User login
A practical guide to prostate cancer diagnosis and management
Prostate cancer screening, diagnosis, and treatment present challenges to internists, urologists, and oncologists. For the internist, there is the ongoing debate about when and how often to screen with prostate-specific antigen (PSA) testing, as well as about how to interpret the results. For urologists and oncologists, there is no consensus on how to treat prostate cancer with the growing array of options, from surgery to cryoablation. Most therapies have not been compared in head-to-head trials, and anxious patients often approach their internist for help in navigating the maze of options.
This review summarizes current American Urological Association (AUA) guidelines,1 as well as current practice patterns at the Glickman Urological and Kidney Institute of Cleveland Clinic regarding screening, diagnosis, risk assessment, treatment, and posttreatment management of prostate cancer. We try to explain the approved and the experimental treatments, outlining what we know about their advantages and disadvantages.
SCREENING: WHEN AND HOW
Screening for prostate cancer should involve both a digital rectal examination (DRE) and measurement of the serum PSA level. But when should screening start?
The AUA recommends annual screening with DRE and serum PSA test starting at age 40 for all men with a life expectancy of more than 10 years.1
The American Cancer Society2 and the American College of Physicians,3 in contrast, recommend that men who choose to undergo screening should begin at age 50, or at age 45 if they are black or have a family history of prostate cancer in a primary relative diagnosed before age 65. They also recommend that screening with PSA and DRE be stopped at age 75, given the low likelihood of death from de novo prostate cancer after this age. The AUA recommends that screening be stopped at age 75, but may be continued beyond age 75 if the patient has a life expectancy of 10 years or more.
Before being screened, patients should understand the benefits and the risks of testing. While a small subset of prostate cancers behave aggressively, the majority are slow-growing and pose minimal risk for the development of fatal disease.
A discussion of the rationale for these guidelines and their differences is beyond the scope of this review. Differences stem from the observation that most men treated for prostate cancer will likely not die from prostate cancer, but rather from another condition.
Digital rectal examination’s role and limitations
The utility of DRE is limited to the detection of nodules, gross asymmetry, and gland fixation. DRE is not highly specific: only 40% to 50% of men who have abnormal findings on DRE have prostate cancer on biopsy.5 Anyone who has an abnormal finding on DRE should undergo prostate biopsy. However, if a rectal mass is palpated or if the prostate is exquisitely sensitive, biopsy is not indicated.
DRE is highly inaccurate for estimating gland volume; it should not be used to gauge cancer risk.
Prostate-specific antigen: Caveats
PSA measurement was introduced as a clinical screening test for prostate cancer in the early 1990s, and it serves as the foundation for early detection.
PSA, a protein involved in seminal coagulation, is produced by the prostate epithelium and is mostly confined within the prostatic ducts. Cancer cells secrete PSA into the bloodstream at increased levels via a disrupted basement membrane in tumor-affected areas of the gland. Elevated PSA can also result from benign prostatic hypertrophy, prostatitis, and prostate biopsy.
PSA levels represent a continuum of prostate cancer risk, and no single PSA value is sensitive and specific enough to predict the presence of cancer.6 Abnormal PSA cutoffs have been defined from 2.5 μg/L to 4 μg/L, and much debate surrounds this topic. Men who present with an elevated PSA (ie, > 2.5 μg/L) should be tested again. If the value remains high, then prostate biopsy should be considered. An elevated PSA level in older men with benign prostatic hypertrophy is not unexpected, and in these patients observation of the PSA value over time may prove valuable to assess the need for biopsy.
A useful adjunct in men with elevated PSA and benign prostatic hypertrophy is the percentage of serum PSA that is free rather than bound.7 PSA produced by prostate cancer binds more avidly with serum proteins (alpha-1 chymotrypsin and alpha-2 macroglobulin), resulting in a lower percentage of free PSA. In men with an elevated PSA (ie, 4.1–10.0 μg/L), the percentage of free PSA provides an indication of whether the elevation is due to benign prostatic hypertrophy or to cancer: the lower the percent free PSA, the more likely an elevated total PSA represents cancer and not benign prostatic hypertrophy. The sensitivity of a free PSA less than 15% to detect prostate cancer is about 85%, and its use as a screening tool is under study.
Much attention has also been given to other PSA indices, namely, the PSA density (the PSA level divided by the prostate volume), the PSA velocity (the rate of increase in the PSA level over time), and the PSA doubling time. While these nuanced PSA measures are useful to predict disease severity and behavior, they are not routinely used in screening.
BIOPSY IS INDICATED IF EITHER TEST IS ABNORMAL
In the past, imaging of the prostate with transrectal ultrasonography was used as a screening tool to detect prostate cancer. Further research showed that only 15% to 20% of hypoechoic lesions detected on ultrasonography contained cancer.8 Because of its low sensitivity and specificity, primary ultrasonographic screening (ie, transrectal ultrasonography alone) is not acceptable for screening or for diagnosis. Its main role is in guiding prostate biopsy.
Biopsy of the prostate with transrectal ultrasonographic guidance is indicated if either the DRE or the PSA level is abnormal. The standard of care is to use an 18-gauge biopsy needle-gun to obtain two to three tissue samples from each of six regions of the prostate, focusing on the outer peripheral zone, specifically the right and left bases, the mid-gland, and the apex.
Pathologic analysis of each tissue core takes into consideration the presence or absence of cancer, the Gleason score, and the percentage of the tissue sample volume that is occupied by cancer.
The Gleason grading system is based on the histologic appearance and reflects the degree of differentiation and aggressiveness of the cancer. The two most prominent tumor grades present are added to give a final Gleason score. For instance, a Gleason grade of 4+3=7 indicates a tumor with predominant Gleason grade 4 disease with a lesser amount of grade 3 disease. The number of positive core samples and the volume of cancer provide information on the severity of the cancer.
If the PSA is high but biopsy is negative
Prostate biopsy misses up to 30% of small cancers. Many of these are clinically insignificant, but about 20% of those missed cancers can be high-risk and thus merit identification. There should be a low threshold for repeating biopsy 1 year later in men who have a persistently high PSA or a rising PSA.
High-grade prostatic intraepithelial neoplasia is a common finding on biopsy. The incidence of de novo prostate cancer at 5 years in men with this finding is 22% to 26%.9 Patients with multifocal high-grade prostatic intraepithelial neoplasia should be monitored with PSA testing and DRE every 6 to 12 months and should be considered for repeat “saturation” biopsy (ie, obtaining as many as 36 core samples).
IF CANCER IS FOUND, HOW RISKY IS IT?
Patients with a new diagnosis of prostate cancer must decide on a treatment plan. This decision is highly individualized, based on the patient’s personal preferences, lifestyle, performance status (ie, his general well-being), disease severity, continence status, and sexual function.
When counseling patients about their disease and the treatment options, we consider three main factors:
- The severity of disease on biopsy
- The patient’s current state of health and performance status
- The patient’s understanding of and willingness to accept the adverse effects of the various treatments.
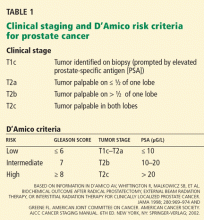
Pathologic features, the PSA level, and clinical stage determined by DRE are used to predict the severity of disease. Most data on the efficacy of treatments for prostate cancer are based on the incidence of biochemical recurrence, ie, a rise in PSA level after primary therapy. The AUA and the D’Amico risk criteria use biopsy pathology, clinical stage, and the pretreatment PSA level to predict the likelihood of biochemical recurrence (Table 1).10,11
DISCUSSING TREATMENT OPTIONS WITH THE PATIENT
In our practice, we usually do not recommend treatment in men with low-risk or intermediate-risk prostate cancer who have a life expectancy of less than 10 years, as most of them will likely die of a cause other than prostate cancer. For patients with poor baseline performance status, surveillance or radiation therapy may be preferable to surgery. In younger patients, surgery may confer a more durable benefit.
- No prospective, randomized clinical trials have directly compared these treatments
- Prostate cancer progresses slowly
- Definitions of treatment failure used in various studies have been inconsistent
- Clinical studies have been subject to selection bias.
ACTIVE SURVEILLANCE IS ACCEPTABLE FOR LOW-RISK PROSTATE CANCER
Active surveillance is an acceptable option for patients with low-risk prostate cancer (ie, if the Gleason score is ≤ 6, the tumor stage is T1c or T2a, and the PSA level is ≤ 10 μg/L). To rule out high-risk disease before starting a program of surveillance, repeat biopsy is advisable, although optional.
Active surveillance consists of PSA testing and DRE every 6 to 12 months, followed by repeat biopsy if significant changes are noted in either test. Some centers advocate biopsy with transrectal ultrasonographic guidance every year regardless of the PSA or DRE findings.
Whether a change in the PSA level is significant is subjective, but a recent phase 2 study in 453 patients23 on a program of active surveillance used a PSA doubling time of less than 3 years as a criterion for repeat biopsy. Thirty-eight percent of the men had to undergo radiation therapy or surgery within 10 years, and 5 patients (1%) died of prostate cancer. The authors concluded that active surveillance did not put these patients at undue risk, and that this approach prevented overtreatment of clinically insignificant prostate cancer.23
The risks of surveillance include the chance that cancer could progress to an incurable state during the surveillance period, greater anxiety for the patient, and, if prostatectomy becomes necessary, greater technical difficulty due to scarring from repeat biopsies. The benefit is postponement or complete avoidance of the adverse effects of treatment.
Debate continues over the potential dangers of deferred treatment of prostate cancer, but in certain patients it is an acceptable option. Patient education, accurate disease assessment, and compliance with monitoring are critical considerations.
RADICAL PROSTATECTOMY: SEVERAL OPTIONS, EQUIVALENT EFFICACY
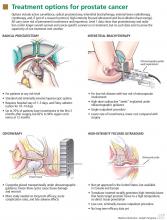
Radical prostatectomy is widely used for treating prostate cancer of any risk level. The operation entails removing the prostate and seminal vesicles, as well as the pelvic lymph nodes in patients with intermediate or high-risk cancer.
This procedure was increasingly used in the 1990s with the introduction of PSA screening and nerve-sparing surgical techniques that preserve continence and erectile function.
Radical prostatectomy can be done via a standard open approach or a minimally invasive laparoscopic approach with or without robotic assistance. Open surgery, laparoscopic surgery, and robotic prostatectomy offer equivalent rates of oncologic efficacy, continence, and potency.24 The more experienced the surgeon, the better the outcome is likely to be.
The average biochemical recurrence rate at 5 years after radical prostatectomy is approximately 6% for patients with low-risk cancer, 23% for those with intermediate-risk cancer, and 45% for those with high-risk cancer.25 The rate of death from prostate cancer at 10 years is about 1% for patients with low-risk cancer, 4% for those with intermediate-risk cancer, and 8% for those with high-risk cancer.12
Secondary therapy
Pathologic staging of the surgical specimen after radical prostatectomy yields information that can be beneficial in terms of initiating early secondary therapy.
Patients with node-positive disease should immediately undergo androgen deprivation treatment.26
Evidence of positive surgical margins, seminal vesicle invasion, bladder neck invasion, and extracapsular extension also increase the risk of recurrence. This additional risk can be ascertained via the use of a postoperative nomogram. Patients at high risk of recurrence should be considered for early adjuvant external beam radiotherapy to the surgical field 3 to 6 months after surgery.
Advantages and disadvantages of radical prostatectomy
Advantages of radical prostatectomy include the ability to accurately stage the cancer with the surgical specimen and the ability to remove the pelvic lymph nodes in patients at intermediate and high risk. Another advantage is that postoperative surveillance is straightforward: PSA should become undetectable after surgery, and a measurable increase in PSA represents disease recurrence.
Disadvantages include:
- The risk of surgical complications (reported in 3% to 17% of cases)24
- An average hospital stay of 1 to 3 days (and a typical 3 to 6 weeks before returning to work)
- The need for a Foley catheter for 10 to 14 days
- The risk of incontinence and impotence, which are very distressing to patients.
Postoperative incontinence is typically defined as the need for any type of protective pad for leakage. Up to 70% of patients have incontinence in the first 3 months after surgery, but 82% to 94% of patients regain continence by 12 months.24 A small percentage of patients (3% to 5%) have significant permanent incontinence.
Counseling about postoperative erectile dysfunction
All patients should be counseled about the risk of a postoperative decrease in erectile function, especially those with pre-existing erectile dysfunction. Potency is defined as the ability to have an erection suitable for intercourse (with or without phosphodiesterase type 5 inhibitors) more than 50% of the time. In men with bilateral nerve-sparing open prostatectomy, potency rates at 12 months have been reported between 63% and 81%.13
Data on potency rates vary widely because of differences in how potency was defined, selection bias, and the multifactorial nature of erectile dysfunction. Also, because single-institution, single-surgeon reports and advertisements tend to underestimate rates of impotence after radical prostatectomy by any approach, many patients have false expectations.
INTERSTITIAL BRACHYTHERAPY FOR LOW-RISK CANCERS
Interstitial brachytherapy delivers a localized, high dose (125 to 145 Gy) of radiation to the prostate, with minimal radiation dosing to the bladder, rectum, or other adjacent organs and tissues. “Seeds” or small pellets containing a radioisotope (iodine 125 or palladium 103) are stereotactically implanted through the perineum into the prostate under ultrasonographic guidance. Computerized mapping done before or during surgery helps determine the optimal placement of the seeds, the object being to cover at least 90% of the prostate with 100% of the radiation dose.
In permanent brachytherapy, the implants give off radiation at a low dose rate over weeks to months and are left in place permanently. In temporary brachytherapy, seeds are implanted to deliver a low or high dose rate for a specified period, and then they are removed.
“Implant quality,” ie, delivery of more than 90% of the radiation dose, is a major predictor of success and can depend on both the available instrumentation and the skill of the operator.
Caveats about brachytherapy
The evidence in support of combining androgen deprivation therapy and interstitial brachytherapy is poor, and there is some evidence of increased rates of irritative voiding symptoms,27 so this is generally not recommended.
Interstitial brachytherapy as monotherapy has usually been reserved for patients with low-risk cancer with a low likelihood of extracapsular disease extension or pelvic lymph node involvement. No randomized controlled clinical trial has compared brachytherapy with radical prostatectomy or external beam radiotherapy. One large long-term study reported an 8-year biochemical recurrence rate of 18% in patients with low-risk cancer and 30% in patients with intermediate-risk cancer.28 The long-term efficacy of brachytherapy for intermediate- and high-risk prostate cancer is still under investigation.
Advantages and disadvantages of interstitial brachytherapy
Advantages. Interstitial brachytherapy is done as a single outpatient procedure. It can deliver a targeted high dose of radiation. And it is associated with a lower rate of posttreatment incontinence than radical prostatectomy, and a lower cost.
Disadvantages. There are limited data to support long-term cancer control in intermediate- and high-risk disease. Short-term adverse effects include dysuria, hematuria, urinary urgency, and urinary frequency in up to 80% of patients.29 Voiding symptoms typically peak 1 to 3 months after the procedure and subside after 8 to 12 months. Erectile dysfunction has been reported in 30% to 35% of men at 5 years after the procedure. Other possible adverse effects include urethral stricture, incontinence, recurrent hematuria, rectal bleeding, proctitis, and the development of bladder cancer and other secondary cancers.
EXTERNAL BEAM RADIOTHERAPY
In external beam radiotherapy, radiation is delivered to the prostate and surrounding tissues via an external energy source. Electrons, protons, or neutrons are used, and although each has theoretical advantages over the others, all appear to have similar clinical efficacy.
As with brachytherapy, the object—and the challenge—is to deliver an effective dose of radiation to the tumor while sparing adjacent organs. Intensity-modulated delivery is a radiotherapy technique that delivers more of the radiation dose where we want it to go—and less where we don’t want it to go. For prostate cancer, the target dose with intensity-modulated delivery is typically 75 to 85 Gy, in doses of 2 to 2.25 Gy for 30 to 36 days.
Androgen deprivation therapy before or after external beam radiotherapy augments the effects of the radiotherapy, particularly in patients with high-risk disease.30
The oncologic efficacy of intensity-modulated radiotherapy in patients at low and intermediate risk appears commensurate with that of radical prostatectomy. In one study,31 in low-risk cases, biochemical disease-free survival rates were 85% for radiotherapy vs 93% for prostatectomy; in intermediate-risk cases, 82% for radiotherapy and 87% for prostatectomy; and in high-risk cases, 62% for combined androgen deprivation and radiotherapy vs 38% for prostatectomy.31
Advantages and disadvantages of external beam radiotherapy
Advantages. External beam radiotherapy is noninvasive. It can treat the prostate as well as areas outside the prostate in patients with intermediate- and high-risk disease, and it is proven effective for high-risk cancer when used in combination with androgen deprivation.
Disadvantages. On the other hand, radiotherapy requires a series of daily treatments, which can be inconvenient and burdensome to the patient. Its adverse effects are similar to those of brachytherapy, and it is expensive. Long-term adverse effects include irritative voiding symptoms (frequency, urgency, nocturia), hemorrhagic cystitis, bowel symptoms (pain with defecation, tenesmus, bleeding), and a significantly higher lifetime risk of a secondary malignancy, particularly of the bladder and rectum.32
External beam radiotherapy also induces tissue changes in the pelvis that make salvage surgery more difficult. Patients in whom radiotherapy is ineffective as monotherapy and who require salvage prostatectomy typically have poor outcomes in terms of disease control, continence, and potency.
COMBINED RADIATION THERAPY: BETTER, OR OVERTREATMENT?
Many patients are offered a combination of external beam radiotherapy and interstitial brachytherapy. The rationale is that the combination can boost the dose of radiation to the prostate and at the same time treat cancer that has extended beyond the prostate or to the pelvic lymph nodes.
The radiation dose in the combined approach is 45 to 50 Gy (vs 70 to 80 Gy in monotherapy), thereby minimizing toxicity.
This combination has not been shown to improve overall survival or cancer-specific survival compared with either therapy alone, and it likely constitutes overtreatment.33 Adverse effects of combination therapy include erectile dysfunction, rectal and bladder toxicity, and secondary malignancy.
A serious complication associated more often with the combination of external beam radiotherapy and brachytherapy than other treatments is rectoprostatic fistula, a condition that requires complex reconstructive surgery and often requires permanent urinary and fecal diversion.34
CRYOTHERAPY: MORE STUDY NEEDED
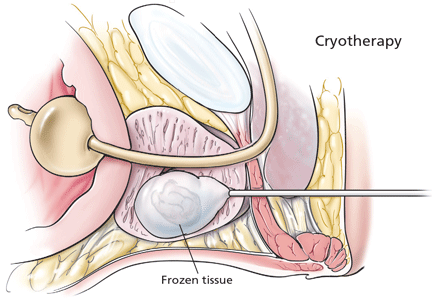
Refinements in cryoablative therapy to destroy prostate tissue have improved the safety and efficacy of this procedure significantly over the past decade. The AUA consensus guidelines recognize cryotherapy as a viable primary cancer monotherapy, but it is most commonly used as a salvage therapy after failure of radiation therapy.
The procedure involves ultrasonographically guided stereotactic placement of cryoprobes into the prostate via a transperineal approach. Argon is pumped through the probes under pressure to initiate ice formation, and repeated freeze-thaw cycles cause tissue damage and necrosis.
Rates of biochemical recurrence at 5 years in patients at low, intermediate, and high risk have been reported at 16%, 27%, and 25%, respectively.35 The presence of viable cancer on biopsy specimens after primary cryoablation has been reported at 15%, compared with 25% after definitive radiation therapy.35
Advantages and disadvantages of cryotherapy
Cryotherapy can destroy cancer tissue in a minimally invasive way. It has no long-term delayed adverse effects, and it is a low-cost and convenient outpatient procedure.
On the other hand, we lack long-term data on its oncologic efficacy, acute complications, and late adverse effects. Acute complications occur in up to 16% of patients and include acute urinary retention requiring prolonged catheterization, hematuria, urethral sloughing, perineal pain, and incontinence.36 Potential late effects include rectoprostatic fistula (< 1%), incontinence (< 5%), persistent hematuria, and chronic pelvic pain.36
Cryoablation therapy appears to have a more significant negative impact on sexual function than does brachytherapy.37
More study of the complications and efficacy of cryotherapy is needed before the procedure can be adopted as routine primary monotherapy.
HIGH-INTENSITY FOCUSED ULTRASOUND: NOT YET FDA-APPROVED
High-intensity focused ultrasound (HIFU) is not yet approved by the US Food and Drug Administration (other than in an approved research protocol) but is used in Canada and in certain countries of Europe and Asia. It involves the insertion of a transducer into the rectum that generates a high-intensity, focused beam that heats target tissue in the prostate to a high temperature. This temperature triggers a heat-shock response that leads to cellular apoptosis and tissue necrosis. The procedure can be done with or without magnetic resonance imaging (MRI) guidance.
Biochemical recurrence rates at 2 years after the procedure have been reported between 23% and 50%, but long-term efficacy data are lacking.38,39
Advantages and disadvantages of ultrasound
HIFU is a minimally invasive, low-cost, outpatient procedure that offers trackless delivery of energy to the prostate: ie, there is no direct mechanical penetration into the tissue.
Complications include rectal-wall injury, fistula, acute urinary retention, hematuria, and urethral stricture.
FOCAL ABLATION: GETTING ATTENTION, BUT STILL UNDER DEVELOPMENT
Focal ablation for prostate cancer has been receiving much attention. This treatment uses heat energy to destroy tumor cells, guided by high-resolution endorectal-coil MRI. The procedure is in the developmental stages and is available only in research protocols.
The procedure has several major hurdles to overcome before becoming acceptable for clinical practice. First, prostate cancer is multifocal, and microscopic tumor foci are likely present that are invisible even to MRI, so ablation of only part of the prostate leaves the rest of the gland at risk of continued or de novo tumor growth.
