User login
A 50-year-old African American woman with type 2 diabetes mellitus, hypertension, hyperlipidemia, and chronic kidney disease presents for a follow-up visit. The patient had been treated with hydrochlorothiazide 25 mg/day and enalapril (Vasotec) 20 mg twice daily until 6 weeks ago. At that time her blood pressure was 160/85 mm Hg, and amlodipine (Norvasc) 10 mg/day was added to her regimen. Her other medications include glipizide (Glucotrol), metformin (Glucophage), lovastatin (Mevacor), fish oils, aspirin, calcium, and vitamin D. Her current blood pressure is 145/80 mm Hg; her serum creatinine level is 1.5 mg/dL, and her urine albumin-to-creatinine ratio is 180 mg/g.
In hypertensive patients who have diabetes or chronic kidney disease, guidelines1 call for intensification of antihypertensive therapy to reach a goal blood pressure of less than 130/80 mm Hg. What data exist to support these guidelines? And what should the clinician do?
IS MORE-INTENSE THERAPY IN THE PATIENT’S BEST INTEREST?
Often, clinicians are faced with hypertensive patients whose blood pressure, despite treatment, is higher than the accepted goal. Often, these patients are elderly and are already taking multiple medications that are costly and have significant potential adverse effects. The dilemma is whether to try to reach a target blood pressure listed in a guideline (by increasing the dosage of the current drugs or by adding a drug of a different class) or to “do no harm,” accept the patient’s blood pressure, and keep the regimen the same.1,2
The current goal blood pressure is less than 140/90 mm Hg for all but the very elderly, with more intense control recommended for patients at high risk, ie, those with diabetes mellitus, chronic kidney disease, or atherosclerotic cardiovascular disease.1
While it appears to be in the patient’s best interests to follow such guidelines, review of available data indicates that this it not necessarily so, and may even be harmful.
OBSERVATIONAL DATA AND EARLY RANDOMIZED TRIALS
Many observational studies have found that the higher one’s blood pressure, the greater one’s risk of cardiovascular events and death. Indeed, meta-analyses of these trials, which involved more than 1.5 million people, demonstrate a strong, positive, log-linear relationship between blood pressure and the incidence of cardiovascular disease and death.3–5
Further, there is no evidence of a threshold pressure below which the risk is not lower (ie, a “J-point”), starting with 115/75 mm Hg. A J-point may exist for diastolic blood pressure in elderly patients with isolated systolic hypertension6 and in patients with coronary artery disease.7 Otherwise, the observation is clear: the lower the blood pressure the better. For every 20 mm Hg lower systolic blood pressure or 10 mm Hg lower diastolic blood pressure, the risk of a cardiovascular event is about 50% less.4,5
Observational analyses also show a strong, graded relationship between blood pressure and future end-stage renal disease.8,9 Post hoc analyses indicate that chronic kidney disease progresses more slowly with lower achieved blood pressures, especially in those with higher degrees of proteinuria.10–12
However, observational data do not prove cause and effect, nor do they guarantee similar results with treatment. This requires randomized controlled trials.
RANDOMIZED TRIALS OF HYPERTENSION TREATMENT
Initial trials were aimed at determining whether hypertension should even be treated. A 1997 meta-analysis of 18 such trials comparing either low-dose diuretic therapy, high-dose diuretic therapy, or beta-blocker therapy with placebo involved 48,000 patients who were followed for an average of 5 years.13 The rates of stroke and congestive heart failure were consistently reduced, although only low-dose diuretic therapy reduced the risk of coronary heart disease and death from any cause.
More recent trials enrolled people not considered hypertensive who were randomized to receive either active drugs or placebo, or no treatment. Other trials attempted to assess non-pressure-related effects of specific agents, using other antihypertensive agents in the control group. Still other randomized controlled trials compared one agent or agents with other agents while attempting to attain equivalent blood pressure between groups. Frequently, however, there was some blood pressure difference.
Meta-analyses of most of these trials conclude that the major benefit of antihypertensive therapy—reducing rates of cardiovascular morbidity and mortality—comes from a lower attained blood pressure, irrespective of which agent is used.14–18 Exceptions exist, however. For example, specific drug classes are indicated after myocardial infarction, and in congestive heart failure and proteinuric chronic kidney disease.10,19–21
16 TRIALS OF DIFFERENT BLOOD PRESSURE TARGETS
The overriding theme of these observational data is that a lower blood pressure, whether attained naturally or with treatment, is better than a higher one from both the cardiovascular and the renal perspective.
What remains unclear is what blood pressure should be aimed for in a particular patient or group of patients. Is it a specific pressure (eg, 140/90 mm Hg), or does the change from baseline count more? Should other factors such as age or comorbidity alter this number?
Several randomized controlled trials have addressed these questions by targeting different levels of blood pressure. We are aware of at least 16 such trials in adults, including 13 with renal or cardiovascular primary end points and three with surrogate primary end points.
An unavoidable design flaw of all of these trials is their unblinded nature. Consequently, nearly all of them carry a Jadad score (a measure of quality, based on randomization and blinding)22 of 3 on a scale of 5.
NINE TRIALS WITH RENAL PRIMARY END POINTS
African American Study of Kidney Disease and Hypertension (AASK)23
Patients: 1,094 African Americans with presumed hypertensive renal disease and a measured glomerular filtration rate between 20 and 65 mL/min/1.73 m2.
Randomized blood pressure goals. Mean arterial pressure 92 mm Hg or less vs 102 to 107 mm Hg.
Results. At 4 years, the two groups had average blood pressures of 128/78 and 141/85 mm Hg, respectively. The groups did not differ in the rates of the primary end points—ie, the rate of change in the measured glomerular filtration rate over time or the composite of a 50% reduction in glomerular filtration rate, the onset of end-stage renal disease, or death.
Comments. Several issues have been raised about the internal validity of this trial.
So-called hypertensive kidney disease in African Americans (as opposed to European Americans) may be a genetic disorder related to polymorphisms of one or more genes on chromosome 22q. Initial data implicated the MYH9 gene, which encodes non-muscle myosin heavy chain II.24,25 More recent data implicate the nearby APOL1 gene encoding apolipoprotein L126 as more relevant. These polymorphisms have a much greater prevalence in African Americans and appear responsible for the higher risk of idiopathic focal segmental glomerulosclerosis and HIV-associated nephropathy in this population.24–26 Therefore, in African Americans, hypertension may in fact be the result of the kidney disease and not its primary cause, which may explain why in this and other African American populations stricter control of blood pressure did not produce a renal benefit.27,28
Also, there is the possibility of misclassification bias. A secondary analysis of data obtained by ambulatory monitoring showed that of the 377 participants whose blood pressure appeared to be under control when measured in the clinic, 70% actually had masked hypertension, ie, uncontrolled hypertension outside the clinic.29 The real difference in blood pressure between groups may well have been different than that determined in the clinic.
In addition, a prespecified secondary analysis showed no difference in the rates of cardiovascular events and death between the groups.30 However, the study was not designed to have the statistical power to detect a difference in cardiovascular events. Moreover fewer cardiovascular events occurred than expected, further reducing the study’s power to detect a difference.
Toto et al31
Toto et al reported similar results in an earlier trial in 87 hypertensive patients (77 randomized), predominantly African American, and similar concerns apply.
Lewis et al32
Patients: 129 patients with type 1 diabetes.
Randomized blood pressure goals. A mean arterial pressure of either no higher than 92 mm Hg or 100 to 107 mm Hg.
Results. At 2 years, despite a difference of 6 mm Hg in mean arterial pressure, the glomerular filtration rate (measured) had declined by the same amount in the two groups. The study was underpowered for this end point. Patients in the group with the lower goal pressure were excreting significantly less protein than those in the other group, but they were received higher doses of an angiotensin-converting enzyme (ACE) inhibitor—in this case, ramipril (Altace).
The Appropriate Blood Pressure Control in Diabetes (ABCD) trials33–35
Patients: 950 patients with type 2 diabetes mellitus and either normal or high blood pressure.
Randomized blood pressure goals. Either intensive or moderate therapy (see Table 1).
Results. At 5 years, creatinine clearance (estimated) had declined by the same amount in the two groups. However, fewer of the hypertensive patients had died in the intensive-therapy group.34 Similarly, normotensive patients had less progression of albuminuria if treated intensively.33
In the ABCD Part 2 with Valsartan (ABCD-2V) trial in normotensive patients,35 therapy with valsartan (Diovan) did not affect creatinine clearance but did reduce albuminuria. However, 75% of the patients in the moderate-treatment group were untreated.
Schrier et al36
Patients. 75 hypertensive patients with autosomal-dominant polycystic kidney disease and left ventricular hypertrophy.
Randomized blood pressure targets. Less than 120/80 mm Hg vs 135/85 to 140/90 mm Hg.
Results. After 7 years, despite a difference in average mean arterial pressure of 11 mm Hg between the groups (90 vs 101 mm Hg), there was no difference in the rate of decline of creatinine clearance. The left ventricular mass index decreased by 21% in the lower-target group and by 35% in the higher-target group (P < .01).
Modification of Diet in Renal Disease (MDRD) trial37,38
Patients: 840 patients whose measured glomerular filtration rate was between 13 and 55 mL/min/1.73 m2.
Randomized blood pressure targets. A target mean arterial pressure of less than 92 mm Hg vs less than 107 mm Hg.11,37
Results. After 2.2 years, the mean difference in mean arterial pressure was 4.7 mm Hg. There was, however, no difference in the rate of decline in the glomerular filtration rate.
In a 6-year follow-up, significantly fewer patients in the lower-blood-pressure group reached the end point of end-stage renal disease or the combined end point of end-stage renal disease or death.38 The rate of death, however, was nearly twice as high in the lower-blood-pressure group (10% vs 6%). The blood pressure and treatment during follow-up were not reported.
Comments. Internal validity is an issue, since the blood pressure and therapy during follow-up were unknown, and more patients received ACE inhibitors in the lower-blood-pressure group during the trial. Further, the higher death rate in the lower-blood-pressure group is worrisome.
The Ramipril Efficacy in Nephropathy (REIN)-2 trial39
Patients: 338 nondiabetic patients who had proteinuria and reduced creatinine clearance.
Treatment and blood pressure goals. All were treated with ramipril and randomized to intensive (< 130/80 mm Hg) vs standard control (diastolic blood pressure < 90 mm Hg) with therapy based on felodipine (Plendil).
Results. The study was terminated early because of futility. Despite a mean difference of 4.1 mm Hg systolic and 2.8 mm Hg diastolic, the groups did not differ in the rate of progression to end-stage renal disease (23% with intensive therapy vs 20% with standard therapy) or in the rate of decline of the measured glomerular filtration rate (0.22 vs 0.24 mL/min/1.73 m2/month).
Comment. The internal validity of this study can be questioned because of the low separation of achieved blood pressure and because of its early termination.
No benefit from a lower blood pressure goal in preserving kidney function
To summarize, these trials all showed no significant benefit from either targeting or achieving lower blood pressure in terms of slowing the decline of kidney function. Overall, they do not define a target and offer little support that a lower goal blood pressure is indicated with respect to the rate of loss of glomerular filtration rate in chronic kidney disease.
However, post hoc analysis of the MDRD trial indicates a statistical interaction between targeted blood pressure and degree of baseline proteinuria. At higher levels of proteinuria (≥ 1 g/day), the group with the lower blood pressure target had better outcomes.
In addition, long-term follow-up (mean of 12.2 years) of the AASK trial, including a 7-year cohort phase with nearly similar blood pressures in both groups, also indicated an interaction with targeted blood pressure and baseline proteinuria.40 Although the overall analysis was negative, there was a significant reduction in the primary end point in the group originally assigned the low target when analysis was restricted to those in the highest tertile of proteinuria. These and other data10 suggest that patients with chronic kidney disease and proteinuria may represent a distinct subset of chronic kidney disease patients who benefit from more intensive blood-pressure-lowering. However, patients in the REIN-2 trial34 and the macroalbuminuric patients in the ABCD hypertensive trial35 did not benefit from a lower targeted blood pressure despite significant proteinuria.
FOUR TRIALS WITH CARDIOVASCULAR END POINTS
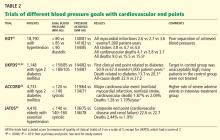
The Hypertension Optimal Treatment (HOT) trial41
Patients: 18,790 patients with diastolic blood pressure between 100 and 115 mm Hg.
Randomized blood pressure goals. Diastolic pressure of equal to or less than 80, 85, or 90 mm Hg.
Results. At an average of 3.8 years, the average blood pressures in the three groups were approximately 140/81, 141/83, and 144/85 mm Hg, respectively. There was no difference between the groups in the rate of the composite primary end point of all myocardial infarctions, all strokes, and cardiovascular death. Any conclusions from this trial were compromised by the small difference in achieved blood pressures between groups.
In the 1,501 patients with diabetes, the incidence of the primary end point was 50% lower with a goal of 80 mm Hg or less than with a goal of 90 mm Hg or less.
The UK Prospective Diabetes Study (UKPDS)42,43
Patients: 1,148 hypertensive patients with type 2 diabetes mellitus.
Randomized blood pressure goals. Either “tight control” (aiming for < 150/85 mm Hg) or “less tight control” (aiming for < 180/105 mm Hg).
Results. At a median follow-up of 8.4 years, the attained blood pressures were 144/82 vs 154/87 mm Hg. The difference produced significant benefits, including a 24% lower rate of any diabetes-related end point, a 32% lower rate of death due to diabetes, and a nonsignificant 18% lower rate of total mortality—all co-primary end points.
The less-tight-control group had many patients with initial blood pressures below 180/105 mm Hg; hence, over 50% of patients received no antihypertensive therapy at the start of the trial. By the end of the trial 9 years later, 20% had still not been treated. This compares with only 5% of patients in the tight-control group who were not treated with antihypertensives throughout the trial. Therefore, this trial serves as better evidence for treating vs not treating, rather than defining a specific goal.
During a 10-year follow-up, blood pressure differences disappeared within 2 years.43 There was no legacy effect, as the significant differences noted during the trial were no longer present 10 years later.
