
- Travers J, Dudgeon DJ, Amjadi K, et al. Mechanisms of exertional dyspnea in patients with cancer. J Appl Physiol 2008; 104:57–66.
- Galbraith S, Fagan P, Perkins P, Lynch A, Booth S. Does the use of a handheld fan improve chronic dyspnea? A randomized, controlled, crossover trial. J Pain Symptom Manage 2010; 39:831–838.
- Abernethy AP, McDonald CF, Frith PA, et al. Effect of palliative oxygen versus room air in relief of breathlessness in patients with refractory dyspnoea: a double-blind, randomised controlled trial. Lancet 2010; 376:784–793.
- Sellner-Pogány T, Lahrmann H. BIPAP-mask-ventilation in terminal amyotrophic lateral sclerosis (ALS) [in German]. Wien Med Wochenschr 2009; 159:604–607.
- Van Meter ME, McKee KY, Kohlwes RJ. Efficacy and safety of tunneled pleural catheters in adults with malignant pleural effusions: a systematic review [published online ahead of print August 10, 2010]. J Gen Intern Med 2011; 26:70–76. doi: 10.1007/s11606-010-1472-0
- Shimoyama N, Shimoyama M. Nebulized furosemide as a novel treatment for dyspnea in terminal cancer patients. J Pain Symptom Manage 2002; 23:73–76.
- Papazisis KT, Kontovinis LF, Papandreou CN, et al. Brain natriuretic peptide precursor (NT-pro-BNP) levels predict for clinical benefit to sunitinib treatment in patients with metastatic renal cell carcinoma. BMC Cancer 2010; 10:489.
- Cavallazzi R, Nair A, Vasu T, Marik PE. Natriuretic peptides in acute pulmonary embolism: a systematic review [published online ahead of print July 15, 2008]. Intensive Care Med 2008; 34:2147–2156. doi: 10.1007/s00134-008-1214-5
- Chen H-J, Yu Y-H, Tu C-Y, Chen C-H, Hsia T-C, Tsai K-D. Ultrasound in peripheral pulmonary air-fluid lesions: color Doppler imaging as an aid in differentiating empyema and abscess. Chest 2009; 135:1426–1432.
- Lichtenstein DA, Mezière GA, Lagoueyte J-F, Biderman P, Goldstein I, Gepner A. A-lines and B-lines: lung ultrasound as a bedside tool for predicting pulmonary artery occlusion pressure in the critically ill. Chest 2009; 136:1014–1020.
- Lichtenstein DA. Ultrasound examination of the lungs in the intensive care unit. Pediatr Crit Care Med 2009; 10:693–698.
- Reissig A, Heyne J-P, Kroegel C. Sonography of lung and pleura in pulmonary embolism: sonomorphologic characterization and comparison with spiral CT scanning. Chest 2001; 120:1977–1983.
- Kallet RH. The role of inhaled opioids and furosemide for the treatment of dyspnea. Respir Care 2007; 52:900–910.
- Clemens KE, Quednau I, Klaschik E. Is there a higher risk of respiratory depression in opioid-naïve palliative care patients during symptomatic therapy of dyspnea with strong opioids? J Palliat Med 2008; 11:204–216.
- Varkey B. Opioids for palliation of refractory dyspnea in chronic obstructive pulmonary disease patients. Curr Opin Pulm Med 2010; 16:150–154.
- Currow DC, Plummer J, Frith P, Abernethy AP. Can we predict which patients with refractory dyspnea will respond to opioids? J Palliat Med 2007; 10:1031–1036.
- Davis MP. Buprenorphine in cancer pain. Support Care Cancer 2005; 13:878–887.
- Watson PJQ, McQuay HJ, Bullingham RES, Allen MC, Moore RA. Single-dose comparison of buprenorphine 0.3 and 0.6 mg i.v. given after operation: clinical effects and plasma concentrations. Br J Anaesth 1982; 54:37–43.
- Koppert W, Ihmsen H, Körber N, et al. Different profiles of buprenorphine-induced analgesia and antihyperalgesia in a human pain model. Pain 2005; 118:15–22.
- Sittl R, Likar R, Nautrup BP. Equipotent doses of transdermal fentanyl and transdermal buprenorphine in patients with cancer and noncancer pain: results of a retrospective cohort study. Clin Ther 2005; 27:225–237.
- Sittl R. Transdermal buprenorphine in cancer pain and palliative care. Palliat Med 2006; 20 (suppl 1):s25–s30.
Several important developments in the palliative care of patients with lung cancer have occurred over the past few years, including publication of a landmark study comparing early with as-needed palliative care, the release of new data on the treatment of cancer-related anorexia, elucidation of new mechanisms and treatment options for dyspnea, and the availability of buprenorphine. This article reviews these emerging concepts.
LUNG CANCER SYMPTOMS: COMMON AND SEVERE
The symptom burden of lung cancer is usually great. At least 80% of patients experience fatigue, 65% suffer loss of appetite, 77% have cough, 73% report dyspnea (both from local symptoms and weight loss), 57% have chest pain, and 17% have hemoptysis.1
When symptoms are present, they are usually severe. Thirty-eight percent of the patients who report fatigue have severe fatigue, 47% have inadequate appetite to the point of requiring intervention, and more than one-half of patients who have chest pain require opioids for relief.1
Symptom frequency and severity are worse in individuals who survive 3 months or less.1 Increasing symptom burden is therefore prognostically important, particularly in patients with advanced stages of lung cancer. As a result, self-assessment of quality of life has a significant ability to predict survival in patients with advanced non–small cell lung cancer (NSCLC).2
Patients with lung cancer tend to suffer from groups of symptoms or symptom clusters. Lutz et al1 found that 79% of patients reported three or more symptoms; these results were similar to the findings of a study by Hollen et al,3 in which 81% of patients suffered from three or more symptoms, all them severe except for cough.
EARLY PALLIATIVE CARE HAS CLINICAL BENEFITS
A landmark study by Temel et al4 examined the benefits of early palliative care integrated with standard oncologic care versus standard oncologic care and palliative care only “as needed” on patient-reported outcomes, the use of health services, and the quality of end-of-life care among patients with metastatic NSCLC. The study was a prospective, nonblinded, randomized, controlled trial of outpatients conducted at a single center. The intervention was based on guidelines from the National Consensus Project for Quality Palliative Care, with specific attention to symptom management, goals of care, decision-making regarding treatment, and coordination of care. Patients assigned to the intervention met monthly with both a palliative care service and an oncologist, and 90% of the patients randomized to intervention complied with at least 50% of the visits.
Measures of health-related quality of life and mood were obtained using the Functional Assessment of Cancer Therapy-Lung (FACT-L), the Hospital Anxiety and Depression Scale, and the 9-item depression scale of the Patient Health Questionnaire.
Measures of health care service utilization included use of antitumor therapy within 14 days of death, late or no referral to hospice, hospital admissions, and emergency room visits. Patients were considered to have received aggressive care if they met any one of the following three criteria: chemotherapy within 14 days of death, no hospice care, or admission to hospice within 3 days of death.
Compared with standard care, early palliative care was associated with an increase in the number of advance directives, earlier hospice referral (11 days vs 4 days), fewer hospitalizations and emergency room visits, and fewer instances of inappropriate oncologic care (defined as chemotherapy within 14 days of death). The percentage of patients with depressed mood was also lower among those assigned to early palliative care versus standard care (16% vs 38%).
A 2.7-month difference in median survival (P = .02) in favor of the group assigned to early palliative care was also observed, although survival was not a primary end point of the trial. This outcome needs to be validated in future studies.
CANCER-RELATED ANOREXIA AND CACHEXIA: TREATMENT IMPROVES APPETITE
The main hallmark of cancer-related anorexia and cachexia is weight loss; this symptom cluster is most often associated with hypophagia. The coexistence of anorexia and appetite-related anhedonia is common in lung cancer patients, such that 25% of lung cancer patients with anorexia report no distress with not eating, nor do they derive pleasure from eating. Others report that early satiety and changes in taste dramatically affect appetite. To some, anorexia is a distressful reminder of progression of their cancer.
Megestrol acetate and medroxyprogesterone acetate at least partially improve appetite in a subset of anorectic cancer patients. The use of medroxyprogesterone acetate has resulted in weight gain but not muscle mass in some patients with cancer-related anorexia, but has had less effect on fatigue and quality of life in these patients.
Olanzapine is an atypical antipsychotic with an affinity for multiple neurotransmitter receptors. Several of these, such as the serotonin receptors 5-HT2 and 5-HT3, histamine receptors, and dopamine receptors, are implicated in anorexia, nausea, and vomiting. Case reports suggest that olanzapine has antiemetic activity in patients with advanced cancer and usefulness as prophylaxis against chemotherapy-related nausea and vomiting.5 Reduced risk of extrapyramidal symptoms compared with standard antiemetics enhances the value of olanzapine for prevention of cancer-related anorexia.
Navari et al6 conducted a randomized trial to determine the effectiveness of megestrol acetate and olanzapine for the treatment of cancer-related anorexia. Eighty patients were randomized to receive oral megestrol acetate 800 mg/d, or oral megestrol acetate 800 mg/d plus olanzapine 5 mg once nightly, for 8 weeks. Patients were removed from the study if they did not take the study medication for a 48-hour period or if intolerable toxicity developed that was attributable to the study agents.
The MD Anderson Symptom Inventory (MDASI) was completed weekly to assess key symptom outcome variables. A change of 3 cm on the visual analog scale over two separate time periods for a symptom was considered sufficient to define a change in the symptom.
Quality of life was measured using a valid 28-item self-reported instrument (Functional Assessment of Cancer Therapy-General). Patients were examined by their physicians every 2 weeks.
In the group assigned to megestrol acetate, 15 patients had a weight gain of at least 5%—a change that was considered significant. Appetite improved in two patients, nausea decreased in three patients, and quality of life improved in five patients at both 4 weeks and 8 weeks. The improvements in appetite, nausea, and quality of life for the whole group on megestrol acetate alone were not significant, and there was no improvement in mean symptom scores measured by the MDASI.
There were incremental improvements of all measures in patients randomized to megestrol acetate plus olanzapine. Among patients receiving the combination, 33 had a weight gain of at least 5%; 25 reported an improvement in appetite, 21 experienced a reduction in nausea, and 23 had an improvement in quality of life at both 4 weeks and 8 weeks. All outcome variables were improved on the MDASI
CANCER AND DYSPNEA: NUMEROUS INTERVENTIONS HAVE BEEN ASSESSED
Reduced inspiratory capacity caused by weakened inspiratory muscles results in an increased Borg rating of perceived exertion (RPE) relative to oxygen levels. Both central nervous system activation of muscle and loss of muscle tissue contribute to dyspnea and fatigue in lung cancer patients.7 Cancer fatigue, also measured by the Borg RPE scale, appears to be a “central” mechanism that stems from a mismatch between efferent output for afferent inputs in the setting of ventilatory muscle weakness, thereby increasing the perception of dyspnea. Several interventions have been used to relieve dyspnea, ranging from oxygen therapy to treatment with opioids.
Oxygen saturation
The association between hypoxemia and dyspnea is poor.8 In a randomized prospective trial, Abernethy et al9 found no benefit to oxygen therapy compared with medical air without added supplemental oxygen in individuals who had normal oxygen saturation but symptomatic dyspnea.
Bilevel positive airway pressure
Bilevel positive airway pressure has been shown to reduce the need for invasive ventilation; improve oxygen saturation; and reduce dynamic hyperinflation, thus relieving dyspnea.10 It has been effective in dyspneic patients with motor neuron disease, cancer, heart failure, status asthmaticus, stroke, drug overdose, and interstitial lung disease.
Indwelling pleural catheters
Tunneled pleural catheters reduce the severity of dyspnea in 95% of patients.11 These catheters are inserted on an outpatient basis, allowing for outpatient drainage. Autopleurodesis occurs in about 45% of patients, in which case the catheter can be removed. Adverse reactions are few (incidence < 10%), but consist of empyema, pneumothorax, cellulitis, or catheter obstruction. The disadvantage is the expense of catheter maintenance.
Nebulized furosemide
Case reports suggest that inhalation of nebulized furosemide, 20 mg four times daily, dramatically improves dyspnea in patients with advanced cancer and severe shortness of breath that is unresponsive to opioids.12 Nebulized furosemide appears to have a direct effect on either pulmonary stretch receptors or irritant receptors in the airways; it also has a diuretic effect. Response occurs quickly with an onset of effect in 20 to 30 minutes.
B-type natriuretic peptide
The level of N-terminal precursor of B-type natriuretic peptide (NT-pro-BNP) can predict response to sunitinib in renal cancer,13 and the BNP level predicts 30-day mortality in pulmonary embolism.14 Measurement of BNP to detect dyspnea in patients with lung cancer is not useful, however, because the BNP level increases with cardiac and pericardial metastases. The BNP level is also persistently elevated after chest radiation therapy, and it increases with anthracycline cardiotoxicity. It is not a useful marker for distinguishing pulmonary from nonpulmonary or cardiac from noncardiac causes of dyspnea.
Lung ultrasound
Portable diagnostic lung ultrasound can be used to detect pneumonia, pleural effusions, pulmonary emboli, pneumothorax, atelectasis, and lung abscesses as potential causes of dyspnea.15–18 In addition to the advantage of portability, there is no radiation exposure and the technology permits echocardiography to be conducted.
Opioids
Evidence supports opioids for pharmacologic relief of dyspnea in the palliative care of patients with chronic obstructive pulmonary disease and cancer. Studies have been conducted with morphine sulfate, hydromorphone, dihydrocodeine, intranasal and transmucosal fentanyl, oxycodone, and diamorphine.19–21
The response to opioids is unrelated to the severity of dyspnea.22 Responses and safe administration occur even in patients with reduced oxygen saturation or elevated carbon dioxide partial pressure.20 Opioids can be used safely in the opioid-naïve population.20 Recommended dosages in these patients are 2.5 to 5.0 mg of morphine sulfate every 4 hours, 5 mg of oxycodone every 4 hours as needed, and 1 mg of hydromorphone every 4 hours in the opioid-naïve. In opioid-tolerant patients, it is recommended that therapy start with these doses and then be increased in 25% increments every 24 hours, as needed.
BUPRENORPHINE: UNIQUE OPIOID
Buprenorphine is a mu- and nociceptin (ORL-1)-receptor partial agonist with intravenous, subcutaneous, sublingual, transdermal, and intranasal routes of delivery.23 An agent that acts as an ORL-1 agonist can induce analgesia by blocking nociceptive responses at the level of the spinal cord. It is a kappa antagonist (depending upon the kappa ligand used in the assay), which may contribute to its antihyperalgesia. The parent drug has a high affinity and low intrinsic efficacy for the mu receptor. The main metabolite, norbuprenorphine, is a delta opioid-receptor agonist.
Secondary hyperalgesia is an increased sensitivity to painful stimuli around an area of injury and occurs frequently following injury. The increased pain sensation is a result of central sensitization derived from brainstem neurons that facilitate pain; it is not derived from afferent signals from the primary site. Secondary hyperalgesia is less responsive to opioids than primary hyperalgesia at the site of injury.
Pain is improved with buprenorphine predominantly through modulation of central sensitization and less so at the primary site. Koppert et al25 demonstrated in human volunteers that buprenorphine reduced the area and duration of secondary hyperalgesia more than pain at the site of injury (half-life of 171 minutes vs 288 minutes, respectively). Buprenorphine had a much greater antihyperalgesic effect than analgesic effect compared with potent opioids such as fentanyl. In contrast, the analgesic effects with fentanyl and alfentanil were much greater than their antihyperalgesic effects (Figure), suggesting the possibility of a combination of opioid therapy for superior pain relief or choices based on pain phenotype (eg, secondary or primary hyperalgesia).
SUMMARY
Early palliative care improves quality of life and decision-making in patients with advanced lung cancer and may improve survival, although survival data need to be confirmed. Olanzapine and megestrol acetate are superior to megestrol acetate alone for the treatment of anorexia. Oxygen is no better than medical air in the management of dyspnea associated with normal oxygen saturation. Buprenorphine is a unique opioid that has value for pharmacologic relief in patients at risk for respiratory depression.
Several important developments in the palliative care of patients with lung cancer have occurred over the past few years, including publication of a landmark study comparing early with as-needed palliative care, the release of new data on the treatment of cancer-related anorexia, elucidation of new mechanisms and treatment options for dyspnea, and the availability of buprenorphine. This article reviews these emerging concepts.
LUNG CANCER SYMPTOMS: COMMON AND SEVERE
The symptom burden of lung cancer is usually great. At least 80% of patients experience fatigue, 65% suffer loss of appetite, 77% have cough, 73% report dyspnea (both from local symptoms and weight loss), 57% have chest pain, and 17% have hemoptysis.1
When symptoms are present, they are usually severe. Thirty-eight percent of the patients who report fatigue have severe fatigue, 47% have inadequate appetite to the point of requiring intervention, and more than one-half of patients who have chest pain require opioids for relief.1
Symptom frequency and severity are worse in individuals who survive 3 months or less.1 Increasing symptom burden is therefore prognostically important, particularly in patients with advanced stages of lung cancer. As a result, self-assessment of quality of life has a significant ability to predict survival in patients with advanced non–small cell lung cancer (NSCLC).2
Patients with lung cancer tend to suffer from groups of symptoms or symptom clusters. Lutz et al1 found that 79% of patients reported three or more symptoms; these results were similar to the findings of a study by Hollen et al,3 in which 81% of patients suffered from three or more symptoms, all them severe except for cough.
EARLY PALLIATIVE CARE HAS CLINICAL BENEFITS
A landmark study by Temel et al4 examined the benefits of early palliative care integrated with standard oncologic care versus standard oncologic care and palliative care only “as needed” on patient-reported outcomes, the use of health services, and the quality of end-of-life care among patients with metastatic NSCLC. The study was a prospective, nonblinded, randomized, controlled trial of outpatients conducted at a single center. The intervention was based on guidelines from the National Consensus Project for Quality Palliative Care, with specific attention to symptom management, goals of care, decision-making regarding treatment, and coordination of care. Patients assigned to the intervention met monthly with both a palliative care service and an oncologist, and 90% of the patients randomized to intervention complied with at least 50% of the visits.
Measures of health-related quality of life and mood were obtained using the Functional Assessment of Cancer Therapy-Lung (FACT-L), the Hospital Anxiety and Depression Scale, and the 9-item depression scale of the Patient Health Questionnaire.
Measures of health care service utilization included use of antitumor therapy within 14 days of death, late or no referral to hospice, hospital admissions, and emergency room visits. Patients were considered to have received aggressive care if they met any one of the following three criteria: chemotherapy within 14 days of death, no hospice care, or admission to hospice within 3 days of death.