Second, a wide range of sensitivities and specificities have been reported for endorectal coil MRI for detecting prostate cancer: its sensitivity has ranged from 27% to 100%, and its specificity has ranged from 32% to 99%.40
ANDROGEN DEPRIVATION, AN ADJUVANT THERAPY
Androgen deprivation therapy (medical castration) is not effective as a monotherapy for prostate cancer. A large population-based study in men with localized prostate cancer showed no higher rate of overall survival at 10 years with primary androgen deprivation therapy than with conservative management.41
Androgen deprivation is achieved with a leutinizing hormone-releasing hormone agonist such as leuprolide (Lupron) or goserelin (Zoladex), or an antiandrogen drug such as flutamide or bicalutamide (Casodex), or a combination of each.
Adverse effects include hot flashes, gynecomastia, decreased libido, erectile dysfunction, weight gain, and hyperlipidemia. Long-term effects include osteoporosis and a significantly higher risk of cardiac events, new-onset type 2 diabetes mellitus, and stroke.
Currently, the only recognized role for androgen deprivation therapy in prostate cancer is as an adjunct to external beam radiotherapy or as a treatment of metastatic prostate cancer.
Orchiectomy
The other way to eliminate testicular production of testosterone is surgical castration. Bilateral orchiectomy has advantages over medical androgen deprivation therapy in that it costs less, is highly reliable, and is done as a single treatment on an outpatient basis. Disadvantages include surgery-related morbidity and the irreversible nature of the procedure. The adverse effects are similar to those of androgen deprivation therapy.
POSTTREATMENT MONITORING
The management of patients with recurrent prostate cancer can be complex, and these patients should be referred to a medical or urologic oncologist.42,43
Often, a rise in PSA after primary therapy represents a regrowth of cancer; 30% to 60% of patients with a recurrence have metastasis, and nearly 20% will die from the disease. The average time from documentation of biochemical recurrence to metastatic progression is 8 years. The average time from metastatic progression to death is 5 years.44,45
After radical prostatectomy, the PSA level should be checked every 6 to 12 months for the first 2 years, then annually until the patient’s life expectancy is only 10 years even without prostate cancer. PSA should reach undetectable levels within 4 to 6 weeks after surgery. Biochemical recurrence after surgery is defined as a PSA level of 0.2 μg/L or higher in two serial studies.
After radiation therapy or cryotherapy, monitoring is complicated by the presence of viable prostatic epithelium that continues to produce PSA. During the first 1 to 2 years after radiation therapy, a PSA “bounce” phenomenon is observed whereby PSA levels rise or fluctuate significantly. This bounce should not be mistaken for a recurrence of cancer. The most widely accepted definition of biochemical recurrence is based on the American Society for Therapeutic Radiology and Oncology “Phoenix” criteria, defined as the nadir PSA level plus 2.0 μg/L.46
- Thompson I, Thrasher JB, Aus G, et al; AUA Prostate Cancer Clinical Guideline Update Panel. Guideline for the management of clinically localized prostate cancer: 2007 update. J Urol 2007; 177:2106–2131.
- Brooks DD, Wolf A, Smith RA, Dash C, Guessous I. Prostate cancer screening 2010: updated recommendations from the American Cancer Society. J Natl Med Assoc 2010; 102:423–429.
- US Preventive Services Task Force. Screening for prostate cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med 2008; 149:185–191.
- Okotie OT, Roehl KA, Han M, Loeb S, Gashti SN, Catalona WJ. Characteristics of prostate cancer detected by digital rectal examination only. Urology 2007; 70:1117–1120.
- Philip J, Dutta Roy S, Ballal M, Foster CS, Javlé P. Is a digital rectal examination necessary in the diagnosis and clinical staging of early prostate cancer? BJU Int 2005; 95:969–971.
- Thompson IM, Ankerst DP, Chi C, et al. Assessing prostate cancer risk: results from the Prostate Cancer Prevention Trial. J Natl Cancer Inst 2006; 98:529–534.
- Catalona WJ, Partin AW, Slawin KM, et al. Use of the percentage of free prostate-specific antigen to enhance differentiation of prostate cancer from benign prostatic disease: a prospective multicenter clinical trial. JAMA 1998; 279:1542–1547.
- Terris MK, Freiha FS, McNeal JE, Stamey TA. Efficacy of transrectal ultrasound for identification of clinically undetected prostate cancer. J Urol 1991; 146:78–83.
- Epstein JI, Herawi M. Prostate needle biopsies containing prostatic intraepithelial neoplasia or atypical foci suspicious for carcinoma: implications for patient care. J Urol 2006 Mar; 175( 3 Pt1):820–34.
- D’Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA 1998; 280:969–974.
- Greene FL. American Joint Committee on Cancer. American Cancer Society. AJCC cancer staging manual. 6th ed. New York, NY: Springer-Verlag; 2002.
- Stephenson AJ, Kattan MW, Eastham JA, et al. Prostate cancer-specific mortality after radical prostatectomy for patients treated in the prostate-specific antigen era. J Clin Oncol 2009; 27:4300–4305.
- Eastham JA, Scardino PT, Kattan MW. Predicting an optimal outcome after radical prostatectomy: the trifecta nomogram. J Urol 2008; 179:2207–2210.
- Stephenson AJ, Scardino PT, Eastham JA, et al. Preoperative nomogram predicting the 10-year probability of prostate cancer recurrence after radical prostatectomy. J Natl Cancer Inst 2006; 98:715–717.
- Potters L, Roach M, Davis BJ, et al. Postoperative nomogram predicting the 9-year probability of prostate cancer recurrence after permanent prostate brachytherapy using radiation dose as a prognostic variable. Int J Radiat Oncol Biol Phys 2010; 76:1061–1065.
- Zelefsky MJ, Kattan MW, Fearn P, et al. Pretreatment nomogram predicting ten-year biochemical outcome of three-dimensional conformal radiotherapy and intensity-modulated radiotherapy for prostate cancer. Urology 2007; 70:283–287.
- Walz J, Gallina A, Saad F, et al. A nomogram predicting 10-year life expectancy in candidates for radical prostatectomy or radiotherapy for prostate cancer. J Clin Oncol 2007; 25:3576–3581.
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40:373–383.
- Hall WH, Ramachandran R, Narayan S, Jani AB, Vijayakumar S. An electronic application for rapidly calculating Charlson comorbidity score. BMC Cancer 2004; 4:94.
- Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982; 5:649–655.
- Bill-Axelson A, Holmberg L, Ruutu M, et al; Scandinavian Prostate Cancer Group Study No. 4. Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med 2005; 352:1977–1984.
- Widmark A, Klepp O, Solberg A, et al; Scandinavian Prostate Cancer Group Study 7. Endocrine treatment, with or without radiotherapy, in locally advanced prostate cancer (SPCG-7/SFUO-3): an open randomised phase III trial. Lancet 2009; 373:301–308.
- Krakowsky Y, Loblaw A, Klotz L. Prostate cancer death of men treated with initial active surveillance: clinical and biochemical characteristics. J Urol 2010; 184:131–135.
- Ficarra V, Novara G, Artibani W, et al. Retropubic, laparoscopic, and robot-assisted radical prostatectomy: a systematic review and cumulative analysis of comparative studies. Eur Urol 2009; 55:1037–1063.
- Hernandez DJ, Nielsen ME, Han M, Partin AW. Contemporary evaluation of the D’amico risk classification of prostate cancer. Urology 2007; 70:931–935.
- Messing EM, Manola J, Yao J, et al; Eastern Cooperative Oncology Group study EST 3886. Immediate versus deferred androgen deprivation treatment in patients with node-positive prostate cancer after radical prostatectomy and pelvic lymphadenectomy. Lancet Oncol 2006; 7:472–479.
- Beyer DC, McKeough T, Thomas T. Impact of short course hormonal therapy on overall and cancer specific survival after permanent prostate brachytherapy. Int J Radiat Oncol Biol Phys 2005; 61:1299–1305.
- Zelefsky MJ, Kuban DA, Levy LB, et al. Multi-institutional analysis of long-term outcome for stages T1-T2 prostate cancer treated with permanent seed implantation. Int J Radiat Oncol Biol Phys 2007; 67:327–333.
- Gelblum DY, Potters L, Ashley R, Waldbaum R, Wang XH, Leibel S. Urinary morbidity following ultrasound-guided transperineal prostate seed implantation. Int J Radiat Oncol Biol Phys 1999; 45:59–67.
- Bolla M, Collette L, Blank L, et al. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): a phase III randomised trial. Lancet 2002; 360:103–106.
- Aizer AA, Yu JB, Colberg JW, McKeon AM, Decker RH, Peschel RE. Radical prostatectomy vs intensity-modulated radiation therapy in the management of localized prostate adenocarcinoma. Radiother Oncol 2009; 93:185–191.
- Moon K, Stukenborg GJ, Keim J, Theodorescu D. Cancer incidence after localized therapy for prostate cancer. Cancer 2006; 107:991–998.
- Terakedis BE, Rossi PJ, Liauw SL, Johnstone PA, Jani AB. A surveillance, epidemiology, and end results registry analysis of prostate cancer modality time trends by age. Am J Clin Oncol 2010; 33:619–623.
- Lane BR, Stein DE, Remzi FH, Strong SA, Fazio VW, Angermeier KW. Management of radiotherapy induced rectourethral fistula. J Urol 2006; 175:1382–1387.
- Jones JS, Rewcastle JC, Donnelly BJ, Lugnani FM, Pisters LL, Katz AE. Whole gland primary prostate cryoablation: initial results from the cryo on-line data registry. J Urol 2008; 180:554–558.
- Hubosky SG, Fabrizio MD, Schellhammer PF, Barone BB, Tepera CM, Given RW. Single center experience with third-generation cryosurgery for management of organ-confined prostate cancer: critical evaluation of short-term outcomes, complications, and patient quality of life. J Endourol 2007; 21:1521–1531.
- Malcolm JB, Fabrizio MD, Barone BB, et al. Quality of life after open or robotic prostatectomy, cryoablation or brachytherapy for localized prostate cancer. J Urol 2010; 183:1822–1828.
- Ficarra V, Antoniolli SZ, Novara G, et al. Short-term outcome after high-intensity focused ultrasound in the treatment of patients with high-risk prostate cancer. BJU Int 2006; 98:1193–1198.
- Challacombe BJ, Murphy DG, Zakri R, Cahill DJ. High-intensity focused ultrasound for localized prostate cancer: initial experience with a 2-year follow-up. BJU Int 2009; 104:200–204.
- Bouchelouche K, Turkbey B, Choyke P, Capala J. Imaging prostate cancer: an update on positron emission tomography and magnetic resonance imaging. Curr Urol Rep 2010; 11:180–190.
- Lu-Yao GL, Albertsen PC, Moore DF, et al. Survival following primary androgen deprivation therapy among men with localized prostate cancer. JAMA 2008; 300:173–181.
- Simmons MN, Stephenson AJ, Klein EA. Natural history of biochemical recurrence after radical prostatectomy: risk assessment for secondary therapy. Eur Urol 2007; 51:1175–1184.
- Boukaram C, Hannoun-Levi JM. Management of prostate cancer recurrence after definitive radiation therapy. Cancer Treat Rev 2010; 36:91–100.
- Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC. Natural history of progression after PSA elevation following radical prostatectomy. JAMA 1999; 281:1591–1597.
- Freedland SJ, Humphreys EB, Mangold LA, et al. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA 2005; 294:433–439.
- Roach M, Hanks G, Thames H, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys 2006; 65:965–974.
Prostate cancer screening, diagnosis, and treatment present challenges to internists, urologists, and oncologists. For the internist, there is the ongoing debate about when and how often to screen with prostate-specific antigen (PSA) testing, as well as about how to interpret the results. For urologists and oncologists, there is no consensus on how to treat prostate cancer with the growing array of options, from surgery to cryoablation. Most therapies have not been compared in head-to-head trials, and anxious patients often approach their internist for help in navigating the maze of options.
This review summarizes current American Urological Association (AUA) guidelines,1 as well as current practice patterns at the Glickman Urological and Kidney Institute of Cleveland Clinic regarding screening, diagnosis, risk assessment, treatment, and posttreatment management of prostate cancer. We try to explain the approved and the experimental treatments, outlining what we know about their advantages and disadvantages.
SCREENING: WHEN AND HOW
Screening for prostate cancer should involve both a digital rectal examination (DRE) and measurement of the serum PSA level. But when should screening start?
The AUA recommends annual screening with DRE and serum PSA test starting at age 40 for all men with a life expectancy of more than 10 years.1
The American Cancer Society2 and the American College of Physicians,3 in contrast, recommend that men who choose to undergo screening should begin at age 50, or at age 45 if they are black or have a family history of prostate cancer in a primary relative diagnosed before age 65. They also recommend that screening with PSA and DRE be stopped at age 75, given the low likelihood of death from de novo prostate cancer after this age. The AUA recommends that screening be stopped at age 75, but may be continued beyond age 75 if the patient has a life expectancy of 10 years or more.
Before being screened, patients should understand the benefits and the risks of testing. While a small subset of prostate cancers behave aggressively, the majority are slow-growing and pose minimal risk for the development of fatal disease.
A discussion of the rationale for these guidelines and their differences is beyond the scope of this review. Differences stem from the observation that most men treated for prostate cancer will likely not die from prostate cancer, but rather from another condition.
Digital rectal examination’s role and limitations
The utility of DRE is limited to the detection of nodules, gross asymmetry, and gland fixation. DRE is not highly specific: only 40% to 50% of men who have abnormal findings on DRE have prostate cancer on biopsy.5 Anyone who has an abnormal finding on DRE should undergo prostate biopsy. However, if a rectal mass is palpated or if the prostate is exquisitely sensitive, biopsy is not indicated.
DRE is highly inaccurate for estimating gland volume; it should not be used to gauge cancer risk.
Prostate-specific antigen: Caveats
PSA measurement was introduced as a clinical screening test for prostate cancer in the early 1990s, and it serves as the foundation for early detection.
PSA, a protein involved in seminal coagulation, is produced by the prostate epithelium and is mostly confined within the prostatic ducts. Cancer cells secrete PSA into the bloodstream at increased levels via a disrupted basement membrane in tumor-affected areas of the gland. Elevated PSA can also result from benign prostatic hypertrophy, prostatitis, and prostate biopsy.
PSA levels represent a continuum of prostate cancer risk, and no single PSA value is sensitive and specific enough to predict the presence of cancer.6 Abnormal PSA cutoffs have been defined from 2.5 μg/L to 4 μg/L, and much debate surrounds this topic. Men who present with an elevated PSA (ie, > 2.5 μg/L) should be tested again. If the value remains high, then prostate biopsy should be considered. An elevated PSA level in older men with benign prostatic hypertrophy is not unexpected, and in these patients observation of the PSA value over time may prove valuable to assess the need for biopsy.
A useful adjunct in men with elevated PSA and benign prostatic hypertrophy is the percentage of serum PSA that is free rather than bound.7 PSA produced by prostate cancer binds more avidly with serum proteins (alpha-1 chymotrypsin and alpha-2 macroglobulin), resulting in a lower percentage of free PSA. In men with an elevated PSA (ie, 4.1–10.0 μg/L), the percentage of free PSA provides an indication of whether the elevation is due to benign prostatic hypertrophy or to cancer: the lower the percent free PSA, the more likely an elevated total PSA represents cancer and not benign prostatic hypertrophy. The sensitivity of a free PSA less than 15% to detect prostate cancer is about 85%, and its use as a screening tool is under study.
Much attention has also been given to other PSA indices, namely, the PSA density (the PSA level divided by the prostate volume), the PSA velocity (the rate of increase in the PSA level over time), and the PSA doubling time. While these nuanced PSA measures are useful to predict disease severity and behavior, they are not routinely used in screening.
BIOPSY IS INDICATED IF EITHER TEST IS ABNORMAL
In the past, imaging of the prostate with transrectal ultrasonography was used as a screening tool to detect prostate cancer. Further research showed that only 15% to 20% of hypoechoic lesions detected on ultrasonography contained cancer.8 Because of its low sensitivity and specificity, primary ultrasonographic screening (ie, transrectal ultrasonography alone) is not acceptable for screening or for diagnosis. Its main role is in guiding prostate biopsy.
Biopsy of the prostate with transrectal ultrasonographic guidance is indicated if either the DRE or the PSA level is abnormal. The standard of care is to use an 18-gauge biopsy needle-gun to obtain two to three tissue samples from each of six regions of the prostate, focusing on the outer peripheral zone, specifically the right and left bases, the mid-gland, and the apex.
Pathologic analysis of each tissue core takes into consideration the presence or absence of cancer, the Gleason score, and the percentage of the tissue sample volume that is occupied by cancer.
The Gleason grading system is based on the histologic appearance and reflects the degree of differentiation and aggressiveness of the cancer. The two most prominent tumor grades present are added to give a final Gleason score. For instance, a Gleason grade of 4+3=7 indicates a tumor with predominant Gleason grade 4 disease with a lesser amount of grade 3 disease. The number of positive core samples and the volume of cancer provide information on the severity of the cancer.
If the PSA is high but biopsy is negative
Prostate biopsy misses up to 30% of small cancers. Many of these are clinically insignificant, but about 20% of those missed cancers can be high-risk and thus merit identification. There should be a low threshold for repeating biopsy 1 year later in men who have a persistently high PSA or a rising PSA.
High-grade prostatic intraepithelial neoplasia is a common finding on biopsy. The incidence of de novo prostate cancer at 5 years in men with this finding is 22% to 26%.9 Patients with multifocal high-grade prostatic intraepithelial neoplasia should be monitored with PSA testing and DRE every 6 to 12 months and should be considered for repeat “saturation” biopsy (ie, obtaining as many as 36 core samples).
IF CANCER IS FOUND, HOW RISKY IS IT?
Patients with a new diagnosis of prostate cancer must decide on a treatment plan. This decision is highly individualized, based on the patient’s personal preferences, lifestyle, performance status (ie, his general well-being), disease severity, continence status, and sexual function.
When counseling patients about their disease and the treatment options, we consider three main factors:
- The severity of disease on biopsy
- The patient’s current state of health and performance status
- The patient’s understanding of and willingness to accept the adverse effects of the various treatments.
Pathologic features, the PSA level, and clinical stage determined by DRE are used to predict the severity of disease. Most data on the efficacy of treatments for prostate cancer are based on the incidence of biochemical recurrence, ie, a rise in PSA level after primary therapy. The AUA and the D’Amico risk criteria use biopsy pathology, clinical stage, and the pretreatment PSA level to predict the likelihood of biochemical recurrence (Table 1).10,11
DISCUSSING TREATMENT OPTIONS WITH THE PATIENT
In our practice, we usually do not recommend treatment in men with low-risk or intermediate-risk prostate cancer who have a life expectancy of less than 10 years, as most of them will likely die of a cause other than prostate cancer. For patients with poor baseline performance status, surveillance or radiation therapy may be preferable to surgery. In younger patients, surgery may confer a more durable benefit.
- No prospective, randomized clinical trials have directly compared these treatments
- Prostate cancer progresses slowly
- Definitions of treatment failure used in various studies have been inconsistent
- Clinical studies have been subject to selection bias.
ACTIVE SURVEILLANCE IS ACCEPTABLE FOR LOW-RISK PROSTATE CANCER
Active surveillance is an acceptable option for patients with low-risk prostate cancer (ie, if the Gleason score is ≤ 6, the tumor stage is T1c or T2a, and the PSA level is ≤ 10 μg/L). To rule out high-risk disease before starting a program of surveillance, repeat biopsy is advisable, although optional.
Active surveillance consists of PSA testing and DRE every 6 to 12 months, followed by repeat biopsy if significant changes are noted in either test. Some centers advocate biopsy with transrectal ultrasonographic guidance every year regardless of the PSA or DRE findings.
Whether a change in the PSA level is significant is subjective, but a recent phase 2 study in 453 patients23 on a program of active surveillance used a PSA doubling time of less than 3 years as a criterion for repeat biopsy. Thirty-eight percent of the men had to undergo radiation therapy or surgery within 10 years, and 5 patients (1%) died of prostate cancer. The authors concluded that active surveillance did not put these patients at undue risk, and that this approach prevented overtreatment of clinically insignificant prostate cancer.23
The risks of surveillance include the chance that cancer could progress to an incurable state during the surveillance period, greater anxiety for the patient, and, if prostatectomy becomes necessary, greater technical difficulty due to scarring from repeat biopsies. The benefit is postponement or complete avoidance of the adverse effects of treatment.
Debate continues over the potential dangers of deferred treatment of prostate cancer, but in certain patients it is an acceptable option. Patient education, accurate disease assessment, and compliance with monitoring are critical considerations.
RADICAL PROSTATECTOMY: SEVERAL OPTIONS, EQUIVALENT EFFICACY
Radical prostatectomy is widely used for treating prostate cancer of any risk level. The operation entails removing the prostate and seminal vesicles, as well as the pelvic lymph nodes in patients with intermediate or high-risk cancer.
This procedure was increasingly used in the 1990s with the introduction of PSA screening and nerve-sparing surgical techniques that preserve continence and erectile function.
Radical prostatectomy can be done via a standard open approach or a minimally invasive laparoscopic approach with or without robotic assistance. Open surgery, laparoscopic surgery, and robotic prostatectomy offer equivalent rates of oncologic efficacy, continence, and potency.24 The more experienced the surgeon, the better the outcome is likely to be.
The average biochemical recurrence rate at 5 years after radical prostatectomy is approximately 6% for patients with low-risk cancer, 23% for those with intermediate-risk cancer, and 45% for those with high-risk cancer.25 The rate of death from prostate cancer at 10 years is about 1% for patients with low-risk cancer, 4% for those with intermediate-risk cancer, and 8% for those with high-risk cancer.12
Secondary therapy
Pathologic staging of the surgical specimen after radical prostatectomy yields information that can be beneficial in terms of initiating early secondary therapy.
Patients with node-positive disease should immediately undergo androgen deprivation treatment.26
Evidence of positive surgical margins, seminal vesicle invasion, bladder neck invasion, and extracapsular extension also increase the risk of recurrence. This additional risk can be ascertained via the use of a postoperative nomogram. Patients at high risk of recurrence should be considered for early adjuvant external beam radiotherapy to the surgical field 3 to 6 months after surgery.
