User login
CASE Woman who has undergone recent cesarean delivery
A 23-year-old woman had a primary cesarean delivery 72 hours ago due to an arrest of dilation at 6 cm. She was in labor for 22 hours, and her membranes were ruptured for 18 hours. She had 10 internal vaginal examinations, and the duration of internal fetal monitoring was 12 hours; 24 hours after delivery, she developed a fever of 39°C, in association with lower abdominal pain and tenderness. She was presumptively treated for endometritis with cefepime; 48 hours after the initiation of antibiotics, she remains febrile and symptomatic.
- What are the most likely causes of her persistent fever?
- What should be the next steps in her evaluation?
Cesarean delivery background
Cesarean delivery is now the most common major operation performed in US hospitals. Cesarean delivery rates hover between 25% and 30% in most medical centers in the United States.1 The most common postoperative complication of cesarean delivery is infection. Infection typically takes 1 of 3 forms: endometritis (organ space infection), wound infection (surgical site infection), and urinary tract infection (UTI).1 This article will review the initial differential diagnosis, evaluation, and management of the patient with a postoperative fever and also will describe the appropriate assessment and treatment of the patient who has a persistent postoperative fever despite therapy. The article will also highlight key interventions that help to prevent postoperative infections.
Initial evaluation of the febrile patient
In the first 24 to 48 hours after cesarean delivery, the most common cause of fever is endometritis (organ space infection). This condition is a polymicrobial, mixed aerobic-anaerobic infection (FIGURE). The principal pathogens include anaerobic gram-positive cocci (
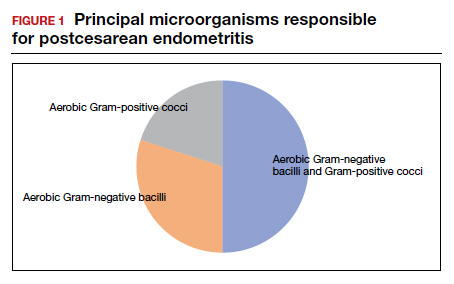
The major risk factors for postcesarean endometritis are extended duration of labor and ruptured membranes, multiple internal vaginal examinations, invasive fetal monitoring, and pre-existing colonization with group B Streptococcus and/or the organisms that cause bacterial vaginosis. Affected patients typically have a fever in the range of 38 to 39°C, tachycardia, mild tachypnea, lower abdominal pain and tenderness, and purulent lochia in some individuals.1
Differential for postoperative fever
The initial differential diagnosis of postoperative fever is relatively limited (TABLE 1). In addition to endometritis, it includes extensive atelectasis, perhaps resulting from general anesthesia; lower respiratory tract infection, either viral influenza or bacterial pneumonia; and acute pyelonephritis. A simple infection of the bladder (cystitis or asymptomatic bacteriuria) should not cause a substantial temperature elevation and systemic symptoms.1
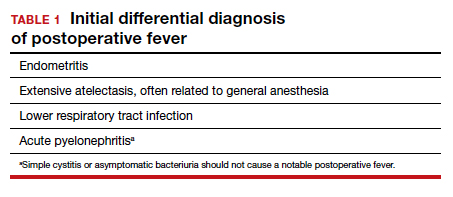
Differentiation between these entities usually is possible based on physical examination and a few laboratory tests. The peripheral white blood cell count usually is elevated, and a left shift may be evident. If a respiratory tract infection is suspected, chest radiography is indicated. A urine culture should be obtained if acute pyelonephritis strongly is considered. Lower genital tract cultures are rarely of value, and uncontaminated upper tract cultures are difficult to obtain. I do not believe that blood cultures should be performed as a matter of routine. They are expensive, and the results are often not available until after the patient has cleared her infection and left the hospital. However, I would obtain blood cultures in patients who meet one of these criteria1,2:
- They are immunocompromised (eg, HIV infection).
- They have a cardiac or vascular prosthesis and, thus, are at increased risk of complications related to bacteremia.
- They seem critically ill at the onset of evaluation.
- They fail to respond appropriately to initial therapy.
The cornerstone of therapy is broad spectrum antibiotics that target the multiple organisms responsible for endometritis.3 There are several single agents and several combination antibiotic regimens that provide excellent coverage against the usual pelvic pathogens (TABLE 2). I personally favor the generic combination regimen (clindamycin plus gentamicin) because it is relatively inexpensive and has been very well validated in multiple studies. In patients who have underlying renal dysfunction, aztreonam can be substituted for gentamicin.
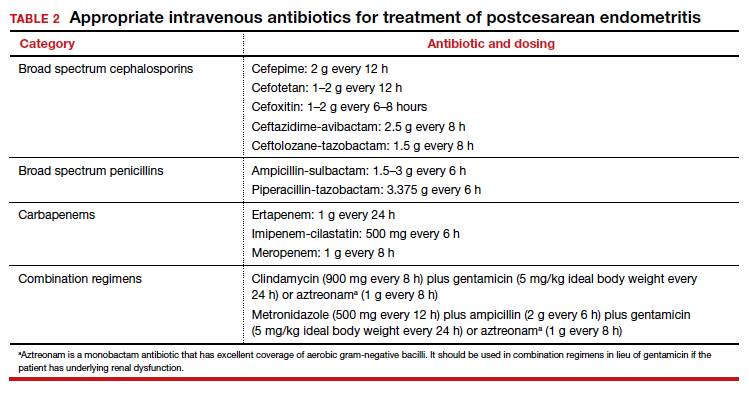
Approximately 90% of patients will show clear evidence of clinical improvement (ie, decrease in temperature and resolution of abdominopelvic pain) within 48 hours of starting antibiotics. Patients should then continue therapy until they have been afebrile and asymptomatic for approximately 24 hours. At that point, antibiotics should be discontinued, and the patient can be discharged. With rare exceptions, there is no indication for administration of oral antibiotics on an outpatient basis.1,4
Continue to: Persistent postoperative fever...