Compared with standard care, early palliative care was associated with an increase in the number of advance directives, earlier hospice referral (11 days vs 4 days), fewer hospitalizations and emergency room visits, and fewer instances of inappropriate oncologic care (defined as chemotherapy within 14 days of death). The percentage of patients with depressed mood was also lower among those assigned to early palliative care versus standard care (16% vs 38%).
A 2.7-month difference in median survival (P = .02) in favor of the group assigned to early palliative care was also observed, although survival was not a primary end point of the trial. This outcome needs to be validated in future studies.
CANCER-RELATED ANOREXIA AND CACHEXIA: TREATMENT IMPROVES APPETITE
The main hallmark of cancer-related anorexia and cachexia is weight loss; this symptom cluster is most often associated with hypophagia. The coexistence of anorexia and appetite-related anhedonia is common in lung cancer patients, such that 25% of lung cancer patients with anorexia report no distress with not eating, nor do they derive pleasure from eating. Others report that early satiety and changes in taste dramatically affect appetite. To some, anorexia is a distressful reminder of progression of their cancer.
Megestrol acetate and medroxyprogesterone acetate at least partially improve appetite in a subset of anorectic cancer patients. The use of medroxyprogesterone acetate has resulted in weight gain but not muscle mass in some patients with cancer-related anorexia, but has had less effect on fatigue and quality of life in these patients.
Olanzapine is an atypical antipsychotic with an affinity for multiple neurotransmitter receptors. Several of these, such as the serotonin receptors 5-HT2 and 5-HT3, histamine receptors, and dopamine receptors, are implicated in anorexia, nausea, and vomiting. Case reports suggest that olanzapine has antiemetic activity in patients with advanced cancer and usefulness as prophylaxis against chemotherapy-related nausea and vomiting.5 Reduced risk of extrapyramidal symptoms compared with standard antiemetics enhances the value of olanzapine for prevention of cancer-related anorexia.
Navari et al6 conducted a randomized trial to determine the effectiveness of megestrol acetate and olanzapine for the treatment of cancer-related anorexia. Eighty patients were randomized to receive oral megestrol acetate 800 mg/d, or oral megestrol acetate 800 mg/d plus olanzapine 5 mg once nightly, for 8 weeks. Patients were removed from the study if they did not take the study medication for a 48-hour period or if intolerable toxicity developed that was attributable to the study agents.
The MD Anderson Symptom Inventory (MDASI) was completed weekly to assess key symptom outcome variables. A change of 3 cm on the visual analog scale over two separate time periods for a symptom was considered sufficient to define a change in the symptom.
Quality of life was measured using a valid 28-item self-reported instrument (Functional Assessment of Cancer Therapy-General). Patients were examined by their physicians every 2 weeks.
In the group assigned to megestrol acetate, 15 patients had a weight gain of at least 5%—a change that was considered significant. Appetite improved in two patients, nausea decreased in three patients, and quality of life improved in five patients at both 4 weeks and 8 weeks. The improvements in appetite, nausea, and quality of life for the whole group on megestrol acetate alone were not significant, and there was no improvement in mean symptom scores measured by the MDASI.
There were incremental improvements of all measures in patients randomized to megestrol acetate plus olanzapine. Among patients receiving the combination, 33 had a weight gain of at least 5%; 25 reported an improvement in appetite, 21 experienced a reduction in nausea, and 23 had an improvement in quality of life at both 4 weeks and 8 weeks. All outcome variables were improved on the MDASI
CANCER AND DYSPNEA: NUMEROUS INTERVENTIONS HAVE BEEN ASSESSED
Reduced inspiratory capacity caused by weakened inspiratory muscles results in an increased Borg rating of perceived exertion (RPE) relative to oxygen levels. Both central nervous system activation of muscle and loss of muscle tissue contribute to dyspnea and fatigue in lung cancer patients.7 Cancer fatigue, also measured by the Borg RPE scale, appears to be a “central” mechanism that stems from a mismatch between efferent output for afferent inputs in the setting of ventilatory muscle weakness, thereby increasing the perception of dyspnea. Several interventions have been used to relieve dyspnea, ranging from oxygen therapy to treatment with opioids.
Oxygen saturation
The association between hypoxemia and dyspnea is poor.8 In a randomized prospective trial, Abernethy et al9 found no benefit to oxygen therapy compared with medical air without added supplemental oxygen in individuals who had normal oxygen saturation but symptomatic dyspnea.
Bilevel positive airway pressure
Bilevel positive airway pressure has been shown to reduce the need for invasive ventilation; improve oxygen saturation; and reduce dynamic hyperinflation, thus relieving dyspnea.10 It has been effective in dyspneic patients with motor neuron disease, cancer, heart failure, status asthmaticus, stroke, drug overdose, and interstitial lung disease.
Indwelling pleural catheters
Tunneled pleural catheters reduce the severity of dyspnea in 95% of patients.11 These catheters are inserted on an outpatient basis, allowing for outpatient drainage. Autopleurodesis occurs in about 45% of patients, in which case the catheter can be removed. Adverse reactions are few (incidence < 10%), but consist of empyema, pneumothorax, cellulitis, or catheter obstruction. The disadvantage is the expense of catheter maintenance.
Nebulized furosemide
Case reports suggest that inhalation of nebulized furosemide, 20 mg four times daily, dramatically improves dyspnea in patients with advanced cancer and severe shortness of breath that is unresponsive to opioids.12 Nebulized furosemide appears to have a direct effect on either pulmonary stretch receptors or irritant receptors in the airways; it also has a diuretic effect. Response occurs quickly with an onset of effect in 20 to 30 minutes.
B-type natriuretic peptide
The level of N-terminal precursor of B-type natriuretic peptide (NT-pro-BNP) can predict response to sunitinib in renal cancer,13 and the BNP level predicts 30-day mortality in pulmonary embolism.14 Measurement of BNP to detect dyspnea in patients with lung cancer is not useful, however, because the BNP level increases with cardiac and pericardial metastases. The BNP level is also persistently elevated after chest radiation therapy, and it increases with anthracycline cardiotoxicity. It is not a useful marker for distinguishing pulmonary from nonpulmonary or cardiac from noncardiac causes of dyspnea.
Lung ultrasound
Portable diagnostic lung ultrasound can be used to detect pneumonia, pleural effusions, pulmonary emboli, pneumothorax, atelectasis, and lung abscesses as potential causes of dyspnea.15–18 In addition to the advantage of portability, there is no radiation exposure and the technology permits echocardiography to be conducted.
Opioids
Evidence supports opioids for pharmacologic relief of dyspnea in the palliative care of patients with chronic obstructive pulmonary disease and cancer. Studies have been conducted with morphine sulfate, hydromorphone, dihydrocodeine, intranasal and transmucosal fentanyl, oxycodone, and diamorphine.19–21
The response to opioids is unrelated to the severity of dyspnea.22 Responses and safe administration occur even in patients with reduced oxygen saturation or elevated carbon dioxide partial pressure.20 Opioids can be used safely in the opioid-naïve population.20 Recommended dosages in these patients are 2.5 to 5.0 mg of morphine sulfate every 4 hours, 5 mg of oxycodone every 4 hours as needed, and 1 mg of hydromorphone every 4 hours in the opioid-naïve. In opioid-tolerant patients, it is recommended that therapy start with these doses and then be increased in 25% increments every 24 hours, as needed.
BUPRENORPHINE: UNIQUE OPIOID
Buprenorphine is a mu- and nociceptin (ORL-1)-receptor partial agonist with intravenous, subcutaneous, sublingual, transdermal, and intranasal routes of delivery.23 An agent that acts as an ORL-1 agonist can induce analgesia by blocking nociceptive responses at the level of the spinal cord. It is a kappa antagonist (depending upon the kappa ligand used in the assay), which may contribute to its antihyperalgesia. The parent drug has a high affinity and low intrinsic efficacy for the mu receptor. The main metabolite, norbuprenorphine, is a delta opioid-receptor agonist.
Secondary hyperalgesia is an increased sensitivity to painful stimuli around an area of injury and occurs frequently following injury. The increased pain sensation is a result of central sensitization derived from brainstem neurons that facilitate pain; it is not derived from afferent signals from the primary site. Secondary hyperalgesia is less responsive to opioids than primary hyperalgesia at the site of injury.
Pain is improved with buprenorphine predominantly through modulation of central sensitization and less so at the primary site. Koppert et al25 demonstrated in human volunteers that buprenorphine reduced the area and duration of secondary hyperalgesia more than pain at the site of injury (half-life of 171 minutes vs 288 minutes, respectively). Buprenorphine had a much greater antihyperalgesic effect than analgesic effect compared with potent opioids such as fentanyl. In contrast, the analgesic effects with fentanyl and alfentanil were much greater than their antihyperalgesic effects (Figure), suggesting the possibility of a combination of opioid therapy for superior pain relief or choices based on pain phenotype (eg, secondary or primary hyperalgesia).
SUMMARY
Early palliative care improves quality of life and decision-making in patients with advanced lung cancer and may improve survival, although survival data need to be confirmed. Olanzapine and megestrol acetate are superior to megestrol acetate alone for the treatment of anorexia. Oxygen is no better than medical air in the management of dyspnea associated with normal oxygen saturation. Buprenorphine is a unique opioid that has value for pharmacologic relief in patients at risk for respiratory depression.
- Lutz S, Norrell R, Bertucio C, et al. Symptom frequency and severity in patients with metastatic or locally recurrent lung cancer: a prospective study using the Lung Cancer Symptom Scale in a community hospital. J Palliat Med 2001; 4:157–165.
- Perrone F, Gridelli C, Cigolari S, et al. Baseline assessment of quality of life (QOL) is a strong prognostic factor for survival of elderly patients with advanced non-small cell lung cancer (NSCLC): a secondary analysis of the MILES study [ASCO abstract 1346]. Proc Am Soc Clin Oncol 2002;21.
- Hollen PJ, Gralla RJ, Kris MG, et al. Measurement of quality of life in patients with lung cancer in multicenter trials of new therapies: psychometric assessment of the Lung Cancer Symptom Scale. Cancer 1994; 73:2087–2098.
- Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non–small-cell lung cancer. N Engl J Med 2010; 363:733–742.
- Navari RM, Einhorn LH, Loehrer PJ, et al. A phase II trial of olanzapine, dexamethasone, and palonosetron for the prevention of chemotherapy-induced nausea and vomiting: a Hoosier oncology group study [published online ahead of print March 21, 2007]. Support Care Cancer 2007; 15:1285–1291. doi: 10.1007/s00520-007-0248-5
- Navari RM, Brenner MC. Treatment of cancer-related anorexia with olanzapine and megestrol acetate: a randomized trial. Support Care Cancer 2010; 18:951–956.
- Travers J, Dudgeon DJ, Amjadi K, et al. Mechanisms of exertional dyspnea in patients with cancer. J Appl Physiol 2008; 104:57–66.
- Galbraith S, Fagan P, Perkins P, Lynch A, Booth S. Does the use of a handheld fan improve chronic dyspnea? A randomized, controlled, crossover trial. J Pain Symptom Manage 2010; 39:831–838.
- Abernethy AP, McDonald CF, Frith PA, et al. Effect of palliative oxygen versus room air in relief of breathlessness in patients with refractory dyspnoea: a double-blind, randomised controlled trial. Lancet 2010; 376:784–793.
- Sellner-Pogány T, Lahrmann H. BIPAP-mask-ventilation in terminal amyotrophic lateral sclerosis (ALS) [in German]. Wien Med Wochenschr 2009; 159:604–607.
- Van Meter ME, McKee KY, Kohlwes RJ. Efficacy and safety of tunneled pleural catheters in adults with malignant pleural effusions: a systematic review [published online ahead of print August 10, 2010]. J Gen Intern Med 2011; 26:70–76. doi: 10.1007/s11606-010-1472-0
- Shimoyama N, Shimoyama M. Nebulized furosemide as a novel treatment for dyspnea in terminal cancer patients. J Pain Symptom Manage 2002; 23:73–76.
- Papazisis KT, Kontovinis LF, Papandreou CN, et al. Brain natriuretic peptide precursor (NT-pro-BNP) levels predict for clinical benefit to sunitinib treatment in patients with metastatic renal cell carcinoma. BMC Cancer 2010; 10:489.
- Cavallazzi R, Nair A, Vasu T, Marik PE. Natriuretic peptides in acute pulmonary embolism: a systematic review [published online ahead of print July 15, 2008]. Intensive Care Med 2008; 34:2147–2156. doi: 10.1007/s00134-008-1214-5
- Chen H-J, Yu Y-H, Tu C-Y, Chen C-H, Hsia T-C, Tsai K-D. Ultrasound in peripheral pulmonary air-fluid lesions: color Doppler imaging as an aid in differentiating empyema and abscess. Chest 2009; 135:1426–1432.
- Lichtenstein DA, Mezière GA, Lagoueyte J-F, Biderman P, Goldstein I, Gepner A. A-lines and B-lines: lung ultrasound as a bedside tool for predicting pulmonary artery occlusion pressure in the critically ill. Chest 2009; 136:1014–1020.
- Lichtenstein DA. Ultrasound examination of the lungs in the intensive care unit. Pediatr Crit Care Med 2009; 10:693–698.
- Reissig A, Heyne J-P, Kroegel C. Sonography of lung and pleura in pulmonary embolism: sonomorphologic characterization and comparison with spiral CT scanning. Chest 2001; 120:1977–1983.
- Kallet RH. The role of inhaled opioids and furosemide for the treatment of dyspnea. Respir Care 2007; 52:900–910.
- Clemens KE, Quednau I, Klaschik E. Is there a higher risk of respiratory depression in opioid-naïve palliative care patients during symptomatic therapy of dyspnea with strong opioids? J Palliat Med 2008; 11:204–216.
- Varkey B. Opioids for palliation of refractory dyspnea in chronic obstructive pulmonary disease patients. Curr Opin Pulm Med 2010; 16:150–154.
- Currow DC, Plummer J, Frith P, Abernethy AP. Can we predict which patients with refractory dyspnea will respond to opioids? J Palliat Med 2007; 10:1031–1036.
- Davis MP. Buprenorphine in cancer pain. Support Care Cancer 2005; 13:878–887.
- Watson PJQ, McQuay HJ, Bullingham RES, Allen MC, Moore RA. Single-dose comparison of buprenorphine 0.3 and 0.6 mg i.v. given after operation: clinical effects and plasma concentrations. Br J Anaesth 1982; 54:37–43.
- Koppert W, Ihmsen H, Körber N, et al. Different profiles of buprenorphine-induced analgesia and antihyperalgesia in a human pain model. Pain 2005; 118:15–22.
- Sittl R, Likar R, Nautrup BP. Equipotent doses of transdermal fentanyl and transdermal buprenorphine in patients with cancer and noncancer pain: results of a retrospective cohort study. Clin Ther 2005; 27:225–237.
- Sittl R. Transdermal buprenorphine in cancer pain and palliative care. Palliat Med 2006; 20 (suppl 1):s25–s30.
- Lutz S, Norrell R, Bertucio C, et al. Symptom frequency and severity in patients with metastatic or locally recurrent lung cancer: a prospective study using the Lung Cancer Symptom Scale in a community hospital. J Palliat Med 2001; 4:157–165.
- Perrone F, Gridelli C, Cigolari S, et al. Baseline assessment of quality of life (QOL) is a strong prognostic factor for survival of elderly patients with advanced non-small cell lung cancer (NSCLC): a secondary analysis of the MILES study [ASCO abstract 1346]. Proc Am Soc Clin Oncol 2002;21.
- Hollen PJ, Gralla RJ, Kris MG, et al. Measurement of quality of life in patients with lung cancer in multicenter trials of new therapies: psychometric assessment of the Lung Cancer Symptom Scale. Cancer 1994; 73:2087–2098.
- Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non–small-cell lung cancer. N Engl J Med 2010; 363:733–742.
- Navari RM, Einhorn LH, Loehrer PJ, et al. A phase II trial of olanzapine, dexamethasone, and palonosetron for the prevention of chemotherapy-induced nausea and vomiting: a Hoosier oncology group study [published online ahead of print March 21, 2007]. Support Care Cancer 2007; 15:1285–1291. doi: 10.1007/s00520-007-0248-5
- Navari RM, Brenner MC. Treatment of cancer-related anorexia with olanzapine and megestrol acetate: a randomized trial. Support Care Cancer 2010; 18:951–956.
- Travers J, Dudgeon DJ, Amjadi K, et al. Mechanisms of exertional dyspnea in patients with cancer. J Appl Physiol 2008; 104:57–66.
- Galbraith S, Fagan P, Perkins P, Lynch A, Booth S. Does the use of a handheld fan improve chronic dyspnea? A randomized, controlled, crossover trial. J Pain Symptom Manage 2010; 39:831–838.
- Abernethy AP, McDonald CF, Frith PA, et al. Effect of palliative oxygen versus room air in relief of breathlessness in patients with refractory dyspnoea: a double-blind, randomised controlled trial. Lancet 2010; 376:784–793.
- Sellner-Pogány T, Lahrmann H. BIPAP-mask-ventilation in terminal amyotrophic lateral sclerosis (ALS) [in German]. Wien Med Wochenschr 2009; 159:604–607.
- Van Meter ME, McKee KY, Kohlwes RJ. Efficacy and safety of tunneled pleural catheters in adults with malignant pleural effusions: a systematic review [published online ahead of print August 10, 2010]. J Gen Intern Med 2011; 26:70–76. doi: 10.1007/s11606-010-1472-0
- Shimoyama N, Shimoyama M. Nebulized furosemide as a novel treatment for dyspnea in terminal cancer patients. J Pain Symptom Manage 2002; 23:73–76.
- Papazisis KT, Kontovinis LF, Papandreou CN, et al. Brain natriuretic peptide precursor (NT-pro-BNP) levels predict for clinical benefit to sunitinib treatment in patients with metastatic renal cell carcinoma. BMC Cancer 2010; 10:489.
- Cavallazzi R, Nair A, Vasu T, Marik PE. Natriuretic peptides in acute pulmonary embolism: a systematic review [published online ahead of print July 15, 2008]. Intensive Care Med 2008; 34:2147–2156. doi: 10.1007/s00134-008-1214-5
- Chen H-J, Yu Y-H, Tu C-Y, Chen C-H, Hsia T-C, Tsai K-D. Ultrasound in peripheral pulmonary air-fluid lesions: color Doppler imaging as an aid in differentiating empyema and abscess. Chest 2009; 135:1426–1432.
- Lichtenstein DA, Mezière GA, Lagoueyte J-F, Biderman P, Goldstein I, Gepner A. A-lines and B-lines: lung ultrasound as a bedside tool for predicting pulmonary artery occlusion pressure in the critically ill. Chest 2009; 136:1014–1020.