Advantages and disadvantages of radical prostatectomy
Advantages of radical prostatectomy include the ability to accurately stage the cancer with the surgical specimen and the ability to remove the pelvic lymph nodes in patients at intermediate and high risk. Another advantage is that postoperative surveillance is straightforward: PSA should become undetectable after surgery, and a measurable increase in PSA represents disease recurrence.
Disadvantages include:
- The risk of surgical complications (reported in 3% to 17% of cases)24
- An average hospital stay of 1 to 3 days (and a typical 3 to 6 weeks before returning to work)
- The need for a Foley catheter for 10 to 14 days
- The risk of incontinence and impotence, which are very distressing to patients.
Postoperative incontinence is typically defined as the need for any type of protective pad for leakage. Up to 70% of patients have incontinence in the first 3 months after surgery, but 82% to 94% of patients regain continence by 12 months.24 A small percentage of patients (3% to 5%) have significant permanent incontinence.
Counseling about postoperative erectile dysfunction
All patients should be counseled about the risk of a postoperative decrease in erectile function, especially those with pre-existing erectile dysfunction. Potency is defined as the ability to have an erection suitable for intercourse (with or without phosphodiesterase type 5 inhibitors) more than 50% of the time. In men with bilateral nerve-sparing open prostatectomy, potency rates at 12 months have been reported between 63% and 81%.13
Data on potency rates vary widely because of differences in how potency was defined, selection bias, and the multifactorial nature of erectile dysfunction. Also, because single-institution, single-surgeon reports and advertisements tend to underestimate rates of impotence after radical prostatectomy by any approach, many patients have false expectations.
INTERSTITIAL BRACHYTHERAPY FOR LOW-RISK CANCERS
Interstitial brachytherapy delivers a localized, high dose (125 to 145 Gy) of radiation to the prostate, with minimal radiation dosing to the bladder, rectum, or other adjacent organs and tissues. “Seeds” or small pellets containing a radioisotope (iodine 125 or palladium 103) are stereotactically implanted through the perineum into the prostate under ultrasonographic guidance. Computerized mapping done before or during surgery helps determine the optimal placement of the seeds, the object being to cover at least 90% of the prostate with 100% of the radiation dose.
In permanent brachytherapy, the implants give off radiation at a low dose rate over weeks to months and are left in place permanently. In temporary brachytherapy, seeds are implanted to deliver a low or high dose rate for a specified period, and then they are removed.
“Implant quality,” ie, delivery of more than 90% of the radiation dose, is a major predictor of success and can depend on both the available instrumentation and the skill of the operator.
Caveats about brachytherapy
The evidence in support of combining androgen deprivation therapy and interstitial brachytherapy is poor, and there is some evidence of increased rates of irritative voiding symptoms,27 so this is generally not recommended.
Interstitial brachytherapy as monotherapy has usually been reserved for patients with low-risk cancer with a low likelihood of extracapsular disease extension or pelvic lymph node involvement. No randomized controlled clinical trial has compared brachytherapy with radical prostatectomy or external beam radiotherapy. One large long-term study reported an 8-year biochemical recurrence rate of 18% in patients with low-risk cancer and 30% in patients with intermediate-risk cancer.28 The long-term efficacy of brachytherapy for intermediate- and high-risk prostate cancer is still under investigation.
Advantages and disadvantages of interstitial brachytherapy
Advantages. Interstitial brachytherapy is done as a single outpatient procedure. It can deliver a targeted high dose of radiation. And it is associated with a lower rate of posttreatment incontinence than radical prostatectomy, and a lower cost.
Disadvantages. There are limited data to support long-term cancer control in intermediate- and high-risk disease. Short-term adverse effects include dysuria, hematuria, urinary urgency, and urinary frequency in up to 80% of patients.29 Voiding symptoms typically peak 1 to 3 months after the procedure and subside after 8 to 12 months. Erectile dysfunction has been reported in 30% to 35% of men at 5 years after the procedure. Other possible adverse effects include urethral stricture, incontinence, recurrent hematuria, rectal bleeding, proctitis, and the development of bladder cancer and other secondary cancers.
EXTERNAL BEAM RADIOTHERAPY
In external beam radiotherapy, radiation is delivered to the prostate and surrounding tissues via an external energy source. Electrons, protons, or neutrons are used, and although each has theoretical advantages over the others, all appear to have similar clinical efficacy.
As with brachytherapy, the object—and the challenge—is to deliver an effective dose of radiation to the tumor while sparing adjacent organs. Intensity-modulated delivery is a radiotherapy technique that delivers more of the radiation dose where we want it to go—and less where we don’t want it to go. For prostate cancer, the target dose with intensity-modulated delivery is typically 75 to 85 Gy, in doses of 2 to 2.25 Gy for 30 to 36 days.
Androgen deprivation therapy before or after external beam radiotherapy augments the effects of the radiotherapy, particularly in patients with high-risk disease.30
The oncologic efficacy of intensity-modulated radiotherapy in patients at low and intermediate risk appears commensurate with that of radical prostatectomy. In one study,31 in low-risk cases, biochemical disease-free survival rates were 85% for radiotherapy vs 93% for prostatectomy; in intermediate-risk cases, 82% for radiotherapy and 87% for prostatectomy; and in high-risk cases, 62% for combined androgen deprivation and radiotherapy vs 38% for prostatectomy.31
Advantages and disadvantages of external beam radiotherapy
Advantages. External beam radiotherapy is noninvasive. It can treat the prostate as well as areas outside the prostate in patients with intermediate- and high-risk disease, and it is proven effective for high-risk cancer when used in combination with androgen deprivation.
Disadvantages. On the other hand, radiotherapy requires a series of daily treatments, which can be inconvenient and burdensome to the patient. Its adverse effects are similar to those of brachytherapy, and it is expensive. Long-term adverse effects include irritative voiding symptoms (frequency, urgency, nocturia), hemorrhagic cystitis, bowel symptoms (pain with defecation, tenesmus, bleeding), and a significantly higher lifetime risk of a secondary malignancy, particularly of the bladder and rectum.32
External beam radiotherapy also induces tissue changes in the pelvis that make salvage surgery more difficult. Patients in whom radiotherapy is ineffective as monotherapy and who require salvage prostatectomy typically have poor outcomes in terms of disease control, continence, and potency.
COMBINED RADIATION THERAPY: BETTER, OR OVERTREATMENT?
Many patients are offered a combination of external beam radiotherapy and interstitial brachytherapy. The rationale is that the combination can boost the dose of radiation to the prostate and at the same time treat cancer that has extended beyond the prostate or to the pelvic lymph nodes.
The radiation dose in the combined approach is 45 to 50 Gy (vs 70 to 80 Gy in monotherapy), thereby minimizing toxicity.
This combination has not been shown to improve overall survival or cancer-specific survival compared with either therapy alone, and it likely constitutes overtreatment.33 Adverse effects of combination therapy include erectile dysfunction, rectal and bladder toxicity, and secondary malignancy.
A serious complication associated more often with the combination of external beam radiotherapy and brachytherapy than other treatments is rectoprostatic fistula, a condition that requires complex reconstructive surgery and often requires permanent urinary and fecal diversion.34
CRYOTHERAPY: MORE STUDY NEEDED
Refinements in cryoablative therapy to destroy prostate tissue have improved the safety and efficacy of this procedure significantly over the past decade. The AUA consensus guidelines recognize cryotherapy as a viable primary cancer monotherapy, but it is most commonly used as a salvage therapy after failure of radiation therapy.
The procedure involves ultrasonographically guided stereotactic placement of cryoprobes into the prostate via a transperineal approach. Argon is pumped through the probes under pressure to initiate ice formation, and repeated freeze-thaw cycles cause tissue damage and necrosis.
Rates of biochemical recurrence at 5 years in patients at low, intermediate, and high risk have been reported at 16%, 27%, and 25%, respectively.35 The presence of viable cancer on biopsy specimens after primary cryoablation has been reported at 15%, compared with 25% after definitive radiation therapy.35
Advantages and disadvantages of cryotherapy
Cryotherapy can destroy cancer tissue in a minimally invasive way. It has no long-term delayed adverse effects, and it is a low-cost and convenient outpatient procedure.
On the other hand, we lack long-term data on its oncologic efficacy, acute complications, and late adverse effects. Acute complications occur in up to 16% of patients and include acute urinary retention requiring prolonged catheterization, hematuria, urethral sloughing, perineal pain, and incontinence.36 Potential late effects include rectoprostatic fistula (< 1%), incontinence (< 5%), persistent hematuria, and chronic pelvic pain.36
Cryoablation therapy appears to have a more significant negative impact on sexual function than does brachytherapy.37
More study of the complications and efficacy of cryotherapy is needed before the procedure can be adopted as routine primary monotherapy.
HIGH-INTENSITY FOCUSED ULTRASOUND: NOT YET FDA-APPROVED
High-intensity focused ultrasound (HIFU) is not yet approved by the US Food and Drug Administration (other than in an approved research protocol) but is used in Canada and in certain countries of Europe and Asia. It involves the insertion of a transducer into the rectum that generates a high-intensity, focused beam that heats target tissue in the prostate to a high temperature. This temperature triggers a heat-shock response that leads to cellular apoptosis and tissue necrosis. The procedure can be done with or without magnetic resonance imaging (MRI) guidance.
Biochemical recurrence rates at 2 years after the procedure have been reported between 23% and 50%, but long-term efficacy data are lacking.38,39
Advantages and disadvantages of ultrasound
HIFU is a minimally invasive, low-cost, outpatient procedure that offers trackless delivery of energy to the prostate: ie, there is no direct mechanical penetration into the tissue.
Complications include rectal-wall injury, fistula, acute urinary retention, hematuria, and urethral stricture.
FOCAL ABLATION: GETTING ATTENTION, BUT STILL UNDER DEVELOPMENT
Focal ablation for prostate cancer has been receiving much attention. This treatment uses heat energy to destroy tumor cells, guided by high-resolution endorectal-coil MRI. The procedure is in the developmental stages and is available only in research protocols.
The procedure has several major hurdles to overcome before becoming acceptable for clinical practice. First, prostate cancer is multifocal, and microscopic tumor foci are likely present that are invisible even to MRI, so ablation of only part of the prostate leaves the rest of the gland at risk of continued or de novo tumor growth.
Second, a wide range of sensitivities and specificities have been reported for endorectal coil MRI for detecting prostate cancer: its sensitivity has ranged from 27% to 100%, and its specificity has ranged from 32% to 99%.40
ANDROGEN DEPRIVATION, AN ADJUVANT THERAPY
Androgen deprivation therapy (medical castration) is not effective as a monotherapy for prostate cancer. A large population-based study in men with localized prostate cancer showed no higher rate of overall survival at 10 years with primary androgen deprivation therapy than with conservative management.41
Androgen deprivation is achieved with a leutinizing hormone-releasing hormone agonist such as leuprolide (Lupron) or goserelin (Zoladex), or an antiandrogen drug such as flutamide or bicalutamide (Casodex), or a combination of each.
Adverse effects include hot flashes, gynecomastia, decreased libido, erectile dysfunction, weight gain, and hyperlipidemia. Long-term effects include osteoporosis and a significantly higher risk of cardiac events, new-onset type 2 diabetes mellitus, and stroke.
Currently, the only recognized role for androgen deprivation therapy in prostate cancer is as an adjunct to external beam radiotherapy or as a treatment of metastatic prostate cancer.
Orchiectomy
The other way to eliminate testicular production of testosterone is surgical castration. Bilateral orchiectomy has advantages over medical androgen deprivation therapy in that it costs less, is highly reliable, and is done as a single treatment on an outpatient basis. Disadvantages include surgery-related morbidity and the irreversible nature of the procedure. The adverse effects are similar to those of androgen deprivation therapy.
POSTTREATMENT MONITORING
The management of patients with recurrent prostate cancer can be complex, and these patients should be referred to a medical or urologic oncologist.42,43
Often, a rise in PSA after primary therapy represents a regrowth of cancer; 30% to 60% of patients with a recurrence have metastasis, and nearly 20% will die from the disease. The average time from documentation of biochemical recurrence to metastatic progression is 8 years. The average time from metastatic progression to death is 5 years.44,45
After radical prostatectomy, the PSA level should be checked every 6 to 12 months for the first 2 years, then annually until the patient’s life expectancy is only 10 years even without prostate cancer. PSA should reach undetectable levels within 4 to 6 weeks after surgery. Biochemical recurrence after surgery is defined as a PSA level of 0.2 μg/L or higher in two serial studies.
After radiation therapy or cryotherapy, monitoring is complicated by the presence of viable prostatic epithelium that continues to produce PSA. During the first 1 to 2 years after radiation therapy, a PSA “bounce” phenomenon is observed whereby PSA levels rise or fluctuate significantly. This bounce should not be mistaken for a recurrence of cancer. The most widely accepted definition of biochemical recurrence is based on the American Society for Therapeutic Radiology and Oncology “Phoenix” criteria, defined as the nadir PSA level plus 2.0 μg/L.46
Prostate cancer screening, diagnosis, and treatment present challenges to internists, urologists, and oncologists. For the internist, there is the ongoing debate about when and how often to screen with prostate-specific antigen (PSA) testing, as well as about how to interpret the results. For urologists and oncologists, there is no consensus on how to treat prostate cancer with the growing array of options, from surgery to cryoablation. Most therapies have not been compared in head-to-head trials, and anxious patients often approach their internist for help in navigating the maze of options.
This review summarizes current American Urological Association (AUA) guidelines,1 as well as current practice patterns at the Glickman Urological and Kidney Institute of Cleveland Clinic regarding screening, diagnosis, risk assessment, treatment, and posttreatment management of prostate cancer. We try to explain the approved and the experimental treatments, outlining what we know about their advantages and disadvantages.
SCREENING: WHEN AND HOW
Screening for prostate cancer should involve both a digital rectal examination (DRE) and measurement of the serum PSA level. But when should screening start?
The AUA recommends annual screening with DRE and serum PSA test starting at age 40 for all men with a life expectancy of more than 10 years.1
The American Cancer Society2 and the American College of Physicians,3 in contrast, recommend that men who choose to undergo screening should begin at age 50, or at age 45 if they are black or have a family history of prostate cancer in a primary relative diagnosed before age 65. They also recommend that screening with PSA and DRE be stopped at age 75, given the low likelihood of death from de novo prostate cancer after this age. The AUA recommends that screening be stopped at age 75, but may be continued beyond age 75 if the patient has a life expectancy of 10 years or more.
Before being screened, patients should understand the benefits and the risks of testing. While a small subset of prostate cancers behave aggressively, the majority are slow-growing and pose minimal risk for the development of fatal disease.
A discussion of the rationale for these guidelines and their differences is beyond the scope of this review. Differences stem from the observation that most men treated for prostate cancer will likely not die from prostate cancer, but rather from another condition.
Digital rectal examination’s role and limitations
The utility of DRE is limited to the detection of nodules, gross asymmetry, and gland fixation. DRE is not highly specific: only 40% to 50% of men who have abnormal findings on DRE have prostate cancer on biopsy.5 Anyone who has an abnormal finding on DRE should undergo prostate biopsy. However, if a rectal mass is palpated or if the prostate is exquisitely sensitive, biopsy is not indicated.
DRE is highly inaccurate for estimating gland volume; it should not be used to gauge cancer risk.
Prostate-specific antigen: Caveats
PSA measurement was introduced as a clinical screening test for prostate cancer in the early 1990s, and it serves as the foundation for early detection.
PSA, a protein involved in seminal coagulation, is produced by the prostate epithelium and is mostly confined within the prostatic ducts. Cancer cells secrete PSA into the bloodstream at increased levels via a disrupted basement membrane in tumor-affected areas of the gland. Elevated PSA can also result from benign prostatic hypertrophy, prostatitis, and prostate biopsy.
PSA levels represent a continuum of prostate cancer risk, and no single PSA value is sensitive and specific enough to predict the presence of cancer.6 Abnormal PSA cutoffs have been defined from 2.5 μg/L to 4 μg/L, and much debate surrounds this topic. Men who present with an elevated PSA (ie, > 2.5 μg/L) should be tested again. If the value remains high, then prostate biopsy should be considered. An elevated PSA level in older men with benign prostatic hypertrophy is not unexpected, and in these patients observation of the PSA value over time may prove valuable to assess the need for biopsy.
A useful adjunct in men with elevated PSA and benign prostatic hypertrophy is the percentage of serum PSA that is free rather than bound.7 PSA produced by prostate cancer binds more avidly with serum proteins (alpha-1 chymotrypsin and alpha-2 macroglobulin), resulting in a lower percentage of free PSA. In men with an elevated PSA (ie, 4.1–10.0 μg/L), the percentage of free PSA provides an indication of whether the elevation is due to benign prostatic hypertrophy or to cancer: the lower the percent free PSA, the more likely an elevated total PSA represents cancer and not benign prostatic hypertrophy. The sensitivity of a free PSA less than 15% to detect prostate cancer is about 85%, and its use as a screening tool is under study.
Much attention has also been given to other PSA indices, namely, the PSA density (the PSA level divided by the prostate volume), the PSA velocity (the rate of increase in the PSA level over time), and the PSA doubling time. While these nuanced PSA measures are useful to predict disease severity and behavior, they are not routinely used in screening.
BIOPSY IS INDICATED IF EITHER TEST IS ABNORMAL
In the past, imaging of the prostate with transrectal ultrasonography was used as a screening tool to detect prostate cancer. Further research showed that only 15% to 20% of hypoechoic lesions detected on ultrasonography contained cancer.8 Because of its low sensitivity and specificity, primary ultrasonographic screening (ie, transrectal ultrasonography alone) is not acceptable for screening or for diagnosis. Its main role is in guiding prostate biopsy.
Biopsy of the prostate with transrectal ultrasonographic guidance is indicated if either the DRE or the PSA level is abnormal. The standard of care is to use an 18-gauge biopsy needle-gun to obtain two to three tissue samples from each of six regions of the prostate, focusing on the outer peripheral zone, specifically the right and left bases, the mid-gland, and the apex.
Pathologic analysis of each tissue core takes into consideration the presence or absence of cancer, the Gleason score, and the percentage of the tissue sample volume that is occupied by cancer.
The Gleason grading system is based on the histologic appearance and reflects the degree of differentiation and aggressiveness of the cancer. The two most prominent tumor grades present are added to give a final Gleason score. For instance, a Gleason grade of 4+3=7 indicates a tumor with predominant Gleason grade 4 disease with a lesser amount of grade 3 disease. The number of positive core samples and the volume of cancer provide information on the severity of the cancer.
If the PSA is high but biopsy is negative
Prostate biopsy misses up to 30% of small cancers. Many of these are clinically insignificant, but about 20% of those missed cancers can be high-risk and thus merit identification. There should be a low threshold for repeating biopsy 1 year later in men who have a persistently high PSA or a rising PSA.
High-grade prostatic intraepithelial neoplasia is a common finding on biopsy. The incidence of de novo prostate cancer at 5 years in men with this finding is 22% to 26%.9 Patients with multifocal high-grade prostatic intraepithelial neoplasia should be monitored with PSA testing and DRE every 6 to 12 months and should be considered for repeat “saturation” biopsy (ie, obtaining as many as 36 core samples).
IF CANCER IS FOUND, HOW RISKY IS IT?
Patients with a new diagnosis of prostate cancer must decide on a treatment plan. This decision is highly individualized, based on the patient’s personal preferences, lifestyle, performance status (ie, his general well-being), disease severity, continence status, and sexual function.
When counseling patients about their disease and the treatment options, we consider three main factors:
- The severity of disease on biopsy
- The patient’s current state of health and performance status
- The patient’s understanding of and willingness to accept the adverse effects of the various treatments.
Pathologic features, the PSA level, and clinical stage determined by DRE are used to predict the severity of disease. Most data on the efficacy of treatments for prostate cancer are based on the incidence of biochemical recurrence, ie, a rise in PSA level after primary therapy. The AUA and the D’Amico risk criteria use biopsy pathology, clinical stage, and the pretreatment PSA level to predict the likelihood of biochemical recurrence (Table 1).10,11
DISCUSSING TREATMENT OPTIONS WITH THE PATIENT
In our practice, we usually do not recommend treatment in men with low-risk or intermediate-risk prostate cancer who have a life expectancy of less than 10 years, as most of them will likely die of a cause other than prostate cancer. For patients with poor baseline performance status, surveillance or radiation therapy may be preferable to surgery. In younger patients, surgery may confer a more durable benefit.
- No prospective, randomized clinical trials have directly compared these treatments
- Prostate cancer progresses slowly
- Definitions of treatment failure used in various studies have been inconsistent
- Clinical studies have been subject to selection bias.
ACTIVE SURVEILLANCE IS ACCEPTABLE FOR LOW-RISK PROSTATE CANCER
Active surveillance is an acceptable option for patients with low-risk prostate cancer (ie, if the Gleason score is ≤ 6, the tumor stage is T1c or T2a, and the PSA level is ≤ 10 μg/L). To rule out high-risk disease before starting a program of surveillance, repeat biopsy is advisable, although optional.
Active surveillance consists of PSA testing and DRE every 6 to 12 months, followed by repeat biopsy if significant changes are noted in either test. Some centers advocate biopsy with transrectal ultrasonographic guidance every year regardless of the PSA or DRE findings.
Whether a change in the PSA level is significant is subjective, but a recent phase 2 study in 453 patients23 on a program of active surveillance used a PSA doubling time of less than 3 years as a criterion for repeat biopsy. Thirty-eight percent of the men had to undergo radiation therapy or surgery within 10 years, and 5 patients (1%) died of prostate cancer. The authors concluded that active surveillance did not put these patients at undue risk, and that this approach prevented overtreatment of clinically insignificant prostate cancer.23
The risks of surveillance include the chance that cancer could progress to an incurable state during the surveillance period, greater anxiety for the patient, and, if prostatectomy becomes necessary, greater technical difficulty due to scarring from repeat biopsies. The benefit is postponement or complete avoidance of the adverse effects of treatment.
