User login
- The value of this new technology might not be so much the prediction of acidosis but identification of the well-oxygenated fetus so that labor may be safely continued in the presence of a concerning—but not ominous—fetal heart rate tracing.
- The only randomized study published so far did not determine whether clinical decisions can be based solely on fetal pulse oximetry. The investigators did suggest that sensitivity and specificity for metabolic acidemia was improved in the intervention group—a promising appraisal, in contrast with previous observational data.
When a teenage nullipara underwent labor induction for preeclampsia at 37 weeks, she was given epidural analgesia and seizure prophylaxis with magnesium sulfate. Her electronic fetal heart rate (FHR) tracing was initially reassuring, with only occasional variable decelerations, but subsequently revealed a baseline of 140 beats per minute (bpm), minimal to absent variability, no accelerations, and variable decelerations to 90 bpm with rapid return to baseline.
The tracing was interpreted as nonreassuring, and a fetal pulse oximeter was inserted. It revealed a fetal oxygen saturation rate between 45% and 50%, and labor was allowed to continue. After 3.5 hours in the second stage, the patient was delivered by outlet forceps. Her infant had Apgar scores of 8 at 1 minute and 9 at 5 minutes. The umbilical arterial pH was 7.25, and base excess was–4.9.
Fetal pulse oximetry made it possible to manage this case without resorting to emergent cesarean. But is this noninvasive technology truly a step forward in intrapartum assessment of fetal well-being?
We describe what the evidence (a single randomized study and a number of observational studies) reveals about these questions:
- How accurately does fetal pulse oximetry reflect the fetal condition?
- What is the critical threshold for fetal oxygen desaturation?
- Is a single reading reliable?
- Does oximetry correlate with acid-base status?
- Does the combination of oximetry and electronic monitoring improve accuracy?
- Will fetal pulse oximetry improve neonatal outcomes?
- How precise is it?
- Is it easy to use?
Needed: Effective adjunct to electronic monitoring
Except in the chronically hypoxic fetus (which is affected by the time labor begins), the pathophysiology of acute intrapartum events is a continuum, from hypoxemia to respiratory acidosis to metabolic acidosis and, ultimately, clinical impairment. The goal of intrapartum surveillance is to detect fetal hypoxemia before it progresses to asphyxia and perinatal mortality or long-term morbidity.
Although it is approved as an adjunct to electronic fetal monitoring (EFM), fetal pulse oximetry has gained only sporadic use since it became available in the United States in 2000—even though EFM has proved disappointing as a tool for predicting fetal hypoxia. Only about 10% of US obstetrical units had fetal pulse oximetry technology as of 2002.1
Clinicians began questioning the reliability of subjective interpretation of fetal heart tracings soon after EFM went into general use. Thirty years later, a meta-analysis of 12 randomized clinical trials involving 58,855 gravidas cast doubt on the benefits of EFM,2 which is associated with an increase in operative deliveries as a result of high sensitivity but low specificity in predicting fetal hypoxia and acidosis.
FDA approval was based on sole randomized trial
The only commercially available fetal oximetry sensor, the Nellcor N-400 (Nellcor, Pleasanton, Calif), obtained US Food and Drug Administration (FDA) approval as an adjunct to EFM when the latter indicates a nonreassuring FHR pattern. That approval was based on the only randomized study3 of fetal pulse oximetry conducted, which involved 1,010 women with predefined nonreassuring FHR patterns in labor.
Goal: Reduced cesarean rate with comparable outcomes. Investigators hypothesized that adjunctive fetal oximetry would improve assessment and reduce the cesarean rate without altering neonatal outcome. Indeed, in the oximetry group, the rate of cesarean delivery performed for a nonreassuring FHR tracing (4.5% versus 10.2%; P = .007) was significantly reduced. Other findings:
- Same neonatal outcomes, with no significant differences between the 2 groups.
- Higher cesarean rate for dystocia in the intervention group, offsetting any advantage in the overall cesarean delivery rate (29% versus 26%). This unexpected increase in cesarean deliveries raises several possibilities:
- Given the unblinded design, it is possible that clinicians, circumspect of the pulse oximetry, continued to perform cesareans for nonreassuring FHR, but labeled the indication for surgery differently. The validity of the dystocia diagnosis was discredited by a subsequent partogram analysis that showed a similar rate of arrested labor in both groups.
- A nonreassuring FHR in conditions of normal fetal oxygenation is predictive of dystocia. Previous randomized studies of EFM have suggested the same thing.4
- Dystocia is the consequence of the device itself. Anecdotal observations suggest a higher rate of persistent occiput posterior positions with fetal oximetry.
Other trials underway. The ongoing Fetal Oximetry (FOX) trial of the National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network, involving 10,000 nulliparous participants, is comparing cesarean delivery rates and safety outcomes in patients monitored for FHR plus pulse oximetry with a group in which the clinicians are blinded to the pulse oximetry readings. Another randomized controlled trial of fetal pulse oximetry is underway in Australia.
Potential for increased costs. The American College of Obstetricians and Gynecologists (ACOG) has raised concerns about the potential increase in costs without demonstrable improvement in outcome.5 ACOG has not endorsed fetal pulse oximetry for general practice.
Question 1How accurately does pulse oximetry reflect the fetal condition?
It yields only indirect information on the partial pressure of oxygen in the blood and no data on perfusion or acid-base status.
In other clinical settings, oxygen saturation is not an acceptable substitute for arterial blood gas analysis. The pulse oximeter is not a hemoximeter—only that device directly and reliably determines blood oxygen saturation by spectrophotometry.6 Even the calculated oxygen saturation values provided automatically by modern blood gas analyzers are inaccurate.7
Studies report varying results. In a comparison8 of fetal oxygen saturation by hemoximetry in a fetal scalp blood (FSB) sample and fetal arterial oxyhemoglobin saturation (FSpO2) by pulse oximetry immediately before the blood sampling, the FSpO2 medians were always higher than the FSB hemoximetry saturation—which led to false-negative results in hypoxic babies.
In animal studies, pulse oximetry correlated well with simultaneously measured arterial oxygen saturation (r = 0.98, P = .01),9 but data from human studies are inconsistent. While McNamara et al10 reported good correlation between FSpO2 measurements and umbilical artery blood oxygen saturation at birth (r = 0.59, P <.001), Langer et al11 found no relationship between FSpO2 levels determined during pushing efforts and oxygen saturation in umbilical vein blood at birth.
Possible reasons for the ambiguous findings:
- differences in practice, such as use of umbilical venous versus arterial blood, or measurement during pushing versus between pushes,
- different intervals from FSpO2 reading to umbilical blood sampling, or
- incomparable groups, such as all women in labor versus those with abnormal FHR.
Limitations. Fetal pulse oximetry measures arterial oxygen saturation during the systolic pulse wave in the skin microcirculation at head level. In the fetus, this is part of the preductal circulation, with oxygen saturation levels somewhere between umbilical arterial and umbilical venous blood oxygen saturation.
Theoretically, FSpO2 should be closer to FSB than to umbilical blood. Although FSB samples consist of capillary blood, which is not exactly central arterial blood, the differences are small, at least in the neonate.12 In the intrapartum period, however, several variables with unknown effect may weaken relationships:
- different intervals between the last oximetry signal and blood sampling after delivery
- differences in local tissue perfusion status13
- perfusion changes during fetal compromise, as the fetus centralizes its blood flow, with vasoconstriction in the skin circulation
Question 2What is the critical threshold for fetal oxygen desaturation?
Human studies indicate that an FSpO2 of 33% is approximately the 10th percentile on the normal distribution, and an FSpO2 of 29% to 30% represents the third to fifth percentiles in normal-outcome labor.14 Studies in catheterized fetal sheep suggest that the level below which metabolic acidosis can be anticipated is an FSpO2 of about 30%.15
The 30% threshold also is supported by prospective human data from a multicenter trial.16 According to those data, an FSpO2 of less than 30% has 100% sensitivity in predicting an FSB pH below 7.20. FSpO2 of less than 30% also correlated with a lack of variability on the FHR tracing.17
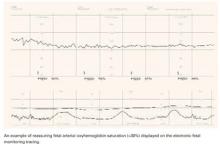
The cutoff of 30% should not be interpreted as an indication of fetal distress, however. Rather, it represents a threshold below which increasing fetal acidosis will be encountered ( FIGURE 1). Oxygen saturation is a dynamic biologic parameter with broad variation.
FIGURE 1 Tracking fetal arterial oxyhemoglobin saturation
Question 3Is a single reading reliable?
The normal fetus has a remarkable capacity to compensate for transient episodes of desaturation. Thus, a single reading cannot reflect the fetal condition; the trend in FSpO2 must be taken into account. Research indicates only FSpO2 levels below 30% for more than 2 minutes18 or more than 10 minutes19 are likely to be associated with intrapartum acidosis.
ACOG has raised concerns about the potential increase in costs without demonstrable improvement in outcome.
Gorenberg et al20 retrospectively correlated FSpO2 with umbilical artery pH and found that neither the 30% threshold alone nor the duration of FSpO2 below 30% correlated with fetal acidemia (pH below 7.20). Rather, the repetition of such episodes was more predictive. The authors concluded that more than 10 episodes of FSpO2below 30% would overcome the ability of the fetus to compensate.
The study was underpowered to detect a significant difference in acidemia, and did not allow sufficient observation time to detect the natural progression of hypoxia to metabolic acidosis, a better indicator of fetal compromise. Additional research is needed.
Question 4Does oximetry correlate with acid-base status?
Many of the studies mentioned here assumed a correlation. Whenever oxygen saturation in the umbilical artery is 30% or more, acidosis (pH below 7.13) in the same blood is rare—only 1%.21 However, the correlation between fetal pulse oximetry values and acid-base status is much weaker.8.
Leszczynska-Gorzelak et al22 found no relationship between FSpO2 levels in the first or second stage of labor and pH or partial pressure of oxygen in umbilical vein blood at delivery. Other investigators concluded similarly, considering intrapartum FSpO2 of limited use for predicting acidosis at birth, irrespective of FSpO2 cutoff.23,24
Rijnders et al24 found no significant correlation between fetal scalp or umbilical artery blood pH and mean FSpO2 for the last 30 minutes before sampling (r = 0.02, P = .9). Even the lowest FSpO2 level did not correlate with arterial pH (r = .04, P = .84). None of the study’s 3 cases of umbilical pH below 7.05 would have been detected using the mean FSpO2 before delivery, and only 1 would have been detected using the lowest FSpO2.
In another multicenter study involving the Nellcor system in 164 cases with abnormal FHR, a correlation between oximetry and FSB sampling (r = 0.29, P < .01) was noted in the first stage of labor, but second-stage FSpO2 readings did not correlate with oxygen saturation, partial pressure of oxygen, pH, or bicarbonate level in the umbilical artery at birth.25
An observational series26 of 128 fetuses with nonreassuring FHR patterns concluded that fetal distress was insufficiently identified by oximetry. Only 2 of the 11 cases with umbilical artery pH below 7.20 were detected by pulse oximetry recordings below 30% during the last 30 minutes of the second stage, and out of 5 cases with hypoxic readings in the second stage, only 2 were acidotic at birth. The calculated sensitivity was 18%, specificity 92%, positive predictive value (PPV) 40%, and negative predictive value (NPV) 80%. A low Apgar score was never predicted by fetal pulse oximetry.
