User login
Gouty arthritis is a common form of inflammatory arthritis, occurring more frequently in men than in women. The condition has a male–female ratio of 3 or 4 to 1, although that ratio narrows as adults age; because the uricosuric effects of estrogen decline with menopause, the risk for gout increases in postmenopausal women.1,2 Mean age at disease onset is 40 to 60 in men,3,4 with onset in women averaging seven years later.5
Apart from the pain and loss of function associated with this disorder of purine metabolism6 and the risk for a chronic form of the disease, gout is almost universally linked with serious comorbidities that require timely intervention. These include hypertension, dyslipidemia, hyperglycemia and diabetes, obesity, metabolic syndrome, cardiovascular disease (CVD), renal insufficiency, and coronary heart disease (CHD).2,3,7 The presence of gout is independently associated with a risk for acute myocardial infarction (AMI) and increased rates of all-cause mortality.3,8-10
EPIDEMIOLOGY
In 2007, the National Arthritis Data Workgroup11,12 estimated that about three million US adults had had “self-reported gout” in the previous year. An estimated six million US adults have been diagnosed with gout,2,11 and its incidence and prevalence are increasing. The incidence of primary gout more than doubled between 1977-1978 and 1995-1996,13 especially affecting the aging population. The prevalence of gout among 1,000 managed care patients ages 65 to 74 increased by at least 30% between 1990 and 1999, while prevalence among those older than 75 almost doubled during this same period.14
Numerous factors appear to contribute to these trends, including aging of the population, dietary trends (ie, increased consumption of red meat, organ food, game, and shellfish and reduced consumption of low-fat dairy products), presence of certain comorbid conditions (ie, hypertension, dyslipidemia, diabetes, metabolic syndrome, end-stage renal disease), the increasing prevalence of obesity in younger adults, use of specific prescription medications, and increased incidence of organ transplantation.1,7,8,15-19
The body’s underexcretion or overproduction of uric acid (a byproduct of purine metabolism12) can lead to hyperuricemia. This condition, defined as a serum urate level exceeding 7.0 mg/dL in men or 6.0 mg/dL in women20,21 (levels above 9.0 mg/dL are considered very high22), is the primary risk factor for gout.8,23,24 As with gout, the incidence of hyperuricemia has increased in recent years,20 with researchers attributing the trend to worldwide popularization of the Westernized diet (particularly use of high-fructose corn syrup20,25) and increased use of certain medications, including thiazide diuretics, cyclosporine, and low-dose aspirin.2,20,25,26
As serum urate levels rise, the patient with hyperuricemia may experience urate supersaturation, often followed by crystallization of the excess urate into monosodium urate (MSU) crystals. Subsequently, circulating MSU crystals may deposit in body tissues, especially in the joint spaces. The body’s ensuing inflammatory response to the MSU deposits is gout.20
In addition to hyperuricemia, risk factors for gout include a high-purine diet, habitual alcohol consumption (especially beer and fortified wines27), diuretic therapy (particularly in patients with heart failure or renal insufficiency), obesity, hypertension, and high levels of fructose consumption.7,28 Additionally, cyclosporine use in an organ transplant recipient, poorly controlled uric acid levels, and a long history of gout increase the patient’s risk for chronic tophaceous gout.24,29 Tophi may be more common in a patient with a history of organ transplantation.16
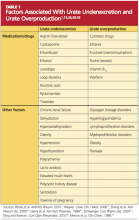
Genetic variants are currently being investigated to possibly identify a predisposition to gout. The most significant genetic factors appear to involve mechanisms that regulate serum uric acid levels—particularly urate underexcretion.23 Other factors that contribute to underexcretion or overproduction of uric acid are shown in Table 1.1,15,26,30-33
A dynamic relationship exists between gout and a number of pathologic processes. According to researchers investigating nearly 178,000 patients with gout in a managed care database, 36% had hypertension, 27% had dyslipidemia, and 15% had diabetes.8 In a smaller cohort study conducted in Spain and Mexico, it was demonstrated that 93% of patients with gout had one or more associated diseases, in order of decreasing frequency: hypertriglyceridemia, obesity, hypertension, metabolic syndrome, hyperglycemia, chronic renal failure, diabetes, and ischemic heart disease.3
Of particular clinical importance in this study was a finding that the first gout attack generally preceded the diagnosis of the associated diseases.3 Thus, a diagnosis of gout should lead the primary care provider to discuss modifiable risk factors with the patient—but also to investigate for comorbid illnesses that may require timely management.2
In a 12-year-long prospective study of more than 50,000 men participating in the Health Professionals Follow-Up Study,9 it was found that men with gout had a 28% increased risk for all-cause mortality, a 38% increased risk for CVD-related death, and a 55% increased risk for CHD-related death, compared with men who did not have gout (excluding other risk factors).9 Similarly, researchers for the Multiple Risk Factor Intervention Trial10 demonstrated a clinically significant association between gout and an increased risk for AMI: 10.5% of men with gout, compared with 8.43% of men without gout, had an AMI during mean follow-up of 6.5 years.10
THE STAGES
The four stages of gout are asymptomatic hyperuricemia, acute gout, intercritical gout, and chronic tophaceous gout.20
Only a small percentage (0.5% to 4.5%) of patients with asymptomatic hyperuricemia will develop acute gout.28 Nevertheless, any patient with serum urate greater than 6.8 mg/dL is at risk for the deposition of MSU crystals into body tissues and the potential associated organ damage—even patients without symptoms. There is currently no evidence-based method to determine which patients with asymptomatic hyperuricemia will experience disease progression.16
Acute gout develops when deposition of MSU crystals in the joints initiates an inflammatory response. In the typical history, the patient experiences sudden-onset severe pain, swelling, and erythema. The pain often starts in the middle of the night or early morning,34 waking the patient from sleep and peaking within 24 hours of onset. At this time, the patient is often unable to bear weight comfortably on the affected joint. The patient may also report fever and flu-like malaise resulting from the release of interleukin 1- (IL-1), IL-1 receptor, cytokines, and prostaglandins.16,24,35 Usually in these early attacks, symptoms resolve spontaneously within three to 14 days.16,24
After resolution of an acute attack, the patient enters the intercritical stage, another asymptomatic stage that may last for months or years—or indefinitely. During the intercritical stage, MSU crystal deposition continues, adding crystals in and around the affected joint or joints, possibly continuing to inflict damage (in some patients, substantial), and in many cases resulting in additional attacks and pain.16 Any subsequent acute gout attacks the patient may experience are likely to last longer than the initial attack and to involve additional joints or tendons.24
Some patients, especially those who do not receive adequate treatment for hyperuricemia,2 progress to develop chronic tophaceous gout. This is a deforming disease process in which the joints may become stiff and swollen, and subcutaneous nodules or whitish-yellow intradermal deposits may be present under taut skin, anywhere in the body.16
PATIENT PRESENTATION AND HISTORY
Typically, a patient with gout will present with a chief complaint of a painful, tender, inflamed joint (classically described in Latin as calor, rubor, dolor, et tumor6). However, clinicians must also be aware of unusual presentations and consider gout in the differential whenever a patient with a history of gout or pertinent risk factors presents with unexplained clinical findings.32 The history of present illness will vary according to the stage of the disease.
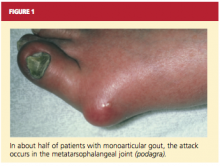
About 90% of recognized initial attacks of gout are monoarticular, usually occurring in one of the lower extremities.16 While the first metatarsophalangeal (MTP) joint is affected in about 50% of gout cases (podagra, the Greek term for gout),2,35 eventually patients with gout have a 90% chance of involvement with the MTP joint (see Figure 1). According to Zhang et al,26 patients with hyperuricemia and an affected MTP joint have an 82% chance of having gout.2,26
Because such a large proportion of patients have the classic presentation of rapid-onset warmth, redness, and tenderness at the MTP, knee, or ankle and surrounding soft tissue, cases with a differing presentation are likely to be misdiagnosed or overlooked, or a correct diagnosis is delayed.26 In many documented cases, gout was the ultimate diagnosis—but one that was reached only incidentally because of unusual clinical presentation, ranging from entrapment neuropathy to a pancreatic mass.32
The presence of hyperuricemia and other risk factors must be investigated. Also relevant in the history of an acute gout attack may be a preceding event that has caused damage or stress to the joint, such as infection, trauma, or surgery. Other possible triggers for an attack include alcohol ingestion, acidosis, use of IV contrast media, diuretic therapy, chemotherapy, recent hospitalization or surgery, and initiation or termination of urate-lowering therapy with the xanthine oxidase inhibitor allopurinol.2,30 According to Primatesta et al,8 the risk for flares is increased in patients with cardiometabolic comorbidities.
The medication history of a patient with gout may include low-dose aspirin (but not standard-dose aspirin, which is uricosuric2), diuretics, cyclosporine, cytotoxic agents, and vitamin B12, which may contribute to hyperuricemia.24 Additionally, ethambutol, pyrazinamide, levodopa, nicotinic acid, didanosine, niacin, and warfarin may raise uric acid levels.15,30,33
Medical history should include a thorough assessment of the comorbidities associated with gout. In addition to the conditions mentioned previously, patients with a history of polycystic kidney disease, dehydration, lactic acidosis, hyperparathyroidism, toxemia of pregnancy, hypothyroidism, or sarcoidosis may have elevated urate levels due to underexcretion—and thus may be vulnerable to gout.30 History of gout in a first-degree relative is associated with an increased risk for gout.36
The social history should address alcohol use or abuse. The clinician should also inquire about how gout is impacting the daily life of the patient. Diet and exercise habits should be assessed37 (see “Patient Education,” below).
PHYSICAL EXAMINATION
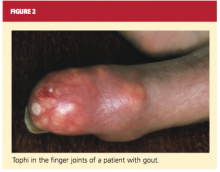
The physical exam begins with evaluation of the skin and extremities for the classic features of gout. Affected joints will be exquisitely tender, and patients may be febrile. Most cases are monoarticular, but polyarticular involvement is likely in patients with advanced disease (and can, particularly in women, be mistaken for rheumatoid arthritis).2,16 In patients with chronic tophaceous gout, there may be whitish-yellow skin deposits, subcutaneous nodules, and areas of taut skin. The lower-extremity joints and tendons, as well as the wrists, fingers, and elbows, are commonly affected16 (see Figure 2).
DIAGNOSIS
Diagnostic criteria that are currently available (and have long been in use) include the American College of Rheumatology/American Rheumatism Association (ACR/ARA) preliminary criteria,34 the New York criteria,21 and the Rome criteria.38 The specifics of each are listed in Table 2.21,34,38,39
The gold standard for gout diagnosis is detection of MSU crystals in a sample of synovial fluid aspirated from the affected joint or from a tophus and examined by polarized light microscopy.24 This is of significant importance to the clinician who is faced with a questionable diagnosis.16 However, crystal visualization is not ordinarily available to the primary care clinician,2,17,40 and it is not always necessary if a careful history and physical exam are conducted in a patient with hyperuricemia or other risk factors for gout. A presumptive diagnosis may be acceptable in a patient with the classic presentation of acute gout: rapid onset of severe pain in a swollen, erythematous joint and symptoms peaking within 24 hours. The presence of tophi is pathognomonic for chronic tophaceous gout.41
In cases of questionable or unusual manifestation of gout, however, various imaging techniques and crystal visualization may be indicated.32
In order to compare the effectiveness of the latter technique with conventional diagnostic criteria for gout, Malik et al39 conducted a pilot study involving 82 patients who had undergone synovial fluid analysis with polarized light microscopy. Patients were surveyed about the clinical features of their disease, as listed in the three standard sets of criteria for diagnosis of gout. Compared with the “gold standard” of urate crystal detection (which is one of the Rome criteria38), the study authors found the ACR/ARA preliminary criteria,34 the New York criteria,21 and the Rome criteria38 generally unsatisfactory.
