User login
Although there are some glimmers of hope that U.S. birthweights may be declining, the average infant birthweight has remained significantly tilted toward obesity. Moreover, and alarming number of infants, children, and adolescents are obese.
In 2007-2008, 9.5% of infants and toddlers were at or above the 95th percentile of the weight-for-recumbent-length growth charts. Among children and adolescents aged 2-19 years, 11.9% were at or above the 97th percentile of the body-mass-index-for-age growth charts; 16.9% were at or above the 95th percentile; and 31.7% were at or above the 85th percentile of BMI for age (JAMA 2010;303:242-9).
While more recent reports of obesity in children indicate a modest decline in obesity among 2- to 5-year-olds (JAMA 2014;311:806-14), an alarming number of infants and children have excess adiposity (roughly twice what is expected). In addition, cardiovascular mortality later in life continues to rise.
The question arises, have childhood and adult obesity rates remained high because mothers are feeding their children the wrong foods or because these children were born obese? One also wonders, with respect to cardiovascular mortality in adulthood, is the in utero environment playing a role?
Old lessons, growing relevance
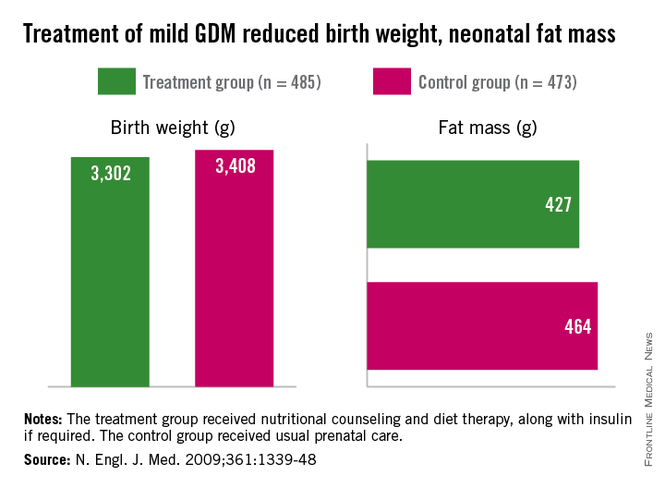
More than 3 decades ago, the late British physician Dr. David Barker got us thinking about how a challenging life in the womb can set us up for downstream ill health. He studied births from 1910 to 1945 and found that the cardiovascular mortality of individuals born during that time was inversely related to birthweight. Smaller babies, he found, could have cardiovascular mortality risks that were double or even quadruple the risks of larger babies.
Dr. Barker theorized that, when faced with undernutrition, the fetus adapts by sending more blood to the brain and sacrificing blood flow to less essential tissues. His theory about how growth and nutrition before birth may affect the heart became known as the "Barker Hypothesis." It was initially controversial, but it led to an explosion of research – especially since 2000 – on various downstream effects of the intrauterine environment.
Investigators have learned that it is not only cardiovascular mortality that is affected by low birthweight, but also the risk of developing diabetes and being overweight. This is because the fetus makes less essential systems insulin resistant. Insulin resistance persists in the womb and after birth as well, predisposing individuals to insulin resistance and obesity, both of which are closely linked to the risk of metabolic syndrome – a group of risk factors that raises the likelihood of developing heart disease, stroke, and diabetes.
In fact, further research on cohorts of Barker children – individuals who had low birthweights – has shown that not only have they had higher rates of cardiovascular disease, but they have had higher blood sugars and higher rates of insulin resistance as well.
Today, we appreciate a fuller picture of the Barker data, one that shows a reversal of this trend when birthweights reach 4,000-4,500 grams. At this point, what was a progressively downward slope of cardiovascular mortality rates with increasing birthweight suddenly shoots upward again when birthweight exceeds 4,000 g.
It is this end of the curve that is most relevant – and most concerning – for ob.gyns. today. Our problem in the United States is not so much one of starving or growth-restricted newborns, as these babies account for 5% or less of all births. It is one of overweight and obese newborns who now represent as many as 1 in 7 births. Just like the Barker babies who were growth restricted, these newborns have high insulin levels and increased risk of cardiovascular disease as adults.