Others used the same Nellcor system over the final 30 minutes of labor and a cutoff for umbilical blood acidemia of pH below 7.13 and reported similar numbers: sensitivity 28%, specificity 94%, PPV 40%, and NPV 80%.23
Vitoratos et al27 analyzed FSpO2 readings in active labor (not limited to the last 30 minutes before delivery) and obtained somewhat better values: sensitivity 72%, specificity 93%, PPV 61.5%, and NPV 95.8% for an umbilical artery blood pH below 7.15.
The impression that the validity of fetal pulse oximetry is higher in earlier labor than in the second stage is supported by data from Stiller et al. 28 Leszczynska-Gorzelak et al29 found a significant decrease in mean FSpO2 from the first stage to the second stage of normal labor (51.9% versus 43.8%, P < .001), and Dildy et al14 noted a similar difference upon analyzing 160 normal labors (59% versus 53%), but other studies failed to verify such differences.25,30
Observational studies had unrealistic pH cutoff. All the evidence presented thus far on the validity of fetal pulse oximetry in predicting acidemia is based on observational data. A common deficiency is the unrealistic cutoff for pathologic fetal acidemia—a pH of less than 7.13 to 7.20—when it is widely accepted that “pathologic fetal acidemia” reflects an umbilical artery blood pH below 7.31 Even in this group, two thirds of neonates are unaffected by morbidity.
Need to identify metabolic acidosis. It also is accepted that the presence of a metabolic component to fetal acidemia may be as important—if not more important—than a single pH cutoff.31 Only a few human studies of pulse oximetry have distinguished between respiratory and metabolic acidemia. When they did, intrapartum fetal pulse oximetry was unable to predict umbilical artery base excess.23,25
The only randomized study failed to determine whether clinical decisions can be based solely on fetal pulse oximetry. 3 The investigators did suggest that sensitivity and specificity for metabolic acidemia was improved in the intervention group—a promising appraisal, in contrast with previous observational data.
In the study, 7 neonates (3 in the intervention group and 4 controls) had umbilical artery blood pH below 7. All 4 controls had vaginal delivery. There also were 6 cases of elevated base excess (ie, -16 mEq/L or below) among controls. None were recorded in the intervention group, and the 3 cases of acidemia were recognized antepartum and led to cesareans.
Unfortunately, the study design did not guarantee that patient management was based exclusively on EFM with or without fetal pulse oximetry. Vibro-acoustic stimulation or FSB sampling was required before proceeding to cesarean delivery in both groups.
It appears that the negative predictive value of fetal oximetry is of greater practical value than other attributes.
When FSpO2 was less than 30% for the entire interval between 2 contractions, or was unobtainable, the physician was supposed to revert to interpretation of EFM. When that was persistently nonreassuring, the physician was given the option of scalp stimulation or FSB sampling. Thus, it was not determined whether clinical decisions can be based exclusively on fetal pulse oximetry. Schmidt et al26 suggested that such exclusive application of fetal pulse oximetry might actually jeopardize fetal health.
Question 5 Does the combination of oximetry and EFM improve accuracy?
Fetal pulse oximetry was not used independently in any of the studies discussed here, but in association with EFM, which has a sensitivity for fetal acidosis of 93%, specificity of 29%, PPV of 2.6%, and NPV of 99.5%.32
From a statistical point of view, whenever 2 evaluation methods with the same endpoint (fetal acidosis) are combined, sensitivity decreases while specificity increases, theoretically resulting in less unnecessary intervention. That is exactly what investigators have reported: sensitivity as low as 18%26 for fetal oximetry, and specificity as high as 94%.23 However, the value of this new technology might not be so much the prediction of acidosis but identification of the well-oxygenated fetus so that labor may be safely continued in the presence of a concerning—but not ominous—FHR tracing.
Fetal pulse oximetry employs principles of optical spectrophotometry and plethysmography to provide information on the percentage of oxygen bound to hemoglobin. Oxyhemoglobin (oxygenated hemoglobin) and deoxyhemoglobin (hemoglobin without oxygen) absorb red and infrared light differently: more red absorption by deoxyhemoglobin, and more infrared absorption by oxyhemoglobin.
By measuring the relative absorption at each wavelength, the fraction of hemoglobin that carries oxygen can be determined. The arterial oxygen saturation is expressed as a percentage. The technology has been refined to measure fetal arterial oxyhemoglobin saturation during labor.
Pulse oximetry sensors must be calibrated for fetal biological values. In the fetus, normal oxygen saturation is much lower than in the adult or neonate; hemoglobin has a higher affinity for oxygen and is in higher concentration; and there are more capillaries per unit of tissue, higher cardiac output, and a higher heart rate.
In the adult or neonate, pulse oximetry sensors can be attached to fingers, toes, ears, or the bridge of the nose, but such stable placement is not feasible in utero. Further, good contact between sensor and fetal skin is a prerequisite for avoidance of artifacts. This last aspect has presented a sizeable challenge.
Fetal sensors measure reflected light. There is disagreement about the merits of the 2 sensor types, reflectance and transmission. Both include 2 light emitters (for red and infrared light) and a detector. In the transmission sensor (the adult or neonatal type), the light produced by the light-emitting diodes (LED) is picked up by the detector after traversing the interposed tissues. Since tissue interposition is not possible in the fetus, most fetal studies have used reflectance sensors, in which the LED and detector are placed side by side, and the light to be analyzed is reflected by the tissues. This design adds variance depending on the light’s depth of tissue penetration and device position changes.
Placement of the sensor. The Nellcor N-400 includes a reflectance sensor housed in a smooth, pliable head that is advanced through the cervix with the aid of a handle. The handle has a removable stylet to stiffen it during placement.
The sensor is placed against the fetal temple, cheek, or forehead and is held in place by the uterine wall. Placement is similar to that of an internal pressure catheter. Once the stylet is removed, it should not be reinserted.
Because the sensor usually descends and rotates with the fetal head, displacements are frequent and adjustments in sensor placement may be necessary. Placement adjustments can be attempted without the stylet and, if unsuccessful, a new device can be inserted. The Nellcor sensor is not reusable.
The prerequisites for insertion are dilatation of at least 2 cm, ruptured membranes, cephalic presentation, single fetus, gestational age of at least 36 weeks, and no placenta previa.
The manufacturer reports that active genital herpes, HIV, and hepatitis B or E seropositivity preclude fetal pulse oximetry monitoring.
Placement may be impossible when the presenting part is at high station (-3 or above) or low station (+2 or below).
The Nellcor N-400 system has been commercially available in many European countries since 1995, and in Canada since 1998. It was approved for sale in the United States in early 2003.
Question 6Will it improve neonatal outcomes?
Neonatal outcome is the ultimate endpoint in obstetrical care. In the randomized trial by Garite et al,3 there was no difference in neonatal outcome between the groups using or not using fetal pulse oximetry. According to Chua et al,33 FSpO2 levels measured even 10 minutes before delivery have no relation to neonatal outcome.
Leszczynska-Gorzelak et al22 believe FSpO2 is more predictive of neonatal outcome in the first stage than the second. However, Apgar score had no relationship with FSpO2readings in the first or second stage. Butterwegge34 reported 6 cases of FSpO2 below 30% for more than 30 minutes, all with good neonatal outcome, and Alshimmiri et al23 noted that only normal FSpO2 correlates with fetal well-being. Thus, it appears that the NPV of fetal oximetry is of greater practical value than other attributes.
Question 7How precise is it?
The Nellcor system monitors the quality of FSpO2 measurement; no value is displayed if the signal lacks the characteristics of a fetal arterial plethysmographic curve or if contact between sensor and skin is insufficient. Because of fetal movements and other artifacts, posting time is always less than 100%.
In the French multicenter study,25 the mean reliable signal time in the first stage of labor was only 64.7%—even less in the second stage (54%). Signal retention was 67% in the randomized trial by Garite et al. 3
Many artifacts may impede signal acquisition and impact the reliability of a reading:
- The sensor’s position on the fetal head. For example, the difference in FSpO2 readings between the forehead and occiput may be as much as 13.4%. (The sensor is designed to go against the fetal cheek, but may move around.)
- Incomplete sensor-to-skin contact, such as with high fetal head station, -2 or above.
- Marked caput formation.
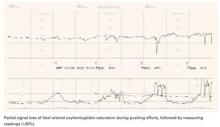
- Increased intrauterine pressure accompanying contractions, especially at presentation stations of +2 or below (FIGURE 2). FSpO2 monitoring requires detection of fetal pulses, which may be undetectable when the surrounding pressure is high, resulting in a loss of signal.
- Interposition of vernix or fetal hair.
- Presence of meconium, which behaves like a red-light filter, altering the ratio of red to infrared light and resulting in artificially low values.35 This theoretical concern is rejected by Yam et al,36 who did not observe any effect of meconium on FSpO2 values. (When the amniotic fluid is meconium-stained, Carbonne et al37 showed that fetal oximetry is a better predictor of meconium aspiration syndrome than FSB sampling.) The data on the influence of meconium on FSpO2 readings remain contradictory.
All these conditions may impair precision and contribute to poor sensitivity.
FIGURE 2 A weakening signal during pushing
Question 8Is it easy to use?
An Australian survey38 assessed clinicians’ perceptions during placement of the oximetry sensor. Ease of placement was rated as good or excellent in 71% of cases, and the patient’s comfort was rated as good or excellent in 90% of cases. Chua et al39 reported a mean insertion time of 90 seconds, with a reliable signal obtained within 5 minutes in 87% of placements. The French multicenter study25 mentioned earlier concluded that the procedure is satisfactory and easier than FSB sampling. The device itself was harmless to both mother and fetus.40
Potential research directions
Fetal pulse oximetry may be an effective tool in clinical scenarios such as:
- fetal arrhythmias with uninterpretable FHR tracing
- fetal tachycardia associated with maternal fever, thyrotoxicosis, or fetal supraventricular tachycardia, when distinguishing other contributions to tachycardia may be difficult
- fetal bradycardia caused by a complete heart block, which may render EFM undecipherable
- when amnioinfusion is attempted for variable decelerations and it is necessary to differentiate a nonreassuring FHR tracing related to transient in utero stress (eg, umbilical cord compressions) from ominous tracings
Another area not yet addressed is cost-effectiveness beyond the immediate direct costs (approximately $11,000 for the monitor and $150 for each disposable sensor). Also uncertain is whether laboring women will accept the device (how disturbing or invasive it is perceived to be) and how acceptable or applicable it is outside tertiary institutions.
Dr. Vidaeff reports no financial relationships relevant to this article. Dr. Ramin reports grant support from the US National Institutes of Health.
1. Dildy GA. A guest editorial: fetal pulse oximetry. Obstet Gynecol Surv. 2003;58:225-226.
2. Thacker SB, Stroup DF, Peterson HB. Efficacy and safety of intrapartum electronic fetal monitoring: an update. Obstet Gynecol. 1995;86:613-620.
3. Garite TJ, Dildy GA, McNamara H, et al. A multicenter controlled trial of fetal pulse oximetry in the intrapartum management of nonreassuring fetal heart rate patterns. Am J Obstet Gynecol. 2000;183:1049-1058.
4. Haverkamp AD, Orleans M, Langendoerfer S, et al. A controlled trial of the differential effects of continuous fetal heart rate monitoring in labor: a randomized trial. Am J Obstet Gynecol. 1979;134:399-412.
5. American College of Obstetricians and Gynecologists. Committee opinion: fetal pulse oximetry. Number 258, September 2001. Obstet Gynecol. 2001;98:523-524.
