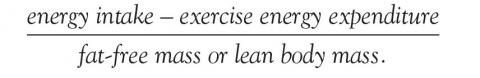User login
Striving for athletic excellence, many young women—and some young men—create an energy deficit from increased exercise, decreased intake, or both. In women, the resulting energy deficit can suppress the menstrual cycle and in turn lead to bone demineralization in a syndrome called the female athlete triad.
Primary care physicians should be aware of this syndrome because it can lead to short-term and long-term health complications, and they are in a good position to screen for, diagnose, and treat it. However, a study of 931 US physicians in 2015 found that only 37% had heard of it.1
DEFINITION HAS CHANGED: ONLY 1 OF 3 COMPONENTS NEEDED
In 1972, Title IX of the Education Amendment Act was passed, prohibiting sex discrimination in any higher education program or activity receiving federal financial aid. Since then, female athletic participation in the United States has increased more than 10-fold.2
Also increasing has been awareness of the link between athletics, eating disorders, and amenorrhea. The American College of Sports Medicine coined the term female athlete triad in 1992, describing it as the constellation of disordered eating, amenorrhea, and osteoporosis (all 3 needed to be present).3 They broadened the definition in 2007 so that the syndrome can be diagnosed if any of the following is present4:
- Low energy availability (with or without an eating disorder)
- Menstrual dysfunction
- Decreased bone mineral density.
Recognizing that low energy availability can affect athletes of either sex and have consequences beyond the female reproductive system and skeleton, in 2014 the International Olympic Committee introduced a broader term called relative energy deficiency in sport.5,6 Like the triad, this condition occurs when energy intake falls below energy output to the point that it negatively affects an athlete’s physical and mental health.
THE COMPONENTS ARE COMMON
The female athlete triad can be seen in high school, collegiate, and elite athletes7 and is especially common in sports with subjective judging (gymnastics, figure skating) or endurance sports that emphasize leanness (eg, running).8
In a review of 65 studies, Gibbs et al9 found that the prevalence of any one of the triad conditions in exercising women and female athletes ranged from 16.0% to 60.0%, the prevalence of any 2 ranged from 2.7% to 27.0%, and the prevalence of all 3 ranged from 0% to 15.9%.
Low energy availability is categorized as either intentional (ie, due to disordered eating) or unintentional (ie, due to activities not associated with eating). Sustained low energy availability is often associated with eating disorders and subsequent low self-esteem, depression, and anxiety disorders.4
The prevalence of eating disorders is high in female athletes—31% and 20% in 2 large studies of elite female athletes, compared with 5.5% and 9%, respectively, in the general population.10,11 Another study found that the prevalence of disordered eating was 46.7% in sports that emphasize leanness, such as track and gymnastics, compared with 19.8% in sports that did not, such as basketball and soccer.12
Calorie restriction is common. In a study of 15 elite ballet dancers and 15 matched controls, the dancers were found to consume only about 3/4 as many calories per day as the controls (1,577 vs 2,075 kcal/day, P ≤ .01).13
Menstrual dysfunction. In small studies, the prevalence of secondary amenorrhea was as high as 69% in dancers and 65% in long-distance runners.4,14–16
Decreased bone mineral density. According to a systematic review, the prevalence of osteopenia in amenorrheic athletes ranged between 22% and 50% and the prevalence of osteoporosis was 0% to 13%, compared with 12% and 2.3%, respectively, in the general population.17
THE COMPONENTS ARE LINKED
dysfunction play causative roles in bone mineral density pathology. Within each component of the triad a spectrum of dysfunction exists, with all 3 components exhibiting serious health end points including low energy availability, functional hypothalamic amenorrhea, and osteoporosis.
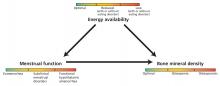
The 3 components of the female athlete triad—low energy availability, menstrual dysfunction, and decreased bone mineral density—are linked, and each exists on a spectrum (Figure 1). The long-term consequences are far-reaching and can affect the cardiovascular, endocrine, reproductive, skeletal, gastrointestinal, renal, and central nervous systems.
Low energy availability is the driving force of the triad, causing menstrual irregularity and subsequent low bone mineral density.
Menstrual dysfunction. Low energy availability can contribute to menstrual disturbances because the body suppresses reproductive function to prevent pregnancy. Functional hypothalamic amenorrhea results from decreased gonadotropin-releasing hormone leading to decreased gonadotropin release from the pituitary gland and, ultimately, to low circulating estrogen levels.18 Menstrual irregularities related to the triad include:
- Primary amenorrhea (a delay in menarche)
- Oligomenorrhea (menstrual cycles occurring at intervals greater than every 35 days)
- Secondary amenorrhea (cessation of menstruation for 3 consecutive months).
(Primary amenorrhea is defined as no menses by age 15 in the presence of normal secondary sexual development or within 5 years after breast development if that occurs before the age of 10. Secondary amenorrhea is defined as the loss of menses for 90 or more days after menarche.19)
In animal studies, reducing dietary intake by more than 30% resulted in infertility.4 Menstrual abnormalities can present as early as 5 days after a patient enters a state of low energy availability.20 Symptoms of menstrual dysfunction are largely indicative of hypogonadism and include vaginal dryness, infertility, and impaired bone health.
Bone health in women with the female athlete triad can range from optimal to osteoporosis.
Low bone mineral density is a result of low energy availability and menstrual dysfunction leading to estrogen deficiency.21,22 Specifically, menstrual abnormalities can result in low estrogen and overactivity of osteoclasts, while low energy availability alters the metabolic environment, inducing changes in insulinlike growth factor 1, leptin, and peptide YY, resulting in deficiencies in vitamin D and calcium—nutrients necessary for bone mineralization. In turn, bone health and density are compromised.21,22
Ninety percent of peak bone mass is attained by age 18. Those who have low bone mineral density as part of the female athlete triad can suffer from long-lasting effects on their bone health.
SCREENING
Untreated, the triad can lead to fatigue, poor sports performance, and a number of serious comorbid conditions such as osteopenia and osteoporosis (leading to stress fractures) anemia, heart arrhythmias, and amenorrhea. Therefore, it is important for primary care providers to screen female athletes for the triad during routine office visits.
In 2014, the Triad Consensus Panel recommended screening female athletes at the high school and collegiate levels during a preparticipation physical evaluation and then every year by a primary care physician, athletic trainer, team physician, or coach.23
Risk factors include signs of dietary restriction, low body mass index, delayed menarche, oligomenorrhea or amenorrhea, and bone stress reactions or fractures.23 Athletes should be questioned about their menstrual history (age of menarche, frequency, and duration of menstrual cycles), history of stress fractures, medication history, family history (osteoporosis, eating disorders, and fractures),24,25 and dietary habits.
Physical findings include low body mass index, recent weight loss, orthostatic hypotension, lanugo, hypercarotenemia, and signs of eating disorders (restrictive, binging, purging) (Table 1).25–27
Additionally, it is important to ascertain if the patient receives critical comments regarding performance or body image from coaches, parents, or teammates and if sport-specific training began early in life.
Certain personality factors and behaviors are clues, such as perfectionism, obsessiveness, frequent weight cycling, and overtraining.4,25 If any of the triad components are apparent, a deeper evaluation can be completed.
Specific screening questions
The Female Athlete Triad Coalition recommends asking 11 screening questions and having prompt discussions regarding the athlete’s nutritional status and body image.23 If the patient gives a worrisome response to a screening question, further workup for a formal diagnosis should be initiated.
Questions about nutritional status.
- Do you worry about your weight?
- Are you trying to gain or lose weight, or has anyone recommended that you do so?
- Are you on a special diet or do you avoid certain types of foods or food groups?
- Have you ever had an eating disorder?
Questions about menstrual function.
- Have you ever had a menstrual period?
- How old were you when you had your first menstrual period?
- When was your most recent menstrual period?
- How many periods have you had in the last 12 months?
- Are you presently taking any female hormones (estrogen, progesterone, birth control pills)?
Questions about bone health.
- Have you ever had a stress fracture?
- Have you ever been told you have low bone density (osteopenia or osteoporosis)?
Along similar lines, the American Academy of Pediatrics, American Academy of Family Physicians, and American College of Sports Medicine28 have a list of 7 questions:
- Do you worry about your weight?
- Do you limit the foods you eat?
- Do you lose weight to meet image requirements for sports?
- Have you ever suffered from an eating disorder?
- How old were you when you had your first menstrual period?
- How many menstrual cycles have you had in the past 12 months?
- Have you ever had a stress fracture?
These questions are not being widely used. A study of the National Collegiate Athletic Association Division I universities found that only 9% of universities included 9 or more of the recommended 12 questions that the Female Athlete Triad Coalition was recommending at that time, and 22% asked only 1 or 2 of the questions. None of the universities included all 12.29 These findings are not surprising, given that screening for the triad is not state-mandated. Screening discrepancies among providers largely stem from knowledge gaps, nonstandardized questionnaires, lack of time at appointments, and the sensitive nature of the questioning (eg, disordered eating).30
DIAGNOSING THE TRIAD
Given that the signs of low energy availability and menstrual dysfunction are often subtle, the diagnosis of the triad for those at risk requires input from a multidisciplinary team including a physician, sports dietitian, mental health professional, exercise physiologist, and other medical consultants.
Table 2 lists diagnostic tests the primary care provider should consider.
Diagnosing low energy availability
Energy availability is the dietary energy remaining after exercise energy expenditure; it is normalized to fat-free (lean) mass to account for resting energy expenditure. It is a product of energy intake, energy expenditure, and stored energy, and is calculated as:
An optimal value is at least 45 kcal/kg/day, while physiologic changes start to occur at less than 30 kcal/kg/day.4,31 Low energy is often seen in adult patients with a body mass index less than 17.5 kg/m2 and adolescent patients who are less than 80% of expected body weight.
Energy availability is hard to calculate, but certain assessments can be performed in a primary care setting to approximate it. To assess dietary intake, patients can bring in a 3-, 4-, or 7-day dietary log or complete a 24-hour food recall or food-frequency questionnaire in the office. To objectively document energy expenditure, patients can use heart rate monitors, accelerometers, an exercise diary, and web-based calculators. The fat-free mass can be calculated using a bioelectric impedance scale and skinfold caliper measurements.26
Those with chronic energy deficiency states may have reduced resting metabolic rates, with measured rates less than 90% of predicted and low triiodothyronine (T3) levels.31
Diagnosing menstrual dysfunction
When evaluating patients with menstrual dysfunction, it is important to first rule out pregnancy and endocrinopathies. These include thyroid dysfunction, hyperprolactinemia, primary ovarian insufficiency, other hypothalamic and pituitary disorders, and hyperandrogenic conditions such as polycystic ovarian syndrome, ovarian tumor, adrenal tumor, nonclassic congenital adrenal hyperplasia, and Cushing syndrome.
