User login
Guanfacine extended release (GXR)—a selective α-2 adrenergic agonist FDA-approved for the treatment of attention-deficit/hyperactivity disorder (ADHD)—has demonstrated efficacy for inattentive and hyperactive/impulsive symptom domains in 2 large trials lasting 8 and 9 weeks.1,2 GXR’s once-daily formulation may increase adherence and deliver consistent control of symptoms across a full day ( Table 1 ).
Table 1
Guanfacine extended release: Fast facts
| Brand name: Intuniv |
| Indication: Attention-deficit/hyperactivity disorder |
| Approval date: September 3, 2009 |
| Availability date: November 2009 |
| Manufacturer: Shire |
| Dosing forms: 1-mg, 2-mg, 3-mg, and 4-mg extended-release tablets |
| Recommended dosage: 0.05 to 0.12 mg/kg once daily |
Clinical implications
GXR exhibits enhancement of noradrenergic pathways through selective direct receptor action in the prefrontal cortex.3 This mechanism of action is different from that of other FDA-approved ADHD medications. GXR can be used alone or in combination with stimulants or atomoxetine for treating complex ADHD, such as cases accompanied by oppositional features and emotional dysregulation or characterized by partial stimulant response.
How it works
Guanfacine—originally developed as an immediate-release (IR) antihypertensive—reduces sympathetic tone, causing centrally mediated vasodilation and reduced heart rate. Although GXR’s mechanism of action in ADHD is not known, the drug is a selective α-2A receptor agonist thought to directly engage postsynaptic receptors in the prefrontal cortex (PFC), an area of the brain believed to play a major role in attentional and organizational functions that preclinical research has linked to ADHD.3
The postsynaptic α-2A receptor is thought to play a central role in the optimal functioning of the PFC as illustrated by the “inverted U hypothesis of PFC activation.”4 In this model, cyclic adenosine monophosphate (cAMP) levels build within the prefrontal cortical neurons and cause specific ion channels—hyperpolarization-activated cyclic nucleotide gated (HCN) channels—to open on dendritic spines of these neurons.5 Activation of HCN channels effectively reduces membrane resistance, cutting off synaptic inputs and disconnecting PFC network connections. Because α-2A receptors are located in proximity to HCN channels, their stimulation by GXR closes HCN channels, inhibits further production of cAMP, and reestablishes synaptic function and the resulting network connectivity.5 Blockade of α-2A receptors by yohimbine reverses this process, eroding network connectivity, and in monkeys has been demonstrated to impair working memory,6 damage inhibition/impulse control, and produce locomotor hyperactivity.
Direct stimulation by GXR of the postsynaptic α-2A receptors is thought to:
- strengthen working memory
- reduce susceptibility to distraction
- improve attention regulation
- improve behavioral inhibition
- enhance impulse control.7
Pharmacokinetics
GXR offers enhanced pharmaceutics relative to IR guanfacine. IR guanfacine exhibits poor absorption characteristics—peak plasma concentration is achieved too rapidly and then declines precipitously, with considerable inter-individual variation.
GXR’s once-daily formulation is implemented by a proprietary enteric-coated sustained release mechanism8 that is meant to:
- control absorption
- provide a broad but flat plasma concentration profile
- reduce inter-individual variation of guanfacine exposure.
Compared with IR guanfacine, GXR exhibits delayed time of maximum concentration (Tmax) and reduced maximum concentration (Cmax). Therapeutic concentrations can be sustained over longer periods with reduced peak-to-trough fluctuation,8 which tends to improve tolerability and symptom control throughout the day. The convenience of once-daily dosing also may increase adherence.
GXR’s pharmacokinetic characteristics do not change with dose, but high-fat meals will increase absorption of the drug—Cmax increases by 75% and area under the plasma concentration time curve increases by 40%. Because GXR primarily is metabolized through cytochrome P450 (CYP) 3A4, CYP3A4 inhibitors such as ketoconazole will increase guanfacine plasma concentrations and elevate the risk of adverse events such as bradycardia, hypotension, and sedation. Conversely, CYP3A4 inducers such as rifampin will significantly reduce total guanfacine exposure. Coadministration of valproic acid with GXR can result in increased valproic acid levels, producing additive CNS side effects.
Efficacy
GXR reduced both inattentive and hyperactive/impulsive symptoms in 2 phase III, forced-dose, parallel-design, randomized, placebo-controlled trials ( Table 2 ). In the first trial,1 345 children age 6 to 17 received placebo or GXR, 2 mg, 3 mg, or 4 mg once daily for 8 weeks. In the second study,2 324 children age 6 to 17 received placebo or GXR, 1 mg, 2 mg, 3 mg, or 4 mg, once daily for 9 weeks; the 1-mg dose was given only to patients weighing <50 kg (<110 lbs).
In both trials, doses were increased in increments of 1 mg/week, and investigators evaluated participants’ ADHD signs and symptoms once a week using the clinician administered and scored ADHD Rating Scale-IV (ADHD-RS-IV). The primary outcome was change in total ADHD-RS-IV score from baseline to endpoint.
In both trials, patients taking GXR demonstrated statistically signifcant improvements in ADHD-RS-IV score starting 1 to 2 weeks after they began receiving once-daily GXR:
- In the first trial, the mean reduction in ADHD-RS-IV total score at endpoint was –16.7 for GXR compared with –8.9 for placebo (P < .0001).
- In the second, the reduction was –19.6 for GXR and –12.2 for placebo (P=.004).
Placebo-adjusted least squares mean changes from baseline were statistically significant for all GXR doses in the randomized treatment groups in both studies.
Secondary efficacy outcome measures included the Conners’ Parent Rating Scale-Revised: Short Form (CPRS-R) and the Conners’ Teacher Rating Scale-Revised: Short Form (CTRS-R).
Significant improvements were seen on both scales. On the CPRS-R, parents reported significant improvement across a full day (as measured at 6 PM, 8 PM, and 6 AM the next day). On the CTRS-R—which was used only in the first trial—teachers reported significant improvement throughout the school day (as measured at 10 AM and 2 PM).
Treating oppositional symptoms. In a collateral study,9 GXR was evaluated in complex ADHD patients age 6 to 12 who exhibited oppositional symptoms. The primary efficacy measure was change from baseline to endpoint in the oppositional subscale of the Conners’ Parent Rating Scale-Revised: Long Form (CPRS-R:L) score.
