User login
Prostate-specific antigen (PSA) testing has been mired in controversy throughout the short time it has been a clinical tool for detecting prostate cancer. During the first decade after it was approved for prostate cancer screening, the dogma prevailed that the upper limit of normal was 4.0 μg/L. Healthy patients with values above this cutoff were believed to be at risk of prostate cancer and were usually advised to undergo biopsy. Patients with levels below this threshold were told they had normal reading sand were reassured that they did not have prostate cancer.
NO PSA VALUE RULES CANCER IN OR OUT
But more men have prostate cancer than we thought. Initial reports suggested that men with a slightly elevated PSA value (4.0–9.9 μg/L) had a 22% chance of having prostate cancer, and those with a significant elevation (values of 10.0 μg/L or higher) had a 67% risk.1 However, these numbers were based on“sextant” biopsies (ie, in which only six samples are taken)—a technique fraught with a high false-negative detection rate. If more biopsy samples per patient are taken, approximately twice as many men with PSA levels above 4.0 are found to harbor prostate cancer, in the range of 40% to 50%.2
An individual patient’s PSA value is only part of the equation. Other risk factors need to be considered, such as his age, race, family history, findings on digital rectal examination, prostate size, results of earlier prostate biopsies, percent free PSA ratio, and whether he takes a 5-alpha reductase inhibitor. Moreover, PSA levels in men who have undergone treatment for prostate cancer are completely independent of the reference ranges in widespread laboratory use, making such references and thresholds even more meaningless in this setting.
Depending on their other risk factors, two men with identical PSA levels can have very different risks of prostate cancer. Conversely, if all their other risk factors are the same and one man has a PSA level of 3.9 μg/L and th eother has a level of 4.1, their risk is essentially identical.
Many patients and physicians are perplexed about PSA testing, often framing their concern in terms of “inaccuracy.” However, accuracy is not the problem with PSA testing. Rather, the problem is that physicians insist on categorizing patients as either normal or abnormal, when it is abundantly clear that such a dichotomy does not exist. Nevertheless, laboratories around the world continue to foster this false division by printing a PSA reference range of less than 4.0 μg/L on their reports.
A MORE MEANINGFUL LABORATORY REPORT
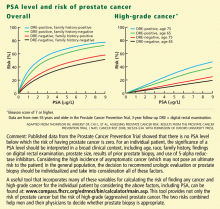
In view of the unequivocal data, urologists at Cleveland Clinic Glickman Urological and Kidney Institute, in collaboration with the Department of Laboratory Medicine, have eliminated 4.0 μg/L as the upper limit of normal from our PSA reports. Instead of a normal range, our reports now include risk ranges from large series (as shown in Figure 1), which provide a more meaningful clinical picture than the categories normal or abnormal.3–5 Moreover, the report also carries an explanation, including a reference to a risk calculator, to assist the patient and physician in interpretingthe reading.
Interpreting these data will inevitably create new controversy, like any major challenge to the status quo. Nevertheless, it is certainly more appropriate to inform patients of their actual risk of having prostate cancer than it is to tell them that their PSA level is normal or abnormal.
Moreover, because many older men have asymptomatic cancer that may never pose a problem in their lifetime, the decision to recommend urologic evaluation or prostate biopsy should be individualized. Indeed, this will take greater consideration than it did with the old 4.0 cutoff: interpreting PSA levels is more complex than once believed.
This change is long overdue and will require substantial education of both physicians and patients. They need to know that there is no PSA level at which a biopsy is mandated, and that the decision to consider biopsy should be based on solid information:ie, what are the odds that this patient has cancer? And what are the odds that he has high-grade, aggressive cancer, as opposed to a more indolent form that might be appropriately ignored?
We anticipate concern that changing the way we report PSA values may increase patients’ anxiety and lead to more prostate biopsies being performed. That is emphatically not the intent of this initiative. Rather, our intent is to accurately report PSA values with a meaningful interpretation of their implications instead of reporting an artificial—and relatively meaningless—cutoff. Interpreting PSA levels in the context of all the above factors will help advance the understanding and management of prostate cancer risk and diagnosis.
- Catalona WJ, Smith DS, Ratliff TL, et al. Measurement of prostate-specific antigen in serum as a screening test for prostate cancer. N Engl J Med 1991; 324:1156–1161.
- Jones JS, Patel A, Schoenfield L, Rabets JC, Zippe CD, Magi-Galluzzi C. Saturation technique does not improve cancer detection as an initial prostate biopsy strategy. J Urol 2006; 175:485–488.
- Thompson IM, Pauler DK, Goodman PJ, et al. Prevalence of prostate cancer among men with a prostate-specific antigen level < or = 4.0 ng per milliliter. N Engl J Med 2004; 350:2239–2246.
- Thompson IM, Ankerst DP, Chi C, et al. Assessing prostate cancer risk: results from the Prostate Cancer Prevention Trial. J Natl Cancer Inst 2006; 98:529–534.
- Presti JC Jr, O’Dowd GJ, Miller MC, et al. Extended peripheral zone biopsy schemes increase cancer detection rates and minimize variance in prostate specific antigen and age related cancer rates: results of a community multi-practice study. J Urol 2003; 169:125–129.
