User login
Gestational diabetes and the Barker Hypothesis
Although there are some glimmers of hope that U.S. birthweights may be declining, the average infant birthweight has remained significantly tilted toward obesity. Moreover, and alarming number of infants, children, and adolescents are obese.
In 2007-2008, 9.5% of infants and toddlers were at or above the 95th percentile of the weight-for-recumbent-length growth charts. Among children and adolescents aged 2-19 years, 11.9% were at or above the 97th percentile of the body-mass-index-for-age growth charts; 16.9% were at or above the 95th percentile; and 31.7% were at or above the 85th percentile of BMI for age (JAMA 2010;303:242-9).
While more recent reports of obesity in children indicate a modest decline in obesity among 2- to 5-year-olds (JAMA 2014;311:806-14), an alarming number of infants and children have excess adiposity (roughly twice what is expected). In addition, cardiovascular mortality later in life continues to rise.
The question arises, have childhood and adult obesity rates remained high because mothers are feeding their children the wrong foods or because these children were born obese? One also wonders, with respect to cardiovascular mortality in adulthood, is the in utero environment playing a role?
Old lessons, growing relevance
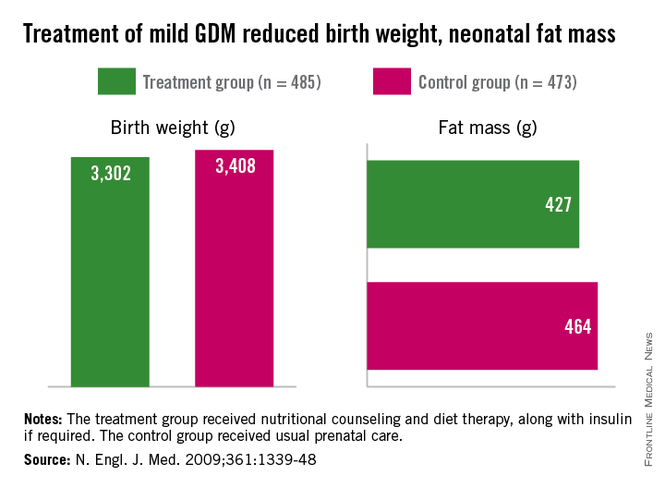
More than 3 decades ago, the late British physician Dr. David Barker got us thinking about how a challenging life in the womb can set us up for downstream ill health. He studied births from 1910 to 1945 and found that the cardiovascular mortality of individuals born during that time was inversely related to birthweight. Smaller babies, he found, could have cardiovascular mortality risks that were double or even quadruple the risks of larger babies.
Dr. Barker theorized that, when faced with undernutrition, the fetus adapts by sending more blood to the brain and sacrificing blood flow to less essential tissues. His theory about how growth and nutrition before birth may affect the heart became known as the "Barker Hypothesis." It was initially controversial, but it led to an explosion of research – especially since 2000 – on various downstream effects of the intrauterine environment.
Investigators have learned that it is not only cardiovascular mortality that is affected by low birthweight, but also the risk of developing diabetes and being overweight. This is because the fetus makes less essential systems insulin resistant. Insulin resistance persists in the womb and after birth as well, predisposing individuals to insulin resistance and obesity, both of which are closely linked to the risk of metabolic syndrome – a group of risk factors that raises the likelihood of developing heart disease, stroke, and diabetes.
In fact, further research on cohorts of Barker children – individuals who had low birthweights – has shown that not only have they had higher rates of cardiovascular disease, but they have had higher blood sugars and higher rates of insulin resistance as well.
Today, we appreciate a fuller picture of the Barker data, one that shows a reversal of this trend when birthweights reach 4,000-4,500 grams. At this point, what was a progressively downward slope of cardiovascular mortality rates with increasing birthweight suddenly shoots upward again when birthweight exceeds 4,000 g.
It is this end of the curve that is most relevant – and most concerning – for ob.gyns. today. Our problem in the United States is not so much one of starving or growth-restricted newborns, as these babies account for 5% or less of all births. It is one of overweight and obese newborns who now represent as many as 1 in 7 births. Just like the Barker babies who were growth restricted, these newborns have high insulin levels and increased risk of cardiovascular disease as adults.
Changing the trajectory
Both maternal obesity and gestational diabetes get at the heart of the Barker Hypothesis, albeit a twist, in that excessive maternal adiposity and associated insulin resistance results in high maternal blood glucose, transferring excessive nutrients to the fetus. This causes accumulation of fat in the fetus and programs the fetus for an increased and persistent risk of adiposity after birth, early-onset metabolic syndrome, and downstream cardiovascular disease in adulthood.
Dr. Dana Dabelea’s sibling study of almost 15 years ago demonstrated the long-term impact of the adverse intrauterine environment associated with maternal diabetes. Matched siblings who were born after their mothers had developed diabetes had almost double the rate of obesity as adolescents, compared with the siblings born before their mothers were diagnosed with diabetes. In childhood, these siblings ate at the same table and came from the same gene pools (with the same fathers), but they experienced dramatically different health outcomes (Diabetes 2000:49:2208-11).
This landmark study has been reproduced by other investigators who have compared children of mothers who had gestational diabetes and/or were overweight, with children whose mothers did not have gestational diabetes mellitus (GDM) or were of normal weight. Such studies have consistently shown that, faced with either or both maternal obesity and diabetes in utero, offspring were significantly more likely to become overweight children and adults with insulin resistance and other components of the metabolic syndrome.
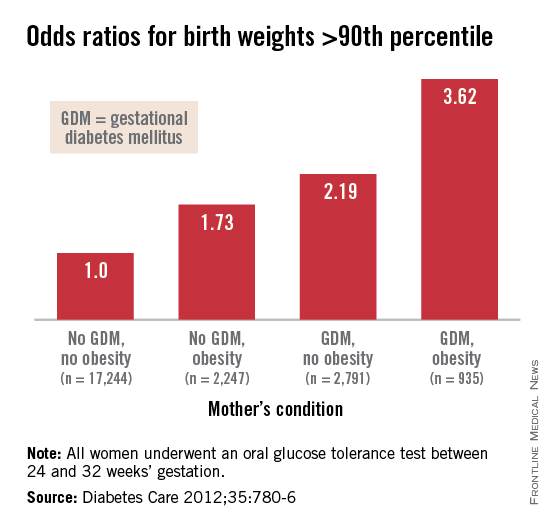
Importantly, we have evidence from randomized trials that interventions to treat GDM can effectively reduce rates of newborn obesity. While differences in birthweight between treatment and no-treatment arms have been modest, reductions in neonatal body fat, as measured by skin-fold thickness, the ponderal index, and birthweight percentile, have been highly significant.
The offspring of mothers who were treated in these trials, the Australian Carbohydrate Intolerance Study in Pregnant Women (N. Engl. J. Med. 2005;352:2477-86), and a study by Dr. Mark B. Landon and his colleagues (N. Engl. J. Med. 2009;361:1339-48), had approximately half of the newborn adiposity than did offspring of mothers who were not treated. In the latter study, maternal dietary measures alone were successful in reducing neonatal adiposity in over 80% of infants.
While published follow-up data of the offspring in these cohorts have covered only 5-8 years (showing persistently less adiposity in the treated groups), the offspring in the Australian cohort are still being monitored. Based on the cohort and case-control studies summarized above, it seems fair to expect that the children of mothers who were treated for GDM will have significantly better health profiles into and through adulthood.
We know from the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study that what were formerly considered mild and inconsequential maternal blood glucose levels are instead potentially quite harmful. The study showed a clear linear relationship between maternal fasting blood glucose levels, fetal cord blood insulin concentrations (a reflection of fetal glucose levels), and newborn body fat percentage (N. Engl. J. Med. 2008;358:1991-2002).
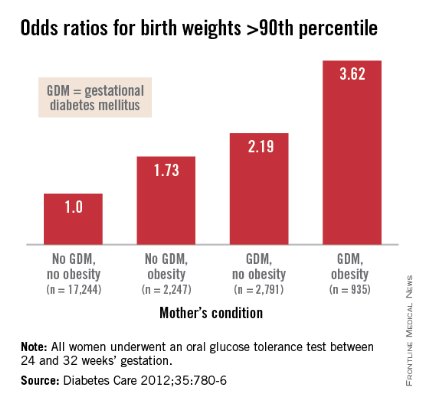
Interestingly, Dr. Patrick Catalano’s analysis of data from the HAPO study (Diabetes Care 2012;35:780-6) shows us more: Maternal obesity is almost as strong a driver of newborn obesity as is GDM. Compared with GDM (which increased the percentage of infant birthweights to greater than the 90th percentile by a factor of 2.19), maternal obesity alone increased the frequency of LGA by a factor of 1.73, and maternal obesity and GDM together increased LGA newborns by 3.62-fold.
In light of these recent findings, it is critical that we not only treat our patients who have GDM, but that we attempt to interrupt the chain of obesity that passes from mother to fetus, and from obese newborns onto their subsequent offspring.
A growing proportion of women across all race and ethnicity groups gain more than 40 pounds during pregnancy for singleton births, and many of them do not lose the weight between pregnancies. Increasingly, we have patients whose first child may not have been exposed to obesity in utero, but whose second child is exposed to overweight or obesity and higher levels of insulin resistance and glycemia.
The Institute of Medicine documented these issues in its 2009 report, "Weight Gain During Pregnancy: Reexamining the Guidelines." Data on maternal postpartum weights are not widely available, but data that have been collected suggest that gaining above recommended ranges is associated with excess maternal weight retention post partum, regardless of prepregnancy BMI. Women who gained above the range recommended by the IOM in 1990 had postpartum weight retention of 15-20 pounds. Among women who gained excessive amounts of weight, moreover, more than 40% retained more than 20 pounds, according to the report.
We must break the intergenerational transfer of obesity and insulin resistance by liberally treating GDM and optimizing glucose control during pregnancy. More importantly, we must emphasize to women the importance of having healthy weights at the time of conception. Recent research affirms that moderately simple interventions, such as dietary improvements and exercise can go a long way to achieving these goals. If we don’t – in keeping with the knowledge spurred on by Dr. Barker – we will be programming more newborns for life with insulin resistance, obesity, and disease.
Dr. Moore is a perinatologist who is chair of the department of reproductive medicine at the University of California, San Diego. He said he had no relevant financial disclosures.
Although there are some glimmers of hope that U.S. birthweights may be declining, the average infant birthweight has remained significantly tilted toward obesity. Moreover, and alarming number of infants, children, and adolescents are obese.
In 2007-2008, 9.5% of infants and toddlers were at or above the 95th percentile of the weight-for-recumbent-length growth charts. Among children and adolescents aged 2-19 years, 11.9% were at or above the 97th percentile of the body-mass-index-for-age growth charts; 16.9% were at or above the 95th percentile; and 31.7% were at or above the 85th percentile of BMI for age (JAMA 2010;303:242-9).

While more recent reports of obesity in children indicate a modest decline in obesity among 2- to 5-year-olds (JAMA 2014;311:806-14), an alarming number of infants and children have excess adiposity (roughly twice what is expected). In addition, cardiovascular mortality later in life continues to rise.
The question arises, have childhood and adult obesity rates remained high because mothers are feeding their children the wrong foods or because these children were born obese? One also wonders, with respect to cardiovascular mortality in adulthood, is the in utero environment playing a role?
