User login
In cutaneous field cancerization, focal treatments such as cryotherapy are impractical, thus necessitating the use of field-directed therapies over the lesion and the surrounding skin field. Although evidence-based guidelines do not exist, field-directed therapy has been proposed in cases of 3 or more actinic keratoses (AKs) in a 25-cm2 area or larger.1 It can be further speculated that patients who are vulnerable to aggressive phenotypes of cutaneous malignancies, such as those with a genodermatosis or who are immunocompromised, necessitate a higher index of suspicion for field effect with even 1 or 2 AKs.
Current field-directed therapies include topical agents (imiquimod, fluorouracil, ingenol mebutate, and diclo-fenac), photodynamic therapy (PDT), and resurfacing procedures (lasers, chemical peels, dermabrasion). Although topical agents and PDT currently are gold standards in field treatment, the use of energy-based devices (ie, ablative and nonablative lasers) are attractive options as monotherapy or as part of a combination therapy. These devices are attractive options for field-directed therapy because they offer defined, customizable control of settings, allowing for optimal cosmesis and precision of therapy.
Principally, lasers function by damaging skin tissue to induce resurfacing, neocollagenesis, and vascular restructuring. Fractional versions of ablative and nonablative systems are available to target a fraction of the treatment area in evenly spaced microthermal zones and to minimize overall thermal damage.2
Given recent advances in laser systems and numerous investigations reported in the literature, a review of ablative and nonablative lasers that have been studied as treatment options for cutaneous field cancerization is provided, with a focus on treatment efficacy.
Ablative Lasers
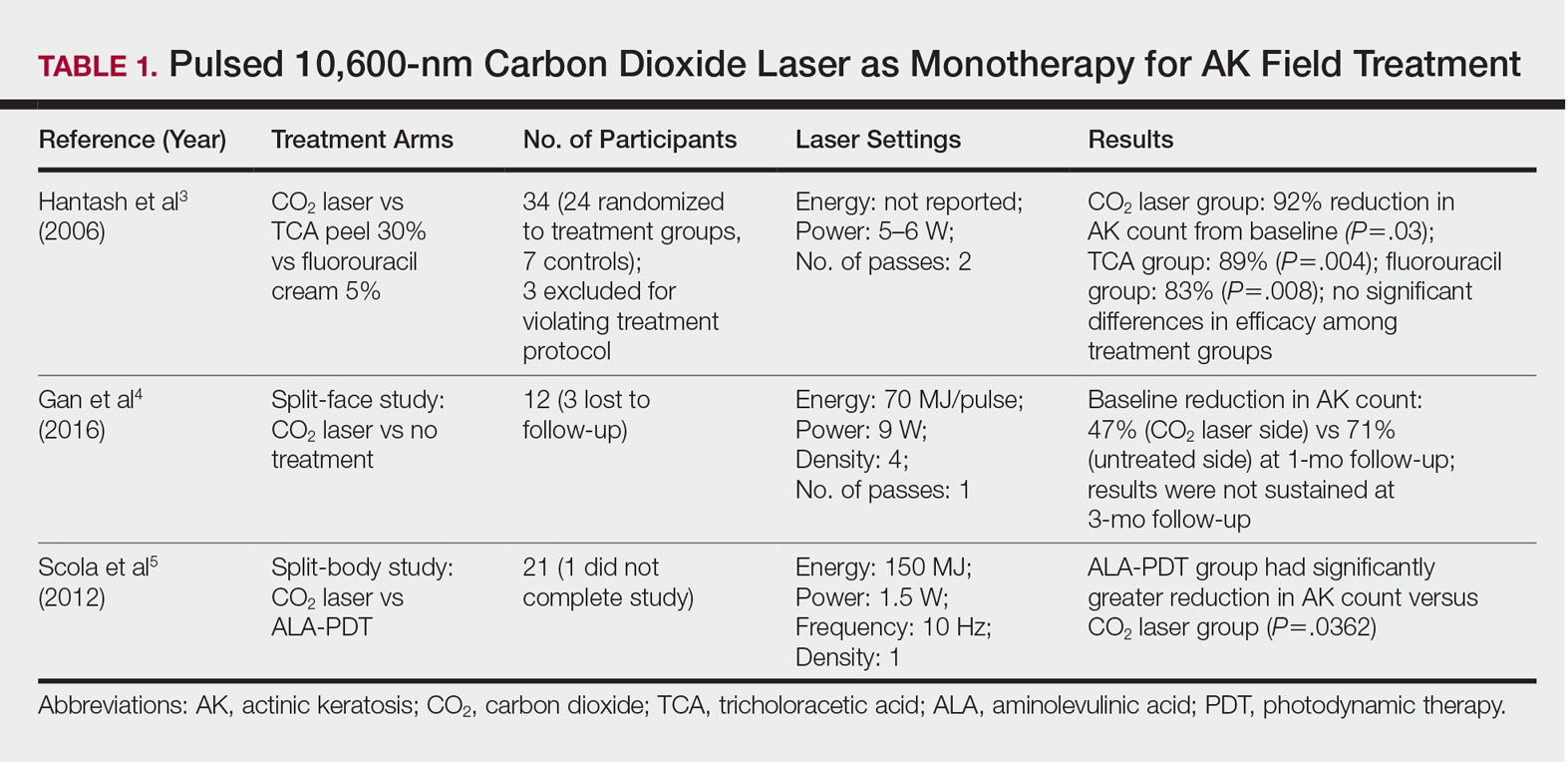
Ablative lasers operate at higher wavelengths than nonablative lasers to destroy epidermal and dermal tissue. The 10,600-nm carbon dioxide (CO2) and 2940-nm Er:YAG lasers have been heavily investigated for field therapy for multiple AKs, both as monotherapies (Table 1) and in combination with PDT (Table 2).
Monotherapy
One randomized trial with 5-year follow-up compared the efficacy of full-face pulsed CO2 laser therapy, full-face trichloroacetic acid (TCA) peel 30%, and fluorouracil cream 5% (twice daily for 3 weeks) on AKs on the face and head.3 Thirty-one participants were randomized to the 3 treatment arms and a negative control arm. The mean AK counts at baseline for the CO2, TCA, and fluorouracil treatment groups were 78.0, 83.7, and 61.8, respectively. At 3-month follow-up, all treatment groups had significant reductions in the mean AK count from baseline (CO2 group, 92% [P=.03]; TCA group, 89% [P=.004]; fluorouracil group, 83% [P=.008]). No significant differences in efficacy among the treatment groups were noted. All 3 treatment groups had a demonstrably lower incidence of nonmelanoma skin cancer over 5-year follow-up compared to the control group (P<.001).3
In contrast to these promising results, the pulsed CO2 laser showed only short-term efficacy in a split-face study of 12 participants with at least 5 facial or scalp AKs on each of 2 symmetric facial sides who were randomized to 1 treatment side.4 At 1-month follow-up, the treatment side exhibited significantly fewer AKs compared to the control side (47% vs 71% at baseline; P=.01), but the improvement was not sustained at 3-month follow-up (49% vs 57%; P=.47).4
In another study, the CO2 laser was found to be inferior to 5-aminolevulinic acid PDT.5 Twenty-one participants who had at least 4 AKs in each symmetric half of a body region (head, hands, forearms) were randomized to PDT on 1 side and CO2 laser therapy on the other. Median baseline AK counts for the PDT and CO2 laser groups were 6 and 8, respectively. Both treatment groups exhibited significant median AK reduction from baseline 4 weeks posttreatment (PDT group, 82.1% [P<.05], CO2 laser group, 100% [P<.05]); however. at 3 months posttreatment the PDT group had significantly higher absolute (P=.0155) and relative (P=.0362) reductions in AK count compared to the CO2 laser group. One participant received a topical antibiotic for superficial infection on the PDT treatment side.5
Many questions remain regarding the practical application of laser ablation monotherapy for multiple AKs. More studies are needed to determine the practicality and long-term clinical efficacy of these devices.