- Lichtenstein DA. Ultrasound examination of the lungs in the intensive care unit. Pediatr Crit Care Med 2009; 10:693–698.
- Reissig A, Heyne J-P, Kroegel C. Sonography of lung and pleura in pulmonary embolism: sonomorphologic characterization and comparison with spiral CT scanning. Chest 2001; 120:1977–1983.
- Kallet RH. The role of inhaled opioids and furosemide for the treatment of dyspnea. Respir Care 2007; 52:900–910.
- Clemens KE, Quednau I, Klaschik E. Is there a higher risk of respiratory depression in opioid-naïve palliative care patients during symptomatic therapy of dyspnea with strong opioids? J Palliat Med 2008; 11:204–216.
- Varkey B. Opioids for palliation of refractory dyspnea in chronic obstructive pulmonary disease patients. Curr Opin Pulm Med 2010; 16:150–154.
- Currow DC, Plummer J, Frith P, Abernethy AP. Can we predict which patients with refractory dyspnea will respond to opioids? J Palliat Med 2007; 10:1031–1036.
- Davis MP. Buprenorphine in cancer pain. Support Care Cancer 2005; 13:878–887.
- Watson PJQ, McQuay HJ, Bullingham RES, Allen MC, Moore RA. Single-dose comparison of buprenorphine 0.3 and 0.6 mg i.v. given after operation: clinical effects and plasma concentrations. Br J Anaesth 1982; 54:37–43.
- Koppert W, Ihmsen H, Körber N, et al. Different profiles of buprenorphine-induced analgesia and antihyperalgesia in a human pain model. Pain 2005; 118:15–22.
- Sittl R, Likar R, Nautrup BP. Equipotent doses of transdermal fentanyl and transdermal buprenorphine in patients with cancer and noncancer pain: results of a retrospective cohort study. Clin Ther 2005; 27:225–237.
- Sittl R. Transdermal buprenorphine in cancer pain and palliative care. Palliat Med 2006; 20 (suppl 1):s25–s30.
Malignant bowel obstruction: Individualized treatment near the end of life
Malignant bowel obstruction occurs in 5% to 51% of women with ovarian cancer and in 10% to 28% of patients with gastrointestinal cancer, predominantly in the advanced stages.1 Median survival after its onset ranges from 30 to 90 days.2–5
Its symptoms are challenging to manage, since nausea, vomiting, colic, and abdominal pain, which are common, cause significant physical distress and demoralization. The decision whether to correct it with surgery requires an individualized approach and a clear understanding of the goals of care and expected survival in the individual patient.
This review focuses on the management of inoperable malignant bowel obstruction and includes discussion of hydration, nutrition, and endoscopic palliative options.
WHAT ARE THE DIFFERENT TYPES OF OBSTRUCTION?
Bowel obstruction may be mechanical or functional, partial or complete, and may occur at one or at many sites. Tumors can impair bowel function in several ways6–8:
- Intraluminal tumors can occlude the lumen or act as a point of intussusception.
- Intramural tumors can extend to the mucosa and obstruct the lumen or impair peristalsis.
- Mesenteric and omental masses or malignant adhesions can kink or angulate the bowel, creating an extramural obstruction.
- Tumors that infiltrate into the mesentery, bowel muscle, or the enteric or celiac plexus can cause dysmotility.
Cholangiocarcinoma, pancreatic carcinoma, and gallbladder carcinoma are the most common tumors causing duodenal obstruction.9 Distal obstruction is caused mainly by colon and ovarian cancer.
Obstruction can be due to treatment
In a minority of patients, obstruction is unrelated to the cancer and is instead due to adhesions arising from surgery, radiation therapy (causing enteritis and strictures), desmoplastic reactions to intraperitoneal chemotherapy, torsion, or internal hernias.10–12
In rare cases, a patient has intestinal pseudo-obstruction from paraneoplastic destruction of enteric neurons, or severe ileus from anticholinergic or sympathomimetic drugs, as seen with acute colonic pseudo-obstruction (Ogilvie syndrome).13
Physiologic reactions to obstruction
Malignant bowel obstruction stimulates gastric, biliary, pancreatic, and intestinal secretions, decreases intraluminal sodium and water reabsorption, and increases mucosal sodium and water secretion.6,14 In response to the obstruction, peristalsis increases, and prostaglandin, vasoactive intestinal peptide, and nociceptive mediators are released. Vasoactive intestinal polypeptide perpetuates a cycle of secretion, distention, and contraction that leads to intestinal hyperemia, bowel edema, and accumulation of fluid in the lumen.8,10,11,15
Signs and symptoms depend on the site
The site of obstruction determines the signs and symptoms patients experience.7,14 Obstructions high in the gastrointestinal tract are associated with greater symptoms but fewer signs than colonic obstructions.1 Patients with proximal small-bowel obstruction have more severe nausea and a greater number of episodes of emesis, but they have relatively normal plain radiographs of the abdomen, which do not have the characteristic air-fluid levels commonly seen with distal small-bowel obstruction.
Most malignant obstructions remain partial, but increasing abdominal distention, worsening nausea, vomiting, abdominal pain, and obstipation over 1 to 2 weeks1 suggest progression to complete obstruction.
IMAGING TESTS FOR MALIGNANT BOWEL OBSTRUCTION
What is the value of plain radiography?
Despite these limitations, plain radiography is useful in assessing constipation and its severity as a potential cause of symptoms, and thus it remains an important initial imaging study in almost all patients with suspected malignant bowel obstruction.17,18 It is also used to assess response to treatment.
When do you need contrast radiographs?
Contrast radiography (barium swallow or barium or Gastrograffin enema) is helpful in patients with symptoms of dysmotility from suspected bowel obstruction. It defines the site or sites of obstruction and the extent of the obstruction with a fair degree of accuracy.7,19 Single-contrast studies, if positive, exclude opioid-induced bowel dysfunction or pseudo-obstruction in 83% of patients, with a sensitivity and specificity of 96% and 98%, respectively.8,20 Small-bowel follow-through with barium is more appropriate for low-grade obstructions or for symptomatic patients with a normal kidney, ureter, bladder radiograph.19
However, contrast radiography is limited by the patient’s ability to swallow barium or water-soluble contrast agents, and it can worsen nausea or vomiting.17,18 Also, barium is not absorbed systemically and may interfere with subsequent radiologic studies. Large volumes of contrast agents increase the risk of aspiration pneumonia in patients with poorly controlled nausea and can lead to severe impaction proximal to the obstructed site.8
Is enteroclysis better than barium swallow?
Enteroclysis, ie, injecting radiographic contrast into the bowel via a nasoduodenal tube, has some advantages over the barium swallow technique for detecting partial small-bowel obstruction, since it bypasses the stomach and allows for therapeutic decompression as well as direct visualization of the area of concern.17,18 Enteroclysis radiography objectively gauges severity of intestinal obstruction and bowel wall distensibility, which is an advantage over other imaging studies. Its sensitivity is 100% and specificity 88% in experienced hands.17 Enteroclysis studies also detect nonobstructing intraluminal tumors when computed tomography (CT) is not diagnostic.17,18,21
The drawbacks to enteroclysis are that it is technically difficult to perform and that few radiologists are trained in it.
When is CT useful?
CT is the primary imaging study for patients with obstructive symptoms and a history of abdominal malignancy or a palpable abdominal mass17,20,22,23 (Figure 1). It has a specificity of 100% and a sensitivity of 94%. It plays a major role in decision-making regarding surgery, endoscopy, or palliative interventions,7,19 as it locates the obstruction and differentiates benign from malignant causes with a fair degree of precision.22
CT findings in malignant bowel obstruction may include:
- A mass at the site of obstruction or within the original surgical field
- Lymphadenopathy
- Abrupt transitions in luminal diameter or irregular thickening of the bowel wall at the site or sites of obstruction.7
SURGERY: A DIFFICULT DECISION
Is the patient fit for surgery?
Surgery for malignant bowel obstruction should not be done in patients who have advanced malignancies with bulky intra-abdominal metastases or cancer that has spread outside the abdominal cavity without taking into account treatment options for the cancer, the patient’s nutritional status, and the goals of care.
The role of abdominal surgery (debulking, resection, or bypass) in advanced cancer remains unclear and controversial.24 From 42% to 80% of patients report that symptoms improve after surgery, but recurrent obstruction occurs in 10% to 50%.10 Even in patients with low tumor bulk and good nutritional status, 30-day mortality rates range from 5% to 40%, and complication rates range from 9% to 90%.3,4,6,7,10,14
Outcomes after surgery depend on patient selection criteria perhaps as much as on the surgeon’s experience and skill. Patients with more advanced cancer who have had multiple surgical procedures and those with cancer that does not respond to chemotherapy and radiation present the greatest challenge to surgeons.23
What is the benefit of surgery?
Reports of palliative surgery have included information about 60-day survival rates after the operation, but a number of factors may be more meaningful in this context, such as postoperative symptoms, the patient’s overall wellbeing, how the original symptoms respond to the surgery, complications, and length of hospitalization.14 The paucity of published, validated, patient-related outcome data on which to gauge the value of surgery and the lack of a standard definition of “benefit” further confuse the objective determination of whether these patients benefit from surgery.
In a cohort with advanced ovarian cancer and bowel obstruction, surgery was detrimental to survival and quality of life for all subgroups, and most patients died in the hospital.6
The risk of surgery for malignant bowel obstruction is presumably higher than for abdominal surgery for other indications, since many of the patients are debilitated from their cancer and chemotherapy, and many are malnourished.23 Even when taking into account a potential selection bias in favor of surgery, several studies have reported no significant difference in 30-day mortality rates or median survival between operative and nonoperative groups.2,12 Neither the type of obstruction nor the extent of the surgery influenced outcomes. Surgical outcomes are best in patients with a benign cause of obstruction; little benefit is seen in operating on those with abdominal carcinomatosis.12
Nevertheless, surgery is beneficial in a select few. For patients with a good performance status, slowly progressive cancer, and an expected survival of more than 6 months, surgical bypass or resection is preferred.7,12,25 The challenge is to identify these surgical candidates, taking into account prognostic factors such as nutritional status, tumor burden, performance status, presence of ascites, advanced age, extensive prior chemotherapy or radiotherapy, and diffuse carcinomatosis.3,10,12,20,23
Is surgery consistent with the goals of care?
Crucial to decision-making are the goals of care. Since palliative surgery carries a low level of evidence for benefit in terms of quality of life and survival, time should be set aside to thoroughly review the patient’s medical condition, to explore options, and to clarify expectations and goals of care.3,10 Family members should be invited to be present during these discussions and to be involved in the decision-making process.
WHAT IS THE BENEFIT OF GASTRIC OR COLONIC STENTING?
Endoscopic procedures are alternatives to surgery and offer a palliative option in malignant bowel obstruction. Endoscopic procedures are associated with a shorter hospital stay and quicker recovery than after laparotomy.9,26–30 In certain situations, stenting serves as a bridge to surgery, allowing time to mitigate comorbid conditions, to enhance nutrition, and to complete staging, while relieving symptoms.27–29,31,32 Definitive surgery can be done as a single-stage procedure without a diverting enterostomy.
Self-expanding metal stents for gastric outlet, small-bowel, and colonic obstructions are an option in patients who have incurable metastatic disease who are unfit for surgery, in patients with a single point of obstruction or locally extensive disease, or in patients who do not want to undergo laparotomy.28–30
Technical and clinical success rates for colorectal stenting are high (88% to 93%).26,27 Stenting is more successful for left-sided colonic obstructions than for proximal colonic obstructions. Even for patients with extracolonic malignancies such as ovarian cancer, the technical success rate of colorectal stenting is 87%.26 However, patients with unrecognized peritoneal carcinomatosis or multifocal bowel obstruction are less likely to have symptomatic relief even after successful stenting.6,9
Contraindications to stenting
Absolute contraindications to stenting are colonic or tumor perforation with peritonitis. A relative contraindication is a rectal tumor within 2 cm of the anal margin. Stenting in this circumstance leads to tenesmus and incontinence.33
Complications of stenting
Death rates during colorectal stent insertion are less than 1%. The hospital stay and incidence of complications are significantly less than with surgery.26,30
Stent migration occurs in 10% of cases and is asymptomatic, but half of patients with this complication require a repeat intervention. The risk of migration is greater if chemotherapy or radiation therapy succeeds in shrinking the tumor.
Bleeding occurs in 5% of cases, usually from the underlying tumor.
Perforation occurs in 4%, but the rate increases to 10% with the use of dilatation before stent placement.
The rate of recurrent obstruction from tumor ingrowth, overgrowth, or fecal impaction is 10%.9,26,29 Recurrent obstruction may be treated with additional stents inserted within the original stent.9
GASTRIC OUTLET OBSTRUCTION: SURGERY VS STENTING
Gastrojejunostomy has in the past been the treatment of choice for gastric outlet obstruction. Certainly, patients with slow-growing tumors and an expected survival of greater than 60 days may be considered for this bypass procedure; those with a short tumor length, a single site of obstruction (preferably in the pylorus or proximal duodenum), a good performance status, and a life expectancy greater than 30 days are good candidates.7 Nevertheless, for patients with advanced cancer and poor performance status, gastroenterostomy carries a significant risk of morbidity and death.28
Endoscopic stenting of gastric outlet obstruction has a greater success rate, a shorter time to oral intake, a lower morbidity rate, a lower incidence of delayed gastric emptying, and a shorter hospital stay compared with gastroenterostomy.28,29 Technical success rates of stenting are 90%, and 75% of patients have resolution of nausea and vomiting.7 Stenting is generally not possible if the obstruction occurs beyond the ligament of Treitz.
Patients who are expected to survive less than 1 month or who have rapidly progressive disease, overt ascites, carcinomatosis, or multiple sites of obstruction should be managed with percutaneous, endoscopically placed gastrostomy tubes.7
Late complications of stenting for gastric outlet obstruction are occlusion with food or ingrowth of tumor through or around the wire mesh.7 This may require laser therapy or placement of a second stent, or both.
DRUG THERAPY
Medical therapy can palliate symptoms of malignant bowel obstruction for most patients.34 Recommendations have been published by the Working Group of the European Association for Palliative Care.24 Symptom management is focused on pain, nausea, and vomiting.
Which drugs can I use for abdominal pain?
Patients experience two types of abdominal pain: continuous and colic. Each type of pain requires different treatment approaches and classes of drugs.
Potent opioids such as morphine, hydromorphone (Dilaudid), and fentanyl (Fentora) are used to relieve continuous abdominal pain.7 The dose is titrated for adequate relief. Subcutaneous, intravenous, sublingual, and transdermal routes can be used if nausea and vomiting prevent oral administration.
However, opioids can aggravate colic by stimulating circular smooth muscle, leading to segmental contractions. Opioid-sparing adjuvant drugs such as ketorolac (Toradol) may improve colic and continuous pain and prevent a partial obstruction from becoming a complete obstruction by sparing opioid doses.35
Colic may persist or worsen with the use of opioids. Drugs that reduce colic include the scopolamine drugs hyoscine butylbromide and hyoscine hydrobromide, glycopyrrolate (Robinul), and octreotide (Sandostatin).7,34–37
Which drugs are appropriate for reducing nausea and vomiting?
Phenothiazines reduce nausea and control vomiting. Chlorpromazine (Thorazine), prochlorperazine (Compro, Compazine), and promethazine (Phenergan) have all been reported to treat nausea successfully.35,37
Haloperidol (Haldol), a butyrophenone selective dopamine D2-receptor antagonist, has negligible anticholinergic activity. At low doses it produces less sedation than phenothiazines and is an ideal agent for patients with nausea and delirium.35 Doses range from 5 to 15 mg/day, given in divided doses or as intermittent or continuous intravenous infusions.
Anticholinergics, with or without somatostatin analogues, reduce gastrointestinal secretions, fluid accumulation, and vomiting. Anticholinergics bind to muscarinic receptors on enteric neurons in the myenteric and the submucosal plexus. Dosages:
- Hyoscine butylbromide 40 to 120 mg/day.
- Hyoscine hydrobromide 0.2 to 0.9 mg/day.7,34
Glycopyrrolate, a quaternary ammonium anticholinergic, has minimal central nervous system penetration and is less likely to cause delirium or cardiac side effects compared with tertiary amine anticholinergics such as atropine and scopolamine.38 The recommended dose is 0.1 to 0.2 mg subcutaneously or intravenously three to four times daily.
Octreotide, an analogue of somatostatin, blocks the release of vasoactive intestinal polypeptide, which is increased in malignant bowel obstruction.14,15 It reduces the excretion of water, sodium, and chloride into the bowel lumen and increases the absorption of electrolytes and water. It also inhibits pancreatic enzyme secretion and splanchnic blood flow. The result of all these effects is reduced luminal content, reduced motility, reduced vascular congestion of the bowel wall, and, in certain circumstances, reduced ascites.39
In small randomized trials, octreotide was more successful than anticholinergics at improving nausea, vomiting, and colic in patients requiring a nasogastric tube and in those whose symptoms were refractory to standard medical treatment.5,34,40–43 A recent case report found octreotide helpful in resolving symptoms of partial bowel obstruction that were unresponsive to standard measures.44
Octreotide is well tolerated and reduces the time patients require a nasogastric tube without significantly worsening xerostomia. High cost limits its use in American hospice care due to the Medicare capitated system of reimbursement for drugs and services, and as a result it is a second-tier drug despite evidence of its efficacy.
Octreotide doses are 100 to 200 mg every 8 hours.
Metoclopramide (Reglan), a dopaminergic antagonist, a 5HT4 receptor agonist, and a 5HT3 receptor blocker at doses greater than 120 mg/day, combines the action of a phenothiazine, which blocks D2 receptors in the central chemoreceptor trigger zone, with promotility actions through serotonin receptors (5HT4).35,37
Metoclopramide should not be used with anticholinergics or in patients with colic or complete obstruction.35,45 In some centers it is the first-line drug for functional or partial bowel obstruction.7 Dosages range from 40 to 240 mg/day.
Olanzapine (Zyprexa), an atypical antipsychotic, blocks multiple neurotransmitter receptors (D2, H1, Ach, 5HT3) responsible for initiating emesis. It is an option in patients whose nausea and vomiting fail to respond to standard antiemetics.46 Dosages range from 2.5 to 20 mg/day.
Dissolvable tablets are given sublingually, which makes olanzapine a versatile antiemetic in cases of intractable nausea. Our unpublished experience is that the sublingual route reduces nausea associated with malignant bowel obstruction and obviates the need for subcutaneous injections or intravenous antiemetic infusions.
Corticosteroids. Although how corticosteroids relieve malignant bowel obstruction is unknown, they are presumed to act centrally.37,45 In addition, they reduce peritumoral edema and luminal salt and water, and they also have antiemetic and analgesic properties.