Changing the trajectory
Both maternal obesity and gestational diabetes get at the heart of the Barker Hypothesis, albeit a twist, in that excessive maternal adiposity and associated insulin resistance results in high maternal blood glucose, transferring excessive nutrients to the fetus. This causes accumulation of fat in the fetus and programs the fetus for an increased and persistent risk of adiposity after birth, early-onset metabolic syndrome, and downstream cardiovascular disease in adulthood.
Dr. Dana Dabelea’s sibling study of almost 15 years ago demonstrated the long-term impact of the adverse intrauterine environment associated with maternal diabetes. Matched siblings who were born after their mothers had developed diabetes had almost double the rate of obesity as adolescents, compared with the siblings born before their mothers were diagnosed with diabetes. In childhood, these siblings ate at the same table and came from the same gene pools (with the same fathers), but they experienced dramatically different health outcomes (Diabetes 2000:49:2208-11).
This landmark study has been reproduced by other investigators who have compared children of mothers who had gestational diabetes and/or were overweight, with children whose mothers did not have gestational diabetes mellitus (GDM) or were of normal weight. Such studies have consistently shown that, faced with either or both maternal obesity and diabetes in utero, offspring were significantly more likely to become overweight children and adults with insulin resistance and other components of the metabolic syndrome.
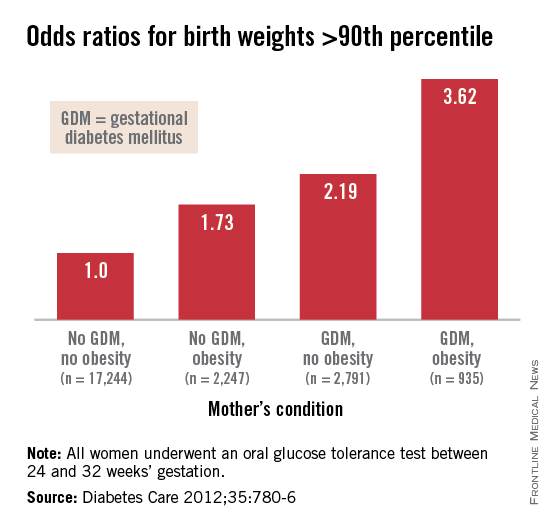
Importantly, we have evidence from randomized trials that interventions to treat GDM can effectively reduce rates of newborn obesity. While differences in birthweight between treatment and no-treatment arms have been modest, reductions in neonatal body fat, as measured by skin-fold thickness, the ponderal index, and birthweight percentile, have been highly significant.
The offspring of mothers who were treated in these trials, the Australian Carbohydrate Intolerance Study in Pregnant Women (N. Engl. J. Med. 2005;352:2477-86), and a study by Dr. Mark B. Landon and his colleagues (N. Engl. J. Med. 2009;361:1339-48), had approximately half of the newborn adiposity than did offspring of mothers who were not treated. In the latter study, maternal dietary measures alone were successful in reducing neonatal adiposity in over 80% of infants.
While published follow-up data of the offspring in these cohorts have covered only 5-8 years (showing persistently less adiposity in the treated groups), the offspring in the Australian cohort are still being monitored. Based on the cohort and case-control studies summarized above, it seems fair to expect that the children of mothers who were treated for GDM will have significantly better health profiles into and through adulthood.
We know from the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study that what were formerly considered mild and inconsequential maternal blood glucose levels are instead potentially quite harmful. The study showed a clear linear relationship between maternal fasting blood glucose levels, fetal cord blood insulin concentrations (a reflection of fetal glucose levels), and newborn body fat percentage (N. Engl. J. Med. 2008;358:1991-2002).
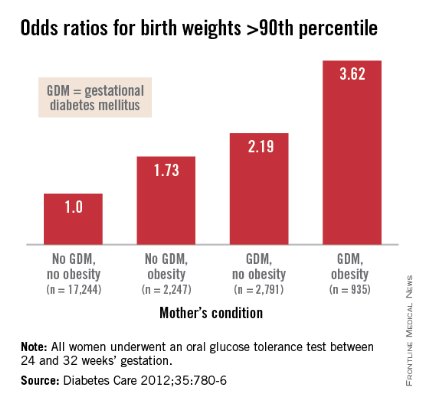
Interestingly, Dr. Patrick Catalano’s analysis of data from the HAPO study (Diabetes Care 2012;35:780-6) shows us more: Maternal obesity is almost as strong a driver of newborn obesity as is GDM. Compared with GDM (which increased the percentage of infant birthweights to greater than the 90th percentile by a factor of 2.19), maternal obesity alone increased the frequency of LGA by a factor of 1.73, and maternal obesity and GDM together increased LGA newborns by 3.62-fold.