PDT Combination Therapy
Laser ablation may be combined with PDT to increase efficacy and prolong remission rates. In fact, laser ablation may be thought of as a physical drug-delivery system to boost uptake of topical agents—in this case, aminolevulinic acid and methyl aminolevulinate (MAL)—given that it disrupts the skin barrier.
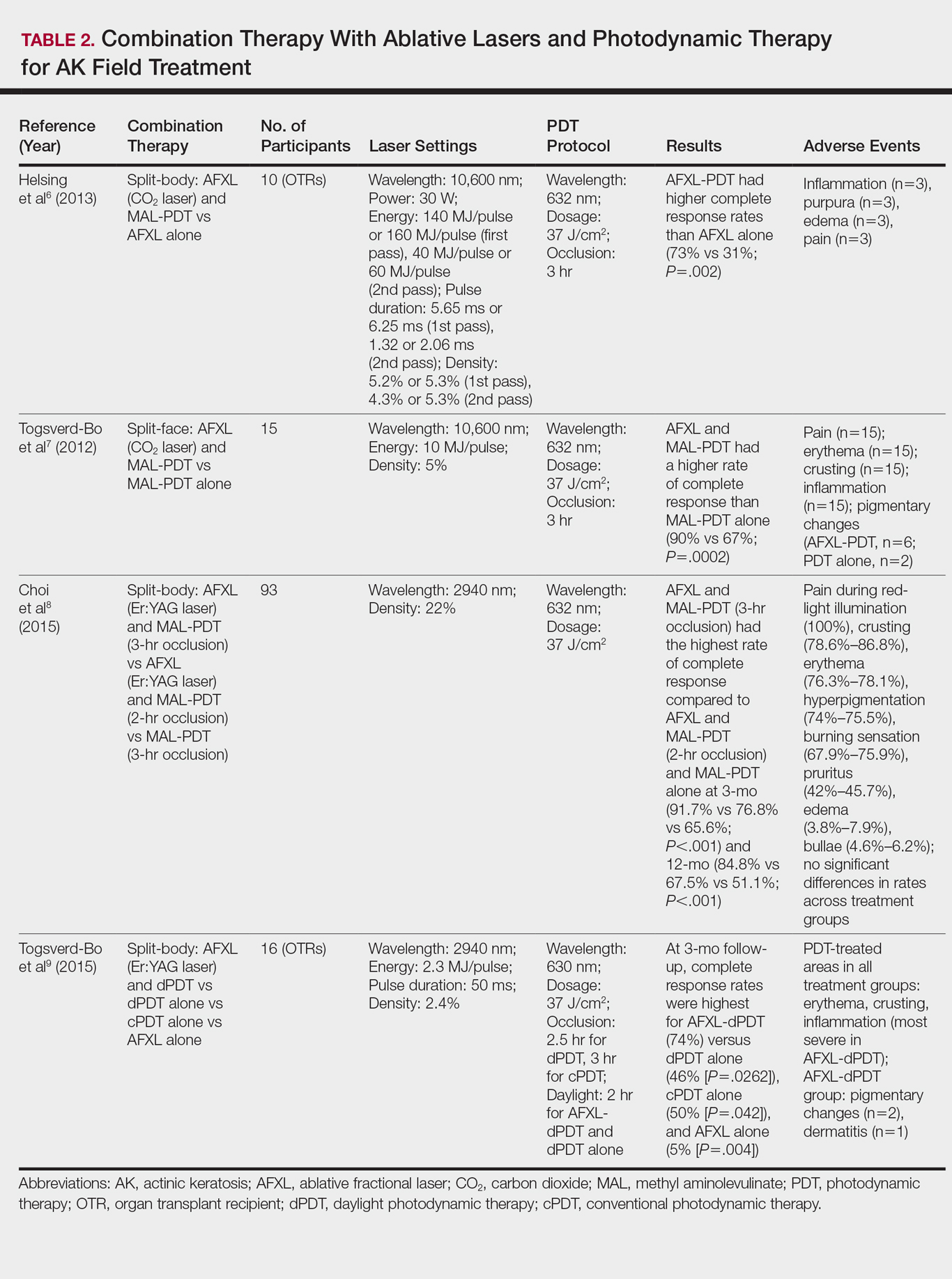
In a comparative study of ablative fractional laser (AFXL)–assisted PDT and AFXL alone in 10 organ transplant recipients on immunosuppression with at least 5 AKs on each dorsal hand, participants were randomized to AFXL-PDT on one treatment side and PDT on the other side.6 Participants received AFXL in an initial lesion-directed pass and then a second field-directed pass of a fractional CO2 laser. After AFXL exposure, methyl aminolevulinate was applied to the AFXL-PDT treatment side, with 3-hour occlusion. A total of 680 AKs were treated (335 in the AFXL-PDT group, 345 in the PDT group); results were stratified by the clinical grade of the lesion (1, slightly palpable; 2, moderately thick; 3, very thick or obvious). At 4-month follow-up, the AFXL-PDT group had a significantly higher median complete response rate of 73% compared to 31% in the AFXL group (P=.002). Interestingly, AFXL-PDT was also significantly more efficacious compared to AFXL for grades 1 (80% vs 37%; P=.02) and 2 (53% vs 7%, P=.009) AKs but not grade 3 AKs (4% vs 0%, P=.17).6
The combination of fractional CO2 laser and PDT also demonstrated superiority to PDT.7 In a split-face investigation, 15 participants with bilateral symmetric areas of 2 to 10 AKs on the face or scalp were randomized to receive fractional CO2 laser and MAL-PDT combination therapy on 1 treatment side and conventional MAL-PDT on the other side.7 The AFXL-PDT treatment side received laser ablation with immediate subsequent application of MAL to both treatment sides under 3-hour occlusion. At baseline, 103 AKs were treated by AFXL-PDT and 109 AKs were treated with conventional PDT. At 3-month follow-up, the AFXL-PDT treatment group exhibited a significantly higher rate of complete response (90%) compared to the conventional PDT group (67%)(P=.0002).7
Like the CO2 laser, the Er:YAG laser has demonstrated superior results when used in combination with PDT to treat field cancerization compared to either treatment alone. In a comparison study, 93 patients with 2 to 10 AK lesions on the face or scalp were randomized to treatment with AFXL (Er:YAG laser) and MAL-PDT with 3-hour occlusion, AFXL (Er:YAG laser) and MAL-PDT with 2-hour occlusion, and MAL-PDT with 3-hour occlusion.8 A total of 440 baseline AK lesions on the face or scalp were treated. At 3-month follow-up, the AFXL-PDT (3-hour occlusion) group had the highest rate of complete response (91.7%), compared to 76.8% (P=.001) in the AFXL-PDT (2-hour occlusion) and 65.6% (P=.001) in the PDT groups, regardless of the grade of AK lesion. The AFXL-PDT (2-hour occlusion) treatment was also superior to PDT alone (P=.038). These findings were sustained at 12-month follow-up (84.8% in the AFXL-PDT [3-hour occlusion] group [P<.001, compared to others]; 67.5% in the AFXL-PDT [2-hour occlusion] group [P<.001, compared to 3-hour PDT]; 51.1% in the PDT group). Importantly, the AK lesion recurrence rate was also lowest in the AFL-PDT (3-hour occlusion) group (7.5% vs 12.1% and 22.1% in the AFXL-PDT [2-hour occlusion] and PDT groups, respectively; P=.007).8