Evidence from a meta-analysis found that 6 to 16 mg of parenteral dexamethasone per day reduced symptoms and improved bowel function in 60% of patients but did not change the prognosis.11
A trial of 4 or 5 days is adequate to determine response. If there is no response, the corticosteroid should be rapidly tapered. Side effects are minimal when corticosteroids are used short-term.
Combination therapy. Only rarely does a single drug resolve symptoms of malignant bowel obstruction. Antiemetics, analgesics, corticosteroids, antisecretory anticholinergics, and octreotide are often required in combination to achieve acceptable symptom relief.3,5,7,47
In a small prospective case series, the combination of metoclopramide 60 mg/day, octreotide 0.3 mg/day, and dexamethasone 12 mg/day with a single bolus of amidotrizoic acid (a contrast agent) improved intestinal transit within 1 to 5 days and resolved vomiting within 24 hours.45
Compatibility and the route of administration of medications are key considerations when choosing drug combinations.
WHEN TO CONSIDER A VENTING GASTROSTOMY
Patients with a poor performance status, rapidly progressive disease, peritoneal carcinomatosis, a life expectancy of less than 30 days, or multiple levels of obstruction benefit from placement of a percutaneous endoscopic gastrostomy tube (ie, a venting gastrostomy) rather than surgery if symptoms do not respond to drug therapy.7,48 There is compelling evidence that this procedure relieves nausea and vomiting in 80% to 90% of patients and restores some level of oral intake in many.5,6,48,49 A venting gastrostomy tube can be placed during surgical exploration, percutaneously with fluoroscopy, or endoscopically.9
There are no absolute contraindications to gastrostomy tube placement. It is feasible even in patients with tumors encasing the stomach, diffuse carcinomatosis, and ascites.48 However, massive ascites, previous upper abdominal surgery, or a large mass attached to the abdominal wall make tube placement difficult.
Complications are often local. Patients experience transient abdominal wall pain after the procedure. Dislodgement, bleeding, catheter migration, peritonitis, and necrotizing fasciitis are early complications. Others include skin excoriation from leakage of gastric contents, leakage of ascitic fluid from the site, and obstruction or dislodgement of the tube.48,49
Patients can be discharged from the hospital soon after the tube is placed, usually with fewer medications than for patients who undergo surgery.48 This is particularly important for patients with a short expected survival. Some patients at home benefit from hydration (less than 2 L/day) via an existing central venous port or peripherally inserted central catheter, or by hypodermoclysis.
WHEN IS A NASOGASTRIC TUBE APPROPRIATE?
Some patients with malignant bowel obstruction require a nasogastric tube early in their hospital course.12 Unfortunately, nasogastric tubes, if left in place, cause nose and throat pain, sinusitis, abscess formation, erosion of nasal cartilage, aspiration, esophageal erosion, pharyngitis, and social isolation.5,6
Nasogastric tubes should be a temporizing measure to vent gastrointestinal secretions, reduce abdominal distension, and improve nausea and vomiting while a decision about surgery is being made.13,24 If surgery is not feasible, one can avoid the long-term complications and discomfort of a nasogastric tube via medical management and earlier evaluation for venting gastrostomy in those with symptoms that respond poorly to optimal medical management.49
WHICH PATIENTS BENEFIT FROM TOTAL PARENTERAL NUTRITION?
The use of total parenteral nutrition in patients with incurable malignancies is controversial. Enteral and parenteral feeding can increase muscle mass and improve functional status and quality of life in a subset of patients who are not suffering from cancer-related cachexia.2,50,51 However, for those whose weight loss and malnutrition are consequences of tumor-mediated cachexia, as demonstrated by anorexia and an elevated C-reactive protein level, parenteral nutrition is unlikely to improve the outcome.51 For most terminally ill patients, retrospective studies have failed to show that parenteral nutrition improves overall survival, performance status, or quality of life.2,48,50–54
Total parenteral nutrition poses risks: it is invasive and requires central venous access, which predisposes to infection; it requires frequent monitoring of hydration and electrolytes; and it predisposes to thrombosis, diarrhea, hyperglycemia, and liver failure.50–56
Total parenteral nutrition may be justified in patients with minimal tumor burden who are candidates for definitive surgery, or in those with a good performance status early in the disease course who have not had chemotherapy or whose cancer responds to chemotherapy.2,50–56
The American College of Physicians discourages the routine use of parenteral nutrition in those with advanced cancer who are undergoing palliative chemotherapy, since few patients benefit and many experience side effects.53
Total parenteral nutrition is much like a medical intervention in that it should be offered or continued only if it provides benefit. Conversations at the time that it is begun must include adverse effects that will lead to its discontinuation, and criteria for response. In certain situations, a limited trial of parenteral nutrition may be considered for patients with an uncertain prognosis or for those who have potentially reversible conditions that limit oral intake.51 In such cases, there should be a clear understanding between patient and physician that parenteral nutrition will be discontinued if it fails to show benefit.53
ADDITIONAL CONCERNS OF PATIENTS AND FAMILIES
‘Will I starve to death?’
Starvation is a fear echoed by patients and families. Ethical discourse on the continuation of nutrition and hydration for the terminally ill has been polarizing.57–60 Withdrawal of nutrition can be perceived as euthanasia.
Advanced cancer patients in general do not experience hunger, and those who do require only small amounts of food for satiation.61 In one report, most patients died of their advanced cancer and not from starvation.52 Artificial hydration and nutrition will thus not influence survival and can even be a burden without benefit in the imminently dying.60 These patients should be encouraged to take food orally for pleasure, as long as it is tolerated, without consideration of end points such as weight gain, body mass index, or albumin levels.
Complaints of thirst and dryness of the mouth are relieved with mouth care, ice chips, lubrication to the lips, and sips of fluid, rather than by parenteral nutrition.59 Patients with a terminal illness experience relief from thirst with minimal intake. The symptom of thirst may be relieved without hydration.34,61 Adequate hydration requires smaller fluid volumes due to decreased body weight, decreased renal clearance of free water, and decreased insensible water losses from reduced physical activity.58
‘Can we continue intravenous hydration so he won’t die of thirst?’
Overzealous intravenous hydration may worsen the symptoms of malignant bowel obstruction. Overhydration can increase secretions in the gut lumen and worsen the secretion-distention-contraction cycle, leading to greater abdominal pain and to nausea and vomiting.7 There is a greater risk of fluid overload in these patients, since they have edema and excessive interstitial fluid. Most have a low serum albumin level, which results in movement of fluid from intravascular to interstitial spaces due to reduced colloid osmotic pressure. In these instances, overzealous hydration can lead to respiratory insufficiency and worsening edema.
In spite of numerous discussions in the medical literature of the benefits and burdens of continual hydration, there is no consensus or guideline. When a patient has limited oral intake, the decision to hydrate should be individualized, with careful assessment of the risks and benefits and in accordance with the patient’s or family’s wishes.57,58
Is treatment at home feasible?
Discharging patients with inoperable malignant bowel obstruction requires careful planning. Patients and family members need to be educated on the use of around-the-clock medications and symptom-targeted, as-needed drugs. Days before discharge, questions about diet need to be clarified. Education about total parenteral nutrition and gastrostomy tube care should be completed before discharge from the hospital.
Drug management should be simplified, or compatible medications should be combined into a single infusion. For example, morphine, glycopyrrolate, and haloperidol or metoclopramide are chemically compatible in standard intravenous solutions and can be combined.
Families feel less anxious about the foreseen and the possible unforeseen course of the illness if they can talk with hospice workers early on. This early involvement also facilitates the transition to home hospice care.
SUMMARY OF IMPORTANT POINTS
- Patients with malignant bowel obstruction need a highly individualized approach, tailored to their medical condition, the prognosis, and the goals of care.
- Surgery should not be routinely undertaken; less-invasive approaches such as gastric or colonic stenting should be considered first.
- Combinations of analgesics, antisecretory drugs, and antiemetics can provide acceptable symptom relief in the inoperable patient.
- A venting gastrostomy should be considered if drug therapy fails to reduce nausea and vomiting to an acceptable level.
- A nasogastric tube should be used only as a temporizing measure, until symptoms are controlled medically or a venting gastrostomy is placed.
- Total parenteral nutrition is of benefit only in patients with intermediate life expectancy who may otherwise die of starvation rather than from the cancer itself.
- Mercadante S. Intestinal dysfunction and obstruction. In:Walsh D, editor. Palliative Medicine. Philadelphia, PA: Saunders/Elsevier, 2009:1267–1275.
- Pasanisi F, Orban A, Scalfi L, et al. Predictors of survival in terminal-cancer patients with irreversible bowel obstruction receiving home parenteral nutrition. Nutrition 2001; 17:581–584.
- Pameijer CR, Mahvi DM, Stewart JA, Weber SM. Bowel obstruction in patients with metastatic cancer: does intervention influence outcome? Int J Gastrointest Cancer 2005; 35:127–133.
- Bais JMJ, Schilthuis MS, Ansink AC. Palliative management of intestinal obstruction in patients with advanced gynaecological cancer. J Gynecol Oncol 2002; 7:299–305.
- Laval G, Arvieux C, Stefani L, Villard ML, Mestrallet JP, Cardin N. Protocol for the treatment of malignant inoperable bowel obstruction: a prospective study of 80 cases at Grenoble University Hospital Center. J Pain Symptom Manage 2006; 31:502–512.
- Jatoi A, Podratz KC, Gill P, Hartmann LC. Pathophysiology and palliation of inoperable bowel obstruction in patients with ovarian cancer. J Support Oncol 2004; 2:323–334.
- Ripamonti CI, Easson AM, Gerdes H. Management of malignant bowel obstruction. Eur J Cancer 2008; 44:1105–1115.
- Roeland E, von Gunten CF. Current concepts in malignant bowel obstruction management. Curr Oncol Rep 2009; 11:298–303.
- Baron TH. Interventional palliative strategies for malignant bowel obstruction. Curr Oncol Rep 2009; 11:293–297.
- Feuer DJ, Broadley KE, Shepherd JH, Barton DP. Surgery for the resolution of symptoms in malignant bowel obstruction in advanced gynaecological and gastrointestinal cancer. Cochrane Database Syst Rev 2000;CD002764.
- Feuer DJ, Broadley KE. Corticosteroids for the resolution of malignant bowel obstruction in advanced gynaecological and gastrointestinal cancer. Cochrane Database Syst Rev 2000;CD001219.
- Woolfson RG, Jennings K, Whalen GF. Management of bowel obstruction in patients with abdominal cancer. Arch Surg 1997; 132:1093–1097.
- Saunders MD, Kimmey MB. Systematic review: acute colonic pseudo-obstruction. Aliment Pharmacol Ther 2005; 22:917–925.
- Ripamonti C, Bruera E. Palliative management of malignant bowel obstruction. Int J Gynecol Cancer 2002; 12:135–143.
- Nellgård P, Bojö L, Cassuto J. Importance of vasoactive intestinal peptide and somatostatin for fluid losses in small-bowel obstruction. Scand J Gastroenterol 1995; 30:464–469.
- Böhner H, Yang Q, Franke C, Verreet PR, Ohmann C. Simple data from history and physical examination help to exclude bowel obstruction and to avoid radiographic studies in patients with acute abdominal pain. Eur J Surg 1998; 164:777–784.
- Maglinte DD, Kelvin FM, Sandrasegaran K, et al. Radiology of small bowel obstruction: contemporary approach and controversies. Abdom Imaging 2005; 30:160–178.
- Maglinte DD, Howard TJ, Lillemoe KD, Sandrasegaran K, Rex DK. Small-bowel obstruction: state-of-the-art imaging and its role in clinical management. Clin Gastroenterol Hepatol 2008; 6:130–139.
- Silva AC, Pimenta M, Guimarães LS. Small bowel obstruction: what to look for. Radiographics 2009; 29:423–439.
- Finan PJ, Campbell S, Verma R, et al. The management of malignant large bowel obstruction: ACPGBI position statement. Colorectal Dis 2007; 9(suppl 4):1–17.
- Kohli MD, Maglinte DD. CT enteroclysis in incomplete small bowel obstruction. Abdom Imaging 2009; 34:321–327.
- Ha HK, Shin BS, Lee SI, et al. Usefulness of CT in patients with intestinal obstruction who have undergone abdominal surgery for malignancy. AJR Am J Roentgenol 1998; 171:1587–1593.
- DeBernardo R. Surgical management of malignant bowel obstruction: strategies toward palliation of patients with advanced cancer. Curr Oncol Rep 2009; 11:287–292.
- Ripamonti C, Twycross R, Baines M, et al; Working Group of the European Association for Palliative Care. Clinical-practice recommendations for the management of bowel obstruction in patients with end-stage cancer. Support Care Cancer 2001; 9:223–233.
- Mangili G, Aletti G, Frigerio L, et al. Palliative care for intestinal obstruction in recurrent ovarian cancer: a multivariate analysis. Int J Gynecol Cancer 2005; 15:830–835.
- Turner J, Cummin T, Bennett A, Swift G, Green J. Stents and stentability: treatment for malignant bowel obstruction. Br J Hosp Med (Lond) 2008; 69:676–680.
- Khot UP, Lang AW, Murali K, Parker MC. Systematic review of the efficacy and safety of colorectal stents. Br J Surg 2002; 89:1096–1102.
- Hosono S, Ohtani H, Arimoto Y, Kanamiya Y. Endoscopic stenting versus surgical gastroenterostomy for palliation of malignant gastroduodenal obstruction: a meta-analysis. J Gastroenterol 2007; 42:283–290.
- Del Piano M, Ballarè M, Montino F, et al. Endoscopy or surgery for malignant GI outlet obstruction? Gastrointest Endosc 2005; 61:421–426.
- Tilney HS, Lovegrove RE, Purkayastha S, et al. Comparison of colonic stenting and open surgery for malignant large bowel obstruction. Surg Endosc 2007; 21:225–233.
- Holt AP, Patel M, Ahmed MM. Palliation of patients with malignant gastroduodenal obstruction with self-expanding metallic stents: the treatment of choice? Gastrointest Endosc 2004; 60:1010–1017.
- Dastur JK, Forshaw MJ, Modarai B, Solkar MM, Raymond T, Parker MC. Comparison of short-and long-term outcomes following either insertion of self-expanding metallic stents or emergency surgery in malignant large bowel obstruction. Tech Coloproctol 2008; 12:51–55.
- Turner J, Cummin T, Bennett A, Swift G, Green J. Stents and stentability: treatment for malignant bowel obstruction. Br J Hosp Med (Lond) 2008; 69:676–680.
- Ripamonti C, Mercadante S, Groff L, Zecca E, De Conno F, Casuccio A. Role of octreotide, scopolamine butylbromide, and hydration in symptom control of patients with inoperable bowel obstruction and nasogastric tubes: a prospective randomized trial. J Pain Symptom Manage 2000; 19:23–34.
- Davis MP, Walsh D. Treatment of nausea and vomiting in advanced cancer. Support Care Cancer 2000; 8:444–452.
- Bicanovsky LK, Lagman RL, Davis MP, Walsh D. Managing nonmalignant chronic abdominal pain and malignant bowel obstruction. Gastroenterol Clin North Am 2006; 35:131–142.
- Glare P, Pereira G, Kristjanson LJ, Stockler M, Tattersall M. Systematic review of the efficacy of antiemetics in the treatment of nausea in patients with far-advanced cancer. Support Care Cancer 2004; 12:432–440.
- Davis MP, Furste A. Glycopyrrolate: a useful drug in the palliation of mechanical bowel obstruction. J Pain Symptom Manage 1999; 18:153–154.
- Ripamonti C, Mercadante S. How to use octreotide for malignant bowel obstruction. J Support Oncol 2004; 2:357–364.
- Shima Y, Ohtsu A, Shirao K, Sasaki Y. Clinical efficacy and safety of octreotide (SMS201-995) in terminally ill Japanese cancer patients with malignant bowel obstruction. Jpn J Clin Oncol 2008; 38:354–359.
- Mercadante S, Casuccio A, Mangione S. Medical treatment for inoperable malignant bowel obstruction: a qualitative systematic review. J Pain Symptom Manage 2007; 33:217–223.
- Mystakidou K, Tsilika E, Kalaidopoulou O, Chondros K, Georgaki S, Papadimitriou L. Comparison of octreotide administration vs conservative treatment in the management of inoperable bowel obstruction in patients with far advanced cancer: a randomized, double-blind, controlled clinical trial. Anticancer Res 2002; 22:1187–1192.
- Mercadante S, Ripamonti C, Casuccio A, Zecca E, Groff L. Comparison of octreotide and hyoscine butylbromide in controlling gastrointestinal symptoms due to malignant inoperable bowel obstruction. Support Care Cancer 2000; 8:188–191.
- Myers J, Tamber A, Farhadian M. Management of treatment-related intermittent partial small bowel obstruction: the use of octreotide. J Pain Symptom Manage 2010; 39:e1–e3.
- Mercadante S, Ferrera P, Villari P, Marrazzo A. Aggressive pharmacological treatment for reversing malignant bowel obstruction. J Pain Symptom Manage 2004; 28:412–416.
- Srivastava M, Brito-Dellan N, Davis MP, Leach M, Lagman R. Olanzapine as an antiemetic in refractory nausea and vomiting in advanced cancer. J Pain Symptom Manage 2003; 25:578–582.
- Bentley A, Boyd K. Use of clinical pictures in the management of nausea and vomiting: a prospective audit. Palliat Med 2001; 15:247–253.
- Pothuri B, Montemarano M, Gerardi M, et al. Percutaneous endoscopic gastrostomy tube placement in patients with malignant bowel obstruction due to ovarian carcinoma. Gynecol Oncol 2005; 96:330–334.
- Brooksbank MA, Game PA, Ashby MA. Palliative venting gastrostomy in malignant intestinal obstruction. Palliat Med 2002; 16:520–526.
- Wang MY, Wu MH, Hsieh DY, et al. Home parenteral nutrition support in adults: experience of a medical center in Asia. JPEN J Parenter Enteral Nutr 2007; 31:306–310.
- Dy SM. Enteral and parenteral nutrition in terminally ill cancer patients: a review of the literature. Am J Hosp Palliat Care 2006; 23:369–377.
- Whitworth MK, Whitfield A, Holm S, Shaffer J, Makin W, Jayson GC. Doctor, does this mean I’m going to starve to death? J Clin Oncol 2004; 22:199–201.
- Hoda D, Jatoi A, Burnes J, Loprinzi C, Kelly D. Should patients with advanced, incurable cancers ever be sent home with total parenteral nutrition? A single institution’s 20-year experience. Cancer 2005; 103:863–868.
- Philip J, Depczynski B. The role of total parenteral nutrition for patients with irreversible bowel obstruction secondary to gynecological malignancy. J Pain Symptom Manage 1997; 13:104–111.