6. Zijlstra WG, Buursma A, Zwart A. Performance of an automated six-wavelength photometer (radiometer OSM3) for routine measurement of hemoglobin derivatives. Clin Chem. 1988;34:149-152.
7. Porath M, Sinah P, Dudenhausen JW, Luttkus AK. Systematic instrumental errors between oxygen saturation analysers in fetal blood during deep hypoxemia. Clin Chim Acta. 2001;307:151-157.
8. Luttkus AK, Lübke M, Büscher U, Porath M, Dudenhausen JW. Accuracy of fetal pulse oximetry. Acta Obstet Gynecol Scand. 2002;81:417-423.
9. Carter AM, Stiller R, König V, Jorgensen JS, Svendsen P, Huch R. Calibration of a reflectance pulse oximeter in fetal lambs for arterial oxygen saturations below 70%. J Soc Gynecol Invest. 1998;5:255-259.
10. McNamara H, Cung CD, Lilford R, Johnson N. Do fetal pulse oximetry readings at delivery correlate with cord blood oxygenation and acidemia? Br J Obstet Gynaecol. 1992;99:735-738.
11. Langer B, Boudier E, Haddad J, Pain L, Schlaeder G. Fetal pulse oximetry during labor of 62 patients. Fetal Diagn Ther. 1996;11:37-45.
12. Harrison AM, Lynch JM, Dean JM, Witte MK. Comparison of simultaneously obtained arterial and capillary blood gases in pediatric intensive care unit patients. Crit Care Med. 1997;25:1904-1908.
13. Nijland R, Jongsma HW, Nijhuis JG, Oeseburg B. Accuracy of fetal pulse oximetry and pitfalls in measurements. Eur J Obstet Gynecol Reprod Biol. 1997;72:S21-S27.
14. Dildy GA, van den Berg PP, Katz M, et al. Intrapartum fetal pulse oximetry: fetal oxygen saturation trends during labor and relation to delivery outcome. Am J Obstet Gynecol. 1994;171:679-684.
15. Nijland R, Jongsma H, Nijhuis J, van den Berg P, Oeseburg B. Arterial saturation in relation to metabolic acidosis in fetal lambs. Am J Obstet Gynecol. 1995;172:810-819.
16. Kühnert M, Seelbach-Göbel B, Butterwegge M. Predictive agreement between fetal arterial oxygen saturation and fetal scalp pH: results of the German multicenter study. Am J Obstet Gynecol. 1998;178:330-335.
17. Salamalekis E, Thomopoulos P, Giannaris D, et al. Computerised intrapartum diagnosis of fetal hypoxia based on heart rate monitoring and fetal pulse oximetry recordings utilizing wavelet analysis and neural networks. Br J Obstet Gynaecol. 2002;109:1137-1142.
18. Bloom SL, Swindle RG, McIntire DD, Leveno KJ. Fetal pulse oximetry: duration of desaturation and intrapartum outcome. Obstet Gynecol. 1999;93:1036-1040.
19. Seelbach-Göbel B, Heupel M, Kühnert M, et al. The prediction of fetal acidosis by means of intrapartum fetal pulse oximetry. Am J Obstet Gynecol. 1999;180:73-81.
20. Gorenberg DM, Patillo C, Hendi P, Rumney PJ, Garite TJ. Fetal pulse oximetry: correlation between oxygen desaturation, duration, and frequency and neonatal outcomes. Am J Obstet Gynecol. 2003;189:136-138.
21. Dildy GA, Thorp JA, Yeast JD, Clark SL. The relationship between oxygen saturation and pH in umbilical blood: implications for intrapartum fetal oxygen saturation monitoring. Am J Obstet Gynecol. 1996;175:682-687.
22. Leszczynska-Gorzelak B, Poniedzialek-Czajkowska E, Oleszczuk J. Fetal blood saturation during the 1st and 2nd stage of labor and its relation to the neonatal outcome. Gynecol Obstet Invest. 2002;54:159-163.
23. Alshimmiri M, Bocking AD, Gagnon R, Natale R, Richardson BS. Prediction of umbilical artery base excess by intrapartum fetal oxygen saturation monitoring. Am J Obstet Gynecol. 1997;177:775-779.
24. Rijnders RJ, Mol BW, Reuwer PJ, Drogtrop AP, Vernooij MM, Visser GH. Is the correlation between fetal oxygen saturation and blood pH sufficient for the use of fetal pulse oximetry? J Matern Fetal Neonatal Med. 2002;11:80-83.
25. Goffinet F, Langer B, Carbonne B, et al. Multicenter study on the clinical value of fetal pulse oximetry. I. Methodologic evaluation. The French Study Group on Fetal Pulse Oximetry. Am J Obstet Gynecol. 1997;177:1238-1246.
26. Schmidt S, Koslowski S, Sierra F, et al. Clinical usefulness of pulse oximetry in the fetus with non-reassuring heart rate pattern? J Perinat Med. 2000;28:298-305.
27. Vitoratos N, Salamalekis E, Saloum J, Makrakis E, Creatsas G. Abnormal fetal heart rate patterns during the active phase of labor and the value of fetal oxygen saturation. J Matern Fetal Neonatal Med. 2002;11:46-49.
28. Stiller R, von Mering R, König V, et al. How well does reflectance pulse oximetry reflect intrapartum fetal acidosis? Am J Obstet Gynecol. 2002;186:1351-1357.
29. Leszczynska-Gorzelak B, Poniedzialek-Czajkowska E, Zych I, Grzechnik M, Oleszczuk J. The usefulness of the intrapartum fetal pulse oximetry in anticipating the neonatal outcome. Ginekol Pol. 2001;72:1183-1188.
30. Nikolov A, Dimitrov A, Vakrilkova L, Iarkova N. Fetal oxygen saturation during normal delivery. Akush Ginekol. 2000;40:3-6.
31. Goldaber KG, Gilstrap LC III, Leveno K, Dax JS, McIntire DD. Pathologic fetal acidemia. Obstet Gynecol. 1991;78:1103-1107.
32. Low JA, Victory R, Derrick EJ. Predictive value of electronic fetal monitoring for intra-partum fetal asphyxia and metabolic acidosis. Obstet Gynecol. 1999;93:285-291.
33. Chua S, Yeong SM, Razvi K, Arulkumaran S. Fetal oxygen saturation during labour. Br J Obstet Gynaecol. 1997;104:1080-1083.
34. Butterwegge M. Fetal pulse oximetry and non-reassuring heart rate. Eur J Obstet Gynecol Reprod Biol. 1997;72:S63-S66.
35. Johnson N, Johnson VA, Bannister J, McNamara H. The effect of meconium on neonatal and fetal reflectance pulse oximetry. J Perinat Med. 1990;18:351-355.
36. Yam J, Chua S, Arulkumaran S. Intrapartum pulse oximetry: Part 1: Principles and technical issues. Obstet Gynecol Surv. 2000;55:163-172.
37. Carbonne B, Cudeville C, Sivan H, Cabrol D, Papiernik E. Fetal oxygen saturation measured by pulse oximetry during labor with clear and meconium-stained amniotic fluid. Eur J Obstet Gynecol Reprod Biol. 1997;72:S51-S55.
38. East CE, Colditz PB. Clinicians’ perceptions of placing a fetal oximetry sensor. J Qual Clin Pract. 2000;20:161-163.
39. Chua S, Yam J, Razvi K, Yeong SM, Arulkumaran S. Intrapartum fetal oxygen saturation monitoring in a busy labour ward. Eur J Obstet Gynecol Reprod Biol. 1999;82:185-189.
40. Luttkus AK, Friedmann W, Thomas S, Dimer JA, Dudenhausen JW. The safety of fetal pulse oximetry in parturients requiring fetal scalp blood sample. Obstet Gynecol. 1997;90:533-537.
- The value of this new technology might not be so much the prediction of acidosis but identification of the well-oxygenated fetus so that labor may be safely continued in the presence of a concerning—but not ominous—fetal heart rate tracing.
- The only randomized study published so far did not determine whether clinical decisions can be based solely on fetal pulse oximetry. The investigators did suggest that sensitivity and specificity for metabolic acidemia was improved in the intervention group—a promising appraisal, in contrast with previous observational data.
When a teenage nullipara underwent labor induction for preeclampsia at 37 weeks, she was given epidural analgesia and seizure prophylaxis with magnesium sulfate. Her electronic fetal heart rate (FHR) tracing was initially reassuring, with only occasional variable decelerations, but subsequently revealed a baseline of 140 beats per minute (bpm), minimal to absent variability, no accelerations, and variable decelerations to 90 bpm with rapid return to baseline.
The tracing was interpreted as nonreassuring, and a fetal pulse oximeter was inserted. It revealed a fetal oxygen saturation rate between 45% and 50%, and labor was allowed to continue. After 3.5 hours in the second stage, the patient was delivered by outlet forceps. Her infant had Apgar scores of 8 at 1 minute and 9 at 5 minutes. The umbilical arterial pH was 7.25, and base excess was–4.9.
Fetal pulse oximetry made it possible to manage this case without resorting to emergent cesarean. But is this noninvasive technology truly a step forward in intrapartum assessment of fetal well-being?
We describe what the evidence (a single randomized study and a number of observational studies) reveals about these questions:
- How accurately does fetal pulse oximetry reflect the fetal condition?
- What is the critical threshold for fetal oxygen desaturation?
- Is a single reading reliable?
- Does oximetry correlate with acid-base status?
- Does the combination of oximetry and electronic monitoring improve accuracy?
- Will fetal pulse oximetry improve neonatal outcomes?
- How precise is it?
- Is it easy to use?
Needed: Effective adjunct to electronic monitoring
Except in the chronically hypoxic fetus (which is affected by the time labor begins), the pathophysiology of acute intrapartum events is a continuum, from hypoxemia to respiratory acidosis to metabolic acidosis and, ultimately, clinical impairment. The goal of intrapartum surveillance is to detect fetal hypoxemia before it progresses to asphyxia and perinatal mortality or long-term morbidity.
Although it is approved as an adjunct to electronic fetal monitoring (EFM), fetal pulse oximetry has gained only sporadic use since it became available in the United States in 2000—even though EFM has proved disappointing as a tool for predicting fetal hypoxia. Only about 10% of US obstetrical units had fetal pulse oximetry technology as of 2002.1
Clinicians began questioning the reliability of subjective interpretation of fetal heart tracings soon after EFM went into general use. Thirty years later, a meta-analysis of 12 randomized clinical trials involving 58,855 gravidas cast doubt on the benefits of EFM,2 which is associated with an increase in operative deliveries as a result of high sensitivity but low specificity in predicting fetal hypoxia and acidosis.
FDA approval was based on sole randomized trial
The only commercially available fetal oximetry sensor, the Nellcor N-400 (Nellcor, Pleasanton, Calif), obtained US Food and Drug Administration (FDA) approval as an adjunct to EFM when the latter indicates a nonreassuring FHR pattern. That approval was based on the only randomized study3 of fetal pulse oximetry conducted, which involved 1,010 women with predefined nonreassuring FHR patterns in labor.
Goal: Reduced cesarean rate with comparable outcomes. Investigators hypothesized that adjunctive fetal oximetry would improve assessment and reduce the cesarean rate without altering neonatal outcome. Indeed, in the oximetry group, the rate of cesarean delivery performed for a nonreassuring FHR tracing (4.5% versus 10.2%; P = .007) was significantly reduced. Other findings:
- Same neonatal outcomes, with no significant differences between the 2 groups.
