User login
Early detection of melanoma, which is known to improve survival rates, remains a challenge for dermatologists. Suspicious pigmented lesions typically are evaluated via clinical examination and dermoscopy; however, new technologies are being developed to provide additional objective information for clinicians to incorporate into their biopsy decisions.
Multispectral digital skin lesion analysis (MSDSLA) uses 10 bands of visible and near-infrared light (430–950 nm) to image and analyze pigmented skin lesions (PSLs) down to 2.5 mm below the skin surface and measures the distribution of melanin using 75 unique algorithms to determine the degree of the morphologic disorder. Using a logical regression model previously validated on a set of 1632 PSLs, the probability of melanoma and probability of being a melanoma/PSL of high-risk malignant potential are then provided to the clinician.1
In this study, we analyzed aggregate data from 7 prior studies2-8 to better determine how MSDSLA impacts the biopsy decisions of dermatologists and nondermatologists following clinical examination and dermoscopic evaluation of PSLs.
Methods
Results
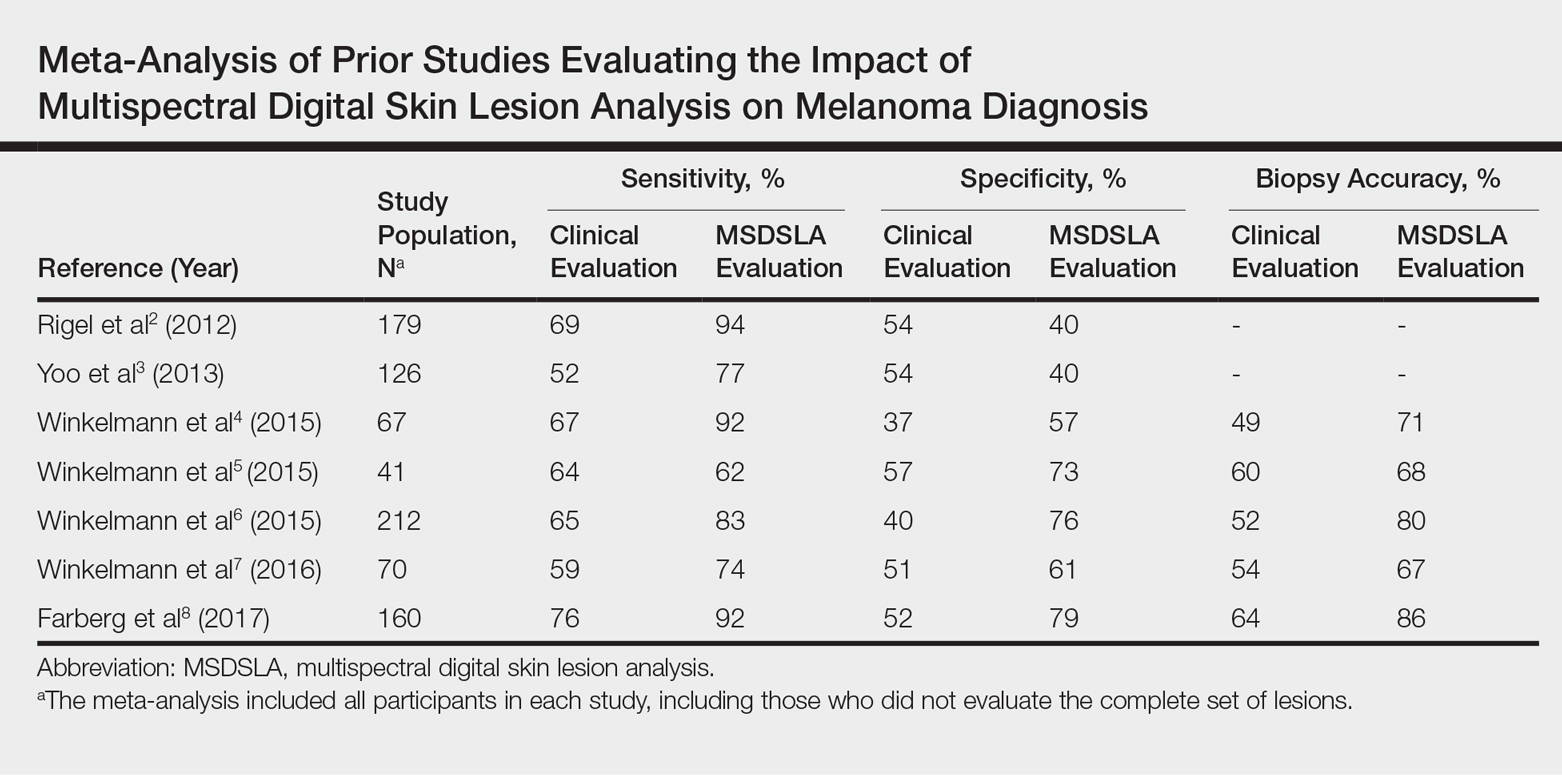
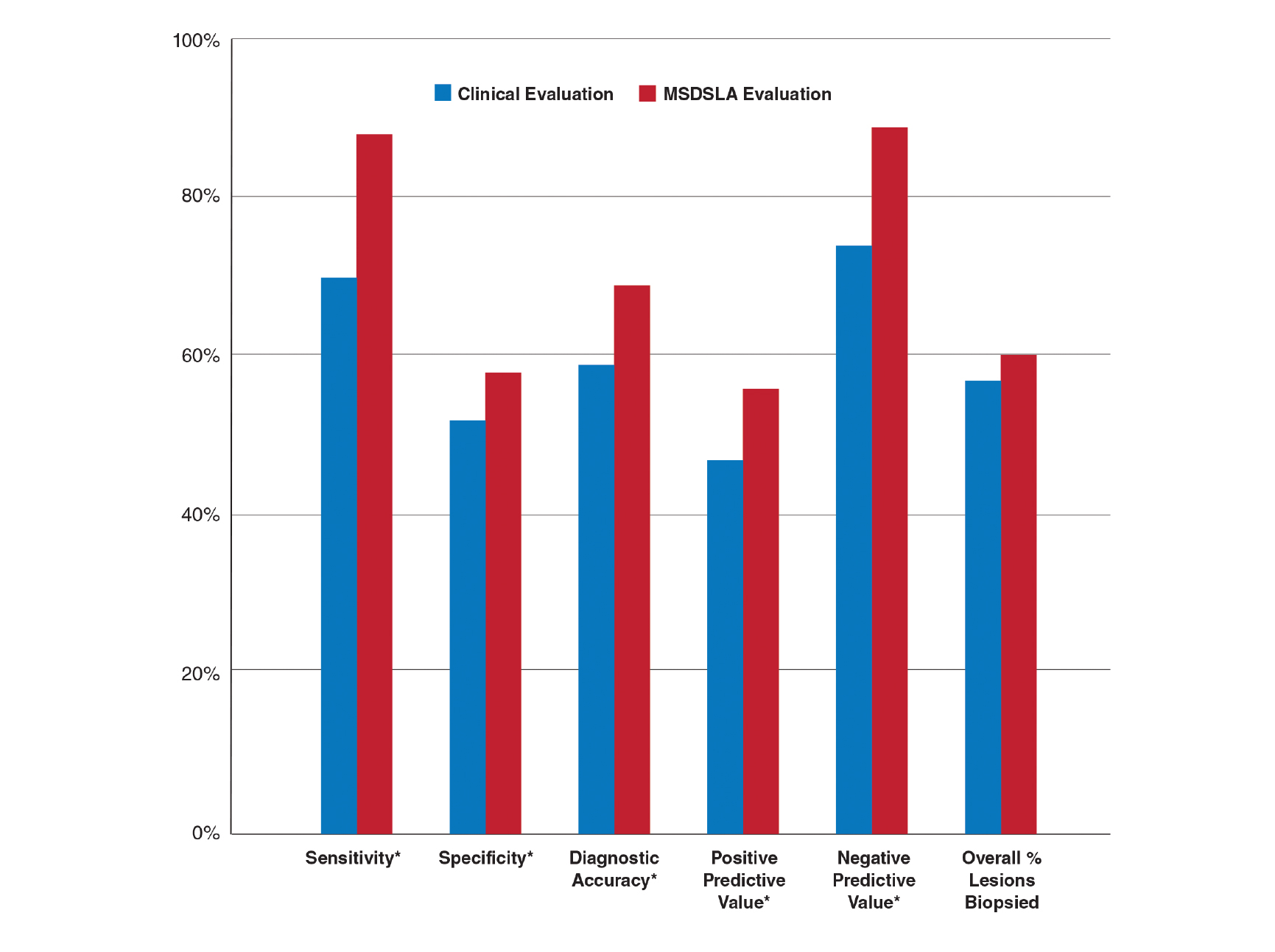
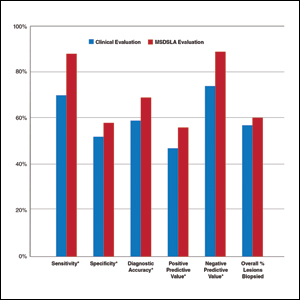
Overall sensitivity for the detection of melanoma or other high-grade PSLs improved from 70% on clinical and dermoscopic evaluation to 88% after MSDSLA information was provided (P<.0001), and specificity increased from 52% to 58% (P<.001). Diagnostic accuracy also improved from 59% on clinical evaluation to 69% after