Persistent postoperative fever
Resistant microorganism
The most common cause of a persistent fever after initiating antibiotic therapy is a resistant microorganism. There are potential gaps in coverage for the antibiotic regimens commonly used to treat postcesarean endometritis (TABLE 3).1,4 Assuming there is no other obvious cause for treatment failure, I recommend that therapy be changed to the triple combination of metronidazole plus ampicillin plus gentamicin (or aztreonam). The first drug provides superb coverage against anaerobes; the second covers enterococci. Gentamicin or aztreonam cover virtually all aerobic Gram-negative bacilli likely to cause postcesarean infection. I prefer metronidazole rather than clindamycin in this regimen because, unlike clindamycin, it is less likely to trigger diarrhea when used in combination with ampicillin. The 3-drug regimen should be continued until the patient has been afebrile and asymptomatic for approximately 24 hours.1,3,4
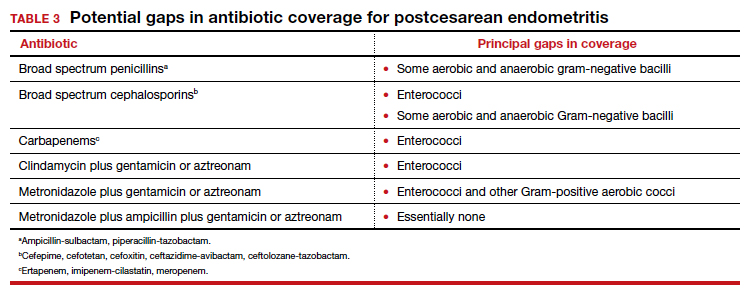
Wound infection
The second most common reason for a poor response to initial antibiotic therapy is a wound (surgical site) infection. Wound infections are caused by many of the same pelvic pathogens responsible for endometritis combined with skin flora, notably Streptococcus and Staphylococcus species, including methicillin-resistant Staphylococcus aureus (MRSA).1,4
Wound infections typically take one of two forms. The first is an actual incisional abscess. The patient is febrile; the margins of the wound are warm, indurated, erythematous, and tender; and purulent material drains from the incision. In this situation, the wound should be opened widely to drain the purulent collection. The fascia should then be probed to be certain that dehiscence has not occurred. In addition, intravenous vancomycin (1 g every 12 h) should be included in the antibiotic regimen to ensure adequate coverage of hospital-acquired MRSA.1,4
The second common presentation of a wound infection is cellulitis. The patient is febrile, and there is a spreading area of erythema, warmth, and exquisite tenderness extending from the edges of the incision; however, no purulent drainage is apparent. In this second scenario, the wound should not be opened, but intravenous vancomycin should be added to the treatment regimen.1,3,4
A third and very rare form of wound infection is necrotizing fasciitis. In affected patients, the margins of the wound are darkened and necrotic rather than erythematous and indurated. Two other key physical findings are crepitance and loss of sensation along the margins of the wound. Necrotizing fasciitis is truly a life-threatening emergency and requires immediate and extensive debridement of the devitalized tissue, combined with broad spectrum therapy with antibiotics that provide excellent coverage against anaerobes, aerobic streptococci (particularly group A streptococci), and staphylococci. The requirement for debridement may be so extensive that a skin graft subsequently is necessary to close the defect.1,4
Continue to: Unusual causes of persistent postoperative fever...
Unusual causes of persistent postoperative fever
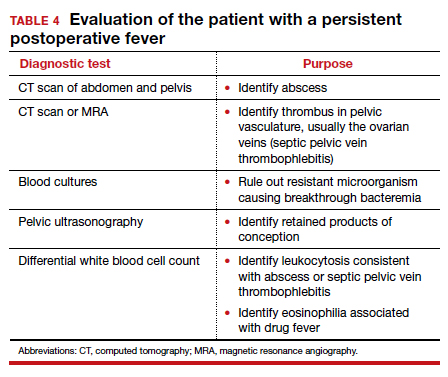
If a resistant microorganism and wound infection can be excluded, the clinician then must begin a diligent search for “zebras” (ie, uncommon but potentially serious causes of persistent fever).1,4 One possible cause is a pelvic abscess. These purulent collections typically form in the retrovesicle space as a result of infection of a hematoma that formed between the posterior bladder wall and the lower uterine segment, in the leaves of the broad ligament, or in the posterior cul-de-sac. The abscess may or may not be palpable. The patient’s peripheral white blood cell count usually is elevated, with a preponderance of neutrophils. The best imaging test for an abscess is a computed tomography (CT) scan. Abscesses require drainage, which usually can be accomplished by insertion of a percutaneous drain under ultrasonographic or CT guidance.
A second unusual cause of persistent fever is septic pelvic vein thrombophlebitis. The infected venous emboli usually are present in the ovarian veins, with the right side predominant. The patient’s peripheral white blood cell count usually is elevated, and the infected clots are best imaged by CT scan with contrast or magnetic resonance angiography. The appropriate treatment is continuation of broad-spectrum antibiotics and administration of therapeutic doses of parenteral anticoagulants such as enoxaparin or unfractionated heparin.
A third explanation for persistent fever is retained products of conception. This diagnosis is best made by ultrasonography. The placental fragments should be removed by sharp curettage.
A fourth consideration when evaluating the patient with persistent fever is an allergic drug reaction. In most instances, the increase in the patient’s temperature will correspond with administration of the offending antibiotic(s). Affected patients typically have an increased number of eosinophils in their peripheral white blood cell count. The appropriate management of drug fever is discontinuation of antibiotics.
A final and distinctly unusual consideration is recrudescence of a connective tissue disorder such as systemic lupus erythematosus. The best test to confirm this diagnosis is the serum complement assay, which will demonstrate a decreased serum concentration of complement, reflecting consumption of this serum protein during the inflammatory process. The correct management for this condition is administration of a short course of systemic glucocorticoids. TABLE 4 summarizes a simple, systematic plan for evaluation of the patient with a persistent postoperative fever.
Preventive measures
We all remember the simple but profound statement by Benjamin Franklin, “An ounce of prevention is worth a pound of cure.” That folksy adage rings true with respect to postoperative infection because this complication extends hospital stay, increases hospital expense, and causes considerable discomfort and inconvenience for the patient. Therefore, we would do well to prevent as many instances of postoperative infection as possible.