- Higher cesarean rate for dystocia in the intervention group, offsetting any advantage in the overall cesarean delivery rate (29% versus 26%). This unexpected increase in cesarean deliveries raises several possibilities:
- Given the unblinded design, it is possible that clinicians, circumspect of the pulse oximetry, continued to perform cesareans for nonreassuring FHR, but labeled the indication for surgery differently. The validity of the dystocia diagnosis was discredited by a subsequent partogram analysis that showed a similar rate of arrested labor in both groups.
- A nonreassuring FHR in conditions of normal fetal oxygenation is predictive of dystocia. Previous randomized studies of EFM have suggested the same thing.4
- Dystocia is the consequence of the device itself. Anecdotal observations suggest a higher rate of persistent occiput posterior positions with fetal oximetry.
Other trials underway. The ongoing Fetal Oximetry (FOX) trial of the National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network, involving 10,000 nulliparous participants, is comparing cesarean delivery rates and safety outcomes in patients monitored for FHR plus pulse oximetry with a group in which the clinicians are blinded to the pulse oximetry readings. Another randomized controlled trial of fetal pulse oximetry is underway in Australia.
Potential for increased costs. The American College of Obstetricians and Gynecologists (ACOG) has raised concerns about the potential increase in costs without demonstrable improvement in outcome.5 ACOG has not endorsed fetal pulse oximetry for general practice.
Question 1How accurately does pulse oximetry reflect the fetal condition?
It yields only indirect information on the partial pressure of oxygen in the blood and no data on perfusion or acid-base status.
In other clinical settings, oxygen saturation is not an acceptable substitute for arterial blood gas analysis. The pulse oximeter is not a hemoximeter—only that device directly and reliably determines blood oxygen saturation by spectrophotometry.6 Even the calculated oxygen saturation values provided automatically by modern blood gas analyzers are inaccurate.7
Studies report varying results. In a comparison8 of fetal oxygen saturation by hemoximetry in a fetal scalp blood (FSB) sample and fetal arterial oxyhemoglobin saturation (FSpO2) by pulse oximetry immediately before the blood sampling, the FSpO2 medians were always higher than the FSB hemoximetry saturation—which led to false-negative results in hypoxic babies.
In animal studies, pulse oximetry correlated well with simultaneously measured arterial oxygen saturation (r = 0.98, P = .01),9 but data from human studies are inconsistent. While McNamara et al10 reported good correlation between FSpO2 measurements and umbilical artery blood oxygen saturation at birth (r = 0.59, P <.001), Langer et al11 found no relationship between FSpO2 levels determined during pushing efforts and oxygen saturation in umbilical vein blood at birth.
Possible reasons for the ambiguous findings:
- differences in practice, such as use of umbilical venous versus arterial blood, or measurement during pushing versus between pushes,
- different intervals from FSpO2 reading to umbilical blood sampling, or
- incomparable groups, such as all women in labor versus those with abnormal FHR.
Limitations. Fetal pulse oximetry measures arterial oxygen saturation during the systolic pulse wave in the skin microcirculation at head level. In the fetus, this is part of the preductal circulation, with oxygen saturation levels somewhere between umbilical arterial and umbilical venous blood oxygen saturation.
Theoretically, FSpO2 should be closer to FSB than to umbilical blood. Although FSB samples consist of capillary blood, which is not exactly central arterial blood, the differences are small, at least in the neonate.12 In the intrapartum period, however, several variables with unknown effect may weaken relationships:
- different intervals between the last oximetry signal and blood sampling after delivery
- differences in local tissue perfusion status13
- perfusion changes during fetal compromise, as the fetus centralizes its blood flow, with vasoconstriction in the skin circulation
Question 2What is the critical threshold for fetal oxygen desaturation?
Human studies indicate that an FSpO2 of 33% is approximately the 10th percentile on the normal distribution, and an FSpO2 of 29% to 30% represents the third to fifth percentiles in normal-outcome labor.14 Studies in catheterized fetal sheep suggest that the level below which metabolic acidosis can be anticipated is an FSpO2 of about 30%.15
The 30% threshold also is supported by prospective human data from a multicenter trial.16 According to those data, an FSpO2 of less than 30% has 100% sensitivity in predicting an FSB pH below 7.20. FSpO2 of less than 30% also correlated with a lack of variability on the FHR tracing.17
The cutoff of 30% should not be interpreted as an indication of fetal distress, however. Rather, it represents a threshold below which increasing fetal acidosis will be encountered ( FIGURE 1). Oxygen saturation is a dynamic biologic parameter with broad variation.
FIGURE 1 Tracking fetal arterial oxyhemoglobin saturation
Question 3Is a single reading reliable?
The normal fetus has a remarkable capacity to compensate for transient episodes of desaturation. Thus, a single reading cannot reflect the fetal condition; the trend in FSpO2 must be taken into account. Research indicates only FSpO2 levels below 30% for more than 2 minutes18 or more than 10 minutes19 are likely to be associated with intrapartum acidosis.
ACOG has raised concerns about the potential increase in costs without demonstrable improvement in outcome.
Gorenberg et al20 retrospectively correlated FSpO2 with umbilical artery pH and found that neither the 30% threshold alone nor the duration of FSpO2 below 30% correlated with fetal acidemia (pH below 7.20). Rather, the repetition of such episodes was more predictive. The authors concluded that more than 10 episodes of FSpO2below 30% would overcome the ability of the fetus to compensate.
The study was underpowered to detect a significant difference in acidemia, and did not allow sufficient observation time to detect the natural progression of hypoxia to metabolic acidosis, a better indicator of fetal compromise. Additional research is needed.
Question 4Does oximetry correlate with acid-base status?
Many of the studies mentioned here assumed a correlation. Whenever oxygen saturation in the umbilical artery is 30% or more, acidosis (pH below 7.13) in the same blood is rare—only 1%.21 However, the correlation between fetal pulse oximetry values and acid-base status is much weaker.8.
Leszczynska-Gorzelak et al22 found no relationship between FSpO2 levels in the first or second stage of labor and pH or partial pressure of oxygen in umbilical vein blood at delivery. Other investigators concluded similarly, considering intrapartum FSpO2 of limited use for predicting acidosis at birth, irrespective of FSpO2 cutoff.23,24
Rijnders et al24 found no significant correlation between fetal scalp or umbilical artery blood pH and mean FSpO2 for the last 30 minutes before sampling (r = 0.02, P = .9). Even the lowest FSpO2 level did not correlate with arterial pH (r = .04, P = .84). None of the study’s 3 cases of umbilical pH below 7.05 would have been detected using the mean FSpO2 before delivery, and only 1 would have been detected using the lowest FSpO2.
In another multicenter study involving the Nellcor system in 164 cases with abnormal FHR, a correlation between oximetry and FSB sampling (r = 0.29, P < .01) was noted in the first stage of labor, but second-stage FSpO2 readings did not correlate with oxygen saturation, partial pressure of oxygen, pH, or bicarbonate level in the umbilical artery at birth.25
An observational series26 of 128 fetuses with nonreassuring FHR patterns concluded that fetal distress was insufficiently identified by oximetry. Only 2 of the 11 cases with umbilical artery pH below 7.20 were detected by pulse oximetry recordings below 30% during the last 30 minutes of the second stage, and out of 5 cases with hypoxic readings in the second stage, only 2 were acidotic at birth. The calculated sensitivity was 18%, specificity 92%, positive predictive value (PPV) 40%, and negative predictive value (NPV) 80%. A low Apgar score was never predicted by fetal pulse oximetry.
Others used the same Nellcor system over the final 30 minutes of labor and a cutoff for umbilical blood acidemia of pH below 7.13 and reported similar numbers: sensitivity 28%, specificity 94%, PPV 40%, and NPV 80%.23
Vitoratos et al27 analyzed FSpO2 readings in active labor (not limited to the last 30 minutes before delivery) and obtained somewhat better values: sensitivity 72%, specificity 93%, PPV 61.5%, and NPV 95.8% for an umbilical artery blood pH below 7.15.
The impression that the validity of fetal pulse oximetry is higher in earlier labor than in the second stage is supported by data from Stiller et al. 28 Leszczynska-Gorzelak et al29 found a significant decrease in mean FSpO2 from the first stage to the second stage of normal labor (51.9% versus 43.8%, P < .001), and Dildy et al14 noted a similar difference upon analyzing 160 normal labors (59% versus 53%), but other studies failed to verify such differences.25,30
Observational studies had unrealistic pH cutoff. All the evidence presented thus far on the validity of fetal pulse oximetry in predicting acidemia is based on observational data. A common deficiency is the unrealistic cutoff for pathologic fetal acidemia—a pH of less than 7.13 to 7.20—when it is widely accepted that “pathologic fetal acidemia” reflects an umbilical artery blood pH below 7.31 Even in this group, two thirds of neonates are unaffected by morbidity.
Need to identify metabolic acidosis. It also is accepted that the presence of a metabolic component to fetal acidemia may be as important—if not more important—than a single pH cutoff.31 Only a few human studies of pulse oximetry have distinguished between respiratory and metabolic acidemia. When they did, intrapartum fetal pulse oximetry was unable to predict umbilical artery base excess.23,25
The only randomized study failed to determine whether clinical decisions can be based solely on fetal pulse oximetry. 3 The investigators did suggest that sensitivity and specificity for metabolic acidemia was improved in the intervention group—a promising appraisal, in contrast with previous observational data.
In the study, 7 neonates (3 in the intervention group and 4 controls) had umbilical artery blood pH below 7. All 4 controls had vaginal delivery. There also were 6 cases of elevated base excess (ie, -16 mEq/L or below) among controls. None were recorded in the intervention group, and the 3 cases of acidemia were recognized antepartum and led to cesareans.
Unfortunately, the study design did not guarantee that patient management was based exclusively on EFM with or without fetal pulse oximetry. Vibro-acoustic stimulation or FSB sampling was required before proceeding to cesarean delivery in both groups.
It appears that the negative predictive value of fetal oximetry is of greater practical value than other attributes.
When FSpO2 was less than 30% for the entire interval between 2 contractions, or was unobtainable, the physician was supposed to revert to interpretation of EFM. When that was persistently nonreassuring, the physician was given the option of scalp stimulation or FSB sampling. Thus, it was not determined whether clinical decisions can be based exclusively on fetal pulse oximetry. Schmidt et al26 suggested that such exclusive application of fetal pulse oximetry might actually jeopardize fetal health.
Question 5 Does the combination of oximetry and EFM improve accuracy?
Fetal pulse oximetry was not used independently in any of the studies discussed here, but in association with EFM, which has a sensitivity for fetal acidosis of 93%, specificity of 29%, PPV of 2.6%, and NPV of 99.5%.32
From a statistical point of view, whenever 2 evaluation methods with the same endpoint (fetal acidosis) are combined, sensitivity decreases while specificity increases, theoretically resulting in less unnecessary intervention. That is exactly what investigators have reported: sensitivity as low as 18%26 for fetal oximetry, and specificity as high as 94%.23 However, the value of this new technology might not be so much the prediction of acidosis but identification of the well-oxygenated fetus so that labor may be safely continued in the presence of a concerning—but not ominous—FHR tracing.