In the study, among patients with confirmed presence of MSU crystals:
• 87% reported more than one attack of acute arthritis (ACR/ARA34)
• 86% reported monoarthritis attack (ACR/ARA34)
• 89% had hyperuricemia (ACR/ARA34 and Rome,38 with the latter giving effective, specific parameters)
• 100% had negative results on joint fluid culture (ACR/ARA34)
• 90% reported an attack starting at night (ACR/ARA34).
The positive predictive values for these signs and symptoms are 38%, 39%, 74%, 50%, and 45%, respectively, according to Malik et al.39 The presence of tophi (cited by all three sets of criteria but “proven or suspected” in the ACR/ARA34) had the highest positive predictive value for gout (91%) and a likelihood ratio of 15.56, which was at least three times higher than any of the other listed criteria. A verified response to colchicine, one of the New York criteria,21 had the second highest positive predictive value at 86%.39
In summary, the ACR/ARA,34 the New York,21 and the Rome criteria38 had specificity of 79%, 83%, and 89%, respectively; sensitivity of 70%, 70%, and 67%, respectively; and positive predictive values for gout of 66%, 70%, and 77%, respectively. The Rome criteria38 had the highest specificity and highest positive predictive value, perhaps making them most helpful for clinicians who lack access to synovial fluid analysis.
DIFFERENTIAL DIAGNOSIS
Conditions to be considered and ruled out before a diagnosis of gout can be made are:
• Pseudogout
• Septic arthritis
• Psoriatic arthritis
• Rheumatoid arthritis
• Erosive osteoarthritis
• Bacterial cellulitis
• Sarcoid arthropathy.16,28,42,43
Unlike gout (in which compensated polarized light microscopy reveals needle-shaped urate crystals with strong negative birefringence), pseudogout is characterized by calcium pyrophosphate dihydrate crystals; these are rhomboid-shaped, with weak positive birefringence.42 Additionally, radiographic imaging will reveal soft tissue swelling and chondrocalcinosis of the joint in pseudogout.44
The patient with septic arthritis, most likely affecting the knee, will have a white blood cell (WBC) count exceeding 50,000/mm3 and a positive culture of the synovial fluid, with absence of crystals.28
Chronic tophaceous gout can mimic rheumatoid arthritis in appearance and joint distribution, and patients affected by either condition may develop a positive rheumatoid factor. Examination of synovial fluid for MSU crystals and radiographic imaging will be of value in making a distinction.
Osteoarthritis is usually evidenced by joint space narrowing on x-ray.42
Bacterial cellulitis will present similarly to gout, but the erythema of bacterial cellulitis will more likely extend beyond the involved joint.16
Sarcoid arthropathy often presents as a polyarthritis, as in advanced gouty arthritis. However, in sarcoid arthropathy, serum calcium and angiotensin-converting enzyme will likely be elevated.43 Synovial or tendon sheath biopsy will show non-caseating granulomas, which are the hallmark for sarcoid disease. Additionally, joint fluid analysis will demonstrate a predominance of mononuclear or polymorphonuclear cells.43
Diagnostic Tests
Diagnostic tests to consider are analysis and culture of the synovial fluid, complete blood count (CBC), blood urea nitrogen (BUN), creatinine, radiography, ultrasonography, serum uric acid, and blood culture if septic arthritis is suspected.28 While serum urate levels may be normal during an acute gout attack, measurement may still be helpful for comparison, since elevation is a likely finding two weeks after an attack—if the patient was, in fact, experiencing an acute gout attack.42
Since renal dialysis increases the risk for gout, pseudogout, and septic arthritis, synovial fluid analysis is essential in patients undergoing renal dialysis.42
Various imaging techniques may aid in confirming a diagnosis of gout and monitoring its progression, but further studies are needed to more clearly define the role of these techniques in management of gout.45 Plain radiographic evidence of asymmetric swelling in a joint (one of the ACR/ARA preliminary criteria34) was shown to have a 60% positive predictive value for a diagnosis of gout.39 Late in the disease process, an affected joint may be affected by characteristic “punched out” intra-articular lesions, with a normal amount of joint space.45
Ultrasound is a safe and inexpensive test that can reveal soft tissue edema and increased vascularity during an acute gout attack. Chronic changes include the double contour sign and tophus-like lesions surrounded by a thin, anechoic rim.45
CT will also show tophi and bony erosion. While CT is more specific than other techniques, it is also more expensive and exposes the patient to increased radiation. MRI can help monitor the complications of gout, especially entrapment neuropathies.45
TREATMENT/MANAGEMENT
According to current evidence, treatment is not indicated for asymptomatic hyperuricemia.16
Acute Gout Management
Pharmacologic treatments available for an acute gout attack include NSAIDs, colchicine, and local or systemic corticosteroids.24,46 At the onset of an attack, patients should start high-dose NSAID therapy, and continue for two to three days after symptoms are resolved.6 Oral indomethacin (50 mg tid) or oral ibuprofen (800 mg tid) are both reasonable options.6 It may be prudent to consider a proton pump inhibitor (eg, omeprazole) to protect the gastric mucosa in patients who are susceptible to gastrointestinal problems.27
In addition to high-dose NSAID therapy, adding colchicine (1.2 mg by mouth at onset of symptoms, followed by 0.6 mg one hour later) has proven to be effective in relieving the symptoms of gout, but its serious gastrointestinal adverse effects, particularly diarrhea, must be considered.6,47
In patients with monoarticular gout who cannot tolerate NSAIDs, intra-articular aspiration and corticosteroid injections may provide relief.
Long-acting triamcinolone, administered by intra-articular injection, has been found to relieve pain and inflammation in patients with gout. Septic arthritis must be ruled out by way of joint aspiration and culture before injection of corticosteroids.47
Oral or IM-administered corticosteroids may be considered for patients with polyarticular involvement. Prednisone (60 mg/d, tapered over 10 days) is an appropriate option for outpatients or inpatients; methylprednisone (80 to 120 mg IM) may be suitable for inpatients.6 Again, septic arthritis must be ruled out before corticosteroids are administered.47
For the patient who is currently taking a thiazide diuretic for hypertension, substituting a different medication may be warranted; the angiotensin receptor blocker losartan, for example, has uricosuric action.27,29,47 Nonpharmacologic strategies, such as rest, ice, elevation, and avoiding trauma to the affected joint, are also recommended.27
Of note, allopurinol therapy should be neither initiated nor discontinued during an acute gout attack.27
Management of Chronic and Intercritical Gout
Urate-lowering therapy, such as allopurinol (50 to 300 mg/d29), should be considered for patients who experience frequent attacks (ie, three or more per year), patients with chronic tophi, patients with radiographically demonstrated joint damage,47 or patients with a documented state of uric acid overproduction.29
Allopurinol dosage should be adjusted based on creatinine clearance; dosing as high as 800 mg/d has been recommended in patients with normal renal function.2 Again, allopurinol should never be started or discontinued during an acute attack,27 because abrupt fluctuations in uric acid levels may heighten the inflammation. The target serum urate level is 6.0 mg/dL.29
Febuxostat, which received FDA approval in 2009, was the first oral urate-lowering treatment to be approved since the 1960s. Like allopurinol, this nonpurine xanthine oxidase inhibitor blocks uric acid synthesis.48,49 In a trial reported by Becker et al,50 67% of patients who took febuxostat 80 mg/d reached the target serum urate level (ie, < 6.0 mg/dL), compared with 45% of those who took 40 mg/d of febuxostat and 42% of those taking 300 mg/d of allopurinol. While incidence of adverse events was low in all treatment groups, Hu and Tomlinson51 report that febuxostat is tolerable in patients who are hypersensitive to allopurinol. As with other urate-lowering medications, gout flares are common during the early period of febuxostat use.51
For patients with gout that does not respond to conventional urate-lowering therapy, new options are being introduced. Two agents, each a recombinant form of the enzyme urate oxidase, are designed to convert uric acid into allantoin, which can then be excreted in the urine. Late in 2010, one of these agents, pegloticase, was approved for use in patients with refractory gout.48 In one clinical trial, tophi were reported dissolved in 40% of patients who took pegloticase, but 58% of patients did not achieve the targeted response (ie, serum urate < 6.0 mg/dL), and 77% of patients experienced gout flares.52 Infusion reactions occurred in 26% to 31% of patients, and Reinders and Jansen52 recommended the clinical evaluation of glucocorticoids and other anti-inflammatory agents to prevent the formation of antibodies involved in these reactions.
The second agent, rasburicase, has been approved for treatment and prevention of acute hyperuricemia in adult cancer patients. Rasburicase is now being investigated for use in patients with nonresponsive tophaceous gout.53-55 It can be administered in the form of monthly infusions.54
Patient Education
Educating the patient about modifiable risk factors, such as diet, alcohol consumption, and adherence to the medication regimen, should be a priority.
Patients should be encouraged to target and maintain an ideal body weight, through diet and moderate physical exercise, as a strategy to normalize serum urate levels.27,47 However, they should be advised to avoid “crash dieting,” as this may precipitate a gout attack.27 In the recommended low-purine diet, consumption of red meat and shellfish is restricted,17 whereas consumption of soy, nonfat milk and other low-fat dairy products, cherries and other fruits, and increased vegetable protein is encouraged.31,37 Consumption of alcohol, especially beer and fortified wines, should be limited.27,47
Avoiding trauma to joints affected by gout (including the stress of bearing excess weight) can help patients limit future attacks.7,27
CONCLUSION
Patients with gout often have the characteristic presentation of an acutely tender, inflamed joint, but since gout is a systemic disorder, the clinician must also consider the possibility of gout in almost any organ system. Gout is a common disease, and its diagnosis can alert the astute clinician to investigate for certain metabolic disorders requiring intervention. Hyperlipidemia, metabolic syndrome, hypertension, chronic kidney disease, obesity, cardiovascular disease, and diabetes are all conditions associated with gout.
Recognizing the opportunity to offer preventive care measures and recommend lifestyle modifications to the patient with gout allows the clinician to play an important role in the patient’s care.
REFERENCES
1. Bhole V, de Vera M, Rahman MM, et al. Epidemiology of gout in women: fifty-two–year followup of a prospective cohort. Arthritis Rheum. 2010;62(4):1069-1076.
2. Neogi T. Clinical practice: gout. N Engl J Med. 2011;364(5):443-452.
3. Hernández-Cuevas CB, Roque LH, Huerta-Sil G, et al. First acute gout attacks commonly precede features of the metabolic syndrome. J Clin Rheumatol. 2009;15(2):65-67.
4. Louthrenoo W, Kasitanon N, Sukitawut W, Wichainun R. A clinical study of crystal-proven gouty arthritis in a university hospital. J Med Assoc Thai. 2003;86(9):868-875.
5. De Souza AW, Fernandes V, Ferrari AJ. Female gout: clinical and laboratory features. J Rheumatol. 2005;32(11):2186-2188.
6. Kurakula PC, Keenan RT. Diagnosis and management of gout: an update. J Musculoskel Med. 2010;27(10). www.musculoskeletalnet work.com/display/article/1145622/1692895. Accessed June 14, 2011.
7. Choi HK, Atkinson K, Karlson EW, Curhan G. Obesity, weight change, hypertension, diuretic use, and risk of gout in men: the Health Professionals Follow-up Study. Arch Intern Med. 2005;165(7):742-748.
8. Primatesta P, Plana E, Rothenbacher D. Gout treatment and comorbidities: a retrospective cohort study in a large US managed care population. BMC Musculoskelet Disord. 2011 May 20;12(1):103. [Epub ahead of print]
9. Choi HK, Curhan G. Independent impact of gout on mortality and risk for coronary heart disease. Circulation. 2007;116(8):894-900.