All subjects randomized to GXR started on a dose of 1 mg/d—which could be titrated by 1 mg/week during the 5-week, dose-optimization period to a maximum of 4 mg/d—and were maintained at their optimal doses for 3 additional weeks. Among the 217 subjects enrolled, 138 received GXR and 79, placebo.
Least-squares mean reductions from baseline to endpoint in CPRS-R:L oppositional subscale scores were –10.9 in the GXR group compared with –6.8 in the placebo group (P < .001; effect size 0.590). The GXR-treated group showed a significantly greater reduction in ADHD-RS-IV total score from baseline to endpoint compared with the placebo group (–23.8 vs –11.4, respectively, P < .001; effect size 0.916).
Table 2
Randomized, controlled trials supporting GXR’s effectiveness
for treating ADHD symptoms
| Study | Subjects | GXR dosages | Results |
|---|---|---|---|
| Biederman et al, 20087 ; phase III, forced-dose parallel-design | 345 ADHD patients age 6 to 17 | 2, 3, or 4 mg given once daily for 8 weeks | GXR was associated with significantly lower ADHD-RS-IV score compared with placebo (-16.7 vs -8.9) |
| Sallee et al, 20098 ; phase III, forced-dose parallel-design | 324 ADHD patients age 6 to 17 | 1,* 2, 3, or 4 mg given once daily for 9 weeks | GXR was associated with significantly lower ADHD-RS-IV score compared with placebo (-19.6 vs -12.2) |
| Connor et al, 20099 ; collateral study | 217 complex ADHD patients age 6 to 12 with oppositional symptoms | Starting dose 1 mg/d, titrated to a maximum of 4 mg/d for a total of 8 weeks | GXR was associated with significantly lower scores on CPRS-R:L oppositional subscale (-10.9 vs -6.8) and ADHD-RS-IV (-23.8 vs -11.4) compared with placebo |
| *1-mg dose was given only to subjects weighing <50 kg (<110 lbs) | |||
| ADHD: attention-deficit/hyperactivity disorder; ADHD-RS-IV: Attention-Deficit/Hyperactivity Disorder Rating Scale-IV; CPRS-R:L: Conners’ Parent Rating Scale-Revised: Long Form; GXR: guanfacine extended release | |||
Tolerability
In the phase III trials, the most commonly reported drug-related adverse reactions (occurring in ≥2% of patients) were:
- somnolence (38%)
- headache (24%)
- fatigue (14%)
- upper abdominal pain (10%)
- nausea, lethargy, dizziness, hypotension/decreased blood pressure, irritability (6% for each)
- decreased appetite (5%)
- dry mouth (4%)
- constipation (3%).
Many of these adverse reactions appear to be dose-related, particularly somnolence, sedation, abdominal pain, dizziness, and hypotension/decreased blood pressure.
Overall, GXR was well tolerated; clinicians rated most events as mild to moderate. Twelve percent of GXR patients discontinued the clinical studies because of adverse events, compared with 4% in the placebo groups. The most common adverse reactions leading to discontinuation were somnolence/sedation (6%) and fatigue (2%). Less common adverse reactions leading to discontinuation (occurring in 1% of patients) included hypotension/decreased blood pressure, headache, and dizziness.
Open-label safety trial. Sallee et al10 conducted a longer-term, open-label, flexible-dose safety continuation study of 259 GXR-treated patients (mean exposure 10 months), some of whom also received a psychostimulant. Common adverse reactions (occurring in ≥5% of subjects) included somnolence (45%), headache (26%), fatigue (16%), upper abdominal pain (11%), hypotension/decreased blood pressure (10%), vomiting (9%), dizziness (7%), nausea (7%), weight gain (7%), and irritability (6%).10 In a subset of patients, the onset of sedative events typically occurred within the first 3 weeks of GXR treatment and then declined with maintenance to a frequency of approximately 16%. The rates of somnolence, sedation, or fatigue were lowest among patients who also received a psychostimulant ( Figure ).
Distribution of GXR doses before the end of this study was 37% of patients on 4 mg, 33% on 3 mg, 27% on 2 mg, and 3% on 1 mg, suggesting a preference for maintenance doses of 3 to 4 mg/d. The most frequent adverse reactions leading to discontinuation were somnolence (3%), syncopal events (2%), increased weight (2%), depression (2%), and fatigue (2%). Other adverse reactions leading to discontinuation (occurring in approximately 1% of patients) included hypotension/decreased blood pressure, sedation, headache, and lethargy. Serious adverse reactions in the longer-term study in >1 patient included syncope (2%) and convulsion (0.4%).
Figure: Incidence of somnolence, sedation, and fatigue in study patients receiving GXR
with or without psychostimulants
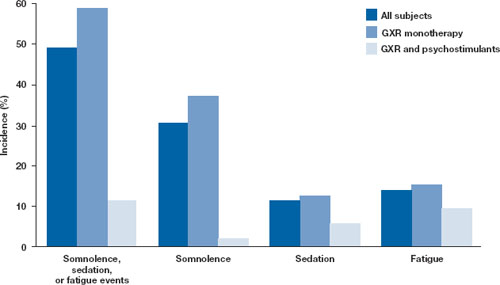
In an open-label continuation study of 259 patients treated with guanfacine extended release (GXR), somnolence, sedation, or fatigue was reported by 49% of subjects overall, 59% of those who received GXR monotherapy, and 11% of those given GXR with a psychostimulant.
GXR: guanfacine extended release
Source: Reprinted with permission from Sallee FR, Lyne A, Wigal T, et al. Long-term safety and efficacy of guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2009;19(3):215-226 Safety warnings relating to the likelihood of hypotension, bradycardia, and possible syncope when prescribing GXR should be understood in the context of its pharmacologic action to lower heart rate and blood pressure. In the short-term (8 to 9 weeks) controlled trials, the maximum mean changes from baseline in systolic blood pressure, diastolic blood pressure, and pulse were -5 mm Hg, -3 mm Hg, and -6 bpm, respectively, for all dose groups combined. These changes, which generally occurred 1 week after reaching target doses of 1 to 4 mg/d, were dose-dependent but usually modest and did not cause other symptoms; however, hypotension and bradycardia can occur.