In light of these recent findings, it is critical that we not only treat our patients who have GDM, but that we attempt to interrupt the chain of obesity that passes from mother to fetus, and from obese newborns onto their subsequent offspring.
A growing proportion of women across all race and ethnicity groups gain more than 40 pounds during pregnancy for singleton births, and many of them do not lose the weight between pregnancies. Increasingly, we have patients whose first child may not have been exposed to obesity in utero, but whose second child is exposed to overweight or obesity and higher levels of insulin resistance and glycemia.
The Institute of Medicine documented these issues in its 2009 report, "Weight Gain During Pregnancy: Reexamining the Guidelines." Data on maternal postpartum weights are not widely available, but data that have been collected suggest that gaining above recommended ranges is associated with excess maternal weight retention post partum, regardless of prepregnancy BMI. Women who gained above the range recommended by the IOM in 1990 had postpartum weight retention of 15-20 pounds. Among women who gained excessive amounts of weight, moreover, more than 40% retained more than 20 pounds, according to the report.
We must break the intergenerational transfer of obesity and insulin resistance by liberally treating GDM and optimizing glucose control during pregnancy. More importantly, we must emphasize to women the importance of having healthy weights at the time of conception. Recent research affirms that moderately simple interventions, such as dietary improvements and exercise can go a long way to achieving these goals. If we don’t – in keeping with the knowledge spurred on by Dr. Barker – we will be programming more newborns for life with insulin resistance, obesity, and disease.
Dr. Moore is a perinatologist who is chair of the department of reproductive medicine at the University of California, San Diego. He said he had no relevant financial disclosures.
Although there are some glimmers of hope that U.S. birthweights may be declining, the average infant birthweight has remained significantly tilted toward obesity. Moreover, and alarming number of infants, children, and adolescents are obese.
In 2007-2008, 9.5% of infants and toddlers were at or above the 95th percentile of the weight-for-recumbent-length growth charts. Among children and adolescents aged 2-19 years, 11.9% were at or above the 97th percentile of the body-mass-index-for-age growth charts; 16.9% were at or above the 95th percentile; and 31.7% were at or above the 85th percentile of BMI for age (JAMA 2010;303:242-9).

While more recent reports of obesity in children indicate a modest decline in obesity among 2- to 5-year-olds (JAMA 2014;311:806-14), an alarming number of infants and children have excess adiposity (roughly twice what is expected). In addition, cardiovascular mortality later in life continues to rise.
The question arises, have childhood and adult obesity rates remained high because mothers are feeding their children the wrong foods or because these children were born obese? One also wonders, with respect to cardiovascular mortality in adulthood, is the in utero environment playing a role?
Old lessons, growing relevance
More than 3 decades ago, the late British physician Dr. David Barker got us thinking about how a challenging life in the womb can set us up for downstream ill health. He studied births from 1910 to 1945 and found that the cardiovascular mortality of individuals born during that time was inversely related to birthweight. Smaller babies, he found, could have cardiovascular mortality risks that were double or even quadruple the risks of larger babies.

Dr. Barker theorized that, when faced with undernutrition, the fetus adapts by sending more blood to the brain and sacrificing blood flow to less essential tissues. His theory about how growth and nutrition before birth may affect the heart became known as the "Barker Hypothesis." It was initially controversial, but it led to an explosion of research – especially since 2000 – on various downstream effects of the intrauterine environment.
Investigators have learned that it is not only cardiovascular mortality that is affected by low birthweight, but also the risk of developing diabetes and being overweight. This is because the fetus makes less essential systems insulin resistant. Insulin resistance persists in the womb and after birth as well, predisposing individuals to insulin resistance and obesity, both of which are closely linked to the risk of metabolic syndrome – a group of risk factors that raises the likelihood of developing heart disease, stroke, and diabetes.
In fact, further research on cohorts of Barker children – individuals who had low birthweights – has shown that not only have they had higher rates of cardiovascular disease, but they have had higher blood sugars and higher rates of insulin resistance as well.
Today, we appreciate a fuller picture of the Barker data, one that shows a reversal of this trend when birthweights reach 4,000-4,500 grams. At this point, what was a progressively downward slope of cardiovascular mortality rates with increasing birthweight suddenly shoots upward again when birthweight exceeds 4,000 g.