Action to Control Cardiovascular Risk in Diabetes (ACCORD)44
Patients: 4,733 patients with type 2 diabetes.
Randomized blood pressure goals. Systolic blood pressure lower than either 120 or 140 mm Hg.
Results. At 4.7 years, despite a significant difference in mean systolic blood pressure of 14.2 mm Hg after the first year (119.3 vs 133.5 mm Hg), there was no difference in the primary end point of nonfatal myocardial infarction, nonfatal stroke, or cardiovascular death. There were fewer strokes in the lower-pressure group but no difference in myocardial infarctions, which were five times more common than strokes. Serious adverse events attributed to antihypertensive treatment occurred more frequently in the intensive-therapy group (3.3% vs 1.3%, P < .001).
Comment. There were fewer events than expected, possibly limiting the trial’s ability to detect a statistical difference. Compared with both the UKPDS and the diabetic population of HOT, ACCORD is much larger and more internally valid (unlike in UKPDS, nearly all patients in both groups were treated, and compared with HOT there was much greater separation of achieved pressure). It is more recent and better reflects current overall practice. It indicates that when specifically aiming for a target blood pressure, lower is not always better and comes at a price (more severe adverse events).
Japanese Trial to Assess Optimal Systolic Blood Pressure in Elderly Hypertensive Patients (JATOS)45
Patients: 4,418 patients, age 65 to 85 years, with a pretreatment systolic blood pressure above 160 mm Hg.
Randomized blood pressure goals. Systolic pressure either lower than 140 mm Hg or 140 to 160 mm Hg.
Results. At 2 years, despite a difference of 9.7/3.3 mm Hg, there was no difference in the primary end point (the combined incidence of cerebrovascular disease, cardiac and vascular disease, and renal failure). Fifty-four patients had died in the strict-treatment group and 42 in the mild-treatment group; the difference was not statistically significant.
Three other trials
Three other trials46–48 had surrogate end points, but only one of them reported a composite cardiovascular secondary end point.46 We will not discuss the other two.47,48
Cardio-Sis. In the Studio Italiano Sugli Effetti Cardiovascolari del Controllo della Pressione Arteriosa Sistolica (Cardio-Sis) trial,46 1,111 people without diabetes with systolic pressure higher than 150 mm Hg were randomized to tight control (systolic pressure < 130 mm Hg) vs usual control (systolic pressure < 140 mm Hg) and followed for 2 years with electrocardiography to detect left ventricular hypertrophy.
At a median of 2 years, the systolic blood pressure had declined by an average of 3.8 mm Hg more in the tight-control group than in the usual-control group, and the diastolic pressure by an average of 1.5 mm Hg. There was significantly less left ventricular hypertrophy in the tight-control group. The incidence of the secondary end point of a composite of cardiovascular and renal events was also significantly lower. There was no difference individually in the rates of myocardial infarction, stroke, transient ischemic attack, admission for congestive heart failure, or death.
DISCUSSION: THE DILEMMA OF TREATING AN INDIVIDUAL PATIENT
These data illustrate the dilemma of treating an individual patient whose blood pressure is not at the currently accepted goal while on multiple antihypertensive medications. According to guidelines, therapy should be intensified in this situation. Observational data show a strong graded relationship between blood pressure and cardiovascular events and death, starting with a blood pressure of 115/75 mm Hg. The observational data relating blood pressure to kidney disease are similar. These data support the guideline recommendations that additional medications should be added to reach the promulgated target. Unfortunately, the targeting trials do not define a target, nor do they support the concept that lower is better.
Possible explanations for the negative results
Why does targeting a lower blood pressure not produce the benefit that the observational data lead us to expect?
One possibility is that blood pressure is merely a marker of cardiovascular risk, not a cause of it. This is unlikely, given the temporal relationship, reproducibility, and biologic plausibility that is supported by a very large body of experimental data. However, blood pressure is only one of multiple factors involved in the pathogenesis of vascular and renal disease, and perhaps better attention to other factors such as lipids and smoking may have made the targeting trials underpowered.
Another possibility is that these trials had such strict inclusion and exclusion criteria that they do not represent the general hypertensive population, reducing their external validity.49 However, the trials generally enrolled populations at higher risk, in which end points were more likely to occur. This would have enhanced the chance to show a positive effect rather than mask it.
It is possible that antihypertensive medications themselves have unwanted side effects that offset their potential benefit. Medication-related side effects could directly contribute to vascular disease despite their beneficial effect of lowering pressure. There could also be reduced tissue perfusion due to lower blood pressure per se in the face of a diseased vasculature, with the lower pressure directly contributing to organ dysfunction.
Finally, these trials measured brachial pressures to monitor blood pressure. Brachial pressure does not always correlate with central aortic pressure, which is probably a better marker of the overall pressure burden.50 It is possible that in these targeting trials, the peripheral blood pressure did not reflect the true central blood pressure and, therefore, significant separation of blood pressures may not have actually occurred.
Targeted vs achieved blood pressures: Analogies with other markers
This contradiction is not an exceptional circumstance in medicine.
For example, in chronic kidney disease, a graded observational relationship exists between decreasing levels of hemoglobin and various adverse outcomes.51–53 However, targeting a more normal level of hemoglobin compared with a lower one has been shown to be detrimental.54–57 This implies either that anemia is merely a marker of higher risk or, more likely, that the actual measures used to raise the hemoglobin to higher levels are the culprit. Notably, although targeting a higher hemoglobin concentration vs a lower one was detrimental, achieving a higher hemoglobin was beneficial within each targeted group.54,58
Another example of harm caused by targeting goals based on observational data is tight glucose control, both acutely in the critically ill59 and chronically in patients with type 2 diabetes.60 In both cases higher mortality rates ensued.
The same concept may apply to lowering blood pressure. While achieving a lower blood pressure may be more beneficial, targeting a specific goal may be harmful. Given that perhaps 20% of those labeled as hypertensive have resistant hypertension,61 millions of patients are susceptible to potential harm from targeting a specific goal based solely on observational data. If lower is always better, the randomized trials outlined above should have had more positive outcomes.
It becomes problematic to assign a specific goal for all patients or even groups of patients. The targeting trials do not provide the answer. Based on the observational data it would be optimal to have a blood pressure less than 120/80 mm Hg. This is an observation, not a recommendation. Patients should be assessed on an individual basis, taking into consideration their starting blood pressure, age, medication burden (antihypertensive and otherwise), comorbidities, and ability to comply with a regimen. Given the available data, it is hard to be more specific. In the future it may be possible to identify specific blood pressure targets based on the patient’s genetic makeup, but today that is not possible. Even patients with lower initial blood pressure may benefit from therapy,62,63 and some experts have advocated blood-pressure-lowering in all, irrespective of the baseline value.14
Avoid misclassification
The first step in treating hypertension should be to avoid misclassification. Make sure the clinic blood pressure is measured correctly, using an appropriately sized cuff, positioning the patient properly, and following all the other recommendations.64
However, the clinic blood pressure may not reflect true blood pressure load in up to one-third of all patients.65 We recommend 24-hour ambulatory blood pressure monitoring66 or home self-measurement, or both,67 to better assess true blood pressure burden in several circumstances, including in patients with resistant hypertension (any patient who has not achieved acceptable clinic blood pressure on three or more antihypertensive medications including a diuretic or who requires four or more medications for adequate control), suspicion of white-coat hypertension (or effect), and any patient who has achieved acceptable clinic blood pressure but either has symptoms of hypotension or progressive end-organ damage.
Currently, we base therapy on out-of-office blood pressure (self-measured or by ambulatory monitoring) whenever there is a discrepancy with clinic blood pressure.
Whether therapy should be altered by other less traditional measures of blood pressure such as assessment of central aortic pressure by radial applanation tonometry,68,69 or 24-hour ambulatory monitoring to assess nighttime blood pressures (specifically, “dipping”),70 morning surge,71 or blood pressure variability72,73 remains unclear and in need of randomized controlled trials.
In any patient requiring blood-pressure-lowering, we recommend lifestyle modifications.1,2 These include exercise, weight loss, salt and alcohol restriction, evaluation for sleep apnea, and avoidance of medications known to elevate blood pressure such as nonsteroidal anti-inflammatory drugs and sympathomimetic decongestants.
Much needs to be learned
For the individual patient with unacceptably high blood pressure who is already taking multiple antihypertensive medications of different classes, it is unclear what to do. This type of patient with resistant hypertension would be an excellent candidate for a future targeting trial. Other cardiovascular risk factors should be appropriately addressed, including obesity, lipids, smoking, and poor glycemic control.74 Each patient should be individually assessed with consideration of both global cardiovascular risk and quality-of-life issues.
Much still needs to be learned about the treatment of hypertension. The facts demonstrate that blood pressure is a strong modifiable risk factor of cardiovascular morbidity and mortality. Lowering it clearly produces benefits. It is unclear what treatment goals should be promulgated by official guidelines for large groups of patients. The resistant case remains a therapeutic dilemma with the potential for harm from overly aggressive treatment. The truly optimal level for an individual patient remains difficult to define. We anxiously await results of ongoing and future targeting trials.
CASE REVISITED
Regarding the initial case vignette, the patient is clearly not at her recommended goal blood pressure, especially given her high-risk status (diabetes mellitus and chronic kidney disease). Observational data support intensification of therapy, whereas targeting trials are essentially negative and indicate the potential for harm with overly aggressive treatment. Thus, we remain uncertain about what is correct or incorrect in terms of a targeted blood pressure, especially when applied to the individual patient.
Our approach would be to emphasize lifestyle modifications, to ensure accurate determination of her true blood pressure load (self-measurement at home or ambulatory blood pressure monitoring), to consider secondary causes of hypertension, and to educate the patient about the benefits and consequences of intensifying therapy with the aim of involving her in the decision.
- Chobanian AV, Bakris GL, Black HR, et al; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289:2560–2572.
- Mancia G, Laurent S, Agabiti-Rosei E, et al; European Society of Hypertension. Reappraisal of European guidelines on hypertension management: a European Society of Hypertension Task Force document. J Hypertens 2009; 27:2121–2158.
- MacMahon S, Peto R, Cutler J, et al. Blood pressure, stroke, and coronary heart disease. Part 1, prolonged differences in blood pressure: prospective observational studies corrected for the regression dilution bias. Lancet 1990; 335:765–774.
- Lewington S, Clarke R, Qizilbash N, Peto R, Collins R; Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002; 360:1903–1913.
- Lawes CM, Rodgers A, Bennett DA, et al; Asia Pacific Cohort Studies Collaboration. Blood pressure and cardiovascular disease in the Asia Pacific region. J Hypertens 2003; 21:707–716.
- Staessen JA, Gasowski J, Wang JG, et al. Risks of untreated and treated isolated systolic hypertension in the elderly: meta-analysis of outcome trials. Lancet 2000; 355:865–872.
- Messerli FH, Mancia G, Conti CR, et al. Dogma disputed: can aggressively lowering blood pressure in hypertensive patients with coronary artery disease be dangerous? Ann Intern Med 2006; 144:884–893.
- Klag MJ, Whelton PK, Randall BL, et al. Blood pressure and end-stage renal disease in men. N Engl J Med 1996; 334:13–18.
- Tozawa M, Iseki K, Iseki C, Kinjo K, Ikemiya Y, Takishita S. Blood pressure predicts risk of developing end-stage renal disease in men and women. Hypertension 2003; 41:1341–1345.
- Jafar TH, Stark PC, Schmid CH, et al; AIPRD Study Group. Progression of chronic kidney disease: the role of blood pressure control, proteinuria, and angiotensin-converting enzyme inhibition: a patient-level meta-analysis. Ann Intern Med 2003; 139:244–252.
- Peterson JC, Adler S, Burkart JM, et al. Blood pressure control, proteinuria, and the progression of renal disease. The Modification of Diet in Renal Disease Study. Ann Intern Med 1995; 123:754–762.
- Pohl MA, Blumenthal S, Cordonnier DJ, et al. Independent and additive impact of blood pressure control and angiotensin II receptor blockade on renal outcomes in the irbesartan diabetic nephropathy trial: clinical implications and limitations. J Am Soc Nephrol 2005; 16:3027–3037.
- Psaty BM, Smith NL, Siscovick DS, et al. Health outcomes associated with antihypertensive therapies used as first-line agents. A systematic review and meta-analysis. JAMA 1997; 277:739–745.
- Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ 2009; 338:b1665.
- Turnbull F; Blood Pressure Lowering Treatment Trialists’ Collaboration. Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trials. Lancet 2003; 362:1527–1535.
- Blood Pressure Lowering Treatment Trialists’ Collaboration; Turnbull F, Neal B, Ninomiya T, et al. Effects of different regimens to lower blood pressure on major cardiovascular events in older and younger adults: meta-analysis of randomised trials. BMJ 2008; 336:1121–1123.
- Staessen JA, Wang JG, Thijs L. Cardiovascular prevention and blood pressure reduction: a quantitative overview updated until 1 March 2003. J Hypertens 2003; 21:1055–1076.
- Psaty BM, Lumley T, Furberg CD, et al. Health outcomes associated with various antihypertensive therapies used as first-line agents: a network meta-analysis. JAMA 2003; 289:2534–2544.
- Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society.—Summary Article. Circulation 2005; 112:e154–e235.
- Brenner BM, Cooper ME, de Zeeuw D, et al; RENAAL Study Investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345:861–869.
- Lewis EJ, Hunsicker LG, Clarke WR, et al; Collaborative Study Group. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 2001; 345:851–860.
- Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996; 17:1–12.
- Wright JT, Bakris G, Greene T, et al; African American Study of Kidney Disease and Hypertension Study Group. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA 2002; 288:2421–2431.
- Freedman BI, Hicks PJ, Bostrom MA, et al. Polymorphisms in the non-muscle myosin heavy chain 9 gene (MYH9) are strongly associated with end-stage renal disease historically attributed to hypertension in African Americans. Kidney Int 2009; 75:736–745.
- Kopp JB, Smith MW, Nelson GW, et al. MYH9 is a major-effect risk gene for focal segmental glomerulosclerosis. Nat Genet 2008; 40:1175–1184.