In the longer-term, open-label safety study,10 maximum decreases in systolic and diastolic blood pressure occurred in the first month of treatment; decreases were less pronounced over time. Syncope occurred in 1% of pediatric subjects but was not dose-dependent. Guanfacine IR can increase QT interval but not in a dose-dependent fashion.
Dosing
The approved dose range for GXR is 1 to 4 mg once daily in the morning. Initiate treatment at 1 mg/d, and adjust the dose in increments of no more than 1 mg/week, evaluating the patient weekly. GXR maintenance therapy is frequently in the range of 2 to 4 mg/d.
Because adverse events such as hypotension, bradycardia, and sedation are dose-related, evaluate benefit and risk using mg/kg range approximation. GXR efficacy on a weight-adjusted (mg/kg) basis is consistent across a dosage range of 0.01 to 0.17 mg/kg/d. Clinically relevant improvements are usually observed beginning at doses of 0.05 to 0.08 mg/kg/d. In clinical trials, efficacy increased with increasing weight-adjusted dose (mg/kg), so if GXR is well-tolerated, doses up to 0.12 mg/kg once daily may provide additional benefit up to the maximum of 4 mg/d.
Instruct patients to swallow GXR whole because crushing, chewing, or otherwise breaking the tablet’s enteric coating will markedly enhance guanfacine release.
Abruptly discontinuing GXR is associated with infrequent, transient elevations in blood pressure above the patient’s baseline (ie, rebound). To minimize these effects, GXR should be gradually tapered in decrements of no more than 1 mg every 3 to 7 days. Isolated missed doses of GXR generally are not a problem, but ≥2 consecutive missed doses may warrant reinitiation of the titration schedule.
Related resource
- Guanfacine extended release (Intuniv) prescribing information. www.intuniv.com/documents/INTUNIV_Full_Prescribing_Information.pdf.
Drug brand names
- Atomoxetine • Strattera
- Guanfacine extended release • Intuniv
- Guanfacine immediate release • Tenex
- Ketoconazole • Nizoral
- Rifampin • Rifadin, Rimactane
- Valproic acid • Depakene, Depakote
Disclosure
Dr. Sallee receives grant/research support from the National Institutes of Health. He is a consultant to Otsuka, Nextwave, and Sepracor and a consultant to and speaker for Shire. Dr. Sallee is a consultant to, shareholder of, and member of the board of directors of P2D Inc. and a principal in Satiety Solutions.
1. Biederman J, Melmed RD, Patel A, et al. A randomized, double-blind, placebo-controlled study of guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder. Pediatrics. 2008;121:e73-e84.
2. Sallee F, McGough J, Wigal T, et al. For the SPD503 Study Group Guanfacine extended release in children and adolescents with attention deficit hyperactivity disorder: a placebo-controlled trial. J Am Acad Child Adolesc Psychiatry. 2009;48(2):155-165.
3. Arnsten AF, Cai JX, Goldman-Rakic PS. The α-2 adrenergic agonist guanfacine improves memory in aged monkeys without sedative or hypotensive side effects: evidence for α-2 receptor subtypes. J Neurosci. 1988;8:4287-4298.
4. Vijayraghavan S, Wang M, Birnbaum SG, et al. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat Neurosci. 2007;10:376-384.
5. Wang M, Ramos BP, Paspalas CD, et al. α 2-A adrenoceptors strengthen working memory networks by inhibiting cAMP-HCN channel signaling in prefrontal cortex. Cell. 2007;129:397-410.
6. Li BM, Mei ZT. Delayed-response deficit induced by local injection of the α 2-adrenergic antagonist yohimbine into the dorsolateral prefrontal cortex in young adult monkeys. Behav Neural Biol. 1994;62:134-139.
7. Scahill L, Chappell PB, Kim YS, et al. A placebo-controlled study of guanfacine in the treatment of children with tic disorders and attention deficit hyperactivity disorder. Am J Psychiatry. 2001;158:1067-1074.
8. Swearingen D, Pennick M, Shojaei A, et al. A phase I, randomized, open-label, crossover study of the single-dose pharmacokinetic properties of guanfacine extended-release 1-, 2-, and 4-mg tablets in healthy adults. Clin Ther. 2007;29:617-625.
9. Connor D, Spencer T, Kratochvil C, et al. Effects of guanfacine extended release on secondary measures in children with attention-deficit/hyperactivity disorder and oppositional symptoms. Abstract presented at: Annual Meeting of the American Psychiatric Association; May 18, 2009; San Francisco, CA.
10. Sallee FR, Lyne A, Wigal T, et al. Long-term safety and efficacy of guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2009;19(3):215-226.
Guanfacine extended release (GXR)—a selective α-2 adrenergic agonist FDA-approved for the treatment of attention-deficit/hyperactivity disorder (ADHD)—has demonstrated efficacy for inattentive and hyperactive/impulsive symptom domains in 2 large trials lasting 8 and 9 weeks.1,2 GXR’s once-daily formulation may increase adherence and deliver consistent control of symptoms across a full day ( Table 1 ).
Table 1
Guanfacine extended release: Fast facts
| Brand name: Intuniv |
| Indication: Attention-deficit/hyperactivity disorder |
| Approval date: September 3, 2009 |
| Availability date: November 2009 |
| Manufacturer: Shire |
| Dosing forms: 1-mg, 2-mg, 3-mg, and 4-mg extended-release tablets |
| Recommended dosage: 0.05 to 0.12 mg/kg once daily |
Clinical implications
GXR exhibits enhancement of noradrenergic pathways through selective direct receptor action in the prefrontal cortex.3 This mechanism of action is different from that of other FDA-approved ADHD medications. GXR can be used alone or in combination with stimulants or atomoxetine for treating complex ADHD, such as cases accompanied by oppositional features and emotional dysregulation or characterized by partial stimulant response.