Old lessons, growing relevance
More than 3 decades ago, the late British physician Dr. David Barker got us thinking about how a challenging life in the womb can set us up for downstream ill health. He studied births from 1910 to 1945 and found that the cardiovascular mortality of individuals born during that time was inversely related to birthweight. Smaller babies, he found, could have cardiovascular mortality risks that were double or even quadruple the risks of larger babies.

Dr. Barker theorized that, when faced with undernutrition, the fetus adapts by sending more blood to the brain and sacrificing blood flow to less essential tissues. His theory about how growth and nutrition before birth may affect the heart became known as the "Barker Hypothesis." It was initially controversial, but it led to an explosion of research – especially since 2000 – on various downstream effects of the intrauterine environment.
Investigators have learned that it is not only cardiovascular mortality that is affected by low birthweight, but also the risk of developing diabetes and being overweight. This is because the fetus makes less essential systems insulin resistant. Insulin resistance persists in the womb and after birth as well, predisposing individuals to insulin resistance and obesity, both of which are closely linked to the risk of metabolic syndrome – a group of risk factors that raises the likelihood of developing heart disease, stroke, and diabetes.
In fact, further research on cohorts of Barker children – individuals who had low birthweights – has shown that not only have they had higher rates of cardiovascular disease, but they have had higher blood sugars and higher rates of insulin resistance as well.
Today, we appreciate a fuller picture of the Barker data, one that shows a reversal of this trend when birthweights reach 4,000-4,500 grams. At this point, what was a progressively downward slope of cardiovascular mortality rates with increasing birthweight suddenly shoots upward again when birthweight exceeds 4,000 g.
It is this end of the curve that is most relevant – and most concerning – for ob.gyns. today. Our problem in the United States is not so much one of starving or growth-restricted newborns, as these babies account for 5% or less of all births. It is one of overweight and obese newborns who now represent as many as 1 in 7 births. Just like the Barker babies who were growth restricted, these newborns have high insulin levels and increased risk of cardiovascular disease as adults.
Changing the trajectory
Both maternal obesity and gestational diabetes get at the heart of the Barker Hypothesis, albeit a twist, in that excessive maternal adiposity and associated insulin resistance results in high maternal blood glucose, transferring excessive nutrients to the fetus. This causes accumulation of fat in the fetus and programs the fetus for an increased and persistent risk of adiposity after birth, early-onset metabolic syndrome, and downstream cardiovascular disease in adulthood.

Dr. Dana Dabelea’s sibling study of almost 15 years ago demonstrated the long-term impact of the adverse intrauterine environment associated with maternal diabetes. Matched siblings who were born after their mothers had developed diabetes had almost double the rate of obesity as adolescents, compared with the siblings born before their mothers were diagnosed with diabetes. In childhood, these siblings ate at the same table and came from the same gene pools (with the same fathers), but they experienced dramatically different health outcomes (Diabetes 2000:49:2208-11).
This landmark study has been reproduced by other investigators who have compared children of mothers who had gestational diabetes and/or were overweight, with children whose mothers did not have gestational diabetes mellitus (GDM) or were of normal weight. Such studies have consistently shown that, faced with either or both maternal obesity and diabetes in utero, offspring were significantly more likely to become overweight children and adults with insulin resistance and other components of the metabolic syndrome.
Importantly, we have evidence from randomized trials that interventions to treat GDM can effectively reduce rates of newborn obesity. While differences in birthweight between treatment and no-treatment arms have been modest, reductions in neonatal body fat, as measured by skin-fold thickness, the ponderal index, and birthweight percentile, have been highly significant.
The offspring of mothers who were treated in these trials, the Australian Carbohydrate Intolerance Study in Pregnant Women (N. Engl. J. Med. 2005;352:2477-86), and a study by Dr. Mark B. Landon and his colleagues (N. Engl. J. Med. 2009;361:1339-48), had approximately half of the newborn adiposity than did offspring of mothers who were not treated. In the latter study, maternal dietary measures alone were successful in reducing neonatal adiposity in over 80% of infants.
While published follow-up data of the offspring in these cohorts have covered only 5-8 years (showing persistently less adiposity in the treated groups), the offspring in the Australian cohort are still being monitored. Based on the cohort and case-control studies summarized above, it seems fair to expect that the children of mothers who were treated for GDM will have significantly better health profiles into and through adulthood.
We know from the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study that what were formerly considered mild and inconsequential maternal blood glucose levels are instead potentially quite harmful. The study showed a clear linear relationship between maternal fasting blood glucose levels, fetal cord blood insulin concentrations (a reflection of fetal glucose levels), and newborn body fat percentage (N. Engl. J. Med. 2008;358:1991-2002).
Interestingly, Dr. Patrick Catalano’s analysis of data from the HAPO study (Diabetes Care 2012;35:780-6) shows us more: Maternal obesity is almost as strong a driver of newborn obesity as is GDM. Compared with GDM (which increased the percentage of infant birthweights to greater than the 90th percentile by a factor of 2.19), maternal obesity alone increased the frequency of LGA by a factor of 1.73, and maternal obesity and GDM together increased LGA newborns by 3.62-fold.
In light of these recent findings, it is critical that we not only treat our patients who have GDM, but that we attempt to interrupt the chain of obesity that passes from mother to fetus, and from obese newborns onto their subsequent offspring.
A growing proportion of women across all race and ethnicity groups gain more than 40 pounds during pregnancy for singleton births, and many of them do not lose the weight between pregnancies. Increasingly, we have patients whose first child may not have been exposed to obesity in utero, but whose second child is exposed to overweight or obesity and higher levels of insulin resistance and glycemia.
The Institute of Medicine documented these issues in its 2009 report, "Weight Gain During Pregnancy: Reexamining the Guidelines." Data on maternal postpartum weights are not widely available, but data that have been collected suggest that gaining above recommended ranges is associated with excess maternal weight retention post partum, regardless of prepregnancy BMI. Women who gained above the range recommended by the IOM in 1990 had postpartum weight retention of 15-20 pounds. Among women who gained excessive amounts of weight, moreover, more than 40% retained more than 20 pounds, according to the report.
We must break the intergenerational transfer of obesity and insulin resistance by liberally treating GDM and optimizing glucose control during pregnancy. More importantly, we must emphasize to women the importance of having healthy weights at the time of conception. Recent research affirms that moderately simple interventions, such as dietary improvements and exercise can go a long way to achieving these goals. If we don’t – in keeping with the knowledge spurred on by Dr. Barker – we will be programming more newborns for life with insulin resistance, obesity, and disease.
Dr. Moore is a perinatologist who is chair of the department of reproductive medicine at the University of California, San Diego. He said he had no relevant financial disclosures.
Although there are some glimmers of hope that U.S. birthweights may be declining, the average infant birthweight has remained significantly tilted toward obesity. Moreover, and alarming number of infants, children, and adolescents are obese.
In 2007-2008, 9.5% of infants and toddlers were at or above the 95th percentile of the weight-for-recumbent-length growth charts. Among children and adolescents aged 2-19 years, 11.9% were at or above the 97th percentile of the body-mass-index-for-age growth charts; 16.9% were at or above the 95th percentile; and 31.7% were at or above the 85th percentile of BMI for age (JAMA 2010;303:242-9).

While more recent reports of obesity in children indicate a modest decline in obesity among 2- to 5-year-olds (JAMA 2014;311:806-14), an alarming number of infants and children have excess adiposity (roughly twice what is expected). In addition, cardiovascular mortality later in life continues to rise.
The question arises, have childhood and adult obesity rates remained high because mothers are feeding their children the wrong foods or because these children were born obese? One also wonders, with respect to cardiovascular mortality in adulthood, is the in utero environment playing a role?
Old lessons, growing relevance
More than 3 decades ago, the late British physician Dr. David Barker got us thinking about how a challenging life in the womb can set us up for downstream ill health. He studied births from 1910 to 1945 and found that the cardiovascular mortality of individuals born during that time was inversely related to birthweight. Smaller babies, he found, could have cardiovascular mortality risks that were double or even quadruple the risks of larger babies.

Dr. Barker theorized that, when faced with undernutrition, the fetus adapts by sending more blood to the brain and sacrificing blood flow to less essential tissues. His theory about how growth and nutrition before birth may affect the heart became known as the "Barker Hypothesis." It was initially controversial, but it led to an explosion of research – especially since 2000 – on various downstream effects of the intrauterine environment.
Investigators have learned that it is not only cardiovascular mortality that is affected by low birthweight, but also the risk of developing diabetes and being overweight. This is because the fetus makes less essential systems insulin resistant. Insulin resistance persists in the womb and after birth as well, predisposing individuals to insulin resistance and obesity, both of which are closely linked to the risk of metabolic syndrome – a group of risk factors that raises the likelihood of developing heart disease, stroke, and diabetes.
In fact, further research on cohorts of Barker children – individuals who had low birthweights – has shown that not only have they had higher rates of cardiovascular disease, but they have had higher blood sugars and higher rates of insulin resistance as well.
Today, we appreciate a fuller picture of the Barker data, one that shows a reversal of this trend when birthweights reach 4,000-4,500 grams. At this point, what was a progressively downward slope of cardiovascular mortality rates with increasing birthweight suddenly shoots upward again when birthweight exceeds 4,000 g.
It is this end of the curve that is most relevant – and most concerning – for ob.gyns. today. Our problem in the United States is not so much one of starving or growth-restricted newborns, as these babies account for 5% or less of all births. It is one of overweight and obese newborns who now represent as many as 1 in 7 births. Just like the Barker babies who were growth restricted, these newborns have high insulin levels and increased risk of cardiovascular disease as adults.
Changing the trajectory
Both maternal obesity and gestational diabetes get at the heart of the Barker Hypothesis, albeit a twist, in that excessive maternal adiposity and associated insulin resistance results in high maternal blood glucose, transferring excessive nutrients to the fetus. This causes accumulation of fat in the fetus and programs the fetus for an increased and persistent risk of adiposity after birth, early-onset metabolic syndrome, and downstream cardiovascular disease in adulthood.

Dr. Dana Dabelea’s sibling study of almost 15 years ago demonstrated the long-term impact of the adverse intrauterine environment associated with maternal diabetes. Matched siblings who were born after their mothers had developed diabetes had almost double the rate of obesity as adolescents, compared with the siblings born before their mothers were diagnosed with diabetes. In childhood, these siblings ate at the same table and came from the same gene pools (with the same fathers), but they experienced dramatically different health outcomes (Diabetes 2000:49:2208-11).
This landmark study has been reproduced by other investigators who have compared children of mothers who had gestational diabetes and/or were overweight, with children whose mothers did not have gestational diabetes mellitus (GDM) or were of normal weight. Such studies have consistently shown that, faced with either or both maternal obesity and diabetes in utero, offspring were significantly more likely to become overweight children and adults with insulin resistance and other components of the metabolic syndrome.
Importantly, we have evidence from randomized trials that interventions to treat GDM can effectively reduce rates of newborn obesity. While differences in birthweight between treatment and no-treatment arms have been modest, reductions in neonatal body fat, as measured by skin-fold thickness, the ponderal index, and birthweight percentile, have been highly significant.
The offspring of mothers who were treated in these trials, the Australian Carbohydrate Intolerance Study in Pregnant Women (N. Engl. J. Med. 2005;352:2477-86), and a study by Dr. Mark B. Landon and his colleagues (N. Engl. J. Med. 2009;361:1339-48), had approximately half of the newborn adiposity than did offspring of mothers who were not treated. In the latter study, maternal dietary measures alone were successful in reducing neonatal adiposity in over 80% of infants.