Fetal pulse oximetry employs principles of optical spectrophotometry and plethysmography to provide information on the percentage of oxygen bound to hemoglobin. Oxyhemoglobin (oxygenated hemoglobin) and deoxyhemoglobin (hemoglobin without oxygen) absorb red and infrared light differently: more red absorption by deoxyhemoglobin, and more infrared absorption by oxyhemoglobin.
By measuring the relative absorption at each wavelength, the fraction of hemoglobin that carries oxygen can be determined. The arterial oxygen saturation is expressed as a percentage. The technology has been refined to measure fetal arterial oxyhemoglobin saturation during labor.
Pulse oximetry sensors must be calibrated for fetal biological values. In the fetus, normal oxygen saturation is much lower than in the adult or neonate; hemoglobin has a higher affinity for oxygen and is in higher concentration; and there are more capillaries per unit of tissue, higher cardiac output, and a higher heart rate.
In the adult or neonate, pulse oximetry sensors can be attached to fingers, toes, ears, or the bridge of the nose, but such stable placement is not feasible in utero. Further, good contact between sensor and fetal skin is a prerequisite for avoidance of artifacts. This last aspect has presented a sizeable challenge.
Fetal sensors measure reflected light. There is disagreement about the merits of the 2 sensor types, reflectance and transmission. Both include 2 light emitters (for red and infrared light) and a detector. In the transmission sensor (the adult or neonatal type), the light produced by the light-emitting diodes (LED) is picked up by the detector after traversing the interposed tissues. Since tissue interposition is not possible in the fetus, most fetal studies have used reflectance sensors, in which the LED and detector are placed side by side, and the light to be analyzed is reflected by the tissues. This design adds variance depending on the light’s depth of tissue penetration and device position changes.
Placement of the sensor. The Nellcor N-400 includes a reflectance sensor housed in a smooth, pliable head that is advanced through the cervix with the aid of a handle. The handle has a removable stylet to stiffen it during placement.
The sensor is placed against the fetal temple, cheek, or forehead and is held in place by the uterine wall. Placement is similar to that of an internal pressure catheter. Once the stylet is removed, it should not be reinserted.
Because the sensor usually descends and rotates with the fetal head, displacements are frequent and adjustments in sensor placement may be necessary. Placement adjustments can be attempted without the stylet and, if unsuccessful, a new device can be inserted. The Nellcor sensor is not reusable.
The prerequisites for insertion are dilatation of at least 2 cm, ruptured membranes, cephalic presentation, single fetus, gestational age of at least 36 weeks, and no placenta previa.
The manufacturer reports that active genital herpes, HIV, and hepatitis B or E seropositivity preclude fetal pulse oximetry monitoring.
Placement may be impossible when the presenting part is at high station (-3 or above) or low station (+2 or below).
The Nellcor N-400 system has been commercially available in many European countries since 1995, and in Canada since 1998. It was approved for sale in the United States in early 2003.
Question 6Will it improve neonatal outcomes?
Neonatal outcome is the ultimate endpoint in obstetrical care. In the randomized trial by Garite et al,3 there was no difference in neonatal outcome between the groups using or not using fetal pulse oximetry. According to Chua et al,33 FSpO2 levels measured even 10 minutes before delivery have no relation to neonatal outcome.
Leszczynska-Gorzelak et al22 believe FSpO2 is more predictive of neonatal outcome in the first stage than the second. However, Apgar score had no relationship with FSpO2readings in the first or second stage. Butterwegge34 reported 6 cases of FSpO2 below 30% for more than 30 minutes, all with good neonatal outcome, and Alshimmiri et al23 noted that only normal FSpO2 correlates with fetal well-being. Thus, it appears that the NPV of fetal oximetry is of greater practical value than other attributes.
Question 7How precise is it?
The Nellcor system monitors the quality of FSpO2 measurement; no value is displayed if the signal lacks the characteristics of a fetal arterial plethysmographic curve or if contact between sensor and skin is insufficient. Because of fetal movements and other artifacts, posting time is always less than 100%.
In the French multicenter study,25 the mean reliable signal time in the first stage of labor was only 64.7%—even less in the second stage (54%). Signal retention was 67% in the randomized trial by Garite et al. 3
Many artifacts may impede signal acquisition and impact the reliability of a reading:
- The sensor’s position on the fetal head. For example, the difference in FSpO2 readings between the forehead and occiput may be as much as 13.4%. (The sensor is designed to go against the fetal cheek, but may move around.)
- Incomplete sensor-to-skin contact, such as with high fetal head station, -2 or above.
- Marked caput formation.
- Increased intrauterine pressure accompanying contractions, especially at presentation stations of +2 or below (FIGURE 2). FSpO2 monitoring requires detection of fetal pulses, which may be undetectable when the surrounding pressure is high, resulting in a loss of signal.
- Interposition of vernix or fetal hair.
- Presence of meconium, which behaves like a red-light filter, altering the ratio of red to infrared light and resulting in artificially low values.35 This theoretical concern is rejected by Yam et al,36 who did not observe any effect of meconium on FSpO2 values. (When the amniotic fluid is meconium-stained, Carbonne et al37 showed that fetal oximetry is a better predictor of meconium aspiration syndrome than FSB sampling.) The data on the influence of meconium on FSpO2 readings remain contradictory.
All these conditions may impair precision and contribute to poor sensitivity.
FIGURE 2 A weakening signal during pushing
Question 8Is it easy to use?
An Australian survey38 assessed clinicians’ perceptions during placement of the oximetry sensor. Ease of placement was rated as good or excellent in 71% of cases, and the patient’s comfort was rated as good or excellent in 90% of cases. Chua et al39 reported a mean insertion time of 90 seconds, with a reliable signal obtained within 5 minutes in 87% of placements. The French multicenter study25 mentioned earlier concluded that the procedure is satisfactory and easier than FSB sampling. The device itself was harmless to both mother and fetus.40
Potential research directions
Fetal pulse oximetry may be an effective tool in clinical scenarios such as:
- fetal arrhythmias with uninterpretable FHR tracing
- fetal tachycardia associated with maternal fever, thyrotoxicosis, or fetal supraventricular tachycardia, when distinguishing other contributions to tachycardia may be difficult
- fetal bradycardia caused by a complete heart block, which may render EFM undecipherable
- when amnioinfusion is attempted for variable decelerations and it is necessary to differentiate a nonreassuring FHR tracing related to transient in utero stress (eg, umbilical cord compressions) from ominous tracings
Another area not yet addressed is cost-effectiveness beyond the immediate direct costs (approximately $11,000 for the monitor and $150 for each disposable sensor). Also uncertain is whether laboring women will accept the device (how disturbing or invasive it is perceived to be) and how acceptable or applicable it is outside tertiary institutions.
Dr. Vidaeff reports no financial relationships relevant to this article. Dr. Ramin reports grant support from the US National Institutes of Health.
- The value of this new technology might not be so much the prediction of acidosis but identification of the well-oxygenated fetus so that labor may be safely continued in the presence of a concerning—but not ominous—fetal heart rate tracing.
- The only randomized study published so far did not determine whether clinical decisions can be based solely on fetal pulse oximetry. The investigators did suggest that sensitivity and specificity for metabolic acidemia was improved in the intervention group—a promising appraisal, in contrast with previous observational data.
When a teenage nullipara underwent labor induction for preeclampsia at 37 weeks, she was given epidural analgesia and seizure prophylaxis with magnesium sulfate. Her electronic fetal heart rate (FHR) tracing was initially reassuring, with only occasional variable decelerations, but subsequently revealed a baseline of 140 beats per minute (bpm), minimal to absent variability, no accelerations, and variable decelerations to 90 bpm with rapid return to baseline.
The tracing was interpreted as nonreassuring, and a fetal pulse oximeter was inserted. It revealed a fetal oxygen saturation rate between 45% and 50%, and labor was allowed to continue. After 3.5 hours in the second stage, the patient was delivered by outlet forceps. Her infant had Apgar scores of 8 at 1 minute and 9 at 5 minutes. The umbilical arterial pH was 7.25, and base excess was–4.9.
Fetal pulse oximetry made it possible to manage this case without resorting to emergent cesarean. But is this noninvasive technology truly a step forward in intrapartum assessment of fetal well-being?
We describe what the evidence (a single randomized study and a number of observational studies) reveals about these questions:
- How accurately does fetal pulse oximetry reflect the fetal condition?
- What is the critical threshold for fetal oxygen desaturation?
- Is a single reading reliable?
- Does oximetry correlate with acid-base status?
- Does the combination of oximetry and electronic monitoring improve accuracy?
- Will fetal pulse oximetry improve neonatal outcomes?
- How precise is it?
- Is it easy to use?
Needed: Effective adjunct to electronic monitoring
Except in the chronically hypoxic fetus (which is affected by the time labor begins), the pathophysiology of acute intrapartum events is a continuum, from hypoxemia to respiratory acidosis to metabolic acidosis and, ultimately, clinical impairment. The goal of intrapartum surveillance is to detect fetal hypoxemia before it progresses to asphyxia and perinatal mortality or long-term morbidity.
Although it is approved as an adjunct to electronic fetal monitoring (EFM), fetal pulse oximetry has gained only sporadic use since it became available in the United States in 2000—even though EFM has proved disappointing as a tool for predicting fetal hypoxia. Only about 10% of US obstetrical units had fetal pulse oximetry technology as of 2002.1
Clinicians began questioning the reliability of subjective interpretation of fetal heart tracings soon after EFM went into general use. Thirty years later, a meta-analysis of 12 randomized clinical trials involving 58,855 gravidas cast doubt on the benefits of EFM,2 which is associated with an increase in operative deliveries as a result of high sensitivity but low specificity in predicting fetal hypoxia and acidosis.
FDA approval was based on sole randomized trial
The only commercially available fetal oximetry sensor, the Nellcor N-400 (Nellcor, Pleasanton, Calif), obtained US Food and Drug Administration (FDA) approval as an adjunct to EFM when the latter indicates a nonreassuring FHR pattern. That approval was based on the only randomized study3 of fetal pulse oximetry conducted, which involved 1,010 women with predefined nonreassuring FHR patterns in labor.
Goal: Reduced cesarean rate with comparable outcomes. Investigators hypothesized that adjunctive fetal oximetry would improve assessment and reduce the cesarean rate without altering neonatal outcome. Indeed, in the oximetry group, the rate of cesarean delivery performed for a nonreassuring FHR tracing (4.5% versus 10.2%; P = .007) was significantly reduced. Other findings:
- Same neonatal outcomes, with no significant differences between the 2 groups.
- Higher cesarean rate for dystocia in the intervention group, offsetting any advantage in the overall cesarean delivery rate (29% versus 26%). This unexpected increase in cesarean deliveries raises several possibilities:
- Given the unblinded design, it is possible that clinicians, circumspect of the pulse oximetry, continued to perform cesareans for nonreassuring FHR, but labeled the indication for surgery differently. The validity of the dystocia diagnosis was discredited by a subsequent partogram analysis that showed a similar rate of arrested labor in both groups.
- A nonreassuring FHR in conditions of normal fetal oxygenation is predictive of dystocia. Previous randomized studies of EFM have suggested the same thing.4
- Dystocia is the consequence of the device itself. Anecdotal observations suggest a higher rate of persistent occiput posterior positions with fetal oximetry.
Other trials underway. The ongoing Fetal Oximetry (FOX) trial of the National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network, involving 10,000 nulliparous participants, is comparing cesarean delivery rates and safety outcomes in patients monitored for FHR plus pulse oximetry with a group in which the clinicians are blinded to the pulse oximetry readings. Another randomized controlled trial of fetal pulse oximetry is underway in Australia.
