User login
IN THIS ARTICLE
• The “why” of endometriosis
• Environmental factors, estrogen, and endometriosis
• Is imaging useful?
• When is diagnostic laparoscopy clearly indicated?
CASE M.L. is a 32-year-old nulliparous woman who is referred to your office by her primary care provider for chronic pelvic pain. She reports severe dysmenorrhea as her main symptom, but she also mentions dyspareunia. She says these symptoms have been present for several years but have increased in intensity gradually. She asks what you consider to be the most likely diagnosis.
What potential diagnoses do you mention to her? And how do you identify the cause of her pain?
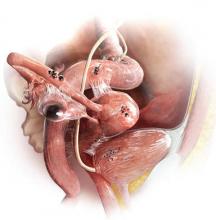
Although endometriosis—the presence of endometrial tissue outside the uterus—affects at least 5 million women of reproductive age in the United States alone, it can be a challenging diagnosis for several reasons.
“Endometriosis is a great masquerader,” says Linda Giudice, MD, PhD. “It presents with a variety of pain patterns, intensities, and triggers. It can also involve symptoms that overlap those of other disorders, including disorders of the gastrointestinal and urinary tracts.”
Although endometriosis falls within the differential diagnosis of chronic pelvic pain, “it is usually not high on the list in the primary care setting (adult and adolescent),” adds Dr. Giudice.
John R. Lue, MD, MPH, an author of the most recent practice bulletin on endometriosis from the American College of Obstetricians and Gynecologists,1 sees the situation similarly.
“The main challenge in the diagnosis of endometriosis is that its presentation mimics other causes of chronic pelvic pain,” he says. “Pelvic pain due to endometriosis is usually chronic (lasting ≥ 6 months). It is associated with dysmenorrhea in 50% to 90% of cases, as well as with dyspareunia, deep pelvic pain, and lower abdominal pain with or without back and loin pain. The pain can occur unpredictably and intermittently throughout the menstrual cycle or it can be continuous. In addition, it can be dull, throbbing, or sharp and may be exacerbated by physical activity.2,3 Up to 20% of women with endometriosis have concurrent pain conditions.”4
Among other diseases of the female pelvis that have a relatively similar presentation, Dr. Lue adds, are pathologies of the
• Uterus (adenomyosis, fibroids)
• Fallopian tube (hydrosalpinx)
• Ovaries (ovarian cysts)
• Bladder (interstitial cystitis)
• Bowel (irritable bowel syndrome)
• Musculoskeletal system (piriformis syndrome).
Before pelvic pain is attributed to endometriosis, he says, the provider should rule out bowel, bladder, musculoskeletal, and psychiatric causes.
This article focuses on seven questions, the answers to which are critical to narrowing in on the diagnosis of endometriosis, including essential factors to consider in the patient history, imaging and other diagnostic tools, and considerations in surgical exploration.
1. WHY SUCH A LONG DELAY IN DIAGNOSIS?
Investigators exploring the length of time from a patient’s presentation with symptoms to diagnosis have found it to be particularly long for endometriosis, ranging from six to 11 years.
Because endometriosis is usually not high on the list of differential diagnoses for chronic pelvic pain in the primary care setting, a patient may not be referred to a gynecologist unless those symptoms include severe dysmenorrhea, dyspareunia, or similar findings. Once the referral is made, the gynecologist “will usually try contraceptive steroids, NSAIDs, or second-line progestins before a diagnosis is made,” says Dr. Giudice.5
The delay in diagnosis “is astounding,” she adds, “and has its roots in empiric medical therapies and a combination of patients fearing a diagnosis of cancer and reluctance of gynecologists to perform laparoscopy on adolescents.”6 Another possible cause of diagnostic delay: Some adolescent girls may not realize when their pain is severe. Because they may have always experienced a high degree of pain since menarche, they may assume it to be a normal aspect of womanhood and delay seeking help, says Pamela Stratton, MD.
Continue to learn any biomarkers proved to be useful diagnostic tools >>
2. HAVE ANY BIOMARKERS PROVED TO BE USEFUL DIAGNOSTIC TOOLS?
Any biomarker proven to reliably identify endometriosis would be a boon to medicine, as it would provide a noninvasive or minimally invasive alternative to diagnostic laparoscopy, the current gold standard. Regrettably, the search for such a biomarker has produced “disappointing results,” says Dr. Giudice.
“Recent systematic reviews of all proposed endometriosis-related biomarkers over the past 25 years in serum, plasma, urine, and endometrium could not identify an unequivocally clinically useful biomarker or panel of biomarkers,” she notes.7,8 “This is due mainly to low numbers of subjects, small populations for validations, cycle/hormonal- and disease stage–dependence, poorly defined controls, and low sensitivity and specificity.”
One hopeful development: “Whole genome transcriptomics of archived endometrial tissue and machine learning found several classifiers to diagnose and stage endometriosis with high accuracy that were validated on an independent sample set,” says Dr. Giudice.9 “However, these data now warrant a prospective, multisite study for further validation.”
Continue for key aspects of patient history >>
3. WHAT ASPECTS OF THE PATIENT HISTORY ARE KEY?
Dr. Stratton recommends that clinicians begin their evaluation of the patient with pain by asking her to describe that pain: how long she has had it, when it occurs, and which areas are affected.
“Most women with endometriosis-associated pain have chronic pelvic pain,” Dr. Stratton continues.5 “Up to 90% of those have dysmenorrhea or cyclic pain with menses.”10 In addition, women with endometriosis “commonly report having pain with any bleeding or spotting. About 30% of women diagnosed with endometriosis initially present to their gynecologist with dyspareunia.”11
“Episodic pain with menses may become more constant, lasting for many days of the month,” says Dr. Stratton. “Women with dyschezia or dysuria may have endometriosis lesions associated with the bowel or bladder, respectively.12 When women with these symptoms do not have lesions on the bowel or bladder, these pain symptoms may occur because of higher peritoneal hormone and inflammatory factor levels or because adjacent organs share the neural networks.”
Dr. Giudice views the history similarly: “I believe listening to the patient is essential in evaluating the possibility of her having endometriosis. This involves asking her to describe where her pain is, grading it on a scale of 1 to 10, identifying when in her cycle it occurs, and learning what makes it better or worse.”
“It also is important to assess the quality of the pain,” she adds. “Does it radiate, does it limit her daily activities, does it interfere with her relationships, intercourse, work, school? Is it associated with bowel movements, urination, other pain syndromes?”
“Having a pain questionnaire is a great help so that patients have a chance to reflect on these and other questions that help to frame the pain associated with endometriosis when they come for consultation,” she adds.
By determining if pain is associated with menstruation or spotting, the clinician is better informed about the value of menstrual suppression, says Dr. Stratton. “Determining what makes the pain better or worse can help define triggers which, if treated, can decrease the likelihood of episodes of pain.”
“A detailed history of any medical or surgical treatments and their outcome is helpful in guiding future treatment,” she adds. “While hormonal therapy has been a mainstay of treatment, in some women, some hormonal treatments may worsen pain or have unacceptable adverse effects, such as worsening depression or anxiety. In addition, some pain—especially that associated with deep lesions—may be relieved by surgical treatment;13,14 pain that worsened after surgery may suggest neural damage.”
“As there is an engagement of the central nervous system, endometriosis is considered a central sensitivity syndrome in which women may also have other sites of pain,” Dr. Stratton says. “Thus, obtaining a history about current symptoms or prior diagnosis of irritable bowel syndrome, interstitial cystitis/painful bladder, migraines, fibromyalgia, or chronic fatigue syndrome is beneficial.10,15-17 Facilitating treatment for these comorbidities is a key principle in helping women with endometriosis-associated pain, as any condition that triggers or perpetuates pain warrants treatment.”