- August DA, Thorn D, Fisher RL, Welchek CM. Home parenteral nutrition for patients with inoperable malignant bowel obstruction. JPEN J Parenter Enteral Nutr 1991; 15:323–327.
- Abu-Rustum NR, Barakat RR, Venkatraman E, Spriggs D. Chemotherapy and total parenteral nutrition for advanced ovarian cancer with bowel obstruction. Gynecol Oncol 1997; 64:493–495.
- Fainsinger RL, Bruera E. When to treat dehydration in a terminally ill patient? Support Care Cancer 1997; 5:205–211.
- Steiner N, Bruera E. Methods of hydration in palliative care patients. J Palliat Care 1998; 14:6–13.
- Slomka J. Withholding nutrition at the end of life: clinical and ethical issues. Cleve Clin J Med 2003; 70:548–552.
- Chiu TY, Hu WY, Chuang RB, Chen CY. Nutrition and hydration for terminal cancer patients in Taiwan. Support Care Cancer 2002; 10:630–636.
- McCann RM, Hall WJ, Groth-Juncker A. Comfort care for terminally ill patients. The appropriate use of nutrition and hydration. JAMA 1994; 272:1263–1266.
Malignant bowel obstruction occurs in 5% to 51% of women with ovarian cancer and in 10% to 28% of patients with gastrointestinal cancer, predominantly in the advanced stages.1 Median survival after its onset ranges from 30 to 90 days.2–5
Its symptoms are challenging to manage, since nausea, vomiting, colic, and abdominal pain, which are common, cause significant physical distress and demoralization. The decision whether to correct it with surgery requires an individualized approach and a clear understanding of the goals of care and expected survival in the individual patient.
This review focuses on the management of inoperable malignant bowel obstruction and includes discussion of hydration, nutrition, and endoscopic palliative options.
WHAT ARE THE DIFFERENT TYPES OF OBSTRUCTION?
Bowel obstruction may be mechanical or functional, partial or complete, and may occur at one or at many sites. Tumors can impair bowel function in several ways6–8:
- Intraluminal tumors can occlude the lumen or act as a point of intussusception.
- Intramural tumors can extend to the mucosa and obstruct the lumen or impair peristalsis.
- Mesenteric and omental masses or malignant adhesions can kink or angulate the bowel, creating an extramural obstruction.
- Tumors that infiltrate into the mesentery, bowel muscle, or the enteric or celiac plexus can cause dysmotility.
Cholangiocarcinoma, pancreatic carcinoma, and gallbladder carcinoma are the most common tumors causing duodenal obstruction.9 Distal obstruction is caused mainly by colon and ovarian cancer.
Obstruction can be due to treatment
In a minority of patients, obstruction is unrelated to the cancer and is instead due to adhesions arising from surgery, radiation therapy (causing enteritis and strictures), desmoplastic reactions to intraperitoneal chemotherapy, torsion, or internal hernias.10–12
In rare cases, a patient has intestinal pseudo-obstruction from paraneoplastic destruction of enteric neurons, or severe ileus from anticholinergic or sympathomimetic drugs, as seen with acute colonic pseudo-obstruction (Ogilvie syndrome).13
Physiologic reactions to obstruction
Malignant bowel obstruction stimulates gastric, biliary, pancreatic, and intestinal secretions, decreases intraluminal sodium and water reabsorption, and increases mucosal sodium and water secretion.6,14 In response to the obstruction, peristalsis increases, and prostaglandin, vasoactive intestinal peptide, and nociceptive mediators are released. Vasoactive intestinal polypeptide perpetuates a cycle of secretion, distention, and contraction that leads to intestinal hyperemia, bowel edema, and accumulation of fluid in the lumen.8,10,11,15
Signs and symptoms depend on the site
The site of obstruction determines the signs and symptoms patients experience.7,14 Obstructions high in the gastrointestinal tract are associated with greater symptoms but fewer signs than colonic obstructions.1 Patients with proximal small-bowel obstruction have more severe nausea and a greater number of episodes of emesis, but they have relatively normal plain radiographs of the abdomen, which do not have the characteristic air-fluid levels commonly seen with distal small-bowel obstruction.
Most malignant obstructions remain partial, but increasing abdominal distention, worsening nausea, vomiting, abdominal pain, and obstipation over 1 to 2 weeks1 suggest progression to complete obstruction.
IMAGING TESTS FOR MALIGNANT BOWEL OBSTRUCTION
What is the value of plain radiography?
Despite these limitations, plain radiography is useful in assessing constipation and its severity as a potential cause of symptoms, and thus it remains an important initial imaging study in almost all patients with suspected malignant bowel obstruction.17,18 It is also used to assess response to treatment.
When do you need contrast radiographs?
Contrast radiography (barium swallow or barium or Gastrograffin enema) is helpful in patients with symptoms of dysmotility from suspected bowel obstruction. It defines the site or sites of obstruction and the extent of the obstruction with a fair degree of accuracy.7,19 Single-contrast studies, if positive, exclude opioid-induced bowel dysfunction or pseudo-obstruction in 83% of patients, with a sensitivity and specificity of 96% and 98%, respectively.8,20 Small-bowel follow-through with barium is more appropriate for low-grade obstructions or for symptomatic patients with a normal kidney, ureter, bladder radiograph.19
However, contrast radiography is limited by the patient’s ability to swallow barium or water-soluble contrast agents, and it can worsen nausea or vomiting.17,18 Also, barium is not absorbed systemically and may interfere with subsequent radiologic studies. Large volumes of contrast agents increase the risk of aspiration pneumonia in patients with poorly controlled nausea and can lead to severe impaction proximal to the obstructed site.8
Is enteroclysis better than barium swallow?
Enteroclysis, ie, injecting radiographic contrast into the bowel via a nasoduodenal tube, has some advantages over the barium swallow technique for detecting partial small-bowel obstruction, since it bypasses the stomach and allows for therapeutic decompression as well as direct visualization of the area of concern.17,18 Enteroclysis radiography objectively gauges severity of intestinal obstruction and bowel wall distensibility, which is an advantage over other imaging studies. Its sensitivity is 100% and specificity 88% in experienced hands.17 Enteroclysis studies also detect nonobstructing intraluminal tumors when computed tomography (CT) is not diagnostic.17,18,21
The drawbacks to enteroclysis are that it is technically difficult to perform and that few radiologists are trained in it.
When is CT useful?
CT is the primary imaging study for patients with obstructive symptoms and a history of abdominal malignancy or a palpable abdominal mass17,20,22,23 (Figure 1). It has a specificity of 100% and a sensitivity of 94%. It plays a major role in decision-making regarding surgery, endoscopy, or palliative interventions,7,19 as it locates the obstruction and differentiates benign from malignant causes with a fair degree of precision.22
CT findings in malignant bowel obstruction may include:
- A mass at the site of obstruction or within the original surgical field
- Lymphadenopathy
- Abrupt transitions in luminal diameter or irregular thickening of the bowel wall at the site or sites of obstruction.7
SURGERY: A DIFFICULT DECISION
Is the patient fit for surgery?
Surgery for malignant bowel obstruction should not be done in patients who have advanced malignancies with bulky intra-abdominal metastases or cancer that has spread outside the abdominal cavity without taking into account treatment options for the cancer, the patient’s nutritional status, and the goals of care.
The role of abdominal surgery (debulking, resection, or bypass) in advanced cancer remains unclear and controversial.24 From 42% to 80% of patients report that symptoms improve after surgery, but recurrent obstruction occurs in 10% to 50%.10 Even in patients with low tumor bulk and good nutritional status, 30-day mortality rates range from 5% to 40%, and complication rates range from 9% to 90%.3,4,6,7,10,14
Outcomes after surgery depend on patient selection criteria perhaps as much as on the surgeon’s experience and skill. Patients with more advanced cancer who have had multiple surgical procedures and those with cancer that does not respond to chemotherapy and radiation present the greatest challenge to surgeons.23
What is the benefit of surgery?
Reports of palliative surgery have included information about 60-day survival rates after the operation, but a number of factors may be more meaningful in this context, such as postoperative symptoms, the patient’s overall wellbeing, how the original symptoms respond to the surgery, complications, and length of hospitalization.14 The paucity of published, validated, patient-related outcome data on which to gauge the value of surgery and the lack of a standard definition of “benefit” further confuse the objective determination of whether these patients benefit from surgery.
In a cohort with advanced ovarian cancer and bowel obstruction, surgery was detrimental to survival and quality of life for all subgroups, and most patients died in the hospital.6
The risk of surgery for malignant bowel obstruction is presumably higher than for abdominal surgery for other indications, since many of the patients are debilitated from their cancer and chemotherapy, and many are malnourished.23 Even when taking into account a potential selection bias in favor of surgery, several studies have reported no significant difference in 30-day mortality rates or median survival between operative and nonoperative groups.2,12 Neither the type of obstruction nor the extent of the surgery influenced outcomes. Surgical outcomes are best in patients with a benign cause of obstruction; little benefit is seen in operating on those with abdominal carcinomatosis.12
Nevertheless, surgery is beneficial in a select few. For patients with a good performance status, slowly progressive cancer, and an expected survival of more than 6 months, surgical bypass or resection is preferred.7,12,25 The challenge is to identify these surgical candidates, taking into account prognostic factors such as nutritional status, tumor burden, performance status, presence of ascites, advanced age, extensive prior chemotherapy or radiotherapy, and diffuse carcinomatosis.3,10,12,20,23
Is surgery consistent with the goals of care?
Crucial to decision-making are the goals of care. Since palliative surgery carries a low level of evidence for benefit in terms of quality of life and survival, time should be set aside to thoroughly review the patient’s medical condition, to explore options, and to clarify expectations and goals of care.3,10 Family members should be invited to be present during these discussions and to be involved in the decision-making process.
WHAT IS THE BENEFIT OF GASTRIC OR COLONIC STENTING?
Endoscopic procedures are alternatives to surgery and offer a palliative option in malignant bowel obstruction. Endoscopic procedures are associated with a shorter hospital stay and quicker recovery than after laparotomy.9,26–30 In certain situations, stenting serves as a bridge to surgery, allowing time to mitigate comorbid conditions, to enhance nutrition, and to complete staging, while relieving symptoms.27–29,31,32 Definitive surgery can be done as a single-stage procedure without a diverting enterostomy.
Self-expanding metal stents for gastric outlet, small-bowel, and colonic obstructions are an option in patients who have incurable metastatic disease who are unfit for surgery, in patients with a single point of obstruction or locally extensive disease, or in patients who do not want to undergo laparotomy.28–30
Technical and clinical success rates for colorectal stenting are high (88% to 93%).26,27 Stenting is more successful for left-sided colonic obstructions than for proximal colonic obstructions. Even for patients with extracolonic malignancies such as ovarian cancer, the technical success rate of colorectal stenting is 87%.26 However, patients with unrecognized peritoneal carcinomatosis or multifocal bowel obstruction are less likely to have symptomatic relief even after successful stenting.6,9
Contraindications to stenting
Absolute contraindications to stenting are colonic or tumor perforation with peritonitis. A relative contraindication is a rectal tumor within 2 cm of the anal margin. Stenting in this circumstance leads to tenesmus and incontinence.33
Complications of stenting
Death rates during colorectal stent insertion are less than 1%. The hospital stay and incidence of complications are significantly less than with surgery.26,30
Stent migration occurs in 10% of cases and is asymptomatic, but half of patients with this complication require a repeat intervention. The risk of migration is greater if chemotherapy or radiation therapy succeeds in shrinking the tumor.
Bleeding occurs in 5% of cases, usually from the underlying tumor.
Perforation occurs in 4%, but the rate increases to 10% with the use of dilatation before stent placement.
The rate of recurrent obstruction from tumor ingrowth, overgrowth, or fecal impaction is 10%.9,26,29 Recurrent obstruction may be treated with additional stents inserted within the original stent.9
GASTRIC OUTLET OBSTRUCTION: SURGERY VS STENTING
Gastrojejunostomy has in the past been the treatment of choice for gastric outlet obstruction. Certainly, patients with slow-growing tumors and an expected survival of greater than 60 days may be considered for this bypass procedure; those with a short tumor length, a single site of obstruction (preferably in the pylorus or proximal duodenum), a good performance status, and a life expectancy greater than 30 days are good candidates.7 Nevertheless, for patients with advanced cancer and poor performance status, gastroenterostomy carries a significant risk of morbidity and death.28
Endoscopic stenting of gastric outlet obstruction has a greater success rate, a shorter time to oral intake, a lower morbidity rate, a lower incidence of delayed gastric emptying, and a shorter hospital stay compared with gastroenterostomy.28,29 Technical success rates of stenting are 90%, and 75% of patients have resolution of nausea and vomiting.7 Stenting is generally not possible if the obstruction occurs beyond the ligament of Treitz.
Patients who are expected to survive less than 1 month or who have rapidly progressive disease, overt ascites, carcinomatosis, or multiple sites of obstruction should be managed with percutaneous, endoscopically placed gastrostomy tubes.7
Late complications of stenting for gastric outlet obstruction are occlusion with food or ingrowth of tumor through or around the wire mesh.7 This may require laser therapy or placement of a second stent, or both.
DRUG THERAPY
Medical therapy can palliate symptoms of malignant bowel obstruction for most patients.34 Recommendations have been published by the Working Group of the European Association for Palliative Care.24 Symptom management is focused on pain, nausea, and vomiting.
Which drugs can I use for abdominal pain?
Patients experience two types of abdominal pain: continuous and colic. Each type of pain requires different treatment approaches and classes of drugs.
Potent opioids such as morphine, hydromorphone (Dilaudid), and fentanyl (Fentora) are used to relieve continuous abdominal pain.7 The dose is titrated for adequate relief. Subcutaneous, intravenous, sublingual, and transdermal routes can be used if nausea and vomiting prevent oral administration.
However, opioids can aggravate colic by stimulating circular smooth muscle, leading to segmental contractions. Opioid-sparing adjuvant drugs such as ketorolac (Toradol) may improve colic and continuous pain and prevent a partial obstruction from becoming a complete obstruction by sparing opioid doses.35
Colic may persist or worsen with the use of opioids. Drugs that reduce colic include the scopolamine drugs hyoscine butylbromide and hyoscine hydrobromide, glycopyrrolate (Robinul), and octreotide (Sandostatin).7,34–37
Which drugs are appropriate for reducing nausea and vomiting?
Phenothiazines reduce nausea and control vomiting. Chlorpromazine (Thorazine), prochlorperazine (Compro, Compazine), and promethazine (Phenergan) have all been reported to treat nausea successfully.35,37
Haloperidol (Haldol), a butyrophenone selective dopamine D2-receptor antagonist, has negligible anticholinergic activity. At low doses it produces less sedation than phenothiazines and is an ideal agent for patients with nausea and delirium.35 Doses range from 5 to 15 mg/day, given in divided doses or as intermittent or continuous intravenous infusions.
Anticholinergics, with or without somatostatin analogues, reduce gastrointestinal secretions, fluid accumulation, and vomiting. Anticholinergics bind to muscarinic receptors on enteric neurons in the myenteric and the submucosal plexus. Dosages:
- Hyoscine butylbromide 40 to 120 mg/day.
- Hyoscine hydrobromide 0.2 to 0.9 mg/day.7,34
Glycopyrrolate, a quaternary ammonium anticholinergic, has minimal central nervous system penetration and is less likely to cause delirium or cardiac side effects compared with tertiary amine anticholinergics such as atropine and scopolamine.38 The recommended dose is 0.1 to 0.2 mg subcutaneously or intravenously three to four times daily.
Octreotide, an analogue of somatostatin, blocks the release of vasoactive intestinal polypeptide, which is increased in malignant bowel obstruction.14,15 It reduces the excretion of water, sodium, and chloride into the bowel lumen and increases the absorption of electrolytes and water. It also inhibits pancreatic enzyme secretion and splanchnic blood flow. The result of all these effects is reduced luminal content, reduced motility, reduced vascular congestion of the bowel wall, and, in certain circumstances, reduced ascites.39
In small randomized trials, octreotide was more successful than anticholinergics at improving nausea, vomiting, and colic in patients requiring a nasogastric tube and in those whose symptoms were refractory to standard medical treatment.5,34,40–43 A recent case report found octreotide helpful in resolving symptoms of partial bowel obstruction that were unresponsive to standard measures.44
Octreotide is well tolerated and reduces the time patients require a nasogastric tube without significantly worsening xerostomia. High cost limits its use in American hospice care due to the Medicare capitated system of reimbursement for drugs and services, and as a result it is a second-tier drug despite evidence of its efficacy.
Octreotide doses are 100 to 200 mg every 8 hours.
Metoclopramide (Reglan), a dopaminergic antagonist, a 5HT4 receptor agonist, and a 5HT3 receptor blocker at doses greater than 120 mg/day, combines the action of a phenothiazine, which blocks D2 receptors in the central chemoreceptor trigger zone, with promotility actions through serotonin receptors (5HT4).35,37
Metoclopramide should not be used with anticholinergics or in patients with colic or complete obstruction.35,45 In some centers it is the first-line drug for functional or partial bowel obstruction.7 Dosages range from 40 to 240 mg/day.
Olanzapine (Zyprexa), an atypical antipsychotic, blocks multiple neurotransmitter receptors (D2, H1, Ach, 5HT3) responsible for initiating emesis. It is an option in patients whose nausea and vomiting fail to respond to standard antiemetics.46 Dosages range from 2.5 to 20 mg/day.
Dissolvable tablets are given sublingually, which makes olanzapine a versatile antiemetic in cases of intractable nausea. Our unpublished experience is that the sublingual route reduces nausea associated with malignant bowel obstruction and obviates the need for subcutaneous injections or intravenous antiemetic infusions.
Corticosteroids. Although how corticosteroids relieve malignant bowel obstruction is unknown, they are presumed to act centrally.37,45 In addition, they reduce peritumoral edema and luminal salt and water, and they also have antiemetic and analgesic properties.
Evidence from a meta-analysis found that 6 to 16 mg of parenteral dexamethasone per day reduced symptoms and improved bowel function in 60% of patients but did not change the prognosis.11
A trial of 4 or 5 days is adequate to determine response. If there is no response, the corticosteroid should be rapidly tapered. Side effects are minimal when corticosteroids are used short-term.
Combination therapy. Only rarely does a single drug resolve symptoms of malignant bowel obstruction. Antiemetics, analgesics, corticosteroids, antisecretory anticholinergics, and octreotide are often required in combination to achieve acceptable symptom relief.3,5,7,47
In a small prospective case series, the combination of metoclopramide 60 mg/day, octreotide 0.3 mg/day, and dexamethasone 12 mg/day with a single bolus of amidotrizoic acid (a contrast agent) improved intestinal transit within 1 to 5 days and resolved vomiting within 24 hours.45
Compatibility and the route of administration of medications are key considerations when choosing drug combinations.
