User login
Patients with poorly controlled diabetes mellitus and an infectious source can be predisposed to infectious aortitis.
Aortitis is the all-encompassing term ascribed to the inflammatory process in the aortic wall that can be either infective or noninfective in origin, commonly autoimmune or inflammatory large-vessel vasculitis.1 Infectious aortitis, also known as bacterial, microbial, or cryptogenic aortitis, as well as mycotic or infected aneurysm, is a rare entity in the current antibiotic era but potentially a life-threatening disorder.2 The potential complications of infectious aortitis include emphysematous aortitis (EA), pseudoaneurysm, aortic rupture, septic emboli, and fistula formation (eg, aorto-enteric fistula).2,3
EA is a rare but serious inflammatory condition of the aorta with a nonspecific clinical presentation associated with high morbidity and mortality.2-6 The condition is characterized by a localized collection of gas and purulent exudate at the aortic wall.1,3 A few cases of EA have previously been reported; however, no known cases have been reported in the literature due to Klebsiella pneumoniae (K pneumoniae).
The pathophysiology of EA is the presence of underlying damage to the arterial wall caused by a hematogenously inoculated gas-producing organism.2,3 Most reported cases of EA are due to endovascular graft complications. Under normal circumstances, the aortic intima is highly resistant to infectious pathogens; however, certain risk factors, such as diabetes mellitus (DM), atherosclerotic disease, preexisting aneurysm, cystic medial necrosis, vascular malformation, presence of medical devices, surgery, or impaired immunity can alter the integrity of the aortic intimal layer and predispose the aortic intima to infection.1,4-7 Bacteria are the most common causative organisms that can infect the aorta, especially Staphylococcus, Enterococcus, Streptococcus, Salmonella, and spirochete Treponema pallidum (syphilis).1,2,4,8 The site of the primary infection remains unclear in some patients.2,3,5,6 Infection of the aorta can arise by several mechanisms: direct extension of a local infection to an existing intimal injury or atherosclerotic plaque (the most common mechanism), septic embolism from endocarditis, direct bacterial inoculation from traumatic contamination, contiguous infection extending to the aorta wall, or a distant source of bacteremia.2,3
Clinical manifestations of EA depend on the site and the extent of infection. The diagnosis should be considered in patients with atherosclerosis, fever, abdominal pain, and leukocytosis.2,4-8 The differential diagnosis for EA includes (1) noninfective causes of aortitis, including rheumatoid arthritis and systemic lupus erythematosus; (2) tuberculous aortitis; (3) syphilitic aortitis; and (4) idiopathic isolated aortitis. Establishing an early diagnosis of infectious aortitis is extremely important because this condition is associated with a high rate of morbidity and mortality secondary to aortic rupture.2-7
Imaging is critical for a reliable and quick diagnosis of acute aortic pathology. Noninvasive cross-sectional imaging modalities, such as contrast-enhanced computed tomography (CT), magnetic resonance imaging, nuclear medicine, or positron emission tomography, are used for both the initial diagnosis and follow-up of aortitis.1 CT is the primary imaging method in most medical centers because it is widely available with short acquisition time in critically ill patients.3 CT allows rapid detection of abnormalities in wall thickness, diameter, and density, and enhancement of periaortic structures, enabling reliable exclusion of other aortic pathologies that may resemble acute aortitis. Also, CT aids in planning the optimal therapeutic approach.1,3,5-8
This case illustrates EA associated with infection by K pneumoniae in a patient with poorly controlled type 2 DM (T2DM). In this single case, our patient presented to the Bay Pines Veterans Affairs Healthcare System (BPVAHS) in Florida with recent superficial soft tissue injury, severe hyperglycemia, worsening abdominal pain, and leukocytosis without fever or chills. The correct diagnosis of EA was confirmed by characteristic CT findings.
Case Presentation
A 72-year-old male with a history of T2DM, hypertension, atherosclerotic vascular disease, obstructive lung disease, and smoking 1.5 packs per day for 40 years presented with diabetic ketoacidosis, a urinary tract infection, and abdominal pain of 1-week duration that started to worsen the morning he arrived at the BPVAHS emergency department. He reported no nausea, vomiting, diarrhea, constipation, chest pain, shortness of breath, fever, chills, fatigue, or dysuria. He had a nonhealing laceration on his left medial foot that occurred 18 days before admission and was treated at an outside hospital.
The patient’s surgical history included a left common femoral endarterectomy and a left femoral popliteal above-knee reverse saphenous vein bypass 4 years ago for severe critical limb ischemia due to occlusion of his left superficial femoral artery with distal embolization to the first and fifth toes. About 6 months later, he developed disabling claudication in his left lower extremity due to distal popliteal artery occlusion and had another bypass surgery to the below-knee popliteal artery with a reverse saphenous vein graft harvested from the right thigh.
On initial examination, his vital signs were within normal limits except for a blood pressure of 177/87 mm Hg. His physical examination demonstrated a nondistended abdomen with normal bowel sounds, mild lower quadrant tenderness on the left more than on the right, intermittent abdominal pain located around umbilicus with radiation to the back, and a negative psoas sign. His left medial foot had a nonhealing laceration with black sutures in place, with minimal erythema in the surrounding tissue and scab formation. He also had mild costovertebral tenderness on the left.