Potential for increased costs. The American College of Obstetricians and Gynecologists (ACOG) has raised concerns about the potential increase in costs without demonstrable improvement in outcome.5 ACOG has not endorsed fetal pulse oximetry for general practice.
Question 1How accurately does pulse oximetry reflect the fetal condition?
It yields only indirect information on the partial pressure of oxygen in the blood and no data on perfusion or acid-base status.
In other clinical settings, oxygen saturation is not an acceptable substitute for arterial blood gas analysis. The pulse oximeter is not a hemoximeter—only that device directly and reliably determines blood oxygen saturation by spectrophotometry.6 Even the calculated oxygen saturation values provided automatically by modern blood gas analyzers are inaccurate.7
Studies report varying results. In a comparison8 of fetal oxygen saturation by hemoximetry in a fetal scalp blood (FSB) sample and fetal arterial oxyhemoglobin saturation (FSpO2) by pulse oximetry immediately before the blood sampling, the FSpO2 medians were always higher than the FSB hemoximetry saturation—which led to false-negative results in hypoxic babies.
In animal studies, pulse oximetry correlated well with simultaneously measured arterial oxygen saturation (r = 0.98, P = .01),9 but data from human studies are inconsistent. While McNamara et al10 reported good correlation between FSpO2 measurements and umbilical artery blood oxygen saturation at birth (r = 0.59, P <.001), Langer et al11 found no relationship between FSpO2 levels determined during pushing efforts and oxygen saturation in umbilical vein blood at birth.
Possible reasons for the ambiguous findings:
- differences in practice, such as use of umbilical venous versus arterial blood, or measurement during pushing versus between pushes,
- different intervals from FSpO2 reading to umbilical blood sampling, or
- incomparable groups, such as all women in labor versus those with abnormal FHR.
Limitations. Fetal pulse oximetry measures arterial oxygen saturation during the systolic pulse wave in the skin microcirculation at head level. In the fetus, this is part of the preductal circulation, with oxygen saturation levels somewhere between umbilical arterial and umbilical venous blood oxygen saturation.
Theoretically, FSpO2 should be closer to FSB than to umbilical blood. Although FSB samples consist of capillary blood, which is not exactly central arterial blood, the differences are small, at least in the neonate.12 In the intrapartum period, however, several variables with unknown effect may weaken relationships:
- different intervals between the last oximetry signal and blood sampling after delivery
- differences in local tissue perfusion status13
- perfusion changes during fetal compromise, as the fetus centralizes its blood flow, with vasoconstriction in the skin circulation
Question 2What is the critical threshold for fetal oxygen desaturation?
Human studies indicate that an FSpO2 of 33% is approximately the 10th percentile on the normal distribution, and an FSpO2 of 29% to 30% represents the third to fifth percentiles in normal-outcome labor.14 Studies in catheterized fetal sheep suggest that the level below which metabolic acidosis can be anticipated is an FSpO2 of about 30%.15
The 30% threshold also is supported by prospective human data from a multicenter trial.16 According to those data, an FSpO2 of less than 30% has 100% sensitivity in predicting an FSB pH below 7.20. FSpO2 of less than 30% also correlated with a lack of variability on the FHR tracing.17
The cutoff of 30% should not be interpreted as an indication of fetal distress, however. Rather, it represents a threshold below which increasing fetal acidosis will be encountered ( FIGURE 1). Oxygen saturation is a dynamic biologic parameter with broad variation.
FIGURE 1 Tracking fetal arterial oxyhemoglobin saturation
Question 3Is a single reading reliable?
The normal fetus has a remarkable capacity to compensate for transient episodes of desaturation. Thus, a single reading cannot reflect the fetal condition; the trend in FSpO2 must be taken into account. Research indicates only FSpO2 levels below 30% for more than 2 minutes18 or more than 10 minutes19 are likely to be associated with intrapartum acidosis.
ACOG has raised concerns about the potential increase in costs without demonstrable improvement in outcome.
Gorenberg et al20 retrospectively correlated FSpO2 with umbilical artery pH and found that neither the 30% threshold alone nor the duration of FSpO2 below 30% correlated with fetal acidemia (pH below 7.20). Rather, the repetition of such episodes was more predictive. The authors concluded that more than 10 episodes of FSpO2below 30% would overcome the ability of the fetus to compensate.
The study was underpowered to detect a significant difference in acidemia, and did not allow sufficient observation time to detect the natural progression of hypoxia to metabolic acidosis, a better indicator of fetal compromise. Additional research is needed.
Question 4Does oximetry correlate with acid-base status?
Many of the studies mentioned here assumed a correlation. Whenever oxygen saturation in the umbilical artery is 30% or more, acidosis (pH below 7.13) in the same blood is rare—only 1%.21 However, the correlation between fetal pulse oximetry values and acid-base status is much weaker.8.
Leszczynska-Gorzelak et al22 found no relationship between FSpO2 levels in the first or second stage of labor and pH or partial pressure of oxygen in umbilical vein blood at delivery. Other investigators concluded similarly, considering intrapartum FSpO2 of limited use for predicting acidosis at birth, irrespective of FSpO2 cutoff.23,24
Rijnders et al24 found no significant correlation between fetal scalp or umbilical artery blood pH and mean FSpO2 for the last 30 minutes before sampling (r = 0.02, P = .9). Even the lowest FSpO2 level did not correlate with arterial pH (r = .04, P = .84). None of the study’s 3 cases of umbilical pH below 7.05 would have been detected using the mean FSpO2 before delivery, and only 1 would have been detected using the lowest FSpO2.
In another multicenter study involving the Nellcor system in 164 cases with abnormal FHR, a correlation between oximetry and FSB sampling (r = 0.29, P < .01) was noted in the first stage of labor, but second-stage FSpO2 readings did not correlate with oxygen saturation, partial pressure of oxygen, pH, or bicarbonate level in the umbilical artery at birth.25
An observational series26 of 128 fetuses with nonreassuring FHR patterns concluded that fetal distress was insufficiently identified by oximetry. Only 2 of the 11 cases with umbilical artery pH below 7.20 were detected by pulse oximetry recordings below 30% during the last 30 minutes of the second stage, and out of 5 cases with hypoxic readings in the second stage, only 2 were acidotic at birth. The calculated sensitivity was 18%, specificity 92%, positive predictive value (PPV) 40%, and negative predictive value (NPV) 80%. A low Apgar score was never predicted by fetal pulse oximetry.
Others used the same Nellcor system over the final 30 minutes of labor and a cutoff for umbilical blood acidemia of pH below 7.13 and reported similar numbers: sensitivity 28%, specificity 94%, PPV 40%, and NPV 80%.23
Vitoratos et al27 analyzed FSpO2 readings in active labor (not limited to the last 30 minutes before delivery) and obtained somewhat better values: sensitivity 72%, specificity 93%, PPV 61.5%, and NPV 95.8% for an umbilical artery blood pH below 7.15.
The impression that the validity of fetal pulse oximetry is higher in earlier labor than in the second stage is supported by data from Stiller et al. 28 Leszczynska-Gorzelak et al29 found a significant decrease in mean FSpO2 from the first stage to the second stage of normal labor (51.9% versus 43.8%, P < .001), and Dildy et al14 noted a similar difference upon analyzing 160 normal labors (59% versus 53%), but other studies failed to verify such differences.25,30
Observational studies had unrealistic pH cutoff. All the evidence presented thus far on the validity of fetal pulse oximetry in predicting acidemia is based on observational data. A common deficiency is the unrealistic cutoff for pathologic fetal acidemia—a pH of less than 7.13 to 7.20—when it is widely accepted that “pathologic fetal acidemia” reflects an umbilical artery blood pH below 7.31 Even in this group, two thirds of neonates are unaffected by morbidity.
Need to identify metabolic acidosis. It also is accepted that the presence of a metabolic component to fetal acidemia may be as important—if not more important—than a single pH cutoff.31 Only a few human studies of pulse oximetry have distinguished between respiratory and metabolic acidemia. When they did, intrapartum fetal pulse oximetry was unable to predict umbilical artery base excess.23,25
The only randomized study failed to determine whether clinical decisions can be based solely on fetal pulse oximetry. 3 The investigators did suggest that sensitivity and specificity for metabolic acidemia was improved in the intervention group—a promising appraisal, in contrast with previous observational data.
In the study, 7 neonates (3 in the intervention group and 4 controls) had umbilical artery blood pH below 7. All 4 controls had vaginal delivery. There also were 6 cases of elevated base excess (ie, -16 mEq/L or below) among controls. None were recorded in the intervention group, and the 3 cases of acidemia were recognized antepartum and led to cesareans.
Unfortunately, the study design did not guarantee that patient management was based exclusively on EFM with or without fetal pulse oximetry. Vibro-acoustic stimulation or FSB sampling was required before proceeding to cesarean delivery in both groups.
It appears that the negative predictive value of fetal oximetry is of greater practical value than other attributes.
When FSpO2 was less than 30% for the entire interval between 2 contractions, or was unobtainable, the physician was supposed to revert to interpretation of EFM. When that was persistently nonreassuring, the physician was given the option of scalp stimulation or FSB sampling. Thus, it was not determined whether clinical decisions can be based exclusively on fetal pulse oximetry. Schmidt et al26 suggested that such exclusive application of fetal pulse oximetry might actually jeopardize fetal health.
Question 5 Does the combination of oximetry and EFM improve accuracy?
Fetal pulse oximetry was not used independently in any of the studies discussed here, but in association with EFM, which has a sensitivity for fetal acidosis of 93%, specificity of 29%, PPV of 2.6%, and NPV of 99.5%.32
From a statistical point of view, whenever 2 evaluation methods with the same endpoint (fetal acidosis) are combined, sensitivity decreases while specificity increases, theoretically resulting in less unnecessary intervention. That is exactly what investigators have reported: sensitivity as low as 18%26 for fetal oximetry, and specificity as high as 94%.23 However, the value of this new technology might not be so much the prediction of acidosis but identification of the well-oxygenated fetus so that labor may be safely continued in the presence of a concerning—but not ominous—FHR tracing.
Fetal pulse oximetry employs principles of optical spectrophotometry and plethysmography to provide information on the percentage of oxygen bound to hemoglobin. Oxyhemoglobin (oxygenated hemoglobin) and deoxyhemoglobin (hemoglobin without oxygen) absorb red and infrared light differently: more red absorption by deoxyhemoglobin, and more infrared absorption by oxyhemoglobin.
By measuring the relative absorption at each wavelength, the fraction of hemoglobin that carries oxygen can be determined. The arterial oxygen saturation is expressed as a percentage. The technology has been refined to measure fetal arterial oxyhemoglobin saturation during labor.
Pulse oximetry sensors must be calibrated for fetal biological values. In the fetus, normal oxygen saturation is much lower than in the adult or neonate; hemoglobin has a higher affinity for oxygen and is in higher concentration; and there are more capillaries per unit of tissue, higher cardiac output, and a higher heart rate.
In the adult or neonate, pulse oximetry sensors can be attached to fingers, toes, ears, or the bridge of the nose, but such stable placement is not feasible in utero. Further, good contact between sensor and fetal skin is a prerequisite for avoidance of artifacts. This last aspect has presented a sizeable challenge.