- Freedman BI, Kopp JB, Langefeld CD, et al. The apolipoprotein L1 (APOL1) gene and nondiabetic nephropathy in African Americans. J Am Soc Nephrol 2010; 21:1422–1426.
- Rostand SG, Brown G, Kirk KA, Rutsky EA, Dustan HP. Renal insufficiency in treated essential hypertension. N Engl J Med 1989; 320:684–688.
- Walker WG, Neaton JD, Cutler JA, Neuwirth R, Cohen JD. Renal function change in hypertensive members of the Multiple Risk Factor Intervention Trial. Racial and treatment effects. The MRFIT Research Group. JAMA 1992; 268:3085–3091.
- Pogue V, Rahman M, Lipkowitz M, et al; African American Study of Kidney Disease and Hypertension Collaborative Research Group. Disparate estimates of hypertension control from ambulatory and clinic blood pressure measurements in hypertensive kidney disease. Hypertension 2009; 53:20–27.
- Norris K, Bourgoigne J, Gassman J, et al; AASK Study Group. Cardiovascular outcomes in the African American Study of Kidney Disease and Hypertension (AASK) Trial. Am J Kidney Dis 2006; 48:739–751.
- Toto RD, Mitchell HC, Smith RD, Lee HC, McIntire D, Pettinger WA. “Strict” blood pressure control and progression of renal disease in hypertensive nephrosclerosis. Kidney Int 1995; 48:851–859.
- Lewis JB, Berl T, Bain RP, Rohde RD, Lewis EJ. Effect of intensive blood pressure control on the course of type 1 diabetic nephropathy. Collaborative Study Group. Am J Kidney Dis 1999; 34:809–817.
- Schrier RW, Estacio RO, Esler A, Mehler P. Effects of aggressive blood pressure control in normotensive type 2 diabetic patients on albuminuria, retinopathy and strokes. Kidney Int 2002; 61:1086–1097.
- Estacio RO, Jeffers BW, Gifford N, Schrier RW. Effect of blood pressure control on diabetic microvascular complications in patients with hypertension and type 2 diabetes. Diabetes Care 2000; 23( suppl 2):B54–B64.
- Estacio RO, Coll JR, Tran ZV, Schrier RW. Effect of intensive blood pressure control with valsartan on urinary albumin excretion in normotensive patients with type 2 diabetes. Am J Hypertens 2006; 19:1241–1248.
- Schrier R, McFann K, Johnson A, et al. Cardiac and renal effects of standard versus rigorous blood pressure control in autosomal-dominant polycystic kidney disease: results of a seven-year prospective randomized study. J Am Soc Nephrol 2002; 13:1733–1739.
- Klahr S, Levey AS, Beck GJ, et al. The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. Modification of Diet in Renal Disease Study Group. N Engl J Med 1994; 330:877–884.
- Sarnak MJ, Greene T, Wang X, et al. The effect of a lower target blood pressure on the progression of kidney disease: long-term follow-up of the modification of diet in renal disease study. Ann Intern Med 2005; 142:342–351.
- Ruggenenti P, Perna A, Loriga G, et al; REIN-2 Study Group. Blood-pressure control for renoprotection in patients with non-diabetic chronic renal disease (REIN-2): multicentre, randomised controlled trial. Lancet 2005; 365:939–946.
- Appel LJ, Wright JT, Greene T, et al; AASK Collaborative Research Group. Intensive blood-pressure control in hypertensive chronic kidney disease. N Engl J Med 2010; 363:918–929.
- Hansson L, Zanchetti A, Carruthers SG, et al. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet 1998; 351:1755–1762.
- Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ 1998; 317:703–713.
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359:1577–1589.
- ACCORD Study Group, Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010; 362:1575–1585.
- JATOS Study Group. Principal results of the Japanese trial to assess optimal systolic blood pressure in elderly hypertensive patients (JATOS). Hypertens Res 2008; 31:2115–2127.
- Verdecchia P, Staessen JA, Angeli F, et al; Cardio-Sis investigators. Usual versus tight control of systolic blood pressure in non-diabetic patients with hypertension (Cardio-Sis): an open-label randomised trial. Lancet 2009; 374:525–533.
- Solomon SD, Verma A, Desai A, et al; Exforge Intensive Control of Hypertension to Evaluate Efficacy in Diastolic Dysfunction Investigators. Effect of intensive versus standard blood pressure lowering on diastolic function in patients with uncontrolled hypertension and diastolic dysfunction. Hypertension 2010; 55:241–248.
- Ichihara A, Hayashi M, Koura Y, Tada Y, Hirota N, Saruta T. Long-term effects of intensive blood-pressure lowering on arterial wall stiffness in hypertensive patients. Am J Hypertens 2003; 16:959–965.
- Rothwell PM. External validity of randomised controlled trials: “to whom do the results of this trial apply?” Lancet 2005; 365:82–93.
- Townsend RR, Roman MJ, Najjar SS, Cockcroft JR, Feig PU, Stockbridge NL. Central blood pressure measurements-an opportunity for efficacy and safety in drug development? J Am Soc Hypertens 2010; 4:211–214.
- Xia H, Ebben J, Ma JZ, Collins AJ. Hematocrit levels and hospitalization risks in hemodialysis patients. J Am Soc Nephrol 1999; 10:1309–1316.
- Ma JZ, Ebben J, Xia H, Collins AJ. Hematocrit level and associated mortality in hemodialysis patients. J Am Soc Nephrol 1999; 10:610–619.
- Ofsthun N, Labrecque J, Lacson E, Keen M, Lazarus JM. The effects of higher hemoglobin levels on mortality and hospitalization in hemodialysis patients. Kidney Int 2003; 63:1908–1914.
- Besarab A, Bolton WK, Browne JK, et al. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med 1998; 339:584–590.
- Drüeke TB, Locatelli F, Clyne N, et al; CREATE Investigators. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med 2006; 355:2071–2084.
- Singh AK, Szczech L, Tang KL, et al; CHOIR Investigators. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med 2006; 355:2085–2098.
- Pfeffer MA, Burdmann EA, Chen CY, et al; TREAT Investigators. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med 2009; 361:2019–2032.
- Szczech LA, Barnhart HX, Inrig JK, et al. Secondary analysis of the CHOIR trial epoetin-alpha dose and achieved hemoglobin outcomes. Kidney Int 2008; 74:791–798.
- Finfer S, Chittock DR, Su SY, et al; NICE-SUGAR Study Investigators Intensive versus conventional glucose control in critically ill patients. N Engl J Med 2009; 360:1283–1297.
- Action to Control Cardiovascular Risk in Diabetes Study Group; Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358:2545–2559.
- Calhoun DA, Jones D, Textor S, et al. Resistant hypertension: diagnosis, evaluation, and treatment. A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension 2008; 51:1403–1419.
- Nissen SE, Tuzcu EM, Libby P, et al; CAMELOT Investigators. Effect of antihypertensive agents on cardiovascular events in patients with coronary disease and normal blood pressure: the CAMELOT study, a randomized controlled trial. JAMA 2004; 292:2217–2225.
- Patel A; ADVANCE Collaborative Group; MacMahon S, Chalmers J, Neal B. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet 2007; 370:829–840.
- Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation 2005; 111:697–716.
- Fagard RH, Cornelissen VA. Incidence of cardiovascular events in white-coat, masked and sustained hypertension versus true normotension: a meta-analysis. J Hypertens 2007; 25:2193–2198.
- Pickering TG, White WB, Giles TD, et al. When and how to use self (home) and ambulatory blood pressure monitoring. J Am Soc Hypertens 2010; 4:56–61.
- Hänninen MR, Niiranen TJ, Puukka PJ, Jula AM. Comparison of home and ambulatory blood pressure measurement in the diagnosis of masked hypertension. J Hypertens 2010; 28:709–714.
- Roman MJ, Devereux RB, Kizer JR, et al. Central pressure more strongly relates to vascular disease and outcome than does brachial pressure: the Strong Heart Study. Hypertension 2007; 50:197–203.
- Williams B, Lacy PS, Thom SM, et al; CAFE Investigators; Anglo-Scandinavian Cardiac Outcomes Trial Investigators; CAFE Steering Committee and Writing Committee. Differential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes: principal results of the Conduit Artery Function Evaluation (CAFE) study. Circulation 2006; 113:1213–1225.
- Ben-Dov IZ, Kark JD, Ben-Ishay D, Mekler J, Ben-Arie L, Bursztyn M. Predictors of all-cause mortality in clinical ambulatory monitoring: unique aspects of blood pressure during sleep. Hypertension 2007; 49:1235–1241.
- Li Y, Thijs L, Hansen TW, et al; International Database on Ambulatory Blood Pressure Monitoring in Relation to Cardiovascular Outcomes Investigators. Prognostic value of the morning blood pressure surge in 5645 subjects from 8 populations. Hypertension 2010; 55:1040–1048.
- Rothwell PM. Limitations of the usual blood-pressure hypothesis and importance of variability, instability, and episodic hypertension. Lancet 2010; 375:938–948.
- Hansen TW, Thijs L, Li Y, et al; International Database on Ambulatory Blood Pressure in Relation to Cardiovascular Outcomes Investigators. Prognostic value of reading-to-reading blood pressure variability over 24 hours in 8938 subjects from 11 populations. Hypertension 2010; 55:1049–1057.
- Jackson R, Lawes CM, Bennett DA, Milne RJ, Rodgers A. Treatment with drugs to lower blood pressure and blood cholesterol based on an individual’s absolute cardiovascular risk. Lancet 2005; 365:434–441.
A 50-year-old African American woman with type 2 diabetes mellitus, hypertension, hyperlipidemia, and chronic kidney disease presents for a follow-up visit. The patient had been treated with hydrochlorothiazide 25 mg/day and enalapril (Vasotec) 20 mg twice daily until 6 weeks ago. At that time her blood pressure was 160/85 mm Hg, and amlodipine (Norvasc) 10 mg/day was added to her regimen. Her other medications include glipizide (Glucotrol), metformin (Glucophage), lovastatin (Mevacor), fish oils, aspirin, calcium, and vitamin D. Her current blood pressure is 145/80 mm Hg; her serum creatinine level is 1.5 mg/dL, and her urine albumin-to-creatinine ratio is 180 mg/g.
In hypertensive patients who have diabetes or chronic kidney disease, guidelines1 call for intensification of antihypertensive therapy to reach a goal blood pressure of less than 130/80 mm Hg. What data exist to support these guidelines? And what should the clinician do?
IS MORE-INTENSE THERAPY IN THE PATIENT’S BEST INTEREST?
Often, clinicians are faced with hypertensive patients whose blood pressure, despite treatment, is higher than the accepted goal. Often, these patients are elderly and are already taking multiple medications that are costly and have significant potential adverse effects. The dilemma is whether to try to reach a target blood pressure listed in a guideline (by increasing the dosage of the current drugs or by adding a drug of a different class) or to “do no harm,” accept the patient’s blood pressure, and keep the regimen the same.1,2
The current goal blood pressure is less than 140/90 mm Hg for all but the very elderly, with more intense control recommended for patients at high risk, ie, those with diabetes mellitus, chronic kidney disease, or atherosclerotic cardiovascular disease.1
While it appears to be in the patient’s best interests to follow such guidelines, review of available data indicates that this it not necessarily so, and may even be harmful.
OBSERVATIONAL DATA AND EARLY RANDOMIZED TRIALS
Many observational studies have found that the higher one’s blood pressure, the greater one’s risk of cardiovascular events and death. Indeed, meta-analyses of these trials, which involved more than 1.5 million people, demonstrate a strong, positive, log-linear relationship between blood pressure and the incidence of cardiovascular disease and death.3–5
Further, there is no evidence of a threshold pressure below which the risk is not lower (ie, a “J-point”), starting with 115/75 mm Hg. A J-point may exist for diastolic blood pressure in elderly patients with isolated systolic hypertension6 and in patients with coronary artery disease.7 Otherwise, the observation is clear: the lower the blood pressure the better. For every 20 mm Hg lower systolic blood pressure or 10 mm Hg lower diastolic blood pressure, the risk of a cardiovascular event is about 50% less.4,5
Observational analyses also show a strong, graded relationship between blood pressure and future end-stage renal disease.8,9 Post hoc analyses indicate that chronic kidney disease progresses more slowly with lower achieved blood pressures, especially in those with higher degrees of proteinuria.10–12
However, observational data do not prove cause and effect, nor do they guarantee similar results with treatment. This requires randomized controlled trials.
RANDOMIZED TRIALS OF HYPERTENSION TREATMENT
Initial trials were aimed at determining whether hypertension should even be treated. A 1997 meta-analysis of 18 such trials comparing either low-dose diuretic therapy, high-dose diuretic therapy, or beta-blocker therapy with placebo involved 48,000 patients who were followed for an average of 5 years.13 The rates of stroke and congestive heart failure were consistently reduced, although only low-dose diuretic therapy reduced the risk of coronary heart disease and death from any cause.
More recent trials enrolled people not considered hypertensive who were randomized to receive either active drugs or placebo, or no treatment. Other trials attempted to assess non-pressure-related effects of specific agents, using other antihypertensive agents in the control group. Still other randomized controlled trials compared one agent or agents with other agents while attempting to attain equivalent blood pressure between groups. Frequently, however, there was some blood pressure difference.
Meta-analyses of most of these trials conclude that the major benefit of antihypertensive therapy—reducing rates of cardiovascular morbidity and mortality—comes from a lower attained blood pressure, irrespective of which agent is used.14–18 Exceptions exist, however. For example, specific drug classes are indicated after myocardial infarction, and in congestive heart failure and proteinuric chronic kidney disease.10,19–21
16 TRIALS OF DIFFERENT BLOOD PRESSURE TARGETS
The overriding theme of these observational data is that a lower blood pressure, whether attained naturally or with treatment, is better than a higher one from both the cardiovascular and the renal perspective.
What remains unclear is what blood pressure should be aimed for in a particular patient or group of patients. Is it a specific pressure (eg, 140/90 mm Hg), or does the change from baseline count more? Should other factors such as age or comorbidity alter this number?
Several randomized controlled trials have addressed these questions by targeting different levels of blood pressure. We are aware of at least 16 such trials in adults, including 13 with renal or cardiovascular primary end points and three with surrogate primary end points.