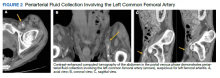
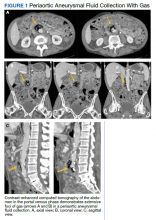
Initial laboratory investigation results were notable for a glucose level of 609 mg/dL and a white blood cell count of 14.6 × 103 cells/mcL with 86.5% of neutrophils. A CT scan of his abdomen revealed extensive atherosclerosis of the abdominal aorta and periaortic aneurysmal fluid collection with multiple foci of gas (Figure 1). Additionally, the aneurysmal fluid collection involved the proximal segment of the left common femoral artery, suspicious for left femoral arteritis (Figure 2). The patient was started on broad-spectrum antibiotics, morphine, and an insulin drip. Both urine and blood cultures were positive for K pneumoniae susceptible to multiple antibiotics. He was transferred to a tertiary medical center and was referred for a vascular surgery consultation.
The patient underwent surgical resection of the infected infrarenal EA and infected left common femoral artery with right axillary-bifemoral bypass with an 8-mm PTFE (polytetrafluoroethylene) graft. During the surgery, excision of the wall of the left common femoral artery and infrarenal aorta revealed frank pus with purulent fluid, which was sent to cytology for analysis and culture. His intraoperative cultures grew K pneumoniae sensitive to multiple antibiotics, including ceftriaxone, sulfamethoxazole/trimethoprim, and ampicillin/sulbactam. The vascular surgery team recommended inpatient admission and administration of 6 weeks of IV antibiotics postoperatively with ceftriaxone, followed by outpatient oral suppression therapy after discharge. The patient tolerated the surgery well and was discharged after 6 weeks of IV ceftriaxone followed by outpatient oral suppression therapy. However, the patient was transferred back to BPVAHS for continued care and rehabilitation placement.
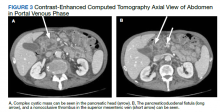
The patient’s subsequent course was complicated by multiple hospital admissions, including aspiration pneumonia, hypoglycemia, diarrhea, and anemia. On one of his CT abdomen/pelvic examinations, a cysticlike mass was noted in the pancreatic head with a possible pancreatic duodenal fistula (this mass was not mentioned on the initial presurgical CT, although it can be seen in retrospect (Figure 3). Gastroenterology was consulted.
An upper endoscopy was performed that confirmed the fistula at the second portion of the duodenum. Findings from an endoscopic ultrasonography performed at an outside institution were concerning for a main duct intraductal papillary mucinous neoplasm (IPMN) with fistula, with biopsy results pending.
Discussion
This case contributes to the evidence that poorly controlled T2DM can be a predisposing factor for multiple vascular complications, including the infection of the aortic wall with progression to EA. Klebsiella species are considered opportunistic, Gram-negative pathogens that may disseminate to other tissues, causing life-threatening infections, including pneumonia, UTIs, bacteremia, and sepsis.9K pneumoniae infections are particularly challenging in neonates, the elderly, and immunocompromised individuals.9 CT is sensitive and specific in the detection of this pathologic entity.1,3 In patients with a suspected infectious etiology, the presence of foci of gas on CT in solid organ tissues is usually associated with an anaerobic infection. Gas can also be produced by Gram-negative facultative anaerobes that can ferment glucose in necrotic tissues.9
Although any microorganism can infect the aorta, K pneumoniae cultured from the blood specimen, urine culture, and intraoperative specimens in our patient was responsible for the formed gas in the aortic wall. Occurrence of spontaneous gas by this microorganism is usually associated with conditions leading to either increased vulnerability to infections and/or enhanced bacterial virulence.9 Although a relationship between EA and T2DM has not been proved, it is well known that patients with T2DM have a defect in their host-defense mechanisms, making them more susceptible to infections such as EA. Furthermore, because patients with T2DM are prone to the development of Gram-negative sepsis, organisms such as K pneumoniae would tend to emerge. Patients with poorly controlled T2DM and the presence of an infectious source can be predisposed to infectious aortitis, eventually leading to a gas-forming infection of the aorta.5,7
We postulate that the hematogenous spread of bacteria from a laceration in the leg as well as the presence of the pancreaticoduodenal fistula was likely the cause of the infectious EA in this case, considering the patient’s underlying uncontrolled T2DM. The patient’s prior left lower extremity vascular graft also may have provided a nidus for spreading to the aorta. Other reported underlying diseases of EA include aortic atherosclerosis, T2DM, diverticulitis, colon cancer, underlying aneurysm, immune-compromised status, and the presence of a medical device or open surgery.4-7,9
To our knowledge, this is the first case of EA associated with a pancreaticoduodenal fistula related to intraductal papillary mucinous neoplasm (IPMN). Fistulation of a main duct IPMN is rare, occurring in just 6.6% of cases.10 It can occur both before and after malignant degeneration.
