User login
Think of anxiety disorders as an overactive brain alarm that psychotropics and exposure therapy quiet via separate mechanisms. Psychotropics “cool off” the alarm by curtailing excitability of the amygdala, brainstem nuclei, and hypothalamus.1 Psychological treatments, particularly exposure therapy, seek to teach the brain not to fear the dreaded object.2
One would assume that combining medication and exposure therapy for anxiety would be beneficial, but results have been disappointing.3 Anxiolytics do not enhance—and many impede—learning that occurs during psychotherapy. When the medications are tapered, patients who receive psychotherapy plus placebo typically experience more-enduring benefit than those receiving psychotherapy plus active medication.4-6
An unlikely candidate—a glutamatergic tuberculosis (TB) drug—may offer a solution. The drug and others in its class may potentiate psychotherapy’s effects by enhancing learning rather than relief.7
Glutamate/learning link
Glutamate neurons have 3 types of glutamatergic receptors, with the NMDA and AMPA types perceived as most important because of their possible role in memory development. Creating new memories may involve strengthening signals between glutamate neurons. The exact cellular mechanism is unknown, but it may involve greater release of neurotransmitters or formation of new synapses.
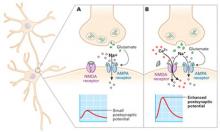
Stronger signaling—and hence learning—may depend on opening the NMDA receptor to enhance postsynaptic potential.8 Opening both NMDA and AMPA receptors generates a stronger signal and allows calcium influx, compared with opening the AMPA receptor alone (Figure). This combination can activate genes that control protein synthesis and result in structural changes necessary for developing long-term memories.
D-cycloserine—a partial agonist at the NMDA receptor—is usually used as an antibiotic to treat TB. The drug also has been shown to enhance the learning process that underlies fear extinction in rats. A group at Emory University studied the effect of adding the medication to exposure therapy in humans with acrophobia.7
FigureNMDA receptor agonists may enhance learning in psychotherapy
Normally, only glutamate neurons’ AMPA receptors activate in response to glutamate release (A). Both AMPA and NMDA receptors open in response to NMDA receptor agonists like D-cycloserine (B). Consequent stronger signaling may improve memory.
Going ‘up’
The researchers developed a virtual reality exposure system in which participants felt as if they were standing in an elevator, watching the floors recede as they rose 19 stories. Exposure therapy—seven weekly 35- to 45-minute sessions—has been shown to reduce the fear patients with acrophobia experience in virtual elevators.
Of 27 subjects, 10 received placebo and 17 received D-cycloserine, 50 mg or 500 mg. Subjects took their pills 2 to 4 hours before an exposure session. All participants experienced 2 virtual exposure sessions 1 to 2 weeks apart, which is considered suboptimal treatment for acrophobia.
Three months after the study, the D-cycloserine groups showed a markedly reduced fear of heights on the virtual elevator, while the placebo group showed no change from baseline. Fear levels were measured by subjective report of discomfort at each “floor.” D-cycloserine subjects also reported significantly greater reductions in measures of acrophobia in their daily lives.
Interestingly, both the D-cycloserine and placebo groups were equally frightened during virtual reality exposure. Only later did the D-cycloserine groups report less fear when exposed to heights, indicating that D-cycloserine enhanced learning that occurred during exposure therapy.
It’s exciting to think that medications could enhance and accelerate healing by activating the appropriate receptors during psychotherapy and give patients enduring benefits without the need for continued treatment.
If shown to be effective in larger studies, glutamatergic medications plus psychotherapy could provide more effective therapy for anxiety disorder. This approach is reported to be under investigation for treating anorexia nervosa, social phobia, panic disorder, and obsessive-compulsive disorder.9
1. Lydiard RB. Break the ‘fear circuit’ in resistant panic disorder. Current Psychiatry 2003;2(11):12-22.
2. Tynes LL, Tynes SF. Panic attacks: help sufferers recover with cognitive-behavioral therapy. Current Psychiatry 2005;4(11):51-60.