Continue for what the physical exam entails >>
4. WHAT SHOULD THE PHYSICAL EXAM ENTAIL?
“An abdominal exam and a pelvic exam are essential in evaluating pain in a woman when endometriosis is suspected,” says Dr. Giudice. “Sometimes the latter is challenging in young teens and can be deferred.” Overall, however, “the pelvic exam can give insight into pain triggers, adnexal masses (possible endometriomas), and mobility of pelvic organs. A rectovaginal exam is important in evaluating deep infiltrating disease and to gauge the pelvic pain landscape overall. In addition, palpating the pelvic floor musculature is important to distinguish pelvic floor muscle spasm from endometriosis pain.”
“The challenge for clinicians is to think beyond the endometrial implants, taking into account multiple factors that influence pain perception,” says Dr. Stratton. During the examination, the clinician should begin by mapping the regions of pain in the abdomen and back, “distinguishing musculoskeletal pain from deep pain. Determining whether pains are focused or diffuse is also important.”
Dr. Stratton recommends that the routine pelvic exam be modified because a standard bimanual exam “confuses pain signals from the pelvic floor, abdominal wall, bladder, and other viscera. For this reason, a pain-oriented assessment is mandatory.”
Begin with a single digital examination to map tender areas, Dr. Stratton advises. Then consider the size, shape, and mobility of reproductive and pelvic organs. “A bimanual exam will help identify adnexal masses like endometriomas,” she says.
Endometriomas usually are not associated with pain, she adds, but “they are associated with deep infiltrating lesions. Nodularity along the uterosacral ligaments, limited reproductive organ mobility, and thickening of the rectovaginal septum also suggest deep infiltrating lesions. Importantly, deep infiltrating lesions are the lesion type most associated with pain.”18,19
Continue to learn if imaging is useful in the diagnosis of endometriosis >>
5. IS IMAGING USEFUL IN THE DIAGNOSIS OF ENDOMETRIOSIS?
Laparoscopy remains the gold standard for diagnosis of endometriosis, observes Steven R. Goldstein, MD. Visualization of endometriotic implants at the time of surgery—with histologic assessment—offers definitive confirmation of the diagnosis. The physical examination, too, can offer a strong suggestion of endometriosis, he says.
“In the past, the pelvic examination and history often were the sine qua non for patients with pain,” Dr. Goldstein says. “Extreme dysmenorrhea and pain between periods, especially with intercourse, defecation, and exercise, all increased the suspicion of endometriosis. People used to talk about feeling nodularity in the uterosacral ligaments and finding decreased mobility of pelvic structures—but I don’t have any question that the skill of today’s gynecologists in doing a bimanual pelvic exam is a fraction of what it was in years gone by, because they haven’t had the necessity of experience. The first thing they do if there’s any question is send the patient for an ultrasound.”
Of course, ultrasound can be especially helpful in identifying endometriomas—sometimes called chocolate cysts—in the ovary. Endometriomas can have a solid appearance on ultrasound, says Dr. Goldstein, because the fluid they contain (dried blood) is sonolucent or pure black on ultrasound, similar to amniotic fluid or the fluid seen in the bladder. “This ‘chocolate’ fluid contained in endometriomas is homogeneous, particulate, and very monotonous in its appearance, in contrast to the internal echoes observed in hemorrhagic corpus lutea, which are very cobweb-like and can sometimes mimic papillary projections,” he adds.
“What’s absolutely essential when imaging a suspected endometrioma by ultrasound is that there be no evidence of any blood flow contained within that structure. Because it’s dried blood, it shouldn’t have any vascularity. If you see blood flow inside what you would call an endometrioma, you need to rethink your diagnosis,” he says.
In some cases, a supposed endometrioma lacks a black, sonolucent appearance, but “the clinician often can tell that it’s a cystic structure by the very bright posterior wall—what we call posterior wall acoustic enhancement—even though the interior of the structure may appear sort of grayish or whitish rather than the pure black of a simple cyst. It’s still fluid-filled,” Dr. Goldstein says.
In some instances, even endometriotic nodules can be imaged by ultrasound, he adds. “There’s an increasing body of literature that suggests that, if you look carefully in people with deep infiltrating endometriosis, you can often see solid-appearing nodules in the rectovaginal septum or between the uterus and bladder. With the kind of resolution that we now have with the vaginal probe, some of these nodules can be seen. That’s somewhat new, and it’s a function of two things—people looking for endometriosis and the better resolution of more modern equipment.”
Dr. Goldstein believes that MRI is “almost never” indicated in the diagnosis of endometriosis. A more helpful approach would be a consultative ultrasound with someone with more experience. However, when that is not available, or “in areas where you have excellent backup in terms of pelvic MRI, that may be the way to go. I don’t think so,” he demurs, “and some of my colleagues would be very upset at the thought of needing to use MRI to diagnose endometriosis. But in the occasional confusing or difficult case, depending on the quality of the referral pattern you have, it might make sense."
Continue to learn when diagnostic laparoscopy is clearly indicated >>
6. WHEN IS DIAGNOSTIC LAPAROSCOPY CLEARLY INDICATED?
Dr. Giudice believes that laparoscopy—with the intention to treat endometriosis, if present—“is essential when firstline medical therapy fails or when pain is acute and severe.”5
Dr. Stratton concurs. “Any woman with chronic pain wants to know what is causing the pain,” she says. Therefore, “women report a benefit from knowing that their pain is associated with endometriosis.6 However, diagnostic laparoscopy alone, with the sole purpose of determining the presence of endometriosis but not treating the lesions, is no longer performed, as it poses little benefit to the patient other than peace of mind.”
“The general trend in the US has been to first use hormonal treatments when the diagnosis of endometriosis is suspected, prior to performing surgery,” Dr. Stratton says.1 In many cases, by using cyclic combined hormonal contraceptives to reduce menstrual flow or “suppressing menstruation with continuous combined hormonal contraceptives,” gonadotropin-releasing hormone analogues (combined with progestin to prevent bone loss) “or continuous progestin alone may be effective in decreasing pain. Not surprisingly, these hormonal approaches are effective for any chronic pelvic pain, even for women who do not have the surgical diagnosis of endometriosis.”20
“When the firstline approach to chronic pelvic pain is hormonal treatment, laparoscopy is considered when these medical treatments have failed to control the pain or are poorly tolerated, or when the diagnosis of endometriosis is in question,” Dr. Stratton says.
“Laparoscopy to treat endometriomas is indicated if an endometrioma is enlarging or measures more than 4 cm in diameter, or if the diagnosis of an ovarian mass is in question,” she explains. “While surgeons have previously been aggressive in removing endometriomas, this practice may have negative consequences on ovarian function. Because endometriomas are pseudocysts, removing them completely leads to the removal of viable ovarian tissue and may diminish ovarian reserve.”21,22
Continue for the surgical appearance of endometriosis >>
7. WHAT IS THE SURGICAL APPEARANCE OF ENDOMETRIOSIS?
Dr. Giudice returns to the enigmatic nature of endometriosis in addressing this question, mentioning its “many faces” at the time of surgery. “It is imperative that the surgeon recognize the disease in its many forms,” she says. “Also, it is especially helpful at the time of surgery if suspected lesions are biopsied and sent to pathology to have the diagnosis made unequivocally.”5
As for the surgical appearance of endometriosis, Dr. Stratton notes that there are three types of lesions—“superficial lesions, deep infiltrating lesions, and endometriomas. Endometriomas occur almost exclusively in the ovary and are pseudocysts without an identifiable cystic lining. They vary in dimension from a few millimeters to several centimeters.”
“Superficial peritoneal endometriosis lesions have a variable appearance, with some lesions being clear or red; some brown, blue or black; and some having a white appearance, like a scar,” says Dr. Stratton. “Endometriosis can be diagnosed on histologic examination of any of these lesion types."
“Overall, single-color lesions have similar frequencies of biopsy-confirmed endometriosis (59% to 62%),” she says.23 “These lesion appearances likely represent different stages of development of endometriosis, with red or clear lesions occurring first, soon after endometrial tissue implantation; black, blue, or brown lesions occurring later, in response to the hormones varying in the menstrual cycle; and white lesions occurring as the lesions age. Deep infiltrating lesions generally have blue/black or white features.”