Depending on the patient’s age, laboratory tests can include follicle-stimulating hormone, luteinizing hormone, prolactin, serum estradiol, and a progesterone challenge.32 For hyperandrogenic symptoms, measuring total and free testosterone, dehydroepiandrosterone sulfate, 24-hour urine cortisol, and 17-hydroxyprogesterone levels may be helpful.
An endocrinologist should be consulted to evaluate the underlying cause of amenorrhea and address any associated hormonal imbalances. Attributing menstrual dysfunction to low energy availability is generally a diagnosis of exclusion. Additionally, outflow tract obstruction should be considered and ruled out with transvaginal ultrasonography in patients with primary amenorrhea.
A patient with hypoestrogenemia and amenorrhea may have the same steroid hormone profile as that of a menopausal woman. Lack of estrogen results in impaired endothelial cell function and arterial dilation, with accelerated development of atherosclerosis and subsequent cardiovascular events.33,34 Further, low energy availability has been linked to negative cardiovascular effects such as decreased vessel dilation leading to decreased tissue perfusion and hastened development of atherosclerosis.33 Female athletes with hypoestrogenism may show reduced perfusion of working muscle, impaired aerobic metabolism in skeletal muscle, elevated low-density lipoprotein cholesterol, and vaginal dryness.4
Diagnosing low bone mineral density
The most common clinical manifestations of low bone mineral density in female athletes are bone stress reactions such as stress fractures. In a study of 311 female high school athletes, 65.6% suffered from musculoskeletal injury from trauma or overuse including stress fractures and the patellofemoral syndrome.35 Many athletes seek medical attention from their primary care physician for stress reactions, providing an opportunity for triad screening.36
In postmenopausal women, osteopenia and osteoporosis are defined using the T score. However, in premenopausal women and adolescents, the International Society for Clinical Densitometry recommends using the Z score. A Z score less than –2.0 is described as “low bone density for chronological age.”14 For the diagnosis of osteoporosis in children and premenopausal women, the Society recommends using a Z score less than –2.0 along with the presence of a secondary risk factor for fracture such as undernutrition, hypogonadism, or a history of fracture.
Table 2 summarizes the diagnosis of low bone mineral density and osteoporosis in premenopausal women, adolescents, and children as well as when to order dual-energy x-ray absorptiometry (DEXA).37,38
Adolescents with low bone mineral density should have an annual DEXA scan of the total hip and lumbar spine.22 Amenorrheic athletes typically present with low areal density at the lumbar spine, reduced trabecular volumetric bone mineral density and bone strength index at the distal radius, and deterioration of the distal tibia.39
EARLY INTERVENTION IS ESSENTIAL
Early intervention is essential in patients with any component of the female athlete triad to prevent long-term adverse health effects. Successful treatment is strongly correlated with a trusting relationship between the athlete and the multidisciplinary team involved in her treatment.40
If needed, selective serotonin reuptake inhibitors and other psychotropic medications can be prescribed for comorbid conditions including bulimia nervosa, anxiety, depression, and obsessive-compulsive disorder. Primary providers can identify risk factors that prompt the evaluation and diagnosis of the triad, as well as support the goals of treatment and help manage comorbid conditions.
Eat more, exercise less
The primary goal is to restore body weight, maximizing nutritional and energy status by modifying the diet and adjusting exercise behavior to increase energy availability.41 Creating an energy-positive state by increasing intake, decreasing energy expenditure, or both increases energy availability, subsequently improves bone mineral density, and normalizes menstrual function.40
To sustain normal physiologic function, an energy availability of at least 45 kcal/kg/day is recommended.42 Patients should consume a minimum of 2,000 kcal/day, although energy needs may far exceed that, depending on energy expenditure. Olympic athletes participating in women’s crew and other sports have been anecdotally known to require over 12,000 kcal a day to maintain weight and performance. Goals include a body mass index of at least 18.5 kg/m2 in adults and a body weight of at least 90% of predicted in adolescents.
Involving a dietitian in the care team can help ensure that the patient consumes an adequate amount of macronutrients and micronutrients necessary for bone growth; these include calcium, vitamin D, iron, zinc, and vitamin K.4,32 For patients with disordered eating, referral to a mental health professional is important to help them avoid pathologic eating behaviors, reduce dieting attempts, and alter negative emotions associated with food and body image.
Once treatment begins, patients must undergo standardized periodic monitoring of their body weight. Although positive effects such as normalization of metabolic hormones (eg, insulinlike growth factor 1) may be seen in days to weeks by reversing low energy availability, it may take several months for menstrual function to improve and years for measurable improvement in bone mineral density to occur.23
Menstrual function should improve with weight gain
Normalizing menses in patients with the female athlete triad depends on improving the low energy availability and inducing weight gain.
Pharmacotherapy such as combined oral contraceptives can treat symptoms of hypogonadism.25 However, combined oral contraceptives do not restore spontaneous menses but rather induce withdrawal bleeding, which can lead to a false sense of security.23 While there are some benefits to prescribing combined oral contraceptives to treat hypogonadism, nonpharmacologic methods should be tried initially to restore menses, including increasing caloric intake and body weight. Golden et al showed that hormone replacement with combined oral contraceptives did not improve bone density in women with low estrogen states (eg, anorexia nervosa, osteopenia).43 Further, combined oral contraceptives may worsen bone health, as oral estrogen suppresses hepatic production of insulinlike growth factor 1, a bone trophic hormone.23
Treating low bone mineral density
Improving energy availability and menstrual function can help improve bone mineral density. Nutritional enhancement is recommended for mineralization of trabecular bone and growth of cortical bone. Supplemental calcium (1,000–1,500 mg daily) and vitamin D (600–1,000 IU daily) should be incorporated into the treatment of low bone mineral density.15,19
Additionally, weight gain has a positive effect on bone mineral density independent of its effect on the resumption of menses. However, weight gain alone does not normalize bone mineral density. Resuming normal physiologic production of hormones with estrogen-dependent effects on bone health is integral to normalization as well.23
Resistance training is encouraged to increase lean mass, although caution must be used to prevent fractures during high-impact activity. Bone mineral density may take up to several years to improve and may not be fully reversible.4,25,39
Pharmacologic therapy for low bone mineral density has unclear outcomes in women under age 50. The decision to treat should be based on bone mineral density along with fracture history. Given their unknown effects on the human fetal skeleton, bisphosphonates and denosumab should be used with caution in women of childbearing potential.24 No studies have used denosumab or teriparatide in women with the female athlete triad. Despite concerns regarding use of these drugs in premenopausal women, drug therapy should be strongly considered in women with a history of fracture and those with a high risk of subsequent fracture. The decision to treat can be made in conjunction with the athlete’s endocrinologist.
RETURN TO PLAY
If an athlete is noted to be at risk for or diagnosed with the female athlete triad, it is important to formulate a plan for her to return to play once her health improves.
De Souza et al provided a cumulative risk assessment for risk stratification and made recommendations on when an athlete should return to play depending on her level of risk.23 Using this grading system, primary care physicians can risk-stratify their patients. Those at low risk may be fully cleared to return to play, while those at moderate to high risk must first follow up with a multidisciplinary team to develop treatment strategies for improving their health.
Once a patient reaches her established goals, she may provisionally return to play under the close supervision of a team physician or primary care physician. A written treatment contract, including the goals set by the multidisciplinary team, should be followed closely as the athlete continues to participate in the sport.23;
- Curry EJ, Logan C, Ackerman K, McInnis KC, Matzkin EG. Female athlete triad awareness among multispecialty physicians. Sports Med Open 2015; 1(1):38. doi:10.1186/s40798-015-0037-5
- National Federation of State High School Associations. 2012–13 high school athletics participation survey. http://old.nfhs.org/content.aspx?id=3282. Accessed February 1, 2018.
- Otis CL, Drinkwater B, Johnson M, Loucks A, Wilmore J. American College of Sports Medicine position stand. The female athlete triad. Med Sci Sports Exerc 1997; 29(5):i–ix.
- Nattiv A, Loucks AB, Manore MM, Sanborn CF, Sundgot-Borgen J, Warren MP; American College of Sports Medicine. American College of Sports Medicine position stand. The female athlete triad. Med Sci Sports Exerc 2007; 39(10):1867–1882. doi:10.1249/mss.0b013e318149f111
- Mountjoy M, Sundgot-Borgen J, Burke L, et al. The IOC consensus statement: beyond the female athlete triad—relative energy deficiency in sport (RED-S). Br J Sports Med 2014; 48(7):491–497. doi:10.1136/bjsports-2014-093502
- Tenforde AS, Barrack MT, Nattiv A, Fredericson M. Parallels with the female athlete triad in male athletes. Sports Med 2016; 46(2):171–182. doi:10.1007/s40279-015-0411-y
- Thein-Nissenbaum JM, Carr KE. Female athlete triad syndrome in the high school athlete. Phys Ther Sport 2011; 12(3):108–116. doi:10.1016/j.ptsp.2011.04.002
- Matzkin E, Curry EJ, Whitlock K. Female athlete triad: past, present, and future. J Am Acad Orthop Surg 2015; 23(7):424–432. doi:10.5435/JAAOS-D-14-00168
- Gibbs JC, Williams NI, De Souza MJ. Prevalence of individual and combined components of the female athlete triad. Med Sci Sports Exerc 2013; 45(5):985–996. doi:10.1249/MSS.0b013e31827e1bdc
- Byrne S, McLean N. Elite athletes: effects of the pressure to be thin. J Sci Med Sport 2002; 5(2):80–94. doi:10.1016/S1440-2440(02)80029-9
- Sundgot-Borgen J, Torstveit MK. Prevalence of eating disorders in elite athletes is higher than in the general population. Clin J Sport Med 2004; 14(1):25–32.
