User login
In clinical practice, for the majority of patients with psoriasis superpotent topical corticosteroids (TCSs) are used as initial therapy as well as ongoing breakthrough therapy to achieve quick resolution of target lesions. However, safe and effective long-term treatment and maintenance options are required for managing the chronic nature of psoriasis to improve patient satisfaction, adherence, and quality of life, especially given that package inserts advise no more than 2 to 4 weeks of continuous use to limit side effects. The long-term use of superpotent TCSs can have a multitude of unwanted cutaneous side effects, such as skin atrophy, telangiectases, striae, and allergic vehicle responses.1,2 Tachyphylaxis, a decreased response to treatment over time, has been more controversial and may not occur with halobetasol propionate (HP) ointment 0.05%.3 In addition, TCSs are associated with relapse or rebound on withdrawal, which can be problematic but are poorly characterized.
We review the clinical data on HP, a superpotent TCS, in the treatment of psoriasis. We also explore both recent formulation developments and fixed-combination approaches to providing optimal treatment.
Clinical Experience With HP 0.05% in Various Formulations
Halobetasol propionate is a superpotent TCS with extensive clinical experience in treating psoriasis spanning nearly 30 years.1,2,3-7 Most recently, a twice-daily HP lotion 0.05% formulation was evaluated in patients with moderate to severe disease.8 Halobetasol propionate lotion 0.05% applied morning and night was shown to be significantly more effective than vehicle after 2 weeks of treatment (P<.001) in 2 parallel-group studies of 443 patients.9 Treatment success (ie, at least a 2-grade improvement in investigator global assessment [IGA] and IGA score of clear or almost clear) was achieved in 44.5% of patients treated with HP lotion 0.05% compared to 6.3% and 7.1% in the 2 vehicle arms. Treatment-related adverse events (AEs) were uncommon, with application-site pain reported in 2 patients treated with HP lotion 0.05% compared to 5 patients treated with vehicle.9
Several earlier studies have evaluated the short-term efficacy of twice-daily HP cream 0.05% and HP ointment 0.05% in the treatment of plaque psoriasis, but only 2 placebo-controlled trials have been reported, and data are limited.
Two 2-week studies of twice-daily HP ointment 0.05% (paired-comparison and parallel-group designs) in 204 patients with moderate plaque psoriasis reported improvement in plaque elevation, erythema, and scaling compared to vehicle. Patient global responses and physician global evaluation favored HP ointment 0.05%, and reports of stinging and burning were similar with active treatment and vehicle.4
Similarly, HP cream 0.05% applied twice daily was shown to be significantly superior to vehicle in reducing overall disease severity, erythema, plaque elevation, and scaling after 1 and 2 weeks of treatment in a paired-comparison study of 110 patients (P=.0001).5 A clinically significant reduction (at least a 1-grade improvement) in erythema, plaque elevation, pruritus, and scaling was noted in 81% to 92% of patients (P=.0001). Patients’ self-assessment of effectiveness rated HP cream 0.05% as excellent, very good, or good in 69% of patients compared to 20% for vehicle. Treatment-related AEs were reported by 4 patients.5
A small, noncontrolled, 2-week pediatric study (N=11) demonstrated the efficacy of combined therapy with HP cream 0.05% every morning and HP ointment 0.05% every night due to the then-perceived preference for creams as being more pleasant to apply during the day and ointments being more efficacious. Reported side effects were relatively mild, with application-site burning being the most common.10
Potential local AEs associated with HP are similar to those seen with other superpotent TCSs. Overall, they were reported in 0% to 13% of patients. The most common AEs were burning, pruritus, erythema, hypopigmentation, dryness, and folliculitis.5-8,10-14 Isolated cases of moderate telangiectasia and mild atrophy also have been reported.8,10
Comparative Studies With Other TCSs
In comparative studies of patients with severe localized plaque psoriasis, HP ointment 0.05% applied twice daily for up to 4 weeks was significantly superior compared to clobetasol propionate ointment 0.05% for the number of patients with none or mild disease (P=.0237) or comparisons of global evaluation scores (P=.01315) at week 2, or compared to betamethasone valerate ointment 0.1% (P=.02).6 It also was more effective than betamethasone dipropionate ointment 0.05% with healing seen in 40% of patients treated with HP ointment 0.05% within 24 days compared to 25% of patients treated with betamethasone dipropionate ointment 0.05%.8 Patient acceptance of HP ointment 0.05% based on cosmetic acceptability and ease of application was better (very good in 90% vs 80% of patients7) or significantly better compared to clobetasol propionate ointment 0.05% (P=.042 and P=.01915) and betamethasone dipropionate ointment 0.05% (P=.02).8
Evolving Management Strategies
A number of management strategies have been proposed to improve the safety and efficacy of long-term therapy with TCSs, including weekend-only or pulse therapy, dose reduction, rotating to another therapy, or combining with other topical therapies. Maintenance efficacy data are sparse. A small double-blind study in 44 patients with mild to moderate psoriasis was conducted wherein patients were treated with calcipotriene ointment in the morning and HP ointment in the evening for 2 weeks.16 Those patients who achieved at least a 50% improvement in disease severity (N=40) were randomized to receive HP ointment twice daily on weekends and calcipotriene ointment or placebo twice daily on weekdays for 6 months. Seventy-six percent of those patients treated with a HP/calcipotriene pulsed therapy maintained remission (achieving and maintaining a 75% improvement in physician global assessment) compared to 40% of those patients treated with HP only (P=.045). Mild AEs were reported in 4 patients treated with the combination regimen and 1 patient treated with HP only. No AE-related discontinuations occurred.16
In a real-world setting, a maintenance regimen that is less complicated enhances the potential for increased patient adherence and successful outcomes.17 After an initial 2-week regimen of twice-daily HP ointment 0.05% in combination with ammonium lactate lotion in patients with mild to moderate psoriasis (N=55), those rated clear or almost clear (41/55 [74.6%]) entered a maintenance phase, applying ammonium lactate lotion twice daily and either HP or placebo ointment twice daily on weekends. The probability of disease worsening by week 14 was 29% in the HP-treated group compared to 100% in the placebo group (P<.0001). By week 24, 12 patients (29.2%) remained clear or almost clear.17
Development of HP Lotion 0.01%
There are numerous examples in dermatology where advances in formulation development have made it possible to reduce the strength of active ingredients without compromising efficacy. Formulation advances also afford improved safety profiles that can extend a product’s utility. The vehicle affects not only the potency of an agent but also patient compliance, which is crucial for adequate response. Patients prefer lighter vehicles, such as lotions, over heavy ointments and creams.18,19
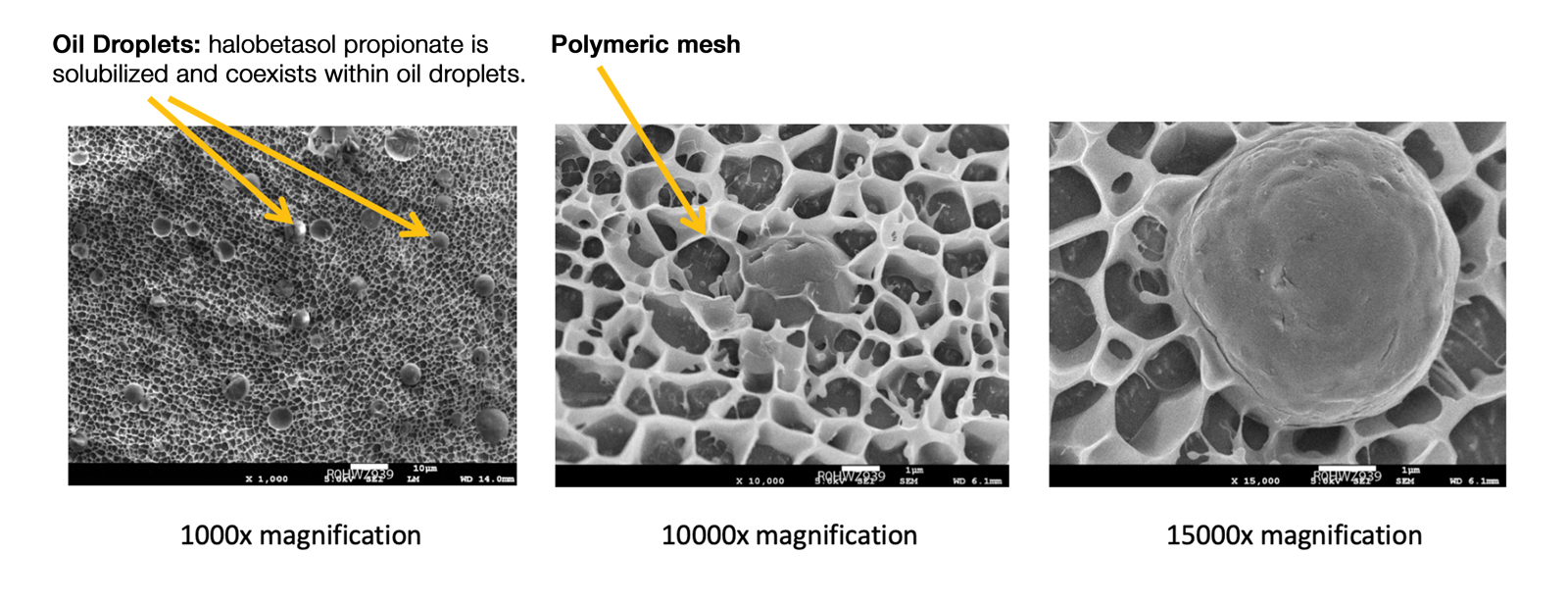
Recently, a polymeric honeycomb matrix (carbomer cross-linked polymers), which helps structure the oil emulsion and provide a uniform distribution of both active and moisturizing/hydrating ingredients (ie, sorbitol, light mineral oil, diethyl sebacate) at the surface of the skin, has been deployed for topical delivery of HP (eFigure 1). Ninety percent of the oil droplets containing solubilized halobetasol are 13 µm or smaller, an ideal size for penetration through follicular openings (unpublished data, Bausch Health, 2018).