Combination therapy with AFXL and daylight PDT (dPDT) may improve the tolerability of PDT and the efficacy rate of field therapy in organ transplant recipients. One study demonstrated the superiority of this combination therapy in a population of 16 organ transplant recipients on immunosuppressants with at least 2 moderate to severely thick AKs in each of 4 comparable areas in the same anatomic region.9 The 4 areas were randomized to a single session of AFXL-dPDT, dPDT alone, conventional PDT, or AFXL alone. Ablation was performed with a fractional Er:YAG laser. The AFXL-dPDT and dPDT alone groups received MAL for 2.5 hours without occlusion, and the conventional PDT group received MAL for 3 hours with occlusion. Daylight exposure in dPDT groups was initiated 30 minutes after MAL application for 2 hours total. A baseline total of 542 AKs were treated. At 3-month follow-up, the complete response rate was highest for the AFXL-dPDT group (74%) compared to dPDT alone (46%; P=.0262), conventional PDT (50%; P=.042), and AFXL alone (5%; P=.004). Pain scores for AFXL–dPDT and dPDT alone were significantly lower than for conventional PDT and AFXL alone (P<.001).9
Nonablative Lasers
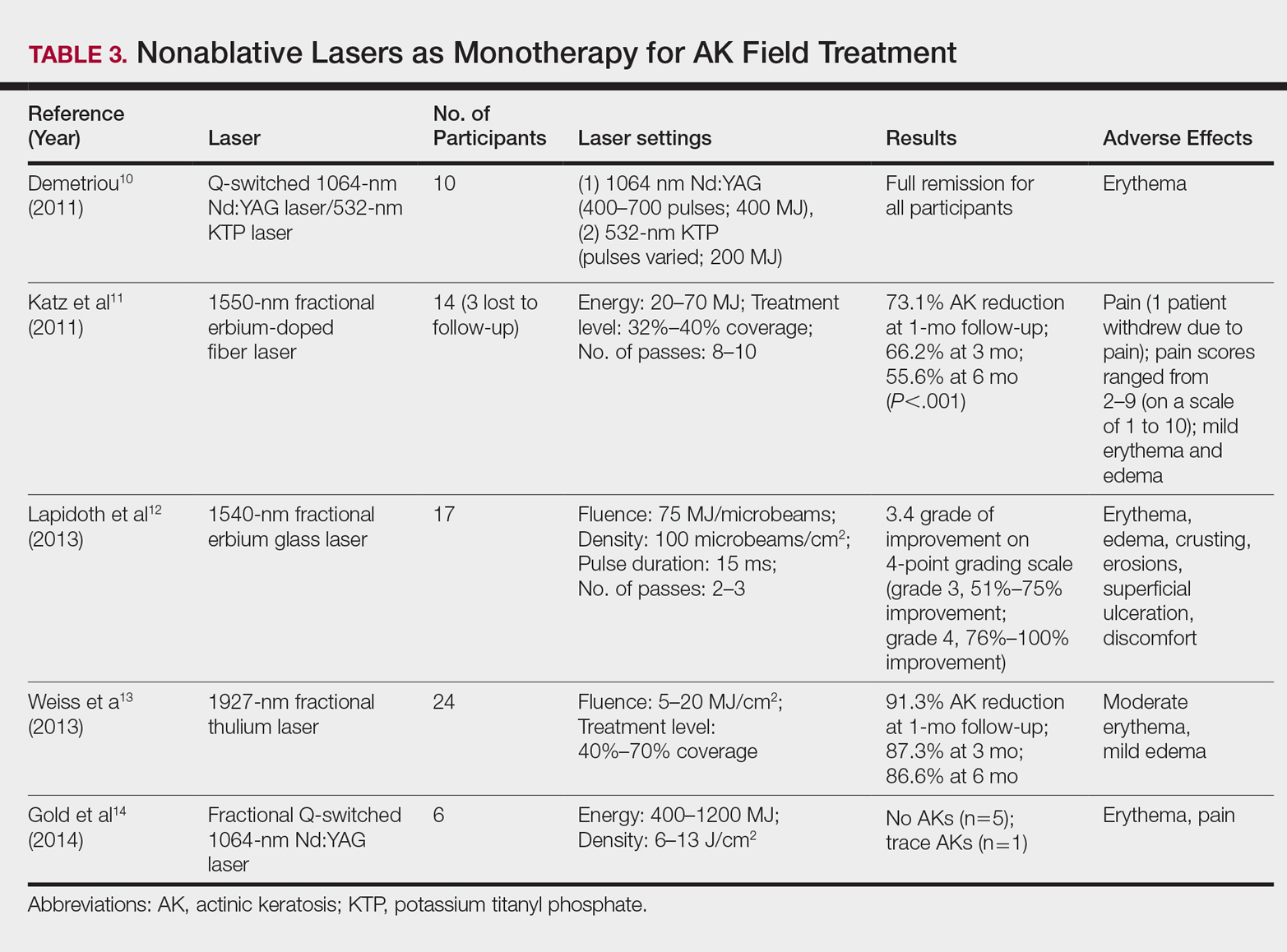
By heating the dermis to induce neogenesis without destruction, nonablative lasers offer superior healing times compared to their ablative counterparts. Multiple treatments with nonablative lasers may be necessary for maximal effect. Four nonablative laser devices have demonstrated efficacy in the treatment of multiple AKs10-14: (1) the Q-switched 1064-nm Nd:YAG laser, with or without a 532-nm potassium titanyl phosphate (KTP) laser; (2) the 1540-nm fractional erbium glass laser; (3) the 1550-nm fractional erbium-doped fiber laser; and (4) the 1927-nm fractional thulium laser (Table 3).
In a proof-of-concept study of the Q-switched Nd:YAG laser with the 532-nm KTP laser, 1 treatment session induced full remission of AKs in 10 patients at follow-up day 20, although the investigator did not grade improvement on a numerical scale.10 In a study of the fractional Q-switched 1064-nm Nd:YAG laser alone, 6 patients with trace or mild AKs received 4 treatment sessions at approximately 2-week intervals.14 All but 1 patient (who had trace AKs) had no AKs at 3-month follow-up.
The efficacy of the 1540-nm fractional erbium glass laser was examined in 17 participants with investigator-rated moderate-to-severe AK involvement of the scalp and face.12 Participants were given 2 or 3 treatment sessions at 3- to 4-week intervals and were graded by blinded dermatologists on a quartile scale of 0 (no improvement), 1 (1%–25% improvement), 2 (26%–50% improvement), 3 (51%–75% improvement), or 4 (76%–100% improvement). At 3 months posttreatment, the average grade of improvement was 3.4.12
The 1550-nm fractional erbium-doped fiber laser was tested in 14 men with multiple facial AKs (range, 9–44 AKs [mean, 22.1 AKs]).11 Participants received 5 treatment sessions at 2- to 4-week intervals, with majority energies used at 70 MJ and treatment level 11. The mean AK count was reduced significantly by 73.1%, 66.2%, and 55.6% at 1-, 3-, and 6-month follow-up, respectively (P<.001).11
The 1927-nm fractional thulium laser showed promising results in 24 participants with facial AKs.13 Participants received up to 4 treatment sessions at intervals from 2 to 6 weeks at the investigators’ discretion. At baseline, patients had an average of 14.04 facial AKs. At 1-, 3-, and 6-month follow-up, participants exhibited 91.3%, 87.3%, and 86.6% reduction in AK counts, respectively. The mean AK count at 3-month follow-up was 1.88.13
Due to limited sample sizes and/or lack of quantifiable results and controls in these studies, more studies are needed to fully elucidate the role of nonablative lasers in the treatment of AK.