An unavoidable design flaw of all of these trials is their unblinded nature. Consequently, nearly all of them carry a Jadad score (a measure of quality, based on randomization and blinding)22 of 3 on a scale of 5.
NINE TRIALS WITH RENAL PRIMARY END POINTS
African American Study of Kidney Disease and Hypertension (AASK)23
Patients: 1,094 African Americans with presumed hypertensive renal disease and a measured glomerular filtration rate between 20 and 65 mL/min/1.73 m2.
Randomized blood pressure goals. Mean arterial pressure 92 mm Hg or less vs 102 to 107 mm Hg.
Results. At 4 years, the two groups had average blood pressures of 128/78 and 141/85 mm Hg, respectively. The groups did not differ in the rates of the primary end points—ie, the rate of change in the measured glomerular filtration rate over time or the composite of a 50% reduction in glomerular filtration rate, the onset of end-stage renal disease, or death.
Comments. Several issues have been raised about the internal validity of this trial.
So-called hypertensive kidney disease in African Americans (as opposed to European Americans) may be a genetic disorder related to polymorphisms of one or more genes on chromosome 22q. Initial data implicated the MYH9 gene, which encodes non-muscle myosin heavy chain II.24,25 More recent data implicate the nearby APOL1 gene encoding apolipoprotein L126 as more relevant. These polymorphisms have a much greater prevalence in African Americans and appear responsible for the higher risk of idiopathic focal segmental glomerulosclerosis and HIV-associated nephropathy in this population.24–26 Therefore, in African Americans, hypertension may in fact be the result of the kidney disease and not its primary cause, which may explain why in this and other African American populations stricter control of blood pressure did not produce a renal benefit.27,28
Also, there is the possibility of misclassification bias. A secondary analysis of data obtained by ambulatory monitoring showed that of the 377 participants whose blood pressure appeared to be under control when measured in the clinic, 70% actually had masked hypertension, ie, uncontrolled hypertension outside the clinic.29 The real difference in blood pressure between groups may well have been different than that determined in the clinic.
In addition, a prespecified secondary analysis showed no difference in the rates of cardiovascular events and death between the groups.30 However, the study was not designed to have the statistical power to detect a difference in cardiovascular events. Moreover fewer cardiovascular events occurred than expected, further reducing the study’s power to detect a difference.
Toto et al31
Toto et al reported similar results in an earlier trial in 87 hypertensive patients (77 randomized), predominantly African American, and similar concerns apply.
Lewis et al32
Patients: 129 patients with type 1 diabetes.
Randomized blood pressure goals. A mean arterial pressure of either no higher than 92 mm Hg or 100 to 107 mm Hg.
Results. At 2 years, despite a difference of 6 mm Hg in mean arterial pressure, the glomerular filtration rate (measured) had declined by the same amount in the two groups. The study was underpowered for this end point. Patients in the group with the lower goal pressure were excreting significantly less protein than those in the other group, but they were received higher doses of an angiotensin-converting enzyme (ACE) inhibitor—in this case, ramipril (Altace).
The Appropriate Blood Pressure Control in Diabetes (ABCD) trials33–35
Patients: 950 patients with type 2 diabetes mellitus and either normal or high blood pressure.
Randomized blood pressure goals. Either intensive or moderate therapy (see Table 1).
Results. At 5 years, creatinine clearance (estimated) had declined by the same amount in the two groups. However, fewer of the hypertensive patients had died in the intensive-therapy group.34 Similarly, normotensive patients had less progression of albuminuria if treated intensively.33
In the ABCD Part 2 with Valsartan (ABCD-2V) trial in normotensive patients,35 therapy with valsartan (Diovan) did not affect creatinine clearance but did reduce albuminuria. However, 75% of the patients in the moderate-treatment group were untreated.
Schrier et al36
Patients. 75 hypertensive patients with autosomal-dominant polycystic kidney disease and left ventricular hypertrophy.
Randomized blood pressure targets. Less than 120/80 mm Hg vs 135/85 to 140/90 mm Hg.
Results. After 7 years, despite a difference in average mean arterial pressure of 11 mm Hg between the groups (90 vs 101 mm Hg), there was no difference in the rate of decline of creatinine clearance. The left ventricular mass index decreased by 21% in the lower-target group and by 35% in the higher-target group (P < .01).
Modification of Diet in Renal Disease (MDRD) trial37,38
Patients: 840 patients whose measured glomerular filtration rate was between 13 and 55 mL/min/1.73 m2.
Randomized blood pressure targets. A target mean arterial pressure of less than 92 mm Hg vs less than 107 mm Hg.11,37
Results. After 2.2 years, the mean difference in mean arterial pressure was 4.7 mm Hg. There was, however, no difference in the rate of decline in the glomerular filtration rate.
In a 6-year follow-up, significantly fewer patients in the lower-blood-pressure group reached the end point of end-stage renal disease or the combined end point of end-stage renal disease or death.38 The rate of death, however, was nearly twice as high in the lower-blood-pressure group (10% vs 6%). The blood pressure and treatment during follow-up were not reported.
Comments. Internal validity is an issue, since the blood pressure and therapy during follow-up were unknown, and more patients received ACE inhibitors in the lower-blood-pressure group during the trial. Further, the higher death rate in the lower-blood-pressure group is worrisome.
The Ramipril Efficacy in Nephropathy (REIN)-2 trial39
Patients: 338 nondiabetic patients who had proteinuria and reduced creatinine clearance.
Treatment and blood pressure goals. All were treated with ramipril and randomized to intensive (< 130/80 mm Hg) vs standard control (diastolic blood pressure < 90 mm Hg) with therapy based on felodipine (Plendil).
Results. The study was terminated early because of futility. Despite a mean difference of 4.1 mm Hg systolic and 2.8 mm Hg diastolic, the groups did not differ in the rate of progression to end-stage renal disease (23% with intensive therapy vs 20% with standard therapy) or in the rate of decline of the measured glomerular filtration rate (0.22 vs 0.24 mL/min/1.73 m2/month).
Comment. The internal validity of this study can be questioned because of the low separation of achieved blood pressure and because of its early termination.
No benefit from a lower blood pressure goal in preserving kidney function
To summarize, these trials all showed no significant benefit from either targeting or achieving lower blood pressure in terms of slowing the decline of kidney function. Overall, they do not define a target and offer little support that a lower goal blood pressure is indicated with respect to the rate of loss of glomerular filtration rate in chronic kidney disease.
However, post hoc analysis of the MDRD trial indicates a statistical interaction between targeted blood pressure and degree of baseline proteinuria. At higher levels of proteinuria (≥ 1 g/day), the group with the lower blood pressure target had better outcomes.
In addition, long-term follow-up (mean of 12.2 years) of the AASK trial, including a 7-year cohort phase with nearly similar blood pressures in both groups, also indicated an interaction with targeted blood pressure and baseline proteinuria.40 Although the overall analysis was negative, there was a significant reduction in the primary end point in the group originally assigned the low target when analysis was restricted to those in the highest tertile of proteinuria. These and other data10 suggest that patients with chronic kidney disease and proteinuria may represent a distinct subset of chronic kidney disease patients who benefit from more intensive blood-pressure-lowering. However, patients in the REIN-2 trial34 and the macroalbuminuric patients in the ABCD hypertensive trial35 did not benefit from a lower targeted blood pressure despite significant proteinuria.
FOUR TRIALS WITH CARDIOVASCULAR END POINTS
The Hypertension Optimal Treatment (HOT) trial41
Patients: 18,790 patients with diastolic blood pressure between 100 and 115 mm Hg.
Randomized blood pressure goals. Diastolic pressure of equal to or less than 80, 85, or 90 mm Hg.
Results. At an average of 3.8 years, the average blood pressures in the three groups were approximately 140/81, 141/83, and 144/85 mm Hg, respectively. There was no difference between the groups in the rate of the composite primary end point of all myocardial infarctions, all strokes, and cardiovascular death. Any conclusions from this trial were compromised by the small difference in achieved blood pressures between groups.
In the 1,501 patients with diabetes, the incidence of the primary end point was 50% lower with a goal of 80 mm Hg or less than with a goal of 90 mm Hg or less.
The UK Prospective Diabetes Study (UKPDS)42,43
Patients: 1,148 hypertensive patients with type 2 diabetes mellitus.
Randomized blood pressure goals. Either “tight control” (aiming for < 150/85 mm Hg) or “less tight control” (aiming for < 180/105 mm Hg).
Results. At a median follow-up of 8.4 years, the attained blood pressures were 144/82 vs 154/87 mm Hg. The difference produced significant benefits, including a 24% lower rate of any diabetes-related end point, a 32% lower rate of death due to diabetes, and a nonsignificant 18% lower rate of total mortality—all co-primary end points.
The less-tight-control group had many patients with initial blood pressures below 180/105 mm Hg; hence, over 50% of patients received no antihypertensive therapy at the start of the trial. By the end of the trial 9 years later, 20% had still not been treated. This compares with only 5% of patients in the tight-control group who were not treated with antihypertensives throughout the trial. Therefore, this trial serves as better evidence for treating vs not treating, rather than defining a specific goal.
During a 10-year follow-up, blood pressure differences disappeared within 2 years.43 There was no legacy effect, as the significant differences noted during the trial were no longer present 10 years later.
Action to Control Cardiovascular Risk in Diabetes (ACCORD)44
Patients: 4,733 patients with type 2 diabetes.
Randomized blood pressure goals. Systolic blood pressure lower than either 120 or 140 mm Hg.
Results. At 4.7 years, despite a significant difference in mean systolic blood pressure of 14.2 mm Hg after the first year (119.3 vs 133.5 mm Hg), there was no difference in the primary end point of nonfatal myocardial infarction, nonfatal stroke, or cardiovascular death. There were fewer strokes in the lower-pressure group but no difference in myocardial infarctions, which were five times more common than strokes. Serious adverse events attributed to antihypertensive treatment occurred more frequently in the intensive-therapy group (3.3% vs 1.3%, P < .001).
Comment. There were fewer events than expected, possibly limiting the trial’s ability to detect a statistical difference. Compared with both the UKPDS and the diabetic population of HOT, ACCORD is much larger and more internally valid (unlike in UKPDS, nearly all patients in both groups were treated, and compared with HOT there was much greater separation of achieved pressure). It is more recent and better reflects current overall practice. It indicates that when specifically aiming for a target blood pressure, lower is not always better and comes at a price (more severe adverse events).
Japanese Trial to Assess Optimal Systolic Blood Pressure in Elderly Hypertensive Patients (JATOS)45
Patients: 4,418 patients, age 65 to 85 years, with a pretreatment systolic blood pressure above 160 mm Hg.
Randomized blood pressure goals. Systolic pressure either lower than 140 mm Hg or 140 to 160 mm Hg.
Results. At 2 years, despite a difference of 9.7/3.3 mm Hg, there was no difference in the primary end point (the combined incidence of cerebrovascular disease, cardiac and vascular disease, and renal failure). Fifty-four patients had died in the strict-treatment group and 42 in the mild-treatment group; the difference was not statistically significant.
Three other trials
Three other trials46–48 had surrogate end points, but only one of them reported a composite cardiovascular secondary end point.46 We will not discuss the other two.47,48
Cardio-Sis. In the Studio Italiano Sugli Effetti Cardiovascolari del Controllo della Pressione Arteriosa Sistolica (Cardio-Sis) trial,46 1,111 people without diabetes with systolic pressure higher than 150 mm Hg were randomized to tight control (systolic pressure < 130 mm Hg) vs usual control (systolic pressure < 140 mm Hg) and followed for 2 years with electrocardiography to detect left ventricular hypertrophy.
At a median of 2 years, the systolic blood pressure had declined by an average of 3.8 mm Hg more in the tight-control group than in the usual-control group, and the diastolic pressure by an average of 1.5 mm Hg. There was significantly less left ventricular hypertrophy in the tight-control group. The incidence of the secondary end point of a composite of cardiovascular and renal events was also significantly lower. There was no difference individually in the rates of myocardial infarction, stroke, transient ischemic attack, admission for congestive heart failure, or death.
DISCUSSION: THE DILEMMA OF TREATING AN INDIVIDUAL PATIENT
These data illustrate the dilemma of treating an individual patient whose blood pressure is not at the currently accepted goal while on multiple antihypertensive medications. According to guidelines, therapy should be intensified in this situation. Observational data show a strong graded relationship between blood pressure and cardiovascular events and death, starting with a blood pressure of 115/75 mm Hg. The observational data relating blood pressure to kidney disease are similar. These data support the guideline recommendations that additional medications should be added to reach the promulgated target. Unfortunately, the targeting trials do not define a target, nor do they support the concept that lower is better.
Possible explanations for the negative results
Why does targeting a lower blood pressure not produce the benefit that the observational data lead us to expect?
One possibility is that blood pressure is merely a marker of cardiovascular risk, not a cause of it. This is unlikely, given the temporal relationship, reproducibility, and biologic plausibility that is supported by a very large body of experimental data. However, blood pressure is only one of multiple factors involved in the pathogenesis of vascular and renal disease, and perhaps better attention to other factors such as lipids and smoking may have made the targeting trials underpowered.
Another possibility is that these trials had such strict inclusion and exclusion criteria that they do not represent the general hypertensive population, reducing their external validity.49 However, the trials generally enrolled populations at higher risk, in which end points were more likely to occur. This would have enhanced the chance to show a positive effect rather than mask it.
It is possible that antihypertensive medications themselves have unwanted side effects that offset their potential benefit. Medication-related side effects could directly contribute to vascular disease despite their beneficial effect of lowering pressure. There could also be reduced tissue perfusion due to lower blood pressure per se in the face of a diseased vasculature, with the lower pressure directly contributing to organ dysfunction.
Finally, these trials measured brachial pressures to monitor blood pressure. Brachial pressure does not always correlate with central aortic pressure, which is probably a better marker of the overall pressure burden.50 It is possible that in these targeting trials, the peripheral blood pressure did not reflect the true central blood pressure and, therefore, significant separation of blood pressures may not have actually occurred.
Targeted vs achieved blood pressures: Analogies with other markers
This contradiction is not an exceptional circumstance in medicine.