It is this end of the curve that is most relevant – and most concerning – for ob.gyns. today. Our problem in the United States is not so much one of starving or growth-restricted newborns, as these babies account for 5% or less of all births. It is one of overweight and obese newborns who now represent as many as 1 in 7 births. Just like the Barker babies who were growth restricted, these newborns have high insulin levels and increased risk of cardiovascular disease as adults.
Changing the trajectory
Both maternal obesity and gestational diabetes get at the heart of the Barker Hypothesis, albeit a twist, in that excessive maternal adiposity and associated insulin resistance results in high maternal blood glucose, transferring excessive nutrients to the fetus. This causes accumulation of fat in the fetus and programs the fetus for an increased and persistent risk of adiposity after birth, early-onset metabolic syndrome, and downstream cardiovascular disease in adulthood.

Dr. Dana Dabelea’s sibling study of almost 15 years ago demonstrated the long-term impact of the adverse intrauterine environment associated with maternal diabetes. Matched siblings who were born after their mothers had developed diabetes had almost double the rate of obesity as adolescents, compared with the siblings born before their mothers were diagnosed with diabetes. In childhood, these siblings ate at the same table and came from the same gene pools (with the same fathers), but they experienced dramatically different health outcomes (Diabetes 2000:49:2208-11).
This landmark study has been reproduced by other investigators who have compared children of mothers who had gestational diabetes and/or were overweight, with children whose mothers did not have gestational diabetes mellitus (GDM) or were of normal weight. Such studies have consistently shown that, faced with either or both maternal obesity and diabetes in utero, offspring were significantly more likely to become overweight children and adults with insulin resistance and other components of the metabolic syndrome.
Importantly, we have evidence from randomized trials that interventions to treat GDM can effectively reduce rates of newborn obesity. While differences in birthweight between treatment and no-treatment arms have been modest, reductions in neonatal body fat, as measured by skin-fold thickness, the ponderal index, and birthweight percentile, have been highly significant.
The offspring of mothers who were treated in these trials, the Australian Carbohydrate Intolerance Study in Pregnant Women (N. Engl. J. Med. 2005;352:2477-86), and a study by Dr. Mark B. Landon and his colleagues (N. Engl. J. Med. 2009;361:1339-48), had approximately half of the newborn adiposity than did offspring of mothers who were not treated. In the latter study, maternal dietary measures alone were successful in reducing neonatal adiposity in over 80% of infants.
While published follow-up data of the offspring in these cohorts have covered only 5-8 years (showing persistently less adiposity in the treated groups), the offspring in the Australian cohort are still being monitored. Based on the cohort and case-control studies summarized above, it seems fair to expect that the children of mothers who were treated for GDM will have significantly better health profiles into and through adulthood.
We know from the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study that what were formerly considered mild and inconsequential maternal blood glucose levels are instead potentially quite harmful. The study showed a clear linear relationship between maternal fasting blood glucose levels, fetal cord blood insulin concentrations (a reflection of fetal glucose levels), and newborn body fat percentage (N. Engl. J. Med. 2008;358:1991-2002).
Interestingly, Dr. Patrick Catalano’s analysis of data from the HAPO study (Diabetes Care 2012;35:780-6) shows us more: Maternal obesity is almost as strong a driver of newborn obesity as is GDM. Compared with GDM (which increased the percentage of infant birthweights to greater than the 90th percentile by a factor of 2.19), maternal obesity alone increased the frequency of LGA by a factor of 1.73, and maternal obesity and GDM together increased LGA newborns by 3.62-fold.
In light of these recent findings, it is critical that we not only treat our patients who have GDM, but that we attempt to interrupt the chain of obesity that passes from mother to fetus, and from obese newborns onto their subsequent offspring.
A growing proportion of women across all race and ethnicity groups gain more than 40 pounds during pregnancy for singleton births, and many of them do not lose the weight between pregnancies. Increasingly, we have patients whose first child may not have been exposed to obesity in utero, but whose second child is exposed to overweight or obesity and higher levels of insulin resistance and glycemia.