While published follow-up data of the offspring in these cohorts have covered only 5-8 years (showing persistently less adiposity in the treated groups), the offspring in the Australian cohort are still being monitored. Based on the cohort and case-control studies summarized above, it seems fair to expect that the children of mothers who were treated for GDM will have significantly better health profiles into and through adulthood.
We know from the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study that what were formerly considered mild and inconsequential maternal blood glucose levels are instead potentially quite harmful. The study showed a clear linear relationship between maternal fasting blood glucose levels, fetal cord blood insulin concentrations (a reflection of fetal glucose levels), and newborn body fat percentage (N. Engl. J. Med. 2008;358:1991-2002).
Interestingly, Dr. Patrick Catalano’s analysis of data from the HAPO study (Diabetes Care 2012;35:780-6) shows us more: Maternal obesity is almost as strong a driver of newborn obesity as is GDM. Compared with GDM (which increased the percentage of infant birthweights to greater than the 90th percentile by a factor of 2.19), maternal obesity alone increased the frequency of LGA by a factor of 1.73, and maternal obesity and GDM together increased LGA newborns by 3.62-fold.
In light of these recent findings, it is critical that we not only treat our patients who have GDM, but that we attempt to interrupt the chain of obesity that passes from mother to fetus, and from obese newborns onto their subsequent offspring.
A growing proportion of women across all race and ethnicity groups gain more than 40 pounds during pregnancy for singleton births, and many of them do not lose the weight between pregnancies. Increasingly, we have patients whose first child may not have been exposed to obesity in utero, but whose second child is exposed to overweight or obesity and higher levels of insulin resistance and glycemia.
The Institute of Medicine documented these issues in its 2009 report, "Weight Gain During Pregnancy: Reexamining the Guidelines." Data on maternal postpartum weights are not widely available, but data that have been collected suggest that gaining above recommended ranges is associated with excess maternal weight retention post partum, regardless of prepregnancy BMI. Women who gained above the range recommended by the IOM in 1990 had postpartum weight retention of 15-20 pounds. Among women who gained excessive amounts of weight, moreover, more than 40% retained more than 20 pounds, according to the report.
We must break the intergenerational transfer of obesity and insulin resistance by liberally treating GDM and optimizing glucose control during pregnancy. More importantly, we must emphasize to women the importance of having healthy weights at the time of conception. Recent research affirms that moderately simple interventions, such as dietary improvements and exercise can go a long way to achieving these goals. If we don’t – in keeping with the knowledge spurred on by Dr. Barker – we will be programming more newborns for life with insulin resistance, obesity, and disease.
Dr. Moore is a perinatologist who is chair of the department of reproductive medicine at the University of California, San Diego. He said he had no relevant financial disclosures.
Managing Gestational Diabetes Mellitus
We now know that gestational diabetes mellitus is a serious condition that, if not properly diagnosed and managed, can have cyclic, intergenerational consequences. Newborns exposed to maternal hyperglycemia during pregnancy have a high risk of being born overweight and of eventually becoming obese children and adults. These newborns also are at a high risk of developing diabetes themselves later in life.
The prevalence of gestational diabetes mellitus (GDM) is increasing in every ethnic group. In the Kaiser Permanente system in Colorado, a state which has traditionally had the lowest obesity rate of any state in the United States, the prevalence of GDM doubled from 1994 to 2002, with significant increases in all racial/ethnic groups (Diabetes Care 2005;28:579-84). Such increases in GDM prevalence are happening worldwide – one part of a worldwide epidemic of obesity and diabetes that is overtaking our youth.
It was not long ago when we used to tell our patients not to worry too much about GDM, that the condition would resolve on its own after childbirth and all would be fine. Then we learned that GDM is one sign post on the way to the development of overt type 2 diabetes. Indeed, a majority of women with GDM will acquire diabetes within 5 years.
In the last decade or so, our clinical research focus has centered on the in utero risks to the fetus. In a striking study of the potential impact of intrauterine hyperglycemia exposure on later development, Dr. D. Dabelea and coinvestigators compared siblings in the Pima Indian population who were born before and after their mothers were diagnosed with diabetes. The children who were born after their mothers had developed diabetes had almost double the rate of obesity as adolescents than their siblings who were born before their mother’s diagnosis of diabetes. Even though these siblings ate the same diet and came from the same gene pools (with the same fathers), they experienced dramatically different health outcomes in adolescence as a result of the differing intrauterine environments (Diabetes 2000;49:2208-11).
This and other studies have given us a body of supplementary science showing that exposure to high blood glucose in utero causes accumulation of fat in the fetus. Even though that baby fat might be lost in early childhood, prenatal exposure nevertheless genetically programs the fetus for a higher risk of developing fatness as an adult.
As I detailed in the last Master Class in obstetrics (see Ob.Gyn News, July 2011, pp. 24-25) we now also have evidence from two randomized controlled trials that interventions to control blood glucose are effective in reducing rates of newborn obesity and therefore should improve adolescent and adult health downstream.
The two randomized trials – the Australian Carbohydrate Intolerance Study in Pregnant Women (N. Engl. J. Med. 2005;352:2477-86) and a study published several years later by Dr. Mark B. Landon and his colleagues (N. Engl. J. Med. 2009; 361:1339-48) – demonstrated the positive impact of treating even mild forms of GDM, with the largest effects being on reducing newborn obesity. Although the offspring of mothers who were treated and not treated in those studies have not yet been followed into adulthood, it seems fair to expect that the children of mothers who were treated for GDM will have significantly better health profiles downstream.
Treating GDM, and learning how to maximize glucose control, has thus moved to center stage in obstetric practice.
Trials of Dietary Change
In Dr. Landon’s landmark study, more than 90% of the women randomized to the treatment group (versus usual prenatal care) needed only dietary counseling and education about blood glucose control for effective treatment of abnormal blood glucose levels. Surprisingly, fewer than 10% needed insulin as well.
That we can manage many of our patients with diet alone is welcome good news. To be successful with this approach, however, we must be vigilant in monitoring the effectiveness of dietary counseling and identifying early on those patients for whom dietary treatment is not enough.
We also must be more vigilant in detecting GDM, because the maximal time of fetal fat accretion is at about 32-34 weeks’ gestation. GDM is typically diagnosed at about 28 weeks’ gestation, and patients usually are not engaged in a regime of blood sugar testing and dietary change until about 30-31 weeks. If we wait until 34-35 weeks’ gestation to change course with treatment – adding insulin or oral hypoglycemic agents – significant body fat accumulation by the fetus already will have occurred.
Screening for GDM even earlier than currently recommended, at 26 weeks’ gestation if possible, and providing dietary counseling as early as possible are worthwhile goals. Our advice is that patients be moved on to a medication regimen if more than one-third of their blood glucose measurements are still abnormal after 2 weeks of dietary change. A more stringent standard may be more prudent, but for now we believe there is enough evidence to warrant this modest change in practice, and we find that it is a rule that most patients can understand.
We also must caution that the effectiveness of dietary change may be significantly less in many populations than it was in Dr. Landon’s study because his study focused on a subset of women who had only mild glucose intolerance. In our patient population, for example, we can achieve good glucose control with diet alone in about 60%-70% of cases.
The Science on Glyburide
Pharmacologic therapy for patients in whom dietary measures fail is no longer limited to insulin. Insulin is certainly still an option as a first-line therapy, and is necessary as an adjunct therapy in patients who are not achieving glucose targets with another agent. It has proven efficacy and well-studied pharmacokinetics. It does not cross the placenta, and research has shown that it may be beneficial by "resting" pancreatic islet cells. Moreover, several forms of insulin – short acting, intermediate, and long-acting – are available, so therapy can be fairly customizable.
Insulin is not an optimal therapy for GDM for several reasons, however. Many patients find it cumbersome to use, and most offices are not equipped for, or used to, teaching women how to give themselves the insulin injections. Insulin itself is also unfamiliar to many patients and can even be scary; some of the families we care for see insulin as a stigma, believing that a person who takes insulin has diabetes while a person who takes a pill does not truly have the condition.
In our practice, we have found that women who take oral hypoglycemics are more likely to have better glycemic control, probably because their drug compliance is better. With insulin, our patients tend to be suboptimally compliant.
Glyburide, one of the oral anti-hyperglycemic drugs that we have been able to transfer from use in the nonpregnant diabetic population to use during pregnancy, has been well-used and studied by this point in time.
When Dr. Oded Langer and his colleagues led the first and only randomized trial comparing glyburide and insulin more than a decade ago, women with GDM were rarely treated with a sulfonylurea drug largely because of reports of prolonged severe hypoglycemia in neonates born to mothers who were receiving the drug at the time of delivery. There were also questions about whether glyburide, a second-generation sulfonylurea, could effectively control postprandial peaks in blood glucose while avoiding periods of hypoglycemia in the mother.
In the nonpregnant population, glyburide has been used for decades as a twice-daily oral medication. After months of use, patients develop active metabolites that prolong the drug’s half life and enable it to last for 12 hours, at least.
Glyburide use in pregnancy is a slightly different story, however. Patients take the medication for a relatively short time and consequently may not build up the active metabolites that nonpregnant patients acquire. The metabolic changes in pregnancy also make women vulnerable to hypoglycemia at certain times of the day, typically in the late morning, the late afternoon, and between 3 a.m. and 4 a.m.
Dr. Langer’s trial, which randomized 404 women with GDM to receive glyburide or insulin, demonstrated similar outcomes in the insulin and glyburide groups. There were no differences in mean birth weight, the percentage of large for gestational age newborns, macrosomia, fetal anomalies, or newborn hypoglycemia. The rate of maternal hypoglycemia, however, was much higher in the insulin-treated group; 20% of the women receiving insulin experienced symptomatic hypoglycemia, compared with only 2% of the women taking glyburide.
In short, glyburide was just as effective as insulin in achieving desired levels of glycemic control (a fasting blood glucose less than 90 mg/dL and 2-hour postprandial glucose of 120 mg/dL) and controlling fetal obesity, while being significantly less likely to cause hypoglycemia in the mothers. (N. Engl. J. Med. 2000;343:1134-8).
Glyburide dosing in Dr. Langer’s trial was increased weekly, as needed, to a maximum of 20 mg per day; women took the drug twice a day. Insulin was administered per a standard intensified schedule of regular NPH (intermediate-acting, lasting 6-12 hours) and regular TID (lasting 2-4 hours).
Despite the impressive findings from the trial, some have contended that the results of one randomized trial are insufficient for adopting glyburide as a first-line therapy. However, numerous retrospective or case-controlled studies also have since shown glyburide to be a clinically effective alternative to insulin therapy, with no adverse neonatal or fetal effects. These studies have shown, moreover, that it can be easier to avoid hypoglycemia and achieve optimal glycemic control with glyburide than with insulin.
One of the best large retrospective studies looked at 584 women at Kaiser Permanente Northern California and found that glyburide was at least as effective as insulin in achieving glycemic control and resulted in similar birth weights in women with GDM who had failed diet therapy alone (Am. J. Obstet. Gynecol. 2005;193:118-24).
Several recent reviews of glyburide studies, such as one that looked at nine glyburide studies covering 745 patients taking glyburide and 645 patients taking insulin, also have been published (Ann. Pharmacother. 2008;42:483-90). In 2007, moreover, the 5th International Workshop-Conference on GDM concluded that glyburide is a legitimate alternative to insulin for GDM (Diabetes Care 2007;30:S251-60).