Future Directions
Iontophoresis involves the noninvasive induction of an electrical current to facilitate ion movement through the skin and may be a novel method to boost the efficacy of current field therapies. In the first known study of its kisnd, iontophoresis-assisted AFXL-PDT was found to be noninferior to conventional AFXL-PDT15; however, additional studies demonstrating its superiority are needed before more widespread clinical use is considered.
Pretreatment with AFXL prior to topical field-directed therapies also has been proposed.16 In a case series of 13 patients, combination therapy with AFXL and ingenol mebutate was shown to be superior to ingenol mebutate alone (AK clearance rate, 89.2% vs 72.1%, respectively; P<.001).16 Randomized studies with longer follow-up time are needed.
Conclusion
Ablative and nonablative laser systems have yielded limited data about their potential as monotherapies for treatment of multiple AKs and are unlikely to replace topical agents and PDT as a first-line modality in field-directed treatment at this time. More studies with a larger number of participants and long-term follow-up are needed for further clarification of efficacy, safety, and clinical feasibility. Nevertheless, fractional ablative lasers in combination with PDT have shown robust efficacy and a favorable safety profile for treatment of multiple AKs.6-9 Further, this combination therapy exhibited a superior clearance rate and lower lesion recurrence in organ transplant recipients—a demographic that classically is difficult to treat.6-9
With continued rapid evolution of laser systems and more widespread use in dermatology, monotherapy and combination therapy may offer a dynamic new option in field cancerization that can decrease disease burden and treatment frequency.
- Peris K, Calzavara-Pinton PG, Neri L, et al. Italian expert consensus for the management of actinic keratosis in immunocompetent patients. J Eur Acad Dermatol Venereol. 2016;30:1077-1084.
- Alexiades-Armenakas MR, Dover JS, Arndt KA. The spectrum of laser skin resurfacing: nonablative, fractional, and ablative laser resurfacing. J Am Acad Dermatol. 2008;58:719-737; quiz 738-740.
- Hantash BM, Stewart DB, Cooper ZA, et al. Facial resurfacing for nonmelanoma skin cancer prophylaxis. Arch Dermatol. 2006;142:976-982.
- Gan SD, Hsu SH, Chuang G, et al. Ablative fractional laser therapy for the treatment of actinic keratosis: a split-face study. J Am Acad Dermatol. 2016;74:387-389.
- Scola N, Terras S, Georgas D, et al. A randomized, half-side comparative study of aminolaevulinate photodynamic therapy vs. CO(2) laser ablation in immunocompetent patients with multiple actinic keratoses. Br J Dermatol. 2012;167:1366-1373.
- Helsing P, Togsverd-Bo K, Veierod MB, et al. Intensified fractional CO2 laser-assisted photodynamic therapy vs. laser alone for organ transplant recipients with multiple actinic keratoses and wart-like lesions: a randomized half-side comparative trial on dorsal hands. Br J Dermatol. 2013;169:1087-1092.
- Togsverd-Bo K, Haak CS, Thaysen-Petersen D, et al. Intensified photodynamic therapy of actinic keratoses with fractional CO2 laser: a randomized clinical trial. Br J Dermatol. 2012;166:1262-1269.
- Choi SH, Kim KH, Song KH. Efficacy of ablative fractional laser-assisted photodynamic therapy with short-incubation time for the treatment of facial and scalp actinic keratosis: 12-month follow-up results of a randomized, prospective, comparative trial. J Eur Acad Dermatol Venereol. 2015;29:1598-1605.
- Togsverd-Bo K, Lei U, Erlendsson AM, et al. Combination of ablative fractional laser and daylight-mediated photodynamic therapy for actinic keratosis in organ transplant recipients—a randomized controlled trial. Br J Dermatol. 2015;172:467-474.
- Demetriou C. Reversing precancerous actinic damage by mixing wavelengths (1064 nm, 532 nm). J Cosmet Laser Ther. 2011;13:113-119.
- Katz TM, Goldberg LH, Marquez D, et al. Nonablative fractional photothermolysis for facial actinic keratoses: 6-month follow-up with histologic evaluation. J Am Acad Dermatol. 2011;65:349-356.
- Lapidoth M, Adatto M, Halachmi S. Treatment of actinic keratoses and photodamage with non-contact fractional 1540-nm laser quasi-ablation: an ex vivo and clinical evaluation. Lasers Med Sci. 2013;28:537-542.
- Weiss ET, Brauer JA, Anolik R, et al. 1927-nm fractional resurfacing of facial actinic keratoses: a promising new therapeutic option. J Am Acad Dermatol. 2013;68:98-102.
- Gold MH, Sensing W, Biron J. Fractional Q-switched 1,064-nm laser for the treatment of photoaged-photodamaged skin. J Cosmet Laser Ther. 2014;16:69-76.
- Choi SH, Kim TH, Song KH. Efficacy of iontophoresis-assisted ablative fractional laser photodynamic therapy with short incubation time for the treatment of actinic keratosis: 12-month follow-up results of a prospective, randomised, comparative trial. Photodiagnosis Photodyn Ther. 2017;18:105-110.
- Nisticò S, Sannino M, Del Duca E, et al. Ablative fractional laser improves treatment of actinic keratoses with ingenol mebutate. Eur J Inflamm. 2016;14:200-205.
In cutaneous field cancerization, focal treatments such as cryotherapy are impractical, thus necessitating the use of field-directed therapies over the lesion and the surrounding skin field. Although evidence-based guidelines do not exist, field-directed therapy has been proposed in cases of 3 or more actinic keratoses (AKs) in a 25-cm2 area or larger.1 It can be further speculated that patients who are vulnerable to aggressive phenotypes of cutaneous malignancies, such as those with a genodermatosis or who are immunocompromised, necessitate a higher index of suspicion for field effect with even 1 or 2 AKs.
Current field-directed therapies include topical agents (imiquimod, fluorouracil, ingenol mebutate, and diclo-fenac), photodynamic therapy (PDT), and resurfacing procedures (lasers, chemical peels, dermabrasion). Although topical agents and PDT currently are gold standards in field treatment, the use of energy-based devices (ie, ablative and nonablative lasers) are attractive options as monotherapy or as part of a combination therapy. These devices are attractive options for field-directed therapy because they offer defined, customizable control of settings, allowing for optimal cosmesis and precision of therapy.
Principally, lasers function by damaging skin tissue to induce resurfacing, neocollagenesis, and vascular restructuring. Fractional versions of ablative and nonablative systems are available to target a fraction of the treatment area in evenly spaced microthermal zones and to minimize overall thermal damage.2
Given recent advances in laser systems and numerous investigations reported in the literature, a review of ablative and nonablative lasers that have been studied as treatment options for cutaneous field cancerization is provided, with a focus on treatment efficacy.
Ablative Lasers
Ablative lasers operate at higher wavelengths than nonablative lasers to destroy epidermal and dermal tissue. The 10,600-nm carbon dioxide (CO2) and 2940-nm Er:YAG lasers have been heavily investigated for field therapy for multiple AKs, both as monotherapies (Table 1) and in combination with PDT (Table 2).