Endometritis
On the basis of well-designed, prospective, randomized trials (Level 1 evidence), 3 interventions have proven effective in reducing the frequency of postcesarean endometritis. The first is irrigation of the vaginal canal preoperatively with an iodophor solution.5,6 The second is preoperative administration of systemic antibiotics.7-9 The combination of cefazolin (2 g IV within 30 minutes of incision) plus azithromycin (500 mg IV over 1 hour prior to incision) is superior to cefazolin alone.10,11 The third important preventive measure is removing the placenta by traction on the umbilical cord rather than by manual extraction.12,13
Wound infection
Several interventions are of proven effectiveness in reducing the frequency of postcesarean wound (surgical site) infection. The first is removal of hair at the incision site by clipping rather than by shaving (Level 2 evidence).14 The second is cleansing of the skin with chlorhexidine rather than iodophor (Level 1 evidence).15 The third is closing of the deep subcutaneous layer of the incision if it exceeds 2 cm in depth (Level 1 evidence).16,17 The fourth is closure of the skin with subcutaneous sutures rather than staples (Level 1 evidence).18 The monofilament suture poliglecaprone 25 is superior to the multifilament suture polyglactin 910 for this purpose (Level 1 evidence).19 Finally, in obese patients (body mass index >30 kg/m2), application of a negative pressure wound vacuum dressing may offer additional protection against infection (Level 1 evidence).20 Such dressings are too expensive, however, to be used routinely in all patients.
Urinary tract infection
The most important measures for preventing postoperative UTIs are identifying and clearing asymptomatic bacteriuria prior to delivery, inserting the urinary catheter prior to surgery using strict sterile technique, and removing the catheter as soon as possible after surgery, ideally within 12 hours.1,4
CASE Resolved
The 2 most likely causes for this patient’s poor response to initial therapy are resistant microorganism and wound infection. If a wound infection can be excluded by physical examination, the patient’s antibiotic regimen should be changed to metronidazole plus ampicillin plus gentamicin (or aztreonam). If an incisional abscess is identified, the incision should be opened and drained, and vancomycin should be added to the treatment regimen. If a wound cellulitis is evident, the incision should not be opened, but vancomycin should be added to the treatment regimen to enhance coverage against aerobic Streptococcus and Staphylococcus species. ●
- Duff WP. Maternal and perinatal infection in pregnancy: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al, eds. Gabbe’s Obstetrics: Normal and Problem Pregnancies. 8th ed. Elsevier; 2020:1124-1146.
- Locksmith GJ, Duff P. Assessment of the value of routine blood cultures in the evaluation and treatment of patients with chorioamnionitis. Infect Dis Obstet Gynecol. 1994;2:111-114.
- Duff P. Antibiotic selection in obstetric patients. Infect Dis Clin N Am. 1997;11:1-12.
- Duff P. Maternal and fetal infections. In: Creasy RK, Resnik R, Iams, JD, et al, eds. Creasy & Resnik’s Maternal Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862-919.
- Haas DM, Morgan S, Contreras K. Vaginal preparation with antiseptic solution before cesarean section for preventing postoperative infections. Cochrane Database Syst Rev. 2014;12:CD007892.
- Caissutti C, Saccone G, Zullo F, et al. Vaginal cleansing before cesarean delivery. a systematic review and meta-analysis. Obstet Gynecol. 2017;130:527-538.
- Sullivan SA, Smith T, Change E, et al. Administration of cefazolin prior to skin incision is superior to cefazolin at cord clamping in preventing postcesarean infectious morbidity; a randomized controlled trial. Am J Obstet Gynecol. 2007;196:455.e1-455.e5.
- Tita ATN, Hauth JC, Grimes A, et al. Decreasing incidence of postcesarean endometritis with extended-spectrum antibiotic prophylaxis. Obstet Gynecol. 2008;111:51-56.
- Tita ATN, Owen J, Stamm AM, et al. Impact of extended-spectrum antibiotic prophylaxis on incidence of postcesarean surgical wound infection. Am J Obstet Gynecol. 2008;199: 303.e1-303.e3.
- Tita ATN, Szchowski JM, Boggess K, et al. Two antibiotics before cesarean delivery reduce infection rates further than one agent. N Engl J Med. 2016;375:1231-1241.
- Harper LM, Kilgore M, Szychowski JM, et al. Economic evaluation of adjunctive azithromycin prophylaxis for cesarean delivery. Obstet Gynecol. 2017;130:328-334.
- Lasley DS, Eblen A, Yancey MK, et al. The effect of placental removal method on the incidence of postcesarean infections. Am J Obstet Gynecol. 1997;176:1250-1254.
- Anorlu RI, Maholwana B, Hofmeyr GJ. Methods of delivering the placenta at cesarean section. Cochrane Database Syst Rev. 2008;3:CD004737.
- Cruse PJ, Foord R. A five-year prospective study of 23,649 surgical wounds. Arch Surg. 1973;107:206-209.
- Tuuli MG, Liu J, Stout MJ, et al. A randomized trial comparing skin antiseptic agents at cesarean delivery. N Engl J Med. 2016;374:657-665.
- Del Valle GO, Combs P, Qualls C, et al. Does closure of camper fascia reduce the incidence of post-cesarean superficial wound disruption? Obstet Gynecol. 1992;80:1013-1016.
- Chelmow D. Rodriguez EJ, Sabatini MM. Suture closure of subcutaneous fat and wound disruption after cesarean delivery: a meta-analysis. Obstet Gynecol. 2004;103:974-980.
- Tuuli MG, Rampersod RM, Carbone JF, et al. Staples compared with subcuticular suture for skin closure after cesarean delivery. a systematic review and meta-analysis. Obstet Gynecol. 2011;117:682-690.
- Buresch AM, Arsdale AV, Ferzli M, et al. Comparison of subcuticular suture type for skin closure after cesarean delivery. a randomized controlled trial. Obstet Gynecol. 2017;130:521-526.
- Yu L, Kronen RJ, Simon LE, et al. Prophylactic negative-pressure wound therapy after cesarean is associated with reduced risk of surgical site infection: a systematic review and meta-analysis. Am J Obstet Gynecol. 2018;218:200-210.