The Institute of Medicine documented these issues in its 2009 report, "Weight Gain During Pregnancy: Reexamining the Guidelines." Data on maternal postpartum weights are not widely available, but data that have been collected suggest that gaining above recommended ranges is associated with excess maternal weight retention post partum, regardless of prepregnancy BMI. Women who gained above the range recommended by the IOM in 1990 had postpartum weight retention of 15-20 pounds. Among women who gained excessive amounts of weight, moreover, more than 40% retained more than 20 pounds, according to the report.
We must break the intergenerational transfer of obesity and insulin resistance by liberally treating GDM and optimizing glucose control during pregnancy. More importantly, we must emphasize to women the importance of having healthy weights at the time of conception. Recent research affirms that moderately simple interventions, such as dietary improvements and exercise can go a long way to achieving these goals. If we don’t – in keeping with the knowledge spurred on by Dr. Barker – we will be programming more newborns for life with insulin resistance, obesity, and disease.
Dr. Moore is a perinatologist who is chair of the department of reproductive medicine at the University of California, San Diego. He said he had no relevant financial disclosures.
Although there are some glimmers of hope that U.S. birthweights may be declining, the average infant birthweight has remained significantly tilted toward obesity. Moreover, and alarming number of infants, children, and adolescents are obese.
In 2007-2008, 9.5% of infants and toddlers were at or above the 95th percentile of the weight-for-recumbent-length growth charts. Among children and adolescents aged 2-19 years, 11.9% were at or above the 97th percentile of the body-mass-index-for-age growth charts; 16.9% were at or above the 95th percentile; and 31.7% were at or above the 85th percentile of BMI for age (JAMA 2010;303:242-9).

While more recent reports of obesity in children indicate a modest decline in obesity among 2- to 5-year-olds (JAMA 2014;311:806-14), an alarming number of infants and children have excess adiposity (roughly twice what is expected). In addition, cardiovascular mortality later in life continues to rise.
The question arises, have childhood and adult obesity rates remained high because mothers are feeding their children the wrong foods or because these children were born obese? One also wonders, with respect to cardiovascular mortality in adulthood, is the in utero environment playing a role?
Old lessons, growing relevance
More than 3 decades ago, the late British physician Dr. David Barker got us thinking about how a challenging life in the womb can set us up for downstream ill health. He studied births from 1910 to 1945 and found that the cardiovascular mortality of individuals born during that time was inversely related to birthweight. Smaller babies, he found, could have cardiovascular mortality risks that were double or even quadruple the risks of larger babies.

Dr. Barker theorized that, when faced with undernutrition, the fetus adapts by sending more blood to the brain and sacrificing blood flow to less essential tissues. His theory about how growth and nutrition before birth may affect the heart became known as the "Barker Hypothesis." It was initially controversial, but it led to an explosion of research – especially since 2000 – on various downstream effects of the intrauterine environment.
Investigators have learned that it is not only cardiovascular mortality that is affected by low birthweight, but also the risk of developing diabetes and being overweight. This is because the fetus makes less essential systems insulin resistant. Insulin resistance persists in the womb and after birth as well, predisposing individuals to insulin resistance and obesity, both of which are closely linked to the risk of metabolic syndrome – a group of risk factors that raises the likelihood of developing heart disease, stroke, and diabetes.
In fact, further research on cohorts of Barker children – individuals who had low birthweights – has shown that not only have they had higher rates of cardiovascular disease, but they have had higher blood sugars and higher rates of insulin resistance as well.
Today, we appreciate a fuller picture of the Barker data, one that shows a reversal of this trend when birthweights reach 4,000-4,500 grams. At this point, what was a progressively downward slope of cardiovascular mortality rates with increasing birthweight suddenly shoots upward again when birthweight exceeds 4,000 g.
It is this end of the curve that is most relevant – and most concerning – for ob.gyns. today. Our problem in the United States is not so much one of starving or growth-restricted newborns, as these babies account for 5% or less of all births. It is one of overweight and obese newborns who now represent as many as 1 in 7 births. Just like the Barker babies who were growth restricted, these newborns have high insulin levels and increased risk of cardiovascular disease as adults.
Changing the trajectory
Both maternal obesity and gestational diabetes get at the heart of the Barker Hypothesis, albeit a twist, in that excessive maternal adiposity and associated insulin resistance results in high maternal blood glucose, transferring excessive nutrients to the fetus. This causes accumulation of fat in the fetus and programs the fetus for an increased and persistent risk of adiposity after birth, early-onset metabolic syndrome, and downstream cardiovascular disease in adulthood.