Debate continues over the potential dangers of deferred treatment of prostate cancer, but in certain patients it is an acceptable option. Patient education, accurate disease assessment, and compliance with monitoring are critical considerations.
RADICAL PROSTATECTOMY: SEVERAL OPTIONS, EQUIVALENT EFFICACY
Radical prostatectomy is widely used for treating prostate cancer of any risk level. The operation entails removing the prostate and seminal vesicles, as well as the pelvic lymph nodes in patients with intermediate or high-risk cancer.
This procedure was increasingly used in the 1990s with the introduction of PSA screening and nerve-sparing surgical techniques that preserve continence and erectile function.
Radical prostatectomy can be done via a standard open approach or a minimally invasive laparoscopic approach with or without robotic assistance. Open surgery, laparoscopic surgery, and robotic prostatectomy offer equivalent rates of oncologic efficacy, continence, and potency.24 The more experienced the surgeon, the better the outcome is likely to be.
The average biochemical recurrence rate at 5 years after radical prostatectomy is approximately 6% for patients with low-risk cancer, 23% for those with intermediate-risk cancer, and 45% for those with high-risk cancer.25 The rate of death from prostate cancer at 10 years is about 1% for patients with low-risk cancer, 4% for those with intermediate-risk cancer, and 8% for those with high-risk cancer.12
Secondary therapy
Pathologic staging of the surgical specimen after radical prostatectomy yields information that can be beneficial in terms of initiating early secondary therapy.
Patients with node-positive disease should immediately undergo androgen deprivation treatment.26
Evidence of positive surgical margins, seminal vesicle invasion, bladder neck invasion, and extracapsular extension also increase the risk of recurrence. This additional risk can be ascertained via the use of a postoperative nomogram. Patients at high risk of recurrence should be considered for early adjuvant external beam radiotherapy to the surgical field 3 to 6 months after surgery.
Advantages and disadvantages of radical prostatectomy
Advantages of radical prostatectomy include the ability to accurately stage the cancer with the surgical specimen and the ability to remove the pelvic lymph nodes in patients at intermediate and high risk. Another advantage is that postoperative surveillance is straightforward: PSA should become undetectable after surgery, and a measurable increase in PSA represents disease recurrence.
Disadvantages include:
- The risk of surgical complications (reported in 3% to 17% of cases)24
- An average hospital stay of 1 to 3 days (and a typical 3 to 6 weeks before returning to work)
- The need for a Foley catheter for 10 to 14 days
- The risk of incontinence and impotence, which are very distressing to patients.
Postoperative incontinence is typically defined as the need for any type of protective pad for leakage. Up to 70% of patients have incontinence in the first 3 months after surgery, but 82% to 94% of patients regain continence by 12 months.24 A small percentage of patients (3% to 5%) have significant permanent incontinence.
Counseling about postoperative erectile dysfunction
All patients should be counseled about the risk of a postoperative decrease in erectile function, especially those with pre-existing erectile dysfunction. Potency is defined as the ability to have an erection suitable for intercourse (with or without phosphodiesterase type 5 inhibitors) more than 50% of the time. In men with bilateral nerve-sparing open prostatectomy, potency rates at 12 months have been reported between 63% and 81%.13
Data on potency rates vary widely because of differences in how potency was defined, selection bias, and the multifactorial nature of erectile dysfunction. Also, because single-institution, single-surgeon reports and advertisements tend to underestimate rates of impotence after radical prostatectomy by any approach, many patients have false expectations.
INTERSTITIAL BRACHYTHERAPY FOR LOW-RISK CANCERS
Interstitial brachytherapy delivers a localized, high dose (125 to 145 Gy) of radiation to the prostate, with minimal radiation dosing to the bladder, rectum, or other adjacent organs and tissues. “Seeds” or small pellets containing a radioisotope (iodine 125 or palladium 103) are stereotactically implanted through the perineum into the prostate under ultrasonographic guidance. Computerized mapping done before or during surgery helps determine the optimal placement of the seeds, the object being to cover at least 90% of the prostate with 100% of the radiation dose.
In permanent brachytherapy, the implants give off radiation at a low dose rate over weeks to months and are left in place permanently. In temporary brachytherapy, seeds are implanted to deliver a low or high dose rate for a specified period, and then they are removed.
“Implant quality,” ie, delivery of more than 90% of the radiation dose, is a major predictor of success and can depend on both the available instrumentation and the skill of the operator.
Caveats about brachytherapy
The evidence in support of combining androgen deprivation therapy and interstitial brachytherapy is poor, and there is some evidence of increased rates of irritative voiding symptoms,27 so this is generally not recommended.
Interstitial brachytherapy as monotherapy has usually been reserved for patients with low-risk cancer with a low likelihood of extracapsular disease extension or pelvic lymph node involvement. No randomized controlled clinical trial has compared brachytherapy with radical prostatectomy or external beam radiotherapy. One large long-term study reported an 8-year biochemical recurrence rate of 18% in patients with low-risk cancer and 30% in patients with intermediate-risk cancer.28 The long-term efficacy of brachytherapy for intermediate- and high-risk prostate cancer is still under investigation.
Advantages and disadvantages of interstitial brachytherapy
Advantages. Interstitial brachytherapy is done as a single outpatient procedure. It can deliver a targeted high dose of radiation. And it is associated with a lower rate of posttreatment incontinence than radical prostatectomy, and a lower cost.
Disadvantages. There are limited data to support long-term cancer control in intermediate- and high-risk disease. Short-term adverse effects include dysuria, hematuria, urinary urgency, and urinary frequency in up to 80% of patients.29 Voiding symptoms typically peak 1 to 3 months after the procedure and subside after 8 to 12 months. Erectile dysfunction has been reported in 30% to 35% of men at 5 years after the procedure. Other possible adverse effects include urethral stricture, incontinence, recurrent hematuria, rectal bleeding, proctitis, and the development of bladder cancer and other secondary cancers.
EXTERNAL BEAM RADIOTHERAPY
In external beam radiotherapy, radiation is delivered to the prostate and surrounding tissues via an external energy source. Electrons, protons, or neutrons are used, and although each has theoretical advantages over the others, all appear to have similar clinical efficacy.
As with brachytherapy, the object—and the challenge—is to deliver an effective dose of radiation to the tumor while sparing adjacent organs. Intensity-modulated delivery is a radiotherapy technique that delivers more of the radiation dose where we want it to go—and less where we don’t want it to go. For prostate cancer, the target dose with intensity-modulated delivery is typically 75 to 85 Gy, in doses of 2 to 2.25 Gy for 30 to 36 days.
Androgen deprivation therapy before or after external beam radiotherapy augments the effects of the radiotherapy, particularly in patients with high-risk disease.30
The oncologic efficacy of intensity-modulated radiotherapy in patients at low and intermediate risk appears commensurate with that of radical prostatectomy. In one study,31 in low-risk cases, biochemical disease-free survival rates were 85% for radiotherapy vs 93% for prostatectomy; in intermediate-risk cases, 82% for radiotherapy and 87% for prostatectomy; and in high-risk cases, 62% for combined androgen deprivation and radiotherapy vs 38% for prostatectomy.31
Advantages and disadvantages of external beam radiotherapy
Advantages. External beam radiotherapy is noninvasive. It can treat the prostate as well as areas outside the prostate in patients with intermediate- and high-risk disease, and it is proven effective for high-risk cancer when used in combination with androgen deprivation.
Disadvantages. On the other hand, radiotherapy requires a series of daily treatments, which can be inconvenient and burdensome to the patient. Its adverse effects are similar to those of brachytherapy, and it is expensive. Long-term adverse effects include irritative voiding symptoms (frequency, urgency, nocturia), hemorrhagic cystitis, bowel symptoms (pain with defecation, tenesmus, bleeding), and a significantly higher lifetime risk of a secondary malignancy, particularly of the bladder and rectum.32
External beam radiotherapy also induces tissue changes in the pelvis that make salvage surgery more difficult. Patients in whom radiotherapy is ineffective as monotherapy and who require salvage prostatectomy typically have poor outcomes in terms of disease control, continence, and potency.
COMBINED RADIATION THERAPY: BETTER, OR OVERTREATMENT?
Many patients are offered a combination of external beam radiotherapy and interstitial brachytherapy. The rationale is that the combination can boost the dose of radiation to the prostate and at the same time treat cancer that has extended beyond the prostate or to the pelvic lymph nodes.
The radiation dose in the combined approach is 45 to 50 Gy (vs 70 to 80 Gy in monotherapy), thereby minimizing toxicity.
This combination has not been shown to improve overall survival or cancer-specific survival compared with either therapy alone, and it likely constitutes overtreatment.33 Adverse effects of combination therapy include erectile dysfunction, rectal and bladder toxicity, and secondary malignancy.
A serious complication associated more often with the combination of external beam radiotherapy and brachytherapy than other treatments is rectoprostatic fistula, a condition that requires complex reconstructive surgery and often requires permanent urinary and fecal diversion.34
CRYOTHERAPY: MORE STUDY NEEDED
Refinements in cryoablative therapy to destroy prostate tissue have improved the safety and efficacy of this procedure significantly over the past decade. The AUA consensus guidelines recognize cryotherapy as a viable primary cancer monotherapy, but it is most commonly used as a salvage therapy after failure of radiation therapy.
The procedure involves ultrasonographically guided stereotactic placement of cryoprobes into the prostate via a transperineal approach. Argon is pumped through the probes under pressure to initiate ice formation, and repeated freeze-thaw cycles cause tissue damage and necrosis.
Rates of biochemical recurrence at 5 years in patients at low, intermediate, and high risk have been reported at 16%, 27%, and 25%, respectively.35 The presence of viable cancer on biopsy specimens after primary cryoablation has been reported at 15%, compared with 25% after definitive radiation therapy.35
Advantages and disadvantages of cryotherapy
Cryotherapy can destroy cancer tissue in a minimally invasive way. It has no long-term delayed adverse effects, and it is a low-cost and convenient outpatient procedure.
On the other hand, we lack long-term data on its oncologic efficacy, acute complications, and late adverse effects. Acute complications occur in up to 16% of patients and include acute urinary retention requiring prolonged catheterization, hematuria, urethral sloughing, perineal pain, and incontinence.36 Potential late effects include rectoprostatic fistula (< 1%), incontinence (< 5%), persistent hematuria, and chronic pelvic pain.36
Cryoablation therapy appears to have a more significant negative impact on sexual function than does brachytherapy.37
More study of the complications and efficacy of cryotherapy is needed before the procedure can be adopted as routine primary monotherapy.
HIGH-INTENSITY FOCUSED ULTRASOUND: NOT YET FDA-APPROVED
High-intensity focused ultrasound (HIFU) is not yet approved by the US Food and Drug Administration (other than in an approved research protocol) but is used in Canada and in certain countries of Europe and Asia. It involves the insertion of a transducer into the rectum that generates a high-intensity, focused beam that heats target tissue in the prostate to a high temperature. This temperature triggers a heat-shock response that leads to cellular apoptosis and tissue necrosis. The procedure can be done with or without magnetic resonance imaging (MRI) guidance.
Biochemical recurrence rates at 2 years after the procedure have been reported between 23% and 50%, but long-term efficacy data are lacking.38,39
Advantages and disadvantages of ultrasound
HIFU is a minimally invasive, low-cost, outpatient procedure that offers trackless delivery of energy to the prostate: ie, there is no direct mechanical penetration into the tissue.
Complications include rectal-wall injury, fistula, acute urinary retention, hematuria, and urethral stricture.
FOCAL ABLATION: GETTING ATTENTION, BUT STILL UNDER DEVELOPMENT
Focal ablation for prostate cancer has been receiving much attention. This treatment uses heat energy to destroy tumor cells, guided by high-resolution endorectal-coil MRI. The procedure is in the developmental stages and is available only in research protocols.
The procedure has several major hurdles to overcome before becoming acceptable for clinical practice. First, prostate cancer is multifocal, and microscopic tumor foci are likely present that are invisible even to MRI, so ablation of only part of the prostate leaves the rest of the gland at risk of continued or de novo tumor growth.
Second, a wide range of sensitivities and specificities have been reported for endorectal coil MRI for detecting prostate cancer: its sensitivity has ranged from 27% to 100%, and its specificity has ranged from 32% to 99%.40
ANDROGEN DEPRIVATION, AN ADJUVANT THERAPY
Androgen deprivation therapy (medical castration) is not effective as a monotherapy for prostate cancer. A large population-based study in men with localized prostate cancer showed no higher rate of overall survival at 10 years with primary androgen deprivation therapy than with conservative management.41
Androgen deprivation is achieved with a leutinizing hormone-releasing hormone agonist such as leuprolide (Lupron) or goserelin (Zoladex), or an antiandrogen drug such as flutamide or bicalutamide (Casodex), or a combination of each.
Adverse effects include hot flashes, gynecomastia, decreased libido, erectile dysfunction, weight gain, and hyperlipidemia. Long-term effects include osteoporosis and a significantly higher risk of cardiac events, new-onset type 2 diabetes mellitus, and stroke.
Currently, the only recognized role for androgen deprivation therapy in prostate cancer is as an adjunct to external beam radiotherapy or as a treatment of metastatic prostate cancer.
Orchiectomy
The other way to eliminate testicular production of testosterone is surgical castration. Bilateral orchiectomy has advantages over medical androgen deprivation therapy in that it costs less, is highly reliable, and is done as a single treatment on an outpatient basis. Disadvantages include surgery-related morbidity and the irreversible nature of the procedure. The adverse effects are similar to those of androgen deprivation therapy.
POSTTREATMENT MONITORING
The management of patients with recurrent prostate cancer can be complex, and these patients should be referred to a medical or urologic oncologist.42,43
Often, a rise in PSA after primary therapy represents a regrowth of cancer; 30% to 60% of patients with a recurrence have metastasis, and nearly 20% will die from the disease. The average time from documentation of biochemical recurrence to metastatic progression is 8 years. The average time from metastatic progression to death is 5 years.44,45
After radical prostatectomy, the PSA level should be checked every 6 to 12 months for the first 2 years, then annually until the patient’s life expectancy is only 10 years even without prostate cancer. PSA should reach undetectable levels within 4 to 6 weeks after surgery. Biochemical recurrence after surgery is defined as a PSA level of 0.2 μg/L or higher in two serial studies.
After radiation therapy or cryotherapy, monitoring is complicated by the presence of viable prostatic epithelium that continues to produce PSA. During the first 1 to 2 years after radiation therapy, a PSA “bounce” phenomenon is observed whereby PSA levels rise or fluctuate significantly. This bounce should not be mistaken for a recurrence of cancer. The most widely accepted definition of biochemical recurrence is based on the American Society for Therapeutic Radiology and Oncology “Phoenix” criteria, defined as the nadir PSA level plus 2.0 μg/L.46
- Thompson I, Thrasher JB, Aus G, et al; AUA Prostate Cancer Clinical Guideline Update Panel. Guideline for the management of clinically localized prostate cancer: 2007 update. J Urol 2007; 177:2106–2131.
- Brooks DD, Wolf A, Smith RA, Dash C, Guessous I. Prostate cancer screening 2010: updated recommendations from the American Cancer Society. J Natl Med Assoc 2010; 102:423–429.
- US Preventive Services Task Force. Screening for prostate cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med 2008; 149:185–191.
- Okotie OT, Roehl KA, Han M, Loeb S, Gashti SN, Catalona WJ. Characteristics of prostate cancer detected by digital rectal examination only. Urology 2007; 70:1117–1120.
- Philip J, Dutta Roy S, Ballal M, Foster CS, Javlé P. Is a digital rectal examination necessary in the diagnosis and clinical staging of early prostate cancer? BJU Int 2005; 95:969–971.
- Thompson IM, Ankerst DP, Chi C, et al. Assessing prostate cancer risk: results from the Prostate Cancer Prevention Trial. J Natl Cancer Inst 2006; 98:529–534.
- Catalona WJ, Partin AW, Slawin KM, et al. Use of the percentage of free prostate-specific antigen to enhance differentiation of prostate cancer from benign prostatic disease: a prospective multicenter clinical trial. JAMA 1998; 279:1542–1547.
- Terris MK, Freiha FS, McNeal JE, Stamey TA. Efficacy of transrectal ultrasound for identification of clinically undetected prostate cancer. J Urol 1991; 146:78–83.
- Epstein JI, Herawi M. Prostate needle biopsies containing prostatic intraepithelial neoplasia or atypical foci suspicious for carcinoma: implications for patient care. J Urol 2006 Mar; 175( 3 Pt1):820–34.
- D’Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA 1998; 280:969–974.
- Greene FL. American Joint Committee on Cancer. American Cancer Society. AJCC cancer staging manual. 6th ed. New York, NY: Springer-Verlag; 2002.
- Stephenson AJ, Kattan MW, Eastham JA, et al. Prostate cancer-specific mortality after radical prostatectomy for patients treated in the prostate-specific antigen era. J Clin Oncol 2009; 27:4300–4305.
- Eastham JA, Scardino PT, Kattan MW. Predicting an optimal outcome after radical prostatectomy: the trifecta nomogram. J Urol 2008; 179:2207–2210.
- Stephenson AJ, Scardino PT, Eastham JA, et al. Preoperative nomogram predicting the 10-year probability of prostate cancer recurrence after radical prostatectomy. J Natl Cancer Inst 2006; 98:715–717.
- Potters L, Roach M, Davis BJ, et al. Postoperative nomogram predicting the 9-year probability of prostate cancer recurrence after permanent prostate brachytherapy using radiation dose as a prognostic variable. Int J Radiat Oncol Biol Phys 2010; 76:1061–1065.
- Zelefsky MJ, Kattan MW, Fearn P, et al. Pretreatment nomogram predicting ten-year biochemical outcome of three-dimensional conformal radiotherapy and intensity-modulated radiotherapy for prostate cancer. Urology 2007; 70:283–287.
- Walz J, Gallina A, Saad F, et al. A nomogram predicting 10-year life expectancy in candidates for radical prostatectomy or radiotherapy for prostate cancer. J Clin Oncol 2007; 25:3576–3581.
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40:373–383.
- Hall WH, Ramachandran R, Narayan S, Jani AB, Vijayakumar S. An electronic application for rapidly calculating Charlson comorbidity score. BMC Cancer 2004; 4:94.
- Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982; 5:649–655.
- Bill-Axelson A, Holmberg L, Ruutu M, et al; Scandinavian Prostate Cancer Group Study No. 4. Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med 2005; 352:1977–1984.
- Widmark A, Klepp O, Solberg A, et al; Scandinavian Prostate Cancer Group Study 7. Endocrine treatment, with or without radiotherapy, in locally advanced prostate cancer (SPCG-7/SFUO-3): an open randomised phase III trial. Lancet 2009; 373:301–308.
- Krakowsky Y, Loblaw A, Klotz L. Prostate cancer death of men treated with initial active surveillance: clinical and biochemical characteristics. J Urol 2010; 184:131–135.
- Ficarra V, Novara G, Artibani W, et al. Retropubic, laparoscopic, and robot-assisted radical prostatectomy: a systematic review and cumulative analysis of comparative studies. Eur Urol 2009; 55:1037–1063.
- Hernandez DJ, Nielsen ME, Han M, Partin AW. Contemporary evaluation of the D’amico risk classification of prostate cancer. Urology 2007; 70:931–935.
- Messing EM, Manola J, Yao J, et al; Eastern Cooperative Oncology Group study EST 3886. Immediate versus deferred androgen deprivation treatment in patients with node-positive prostate cancer after radical prostatectomy and pelvic lymphadenectomy. Lancet Oncol 2006; 7:472–479.
- Beyer DC, McKeough T, Thomas T. Impact of short course hormonal therapy on overall and cancer specific survival after permanent prostate brachytherapy. Int J Radiat Oncol Biol Phys 2005; 61:1299–1305.
- Zelefsky MJ, Kuban DA, Levy LB, et al. Multi-institutional analysis of long-term outcome for stages T1-T2 prostate cancer treated with permanent seed implantation. Int J Radiat Oncol Biol Phys 2007; 67:327–333.
- Gelblum DY, Potters L, Ashley R, Waldbaum R, Wang XH, Leibel S. Urinary morbidity following ultrasound-guided transperineal prostate seed implantation. Int J Radiat Oncol Biol Phys 1999; 45:59–67.
- Bolla M, Collette L, Blank L, et al. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): a phase III randomised trial. Lancet 2002; 360:103–106.
- Aizer AA, Yu JB, Colberg JW, McKeon AM, Decker RH, Peschel RE. Radical prostatectomy vs intensity-modulated radiation therapy in the management of localized prostate adenocarcinoma. Radiother Oncol 2009; 93:185–191.
- Moon K, Stukenborg GJ, Keim J, Theodorescu D. Cancer incidence after localized therapy for prostate cancer. Cancer 2006; 107:991–998.
- Terakedis BE, Rossi PJ, Liauw SL, Johnstone PA, Jani AB. A surveillance, epidemiology, and end results registry analysis of prostate cancer modality time trends by age. Am J Clin Oncol 2010; 33:619–623.
- Lane BR, Stein DE, Remzi FH, Strong SA, Fazio VW, Angermeier KW. Management of radiotherapy induced rectourethral fistula. J Urol 2006; 175:1382–1387.
- Jones JS, Rewcastle JC, Donnelly BJ, Lugnani FM, Pisters LL, Katz AE. Whole gland primary prostate cryoablation: initial results from the cryo on-line data registry. J Urol 2008; 180:554–558.
- Hubosky SG, Fabrizio MD, Schellhammer PF, Barone BB, Tepera CM, Given RW. Single center experience with third-generation cryosurgery for management of organ-confined prostate cancer: critical evaluation of short-term outcomes, complications, and patient quality of life. J Endourol 2007; 21:1521–1531.