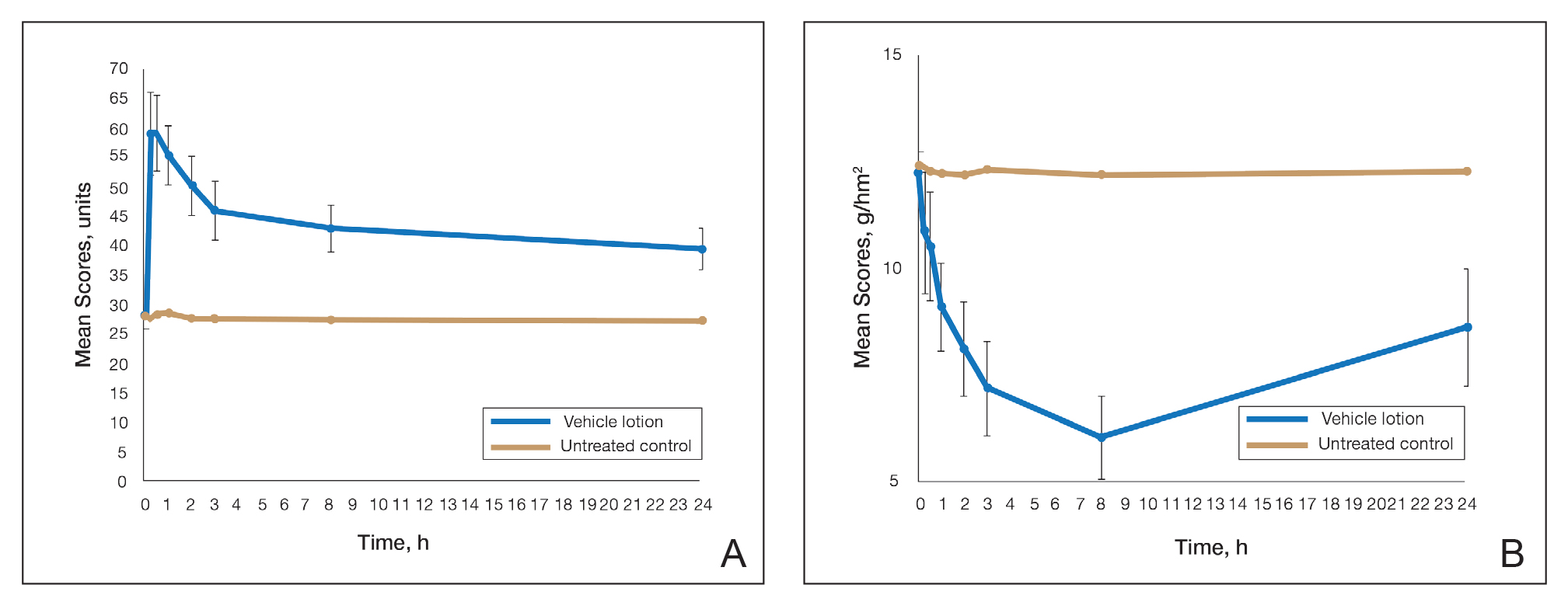
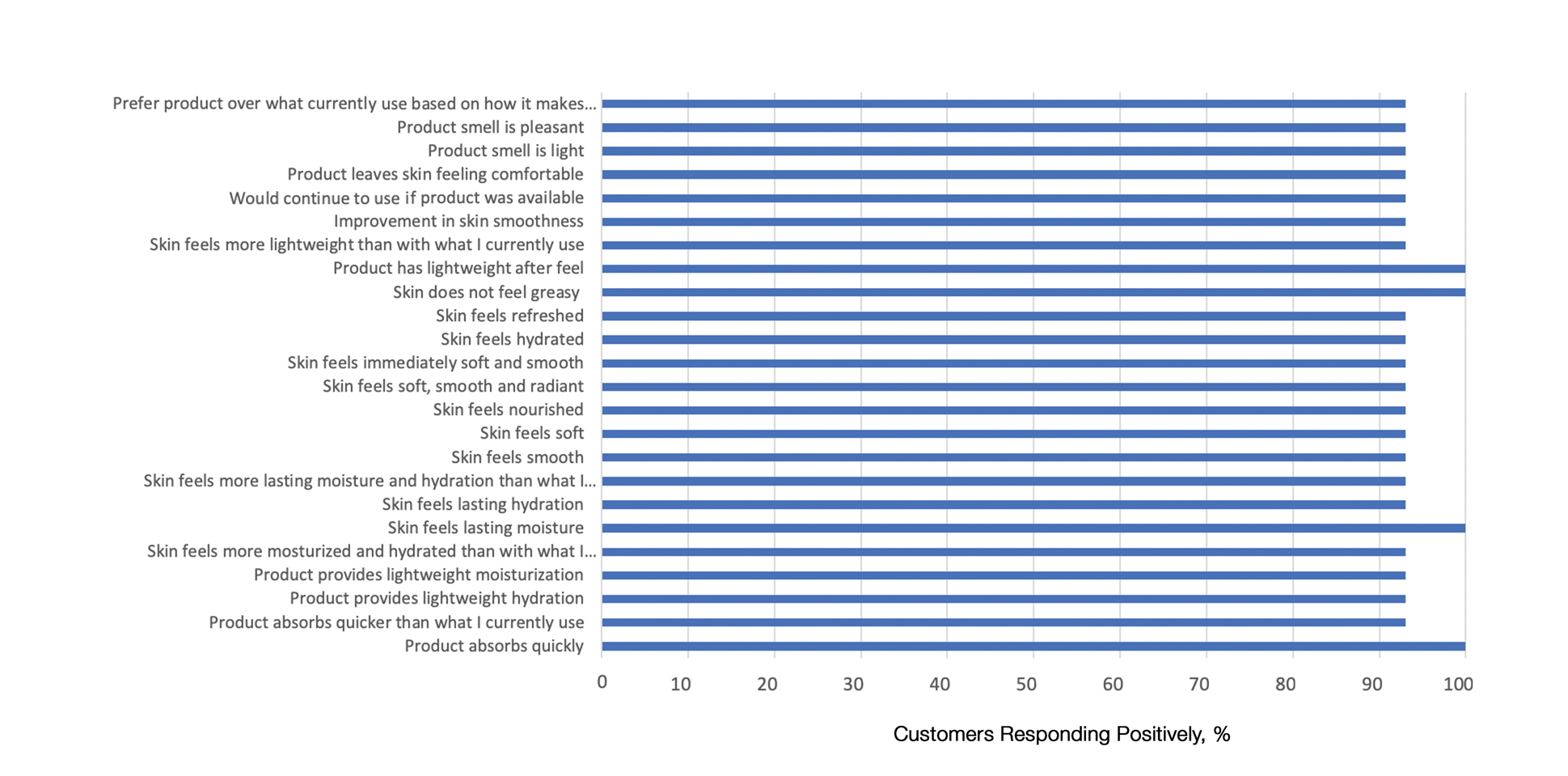
This polymerized emulsion also forms a barrier by reducing epidermal water loss and improving skin hydration. Skin hydration and barrier protection of the lotion were assessed through corneometry and transepidermal water loss (TEWL) in 30 healthy female volunteers (aged 35–65 years) over 24 hours. The test material was applied to the volar forearm, with an untreated site serving as a control. Measurements using Tewameter and Corneometer were taken at baseline; 15 and 30 minutes; and 1, 2, 3, 8, and 24 hours postapplication. In addition, for the 8-hour study period, 15 patients applied the test material to the right side of the face and completed a customer-perception evaluation. Adverse events were noted throughout and irritation was assessed preapplication and postapplication. There were no AEs or skin irritation reported throughout the study. At baseline, mean (standard deviation [SD]) corneometry scores were 28.9 (2.9) and 28.1 (2.7) units for the test material and untreated control, respectively. There was an immediate improvement in water content that was maintained throughout the study. After 15 minutes, the mean (SD) score had increased to 59.1 (7.1) units in the vehicle lotion group (eFigure 2A). There was no improvement at the control site, and differences were significant at all postapplication assessments (P<.001). At baseline, mean (SD) TEWL scores were 12.26 (0.48) and 12.42 (0.44) g/hm2, respectively (eFigure 2B). There was an immediate improvement in TEWL with a mean (SD) score of 6.04 (0.99) after 8 hours in the vehicle lotion group, a 50.7% change over baseline. There was no improvement at the control site, and differences were significant at all postapplication assessments (P<.001). Customer perception of the novel lotion formulation was positive, with the majority of patients (93%–100%) responding favorably to all questions about the various attributes of the test material (eFigure 3)(unpublished data, Bausch Health, 2018).
