User login
Malignant bowel obstruction occurs in 5% to 51% of women with ovarian cancer and in 10% to 28% of patients with gastrointestinal cancer, predominantly in the advanced stages.1 Median survival after its onset ranges from 30 to 90 days.2–5
Its symptoms are challenging to manage, since nausea, vomiting, colic, and abdominal pain, which are common, cause significant physical distress and demoralization. The decision whether to correct it with surgery requires an individualized approach and a clear understanding of the goals of care and expected survival in the individual patient.
This review focuses on the management of inoperable malignant bowel obstruction and includes discussion of hydration, nutrition, and endoscopic palliative options.
WHAT ARE THE DIFFERENT TYPES OF OBSTRUCTION?
Bowel obstruction may be mechanical or functional, partial or complete, and may occur at one or at many sites. Tumors can impair bowel function in several ways6–8:
- Intraluminal tumors can occlude the lumen or act as a point of intussusception.
- Intramural tumors can extend to the mucosa and obstruct the lumen or impair peristalsis.
- Mesenteric and omental masses or malignant adhesions can kink or angulate the bowel, creating an extramural obstruction.
- Tumors that infiltrate into the mesentery, bowel muscle, or the enteric or celiac plexus can cause dysmotility.
Cholangiocarcinoma, pancreatic carcinoma, and gallbladder carcinoma are the most common tumors causing duodenal obstruction.9 Distal obstruction is caused mainly by colon and ovarian cancer.
Obstruction can be due to treatment
In a minority of patients, obstruction is unrelated to the cancer and is instead due to adhesions arising from surgery, radiation therapy (causing enteritis and strictures), desmoplastic reactions to intraperitoneal chemotherapy, torsion, or internal hernias.10–12
In rare cases, a patient has intestinal pseudo-obstruction from paraneoplastic destruction of enteric neurons, or severe ileus from anticholinergic or sympathomimetic drugs, as seen with acute colonic pseudo-obstruction (Ogilvie syndrome).13
Physiologic reactions to obstruction
Malignant bowel obstruction stimulates gastric, biliary, pancreatic, and intestinal secretions, decreases intraluminal sodium and water reabsorption, and increases mucosal sodium and water secretion.6,14 In response to the obstruction, peristalsis increases, and prostaglandin, vasoactive intestinal peptide, and nociceptive mediators are released. Vasoactive intestinal polypeptide perpetuates a cycle of secretion, distention, and contraction that leads to intestinal hyperemia, bowel edema, and accumulation of fluid in the lumen.8,10,11,15
Signs and symptoms depend on the site
The site of obstruction determines the signs and symptoms patients experience.7,14 Obstructions high in the gastrointestinal tract are associated with greater symptoms but fewer signs than colonic obstructions.1 Patients with proximal small-bowel obstruction have more severe nausea and a greater number of episodes of emesis, but they have relatively normal plain radiographs of the abdomen, which do not have the characteristic air-fluid levels commonly seen with distal small-bowel obstruction.
Most malignant obstructions remain partial, but increasing abdominal distention, worsening nausea, vomiting, abdominal pain, and obstipation over 1 to 2 weeks1 suggest progression to complete obstruction.
IMAGING TESTS FOR MALIGNANT BOWEL OBSTRUCTION
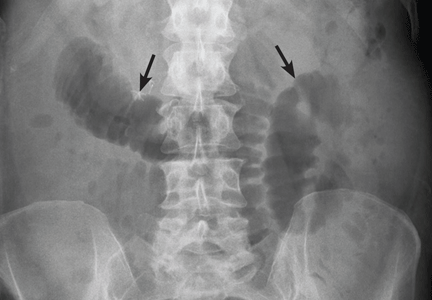
What is the value of plain radiography?
Despite these limitations, plain radiography is useful in assessing constipation and its severity as a potential cause of symptoms, and thus it remains an important initial imaging study in almost all patients with suspected malignant bowel obstruction.17,18 It is also used to assess response to treatment.
When do you need contrast radiographs?
Contrast radiography (barium swallow or barium or Gastrograffin enema) is helpful in patients with symptoms of dysmotility from suspected bowel obstruction. It defines the site or sites of obstruction and the extent of the obstruction with a fair degree of accuracy.7,19 Single-contrast studies, if positive, exclude opioid-induced bowel dysfunction or pseudo-obstruction in 83% of patients, with a sensitivity and specificity of 96% and 98%, respectively.8,20 Small-bowel follow-through with barium is more appropriate for low-grade obstructions or for symptomatic patients with a normal kidney, ureter, bladder radiograph.19
However, contrast radiography is limited by the patient’s ability to swallow barium or water-soluble contrast agents, and it can worsen nausea or vomiting.17,18 Also, barium is not absorbed systemically and may interfere with subsequent radiologic studies. Large volumes of contrast agents increase the risk of aspiration pneumonia in patients with poorly controlled nausea and can lead to severe impaction proximal to the obstructed site.8
Is enteroclysis better than barium swallow?
Enteroclysis, ie, injecting radiographic contrast into the bowel via a nasoduodenal tube, has some advantages over the barium swallow technique for detecting partial small-bowel obstruction, since it bypasses the stomach and allows for therapeutic decompression as well as direct visualization of the area of concern.17,18 Enteroclysis radiography objectively gauges severity of intestinal obstruction and bowel wall distensibility, which is an advantage over other imaging studies. Its sensitivity is 100% and specificity 88% in experienced hands.17 Enteroclysis studies also detect nonobstructing intraluminal tumors when computed tomography (CT) is not diagnostic.17,18,21
The drawbacks to enteroclysis are that it is technically difficult to perform and that few radiologists are trained in it.
When is CT useful?
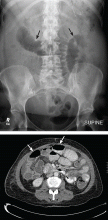
CT is the primary imaging study for patients with obstructive symptoms and a history of abdominal malignancy or a palpable abdominal mass17,20,22,23 (Figure 1). It has a specificity of 100% and a sensitivity of 94%. It plays a major role in decision-making regarding surgery, endoscopy, or palliative interventions,7,19 as it locates the obstruction and differentiates benign from malignant causes with a fair degree of precision.22
CT findings in malignant bowel obstruction may include:
- A mass at the site of obstruction or within the original surgical field
- Lymphadenopathy
- Abrupt transitions in luminal diameter or irregular thickening of the bowel wall at the site or sites of obstruction.7
SURGERY: A DIFFICULT DECISION
Is the patient fit for surgery?
Surgery for malignant bowel obstruction should not be done in patients who have advanced malignancies with bulky intra-abdominal metastases or cancer that has spread outside the abdominal cavity without taking into account treatment options for the cancer, the patient’s nutritional status, and the goals of care.
The role of abdominal surgery (debulking, resection, or bypass) in advanced cancer remains unclear and controversial.24 From 42% to 80% of patients report that symptoms improve after surgery, but recurrent obstruction occurs in 10% to 50%.10 Even in patients with low tumor bulk and good nutritional status, 30-day mortality rates range from 5% to 40%, and complication rates range from 9% to 90%.3,4,6,7,10,14
Outcomes after surgery depend on patient selection criteria perhaps as much as on the surgeon’s experience and skill. Patients with more advanced cancer who have had multiple surgical procedures and those with cancer that does not respond to chemotherapy and radiation present the greatest challenge to surgeons.23
What is the benefit of surgery?
Reports of palliative surgery have included information about 60-day survival rates after the operation, but a number of factors may be more meaningful in this context, such as postoperative symptoms, the patient’s overall wellbeing, how the original symptoms respond to the surgery, complications, and length of hospitalization.14 The paucity of published, validated, patient-related outcome data on which to gauge the value of surgery and the lack of a standard definition of “benefit” further confuse the objective determination of whether these patients benefit from surgery.
In a cohort with advanced ovarian cancer and bowel obstruction, surgery was detrimental to survival and quality of life for all subgroups, and most patients died in the hospital.6
The risk of surgery for malignant bowel obstruction is presumably higher than for abdominal surgery for other indications, since many of the patients are debilitated from their cancer and chemotherapy, and many are malnourished.23 Even when taking into account a potential selection bias in favor of surgery, several studies have reported no significant difference in 30-day mortality rates or median survival between operative and nonoperative groups.2,12 Neither the type of obstruction nor the extent of the surgery influenced outcomes. Surgical outcomes are best in patients with a benign cause of obstruction; little benefit is seen in operating on those with abdominal carcinomatosis.12
Nevertheless, surgery is beneficial in a select few. For patients with a good performance status, slowly progressive cancer, and an expected survival of more than 6 months, surgical bypass or resection is preferred.7,12,25 The challenge is to identify these surgical candidates, taking into account prognostic factors such as nutritional status, tumor burden, performance status, presence of ascites, advanced age, extensive prior chemotherapy or radiotherapy, and diffuse carcinomatosis.3,10,12,20,23
Is surgery consistent with the goals of care?
Crucial to decision-making are the goals of care. Since palliative surgery carries a low level of evidence for benefit in terms of quality of life and survival, time should be set aside to thoroughly review the patient’s medical condition, to explore options, and to clarify expectations and goals of care.3,10 Family members should be invited to be present during these discussions and to be involved in the decision-making process.
WHAT IS THE BENEFIT OF GASTRIC OR COLONIC STENTING?
Endoscopic procedures are alternatives to surgery and offer a palliative option in malignant bowel obstruction. Endoscopic procedures are associated with a shorter hospital stay and quicker recovery than after laparotomy.9,26–30 In certain situations, stenting serves as a bridge to surgery, allowing time to mitigate comorbid conditions, to enhance nutrition, and to complete staging, while relieving symptoms.27–29,31,32 Definitive surgery can be done as a single-stage procedure without a diverting enterostomy.
Self-expanding metal stents for gastric outlet, small-bowel, and colonic obstructions are an option in patients who have incurable metastatic disease who are unfit for surgery, in patients with a single point of obstruction or locally extensive disease, or in patients who do not want to undergo laparotomy.28–30
Technical and clinical success rates for colorectal stenting are high (88% to 93%).26,27 Stenting is more successful for left-sided colonic obstructions than for proximal colonic obstructions. Even for patients with extracolonic malignancies such as ovarian cancer, the technical success rate of colorectal stenting is 87%.26 However, patients with unrecognized peritoneal carcinomatosis or multifocal bowel obstruction are less likely to have symptomatic relief even after successful stenting.6,9
Contraindications to stenting
Absolute contraindications to stenting are colonic or tumor perforation with peritonitis. A relative contraindication is a rectal tumor within 2 cm of the anal margin. Stenting in this circumstance leads to tenesmus and incontinence.33
Complications of stenting
Death rates during colorectal stent insertion are less than 1%. The hospital stay and incidence of complications are significantly less than with surgery.26,30
Stent migration occurs in 10% of cases and is asymptomatic, but half of patients with this complication require a repeat intervention. The risk of migration is greater if chemotherapy or radiation therapy succeeds in shrinking the tumor.
Bleeding occurs in 5% of cases, usually from the underlying tumor.
Perforation occurs in 4%, but the rate increases to 10% with the use of dilatation before stent placement.
The rate of recurrent obstruction from tumor ingrowth, overgrowth, or fecal impaction is 10%.9,26,29 Recurrent obstruction may be treated with additional stents inserted within the original stent.9
GASTRIC OUTLET OBSTRUCTION: SURGERY VS STENTING
Gastrojejunostomy has in the past been the treatment of choice for gastric outlet obstruction. Certainly, patients with slow-growing tumors and an expected survival of greater than 60 days may be considered for this bypass procedure; those with a short tumor length, a single site of obstruction (preferably in the pylorus or proximal duodenum), a good performance status, and a life expectancy greater than 30 days are good candidates.7 Nevertheless, for patients with advanced cancer and poor performance status, gastroenterostomy carries a significant risk of morbidity and death.28
Endoscopic stenting of gastric outlet obstruction has a greater success rate, a shorter time to oral intake, a lower morbidity rate, a lower incidence of delayed gastric emptying, and a shorter hospital stay compared with gastroenterostomy.28,29 Technical success rates of stenting are 90%, and 75% of patients have resolution of nausea and vomiting.7 Stenting is generally not possible if the obstruction occurs beyond the ligament of Treitz.
Patients who are expected to survive less than 1 month or who have rapidly progressive disease, overt ascites, carcinomatosis, or multiple sites of obstruction should be managed with percutaneous, endoscopically placed gastrostomy tubes.7
Late complications of stenting for gastric outlet obstruction are occlusion with food or ingrowth of tumor through or around the wire mesh.7 This may require laser therapy or placement of a second stent, or both.
DRUG THERAPY
Medical therapy can palliate symptoms of malignant bowel obstruction for most patients.34 Recommendations have been published by the Working Group of the European Association for Palliative Care.24 Symptom management is focused on pain, nausea, and vomiting.
Which drugs can I use for abdominal pain?
Patients experience two types of abdominal pain: continuous and colic. Each type of pain requires different treatment approaches and classes of drugs.
Potent opioids such as morphine, hydromorphone (Dilaudid), and fentanyl (Fentora) are used to relieve continuous abdominal pain.7 The dose is titrated for adequate relief. Subcutaneous, intravenous, sublingual, and transdermal routes can be used if nausea and vomiting prevent oral administration.
However, opioids can aggravate colic by stimulating circular smooth muscle, leading to segmental contractions. Opioid-sparing adjuvant drugs such as ketorolac (Toradol) may improve colic and continuous pain and prevent a partial obstruction from becoming a complete obstruction by sparing opioid doses.35
Colic may persist or worsen with the use of opioids. Drugs that reduce colic include the scopolamine drugs hyoscine butylbromide and hyoscine hydrobromide, glycopyrrolate (Robinul), and octreotide (Sandostatin).7,34–37
Which drugs are appropriate for reducing nausea and vomiting?