WHEN TO CONSIDER A VENTING GASTROSTOMY
Patients with a poor performance status, rapidly progressive disease, peritoneal carcinomatosis, a life expectancy of less than 30 days, or multiple levels of obstruction benefit from placement of a percutaneous endoscopic gastrostomy tube (ie, a venting gastrostomy) rather than surgery if symptoms do not respond to drug therapy.7,48 There is compelling evidence that this procedure relieves nausea and vomiting in 80% to 90% of patients and restores some level of oral intake in many.5,6,48,49 A venting gastrostomy tube can be placed during surgical exploration, percutaneously with fluoroscopy, or endoscopically.9
There are no absolute contraindications to gastrostomy tube placement. It is feasible even in patients with tumors encasing the stomach, diffuse carcinomatosis, and ascites.48 However, massive ascites, previous upper abdominal surgery, or a large mass attached to the abdominal wall make tube placement difficult.
Complications are often local. Patients experience transient abdominal wall pain after the procedure. Dislodgement, bleeding, catheter migration, peritonitis, and necrotizing fasciitis are early complications. Others include skin excoriation from leakage of gastric contents, leakage of ascitic fluid from the site, and obstruction or dislodgement of the tube.48,49
Patients can be discharged from the hospital soon after the tube is placed, usually with fewer medications than for patients who undergo surgery.48 This is particularly important for patients with a short expected survival. Some patients at home benefit from hydration (less than 2 L/day) via an existing central venous port or peripherally inserted central catheter, or by hypodermoclysis.
WHEN IS A NASOGASTRIC TUBE APPROPRIATE?
Some patients with malignant bowel obstruction require a nasogastric tube early in their hospital course.12 Unfortunately, nasogastric tubes, if left in place, cause nose and throat pain, sinusitis, abscess formation, erosion of nasal cartilage, aspiration, esophageal erosion, pharyngitis, and social isolation.5,6
Nasogastric tubes should be a temporizing measure to vent gastrointestinal secretions, reduce abdominal distension, and improve nausea and vomiting while a decision about surgery is being made.13,24 If surgery is not feasible, one can avoid the long-term complications and discomfort of a nasogastric tube via medical management and earlier evaluation for venting gastrostomy in those with symptoms that respond poorly to optimal medical management.49
WHICH PATIENTS BENEFIT FROM TOTAL PARENTERAL NUTRITION?
The use of total parenteral nutrition in patients with incurable malignancies is controversial. Enteral and parenteral feeding can increase muscle mass and improve functional status and quality of life in a subset of patients who are not suffering from cancer-related cachexia.2,50,51 However, for those whose weight loss and malnutrition are consequences of tumor-mediated cachexia, as demonstrated by anorexia and an elevated C-reactive protein level, parenteral nutrition is unlikely to improve the outcome.51 For most terminally ill patients, retrospective studies have failed to show that parenteral nutrition improves overall survival, performance status, or quality of life.2,48,50–54
Total parenteral nutrition poses risks: it is invasive and requires central venous access, which predisposes to infection; it requires frequent monitoring of hydration and electrolytes; and it predisposes to thrombosis, diarrhea, hyperglycemia, and liver failure.50–56
Total parenteral nutrition may be justified in patients with minimal tumor burden who are candidates for definitive surgery, or in those with a good performance status early in the disease course who have not had chemotherapy or whose cancer responds to chemotherapy.2,50–56
The American College of Physicians discourages the routine use of parenteral nutrition in those with advanced cancer who are undergoing palliative chemotherapy, since few patients benefit and many experience side effects.53
Total parenteral nutrition is much like a medical intervention in that it should be offered or continued only if it provides benefit. Conversations at the time that it is begun must include adverse effects that will lead to its discontinuation, and criteria for response. In certain situations, a limited trial of parenteral nutrition may be considered for patients with an uncertain prognosis or for those who have potentially reversible conditions that limit oral intake.51 In such cases, there should be a clear understanding between patient and physician that parenteral nutrition will be discontinued if it fails to show benefit.53
ADDITIONAL CONCERNS OF PATIENTS AND FAMILIES
‘Will I starve to death?’
Starvation is a fear echoed by patients and families. Ethical discourse on the continuation of nutrition and hydration for the terminally ill has been polarizing.57–60 Withdrawal of nutrition can be perceived as euthanasia.
Advanced cancer patients in general do not experience hunger, and those who do require only small amounts of food for satiation.61 In one report, most patients died of their advanced cancer and not from starvation.52 Artificial hydration and nutrition will thus not influence survival and can even be a burden without benefit in the imminently dying.60 These patients should be encouraged to take food orally for pleasure, as long as it is tolerated, without consideration of end points such as weight gain, body mass index, or albumin levels.
Complaints of thirst and dryness of the mouth are relieved with mouth care, ice chips, lubrication to the lips, and sips of fluid, rather than by parenteral nutrition.59 Patients with a terminal illness experience relief from thirst with minimal intake. The symptom of thirst may be relieved without hydration.34,61 Adequate hydration requires smaller fluid volumes due to decreased body weight, decreased renal clearance of free water, and decreased insensible water losses from reduced physical activity.58
‘Can we continue intravenous hydration so he won’t die of thirst?’
Overzealous intravenous hydration may worsen the symptoms of malignant bowel obstruction. Overhydration can increase secretions in the gut lumen and worsen the secretion-distention-contraction cycle, leading to greater abdominal pain and to nausea and vomiting.7 There is a greater risk of fluid overload in these patients, since they have edema and excessive interstitial fluid. Most have a low serum albumin level, which results in movement of fluid from intravascular to interstitial spaces due to reduced colloid osmotic pressure. In these instances, overzealous hydration can lead to respiratory insufficiency and worsening edema.
In spite of numerous discussions in the medical literature of the benefits and burdens of continual hydration, there is no consensus or guideline. When a patient has limited oral intake, the decision to hydrate should be individualized, with careful assessment of the risks and benefits and in accordance with the patient’s or family’s wishes.57,58
Is treatment at home feasible?
Discharging patients with inoperable malignant bowel obstruction requires careful planning. Patients and family members need to be educated on the use of around-the-clock medications and symptom-targeted, as-needed drugs. Days before discharge, questions about diet need to be clarified. Education about total parenteral nutrition and gastrostomy tube care should be completed before discharge from the hospital.
Drug management should be simplified, or compatible medications should be combined into a single infusion. For example, morphine, glycopyrrolate, and haloperidol or metoclopramide are chemically compatible in standard intravenous solutions and can be combined.
Families feel less anxious about the foreseen and the possible unforeseen course of the illness if they can talk with hospice workers early on. This early involvement also facilitates the transition to home hospice care.
SUMMARY OF IMPORTANT POINTS
- Patients with malignant bowel obstruction need a highly individualized approach, tailored to their medical condition, the prognosis, and the goals of care.
- Surgery should not be routinely undertaken; less-invasive approaches such as gastric or colonic stenting should be considered first.
- Combinations of analgesics, antisecretory drugs, and antiemetics can provide acceptable symptom relief in the inoperable patient.
- A venting gastrostomy should be considered if drug therapy fails to reduce nausea and vomiting to an acceptable level.
- A nasogastric tube should be used only as a temporizing measure, until symptoms are controlled medically or a venting gastrostomy is placed.
- Total parenteral nutrition is of benefit only in patients with intermediate life expectancy who may otherwise die of starvation rather than from the cancer itself.
Malignant bowel obstruction occurs in 5% to 51% of women with ovarian cancer and in 10% to 28% of patients with gastrointestinal cancer, predominantly in the advanced stages.1 Median survival after its onset ranges from 30 to 90 days.2–5
Its symptoms are challenging to manage, since nausea, vomiting, colic, and abdominal pain, which are common, cause significant physical distress and demoralization. The decision whether to correct it with surgery requires an individualized approach and a clear understanding of the goals of care and expected survival in the individual patient.
This review focuses on the management of inoperable malignant bowel obstruction and includes discussion of hydration, nutrition, and endoscopic palliative options.
WHAT ARE THE DIFFERENT TYPES OF OBSTRUCTION?
Bowel obstruction may be mechanical or functional, partial or complete, and may occur at one or at many sites. Tumors can impair bowel function in several ways6–8:
- Intraluminal tumors can occlude the lumen or act as a point of intussusception.
- Intramural tumors can extend to the mucosa and obstruct the lumen or impair peristalsis.
- Mesenteric and omental masses or malignant adhesions can kink or angulate the bowel, creating an extramural obstruction.
- Tumors that infiltrate into the mesentery, bowel muscle, or the enteric or celiac plexus can cause dysmotility.
Cholangiocarcinoma, pancreatic carcinoma, and gallbladder carcinoma are the most common tumors causing duodenal obstruction.9 Distal obstruction is caused mainly by colon and ovarian cancer.
Obstruction can be due to treatment
In a minority of patients, obstruction is unrelated to the cancer and is instead due to adhesions arising from surgery, radiation therapy (causing enteritis and strictures), desmoplastic reactions to intraperitoneal chemotherapy, torsion, or internal hernias.10–12
In rare cases, a patient has intestinal pseudo-obstruction from paraneoplastic destruction of enteric neurons, or severe ileus from anticholinergic or sympathomimetic drugs, as seen with acute colonic pseudo-obstruction (Ogilvie syndrome).13
Physiologic reactions to obstruction
Malignant bowel obstruction stimulates gastric, biliary, pancreatic, and intestinal secretions, decreases intraluminal sodium and water reabsorption, and increases mucosal sodium and water secretion.6,14 In response to the obstruction, peristalsis increases, and prostaglandin, vasoactive intestinal peptide, and nociceptive mediators are released. Vasoactive intestinal polypeptide perpetuates a cycle of secretion, distention, and contraction that leads to intestinal hyperemia, bowel edema, and accumulation of fluid in the lumen.8,10,11,15
Signs and symptoms depend on the site
The site of obstruction determines the signs and symptoms patients experience.7,14 Obstructions high in the gastrointestinal tract are associated with greater symptoms but fewer signs than colonic obstructions.1 Patients with proximal small-bowel obstruction have more severe nausea and a greater number of episodes of emesis, but they have relatively normal plain radiographs of the abdomen, which do not have the characteristic air-fluid levels commonly seen with distal small-bowel obstruction.
Most malignant obstructions remain partial, but increasing abdominal distention, worsening nausea, vomiting, abdominal pain, and obstipation over 1 to 2 weeks1 suggest progression to complete obstruction.
IMAGING TESTS FOR MALIGNANT BOWEL OBSTRUCTION
What is the value of plain radiography?
Despite these limitations, plain radiography is useful in assessing constipation and its severity as a potential cause of symptoms, and thus it remains an important initial imaging study in almost all patients with suspected malignant bowel obstruction.17,18 It is also used to assess response to treatment.
When do you need contrast radiographs?
Contrast radiography (barium swallow or barium or Gastrograffin enema) is helpful in patients with symptoms of dysmotility from suspected bowel obstruction. It defines the site or sites of obstruction and the extent of the obstruction with a fair degree of accuracy.7,19 Single-contrast studies, if positive, exclude opioid-induced bowel dysfunction or pseudo-obstruction in 83% of patients, with a sensitivity and specificity of 96% and 98%, respectively.8,20 Small-bowel follow-through with barium is more appropriate for low-grade obstructions or for symptomatic patients with a normal kidney, ureter, bladder radiograph.19
However, contrast radiography is limited by the patient’s ability to swallow barium or water-soluble contrast agents, and it can worsen nausea or vomiting.17,18 Also, barium is not absorbed systemically and may interfere with subsequent radiologic studies. Large volumes of contrast agents increase the risk of aspiration pneumonia in patients with poorly controlled nausea and can lead to severe impaction proximal to the obstructed site.8
Is enteroclysis better than barium swallow?
Enteroclysis, ie, injecting radiographic contrast into the bowel via a nasoduodenal tube, has some advantages over the barium swallow technique for detecting partial small-bowel obstruction, since it bypasses the stomach and allows for therapeutic decompression as well as direct visualization of the area of concern.17,18 Enteroclysis radiography objectively gauges severity of intestinal obstruction and bowel wall distensibility, which is an advantage over other imaging studies. Its sensitivity is 100% and specificity 88% in experienced hands.17 Enteroclysis studies also detect nonobstructing intraluminal tumors when computed tomography (CT) is not diagnostic.17,18,21
The drawbacks to enteroclysis are that it is technically difficult to perform and that few radiologists are trained in it.
When is CT useful?
CT is the primary imaging study for patients with obstructive symptoms and a history of abdominal malignancy or a palpable abdominal mass17,20,22,23 (Figure 1). It has a specificity of 100% and a sensitivity of 94%. It plays a major role in decision-making regarding surgery, endoscopy, or palliative interventions,7,19 as it locates the obstruction and differentiates benign from malignant causes with a fair degree of precision.22
CT findings in malignant bowel obstruction may include:
- A mass at the site of obstruction or within the original surgical field
- Lymphadenopathy
- Abrupt transitions in luminal diameter or irregular thickening of the bowel wall at the site or sites of obstruction.7
SURGERY: A DIFFICULT DECISION
Is the patient fit for surgery?
Surgery for malignant bowel obstruction should not be done in patients who have advanced malignancies with bulky intra-abdominal metastases or cancer that has spread outside the abdominal cavity without taking into account treatment options for the cancer, the patient’s nutritional status, and the goals of care.
The role of abdominal surgery (debulking, resection, or bypass) in advanced cancer remains unclear and controversial.24 From 42% to 80% of patients report that symptoms improve after surgery, but recurrent obstruction occurs in 10% to 50%.10 Even in patients with low tumor bulk and good nutritional status, 30-day mortality rates range from 5% to 40%, and complication rates range from 9% to 90%.3,4,6,7,10,14
Outcomes after surgery depend on patient selection criteria perhaps as much as on the surgeon’s experience and skill. Patients with more advanced cancer who have had multiple surgical procedures and those with cancer that does not respond to chemotherapy and radiation present the greatest challenge to surgeons.23
What is the benefit of surgery?
Reports of palliative surgery have included information about 60-day survival rates after the operation, but a number of factors may be more meaningful in this context, such as postoperative symptoms, the patient’s overall wellbeing, how the original symptoms respond to the surgery, complications, and length of hospitalization.14 The paucity of published, validated, patient-related outcome data on which to gauge the value of surgery and the lack of a standard definition of “benefit” further confuse the objective determination of whether these patients benefit from surgery.
In a cohort with advanced ovarian cancer and bowel obstruction, surgery was detrimental to survival and quality of life for all subgroups, and most patients died in the hospital.6
The risk of surgery for malignant bowel obstruction is presumably higher than for abdominal surgery for other indications, since many of the patients are debilitated from their cancer and chemotherapy, and many are malnourished.23 Even when taking into account a potential selection bias in favor of surgery, several studies have reported no significant difference in 30-day mortality rates or median survival between operative and nonoperative groups.2,12 Neither the type of obstruction nor the extent of the surgery influenced outcomes. Surgical outcomes are best in patients with a benign cause of obstruction; little benefit is seen in operating on those with abdominal carcinomatosis.12
Nevertheless, surgery is beneficial in a select few. For patients with a good performance status, slowly progressive cancer, and an expected survival of more than 6 months, surgical bypass or resection is preferred.7,12,25 The challenge is to identify these surgical candidates, taking into account prognostic factors such as nutritional status, tumor burden, performance status, presence of ascites, advanced age, extensive prior chemotherapy or radiotherapy, and diffuse carcinomatosis.3,10,12,20,23
Is surgery consistent with the goals of care?
Crucial to decision-making are the goals of care. Since palliative surgery carries a low level of evidence for benefit in terms of quality of life and survival, time should be set aside to thoroughly review the patient’s medical condition, to explore options, and to clarify expectations and goals of care.3,10 Family members should be invited to be present during these discussions and to be involved in the decision-making process.
WHAT IS THE BENEFIT OF GASTRIC OR COLONIC STENTING?
Endoscopic procedures are alternatives to surgery and offer a palliative option in malignant bowel obstruction. Endoscopic procedures are associated with a shorter hospital stay and quicker recovery than after laparotomy.9,26–30 In certain situations, stenting serves as a bridge to surgery, allowing time to mitigate comorbid conditions, to enhance nutrition, and to complete staging, while relieving symptoms.27–29,31,32 Definitive surgery can be done as a single-stage procedure without a diverting enterostomy.
Self-expanding metal stents for gastric outlet, small-bowel, and colonic obstructions are an option in patients who have incurable metastatic disease who are unfit for surgery, in patients with a single point of obstruction or locally extensive disease, or in patients who do not want to undergo laparotomy.28–30
Technical and clinical success rates for colorectal stenting are high (88% to 93%).26,27 Stenting is more successful for left-sided colonic obstructions than for proximal colonic obstructions. Even for patients with extracolonic malignancies such as ovarian cancer, the technical success rate of colorectal stenting is 87%.26 However, patients with unrecognized peritoneal carcinomatosis or multifocal bowel obstruction are less likely to have symptomatic relief even after successful stenting.6,9
Contraindications to stenting
Absolute contraindications to stenting are colonic or tumor perforation with peritonitis. A relative contraindication is a rectal tumor within 2 cm of the anal margin. Stenting in this circumstance leads to tenesmus and incontinence.33
Complications of stenting
Death rates during colorectal stent insertion are less than 1%. The hospital stay and incidence of complications are significantly less than with surgery.26,30
Stent migration occurs in 10% of cases and is asymptomatic, but half of patients with this complication require a repeat intervention. The risk of migration is greater if chemotherapy or radiation therapy succeeds in shrinking the tumor.
Bleeding occurs in 5% of cases, usually from the underlying tumor.
Perforation occurs in 4%, but the rate increases to 10% with the use of dilatation before stent placement.
The rate of recurrent obstruction from tumor ingrowth, overgrowth, or fecal impaction is 10%.9,26,29 Recurrent obstruction may be treated with additional stents inserted within the original stent.9
GASTRIC OUTLET OBSTRUCTION: SURGERY VS STENTING
Gastrojejunostomy has in the past been the treatment of choice for gastric outlet obstruction. Certainly, patients with slow-growing tumors and an expected survival of greater than 60 days may be considered for this bypass procedure; those with a short tumor length, a single site of obstruction (preferably in the pylorus or proximal duodenum), a good performance status, and a life expectancy greater than 30 days are good candidates.7 Nevertheless, for patients with advanced cancer and poor performance status, gastroenterostomy carries a significant risk of morbidity and death.28
Endoscopic stenting of gastric outlet obstruction has a greater success rate, a shorter time to oral intake, a lower morbidity rate, a lower incidence of delayed gastric emptying, and a shorter hospital stay compared with gastroenterostomy.28,29 Technical success rates of stenting are 90%, and 75% of patients have resolution of nausea and vomiting.7 Stenting is generally not possible if the obstruction occurs beyond the ligament of Treitz.