review of MSDSLA findings (P<.0001). The positive predictive value of biopsy decisions was 47% following clinical evaluation, which improved to 56% after evaluation of MSDSLA findings (P<.001), and the negative predictive value increased from 74% to 89% (P<.0001). The overall percentage of lesions selected for biopsy did not significantly change following MSDSLA data integration (57% vs 60%)(Figure). Given that similar numbers of lesions were biopsied with improved sensitivity and specificity, the integration of MSDSLA data into the biopsy decision led to an improved biopsy ratio (ratio of melanomas biopsied to total biopsies) and fewer unnecessary biopsies.
Comment
Our broad analysis further supported the findings of prior studies that decisions to biopsy clinically suspicious PSLs are more sensitive, specific, and accurate when practitioners are provided MSDSLA information following clinical examination.2-8
Given the evolution in health care economics, it is clear that greater emphasis will continue to be placed on superior, evidence-based, effective care. The reported diagnostic sensitivities and specificities of clinical evaluation and dermoscopy for melanoma detection vary widely throughout the literature, with sensitivities ranging from 58% to over 90% and specificities ranging from 77% to 99%.9-11
Our study had several limitations. For this analysis to be more representative of lesion biopsy selection in the clinical setting, biopsy sensitivity (correctly identifying lesions appropriate for biopsy) vs melanoma sensitivity (identifying a lesion as