CASE Woman who has undergone recent cesarean delivery
A 23-year-old woman had a primary cesarean delivery 72 hours ago due to an arrest of dilation at 6 cm. She was in labor for 22 hours, and her membranes were ruptured for 18 hours. She had 10 internal vaginal examinations, and the duration of internal fetal monitoring was 12 hours; 24 hours after delivery, she developed a fever of 39°C, in association with lower abdominal pain and tenderness. She was presumptively treated for endometritis with cefepime; 48 hours after the initiation of antibiotics, she remains febrile and symptomatic.
- What are the most likely causes of her persistent fever?
- What should be the next steps in her evaluation?
Cesarean delivery background
Cesarean delivery is now the most common major operation performed in US hospitals. Cesarean delivery rates hover between 25% and 30% in most medical centers in the United States.1 The most common postoperative complication of cesarean delivery is infection. Infection typically takes 1 of 3 forms: endometritis (organ space infection), wound infection (surgical site infection), and urinary tract infection (UTI).1 This article will review the initial differential diagnosis, evaluation, and management of the patient with a postoperative fever and also will describe the appropriate assessment and treatment of the patient who has a persistent postoperative fever despite therapy. The article will also highlight key interventions that help to prevent postoperative infections.
Initial evaluation of the febrile patient
In the first 24 to 48 hours after cesarean delivery, the most common cause of fever is endometritis (organ space infection). This condition is a polymicrobial, mixed aerobic-anaerobic infection (FIGURE). The principal pathogens include anaerobic gram-positive cocci (

The major risk factors for postcesarean endometritis are extended duration of labor and ruptured membranes, multiple internal vaginal examinations, invasive fetal monitoring, and pre-existing colonization with group B Streptococcus and/or the organisms that cause bacterial vaginosis. Affected patients typically have a fever in the range of 38 to 39°C, tachycardia, mild tachypnea, lower abdominal pain and tenderness, and purulent lochia in some individuals.1
Differential for postoperative fever
The initial differential diagnosis of postoperative fever is relatively limited (TABLE 1). In addition to endometritis, it includes extensive atelectasis, perhaps resulting from general anesthesia; lower respiratory tract infection, either viral influenza or bacterial pneumonia; and acute pyelonephritis. A simple infection of the bladder (cystitis or asymptomatic bacteriuria) should not cause a substantial temperature elevation and systemic symptoms.1

Differentiation between these entities usually is possible based on physical examination and a few laboratory tests. The peripheral white blood cell count usually is elevated, and a left shift may be evident. If a respiratory tract infection is suspected, chest radiography is indicated. A urine culture should be obtained if acute pyelonephritis strongly is considered. Lower genital tract cultures are rarely of value, and uncontaminated upper tract cultures are difficult to obtain. I do not believe that blood cultures should be performed as a matter of routine. They are expensive, and the results are often not available until after the patient has cleared her infection and left the hospital. However, I would obtain blood cultures in patients who meet one of these criteria1,2:
- They are immunocompromised (eg, HIV infection).
- They have a cardiac or vascular prosthesis and, thus, are at increased risk of complications related to bacteremia.
- They seem critically ill at the onset of evaluation.
- They fail to respond appropriately to initial therapy.
The cornerstone of therapy is broad spectrum antibiotics that target the multiple organisms responsible for endometritis.3 There are several single agents and several combination antibiotic regimens that provide excellent coverage against the usual pelvic pathogens (TABLE 2). I personally favor the generic combination regimen (clindamycin plus gentamicin) because it is relatively inexpensive and has been very well validated in multiple studies. In patients who have underlying renal dysfunction, aztreonam can be substituted for gentamicin.

Approximately 90% of patients will show clear evidence of clinical improvement (ie, decrease in temperature and resolution of abdominopelvic pain) within 48 hours of starting antibiotics. Patients should then continue therapy until they have been afebrile and asymptomatic for approximately 24 hours. At that point, antibiotics should be discontinued, and the patient can be discharged. With rare exceptions, there is no indication for administration of oral antibiotics on an outpatient basis.1,4
Continue to: Persistent postoperative fever...
Persistent postoperative fever
Resistant microorganism
The most common cause of a persistent fever after initiating antibiotic therapy is a resistant microorganism. There are potential gaps in coverage for the antibiotic regimens commonly used to treat postcesarean endometritis (TABLE 3).1,4 Assuming there is no other obvious cause for treatment failure, I recommend that therapy be changed to the triple combination of metronidazole plus ampicillin plus gentamicin (or aztreonam). The first drug provides superb coverage against anaerobes; the second covers enterococci. Gentamicin or aztreonam cover virtually all aerobic Gram-negative bacilli likely to cause postcesarean infection. I prefer metronidazole rather than clindamycin in this regimen because, unlike clindamycin, it is less likely to trigger diarrhea when used in combination with ampicillin. The 3-drug regimen should be continued until the patient has been afebrile and asymptomatic for approximately 24 hours.1,3,4

Wound infection
The second most common reason for a poor response to initial antibiotic therapy is a wound (surgical site) infection. Wound infections are caused by many of the same pelvic pathogens responsible for endometritis combined with skin flora, notably Streptococcus and Staphylococcus species, including methicillin-resistant Staphylococcus aureus (MRSA).1,4
Wound infections typically take one of two forms. The first is an actual incisional abscess. The patient is febrile; the margins of the wound are warm, indurated, erythematous, and tender; and purulent material drains from the incision. In this situation, the wound should be opened widely to drain the purulent collection. The fascia should then be probed to be certain that dehiscence has not occurred. In addition, intravenous vancomycin (1 g every 12 h) should be included in the antibiotic regimen to ensure adequate coverage of hospital-acquired MRSA.1,4
The second common presentation of a wound infection is cellulitis. The patient is febrile, and there is a spreading area of erythema, warmth, and exquisite tenderness extending from the edges of the incision; however, no purulent drainage is apparent. In this second scenario, the wound should not be opened, but intravenous vancomycin should be added to the treatment regimen.1,3,4
A third and very rare form of wound infection is necrotizing fasciitis. In affected patients, the margins of the wound are darkened and necrotic rather than erythematous and indurated. Two other key physical findings are crepitance and loss of sensation along the margins of the wound. Necrotizing fasciitis is truly a life-threatening emergency and requires immediate and extensive debridement of the devitalized tissue, combined with broad spectrum therapy with antibiotics that provide excellent coverage against anaerobes, aerobic streptococci (particularly group A streptococci), and staphylococci. The requirement for debridement may be so extensive that a skin graft subsequently is necessary to close the defect.1,4
Continue to: Unusual causes of persistent postoperative fever...
