User login
Comprehensive wound malodor management: Win the RACE
Wounds that fail to heal become more than mere skin lesions. Pain, malodor, and the accompanying psychological distress often complicate nonhealing wounds and impair quality of life.1 Management of malodor requires perseverance, sensitivity, and familiarity with tools and procedures that range from surgical debridement to medical-grade honey.
Chronic, nonhealing wounds are defined as persisting for more than 6 months.2 These lesions are incapable of undergoing anatomic and functional repair on their own. Commonly encountered nonhealing wounds include pressure ulcers, venous stasis ulcers, arterial insufficiency ulcers, and malignant cutaneous wounds.
Typically, the patient with a nonhealing wound is frail, debilitated, medically complex, and often faced with one or more life-limiting illnesses. Complete wound healing may therefore be unrealistic, and optimal wound management becomes the goal of care.3,4
Healthcare providers encounter nonhealing wounds in varied settings—acute inpatient, outpatient, long-term, and home care. For instance, in the home care setting, a study of 383 patients enrolled in hospice found that 35% had skin ulcers and wounds.3 Half of those affected had pressure ulcers, 20% had ischemic ulcers, and 30% had other skin disorders such as stasis ulcers, burns, skin tears, and tumors. A larger study, also in hospice patients, found that 26% had pressure ulcers and 10% more developed them within 6 months.5
While pressure ulcers are the most common nonhealing wounds, malignant or fungating wounds are found in 5% to 10% of patients with metastatic disease, usually with cancers of the breast, head, and neck.6
Maximizing wound care provides comfort, relieves suffering, and promotes quality of life.3,7 To achieve these goals, clinicians must be familiar with strategies to manage complications associated with nonhealing wounds such as pain, malodor, and psychosocial adverse effects. Of these complications, malodor has been pointed out by both patients and caregivers as the most distressing.8
This article focuses on wound malodor, discusses the processes that cause wounds to emit an offensive smell, and outlines a comprehensive management approach.
MRS. A., AGE 61, WITH STAGE IV BREAST CANCER
Mrs. A., 61 years old, had a fungating mass in her left breast, which began as a small nodule and progressively enlarged to deform her breast over several months. Her oncologist subsequently staged the extent of her cancer as stage IV after workup revealed lung metastasis. Mrs. A. and her family decided to forgo cancer treatment, including radiotherapy, and to transition to hospice care after discussions with the oncologist.
Mrs. A. lived at home with her husband. Her daughter and three grandchildren all lived nearby.
When her hospice physician arrived at her home to meet her, a strong, pungent, and nauseating smell greeted him as he entered her bedroom. The patient said that for the past few months she had been increasingly distressed by the revolting odor. She rarely left home and had been ashamed to have people visit her, including her family.
On examination, the physician noticed a large fungating mass with yellowish discharge and necrotic tissue in her left breast. In addition to mild pain, she was immensely bothered by the strong odor coming from her breast.
THE IMPACT OF MALODOR
As seen in the case of Mrs. A., malodor has grave effects, both physical and psychological. Patients experience impaired or socially unacceptable body image, social rejection, personal shame, and embarrassment.9,10 Feelings of fear, anxiety, and depression are common. If left uncontrolled, malodor results in social isolation, reluctance to engage in social activities, diminished appetite, and nausea. In addition, malodor is a constant reminder of patients’ pain and cancer, and it results in further suffering.11
Reactions of family members and caregivers can worsen the situation.9,12 Expressions of revulsion limit contact and inhibit intimacy, especially near the end of life. Caregivers are often frustrated and distressed over their inability to control the malodor. The environment becomes uninhabitable, and the malodor can permeate clothing, furniture, and living quarters.
Managing malodor can be emotionally draining, physically daunting, and frustrating for healthcare professionals, as several methods are usually employed, often in a trial-and-error approach, to achieve an acceptable degree of odor control. In addition, clinicians must face the challenge of treating malodorous wounds at very close distance without reacting in a way that offends or alarms patients and family members.13
MALODOR PRODUCTION: WHERE IS THAT SMELL COMING FROM?
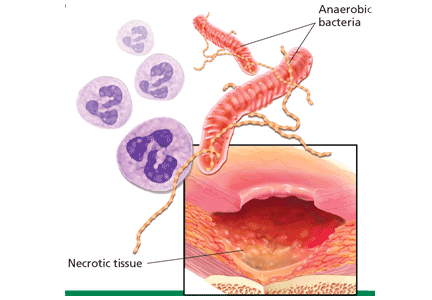
All wounds can produce an odor.14 Wounds that are expected to heal typically emit a faint but not unpleasant odor, akin to fresh blood. Wounds colonized by Pseudomonas aeruginosa produce a fruity or grapelike odor that is tolerable. Malodor occurs with wounds infected by other gram-negative organisms or anaerobic bacteria.15 Similarly, wounds covered by necrotic tissue smell like decaying flesh.
Three major causes
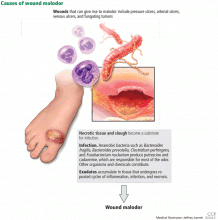
The three major causes of wound malodor are slough, infection, and exudate (Figure 1).
Slough is dead or necrotic tissue, usually resulting from vascular compromise. Arterial ulcers, pressure ulcers, and malignant wounds all form slough from capillary occlusion, subsequent ischemia, and tissue necrosis.
Infection. Devitalized tissue, an ideal medium in which bacteria thrive, becomes the source of infection. Anaerobic bacteria are usually implicated in malodor. These include Bacteroides fragilis, Bacteroides prevotella, Clostridium perfringens, and Fusobacterium nucleatum.16,17 Anaerobic organisms produce putrescine and cadaverine, which are largely responsible for the offensive odor.16,18 Volatile fatty acids such as propionic, butyric, isovaleric, and valeric acid are formed from lipid catabolism by anaerobes and add to malodor.17 Aerobic bacteria such as Proteus, Klebsiella, and Pseudomonas species supercolonize necrotic tissue as well and contribute to malodor.17,18
Exudate. Since nonhealing wounds undergo repeated cycles of inflammation, infection, and necrosis, accumulation of exudate becomes inevitable. Exudate typically is a pus-like fluid containing serum, fibrin, and white blood cells, which leak from blood vessels. In addition, bacteria that colonize chronic wounds filled with necrotic tissue activate proteases that degrade and liquefy dead tissue, thereby forming extensive amounts of exudate.19
Apart from slough, infection, and exudate, poor general hygiene and dressings left on for too long may contribute to malodor.16 Moisture-retentive dressings such as hydrocolloids leave an odor after removal. Dressings that liquefy upon contact with the wound surface leave a pus-like, potentially malodorous material.
MALODOR ASSESSMENT: DO YOU SMELL SOMETHING?
Various ways to document wound malodor can prove useful in guiding assessment and treatment. Descriptions such as “foul,” “putrid,” “fishy,” or “filled the room” vividly portray the initial presentation. A 10-point numerical scale similar to a numerical pain scale or a visual analogue scale can be used as a subjective measure.
Other grading methods, which to the authors’ knowledge are not validated, may be helpful. In a study that focused on patients suffering from malodorous gynecologic malignancies, von Gruenigen et al20 used a 0-to-3 scale:
- 0 Absent
- 1 Not offensive
- 2 Offensive but tolerable
- 3 Offensive and intolerable.
A scale often adapted by other authors was devised by Baker and Haig,21 which clearly defines four classes:
- 1 Strong—odor is evident upon entering the room (6 to 10 feet from the patient) with the dressing intact
- 2 Moderate—odor is evident upon entering the room with dressing removed
- 3 Slight—odor is evident at close proximity to the patient when the dressing is removed
- 4 No odor—no odor is evident, even at the patient’s bedside with the dressing removed.
COMPREHENSIVE MANAGEMENT: HOW DO WE WIN THE ‘RACE’?
The acronym RACE outlines an approach to dealing with malodor. It stands for removal of necrotic tissue; antibacterials; odor concealers; and education and support (Table 1).
Remove necrotic tissue
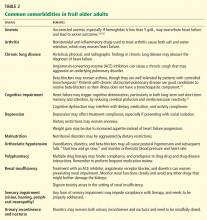
An important step in eliminating malodor is to remove necrotic tissue. This starts with debridement, which decreases the incidence of infection and hastens wound closure.22,23 Table 2 compares the different types of debridement.
Sharp or surgical debridement involves the use of a scalpel or scissors. This type of debridement may increase the risk of bleeding, pain, and malignant cell seeding in fungating wounds.4,24
Enzymatic debridement employs chemicals with proteolytic action (eg, collagenase) to digest extracellular proteins in wounds.18,25
Mechanical debridement involves aggressive therapies such as forceful irrigation and hydrotherapy, which may fail to discriminate between necrotic and viable tissues.18,26
Biological debridement using maggots, which ingest bacteria and devitalized tissue, may cause increased wound bleeding and may be unacceptable for patients and families.24,27
Autolytic debridement is often recommended, particularly if complete healing is not the primary goal.17,24,28,29 Autolysis uses proteolytic enzymes and phagocytic cells present in the wound bed and wound fluid to clear devitalized tissue. It is easy, inexpensive, noninvasive, and painless,4 and it requires less frequent dressing changes relative to standard dressing or wet-to-dry dressing.
Autolytic debridement is commonly accomplished using hydrocolloid and hydrogel dressings.15,29 Hydrocolloids are adhesive, occlusive, and conformable dressings that are suitable for wounds with low to moderate amounts of exudate. Upon contact with the wound surface, the dressing absorbs the exudate, forms a gel layer, and maintains a moist environment. Hydrocolloids are not recommended for infected wounds or for those with copious exudate as they may lead to maceration around the wound. A disadvantage of hydrocolloid dressings is their tendency to generate brown, often malodorous exudate when removed.
On the other hand, hydrogels in amorphous gel, dressing, sheet, or impregnated gauze form are water-based products that create a moist environment similar to hydrocolloids. Aside from causing minimal trauma to the wound bed when removed, the dressing’s cooling effect may bring some pain relief. Hydrogels are appropriate for dry wounds and for those with minimal exudate.
After debridement, the wound is cleansed and irrigated. A number of cleansers and solutions are available, but normal saline is a cheap alternative. To irrigate, experts recommend an 18- or 20-gauge intravenous catheter attached to a 30- or 60-mL syringe.15 This technique provides 8 to 15 psi of pressure, enough to cleanse the wound without causing tissue trauma.
Antibacterials and absorption
Antibacterials. Topical antibiotics have several advantages over systemic antibiotics in treating chronic wounds.30,31 These include a high and sustained concentration of the antimicrobial at the site of infection, limited potential for systemic absorption and toxicity, reduced potential for antibiotic resistance, and drawing of the patient’s and caregiver’s attention to the wound.
Metronidazole is the most widely used topical antibacterial for malodor management. Its efficacy is likely due to the predominant involvement of anaerobic bacteria in foul-smelling wounds. Topical metronidazole is available as a gel and as a cream. A systematic review showed that on average, topical metronidazole was used once daily for 14 consecutive days.19 The layer of topical metronidazole is typically covered with a nonadherent primary dressing followed by an absorbent secondary dressing.
The best clinical evidence for topical metronidazole consists of case reports and series.32–35 The largest of these studies was done by Finlay et al, who treated 47 patients with malodorous benign and malignant cutaneous wounds with 0.75% metronidazole gel daily.32 Forty-five (96%) of the patients reported significantly decreased odor by 14 days, as well as decreased pain, discharge, and surrounding cellulitis.
A randomized, placebo-controlled trial conducted by Bale et al had equivocal findings.9 All 41 patients who received metronidazole gel reported a decrease in malodor within 3 days of starting it. However, 76% of patients who received placebo also reported malodor control; in the final analysis, no significant difference was noted in the success rate between the two groups.
Metronidazole tablets can be crushed and sprinkled over the wound. As with metronidazole gel or cream, the crushed tablets are applied daily and covered by a primary nonadherent dressing and an absorbent secondary dressing. This off-label use of metronidazole serves as a cheaper alternative to commercially available topical preparations. To our knowledge, there has been no head-to-head trial comparing the two topical strategies.
Systemic metronidazole, often given orally, has been recommended if evidence of deep tissue or systemic infection is noted15 and in cases of fungating wounds with fistulas invading either the gastrointestinal or genitourinary tracts.18 Side effects such as nausea, neuropathy, and alcohol intolerance (ie, disulfiram reaction) may occur, which are not seen with topical metronidazole.
Both topical and systemic metronidazole can be used together on a time-limited basis for extensive malodorous wounds, such as fungating malignant wounds or stage IV sacral pressure ulcers.
Other antimicrobial agents used to treat malodor include silver-containing products, iodine-containing topical agents, mupirocin, bacitracin, neomycin, and polymyxin B.
Honey was used for wound care by the ancient Egyptians, and it is still used.36 Its beneficial effects include antimicrobial, debriding, deodorizing, anti-inflammatory, and granulation tissue-stimulating. Honey has even been shown to significantly decrease skin colonization with various kinds of bacteria, including methicillin-resistant Staphylococcus aureus.37 Medical-grade honey is preferred over table honey, as the latter is nonsterile and can contain Clostridium spores, which contaminate the wound.38
Yogurt and buttermilk lower the pH of the wound and control bacterial proliferation to control malodor.39,40 Either is applied for 10 to 15 minutes after the wound is cleansed and is then washed off thoroughly.
Absorbent dressings are used either over a layer of topical metronidazole and a nonadherent primary dressing or as a primary dressing itself. An absorbent dressing containing activated charcoal is used for rapid improvement, although cost may be prohibitive, especially in developing countries.13,19 Another type of absorbent dressing, composed of polyester impregnated with sodium chloride, has been found to be useful in malodor control.41 An important pointer is to maintain a tight seal around the absorbent dressing to prevent leakage of exudate.
Concealers
Aromatics used to conceal malodor include scented candles, incense, fragrant flowers and plants, and air-freshener sprays. When circumstances allow, candles are good options since they conceal malodor by emitting fragrance, and the flame burns off foul-smelling chemicals. Aromatics such as coffee beans, vanilla beans, and cider vinegar can be placed in a pan and left under the patient’s bed or close to it. Drops of peppermint oil or oil of wintergreen can be placed on wound dressings.
Other odor concealers are adsorbent materials that attract and cause ions and molecules to adhere to their surface. Examples are charcoal, baking soda, and cat litter. As with other aromatics, these materials are placed in pans and left under the bed or near the patient.
Aromatics can have disadvantages, as certain scents, especially strong ones, can be nauseating for patients. Some fragrances trigger asthma or skin irritation. Patients and caregivers can be left with an unpleasant association of certain fragrances with malodor by conditioning.15,17,18
Education and support
Concerns of the patient and family members need to be heard, addressed promptly, and reassessed with each visit, since uncontrolled malodor can be a chief source of caregiver fatigue.
Foremost in formulating a patient- and family-centered malodor management strategy is to commit to controlling malodor as much as possible. Regular follow-up appointments should be made, whether in the office or at home, to check on the patient’s progress and address new and ongoing concerns. Symptoms accompanying malodor, such as pain, bleeding, and sleep disturbance, need to be addressed, as they all affect quality of life.1 Audience-appropriate educational materials should be made available.26 Online resources that patients and families can explore include the websites of the Wound Ostomy and Continence Nurses Society (www.wocn.org) and the Association for the Advancement of Wound Care (aawconline.org).
Healthcare professionals need to be prepared to deal with problems and complications involving patients and family members that may arise in the course of treatment.12 Problems include the cost and local unavailability of dressing supplies, insurance coverage for dressings and topical agents, lack of assistance at home, and fear of changing dressings. A cardinal rule for healthcare providers is to avoid expressing distress at odors in front of or within hearing of patients and families.
OTHER STRATEGIES: WHAT ELSE CAN WE DO?
Curcumin, the main biologically active compound in the herb turmeric, applied directly to wounds three times daily as an ointment, has been shown to have odor-controlling properties.42
Sugar paste has been reported to control malodor by drawing out exudative and tissue fluid osmotically, and inhibiting bacterial growth.16,17 Water is mixed with sugar (ie, granulated, caster, or powdered) to form a paste, with additives like glycerin and polyethylene glycol used to alter the consistency. Thick clay-like paste is good for wounds with large cavities, while thin paste is useful for wounds with small or superficial openings. The paste is applied twice daily and is covered by an absorbent dressing.
Pressure relief is vital in managing pressure ulcers.18,43 Repositioning every 2 hours and using special devices, such as mattress overlays, alternating pressure mattresses, and low air loss mattresses, are frequently employed techniques.
If circumstances permit and when congruent with the patient’s goals of care, intra-arterial chemotherapy and radiotherapy can be contemplated for malignant fungating wounds.44,45
Other strategies include opening the windows during dressing changes, increasing the frequency of dressing changes, promptly removing used dressings from the house, and ensuring good general hygiene.
CASE RESOLUTION
After telling her that he was committed to control the malodor or, if possible, eliminate it, Mrs. A.’s doctor prepared two lists of materials—one for himself and one for Mrs. A.’s husband. He returned the next day, brought out his supplies, asked Mrs. A. to lie in bed, and invited her husband to assist him.
He cleansed and irrigated the breast lesion with normal saline, making sure to remove as much dead tissue as he could. He applied a layer of metronidazole cream to the wound cavity, then covered it with a nonadherent dressing. He then covered the wound with gauze, sealed the edges with medical adhesive tape, and applied a few drops of oil of wintergreen to the surface. A pan of charcoal briquettes was put under the bed, and a candle with Mrs. A.’s favorite scent was lit by the bedside. The physician then instructed Mrs. A.’s husband to repeat the procedure once daily for 1 week.
After 2 weeks, Mrs. A. and her husband said the foul odor had greatly decreased. She appeared more cheerful and energetic, especially after her grandchildren visited a few days earlier. The physician then instructed the husband to stop using metronidazole cream and to apply a hydrocolloid dressing every 3 days instead. He advised them to continue the rest of the process of applying a few drops of oil of wintergreen on the dressing surface, placing a pan of charcoal briquettes under the bed, and lighting a scented candle by the bedside.
FINISH THE RACE!
Complex nonhealing wounds are encountered across various healthcare settings. Wound malodor is an important component of nonhealing wounds, which adversely affects patients, families, and healthcare providers. Infection, slough, and exudate are the major causes of wound malodor. The essential steps to reduce malodor are to remove necrotic tissue, use antibacterial and odor-absorbing agents, apply appropriate odor “concealers,” educate families, and formulate a patient- and family-centered strategy (Table 1).
Acknowledgment: The authors would like to thank Sue Reif, CNP, for her assistance in completing the manuscript.
- Lo SF, Hayter M, Hu WY, Tai CY, Hsu MY, Li YF. Symptom burden and quality of life in patients with malignant fungating wounds. J Adv Nurs 2012; 68:1312–1321.
- Lazarus GS, Cooper DM, Knighton DR, et al. Definitions and guidelines for assessment of wounds and evaluation of healing. Arch Dermatol 1994; 130:489–493.
- Tippett AW. Wounds at the end of life. Wounds 2005; 17:91–98.
- Burt T. Palliative care of pressure ulcers in long-term care. Ann Long-Term Care 2013; 21:20–28.
- Reifsnyder J, Magee HS. Development of pressure ulcers in patients receiving home hospice care. Wounds 2005; 17:74–79.
- Haisfield-Wolfe ME, Rund C. Malignant cutaneous wounds: a management protocol. Ostomy Wound Manage 1997; 43:56–66.
- O’Brien C. Malignant wounds: managing odour. Can Fam Physician 2012; 58:272–274.
- Gethin G, Grocott P, Probst S, Clarke E. Current practice in the management of wound odour: an international survey. Int J Nurs Stud 2014; 51:865–874.
- Bale S, Tebble N, Price P. A topical metronidazole gel used to treat malodorous wounds. Br J Nurs 2004; 13:S4–S11.
- Hack A. Malodorous wounds—taking the patient’s perspective into account. J Wound Care 2003; 12:319–321.
- Price E. Wound care. The stigma of smell. Nurs Times 1996; 92:71–72.
- Paul JC, Pieper BA. Topical metronidazole for the treatment of wound odor: a review of the literature. Ostomy Wound Manage 2008; 54:18–27.
- Lee G, Anand SC, Rajendran S, Walker I. Overview of current practice and future trends in the evaluation of dressings for malodorous wounds. J Wound Care 2006; 15:344–346.
- Cutting K, Harding K. Criteria for identifying wound infection. J Wound Care 1994; 3:198–201.
- McDonald A, Lesage P. Palliative management of pressure ulcers and malignant wounds in patients with advanced illness. J Palliat Med 2006; 9:285–295.
- Holloway S. Recognising and treating the causes of chronic malodorous wounds. Prof Nurse 2004; 19:380–384.
- Haughton W, Young T. Common problems in wound care: malodorous wounds. Br J Nurs 1995; 4:959–963.
- Alvarez OM, Kalinski C, Nusbaum J, et al. Incorporating wound healing strategies to improve palliation (symptom management) in patients with chronic wounds. J Palliat Med 2007; 10:1161–1189.
- da Costa Santos CM, de Mattos Pimenta CA, Nobre MR. A systematic review of topical treatments to control the odor of malignant fungating wounds. J Pain Symptom Manage 2010; 39:1065–1076.
- Von Gruenigen VE, Coleman RL, et al. Bacteriology and treatment of malodorous lower reproductive tract in gynecologic cancer patients. Obstet Gynecol 2000; 96:23–27.
- Baker PG, Haig G. Metronidazole in the treatment of chronic pressure sores and ulcers: a comparison with standard treatment in general practice. Practitioner 1981; 225:569–573.
- Whitney J, Phillips L, Aslam R, et al. Guidelines for the treatment of pressure ulcers. Wound Repair Regen 2006; 14:663–679.
- Williams D, Enoch S, Miller D, Harris K, Price P, Harding KG. Effect of sharp debridement using curette on recalcitrant nonhealing venous ulcers: a concurrently controlled, prospective cohort study. Wound Repair Regen 2005; 13:131–137.
- Bergstrom KJ. Assessment and management of fungating wounds. J Wound Ostomy Continence Nurs 2011: 38:31–37.
- Sinclair RD, Ryan TJ. Proteolytic enzymes in wound healing: the role of enzymatic debridement. Australas J Dermatol 1994; 35:35–41.
- Enoch S, Harding KG. Wound bed preparation: the science behind the removal of barriers to healing. Wounds 2003;15:213–229.
- Mumcuoglu KY. Clinical applications for maggots in wound care. Am J Clin Dermatol 2001; 2:219–227.
- Langemo DK, Black J; National Pressure Ulcer Advisory Panel. Pressure ulcers in individuals receiving palliative care: a National Pressure Ulcer Advisory Panel white paper. Adv Skin Wound Care 2010; 23:59–72.
- Fonder MA, Lazarus GS, Cowan DA, Aronson-Cook B, Kohli AR, Mamelak AJ. Treating the chronic wound: a practical approach to the care of nonhealing wounds and wound care dressings. J Am Acad Dermatol 2008; 58:185–206.
- Lio PA, Kaye ET. Topical antibacterial agents. Infect Dis Clin North Am 2004; 18:717–733.
- Gelmetti C. Local antibiotics in dermatology. Dermatol Ther 2008; 21:187–195.
- Finlay IG, Bowszyc J, Ramlau C, Gwiezdzinski Z. The effect of topical 0.75% metronidazole gel on malodorous cutaneous ulcers. J Pain Symptom Manage 1996; 11:158–162.
- Bower M, Stein R, Evans TR, Hedley A, Pert P, Coombes RC. A double-blind study of the efficacy of metronidazole gel in the treatment of malodorous fungating tumours. Eur J Cancer 1992; 28A:888–889.
- Kalinski C, Schnepf M, Laboy D, et al. Effectiveness of a topical formulation containing metronidazole for wound odor and exudate control. Wounds 2005; 17:84–90.
- Kuge S, Tokuda Y, Ohta M, et al. Use of metronidazole gel to control malodor in advanced and recurrent breast cancer. Jpn J Clin Oncol 1996; 26:207–210.
- Belcher J. A review of medical-grade honey in wound care. Br J Nurs 2012: 21:S4–S9.
- Kwakman PH, Van den Akker JP, Güçlü A, et al. Medical-grade honey kills antibiotic-resistant bacteria in vitro and eradicates skin colonization. Clin Infect Dis 2008; 46:1677–1682.
- Cooper RA, Jenkins L. A comparison between medical grade honey and table honeys in relation to antimicrobial efficacy. Wounds 2009; 21:29–36.
- Patel B, Cox-Hayley D. Managing wound odor #218. J Palliat Med 2010; 13:1286–1287.
- Schulte MJ. Yogurt helps to control wound odor. Oncol Nurs Forum 1993; 20:1262.
- Upright CA, Salton C, Roberts F, Murphy J. Evaluation of Mesalt dressings and continuous wet saline dressings in ulcerating metastatic skin lesions. Cancer Nurs 1994; 17:149–155.
- Kuttan R, Sudheeran PC, Josph CD. Turmeric and curcumin as topical agents in cancer therapy. Tumori 1987; 73:29–31.
- Bass MJ, Phillips LG. Pressure sores. Curr Probl Surg 2007; 44:101–143.
- Bufill JA, Grace WR, Neff R. Intra-arterial chemotherapy for palliation of fungating breast cancer: a case report and review of the literature. Am J Clin Oncol 1994; 17:118–124.
- Murakami M, Kuroda Y, Sano A, et al. Validity of local treatment including intraarterial infusion chemotherapy and radiotherapy for fungating adenocarcinoma of the breast: case report of more than 8-year survival. Am J Clin Oncol 2001; 24:388–391.
Wounds that fail to heal become more than mere skin lesions. Pain, malodor, and the accompanying psychological distress often complicate nonhealing wounds and impair quality of life.1 Management of malodor requires perseverance, sensitivity, and familiarity with tools and procedures that range from surgical debridement to medical-grade honey.
Chronic, nonhealing wounds are defined as persisting for more than 6 months.2 These lesions are incapable of undergoing anatomic and functional repair on their own. Commonly encountered nonhealing wounds include pressure ulcers, venous stasis ulcers, arterial insufficiency ulcers, and malignant cutaneous wounds.
Typically, the patient with a nonhealing wound is frail, debilitated, medically complex, and often faced with one or more life-limiting illnesses. Complete wound healing may therefore be unrealistic, and optimal wound management becomes the goal of care.3,4
Healthcare providers encounter nonhealing wounds in varied settings—acute inpatient, outpatient, long-term, and home care. For instance, in the home care setting, a study of 383 patients enrolled in hospice found that 35% had skin ulcers and wounds.3 Half of those affected had pressure ulcers, 20% had ischemic ulcers, and 30% had other skin disorders such as stasis ulcers, burns, skin tears, and tumors. A larger study, also in hospice patients, found that 26% had pressure ulcers and 10% more developed them within 6 months.5
While pressure ulcers are the most common nonhealing wounds, malignant or fungating wounds are found in 5% to 10% of patients with metastatic disease, usually with cancers of the breast, head, and neck.6
Maximizing wound care provides comfort, relieves suffering, and promotes quality of life.3,7 To achieve these goals, clinicians must be familiar with strategies to manage complications associated with nonhealing wounds such as pain, malodor, and psychosocial adverse effects. Of these complications, malodor has been pointed out by both patients and caregivers as the most distressing.8
This article focuses on wound malodor, discusses the processes that cause wounds to emit an offensive smell, and outlines a comprehensive management approach.