How it works
Guanfacine—originally developed as an immediate-release (IR) antihypertensive—reduces sympathetic tone, causing centrally mediated vasodilation and reduced heart rate. Although GXR’s mechanism of action in ADHD is not known, the drug is a selective α-2A receptor agonist thought to directly engage postsynaptic receptors in the prefrontal cortex (PFC), an area of the brain believed to play a major role in attentional and organizational functions that preclinical research has linked to ADHD.3
The postsynaptic α-2A receptor is thought to play a central role in the optimal functioning of the PFC as illustrated by the “inverted U hypothesis of PFC activation.”4 In this model, cyclic adenosine monophosphate (cAMP) levels build within the prefrontal cortical neurons and cause specific ion channels—hyperpolarization-activated cyclic nucleotide gated (HCN) channels—to open on dendritic spines of these neurons.5 Activation of HCN channels effectively reduces membrane resistance, cutting off synaptic inputs and disconnecting PFC network connections. Because α-2A receptors are located in proximity to HCN channels, their stimulation by GXR closes HCN channels, inhibits further production of cAMP, and reestablishes synaptic function and the resulting network connectivity.5 Blockade of α-2A receptors by yohimbine reverses this process, eroding network connectivity, and in monkeys has been demonstrated to impair working memory,6 damage inhibition/impulse control, and produce locomotor hyperactivity.
Direct stimulation by GXR of the postsynaptic α-2A receptors is thought to:
- strengthen working memory
- reduce susceptibility to distraction
- improve attention regulation
- improve behavioral inhibition
- enhance impulse control.7
Pharmacokinetics
GXR offers enhanced pharmaceutics relative to IR guanfacine. IR guanfacine exhibits poor absorption characteristics—peak plasma concentration is achieved too rapidly and then declines precipitously, with considerable inter-individual variation.
GXR’s once-daily formulation is implemented by a proprietary enteric-coated sustained release mechanism8 that is meant to:
- control absorption
- provide a broad but flat plasma concentration profile
- reduce inter-individual variation of guanfacine exposure.
Compared with IR guanfacine, GXR exhibits delayed time of maximum concentration (Tmax) and reduced maximum concentration (Cmax). Therapeutic concentrations can be sustained over longer periods with reduced peak-to-trough fluctuation,8 which tends to improve tolerability and symptom control throughout the day. The convenience of once-daily dosing also may increase adherence.
GXR’s pharmacokinetic characteristics do not change with dose, but high-fat meals will increase absorption of the drug—Cmax increases by 75% and area under the plasma concentration time curve increases by 40%. Because GXR primarily is metabolized through cytochrome P450 (CYP) 3A4, CYP3A4 inhibitors such as ketoconazole will increase guanfacine plasma concentrations and elevate the risk of adverse events such as bradycardia, hypotension, and sedation. Conversely, CYP3A4 inducers such as rifampin will significantly reduce total guanfacine exposure. Coadministration of valproic acid with GXR can result in increased valproic acid levels, producing additive CNS side effects.
Efficacy
GXR reduced both inattentive and hyperactive/impulsive symptoms in 2 phase III, forced-dose, parallel-design, randomized, placebo-controlled trials ( Table 2 ). In the first trial,1 345 children age 6 to 17 received placebo or GXR, 2 mg, 3 mg, or 4 mg once daily for 8 weeks. In the second study,2 324 children age 6 to 17 received placebo or GXR, 1 mg, 2 mg, 3 mg, or 4 mg, once daily for 9 weeks; the 1-mg dose was given only to patients weighing <50 kg (<110 lbs).
In both trials, doses were increased in increments of 1 mg/week, and investigators evaluated participants’ ADHD signs and symptoms once a week using the clinician administered and scored ADHD Rating Scale-IV (ADHD-RS-IV). The primary outcome was change in total ADHD-RS-IV score from baseline to endpoint.
In both trials, patients taking GXR demonstrated statistically signifcant improvements in ADHD-RS-IV score starting 1 to 2 weeks after they began receiving once-daily GXR:
- In the first trial, the mean reduction in ADHD-RS-IV total score at endpoint was –16.7 for GXR compared with –8.9 for placebo (P < .0001).
- In the second, the reduction was –19.6 for GXR and –12.2 for placebo (P=.004).
Placebo-adjusted least squares mean changes from baseline were statistically significant for all GXR doses in the randomized treatment groups in both studies.
Secondary efficacy outcome measures included the Conners’ Parent Rating Scale-Revised: Short Form (CPRS-R) and the Conners’ Teacher Rating Scale-Revised: Short Form (CTRS-R).
Significant improvements were seen on both scales. On the CPRS-R, parents reported significant improvement across a full day (as measured at 6 PM, 8 PM, and 6 AM the next day). On the CTRS-R—which was used only in the first trial—teachers reported significant improvement throughout the school day (as measured at 10 AM and 2 PM).
Treating oppositional symptoms. In a collateral study,9 GXR was evaluated in complex ADHD patients age 6 to 12 who exhibited oppositional symptoms. The primary efficacy measure was change from baseline to endpoint in the oppositional subscale of the Conners’ Parent Rating Scale-Revised: Long Form (CPRS-R:L) score.
All subjects randomized to GXR started on a dose of 1 mg/d—which could be titrated by 1 mg/week during the 5-week, dose-optimization period to a maximum of 4 mg/d—and were maintained at their optimal doses for 3 additional weeks. Among the 217 subjects enrolled, 138 received GXR and 79, placebo.
Least-squares mean reductions from baseline to endpoint in CPRS-R:L oppositional subscale scores were –10.9 in the GXR group compared with –6.8 in the placebo group (P < .001; effect size 0.590). The GXR-treated group showed a significantly greater reduction in ADHD-RS-IV total score from baseline to endpoint compared with the placebo group (–23.8 vs –11.4, respectively, P < .001; effect size 0.916).
Table 2
Randomized, controlled trials supporting GXR’s effectiveness
for treating ADHD symptoms
| Study | Subjects | GXR dosages | Results |
|---|---|---|---|
| Biederman et al, 20087 ; phase III, forced-dose parallel-design | 345 ADHD patients age 6 to 17 | 2, 3, or 4 mg given once daily for 8 weeks | GXR was associated with significantly lower ADHD-RS-IV score compared with placebo (-16.7 vs -8.9) |
| Sallee et al, 20098 ; phase III, forced-dose parallel-design | 324 ADHD patients age 6 to 17 | 1,* 2, 3, or 4 mg given once daily for 9 weeks | GXR was associated with significantly lower ADHD-RS-IV score compared with placebo (-19.6 vs -12.2) |
| Connor et al, 20099 ; collateral study | 217 complex ADHD patients age 6 to 12 with oppositional symptoms | Starting dose 1 mg/d, titrated to a maximum of 4 mg/d for a total of 8 weeks | GXR was associated with significantly lower scores on CPRS-R:L oppositional subscale (-10.9 vs -6.8) and ADHD-RS-IV (-23.8 vs -11.4) compared with placebo |
| *1-mg dose was given only to subjects weighing <50 kg (<110 lbs) | |||
| ADHD: attention-deficit/hyperactivity disorder; ADHD-RS-IV: Attention-Deficit/Hyperactivity Disorder Rating Scale-IV; CPRS-R:L: Conners’ Parent Rating Scale-Revised: Long Form; GXR: guanfacine extended release | |||
Tolerability
In the phase III trials, the most commonly reported drug-related adverse reactions (occurring in ≥2% of patients) were:
- somnolence (38%)
- headache (24%)
- fatigue (14%)
- upper abdominal pain (10%)
- nausea, lethargy, dizziness, hypotension/decreased blood pressure, irritability (6% for each)
- decreased appetite (5%)
- dry mouth (4%)
- constipation (3%).