Phenothiazines reduce nausea and control vomiting. Chlorpromazine (Thorazine), prochlorperazine (Compro, Compazine), and promethazine (Phenergan) have all been reported to treat nausea successfully.35,37
Haloperidol (Haldol), a butyrophenone selective dopamine D2-receptor antagonist, has negligible anticholinergic activity. At low doses it produces less sedation than phenothiazines and is an ideal agent for patients with nausea and delirium.35 Doses range from 5 to 15 mg/day, given in divided doses or as intermittent or continuous intravenous infusions.
Anticholinergics, with or without somatostatin analogues, reduce gastrointestinal secretions, fluid accumulation, and vomiting. Anticholinergics bind to muscarinic receptors on enteric neurons in the myenteric and the submucosal plexus. Dosages:
- Hyoscine butylbromide 40 to 120 mg/day.
- Hyoscine hydrobromide 0.2 to 0.9 mg/day.7,34
Glycopyrrolate, a quaternary ammonium anticholinergic, has minimal central nervous system penetration and is less likely to cause delirium or cardiac side effects compared with tertiary amine anticholinergics such as atropine and scopolamine.38 The recommended dose is 0.1 to 0.2 mg subcutaneously or intravenously three to four times daily.
Octreotide, an analogue of somatostatin, blocks the release of vasoactive intestinal polypeptide, which is increased in malignant bowel obstruction.14,15 It reduces the excretion of water, sodium, and chloride into the bowel lumen and increases the absorption of electrolytes and water. It also inhibits pancreatic enzyme secretion and splanchnic blood flow. The result of all these effects is reduced luminal content, reduced motility, reduced vascular congestion of the bowel wall, and, in certain circumstances, reduced ascites.39
In small randomized trials, octreotide was more successful than anticholinergics at improving nausea, vomiting, and colic in patients requiring a nasogastric tube and in those whose symptoms were refractory to standard medical treatment.5,34,40–43 A recent case report found octreotide helpful in resolving symptoms of partial bowel obstruction that were unresponsive to standard measures.44
Octreotide is well tolerated and reduces the time patients require a nasogastric tube without significantly worsening xerostomia. High cost limits its use in American hospice care due to the Medicare capitated system of reimbursement for drugs and services, and as a result it is a second-tier drug despite evidence of its efficacy.
Octreotide doses are 100 to 200 mg every 8 hours.
Metoclopramide (Reglan), a dopaminergic antagonist, a 5HT4 receptor agonist, and a 5HT3 receptor blocker at doses greater than 120 mg/day, combines the action of a phenothiazine, which blocks D2 receptors in the central chemoreceptor trigger zone, with promotility actions through serotonin receptors (5HT4).35,37
Metoclopramide should not be used with anticholinergics or in patients with colic or complete obstruction.35,45 In some centers it is the first-line drug for functional or partial bowel obstruction.7 Dosages range from 40 to 240 mg/day.
Olanzapine (Zyprexa), an atypical antipsychotic, blocks multiple neurotransmitter receptors (D2, H1, Ach, 5HT3) responsible for initiating emesis. It is an option in patients whose nausea and vomiting fail to respond to standard antiemetics.46 Dosages range from 2.5 to 20 mg/day.
Dissolvable tablets are given sublingually, which makes olanzapine a versatile antiemetic in cases of intractable nausea. Our unpublished experience is that the sublingual route reduces nausea associated with malignant bowel obstruction and obviates the need for subcutaneous injections or intravenous antiemetic infusions.
Corticosteroids. Although how corticosteroids relieve malignant bowel obstruction is unknown, they are presumed to act centrally.37,45 In addition, they reduce peritumoral edema and luminal salt and water, and they also have antiemetic and analgesic properties.
Evidence from a meta-analysis found that 6 to 16 mg of parenteral dexamethasone per day reduced symptoms and improved bowel function in 60% of patients but did not change the prognosis.11
A trial of 4 or 5 days is adequate to determine response. If there is no response, the corticosteroid should be rapidly tapered. Side effects are minimal when corticosteroids are used short-term.
Combination therapy. Only rarely does a single drug resolve symptoms of malignant bowel obstruction. Antiemetics, analgesics, corticosteroids, antisecretory anticholinergics, and octreotide are often required in combination to achieve acceptable symptom relief.3,5,7,47
In a small prospective case series, the combination of metoclopramide 60 mg/day, octreotide 0.3 mg/day, and dexamethasone 12 mg/day with a single bolus of amidotrizoic acid (a contrast agent) improved intestinal transit within 1 to 5 days and resolved vomiting within 24 hours.45
Compatibility and the route of administration of medications are key considerations when choosing drug combinations.
WHEN TO CONSIDER A VENTING GASTROSTOMY
Patients with a poor performance status, rapidly progressive disease, peritoneal carcinomatosis, a life expectancy of less than 30 days, or multiple levels of obstruction benefit from placement of a percutaneous endoscopic gastrostomy tube (ie, a venting gastrostomy) rather than surgery if symptoms do not respond to drug therapy.7,48 There is compelling evidence that this procedure relieves nausea and vomiting in 80% to 90% of patients and restores some level of oral intake in many.5,6,48,49 A venting gastrostomy tube can be placed during surgical exploration, percutaneously with fluoroscopy, or endoscopically.9
There are no absolute contraindications to gastrostomy tube placement. It is feasible even in patients with tumors encasing the stomach, diffuse carcinomatosis, and ascites.48 However, massive ascites, previous upper abdominal surgery, or a large mass attached to the abdominal wall make tube placement difficult.
Complications are often local. Patients experience transient abdominal wall pain after the procedure. Dislodgement, bleeding, catheter migration, peritonitis, and necrotizing fasciitis are early complications. Others include skin excoriation from leakage of gastric contents, leakage of ascitic fluid from the site, and obstruction or dislodgement of the tube.48,49
Patients can be discharged from the hospital soon after the tube is placed, usually with fewer medications than for patients who undergo surgery.48 This is particularly important for patients with a short expected survival. Some patients at home benefit from hydration (less than 2 L/day) via an existing central venous port or peripherally inserted central catheter, or by hypodermoclysis.
WHEN IS A NASOGASTRIC TUBE APPROPRIATE?
Some patients with malignant bowel obstruction require a nasogastric tube early in their hospital course.12 Unfortunately, nasogastric tubes, if left in place, cause nose and throat pain, sinusitis, abscess formation, erosion of nasal cartilage, aspiration, esophageal erosion, pharyngitis, and social isolation.5,6
Nasogastric tubes should be a temporizing measure to vent gastrointestinal secretions, reduce abdominal distension, and improve nausea and vomiting while a decision about surgery is being made.13,24 If surgery is not feasible, one can avoid the long-term complications and discomfort of a nasogastric tube via medical management and earlier evaluation for venting gastrostomy in those with symptoms that respond poorly to optimal medical management.49
WHICH PATIENTS BENEFIT FROM TOTAL PARENTERAL NUTRITION?
The use of total parenteral nutrition in patients with incurable malignancies is controversial. Enteral and parenteral feeding can increase muscle mass and improve functional status and quality of life in a subset of patients who are not suffering from cancer-related cachexia.2,50,51 However, for those whose weight loss and malnutrition are consequences of tumor-mediated cachexia, as demonstrated by anorexia and an elevated C-reactive protein level, parenteral nutrition is unlikely to improve the outcome.51 For most terminally ill patients, retrospective studies have failed to show that parenteral nutrition improves overall survival, performance status, or quality of life.2,48,50–54
Total parenteral nutrition poses risks: it is invasive and requires central venous access, which predisposes to infection; it requires frequent monitoring of hydration and electrolytes; and it predisposes to thrombosis, diarrhea, hyperglycemia, and liver failure.50–56
Total parenteral nutrition may be justified in patients with minimal tumor burden who are candidates for definitive surgery, or in those with a good performance status early in the disease course who have not had chemotherapy or whose cancer responds to chemotherapy.2,50–56
The American College of Physicians discourages the routine use of parenteral nutrition in those with advanced cancer who are undergoing palliative chemotherapy, since few patients benefit and many experience side effects.53
Total parenteral nutrition is much like a medical intervention in that it should be offered or continued only if it provides benefit. Conversations at the time that it is begun must include adverse effects that will lead to its discontinuation, and criteria for response. In certain situations, a limited trial of parenteral nutrition may be considered for patients with an uncertain prognosis or for those who have potentially reversible conditions that limit oral intake.51 In such cases, there should be a clear understanding between patient and physician that parenteral nutrition will be discontinued if it fails to show benefit.53
ADDITIONAL CONCERNS OF PATIENTS AND FAMILIES
‘Will I starve to death?’
Starvation is a fear echoed by patients and families. Ethical discourse on the continuation of nutrition and hydration for the terminally ill has been polarizing.57–60 Withdrawal of nutrition can be perceived as euthanasia.
Advanced cancer patients in general do not experience hunger, and those who do require only small amounts of food for satiation.61 In one report, most patients died of their advanced cancer and not from starvation.52 Artificial hydration and nutrition will thus not influence survival and can even be a burden without benefit in the imminently dying.60 These patients should be encouraged to take food orally for pleasure, as long as it is tolerated, without consideration of end points such as weight gain, body mass index, or albumin levels.
Complaints of thirst and dryness of the mouth are relieved with mouth care, ice chips, lubrication to the lips, and sips of fluid, rather than by parenteral nutrition.59 Patients with a terminal illness experience relief from thirst with minimal intake. The symptom of thirst may be relieved without hydration.34,61 Adequate hydration requires smaller fluid volumes due to decreased body weight, decreased renal clearance of free water, and decreased insensible water losses from reduced physical activity.58
‘Can we continue intravenous hydration so he won’t die of thirst?’
Overzealous intravenous hydration may worsen the symptoms of malignant bowel obstruction. Overhydration can increase secretions in the gut lumen and worsen the secretion-distention-contraction cycle, leading to greater abdominal pain and to nausea and vomiting.7 There is a greater risk of fluid overload in these patients, since they have edema and excessive interstitial fluid. Most have a low serum albumin level, which results in movement of fluid from intravascular to interstitial spaces due to reduced colloid osmotic pressure. In these instances, overzealous hydration can lead to respiratory insufficiency and worsening edema.
In spite of numerous discussions in the medical literature of the benefits and burdens of continual hydration, there is no consensus or guideline. When a patient has limited oral intake, the decision to hydrate should be individualized, with careful assessment of the risks and benefits and in accordance with the patient’s or family’s wishes.57,58
Is treatment at home feasible?
Discharging patients with inoperable malignant bowel obstruction requires careful planning. Patients and family members need to be educated on the use of around-the-clock medications and symptom-targeted, as-needed drugs. Days before discharge, questions about diet need to be clarified. Education about total parenteral nutrition and gastrostomy tube care should be completed before discharge from the hospital.
Drug management should be simplified, or compatible medications should be combined into a single infusion. For example, morphine, glycopyrrolate, and haloperidol or metoclopramide are chemically compatible in standard intravenous solutions and can be combined.
Families feel less anxious about the foreseen and the possible unforeseen course of the illness if they can talk with hospice workers early on. This early involvement also facilitates the transition to home hospice care.
SUMMARY OF IMPORTANT POINTS
- Patients with malignant bowel obstruction need a highly individualized approach, tailored to their medical condition, the prognosis, and the goals of care.
- Surgery should not be routinely undertaken; less-invasive approaches such as gastric or colonic stenting should be considered first.
- Combinations of analgesics, antisecretory drugs, and antiemetics can provide acceptable symptom relief in the inoperable patient.
- A venting gastrostomy should be considered if drug therapy fails to reduce nausea and vomiting to an acceptable level.
- A nasogastric tube should be used only as a temporizing measure, until symptoms are controlled medically or a venting gastrostomy is placed.
- Total parenteral nutrition is of benefit only in patients with intermediate life expectancy who may otherwise die of starvation rather than from the cancer itself.
- Mercadante S. Intestinal dysfunction and obstruction. In:Walsh D, editor. Palliative Medicine. Philadelphia, PA: Saunders/Elsevier, 2009:1267–1275.
- Pasanisi F, Orban A, Scalfi L, et al. Predictors of survival in terminal-cancer patients with irreversible bowel obstruction receiving home parenteral nutrition. Nutrition 2001; 17:581–584.
- Pameijer CR, Mahvi DM, Stewart JA, Weber SM. Bowel obstruction in patients with metastatic cancer: does intervention influence outcome? Int J Gastrointest Cancer 2005; 35:127–133.
- Bais JMJ, Schilthuis MS, Ansink AC. Palliative management of intestinal obstruction in patients with advanced gynaecological cancer. J Gynecol Oncol 2002; 7:299–305.
- Laval G, Arvieux C, Stefani L, Villard ML, Mestrallet JP, Cardin N. Protocol for the treatment of malignant inoperable bowel obstruction: a prospective study of 80 cases at Grenoble University Hospital Center. J Pain Symptom Manage 2006; 31:502–512.
- Jatoi A, Podratz KC, Gill P, Hartmann LC. Pathophysiology and palliation of inoperable bowel obstruction in patients with ovarian cancer. J Support Oncol 2004; 2:323–334.
- Ripamonti CI, Easson AM, Gerdes H. Management of malignant bowel obstruction. Eur J Cancer 2008; 44:1105–1115.
- Roeland E, von Gunten CF. Current concepts in malignant bowel obstruction management. Curr Oncol Rep 2009; 11:298–303.
- Baron TH. Interventional palliative strategies for malignant bowel obstruction. Curr Oncol Rep 2009; 11:293–297.
- Feuer DJ, Broadley KE, Shepherd JH, Barton DP. Surgery for the resolution of symptoms in malignant bowel obstruction in advanced gynaecological and gastrointestinal cancer. Cochrane Database Syst Rev 2000;CD002764.
- Feuer DJ, Broadley KE. Corticosteroids for the resolution of malignant bowel obstruction in advanced gynaecological and gastrointestinal cancer. Cochrane Database Syst Rev 2000;CD001219.
- Woolfson RG, Jennings K, Whalen GF. Management of bowel obstruction in patients with abdominal cancer. Arch Surg 1997; 132:1093–1097.