EA requires rapid diagnosis, antibiotic therapy, and consultation with a vascular surgeon for immediate resection of the infected artery and graft bypass. The initial treatment of suspected infectious aortitis is IV antibiotics with broad antimicrobial coverage of the most likely pathologic organisms, particularly staphylococcal species and Gram-negative rods. Surgical debridement and revascularization should be completed early because of the high mortality rate of this condition. The intent of surgery is to control sepsis and reconstruct the arterial vasculature. Patients should remain on parenteral or oral antibiotics for at least 6 weeks to ensure full clearance of the infection.8 They should be followed up closely with serial blood cultures and CT scans.8 The rarity of the disorder, low level of awareness, varying presentations, and lack of evidence delineating pathogenesis and causality contribute to the challenge of recognizing, diagnosing, and treating EA in patients with T2DM and inflammation.
Conclusions
This case report can help bring awareness of this rare and potentially life-threatening condition in patients with T2DM. Clinicians should be aware of the risk of AE, particularly in patients with several additional risk factors: recent skin/soft tissue trauma, prior vascular graft surgery, and an underlying pancreatic mass. CT is the imaging method of choice that helps to rapidly choose a necessary emergent treatment approach.
1. Litmanovich DE, Yıldırım A, Bankier AA. Insights into imaging of aortitis. Insights Imaging. 2012;3(6):545-560. doi:10.1007/s13244-012-0192-x
2. Lopes RJ, Almeida J, Dias PJ, Pinho P, Maciel MJ. Infectious thoracic aortitis: a literature review. Clin Cardiol. 2009;32(9):488-490. doi:10.1002/clc.20578
3. Murphy DJ, Keraliya AR, Agrawal MD, Aghayev A, Steigner ML. Cross-sectional imaging of aortic infections. Insights Imaging. 2016;7(6):801-818. doi:10.1007/s13244-016-0522-5
4. Md Noh MSF, Abdul Rashid AM, Ar A, B N, Mohammed Y, A RE. Emphysematous aortitis: report of two cases and CT imaging findings. BJR Case Rep. 2017;3(3):20170006. doi:10.1259/bjrcr.20170006
5. Harris C, Geffen J, Rizg K, et al. A rare report of infectious emphysematous aortitis secondary to Clostridium septicum without prior vascular intervention. Case Rep Vasc Med. 2017;2017:4984325. doi:10.1155/2017/4984325
6. Ito F, Inokuchi R, Matsumoto A, et al. Presence of periaortic gas in Clostridium septicum-infected aortic aneurysm aids in early diagnosis: a case report and systematic review of the literature. J Med Case Rep. 2017;11(1):268. doi:10.1186/s13256-017-1422-0
7. Urgiles S, Matos-Casano H, Win KZ, Berardo J, Bhatt U, Shah J. Emphysematous aortitis due to Clostridium septicum in an 89-year-old female with ileus. Case Rep Infect Dis. 2019;2019:1094837. doi:10.1155/2019/1094837
8. Foote EA, Postier RG, Greenfield RA, Bronze MS. Infectious aortitis. Curr Treat Options Cardiovasc Med. 2005;7(2):89-97. doi:10.1007/s11936-005-0010-6
9. Paczosa MK, Mecsas J. Klebsiella pneumoniae: going on the offense with a strong defense. Microbiol Mol Biol Rev. 2016;80(3):629-661. doi:10.1128/mmbr.00078-15
10. Kobayashi G, Fujita N, Noda Y, et al. Intraductal papillary mucinous neoplasms of the pancreas showing fistula formation into other organs. J Gastroenterol. 2010;45(10):1080-1089. doi:10.1007/s00535-010-0263-z
Patients with poorly controlled diabetes mellitus and an infectious source can be predisposed to infectious aortitis.
Patients with poorly controlled diabetes mellitus and an infectious source can be predisposed to infectious aortitis.
Aortitis is the all-encompassing term ascribed to the inflammatory process in the aortic wall that can be either infective or noninfective in origin, commonly autoimmune or inflammatory large-vessel vasculitis.1 Infectious aortitis, also known as bacterial, microbial, or cryptogenic aortitis, as well as mycotic or infected aneurysm, is a rare entity in the current antibiotic era but potentially a life-threatening disorder.2 The potential complications of infectious aortitis include emphysematous aortitis (EA), pseudoaneurysm, aortic rupture, septic emboli, and fistula formation (eg, aorto-enteric fistula).2,3
EA is a rare but serious inflammatory condition of the aorta with a nonspecific clinical presentation associated with high morbidity and mortality.2-6 The condition is characterized by a localized collection of gas and purulent exudate at the aortic wall.1,3 A few cases of EA have previously been reported; however, no known cases have been reported in the literature due to Klebsiella pneumoniae (K pneumoniae).