“Wide, deep, multiple-color lesions in the cul-de-sac, ovarian fossa, or uterosacral ligaments are most likely endometriosis,” Dr. Stratton adds.23 Only lesions with multiple colors have a significantly higher percentage of positive biopsies (76%). Importantly, more than half of women with only subtle lesions (small red or white lesions) have endometriosis.
You tell the patient that endometriosis is one of the possible diagnoses for her chronic pelvic pain, and you take a focused history. During a pelvic examination, you observe that her right ovary lacks mobility, and you map a number of trigger points for her pain. Transvaginal ultrasound results suggest the presence of nodules in the rectovaginal septum. You begin empiric treatment with continuous combined hormonal contraceptives to suppress menstruation. On her next visit, M.L. reports reduced but still bothersome pain. Laparoscopy reveals a 2-cm endometrioma in the right ovary and deep infiltrating lesions in the cul-de-sac. The endometrioma is resected. Histology confirms the diagnosis of endometriosis.
REFERENCES
1. American College of Obstetricians and Gynecologists. Practice Bulletin #114: Management of endometriosis. Obstet Gynecol. 2010; 116(1):223-236.
2. Sanfilippo JS, Wakim NG, Schikler KN, Yussman MA. Endometriosis in association with uterine anomaly. Am J Obstet Gynecol. 1986; 154(1):39-43.
3. Taylor HS, Bagot C, Kardana A. HOX gene expression is altered in the endometrium of women with endometriosis. Hum Reprod. 1999; 14(5):1328-1331.
4. Berkley KJ, Stratton P. Mechanisms: lessons from translational studies of endometriosis. In: Giamberardino MA, ed. Visceral Pain: Clinical, Pathophysiological and Therapeutic Aspects. Oxford, UK: Oxford University Press; 2009:39-50.
5. Giudice LC. Clinical practice: endometriosis. N Engl J Med. 2010;362(25): 2389-2398.
6. Ballard K, Lowton K, Wright J. What’s the delay: a qualitative study of women’s experiences of reaching a diagnosis of endometriosis. Fertil Steril. 2006;86(5):1296-1301.
7. May KE, Conduit-Hulbert SA, Villar J, et al. Peripheral biomarkers of endometriosis: a systematic review. Hum Reprod Update. 2010; 16(6):651-674.
8. May KE, Villar J, Kirtley S, et al. Endometrial alterations in endometriosis: a systematic review of putative biomarkers. Hum Reprod Update. 2011; 17(5):637-653.
9. Tamaresis JS, Irwin JC, Goldfien GA, et al. Molecular classification of endometriosis and disease stage using high-dimensional genomic data. Endocrinology. 2014;155(12):4986-4999.
10. Sinaii N, Cleary SD, Ballweg ML, et al. High rates of autoimmune and endocrine disorders, fibromyalgia, chronic fatigue syndrome and atopic diseases among women with endometriosis: a survey analysis. Hum Reprod. 2002;17(10):2715-2724.
11. De Graaff AA, D’Hooghe TM, Dunselman GA, et al. The significant effect of endometriosis on physical, mental and social wellbeing: results from an international cross-sectional survey. Hum Reprod. 2013;28(10):2677-2685.
12. Lafay Pillet MC, Huchon C, Santulli P, et al. A clinical score can predict associated deep infiltrating endometriosis before surgery for an endometrioma. Hum Reprod. 2014;29(8):1666-1676.
13. Healey M, Cheng C, Kaur H. To excise or ablate endometriosis? A prospective randomized double-blinded trial after 5-year follow-up. J Minim Invasive Gynecol. 2014;21(6):999-1004.
14. Anaf V, El Nakadi I, De Moor V, et al. Increased nerve density in deep infiltrating endometriotic nodules. Gynecol Obstet Invest. 2011;71(2):112-117.
15. Stratton P, Berkley KJ. Chronic pelvic pain and endometriosis: translational evidence of the relationship and implications. Hum Reprod Update. 2011;17(3):327-346.
16. Karp BI, Sinaii N, Nieman LK, et al. Migraine in women with chronic pelvic pain with and without endometriosis. Fertil Steril. 2011;95(3):895-899.
17. Berkley KJ. A life of pelvic pain. Physiol Behav. 2005;86(3):272-280.
18. Fauconnier A, Chapron C. Endometriosis and pelvic pain: epidemiological evidence of the relationship and implications. Hum Reprod Update. 2005;11(6):595-606.
19. Vercellini P, Fedele L, Aimi G, et al. Association between endometriosis stage, lesion type, patient characteristics and severity of pelvic pain symptoms: a multivariate analysis of over 1000 patients. Hum Reprod. 2007;22(1):266-271.
20. Ling FW. Randomized controlled trial of depot leuprolide in patients with chronic pelvic pain and clinically suspected endometriosis. Pelvic Pain Study Group. Obstet Gynecol. 1999;93(1):51-58.
21. Muzii L, Di Tucci C, Di Feliciantonio M, et al. The effect of surgery for endometrioma on ovarian reserve evaluated by antral follicle count: a systematic review and meta-analysis. Hum Reprod. 2014;29(10):2190-2198.
22. Muzii L, Luciano AA, Zupi E, Panici PB. Effect of surgery for endometrioma on ovarian function: a different point of view. J Minim Invasive Gynecol. 2014;21(4):531-533.
23. Stegmann BJ, Sinaii N, Liu S, et al. Using location, color, size, and depth to characterize and identify endometriosis lesions in a cohort of 133 women. Fertil Steril. 2008;89(6):1632-1636.
IN THIS ARTICLE
• The “why” of endometriosis
• Environmental factors, estrogen, and endometriosis
• Is imaging useful?
• When is diagnostic laparoscopy clearly indicated?
CASE M.L. is a 32-year-old nulliparous woman who is referred to your office by her primary care provider for chronic pelvic pain. She reports severe dysmenorrhea as her main symptom, but she also mentions dyspareunia. She says these symptoms have been present for several years but have increased in intensity gradually. She asks what you consider to be the most likely diagnosis.
What potential diagnoses do you mention to her? And how do you identify the cause of her pain?
Although endometriosis—the presence of endometrial tissue outside the uterus—affects at least 5 million women of reproductive age in the United States alone, it can be a challenging diagnosis for several reasons.
“Endometriosis is a great masquerader,” says Linda Giudice, MD, PhD. “It presents with a variety of pain patterns, intensities, and triggers. It can also involve symptoms that overlap those of other disorders, including disorders of the gastrointestinal and urinary tracts.”
Although endometriosis falls within the differential diagnosis of chronic pelvic pain, “it is usually not high on the list in the primary care setting (adult and adolescent),” adds Dr. Giudice.
John R. Lue, MD, MPH, an author of the most recent practice bulletin on endometriosis from the American College of Obstetricians and Gynecologists,1 sees the situation similarly.
“The main challenge in the diagnosis of endometriosis is that its presentation mimics other causes of chronic pelvic pain,” he says. “Pelvic pain due to endometriosis is usually chronic (lasting ≥ 6 months). It is associated with dysmenorrhea in 50% to 90% of cases, as well as with dyspareunia, deep pelvic pain, and lower abdominal pain with or without back and loin pain. The pain can occur unpredictably and intermittently throughout the menstrual cycle or it can be continuous. In addition, it can be dull, throbbing, or sharp and may be exacerbated by physical activity.2,3 Up to 20% of women with endometriosis have concurrent pain conditions.”4
Among other diseases of the female pelvis that have a relatively similar presentation, Dr. Lue adds, are pathologies of the
• Uterus (adenomyosis, fibroids)
• Fallopian tube (hydrosalpinx)
• Ovaries (ovarian cysts)
• Bladder (interstitial cystitis)
• Bowel (irritable bowel syndrome)
• Musculoskeletal system (piriformis syndrome).
Before pelvic pain is attributed to endometriosis, he says, the provider should rule out bowel, bladder, musculoskeletal, and psychiatric causes.
This article focuses on seven questions, the answers to which are critical to narrowing in on the diagnosis of endometriosis, including essential factors to consider in the patient history, imaging and other diagnostic tools, and considerations in surgical exploration.