melanoma) was used.13 The overall sensitivity found was within the range of prior studies,2-8 but this approach may have potentially led to a lower specificity due to an increased number of lesions biopsied. Additionally, the melanomas selected for these studies were early (malignant melanoma in situ or mean thickness of invasive malignant melanoma of 0.3 mm), and the nonmelanomas (including low-grade dysplastic nevi) were not necessarily diagnostically straightforward. This may have led to the clinical and dermoscopic sensitivity and specificity noted being lower than in some prior studies.9-11
The risk of missing a melanoma with MSDSLA devices has led manufacturers to strive for a high sensitivity for their devices, leading to lower specificity as a consequence. For this reason and other ambiguous practical considerations (eg, device and patient costs, difficulty with insurance reimbursement), the adoption of this technology into routine clinical practice has remained relatively static; however, using enhanced diagnostic technologies such as MSDSLA may help with more accurate identification of high-risk PSLs, thereby leading to earlier detection and overall less expensive, more cost-effective treatment of melanoma.
- Monheit G, Cognetta AB, Ferris L, et al. The performance of MelaFind: a prospective multicenter study. Arch Dermatol. 2011;147:188-194.
- Rigel DS, Roy M, Yoo J, et al. Impact of guidance from a computer-aided multispectral digital skin lesion analysis device on decision to biopsy lesions clinically suggestive of melanoma. Arch Dermatol. 2012;148:541-543.