10. Krishnan E, Baker JF, Furst DE, Schumacher HR. Gout and the risk of acute myocardial infarction. Arthritis Rheum. 2006;54(8):2688-2696.
11. Lawrence RC, Felson DT, Helmick CG, et al; National Arthritis Data Workgroup. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58(1):26-35.
12. CDC. Gout. www.cdc.gov/arthritis/basics/gout.htm. Accessed June 14, 2011.
13. Arromdee E, Michet CJ, Crowson CS, et al. Epidemiology of gout: is the incidence rising? J Rheumatol. 2002;29(11):2403-2406.
14. Wallace KL, Riedel AA, Joseph-Ridge N, Wortmann R. Increasing prevalence of gout and hyperuricemia over 10 years among older adults in a managed care population. J Rheumatol. 2004; 31(8):1582-1587.
15. Weaver AL. Epidemiology of gout. Cleve Clin J Med. 2008;75 suppl 5:S9-S12.
16. Mandell BF. Clinical manifestations of hyperuricemia and gout. Cleve Clin J Med. 2008;75 suppl 5:S5-S8.
17. Choi HK, Atkinson K, Karlson EW, et al. Purine-rich foods, dairy and protein intake, and the risk of gout in men. N Engl J Med. 2004;350(11):1093-1103.
18. Demarco MA, Maynard JW, Huizinga MM, et al. Younger age at gout onset is related to obesity in a community-based cohort. Arthritis Care Res (Hoboken). 2011 Apr 11; [Epub ahead of print].
19. Brook RA, Forsythe A, Smeeding JE, Lawrence Edwards N. Chronic gout: epidemiology, disease progression treatment and disease burden. Curr Med Res Opin. 2010;26(12):2813-2821.
20. Sachs L, Batra KL, Zimmermann B. Medical implications of hyperuricemia. Med Health R I. 2009;92(11):353-355.
21. Kellgren JH, Jeffrey MR, Ball J, eds. The Epidemiology of Chronic Rheumatism: Atlas of Standard Radiographs of Arthritis. Oxford: Blackwell; 1963:327.
22. Wu EQ, Patel PA, Mody RR, et al. Frequency, risk, and cost of gout-related episodes among the elderly: does serum uric acid level matter? J Rheumatol. 2009;36(5):1032-1040.
23. Riches PL, Wright AF, Ralston SH. Recent insights into the pathogenesis of hyperuricaemia and gout. Hum Mol Genet. 2009;18(R2):R177-R184.
24. Schumacher HR Jr. The pathogenesis of gout. Cleve Clin J Med. 2008;75 suppl 5:S2-S4.
25. Nakagawa T, Hu H, Zharikov S, et al. A causal role for uric acid in fructose-induced metabolic syndrome. Am J Physiol Renal Physiol. 2006;290(3):F625-F631.
26. Zhang W, Doherty M, Pascual E, et al; EULAR (European League Against Rheumatism) Standing Committee for International Clinical Studies Including Therapeutics. EULAR evidence based recommendations for gout. Part I: Diagnosis. Ann Rheum Dis. 2006;65(10):1301-1311.
27. Jordan KM, Cameron JS, Snaith M, et al. British Society for Rheumatology and British Health Professionals in Rheumatology guideline for the management of gout. Rheumatology. 2007;46(8):1372-1374.
28. Eggebeen AT. Gout: an update. Am Fam Physician. 2007;76(6):801-808.
29. Terkeltaub RA. Gout. N Engl J Med. 2003; 349(17):1647-1655.
30. Harris MD, Siegel LB, Alloway JA. Gout and hyperuricemia. Am Fam Physician. 1999;59(4): 925-934.
31. Schlesinger N. Dietary factors and hyperuricemia. Curr Pharm Des. 2005;11(32):4133-4138.
32. Ning TC, Keenan RT. Unusual presentations of gout. Curr Opin Rheumatol. 2010;22(2): 181-187.
33. Menon RK, Mikhailidis DP, Bell JL, et al. Warfarin administration increases uric acid concentrations in plasma. Clin Chem. 1986;32(8):
1557-1559.
34. Wallace SL, Robinson H, Masi AT, et al. Preliminary criteria for the classification of the acute arthritis of primary gout. Arthritis Rheum. 1977;20(3):895-900.
35. Martinon F, Glincher LH. Gout: new insights into an old disease. J Clin Invest. 2006;116 (8):2073-2075.
36. Zampogna G, Andracco R, Parodi M, Cimmino MA. Clinical features of gout in a cohort of Italian patients [in Italian]. Reumatismo. 2009; 61(1):41-47.
37. Choi HK. A prescription for lifestyle change in patients with hyperuricemia and gout. Curr Opin Rheumatol. 2010;22(2):165-172.
38. Bennett PH, Wood PH, eds. Population studies of the rheumatic diseases: proceedings of the Third International Symposium; June 5-10, 1966; New York, NY. Amsterdam: Excerpta Medica Foundation; 1968:457-458.
39. Malik A, Schumacher HR, Dinnella JE, Clayburne GM. Clinical diagnostic criteria for gout: comparison with the gold standard of synovial fluid crystal analysis. J Clin Rheumatol. 2009;15 (1):22-24.
40. Wijnands JMA, Boonen A, Arts ICW, et al. Large epidemiologic studies of gout: challenges in diagnosis and diagnostic criteria. Curr Rheumatol Rep. 2011;13(2):167-174.
41. Dodd LG, Major NM. Fine-needle aspiration cytology of articular and periarticular lesions. Cancer. 2002;96(3):157-165.
42. Dore RK. The gout diagnosis. Cleve Clin J Med. 2008;75 suppl 5:S17-S21.
43. Pettersson T. Sarcoid and erythema nodosum arthropathies. Baillieres Best Pract Res Clin Rheumatol. 2000;14(3):461-476.
44. Córdoba-Fernández A, Rayo-Rosado R. Pseudogout of the first metatarsophalangeal joint associated with hallux valgus: an atypical bilateral case. J Am Podiatr Med Assoc. 2010;100(2):138-142.
45. Dalbeth N, McQueen FM. Use of imaging to evaluate gout and other crystal deposition disorders. Curr Opin Rheumatol. 2009;21(2):124-131.
46. Wu EQ, Forsythe A, Guérin A, et al. Comorbidity burden healthcare resource utilization, and costs in chronic gout patients refractory to conventional urate-lowering therapy. Am J Ther. 2011 Feb 10; [Epub ahead of print].
47. Zhang W, Doherty M, Pascual E, et al; EULAR (European League Against Rheumatism) Standing Committee for International Clinical Studies Including Therapeutics. EULAR evidence based recommendations for gout. Part II: Management. Ann Rheum Dis. 2006;65(10):1312-1324.
48. Schlesinger N, Yasothan U, Kirkpatrick P. Pegloticase [published correction appears in Nat Rev Drug Discov. 2011;10(2):156]. Nat Rev Drug Discov. 2011;10(1):17-18.
49. Pascual E, Sivera F, Yasothan U, Kurkpatrick P. Febuxostat. Nat Rev Drug Discov. 2009;8(3): 191-192.
50. Becker MA, Schumacher HR, Espinoza LR, et al. The urate-lowering efficacy and safety of febuxostat in the treatment of the hyperuricemia of gout; the CONFIRMS trial. Arthritis Res Ther. 2010;12(2):R63.
51. Hu M, Tomlinson B. Febuxostat in the management of hyperuricemia and chronic gout: a review. Ther Clin Risk Manag. 2008;4(6):1209-1220.
52. Reinders MK, Jansen TL. New advances in the treatment of gout: review of pegloticase. Ther Clin Risk Manag. 2010;6:543-550.
53. Cammalleri L, Malaguarnera M. Rasburicase represents a new tool for hyperuricemia in tumor lysis syndrome and in gout. Int J Med Sci. 2007; 4(2):83-93.
54. Richette P, Brière C, Hoenen-Clavert V, et al. Rasburicase for topaceous gout not treatable with allopurinol: an exploratory study. J Rheumatol. 2007;34(10):2093-2098.
55. Moolenburgh JD, Reinders MK, Jansen TL. Rasburicase treatment in severe tophaceous gout: a novel therapeutic option. Clin Rheumatol. 2006;25(5):749-752.
Gouty arthritis is a common form of inflammatory arthritis, occurring more frequently in men than in women. The condition has a male–female ratio of 3 or 4 to 1, although that ratio narrows as adults age; because the uricosuric effects of estrogen decline with menopause, the risk for gout increases in postmenopausal women.1,2 Mean age at disease onset is 40 to 60 in men,3,4 with onset in women averaging seven years later.5
Apart from the pain and loss of function associated with this disorder of purine metabolism6 and the risk for a chronic form of the disease, gout is almost universally linked with serious comorbidities that require timely intervention. These include hypertension, dyslipidemia, hyperglycemia and diabetes, obesity, metabolic syndrome, cardiovascular disease (CVD), renal insufficiency, and coronary heart disease (CHD).2,3,7 The presence of gout is independently associated with a risk for acute myocardial infarction (AMI) and increased rates of all-cause mortality.3,8-10
EPIDEMIOLOGY
In 2007, the National Arthritis Data Workgroup11,12 estimated that about three million US adults had had “self-reported gout” in the previous year. An estimated six million US adults have been diagnosed with gout,2,11 and its incidence and prevalence are increasing. The incidence of primary gout more than doubled between 1977-1978 and 1995-1996,13 especially affecting the aging population. The prevalence of gout among 1,000 managed care patients ages 65 to 74 increased by at least 30% between 1990 and 1999, while prevalence among those older than 75 almost doubled during this same period.14
Numerous factors appear to contribute to these trends, including aging of the population, dietary trends (ie, increased consumption of red meat, organ food, game, and shellfish and reduced consumption of low-fat dairy products), presence of certain comorbid conditions (ie, hypertension, dyslipidemia, diabetes, metabolic syndrome, end-stage renal disease), the increasing prevalence of obesity in younger adults, use of specific prescription medications, and increased incidence of organ transplantation.1,7,8,15-19
The body’s underexcretion or overproduction of uric acid (a byproduct of purine metabolism12) can lead to hyperuricemia. This condition, defined as a serum urate level exceeding 7.0 mg/dL in men or 6.0 mg/dL in women20,21 (levels above 9.0 mg/dL are considered very high22), is the primary risk factor for gout.8,23,24 As with gout, the incidence of hyperuricemia has increased in recent years,20 with researchers attributing the trend to worldwide popularization of the Westernized diet (particularly use of high-fructose corn syrup20,25) and increased use of certain medications, including thiazide diuretics, cyclosporine, and low-dose aspirin.2,20,25,26
As serum urate levels rise, the patient with hyperuricemia may experience urate supersaturation, often followed by crystallization of the excess urate into monosodium urate (MSU) crystals. Subsequently, circulating MSU crystals may deposit in body tissues, especially in the joint spaces. The body’s ensuing inflammatory response to the MSU deposits is gout.20
In addition to hyperuricemia, risk factors for gout include a high-purine diet, habitual alcohol consumption (especially beer and fortified wines27), diuretic therapy (particularly in patients with heart failure or renal insufficiency), obesity, hypertension, and high levels of fructose consumption.7,28 Additionally, cyclosporine use in an organ transplant recipient, poorly controlled uric acid levels, and a long history of gout increase the patient’s risk for chronic tophaceous gout.24,29 Tophi may be more common in a patient with a history of organ transplantation.16
Genetic variants are currently being investigated to possibly identify a predisposition to gout. The most significant genetic factors appear to involve mechanisms that regulate serum uric acid levels—particularly urate underexcretion.23 Other factors that contribute to underexcretion or overproduction of uric acid are shown in Table 1.1,15,26,30-33
A dynamic relationship exists between gout and a number of pathologic processes. According to researchers investigating nearly 178,000 patients with gout in a managed care database, 36% had hypertension, 27% had dyslipidemia, and 15% had diabetes.8 In a smaller cohort study conducted in Spain and Mexico, it was demonstrated that 93% of patients with gout had one or more associated diseases, in order of decreasing frequency: hypertriglyceridemia, obesity, hypertension, metabolic syndrome, hyperglycemia, chronic renal failure, diabetes, and ischemic heart disease.3
Of particular clinical importance in this study was a finding that the first gout attack generally preceded the diagnosis of the associated diseases.3 Thus, a diagnosis of gout should lead the primary care provider to discuss modifiable risk factors with the patient—but also to investigate for comorbid illnesses that may require timely management.2
In a 12-year-long prospective study of more than 50,000 men participating in the Health Professionals Follow-Up Study,9 it was found that men with gout had a 28% increased risk for all-cause mortality, a 38% increased risk for CVD-related death, and a 55% increased risk for CHD-related death, compared with men who did not have gout (excluding other risk factors).9 Similarly, researchers for the Multiple Risk Factor Intervention Trial10 demonstrated a clinically significant association between gout and an increased risk for AMI: 10.5% of men with gout, compared with 8.43% of men without gout, had an AMI during mean follow-up of 6.5 years.10
THE STAGES
The four stages of gout are asymptomatic hyperuricemia, acute gout, intercritical gout, and chronic tophaceous gout.20
Only a small percentage (0.5% to 4.5%) of patients with asymptomatic hyperuricemia will develop acute gout.28 Nevertheless, any patient with serum urate greater than 6.8 mg/dL is at risk for the deposition of MSU crystals into body tissues and the potential associated organ damage—even patients without symptoms. There is currently no evidence-based method to determine which patients with asymptomatic hyperuricemia will experience disease progression.16
Acute gout develops when deposition of MSU crystals in the joints initiates an inflammatory response. In the typical history, the patient experiences sudden-onset severe pain, swelling, and erythema. The pain often starts in the middle of the night or early morning,34 waking the patient from sleep and peaking within 24 hours of onset. At this time, the patient is often unable to bear weight comfortably on the affected joint. The patient may also report fever and flu-like malaise resulting from the release of interleukin 1- (IL-1), IL-1 receptor, cytokines, and prostaglandins.16,24,35 Usually in these early attacks, symptoms resolve spontaneously within three to 14 days.16,24
After resolution of an acute attack, the patient enters the intercritical stage, another asymptomatic stage that may last for months or years—or indefinitely. During the intercritical stage, MSU crystal deposition continues, adding crystals in and around the affected joint or joints, possibly continuing to inflict damage (in some patients, substantial), and in many cases resulting in additional attacks and pain.16 Any subsequent acute gout attacks the patient may experience are likely to last longer than the initial attack and to involve additional joints or tendons.24
Some patients, especially those who do not receive adequate treatment for hyperuricemia,2 progress to develop chronic tophaceous gout. This is a deforming disease process in which the joints may become stiff and swollen, and subcutaneous nodules or whitish-yellow intradermal deposits may be present under taut skin, anywhere in the body.16
PATIENT PRESENTATION AND HISTORY
Typically, a patient with gout will present with a chief complaint of a painful, tender, inflamed joint (classically described in Latin as calor, rubor, dolor, et tumor6). However, clinicians must also be aware of unusual presentations and consider gout in the differential whenever a patient with a history of gout or pertinent risk factors presents with unexplained clinical findings.32 The history of present illness will vary according to the stage of the disease.