1. WHY SUCH A LONG DELAY IN DIAGNOSIS?
Investigators exploring the length of time from a patient’s presentation with symptoms to diagnosis have found it to be particularly long for endometriosis, ranging from six to 11 years.
Because endometriosis is usually not high on the list of differential diagnoses for chronic pelvic pain in the primary care setting, a patient may not be referred to a gynecologist unless those symptoms include severe dysmenorrhea, dyspareunia, or similar findings. Once the referral is made, the gynecologist “will usually try contraceptive steroids, NSAIDs, or second-line progestins before a diagnosis is made,” says Dr. Giudice.5
The delay in diagnosis “is astounding,” she adds, “and has its roots in empiric medical therapies and a combination of patients fearing a diagnosis of cancer and reluctance of gynecologists to perform laparoscopy on adolescents.”6 Another possible cause of diagnostic delay: Some adolescent girls may not realize when their pain is severe. Because they may have always experienced a high degree of pain since menarche, they may assume it to be a normal aspect of womanhood and delay seeking help, says Pamela Stratton, MD.
Continue to learn any biomarkers proved to be useful diagnostic tools >>
2. HAVE ANY BIOMARKERS PROVED TO BE USEFUL DIAGNOSTIC TOOLS?
Any biomarker proven to reliably identify endometriosis would be a boon to medicine, as it would provide a noninvasive or minimally invasive alternative to diagnostic laparoscopy, the current gold standard. Regrettably, the search for such a biomarker has produced “disappointing results,” says Dr. Giudice.
“Recent systematic reviews of all proposed endometriosis-related biomarkers over the past 25 years in serum, plasma, urine, and endometrium could not identify an unequivocally clinically useful biomarker or panel of biomarkers,” she notes.7,8 “This is due mainly to low numbers of subjects, small populations for validations, cycle/hormonal- and disease stage–dependence, poorly defined controls, and low sensitivity and specificity.”
One hopeful development: “Whole genome transcriptomics of archived endometrial tissue and machine learning found several classifiers to diagnose and stage endometriosis with high accuracy that were validated on an independent sample set,” says Dr. Giudice.9 “However, these data now warrant a prospective, multisite study for further validation.”
Continue for key aspects of patient history >>
3. WHAT ASPECTS OF THE PATIENT HISTORY ARE KEY?
Dr. Stratton recommends that clinicians begin their evaluation of the patient with pain by asking her to describe that pain: how long she has had it, when it occurs, and which areas are affected.
“Most women with endometriosis-associated pain have chronic pelvic pain,” Dr. Stratton continues.5 “Up to 90% of those have dysmenorrhea or cyclic pain with menses.”10 In addition, women with endometriosis “commonly report having pain with any bleeding or spotting. About 30% of women diagnosed with endometriosis initially present to their gynecologist with dyspareunia.”11
“Episodic pain with menses may become more constant, lasting for many days of the month,” says Dr. Stratton. “Women with dyschezia or dysuria may have endometriosis lesions associated with the bowel or bladder, respectively.12 When women with these symptoms do not have lesions on the bowel or bladder, these pain symptoms may occur because of higher peritoneal hormone and inflammatory factor levels or because adjacent organs share the neural networks.”
Dr. Giudice views the history similarly: “I believe listening to the patient is essential in evaluating the possibility of her having endometriosis. This involves asking her to describe where her pain is, grading it on a scale of 1 to 10, identifying when in her cycle it occurs, and learning what makes it better or worse.”
“It also is important to assess the quality of the pain,” she adds. “Does it radiate, does it limit her daily activities, does it interfere with her relationships, intercourse, work, school? Is it associated with bowel movements, urination, other pain syndromes?”
“Having a pain questionnaire is a great help so that patients have a chance to reflect on these and other questions that help to frame the pain associated with endometriosis when they come for consultation,” she adds.
By determining if pain is associated with menstruation or spotting, the clinician is better informed about the value of menstrual suppression, says Dr. Stratton. “Determining what makes the pain better or worse can help define triggers which, if treated, can decrease the likelihood of episodes of pain.”
“A detailed history of any medical or surgical treatments and their outcome is helpful in guiding future treatment,” she adds. “While hormonal therapy has been a mainstay of treatment, in some women, some hormonal treatments may worsen pain or have unacceptable adverse effects, such as worsening depression or anxiety. In addition, some pain—especially that associated with deep lesions—may be relieved by surgical treatment;13,14 pain that worsened after surgery may suggest neural damage.”
“As there is an engagement of the central nervous system, endometriosis is considered a central sensitivity syndrome in which women may also have other sites of pain,” Dr. Stratton says. “Thus, obtaining a history about current symptoms or prior diagnosis of irritable bowel syndrome, interstitial cystitis/painful bladder, migraines, fibromyalgia, or chronic fatigue syndrome is beneficial.10,15-17 Facilitating treatment for these comorbidities is a key principle in helping women with endometriosis-associated pain, as any condition that triggers or perpetuates pain warrants treatment.”
Continue for what the physical exam entails >>
4. WHAT SHOULD THE PHYSICAL EXAM ENTAIL?
“An abdominal exam and a pelvic exam are essential in evaluating pain in a woman when endometriosis is suspected,” says Dr. Giudice. “Sometimes the latter is challenging in young teens and can be deferred.” Overall, however, “the pelvic exam can give insight into pain triggers, adnexal masses (possible endometriomas), and mobility of pelvic organs. A rectovaginal exam is important in evaluating deep infiltrating disease and to gauge the pelvic pain landscape overall. In addition, palpating the pelvic floor musculature is important to distinguish pelvic floor muscle spasm from endometriosis pain.”
“The challenge for clinicians is to think beyond the endometrial implants, taking into account multiple factors that influence pain perception,” says Dr. Stratton. During the examination, the clinician should begin by mapping the regions of pain in the abdomen and back, “distinguishing musculoskeletal pain from deep pain. Determining whether pains are focused or diffuse is also important.”
Dr. Stratton recommends that the routine pelvic exam be modified because a standard bimanual exam “confuses pain signals from the pelvic floor, abdominal wall, bladder, and other viscera. For this reason, a pain-oriented assessment is mandatory.”
Begin with a single digital examination to map tender areas, Dr. Stratton advises. Then consider the size, shape, and mobility of reproductive and pelvic organs. “A bimanual exam will help identify adnexal masses like endometriomas,” she says.
Endometriomas usually are not associated with pain, she adds, but “they are associated with deep infiltrating lesions. Nodularity along the uterosacral ligaments, limited reproductive organ mobility, and thickening of the rectovaginal septum also suggest deep infiltrating lesions. Importantly, deep infiltrating lesions are the lesion type most associated with pain.”18,19
Continue to learn if imaging is useful in the diagnosis of endometriosis >>
5. IS IMAGING USEFUL IN THE DIAGNOSIS OF ENDOMETRIOSIS?
Laparoscopy remains the gold standard for diagnosis of endometriosis, observes Steven R. Goldstein, MD. Visualization of endometriotic implants at the time of surgery—with histologic assessment—offers definitive confirmation of the diagnosis. The physical examination, too, can offer a strong suggestion of endometriosis, he says.
“In the past, the pelvic examination and history often were the sine qua non for patients with pain,” Dr. Goldstein says. “Extreme dysmenorrhea and pain between periods, especially with intercourse, defecation, and exercise, all increased the suspicion of endometriosis. People used to talk about feeling nodularity in the uterosacral ligaments and finding decreased mobility of pelvic structures—but I don’t have any question that the skill of today’s gynecologists in doing a bimanual pelvic exam is a fraction of what it was in years gone by, because they haven’t had the necessity of experience. The first thing they do if there’s any question is send the patient for an ultrasound.”