The pathophysiology of EA is the presence of underlying damage to the arterial wall caused by a hematogenously inoculated gas-producing organism.2,3 Most reported cases of EA are due to endovascular graft complications. Under normal circumstances, the aortic intima is highly resistant to infectious pathogens; however, certain risk factors, such as diabetes mellitus (DM), atherosclerotic disease, preexisting aneurysm, cystic medial necrosis, vascular malformation, presence of medical devices, surgery, or impaired immunity can alter the integrity of the aortic intimal layer and predispose the aortic intima to infection.1,4-7 Bacteria are the most common causative organisms that can infect the aorta, especially Staphylococcus, Enterococcus, Streptococcus, Salmonella, and spirochete Treponema pallidum (syphilis).1,2,4,8 The site of the primary infection remains unclear in some patients.2,3,5,6 Infection of the aorta can arise by several mechanisms: direct extension of a local infection to an existing intimal injury or atherosclerotic plaque (the most common mechanism), septic embolism from endocarditis, direct bacterial inoculation from traumatic contamination, contiguous infection extending to the aorta wall, or a distant source of bacteremia.2,3
Clinical manifestations of EA depend on the site and the extent of infection. The diagnosis should be considered in patients with atherosclerosis, fever, abdominal pain, and leukocytosis.2,4-8 The differential diagnosis for EA includes (1) noninfective causes of aortitis, including rheumatoid arthritis and systemic lupus erythematosus; (2) tuberculous aortitis; (3) syphilitic aortitis; and (4) idiopathic isolated aortitis. Establishing an early diagnosis of infectious aortitis is extremely important because this condition is associated with a high rate of morbidity and mortality secondary to aortic rupture.2-7
Imaging is critical for a reliable and quick diagnosis of acute aortic pathology. Noninvasive cross-sectional imaging modalities, such as contrast-enhanced computed tomography (CT), magnetic resonance imaging, nuclear medicine, or positron emission tomography, are used for both the initial diagnosis and follow-up of aortitis.1 CT is the primary imaging method in most medical centers because it is widely available with short acquisition time in critically ill patients.3 CT allows rapid detection of abnormalities in wall thickness, diameter, and density, and enhancement of periaortic structures, enabling reliable exclusion of other aortic pathologies that may resemble acute aortitis. Also, CT aids in planning the optimal therapeutic approach.1,3,5-8
This case illustrates EA associated with infection by K pneumoniae in a patient with poorly controlled type 2 DM (T2DM). In this single case, our patient presented to the Bay Pines Veterans Affairs Healthcare System (BPVAHS) in Florida with recent superficial soft tissue injury, severe hyperglycemia, worsening abdominal pain, and leukocytosis without fever or chills. The correct diagnosis of EA was confirmed by characteristic CT findings.
Case Presentation
A 72-year-old male with a history of T2DM, hypertension, atherosclerotic vascular disease, obstructive lung disease, and smoking 1.5 packs per day for 40 years presented with diabetic ketoacidosis, a urinary tract infection, and abdominal pain of 1-week duration that started to worsen the morning he arrived at the BPVAHS emergency department. He reported no nausea, vomiting, diarrhea, constipation, chest pain, shortness of breath, fever, chills, fatigue, or dysuria. He had a nonhealing laceration on his left medial foot that occurred 18 days before admission and was treated at an outside hospital.
The patient’s surgical history included a left common femoral endarterectomy and a left femoral popliteal above-knee reverse saphenous vein bypass 4 years ago for severe critical limb ischemia due to occlusion of his left superficial femoral artery with distal embolization to the first and fifth toes. About 6 months later, he developed disabling claudication in his left lower extremity due to distal popliteal artery occlusion and had another bypass surgery to the below-knee popliteal artery with a reverse saphenous vein graft harvested from the right thigh.
On initial examination, his vital signs were within normal limits except for a blood pressure of 177/87 mm Hg. His physical examination demonstrated a nondistended abdomen with normal bowel sounds, mild lower quadrant tenderness on the left more than on the right, intermittent abdominal pain located around umbilicus with radiation to the back, and a negative psoas sign. His left medial foot had a nonhealing laceration with black sutures in place, with minimal erythema in the surrounding tissue and scab formation. He also had mild costovertebral tenderness on the left.
Initial laboratory investigation results were notable for a glucose level of 609 mg/dL and a white blood cell count of 14.6 × 103 cells/mcL with 86.5% of neutrophils. A CT scan of his abdomen revealed extensive atherosclerosis of the abdominal aorta and periaortic aneurysmal fluid collection with multiple foci of gas (Figure 1). Additionally, the aneurysmal fluid collection involved the proximal segment of the left common femoral artery, suspicious for left femoral arteritis (Figure 2). The patient was started on broad-spectrum antibiotics, morphine, and an insulin drip. Both urine and blood cultures were positive for K pneumoniae susceptible to multiple antibiotics. He was transferred to a tertiary medical center and was referred for a vascular surgery consultation.
The patient underwent surgical resection of the infected infrarenal EA and infected left common femoral artery with right axillary-bifemoral bypass with an 8-mm PTFE (polytetrafluoroethylene) graft. During the surgery, excision of the wall of the left common femoral artery and infrarenal aorta revealed frank pus with purulent fluid, which was sent to cytology for analysis and culture. His intraoperative cultures grew K pneumoniae sensitive to multiple antibiotics, including ceftriaxone, sulfamethoxazole/trimethoprim, and ampicillin/sulbactam. The vascular surgery team recommended inpatient admission and administration of 6 weeks of IV antibiotics postoperatively with ceftriaxone, followed by outpatient oral suppression therapy after discharge. The patient tolerated the surgery well and was discharged after 6 weeks of IV ceftriaxone followed by outpatient oral suppression therapy. However, the patient was transferred back to BPVAHS for continued care and rehabilitation placement.