We also now know that unlike other, first-generation sulfonylureas that tend to cross the placenta freely, glyburide is 99.8% protein-bound and thus crosses the placenta only minimally.
Theoretically, there is one potential problem with glyburide. Because the drug acts by stimulating maternal pancreatic insulin production, it could potentially promote "pancreatic burnout," thus shortening the time to development of overt diabetes in women whose pancreas is struggling to begin with. Women who are obese and have significant insulin resistance at the start of their pregnancies thus might be susceptible to pancreatic burnout. Although this potential effect has not been demonstrated in any trials, it must be kept in mind.
It would be informative to conduct long-term follow-up studies that track the children of mothers who used glyburide during their pregnancies, but at this point it is unclear if such studies will be designed and carried out. The likelihood of additional randomized trials being conducted is practically nil, given the extent to which women already are choosing the oral hypoglycemics over insulin.
Glyburide in Practice
As clinicians, we must appreciate that the pharmacodynamics of glyburide are quite different in pregnant women, with important dosing implications for our patients. Indeed, for pregnant women, glyburide is not the 12-hour medication that it is in nonpregnant women.
During pregnancy, glyburide action peaks about 2.5 hours after it’s taken, and the increased renal clearance and metabolism of pregnancy (in addition to the short duration of therapy in this patient population) leave the drug with a "useful" life of only about 6-8 hours.
Because blood glucose peaks 60-90 minutes after a meal, we instruct our patients to take a glyburide dose a full hour before a planned meal. Otherwise, postprandial glucose peaks will not be controlled. Usually, a dose taken an hour before breakfast will help control postprandial peaks after breakfast and lunch but will not last for dinner. Another dose 1 hour before an evening meal can be given.
To effectively control fasting blood glucose, we instruct patients to take a glyburide dose between 10 p.m. and midnight so that the drug will still be active in the early morning when it is needed. If the dose is taken too early at night – at 8-9 p.m., for instance – it will peak between 10 p.m. and midnight, and will not be working at 6 a.m.
As it is with insulin, careful glucose monitoring is critical for determining optimal administration of glyburide and for balancing glyburide action with meals and snacks. Individual glycemic profiles should be analyzed each week, with the goal of keeping fasting blood glucose below 90 mg/dL, and postprandial levels below 130 mg/dL, while preventing maternal hypoglycemia.
Attention must be paid not only to times of consistent elevation in blood glucose levels, but also to the potential for dosage overlap – for instance, a prelunch dosage administered to correct consistently high postprandial glucose levels after the mid-day meal could lead to low blood glucose levels at about 4-5 p.m. as its action overlaps with the end duration of a morning dose.
Patients should always be prepared for vulnerable times and have a glucose tablet, juice box, or food with them to correct any periods of hypoglycemia.
Insulin should be added if more than 30% of blood glucose readings are above target with administration of 15-20 mg/day of glyburide.
Metformin as an Option
As ob.gyns, our experience with metformin, the other oral anti-hyperglycemic agent now available for treating GDM, came originally from its use as an infertility treatment in women with polycystic ovary syndrome (PCOS).
Metformin is frequently prescribed for women with PCOS to improve ovulation. These women have significant insulin resistance and are at high risk for developing GDM during their pregnancies. The main concern in this population, however, has been infertility, and studies have shown that metformin induces ovulation in women with PCOS.
Although metformin crosses the placenta, numerous studies have shown no increase in birth anomalies in women who conceive while taking the agent.
A study published a decade ago in women who chose whether or not to continue metformin treatment throughout their pregnancies showed that of those who discontinued metformin, 31% developed GDM, compared with only 3% of those who continued their metformin treatment (Fertil. Steril. 2002;77:520-5). These results helped fuel the idea that the agent may be a logical treatment for women with GDM.
Metformin also has a theoretical advantage over glyburide since its mechanism of action gets directly to the root of the problem of GDM. Metformin is an insulin sensitizer, and the root cause of GDM is resistance to insulin, or insulin insensitivity, at the tissue level.
In a study by Dr. J.A. Rowan published in 2008 that randomized more than 700 patients to either insulin or metformin, there were no appreciable differences in neonatal and maternal outcomes – from birth weight and neonatal morbidity to maternal hypoglycemia and glycemic control (N. Engl J. Med. 2008;358:2003-15). However, whereas 4% of the glyburide group in Dr. Langer’s trial had to eventually add insulin (and up to 10%-20% in other studies), 47% of the patients taking metformin in this trial had to add insulin to maintain glycemic control.
Indeed, the downside to metformin, this and other studies have shown, is a high so-called failure rate – the need for supplementary insulin, which in this case typically occurs later in the pregnancy – of between 30% and 50%.
On the other hand, patients generally will be more satisfied starting treatment with metformin than insulin. In weighing glyburide and metformin, patients should be counseled about their chances of needing insulin later in the pregnancy: about 10% with glyburide and closer to 50% with metformin.
In terms of glycemic control and other outcomes, several smaller, recent studies comparing the two agents have shown no statistical difference between them. Interestingly, most studies have shown less maternal weight gain in patients taking metformin than glyburide – about 6 pounds – but the significance of this difference is unclear since the babies’ birth weights were not appreciably different.
Dr. Moore said he had no relevant financial disclosures.
We now know that gestational diabetes mellitus is a serious condition that, if not properly diagnosed and managed, can have cyclic, intergenerational consequences. Newborns exposed to maternal hyperglycemia during pregnancy have a high risk of being born overweight and of eventually becoming obese children and adults. These newborns also are at a high risk of developing diabetes themselves later in life.
The prevalence of gestational diabetes mellitus (GDM) is increasing in every ethnic group. In the Kaiser Permanente system in Colorado, a state which has traditionally had the lowest obesity rate of any state in the United States, the prevalence of GDM doubled from 1994 to 2002, with significant increases in all racial/ethnic groups (Diabetes Care 2005;28:579-84). Such increases in GDM prevalence are happening worldwide – one part of a worldwide epidemic of obesity and diabetes that is overtaking our youth.
It was not long ago when we used to tell our patients not to worry too much about GDM, that the condition would resolve on its own after childbirth and all would be fine. Then we learned that GDM is one sign post on the way to the development of overt type 2 diabetes. Indeed, a majority of women with GDM will acquire diabetes within 5 years.
In the last decade or so, our clinical research focus has centered on the in utero risks to the fetus. In a striking study of the potential impact of intrauterine hyperglycemia exposure on later development, Dr. D. Dabelea and coinvestigators compared siblings in the Pima Indian population who were born before and after their mothers were diagnosed with diabetes. The children who were born after their mothers had developed diabetes had almost double the rate of obesity as adolescents than their siblings who were born before their mother’s diagnosis of diabetes. Even though these siblings ate the same diet and came from the same gene pools (with the same fathers), they experienced dramatically different health outcomes in adolescence as a result of the differing intrauterine environments (Diabetes 2000;49:2208-11).
This and other studies have given us a body of supplementary science showing that exposure to high blood glucose in utero causes accumulation of fat in the fetus. Even though that baby fat might be lost in early childhood, prenatal exposure nevertheless genetically programs the fetus for a higher risk of developing fatness as an adult.
As I detailed in the last Master Class in obstetrics (see Ob.Gyn News, July 2011, pp. 24-25) we now also have evidence from two randomized controlled trials that interventions to control blood glucose are effective in reducing rates of newborn obesity and therefore should improve adolescent and adult health downstream.
The two randomized trials – the Australian Carbohydrate Intolerance Study in Pregnant Women (N. Engl. J. Med. 2005;352:2477-86) and a study published several years later by Dr. Mark B. Landon and his colleagues (N. Engl. J. Med. 2009; 361:1339-48) – demonstrated the positive impact of treating even mild forms of GDM, with the largest effects being on reducing newborn obesity. Although the offspring of mothers who were treated and not treated in those studies have not yet been followed into adulthood, it seems fair to expect that the children of mothers who were treated for GDM will have significantly better health profiles downstream.
Treating GDM, and learning how to maximize glucose control, has thus moved to center stage in obstetric practice.
Trials of Dietary Change
In Dr. Landon’s landmark study, more than 90% of the women randomized to the treatment group (versus usual prenatal care) needed only dietary counseling and education about blood glucose control for effective treatment of abnormal blood glucose levels. Surprisingly, fewer than 10% needed insulin as well.
That we can manage many of our patients with diet alone is welcome good news. To be successful with this approach, however, we must be vigilant in monitoring the effectiveness of dietary counseling and identifying early on those patients for whom dietary treatment is not enough.
We also must be more vigilant in detecting GDM, because the maximal time of fetal fat accretion is at about 32-34 weeks’ gestation. GDM is typically diagnosed at about 28 weeks’ gestation, and patients usually are not engaged in a regime of blood sugar testing and dietary change until about 30-31 weeks. If we wait until 34-35 weeks’ gestation to change course with treatment – adding insulin or oral hypoglycemic agents – significant body fat accumulation by the fetus already will have occurred.
Screening for GDM even earlier than currently recommended, at 26 weeks’ gestation if possible, and providing dietary counseling as early as possible are worthwhile goals. Our advice is that patients be moved on to a medication regimen if more than one-third of their blood glucose measurements are still abnormal after 2 weeks of dietary change. A more stringent standard may be more prudent, but for now we believe there is enough evidence to warrant this modest change in practice, and we find that it is a rule that most patients can understand.
We also must caution that the effectiveness of dietary change may be significantly less in many populations than it was in Dr. Landon’s study because his study focused on a subset of women who had only mild glucose intolerance. In our patient population, for example, we can achieve good glucose control with diet alone in about 60%-70% of cases.
The Science on Glyburide
Pharmacologic therapy for patients in whom dietary measures fail is no longer limited to insulin. Insulin is certainly still an option as a first-line therapy, and is necessary as an adjunct therapy in patients who are not achieving glucose targets with another agent. It has proven efficacy and well-studied pharmacokinetics. It does not cross the placenta, and research has shown that it may be beneficial by "resting" pancreatic islet cells. Moreover, several forms of insulin – short acting, intermediate, and long-acting – are available, so therapy can be fairly customizable.
Insulin is not an optimal therapy for GDM for several reasons, however. Many patients find it cumbersome to use, and most offices are not equipped for, or used to, teaching women how to give themselves the insulin injections. Insulin itself is also unfamiliar to many patients and can even be scary; some of the families we care for see insulin as a stigma, believing that a person who takes insulin has diabetes while a person who takes a pill does not truly have the condition.
In our practice, we have found that women who take oral hypoglycemics are more likely to have better glycemic control, probably because their drug compliance is better. With insulin, our patients tend to be suboptimally compliant.
Glyburide, one of the oral anti-hyperglycemic drugs that we have been able to transfer from use in the nonpregnant diabetic population to use during pregnancy, has been well-used and studied by this point in time.
When Dr. Oded Langer and his colleagues led the first and only randomized trial comparing glyburide and insulin more than a decade ago, women with GDM were rarely treated with a sulfonylurea drug largely because of reports of prolonged severe hypoglycemia in neonates born to mothers who were receiving the drug at the time of delivery. There were also questions about whether glyburide, a second-generation sulfonylurea, could effectively control postprandial peaks in blood glucose while avoiding periods of hypoglycemia in the mother.
In the nonpregnant population, glyburide has been used for decades as a twice-daily oral medication. After months of use, patients develop active metabolites that prolong the drug’s half life and enable it to last for 12 hours, at least.