Prostate-specific antigen (PSA) testing has been mired in controversy throughout the short time it has been a clinical tool for detecting prostate cancer. During the first decade after it was approved for prostate cancer screening, the dogma prevailed that the upper limit of normal was 4.0 μg/L. Healthy patients with values above this cutoff were believed to be at risk of prostate cancer and were usually advised to undergo biopsy. Patients with levels below this threshold were told they had normal reading sand were reassured that they did not have prostate cancer.
NO PSA VALUE RULES CANCER IN OR OUT
But more men have prostate cancer than we thought. Initial reports suggested that men with a slightly elevated PSA value (4.0–9.9 μg/L) had a 22% chance of having prostate cancer, and those with a significant elevation (values of 10.0 μg/L or higher) had a 67% risk.1 However, these numbers were based on“sextant” biopsies (ie, in which only six samples are taken)—a technique fraught with a high false-negative detection rate. If more biopsy samples per patient are taken, approximately twice as many men with PSA levels above 4.0 are found to harbor prostate cancer, in the range of 40% to 50%.2
An individual patient’s PSA value is only part of the equation. Other risk factors need to be considered, such as his age, race, family history, findings on digital rectal examination, prostate size, results of earlier prostate biopsies, percent free PSA ratio, and whether he takes a 5-alpha reductase inhibitor. Moreover, PSA levels in men who have undergone treatment for prostate cancer are completely independent of the reference ranges in widespread laboratory use, making such references and thresholds even more meaningless in this setting.
Depending on their other risk factors, two men with identical PSA levels can have very different risks of prostate cancer. Conversely, if all their other risk factors are the same and one man has a PSA level of 3.9 μg/L and th eother has a level of 4.1, their risk is essentially identical.
Many patients and physicians are perplexed about PSA testing, often framing their concern in terms of “inaccuracy.” However, accuracy is not the problem with PSA testing. Rather, the problem is that physicians insist on categorizing patients as either normal or abnormal, when it is abundantly clear that such a dichotomy does not exist. Nevertheless, laboratories around the world continue to foster this false division by printing a PSA reference range of less than 4.0 μg/L on their reports.
A MORE MEANINGFUL LABORATORY REPORT
In view of the unequivocal data, urologists at Cleveland Clinic Glickman Urological and Kidney Institute, in collaboration with the Department of Laboratory Medicine, have eliminated 4.0 μg/L as the upper limit of normal from our PSA reports. Instead of a normal range, our reports now include risk ranges from large series (as shown in Figure 1), which provide a more meaningful clinical picture than the categories normal or abnormal.3–5 Moreover, the report also carries an explanation, including a reference to a risk calculator, to assist the patient and physician in interpretingthe reading.
Interpreting these data will inevitably create new controversy, like any major challenge to the status quo. Nevertheless, it is certainly more appropriate to inform patients of their actual risk of having prostate cancer than it is to tell them that their PSA level is normal or abnormal.
Moreover, because many older men have asymptomatic cancer that may never pose a problem in their lifetime, the decision to recommend urologic evaluation or prostate biopsy should be individualized. Indeed, this will take greater consideration than it did with the old 4.0 cutoff: interpreting PSA levels is more complex than once believed.
This change is long overdue and will require substantial education of both physicians and patients. They need to know that there is no PSA level at which a biopsy is mandated, and that the decision to consider biopsy should be based on solid information:ie, what are the odds that this patient has cancer? And what are the odds that he has high-grade, aggressive cancer, as opposed to a more indolent form that might be appropriately ignored?
We anticipate concern that changing the way we report PSA values may increase patients’ anxiety and lead to more prostate biopsies being performed. That is emphatically not the intent of this initiative. Rather, our intent is to accurately report PSA values with a meaningful interpretation of their implications instead of reporting an artificial—and relatively meaningless—cutoff. Interpreting PSA levels in the context of all the above factors will help advance the understanding and management of prostate cancer risk and diagnosis.
Prostate-specific antigen (PSA) testing has been mired in controversy throughout the short time it has been a clinical tool for detecting prostate cancer. During the first decade after it was approved for prostate cancer screening, the dogma prevailed that the upper limit of normal was 4.0 μg/L. Healthy patients with values above this cutoff were believed to be at risk of prostate cancer and were usually advised to undergo biopsy. Patients with levels below this threshold were told they had normal reading sand were reassured that they did not have prostate cancer.
NO PSA VALUE RULES CANCER IN OR OUT
But more men have prostate cancer than we thought. Initial reports suggested that men with a slightly elevated PSA value (4.0–9.9 μg/L) had a 22% chance of having prostate cancer, and those with a significant elevation (values of 10.0 μg/L or higher) had a 67% risk.1 However, these numbers were based on“sextant” biopsies (ie, in which only six samples are taken)—a technique fraught with a high false-negative detection rate. If more biopsy samples per patient are taken, approximately twice as many men with PSA levels above 4.0 are found to harbor prostate cancer, in the range of 40% to 50%.2
An individual patient’s PSA value is only part of the equation. Other risk factors need to be considered, such as his age, race, family history, findings on digital rectal examination, prostate size, results of earlier prostate biopsies, percent free PSA ratio, and whether he takes a 5-alpha reductase inhibitor. Moreover, PSA levels in men who have undergone treatment for prostate cancer are completely independent of the reference ranges in widespread laboratory use, making such references and thresholds even more meaningless in this setting.