Of course, ultrasound can be especially helpful in identifying endometriomas—sometimes called chocolate cysts—in the ovary. Endometriomas can have a solid appearance on ultrasound, says Dr. Goldstein, because the fluid they contain (dried blood) is sonolucent or pure black on ultrasound, similar to amniotic fluid or the fluid seen in the bladder. “This ‘chocolate’ fluid contained in endometriomas is homogeneous, particulate, and very monotonous in its appearance, in contrast to the internal echoes observed in hemorrhagic corpus lutea, which are very cobweb-like and can sometimes mimic papillary projections,” he adds.
“What’s absolutely essential when imaging a suspected endometrioma by ultrasound is that there be no evidence of any blood flow contained within that structure. Because it’s dried blood, it shouldn’t have any vascularity. If you see blood flow inside what you would call an endometrioma, you need to rethink your diagnosis,” he says.
In some cases, a supposed endometrioma lacks a black, sonolucent appearance, but “the clinician often can tell that it’s a cystic structure by the very bright posterior wall—what we call posterior wall acoustic enhancement—even though the interior of the structure may appear sort of grayish or whitish rather than the pure black of a simple cyst. It’s still fluid-filled,” Dr. Goldstein says.
In some instances, even endometriotic nodules can be imaged by ultrasound, he adds. “There’s an increasing body of literature that suggests that, if you look carefully in people with deep infiltrating endometriosis, you can often see solid-appearing nodules in the rectovaginal septum or between the uterus and bladder. With the kind of resolution that we now have with the vaginal probe, some of these nodules can be seen. That’s somewhat new, and it’s a function of two things—people looking for endometriosis and the better resolution of more modern equipment.”
Dr. Goldstein believes that MRI is “almost never” indicated in the diagnosis of endometriosis. A more helpful approach would be a consultative ultrasound with someone with more experience. However, when that is not available, or “in areas where you have excellent backup in terms of pelvic MRI, that may be the way to go. I don’t think so,” he demurs, “and some of my colleagues would be very upset at the thought of needing to use MRI to diagnose endometriosis. But in the occasional confusing or difficult case, depending on the quality of the referral pattern you have, it might make sense."
Continue to learn when diagnostic laparoscopy is clearly indicated >>
6. WHEN IS DIAGNOSTIC LAPAROSCOPY CLEARLY INDICATED?
Dr. Giudice believes that laparoscopy—with the intention to treat endometriosis, if present—“is essential when firstline medical therapy fails or when pain is acute and severe.”5
Dr. Stratton concurs. “Any woman with chronic pain wants to know what is causing the pain,” she says. Therefore, “women report a benefit from knowing that their pain is associated with endometriosis.6 However, diagnostic laparoscopy alone, with the sole purpose of determining the presence of endometriosis but not treating the lesions, is no longer performed, as it poses little benefit to the patient other than peace of mind.”
“The general trend in the US has been to first use hormonal treatments when the diagnosis of endometriosis is suspected, prior to performing surgery,” Dr. Stratton says.1 In many cases, by using cyclic combined hormonal contraceptives to reduce menstrual flow or “suppressing menstruation with continuous combined hormonal contraceptives,” gonadotropin-releasing hormone analogues (combined with progestin to prevent bone loss) “or continuous progestin alone may be effective in decreasing pain. Not surprisingly, these hormonal approaches are effective for any chronic pelvic pain, even for women who do not have the surgical diagnosis of endometriosis.”20
“When the firstline approach to chronic pelvic pain is hormonal treatment, laparoscopy is considered when these medical treatments have failed to control the pain or are poorly tolerated, or when the diagnosis of endometriosis is in question,” Dr. Stratton says.
“Laparoscopy to treat endometriomas is indicated if an endometrioma is enlarging or measures more than 4 cm in diameter, or if the diagnosis of an ovarian mass is in question,” she explains. “While surgeons have previously been aggressive in removing endometriomas, this practice may have negative consequences on ovarian function. Because endometriomas are pseudocysts, removing them completely leads to the removal of viable ovarian tissue and may diminish ovarian reserve.”21,22
Continue for the surgical appearance of endometriosis >>
7. WHAT IS THE SURGICAL APPEARANCE OF ENDOMETRIOSIS?
Dr. Giudice returns to the enigmatic nature of endometriosis in addressing this question, mentioning its “many faces” at the time of surgery. “It is imperative that the surgeon recognize the disease in its many forms,” she says. “Also, it is especially helpful at the time of surgery if suspected lesions are biopsied and sent to pathology to have the diagnosis made unequivocally.”5
As for the surgical appearance of endometriosis, Dr. Stratton notes that there are three types of lesions—“superficial lesions, deep infiltrating lesions, and endometriomas. Endometriomas occur almost exclusively in the ovary and are pseudocysts without an identifiable cystic lining. They vary in dimension from a few millimeters to several centimeters.”
“Superficial peritoneal endometriosis lesions have a variable appearance, with some lesions being clear or red; some brown, blue or black; and some having a white appearance, like a scar,” says Dr. Stratton. “Endometriosis can be diagnosed on histologic examination of any of these lesion types."
“Overall, single-color lesions have similar frequencies of biopsy-confirmed endometriosis (59% to 62%),” she says.23 “These lesion appearances likely represent different stages of development of endometriosis, with red or clear lesions occurring first, soon after endometrial tissue implantation; black, blue, or brown lesions occurring later, in response to the hormones varying in the menstrual cycle; and white lesions occurring as the lesions age. Deep infiltrating lesions generally have blue/black or white features.”
“Wide, deep, multiple-color lesions in the cul-de-sac, ovarian fossa, or uterosacral ligaments are most likely endometriosis,” Dr. Stratton adds.23 Only lesions with multiple colors have a significantly higher percentage of positive biopsies (76%). Importantly, more than half of women with only subtle lesions (small red or white lesions) have endometriosis.
You tell the patient that endometriosis is one of the possible diagnoses for her chronic pelvic pain, and you take a focused history. During a pelvic examination, you observe that her right ovary lacks mobility, and you map a number of trigger points for her pain. Transvaginal ultrasound results suggest the presence of nodules in the rectovaginal septum. You begin empiric treatment with continuous combined hormonal contraceptives to suppress menstruation. On her next visit, M.L. reports reduced but still bothersome pain. Laparoscopy reveals a 2-cm endometrioma in the right ovary and deep infiltrating lesions in the cul-de-sac. The endometrioma is resected. Histology confirms the diagnosis of endometriosis.
REFERENCES
1. American College of Obstetricians and Gynecologists. Practice Bulletin #114: Management of endometriosis. Obstet Gynecol. 2010; 116(1):223-236.
2. Sanfilippo JS, Wakim NG, Schikler KN, Yussman MA. Endometriosis in association with uterine anomaly. Am J Obstet Gynecol. 1986; 154(1):39-43.
3. Taylor HS, Bagot C, Kardana A. HOX gene expression is altered in the endometrium of women with endometriosis. Hum Reprod. 1999; 14(5):1328-1331.
4. Berkley KJ, Stratton P. Mechanisms: lessons from translational studies of endometriosis. In: Giamberardino MA, ed. Visceral Pain: Clinical, Pathophysiological and Therapeutic Aspects. Oxford, UK: Oxford University Press; 2009:39-50.
5. Giudice LC. Clinical practice: endometriosis. N Engl J Med. 2010;362(25): 2389-2398.
6. Ballard K, Lowton K, Wright J. What’s the delay: a qualitative study of women’s experiences of reaching a diagnosis of endometriosis. Fertil Steril. 2006;86(5):1296-1301.
7. May KE, Conduit-Hulbert SA, Villar J, et al. Peripheral biomarkers of endometriosis: a systematic review. Hum Reprod Update. 2010; 16(6):651-674.
8. May KE, Villar J, Kirtley S, et al. Endometrial alterations in endometriosis: a systematic review of putative biomarkers. Hum Reprod Update. 2011; 17(5):637-653.
9. Tamaresis JS, Irwin JC, Goldfien GA, et al. Molecular classification of endometriosis and disease stage using high-dimensional genomic data. Endocrinology. 2014;155(12):4986-4999.