Comparison of Skin Penetration of HP Lotion 0.01% vs HP Cream 0.05%
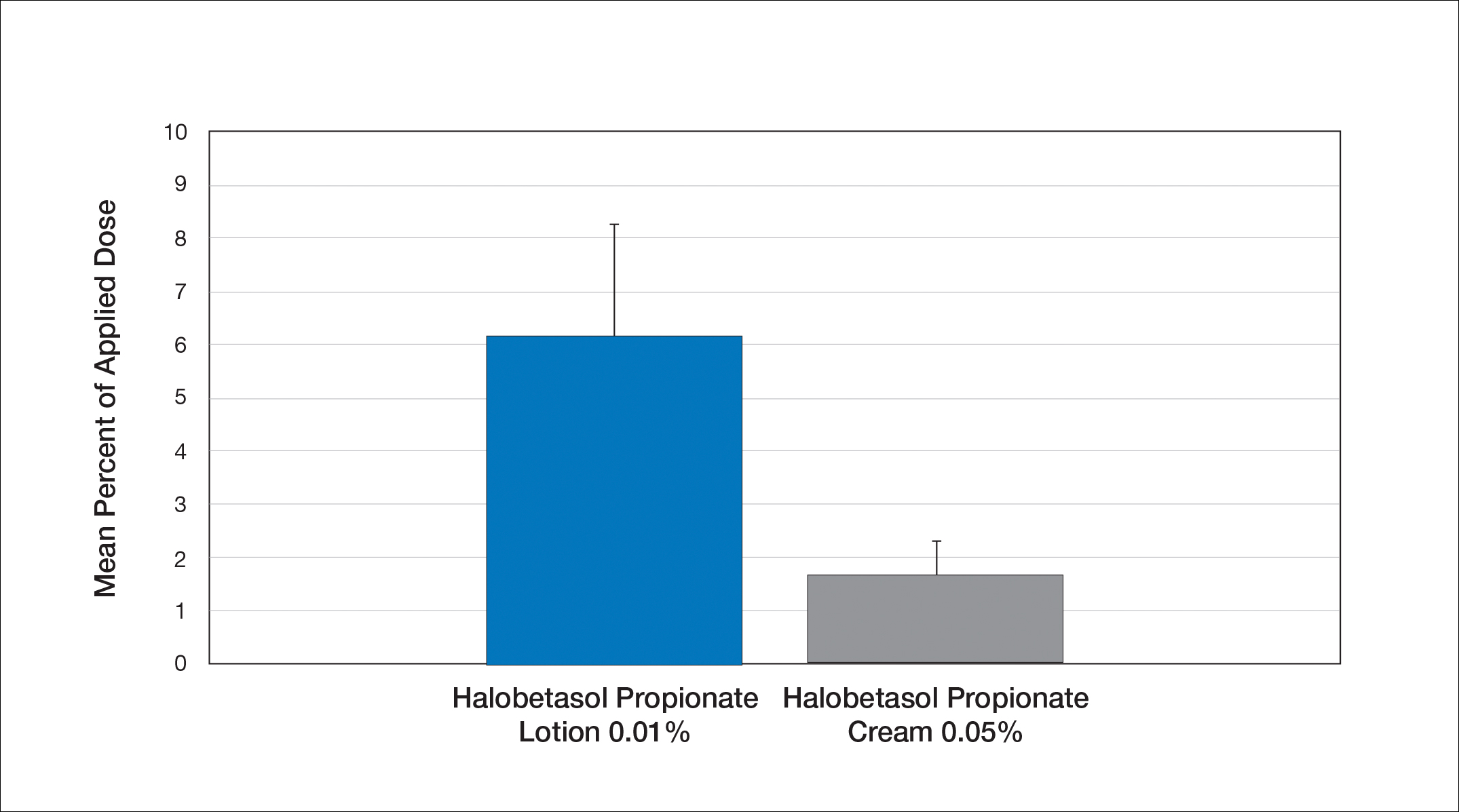
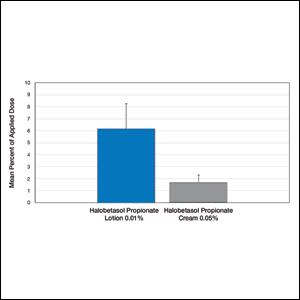
Comparative percutaneous absorption of 2 HP formulations—0.01% lotion and 0.05% cream—was evaluated in vitro using human tissue from a single donor mounted on Bronaugh flow-through diffusion cells. Receptor phase samples were collected over the 24-hour study period and HP content assessed using liquid chromatography–mass spectrometry analysis. Halobetasol propionate lotion 0.01% demonstrated faster tissue permeation, with receptor phase levels of 0.91% of the applied dose at 24 hours compared to 0.28% of the applied dose with HP cream 0.05%. Although there was little differentiation of cumulative receptor fluid levels of HP at 6 hours, there was significant differentiation at 12 hours. Levels of HP were lowest in the receptor phase and highest in the epidermal layers of the skin, indicating limited permeation through the epidermis to the dermis. The mean (SD) for epidermal deposition of HP following the 24-hour duration of exposure was 6.17% (2.07%) and 1.72% (0.76%) for the 0.01% lotion and 0.05% cream, respectively (Figure 1)(unpublished data, Bausch Health, 2018).
Efficacy and Safety of HP Lotion 0.01% in Moderate to Severe Plaque Psoriasis
Two articles have been published on the use of HP lotion 0.01% in moderate to severe psoriasis: 2 pivotal studies comparing once-daily application with vehicle lotion over 8 weeks (N=430),20 and a comparative “label-restricted” 2-week study with HP lotion 0.01% and HP cream 0.05% (N=150).21
HP Lotion 0.01% Compared to Vehicle
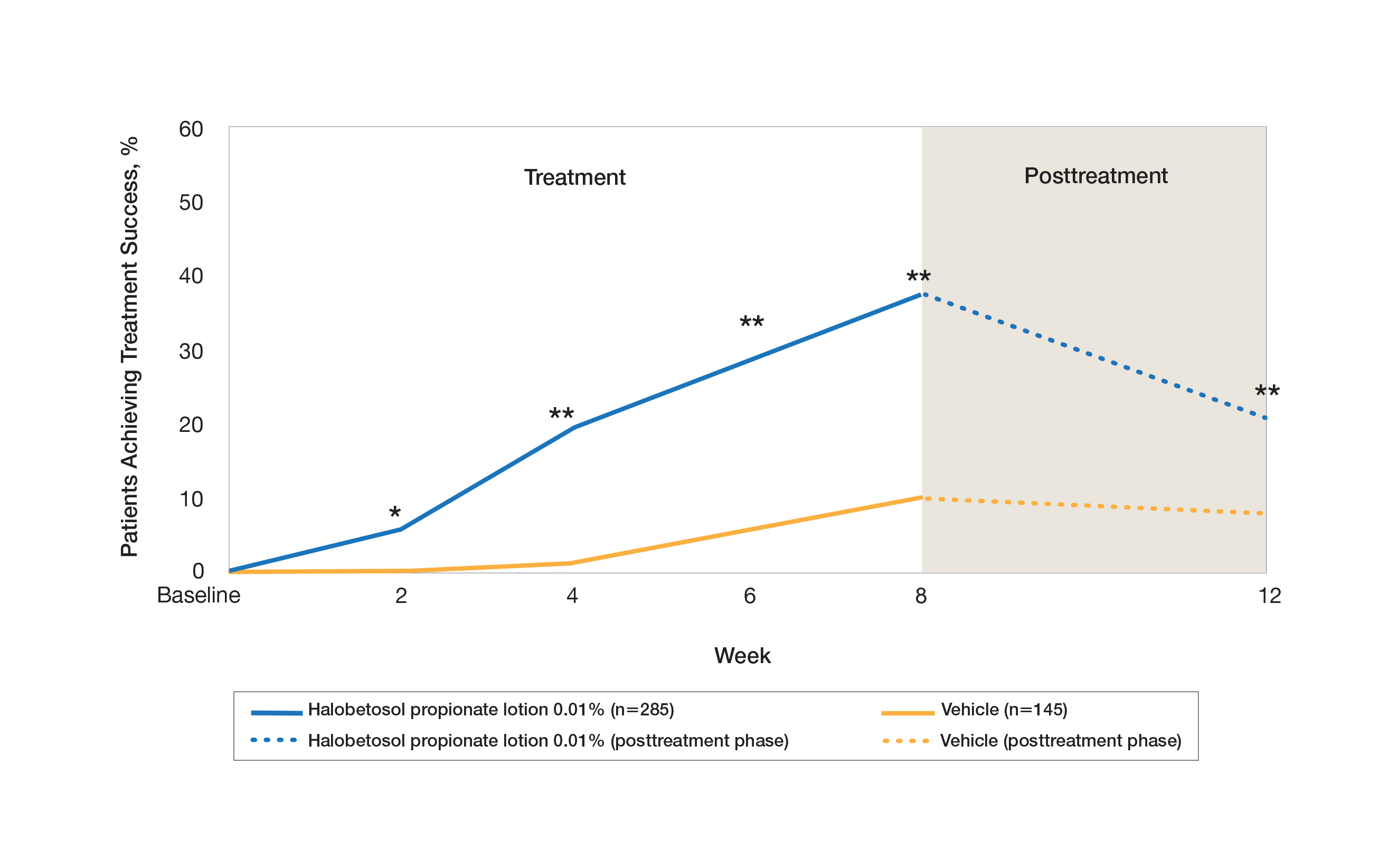
Two multicenter, randomized, double-blind, vehicle-controlled phase 3 studies investigated the safety and efficacy of once-daily HP lotion 0.01% in moderate to severe plaque psoriasis (N=430).20 Patients were treated with HP lotion 0.01% or vehicle (randomized in a 2:1 ratio) for 8 weeks, with a 4-week posttreatment follow-up. Treatment success (defined as at least a 2-grade improvement in baseline IGA score and a score equating to clear or almost clear) was significantly greater with HP lotion 0.01% at all assessment points (Figure 2)(P=.003 for week 2; P<.001 for other time points). At week 8, 37.4% of patients receiving HP lotion 0.01% were treatment successes compared to 10.0% of patients receiving vehicle (P<.001). Additionally, a 2-grade improvement from baseline for each psoriasis sign—erythema, plaque elevation, and scaling—was achieved by 42.2% of patients receiving HP lotion 0.01% at week 8 compared to 11.4% of patients receiving vehicle (P<.001). Good efficacy was maintained posttreatment that was significant compared to vehicle (P<.001).20
There were corresponding reductions in body surface area (BSA) affected following treatment with HP lotion 0.01%.20 At baseline, the mean BSA was 6.1 (range, 3–12). By week 8, there was a 35.2% reduction in BSA compared to 5.9% with vehicle. Again, a significant reduction in BSA was maintained posttreatment compared to vehicle (P<.001).20
Halobetasol propionate lotion 0.01% was well tolerated with few treatment-related AEs.20 Most AEs were application-site reactions such as dermatitis (0.7%), infection, pruritus, and discoloration (0.4% each). Mild to moderate itching, dryness, burning, and stinging present at baseline all improved with treatment, and severity of local skin reactions was significantly lower than with vehicle at week 8 (P<.001). Quality-of-life data also highlighted the benefits of active treatment compared to vehicle for cutaneous tolerability. The Dermatology Life Quality Index (DLQI) is a 10-item patient-reported questionnaire consisting of questions concerning symptoms and feelings, daily activities, leisure, work and school, personal relationships, and treatment.22 Change from baseline for DLQI (how itchy, sore, painful, stinging) was significantly greater with HP lotion 0.01% at weeks 4 and 8 (P<.001). Changes in the overall DLQI score also were significantly greater with HP lotion 0.01% at both study visits (P=.006 and P=.014 at week 4 and P=.001 and P=.004 at week 8 for study 1 and study 2, respectively).20
HP Lotion 0.01% Compared to HP Cream 0.05%
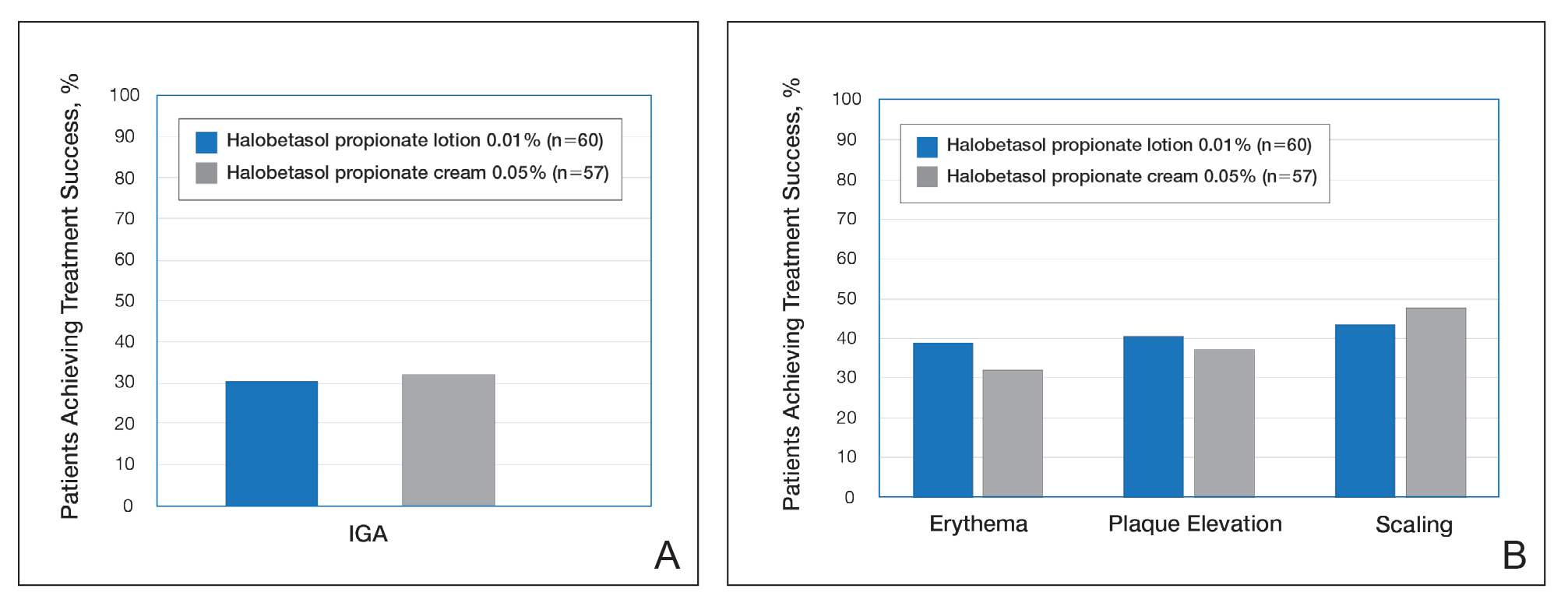
Treatment success with HP lotion 0.01% also was shown to be comparable to the higher-concentration HP cream 0.05% in patients with moderate to severe psoriasis over a 2-week “label-restricted” treatment period (Figure 3). Both products were well tolerated over the 2-week treatment period. One patient reported application-site dermatitis (1.7%) with HP lotion 0.01%.21
Conclusion
Halobetasol propionate 0.05%—cream, ointment, and lotion—has been shown to be a highly effective short-term topical treatment for psoriasis. Longer-term treatment strategies using HP, which are important when considering management of a chronic condition, have been limited by safety concerns and labelling. However, there are data to suggest weekend or pulsed therapy may be an option.
A novel formulation of HP lotion 0.01% has been developed using a polymerized matrix with active ingredients and moisturizing excipients suspended in oil droplets. The polymerized honeycomb matrix and vehicle formulation form a barrier by reducing epidermal water loss and improving skin hydration. The oil droplets deliver uniform amounts of active ingredient in an optimal size for follicular penetration. Skin penetration has been shown to be quicker with greater retention in the epidermis with HP lotion 0.01% compared to HP cream 0.05%, with corresponding considerably lower penetration into the dermis.