Glyburide use in pregnancy is a slightly different story, however. Patients take the medication for a relatively short time and consequently may not build up the active metabolites that nonpregnant patients acquire. The metabolic changes in pregnancy also make women vulnerable to hypoglycemia at certain times of the day, typically in the late morning, the late afternoon, and between 3 a.m. and 4 a.m.
Dr. Langer’s trial, which randomized 404 women with GDM to receive glyburide or insulin, demonstrated similar outcomes in the insulin and glyburide groups. There were no differences in mean birth weight, the percentage of large for gestational age newborns, macrosomia, fetal anomalies, or newborn hypoglycemia. The rate of maternal hypoglycemia, however, was much higher in the insulin-treated group; 20% of the women receiving insulin experienced symptomatic hypoglycemia, compared with only 2% of the women taking glyburide.
In short, glyburide was just as effective as insulin in achieving desired levels of glycemic control (a fasting blood glucose less than 90 mg/dL and 2-hour postprandial glucose of 120 mg/dL) and controlling fetal obesity, while being significantly less likely to cause hypoglycemia in the mothers. (N. Engl. J. Med. 2000;343:1134-8).
Glyburide dosing in Dr. Langer’s trial was increased weekly, as needed, to a maximum of 20 mg per day; women took the drug twice a day. Insulin was administered per a standard intensified schedule of regular NPH (intermediate-acting, lasting 6-12 hours) and regular TID (lasting 2-4 hours).
Despite the impressive findings from the trial, some have contended that the results of one randomized trial are insufficient for adopting glyburide as a first-line therapy. However, numerous retrospective or case-controlled studies also have since shown glyburide to be a clinically effective alternative to insulin therapy, with no adverse neonatal or fetal effects. These studies have shown, moreover, that it can be easier to avoid hypoglycemia and achieve optimal glycemic control with glyburide than with insulin.
One of the best large retrospective studies looked at 584 women at Kaiser Permanente Northern California and found that glyburide was at least as effective as insulin in achieving glycemic control and resulted in similar birth weights in women with GDM who had failed diet therapy alone (Am. J. Obstet. Gynecol. 2005;193:118-24).
Several recent reviews of glyburide studies, such as one that looked at nine glyburide studies covering 745 patients taking glyburide and 645 patients taking insulin, also have been published (Ann. Pharmacother. 2008;42:483-90). In 2007, moreover, the 5th International Workshop-Conference on GDM concluded that glyburide is a legitimate alternative to insulin for GDM (Diabetes Care 2007;30:S251-60).
We also now know that unlike other, first-generation sulfonylureas that tend to cross the placenta freely, glyburide is 99.8% protein-bound and thus crosses the placenta only minimally.
Theoretically, there is one potential problem with glyburide. Because the drug acts by stimulating maternal pancreatic insulin production, it could potentially promote "pancreatic burnout," thus shortening the time to development of overt diabetes in women whose pancreas is struggling to begin with. Women who are obese and have significant insulin resistance at the start of their pregnancies thus might be susceptible to pancreatic burnout. Although this potential effect has not been demonstrated in any trials, it must be kept in mind.
It would be informative to conduct long-term follow-up studies that track the children of mothers who used glyburide during their pregnancies, but at this point it is unclear if such studies will be designed and carried out. The likelihood of additional randomized trials being conducted is practically nil, given the extent to which women already are choosing the oral hypoglycemics over insulin.
Glyburide in Practice
As clinicians, we must appreciate that the pharmacodynamics of glyburide are quite different in pregnant women, with important dosing implications for our patients. Indeed, for pregnant women, glyburide is not the 12-hour medication that it is in nonpregnant women.
During pregnancy, glyburide action peaks about 2.5 hours after it’s taken, and the increased renal clearance and metabolism of pregnancy (in addition to the short duration of therapy in this patient population) leave the drug with a "useful" life of only about 6-8 hours.
Because blood glucose peaks 60-90 minutes after a meal, we instruct our patients to take a glyburide dose a full hour before a planned meal. Otherwise, postprandial glucose peaks will not be controlled. Usually, a dose taken an hour before breakfast will help control postprandial peaks after breakfast and lunch but will not last for dinner. Another dose 1 hour before an evening meal can be given.
To effectively control fasting blood glucose, we instruct patients to take a glyburide dose between 10 p.m. and midnight so that the drug will still be active in the early morning when it is needed. If the dose is taken too early at night – at 8-9 p.m., for instance – it will peak between 10 p.m. and midnight, and will not be working at 6 a.m.
As it is with insulin, careful glucose monitoring is critical for determining optimal administration of glyburide and for balancing glyburide action with meals and snacks. Individual glycemic profiles should be analyzed each week, with the goal of keeping fasting blood glucose below 90 mg/dL, and postprandial levels below 130 mg/dL, while preventing maternal hypoglycemia.
Attention must be paid not only to times of consistent elevation in blood glucose levels, but also to the potential for dosage overlap – for instance, a prelunch dosage administered to correct consistently high postprandial glucose levels after the mid-day meal could lead to low blood glucose levels at about 4-5 p.m. as its action overlaps with the end duration of a morning dose.
Patients should always be prepared for vulnerable times and have a glucose tablet, juice box, or food with them to correct any periods of hypoglycemia.
Insulin should be added if more than 30% of blood glucose readings are above target with administration of 15-20 mg/day of glyburide.
Metformin as an Option
As ob.gyns, our experience with metformin, the other oral anti-hyperglycemic agent now available for treating GDM, came originally from its use as an infertility treatment in women with polycystic ovary syndrome (PCOS).
Metformin is frequently prescribed for women with PCOS to improve ovulation. These women have significant insulin resistance and are at high risk for developing GDM during their pregnancies. The main concern in this population, however, has been infertility, and studies have shown that metformin induces ovulation in women with PCOS.
Although metformin crosses the placenta, numerous studies have shown no increase in birth anomalies in women who conceive while taking the agent.
A study published a decade ago in women who chose whether or not to continue metformin treatment throughout their pregnancies showed that of those who discontinued metformin, 31% developed GDM, compared with only 3% of those who continued their metformin treatment (Fertil. Steril. 2002;77:520-5). These results helped fuel the idea that the agent may be a logical treatment for women with GDM.
Metformin also has a theoretical advantage over glyburide since its mechanism of action gets directly to the root of the problem of GDM. Metformin is an insulin sensitizer, and the root cause of GDM is resistance to insulin, or insulin insensitivity, at the tissue level.
In a study by Dr. J.A. Rowan published in 2008 that randomized more than 700 patients to either insulin or metformin, there were no appreciable differences in neonatal and maternal outcomes – from birth weight and neonatal morbidity to maternal hypoglycemia and glycemic control (N. Engl J. Med. 2008;358:2003-15). However, whereas 4% of the glyburide group in Dr. Langer’s trial had to eventually add insulin (and up to 10%-20% in other studies), 47% of the patients taking metformin in this trial had to add insulin to maintain glycemic control.
Indeed, the downside to metformin, this and other studies have shown, is a high so-called failure rate – the need for supplementary insulin, which in this case typically occurs later in the pregnancy – of between 30% and 50%.
On the other hand, patients generally will be more satisfied starting treatment with metformin than insulin. In weighing glyburide and metformin, patients should be counseled about their chances of needing insulin later in the pregnancy: about 10% with glyburide and closer to 50% with metformin.
In terms of glycemic control and other outcomes, several smaller, recent studies comparing the two agents have shown no statistical difference between them. Interestingly, most studies have shown less maternal weight gain in patients taking metformin than glyburide – about 6 pounds – but the significance of this difference is unclear since the babies’ birth weights were not appreciably different.
Dr. Moore said he had no relevant financial disclosures.
We now know that gestational diabetes mellitus is a serious condition that, if not properly diagnosed and managed, can have cyclic, intergenerational consequences. Newborns exposed to maternal hyperglycemia during pregnancy have a high risk of being born overweight and of eventually becoming obese children and adults. These newborns also are at a high risk of developing diabetes themselves later in life.
The prevalence of gestational diabetes mellitus (GDM) is increasing in every ethnic group. In the Kaiser Permanente system in Colorado, a state which has traditionally had the lowest obesity rate of any state in the United States, the prevalence of GDM doubled from 1994 to 2002, with significant increases in all racial/ethnic groups (Diabetes Care 2005;28:579-84). Such increases in GDM prevalence are happening worldwide – one part of a worldwide epidemic of obesity and diabetes that is overtaking our youth.
It was not long ago when we used to tell our patients not to worry too much about GDM, that the condition would resolve on its own after childbirth and all would be fine. Then we learned that GDM is one sign post on the way to the development of overt type 2 diabetes. Indeed, a majority of women with GDM will acquire diabetes within 5 years.
In the last decade or so, our clinical research focus has centered on the in utero risks to the fetus. In a striking study of the potential impact of intrauterine hyperglycemia exposure on later development, Dr. D. Dabelea and coinvestigators compared siblings in the Pima Indian population who were born before and after their mothers were diagnosed with diabetes. The children who were born after their mothers had developed diabetes had almost double the rate of obesity as adolescents than their siblings who were born before their mother’s diagnosis of diabetes. Even though these siblings ate the same diet and came from the same gene pools (with the same fathers), they experienced dramatically different health outcomes in adolescence as a result of the differing intrauterine environments (Diabetes 2000;49:2208-11).
This and other studies have given us a body of supplementary science showing that exposure to high blood glucose in utero causes accumulation of fat in the fetus. Even though that baby fat might be lost in early childhood, prenatal exposure nevertheless genetically programs the fetus for a higher risk of developing fatness as an adult.
As I detailed in the last Master Class in obstetrics (see Ob.Gyn News, July 2011, pp. 24-25) we now also have evidence from two randomized controlled trials that interventions to control blood glucose are effective in reducing rates of newborn obesity and therefore should improve adolescent and adult health downstream.
The two randomized trials – the Australian Carbohydrate Intolerance Study in Pregnant Women (N. Engl. J. Med. 2005;352:2477-86) and a study published several years later by Dr. Mark B. Landon and his colleagues (N. Engl. J. Med. 2009; 361:1339-48) – demonstrated the positive impact of treating even mild forms of GDM, with the largest effects being on reducing newborn obesity. Although the offspring of mothers who were treated and not treated in those studies have not yet been followed into adulthood, it seems fair to expect that the children of mothers who were treated for GDM will have significantly better health profiles downstream.
Treating GDM, and learning how to maximize glucose control, has thus moved to center stage in obstetric practice.
Trials of Dietary Change
In Dr. Landon’s landmark study, more than 90% of the women randomized to the treatment group (versus usual prenatal care) needed only dietary counseling and education about blood glucose control for effective treatment of abnormal blood glucose levels. Surprisingly, fewer than 10% needed insulin as well.
That we can manage many of our patients with diet alone is welcome good news. To be successful with this approach, however, we must be vigilant in monitoring the effectiveness of dietary counseling and identifying early on those patients for whom dietary treatment is not enough.
We also must be more vigilant in detecting GDM, because the maximal time of fetal fat accretion is at about 32-34 weeks’ gestation. GDM is typically diagnosed at about 28 weeks’ gestation, and patients usually are not engaged in a regime of blood sugar testing and dietary change until about 30-31 weeks. If we wait until 34-35 weeks’ gestation to change course with treatment – adding insulin or oral hypoglycemic agents – significant body fat accumulation by the fetus already will have occurred.