The patient’s subsequent course was complicated by multiple hospital admissions, including aspiration pneumonia, hypoglycemia, diarrhea, and anemia. On one of his CT abdomen/pelvic examinations, a cysticlike mass was noted in the pancreatic head with a possible pancreatic duodenal fistula (this mass was not mentioned on the initial presurgical CT, although it can be seen in retrospect (Figure 3). Gastroenterology was consulted.
An upper endoscopy was performed that confirmed the fistula at the second portion of the duodenum. Findings from an endoscopic ultrasonography performed at an outside institution were concerning for a main duct intraductal papillary mucinous neoplasm (IPMN) with fistula, with biopsy results pending.
Discussion
This case contributes to the evidence that poorly controlled T2DM can be a predisposing factor for multiple vascular complications, including the infection of the aortic wall with progression to EA. Klebsiella species are considered opportunistic, Gram-negative pathogens that may disseminate to other tissues, causing life-threatening infections, including pneumonia, UTIs, bacteremia, and sepsis.9K pneumoniae infections are particularly challenging in neonates, the elderly, and immunocompromised individuals.9 CT is sensitive and specific in the detection of this pathologic entity.1,3 In patients with a suspected infectious etiology, the presence of foci of gas on CT in solid organ tissues is usually associated with an anaerobic infection. Gas can also be produced by Gram-negative facultative anaerobes that can ferment glucose in necrotic tissues.9
Although any microorganism can infect the aorta, K pneumoniae cultured from the blood specimen, urine culture, and intraoperative specimens in our patient was responsible for the formed gas in the aortic wall. Occurrence of spontaneous gas by this microorganism is usually associated with conditions leading to either increased vulnerability to infections and/or enhanced bacterial virulence.9 Although a relationship between EA and T2DM has not been proved, it is well known that patients with T2DM have a defect in their host-defense mechanisms, making them more susceptible to infections such as EA. Furthermore, because patients with T2DM are prone to the development of Gram-negative sepsis, organisms such as K pneumoniae would tend to emerge. Patients with poorly controlled T2DM and the presence of an infectious source can be predisposed to infectious aortitis, eventually leading to a gas-forming infection of the aorta.5,7
We postulate that the hematogenous spread of bacteria from a laceration in the leg as well as the presence of the pancreaticoduodenal fistula was likely the cause of the infectious EA in this case, considering the patient’s underlying uncontrolled T2DM. The patient’s prior left lower extremity vascular graft also may have provided a nidus for spreading to the aorta. Other reported underlying diseases of EA include aortic atherosclerosis, T2DM, diverticulitis, colon cancer, underlying aneurysm, immune-compromised status, and the presence of a medical device or open surgery.4-7,9
To our knowledge, this is the first case of EA associated with a pancreaticoduodenal fistula related to intraductal papillary mucinous neoplasm (IPMN). Fistulation of a main duct IPMN is rare, occurring in just 6.6% of cases.10 It can occur both before and after malignant degeneration.
EA requires rapid diagnosis, antibiotic therapy, and consultation with a vascular surgeon for immediate resection of the infected artery and graft bypass. The initial treatment of suspected infectious aortitis is IV antibiotics with broad antimicrobial coverage of the most likely pathologic organisms, particularly staphylococcal species and Gram-negative rods. Surgical debridement and revascularization should be completed early because of the high mortality rate of this condition. The intent of surgery is to control sepsis and reconstruct the arterial vasculature. Patients should remain on parenteral or oral antibiotics for at least 6 weeks to ensure full clearance of the infection.8 They should be followed up closely with serial blood cultures and CT scans.8 The rarity of the disorder, low level of awareness, varying presentations, and lack of evidence delineating pathogenesis and causality contribute to the challenge of recognizing, diagnosing, and treating EA in patients with T2DM and inflammation.
Conclusions
This case report can help bring awareness of this rare and potentially life-threatening condition in patients with T2DM. Clinicians should be aware of the risk of AE, particularly in patients with several additional risk factors: recent skin/soft tissue trauma, prior vascular graft surgery, and an underlying pancreatic mass. CT is the imaging method of choice that helps to rapidly choose a necessary emergent treatment approach.
Aortitis is the all-encompassing term ascribed to the inflammatory process in the aortic wall that can be either infective or noninfective in origin, commonly autoimmune or inflammatory large-vessel vasculitis.1 Infectious aortitis, also known as bacterial, microbial, or cryptogenic aortitis, as well as mycotic or infected aneurysm, is a rare entity in the current antibiotic era but potentially a life-threatening disorder.2 The potential complications of infectious aortitis include emphysematous aortitis (EA), pseudoaneurysm, aortic rupture, septic emboli, and fistula formation (eg, aorto-enteric fistula).2,3
EA is a rare but serious inflammatory condition of the aorta with a nonspecific clinical presentation associated with high morbidity and mortality.2-6 The condition is characterized by a localized collection of gas and purulent exudate at the aortic wall.1,3 A few cases of EA have previously been reported; however, no known cases have been reported in the literature due to Klebsiella pneumoniae (K pneumoniae).