About 90% of recognized initial attacks of gout are monoarticular, usually occurring in one of the lower extremities.16 While the first metatarsophalangeal (MTP) joint is affected in about 50% of gout cases (podagra, the Greek term for gout),2,35 eventually patients with gout have a 90% chance of involvement with the MTP joint (see Figure 1). According to Zhang et al,26 patients with hyperuricemia and an affected MTP joint have an 82% chance of having gout.2,26
Because such a large proportion of patients have the classic presentation of rapid-onset warmth, redness, and tenderness at the MTP, knee, or ankle and surrounding soft tissue, cases with a differing presentation are likely to be misdiagnosed or overlooked, or a correct diagnosis is delayed.26 In many documented cases, gout was the ultimate diagnosis—but one that was reached only incidentally because of unusual clinical presentation, ranging from entrapment neuropathy to a pancreatic mass.32
The presence of hyperuricemia and other risk factors must be investigated. Also relevant in the history of an acute gout attack may be a preceding event that has caused damage or stress to the joint, such as infection, trauma, or surgery. Other possible triggers for an attack include alcohol ingestion, acidosis, use of IV contrast media, diuretic therapy, chemotherapy, recent hospitalization or surgery, and initiation or termination of urate-lowering therapy with the xanthine oxidase inhibitor allopurinol.2,30 According to Primatesta et al,8 the risk for flares is increased in patients with cardiometabolic comorbidities.
The medication history of a patient with gout may include low-dose aspirin (but not standard-dose aspirin, which is uricosuric2), diuretics, cyclosporine, cytotoxic agents, and vitamin B12, which may contribute to hyperuricemia.24 Additionally, ethambutol, pyrazinamide, levodopa, nicotinic acid, didanosine, niacin, and warfarin may raise uric acid levels.15,30,33
Medical history should include a thorough assessment of the comorbidities associated with gout. In addition to the conditions mentioned previously, patients with a history of polycystic kidney disease, dehydration, lactic acidosis, hyperparathyroidism, toxemia of pregnancy, hypothyroidism, or sarcoidosis may have elevated urate levels due to underexcretion—and thus may be vulnerable to gout.30 History of gout in a first-degree relative is associated with an increased risk for gout.36
The social history should address alcohol use or abuse. The clinician should also inquire about how gout is impacting the daily life of the patient. Diet and exercise habits should be assessed37 (see “Patient Education,” below).
PHYSICAL EXAMINATION
The physical exam begins with evaluation of the skin and extremities for the classic features of gout. Affected joints will be exquisitely tender, and patients may be febrile. Most cases are monoarticular, but polyarticular involvement is likely in patients with advanced disease (and can, particularly in women, be mistaken for rheumatoid arthritis).2,16 In patients with chronic tophaceous gout, there may be whitish-yellow skin deposits, subcutaneous nodules, and areas of taut skin. The lower-extremity joints and tendons, as well as the wrists, fingers, and elbows, are commonly affected16 (see Figure 2).
DIAGNOSIS
Diagnostic criteria that are currently available (and have long been in use) include the American College of Rheumatology/American Rheumatism Association (ACR/ARA) preliminary criteria,34 the New York criteria,21 and the Rome criteria.38 The specifics of each are listed in Table 2.21,34,38,39
The gold standard for gout diagnosis is detection of MSU crystals in a sample of synovial fluid aspirated from the affected joint or from a tophus and examined by polarized light microscopy.24 This is of significant importance to the clinician who is faced with a questionable diagnosis.16 However, crystal visualization is not ordinarily available to the primary care clinician,2,17,40 and it is not always necessary if a careful history and physical exam are conducted in a patient with hyperuricemia or other risk factors for gout. A presumptive diagnosis may be acceptable in a patient with the classic presentation of acute gout: rapid onset of severe pain in a swollen, erythematous joint and symptoms peaking within 24 hours. The presence of tophi is pathognomonic for chronic tophaceous gout.41
In cases of questionable or unusual manifestation of gout, however, various imaging techniques and crystal visualization may be indicated.32
In order to compare the effectiveness of the latter technique with conventional diagnostic criteria for gout, Malik et al39 conducted a pilot study involving 82 patients who had undergone synovial fluid analysis with polarized light microscopy. Patients were surveyed about the clinical features of their disease, as listed in the three standard sets of criteria for diagnosis of gout. Compared with the “gold standard” of urate crystal detection (which is one of the Rome criteria38), the study authors found the ACR/ARA preliminary criteria,34 the New York criteria,21 and the Rome criteria38 generally unsatisfactory.
In the study, among patients with confirmed presence of MSU crystals:
• 87% reported more than one attack of acute arthritis (ACR/ARA34)
• 86% reported monoarthritis attack (ACR/ARA34)
• 89% had hyperuricemia (ACR/ARA34 and Rome,38 with the latter giving effective, specific parameters)
• 100% had negative results on joint fluid culture (ACR/ARA34)
• 90% reported an attack starting at night (ACR/ARA34).
The positive predictive values for these signs and symptoms are 38%, 39%, 74%, 50%, and 45%, respectively, according to Malik et al.39 The presence of tophi (cited by all three sets of criteria but “proven or suspected” in the ACR/ARA34) had the highest positive predictive value for gout (91%) and a likelihood ratio of 15.56, which was at least three times higher than any of the other listed criteria. A verified response to colchicine, one of the New York criteria,21 had the second highest positive predictive value at 86%.39
In summary, the ACR/ARA,34 the New York,21 and the Rome criteria38 had specificity of 79%, 83%, and 89%, respectively; sensitivity of 70%, 70%, and 67%, respectively; and positive predictive values for gout of 66%, 70%, and 77%, respectively. The Rome criteria38 had the highest specificity and highest positive predictive value, perhaps making them most helpful for clinicians who lack access to synovial fluid analysis.
DIFFERENTIAL DIAGNOSIS
Conditions to be considered and ruled out before a diagnosis of gout can be made are:
• Pseudogout
• Septic arthritis
• Psoriatic arthritis
• Rheumatoid arthritis
• Erosive osteoarthritis
• Bacterial cellulitis
• Sarcoid arthropathy.16,28,42,43
Unlike gout (in which compensated polarized light microscopy reveals needle-shaped urate crystals with strong negative birefringence), pseudogout is characterized by calcium pyrophosphate dihydrate crystals; these are rhomboid-shaped, with weak positive birefringence.42 Additionally, radiographic imaging will reveal soft tissue swelling and chondrocalcinosis of the joint in pseudogout.44
The patient with septic arthritis, most likely affecting the knee, will have a white blood cell (WBC) count exceeding 50,000/mm3 and a positive culture of the synovial fluid, with absence of crystals.28
Chronic tophaceous gout can mimic rheumatoid arthritis in appearance and joint distribution, and patients affected by either condition may develop a positive rheumatoid factor. Examination of synovial fluid for MSU crystals and radiographic imaging will be of value in making a distinction.
Osteoarthritis is usually evidenced by joint space narrowing on x-ray.42
Bacterial cellulitis will present similarly to gout, but the erythema of bacterial cellulitis will more likely extend beyond the involved joint.16
Sarcoid arthropathy often presents as a polyarthritis, as in advanced gouty arthritis. However, in sarcoid arthropathy, serum calcium and angiotensin-converting enzyme will likely be elevated.43 Synovial or tendon sheath biopsy will show non-caseating granulomas, which are the hallmark for sarcoid disease. Additionally, joint fluid analysis will demonstrate a predominance of mononuclear or polymorphonuclear cells.43
Diagnostic Tests
Diagnostic tests to consider are analysis and culture of the synovial fluid, complete blood count (CBC), blood urea nitrogen (BUN), creatinine, radiography, ultrasonography, serum uric acid, and blood culture if septic arthritis is suspected.28 While serum urate levels may be normal during an acute gout attack, measurement may still be helpful for comparison, since elevation is a likely finding two weeks after an attack—if the patient was, in fact, experiencing an acute gout attack.42
Since renal dialysis increases the risk for gout, pseudogout, and septic arthritis, synovial fluid analysis is essential in patients undergoing renal dialysis.42
Various imaging techniques may aid in confirming a diagnosis of gout and monitoring its progression, but further studies are needed to more clearly define the role of these techniques in management of gout.45 Plain radiographic evidence of asymmetric swelling in a joint (one of the ACR/ARA preliminary criteria34) was shown to have a 60% positive predictive value for a diagnosis of gout.39 Late in the disease process, an affected joint may be affected by characteristic “punched out” intra-articular lesions, with a normal amount of joint space.45
Ultrasound is a safe and inexpensive test that can reveal soft tissue edema and increased vascularity during an acute gout attack. Chronic changes include the double contour sign and tophus-like lesions surrounded by a thin, anechoic rim.45
CT will also show tophi and bony erosion. While CT is more specific than other techniques, it is also more expensive and exposes the patient to increased radiation. MRI can help monitor the complications of gout, especially entrapment neuropathies.45
TREATMENT/MANAGEMENT
According to current evidence, treatment is not indicated for asymptomatic hyperuricemia.16
Acute Gout Management
Pharmacologic treatments available for an acute gout attack include NSAIDs, colchicine, and local or systemic corticosteroids.24,46 At the onset of an attack, patients should start high-dose NSAID therapy, and continue for two to three days after symptoms are resolved.6 Oral indomethacin (50 mg tid) or oral ibuprofen (800 mg tid) are both reasonable options.6 It may be prudent to consider a proton pump inhibitor (eg, omeprazole) to protect the gastric mucosa in patients who are susceptible to gastrointestinal problems.27
In addition to high-dose NSAID therapy, adding colchicine (1.2 mg by mouth at onset of symptoms, followed by 0.6 mg one hour later) has proven to be effective in relieving the symptoms of gout, but its serious gastrointestinal adverse effects, particularly diarrhea, must be considered.6,47
In patients with monoarticular gout who cannot tolerate NSAIDs, intra-articular aspiration and corticosteroid injections may provide relief.