Monotherapy
One randomized trial with 5-year follow-up compared the efficacy of full-face pulsed CO2 laser therapy, full-face trichloroacetic acid (TCA) peel 30%, and fluorouracil cream 5% (twice daily for 3 weeks) on AKs on the face and head.3 Thirty-one participants were randomized to the 3 treatment arms and a negative control arm. The mean AK counts at baseline for the CO2, TCA, and fluorouracil treatment groups were 78.0, 83.7, and 61.8, respectively. At 3-month follow-up, all treatment groups had significant reductions in the mean AK count from baseline (CO2 group, 92% [P=.03]; TCA group, 89% [P=.004]; fluorouracil group, 83% [P=.008]). No significant differences in efficacy among the treatment groups were noted. All 3 treatment groups had a demonstrably lower incidence of nonmelanoma skin cancer over 5-year follow-up compared to the control group (P<.001).3
In contrast to these promising results, the pulsed CO2 laser showed only short-term efficacy in a split-face study of 12 participants with at least 5 facial or scalp AKs on each of 2 symmetric facial sides who were randomized to 1 treatment side.4 At 1-month follow-up, the treatment side exhibited significantly fewer AKs compared to the control side (47% vs 71% at baseline; P=.01), but the improvement was not sustained at 3-month follow-up (49% vs 57%; P=.47).4
In another study, the CO2 laser was found to be inferior to 5-aminolevulinic acid PDT.5 Twenty-one participants who had at least 4 AKs in each symmetric half of a body region (head, hands, forearms) were randomized to PDT on 1 side and CO2 laser therapy on the other. Median baseline AK counts for the PDT and CO2 laser groups were 6 and 8, respectively. Both treatment groups exhibited significant median AK reduction from baseline 4 weeks posttreatment (PDT group, 82.1% [P<.05], CO2 laser group, 100% [P<.05]); however. at 3 months posttreatment the PDT group had significantly higher absolute (P=.0155) and relative (P=.0362) reductions in AK count compared to the CO2 laser group. One participant received a topical antibiotic for superficial infection on the PDT treatment side.5
Many questions remain regarding the practical application of laser ablation monotherapy for multiple AKs. More studies are needed to determine the practicality and long-term clinical efficacy of these devices.
PDT Combination Therapy
Laser ablation may be combined with PDT to increase efficacy and prolong remission rates. In fact, laser ablation may be thought of as a physical drug-delivery system to boost uptake of topical agents—in this case, aminolevulinic acid and methyl aminolevulinate (MAL)—given that it disrupts the skin barrier.
In a comparative study of ablative fractional laser (AFXL)–assisted PDT and AFXL alone in 10 organ transplant recipients on immunosuppression with at least 5 AKs on each dorsal hand, participants were randomized to AFXL-PDT on one treatment side and PDT on the other side.6 Participants received AFXL in an initial lesion-directed pass and then a second field-directed pass of a fractional CO2 laser. After AFXL exposure, methyl aminolevulinate was applied to the AFXL-PDT treatment side, with 3-hour occlusion. A total of 680 AKs were treated (335 in the AFXL-PDT group, 345 in the PDT group); results were stratified by the clinical grade of the lesion (1, slightly palpable; 2, moderately thick; 3, very thick or obvious). At 4-month follow-up, the AFXL-PDT group had a significantly higher median complete response rate of 73% compared to 31% in the AFXL group (P=.002). Interestingly, AFXL-PDT was also significantly more efficacious compared to AFXL for grades 1 (80% vs 37%; P=.02) and 2 (53% vs 7%, P=.009) AKs but not grade 3 AKs (4% vs 0%, P=.17).6
The combination of fractional CO2 laser and PDT also demonstrated superiority to PDT.7 In a split-face investigation, 15 participants with bilateral symmetric areas of 2 to 10 AKs on the face or scalp were randomized to receive fractional CO2 laser and MAL-PDT combination therapy on 1 treatment side and conventional MAL-PDT on the other side.7 The AFXL-PDT treatment side received laser ablation with immediate subsequent application of MAL to both treatment sides under 3-hour occlusion. At baseline, 103 AKs were treated by AFXL-PDT and 109 AKs were treated with conventional PDT. At 3-month follow-up, the AFXL-PDT treatment group exhibited a significantly higher rate of complete response (90%) compared to the conventional PDT group (67%)(P=.0002).7
Like the CO2 laser, the Er:YAG laser has demonstrated superior results when used in combination with PDT to treat field cancerization compared to either treatment alone. In a comparison study, 93 patients with 2 to 10 AK lesions on the face or scalp were randomized to treatment with AFXL (Er:YAG laser) and MAL-PDT with 3-hour occlusion, AFXL (Er:YAG laser) and MAL-PDT with 2-hour occlusion, and MAL-PDT with 3-hour occlusion.8 A total of 440 baseline AK lesions on the face or scalp were treated. At 3-month follow-up, the AFXL-PDT (3-hour occlusion) group had the highest rate of complete response (91.7%), compared to 76.8% (P=.001) in the AFXL-PDT (2-hour occlusion) and 65.6% (P=.001) in the PDT groups, regardless of the grade of AK lesion. The AFXL-PDT (2-hour occlusion) treatment was also superior to PDT alone (P=.038). These findings were sustained at 12-month follow-up (84.8% in the AFXL-PDT [3-hour occlusion] group [P<.001, compared to others]; 67.5% in the AFXL-PDT [2-hour occlusion] group [P<.001, compared to 3-hour PDT]; 51.1% in the PDT group). Importantly, the AK lesion recurrence rate was also lowest in the AFL-PDT (3-hour occlusion) group (7.5% vs 12.1% and 22.1% in the AFXL-PDT [2-hour occlusion] and PDT groups, respectively; P=.007).8
Combination therapy with AFXL and daylight PDT (dPDT) may improve the tolerability of PDT and the efficacy rate of field therapy in organ transplant recipients. One study demonstrated the superiority of this combination therapy in a population of 16 organ transplant recipients on immunosuppressants with at least 2 moderate to severely thick AKs in each of 4 comparable areas in the same anatomic region.9 The 4 areas were randomized to a single session of AFXL-dPDT, dPDT alone, conventional PDT, or AFXL alone. Ablation was performed with a fractional Er:YAG laser. The AFXL-dPDT and dPDT alone groups received MAL for 2.5 hours without occlusion, and the conventional PDT group received MAL for 3 hours with occlusion. Daylight exposure in dPDT groups was initiated 30 minutes after MAL application for 2 hours total. A baseline total of 542 AKs were treated. At 3-month follow-up, the complete response rate was highest for the AFXL-dPDT group (74%) compared to dPDT alone (46%; P=.0262), conventional PDT (50%; P=.042), and AFXL alone (5%; P=.004). Pain scores for AFXL–dPDT and dPDT alone were significantly lower than for conventional PDT and AFXL alone (P<.001).9
Nonablative Lasers
By heating the dermis to induce neogenesis without destruction, nonablative lasers offer superior healing times compared to their ablative counterparts. Multiple treatments with nonablative lasers may be necessary for maximal effect. Four nonablative laser devices have demonstrated efficacy in the treatment of multiple AKs10-14: (1) the Q-switched 1064-nm Nd:YAG laser, with or without a 532-nm potassium titanyl phosphate (KTP) laser; (2) the 1540-nm fractional erbium glass laser; (3) the 1550-nm fractional erbium-doped fiber laser; and (4) the 1927-nm fractional thulium laser (Table 3).
In a proof-of-concept study of the Q-switched Nd:YAG laser with the 532-nm KTP laser, 1 treatment session induced full remission of AKs in 10 patients at follow-up day 20, although the investigator did not grade improvement on a numerical scale.10 In a study of the fractional Q-switched 1064-nm Nd:YAG laser alone, 6 patients with trace or mild AKs received 4 treatment sessions at approximately 2-week intervals.14 All but 1 patient (who had trace AKs) had no AKs at 3-month follow-up.
