User login
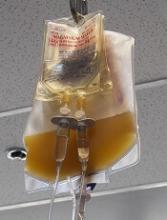
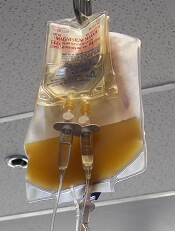
The U.S. Food and Drug Administration (FDA) recently released a draft guidance on reducing the risk of bacterial contamination in platelets destined for transfusion, especially those stored at room temperature.
The recommendations in this guidance incorporate ideas put forth during a July meeting of the Blood Products Advisory Committee.
Committee members were asked to discuss the advantages and disadvantages of various strategies to control the risk of bacterial contamination in platelets, including the scientific evidence and the operational considerations involved.
Since the last guidance document on this topic was issued in 2016, there have been new developments that could potentially reduce the risk of bacterial contamination of platelets and permit extension of platelet dating up to 7 days.
These developments include bacterial testing strategies using culture-based devices, rapid bacterial detection devices, and the implementation of pathogen-reduction technology.
The new draft guidance “further advances the potential for technology to be used to reduce the risk of contamination of the blood supply from known and emerging pathogens and to measurably increase the availability of safe blood products while ultimately reducing cost overall,” said FDA Commissioner Scott Gottlieb, MD.
“The U.S. has one of the world’s safest blood supplies, but there remains risk, albeit uncommon, of contamination with infectious diseases, particularly with blood products that are stored at room temperature. While we’ve made great strides in reducing the risk of blood contamination through donor screening and laboratory testing, we continue to support innovations and blood product alternatives that can better keep pace with emerging pathogens and reduce some of the logistical challenges and costs associated with ensuring the safety of blood products.”
The draft guidance, “Bacterial Risk Control Strategies for Blood Collection Establishments and Transfusion Services to Enhance the Safety and Availability of Platelets for Transfusion,” will be open for public comment through February 4, 2019.
Comments may be submitted online at https://www.regulations.gov/ or by mail to the Dockets Management Staff (HFA-305), Food and Drug Administration, 5630 Fishers Lane, Rm. 1061, Rockville, MD 20852.
The U.S. Food and Drug Administration (FDA) recently released a draft guidance on reducing the risk of bacterial contamination in platelets destined for transfusion, especially those stored at room temperature.
The recommendations in this guidance incorporate ideas put forth during a July meeting of the Blood Products Advisory Committee.
Committee members were asked to discuss the advantages and disadvantages of various strategies to control the risk of bacterial contamination in platelets, including the scientific evidence and the operational considerations involved.
Since the last guidance document on this topic was issued in 2016, there have been new developments that could potentially reduce the risk of bacterial contamination of platelets and permit extension of platelet dating up to 7 days.
These developments include bacterial testing strategies using culture-based devices, rapid bacterial detection devices, and the implementation of pathogen-reduction technology.
The new draft guidance “further advances the potential for technology to be used to reduce the risk of contamination of the blood supply from known and emerging pathogens and to measurably increase the availability of safe blood products while ultimately reducing cost overall,” said FDA Commissioner Scott Gottlieb, MD.
“The U.S. has one of the world’s safest blood supplies, but there remains risk, albeit uncommon, of contamination with infectious diseases, particularly with blood products that are stored at room temperature. While we’ve made great strides in reducing the risk of blood contamination through donor screening and laboratory testing, we continue to support innovations and blood product alternatives that can better keep pace with emerging pathogens and reduce some of the logistical challenges and costs associated with ensuring the safety of blood products.”
The draft guidance, “Bacterial Risk Control Strategies for Blood Collection Establishments and Transfusion Services to Enhance the Safety and Availability of Platelets for Transfusion,” will be open for public comment through February 4, 2019.
Comments may be submitted online at https://www.regulations.gov/ or by mail to the Dockets Management Staff (HFA-305), Food and Drug Administration, 5630 Fishers Lane, Rm. 1061, Rockville, MD 20852.
The U.S. Food and Drug Administration (FDA) recently released a draft guidance on reducing the risk of bacterial contamination in platelets destined for transfusion, especially those stored at room temperature.
The recommendations in this guidance incorporate ideas put forth during a July meeting of the Blood Products Advisory Committee.
Committee members were asked to discuss the advantages and disadvantages of various strategies to control the risk of bacterial contamination in platelets, including the scientific evidence and the operational considerations involved.
Since the last guidance document on this topic was issued in 2016, there have been new developments that could potentially reduce the risk of bacterial contamination of platelets and permit extension of platelet dating up to 7 days.
These developments include bacterial testing strategies using culture-based devices, rapid bacterial detection devices, and the implementation of pathogen-reduction technology.
The new draft guidance “further advances the potential for technology to be used to reduce the risk of contamination of the blood supply from known and emerging pathogens and to measurably increase the availability of safe blood products while ultimately reducing cost overall,” said FDA Commissioner Scott Gottlieb, MD.
“The U.S. has one of the world’s safest blood supplies, but there remains risk, albeit uncommon, of contamination with infectious diseases, particularly with blood products that are stored at room temperature. While we’ve made great strides in reducing the risk of blood contamination through donor screening and laboratory testing, we continue to support innovations and blood product alternatives that can better keep pace with emerging pathogens and reduce some of the logistical challenges and costs associated with ensuring the safety of blood products.”
The draft guidance, “Bacterial Risk Control Strategies for Blood Collection Establishments and Transfusion Services to Enhance the Safety and Availability of Platelets for Transfusion,” will be open for public comment through February 4, 2019.
Comments may be submitted online at https://www.regulations.gov/ or by mail to the Dockets Management Staff (HFA-305), Food and Drug Administration, 5630 Fishers Lane, Rm. 1061, Rockville, MD 20852.