Although there have been a number of clinical studies of HP for psoriasis, until recently there have been no comparative trials, with studies label restricted to a 2- to 4-week duration. Three clinical studies with HP lotion 0.01% have now been reported.Not only has HP lotion 0.01% been shown to be as effective as HP cream 0.05% in a 2-week comparative study (despite having one-fifth the concentration of HP), it also has been shown to be very effective and well tolerated following 8 weeks of daily use.20,21 Further studies involving longer treatment durations are required to better elucidate AEs, but HP lotion 0.01% may provide the first longer-term TCS treatment solution for moderate to severe psoriasis.
Acknowledgments
We thank Brian Bulley, MSc (Konic Limited, United Kingdom), for assistance with the preparation of the manuscript. Ortho Dermatologics funded Konic’s activities pertaining to this manuscript.
- Kamili QU, Menter A. Topical treatment of psoriasis. Curr Probl Dermatol. 2009;38:37-58.
- Bailey J, Whitehair B. Topical treatments for chronic plaque psoriasis. Am Fam Physician. 2010;81:596.
- Czarnowicki T, Linkner RV, Suarez-Farinas M, et al. An investigator-initiated, double-blind, vehicle-controlled pilot study: assessment for tachyphylaxis to topically occluded halobetasol 0.05% ointment in the treatment of psoriasis. J Am Acad Dermatol. 2014;71:954-959.
- Bernhard J, Whitmore C, Guzzo C, et al. Evaluation of halobetasol propionate ointment in the treatment of plaque psoriasis: report on two double-blind, vehicle-controlled studies. J Am Acad Dermatol. 1991;25:1170-1174.
- Katz HI, Gross E, Buxman M, et al. A double-blind, vehicle-controlled paired comparison of halobetasol propionate cream on patients with plaque psoriasis. J Am Acad Dermatol. 1991;25:1175-1178.
- Blum G, Yawalkar S. A comparative, multicenter, double blind trial of 0.05% halobetasol propionate ointment and 0.1% betamethasone valerate ointment in the treatment of patients with chronic, localized plaque psoriasis. J Am Acad Dermatol. 1991;25:1153-1156.
- Goldberg B, Hartdegen R, Presbury D, et al. A double-blind, multicenter comparison of 0.05% halobetasol propionate ointment and 0.05% clobetasol propionate ointment in patients with chronic, localized plaque psoriasis. J Am Acad Dermatol. 1991;25:1145-1148.
- Mensing H, Korsukewitz G, Yawalkar S. A double-blind, multicenter comparison between 0.05% halobetasol propionate ointment and 0.05% betamethasone dipropionate ointment in chronic plaque psoriasis. J Am Acad Dermatol. 1991;25:1149-1152.
- Pariser D, Bukhalo M, Guenthner S, et al. Two multicenter, randomized, double-blind, parallel group comparison studies of a novel enhanced lotion formulation of halobetasol propionate, 0.05% versus its vehicle in adult subjects with plaque psoriasis. J Drugs Dermatol. 2017;16:234-240.
- Herz G, Blum G, Yawalkar S. Halobetasol propionate cream by day and halobetasol propionate ointment at night for the treatment of pediatric patients with chronic, localized psoriasis and atopic dermatitis. J Am Acad Dermatol. 1991;25:1166-1169.
- Datz B, Yawalkar S. A double-blind, multicenter trial of 0.05% halobetasol propionate ointment and 0.05% clobetasol 17-propionate ointment in the treatment of patients with chronic, localized atopic dermatitis or lichen simplex chronicus. J Am Acad Dermatol. 1991;25:1157-1160.
- Kantor I, Cook PR, Cullen SI, et al. Double-blind bilateral paired comparison of 0.05% halobetasol propionate cream and its vehicle in patients with chronic atopic dermatitis and other eczematous dermatoses. J Am Acad Dermatol. 1991;25:1184-1186.
- Yawalkar SJ, Schwerzmann L. Double-blind, comparative clinical trials with halobetasol propionate cream in patients with atopic dermatitis. J Am Acad Dermatol. 1991;25:1163-1166.
- Watson WA, Kalb RE, Siskin SB, et al. The safety of halobetasol 0.05% ointment in the treatment of psoriasis. Pharmacotherapy. 1990;10:107-111.
- Dhurat R, Aj K, Vishwanath V, et al. Evaluation of the efficacy and safety of 0.05% halobetasol propionate ointment and 0.05% clobetasol propionate ointment in chronic, localized plaque psoriasis. Asian J Pharm Clin Res. 2016;9:288-291.
- Lebwohl M, Yoles A, Lombardi K, et al. Calcipotriene ointment and halobetasol ointment in the long-term treatment of psoriasis: effects on the duration of improvement. J Am Acad Dermatol. 1998;39:447-450.
- Feldman SR, Horn EJ, Balkrishnan R, et al. Psoriasis: improvingadherence to topical therapy. J Am Acad Dermatol. 2008;59:1009-1016.
- Housman TS, Mellen BG, Rapp SR, et al. Patients with psoriasis prefer solution and foam vehicles: a quantitative assessment of vehicle preference. Cutis. 2002;70:327-332.
- Eastman WJ, Malahias S, Delconte J, et al. Assessing attributes of topical vehicles for the treatment of acne, atopic dermatitis, and plaque psoriasis. Cutis. 2014;94:46-53.
- Green LJ, Kerdel FA, Cook-Bolden FE, et al. Safety and efficacy of halobetasol propionate 0.01% lotion in the treatment of moderate-to-severe plaque psoriasis: results of 2 phase III randomized controlled trials. J Drugs Dermatol. 2018;17:1062-1069.
- Kerdel FA, Draelos ZD, Tyring SK, et al. A phase 2, multicenter, double-blind, randomized, vehicle controlled clinical study to compare the safety and efficacy of halobetasol propionate 0.01% lotion and halobetasol propionate 0.05% cream in the treatment of plaque psoriasis [published online November 5, 2018].J Dermatolog Treat. 2019;30:333-339.