Many of these adverse reactions appear to be dose-related, particularly somnolence, sedation, abdominal pain, dizziness, and hypotension/decreased blood pressure.
Overall, GXR was well tolerated; clinicians rated most events as mild to moderate. Twelve percent of GXR patients discontinued the clinical studies because of adverse events, compared with 4% in the placebo groups. The most common adverse reactions leading to discontinuation were somnolence/sedation (6%) and fatigue (2%). Less common adverse reactions leading to discontinuation (occurring in 1% of patients) included hypotension/decreased blood pressure, headache, and dizziness.
Open-label safety trial. Sallee et al10 conducted a longer-term, open-label, flexible-dose safety continuation study of 259 GXR-treated patients (mean exposure 10 months), some of whom also received a psychostimulant. Common adverse reactions (occurring in ≥5% of subjects) included somnolence (45%), headache (26%), fatigue (16%), upper abdominal pain (11%), hypotension/decreased blood pressure (10%), vomiting (9%), dizziness (7%), nausea (7%), weight gain (7%), and irritability (6%).10 In a subset of patients, the onset of sedative events typically occurred within the first 3 weeks of GXR treatment and then declined with maintenance to a frequency of approximately 16%. The rates of somnolence, sedation, or fatigue were lowest among patients who also received a psychostimulant ( Figure ).
Distribution of GXR doses before the end of this study was 37% of patients on 4 mg, 33% on 3 mg, 27% on 2 mg, and 3% on 1 mg, suggesting a preference for maintenance doses of 3 to 4 mg/d. The most frequent adverse reactions leading to discontinuation were somnolence (3%), syncopal events (2%), increased weight (2%), depression (2%), and fatigue (2%). Other adverse reactions leading to discontinuation (occurring in approximately 1% of patients) included hypotension/decreased blood pressure, sedation, headache, and lethargy. Serious adverse reactions in the longer-term study in >1 patient included syncope (2%) and convulsion (0.4%).
Figure: Incidence of somnolence, sedation, and fatigue in study patients receiving GXR
with or without psychostimulants

In an open-label continuation study of 259 patients treated with guanfacine extended release (GXR), somnolence, sedation, or fatigue was reported by 49% of subjects overall, 59% of those who received GXR monotherapy, and 11% of those given GXR with a psychostimulant.
GXR: guanfacine extended release
Source: Reprinted with permission from Sallee FR, Lyne A, Wigal T, et al. Long-term safety and efficacy of guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2009;19(3):215-226 Safety warnings relating to the likelihood of hypotension, bradycardia, and possible syncope when prescribing GXR should be understood in the context of its pharmacologic action to lower heart rate and blood pressure. In the short-term (8 to 9 weeks) controlled trials, the maximum mean changes from baseline in systolic blood pressure, diastolic blood pressure, and pulse were -5 mm Hg, -3 mm Hg, and -6 bpm, respectively, for all dose groups combined. These changes, which generally occurred 1 week after reaching target doses of 1 to 4 mg/d, were dose-dependent but usually modest and did not cause other symptoms; however, hypotension and bradycardia can occur.
In the longer-term, open-label safety study,10 maximum decreases in systolic and diastolic blood pressure occurred in the first month of treatment; decreases were less pronounced over time. Syncope occurred in 1% of pediatric subjects but was not dose-dependent. Guanfacine IR can increase QT interval but not in a dose-dependent fashion.
Dosing
The approved dose range for GXR is 1 to 4 mg once daily in the morning. Initiate treatment at 1 mg/d, and adjust the dose in increments of no more than 1 mg/week, evaluating the patient weekly. GXR maintenance therapy is frequently in the range of 2 to 4 mg/d.
Because adverse events such as hypotension, bradycardia, and sedation are dose-related, evaluate benefit and risk using mg/kg range approximation. GXR efficacy on a weight-adjusted (mg/kg) basis is consistent across a dosage range of 0.01 to 0.17 mg/kg/d. Clinically relevant improvements are usually observed beginning at doses of 0.05 to 0.08 mg/kg/d. In clinical trials, efficacy increased with increasing weight-adjusted dose (mg/kg), so if GXR is well-tolerated, doses up to 0.12 mg/kg once daily may provide additional benefit up to the maximum of 4 mg/d.
Instruct patients to swallow GXR whole because crushing, chewing, or otherwise breaking the tablet’s enteric coating will markedly enhance guanfacine release.
Abruptly discontinuing GXR is associated with infrequent, transient elevations in blood pressure above the patient’s baseline (ie, rebound). To minimize these effects, GXR should be gradually tapered in decrements of no more than 1 mg every 3 to 7 days. Isolated missed doses of GXR generally are not a problem, but ≥2 consecutive missed doses may warrant reinitiation of the titration schedule.
Related resource
- Guanfacine extended release (Intuniv) prescribing information. www.intuniv.com/documents/INTUNIV_Full_Prescribing_Information.pdf.
Drug brand names
- Atomoxetine • Strattera
- Guanfacine extended release • Intuniv
- Guanfacine immediate release • Tenex
- Ketoconazole • Nizoral
- Rifampin • Rifadin, Rimactane
- Valproic acid • Depakene, Depakote
Disclosure
Dr. Sallee receives grant/research support from the National Institutes of Health. He is a consultant to Otsuka, Nextwave, and Sepracor and a consultant to and speaker for Shire. Dr. Sallee is a consultant to, shareholder of, and member of the board of directors of P2D Inc. and a principal in Satiety Solutions.