MRS. A., AGE 61, WITH STAGE IV BREAST CANCER
Mrs. A., 61 years old, had a fungating mass in her left breast, which began as a small nodule and progressively enlarged to deform her breast over several months. Her oncologist subsequently staged the extent of her cancer as stage IV after workup revealed lung metastasis. Mrs. A. and her family decided to forgo cancer treatment, including radiotherapy, and to transition to hospice care after discussions with the oncologist.
Mrs. A. lived at home with her husband. Her daughter and three grandchildren all lived nearby.
When her hospice physician arrived at her home to meet her, a strong, pungent, and nauseating smell greeted him as he entered her bedroom. The patient said that for the past few months she had been increasingly distressed by the revolting odor. She rarely left home and had been ashamed to have people visit her, including her family.
On examination, the physician noticed a large fungating mass with yellowish discharge and necrotic tissue in her left breast. In addition to mild pain, she was immensely bothered by the strong odor coming from her breast.
THE IMPACT OF MALODOR
As seen in the case of Mrs. A., malodor has grave effects, both physical and psychological. Patients experience impaired or socially unacceptable body image, social rejection, personal shame, and embarrassment.9,10 Feelings of fear, anxiety, and depression are common. If left uncontrolled, malodor results in social isolation, reluctance to engage in social activities, diminished appetite, and nausea. In addition, malodor is a constant reminder of patients’ pain and cancer, and it results in further suffering.11
Reactions of family members and caregivers can worsen the situation.9,12 Expressions of revulsion limit contact and inhibit intimacy, especially near the end of life. Caregivers are often frustrated and distressed over their inability to control the malodor. The environment becomes uninhabitable, and the malodor can permeate clothing, furniture, and living quarters.
Managing malodor can be emotionally draining, physically daunting, and frustrating for healthcare professionals, as several methods are usually employed, often in a trial-and-error approach, to achieve an acceptable degree of odor control. In addition, clinicians must face the challenge of treating malodorous wounds at very close distance without reacting in a way that offends or alarms patients and family members.13
MALODOR PRODUCTION: WHERE IS THAT SMELL COMING FROM?
All wounds can produce an odor.14 Wounds that are expected to heal typically emit a faint but not unpleasant odor, akin to fresh blood. Wounds colonized by Pseudomonas aeruginosa produce a fruity or grapelike odor that is tolerable. Malodor occurs with wounds infected by other gram-negative organisms or anaerobic bacteria.15 Similarly, wounds covered by necrotic tissue smell like decaying flesh.
Three major causes
The three major causes of wound malodor are slough, infection, and exudate (Figure 1).
Slough is dead or necrotic tissue, usually resulting from vascular compromise. Arterial ulcers, pressure ulcers, and malignant wounds all form slough from capillary occlusion, subsequent ischemia, and tissue necrosis.
Infection. Devitalized tissue, an ideal medium in which bacteria thrive, becomes the source of infection. Anaerobic bacteria are usually implicated in malodor. These include Bacteroides fragilis, Bacteroides prevotella, Clostridium perfringens, and Fusobacterium nucleatum.16,17 Anaerobic organisms produce putrescine and cadaverine, which are largely responsible for the offensive odor.16,18 Volatile fatty acids such as propionic, butyric, isovaleric, and valeric acid are formed from lipid catabolism by anaerobes and add to malodor.17 Aerobic bacteria such as Proteus, Klebsiella, and Pseudomonas species supercolonize necrotic tissue as well and contribute to malodor.17,18
Exudate. Since nonhealing wounds undergo repeated cycles of inflammation, infection, and necrosis, accumulation of exudate becomes inevitable. Exudate typically is a pus-like fluid containing serum, fibrin, and white blood cells, which leak from blood vessels. In addition, bacteria that colonize chronic wounds filled with necrotic tissue activate proteases that degrade and liquefy dead tissue, thereby forming extensive amounts of exudate.19
Apart from slough, infection, and exudate, poor general hygiene and dressings left on for too long may contribute to malodor.16 Moisture-retentive dressings such as hydrocolloids leave an odor after removal. Dressings that liquefy upon contact with the wound surface leave a pus-like, potentially malodorous material.
MALODOR ASSESSMENT: DO YOU SMELL SOMETHING?
Various ways to document wound malodor can prove useful in guiding assessment and treatment. Descriptions such as “foul,” “putrid,” “fishy,” or “filled the room” vividly portray the initial presentation. A 10-point numerical scale similar to a numerical pain scale or a visual analogue scale can be used as a subjective measure.
Other grading methods, which to the authors’ knowledge are not validated, may be helpful. In a study that focused on patients suffering from malodorous gynecologic malignancies, von Gruenigen et al20 used a 0-to-3 scale:
- 0 Absent
- 1 Not offensive
- 2 Offensive but tolerable
- 3 Offensive and intolerable.
A scale often adapted by other authors was devised by Baker and Haig,21 which clearly defines four classes:
- 1 Strong—odor is evident upon entering the room (6 to 10 feet from the patient) with the dressing intact
- 2 Moderate—odor is evident upon entering the room with dressing removed
- 3 Slight—odor is evident at close proximity to the patient when the dressing is removed
- 4 No odor—no odor is evident, even at the patient’s bedside with the dressing removed.
COMPREHENSIVE MANAGEMENT: HOW DO WE WIN THE ‘RACE’?
The acronym RACE outlines an approach to dealing with malodor. It stands for removal of necrotic tissue; antibacterials; odor concealers; and education and support (Table 1).
Remove necrotic tissue
An important step in eliminating malodor is to remove necrotic tissue. This starts with debridement, which decreases the incidence of infection and hastens wound closure.22,23 Table 2 compares the different types of debridement.
Sharp or surgical debridement involves the use of a scalpel or scissors. This type of debridement may increase the risk of bleeding, pain, and malignant cell seeding in fungating wounds.4,24
Enzymatic debridement employs chemicals with proteolytic action (eg, collagenase) to digest extracellular proteins in wounds.18,25
Mechanical debridement involves aggressive therapies such as forceful irrigation and hydrotherapy, which may fail to discriminate between necrotic and viable tissues.18,26
Biological debridement using maggots, which ingest bacteria and devitalized tissue, may cause increased wound bleeding and may be unacceptable for patients and families.24,27
Autolytic debridement is often recommended, particularly if complete healing is not the primary goal.17,24,28,29 Autolysis uses proteolytic enzymes and phagocytic cells present in the wound bed and wound fluid to clear devitalized tissue. It is easy, inexpensive, noninvasive, and painless,4 and it requires less frequent dressing changes relative to standard dressing or wet-to-dry dressing.
Autolytic debridement is commonly accomplished using hydrocolloid and hydrogel dressings.15,29 Hydrocolloids are adhesive, occlusive, and conformable dressings that are suitable for wounds with low to moderate amounts of exudate. Upon contact with the wound surface, the dressing absorbs the exudate, forms a gel layer, and maintains a moist environment. Hydrocolloids are not recommended for infected wounds or for those with copious exudate as they may lead to maceration around the wound. A disadvantage of hydrocolloid dressings is their tendency to generate brown, often malodorous exudate when removed.
On the other hand, hydrogels in amorphous gel, dressing, sheet, or impregnated gauze form are water-based products that create a moist environment similar to hydrocolloids. Aside from causing minimal trauma to the wound bed when removed, the dressing’s cooling effect may bring some pain relief. Hydrogels are appropriate for dry wounds and for those with minimal exudate.
After debridement, the wound is cleansed and irrigated. A number of cleansers and solutions are available, but normal saline is a cheap alternative. To irrigate, experts recommend an 18- or 20-gauge intravenous catheter attached to a 30- or 60-mL syringe.15 This technique provides 8 to 15 psi of pressure, enough to cleanse the wound without causing tissue trauma.
Antibacterials and absorption
Antibacterials. Topical antibiotics have several advantages over systemic antibiotics in treating chronic wounds.30,31 These include a high and sustained concentration of the antimicrobial at the site of infection, limited potential for systemic absorption and toxicity, reduced potential for antibiotic resistance, and drawing of the patient’s and caregiver’s attention to the wound.
Metronidazole is the most widely used topical antibacterial for malodor management. Its efficacy is likely due to the predominant involvement of anaerobic bacteria in foul-smelling wounds. Topical metronidazole is available as a gel and as a cream. A systematic review showed that on average, topical metronidazole was used once daily for 14 consecutive days.19 The layer of topical metronidazole is typically covered with a nonadherent primary dressing followed by an absorbent secondary dressing.
The best clinical evidence for topical metronidazole consists of case reports and series.32–35 The largest of these studies was done by Finlay et al, who treated 47 patients with malodorous benign and malignant cutaneous wounds with 0.75% metronidazole gel daily.32 Forty-five (96%) of the patients reported significantly decreased odor by 14 days, as well as decreased pain, discharge, and surrounding cellulitis.
A randomized, placebo-controlled trial conducted by Bale et al had equivocal findings.9 All 41 patients who received metronidazole gel reported a decrease in malodor within 3 days of starting it. However, 76% of patients who received placebo also reported malodor control; in the final analysis, no significant difference was noted in the success rate between the two groups.
Metronidazole tablets can be crushed and sprinkled over the wound. As with metronidazole gel or cream, the crushed tablets are applied daily and covered by a primary nonadherent dressing and an absorbent secondary dressing. This off-label use of metronidazole serves as a cheaper alternative to commercially available topical preparations. To our knowledge, there has been no head-to-head trial comparing the two topical strategies.
Systemic metronidazole, often given orally, has been recommended if evidence of deep tissue or systemic infection is noted15 and in cases of fungating wounds with fistulas invading either the gastrointestinal or genitourinary tracts.18 Side effects such as nausea, neuropathy, and alcohol intolerance (ie, disulfiram reaction) may occur, which are not seen with topical metronidazole.
Both topical and systemic metronidazole can be used together on a time-limited basis for extensive malodorous wounds, such as fungating malignant wounds or stage IV sacral pressure ulcers.
Other antimicrobial agents used to treat malodor include silver-containing products, iodine-containing topical agents, mupirocin, bacitracin, neomycin, and polymyxin B.
Honey was used for wound care by the ancient Egyptians, and it is still used.36 Its beneficial effects include antimicrobial, debriding, deodorizing, anti-inflammatory, and granulation tissue-stimulating. Honey has even been shown to significantly decrease skin colonization with various kinds of bacteria, including methicillin-resistant Staphylococcus aureus.37 Medical-grade honey is preferred over table honey, as the latter is nonsterile and can contain Clostridium spores, which contaminate the wound.38
Yogurt and buttermilk lower the pH of the wound and control bacterial proliferation to control malodor.39,40 Either is applied for 10 to 15 minutes after the wound is cleansed and is then washed off thoroughly.
Absorbent dressings are used either over a layer of topical metronidazole and a nonadherent primary dressing or as a primary dressing itself. An absorbent dressing containing activated charcoal is used for rapid improvement, although cost may be prohibitive, especially in developing countries.13,19 Another type of absorbent dressing, composed of polyester impregnated with sodium chloride, has been found to be useful in malodor control.41 An important pointer is to maintain a tight seal around the absorbent dressing to prevent leakage of exudate.
Concealers
Aromatics used to conceal malodor include scented candles, incense, fragrant flowers and plants, and air-freshener sprays. When circumstances allow, candles are good options since they conceal malodor by emitting fragrance, and the flame burns off foul-smelling chemicals. Aromatics such as coffee beans, vanilla beans, and cider vinegar can be placed in a pan and left under the patient’s bed or close to it. Drops of peppermint oil or oil of wintergreen can be placed on wound dressings.
Other odor concealers are adsorbent materials that attract and cause ions and molecules to adhere to their surface. Examples are charcoal, baking soda, and cat litter. As with other aromatics, these materials are placed in pans and left under the bed or near the patient.
Aromatics can have disadvantages, as certain scents, especially strong ones, can be nauseating for patients. Some fragrances trigger asthma or skin irritation. Patients and caregivers can be left with an unpleasant association of certain fragrances with malodor by conditioning.15,17,18
Education and support
Concerns of the patient and family members need to be heard, addressed promptly, and reassessed with each visit, since uncontrolled malodor can be a chief source of caregiver fatigue.
Foremost in formulating a patient- and family-centered malodor management strategy is to commit to controlling malodor as much as possible. Regular follow-up appointments should be made, whether in the office or at home, to check on the patient’s progress and address new and ongoing concerns. Symptoms accompanying malodor, such as pain, bleeding, and sleep disturbance, need to be addressed, as they all affect quality of life.1 Audience-appropriate educational materials should be made available.26 Online resources that patients and families can explore include the websites of the Wound Ostomy and Continence Nurses Society (www.wocn.org) and the Association for the Advancement of Wound Care (aawconline.org).
Healthcare professionals need to be prepared to deal with problems and complications involving patients and family members that may arise in the course of treatment.12 Problems include the cost and local unavailability of dressing supplies, insurance coverage for dressings and topical agents, lack of assistance at home, and fear of changing dressings. A cardinal rule for healthcare providers is to avoid expressing distress at odors in front of or within hearing of patients and families.
OTHER STRATEGIES: WHAT ELSE CAN WE DO?
Curcumin, the main biologically active compound in the herb turmeric, applied directly to wounds three times daily as an ointment, has been shown to have odor-controlling properties.42
Sugar paste has been reported to control malodor by drawing out exudative and tissue fluid osmotically, and inhibiting bacterial growth.16,17 Water is mixed with sugar (ie, granulated, caster, or powdered) to form a paste, with additives like glycerin and polyethylene glycol used to alter the consistency. Thick clay-like paste is good for wounds with large cavities, while thin paste is useful for wounds with small or superficial openings. The paste is applied twice daily and is covered by an absorbent dressing.
Pressure relief is vital in managing pressure ulcers.18,43 Repositioning every 2 hours and using special devices, such as mattress overlays, alternating pressure mattresses, and low air loss mattresses, are frequently employed techniques.
If circumstances permit and when congruent with the patient’s goals of care, intra-arterial chemotherapy and radiotherapy can be contemplated for malignant fungating wounds.44,45
Other strategies include opening the windows during dressing changes, increasing the frequency of dressing changes, promptly removing used dressings from the house, and ensuring good general hygiene.
CASE RESOLUTION
After telling her that he was committed to control the malodor or, if possible, eliminate it, Mrs. A.’s doctor prepared two lists of materials—one for himself and one for Mrs. A.’s husband. He returned the next day, brought out his supplies, asked Mrs. A. to lie in bed, and invited her husband to assist him.
He cleansed and irrigated the breast lesion with normal saline, making sure to remove as much dead tissue as he could. He applied a layer of metronidazole cream to the wound cavity, then covered it with a nonadherent dressing. He then covered the wound with gauze, sealed the edges with medical adhesive tape, and applied a few drops of oil of wintergreen to the surface. A pan of charcoal briquettes was put under the bed, and a candle with Mrs. A.’s favorite scent was lit by the bedside. The physician then instructed Mrs. A.’s husband to repeat the procedure once daily for 1 week.
After 2 weeks, Mrs. A. and her husband said the foul odor had greatly decreased. She appeared more cheerful and energetic, especially after her grandchildren visited a few days earlier. The physician then instructed the husband to stop using metronidazole cream and to apply a hydrocolloid dressing every 3 days instead. He advised them to continue the rest of the process of applying a few drops of oil of wintergreen on the dressing surface, placing a pan of charcoal briquettes under the bed, and lighting a scented candle by the bedside.
FINISH THE RACE!
Complex nonhealing wounds are encountered across various healthcare settings. Wound malodor is an important component of nonhealing wounds, which adversely affects patients, families, and healthcare providers. Infection, slough, and exudate are the major causes of wound malodor. The essential steps to reduce malodor are to remove necrotic tissue, use antibacterial and odor-absorbing agents, apply appropriate odor “concealers,” educate families, and formulate a patient- and family-centered strategy (Table 1).
Acknowledgment: The authors would like to thank Sue Reif, CNP, for her assistance in completing the manuscript.
Wounds that fail to heal become more than mere skin lesions. Pain, malodor, and the accompanying psychological distress often complicate nonhealing wounds and impair quality of life.1 Management of malodor requires perseverance, sensitivity, and familiarity with tools and procedures that range from surgical debridement to medical-grade honey.
Chronic, nonhealing wounds are defined as persisting for more than 6 months.2 These lesions are incapable of undergoing anatomic and functional repair on their own. Commonly encountered nonhealing wounds include pressure ulcers, venous stasis ulcers, arterial insufficiency ulcers, and malignant cutaneous wounds.
Typically, the patient with a nonhealing wound is frail, debilitated, medically complex, and often faced with one or more life-limiting illnesses. Complete wound healing may therefore be unrealistic, and optimal wound management becomes the goal of care.3,4
Healthcare providers encounter nonhealing wounds in varied settings—acute inpatient, outpatient, long-term, and home care. For instance, in the home care setting, a study of 383 patients enrolled in hospice found that 35% had skin ulcers and wounds.3 Half of those affected had pressure ulcers, 20% had ischemic ulcers, and 30% had other skin disorders such as stasis ulcers, burns, skin tears, and tumors. A larger study, also in hospice patients, found that 26% had pressure ulcers and 10% more developed them within 6 months.5
While pressure ulcers are the most common nonhealing wounds, malignant or fungating wounds are found in 5% to 10% of patients with metastatic disease, usually with cancers of the breast, head, and neck.6
Maximizing wound care provides comfort, relieves suffering, and promotes quality of life.3,7 To achieve these goals, clinicians must be familiar with strategies to manage complications associated with nonhealing wounds such as pain, malodor, and psychosocial adverse effects. Of these complications, malodor has been pointed out by both patients and caregivers as the most distressing.8
This article focuses on wound malodor, discusses the processes that cause wounds to emit an offensive smell, and outlines a comprehensive management approach.
MRS. A., AGE 61, WITH STAGE IV BREAST CANCER
Mrs. A., 61 years old, had a fungating mass in her left breast, which began as a small nodule and progressively enlarged to deform her breast over several months. Her oncologist subsequently staged the extent of her cancer as stage IV after workup revealed lung metastasis. Mrs. A. and her family decided to forgo cancer treatment, including radiotherapy, and to transition to hospice care after discussions with the oncologist.
Mrs. A. lived at home with her husband. Her daughter and three grandchildren all lived nearby.
When her hospice physician arrived at her home to meet her, a strong, pungent, and nauseating smell greeted him as he entered her bedroom. The patient said that for the past few months she had been increasingly distressed by the revolting odor. She rarely left home and had been ashamed to have people visit her, including her family.
On examination, the physician noticed a large fungating mass with yellowish discharge and necrotic tissue in her left breast. In addition to mild pain, she was immensely bothered by the strong odor coming from her breast.
THE IMPACT OF MALODOR
As seen in the case of Mrs. A., malodor has grave effects, both physical and psychological. Patients experience impaired or socially unacceptable body image, social rejection, personal shame, and embarrassment.9,10 Feelings of fear, anxiety, and depression are common. If left uncontrolled, malodor results in social isolation, reluctance to engage in social activities, diminished appetite, and nausea. In addition, malodor is a constant reminder of patients’ pain and cancer, and it results in further suffering.11
Reactions of family members and caregivers can worsen the situation.9,12 Expressions of revulsion limit contact and inhibit intimacy, especially near the end of life. Caregivers are often frustrated and distressed over their inability to control the malodor. The environment becomes uninhabitable, and the malodor can permeate clothing, furniture, and living quarters.
Managing malodor can be emotionally draining, physically daunting, and frustrating for healthcare professionals, as several methods are usually employed, often in a trial-and-error approach, to achieve an acceptable degree of odor control. In addition, clinicians must face the challenge of treating malodorous wounds at very close distance without reacting in a way that offends or alarms patients and family members.13
MALODOR PRODUCTION: WHERE IS THAT SMELL COMING FROM?
All wounds can produce an odor.14 Wounds that are expected to heal typically emit a faint but not unpleasant odor, akin to fresh blood. Wounds colonized by Pseudomonas aeruginosa produce a fruity or grapelike odor that is tolerable. Malodor occurs with wounds infected by other gram-negative organisms or anaerobic bacteria.15 Similarly, wounds covered by necrotic tissue smell like decaying flesh.
Three major causes
The three major causes of wound malodor are slough, infection, and exudate (Figure 1).
Slough is dead or necrotic tissue, usually resulting from vascular compromise. Arterial ulcers, pressure ulcers, and malignant wounds all form slough from capillary occlusion, subsequent ischemia, and tissue necrosis.
Infection. Devitalized tissue, an ideal medium in which bacteria thrive, becomes the source of infection. Anaerobic bacteria are usually implicated in malodor. These include Bacteroides fragilis, Bacteroides prevotella, Clostridium perfringens, and Fusobacterium nucleatum.16,17 Anaerobic organisms produce putrescine and cadaverine, which are largely responsible for the offensive odor.16,18 Volatile fatty acids such as propionic, butyric, isovaleric, and valeric acid are formed from lipid catabolism by anaerobes and add to malodor.17 Aerobic bacteria such as Proteus, Klebsiella, and Pseudomonas species supercolonize necrotic tissue as well and contribute to malodor.17,18
Exudate. Since nonhealing wounds undergo repeated cycles of inflammation, infection, and necrosis, accumulation of exudate becomes inevitable. Exudate typically is a pus-like fluid containing serum, fibrin, and white blood cells, which leak from blood vessels. In addition, bacteria that colonize chronic wounds filled with necrotic tissue activate proteases that degrade and liquefy dead tissue, thereby forming extensive amounts of exudate.19
Apart from slough, infection, and exudate, poor general hygiene and dressings left on for too long may contribute to malodor.16 Moisture-retentive dressings such as hydrocolloids leave an odor after removal. Dressings that liquefy upon contact with the wound surface leave a pus-like, potentially malodorous material.
MALODOR ASSESSMENT: DO YOU SMELL SOMETHING?
Various ways to document wound malodor can prove useful in guiding assessment and treatment. Descriptions such as “foul,” “putrid,” “fishy,” or “filled the room” vividly portray the initial presentation. A 10-point numerical scale similar to a numerical pain scale or a visual analogue scale can be used as a subjective measure.
Other grading methods, which to the authors’ knowledge are not validated, may be helpful. In a study that focused on patients suffering from malodorous gynecologic malignancies, von Gruenigen et al20 used a 0-to-3 scale:
- 0 Absent
- 1 Not offensive
- 2 Offensive but tolerable
- 3 Offensive and intolerable.
A scale often adapted by other authors was devised by Baker and Haig,21 which clearly defines four classes:
- 1 Strong—odor is evident upon entering the room (6 to 10 feet from the patient) with the dressing intact
- 2 Moderate—odor is evident upon entering the room with dressing removed
- 3 Slight—odor is evident at close proximity to the patient when the dressing is removed
- 4 No odor—no odor is evident, even at the patient’s bedside with the dressing removed.
COMPREHENSIVE MANAGEMENT: HOW DO WE WIN THE ‘RACE’?
The acronym RACE outlines an approach to dealing with malodor. It stands for removal of necrotic tissue; antibacterials; odor concealers; and education and support (Table 1).
Remove necrotic tissue
An important step in eliminating malodor is to remove necrotic tissue. This starts with debridement, which decreases the incidence of infection and hastens wound closure.22,23 Table 2 compares the different types of debridement.
Sharp or surgical debridement involves the use of a scalpel or scissors. This type of debridement may increase the risk of bleeding, pain, and malignant cell seeding in fungating wounds.4,24
Enzymatic debridement employs chemicals with proteolytic action (eg, collagenase) to digest extracellular proteins in wounds.18,25
Mechanical debridement involves aggressive therapies such as forceful irrigation and hydrotherapy, which may fail to discriminate between necrotic and viable tissues.18,26
Biological debridement using maggots, which ingest bacteria and devitalized tissue, may cause increased wound bleeding and may be unacceptable for patients and families.24,27
Autolytic debridement is often recommended, particularly if complete healing is not the primary goal.17,24,28,29 Autolysis uses proteolytic enzymes and phagocytic cells present in the wound bed and wound fluid to clear devitalized tissue. It is easy, inexpensive, noninvasive, and painless,4 and it requires less frequent dressing changes relative to standard dressing or wet-to-dry dressing.
Autolytic debridement is commonly accomplished using hydrocolloid and hydrogel dressings.15,29 Hydrocolloids are adhesive, occlusive, and conformable dressings that are suitable for wounds with low to moderate amounts of exudate. Upon contact with the wound surface, the dressing absorbs the exudate, forms a gel layer, and maintains a moist environment. Hydrocolloids are not recommended for infected wounds or for those with copious exudate as they may lead to maceration around the wound. A disadvantage of hydrocolloid dressings is their tendency to generate brown, often malodorous exudate when removed.
On the other hand, hydrogels in amorphous gel, dressing, sheet, or impregnated gauze form are water-based products that create a moist environment similar to hydrocolloids. Aside from causing minimal trauma to the wound bed when removed, the dressing’s cooling effect may bring some pain relief. Hydrogels are appropriate for dry wounds and for those with minimal exudate.
After debridement, the wound is cleansed and irrigated. A number of cleansers and solutions are available, but normal saline is a cheap alternative. To irrigate, experts recommend an 18- or 20-gauge intravenous catheter attached to a 30- or 60-mL syringe.15 This technique provides 8 to 15 psi of pressure, enough to cleanse the wound without causing tissue trauma.
Antibacterials and absorption
Antibacterials. Topical antibiotics have several advantages over systemic antibiotics in treating chronic wounds.30,31 These include a high and sustained concentration of the antimicrobial at the site of infection, limited potential for systemic absorption and toxicity, reduced potential for antibiotic resistance, and drawing of the patient’s and caregiver’s attention to the wound.
Metronidazole is the most widely used topical antibacterial for malodor management. Its efficacy is likely due to the predominant involvement of anaerobic bacteria in foul-smelling wounds. Topical metronidazole is available as a gel and as a cream. A systematic review showed that on average, topical metronidazole was used once daily for 14 consecutive days.19 The layer of topical metronidazole is typically covered with a nonadherent primary dressing followed by an absorbent secondary dressing.
The best clinical evidence for topical metronidazole consists of case reports and series.32–35 The largest of these studies was done by Finlay et al, who treated 47 patients with malodorous benign and malignant cutaneous wounds with 0.75% metronidazole gel daily.32 Forty-five (96%) of the patients reported significantly decreased odor by 14 days, as well as decreased pain, discharge, and surrounding cellulitis.
A randomized, placebo-controlled trial conducted by Bale et al had equivocal findings.9 All 41 patients who received metronidazole gel reported a decrease in malodor within 3 days of starting it. However, 76% of patients who received placebo also reported malodor control; in the final analysis, no significant difference was noted in the success rate between the two groups.
Metronidazole tablets can be crushed and sprinkled over the wound. As with metronidazole gel or cream, the crushed tablets are applied daily and covered by a primary nonadherent dressing and an absorbent secondary dressing. This off-label use of metronidazole serves as a cheaper alternative to commercially available topical preparations. To our knowledge, there has been no head-to-head trial comparing the two topical strategies.
Systemic metronidazole, often given orally, has been recommended if evidence of deep tissue or systemic infection is noted15 and in cases of fungating wounds with fistulas invading either the gastrointestinal or genitourinary tracts.18 Side effects such as nausea, neuropathy, and alcohol intolerance (ie, disulfiram reaction) may occur, which are not seen with topical metronidazole.
Both topical and systemic metronidazole can be used together on a time-limited basis for extensive malodorous wounds, such as fungating malignant wounds or stage IV sacral pressure ulcers.