- Saunders MD, Kimmey MB. Systematic review: acute colonic pseudo-obstruction. Aliment Pharmacol Ther 2005; 22:917–925.
- Ripamonti C, Bruera E. Palliative management of malignant bowel obstruction. Int J Gynecol Cancer 2002; 12:135–143.
- Nellgård P, Bojö L, Cassuto J. Importance of vasoactive intestinal peptide and somatostatin for fluid losses in small-bowel obstruction. Scand J Gastroenterol 1995; 30:464–469.
- Böhner H, Yang Q, Franke C, Verreet PR, Ohmann C. Simple data from history and physical examination help to exclude bowel obstruction and to avoid radiographic studies in patients with acute abdominal pain. Eur J Surg 1998; 164:777–784.
- Maglinte DD, Kelvin FM, Sandrasegaran K, et al. Radiology of small bowel obstruction: contemporary approach and controversies. Abdom Imaging 2005; 30:160–178.
- Maglinte DD, Howard TJ, Lillemoe KD, Sandrasegaran K, Rex DK. Small-bowel obstruction: state-of-the-art imaging and its role in clinical management. Clin Gastroenterol Hepatol 2008; 6:130–139.
- Silva AC, Pimenta M, Guimarães LS. Small bowel obstruction: what to look for. Radiographics 2009; 29:423–439.
- Finan PJ, Campbell S, Verma R, et al. The management of malignant large bowel obstruction: ACPGBI position statement. Colorectal Dis 2007; 9(suppl 4):1–17.
- Kohli MD, Maglinte DD. CT enteroclysis in incomplete small bowel obstruction. Abdom Imaging 2009; 34:321–327.
- Ha HK, Shin BS, Lee SI, et al. Usefulness of CT in patients with intestinal obstruction who have undergone abdominal surgery for malignancy. AJR Am J Roentgenol 1998; 171:1587–1593.
- DeBernardo R. Surgical management of malignant bowel obstruction: strategies toward palliation of patients with advanced cancer. Curr Oncol Rep 2009; 11:287–292.
- Ripamonti C, Twycross R, Baines M, et al; Working Group of the European Association for Palliative Care. Clinical-practice recommendations for the management of bowel obstruction in patients with end-stage cancer. Support Care Cancer 2001; 9:223–233.
- Mangili G, Aletti G, Frigerio L, et al. Palliative care for intestinal obstruction in recurrent ovarian cancer: a multivariate analysis. Int J Gynecol Cancer 2005; 15:830–835.
- Turner J, Cummin T, Bennett A, Swift G, Green J. Stents and stentability: treatment for malignant bowel obstruction. Br J Hosp Med (Lond) 2008; 69:676–680.
- Khot UP, Lang AW, Murali K, Parker MC. Systematic review of the efficacy and safety of colorectal stents. Br J Surg 2002; 89:1096–1102.
- Hosono S, Ohtani H, Arimoto Y, Kanamiya Y. Endoscopic stenting versus surgical gastroenterostomy for palliation of malignant gastroduodenal obstruction: a meta-analysis. J Gastroenterol 2007; 42:283–290.
- Del Piano M, Ballarè M, Montino F, et al. Endoscopy or surgery for malignant GI outlet obstruction? Gastrointest Endosc 2005; 61:421–426.
- Tilney HS, Lovegrove RE, Purkayastha S, et al. Comparison of colonic stenting and open surgery for malignant large bowel obstruction. Surg Endosc 2007; 21:225–233.
- Holt AP, Patel M, Ahmed MM. Palliation of patients with malignant gastroduodenal obstruction with self-expanding metallic stents: the treatment of choice? Gastrointest Endosc 2004; 60:1010–1017.
- Dastur JK, Forshaw MJ, Modarai B, Solkar MM, Raymond T, Parker MC. Comparison of short-and long-term outcomes following either insertion of self-expanding metallic stents or emergency surgery in malignant large bowel obstruction. Tech Coloproctol 2008; 12:51–55.
- Turner J, Cummin T, Bennett A, Swift G, Green J. Stents and stentability: treatment for malignant bowel obstruction. Br J Hosp Med (Lond) 2008; 69:676–680.
- Ripamonti C, Mercadante S, Groff L, Zecca E, De Conno F, Casuccio A. Role of octreotide, scopolamine butylbromide, and hydration in symptom control of patients with inoperable bowel obstruction and nasogastric tubes: a prospective randomized trial. J Pain Symptom Manage 2000; 19:23–34.
- Davis MP, Walsh D. Treatment of nausea and vomiting in advanced cancer. Support Care Cancer 2000; 8:444–452.
- Bicanovsky LK, Lagman RL, Davis MP, Walsh D. Managing nonmalignant chronic abdominal pain and malignant bowel obstruction. Gastroenterol Clin North Am 2006; 35:131–142.
- Glare P, Pereira G, Kristjanson LJ, Stockler M, Tattersall M. Systematic review of the efficacy of antiemetics in the treatment of nausea in patients with far-advanced cancer. Support Care Cancer 2004; 12:432–440.
- Davis MP, Furste A. Glycopyrrolate: a useful drug in the palliation of mechanical bowel obstruction. J Pain Symptom Manage 1999; 18:153–154.
- Ripamonti C, Mercadante S. How to use octreotide for malignant bowel obstruction. J Support Oncol 2004; 2:357–364.
- Shima Y, Ohtsu A, Shirao K, Sasaki Y. Clinical efficacy and safety of octreotide (SMS201-995) in terminally ill Japanese cancer patients with malignant bowel obstruction. Jpn J Clin Oncol 2008; 38:354–359.
- Mercadante S, Casuccio A, Mangione S. Medical treatment for inoperable malignant bowel obstruction: a qualitative systematic review. J Pain Symptom Manage 2007; 33:217–223.
- Mystakidou K, Tsilika E, Kalaidopoulou O, Chondros K, Georgaki S, Papadimitriou L. Comparison of octreotide administration vs conservative treatment in the management of inoperable bowel obstruction in patients with far advanced cancer: a randomized, double-blind, controlled clinical trial. Anticancer Res 2002; 22:1187–1192.
- Mercadante S, Ripamonti C, Casuccio A, Zecca E, Groff L. Comparison of octreotide and hyoscine butylbromide in controlling gastrointestinal symptoms due to malignant inoperable bowel obstruction. Support Care Cancer 2000; 8:188–191.
- Myers J, Tamber A, Farhadian M. Management of treatment-related intermittent partial small bowel obstruction: the use of octreotide. J Pain Symptom Manage 2010; 39:e1–e3.
- Mercadante S, Ferrera P, Villari P, Marrazzo A. Aggressive pharmacological treatment for reversing malignant bowel obstruction. J Pain Symptom Manage 2004; 28:412–416.
- Srivastava M, Brito-Dellan N, Davis MP, Leach M, Lagman R. Olanzapine as an antiemetic in refractory nausea and vomiting in advanced cancer. J Pain Symptom Manage 2003; 25:578–582.
- Bentley A, Boyd K. Use of clinical pictures in the management of nausea and vomiting: a prospective audit. Palliat Med 2001; 15:247–253.
- Pothuri B, Montemarano M, Gerardi M, et al. Percutaneous endoscopic gastrostomy tube placement in patients with malignant bowel obstruction due to ovarian carcinoma. Gynecol Oncol 2005; 96:330–334.
- Brooksbank MA, Game PA, Ashby MA. Palliative venting gastrostomy in malignant intestinal obstruction. Palliat Med 2002; 16:520–526.
- Wang MY, Wu MH, Hsieh DY, et al. Home parenteral nutrition support in adults: experience of a medical center in Asia. JPEN J Parenter Enteral Nutr 2007; 31:306–310.
- Dy SM. Enteral and parenteral nutrition in terminally ill cancer patients: a review of the literature. Am J Hosp Palliat Care 2006; 23:369–377.
- Whitworth MK, Whitfield A, Holm S, Shaffer J, Makin W, Jayson GC. Doctor, does this mean I’m going to starve to death? J Clin Oncol 2004; 22:199–201.
- Hoda D, Jatoi A, Burnes J, Loprinzi C, Kelly D. Should patients with advanced, incurable cancers ever be sent home with total parenteral nutrition? A single institution’s 20-year experience. Cancer 2005; 103:863–868.
- Philip J, Depczynski B. The role of total parenteral nutrition for patients with irreversible bowel obstruction secondary to gynecological malignancy. J Pain Symptom Manage 1997; 13:104–111.
- August DA, Thorn D, Fisher RL, Welchek CM. Home parenteral nutrition for patients with inoperable malignant bowel obstruction. JPEN J Parenter Enteral Nutr 1991; 15:323–327.
- Abu-Rustum NR, Barakat RR, Venkatraman E, Spriggs D. Chemotherapy and total parenteral nutrition for advanced ovarian cancer with bowel obstruction. Gynecol Oncol 1997; 64:493–495.
- Fainsinger RL, Bruera E. When to treat dehydration in a terminally ill patient? Support Care Cancer 1997; 5:205–211.
- Steiner N, Bruera E. Methods of hydration in palliative care patients. J Palliat Care 1998; 14:6–13.
- Slomka J. Withholding nutrition at the end of life: clinical and ethical issues. Cleve Clin J Med 2003; 70:548–552.
- Chiu TY, Hu WY, Chuang RB, Chen CY. Nutrition and hydration for terminal cancer patients in Taiwan. Support Care Cancer 2002; 10:630–636.
- McCann RM, Hall WJ, Groth-Juncker A. Comfort care for terminally ill patients. The appropriate use of nutrition and hydration. JAMA 1994; 272:1263–1266.
Malignant bowel obstruction occurs in 5% to 51% of women with ovarian cancer and in 10% to 28% of patients with gastrointestinal cancer, predominantly in the advanced stages.1 Median survival after its onset ranges from 30 to 90 days.2–5
Its symptoms are challenging to manage, since nausea, vomiting, colic, and abdominal pain, which are common, cause significant physical distress and demoralization. The decision whether to correct it with surgery requires an individualized approach and a clear understanding of the goals of care and expected survival in the individual patient.
This review focuses on the management of inoperable malignant bowel obstruction and includes discussion of hydration, nutrition, and endoscopic palliative options.
WHAT ARE THE DIFFERENT TYPES OF OBSTRUCTION?
Bowel obstruction may be mechanical or functional, partial or complete, and may occur at one or at many sites. Tumors can impair bowel function in several ways6–8:
- Intraluminal tumors can occlude the lumen or act as a point of intussusception.
- Intramural tumors can extend to the mucosa and obstruct the lumen or impair peristalsis.
- Mesenteric and omental masses or malignant adhesions can kink or angulate the bowel, creating an extramural obstruction.
- Tumors that infiltrate into the mesentery, bowel muscle, or the enteric or celiac plexus can cause dysmotility.
Cholangiocarcinoma, pancreatic carcinoma, and gallbladder carcinoma are the most common tumors causing duodenal obstruction.9 Distal obstruction is caused mainly by colon and ovarian cancer.
Obstruction can be due to treatment
In a minority of patients, obstruction is unrelated to the cancer and is instead due to adhesions arising from surgery, radiation therapy (causing enteritis and strictures), desmoplastic reactions to intraperitoneal chemotherapy, torsion, or internal hernias.10–12
In rare cases, a patient has intestinal pseudo-obstruction from paraneoplastic destruction of enteric neurons, or severe ileus from anticholinergic or sympathomimetic drugs, as seen with acute colonic pseudo-obstruction (Ogilvie syndrome).13
Physiologic reactions to obstruction
Malignant bowel obstruction stimulates gastric, biliary, pancreatic, and intestinal secretions, decreases intraluminal sodium and water reabsorption, and increases mucosal sodium and water secretion.6,14 In response to the obstruction, peristalsis increases, and prostaglandin, vasoactive intestinal peptide, and nociceptive mediators are released. Vasoactive intestinal polypeptide perpetuates a cycle of secretion, distention, and contraction that leads to intestinal hyperemia, bowel edema, and accumulation of fluid in the lumen.8,10,11,15
Signs and symptoms depend on the site
The site of obstruction determines the signs and symptoms patients experience.7,14 Obstructions high in the gastrointestinal tract are associated with greater symptoms but fewer signs than colonic obstructions.1 Patients with proximal small-bowel obstruction have more severe nausea and a greater number of episodes of emesis, but they have relatively normal plain radiographs of the abdomen, which do not have the characteristic air-fluid levels commonly seen with distal small-bowel obstruction.
Most malignant obstructions remain partial, but increasing abdominal distention, worsening nausea, vomiting, abdominal pain, and obstipation over 1 to 2 weeks1 suggest progression to complete obstruction.
IMAGING TESTS FOR MALIGNANT BOWEL OBSTRUCTION
What is the value of plain radiography?
Despite these limitations, plain radiography is useful in assessing constipation and its severity as a potential cause of symptoms, and thus it remains an important initial imaging study in almost all patients with suspected malignant bowel obstruction.17,18 It is also used to assess response to treatment.
When do you need contrast radiographs?
Contrast radiography (barium swallow or barium or Gastrograffin enema) is helpful in patients with symptoms of dysmotility from suspected bowel obstruction. It defines the site or sites of obstruction and the extent of the obstruction with a fair degree of accuracy.7,19 Single-contrast studies, if positive, exclude opioid-induced bowel dysfunction or pseudo-obstruction in 83% of patients, with a sensitivity and specificity of 96% and 98%, respectively.8,20 Small-bowel follow-through with barium is more appropriate for low-grade obstructions or for symptomatic patients with a normal kidney, ureter, bladder radiograph.19
However, contrast radiography is limited by the patient’s ability to swallow barium or water-soluble contrast agents, and it can worsen nausea or vomiting.17,18 Also, barium is not absorbed systemically and may interfere with subsequent radiologic studies. Large volumes of contrast agents increase the risk of aspiration pneumonia in patients with poorly controlled nausea and can lead to severe impaction proximal to the obstructed site.8
Is enteroclysis better than barium swallow?