For example, in chronic kidney disease, a graded observational relationship exists between decreasing levels of hemoglobin and various adverse outcomes.51–53 However, targeting a more normal level of hemoglobin compared with a lower one has been shown to be detrimental.54–57 This implies either that anemia is merely a marker of higher risk or, more likely, that the actual measures used to raise the hemoglobin to higher levels are the culprit. Notably, although targeting a higher hemoglobin concentration vs a lower one was detrimental, achieving a higher hemoglobin was beneficial within each targeted group.54,58
Another example of harm caused by targeting goals based on observational data is tight glucose control, both acutely in the critically ill59 and chronically in patients with type 2 diabetes.60 In both cases higher mortality rates ensued.
The same concept may apply to lowering blood pressure. While achieving a lower blood pressure may be more beneficial, targeting a specific goal may be harmful. Given that perhaps 20% of those labeled as hypertensive have resistant hypertension,61 millions of patients are susceptible to potential harm from targeting a specific goal based solely on observational data. If lower is always better, the randomized trials outlined above should have had more positive outcomes.
It becomes problematic to assign a specific goal for all patients or even groups of patients. The targeting trials do not provide the answer. Based on the observational data it would be optimal to have a blood pressure less than 120/80 mm Hg. This is an observation, not a recommendation. Patients should be assessed on an individual basis, taking into consideration their starting blood pressure, age, medication burden (antihypertensive and otherwise), comorbidities, and ability to comply with a regimen. Given the available data, it is hard to be more specific. In the future it may be possible to identify specific blood pressure targets based on the patient’s genetic makeup, but today that is not possible. Even patients with lower initial blood pressure may benefit from therapy,62,63 and some experts have advocated blood-pressure-lowering in all, irrespective of the baseline value.14
Avoid misclassification
The first step in treating hypertension should be to avoid misclassification. Make sure the clinic blood pressure is measured correctly, using an appropriately sized cuff, positioning the patient properly, and following all the other recommendations.64
However, the clinic blood pressure may not reflect true blood pressure load in up to one-third of all patients.65 We recommend 24-hour ambulatory blood pressure monitoring66 or home self-measurement, or both,67 to better assess true blood pressure burden in several circumstances, including in patients with resistant hypertension (any patient who has not achieved acceptable clinic blood pressure on three or more antihypertensive medications including a diuretic or who requires four or more medications for adequate control), suspicion of white-coat hypertension (or effect), and any patient who has achieved acceptable clinic blood pressure but either has symptoms of hypotension or progressive end-organ damage.
Currently, we base therapy on out-of-office blood pressure (self-measured or by ambulatory monitoring) whenever there is a discrepancy with clinic blood pressure.
Whether therapy should be altered by other less traditional measures of blood pressure such as assessment of central aortic pressure by radial applanation tonometry,68,69 or 24-hour ambulatory monitoring to assess nighttime blood pressures (specifically, “dipping”),70 morning surge,71 or blood pressure variability72,73 remains unclear and in need of randomized controlled trials.
In any patient requiring blood-pressure-lowering, we recommend lifestyle modifications.1,2 These include exercise, weight loss, salt and alcohol restriction, evaluation for sleep apnea, and avoidance of medications known to elevate blood pressure such as nonsteroidal anti-inflammatory drugs and sympathomimetic decongestants.
Much needs to be learned
For the individual patient with unacceptably high blood pressure who is already taking multiple antihypertensive medications of different classes, it is unclear what to do. This type of patient with resistant hypertension would be an excellent candidate for a future targeting trial. Other cardiovascular risk factors should be appropriately addressed, including obesity, lipids, smoking, and poor glycemic control.74 Each patient should be individually assessed with consideration of both global cardiovascular risk and quality-of-life issues.
Much still needs to be learned about the treatment of hypertension. The facts demonstrate that blood pressure is a strong modifiable risk factor of cardiovascular morbidity and mortality. Lowering it clearly produces benefits. It is unclear what treatment goals should be promulgated by official guidelines for large groups of patients. The resistant case remains a therapeutic dilemma with the potential for harm from overly aggressive treatment. The truly optimal level for an individual patient remains difficult to define. We anxiously await results of ongoing and future targeting trials.
CASE REVISITED
Regarding the initial case vignette, the patient is clearly not at her recommended goal blood pressure, especially given her high-risk status (diabetes mellitus and chronic kidney disease). Observational data support intensification of therapy, whereas targeting trials are essentially negative and indicate the potential for harm with overly aggressive treatment. Thus, we remain uncertain about what is correct or incorrect in terms of a targeted blood pressure, especially when applied to the individual patient.
Our approach would be to emphasize lifestyle modifications, to ensure accurate determination of her true blood pressure load (self-measurement at home or ambulatory blood pressure monitoring), to consider secondary causes of hypertension, and to educate the patient about the benefits and consequences of intensifying therapy with the aim of involving her in the decision.
A 50-year-old African American woman with type 2 diabetes mellitus, hypertension, hyperlipidemia, and chronic kidney disease presents for a follow-up visit. The patient had been treated with hydrochlorothiazide 25 mg/day and enalapril (Vasotec) 20 mg twice daily until 6 weeks ago. At that time her blood pressure was 160/85 mm Hg, and amlodipine (Norvasc) 10 mg/day was added to her regimen. Her other medications include glipizide (Glucotrol), metformin (Glucophage), lovastatin (Mevacor), fish oils, aspirin, calcium, and vitamin D. Her current blood pressure is 145/80 mm Hg; her serum creatinine level is 1.5 mg/dL, and her urine albumin-to-creatinine ratio is 180 mg/g.
In hypertensive patients who have diabetes or chronic kidney disease, guidelines1 call for intensification of antihypertensive therapy to reach a goal blood pressure of less than 130/80 mm Hg. What data exist to support these guidelines? And what should the clinician do?
IS MORE-INTENSE THERAPY IN THE PATIENT’S BEST INTEREST?
Often, clinicians are faced with hypertensive patients whose blood pressure, despite treatment, is higher than the accepted goal. Often, these patients are elderly and are already taking multiple medications that are costly and have significant potential adverse effects. The dilemma is whether to try to reach a target blood pressure listed in a guideline (by increasing the dosage of the current drugs or by adding a drug of a different class) or to “do no harm,” accept the patient’s blood pressure, and keep the regimen the same.1,2
The current goal blood pressure is less than 140/90 mm Hg for all but the very elderly, with more intense control recommended for patients at high risk, ie, those with diabetes mellitus, chronic kidney disease, or atherosclerotic cardiovascular disease.1
While it appears to be in the patient’s best interests to follow such guidelines, review of available data indicates that this it not necessarily so, and may even be harmful.
OBSERVATIONAL DATA AND EARLY RANDOMIZED TRIALS
Many observational studies have found that the higher one’s blood pressure, the greater one’s risk of cardiovascular events and death. Indeed, meta-analyses of these trials, which involved more than 1.5 million people, demonstrate a strong, positive, log-linear relationship between blood pressure and the incidence of cardiovascular disease and death.3–5
Further, there is no evidence of a threshold pressure below which the risk is not lower (ie, a “J-point”), starting with 115/75 mm Hg. A J-point may exist for diastolic blood pressure in elderly patients with isolated systolic hypertension6 and in patients with coronary artery disease.7 Otherwise, the observation is clear: the lower the blood pressure the better. For every 20 mm Hg lower systolic blood pressure or 10 mm Hg lower diastolic blood pressure, the risk of a cardiovascular event is about 50% less.4,5
Observational analyses also show a strong, graded relationship between blood pressure and future end-stage renal disease.8,9 Post hoc analyses indicate that chronic kidney disease progresses more slowly with lower achieved blood pressures, especially in those with higher degrees of proteinuria.10–12
However, observational data do not prove cause and effect, nor do they guarantee similar results with treatment. This requires randomized controlled trials.
RANDOMIZED TRIALS OF HYPERTENSION TREATMENT
Initial trials were aimed at determining whether hypertension should even be treated. A 1997 meta-analysis of 18 such trials comparing either low-dose diuretic therapy, high-dose diuretic therapy, or beta-blocker therapy with placebo involved 48,000 patients who were followed for an average of 5 years.13 The rates of stroke and congestive heart failure were consistently reduced, although only low-dose diuretic therapy reduced the risk of coronary heart disease and death from any cause.
More recent trials enrolled people not considered hypertensive who were randomized to receive either active drugs or placebo, or no treatment. Other trials attempted to assess non-pressure-related effects of specific agents, using other antihypertensive agents in the control group. Still other randomized controlled trials compared one agent or agents with other agents while attempting to attain equivalent blood pressure between groups. Frequently, however, there was some blood pressure difference.
Meta-analyses of most of these trials conclude that the major benefit of antihypertensive therapy—reducing rates of cardiovascular morbidity and mortality—comes from a lower attained blood pressure, irrespective of which agent is used.14–18 Exceptions exist, however. For example, specific drug classes are indicated after myocardial infarction, and in congestive heart failure and proteinuric chronic kidney disease.10,19–21
16 TRIALS OF DIFFERENT BLOOD PRESSURE TARGETS
The overriding theme of these observational data is that a lower blood pressure, whether attained naturally or with treatment, is better than a higher one from both the cardiovascular and the renal perspective.
What remains unclear is what blood pressure should be aimed for in a particular patient or group of patients. Is it a specific pressure (eg, 140/90 mm Hg), or does the change from baseline count more? Should other factors such as age or comorbidity alter this number?
Several randomized controlled trials have addressed these questions by targeting different levels of blood pressure. We are aware of at least 16 such trials in adults, including 13 with renal or cardiovascular primary end points and three with surrogate primary end points.
An unavoidable design flaw of all of these trials is their unblinded nature. Consequently, nearly all of them carry a Jadad score (a measure of quality, based on randomization and blinding)22 of 3 on a scale of 5.
NINE TRIALS WITH RENAL PRIMARY END POINTS
African American Study of Kidney Disease and Hypertension (AASK)23
Patients: 1,094 African Americans with presumed hypertensive renal disease and a measured glomerular filtration rate between 20 and 65 mL/min/1.73 m2.
Randomized blood pressure goals. Mean arterial pressure 92 mm Hg or less vs 102 to 107 mm Hg.
Results. At 4 years, the two groups had average blood pressures of 128/78 and 141/85 mm Hg, respectively. The groups did not differ in the rates of the primary end points—ie, the rate of change in the measured glomerular filtration rate over time or the composite of a 50% reduction in glomerular filtration rate, the onset of end-stage renal disease, or death.
Comments. Several issues have been raised about the internal validity of this trial.
So-called hypertensive kidney disease in African Americans (as opposed to European Americans) may be a genetic disorder related to polymorphisms of one or more genes on chromosome 22q. Initial data implicated the MYH9 gene, which encodes non-muscle myosin heavy chain II.24,25 More recent data implicate the nearby APOL1 gene encoding apolipoprotein L126 as more relevant. These polymorphisms have a much greater prevalence in African Americans and appear responsible for the higher risk of idiopathic focal segmental glomerulosclerosis and HIV-associated nephropathy in this population.24–26 Therefore, in African Americans, hypertension may in fact be the result of the kidney disease and not its primary cause, which may explain why in this and other African American populations stricter control of blood pressure did not produce a renal benefit.27,28
Also, there is the possibility of misclassification bias. A secondary analysis of data obtained by ambulatory monitoring showed that of the 377 participants whose blood pressure appeared to be under control when measured in the clinic, 70% actually had masked hypertension, ie, uncontrolled hypertension outside the clinic.29 The real difference in blood pressure between groups may well have been different than that determined in the clinic.
In addition, a prespecified secondary analysis showed no difference in the rates of cardiovascular events and death between the groups.30 However, the study was not designed to have the statistical power to detect a difference in cardiovascular events. Moreover fewer cardiovascular events occurred than expected, further reducing the study’s power to detect a difference.
Toto et al31
Toto et al reported similar results in an earlier trial in 87 hypertensive patients (77 randomized), predominantly African American, and similar concerns apply.
Lewis et al32
Patients: 129 patients with type 1 diabetes.
Randomized blood pressure goals. A mean arterial pressure of either no higher than 92 mm Hg or 100 to 107 mm Hg.
Results. At 2 years, despite a difference of 6 mm Hg in mean arterial pressure, the glomerular filtration rate (measured) had declined by the same amount in the two groups. The study was underpowered for this end point. Patients in the group with the lower goal pressure were excreting significantly less protein than those in the other group, but they were received higher doses of an angiotensin-converting enzyme (ACE) inhibitor—in this case, ramipril (Altace).
The Appropriate Blood Pressure Control in Diabetes (ABCD) trials33–35
Patients: 950 patients with type 2 diabetes mellitus and either normal or high blood pressure.
Randomized blood pressure goals. Either intensive or moderate therapy (see Table 1).
Results. At 5 years, creatinine clearance (estimated) had declined by the same amount in the two groups. However, fewer of the hypertensive patients had died in the intensive-therapy group.34 Similarly, normotensive patients had less progression of albuminuria if treated intensively.33
In the ABCD Part 2 with Valsartan (ABCD-2V) trial in normotensive patients,35 therapy with valsartan (Diovan) did not affect creatinine clearance but did reduce albuminuria. However, 75% of the patients in the moderate-treatment group were untreated.
Schrier et al36
Patients. 75 hypertensive patients with autosomal-dominant polycystic kidney disease and left ventricular hypertrophy.
Randomized blood pressure targets. Less than 120/80 mm Hg vs 135/85 to 140/90 mm Hg.
Results. After 7 years, despite a difference in average mean arterial pressure of 11 mm Hg between the groups (90 vs 101 mm Hg), there was no difference in the rate of decline of creatinine clearance. The left ventricular mass index decreased by 21% in the lower-target group and by 35% in the higher-target group (P < .01).
Modification of Diet in Renal Disease (MDRD) trial37,38
Patients: 840 patients whose measured glomerular filtration rate was between 13 and 55 mL/min/1.73 m2.
Randomized blood pressure targets. A target mean arterial pressure of less than 92 mm Hg vs less than 107 mm Hg.11,37
Results. After 2.2 years, the mean difference in mean arterial pressure was 4.7 mm Hg. There was, however, no difference in the rate of decline in the glomerular filtration rate.