Other antimicrobial agents used to treat malodor include silver-containing products, iodine-containing topical agents, mupirocin, bacitracin, neomycin, and polymyxin B.
Honey was used for wound care by the ancient Egyptians, and it is still used.36 Its beneficial effects include antimicrobial, debriding, deodorizing, anti-inflammatory, and granulation tissue-stimulating. Honey has even been shown to significantly decrease skin colonization with various kinds of bacteria, including methicillin-resistant Staphylococcus aureus.37 Medical-grade honey is preferred over table honey, as the latter is nonsterile and can contain Clostridium spores, which contaminate the wound.38
Yogurt and buttermilk lower the pH of the wound and control bacterial proliferation to control malodor.39,40 Either is applied for 10 to 15 minutes after the wound is cleansed and is then washed off thoroughly.
Absorbent dressings are used either over a layer of topical metronidazole and a nonadherent primary dressing or as a primary dressing itself. An absorbent dressing containing activated charcoal is used for rapid improvement, although cost may be prohibitive, especially in developing countries.13,19 Another type of absorbent dressing, composed of polyester impregnated with sodium chloride, has been found to be useful in malodor control.41 An important pointer is to maintain a tight seal around the absorbent dressing to prevent leakage of exudate.
Concealers
Aromatics used to conceal malodor include scented candles, incense, fragrant flowers and plants, and air-freshener sprays. When circumstances allow, candles are good options since they conceal malodor by emitting fragrance, and the flame burns off foul-smelling chemicals. Aromatics such as coffee beans, vanilla beans, and cider vinegar can be placed in a pan and left under the patient’s bed or close to it. Drops of peppermint oil or oil of wintergreen can be placed on wound dressings.
Other odor concealers are adsorbent materials that attract and cause ions and molecules to adhere to their surface. Examples are charcoal, baking soda, and cat litter. As with other aromatics, these materials are placed in pans and left under the bed or near the patient.
Aromatics can have disadvantages, as certain scents, especially strong ones, can be nauseating for patients. Some fragrances trigger asthma or skin irritation. Patients and caregivers can be left with an unpleasant association of certain fragrances with malodor by conditioning.15,17,18
Education and support
Concerns of the patient and family members need to be heard, addressed promptly, and reassessed with each visit, since uncontrolled malodor can be a chief source of caregiver fatigue.
Foremost in formulating a patient- and family-centered malodor management strategy is to commit to controlling malodor as much as possible. Regular follow-up appointments should be made, whether in the office or at home, to check on the patient’s progress and address new and ongoing concerns. Symptoms accompanying malodor, such as pain, bleeding, and sleep disturbance, need to be addressed, as they all affect quality of life.1 Audience-appropriate educational materials should be made available.26 Online resources that patients and families can explore include the websites of the Wound Ostomy and Continence Nurses Society (www.wocn.org) and the Association for the Advancement of Wound Care (aawconline.org).
Healthcare professionals need to be prepared to deal with problems and complications involving patients and family members that may arise in the course of treatment.12 Problems include the cost and local unavailability of dressing supplies, insurance coverage for dressings and topical agents, lack of assistance at home, and fear of changing dressings. A cardinal rule for healthcare providers is to avoid expressing distress at odors in front of or within hearing of patients and families.
OTHER STRATEGIES: WHAT ELSE CAN WE DO?
Curcumin, the main biologically active compound in the herb turmeric, applied directly to wounds three times daily as an ointment, has been shown to have odor-controlling properties.42
Sugar paste has been reported to control malodor by drawing out exudative and tissue fluid osmotically, and inhibiting bacterial growth.16,17 Water is mixed with sugar (ie, granulated, caster, or powdered) to form a paste, with additives like glycerin and polyethylene glycol used to alter the consistency. Thick clay-like paste is good for wounds with large cavities, while thin paste is useful for wounds with small or superficial openings. The paste is applied twice daily and is covered by an absorbent dressing.
Pressure relief is vital in managing pressure ulcers.18,43 Repositioning every 2 hours and using special devices, such as mattress overlays, alternating pressure mattresses, and low air loss mattresses, are frequently employed techniques.
If circumstances permit and when congruent with the patient’s goals of care, intra-arterial chemotherapy and radiotherapy can be contemplated for malignant fungating wounds.44,45
Other strategies include opening the windows during dressing changes, increasing the frequency of dressing changes, promptly removing used dressings from the house, and ensuring good general hygiene.
CASE RESOLUTION
After telling her that he was committed to control the malodor or, if possible, eliminate it, Mrs. A.’s doctor prepared two lists of materials—one for himself and one for Mrs. A.’s husband. He returned the next day, brought out his supplies, asked Mrs. A. to lie in bed, and invited her husband to assist him.
He cleansed and irrigated the breast lesion with normal saline, making sure to remove as much dead tissue as he could. He applied a layer of metronidazole cream to the wound cavity, then covered it with a nonadherent dressing. He then covered the wound with gauze, sealed the edges with medical adhesive tape, and applied a few drops of oil of wintergreen to the surface. A pan of charcoal briquettes was put under the bed, and a candle with Mrs. A.’s favorite scent was lit by the bedside. The physician then instructed Mrs. A.’s husband to repeat the procedure once daily for 1 week.
After 2 weeks, Mrs. A. and her husband said the foul odor had greatly decreased. She appeared more cheerful and energetic, especially after her grandchildren visited a few days earlier. The physician then instructed the husband to stop using metronidazole cream and to apply a hydrocolloid dressing every 3 days instead. He advised them to continue the rest of the process of applying a few drops of oil of wintergreen on the dressing surface, placing a pan of charcoal briquettes under the bed, and lighting a scented candle by the bedside.
FINISH THE RACE!
Complex nonhealing wounds are encountered across various healthcare settings. Wound malodor is an important component of nonhealing wounds, which adversely affects patients, families, and healthcare providers. Infection, slough, and exudate are the major causes of wound malodor. The essential steps to reduce malodor are to remove necrotic tissue, use antibacterial and odor-absorbing agents, apply appropriate odor “concealers,” educate families, and formulate a patient- and family-centered strategy (Table 1).
Acknowledgment: The authors would like to thank Sue Reif, CNP, for her assistance in completing the manuscript.
- Lo SF, Hayter M, Hu WY, Tai CY, Hsu MY, Li YF. Symptom burden and quality of life in patients with malignant fungating wounds. J Adv Nurs 2012; 68:1312–1321.
- Lazarus GS, Cooper DM, Knighton DR, et al. Definitions and guidelines for assessment of wounds and evaluation of healing. Arch Dermatol 1994; 130:489–493.
- Tippett AW. Wounds at the end of life. Wounds 2005; 17:91–98.
- Burt T. Palliative care of pressure ulcers in long-term care. Ann Long-Term Care 2013; 21:20–28.
- Reifsnyder J, Magee HS. Development of pressure ulcers in patients receiving home hospice care. Wounds 2005; 17:74–79.
- Haisfield-Wolfe ME, Rund C. Malignant cutaneous wounds: a management protocol. Ostomy Wound Manage 1997; 43:56–66.
- O’Brien C. Malignant wounds: managing odour. Can Fam Physician 2012; 58:272–274.
- Gethin G, Grocott P, Probst S, Clarke E. Current practice in the management of wound odour: an international survey. Int J Nurs Stud 2014; 51:865–874.
- Bale S, Tebble N, Price P. A topical metronidazole gel used to treat malodorous wounds. Br J Nurs 2004; 13:S4–S11.
- Hack A. Malodorous wounds—taking the patient’s perspective into account. J Wound Care 2003; 12:319–321.
- Price E. Wound care. The stigma of smell. Nurs Times 1996; 92:71–72.
- Paul JC, Pieper BA. Topical metronidazole for the treatment of wound odor: a review of the literature. Ostomy Wound Manage 2008; 54:18–27.
- Lee G, Anand SC, Rajendran S, Walker I. Overview of current practice and future trends in the evaluation of dressings for malodorous wounds. J Wound Care 2006; 15:344–346.
- Cutting K, Harding K. Criteria for identifying wound infection. J Wound Care 1994; 3:198–201.
- McDonald A, Lesage P. Palliative management of pressure ulcers and malignant wounds in patients with advanced illness. J Palliat Med 2006; 9:285–295.
- Holloway S. Recognising and treating the causes of chronic malodorous wounds. Prof Nurse 2004; 19:380–384.
- Haughton W, Young T. Common problems in wound care: malodorous wounds. Br J Nurs 1995; 4:959–963.
- Alvarez OM, Kalinski C, Nusbaum J, et al. Incorporating wound healing strategies to improve palliation (symptom management) in patients with chronic wounds. J Palliat Med 2007; 10:1161–1189.
- da Costa Santos CM, de Mattos Pimenta CA, Nobre MR. A systematic review of topical treatments to control the odor of malignant fungating wounds. J Pain Symptom Manage 2010; 39:1065–1076.
- Von Gruenigen VE, Coleman RL, et al. Bacteriology and treatment of malodorous lower reproductive tract in gynecologic cancer patients. Obstet Gynecol 2000; 96:23–27.
- Baker PG, Haig G. Metronidazole in the treatment of chronic pressure sores and ulcers: a comparison with standard treatment in general practice. Practitioner 1981; 225:569–573.
- Whitney J, Phillips L, Aslam R, et al. Guidelines for the treatment of pressure ulcers. Wound Repair Regen 2006; 14:663–679.
- Williams D, Enoch S, Miller D, Harris K, Price P, Harding KG. Effect of sharp debridement using curette on recalcitrant nonhealing venous ulcers: a concurrently controlled, prospective cohort study. Wound Repair Regen 2005; 13:131–137.
- Bergstrom KJ. Assessment and management of fungating wounds. J Wound Ostomy Continence Nurs 2011: 38:31–37.
- Sinclair RD, Ryan TJ. Proteolytic enzymes in wound healing: the role of enzymatic debridement. Australas J Dermatol 1994; 35:35–41.
- Enoch S, Harding KG. Wound bed preparation: the science behind the removal of barriers to healing. Wounds 2003;15:213–229.
- Mumcuoglu KY. Clinical applications for maggots in wound care. Am J Clin Dermatol 2001; 2:219–227.
- Langemo DK, Black J; National Pressure Ulcer Advisory Panel. Pressure ulcers in individuals receiving palliative care: a National Pressure Ulcer Advisory Panel white paper. Adv Skin Wound Care 2010; 23:59–72.
- Fonder MA, Lazarus GS, Cowan DA, Aronson-Cook B, Kohli AR, Mamelak AJ. Treating the chronic wound: a practical approach to the care of nonhealing wounds and wound care dressings. J Am Acad Dermatol 2008; 58:185–206.
- Lio PA, Kaye ET. Topical antibacterial agents. Infect Dis Clin North Am 2004; 18:717–733.
- Gelmetti C. Local antibiotics in dermatology. Dermatol Ther 2008; 21:187–195.
- Finlay IG, Bowszyc J, Ramlau C, Gwiezdzinski Z. The effect of topical 0.75% metronidazole gel on malodorous cutaneous ulcers. J Pain Symptom Manage 1996; 11:158–162.
- Bower M, Stein R, Evans TR, Hedley A, Pert P, Coombes RC. A double-blind study of the efficacy of metronidazole gel in the treatment of malodorous fungating tumours. Eur J Cancer 1992; 28A:888–889.
- Kalinski C, Schnepf M, Laboy D, et al. Effectiveness of a topical formulation containing metronidazole for wound odor and exudate control. Wounds 2005; 17:84–90.
- Kuge S, Tokuda Y, Ohta M, et al. Use of metronidazole gel to control malodor in advanced and recurrent breast cancer. Jpn J Clin Oncol 1996; 26:207–210.
- Belcher J. A review of medical-grade honey in wound care. Br J Nurs 2012: 21:S4–S9.
- Kwakman PH, Van den Akker JP, Güçlü A, et al. Medical-grade honey kills antibiotic-resistant bacteria in vitro and eradicates skin colonization. Clin Infect Dis 2008; 46:1677–1682.
- Cooper RA, Jenkins L. A comparison between medical grade honey and table honeys in relation to antimicrobial efficacy. Wounds 2009; 21:29–36.
- Patel B, Cox-Hayley D. Managing wound odor #218. J Palliat Med 2010; 13:1286–1287.
- Schulte MJ. Yogurt helps to control wound odor. Oncol Nurs Forum 1993; 20:1262.
- Upright CA, Salton C, Roberts F, Murphy J. Evaluation of Mesalt dressings and continuous wet saline dressings in ulcerating metastatic skin lesions. Cancer Nurs 1994; 17:149–155.
- Kuttan R, Sudheeran PC, Josph CD. Turmeric and curcumin as topical agents in cancer therapy. Tumori 1987; 73:29–31.
- Bass MJ, Phillips LG. Pressure sores. Curr Probl Surg 2007; 44:101–143.
- Bufill JA, Grace WR, Neff R. Intra-arterial chemotherapy for palliation of fungating breast cancer: a case report and review of the literature. Am J Clin Oncol 1994; 17:118–124.
- Murakami M, Kuroda Y, Sano A, et al. Validity of local treatment including intraarterial infusion chemotherapy and radiotherapy for fungating adenocarcinoma of the breast: case report of more than 8-year survival. Am J Clin Oncol 2001; 24:388–391.
- Lo SF, Hayter M, Hu WY, Tai CY, Hsu MY, Li YF. Symptom burden and quality of life in patients with malignant fungating wounds. J Adv Nurs 2012; 68:1312–1321.
- Lazarus GS, Cooper DM, Knighton DR, et al. Definitions and guidelines for assessment of wounds and evaluation of healing. Arch Dermatol 1994; 130:489–493.
- Tippett AW. Wounds at the end of life. Wounds 2005; 17:91–98.
- Burt T. Palliative care of pressure ulcers in long-term care. Ann Long-Term Care 2013; 21:20–28.
- Reifsnyder J, Magee HS. Development of pressure ulcers in patients receiving home hospice care. Wounds 2005; 17:74–79.
- Haisfield-Wolfe ME, Rund C. Malignant cutaneous wounds: a management protocol. Ostomy Wound Manage 1997; 43:56–66.
- O’Brien C. Malignant wounds: managing odour. Can Fam Physician 2012; 58:272–274.
- Gethin G, Grocott P, Probst S, Clarke E. Current practice in the management of wound odour: an international survey. Int J Nurs Stud 2014; 51:865–874.
- Bale S, Tebble N, Price P. A topical metronidazole gel used to treat malodorous wounds. Br J Nurs 2004; 13:S4–S11.
- Hack A. Malodorous wounds—taking the patient’s perspective into account. J Wound Care 2003; 12:319–321.
- Price E. Wound care. The stigma of smell. Nurs Times 1996; 92:71–72.
- Paul JC, Pieper BA. Topical metronidazole for the treatment of wound odor: a review of the literature. Ostomy Wound Manage 2008; 54:18–27.
- Lee G, Anand SC, Rajendran S, Walker I. Overview of current practice and future trends in the evaluation of dressings for malodorous wounds. J Wound Care 2006; 15:344–346.
- Cutting K, Harding K. Criteria for identifying wound infection. J Wound Care 1994; 3:198–201.
- McDonald A, Lesage P. Palliative management of pressure ulcers and malignant wounds in patients with advanced illness. J Palliat Med 2006; 9:285–295.
- Holloway S. Recognising and treating the causes of chronic malodorous wounds. Prof Nurse 2004; 19:380–384.
- Haughton W, Young T. Common problems in wound care: malodorous wounds. Br J Nurs 1995; 4:959–963.
- Alvarez OM, Kalinski C, Nusbaum J, et al. Incorporating wound healing strategies to improve palliation (symptom management) in patients with chronic wounds. J Palliat Med 2007; 10:1161–1189.
- da Costa Santos CM, de Mattos Pimenta CA, Nobre MR. A systematic review of topical treatments to control the odor of malignant fungating wounds. J Pain Symptom Manage 2010; 39:1065–1076.
- Von Gruenigen VE, Coleman RL, et al. Bacteriology and treatment of malodorous lower reproductive tract in gynecologic cancer patients. Obstet Gynecol 2000; 96:23–27.
- Baker PG, Haig G. Metronidazole in the treatment of chronic pressure sores and ulcers: a comparison with standard treatment in general practice. Practitioner 1981; 225:569–573.
- Whitney J, Phillips L, Aslam R, et al. Guidelines for the treatment of pressure ulcers. Wound Repair Regen 2006; 14:663–679.
- Williams D, Enoch S, Miller D, Harris K, Price P, Harding KG. Effect of sharp debridement using curette on recalcitrant nonhealing venous ulcers: a concurrently controlled, prospective cohort study. Wound Repair Regen 2005; 13:131–137.
- Bergstrom KJ. Assessment and management of fungating wounds. J Wound Ostomy Continence Nurs 2011: 38:31–37.
- Sinclair RD, Ryan TJ. Proteolytic enzymes in wound healing: the role of enzymatic debridement. Australas J Dermatol 1994; 35:35–41.
- Enoch S, Harding KG. Wound bed preparation: the science behind the removal of barriers to healing. Wounds 2003;15:213–229.
- Mumcuoglu KY. Clinical applications for maggots in wound care. Am J Clin Dermatol 2001; 2:219–227.
- Langemo DK, Black J; National Pressure Ulcer Advisory Panel. Pressure ulcers in individuals receiving palliative care: a National Pressure Ulcer Advisory Panel white paper. Adv Skin Wound Care 2010; 23:59–72.
- Fonder MA, Lazarus GS, Cowan DA, Aronson-Cook B, Kohli AR, Mamelak AJ. Treating the chronic wound: a practical approach to the care of nonhealing wounds and wound care dressings. J Am Acad Dermatol 2008; 58:185–206.
- Lio PA, Kaye ET. Topical antibacterial agents. Infect Dis Clin North Am 2004; 18:717–733.
- Gelmetti C. Local antibiotics in dermatology. Dermatol Ther 2008; 21:187–195.
- Finlay IG, Bowszyc J, Ramlau C, Gwiezdzinski Z. The effect of topical 0.75% metronidazole gel on malodorous cutaneous ulcers. J Pain Symptom Manage 1996; 11:158–162.
- Bower M, Stein R, Evans TR, Hedley A, Pert P, Coombes RC. A double-blind study of the efficacy of metronidazole gel in the treatment of malodorous fungating tumours. Eur J Cancer 1992; 28A:888–889.
- Kalinski C, Schnepf M, Laboy D, et al. Effectiveness of a topical formulation containing metronidazole for wound odor and exudate control. Wounds 2005; 17:84–90.
- Kuge S, Tokuda Y, Ohta M, et al. Use of metronidazole gel to control malodor in advanced and recurrent breast cancer. Jpn J Clin Oncol 1996; 26:207–210.
- Belcher J. A review of medical-grade honey in wound care. Br J Nurs 2012: 21:S4–S9.
- Kwakman PH, Van den Akker JP, Güçlü A, et al. Medical-grade honey kills antibiotic-resistant bacteria in vitro and eradicates skin colonization. Clin Infect Dis 2008; 46:1677–1682.
- Cooper RA, Jenkins L. A comparison between medical grade honey and table honeys in relation to antimicrobial efficacy. Wounds 2009; 21:29–36.
- Patel B, Cox-Hayley D. Managing wound odor #218. J Palliat Med 2010; 13:1286–1287.
- Schulte MJ. Yogurt helps to control wound odor. Oncol Nurs Forum 1993; 20:1262.
- Upright CA, Salton C, Roberts F, Murphy J. Evaluation of Mesalt dressings and continuous wet saline dressings in ulcerating metastatic skin lesions. Cancer Nurs 1994; 17:149–155.
- Kuttan R, Sudheeran PC, Josph CD. Turmeric and curcumin as topical agents in cancer therapy. Tumori 1987; 73:29–31.
- Bass MJ, Phillips LG. Pressure sores. Curr Probl Surg 2007; 44:101–143.
- Bufill JA, Grace WR, Neff R. Intra-arterial chemotherapy for palliation of fungating breast cancer: a case report and review of the literature. Am J Clin Oncol 1994; 17:118–124.
- Murakami M, Kuroda Y, Sano A, et al. Validity of local treatment including intraarterial infusion chemotherapy and radiotherapy for fungating adenocarcinoma of the breast: case report of more than 8-year survival. Am J Clin Oncol 2001; 24:388–391.
KEY POINTS
- Necrotic tissue is a substrate for bacterial growth and should be debrided. A variety of methods can be used.
- Malodor is most often from infection with anaerobic organisms, which topical metronidazole and other agents can help control.
- An absorbent dressing should be used either as a primary dressing, or over a layer of topical metronidazole and a nonadherent primary dressing.
- Foremost in formulating a patient- and family-centered malodor management strategy is to commit to controlling it as much as possible.
Heart failure in frail, older patients: We can do ‘MORE’
Mr. R. is an 85-year-old with congestive heart failure; the last time his ejection fraction was measured it was 30%. He also has hypertension, coronary artery disease (for which he underwent triple-vessel coronary artery bypass grafting), osteoarthritis, hyperlipidemia, and chronic obstructive pulmonary disease. He currently takes lisinopril (Zestril), carvedilol (Coreg), aspirin, clopidogrel (Plavix), digoxin, simvastatin (Zocor), furosemide (Lasix), an albuterol inhaler (Proventil), and over-the-counter naproxen (Naprosyn), the last two taken as needed.
Accompanied by his daughter, Mr. R. comes to see his primary care physician for a routine follow-up visit. He says he feels fine and has no shortness of breath or chest pain, but he feels light-headed at times, especially when he gets out of bed. He also mentions that he is bothered with having to get up three to four times at night to urinate.
On further questioning, he relates that he uses a cane to walk around the house and gets short of breath when walking from his bed to the bathroom and from one room to the next. He can feed himself, but he needs assistance with bathing and getting dressed.
Mr. R. admits that he has been feeling lonely since his wife died about a year ago. He now lives with his daughter and her family, and they all get along well. His daughter mentions that over the last 6 months he has not been eating well, that he appears to have lost interest in doing some of the things that he used to enjoy, and that he has lost weight. She adds that he has fallen twice in the last month.
On physical examination, Mr. R. is without distress but appears weak. He answers all questions appropriately, although his affect is flat and his daughter fills in some of the details.
Supine, his blood pressure is 160/90 mm Hg and his heart rate is 75; immediately after standing up he feels dizzy and his blood pressure drops to 120/60 mm Hg with a heart rate of 110. Three months ago he weighed 155 pounds (70.3 kg); today he weighs 145 pounds (65.9 kg).
His neck veins are not distended. On chest auscultation, bibasilar coarse crackles are heard, as well as a systolic murmur (grade 2 on a scale of 6), loudest in the second intercostal space at the right parasternal border. No peripheral edema is detected. His Mini-Mental State Exam score is 22 out of 30.
What changes, if any, should be made in Mr. R.’s management? What advice should the primary care physician give Mr. R. and his daughter about the course of his heart failure?
THE IMPORTANCE OF COMPLETE CARE
Mr. R. has multiple convoluted medical issues that plague many elderly patients with heart failure. To provide optimal care to patients like him, physicians need to draw on knowledge from the fields of internal medicine, geriatrics, and cardiology.
In this paper, we discuss how diagnosing and managing heart failure is different in elderly patients. We emphasize the importance of complete care of frail elderly patients, highlighting the pharmacologic and nonpharmacologic interventions that are available. Finally, we will return to Mr. R. and discuss a comprehensive plan for him.
HEART FAILURE, FRAILTY, DISABILITY ARE ALL CONNECTED
The ability to bounce back from physical insults, chiefly medical illnesses, sharply declines in old age. As various stressors accumulate, physical deterioration becomes inevitable. While some older adults can avoid going down this path of morbidity, in an increasing number of frail elderly patients, congestive heart failure inescapably assumes a complicated course.
Frailty is a state of increased vulnerability to stressors due to age-related declines in physiologic reserve.1 Two elements intimately related to frailty are comorbidity and disability.
Fried et al2 analyzed data from more than 5,000 older men and women in the Cardiovascular Health Study and concluded that comorbidity (ie, having two or more chronic diseases) is a risk factor for frailty, which in turn results in disability, falls, hospitalizations, and death.
The relationship between congestive heart failure and frailty is complex. Not only does heart failure itself result in frailty, but its multiple therapies can put additional stress on a frail patient. In addition, the heart failure and its treatments can negatively affect coexisting disorders (Figure 1).
BY THE NUMBERS
Heart failure is largely a disorder of the elderly, and as the US population ages, heart failure is rising in prevalence to epidemic numbers.3 The median age of patients admitted to the hospital because of heart failure is 75,4 and patients age 65 and older account for more than 75% of heart failure hospitalizations.5 Every year, in every 1,000 people over age 65, nearly 10 new cases of heart failure are diagnosed.6
Before age 70, men are affected more than women, but the opposite is true at age 70 and beyond. The reason for this reversal is that women live longer and have a better prognosis, as the cause of heart failure in most women is diastolic dysfunction secondary to hypertension rather than systolic dysfunction due to coronary artery disease, as in most men.7
Heart failure is costly and generally has a poor prognosis. The total cost of treating it reached a staggering $37.2 billion in 2009, and it was the leading cause of Medicare hospital admissions.6 Heart failure is the primary cause or a contributory cause of death in about 290,000 patients each year, and the rate of death at 1 year is an astonishing 1 in 5.6 The median survival time after diagnosis is 2.3 to 3.6 years in patients ages 67 to 74, and it is considerably shorter—1.1 to 1.6 years—in patients age 85 and older.8
THE BROKEN HEART
In 2005, the American College of Cardiology and the American Heart Association defined congestive heart failure as “a complex clinical syndrome that can result from any structural or functional cardiac disorder that impairs the ability of the ventricle to fill with or eject blood.”9 This characterization captures the intricate nature of the disease: its spectrum of symptoms, its many causes (eg, coronary artery disease, hypertension, nonischemic or idiopathic cardiomyopathy, and valvular heart disease), and the dual pathophysiologic features of systolic and diastolic impairment.
Systolic vs diastolic failure
Of the various ways of classifying heart failure, the most important is systolic vs diastolic.
The hallmark of systolic heart failure is a decreased left ventricular ejection fraction, and it is characterized by a large thin-walled ventricle that is weak and unable to eject enough blood to generate a normal cardiac output.
In contrast, the ejection fraction is normal or nearly normal in diastolic heart failure, but the end-diastolic volume is decreased because the ventricle is hypertrophied and thick-walled. The resultant chamber has become small and stiff and does not have enough volume for sufficient cardiac output.
QUIRKS IN THE HISTORY AND PHYSICAL EXAMINATION
The combination of inactivity and coexisting illnesses in a frail older adult may obscure some of the usual clinical manifestations of heart failure. While shortness of breath on mild exertion, easy fatigability, and leg swelling are common in younger heart failure patients, these symptoms may be due to normal aging in a much older patient. Let us consider some important aspects of the common signs and symptoms associated with heart failure.
Dyspnea on exertion is one of the earliest and most prominent symptoms. The usual question asked of patients to elicit whether this key manifestation is present is, “Do you get short of breath after walking a block?” However, this question may not be appropriate for a frail elderly person whose activity is restricted by comorbidities such as severe arthritis, coronary artery disease, or peripheral arterial disease. For a patient like this, ask instead if he or she gets short of breath after milder forms of exertion, such as making the bed, walking to the bathroom, or changing clothes.10 Also, keep in mind that dyspnea on exertion may be due to other conditions, such as renal failure, lung disease, depression, anemia, or deconditioning.