Enteroclysis, ie, injecting radiographic contrast into the bowel via a nasoduodenal tube, has some advantages over the barium swallow technique for detecting partial small-bowel obstruction, since it bypasses the stomach and allows for therapeutic decompression as well as direct visualization of the area of concern.17,18 Enteroclysis radiography objectively gauges severity of intestinal obstruction and bowel wall distensibility, which is an advantage over other imaging studies. Its sensitivity is 100% and specificity 88% in experienced hands.17 Enteroclysis studies also detect nonobstructing intraluminal tumors when computed tomography (CT) is not diagnostic.17,18,21
The drawbacks to enteroclysis are that it is technically difficult to perform and that few radiologists are trained in it.
When is CT useful?
CT is the primary imaging study for patients with obstructive symptoms and a history of abdominal malignancy or a palpable abdominal mass17,20,22,23 (Figure 1). It has a specificity of 100% and a sensitivity of 94%. It plays a major role in decision-making regarding surgery, endoscopy, or palliative interventions,7,19 as it locates the obstruction and differentiates benign from malignant causes with a fair degree of precision.22
CT findings in malignant bowel obstruction may include:
- A mass at the site of obstruction or within the original surgical field
- Lymphadenopathy
- Abrupt transitions in luminal diameter or irregular thickening of the bowel wall at the site or sites of obstruction.7
SURGERY: A DIFFICULT DECISION
Is the patient fit for surgery?
Surgery for malignant bowel obstruction should not be done in patients who have advanced malignancies with bulky intra-abdominal metastases or cancer that has spread outside the abdominal cavity without taking into account treatment options for the cancer, the patient’s nutritional status, and the goals of care.
The role of abdominal surgery (debulking, resection, or bypass) in advanced cancer remains unclear and controversial.24 From 42% to 80% of patients report that symptoms improve after surgery, but recurrent obstruction occurs in 10% to 50%.10 Even in patients with low tumor bulk and good nutritional status, 30-day mortality rates range from 5% to 40%, and complication rates range from 9% to 90%.3,4,6,7,10,14
Outcomes after surgery depend on patient selection criteria perhaps as much as on the surgeon’s experience and skill. Patients with more advanced cancer who have had multiple surgical procedures and those with cancer that does not respond to chemotherapy and radiation present the greatest challenge to surgeons.23
What is the benefit of surgery?
Reports of palliative surgery have included information about 60-day survival rates after the operation, but a number of factors may be more meaningful in this context, such as postoperative symptoms, the patient’s overall wellbeing, how the original symptoms respond to the surgery, complications, and length of hospitalization.14 The paucity of published, validated, patient-related outcome data on which to gauge the value of surgery and the lack of a standard definition of “benefit” further confuse the objective determination of whether these patients benefit from surgery.
In a cohort with advanced ovarian cancer and bowel obstruction, surgery was detrimental to survival and quality of life for all subgroups, and most patients died in the hospital.6
The risk of surgery for malignant bowel obstruction is presumably higher than for abdominal surgery for other indications, since many of the patients are debilitated from their cancer and chemotherapy, and many are malnourished.23 Even when taking into account a potential selection bias in favor of surgery, several studies have reported no significant difference in 30-day mortality rates or median survival between operative and nonoperative groups.2,12 Neither the type of obstruction nor the extent of the surgery influenced outcomes. Surgical outcomes are best in patients with a benign cause of obstruction; little benefit is seen in operating on those with abdominal carcinomatosis.12
Nevertheless, surgery is beneficial in a select few. For patients with a good performance status, slowly progressive cancer, and an expected survival of more than 6 months, surgical bypass or resection is preferred.7,12,25 The challenge is to identify these surgical candidates, taking into account prognostic factors such as nutritional status, tumor burden, performance status, presence of ascites, advanced age, extensive prior chemotherapy or radiotherapy, and diffuse carcinomatosis.3,10,12,20,23
Is surgery consistent with the goals of care?
Crucial to decision-making are the goals of care. Since palliative surgery carries a low level of evidence for benefit in terms of quality of life and survival, time should be set aside to thoroughly review the patient’s medical condition, to explore options, and to clarify expectations and goals of care.3,10 Family members should be invited to be present during these discussions and to be involved in the decision-making process.
WHAT IS THE BENEFIT OF GASTRIC OR COLONIC STENTING?
Endoscopic procedures are alternatives to surgery and offer a palliative option in malignant bowel obstruction. Endoscopic procedures are associated with a shorter hospital stay and quicker recovery than after laparotomy.9,26–30 In certain situations, stenting serves as a bridge to surgery, allowing time to mitigate comorbid conditions, to enhance nutrition, and to complete staging, while relieving symptoms.27–29,31,32 Definitive surgery can be done as a single-stage procedure without a diverting enterostomy.
Self-expanding metal stents for gastric outlet, small-bowel, and colonic obstructions are an option in patients who have incurable metastatic disease who are unfit for surgery, in patients with a single point of obstruction or locally extensive disease, or in patients who do not want to undergo laparotomy.28–30
Technical and clinical success rates for colorectal stenting are high (88% to 93%).26,27 Stenting is more successful for left-sided colonic obstructions than for proximal colonic obstructions. Even for patients with extracolonic malignancies such as ovarian cancer, the technical success rate of colorectal stenting is 87%.26 However, patients with unrecognized peritoneal carcinomatosis or multifocal bowel obstruction are less likely to have symptomatic relief even after successful stenting.6,9
Contraindications to stenting
Absolute contraindications to stenting are colonic or tumor perforation with peritonitis. A relative contraindication is a rectal tumor within 2 cm of the anal margin. Stenting in this circumstance leads to tenesmus and incontinence.33
Complications of stenting
Death rates during colorectal stent insertion are less than 1%. The hospital stay and incidence of complications are significantly less than with surgery.26,30
Stent migration occurs in 10% of cases and is asymptomatic, but half of patients with this complication require a repeat intervention. The risk of migration is greater if chemotherapy or radiation therapy succeeds in shrinking the tumor.
Bleeding occurs in 5% of cases, usually from the underlying tumor.
Perforation occurs in 4%, but the rate increases to 10% with the use of dilatation before stent placement.
The rate of recurrent obstruction from tumor ingrowth, overgrowth, or fecal impaction is 10%.9,26,29 Recurrent obstruction may be treated with additional stents inserted within the original stent.9
GASTRIC OUTLET OBSTRUCTION: SURGERY VS STENTING
Gastrojejunostomy has in the past been the treatment of choice for gastric outlet obstruction. Certainly, patients with slow-growing tumors and an expected survival of greater than 60 days may be considered for this bypass procedure; those with a short tumor length, a single site of obstruction (preferably in the pylorus or proximal duodenum), a good performance status, and a life expectancy greater than 30 days are good candidates.7 Nevertheless, for patients with advanced cancer and poor performance status, gastroenterostomy carries a significant risk of morbidity and death.28
Endoscopic stenting of gastric outlet obstruction has a greater success rate, a shorter time to oral intake, a lower morbidity rate, a lower incidence of delayed gastric emptying, and a shorter hospital stay compared with gastroenterostomy.28,29 Technical success rates of stenting are 90%, and 75% of patients have resolution of nausea and vomiting.7 Stenting is generally not possible if the obstruction occurs beyond the ligament of Treitz.
Patients who are expected to survive less than 1 month or who have rapidly progressive disease, overt ascites, carcinomatosis, or multiple sites of obstruction should be managed with percutaneous, endoscopically placed gastrostomy tubes.7
Late complications of stenting for gastric outlet obstruction are occlusion with food or ingrowth of tumor through or around the wire mesh.7 This may require laser therapy or placement of a second stent, or both.
DRUG THERAPY
Medical therapy can palliate symptoms of malignant bowel obstruction for most patients.34 Recommendations have been published by the Working Group of the European Association for Palliative Care.24 Symptom management is focused on pain, nausea, and vomiting.
Which drugs can I use for abdominal pain?
Patients experience two types of abdominal pain: continuous and colic. Each type of pain requires different treatment approaches and classes of drugs.
Potent opioids such as morphine, hydromorphone (Dilaudid), and fentanyl (Fentora) are used to relieve continuous abdominal pain.7 The dose is titrated for adequate relief. Subcutaneous, intravenous, sublingual, and transdermal routes can be used if nausea and vomiting prevent oral administration.
However, opioids can aggravate colic by stimulating circular smooth muscle, leading to segmental contractions. Opioid-sparing adjuvant drugs such as ketorolac (Toradol) may improve colic and continuous pain and prevent a partial obstruction from becoming a complete obstruction by sparing opioid doses.35
Colic may persist or worsen with the use of opioids. Drugs that reduce colic include the scopolamine drugs hyoscine butylbromide and hyoscine hydrobromide, glycopyrrolate (Robinul), and octreotide (Sandostatin).7,34–37
Which drugs are appropriate for reducing nausea and vomiting?
Phenothiazines reduce nausea and control vomiting. Chlorpromazine (Thorazine), prochlorperazine (Compro, Compazine), and promethazine (Phenergan) have all been reported to treat nausea successfully.35,37
Haloperidol (Haldol), a butyrophenone selective dopamine D2-receptor antagonist, has negligible anticholinergic activity. At low doses it produces less sedation than phenothiazines and is an ideal agent for patients with nausea and delirium.35 Doses range from 5 to 15 mg/day, given in divided doses or as intermittent or continuous intravenous infusions.
Anticholinergics, with or without somatostatin analogues, reduce gastrointestinal secretions, fluid accumulation, and vomiting. Anticholinergics bind to muscarinic receptors on enteric neurons in the myenteric and the submucosal plexus. Dosages:
- Hyoscine butylbromide 40 to 120 mg/day.
- Hyoscine hydrobromide 0.2 to 0.9 mg/day.7,34
Glycopyrrolate, a quaternary ammonium anticholinergic, has minimal central nervous system penetration and is less likely to cause delirium or cardiac side effects compared with tertiary amine anticholinergics such as atropine and scopolamine.38 The recommended dose is 0.1 to 0.2 mg subcutaneously or intravenously three to four times daily.
Octreotide, an analogue of somatostatin, blocks the release of vasoactive intestinal polypeptide, which is increased in malignant bowel obstruction.14,15 It reduces the excretion of water, sodium, and chloride into the bowel lumen and increases the absorption of electrolytes and water. It also inhibits pancreatic enzyme secretion and splanchnic blood flow. The result of all these effects is reduced luminal content, reduced motility, reduced vascular congestion of the bowel wall, and, in certain circumstances, reduced ascites.39
In small randomized trials, octreotide was more successful than anticholinergics at improving nausea, vomiting, and colic in patients requiring a nasogastric tube and in those whose symptoms were refractory to standard medical treatment.5,34,40–43 A recent case report found octreotide helpful in resolving symptoms of partial bowel obstruction that were unresponsive to standard measures.44
Octreotide is well tolerated and reduces the time patients require a nasogastric tube without significantly worsening xerostomia. High cost limits its use in American hospice care due to the Medicare capitated system of reimbursement for drugs and services, and as a result it is a second-tier drug despite evidence of its efficacy.
Octreotide doses are 100 to 200 mg every 8 hours.
Metoclopramide (Reglan), a dopaminergic antagonist, a 5HT4 receptor agonist, and a 5HT3 receptor blocker at doses greater than 120 mg/day, combines the action of a phenothiazine, which blocks D2 receptors in the central chemoreceptor trigger zone, with promotility actions through serotonin receptors (5HT4).35,37
Metoclopramide should not be used with anticholinergics or in patients with colic or complete obstruction.35,45 In some centers it is the first-line drug for functional or partial bowel obstruction.7 Dosages range from 40 to 240 mg/day.
Olanzapine (Zyprexa), an atypical antipsychotic, blocks multiple neurotransmitter receptors (D2, H1, Ach, 5HT3) responsible for initiating emesis. It is an option in patients whose nausea and vomiting fail to respond to standard antiemetics.46 Dosages range from 2.5 to 20 mg/day.
Dissolvable tablets are given sublingually, which makes olanzapine a versatile antiemetic in cases of intractable nausea. Our unpublished experience is that the sublingual route reduces nausea associated with malignant bowel obstruction and obviates the need for subcutaneous injections or intravenous antiemetic infusions.
Corticosteroids. Although how corticosteroids relieve malignant bowel obstruction is unknown, they are presumed to act centrally.37,45 In addition, they reduce peritumoral edema and luminal salt and water, and they also have antiemetic and analgesic properties.
Evidence from a meta-analysis found that 6 to 16 mg of parenteral dexamethasone per day reduced symptoms and improved bowel function in 60% of patients but did not change the prognosis.11
A trial of 4 or 5 days is adequate to determine response. If there is no response, the corticosteroid should be rapidly tapered. Side effects are minimal when corticosteroids are used short-term.
Combination therapy. Only rarely does a single drug resolve symptoms of malignant bowel obstruction. Antiemetics, analgesics, corticosteroids, antisecretory anticholinergics, and octreotide are often required in combination to achieve acceptable symptom relief.3,5,7,47
In a small prospective case series, the combination of metoclopramide 60 mg/day, octreotide 0.3 mg/day, and dexamethasone 12 mg/day with a single bolus of amidotrizoic acid (a contrast agent) improved intestinal transit within 1 to 5 days and resolved vomiting within 24 hours.45
Compatibility and the route of administration of medications are key considerations when choosing drug combinations.
WHEN TO CONSIDER A VENTING GASTROSTOMY
Patients with a poor performance status, rapidly progressive disease, peritoneal carcinomatosis, a life expectancy of less than 30 days, or multiple levels of obstruction benefit from placement of a percutaneous endoscopic gastrostomy tube (ie, a venting gastrostomy) rather than surgery if symptoms do not respond to drug therapy.7,48 There is compelling evidence that this procedure relieves nausea and vomiting in 80% to 90% of patients and restores some level of oral intake in many.5,6,48,49 A venting gastrostomy tube can be placed during surgical exploration, percutaneously with fluoroscopy, or endoscopically.9
There are no absolute contraindications to gastrostomy tube placement. It is feasible even in patients with tumors encasing the stomach, diffuse carcinomatosis, and ascites.48 However, massive ascites, previous upper abdominal surgery, or a large mass attached to the abdominal wall make tube placement difficult.