Guanfacine extended release (GXR)—a selective α-2 adrenergic agonist FDA-approved for the treatment of attention-deficit/hyperactivity disorder (ADHD)—has demonstrated efficacy for inattentive and hyperactive/impulsive symptom domains in 2 large trials lasting 8 and 9 weeks.1,2 GXR’s once-daily formulation may increase adherence and deliver consistent control of symptoms across a full day ( Table 1 ).
Table 1
Guanfacine extended release: Fast facts
| Brand name: Intuniv |
| Indication: Attention-deficit/hyperactivity disorder |
| Approval date: September 3, 2009 |
| Availability date: November 2009 |
| Manufacturer: Shire |
| Dosing forms: 1-mg, 2-mg, 3-mg, and 4-mg extended-release tablets |
| Recommended dosage: 0.05 to 0.12 mg/kg once daily |
Clinical implications
GXR exhibits enhancement of noradrenergic pathways through selective direct receptor action in the prefrontal cortex.3 This mechanism of action is different from that of other FDA-approved ADHD medications. GXR can be used alone or in combination with stimulants or atomoxetine for treating complex ADHD, such as cases accompanied by oppositional features and emotional dysregulation or characterized by partial stimulant response.
How it works
Guanfacine—originally developed as an immediate-release (IR) antihypertensive—reduces sympathetic tone, causing centrally mediated vasodilation and reduced heart rate. Although GXR’s mechanism of action in ADHD is not known, the drug is a selective α-2A receptor agonist thought to directly engage postsynaptic receptors in the prefrontal cortex (PFC), an area of the brain believed to play a major role in attentional and organizational functions that preclinical research has linked to ADHD.3
The postsynaptic α-2A receptor is thought to play a central role in the optimal functioning of the PFC as illustrated by the “inverted U hypothesis of PFC activation.”4 In this model, cyclic adenosine monophosphate (cAMP) levels build within the prefrontal cortical neurons and cause specific ion channels—hyperpolarization-activated cyclic nucleotide gated (HCN) channels—to open on dendritic spines of these neurons.5 Activation of HCN channels effectively reduces membrane resistance, cutting off synaptic inputs and disconnecting PFC network connections. Because α-2A receptors are located in proximity to HCN channels, their stimulation by GXR closes HCN channels, inhibits further production of cAMP, and reestablishes synaptic function and the resulting network connectivity.5 Blockade of α-2A receptors by yohimbine reverses this process, eroding network connectivity, and in monkeys has been demonstrated to impair working memory,6 damage inhibition/impulse control, and produce locomotor hyperactivity.
Direct stimulation by GXR of the postsynaptic α-2A receptors is thought to:
- strengthen working memory
- reduce susceptibility to distraction
- improve attention regulation
- improve behavioral inhibition
- enhance impulse control.7
Pharmacokinetics
GXR offers enhanced pharmaceutics relative to IR guanfacine. IR guanfacine exhibits poor absorption characteristics—peak plasma concentration is achieved too rapidly and then declines precipitously, with considerable inter-individual variation.
GXR’s once-daily formulation is implemented by a proprietary enteric-coated sustained release mechanism8 that is meant to:
- control absorption
- provide a broad but flat plasma concentration profile
- reduce inter-individual variation of guanfacine exposure.
Compared with IR guanfacine, GXR exhibits delayed time of maximum concentration (Tmax) and reduced maximum concentration (Cmax). Therapeutic concentrations can be sustained over longer periods with reduced peak-to-trough fluctuation,8 which tends to improve tolerability and symptom control throughout the day. The convenience of once-daily dosing also may increase adherence.
GXR’s pharmacokinetic characteristics do not change with dose, but high-fat meals will increase absorption of the drug—Cmax increases by 75% and area under the plasma concentration time curve increases by 40%. Because GXR primarily is metabolized through cytochrome P450 (CYP) 3A4, CYP3A4 inhibitors such as ketoconazole will increase guanfacine plasma concentrations and elevate the risk of adverse events such as bradycardia, hypotension, and sedation. Conversely, CYP3A4 inducers such as rifampin will significantly reduce total guanfacine exposure. Coadministration of valproic acid with GXR can result in increased valproic acid levels, producing additive CNS side effects.
Efficacy
GXR reduced both inattentive and hyperactive/impulsive symptoms in 2 phase III, forced-dose, parallel-design, randomized, placebo-controlled trials ( Table 2 ). In the first trial,1 345 children age 6 to 17 received placebo or GXR, 2 mg, 3 mg, or 4 mg once daily for 8 weeks. In the second study,2 324 children age 6 to 17 received placebo or GXR, 1 mg, 2 mg, 3 mg, or 4 mg, once daily for 9 weeks; the 1-mg dose was given only to patients weighing <50 kg (<110 lbs).
In both trials, doses were increased in increments of 1 mg/week, and investigators evaluated participants’ ADHD signs and symptoms once a week using the clinician administered and scored ADHD Rating Scale-IV (ADHD-RS-IV). The primary outcome was change in total ADHD-RS-IV score from baseline to endpoint.
In both trials, patients taking GXR demonstrated statistically signifcant improvements in ADHD-RS-IV score starting 1 to 2 weeks after they began receiving once-daily GXR:
- In the first trial, the mean reduction in ADHD-RS-IV total score at endpoint was –16.7 for GXR compared with –8.9 for placebo (P < .0001).
- In the second, the reduction was –19.6 for GXR and –12.2 for placebo (P=.004).
Placebo-adjusted least squares mean changes from baseline were statistically significant for all GXR doses in the randomized treatment groups in both studies.
Secondary efficacy outcome measures included the Conners’ Parent Rating Scale-Revised: Short Form (CPRS-R) and the Conners’ Teacher Rating Scale-Revised: Short Form (CTRS-R).
Significant improvements were seen on both scales. On the CPRS-R, parents reported significant improvement across a full day (as measured at 6 PM, 8 PM, and 6 AM the next day). On the CTRS-R—which was used only in the first trial—teachers reported significant improvement throughout the school day (as measured at 10 AM and 2 PM).
Treating oppositional symptoms. In a collateral study,9 GXR was evaluated in complex ADHD patients age 6 to 12 who exhibited oppositional symptoms. The primary efficacy measure was change from baseline to endpoint in the oppositional subscale of the Conners’ Parent Rating Scale-Revised: Long Form (CPRS-R:L) score.