Orthopnea and paroxysmal nocturnal dyspnea may not be volunteered or elicited if a patient is sleeping in a chair or a recliner.
Leg swelling is less specific in older adults than in younger patients because chronic venous insufficiency is common in older people.
Weight gain almost always accompanies symptomatic heart failure but may also be due to increased appetite secondary to depression.
A change in mental status is common in elderly people with heart failure, especially those with vascular dementia with extensive cerebrovascular atherosclerosis or those who have latent Alzheimer disease.10
Cough, a symptom of a multitude of disorders, may be an early or the only manifestation of heart failure.
Pulmonary crackles are typically detected in most heart failure patients, but they may not be as characteristic in older adults, as they may also be noted in bronchitis, pneumonia, and other chronic lung diseases.
Additional symptoms to watch for include fatigue, syncope, angina, nocturia, and oliguria.
The bottom line is to integrate individual findings with other elements of the history and physical examination in diagnosing heart failure and tracking its progression.
CLINCHING THE DIAGNOSIS
Congestive heart failure is essentially a clinical diagnosis best established even before ordering tests, especially during times and situations in which these tests are not always readily available, such as outside office hours and in a long-term care setting.
A reliable and thorough history and physical examination is the most important component of the diagnostic process.
An echocardiogram is obtained next to measure the ejection fraction, which has both prognostic and therapeutic significance. Echocardiography can also uncover potential contributory cardiac structural abnormalities.
A chest radiograph is also typically obtained to look for pulmonary congestion, but in older adults its interpretation may be skewed by chronic lung disease or spinal deformities such as scoliosis and kyphosis.
The B-type natriuretic peptide (BNP) level is a popular blood test. BNP is commonly elevated in patients with heart failure. However, an elevated level in older adults should always be evaluated within the context of other clinical findings, as it can also result from advancing age and diseases other than heart failure, such as coronary artery disease, chronic pulmonary disease, pulmonary embolism, and renal insufficiency.11,12
PHARMACOTHERAPY PEARLS
Drug treatment for heart failure has evolved rapidly. Robust and sophisticated clinical trials have led to guidelines that call for specific medications. Unfortunately, older patients, particularly the very old and frail, have been poorly represented in these studies.9 Nonetheless, the type and choice of drugs for the young and old are similar.
Take into account age-associated changes in pharmacokinetics
Age-associated changes in pharmacokinetics must be taken into account when prescribing drugs for heart failure.13
Oral absorption of cardiovascular drugs is not significantly affected by the various changes that occur in older adults (eg, reduced gastric acid production, gastric emptying rate, gastrointestinal blood flow, and mobility). However, reductions in both lean body mass and total body water that come with aging result in lower volumes of distribution and higher plasma concentrations of hydrophilic drugs, most notably angiotensin-converting enzyme (ACE) inhibitors and digoxin. In contrast, the plasma concentrations of lipophilic drugs such as beta-blockers and central alpha-agonists tend to decrease as the proportion of body fat increases in older adults.
As the plasma albumin level diminishes with age, the free-drug concentration of salicylates and warfarin (Coumadin), which are extensively albumin-bound, may increase.
The serum concentrations of cardiovascular drugs metabolized in the liver—eg, propranolol (Inderal), lidocaine, labetalol (Trandate), verapamil (Calan), diltiazem (Cardizem), nitrates, and warfarin—may be elevated due to reduced hepatic blood flow, mass, volume, and overall metabolic capacity.
Declines in renal blood flow, glomerular filtration, and tubular function may cause accumulation of drugs that are excreted through the kidneys.
Beware of toxicities
Table 1 lists some of the drugs used in treating heart failure, common adverse affects to watch for, and recommendations for their use.
WIELDING THE SCALPEL
A tenet of heart failure management is to correct the underlying cardiac structural abnormality. This often calls for invasive intervention along with optimization of drug therapy.
For example:
- Diseased coronary arteries may be amenable to revascularization, either by percutaneous coronary intervention or by the much more involved coronary artery bypass grafting, with the aim of enhancing cardiac function.
- Valves can be repaired or replaced in patients with valvular heart disease.
- A pacemaker can be implanted to remedy sick sinus syndrome, especially with concurrent use of heart-rate-lowering agents such as beta-blockers.
- Placement of an implantable cardioverter-defibrillator has been found to be effective in preventing death due to ventricular tachyarrhythmias in patients with an ejection fraction of less than 30%.9
- Cardiac resynchronization with a biventricular pacemaker may increase the ejection fraction and cardiac output by eliminating dyssynchronous contraction of the left and right ventricles.14
In frail older adults, consideration of these invasive therapies must be individualized. While procedures such as percutaneous coronary intervention and pacemaker placement may not be as physically taxing as bypass grafting or valve replacement, the potential for surgical complications must be seriously considered, particularly if the patient has diminished physiologic reserve. Case-to-case consideration is also crucial in cardioverter-defibrillator insertion, as the survival benefit may be diminished in older adults, who likely have coexisting illnesses that predispose them to die of a noncardiac cause.15,16
The bottom line is to contemplate multiple factors—severity of the heart failure, comorbidities, baseline functional status, and social support—when assessing the appropriateness of an invasive intervention.
BEYOND DRUGS AND DEVICES: WE CAN DO ‘MORE’
Much of the spotlight has been on the various drugs and devices used to treat heart failure, but of equal importance for frail elderly patients are complementary approaches that can be used to ease disease progression and boost the quality of life. The acronym MORE highlights these strategies.
M: Multidisciplinary management programs
Heart failure disease-management programs are designed to provide comprehensive multidisciplinary care across different settings (ie, home, outpatient, and inpatient) to high-risk patients who often have multiple medical, social, and behavioral issues.9 Interventions usually include intensive patient education, encouraging patients to be more aggressive participants in their care, closely monitoring patients through telephone follow-up or home nursing, carefully reviewing medications to improve adherence to evidence-based guidelines, and multidisciplinary care with nurse case management directed by a physician.
Studies have shown that management programs, which were largely nurse-directed and targeted at older adults and patients with advanced disease, can improve quality of life and functional status, decrease hospitalizations for both heart failure and other causes, and decrease medical costs.17–19
O: Other diseases
R: Restrictions
Specific limitations in the intake of certain dietary elements are a valuable adjunct in heart failure management.
Sodium intake should be restricted to less than 3 g/day by not adding salt to meals and by avoiding salt-rich foods (eg, canned and processed foods).24 During times of distressing volume overload, a tighter sodium limit of 2 g/day is necessary, and diuretics may be less effective if this restriction is not implemented.
Fluid restriction depends on the patient’s clinical status.25 While it is not necessary to limit fluid intake in the absence of retention, a limit of 2 L/day is recommended if edema is detected. If volume overload is severe, the limit should be 1 L/day.
Alcohol is a myocardial depressant that reduces the left ventricular ejection fraction.26 Abstinence is a must for patients with alcohol-induced heart failure; otherwise, a limit of 1 drink (8 oz of beer, 4 oz of wine, or 1 oz of hard liquor) per day is suggested.24
Calories and fat intake are both important to watch, particularly in patients with obesity, hyperlipidemia, hypertension, or coronary artery disease.
E: End-of-life issues
Usual causes of death in patients with heart failure include sudden cardiac death, arrhythmias, hypotension, end-organ hypoperfusion, and metabolic derangement.27,28
Given the life-limiting nature of the disease in frail older adults, it is very important for clinicians to discuss end-of-life matters with patients and their families as early as possible. Needed are effective communication skills that foster respect, empathy, and mutual understanding.
Advance directives. The primary task is to encourage patients to develop advance health directives. These are legal documents that represent patients’ preferences about interventions available toward the end of life such as do-not-resuscitate orders, appointment of surrogate decision-makers, and use of life-sustaining interventions (eg, a feeding tube, dialysis, blood transfusions). Establishing these directives early on will help ease the transition from one mode of care to another (eg, from acute care to hospice care), prevent pointless use of resources (eg, emergency room visits, hospital admissions), and ensure that the patient’s wishes are carried out.
Palliative measures that aim to alleviate suffering and promote quality of life and dignity are available for patients with severe symptoms. For varying degrees of dyspnea, diuretics, nitrates, morphine, and positive inotropic agents such as dobutamine (Dobutrex) and milrinone (Primacor) can be tried. Thoracentesis is done in patients with extensive pleural effusion. Fatigue and anorexia are due to a combination of factors, namely, decreased cardiac output, increased neurohormone levels, deconditioning, depression, decreased sleep, and anxiety.29 Opioids, caffeine, exercise, oxygen, fluid and salt restriction, and correction of anemia and depression may help ease these symptoms.
For patients with an implantable cardioverter-defibrillator, deactivation is an important matter that needs to be addressed. Deactivation can be carried out with certainty once the goal of care has shifted away from curative efforts and either the patient or a surrogate decision-maker has made the informed decision to turn the device off. Berger30 raised three points that the clinician and decision-maker can discuss in trying to achieve a resolution during times of doubt and indecision:
- The patient may no longer value continued survival
- The device may no longer offer the prospect of increased survival
- The device may impede active dying.
The idea of hospice care should be gradually and gently explored to ensure a prompt and seamless transition when the time comes. The patient and family need to know that the goal of hospice care is to ensure comfort and that they can benefit the most by enrolling early during the course of the terminal illness.
The Medicare hospice benefit is granted to patients who have been certified by two physicians to have a life expectancy of 6 months or less if their terminal illness runs its natural course. The criteria for determining that heart failure is terminal are:
- New York Heart Association class III (symptomatic with less than ordinary activities) or IV (symptomatic at rest)
- Left ventricular ejection fraction less than or equal to 20%
- Persistent symptoms despite optimal medical management
- Inability to tolerate optional management due to hypotension with or without renal failure.31
WHAT CAN WE DO FOR MR. R.?
Mr. R. has systolic heart failure stemming from coronary artery disease, and his symptoms put him in New York Heart Association class III. He is well managed with drugs of different appropriate classes: an ACE inhibitor, a beta-blocker, digoxin, an aldosterone antagonist, and a diuretic. His other drugs all have well-defined indications.
Since he does not have fluid overload, his furosemide can be stopped, and this change will likely relieve his orthostatic hypotension and nocturia. His systolic blood pressure target can be liberalized to 150 mm Hg or less, as tighter control might exacerbate orthostatic hypotension. This change, along with having him start using a walker instead of a cane, will hopefully prevent future falls. Furthermore, his naproxen should be discontinued, as it can worsen heart failure.
Mr. R. has symptoms of depression and thus needs to be started on an antidepressant and encouraged to engage in social activities as much as he can tolerate. These interventions may also help with his mild dementia, which is evidenced by a Mini-Mental State Exam score of 22. He will not benefit from sodium and fat restriction, as he has actually been losing weight.
To keep Mr. R.’s cognitive impairment and overall decline in function from compromising his compliance with his treatment, he will need a substantial amount of assistance, which his daughter alone may not be able to provide. To tackle this concern, a discussion about participating in a heart failure management program can be started with Mr. R. and his family.
More importantly, his advanced directives, including delegating a surrogate decision-maker and deciding on do-not-resuscitate status, have to be clarified. Finally, it would be prudent to introduce the concept of hospice care to the patient and his daughter while he is still coherent and able to state his preferences.
- Walston J, Hadley EC, Ferruci L, et al. Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J Am Geriatr Soc 2006; 54:991–1001.
- Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56:M146–M156.
- Schocken DD, Arrieta MI, Leaverton PE, Ross EA. Prevalence and mortality rate of congestive heart failure in the United States. J Am Coll Cardiol 1992; 20:301–306.
- Popovic JR, 1999 National Hospital Discharge Survey: annual summary with detailed diagnosis and procedure data. National Center for Health Statistics. Vital Health Stat 2001; 13:1–206.
- DeFrances CJ, Hall MJ, Podgornik MN. 2003 National Hospital Discharge Survey. Advance data from vital and health statistics; no. 359. Hyattsville (MD): National Center for Health Statistics, 2005.
- American Heart Association. Heart disease and stroke statistics—2009 update: a report From the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2009; 119:e21–e181.
- Levy D, Larson MG, Vasan RS, et al. The progression from hypertension to congestive heart failure. JAMA 1996; 275:1557–1562.
- Croft JB, Giles WH, Pollard RA, et al. Heart failure survival among older adults in the United States: a poor prognosis for an emerging epidemic in the Medicare population. Arch Intern Med 1999; 159:505–510.
- Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure). Circulation 2005; 112:e154–e235.
- Ahmed A. Clinical manifestations, diagnostic assessment, and etiology of heart failure in older adults. Clin Geriatr Med 2007; 23:11–30.
- Redfield MM, Rodeheffer RJ, Jacobsen SJ, et al. Plasma brain natriuretic peptide concentration: impact of age and gender. J Am Coll Cardiol 2002; 40:976–982.
- Wang TJ, Larson MG, Levy D, et al. Impact of age and sex on plasma natriuretic peptide levels in healthy adults. Am J Cardiol 2002; 90:254–258.
- Aronow WS, Frishman WH, Cheng-Lai A. Cardiovascular drug therapy in the elderly. Cardiol Rev 2007; 15:195–215.
- Bakker P, Meijburg H, de Bries J, et al. Biventricular pacing in end-stage heart failure improves functional capacity and left ventricular function. J Interv Card Electrophysiol 2000; 4:395–404.
- Healey JS, Hallstrom AP, Kuck KH, et al. Role of the implantable defibrillator among elderly patients with a history of life-threatening ventricular arrhythmias. Eur Heart J 2007; 28:1746–1749.
- Lee DS, Tu JV, Austin PC, et al. Effect of cardiac and noncardiac conditions on survival after defibrillator implantation. J Am Coll Cardiol 2007; 49:2408–2415.
- Rich MW, Beckham V, Wittenberg C, et al. A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure. N Engl J Med 1995; 333:1190–1195.
- Fonarow GC, Stevenson LW, Walden JA, et al. Impact of a comprehensive heart failure management program on hospital readmission and functional status of patients with advanced heart failure. J Am Coll Cardiol 1997; 30:725–732.
- McAlister F, Stewart S, Ferrua S, McMurray JJ. Multidisciplinary strategies for the management of heart failure patients at high risk for admission: a systematic review of randomized trials. J Am Coll Cardiol 2004; 44:810–819.
- Horwich TB, Fonarow GC, Hamilton MA, et al. Anemia is associated with worse symptoms, greater impairment in functional capacity and a significant increase in mortality in patients with advanced heart failure. J Am Coll Cardiol 2002; 39:1780–1786.
- Al-Ahmad A, Rand WM, Manjunath G, et al. Reduced kidney function and anemia as risk factors for mortality in patients with left ventricular dysfunction. J Am Coll Cardiol 2001; 38:955–962.
- Singh SN, Fisher SG, Deedwania PC, et al. Pulmonary effect of amiodarone in patients with heart failure: the Congestive Heart Failure-Survival Trial of Antiarrhythmic Therapy (CHF-STAT) Investigators (Veterans Affairs Cooperative Study No. 320). J Am Coll Cardiol 1997; 30:514–517.
- Cohen MB, Mather PJ. A review of the association between congestive heart failure and cognitive impairment. Am J Geriatr Cardiol 2007; 16:171–174.
- Dracup K, Baker DW, Dunbar SB, et al. Management of heart failure. II. Counseling, education, and lifestyle modifications. JAMA 1994; 272:1442–1446.
- Lenihan DJ, Uretsky BF. Non-pharmacologic treatment of heart failure in the elderly. Clin Geriatr Med 2000; 16:477–488.
- Regan TJ. Alcohol and the cardiovascular system. JAMA 1990; 264:377–381.
- Teuteberg JJ, Lewis EF, Nohria A, et al. Characteristics of patients who die with heart failure and a low ejection fraction in the new millennium. J Card Fail 2006; 12:47–53.
- Derfler MC, Jacob M, Wolf RE, et al. Mode of death from congestive heart failure: implications for clinical management. Am J Geriatr Cardiol 2004; 13:299–304.
- Evangelista LS, Moser DK, Westlake C, et al. Correlates of fatigue in patients with heart failure. Prog Cardiovasc Nurs 2008; 23:12–17.
- Berger JT. The ethics of deactivating implanted cardioverter defibrillators. Ann Intern Med 2005; 142:631–634.
- Stuart B, Connor S, Kinzbrunner BM, et al. Medical guidelines for determining prognosis in selected non-cancer diseases, 2nd ed. Arlington VA, National Hospice Organization; 1996.
Mr. R. is an 85-year-old with congestive heart failure; the last time his ejection fraction was measured it was 30%. He also has hypertension, coronary artery disease (for which he underwent triple-vessel coronary artery bypass grafting), osteoarthritis, hyperlipidemia, and chronic obstructive pulmonary disease. He currently takes lisinopril (Zestril), carvedilol (Coreg), aspirin, clopidogrel (Plavix), digoxin, simvastatin (Zocor), furosemide (Lasix), an albuterol inhaler (Proventil), and over-the-counter naproxen (Naprosyn), the last two taken as needed.
Accompanied by his daughter, Mr. R. comes to see his primary care physician for a routine follow-up visit. He says he feels fine and has no shortness of breath or chest pain, but he feels light-headed at times, especially when he gets out of bed. He also mentions that he is bothered with having to get up three to four times at night to urinate.
On further questioning, he relates that he uses a cane to walk around the house and gets short of breath when walking from his bed to the bathroom and from one room to the next. He can feed himself, but he needs assistance with bathing and getting dressed.
Mr. R. admits that he has been feeling lonely since his wife died about a year ago. He now lives with his daughter and her family, and they all get along well. His daughter mentions that over the last 6 months he has not been eating well, that he appears to have lost interest in doing some of the things that he used to enjoy, and that he has lost weight. She adds that he has fallen twice in the last month.
On physical examination, Mr. R. is without distress but appears weak. He answers all questions appropriately, although his affect is flat and his daughter fills in some of the details.
Supine, his blood pressure is 160/90 mm Hg and his heart rate is 75; immediately after standing up he feels dizzy and his blood pressure drops to 120/60 mm Hg with a heart rate of 110. Three months ago he weighed 155 pounds (70.3 kg); today he weighs 145 pounds (65.9 kg).
His neck veins are not distended. On chest auscultation, bibasilar coarse crackles are heard, as well as a systolic murmur (grade 2 on a scale of 6), loudest in the second intercostal space at the right parasternal border. No peripheral edema is detected. His Mini-Mental State Exam score is 22 out of 30.
What changes, if any, should be made in Mr. R.’s management? What advice should the primary care physician give Mr. R. and his daughter about the course of his heart failure?
THE IMPORTANCE OF COMPLETE CARE
Mr. R. has multiple convoluted medical issues that plague many elderly patients with heart failure. To provide optimal care to patients like him, physicians need to draw on knowledge from the fields of internal medicine, geriatrics, and cardiology.
In this paper, we discuss how diagnosing and managing heart failure is different in elderly patients. We emphasize the importance of complete care of frail elderly patients, highlighting the pharmacologic and nonpharmacologic interventions that are available. Finally, we will return to Mr. R. and discuss a comprehensive plan for him.
HEART FAILURE, FRAILTY, DISABILITY ARE ALL CONNECTED
The ability to bounce back from physical insults, chiefly medical illnesses, sharply declines in old age. As various stressors accumulate, physical deterioration becomes inevitable. While some older adults can avoid going down this path of morbidity, in an increasing number of frail elderly patients, congestive heart failure inescapably assumes a complicated course.
Frailty is a state of increased vulnerability to stressors due to age-related declines in physiologic reserve.1 Two elements intimately related to frailty are comorbidity and disability.
Fried et al2 analyzed data from more than 5,000 older men and women in the Cardiovascular Health Study and concluded that comorbidity (ie, having two or more chronic diseases) is a risk factor for frailty, which in turn results in disability, falls, hospitalizations, and death.
The relationship between congestive heart failure and frailty is complex. Not only does heart failure itself result in frailty, but its multiple therapies can put additional stress on a frail patient. In addition, the heart failure and its treatments can negatively affect coexisting disorders (Figure 1).
BY THE NUMBERS
Heart failure is largely a disorder of the elderly, and as the US population ages, heart failure is rising in prevalence to epidemic numbers.3 The median age of patients admitted to the hospital because of heart failure is 75,4 and patients age 65 and older account for more than 75% of heart failure hospitalizations.5 Every year, in every 1,000 people over age 65, nearly 10 new cases of heart failure are diagnosed.6
Before age 70, men are affected more than women, but the opposite is true at age 70 and beyond. The reason for this reversal is that women live longer and have a better prognosis, as the cause of heart failure in most women is diastolic dysfunction secondary to hypertension rather than systolic dysfunction due to coronary artery disease, as in most men.7
Heart failure is costly and generally has a poor prognosis. The total cost of treating it reached a staggering $37.2 billion in 2009, and it was the leading cause of Medicare hospital admissions.6 Heart failure is the primary cause or a contributory cause of death in about 290,000 patients each year, and the rate of death at 1 year is an astonishing 1 in 5.6 The median survival time after diagnosis is 2.3 to 3.6 years in patients ages 67 to 74, and it is considerably shorter—1.1 to 1.6 years—in patients age 85 and older.8
THE BROKEN HEART
In 2005, the American College of Cardiology and the American Heart Association defined congestive heart failure as “a complex clinical syndrome that can result from any structural or functional cardiac disorder that impairs the ability of the ventricle to fill with or eject blood.”9 This characterization captures the intricate nature of the disease: its spectrum of symptoms, its many causes (eg, coronary artery disease, hypertension, nonischemic or idiopathic cardiomyopathy, and valvular heart disease), and the dual pathophysiologic features of systolic and diastolic impairment.
Systolic vs diastolic failure
Of the various ways of classifying heart failure, the most important is systolic vs diastolic.
The hallmark of systolic heart failure is a decreased left ventricular ejection fraction, and it is characterized by a large thin-walled ventricle that is weak and unable to eject enough blood to generate a normal cardiac output.
In contrast, the ejection fraction is normal or nearly normal in diastolic heart failure, but the end-diastolic volume is decreased because the ventricle is hypertrophied and thick-walled. The resultant chamber has become small and stiff and does not have enough volume for sufficient cardiac output.
QUIRKS IN THE HISTORY AND PHYSICAL EXAMINATION
The combination of inactivity and coexisting illnesses in a frail older adult may obscure some of the usual clinical manifestations of heart failure. While shortness of breath on mild exertion, easy fatigability, and leg swelling are common in younger heart failure patients, these symptoms may be due to normal aging in a much older patient. Let us consider some important aspects of the common signs and symptoms associated with heart failure.
Dyspnea on exertion is one of the earliest and most prominent symptoms. The usual question asked of patients to elicit whether this key manifestation is present is, “Do you get short of breath after walking a block?” However, this question may not be appropriate for a frail elderly person whose activity is restricted by comorbidities such as severe arthritis, coronary artery disease, or peripheral arterial disease. For a patient like this, ask instead if he or she gets short of breath after milder forms of exertion, such as making the bed, walking to the bathroom, or changing clothes.10 Also, keep in mind that dyspnea on exertion may be due to other conditions, such as renal failure, lung disease, depression, anemia, or deconditioning.
Orthopnea and paroxysmal nocturnal dyspnea may not be volunteered or elicited if a patient is sleeping in a chair or a recliner.
Leg swelling is less specific in older adults than in younger patients because chronic venous insufficiency is common in older people.
Weight gain almost always accompanies symptomatic heart failure but may also be due to increased appetite secondary to depression.
A change in mental status is common in elderly people with heart failure, especially those with vascular dementia with extensive cerebrovascular atherosclerosis or those who have latent Alzheimer disease.10
Cough, a symptom of a multitude of disorders, may be an early or the only manifestation of heart failure.
Pulmonary crackles are typically detected in most heart failure patients, but they may not be as characteristic in older adults, as they may also be noted in bronchitis, pneumonia, and other chronic lung diseases.
Additional symptoms to watch for include fatigue, syncope, angina, nocturia, and oliguria.
The bottom line is to integrate individual findings with other elements of the history and physical examination in diagnosing heart failure and tracking its progression.
CLINCHING THE DIAGNOSIS
Congestive heart failure is essentially a clinical diagnosis best established even before ordering tests, especially during times and situations in which these tests are not always readily available, such as outside office hours and in a long-term care setting.
A reliable and thorough history and physical examination is the most important component of the diagnostic process.
An echocardiogram is obtained next to measure the ejection fraction, which has both prognostic and therapeutic significance. Echocardiography can also uncover potential contributory cardiac structural abnormalities.
A chest radiograph is also typically obtained to look for pulmonary congestion, but in older adults its interpretation may be skewed by chronic lung disease or spinal deformities such as scoliosis and kyphosis.
The B-type natriuretic peptide (BNP) level is a popular blood test. BNP is commonly elevated in patients with heart failure. However, an elevated level in older adults should always be evaluated within the context of other clinical findings, as it can also result from advancing age and diseases other than heart failure, such as coronary artery disease, chronic pulmonary disease, pulmonary embolism, and renal insufficiency.11,12
PHARMACOTHERAPY PEARLS
Drug treatment for heart failure has evolved rapidly. Robust and sophisticated clinical trials have led to guidelines that call for specific medications. Unfortunately, older patients, particularly the very old and frail, have been poorly represented in these studies.9 Nonetheless, the type and choice of drugs for the young and old are similar.
Take into account age-associated changes in pharmacokinetics
Age-associated changes in pharmacokinetics must be taken into account when prescribing drugs for heart failure.13
Oral absorption of cardiovascular drugs is not significantly affected by the various changes that occur in older adults (eg, reduced gastric acid production, gastric emptying rate, gastrointestinal blood flow, and mobility). However, reductions in both lean body mass and total body water that come with aging result in lower volumes of distribution and higher plasma concentrations of hydrophilic drugs, most notably angiotensin-converting enzyme (ACE) inhibitors and digoxin. In contrast, the plasma concentrations of lipophilic drugs such as beta-blockers and central alpha-agonists tend to decrease as the proportion of body fat increases in older adults.
As the plasma albumin level diminishes with age, the free-drug concentration of salicylates and warfarin (Coumadin), which are extensively albumin-bound, may increase.
The serum concentrations of cardiovascular drugs metabolized in the liver—eg, propranolol (Inderal), lidocaine, labetalol (Trandate), verapamil (Calan), diltiazem (Cardizem), nitrates, and warfarin—may be elevated due to reduced hepatic blood flow, mass, volume, and overall metabolic capacity.
Declines in renal blood flow, glomerular filtration, and tubular function may cause accumulation of drugs that are excreted through the kidneys.
Beware of toxicities
Table 1 lists some of the drugs used in treating heart failure, common adverse affects to watch for, and recommendations for their use.
WIELDING THE SCALPEL
A tenet of heart failure management is to correct the underlying cardiac structural abnormality. This often calls for invasive intervention along with optimization of drug therapy.
For example:
- Diseased coronary arteries may be amenable to revascularization, either by percutaneous coronary intervention or by the much more involved coronary artery bypass grafting, with the aim of enhancing cardiac function.
- Valves can be repaired or replaced in patients with valvular heart disease.
- A pacemaker can be implanted to remedy sick sinus syndrome, especially with concurrent use of heart-rate-lowering agents such as beta-blockers.