- Yoo J, Rigel DS, Roy M, et al. Impact of guidance from a multispectral digital skin lesion analysis device on dermatology residents decisions to biopsy lesions clinically suggestive of melanoma. J Am Acad Dermatol. 2013;68:AB152.
- Winkelmann RR, Yoo J, Tucker N, et al. Impact of guidance provided by a multispectral digital skin lesion analysis device following dermoscopy on decisions to biopsy atypical melanocytic lesions. J Clin Aesthet Dermatol. 2015;8:21-24.
- Winkelmann RR, Hauschild A, Tucker N, et al. The impact of multispectral digital skin lesion analysis on German dermatologist decisions to biopsy atypical pigmented lesions with clinical characteristics of melanoma. J Clin Aesthet Dermatol. 2015;8:27-29.
- Winkelmann RR, Tucker N, White R, et al. Pigmented skin lesion biopsies after computer-aided multispectral digital skin lesion analysis. J Am Osteopath Assoc. 2015;115:666-669.
- Winkelmann RR, Farberg AS, Tucker N, et al. Enhancement of international dermatologists’ pigmented skin lesion biopsy decisions following dermoscopy with subsequent integration of multispectral digital skin lesion analysis [published online July 1, 2016]. J Clin Aesthet Dermatol. 2016;9:53-55.
- Farberg AS, Winkelmann RR, Tucker N, et al. The impact of quantitative data provided by a multi-spectral digital skin lesion analysis device on dermatologists’ decisions to biopsy pigmented lesions [published online September 1, 2017]. J Clin Aesthet Dermatol. 2017;10:24-26.
- Wolf IH, Smolle J, Soyer HP, et al. Sensitivity in the clinical diagnosis of malignant melanoma. Melanoma Res. 1998;8:425-429.
- Kittler H, Pehamberger H, Wolff K, et al. Diagnostic accuracy of dermoscopy. Lancet Oncol. 2002;3:159-165.
- Ascierto PA, Palmieri G, Celentano E, et al. Sensitivity and specificity of epiluminescence microscopy: evaluation on a sample of 2731 excised cutaneous pigmented lesions: the Melanoma Cooperative Study. Br J Dermatol. 2000;142:893-898.
- Carli P, Nardini P, Crocetti E, et al. Frequency and characteristics of melanomas missed at a pigmented lesion clinic: a registry-based study. Melanoma Res. 2004;14:403-407.
- Friedman RJ, Gutkowicz-Krusin D, Farber MJ, et al. The diagnostic performance of expert dermoscopists vs a computer-vision system on small-diameter melanomas. Arch Dermatol. 2008;144:476-482.
Early detection of melanoma, which is known to improve survival rates, remains a challenge for dermatologists. Suspicious pigmented lesions typically are evaluated via clinical examination and dermoscopy; however, new technologies are being developed to provide additional objective information for clinicians to incorporate into their biopsy decisions.
Multispectral digital skin lesion analysis (MSDSLA) uses 10 bands of visible and near-infrared light (430–950 nm) to image and analyze pigmented skin lesions (PSLs) down to 2.5 mm below the skin surface and measures the distribution of melanin using 75 unique algorithms to determine the degree of the morphologic disorder. Using a logical regression model previously validated on a set of 1632 PSLs, the probability of melanoma and probability of being a melanoma/PSL of high-risk malignant potential are then provided to the clinician.1
In this study, we analyzed aggregate data from 7 prior studies2-8 to better determine how MSDSLA impacts the biopsy decisions of dermatologists and nondermatologists following clinical examination and dermoscopic evaluation of PSLs.