The pathophysiology of EA is the presence of underlying damage to the arterial wall caused by a hematogenously inoculated gas-producing organism.2,3 Most reported cases of EA are due to endovascular graft complications. Under normal circumstances, the aortic intima is highly resistant to infectious pathogens; however, certain risk factors, such as diabetes mellitus (DM), atherosclerotic disease, preexisting aneurysm, cystic medial necrosis, vascular malformation, presence of medical devices, surgery, or impaired immunity can alter the integrity of the aortic intimal layer and predispose the aortic intima to infection.1,4-7 Bacteria are the most common causative organisms that can infect the aorta, especially Staphylococcus, Enterococcus, Streptococcus, Salmonella, and spirochete Treponema pallidum (syphilis).1,2,4,8 The site of the primary infection remains unclear in some patients.2,3,5,6 Infection of the aorta can arise by several mechanisms: direct extension of a local infection to an existing intimal injury or atherosclerotic plaque (the most common mechanism), septic embolism from endocarditis, direct bacterial inoculation from traumatic contamination, contiguous infection extending to the aorta wall, or a distant source of bacteremia.2,3
Clinical manifestations of EA depend on the site and the extent of infection. The diagnosis should be considered in patients with atherosclerosis, fever, abdominal pain, and leukocytosis.2,4-8 The differential diagnosis for EA includes (1) noninfective causes of aortitis, including rheumatoid arthritis and systemic lupus erythematosus; (2) tuberculous aortitis; (3) syphilitic aortitis; and (4) idiopathic isolated aortitis. Establishing an early diagnosis of infectious aortitis is extremely important because this condition is associated with a high rate of morbidity and mortality secondary to aortic rupture.2-7
Imaging is critical for a reliable and quick diagnosis of acute aortic pathology. Noninvasive cross-sectional imaging modalities, such as contrast-enhanced computed tomography (CT), magnetic resonance imaging, nuclear medicine, or positron emission tomography, are used for both the initial diagnosis and follow-up of aortitis.1 CT is the primary imaging method in most medical centers because it is widely available with short acquisition time in critically ill patients.3 CT allows rapid detection of abnormalities in wall thickness, diameter, and density, and enhancement of periaortic structures, enabling reliable exclusion of other aortic pathologies that may resemble acute aortitis. Also, CT aids in planning the optimal therapeutic approach.1,3,5-8
This case illustrates EA associated with infection by K pneumoniae in a patient with poorly controlled type 2 DM (T2DM). In this single case, our patient presented to the Bay Pines Veterans Affairs Healthcare System (BPVAHS) in Florida with recent superficial soft tissue injury, severe hyperglycemia, worsening abdominal pain, and leukocytosis without fever or chills. The correct diagnosis of EA was confirmed by characteristic CT findings.
Case Presentation
A 72-year-old male with a history of T2DM, hypertension, atherosclerotic vascular disease, obstructive lung disease, and smoking 1.5 packs per day for 40 years presented with diabetic ketoacidosis, a urinary tract infection, and abdominal pain of 1-week duration that started to worsen the morning he arrived at the BPVAHS emergency department. He reported no nausea, vomiting, diarrhea, constipation, chest pain, shortness of breath, fever, chills, fatigue, or dysuria. He had a nonhealing laceration on his left medial foot that occurred 18 days before admission and was treated at an outside hospital.
The patient’s surgical history included a left common femoral endarterectomy and a left femoral popliteal above-knee reverse saphenous vein bypass 4 years ago for severe critical limb ischemia due to occlusion of his left superficial femoral artery with distal embolization to the first and fifth toes. About 6 months later, he developed disabling claudication in his left lower extremity due to distal popliteal artery occlusion and had another bypass surgery to the below-knee popliteal artery with a reverse saphenous vein graft harvested from the right thigh.
On initial examination, his vital signs were within normal limits except for a blood pressure of 177/87 mm Hg. His physical examination demonstrated a nondistended abdomen with normal bowel sounds, mild lower quadrant tenderness on the left more than on the right, intermittent abdominal pain located around umbilicus with radiation to the back, and a negative psoas sign. His left medial foot had a nonhealing laceration with black sutures in place, with minimal erythema in the surrounding tissue and scab formation. He also had mild costovertebral tenderness on the left.
Initial laboratory investigation results were notable for a glucose level of 609 mg/dL and a white blood cell count of 14.6 × 103 cells/mcL with 86.5% of neutrophils. A CT scan of his abdomen revealed extensive atherosclerosis of the abdominal aorta and periaortic aneurysmal fluid collection with multiple foci of gas (Figure 1). Additionally, the aneurysmal fluid collection involved the proximal segment of the left common femoral artery, suspicious for left femoral arteritis (Figure 2). The patient was started on broad-spectrum antibiotics, morphine, and an insulin drip. Both urine and blood cultures were positive for K pneumoniae susceptible to multiple antibiotics. He was transferred to a tertiary medical center and was referred for a vascular surgery consultation.