Complications are often local. Patients experience transient abdominal wall pain after the procedure. Dislodgement, bleeding, catheter migration, peritonitis, and necrotizing fasciitis are early complications. Others include skin excoriation from leakage of gastric contents, leakage of ascitic fluid from the site, and obstruction or dislodgement of the tube.48,49
Patients can be discharged from the hospital soon after the tube is placed, usually with fewer medications than for patients who undergo surgery.48 This is particularly important for patients with a short expected survival. Some patients at home benefit from hydration (less than 2 L/day) via an existing central venous port or peripherally inserted central catheter, or by hypodermoclysis.
WHEN IS A NASOGASTRIC TUBE APPROPRIATE?
Some patients with malignant bowel obstruction require a nasogastric tube early in their hospital course.12 Unfortunately, nasogastric tubes, if left in place, cause nose and throat pain, sinusitis, abscess formation, erosion of nasal cartilage, aspiration, esophageal erosion, pharyngitis, and social isolation.5,6
Nasogastric tubes should be a temporizing measure to vent gastrointestinal secretions, reduce abdominal distension, and improve nausea and vomiting while a decision about surgery is being made.13,24 If surgery is not feasible, one can avoid the long-term complications and discomfort of a nasogastric tube via medical management and earlier evaluation for venting gastrostomy in those with symptoms that respond poorly to optimal medical management.49
WHICH PATIENTS BENEFIT FROM TOTAL PARENTERAL NUTRITION?
The use of total parenteral nutrition in patients with incurable malignancies is controversial. Enteral and parenteral feeding can increase muscle mass and improve functional status and quality of life in a subset of patients who are not suffering from cancer-related cachexia.2,50,51 However, for those whose weight loss and malnutrition are consequences of tumor-mediated cachexia, as demonstrated by anorexia and an elevated C-reactive protein level, parenteral nutrition is unlikely to improve the outcome.51 For most terminally ill patients, retrospective studies have failed to show that parenteral nutrition improves overall survival, performance status, or quality of life.2,48,50–54
Total parenteral nutrition poses risks: it is invasive and requires central venous access, which predisposes to infection; it requires frequent monitoring of hydration and electrolytes; and it predisposes to thrombosis, diarrhea, hyperglycemia, and liver failure.50–56
Total parenteral nutrition may be justified in patients with minimal tumor burden who are candidates for definitive surgery, or in those with a good performance status early in the disease course who have not had chemotherapy or whose cancer responds to chemotherapy.2,50–56
The American College of Physicians discourages the routine use of parenteral nutrition in those with advanced cancer who are undergoing palliative chemotherapy, since few patients benefit and many experience side effects.53
Total parenteral nutrition is much like a medical intervention in that it should be offered or continued only if it provides benefit. Conversations at the time that it is begun must include adverse effects that will lead to its discontinuation, and criteria for response. In certain situations, a limited trial of parenteral nutrition may be considered for patients with an uncertain prognosis or for those who have potentially reversible conditions that limit oral intake.51 In such cases, there should be a clear understanding between patient and physician that parenteral nutrition will be discontinued if it fails to show benefit.53
ADDITIONAL CONCERNS OF PATIENTS AND FAMILIES
‘Will I starve to death?’
Starvation is a fear echoed by patients and families. Ethical discourse on the continuation of nutrition and hydration for the terminally ill has been polarizing.57–60 Withdrawal of nutrition can be perceived as euthanasia.
Advanced cancer patients in general do not experience hunger, and those who do require only small amounts of food for satiation.61 In one report, most patients died of their advanced cancer and not from starvation.52 Artificial hydration and nutrition will thus not influence survival and can even be a burden without benefit in the imminently dying.60 These patients should be encouraged to take food orally for pleasure, as long as it is tolerated, without consideration of end points such as weight gain, body mass index, or albumin levels.
Complaints of thirst and dryness of the mouth are relieved with mouth care, ice chips, lubrication to the lips, and sips of fluid, rather than by parenteral nutrition.59 Patients with a terminal illness experience relief from thirst with minimal intake. The symptom of thirst may be relieved without hydration.34,61 Adequate hydration requires smaller fluid volumes due to decreased body weight, decreased renal clearance of free water, and decreased insensible water losses from reduced physical activity.58
‘Can we continue intravenous hydration so he won’t die of thirst?’
Overzealous intravenous hydration may worsen the symptoms of malignant bowel obstruction. Overhydration can increase secretions in the gut lumen and worsen the secretion-distention-contraction cycle, leading to greater abdominal pain and to nausea and vomiting.7 There is a greater risk of fluid overload in these patients, since they have edema and excessive interstitial fluid. Most have a low serum albumin level, which results in movement of fluid from intravascular to interstitial spaces due to reduced colloid osmotic pressure. In these instances, overzealous hydration can lead to respiratory insufficiency and worsening edema.
In spite of numerous discussions in the medical literature of the benefits and burdens of continual hydration, there is no consensus or guideline. When a patient has limited oral intake, the decision to hydrate should be individualized, with careful assessment of the risks and benefits and in accordance with the patient’s or family’s wishes.57,58
Is treatment at home feasible?
Discharging patients with inoperable malignant bowel obstruction requires careful planning. Patients and family members need to be educated on the use of around-the-clock medications and symptom-targeted, as-needed drugs. Days before discharge, questions about diet need to be clarified. Education about total parenteral nutrition and gastrostomy tube care should be completed before discharge from the hospital.
Drug management should be simplified, or compatible medications should be combined into a single infusion. For example, morphine, glycopyrrolate, and haloperidol or metoclopramide are chemically compatible in standard intravenous solutions and can be combined.
Families feel less anxious about the foreseen and the possible unforeseen course of the illness if they can talk with hospice workers early on. This early involvement also facilitates the transition to home hospice care.
SUMMARY OF IMPORTANT POINTS
- Patients with malignant bowel obstruction need a highly individualized approach, tailored to their medical condition, the prognosis, and the goals of care.
- Surgery should not be routinely undertaken; less-invasive approaches such as gastric or colonic stenting should be considered first.
- Combinations of analgesics, antisecretory drugs, and antiemetics can provide acceptable symptom relief in the inoperable patient.
- A venting gastrostomy should be considered if drug therapy fails to reduce nausea and vomiting to an acceptable level.
- A nasogastric tube should be used only as a temporizing measure, until symptoms are controlled medically or a venting gastrostomy is placed.
- Total parenteral nutrition is of benefit only in patients with intermediate life expectancy who may otherwise die of starvation rather than from the cancer itself.
Malignant bowel obstruction occurs in 5% to 51% of women with ovarian cancer and in 10% to 28% of patients with gastrointestinal cancer, predominantly in the advanced stages.1 Median survival after its onset ranges from 30 to 90 days.2–5
Its symptoms are challenging to manage, since nausea, vomiting, colic, and abdominal pain, which are common, cause significant physical distress and demoralization. The decision whether to correct it with surgery requires an individualized approach and a clear understanding of the goals of care and expected survival in the individual patient.
This review focuses on the management of inoperable malignant bowel obstruction and includes discussion of hydration, nutrition, and endoscopic palliative options.
WHAT ARE THE DIFFERENT TYPES OF OBSTRUCTION?
Bowel obstruction may be mechanical or functional, partial or complete, and may occur at one or at many sites. Tumors can impair bowel function in several ways6–8:
- Intraluminal tumors can occlude the lumen or act as a point of intussusception.
- Intramural tumors can extend to the mucosa and obstruct the lumen or impair peristalsis.
- Mesenteric and omental masses or malignant adhesions can kink or angulate the bowel, creating an extramural obstruction.
- Tumors that infiltrate into the mesentery, bowel muscle, or the enteric or celiac plexus can cause dysmotility.
Cholangiocarcinoma, pancreatic carcinoma, and gallbladder carcinoma are the most common tumors causing duodenal obstruction.9 Distal obstruction is caused mainly by colon and ovarian cancer.
Obstruction can be due to treatment
In a minority of patients, obstruction is unrelated to the cancer and is instead due to adhesions arising from surgery, radiation therapy (causing enteritis and strictures), desmoplastic reactions to intraperitoneal chemotherapy, torsion, or internal hernias.10–12
In rare cases, a patient has intestinal pseudo-obstruction from paraneoplastic destruction of enteric neurons, or severe ileus from anticholinergic or sympathomimetic drugs, as seen with acute colonic pseudo-obstruction (Ogilvie syndrome).13
Physiologic reactions to obstruction
Malignant bowel obstruction stimulates gastric, biliary, pancreatic, and intestinal secretions, decreases intraluminal sodium and water reabsorption, and increases mucosal sodium and water secretion.6,14 In response to the obstruction, peristalsis increases, and prostaglandin, vasoactive intestinal peptide, and nociceptive mediators are released. Vasoactive intestinal polypeptide perpetuates a cycle of secretion, distention, and contraction that leads to intestinal hyperemia, bowel edema, and accumulation of fluid in the lumen.8,10,11,15
Signs and symptoms depend on the site
The site of obstruction determines the signs and symptoms patients experience.7,14 Obstructions high in the gastrointestinal tract are associated with greater symptoms but fewer signs than colonic obstructions.1 Patients with proximal small-bowel obstruction have more severe nausea and a greater number of episodes of emesis, but they have relatively normal plain radiographs of the abdomen, which do not have the characteristic air-fluid levels commonly seen with distal small-bowel obstruction.
Most malignant obstructions remain partial, but increasing abdominal distention, worsening nausea, vomiting, abdominal pain, and obstipation over 1 to 2 weeks1 suggest progression to complete obstruction.
IMAGING TESTS FOR MALIGNANT BOWEL OBSTRUCTION
What is the value of plain radiography?
Despite these limitations, plain radiography is useful in assessing constipation and its severity as a potential cause of symptoms, and thus it remains an important initial imaging study in almost all patients with suspected malignant bowel obstruction.17,18 It is also used to assess response to treatment.
When do you need contrast radiographs?
Contrast radiography (barium swallow or barium or Gastrograffin enema) is helpful in patients with symptoms of dysmotility from suspected bowel obstruction. It defines the site or sites of obstruction and the extent of the obstruction with a fair degree of accuracy.7,19 Single-contrast studies, if positive, exclude opioid-induced bowel dysfunction or pseudo-obstruction in 83% of patients, with a sensitivity and specificity of 96% and 98%, respectively.8,20 Small-bowel follow-through with barium is more appropriate for low-grade obstructions or for symptomatic patients with a normal kidney, ureter, bladder radiograph.19
However, contrast radiography is limited by the patient’s ability to swallow barium or water-soluble contrast agents, and it can worsen nausea or vomiting.17,18 Also, barium is not absorbed systemically and may interfere with subsequent radiologic studies. Large volumes of contrast agents increase the risk of aspiration pneumonia in patients with poorly controlled nausea and can lead to severe impaction proximal to the obstructed site.8
Is enteroclysis better than barium swallow?
Enteroclysis, ie, injecting radiographic contrast into the bowel via a nasoduodenal tube, has some advantages over the barium swallow technique for detecting partial small-bowel obstruction, since it bypasses the stomach and allows for therapeutic decompression as well as direct visualization of the area of concern.17,18 Enteroclysis radiography objectively gauges severity of intestinal obstruction and bowel wall distensibility, which is an advantage over other imaging studies. Its sensitivity is 100% and specificity 88% in experienced hands.17 Enteroclysis studies also detect nonobstructing intraluminal tumors when computed tomography (CT) is not diagnostic.17,18,21
The drawbacks to enteroclysis are that it is technically difficult to perform and that few radiologists are trained in it.
When is CT useful?
CT is the primary imaging study for patients with obstructive symptoms and a history of abdominal malignancy or a palpable abdominal mass17,20,22,23 (Figure 1). It has a specificity of 100% and a sensitivity of 94%. It plays a major role in decision-making regarding surgery, endoscopy, or palliative interventions,7,19 as it locates the obstruction and differentiates benign from malignant causes with a fair degree of precision.22
CT findings in malignant bowel obstruction may include:
- A mass at the site of obstruction or within the original surgical field
- Lymphadenopathy
- Abrupt transitions in luminal diameter or irregular thickening of the bowel wall at the site or sites of obstruction.7
SURGERY: A DIFFICULT DECISION
Is the patient fit for surgery?
Surgery for malignant bowel obstruction should not be done in patients who have advanced malignancies with bulky intra-abdominal metastases or cancer that has spread outside the abdominal cavity without taking into account treatment options for the cancer, the patient’s nutritional status, and the goals of care.
The role of abdominal surgery (debulking, resection, or bypass) in advanced cancer remains unclear and controversial.24 From 42% to 80% of patients report that symptoms improve after surgery, but recurrent obstruction occurs in 10% to 50%.10 Even in patients with low tumor bulk and good nutritional status, 30-day mortality rates range from 5% to 40%, and complication rates range from 9% to 90%.3,4,6,7,10,14
Outcomes after surgery depend on patient selection criteria perhaps as much as on the surgeon’s experience and skill. Patients with more advanced cancer who have had multiple surgical procedures and those with cancer that does not respond to chemotherapy and radiation present the greatest challenge to surgeons.23
What is the benefit of surgery?
Reports of palliative surgery have included information about 60-day survival rates after the operation, but a number of factors may be more meaningful in this context, such as postoperative symptoms, the patient’s overall wellbeing, how the original symptoms respond to the surgery, complications, and length of hospitalization.14 The paucity of published, validated, patient-related outcome data on which to gauge the value of surgery and the lack of a standard definition of “benefit” further confuse the objective determination of whether these patients benefit from surgery.