Methods
Results
Overall sensitivity for the detection of melanoma or other high-grade PSLs improved from 70% on clinical and dermoscopic evaluation to 88% after MSDSLA information was provided (P<.0001), and specificity increased from 52% to 58% (P<.001). Diagnostic accuracy also improved from 59% on clinical evaluation to 69% after review of MSDSLA findings (P<.0001). The positive predictive value of biopsy decisions was 47% following clinical evaluation, which improved to 56% after evaluation of MSDSLA findings (P<.001), and the negative predictive value increased from 74% to 89% (P<.0001). The overall percentage of lesions selected for biopsy did not significantly change following MSDSLA data integration (57% vs 60%)(Figure). Given that similar numbers of lesions were biopsied with improved sensitivity and specificity, the integration of MSDSLA data into the biopsy decision led to an improved biopsy ratio (ratio of melanomas biopsied to total biopsies) and fewer unnecessary biopsies.
Comment
Our broad analysis further supported the findings of prior studies that decisions to biopsy clinically suspicious PSLs are more sensitive, specific, and accurate when practitioners are provided MSDSLA information following clinical examination.2-8
Given the evolution in health care economics, it is clear that greater emphasis will continue to be placed on superior, evidence-based, effective care. The reported diagnostic sensitivities and specificities of clinical evaluation and dermoscopy for melanoma detection vary widely throughout the literature, with sensitivities ranging from 58% to over 90% and specificities ranging from 77% to 99%.9-11
Our study had several limitations. For this analysis to be more representative of lesion biopsy selection in the clinical setting, biopsy sensitivity (correctly identifying lesions appropriate for biopsy) vs melanoma sensitivity (identifying a lesion as melanoma) was used.13 The overall sensitivity found was within the range of prior studies,2-8 but this approach may have potentially led to a lower specificity due to an increased number of lesions biopsied. Additionally, the melanomas selected for these studies were early (malignant melanoma in situ or mean thickness of invasive malignant melanoma of 0.3 mm), and the nonmelanomas (including low-grade dysplastic nevi) were not necessarily diagnostically straightforward. This may have led to the clinical and dermoscopic sensitivity and specificity noted being lower than in some prior studies.9-11
The risk of missing a melanoma with MSDSLA devices has led manufacturers to strive for a high sensitivity for their devices, leading to lower specificity as a consequence. For this reason and other ambiguous practical considerations (eg, device and patient costs, difficulty with insurance reimbursement), the adoption of this technology into routine clinical practice has remained relatively static; however, using enhanced diagnostic technologies such as MSDSLA may help with more accurate identification of high-risk PSLs, thereby leading to earlier detection and overall less expensive, more cost-effective treatment of melanoma.
Early detection of melanoma, which is known to improve survival rates, remains a challenge for dermatologists. Suspicious pigmented lesions typically are evaluated via clinical examination and dermoscopy; however, new technologies are being developed to provide additional objective information for clinicians to incorporate into their biopsy decisions.
Multispectral digital skin lesion analysis (MSDSLA) uses 10 bands of visible and near-infrared light (430–950 nm) to image and analyze pigmented skin lesions (PSLs) down to 2.5 mm below the skin surface and measures the distribution of melanin using 75 unique algorithms to determine the degree of the morphologic disorder. Using a logical regression model previously validated on a set of 1632 PSLs, the probability of melanoma and probability of being a melanoma/PSL of high-risk malignant potential are then provided to the clinician.1
In this study, we analyzed aggregate data from 7 prior studies2-8 to better determine how MSDSLA impacts the biopsy decisions of dermatologists and nondermatologists following clinical examination and dermoscopic evaluation of PSLs.
Methods
Results
Overall sensitivity for the detection of melanoma or other high-grade PSLs improved from 70% on clinical and dermoscopic evaluation to 88% after MSDSLA information was provided (P<.0001), and specificity increased from 52% to 58% (P<.001). Diagnostic accuracy also improved from 59% on clinical evaluation to 69% after review of MSDSLA findings (P<.0001). The positive predictive value of biopsy decisions was 47% following clinical evaluation, which improved to 56% after evaluation of MSDSLA findings (P<.001), and the negative predictive value increased from 74% to 89% (P<.0001). The overall percentage of lesions selected for biopsy did not significantly change following MSDSLA data integration (57% vs 60%)(Figure). Given that similar numbers of lesions were biopsied with improved sensitivity and specificity, the integration of MSDSLA data into the biopsy decision led to an improved biopsy ratio (ratio of melanomas biopsied to total biopsies) and fewer unnecessary biopsies.