- Placement of an implantable cardioverter-defibrillator has been found to be effective in preventing death due to ventricular tachyarrhythmias in patients with an ejection fraction of less than 30%.9
- Cardiac resynchronization with a biventricular pacemaker may increase the ejection fraction and cardiac output by eliminating dyssynchronous contraction of the left and right ventricles.14
In frail older adults, consideration of these invasive therapies must be individualized. While procedures such as percutaneous coronary intervention and pacemaker placement may not be as physically taxing as bypass grafting or valve replacement, the potential for surgical complications must be seriously considered, particularly if the patient has diminished physiologic reserve. Case-to-case consideration is also crucial in cardioverter-defibrillator insertion, as the survival benefit may be diminished in older adults, who likely have coexisting illnesses that predispose them to die of a noncardiac cause.15,16
The bottom line is to contemplate multiple factors—severity of the heart failure, comorbidities, baseline functional status, and social support—when assessing the appropriateness of an invasive intervention.
BEYOND DRUGS AND DEVICES: WE CAN DO ‘MORE’
Much of the spotlight has been on the various drugs and devices used to treat heart failure, but of equal importance for frail elderly patients are complementary approaches that can be used to ease disease progression and boost the quality of life. The acronym MORE highlights these strategies.
M: Multidisciplinary management programs
Heart failure disease-management programs are designed to provide comprehensive multidisciplinary care across different settings (ie, home, outpatient, and inpatient) to high-risk patients who often have multiple medical, social, and behavioral issues.9 Interventions usually include intensive patient education, encouraging patients to be more aggressive participants in their care, closely monitoring patients through telephone follow-up or home nursing, carefully reviewing medications to improve adherence to evidence-based guidelines, and multidisciplinary care with nurse case management directed by a physician.
Studies have shown that management programs, which were largely nurse-directed and targeted at older adults and patients with advanced disease, can improve quality of life and functional status, decrease hospitalizations for both heart failure and other causes, and decrease medical costs.17–19
O: Other diseases
R: Restrictions
Specific limitations in the intake of certain dietary elements are a valuable adjunct in heart failure management.
Sodium intake should be restricted to less than 3 g/day by not adding salt to meals and by avoiding salt-rich foods (eg, canned and processed foods).24 During times of distressing volume overload, a tighter sodium limit of 2 g/day is necessary, and diuretics may be less effective if this restriction is not implemented.
Fluid restriction depends on the patient’s clinical status.25 While it is not necessary to limit fluid intake in the absence of retention, a limit of 2 L/day is recommended if edema is detected. If volume overload is severe, the limit should be 1 L/day.
Alcohol is a myocardial depressant that reduces the left ventricular ejection fraction.26 Abstinence is a must for patients with alcohol-induced heart failure; otherwise, a limit of 1 drink (8 oz of beer, 4 oz of wine, or 1 oz of hard liquor) per day is suggested.24
Calories and fat intake are both important to watch, particularly in patients with obesity, hyperlipidemia, hypertension, or coronary artery disease.
E: End-of-life issues
Usual causes of death in patients with heart failure include sudden cardiac death, arrhythmias, hypotension, end-organ hypoperfusion, and metabolic derangement.27,28
Given the life-limiting nature of the disease in frail older adults, it is very important for clinicians to discuss end-of-life matters with patients and their families as early as possible. Needed are effective communication skills that foster respect, empathy, and mutual understanding.
Advance directives. The primary task is to encourage patients to develop advance health directives. These are legal documents that represent patients’ preferences about interventions available toward the end of life such as do-not-resuscitate orders, appointment of surrogate decision-makers, and use of life-sustaining interventions (eg, a feeding tube, dialysis, blood transfusions). Establishing these directives early on will help ease the transition from one mode of care to another (eg, from acute care to hospice care), prevent pointless use of resources (eg, emergency room visits, hospital admissions), and ensure that the patient’s wishes are carried out.
Palliative measures that aim to alleviate suffering and promote quality of life and dignity are available for patients with severe symptoms. For varying degrees of dyspnea, diuretics, nitrates, morphine, and positive inotropic agents such as dobutamine (Dobutrex) and milrinone (Primacor) can be tried. Thoracentesis is done in patients with extensive pleural effusion. Fatigue and anorexia are due to a combination of factors, namely, decreased cardiac output, increased neurohormone levels, deconditioning, depression, decreased sleep, and anxiety.29 Opioids, caffeine, exercise, oxygen, fluid and salt restriction, and correction of anemia and depression may help ease these symptoms.
For patients with an implantable cardioverter-defibrillator, deactivation is an important matter that needs to be addressed. Deactivation can be carried out with certainty once the goal of care has shifted away from curative efforts and either the patient or a surrogate decision-maker has made the informed decision to turn the device off. Berger30 raised three points that the clinician and decision-maker can discuss in trying to achieve a resolution during times of doubt and indecision:
- The patient may no longer value continued survival
- The device may no longer offer the prospect of increased survival
- The device may impede active dying.
The idea of hospice care should be gradually and gently explored to ensure a prompt and seamless transition when the time comes. The patient and family need to know that the goal of hospice care is to ensure comfort and that they can benefit the most by enrolling early during the course of the terminal illness.
The Medicare hospice benefit is granted to patients who have been certified by two physicians to have a life expectancy of 6 months or less if their terminal illness runs its natural course. The criteria for determining that heart failure is terminal are:
- New York Heart Association class III (symptomatic with less than ordinary activities) or IV (symptomatic at rest)
- Left ventricular ejection fraction less than or equal to 20%
- Persistent symptoms despite optimal medical management
- Inability to tolerate optional management due to hypotension with or without renal failure.31
WHAT CAN WE DO FOR MR. R.?
Mr. R. has systolic heart failure stemming from coronary artery disease, and his symptoms put him in New York Heart Association class III. He is well managed with drugs of different appropriate classes: an ACE inhibitor, a beta-blocker, digoxin, an aldosterone antagonist, and a diuretic. His other drugs all have well-defined indications.
Since he does not have fluid overload, his furosemide can be stopped, and this change will likely relieve his orthostatic hypotension and nocturia. His systolic blood pressure target can be liberalized to 150 mm Hg or less, as tighter control might exacerbate orthostatic hypotension. This change, along with having him start using a walker instead of a cane, will hopefully prevent future falls. Furthermore, his naproxen should be discontinued, as it can worsen heart failure.
Mr. R. has symptoms of depression and thus needs to be started on an antidepressant and encouraged to engage in social activities as much as he can tolerate. These interventions may also help with his mild dementia, which is evidenced by a Mini-Mental State Exam score of 22. He will not benefit from sodium and fat restriction, as he has actually been losing weight.
To keep Mr. R.’s cognitive impairment and overall decline in function from compromising his compliance with his treatment, he will need a substantial amount of assistance, which his daughter alone may not be able to provide. To tackle this concern, a discussion about participating in a heart failure management program can be started with Mr. R. and his family.
More importantly, his advanced directives, including delegating a surrogate decision-maker and deciding on do-not-resuscitate status, have to be clarified. Finally, it would be prudent to introduce the concept of hospice care to the patient and his daughter while he is still coherent and able to state his preferences.
Mr. R. is an 85-year-old with congestive heart failure; the last time his ejection fraction was measured it was 30%. He also has hypertension, coronary artery disease (for which he underwent triple-vessel coronary artery bypass grafting), osteoarthritis, hyperlipidemia, and chronic obstructive pulmonary disease. He currently takes lisinopril (Zestril), carvedilol (Coreg), aspirin, clopidogrel (Plavix), digoxin, simvastatin (Zocor), furosemide (Lasix), an albuterol inhaler (Proventil), and over-the-counter naproxen (Naprosyn), the last two taken as needed.
Accompanied by his daughter, Mr. R. comes to see his primary care physician for a routine follow-up visit. He says he feels fine and has no shortness of breath or chest pain, but he feels light-headed at times, especially when he gets out of bed. He also mentions that he is bothered with having to get up three to four times at night to urinate.
On further questioning, he relates that he uses a cane to walk around the house and gets short of breath when walking from his bed to the bathroom and from one room to the next. He can feed himself, but he needs assistance with bathing and getting dressed.
Mr. R. admits that he has been feeling lonely since his wife died about a year ago. He now lives with his daughter and her family, and they all get along well. His daughter mentions that over the last 6 months he has not been eating well, that he appears to have lost interest in doing some of the things that he used to enjoy, and that he has lost weight. She adds that he has fallen twice in the last month.
On physical examination, Mr. R. is without distress but appears weak. He answers all questions appropriately, although his affect is flat and his daughter fills in some of the details.
Supine, his blood pressure is 160/90 mm Hg and his heart rate is 75; immediately after standing up he feels dizzy and his blood pressure drops to 120/60 mm Hg with a heart rate of 110. Three months ago he weighed 155 pounds (70.3 kg); today he weighs 145 pounds (65.9 kg).
His neck veins are not distended. On chest auscultation, bibasilar coarse crackles are heard, as well as a systolic murmur (grade 2 on a scale of 6), loudest in the second intercostal space at the right parasternal border. No peripheral edema is detected. His Mini-Mental State Exam score is 22 out of 30.
What changes, if any, should be made in Mr. R.’s management? What advice should the primary care physician give Mr. R. and his daughter about the course of his heart failure?
THE IMPORTANCE OF COMPLETE CARE
Mr. R. has multiple convoluted medical issues that plague many elderly patients with heart failure. To provide optimal care to patients like him, physicians need to draw on knowledge from the fields of internal medicine, geriatrics, and cardiology.
In this paper, we discuss how diagnosing and managing heart failure is different in elderly patients. We emphasize the importance of complete care of frail elderly patients, highlighting the pharmacologic and nonpharmacologic interventions that are available. Finally, we will return to Mr. R. and discuss a comprehensive plan for him.
HEART FAILURE, FRAILTY, DISABILITY ARE ALL CONNECTED
The ability to bounce back from physical insults, chiefly medical illnesses, sharply declines in old age. As various stressors accumulate, physical deterioration becomes inevitable. While some older adults can avoid going down this path of morbidity, in an increasing number of frail elderly patients, congestive heart failure inescapably assumes a complicated course.
Frailty is a state of increased vulnerability to stressors due to age-related declines in physiologic reserve.1 Two elements intimately related to frailty are comorbidity and disability.
Fried et al2 analyzed data from more than 5,000 older men and women in the Cardiovascular Health Study and concluded that comorbidity (ie, having two or more chronic diseases) is a risk factor for frailty, which in turn results in disability, falls, hospitalizations, and death.
The relationship between congestive heart failure and frailty is complex. Not only does heart failure itself result in frailty, but its multiple therapies can put additional stress on a frail patient. In addition, the heart failure and its treatments can negatively affect coexisting disorders (Figure 1).
BY THE NUMBERS
Heart failure is largely a disorder of the elderly, and as the US population ages, heart failure is rising in prevalence to epidemic numbers.3 The median age of patients admitted to the hospital because of heart failure is 75,4 and patients age 65 and older account for more than 75% of heart failure hospitalizations.5 Every year, in every 1,000 people over age 65, nearly 10 new cases of heart failure are diagnosed.6
Before age 70, men are affected more than women, but the opposite is true at age 70 and beyond. The reason for this reversal is that women live longer and have a better prognosis, as the cause of heart failure in most women is diastolic dysfunction secondary to hypertension rather than systolic dysfunction due to coronary artery disease, as in most men.7
Heart failure is costly and generally has a poor prognosis. The total cost of treating it reached a staggering $37.2 billion in 2009, and it was the leading cause of Medicare hospital admissions.6 Heart failure is the primary cause or a contributory cause of death in about 290,000 patients each year, and the rate of death at 1 year is an astonishing 1 in 5.6 The median survival time after diagnosis is 2.3 to 3.6 years in patients ages 67 to 74, and it is considerably shorter—1.1 to 1.6 years—in patients age 85 and older.8
THE BROKEN HEART
In 2005, the American College of Cardiology and the American Heart Association defined congestive heart failure as “a complex clinical syndrome that can result from any structural or functional cardiac disorder that impairs the ability of the ventricle to fill with or eject blood.”9 This characterization captures the intricate nature of the disease: its spectrum of symptoms, its many causes (eg, coronary artery disease, hypertension, nonischemic or idiopathic cardiomyopathy, and valvular heart disease), and the dual pathophysiologic features of systolic and diastolic impairment.
Systolic vs diastolic failure
Of the various ways of classifying heart failure, the most important is systolic vs diastolic.
The hallmark of systolic heart failure is a decreased left ventricular ejection fraction, and it is characterized by a large thin-walled ventricle that is weak and unable to eject enough blood to generate a normal cardiac output.
In contrast, the ejection fraction is normal or nearly normal in diastolic heart failure, but the end-diastolic volume is decreased because the ventricle is hypertrophied and thick-walled. The resultant chamber has become small and stiff and does not have enough volume for sufficient cardiac output.
QUIRKS IN THE HISTORY AND PHYSICAL EXAMINATION
The combination of inactivity and coexisting illnesses in a frail older adult may obscure some of the usual clinical manifestations of heart failure. While shortness of breath on mild exertion, easy fatigability, and leg swelling are common in younger heart failure patients, these symptoms may be due to normal aging in a much older patient. Let us consider some important aspects of the common signs and symptoms associated with heart failure.
Dyspnea on exertion is one of the earliest and most prominent symptoms. The usual question asked of patients to elicit whether this key manifestation is present is, “Do you get short of breath after walking a block?” However, this question may not be appropriate for a frail elderly person whose activity is restricted by comorbidities such as severe arthritis, coronary artery disease, or peripheral arterial disease. For a patient like this, ask instead if he or she gets short of breath after milder forms of exertion, such as making the bed, walking to the bathroom, or changing clothes.10 Also, keep in mind that dyspnea on exertion may be due to other conditions, such as renal failure, lung disease, depression, anemia, or deconditioning.
Orthopnea and paroxysmal nocturnal dyspnea may not be volunteered or elicited if a patient is sleeping in a chair or a recliner.
Leg swelling is less specific in older adults than in younger patients because chronic venous insufficiency is common in older people.
Weight gain almost always accompanies symptomatic heart failure but may also be due to increased appetite secondary to depression.
A change in mental status is common in elderly people with heart failure, especially those with vascular dementia with extensive cerebrovascular atherosclerosis or those who have latent Alzheimer disease.10
Cough, a symptom of a multitude of disorders, may be an early or the only manifestation of heart failure.
Pulmonary crackles are typically detected in most heart failure patients, but they may not be as characteristic in older adults, as they may also be noted in bronchitis, pneumonia, and other chronic lung diseases.
Additional symptoms to watch for include fatigue, syncope, angina, nocturia, and oliguria.
The bottom line is to integrate individual findings with other elements of the history and physical examination in diagnosing heart failure and tracking its progression.
CLINCHING THE DIAGNOSIS
Congestive heart failure is essentially a clinical diagnosis best established even before ordering tests, especially during times and situations in which these tests are not always readily available, such as outside office hours and in a long-term care setting.
A reliable and thorough history and physical examination is the most important component of the diagnostic process.
An echocardiogram is obtained next to measure the ejection fraction, which has both prognostic and therapeutic significance. Echocardiography can also uncover potential contributory cardiac structural abnormalities.
A chest radiograph is also typically obtained to look for pulmonary congestion, but in older adults its interpretation may be skewed by chronic lung disease or spinal deformities such as scoliosis and kyphosis.
The B-type natriuretic peptide (BNP) level is a popular blood test. BNP is commonly elevated in patients with heart failure. However, an elevated level in older adults should always be evaluated within the context of other clinical findings, as it can also result from advancing age and diseases other than heart failure, such as coronary artery disease, chronic pulmonary disease, pulmonary embolism, and renal insufficiency.11,12
PHARMACOTHERAPY PEARLS
Drug treatment for heart failure has evolved rapidly. Robust and sophisticated clinical trials have led to guidelines that call for specific medications. Unfortunately, older patients, particularly the very old and frail, have been poorly represented in these studies.9 Nonetheless, the type and choice of drugs for the young and old are similar.
Take into account age-associated changes in pharmacokinetics
Age-associated changes in pharmacokinetics must be taken into account when prescribing drugs for heart failure.13
Oral absorption of cardiovascular drugs is not significantly affected by the various changes that occur in older adults (eg, reduced gastric acid production, gastric emptying rate, gastrointestinal blood flow, and mobility). However, reductions in both lean body mass and total body water that come with aging result in lower volumes of distribution and higher plasma concentrations of hydrophilic drugs, most notably angiotensin-converting enzyme (ACE) inhibitors and digoxin. In contrast, the plasma concentrations of lipophilic drugs such as beta-blockers and central alpha-agonists tend to decrease as the proportion of body fat increases in older adults.
As the plasma albumin level diminishes with age, the free-drug concentration of salicylates and warfarin (Coumadin), which are extensively albumin-bound, may increase.
The serum concentrations of cardiovascular drugs metabolized in the liver—eg, propranolol (Inderal), lidocaine, labetalol (Trandate), verapamil (Calan), diltiazem (Cardizem), nitrates, and warfarin—may be elevated due to reduced hepatic blood flow, mass, volume, and overall metabolic capacity.
Declines in renal blood flow, glomerular filtration, and tubular function may cause accumulation of drugs that are excreted through the kidneys.
Beware of toxicities
Table 1 lists some of the drugs used in treating heart failure, common adverse affects to watch for, and recommendations for their use.
WIELDING THE SCALPEL
A tenet of heart failure management is to correct the underlying cardiac structural abnormality. This often calls for invasive intervention along with optimization of drug therapy.
For example:
- Diseased coronary arteries may be amenable to revascularization, either by percutaneous coronary intervention or by the much more involved coronary artery bypass grafting, with the aim of enhancing cardiac function.
- Valves can be repaired or replaced in patients with valvular heart disease.
- A pacemaker can be implanted to remedy sick sinus syndrome, especially with concurrent use of heart-rate-lowering agents such as beta-blockers.
- Placement of an implantable cardioverter-defibrillator has been found to be effective in preventing death due to ventricular tachyarrhythmias in patients with an ejection fraction of less than 30%.9
- Cardiac resynchronization with a biventricular pacemaker may increase the ejection fraction and cardiac output by eliminating dyssynchronous contraction of the left and right ventricles.14
In frail older adults, consideration of these invasive therapies must be individualized. While procedures such as percutaneous coronary intervention and pacemaker placement may not be as physically taxing as bypass grafting or valve replacement, the potential for surgical complications must be seriously considered, particularly if the patient has diminished physiologic reserve. Case-to-case consideration is also crucial in cardioverter-defibrillator insertion, as the survival benefit may be diminished in older adults, who likely have coexisting illnesses that predispose them to die of a noncardiac cause.15,16
The bottom line is to contemplate multiple factors—severity of the heart failure, comorbidities, baseline functional status, and social support—when assessing the appropriateness of an invasive intervention.
BEYOND DRUGS AND DEVICES: WE CAN DO ‘MORE’
Much of the spotlight has been on the various drugs and devices used to treat heart failure, but of equal importance for frail elderly patients are complementary approaches that can be used to ease disease progression and boost the quality of life. The acronym MORE highlights these strategies.
M: Multidisciplinary management programs
Heart failure disease-management programs are designed to provide comprehensive multidisciplinary care across different settings (ie, home, outpatient, and inpatient) to high-risk patients who often have multiple medical, social, and behavioral issues.9 Interventions usually include intensive patient education, encouraging patients to be more aggressive participants in their care, closely monitoring patients through telephone follow-up or home nursing, carefully reviewing medications to improve adherence to evidence-based guidelines, and multidisciplinary care with nurse case management directed by a physician.
Studies have shown that management programs, which were largely nurse-directed and targeted at older adults and patients with advanced disease, can improve quality of life and functional status, decrease hospitalizations for both heart failure and other causes, and decrease medical costs.17–19
O: Other diseases
R: Restrictions
Specific limitations in the intake of certain dietary elements are a valuable adjunct in heart failure management.
Sodium intake should be restricted to less than 3 g/day by not adding salt to meals and by avoiding salt-rich foods (eg, canned and processed foods).24 During times of distressing volume overload, a tighter sodium limit of 2 g/day is necessary, and diuretics may be less effective if this restriction is not implemented.
Fluid restriction depends on the patient’s clinical status.25 While it is not necessary to limit fluid intake in the absence of retention, a limit of 2 L/day is recommended if edema is detected. If volume overload is severe, the limit should be 1 L/day.
Alcohol is a myocardial depressant that reduces the left ventricular ejection fraction.26 Abstinence is a must for patients with alcohol-induced heart failure; otherwise, a limit of 1 drink (8 oz of beer, 4 oz of wine, or 1 oz of hard liquor) per day is suggested.24
Calories and fat intake are both important to watch, particularly in patients with obesity, hyperlipidemia, hypertension, or coronary artery disease.
E: End-of-life issues
Usual causes of death in patients with heart failure include sudden cardiac death, arrhythmias, hypotension, end-organ hypoperfusion, and metabolic derangement.27,28
Given the life-limiting nature of the disease in frail older adults, it is very important for clinicians to discuss end-of-life matters with patients and their families as early as possible. Needed are effective communication skills that foster respect, empathy, and mutual understanding.
Advance directives. The primary task is to encourage patients to develop advance health directives. These are legal documents that represent patients’ preferences about interventions available toward the end of life such as do-not-resuscitate orders, appointment of surrogate decision-makers, and use of life-sustaining interventions (eg, a feeding tube, dialysis, blood transfusions). Establishing these directives early on will help ease the transition from one mode of care to another (eg, from acute care to hospice care), prevent pointless use of resources (eg, emergency room visits, hospital admissions), and ensure that the patient’s wishes are carried out.
Palliative measures that aim to alleviate suffering and promote quality of life and dignity are available for patients with severe symptoms. For varying degrees of dyspnea, diuretics, nitrates, morphine, and positive inotropic agents such as dobutamine (Dobutrex) and milrinone (Primacor) can be tried. Thoracentesis is done in patients with extensive pleural effusion. Fatigue and anorexia are due to a combination of factors, namely, decreased cardiac output, increased neurohormone levels, deconditioning, depression, decreased sleep, and anxiety.29 Opioids, caffeine, exercise, oxygen, fluid and salt restriction, and correction of anemia and depression may help ease these symptoms.
For patients with an implantable cardioverter-defibrillator, deactivation is an important matter that needs to be addressed. Deactivation can be carried out with certainty once the goal of care has shifted away from curative efforts and either the patient or a surrogate decision-maker has made the informed decision to turn the device off. Berger30 raised three points that the clinician and decision-maker can discuss in trying to achieve a resolution during times of doubt and indecision:
- The patient may no longer value continued survival
- The device may no longer offer the prospect of increased survival
- The device may impede active dying.
The idea of hospice care should be gradually and gently explored to ensure a prompt and seamless transition when the time comes. The patient and family need to know that the goal of hospice care is to ensure comfort and that they can benefit the most by enrolling early during the course of the terminal illness.
The Medicare hospice benefit is granted to patients who have been certified by two physicians to have a life expectancy of 6 months or less if their terminal illness runs its natural course. The criteria for determining that heart failure is terminal are:
- New York Heart Association class III (symptomatic with less than ordinary activities) or IV (symptomatic at rest)
- Left ventricular ejection fraction less than or equal to 20%
- Persistent symptoms despite optimal medical management
- Inability to tolerate optional management due to hypotension with or without renal failure.31
WHAT CAN WE DO FOR MR. R.?
Mr. R. has systolic heart failure stemming from coronary artery disease, and his symptoms put him in New York Heart Association class III. He is well managed with drugs of different appropriate classes: an ACE inhibitor, a beta-blocker, digoxin, an aldosterone antagonist, and a diuretic. His other drugs all have well-defined indications.
Since he does not have fluid overload, his furosemide can be stopped, and this change will likely relieve his orthostatic hypotension and nocturia. His systolic blood pressure target can be liberalized to 150 mm Hg or less, as tighter control might exacerbate orthostatic hypotension. This change, along with having him start using a walker instead of a cane, will hopefully prevent future falls. Furthermore, his naproxen should be discontinued, as it can worsen heart failure.
Mr. R. has symptoms of depression and thus needs to be started on an antidepressant and encouraged to engage in social activities as much as he can tolerate. These interventions may also help with his mild dementia, which is evidenced by a Mini-Mental State Exam score of 22. He will not benefit from sodium and fat restriction, as he has actually been losing weight.
To keep Mr. R.’s cognitive impairment and overall decline in function from compromising his compliance with his treatment, he will need a substantial amount of assistance, which his daughter alone may not be able to provide. To tackle this concern, a discussion about participating in a heart failure management program can be started with Mr. R. and his family.
More importantly, his advanced directives, including delegating a surrogate decision-maker and deciding on do-not-resuscitate status, have to be clarified. Finally, it would be prudent to introduce the concept of hospice care to the patient and his daughter while he is still coherent and able to state his preferences.
- Walston J, Hadley EC, Ferruci L, et al. Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J Am Geriatr Soc 2006; 54:991–1001.
- Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56:M146–M156.
- Schocken DD, Arrieta MI, Leaverton PE, Ross EA. Prevalence and mortality rate of congestive heart failure in the United States. J Am Coll Cardiol 1992; 20:301–306.
- Popovic JR, 1999 National Hospital Discharge Survey: annual summary with detailed diagnosis and procedure data. National Center for Health Statistics. Vital Health Stat 2001; 13:1–206.
- DeFrances CJ, Hall MJ, Podgornik MN. 2003 National Hospital Discharge Survey. Advance data from vital and health statistics; no. 359. Hyattsville (MD): National Center for Health Statistics, 2005.
- American Heart Association. Heart disease and stroke statistics—2009 update: a report From the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2009; 119:e21–e181.
- Levy D, Larson MG, Vasan RS, et al. The progression from hypertension to congestive heart failure. JAMA 1996; 275:1557–1562.
- Croft JB, Giles WH, Pollard RA, et al. Heart failure survival among older adults in the United States: a poor prognosis for an emerging epidemic in the Medicare population. Arch Intern Med 1999; 159:505–510.
- Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure). Circulation 2005; 112:e154–e235.
- Ahmed A. Clinical manifestations, diagnostic assessment, and etiology of heart failure in older adults. Clin Geriatr Med 2007; 23:11–30.
- Redfield MM, Rodeheffer RJ, Jacobsen SJ, et al. Plasma brain natriuretic peptide concentration: impact of age and gender. J Am Coll Cardiol 2002; 40:976–982.
- Wang TJ, Larson MG, Levy D, et al. Impact of age and sex on plasma natriuretic peptide levels in healthy adults. Am J Cardiol 2002; 90:254–258.
- Aronow WS, Frishman WH, Cheng-Lai A. Cardiovascular drug therapy in the elderly. Cardiol Rev 2007; 15:195–215.
- Bakker P, Meijburg H, de Bries J, et al. Biventricular pacing in end-stage heart failure improves functional capacity and left ventricular function. J Interv Card Electrophysiol 2000; 4:395–404.
- Healey JS, Hallstrom AP, Kuck KH, et al. Role of the implantable defibrillator among elderly patients with a history of life-threatening ventricular arrhythmias. Eur Heart J 2007; 28:1746–1749.
- Lee DS, Tu JV, Austin PC, et al. Effect of cardiac and noncardiac conditions on survival after defibrillator implantation. J Am Coll Cardiol 2007; 49:2408–2415.
- Rich MW, Beckham V, Wittenberg C, et al. A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure. N Engl J Med 1995; 333:1190–1195.
- Fonarow GC, Stevenson LW, Walden JA, et al. Impact of a comprehensive heart failure management program on hospital readmission and functional status of patients with advanced heart failure. J Am Coll Cardiol 1997; 30:725–732.