Fetal sensors measure reflected light. There is disagreement about the merits of the 2 sensor types, reflectance and transmission. Both include 2 light emitters (for red and infrared light) and a detector. In the transmission sensor (the adult or neonatal type), the light produced by the light-emitting diodes (LED) is picked up by the detector after traversing the interposed tissues. Since tissue interposition is not possible in the fetus, most fetal studies have used reflectance sensors, in which the LED and detector are placed side by side, and the light to be analyzed is reflected by the tissues. This design adds variance depending on the light’s depth of tissue penetration and device position changes.
Placement of the sensor. The Nellcor N-400 includes a reflectance sensor housed in a smooth, pliable head that is advanced through the cervix with the aid of a handle. The handle has a removable stylet to stiffen it during placement.
The sensor is placed against the fetal temple, cheek, or forehead and is held in place by the uterine wall. Placement is similar to that of an internal pressure catheter. Once the stylet is removed, it should not be reinserted.
Because the sensor usually descends and rotates with the fetal head, displacements are frequent and adjustments in sensor placement may be necessary. Placement adjustments can be attempted without the stylet and, if unsuccessful, a new device can be inserted. The Nellcor sensor is not reusable.
The prerequisites for insertion are dilatation of at least 2 cm, ruptured membranes, cephalic presentation, single fetus, gestational age of at least 36 weeks, and no placenta previa.
The manufacturer reports that active genital herpes, HIV, and hepatitis B or E seropositivity preclude fetal pulse oximetry monitoring.
Placement may be impossible when the presenting part is at high station (-3 or above) or low station (+2 or below).
The Nellcor N-400 system has been commercially available in many European countries since 1995, and in Canada since 1998. It was approved for sale in the United States in early 2003.
Question 6Will it improve neonatal outcomes?
Neonatal outcome is the ultimate endpoint in obstetrical care. In the randomized trial by Garite et al,3 there was no difference in neonatal outcome between the groups using or not using fetal pulse oximetry. According to Chua et al,33 FSpO2 levels measured even 10 minutes before delivery have no relation to neonatal outcome.
Leszczynska-Gorzelak et al22 believe FSpO2 is more predictive of neonatal outcome in the first stage than the second. However, Apgar score had no relationship with FSpO2readings in the first or second stage. Butterwegge34 reported 6 cases of FSpO2 below 30% for more than 30 minutes, all with good neonatal outcome, and Alshimmiri et al23 noted that only normal FSpO2 correlates with fetal well-being. Thus, it appears that the NPV of fetal oximetry is of greater practical value than other attributes.
Question 7How precise is it?
The Nellcor system monitors the quality of FSpO2 measurement; no value is displayed if the signal lacks the characteristics of a fetal arterial plethysmographic curve or if contact between sensor and skin is insufficient. Because of fetal movements and other artifacts, posting time is always less than 100%.
In the French multicenter study,25 the mean reliable signal time in the first stage of labor was only 64.7%—even less in the second stage (54%). Signal retention was 67% in the randomized trial by Garite et al. 3
Many artifacts may impede signal acquisition and impact the reliability of a reading:
- The sensor’s position on the fetal head. For example, the difference in FSpO2 readings between the forehead and occiput may be as much as 13.4%. (The sensor is designed to go against the fetal cheek, but may move around.)
- Incomplete sensor-to-skin contact, such as with high fetal head station, -2 or above.
- Marked caput formation.
- Increased intrauterine pressure accompanying contractions, especially at presentation stations of +2 or below (FIGURE 2). FSpO2 monitoring requires detection of fetal pulses, which may be undetectable when the surrounding pressure is high, resulting in a loss of signal.
- Interposition of vernix or fetal hair.
- Presence of meconium, which behaves like a red-light filter, altering the ratio of red to infrared light and resulting in artificially low values.35 This theoretical concern is rejected by Yam et al,36 who did not observe any effect of meconium on FSpO2 values. (When the amniotic fluid is meconium-stained, Carbonne et al37 showed that fetal oximetry is a better predictor of meconium aspiration syndrome than FSB sampling.) The data on the influence of meconium on FSpO2 readings remain contradictory.
All these conditions may impair precision and contribute to poor sensitivity.
FIGURE 2 A weakening signal during pushing
Question 8Is it easy to use?
An Australian survey38 assessed clinicians’ perceptions during placement of the oximetry sensor. Ease of placement was rated as good or excellent in 71% of cases, and the patient’s comfort was rated as good or excellent in 90% of cases. Chua et al39 reported a mean insertion time of 90 seconds, with a reliable signal obtained within 5 minutes in 87% of placements. The French multicenter study25 mentioned earlier concluded that the procedure is satisfactory and easier than FSB sampling. The device itself was harmless to both mother and fetus.40
Potential research directions
Fetal pulse oximetry may be an effective tool in clinical scenarios such as:
- fetal arrhythmias with uninterpretable FHR tracing
- fetal tachycardia associated with maternal fever, thyrotoxicosis, or fetal supraventricular tachycardia, when distinguishing other contributions to tachycardia may be difficult
- fetal bradycardia caused by a complete heart block, which may render EFM undecipherable
- when amnioinfusion is attempted for variable decelerations and it is necessary to differentiate a nonreassuring FHR tracing related to transient in utero stress (eg, umbilical cord compressions) from ominous tracings
Another area not yet addressed is cost-effectiveness beyond the immediate direct costs (approximately $11,000 for the monitor and $150 for each disposable sensor). Also uncertain is whether laboring women will accept the device (how disturbing or invasive it is perceived to be) and how acceptable or applicable it is outside tertiary institutions.
Dr. Vidaeff reports no financial relationships relevant to this article. Dr. Ramin reports grant support from the US National Institutes of Health.
1. Dildy GA. A guest editorial: fetal pulse oximetry. Obstet Gynecol Surv. 2003;58:225-226.
2. Thacker SB, Stroup DF, Peterson HB. Efficacy and safety of intrapartum electronic fetal monitoring: an update. Obstet Gynecol. 1995;86:613-620.
3. Garite TJ, Dildy GA, McNamara H, et al. A multicenter controlled trial of fetal pulse oximetry in the intrapartum management of nonreassuring fetal heart rate patterns. Am J Obstet Gynecol. 2000;183:1049-1058.
4. Haverkamp AD, Orleans M, Langendoerfer S, et al. A controlled trial of the differential effects of continuous fetal heart rate monitoring in labor: a randomized trial. Am J Obstet Gynecol. 1979;134:399-412.
5. American College of Obstetricians and Gynecologists. Committee opinion: fetal pulse oximetry. Number 258, September 2001. Obstet Gynecol. 2001;98:523-524.
6. Zijlstra WG, Buursma A, Zwart A. Performance of an automated six-wavelength photometer (radiometer OSM3) for routine measurement of hemoglobin derivatives. Clin Chem. 1988;34:149-152.
7. Porath M, Sinah P, Dudenhausen JW, Luttkus AK. Systematic instrumental errors between oxygen saturation analysers in fetal blood during deep hypoxemia. Clin Chim Acta. 2001;307:151-157.
8. Luttkus AK, Lübke M, Büscher U, Porath M, Dudenhausen JW. Accuracy of fetal pulse oximetry. Acta Obstet Gynecol Scand. 2002;81:417-423.
9. Carter AM, Stiller R, König V, Jorgensen JS, Svendsen P, Huch R. Calibration of a reflectance pulse oximeter in fetal lambs for arterial oxygen saturations below 70%. J Soc Gynecol Invest. 1998;5:255-259.
10. McNamara H, Cung CD, Lilford R, Johnson N. Do fetal pulse oximetry readings at delivery correlate with cord blood oxygenation and acidemia? Br J Obstet Gynaecol. 1992;99:735-738.
11. Langer B, Boudier E, Haddad J, Pain L, Schlaeder G. Fetal pulse oximetry during labor of 62 patients. Fetal Diagn Ther. 1996;11:37-45.
12. Harrison AM, Lynch JM, Dean JM, Witte MK. Comparison of simultaneously obtained arterial and capillary blood gases in pediatric intensive care unit patients. Crit Care Med. 1997;25:1904-1908.
13. Nijland R, Jongsma HW, Nijhuis JG, Oeseburg B. Accuracy of fetal pulse oximetry and pitfalls in measurements. Eur J Obstet Gynecol Reprod Biol. 1997;72:S21-S27.
14. Dildy GA, van den Berg PP, Katz M, et al. Intrapartum fetal pulse oximetry: fetal oxygen saturation trends during labor and relation to delivery outcome. Am J Obstet Gynecol. 1994;171:679-684.
15. Nijland R, Jongsma H, Nijhuis J, van den Berg P, Oeseburg B. Arterial saturation in relation to metabolic acidosis in fetal lambs. Am J Obstet Gynecol. 1995;172:810-819.
16. Kühnert M, Seelbach-Göbel B, Butterwegge M. Predictive agreement between fetal arterial oxygen saturation and fetal scalp pH: results of the German multicenter study. Am J Obstet Gynecol. 1998;178:330-335.
17. Salamalekis E, Thomopoulos P, Giannaris D, et al. Computerised intrapartum diagnosis of fetal hypoxia based on heart rate monitoring and fetal pulse oximetry recordings utilizing wavelet analysis and neural networks. Br J Obstet Gynaecol. 2002;109:1137-1142.
18. Bloom SL, Swindle RG, McIntire DD, Leveno KJ. Fetal pulse oximetry: duration of desaturation and intrapartum outcome. Obstet Gynecol. 1999;93:1036-1040.
19. Seelbach-Göbel B, Heupel M, Kühnert M, et al. The prediction of fetal acidosis by means of intrapartum fetal pulse oximetry. Am J Obstet Gynecol. 1999;180:73-81.
20. Gorenberg DM, Patillo C, Hendi P, Rumney PJ, Garite TJ. Fetal pulse oximetry: correlation between oxygen desaturation, duration, and frequency and neonatal outcomes. Am J Obstet Gynecol. 2003;189:136-138.
21. Dildy GA, Thorp JA, Yeast JD, Clark SL. The relationship between oxygen saturation and pH in umbilical blood: implications for intrapartum fetal oxygen saturation monitoring. Am J Obstet Gynecol. 1996;175:682-687.
22. Leszczynska-Gorzelak B, Poniedzialek-Czajkowska E, Oleszczuk J. Fetal blood saturation during the 1st and 2nd stage of labor and its relation to the neonatal outcome. Gynecol Obstet Invest. 2002;54:159-163.
23. Alshimmiri M, Bocking AD, Gagnon R, Natale R, Richardson BS. Prediction of umbilical artery base excess by intrapartum fetal oxygen saturation monitoring. Am J Obstet Gynecol. 1997;177:775-779.
24. Rijnders RJ, Mol BW, Reuwer PJ, Drogtrop AP, Vernooij MM, Visser GH. Is the correlation between fetal oxygen saturation and blood pH sufficient for the use of fetal pulse oximetry? J Matern Fetal Neonatal Med. 2002;11:80-83.
25. Goffinet F, Langer B, Carbonne B, et al. Multicenter study on the clinical value of fetal pulse oximetry. I. Methodologic evaluation. The French Study Group on Fetal Pulse Oximetry. Am J Obstet Gynecol. 1997;177:1238-1246.
26. Schmidt S, Koslowski S, Sierra F, et al. Clinical usefulness of pulse oximetry in the fetus with non-reassuring heart rate pattern? J Perinat Med. 2000;28:298-305.