3. Otto MW, Smits JAJ, Reese HE. Combined psychotherapy and pharmacotherapy for mood and anxiety disorders in adults: review and analysis. Clinical Psychology: Science & Practice 2005;12(1):72-86.
4. Barlow DH, Gorman JM, Shear MK, Woods SW. Cognitive-behavioral therapy, imipramine, or their combination for panic disorder: A randomized controlled trial. JAMA 2000;283(19):2529-36.
5. Haug TT, Blomhoff S, Hellstrom K, et al. Exposure therapy and sertraline in social phobia: I-year follow-up of a randomised controlled trial. Br J Psychiatry 2003;182:312-8.
6. Marks IM, Swinson RP, Basoglu M, et al. Alprazolam and exposure alone and combined in panic disorder with agoraphobia. A controlled study in London and Toronto. Br J Psychiatry 1993;162:776-87.
7. Ressler KJ, Rothbaum BO, Tannenbaum L, et al. Cognitive enhancers as adjuncts to psychotherapy: use of D-cycloserine in phobic individuals to facilitate extinction of fear. Arch Gen Psychiatry 2004;61(11):1136-44.
8. Purves D, Augustine GJ, Fitzpatrick D, et al. Plasticity of mature synapses and circuits. In: Neuroscience (3rd ed). Sunderland, MA: Sinauer; 2004:575-610.
9. O’Connor A. A pill that helps ease grip of irrational fears. New York Times March 22, 2005.
Think of anxiety disorders as an overactive brain alarm that psychotropics and exposure therapy quiet via separate mechanisms. Psychotropics “cool off” the alarm by curtailing excitability of the amygdala, brainstem nuclei, and hypothalamus.1 Psychological treatments, particularly exposure therapy, seek to teach the brain not to fear the dreaded object.2
One would assume that combining medication and exposure therapy for anxiety would be beneficial, but results have been disappointing.3 Anxiolytics do not enhance—and many impede—learning that occurs during psychotherapy. When the medications are tapered, patients who receive psychotherapy plus placebo typically experience more-enduring benefit than those receiving psychotherapy plus active medication.4-6
An unlikely candidate—a glutamatergic tuberculosis (TB) drug—may offer a solution. The drug and others in its class may potentiate psychotherapy’s effects by enhancing learning rather than relief.7
Glutamate/learning link
Glutamate neurons have 3 types of glutamatergic receptors, with the NMDA and AMPA types perceived as most important because of their possible role in memory development. Creating new memories may involve strengthening signals between glutamate neurons. The exact cellular mechanism is unknown, but it may involve greater release of neurotransmitters or formation of new synapses.
Stronger signaling—and hence learning—may depend on opening the NMDA receptor to enhance postsynaptic potential.8 Opening both NMDA and AMPA receptors generates a stronger signal and allows calcium influx, compared with opening the AMPA receptor alone (Figure). This combination can activate genes that control protein synthesis and result in structural changes necessary for developing long-term memories.
D-cycloserine—a partial agonist at the NMDA receptor—is usually used as an antibiotic to treat TB. The drug also has been shown to enhance the learning process that underlies fear extinction in rats. A group at Emory University studied the effect of adding the medication to exposure therapy in humans with acrophobia.7
FigureNMDA receptor agonists may enhance learning in psychotherapy
Normally, only glutamate neurons’ AMPA receptors activate in response to glutamate release (A). Both AMPA and NMDA receptors open in response to NMDA receptor agonists like D-cycloserine (B). Consequent stronger signaling may improve memory.
Going ‘up’
The researchers developed a virtual reality exposure system in which participants felt as if they were standing in an elevator, watching the floors recede as they rose 19 stories. Exposure therapy—seven weekly 35- to 45-minute sessions—has been shown to reduce the fear patients with acrophobia experience in virtual elevators.
Of 27 subjects, 10 received placebo and 17 received D-cycloserine, 50 mg or 500 mg. Subjects took their pills 2 to 4 hours before an exposure session. All participants experienced 2 virtual exposure sessions 1 to 2 weeks apart, which is considered suboptimal treatment for acrophobia.