In a 6-year follow-up, significantly fewer patients in the lower-blood-pressure group reached the end point of end-stage renal disease or the combined end point of end-stage renal disease or death.38 The rate of death, however, was nearly twice as high in the lower-blood-pressure group (10% vs 6%). The blood pressure and treatment during follow-up were not reported.
Comments. Internal validity is an issue, since the blood pressure and therapy during follow-up were unknown, and more patients received ACE inhibitors in the lower-blood-pressure group during the trial. Further, the higher death rate in the lower-blood-pressure group is worrisome.
The Ramipril Efficacy in Nephropathy (REIN)-2 trial39
Patients: 338 nondiabetic patients who had proteinuria and reduced creatinine clearance.
Treatment and blood pressure goals. All were treated with ramipril and randomized to intensive (< 130/80 mm Hg) vs standard control (diastolic blood pressure < 90 mm Hg) with therapy based on felodipine (Plendil).
Results. The study was terminated early because of futility. Despite a mean difference of 4.1 mm Hg systolic and 2.8 mm Hg diastolic, the groups did not differ in the rate of progression to end-stage renal disease (23% with intensive therapy vs 20% with standard therapy) or in the rate of decline of the measured glomerular filtration rate (0.22 vs 0.24 mL/min/1.73 m2/month).
Comment. The internal validity of this study can be questioned because of the low separation of achieved blood pressure and because of its early termination.
No benefit from a lower blood pressure goal in preserving kidney function
To summarize, these trials all showed no significant benefit from either targeting or achieving lower blood pressure in terms of slowing the decline of kidney function. Overall, they do not define a target and offer little support that a lower goal blood pressure is indicated with respect to the rate of loss of glomerular filtration rate in chronic kidney disease.
However, post hoc analysis of the MDRD trial indicates a statistical interaction between targeted blood pressure and degree of baseline proteinuria. At higher levels of proteinuria (≥ 1 g/day), the group with the lower blood pressure target had better outcomes.
In addition, long-term follow-up (mean of 12.2 years) of the AASK trial, including a 7-year cohort phase with nearly similar blood pressures in both groups, also indicated an interaction with targeted blood pressure and baseline proteinuria.40 Although the overall analysis was negative, there was a significant reduction in the primary end point in the group originally assigned the low target when analysis was restricted to those in the highest tertile of proteinuria. These and other data10 suggest that patients with chronic kidney disease and proteinuria may represent a distinct subset of chronic kidney disease patients who benefit from more intensive blood-pressure-lowering. However, patients in the REIN-2 trial34 and the macroalbuminuric patients in the ABCD hypertensive trial35 did not benefit from a lower targeted blood pressure despite significant proteinuria.
FOUR TRIALS WITH CARDIOVASCULAR END POINTS
The Hypertension Optimal Treatment (HOT) trial41
Patients: 18,790 patients with diastolic blood pressure between 100 and 115 mm Hg.
Randomized blood pressure goals. Diastolic pressure of equal to or less than 80, 85, or 90 mm Hg.
Results. At an average of 3.8 years, the average blood pressures in the three groups were approximately 140/81, 141/83, and 144/85 mm Hg, respectively. There was no difference between the groups in the rate of the composite primary end point of all myocardial infarctions, all strokes, and cardiovascular death. Any conclusions from this trial were compromised by the small difference in achieved blood pressures between groups.
In the 1,501 patients with diabetes, the incidence of the primary end point was 50% lower with a goal of 80 mm Hg or less than with a goal of 90 mm Hg or less.
The UK Prospective Diabetes Study (UKPDS)42,43
Patients: 1,148 hypertensive patients with type 2 diabetes mellitus.
Randomized blood pressure goals. Either “tight control” (aiming for < 150/85 mm Hg) or “less tight control” (aiming for < 180/105 mm Hg).
Results. At a median follow-up of 8.4 years, the attained blood pressures were 144/82 vs 154/87 mm Hg. The difference produced significant benefits, including a 24% lower rate of any diabetes-related end point, a 32% lower rate of death due to diabetes, and a nonsignificant 18% lower rate of total mortality—all co-primary end points.
The less-tight-control group had many patients with initial blood pressures below 180/105 mm Hg; hence, over 50% of patients received no antihypertensive therapy at the start of the trial. By the end of the trial 9 years later, 20% had still not been treated. This compares with only 5% of patients in the tight-control group who were not treated with antihypertensives throughout the trial. Therefore, this trial serves as better evidence for treating vs not treating, rather than defining a specific goal.
During a 10-year follow-up, blood pressure differences disappeared within 2 years.43 There was no legacy effect, as the significant differences noted during the trial were no longer present 10 years later.
Action to Control Cardiovascular Risk in Diabetes (ACCORD)44
Patients: 4,733 patients with type 2 diabetes.
Randomized blood pressure goals. Systolic blood pressure lower than either 120 or 140 mm Hg.
Results. At 4.7 years, despite a significant difference in mean systolic blood pressure of 14.2 mm Hg after the first year (119.3 vs 133.5 mm Hg), there was no difference in the primary end point of nonfatal myocardial infarction, nonfatal stroke, or cardiovascular death. There were fewer strokes in the lower-pressure group but no difference in myocardial infarctions, which were five times more common than strokes. Serious adverse events attributed to antihypertensive treatment occurred more frequently in the intensive-therapy group (3.3% vs 1.3%, P < .001).
Comment. There were fewer events than expected, possibly limiting the trial’s ability to detect a statistical difference. Compared with both the UKPDS and the diabetic population of HOT, ACCORD is much larger and more internally valid (unlike in UKPDS, nearly all patients in both groups were treated, and compared with HOT there was much greater separation of achieved pressure). It is more recent and better reflects current overall practice. It indicates that when specifically aiming for a target blood pressure, lower is not always better and comes at a price (more severe adverse events).
Japanese Trial to Assess Optimal Systolic Blood Pressure in Elderly Hypertensive Patients (JATOS)45
Patients: 4,418 patients, age 65 to 85 years, with a pretreatment systolic blood pressure above 160 mm Hg.
Randomized blood pressure goals. Systolic pressure either lower than 140 mm Hg or 140 to 160 mm Hg.
Results. At 2 years, despite a difference of 9.7/3.3 mm Hg, there was no difference in the primary end point (the combined incidence of cerebrovascular disease, cardiac and vascular disease, and renal failure). Fifty-four patients had died in the strict-treatment group and 42 in the mild-treatment group; the difference was not statistically significant.
Three other trials
Three other trials46–48 had surrogate end points, but only one of them reported a composite cardiovascular secondary end point.46 We will not discuss the other two.47,48
Cardio-Sis. In the Studio Italiano Sugli Effetti Cardiovascolari del Controllo della Pressione Arteriosa Sistolica (Cardio-Sis) trial,46 1,111 people without diabetes with systolic pressure higher than 150 mm Hg were randomized to tight control (systolic pressure < 130 mm Hg) vs usual control (systolic pressure < 140 mm Hg) and followed for 2 years with electrocardiography to detect left ventricular hypertrophy.
At a median of 2 years, the systolic blood pressure had declined by an average of 3.8 mm Hg more in the tight-control group than in the usual-control group, and the diastolic pressure by an average of 1.5 mm Hg. There was significantly less left ventricular hypertrophy in the tight-control group. The incidence of the secondary end point of a composite of cardiovascular and renal events was also significantly lower. There was no difference individually in the rates of myocardial infarction, stroke, transient ischemic attack, admission for congestive heart failure, or death.
DISCUSSION: THE DILEMMA OF TREATING AN INDIVIDUAL PATIENT
These data illustrate the dilemma of treating an individual patient whose blood pressure is not at the currently accepted goal while on multiple antihypertensive medications. According to guidelines, therapy should be intensified in this situation. Observational data show a strong graded relationship between blood pressure and cardiovascular events and death, starting with a blood pressure of 115/75 mm Hg. The observational data relating blood pressure to kidney disease are similar. These data support the guideline recommendations that additional medications should be added to reach the promulgated target. Unfortunately, the targeting trials do not define a target, nor do they support the concept that lower is better.
Possible explanations for the negative results
Why does targeting a lower blood pressure not produce the benefit that the observational data lead us to expect?
One possibility is that blood pressure is merely a marker of cardiovascular risk, not a cause of it. This is unlikely, given the temporal relationship, reproducibility, and biologic plausibility that is supported by a very large body of experimental data. However, blood pressure is only one of multiple factors involved in the pathogenesis of vascular and renal disease, and perhaps better attention to other factors such as lipids and smoking may have made the targeting trials underpowered.
Another possibility is that these trials had such strict inclusion and exclusion criteria that they do not represent the general hypertensive population, reducing their external validity.49 However, the trials generally enrolled populations at higher risk, in which end points were more likely to occur. This would have enhanced the chance to show a positive effect rather than mask it.
It is possible that antihypertensive medications themselves have unwanted side effects that offset their potential benefit. Medication-related side effects could directly contribute to vascular disease despite their beneficial effect of lowering pressure. There could also be reduced tissue perfusion due to lower blood pressure per se in the face of a diseased vasculature, with the lower pressure directly contributing to organ dysfunction.
Finally, these trials measured brachial pressures to monitor blood pressure. Brachial pressure does not always correlate with central aortic pressure, which is probably a better marker of the overall pressure burden.50 It is possible that in these targeting trials, the peripheral blood pressure did not reflect the true central blood pressure and, therefore, significant separation of blood pressures may not have actually occurred.
Targeted vs achieved blood pressures: Analogies with other markers
This contradiction is not an exceptional circumstance in medicine.
For example, in chronic kidney disease, a graded observational relationship exists between decreasing levels of hemoglobin and various adverse outcomes.51–53 However, targeting a more normal level of hemoglobin compared with a lower one has been shown to be detrimental.54–57 This implies either that anemia is merely a marker of higher risk or, more likely, that the actual measures used to raise the hemoglobin to higher levels are the culprit. Notably, although targeting a higher hemoglobin concentration vs a lower one was detrimental, achieving a higher hemoglobin was beneficial within each targeted group.54,58
Another example of harm caused by targeting goals based on observational data is tight glucose control, both acutely in the critically ill59 and chronically in patients with type 2 diabetes.60 In both cases higher mortality rates ensued.
The same concept may apply to lowering blood pressure. While achieving a lower blood pressure may be more beneficial, targeting a specific goal may be harmful. Given that perhaps 20% of those labeled as hypertensive have resistant hypertension,61 millions of patients are susceptible to potential harm from targeting a specific goal based solely on observational data. If lower is always better, the randomized trials outlined above should have had more positive outcomes.
It becomes problematic to assign a specific goal for all patients or even groups of patients. The targeting trials do not provide the answer. Based on the observational data it would be optimal to have a blood pressure less than 120/80 mm Hg. This is an observation, not a recommendation. Patients should be assessed on an individual basis, taking into consideration their starting blood pressure, age, medication burden (antihypertensive and otherwise), comorbidities, and ability to comply with a regimen. Given the available data, it is hard to be more specific. In the future it may be possible to identify specific blood pressure targets based on the patient’s genetic makeup, but today that is not possible. Even patients with lower initial blood pressure may benefit from therapy,62,63 and some experts have advocated blood-pressure-lowering in all, irrespective of the baseline value.14
Avoid misclassification
The first step in treating hypertension should be to avoid misclassification. Make sure the clinic blood pressure is measured correctly, using an appropriately sized cuff, positioning the patient properly, and following all the other recommendations.64
However, the clinic blood pressure may not reflect true blood pressure load in up to one-third of all patients.65 We recommend 24-hour ambulatory blood pressure monitoring66 or home self-measurement, or both,67 to better assess true blood pressure burden in several circumstances, including in patients with resistant hypertension (any patient who has not achieved acceptable clinic blood pressure on three or more antihypertensive medications including a diuretic or who requires four or more medications for adequate control), suspicion of white-coat hypertension (or effect), and any patient who has achieved acceptable clinic blood pressure but either has symptoms of hypotension or progressive end-organ damage.
Currently, we base therapy on out-of-office blood pressure (self-measured or by ambulatory monitoring) whenever there is a discrepancy with clinic blood pressure.
Whether therapy should be altered by other less traditional measures of blood pressure such as assessment of central aortic pressure by radial applanation tonometry,68,69 or 24-hour ambulatory monitoring to assess nighttime blood pressures (specifically, “dipping”),70 morning surge,71 or blood pressure variability72,73 remains unclear and in need of randomized controlled trials.
In any patient requiring blood-pressure-lowering, we recommend lifestyle modifications.1,2 These include exercise, weight loss, salt and alcohol restriction, evaluation for sleep apnea, and avoidance of medications known to elevate blood pressure such as nonsteroidal anti-inflammatory drugs and sympathomimetic decongestants.
Much needs to be learned
For the individual patient with unacceptably high blood pressure who is already taking multiple antihypertensive medications of different classes, it is unclear what to do. This type of patient with resistant hypertension would be an excellent candidate for a future targeting trial. Other cardiovascular risk factors should be appropriately addressed, including obesity, lipids, smoking, and poor glycemic control.74 Each patient should be individually assessed with consideration of both global cardiovascular risk and quality-of-life issues.
Much still needs to be learned about the treatment of hypertension. The facts demonstrate that blood pressure is a strong modifiable risk factor of cardiovascular morbidity and mortality. Lowering it clearly produces benefits. It is unclear what treatment goals should be promulgated by official guidelines for large groups of patients. The resistant case remains a therapeutic dilemma with the potential for harm from overly aggressive treatment. The truly optimal level for an individual patient remains difficult to define. We anxiously await results of ongoing and future targeting trials.
CASE REVISITED
Regarding the initial case vignette, the patient is clearly not at her recommended goal blood pressure, especially given her high-risk status (diabetes mellitus and chronic kidney disease). Observational data support intensification of therapy, whereas targeting trials are essentially negative and indicate the potential for harm with overly aggressive treatment. Thus, we remain uncertain about what is correct or incorrect in terms of a targeted blood pressure, especially when applied to the individual patient.