10. Sinaii N, Cleary SD, Ballweg ML, et al. High rates of autoimmune and endocrine disorders, fibromyalgia, chronic fatigue syndrome and atopic diseases among women with endometriosis: a survey analysis. Hum Reprod. 2002;17(10):2715-2724.
11. De Graaff AA, D’Hooghe TM, Dunselman GA, et al. The significant effect of endometriosis on physical, mental and social wellbeing: results from an international cross-sectional survey. Hum Reprod. 2013;28(10):2677-2685.
12. Lafay Pillet MC, Huchon C, Santulli P, et al. A clinical score can predict associated deep infiltrating endometriosis before surgery for an endometrioma. Hum Reprod. 2014;29(8):1666-1676.
13. Healey M, Cheng C, Kaur H. To excise or ablate endometriosis? A prospective randomized double-blinded trial after 5-year follow-up. J Minim Invasive Gynecol. 2014;21(6):999-1004.
14. Anaf V, El Nakadi I, De Moor V, et al. Increased nerve density in deep infiltrating endometriotic nodules. Gynecol Obstet Invest. 2011;71(2):112-117.
15. Stratton P, Berkley KJ. Chronic pelvic pain and endometriosis: translational evidence of the relationship and implications. Hum Reprod Update. 2011;17(3):327-346.
16. Karp BI, Sinaii N, Nieman LK, et al. Migraine in women with chronic pelvic pain with and without endometriosis. Fertil Steril. 2011;95(3):895-899.
17. Berkley KJ. A life of pelvic pain. Physiol Behav. 2005;86(3):272-280.
18. Fauconnier A, Chapron C. Endometriosis and pelvic pain: epidemiological evidence of the relationship and implications. Hum Reprod Update. 2005;11(6):595-606.
19. Vercellini P, Fedele L, Aimi G, et al. Association between endometriosis stage, lesion type, patient characteristics and severity of pelvic pain symptoms: a multivariate analysis of over 1000 patients. Hum Reprod. 2007;22(1):266-271.
20. Ling FW. Randomized controlled trial of depot leuprolide in patients with chronic pelvic pain and clinically suspected endometriosis. Pelvic Pain Study Group. Obstet Gynecol. 1999;93(1):51-58.
21. Muzii L, Di Tucci C, Di Feliciantonio M, et al. The effect of surgery for endometrioma on ovarian reserve evaluated by antral follicle count: a systematic review and meta-analysis. Hum Reprod. 2014;29(10):2190-2198.
22. Muzii L, Luciano AA, Zupi E, Panici PB. Effect of surgery for endometrioma on ovarian function: a different point of view. J Minim Invasive Gynecol. 2014;21(4):531-533.
23. Stegmann BJ, Sinaii N, Liu S, et al. Using location, color, size, and depth to characterize and identify endometriosis lesions in a cohort of 133 women. Fertil Steril. 2008;89(6):1632-1636.
IN THIS ARTICLE
• The “why” of endometriosis
• Environmental factors, estrogen, and endometriosis
• Is imaging useful?
• When is diagnostic laparoscopy clearly indicated?
CASE M.L. is a 32-year-old nulliparous woman who is referred to your office by her primary care provider for chronic pelvic pain. She reports severe dysmenorrhea as her main symptom, but she also mentions dyspareunia. She says these symptoms have been present for several years but have increased in intensity gradually. She asks what you consider to be the most likely diagnosis.
What potential diagnoses do you mention to her? And how do you identify the cause of her pain?
Although endometriosis—the presence of endometrial tissue outside the uterus—affects at least 5 million women of reproductive age in the United States alone, it can be a challenging diagnosis for several reasons.
“Endometriosis is a great masquerader,” says Linda Giudice, MD, PhD. “It presents with a variety of pain patterns, intensities, and triggers. It can also involve symptoms that overlap those of other disorders, including disorders of the gastrointestinal and urinary tracts.”
Although endometriosis falls within the differential diagnosis of chronic pelvic pain, “it is usually not high on the list in the primary care setting (adult and adolescent),” adds Dr. Giudice.
John R. Lue, MD, MPH, an author of the most recent practice bulletin on endometriosis from the American College of Obstetricians and Gynecologists,1 sees the situation similarly.
“The main challenge in the diagnosis of endometriosis is that its presentation mimics other causes of chronic pelvic pain,” he says. “Pelvic pain due to endometriosis is usually chronic (lasting ≥ 6 months). It is associated with dysmenorrhea in 50% to 90% of cases, as well as with dyspareunia, deep pelvic pain, and lower abdominal pain with or without back and loin pain. The pain can occur unpredictably and intermittently throughout the menstrual cycle or it can be continuous. In addition, it can be dull, throbbing, or sharp and may be exacerbated by physical activity.2,3 Up to 20% of women with endometriosis have concurrent pain conditions.”4
Among other diseases of the female pelvis that have a relatively similar presentation, Dr. Lue adds, are pathologies of the
• Uterus (adenomyosis, fibroids)
• Fallopian tube (hydrosalpinx)
• Ovaries (ovarian cysts)
• Bladder (interstitial cystitis)
• Bowel (irritable bowel syndrome)
• Musculoskeletal system (piriformis syndrome).
Before pelvic pain is attributed to endometriosis, he says, the provider should rule out bowel, bladder, musculoskeletal, and psychiatric causes.
This article focuses on seven questions, the answers to which are critical to narrowing in on the diagnosis of endometriosis, including essential factors to consider in the patient history, imaging and other diagnostic tools, and considerations in surgical exploration.
1. WHY SUCH A LONG DELAY IN DIAGNOSIS?
Investigators exploring the length of time from a patient’s presentation with symptoms to diagnosis have found it to be particularly long for endometriosis, ranging from six to 11 years.
Because endometriosis is usually not high on the list of differential diagnoses for chronic pelvic pain in the primary care setting, a patient may not be referred to a gynecologist unless those symptoms include severe dysmenorrhea, dyspareunia, or similar findings. Once the referral is made, the gynecologist “will usually try contraceptive steroids, NSAIDs, or second-line progestins before a diagnosis is made,” says Dr. Giudice.5
The delay in diagnosis “is astounding,” she adds, “and has its roots in empiric medical therapies and a combination of patients fearing a diagnosis of cancer and reluctance of gynecologists to perform laparoscopy on adolescents.”6 Another possible cause of diagnostic delay: Some adolescent girls may not realize when their pain is severe. Because they may have always experienced a high degree of pain since menarche, they may assume it to be a normal aspect of womanhood and delay seeking help, says Pamela Stratton, MD.
Continue to learn any biomarkers proved to be useful diagnostic tools >>
2. HAVE ANY BIOMARKERS PROVED TO BE USEFUL DIAGNOSTIC TOOLS?
Any biomarker proven to reliably identify endometriosis would be a boon to medicine, as it would provide a noninvasive or minimally invasive alternative to diagnostic laparoscopy, the current gold standard. Regrettably, the search for such a biomarker has produced “disappointing results,” says Dr. Giudice.
“Recent systematic reviews of all proposed endometriosis-related biomarkers over the past 25 years in serum, plasma, urine, and endometrium could not identify an unequivocally clinically useful biomarker or panel of biomarkers,” she notes.7,8 “This is due mainly to low numbers of subjects, small populations for validations, cycle/hormonal- and disease stage–dependence, poorly defined controls, and low sensitivity and specificity.”
One hopeful development: “Whole genome transcriptomics of archived endometrial tissue and machine learning found several classifiers to diagnose and stage endometriosis with high accuracy that were validated on an independent sample set,” says Dr. Giudice.9 “However, these data now warrant a prospective, multisite study for further validation.”
Continue for key aspects of patient history >>
3. WHAT ASPECTS OF THE PATIENT HISTORY ARE KEY?
Dr. Stratton recommends that clinicians begin their evaluation of the patient with pain by asking her to describe that pain: how long she has had it, when it occurs, and which areas are affected.