- Lewis V, Finlay AY. 10 years’ experience of the Dermatology Life Quality Index (DLQI). J Investig Dermatol Symp Proc. 2004;9:169-180.
In clinical practice, for the majority of patients with psoriasis superpotent topical corticosteroids (TCSs) are used as initial therapy as well as ongoing breakthrough therapy to achieve quick resolution of target lesions. However, safe and effective long-term treatment and maintenance options are required for managing the chronic nature of psoriasis to improve patient satisfaction, adherence, and quality of life, especially given that package inserts advise no more than 2 to 4 weeks of continuous use to limit side effects. The long-term use of superpotent TCSs can have a multitude of unwanted cutaneous side effects, such as skin atrophy, telangiectases, striae, and allergic vehicle responses.1,2 Tachyphylaxis, a decreased response to treatment over time, has been more controversial and may not occur with halobetasol propionate (HP) ointment 0.05%.3 In addition, TCSs are associated with relapse or rebound on withdrawal, which can be problematic but are poorly characterized.
We review the clinical data on HP, a superpotent TCS, in the treatment of psoriasis. We also explore both recent formulation developments and fixed-combination approaches to providing optimal treatment.
Clinical Experience With HP 0.05% in Various Formulations
Halobetasol propionate is a superpotent TCS with extensive clinical experience in treating psoriasis spanning nearly 30 years.1,2,3-7 Most recently, a twice-daily HP lotion 0.05% formulation was evaluated in patients with moderate to severe disease.8 Halobetasol propionate lotion 0.05% applied morning and night was shown to be significantly more effective than vehicle after 2 weeks of treatment (P<.001) in 2 parallel-group studies of 443 patients.9 Treatment success (ie, at least a 2-grade improvement in investigator global assessment [IGA] and IGA score of clear or almost clear) was achieved in 44.5% of patients treated with HP lotion 0.05% compared to 6.3% and 7.1% in the 2 vehicle arms. Treatment-related adverse events (AEs) were uncommon, with application-site pain reported in 2 patients treated with HP lotion 0.05% compared to 5 patients treated with vehicle.9
Several earlier studies have evaluated the short-term efficacy of twice-daily HP cream 0.05% and HP ointment 0.05% in the treatment of plaque psoriasis, but only 2 placebo-controlled trials have been reported, and data are limited.
Two 2-week studies of twice-daily HP ointment 0.05% (paired-comparison and parallel-group designs) in 204 patients with moderate plaque psoriasis reported improvement in plaque elevation, erythema, and scaling compared to vehicle. Patient global responses and physician global evaluation favored HP ointment 0.05%, and reports of stinging and burning were similar with active treatment and vehicle.4
Similarly, HP cream 0.05% applied twice daily was shown to be significantly superior to vehicle in reducing overall disease severity, erythema, plaque elevation, and scaling after 1 and 2 weeks of treatment in a paired-comparison study of 110 patients (P=.0001).5 A clinically significant reduction (at least a 1-grade improvement) in erythema, plaque elevation, pruritus, and scaling was noted in 81% to 92% of patients (P=.0001). Patients’ self-assessment of effectiveness rated HP cream 0.05% as excellent, very good, or good in 69% of patients compared to 20% for vehicle. Treatment-related AEs were reported by 4 patients.5
A small, noncontrolled, 2-week pediatric study (N=11) demonstrated the efficacy of combined therapy with HP cream 0.05% every morning and HP ointment 0.05% every night due to the then-perceived preference for creams as being more pleasant to apply during the day and ointments being more efficacious. Reported side effects were relatively mild, with application-site burning being the most common.10
Potential local AEs associated with HP are similar to those seen with other superpotent TCSs. Overall, they were reported in 0% to 13% of patients. The most common AEs were burning, pruritus, erythema, hypopigmentation, dryness, and folliculitis.5-8,10-14 Isolated cases of moderate telangiectasia and mild atrophy also have been reported.8,10
Comparative Studies With Other TCSs
In comparative studies of patients with severe localized plaque psoriasis, HP ointment 0.05% applied twice daily for up to 4 weeks was significantly superior compared to clobetasol propionate ointment 0.05% for the number of patients with none or mild disease (P=.0237) or comparisons of global evaluation scores (P=.01315) at week 2, or compared to betamethasone valerate ointment 0.1% (P=.02).6 It also was more effective than betamethasone dipropionate ointment 0.05% with healing seen in 40% of patients treated with HP ointment 0.05% within 24 days compared to 25% of patients treated with betamethasone dipropionate ointment 0.05%.8 Patient acceptance of HP ointment 0.05% based on cosmetic acceptability and ease of application was better (very good in 90% vs 80% of patients7) or significantly better compared to clobetasol propionate ointment 0.05% (P=.042 and P=.01915) and betamethasone dipropionate ointment 0.05% (P=.02).8
Evolving Management Strategies
A number of management strategies have been proposed to improve the safety and efficacy of long-term therapy with TCSs, including weekend-only or pulse therapy, dose reduction, rotating to another therapy, or combining with other topical therapies. Maintenance efficacy data are sparse. A small double-blind study in 44 patients with mild to moderate psoriasis was conducted wherein patients were treated with calcipotriene ointment in the morning and HP ointment in the evening for 2 weeks.16 Those patients who achieved at least a 50% improvement in disease severity (N=40) were randomized to receive HP ointment twice daily on weekends and calcipotriene ointment or placebo twice daily on weekdays for 6 months. Seventy-six percent of those patients treated with a HP/calcipotriene pulsed therapy maintained remission (achieving and maintaining a 75% improvement in physician global assessment) compared to 40% of those patients treated with HP only (P=.045). Mild AEs were reported in 4 patients treated with the combination regimen and 1 patient treated with HP only. No AE-related discontinuations occurred.16
In a real-world setting, a maintenance regimen that is less complicated enhances the potential for increased patient adherence and successful outcomes.17 After an initial 2-week regimen of twice-daily HP ointment 0.05% in combination with ammonium lactate lotion in patients with mild to moderate psoriasis (N=55), those rated clear or almost clear (41/55 [74.6%]) entered a maintenance phase, applying ammonium lactate lotion twice daily and either HP or placebo ointment twice daily on weekends. The probability of disease worsening by week 14 was 29% in the HP-treated group compared to 100% in the placebo group (P<.0001). By week 24, 12 patients (29.2%) remained clear or almost clear.17
Development of HP Lotion 0.01%
There are numerous examples in dermatology where advances in formulation development have made it possible to reduce the strength of active ingredients without compromising efficacy. Formulation advances also afford improved safety profiles that can extend a product’s utility. The vehicle affects not only the potency of an agent but also patient compliance, which is crucial for adequate response. Patients prefer lighter vehicles, such as lotions, over heavy ointments and creams.18,19
Recently, a polymeric honeycomb matrix (carbomer cross-linked polymers), which helps structure the oil emulsion and provide a uniform distribution of both active and moisturizing/hydrating ingredients (ie, sorbitol, light mineral oil, diethyl sebacate) at the surface of the skin, has been deployed for topical delivery of HP (eFigure 1). Ninety percent of the oil droplets containing solubilized halobetasol are 13 µm or smaller, an ideal size for penetration through follicular openings (unpublished data, Bausch Health, 2018).
This polymerized emulsion also forms a barrier by reducing epidermal water loss and improving skin hydration. Skin hydration and barrier protection of the lotion were assessed through corneometry and transepidermal water loss (TEWL) in 30 healthy female volunteers (aged 35–65 years) over 24 hours. The test material was applied to the volar forearm, with an untreated site serving as a control. Measurements using Tewameter and Corneometer were taken at baseline; 15 and 30 minutes; and 1, 2, 3, 8, and 24 hours postapplication. In addition, for the 8-hour study period, 15 patients applied the test material to the right side of the face and completed a customer-perception evaluation. Adverse events were noted throughout and irritation was assessed preapplication and postapplication. There were no AEs or skin irritation reported throughout the study. At baseline, mean (standard deviation [SD]) corneometry scores were 28.9 (2.9) and 28.1 (2.7) units for the test material and untreated control, respectively. There was an immediate improvement in water content that was maintained throughout the study. After 15 minutes, the mean (SD) score had increased to 59.1 (7.1) units in the vehicle lotion group (eFigure 2A). There was no improvement at the control site, and differences were significant at all postapplication assessments (P<.001). At baseline, mean (SD) TEWL scores were 12.26 (0.48) and 12.42 (0.44) g/hm2, respectively (eFigure 2B). There was an immediate improvement in TEWL with a mean (SD) score of 6.04 (0.99) after 8 hours in the vehicle lotion group, a 50.7% change over baseline. There was no improvement at the control site, and differences were significant at all postapplication assessments (P<.001). Customer perception of the novel lotion formulation was positive, with the majority of patients (93%–100%) responding favorably to all questions about the various attributes of the test material (eFigure 3)(unpublished data, Bausch Health, 2018).
Comparison of Skin Penetration of HP Lotion 0.01% vs HP Cream 0.05%
Comparative percutaneous absorption of 2 HP formulations—0.01% lotion and 0.05% cream—was evaluated in vitro using human tissue from a single donor mounted on Bronaugh flow-through diffusion cells. Receptor phase samples were collected over the 24-hour study period and HP content assessed using liquid chromatography–mass spectrometry analysis. Halobetasol propionate lotion 0.01% demonstrated faster tissue permeation, with receptor phase levels of 0.91% of the applied dose at 24 hours compared to 0.28% of the applied dose with HP cream 0.05%. Although there was little differentiation of cumulative receptor fluid levels of HP at 6 hours, there was significant differentiation at 12 hours. Levels of HP were lowest in the receptor phase and highest in the epidermal layers of the skin, indicating limited permeation through the epidermis to the dermis. The mean (SD) for epidermal deposition of HP following the 24-hour duration of exposure was 6.17% (2.07%) and 1.72% (0.76%) for the 0.01% lotion and 0.05% cream, respectively (Figure 1)(unpublished data, Bausch Health, 2018).