- McAlister F, Stewart S, Ferrua S, McMurray JJ. Multidisciplinary strategies for the management of heart failure patients at high risk for admission: a systematic review of randomized trials. J Am Coll Cardiol 2004; 44:810–819.
- Horwich TB, Fonarow GC, Hamilton MA, et al. Anemia is associated with worse symptoms, greater impairment in functional capacity and a significant increase in mortality in patients with advanced heart failure. J Am Coll Cardiol 2002; 39:1780–1786.
- Al-Ahmad A, Rand WM, Manjunath G, et al. Reduced kidney function and anemia as risk factors for mortality in patients with left ventricular dysfunction. J Am Coll Cardiol 2001; 38:955–962.
- Singh SN, Fisher SG, Deedwania PC, et al. Pulmonary effect of amiodarone in patients with heart failure: the Congestive Heart Failure-Survival Trial of Antiarrhythmic Therapy (CHF-STAT) Investigators (Veterans Affairs Cooperative Study No. 320). J Am Coll Cardiol 1997; 30:514–517.
- Cohen MB, Mather PJ. A review of the association between congestive heart failure and cognitive impairment. Am J Geriatr Cardiol 2007; 16:171–174.
- Dracup K, Baker DW, Dunbar SB, et al. Management of heart failure. II. Counseling, education, and lifestyle modifications. JAMA 1994; 272:1442–1446.
- Lenihan DJ, Uretsky BF. Non-pharmacologic treatment of heart failure in the elderly. Clin Geriatr Med 2000; 16:477–488.
- Regan TJ. Alcohol and the cardiovascular system. JAMA 1990; 264:377–381.
- Teuteberg JJ, Lewis EF, Nohria A, et al. Characteristics of patients who die with heart failure and a low ejection fraction in the new millennium. J Card Fail 2006; 12:47–53.
- Derfler MC, Jacob M, Wolf RE, et al. Mode of death from congestive heart failure: implications for clinical management. Am J Geriatr Cardiol 2004; 13:299–304.
- Evangelista LS, Moser DK, Westlake C, et al. Correlates of fatigue in patients with heart failure. Prog Cardiovasc Nurs 2008; 23:12–17.
- Berger JT. The ethics of deactivating implanted cardioverter defibrillators. Ann Intern Med 2005; 142:631–634.
- Stuart B, Connor S, Kinzbrunner BM, et al. Medical guidelines for determining prognosis in selected non-cancer diseases, 2nd ed. Arlington VA, National Hospice Organization; 1996.
- Walston J, Hadley EC, Ferruci L, et al. Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J Am Geriatr Soc 2006; 54:991–1001.
- Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56:M146–M156.
- Schocken DD, Arrieta MI, Leaverton PE, Ross EA. Prevalence and mortality rate of congestive heart failure in the United States. J Am Coll Cardiol 1992; 20:301–306.
- Popovic JR, 1999 National Hospital Discharge Survey: annual summary with detailed diagnosis and procedure data. National Center for Health Statistics. Vital Health Stat 2001; 13:1–206.
- DeFrances CJ, Hall MJ, Podgornik MN. 2003 National Hospital Discharge Survey. Advance data from vital and health statistics; no. 359. Hyattsville (MD): National Center for Health Statistics, 2005.
- American Heart Association. Heart disease and stroke statistics—2009 update: a report From the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2009; 119:e21–e181.
- Levy D, Larson MG, Vasan RS, et al. The progression from hypertension to congestive heart failure. JAMA 1996; 275:1557–1562.
- Croft JB, Giles WH, Pollard RA, et al. Heart failure survival among older adults in the United States: a poor prognosis for an emerging epidemic in the Medicare population. Arch Intern Med 1999; 159:505–510.
- Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure). Circulation 2005; 112:e154–e235.
- Ahmed A. Clinical manifestations, diagnostic assessment, and etiology of heart failure in older adults. Clin Geriatr Med 2007; 23:11–30.
- Redfield MM, Rodeheffer RJ, Jacobsen SJ, et al. Plasma brain natriuretic peptide concentration: impact of age and gender. J Am Coll Cardiol 2002; 40:976–982.
- Wang TJ, Larson MG, Levy D, et al. Impact of age and sex on plasma natriuretic peptide levels in healthy adults. Am J Cardiol 2002; 90:254–258.
- Aronow WS, Frishman WH, Cheng-Lai A. Cardiovascular drug therapy in the elderly. Cardiol Rev 2007; 15:195–215.
- Bakker P, Meijburg H, de Bries J, et al. Biventricular pacing in end-stage heart failure improves functional capacity and left ventricular function. J Interv Card Electrophysiol 2000; 4:395–404.
- Healey JS, Hallstrom AP, Kuck KH, et al. Role of the implantable defibrillator among elderly patients with a history of life-threatening ventricular arrhythmias. Eur Heart J 2007; 28:1746–1749.
- Lee DS, Tu JV, Austin PC, et al. Effect of cardiac and noncardiac conditions on survival after defibrillator implantation. J Am Coll Cardiol 2007; 49:2408–2415.
- Rich MW, Beckham V, Wittenberg C, et al. A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure. N Engl J Med 1995; 333:1190–1195.
- Fonarow GC, Stevenson LW, Walden JA, et al. Impact of a comprehensive heart failure management program on hospital readmission and functional status of patients with advanced heart failure. J Am Coll Cardiol 1997; 30:725–732.
- McAlister F, Stewart S, Ferrua S, McMurray JJ. Multidisciplinary strategies for the management of heart failure patients at high risk for admission: a systematic review of randomized trials. J Am Coll Cardiol 2004; 44:810–819.
- Horwich TB, Fonarow GC, Hamilton MA, et al. Anemia is associated with worse symptoms, greater impairment in functional capacity and a significant increase in mortality in patients with advanced heart failure. J Am Coll Cardiol 2002; 39:1780–1786.
- Al-Ahmad A, Rand WM, Manjunath G, et al. Reduced kidney function and anemia as risk factors for mortality in patients with left ventricular dysfunction. J Am Coll Cardiol 2001; 38:955–962.
- Singh SN, Fisher SG, Deedwania PC, et al. Pulmonary effect of amiodarone in patients with heart failure: the Congestive Heart Failure-Survival Trial of Antiarrhythmic Therapy (CHF-STAT) Investigators (Veterans Affairs Cooperative Study No. 320). J Am Coll Cardiol 1997; 30:514–517.
- Cohen MB, Mather PJ. A review of the association between congestive heart failure and cognitive impairment. Am J Geriatr Cardiol 2007; 16:171–174.
- Dracup K, Baker DW, Dunbar SB, et al. Management of heart failure. II. Counseling, education, and lifestyle modifications. JAMA 1994; 272:1442–1446.
- Lenihan DJ, Uretsky BF. Non-pharmacologic treatment of heart failure in the elderly. Clin Geriatr Med 2000; 16:477–488.
- Regan TJ. Alcohol and the cardiovascular system. JAMA 1990; 264:377–381.
- Teuteberg JJ, Lewis EF, Nohria A, et al. Characteristics of patients who die with heart failure and a low ejection fraction in the new millennium. J Card Fail 2006; 12:47–53.
- Derfler MC, Jacob M, Wolf RE, et al. Mode of death from congestive heart failure: implications for clinical management. Am J Geriatr Cardiol 2004; 13:299–304.
- Evangelista LS, Moser DK, Westlake C, et al. Correlates of fatigue in patients with heart failure. Prog Cardiovasc Nurs 2008; 23:12–17.
- Berger JT. The ethics of deactivating implanted cardioverter defibrillators. Ann Intern Med 2005; 142:631–634.
- Stuart B, Connor S, Kinzbrunner BM, et al. Medical guidelines for determining prognosis in selected non-cancer diseases, 2nd ed. Arlington VA, National Hospice Organization; 1996.
KEY POINTS
- Not only does heart failure itself result in frailty, but its treatment can also put additional stress on an already frail patient. In addition, the illness and its treatments can negatively affect coexisting disorders.
- Common signs and symptoms of heart failure are less specific in older adults, and atypical symptoms may predominate.
- Age-associated changes in pharmacokinetics must be taken into account when prescribing drugs for heart failure.
- Effective communication among health professionals, patients, and families is necessary.
- Given the life-limiting nature of heart failure in frail older adults, it is critical for clinicians to discuss end-of-life issues with patients and their families as soon as possible.
Nocturia in the elderly: A wake-up call
Nocturia is common, but elderly patients infrequently volunteer this complaint, and even when they do, some clinicians may dismiss it as simply a part of aging. Nevertheless, nocturia causes significant distress and impairment of quality of life. It is associated with very serious consequences such as depression, social isolation, and a higher risk of death.
In this article, we review the concepts behind frequent nighttime voiding in older adults. We will start with two case scenarios to aid in understanding these concepts; near the end of the article, we will discuss the most appropriate management strategies for these two patients.
CASE SCENARIOS
Case 1: An 82-year-old man with fatigue
An 82-year-old obese white man with a history of hypertension, diabetes, and benign prostatic hyperplasia comes in to see his primary care provider, complaining of fatigue. He wakes up tired and has difficulty completing his daytime tasks. He gets up every 1 to 2 hours at night to urinate and has slow urinary flow and a feeling of incomplete bladder emptying.
See related patient education material
He says his wife has been increasingly bothered by his loud snoring. Recently, he had a car accident when he fell asleep while driving.
Case 2: An 85-year-old woman with incontinence
An 85-year-old white woman is in her family physician’s office with a primary complaint of waking up at least four times at night to urinate, and often ends up soaking her bed or adult diapers. She is bothered by urinary urgency and frequency during the day as well. She denies dysuria and hematuria.
She has a history of hypertension and urinary incontinence, and she has seven children. Her current medications are diltiazem (Cardizem), metoprolol (Toprol), and oxybutynin (Ditropan).
In these two cases, what would account for the nocturia? What would be the best way to help these patients?
THE NORM, NOT THE EXCEPTION
Although nocturia is defined as an awakening by the need to urinate even once in a night, many experts consider that it begins to be clinically significant only when the patient voids at least twice during the night.1
In older adults, nocturia is the norm rather than the exception. Studies done between 1990 and 2009 found that 68.9% to 93% of men age 70 and older get up at least once a night to void. The prevalence in women is somewhat lower, at 74.1% to 77.1%.2 Clinically significant nocturia is present in a majority of the elderly: more than 60% of both men and women.3
An Austrian study4 reported that elderly men got up to urinate a mean of 2.8 times per night, while women got up significantly more often—3.1 times. Women were also bothered more by this symptom, and their quality of life was significantly more decreased.
In another study,5 whites had a significantly higher nocturia ratio (ratio of nighttime urine volume to the 24-hour urine volume) than Asians. Asians, on the other hand, had a significantly higher nocturnal bladder capacity index than whites. (See below for definitions of the various indices of nocturia.) This information implies that nocturia may be a more prominent problem for elderly whites than for other racial groups.
In an epidemiologic study in Sweden,6 the death rate was as much as twice as high in both men and women who had three or more nocturnal voids, even after taking into account the influence of cardiac disease, diabetes mellitus, and stroke.
If nocturia is not addressed in the physician-patient encounter, patients may try to “self-manage” it by restricting their fluid intake or by limiting their social exposure,7 with limited success and with unwanted social isolation.
WHAT CAUSES NOCTURIA?
Advancing age is primary among these factors. Age-related structural changes in the urinary system include decreased functional bladder capacity, a decreased maximum urinary flow rate,6 a decreased ability to postpone urination,8 and an age-related increase in postvoiding residual urine volume.9 The aging kidney is also less able to concentrate urine. Also implicated are histologic changes in the detrusor muscle10 that lead to diminished bladder compliance and, together with detrusor overactivity, result in increased urinary frequency.
Nocturnal polyuria or nocturnal urine overproduction is common in patients with nocturia.11
Although the pathophysiology of nocturnal polyuria is still unclear, some investigators believe that low levels of antidiuretic hormone (ADH) at night are involved, reflecting an alteration in the circadian rhythm seen in diurnal plasma arginine vasopressin levels.12 In patients with nocturnal polyuria, ADH levels drop to very low or undetectable levels at night, which increases nocturnal urine output. In some extreme cases, the low to absent levels of ADH increase nocturnal voiding to 85% of the total 24-hour urine volume.13
Other causes of nocturnal polyuria include mobilization of fluids in patients with edema,14 and autonomic dysfunction. Other biochemical changes that contribute to nocturia include a decrease in nighttime plasma melatonin levels, an increase in nighttime plasma catecholamine levels, an increase in nighttime plasma natriuretic peptide levels, an increase in blood pressure, and an increase in total urine volume.15
A decreased ability to store urine also leads to nocturia. This is caused by decreased nocturnal bladder capacity, more irritative symptoms, and comorbid conditions such as overactive bladder, pelvic floor laxity resulting in pelvic organ prolapse, and, in men, benign prostatic hyperplasia.
Neural inputs to the bladder can also be impaired, as in patients who have diabetes mellitus or spinal stenosis, leading to chronic urinary retention, detrusor dysfunction, nocturia, and incontinence.
WHICH PATIENTS ARE AT RISK?
Obesity is associated with a higher incidence of moderate to severe nocturia.15 Studies have shown that the higher the body mass index, the greater the number of nighttime voids, especially in women.16
Habitually eating at night, with poor daytime appetite, is shown to be associated with increased nighttime diuresis.
Obstructive sleep apnea17 and untreated depressive symptoms such as frequent napping18 are also associated with moderate to severe nocturia.19
Higher systolic blood pressures are associated with more urine production at night. Plasma ADH regulation is also altered, which contributes to nocturnal polyuria.21
Other comorbid conditions associated with nocturia include recurrent cystitis, lung disease, congestive heart failure, neurodegenerative conditions (eg, Alzheimer disease and parkinsonism), and chronic kidney disease.21
Drugs associated with nocturia include cholinesterase inhibitors (for dementia),22 beta-blockers,23 and calcium channel blockers.24
Lifestyle factors. Alcohol and coffee have shown either no or only a mild diuretic effect. Smoking has not been shown to be associated with nocturia.15
Seasonal differences also exist, with increased frequency of nocturia in the winter.25
WHAT ARE THE CLINICAL CONSEQUENCES OF NOCTURIA?
Quality of life can be profoundly affected, and if nocturia is left untreated, it may lead to morbidity and even death. Elderly patients may feel simultaneously debilitated, frustrated, distressed, and puzzled. Nocturia may also increase their fear of falling and may negatively affect personal relationships.26
Falls, injuries. Nocturia exposes elderly patients to injuries such as hip fractures due to falling, significantly increasing the incidence of this injury.26 This occurs as elderly patients get up from bed and walk to the bathroom to void.27 In addition, during the day, superficial and fragmented sleep leads to daytime sleepiness and impaired perception and balance, also increasing the risk of falls.28 The complications of immobility and the need for surgery in many cases lead to debility, increased risk of infections, decubitus ulcers, and death. The risk of hip fractures can lead elderly patients with nocturia to associate this symptom with a fear of falling and can alter their concept of their own age (“Nocturia makes me feel old”),29 further diminishing quality of life.
The estimated medical cost of nocturia-associated falls in the elderly is about $1.5 billion per year, part of the $61 billion in lost productivity due to nocturia in adults.30
Long-term complications (eg, debilitation, poor sleep, obesity, decreased energy), increase the overall mortality rate, especially in patients who report voiding more than three times per night.29 Elderly patients with nocturia also have a greater need for emergency care.31
Nocturia also complicates other comorbid conditions, such as dementia, which increases the risk of urinary incontinence.32 In patients who have had a stroke, nocturia is the most frequent lower urinary tract symptom, and represents a major impact on daily life.33
Sleep disturbance is another important consequence. In one survey,34 nocturia was cited as a cause of poor sleep four times more often than the cause cited next most often, ie, pain. Because the elderly patient is awakened from sleep numerous times throughout the night, nocturia leads to more fatigue,35 lower energy levels, and poorer quality of sleep.36 Depression may be linked to poor sleep, as men with two or more nocturnal episodes were shown to be six times more likely to experience depression.
The patient is not the only person who loses sleep: so do the patient’s family members or sleeping partner.7 It is therefore not surprising that sleep disruption caused by nocturia has been cited as a principal reason for admitting older relatives to care homes.37
The risk of death is higher for elderly patients with coronary heart disease if they have nocturia. The causative link is the hemodynamic changes (increases in blood pressure and heart rate) that accompany awakening and arising, which may cause cardiovascular strain and lead to cardiovascular events. The 12-year survival rate has been shown to be significantly lower in patients with nighttime voiding, making nocturia a highly significant independent predictor of death in coronary heart disease patients.38
HOW TO EVALUATE AN OLDER ADULT WHO PRESENTS WITH NOCTURIA
A thorough history and physical examination are crucial in diagnosing nocturia. The goal is to identify any treatable underlying condition, such as diabetes mellitus, obstructive sleep apnea, diabetes insipidus, overactive bladder, benign prostatic hyperplasia, urinary tract infection, and congestive heart failure. Laboratory tests and imaging studies can help rule out these underlying conditions.
Other important facets in the history that must be elicited are medication use, patterns of fluid intake, and a history of other urinary complaints.39
A voiding diary and indices of nocturia
A voiding diary is extremely useful and should be used whenever possible. Episodes of incontinence, time of voids, volume voided, and frequency and volume of fluid intake are recorded. From the raw data, one can determine the following:
Total nocturnal urine volume, ie, the sum volume of the nighttime voids
Maximum voided volume, ie, the largest single recorded volume voided in a 24-hour period
Nocturia index, ie, the total nocturnal urine volume divided by the maximum voided volume. A nocturia index greater than 1 shows that nocturnal urine production is greater than the functional bladder capacity. Clinically significant nocturia is observed in patients with a nocturia index of 2.1 or greater.
Nocturnal polyuria index, ie, total nocturnal urine volume divided by the 24-hour urine output. A nocturnal polyuria index higher than 33% implies nocturnal polyuria.40
Nocturnal bladder capacity index, ie, the actual number of nightly voids minus the predicted number of nightly voids, which in turn is calculated as the nocturia index minus 1.
It is especially important to encourage patients to make a voiding diary, as some patients may find this cumbersome, and compliance can be low unless its importance is emphasized. A diary over 7 days usually gives meaningful data. The results from the diary typically confirm the presence of nocturnal polyuria or a decrease in bladder capacity, influencing management.41
WHAT ARE THE TREATMENT OPTIONS?
Therapy must be directed at the primary cause, addressing any underlying conditions that can contribute to nocturia. Examples39:
- Tight control of blood sugar for patients with diabetes mellitus
- Treatment of diabetes insipidus
- Referral for patients with primary polydipsia
- Management of hypercalcemia and hypokalemia
- A survey of medications
- Treatment of infections.
Nonpharmacologic measures
Tailored behavioral therapy can also be instituted, but the patient needs to have realistic expectations, as these measures are rarely effective alone.
Avoiding nighttime fluid intake, including alcohol and caffeine, has shown promise.
Wearing compression stockings and elevating the legs in the afternoon decrease the retention of fluid that otherwise would return to the circulation at night.
Identifying and eliminating nighttime influences that disturb sleep has variable efficacy. The use of continuous positive airway pressure helps to treat sleep apnea. Moderate exercise, reducing nonsleep time spent in bed,42 and sleeping in a warm bed43 to decrease cold diuresis have also been shown to improve sleep quality.44 Patients with nocturia may have a disrupted circadian rhythm, and phototherapy may help resynchronize the diurnal rhythm and melatonin secretion.
Pharmacotherapy
Pharmacotherapy of nocturia includes desmopressin (DDAVP) to manage nocturnal polyuria and antimuscarinic agents to manage the patient’s decreased ability to store urine. Alpha-blockers such as tamsulosin (Flomax) and 5-alpha-reductase inhibitors such as finasteride (Proscar) are used for men with benign prostatic hyperplasia. Novel and second-line therapies include diuretics such as furosemide (Lasix), cyclooxygenase-2 inhibitors, as well as botulinum toxin injected directly into the detrusor muscle for overactive bladder.45
Desmopressin in a low oral dose (0.1–0.4 mg) at bedtime can be initiated and the response assessed. Patients with nocturnal polyuria and disorders of the vasopressin system have been found to be more sensitive to desmopressin therapy.46 Fluid retention and hyponatremia can complicate therapy, and desmopressin must be avoided in patients with liver cirrhosis, renal failure, or congestive heart failure.47
Antimuscarinic agents are effective for patients who have lower urinary tract symptoms and for those with a diminished ability to store urine. They act by decreasing both voluntary and involuntary bladder contractions by blocking muscarinic receptors on the detrusor muscle. This reduces the bladder’s ability to contract and the urge to urinate, thereby increasing bladder capacity.48 These agents include oxybutynin (Ditropan), tolterodine (Detrol), solifenacin (Vesicare), and propiverine (not available in the United States).
Diuretics are being used as second-line agents or for patients who cannot tolerate desmopressin.49 Hydrochlorothiazide is taken 8 hours before bedtime to prevent water accumulation before the early sleeping hours.50 Furosemide has also led to a reduction in the mean number of nocturnal voids.51 The effect of these drugs on nocturia are especially beneficial to patients with concomitant hypertension or cardiovascular disease.
Cyclo-oxygenase-2 inhibitors such as celecoxib (Celebrex)52 and other nonsteroidal anti-inflammatory drugs such as diclofenac (Voltaren, others)53 and loxoprofen (not available in the United States)54 have been shown to decrease urine production, detrusor muscle tone, and inflammation, especially in men with benign prostatic hyperplasia.
Botulinum toxin has been used, usually in patients refractory to first-line treatment.44
Table 4 summarizes the treatment strategies for nocturia.
CASES REVISITED
The first patient described above has nocturia caused by several concomitant diseases, ie, hypertension, diabetes, benign prostatic hyperplasia, and obstructive sleep apnea. In addition to controlling his blood pressure and blood sugar, his primary care provider referred him to a pulmonologist, who confirmed obstructive sleep apnea with polysomnography and prescribed nightly use of a continuous positive airway pressure apparatus. A few weeks later, the patient’s nocturia had improved significantly, and his level of fatigue had decreased.
Apart from hypertension, the second patient’s nocturia was mostly attributed to her existing urinary incontinence. Recognizing that her current antihypertensive regimen may worsen nocturia, her family physician changed it to enalapril (Vasotec) and doxazosin (Cardura) and counseled her to restrict her fluid intake 2 hours before bedtime. She was also referred to a gynecologist, who found a moderate degree of cystocele and treated her with a collagen injection. Her nocturia improved significantly.
- Abrams P. Nocturia: the major problem in patients with lower urinary tract symptoms suggestive of benign prostatic obstruction (LUTS/BPO). Eur Urol Suppl 2005; 3(6):8–16.
- Bosch JL, Weiss J. The prevalence and causes of nocturia. J Urol 2010; 184:440–446.
- Tikkinen KA, Johnson TM, Tammela TL, et al. Nocturia frequency, bother, and quality of life: how often is too often? A population-based study in Finland. Eur Urol 2010; 57:488–496.
- Klingler HG, Heidler H, Madersbacher H, Primus G. Nocturia: an Austrian study on the multifactorial etiology of this symptom. Neurourol Urodyn 2009; 28:427–431.
- Mariappan P, Turner KJ, Sothilingam S, Rajan P, Sundram M, Steward LH. Nocturia, nocturia indices and variables from frequency-volume charts are significantly different in Asian and Caucasian men with lower urinary tract symptoms: a prospective comparison study. BJU Int 2007; 100:332–336.
- Asplund R. Mortality in the elderly in relation to nocturnal micturition. BJU Int 1999; 84:297–301.
- Booth J, O’Neil K, Lawrence M, et al. Advancing community nursing practice: detecting and managing nocturia in community-living older people. Final report. 2008. Queens Nursing Institute, Scotland. http://www.qnis.co.uk/documents/Item3.2-finalreportnocturiav2.doc. Accessed 8/22/11
- Kawauchi A, Tanaka Y, Soh J, Ukimura O, Kojima M, Miki T. Causes of nocturnal urinary frequency and reasons for its increase with age in healthy older men. J Urol 2000; 163:81–84.
- Madersbacher S, Pycha A, Schatzl G, Mian C, Klingler CH, Marberger M. The aging lower urinary tract: a comparative urodynamics study of men and women. Urology 1998; 51:206–212.
- Elbedawi A, Yalla SV, Resnick NM. Structural basis of geriatric voiding dysfunction. I: methods of a prospective ultra structural/urodynamics study and an overview of the findings. J Urol 1993; 150:1650–1656.
- Weiss JP, Blaivas JG, Jones M, Wang JT, Guan Z; 037 Study Group. Age related pathogenesis of nocturia in patients with overactive bladder. J Urol 2007; 178:548–551.
- Natsume O, Kaneko Y, Hirayama A, Fujimoto K, Hirao Y. Fluid control in elderly patients with nocturia. Int J Urol 2009; 16:307–313.
- Asplund R. Pharmacotherapy for nocturia in the elderly patient. Drugs Aging 2007; 24:325–343.
- Sugaya K, Nishijima S, Oda M, Owan T, Miyazato M, Ogawa Y. Biochemical and body composition analysis of nocturia in the elderly. Neurourol Urodyn 2008; 27:205–211.
- Shiri R, Hakama M, Häkkinen J, et al. The effects of lifestyle factors on the incidence of nocturia. J Urol 2008; 180:2059–2062.
- Asplund R. Obesity in elderly people with nocturia: cause or consequence? Can J Urol 2007; 14:3424–3428.
- Hardin-Fanning F, Gross JC. The effects of sleep-disordered breathing symptoms on voiding patterns in stroke patients. Urol Nurs 2007; 27:221–229.
- Foley DJ, Vitiello MV, Bliwise DL, Ancoli-Israel S, Monjan AA, Walsh JK. Frequent napping is associated with excessive daytime sleepiness, depression, pain, and nocturia in older adults: findings from the National Sleep Foundation ‘2003 Sleep in America’ Poll. Am J Geriatr Psychiatry 2007; 15:344–350.
- Häkkinen JT, Shiri R, Koskimäki J, Tammela TL, Auvinen A, Hakama M. Depressive symptoms increase the incidence of nocturia: Tampere Aging Male Urologic Study (TAMUS). J Urol 2008; 179:1897–1901.
- Natsume O, Kaneko Y, Hirayama A, Fujimoto K, Hirao Y. Fluid control in elderly patients with nocturia. Int J Urol 2009; 16:307–313.
- Kujubu DA, Aboseif SR. An overview of nocturia and the syndrome of nocturnal polyuria in the elderly. Nat Clin Pract Nephrol 2008; 4:426–435.
- Hashimoto M, Imamura T, Tanimukai S, Kazui H, Mori E. Urinary incontinence: an unrecognized adverse effect with donepezil (letter). Lancet 2000; 356:568.
- Wagg A, Cohen M. Medical therapy for the overactive bladder in the elderly. Age Ageing 2002; 31:241–246.
- Williams G, Donaldson RM. Nifedipine and nocturia. Lancet 1986: 1:738.
- Yoshimura K, Kamoto T, Tsukamoto T, Oshiro K, Kinukawa N, Ogawa O. Seasonal alterations in nocturia and other storage symptoms in three Japanese communities. Urology 2007; 69:864–870.
- Asplund R. Hip fractures, nocturia, and nocturnal polyuria in the elderly. Arch Gerontol Geriatr 2006; 43:319–326.
- Stewart RB, Moore MT, May FE, Marks RG, Hale WE. Nocturia: a risk factor for falls in the elderly. J Am Geriatr Soc 1992; 40:1217–1220.