In a cohort with advanced ovarian cancer and bowel obstruction, surgery was detrimental to survival and quality of life for all subgroups, and most patients died in the hospital.6
The risk of surgery for malignant bowel obstruction is presumably higher than for abdominal surgery for other indications, since many of the patients are debilitated from their cancer and chemotherapy, and many are malnourished.23 Even when taking into account a potential selection bias in favor of surgery, several studies have reported no significant difference in 30-day mortality rates or median survival between operative and nonoperative groups.2,12 Neither the type of obstruction nor the extent of the surgery influenced outcomes. Surgical outcomes are best in patients with a benign cause of obstruction; little benefit is seen in operating on those with abdominal carcinomatosis.12
Nevertheless, surgery is beneficial in a select few. For patients with a good performance status, slowly progressive cancer, and an expected survival of more than 6 months, surgical bypass or resection is preferred.7,12,25 The challenge is to identify these surgical candidates, taking into account prognostic factors such as nutritional status, tumor burden, performance status, presence of ascites, advanced age, extensive prior chemotherapy or radiotherapy, and diffuse carcinomatosis.3,10,12,20,23
Is surgery consistent with the goals of care?
Crucial to decision-making are the goals of care. Since palliative surgery carries a low level of evidence for benefit in terms of quality of life and survival, time should be set aside to thoroughly review the patient’s medical condition, to explore options, and to clarify expectations and goals of care.3,10 Family members should be invited to be present during these discussions and to be involved in the decision-making process.
WHAT IS THE BENEFIT OF GASTRIC OR COLONIC STENTING?
Endoscopic procedures are alternatives to surgery and offer a palliative option in malignant bowel obstruction. Endoscopic procedures are associated with a shorter hospital stay and quicker recovery than after laparotomy.9,26–30 In certain situations, stenting serves as a bridge to surgery, allowing time to mitigate comorbid conditions, to enhance nutrition, and to complete staging, while relieving symptoms.27–29,31,32 Definitive surgery can be done as a single-stage procedure without a diverting enterostomy.
Self-expanding metal stents for gastric outlet, small-bowel, and colonic obstructions are an option in patients who have incurable metastatic disease who are unfit for surgery, in patients with a single point of obstruction or locally extensive disease, or in patients who do not want to undergo laparotomy.28–30
Technical and clinical success rates for colorectal stenting are high (88% to 93%).26,27 Stenting is more successful for left-sided colonic obstructions than for proximal colonic obstructions. Even for patients with extracolonic malignancies such as ovarian cancer, the technical success rate of colorectal stenting is 87%.26 However, patients with unrecognized peritoneal carcinomatosis or multifocal bowel obstruction are less likely to have symptomatic relief even after successful stenting.6,9
Contraindications to stenting
Absolute contraindications to stenting are colonic or tumor perforation with peritonitis. A relative contraindication is a rectal tumor within 2 cm of the anal margin. Stenting in this circumstance leads to tenesmus and incontinence.33
Complications of stenting
Death rates during colorectal stent insertion are less than 1%. The hospital stay and incidence of complications are significantly less than with surgery.26,30
Stent migration occurs in 10% of cases and is asymptomatic, but half of patients with this complication require a repeat intervention. The risk of migration is greater if chemotherapy or radiation therapy succeeds in shrinking the tumor.
Bleeding occurs in 5% of cases, usually from the underlying tumor.
Perforation occurs in 4%, but the rate increases to 10% with the use of dilatation before stent placement.
The rate of recurrent obstruction from tumor ingrowth, overgrowth, or fecal impaction is 10%.9,26,29 Recurrent obstruction may be treated with additional stents inserted within the original stent.9
GASTRIC OUTLET OBSTRUCTION: SURGERY VS STENTING
Gastrojejunostomy has in the past been the treatment of choice for gastric outlet obstruction. Certainly, patients with slow-growing tumors and an expected survival of greater than 60 days may be considered for this bypass procedure; those with a short tumor length, a single site of obstruction (preferably in the pylorus or proximal duodenum), a good performance status, and a life expectancy greater than 30 days are good candidates.7 Nevertheless, for patients with advanced cancer and poor performance status, gastroenterostomy carries a significant risk of morbidity and death.28
Endoscopic stenting of gastric outlet obstruction has a greater success rate, a shorter time to oral intake, a lower morbidity rate, a lower incidence of delayed gastric emptying, and a shorter hospital stay compared with gastroenterostomy.28,29 Technical success rates of stenting are 90%, and 75% of patients have resolution of nausea and vomiting.7 Stenting is generally not possible if the obstruction occurs beyond the ligament of Treitz.
Patients who are expected to survive less than 1 month or who have rapidly progressive disease, overt ascites, carcinomatosis, or multiple sites of obstruction should be managed with percutaneous, endoscopically placed gastrostomy tubes.7
Late complications of stenting for gastric outlet obstruction are occlusion with food or ingrowth of tumor through or around the wire mesh.7 This may require laser therapy or placement of a second stent, or both.
DRUG THERAPY
Medical therapy can palliate symptoms of malignant bowel obstruction for most patients.34 Recommendations have been published by the Working Group of the European Association for Palliative Care.24 Symptom management is focused on pain, nausea, and vomiting.
Which drugs can I use for abdominal pain?
Patients experience two types of abdominal pain: continuous and colic. Each type of pain requires different treatment approaches and classes of drugs.
Potent opioids such as morphine, hydromorphone (Dilaudid), and fentanyl (Fentora) are used to relieve continuous abdominal pain.7 The dose is titrated for adequate relief. Subcutaneous, intravenous, sublingual, and transdermal routes can be used if nausea and vomiting prevent oral administration.
However, opioids can aggravate colic by stimulating circular smooth muscle, leading to segmental contractions. Opioid-sparing adjuvant drugs such as ketorolac (Toradol) may improve colic and continuous pain and prevent a partial obstruction from becoming a complete obstruction by sparing opioid doses.35
Colic may persist or worsen with the use of opioids. Drugs that reduce colic include the scopolamine drugs hyoscine butylbromide and hyoscine hydrobromide, glycopyrrolate (Robinul), and octreotide (Sandostatin).7,34–37
Which drugs are appropriate for reducing nausea and vomiting?
Phenothiazines reduce nausea and control vomiting. Chlorpromazine (Thorazine), prochlorperazine (Compro, Compazine), and promethazine (Phenergan) have all been reported to treat nausea successfully.35,37
Haloperidol (Haldol), a butyrophenone selective dopamine D2-receptor antagonist, has negligible anticholinergic activity. At low doses it produces less sedation than phenothiazines and is an ideal agent for patients with nausea and delirium.35 Doses range from 5 to 15 mg/day, given in divided doses or as intermittent or continuous intravenous infusions.
Anticholinergics, with or without somatostatin analogues, reduce gastrointestinal secretions, fluid accumulation, and vomiting. Anticholinergics bind to muscarinic receptors on enteric neurons in the myenteric and the submucosal plexus. Dosages:
- Hyoscine butylbromide 40 to 120 mg/day.
- Hyoscine hydrobromide 0.2 to 0.9 mg/day.7,34
Glycopyrrolate, a quaternary ammonium anticholinergic, has minimal central nervous system penetration and is less likely to cause delirium or cardiac side effects compared with tertiary amine anticholinergics such as atropine and scopolamine.38 The recommended dose is 0.1 to 0.2 mg subcutaneously or intravenously three to four times daily.
Octreotide, an analogue of somatostatin, blocks the release of vasoactive intestinal polypeptide, which is increased in malignant bowel obstruction.14,15 It reduces the excretion of water, sodium, and chloride into the bowel lumen and increases the absorption of electrolytes and water. It also inhibits pancreatic enzyme secretion and splanchnic blood flow. The result of all these effects is reduced luminal content, reduced motility, reduced vascular congestion of the bowel wall, and, in certain circumstances, reduced ascites.39
In small randomized trials, octreotide was more successful than anticholinergics at improving nausea, vomiting, and colic in patients requiring a nasogastric tube and in those whose symptoms were refractory to standard medical treatment.5,34,40–43 A recent case report found octreotide helpful in resolving symptoms of partial bowel obstruction that were unresponsive to standard measures.44
Octreotide is well tolerated and reduces the time patients require a nasogastric tube without significantly worsening xerostomia. High cost limits its use in American hospice care due to the Medicare capitated system of reimbursement for drugs and services, and as a result it is a second-tier drug despite evidence of its efficacy.
Octreotide doses are 100 to 200 mg every 8 hours.
Metoclopramide (Reglan), a dopaminergic antagonist, a 5HT4 receptor agonist, and a 5HT3 receptor blocker at doses greater than 120 mg/day, combines the action of a phenothiazine, which blocks D2 receptors in the central chemoreceptor trigger zone, with promotility actions through serotonin receptors (5HT4).35,37
Metoclopramide should not be used with anticholinergics or in patients with colic or complete obstruction.35,45 In some centers it is the first-line drug for functional or partial bowel obstruction.7 Dosages range from 40 to 240 mg/day.
Olanzapine (Zyprexa), an atypical antipsychotic, blocks multiple neurotransmitter receptors (D2, H1, Ach, 5HT3) responsible for initiating emesis. It is an option in patients whose nausea and vomiting fail to respond to standard antiemetics.46 Dosages range from 2.5 to 20 mg/day.
Dissolvable tablets are given sublingually, which makes olanzapine a versatile antiemetic in cases of intractable nausea. Our unpublished experience is that the sublingual route reduces nausea associated with malignant bowel obstruction and obviates the need for subcutaneous injections or intravenous antiemetic infusions.
Corticosteroids. Although how corticosteroids relieve malignant bowel obstruction is unknown, they are presumed to act centrally.37,45 In addition, they reduce peritumoral edema and luminal salt and water, and they also have antiemetic and analgesic properties.
Evidence from a meta-analysis found that 6 to 16 mg of parenteral dexamethasone per day reduced symptoms and improved bowel function in 60% of patients but did not change the prognosis.11
A trial of 4 or 5 days is adequate to determine response. If there is no response, the corticosteroid should be rapidly tapered. Side effects are minimal when corticosteroids are used short-term.
Combination therapy. Only rarely does a single drug resolve symptoms of malignant bowel obstruction. Antiemetics, analgesics, corticosteroids, antisecretory anticholinergics, and octreotide are often required in combination to achieve acceptable symptom relief.3,5,7,47
In a small prospective case series, the combination of metoclopramide 60 mg/day, octreotide 0.3 mg/day, and dexamethasone 12 mg/day with a single bolus of amidotrizoic acid (a contrast agent) improved intestinal transit within 1 to 5 days and resolved vomiting within 24 hours.45
Compatibility and the route of administration of medications are key considerations when choosing drug combinations.
WHEN TO CONSIDER A VENTING GASTROSTOMY
Patients with a poor performance status, rapidly progressive disease, peritoneal carcinomatosis, a life expectancy of less than 30 days, or multiple levels of obstruction benefit from placement of a percutaneous endoscopic gastrostomy tube (ie, a venting gastrostomy) rather than surgery if symptoms do not respond to drug therapy.7,48 There is compelling evidence that this procedure relieves nausea and vomiting in 80% to 90% of patients and restores some level of oral intake in many.5,6,48,49 A venting gastrostomy tube can be placed during surgical exploration, percutaneously with fluoroscopy, or endoscopically.9
There are no absolute contraindications to gastrostomy tube placement. It is feasible even in patients with tumors encasing the stomach, diffuse carcinomatosis, and ascites.48 However, massive ascites, previous upper abdominal surgery, or a large mass attached to the abdominal wall make tube placement difficult.
Complications are often local. Patients experience transient abdominal wall pain after the procedure. Dislodgement, bleeding, catheter migration, peritonitis, and necrotizing fasciitis are early complications. Others include skin excoriation from leakage of gastric contents, leakage of ascitic fluid from the site, and obstruction or dislodgement of the tube.48,49
Patients can be discharged from the hospital soon after the tube is placed, usually with fewer medications than for patients who undergo surgery.48 This is particularly important for patients with a short expected survival. Some patients at home benefit from hydration (less than 2 L/day) via an existing central venous port or peripherally inserted central catheter, or by hypodermoclysis.
WHEN IS A NASOGASTRIC TUBE APPROPRIATE?
Some patients with malignant bowel obstruction require a nasogastric tube early in their hospital course.12 Unfortunately, nasogastric tubes, if left in place, cause nose and throat pain, sinusitis, abscess formation, erosion of nasal cartilage, aspiration, esophageal erosion, pharyngitis, and social isolation.5,6
Nasogastric tubes should be a temporizing measure to vent gastrointestinal secretions, reduce abdominal distension, and improve nausea and vomiting while a decision about surgery is being made.13,24 If surgery is not feasible, one can avoid the long-term complications and discomfort of a nasogastric tube via medical management and earlier evaluation for venting gastrostomy in those with symptoms that respond poorly to optimal medical management.49
WHICH PATIENTS BENEFIT FROM TOTAL PARENTERAL NUTRITION?
The use of total parenteral nutrition in patients with incurable malignancies is controversial. Enteral and parenteral feeding can increase muscle mass and improve functional status and quality of life in a subset of patients who are not suffering from cancer-related cachexia.2,50,51 However, for those whose weight loss and malnutrition are consequences of tumor-mediated cachexia, as demonstrated by anorexia and an elevated C-reactive protein level, parenteral nutrition is unlikely to improve the outcome.51 For most terminally ill patients, retrospective studies have failed to show that parenteral nutrition improves overall survival, performance status, or quality of life.2,48,50–54
Total parenteral nutrition poses risks: it is invasive and requires central venous access, which predisposes to infection; it requires frequent monitoring of hydration and electrolytes; and it predisposes to thrombosis, diarrhea, hyperglycemia, and liver failure.50–56
Total parenteral nutrition may be justified in patients with minimal tumor burden who are candidates for definitive surgery, or in those with a good performance status early in the disease course who have not had chemotherapy or whose cancer responds to chemotherapy.2,50–56
The American College of Physicians discourages the routine use of parenteral nutrition in those with advanced cancer who are undergoing palliative chemotherapy, since few patients benefit and many experience side effects.53
Total parenteral nutrition is much like a medical intervention in that it should be offered or continued only if it provides benefit. Conversations at the time that it is begun must include adverse effects that will lead to its discontinuation, and criteria for response. In certain situations, a limited trial of parenteral nutrition may be considered for patients with an uncertain prognosis or for those who have potentially reversible conditions that limit oral intake.51 In such cases, there should be a clear understanding between patient and physician that parenteral nutrition will be discontinued if it fails to show benefit.53
ADDITIONAL CONCERNS OF PATIENTS AND FAMILIES
‘Will I starve to death?’