Our approach would be to emphasize lifestyle modifications, to ensure accurate determination of her true blood pressure load (self-measurement at home or ambulatory blood pressure monitoring), to consider secondary causes of hypertension, and to educate the patient about the benefits and consequences of intensifying therapy with the aim of involving her in the decision.
- Chobanian AV, Bakris GL, Black HR, et al; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289:2560–2572.
- Mancia G, Laurent S, Agabiti-Rosei E, et al; European Society of Hypertension. Reappraisal of European guidelines on hypertension management: a European Society of Hypertension Task Force document. J Hypertens 2009; 27:2121–2158.
- MacMahon S, Peto R, Cutler J, et al. Blood pressure, stroke, and coronary heart disease. Part 1, prolonged differences in blood pressure: prospective observational studies corrected for the regression dilution bias. Lancet 1990; 335:765–774.
- Lewington S, Clarke R, Qizilbash N, Peto R, Collins R; Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002; 360:1903–1913.
- Lawes CM, Rodgers A, Bennett DA, et al; Asia Pacific Cohort Studies Collaboration. Blood pressure and cardiovascular disease in the Asia Pacific region. J Hypertens 2003; 21:707–716.
- Staessen JA, Gasowski J, Wang JG, et al. Risks of untreated and treated isolated systolic hypertension in the elderly: meta-analysis of outcome trials. Lancet 2000; 355:865–872.
- Messerli FH, Mancia G, Conti CR, et al. Dogma disputed: can aggressively lowering blood pressure in hypertensive patients with coronary artery disease be dangerous? Ann Intern Med 2006; 144:884–893.
- Klag MJ, Whelton PK, Randall BL, et al. Blood pressure and end-stage renal disease in men. N Engl J Med 1996; 334:13–18.
- Tozawa M, Iseki K, Iseki C, Kinjo K, Ikemiya Y, Takishita S. Blood pressure predicts risk of developing end-stage renal disease in men and women. Hypertension 2003; 41:1341–1345.
- Jafar TH, Stark PC, Schmid CH, et al; AIPRD Study Group. Progression of chronic kidney disease: the role of blood pressure control, proteinuria, and angiotensin-converting enzyme inhibition: a patient-level meta-analysis. Ann Intern Med 2003; 139:244–252.
- Peterson JC, Adler S, Burkart JM, et al. Blood pressure control, proteinuria, and the progression of renal disease. The Modification of Diet in Renal Disease Study. Ann Intern Med 1995; 123:754–762.
- Pohl MA, Blumenthal S, Cordonnier DJ, et al. Independent and additive impact of blood pressure control and angiotensin II receptor blockade on renal outcomes in the irbesartan diabetic nephropathy trial: clinical implications and limitations. J Am Soc Nephrol 2005; 16:3027–3037.
- Psaty BM, Smith NL, Siscovick DS, et al. Health outcomes associated with antihypertensive therapies used as first-line agents. A systematic review and meta-analysis. JAMA 1997; 277:739–745.
- Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ 2009; 338:b1665.
- Turnbull F; Blood Pressure Lowering Treatment Trialists’ Collaboration. Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trials. Lancet 2003; 362:1527–1535.
- Blood Pressure Lowering Treatment Trialists’ Collaboration; Turnbull F, Neal B, Ninomiya T, et al. Effects of different regimens to lower blood pressure on major cardiovascular events in older and younger adults: meta-analysis of randomised trials. BMJ 2008; 336:1121–1123.
- Staessen JA, Wang JG, Thijs L. Cardiovascular prevention and blood pressure reduction: a quantitative overview updated until 1 March 2003. J Hypertens 2003; 21:1055–1076.
- Psaty BM, Lumley T, Furberg CD, et al. Health outcomes associated with various antihypertensive therapies used as first-line agents: a network meta-analysis. JAMA 2003; 289:2534–2544.
- Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society.—Summary Article. Circulation 2005; 112:e154–e235.
- Brenner BM, Cooper ME, de Zeeuw D, et al; RENAAL Study Investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345:861–869.
- Lewis EJ, Hunsicker LG, Clarke WR, et al; Collaborative Study Group. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 2001; 345:851–860.
- Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996; 17:1–12.
- Wright JT, Bakris G, Greene T, et al; African American Study of Kidney Disease and Hypertension Study Group. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA 2002; 288:2421–2431.
- Freedman BI, Hicks PJ, Bostrom MA, et al. Polymorphisms in the non-muscle myosin heavy chain 9 gene (MYH9) are strongly associated with end-stage renal disease historically attributed to hypertension in African Americans. Kidney Int 2009; 75:736–745.
- Kopp JB, Smith MW, Nelson GW, et al. MYH9 is a major-effect risk gene for focal segmental glomerulosclerosis. Nat Genet 2008; 40:1175–1184.
- Freedman BI, Kopp JB, Langefeld CD, et al. The apolipoprotein L1 (APOL1) gene and nondiabetic nephropathy in African Americans. J Am Soc Nephrol 2010; 21:1422–1426.
- Rostand SG, Brown G, Kirk KA, Rutsky EA, Dustan HP. Renal insufficiency in treated essential hypertension. N Engl J Med 1989; 320:684–688.
- Walker WG, Neaton JD, Cutler JA, Neuwirth R, Cohen JD. Renal function change in hypertensive members of the Multiple Risk Factor Intervention Trial. Racial and treatment effects. The MRFIT Research Group. JAMA 1992; 268:3085–3091.
- Pogue V, Rahman M, Lipkowitz M, et al; African American Study of Kidney Disease and Hypertension Collaborative Research Group. Disparate estimates of hypertension control from ambulatory and clinic blood pressure measurements in hypertensive kidney disease. Hypertension 2009; 53:20–27.
- Norris K, Bourgoigne J, Gassman J, et al; AASK Study Group. Cardiovascular outcomes in the African American Study of Kidney Disease and Hypertension (AASK) Trial. Am J Kidney Dis 2006; 48:739–751.
- Toto RD, Mitchell HC, Smith RD, Lee HC, McIntire D, Pettinger WA. “Strict” blood pressure control and progression of renal disease in hypertensive nephrosclerosis. Kidney Int 1995; 48:851–859.
- Lewis JB, Berl T, Bain RP, Rohde RD, Lewis EJ. Effect of intensive blood pressure control on the course of type 1 diabetic nephropathy. Collaborative Study Group. Am J Kidney Dis 1999; 34:809–817.
- Schrier RW, Estacio RO, Esler A, Mehler P. Effects of aggressive blood pressure control in normotensive type 2 diabetic patients on albuminuria, retinopathy and strokes. Kidney Int 2002; 61:1086–1097.
- Estacio RO, Jeffers BW, Gifford N, Schrier RW. Effect of blood pressure control on diabetic microvascular complications in patients with hypertension and type 2 diabetes. Diabetes Care 2000; 23( suppl 2):B54–B64.
- Estacio RO, Coll JR, Tran ZV, Schrier RW. Effect of intensive blood pressure control with valsartan on urinary albumin excretion in normotensive patients with type 2 diabetes. Am J Hypertens 2006; 19:1241–1248.
- Schrier R, McFann K, Johnson A, et al. Cardiac and renal effects of standard versus rigorous blood pressure control in autosomal-dominant polycystic kidney disease: results of a seven-year prospective randomized study. J Am Soc Nephrol 2002; 13:1733–1739.
- Klahr S, Levey AS, Beck GJ, et al. The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. Modification of Diet in Renal Disease Study Group. N Engl J Med 1994; 330:877–884.
- Sarnak MJ, Greene T, Wang X, et al. The effect of a lower target blood pressure on the progression of kidney disease: long-term follow-up of the modification of diet in renal disease study. Ann Intern Med 2005; 142:342–351.
- Ruggenenti P, Perna A, Loriga G, et al; REIN-2 Study Group. Blood-pressure control for renoprotection in patients with non-diabetic chronic renal disease (REIN-2): multicentre, randomised controlled trial. Lancet 2005; 365:939–946.
- Appel LJ, Wright JT, Greene T, et al; AASK Collaborative Research Group. Intensive blood-pressure control in hypertensive chronic kidney disease. N Engl J Med 2010; 363:918–929.
- Hansson L, Zanchetti A, Carruthers SG, et al. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet 1998; 351:1755–1762.
- Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ 1998; 317:703–713.
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359:1577–1589.
- ACCORD Study Group, Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010; 362:1575–1585.
- JATOS Study Group. Principal results of the Japanese trial to assess optimal systolic blood pressure in elderly hypertensive patients (JATOS). Hypertens Res 2008; 31:2115–2127.
- Verdecchia P, Staessen JA, Angeli F, et al; Cardio-Sis investigators. Usual versus tight control of systolic blood pressure in non-diabetic patients with hypertension (Cardio-Sis): an open-label randomised trial. Lancet 2009; 374:525–533.
- Solomon SD, Verma A, Desai A, et al; Exforge Intensive Control of Hypertension to Evaluate Efficacy in Diastolic Dysfunction Investigators. Effect of intensive versus standard blood pressure lowering on diastolic function in patients with uncontrolled hypertension and diastolic dysfunction. Hypertension 2010; 55:241–248.
- Ichihara A, Hayashi M, Koura Y, Tada Y, Hirota N, Saruta T. Long-term effects of intensive blood-pressure lowering on arterial wall stiffness in hypertensive patients. Am J Hypertens 2003; 16:959–965.
- Rothwell PM. External validity of randomised controlled trials: “to whom do the results of this trial apply?” Lancet 2005; 365:82–93.
- Townsend RR, Roman MJ, Najjar SS, Cockcroft JR, Feig PU, Stockbridge NL. Central blood pressure measurements-an opportunity for efficacy and safety in drug development? J Am Soc Hypertens 2010; 4:211–214.
- Xia H, Ebben J, Ma JZ, Collins AJ. Hematocrit levels and hospitalization risks in hemodialysis patients. J Am Soc Nephrol 1999; 10:1309–1316.
- Ma JZ, Ebben J, Xia H, Collins AJ. Hematocrit level and associated mortality in hemodialysis patients. J Am Soc Nephrol 1999; 10:610–619.
- Ofsthun N, Labrecque J, Lacson E, Keen M, Lazarus JM. The effects of higher hemoglobin levels on mortality and hospitalization in hemodialysis patients. Kidney Int 2003; 63:1908–1914.
- Besarab A, Bolton WK, Browne JK, et al. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med 1998; 339:584–590.
- Drüeke TB, Locatelli F, Clyne N, et al; CREATE Investigators. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med 2006; 355:2071–2084.
- Singh AK, Szczech L, Tang KL, et al; CHOIR Investigators. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med 2006; 355:2085–2098.
- Pfeffer MA, Burdmann EA, Chen CY, et al; TREAT Investigators. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med 2009; 361:2019–2032.
- Szczech LA, Barnhart HX, Inrig JK, et al. Secondary analysis of the CHOIR trial epoetin-alpha dose and achieved hemoglobin outcomes. Kidney Int 2008; 74:791–798.
- Finfer S, Chittock DR, Su SY, et al; NICE-SUGAR Study Investigators Intensive versus conventional glucose control in critically ill patients. N Engl J Med 2009; 360:1283–1297.
- Action to Control Cardiovascular Risk in Diabetes Study Group; Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358:2545–2559.
- Calhoun DA, Jones D, Textor S, et al. Resistant hypertension: diagnosis, evaluation, and treatment. A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension 2008; 51:1403–1419.
- Nissen SE, Tuzcu EM, Libby P, et al; CAMELOT Investigators. Effect of antihypertensive agents on cardiovascular events in patients with coronary disease and normal blood pressure: the CAMELOT study, a randomized controlled trial. JAMA 2004; 292:2217–2225.
- Patel A; ADVANCE Collaborative Group; MacMahon S, Chalmers J, Neal B. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet 2007; 370:829–840.
- Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation 2005; 111:697–716.
- Fagard RH, Cornelissen VA. Incidence of cardiovascular events in white-coat, masked and sustained hypertension versus true normotension: a meta-analysis. J Hypertens 2007; 25:2193–2198.
- Pickering TG, White WB, Giles TD, et al. When and how to use self (home) and ambulatory blood pressure monitoring. J Am Soc Hypertens 2010; 4:56–61.
- Hänninen MR, Niiranen TJ, Puukka PJ, Jula AM. Comparison of home and ambulatory blood pressure measurement in the diagnosis of masked hypertension. J Hypertens 2010; 28:709–714.
- Roman MJ, Devereux RB, Kizer JR, et al. Central pressure more strongly relates to vascular disease and outcome than does brachial pressure: the Strong Heart Study. Hypertension 2007; 50:197–203.
- Williams B, Lacy PS, Thom SM, et al; CAFE Investigators; Anglo-Scandinavian Cardiac Outcomes Trial Investigators; CAFE Steering Committee and Writing Committee. Differential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes: principal results of the Conduit Artery Function Evaluation (CAFE) study. Circulation 2006; 113:1213–1225.
- Ben-Dov IZ, Kark JD, Ben-Ishay D, Mekler J, Ben-Arie L, Bursztyn M. Predictors of all-cause mortality in clinical ambulatory monitoring: unique aspects of blood pressure during sleep. Hypertension 2007; 49:1235–1241.
- Li Y, Thijs L, Hansen TW, et al; International Database on Ambulatory Blood Pressure Monitoring in Relation to Cardiovascular Outcomes Investigators. Prognostic value of the morning blood pressure surge in 5645 subjects from 8 populations. Hypertension 2010; 55:1040–1048.
- Rothwell PM. Limitations of the usual blood-pressure hypothesis and importance of variability, instability, and episodic hypertension. Lancet 2010; 375:938–948.
- Hansen TW, Thijs L, Li Y, et al; International Database on Ambulatory Blood Pressure in Relation to Cardiovascular Outcomes Investigators. Prognostic value of reading-to-reading blood pressure variability over 24 hours in 8938 subjects from 11 populations. Hypertension 2010; 55:1049–1057.
- Jackson R, Lawes CM, Bennett DA, Milne RJ, Rodgers A. Treatment with drugs to lower blood pressure and blood cholesterol based on an individual’s absolute cardiovascular risk. Lancet 2005; 365:434–441.
- Chobanian AV, Bakris GL, Black HR, et al; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289:2560–2572.