Screening for GDM even earlier than currently recommended, at 26 weeks’ gestation if possible, and providing dietary counseling as early as possible are worthwhile goals. Our advice is that patients be moved on to a medication regimen if more than one-third of their blood glucose measurements are still abnormal after 2 weeks of dietary change. A more stringent standard may be more prudent, but for now we believe there is enough evidence to warrant this modest change in practice, and we find that it is a rule that most patients can understand.
We also must caution that the effectiveness of dietary change may be significantly less in many populations than it was in Dr. Landon’s study because his study focused on a subset of women who had only mild glucose intolerance. In our patient population, for example, we can achieve good glucose control with diet alone in about 60%-70% of cases.
The Science on Glyburide
Pharmacologic therapy for patients in whom dietary measures fail is no longer limited to insulin. Insulin is certainly still an option as a first-line therapy, and is necessary as an adjunct therapy in patients who are not achieving glucose targets with another agent. It has proven efficacy and well-studied pharmacokinetics. It does not cross the placenta, and research has shown that it may be beneficial by "resting" pancreatic islet cells. Moreover, several forms of insulin – short acting, intermediate, and long-acting – are available, so therapy can be fairly customizable.
Insulin is not an optimal therapy for GDM for several reasons, however. Many patients find it cumbersome to use, and most offices are not equipped for, or used to, teaching women how to give themselves the insulin injections. Insulin itself is also unfamiliar to many patients and can even be scary; some of the families we care for see insulin as a stigma, believing that a person who takes insulin has diabetes while a person who takes a pill does not truly have the condition.
In our practice, we have found that women who take oral hypoglycemics are more likely to have better glycemic control, probably because their drug compliance is better. With insulin, our patients tend to be suboptimally compliant.
Glyburide, one of the oral anti-hyperglycemic drugs that we have been able to transfer from use in the nonpregnant diabetic population to use during pregnancy, has been well-used and studied by this point in time.
When Dr. Oded Langer and his colleagues led the first and only randomized trial comparing glyburide and insulin more than a decade ago, women with GDM were rarely treated with a sulfonylurea drug largely because of reports of prolonged severe hypoglycemia in neonates born to mothers who were receiving the drug at the time of delivery. There were also questions about whether glyburide, a second-generation sulfonylurea, could effectively control postprandial peaks in blood glucose while avoiding periods of hypoglycemia in the mother.
In the nonpregnant population, glyburide has been used for decades as a twice-daily oral medication. After months of use, patients develop active metabolites that prolong the drug’s half life and enable it to last for 12 hours, at least.
Glyburide use in pregnancy is a slightly different story, however. Patients take the medication for a relatively short time and consequently may not build up the active metabolites that nonpregnant patients acquire. The metabolic changes in pregnancy also make women vulnerable to hypoglycemia at certain times of the day, typically in the late morning, the late afternoon, and between 3 a.m. and 4 a.m.
Dr. Langer’s trial, which randomized 404 women with GDM to receive glyburide or insulin, demonstrated similar outcomes in the insulin and glyburide groups. There were no differences in mean birth weight, the percentage of large for gestational age newborns, macrosomia, fetal anomalies, or newborn hypoglycemia. The rate of maternal hypoglycemia, however, was much higher in the insulin-treated group; 20% of the women receiving insulin experienced symptomatic hypoglycemia, compared with only 2% of the women taking glyburide.
In short, glyburide was just as effective as insulin in achieving desired levels of glycemic control (a fasting blood glucose less than 90 mg/dL and 2-hour postprandial glucose of 120 mg/dL) and controlling fetal obesity, while being significantly less likely to cause hypoglycemia in the mothers. (N. Engl. J. Med. 2000;343:1134-8).
Glyburide dosing in Dr. Langer’s trial was increased weekly, as needed, to a maximum of 20 mg per day; women took the drug twice a day. Insulin was administered per a standard intensified schedule of regular NPH (intermediate-acting, lasting 6-12 hours) and regular TID (lasting 2-4 hours).
Despite the impressive findings from the trial, some have contended that the results of one randomized trial are insufficient for adopting glyburide as a first-line therapy. However, numerous retrospective or case-controlled studies also have since shown glyburide to be a clinically effective alternative to insulin therapy, with no adverse neonatal or fetal effects. These studies have shown, moreover, that it can be easier to avoid hypoglycemia and achieve optimal glycemic control with glyburide than with insulin.
One of the best large retrospective studies looked at 584 women at Kaiser Permanente Northern California and found that glyburide was at least as effective as insulin in achieving glycemic control and resulted in similar birth weights in women with GDM who had failed diet therapy alone (Am. J. Obstet. Gynecol. 2005;193:118-24).
Several recent reviews of glyburide studies, such as one that looked at nine glyburide studies covering 745 patients taking glyburide and 645 patients taking insulin, also have been published (Ann. Pharmacother. 2008;42:483-90). In 2007, moreover, the 5th International Workshop-Conference on GDM concluded that glyburide is a legitimate alternative to insulin for GDM (Diabetes Care 2007;30:S251-60).
We also now know that unlike other, first-generation sulfonylureas that tend to cross the placenta freely, glyburide is 99.8% protein-bound and thus crosses the placenta only minimally.
Theoretically, there is one potential problem with glyburide. Because the drug acts by stimulating maternal pancreatic insulin production, it could potentially promote "pancreatic burnout," thus shortening the time to development of overt diabetes in women whose pancreas is struggling to begin with. Women who are obese and have significant insulin resistance at the start of their pregnancies thus might be susceptible to pancreatic burnout. Although this potential effect has not been demonstrated in any trials, it must be kept in mind.
It would be informative to conduct long-term follow-up studies that track the children of mothers who used glyburide during their pregnancies, but at this point it is unclear if such studies will be designed and carried out. The likelihood of additional randomized trials being conducted is practically nil, given the extent to which women already are choosing the oral hypoglycemics over insulin.
Glyburide in Practice
As clinicians, we must appreciate that the pharmacodynamics of glyburide are quite different in pregnant women, with important dosing implications for our patients. Indeed, for pregnant women, glyburide is not the 12-hour medication that it is in nonpregnant women.
During pregnancy, glyburide action peaks about 2.5 hours after it’s taken, and the increased renal clearance and metabolism of pregnancy (in addition to the short duration of therapy in this patient population) leave the drug with a "useful" life of only about 6-8 hours.
Because blood glucose peaks 60-90 minutes after a meal, we instruct our patients to take a glyburide dose a full hour before a planned meal. Otherwise, postprandial glucose peaks will not be controlled. Usually, a dose taken an hour before breakfast will help control postprandial peaks after breakfast and lunch but will not last for dinner. Another dose 1 hour before an evening meal can be given.
To effectively control fasting blood glucose, we instruct patients to take a glyburide dose between 10 p.m. and midnight so that the drug will still be active in the early morning when it is needed. If the dose is taken too early at night – at 8-9 p.m., for instance – it will peak between 10 p.m. and midnight, and will not be working at 6 a.m.
As it is with insulin, careful glucose monitoring is critical for determining optimal administration of glyburide and for balancing glyburide action with meals and snacks. Individual glycemic profiles should be analyzed each week, with the goal of keeping fasting blood glucose below 90 mg/dL, and postprandial levels below 130 mg/dL, while preventing maternal hypoglycemia.
Attention must be paid not only to times of consistent elevation in blood glucose levels, but also to the potential for dosage overlap – for instance, a prelunch dosage administered to correct consistently high postprandial glucose levels after the mid-day meal could lead to low blood glucose levels at about 4-5 p.m. as its action overlaps with the end duration of a morning dose.
Patients should always be prepared for vulnerable times and have a glucose tablet, juice box, or food with them to correct any periods of hypoglycemia.
Insulin should be added if more than 30% of blood glucose readings are above target with administration of 15-20 mg/day of glyburide.
Metformin as an Option
As ob.gyns, our experience with metformin, the other oral anti-hyperglycemic agent now available for treating GDM, came originally from its use as an infertility treatment in women with polycystic ovary syndrome (PCOS).
Metformin is frequently prescribed for women with PCOS to improve ovulation. These women have significant insulin resistance and are at high risk for developing GDM during their pregnancies. The main concern in this population, however, has been infertility, and studies have shown that metformin induces ovulation in women with PCOS.
Although metformin crosses the placenta, numerous studies have shown no increase in birth anomalies in women who conceive while taking the agent.
A study published a decade ago in women who chose whether or not to continue metformin treatment throughout their pregnancies showed that of those who discontinued metformin, 31% developed GDM, compared with only 3% of those who continued their metformin treatment (Fertil. Steril. 2002;77:520-5). These results helped fuel the idea that the agent may be a logical treatment for women with GDM.
Metformin also has a theoretical advantage over glyburide since its mechanism of action gets directly to the root of the problem of GDM. Metformin is an insulin sensitizer, and the root cause of GDM is resistance to insulin, or insulin insensitivity, at the tissue level.
In a study by Dr. J.A. Rowan published in 2008 that randomized more than 700 patients to either insulin or metformin, there were no appreciable differences in neonatal and maternal outcomes – from birth weight and neonatal morbidity to maternal hypoglycemia and glycemic control (N. Engl J. Med. 2008;358:2003-15). However, whereas 4% of the glyburide group in Dr. Langer’s trial had to eventually add insulin (and up to 10%-20% in other studies), 47% of the patients taking metformin in this trial had to add insulin to maintain glycemic control.
Indeed, the downside to metformin, this and other studies have shown, is a high so-called failure rate – the need for supplementary insulin, which in this case typically occurs later in the pregnancy – of between 30% and 50%.
On the other hand, patients generally will be more satisfied starting treatment with metformin than insulin. In weighing glyburide and metformin, patients should be counseled about their chances of needing insulin later in the pregnancy: about 10% with glyburide and closer to 50% with metformin.
In terms of glycemic control and other outcomes, several smaller, recent studies comparing the two agents have shown no statistical difference between them. Interestingly, most studies have shown less maternal weight gain in patients taking metformin than glyburide – about 6 pounds – but the significance of this difference is unclear since the babies’ birth weights were not appreciably different.
Dr. Moore said he had no relevant financial disclosures.
Point/Counterpoint: Glucose Sensors and Insulin Pumps for Diabetic Pregnancies?
Yes – Continuous Glucose Monitoring and Insulin Pumps Improve Glycemic Control
Consider the case of a 33-year-old teacher who has a toddler in preschool, regularly attends Pilates classes, and is in her second pregnancy. Her privacy is limited, and her lunchtime varies by at least 30 minutes. In her first pregnancy, when she used multiple daily injections (MDI) for insulin injection, her hemoglobin A1c at 36 weeks’ gestation was 7.2%, and her baby’s birth weight was 4,200 g. This time around, she cannot get her fasting blood glucose under 110 mg/dL without experiencing marked hypoglycemia around 3:30 a.m.
In her case, the demonstrated benefits of continuous glucose monitoring (CGM) and an insulin pump (for continuous subcutaneous insulin infusion, or CSII) make this method of insulin administration a compelling – and even obvious – choice over MDI. A glucose sensor and insulin pump would improve her glucose control, bring down her HbA1c, and lessen the frequency and severity of hypoglycemic episodes, while better meshing with her lifestyle.
She is far from the only type of patient who would benefit, however. Modern CSII pumps (with Bolus Wizard calculators) have been around for a decade, yet we significantly underutilize them. Many, if not most, insulin-requiring pregnant women are good candidates for glucose sensors and insulin pumps.