- van Balen R, Steyerberg EW, Polder JJ, Ribbers TL, Habbema JD, Cools HJ. Hip fracture in elderly patients: outcomes for function, quality of life, and type of residence. Clin Orthop Relat Res 2001; 390:232–243.
- Mock LL, Parmelee PA, Kutner N, Scott J, Johnson TM. Content validation of symptom-specific nocturia quality-of-life instrument developed in men: issues expressed by women, as well as men. Urology 2008; 72:736–742.
- Holm-Larsen T, Weiss J, Langkilde LK. Economic burden of nocturia in the US adult population. J Urol Suppl 2010; 100:332–336.
- Ali A, Snape J. Nocturia in older people: a review of causes, consequences, assessment, and management. Int J Clin Pract 2004; 58:366–373.
- Miu DK, Lau S, Szeto SS. Etiology and predictors of urinary incontinence and its effect on quality of life. Geriatr Gerontol Int 2010; 10:177–182.
- Tibaek S, Gard G, Klarskov P, Iversen HK, Dehlendorff C, Jensen R. Prevalence of lower urinary tract symptoms (LUTS) in stroke patients: a cross-sectional, clinical survey. Neurourol Urodyn 2008; 27:763–771.
- Bliwise DL, Foley DJ, Vitiello MV, Ansari FP, Ancoli-Israel S, Walsh JK. Nocturia and disturbed sleep in the elderly. Sleep Med 2009; 10:540–548.
- Asplund R. Nocturia: consequences for sleep and daytime activities and associated risks. Eur Urol Suppl 2005; 3(6):24–32.
- Hernández C, Estivill E, Prieto M, Badia X. Nocturia in Spanish patients with lower urinary tract symptoms suggestive of benign prostatic hyperplasia (LUTS/BPH). Curr Med Res Opin 2008; 24:1033–1038.
- Pollak CP, Perlick D, Linsner JP, Wenston J, Hsieh F. Sleep problems in the community elderly as predictors of death and nursing home placement. J Community Health 1990; 15:123–135.
- Bursztyn M, Jacob J, Stessman J. Usefulness of nocturia as a mortality risk factor for coronary heart disease among persons born in 1920 or 1921. Am J Cardiol 2006; 98:1311–1315.
- Appell RA, Sand PK. Nocturia: etiology, diagnosis, and treatment. Neurourol Urodyn 2008; 27:34–39.
- Weiss JP, Blaivas JG, Stember DS, Chaikin DC. Evaluation of the etiology of nocturia in men: the nocturia and nocturnal bladder capacity indices. Neurourol Urodyn 1999; 18:559–565.
- Jaffe JS, Ginsberg PC, Silverberg DM, Harkaway RC. The need for voiding diaries in the evaluation of men with nocturia. J Am Osteopath Assoc 2002; 102:261–265.
- Yoshimura K, Terai A. Classification and distribution of symptomatic nocturia with special attention to duration of time in bed: a patient-based study. BJU Int 2005; 95:1259–1262.
- Polderman KH. Mechanisms of action, physiological effects, and complications of hypothermia. Crit Care Med 2009; 37:S186–S202.
- Soda T, Masui K, Okuno H, Terai A, Ogawa O, Yoshimura K. Efficacy of nondrug lifestyle measures for the treatment of nocturia. J Urol 2010; 184:1000–1004.
- Flynn MK, Amundsen CL, Perevich M, Liu F, Webster GD. Outcome of a randomized, double-blind, placebo controlled trial of botulinum A toxin for refractory overactive bladder. J Urol 2009; 181:2608–2615.
- Asplund R, Sundberg B, Bengtsson P. Desmopressin for the treatment of nocturnal polyuria in the elderly: a dose titration study. Br J Urol 1998; 82:642–646.
- Abrams P, Mattiasson A, Lose GR, Robertson GL. The role of desmopressin treatment in adult nocturia. BJU Int 2002; 90:32–36.
- Andersson K. Treatment of the overactive bladder syndrome and detrusor overactivity with antimuscarinic drugs. Continence 2005; 1:1–8.
- Reynard JM, Cannon A, Yang Q, Abrams P. A novel therapy for nocturnal polyuria: a double-blind randomized trial of frusemide against placebo. Br J Urol 1998; 81:215–218.
- Cho MC, Ku JH, Paick JS. Alpha-blocker plus diuretic combination therapy as second-line treatment for nocturia in men with LUTS: a pilot study. Urology 2009; 73:549–553.
- Fu FG, Lavery HJ, Wu DL. Reducing nocturia in the elderly: a randomized placebo-controlled trial of staggered furosemide and desmopressin. Neurourol Urodyn 2011; 30:312–316.
- Falahatkar S, Mokhtari G, Pourezza F, Asgari SA, Kamran AN. Celecoxib for treatment of nocturia caused by benign prostatic hyperplasia: a prospective, randomized, double-blind, placebo-controlled study. Urology 2008; 72:813–816.
- Addla SK, Adeyoju AB, Neilson D, O’Reilly P. Diclofenac for treatment of nocturia caused by nocturnal polyuria: a prospective, randomised, double-blind, placebo-controlled crossover study. Eur Urol 2006; 49:720–725.
- Saito M, Kawatani M, Kinoshita Y, Satoh K, Miyagawa I. Effectiveness of an anti-inflammatory drug, loxoprofen, for patients with nocturia. Int J Urol 2005; 12:779–782.
Nocturia is common, but elderly patients infrequently volunteer this complaint, and even when they do, some clinicians may dismiss it as simply a part of aging. Nevertheless, nocturia causes significant distress and impairment of quality of life. It is associated with very serious consequences such as depression, social isolation, and a higher risk of death.
In this article, we review the concepts behind frequent nighttime voiding in older adults. We will start with two case scenarios to aid in understanding these concepts; near the end of the article, we will discuss the most appropriate management strategies for these two patients.
CASE SCENARIOS
Case 1: An 82-year-old man with fatigue
An 82-year-old obese white man with a history of hypertension, diabetes, and benign prostatic hyperplasia comes in to see his primary care provider, complaining of fatigue. He wakes up tired and has difficulty completing his daytime tasks. He gets up every 1 to 2 hours at night to urinate and has slow urinary flow and a feeling of incomplete bladder emptying.
See related patient education material
He says his wife has been increasingly bothered by his loud snoring. Recently, he had a car accident when he fell asleep while driving.
Case 2: An 85-year-old woman with incontinence
An 85-year-old white woman is in her family physician’s office with a primary complaint of waking up at least four times at night to urinate, and often ends up soaking her bed or adult diapers. She is bothered by urinary urgency and frequency during the day as well. She denies dysuria and hematuria.
She has a history of hypertension and urinary incontinence, and she has seven children. Her current medications are diltiazem (Cardizem), metoprolol (Toprol), and oxybutynin (Ditropan).
In these two cases, what would account for the nocturia? What would be the best way to help these patients?
THE NORM, NOT THE EXCEPTION
Although nocturia is defined as an awakening by the need to urinate even once in a night, many experts consider that it begins to be clinically significant only when the patient voids at least twice during the night.1
In older adults, nocturia is the norm rather than the exception. Studies done between 1990 and 2009 found that 68.9% to 93% of men age 70 and older get up at least once a night to void. The prevalence in women is somewhat lower, at 74.1% to 77.1%.2 Clinically significant nocturia is present in a majority of the elderly: more than 60% of both men and women.3
An Austrian study4 reported that elderly men got up to urinate a mean of 2.8 times per night, while women got up significantly more often—3.1 times. Women were also bothered more by this symptom, and their quality of life was significantly more decreased.
In another study,5 whites had a significantly higher nocturia ratio (ratio of nighttime urine volume to the 24-hour urine volume) than Asians. Asians, on the other hand, had a significantly higher nocturnal bladder capacity index than whites. (See below for definitions of the various indices of nocturia.) This information implies that nocturia may be a more prominent problem for elderly whites than for other racial groups.
In an epidemiologic study in Sweden,6 the death rate was as much as twice as high in both men and women who had three or more nocturnal voids, even after taking into account the influence of cardiac disease, diabetes mellitus, and stroke.
If nocturia is not addressed in the physician-patient encounter, patients may try to “self-manage” it by restricting their fluid intake or by limiting their social exposure,7 with limited success and with unwanted social isolation.
WHAT CAUSES NOCTURIA?
Advancing age is primary among these factors. Age-related structural changes in the urinary system include decreased functional bladder capacity, a decreased maximum urinary flow rate,6 a decreased ability to postpone urination,8 and an age-related increase in postvoiding residual urine volume.9 The aging kidney is also less able to concentrate urine. Also implicated are histologic changes in the detrusor muscle10 that lead to diminished bladder compliance and, together with detrusor overactivity, result in increased urinary frequency.
Nocturnal polyuria or nocturnal urine overproduction is common in patients with nocturia.11
Although the pathophysiology of nocturnal polyuria is still unclear, some investigators believe that low levels of antidiuretic hormone (ADH) at night are involved, reflecting an alteration in the circadian rhythm seen in diurnal plasma arginine vasopressin levels.12 In patients with nocturnal polyuria, ADH levels drop to very low or undetectable levels at night, which increases nocturnal urine output. In some extreme cases, the low to absent levels of ADH increase nocturnal voiding to 85% of the total 24-hour urine volume.13
Other causes of nocturnal polyuria include mobilization of fluids in patients with edema,14 and autonomic dysfunction. Other biochemical changes that contribute to nocturia include a decrease in nighttime plasma melatonin levels, an increase in nighttime plasma catecholamine levels, an increase in nighttime plasma natriuretic peptide levels, an increase in blood pressure, and an increase in total urine volume.15
A decreased ability to store urine also leads to nocturia. This is caused by decreased nocturnal bladder capacity, more irritative symptoms, and comorbid conditions such as overactive bladder, pelvic floor laxity resulting in pelvic organ prolapse, and, in men, benign prostatic hyperplasia.
Neural inputs to the bladder can also be impaired, as in patients who have diabetes mellitus or spinal stenosis, leading to chronic urinary retention, detrusor dysfunction, nocturia, and incontinence.
WHICH PATIENTS ARE AT RISK?
Obesity is associated with a higher incidence of moderate to severe nocturia.15 Studies have shown that the higher the body mass index, the greater the number of nighttime voids, especially in women.16
Habitually eating at night, with poor daytime appetite, is shown to be associated with increased nighttime diuresis.
Obstructive sleep apnea17 and untreated depressive symptoms such as frequent napping18 are also associated with moderate to severe nocturia.19
Higher systolic blood pressures are associated with more urine production at night. Plasma ADH regulation is also altered, which contributes to nocturnal polyuria.21
Other comorbid conditions associated with nocturia include recurrent cystitis, lung disease, congestive heart failure, neurodegenerative conditions (eg, Alzheimer disease and parkinsonism), and chronic kidney disease.21
Drugs associated with nocturia include cholinesterase inhibitors (for dementia),22 beta-blockers,23 and calcium channel blockers.24
Lifestyle factors. Alcohol and coffee have shown either no or only a mild diuretic effect. Smoking has not been shown to be associated with nocturia.15
Seasonal differences also exist, with increased frequency of nocturia in the winter.25
WHAT ARE THE CLINICAL CONSEQUENCES OF NOCTURIA?
Quality of life can be profoundly affected, and if nocturia is left untreated, it may lead to morbidity and even death. Elderly patients may feel simultaneously debilitated, frustrated, distressed, and puzzled. Nocturia may also increase their fear of falling and may negatively affect personal relationships.26
Falls, injuries. Nocturia exposes elderly patients to injuries such as hip fractures due to falling, significantly increasing the incidence of this injury.26 This occurs as elderly patients get up from bed and walk to the bathroom to void.27 In addition, during the day, superficial and fragmented sleep leads to daytime sleepiness and impaired perception and balance, also increasing the risk of falls.28 The complications of immobility and the need for surgery in many cases lead to debility, increased risk of infections, decubitus ulcers, and death. The risk of hip fractures can lead elderly patients with nocturia to associate this symptom with a fear of falling and can alter their concept of their own age (“Nocturia makes me feel old”),29 further diminishing quality of life.
The estimated medical cost of nocturia-associated falls in the elderly is about $1.5 billion per year, part of the $61 billion in lost productivity due to nocturia in adults.30
Long-term complications (eg, debilitation, poor sleep, obesity, decreased energy), increase the overall mortality rate, especially in patients who report voiding more than three times per night.29 Elderly patients with nocturia also have a greater need for emergency care.31
Nocturia also complicates other comorbid conditions, such as dementia, which increases the risk of urinary incontinence.32 In patients who have had a stroke, nocturia is the most frequent lower urinary tract symptom, and represents a major impact on daily life.33
Sleep disturbance is another important consequence. In one survey,34 nocturia was cited as a cause of poor sleep four times more often than the cause cited next most often, ie, pain. Because the elderly patient is awakened from sleep numerous times throughout the night, nocturia leads to more fatigue,35 lower energy levels, and poorer quality of sleep.36 Depression may be linked to poor sleep, as men with two or more nocturnal episodes were shown to be six times more likely to experience depression.
The patient is not the only person who loses sleep: so do the patient’s family members or sleeping partner.7 It is therefore not surprising that sleep disruption caused by nocturia has been cited as a principal reason for admitting older relatives to care homes.37
The risk of death is higher for elderly patients with coronary heart disease if they have nocturia. The causative link is the hemodynamic changes (increases in blood pressure and heart rate) that accompany awakening and arising, which may cause cardiovascular strain and lead to cardiovascular events. The 12-year survival rate has been shown to be significantly lower in patients with nighttime voiding, making nocturia a highly significant independent predictor of death in coronary heart disease patients.38
HOW TO EVALUATE AN OLDER ADULT WHO PRESENTS WITH NOCTURIA
A thorough history and physical examination are crucial in diagnosing nocturia. The goal is to identify any treatable underlying condition, such as diabetes mellitus, obstructive sleep apnea, diabetes insipidus, overactive bladder, benign prostatic hyperplasia, urinary tract infection, and congestive heart failure. Laboratory tests and imaging studies can help rule out these underlying conditions.
Other important facets in the history that must be elicited are medication use, patterns of fluid intake, and a history of other urinary complaints.39
A voiding diary and indices of nocturia
A voiding diary is extremely useful and should be used whenever possible. Episodes of incontinence, time of voids, volume voided, and frequency and volume of fluid intake are recorded. From the raw data, one can determine the following:
Total nocturnal urine volume, ie, the sum volume of the nighttime voids
Maximum voided volume, ie, the largest single recorded volume voided in a 24-hour period
Nocturia index, ie, the total nocturnal urine volume divided by the maximum voided volume. A nocturia index greater than 1 shows that nocturnal urine production is greater than the functional bladder capacity. Clinically significant nocturia is observed in patients with a nocturia index of 2.1 or greater.
Nocturnal polyuria index, ie, total nocturnal urine volume divided by the 24-hour urine output. A nocturnal polyuria index higher than 33% implies nocturnal polyuria.40
Nocturnal bladder capacity index, ie, the actual number of nightly voids minus the predicted number of nightly voids, which in turn is calculated as the nocturia index minus 1.
It is especially important to encourage patients to make a voiding diary, as some patients may find this cumbersome, and compliance can be low unless its importance is emphasized. A diary over 7 days usually gives meaningful data. The results from the diary typically confirm the presence of nocturnal polyuria or a decrease in bladder capacity, influencing management.41
WHAT ARE THE TREATMENT OPTIONS?
Therapy must be directed at the primary cause, addressing any underlying conditions that can contribute to nocturia. Examples39:
- Tight control of blood sugar for patients with diabetes mellitus
- Treatment of diabetes insipidus
- Referral for patients with primary polydipsia
- Management of hypercalcemia and hypokalemia
- A survey of medications
- Treatment of infections.
Nonpharmacologic measures
Tailored behavioral therapy can also be instituted, but the patient needs to have realistic expectations, as these measures are rarely effective alone.
Avoiding nighttime fluid intake, including alcohol and caffeine, has shown promise.
Wearing compression stockings and elevating the legs in the afternoon decrease the retention of fluid that otherwise would return to the circulation at night.
Identifying and eliminating nighttime influences that disturb sleep has variable efficacy. The use of continuous positive airway pressure helps to treat sleep apnea. Moderate exercise, reducing nonsleep time spent in bed,42 and sleeping in a warm bed43 to decrease cold diuresis have also been shown to improve sleep quality.44 Patients with nocturia may have a disrupted circadian rhythm, and phototherapy may help resynchronize the diurnal rhythm and melatonin secretion.
Pharmacotherapy
Pharmacotherapy of nocturia includes desmopressin (DDAVP) to manage nocturnal polyuria and antimuscarinic agents to manage the patient’s decreased ability to store urine. Alpha-blockers such as tamsulosin (Flomax) and 5-alpha-reductase inhibitors such as finasteride (Proscar) are used for men with benign prostatic hyperplasia. Novel and second-line therapies include diuretics such as furosemide (Lasix), cyclooxygenase-2 inhibitors, as well as botulinum toxin injected directly into the detrusor muscle for overactive bladder.45
Desmopressin in a low oral dose (0.1–0.4 mg) at bedtime can be initiated and the response assessed. Patients with nocturnal polyuria and disorders of the vasopressin system have been found to be more sensitive to desmopressin therapy.46 Fluid retention and hyponatremia can complicate therapy, and desmopressin must be avoided in patients with liver cirrhosis, renal failure, or congestive heart failure.47
Antimuscarinic agents are effective for patients who have lower urinary tract symptoms and for those with a diminished ability to store urine. They act by decreasing both voluntary and involuntary bladder contractions by blocking muscarinic receptors on the detrusor muscle. This reduces the bladder’s ability to contract and the urge to urinate, thereby increasing bladder capacity.48 These agents include oxybutynin (Ditropan), tolterodine (Detrol), solifenacin (Vesicare), and propiverine (not available in the United States).
Diuretics are being used as second-line agents or for patients who cannot tolerate desmopressin.49 Hydrochlorothiazide is taken 8 hours before bedtime to prevent water accumulation before the early sleeping hours.50 Furosemide has also led to a reduction in the mean number of nocturnal voids.51 The effect of these drugs on nocturia are especially beneficial to patients with concomitant hypertension or cardiovascular disease.
Cyclo-oxygenase-2 inhibitors such as celecoxib (Celebrex)52 and other nonsteroidal anti-inflammatory drugs such as diclofenac (Voltaren, others)53 and loxoprofen (not available in the United States)54 have been shown to decrease urine production, detrusor muscle tone, and inflammation, especially in men with benign prostatic hyperplasia.
Botulinum toxin has been used, usually in patients refractory to first-line treatment.44
Table 4 summarizes the treatment strategies for nocturia.
CASES REVISITED
The first patient described above has nocturia caused by several concomitant diseases, ie, hypertension, diabetes, benign prostatic hyperplasia, and obstructive sleep apnea. In addition to controlling his blood pressure and blood sugar, his primary care provider referred him to a pulmonologist, who confirmed obstructive sleep apnea with polysomnography and prescribed nightly use of a continuous positive airway pressure apparatus. A few weeks later, the patient’s nocturia had improved significantly, and his level of fatigue had decreased.
Apart from hypertension, the second patient’s nocturia was mostly attributed to her existing urinary incontinence. Recognizing that her current antihypertensive regimen may worsen nocturia, her family physician changed it to enalapril (Vasotec) and doxazosin (Cardura) and counseled her to restrict her fluid intake 2 hours before bedtime. She was also referred to a gynecologist, who found a moderate degree of cystocele and treated her with a collagen injection. Her nocturia improved significantly.
Nocturia is common, but elderly patients infrequently volunteer this complaint, and even when they do, some clinicians may dismiss it as simply a part of aging. Nevertheless, nocturia causes significant distress and impairment of quality of life. It is associated with very serious consequences such as depression, social isolation, and a higher risk of death.
In this article, we review the concepts behind frequent nighttime voiding in older adults. We will start with two case scenarios to aid in understanding these concepts; near the end of the article, we will discuss the most appropriate management strategies for these two patients.
CASE SCENARIOS
Case 1: An 82-year-old man with fatigue
An 82-year-old obese white man with a history of hypertension, diabetes, and benign prostatic hyperplasia comes in to see his primary care provider, complaining of fatigue. He wakes up tired and has difficulty completing his daytime tasks. He gets up every 1 to 2 hours at night to urinate and has slow urinary flow and a feeling of incomplete bladder emptying.
See related patient education material
He says his wife has been increasingly bothered by his loud snoring. Recently, he had a car accident when he fell asleep while driving.
Case 2: An 85-year-old woman with incontinence
An 85-year-old white woman is in her family physician’s office with a primary complaint of waking up at least four times at night to urinate, and often ends up soaking her bed or adult diapers. She is bothered by urinary urgency and frequency during the day as well. She denies dysuria and hematuria.
She has a history of hypertension and urinary incontinence, and she has seven children. Her current medications are diltiazem (Cardizem), metoprolol (Toprol), and oxybutynin (Ditropan).
In these two cases, what would account for the nocturia? What would be the best way to help these patients?
THE NORM, NOT THE EXCEPTION
Although nocturia is defined as an awakening by the need to urinate even once in a night, many experts consider that it begins to be clinically significant only when the patient voids at least twice during the night.1
In older adults, nocturia is the norm rather than the exception. Studies done between 1990 and 2009 found that 68.9% to 93% of men age 70 and older get up at least once a night to void. The prevalence in women is somewhat lower, at 74.1% to 77.1%.2 Clinically significant nocturia is present in a majority of the elderly: more than 60% of both men and women.3
An Austrian study4 reported that elderly men got up to urinate a mean of 2.8 times per night, while women got up significantly more often—3.1 times. Women were also bothered more by this symptom, and their quality of life was significantly more decreased.
In another study,5 whites had a significantly higher nocturia ratio (ratio of nighttime urine volume to the 24-hour urine volume) than Asians. Asians, on the other hand, had a significantly higher nocturnal bladder capacity index than whites. (See below for definitions of the various indices of nocturia.) This information implies that nocturia may be a more prominent problem for elderly whites than for other racial groups.
In an epidemiologic study in Sweden,6 the death rate was as much as twice as high in both men and women who had three or more nocturnal voids, even after taking into account the influence of cardiac disease, diabetes mellitus, and stroke.
If nocturia is not addressed in the physician-patient encounter, patients may try to “self-manage” it by restricting their fluid intake or by limiting their social exposure,7 with limited success and with unwanted social isolation.
WHAT CAUSES NOCTURIA?
Advancing age is primary among these factors. Age-related structural changes in the urinary system include decreased functional bladder capacity, a decreased maximum urinary flow rate,6 a decreased ability to postpone urination,8 and an age-related increase in postvoiding residual urine volume.9 The aging kidney is also less able to concentrate urine. Also implicated are histologic changes in the detrusor muscle10 that lead to diminished bladder compliance and, together with detrusor overactivity, result in increased urinary frequency.
Nocturnal polyuria or nocturnal urine overproduction is common in patients with nocturia.11
Although the pathophysiology of nocturnal polyuria is still unclear, some investigators believe that low levels of antidiuretic hormone (ADH) at night are involved, reflecting an alteration in the circadian rhythm seen in diurnal plasma arginine vasopressin levels.12 In patients with nocturnal polyuria, ADH levels drop to very low or undetectable levels at night, which increases nocturnal urine output. In some extreme cases, the low to absent levels of ADH increase nocturnal voiding to 85% of the total 24-hour urine volume.13
Other causes of nocturnal polyuria include mobilization of fluids in patients with edema,14 and autonomic dysfunction. Other biochemical changes that contribute to nocturia include a decrease in nighttime plasma melatonin levels, an increase in nighttime plasma catecholamine levels, an increase in nighttime plasma natriuretic peptide levels, an increase in blood pressure, and an increase in total urine volume.15
A decreased ability to store urine also leads to nocturia. This is caused by decreased nocturnal bladder capacity, more irritative symptoms, and comorbid conditions such as overactive bladder, pelvic floor laxity resulting in pelvic organ prolapse, and, in men, benign prostatic hyperplasia.
Neural inputs to the bladder can also be impaired, as in patients who have diabetes mellitus or spinal stenosis, leading to chronic urinary retention, detrusor dysfunction, nocturia, and incontinence.
WHICH PATIENTS ARE AT RISK?
Obesity is associated with a higher incidence of moderate to severe nocturia.15 Studies have shown that the higher the body mass index, the greater the number of nighttime voids, especially in women.16
Habitually eating at night, with poor daytime appetite, is shown to be associated with increased nighttime diuresis.
Obstructive sleep apnea17 and untreated depressive symptoms such as frequent napping18 are also associated with moderate to severe nocturia.19
Higher systolic blood pressures are associated with more urine production at night. Plasma ADH regulation is also altered, which contributes to nocturnal polyuria.21
Other comorbid conditions associated with nocturia include recurrent cystitis, lung disease, congestive heart failure, neurodegenerative conditions (eg, Alzheimer disease and parkinsonism), and chronic kidney disease.21
Drugs associated with nocturia include cholinesterase inhibitors (for dementia),22 beta-blockers,23 and calcium channel blockers.24
Lifestyle factors. Alcohol and coffee have shown either no or only a mild diuretic effect. Smoking has not been shown to be associated with nocturia.15
Seasonal differences also exist, with increased frequency of nocturia in the winter.25
WHAT ARE THE CLINICAL CONSEQUENCES OF NOCTURIA?
Quality of life can be profoundly affected, and if nocturia is left untreated, it may lead to morbidity and even death. Elderly patients may feel simultaneously debilitated, frustrated, distressed, and puzzled. Nocturia may also increase their fear of falling and may negatively affect personal relationships.26
Falls, injuries. Nocturia exposes elderly patients to injuries such as hip fractures due to falling, significantly increasing the incidence of this injury.26 This occurs as elderly patients get up from bed and walk to the bathroom to void.27 In addition, during the day, superficial and fragmented sleep leads to daytime sleepiness and impaired perception and balance, also increasing the risk of falls.28 The complications of immobility and the need for surgery in many cases lead to debility, increased risk of infections, decubitus ulcers, and death. The risk of hip fractures can lead elderly patients with nocturia to associate this symptom with a fear of falling and can alter their concept of their own age (“Nocturia makes me feel old”),29 further diminishing quality of life.
The estimated medical cost of nocturia-associated falls in the elderly is about $1.5 billion per year, part of the $61 billion in lost productivity due to nocturia in adults.30
Long-term complications (eg, debilitation, poor sleep, obesity, decreased energy), increase the overall mortality rate, especially in patients who report voiding more than three times per night.29 Elderly patients with nocturia also have a greater need for emergency care.31
Nocturia also complicates other comorbid conditions, such as dementia, which increases the risk of urinary incontinence.32 In patients who have had a stroke, nocturia is the most frequent lower urinary tract symptom, and represents a major impact on daily life.33
Sleep disturbance is another important consequence. In one survey,34 nocturia was cited as a cause of poor sleep four times more often than the cause cited next most often, ie, pain. Because the elderly patient is awakened from sleep numerous times throughout the night, nocturia leads to more fatigue,35 lower energy levels, and poorer quality of sleep.36 Depression may be linked to poor sleep, as men with two or more nocturnal episodes were shown to be six times more likely to experience depression.