- Mancia G, Laurent S, Agabiti-Rosei E, et al; European Society of Hypertension. Reappraisal of European guidelines on hypertension management: a European Society of Hypertension Task Force document. J Hypertens 2009; 27:2121–2158.
- MacMahon S, Peto R, Cutler J, et al. Blood pressure, stroke, and coronary heart disease. Part 1, prolonged differences in blood pressure: prospective observational studies corrected for the regression dilution bias. Lancet 1990; 335:765–774.
- Lewington S, Clarke R, Qizilbash N, Peto R, Collins R; Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002; 360:1903–1913.
- Lawes CM, Rodgers A, Bennett DA, et al; Asia Pacific Cohort Studies Collaboration. Blood pressure and cardiovascular disease in the Asia Pacific region. J Hypertens 2003; 21:707–716.
- Staessen JA, Gasowski J, Wang JG, et al. Risks of untreated and treated isolated systolic hypertension in the elderly: meta-analysis of outcome trials. Lancet 2000; 355:865–872.
- Messerli FH, Mancia G, Conti CR, et al. Dogma disputed: can aggressively lowering blood pressure in hypertensive patients with coronary artery disease be dangerous? Ann Intern Med 2006; 144:884–893.
- Klag MJ, Whelton PK, Randall BL, et al. Blood pressure and end-stage renal disease in men. N Engl J Med 1996; 334:13–18.
- Tozawa M, Iseki K, Iseki C, Kinjo K, Ikemiya Y, Takishita S. Blood pressure predicts risk of developing end-stage renal disease in men and women. Hypertension 2003; 41:1341–1345.
- Jafar TH, Stark PC, Schmid CH, et al; AIPRD Study Group. Progression of chronic kidney disease: the role of blood pressure control, proteinuria, and angiotensin-converting enzyme inhibition: a patient-level meta-analysis. Ann Intern Med 2003; 139:244–252.
- Peterson JC, Adler S, Burkart JM, et al. Blood pressure control, proteinuria, and the progression of renal disease. The Modification of Diet in Renal Disease Study. Ann Intern Med 1995; 123:754–762.
- Pohl MA, Blumenthal S, Cordonnier DJ, et al. Independent and additive impact of blood pressure control and angiotensin II receptor blockade on renal outcomes in the irbesartan diabetic nephropathy trial: clinical implications and limitations. J Am Soc Nephrol 2005; 16:3027–3037.
- Psaty BM, Smith NL, Siscovick DS, et al. Health outcomes associated with antihypertensive therapies used as first-line agents. A systematic review and meta-analysis. JAMA 1997; 277:739–745.
- Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ 2009; 338:b1665.
- Turnbull F; Blood Pressure Lowering Treatment Trialists’ Collaboration. Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trials. Lancet 2003; 362:1527–1535.
- Blood Pressure Lowering Treatment Trialists’ Collaboration; Turnbull F, Neal B, Ninomiya T, et al. Effects of different regimens to lower blood pressure on major cardiovascular events in older and younger adults: meta-analysis of randomised trials. BMJ 2008; 336:1121–1123.
- Staessen JA, Wang JG, Thijs L. Cardiovascular prevention and blood pressure reduction: a quantitative overview updated until 1 March 2003. J Hypertens 2003; 21:1055–1076.
- Psaty BM, Lumley T, Furberg CD, et al. Health outcomes associated with various antihypertensive therapies used as first-line agents: a network meta-analysis. JAMA 2003; 289:2534–2544.
- Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society.—Summary Article. Circulation 2005; 112:e154–e235.
- Brenner BM, Cooper ME, de Zeeuw D, et al; RENAAL Study Investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345:861–869.
- Lewis EJ, Hunsicker LG, Clarke WR, et al; Collaborative Study Group. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 2001; 345:851–860.
- Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996; 17:1–12.
- Wright JT, Bakris G, Greene T, et al; African American Study of Kidney Disease and Hypertension Study Group. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA 2002; 288:2421–2431.
- Freedman BI, Hicks PJ, Bostrom MA, et al. Polymorphisms in the non-muscle myosin heavy chain 9 gene (MYH9) are strongly associated with end-stage renal disease historically attributed to hypertension in African Americans. Kidney Int 2009; 75:736–745.
- Kopp JB, Smith MW, Nelson GW, et al. MYH9 is a major-effect risk gene for focal segmental glomerulosclerosis. Nat Genet 2008; 40:1175–1184.
- Freedman BI, Kopp JB, Langefeld CD, et al. The apolipoprotein L1 (APOL1) gene and nondiabetic nephropathy in African Americans. J Am Soc Nephrol 2010; 21:1422–1426.
- Rostand SG, Brown G, Kirk KA, Rutsky EA, Dustan HP. Renal insufficiency in treated essential hypertension. N Engl J Med 1989; 320:684–688.
- Walker WG, Neaton JD, Cutler JA, Neuwirth R, Cohen JD. Renal function change in hypertensive members of the Multiple Risk Factor Intervention Trial. Racial and treatment effects. The MRFIT Research Group. JAMA 1992; 268:3085–3091.
- Pogue V, Rahman M, Lipkowitz M, et al; African American Study of Kidney Disease and Hypertension Collaborative Research Group. Disparate estimates of hypertension control from ambulatory and clinic blood pressure measurements in hypertensive kidney disease. Hypertension 2009; 53:20–27.
- Norris K, Bourgoigne J, Gassman J, et al; AASK Study Group. Cardiovascular outcomes in the African American Study of Kidney Disease and Hypertension (AASK) Trial. Am J Kidney Dis 2006; 48:739–751.
- Toto RD, Mitchell HC, Smith RD, Lee HC, McIntire D, Pettinger WA. “Strict” blood pressure control and progression of renal disease in hypertensive nephrosclerosis. Kidney Int 1995; 48:851–859.
- Lewis JB, Berl T, Bain RP, Rohde RD, Lewis EJ. Effect of intensive blood pressure control on the course of type 1 diabetic nephropathy. Collaborative Study Group. Am J Kidney Dis 1999; 34:809–817.
- Schrier RW, Estacio RO, Esler A, Mehler P. Effects of aggressive blood pressure control in normotensive type 2 diabetic patients on albuminuria, retinopathy and strokes. Kidney Int 2002; 61:1086–1097.
- Estacio RO, Jeffers BW, Gifford N, Schrier RW. Effect of blood pressure control on diabetic microvascular complications in patients with hypertension and type 2 diabetes. Diabetes Care 2000; 23( suppl 2):B54–B64.
- Estacio RO, Coll JR, Tran ZV, Schrier RW. Effect of intensive blood pressure control with valsartan on urinary albumin excretion in normotensive patients with type 2 diabetes. Am J Hypertens 2006; 19:1241–1248.
- Schrier R, McFann K, Johnson A, et al. Cardiac and renal effects of standard versus rigorous blood pressure control in autosomal-dominant polycystic kidney disease: results of a seven-year prospective randomized study. J Am Soc Nephrol 2002; 13:1733–1739.
- Klahr S, Levey AS, Beck GJ, et al. The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. Modification of Diet in Renal Disease Study Group. N Engl J Med 1994; 330:877–884.
- Sarnak MJ, Greene T, Wang X, et al. The effect of a lower target blood pressure on the progression of kidney disease: long-term follow-up of the modification of diet in renal disease study. Ann Intern Med 2005; 142:342–351.
- Ruggenenti P, Perna A, Loriga G, et al; REIN-2 Study Group. Blood-pressure control for renoprotection in patients with non-diabetic chronic renal disease (REIN-2): multicentre, randomised controlled trial. Lancet 2005; 365:939–946.
- Appel LJ, Wright JT, Greene T, et al; AASK Collaborative Research Group. Intensive blood-pressure control in hypertensive chronic kidney disease. N Engl J Med 2010; 363:918–929.
- Hansson L, Zanchetti A, Carruthers SG, et al. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet 1998; 351:1755–1762.
- Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ 1998; 317:703–713.
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359:1577–1589.
- ACCORD Study Group, Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010; 362:1575–1585.
- JATOS Study Group. Principal results of the Japanese trial to assess optimal systolic blood pressure in elderly hypertensive patients (JATOS). Hypertens Res 2008; 31:2115–2127.
- Verdecchia P, Staessen JA, Angeli F, et al; Cardio-Sis investigators. Usual versus tight control of systolic blood pressure in non-diabetic patients with hypertension (Cardio-Sis): an open-label randomised trial. Lancet 2009; 374:525–533.
- Solomon SD, Verma A, Desai A, et al; Exforge Intensive Control of Hypertension to Evaluate Efficacy in Diastolic Dysfunction Investigators. Effect of intensive versus standard blood pressure lowering on diastolic function in patients with uncontrolled hypertension and diastolic dysfunction. Hypertension 2010; 55:241–248.
- Ichihara A, Hayashi M, Koura Y, Tada Y, Hirota N, Saruta T. Long-term effects of intensive blood-pressure lowering on arterial wall stiffness in hypertensive patients. Am J Hypertens 2003; 16:959–965.
- Rothwell PM. External validity of randomised controlled trials: “to whom do the results of this trial apply?” Lancet 2005; 365:82–93.
- Townsend RR, Roman MJ, Najjar SS, Cockcroft JR, Feig PU, Stockbridge NL. Central blood pressure measurements-an opportunity for efficacy and safety in drug development? J Am Soc Hypertens 2010; 4:211–214.
- Xia H, Ebben J, Ma JZ, Collins AJ. Hematocrit levels and hospitalization risks in hemodialysis patients. J Am Soc Nephrol 1999; 10:1309–1316.
- Ma JZ, Ebben J, Xia H, Collins AJ. Hematocrit level and associated mortality in hemodialysis patients. J Am Soc Nephrol 1999; 10:610–619.
- Ofsthun N, Labrecque J, Lacson E, Keen M, Lazarus JM. The effects of higher hemoglobin levels on mortality and hospitalization in hemodialysis patients. Kidney Int 2003; 63:1908–1914.
- Besarab A, Bolton WK, Browne JK, et al. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med 1998; 339:584–590.
- Drüeke TB, Locatelli F, Clyne N, et al; CREATE Investigators. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med 2006; 355:2071–2084.
- Singh AK, Szczech L, Tang KL, et al; CHOIR Investigators. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med 2006; 355:2085–2098.
- Pfeffer MA, Burdmann EA, Chen CY, et al; TREAT Investigators. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med 2009; 361:2019–2032.
- Szczech LA, Barnhart HX, Inrig JK, et al. Secondary analysis of the CHOIR trial epoetin-alpha dose and achieved hemoglobin outcomes. Kidney Int 2008; 74:791–798.
- Finfer S, Chittock DR, Su SY, et al; NICE-SUGAR Study Investigators Intensive versus conventional glucose control in critically ill patients. N Engl J Med 2009; 360:1283–1297.
- Action to Control Cardiovascular Risk in Diabetes Study Group; Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358:2545–2559.
- Calhoun DA, Jones D, Textor S, et al. Resistant hypertension: diagnosis, evaluation, and treatment. A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension 2008; 51:1403–1419.
- Nissen SE, Tuzcu EM, Libby P, et al; CAMELOT Investigators. Effect of antihypertensive agents on cardiovascular events in patients with coronary disease and normal blood pressure: the CAMELOT study, a randomized controlled trial. JAMA 2004; 292:2217–2225.
- Patel A; ADVANCE Collaborative Group; MacMahon S, Chalmers J, Neal B. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet 2007; 370:829–840.
- Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation 2005; 111:697–716.
- Fagard RH, Cornelissen VA. Incidence of cardiovascular events in white-coat, masked and sustained hypertension versus true normotension: a meta-analysis. J Hypertens 2007; 25:2193–2198.
- Pickering TG, White WB, Giles TD, et al. When and how to use self (home) and ambulatory blood pressure monitoring. J Am Soc Hypertens 2010; 4:56–61.
- Hänninen MR, Niiranen TJ, Puukka PJ, Jula AM. Comparison of home and ambulatory blood pressure measurement in the diagnosis of masked hypertension. J Hypertens 2010; 28:709–714.
- Roman MJ, Devereux RB, Kizer JR, et al. Central pressure more strongly relates to vascular disease and outcome than does brachial pressure: the Strong Heart Study. Hypertension 2007; 50:197–203.
- Williams B, Lacy PS, Thom SM, et al; CAFE Investigators; Anglo-Scandinavian Cardiac Outcomes Trial Investigators; CAFE Steering Committee and Writing Committee. Differential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes: principal results of the Conduit Artery Function Evaluation (CAFE) study. Circulation 2006; 113:1213–1225.
- Ben-Dov IZ, Kark JD, Ben-Ishay D, Mekler J, Ben-Arie L, Bursztyn M. Predictors of all-cause mortality in clinical ambulatory monitoring: unique aspects of blood pressure during sleep. Hypertension 2007; 49:1235–1241.
- Li Y, Thijs L, Hansen TW, et al; International Database on Ambulatory Blood Pressure Monitoring in Relation to Cardiovascular Outcomes Investigators. Prognostic value of the morning blood pressure surge in 5645 subjects from 8 populations. Hypertension 2010; 55:1040–1048.
- Rothwell PM. Limitations of the usual blood-pressure hypothesis and importance of variability, instability, and episodic hypertension. Lancet 2010; 375:938–948.
- Hansen TW, Thijs L, Li Y, et al; International Database on Ambulatory Blood Pressure in Relation to Cardiovascular Outcomes Investigators. Prognostic value of reading-to-reading blood pressure variability over 24 hours in 8938 subjects from 11 populations. Hypertension 2010; 55:1049–1057.
- Jackson R, Lawes CM, Bennett DA, Milne RJ, Rodgers A. Treatment with drugs to lower blood pressure and blood cholesterol based on an individual’s absolute cardiovascular risk. Lancet 2005; 365:434–441.
KEY POINTS
- Observational data indicate that lower blood pressure is better than higher, and many trials have confirmed that treatment of hypertension is beneficial. Guidelines have set specific goals based on the observational data.
- Surprisingly, randomized controlled trials have not shown a lower target to offer significant clinical benefit, and suggest the potential for harm with overly aggressive therapy.
- The optimal blood pressure on treatment for an individual patient remains unclear.