All subjects randomized to GXR started on a dose of 1 mg/d—which could be titrated by 1 mg/week during the 5-week, dose-optimization period to a maximum of 4 mg/d—and were maintained at their optimal doses for 3 additional weeks. Among the 217 subjects enrolled, 138 received GXR and 79, placebo.
Least-squares mean reductions from baseline to endpoint in CPRS-R:L oppositional subscale scores were –10.9 in the GXR group compared with –6.8 in the placebo group (P < .001; effect size 0.590). The GXR-treated group showed a significantly greater reduction in ADHD-RS-IV total score from baseline to endpoint compared with the placebo group (–23.8 vs –11.4, respectively, P < .001; effect size 0.916).
Table 2
Randomized, controlled trials supporting GXR’s effectiveness
for treating ADHD symptoms
| Study | Subjects | GXR dosages | Results |
|---|---|---|---|
| Biederman et al, 20087 ; phase III, forced-dose parallel-design | 345 ADHD patients age 6 to 17 | 2, 3, or 4 mg given once daily for 8 weeks | GXR was associated with significantly lower ADHD-RS-IV score compared with placebo (-16.7 vs -8.9) |
| Sallee et al, 20098 ; phase III, forced-dose parallel-design | 324 ADHD patients age 6 to 17 | 1,* 2, 3, or 4 mg given once daily for 9 weeks | GXR was associated with significantly lower ADHD-RS-IV score compared with placebo (-19.6 vs -12.2) |
| Connor et al, 20099 ; collateral study | 217 complex ADHD patients age 6 to 12 with oppositional symptoms | Starting dose 1 mg/d, titrated to a maximum of 4 mg/d for a total of 8 weeks | GXR was associated with significantly lower scores on CPRS-R:L oppositional subscale (-10.9 vs -6.8) and ADHD-RS-IV (-23.8 vs -11.4) compared with placebo |
| *1-mg dose was given only to subjects weighing <50 kg (<110 lbs) | |||
| ADHD: attention-deficit/hyperactivity disorder; ADHD-RS-IV: Attention-Deficit/Hyperactivity Disorder Rating Scale-IV; CPRS-R:L: Conners’ Parent Rating Scale-Revised: Long Form; GXR: guanfacine extended release | |||
Tolerability
In the phase III trials, the most commonly reported drug-related adverse reactions (occurring in ≥2% of patients) were:
- somnolence (38%)
- headache (24%)
- fatigue (14%)
- upper abdominal pain (10%)
- nausea, lethargy, dizziness, hypotension/decreased blood pressure, irritability (6% for each)
- decreased appetite (5%)
- dry mouth (4%)
- constipation (3%).
Many of these adverse reactions appear to be dose-related, particularly somnolence, sedation, abdominal pain, dizziness, and hypotension/decreased blood pressure.
Overall, GXR was well tolerated; clinicians rated most events as mild to moderate. Twelve percent of GXR patients discontinued the clinical studies because of adverse events, compared with 4% in the placebo groups. The most common adverse reactions leading to discontinuation were somnolence/sedation (6%) and fatigue (2%). Less common adverse reactions leading to discontinuation (occurring in 1% of patients) included hypotension/decreased blood pressure, headache, and dizziness.
Open-label safety trial. Sallee et al10 conducted a longer-term, open-label, flexible-dose safety continuation study of 259 GXR-treated patients (mean exposure 10 months), some of whom also received a psychostimulant. Common adverse reactions (occurring in ≥5% of subjects) included somnolence (45%), headache (26%), fatigue (16%), upper abdominal pain (11%), hypotension/decreased blood pressure (10%), vomiting (9%), dizziness (7%), nausea (7%), weight gain (7%), and irritability (6%).10 In a subset of patients, the onset of sedative events typically occurred within the first 3 weeks of GXR treatment and then declined with maintenance to a frequency of approximately 16%. The rates of somnolence, sedation, or fatigue were lowest among patients who also received a psychostimulant ( Figure ).
Distribution of GXR doses before the end of this study was 37% of patients on 4 mg, 33% on 3 mg, 27% on 2 mg, and 3% on 1 mg, suggesting a preference for maintenance doses of 3 to 4 mg/d. The most frequent adverse reactions leading to discontinuation were somnolence (3%), syncopal events (2%), increased weight (2%), depression (2%), and fatigue (2%). Other adverse reactions leading to discontinuation (occurring in approximately 1% of patients) included hypotension/decreased blood pressure, sedation, headache, and lethargy. Serious adverse reactions in the longer-term study in >1 patient included syncope (2%) and convulsion (0.4%).
Figure: Incidence of somnolence, sedation, and fatigue in study patients receiving GXR
with or without psychostimulants

In an open-label continuation study of 259 patients treated with guanfacine extended release (GXR), somnolence, sedation, or fatigue was reported by 49% of subjects overall, 59% of those who received GXR monotherapy, and 11% of those given GXR with a psychostimulant.
GXR: guanfacine extended release
Source: Reprinted with permission from Sallee FR, Lyne A, Wigal T, et al. Long-term safety and efficacy of guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2009;19(3):215-226 Safety warnings relating to the likelihood of hypotension, bradycardia, and possible syncope when prescribing GXR should be understood in the context of its pharmacologic action to lower heart rate and blood pressure. In the short-term (8 to 9 weeks) controlled trials, the maximum mean changes from baseline in systolic blood pressure, diastolic blood pressure, and pulse were -5 mm Hg, -3 mm Hg, and -6 bpm, respectively, for all dose groups combined. These changes, which generally occurred 1 week after reaching target doses of 1 to 4 mg/d, were dose-dependent but usually modest and did not cause other symptoms; however, hypotension and bradycardia can occur.
In the longer-term, open-label safety study,10 maximum decreases in systolic and diastolic blood pressure occurred in the first month of treatment; decreases were less pronounced over time. Syncope occurred in 1% of pediatric subjects but was not dose-dependent. Guanfacine IR can increase QT interval but not in a dose-dependent fashion.
Dosing
The approved dose range for GXR is 1 to 4 mg once daily in the morning. Initiate treatment at 1 mg/d, and adjust the dose in increments of no more than 1 mg/week, evaluating the patient weekly. GXR maintenance therapy is frequently in the range of 2 to 4 mg/d.