The patient underwent surgical resection of the infected infrarenal EA and infected left common femoral artery with right axillary-bifemoral bypass with an 8-mm PTFE (polytetrafluoroethylene) graft. During the surgery, excision of the wall of the left common femoral artery and infrarenal aorta revealed frank pus with purulent fluid, which was sent to cytology for analysis and culture. His intraoperative cultures grew K pneumoniae sensitive to multiple antibiotics, including ceftriaxone, sulfamethoxazole/trimethoprim, and ampicillin/sulbactam. The vascular surgery team recommended inpatient admission and administration of 6 weeks of IV antibiotics postoperatively with ceftriaxone, followed by outpatient oral suppression therapy after discharge. The patient tolerated the surgery well and was discharged after 6 weeks of IV ceftriaxone followed by outpatient oral suppression therapy. However, the patient was transferred back to BPVAHS for continued care and rehabilitation placement.
The patient’s subsequent course was complicated by multiple hospital admissions, including aspiration pneumonia, hypoglycemia, diarrhea, and anemia. On one of his CT abdomen/pelvic examinations, a cysticlike mass was noted in the pancreatic head with a possible pancreatic duodenal fistula (this mass was not mentioned on the initial presurgical CT, although it can be seen in retrospect (Figure 3). Gastroenterology was consulted.
An upper endoscopy was performed that confirmed the fistula at the second portion of the duodenum. Findings from an endoscopic ultrasonography performed at an outside institution were concerning for a main duct intraductal papillary mucinous neoplasm (IPMN) with fistula, with biopsy results pending.
Discussion
This case contributes to the evidence that poorly controlled T2DM can be a predisposing factor for multiple vascular complications, including the infection of the aortic wall with progression to EA. Klebsiella species are considered opportunistic, Gram-negative pathogens that may disseminate to other tissues, causing life-threatening infections, including pneumonia, UTIs, bacteremia, and sepsis.9K pneumoniae infections are particularly challenging in neonates, the elderly, and immunocompromised individuals.9 CT is sensitive and specific in the detection of this pathologic entity.1,3 In patients with a suspected infectious etiology, the presence of foci of gas on CT in solid organ tissues is usually associated with an anaerobic infection. Gas can also be produced by Gram-negative facultative anaerobes that can ferment glucose in necrotic tissues.9
Although any microorganism can infect the aorta, K pneumoniae cultured from the blood specimen, urine culture, and intraoperative specimens in our patient was responsible for the formed gas in the aortic wall. Occurrence of spontaneous gas by this microorganism is usually associated with conditions leading to either increased vulnerability to infections and/or enhanced bacterial virulence.9 Although a relationship between EA and T2DM has not been proved, it is well known that patients with T2DM have a defect in their host-defense mechanisms, making them more susceptible to infections such as EA. Furthermore, because patients with T2DM are prone to the development of Gram-negative sepsis, organisms such as K pneumoniae would tend to emerge. Patients with poorly controlled T2DM and the presence of an infectious source can be predisposed to infectious aortitis, eventually leading to a gas-forming infection of the aorta.5,7
We postulate that the hematogenous spread of bacteria from a laceration in the leg as well as the presence of the pancreaticoduodenal fistula was likely the cause of the infectious EA in this case, considering the patient’s underlying uncontrolled T2DM. The patient’s prior left lower extremity vascular graft also may have provided a nidus for spreading to the aorta. Other reported underlying diseases of EA include aortic atherosclerosis, T2DM, diverticulitis, colon cancer, underlying aneurysm, immune-compromised status, and the presence of a medical device or open surgery.4-7,9
To our knowledge, this is the first case of EA associated with a pancreaticoduodenal fistula related to intraductal papillary mucinous neoplasm (IPMN). Fistulation of a main duct IPMN is rare, occurring in just 6.6% of cases.10 It can occur both before and after malignant degeneration.
EA requires rapid diagnosis, antibiotic therapy, and consultation with a vascular surgeon for immediate resection of the infected artery and graft bypass. The initial treatment of suspected infectious aortitis is IV antibiotics with broad antimicrobial coverage of the most likely pathologic organisms, particularly staphylococcal species and Gram-negative rods. Surgical debridement and revascularization should be completed early because of the high mortality rate of this condition. The intent of surgery is to control sepsis and reconstruct the arterial vasculature. Patients should remain on parenteral or oral antibiotics for at least 6 weeks to ensure full clearance of the infection.8 They should be followed up closely with serial blood cultures and CT scans.8 The rarity of the disorder, low level of awareness, varying presentations, and lack of evidence delineating pathogenesis and causality contribute to the challenge of recognizing, diagnosing, and treating EA in patients with T2DM and inflammation.