The efficacy of the 1540-nm fractional erbium glass laser was examined in 17 participants with investigator-rated moderate-to-severe AK involvement of the scalp and face.12 Participants were given 2 or 3 treatment sessions at 3- to 4-week intervals and were graded by blinded dermatologists on a quartile scale of 0 (no improvement), 1 (1%–25% improvement), 2 (26%–50% improvement), 3 (51%–75% improvement), or 4 (76%–100% improvement). At 3 months posttreatment, the average grade of improvement was 3.4.12
The 1550-nm fractional erbium-doped fiber laser was tested in 14 men with multiple facial AKs (range, 9–44 AKs [mean, 22.1 AKs]).11 Participants received 5 treatment sessions at 2- to 4-week intervals, with majority energies used at 70 MJ and treatment level 11. The mean AK count was reduced significantly by 73.1%, 66.2%, and 55.6% at 1-, 3-, and 6-month follow-up, respectively (P<.001).11
The 1927-nm fractional thulium laser showed promising results in 24 participants with facial AKs.13 Participants received up to 4 treatment sessions at intervals from 2 to 6 weeks at the investigators’ discretion. At baseline, patients had an average of 14.04 facial AKs. At 1-, 3-, and 6-month follow-up, participants exhibited 91.3%, 87.3%, and 86.6% reduction in AK counts, respectively. The mean AK count at 3-month follow-up was 1.88.13
Due to limited sample sizes and/or lack of quantifiable results and controls in these studies, more studies are needed to fully elucidate the role of nonablative lasers in the treatment of AK.
Future Directions
Iontophoresis involves the noninvasive induction of an electrical current to facilitate ion movement through the skin and may be a novel method to boost the efficacy of current field therapies. In the first known study of its kisnd, iontophoresis-assisted AFXL-PDT was found to be noninferior to conventional AFXL-PDT15; however, additional studies demonstrating its superiority are needed before more widespread clinical use is considered.
Pretreatment with AFXL prior to topical field-directed therapies also has been proposed.16 In a case series of 13 patients, combination therapy with AFXL and ingenol mebutate was shown to be superior to ingenol mebutate alone (AK clearance rate, 89.2% vs 72.1%, respectively; P<.001).16 Randomized studies with longer follow-up time are needed.
Conclusion
Ablative and nonablative laser systems have yielded limited data about their potential as monotherapies for treatment of multiple AKs and are unlikely to replace topical agents and PDT as a first-line modality in field-directed treatment at this time. More studies with a larger number of participants and long-term follow-up are needed for further clarification of efficacy, safety, and clinical feasibility. Nevertheless, fractional ablative lasers in combination with PDT have shown robust efficacy and a favorable safety profile for treatment of multiple AKs.6-9 Further, this combination therapy exhibited a superior clearance rate and lower lesion recurrence in organ transplant recipients—a demographic that classically is difficult to treat.6-9
With continued rapid evolution of laser systems and more widespread use in dermatology, monotherapy and combination therapy may offer a dynamic new option in field cancerization that can decrease disease burden and treatment frequency.
In cutaneous field cancerization, focal treatments such as cryotherapy are impractical, thus necessitating the use of field-directed therapies over the lesion and the surrounding skin field. Although evidence-based guidelines do not exist, field-directed therapy has been proposed in cases of 3 or more actinic keratoses (AKs) in a 25-cm2 area or larger.1 It can be further speculated that patients who are vulnerable to aggressive phenotypes of cutaneous malignancies, such as those with a genodermatosis or who are immunocompromised, necessitate a higher index of suspicion for field effect with even 1 or 2 AKs.
Current field-directed therapies include topical agents (imiquimod, fluorouracil, ingenol mebutate, and diclo-fenac), photodynamic therapy (PDT), and resurfacing procedures (lasers, chemical peels, dermabrasion). Although topical agents and PDT currently are gold standards in field treatment, the use of energy-based devices (ie, ablative and nonablative lasers) are attractive options as monotherapy or as part of a combination therapy. These devices are attractive options for field-directed therapy because they offer defined, customizable control of settings, allowing for optimal cosmesis and precision of therapy.
Principally, lasers function by damaging skin tissue to induce resurfacing, neocollagenesis, and vascular restructuring. Fractional versions of ablative and nonablative systems are available to target a fraction of the treatment area in evenly spaced microthermal zones and to minimize overall thermal damage.2
Given recent advances in laser systems and numerous investigations reported in the literature, a review of ablative and nonablative lasers that have been studied as treatment options for cutaneous field cancerization is provided, with a focus on treatment efficacy.
Ablative Lasers
Ablative lasers operate at higher wavelengths than nonablative lasers to destroy epidermal and dermal tissue. The 10,600-nm carbon dioxide (CO2) and 2940-nm Er:YAG lasers have been heavily investigated for field therapy for multiple AKs, both as monotherapies (Table 1) and in combination with PDT (Table 2).
Monotherapy
One randomized trial with 5-year follow-up compared the efficacy of full-face pulsed CO2 laser therapy, full-face trichloroacetic acid (TCA) peel 30%, and fluorouracil cream 5% (twice daily for 3 weeks) on AKs on the face and head.3 Thirty-one participants were randomized to the 3 treatment arms and a negative control arm. The mean AK counts at baseline for the CO2, TCA, and fluorouracil treatment groups were 78.0, 83.7, and 61.8, respectively. At 3-month follow-up, all treatment groups had significant reductions in the mean AK count from baseline (CO2 group, 92% [P=.03]; TCA group, 89% [P=.004]; fluorouracil group, 83% [P=.008]). No significant differences in efficacy among the treatment groups were noted. All 3 treatment groups had a demonstrably lower incidence of nonmelanoma skin cancer over 5-year follow-up compared to the control group (P<.001).3
In contrast to these promising results, the pulsed CO2 laser showed only short-term efficacy in a split-face study of 12 participants with at least 5 facial or scalp AKs on each of 2 symmetric facial sides who were randomized to 1 treatment side.4 At 1-month follow-up, the treatment side exhibited significantly fewer AKs compared to the control side (47% vs 71% at baseline; P=.01), but the improvement was not sustained at 3-month follow-up (49% vs 57%; P=.47).4
In another study, the CO2 laser was found to be inferior to 5-aminolevulinic acid PDT.5 Twenty-one participants who had at least 4 AKs in each symmetric half of a body region (head, hands, forearms) were randomized to PDT on 1 side and CO2 laser therapy on the other. Median baseline AK counts for the PDT and CO2 laser groups were 6 and 8, respectively. Both treatment groups exhibited significant median AK reduction from baseline 4 weeks posttreatment (PDT group, 82.1% [P<.05], CO2 laser group, 100% [P<.05]); however. at 3 months posttreatment the PDT group had significantly higher absolute (P=.0155) and relative (P=.0362) reductions in AK count compared to the CO2 laser group. One participant received a topical antibiotic for superficial infection on the PDT treatment side.5
Many questions remain regarding the practical application of laser ablation monotherapy for multiple AKs. More studies are needed to determine the practicality and long-term clinical efficacy of these devices.
PDT Combination Therapy
Laser ablation may be combined with PDT to increase efficacy and prolong remission rates. In fact, laser ablation may be thought of as a physical drug-delivery system to boost uptake of topical agents—in this case, aminolevulinic acid and methyl aminolevulinate (MAL)—given that it disrupts the skin barrier.