Efficacy and Safety of HP Lotion 0.01% in Moderate to Severe Plaque Psoriasis
Two articles have been published on the use of HP lotion 0.01% in moderate to severe psoriasis: 2 pivotal studies comparing once-daily application with vehicle lotion over 8 weeks (N=430),20 and a comparative “label-restricted” 2-week study with HP lotion 0.01% and HP cream 0.05% (N=150).21
HP Lotion 0.01% Compared to Vehicle
Two multicenter, randomized, double-blind, vehicle-controlled phase 3 studies investigated the safety and efficacy of once-daily HP lotion 0.01% in moderate to severe plaque psoriasis (N=430).20 Patients were treated with HP lotion 0.01% or vehicle (randomized in a 2:1 ratio) for 8 weeks, with a 4-week posttreatment follow-up. Treatment success (defined as at least a 2-grade improvement in baseline IGA score and a score equating to clear or almost clear) was significantly greater with HP lotion 0.01% at all assessment points (Figure 2)(P=.003 for week 2; P<.001 for other time points). At week 8, 37.4% of patients receiving HP lotion 0.01% were treatment successes compared to 10.0% of patients receiving vehicle (P<.001). Additionally, a 2-grade improvement from baseline for each psoriasis sign—erythema, plaque elevation, and scaling—was achieved by 42.2% of patients receiving HP lotion 0.01% at week 8 compared to 11.4% of patients receiving vehicle (P<.001). Good efficacy was maintained posttreatment that was significant compared to vehicle (P<.001).20
There were corresponding reductions in body surface area (BSA) affected following treatment with HP lotion 0.01%.20 At baseline, the mean BSA was 6.1 (range, 3–12). By week 8, there was a 35.2% reduction in BSA compared to 5.9% with vehicle. Again, a significant reduction in BSA was maintained posttreatment compared to vehicle (P<.001).20
Halobetasol propionate lotion 0.01% was well tolerated with few treatment-related AEs.20 Most AEs were application-site reactions such as dermatitis (0.7%), infection, pruritus, and discoloration (0.4% each). Mild to moderate itching, dryness, burning, and stinging present at baseline all improved with treatment, and severity of local skin reactions was significantly lower than with vehicle at week 8 (P<.001). Quality-of-life data also highlighted the benefits of active treatment compared to vehicle for cutaneous tolerability. The Dermatology Life Quality Index (DLQI) is a 10-item patient-reported questionnaire consisting of questions concerning symptoms and feelings, daily activities, leisure, work and school, personal relationships, and treatment.22 Change from baseline for DLQI (how itchy, sore, painful, stinging) was significantly greater with HP lotion 0.01% at weeks 4 and 8 (P<.001). Changes in the overall DLQI score also were significantly greater with HP lotion 0.01% at both study visits (P=.006 and P=.014 at week 4 and P=.001 and P=.004 at week 8 for study 1 and study 2, respectively).20
HP Lotion 0.01% Compared to HP Cream 0.05%
Treatment success with HP lotion 0.01% also was shown to be comparable to the higher-concentration HP cream 0.05% in patients with moderate to severe psoriasis over a 2-week “label-restricted” treatment period (Figure 3). Both products were well tolerated over the 2-week treatment period. One patient reported application-site dermatitis (1.7%) with HP lotion 0.01%.21
Conclusion
Halobetasol propionate 0.05%—cream, ointment, and lotion—has been shown to be a highly effective short-term topical treatment for psoriasis. Longer-term treatment strategies using HP, which are important when considering management of a chronic condition, have been limited by safety concerns and labelling. However, there are data to suggest weekend or pulsed therapy may be an option.
A novel formulation of HP lotion 0.01% has been developed using a polymerized matrix with active ingredients and moisturizing excipients suspended in oil droplets. The polymerized honeycomb matrix and vehicle formulation form a barrier by reducing epidermal water loss and improving skin hydration. The oil droplets deliver uniform amounts of active ingredient in an optimal size for follicular penetration. Skin penetration has been shown to be quicker with greater retention in the epidermis with HP lotion 0.01% compared to HP cream 0.05%, with corresponding considerably lower penetration into the dermis.
Although there have been a number of clinical studies of HP for psoriasis, until recently there have been no comparative trials, with studies label restricted to a 2- to 4-week duration. Three clinical studies with HP lotion 0.01% have now been reported.Not only has HP lotion 0.01% been shown to be as effective as HP cream 0.05% in a 2-week comparative study (despite having one-fifth the concentration of HP), it also has been shown to be very effective and well tolerated following 8 weeks of daily use.20,21 Further studies involving longer treatment durations are required to better elucidate AEs, but HP lotion 0.01% may provide the first longer-term TCS treatment solution for moderate to severe psoriasis.
Acknowledgments
We thank Brian Bulley, MSc (Konic Limited, United Kingdom), for assistance with the preparation of the manuscript. Ortho Dermatologics funded Konic’s activities pertaining to this manuscript.
In clinical practice, for the majority of patients with psoriasis superpotent topical corticosteroids (TCSs) are used as initial therapy as well as ongoing breakthrough therapy to achieve quick resolution of target lesions. However, safe and effective long-term treatment and maintenance options are required for managing the chronic nature of psoriasis to improve patient satisfaction, adherence, and quality of life, especially given that package inserts advise no more than 2 to 4 weeks of continuous use to limit side effects. The long-term use of superpotent TCSs can have a multitude of unwanted cutaneous side effects, such as skin atrophy, telangiectases, striae, and allergic vehicle responses.1,2 Tachyphylaxis, a decreased response to treatment over time, has been more controversial and may not occur with halobetasol propionate (HP) ointment 0.05%.3 In addition, TCSs are associated with relapse or rebound on withdrawal, which can be problematic but are poorly characterized.
We review the clinical data on HP, a superpotent TCS, in the treatment of psoriasis. We also explore both recent formulation developments and fixed-combination approaches to providing optimal treatment.
Clinical Experience With HP 0.05% in Various Formulations
Halobetasol propionate is a superpotent TCS with extensive clinical experience in treating psoriasis spanning nearly 30 years.1,2,3-7 Most recently, a twice-daily HP lotion 0.05% formulation was evaluated in patients with moderate to severe disease.8 Halobetasol propionate lotion 0.05% applied morning and night was shown to be significantly more effective than vehicle after 2 weeks of treatment (P<.001) in 2 parallel-group studies of 443 patients.9 Treatment success (ie, at least a 2-grade improvement in investigator global assessment [IGA] and IGA score of clear or almost clear) was achieved in 44.5% of patients treated with HP lotion 0.05% compared to 6.3% and 7.1% in the 2 vehicle arms. Treatment-related adverse events (AEs) were uncommon, with application-site pain reported in 2 patients treated with HP lotion 0.05% compared to 5 patients treated with vehicle.9
Several earlier studies have evaluated the short-term efficacy of twice-daily HP cream 0.05% and HP ointment 0.05% in the treatment of plaque psoriasis, but only 2 placebo-controlled trials have been reported, and data are limited.
Two 2-week studies of twice-daily HP ointment 0.05% (paired-comparison and parallel-group designs) in 204 patients with moderate plaque psoriasis reported improvement in plaque elevation, erythema, and scaling compared to vehicle. Patient global responses and physician global evaluation favored HP ointment 0.05%, and reports of stinging and burning were similar with active treatment and vehicle.4
Similarly, HP cream 0.05% applied twice daily was shown to be significantly superior to vehicle in reducing overall disease severity, erythema, plaque elevation, and scaling after 1 and 2 weeks of treatment in a paired-comparison study of 110 patients (P=.0001).5 A clinically significant reduction (at least a 1-grade improvement) in erythema, plaque elevation, pruritus, and scaling was noted in 81% to 92% of patients (P=.0001). Patients’ self-assessment of effectiveness rated HP cream 0.05% as excellent, very good, or good in 69% of patients compared to 20% for vehicle. Treatment-related AEs were reported by 4 patients.5
A small, noncontrolled, 2-week pediatric study (N=11) demonstrated the efficacy of combined therapy with HP cream 0.05% every morning and HP ointment 0.05% every night due to the then-perceived preference for creams as being more pleasant to apply during the day and ointments being more efficacious. Reported side effects were relatively mild, with application-site burning being the most common.10
Potential local AEs associated with HP are similar to those seen with other superpotent TCSs. Overall, they were reported in 0% to 13% of patients. The most common AEs were burning, pruritus, erythema, hypopigmentation, dryness, and folliculitis.5-8,10-14 Isolated cases of moderate telangiectasia and mild atrophy also have been reported.8,10
Comparative Studies With Other TCSs
In comparative studies of patients with severe localized plaque psoriasis, HP ointment 0.05% applied twice daily for up to 4 weeks was significantly superior compared to clobetasol propionate ointment 0.05% for the number of patients with none or mild disease (P=.0237) or comparisons of global evaluation scores (P=.01315) at week 2, or compared to betamethasone valerate ointment 0.1% (P=.02).6 It also was more effective than betamethasone dipropionate ointment 0.05% with healing seen in 40% of patients treated with HP ointment 0.05% within 24 days compared to 25% of patients treated with betamethasone dipropionate ointment 0.05%.8 Patient acceptance of HP ointment 0.05% based on cosmetic acceptability and ease of application was better (very good in 90% vs 80% of patients7) or significantly better compared to clobetasol propionate ointment 0.05% (P=.042 and P=.01915) and betamethasone dipropionate ointment 0.05% (P=.02).8
Evolving Management Strategies
A number of management strategies have been proposed to improve the safety and efficacy of long-term therapy with TCSs, including weekend-only or pulse therapy, dose reduction, rotating to another therapy, or combining with other topical therapies. Maintenance efficacy data are sparse. A small double-blind study in 44 patients with mild to moderate psoriasis was conducted wherein patients were treated with calcipotriene ointment in the morning and HP ointment in the evening for 2 weeks.16 Those patients who achieved at least a 50% improvement in disease severity (N=40) were randomized to receive HP ointment twice daily on weekends and calcipotriene ointment or placebo twice daily on weekdays for 6 months. Seventy-six percent of those patients treated with a HP/calcipotriene pulsed therapy maintained remission (achieving and maintaining a 75% improvement in physician global assessment) compared to 40% of those patients treated with HP only (P=.045). Mild AEs were reported in 4 patients treated with the combination regimen and 1 patient treated with HP only. No AE-related discontinuations occurred.16
In a real-world setting, a maintenance regimen that is less complicated enhances the potential for increased patient adherence and successful outcomes.17 After an initial 2-week regimen of twice-daily HP ointment 0.05% in combination with ammonium lactate lotion in patients with mild to moderate psoriasis (N=55), those rated clear or almost clear (41/55 [74.6%]) entered a maintenance phase, applying ammonium lactate lotion twice daily and either HP or placebo ointment twice daily on weekends. The probability of disease worsening by week 14 was 29% in the HP-treated group compared to 100% in the placebo group (P<.0001). By week 24, 12 patients (29.2%) remained clear or almost clear.17
Development of HP Lotion 0.01%
There are numerous examples in dermatology where advances in formulation development have made it possible to reduce the strength of active ingredients without compromising efficacy. Formulation advances also afford improved safety profiles that can extend a product’s utility. The vehicle affects not only the potency of an agent but also patient compliance, which is crucial for adequate response. Patients prefer lighter vehicles, such as lotions, over heavy ointments and creams.18,19
Recently, a polymeric honeycomb matrix (carbomer cross-linked polymers), which helps structure the oil emulsion and provide a uniform distribution of both active and moisturizing/hydrating ingredients (ie, sorbitol, light mineral oil, diethyl sebacate) at the surface of the skin, has been deployed for topical delivery of HP (eFigure 1). Ninety percent of the oil droplets containing solubilized halobetasol are 13 µm or smaller, an ideal size for penetration through follicular openings (unpublished data, Bausch Health, 2018).