- Lynch SL, Hoch AZ. The female runner: gender specifics. Clin Sports Med 2010; 29(3):477–498. doi:10.1016/j.csm.2010.03.003
- Doyle-Lucas AF, Akers JD, Davy BM. Energetic efficiency, menstrual irregularity, and bone mineral density in elite professional female ballet dancers. J Dance Med Sci 2010; 14(4):146–154.
- Thein-Nissenbaum J. Long term consequences of the female athlete triad. Maturitas 2013; 75(2):107–112. doi:10.1016/j.maturitas.2013.02.010
- Hilibrand MJ, Hammoud S, Bishop M, Woods D, Fredrick RW, Dodson CC. Common injuries and ailments of the female athlete; pathophysiology, treatment and prevention. Phys Sportsmed 2015; 43(4):403–411. doi:10.1080/00913847.2015.1092856
- Demorest RA, Hergenroeder AC. Preface. Sports medicine and sports injuries. Adolesc Med State Art Rev 2015; 26(1):xv–xvi.
- Khan KM, Liu-Ambrose T, Sran MM, Ashe MC, Donaldson MG, Wark JD. New criteria for female athlete triad syndrome? Br J Sports Med 2002; 36(1):10–13. doi:10.1136/bjsm.36.1.10
- Fontana R, Della Torre S. The deep correlation between energy metabolism and reproduction: a view of the effects of nutrition for women fertility. Nutrients 2016; 8:87. www.ncbi.nlm.nih.gov/pmc/articles/PMC4772050/. Accessed February 2, 2018.
- Nazem TG, Ackerman K. The female athlete triad. Sports Health 2012; 4(4):302–311. doi:10.1177/1941738112439685
- Pantano KJ. Coaching concerns in physically active girls and young women—part 1: the female athlete triad. Strength Conditioning J 2009; 31(6):38–43. doi:10.1519/SSC.0b013e3181c105dd
- Micklesfield LK, Hugo J, Johnson C, Noakes TD, Lambert EV. Factors associated with menstrual dysfunction and self-reported bone stress injuries in female runners in the ultra- and half-marathons of the Two Oceans. Br J Sports Med 2007; 41(10):679–683. doi:10.1136/bjsm.2007.037077
- Lambrinoudaki I, Papadimitriou D. Pathophysiology of bone loss in the female athlete. Ann N Y Acad Sci 2010; 1205:45–50. doi:10.1111/j.1749-6632.2010.05681.x
- De Souza MJ, Nattiv A, Joy E, et al. 2014 Female Athlete Triad Coalition consensus statement on treatment and return to play of the female athlete triad: 1st International Conference held in San Francisco, CA, May 2012, and 2nd International Conference held in Indianapolis, IN, May 2013. Clin J Sport Med 2014; 24(2):96–119. doi:10.1136/bjsports-2013-093218
- Horn E, Gergen N, McGarry KA. The female athlete triad. R I Med J (2013) 2014; 97(11):18–21.
- Joy E, De Souza MJ, Nattiv A, et al. 2014 Female Athlete Triad Coalition consensus statement on treatment and return to play of the female athlete triad. Curr Sports Med Rep 2014; 13(4):219–232. doi:10.1249/JSR.0000000000000077
- Temme KE, Hoch AZ. Recognition and rehabilitation of the female athlete triad/tetrad: a multidisciplinary approach. Curr Sports Med Rep 2013; 12(3):190–199. doi:10.1249/JSR.0b013e318296190b
- Pritts SD, Susman J. Diagnosis of eating disorders in primary care. Am Fam Physician 2003; 67(2):297–304.
- Berhardt DR, Roberts WO, editors. Preparticipation Physical Evaluation, 4th Ed. American Academy of Pediatrics, Elk Grove Village, IL, 2010.
- Mencias T, Noon M, Hoch AZ. Female athlete triad screening in National Collegiate Athletic Association Division I athletes: is the preparticipation evaluation form effective? Clin J Sport Med 2012; 22(2):122–125. doi:10.1097/JSM.0b013e3182425aee
- Javed A, Tebben PJ, Fischer PR, Lteif AN. Female athlete triad and its components: toward improved screening and management. Mayo Clin Proc 2013; 88(9):996–1009. doi:10.1016/j.mayocp.2013.07.001
- Melin A, Tornberg AB, Skouby S, et al. Energy availability and the female athlete triad in elite endurance athletes. Scand J Med Sci Sports 2015; 25(5):610–622. doi:10.1111/sms.12261
- Warr BJ, Woolf K. The female athlete triad: patients do best with a team approach to care. JAAPA 2011; 24(4):50–55.
- Hoch AZ, Lal S, Jurva JW, Gutterman DD. The female athlete triad and cardiovascular dysfunction. Phys Med Rehabil Clin North Am 2007; 18(3):385–400. doi:10.1016/j.pmr.2007.05.001
- Lanser EM, Zach KN, Hoch AZ. The female athlete triad and endothelial dysfunction. PM R 2011; 3(5):458–465. doi:10.1016/j.pmrj.2010.12.024
- Thein-Nissenbaum JM, Rauh MJ, Carr KE, Loud KJ, McGuine TA. Associations between disordered eating, menstrual dysfunction, and musculoskeletal injury among high school athletes. J Orthop Sports Phys Ther 2011; 41(2):60–69. doi:10.2519/jospt.2011.3312
- Ducher G, Turner AI, Kukuljan S, et al. Obstacles in the optimization of bone health outcomes in the female athlete triad. Sports Med 2011; 41(7):587–607. doi:10.2165/11588770-000000000-00000
- Mendelsohn FA, Warren MP. Anorexia, bulimia, and the female athlete triad: evaluation and management. Endocrinol Metab Clin North Am 2010; 39(1):155–167. doi:10.1016/j.ecl.2009.11.002
- House S, Loud K, Shubkin C. Female athlete triad for the primary care pediatrician. Curr Opin Pediatr 2013; 25(6):755–761. doi:10.1097/MOP.0000000000000033
- Mallinson RJ, De Souza MJ. Current perspectives on the etiology and manifestation of the “silent” component of the female athlete triad. Int J Womens Health 2014; 6:451–467. doi:10.2147/IJWH.S38603
- Deimel JF, Dunlap BJ. The female athlete triad. Clin Sports Med 2012; 31(2):247–254. doi:10.1016/j.csm.2011.09.007
- Manore MM, Kam LC, Loucks AB; International Association of Athletics Federations. The female athlete triad: components, nutrition issues, and health consequences. J Sports Sci 2007; 25(suppl 1):S61–S71. doi:10.1080/02640410701607320
- Witkop CT, Warren MP. Understanding the spectrum of the female athlete triad. Obstet Gynecol 2010; 116(6):1444–1448. doi:10.1097/AOG.0b013e3181fbed40
- Golden NH, Lanzkowsky L, Schebendach J, Palestro CJ, Jacobson MS, Shenker IR. The effect of estrogen-progestin treatment on bone mineral density in anorexia nervosa. J Pediatr Adolesc Gynecol 2002; 15(3):135–143. doi:10.1016/S1083-3188(02)00145-6
Striving for athletic excellence, many young women—and some young men—create an energy deficit from increased exercise, decreased intake, or both. In women, the resulting energy deficit can suppress the menstrual cycle and in turn lead to bone demineralization in a syndrome called the female athlete triad.
Primary care physicians should be aware of this syndrome because it can lead to short-term and long-term health complications, and they are in a good position to screen for, diagnose, and treat it. However, a study of 931 US physicians in 2015 found that only 37% had heard of it.1
DEFINITION HAS CHANGED: ONLY 1 OF 3 COMPONENTS NEEDED
In 1972, Title IX of the Education Amendment Act was passed, prohibiting sex discrimination in any higher education program or activity receiving federal financial aid. Since then, female athletic participation in the United States has increased more than 10-fold.2
Also increasing has been awareness of the link between athletics, eating disorders, and amenorrhea. The American College of Sports Medicine coined the term female athlete triad in 1992, describing it as the constellation of disordered eating, amenorrhea, and osteoporosis (all 3 needed to be present).3 They broadened the definition in 2007 so that the syndrome can be diagnosed if any of the following is present4:
- Low energy availability (with or without an eating disorder)
- Menstrual dysfunction
- Decreased bone mineral density.
Recognizing that low energy availability can affect athletes of either sex and have consequences beyond the female reproductive system and skeleton, in 2014 the International Olympic Committee introduced a broader term called relative energy deficiency in sport.5,6 Like the triad, this condition occurs when energy intake falls below energy output to the point that it negatively affects an athlete’s physical and mental health.
THE COMPONENTS ARE COMMON
The female athlete triad can be seen in high school, collegiate, and elite athletes7 and is especially common in sports with subjective judging (gymnastics, figure skating) or endurance sports that emphasize leanness (eg, running).8
In a review of 65 studies, Gibbs et al9 found that the prevalence of any one of the triad conditions in exercising women and female athletes ranged from 16.0% to 60.0%, the prevalence of any 2 ranged from 2.7% to 27.0%, and the prevalence of all 3 ranged from 0% to 15.9%.
Low energy availability is categorized as either intentional (ie, due to disordered eating) or unintentional (ie, due to activities not associated with eating). Sustained low energy availability is often associated with eating disorders and subsequent low self-esteem, depression, and anxiety disorders.4
The prevalence of eating disorders is high in female athletes—31% and 20% in 2 large studies of elite female athletes, compared with 5.5% and 9%, respectively, in the general population.10,11 Another study found that the prevalence of disordered eating was 46.7% in sports that emphasize leanness, such as track and gymnastics, compared with 19.8% in sports that did not, such as basketball and soccer.12
Calorie restriction is common. In a study of 15 elite ballet dancers and 15 matched controls, the dancers were found to consume only about 3/4 as many calories per day as the controls (1,577 vs 2,075 kcal/day, P ≤ .01).13
Menstrual dysfunction. In small studies, the prevalence of secondary amenorrhea was as high as 69% in dancers and 65% in long-distance runners.4,14–16
Decreased bone mineral density. According to a systematic review, the prevalence of osteopenia in amenorrheic athletes ranged between 22% and 50% and the prevalence of osteoporosis was 0% to 13%, compared with 12% and 2.3%, respectively, in the general population.17
THE COMPONENTS ARE LINKED
dysfunction play causative roles in bone mineral density pathology. Within each component of the triad a spectrum of dysfunction exists, with all 3 components exhibiting serious health end points including low energy availability, functional hypothalamic amenorrhea, and osteoporosis.