“Most women with endometriosis-associated pain have chronic pelvic pain,” Dr. Stratton continues.5 “Up to 90% of those have dysmenorrhea or cyclic pain with menses.”10 In addition, women with endometriosis “commonly report having pain with any bleeding or spotting. About 30% of women diagnosed with endometriosis initially present to their gynecologist with dyspareunia.”11
“Episodic pain with menses may become more constant, lasting for many days of the month,” says Dr. Stratton. “Women with dyschezia or dysuria may have endometriosis lesions associated with the bowel or bladder, respectively.12 When women with these symptoms do not have lesions on the bowel or bladder, these pain symptoms may occur because of higher peritoneal hormone and inflammatory factor levels or because adjacent organs share the neural networks.”
Dr. Giudice views the history similarly: “I believe listening to the patient is essential in evaluating the possibility of her having endometriosis. This involves asking her to describe where her pain is, grading it on a scale of 1 to 10, identifying when in her cycle it occurs, and learning what makes it better or worse.”
“It also is important to assess the quality of the pain,” she adds. “Does it radiate, does it limit her daily activities, does it interfere with her relationships, intercourse, work, school? Is it associated with bowel movements, urination, other pain syndromes?”
“Having a pain questionnaire is a great help so that patients have a chance to reflect on these and other questions that help to frame the pain associated with endometriosis when they come for consultation,” she adds.
By determining if pain is associated with menstruation or spotting, the clinician is better informed about the value of menstrual suppression, says Dr. Stratton. “Determining what makes the pain better or worse can help define triggers which, if treated, can decrease the likelihood of episodes of pain.”
“A detailed history of any medical or surgical treatments and their outcome is helpful in guiding future treatment,” she adds. “While hormonal therapy has been a mainstay of treatment, in some women, some hormonal treatments may worsen pain or have unacceptable adverse effects, such as worsening depression or anxiety. In addition, some pain—especially that associated with deep lesions—may be relieved by surgical treatment;13,14 pain that worsened after surgery may suggest neural damage.”
“As there is an engagement of the central nervous system, endometriosis is considered a central sensitivity syndrome in which women may also have other sites of pain,” Dr. Stratton says. “Thus, obtaining a history about current symptoms or prior diagnosis of irritable bowel syndrome, interstitial cystitis/painful bladder, migraines, fibromyalgia, or chronic fatigue syndrome is beneficial.10,15-17 Facilitating treatment for these comorbidities is a key principle in helping women with endometriosis-associated pain, as any condition that triggers or perpetuates pain warrants treatment.”
Continue for what the physical exam entails >>
4. WHAT SHOULD THE PHYSICAL EXAM ENTAIL?
“An abdominal exam and a pelvic exam are essential in evaluating pain in a woman when endometriosis is suspected,” says Dr. Giudice. “Sometimes the latter is challenging in young teens and can be deferred.” Overall, however, “the pelvic exam can give insight into pain triggers, adnexal masses (possible endometriomas), and mobility of pelvic organs. A rectovaginal exam is important in evaluating deep infiltrating disease and to gauge the pelvic pain landscape overall. In addition, palpating the pelvic floor musculature is important to distinguish pelvic floor muscle spasm from endometriosis pain.”
“The challenge for clinicians is to think beyond the endometrial implants, taking into account multiple factors that influence pain perception,” says Dr. Stratton. During the examination, the clinician should begin by mapping the regions of pain in the abdomen and back, “distinguishing musculoskeletal pain from deep pain. Determining whether pains are focused or diffuse is also important.”
Dr. Stratton recommends that the routine pelvic exam be modified because a standard bimanual exam “confuses pain signals from the pelvic floor, abdominal wall, bladder, and other viscera. For this reason, a pain-oriented assessment is mandatory.”
Begin with a single digital examination to map tender areas, Dr. Stratton advises. Then consider the size, shape, and mobility of reproductive and pelvic organs. “A bimanual exam will help identify adnexal masses like endometriomas,” she says.
Endometriomas usually are not associated with pain, she adds, but “they are associated with deep infiltrating lesions. Nodularity along the uterosacral ligaments, limited reproductive organ mobility, and thickening of the rectovaginal septum also suggest deep infiltrating lesions. Importantly, deep infiltrating lesions are the lesion type most associated with pain.”18,19
Continue to learn if imaging is useful in the diagnosis of endometriosis >>
5. IS IMAGING USEFUL IN THE DIAGNOSIS OF ENDOMETRIOSIS?
Laparoscopy remains the gold standard for diagnosis of endometriosis, observes Steven R. Goldstein, MD. Visualization of endometriotic implants at the time of surgery—with histologic assessment—offers definitive confirmation of the diagnosis. The physical examination, too, can offer a strong suggestion of endometriosis, he says.
“In the past, the pelvic examination and history often were the sine qua non for patients with pain,” Dr. Goldstein says. “Extreme dysmenorrhea and pain between periods, especially with intercourse, defecation, and exercise, all increased the suspicion of endometriosis. People used to talk about feeling nodularity in the uterosacral ligaments and finding decreased mobility of pelvic structures—but I don’t have any question that the skill of today’s gynecologists in doing a bimanual pelvic exam is a fraction of what it was in years gone by, because they haven’t had the necessity of experience. The first thing they do if there’s any question is send the patient for an ultrasound.”
Of course, ultrasound can be especially helpful in identifying endometriomas—sometimes called chocolate cysts—in the ovary. Endometriomas can have a solid appearance on ultrasound, says Dr. Goldstein, because the fluid they contain (dried blood) is sonolucent or pure black on ultrasound, similar to amniotic fluid or the fluid seen in the bladder. “This ‘chocolate’ fluid contained in endometriomas is homogeneous, particulate, and very monotonous in its appearance, in contrast to the internal echoes observed in hemorrhagic corpus lutea, which are very cobweb-like and can sometimes mimic papillary projections,” he adds.
“What’s absolutely essential when imaging a suspected endometrioma by ultrasound is that there be no evidence of any blood flow contained within that structure. Because it’s dried blood, it shouldn’t have any vascularity. If you see blood flow inside what you would call an endometrioma, you need to rethink your diagnosis,” he says.
In some cases, a supposed endometrioma lacks a black, sonolucent appearance, but “the clinician often can tell that it’s a cystic structure by the very bright posterior wall—what we call posterior wall acoustic enhancement—even though the interior of the structure may appear sort of grayish or whitish rather than the pure black of a simple cyst. It’s still fluid-filled,” Dr. Goldstein says.
In some instances, even endometriotic nodules can be imaged by ultrasound, he adds. “There’s an increasing body of literature that suggests that, if you look carefully in people with deep infiltrating endometriosis, you can often see solid-appearing nodules in the rectovaginal septum or between the uterus and bladder. With the kind of resolution that we now have with the vaginal probe, some of these nodules can be seen. That’s somewhat new, and it’s a function of two things—people looking for endometriosis and the better resolution of more modern equipment.”
Dr. Goldstein believes that MRI is “almost never” indicated in the diagnosis of endometriosis. A more helpful approach would be a consultative ultrasound with someone with more experience. However, when that is not available, or “in areas where you have excellent backup in terms of pelvic MRI, that may be the way to go. I don’t think so,” he demurs, “and some of my colleagues would be very upset at the thought of needing to use MRI to diagnose endometriosis. But in the occasional confusing or difficult case, depending on the quality of the referral pattern you have, it might make sense."
Continue to learn when diagnostic laparoscopy is clearly indicated >>
6. WHEN IS DIAGNOSTIC LAPAROSCOPY CLEARLY INDICATED?
Dr. Giudice believes that laparoscopy—with the intention to treat endometriosis, if present—“is essential when firstline medical therapy fails or when pain is acute and severe.”5
Dr. Stratton concurs. “Any woman with chronic pain wants to know what is causing the pain,” she says. Therefore, “women report a benefit from knowing that their pain is associated with endometriosis.6 However, diagnostic laparoscopy alone, with the sole purpose of determining the presence of endometriosis but not treating the lesions, is no longer performed, as it poses little benefit to the patient other than peace of mind.”