This polymerized emulsion also forms a barrier by reducing epidermal water loss and improving skin hydration. Skin hydration and barrier protection of the lotion were assessed through corneometry and transepidermal water loss (TEWL) in 30 healthy female volunteers (aged 35–65 years) over 24 hours. The test material was applied to the volar forearm, with an untreated site serving as a control. Measurements using Tewameter and Corneometer were taken at baseline; 15 and 30 minutes; and 1, 2, 3, 8, and 24 hours postapplication. In addition, for the 8-hour study period, 15 patients applied the test material to the right side of the face and completed a customer-perception evaluation. Adverse events were noted throughout and irritation was assessed preapplication and postapplication. There were no AEs or skin irritation reported throughout the study. At baseline, mean (standard deviation [SD]) corneometry scores were 28.9 (2.9) and 28.1 (2.7) units for the test material and untreated control, respectively. There was an immediate improvement in water content that was maintained throughout the study. After 15 minutes, the mean (SD) score had increased to 59.1 (7.1) units in the vehicle lotion group (eFigure 2A). There was no improvement at the control site, and differences were significant at all postapplication assessments (P<.001). At baseline, mean (SD) TEWL scores were 12.26 (0.48) and 12.42 (0.44) g/hm2, respectively (eFigure 2B). There was an immediate improvement in TEWL with a mean (SD) score of 6.04 (0.99) after 8 hours in the vehicle lotion group, a 50.7% change over baseline. There was no improvement at the control site, and differences were significant at all postapplication assessments (P<.001). Customer perception of the novel lotion formulation was positive, with the majority of patients (93%–100%) responding favorably to all questions about the various attributes of the test material (eFigure 3)(unpublished data, Bausch Health, 2018).
Comparison of Skin Penetration of HP Lotion 0.01% vs HP Cream 0.05%
Comparative percutaneous absorption of 2 HP formulations—0.01% lotion and 0.05% cream—was evaluated in vitro using human tissue from a single donor mounted on Bronaugh flow-through diffusion cells. Receptor phase samples were collected over the 24-hour study period and HP content assessed using liquid chromatography–mass spectrometry analysis. Halobetasol propionate lotion 0.01% demonstrated faster tissue permeation, with receptor phase levels of 0.91% of the applied dose at 24 hours compared to 0.28% of the applied dose with HP cream 0.05%. Although there was little differentiation of cumulative receptor fluid levels of HP at 6 hours, there was significant differentiation at 12 hours. Levels of HP were lowest in the receptor phase and highest in the epidermal layers of the skin, indicating limited permeation through the epidermis to the dermis. The mean (SD) for epidermal deposition of HP following the 24-hour duration of exposure was 6.17% (2.07%) and 1.72% (0.76%) for the 0.01% lotion and 0.05% cream, respectively (Figure 1)(unpublished data, Bausch Health, 2018).
Efficacy and Safety of HP Lotion 0.01% in Moderate to Severe Plaque Psoriasis
Two articles have been published on the use of HP lotion 0.01% in moderate to severe psoriasis: 2 pivotal studies comparing once-daily application with vehicle lotion over 8 weeks (N=430),20 and a comparative “label-restricted” 2-week study with HP lotion 0.01% and HP cream 0.05% (N=150).21
HP Lotion 0.01% Compared to Vehicle
Two multicenter, randomized, double-blind, vehicle-controlled phase 3 studies investigated the safety and efficacy of once-daily HP lotion 0.01% in moderate to severe plaque psoriasis (N=430).20 Patients were treated with HP lotion 0.01% or vehicle (randomized in a 2:1 ratio) for 8 weeks, with a 4-week posttreatment follow-up. Treatment success (defined as at least a 2-grade improvement in baseline IGA score and a score equating to clear or almost clear) was significantly greater with HP lotion 0.01% at all assessment points (Figure 2)(P=.003 for week 2; P<.001 for other time points). At week 8, 37.4% of patients receiving HP lotion 0.01% were treatment successes compared to 10.0% of patients receiving vehicle (P<.001). Additionally, a 2-grade improvement from baseline for each psoriasis sign—erythema, plaque elevation, and scaling—was achieved by 42.2% of patients receiving HP lotion 0.01% at week 8 compared to 11.4% of patients receiving vehicle (P<.001). Good efficacy was maintained posttreatment that was significant compared to vehicle (P<.001).20
There were corresponding reductions in body surface area (BSA) affected following treatment with HP lotion 0.01%.20 At baseline, the mean BSA was 6.1 (range, 3–12). By week 8, there was a 35.2% reduction in BSA compared to 5.9% with vehicle. Again, a significant reduction in BSA was maintained posttreatment compared to vehicle (P<.001).20
Halobetasol propionate lotion 0.01% was well tolerated with few treatment-related AEs.20 Most AEs were application-site reactions such as dermatitis (0.7%), infection, pruritus, and discoloration (0.4% each). Mild to moderate itching, dryness, burning, and stinging present at baseline all improved with treatment, and severity of local skin reactions was significantly lower than with vehicle at week 8 (P<.001). Quality-of-life data also highlighted the benefits of active treatment compared to vehicle for cutaneous tolerability. The Dermatology Life Quality Index (DLQI) is a 10-item patient-reported questionnaire consisting of questions concerning symptoms and feelings, daily activities, leisure, work and school, personal relationships, and treatment.22 Change from baseline for DLQI (how itchy, sore, painful, stinging) was significantly greater with HP lotion 0.01% at weeks 4 and 8 (P<.001). Changes in the overall DLQI score also were significantly greater with HP lotion 0.01% at both study visits (P=.006 and P=.014 at week 4 and P=.001 and P=.004 at week 8 for study 1 and study 2, respectively).20
HP Lotion 0.01% Compared to HP Cream 0.05%
Treatment success with HP lotion 0.01% also was shown to be comparable to the higher-concentration HP cream 0.05% in patients with moderate to severe psoriasis over a 2-week “label-restricted” treatment period (Figure 3). Both products were well tolerated over the 2-week treatment period. One patient reported application-site dermatitis (1.7%) with HP lotion 0.01%.21
Conclusion
Halobetasol propionate 0.05%—cream, ointment, and lotion—has been shown to be a highly effective short-term topical treatment for psoriasis. Longer-term treatment strategies using HP, which are important when considering management of a chronic condition, have been limited by safety concerns and labelling. However, there are data to suggest weekend or pulsed therapy may be an option.
A novel formulation of HP lotion 0.01% has been developed using a polymerized matrix with active ingredients and moisturizing excipients suspended in oil droplets. The polymerized honeycomb matrix and vehicle formulation form a barrier by reducing epidermal water loss and improving skin hydration. The oil droplets deliver uniform amounts of active ingredient in an optimal size for follicular penetration. Skin penetration has been shown to be quicker with greater retention in the epidermis with HP lotion 0.01% compared to HP cream 0.05%, with corresponding considerably lower penetration into the dermis.
Although there have been a number of clinical studies of HP for psoriasis, until recently there have been no comparative trials, with studies label restricted to a 2- to 4-week duration. Three clinical studies with HP lotion 0.01% have now been reported.Not only has HP lotion 0.01% been shown to be as effective as HP cream 0.05% in a 2-week comparative study (despite having one-fifth the concentration of HP), it also has been shown to be very effective and well tolerated following 8 weeks of daily use.20,21 Further studies involving longer treatment durations are required to better elucidate AEs, but HP lotion 0.01% may provide the first longer-term TCS treatment solution for moderate to severe psoriasis.
Acknowledgments
We thank Brian Bulley, MSc (Konic Limited, United Kingdom), for assistance with the preparation of the manuscript. Ortho Dermatologics funded Konic’s activities pertaining to this manuscript.
- Kamili QU, Menter A. Topical treatment of psoriasis. Curr Probl Dermatol. 2009;38:37-58.
- Bailey J, Whitehair B. Topical treatments for chronic plaque psoriasis. Am Fam Physician. 2010;81:596.
- Czarnowicki T, Linkner RV, Suarez-Farinas M, et al. An investigator-initiated, double-blind, vehicle-controlled pilot study: assessment for tachyphylaxis to topically occluded halobetasol 0.05% ointment in the treatment of psoriasis. J Am Acad Dermatol. 2014;71:954-959.
- Bernhard J, Whitmore C, Guzzo C, et al. Evaluation of halobetasol propionate ointment in the treatment of plaque psoriasis: report on two double-blind, vehicle-controlled studies. J Am Acad Dermatol. 1991;25:1170-1174.