The 3 components of the female athlete triad—low energy availability, menstrual dysfunction, and decreased bone mineral density—are linked, and each exists on a spectrum (Figure 1). The long-term consequences are far-reaching and can affect the cardiovascular, endocrine, reproductive, skeletal, gastrointestinal, renal, and central nervous systems.
Low energy availability is the driving force of the triad, causing menstrual irregularity and subsequent low bone mineral density.
Menstrual dysfunction. Low energy availability can contribute to menstrual disturbances because the body suppresses reproductive function to prevent pregnancy. Functional hypothalamic amenorrhea results from decreased gonadotropin-releasing hormone leading to decreased gonadotropin release from the pituitary gland and, ultimately, to low circulating estrogen levels.18 Menstrual irregularities related to the triad include:
- Primary amenorrhea (a delay in menarche)
- Oligomenorrhea (menstrual cycles occurring at intervals greater than every 35 days)
- Secondary amenorrhea (cessation of menstruation for 3 consecutive months).
(Primary amenorrhea is defined as no menses by age 15 in the presence of normal secondary sexual development or within 5 years after breast development if that occurs before the age of 10. Secondary amenorrhea is defined as the loss of menses for 90 or more days after menarche.19)
In animal studies, reducing dietary intake by more than 30% resulted in infertility.4 Menstrual abnormalities can present as early as 5 days after a patient enters a state of low energy availability.20 Symptoms of menstrual dysfunction are largely indicative of hypogonadism and include vaginal dryness, infertility, and impaired bone health.
Bone health in women with the female athlete triad can range from optimal to osteoporosis.
Low bone mineral density is a result of low energy availability and menstrual dysfunction leading to estrogen deficiency.21,22 Specifically, menstrual abnormalities can result in low estrogen and overactivity of osteoclasts, while low energy availability alters the metabolic environment, inducing changes in insulinlike growth factor 1, leptin, and peptide YY, resulting in deficiencies in vitamin D and calcium—nutrients necessary for bone mineralization. In turn, bone health and density are compromised.21,22
Ninety percent of peak bone mass is attained by age 18. Those who have low bone mineral density as part of the female athlete triad can suffer from long-lasting effects on their bone health.
SCREENING
Untreated, the triad can lead to fatigue, poor sports performance, and a number of serious comorbid conditions such as osteopenia and osteoporosis (leading to stress fractures) anemia, heart arrhythmias, and amenorrhea. Therefore, it is important for primary care providers to screen female athletes for the triad during routine office visits.
In 2014, the Triad Consensus Panel recommended screening female athletes at the high school and collegiate levels during a preparticipation physical evaluation and then every year by a primary care physician, athletic trainer, team physician, or coach.23
Risk factors include signs of dietary restriction, low body mass index, delayed menarche, oligomenorrhea or amenorrhea, and bone stress reactions or fractures.23 Athletes should be questioned about their menstrual history (age of menarche, frequency, and duration of menstrual cycles), history of stress fractures, medication history, family history (osteoporosis, eating disorders, and fractures),24,25 and dietary habits.
Physical findings include low body mass index, recent weight loss, orthostatic hypotension, lanugo, hypercarotenemia, and signs of eating disorders (restrictive, binging, purging) (Table 1).25–27
Additionally, it is important to ascertain if the patient receives critical comments regarding performance or body image from coaches, parents, or teammates and if sport-specific training began early in life.
Certain personality factors and behaviors are clues, such as perfectionism, obsessiveness, frequent weight cycling, and overtraining.4,25 If any of the triad components are apparent, a deeper evaluation can be completed.
Specific screening questions
The Female Athlete Triad Coalition recommends asking 11 screening questions and having prompt discussions regarding the athlete’s nutritional status and body image.23 If the patient gives a worrisome response to a screening question, further workup for a formal diagnosis should be initiated.
Questions about nutritional status.
- Do you worry about your weight?
- Are you trying to gain or lose weight, or has anyone recommended that you do so?
- Are you on a special diet or do you avoid certain types of foods or food groups?
- Have you ever had an eating disorder?
Questions about menstrual function.
- Have you ever had a menstrual period?
- How old were you when you had your first menstrual period?
- When was your most recent menstrual period?
- How many periods have you had in the last 12 months?
- Are you presently taking any female hormones (estrogen, progesterone, birth control pills)?
Questions about bone health.
- Have you ever had a stress fracture?
- Have you ever been told you have low bone density (osteopenia or osteoporosis)?
Along similar lines, the American Academy of Pediatrics, American Academy of Family Physicians, and American College of Sports Medicine28 have a list of 7 questions:
- Do you worry about your weight?
- Do you limit the foods you eat?
- Do you lose weight to meet image requirements for sports?
- Have you ever suffered from an eating disorder?
- How old were you when you had your first menstrual period?
- How many menstrual cycles have you had in the past 12 months?
- Have you ever had a stress fracture?
These questions are not being widely used. A study of the National Collegiate Athletic Association Division I universities found that only 9% of universities included 9 or more of the recommended 12 questions that the Female Athlete Triad Coalition was recommending at that time, and 22% asked only 1 or 2 of the questions. None of the universities included all 12.29 These findings are not surprising, given that screening for the triad is not state-mandated. Screening discrepancies among providers largely stem from knowledge gaps, nonstandardized questionnaires, lack of time at appointments, and the sensitive nature of the questioning (eg, disordered eating).30
DIAGNOSING THE TRIAD
Given that the signs of low energy availability and menstrual dysfunction are often subtle, the diagnosis of the triad for those at risk requires input from a multidisciplinary team including a physician, sports dietitian, mental health professional, exercise physiologist, and other medical consultants.
Table 2 lists diagnostic tests the primary care provider should consider.
Diagnosing low energy availability
Energy availability is the dietary energy remaining after exercise energy expenditure; it is normalized to fat-free (lean) mass to account for resting energy expenditure. It is a product of energy intake, energy expenditure, and stored energy, and is calculated as:
An optimal value is at least 45 kcal/kg/day, while physiologic changes start to occur at less than 30 kcal/kg/day.4,31 Low energy is often seen in adult patients with a body mass index less than 17.5 kg/m2 and adolescent patients who are less than 80% of expected body weight.
Energy availability is hard to calculate, but certain assessments can be performed in a primary care setting to approximate it. To assess dietary intake, patients can bring in a 3-, 4-, or 7-day dietary log or complete a 24-hour food recall or food-frequency questionnaire in the office. To objectively document energy expenditure, patients can use heart rate monitors, accelerometers, an exercise diary, and web-based calculators. The fat-free mass can be calculated using a bioelectric impedance scale and skinfold caliper measurements.26
Those with chronic energy deficiency states may have reduced resting metabolic rates, with measured rates less than 90% of predicted and low triiodothyronine (T3) levels.31
Diagnosing menstrual dysfunction
When evaluating patients with menstrual dysfunction, it is important to first rule out pregnancy and endocrinopathies. These include thyroid dysfunction, hyperprolactinemia, primary ovarian insufficiency, other hypothalamic and pituitary disorders, and hyperandrogenic conditions such as polycystic ovarian syndrome, ovarian tumor, adrenal tumor, nonclassic congenital adrenal hyperplasia, and Cushing syndrome.
Depending on the patient’s age, laboratory tests can include follicle-stimulating hormone, luteinizing hormone, prolactin, serum estradiol, and a progesterone challenge.32 For hyperandrogenic symptoms, measuring total and free testosterone, dehydroepiandrosterone sulfate, 24-hour urine cortisol, and 17-hydroxyprogesterone levels may be helpful.
An endocrinologist should be consulted to evaluate the underlying cause of amenorrhea and address any associated hormonal imbalances. Attributing menstrual dysfunction to low energy availability is generally a diagnosis of exclusion. Additionally, outflow tract obstruction should be considered and ruled out with transvaginal ultrasonography in patients with primary amenorrhea.
A patient with hypoestrogenemia and amenorrhea may have the same steroid hormone profile as that of a menopausal woman. Lack of estrogen results in impaired endothelial cell function and arterial dilation, with accelerated development of atherosclerosis and subsequent cardiovascular events.33,34 Further, low energy availability has been linked to negative cardiovascular effects such as decreased vessel dilation leading to decreased tissue perfusion and hastened development of atherosclerosis.33 Female athletes with hypoestrogenism may show reduced perfusion of working muscle, impaired aerobic metabolism in skeletal muscle, elevated low-density lipoprotein cholesterol, and vaginal dryness.4
Diagnosing low bone mineral density
The most common clinical manifestations of low bone mineral density in female athletes are bone stress reactions such as stress fractures. In a study of 311 female high school athletes, 65.6% suffered from musculoskeletal injury from trauma or overuse including stress fractures and the patellofemoral syndrome.35 Many athletes seek medical attention from their primary care physician for stress reactions, providing an opportunity for triad screening.36
In postmenopausal women, osteopenia and osteoporosis are defined using the T score. However, in premenopausal women and adolescents, the International Society for Clinical Densitometry recommends using the Z score. A Z score less than –2.0 is described as “low bone density for chronological age.”14 For the diagnosis of osteoporosis in children and premenopausal women, the Society recommends using a Z score less than –2.0 along with the presence of a secondary risk factor for fracture such as undernutrition, hypogonadism, or a history of fracture.
Table 2 summarizes the diagnosis of low bone mineral density and osteoporosis in premenopausal women, adolescents, and children as well as when to order dual-energy x-ray absorptiometry (DEXA).37,38
Adolescents with low bone mineral density should have an annual DEXA scan of the total hip and lumbar spine.22 Amenorrheic athletes typically present with low areal density at the lumbar spine, reduced trabecular volumetric bone mineral density and bone strength index at the distal radius, and deterioration of the distal tibia.39
EARLY INTERVENTION IS ESSENTIAL
Early intervention is essential in patients with any component of the female athlete triad to prevent long-term adverse health effects. Successful treatment is strongly correlated with a trusting relationship between the athlete and the multidisciplinary team involved in her treatment.40
If needed, selective serotonin reuptake inhibitors and other psychotropic medications can be prescribed for comorbid conditions including bulimia nervosa, anxiety, depression, and obsessive-compulsive disorder. Primary providers can identify risk factors that prompt the evaluation and diagnosis of the triad, as well as support the goals of treatment and help manage comorbid conditions.