27. Vitoratos N, Salamalekis E, Saloum J, Makrakis E, Creatsas G. Abnormal fetal heart rate patterns during the active phase of labor and the value of fetal oxygen saturation. J Matern Fetal Neonatal Med. 2002;11:46-49.
28. Stiller R, von Mering R, König V, et al. How well does reflectance pulse oximetry reflect intrapartum fetal acidosis? Am J Obstet Gynecol. 2002;186:1351-1357.
29. Leszczynska-Gorzelak B, Poniedzialek-Czajkowska E, Zych I, Grzechnik M, Oleszczuk J. The usefulness of the intrapartum fetal pulse oximetry in anticipating the neonatal outcome. Ginekol Pol. 2001;72:1183-1188.
30. Nikolov A, Dimitrov A, Vakrilkova L, Iarkova N. Fetal oxygen saturation during normal delivery. Akush Ginekol. 2000;40:3-6.
31. Goldaber KG, Gilstrap LC III, Leveno K, Dax JS, McIntire DD. Pathologic fetal acidemia. Obstet Gynecol. 1991;78:1103-1107.
32. Low JA, Victory R, Derrick EJ. Predictive value of electronic fetal monitoring for intra-partum fetal asphyxia and metabolic acidosis. Obstet Gynecol. 1999;93:285-291.
33. Chua S, Yeong SM, Razvi K, Arulkumaran S. Fetal oxygen saturation during labour. Br J Obstet Gynaecol. 1997;104:1080-1083.
34. Butterwegge M. Fetal pulse oximetry and non-reassuring heart rate. Eur J Obstet Gynecol Reprod Biol. 1997;72:S63-S66.
35. Johnson N, Johnson VA, Bannister J, McNamara H. The effect of meconium on neonatal and fetal reflectance pulse oximetry. J Perinat Med. 1990;18:351-355.
36. Yam J, Chua S, Arulkumaran S. Intrapartum pulse oximetry: Part 1: Principles and technical issues. Obstet Gynecol Surv. 2000;55:163-172.
37. Carbonne B, Cudeville C, Sivan H, Cabrol D, Papiernik E. Fetal oxygen saturation measured by pulse oximetry during labor with clear and meconium-stained amniotic fluid. Eur J Obstet Gynecol Reprod Biol. 1997;72:S51-S55.
38. East CE, Colditz PB. Clinicians’ perceptions of placing a fetal oximetry sensor. J Qual Clin Pract. 2000;20:161-163.
39. Chua S, Yam J, Razvi K, Yeong SM, Arulkumaran S. Intrapartum fetal oxygen saturation monitoring in a busy labour ward. Eur J Obstet Gynecol Reprod Biol. 1999;82:185-189.
40. Luttkus AK, Friedmann W, Thomas S, Dimer JA, Dudenhausen JW. The safety of fetal pulse oximetry in parturients requiring fetal scalp blood sample. Obstet Gynecol. 1997;90:533-537.
1. Dildy GA. A guest editorial: fetal pulse oximetry. Obstet Gynecol Surv. 2003;58:225-226.
2. Thacker SB, Stroup DF, Peterson HB. Efficacy and safety of intrapartum electronic fetal monitoring: an update. Obstet Gynecol. 1995;86:613-620.
3. Garite TJ, Dildy GA, McNamara H, et al. A multicenter controlled trial of fetal pulse oximetry in the intrapartum management of nonreassuring fetal heart rate patterns. Am J Obstet Gynecol. 2000;183:1049-1058.
4. Haverkamp AD, Orleans M, Langendoerfer S, et al. A controlled trial of the differential effects of continuous fetal heart rate monitoring in labor: a randomized trial. Am J Obstet Gynecol. 1979;134:399-412.
5. American College of Obstetricians and Gynecologists. Committee opinion: fetal pulse oximetry. Number 258, September 2001. Obstet Gynecol. 2001;98:523-524.
6. Zijlstra WG, Buursma A, Zwart A. Performance of an automated six-wavelength photometer (radiometer OSM3) for routine measurement of hemoglobin derivatives. Clin Chem. 1988;34:149-152.
7. Porath M, Sinah P, Dudenhausen JW, Luttkus AK. Systematic instrumental errors between oxygen saturation analysers in fetal blood during deep hypoxemia. Clin Chim Acta. 2001;307:151-157.
8. Luttkus AK, Lübke M, Büscher U, Porath M, Dudenhausen JW. Accuracy of fetal pulse oximetry. Acta Obstet Gynecol Scand. 2002;81:417-423.
9. Carter AM, Stiller R, König V, Jorgensen JS, Svendsen P, Huch R. Calibration of a reflectance pulse oximeter in fetal lambs for arterial oxygen saturations below 70%. J Soc Gynecol Invest. 1998;5:255-259.
10. McNamara H, Cung CD, Lilford R, Johnson N. Do fetal pulse oximetry readings at delivery correlate with cord blood oxygenation and acidemia? Br J Obstet Gynaecol. 1992;99:735-738.
11. Langer B, Boudier E, Haddad J, Pain L, Schlaeder G. Fetal pulse oximetry during labor of 62 patients. Fetal Diagn Ther. 1996;11:37-45.
12. Harrison AM, Lynch JM, Dean JM, Witte MK. Comparison of simultaneously obtained arterial and capillary blood gases in pediatric intensive care unit patients. Crit Care Med. 1997;25:1904-1908.
13. Nijland R, Jongsma HW, Nijhuis JG, Oeseburg B. Accuracy of fetal pulse oximetry and pitfalls in measurements. Eur J Obstet Gynecol Reprod Biol. 1997;72:S21-S27.
14. Dildy GA, van den Berg PP, Katz M, et al. Intrapartum fetal pulse oximetry: fetal oxygen saturation trends during labor and relation to delivery outcome. Am J Obstet Gynecol. 1994;171:679-684.
15. Nijland R, Jongsma H, Nijhuis J, van den Berg P, Oeseburg B. Arterial saturation in relation to metabolic acidosis in fetal lambs. Am J Obstet Gynecol. 1995;172:810-819.
16. Kühnert M, Seelbach-Göbel B, Butterwegge M. Predictive agreement between fetal arterial oxygen saturation and fetal scalp pH: results of the German multicenter study. Am J Obstet Gynecol. 1998;178:330-335.
17. Salamalekis E, Thomopoulos P, Giannaris D, et al. Computerised intrapartum diagnosis of fetal hypoxia based on heart rate monitoring and fetal pulse oximetry recordings utilizing wavelet analysis and neural networks. Br J Obstet Gynaecol. 2002;109:1137-1142.
18. Bloom SL, Swindle RG, McIntire DD, Leveno KJ. Fetal pulse oximetry: duration of desaturation and intrapartum outcome. Obstet Gynecol. 1999;93:1036-1040.
19. Seelbach-Göbel B, Heupel M, Kühnert M, et al. The prediction of fetal acidosis by means of intrapartum fetal pulse oximetry. Am J Obstet Gynecol. 1999;180:73-81.
20. Gorenberg DM, Patillo C, Hendi P, Rumney PJ, Garite TJ. Fetal pulse oximetry: correlation between oxygen desaturation, duration, and frequency and neonatal outcomes. Am J Obstet Gynecol. 2003;189:136-138.
21. Dildy GA, Thorp JA, Yeast JD, Clark SL. The relationship between oxygen saturation and pH in umbilical blood: implications for intrapartum fetal oxygen saturation monitoring. Am J Obstet Gynecol. 1996;175:682-687.
22. Leszczynska-Gorzelak B, Poniedzialek-Czajkowska E, Oleszczuk J. Fetal blood saturation during the 1st and 2nd stage of labor and its relation to the neonatal outcome. Gynecol Obstet Invest. 2002;54:159-163.
23. Alshimmiri M, Bocking AD, Gagnon R, Natale R, Richardson BS. Prediction of umbilical artery base excess by intrapartum fetal oxygen saturation monitoring. Am J Obstet Gynecol. 1997;177:775-779.
24. Rijnders RJ, Mol BW, Reuwer PJ, Drogtrop AP, Vernooij MM, Visser GH. Is the correlation between fetal oxygen saturation and blood pH sufficient for the use of fetal pulse oximetry? J Matern Fetal Neonatal Med. 2002;11:80-83.
25. Goffinet F, Langer B, Carbonne B, et al. Multicenter study on the clinical value of fetal pulse oximetry. I. Methodologic evaluation. The French Study Group on Fetal Pulse Oximetry. Am J Obstet Gynecol. 1997;177:1238-1246.
26. Schmidt S, Koslowski S, Sierra F, et al. Clinical usefulness of pulse oximetry in the fetus with non-reassuring heart rate pattern? J Perinat Med. 2000;28:298-305.
27. Vitoratos N, Salamalekis E, Saloum J, Makrakis E, Creatsas G. Abnormal fetal heart rate patterns during the active phase of labor and the value of fetal oxygen saturation. J Matern Fetal Neonatal Med. 2002;11:46-49.
28. Stiller R, von Mering R, König V, et al. How well does reflectance pulse oximetry reflect intrapartum fetal acidosis? Am J Obstet Gynecol. 2002;186:1351-1357.
29. Leszczynska-Gorzelak B, Poniedzialek-Czajkowska E, Zych I, Grzechnik M, Oleszczuk J. The usefulness of the intrapartum fetal pulse oximetry in anticipating the neonatal outcome. Ginekol Pol. 2001;72:1183-1188.
30. Nikolov A, Dimitrov A, Vakrilkova L, Iarkova N. Fetal oxygen saturation during normal delivery. Akush Ginekol. 2000;40:3-6.
31. Goldaber KG, Gilstrap LC III, Leveno K, Dax JS, McIntire DD. Pathologic fetal acidemia. Obstet Gynecol. 1991;78:1103-1107.
32. Low JA, Victory R, Derrick EJ. Predictive value of electronic fetal monitoring for intra-partum fetal asphyxia and metabolic acidosis. Obstet Gynecol. 1999;93:285-291.
33. Chua S, Yeong SM, Razvi K, Arulkumaran S. Fetal oxygen saturation during labour. Br J Obstet Gynaecol. 1997;104:1080-1083.
34. Butterwegge M. Fetal pulse oximetry and non-reassuring heart rate. Eur J Obstet Gynecol Reprod Biol. 1997;72:S63-S66.
35. Johnson N, Johnson VA, Bannister J, McNamara H. The effect of meconium on neonatal and fetal reflectance pulse oximetry. J Perinat Med. 1990;18:351-355.
36. Yam J, Chua S, Arulkumaran S. Intrapartum pulse oximetry: Part 1: Principles and technical issues. Obstet Gynecol Surv. 2000;55:163-172.
37. Carbonne B, Cudeville C, Sivan H, Cabrol D, Papiernik E. Fetal oxygen saturation measured by pulse oximetry during labor with clear and meconium-stained amniotic fluid. Eur J Obstet Gynecol Reprod Biol. 1997;72:S51-S55.
38. East CE, Colditz PB. Clinicians’ perceptions of placing a fetal oximetry sensor. J Qual Clin Pract. 2000;20:161-163.
39. Chua S, Yam J, Razvi K, Yeong SM, Arulkumaran S. Intrapartum fetal oxygen saturation monitoring in a busy labour ward. Eur J Obstet Gynecol Reprod Biol. 1999;82:185-189.
40. Luttkus AK, Friedmann W, Thomas S, Dimer JA, Dudenhausen JW. The safety of fetal pulse oximetry in parturients requiring fetal scalp blood sample. Obstet Gynecol. 1997;90:533-537.