Unusual causes of persistent postoperative fever
If a resistant microorganism and wound infection can be excluded, the clinician then must begin a diligent search for “zebras” (ie, uncommon but potentially serious causes of persistent fever).1,4 One possible cause is a pelvic abscess. These purulent collections typically form in the retrovesicle space as a result of infection of a hematoma that formed between the posterior bladder wall and the lower uterine segment, in the leaves of the broad ligament, or in the posterior cul-de-sac. The abscess may or may not be palpable. The patient’s peripheral white blood cell count usually is elevated, with a preponderance of neutrophils. The best imaging test for an abscess is a computed tomography (CT) scan. Abscesses require drainage, which usually can be accomplished by insertion of a percutaneous drain under ultrasonographic or CT guidance.
A second unusual cause of persistent fever is septic pelvic vein thrombophlebitis. The infected venous emboli usually are present in the ovarian veins, with the right side predominant. The patient’s peripheral white blood cell count usually is elevated, and the infected clots are best imaged by CT scan with contrast or magnetic resonance angiography. The appropriate treatment is continuation of broad-spectrum antibiotics and administration of therapeutic doses of parenteral anticoagulants such as enoxaparin or unfractionated heparin.
A third explanation for persistent fever is retained products of conception. This diagnosis is best made by ultrasonography. The placental fragments should be removed by sharp curettage.
A fourth consideration when evaluating the patient with persistent fever is an allergic drug reaction. In most instances, the increase in the patient’s temperature will correspond with administration of the offending antibiotic(s). Affected patients typically have an increased number of eosinophils in their peripheral white blood cell count. The appropriate management of drug fever is discontinuation of antibiotics.
A final and distinctly unusual consideration is recrudescence of a connective tissue disorder such as systemic lupus erythematosus. The best test to confirm this diagnosis is the serum complement assay, which will demonstrate a decreased serum concentration of complement, reflecting consumption of this serum protein during the inflammatory process. The correct management for this condition is administration of a short course of systemic glucocorticoids. TABLE 4 summarizes a simple, systematic plan for evaluation of the patient with a persistent postoperative fever.

Preventive measures
We all remember the simple but profound statement by Benjamin Franklin, “An ounce of prevention is worth a pound of cure.” That folksy adage rings true with respect to postoperative infection because this complication extends hospital stay, increases hospital expense, and causes considerable discomfort and inconvenience for the patient. Therefore, we would do well to prevent as many instances of postoperative infection as possible.
Endometritis
On the basis of well-designed, prospective, randomized trials (Level 1 evidence), 3 interventions have proven effective in reducing the frequency of postcesarean endometritis. The first is irrigation of the vaginal canal preoperatively with an iodophor solution.5,6 The second is preoperative administration of systemic antibiotics.7-9 The combination of cefazolin (2 g IV within 30 minutes of incision) plus azithromycin (500 mg IV over 1 hour prior to incision) is superior to cefazolin alone.10,11 The third important preventive measure is removing the placenta by traction on the umbilical cord rather than by manual extraction.12,13
Wound infection
Several interventions are of proven effectiveness in reducing the frequency of postcesarean wound (surgical site) infection. The first is removal of hair at the incision site by clipping rather than by shaving (Level 2 evidence).14 The second is cleansing of the skin with chlorhexidine rather than iodophor (Level 1 evidence).15 The third is closing of the deep subcutaneous layer of the incision if it exceeds 2 cm in depth (Level 1 evidence).16,17 The fourth is closure of the skin with subcutaneous sutures rather than staples (Level 1 evidence).18 The monofilament suture poliglecaprone 25 is superior to the multifilament suture polyglactin 910 for this purpose (Level 1 evidence).19 Finally, in obese patients (body mass index >30 kg/m2), application of a negative pressure wound vacuum dressing may offer additional protection against infection (Level 1 evidence).20 Such dressings are too expensive, however, to be used routinely in all patients.
Urinary tract infection
The most important measures for preventing postoperative UTIs are identifying and clearing asymptomatic bacteriuria prior to delivery, inserting the urinary catheter prior to surgery using strict sterile technique, and removing the catheter as soon as possible after surgery, ideally within 12 hours.1,4
CASE Resolved
The 2 most likely causes for this patient’s poor response to initial therapy are resistant microorganism and wound infection. If a wound infection can be excluded by physical examination, the patient’s antibiotic regimen should be changed to metronidazole plus ampicillin plus gentamicin (or aztreonam). If an incisional abscess is identified, the incision should be opened and drained, and vancomycin should be added to the treatment regimen. If a wound cellulitis is evident, the incision should not be opened, but vancomycin should be added to the treatment regimen to enhance coverage against aerobic Streptococcus and Staphylococcus species. ●
CASE Woman who has undergone recent cesarean delivery
A 23-year-old woman had a primary cesarean delivery 72 hours ago due to an arrest of dilation at 6 cm. She was in labor for 22 hours, and her membranes were ruptured for 18 hours. She had 10 internal vaginal examinations, and the duration of internal fetal monitoring was 12 hours; 24 hours after delivery, she developed a fever of 39°C, in association with lower abdominal pain and tenderness. She was presumptively treated for endometritis with cefepime; 48 hours after the initiation of antibiotics, she remains febrile and symptomatic.
- What are the most likely causes of her persistent fever?
- What should be the next steps in her evaluation?