- Malcolm JB, Fabrizio MD, Barone BB, et al. Quality of life after open or robotic prostatectomy, cryoablation or brachytherapy for localized prostate cancer. J Urol 2010; 183:1822–1828.
- Ficarra V, Antoniolli SZ, Novara G, et al. Short-term outcome after high-intensity focused ultrasound in the treatment of patients with high-risk prostate cancer. BJU Int 2006; 98:1193–1198.
- Challacombe BJ, Murphy DG, Zakri R, Cahill DJ. High-intensity focused ultrasound for localized prostate cancer: initial experience with a 2-year follow-up. BJU Int 2009; 104:200–204.
- Bouchelouche K, Turkbey B, Choyke P, Capala J. Imaging prostate cancer: an update on positron emission tomography and magnetic resonance imaging. Curr Urol Rep 2010; 11:180–190.
- Lu-Yao GL, Albertsen PC, Moore DF, et al. Survival following primary androgen deprivation therapy among men with localized prostate cancer. JAMA 2008; 300:173–181.
- Simmons MN, Stephenson AJ, Klein EA. Natural history of biochemical recurrence after radical prostatectomy: risk assessment for secondary therapy. Eur Urol 2007; 51:1175–1184.
- Boukaram C, Hannoun-Levi JM. Management of prostate cancer recurrence after definitive radiation therapy. Cancer Treat Rev 2010; 36:91–100.
- Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC. Natural history of progression after PSA elevation following radical prostatectomy. JAMA 1999; 281:1591–1597.
- Freedland SJ, Humphreys EB, Mangold LA, et al. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA 2005; 294:433–439.
- Roach M, Hanks G, Thames H, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys 2006; 65:965–974.
- Thompson I, Thrasher JB, Aus G, et al; AUA Prostate Cancer Clinical Guideline Update Panel. Guideline for the management of clinically localized prostate cancer: 2007 update. J Urol 2007; 177:2106–2131.
- Brooks DD, Wolf A, Smith RA, Dash C, Guessous I. Prostate cancer screening 2010: updated recommendations from the American Cancer Society. J Natl Med Assoc 2010; 102:423–429.
- US Preventive Services Task Force. Screening for prostate cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med 2008; 149:185–191.
- Okotie OT, Roehl KA, Han M, Loeb S, Gashti SN, Catalona WJ. Characteristics of prostate cancer detected by digital rectal examination only. Urology 2007; 70:1117–1120.
- Philip J, Dutta Roy S, Ballal M, Foster CS, Javlé P. Is a digital rectal examination necessary in the diagnosis and clinical staging of early prostate cancer? BJU Int 2005; 95:969–971.
- Thompson IM, Ankerst DP, Chi C, et al. Assessing prostate cancer risk: results from the Prostate Cancer Prevention Trial. J Natl Cancer Inst 2006; 98:529–534.
- Catalona WJ, Partin AW, Slawin KM, et al. Use of the percentage of free prostate-specific antigen to enhance differentiation of prostate cancer from benign prostatic disease: a prospective multicenter clinical trial. JAMA 1998; 279:1542–1547.
- Terris MK, Freiha FS, McNeal JE, Stamey TA. Efficacy of transrectal ultrasound for identification of clinically undetected prostate cancer. J Urol 1991; 146:78–83.
- Epstein JI, Herawi M. Prostate needle biopsies containing prostatic intraepithelial neoplasia or atypical foci suspicious for carcinoma: implications for patient care. J Urol 2006 Mar; 175( 3 Pt1):820–34.
- D’Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA 1998; 280:969–974.
- Greene FL. American Joint Committee on Cancer. American Cancer Society. AJCC cancer staging manual. 6th ed. New York, NY: Springer-Verlag; 2002.
- Stephenson AJ, Kattan MW, Eastham JA, et al. Prostate cancer-specific mortality after radical prostatectomy for patients treated in the prostate-specific antigen era. J Clin Oncol 2009; 27:4300–4305.
- Eastham JA, Scardino PT, Kattan MW. Predicting an optimal outcome after radical prostatectomy: the trifecta nomogram. J Urol 2008; 179:2207–2210.
- Stephenson AJ, Scardino PT, Eastham JA, et al. Preoperative nomogram predicting the 10-year probability of prostate cancer recurrence after radical prostatectomy. J Natl Cancer Inst 2006; 98:715–717.
- Potters L, Roach M, Davis BJ, et al. Postoperative nomogram predicting the 9-year probability of prostate cancer recurrence after permanent prostate brachytherapy using radiation dose as a prognostic variable. Int J Radiat Oncol Biol Phys 2010; 76:1061–1065.
- Zelefsky MJ, Kattan MW, Fearn P, et al. Pretreatment nomogram predicting ten-year biochemical outcome of three-dimensional conformal radiotherapy and intensity-modulated radiotherapy for prostate cancer. Urology 2007; 70:283–287.
- Walz J, Gallina A, Saad F, et al. A nomogram predicting 10-year life expectancy in candidates for radical prostatectomy or radiotherapy for prostate cancer. J Clin Oncol 2007; 25:3576–3581.
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40:373–383.
- Hall WH, Ramachandran R, Narayan S, Jani AB, Vijayakumar S. An electronic application for rapidly calculating Charlson comorbidity score. BMC Cancer 2004; 4:94.
- Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982; 5:649–655.
- Bill-Axelson A, Holmberg L, Ruutu M, et al; Scandinavian Prostate Cancer Group Study No. 4. Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med 2005; 352:1977–1984.
- Widmark A, Klepp O, Solberg A, et al; Scandinavian Prostate Cancer Group Study 7. Endocrine treatment, with or without radiotherapy, in locally advanced prostate cancer (SPCG-7/SFUO-3): an open randomised phase III trial. Lancet 2009; 373:301–308.
- Krakowsky Y, Loblaw A, Klotz L. Prostate cancer death of men treated with initial active surveillance: clinical and biochemical characteristics. J Urol 2010; 184:131–135.
- Ficarra V, Novara G, Artibani W, et al. Retropubic, laparoscopic, and robot-assisted radical prostatectomy: a systematic review and cumulative analysis of comparative studies. Eur Urol 2009; 55:1037–1063.
- Hernandez DJ, Nielsen ME, Han M, Partin AW. Contemporary evaluation of the D’amico risk classification of prostate cancer. Urology 2007; 70:931–935.
- Messing EM, Manola J, Yao J, et al; Eastern Cooperative Oncology Group study EST 3886. Immediate versus deferred androgen deprivation treatment in patients with node-positive prostate cancer after radical prostatectomy and pelvic lymphadenectomy. Lancet Oncol 2006; 7:472–479.
- Beyer DC, McKeough T, Thomas T. Impact of short course hormonal therapy on overall and cancer specific survival after permanent prostate brachytherapy. Int J Radiat Oncol Biol Phys 2005; 61:1299–1305.
- Zelefsky MJ, Kuban DA, Levy LB, et al. Multi-institutional analysis of long-term outcome for stages T1-T2 prostate cancer treated with permanent seed implantation. Int J Radiat Oncol Biol Phys 2007; 67:327–333.
- Gelblum DY, Potters L, Ashley R, Waldbaum R, Wang XH, Leibel S. Urinary morbidity following ultrasound-guided transperineal prostate seed implantation. Int J Radiat Oncol Biol Phys 1999; 45:59–67.
- Bolla M, Collette L, Blank L, et al. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): a phase III randomised trial. Lancet 2002; 360:103–106.
- Aizer AA, Yu JB, Colberg JW, McKeon AM, Decker RH, Peschel RE. Radical prostatectomy vs intensity-modulated radiation therapy in the management of localized prostate adenocarcinoma. Radiother Oncol 2009; 93:185–191.
- Moon K, Stukenborg GJ, Keim J, Theodorescu D. Cancer incidence after localized therapy for prostate cancer. Cancer 2006; 107:991–998.
- Terakedis BE, Rossi PJ, Liauw SL, Johnstone PA, Jani AB. A surveillance, epidemiology, and end results registry analysis of prostate cancer modality time trends by age. Am J Clin Oncol 2010; 33:619–623.
- Lane BR, Stein DE, Remzi FH, Strong SA, Fazio VW, Angermeier KW. Management of radiotherapy induced rectourethral fistula. J Urol 2006; 175:1382–1387.
- Jones JS, Rewcastle JC, Donnelly BJ, Lugnani FM, Pisters LL, Katz AE. Whole gland primary prostate cryoablation: initial results from the cryo on-line data registry. J Urol 2008; 180:554–558.
- Hubosky SG, Fabrizio MD, Schellhammer PF, Barone BB, Tepera CM, Given RW. Single center experience with third-generation cryosurgery for management of organ-confined prostate cancer: critical evaluation of short-term outcomes, complications, and patient quality of life. J Endourol 2007; 21:1521–1531.
- Malcolm JB, Fabrizio MD, Barone BB, et al. Quality of life after open or robotic prostatectomy, cryoablation or brachytherapy for localized prostate cancer. J Urol 2010; 183:1822–1828.
- Ficarra V, Antoniolli SZ, Novara G, et al. Short-term outcome after high-intensity focused ultrasound in the treatment of patients with high-risk prostate cancer. BJU Int 2006; 98:1193–1198.
- Challacombe BJ, Murphy DG, Zakri R, Cahill DJ. High-intensity focused ultrasound for localized prostate cancer: initial experience with a 2-year follow-up. BJU Int 2009; 104:200–204.
- Bouchelouche K, Turkbey B, Choyke P, Capala J. Imaging prostate cancer: an update on positron emission tomography and magnetic resonance imaging. Curr Urol Rep 2010; 11:180–190.
- Lu-Yao GL, Albertsen PC, Moore DF, et al. Survival following primary androgen deprivation therapy among men with localized prostate cancer. JAMA 2008; 300:173–181.
- Simmons MN, Stephenson AJ, Klein EA. Natural history of biochemical recurrence after radical prostatectomy: risk assessment for secondary therapy. Eur Urol 2007; 51:1175–1184.
- Boukaram C, Hannoun-Levi JM. Management of prostate cancer recurrence after definitive radiation therapy. Cancer Treat Rev 2010; 36:91–100.
- Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC. Natural history of progression after PSA elevation following radical prostatectomy. JAMA 1999; 281:1591–1597.
- Freedland SJ, Humphreys EB, Mangold LA, et al. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA 2005; 294:433–439.
- Roach M, Hanks G, Thames H, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys 2006; 65:965–974.
KEY POINTS
- The AUA recommends annual screening with both digital rectal examination (DRE) and prostate-specific antigen (PSA) testing starting at age 40 for all men whose life expectancy is more than 10 years. Guidelines from other organizations differ somewhat.
- If the DRE is abnormal or if the PSA level is persistently higher than 2.5 μg/L, then biopsy should be considered.
- In low-risk cases, active surveillance may be acceptable in lieu of immediate treatment. Patient education, accurate disease assessment, and compliance with monitoring are critical considerations.
- The most common primary treatments are active surveillance, prostatectomy, interstitial brachytherapy, external beam radiotherapy, and cryotherapy. Newer ablative and focal therapies may offer an advantage in select patients. Which treatment to use is highly patient-dependent.
- Single-institution, single-surgeon reports and advertisements tend to underestimate rates of impotence after prostatectomy, and as a result patients may have false expectations.
In reply: Hematuria evaluation
In Reply: Although a Foley catheter can contribute to macroscopic (gross) hematuria, to assume that it is the cause of the hematuria carries some undefined, but presumably small, risk of missing a more serious diagnosis. A more serious potential for misdiagnosis is in the setting of anticoagulation. As we discuss in our review, hematuria is analagous to hematochezia in the setting of anticoagulants. The possibility that anticoagulation has simply exposed an underlying bleeding source such as a tumor in either setting must not be underestimated, and we recommend that all patients with macroscopic hematuria undergo urologic evaluation unless there is a documented benign physical cause of bleeding such as traumatic catheterization.
In Reply: Although a Foley catheter can contribute to macroscopic (gross) hematuria, to assume that it is the cause of the hematuria carries some undefined, but presumably small, risk of missing a more serious diagnosis. A more serious potential for misdiagnosis is in the setting of anticoagulation. As we discuss in our review, hematuria is analagous to hematochezia in the setting of anticoagulants. The possibility that anticoagulation has simply exposed an underlying bleeding source such as a tumor in either setting must not be underestimated, and we recommend that all patients with macroscopic hematuria undergo urologic evaluation unless there is a documented benign physical cause of bleeding such as traumatic catheterization.
In Reply: Although a Foley catheter can contribute to macroscopic (gross) hematuria, to assume that it is the cause of the hematuria carries some undefined, but presumably small, risk of missing a more serious diagnosis. A more serious potential for misdiagnosis is in the setting of anticoagulation. As we discuss in our review, hematuria is analagous to hematochezia in the setting of anticoagulants. The possibility that anticoagulation has simply exposed an underlying bleeding source such as a tumor in either setting must not be underestimated, and we recommend that all patients with macroscopic hematuria undergo urologic evaluation unless there is a documented benign physical cause of bleeding such as traumatic catheterization.
How to evaluate ‘dipstick hematuria’: What to do before you refer
Many people have some amount of blood in their urine, but relatively few have a serious problem.
In a population-based study in Rochester, Minnesota, red blood cells were found in the urine of 13% of symptom-free adults.1 In other studies, the figure ranged from 9% to 18%.2
Hematuria is sometimes detected during investigation of symptoms such as dysuria, urinary frequency, or flank pain. However, many referrals to urologists are made purely on the basis of the results of a dipstick urinalysis screening test in a patient without symptoms.
The United States Preventive Task Force3 discourages routine testing for hematuria to screen for bladder cancer in patients without symptoms, and the Canadian Task Force on the Periodic Health Examination4 and the American Urological Association (AUA)2,5 do not recommend it either. Nevertheless, approximately 40% of primary care physicians believe in it,6 although the number seems to be declining.7 A reason that dipstick testing is so popular is that it is an inexpensive and simple way to detect glucosuria and medical renal disease.8–11
The risk of significant disease in a patient with microhematuria but without symptoms is low, and the evaluation for hematuria can be costly and invasive. For an individual patient with a hemoglobin-positive dipstick test, the finding should not be ignored, but the patient does not necessarily need a complete evaluation. It is important to determine which patients require urologic studies and consultation, nephrologic evaluation, or no intervention at all.
This review addresses issues related to hematuria for the primary care physician, clarifies some of the important definitions, builds on these terms to delineate which patients should be referred to a urologist, and provides simple recommendations about ancillary studies and their potential role before urologic consultation. Understanding this information will ultimately assure appropriate management of patients without symptoms who have positive dipstick screening tests, leading to decreased use of costly and invasive tests and more appropriate long-term follow-up.
BASIC DEFINITIONS
Gross (macroscopic) hematuria is blood in the urine that is visible without microscopy. This condition almost always warrants urologic evaluation.2,5
Microscopic hematuria, or microhematuria, is the finding of red blood cells in the urine on microscopy. (In contrast, in dipstick hematuria—see below—blood cells may or may not be present in the urine.)
“Dipstick hematuria” and “dipstick microhematuria” are potential misnomers. The dip-stick test for hematuria is a nondiagnostic screening test. A positive result is simply a color change due to oxidation of a test-strip reagent; it does not confirm that blood cells are present. Factors that can cause a false-positive result on a dipstick test include hemoglobinuria, myoglobinuria, concentrated urine, menstrual blood in the urine sample, and rigorous exercise.12 Thus, the diagnosis of hematuria cannot be made by dipstick alone. Unless red blood cells are seen microscopically, the term microhematuria is inappropriate.
Of note, if the specific gravity of the urine is very low (< 1.007), microscopy can fail to detect urinary red blood cells, owing to cell lysis.2 This limitation can be overcome by restricting the patient’s fluid intake and then repeating the urinalysis.
Many patients with a positive dipstick oxidation reaction are labeled as having dip-stick hematuria although microscopic analysis would show that red blood cells are absent. Perhaps the term dipstick pseudohematuria would be more accurate. These patients will not benefit from a costly and invasive urologic workup, so it is crucial to distinguish them from patients with true microhematuria.
SIGNIFICANT HEMATURIA: ≥ 3 RBCs/HPF
Microhematuria is often intermittent, and many healthy patients occasionally have a few red blood cells in the urine.13 However, no cutoff point for the amount of hematuria can be used to rule out the possibility of cancer.14 To account for these complicating factors, the AUA Best Practice Policy Panel on Asymptomatic Hematuria considered the literature and expert opinions to define the amount of microhematuria warranting evaluation in patients with risk factors for significant urologic disease.2,5
The AUA panel defined microhematuria as an average of three or more red blood cells per high-power microscopic field (RBCs/HPF) in two out of three properly collected and prepared specimens. Urine should be collected midstream after wiping the urethral meatus with a disinfectant and voiding the initial portion of urine into the toilet. For proper preparation of the urine sample, 10 mL of urine should be centrifuged at 2,000 rpm for 5 minutes and the supernatant discarded. The sediment should then be resuspended in 0.5 to 1.0 mL of remaining urine, and a drop of this suspension should be examined microscopically. If contamination is suggested (ie, if squamous epithelial cells, bacteria, or both are present), one should consider collecting a specimen through catheterization.2,5
The AUA guidelines do not specify some of the details of management, such as the timing of subsequent microscopic urinalyses, but we recommend that all urinalyses to establish whether significant hematuria is present be done within 3 to 6 months of the screening dip-stick test. If a patient has no risk factors for cancer and has a negative result on the first microscopic urinalysis, the follow-up test can be performed in 1 year. Table 1 shows risk factors for significant urologic disease that warrant evaluation in patients with asymptomatic hematuria.5
Isolated urinary findings that might warrant evaluation by a nephrologist rather than a urologist include proteinuria, red cell casts, and dysmorphic red blood cells, especially if the serum creatinine level is elevated.2,5 Many medical renal conditions (eg, glomerulonephritis) and hemoglobinopathies (eg, sickle cell trait) can cause blood in the urine; when these conditions are accompanied by risk factors for urologic disease, urologic evaluation is indicated as well.
CLINICAL RELEVANCE OF HEMATURIA
Approximately 25% of cases of macroscopic hematuria are due to urologic cancers,14,15 and another 34% are due to other significant urologic diseases14—thus, the recommendation that patients with macroscopic hematuria be evaluated by a urologist. In contrast, in microhematuria, the rates of cancer are much lower, ranging between 1% and 10% in large studies.2,5
The urine dipstick test has been found to be 65% to 99% specific for the presence of blood cells, free hemoglobin, or myoglobin.2,5 If the true specificity is closer to the lower figure and all patients with a positive dipstick test were referred to a urologist, this would mean the urologic workup would be unnecessary in up to 35% of them, because the dipstick result would be falsely positive.
But that is not all. Most causes of hemoglobinuria or myoglobinuria are of limited clinical significance, except for rare conditions that are usually clinically obvious, such as severe burn injury. Further, remember that from 9% to 18% of patients without symptoms have red blood cells in the urine.2,5 In theory, if everyone in the United States had a dipstick test, this would be positive in patients with hematuria as well as in those with hemoglobinuria, myoglobinuria, and other false-positives; if everyone with a positive dipstick result were then referred to a urologist, a substantial portion of the population would receive an unnecessary urologic referral.
Urologic referral and evaluation in these patients not only wastes money: if they undergo imaging studies, they are exposed to radiation and contrast media, with their associated risks, and if they undergo cystoscopy, they face its attendant discomfort and risk of infection.
WHICH PATIENTS WITH HEMATURIA TO REFER TO A UROLOGIST
Gross hematuria
Red or tea-colored urine usually indicates gross hematuria. When there is any doubt—such as in the case of a color-blind patient—the presence of red blood cells can be confirmed or ruled out by a microscopic urinalysis.
Nearly all patients with an episode of gross hematuria should be referred to a urologist. The sole exception to this rule can be made when a woman younger than 40 years experiences gross hematuria in the classic setting of a culture-proven, symptomatic urinary tract infection (UTI) and her infection, symptoms, and hematuria all resolve completely with appropriate antibiotics.2,5 However, bleeding from cancer is classically intermittent. Therefore, one should not skip the urine culture and just prescribe antibiotics empirically: the patient might actually have cancer, but the supposed UTI may appear to resolve with antibiotic therapy. For the same reason, resolution of hematuria in any other setting does not obviate the need for referral.
Another scenario usually associated with a benign cause is bleeding after extreme physical activity—also known as “runner’s hematuria” or “march hematuria” (so named because it sometimes occurs in soldiers after a particularly grueling training march). Importantly, even in this situation, one should still be suspicious and probably refer the patient to a urologist: just because the patient has just run a marathon, it does not mean that he or she does not have cancer.
Depending on the character, timing, location, and many other characteristics of the patient’s bleeding, a variety of studies may or may not be necessary. For example, blood-spotting of the underpants might signify urethral bleeding, and imaging and cytologic studies might not be indicated. In view of the variability in presentation and workup, we recommend that the proper workup for these patients be determined by a urologist.
Symptomatic microhematuria
Patients with true microhematuria (three or more RBCs/HPF) accompanied by bothersome or worrisome symptoms should be referred to a urologist. In a study in Scotland, Sultana et al16 found that cancer was present in 6 (5%) of 126 patients with microhematuria without symptoms compared with 13 (10.5%) of 124 patients with microhematuria and irritative voiding symptoms; the difference, however, was not statistically significant.
Microscopic urinalysis should be part of the evaluation for flank pain or certain urinary symptoms such as frequency, urgency, retention, or dysuria; results of this test at the time of symptoms can later help the urologist distinguish the cause of the symptoms or hematuria. In addition, in combination with dipstick analysis, microscopic analysis can help distinguish patients with UTI or medical renal disease. If the evaluation suggests that the hematuria and symptoms are due to a UTI, then the findings on a repeat microscopic analysis, performed after the infection has cleared, should be normal. If hematuria, defined as three or more RBCs/HPF, persists in two of three subsequent urinalyses, then the guidelines mandate diagnostic evaluation even if the urinalysis subsequently becomes negative.