Depending on their other risk factors, two men with identical PSA levels can have very different risks of prostate cancer. Conversely, if all their other risk factors are the same and one man has a PSA level of 3.9 μg/L and th eother has a level of 4.1, their risk is essentially identical.
Many patients and physicians are perplexed about PSA testing, often framing their concern in terms of “inaccuracy.” However, accuracy is not the problem with PSA testing. Rather, the problem is that physicians insist on categorizing patients as either normal or abnormal, when it is abundantly clear that such a dichotomy does not exist. Nevertheless, laboratories around the world continue to foster this false division by printing a PSA reference range of less than 4.0 μg/L on their reports.
A MORE MEANINGFUL LABORATORY REPORT
In view of the unequivocal data, urologists at Cleveland Clinic Glickman Urological and Kidney Institute, in collaboration with the Department of Laboratory Medicine, have eliminated 4.0 μg/L as the upper limit of normal from our PSA reports. Instead of a normal range, our reports now include risk ranges from large series (as shown in Figure 1), which provide a more meaningful clinical picture than the categories normal or abnormal.3–5 Moreover, the report also carries an explanation, including a reference to a risk calculator, to assist the patient and physician in interpretingthe reading.
Interpreting these data will inevitably create new controversy, like any major challenge to the status quo. Nevertheless, it is certainly more appropriate to inform patients of their actual risk of having prostate cancer than it is to tell them that their PSA level is normal or abnormal.
Moreover, because many older men have asymptomatic cancer that may never pose a problem in their lifetime, the decision to recommend urologic evaluation or prostate biopsy should be individualized. Indeed, this will take greater consideration than it did with the old 4.0 cutoff: interpreting PSA levels is more complex than once believed.
This change is long overdue and will require substantial education of both physicians and patients. They need to know that there is no PSA level at which a biopsy is mandated, and that the decision to consider biopsy should be based on solid information:ie, what are the odds that this patient has cancer? And what are the odds that he has high-grade, aggressive cancer, as opposed to a more indolent form that might be appropriately ignored?
We anticipate concern that changing the way we report PSA values may increase patients’ anxiety and lead to more prostate biopsies being performed. That is emphatically not the intent of this initiative. Rather, our intent is to accurately report PSA values with a meaningful interpretation of their implications instead of reporting an artificial—and relatively meaningless—cutoff. Interpreting PSA levels in the context of all the above factors will help advance the understanding and management of prostate cancer risk and diagnosis.
- Catalona WJ, Smith DS, Ratliff TL, et al. Measurement of prostate-specific antigen in serum as a screening test for prostate cancer. N Engl J Med 1991; 324:1156–1161.
- Jones JS, Patel A, Schoenfield L, Rabets JC, Zippe CD, Magi-Galluzzi C. Saturation technique does not improve cancer detection as an initial prostate biopsy strategy. J Urol 2006; 175:485–488.
- Thompson IM, Pauler DK, Goodman PJ, et al. Prevalence of prostate cancer among men with a prostate-specific antigen level < or = 4.0 ng per milliliter. N Engl J Med 2004; 350:2239–2246.
- Thompson IM, Ankerst DP, Chi C, et al. Assessing prostate cancer risk: results from the Prostate Cancer Prevention Trial. J Natl Cancer Inst 2006; 98:529–534.
- Presti JC Jr, O’Dowd GJ, Miller MC, et al. Extended peripheral zone biopsy schemes increase cancer detection rates and minimize variance in prostate specific antigen and age related cancer rates: results of a community multi-practice study. J Urol 2003; 169:125–129.
- Catalona WJ, Smith DS, Ratliff TL, et al. Measurement of prostate-specific antigen in serum as a screening test for prostate cancer. N Engl J Med 1991; 324:1156–1161.
- Jones JS, Patel A, Schoenfield L, Rabets JC, Zippe CD, Magi-Galluzzi C. Saturation technique does not improve cancer detection as an initial prostate biopsy strategy. J Urol 2006; 175:485–488.
- Thompson IM, Pauler DK, Goodman PJ, et al. Prevalence of prostate cancer among men with a prostate-specific antigen level < or = 4.0 ng per milliliter. N Engl J Med 2004; 350:2239–2246.
- Thompson IM, Ankerst DP, Chi C, et al. Assessing prostate cancer risk: results from the Prostate Cancer Prevention Trial. J Natl Cancer Inst 2006; 98:529–534.
- Presti JC Jr, O’Dowd GJ, Miller MC, et al. Extended peripheral zone biopsy schemes increase cancer detection rates and minimize variance in prostate specific antigen and age related cancer rates: results of a community multi-practice study. J Urol 2003; 169:125–129.