Dr. Dana Dabelea’s sibling study of almost 15 years ago demonstrated the long-term impact of the adverse intrauterine environment associated with maternal diabetes. Matched siblings who were born after their mothers had developed diabetes had almost double the rate of obesity as adolescents, compared with the siblings born before their mothers were diagnosed with diabetes. In childhood, these siblings ate at the same table and came from the same gene pools (with the same fathers), but they experienced dramatically different health outcomes (Diabetes 2000:49:2208-11).
This landmark study has been reproduced by other investigators who have compared children of mothers who had gestational diabetes and/or were overweight, with children whose mothers did not have gestational diabetes mellitus (GDM) or were of normal weight. Such studies have consistently shown that, faced with either or both maternal obesity and diabetes in utero, offspring were significantly more likely to become overweight children and adults with insulin resistance and other components of the metabolic syndrome.
Importantly, we have evidence from randomized trials that interventions to treat GDM can effectively reduce rates of newborn obesity. While differences in birthweight between treatment and no-treatment arms have been modest, reductions in neonatal body fat, as measured by skin-fold thickness, the ponderal index, and birthweight percentile, have been highly significant.
The offspring of mothers who were treated in these trials, the Australian Carbohydrate Intolerance Study in Pregnant Women (N. Engl. J. Med. 2005;352:2477-86), and a study by Dr. Mark B. Landon and his colleagues (N. Engl. J. Med. 2009;361:1339-48), had approximately half of the newborn adiposity than did offspring of mothers who were not treated. In the latter study, maternal dietary measures alone were successful in reducing neonatal adiposity in over 80% of infants.
While published follow-up data of the offspring in these cohorts have covered only 5-8 years (showing persistently less adiposity in the treated groups), the offspring in the Australian cohort are still being monitored. Based on the cohort and case-control studies summarized above, it seems fair to expect that the children of mothers who were treated for GDM will have significantly better health profiles into and through adulthood.
We know from the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study that what were formerly considered mild and inconsequential maternal blood glucose levels are instead potentially quite harmful. The study showed a clear linear relationship between maternal fasting blood glucose levels, fetal cord blood insulin concentrations (a reflection of fetal glucose levels), and newborn body fat percentage (N. Engl. J. Med. 2008;358:1991-2002).
Interestingly, Dr. Patrick Catalano’s analysis of data from the HAPO study (Diabetes Care 2012;35:780-6) shows us more: Maternal obesity is almost as strong a driver of newborn obesity as is GDM. Compared with GDM (which increased the percentage of infant birthweights to greater than the 90th percentile by a factor of 2.19), maternal obesity alone increased the frequency of LGA by a factor of 1.73, and maternal obesity and GDM together increased LGA newborns by 3.62-fold.
In light of these recent findings, it is critical that we not only treat our patients who have GDM, but that we attempt to interrupt the chain of obesity that passes from mother to fetus, and from obese newborns onto their subsequent offspring.
A growing proportion of women across all race and ethnicity groups gain more than 40 pounds during pregnancy for singleton births, and many of them do not lose the weight between pregnancies. Increasingly, we have patients whose first child may not have been exposed to obesity in utero, but whose second child is exposed to overweight or obesity and higher levels of insulin resistance and glycemia.
The Institute of Medicine documented these issues in its 2009 report, "Weight Gain During Pregnancy: Reexamining the Guidelines." Data on maternal postpartum weights are not widely available, but data that have been collected suggest that gaining above recommended ranges is associated with excess maternal weight retention post partum, regardless of prepregnancy BMI. Women who gained above the range recommended by the IOM in 1990 had postpartum weight retention of 15-20 pounds. Among women who gained excessive amounts of weight, moreover, more than 40% retained more than 20 pounds, according to the report.
We must break the intergenerational transfer of obesity and insulin resistance by liberally treating GDM and optimizing glucose control during pregnancy. More importantly, we must emphasize to women the importance of having healthy weights at the time of conception. Recent research affirms that moderately simple interventions, such as dietary improvements and exercise can go a long way to achieving these goals. If we don’t – in keeping with the knowledge spurred on by Dr. Barker – we will be programming more newborns for life with insulin resistance, obesity, and disease.
Dr. Moore is a perinatologist who is chair of the department of reproductive medicine at the University of California, San Diego. He said he had no relevant financial disclosures.