Asymptomatic hematuria
In symptom-free patients with dipstick hematuria found on a screening examination, it is crucial to confirm and document true microhematuria. Per the AUA guidelines, microhematuria worthy of urologic workup is the presence of three or more RBCs/HPF on at least two out of three microscopic urinalyses.2,5 Patients with dipstick pseudohematuria do not meet this criterion and will not benefit from a costly and invasive evaluation. Conversely, patients with higher levels of microhematuria, who have any risk factors for cancer, or who are anxious about the test results might benefit from urologic consultation before a second urinalysis to confirm the first, positive finding.
Many patients younger than age 40 with asymptomatic microhematuria but no other risk factors for urinary tract cancer can be followed conservatively. Khadra et al14 reported that only 1 of 143 patients younger than 40 years with microhematuria had cancer. Similarly, Jones et al17 found, in a prospective study, that no man younger than 40 years with microscopic hematuria had cancer.
Of note: gross or microscopic blood in the urine, even in the setting of anticoagulation, is a marker of urinary tract pathology such as cancer, stones, or infection. Just as patients on anticoagulation therapy who develop gastrointestinal bleeding need a gastrointestinal evaluation, those with hematuria require a urologic evaluation.2,5
STUDIES TO CONSIDER BEFORE CONSULTATION
In symptom-free patients, it is inappropriate to order laboratory or imaging tests on the basis of a dipstick test alone, without confirming that they actually have hematuria. When the blood is confirmed to be present by microscopic examination of centrifuged urine (as described above), benign causes such as UTI should be considered. If a patient does have a UTI with hematuria, urinalysis should be repeated once the infection has cleared up.
Imaging studies
For symptomatic microhematuria. Patients with acute symptoms of renal colic should undergo computed tomography (CT) in a “stone protocol” (without contrast, with 3- to 5-mm cuts of the abdomen and pelvis) to assess for urinary lithiasis. Pregnancy should always be ruled out before radiation exposure; renal ultrasonography is generally the first-choice imaging study for pregnant patients.
For asymptomatic microhematuria. Patients without the classic flank pain of urolithiasis should undergo more extensive studies. For patients at increased risk of cancer, such as heavy smokers, CT urography is the optimal imaging study and is the test least likely to necessitate other follow-up studies.18–20 Other imaging options, including ultrasonography and intravenous pyelography, incompletely assess the upper urinary tracts including both renal parenchyma and urothelial surfaces. CT urography has been shown to find more than 40% of hematuria-causing lesions missed by other studies.18 Because ordering alternative imaging first will often result in redundant studies, CT urography is the preferred initial imaging study in the evaluation of hematuria.
Before exposing a patient to contrast media, one should ascertain that he or she is not allergic to it. In addition, in patients at risk of contrast nephropathy (ie, those older than 60 years, with diabetes, or with preexisting medical renal disease), one should check the serum creatinine concentration. Magnetic resonance urography, a more expensive study, is as accurate as CT for diagnosing many urologic conditions, so it can be performed in lieu of CT urography in patients with renal insufficiency, iodine allergy, or any reason to avoid ionizing radiation. Some clinicians perform plain radiography of the kidneys, ureters, and bladder as well as ultrasonography in this setting, but determination of the appropriate alternative to CT urography, if required, is best left to the urologist.
Other tests
Cytologic testing of the urine can be valuable in patients with gross hematuria and in those with microhematuria who have risk factors for urinary tract cancer. Although its reported median sensitivity for malignancy is only 48%, a positive cytologic test is approximately 94% specific for malignancy.21 Other studies, such as the fluorescence in situ hybridization assay, and the nuclear matrix protein 22 test do not yet have a clear role in the diagnosis of urinary tract disease.22
However, in general, we caution non-urologists not to order special tumor marker or cytologic tests, or to do so only with careful forethought. Although these studies occasionally detect occult cancer in patients at high risk, an “atypical” finding on cytology or a positive tumor marker test can lead to inappropriate referral and unnecessary biopsy or other tests.
WHEN NOT TO REFER A PATIENT WITH HEMATURIA TO A UROLOGIST
Symptom-free patients with a positive dipstick hemoglobin test should not immediately be referred to a urologist: they should have a microscopic urinalysis first to determine whether they actually have microhematuria, unless microscopic laboratory services are unavailable. Only patients with documented true hematuria, as defined by the AUA guidelines, should be referred for urologic evaluation and diagnostic testing. Once a patient is referred for evaluation, the consultant is under clear pressure to perform a complete investigation to fulfill the expectations of the referring physician. Avoiding expensive unnecessary testing and referral in those without hematuria allows appropriate utilization of resources.
Patients with microhematuria associated with a UTI should have a repeat urinalysis after the UTI is successfully treated; if the hematuria clears with the infection, then the patient needs no further evaluation. Patients with dipstick pseudohematuria and significant proteinuria or a predominance of dysmorphic urinary blood cells might benefit from an evaluation by a nephrologist rather than a urologist.2 This is especially true if the patient has an elevated serum creatinine level.
ECONOMIC RELEVANCE
In our tertiary care urology clinic, approximately 75% of patients who are referred to us because of microhematuria have not had a microscopic urinalysis before coming here. On further evaluation, up to 75% of these patients are found to have dipstick pseudohematuria that did not actually require consultation or evaluation.23 It is possible that this occurs even more frequently in the general practice setting.
A Medicare level-4 urologic consultation for hematuria costs $170; the cumulative cost of unwarranted referrals is undoubtedly substantial. Even more money is wasted on CT urography, cytology, and other testing performed before urologic consultation in patients ultimately found not to have true hematuria. The economic and iatrogenic risks of evaluation cannot be justified in patients who do not exhibit findings that can be considered abnormal as defined in this article.
CONCLUSION
It is important to distinguish whether hematuria is microscopic or macroscopic, whether there are associated symptoms, and whether a patient has risk factors for significant urologic disease. While dipstick tests are sensitive, they do not reliably diagnose microhematuria, which is the microscopically proven presence of urinary red blood cells. Positive dipstick tests should always be followed by microscopic urinalysis; failure to do so can result in the unfortunate and unnecessary evaluation of dipstick pseudohematuria, a normal condition.
The AUA defines significant hematuria as three or more RBCs/HPF in two of three properly prepared specimens.2 This should determine whether a symptom-free patient needs urologic referral and evaluation for hematuria.
By following these principles, primary care physicians have a valuable opportunity to direct medical care, increase the efficiency of our health care system, and protect patients from the anxiety, costs, and risks of an unnecessary urologic workup.
- Mohr DN, Offord KP, Owen RA, Melton LJ. Asymptomatic microhematuria and urologic disease. A population-based study. JAMA 1986; 256:224–229.
- Grossfeld GD, Litwin MS, Wolf JS, et al. Evaluation of asymptomatic microscopic hematuria in adults: the American Urological Association best practice policy—part I: definition, detection, prevalence, and etiology. Urology 2001; 57:599–603.
- US Preventive Services Task Force. Screening for Bladder Cancer, updated November 2004. Available at www.ahrq.gov/clinic/uspstf/uspsblad.htm.
- Logsetty S. Screening for bladder cancer. Canadian Task Force on the Periodic Health Examination. Canadian Guide to Clinical Preventive Health Care. 1994: Ottawa: Health Canada,826–836.
- Grossfeld GD, Litwin MS, Wolf JS, et al. Evaluation of asymptomatic microscopic hematuria in adults: The American Urological Association Best Practice Policy—Part II: patient evaluation, cytology, voided markers, imaging cystoscopy, nephrology evaluation, and follow-up. Urology 2001; 57:604–610.
- Prochazka AV, Lundahl K, Pearson W, Oboler SK, Anderson RJ. Support of evidence-based guidelines for the annual physical examination: a survey of primary care providers. Arch Intern Med 2005; 165:1347–1352.
- Chacko KM, Feinberg LE. Laboratory screening at preventive health exams: trend of testing, 1978–2004. Am J Prev Med 2007; 32:59–62.
- Mariani AJ, Luangphinith S, Loo S, Scottolini A, Hodges CV. Dipstick chemical urinalysis: an accurate cost-effective screening test. J Urol 1984; 132:64–66.
- Murphy TE. The urinalysis—inexpensive and informative. J Insur Med 2004; 36:320–326.
- Woolhandler S, Pels RJ, Bor DH, Himmelstein DU, Lawrence RS. Dipstick urinalysis screening of asymptomatic adults for urinary tract disorders. I. Hematuria and proteinuria. JAMA 1989; 262:1214–1219.
- Pels RJ, Bor DH, Woolhandler S, Himmelstein DU, Lawrence RS. Dipstick urinalysis screening of asymptomatic adults for urinary tract disorders. II. Bacteriuria. JAMA 1989; 262:1221–1224.
- Gerber GS, Brendler CBWein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CA. Evaluation of the urologic patient: history, physical examination, and urinalysis. Campbell-Walsh Urology 2007; 9th ed. Saunders Elsevier: Philadelphia:81–110.
- Cohen RA, Brown RS. Clinical practice. Microscopic hematuria. N Engl J Med 2003; 348:2330–2338.
- Khadra MH, Pickard RS, Charlton M, Powell PH, Neal DE. A prospective analysis of 1,930 patients with hematuria to evaluate current diagnostic practice. J Urol 2000; 163:524–527.
- Alishahi S, Byrne D, Goodman CM, Baxby K. Haematuria investigation based on a standard protocol: emphasis on the diagnosis of urological malignancy. J R Coll Surg Edinb 2002; 47:422–427.
- Sultana SR, Goodman CM, Byrne DJ, Baxby K. Microscopic haematuria: urological investigation using a standard protocol. Br J Urol 1996; 78:691–696.
- Jones DJ, Langstaff RJ, Holt SD, Morgans BT. The value of cystourethroscopy in the investigation of microscopic haematuria in adult males under 40 years. A prospective study of 100 patients. Br J Urol 1988; 62:541–545.
- Lang EK, Thomas R, Davis R, et al. Multiphasic helical computerized tomography for the assessment of microscopic hematuria: a prospective study. J Urol 2004; 171:237–243.
- Gray Sears CL, Ward JF, Sears ST, Puckett MF, Kane CJ, Amling CL. Prospective comparison of computerized tomography and excretory urography in the initial evaluation of asymptomatic microhematuria. J Urol 2002; 168:2457–2460.
- Liu W, Mortelé KJ, Silverman SG. Incidental extraurinary findings at MDCT urography in patients with hematuria: prevalence and impact on imaging costs. AJR Am J Roentgenol 2005; 185:1051–1056.
- van Rhijn BW, van der Poel HG, van der Kwast TH. Urine markers for bladder cancer surveillance: a systematic review. Eur Urol 2005; 47:736–748.
- Black PC, Brown GA, Dinney CP. Molecular markers of urothelial cancer and their use in the monitoring of superficial urothelial cancer. J Clin Oncol 2006; 24:5528–5535.
- Rao PR, Jones JS. Retrospective chart review of consultations for hematuria, 2004–2006. Unpublished data. Manuscript in progress.
Many people have some amount of blood in their urine, but relatively few have a serious problem.
In a population-based study in Rochester, Minnesota, red blood cells were found in the urine of 13% of symptom-free adults.1 In other studies, the figure ranged from 9% to 18%.2
Hematuria is sometimes detected during investigation of symptoms such as dysuria, urinary frequency, or flank pain. However, many referrals to urologists are made purely on the basis of the results of a dipstick urinalysis screening test in a patient without symptoms.
The United States Preventive Task Force3 discourages routine testing for hematuria to screen for bladder cancer in patients without symptoms, and the Canadian Task Force on the Periodic Health Examination4 and the American Urological Association (AUA)2,5 do not recommend it either. Nevertheless, approximately 40% of primary care physicians believe in it,6 although the number seems to be declining.7 A reason that dipstick testing is so popular is that it is an inexpensive and simple way to detect glucosuria and medical renal disease.8–11
The risk of significant disease in a patient with microhematuria but without symptoms is low, and the evaluation for hematuria can be costly and invasive. For an individual patient with a hemoglobin-positive dipstick test, the finding should not be ignored, but the patient does not necessarily need a complete evaluation. It is important to determine which patients require urologic studies and consultation, nephrologic evaluation, or no intervention at all.
This review addresses issues related to hematuria for the primary care physician, clarifies some of the important definitions, builds on these terms to delineate which patients should be referred to a urologist, and provides simple recommendations about ancillary studies and their potential role before urologic consultation. Understanding this information will ultimately assure appropriate management of patients without symptoms who have positive dipstick screening tests, leading to decreased use of costly and invasive tests and more appropriate long-term follow-up.
BASIC DEFINITIONS
Gross (macroscopic) hematuria is blood in the urine that is visible without microscopy. This condition almost always warrants urologic evaluation.2,5
Microscopic hematuria, or microhematuria, is the finding of red blood cells in the urine on microscopy. (In contrast, in dipstick hematuria—see below—blood cells may or may not be present in the urine.)
“Dipstick hematuria” and “dipstick microhematuria” are potential misnomers. The dip-stick test for hematuria is a nondiagnostic screening test. A positive result is simply a color change due to oxidation of a test-strip reagent; it does not confirm that blood cells are present. Factors that can cause a false-positive result on a dipstick test include hemoglobinuria, myoglobinuria, concentrated urine, menstrual blood in the urine sample, and rigorous exercise.12 Thus, the diagnosis of hematuria cannot be made by dipstick alone. Unless red blood cells are seen microscopically, the term microhematuria is inappropriate.
Of note, if the specific gravity of the urine is very low (< 1.007), microscopy can fail to detect urinary red blood cells, owing to cell lysis.2 This limitation can be overcome by restricting the patient’s fluid intake and then repeating the urinalysis.
Many patients with a positive dipstick oxidation reaction are labeled as having dip-stick hematuria although microscopic analysis would show that red blood cells are absent. Perhaps the term dipstick pseudohematuria would be more accurate. These patients will not benefit from a costly and invasive urologic workup, so it is crucial to distinguish them from patients with true microhematuria.
SIGNIFICANT HEMATURIA: ≥ 3 RBCs/HPF
Microhematuria is often intermittent, and many healthy patients occasionally have a few red blood cells in the urine.13 However, no cutoff point for the amount of hematuria can be used to rule out the possibility of cancer.14 To account for these complicating factors, the AUA Best Practice Policy Panel on Asymptomatic Hematuria considered the literature and expert opinions to define the amount of microhematuria warranting evaluation in patients with risk factors for significant urologic disease.2,5
The AUA panel defined microhematuria as an average of three or more red blood cells per high-power microscopic field (RBCs/HPF) in two out of three properly collected and prepared specimens. Urine should be collected midstream after wiping the urethral meatus with a disinfectant and voiding the initial portion of urine into the toilet. For proper preparation of the urine sample, 10 mL of urine should be centrifuged at 2,000 rpm for 5 minutes and the supernatant discarded. The sediment should then be resuspended in 0.5 to 1.0 mL of remaining urine, and a drop of this suspension should be examined microscopically. If contamination is suggested (ie, if squamous epithelial cells, bacteria, or both are present), one should consider collecting a specimen through catheterization.2,5
The AUA guidelines do not specify some of the details of management, such as the timing of subsequent microscopic urinalyses, but we recommend that all urinalyses to establish whether significant hematuria is present be done within 3 to 6 months of the screening dip-stick test. If a patient has no risk factors for cancer and has a negative result on the first microscopic urinalysis, the follow-up test can be performed in 1 year. Table 1 shows risk factors for significant urologic disease that warrant evaluation in patients with asymptomatic hematuria.5
Isolated urinary findings that might warrant evaluation by a nephrologist rather than a urologist include proteinuria, red cell casts, and dysmorphic red blood cells, especially if the serum creatinine level is elevated.2,5 Many medical renal conditions (eg, glomerulonephritis) and hemoglobinopathies (eg, sickle cell trait) can cause blood in the urine; when these conditions are accompanied by risk factors for urologic disease, urologic evaluation is indicated as well.
CLINICAL RELEVANCE OF HEMATURIA
Approximately 25% of cases of macroscopic hematuria are due to urologic cancers,14,15 and another 34% are due to other significant urologic diseases14—thus, the recommendation that patients with macroscopic hematuria be evaluated by a urologist. In contrast, in microhematuria, the rates of cancer are much lower, ranging between 1% and 10% in large studies.2,5
The urine dipstick test has been found to be 65% to 99% specific for the presence of blood cells, free hemoglobin, or myoglobin.2,5 If the true specificity is closer to the lower figure and all patients with a positive dipstick test were referred to a urologist, this would mean the urologic workup would be unnecessary in up to 35% of them, because the dipstick result would be falsely positive.
But that is not all. Most causes of hemoglobinuria or myoglobinuria are of limited clinical significance, except for rare conditions that are usually clinically obvious, such as severe burn injury. Further, remember that from 9% to 18% of patients without symptoms have red blood cells in the urine.2,5 In theory, if everyone in the United States had a dipstick test, this would be positive in patients with hematuria as well as in those with hemoglobinuria, myoglobinuria, and other false-positives; if everyone with a positive dipstick result were then referred to a urologist, a substantial portion of the population would receive an unnecessary urologic referral.
Urologic referral and evaluation in these patients not only wastes money: if they undergo imaging studies, they are exposed to radiation and contrast media, with their associated risks, and if they undergo cystoscopy, they face its attendant discomfort and risk of infection.
WHICH PATIENTS WITH HEMATURIA TO REFER TO A UROLOGIST
Gross hematuria
Red or tea-colored urine usually indicates gross hematuria. When there is any doubt—such as in the case of a color-blind patient—the presence of red blood cells can be confirmed or ruled out by a microscopic urinalysis.
Nearly all patients with an episode of gross hematuria should be referred to a urologist. The sole exception to this rule can be made when a woman younger than 40 years experiences gross hematuria in the classic setting of a culture-proven, symptomatic urinary tract infection (UTI) and her infection, symptoms, and hematuria all resolve completely with appropriate antibiotics.2,5 However, bleeding from cancer is classically intermittent. Therefore, one should not skip the urine culture and just prescribe antibiotics empirically: the patient might actually have cancer, but the supposed UTI may appear to resolve with antibiotic therapy. For the same reason, resolution of hematuria in any other setting does not obviate the need for referral.
Another scenario usually associated with a benign cause is bleeding after extreme physical activity—also known as “runner’s hematuria” or “march hematuria” (so named because it sometimes occurs in soldiers after a particularly grueling training march). Importantly, even in this situation, one should still be suspicious and probably refer the patient to a urologist: just because the patient has just run a marathon, it does not mean that he or she does not have cancer.
Depending on the character, timing, location, and many other characteristics of the patient’s bleeding, a variety of studies may or may not be necessary. For example, blood-spotting of the underpants might signify urethral bleeding, and imaging and cytologic studies might not be indicated. In view of the variability in presentation and workup, we recommend that the proper workup for these patients be determined by a urologist.
Symptomatic microhematuria
Patients with true microhematuria (three or more RBCs/HPF) accompanied by bothersome or worrisome symptoms should be referred to a urologist. In a study in Scotland, Sultana et al16 found that cancer was present in 6 (5%) of 126 patients with microhematuria without symptoms compared with 13 (10.5%) of 124 patients with microhematuria and irritative voiding symptoms; the difference, however, was not statistically significant.
Microscopic urinalysis should be part of the evaluation for flank pain or certain urinary symptoms such as frequency, urgency, retention, or dysuria; results of this test at the time of symptoms can later help the urologist distinguish the cause of the symptoms or hematuria. In addition, in combination with dipstick analysis, microscopic analysis can help distinguish patients with UTI or medical renal disease. If the evaluation suggests that the hematuria and symptoms are due to a UTI, then the findings on a repeat microscopic analysis, performed after the infection has cleared, should be normal. If hematuria, defined as three or more RBCs/HPF, persists in two of three subsequent urinalyses, then the guidelines mandate diagnostic evaluation even if the urinalysis subsequently becomes negative.
Asymptomatic hematuria
In symptom-free patients with dipstick hematuria found on a screening examination, it is crucial to confirm and document true microhematuria. Per the AUA guidelines, microhematuria worthy of urologic workup is the presence of three or more RBCs/HPF on at least two out of three microscopic urinalyses.2,5 Patients with dipstick pseudohematuria do not meet this criterion and will not benefit from a costly and invasive evaluation. Conversely, patients with higher levels of microhematuria, who have any risk factors for cancer, or who are anxious about the test results might benefit from urologic consultation before a second urinalysis to confirm the first, positive finding.
Many patients younger than age 40 with asymptomatic microhematuria but no other risk factors for urinary tract cancer can be followed conservatively. Khadra et al14 reported that only 1 of 143 patients younger than 40 years with microhematuria had cancer. Similarly, Jones et al17 found, in a prospective study, that no man younger than 40 years with microscopic hematuria had cancer.
Of note: gross or microscopic blood in the urine, even in the setting of anticoagulation, is a marker of urinary tract pathology such as cancer, stones, or infection. Just as patients on anticoagulation therapy who develop gastrointestinal bleeding need a gastrointestinal evaluation, those with hematuria require a urologic evaluation.2,5
STUDIES TO CONSIDER BEFORE CONSULTATION
In symptom-free patients, it is inappropriate to order laboratory or imaging tests on the basis of a dipstick test alone, without confirming that they actually have hematuria. When the blood is confirmed to be present by microscopic examination of centrifuged urine (as described above), benign causes such as UTI should be considered. If a patient does have a UTI with hematuria, urinalysis should be repeated once the infection has cleared up.
Imaging studies
For symptomatic microhematuria. Patients with acute symptoms of renal colic should undergo computed tomography (CT) in a “stone protocol” (without contrast, with 3- to 5-mm cuts of the abdomen and pelvis) to assess for urinary lithiasis. Pregnancy should always be ruled out before radiation exposure; renal ultrasonography is generally the first-choice imaging study for pregnant patients.