Eat more, exercise less
The primary goal is to restore body weight, maximizing nutritional and energy status by modifying the diet and adjusting exercise behavior to increase energy availability.41 Creating an energy-positive state by increasing intake, decreasing energy expenditure, or both increases energy availability, subsequently improves bone mineral density, and normalizes menstrual function.40
To sustain normal physiologic function, an energy availability of at least 45 kcal/kg/day is recommended.42 Patients should consume a minimum of 2,000 kcal/day, although energy needs may far exceed that, depending on energy expenditure. Olympic athletes participating in women’s crew and other sports have been anecdotally known to require over 12,000 kcal a day to maintain weight and performance. Goals include a body mass index of at least 18.5 kg/m2 in adults and a body weight of at least 90% of predicted in adolescents.
Involving a dietitian in the care team can help ensure that the patient consumes an adequate amount of macronutrients and micronutrients necessary for bone growth; these include calcium, vitamin D, iron, zinc, and vitamin K.4,32 For patients with disordered eating, referral to a mental health professional is important to help them avoid pathologic eating behaviors, reduce dieting attempts, and alter negative emotions associated with food and body image.
Once treatment begins, patients must undergo standardized periodic monitoring of their body weight. Although positive effects such as normalization of metabolic hormones (eg, insulinlike growth factor 1) may be seen in days to weeks by reversing low energy availability, it may take several months for menstrual function to improve and years for measurable improvement in bone mineral density to occur.23
Menstrual function should improve with weight gain
Normalizing menses in patients with the female athlete triad depends on improving the low energy availability and inducing weight gain.
Pharmacotherapy such as combined oral contraceptives can treat symptoms of hypogonadism.25 However, combined oral contraceptives do not restore spontaneous menses but rather induce withdrawal bleeding, which can lead to a false sense of security.23 While there are some benefits to prescribing combined oral contraceptives to treat hypogonadism, nonpharmacologic methods should be tried initially to restore menses, including increasing caloric intake and body weight. Golden et al showed that hormone replacement with combined oral contraceptives did not improve bone density in women with low estrogen states (eg, anorexia nervosa, osteopenia).43 Further, combined oral contraceptives may worsen bone health, as oral estrogen suppresses hepatic production of insulinlike growth factor 1, a bone trophic hormone.23
Treating low bone mineral density
Improving energy availability and menstrual function can help improve bone mineral density. Nutritional enhancement is recommended for mineralization of trabecular bone and growth of cortical bone. Supplemental calcium (1,000–1,500 mg daily) and vitamin D (600–1,000 IU daily) should be incorporated into the treatment of low bone mineral density.15,19
Additionally, weight gain has a positive effect on bone mineral density independent of its effect on the resumption of menses. However, weight gain alone does not normalize bone mineral density. Resuming normal physiologic production of hormones with estrogen-dependent effects on bone health is integral to normalization as well.23
Resistance training is encouraged to increase lean mass, although caution must be used to prevent fractures during high-impact activity. Bone mineral density may take up to several years to improve and may not be fully reversible.4,25,39
Pharmacologic therapy for low bone mineral density has unclear outcomes in women under age 50. The decision to treat should be based on bone mineral density along with fracture history. Given their unknown effects on the human fetal skeleton, bisphosphonates and denosumab should be used with caution in women of childbearing potential.24 No studies have used denosumab or teriparatide in women with the female athlete triad. Despite concerns regarding use of these drugs in premenopausal women, drug therapy should be strongly considered in women with a history of fracture and those with a high risk of subsequent fracture. The decision to treat can be made in conjunction with the athlete’s endocrinologist.
RETURN TO PLAY
If an athlete is noted to be at risk for or diagnosed with the female athlete triad, it is important to formulate a plan for her to return to play once her health improves.
De Souza et al provided a cumulative risk assessment for risk stratification and made recommendations on when an athlete should return to play depending on her level of risk.23 Using this grading system, primary care physicians can risk-stratify their patients. Those at low risk may be fully cleared to return to play, while those at moderate to high risk must first follow up with a multidisciplinary team to develop treatment strategies for improving their health.
Once a patient reaches her established goals, she may provisionally return to play under the close supervision of a team physician or primary care physician. A written treatment contract, including the goals set by the multidisciplinary team, should be followed closely as the athlete continues to participate in the sport.23;
Striving for athletic excellence, many young women—and some young men—create an energy deficit from increased exercise, decreased intake, or both. In women, the resulting energy deficit can suppress the menstrual cycle and in turn lead to bone demineralization in a syndrome called the female athlete triad.
Primary care physicians should be aware of this syndrome because it can lead to short-term and long-term health complications, and they are in a good position to screen for, diagnose, and treat it. However, a study of 931 US physicians in 2015 found that only 37% had heard of it.1
DEFINITION HAS CHANGED: ONLY 1 OF 3 COMPONENTS NEEDED
In 1972, Title IX of the Education Amendment Act was passed, prohibiting sex discrimination in any higher education program or activity receiving federal financial aid. Since then, female athletic participation in the United States has increased more than 10-fold.2
Also increasing has been awareness of the link between athletics, eating disorders, and amenorrhea. The American College of Sports Medicine coined the term female athlete triad in 1992, describing it as the constellation of disordered eating, amenorrhea, and osteoporosis (all 3 needed to be present).3 They broadened the definition in 2007 so that the syndrome can be diagnosed if any of the following is present4:
- Low energy availability (with or without an eating disorder)
- Menstrual dysfunction
- Decreased bone mineral density.
Recognizing that low energy availability can affect athletes of either sex and have consequences beyond the female reproductive system and skeleton, in 2014 the International Olympic Committee introduced a broader term called relative energy deficiency in sport.5,6 Like the triad, this condition occurs when energy intake falls below energy output to the point that it negatively affects an athlete’s physical and mental health.
THE COMPONENTS ARE COMMON
The female athlete triad can be seen in high school, collegiate, and elite athletes7 and is especially common in sports with subjective judging (gymnastics, figure skating) or endurance sports that emphasize leanness (eg, running).8
In a review of 65 studies, Gibbs et al9 found that the prevalence of any one of the triad conditions in exercising women and female athletes ranged from 16.0% to 60.0%, the prevalence of any 2 ranged from 2.7% to 27.0%, and the prevalence of all 3 ranged from 0% to 15.9%.
Low energy availability is categorized as either intentional (ie, due to disordered eating) or unintentional (ie, due to activities not associated with eating). Sustained low energy availability is often associated with eating disorders and subsequent low self-esteem, depression, and anxiety disorders.4
The prevalence of eating disorders is high in female athletes—31% and 20% in 2 large studies of elite female athletes, compared with 5.5% and 9%, respectively, in the general population.10,11 Another study found that the prevalence of disordered eating was 46.7% in sports that emphasize leanness, such as track and gymnastics, compared with 19.8% in sports that did not, such as basketball and soccer.12
Calorie restriction is common. In a study of 15 elite ballet dancers and 15 matched controls, the dancers were found to consume only about 3/4 as many calories per day as the controls (1,577 vs 2,075 kcal/day, P ≤ .01).13
Menstrual dysfunction. In small studies, the prevalence of secondary amenorrhea was as high as 69% in dancers and 65% in long-distance runners.4,14–16
Decreased bone mineral density. According to a systematic review, the prevalence of osteopenia in amenorrheic athletes ranged between 22% and 50% and the prevalence of osteoporosis was 0% to 13%, compared with 12% and 2.3%, respectively, in the general population.17
THE COMPONENTS ARE LINKED
dysfunction play causative roles in bone mineral density pathology. Within each component of the triad a spectrum of dysfunction exists, with all 3 components exhibiting serious health end points including low energy availability, functional hypothalamic amenorrhea, and osteoporosis.
The 3 components of the female athlete triad—low energy availability, menstrual dysfunction, and decreased bone mineral density—are linked, and each exists on a spectrum (Figure 1). The long-term consequences are far-reaching and can affect the cardiovascular, endocrine, reproductive, skeletal, gastrointestinal, renal, and central nervous systems.
Low energy availability is the driving force of the triad, causing menstrual irregularity and subsequent low bone mineral density.
Menstrual dysfunction. Low energy availability can contribute to menstrual disturbances because the body suppresses reproductive function to prevent pregnancy. Functional hypothalamic amenorrhea results from decreased gonadotropin-releasing hormone leading to decreased gonadotropin release from the pituitary gland and, ultimately, to low circulating estrogen levels.18 Menstrual irregularities related to the triad include:
- Primary amenorrhea (a delay in menarche)
- Oligomenorrhea (menstrual cycles occurring at intervals greater than every 35 days)
- Secondary amenorrhea (cessation of menstruation for 3 consecutive months).
(Primary amenorrhea is defined as no menses by age 15 in the presence of normal secondary sexual development or within 5 years after breast development if that occurs before the age of 10. Secondary amenorrhea is defined as the loss of menses for 90 or more days after menarche.19)
In animal studies, reducing dietary intake by more than 30% resulted in infertility.4 Menstrual abnormalities can present as early as 5 days after a patient enters a state of low energy availability.20 Symptoms of menstrual dysfunction are largely indicative of hypogonadism and include vaginal dryness, infertility, and impaired bone health.
Bone health in women with the female athlete triad can range from optimal to osteoporosis.
Low bone mineral density is a result of low energy availability and menstrual dysfunction leading to estrogen deficiency.21,22 Specifically, menstrual abnormalities can result in low estrogen and overactivity of osteoclasts, while low energy availability alters the metabolic environment, inducing changes in insulinlike growth factor 1, leptin, and peptide YY, resulting in deficiencies in vitamin D and calcium—nutrients necessary for bone mineralization. In turn, bone health and density are compromised.21,22
Ninety percent of peak bone mass is attained by age 18. Those who have low bone mineral density as part of the female athlete triad can suffer from long-lasting effects on their bone health.
SCREENING
Untreated, the triad can lead to fatigue, poor sports performance, and a number of serious comorbid conditions such as osteopenia and osteoporosis (leading to stress fractures) anemia, heart arrhythmias, and amenorrhea. Therefore, it is important for primary care providers to screen female athletes for the triad during routine office visits.
In 2014, the Triad Consensus Panel recommended screening female athletes at the high school and collegiate levels during a preparticipation physical evaluation and then every year by a primary care physician, athletic trainer, team physician, or coach.23
Risk factors include signs of dietary restriction, low body mass index, delayed menarche, oligomenorrhea or amenorrhea, and bone stress reactions or fractures.23 Athletes should be questioned about their menstrual history (age of menarche, frequency, and duration of menstrual cycles), history of stress fractures, medication history, family history (osteoporosis, eating disorders, and fractures),24,25 and dietary habits.