Patients who are expected to survive less than 1 month or who have rapidly progressive disease, overt ascites, carcinomatosis, or multiple sites of obstruction should be managed with percutaneous, endoscopically placed gastrostomy tubes.7
Late complications of stenting for gastric outlet obstruction are occlusion with food or ingrowth of tumor through or around the wire mesh.7 This may require laser therapy or placement of a second stent, or both.
DRUG THERAPY
Medical therapy can palliate symptoms of malignant bowel obstruction for most patients.34 Recommendations have been published by the Working Group of the European Association for Palliative Care.24 Symptom management is focused on pain, nausea, and vomiting.
Which drugs can I use for abdominal pain?
Patients experience two types of abdominal pain: continuous and colic. Each type of pain requires different treatment approaches and classes of drugs.
Potent opioids such as morphine, hydromorphone (Dilaudid), and fentanyl (Fentora) are used to relieve continuous abdominal pain.7 The dose is titrated for adequate relief. Subcutaneous, intravenous, sublingual, and transdermal routes can be used if nausea and vomiting prevent oral administration.
However, opioids can aggravate colic by stimulating circular smooth muscle, leading to segmental contractions. Opioid-sparing adjuvant drugs such as ketorolac (Toradol) may improve colic and continuous pain and prevent a partial obstruction from becoming a complete obstruction by sparing opioid doses.35
Colic may persist or worsen with the use of opioids. Drugs that reduce colic include the scopolamine drugs hyoscine butylbromide and hyoscine hydrobromide, glycopyrrolate (Robinul), and octreotide (Sandostatin).7,34–37
Which drugs are appropriate for reducing nausea and vomiting?
Phenothiazines reduce nausea and control vomiting. Chlorpromazine (Thorazine), prochlorperazine (Compro, Compazine), and promethazine (Phenergan) have all been reported to treat nausea successfully.35,37
Haloperidol (Haldol), a butyrophenone selective dopamine D2-receptor antagonist, has negligible anticholinergic activity. At low doses it produces less sedation than phenothiazines and is an ideal agent for patients with nausea and delirium.35 Doses range from 5 to 15 mg/day, given in divided doses or as intermittent or continuous intravenous infusions.
Anticholinergics, with or without somatostatin analogues, reduce gastrointestinal secretions, fluid accumulation, and vomiting. Anticholinergics bind to muscarinic receptors on enteric neurons in the myenteric and the submucosal plexus. Dosages:
- Hyoscine butylbromide 40 to 120 mg/day.
- Hyoscine hydrobromide 0.2 to 0.9 mg/day.7,34
Glycopyrrolate, a quaternary ammonium anticholinergic, has minimal central nervous system penetration and is less likely to cause delirium or cardiac side effects compared with tertiary amine anticholinergics such as atropine and scopolamine.38 The recommended dose is 0.1 to 0.2 mg subcutaneously or intravenously three to four times daily.
Octreotide, an analogue of somatostatin, blocks the release of vasoactive intestinal polypeptide, which is increased in malignant bowel obstruction.14,15 It reduces the excretion of water, sodium, and chloride into the bowel lumen and increases the absorption of electrolytes and water. It also inhibits pancreatic enzyme secretion and splanchnic blood flow. The result of all these effects is reduced luminal content, reduced motility, reduced vascular congestion of the bowel wall, and, in certain circumstances, reduced ascites.39
In small randomized trials, octreotide was more successful than anticholinergics at improving nausea, vomiting, and colic in patients requiring a nasogastric tube and in those whose symptoms were refractory to standard medical treatment.5,34,40–43 A recent case report found octreotide helpful in resolving symptoms of partial bowel obstruction that were unresponsive to standard measures.44
Octreotide is well tolerated and reduces the time patients require a nasogastric tube without significantly worsening xerostomia. High cost limits its use in American hospice care due to the Medicare capitated system of reimbursement for drugs and services, and as a result it is a second-tier drug despite evidence of its efficacy.
Octreotide doses are 100 to 200 mg every 8 hours.
Metoclopramide (Reglan), a dopaminergic antagonist, a 5HT4 receptor agonist, and a 5HT3 receptor blocker at doses greater than 120 mg/day, combines the action of a phenothiazine, which blocks D2 receptors in the central chemoreceptor trigger zone, with promotility actions through serotonin receptors (5HT4).35,37
Metoclopramide should not be used with anticholinergics or in patients with colic or complete obstruction.35,45 In some centers it is the first-line drug for functional or partial bowel obstruction.7 Dosages range from 40 to 240 mg/day.
Olanzapine (Zyprexa), an atypical antipsychotic, blocks multiple neurotransmitter receptors (D2, H1, Ach, 5HT3) responsible for initiating emesis. It is an option in patients whose nausea and vomiting fail to respond to standard antiemetics.46 Dosages range from 2.5 to 20 mg/day.
Dissolvable tablets are given sublingually, which makes olanzapine a versatile antiemetic in cases of intractable nausea. Our unpublished experience is that the sublingual route reduces nausea associated with malignant bowel obstruction and obviates the need for subcutaneous injections or intravenous antiemetic infusions.
Corticosteroids. Although how corticosteroids relieve malignant bowel obstruction is unknown, they are presumed to act centrally.37,45 In addition, they reduce peritumoral edema and luminal salt and water, and they also have antiemetic and analgesic properties.
Evidence from a meta-analysis found that 6 to 16 mg of parenteral dexamethasone per day reduced symptoms and improved bowel function in 60% of patients but did not change the prognosis.11
A trial of 4 or 5 days is adequate to determine response. If there is no response, the corticosteroid should be rapidly tapered. Side effects are minimal when corticosteroids are used short-term.
Combination therapy. Only rarely does a single drug resolve symptoms of malignant bowel obstruction. Antiemetics, analgesics, corticosteroids, antisecretory anticholinergics, and octreotide are often required in combination to achieve acceptable symptom relief.3,5,7,47
In a small prospective case series, the combination of metoclopramide 60 mg/day, octreotide 0.3 mg/day, and dexamethasone 12 mg/day with a single bolus of amidotrizoic acid (a contrast agent) improved intestinal transit within 1 to 5 days and resolved vomiting within 24 hours.45
Compatibility and the route of administration of medications are key considerations when choosing drug combinations.
WHEN TO CONSIDER A VENTING GASTROSTOMY
Patients with a poor performance status, rapidly progressive disease, peritoneal carcinomatosis, a life expectancy of less than 30 days, or multiple levels of obstruction benefit from placement of a percutaneous endoscopic gastrostomy tube (ie, a venting gastrostomy) rather than surgery if symptoms do not respond to drug therapy.7,48 There is compelling evidence that this procedure relieves nausea and vomiting in 80% to 90% of patients and restores some level of oral intake in many.5,6,48,49 A venting gastrostomy tube can be placed during surgical exploration, percutaneously with fluoroscopy, or endoscopically.9
There are no absolute contraindications to gastrostomy tube placement. It is feasible even in patients with tumors encasing the stomach, diffuse carcinomatosis, and ascites.48 However, massive ascites, previous upper abdominal surgery, or a large mass attached to the abdominal wall make tube placement difficult.
Complications are often local. Patients experience transient abdominal wall pain after the procedure. Dislodgement, bleeding, catheter migration, peritonitis, and necrotizing fasciitis are early complications. Others include skin excoriation from leakage of gastric contents, leakage of ascitic fluid from the site, and obstruction or dislodgement of the tube.48,49
Patients can be discharged from the hospital soon after the tube is placed, usually with fewer medications than for patients who undergo surgery.48 This is particularly important for patients with a short expected survival. Some patients at home benefit from hydration (less than 2 L/day) via an existing central venous port or peripherally inserted central catheter, or by hypodermoclysis.
WHEN IS A NASOGASTRIC TUBE APPROPRIATE?
Some patients with malignant bowel obstruction require a nasogastric tube early in their hospital course.12 Unfortunately, nasogastric tubes, if left in place, cause nose and throat pain, sinusitis, abscess formation, erosion of nasal cartilage, aspiration, esophageal erosion, pharyngitis, and social isolation.5,6
Nasogastric tubes should be a temporizing measure to vent gastrointestinal secretions, reduce abdominal distension, and improve nausea and vomiting while a decision about surgery is being made.13,24 If surgery is not feasible, one can avoid the long-term complications and discomfort of a nasogastric tube via medical management and earlier evaluation for venting gastrostomy in those with symptoms that respond poorly to optimal medical management.49
WHICH PATIENTS BENEFIT FROM TOTAL PARENTERAL NUTRITION?
The use of total parenteral nutrition in patients with incurable malignancies is controversial. Enteral and parenteral feeding can increase muscle mass and improve functional status and quality of life in a subset of patients who are not suffering from cancer-related cachexia.2,50,51 However, for those whose weight loss and malnutrition are consequences of tumor-mediated cachexia, as demonstrated by anorexia and an elevated C-reactive protein level, parenteral nutrition is unlikely to improve the outcome.51 For most terminally ill patients, retrospective studies have failed to show that parenteral nutrition improves overall survival, performance status, or quality of life.2,48,50–54
Total parenteral nutrition poses risks: it is invasive and requires central venous access, which predisposes to infection; it requires frequent monitoring of hydration and electrolytes; and it predisposes to thrombosis, diarrhea, hyperglycemia, and liver failure.50–56
Total parenteral nutrition may be justified in patients with minimal tumor burden who are candidates for definitive surgery, or in those with a good performance status early in the disease course who have not had chemotherapy or whose cancer responds to chemotherapy.2,50–56
The American College of Physicians discourages the routine use of parenteral nutrition in those with advanced cancer who are undergoing palliative chemotherapy, since few patients benefit and many experience side effects.53
Total parenteral nutrition is much like a medical intervention in that it should be offered or continued only if it provides benefit. Conversations at the time that it is begun must include adverse effects that will lead to its discontinuation, and criteria for response. In certain situations, a limited trial of parenteral nutrition may be considered for patients with an uncertain prognosis or for those who have potentially reversible conditions that limit oral intake.51 In such cases, there should be a clear understanding between patient and physician that parenteral nutrition will be discontinued if it fails to show benefit.53
ADDITIONAL CONCERNS OF PATIENTS AND FAMILIES
‘Will I starve to death?’
Starvation is a fear echoed by patients and families. Ethical discourse on the continuation of nutrition and hydration for the terminally ill has been polarizing.57–60 Withdrawal of nutrition can be perceived as euthanasia.
Advanced cancer patients in general do not experience hunger, and those who do require only small amounts of food for satiation.61 In one report, most patients died of their advanced cancer and not from starvation.52 Artificial hydration and nutrition will thus not influence survival and can even be a burden without benefit in the imminently dying.60 These patients should be encouraged to take food orally for pleasure, as long as it is tolerated, without consideration of end points such as weight gain, body mass index, or albumin levels.
Complaints of thirst and dryness of the mouth are relieved with mouth care, ice chips, lubrication to the lips, and sips of fluid, rather than by parenteral nutrition.59 Patients with a terminal illness experience relief from thirst with minimal intake. The symptom of thirst may be relieved without hydration.34,61 Adequate hydration requires smaller fluid volumes due to decreased body weight, decreased renal clearance of free water, and decreased insensible water losses from reduced physical activity.58
‘Can we continue intravenous hydration so he won’t die of thirst?’
Overzealous intravenous hydration may worsen the symptoms of malignant bowel obstruction. Overhydration can increase secretions in the gut lumen and worsen the secretion-distention-contraction cycle, leading to greater abdominal pain and to nausea and vomiting.7 There is a greater risk of fluid overload in these patients, since they have edema and excessive interstitial fluid. Most have a low serum albumin level, which results in movement of fluid from intravascular to interstitial spaces due to reduced colloid osmotic pressure. In these instances, overzealous hydration can lead to respiratory insufficiency and worsening edema.
In spite of numerous discussions in the medical literature of the benefits and burdens of continual hydration, there is no consensus or guideline. When a patient has limited oral intake, the decision to hydrate should be individualized, with careful assessment of the risks and benefits and in accordance with the patient’s or family’s wishes.57,58
Is treatment at home feasible?
Discharging patients with inoperable malignant bowel obstruction requires careful planning. Patients and family members need to be educated on the use of around-the-clock medications and symptom-targeted, as-needed drugs. Days before discharge, questions about diet need to be clarified. Education about total parenteral nutrition and gastrostomy tube care should be completed before discharge from the hospital.
Drug management should be simplified, or compatible medications should be combined into a single infusion. For example, morphine, glycopyrrolate, and haloperidol or metoclopramide are chemically compatible in standard intravenous solutions and can be combined.
Families feel less anxious about the foreseen and the possible unforeseen course of the illness if they can talk with hospice workers early on. This early involvement also facilitates the transition to home hospice care.
SUMMARY OF IMPORTANT POINTS
- Patients with malignant bowel obstruction need a highly individualized approach, tailored to their medical condition, the prognosis, and the goals of care.
- Surgery should not be routinely undertaken; less-invasive approaches such as gastric or colonic stenting should be considered first.
- Combinations of analgesics, antisecretory drugs, and antiemetics can provide acceptable symptom relief in the inoperable patient.
- A venting gastrostomy should be considered if drug therapy fails to reduce nausea and vomiting to an acceptable level.
- A nasogastric tube should be used only as a temporizing measure, until symptoms are controlled medically or a venting gastrostomy is placed.
- Total parenteral nutrition is of benefit only in patients with intermediate life expectancy who may otherwise die of starvation rather than from the cancer itself.
- Mercadante S. Intestinal dysfunction and obstruction. In:Walsh D, editor. Palliative Medicine. Philadelphia, PA: Saunders/Elsevier, 2009:1267–1275.
- Pasanisi F, Orban A, Scalfi L, et al. Predictors of survival in terminal-cancer patients with irreversible bowel obstruction receiving home parenteral nutrition. Nutrition 2001; 17:581–584.
- Pameijer CR, Mahvi DM, Stewart JA, Weber SM. Bowel obstruction in patients with metastatic cancer: does intervention influence outcome? Int J Gastrointest Cancer 2005; 35:127–133.
- Bais JMJ, Schilthuis MS, Ansink AC. Palliative management of intestinal obstruction in patients with advanced gynaecological cancer. J Gynecol Oncol 2002; 7:299–305.
- Laval G, Arvieux C, Stefani L, Villard ML, Mestrallet JP, Cardin N. Protocol for the treatment of malignant inoperable bowel obstruction: a prospective study of 80 cases at Grenoble University Hospital Center. J Pain Symptom Manage 2006; 31:502–512.
- Jatoi A, Podratz KC, Gill P, Hartmann LC. Pathophysiology and palliation of inoperable bowel obstruction in patients with ovarian cancer. J Support Oncol 2004; 2:323–334.
- Ripamonti CI, Easson AM, Gerdes H. Management of malignant bowel obstruction. Eur J Cancer 2008; 44:1105–1115.
- Roeland E, von Gunten CF. Current concepts in malignant bowel obstruction management. Curr Oncol Rep 2009; 11:298–303.
- Baron TH. Interventional palliative strategies for malignant bowel obstruction. Curr Oncol Rep 2009; 11:293–297.
- Feuer DJ, Broadley KE, Shepherd JH, Barton DP. Surgery for the resolution of symptoms in malignant bowel obstruction in advanced gynaecological and gastrointestinal cancer. Cochrane Database Syst Rev 2000;CD002764.
- Feuer DJ, Broadley KE. Corticosteroids for the resolution of malignant bowel obstruction in advanced gynaecological and gastrointestinal cancer. Cochrane Database Syst Rev 2000;CD001219.
- Woolfson RG, Jennings K, Whalen GF. Management of bowel obstruction in patients with abdominal cancer. Arch Surg 1997; 132:1093–1097.
- Saunders MD, Kimmey MB. Systematic review: acute colonic pseudo-obstruction. Aliment Pharmacol Ther 2005; 22:917–925.
- Ripamonti C, Bruera E. Palliative management of malignant bowel obstruction. Int J Gynecol Cancer 2002; 12:135–143.
- Nellgård P, Bojö L, Cassuto J. Importance of vasoactive intestinal peptide and somatostatin for fluid losses in small-bowel obstruction. Scand J Gastroenterol 1995; 30:464–469.
- Böhner H, Yang Q, Franke C, Verreet PR, Ohmann C. Simple data from history and physical examination help to exclude bowel obstruction and to avoid radiographic studies in patients with acute abdominal pain. Eur J Surg 1998; 164:777–784.
- Maglinte DD, Kelvin FM, Sandrasegaran K, et al. Radiology of small bowel obstruction: contemporary approach and controversies. Abdom Imaging 2005; 30:160–178.
- Maglinte DD, Howard TJ, Lillemoe KD, Sandrasegaran K, Rex DK. Small-bowel obstruction: state-of-the-art imaging and its role in clinical management. Clin Gastroenterol Hepatol 2008; 6:130–139.
- Silva AC, Pimenta M, Guimarães LS. Small bowel obstruction: what to look for. Radiographics 2009; 29:423–439.
- Finan PJ, Campbell S, Verma R, et al. The management of malignant large bowel obstruction: ACPGBI position statement. Colorectal Dis 2007; 9(suppl 4):1–17.
- Kohli MD, Maglinte DD. CT enteroclysis in incomplete small bowel obstruction. Abdom Imaging 2009; 34:321–327.
- Ha HK, Shin BS, Lee SI, et al. Usefulness of CT in patients with intestinal obstruction who have undergone abdominal surgery for malignancy. AJR Am J Roentgenol 1998; 171:1587–1593.
- DeBernardo R. Surgical management of malignant bowel obstruction: strategies toward palliation of patients with advanced cancer. Curr Oncol Rep 2009; 11:287–292.
- Ripamonti C, Twycross R, Baines M, et al; Working Group of the European Association for Palliative Care. Clinical-practice recommendations for the management of bowel obstruction in patients with end-stage cancer. Support Care Cancer 2001; 9:223–233.
- Mangili G, Aletti G, Frigerio L, et al. Palliative care for intestinal obstruction in recurrent ovarian cancer: a multivariate analysis. Int J Gynecol Cancer 2005; 15:830–835.
- Turner J, Cummin T, Bennett A, Swift G, Green J. Stents and stentability: treatment for malignant bowel obstruction. Br J Hosp Med (Lond) 2008; 69:676–680.
- Khot UP, Lang AW, Murali K, Parker MC. Systematic review of the efficacy and safety of colorectal stents. Br J Surg 2002; 89:1096–1102.
- Hosono S, Ohtani H, Arimoto Y, Kanamiya Y. Endoscopic stenting versus surgical gastroenterostomy for palliation of malignant gastroduodenal obstruction: a meta-analysis. J Gastroenterol 2007; 42:283–290.