Because adverse events such as hypotension, bradycardia, and sedation are dose-related, evaluate benefit and risk using mg/kg range approximation. GXR efficacy on a weight-adjusted (mg/kg) basis is consistent across a dosage range of 0.01 to 0.17 mg/kg/d. Clinically relevant improvements are usually observed beginning at doses of 0.05 to 0.08 mg/kg/d. In clinical trials, efficacy increased with increasing weight-adjusted dose (mg/kg), so if GXR is well-tolerated, doses up to 0.12 mg/kg once daily may provide additional benefit up to the maximum of 4 mg/d.
Instruct patients to swallow GXR whole because crushing, chewing, or otherwise breaking the tablet’s enteric coating will markedly enhance guanfacine release.
Abruptly discontinuing GXR is associated with infrequent, transient elevations in blood pressure above the patient’s baseline (ie, rebound). To minimize these effects, GXR should be gradually tapered in decrements of no more than 1 mg every 3 to 7 days. Isolated missed doses of GXR generally are not a problem, but ≥2 consecutive missed doses may warrant reinitiation of the titration schedule.
Related resource
- Guanfacine extended release (Intuniv) prescribing information. www.intuniv.com/documents/INTUNIV_Full_Prescribing_Information.pdf.
Drug brand names
- Atomoxetine • Strattera
- Guanfacine extended release • Intuniv
- Guanfacine immediate release • Tenex
- Ketoconazole • Nizoral
- Rifampin • Rifadin, Rimactane
- Valproic acid • Depakene, Depakote
Disclosure
Dr. Sallee receives grant/research support from the National Institutes of Health. He is a consultant to Otsuka, Nextwave, and Sepracor and a consultant to and speaker for Shire. Dr. Sallee is a consultant to, shareholder of, and member of the board of directors of P2D Inc. and a principal in Satiety Solutions.
1. Biederman J, Melmed RD, Patel A, et al. A randomized, double-blind, placebo-controlled study of guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder. Pediatrics. 2008;121:e73-e84.
2. Sallee F, McGough J, Wigal T, et al. For the SPD503 Study Group Guanfacine extended release in children and adolescents with attention deficit hyperactivity disorder: a placebo-controlled trial. J Am Acad Child Adolesc Psychiatry. 2009;48(2):155-165.
3. Arnsten AF, Cai JX, Goldman-Rakic PS. The α-2 adrenergic agonist guanfacine improves memory in aged monkeys without sedative or hypotensive side effects: evidence for α-2 receptor subtypes. J Neurosci. 1988;8:4287-4298.
4. Vijayraghavan S, Wang M, Birnbaum SG, et al. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat Neurosci. 2007;10:376-384.
5. Wang M, Ramos BP, Paspalas CD, et al. α 2-A adrenoceptors strengthen working memory networks by inhibiting cAMP-HCN channel signaling in prefrontal cortex. Cell. 2007;129:397-410.
6. Li BM, Mei ZT. Delayed-response deficit induced by local injection of the α 2-adrenergic antagonist yohimbine into the dorsolateral prefrontal cortex in young adult monkeys. Behav Neural Biol. 1994;62:134-139.
7. Scahill L, Chappell PB, Kim YS, et al. A placebo-controlled study of guanfacine in the treatment of children with tic disorders and attention deficit hyperactivity disorder. Am J Psychiatry. 2001;158:1067-1074.
8. Swearingen D, Pennick M, Shojaei A, et al. A phase I, randomized, open-label, crossover study of the single-dose pharmacokinetic properties of guanfacine extended-release 1-, 2-, and 4-mg tablets in healthy adults. Clin Ther. 2007;29:617-625.
9. Connor D, Spencer T, Kratochvil C, et al. Effects of guanfacine extended release on secondary measures in children with attention-deficit/hyperactivity disorder and oppositional symptoms. Abstract presented at: Annual Meeting of the American Psychiatric Association; May 18, 2009; San Francisco, CA.
10. Sallee FR, Lyne A, Wigal T, et al. Long-term safety and efficacy of guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2009;19(3):215-226.
1. Biederman J, Melmed RD, Patel A, et al. A randomized, double-blind, placebo-controlled study of guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder. Pediatrics. 2008;121:e73-e84.
2. Sallee F, McGough J, Wigal T, et al. For the SPD503 Study Group Guanfacine extended release in children and adolescents with attention deficit hyperactivity disorder: a placebo-controlled trial. J Am Acad Child Adolesc Psychiatry. 2009;48(2):155-165.
3. Arnsten AF, Cai JX, Goldman-Rakic PS. The α-2 adrenergic agonist guanfacine improves memory in aged monkeys without sedative or hypotensive side effects: evidence for α-2 receptor subtypes. J Neurosci. 1988;8:4287-4298.
4. Vijayraghavan S, Wang M, Birnbaum SG, et al. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat Neurosci. 2007;10:376-384.
5. Wang M, Ramos BP, Paspalas CD, et al. α 2-A adrenoceptors strengthen working memory networks by inhibiting cAMP-HCN channel signaling in prefrontal cortex. Cell. 2007;129:397-410.
6. Li BM, Mei ZT. Delayed-response deficit induced by local injection of the α 2-adrenergic antagonist yohimbine into the dorsolateral prefrontal cortex in young adult monkeys. Behav Neural Biol. 1994;62:134-139.
7. Scahill L, Chappell PB, Kim YS, et al. A placebo-controlled study of guanfacine in the treatment of children with tic disorders and attention deficit hyperactivity disorder. Am J Psychiatry. 2001;158:1067-1074.
8. Swearingen D, Pennick M, Shojaei A, et al. A phase I, randomized, open-label, crossover study of the single-dose pharmacokinetic properties of guanfacine extended-release 1-, 2-, and 4-mg tablets in healthy adults. Clin Ther. 2007;29:617-625.
9. Connor D, Spencer T, Kratochvil C, et al. Effects of guanfacine extended release on secondary measures in children with attention-deficit/hyperactivity disorder and oppositional symptoms. Abstract presented at: Annual Meeting of the American Psychiatric Association; May 18, 2009; San Francisco, CA.
10. Sallee FR, Lyne A, Wigal T, et al. Long-term safety and efficacy of guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2009;19(3):215-226.