Three months after the study, the D-cycloserine groups showed a markedly reduced fear of heights on the virtual elevator, while the placebo group showed no change from baseline. Fear levels were measured by subjective report of discomfort at each “floor.” D-cycloserine subjects also reported significantly greater reductions in measures of acrophobia in their daily lives.
Interestingly, both the D-cycloserine and placebo groups were equally frightened during virtual reality exposure. Only later did the D-cycloserine groups report less fear when exposed to heights, indicating that D-cycloserine enhanced learning that occurred during exposure therapy.
It’s exciting to think that medications could enhance and accelerate healing by activating the appropriate receptors during psychotherapy and give patients enduring benefits without the need for continued treatment.
If shown to be effective in larger studies, glutamatergic medications plus psychotherapy could provide more effective therapy for anxiety disorder. This approach is reported to be under investigation for treating anorexia nervosa, social phobia, panic disorder, and obsessive-compulsive disorder.9
Think of anxiety disorders as an overactive brain alarm that psychotropics and exposure therapy quiet via separate mechanisms. Psychotropics “cool off” the alarm by curtailing excitability of the amygdala, brainstem nuclei, and hypothalamus.1 Psychological treatments, particularly exposure therapy, seek to teach the brain not to fear the dreaded object.2
One would assume that combining medication and exposure therapy for anxiety would be beneficial, but results have been disappointing.3 Anxiolytics do not enhance—and many impede—learning that occurs during psychotherapy. When the medications are tapered, patients who receive psychotherapy plus placebo typically experience more-enduring benefit than those receiving psychotherapy plus active medication.4-6
An unlikely candidate—a glutamatergic tuberculosis (TB) drug—may offer a solution. The drug and others in its class may potentiate psychotherapy’s effects by enhancing learning rather than relief.7
Glutamate/learning link
Glutamate neurons have 3 types of glutamatergic receptors, with the NMDA and AMPA types perceived as most important because of their possible role in memory development. Creating new memories may involve strengthening signals between glutamate neurons. The exact cellular mechanism is unknown, but it may involve greater release of neurotransmitters or formation of new synapses.
Stronger signaling—and hence learning—may depend on opening the NMDA receptor to enhance postsynaptic potential.8 Opening both NMDA and AMPA receptors generates a stronger signal and allows calcium influx, compared with opening the AMPA receptor alone (Figure). This combination can activate genes that control protein synthesis and result in structural changes necessary for developing long-term memories.
D-cycloserine—a partial agonist at the NMDA receptor—is usually used as an antibiotic to treat TB. The drug also has been shown to enhance the learning process that underlies fear extinction in rats. A group at Emory University studied the effect of adding the medication to exposure therapy in humans with acrophobia.7
FigureNMDA receptor agonists may enhance learning in psychotherapy
Normally, only glutamate neurons’ AMPA receptors activate in response to glutamate release (A). Both AMPA and NMDA receptors open in response to NMDA receptor agonists like D-cycloserine (B). Consequent stronger signaling may improve memory.
Going ‘up’
The researchers developed a virtual reality exposure system in which participants felt as if they were standing in an elevator, watching the floors recede as they rose 19 stories. Exposure therapy—seven weekly 35- to 45-minute sessions—has been shown to reduce the fear patients with acrophobia experience in virtual elevators.
Of 27 subjects, 10 received placebo and 17 received D-cycloserine, 50 mg or 500 mg. Subjects took their pills 2 to 4 hours before an exposure session. All participants experienced 2 virtual exposure sessions 1 to 2 weeks apart, which is considered suboptimal treatment for acrophobia.
Three months after the study, the D-cycloserine groups showed a markedly reduced fear of heights on the virtual elevator, while the placebo group showed no change from baseline. Fear levels were measured by subjective report of discomfort at each “floor.” D-cycloserine subjects also reported significantly greater reductions in measures of acrophobia in their daily lives.