For asymptomatic microhematuria. Patients without the classic flank pain of urolithiasis should undergo more extensive studies. For patients at increased risk of cancer, such as heavy smokers, CT urography is the optimal imaging study and is the test least likely to necessitate other follow-up studies.18–20 Other imaging options, including ultrasonography and intravenous pyelography, incompletely assess the upper urinary tracts including both renal parenchyma and urothelial surfaces. CT urography has been shown to find more than 40% of hematuria-causing lesions missed by other studies.18 Because ordering alternative imaging first will often result in redundant studies, CT urography is the preferred initial imaging study in the evaluation of hematuria.
Before exposing a patient to contrast media, one should ascertain that he or she is not allergic to it. In addition, in patients at risk of contrast nephropathy (ie, those older than 60 years, with diabetes, or with preexisting medical renal disease), one should check the serum creatinine concentration. Magnetic resonance urography, a more expensive study, is as accurate as CT for diagnosing many urologic conditions, so it can be performed in lieu of CT urography in patients with renal insufficiency, iodine allergy, or any reason to avoid ionizing radiation. Some clinicians perform plain radiography of the kidneys, ureters, and bladder as well as ultrasonography in this setting, but determination of the appropriate alternative to CT urography, if required, is best left to the urologist.
Other tests
Cytologic testing of the urine can be valuable in patients with gross hematuria and in those with microhematuria who have risk factors for urinary tract cancer. Although its reported median sensitivity for malignancy is only 48%, a positive cytologic test is approximately 94% specific for malignancy.21 Other studies, such as the fluorescence in situ hybridization assay, and the nuclear matrix protein 22 test do not yet have a clear role in the diagnosis of urinary tract disease.22
However, in general, we caution non-urologists not to order special tumor marker or cytologic tests, or to do so only with careful forethought. Although these studies occasionally detect occult cancer in patients at high risk, an “atypical” finding on cytology or a positive tumor marker test can lead to inappropriate referral and unnecessary biopsy or other tests.
WHEN NOT TO REFER A PATIENT WITH HEMATURIA TO A UROLOGIST
Symptom-free patients with a positive dipstick hemoglobin test should not immediately be referred to a urologist: they should have a microscopic urinalysis first to determine whether they actually have microhematuria, unless microscopic laboratory services are unavailable. Only patients with documented true hematuria, as defined by the AUA guidelines, should be referred for urologic evaluation and diagnostic testing. Once a patient is referred for evaluation, the consultant is under clear pressure to perform a complete investigation to fulfill the expectations of the referring physician. Avoiding expensive unnecessary testing and referral in those without hematuria allows appropriate utilization of resources.
Patients with microhematuria associated with a UTI should have a repeat urinalysis after the UTI is successfully treated; if the hematuria clears with the infection, then the patient needs no further evaluation. Patients with dipstick pseudohematuria and significant proteinuria or a predominance of dysmorphic urinary blood cells might benefit from an evaluation by a nephrologist rather than a urologist.2 This is especially true if the patient has an elevated serum creatinine level.
ECONOMIC RELEVANCE
In our tertiary care urology clinic, approximately 75% of patients who are referred to us because of microhematuria have not had a microscopic urinalysis before coming here. On further evaluation, up to 75% of these patients are found to have dipstick pseudohematuria that did not actually require consultation or evaluation.23 It is possible that this occurs even more frequently in the general practice setting.
A Medicare level-4 urologic consultation for hematuria costs $170; the cumulative cost of unwarranted referrals is undoubtedly substantial. Even more money is wasted on CT urography, cytology, and other testing performed before urologic consultation in patients ultimately found not to have true hematuria. The economic and iatrogenic risks of evaluation cannot be justified in patients who do not exhibit findings that can be considered abnormal as defined in this article.
CONCLUSION
It is important to distinguish whether hematuria is microscopic or macroscopic, whether there are associated symptoms, and whether a patient has risk factors for significant urologic disease. While dipstick tests are sensitive, they do not reliably diagnose microhematuria, which is the microscopically proven presence of urinary red blood cells. Positive dipstick tests should always be followed by microscopic urinalysis; failure to do so can result in the unfortunate and unnecessary evaluation of dipstick pseudohematuria, a normal condition.
The AUA defines significant hematuria as three or more RBCs/HPF in two of three properly prepared specimens.2 This should determine whether a symptom-free patient needs urologic referral and evaluation for hematuria.
By following these principles, primary care physicians have a valuable opportunity to direct medical care, increase the efficiency of our health care system, and protect patients from the anxiety, costs, and risks of an unnecessary urologic workup.
Many people have some amount of blood in their urine, but relatively few have a serious problem.
In a population-based study in Rochester, Minnesota, red blood cells were found in the urine of 13% of symptom-free adults.1 In other studies, the figure ranged from 9% to 18%.2
Hematuria is sometimes detected during investigation of symptoms such as dysuria, urinary frequency, or flank pain. However, many referrals to urologists are made purely on the basis of the results of a dipstick urinalysis screening test in a patient without symptoms.
The United States Preventive Task Force3 discourages routine testing for hematuria to screen for bladder cancer in patients without symptoms, and the Canadian Task Force on the Periodic Health Examination4 and the American Urological Association (AUA)2,5 do not recommend it either. Nevertheless, approximately 40% of primary care physicians believe in it,6 although the number seems to be declining.7 A reason that dipstick testing is so popular is that it is an inexpensive and simple way to detect glucosuria and medical renal disease.8–11
The risk of significant disease in a patient with microhematuria but without symptoms is low, and the evaluation for hematuria can be costly and invasive. For an individual patient with a hemoglobin-positive dipstick test, the finding should not be ignored, but the patient does not necessarily need a complete evaluation. It is important to determine which patients require urologic studies and consultation, nephrologic evaluation, or no intervention at all.
This review addresses issues related to hematuria for the primary care physician, clarifies some of the important definitions, builds on these terms to delineate which patients should be referred to a urologist, and provides simple recommendations about ancillary studies and their potential role before urologic consultation. Understanding this information will ultimately assure appropriate management of patients without symptoms who have positive dipstick screening tests, leading to decreased use of costly and invasive tests and more appropriate long-term follow-up.
BASIC DEFINITIONS
Gross (macroscopic) hematuria is blood in the urine that is visible without microscopy. This condition almost always warrants urologic evaluation.2,5
Microscopic hematuria, or microhematuria, is the finding of red blood cells in the urine on microscopy. (In contrast, in dipstick hematuria—see below—blood cells may or may not be present in the urine.)
“Dipstick hematuria” and “dipstick microhematuria” are potential misnomers. The dip-stick test for hematuria is a nondiagnostic screening test. A positive result is simply a color change due to oxidation of a test-strip reagent; it does not confirm that blood cells are present. Factors that can cause a false-positive result on a dipstick test include hemoglobinuria, myoglobinuria, concentrated urine, menstrual blood in the urine sample, and rigorous exercise.12 Thus, the diagnosis of hematuria cannot be made by dipstick alone. Unless red blood cells are seen microscopically, the term microhematuria is inappropriate.
Of note, if the specific gravity of the urine is very low (< 1.007), microscopy can fail to detect urinary red blood cells, owing to cell lysis.2 This limitation can be overcome by restricting the patient’s fluid intake and then repeating the urinalysis.
Many patients with a positive dipstick oxidation reaction are labeled as having dip-stick hematuria although microscopic analysis would show that red blood cells are absent. Perhaps the term dipstick pseudohematuria would be more accurate. These patients will not benefit from a costly and invasive urologic workup, so it is crucial to distinguish them from patients with true microhematuria.
SIGNIFICANT HEMATURIA: ≥ 3 RBCs/HPF
Microhematuria is often intermittent, and many healthy patients occasionally have a few red blood cells in the urine.13 However, no cutoff point for the amount of hematuria can be used to rule out the possibility of cancer.14 To account for these complicating factors, the AUA Best Practice Policy Panel on Asymptomatic Hematuria considered the literature and expert opinions to define the amount of microhematuria warranting evaluation in patients with risk factors for significant urologic disease.2,5
The AUA panel defined microhematuria as an average of three or more red blood cells per high-power microscopic field (RBCs/HPF) in two out of three properly collected and prepared specimens. Urine should be collected midstream after wiping the urethral meatus with a disinfectant and voiding the initial portion of urine into the toilet. For proper preparation of the urine sample, 10 mL of urine should be centrifuged at 2,000 rpm for 5 minutes and the supernatant discarded. The sediment should then be resuspended in 0.5 to 1.0 mL of remaining urine, and a drop of this suspension should be examined microscopically. If contamination is suggested (ie, if squamous epithelial cells, bacteria, or both are present), one should consider collecting a specimen through catheterization.2,5
The AUA guidelines do not specify some of the details of management, such as the timing of subsequent microscopic urinalyses, but we recommend that all urinalyses to establish whether significant hematuria is present be done within 3 to 6 months of the screening dip-stick test. If a patient has no risk factors for cancer and has a negative result on the first microscopic urinalysis, the follow-up test can be performed in 1 year. Table 1 shows risk factors for significant urologic disease that warrant evaluation in patients with asymptomatic hematuria.5
Isolated urinary findings that might warrant evaluation by a nephrologist rather than a urologist include proteinuria, red cell casts, and dysmorphic red blood cells, especially if the serum creatinine level is elevated.2,5 Many medical renal conditions (eg, glomerulonephritis) and hemoglobinopathies (eg, sickle cell trait) can cause blood in the urine; when these conditions are accompanied by risk factors for urologic disease, urologic evaluation is indicated as well.
CLINICAL RELEVANCE OF HEMATURIA
Approximately 25% of cases of macroscopic hematuria are due to urologic cancers,14,15 and another 34% are due to other significant urologic diseases14—thus, the recommendation that patients with macroscopic hematuria be evaluated by a urologist. In contrast, in microhematuria, the rates of cancer are much lower, ranging between 1% and 10% in large studies.2,5
The urine dipstick test has been found to be 65% to 99% specific for the presence of blood cells, free hemoglobin, or myoglobin.2,5 If the true specificity is closer to the lower figure and all patients with a positive dipstick test were referred to a urologist, this would mean the urologic workup would be unnecessary in up to 35% of them, because the dipstick result would be falsely positive.
But that is not all. Most causes of hemoglobinuria or myoglobinuria are of limited clinical significance, except for rare conditions that are usually clinically obvious, such as severe burn injury. Further, remember that from 9% to 18% of patients without symptoms have red blood cells in the urine.2,5 In theory, if everyone in the United States had a dipstick test, this would be positive in patients with hematuria as well as in those with hemoglobinuria, myoglobinuria, and other false-positives; if everyone with a positive dipstick result were then referred to a urologist, a substantial portion of the population would receive an unnecessary urologic referral.
Urologic referral and evaluation in these patients not only wastes money: if they undergo imaging studies, they are exposed to radiation and contrast media, with their associated risks, and if they undergo cystoscopy, they face its attendant discomfort and risk of infection.
WHICH PATIENTS WITH HEMATURIA TO REFER TO A UROLOGIST
Gross hematuria
Red or tea-colored urine usually indicates gross hematuria. When there is any doubt—such as in the case of a color-blind patient—the presence of red blood cells can be confirmed or ruled out by a microscopic urinalysis.
Nearly all patients with an episode of gross hematuria should be referred to a urologist. The sole exception to this rule can be made when a woman younger than 40 years experiences gross hematuria in the classic setting of a culture-proven, symptomatic urinary tract infection (UTI) and her infection, symptoms, and hematuria all resolve completely with appropriate antibiotics.2,5 However, bleeding from cancer is classically intermittent. Therefore, one should not skip the urine culture and just prescribe antibiotics empirically: the patient might actually have cancer, but the supposed UTI may appear to resolve with antibiotic therapy. For the same reason, resolution of hematuria in any other setting does not obviate the need for referral.
Another scenario usually associated with a benign cause is bleeding after extreme physical activity—also known as “runner’s hematuria” or “march hematuria” (so named because it sometimes occurs in soldiers after a particularly grueling training march). Importantly, even in this situation, one should still be suspicious and probably refer the patient to a urologist: just because the patient has just run a marathon, it does not mean that he or she does not have cancer.
Depending on the character, timing, location, and many other characteristics of the patient’s bleeding, a variety of studies may or may not be necessary. For example, blood-spotting of the underpants might signify urethral bleeding, and imaging and cytologic studies might not be indicated. In view of the variability in presentation and workup, we recommend that the proper workup for these patients be determined by a urologist.
Symptomatic microhematuria
Patients with true microhematuria (three or more RBCs/HPF) accompanied by bothersome or worrisome symptoms should be referred to a urologist. In a study in Scotland, Sultana et al16 found that cancer was present in 6 (5%) of 126 patients with microhematuria without symptoms compared with 13 (10.5%) of 124 patients with microhematuria and irritative voiding symptoms; the difference, however, was not statistically significant.
Microscopic urinalysis should be part of the evaluation for flank pain or certain urinary symptoms such as frequency, urgency, retention, or dysuria; results of this test at the time of symptoms can later help the urologist distinguish the cause of the symptoms or hematuria. In addition, in combination with dipstick analysis, microscopic analysis can help distinguish patients with UTI or medical renal disease. If the evaluation suggests that the hematuria and symptoms are due to a UTI, then the findings on a repeat microscopic analysis, performed after the infection has cleared, should be normal. If hematuria, defined as three or more RBCs/HPF, persists in two of three subsequent urinalyses, then the guidelines mandate diagnostic evaluation even if the urinalysis subsequently becomes negative.
Asymptomatic hematuria
In symptom-free patients with dipstick hematuria found on a screening examination, it is crucial to confirm and document true microhematuria. Per the AUA guidelines, microhematuria worthy of urologic workup is the presence of three or more RBCs/HPF on at least two out of three microscopic urinalyses.2,5 Patients with dipstick pseudohematuria do not meet this criterion and will not benefit from a costly and invasive evaluation. Conversely, patients with higher levels of microhematuria, who have any risk factors for cancer, or who are anxious about the test results might benefit from urologic consultation before a second urinalysis to confirm the first, positive finding.
Many patients younger than age 40 with asymptomatic microhematuria but no other risk factors for urinary tract cancer can be followed conservatively. Khadra et al14 reported that only 1 of 143 patients younger than 40 years with microhematuria had cancer. Similarly, Jones et al17 found, in a prospective study, that no man younger than 40 years with microscopic hematuria had cancer.
Of note: gross or microscopic blood in the urine, even in the setting of anticoagulation, is a marker of urinary tract pathology such as cancer, stones, or infection. Just as patients on anticoagulation therapy who develop gastrointestinal bleeding need a gastrointestinal evaluation, those with hematuria require a urologic evaluation.2,5
STUDIES TO CONSIDER BEFORE CONSULTATION
In symptom-free patients, it is inappropriate to order laboratory or imaging tests on the basis of a dipstick test alone, without confirming that they actually have hematuria. When the blood is confirmed to be present by microscopic examination of centrifuged urine (as described above), benign causes such as UTI should be considered. If a patient does have a UTI with hematuria, urinalysis should be repeated once the infection has cleared up.
Imaging studies
For symptomatic microhematuria. Patients with acute symptoms of renal colic should undergo computed tomography (CT) in a “stone protocol” (without contrast, with 3- to 5-mm cuts of the abdomen and pelvis) to assess for urinary lithiasis. Pregnancy should always be ruled out before radiation exposure; renal ultrasonography is generally the first-choice imaging study for pregnant patients.
For asymptomatic microhematuria. Patients without the classic flank pain of urolithiasis should undergo more extensive studies. For patients at increased risk of cancer, such as heavy smokers, CT urography is the optimal imaging study and is the test least likely to necessitate other follow-up studies.18–20 Other imaging options, including ultrasonography and intravenous pyelography, incompletely assess the upper urinary tracts including both renal parenchyma and urothelial surfaces. CT urography has been shown to find more than 40% of hematuria-causing lesions missed by other studies.18 Because ordering alternative imaging first will often result in redundant studies, CT urography is the preferred initial imaging study in the evaluation of hematuria.
Before exposing a patient to contrast media, one should ascertain that he or she is not allergic to it. In addition, in patients at risk of contrast nephropathy (ie, those older than 60 years, with diabetes, or with preexisting medical renal disease), one should check the serum creatinine concentration. Magnetic resonance urography, a more expensive study, is as accurate as CT for diagnosing many urologic conditions, so it can be performed in lieu of CT urography in patients with renal insufficiency, iodine allergy, or any reason to avoid ionizing radiation. Some clinicians perform plain radiography of the kidneys, ureters, and bladder as well as ultrasonography in this setting, but determination of the appropriate alternative to CT urography, if required, is best left to the urologist.
Other tests
Cytologic testing of the urine can be valuable in patients with gross hematuria and in those with microhematuria who have risk factors for urinary tract cancer. Although its reported median sensitivity for malignancy is only 48%, a positive cytologic test is approximately 94% specific for malignancy.21 Other studies, such as the fluorescence in situ hybridization assay, and the nuclear matrix protein 22 test do not yet have a clear role in the diagnosis of urinary tract disease.22
However, in general, we caution non-urologists not to order special tumor marker or cytologic tests, or to do so only with careful forethought. Although these studies occasionally detect occult cancer in patients at high risk, an “atypical” finding on cytology or a positive tumor marker test can lead to inappropriate referral and unnecessary biopsy or other tests.
WHEN NOT TO REFER A PATIENT WITH HEMATURIA TO A UROLOGIST
Symptom-free patients with a positive dipstick hemoglobin test should not immediately be referred to a urologist: they should have a microscopic urinalysis first to determine whether they actually have microhematuria, unless microscopic laboratory services are unavailable. Only patients with documented true hematuria, as defined by the AUA guidelines, should be referred for urologic evaluation and diagnostic testing. Once a patient is referred for evaluation, the consultant is under clear pressure to perform a complete investigation to fulfill the expectations of the referring physician. Avoiding expensive unnecessary testing and referral in those without hematuria allows appropriate utilization of resources.
Patients with microhematuria associated with a UTI should have a repeat urinalysis after the UTI is successfully treated; if the hematuria clears with the infection, then the patient needs no further evaluation. Patients with dipstick pseudohematuria and significant proteinuria or a predominance of dysmorphic urinary blood cells might benefit from an evaluation by a nephrologist rather than a urologist.2 This is especially true if the patient has an elevated serum creatinine level.
ECONOMIC RELEVANCE
In our tertiary care urology clinic, approximately 75% of patients who are referred to us because of microhematuria have not had a microscopic urinalysis before coming here. On further evaluation, up to 75% of these patients are found to have dipstick pseudohematuria that did not actually require consultation or evaluation.23 It is possible that this occurs even more frequently in the general practice setting.
A Medicare level-4 urologic consultation for hematuria costs $170; the cumulative cost of unwarranted referrals is undoubtedly substantial. Even more money is wasted on CT urography, cytology, and other testing performed before urologic consultation in patients ultimately found not to have true hematuria. The economic and iatrogenic risks of evaluation cannot be justified in patients who do not exhibit findings that can be considered abnormal as defined in this article.
CONCLUSION
It is important to distinguish whether hematuria is microscopic or macroscopic, whether there are associated symptoms, and whether a patient has risk factors for significant urologic disease. While dipstick tests are sensitive, they do not reliably diagnose microhematuria, which is the microscopically proven presence of urinary red blood cells. Positive dipstick tests should always be followed by microscopic urinalysis; failure to do so can result in the unfortunate and unnecessary evaluation of dipstick pseudohematuria, a normal condition.
The AUA defines significant hematuria as three or more RBCs/HPF in two of three properly prepared specimens.2 This should determine whether a symptom-free patient needs urologic referral and evaluation for hematuria.
By following these principles, primary care physicians have a valuable opportunity to direct medical care, increase the efficiency of our health care system, and protect patients from the anxiety, costs, and risks of an unnecessary urologic workup.
- Mohr DN, Offord KP, Owen RA, Melton LJ. Asymptomatic microhematuria and urologic disease. A population-based study. JAMA 1986; 256:224–229.
- Grossfeld GD, Litwin MS, Wolf JS, et al. Evaluation of asymptomatic microscopic hematuria in adults: the American Urological Association best practice policy—part I: definition, detection, prevalence, and etiology. Urology 2001; 57:599–603.
- US Preventive Services Task Force. Screening for Bladder Cancer, updated November 2004. Available at www.ahrq.gov/clinic/uspstf/uspsblad.htm.
- Logsetty S. Screening for bladder cancer. Canadian Task Force on the Periodic Health Examination. Canadian Guide to Clinical Preventive Health Care. 1994: Ottawa: Health Canada,826–836.
- Grossfeld GD, Litwin MS, Wolf JS, et al. Evaluation of asymptomatic microscopic hematuria in adults: The American Urological Association Best Practice Policy—Part II: patient evaluation, cytology, voided markers, imaging cystoscopy, nephrology evaluation, and follow-up. Urology 2001; 57:604–610.
- Prochazka AV, Lundahl K, Pearson W, Oboler SK, Anderson RJ. Support of evidence-based guidelines for the annual physical examination: a survey of primary care providers. Arch Intern Med 2005; 165:1347–1352.
- Chacko KM, Feinberg LE. Laboratory screening at preventive health exams: trend of testing, 1978–2004. Am J Prev Med 2007; 32:59–62.
- Mariani AJ, Luangphinith S, Loo S, Scottolini A, Hodges CV. Dipstick chemical urinalysis: an accurate cost-effective screening test. J Urol 1984; 132:64–66.
- Murphy TE. The urinalysis—inexpensive and informative. J Insur Med 2004; 36:320–326.
- Woolhandler S, Pels RJ, Bor DH, Himmelstein DU, Lawrence RS. Dipstick urinalysis screening of asymptomatic adults for urinary tract disorders. I. Hematuria and proteinuria. JAMA 1989; 262:1214–1219.
- Pels RJ, Bor DH, Woolhandler S, Himmelstein DU, Lawrence RS. Dipstick urinalysis screening of asymptomatic adults for urinary tract disorders. II. Bacteriuria. JAMA 1989; 262:1221–1224.
- Gerber GS, Brendler CBWein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CA. Evaluation of the urologic patient: history, physical examination, and urinalysis. Campbell-Walsh Urology 2007; 9th ed. Saunders Elsevier: Philadelphia:81–110.