The patient is not the only person who loses sleep: so do the patient’s family members or sleeping partner.7 It is therefore not surprising that sleep disruption caused by nocturia has been cited as a principal reason for admitting older relatives to care homes.37
The risk of death is higher for elderly patients with coronary heart disease if they have nocturia. The causative link is the hemodynamic changes (increases in blood pressure and heart rate) that accompany awakening and arising, which may cause cardiovascular strain and lead to cardiovascular events. The 12-year survival rate has been shown to be significantly lower in patients with nighttime voiding, making nocturia a highly significant independent predictor of death in coronary heart disease patients.38
HOW TO EVALUATE AN OLDER ADULT WHO PRESENTS WITH NOCTURIA
A thorough history and physical examination are crucial in diagnosing nocturia. The goal is to identify any treatable underlying condition, such as diabetes mellitus, obstructive sleep apnea, diabetes insipidus, overactive bladder, benign prostatic hyperplasia, urinary tract infection, and congestive heart failure. Laboratory tests and imaging studies can help rule out these underlying conditions.
Other important facets in the history that must be elicited are medication use, patterns of fluid intake, and a history of other urinary complaints.39
A voiding diary and indices of nocturia
A voiding diary is extremely useful and should be used whenever possible. Episodes of incontinence, time of voids, volume voided, and frequency and volume of fluid intake are recorded. From the raw data, one can determine the following:
Total nocturnal urine volume, ie, the sum volume of the nighttime voids
Maximum voided volume, ie, the largest single recorded volume voided in a 24-hour period
Nocturia index, ie, the total nocturnal urine volume divided by the maximum voided volume. A nocturia index greater than 1 shows that nocturnal urine production is greater than the functional bladder capacity. Clinically significant nocturia is observed in patients with a nocturia index of 2.1 or greater.
Nocturnal polyuria index, ie, total nocturnal urine volume divided by the 24-hour urine output. A nocturnal polyuria index higher than 33% implies nocturnal polyuria.40
Nocturnal bladder capacity index, ie, the actual number of nightly voids minus the predicted number of nightly voids, which in turn is calculated as the nocturia index minus 1.
It is especially important to encourage patients to make a voiding diary, as some patients may find this cumbersome, and compliance can be low unless its importance is emphasized. A diary over 7 days usually gives meaningful data. The results from the diary typically confirm the presence of nocturnal polyuria or a decrease in bladder capacity, influencing management.41
WHAT ARE THE TREATMENT OPTIONS?
Therapy must be directed at the primary cause, addressing any underlying conditions that can contribute to nocturia. Examples39:
- Tight control of blood sugar for patients with diabetes mellitus
- Treatment of diabetes insipidus
- Referral for patients with primary polydipsia
- Management of hypercalcemia and hypokalemia
- A survey of medications
- Treatment of infections.
Nonpharmacologic measures
Tailored behavioral therapy can also be instituted, but the patient needs to have realistic expectations, as these measures are rarely effective alone.
Avoiding nighttime fluid intake, including alcohol and caffeine, has shown promise.
Wearing compression stockings and elevating the legs in the afternoon decrease the retention of fluid that otherwise would return to the circulation at night.
Identifying and eliminating nighttime influences that disturb sleep has variable efficacy. The use of continuous positive airway pressure helps to treat sleep apnea. Moderate exercise, reducing nonsleep time spent in bed,42 and sleeping in a warm bed43 to decrease cold diuresis have also been shown to improve sleep quality.44 Patients with nocturia may have a disrupted circadian rhythm, and phototherapy may help resynchronize the diurnal rhythm and melatonin secretion.
Pharmacotherapy
Pharmacotherapy of nocturia includes desmopressin (DDAVP) to manage nocturnal polyuria and antimuscarinic agents to manage the patient’s decreased ability to store urine. Alpha-blockers such as tamsulosin (Flomax) and 5-alpha-reductase inhibitors such as finasteride (Proscar) are used for men with benign prostatic hyperplasia. Novel and second-line therapies include diuretics such as furosemide (Lasix), cyclooxygenase-2 inhibitors, as well as botulinum toxin injected directly into the detrusor muscle for overactive bladder.45
Desmopressin in a low oral dose (0.1–0.4 mg) at bedtime can be initiated and the response assessed. Patients with nocturnal polyuria and disorders of the vasopressin system have been found to be more sensitive to desmopressin therapy.46 Fluid retention and hyponatremia can complicate therapy, and desmopressin must be avoided in patients with liver cirrhosis, renal failure, or congestive heart failure.47
Antimuscarinic agents are effective for patients who have lower urinary tract symptoms and for those with a diminished ability to store urine. They act by decreasing both voluntary and involuntary bladder contractions by blocking muscarinic receptors on the detrusor muscle. This reduces the bladder’s ability to contract and the urge to urinate, thereby increasing bladder capacity.48 These agents include oxybutynin (Ditropan), tolterodine (Detrol), solifenacin (Vesicare), and propiverine (not available in the United States).
Diuretics are being used as second-line agents or for patients who cannot tolerate desmopressin.49 Hydrochlorothiazide is taken 8 hours before bedtime to prevent water accumulation before the early sleeping hours.50 Furosemide has also led to a reduction in the mean number of nocturnal voids.51 The effect of these drugs on nocturia are especially beneficial to patients with concomitant hypertension or cardiovascular disease.
Cyclo-oxygenase-2 inhibitors such as celecoxib (Celebrex)52 and other nonsteroidal anti-inflammatory drugs such as diclofenac (Voltaren, others)53 and loxoprofen (not available in the United States)54 have been shown to decrease urine production, detrusor muscle tone, and inflammation, especially in men with benign prostatic hyperplasia.
Botulinum toxin has been used, usually in patients refractory to first-line treatment.44
Table 4 summarizes the treatment strategies for nocturia.
CASES REVISITED
The first patient described above has nocturia caused by several concomitant diseases, ie, hypertension, diabetes, benign prostatic hyperplasia, and obstructive sleep apnea. In addition to controlling his blood pressure and blood sugar, his primary care provider referred him to a pulmonologist, who confirmed obstructive sleep apnea with polysomnography and prescribed nightly use of a continuous positive airway pressure apparatus. A few weeks later, the patient’s nocturia had improved significantly, and his level of fatigue had decreased.
Apart from hypertension, the second patient’s nocturia was mostly attributed to her existing urinary incontinence. Recognizing that her current antihypertensive regimen may worsen nocturia, her family physician changed it to enalapril (Vasotec) and doxazosin (Cardura) and counseled her to restrict her fluid intake 2 hours before bedtime. She was also referred to a gynecologist, who found a moderate degree of cystocele and treated her with a collagen injection. Her nocturia improved significantly.
- Abrams P. Nocturia: the major problem in patients with lower urinary tract symptoms suggestive of benign prostatic obstruction (LUTS/BPO). Eur Urol Suppl 2005; 3(6):8–16.
- Bosch JL, Weiss J. The prevalence and causes of nocturia. J Urol 2010; 184:440–446.
- Tikkinen KA, Johnson TM, Tammela TL, et al. Nocturia frequency, bother, and quality of life: how often is too often? A population-based study in Finland. Eur Urol 2010; 57:488–496.
- Klingler HG, Heidler H, Madersbacher H, Primus G. Nocturia: an Austrian study on the multifactorial etiology of this symptom. Neurourol Urodyn 2009; 28:427–431.
- Mariappan P, Turner KJ, Sothilingam S, Rajan P, Sundram M, Steward LH. Nocturia, nocturia indices and variables from frequency-volume charts are significantly different in Asian and Caucasian men with lower urinary tract symptoms: a prospective comparison study. BJU Int 2007; 100:332–336.
- Asplund R. Mortality in the elderly in relation to nocturnal micturition. BJU Int 1999; 84:297–301.
- Booth J, O’Neil K, Lawrence M, et al. Advancing community nursing practice: detecting and managing nocturia in community-living older people. Final report. 2008. Queens Nursing Institute, Scotland. http://www.qnis.co.uk/documents/Item3.2-finalreportnocturiav2.doc. Accessed 8/22/11
- Kawauchi A, Tanaka Y, Soh J, Ukimura O, Kojima M, Miki T. Causes of nocturnal urinary frequency and reasons for its increase with age in healthy older men. J Urol 2000; 163:81–84.
- Madersbacher S, Pycha A, Schatzl G, Mian C, Klingler CH, Marberger M. The aging lower urinary tract: a comparative urodynamics study of men and women. Urology 1998; 51:206–212.
- Elbedawi A, Yalla SV, Resnick NM. Structural basis of geriatric voiding dysfunction. I: methods of a prospective ultra structural/urodynamics study and an overview of the findings. J Urol 1993; 150:1650–1656.
- Weiss JP, Blaivas JG, Jones M, Wang JT, Guan Z; 037 Study Group. Age related pathogenesis of nocturia in patients with overactive bladder. J Urol 2007; 178:548–551.
- Natsume O, Kaneko Y, Hirayama A, Fujimoto K, Hirao Y. Fluid control in elderly patients with nocturia. Int J Urol 2009; 16:307–313.
- Asplund R. Pharmacotherapy for nocturia in the elderly patient. Drugs Aging 2007; 24:325–343.
- Sugaya K, Nishijima S, Oda M, Owan T, Miyazato M, Ogawa Y. Biochemical and body composition analysis of nocturia in the elderly. Neurourol Urodyn 2008; 27:205–211.
- Shiri R, Hakama M, Häkkinen J, et al. The effects of lifestyle factors on the incidence of nocturia. J Urol 2008; 180:2059–2062.
- Asplund R. Obesity in elderly people with nocturia: cause or consequence? Can J Urol 2007; 14:3424–3428.
- Hardin-Fanning F, Gross JC. The effects of sleep-disordered breathing symptoms on voiding patterns in stroke patients. Urol Nurs 2007; 27:221–229.
- Foley DJ, Vitiello MV, Bliwise DL, Ancoli-Israel S, Monjan AA, Walsh JK. Frequent napping is associated with excessive daytime sleepiness, depression, pain, and nocturia in older adults: findings from the National Sleep Foundation ‘2003 Sleep in America’ Poll. Am J Geriatr Psychiatry 2007; 15:344–350.
- Häkkinen JT, Shiri R, Koskimäki J, Tammela TL, Auvinen A, Hakama M. Depressive symptoms increase the incidence of nocturia: Tampere Aging Male Urologic Study (TAMUS). J Urol 2008; 179:1897–1901.
- Natsume O, Kaneko Y, Hirayama A, Fujimoto K, Hirao Y. Fluid control in elderly patients with nocturia. Int J Urol 2009; 16:307–313.
- Kujubu DA, Aboseif SR. An overview of nocturia and the syndrome of nocturnal polyuria in the elderly. Nat Clin Pract Nephrol 2008; 4:426–435.
- Hashimoto M, Imamura T, Tanimukai S, Kazui H, Mori E. Urinary incontinence: an unrecognized adverse effect with donepezil (letter). Lancet 2000; 356:568.
- Wagg A, Cohen M. Medical therapy for the overactive bladder in the elderly. Age Ageing 2002; 31:241–246.
- Williams G, Donaldson RM. Nifedipine and nocturia. Lancet 1986: 1:738.
- Yoshimura K, Kamoto T, Tsukamoto T, Oshiro K, Kinukawa N, Ogawa O. Seasonal alterations in nocturia and other storage symptoms in three Japanese communities. Urology 2007; 69:864–870.
- Asplund R. Hip fractures, nocturia, and nocturnal polyuria in the elderly. Arch Gerontol Geriatr 2006; 43:319–326.
- Stewart RB, Moore MT, May FE, Marks RG, Hale WE. Nocturia: a risk factor for falls in the elderly. J Am Geriatr Soc 1992; 40:1217–1220.
- van Balen R, Steyerberg EW, Polder JJ, Ribbers TL, Habbema JD, Cools HJ. Hip fracture in elderly patients: outcomes for function, quality of life, and type of residence. Clin Orthop Relat Res 2001; 390:232–243.
- Mock LL, Parmelee PA, Kutner N, Scott J, Johnson TM. Content validation of symptom-specific nocturia quality-of-life instrument developed in men: issues expressed by women, as well as men. Urology 2008; 72:736–742.
- Holm-Larsen T, Weiss J, Langkilde LK. Economic burden of nocturia in the US adult population. J Urol Suppl 2010; 100:332–336.
- Ali A, Snape J. Nocturia in older people: a review of causes, consequences, assessment, and management. Int J Clin Pract 2004; 58:366–373.
- Miu DK, Lau S, Szeto SS. Etiology and predictors of urinary incontinence and its effect on quality of life. Geriatr Gerontol Int 2010; 10:177–182.
- Tibaek S, Gard G, Klarskov P, Iversen HK, Dehlendorff C, Jensen R. Prevalence of lower urinary tract symptoms (LUTS) in stroke patients: a cross-sectional, clinical survey. Neurourol Urodyn 2008; 27:763–771.
- Bliwise DL, Foley DJ, Vitiello MV, Ansari FP, Ancoli-Israel S, Walsh JK. Nocturia and disturbed sleep in the elderly. Sleep Med 2009; 10:540–548.
- Asplund R. Nocturia: consequences for sleep and daytime activities and associated risks. Eur Urol Suppl 2005; 3(6):24–32.
- Hernández C, Estivill E, Prieto M, Badia X. Nocturia in Spanish patients with lower urinary tract symptoms suggestive of benign prostatic hyperplasia (LUTS/BPH). Curr Med Res Opin 2008; 24:1033–1038.
- Pollak CP, Perlick D, Linsner JP, Wenston J, Hsieh F. Sleep problems in the community elderly as predictors of death and nursing home placement. J Community Health 1990; 15:123–135.
- Bursztyn M, Jacob J, Stessman J. Usefulness of nocturia as a mortality risk factor for coronary heart disease among persons born in 1920 or 1921. Am J Cardiol 2006; 98:1311–1315.
- Appell RA, Sand PK. Nocturia: etiology, diagnosis, and treatment. Neurourol Urodyn 2008; 27:34–39.
- Weiss JP, Blaivas JG, Stember DS, Chaikin DC. Evaluation of the etiology of nocturia in men: the nocturia and nocturnal bladder capacity indices. Neurourol Urodyn 1999; 18:559–565.
- Jaffe JS, Ginsberg PC, Silverberg DM, Harkaway RC. The need for voiding diaries in the evaluation of men with nocturia. J Am Osteopath Assoc 2002; 102:261–265.
- Yoshimura K, Terai A. Classification and distribution of symptomatic nocturia with special attention to duration of time in bed: a patient-based study. BJU Int 2005; 95:1259–1262.
- Polderman KH. Mechanisms of action, physiological effects, and complications of hypothermia. Crit Care Med 2009; 37:S186–S202.
- Soda T, Masui K, Okuno H, Terai A, Ogawa O, Yoshimura K. Efficacy of nondrug lifestyle measures for the treatment of nocturia. J Urol 2010; 184:1000–1004.
- Flynn MK, Amundsen CL, Perevich M, Liu F, Webster GD. Outcome of a randomized, double-blind, placebo controlled trial of botulinum A toxin for refractory overactive bladder. J Urol 2009; 181:2608–2615.
- Asplund R, Sundberg B, Bengtsson P. Desmopressin for the treatment of nocturnal polyuria in the elderly: a dose titration study. Br J Urol 1998; 82:642–646.
- Abrams P, Mattiasson A, Lose GR, Robertson GL. The role of desmopressin treatment in adult nocturia. BJU Int 2002; 90:32–36.
- Andersson K. Treatment of the overactive bladder syndrome and detrusor overactivity with antimuscarinic drugs. Continence 2005; 1:1–8.
- Reynard JM, Cannon A, Yang Q, Abrams P. A novel therapy for nocturnal polyuria: a double-blind randomized trial of frusemide against placebo. Br J Urol 1998; 81:215–218.
- Cho MC, Ku JH, Paick JS. Alpha-blocker plus diuretic combination therapy as second-line treatment for nocturia in men with LUTS: a pilot study. Urology 2009; 73:549–553.
- Fu FG, Lavery HJ, Wu DL. Reducing nocturia in the elderly: a randomized placebo-controlled trial of staggered furosemide and desmopressin. Neurourol Urodyn 2011; 30:312–316.
- Falahatkar S, Mokhtari G, Pourezza F, Asgari SA, Kamran AN. Celecoxib for treatment of nocturia caused by benign prostatic hyperplasia: a prospective, randomized, double-blind, placebo-controlled study. Urology 2008; 72:813–816.
- Addla SK, Adeyoju AB, Neilson D, O’Reilly P. Diclofenac for treatment of nocturia caused by nocturnal polyuria: a prospective, randomised, double-blind, placebo-controlled crossover study. Eur Urol 2006; 49:720–725.
- Saito M, Kawatani M, Kinoshita Y, Satoh K, Miyagawa I. Effectiveness of an anti-inflammatory drug, loxoprofen, for patients with nocturia. Int J Urol 2005; 12:779–782.
- Abrams P. Nocturia: the major problem in patients with lower urinary tract symptoms suggestive of benign prostatic obstruction (LUTS/BPO). Eur Urol Suppl 2005; 3(6):8–16.
- Bosch JL, Weiss J. The prevalence and causes of nocturia. J Urol 2010; 184:440–446.
- Tikkinen KA, Johnson TM, Tammela TL, et al. Nocturia frequency, bother, and quality of life: how often is too often? A population-based study in Finland. Eur Urol 2010; 57:488–496.
- Klingler HG, Heidler H, Madersbacher H, Primus G. Nocturia: an Austrian study on the multifactorial etiology of this symptom. Neurourol Urodyn 2009; 28:427–431.
- Mariappan P, Turner KJ, Sothilingam S, Rajan P, Sundram M, Steward LH. Nocturia, nocturia indices and variables from frequency-volume charts are significantly different in Asian and Caucasian men with lower urinary tract symptoms: a prospective comparison study. BJU Int 2007; 100:332–336.
- Asplund R. Mortality in the elderly in relation to nocturnal micturition. BJU Int 1999; 84:297–301.
- Booth J, O’Neil K, Lawrence M, et al. Advancing community nursing practice: detecting and managing nocturia in community-living older people. Final report. 2008. Queens Nursing Institute, Scotland. http://www.qnis.co.uk/documents/Item3.2-finalreportnocturiav2.doc. Accessed 8/22/11
- Kawauchi A, Tanaka Y, Soh J, Ukimura O, Kojima M, Miki T. Causes of nocturnal urinary frequency and reasons for its increase with age in healthy older men. J Urol 2000; 163:81–84.
- Madersbacher S, Pycha A, Schatzl G, Mian C, Klingler CH, Marberger M. The aging lower urinary tract: a comparative urodynamics study of men and women. Urology 1998; 51:206–212.
- Elbedawi A, Yalla SV, Resnick NM. Structural basis of geriatric voiding dysfunction. I: methods of a prospective ultra structural/urodynamics study and an overview of the findings. J Urol 1993; 150:1650–1656.
- Weiss JP, Blaivas JG, Jones M, Wang JT, Guan Z; 037 Study Group. Age related pathogenesis of nocturia in patients with overactive bladder. J Urol 2007; 178:548–551.
- Natsume O, Kaneko Y, Hirayama A, Fujimoto K, Hirao Y. Fluid control in elderly patients with nocturia. Int J Urol 2009; 16:307–313.
- Asplund R. Pharmacotherapy for nocturia in the elderly patient. Drugs Aging 2007; 24:325–343.
- Sugaya K, Nishijima S, Oda M, Owan T, Miyazato M, Ogawa Y. Biochemical and body composition analysis of nocturia in the elderly. Neurourol Urodyn 2008; 27:205–211.
- Shiri R, Hakama M, Häkkinen J, et al. The effects of lifestyle factors on the incidence of nocturia. J Urol 2008; 180:2059–2062.
- Asplund R. Obesity in elderly people with nocturia: cause or consequence? Can J Urol 2007; 14:3424–3428.
- Hardin-Fanning F, Gross JC. The effects of sleep-disordered breathing symptoms on voiding patterns in stroke patients. Urol Nurs 2007; 27:221–229.
- Foley DJ, Vitiello MV, Bliwise DL, Ancoli-Israel S, Monjan AA, Walsh JK. Frequent napping is associated with excessive daytime sleepiness, depression, pain, and nocturia in older adults: findings from the National Sleep Foundation ‘2003 Sleep in America’ Poll. Am J Geriatr Psychiatry 2007; 15:344–350.
- Häkkinen JT, Shiri R, Koskimäki J, Tammela TL, Auvinen A, Hakama M. Depressive symptoms increase the incidence of nocturia: Tampere Aging Male Urologic Study (TAMUS). J Urol 2008; 179:1897–1901.
- Natsume O, Kaneko Y, Hirayama A, Fujimoto K, Hirao Y. Fluid control in elderly patients with nocturia. Int J Urol 2009; 16:307–313.
- Kujubu DA, Aboseif SR. An overview of nocturia and the syndrome of nocturnal polyuria in the elderly. Nat Clin Pract Nephrol 2008; 4:426–435.
- Hashimoto M, Imamura T, Tanimukai S, Kazui H, Mori E. Urinary incontinence: an unrecognized adverse effect with donepezil (letter). Lancet 2000; 356:568.
- Wagg A, Cohen M. Medical therapy for the overactive bladder in the elderly. Age Ageing 2002; 31:241–246.
- Williams G, Donaldson RM. Nifedipine and nocturia. Lancet 1986: 1:738.
- Yoshimura K, Kamoto T, Tsukamoto T, Oshiro K, Kinukawa N, Ogawa O. Seasonal alterations in nocturia and other storage symptoms in three Japanese communities. Urology 2007; 69:864–870.
- Asplund R. Hip fractures, nocturia, and nocturnal polyuria in the elderly. Arch Gerontol Geriatr 2006; 43:319–326.
- Stewart RB, Moore MT, May FE, Marks RG, Hale WE. Nocturia: a risk factor for falls in the elderly. J Am Geriatr Soc 1992; 40:1217–1220.
- van Balen R, Steyerberg EW, Polder JJ, Ribbers TL, Habbema JD, Cools HJ. Hip fracture in elderly patients: outcomes for function, quality of life, and type of residence. Clin Orthop Relat Res 2001; 390:232–243.
- Mock LL, Parmelee PA, Kutner N, Scott J, Johnson TM. Content validation of symptom-specific nocturia quality-of-life instrument developed in men: issues expressed by women, as well as men. Urology 2008; 72:736–742.
- Holm-Larsen T, Weiss J, Langkilde LK. Economic burden of nocturia in the US adult population. J Urol Suppl 2010; 100:332–336.
- Ali A, Snape J. Nocturia in older people: a review of causes, consequences, assessment, and management. Int J Clin Pract 2004; 58:366–373.
- Miu DK, Lau S, Szeto SS. Etiology and predictors of urinary incontinence and its effect on quality of life. Geriatr Gerontol Int 2010; 10:177–182.
- Tibaek S, Gard G, Klarskov P, Iversen HK, Dehlendorff C, Jensen R. Prevalence of lower urinary tract symptoms (LUTS) in stroke patients: a cross-sectional, clinical survey. Neurourol Urodyn 2008; 27:763–771.
- Bliwise DL, Foley DJ, Vitiello MV, Ansari FP, Ancoli-Israel S, Walsh JK. Nocturia and disturbed sleep in the elderly. Sleep Med 2009; 10:540–548.
- Asplund R. Nocturia: consequences for sleep and daytime activities and associated risks. Eur Urol Suppl 2005; 3(6):24–32.
- Hernández C, Estivill E, Prieto M, Badia X. Nocturia in Spanish patients with lower urinary tract symptoms suggestive of benign prostatic hyperplasia (LUTS/BPH). Curr Med Res Opin 2008; 24:1033–1038.
- Pollak CP, Perlick D, Linsner JP, Wenston J, Hsieh F. Sleep problems in the community elderly as predictors of death and nursing home placement. J Community Health 1990; 15:123–135.
- Bursztyn M, Jacob J, Stessman J. Usefulness of nocturia as a mortality risk factor for coronary heart disease among persons born in 1920 or 1921. Am J Cardiol 2006; 98:1311–1315.
- Appell RA, Sand PK. Nocturia: etiology, diagnosis, and treatment. Neurourol Urodyn 2008; 27:34–39.
- Weiss JP, Blaivas JG, Stember DS, Chaikin DC. Evaluation of the etiology of nocturia in men: the nocturia and nocturnal bladder capacity indices. Neurourol Urodyn 1999; 18:559–565.
- Jaffe JS, Ginsberg PC, Silverberg DM, Harkaway RC. The need for voiding diaries in the evaluation of men with nocturia. J Am Osteopath Assoc 2002; 102:261–265.
- Yoshimura K, Terai A. Classification and distribution of symptomatic nocturia with special attention to duration of time in bed: a patient-based study. BJU Int 2005; 95:1259–1262.
- Polderman KH. Mechanisms of action, physiological effects, and complications of hypothermia. Crit Care Med 2009; 37:S186–S202.
- Soda T, Masui K, Okuno H, Terai A, Ogawa O, Yoshimura K. Efficacy of nondrug lifestyle measures for the treatment of nocturia. J Urol 2010; 184:1000–1004.
- Flynn MK, Amundsen CL, Perevich M, Liu F, Webster GD. Outcome of a randomized, double-blind, placebo controlled trial of botulinum A toxin for refractory overactive bladder. J Urol 2009; 181:2608–2615.
- Asplund R, Sundberg B, Bengtsson P. Desmopressin for the treatment of nocturnal polyuria in the elderly: a dose titration study. Br J Urol 1998; 82:642–646.
- Abrams P, Mattiasson A, Lose GR, Robertson GL. The role of desmopressin treatment in adult nocturia. BJU Int 2002; 90:32–36.
- Andersson K. Treatment of the overactive bladder syndrome and detrusor overactivity with antimuscarinic drugs. Continence 2005; 1:1–8.
- Reynard JM, Cannon A, Yang Q, Abrams P. A novel therapy for nocturnal polyuria: a double-blind randomized trial of frusemide against placebo. Br J Urol 1998; 81:215–218.
- Cho MC, Ku JH, Paick JS. Alpha-blocker plus diuretic combination therapy as second-line treatment for nocturia in men with LUTS: a pilot study. Urology 2009; 73:549–553.
- Fu FG, Lavery HJ, Wu DL. Reducing nocturia in the elderly: a randomized placebo-controlled trial of staggered furosemide and desmopressin. Neurourol Urodyn 2011; 30:312–316.
- Falahatkar S, Mokhtari G, Pourezza F, Asgari SA, Kamran AN. Celecoxib for treatment of nocturia caused by benign prostatic hyperplasia: a prospective, randomized, double-blind, placebo-controlled study. Urology 2008; 72:813–816.
- Addla SK, Adeyoju AB, Neilson D, O’Reilly P. Diclofenac for treatment of nocturia caused by nocturnal polyuria: a prospective, randomised, double-blind, placebo-controlled crossover study. Eur Urol 2006; 49:720–725.
- Saito M, Kawatani M, Kinoshita Y, Satoh K, Miyagawa I. Effectiveness of an anti-inflammatory drug, loxoprofen, for patients with nocturia. Int J Urol 2005; 12:779–782.
KEY POINTS
- Nocturia is multifactorial and is caused by factors that increase urine production and others that decrease the bladder’s ability to hold urine.
- The first priority in treating nocturia is to identify and treat concomitant conditions that may be contributing to it, such as diabetes mellitus, diabetes insipidus, urinary tract infections, hypercalcemia, and hypokalemia.
- Nonpharmacologic measures can help, but by themselves usually do not solve the problem.
- Drug therapies for nocturia include desmopressin (DDAVP), antimuscarinic agents, alpha-blockers, and 5-alpha reductase inhibitors.