Interestingly, both the D-cycloserine and placebo groups were equally frightened during virtual reality exposure. Only later did the D-cycloserine groups report less fear when exposed to heights, indicating that D-cycloserine enhanced learning that occurred during exposure therapy.
It’s exciting to think that medications could enhance and accelerate healing by activating the appropriate receptors during psychotherapy and give patients enduring benefits without the need for continued treatment.
If shown to be effective in larger studies, glutamatergic medications plus psychotherapy could provide more effective therapy for anxiety disorder. This approach is reported to be under investigation for treating anorexia nervosa, social phobia, panic disorder, and obsessive-compulsive disorder.9
1. Lydiard RB. Break the ‘fear circuit’ in resistant panic disorder. Current Psychiatry 2003;2(11):12-22.
2. Tynes LL, Tynes SF. Panic attacks: help sufferers recover with cognitive-behavioral therapy. Current Psychiatry 2005;4(11):51-60.
3. Otto MW, Smits JAJ, Reese HE. Combined psychotherapy and pharmacotherapy for mood and anxiety disorders in adults: review and analysis. Clinical Psychology: Science & Practice 2005;12(1):72-86.
4. Barlow DH, Gorman JM, Shear MK, Woods SW. Cognitive-behavioral therapy, imipramine, or their combination for panic disorder: A randomized controlled trial. JAMA 2000;283(19):2529-36.
5. Haug TT, Blomhoff S, Hellstrom K, et al. Exposure therapy and sertraline in social phobia: I-year follow-up of a randomised controlled trial. Br J Psychiatry 2003;182:312-8.
6. Marks IM, Swinson RP, Basoglu M, et al. Alprazolam and exposure alone and combined in panic disorder with agoraphobia. A controlled study in London and Toronto. Br J Psychiatry 1993;162:776-87.
7. Ressler KJ, Rothbaum BO, Tannenbaum L, et al. Cognitive enhancers as adjuncts to psychotherapy: use of D-cycloserine in phobic individuals to facilitate extinction of fear. Arch Gen Psychiatry 2004;61(11):1136-44.
8. Purves D, Augustine GJ, Fitzpatrick D, et al. Plasticity of mature synapses and circuits. In: Neuroscience (3rd ed). Sunderland, MA: Sinauer; 2004:575-610.
9. O’Connor A. A pill that helps ease grip of irrational fears. New York Times March 22, 2005.
1. Lydiard RB. Break the ‘fear circuit’ in resistant panic disorder. Current Psychiatry 2003;2(11):12-22.
2. Tynes LL, Tynes SF. Panic attacks: help sufferers recover with cognitive-behavioral therapy. Current Psychiatry 2005;4(11):51-60.
3. Otto MW, Smits JAJ, Reese HE. Combined psychotherapy and pharmacotherapy for mood and anxiety disorders in adults: review and analysis. Clinical Psychology: Science & Practice 2005;12(1):72-86.
4. Barlow DH, Gorman JM, Shear MK, Woods SW. Cognitive-behavioral therapy, imipramine, or their combination for panic disorder: A randomized controlled trial. JAMA 2000;283(19):2529-36.
5. Haug TT, Blomhoff S, Hellstrom K, et al. Exposure therapy and sertraline in social phobia: I-year follow-up of a randomised controlled trial. Br J Psychiatry 2003;182:312-8.
6. Marks IM, Swinson RP, Basoglu M, et al. Alprazolam and exposure alone and combined in panic disorder with agoraphobia. A controlled study in London and Toronto. Br J Psychiatry 1993;162:776-87.
7. Ressler KJ, Rothbaum BO, Tannenbaum L, et al. Cognitive enhancers as adjuncts to psychotherapy: use of D-cycloserine in phobic individuals to facilitate extinction of fear. Arch Gen Psychiatry 2004;61(11):1136-44.
8. Purves D, Augustine GJ, Fitzpatrick D, et al. Plasticity of mature synapses and circuits. In: Neuroscience (3rd ed). Sunderland, MA: Sinauer; 2004:575-610.
9. O’Connor A. A pill that helps ease grip of irrational fears. New York Times March 22, 2005.