Physical findings include low body mass index, recent weight loss, orthostatic hypotension, lanugo, hypercarotenemia, and signs of eating disorders (restrictive, binging, purging) (Table 1).25–27
Additionally, it is important to ascertain if the patient receives critical comments regarding performance or body image from coaches, parents, or teammates and if sport-specific training began early in life.
Certain personality factors and behaviors are clues, such as perfectionism, obsessiveness, frequent weight cycling, and overtraining.4,25 If any of the triad components are apparent, a deeper evaluation can be completed.
Specific screening questions
The Female Athlete Triad Coalition recommends asking 11 screening questions and having prompt discussions regarding the athlete’s nutritional status and body image.23 If the patient gives a worrisome response to a screening question, further workup for a formal diagnosis should be initiated.
Questions about nutritional status.
- Do you worry about your weight?
- Are you trying to gain or lose weight, or has anyone recommended that you do so?
- Are you on a special diet or do you avoid certain types of foods or food groups?
- Have you ever had an eating disorder?
Questions about menstrual function.
- Have you ever had a menstrual period?
- How old were you when you had your first menstrual period?
- When was your most recent menstrual period?
- How many periods have you had in the last 12 months?
- Are you presently taking any female hormones (estrogen, progesterone, birth control pills)?
Questions about bone health.
- Have you ever had a stress fracture?
- Have you ever been told you have low bone density (osteopenia or osteoporosis)?
Along similar lines, the American Academy of Pediatrics, American Academy of Family Physicians, and American College of Sports Medicine28 have a list of 7 questions:
- Do you worry about your weight?
- Do you limit the foods you eat?
- Do you lose weight to meet image requirements for sports?
- Have you ever suffered from an eating disorder?
- How old were you when you had your first menstrual period?
- How many menstrual cycles have you had in the past 12 months?
- Have you ever had a stress fracture?
These questions are not being widely used. A study of the National Collegiate Athletic Association Division I universities found that only 9% of universities included 9 or more of the recommended 12 questions that the Female Athlete Triad Coalition was recommending at that time, and 22% asked only 1 or 2 of the questions. None of the universities included all 12.29 These findings are not surprising, given that screening for the triad is not state-mandated. Screening discrepancies among providers largely stem from knowledge gaps, nonstandardized questionnaires, lack of time at appointments, and the sensitive nature of the questioning (eg, disordered eating).30
DIAGNOSING THE TRIAD
Given that the signs of low energy availability and menstrual dysfunction are often subtle, the diagnosis of the triad for those at risk requires input from a multidisciplinary team including a physician, sports dietitian, mental health professional, exercise physiologist, and other medical consultants.
Table 2 lists diagnostic tests the primary care provider should consider.
Diagnosing low energy availability
Energy availability is the dietary energy remaining after exercise energy expenditure; it is normalized to fat-free (lean) mass to account for resting energy expenditure. It is a product of energy intake, energy expenditure, and stored energy, and is calculated as:
An optimal value is at least 45 kcal/kg/day, while physiologic changes start to occur at less than 30 kcal/kg/day.4,31 Low energy is often seen in adult patients with a body mass index less than 17.5 kg/m2 and adolescent patients who are less than 80% of expected body weight.
Energy availability is hard to calculate, but certain assessments can be performed in a primary care setting to approximate it. To assess dietary intake, patients can bring in a 3-, 4-, or 7-day dietary log or complete a 24-hour food recall or food-frequency questionnaire in the office. To objectively document energy expenditure, patients can use heart rate monitors, accelerometers, an exercise diary, and web-based calculators. The fat-free mass can be calculated using a bioelectric impedance scale and skinfold caliper measurements.26
Those with chronic energy deficiency states may have reduced resting metabolic rates, with measured rates less than 90% of predicted and low triiodothyronine (T3) levels.31
Diagnosing menstrual dysfunction
When evaluating patients with menstrual dysfunction, it is important to first rule out pregnancy and endocrinopathies. These include thyroid dysfunction, hyperprolactinemia, primary ovarian insufficiency, other hypothalamic and pituitary disorders, and hyperandrogenic conditions such as polycystic ovarian syndrome, ovarian tumor, adrenal tumor, nonclassic congenital adrenal hyperplasia, and Cushing syndrome.
Depending on the patient’s age, laboratory tests can include follicle-stimulating hormone, luteinizing hormone, prolactin, serum estradiol, and a progesterone challenge.32 For hyperandrogenic symptoms, measuring total and free testosterone, dehydroepiandrosterone sulfate, 24-hour urine cortisol, and 17-hydroxyprogesterone levels may be helpful.
An endocrinologist should be consulted to evaluate the underlying cause of amenorrhea and address any associated hormonal imbalances. Attributing menstrual dysfunction to low energy availability is generally a diagnosis of exclusion. Additionally, outflow tract obstruction should be considered and ruled out with transvaginal ultrasonography in patients with primary amenorrhea.
A patient with hypoestrogenemia and amenorrhea may have the same steroid hormone profile as that of a menopausal woman. Lack of estrogen results in impaired endothelial cell function and arterial dilation, with accelerated development of atherosclerosis and subsequent cardiovascular events.33,34 Further, low energy availability has been linked to negative cardiovascular effects such as decreased vessel dilation leading to decreased tissue perfusion and hastened development of atherosclerosis.33 Female athletes with hypoestrogenism may show reduced perfusion of working muscle, impaired aerobic metabolism in skeletal muscle, elevated low-density lipoprotein cholesterol, and vaginal dryness.4
Diagnosing low bone mineral density
The most common clinical manifestations of low bone mineral density in female athletes are bone stress reactions such as stress fractures. In a study of 311 female high school athletes, 65.6% suffered from musculoskeletal injury from trauma or overuse including stress fractures and the patellofemoral syndrome.35 Many athletes seek medical attention from their primary care physician for stress reactions, providing an opportunity for triad screening.36
In postmenopausal women, osteopenia and osteoporosis are defined using the T score. However, in premenopausal women and adolescents, the International Society for Clinical Densitometry recommends using the Z score. A Z score less than –2.0 is described as “low bone density for chronological age.”14 For the diagnosis of osteoporosis in children and premenopausal women, the Society recommends using a Z score less than –2.0 along with the presence of a secondary risk factor for fracture such as undernutrition, hypogonadism, or a history of fracture.
Table 2 summarizes the diagnosis of low bone mineral density and osteoporosis in premenopausal women, adolescents, and children as well as when to order dual-energy x-ray absorptiometry (DEXA).37,38
Adolescents with low bone mineral density should have an annual DEXA scan of the total hip and lumbar spine.22 Amenorrheic athletes typically present with low areal density at the lumbar spine, reduced trabecular volumetric bone mineral density and bone strength index at the distal radius, and deterioration of the distal tibia.39
EARLY INTERVENTION IS ESSENTIAL
Early intervention is essential in patients with any component of the female athlete triad to prevent long-term adverse health effects. Successful treatment is strongly correlated with a trusting relationship between the athlete and the multidisciplinary team involved in her treatment.40
If needed, selective serotonin reuptake inhibitors and other psychotropic medications can be prescribed for comorbid conditions including bulimia nervosa, anxiety, depression, and obsessive-compulsive disorder. Primary providers can identify risk factors that prompt the evaluation and diagnosis of the triad, as well as support the goals of treatment and help manage comorbid conditions.
Eat more, exercise less
The primary goal is to restore body weight, maximizing nutritional and energy status by modifying the diet and adjusting exercise behavior to increase energy availability.41 Creating an energy-positive state by increasing intake, decreasing energy expenditure, or both increases energy availability, subsequently improves bone mineral density, and normalizes menstrual function.40
To sustain normal physiologic function, an energy availability of at least 45 kcal/kg/day is recommended.42 Patients should consume a minimum of 2,000 kcal/day, although energy needs may far exceed that, depending on energy expenditure. Olympic athletes participating in women’s crew and other sports have been anecdotally known to require over 12,000 kcal a day to maintain weight and performance. Goals include a body mass index of at least 18.5 kg/m2 in adults and a body weight of at least 90% of predicted in adolescents.
Involving a dietitian in the care team can help ensure that the patient consumes an adequate amount of macronutrients and micronutrients necessary for bone growth; these include calcium, vitamin D, iron, zinc, and vitamin K.4,32 For patients with disordered eating, referral to a mental health professional is important to help them avoid pathologic eating behaviors, reduce dieting attempts, and alter negative emotions associated with food and body image.
Once treatment begins, patients must undergo standardized periodic monitoring of their body weight. Although positive effects such as normalization of metabolic hormones (eg, insulinlike growth factor 1) may be seen in days to weeks by reversing low energy availability, it may take several months for menstrual function to improve and years for measurable improvement in bone mineral density to occur.23
Menstrual function should improve with weight gain
Normalizing menses in patients with the female athlete triad depends on improving the low energy availability and inducing weight gain.
Pharmacotherapy such as combined oral contraceptives can treat symptoms of hypogonadism.25 However, combined oral contraceptives do not restore spontaneous menses but rather induce withdrawal bleeding, which can lead to a false sense of security.23 While there are some benefits to prescribing combined oral contraceptives to treat hypogonadism, nonpharmacologic methods should be tried initially to restore menses, including increasing caloric intake and body weight. Golden et al showed that hormone replacement with combined oral contraceptives did not improve bone density in women with low estrogen states (eg, anorexia nervosa, osteopenia).43 Further, combined oral contraceptives may worsen bone health, as oral estrogen suppresses hepatic production of insulinlike growth factor 1, a bone trophic hormone.23
Treating low bone mineral density
Improving energy availability and menstrual function can help improve bone mineral density. Nutritional enhancement is recommended for mineralization of trabecular bone and growth of cortical bone. Supplemental calcium (1,000–1,500 mg daily) and vitamin D (600–1,000 IU daily) should be incorporated into the treatment of low bone mineral density.15,19
Additionally, weight gain has a positive effect on bone mineral density independent of its effect on the resumption of menses. However, weight gain alone does not normalize bone mineral density. Resuming normal physiologic production of hormones with estrogen-dependent effects on bone health is integral to normalization as well.23
Resistance training is encouraged to increase lean mass, although caution must be used to prevent fractures during high-impact activity. Bone mineral density may take up to several years to improve and may not be fully reversible.4,25,39
Pharmacologic therapy for low bone mineral density has unclear outcomes in women under age 50. The decision to treat should be based on bone mineral density along with fracture history. Given their unknown effects on the human fetal skeleton, bisphosphonates and denosumab should be used with caution in women of childbearing potential.24 No studies have used denosumab or teriparatide in women with the female athlete triad. Despite concerns regarding use of these drugs in premenopausal women, drug therapy should be strongly considered in women with a history of fracture and those with a high risk of subsequent fracture. The decision to treat can be made in conjunction with the athlete’s endocrinologist.