Conclusions
This case report can help bring awareness of this rare and potentially life-threatening condition in patients with T2DM. Clinicians should be aware of the risk of AE, particularly in patients with several additional risk factors: recent skin/soft tissue trauma, prior vascular graft surgery, and an underlying pancreatic mass. CT is the imaging method of choice that helps to rapidly choose a necessary emergent treatment approach.
1. Litmanovich DE, Yıldırım A, Bankier AA. Insights into imaging of aortitis. Insights Imaging. 2012;3(6):545-560. doi:10.1007/s13244-012-0192-x
2. Lopes RJ, Almeida J, Dias PJ, Pinho P, Maciel MJ. Infectious thoracic aortitis: a literature review. Clin Cardiol. 2009;32(9):488-490. doi:10.1002/clc.20578
3. Murphy DJ, Keraliya AR, Agrawal MD, Aghayev A, Steigner ML. Cross-sectional imaging of aortic infections. Insights Imaging. 2016;7(6):801-818. doi:10.1007/s13244-016-0522-5
4. Md Noh MSF, Abdul Rashid AM, Ar A, B N, Mohammed Y, A RE. Emphysematous aortitis: report of two cases and CT imaging findings. BJR Case Rep. 2017;3(3):20170006. doi:10.1259/bjrcr.20170006
5. Harris C, Geffen J, Rizg K, et al. A rare report of infectious emphysematous aortitis secondary to Clostridium septicum without prior vascular intervention. Case Rep Vasc Med. 2017;2017:4984325. doi:10.1155/2017/4984325
6. Ito F, Inokuchi R, Matsumoto A, et al. Presence of periaortic gas in Clostridium septicum-infected aortic aneurysm aids in early diagnosis: a case report and systematic review of the literature. J Med Case Rep. 2017;11(1):268. doi:10.1186/s13256-017-1422-0
7. Urgiles S, Matos-Casano H, Win KZ, Berardo J, Bhatt U, Shah J. Emphysematous aortitis due to Clostridium septicum in an 89-year-old female with ileus. Case Rep Infect Dis. 2019;2019:1094837. doi:10.1155/2019/1094837
8. Foote EA, Postier RG, Greenfield RA, Bronze MS. Infectious aortitis. Curr Treat Options Cardiovasc Med. 2005;7(2):89-97. doi:10.1007/s11936-005-0010-6
9. Paczosa MK, Mecsas J. Klebsiella pneumoniae: going on the offense with a strong defense. Microbiol Mol Biol Rev. 2016;80(3):629-661. doi:10.1128/mmbr.00078-15
10. Kobayashi G, Fujita N, Noda Y, et al. Intraductal papillary mucinous neoplasms of the pancreas showing fistula formation into other organs. J Gastroenterol. 2010;45(10):1080-1089. doi:10.1007/s00535-010-0263-z
1. Litmanovich DE, Yıldırım A, Bankier AA. Insights into imaging of aortitis. Insights Imaging. 2012;3(6):545-560. doi:10.1007/s13244-012-0192-x
2. Lopes RJ, Almeida J, Dias PJ, Pinho P, Maciel MJ. Infectious thoracic aortitis: a literature review. Clin Cardiol. 2009;32(9):488-490. doi:10.1002/clc.20578
3. Murphy DJ, Keraliya AR, Agrawal MD, Aghayev A, Steigner ML. Cross-sectional imaging of aortic infections. Insights Imaging. 2016;7(6):801-818. doi:10.1007/s13244-016-0522-5
4. Md Noh MSF, Abdul Rashid AM, Ar A, B N, Mohammed Y, A RE. Emphysematous aortitis: report of two cases and CT imaging findings. BJR Case Rep. 2017;3(3):20170006. doi:10.1259/bjrcr.20170006
5. Harris C, Geffen J, Rizg K, et al. A rare report of infectious emphysematous aortitis secondary to Clostridium septicum without prior vascular intervention. Case Rep Vasc Med. 2017;2017:4984325. doi:10.1155/2017/4984325
6. Ito F, Inokuchi R, Matsumoto A, et al. Presence of periaortic gas in Clostridium septicum-infected aortic aneurysm aids in early diagnosis: a case report and systematic review of the literature. J Med Case Rep. 2017;11(1):268. doi:10.1186/s13256-017-1422-0
7. Urgiles S, Matos-Casano H, Win KZ, Berardo J, Bhatt U, Shah J. Emphysematous aortitis due to Clostridium septicum in an 89-year-old female with ileus. Case Rep Infect Dis. 2019;2019:1094837. doi:10.1155/2019/1094837
8. Foote EA, Postier RG, Greenfield RA, Bronze MS. Infectious aortitis. Curr Treat Options Cardiovasc Med. 2005;7(2):89-97. doi:10.1007/s11936-005-0010-6
9. Paczosa MK, Mecsas J. Klebsiella pneumoniae: going on the offense with a strong defense. Microbiol Mol Biol Rev. 2016;80(3):629-661. doi:10.1128/mmbr.00078-15
10. Kobayashi G, Fujita N, Noda Y, et al. Intraductal papillary mucinous neoplasms of the pancreas showing fistula formation into other organs. J Gastroenterol. 2010;45(10):1080-1089. doi:10.1007/s00535-010-0263-z