“The general trend in the US has been to first use hormonal treatments when the diagnosis of endometriosis is suspected, prior to performing surgery,” Dr. Stratton says.1 In many cases, by using cyclic combined hormonal contraceptives to reduce menstrual flow or “suppressing menstruation with continuous combined hormonal contraceptives,” gonadotropin-releasing hormone analogues (combined with progestin to prevent bone loss) “or continuous progestin alone may be effective in decreasing pain. Not surprisingly, these hormonal approaches are effective for any chronic pelvic pain, even for women who do not have the surgical diagnosis of endometriosis.”20
“When the firstline approach to chronic pelvic pain is hormonal treatment, laparoscopy is considered when these medical treatments have failed to control the pain or are poorly tolerated, or when the diagnosis of endometriosis is in question,” Dr. Stratton says.
“Laparoscopy to treat endometriomas is indicated if an endometrioma is enlarging or measures more than 4 cm in diameter, or if the diagnosis of an ovarian mass is in question,” she explains. “While surgeons have previously been aggressive in removing endometriomas, this practice may have negative consequences on ovarian function. Because endometriomas are pseudocysts, removing them completely leads to the removal of viable ovarian tissue and may diminish ovarian reserve.”21,22
Continue for the surgical appearance of endometriosis >>
7. WHAT IS THE SURGICAL APPEARANCE OF ENDOMETRIOSIS?
Dr. Giudice returns to the enigmatic nature of endometriosis in addressing this question, mentioning its “many faces” at the time of surgery. “It is imperative that the surgeon recognize the disease in its many forms,” she says. “Also, it is especially helpful at the time of surgery if suspected lesions are biopsied and sent to pathology to have the diagnosis made unequivocally.”5
As for the surgical appearance of endometriosis, Dr. Stratton notes that there are three types of lesions—“superficial lesions, deep infiltrating lesions, and endometriomas. Endometriomas occur almost exclusively in the ovary and are pseudocysts without an identifiable cystic lining. They vary in dimension from a few millimeters to several centimeters.”
“Superficial peritoneal endometriosis lesions have a variable appearance, with some lesions being clear or red; some brown, blue or black; and some having a white appearance, like a scar,” says Dr. Stratton. “Endometriosis can be diagnosed on histologic examination of any of these lesion types."
“Overall, single-color lesions have similar frequencies of biopsy-confirmed endometriosis (59% to 62%),” she says.23 “These lesion appearances likely represent different stages of development of endometriosis, with red or clear lesions occurring first, soon after endometrial tissue implantation; black, blue, or brown lesions occurring later, in response to the hormones varying in the menstrual cycle; and white lesions occurring as the lesions age. Deep infiltrating lesions generally have blue/black or white features.”
“Wide, deep, multiple-color lesions in the cul-de-sac, ovarian fossa, or uterosacral ligaments are most likely endometriosis,” Dr. Stratton adds.23 Only lesions with multiple colors have a significantly higher percentage of positive biopsies (76%). Importantly, more than half of women with only subtle lesions (small red or white lesions) have endometriosis.
You tell the patient that endometriosis is one of the possible diagnoses for her chronic pelvic pain, and you take a focused history. During a pelvic examination, you observe that her right ovary lacks mobility, and you map a number of trigger points for her pain. Transvaginal ultrasound results suggest the presence of nodules in the rectovaginal septum. You begin empiric treatment with continuous combined hormonal contraceptives to suppress menstruation. On her next visit, M.L. reports reduced but still bothersome pain. Laparoscopy reveals a 2-cm endometrioma in the right ovary and deep infiltrating lesions in the cul-de-sac. The endometrioma is resected. Histology confirms the diagnosis of endometriosis.
REFERENCES
1. American College of Obstetricians and Gynecologists. Practice Bulletin #114: Management of endometriosis. Obstet Gynecol. 2010; 116(1):223-236.
2. Sanfilippo JS, Wakim NG, Schikler KN, Yussman MA. Endometriosis in association with uterine anomaly. Am J Obstet Gynecol. 1986; 154(1):39-43.
3. Taylor HS, Bagot C, Kardana A. HOX gene expression is altered in the endometrium of women with endometriosis. Hum Reprod. 1999; 14(5):1328-1331.
4. Berkley KJ, Stratton P. Mechanisms: lessons from translational studies of endometriosis. In: Giamberardino MA, ed. Visceral Pain: Clinical, Pathophysiological and Therapeutic Aspects. Oxford, UK: Oxford University Press; 2009:39-50.
5. Giudice LC. Clinical practice: endometriosis. N Engl J Med. 2010;362(25): 2389-2398.
6. Ballard K, Lowton K, Wright J. What’s the delay: a qualitative study of women’s experiences of reaching a diagnosis of endometriosis. Fertil Steril. 2006;86(5):1296-1301.
7. May KE, Conduit-Hulbert SA, Villar J, et al. Peripheral biomarkers of endometriosis: a systematic review. Hum Reprod Update. 2010; 16(6):651-674.
8. May KE, Villar J, Kirtley S, et al. Endometrial alterations in endometriosis: a systematic review of putative biomarkers. Hum Reprod Update. 2011; 17(5):637-653.
9. Tamaresis JS, Irwin JC, Goldfien GA, et al. Molecular classification of endometriosis and disease stage using high-dimensional genomic data. Endocrinology. 2014;155(12):4986-4999.
10. Sinaii N, Cleary SD, Ballweg ML, et al. High rates of autoimmune and endocrine disorders, fibromyalgia, chronic fatigue syndrome and atopic diseases among women with endometriosis: a survey analysis. Hum Reprod. 2002;17(10):2715-2724.
11. De Graaff AA, D’Hooghe TM, Dunselman GA, et al. The significant effect of endometriosis on physical, mental and social wellbeing: results from an international cross-sectional survey. Hum Reprod. 2013;28(10):2677-2685.
12. Lafay Pillet MC, Huchon C, Santulli P, et al. A clinical score can predict associated deep infiltrating endometriosis before surgery for an endometrioma. Hum Reprod. 2014;29(8):1666-1676.
13. Healey M, Cheng C, Kaur H. To excise or ablate endometriosis? A prospective randomized double-blinded trial after 5-year follow-up. J Minim Invasive Gynecol. 2014;21(6):999-1004.
14. Anaf V, El Nakadi I, De Moor V, et al. Increased nerve density in deep infiltrating endometriotic nodules. Gynecol Obstet Invest. 2011;71(2):112-117.
15. Stratton P, Berkley KJ. Chronic pelvic pain and endometriosis: translational evidence of the relationship and implications. Hum Reprod Update. 2011;17(3):327-346.
16. Karp BI, Sinaii N, Nieman LK, et al. Migraine in women with chronic pelvic pain with and without endometriosis. Fertil Steril. 2011;95(3):895-899.
17. Berkley KJ. A life of pelvic pain. Physiol Behav. 2005;86(3):272-280.
18. Fauconnier A, Chapron C. Endometriosis and pelvic pain: epidemiological evidence of the relationship and implications. Hum Reprod Update. 2005;11(6):595-606.
19. Vercellini P, Fedele L, Aimi G, et al. Association between endometriosis stage, lesion type, patient characteristics and severity of pelvic pain symptoms: a multivariate analysis of over 1000 patients. Hum Reprod. 2007;22(1):266-271.
20. Ling FW. Randomized controlled trial of depot leuprolide in patients with chronic pelvic pain and clinically suspected endometriosis. Pelvic Pain Study Group. Obstet Gynecol. 1999;93(1):51-58.
21. Muzii L, Di Tucci C, Di Feliciantonio M, et al. The effect of surgery for endometrioma on ovarian reserve evaluated by antral follicle count: a systematic review and meta-analysis. Hum Reprod. 2014;29(10):2190-2198.
22. Muzii L, Luciano AA, Zupi E, Panici PB. Effect of surgery for endometrioma on ovarian function: a different point of view. J Minim Invasive Gynecol. 2014;21(4):531-533.
23. Stegmann BJ, Sinaii N, Liu S, et al. Using location, color, size, and depth to characterize and identify endometriosis lesions in a cohort of 133 women. Fertil Steril. 2008;89(6):1632-1636.