Long-acting triamcinolone, administered by intra-articular injection, has been found to relieve pain and inflammation in patients with gout. Septic arthritis must be ruled out by way of joint aspiration and culture before injection of corticosteroids.47
Oral or IM-administered corticosteroids may be considered for patients with polyarticular involvement. Prednisone (60 mg/d, tapered over 10 days) is an appropriate option for outpatients or inpatients; methylprednisone (80 to 120 mg IM) may be suitable for inpatients.6 Again, septic arthritis must be ruled out before corticosteroids are administered.47
For the patient who is currently taking a thiazide diuretic for hypertension, substituting a different medication may be warranted; the angiotensin receptor blocker losartan, for example, has uricosuric action.27,29,47 Nonpharmacologic strategies, such as rest, ice, elevation, and avoiding trauma to the affected joint, are also recommended.27
Of note, allopurinol therapy should be neither initiated nor discontinued during an acute gout attack.27
Management of Chronic and Intercritical Gout
Urate-lowering therapy, such as allopurinol (50 to 300 mg/d29), should be considered for patients who experience frequent attacks (ie, three or more per year), patients with chronic tophi, patients with radiographically demonstrated joint damage,47 or patients with a documented state of uric acid overproduction.29
Allopurinol dosage should be adjusted based on creatinine clearance; dosing as high as 800 mg/d has been recommended in patients with normal renal function.2 Again, allopurinol should never be started or discontinued during an acute attack,27 because abrupt fluctuations in uric acid levels may heighten the inflammation. The target serum urate level is 6.0 mg/dL.29
Febuxostat, which received FDA approval in 2009, was the first oral urate-lowering treatment to be approved since the 1960s. Like allopurinol, this nonpurine xanthine oxidase inhibitor blocks uric acid synthesis.48,49 In a trial reported by Becker et al,50 67% of patients who took febuxostat 80 mg/d reached the target serum urate level (ie, < 6.0 mg/dL), compared with 45% of those who took 40 mg/d of febuxostat and 42% of those taking 300 mg/d of allopurinol. While incidence of adverse events was low in all treatment groups, Hu and Tomlinson51 report that febuxostat is tolerable in patients who are hypersensitive to allopurinol. As with other urate-lowering medications, gout flares are common during the early period of febuxostat use.51
For patients with gout that does not respond to conventional urate-lowering therapy, new options are being introduced. Two agents, each a recombinant form of the enzyme urate oxidase, are designed to convert uric acid into allantoin, which can then be excreted in the urine. Late in 2010, one of these agents, pegloticase, was approved for use in patients with refractory gout.48 In one clinical trial, tophi were reported dissolved in 40% of patients who took pegloticase, but 58% of patients did not achieve the targeted response (ie, serum urate < 6.0 mg/dL), and 77% of patients experienced gout flares.52 Infusion reactions occurred in 26% to 31% of patients, and Reinders and Jansen52 recommended the clinical evaluation of glucocorticoids and other anti-inflammatory agents to prevent the formation of antibodies involved in these reactions.
The second agent, rasburicase, has been approved for treatment and prevention of acute hyperuricemia in adult cancer patients. Rasburicase is now being investigated for use in patients with nonresponsive tophaceous gout.53-55 It can be administered in the form of monthly infusions.54
Patient Education
Educating the patient about modifiable risk factors, such as diet, alcohol consumption, and adherence to the medication regimen, should be a priority.
Patients should be encouraged to target and maintain an ideal body weight, through diet and moderate physical exercise, as a strategy to normalize serum urate levels.27,47 However, they should be advised to avoid “crash dieting,” as this may precipitate a gout attack.27 In the recommended low-purine diet, consumption of red meat and shellfish is restricted,17 whereas consumption of soy, nonfat milk and other low-fat dairy products, cherries and other fruits, and increased vegetable protein is encouraged.31,37 Consumption of alcohol, especially beer and fortified wines, should be limited.27,47
Avoiding trauma to joints affected by gout (including the stress of bearing excess weight) can help patients limit future attacks.7,27
CONCLUSION
Patients with gout often have the characteristic presentation of an acutely tender, inflamed joint, but since gout is a systemic disorder, the clinician must also consider the possibility of gout in almost any organ system. Gout is a common disease, and its diagnosis can alert the astute clinician to investigate for certain metabolic disorders requiring intervention. Hyperlipidemia, metabolic syndrome, hypertension, chronic kidney disease, obesity, cardiovascular disease, and diabetes are all conditions associated with gout.
Recognizing the opportunity to offer preventive care measures and recommend lifestyle modifications to the patient with gout allows the clinician to play an important role in the patient’s care.
REFERENCES
1. Bhole V, de Vera M, Rahman MM, et al. Epidemiology of gout in women: fifty-two–year followup of a prospective cohort. Arthritis Rheum. 2010;62(4):1069-1076.
2. Neogi T. Clinical practice: gout. N Engl J Med. 2011;364(5):443-452.
3. Hernández-Cuevas CB, Roque LH, Huerta-Sil G, et al. First acute gout attacks commonly precede features of the metabolic syndrome. J Clin Rheumatol. 2009;15(2):65-67.
4. Louthrenoo W, Kasitanon N, Sukitawut W, Wichainun R. A clinical study of crystal-proven gouty arthritis in a university hospital. J Med Assoc Thai. 2003;86(9):868-875.
5. De Souza AW, Fernandes V, Ferrari AJ. Female gout: clinical and laboratory features. J Rheumatol. 2005;32(11):2186-2188.
6. Kurakula PC, Keenan RT. Diagnosis and management of gout: an update. J Musculoskel Med. 2010;27(10). www.musculoskeletalnet work.com/display/article/1145622/1692895. Accessed June 14, 2011.
7. Choi HK, Atkinson K, Karlson EW, Curhan G. Obesity, weight change, hypertension, diuretic use, and risk of gout in men: the Health Professionals Follow-up Study. Arch Intern Med. 2005;165(7):742-748.
8. Primatesta P, Plana E, Rothenbacher D. Gout treatment and comorbidities: a retrospective cohort study in a large US managed care population. BMC Musculoskelet Disord. 2011 May 20;12(1):103. [Epub ahead of print]
9. Choi HK, Curhan G. Independent impact of gout on mortality and risk for coronary heart disease. Circulation. 2007;116(8):894-900.
10. Krishnan E, Baker JF, Furst DE, Schumacher HR. Gout and the risk of acute myocardial infarction. Arthritis Rheum. 2006;54(8):2688-2696.
11. Lawrence RC, Felson DT, Helmick CG, et al; National Arthritis Data Workgroup. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58(1):26-35.
12. CDC. Gout. www.cdc.gov/arthritis/basics/gout.htm. Accessed June 14, 2011.
13. Arromdee E, Michet CJ, Crowson CS, et al. Epidemiology of gout: is the incidence rising? J Rheumatol. 2002;29(11):2403-2406.
14. Wallace KL, Riedel AA, Joseph-Ridge N, Wortmann R. Increasing prevalence of gout and hyperuricemia over 10 years among older adults in a managed care population. J Rheumatol. 2004; 31(8):1582-1587.
15. Weaver AL. Epidemiology of gout. Cleve Clin J Med. 2008;75 suppl 5:S9-S12.
16. Mandell BF. Clinical manifestations of hyperuricemia and gout. Cleve Clin J Med. 2008;75 suppl 5:S5-S8.
17. Choi HK, Atkinson K, Karlson EW, et al. Purine-rich foods, dairy and protein intake, and the risk of gout in men. N Engl J Med. 2004;350(11):1093-1103.
18. Demarco MA, Maynard JW, Huizinga MM, et al. Younger age at gout onset is related to obesity in a community-based cohort. Arthritis Care Res (Hoboken). 2011 Apr 11; [Epub ahead of print].
19. Brook RA, Forsythe A, Smeeding JE, Lawrence Edwards N. Chronic gout: epidemiology, disease progression treatment and disease burden. Curr Med Res Opin. 2010;26(12):2813-2821.
20. Sachs L, Batra KL, Zimmermann B. Medical implications of hyperuricemia. Med Health R I. 2009;92(11):353-355.
21. Kellgren JH, Jeffrey MR, Ball J, eds. The Epidemiology of Chronic Rheumatism: Atlas of Standard Radiographs of Arthritis. Oxford: Blackwell; 1963:327.
22. Wu EQ, Patel PA, Mody RR, et al. Frequency, risk, and cost of gout-related episodes among the elderly: does serum uric acid level matter? J Rheumatol. 2009;36(5):1032-1040.
23. Riches PL, Wright AF, Ralston SH. Recent insights into the pathogenesis of hyperuricaemia and gout. Hum Mol Genet. 2009;18(R2):R177-R184.
24. Schumacher HR Jr. The pathogenesis of gout. Cleve Clin J Med. 2008;75 suppl 5:S2-S4.
25. Nakagawa T, Hu H, Zharikov S, et al. A causal role for uric acid in fructose-induced metabolic syndrome. Am J Physiol Renal Physiol. 2006;290(3):F625-F631.
26. Zhang W, Doherty M, Pascual E, et al; EULAR (European League Against Rheumatism) Standing Committee for International Clinical Studies Including Therapeutics. EULAR evidence based recommendations for gout. Part I: Diagnosis. Ann Rheum Dis. 2006;65(10):1301-1311.
27. Jordan KM, Cameron JS, Snaith M, et al. British Society for Rheumatology and British Health Professionals in Rheumatology guideline for the management of gout. Rheumatology. 2007;46(8):1372-1374.
28. Eggebeen AT. Gout: an update. Am Fam Physician. 2007;76(6):801-808.
29. Terkeltaub RA. Gout. N Engl J Med. 2003; 349(17):1647-1655.
30. Harris MD, Siegel LB, Alloway JA. Gout and hyperuricemia. Am Fam Physician. 1999;59(4): 925-934.
31. Schlesinger N. Dietary factors and hyperuricemia. Curr Pharm Des. 2005;11(32):4133-4138.
32. Ning TC, Keenan RT. Unusual presentations of gout. Curr Opin Rheumatol. 2010;22(2): 181-187.
33. Menon RK, Mikhailidis DP, Bell JL, et al. Warfarin administration increases uric acid concentrations in plasma. Clin Chem. 1986;32(8):
1557-1559.
34. Wallace SL, Robinson H, Masi AT, et al. Preliminary criteria for the classification of the acute arthritis of primary gout. Arthritis Rheum. 1977;20(3):895-900.