CSII improves glucose control by providing "background" changes in insulin dosing to match the complexities of metabolism in pregnancy, and CGM (as opposed to self–blood glucose monitoring) improves glucose control by removing the fear of unexpected lows, which is a big issue in my patients, and by warning of impending highs.
Together, today’s equipment also improves patients’ ability to administer insulin in social and business settings. The equipment is also easily programmable and understandable to women who routinely use electronic devices.
A Cochrane systematic review of CSII vs. MDI in pregnancy, published in 2007, unfortunately was not that telling for obstetrics, as only two of the seven available randomized trials were deemed appropriate for meta-analysis. While the mean birth weight was higher in the CSII group, there were no significant differences in macrosomia or any other outcomes, and the reviewers warned they could not draw any conclusions because of the dearth of data. It is important to note, too, that the studies involved ’90s-style pumps (Cochrane Database Syst. Rev. 2007 [doi:10.1002/14651858.CD005542.pub2]).
Fortunately, outside of pregnant populations, we see quite a bit of data on CSII v. MDI. For instance, a 2010 Cochrane systematic review of CSII v. MDI covering 23 randomized controlled trials of 976 nonpregnant patients with type 1 diabetes found a statistically significant difference in HbA1c favoring CSII (–0.3%), as well as a reduction in severe hypoglycemia and higher quality of life scores (Cochrane Database Syst. Rev. 2010 [doi:10.1002/14651858.CD005103.pub2]).
A randomized, controlled, crossover trial published in 2006 called the 5-Nations Trial similarly showed that CSII treatment in individuals with type 1 diabetes resulted in lower HbA1c, lower mean blood glucose level, less fluctuation in glucose levels, and higher quality of life than MDI (Diabet. Med. 2006;23:141-7).
CGM technology is rapidly evolving. A randomized controlled trial published this year found that patients receiving CGM spent significantly less time in hypoglycemia each day and had a concomitant decrease in HbA1c (again –0.3%, approximately). Normoglycemia was significantly longer (mean of 17.6 v. 16 hours per day). The 120 type 1 patients in the study had been randomly assigned to either receive real-time CGM or perform conventional self-monitoring (while wearing a masked CGM device) (Diabetes Care 2011;34:795-800).
Another smaller study of patients with type 1 diabetes or gestational diabetes mellitus being treated with MDI found that nocturnal hypoglycemic events dropped significantly when the patients switched to CGM.
Granted, the false alarms that occur with glucose sensors can be disconcerting, the equipment is expensive, and patients have to enter a series of finger-stick blood sugar readings into the monitor for calibration. Still, most patients place a value on being able to get real-time blood glucose readings at any time, even when asleep, busy, or distracted. And they can always temporarily suspend their system – to get ready for bed, for example, or to go to Pilates classes.
Dr. Moore is professor and chair of reproductive medicine at the University of California, San Diego. He said he had no relevant financial disclosures.
No – There Is Little Data Showing Improved Outcomes
I use these tools in less than one-third of my patients with type 1 diabetes, and only rarely in patients with type 2 diabetes or gestational diabetes. There is very little evidence about the efficacy and implications of monitoring. Because of this, and because it is expensive, many payers do not reimburse for continuous glucose monitoring (CGM).
The goals of monitoring and treatment are very different. We monitor in order to guide treatment, to guide further diagnostic testing, to predict outcome, and to understand pathophysiology. But when we consider the value of glucose sensors and insulin pumps, we really need to consider the goals of treatment, which include reducing hyperglycemia without tripping over the threshold into hypoglycemia, and reducing short- and long-term neonatal and maternal complications of diabetes.
We have little data to guide us. A 2007 meta-analysis of randomized controlled trials comparing continuous subcutaneous insulin infusion (CSII) to multiple daily injections (MDI) in pregnant patients – an analysis separate from the Cochrane systematic review of 2007 that Dr. Moore mentions – found no significant differences in glycemic control or pregnancy outcomes. The studies included were published between 1986 and 1993, and since then, there have been no randomized trials comparing CSII and MDI in pregnant women (Am. J. Obstet. Gynecol. 2007;197:447-56).
With respect to CGM, a study reported by Dr. Y. Yogev and his associates in 2003 showed some promise: It compared the daily glycemic profiles reflected by continuous and intermittent blood glucose monitoring in pregnant women with type 1 diabetes, and found that CGM recorded high postprandial blood glucose levels and nocturnal hypoglycemic events that were unrecognized by intermittent monitoring. The question remains, however, whether such information leads to clinical interventions that can improve outcomes (Obstet. Gynecol. 2003;101:633-8).
A study published a year later, also by Dr. Yogev, showed that asymptomatic hypoglycemic events were surprisingly common in insulin- and glyburide-treated patients with gestational diabetes mellitus. The findings were eye-opening, but we really don’t know the significance of such hypoglycemic events.
We take it as a matter of faith that it’s bad to be hypoglycemic. In reality we know very little about what’s "normal" and "abnormal" in diabetic pregnant women and nondiabetic pregnant women.
In the only randomized controlled trial of CGM during pregnancy, women with type 1 and type 2 diabetes who were early in their pregnancies were assigned to perform self–blood glucose monitoring seven times a day or to have professional CGM for 1-week periods every 4-6 weeks. Findings in both groups were used to adjust therapy. By 36 weeks’ gestation, the CGM group had significantly lower HbA1c. Birth weights and the rate of macrosomia also were reduced (BMJ 2008;337:a1680).
The findings were impressive, but some of the data are unusual. For instance, the incidence of large-for-gestational-age (LGA) infants was reduced from 60% to 35%; both of these rates are high compared with rates at other centers. Data from the California Sweet Success program – a statewide effort to collect and share data on diabetes in pregnancy – show LGA in patients with type 1, type 2, and gestational diabetes to be 22.5%, 20%, and 11.4%, respectively – 27% of the type 1 patients were on CSII.
The American Association of Clinical Endocrinologists last year published a consensus statement recommending CGM for patients with type 1 diabetes who have certain risk factors, like frequent hypoglycemia or HbA1c over target. The AACE statement also includes those who are pregnant or about to become pregnant as ideal candidates for CGM, but curiously it provides no data to support this.
We have much more to learn. It is telling that in a recently published evaluation of a closed-loop insulin delivery model (an "artificial pancreas") in pregnant women with type 1 diabetes, investigators concluded that "before outpatient closed-loop studies proceed, they must be supported by scientifically rigorous data on safety and efficacy of real-time CGM," as well as more data on the algorithm for adjusting insulin infusion rates based on glycemic levels seen in pregnancy.
Dr. Combs is with Obstetrix Medical Group in San Jose, Calif. He said he had no relevant financial disclosures.
This feature is based on presentations given by Dr. Moore and Dr. Combs at the annual meeting of the Diabetes in Pregnancy Study Group of North America in Washington, D.C.
Yes – Continuous Glucose Monitoring and Insulin Pumps Improve Glycemic Control
Consider the case of a 33-year-old teacher who has a toddler in preschool, regularly attends Pilates classes, and is in her second pregnancy. Her privacy is limited, and her lunchtime varies by at least 30 minutes. In her first pregnancy, when she used multiple daily injections (MDI) for insulin injection, her hemoglobin A1c at 36 weeks’ gestation was 7.2%, and her baby’s birth weight was 4,200 g. This time around, she cannot get her fasting blood glucose under 110 mg/dL without experiencing marked hypoglycemia around 3:30 a.m.
In her case, the demonstrated benefits of continuous glucose monitoring (CGM) and an insulin pump (for continuous subcutaneous insulin infusion, or CSII) make this method of insulin administration a compelling – and even obvious – choice over MDI. A glucose sensor and insulin pump would improve her glucose control, bring down her HbA1c, and lessen the frequency and severity of hypoglycemic episodes, while better meshing with her lifestyle.
She is far from the only type of patient who would benefit, however. Modern CSII pumps (with Bolus Wizard calculators) have been around for a decade, yet we significantly underutilize them. Many, if not most, insulin-requiring pregnant women are good candidates for glucose sensors and insulin pumps.
CSII improves glucose control by providing "background" changes in insulin dosing to match the complexities of metabolism in pregnancy, and CGM (as opposed to self–blood glucose monitoring) improves glucose control by removing the fear of unexpected lows, which is a big issue in my patients, and by warning of impending highs.
Together, today’s equipment also improves patients’ ability to administer insulin in social and business settings. The equipment is also easily programmable and understandable to women who routinely use electronic devices.
A Cochrane systematic review of CSII vs. MDI in pregnancy, published in 2007, unfortunately was not that telling for obstetrics, as only two of the seven available randomized trials were deemed appropriate for meta-analysis. While the mean birth weight was higher in the CSII group, there were no significant differences in macrosomia or any other outcomes, and the reviewers warned they could not draw any conclusions because of the dearth of data. It is important to note, too, that the studies involved ’90s-style pumps (Cochrane Database Syst. Rev. 2007 [doi:10.1002/14651858.CD005542.pub2]).
Fortunately, outside of pregnant populations, we see quite a bit of data on CSII v. MDI. For instance, a 2010 Cochrane systematic review of CSII v. MDI covering 23 randomized controlled trials of 976 nonpregnant patients with type 1 diabetes found a statistically significant difference in HbA1c favoring CSII (–0.3%), as well as a reduction in severe hypoglycemia and higher quality of life scores (Cochrane Database Syst. Rev. 2010 [doi:10.1002/14651858.CD005103.pub2]).
A randomized, controlled, crossover trial published in 2006 called the 5-Nations Trial similarly showed that CSII treatment in individuals with type 1 diabetes resulted in lower HbA1c, lower mean blood glucose level, less fluctuation in glucose levels, and higher quality of life than MDI (Diabet. Med. 2006;23:141-7).
CGM technology is rapidly evolving. A randomized controlled trial published this year found that patients receiving CGM spent significantly less time in hypoglycemia each day and had a concomitant decrease in HbA1c (again –0.3%, approximately). Normoglycemia was significantly longer (mean of 17.6 v. 16 hours per day). The 120 type 1 patients in the study had been randomly assigned to either receive real-time CGM or perform conventional self-monitoring (while wearing a masked CGM device) (Diabetes Care 2011;34:795-800).
Another smaller study of patients with type 1 diabetes or gestational diabetes mellitus being treated with MDI found that nocturnal hypoglycemic events dropped significantly when the patients switched to CGM.
Granted, the false alarms that occur with glucose sensors can be disconcerting, the equipment is expensive, and patients have to enter a series of finger-stick blood sugar readings into the monitor for calibration. Still, most patients place a value on being able to get real-time blood glucose readings at any time, even when asleep, busy, or distracted. And they can always temporarily suspend their system – to get ready for bed, for example, or to go to Pilates classes.
Dr. Moore is professor and chair of reproductive medicine at the University of California, San Diego. He said he had no relevant financial disclosures.
No – There Is Little Data Showing Improved Outcomes
I use these tools in less than one-third of my patients with type 1 diabetes, and only rarely in patients with type 2 diabetes or gestational diabetes. There is very little evidence about the efficacy and implications of monitoring. Because of this, and because it is expensive, many payers do not reimburse for continuous glucose monitoring (CGM).
The goals of monitoring and treatment are very different. We monitor in order to guide treatment, to guide further diagnostic testing, to predict outcome, and to understand pathophysiology. But when we consider the value of glucose sensors and insulin pumps, we really need to consider the goals of treatment, which include reducing hyperglycemia without tripping over the threshold into hypoglycemia, and reducing short- and long-term neonatal and maternal complications of diabetes.