- Katz HI, Gross E, Buxman M, et al. A double-blind, vehicle-controlled paired comparison of halobetasol propionate cream on patients with plaque psoriasis. J Am Acad Dermatol. 1991;25:1175-1178.
- Blum G, Yawalkar S. A comparative, multicenter, double blind trial of 0.05% halobetasol propionate ointment and 0.1% betamethasone valerate ointment in the treatment of patients with chronic, localized plaque psoriasis. J Am Acad Dermatol. 1991;25:1153-1156.
- Goldberg B, Hartdegen R, Presbury D, et al. A double-blind, multicenter comparison of 0.05% halobetasol propionate ointment and 0.05% clobetasol propionate ointment in patients with chronic, localized plaque psoriasis. J Am Acad Dermatol. 1991;25:1145-1148.
- Mensing H, Korsukewitz G, Yawalkar S. A double-blind, multicenter comparison between 0.05% halobetasol propionate ointment and 0.05% betamethasone dipropionate ointment in chronic plaque psoriasis. J Am Acad Dermatol. 1991;25:1149-1152.
- Pariser D, Bukhalo M, Guenthner S, et al. Two multicenter, randomized, double-blind, parallel group comparison studies of a novel enhanced lotion formulation of halobetasol propionate, 0.05% versus its vehicle in adult subjects with plaque psoriasis. J Drugs Dermatol. 2017;16:234-240.
- Herz G, Blum G, Yawalkar S. Halobetasol propionate cream by day and halobetasol propionate ointment at night for the treatment of pediatric patients with chronic, localized psoriasis and atopic dermatitis. J Am Acad Dermatol. 1991;25:1166-1169.
- Datz B, Yawalkar S. A double-blind, multicenter trial of 0.05% halobetasol propionate ointment and 0.05% clobetasol 17-propionate ointment in the treatment of patients with chronic, localized atopic dermatitis or lichen simplex chronicus. J Am Acad Dermatol. 1991;25:1157-1160.
- Kantor I, Cook PR, Cullen SI, et al. Double-blind bilateral paired comparison of 0.05% halobetasol propionate cream and its vehicle in patients with chronic atopic dermatitis and other eczematous dermatoses. J Am Acad Dermatol. 1991;25:1184-1186.
- Yawalkar SJ, Schwerzmann L. Double-blind, comparative clinical trials with halobetasol propionate cream in patients with atopic dermatitis. J Am Acad Dermatol. 1991;25:1163-1166.
- Watson WA, Kalb RE, Siskin SB, et al. The safety of halobetasol 0.05% ointment in the treatment of psoriasis. Pharmacotherapy. 1990;10:107-111.
- Dhurat R, Aj K, Vishwanath V, et al. Evaluation of the efficacy and safety of 0.05% halobetasol propionate ointment and 0.05% clobetasol propionate ointment in chronic, localized plaque psoriasis. Asian J Pharm Clin Res. 2016;9:288-291.
- Lebwohl M, Yoles A, Lombardi K, et al. Calcipotriene ointment and halobetasol ointment in the long-term treatment of psoriasis: effects on the duration of improvement. J Am Acad Dermatol. 1998;39:447-450.
- Feldman SR, Horn EJ, Balkrishnan R, et al. Psoriasis: improvingadherence to topical therapy. J Am Acad Dermatol. 2008;59:1009-1016.
- Housman TS, Mellen BG, Rapp SR, et al. Patients with psoriasis prefer solution and foam vehicles: a quantitative assessment of vehicle preference. Cutis. 2002;70:327-332.
- Eastman WJ, Malahias S, Delconte J, et al. Assessing attributes of topical vehicles for the treatment of acne, atopic dermatitis, and plaque psoriasis. Cutis. 2014;94:46-53.
- Green LJ, Kerdel FA, Cook-Bolden FE, et al. Safety and efficacy of halobetasol propionate 0.01% lotion in the treatment of moderate-to-severe plaque psoriasis: results of 2 phase III randomized controlled trials. J Drugs Dermatol. 2018;17:1062-1069.
- Kerdel FA, Draelos ZD, Tyring SK, et al. A phase 2, multicenter, double-blind, randomized, vehicle controlled clinical study to compare the safety and efficacy of halobetasol propionate 0.01% lotion and halobetasol propionate 0.05% cream in the treatment of plaque psoriasis [published online November 5, 2018].J Dermatolog Treat. 2019;30:333-339.
- Lewis V, Finlay AY. 10 years’ experience of the Dermatology Life Quality Index (DLQI). J Investig Dermatol Symp Proc. 2004;9:169-180.
- Kamili QU, Menter A. Topical treatment of psoriasis. Curr Probl Dermatol. 2009;38:37-58.
- Bailey J, Whitehair B. Topical treatments for chronic plaque psoriasis. Am Fam Physician. 2010;81:596.
- Czarnowicki T, Linkner RV, Suarez-Farinas M, et al. An investigator-initiated, double-blind, vehicle-controlled pilot study: assessment for tachyphylaxis to topically occluded halobetasol 0.05% ointment in the treatment of psoriasis. J Am Acad Dermatol. 2014;71:954-959.
- Bernhard J, Whitmore C, Guzzo C, et al. Evaluation of halobetasol propionate ointment in the treatment of plaque psoriasis: report on two double-blind, vehicle-controlled studies. J Am Acad Dermatol. 1991;25:1170-1174.
- Katz HI, Gross E, Buxman M, et al. A double-blind, vehicle-controlled paired comparison of halobetasol propionate cream on patients with plaque psoriasis. J Am Acad Dermatol. 1991;25:1175-1178.
- Blum G, Yawalkar S. A comparative, multicenter, double blind trial of 0.05% halobetasol propionate ointment and 0.1% betamethasone valerate ointment in the treatment of patients with chronic, localized plaque psoriasis. J Am Acad Dermatol. 1991;25:1153-1156.
- Goldberg B, Hartdegen R, Presbury D, et al. A double-blind, multicenter comparison of 0.05% halobetasol propionate ointment and 0.05% clobetasol propionate ointment in patients with chronic, localized plaque psoriasis. J Am Acad Dermatol. 1991;25:1145-1148.
- Mensing H, Korsukewitz G, Yawalkar S. A double-blind, multicenter comparison between 0.05% halobetasol propionate ointment and 0.05% betamethasone dipropionate ointment in chronic plaque psoriasis. J Am Acad Dermatol. 1991;25:1149-1152.
- Pariser D, Bukhalo M, Guenthner S, et al. Two multicenter, randomized, double-blind, parallel group comparison studies of a novel enhanced lotion formulation of halobetasol propionate, 0.05% versus its vehicle in adult subjects with plaque psoriasis. J Drugs Dermatol. 2017;16:234-240.
- Herz G, Blum G, Yawalkar S. Halobetasol propionate cream by day and halobetasol propionate ointment at night for the treatment of pediatric patients with chronic, localized psoriasis and atopic dermatitis. J Am Acad Dermatol. 1991;25:1166-1169.
- Datz B, Yawalkar S. A double-blind, multicenter trial of 0.05% halobetasol propionate ointment and 0.05% clobetasol 17-propionate ointment in the treatment of patients with chronic, localized atopic dermatitis or lichen simplex chronicus. J Am Acad Dermatol. 1991;25:1157-1160.
- Kantor I, Cook PR, Cullen SI, et al. Double-blind bilateral paired comparison of 0.05% halobetasol propionate cream and its vehicle in patients with chronic atopic dermatitis and other eczematous dermatoses. J Am Acad Dermatol. 1991;25:1184-1186.
- Yawalkar SJ, Schwerzmann L. Double-blind, comparative clinical trials with halobetasol propionate cream in patients with atopic dermatitis. J Am Acad Dermatol. 1991;25:1163-1166.
- Watson WA, Kalb RE, Siskin SB, et al. The safety of halobetasol 0.05% ointment in the treatment of psoriasis. Pharmacotherapy. 1990;10:107-111.
- Dhurat R, Aj K, Vishwanath V, et al. Evaluation of the efficacy and safety of 0.05% halobetasol propionate ointment and 0.05% clobetasol propionate ointment in chronic, localized plaque psoriasis. Asian J Pharm Clin Res. 2016;9:288-291.
- Lebwohl M, Yoles A, Lombardi K, et al. Calcipotriene ointment and halobetasol ointment in the long-term treatment of psoriasis: effects on the duration of improvement. J Am Acad Dermatol. 1998;39:447-450.
- Feldman SR, Horn EJ, Balkrishnan R, et al. Psoriasis: improvingadherence to topical therapy. J Am Acad Dermatol. 2008;59:1009-1016.
- Housman TS, Mellen BG, Rapp SR, et al. Patients with psoriasis prefer solution and foam vehicles: a quantitative assessment of vehicle preference. Cutis. 2002;70:327-332.
- Eastman WJ, Malahias S, Delconte J, et al. Assessing attributes of topical vehicles for the treatment of acne, atopic dermatitis, and plaque psoriasis. Cutis. 2014;94:46-53.
- Green LJ, Kerdel FA, Cook-Bolden FE, et al. Safety and efficacy of halobetasol propionate 0.01% lotion in the treatment of moderate-to-severe plaque psoriasis: results of 2 phase III randomized controlled trials. J Drugs Dermatol. 2018;17:1062-1069.
- Kerdel FA, Draelos ZD, Tyring SK, et al. A phase 2, multicenter, double-blind, randomized, vehicle controlled clinical study to compare the safety and efficacy of halobetasol propionate 0.01% lotion and halobetasol propionate 0.05% cream in the treatment of plaque psoriasis [published online November 5, 2018].J Dermatolog Treat. 2019;30:333-339.
- Lewis V, Finlay AY. 10 years’ experience of the Dermatology Life Quality Index (DLQI). J Investig Dermatol Symp Proc. 2004;9:169-180.
Practice Points
- The widespread use of superpotent topical corticosteroids in treating psoriasis is limited by labelling that restricts short-term use, concerns about side effects, and a paucity of clinical data with longer-term use.
- Long-term management and treatment options are required for managing the chronic nature of psoriasis to improve patient satisfaction, adherence, and quality of life.
- A novel formulation of halobetasol propionate lotion 0.01% has been developed using a polymerized matrix with active ingredients and moisturizing excipients suspended in oil droplets.