Comment
Our broad analysis further supported the findings of prior studies that decisions to biopsy clinically suspicious PSLs are more sensitive, specific, and accurate when practitioners are provided MSDSLA information following clinical examination.2-8
Given the evolution in health care economics, it is clear that greater emphasis will continue to be placed on superior, evidence-based, effective care. The reported diagnostic sensitivities and specificities of clinical evaluation and dermoscopy for melanoma detection vary widely throughout the literature, with sensitivities ranging from 58% to over 90% and specificities ranging from 77% to 99%.9-11
Our study had several limitations. For this analysis to be more representative of lesion biopsy selection in the clinical setting, biopsy sensitivity (correctly identifying lesions appropriate for biopsy) vs melanoma sensitivity (identifying a lesion as melanoma) was used.13 The overall sensitivity found was within the range of prior studies,2-8 but this approach may have potentially led to a lower specificity due to an increased number of lesions biopsied. Additionally, the melanomas selected for these studies were early (malignant melanoma in situ or mean thickness of invasive malignant melanoma of 0.3 mm), and the nonmelanomas (including low-grade dysplastic nevi) were not necessarily diagnostically straightforward. This may have led to the clinical and dermoscopic sensitivity and specificity noted being lower than in some prior studies.9-11
The risk of missing a melanoma with MSDSLA devices has led manufacturers to strive for a high sensitivity for their devices, leading to lower specificity as a consequence. For this reason and other ambiguous practical considerations (eg, device and patient costs, difficulty with insurance reimbursement), the adoption of this technology into routine clinical practice has remained relatively static; however, using enhanced diagnostic technologies such as MSDSLA may help with more accurate identification of high-risk PSLs, thereby leading to earlier detection and overall less expensive, more cost-effective treatment of melanoma.
- Monheit G, Cognetta AB, Ferris L, et al. The performance of MelaFind: a prospective multicenter study. Arch Dermatol. 2011;147:188-194.
- Rigel DS, Roy M, Yoo J, et al. Impact of guidance from a computer-aided multispectral digital skin lesion analysis device on decision to biopsy lesions clinically suggestive of melanoma. Arch Dermatol. 2012;148:541-543.
- Yoo J, Rigel DS, Roy M, et al. Impact of guidance from a multispectral digital skin lesion analysis device on dermatology residents decisions to biopsy lesions clinically suggestive of melanoma. J Am Acad Dermatol. 2013;68:AB152.
- Winkelmann RR, Yoo J, Tucker N, et al. Impact of guidance provided by a multispectral digital skin lesion analysis device following dermoscopy on decisions to biopsy atypical melanocytic lesions. J Clin Aesthet Dermatol. 2015;8:21-24.
- Winkelmann RR, Hauschild A, Tucker N, et al. The impact of multispectral digital skin lesion analysis on German dermatologist decisions to biopsy atypical pigmented lesions with clinical characteristics of melanoma. J Clin Aesthet Dermatol. 2015;8:27-29.
- Winkelmann RR, Tucker N, White R, et al. Pigmented skin lesion biopsies after computer-aided multispectral digital skin lesion analysis. J Am Osteopath Assoc. 2015;115:666-669.
- Winkelmann RR, Farberg AS, Tucker N, et al. Enhancement of international dermatologists’ pigmented skin lesion biopsy decisions following dermoscopy with subsequent integration of multispectral digital skin lesion analysis [published online July 1, 2016]. J Clin Aesthet Dermatol. 2016;9:53-55.
- Farberg AS, Winkelmann RR, Tucker N, et al. The impact of quantitative data provided by a multi-spectral digital skin lesion analysis device on dermatologists’ decisions to biopsy pigmented lesions [published online September 1, 2017]. J Clin Aesthet Dermatol. 2017;10:24-26.
- Wolf IH, Smolle J, Soyer HP, et al. Sensitivity in the clinical diagnosis of malignant melanoma. Melanoma Res. 1998;8:425-429.