- Del Piano M, Ballarè M, Montino F, et al. Endoscopy or surgery for malignant GI outlet obstruction? Gastrointest Endosc 2005; 61:421–426.
- Tilney HS, Lovegrove RE, Purkayastha S, et al. Comparison of colonic stenting and open surgery for malignant large bowel obstruction. Surg Endosc 2007; 21:225–233.
- Holt AP, Patel M, Ahmed MM. Palliation of patients with malignant gastroduodenal obstruction with self-expanding metallic stents: the treatment of choice? Gastrointest Endosc 2004; 60:1010–1017.
- Dastur JK, Forshaw MJ, Modarai B, Solkar MM, Raymond T, Parker MC. Comparison of short-and long-term outcomes following either insertion of self-expanding metallic stents or emergency surgery in malignant large bowel obstruction. Tech Coloproctol 2008; 12:51–55.
- Turner J, Cummin T, Bennett A, Swift G, Green J. Stents and stentability: treatment for malignant bowel obstruction. Br J Hosp Med (Lond) 2008; 69:676–680.
- Ripamonti C, Mercadante S, Groff L, Zecca E, De Conno F, Casuccio A. Role of octreotide, scopolamine butylbromide, and hydration in symptom control of patients with inoperable bowel obstruction and nasogastric tubes: a prospective randomized trial. J Pain Symptom Manage 2000; 19:23–34.
- Davis MP, Walsh D. Treatment of nausea and vomiting in advanced cancer. Support Care Cancer 2000; 8:444–452.
- Bicanovsky LK, Lagman RL, Davis MP, Walsh D. Managing nonmalignant chronic abdominal pain and malignant bowel obstruction. Gastroenterol Clin North Am 2006; 35:131–142.
- Glare P, Pereira G, Kristjanson LJ, Stockler M, Tattersall M. Systematic review of the efficacy of antiemetics in the treatment of nausea in patients with far-advanced cancer. Support Care Cancer 2004; 12:432–440.
- Davis MP, Furste A. Glycopyrrolate: a useful drug in the palliation of mechanical bowel obstruction. J Pain Symptom Manage 1999; 18:153–154.
- Ripamonti C, Mercadante S. How to use octreotide for malignant bowel obstruction. J Support Oncol 2004; 2:357–364.
- Shima Y, Ohtsu A, Shirao K, Sasaki Y. Clinical efficacy and safety of octreotide (SMS201-995) in terminally ill Japanese cancer patients with malignant bowel obstruction. Jpn J Clin Oncol 2008; 38:354–359.
- Mercadante S, Casuccio A, Mangione S. Medical treatment for inoperable malignant bowel obstruction: a qualitative systematic review. J Pain Symptom Manage 2007; 33:217–223.
- Mystakidou K, Tsilika E, Kalaidopoulou O, Chondros K, Georgaki S, Papadimitriou L. Comparison of octreotide administration vs conservative treatment in the management of inoperable bowel obstruction in patients with far advanced cancer: a randomized, double-blind, controlled clinical trial. Anticancer Res 2002; 22:1187–1192.
- Mercadante S, Ripamonti C, Casuccio A, Zecca E, Groff L. Comparison of octreotide and hyoscine butylbromide in controlling gastrointestinal symptoms due to malignant inoperable bowel obstruction. Support Care Cancer 2000; 8:188–191.
- Myers J, Tamber A, Farhadian M. Management of treatment-related intermittent partial small bowel obstruction: the use of octreotide. J Pain Symptom Manage 2010; 39:e1–e3.
- Mercadante S, Ferrera P, Villari P, Marrazzo A. Aggressive pharmacological treatment for reversing malignant bowel obstruction. J Pain Symptom Manage 2004; 28:412–416.
- Srivastava M, Brito-Dellan N, Davis MP, Leach M, Lagman R. Olanzapine as an antiemetic in refractory nausea and vomiting in advanced cancer. J Pain Symptom Manage 2003; 25:578–582.
- Bentley A, Boyd K. Use of clinical pictures in the management of nausea and vomiting: a prospective audit. Palliat Med 2001; 15:247–253.
- Pothuri B, Montemarano M, Gerardi M, et al. Percutaneous endoscopic gastrostomy tube placement in patients with malignant bowel obstruction due to ovarian carcinoma. Gynecol Oncol 2005; 96:330–334.
- Brooksbank MA, Game PA, Ashby MA. Palliative venting gastrostomy in malignant intestinal obstruction. Palliat Med 2002; 16:520–526.
- Wang MY, Wu MH, Hsieh DY, et al. Home parenteral nutrition support in adults: experience of a medical center in Asia. JPEN J Parenter Enteral Nutr 2007; 31:306–310.
- Dy SM. Enteral and parenteral nutrition in terminally ill cancer patients: a review of the literature. Am J Hosp Palliat Care 2006; 23:369–377.
- Whitworth MK, Whitfield A, Holm S, Shaffer J, Makin W, Jayson GC. Doctor, does this mean I’m going to starve to death? J Clin Oncol 2004; 22:199–201.
- Hoda D, Jatoi A, Burnes J, Loprinzi C, Kelly D. Should patients with advanced, incurable cancers ever be sent home with total parenteral nutrition? A single institution’s 20-year experience. Cancer 2005; 103:863–868.
- Philip J, Depczynski B. The role of total parenteral nutrition for patients with irreversible bowel obstruction secondary to gynecological malignancy. J Pain Symptom Manage 1997; 13:104–111.
- August DA, Thorn D, Fisher RL, Welchek CM. Home parenteral nutrition for patients with inoperable malignant bowel obstruction. JPEN J Parenter Enteral Nutr 1991; 15:323–327.
- Abu-Rustum NR, Barakat RR, Venkatraman E, Spriggs D. Chemotherapy and total parenteral nutrition for advanced ovarian cancer with bowel obstruction. Gynecol Oncol 1997; 64:493–495.
- Fainsinger RL, Bruera E. When to treat dehydration in a terminally ill patient? Support Care Cancer 1997; 5:205–211.
- Steiner N, Bruera E. Methods of hydration in palliative care patients. J Palliat Care 1998; 14:6–13.
- Slomka J. Withholding nutrition at the end of life: clinical and ethical issues. Cleve Clin J Med 2003; 70:548–552.
- Chiu TY, Hu WY, Chuang RB, Chen CY. Nutrition and hydration for terminal cancer patients in Taiwan. Support Care Cancer 2002; 10:630–636.
- McCann RM, Hall WJ, Groth-Juncker A. Comfort care for terminally ill patients. The appropriate use of nutrition and hydration. JAMA 1994; 272:1263–1266.
- Mercadante S. Intestinal dysfunction and obstruction. In:Walsh D, editor. Palliative Medicine. Philadelphia, PA: Saunders/Elsevier, 2009:1267–1275.
- Pasanisi F, Orban A, Scalfi L, et al. Predictors of survival in terminal-cancer patients with irreversible bowel obstruction receiving home parenteral nutrition. Nutrition 2001; 17:581–584.
- Pameijer CR, Mahvi DM, Stewart JA, Weber SM. Bowel obstruction in patients with metastatic cancer: does intervention influence outcome? Int J Gastrointest Cancer 2005; 35:127–133.
- Bais JMJ, Schilthuis MS, Ansink AC. Palliative management of intestinal obstruction in patients with advanced gynaecological cancer. J Gynecol Oncol 2002; 7:299–305.
- Laval G, Arvieux C, Stefani L, Villard ML, Mestrallet JP, Cardin N. Protocol for the treatment of malignant inoperable bowel obstruction: a prospective study of 80 cases at Grenoble University Hospital Center. J Pain Symptom Manage 2006; 31:502–512.
- Jatoi A, Podratz KC, Gill P, Hartmann LC. Pathophysiology and palliation of inoperable bowel obstruction in patients with ovarian cancer. J Support Oncol 2004; 2:323–334.
- Ripamonti CI, Easson AM, Gerdes H. Management of malignant bowel obstruction. Eur J Cancer 2008; 44:1105–1115.
- Roeland E, von Gunten CF. Current concepts in malignant bowel obstruction management. Curr Oncol Rep 2009; 11:298–303.
- Baron TH. Interventional palliative strategies for malignant bowel obstruction. Curr Oncol Rep 2009; 11:293–297.
- Feuer DJ, Broadley KE, Shepherd JH, Barton DP. Surgery for the resolution of symptoms in malignant bowel obstruction in advanced gynaecological and gastrointestinal cancer. Cochrane Database Syst Rev 2000;CD002764.
- Feuer DJ, Broadley KE. Corticosteroids for the resolution of malignant bowel obstruction in advanced gynaecological and gastrointestinal cancer. Cochrane Database Syst Rev 2000;CD001219.
- Woolfson RG, Jennings K, Whalen GF. Management of bowel obstruction in patients with abdominal cancer. Arch Surg 1997; 132:1093–1097.
- Saunders MD, Kimmey MB. Systematic review: acute colonic pseudo-obstruction. Aliment Pharmacol Ther 2005; 22:917–925.
- Ripamonti C, Bruera E. Palliative management of malignant bowel obstruction. Int J Gynecol Cancer 2002; 12:135–143.
- Nellgård P, Bojö L, Cassuto J. Importance of vasoactive intestinal peptide and somatostatin for fluid losses in small-bowel obstruction. Scand J Gastroenterol 1995; 30:464–469.
- Böhner H, Yang Q, Franke C, Verreet PR, Ohmann C. Simple data from history and physical examination help to exclude bowel obstruction and to avoid radiographic studies in patients with acute abdominal pain. Eur J Surg 1998; 164:777–784.
- Maglinte DD, Kelvin FM, Sandrasegaran K, et al. Radiology of small bowel obstruction: contemporary approach and controversies. Abdom Imaging 2005; 30:160–178.
- Maglinte DD, Howard TJ, Lillemoe KD, Sandrasegaran K, Rex DK. Small-bowel obstruction: state-of-the-art imaging and its role in clinical management. Clin Gastroenterol Hepatol 2008; 6:130–139.
- Silva AC, Pimenta M, Guimarães LS. Small bowel obstruction: what to look for. Radiographics 2009; 29:423–439.
- Finan PJ, Campbell S, Verma R, et al. The management of malignant large bowel obstruction: ACPGBI position statement. Colorectal Dis 2007; 9(suppl 4):1–17.
- Kohli MD, Maglinte DD. CT enteroclysis in incomplete small bowel obstruction. Abdom Imaging 2009; 34:321–327.
- Ha HK, Shin BS, Lee SI, et al. Usefulness of CT in patients with intestinal obstruction who have undergone abdominal surgery for malignancy. AJR Am J Roentgenol 1998; 171:1587–1593.
- DeBernardo R. Surgical management of malignant bowel obstruction: strategies toward palliation of patients with advanced cancer. Curr Oncol Rep 2009; 11:287–292.
- Ripamonti C, Twycross R, Baines M, et al; Working Group of the European Association for Palliative Care. Clinical-practice recommendations for the management of bowel obstruction in patients with end-stage cancer. Support Care Cancer 2001; 9:223–233.
- Mangili G, Aletti G, Frigerio L, et al. Palliative care for intestinal obstruction in recurrent ovarian cancer: a multivariate analysis. Int J Gynecol Cancer 2005; 15:830–835.
- Turner J, Cummin T, Bennett A, Swift G, Green J. Stents and stentability: treatment for malignant bowel obstruction. Br J Hosp Med (Lond) 2008; 69:676–680.
- Khot UP, Lang AW, Murali K, Parker MC. Systematic review of the efficacy and safety of colorectal stents. Br J Surg 2002; 89:1096–1102.
- Hosono S, Ohtani H, Arimoto Y, Kanamiya Y. Endoscopic stenting versus surgical gastroenterostomy for palliation of malignant gastroduodenal obstruction: a meta-analysis. J Gastroenterol 2007; 42:283–290.
- Del Piano M, Ballarè M, Montino F, et al. Endoscopy or surgery for malignant GI outlet obstruction? Gastrointest Endosc 2005; 61:421–426.
- Tilney HS, Lovegrove RE, Purkayastha S, et al. Comparison of colonic stenting and open surgery for malignant large bowel obstruction. Surg Endosc 2007; 21:225–233.
- Holt AP, Patel M, Ahmed MM. Palliation of patients with malignant gastroduodenal obstruction with self-expanding metallic stents: the treatment of choice? Gastrointest Endosc 2004; 60:1010–1017.
- Dastur JK, Forshaw MJ, Modarai B, Solkar MM, Raymond T, Parker MC. Comparison of short-and long-term outcomes following either insertion of self-expanding metallic stents or emergency surgery in malignant large bowel obstruction. Tech Coloproctol 2008; 12:51–55.
- Turner J, Cummin T, Bennett A, Swift G, Green J. Stents and stentability: treatment for malignant bowel obstruction. Br J Hosp Med (Lond) 2008; 69:676–680.
- Ripamonti C, Mercadante S, Groff L, Zecca E, De Conno F, Casuccio A. Role of octreotide, scopolamine butylbromide, and hydration in symptom control of patients with inoperable bowel obstruction and nasogastric tubes: a prospective randomized trial. J Pain Symptom Manage 2000; 19:23–34.
- Davis MP, Walsh D. Treatment of nausea and vomiting in advanced cancer. Support Care Cancer 2000; 8:444–452.
- Bicanovsky LK, Lagman RL, Davis MP, Walsh D. Managing nonmalignant chronic abdominal pain and malignant bowel obstruction. Gastroenterol Clin North Am 2006; 35:131–142.
- Glare P, Pereira G, Kristjanson LJ, Stockler M, Tattersall M. Systematic review of the efficacy of antiemetics in the treatment of nausea in patients with far-advanced cancer. Support Care Cancer 2004; 12:432–440.
- Davis MP, Furste A. Glycopyrrolate: a useful drug in the palliation of mechanical bowel obstruction. J Pain Symptom Manage 1999; 18:153–154.
- Ripamonti C, Mercadante S. How to use octreotide for malignant bowel obstruction. J Support Oncol 2004; 2:357–364.
- Shima Y, Ohtsu A, Shirao K, Sasaki Y. Clinical efficacy and safety of octreotide (SMS201-995) in terminally ill Japanese cancer patients with malignant bowel obstruction. Jpn J Clin Oncol 2008; 38:354–359.
- Mercadante S, Casuccio A, Mangione S. Medical treatment for inoperable malignant bowel obstruction: a qualitative systematic review. J Pain Symptom Manage 2007; 33:217–223.
- Mystakidou K, Tsilika E, Kalaidopoulou O, Chondros K, Georgaki S, Papadimitriou L. Comparison of octreotide administration vs conservative treatment in the management of inoperable bowel obstruction in patients with far advanced cancer: a randomized, double-blind, controlled clinical trial. Anticancer Res 2002; 22:1187–1192.
- Mercadante S, Ripamonti C, Casuccio A, Zecca E, Groff L. Comparison of octreotide and hyoscine butylbromide in controlling gastrointestinal symptoms due to malignant inoperable bowel obstruction. Support Care Cancer 2000; 8:188–191.
- Myers J, Tamber A, Farhadian M. Management of treatment-related intermittent partial small bowel obstruction: the use of octreotide. J Pain Symptom Manage 2010; 39:e1–e3.
- Mercadante S, Ferrera P, Villari P, Marrazzo A. Aggressive pharmacological treatment for reversing malignant bowel obstruction. J Pain Symptom Manage 2004; 28:412–416.
- Srivastava M, Brito-Dellan N, Davis MP, Leach M, Lagman R. Olanzapine as an antiemetic in refractory nausea and vomiting in advanced cancer. J Pain Symptom Manage 2003; 25:578–582.
- Bentley A, Boyd K. Use of clinical pictures in the management of nausea and vomiting: a prospective audit. Palliat Med 2001; 15:247–253.
- Pothuri B, Montemarano M, Gerardi M, et al. Percutaneous endoscopic gastrostomy tube placement in patients with malignant bowel obstruction due to ovarian carcinoma. Gynecol Oncol 2005; 96:330–334.
- Brooksbank MA, Game PA, Ashby MA. Palliative venting gastrostomy in malignant intestinal obstruction. Palliat Med 2002; 16:520–526.
- Wang MY, Wu MH, Hsieh DY, et al. Home parenteral nutrition support in adults: experience of a medical center in Asia. JPEN J Parenter Enteral Nutr 2007; 31:306–310.
- Dy SM. Enteral and parenteral nutrition in terminally ill cancer patients: a review of the literature. Am J Hosp Palliat Care 2006; 23:369–377.
- Whitworth MK, Whitfield A, Holm S, Shaffer J, Makin W, Jayson GC. Doctor, does this mean I’m going to starve to death? J Clin Oncol 2004; 22:199–201.
- Hoda D, Jatoi A, Burnes J, Loprinzi C, Kelly D. Should patients with advanced, incurable cancers ever be sent home with total parenteral nutrition? A single institution’s 20-year experience. Cancer 2005; 103:863–868.
- Philip J, Depczynski B. The role of total parenteral nutrition for patients with irreversible bowel obstruction secondary to gynecological malignancy. J Pain Symptom Manage 1997; 13:104–111.
- August DA, Thorn D, Fisher RL, Welchek CM. Home parenteral nutrition for patients with inoperable malignant bowel obstruction. JPEN J Parenter Enteral Nutr 1991; 15:323–327.
- Abu-Rustum NR, Barakat RR, Venkatraman E, Spriggs D. Chemotherapy and total parenteral nutrition for advanced ovarian cancer with bowel obstruction. Gynecol Oncol 1997; 64:493–495.
- Fainsinger RL, Bruera E. When to treat dehydration in a terminally ill patient? Support Care Cancer 1997; 5:205–211.
- Steiner N, Bruera E. Methods of hydration in palliative care patients. J Palliat Care 1998; 14:6–13.
- Slomka J. Withholding nutrition at the end of life: clinical and ethical issues. Cleve Clin J Med 2003; 70:548–552.
- Chiu TY, Hu WY, Chuang RB, Chen CY. Nutrition and hydration for terminal cancer patients in Taiwan. Support Care Cancer 2002; 10:630–636.
- McCann RM, Hall WJ, Groth-Juncker A. Comfort care for terminally ill patients. The appropriate use of nutrition and hydration. JAMA 1994; 272:1263–1266.
KEY POINTS
- Combinations of analgesics, antisecretory drugs, and antiemetics can provide acceptable symptom relief.
- A venting gastrostomy should be considered if drug therapy fails to reduce nausea and vomiting to an acceptable level.
- A nasogastric tube should be used only as a temporizing measure, until symptoms are controlled medically or a venting gastrostomy is placed.
- Total parenteral nutrition is beneficial only in patients with intermediate life expectancy who may otherwise die of starvation rather than the cancer itself.