In a comparative study of ablative fractional laser (AFXL)–assisted PDT and AFXL alone in 10 organ transplant recipients on immunosuppression with at least 5 AKs on each dorsal hand, participants were randomized to AFXL-PDT on one treatment side and PDT on the other side.6 Participants received AFXL in an initial lesion-directed pass and then a second field-directed pass of a fractional CO2 laser. After AFXL exposure, methyl aminolevulinate was applied to the AFXL-PDT treatment side, with 3-hour occlusion. A total of 680 AKs were treated (335 in the AFXL-PDT group, 345 in the PDT group); results were stratified by the clinical grade of the lesion (1, slightly palpable; 2, moderately thick; 3, very thick or obvious). At 4-month follow-up, the AFXL-PDT group had a significantly higher median complete response rate of 73% compared to 31% in the AFXL group (P=.002). Interestingly, AFXL-PDT was also significantly more efficacious compared to AFXL for grades 1 (80% vs 37%; P=.02) and 2 (53% vs 7%, P=.009) AKs but not grade 3 AKs (4% vs 0%, P=.17).6
The combination of fractional CO2 laser and PDT also demonstrated superiority to PDT.7 In a split-face investigation, 15 participants with bilateral symmetric areas of 2 to 10 AKs on the face or scalp were randomized to receive fractional CO2 laser and MAL-PDT combination therapy on 1 treatment side and conventional MAL-PDT on the other side.7 The AFXL-PDT treatment side received laser ablation with immediate subsequent application of MAL to both treatment sides under 3-hour occlusion. At baseline, 103 AKs were treated by AFXL-PDT and 109 AKs were treated with conventional PDT. At 3-month follow-up, the AFXL-PDT treatment group exhibited a significantly higher rate of complete response (90%) compared to the conventional PDT group (67%)(P=.0002).7
Like the CO2 laser, the Er:YAG laser has demonstrated superior results when used in combination with PDT to treat field cancerization compared to either treatment alone. In a comparison study, 93 patients with 2 to 10 AK lesions on the face or scalp were randomized to treatment with AFXL (Er:YAG laser) and MAL-PDT with 3-hour occlusion, AFXL (Er:YAG laser) and MAL-PDT with 2-hour occlusion, and MAL-PDT with 3-hour occlusion.8 A total of 440 baseline AK lesions on the face or scalp were treated. At 3-month follow-up, the AFXL-PDT (3-hour occlusion) group had the highest rate of complete response (91.7%), compared to 76.8% (P=.001) in the AFXL-PDT (2-hour occlusion) and 65.6% (P=.001) in the PDT groups, regardless of the grade of AK lesion. The AFXL-PDT (2-hour occlusion) treatment was also superior to PDT alone (P=.038). These findings were sustained at 12-month follow-up (84.8% in the AFXL-PDT [3-hour occlusion] group [P<.001, compared to others]; 67.5% in the AFXL-PDT [2-hour occlusion] group [P<.001, compared to 3-hour PDT]; 51.1% in the PDT group). Importantly, the AK lesion recurrence rate was also lowest in the AFL-PDT (3-hour occlusion) group (7.5% vs 12.1% and 22.1% in the AFXL-PDT [2-hour occlusion] and PDT groups, respectively; P=.007).8
Combination therapy with AFXL and daylight PDT (dPDT) may improve the tolerability of PDT and the efficacy rate of field therapy in organ transplant recipients. One study demonstrated the superiority of this combination therapy in a population of 16 organ transplant recipients on immunosuppressants with at least 2 moderate to severely thick AKs in each of 4 comparable areas in the same anatomic region.9 The 4 areas were randomized to a single session of AFXL-dPDT, dPDT alone, conventional PDT, or AFXL alone. Ablation was performed with a fractional Er:YAG laser. The AFXL-dPDT and dPDT alone groups received MAL for 2.5 hours without occlusion, and the conventional PDT group received MAL for 3 hours with occlusion. Daylight exposure in dPDT groups was initiated 30 minutes after MAL application for 2 hours total. A baseline total of 542 AKs were treated. At 3-month follow-up, the complete response rate was highest for the AFXL-dPDT group (74%) compared to dPDT alone (46%; P=.0262), conventional PDT (50%; P=.042), and AFXL alone (5%; P=.004). Pain scores for AFXL–dPDT and dPDT alone were significantly lower than for conventional PDT and AFXL alone (P<.001).9
Nonablative Lasers
By heating the dermis to induce neogenesis without destruction, nonablative lasers offer superior healing times compared to their ablative counterparts. Multiple treatments with nonablative lasers may be necessary for maximal effect. Four nonablative laser devices have demonstrated efficacy in the treatment of multiple AKs10-14: (1) the Q-switched 1064-nm Nd:YAG laser, with or without a 532-nm potassium titanyl phosphate (KTP) laser; (2) the 1540-nm fractional erbium glass laser; (3) the 1550-nm fractional erbium-doped fiber laser; and (4) the 1927-nm fractional thulium laser (Table 3).
In a proof-of-concept study of the Q-switched Nd:YAG laser with the 532-nm KTP laser, 1 treatment session induced full remission of AKs in 10 patients at follow-up day 20, although the investigator did not grade improvement on a numerical scale.10 In a study of the fractional Q-switched 1064-nm Nd:YAG laser alone, 6 patients with trace or mild AKs received 4 treatment sessions at approximately 2-week intervals.14 All but 1 patient (who had trace AKs) had no AKs at 3-month follow-up.
The efficacy of the 1540-nm fractional erbium glass laser was examined in 17 participants with investigator-rated moderate-to-severe AK involvement of the scalp and face.12 Participants were given 2 or 3 treatment sessions at 3- to 4-week intervals and were graded by blinded dermatologists on a quartile scale of 0 (no improvement), 1 (1%–25% improvement), 2 (26%–50% improvement), 3 (51%–75% improvement), or 4 (76%–100% improvement). At 3 months posttreatment, the average grade of improvement was 3.4.12
The 1550-nm fractional erbium-doped fiber laser was tested in 14 men with multiple facial AKs (range, 9–44 AKs [mean, 22.1 AKs]).11 Participants received 5 treatment sessions at 2- to 4-week intervals, with majority energies used at 70 MJ and treatment level 11. The mean AK count was reduced significantly by 73.1%, 66.2%, and 55.6% at 1-, 3-, and 6-month follow-up, respectively (P<.001).11
The 1927-nm fractional thulium laser showed promising results in 24 participants with facial AKs.13 Participants received up to 4 treatment sessions at intervals from 2 to 6 weeks at the investigators’ discretion. At baseline, patients had an average of 14.04 facial AKs. At 1-, 3-, and 6-month follow-up, participants exhibited 91.3%, 87.3%, and 86.6% reduction in AK counts, respectively. The mean AK count at 3-month follow-up was 1.88.13
Due to limited sample sizes and/or lack of quantifiable results and controls in these studies, more studies are needed to fully elucidate the role of nonablative lasers in the treatment of AK.
Future Directions
Iontophoresis involves the noninvasive induction of an electrical current to facilitate ion movement through the skin and may be a novel method to boost the efficacy of current field therapies. In the first known study of its kisnd, iontophoresis-assisted AFXL-PDT was found to be noninferior to conventional AFXL-PDT15; however, additional studies demonstrating its superiority are needed before more widespread clinical use is considered.
Pretreatment with AFXL prior to topical field-directed therapies also has been proposed.16 In a case series of 13 patients, combination therapy with AFXL and ingenol mebutate was shown to be superior to ingenol mebutate alone (AK clearance rate, 89.2% vs 72.1%, respectively; P<.001).16 Randomized studies with longer follow-up time are needed.
Conclusion
Ablative and nonablative laser systems have yielded limited data about their potential as monotherapies for treatment of multiple AKs and are unlikely to replace topical agents and PDT as a first-line modality in field-directed treatment at this time. More studies with a larger number of participants and long-term follow-up are needed for further clarification of efficacy, safety, and clinical feasibility. Nevertheless, fractional ablative lasers in combination with PDT have shown robust efficacy and a favorable safety profile for treatment of multiple AKs.6-9 Further, this combination therapy exhibited a superior clearance rate and lower lesion recurrence in organ transplant recipients—a demographic that classically is difficult to treat.6-9
With continued rapid evolution of laser systems and more widespread use in dermatology, monotherapy and combination therapy may offer a dynamic new option in field cancerization that can decrease disease burden and treatment frequency.
- Peris K, Calzavara-Pinton PG, Neri L, et al. Italian expert consensus for the management of actinic keratosis in immunocompetent patients. J Eur Acad Dermatol Venereol. 2016;30:1077-1084.