35. Martinon F, Glincher LH. Gout: new insights into an old disease. J Clin Invest. 2006;116 (8):2073-2075.
36. Zampogna G, Andracco R, Parodi M, Cimmino MA. Clinical features of gout in a cohort of Italian patients [in Italian]. Reumatismo. 2009; 61(1):41-47.
37. Choi HK. A prescription for lifestyle change in patients with hyperuricemia and gout. Curr Opin Rheumatol. 2010;22(2):165-172.
38. Bennett PH, Wood PH, eds. Population studies of the rheumatic diseases: proceedings of the Third International Symposium; June 5-10, 1966; New York, NY. Amsterdam: Excerpta Medica Foundation; 1968:457-458.
39. Malik A, Schumacher HR, Dinnella JE, Clayburne GM. Clinical diagnostic criteria for gout: comparison with the gold standard of synovial fluid crystal analysis. J Clin Rheumatol. 2009;15 (1):22-24.
40. Wijnands JMA, Boonen A, Arts ICW, et al. Large epidemiologic studies of gout: challenges in diagnosis and diagnostic criteria. Curr Rheumatol Rep. 2011;13(2):167-174.
41. Dodd LG, Major NM. Fine-needle aspiration cytology of articular and periarticular lesions. Cancer. 2002;96(3):157-165.
42. Dore RK. The gout diagnosis. Cleve Clin J Med. 2008;75 suppl 5:S17-S21.
43. Pettersson T. Sarcoid and erythema nodosum arthropathies. Baillieres Best Pract Res Clin Rheumatol. 2000;14(3):461-476.
44. Córdoba-Fernández A, Rayo-Rosado R. Pseudogout of the first metatarsophalangeal joint associated with hallux valgus: an atypical bilateral case. J Am Podiatr Med Assoc. 2010;100(2):138-142.
45. Dalbeth N, McQueen FM. Use of imaging to evaluate gout and other crystal deposition disorders. Curr Opin Rheumatol. 2009;21(2):124-131.
46. Wu EQ, Forsythe A, Guérin A, et al. Comorbidity burden healthcare resource utilization, and costs in chronic gout patients refractory to conventional urate-lowering therapy. Am J Ther. 2011 Feb 10; [Epub ahead of print].
47. Zhang W, Doherty M, Pascual E, et al; EULAR (European League Against Rheumatism) Standing Committee for International Clinical Studies Including Therapeutics. EULAR evidence based recommendations for gout. Part II: Management. Ann Rheum Dis. 2006;65(10):1312-1324.
48. Schlesinger N, Yasothan U, Kirkpatrick P. Pegloticase [published correction appears in Nat Rev Drug Discov. 2011;10(2):156]. Nat Rev Drug Discov. 2011;10(1):17-18.
49. Pascual E, Sivera F, Yasothan U, Kurkpatrick P. Febuxostat. Nat Rev Drug Discov. 2009;8(3): 191-192.
50. Becker MA, Schumacher HR, Espinoza LR, et al. The urate-lowering efficacy and safety of febuxostat in the treatment of the hyperuricemia of gout; the CONFIRMS trial. Arthritis Res Ther. 2010;12(2):R63.
51. Hu M, Tomlinson B. Febuxostat in the management of hyperuricemia and chronic gout: a review. Ther Clin Risk Manag. 2008;4(6):1209-1220.
52. Reinders MK, Jansen TL. New advances in the treatment of gout: review of pegloticase. Ther Clin Risk Manag. 2010;6:543-550.
53. Cammalleri L, Malaguarnera M. Rasburicase represents a new tool for hyperuricemia in tumor lysis syndrome and in gout. Int J Med Sci. 2007; 4(2):83-93.
54. Richette P, Brière C, Hoenen-Clavert V, et al. Rasburicase for topaceous gout not treatable with allopurinol: an exploratory study. J Rheumatol. 2007;34(10):2093-2098.
55. Moolenburgh JD, Reinders MK, Jansen TL. Rasburicase treatment in severe tophaceous gout: a novel therapeutic option. Clin Rheumatol. 2006;25(5):749-752.
Gouty arthritis is a common form of inflammatory arthritis, occurring more frequently in men than in women. The condition has a male–female ratio of 3 or 4 to 1, although that ratio narrows as adults age; because the uricosuric effects of estrogen decline with menopause, the risk for gout increases in postmenopausal women.1,2 Mean age at disease onset is 40 to 60 in men,3,4 with onset in women averaging seven years later.5
Apart from the pain and loss of function associated with this disorder of purine metabolism6 and the risk for a chronic form of the disease, gout is almost universally linked with serious comorbidities that require timely intervention. These include hypertension, dyslipidemia, hyperglycemia and diabetes, obesity, metabolic syndrome, cardiovascular disease (CVD), renal insufficiency, and coronary heart disease (CHD).2,3,7 The presence of gout is independently associated with a risk for acute myocardial infarction (AMI) and increased rates of all-cause mortality.3,8-10
EPIDEMIOLOGY
In 2007, the National Arthritis Data Workgroup11,12 estimated that about three million US adults had had “self-reported gout” in the previous year. An estimated six million US adults have been diagnosed with gout,2,11 and its incidence and prevalence are increasing. The incidence of primary gout more than doubled between 1977-1978 and 1995-1996,13 especially affecting the aging population. The prevalence of gout among 1,000 managed care patients ages 65 to 74 increased by at least 30% between 1990 and 1999, while prevalence among those older than 75 almost doubled during this same period.14
Numerous factors appear to contribute to these trends, including aging of the population, dietary trends (ie, increased consumption of red meat, organ food, game, and shellfish and reduced consumption of low-fat dairy products), presence of certain comorbid conditions (ie, hypertension, dyslipidemia, diabetes, metabolic syndrome, end-stage renal disease), the increasing prevalence of obesity in younger adults, use of specific prescription medications, and increased incidence of organ transplantation.1,7,8,15-19
The body’s underexcretion or overproduction of uric acid (a byproduct of purine metabolism12) can lead to hyperuricemia. This condition, defined as a serum urate level exceeding 7.0 mg/dL in men or 6.0 mg/dL in women20,21 (levels above 9.0 mg/dL are considered very high22), is the primary risk factor for gout.8,23,24 As with gout, the incidence of hyperuricemia has increased in recent years,20 with researchers attributing the trend to worldwide popularization of the Westernized diet (particularly use of high-fructose corn syrup20,25) and increased use of certain medications, including thiazide diuretics, cyclosporine, and low-dose aspirin.2,20,25,26
As serum urate levels rise, the patient with hyperuricemia may experience urate supersaturation, often followed by crystallization of the excess urate into monosodium urate (MSU) crystals. Subsequently, circulating MSU crystals may deposit in body tissues, especially in the joint spaces. The body’s ensuing inflammatory response to the MSU deposits is gout.20
In addition to hyperuricemia, risk factors for gout include a high-purine diet, habitual alcohol consumption (especially beer and fortified wines27), diuretic therapy (particularly in patients with heart failure or renal insufficiency), obesity, hypertension, and high levels of fructose consumption.7,28 Additionally, cyclosporine use in an organ transplant recipient, poorly controlled uric acid levels, and a long history of gout increase the patient’s risk for chronic tophaceous gout.24,29 Tophi may be more common in a patient with a history of organ transplantation.16
Genetic variants are currently being investigated to possibly identify a predisposition to gout. The most significant genetic factors appear to involve mechanisms that regulate serum uric acid levels—particularly urate underexcretion.23 Other factors that contribute to underexcretion or overproduction of uric acid are shown in Table 1.1,15,26,30-33
A dynamic relationship exists between gout and a number of pathologic processes. According to researchers investigating nearly 178,000 patients with gout in a managed care database, 36% had hypertension, 27% had dyslipidemia, and 15% had diabetes.8 In a smaller cohort study conducted in Spain and Mexico, it was demonstrated that 93% of patients with gout had one or more associated diseases, in order of decreasing frequency: hypertriglyceridemia, obesity, hypertension, metabolic syndrome, hyperglycemia, chronic renal failure, diabetes, and ischemic heart disease.3
Of particular clinical importance in this study was a finding that the first gout attack generally preceded the diagnosis of the associated diseases.3 Thus, a diagnosis of gout should lead the primary care provider to discuss modifiable risk factors with the patient—but also to investigate for comorbid illnesses that may require timely management.2
In a 12-year-long prospective study of more than 50,000 men participating in the Health Professionals Follow-Up Study,9 it was found that men with gout had a 28% increased risk for all-cause mortality, a 38% increased risk for CVD-related death, and a 55% increased risk for CHD-related death, compared with men who did not have gout (excluding other risk factors).9 Similarly, researchers for the Multiple Risk Factor Intervention Trial10 demonstrated a clinically significant association between gout and an increased risk for AMI: 10.5% of men with gout, compared with 8.43% of men without gout, had an AMI during mean follow-up of 6.5 years.10
THE STAGES
The four stages of gout are asymptomatic hyperuricemia, acute gout, intercritical gout, and chronic tophaceous gout.20
Only a small percentage (0.5% to 4.5%) of patients with asymptomatic hyperuricemia will develop acute gout.28 Nevertheless, any patient with serum urate greater than 6.8 mg/dL is at risk for the deposition of MSU crystals into body tissues and the potential associated organ damage—even patients without symptoms. There is currently no evidence-based method to determine which patients with asymptomatic hyperuricemia will experience disease progression.16
Acute gout develops when deposition of MSU crystals in the joints initiates an inflammatory response. In the typical history, the patient experiences sudden-onset severe pain, swelling, and erythema. The pain often starts in the middle of the night or early morning,34 waking the patient from sleep and peaking within 24 hours of onset. At this time, the patient is often unable to bear weight comfortably on the affected joint. The patient may also report fever and flu-like malaise resulting from the release of interleukin 1- (IL-1), IL-1 receptor, cytokines, and prostaglandins.16,24,35 Usually in these early attacks, symptoms resolve spontaneously within three to 14 days.16,24
After resolution of an acute attack, the patient enters the intercritical stage, another asymptomatic stage that may last for months or years—or indefinitely. During the intercritical stage, MSU crystal deposition continues, adding crystals in and around the affected joint or joints, possibly continuing to inflict damage (in some patients, substantial), and in many cases resulting in additional attacks and pain.16 Any subsequent acute gout attacks the patient may experience are likely to last longer than the initial attack and to involve additional joints or tendons.24
Some patients, especially those who do not receive adequate treatment for hyperuricemia,2 progress to develop chronic tophaceous gout. This is a deforming disease process in which the joints may become stiff and swollen, and subcutaneous nodules or whitish-yellow intradermal deposits may be present under taut skin, anywhere in the body.16
PATIENT PRESENTATION AND HISTORY
Typically, a patient with gout will present with a chief complaint of a painful, tender, inflamed joint (classically described in Latin as calor, rubor, dolor, et tumor6). However, clinicians must also be aware of unusual presentations and consider gout in the differential whenever a patient with a history of gout or pertinent risk factors presents with unexplained clinical findings.32 The history of present illness will vary according to the stage of the disease.
About 90% of recognized initial attacks of gout are monoarticular, usually occurring in one of the lower extremities.16 While the first metatarsophalangeal (MTP) joint is affected in about 50% of gout cases (podagra, the Greek term for gout),2,35 eventually patients with gout have a 90% chance of involvement with the MTP joint (see Figure 1). According to Zhang et al,26 patients with hyperuricemia and an affected MTP joint have an 82% chance of having gout.2,26
Because such a large proportion of patients have the classic presentation of rapid-onset warmth, redness, and tenderness at the MTP, knee, or ankle and surrounding soft tissue, cases with a differing presentation are likely to be misdiagnosed or overlooked, or a correct diagnosis is delayed.26 In many documented cases, gout was the ultimate diagnosis—but one that was reached only incidentally because of unusual clinical presentation, ranging from entrapment neuropathy to a pancreatic mass.32
The presence of hyperuricemia and other risk factors must be investigated. Also relevant in the history of an acute gout attack may be a preceding event that has caused damage or stress to the joint, such as infection, trauma, or surgery. Other possible triggers for an attack include alcohol ingestion, acidosis, use of IV contrast media, diuretic therapy, chemotherapy, recent hospitalization or surgery, and initiation or termination of urate-lowering therapy with the xanthine oxidase inhibitor allopurinol.2,30 According to Primatesta et al,8 the risk for flares is increased in patients with cardiometabolic comorbidities.