Starvation is a fear echoed by patients and families. Ethical discourse on the continuation of nutrition and hydration for the terminally ill has been polarizing.57–60 Withdrawal of nutrition can be perceived as euthanasia.
Advanced cancer patients in general do not experience hunger, and those who do require only small amounts of food for satiation.61 In one report, most patients died of their advanced cancer and not from starvation.52 Artificial hydration and nutrition will thus not influence survival and can even be a burden without benefit in the imminently dying.60 These patients should be encouraged to take food orally for pleasure, as long as it is tolerated, without consideration of end points such as weight gain, body mass index, or albumin levels.
Complaints of thirst and dryness of the mouth are relieved with mouth care, ice chips, lubrication to the lips, and sips of fluid, rather than by parenteral nutrition.59 Patients with a terminal illness experience relief from thirst with minimal intake. The symptom of thirst may be relieved without hydration.34,61 Adequate hydration requires smaller fluid volumes due to decreased body weight, decreased renal clearance of free water, and decreased insensible water losses from reduced physical activity.58
‘Can we continue intravenous hydration so he won’t die of thirst?’
Overzealous intravenous hydration may worsen the symptoms of malignant bowel obstruction. Overhydration can increase secretions in the gut lumen and worsen the secretion-distention-contraction cycle, leading to greater abdominal pain and to nausea and vomiting.7 There is a greater risk of fluid overload in these patients, since they have edema and excessive interstitial fluid. Most have a low serum albumin level, which results in movement of fluid from intravascular to interstitial spaces due to reduced colloid osmotic pressure. In these instances, overzealous hydration can lead to respiratory insufficiency and worsening edema.
In spite of numerous discussions in the medical literature of the benefits and burdens of continual hydration, there is no consensus or guideline. When a patient has limited oral intake, the decision to hydrate should be individualized, with careful assessment of the risks and benefits and in accordance with the patient’s or family’s wishes.57,58
Is treatment at home feasible?
Discharging patients with inoperable malignant bowel obstruction requires careful planning. Patients and family members need to be educated on the use of around-the-clock medications and symptom-targeted, as-needed drugs. Days before discharge, questions about diet need to be clarified. Education about total parenteral nutrition and gastrostomy tube care should be completed before discharge from the hospital.
Drug management should be simplified, or compatible medications should be combined into a single infusion. For example, morphine, glycopyrrolate, and haloperidol or metoclopramide are chemically compatible in standard intravenous solutions and can be combined.
Families feel less anxious about the foreseen and the possible unforeseen course of the illness if they can talk with hospice workers early on. This early involvement also facilitates the transition to home hospice care.
SUMMARY OF IMPORTANT POINTS
- Patients with malignant bowel obstruction need a highly individualized approach, tailored to their medical condition, the prognosis, and the goals of care.
- Surgery should not be routinely undertaken; less-invasive approaches such as gastric or colonic stenting should be considered first.
- Combinations of analgesics, antisecretory drugs, and antiemetics can provide acceptable symptom relief in the inoperable patient.
- A venting gastrostomy should be considered if drug therapy fails to reduce nausea and vomiting to an acceptable level.
- A nasogastric tube should be used only as a temporizing measure, until symptoms are controlled medically or a venting gastrostomy is placed.
- Total parenteral nutrition is of benefit only in patients with intermediate life expectancy who may otherwise die of starvation rather than from the cancer itself.
- Mercadante S. Intestinal dysfunction and obstruction. In:Walsh D, editor. Palliative Medicine. Philadelphia, PA: Saunders/Elsevier, 2009:1267–1275.
- Pasanisi F, Orban A, Scalfi L, et al. Predictors of survival in terminal-cancer patients with irreversible bowel obstruction receiving home parenteral nutrition. Nutrition 2001; 17:581–584.
- Pameijer CR, Mahvi DM, Stewart JA, Weber SM. Bowel obstruction in patients with metastatic cancer: does intervention influence outcome? Int J Gastrointest Cancer 2005; 35:127–133.
- Bais JMJ, Schilthuis MS, Ansink AC. Palliative management of intestinal obstruction in patients with advanced gynaecological cancer. J Gynecol Oncol 2002; 7:299–305.
- Laval G, Arvieux C, Stefani L, Villard ML, Mestrallet JP, Cardin N. Protocol for the treatment of malignant inoperable bowel obstruction: a prospective study of 80 cases at Grenoble University Hospital Center. J Pain Symptom Manage 2006; 31:502–512.
- Jatoi A, Podratz KC, Gill P, Hartmann LC. Pathophysiology and palliation of inoperable bowel obstruction in patients with ovarian cancer. J Support Oncol 2004; 2:323–334.
- Ripamonti CI, Easson AM, Gerdes H. Management of malignant bowel obstruction. Eur J Cancer 2008; 44:1105–1115.
- Roeland E, von Gunten CF. Current concepts in malignant bowel obstruction management. Curr Oncol Rep 2009; 11:298–303.
- Baron TH. Interventional palliative strategies for malignant bowel obstruction. Curr Oncol Rep 2009; 11:293–297.
- Feuer DJ, Broadley KE, Shepherd JH, Barton DP. Surgery for the resolution of symptoms in malignant bowel obstruction in advanced gynaecological and gastrointestinal cancer. Cochrane Database Syst Rev 2000;CD002764.
- Feuer DJ, Broadley KE. Corticosteroids for the resolution of malignant bowel obstruction in advanced gynaecological and gastrointestinal cancer. Cochrane Database Syst Rev 2000;CD001219.
- Woolfson RG, Jennings K, Whalen GF. Management of bowel obstruction in patients with abdominal cancer. Arch Surg 1997; 132:1093–1097.
- Saunders MD, Kimmey MB. Systematic review: acute colonic pseudo-obstruction. Aliment Pharmacol Ther 2005; 22:917–925.
- Ripamonti C, Bruera E. Palliative management of malignant bowel obstruction. Int J Gynecol Cancer 2002; 12:135–143.
- Nellgård P, Bojö L, Cassuto J. Importance of vasoactive intestinal peptide and somatostatin for fluid losses in small-bowel obstruction. Scand J Gastroenterol 1995; 30:464–469.
- Böhner H, Yang Q, Franke C, Verreet PR, Ohmann C. Simple data from history and physical examination help to exclude bowel obstruction and to avoid radiographic studies in patients with acute abdominal pain. Eur J Surg 1998; 164:777–784.
- Maglinte DD, Kelvin FM, Sandrasegaran K, et al. Radiology of small bowel obstruction: contemporary approach and controversies. Abdom Imaging 2005; 30:160–178.
- Maglinte DD, Howard TJ, Lillemoe KD, Sandrasegaran K, Rex DK. Small-bowel obstruction: state-of-the-art imaging and its role in clinical management. Clin Gastroenterol Hepatol 2008; 6:130–139.
- Silva AC, Pimenta M, Guimarães LS. Small bowel obstruction: what to look for. Radiographics 2009; 29:423–439.
- Finan PJ, Campbell S, Verma R, et al. The management of malignant large bowel obstruction: ACPGBI position statement. Colorectal Dis 2007; 9(suppl 4):1–17.
- Kohli MD, Maglinte DD. CT enteroclysis in incomplete small bowel obstruction. Abdom Imaging 2009; 34:321–327.
- Ha HK, Shin BS, Lee SI, et al. Usefulness of CT in patients with intestinal obstruction who have undergone abdominal surgery for malignancy. AJR Am J Roentgenol 1998; 171:1587–1593.
- DeBernardo R. Surgical management of malignant bowel obstruction: strategies toward palliation of patients with advanced cancer. Curr Oncol Rep 2009; 11:287–292.
- Ripamonti C, Twycross R, Baines M, et al; Working Group of the European Association for Palliative Care. Clinical-practice recommendations for the management of bowel obstruction in patients with end-stage cancer. Support Care Cancer 2001; 9:223–233.
- Mangili G, Aletti G, Frigerio L, et al. Palliative care for intestinal obstruction in recurrent ovarian cancer: a multivariate analysis. Int J Gynecol Cancer 2005; 15:830–835.
- Turner J, Cummin T, Bennett A, Swift G, Green J. Stents and stentability: treatment for malignant bowel obstruction. Br J Hosp Med (Lond) 2008; 69:676–680.
- Khot UP, Lang AW, Murali K, Parker MC. Systematic review of the efficacy and safety of colorectal stents. Br J Surg 2002; 89:1096–1102.
- Hosono S, Ohtani H, Arimoto Y, Kanamiya Y. Endoscopic stenting versus surgical gastroenterostomy for palliation of malignant gastroduodenal obstruction: a meta-analysis. J Gastroenterol 2007; 42:283–290.
- Del Piano M, Ballarè M, Montino F, et al. Endoscopy or surgery for malignant GI outlet obstruction? Gastrointest Endosc 2005; 61:421–426.
- Tilney HS, Lovegrove RE, Purkayastha S, et al. Comparison of colonic stenting and open surgery for malignant large bowel obstruction. Surg Endosc 2007; 21:225–233.
- Holt AP, Patel M, Ahmed MM. Palliation of patients with malignant gastroduodenal obstruction with self-expanding metallic stents: the treatment of choice? Gastrointest Endosc 2004; 60:1010–1017.
- Dastur JK, Forshaw MJ, Modarai B, Solkar MM, Raymond T, Parker MC. Comparison of short-and long-term outcomes following either insertion of self-expanding metallic stents or emergency surgery in malignant large bowel obstruction. Tech Coloproctol 2008; 12:51–55.
- Turner J, Cummin T, Bennett A, Swift G, Green J. Stents and stentability: treatment for malignant bowel obstruction. Br J Hosp Med (Lond) 2008; 69:676–680.
- Ripamonti C, Mercadante S, Groff L, Zecca E, De Conno F, Casuccio A. Role of octreotide, scopolamine butylbromide, and hydration in symptom control of patients with inoperable bowel obstruction and nasogastric tubes: a prospective randomized trial. J Pain Symptom Manage 2000; 19:23–34.
- Davis MP, Walsh D. Treatment of nausea and vomiting in advanced cancer. Support Care Cancer 2000; 8:444–452.
- Bicanovsky LK, Lagman RL, Davis MP, Walsh D. Managing nonmalignant chronic abdominal pain and malignant bowel obstruction. Gastroenterol Clin North Am 2006; 35:131–142.
- Glare P, Pereira G, Kristjanson LJ, Stockler M, Tattersall M. Systematic review of the efficacy of antiemetics in the treatment of nausea in patients with far-advanced cancer. Support Care Cancer 2004; 12:432–440.
- Davis MP, Furste A. Glycopyrrolate: a useful drug in the palliation of mechanical bowel obstruction. J Pain Symptom Manage 1999; 18:153–154.
- Ripamonti C, Mercadante S. How to use octreotide for malignant bowel obstruction. J Support Oncol 2004; 2:357–364.
- Shima Y, Ohtsu A, Shirao K, Sasaki Y. Clinical efficacy and safety of octreotide (SMS201-995) in terminally ill Japanese cancer patients with malignant bowel obstruction. Jpn J Clin Oncol 2008; 38:354–359.
- Mercadante S, Casuccio A, Mangione S. Medical treatment for inoperable malignant bowel obstruction: a qualitative systematic review. J Pain Symptom Manage 2007; 33:217–223.
- Mystakidou K, Tsilika E, Kalaidopoulou O, Chondros K, Georgaki S, Papadimitriou L. Comparison of octreotide administration vs conservative treatment in the management of inoperable bowel obstruction in patients with far advanced cancer: a randomized, double-blind, controlled clinical trial. Anticancer Res 2002; 22:1187–1192.
- Mercadante S, Ripamonti C, Casuccio A, Zecca E, Groff L. Comparison of octreotide and hyoscine butylbromide in controlling gastrointestinal symptoms due to malignant inoperable bowel obstruction. Support Care Cancer 2000; 8:188–191.
- Myers J, Tamber A, Farhadian M. Management of treatment-related intermittent partial small bowel obstruction: the use of octreotide. J Pain Symptom Manage 2010; 39:e1–e3.
- Mercadante S, Ferrera P, Villari P, Marrazzo A. Aggressive pharmacological treatment for reversing malignant bowel obstruction. J Pain Symptom Manage 2004; 28:412–416.
- Srivastava M, Brito-Dellan N, Davis MP, Leach M, Lagman R. Olanzapine as an antiemetic in refractory nausea and vomiting in advanced cancer. J Pain Symptom Manage 2003; 25:578–582.
- Bentley A, Boyd K. Use of clinical pictures in the management of nausea and vomiting: a prospective audit. Palliat Med 2001; 15:247–253.
- Pothuri B, Montemarano M, Gerardi M, et al. Percutaneous endoscopic gastrostomy tube placement in patients with malignant bowel obstruction due to ovarian carcinoma. Gynecol Oncol 2005; 96:330–334.
- Brooksbank MA, Game PA, Ashby MA. Palliative venting gastrostomy in malignant intestinal obstruction. Palliat Med 2002; 16:520–526.
- Wang MY, Wu MH, Hsieh DY, et al. Home parenteral nutrition support in adults: experience of a medical center in Asia. JPEN J Parenter Enteral Nutr 2007; 31:306–310.
- Dy SM. Enteral and parenteral nutrition in terminally ill cancer patients: a review of the literature. Am J Hosp Palliat Care 2006; 23:369–377.