- Alexiades-Armenakas MR, Dover JS, Arndt KA. The spectrum of laser skin resurfacing: nonablative, fractional, and ablative laser resurfacing. J Am Acad Dermatol. 2008;58:719-737; quiz 738-740.
- Hantash BM, Stewart DB, Cooper ZA, et al. Facial resurfacing for nonmelanoma skin cancer prophylaxis. Arch Dermatol. 2006;142:976-982.
- Gan SD, Hsu SH, Chuang G, et al. Ablative fractional laser therapy for the treatment of actinic keratosis: a split-face study. J Am Acad Dermatol. 2016;74:387-389.
- Scola N, Terras S, Georgas D, et al. A randomized, half-side comparative study of aminolaevulinate photodynamic therapy vs. CO(2) laser ablation in immunocompetent patients with multiple actinic keratoses. Br J Dermatol. 2012;167:1366-1373.
- Helsing P, Togsverd-Bo K, Veierod MB, et al. Intensified fractional CO2 laser-assisted photodynamic therapy vs. laser alone for organ transplant recipients with multiple actinic keratoses and wart-like lesions: a randomized half-side comparative trial on dorsal hands. Br J Dermatol. 2013;169:1087-1092.
- Togsverd-Bo K, Haak CS, Thaysen-Petersen D, et al. Intensified photodynamic therapy of actinic keratoses with fractional CO2 laser: a randomized clinical trial. Br J Dermatol. 2012;166:1262-1269.
- Choi SH, Kim KH, Song KH. Efficacy of ablative fractional laser-assisted photodynamic therapy with short-incubation time for the treatment of facial and scalp actinic keratosis: 12-month follow-up results of a randomized, prospective, comparative trial. J Eur Acad Dermatol Venereol. 2015;29:1598-1605.
- Togsverd-Bo K, Lei U, Erlendsson AM, et al. Combination of ablative fractional laser and daylight-mediated photodynamic therapy for actinic keratosis in organ transplant recipients—a randomized controlled trial. Br J Dermatol. 2015;172:467-474.
- Demetriou C. Reversing precancerous actinic damage by mixing wavelengths (1064 nm, 532 nm). J Cosmet Laser Ther. 2011;13:113-119.
- Katz TM, Goldberg LH, Marquez D, et al. Nonablative fractional photothermolysis for facial actinic keratoses: 6-month follow-up with histologic evaluation. J Am Acad Dermatol. 2011;65:349-356.
- Lapidoth M, Adatto M, Halachmi S. Treatment of actinic keratoses and photodamage with non-contact fractional 1540-nm laser quasi-ablation: an ex vivo and clinical evaluation. Lasers Med Sci. 2013;28:537-542.
- Weiss ET, Brauer JA, Anolik R, et al. 1927-nm fractional resurfacing of facial actinic keratoses: a promising new therapeutic option. J Am Acad Dermatol. 2013;68:98-102.
- Gold MH, Sensing W, Biron J. Fractional Q-switched 1,064-nm laser for the treatment of photoaged-photodamaged skin. J Cosmet Laser Ther. 2014;16:69-76.
- Choi SH, Kim TH, Song KH. Efficacy of iontophoresis-assisted ablative fractional laser photodynamic therapy with short incubation time for the treatment of actinic keratosis: 12-month follow-up results of a prospective, randomised, comparative trial. Photodiagnosis Photodyn Ther. 2017;18:105-110.
- Nisticò S, Sannino M, Del Duca E, et al. Ablative fractional laser improves treatment of actinic keratoses with ingenol mebutate. Eur J Inflamm. 2016;14:200-205.
- Peris K, Calzavara-Pinton PG, Neri L, et al. Italian expert consensus for the management of actinic keratosis in immunocompetent patients. J Eur Acad Dermatol Venereol. 2016;30:1077-1084.
- Alexiades-Armenakas MR, Dover JS, Arndt KA. The spectrum of laser skin resurfacing: nonablative, fractional, and ablative laser resurfacing. J Am Acad Dermatol. 2008;58:719-737; quiz 738-740.
- Hantash BM, Stewart DB, Cooper ZA, et al. Facial resurfacing for nonmelanoma skin cancer prophylaxis. Arch Dermatol. 2006;142:976-982.
- Gan SD, Hsu SH, Chuang G, et al. Ablative fractional laser therapy for the treatment of actinic keratosis: a split-face study. J Am Acad Dermatol. 2016;74:387-389.
- Scola N, Terras S, Georgas D, et al. A randomized, half-side comparative study of aminolaevulinate photodynamic therapy vs. CO(2) laser ablation in immunocompetent patients with multiple actinic keratoses. Br J Dermatol. 2012;167:1366-1373.
- Helsing P, Togsverd-Bo K, Veierod MB, et al. Intensified fractional CO2 laser-assisted photodynamic therapy vs. laser alone for organ transplant recipients with multiple actinic keratoses and wart-like lesions: a randomized half-side comparative trial on dorsal hands. Br J Dermatol. 2013;169:1087-1092.
- Togsverd-Bo K, Haak CS, Thaysen-Petersen D, et al. Intensified photodynamic therapy of actinic keratoses with fractional CO2 laser: a randomized clinical trial. Br J Dermatol. 2012;166:1262-1269.
- Choi SH, Kim KH, Song KH. Efficacy of ablative fractional laser-assisted photodynamic therapy with short-incubation time for the treatment of facial and scalp actinic keratosis: 12-month follow-up results of a randomized, prospective, comparative trial. J Eur Acad Dermatol Venereol. 2015;29:1598-1605.
- Togsverd-Bo K, Lei U, Erlendsson AM, et al. Combination of ablative fractional laser and daylight-mediated photodynamic therapy for actinic keratosis in organ transplant recipients—a randomized controlled trial. Br J Dermatol. 2015;172:467-474.
- Demetriou C. Reversing precancerous actinic damage by mixing wavelengths (1064 nm, 532 nm). J Cosmet Laser Ther. 2011;13:113-119.
- Katz TM, Goldberg LH, Marquez D, et al. Nonablative fractional photothermolysis for facial actinic keratoses: 6-month follow-up with histologic evaluation. J Am Acad Dermatol. 2011;65:349-356.
- Lapidoth M, Adatto M, Halachmi S. Treatment of actinic keratoses and photodamage with non-contact fractional 1540-nm laser quasi-ablation: an ex vivo and clinical evaluation. Lasers Med Sci. 2013;28:537-542.
- Weiss ET, Brauer JA, Anolik R, et al. 1927-nm fractional resurfacing of facial actinic keratoses: a promising new therapeutic option. J Am Acad Dermatol. 2013;68:98-102.
- Gold MH, Sensing W, Biron J. Fractional Q-switched 1,064-nm laser for the treatment of photoaged-photodamaged skin. J Cosmet Laser Ther. 2014;16:69-76.
- Choi SH, Kim TH, Song KH. Efficacy of iontophoresis-assisted ablative fractional laser photodynamic therapy with short incubation time for the treatment of actinic keratosis: 12-month follow-up results of a prospective, randomised, comparative trial. Photodiagnosis Photodyn Ther. 2017;18:105-110.
- Nisticò S, Sannino M, Del Duca E, et al. Ablative fractional laser improves treatment of actinic keratoses with ingenol mebutate. Eur J Inflamm. 2016;14:200-205.
Practice Points
- Ablative fractional laser therapy in combination with photodynamic therapy has demonstrated increased efficacy in treating field actinic keratoses (AKs) for up to 12 months of follow-up over either modality alone.
- Ablative and nonablative lasers as monotherapy in treating field AKs require further studies with larger sample sizes to determine efficacy and safety.