RETURN TO PLAY
If an athlete is noted to be at risk for or diagnosed with the female athlete triad, it is important to formulate a plan for her to return to play once her health improves.
De Souza et al provided a cumulative risk assessment for risk stratification and made recommendations on when an athlete should return to play depending on her level of risk.23 Using this grading system, primary care physicians can risk-stratify their patients. Those at low risk may be fully cleared to return to play, while those at moderate to high risk must first follow up with a multidisciplinary team to develop treatment strategies for improving their health.
Once a patient reaches her established goals, she may provisionally return to play under the close supervision of a team physician or primary care physician. A written treatment contract, including the goals set by the multidisciplinary team, should be followed closely as the athlete continues to participate in the sport.23;
- Curry EJ, Logan C, Ackerman K, McInnis KC, Matzkin EG. Female athlete triad awareness among multispecialty physicians. Sports Med Open 2015; 1(1):38. doi:10.1186/s40798-015-0037-5
- National Federation of State High School Associations. 2012–13 high school athletics participation survey. http://old.nfhs.org/content.aspx?id=3282. Accessed February 1, 2018.
- Otis CL, Drinkwater B, Johnson M, Loucks A, Wilmore J. American College of Sports Medicine position stand. The female athlete triad. Med Sci Sports Exerc 1997; 29(5):i–ix.
- Nattiv A, Loucks AB, Manore MM, Sanborn CF, Sundgot-Borgen J, Warren MP; American College of Sports Medicine. American College of Sports Medicine position stand. The female athlete triad. Med Sci Sports Exerc 2007; 39(10):1867–1882. doi:10.1249/mss.0b013e318149f111
- Mountjoy M, Sundgot-Borgen J, Burke L, et al. The IOC consensus statement: beyond the female athlete triad—relative energy deficiency in sport (RED-S). Br J Sports Med 2014; 48(7):491–497. doi:10.1136/bjsports-2014-093502
- Tenforde AS, Barrack MT, Nattiv A, Fredericson M. Parallels with the female athlete triad in male athletes. Sports Med 2016; 46(2):171–182. doi:10.1007/s40279-015-0411-y
- Thein-Nissenbaum JM, Carr KE. Female athlete triad syndrome in the high school athlete. Phys Ther Sport 2011; 12(3):108–116. doi:10.1016/j.ptsp.2011.04.002
- Matzkin E, Curry EJ, Whitlock K. Female athlete triad: past, present, and future. J Am Acad Orthop Surg 2015; 23(7):424–432. doi:10.5435/JAAOS-D-14-00168
- Gibbs JC, Williams NI, De Souza MJ. Prevalence of individual and combined components of the female athlete triad. Med Sci Sports Exerc 2013; 45(5):985–996. doi:10.1249/MSS.0b013e31827e1bdc
- Byrne S, McLean N. Elite athletes: effects of the pressure to be thin. J Sci Med Sport 2002; 5(2):80–94. doi:10.1016/S1440-2440(02)80029-9
- Sundgot-Borgen J, Torstveit MK. Prevalence of eating disorders in elite athletes is higher than in the general population. Clin J Sport Med 2004; 14(1):25–32.
- Lynch SL, Hoch AZ. The female runner: gender specifics. Clin Sports Med 2010; 29(3):477–498. doi:10.1016/j.csm.2010.03.003
- Doyle-Lucas AF, Akers JD, Davy BM. Energetic efficiency, menstrual irregularity, and bone mineral density in elite professional female ballet dancers. J Dance Med Sci 2010; 14(4):146–154.
- Thein-Nissenbaum J. Long term consequences of the female athlete triad. Maturitas 2013; 75(2):107–112. doi:10.1016/j.maturitas.2013.02.010
- Hilibrand MJ, Hammoud S, Bishop M, Woods D, Fredrick RW, Dodson CC. Common injuries and ailments of the female athlete; pathophysiology, treatment and prevention. Phys Sportsmed 2015; 43(4):403–411. doi:10.1080/00913847.2015.1092856
- Demorest RA, Hergenroeder AC. Preface. Sports medicine and sports injuries. Adolesc Med State Art Rev 2015; 26(1):xv–xvi.
- Khan KM, Liu-Ambrose T, Sran MM, Ashe MC, Donaldson MG, Wark JD. New criteria for female athlete triad syndrome? Br J Sports Med 2002; 36(1):10–13. doi:10.1136/bjsm.36.1.10
- Fontana R, Della Torre S. The deep correlation between energy metabolism and reproduction: a view of the effects of nutrition for women fertility. Nutrients 2016; 8:87. www.ncbi.nlm.nih.gov/pmc/articles/PMC4772050/. Accessed February 2, 2018.
- Nazem TG, Ackerman K. The female athlete triad. Sports Health 2012; 4(4):302–311. doi:10.1177/1941738112439685
- Pantano KJ. Coaching concerns in physically active girls and young women—part 1: the female athlete triad. Strength Conditioning J 2009; 31(6):38–43. doi:10.1519/SSC.0b013e3181c105dd
- Micklesfield LK, Hugo J, Johnson C, Noakes TD, Lambert EV. Factors associated with menstrual dysfunction and self-reported bone stress injuries in female runners in the ultra- and half-marathons of the Two Oceans. Br J Sports Med 2007; 41(10):679–683. doi:10.1136/bjsm.2007.037077
- Lambrinoudaki I, Papadimitriou D. Pathophysiology of bone loss in the female athlete. Ann N Y Acad Sci 2010; 1205:45–50. doi:10.1111/j.1749-6632.2010.05681.x
- De Souza MJ, Nattiv A, Joy E, et al. 2014 Female Athlete Triad Coalition consensus statement on treatment and return to play of the female athlete triad: 1st International Conference held in San Francisco, CA, May 2012, and 2nd International Conference held in Indianapolis, IN, May 2013. Clin J Sport Med 2014; 24(2):96–119. doi:10.1136/bjsports-2013-093218
- Horn E, Gergen N, McGarry KA. The female athlete triad. R I Med J (2013) 2014; 97(11):18–21.
- Joy E, De Souza MJ, Nattiv A, et al. 2014 Female Athlete Triad Coalition consensus statement on treatment and return to play of the female athlete triad. Curr Sports Med Rep 2014; 13(4):219–232. doi:10.1249/JSR.0000000000000077
- Temme KE, Hoch AZ. Recognition and rehabilitation of the female athlete triad/tetrad: a multidisciplinary approach. Curr Sports Med Rep 2013; 12(3):190–199. doi:10.1249/JSR.0b013e318296190b
- Pritts SD, Susman J. Diagnosis of eating disorders in primary care. Am Fam Physician 2003; 67(2):297–304.
- Berhardt DR, Roberts WO, editors. Preparticipation Physical Evaluation, 4th Ed. American Academy of Pediatrics, Elk Grove Village, IL, 2010.
- Mencias T, Noon M, Hoch AZ. Female athlete triad screening in National Collegiate Athletic Association Division I athletes: is the preparticipation evaluation form effective? Clin J Sport Med 2012; 22(2):122–125. doi:10.1097/JSM.0b013e3182425aee
- Javed A, Tebben PJ, Fischer PR, Lteif AN. Female athlete triad and its components: toward improved screening and management. Mayo Clin Proc 2013; 88(9):996–1009. doi:10.1016/j.mayocp.2013.07.001
- Melin A, Tornberg AB, Skouby S, et al. Energy availability and the female athlete triad in elite endurance athletes. Scand J Med Sci Sports 2015; 25(5):610–622. doi:10.1111/sms.12261
- Warr BJ, Woolf K. The female athlete triad: patients do best with a team approach to care. JAAPA 2011; 24(4):50–55.
- Hoch AZ, Lal S, Jurva JW, Gutterman DD. The female athlete triad and cardiovascular dysfunction. Phys Med Rehabil Clin North Am 2007; 18(3):385–400. doi:10.1016/j.pmr.2007.05.001
- Lanser EM, Zach KN, Hoch AZ. The female athlete triad and endothelial dysfunction. PM R 2011; 3(5):458–465. doi:10.1016/j.pmrj.2010.12.024
- Thein-Nissenbaum JM, Rauh MJ, Carr KE, Loud KJ, McGuine TA. Associations between disordered eating, menstrual dysfunction, and musculoskeletal injury among high school athletes. J Orthop Sports Phys Ther 2011; 41(2):60–69. doi:10.2519/jospt.2011.3312
- Ducher G, Turner AI, Kukuljan S, et al. Obstacles in the optimization of bone health outcomes in the female athlete triad. Sports Med 2011; 41(7):587–607. doi:10.2165/11588770-000000000-00000
- Mendelsohn FA, Warren MP. Anorexia, bulimia, and the female athlete triad: evaluation and management. Endocrinol Metab Clin North Am 2010; 39(1):155–167. doi:10.1016/j.ecl.2009.11.002
- House S, Loud K, Shubkin C. Female athlete triad for the primary care pediatrician. Curr Opin Pediatr 2013; 25(6):755–761. doi:10.1097/MOP.0000000000000033
- Mallinson RJ, De Souza MJ. Current perspectives on the etiology and manifestation of the “silent” component of the female athlete triad. Int J Womens Health 2014; 6:451–467. doi:10.2147/IJWH.S38603
- Deimel JF, Dunlap BJ. The female athlete triad. Clin Sports Med 2012; 31(2):247–254. doi:10.1016/j.csm.2011.09.007
- Manore MM, Kam LC, Loucks AB; International Association of Athletics Federations. The female athlete triad: components, nutrition issues, and health consequences. J Sports Sci 2007; 25(suppl 1):S61–S71. doi:10.1080/02640410701607320
- Witkop CT, Warren MP. Understanding the spectrum of the female athlete triad. Obstet Gynecol 2010; 116(6):1444–1448. doi:10.1097/AOG.0b013e3181fbed40
- Golden NH, Lanzkowsky L, Schebendach J, Palestro CJ, Jacobson MS, Shenker IR. The effect of estrogen-progestin treatment on bone mineral density in anorexia nervosa. J Pediatr Adolesc Gynecol 2002; 15(3):135–143. doi:10.1016/S1083-3188(02)00145-6
- Curry EJ, Logan C, Ackerman K, McInnis KC, Matzkin EG. Female athlete triad awareness among multispecialty physicians. Sports Med Open 2015; 1(1):38. doi:10.1186/s40798-015-0037-5
- National Federation of State High School Associations. 2012–13 high school athletics participation survey. http://old.nfhs.org/content.aspx?id=3282. Accessed February 1, 2018.