- Cohen RA, Brown RS. Clinical practice. Microscopic hematuria. N Engl J Med 2003; 348:2330–2338.
- Khadra MH, Pickard RS, Charlton M, Powell PH, Neal DE. A prospective analysis of 1,930 patients with hematuria to evaluate current diagnostic practice. J Urol 2000; 163:524–527.
- Alishahi S, Byrne D, Goodman CM, Baxby K. Haematuria investigation based on a standard protocol: emphasis on the diagnosis of urological malignancy. J R Coll Surg Edinb 2002; 47:422–427.
- Sultana SR, Goodman CM, Byrne DJ, Baxby K. Microscopic haematuria: urological investigation using a standard protocol. Br J Urol 1996; 78:691–696.
- Jones DJ, Langstaff RJ, Holt SD, Morgans BT. The value of cystourethroscopy in the investigation of microscopic haematuria in adult males under 40 years. A prospective study of 100 patients. Br J Urol 1988; 62:541–545.
- Lang EK, Thomas R, Davis R, et al. Multiphasic helical computerized tomography for the assessment of microscopic hematuria: a prospective study. J Urol 2004; 171:237–243.
- Gray Sears CL, Ward JF, Sears ST, Puckett MF, Kane CJ, Amling CL. Prospective comparison of computerized tomography and excretory urography in the initial evaluation of asymptomatic microhematuria. J Urol 2002; 168:2457–2460.
- Liu W, Mortelé KJ, Silverman SG. Incidental extraurinary findings at MDCT urography in patients with hematuria: prevalence and impact on imaging costs. AJR Am J Roentgenol 2005; 185:1051–1056.
- van Rhijn BW, van der Poel HG, van der Kwast TH. Urine markers for bladder cancer surveillance: a systematic review. Eur Urol 2005; 47:736–748.
- Black PC, Brown GA, Dinney CP. Molecular markers of urothelial cancer and their use in the monitoring of superficial urothelial cancer. J Clin Oncol 2006; 24:5528–5535.
- Rao PR, Jones JS. Retrospective chart review of consultations for hematuria, 2004–2006. Unpublished data. Manuscript in progress.
- Mohr DN, Offord KP, Owen RA, Melton LJ. Asymptomatic microhematuria and urologic disease. A population-based study. JAMA 1986; 256:224–229.
- Grossfeld GD, Litwin MS, Wolf JS, et al. Evaluation of asymptomatic microscopic hematuria in adults: the American Urological Association best practice policy—part I: definition, detection, prevalence, and etiology. Urology 2001; 57:599–603.
- US Preventive Services Task Force. Screening for Bladder Cancer, updated November 2004. Available at www.ahrq.gov/clinic/uspstf/uspsblad.htm.
- Logsetty S. Screening for bladder cancer. Canadian Task Force on the Periodic Health Examination. Canadian Guide to Clinical Preventive Health Care. 1994: Ottawa: Health Canada,826–836.
- Grossfeld GD, Litwin MS, Wolf JS, et al. Evaluation of asymptomatic microscopic hematuria in adults: The American Urological Association Best Practice Policy—Part II: patient evaluation, cytology, voided markers, imaging cystoscopy, nephrology evaluation, and follow-up. Urology 2001; 57:604–610.
- Prochazka AV, Lundahl K, Pearson W, Oboler SK, Anderson RJ. Support of evidence-based guidelines for the annual physical examination: a survey of primary care providers. Arch Intern Med 2005; 165:1347–1352.
- Chacko KM, Feinberg LE. Laboratory screening at preventive health exams: trend of testing, 1978–2004. Am J Prev Med 2007; 32:59–62.
- Mariani AJ, Luangphinith S, Loo S, Scottolini A, Hodges CV. Dipstick chemical urinalysis: an accurate cost-effective screening test. J Urol 1984; 132:64–66.
- Murphy TE. The urinalysis—inexpensive and informative. J Insur Med 2004; 36:320–326.
- Woolhandler S, Pels RJ, Bor DH, Himmelstein DU, Lawrence RS. Dipstick urinalysis screening of asymptomatic adults for urinary tract disorders. I. Hematuria and proteinuria. JAMA 1989; 262:1214–1219.
- Pels RJ, Bor DH, Woolhandler S, Himmelstein DU, Lawrence RS. Dipstick urinalysis screening of asymptomatic adults for urinary tract disorders. II. Bacteriuria. JAMA 1989; 262:1221–1224.
- Gerber GS, Brendler CBWein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CA. Evaluation of the urologic patient: history, physical examination, and urinalysis. Campbell-Walsh Urology 2007; 9th ed. Saunders Elsevier: Philadelphia:81–110.
- Cohen RA, Brown RS. Clinical practice. Microscopic hematuria. N Engl J Med 2003; 348:2330–2338.
- Khadra MH, Pickard RS, Charlton M, Powell PH, Neal DE. A prospective analysis of 1,930 patients with hematuria to evaluate current diagnostic practice. J Urol 2000; 163:524–527.
- Alishahi S, Byrne D, Goodman CM, Baxby K. Haematuria investigation based on a standard protocol: emphasis on the diagnosis of urological malignancy. J R Coll Surg Edinb 2002; 47:422–427.
- Sultana SR, Goodman CM, Byrne DJ, Baxby K. Microscopic haematuria: urological investigation using a standard protocol. Br J Urol 1996; 78:691–696.
- Jones DJ, Langstaff RJ, Holt SD, Morgans BT. The value of cystourethroscopy in the investigation of microscopic haematuria in adult males under 40 years. A prospective study of 100 patients. Br J Urol 1988; 62:541–545.
- Lang EK, Thomas R, Davis R, et al. Multiphasic helical computerized tomography for the assessment of microscopic hematuria: a prospective study. J Urol 2004; 171:237–243.
- Gray Sears CL, Ward JF, Sears ST, Puckett MF, Kane CJ, Amling CL. Prospective comparison of computerized tomography and excretory urography in the initial evaluation of asymptomatic microhematuria. J Urol 2002; 168:2457–2460.
- Liu W, Mortelé KJ, Silverman SG. Incidental extraurinary findings at MDCT urography in patients with hematuria: prevalence and impact on imaging costs. AJR Am J Roentgenol 2005; 185:1051–1056.
- van Rhijn BW, van der Poel HG, van der Kwast TH. Urine markers for bladder cancer surveillance: a systematic review. Eur Urol 2005; 47:736–748.
- Black PC, Brown GA, Dinney CP. Molecular markers of urothelial cancer and their use in the monitoring of superficial urothelial cancer. J Clin Oncol 2006; 24:5528–5535.
- Rao PR, Jones JS. Retrospective chart review of consultations for hematuria, 2004–2006. Unpublished data. Manuscript in progress.
KEY POINTS
- Dipstick tests by themselves do not confirm that hematuria is present; thus, “dipstick hematuria” is a potential misnomer. Patients without symptoms who have a positive dipstick test and negative microscopic urinalysis are better described as having dipstick pseudohematuria, a clinically insignificant finding.
- Significant hematuria is defined as three or more red blood cells per high-power field in a properly collected and centrifuged urine specimen; this is the definition that should dictate which patients require further urologic evaluation.
- Since the evaluation for hematuria usually includes cystoscopy and imaging studies, it is crucial to confirm that hematuria is truly present before initiating an invasive and costly evaluation.
Four no more: The 'PSA cutoff era' is over
Prostate-specific antigen (PSA) testing has been mired in controversy throughout the short time it has been a clinical tool for detecting prostate cancer. During the first decade after it was approved for prostate cancer screening, the dogma prevailed that the upper limit of normal was 4.0 μg/L. Healthy patients with values above this cutoff were believed to be at risk of prostate cancer and were usually advised to undergo biopsy. Patients with levels below this threshold were told they had normal reading sand were reassured that they did not have prostate cancer.
NO PSA VALUE RULES CANCER IN OR OUT
But more men have prostate cancer than we thought. Initial reports suggested that men with a slightly elevated PSA value (4.0–9.9 μg/L) had a 22% chance of having prostate cancer, and those with a significant elevation (values of 10.0 μg/L or higher) had a 67% risk.1 However, these numbers were based on“sextant” biopsies (ie, in which only six samples are taken)—a technique fraught with a high false-negative detection rate. If more biopsy samples per patient are taken, approximately twice as many men with PSA levels above 4.0 are found to harbor prostate cancer, in the range of 40% to 50%.2
An individual patient’s PSA value is only part of the equation. Other risk factors need to be considered, such as his age, race, family history, findings on digital rectal examination, prostate size, results of earlier prostate biopsies, percent free PSA ratio, and whether he takes a 5-alpha reductase inhibitor. Moreover, PSA levels in men who have undergone treatment for prostate cancer are completely independent of the reference ranges in widespread laboratory use, making such references and thresholds even more meaningless in this setting.
Depending on their other risk factors, two men with identical PSA levels can have very different risks of prostate cancer. Conversely, if all their other risk factors are the same and one man has a PSA level of 3.9 μg/L and th eother has a level of 4.1, their risk is essentially identical.
Many patients and physicians are perplexed about PSA testing, often framing their concern in terms of “inaccuracy.” However, accuracy is not the problem with PSA testing. Rather, the problem is that physicians insist on categorizing patients as either normal or abnormal, when it is abundantly clear that such a dichotomy does not exist. Nevertheless, laboratories around the world continue to foster this false division by printing a PSA reference range of less than 4.0 μg/L on their reports.
A MORE MEANINGFUL LABORATORY REPORT
In view of the unequivocal data, urologists at Cleveland Clinic Glickman Urological and Kidney Institute, in collaboration with the Department of Laboratory Medicine, have eliminated 4.0 μg/L as the upper limit of normal from our PSA reports. Instead of a normal range, our reports now include risk ranges from large series (as shown in Figure 1), which provide a more meaningful clinical picture than the categories normal or abnormal.3–5 Moreover, the report also carries an explanation, including a reference to a risk calculator, to assist the patient and physician in interpretingthe reading.
Interpreting these data will inevitably create new controversy, like any major challenge to the status quo. Nevertheless, it is certainly more appropriate to inform patients of their actual risk of having prostate cancer than it is to tell them that their PSA level is normal or abnormal.
Moreover, because many older men have asymptomatic cancer that may never pose a problem in their lifetime, the decision to recommend urologic evaluation or prostate biopsy should be individualized. Indeed, this will take greater consideration than it did with the old 4.0 cutoff: interpreting PSA levels is more complex than once believed.
This change is long overdue and will require substantial education of both physicians and patients. They need to know that there is no PSA level at which a biopsy is mandated, and that the decision to consider biopsy should be based on solid information:ie, what are the odds that this patient has cancer? And what are the odds that he has high-grade, aggressive cancer, as opposed to a more indolent form that might be appropriately ignored?
We anticipate concern that changing the way we report PSA values may increase patients’ anxiety and lead to more prostate biopsies being performed. That is emphatically not the intent of this initiative. Rather, our intent is to accurately report PSA values with a meaningful interpretation of their implications instead of reporting an artificial—and relatively meaningless—cutoff. Interpreting PSA levels in the context of all the above factors will help advance the understanding and management of prostate cancer risk and diagnosis.
- Catalona WJ, Smith DS, Ratliff TL, et al. Measurement of prostate-specific antigen in serum as a screening test for prostate cancer. N Engl J Med 1991; 324:1156–1161.
- Jones JS, Patel A, Schoenfield L, Rabets JC, Zippe CD, Magi-Galluzzi C. Saturation technique does not improve cancer detection as an initial prostate biopsy strategy. J Urol 2006; 175:485–488.
- Thompson IM, Pauler DK, Goodman PJ, et al. Prevalence of prostate cancer among men with a prostate-specific antigen level < or = 4.0 ng per milliliter. N Engl J Med 2004; 350:2239–2246.
- Thompson IM, Ankerst DP, Chi C, et al. Assessing prostate cancer risk: results from the Prostate Cancer Prevention Trial. J Natl Cancer Inst 2006; 98:529–534.
- Presti JC Jr, O’Dowd GJ, Miller MC, et al. Extended peripheral zone biopsy schemes increase cancer detection rates and minimize variance in prostate specific antigen and age related cancer rates: results of a community multi-practice study. J Urol 2003; 169:125–129.
Prostate-specific antigen (PSA) testing has been mired in controversy throughout the short time it has been a clinical tool for detecting prostate cancer. During the first decade after it was approved for prostate cancer screening, the dogma prevailed that the upper limit of normal was 4.0 μg/L. Healthy patients with values above this cutoff were believed to be at risk of prostate cancer and were usually advised to undergo biopsy. Patients with levels below this threshold were told they had normal reading sand were reassured that they did not have prostate cancer.
NO PSA VALUE RULES CANCER IN OR OUT
But more men have prostate cancer than we thought. Initial reports suggested that men with a slightly elevated PSA value (4.0–9.9 μg/L) had a 22% chance of having prostate cancer, and those with a significant elevation (values of 10.0 μg/L or higher) had a 67% risk.1 However, these numbers were based on“sextant” biopsies (ie, in which only six samples are taken)—a technique fraught with a high false-negative detection rate. If more biopsy samples per patient are taken, approximately twice as many men with PSA levels above 4.0 are found to harbor prostate cancer, in the range of 40% to 50%.2
An individual patient’s PSA value is only part of the equation. Other risk factors need to be considered, such as his age, race, family history, findings on digital rectal examination, prostate size, results of earlier prostate biopsies, percent free PSA ratio, and whether he takes a 5-alpha reductase inhibitor. Moreover, PSA levels in men who have undergone treatment for prostate cancer are completely independent of the reference ranges in widespread laboratory use, making such references and thresholds even more meaningless in this setting.
Depending on their other risk factors, two men with identical PSA levels can have very different risks of prostate cancer. Conversely, if all their other risk factors are the same and one man has a PSA level of 3.9 μg/L and th eother has a level of 4.1, their risk is essentially identical.
Many patients and physicians are perplexed about PSA testing, often framing their concern in terms of “inaccuracy.” However, accuracy is not the problem with PSA testing. Rather, the problem is that physicians insist on categorizing patients as either normal or abnormal, when it is abundantly clear that such a dichotomy does not exist. Nevertheless, laboratories around the world continue to foster this false division by printing a PSA reference range of less than 4.0 μg/L on their reports.
A MORE MEANINGFUL LABORATORY REPORT
In view of the unequivocal data, urologists at Cleveland Clinic Glickman Urological and Kidney Institute, in collaboration with the Department of Laboratory Medicine, have eliminated 4.0 μg/L as the upper limit of normal from our PSA reports. Instead of a normal range, our reports now include risk ranges from large series (as shown in Figure 1), which provide a more meaningful clinical picture than the categories normal or abnormal.3–5 Moreover, the report also carries an explanation, including a reference to a risk calculator, to assist the patient and physician in interpretingthe reading.
Interpreting these data will inevitably create new controversy, like any major challenge to the status quo. Nevertheless, it is certainly more appropriate to inform patients of their actual risk of having prostate cancer than it is to tell them that their PSA level is normal or abnormal.
Moreover, because many older men have asymptomatic cancer that may never pose a problem in their lifetime, the decision to recommend urologic evaluation or prostate biopsy should be individualized. Indeed, this will take greater consideration than it did with the old 4.0 cutoff: interpreting PSA levels is more complex than once believed.
This change is long overdue and will require substantial education of both physicians and patients. They need to know that there is no PSA level at which a biopsy is mandated, and that the decision to consider biopsy should be based on solid information:ie, what are the odds that this patient has cancer? And what are the odds that he has high-grade, aggressive cancer, as opposed to a more indolent form that might be appropriately ignored?
We anticipate concern that changing the way we report PSA values may increase patients’ anxiety and lead to more prostate biopsies being performed. That is emphatically not the intent of this initiative. Rather, our intent is to accurately report PSA values with a meaningful interpretation of their implications instead of reporting an artificial—and relatively meaningless—cutoff. Interpreting PSA levels in the context of all the above factors will help advance the understanding and management of prostate cancer risk and diagnosis.
Prostate-specific antigen (PSA) testing has been mired in controversy throughout the short time it has been a clinical tool for detecting prostate cancer. During the first decade after it was approved for prostate cancer screening, the dogma prevailed that the upper limit of normal was 4.0 μg/L. Healthy patients with values above this cutoff were believed to be at risk of prostate cancer and were usually advised to undergo biopsy. Patients with levels below this threshold were told they had normal reading sand were reassured that they did not have prostate cancer.
NO PSA VALUE RULES CANCER IN OR OUT
But more men have prostate cancer than we thought. Initial reports suggested that men with a slightly elevated PSA value (4.0–9.9 μg/L) had a 22% chance of having prostate cancer, and those with a significant elevation (values of 10.0 μg/L or higher) had a 67% risk.1 However, these numbers were based on“sextant” biopsies (ie, in which only six samples are taken)—a technique fraught with a high false-negative detection rate. If more biopsy samples per patient are taken, approximately twice as many men with PSA levels above 4.0 are found to harbor prostate cancer, in the range of 40% to 50%.2
An individual patient’s PSA value is only part of the equation. Other risk factors need to be considered, such as his age, race, family history, findings on digital rectal examination, prostate size, results of earlier prostate biopsies, percent free PSA ratio, and whether he takes a 5-alpha reductase inhibitor. Moreover, PSA levels in men who have undergone treatment for prostate cancer are completely independent of the reference ranges in widespread laboratory use, making such references and thresholds even more meaningless in this setting.
Depending on their other risk factors, two men with identical PSA levels can have very different risks of prostate cancer. Conversely, if all their other risk factors are the same and one man has a PSA level of 3.9 μg/L and th eother has a level of 4.1, their risk is essentially identical.
Many patients and physicians are perplexed about PSA testing, often framing their concern in terms of “inaccuracy.” However, accuracy is not the problem with PSA testing. Rather, the problem is that physicians insist on categorizing patients as either normal or abnormal, when it is abundantly clear that such a dichotomy does not exist. Nevertheless, laboratories around the world continue to foster this false division by printing a PSA reference range of less than 4.0 μg/L on their reports.
A MORE MEANINGFUL LABORATORY REPORT
In view of the unequivocal data, urologists at Cleveland Clinic Glickman Urological and Kidney Institute, in collaboration with the Department of Laboratory Medicine, have eliminated 4.0 μg/L as the upper limit of normal from our PSA reports. Instead of a normal range, our reports now include risk ranges from large series (as shown in Figure 1), which provide a more meaningful clinical picture than the categories normal or abnormal.3–5 Moreover, the report also carries an explanation, including a reference to a risk calculator, to assist the patient and physician in interpretingthe reading.
Interpreting these data will inevitably create new controversy, like any major challenge to the status quo. Nevertheless, it is certainly more appropriate to inform patients of their actual risk of having prostate cancer than it is to tell them that their PSA level is normal or abnormal.
Moreover, because many older men have asymptomatic cancer that may never pose a problem in their lifetime, the decision to recommend urologic evaluation or prostate biopsy should be individualized. Indeed, this will take greater consideration than it did with the old 4.0 cutoff: interpreting PSA levels is more complex than once believed.
This change is long overdue and will require substantial education of both physicians and patients. They need to know that there is no PSA level at which a biopsy is mandated, and that the decision to consider biopsy should be based on solid information:ie, what are the odds that this patient has cancer? And what are the odds that he has high-grade, aggressive cancer, as opposed to a more indolent form that might be appropriately ignored?
We anticipate concern that changing the way we report PSA values may increase patients’ anxiety and lead to more prostate biopsies being performed. That is emphatically not the intent of this initiative. Rather, our intent is to accurately report PSA values with a meaningful interpretation of their implications instead of reporting an artificial—and relatively meaningless—cutoff. Interpreting PSA levels in the context of all the above factors will help advance the understanding and management of prostate cancer risk and diagnosis.
- Catalona WJ, Smith DS, Ratliff TL, et al. Measurement of prostate-specific antigen in serum as a screening test for prostate cancer. N Engl J Med 1991; 324:1156–1161.
- Jones JS, Patel A, Schoenfield L, Rabets JC, Zippe CD, Magi-Galluzzi C. Saturation technique does not improve cancer detection as an initial prostate biopsy strategy. J Urol 2006; 175:485–488.
- Thompson IM, Pauler DK, Goodman PJ, et al. Prevalence of prostate cancer among men with a prostate-specific antigen level < or = 4.0 ng per milliliter. N Engl J Med 2004; 350:2239–2246.
- Thompson IM, Ankerst DP, Chi C, et al. Assessing prostate cancer risk: results from the Prostate Cancer Prevention Trial. J Natl Cancer Inst 2006; 98:529–534.
- Presti JC Jr, O’Dowd GJ, Miller MC, et al. Extended peripheral zone biopsy schemes increase cancer detection rates and minimize variance in prostate specific antigen and age related cancer rates: results of a community multi-practice study. J Urol 2003; 169:125–129.
- Catalona WJ, Smith DS, Ratliff TL, et al. Measurement of prostate-specific antigen in serum as a screening test for prostate cancer. N Engl J Med 1991; 324:1156–1161.
- Jones JS, Patel A, Schoenfield L, Rabets JC, Zippe CD, Magi-Galluzzi C. Saturation technique does not improve cancer detection as an initial prostate biopsy strategy. J Urol 2006; 175:485–488.
- Thompson IM, Pauler DK, Goodman PJ, et al. Prevalence of prostate cancer among men with a prostate-specific antigen level < or = 4.0 ng per milliliter. N Engl J Med 2004; 350:2239–2246.
- Thompson IM, Ankerst DP, Chi C, et al. Assessing prostate cancer risk: results from the Prostate Cancer Prevention Trial. J Natl Cancer Inst 2006; 98:529–534.
- Presti JC Jr, O’Dowd GJ, Miller MC, et al. Extended peripheral zone biopsy schemes increase cancer detection rates and minimize variance in prostate specific antigen and age related cancer rates: results of a community multi-practice study. J Urol 2003; 169:125–129.