Cesarean delivery background
Cesarean delivery is now the most common major operation performed in US hospitals. Cesarean delivery rates hover between 25% and 30% in most medical centers in the United States.1 The most common postoperative complication of cesarean delivery is infection. Infection typically takes 1 of 3 forms: endometritis (organ space infection), wound infection (surgical site infection), and urinary tract infection (UTI).1 This article will review the initial differential diagnosis, evaluation, and management of the patient with a postoperative fever and also will describe the appropriate assessment and treatment of the patient who has a persistent postoperative fever despite therapy. The article will also highlight key interventions that help to prevent postoperative infections.
Initial evaluation of the febrile patient
In the first 24 to 48 hours after cesarean delivery, the most common cause of fever is endometritis (organ space infection). This condition is a polymicrobial, mixed aerobic-anaerobic infection (FIGURE). The principal pathogens include anaerobic gram-positive cocci (

The major risk factors for postcesarean endometritis are extended duration of labor and ruptured membranes, multiple internal vaginal examinations, invasive fetal monitoring, and pre-existing colonization with group B Streptococcus and/or the organisms that cause bacterial vaginosis. Affected patients typically have a fever in the range of 38 to 39°C, tachycardia, mild tachypnea, lower abdominal pain and tenderness, and purulent lochia in some individuals.1
Differential for postoperative fever
The initial differential diagnosis of postoperative fever is relatively limited (TABLE 1). In addition to endometritis, it includes extensive atelectasis, perhaps resulting from general anesthesia; lower respiratory tract infection, either viral influenza or bacterial pneumonia; and acute pyelonephritis. A simple infection of the bladder (cystitis or asymptomatic bacteriuria) should not cause a substantial temperature elevation and systemic symptoms.1

Differentiation between these entities usually is possible based on physical examination and a few laboratory tests. The peripheral white blood cell count usually is elevated, and a left shift may be evident. If a respiratory tract infection is suspected, chest radiography is indicated. A urine culture should be obtained if acute pyelonephritis strongly is considered. Lower genital tract cultures are rarely of value, and uncontaminated upper tract cultures are difficult to obtain. I do not believe that blood cultures should be performed as a matter of routine. They are expensive, and the results are often not available until after the patient has cleared her infection and left the hospital. However, I would obtain blood cultures in patients who meet one of these criteria1,2:
- They are immunocompromised (eg, HIV infection).
- They have a cardiac or vascular prosthesis and, thus, are at increased risk of complications related to bacteremia.
- They seem critically ill at the onset of evaluation.
- They fail to respond appropriately to initial therapy.
The cornerstone of therapy is broad spectrum antibiotics that target the multiple organisms responsible for endometritis.3 There are several single agents and several combination antibiotic regimens that provide excellent coverage against the usual pelvic pathogens (TABLE 2). I personally favor the generic combination regimen (clindamycin plus gentamicin) because it is relatively inexpensive and has been very well validated in multiple studies. In patients who have underlying renal dysfunction, aztreonam can be substituted for gentamicin.

Approximately 90% of patients will show clear evidence of clinical improvement (ie, decrease in temperature and resolution of abdominopelvic pain) within 48 hours of starting antibiotics. Patients should then continue therapy until they have been afebrile and asymptomatic for approximately 24 hours. At that point, antibiotics should be discontinued, and the patient can be discharged. With rare exceptions, there is no indication for administration of oral antibiotics on an outpatient basis.1,4
Continue to: Persistent postoperative fever...
Persistent postoperative fever
Resistant microorganism
The most common cause of a persistent fever after initiating antibiotic therapy is a resistant microorganism. There are potential gaps in coverage for the antibiotic regimens commonly used to treat postcesarean endometritis (TABLE 3).1,4 Assuming there is no other obvious cause for treatment failure, I recommend that therapy be changed to the triple combination of metronidazole plus ampicillin plus gentamicin (or aztreonam). The first drug provides superb coverage against anaerobes; the second covers enterococci. Gentamicin or aztreonam cover virtually all aerobic Gram-negative bacilli likely to cause postcesarean infection. I prefer metronidazole rather than clindamycin in this regimen because, unlike clindamycin, it is less likely to trigger diarrhea when used in combination with ampicillin. The 3-drug regimen should be continued until the patient has been afebrile and asymptomatic for approximately 24 hours.1,3,4

Wound infection
The second most common reason for a poor response to initial antibiotic therapy is a wound (surgical site) infection. Wound infections are caused by many of the same pelvic pathogens responsible for endometritis combined with skin flora, notably Streptococcus and Staphylococcus species, including methicillin-resistant Staphylococcus aureus (MRSA).1,4
Wound infections typically take one of two forms. The first is an actual incisional abscess. The patient is febrile; the margins of the wound are warm, indurated, erythematous, and tender; and purulent material drains from the incision. In this situation, the wound should be opened widely to drain the purulent collection. The fascia should then be probed to be certain that dehiscence has not occurred. In addition, intravenous vancomycin (1 g every 12 h) should be included in the antibiotic regimen to ensure adequate coverage of hospital-acquired MRSA.1,4
The second common presentation of a wound infection is cellulitis. The patient is febrile, and there is a spreading area of erythema, warmth, and exquisite tenderness extending from the edges of the incision; however, no purulent drainage is apparent. In this second scenario, the wound should not be opened, but intravenous vancomycin should be added to the treatment regimen.1,3,4
A third and very rare form of wound infection is necrotizing fasciitis. In affected patients, the margins of the wound are darkened and necrotic rather than erythematous and indurated. Two other key physical findings are crepitance and loss of sensation along the margins of the wound. Necrotizing fasciitis is truly a life-threatening emergency and requires immediate and extensive debridement of the devitalized tissue, combined with broad spectrum therapy with antibiotics that provide excellent coverage against anaerobes, aerobic streptococci (particularly group A streptococci), and staphylococci. The requirement for debridement may be so extensive that a skin graft subsequently is necessary to close the defect.1,4
Continue to: Unusual causes of persistent postoperative fever...