The medication history of a patient with gout may include low-dose aspirin (but not standard-dose aspirin, which is uricosuric2), diuretics, cyclosporine, cytotoxic agents, and vitamin B12, which may contribute to hyperuricemia.24 Additionally, ethambutol, pyrazinamide, levodopa, nicotinic acid, didanosine, niacin, and warfarin may raise uric acid levels.15,30,33
Medical history should include a thorough assessment of the comorbidities associated with gout. In addition to the conditions mentioned previously, patients with a history of polycystic kidney disease, dehydration, lactic acidosis, hyperparathyroidism, toxemia of pregnancy, hypothyroidism, or sarcoidosis may have elevated urate levels due to underexcretion—and thus may be vulnerable to gout.30 History of gout in a first-degree relative is associated with an increased risk for gout.36
The social history should address alcohol use or abuse. The clinician should also inquire about how gout is impacting the daily life of the patient. Diet and exercise habits should be assessed37 (see “Patient Education,” below).
PHYSICAL EXAMINATION
The physical exam begins with evaluation of the skin and extremities for the classic features of gout. Affected joints will be exquisitely tender, and patients may be febrile. Most cases are monoarticular, but polyarticular involvement is likely in patients with advanced disease (and can, particularly in women, be mistaken for rheumatoid arthritis).2,16 In patients with chronic tophaceous gout, there may be whitish-yellow skin deposits, subcutaneous nodules, and areas of taut skin. The lower-extremity joints and tendons, as well as the wrists, fingers, and elbows, are commonly affected16 (see Figure 2).
DIAGNOSIS
Diagnostic criteria that are currently available (and have long been in use) include the American College of Rheumatology/American Rheumatism Association (ACR/ARA) preliminary criteria,34 the New York criteria,21 and the Rome criteria.38 The specifics of each are listed in Table 2.21,34,38,39
The gold standard for gout diagnosis is detection of MSU crystals in a sample of synovial fluid aspirated from the affected joint or from a tophus and examined by polarized light microscopy.24 This is of significant importance to the clinician who is faced with a questionable diagnosis.16 However, crystal visualization is not ordinarily available to the primary care clinician,2,17,40 and it is not always necessary if a careful history and physical exam are conducted in a patient with hyperuricemia or other risk factors for gout. A presumptive diagnosis may be acceptable in a patient with the classic presentation of acute gout: rapid onset of severe pain in a swollen, erythematous joint and symptoms peaking within 24 hours. The presence of tophi is pathognomonic for chronic tophaceous gout.41
In cases of questionable or unusual manifestation of gout, however, various imaging techniques and crystal visualization may be indicated.32
In order to compare the effectiveness of the latter technique with conventional diagnostic criteria for gout, Malik et al39 conducted a pilot study involving 82 patients who had undergone synovial fluid analysis with polarized light microscopy. Patients were surveyed about the clinical features of their disease, as listed in the three standard sets of criteria for diagnosis of gout. Compared with the “gold standard” of urate crystal detection (which is one of the Rome criteria38), the study authors found the ACR/ARA preliminary criteria,34 the New York criteria,21 and the Rome criteria38 generally unsatisfactory.
In the study, among patients with confirmed presence of MSU crystals:
• 87% reported more than one attack of acute arthritis (ACR/ARA34)
• 86% reported monoarthritis attack (ACR/ARA34)
• 89% had hyperuricemia (ACR/ARA34 and Rome,38 with the latter giving effective, specific parameters)
• 100% had negative results on joint fluid culture (ACR/ARA34)
• 90% reported an attack starting at night (ACR/ARA34).
The positive predictive values for these signs and symptoms are 38%, 39%, 74%, 50%, and 45%, respectively, according to Malik et al.39 The presence of tophi (cited by all three sets of criteria but “proven or suspected” in the ACR/ARA34) had the highest positive predictive value for gout (91%) and a likelihood ratio of 15.56, which was at least three times higher than any of the other listed criteria. A verified response to colchicine, one of the New York criteria,21 had the second highest positive predictive value at 86%.39
In summary, the ACR/ARA,34 the New York,21 and the Rome criteria38 had specificity of 79%, 83%, and 89%, respectively; sensitivity of 70%, 70%, and 67%, respectively; and positive predictive values for gout of 66%, 70%, and 77%, respectively. The Rome criteria38 had the highest specificity and highest positive predictive value, perhaps making them most helpful for clinicians who lack access to synovial fluid analysis.
DIFFERENTIAL DIAGNOSIS
Conditions to be considered and ruled out before a diagnosis of gout can be made are:
• Pseudogout
• Septic arthritis
• Psoriatic arthritis
• Rheumatoid arthritis
• Erosive osteoarthritis
• Bacterial cellulitis
• Sarcoid arthropathy.16,28,42,43
Unlike gout (in which compensated polarized light microscopy reveals needle-shaped urate crystals with strong negative birefringence), pseudogout is characterized by calcium pyrophosphate dihydrate crystals; these are rhomboid-shaped, with weak positive birefringence.42 Additionally, radiographic imaging will reveal soft tissue swelling and chondrocalcinosis of the joint in pseudogout.44
The patient with septic arthritis, most likely affecting the knee, will have a white blood cell (WBC) count exceeding 50,000/mm3 and a positive culture of the synovial fluid, with absence of crystals.28
Chronic tophaceous gout can mimic rheumatoid arthritis in appearance and joint distribution, and patients affected by either condition may develop a positive rheumatoid factor. Examination of synovial fluid for MSU crystals and radiographic imaging will be of value in making a distinction.
Osteoarthritis is usually evidenced by joint space narrowing on x-ray.42
Bacterial cellulitis will present similarly to gout, but the erythema of bacterial cellulitis will more likely extend beyond the involved joint.16
Sarcoid arthropathy often presents as a polyarthritis, as in advanced gouty arthritis. However, in sarcoid arthropathy, serum calcium and angiotensin-converting enzyme will likely be elevated.43 Synovial or tendon sheath biopsy will show non-caseating granulomas, which are the hallmark for sarcoid disease. Additionally, joint fluid analysis will demonstrate a predominance of mononuclear or polymorphonuclear cells.43
Diagnostic Tests
Diagnostic tests to consider are analysis and culture of the synovial fluid, complete blood count (CBC), blood urea nitrogen (BUN), creatinine, radiography, ultrasonography, serum uric acid, and blood culture if septic arthritis is suspected.28 While serum urate levels may be normal during an acute gout attack, measurement may still be helpful for comparison, since elevation is a likely finding two weeks after an attack—if the patient was, in fact, experiencing an acute gout attack.42
Since renal dialysis increases the risk for gout, pseudogout, and septic arthritis, synovial fluid analysis is essential in patients undergoing renal dialysis.42
Various imaging techniques may aid in confirming a diagnosis of gout and monitoring its progression, but further studies are needed to more clearly define the role of these techniques in management of gout.45 Plain radiographic evidence of asymmetric swelling in a joint (one of the ACR/ARA preliminary criteria34) was shown to have a 60% positive predictive value for a diagnosis of gout.39 Late in the disease process, an affected joint may be affected by characteristic “punched out” intra-articular lesions, with a normal amount of joint space.45
Ultrasound is a safe and inexpensive test that can reveal soft tissue edema and increased vascularity during an acute gout attack. Chronic changes include the double contour sign and tophus-like lesions surrounded by a thin, anechoic rim.45
CT will also show tophi and bony erosion. While CT is more specific than other techniques, it is also more expensive and exposes the patient to increased radiation. MRI can help monitor the complications of gout, especially entrapment neuropathies.45
TREATMENT/MANAGEMENT
According to current evidence, treatment is not indicated for asymptomatic hyperuricemia.16
Acute Gout Management
Pharmacologic treatments available for an acute gout attack include NSAIDs, colchicine, and local or systemic corticosteroids.24,46 At the onset of an attack, patients should start high-dose NSAID therapy, and continue for two to three days after symptoms are resolved.6 Oral indomethacin (50 mg tid) or oral ibuprofen (800 mg tid) are both reasonable options.6 It may be prudent to consider a proton pump inhibitor (eg, omeprazole) to protect the gastric mucosa in patients who are susceptible to gastrointestinal problems.27
In addition to high-dose NSAID therapy, adding colchicine (1.2 mg by mouth at onset of symptoms, followed by 0.6 mg one hour later) has proven to be effective in relieving the symptoms of gout, but its serious gastrointestinal adverse effects, particularly diarrhea, must be considered.6,47
In patients with monoarticular gout who cannot tolerate NSAIDs, intra-articular aspiration and corticosteroid injections may provide relief.
Long-acting triamcinolone, administered by intra-articular injection, has been found to relieve pain and inflammation in patients with gout. Septic arthritis must be ruled out by way of joint aspiration and culture before injection of corticosteroids.47
Oral or IM-administered corticosteroids may be considered for patients with polyarticular involvement. Prednisone (60 mg/d, tapered over 10 days) is an appropriate option for outpatients or inpatients; methylprednisone (80 to 120 mg IM) may be suitable for inpatients.6 Again, septic arthritis must be ruled out before corticosteroids are administered.47
For the patient who is currently taking a thiazide diuretic for hypertension, substituting a different medication may be warranted; the angiotensin receptor blocker losartan, for example, has uricosuric action.27,29,47 Nonpharmacologic strategies, such as rest, ice, elevation, and avoiding trauma to the affected joint, are also recommended.27
Of note, allopurinol therapy should be neither initiated nor discontinued during an acute gout attack.27
Management of Chronic and Intercritical Gout
Urate-lowering therapy, such as allopurinol (50 to 300 mg/d29), should be considered for patients who experience frequent attacks (ie, three or more per year), patients with chronic tophi, patients with radiographically demonstrated joint damage,47 or patients with a documented state of uric acid overproduction.29
Allopurinol dosage should be adjusted based on creatinine clearance; dosing as high as 800 mg/d has been recommended in patients with normal renal function.2 Again, allopurinol should never be started or discontinued during an acute attack,27 because abrupt fluctuations in uric acid levels may heighten the inflammation. The target serum urate level is 6.0 mg/dL.29
Febuxostat, which received FDA approval in 2009, was the first oral urate-lowering treatment to be approved since the 1960s. Like allopurinol, this nonpurine xanthine oxidase inhibitor blocks uric acid synthesis.48,49 In a trial reported by Becker et al,50 67% of patients who took febuxostat 80 mg/d reached the target serum urate level (ie, < 6.0 mg/dL), compared with 45% of those who took 40 mg/d of febuxostat and 42% of those taking 300 mg/d of allopurinol. While incidence of adverse events was low in all treatment groups, Hu and Tomlinson51 report that febuxostat is tolerable in patients who are hypersensitive to allopurinol. As with other urate-lowering medications, gout flares are common during the early period of febuxostat use.51
For patients with gout that does not respond to conventional urate-lowering therapy, new options are being introduced. Two agents, each a recombinant form of the enzyme urate oxidase, are designed to convert uric acid into allantoin, which can then be excreted in the urine. Late in 2010, one of these agents, pegloticase, was approved for use in patients with refractory gout.48 In one clinical trial, tophi were reported dissolved in 40% of patients who took pegloticase, but 58% of patients did not achieve the targeted response (ie, serum urate < 6.0 mg/dL), and 77% of patients experienced gout flares.52 Infusion reactions occurred in 26% to 31% of patients, and Reinders and Jansen52 recommended the clinical evaluation of glucocorticoids and other anti-inflammatory agents to prevent the formation of antibodies involved in these reactions.