- Kittler H, Pehamberger H, Wolff K, et al. Diagnostic accuracy of dermoscopy. Lancet Oncol. 2002;3:159-165.
- Ascierto PA, Palmieri G, Celentano E, et al. Sensitivity and specificity of epiluminescence microscopy: evaluation on a sample of 2731 excised cutaneous pigmented lesions: the Melanoma Cooperative Study. Br J Dermatol. 2000;142:893-898.
- Carli P, Nardini P, Crocetti E, et al. Frequency and characteristics of melanomas missed at a pigmented lesion clinic: a registry-based study. Melanoma Res. 2004;14:403-407.
- Friedman RJ, Gutkowicz-Krusin D, Farber MJ, et al. The diagnostic performance of expert dermoscopists vs a computer-vision system on small-diameter melanomas. Arch Dermatol. 2008;144:476-482.
- Monheit G, Cognetta AB, Ferris L, et al. The performance of MelaFind: a prospective multicenter study. Arch Dermatol. 2011;147:188-194.
- Rigel DS, Roy M, Yoo J, et al. Impact of guidance from a computer-aided multispectral digital skin lesion analysis device on decision to biopsy lesions clinically suggestive of melanoma. Arch Dermatol. 2012;148:541-543.
- Yoo J, Rigel DS, Roy M, et al. Impact of guidance from a multispectral digital skin lesion analysis device on dermatology residents decisions to biopsy lesions clinically suggestive of melanoma. J Am Acad Dermatol. 2013;68:AB152.
- Winkelmann RR, Yoo J, Tucker N, et al. Impact of guidance provided by a multispectral digital skin lesion analysis device following dermoscopy on decisions to biopsy atypical melanocytic lesions. J Clin Aesthet Dermatol. 2015;8:21-24.
- Winkelmann RR, Hauschild A, Tucker N, et al. The impact of multispectral digital skin lesion analysis on German dermatologist decisions to biopsy atypical pigmented lesions with clinical characteristics of melanoma. J Clin Aesthet Dermatol. 2015;8:27-29.
- Winkelmann RR, Tucker N, White R, et al. Pigmented skin lesion biopsies after computer-aided multispectral digital skin lesion analysis. J Am Osteopath Assoc. 2015;115:666-669.
- Winkelmann RR, Farberg AS, Tucker N, et al. Enhancement of international dermatologists’ pigmented skin lesion biopsy decisions following dermoscopy with subsequent integration of multispectral digital skin lesion analysis [published online July 1, 2016]. J Clin Aesthet Dermatol. 2016;9:53-55.
- Farberg AS, Winkelmann RR, Tucker N, et al. The impact of quantitative data provided by a multi-spectral digital skin lesion analysis device on dermatologists’ decisions to biopsy pigmented lesions [published online September 1, 2017]. J Clin Aesthet Dermatol. 2017;10:24-26.
- Wolf IH, Smolle J, Soyer HP, et al. Sensitivity in the clinical diagnosis of malignant melanoma. Melanoma Res. 1998;8:425-429.
- Kittler H, Pehamberger H, Wolff K, et al. Diagnostic accuracy of dermoscopy. Lancet Oncol. 2002;3:159-165.
- Ascierto PA, Palmieri G, Celentano E, et al. Sensitivity and specificity of epiluminescence microscopy: evaluation on a sample of 2731 excised cutaneous pigmented lesions: the Melanoma Cooperative Study. Br J Dermatol. 2000;142:893-898.
- Carli P, Nardini P, Crocetti E, et al. Frequency and characteristics of melanomas missed at a pigmented lesion clinic: a registry-based study. Melanoma Res. 2004;14:403-407.
- Friedman RJ, Gutkowicz-Krusin D, Farber MJ, et al. The diagnostic performance of expert dermoscopists vs a computer-vision system on small-diameter melanomas. Arch Dermatol. 2008;144:476-482.
Practice Points
- Multispectral digital skin lesion analysis (MSDSLA) can be a valuable tool in the evaluation of pigmented skin lesions (PSLs).
- MSDSLA may help to better identify high-risk PSLs and improve cost of care.