- Whitworth MK, Whitfield A, Holm S, Shaffer J, Makin W, Jayson GC. Doctor, does this mean I’m going to starve to death? J Clin Oncol 2004; 22:199–201.
- Hoda D, Jatoi A, Burnes J, Loprinzi C, Kelly D. Should patients with advanced, incurable cancers ever be sent home with total parenteral nutrition? A single institution’s 20-year experience. Cancer 2005; 103:863–868.
- Philip J, Depczynski B. The role of total parenteral nutrition for patients with irreversible bowel obstruction secondary to gynecological malignancy. J Pain Symptom Manage 1997; 13:104–111.
- August DA, Thorn D, Fisher RL, Welchek CM. Home parenteral nutrition for patients with inoperable malignant bowel obstruction. JPEN J Parenter Enteral Nutr 1991; 15:323–327.
- Abu-Rustum NR, Barakat RR, Venkatraman E, Spriggs D. Chemotherapy and total parenteral nutrition for advanced ovarian cancer with bowel obstruction. Gynecol Oncol 1997; 64:493–495.
- Fainsinger RL, Bruera E. When to treat dehydration in a terminally ill patient? Support Care Cancer 1997; 5:205–211.
- Steiner N, Bruera E. Methods of hydration in palliative care patients. J Palliat Care 1998; 14:6–13.
- Slomka J. Withholding nutrition at the end of life: clinical and ethical issues. Cleve Clin J Med 2003; 70:548–552.
- Chiu TY, Hu WY, Chuang RB, Chen CY. Nutrition and hydration for terminal cancer patients in Taiwan. Support Care Cancer 2002; 10:630–636.
- McCann RM, Hall WJ, Groth-Juncker A. Comfort care for terminally ill patients. The appropriate use of nutrition and hydration. JAMA 1994; 272:1263–1266.
- Mercadante S. Intestinal dysfunction and obstruction. In:Walsh D, editor. Palliative Medicine. Philadelphia, PA: Saunders/Elsevier, 2009:1267–1275.
- Pasanisi F, Orban A, Scalfi L, et al. Predictors of survival in terminal-cancer patients with irreversible bowel obstruction receiving home parenteral nutrition. Nutrition 2001; 17:581–584.
- Pameijer CR, Mahvi DM, Stewart JA, Weber SM. Bowel obstruction in patients with metastatic cancer: does intervention influence outcome? Int J Gastrointest Cancer 2005; 35:127–133.
- Bais JMJ, Schilthuis MS, Ansink AC. Palliative management of intestinal obstruction in patients with advanced gynaecological cancer. J Gynecol Oncol 2002; 7:299–305.
- Laval G, Arvieux C, Stefani L, Villard ML, Mestrallet JP, Cardin N. Protocol for the treatment of malignant inoperable bowel obstruction: a prospective study of 80 cases at Grenoble University Hospital Center. J Pain Symptom Manage 2006; 31:502–512.
- Jatoi A, Podratz KC, Gill P, Hartmann LC. Pathophysiology and palliation of inoperable bowel obstruction in patients with ovarian cancer. J Support Oncol 2004; 2:323–334.
- Ripamonti CI, Easson AM, Gerdes H. Management of malignant bowel obstruction. Eur J Cancer 2008; 44:1105–1115.
- Roeland E, von Gunten CF. Current concepts in malignant bowel obstruction management. Curr Oncol Rep 2009; 11:298–303.
- Baron TH. Interventional palliative strategies for malignant bowel obstruction. Curr Oncol Rep 2009; 11:293–297.
- Feuer DJ, Broadley KE, Shepherd JH, Barton DP. Surgery for the resolution of symptoms in malignant bowel obstruction in advanced gynaecological and gastrointestinal cancer. Cochrane Database Syst Rev 2000;CD002764.
- Feuer DJ, Broadley KE. Corticosteroids for the resolution of malignant bowel obstruction in advanced gynaecological and gastrointestinal cancer. Cochrane Database Syst Rev 2000;CD001219.
- Woolfson RG, Jennings K, Whalen GF. Management of bowel obstruction in patients with abdominal cancer. Arch Surg 1997; 132:1093–1097.
- Saunders MD, Kimmey MB. Systematic review: acute colonic pseudo-obstruction. Aliment Pharmacol Ther 2005; 22:917–925.
- Ripamonti C, Bruera E. Palliative management of malignant bowel obstruction. Int J Gynecol Cancer 2002; 12:135–143.
- Nellgård P, Bojö L, Cassuto J. Importance of vasoactive intestinal peptide and somatostatin for fluid losses in small-bowel obstruction. Scand J Gastroenterol 1995; 30:464–469.
- Böhner H, Yang Q, Franke C, Verreet PR, Ohmann C. Simple data from history and physical examination help to exclude bowel obstruction and to avoid radiographic studies in patients with acute abdominal pain. Eur J Surg 1998; 164:777–784.
- Maglinte DD, Kelvin FM, Sandrasegaran K, et al. Radiology of small bowel obstruction: contemporary approach and controversies. Abdom Imaging 2005; 30:160–178.
- Maglinte DD, Howard TJ, Lillemoe KD, Sandrasegaran K, Rex DK. Small-bowel obstruction: state-of-the-art imaging and its role in clinical management. Clin Gastroenterol Hepatol 2008; 6:130–139.
- Silva AC, Pimenta M, Guimarães LS. Small bowel obstruction: what to look for. Radiographics 2009; 29:423–439.
- Finan PJ, Campbell S, Verma R, et al. The management of malignant large bowel obstruction: ACPGBI position statement. Colorectal Dis 2007; 9(suppl 4):1–17.
- Kohli MD, Maglinte DD. CT enteroclysis in incomplete small bowel obstruction. Abdom Imaging 2009; 34:321–327.
- Ha HK, Shin BS, Lee SI, et al. Usefulness of CT in patients with intestinal obstruction who have undergone abdominal surgery for malignancy. AJR Am J Roentgenol 1998; 171:1587–1593.
- DeBernardo R. Surgical management of malignant bowel obstruction: strategies toward palliation of patients with advanced cancer. Curr Oncol Rep 2009; 11:287–292.
- Ripamonti C, Twycross R, Baines M, et al; Working Group of the European Association for Palliative Care. Clinical-practice recommendations for the management of bowel obstruction in patients with end-stage cancer. Support Care Cancer 2001; 9:223–233.
- Mangili G, Aletti G, Frigerio L, et al. Palliative care for intestinal obstruction in recurrent ovarian cancer: a multivariate analysis. Int J Gynecol Cancer 2005; 15:830–835.
- Turner J, Cummin T, Bennett A, Swift G, Green J. Stents and stentability: treatment for malignant bowel obstruction. Br J Hosp Med (Lond) 2008; 69:676–680.
- Khot UP, Lang AW, Murali K, Parker MC. Systematic review of the efficacy and safety of colorectal stents. Br J Surg 2002; 89:1096–1102.
- Hosono S, Ohtani H, Arimoto Y, Kanamiya Y. Endoscopic stenting versus surgical gastroenterostomy for palliation of malignant gastroduodenal obstruction: a meta-analysis. J Gastroenterol 2007; 42:283–290.
- Del Piano M, Ballarè M, Montino F, et al. Endoscopy or surgery for malignant GI outlet obstruction? Gastrointest Endosc 2005; 61:421–426.
- Tilney HS, Lovegrove RE, Purkayastha S, et al. Comparison of colonic stenting and open surgery for malignant large bowel obstruction. Surg Endosc 2007; 21:225–233.
- Holt AP, Patel M, Ahmed MM. Palliation of patients with malignant gastroduodenal obstruction with self-expanding metallic stents: the treatment of choice? Gastrointest Endosc 2004; 60:1010–1017.
- Dastur JK, Forshaw MJ, Modarai B, Solkar MM, Raymond T, Parker MC. Comparison of short-and long-term outcomes following either insertion of self-expanding metallic stents or emergency surgery in malignant large bowel obstruction. Tech Coloproctol 2008; 12:51–55.
- Turner J, Cummin T, Bennett A, Swift G, Green J. Stents and stentability: treatment for malignant bowel obstruction. Br J Hosp Med (Lond) 2008; 69:676–680.
- Ripamonti C, Mercadante S, Groff L, Zecca E, De Conno F, Casuccio A. Role of octreotide, scopolamine butylbromide, and hydration in symptom control of patients with inoperable bowel obstruction and nasogastric tubes: a prospective randomized trial. J Pain Symptom Manage 2000; 19:23–34.
- Davis MP, Walsh D. Treatment of nausea and vomiting in advanced cancer. Support Care Cancer 2000; 8:444–452.
- Bicanovsky LK, Lagman RL, Davis MP, Walsh D. Managing nonmalignant chronic abdominal pain and malignant bowel obstruction. Gastroenterol Clin North Am 2006; 35:131–142.
- Glare P, Pereira G, Kristjanson LJ, Stockler M, Tattersall M. Systematic review of the efficacy of antiemetics in the treatment of nausea in patients with far-advanced cancer. Support Care Cancer 2004; 12:432–440.
- Davis MP, Furste A. Glycopyrrolate: a useful drug in the palliation of mechanical bowel obstruction. J Pain Symptom Manage 1999; 18:153–154.
- Ripamonti C, Mercadante S. How to use octreotide for malignant bowel obstruction. J Support Oncol 2004; 2:357–364.
- Shima Y, Ohtsu A, Shirao K, Sasaki Y. Clinical efficacy and safety of octreotide (SMS201-995) in terminally ill Japanese cancer patients with malignant bowel obstruction. Jpn J Clin Oncol 2008; 38:354–359.
- Mercadante S, Casuccio A, Mangione S. Medical treatment for inoperable malignant bowel obstruction: a qualitative systematic review. J Pain Symptom Manage 2007; 33:217–223.
- Mystakidou K, Tsilika E, Kalaidopoulou O, Chondros K, Georgaki S, Papadimitriou L. Comparison of octreotide administration vs conservative treatment in the management of inoperable bowel obstruction in patients with far advanced cancer: a randomized, double-blind, controlled clinical trial. Anticancer Res 2002; 22:1187–1192.
- Mercadante S, Ripamonti C, Casuccio A, Zecca E, Groff L. Comparison of octreotide and hyoscine butylbromide in controlling gastrointestinal symptoms due to malignant inoperable bowel obstruction. Support Care Cancer 2000; 8:188–191.
- Myers J, Tamber A, Farhadian M. Management of treatment-related intermittent partial small bowel obstruction: the use of octreotide. J Pain Symptom Manage 2010; 39:e1–e3.
- Mercadante S, Ferrera P, Villari P, Marrazzo A. Aggressive pharmacological treatment for reversing malignant bowel obstruction. J Pain Symptom Manage 2004; 28:412–416.
- Srivastava M, Brito-Dellan N, Davis MP, Leach M, Lagman R. Olanzapine as an antiemetic in refractory nausea and vomiting in advanced cancer. J Pain Symptom Manage 2003; 25:578–582.
- Bentley A, Boyd K. Use of clinical pictures in the management of nausea and vomiting: a prospective audit. Palliat Med 2001; 15:247–253.
- Pothuri B, Montemarano M, Gerardi M, et al. Percutaneous endoscopic gastrostomy tube placement in patients with malignant bowel obstruction due to ovarian carcinoma. Gynecol Oncol 2005; 96:330–334.
- Brooksbank MA, Game PA, Ashby MA. Palliative venting gastrostomy in malignant intestinal obstruction. Palliat Med 2002; 16:520–526.
- Wang MY, Wu MH, Hsieh DY, et al. Home parenteral nutrition support in adults: experience of a medical center in Asia. JPEN J Parenter Enteral Nutr 2007; 31:306–310.
- Dy SM. Enteral and parenteral nutrition in terminally ill cancer patients: a review of the literature. Am J Hosp Palliat Care 2006; 23:369–377.
- Whitworth MK, Whitfield A, Holm S, Shaffer J, Makin W, Jayson GC. Doctor, does this mean I’m going to starve to death? J Clin Oncol 2004; 22:199–201.
- Hoda D, Jatoi A, Burnes J, Loprinzi C, Kelly D. Should patients with advanced, incurable cancers ever be sent home with total parenteral nutrition? A single institution’s 20-year experience. Cancer 2005; 103:863–868.
- Philip J, Depczynski B. The role of total parenteral nutrition for patients with irreversible bowel obstruction secondary to gynecological malignancy. J Pain Symptom Manage 1997; 13:104–111.
- August DA, Thorn D, Fisher RL, Welchek CM. Home parenteral nutrition for patients with inoperable malignant bowel obstruction. JPEN J Parenter Enteral Nutr 1991; 15:323–327.
- Abu-Rustum NR, Barakat RR, Venkatraman E, Spriggs D. Chemotherapy and total parenteral nutrition for advanced ovarian cancer with bowel obstruction. Gynecol Oncol 1997; 64:493–495.
- Fainsinger RL, Bruera E. When to treat dehydration in a terminally ill patient? Support Care Cancer 1997; 5:205–211.
- Steiner N, Bruera E. Methods of hydration in palliative care patients. J Palliat Care 1998; 14:6–13.
- Slomka J. Withholding nutrition at the end of life: clinical and ethical issues. Cleve Clin J Med 2003; 70:548–552.
- Chiu TY, Hu WY, Chuang RB, Chen CY. Nutrition and hydration for terminal cancer patients in Taiwan. Support Care Cancer 2002; 10:630–636.
- McCann RM, Hall WJ, Groth-Juncker A. Comfort care for terminally ill patients. The appropriate use of nutrition and hydration. JAMA 1994; 272:1263–1266.
KEY POINTS
- Combinations of analgesics, antisecretory drugs, and antiemetics can provide acceptable symptom relief.
- A venting gastrostomy should be considered if drug therapy fails to reduce nausea and vomiting to an acceptable level.
- A nasogastric tube should be used only as a temporizing measure, until symptoms are controlled medically or a venting gastrostomy is placed.
- Total parenteral nutrition is beneficial only in patients with intermediate life expectancy who may otherwise die of starvation rather than the cancer itself.