We have little data to guide us. A 2007 meta-analysis of randomized controlled trials comparing continuous subcutaneous insulin infusion (CSII) to multiple daily injections (MDI) in pregnant patients – an analysis separate from the Cochrane systematic review of 2007 that Dr. Moore mentions – found no significant differences in glycemic control or pregnancy outcomes. The studies included were published between 1986 and 1993, and since then, there have been no randomized trials comparing CSII and MDI in pregnant women (Am. J. Obstet. Gynecol. 2007;197:447-56).
With respect to CGM, a study reported by Dr. Y. Yogev and his associates in 2003 showed some promise: It compared the daily glycemic profiles reflected by continuous and intermittent blood glucose monitoring in pregnant women with type 1 diabetes, and found that CGM recorded high postprandial blood glucose levels and nocturnal hypoglycemic events that were unrecognized by intermittent monitoring. The question remains, however, whether such information leads to clinical interventions that can improve outcomes (Obstet. Gynecol. 2003;101:633-8).
A study published a year later, also by Dr. Yogev, showed that asymptomatic hypoglycemic events were surprisingly common in insulin- and glyburide-treated patients with gestational diabetes mellitus. The findings were eye-opening, but we really don’t know the significance of such hypoglycemic events.
We take it as a matter of faith that it’s bad to be hypoglycemic. In reality we know very little about what’s "normal" and "abnormal" in diabetic pregnant women and nondiabetic pregnant women.
In the only randomized controlled trial of CGM during pregnancy, women with type 1 and type 2 diabetes who were early in their pregnancies were assigned to perform self–blood glucose monitoring seven times a day or to have professional CGM for 1-week periods every 4-6 weeks. Findings in both groups were used to adjust therapy. By 36 weeks’ gestation, the CGM group had significantly lower HbA1c. Birth weights and the rate of macrosomia also were reduced (BMJ 2008;337:a1680).
The findings were impressive, but some of the data are unusual. For instance, the incidence of large-for-gestational-age (LGA) infants was reduced from 60% to 35%; both of these rates are high compared with rates at other centers. Data from the California Sweet Success program – a statewide effort to collect and share data on diabetes in pregnancy – show LGA in patients with type 1, type 2, and gestational diabetes to be 22.5%, 20%, and 11.4%, respectively – 27% of the type 1 patients were on CSII.
The American Association of Clinical Endocrinologists last year published a consensus statement recommending CGM for patients with type 1 diabetes who have certain risk factors, like frequent hypoglycemia or HbA1c over target. The AACE statement also includes those who are pregnant or about to become pregnant as ideal candidates for CGM, but curiously it provides no data to support this.
We have much more to learn. It is telling that in a recently published evaluation of a closed-loop insulin delivery model (an "artificial pancreas") in pregnant women with type 1 diabetes, investigators concluded that "before outpatient closed-loop studies proceed, they must be supported by scientifically rigorous data on safety and efficacy of real-time CGM," as well as more data on the algorithm for adjusting insulin infusion rates based on glycemic levels seen in pregnancy.
Dr. Combs is with Obstetrix Medical Group in San Jose, Calif. He said he had no relevant financial disclosures.
This feature is based on presentations given by Dr. Moore and Dr. Combs at the annual meeting of the Diabetes in Pregnancy Study Group of North America in Washington, D.C.
Yes – Continuous Glucose Monitoring and Insulin Pumps Improve Glycemic Control
Consider the case of a 33-year-old teacher who has a toddler in preschool, regularly attends Pilates classes, and is in her second pregnancy. Her privacy is limited, and her lunchtime varies by at least 30 minutes. In her first pregnancy, when she used multiple daily injections (MDI) for insulin injection, her hemoglobin A1c at 36 weeks’ gestation was 7.2%, and her baby’s birth weight was 4,200 g. This time around, she cannot get her fasting blood glucose under 110 mg/dL without experiencing marked hypoglycemia around 3:30 a.m.
In her case, the demonstrated benefits of continuous glucose monitoring (CGM) and an insulin pump (for continuous subcutaneous insulin infusion, or CSII) make this method of insulin administration a compelling – and even obvious – choice over MDI. A glucose sensor and insulin pump would improve her glucose control, bring down her HbA1c, and lessen the frequency and severity of hypoglycemic episodes, while better meshing with her lifestyle.
She is far from the only type of patient who would benefit, however. Modern CSII pumps (with Bolus Wizard calculators) have been around for a decade, yet we significantly underutilize them. Many, if not most, insulin-requiring pregnant women are good candidates for glucose sensors and insulin pumps.
CSII improves glucose control by providing "background" changes in insulin dosing to match the complexities of metabolism in pregnancy, and CGM (as opposed to self–blood glucose monitoring) improves glucose control by removing the fear of unexpected lows, which is a big issue in my patients, and by warning of impending highs.
Together, today’s equipment also improves patients’ ability to administer insulin in social and business settings. The equipment is also easily programmable and understandable to women who routinely use electronic devices.
A Cochrane systematic review of CSII vs. MDI in pregnancy, published in 2007, unfortunately was not that telling for obstetrics, as only two of the seven available randomized trials were deemed appropriate for meta-analysis. While the mean birth weight was higher in the CSII group, there were no significant differences in macrosomia or any other outcomes, and the reviewers warned they could not draw any conclusions because of the dearth of data. It is important to note, too, that the studies involved ’90s-style pumps (Cochrane Database Syst. Rev. 2007 [doi:10.1002/14651858.CD005542.pub2]).
Fortunately, outside of pregnant populations, we see quite a bit of data on CSII v. MDI. For instance, a 2010 Cochrane systematic review of CSII v. MDI covering 23 randomized controlled trials of 976 nonpregnant patients with type 1 diabetes found a statistically significant difference in HbA1c favoring CSII (–0.3%), as well as a reduction in severe hypoglycemia and higher quality of life scores (Cochrane Database Syst. Rev. 2010 [doi:10.1002/14651858.CD005103.pub2]).
A randomized, controlled, crossover trial published in 2006 called the 5-Nations Trial similarly showed that CSII treatment in individuals with type 1 diabetes resulted in lower HbA1c, lower mean blood glucose level, less fluctuation in glucose levels, and higher quality of life than MDI (Diabet. Med. 2006;23:141-7).
CGM technology is rapidly evolving. A randomized controlled trial published this year found that patients receiving CGM spent significantly less time in hypoglycemia each day and had a concomitant decrease in HbA1c (again –0.3%, approximately). Normoglycemia was significantly longer (mean of 17.6 v. 16 hours per day). The 120 type 1 patients in the study had been randomly assigned to either receive real-time CGM or perform conventional self-monitoring (while wearing a masked CGM device) (Diabetes Care 2011;34:795-800).
Another smaller study of patients with type 1 diabetes or gestational diabetes mellitus being treated with MDI found that nocturnal hypoglycemic events dropped significantly when the patients switched to CGM.
Granted, the false alarms that occur with glucose sensors can be disconcerting, the equipment is expensive, and patients have to enter a series of finger-stick blood sugar readings into the monitor for calibration. Still, most patients place a value on being able to get real-time blood glucose readings at any time, even when asleep, busy, or distracted. And they can always temporarily suspend their system – to get ready for bed, for example, or to go to Pilates classes.
Dr. Moore is professor and chair of reproductive medicine at the University of California, San Diego. He said he had no relevant financial disclosures.
No – There Is Little Data Showing Improved Outcomes
I use these tools in less than one-third of my patients with type 1 diabetes, and only rarely in patients with type 2 diabetes or gestational diabetes. There is very little evidence about the efficacy and implications of monitoring. Because of this, and because it is expensive, many payers do not reimburse for continuous glucose monitoring (CGM).
The goals of monitoring and treatment are very different. We monitor in order to guide treatment, to guide further diagnostic testing, to predict outcome, and to understand pathophysiology. But when we consider the value of glucose sensors and insulin pumps, we really need to consider the goals of treatment, which include reducing hyperglycemia without tripping over the threshold into hypoglycemia, and reducing short- and long-term neonatal and maternal complications of diabetes.
We have little data to guide us. A 2007 meta-analysis of randomized controlled trials comparing continuous subcutaneous insulin infusion (CSII) to multiple daily injections (MDI) in pregnant patients – an analysis separate from the Cochrane systematic review of 2007 that Dr. Moore mentions – found no significant differences in glycemic control or pregnancy outcomes. The studies included were published between 1986 and 1993, and since then, there have been no randomized trials comparing CSII and MDI in pregnant women (Am. J. Obstet. Gynecol. 2007;197:447-56).
With respect to CGM, a study reported by Dr. Y. Yogev and his associates in 2003 showed some promise: It compared the daily glycemic profiles reflected by continuous and intermittent blood glucose monitoring in pregnant women with type 1 diabetes, and found that CGM recorded high postprandial blood glucose levels and nocturnal hypoglycemic events that were unrecognized by intermittent monitoring. The question remains, however, whether such information leads to clinical interventions that can improve outcomes (Obstet. Gynecol. 2003;101:633-8).
A study published a year later, also by Dr. Yogev, showed that asymptomatic hypoglycemic events were surprisingly common in insulin- and glyburide-treated patients with gestational diabetes mellitus. The findings were eye-opening, but we really don’t know the significance of such hypoglycemic events.
We take it as a matter of faith that it’s bad to be hypoglycemic. In reality we know very little about what’s "normal" and "abnormal" in diabetic pregnant women and nondiabetic pregnant women.
In the only randomized controlled trial of CGM during pregnancy, women with type 1 and type 2 diabetes who were early in their pregnancies were assigned to perform self–blood glucose monitoring seven times a day or to have professional CGM for 1-week periods every 4-6 weeks. Findings in both groups were used to adjust therapy. By 36 weeks’ gestation, the CGM group had significantly lower HbA1c. Birth weights and the rate of macrosomia also were reduced (BMJ 2008;337:a1680).
The findings were impressive, but some of the data are unusual. For instance, the incidence of large-for-gestational-age (LGA) infants was reduced from 60% to 35%; both of these rates are high compared with rates at other centers. Data from the California Sweet Success program – a statewide effort to collect and share data on diabetes in pregnancy – show LGA in patients with type 1, type 2, and gestational diabetes to be 22.5%, 20%, and 11.4%, respectively – 27% of the type 1 patients were on CSII.
The American Association of Clinical Endocrinologists last year published a consensus statement recommending CGM for patients with type 1 diabetes who have certain risk factors, like frequent hypoglycemia or HbA1c over target. The AACE statement also includes those who are pregnant or about to become pregnant as ideal candidates for CGM, but curiously it provides no data to support this.
We have much more to learn. It is telling that in a recently published evaluation of a closed-loop insulin delivery model (an "artificial pancreas") in pregnant women with type 1 diabetes, investigators concluded that "before outpatient closed-loop studies proceed, they must be supported by scientifically rigorous data on safety and efficacy of real-time CGM," as well as more data on the algorithm for adjusting insulin infusion rates based on glycemic levels seen in pregnancy.
Dr. Combs is with Obstetrix Medical Group in San Jose, Calif. He said he had no relevant financial disclosures.
This feature is based on presentations given by Dr. Moore and Dr. Combs at the annual meeting of the Diabetes in Pregnancy Study Group of North America in Washington, D.C.