- Otis CL, Drinkwater B, Johnson M, Loucks A, Wilmore J. American College of Sports Medicine position stand. The female athlete triad. Med Sci Sports Exerc 1997; 29(5):i–ix.
- Nattiv A, Loucks AB, Manore MM, Sanborn CF, Sundgot-Borgen J, Warren MP; American College of Sports Medicine. American College of Sports Medicine position stand. The female athlete triad. Med Sci Sports Exerc 2007; 39(10):1867–1882. doi:10.1249/mss.0b013e318149f111
- Mountjoy M, Sundgot-Borgen J, Burke L, et al. The IOC consensus statement: beyond the female athlete triad—relative energy deficiency in sport (RED-S). Br J Sports Med 2014; 48(7):491–497. doi:10.1136/bjsports-2014-093502
- Tenforde AS, Barrack MT, Nattiv A, Fredericson M. Parallels with the female athlete triad in male athletes. Sports Med 2016; 46(2):171–182. doi:10.1007/s40279-015-0411-y
- Thein-Nissenbaum JM, Carr KE. Female athlete triad syndrome in the high school athlete. Phys Ther Sport 2011; 12(3):108–116. doi:10.1016/j.ptsp.2011.04.002
- Matzkin E, Curry EJ, Whitlock K. Female athlete triad: past, present, and future. J Am Acad Orthop Surg 2015; 23(7):424–432. doi:10.5435/JAAOS-D-14-00168
- Gibbs JC, Williams NI, De Souza MJ. Prevalence of individual and combined components of the female athlete triad. Med Sci Sports Exerc 2013; 45(5):985–996. doi:10.1249/MSS.0b013e31827e1bdc
- Byrne S, McLean N. Elite athletes: effects of the pressure to be thin. J Sci Med Sport 2002; 5(2):80–94. doi:10.1016/S1440-2440(02)80029-9
- Sundgot-Borgen J, Torstveit MK. Prevalence of eating disorders in elite athletes is higher than in the general population. Clin J Sport Med 2004; 14(1):25–32.
- Lynch SL, Hoch AZ. The female runner: gender specifics. Clin Sports Med 2010; 29(3):477–498. doi:10.1016/j.csm.2010.03.003
- Doyle-Lucas AF, Akers JD, Davy BM. Energetic efficiency, menstrual irregularity, and bone mineral density in elite professional female ballet dancers. J Dance Med Sci 2010; 14(4):146–154.
- Thein-Nissenbaum J. Long term consequences of the female athlete triad. Maturitas 2013; 75(2):107–112. doi:10.1016/j.maturitas.2013.02.010
- Hilibrand MJ, Hammoud S, Bishop M, Woods D, Fredrick RW, Dodson CC. Common injuries and ailments of the female athlete; pathophysiology, treatment and prevention. Phys Sportsmed 2015; 43(4):403–411. doi:10.1080/00913847.2015.1092856
- Demorest RA, Hergenroeder AC. Preface. Sports medicine and sports injuries. Adolesc Med State Art Rev 2015; 26(1):xv–xvi.
- Khan KM, Liu-Ambrose T, Sran MM, Ashe MC, Donaldson MG, Wark JD. New criteria for female athlete triad syndrome? Br J Sports Med 2002; 36(1):10–13. doi:10.1136/bjsm.36.1.10
- Fontana R, Della Torre S. The deep correlation between energy metabolism and reproduction: a view of the effects of nutrition for women fertility. Nutrients 2016; 8:87. www.ncbi.nlm.nih.gov/pmc/articles/PMC4772050/. Accessed February 2, 2018.
- Nazem TG, Ackerman K. The female athlete triad. Sports Health 2012; 4(4):302–311. doi:10.1177/1941738112439685
- Pantano KJ. Coaching concerns in physically active girls and young women—part 1: the female athlete triad. Strength Conditioning J 2009; 31(6):38–43. doi:10.1519/SSC.0b013e3181c105dd
- Micklesfield LK, Hugo J, Johnson C, Noakes TD, Lambert EV. Factors associated with menstrual dysfunction and self-reported bone stress injuries in female runners in the ultra- and half-marathons of the Two Oceans. Br J Sports Med 2007; 41(10):679–683. doi:10.1136/bjsm.2007.037077
- Lambrinoudaki I, Papadimitriou D. Pathophysiology of bone loss in the female athlete. Ann N Y Acad Sci 2010; 1205:45–50. doi:10.1111/j.1749-6632.2010.05681.x
- De Souza MJ, Nattiv A, Joy E, et al. 2014 Female Athlete Triad Coalition consensus statement on treatment and return to play of the female athlete triad: 1st International Conference held in San Francisco, CA, May 2012, and 2nd International Conference held in Indianapolis, IN, May 2013. Clin J Sport Med 2014; 24(2):96–119. doi:10.1136/bjsports-2013-093218
- Horn E, Gergen N, McGarry KA. The female athlete triad. R I Med J (2013) 2014; 97(11):18–21.
- Joy E, De Souza MJ, Nattiv A, et al. 2014 Female Athlete Triad Coalition consensus statement on treatment and return to play of the female athlete triad. Curr Sports Med Rep 2014; 13(4):219–232. doi:10.1249/JSR.0000000000000077
- Temme KE, Hoch AZ. Recognition and rehabilitation of the female athlete triad/tetrad: a multidisciplinary approach. Curr Sports Med Rep 2013; 12(3):190–199. doi:10.1249/JSR.0b013e318296190b
- Pritts SD, Susman J. Diagnosis of eating disorders in primary care. Am Fam Physician 2003; 67(2):297–304.
- Berhardt DR, Roberts WO, editors. Preparticipation Physical Evaluation, 4th Ed. American Academy of Pediatrics, Elk Grove Village, IL, 2010.
- Mencias T, Noon M, Hoch AZ. Female athlete triad screening in National Collegiate Athletic Association Division I athletes: is the preparticipation evaluation form effective? Clin J Sport Med 2012; 22(2):122–125. doi:10.1097/JSM.0b013e3182425aee
- Javed A, Tebben PJ, Fischer PR, Lteif AN. Female athlete triad and its components: toward improved screening and management. Mayo Clin Proc 2013; 88(9):996–1009. doi:10.1016/j.mayocp.2013.07.001
- Melin A, Tornberg AB, Skouby S, et al. Energy availability and the female athlete triad in elite endurance athletes. Scand J Med Sci Sports 2015; 25(5):610–622. doi:10.1111/sms.12261
- Warr BJ, Woolf K. The female athlete triad: patients do best with a team approach to care. JAAPA 2011; 24(4):50–55.
- Hoch AZ, Lal S, Jurva JW, Gutterman DD. The female athlete triad and cardiovascular dysfunction. Phys Med Rehabil Clin North Am 2007; 18(3):385–400. doi:10.1016/j.pmr.2007.05.001
- Lanser EM, Zach KN, Hoch AZ. The female athlete triad and endothelial dysfunction. PM R 2011; 3(5):458–465. doi:10.1016/j.pmrj.2010.12.024
- Thein-Nissenbaum JM, Rauh MJ, Carr KE, Loud KJ, McGuine TA. Associations between disordered eating, menstrual dysfunction, and musculoskeletal injury among high school athletes. J Orthop Sports Phys Ther 2011; 41(2):60–69. doi:10.2519/jospt.2011.3312
- Ducher G, Turner AI, Kukuljan S, et al. Obstacles in the optimization of bone health outcomes in the female athlete triad. Sports Med 2011; 41(7):587–607. doi:10.2165/11588770-000000000-00000
- Mendelsohn FA, Warren MP. Anorexia, bulimia, and the female athlete triad: evaluation and management. Endocrinol Metab Clin North Am 2010; 39(1):155–167. doi:10.1016/j.ecl.2009.11.002
- House S, Loud K, Shubkin C. Female athlete triad for the primary care pediatrician. Curr Opin Pediatr 2013; 25(6):755–761. doi:10.1097/MOP.0000000000000033
- Mallinson RJ, De Souza MJ. Current perspectives on the etiology and manifestation of the “silent” component of the female athlete triad. Int J Womens Health 2014; 6:451–467. doi:10.2147/IJWH.S38603
- Deimel JF, Dunlap BJ. The female athlete triad. Clin Sports Med 2012; 31(2):247–254. doi:10.1016/j.csm.2011.09.007
- Manore MM, Kam LC, Loucks AB; International Association of Athletics Federations. The female athlete triad: components, nutrition issues, and health consequences. J Sports Sci 2007; 25(suppl 1):S61–S71. doi:10.1080/02640410701607320
- Witkop CT, Warren MP. Understanding the spectrum of the female athlete triad. Obstet Gynecol 2010; 116(6):1444–1448. doi:10.1097/AOG.0b013e3181fbed40
- Golden NH, Lanzkowsky L, Schebendach J, Palestro CJ, Jacobson MS, Shenker IR. The effect of estrogen-progestin treatment on bone mineral density in anorexia nervosa. J Pediatr Adolesc Gynecol 2002; 15(3):135–143. doi:10.1016/S1083-3188(02)00145-6
KEY POINTS
- Low energy availability is the driving force of the triad, causing menstrual irregularity and subsequent low bone mineral density.
- Recognizing that men as well as women can suffer from energy deficiency and that it can affect more than the female reproductive system and skeleton, the International Olympic Committee has proposed calling the disorder relative energy deficiency in sport.
- Screening for the triad with a specific set of questions is recommended during the preparticipation assessment.
- Early intervention and treatment prevents serious health consequences including life-threatening arrhythmias, amenorrhea, and osteoporosis.