Unusual causes of persistent postoperative fever
If a resistant microorganism and wound infection can be excluded, the clinician then must begin a diligent search for “zebras” (ie, uncommon but potentially serious causes of persistent fever).1,4 One possible cause is a pelvic abscess. These purulent collections typically form in the retrovesicle space as a result of infection of a hematoma that formed between the posterior bladder wall and the lower uterine segment, in the leaves of the broad ligament, or in the posterior cul-de-sac. The abscess may or may not be palpable. The patient’s peripheral white blood cell count usually is elevated, with a preponderance of neutrophils. The best imaging test for an abscess is a computed tomography (CT) scan. Abscesses require drainage, which usually can be accomplished by insertion of a percutaneous drain under ultrasonographic or CT guidance.
A second unusual cause of persistent fever is septic pelvic vein thrombophlebitis. The infected venous emboli usually are present in the ovarian veins, with the right side predominant. The patient’s peripheral white blood cell count usually is elevated, and the infected clots are best imaged by CT scan with contrast or magnetic resonance angiography. The appropriate treatment is continuation of broad-spectrum antibiotics and administration of therapeutic doses of parenteral anticoagulants such as enoxaparin or unfractionated heparin.
A third explanation for persistent fever is retained products of conception. This diagnosis is best made by ultrasonography. The placental fragments should be removed by sharp curettage.
A fourth consideration when evaluating the patient with persistent fever is an allergic drug reaction. In most instances, the increase in the patient’s temperature will correspond with administration of the offending antibiotic(s). Affected patients typically have an increased number of eosinophils in their peripheral white blood cell count. The appropriate management of drug fever is discontinuation of antibiotics.
A final and distinctly unusual consideration is recrudescence of a connective tissue disorder such as systemic lupus erythematosus. The best test to confirm this diagnosis is the serum complement assay, which will demonstrate a decreased serum concentration of complement, reflecting consumption of this serum protein during the inflammatory process. The correct management for this condition is administration of a short course of systemic glucocorticoids. TABLE 4 summarizes a simple, systematic plan for evaluation of the patient with a persistent postoperative fever.

Preventive measures
We all remember the simple but profound statement by Benjamin Franklin, “An ounce of prevention is worth a pound of cure.” That folksy adage rings true with respect to postoperative infection because this complication extends hospital stay, increases hospital expense, and causes considerable discomfort and inconvenience for the patient. Therefore, we would do well to prevent as many instances of postoperative infection as possible.
Endometritis
On the basis of well-designed, prospective, randomized trials (Level 1 evidence), 3 interventions have proven effective in reducing the frequency of postcesarean endometritis. The first is irrigation of the vaginal canal preoperatively with an iodophor solution.5,6 The second is preoperative administration of systemic antibiotics.7-9 The combination of cefazolin (2 g IV within 30 minutes of incision) plus azithromycin (500 mg IV over 1 hour prior to incision) is superior to cefazolin alone.10,11 The third important preventive measure is removing the placenta by traction on the umbilical cord rather than by manual extraction.12,13
Wound infection
Several interventions are of proven effectiveness in reducing the frequency of postcesarean wound (surgical site) infection. The first is removal of hair at the incision site by clipping rather than by shaving (Level 2 evidence).14 The second is cleansing of the skin with chlorhexidine rather than iodophor (Level 1 evidence).15 The third is closing of the deep subcutaneous layer of the incision if it exceeds 2 cm in depth (Level 1 evidence).16,17 The fourth is closure of the skin with subcutaneous sutures rather than staples (Level 1 evidence).18 The monofilament suture poliglecaprone 25 is superior to the multifilament suture polyglactin 910 for this purpose (Level 1 evidence).19 Finally, in obese patients (body mass index >30 kg/m2), application of a negative pressure wound vacuum dressing may offer additional protection against infection (Level 1 evidence).20 Such dressings are too expensive, however, to be used routinely in all patients.
Urinary tract infection
The most important measures for preventing postoperative UTIs are identifying and clearing asymptomatic bacteriuria prior to delivery, inserting the urinary catheter prior to surgery using strict sterile technique, and removing the catheter as soon as possible after surgery, ideally within 12 hours.1,4
CASE Resolved
The 2 most likely causes for this patient’s poor response to initial therapy are resistant microorganism and wound infection. If a wound infection can be excluded by physical examination, the patient’s antibiotic regimen should be changed to metronidazole plus ampicillin plus gentamicin (or aztreonam). If an incisional abscess is identified, the incision should be opened and drained, and vancomycin should be added to the treatment regimen. If a wound cellulitis is evident, the incision should not be opened, but vancomycin should be added to the treatment regimen to enhance coverage against aerobic Streptococcus and Staphylococcus species. ●
- Duff WP. Maternal and perinatal infection in pregnancy: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al, eds. Gabbe’s Obstetrics: Normal and Problem Pregnancies. 8th ed. Elsevier; 2020:1124-1146.
- Locksmith GJ, Duff P. Assessment of the value of routine blood cultures in the evaluation and treatment of patients with chorioamnionitis. Infect Dis Obstet Gynecol. 1994;2:111-114.
- Duff P. Antibiotic selection in obstetric patients. Infect Dis Clin N Am. 1997;11:1-12.
- Duff P. Maternal and fetal infections. In: Creasy RK, Resnik R, Iams, JD, et al, eds. Creasy & Resnik’s Maternal Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862-919.
- Haas DM, Morgan S, Contreras K. Vaginal preparation with antiseptic solution before cesarean section for preventing postoperative infections. Cochrane Database Syst Rev. 2014;12:CD007892.
- Caissutti C, Saccone G, Zullo F, et al. Vaginal cleansing before cesarean delivery. a systematic review and meta-analysis. Obstet Gynecol. 2017;130:527-538.
- Sullivan SA, Smith T, Change E, et al. Administration of cefazolin prior to skin incision is superior to cefazolin at cord clamping in preventing postcesarean infectious morbidity; a randomized controlled trial. Am J Obstet Gynecol. 2007;196:455.e1-455.e5.
- Tita ATN, Hauth JC, Grimes A, et al. Decreasing incidence of postcesarean endometritis with extended-spectrum antibiotic prophylaxis. Obstet Gynecol. 2008;111:51-56.
- Tita ATN, Owen J, Stamm AM, et al. Impact of extended-spectrum antibiotic prophylaxis on incidence of postcesarean surgical wound infection. Am J Obstet Gynecol. 2008;199: 303.e1-303.e3.