The second agent, rasburicase, has been approved for treatment and prevention of acute hyperuricemia in adult cancer patients. Rasburicase is now being investigated for use in patients with nonresponsive tophaceous gout.53-55 It can be administered in the form of monthly infusions.54
Patient Education
Educating the patient about modifiable risk factors, such as diet, alcohol consumption, and adherence to the medication regimen, should be a priority.
Patients should be encouraged to target and maintain an ideal body weight, through diet and moderate physical exercise, as a strategy to normalize serum urate levels.27,47 However, they should be advised to avoid “crash dieting,” as this may precipitate a gout attack.27 In the recommended low-purine diet, consumption of red meat and shellfish is restricted,17 whereas consumption of soy, nonfat milk and other low-fat dairy products, cherries and other fruits, and increased vegetable protein is encouraged.31,37 Consumption of alcohol, especially beer and fortified wines, should be limited.27,47
Avoiding trauma to joints affected by gout (including the stress of bearing excess weight) can help patients limit future attacks.7,27
CONCLUSION
Patients with gout often have the characteristic presentation of an acutely tender, inflamed joint, but since gout is a systemic disorder, the clinician must also consider the possibility of gout in almost any organ system. Gout is a common disease, and its diagnosis can alert the astute clinician to investigate for certain metabolic disorders requiring intervention. Hyperlipidemia, metabolic syndrome, hypertension, chronic kidney disease, obesity, cardiovascular disease, and diabetes are all conditions associated with gout.
Recognizing the opportunity to offer preventive care measures and recommend lifestyle modifications to the patient with gout allows the clinician to play an important role in the patient’s care.
REFERENCES
1. Bhole V, de Vera M, Rahman MM, et al. Epidemiology of gout in women: fifty-two–year followup of a prospective cohort. Arthritis Rheum. 2010;62(4):1069-1076.
2. Neogi T. Clinical practice: gout. N Engl J Med. 2011;364(5):443-452.
3. Hernández-Cuevas CB, Roque LH, Huerta-Sil G, et al. First acute gout attacks commonly precede features of the metabolic syndrome. J Clin Rheumatol. 2009;15(2):65-67.
4. Louthrenoo W, Kasitanon N, Sukitawut W, Wichainun R. A clinical study of crystal-proven gouty arthritis in a university hospital. J Med Assoc Thai. 2003;86(9):868-875.
5. De Souza AW, Fernandes V, Ferrari AJ. Female gout: clinical and laboratory features. J Rheumatol. 2005;32(11):2186-2188.
6. Kurakula PC, Keenan RT. Diagnosis and management of gout: an update. J Musculoskel Med. 2010;27(10). www.musculoskeletalnet work.com/display/article/1145622/1692895. Accessed June 14, 2011.
7. Choi HK, Atkinson K, Karlson EW, Curhan G. Obesity, weight change, hypertension, diuretic use, and risk of gout in men: the Health Professionals Follow-up Study. Arch Intern Med. 2005;165(7):742-748.
8. Primatesta P, Plana E, Rothenbacher D. Gout treatment and comorbidities: a retrospective cohort study in a large US managed care population. BMC Musculoskelet Disord. 2011 May 20;12(1):103. [Epub ahead of print]
9. Choi HK, Curhan G. Independent impact of gout on mortality and risk for coronary heart disease. Circulation. 2007;116(8):894-900.
10. Krishnan E, Baker JF, Furst DE, Schumacher HR. Gout and the risk of acute myocardial infarction. Arthritis Rheum. 2006;54(8):2688-2696.
11. Lawrence RC, Felson DT, Helmick CG, et al; National Arthritis Data Workgroup. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58(1):26-35.
12. CDC. Gout. www.cdc.gov/arthritis/basics/gout.htm. Accessed June 14, 2011.
13. Arromdee E, Michet CJ, Crowson CS, et al. Epidemiology of gout: is the incidence rising? J Rheumatol. 2002;29(11):2403-2406.
14. Wallace KL, Riedel AA, Joseph-Ridge N, Wortmann R. Increasing prevalence of gout and hyperuricemia over 10 years among older adults in a managed care population. J Rheumatol. 2004; 31(8):1582-1587.
15. Weaver AL. Epidemiology of gout. Cleve Clin J Med. 2008;75 suppl 5:S9-S12.
16. Mandell BF. Clinical manifestations of hyperuricemia and gout. Cleve Clin J Med. 2008;75 suppl 5:S5-S8.
17. Choi HK, Atkinson K, Karlson EW, et al. Purine-rich foods, dairy and protein intake, and the risk of gout in men. N Engl J Med. 2004;350(11):1093-1103.
18. Demarco MA, Maynard JW, Huizinga MM, et al. Younger age at gout onset is related to obesity in a community-based cohort. Arthritis Care Res (Hoboken). 2011 Apr 11; [Epub ahead of print].
19. Brook RA, Forsythe A, Smeeding JE, Lawrence Edwards N. Chronic gout: epidemiology, disease progression treatment and disease burden. Curr Med Res Opin. 2010;26(12):2813-2821.
20. Sachs L, Batra KL, Zimmermann B. Medical implications of hyperuricemia. Med Health R I. 2009;92(11):353-355.
21. Kellgren JH, Jeffrey MR, Ball J, eds. The Epidemiology of Chronic Rheumatism: Atlas of Standard Radiographs of Arthritis. Oxford: Blackwell; 1963:327.
22. Wu EQ, Patel PA, Mody RR, et al. Frequency, risk, and cost of gout-related episodes among the elderly: does serum uric acid level matter? J Rheumatol. 2009;36(5):1032-1040.
23. Riches PL, Wright AF, Ralston SH. Recent insights into the pathogenesis of hyperuricaemia and gout. Hum Mol Genet. 2009;18(R2):R177-R184.
24. Schumacher HR Jr. The pathogenesis of gout. Cleve Clin J Med. 2008;75 suppl 5:S2-S4.
25. Nakagawa T, Hu H, Zharikov S, et al. A causal role for uric acid in fructose-induced metabolic syndrome. Am J Physiol Renal Physiol. 2006;290(3):F625-F631.
26. Zhang W, Doherty M, Pascual E, et al; EULAR (European League Against Rheumatism) Standing Committee for International Clinical Studies Including Therapeutics. EULAR evidence based recommendations for gout. Part I: Diagnosis. Ann Rheum Dis. 2006;65(10):1301-1311.
27. Jordan KM, Cameron JS, Snaith M, et al. British Society for Rheumatology and British Health Professionals in Rheumatology guideline for the management of gout. Rheumatology. 2007;46(8):1372-1374.
28. Eggebeen AT. Gout: an update. Am Fam Physician. 2007;76(6):801-808.
29. Terkeltaub RA. Gout. N Engl J Med. 2003; 349(17):1647-1655.
30. Harris MD, Siegel LB, Alloway JA. Gout and hyperuricemia. Am Fam Physician. 1999;59(4): 925-934.
31. Schlesinger N. Dietary factors and hyperuricemia. Curr Pharm Des. 2005;11(32):4133-4138.
32. Ning TC, Keenan RT. Unusual presentations of gout. Curr Opin Rheumatol. 2010;22(2): 181-187.
33. Menon RK, Mikhailidis DP, Bell JL, et al. Warfarin administration increases uric acid concentrations in plasma. Clin Chem. 1986;32(8):
1557-1559.
34. Wallace SL, Robinson H, Masi AT, et al. Preliminary criteria for the classification of the acute arthritis of primary gout. Arthritis Rheum. 1977;20(3):895-900.
35. Martinon F, Glincher LH. Gout: new insights into an old disease. J Clin Invest. 2006;116 (8):2073-2075.
36. Zampogna G, Andracco R, Parodi M, Cimmino MA. Clinical features of gout in a cohort of Italian patients [in Italian]. Reumatismo. 2009; 61(1):41-47.
37. Choi HK. A prescription for lifestyle change in patients with hyperuricemia and gout. Curr Opin Rheumatol. 2010;22(2):165-172.
38. Bennett PH, Wood PH, eds. Population studies of the rheumatic diseases: proceedings of the Third International Symposium; June 5-10, 1966; New York, NY. Amsterdam: Excerpta Medica Foundation; 1968:457-458.
39. Malik A, Schumacher HR, Dinnella JE, Clayburne GM. Clinical diagnostic criteria for gout: comparison with the gold standard of synovial fluid crystal analysis. J Clin Rheumatol. 2009;15 (1):22-24.
40. Wijnands JMA, Boonen A, Arts ICW, et al. Large epidemiologic studies of gout: challenges in diagnosis and diagnostic criteria. Curr Rheumatol Rep. 2011;13(2):167-174.
41. Dodd LG, Major NM. Fine-needle aspiration cytology of articular and periarticular lesions. Cancer. 2002;96(3):157-165.
42. Dore RK. The gout diagnosis. Cleve Clin J Med. 2008;75 suppl 5:S17-S21.
43. Pettersson T. Sarcoid and erythema nodosum arthropathies. Baillieres Best Pract Res Clin Rheumatol. 2000;14(3):461-476.
44. Córdoba-Fernández A, Rayo-Rosado R. Pseudogout of the first metatarsophalangeal joint associated with hallux valgus: an atypical bilateral case. J Am Podiatr Med Assoc. 2010;100(2):138-142.
45. Dalbeth N, McQueen FM. Use of imaging to evaluate gout and other crystal deposition disorders. Curr Opin Rheumatol. 2009;21(2):124-131.
46. Wu EQ, Forsythe A, Guérin A, et al. Comorbidity burden healthcare resource utilization, and costs in chronic gout patients refractory to conventional urate-lowering therapy. Am J Ther. 2011 Feb 10; [Epub ahead of print].
47. Zhang W, Doherty M, Pascual E, et al; EULAR (European League Against Rheumatism) Standing Committee for International Clinical Studies Including Therapeutics. EULAR evidence based recommendations for gout. Part II: Management. Ann Rheum Dis. 2006;65(10):1312-1324.
48. Schlesinger N, Yasothan U, Kirkpatrick P. Pegloticase [published correction appears in Nat Rev Drug Discov. 2011;10(2):156]. Nat Rev Drug Discov. 2011;10(1):17-18.
49. Pascual E, Sivera F, Yasothan U, Kurkpatrick P. Febuxostat. Nat Rev Drug Discov. 2009;8(3): 191-192.
50. Becker MA, Schumacher HR, Espinoza LR, et al. The urate-lowering efficacy and safety of febuxostat in the treatment of the hyperuricemia of gout; the CONFIRMS trial. Arthritis Res Ther. 2010;12(2):R63.
51. Hu M, Tomlinson B. Febuxostat in the management of hyperuricemia and chronic gout: a review. Ther Clin Risk Manag. 2008;4(6):1209-1220.
52. Reinders MK, Jansen TL. New advances in the treatment of gout: review of pegloticase. Ther Clin Risk Manag. 2010;6:543-550.
53. Cammalleri L, Malaguarnera M. Rasburicase represents a new tool for hyperuricemia in tumor lysis syndrome and in gout. Int J Med Sci. 2007; 4(2):83-93.
54. Richette P, Brière C, Hoenen-Clavert V, et al. Rasburicase for topaceous gout not treatable with allopurinol: an exploratory study. J Rheumatol. 2007;34(10):2093-2098.
55. Moolenburgh JD, Reinders MK, Jansen TL. Rasburicase treatment in severe tophaceous gout: a novel therapeutic option. Clin Rheumatol. 2006;25(5):749-752.