- Tita ATN, Szchowski JM, Boggess K, et al. Two antibiotics before cesarean delivery reduce infection rates further than one agent. N Engl J Med. 2016;375:1231-1241.
- Harper LM, Kilgore M, Szychowski JM, et al. Economic evaluation of adjunctive azithromycin prophylaxis for cesarean delivery. Obstet Gynecol. 2017;130:328-334.
- Lasley DS, Eblen A, Yancey MK, et al. The effect of placental removal method on the incidence of postcesarean infections. Am J Obstet Gynecol. 1997;176:1250-1254.
- Anorlu RI, Maholwana B, Hofmeyr GJ. Methods of delivering the placenta at cesarean section. Cochrane Database Syst Rev. 2008;3:CD004737.
- Cruse PJ, Foord R. A five-year prospective study of 23,649 surgical wounds. Arch Surg. 1973;107:206-209.
- Tuuli MG, Liu J, Stout MJ, et al. A randomized trial comparing skin antiseptic agents at cesarean delivery. N Engl J Med. 2016;374:657-665.
- Del Valle GO, Combs P, Qualls C, et al. Does closure of camper fascia reduce the incidence of post-cesarean superficial wound disruption? Obstet Gynecol. 1992;80:1013-1016.
- Chelmow D. Rodriguez EJ, Sabatini MM. Suture closure of subcutaneous fat and wound disruption after cesarean delivery: a meta-analysis. Obstet Gynecol. 2004;103:974-980.
- Tuuli MG, Rampersod RM, Carbone JF, et al. Staples compared with subcuticular suture for skin closure after cesarean delivery. a systematic review and meta-analysis. Obstet Gynecol. 2011;117:682-690.
- Buresch AM, Arsdale AV, Ferzli M, et al. Comparison of subcuticular suture type for skin closure after cesarean delivery. a randomized controlled trial. Obstet Gynecol. 2017;130:521-526.
- Yu L, Kronen RJ, Simon LE, et al. Prophylactic negative-pressure wound therapy after cesarean is associated with reduced risk of surgical site infection: a systematic review and meta-analysis. Am J Obstet Gynecol. 2018;218:200-210.
- Duff WP. Maternal and perinatal infection in pregnancy: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al, eds. Gabbe’s Obstetrics: Normal and Problem Pregnancies. 8th ed. Elsevier; 2020:1124-1146.
- Locksmith GJ, Duff P. Assessment of the value of routine blood cultures in the evaluation and treatment of patients with chorioamnionitis. Infect Dis Obstet Gynecol. 1994;2:111-114.
- Duff P. Antibiotic selection in obstetric patients. Infect Dis Clin N Am. 1997;11:1-12.
- Duff P. Maternal and fetal infections. In: Creasy RK, Resnik R, Iams, JD, et al, eds. Creasy & Resnik’s Maternal Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862-919.
- Haas DM, Morgan S, Contreras K. Vaginal preparation with antiseptic solution before cesarean section for preventing postoperative infections. Cochrane Database Syst Rev. 2014;12:CD007892.
- Caissutti C, Saccone G, Zullo F, et al. Vaginal cleansing before cesarean delivery. a systematic review and meta-analysis. Obstet Gynecol. 2017;130:527-538.
- Sullivan SA, Smith T, Change E, et al. Administration of cefazolin prior to skin incision is superior to cefazolin at cord clamping in preventing postcesarean infectious morbidity; a randomized controlled trial. Am J Obstet Gynecol. 2007;196:455.e1-455.e5.
- Tita ATN, Hauth JC, Grimes A, et al. Decreasing incidence of postcesarean endometritis with extended-spectrum antibiotic prophylaxis. Obstet Gynecol. 2008;111:51-56.
- Tita ATN, Owen J, Stamm AM, et al. Impact of extended-spectrum antibiotic prophylaxis on incidence of postcesarean surgical wound infection. Am J Obstet Gynecol. 2008;199: 303.e1-303.e3.
- Tita ATN, Szchowski JM, Boggess K, et al. Two antibiotics before cesarean delivery reduce infection rates further than one agent. N Engl J Med. 2016;375:1231-1241.
- Harper LM, Kilgore M, Szychowski JM, et al. Economic evaluation of adjunctive azithromycin prophylaxis for cesarean delivery. Obstet Gynecol. 2017;130:328-334.
- Lasley DS, Eblen A, Yancey MK, et al. The effect of placental removal method on the incidence of postcesarean infections. Am J Obstet Gynecol. 1997;176:1250-1254.
- Anorlu RI, Maholwana B, Hofmeyr GJ. Methods of delivering the placenta at cesarean section. Cochrane Database Syst Rev. 2008;3:CD004737.
- Cruse PJ, Foord R. A five-year prospective study of 23,649 surgical wounds. Arch Surg. 1973;107:206-209.
- Tuuli MG, Liu J, Stout MJ, et al. A randomized trial comparing skin antiseptic agents at cesarean delivery. N Engl J Med. 2016;374:657-665.
- Del Valle GO, Combs P, Qualls C, et al. Does closure of camper fascia reduce the incidence of post-cesarean superficial wound disruption? Obstet Gynecol. 1992;80:1013-1016.
- Chelmow D. Rodriguez EJ, Sabatini MM. Suture closure of subcutaneous fat and wound disruption after cesarean delivery: a meta-analysis. Obstet Gynecol. 2004;103:974-980.
- Tuuli MG, Rampersod RM, Carbone JF, et al. Staples compared with subcuticular suture for skin closure after cesarean delivery. a systematic review and meta-analysis. Obstet Gynecol. 2011;117:682-690.
- Buresch AM, Arsdale AV, Ferzli M, et al. Comparison of subcuticular suture type for skin closure after cesarean delivery. a randomized controlled trial. Obstet Gynecol. 2017;130:521-526.
- Yu L, Kronen RJ, Simon LE, et al. Prophylactic negative-pressure wound therapy after cesarean is associated with reduced risk of surgical site infection: a systematic review and meta-analysis. Am J Obstet Gynecol. 2018;218:200-210.
